Note: Descriptions are shown in the official language in which they were submitted.
CA 03230936 2024-03-01
Rapidly disintegrating oral thin-films/foams having a high active-ingredient
content
based on a mixture of polyvinyl alcohols having various molecular weights
Description
The present invention relates to an oral thin film containing at least one
active
pharmaceutical ingredient and to the use of such an oral thin film as a drug.
Oral thin films (OTFs) are thin, flexible films based on a polymer matrix and
loaded
with active ingredients for drug delivery. The oral thin films are taken
orally and
dissolve immediately in the mouth or are applied to the mucosa. They are
placed on
or under the tongue or buccally, where they dissolve or disintegrate.
Oral thin films can be formed as non-foamed films or as foamed films or foam.
OTFs
are sometimes also referred to as oral thin films.
EP 422100 B1 discloses the active ingredient ulipristal acetate. Ulipristal
acetate is a
well-known emergency contraceptive ("morning-after pill"). It is administered
as a
tablet ("EllaOneC)") containing 30 mg micronized ulipristal acetate, and as
further
ingredients lactose monohydrate, povidone, croscarmellose sodium, and
magnesium
stearate. EllaOneC) was approved in the European Union in 2009.
Ketamine is another known active pharmaceutical ingredient that is used more
particularly for the treatment or prevention of pain.
For the administration of some active pharmaceutical ingredients, high drug
loadings of the oral thin film are desirable, e.g. oral thin films with
ulipristal acetate
as the active ingredient with 30 mg per single dose. However, the problem with
such oral thin films or oral foams with high drug loading per single dose is
that
comparatively large amounts of polymer are required to produce a film or foam
that
has acceptable flexibility, so that brittle films are avoided. However, the
high
polymer content leads to an increase in the thickness or basis weight of the
films or
foams. As a result, these formulations exhibit increased disintegration times.
This is
undesirable for films or foams that are supposed to disintegrate very quickly
in the
mouth.
1
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
In the first foam formulations tested, which contained ulipristal acetate as
the active
ingredient and were based on polyvinyl alcohol (PVA) with low molecular weight
(PVA 4-88, MW approx. 31,000 Da), the content of PVA was 63.9%. With this
concentration, it was possible to produce an ulipristal acetate foam with good
haptic
properties (see formulation Al in the experimental section). However, as
described
above, the high polymer concentration led to an increase of the basis weight
(180 g/m2) and the disintegration time.
In addition, known oral thin films with high active ingredient loading have
the
disadvantage that the maximum basis weight and thus the amount of active
pharmaceutical ingredient contained therein is determined by the drying of the
oral
thin film during its manufacture. The higher the basis weight of the oral thin
film,
the more active pharmaceutical ingredient it may contain, but this prolongs
the
drying time of the oral thin film to a time that is no longer economically
acceptable
and can also lead to an inhomogeneous distribution of the active ingredient in
the
oral thin film.
The object of the present invention is to remove the above-mentioned
disadvantages. More particularly, the object of the present invention is to
provide
flexible and stable oral thin films/foams which allow high drug loading while
having
an acceptable film thickness and a rapid disintegration time on application.
This object was surprisingly achieved according to claim 1 by using a mixture
of
polyvinyl alcohols with different molecular weights in the matrix layer. An
additional
improvement was achieved by adding humectants, more particularly certain sugar
alcohols or starch derivatives.
Accordingly, the invention relates to an oral thin film comprising at least
one matrix
layer, wherein the at least one matrix layer comprises at least one polyvinyl
alcohol
having a lower average molecular weight of 10,000 to about 75,000 g/mol,
preferably 10,000 to about 40,000 g/mol, and at least one polyvinyl alcohol
having a
higher average molecular weight of 140,000 to 300,000 g/mol, preferably
140,000
to 220,000 g/mol, and at least one active pharmaceutical ingredient, wherein
said at
least one polyvinyl alcohol having a lower average molecular weight and said
at
least one polyvinyl alcohol having a higher average molecular weight are
present in
the matrix layer in a total amount of 15 to 70 wt%, preferably from 15 to 65
wt%,
based on the total weight of the matrix layer, and the weight ratio of said at
least
one polyvinyl alcohol having a lower average molecular weight to said at least
one
2
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
polyvinyl alcohol having a higher average molecular weight is in the range
from 20:1
to 2:1.
The independent claim relates to the use of the oral thin film according to
the
invention. Preferred embodiments are indicated in the dependent claims.
In the following, PVA is used as an abbreviation for polyvinyl alcohol. The
polyvinyl
alcohol with a lower average molecular weight of 10,000 to about 75,000 g/mol,
preferably 10,000 to about 40,000 g/mol, used according to the invention is
also
referred to below as "low molecular weight polyvinyl alcohol" or "low
molecular
weight PVA". The polyvinyl alcohol with a higher average molecular weight of
140,000 to 300,000 g/mol, preferably 140,000 to 220,000 g/mol, is also
referred to
below as "high molecular weight polyvinyl alcohol" or "high molecular weight
PVA".
In the present specification, "comprising" can also mean "consisting of".
By adding a relatively low concentration, e.g. 6 wt%, of high molecular weight
PVA,
e.g. PVA 40-88, to a low molecular weight PVA, it was surprisingly possible to
significantly reduce the total polymer concentration in the formulation (in
the
example, from 65.8% (formulation Al) to 39% (formulation A2)) without losing
the
dimensional stability of the previous formulations and without noticeably
increasing
the disintegration time. Since the PVA mixture according to the invention,
e.g. PVA
4-88/40-88, allows the use of a lower total polymer concentration, it was also
possible to reduce the basis weight of the oral films significantly, e.g. from
180 to
109.5 g/m2. This improved the disintegration time compared to formulations
containing only low molecular weight PVA.
Without wishing to be bound by any theory, it is assumed that the longer-chain
PVA,
such as PVA 40-88, in this mixture represents a kind of structure-forming
agent that
enables the incorporation of lower PVA concentrations.
The oral thin film according to the invention has at least one matrix layer.
The at
least one matrix layer comprises at least one polyvinyl alcohol having a lower
average molecular weight in the range from 10,000 to about 75,000 g/mol,
preferably 10,000 to 40,000 g/mol, more preferably 25,000 to 35,000 g/mol, and
at
least one polyvinyl alcohol having a higher average molecular weight in the
range
from 140,000 to 300,000 g/mol, preferably 140,000 to 220,000 g/mol, more
preferably 200,000 to 210,000 g/mol.
3
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Unless otherwise stated, all average molecular weights of polymers mentioned
refer
to the weight average molecular weight (Mw) determined by gel permeation
chromatography.
In order to produce PVA, polyvinyl acetate is usually produced first, the
acetate
groups of which are then hydrolyzed. Commercially available polyvinyl alcohols
can
have a degree of hydrolysis of 84 to 92 mol%, preferably 86 to 90 mol%, and
thus
still contain a residual content of acetyl groups; a degree of hydrolysis of
98 to 99
or 86 to 89 mol% are common.
According to the present invention, a combination of at least one polyvinyl
alcohol
having an average molecular weight of about 31,000 g/mol and at least one
polyvinyl alcohol having an average molecular weight of about 205,000 g/mol is
particularly suitable.
In a preferred embodiment, the oral thin film therefore comprises a matrix
layer in
which the at least one polyvinyl alcohol having a lower average molecular
weight is
polyvinyl alcohol 4-88 or polyvinyl alcohol 8-88 and/or the at least one
polyvinyl
alcohol having a higher average molecular weight is polyvinyl alcohol 40-88.
These
polyvinyl alcohols are commercially available. If the molecular weight of the
polyvinyl alcohol with a higher average molecular weight is too high, the
resulting
mixture is too viscous, which makes processing difficult or almost impossible.
If the
molecular weight is too low, no sufficient structuring of the resulting
mixture will be
achieved.
The total amount of the at least one polyvinyl alcohol with a lower average
molecular weight and of the at least one polyvinyl alcohol with a higher
average
molecular weight in the matrix layer is in the range from 15 to 65 wt%,
preferably
from 20 to 60 wt%, more preferably from 22 to 58 wt%, more particularly from
24
to 56 wt% or from 22 to 50 wt% or from 25 to 40 wt%, based on the total weight
of the matrix layer.
The weight ratio (A:B) of the at least one polyvinyl alcohol with a lower
average
molecular weight (A) to the at least one polyvinyl alcohol with a higher
average
molecular weight (B) is in the range from 20:1 to 2:1, preferably from 15:1 to
3:1,
more preferably from 12:1 to 4:1, or from 10:1 to 4:1 or from 10:1 to 2:1,
more
particularly from 9:1 to 7:1.
The amount of the at least one polyvinyl alcohol with a higher average
molecular
weight in the matrix layer can be relatively small, e.g. in the range from 1
to
4
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
18 wt% or from 1 to 16 wt% or from 1 to 12 wt%, preferably from 2 to 10 wt%,
more particularly from 3 to 4 wt%, based on the total weight of the matrix
layer.
It is preferred that the at least one polyvinyl alcohol with a lower average
molecular
weight is a polyvinyl alcohol with a lower average molecular weight and the at
least
one polyvinyl alcohol with a higher average molecular weight is a polyvinyl
alcohol
with a higher average molecular weight.
The polyvinyl alcohols used function as matrix formers for the matrix layer,
which
comprises a polymer matrix. It is preferred that no other polymers, more
particularly no other matrix-forming polymers, are contained in the matrix
layer in
addition to these polyvinyl alcohols. If other polymers are included, the
amount of
polymers other than the polyvinyl alcohols mentioned is preferably not higher
than
15 wt%, more preferably not higher than 10 wt%, based on the total weight of
the
matrix layer. Examples of other polymers that may be used, if applicable,
include
hydroxypropyl methyl cellulose (HPMC), polyvinylpyrrolidone (PVP),
hydroxypropyl
cellulose (HPC), polyethylene glycol (PEG) or mixtures of these polymers.
The matrix layer of the oral thin film according to the invention further
comprises at
least one active pharmaceutical ingredient, e.g. one active pharmaceutical
ingredient or a combination of two or more active pharmaceutical ingredients.
The
at least one active pharmaceutical ingredient is in principle not subject to
any
limitation, but is preferably selected from all active pharmaceutical
ingredients that
can be administered orally and/or transmucosally. According to the present
invention, the active pharmaceutical ingredient also subsumes, for example,
all
pharmaceutically acceptable salts and solvates of the respective active
pharmaceutical ingredient.
The at least one active pharmaceutical ingredient may be selected, for
example,
from active pharmaceutical ingredients of the active ingredient classes of
emergency
contraceptives, analgesics, hormones, hypnotics, sedatives, antiepileptics,
amphetamines, psychoneurotropic agents, neuromuscular-blocking agents,
antispasmodics, antihistamines, antiallergics, cardiotonic agents,
antiarrhythmics,
diuretics, hypotensive agents, vasopressors, antidepressants, antitussives,
expectorants, thyroid hormones, sex hormones, antidiabetics, antitumor agents,
antibiotics, chemotherapeutics and narcotics, wherein the list is not
exhaustive.
The at least one active pharmaceutical ingredient is preferably a particulate
active
pharmaceutical ingredient that is dispersed in the matrix or polymer matrix.
The at
least one active pharmaceutical ingredient may be a water-soluble active
ingredient
or an active ingredient that is sparingly soluble or insoluble in water.
Herein, a
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
water-soluble active ingredient refers to an active ingredient with a
solubility in
water of more than 10 g/L at a temperature of 20 C Herein, a sparingly water-
soluble or water-insoluble active ingredient refers to an active ingredient
with a
solubility in water of not more than 10.0 g/L at a temperature of 20 C. The
specification is derived from the definitions in the European Pharmacopoeia.
The active ingredient may be present in the formulation for the preparation of
the
oral thin film in dissolved form, in suspended form or in both dissolved and
suspended form.
The active ingredient can be present as a starting material in particle form.
If the
starting material of the active ingredient is particulate, it may be
micronized
particles or non-micronized particles. The particle size can range from 0.2 pm
to
1 mm, for example.
In a preferred embodiment, the at least one active pharmaceutical ingredient
is or
comprises ulipristal acetate, preferably micronized ulipristal acetate.
Ulipristal
acetate is 17-acetoxy-11-(4-N,N-dimethylaminophenyI)-19-norpregna-4,9-diene-
3,20-dione) with the following chemical formula:
0
0
N 11101 0
4110*
0
O.
The active ingredient ulipristal acetate is dispersed in the polymer matrix of
the
matrix layer. In the matrix layer, ulipristal acetate is preferably present in
solid
form, more particularly in crystalline form, as particles.
The ulipristal acetate as starting material may be, for example, non-
micronized or
micronized ulipristal acetate. The particle size of the ulipristal acetate may
be in the
range from 0.2 pm to 1 mm, e.g. 0.2 to 100.0 pm or 0.5 to 40.0 pm.
The particle size refers to the volume-weighted particle size d90 and can be
determined, for example, by laser diffraction analysis, dynamic light
scattering or
sieve analysis. The volume-weighted particle size d90 refers to the volume-
weighted
6
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
particle size in which 90% of the distribution has a smaller particle size and
ten
percent has a larger particle size, as is known to one skilled in the art.
In another preferred embodiment, the at least one active pharmaceutical
ingredient
comprises or is ketamine or a pharmaceutically acceptable salt thereof.
Ketamine is
understood here to mean (S)-( )-2-(2-chlorophenyI)-2-(methylamino)cyclohexan-1-
one, (R)-( )-2-(2-chlorophenyI)-2-(methylamino)cyclohexan-1-one, and the
racemate (RS)-( )-2-(2-chlorophenyI)-2-(methylamino)cyclohexan-1-one. The
ketamine preferably is (S)-ketamine or a pharmaceutically acceptable salt
thereof,
wherein ketamine is preferably present as an HCI salt, such as (S)-ketamine
HCI, or
as a free base. such as (S)-ketamine HCI. Ketamine is used as an analgesic,
antidepressant, or anesthetic.
The ketamine as starting material may be present as micronized particles or as
non-
micronized particles. Suitable average particle sizes are, for example, in the
range
from 1 to 1000 pm or from 5 to 500 pm or from 10 to 200 pm. The particle size
can
be determined using an optical microscope, for example.
In another preferred embodiment, the at least one active pharmaceutical
ingredient
comprises or is naloxone, more particularly naloxone HCI dihydrate. Naloxone
is an
opioid antagonist and belongs to the group of pure opioid antagonists that act
as
competitive antagonists on all opioid receptors. Thus, they partially or
completely
cancel out the effects caused by opiates and opioids.
In another preferred embodiment, the at least one active pharmaceutical
ingredient
comprises or is nalmefene, more particularly nalmefene HCI. Nalmefene is an
opioid
antagonist and thus partially or completely reverses the effects caused by
opiates
and opioids. Nalmefene is also used in the treatment of alcoholism.
In another preferred embodiment, the at least one active pharmaceutical
ingredient
comprises or is vortioxetine, more particularly vortioxetine HBr. Vortioxetine
is an
antidepressant drug with a multimodal mechanism of action.
The at least one active pharmaceutical ingredient, or a pharmaceutically
acceptable
salt thereof, is preferably present in the matrix layer in an amount in the
range from
to 60 wt%, preferably from 20 to 55 wt%, more preferably from 30 to 50 wt%,
based on the total weight of the matrix layer.
7
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
In a particularly preferred embodiment, the matrix layer further includes at
least
one hygroscopic additive having more than 3 carbon atoms, which is preferably
selected from sugars, sugar alcohols, starch and starch derivatives, or
mixtures
thereof.
The additional inclusion of a hygroscopic excipient, such as sugar, sugar
alcohols,
starch, and/or starch derivatives, leads to a further noticeable reduction in
the
disintegration time (see formulation A2 in the examples). Without wishing to
be
bound by a theory, it is assumed that the effect is based on the fact that the
hygroscopic excipients quickly "draw" water into the formulation, which then
leads
to a faster disintegration time compared to a reference formulation without
such a
hygroscopic excipient due to the low polymer concentration.
Preferred specific examples of suitable hygroscopic excipients with more than
3
carbon atoms are mannitol, isomalt, lactitol, sorbitol (glucitol) and xylitol,
threitol,
erythritol, starches such as corn or rice starch, including starch
derivatives,
sucralose, or mixtures of two or more of these compounds. A particularly
preferred
hygroscopic additive is sorbitol. The hygroscopic additives can also be
described
here as disintegrants.
It is possible to incorporate one hygroscopic excipient or a combination of
two or
more. The at least one hygroscopic excipient having more than 3 carbon atoms,
if
used, may be present in the matrix layer, for example, in an amount of up to
20 wt%, preferably in an amount of 5 to 20 wt%, more preferably 5 to 15 wt%,
based on the total weight of the matrix layer.
The matrix layer may further comprise at least one excipient, e.g. selected
from the
group consisting of colorants, flavorings, sweeteners, plasticizers, taste-
masking
agents, emulsifiers, enhancers, pH regulators, disintegrants, solubilizers,
preservatives, anti-foaming agents (for film production only) and/or
antioxidants. It
is preferred that the matrix layer comprises one or more plasticizers,
preferably
glycerol.
Each of these excipients, if present, is preferably present in the matrix
layer in an
amount of 0.1 to 15 wt%, preferably 0.1 to 10 wt% or 0.1 to 5 wt%, based on
the
total weight of the matrix layer.
The matrix layer of the oral thin film may be a non-foamed matrix layer or a
foamed
matrix layer, wherein the matrix layer is preferably a foam. The configuration
as a
non-foamed matrix layer or as a foamed matrix layer is known to those skilled
in the
art.
8
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
In the case of a foamed matrix layer, the layer may be in the form of a
solidified
foam that has spaces or cavities filled with a gas, a gas mixture, a liquid,
or a liquid
mixture. Such OTFs are usually referred to as "foam OTFs". The cavities in
foam
OTFs and the associated larger surface area of the films can accelerate the
dissolution of the dosage form and the release of the active ingredient.
In addition to the production of foams, the components of the matrix layer
according to the invention also enable the production of non-foamed OTFs with
a
high loading of active ingredient (e.g. 43%) and an acceptable disintegration
time
(see example of formulation A4). Without the addition of the high-molecular
PVA,
the maximum loading of active ingredient would have to be lower, which would
have
to be compensated for by increasing the basis weight. This in turn would lead
to
longer disintegration times. With non-foamed OTFs, in contrast to foams, there
is
the additional complication that the possible increase in basis weight is very
limited.
If a basis weight of more than 170 g/m2 is reached, the drying process becomes
very time-consuming and therefore unprofitable.
The basis weight of the matrix layer is preferably in the range of 50 to 220
g/m2,
more preferably 50 to 150 g/m2, particularly preferably 70 to 130 g/m2.
The matrix layer of the oral thin film according to the invention has, for
example, a
surface area in the range from 0.3 to 20 cm2, preferably 1 to 10 cm2. The
thickness
of the matrix layer can be in the range, for example, from 10 to 3000 pm,
preferably from 90 pm to 2000 pm, more preferably from 40 to 400 pm.
In a preferred embodiment, the matrix layer comprises at least 1 mg,
preferably at
least 20 mg, more preferably at least 30 mg of active ingredient, e.g. 25 to
150 mg,
preferably 30 to 150 mg, of active ingredient, more particularly ulipristal
acetate,
ketamine, naloxone, nalmefene or vortioxetine, more particularly naloxone HCI
dihydrate or nalmefene HCL or vortioxetine HBr.
Particularly preferably, the weight ratio of the at least one polyvinyl
alcohol having a
lower average molecular weight to the at least one polyvinyl alcohol having a
higher
average molecular weight and the respective total amounts of the at least one
polyvinyl alcohol having a lower average molecular weight and the at least one
polyvinyl alcohol having a higher average molecular weight are adapted to an
active
ingredient contained in the oral thin film.
9
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Thus, it is preferred that in an oral thin film, more particularly as defined
above, in
which ulipristal acetate or a pharmaceutically acceptable salt thereof is
present as
the active pharmaceutical ingredient, the weight ratio of the at least one
polyvinyl
alcohol having a lower average molecular weight to the at least one polyvinyl
alcohol having a higher average molecular weight is in the range of 10:1 to
4:1.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol having a lower average molecular weight is in the range of
20 to
40 wt%, based on the total weight of the matrix layer. Additionally or
independently, it is preferred that the amount of the at least one polyvinyl
alcohol
with a higher average molecular weight is 2 to 10 wt%, based on the total
weight of
the matrix layer. Additionally or independently, it is preferred that the
amount of
ulipristal acetate or the pharmaceutically acceptable salt thereof is 35 to 45
wt%,
based on the total weight of the matrix layer.
Further, it is preferred that in an oral thin film, more particularly as
defined above,
in which ketamine or a pharmaceutically acceptable salt thereof is present as
the
active pharmaceutical ingredient, the weight ratio of the at least one
polyvinyl
alcohol having a lower average molecular weight to the at least one polyvinyl
alcohol having a higher average molecular weight is in the range of 10:1 to
4:1.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol having a lower average molecular weight is in the range of
20 to
40 wt%, preferably of 25 to 35 wt%, based on the total weight of the matrix
layer.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol with a higher average molecular weight is 2 to 10 wt%, based
on
the total weight of the matrix layer. Additionally or independently, it is
preferred
that the amount of ketamine or the pharmaceutically acceptable salt thereof is
35 to
45 wt%, based on the total weight of the matrix layer.
Further, it is preferred that in an oral thin film, more particularly as
defined above,
in which naloxone or a pharmaceutically acceptable salt thereof is present as
the
active pharmaceutical ingredient, the weight ratio of the at least one
polyvinyl
alcohol having a lower average molecular weight to the at least one polyvinyl
alcohol having a higher average molecular weight is in the range of 10:1 to
2:1.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol having a lower average molecular weight is in the range of
45 to
60 wt%, preferably of 50 to 58 wt%, based on the total weight of the matrix
layer.
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol with a higher average molecular weight is 5 to 20 wt%, based
on
the total weight of the matrix layer. Additionally or independently, it is
preferred
that the amount of naloxone or the pharmaceutically acceptable salt thereof is
5 to
15 wt%, preferably about 10 wt%, based on the total weight of the matrix
layer.
Further, it is preferred that in an oral thin film, more particularly as
defined above,
in which nalmefene or a pharmaceutically acceptable salt thereof is present as
the
active pharmaceutical ingredient, the weight ratio of the at least one
polyvinyl
alcohol having a lower average molecular weight to the at least one polyvinyl
alcohol having a higher average molecular weight is in the range of 10:1 to
2:1.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol having a lower average molecular weight is in the range of
45 to
60 wt%, preferably of 50 to 58 wt%, based on the total weight of the matrix
layer.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol with a higher average molecular weight is 5 to 20 wt%, based
on
the total weight of the matrix layer. Additionally or independently, it is
preferred
that the amount of nalmefene or the pharmaceutically acceptable salt thereof
is 5 to
15 wt%, preferably about 10 wt%, based on the total weight of the matrix
layer.
Further, it is preferred that in an oral thin film, more particularly as
defined above,
in which vortioxetine or a pharmaceutically acceptable salt thereof is present
as the
active pharmaceutical ingredient, the weight ratio of the at least one
polyvinyl
alcohol having a lower average molecular weight to the at least one polyvinyl
alcohol having a higher average molecular weight is in the range of 10:1 to
2:1.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol having a lower average molecular weight is in the range of
20 to
40 wt%, preferably of 30 to 40 wt%, based on the total weight of the matrix
layer.
Additionally or independently, it is preferred that the amount of the at least
one
polyvinyl alcohol with a higher average molecular weight is 2 to 12 wt%, based
on
the total weight of the matrix layer. Additionally or independently, it is
preferred
that the amount of vortioxetine or the pharmaceutically acceptable salt
thereof is 25
to 35 wt%, based on the total weight of the matrix layer.
The oral thin film according to the invention is not subject to any
limitations with
regard to its structure. The following configurations are independent of
whether the
matrix layer is foamed or non-foamed.
11
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
The OTF according to the invention may be formed as a single-layer or multi-
layer
film, wherein a single-layer film is preferred. Thus, the oral thin film
according to
the invention may be present as a single-layer oral thin film and thus consist
only of
the matrix layer as defined above.
In another embodiment, the OTF may be present as a multilayer OTF and thus
contain further layers in addition to the matrix layer as defined above. These
additional layers may be laminated directly on top of each other or coated on
top of
each other or bonded with an adhesive layer in between. An adhesive layer is
understood to be a layer that can act as an adhesive, as defined in DIN EN
923:2016-03. A non-adhesive layer can therefore not act as an adhesive as
defined
above.
Other layers can be, for example, buffer layers to adjust the pH, or slowly
soluble or
insoluble layers that protect the oral thin film from premature erosion.
Alternatively,
other matrix layers may be present that contain other active pharmaceutical
ingredients or flavorings or flavor-masking ingredients. Other conceivable
layers are
adhesive layers and protective top layers.
The oral thin film according to the invention can be prepared by conventional
methods known to those skilled in the art. Conventional methods for preparing
the
oral thin film according to the invention comprise, for example, the steps of:
a) preparing of a solution, dispersion or melt comprising the components of
the
matrix layer according to the invention, e.g. a dispersion in water or in a
mixture of
water and alcohol;
al) optionally foaming the solution, dispersion or melt from step a) by
introducing a
gas or gas mixture, by chemical gas generation or by expansion of a dissolved
gas
or with the aid of a foaming machine;
b) spreading or pouring the solution, dispersion or melt from step a) or the
optionally foamed solution, dispersion or melt from step al) onto a surface or
into a
mold and cooling or drying.
The present invention further relates to an oral thin film as described above
for use
as a drug.
In a preferred embodiment, the oral thin film is used as an emergency
contraceptive. In this case, the OTF contains an emergency contraceptive as
the
12
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
active pharmaceutical ingredient, preferably ulipristal acetate. The above
details on
preferred embodiments of ulipristal acetate evidently also apply to the use.
In another preferred embodiment, the oral thin film is used for the treatment
or
prevention of pain and/or depression or as an anesthetic. In this case, the
OTF
contains more particularly an analgesic, an anesthetic or an antidepressant as
the
active pharmaceutical ingredient, preferably ketamine or vortioxetine. The
above
details on preferred embodiments of ketamine or vortioxetine evidently also
apply to
the use.
In another preferred embodiment, the oral thin film is used in the treatment
of
opiate overdosing. In this case, the OTF contains more particularly an opioid
antagonist as the active pharmaceutical ingredient, preferably naloxone, more
particularly naloxone HCI dihydrate, or nalmefene, more particularly nalmefene
HCI.
The invention is explained in more detail below with reference to non-limiting
examples.
13
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Examples
Example 1:
For all of the following formulations in Example 1, the single-dose dosage
forms
resulting from them are meant to have a surface area of 7.04 cm2 and are meant
to
contain, for the examples according to the invention, a single dose of at
least 30 mg
of ulipristal acetate or ketamine.
Formulation Al: Example of a PVA foam formulation without PVA mix and sugar
alcohols
(reference formulation)
_
Ingredient Content in [wt%] Function of the ingredient
Ulipristal acetate 23.7 Drug
PVA 4-88 64.2 Film polymer
Glycerol 8.8 Plasticizer
Saccharin Na 2.2 Sweetener
Sucralose 1.1 Sweetener, disintegrant, and
humectant
_
Basis weight 180 g/m2
Process solvent Water
Since the formulation only contains PVA 4-88, it can only be produced with a
high
polymer content of 64.2%, and therefore with a high basis weight. If the
polymer
content were lower, the film would be too brittle and, in the worst case,
could no
longer be made up. Additionally, the high basis weight has a negative effect
on the
disintegration time.
Formulation A2: Example of a PVA foam formulation with PVA Mix and sugar
alcohols
Ingredient Content in [wt%] Function of the ingredient
Ulipristal acetate 39.0 Drug
14
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
_ _
PVA 4-88 31.0 Film polymer
PVA 40-88 6.0 Film polymer
Xylitol 2.0 Sweetener and disintegrant
Tween 80 1.0 Plasticizer
Sorbitol 9.0 Sweetener and disintegrant
Sucralose 1.0 Sweetener, disintegrant, and
humectant
Glycerol 10.0 Plasticizer
Menthol 1 Flavor
_ _
Basis weight 109.5 g/m2
¨ --
Process solvent Water
¨ -
This formulation has a lower basis weight than the reference formulation. In
addition, it has acceptable haptic properties and a significantly shorter
disintegration time than the reference
Formulation A3: Example of a PVA foam formulation with PVA Mix without sugar
alcohols
Ingredient Content in [wt%] Function of the ingredient
Ulipristal acetate 39.0 Drug
PVA 4-88 38.0 Film polymer
PVA 40-88 8.0 Film polymer
Saccharin Na 2.0 Sweetener
Tween 80 1.0 Plasticizer
Sucralose 1.0 Sweetener, disintegrant, and
humectant
Glycerol 10.0 Plasticizer
Menthol 1.0 Flavor
Basis weight 109.5 g/m2
Process solvent Water
Omitting sorbitol increases the disintegration time of the foam (although
still much
faster than the reference). This shows that sugar alcohols or starch
derivatives in
the formulation improve disintegration. Due to their high hydrophilicity,
sugar
alcohols or starch derivatives draw water into the foam or film, which
improves
solubility.
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Formulation A4: Example of a PVA film formulation with PVA Mix, sugar alcohols
and
rice starch
Ingredient Content in [wt%] Function of the ingredient
Ulipristal acetate 43.0 Drug
PVA 4-88 24.0 Film polymer
PVA 40-88 3.0 Film polymer
Span 85 1.0 Plasticizer
Xylitol 2.0 Sweetener and disintegrant
Tween 80 1.0 Plasticizer
Sorbitol 9.0 Sweetener and disintegrant
Sucralose 1.0 Sweetener, disintegrant, and
humectant
Glycerol 9.0 Plasticizer
Menthol 1.0 Flavor
Rice starch 6.0 Disintegrant
¨ -
Basis weight 99.5 g/m2
Process solvent Water
The film has a high concentration of active ingredients and a low basis
weight.
Although it is a film, the formulation has a shorter disintegration time than
the
reference formulation.
Formulation B1: Example of a PVA film formulation with PVA Mix and sugar
alcohols
Starting materials Composition [wt%] Type / Function
Ketamine HCI 38.0 Active ingredient
PVA 4-88 29.0 Polymer
PVA 40-88 3.0 Polymer
Span 85 1.0 Plasticizer
Xylitol 2.0 Sweetener and disintegrant
Tween 80 1.0 Plasticizer
Sorbitol 9.0 Sweetener and disintegrant
Sucralose 1.0 Sweetener, disintegrant, and
humectant
Glycerol 9.0 Plasticizer
16
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
- -
Menthol 1.0 Flavor
Rice starch 6.0 Disintegrant
Basis weight 112.5 g/m2
Solvent Water
Formulation B2: Example of a PVA foam formulation with PVA Mix and sugar
alcohols
_ _
Starting materials Composition [wt%] Type / Function
Ketamine HCI 39.0 Active ingredient
PVA 4-88 31.0 Polymer
PVA 40-88 6.0 Polymer
Xylitol 2.0 Sweetener and disintegrant
Tween 80 1.0 Plasticizer
Sorbitol 9.0 Sweetener and disintegrant
Sucralose 1.0 Sweetener, disintegrant, and
humectant
_
Glycerol 10.0 Plasticizer
Menthol 1.0 Flavor
_
Basis weight 109.5 g/m2
Solvent Water
Disintegration times of the above formulations
The disintegration time was determined using method Ph. Eur. 2.9.1. with
weight
and plate.
Formulation Disintegration time Disintegration time
measurement 1 measurement 2
Al (reference) 50 seconds 51 seconds
A2 4 seconds 4 seconds
A3 17 seconds 18 seconds
A4 30 seconds 31 seconds
B1 8 seconds 8 seconds
B2 15 seconds 15 seconds
17
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Example 2:
Oral thin films were prepared with naloxone HCI dihydrate and nalmefene HCL as
the active ingredient and their disintegration times were determined according
to
method Ph. Eur. 2.9.1. with weight and plate.
Formulation Cl: PVA foam formulation
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 51 Polymer
PVA 40-88 16 Polymer
Xylitol 2 Sweetener and disintegrant
Tween 80 1 Plasticizer
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 10 Plasticizer
Basis weight 128 g/m2
Solvent Water
Disintegration time 4 s
Formulation C2: PVA film formulation
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 56 Polymer
PVA 40-88 6 Polymer
Xylitol 2 Sweetener and disintegrant
Span 83 1 Emulsifier
Tween 80 1 Plasticizer
Sorbitol 9 Sweetener and disintegrant
18
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
_
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 9 Plasticizer
--
Rice starch 5 Disintegrant
Basis weight 147 g/m2
_ _
Solvent Water
- ¨
Disintegration time 33 s
-
Formulation C3: PVA foam formulation
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 51 Polymer
PVA 40-88 16 Polymer
Xylitol 2 Sweetener and disintegrant
--
Sodium bisulfite 0.1 Antioxidant
--
Tween 80 1 Plasticizer
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 9.9 Plasticizer
Basis weight 176 g/m2
Solvent Water
Disintegration time 5 s
Formulation C4: PVA foam formulation; PVA solutions were adjusted to pH 4
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 51 Polymer
PVA 40-88 16 Polymer
Xylitol 2 Sweetener and disinteg rant
19
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
-- -
Sodium bisulfite 0.1 Antioxidant
Tween 80 1 Plasticizer
--
Sorbitol 9 Sweetener and disinteg rant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 9.9 Plasticizer
_ _
Basis weight 152 g/m2
Solvent Water
Disintegration time 4 s
-
Formulation C5: PVA foam formulation; PVA solutions were adjusted to pH 8
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 51 Polymer
--
PVA 40-88 16 Polymer
Xylitol 2 Sweetener and disintegrant
Sodium bisulfite 0.1 Antioxidant
--
Tween 80 1 Plasticizer
_ _
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 9.9 Plasticizer
- -
Basis weight 153 g/m2
Solvent Water
Disintegration time 7 s
Formulation C6: PVA film formulation
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 56 Polymer
4.
PVA 40-88 6 Polymer
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Xylitol 2 Sweetener and disintegrant
Span 83 1 Emulsifier
Sodium bisulfite 0.1 Antioxidant
Tween 80 1 Plasticizer
_ _
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 8.9 Plasticizer
Rice starch 5 Disintegrant
_ _
Basis weight 147 g/m2
Solvent Water
Disintegration time 20 s
Formulation C7: PVA film formulation; PVA solutions were adjusted to pH 4
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 56 Polymer
--
PVA 40-88 6 Polymer
Xylitol 2 Sweetener and disintegrant
--
Span 83 1 Emulsifier
--
Sodium bisulfite 0.1 Antioxidant
--
Tween 80 1 Plasticizer
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 8.9 Plasticizer
Rice starch 5 Disintegrant
Basis weight 157 g/m2
Solvent Water
Disintegration time 22 s
21
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Formulation C8: PVA film formulation; PVA solutions were adjusted to pH 8
Starting materials Composition [wt%] Type / Function
Naloxone HCI dihydrate 10 Active ingredient
PVA 4-88 56 Polymer
--
PVA 40-88 6 Polymer
Xylitol 2 Sweetener and disintegrant
Span 83 1 Emulsifier
--
Sodium bisulfite 0.1 Antioxidant
--
Tween 80 1 Plasticizer
_
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 8.9 Plasticizer
--
Rice starch 5 Disintegrant
_
Basis weight 154 g/m2
Solvent Water
Disintegration time 28 s
Formulation C9: PVA foam formulation
Starting materials Composition [wt%] Type / Function
Nalmefene HCI 10 Active ingredient
PVA 4-88 51 Polymer
PVA 40-88 16 Polymer
Xylitol 2 Sweetener and disintegrant
Tween 80 1 Plasticizer
Sorbitol 9 Sweetener and disintegrant
_
Sucralose 1 Sweetener, disintegrant, and
humectant
_
Glycerol 10 Plasticizer
Basis weight 164 g/m2
Solvent Water
22
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
_
Disintegration time 10 s
23
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Example 3:
Oral thin films were prepared with vortioxetine HBr as the active ingredient
and
their disintegration times were determined according to method Ph. Eur. 2.9.1.
with
weight and plate.
Formulation Dl: PVA foam formulation
Starting materials Composition [wt%] Type / Function
Vortioxetine HBr 30 Active ingredient
PVA 4-88 36 Polymer
PVA 40-88 11 Polymer
Xylitol 2 Sweetener and disintegrant
Sodium bisulfite Antioxidant
Tween 80 1 Plasticizer
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 10 Plasticizer
Basis weight 167.5 g/m2
Solvent Water
Disintegration time 5 s
Formulation D2: PVA film formulation
Starting materials Composition [wt%] Type / Function
Vortioxetine HBr 30 Active ingredient
PVA 4-88 37 Polymer
PVA 40-88 4 Polymer
Xylitol 2 Sweetener and disintegrant
Span 83 1
Tween 80 1 Plasticizer
24
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
-
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
_
Glycerol 9 Plasticizer
Rice starch 6 Disintegrant
Basis weight 153.6 g/m2
Solvent Water
Disintegration time 19 s
Formulation D3: PVA film formulation
Starting materials Composition [wt%] Type / Function
Vortioxetine HBr 30 Active ingredient
PVA 4-88 36 Polymer
--
PVA 40-88 11 Polymer
Xylitol 2 Sweetener and disintegrant
Tween 80 1 Plasticizer
_
Sorbitol 9 Sweetener and disintegrant
Sucralose 1 Sweetener, disintegrant, and
humectant
Glycerol 7 Plasticizer
Rice starch 3 Disintegrant
_
Basis weight 148.9 g/m2
Solvent Water
Disintegration time 4 s
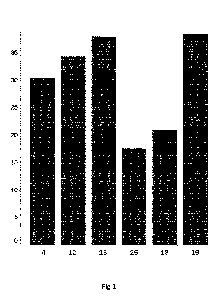
For the above formulations D1 to D3, the in-vitro permeation was carried out
using
Franz diffusion cells (volume 10 mL) at 37 C. At predetermined changeover
times,
the acceptor medium used in each case was completely replaced by a new one,
and
the content of permeated active ingredient in these acceptor solutions was
determined
using HPLC.
Phosphate buffer pH 7.4 was used as the acceptor medium.
Date Recue/Date Received 2024-03-01
CA 03230936 2024-03-01
Dermatomized skin from the esophagus of a pig with a layer thickness of 400 pm
was used as a skin model.
The results are summarized in Figure 1.
26
Date Recue/Date Received 2024-03-01