Note: Descriptions are shown in the official language in which they were submitted.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
1
JAMMED EMULSION TOOTHPASTE COMPOSITIONS
FIELD OF THE INVENTION
The present invention relates to toothpaste compositions comprising jammed
emulsion, such
as jammed oil-in-water emulsion. The present invention also relates to
toothpaste compositions
comprising jammed oil-in-water emulsion with unexpectedly high yield stress
values and/or high
opacity without solid opacifiers. The present invention further relates to
toothpaste compositions
comprising jammed oil-in-water emulsion wherein the hydrophobic phase can have
a particular
droplet size to provide a suitable yield stress and/or opacity.
BACKGROUND OF THE INVENTION
Typically, toothpaste compositions are formulated as a single-phase aqueous
chassis or a
single-phase non-aqueous chassis. In many cases, additional ingredients are
added to toothpaste
composition to impart structure and/or increase the yield stress of the
toothpaste composition.
For example, thickening agents can be added to toothpaste compositions to
increase the yield
stress of the toothpaste so that the toothpaste can be dispensed from a tube
and/or stand-up on the
bristles of a toothbrush (i.e. not sink into the bristles of the toothbrush
upon dispensing). However,
many consumers desire a simpler toothpaste composition with a smaller number
of ingredients.
Additionally, the introduction of additional ingredients can lead to
formulation instability if the added
ingredients interact with other toothpaste components.
Thus, there is a need for a toothpaste composition with a suitable yield
stress and/or opacity
without having to introduce thickening agents, structuring agents, and/or
solid opacifiers.
SUMMARY OF THE INVENTION
Disclosed herein is a jammed oil-in-water toothpaste composition comprising
(a) aqueous
phase; (b) hydrophobic phase; and (c) emulsifier, wherein the hydrophobic
phase has a D[4,3]
equivalent-diameter of droplets of hydrophobic phase is from about 0.001
microns to about 1000
microns.
Also disclosed herein is a jammed oil-in-water toothpaste composition
comprising (a) aqueous
phase; (b) hydrophobic phase; and (c) emulsifier, wherein the toothpaste
composition has a yield stress
of from about 2 Pa to about 5000 Pa.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
2
Also disclosed herein is an opaque jammed oil-in-water toothpaste composition
comprising (a)
aqueous phase; (b) hydrophobic phase; and (c) emulsifier, wherein the
toothpaste composition is
substantially free of, essentially free, and/or free of solid opacifier.
BRIEF DESCRIPTION OF THE DRAWINGS
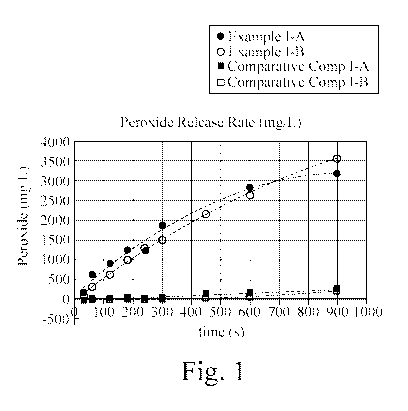
FIG. 1 shows the peroxide release rate of Example I-A and Example I-B.
FIG. 2 shows a photograph of Hydrophobic Phase and Aqueous Phase from TABLE 8,
and
Examples II-C, II-D and II-F.
FIG. 3A shows a photograph of a nurdle of Example II-C dispensed on a
toothbrush.
FIG. 3B shows a photograph of a nurdle of Example II-D dispensed on a
toothbrush.
FIG. 3C shows a photograph of a nurdle of Example II-F dispensed on a
toothbrush.
FIG. 3D shows a photograph of a nurdle of Example II-F dispensed on a
toothbrush.
FIG. 4 shows a photograph of a nurdle of Example I-A dispensed onto a
toothbrush.
FIG. 5 shows the fluoride release rate of Example II-E, Example II-I, and
Comparative
Composition I-C.
FIG. 6A shows a microscope image of Example I-A.
FIG. 6B shows a microscope image of Example II-C.
FIG. 6C shows a microscope image of Example II-D.
FIG. 6D shows a microscope image of Example II-F.
DETAILED DESCRIPTION OF THE INVENTION
Typically, toothpaste compositions are formulated as a single-phase aqueous
chassis or a
single-phase non-aqueous chassis further combined with abrasives and flavors.
In many cases,
thickening agents need to be added to these toothpaste compositions to
increase the yield stress of the
toothpaste so that the toothpaste can be dispensed from a tube and/or stand-up
on the bristles of a
toothbrush (i.e. not sink into the bristles of the toothbrush upon
dispensing).
Surprisingly, a toothpaste composition including jammed oil-in-water emulsion
has a yield
stress that is greater than the aqueous phase and/or the hydrophobic phase
that are used to make the
jammed oil-in-water emulsion. In other words, two components are mixed with a
low yield stress and
the resulting jammed oil-in-water emulsion that is made upon mixing, as
described herein, has an
unexpectedly high yield stress. While not wishing to being bound by theory, it
is believed that the
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
3
jammed oil-in-water emulsion can have a higher than expected yield stress once
the high internal phase
undergoes the jamming transition.
The jammed oil-in-water emulsion can be made through the portion-wise addition
or slow
gradual addition of the hydrophobic phase to the aqueous phase, as described
herein. Upon the making
of the jammed oil-in-water emulsion, the yield stress is greater than the
yield stress of the hydrophobic
phase and/or the aqueous phase. It has also been found that, surprisingly, the
yield stress of the
jammed oil-in-water emulsion can also be manipulated through physical
manipulation, such as, for
example, rate of mixing or shear, after the entirety of the hydrophobic phase
has been added to the
aqueous phase or while the hydrophobic phase is being added to the aqueous
phase. The physical
manipulation, such as through stirring, shaking, vibrating, high shear mixing,
homogenization, etc.,
can lead to additional increases in yield stress of the toothpaste composition
without the need to add
subsequent processing or stabilizing aids, such as thickening agents.
While not wishing to being bound by theory, it is believed as droplets or
regions of
hydrophobic phase are appropriately sized, the yield stress of the composition
will be suitable for use
as a toothpaste. Suitable yield stresses can include 4 Pa to about 1000 Pa,
from about 25 Pa to about
500 Pa, and/or other ranges described further herein. Opacifiers, such as
titanium dioxide, can be
added to the formulation to make the toothpaste opaque in appearance.
Unexpectedly, the toothpaste
composition comprising jammed oil-in-water emulsion can provide an opaque
appearance without the
addition of opacifiers even when the aqueous phase and/or the hydrophobic
phase are not opaque.
While not wishing to being bound by theory, it is believed when droplets or
regions of
hydrophobic phase are appropriately sized to reflect wavelengths of visible
light (from about 400 nm
to about 700 nm), the composition becomes opaque in appearance ¨ surprisingly
even without solid
particulate opacifiers, such as titanium dioxide. Accordingly, the Dv 50
equivalent-diameter, D[4,3]
equivalent-diameter, or D[3,2] equivalent-diameter of the droplets or regions
of hydrophobic phase
may be from about 0.001 to about 1000 microns, or preferably from about 0.01
to about 100 microns.
These compositions may be jammed macro-emulsions.
Surprisingly, when the Dv 50 equivalent-diameter, D[4,3] equivalent-diameter,
or D[3,2]
equivalent-diameter of the droplets or regions of hydrophobic phase is be from
about 0.001 to about
1000 microns or preferably from about 0.01 to about 100 micronsõ the jammed
oil-in-water toothpaste
has a suitable yield stress and a suitable opacity without the need for solid
particulates.
Definitions
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
4
The term "oral care composition", as used herein, includes a product, which in
the ordinary
course of usage, is not intentionally swallowed for purposes of systemic
administration of particular
therapeutic agents, but is rather retained in the oral cavity for a time
sufficient to contact dental surfaces
or oral tissues. Examples of oral care compositions include dentifrice,
toothpaste, tooth gel,
subgingival gel, mouth rinse, mousse, foam, mouth spray, lozenge, chewable
tablet, chewing gum,
tooth whitening strips, floss and floss coatings, breath freshening
dissolvable strips, denture care or
adhesive product, unit-dose composition, and/or fibrous composition. The oral
care composition may
also be incorporated onto strips or films for direct application or attachment
to oral surfaces.
The term "dentifrice composition", as used herein, includes tooth or
subgingival -paste, gel, or
liquid formulations unless otherwise specified. The dentifrice composition may
be a single-phase
composition or may be a combination of two or more separate dentifrice
compositions. The dentifrice
composition may be in any desired form, such as deep striped, surface striped,
multilayered, having a
gel surrounding a paste, or any combination thereof. Each dentifrice
composition in a dentifrice
comprising two or more separate dentifrice compositions may be contained in a
physically separated
compartment of a dispenser or single compartment of a dispenser and dispensed
side-by-side.
The term "toothpaste composition" as used herein means a water-dispersible
composition that
is designed to treat surfaces of the oral cavity, such as through the release
of oral care agents. Suitable
toothpaste compositions can be applied or used using a brush with bristles and
can be rinsed from the
brush. Additionally, toothpaste compositions can include compositions that are
brushed onto teeth and
the excess may be spit out but not rinsed out of the mouth.
The term "immiscible" or "insoluble" as used herein means less than 1 part by
weight of the
substance dissolves in 100 parts by weight of a second substance.
The term "solubility" as used herein is the maximum number of parts by weight
of the
substance that can dissolve in 100 parts by weight of a second substance.
The term "phase" as used herein means a physically distinct region or regions,
which may be
continuous or discontinuous, having one or more properties that are different
from another phase.
Non-limiting examples of properties that may be different between phases
include composition,
viscosity, solubility, hydrophobicity, hydrophilicity, visual characteristics,
and miscibility. Examples
of phases include solids, semisolids, liquids, and gases.
The term "multi-phase oral care composition" as used herein comprises a
mixture of two or
more phases that are immiscible with each other, for example water-in-oil, oil-
in-water emulsions, or
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
mixtures thereof. The phases may be continuous, discontinuous, or combinations
thereof The multi-
phase oral care composition or a phase of the multi-phase oral care
composition may be solid, liquid,
semisolid, or combinations thereof In preferred aspects the multi-phase oral
care composition is
semisolid. Examples of multi-phase oral care compositions also include
compositions where the
5
phases are multi-continuous including bi-continuous, layered, striped,
marbled, ribbons, swirled, and
combinations thereof. Examples of multi-phase oral care compositions also
include compositions
where the phases are tessellated or tiled.
The term "emulsion" as used herein is an example of a toothpaste composition
wherein: 1) at
least one of the phases is discontinuous and 2) at least one of the phases is
continuous. Examples of
emulsions include droplets of oil dispersed in water. In this example, the
water and oil would be
mutually immiscible with each other, oil would be the discontinuous phase, and
the water would be
the continuous phase.
The term "macro-emulsion" as used herein is an example of an emulsion wherein
at least one
of the discontinuous phases is visible under a microscope using light with one
or more wavelengths
from 400 nm to 700 nm. Examples of macro-emulsions include those in which the
Dv 50 equivalent-
diameter, D[4,3] equivalent-diameter, or D[3,2] equivalent-diameter of the
regions of at least one of
the discontinuous phases is larger than the wavelength of light being used,
for instance larger than 0.4,
or 0.7 micron.
The term "micro-emulsion" as used herein is an example of an emulsion wherein
the
discontinuous phases is not visible under a microscope using light with one or
more wavelengths from
400 nm to 700 nm. Examples of micro-emulsions include those in which the
regions of the
discontinuous phases are smaller than the wavelength of light being used, for
instance smaller than
0.4, or 0.7 micron.
The term "oil-in-water emulsion" as used herein is an example of an emulsion
wherein 1) the
continuous phase is aqueous or hydrophilic, and 2) the discontinuous phase is
hydrophobic.
The term "water-in-oil emulsion" as used herein is an example of an emulsion
wherein 1) the
continuous phase is hydrophobic, and 2) the discontinuous phase is aqueous or
hydrophilic.
The term "high internal phase emulsion" as used herein is an example of an
emulsion wherein
the discontinuous phase comprises more than about 74% by weight or volume of
the toothpaste
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
6
composition. High internal phase emulsions may be oil-in-water emulsions,
water-in-oil emulsions,
or mixtures thereof
The term, "jammed emulsion" as used herein, is a high internal phase emulsion
1) wherein the
high internal phase emulsion exhibits no more than 5% macroscopic separation
after 48 hours at 23 C
measured according to the method specified herein, and/or 2) wherein separate
regions of
discontinuous phase influence the shape of one another. Examples of j ammed
emulsions may include
high internal phase emulsions in which adjacent or neighboring regions of
discontinuous phase
influence the shape of one another.
The term "jamming concentration" of a high internal phase emulsion as used
herein is the
minimum concentration of the discontinuous phase above which the high internal
phase emulsion 1)
exhibits no more than 5% macroscopic separation after 48 hours at 23 C
measured according to the
method specified herein, and/or 2) wherein separate regions of discontinuous
phase influence the
shape of one another.
The term "jam" or "jamming" of a high internal phase emulsion as used herein
is the
phenomenon where the high internal phase emulsion transitions to one that 1)
exhibits no more than
5% macroscopic separation after 48 hours at 23 C measured according to the
method specified herein
and/or 2) wherein separate regions of discontinuous phase influence the shape
of one another.
The term "solid" as used herein is a material that, at room temperature, 1)
has defined
dimensions even when it is not constrained in a container, or 2) maintains its
original shape when it is
picked up off a surface and subsequently placed back on the surface.
The term "liquid" as used herein is a material that, at room temperature, 1)
flows under gravity,
or 2) takes the shape of the container it is placed in. Examples of liquids
include mineral oil, water,
and silicone oil. When a liquid is poured into a container, the exposed
surface (the surface that is not
in contact with the walls of the container) of liquids may become horizontal
and flat due to gravity.
Liquids may have a freezing point, melting point or drop melting point as
measured according to
ASTM method D127 or a congealing point as measured according to ASTM method
D938 or a pour
point as measured according to ASTM D97 less than about OC, less than about 23
C, or less than about
40 C. Liquids may have a kinematic viscosity measured according to ASTM D445
at 40 C less than
about 10,000 cSt, less than about 1000 cSt, or less than about 100 cSt.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
7
The term "semisolid" as used herein is a material that, at room temperature,
1) has some solid-
like properties and some liquid-like properties, or 2) whose ability to meet
the above definition of a
solid or liquid may depend on the amount of material being evaluated; for
example, a small amount
of petrolatum placed in a large container may not flow under gravity, and it
may not take the shape of
the container (thus not meeting the definition of a liquid); but a large
amount of petrolatum placed in
an large container may flow under gravity, or it may take the shape of the
container (thus meeting the
definition of a liquid). Examples of semisolids include petrolatum,
toothpaste, silicone gels, butter,
creams, ointments, and jammed emulsions.
The term "lotion" as used herein is a preparation intended for application on
the body, surfaces
of the oral cavity, or mucosal surfaces. Examples of lotions include hand
lotions, skin care lotions,
body lotions, suntan lotions, and jammed emulsions.
The term "aqueous phase" as used herein is a phase that comprises water,
optionally at least
one oral care active agent, and is immiscible with the hydrophobic phase.
The term "hydrophobic phase" as used herein means all components of the
composition that
.. are immiscible with the aqueous phase.
The term "equivalent-diameter" of a region or droplet as used herein means the
diameter of a
sphere having the same volume as the region or droplet.
The term "Dv 50 equivalent-diameter" as used herein is the equivalent-diameter
in microns at
which 50% of the regions of hydrophobic phase or droplets of aqueous phase are
smaller and 50% are
larger. The v in the term Dv 50 shows that this refers to the volume
distribution. The Dv 50 equivalent-
diameter of regions of hydrophobic phase of a multi-phase oral care
composition is measured
according to the method specified herein.
The term "D[4,3] equivalent-diameter" as used herein is the volume-weighted-
mean
equivalent-diameter in microns of the regions of hydrophobic phase or droplets
of aqueous phase. The
D[4,3] equivalent-diameter of regions of hydrophobic phase of a multi-phase
oral care composition
is measured according to the method specified herein.
The term "D[3,2] equivalent-diameter" as used herein is the surface-weighted-
mean
equivalent-diameter in microns of the regions of hydrophobic phase or droplets
of aqueous phase. The
D[3,2] equivalent-diameter of regions of hydrophobic phase of a multi-phase
oral care composition
is measured according to the method specified herein.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
8
The term "cone penetration consistency value" as used herein means the depth,
in tenths of a
millimeter, that a standard cone will penetrate the sample under fixed
conditions of mass, time, and
temperature. The cone penetration consistency value is measured according to
ASTM method D937.
The term "yield stress" as used herein means the critical shear stress at
which the material
begins to flow as a liquid.
The term "rinseable" as used herein means the material can be rinsed from a
surface using
water at a certain temperature in a certain period of time. Examples of
rinseable materials generally
include honey, milk, and compositions comprising oil-in-water emulsions such
as Example I and
Example II below.
The term "dispersible" as used herein means the material can be dispersed in
water at 23 C
2 C. The water-dispersibility of the material is measured according to the
method specified herein.
Examples of water-dispersible materials generally include compositions
comprising oil-in-water
emulsions such as Examples I and Example II below.
The term "macroscopic separation" as used herein is a phenomenon in which at
least a portion
of one or more components or one or more phases of a composition separates out
of the composition.
The macroscopic separation is measured according to the method specified
herein. The lack of
macroscopic separation is a measure of the physical stability of a
composition.
The term "opacifier" as used herein is a solid particulate material that is
opaque and is generally
used to increase the opacity of a composition. Examples of opacifiers include
titanium dioxide powder
and zinc oxide powder.
The term "heterogenous dispersion" as used herein is a heterogenous
combination of two or
more substances Examples of heterogenous dispersions include emulsions such as
oil-in-water
emulsions, and jammed emulsions. Heterogenous dispersions do not include
homogenous dispersions
(such as solutions where a solute is uniformly dissolved in a solvent).
The term "petrolatum" as used herein means a semisolid mixture of
hydrocarbons. Petrolatum
may have a cone penetration consistency value as measured according ASTM
method D937 from
about 10 to about 500, preferably from about 25 to about 300, more preferred
from about 50 to about
250, or more preferred from about 100 to about 200. Petrolatum may have a
melting point or drop
melting point as measured according to ASTM method D127 or a congealing point
as measured
according to ASTM method D938 from about from about 40 C to about 120 C,
preferably from about
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
9
50 C to about 100 C, more preferred from about 50 to about 90 C, or more
preferred from about
60 C to about 80 C.
The term "mineral oil" as used herein means a liquid mixture of hydrocarbons.
Mineral oil
may have a cone penetration consistency value as measured according ASTM
method D937 more than
about 600, preferably more than about 500, or more preferred more than about
400. Mineral oil may
have a freezing point, melting point or drop melting point as measured
according to ASTM method
D127 or a congealing point as measured according to ASTM method D938 or a pour
point as measured
according to ASTM D97 less than about 0 C, less than about 23 C, or less than
about 40 C. Mineral
oil may have a kinematic viscosity measured according to ASTM D445 at 40 C
less than about 10,000
cSt, less than about 1000 cSt, or less than about 100 cSt.
The term "HLB" of an emulsifier is an expression of its Hydrophile-Lipophile
Balance, i.e. the
balance of the size and strength of the hydrophilic (water-loving or polar)
and the lipophilic (oil loving
or non-polar) groups of the emulsifier. The HLB values are quantified as
follows:
A. For non-ionic emulsifiers (except those containing propylene oxide,
butylene oxide,
nitrogen, or sulfur) HLB values are calculated according to the procedure
specified in "The
HLB system ¨ a time-saving guide to emulsifier selection", from ICI Americas,
Wilmington Delaware 19897, which is herein incorporated in its entirety by
reference,
including the various emulsifiers and blends of multiple emulsifiers listed in
it along with
their HLB values.
B. For ionic emulsifiers HLB values are calculated according to the procedure
specified in
1) "A quantitative kinetic theory of emulsion type I, physical chemistry of
the
emulsifying agent" by J.T. Davies J.H. Schulman (Ed.), Proceedings of the 2nd
International Congress on Surface Activity, Academic Press, New York (1957),
2)
Davies, J.T. (1959) Proc. Int. Congr. Surf. Act., 1, 426, and/or 3) Davies,
J.T. and Rideal,
E.K. (1961) Interfacial Phenomena.
For all other emulsifiers and those whose HLB values cannot be calculated
according to
either of the above two procedures, HLB values are measured experimentally
according to
the experimental procedure specified in "The HLB system ¨ a time-saving guide
to
emulsifier selection", from ICI Americas, Wilmington Delaware 19897.
"Active and other ingredients" useful herein may be categorized or described
herein by their
cosmetic and/or therapeutic benefit or their postulated mode of action or
function. However, it is to be
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
understood that the active and other ingredients useful herein can, in some
instances, provide more
than one cosmetic and/or therapeutic benefit or function or operate via more
than one mode of action.
Therefore, classifications herein are made for the sake of convenience and are
not intended to limit an
ingredient to the particularly stated function(s) or activities listed.
5
The term "teeth", as used herein, refers to natural teeth as well as
artificial teeth or dental
prosthesis and is construed to comprise one tooth or multiple teeth. The term
"tooth surface" as used
herein, refers to natural tooth surface(s) as well as artificial tooth
surface(s) or dental prosthesis
surface(s) accordingly.
10
As used herein, the word "or" when used as a connector of two or more
elements is meant to
include the elements individually and in combination; for example X or Y,
means X or Y or both.
"Array" means a display of packages comprising oral care compositions
comprising varying
amounts and identities of actives, such as anticaries drugs. The packages may
have the same brand
and/or sub-brand and/or the same trademark registration and/or having been
manufactured by or for a
common manufacturer and the packages may be available at a common point of
sale (e.g. oriented in
proximity to each other in a given area of a retail store or organized
together on the same web site).
An array is marketed as a line-up of products normally having like packaging
elements (e.g., packaging
material type, film, paper, dominant color, design theme, etc.) that convey to
consumers that the
different individual packages are part of a larger line-up. Arrays often have
the same brand, for
example, "Crest," and same sub-brand, for example, "Pro-Health." A different
product in the array
may have the same brand "Crest" and, optionally a different sub-brand "3D
White." The differences
between the "Pro-Health" product of the array and the "3D White" product in
the array may include
product form, different anticaries drug, different amounts of the anticaries
drug, or other differences
in other active or inactive ingredients. Arrays also often have the same
trademarks, including
trademarks of the brand, sub-brand, and/or features and/or benefits across the
line-up. "On-line Array"
means an "Array" distributed by a common on-line source.
While compositions and methods are described herein in terms of "comprising"
various
components or steps, the compositions and methods can also "consist
essentially of' or "consist of'
the various components or steps, unless stated otherwise.
CA 03231717 2024-03-07
WO 2023/044470 PC
T/US2022/076631
11
The term "substantially free" as used herein refers to the presence of no more
than 0.05%,
preferably no more than 0.01%, and more preferably no more than 0.001%, of an
indicated material
in a composition, by total weight of such composition.
The term "essentially free" as used herein means that the indicated material
is not deliberately
added to the composition, or preferably not present at analytically detectable
levels. It is meant to
include compositions whereby the indicated material is present only as an
impurity of one of the other
materials deliberately added.
All measurements referred to herein are made at about 23 C (i.e. room
temperature) unless
otherwise specified.
The toothpaste composition, as described herein, comprises oral care active
agent, such as
fluoride, peroxide, and/or metal, delivered from a jammed-oil-in-water
emulsion, as described herein.
Additionally, the toothpaste composition can comprise other optional
ingredients, as described below.
The section headers below are provided for convenience only. In some cases, a
compound can fall
within one or more sections. For example, stannous fluoride can be a tin
compound and/or a fluoride
compound.
Jammed Emulsions
The toothpaste comprises a high internal phase emulsion, jammed water-in-oil
emulsion, or
jammed oil-in-water emulsion, such as the jammed oil-in-water emulsions that
are described in U.S.
Patent No. 10,780,032, which is herein incorporated by reference in its
entirety.
Traditional oil-in-water emulsions are multi-phase compositions with a
discontinuous
hydrophobic phase and a continuous aqueous phase. Traditional oil-in-water
emulsions can be
prepared by combining a minority hydrophobic phase with a majority aqueous
phase. Traditional oil-
in-water emulsions are discontinuous droplets of hydrophobic phase suspended
and/or stabilized
within a continuous aqueous phase. As the hydrophobic and aqueous phases are
immiscible, generally
only a small portion of the hydrophobic phase can be stabilized within the
aqueous phase before
macroscopic separation occurs.
A high internal phase emulsion can be either oil-in-water or water-in-oil
emulsion, wherein
there is a high amount of the internal, discontinuous phase, by volume or
weight of the multi-phase
composition, relative to a traditional emulsion. A high internal phase
emulsion can have more of the
internal, discontinuous phase, by volume or weight of the total multi-phase
composition than the
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
12
external, continuous phase, by volume or weight of the multi-phase
composition. However, the
stability of high internal phase emulsions can prove challenging. High
internal phase emulsions can
suffer from macroscopic separation upon mixing or during storage of the high
internal phase emulsions
prior to use by a consumer.
As described herein, a jammed emulsion may be an unexpectedly stable high
internal phase
emulsion. As the concentration of the discontinuous phase of a high internal
phase emulsion is
increased, regions of discontinuous phase can become sufficiently crowded,
such that they can jam
against each other with a region of continuous phase between them and deform
each other with a
region of continuous phase between them. If both the continuous phase and
discontinuous phase are
liquids, the emulsion can transition into an at least partially semisolid
structure once the jamming
transition occurs.
Examples of jammed emulsions include those in which, under a microscope, 1)
regions of
discontinuous phase are or resemble polyhedrons or polygons, with or without
rounded corners, with
visible jamming between regions of discontinuous phase, with continuous phase
sandwiched between
regions of discontinuous phase, 2) regions of discontinuous phase are or
resemble non-spherical
shapes, with visible jamming between regions of discontinuous phase, with
continuous phase
sandwiched between regions of discontinuous phase, 3) regions of discontinuous
phase are in a
tessellated or tiled pattern or resemble one, with continuous phase sandwiched
between regions of
discontinuous phase, or 4) regions of discontinuous phase are in a pattern
that resemble a Voronoi
diagram with continuous phase sandwiched between regions of discontinuous
phase.
The jammed emulsion, as described herein, can be prepared by the portion-wise
addition or
gradual addition or slow addition of the discontinuous phase to the continuous
phase with adequate
energy of mixing. Simply combining the entire discontinuous phase to the
continuous phase will not
necessarily result in jammed emulsion.
Without wishing to be bound by theory, the ratio of the rate of addition of
the discontinuous
phase to the energy of mixing may be a factor to help form a jammed emulsion.
For example,
hypothetically, a slow rate of addition combined with inadequate energy of
mixing may not favor the
formation of a jammed emulsion. In contrast, hypothetically, even a faster
rate of addition combined
with an adequate energy of mixing may favor the formation of a jammed
emulsion.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
13
Without wishing to be bound by theory, it is believed that adding the entire
discontinuous
phase to the continuous phase, macroscopic separation will be more likely to
occur. Instead, by slowly
adding (either by portion-wise addition or a slow and/or steady continuous
addition), the molecules of
the discontinuous phase can associate into discrete regions instead of
separating macroscopically. As
the concentration of the discontinuous phase reaches the jamming
concentration, a jamming transition
can occur where separate regions of the discontinuous phase can influence the
shapes of one another
(for example neighboring or adjacent regions of discontinuous phase), which
can contribute to the
unexpected stability of jammed emulsions. In certain aspects of jammed
emulsions, 1) separate
regions of the discontinuous phase can influence the shape of one another (for
example neighboring
or adjacent regions of discontinuous phase), which can lead to a transition
from substantially spherical
discontinuous regions to at least partially polyhedral discontinuous regions
at the jamming
concentration, or 2) the emulsion can exhibit a Yield Stress or Brookfield
Viscosity greater than that
of the constituent aqueous phase and/or the hydrophobic phase measured
according to the methods
specified herein at 23 C.
The toothpaste composition, as described herein, comprises a jammed emulsion,
such as a
jammed oil-in-water emulsion. The jammed oil-in-water emulsion comprises
discontinuous
hydrophobic phase, continuous aqueous phase, and/or oral care active agent.
Aqueous Phase
The jammed oil-in-water emulsion comprises aqueous phase. The jammed oil-in-
water
emulsion can comprise minority aqueous phase. The aqueous phase can be at
least partially
continuous, essentially continuous, or continuous.
The jammed oil-in-water emulsion can comprise from about 0.01% to about 75%,
from about
0.01% to about 25%, from about 1% to about 20%, from about 2.5% to about 20 %,
from about 1% to
about 20%, or from about 5% to about 15%, by weight or volume of the jammed
oil-in-water emulsion,
of the aqueous phase.
The aqueous phase may also include other water-soluble solvents, such as for
example,
polyalkylene glycols with molecular weights from about 200 to about 20,000,
humectants, or
combinations thereof. Suitable humectants generally include edible polyhydric
alcohols such as
glycerin, sorbitol, xylitol, butylene glycol, and propylene glycol, and
mixtures thereof The aqueous
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
14
phase may comprise at least about 10%, at least about 20%, or at least about
30%, of water, by weight
or volume of the aqueous phase.
The aqueous phase can be in a minority proportion relative to the aqueous
phase present in the
toothpaste composition. As used herein "minority proportion" means that the
percent by weight or
volume of the aqueous phase of the toothpaste composition is less than the
percent by weight or
volume of the hydrophobic phase of the toothpaste composition.
The jammed oil-in-water emulsion may comprise an aqueous solution of a
bleaching agent,
such as hydrogen peroxide, optionally including emulsifier.
Hydrophobic phase
The jammed oil-in-water emulsion comprises hydrophobic phase. The jammed oil-
in-water
emulsion can comprise majority hydrophobic phase. The hydrophobic phase is at
least partially
discontinuous, essentially discontinuous, or preferably discontinuous.
The toothpaste composition comprises a safe and effective amount of a
hydrophobic phase.
The toothpaste composition can comprise at least about 10%, at least about
25%, at least about 50%,
at least about 60%, from about 75% to about 99%, from about 10% to about 99%,
from about 25% to
about 95%, from about 80% to about 99%, greater than about 80%, greater than
about 90%, or from
about 85% to about 95%, by weight or volume of the jammed oil-in-water
emulsion, of the
hydrophobic phase.
The density of the hydrophobic phase used in the toothpaste composition, as
described herein,
may be in the range of from about 0.8 g/cm3 to about 1.0 g/cm3, from about
0.85 g/cm3 to about 0.95
g/cm3, or about 0.9 g/cm3, or any other numerical range, which is narrower,
and which falls within
such broader numerical range, as if such narrower numerical ranges were all
expressly written herein.
While not wishing to being bound by theory, it is believed when the
hydrophobic phase is
appropriately selected based on its refractive index, suitably sized droplets
or regions of hydrophobic
phase can reflect visible light and the composition becomes opaque in
appearance ¨ surprisingly even
without solid particulate opacifiers, such as titanium dioxide. Accordingly,
the refractive index of the
hydrophobic phase or aqueous phase used in the toothpaste composition, as
described herein, may be
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
in the range of from about 1 to about 2, preferably from about 1.1 to about
1.6, more preferably from
about 1.2 to about 1.5, and most preferably from about 1.3 to about 1.5.
The hydrophobic phase can comprise a hydrophobic liquid. The hydrophobic phase
can
comprise an oil, such as edible oil, natural oil, or synthetic oil. The
hydrophobic phase can comprise
5 non-toxic edible oils, aliphatic hydrocarbons, fatty esters, and
combinations thereof
The hydrophobic phase can comprise unsaturated or saturated fatty alcohols,
unsaturated or
preferably saturated triglycerides, unsaturated or saturated fatty acids, or
combinations thereof. The
hydrophobic phase can comprise saturated long chain (greater than 12 carbon
atoms in aliphatic chain)
triglycerides, saturated short chain (less than 6 carbon atoms in aliphatic
chain) triglycerides, saturated
10 medium chain (6 to 12 carbon atoms in aliphatic chain) triglycerides, or
combinations thereof The
hydrophobic phase can comprise saturated long chain fatty acids or alcohols,
saturated short chain
fatty acids or alcohols, saturated medium chain fatty acids or alcohols, or
combinations thereof.
Saturated triglycerides, saturated fatty acids, and saturated fatty alcohols
may be artificially
hydrogenated or naturally saturated. Examples of naturally saturated medium
chain triglycerides
15 include fractionated coconut oil, fractionated palm oil, and/or
triglycerides of saturated medium chain
fatty acids. Examples of naturally saturated medium chain fatty acids include
caproic acid (6 carbons
in aliphatic chain), caprylic acid (8 carbons in aliphatic chain), capric acid
(10 carbons in aliphatic
chain), and/or lauric acid (12 carbons in aliphatic chain).
While not wishing to being bound by theory, it is believed that saturated
compounds are
preferred (vs. unsaturated compounds) as alkene functional groups may be more
reactive to certain
ingredients in toothpaste compositions, such as fluoride and/or peroxide.
Compound I shows an example of a medium-chain triglyceride, containing three
medium chain
fatty acids (two caprylic acids and one capric acid)
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
16
0
CH3
0 CH3
0
CH3
Compound I.
The hydrophobic phase may comprise fractionated coconut oil, fractionated palm
oil, and/or
combinations thereof.
Coconut oil can comprise the following esters listed in TABLE A.
TABLE A. Esters commonly found in Coconut Oil
Ester of:
Caprylic acid (C8) Saturated 7
Capric acid (C10) Saturated 8
Lauric acid (C12) Saturated 48
Myristic acid (C14) Saturated 16
Palmitic acid (C16) Saturated 9.5
Oleic acid (C18:1) Monounsaturated 6.5
Other Polyunsaturated 5
Accordingly, coconut oil can comprise caprylic acid, capric acid, lauric acid,
myrstic acid,
palmitic acid, oleic acid, and/or combinations.
Fractionated coconut oil can include coconut oil where certain long chain
triglycerides have
been removed through a variety of techniques well known to a person of
ordinary skill in the art, such
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
17
as lauric acid, myristic acid, palmitic acid, oleic acid, other
monounsaturated esters, other
polyunsaturated esters, and/or combinations thereof
Certain suppliers of fractionated coconut oil, such as bulkapothecary.com
exclude lauric acid
(C12), stating "...fractionated coconut oil has the long chain triglycerides
like lauric acid removed
retaining the capric and caprylic acids.".
However, while certain suppliers of fractionated coconut oil, such as
bulkapothecary.com,
exclude lauric acid, it is believed that triglyceride esters of lauric acid
are not always considered a long
chain triglyceride.
Medium chain triglycerides found in coconut oil can include triglyceride
esters of acid
molecules including from about 6 carbon atoms to about 14 carbon atoms, from
about 6 carbon atoms
to about 12 carbon atoms, from about 8 carbon atoms to about 12 carbon atoms,
and/or from about 8
carbon atoms to about 10 carbon atoms. Thus, medium chain triglycerides can
include caprylic acid
(8 carbon atoms), capric acid (10 carbon atoms), and/or lauric acid (12 carbon
atoms).
Long chain triglycerides found in coconut oil can include triglyceride esters
of acid molecules
including greater than 10 carbon atoms, greater than 12 carbon atoms, and/or
greater than 14 carbon
atoms.
Accordingly, the hydrophobic phase can comprise triglycerides of caprylic
acid, capric acid,
lauric acid, and/or combinations thereof. While not wishing to being bound by
theory, it is believed
that fractionated oils are preferred (vs. non-fractionated oils) as longer
chain triglycerides are typically
removed through the fractionation process. Many of the longer chain
triglycerides can include mono-
di, and/or polyunsaturated esters. Some suppliers may also remove certain
medium length
triglycerides, such as lauric acid. Thus, the hydrophobic phase can also
comprise triglycerides of
caprylic acid, capric acid, and/or combinations.
The hydrophobic phase can also comprise saturated long chain monoglycerides or
diglycerides, saturated short chain monoglycerides or diglycerides, saturated
medium chain
monoglycerides or diglycerides, and/or combinations thereof
The hydrophobic phase may also comprise silicones, polysiloxanes, and mixtures
thereof.
The hydrophobic phase may comprise mineral oil, petrolatum, and/or
combinations thereof
A suitable petrolatum includes white petrolatum. Other examples of suitable
petrolatum
include Snow White Pet ¨ C from Calumet Specialty Products (Indianapolis, IN),
G-2191 from
Sonneborn (Parsippany, NJ), G-2218 from Sonneborn, G-1958 from Sonneborn, G-
2180 from
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
18
Sonneborn, Snow White V28 EP from Sonneborn, and Snow White V30 from
Sonneborn, G-2494
from Sonneborn, and mixtures thereof.
The hydrophobic phase may comprise plant-based ingredients, for example non-
petrochemical
alternatives to mineral oil or petrolatum such as plant-based oils, plant-
based waxes, and mixtures
thereof. Examples also include castor seed oil, hydrogenated castor oil,
beeswax, and mixtures
thereof
The hydrophobic phase can comprise aliphatic hydrocarbon. The aliphatic
hydrocarbons can
comprise from about 4, 6, 8, 10, 12, 14, or 16 to about 16, 18, 20, 22, 24,
26, 28, 30, 36, 40 carbon
atoms such as decane, 2 ethyldecane, tetradecane, isotetradecane, hexadecane,
eicosane, and
combinations thereof. Medium chain or long chain triglycerides can comprise
vegetable oils, fish oils,
animal fats, hydrogenated vegetable oils, partially hydrogenated vegetable
oils, semi-synthetic
triglycerides, synthetic triglycerides, and mixtures thereof Fractionated,
refined or purified oils of
these types can also be used. Examples of long chain triglyceride-containing
oils include almond oil;
babassu oil; borage oil; black currant seed oil; canola oil; castor oil;
coconut oil; fractionated coconut
oil; liquid coconut oil; corn oil; cottonseed oil; emu oil; evening primrose
oil; flax seed oil; grapeseed
oil; groundnut oil; mustard seed oil; olive oil; palm oil; palm kernel oil;
peanut oil; rapeseed oil;
safflower oil; sesame oil; shark liver oil; soybean oil; sunflower oil;
hydrogenated castor oil;
hydrogenated coconut oil; hydrogenated palm oil; hydrogenated soybean oil;
hydrogenated vegetable
oil; a mixture of hydrogenated cottonseed oil and hydrogenated castor oil;
partially hydrogenated
soybean oil; a mixture of partially hydrogenated soybean oil and partially
hydrogenated cottonseed
oil; glyceryl trioleate; glyceryl trilinoleate; glyceryl trilinolenate; a S23-
polyunsaturated fatty acid
triglyceride containing oil; and mixtures thereof. The long chain triglyceride
containing oils may be
selected from the group consisting of corn oil, olive oil, palm oil, peanut
oil, safflower oil, sesame oil,
soybean oil, castor oil, linseed oil, rape oil, rice bran oil, coconut oil,
hydrogenated castor oil; partially
hydrogenated soybean oil; glyceryl trioleate; glyceryl trilinoleate; a S23-
polyunsaturated fatty acid
triglyceride containing oil; and combinations thereof Examples of medium chain
triglycerides include
fractionated natural oils, such as fractionated coconut oil, as described
further herein.
Saturated or unsaturated fatty alcohols may have from about 6 to about 20
carbon atoms,
cetearyl alcohol, lauryl alcohol, and mixtures thereof. For example, Lipowax
(Cetearyl Alcohol and
Ceteareth-20) are supplied and manufactured by Lipo Chemical.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
19
General information on silicones including silicone fluids, gums and resins,
as well as the
manufacture of silicones, can be found in Encyclopedia of Polymer Science and
Engineering, Volume
15, Second Edition, pp 204-308, John Wiley & Sons Inc. 1989 and Chemistry and
Technology of
Silicones, Walter Noll, Academic Press Inc, (Harcourt Brue Javanovich,
Publishers, New York), 1968,
pp 282-287 and 409-426.
The toothpaste composition, aqueous phase, or hydrophobic phase may be
substantially free
of ingredients, for example acids and/or alcohols, combinations of mineral oil
and
ethylene/propylene/styrene copolymer and/or butylene/ethylene/styrene
copolymer, certain bleaching
agents, fumed silica, polyorganosiloxanes, copolymer condensation products of
silicone resins and
polydiorganosiloxanes, or combinations thereof, silicones, dimethicone,
paraffinum liquidum,
trimethylsiloxysilicate/dimethiconol crosspolymer, or combinations thereof,
molecules with double or
triple covalent bonds between adjacent carbon atoms, molecules with styrene
groups, that at
temperatures (e.g. -7 C, 4 C, 23 C, 25 C, 300C, 40 C,
J 0 C, or 60 C) and conditions that the toothpaste
composition may be exposed to during manufacture, filling, shipping, or
storage (for example 1 day,
2 days, 1 week, 2 weeks, 1 month, 2 months, 3 months, 6 months, 12 months, 18
months, or 24 months)
prior to use by the consumer that 1) may compromise the efficacy, comfort,
usage experience,
concentration of actives or bleaching agents at the tooth surface over time,
active or bleaching
efficiency, or compatibility between ingredients, or 2) may react with other
ingredients or degrade
other ingredients or may cause foam or pressure to build up in the package or
container in which the
toothpaste composition is stored. The toothpaste compositions may comprise
less than 0.001% by
weight of the composition, of any of the compounds recited in this paragraph.
Without being bound
by a theory it is believed that the decrease in surface tension produced by
alcohol may decrease the
retention time of the aqueous phase at the tooth surface, thereby decreasing
the efficacy of the oral
care actives. The presence of acids might contradict with the actives and/or
may produce negative
side effects. Thus, the toothpaste compositions can be free of acids, free of
alcohols, or free of a
mixture thereof.
The hydrophobic phase can be in a predominant or majority proportion relative
to the aqueous
phase present in the toothpaste composition. As used herein "majority
proportion" means that the
percent by weight or volume of the hydrophobic phase of the toothpaste
composition is in excess
relative to the percent by weight or volume of the aqueous phase of the
toothpaste composition.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
The size and number of regions of hydrophobic phase may affect the amount of
oral/topical
irritation and/or tooth sensitivity imparted by the toothpaste composition,
opacity, translucency,
transparency, brightness, whiteness, and/or stability of the toothpaste
composition. The toothpaste
composition can be described in terms of its Dv 50 equivalent-diameter, D[4,3]
equivalent-diameter,
5 or D[3,2] equivalent-diameter of regions of the hydrophobic phase. For
example, the Dv 50
equivalent-diameter, D[4,3] equivalent-diameter, or D[3,2] equivalent-diameter
of regions of the
hydrophobic phase can be from about 0.001 to about 5000, preferably from about
0.001 to 1000, more
preferably from about 0.01 to about 100, more preferably from about 0.1 to
about 100 microns, or
most preferably from about 0.4 to about 100 microns. The multi-phase oral
compositions may be
10 macro-emulsions or micro-emulsions.
Emulsifiers
The jammed oil-in-water can comprise emulsifier. Depending on the design of
jammed oil-
in-water emulsion, the hydrophobic phase can have emulsifying properties.
Thus, the emulsifier and
15 .. the hydrophobic phase can comprise the same compound.
The jammed oil-in-water emulsion, as described herein, can comprise from about
0.001% to
about 20%, from about 0.01% to about 10%, up to about 10%, up to about 5%, or
from about 0.1% to
about 10%, by weight of the jammed oil-in-water emulsion, of the emulsifier.
Classes of surfactants useful as emulsifiers include nonionic surfactant,
anionic surfactant,
20 cationic surfactant, zwitterionic surfactant, amphoteric surfactant,
polymeric surfactant, synthetic
surfactant, and/or combinations thereof. Many suitable nonionic and amphoteric
surfactants are
disclosed by U.S. Pat. No. 3,988,433; U.S. Pat. No. 4,051,234, and many
suitable nonionic surfactants
are also disclosed by U.S. Pat. No. 3,959,458.
The emulsifier can comprise polysorbate, an alkyl sulfate, Lipowax D, or
combinations
thereof. Suitable polysorbate compounds include, polysorbate 20, 40, 60, 80,
or combinations thereof,
such as Tween 20, 40, 60, 80, or combinations thereof.
The emulsifier can comprise natural emulsifiers, such as acacia, gelatin,
lecithin and
cholesterol; finely dispersed solids, such as colloidal clays, bentonite,
veegum (magnesium aluminum
silicate; and synthetic emulsifiers, such as salts of fatty acids, sulfates
such as sorbitan trioleate,
sorbitan tristearate, sucrose distearate, propylene glycol monostearate,
glycerol monostearate,
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
21
propylene glycol monolaurate, sorbitan monostearate, sorbitan monolaurate,
polyoxyethylene-4-lauryl
ether, sodium lauryl sulfate, sulfonates such as dioctyl sosium
sulfosuccinate, glyceryl esters,
polyoxyethylene glycol esters and ethers, diethylene glycol monostearate, PEG
200 distearate, and
sorbitan fatty acid esters, such as sorbitan monopalmitate, and their
polyoxyethylene derivatives,
polyoxyethylene glycol esters such as the monostearate, Polysorbate 80
(ethoxylated sorbitan
monooleate) (supplied by Spectrum, etc.); and combinations thereof.
The emulsifier can be a surfactant that is non-reactive with oral care active
agents. For
example, surfactants that are non-reactive with a bleaching agent may be
substantially free of hydroxy
groups, nitrogen groups and linkages, double or triple covalent bonds between
adjacent carbon atoms,
metals such as Zn, etc., or combinations thereof
The jammed oil-in-water toothpaste composition may be free of, essentially
free of, and/or
substantially free of sulfate, alkyl sulfate, and/or sodium lauryl sulfate, as
some consumers have a
perception that these surfactants may lead to harsh conditions in the oral
cavity.
The jammed oil-in-water may be substantially free of ingredients, for example
reactive
emulsifiers, that at temperatures (e.g. -7 C, 4 C, 23 C, 25 C, 30 C,
40 C, 50 C, or 60 C) and
conditions that the jammed oil-in-water emulsion may be exposed to during
manufacture, filling,
shipping, or storage (for example 1 day, 2 days, 1 week, 2 weeks, 1 month, 2
months, 3 months, 6
months, 12 months, 18 months, or 24 months) prior to use by the consumer, 1)
may compromise the
efficacy, comfort, usage experience, concentration of actives or bleaching
agents at the tooth surface
over time, active or bleaching efficiency, or compatibility between
ingredients, or 2) may react with
other ingredients or degrade other ingredients or may cause foam or pressure
to build up in the package
or container in which the jammed oil-in-water emulsion is stored.
"Substantially free of a reactive
emulsifier" as used herein means that the composition comprises less than
0.001% by weight of a
reactive emulsifier.
The emulsifier may be a non-ionic surfactant. Nonionic surfactants include
polyoxyethylene
sorbitan fatty acid esters, such as, materials sold under the trademark Tween.
The number following
the 'polyoxyethylene' part in the following section refers to the total number
of oxyethylene -
(CH2CH20)- groups found in the molecule. The number following the
'polysorbate' part is related to
the type of fatty acid associated with the polyoxyethylene sorbitan part of
the molecule. Monolaurate
is indicated by 20, monopalmitate is indicated by 40, monostearate by 60, and
monooleate by 80.
Examples of such materials are polyoxyethylene (20) sorbitan monolaurate
(Tween 20),
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
22
polyoxyethylene (20) sorbitan monopalmitate (Tween 40), polyoxyethylene (20)
sorbitan
monostearate (Tween 60), polyoxyethylene (4) sorbitan monostearate (Tween 61),
polyoxyethylene
(20) sorbitan tristearate (Tween 65), polyoxyethylene (20) sorbitan monooleate
(Tween 80),
polyoxyethylene (5) sorbitan monooleate (Tween 81), and polyoxyethlene (20)
sorbitan trioleate
(Tween 85), and mixtures thereof Polyoxyethylene fatty acid esters are also
suitable and examples
include those materials sold under the trademark Myrj such as polyoxyethylene
(8) stearate (Myrj 45)
and polyoxyethylene (40) stearate (Myrj 52), and mixtures thereof. Further
nonionics include,
polyoxyethylene polyoxypropylene block polymers, such as poloxamers and
Pluronics.
Other suitable surfactants include zwitterionic surfactants, such as
cocamidopropyl betaine,
which can be used to improve foaming properties of jammed oil-in-water
emulsions, if desired.
Another suitable class of non-ionic surfactants that can be used in the
emulsifier are
polyoxyethylene fatty ethers, such as, the materials sold under the trademark
Brij. Examples of such
materials are polyoxyethylene (4) lauryl ether (Brij 30), polyoxyethylene (23)
lauryl ether (Brij 35),
polyoxyethylene (2) cetyl ether (Brij 52), polyoxyethylene (10) cetyl ether
(Brij 56), polyoxyethylene
(20) cetyl ether (Brij 58), polyoxyethylene (2) stearyl ether (Brij 72),
polyoxyethylene (10) stearyl
ether (Brij 76), polyoxyethylene (20) stearyl ether (Brij 78), polyoxyethylne
(2) oleyl ether (Brij 93),
polyoxyethylene (10) oleyl ether, and polyoxyethylene (20) oleyl ether (Brij
99), and mixtures thereof.
A portion of a non-ionic surfactant may be substituted with a lipophilic
surfactant, such as,
sorbitan fatty acid esters such as the materials sold under the trademark
Arlacel. Suitable lipophilic
surfactants include sorbitan monolaurate (Arlacel 20), sorbitan monopalmitate
(Arlacel 40), sorbitan
monostearate (Aracel 60), sorbitan monooleate (Arlacel 80), sorbitan
sesquioleate (Arlacel 83), and
sorbitan trioleate (Arlacel 85), and mixtures thereof Typically, from about 2%
to about 90% of the
level of the nonionic surfactant may be substituted by a lipophilic
surfactant, or from about 25% to
about 50%.
Other suitable emulsifiers include sodium lauryl sulfate, sodium lauryl
isethionate, sodium
lauroyl methyl isethionate, sodium cocoyl glutamate, lauryl glucoside
carboxylate, sodium dodecyl
benzene sulfonate, alkali metal or ammonium salts of lauroyl sarcosinate,
myristoyl sarcosinate,
palmitoyl sarcosinate, stearoyl sarcosinate and oleoyl sarcosinate,
polyoxyethylene sorbitan
monostearate, isostearate and laurate, sodium lauryl sulfoacetate, N-lauroyl
sarcosine, the sodium,
potassium, and ethanolamine salts of N-lauroyl, N-myristoyl, or N-palmitoyl
sarcosine, polyethylene
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
23
oxide condensates of alkyl phenols, cocamidopropyl betaine, lauramidopropyl
betaine, palmityl
betaine, sodium cocoyl glutamate, and/or combinations thereof
Each emulsifier and/or blends of multiple emulsifiers can have a hydrophilic-
lipophilic balance
(HLB) value. An emulsifier that is lipophilic in character is assigned a low
HLB number (below about
.. 9), and one that is hydrophilic is assigned a high HLB number (above about
11). In certain
embodiments, the skilled formulator will recognize the importance of selecting
an emulsifier (or blend
of multiple emulsifiers) with a suitable balance of hydrophilic and lipophilic
properties to encourage
the formation of a high internal phase emulsion or preferably a jammed
emulsion. The HLB is
calculated according to the procedure specified previously. Information on
emulsifiers and HLB
values can be found in 1) "Emulsion science and technology" edited by Tharwat
F. Tadros, Wiley
VCH, ISBN: 978-3-527-32525-2, 2) "Classification of surface-active agents by
HLB" by W.C. Griffin
of the Atlas Powder Company in the Journal of Cosmetic Chemists 1949, 3)
"Calculation of HLB of
non-ionic surfactants" by W.C. Griffin in the Journal of Cosmetic Chemists
1954, 4) "Interfacial
phenomena", Chapter 8 "Disperse systems and adhesion" by J. T. Davies and E.
K. Rideal Academic
Press, New York, 1963, 5) "A quantitative kinetic theory of emulsion type I,
physical chemistry of
the emulsifying agent" by J.T. Davies J.H. Schulman (Ed.), Proceedings of the
2nd International
Congress on Surface Activity, 1, Academic Press, New York (1957), 6) "Span and
Tween" brochure
08/10 D1005/1 by Croda Europe Ltd. England, 7) "Food enrichment with Omega-3
fatty acids",
Chapter 5 "Stabilization of omega-3 oils and enriched foods using emulsifiers"
by C. Genot, T.-H.
.. Kabri and A. Meynier, France, Woodhead Publishing, and 8) "Health Care
Prodoct Guide ¨ North
America", brochure "Pharmaceuticals, Dermatology, Delivering your solution,
Animal Health,
Nutraceuticals" by Croda . The emulsifiers and blends of multiple emulsifiers
along with their HLB
values specified in these documents are incorporated herein by reference.
An emulsifier that tends to form a water-in-oil emulsion and an emulsifier
that forms an oil-
.. in-water emulsion may be blended to achieve an HLB suitable for an oil-in-
water emulsion. The
average HLB number of the blend may be calculated from additivity:
HLB of blend = (a) * HLB1 + (b) * HLB2
Wherein a and b are the weight fractions of the two emulsifiers with HLB1 and
HLB2.
For example, to determine the HLB value of a blend comprising 70% of TWEEN 80
(HLB =
15) and 30% Of SPAN 80 (HLB = 4.3), the calculation would be:
The contribution from TWEEN 80 is 70% X 15.0 = 10.5
The contribution from SPAN 80 is 30% X 4.3 = 1.3
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
24
Thus, the HLB of blend is 11.8 (i.e. 10.5+ 1.3)
The HLB values of various emulsifiers and/or blends of multiple emulsifiers
can be from about
are from about 0 to about 60, above 11, from about 11 to about 60, from about
11 to about 40,
preferably from about 11 to about 20, or more preferred from about 16 to about
18, or combinations
thereof; or from about 20 to about 40, or from about 30 to about 40.
The emulsifier or blend of multiple emulsifiers can be hydrophilic, miscible
with water,
immiscible with mineral oil, or combinations thereof
Each emulsifier can comprise at least one hydrophobic tail group and at least
one hydrophilic
head group. There can be from about 6 to about 20, from about 8 to about 16,
or from about 10 to 14
carbon atoms in from about 1 to about 4, from about 1 to about 3, or from
about 1 to about 2
hydrophobic tails, or in 1 hydrophobic tail. Each hydrophobic tail can have up
to about 4, up to about
3, or up to about 1 branch, or 0 branches. Each hydrophobic tail can have up
to about 3, up to about
2, up to about 1, or 0 alkene functional groups (or carbon-carbon double
bonds). The hydrophilic head
group of each emulsifier molecule can comprise from about PEG-4 to about PEG-
40, from about PEG-
8 to about PEG-30, or preferably from about PEG-16 to about PEG-24 attached to
sorbitan. The
emulsifier can comprise from about 4 to about 60, from about 8 to about 30,
from about 16 to about
34 of moles of ethylene oxide in each emulsifier molecule.
The emulsifier or blend of multiple emulsifiers can comprise PEG-20 sorbitan
monolaurate
(Tween 20), PEG-20 sorbitan monooleate (Tween 80), and/or sodium lauryl
sulfate. Preferably, the
emulisifer can comprise PEG-20 sorbitan monolaurate.
The emulsifier (and HLB) may comprise one or more of the following list, and
blends of
multiple emulsifiers may comprise blends of these in any combination thereof:
Span 20 (HLB of 8.6),
Span 40 (6.7), Span 60 (4.7), Span 80 (4.3), Span 83 (3.7), Span 85 (1.8),
Span 120 (4.7), Tween 20
(16.7), Tween 21(13.3), Tween 40 (15.6), Tween 60 (14.9), Tween 61(9.6), Tween
65 (10.5), and
Tween 80 (15).
Yield Stress
Typically, toothpaste compositions are formulated as a single-phase aqueous
chassis or a
single-phase non-aqueous chassis further combined with abrasives and flavors.
In many cases,
thickening agents need to be added to these toothpaste compositions to
increase the yield stress of the
toothpaste so that the toothpaste can be dispensed from a tube and/or stand-up
on the bristles of a
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
toothbrush (i.e. not sink into the bristles or flow down the sides of the
bristles of the toothbrush upon
dispensing).
Surprisingly, a toothpaste composition including jammed oil-in-water emulsion
has a yield
stress that is greater than the aqueous phase and/or the hydrophobic phase
that are used to make the
5 jammed oil-in-water emulsion. In other words, two components are mixed
with a low yield stress and
the resulting jammed oil-in-water emulsion that is made upon mixing, as
described herein, has an
unexpectedly high yield stress. While not wishing to being bound by theory, it
is believed that the
jammed oil-in-water emulsion can have a higher-than-expected yield stress once
the high internal
phase undergoes the jamming transition.
10 Furthermore, it has also been surprisingly found that jammed oil-in-
water emulsions can be
made with a high yield stress by mixing the aqueous phase and hydrophobic
phase at a high rate of
mixing or shear. While not wishing to being bound by theory, it is believed
that the high shear rate
decreases the droplet size of hydrophobic phase which leads to a higher yield
stress.
The jammed oil-in-water emulsion can be made through the portion-wise addition
or gradual
15 .. addition of the hydrophobic phase to the aqueous phase, as described
herein. Upon the making of the
jammed oil-in-water emulsion, the yield stress is greater than the yield
stress of the hydrophobic phase
and/or the aqueous phase. It has also been found that, surprisingly, the yield
stress of the jammed oil-
in-water emulsion can also be manipulated through physical manipulation, such
as, for example, rate
of mixing or shear, after the entirety of the hydrophobic phase has been added
to the aqueous phase or
20 while the hydrophobic phase is being added to the aqueous phase. The
physical manipulation, such
as through stirring, shaking, vibrating, high shear mixing, homogenization,
etc., can lead to additional
increases in yield stress of the toothpaste composition without the need to
add subsequent processing
or stabilizing aids, such as thickening agents.
The multi-phase oral care compositions can be described by its water-
dispersibility according
25 to the method disclosed herein. The water dispersibility of the multi-
phase oral care composition can
be greater than about 5%, greater than about 10%, greater than about 20%,
greater than about 25%, or
greater than about 50% of the total content of the multi-phase oral care
composition, by weight or
volume. Preferably, the water-dispersibility of the multi-phase oral care
compositions can be from
about 20% to 100%, from about 40% to 100%, from about 60% to 100%, or greater
than about 70%,
by weight or volume of the total multi-phase oral care composition.
As yield stress of the jammed oil-in-water emulsion can be manipulated through
physical
processing of the emulsion, an array of toothpaste compositions that differ on
yield stress, but not
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
26
requiring the addition or removal of any ingredients is also disclosed herein.
The array can include a
first toothpaste composition comprising a jammed oil-in-water emulsion and a
second toothpaste
composition comprising a jammed oil-in-water emulsion. The first toothpaste
composition can have
a lower yield stress than the second toothpaste composition, but still a
higher yield stress than the
aqueous phase and/or hydrophobic phase. The first toothpaste composition could
be useful for
consumers that desire the paste to sink into the bristles of a brush, such as
in the case of users of power
brushes, hands free mouthpiece brushes, and/or trays. The second toothpaste
composition can be
useful for consumers of power brushes and/or manual brushes. The yield stress
of the first toothpaste
composition may be up to about 20 Pa, preferably up to about 15 Pa, and most
preferably up to about
10 Pa. The yield stress of the second toothpaste composition may be from about
25 to about 2000 Pas,
from about 25 to about 1000 Pa, preferably from about 25 to about 500 Pa, and
most preferably from
about 25 to about 200 Pa.
The yield stress of the toothpaste composition can be from about 2 Pa to about
5000 Pa, from
about 2 Pa to about 2000 Pa, from about 4 Pa to about 1000 Pa, from about 2 Pa
to about 500 Pa, from
about 2 Pa to about 100 Pa, from about 5 Pa to about 50 Pa, or from about 25
Pa to about 500 Pa, as
measured according to the method specified herein at 23 C.
Opacifiers
Typically, toothpaste compositions are formulated as a single-phase aqueous
chassis or a
single-phase non-aqueous chassis further combined with abrasives and flavors.
In many cases,
ingredients are added to toothpaste compositions that can result in toothpaste
with an unappealing
cloudy appearance ¨ neither completely translucent nor completely opaque.
In some cases, opacifiers, such as titanium dioxide are added to make the
paste a) opaque in
appearance, orb) bright or white in appearance. Unexpectedly, the toothpaste
composition comprising
jammed oil-in-water emulsion can provide a) an opaque appearance, orb) bright
or white appearance
without the addition of opacifiers, such as titanium dioxide, even when the
aqueous phase and/or the
hydrophobic phase are not opaque.
Opacifiers, such as titanium dioxide, can be added to the formulation to make
the toothpaste
opaque in appearance. Unexpectedly, the toothpaste composition comprising
jammed oil-in-water
emulsion can provide an opaque appearance without the addition of opacifiers
even when the aqueous
phase and/or the hydrophobic phase are not opaque.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
27
While not wishing to being bound by theory, it is believed when droplets or
regions of
hydrophobic phase are appropriately sized to reflect wavelengths of visible
light (from about 400 nm
to about 700 nm), the composition can become opaque in appearance ¨
surprisingly even without solid
particulate opacifiers, such as titanium dioxide. Accordingly, the Dv 50
equivalent-diameter, D[4,3]
equivalent-diameter, or D[3,2] equivalent-diameter of the droplets or regions
of hydrophobic phase
may be from about 0.4 to about 1000 microns, preferably from about 0.4 to
about 500 microns, and
most preferably from about 0.4 to about 100 microns. These compositions may be
jammed macro-
emulsions.
The opacity of a substance or composition can be correlated with its L*.
Bright or white
substances or compositions can have a L* of at least 25 units. Surprisingly
the toothpaste composition
comprising jammed oil-in-water emulsion can provide compositions that have a
L* of at least about
25, preferably at least about 50, more preferably at least about 70, or most
preferably at least about 80
units without the addition of opacifiers. Even more surprisingly, the
toothpaste composition
comprising jammed oil-in-water emulsion can provide compositions that have a
L* of at least about
25, preferably at least about 50, more preferably at least about 70, or most
preferably at least about 80
units without the addition of opacifiers and even when the aqueous phase
and/or the hydrophobic
phase have a L* less than about 5, less than about 10, or less than about 25
units.
In traditional toothpastes, solid particulates and solid abrasives can be
carefully selected such
that their refractive index matches that of the surrounding toothpaste matrix
to make the composition
more translucent and "gel-like" in appearance. Unexpectedly, the toothpaste
composition comprising
jammed oil-in-water emulsion can also provide a translucent appearance without
the need for solid
particulates at all. While not wishing to being bound by theory, it is
believed when droplets or regions
of hydrophobic phase of the present invention are small enough to allow
wavelengths of visible light
(less than about 0.4 microns, or less than about 0.7 microns) to pass through,
the composition becomes
translucent in appearance. Accordingly, the Dv 50 equivalent-diameter, D[4,3]
equivalent-diameter,
or D[3,2] equivalent-diameter of the droplets or regions of hydrophobic phase
may be from about
0.001 to about 1 micron, preferably from about 0.001 to about 0.7 microns, and
most preferably from
about 0.001 to about 0.4 microns. These compositions may be jammed micro-
emulsions.
Additionally, the jammed oil-in-water emulsion can have a white appearance
that can be used
without the need for dyes and/or opacifiers. Dyes can be added to the jammed
oil-in-water emulsion
to modify the color of the emulsion.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
28
The toothpaste composition can be free of, essentially free of, and/or
substantially free of
opacifiers, such as titanium dioxide, zinc oxide, calcium salts,
pyrophosphate, other metal oxides,
and/or bismuth oxychloride. The toothpaste composition can comprise less than
0.01%, less than
0.001%, and or less than 0.0001%, by weight of the toothpaste composition, of
opacifiers.
Minimum Number of Ingredients
As traditional toothpaste compositions are typically formulated as a single-
phase aqueous
chassis or a single-phase non-aqueous chassis further combined with abrasives
and flavors, a variety
of thickening agents, opacifiers, stabilizers, and/or surfactants can be added
to keep the active
ingredient, such as fluoride, stable until it reaches the oral cavity. By
instead formulating the
toothpaste as a jammed oil-in-water emulsion, the toothpaste composition can
be free of, essentially
free of, and/or substantially free of many ingredients that are normally
included in toothpaste
formulation, such as opacifiers, sodium lauryl sulfate, abrasive, thickening
agents, dyes, etc., as further
described herein.
Some consumers desire an elegantly designed toothpaste composition that
includes fewer
ingredients. The jammed oil-in-water emulsion may have a maximum of 9, maximum
of 8, maximum
of 7, maximum of 6, or maximum of 5 ingredients. Additionally, many of the
ingredients can be
naturally derived, natural, and/or sustainable ingredients, such as
hydrophobic phase comprising
natural oil.
Abrasive
The toothpaste composition of the present invention can comprise abrasive.
Abrasives can be
added to oral care formulations, such as toothpaste compositions, to help
remove surface stains from
teeth. The abrasive can comprise calcium abrasive, silica abrasive, and/or
alumina abrasive.
The calcium abrasive can be any suitable abrasive compound that can provide
calcium ions in
a toothpaste composition and/or deliver calcium ions to the oral cavity when
the toothpaste
composition is applied to the oral cavity. The toothpaste composition can
comprise from about 5% to
about 70%, from about 10% to about 60%, from about 20% to about 50%, from
about 25% to about
40%, or from about 1% to about 50% of a calcium abrasive. The calcium abrasive
can comprise one
or more calcium abrasive compounds, such as calcium carbonate, precipitated
calcium carbonate
(PCC), ground calcium carbonate (GCC), chalk, dicalcium phosphate, calcium
pyrophosphate, and/or
mixtures thereof
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
29
The toothpaste composition can also comprise silica abrasive, such as silica
gel (by itself, and
of any structure), precipitated silica, amorphous precipitated silica (by
itself, and of any structure as
well), hydrated silica, and/or combinations thereof. The toothpaste
composition can comprise from
about 5% to about 70%, from about 10% to about 60%, from about 10% to about
50%, from about
20% to about 50%, from about 25% to about 40%, or from about 1% to about 50%
of a silica abrasive.
The abrasive can also comprise other abrasives, such as bentonite, perlite,
titanium dioxide,
alumina, hydrated alumina, calcined alumina, aluminum silicate, insoluble
sodium metaphosphate,
insoluble potassium metaphosphate, insoluble magnesium carbonate, zirconium
silicate, solid
particulate thermosetting resins and/or other suitable abrasive materials. The
toothpaste composition
can comprise from about 5% to about 70%, from about 10% to about 60%, from
about 10% to about
50%, from about 20% to about 50%, from about 25% to about 40%, or from about
1% to about 50%
of other abrasives.
The toothpaste composition can be free of, essentially free of, and/or
substantially free of
abrasive. Unexpectedly, the jammed oil-in-water composition can provide
abrasive-free cleaning.
While not wishing to being bound by theory, it is believed that the jammed oil-
in-water emulsion can
provide abrasive-free cleaning because a) it can quickly release cleaning or
bleaching agents that may
be present in the aqueous phase, and b) it can have a majority hydrophobic
phase that can remove or
bleach stains, plaque, tartar, biofilm, and/or bacteria through an oil pulling
or bleaching mechanism.
Oral Care Active Agent
The toothpaste composition can comprise oral care active agent. Suitable oral
care active
agents can include whitening agent, anticaries agent, antibacterial agent,
antisensitivity agent,
dicarboxylic acid, among other components described herein.
Anticaries Agent
The oral care active agent can comprise an anticaries agent. The anticaries
agent can be active
against caries through one of these four mechanisms: i) suppressing acid
formation via antibacterial
action; ii) reducing enamel solubility through a calcium co-ion effect; iii)
reducing enamel solubility
through a fluoride co-ion effect; and iv) reducing enamel solubility through
surface adsorbed
stabilizers. Thus, the anticaries agent can comprise antibacterial agent,
calcium, and/or fluoride.
However, a compound can fall within more than one of these categories, such
as, for example, stannous
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
chloride, which can be antibacterial agent and/or metal or stannous fluoride,
which can be antibacterial
agent, fluoride ion source, and/or metal.
Antibacterial Agent
5
The oral care active agent can comprise antibacterial agent. The
antibacterial agent can be
any agent that suppresses acid formation by the bacteria of dental caries.
Suitable antibacterial agents
include agents that those that can provide at least about an 80%, or about
30%, 60%, 65%, 75%, 85%,
90%, or 95%, reduction in ApH with respect to Crest Cavity Protection that
thereby reduce caries at
least about 9%, or about 1%, 6%, 7%, 8%, 10%, 11%, or 12%, with respect to the
placebo or water
10 control in rat caries experiments.
Suitable antibacterial agents include hops acids, such as hops alpha acids,
hops beta acids,
hydrogenated hops acids, and/or combinations thereof Other suitable
antibacterial agents include
metal ion sources, such as tin ion sources, zinc ion sources, copper ion
sources, and/or combinations
thereof. Other suitable antibacterial agents include triclosan, extracts from
any species within the
15
genus Magnolia, extracts from any species within the genus Humulus. Other
suitable antibacterial
agents include hops acids, tin ion sources, benzyl alcohol, sodium benzoate,
menthylglycyl acetate,
menthyl lactate, L-menthol, o-neomenthol, chlorophyllin copper complex,
phenol, oxyquinoline,
and/or combinations thereof. Other suitable antibacterial agents include one
or more amino acids,
such as basic amino acids.
20
The oral care composition can comprise from about 0.01% to about 10%, from
about 1% to
about 5%, or from about 0.5% to about 15% of an antibacterial agent. Some, but
not all, suitable
antibacterial agents will be discussed separately.
Anti sensitivity Agent
25
The oral care active agent can comprise antisensitivity agent. Suitable
antisensitivity agents
include potassium nitrate, dicarboxylic acid, such as oxalic acid, tin, and/or
combinations thereof
Whitening Agent
The toothpaste composition may comprise from about 0.1% to about 10%, from
about 0.2% to
30
about 5%, from about 1% to about 5%, or from about 1% to about 15%, by
weight of the toothpaste
composition, of whitening agent.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
31
The whitening agent can be a compound suitable for whitening at least one
tooth in the oral
cavity. The whitening agent may include peroxides, metal chlorites,
perborates, percarbonates,
peroxyacids, persulfates, dicarboxylic acids, as described herein, and/or
combinations thereof.
Suitable peroxides include solid peroxides, hydrogen peroxide, urea peroxide,
polyvinylpyrrolidone
peroxide complex, cross-linked polyvinylpyrrolidone peroxide complex, calcium
peroxide, benzoyl
peroxide, sodium peroxide, barium peroxide, inorganic peroxides,
hydroperoxides, organic peroxides,
and mixtures thereof Suitable metal chlorites include calcium chlorite, barium
chlorite, magnesium
chlorite, lithium chlorite, sodium chlorite, and potassium chlorite. Other
suitable whitening agents
include sodium persulfate, potassium persulfate, peroxydone, 6-phthalimido
peroxy hexanoic acid,
Pthalamidoperoxycaproic acid, dicarboxylic acids, such as oxalic acid, malonic
acid, methylmalonic
acid, or mixtures thereof.
Dicarboxylic acid
The toothpaste composition can comprise dicarboxylic acid. The dicarboxylic
acid comprises
a compound with two carboxylic acid functional groups. The dicarboxylic acid
can comprise a
compound or salt thereof defined by Formula I.
0 0
HO ROH
Formula I. Dicarboxylic acid
R can be null, alkyl, alkenyl, allyl, phenyl, benzyl, aliphatic, aromatic,
polyethylene glycol,
polymer, 0, N, P, and/or combinations thereof.
The dicarboxylic acid can comprise oxalic acid, malonic acid, succinic acid,
glutaric acid,
adipic acid, pimelic acid, suberic acid, azerlaic acid, sebacic acid,
undecanedioic acid, dodecanedioic
acid, brassylic acid, thapsic acid, japanic acid, phellogenic acid,
equisetolic acid, malic acid, maleic
acid, tartaric acid, phthalic acid, methylmalonic acid, dimethylmalonic acid,
tartronic acid, mesoxalic
acid, dihydroxymalonic acid, fumaric acid, terephthalic acid, glutaric acid,
salts thereof, or
combinations thereof. The dicarboxylic acid can comprise suitable salts of
dicarboxylic acid, such as,
for example, monoalkali metal oxalate, dialkali metal oxalate, monopotassium
monohydrogen oxalate,
dipotassium oxalate, monosodium monohydrogen oxalate, disodium oxalate,
titanium oxalate, and/or
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
32
other metal salts of oxalate. The dicarboxylic acid can also include hydrates
of the dicarboxylic acid
and/or a hydrate of a salt of the dicarboxylic acid.
The toothpaste composition can comprise from about 0.01% to about 10%, from
about 0.1%
to about 15%, from about 1% to about 5%, or from about 0.0001 to about 25%, by
weight of the
toothpaste composition, of dicarboxylic acid.
Polyphosphate
The toothpaste composition and/or polydentate ligand can comprise
polyphosphate, which can
be provided by a polyphosphate source. A polyphosphate source can comprise one
or more
polyphosphate molecules. Polyphosphates are a class of materials obtained by
the dehydration and
condensation of orthophosphate to yield linear and cyclic polyphosphates, such
as phytic acid, of
varying chain lengths. Thus, polyphosphate molecules are generally identified
with an average
number (n) of polyphosphate molecules, as described below. A polyphosphate is
generally understood
to consist of two or more phosphate molecules arranged primarily in a linear
configuration, although
some cyclic derivatives may be present.
Preferred polyphosphates are those having an average of two or more phosphate
groups so that
surface adsorption at effective concentrations produces sufficient non-bound
phosphate functions,
which enhance the anionic surface charge as well as hydrophilic character of
the surfaces. :Preferred
in this invention are the linear polyphosphates having the formula:
X0(XP03)11X, wherein X is
sodium, potassium, ammonium, or any other alkali metal cations and n averages
from about 2 to about
21, from about 2 to about 14, or from about 2 to about 7. Alkali earth metal
cations, such as calcium,
are not preferred because they tend to foini insoluble fluoride salts from
aqueous solutions comprising
a fluoride ions and alkali earth metal cations. Thus, the toothpaste
compositions disclosed herein can
be free of or substantially free of calcium pyrophosphate.
Some examples of suitable polyphosphate molecules include, for example,
pyrophosphate
(n=2), tripolyphosphate (n=3), tetrapolyphosphate (n=4), sodaphos
polyphosphate (n=6), hexaphos
polyphosphate (n=13), benephos polyphosphate (n=14), hexarnetaphosphate
(n=21), which is also
known as Glass H. Polyphosphates can include those polyphosphate compounds
manufactured by
FMC Corporation, JCL Performance Products, and/or A.staris.
The toothpaste composition can comprise from about 0.01% to about 15%, from
about 0.1%
to about 10%, from about 0.5% to about 5%, from about 1. to about 20%, or
about 10% or less, by
weight of the toothpaste composition, of the polyphosphate source.
Alternatively, the toothpaste
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
33
composition can be essentially free of, substantially free of, or free of
polyphosphate. The toothpaste
composition can be essentially free of, substantially free of, or free of
cyclic polyphosphate, The
toothpaste composition can be essentially free of, substantially free of, or
free of phytic acid, which
can lead to insoluble tin and/or zinc compounds.
Fluoride
The toothpaste composition can comprise fluoride, which can be provided by a
fluoride ion
source. The fluoride ion source can comprise one or more fluoride containing
compounds, such as
stannous fluoride, sodium fluoride, potassium fluoride, amine fluoride, sodium
monofluorophosphate,
zinc fluoride, and/or mixtures thereof.
The fluoride ion source and the tin ion source can be the same compound, such
as for example,
stannous fluoride, which can generate tin ions and fluoride ions.
Additionally, the fluoride ion source
and the tin ion source can be separate compounds, such as when the tin ion
source is stannous chloride
and the fluoride ion source is sodium monofluorophosphate or sodium fluoride.
The fluoride ion source and the zinc ion source can be the same compound, such
as for
example, zinc fluoride, which can generate zinc ions and fluoride ions.
Additionally, the fluoride ion
source and the zinc ion source can be separate compounds, such as when the
zinc ion source is zinc
phosphate and the fluoride ion source is stannous fluoride.
The fluoride ion source can be essentially free of or free of stannous
fluoride. Thus, the
toothpaste composition can comprise sodium fluoride, potassium fluoride, amine
fluoride, sodium
monofluorophosphate, zinc fluoride, and/or mixtures thereof.
The toothpaste composition can comprise a fluoride ion source capable of
providing from
about 50 ppm to about 5000 ppm, and preferably from about 500 ppm to about
3000 ppm of free
fluoride ions. To deliver the desired amount of fluoride ions, the fluoride
ion source may be present
in the toothpaste composition at an amount of from about 0.0025% to about 5%,
from about 0.01% to
about 10%, from about 0.2% to about 1%, from about 0.5% to about 1.5%, or from
about 0.3% to
about 0.6%, by weight of the toothpaste composition. Alternatively, the
toothpaste composition can
comprise less than 0.1%, less than 0.01%, be essentially free of, be
substantially free of, or free of a
fluoride ion source.
Metal
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
34
The toothpaste composition, as described herein, can comprise metal, which can
be provided
by a metal ion source comprising one or more metal ions. The metal ion source
can comprise or be in
addition to the tin ion source and/or the zinc ion source, as described
herein. Suitable metal ion sources
include compounds with metal ions, such as, but not limited to Sn, Zn, Cu, Mn,
Mg, Sr, Ti, Fe, Mo,
B, Ba, Ce, Al, In and/or mixtures thereof. The metal ion source can be any
compound with a suitable
metal and any accompanying ligands and/or anions.
Suitable ligands and/or anions that can be paired with metal ion sources
include, but are not
limited to acetate, ammonium sulfate, benzoate, bromide, borate, carbonate,
chloride, citrate,
gluconate, glycerophosphate, hydroxide, iodide, oxalate, oxide, propionate, D-
lactate, DL-lactate,
orthophosphate, pyrophosphate, sulfate, nitrate, tartrate, and/or mixtures
thereof.
The toothpaste composition can comprise from about 0.01% to about 10%, from
about 1% to
about 5%, or from about 0.5% to about 15% of metal and/or a metal ion source.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
Tin
The toothpaste composition of the present invention can comprise tin, which
can be provided
by a tin ion source. The tin ion source can be any suitable compound that can
provide tin ions in a
toothpaste composition and/or deliver tin ions to the oral cavity when the
toothpaste composition is
5 applied to the oral cavity. The tin ion source can comprise one or more
tin containing compounds,
such as stannous fluoride, stannous chloride, stannous bromide, stannous
iodide, stannous oxide,
stannous oxalate, stannous sulfate, stannous sulfide, stannic fluoride,
stannic chloride, stannic
bromide, stannic iodide, stannic sulfide, and/or mixtures thereof The tin ion
source can comprise
stannous fluoride, stannous chloride, and/or mixture thereof. The tin ion
source can also be a fluoride-
10 free tin ion source, such as stannous chloride.
The toothpaste composition can comprise from about 0.0025% to about 15%, from
about
0.01% to about 10%, from about 0.2% to about 1%, from about 0.4% to about 1%,
or from about 0.3%
to about 0.6%, by weight of the toothpaste composition, of tin and/or a tin
ion source.
15 .. Zinc
The toothpaste composition can comprise zinc, which can be provided by a zinc
ion source.
The zinc ion source can comprise one or more zinc containing compounds, such
as zinc fluoride, zinc
lactate, zinc oxide, zinc phosphate, zinc chloride, zinc acetate, zinc
hexafluorozirconate, zinc sulfate,
zinc tartrate, zinc gluconate, zinc citrate, zinc malate, zinc glycinate, zinc
pyrophosphate, zinc
20 metaphosphate, zinc oxalate, and/or zinc carbonate. The zinc ion source
can be a fluoride-free zinc
ion source, such as zinc phosphate, zinc oxide, and/or zinc citrate.
The zinc and/or zinc ion source may be present in the total toothpaste
composition at an amount
of from about 0.01% to about 10%, from about 0.2% to about 1%, from about 0.5%
to about 1.5%, or
from about 0.3% to about 0.6%, by weight of the composition. Alternatively,
the composition can be
25 essentially free of, substantially free of, or free of zinc.
pH
The pH of the toothpaste compositions as described herein can be from about 4
to about 7,
from about 4.5 to about 6.5, or from about 4.5 to about 5.5. The pH of the
toothpaste compositions,
30 as described herein, can also be at least about 6, at least about 6.5,
or at least about 7. The pH of a
mouthrinse solution can be determined as the pH of the neat solution. The pH
of a dentifrice
composition can be determined as a slurry pH, which is the pH of a mixture of
the dentifrice
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
36
composition and water, such as a 1:4, 1:3, or 1:2 mixture of the dentifrice
composition and water. The
pH of the toothpaste compositions as described herein have a preferred pH of
from about 4 to about
10, from about 5 to about 9, from about 6 to 8, or about 7.
The toothpaste composition can comprise one or more buffering agents.
Buffering agents, as
used herein, refer to agents that can be used to adjust the slurry pH of the
toothpaste compositions.
The buffering agents include alkali metal hydroxides, carbonates,
sesquicarbonates, borates, silicates,
phosphates, imidazole, and mixtures thereof. Specific buffering agents include
monosodium
phosphate, trisodium phosphate, sodium hydroxide, potassium hydroxide, alkali
metal carbonate salts,
sodium carbonate, imidazole, pyrophosphate salts, citric acid, and sodium
citrate. The toothpaste
composition can comprise one or more buffering agents each at a level of from
about 0.1 % to about
30%, from about 1% to about 10%, or from about 1.5% to about 3%, by weight of
the present
composition.
Thickening Agent
The toothpaste composition can comprise one or more thickening agents.
Thickening agents
can be useful in the toothpaste compositions to provide a gelatinous structure
that stabilizes the
toothpaste against phase separation. Suitable thickening agents include
polysaccharides, polymers,
and/or silica thickeners. Some non-limiting examples of polysaccharides
include starch; glycerite of
starch; gums such as gum karaya (sterculia gum), gum tragacanth, gum arabic,
gum ghatti, gum acacia,
xanthan gum, guar gum and cellulose gum; magnesium aluminum silicate (Veegum);
carrageenan;
sodium alginate; agar-agar; pectin; gelatin; cellulose compounds such as
cellulose, carboxymethyl
cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxymethyl
cellulose, hydroxymethyl
carboxypropyl cellulose, methyl cellulose, ethyl cellulose, and sulfated
cellulose; natural and synthetic
clays such as hectorite clays; and mixtures thereof.
The thickening agent can comprise polysaccharides. Polysaccharides that are
suitable for use
herein include carageenans, gellan gum, locust bean gum, xanthan gum,
carbomers, poloxamers,
modified cellulose, and mixtures thereof. Carageenan is a polysaccharide
derived from seaweed.
There are several types of carageenan that may be distinguished by their
seaweed source and/or by
their degree of and position of sulfation. The thickening agent can comprise
kappa carageenans,
modified kappa carageenans, iota carageenans, modified iota carageenans,
lambda carrageenan, and
mixtures thereof. Carageenans suitable for use herein include those
commercially available from the
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
37
FMC Company under the series designation "Viscarin," including but not limited
to Viscarin TP 329,
Viscarin TP 388, and Viscarin TP 389.
The thickening agent can comprise one or more polymers. The polymer can be a
polyethylene
glycol (PEG), a polyvinylpyrrolidone (PVP), polyacrylic acid, a polymer
derived from at least one
acrylic acid monomer, a copolymer of maleic anhydride and methyl vinyl ether,
a crosslinked
polyacrylic acid polymer, of various weight percentages of the toothpaste
composition as well as
various ranges of average molecular ranges. The polymer can comprise
polyacrylate crosspolymer,
such as polyacrylate crosspolymer-6. Suitable sources of polyacrylate
crosspolymer-6 can include
Sepimax Zen Tm commercially available from Seppic.
The thickening agent can comprise inorganic thickening agents. Some non-
limiting examples
of suitable inorganic thickening agents include colloidal magnesium aluminum
silicate, silica
thickeners. Useful silica thickeners include, for example, include, as a non-
limiting example, an
amorphous precipitated silica such as ZEODENT 165 silica. Other non-limiting
silica thickeners
include ZEODENT 153, 163, and 167, and ZEOFREE 177 and 265 silica products,
all available
from Evonik Corporation, and AEROSIL fumed silicas.
The toothpaste composition can comprise from 0.01% to about 15%, from 0.1% to
about 10%,
from about 0.2% to about 5%, or from about 0.5 % to about 2% of one or more
thickening agents.
Alternatively, the toothpaste composition can be free of, essentially free of,
and/or
substantially free of thickening agent as the jammed oil-in-water emulsion has
an unexpectedly high
yield stress relative to its constituent components.
Amino Acid
The toothpaste composition can comprise amino acid. The amino acid can
comprise one or
more amino acids, peptide, and/or polypeptide, as described herein.
Amino acids, as in Formula II, are organic compounds that contain an amine
functional group,
a carboxyl functional group, and a side chain (R in Formula III) specific to
each amino acid. Suitable
amino acids include, for example, amino acids with a positive or negative side
chain, amino acids with
an acidic or basic side chain, amino acids with polar uncharged side chains,
amino acids with
hydrophobic side chains, and/or combinations thereof. Suitable amino acids
also include, for example,
arginine, histidine, lysine, aspartic acid, glutamic acid, serine, threonine,
asparagine, glutamine,
cysteine, selenocysteine, glycine, proline, alanine, valine, isoleucine,
leucine, methionine,
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
38
phenylalanine, tyrosine, tryptophan, citrulline, ornithine, creatine,
diaminobutanoic acid,
diaminoproprionic acid, salts thereof, and/or combinations thereof
Suitable amino acids include the compounds described by Formula III, either
naturally
occurring or synthetically derived. The amino acid can be zwitterionic,
neutral, positively charged, or
negatively charged based on the R group and the environment. The charge of the
amino acid, and
whether particular functional groups, can interact with tin at particular pH
conditions, would be well
known to one of ordinary skill in the art.
C)
H3N
0
Formula III. Amino Acid. R is any suitable functional group
Suitable amino acids include one or more basic amino acids, one or more acidic
amino acids,
one or more neutral amino acids, or combinations thereof.
The toothpaste composition can comprise from about 0.01% to about 20%, from
about 0.1%
to about 10%, from about 0.5% to about 6%, or from about 1% to about 10 % of
amino acid, by weight
of the toothpaste composition.
The term "neutral amino acid" as used herein includes not only naturally
occurring neutral amino
acids, such as alanine, asparagine, cysteine, glutamine, glycine, isoleucine,
leucine, methionine,
phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine, but
also other amino acids
having an isoelectric point in range of pH 5.0 to 7Ø The neutral amino acid
can also be at least
partially water soluble and provide a pH of about 7 or less in an aqueous
solution of 1 g of neutral
amino acid in 1000 mL of distilled water at 25 C.
Accordingly, suitable neutral amino acids can also include alanine,
aminobutyrate, asparagine,
cysteine, cystine, glutamine, glycine, hydroxyproline, isoleucine, leucine,
methionine, phenylalanine,
proline, serine, taurine, threonine, tryptophan, tyrosine, valine, salts
thereof, or mixtures thereof
Preferably, neutral amino acids used in the composition of the present
invention may include
asparagine, glutamine, glycine, salts thereof, and/or mixtures thereof
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
39
Vitamin
The toothpaste composition can comprise one or more vitamins. As used herein,
"vitamin"
includes all natural and/or synthetic analogs of vitamins, vitamers, compounds
and/or derivatives that
exhibit the biologically activity of vitamins, isomers of these compounds,
stereoisomers of these
compounds, salts of these compounds, or combinations thereof.
Suitable vitamins for gum health can include Vitamin A, such as retinoid
compound, Vitamin
B, including Vitamin B1 (thiamine), Vitamin B2 (riboflavin), Vitamin B3
(niacin), Vitamin B5
(pantothenic acid), Vitamin B6, Vitamin B7 (biotin), Vitamin B9 (folic acid
and/or folate), Vitamin
B12 (cyanocobalamin), Vitamin C, Vitamin D, Vitamin E, Vitamin K, and/or
combinations thereof.
Vitamins can also include other vitamin-like compounds, such as choline,
carnitine, or combinations
thereof
The toothpaste composition can comprise from about 0.0001% to about 10%, from
about
0.01% to about 5%, or from about 0.01% to about 2%, by weight of the
composition, of vitamin.
Retinoid Compound
The compositions of the present invention can comprise one or more retinoid
compounds. As
used herein, "retinoid compound" includes all natural and/or synthetic analogs
of Vitamin A or retinol-
like compounds that possess the biological activity of Vitamin A in the skin
as well as the geometric
isomers and stereoisomers of these compounds. The retinoid compound can, for
example, be retinol,
retinyl esters (e.g., C-C alkyl esters of retinol, including retinyl
palmitate, retinyl acetate, retinyl
propionate), retinal, and/or retinoic acid (including all-trans retinoic acid
and/or 13-cis-retinoic acid).
In some embodiments, retinoids other than retinoic acid are used. These
compounds are available in
the art and are commercially available from several sources, e.g., Sigma
Chemical Company (St.
Louis, Mo.), and Boehringer Mannheim (Indianapolis, Ind.). Other suitable
retinoids are tocopheryl-
.. retinoate, tocopherol ester of cis- or trans-retinoic acid, adapalene (6-3-
(1-adamanty1)- 4-
methoxypheny1-2-naphthoic acid), and tazarotene (ethyl 6-2-(4.4-
dimethylthiochroman-6-y1)-
ethynylnicotinate). Desirable retinoids include retinol, retinoic acid,
retinyl palmitate, retinyl acetate,
retinyl propionate, retinal, and combinations thereof
The retinoid compound may be included as the substantially pure material, or
as an extract
obtained by suitable physical and/or chemical isolation from natural (e.g.,
plant) sources. The retinoid
compound can be substantially pure, or essentially pure. The compositions of
this invention may
contain a safe and effective amount of the retinoid compound, such that the
toothpaste composition is
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
safe and effective for regulating or improving the condition of keratinous
tissues and accidental
ingestion since applied to the oral cavity.
The retinoid compound can comprise retinol, retinyl ester, retinal, retinoic
acid, tocopheryl-
retinoate, tocopherol ester of cis- or trans-retinoic acid, isotretinoin,
alitretinoin, etretinate, acitretin,
5 adapalene, bexarotene, tazarotene, or combinations thereof The retinoid
compound can be
pharmaceutical grade, USP, or the like grade, due to use in the oral cavity.
The retinoid compound
and/or the retinol can have a purity of at least about 95%, at least about
97%, at least about 99%, at
least about 99.5%, or at least about 99.9%. The toothpaste composition can
comprise from about
0.0001% to about 10%, from about 0.01% to about 5%, or from about 0.01% to
about 2%, by weight
10 of the composition, of retinoid compound. The toothpaste composition can
comprise from about 1
ppm to about 10,000 ppm, from about 500 ppm to about 5000 ppm, from about 750
ppm to about 5000
ppm, from about 1000 ppm to about 2500 ppm, about 1500 ppm, or about 2250 ppm
of retinoid
compound. Amounts of retinoid compound that are greater than about 5000 ppm
are thought to lead
to toxicity concerns for formulating with toothpaste compositions, which would
not be present in skin
15 care compositions.
The retinoid compound can comprise retinol comprising cis- and/or trans-
alkene functional
groups. The retinol can comprise at least about 80%, at least about 90%, at
least about 95%, and/or at
least about 99% of trans-alkene functional groups.
The retinoid compound can also comprise surfactant, such as anionic
surfactant, cationic
20 surfactant, and/or nonionic surfactant, which can improve gum barrier
permeability. Suitable
surfactants can include polysorbate.
Peptide
The toothpaste composition can comprise peptide. A peptide is a linear organic
polymer
25 consisting of a number of amino-acid residues bonded together in a
chain, forming part of (or the
whole of) a protein molecule. The peptide can comprise from two amino acids to
ten amino acids,
from two amino acids to five amino acids, or from four amino acids to six
amino acids.
Peptides, including but not limited to, di-, tri-, tetra-, and pentapeptides
and derivatives thereof,
may be included in the compositions of the present invention in amounts that
are safe and effective,
30 including safe and effective for ingestion. As used herein, "peptides'
refers to both the naturally
occurring peptides and synthesized peptides. Also, useful herein are naturally
occurring and
commercially available compositions that contain peptides.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
41
Suitable dipeptides for use herein include, for example, Carnosine (beta-ala-
his). Suitable
tripeptides for use herein include, for example, gly-his-lys, arg-lys-arg,
and/or his-gly-gly. Suitable
tripeptide derivatives include palmitoyl-gly-his-lys, which may be purchased
as Biopeptide CLTM
(100 ppm of palmitoyl-gly his-lys commercially available from Sederma,
France); Peptide CK (arg-
lys-arg); Peptide CK(ac-arg-lys-arg-NH2); and a copper derivative of his-gly-
gly sold commercially
as Iamin, from Sigma (St. Louis, Mo.). Suitable tetrapeptides for use herein
include, for example,
Peptide E, arg-ser-arg-lys.
Suitable pentapeptides for use herein include lys-thr-thr-lys-ser. A preferred
commercially
available pentapeptide derivative composition is MatrixylTm, which contains
100 ppm palmitoyl-lys-
thr-thr-lys-ser (commercially available from Sederma France).
The peptide can comprise palmitoyl-lys-thr-thr lys-ser, palmitoyl-gly-his-lys,
beta-ala-his,
their derivatives, and/or combinations thereof In some embodiments, the
peptide comprises
palmitoyl-lys-thr-thr-lys-ser, palmitoyl-gly-his-lys, their derivatives, or
combinations thereof. In
other embodiments, the peptide comprises palmitoyl-lys-thr-thr-lys-ser (pal-
KTTKS) and/or
derivatives thereof. Other suitable peptides include gly-his-ly (GHK), gly-glu-
lys-gly (GEKG), or
combinations thereof.
The toothpaste composition can comprise from about 0.0001% to about 10%, from
about
0.01% to about 5%, from about 0.001% to about 5%, from about 0.01% to about
2%, or from about
0.0001% to about 1%, by weight of the composition of peptide. The toothpaste
composition can
comprise from about 1 ppm to about 1000 ppm, from about 1 ppm to about 100
ppm, from about 3
ppm to about 50 ppm, or from about 1 ppm to about 10000 ppm, by weight of the
toothpaste
composition, of peptide. Amounts of peptide that are greater than about 10000
ppm are thought to
lead to toxicity concerns for formulating with toothpaste compositions, which
would not be present in
skin care compositions.
Humectant
The toothpaste composition can comprise one or more humectants, have low
levels of
humectant, be free of humectant, be substantially free of humectant, and/or
essentially free of
humectant. Humectants serve to add body or "mouth texture" to a toothpaste
composition or dentifrice
as well as preventing the dentifrice from drying out. Suitable humectants
include polyethylene glycol
(at a variety of different molecular weights), propylene glycol, glycerin
(glycerol), erythritol, xylitol,
sorbitol, mannitol, butylene glycol, lactitol, hydrogenated starch
hydrolysates, and/or mixtures
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
42
thereof. The toothpaste composition can comprise one or more humectants each
at a level of from 0
to about 70%, from about 5% to about 50%, from about 10% to about 60%, or from
about 20% to
about 80%, by weight of the toothpaste composition.
Humulus lupulus
The toothpaste compositions of the present invention can comprise hops. The
hops can
comprise at least one hops compound from Formula IV and/or Formula VII. The
compound from
Formula IV and/or Formula VII can be provided by any suitable source, such as
an extract from
Humulus lupulus or Hops, Humulus lupulus itself, a synthetically derived
compound, and/or salts,
prodrugs, or other analogs thereof The hops extract can comprise one or more
hops alpha acids, one
or more hops iso-alpha acids, one or more hops beta acids, one or more hops
oils, one or more
flavonoids, one or more solvents, and/or water. Suitable hops alpha acids
(generically shown in
Formula IV) can include humulone (Formula V), adhumulone, cohumulone,
posthumulone,
prehumulone, and/or mixtures thereof. Suitable hops iso-alpha acids can
include cis-isohumulone
.. and/or trans-isohumulone. The isomerization of humulone into cis-
isohumulone and trans-
isohumulone can be represented by Formula VI.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
43
0
B 0
A
Formula IV. Hops Alpha Acids. A is the acidic hydroxyl functional group in the
alpha position, B
are the acidic hydroxyl functional groups in the beta position, and R is an
alkyl functional group.
OH 0
HO 0
'" OH
Formula V. Humulone
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
44
OH 0 INN.,
0
410 1 0
HO . 0 ________00._
HO i:--
" y OH
Formula VI. Isomerization of Humulone to isohumulone.
Suitable hops beta acids can include lupulone, adlupulone, colupulone, and/or
mixtures
thereof. A suitable hops beta acid can include a compound a described in
Formula VII, VIII, IX,
and/or X.
B 0
R
B 0
.--
$
'\.õ...,..'%-. \.
Formula VII. Hops Beta Acids. B are the acidic hydroxyl functional groups in
the beta position and
R is an alkyl functional group.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
OH 0
Is HO 0
:-
Formula VIII. Lupulone
OH 0
is '--
HO . 0
$
:-.
y,.....,.,
5 Formula IX. Adlupulone
OH 0
op HO 0
;--'
Formula X. Colupulone
10 While hops alpha acids can demonstrate some antibacterial activity, hops
alpha acids also have
a bitter taste. The bitterness provided by hops alpha acids can be suitable
for beer, but are not suitable
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
46
for use in toothpaste compositions. In contrast, hops beta acids can be
associated with a higher
antibacterial and/or anticaries activity, but not as bitter a taste. Thus, a
hops extract with a higher
proportion of beta acids to alpha acids than normally found in nature, can be
suitable for use in
toothpaste compositions for use as an antibacterial and/or anticaries agent.
A natural hops source can comprise from about 2% to about 12%, by weight of
the hops source,
of hops beta acids depending on the variety of hops. Hops extracts used in
other contexts, such as in
the brewing of beer, can comprise from about 15% to about 35%, by weight of
the extract, of hops
beta acids. The hops extract desired herein can comprise at least about 35%,
at least about 40%, at
least about 45%, from about 35% to about 95%, from about 40% to about 90%, or
from about 45% to
about 99%, of hops beta acids. The hops beta acids can be in an acidic form
(i.e. with attached
hydrogen atom(s) to the hydroxl functional group(s)) or as a salt form.
A suitable hops extract is described in detail in U.S. Patent No. 7,910,140,
which is herein
incorporated by reference in its entirety. The hops beta acids desired can be
non-hydrogenated,
partially hydrogenated by a non-naturally occurring chemical reaction, or
hydrogenated by a non-
naturally occurring chemical reaction. The hops beta acid can be essentially
free of or substantially
free of hydrogenated hops beta acid and/or hops acid. A non-naturally
occurring chemical reaction is
a chemical reaction that was conducted with the aid of chemical compound not
found within Humulus
lupulus, such as a chemical hydrogenation reaction conducted with high heat
not normally experienced
by Humulus lupulus in the wild and/or a metal catalyst.
A natural hops source can comprise from about 2% to about 12%, by weight of
the hops source,
of hops alpha acids. Hops extracts used in other contexts, such as in the
brewing of beer, can comprise
from about 15% to about 35%, by weight of the extract, of hops alpha acids.
The hops extract desired
herein can comprise less than about 10%, less than about 5%, less than about
1%, or less than about
0.5%, by weight of the extract, of hops alpha acids.
Hops oils can include terpene hydrocarbons, such as myrcene, humulene,
caryophyllene,
and/or mixtures thereof The hops extract desired herein can comprise less than
5%, less than 2.5%,
or less than 2%, by weight of the extract, of one or more hops oils.
Flavonoids present in the hops extract can include xanthohumol, 8-
prenylnaringenin,
isoxanthohumol, and/or mixtures thereof. The hops extract can be substantially
free of, essentially
free of, free of, or have less than 250 ppm, less than 150 ppm, and/or less
than 100 ppm of one or more
flavonoids.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
47
As described in U.S. Patent No. 5,370,863, hops acids have been previously
added to
toothpaste compositions. However, the toothpaste compositions taught by U.S.
Patent No. 5,370,863
only included up to 0.01%, by weight of the toothpaste composition. While not
wishing to be bound
by theory, it is believed that U.S. Patent No. 5,370,863 could only
incorporate a low amount of hops
acids because of the bitterness of hops alpha acids. A hops extract with a low
level of hops alpha acids
would not have this concern.
The hops compound can be combined with or free from an extract from another
plant, such as
a species from genus Magnolia. The hops compounds can be combined with or free
from triclosan.
The toothpaste composition can comprise from about 0.01% to about 10%, greater
than 0.01%
to about 10%, from about 0.05%, to about 10%, from about 0.1% to about 10%,
from about 0.2% to
about 10%, from about 0.2% to about 10%, from about 0.2% to about 5%, from
about 0.25% to about
2%, from about 0.05% to about 2%, or from greater than 0.25% to about 2%, of
hops, such as hops
beta acid, as described herein. The hops, such as the hops beta acid, can be
provided by a suitable
hops extract, the hops plant itself, or a synthetically derived compound. The
hops, such as hops beta
acid, can be provided as neutral, acidic compounds, and/or as salts with a
suitable counter ion, such as
sodium, potassium, ammonia, or any other suitable counter ion.
The hops can be provided by a hops extract, such as an extract from Humulus
lupulus with at
least 35%, by weight of the extract, of hops beta acid and less than 1%, by
weight of the hops extract,
of hops alpha acid. The toothpaste composition can comprise 0.01% to about
10%, greater than 0.01%
to about 10%, from about 0.05%, to about 10%, from about 0.1% to about 10%,
from about 0.2% to
about 10%, from about 0.2% to about 10%, from about 0.2% to about 5%, from
about 0.25% to about
2%, from about 0.05% to about 2%, or from greater than 0.25% to about 2%, of
hops extract, as
described herein.
Prenylated Flavonoids
The toothpaste composition can comprise prenylated flavonoid. Flavonoids are a
group of
natural substances found in a wide range of fruits, vegetables, grains, bark,
roots, stems, flowers, tea,
and wine. Flavonoids can have a variety of beneficial effects on health, such
as antioxidative, anti-
inflammatory, antimutagenic, anticarcinogenic, and antibacterial benefits.
Prenylated flavonoids are
flavonoids that include at least one prenyl functional group (3-methylbut-2-en-
1-yl, as shown in
Formula XI), which has been previously identified to facilitate attachment to
cell membranes. Thus,
while not wishing to being bound by theory, it is believed that the addition
of a prenyl group, i.e.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
48
prenylation, to a flavonoid can increase the activity of the original
flavonoid by increasing the
lipophilicity of the parent molecule and improving the penetration of the
prenylated molecule into the
bacterial cell membrane. Increasing the lipophilicity to increase penetration
into the cell membrane
can be a double-edged sword because the prenylated flavonoid will tend towards
insolubility at high
Log P values (high lipophilicity). Log P can be an important indicator of
antibacterial efficacy.
As such, the term prenylated flavonoids can include flavonoids found naturally
with one or
more prenyl functional groups, flavonoids with a synthetically added prenyl
functional group, and/or
prenylated flavonoids with additional prenyl functional groups synthetically
added.
Fz
Formula XI. Prenyl Function Group with R representing the other portions of
the molecule
Other suitable functionalities of the parent molecule that improve the
structure-activity
relationship (e.g,. structure-MIC relationship) of the prenylated molecule
include additional
heterocycles containing nitrogen or oxygen, alkylamino chains, or alkyl chains
substituted onto one
or more of the aromatic rings of the parent flavonoid.
Flavonoids can have a 15-carbon skeleton with at least two phenyl rings and at
least one
heterocyclic ring. Some suitable flavonoid backbones can be shown in Formula
XII (flavone
backbone), Formula XIII (i soflavan backbone), and/or Formula XIV (neofl
avonoid backbone).
Formula XII. Flavone Backbone
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
49
Formula XIII. Isoflavan backbone
Formula XIV. Neoflavanoid backbone
Other suitable subgroups of flavonoids include anthocyanidins, anthoxanthins,
flavanones,
flavanonols, flavans, isoflavonoids, chalcones and/or combinations thereof.
Prenylated flavonoids can include naturally isolated prenylated flavonoids or
naturally isolated
flavonoids that are synthetically altered to add one or more prenyl functional
groups through a variety
of synthetic processes that would be known to a person of ordinary skill in
the art of synthetic organic
chemistry.
Other suitable prenylated flavonoids can include Bavachalcone, Bavachin,
Bavachinin,
Corylifol A, Epimedin A, Epimedin Al, Epimedin B, Epimedin C, Icariin,
Icariside I, Icariside II,
Icaritin, Isobavachalcone, Isoxanthohumol, Neobavaisoflavone, 6-
Prenylnaringenin, 8-
Prenylnaringenin, Sophoraflavanone G, (-)-Sophoranone, Xanthohumol, Quercetin,
Macelignan,
Kuraridin, Kurarinone, Kuwanon G, Kuwanon C, Panduratin A, 6-
geranylnaringenin, Australone A,
6,8-Diprenyleriodictyol, dorsmanin C, dorsmanin F, 8-Prenylkaempferol, 7-0-
Methylluteone,
luteone, 6-prenylgenistein, isowighteone, lupiwighteone, and/or combinations
thereof Other suitable
prenylated flavonoids include cannflavins, such as Cannflavin A, Cannflavin B,
and/or Cannflavin C.
Preferably, the prenylated flavonoid has a high probability of having an MIC
of less than about
ppm for S. aureus, a gram-positive bacterium. Suitable prenylated flavonoids
include Bavachin,
Bavachinin, Corylifol A, Icaritin, Isoxanthohumol, Neobavaisoflavone, 6-
Prenylnaringenin, 8-
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
Prenylnaringenin, Sophoraflavanone G, (-)-Sophoranone, Kurarinone, Kuwanon C,
Panduratin A,
and/or combinations thereof
Preferably, the prenylated flavonoid has a high probability of having an MIC
of less than about
25 ppm for E. coil, a gram-negative bacterium. Suitable prenylated flavonoids
include Bavachinin,
5 Isoxanthohumol, 8-Prenylnaringenin, Sophoraflavanone G, Kurarinone,
Panduratin A, and/or
combinations thereof.
Approximately 1000 prenylated flavonoids have been identified from plants.
According to the
number of prenylated flavonoids reported before, prenylated flavonones are the
most common
subclass and prenylated flavanols is the rarest sub-class. Even though natural
prenylated flavonoids
10 .. have been detected to have diversely structural characteristics, they
have a narrow distribution in
plants, which are different to the parent flavonoids as they are present
almost in all plants. Most of
prenylated flavonoids are found in the following families, including
Cannabaceae, Guttiferae,
Leguminosae, Moraceae, Rutaceae and Umbelliferae. Leguminosae and Moraceae,
due to their
consumption as fruits and vegetables, are the most frequently investigated
families and many novel
15 prenylated flavonoids have been explored. Humulus lupulus of the
Cannabaceae include 8-
prenylnaringenin and xanthohumol, which can play a role in the health benefits
of beer.
The prenylated flavonoid can be incorporated through a hops extract,
incorporated in a
separately added extract, or added as a separate component of the toothpaste
compositions disclosed
herein.
20 Suitable prenylated flavonoids can have a particular octanol-water
partitioning coefficient.
The octanol-water partitioning coefficient can be used to predict the
lipophilicity of a compound.
Without wishing to being bound by theory, it is believed that compounds that
fall within the ranges
described herein will be able to enter and/or disrupt the primarily
hydrophobic phospholipid bilayer
that makes up the cell membrane of microorganisms. Thus, the octanol-water
partitioning coefficient
25 .. can be correlated to the antibacterial effect of prenylated flavonoids.
Suitable prenylated flavonoids
can have a log P of at least about 2, at least about 4, from about 2 to about
10, from about 4 to about
10, from about 4 to about 7, or from about 4 to about 6.
The toothpaste composition can comprise at least about 0.001%, from about
0.001% to about
5%, from about 0.01% to about 2%, from about 0.0001% to about 2%, or at least
about 0.05% of
30 prenylated flavonoid.
Other Ingredients
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
51
The toothpaste composition can comprise a variety of other ingredients, such
as flavoring
agents, sweeteners, colorants, preservatives, buffering agents, or other
ingredients suitable for use in
toothpaste compositions, as described below.
Flavoring agents also can be added to the toothpaste composition. Suitable
flavoring agents
include oil of wintergreen, oil of peppermint, oil of spearmint, clove bud
oil, menthol, anethole, methyl
salicylate, eucalyptol, cassia, 1-menthyl acetate, sage, eugenol, parsley oil,
oxanone, alpha-irisone,
marjoram, lemon, orange, propenyl guaethol, cinnamon, vanillin, ethyl
vanillin, heliotropine, 4-cis-
heptenal, diacetyl, methyl-para-tert-butyl phenyl acetate, and mixtures
thereof. Coolants may also be
part of the flavor system. Preferred coolants in the present compositions are
the paramenthan
carboxyamide agents such as N-ethyl-p-menthan-3-carboxamide (known
commercially as "WS-3") or
N-(Ethoxycarbonylmethyl)-3-p-menthanecarboxamide (known commercially as "WS-
5"), and
mixtures thereof. A flavor system is generally used in the compositions at
levels of from about 0.001
% to about 5%, by weight of the toothpaste composition. These flavoring agents
generally comprise
mixtures of aldehydes, ketones, esters, phenols, acids, and aliphatic,
aromatic and other alcohols.
Sweeteners can be added to the toothpaste composition to impart a pleasing
taste to the product.
Suitable sweeteners include saccharin (as sodium, potassium or calcium
saccharin), cyclamate (as a
sodium, potassium or calcium salt), acesulfame-K, thaumatin, neohesperidin
dihydrochalcone,
ammoniated glycyrrhizin, dextrose, levulose, sucrose, mannose, sucralose,
stevia, and glucose.
Colorants can be added to improve the aesthetic appearance of the product.
Suitable colorants
include without limitation those colorants approved by appropriate regulatory
bodies such as the FDA
and those listed in the European Food and Pharmaceutical Directives and
include pigments, such as
TiO2, and colors such as FD&C and D&C dyes.
Preservatives also can be added to the toothpaste compositions to prevent
bacterial growth.
Suitable preservatives approved for use in oral compositions such as
methylparaben, propylparaben,
benzoic acid, and sodium benzoate can be added in safe and effective amounts.
Titanium dioxide may also be added to the present composition. Titanium
dioxide is a white
powder which adds opacity to the compositions. Titanium dioxide generally
comprises from about
0.25% to about 5%, by weight of the toothpaste composition.
Other ingredients can be used in the toothpaste composition, such as
desensitizing agents,
healing agents, other caries preventative agents, chelating/sequestering
agents, vitamins, proteins,
other anti-plaque/anti-calculus agents, antibiotics, anti-enzymes, enzymes, pH
control agents,
oxidizing agents, antioxidants, and the like.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
52
Methods of Using the Compositions and/or Delivery Systems
The jammed oil-in-water emulsion toothpaste composition can be used in the
treatment,
reduction, and/or prevention of caries, cavities, gingivitis, and/or
combinations thereof
The user can be instructed to apply a portion of the toothpaste onto a
toothbrush. The portion
of the toothpaste can be of any suitable shape, such as strip, a pea-sized
amount, or various other
shapes that would fit onto any mechanical and/or manual brush head. The user
can be instructed to
apply a strip of the toothpaste that is at least about 1 inch, at least about
0.5 inch, at least 1 inch, and/or
at least 0.5 inch long to the bristles of a toothbrush, such as soft-bristled
toothbrush.
The user can be instructed to apply pea-sized or grain of rice-sized portion
of the toothpaste to
the bristles of a toothbrush, such as in the case of use by children of less
than 6 years old and/or less
than 2 years old.
The user can be instructed to brush their teeth for at least about 30 seconds,
at least about 1
minute, at least about 90 seconds, at least about 2 minutes, at least 30
seconds, at least 1 minute, at
least 90 seconds, and/or at least 2 minutes.
The user can be instructed to brush their teeth thoroughly and/or as directed
by a physician
and/or dentist.
The user can be instructed to brush their teeth after each meal. The user can
be instructed to
brush their teeth at least once per day, at least twice per day, and/or at
least three times per day. The
user can be instructed to brush their teeth no more than three times a day,
such as to prevent Sn staining.
The user can be instructed to brush their teeth in the morning and/or in the
evening prior to sleeping.
The user can be instructed to not swallow the toothpaste composition due to
the inclusion of
ingredients that are not suitable for ingestion, such as fluoride. The user
may be instructed to
expectorate (or spit out) the toothpaste composition after the cessation of
the brushing cycle.
The usage instructions for the toothpaste, can vary based on age. For example,
adults and
children that are at least 6 or at least 2 can have one usage instruction
while children under 6 or under
2 can have a second usage instruction.
The jammed oil-in-water emulsion toothpaste can be used in a multi-step oral
health regimen.
A first composition comprising jammed oil-in-water emulsion toothpaste
comprising first oral care
active, such as peroxide, fluoride, and/or tin, can be used as the first step
in the oral health regimen.
A second oral care composition comprising second oral care active, such as
peroxide, fluoride, and/or
tin, can be used as the second step in the oral health regimen.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
53
The oral health regimen can comprise: (1) directing a user to apply a first
composition to an
oral cavity of the user, the first composition comprising a jammed oil-in-
water toothpaste composition,
the jammed oil-in-water toothpaste composition comprising fluoride and/or tin
and (2) directing the
user to apply a second composition to the oral cavity of the user, the second
composition comprising
peroxide. The second composition can be a jammed oil-in-water emulsion
composition, a multi-phase
oral care composition, or a single-phase oral care composition. Application of
fluoride and/or tin
followed by peroxide can be particularly advantageous to the user.
The oral health regimen can comprise: (1) directing a user to apply a first
composition to an
oral cavity of the user, the first composition comprising a jammed oil-in-
water toothpaste composition,
the jammed oil-in-water toothpaste composition comprising antisensitivity
compound and (2)
directing the user to apply a second composition to the oral cavity of the
user, the second composition
comprising peroxide. The second composition can be a jammed oil-in-water
emulsion composition,
a multi-phase oral care composition, or a single-phase oral care composition.
Application of
antisensitivity compound followed by peroxide can be particularly advantageous
to the user to
minimize sensitivity that can be experienced by some uses of peroxide.
The present invention can also be applied to the teeth of a consumer in the
dental office by a
dental professional, or the present invention can be applied at home by the
consumer. Generally, the
recommended treatment period is a sufficient period of time to achieve
whitening.
The composition can also be applied with a paint-on device, a syringe or unit
dose syringe,
squeezable tube, a brush, a pen or brush tip applicator, a doe's foot
applicator, swab, lip gloss
applicator, strip that is removed after application, tray that is removed
after application, or the like, or
even with the fingers. The composition can also be combined with a delivery
carrier, such as a strip
of material, a dental tray, or a sponge material, and thereafter applied to
the teeth. In certain aspects,
the compositions or delivery systems herein are almost unnoticeable when
applied to the teeth. After
a desired period of time has elapsed, any residual composition may be easily
removed by wiping,
brushing or rinsing the oral surface.
The described compositions and delivery systems, described herein, may be
combined in a kit
which comprises: 1. present composition and 2. instructions for use; or which
comprises: 1. present
composition, 2. instructions for use, and 3. a delivery carrier. In addition,
if the tooth shall be radiated
by electromagnetic radiation, the kit may further comprise an electromagnetic
radiation source of the
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
54
appropriate wavelength and instruction for use, so that the kit can be used by
consumers in a
convenient manner.
Optional Electromagnetic Radiation Treatment
The multi-phase oral care composition as disclosed herein may be used to
whiten teeth and/or
removing stain from tooth surfaces. In addition, the bleaching efficacy may be
further increased by
directing electromagnetic radiation of a suitable wavelength toward at least
one tooth. A suitable
wavelength may be any wavelength, which corresponds to a maximum absorption
band of the tooth
and/or the tooth stain to be bleached. For example, the multi-phase oral care
composition may be
radiated with an electromagnetic radiation with one or more wavelengths in the
range of from about
200 nm to about 1200 nm. The electromagnetic radiation may be directed toward
at least one tooth.
In addition, more than one tooth may be irradiated. For example, the
electromagnetic radiation may
have a peak intensity at one or more wavelengths in the range of from about 1
nm to about 750 nm,
from about 200 nm to about 700 nm, from about 300 nm to about 700 nm, from
about 400 nm to about
600 nm, from about 400 nm to about 500 nm, or up to about 750 nm.
Additionally, the electromagnetic
radiation may have a peak intensity at one or more wavelengths in the range of
from about 400, 405,
410, 415, 420, 425, 430, 435, 440, or 445, 446 nm to about 450, 455, 460, 465,
470, 475, 480, 481,
485, 490, 495, or 500 nm or any other numerical range, which is narrower and
which falls within such
broader numerical range, as if such narrower numerical ranges were all
expressly written herein. The
electromagnetic radiation can have a peak intensity at a wavelength in the
range of from about 425
nm to about 475 nm, from about 445 nm to about 465 nm, or wherein the peak
intensity wavelength
of the electromagnetic radiation is similar to the wavelength at which the
stain absorbs the most
electromagnetic radiation. Electromagnetic radiation may be directed toward at
least one tooth for
partial or whole wearing time of the composition; or after the composition has
been removed from the
tooth. Electromagnetic radiation may be applied at least for a sufficient
period of time for whitening,
e.g. for at least about 1 minute, for at least about 5 minutes, or for at
least about 10 min. The
electromagnetic radiation may be applied using the procedure disclosed in US
2013/0295525.
Preferably the multi-phase oral care composition as disclosed herein is
applied to at least one tooth
and maintained on the at least one tooth for a first period of time; after the
first period of time
electromagnetic radiation is directed toward the at least one tooth for a
second period of time, wherein
the first period of time has a duration greater than 50%, preferably 80% of a
total duration of the first
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
and second periods of time; and finally, the multi-phase oral care composition
is removed from the at
least one tooth. Suitable sources of electromagnetic radiation include the
sources described herein.
The multi-phase oral care compositions as disclosed herein may be transparent
or translucent
to electromagnetic radiation with wavelengths from about 400nm to about 500nm.
In certain aspects,
5 the multi-phase oral care compositions as disclosed herein when applied
in a thickness of from about
0.0001, 0.001, or 0.01 cm to about 0.01, 0.1, or 0.5 cm thick allow from about
10%, 20%, or 30% to
about 40%, 50%, 60%, 70%, 80%, 90%, or 100% of electromagnetic radiation at
one or more
wavelengths in the range of from about 1 nm to about 750 nm, from about 200 nm
to about 700 nm,
from about 300 nm to about 700 nm, from about 400 nm to about 600 nm, from
about 400 nm to about
10 500 nm, or up to about 750 nm to pass through, as measured by a
spectrophotometer. When a multi-
phase oral care composition is applied in a thickness of about 0.1cm, from
about 80% to about 100%
of electromagnetic radiation from about 400nm to about 500nm can pass through,
as measured by a
spectrophotometer. The multi-phase oral care compositions, as disclosed
herein, may when applied
in an amount from about 0.0001, 0.001, or 0.01 grams to about 0.01, 0.1, 1, or
5 grams, on a delivery
15 carrier or tray with a surface area from about 5cm2 to about 20cm2,
allow from about 10%, 20%, or
30% to about 40%, 50%, 60%, 70%, 80%, 90%, or 100% of electromagnetic
radiation from about 400
nm to about 500 nm to pass through.
The electromagnetic radiation impinging on the surface of the tooth or outer
surface of the
carrier, which may be a strip or tray, at one or more wavelengths in the range
of from about 1 nm to
20 about 750 nm, from about 200 nm to about 700 nm, from about 300 nm to
about 700 nm, from about
400 nm to about 600 nm, from about 400 nm to about 500 nm, or up to about 750
nm. may range in
intensity from about 5, 10, 25, 50, 75, or 100 mW/cm2 to about 10000, 5000,
2000, 1000, 500, 250,
225, 205, 200, 175, 150, 125, 100, 75, 50, 25, 10, or 5 mW/cm2 or any other
numerical range, which
is narrower and which falls within such broader numerical range, as if such
narrower numerical ranges
25 were all expressly written herein.
The intensity of the electromagnetic radiation can be measured using a
spectrometer (USB
2000+ from Ocean Optics) connected to a UV-VIS 200 micron fiber-optic cable
with a cosine
corrector at the tip (OP 200-2-UV-VIS from Ocean Optics). The spectrometer is
connected to a
computer running the spectrometer software (Oceanview 1.3.4 from Ocean
Optics). The tip of the
30 fiber-optic cable is held pointing toward the light source at the
location where the light intensity is to
be measured. The photons collected at the detector surface are guided via the
fiber-optic cable to the
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
56
charge-coupled device in the spectrometer (CCD). The CCD counts photons
arriving to the CCD
during a pre-determined time period at each wavelength from 200 nm to 1100 nm,
and uses a software
algorithm to convert these photon counts to spectral irradiance (mW/cm2/nm).
The spectral irradiance
is integrated from 200 nm to 1100 nm by the software to yield the Absolute
Irradiance (mW/cm2),
which is the intensity of electromagnetic radiation from 200 nm to 1100 nm.
The spectral irradiance
is integrated from 400 nm to 500 nm by the software to yield the Absolute
Irradiance (mW/cm2),
which is the intensity of electromagnetic radiation from 400 nm to 500 nm.
For consumer convenience, the multi-phase oral care composition as disclosed
herein may be
provided as a Kit comprising the bleaching composition as disclosed herein, a
delivery carrier for
easier application, an electromagnetic radiation source emitting
electromagnetic radiation in a suitable
wavelength, and instructions for use.
The electromagnetic radiation source emitting electromagnetic radiation in a
suitable
wavelength can be a device capable of producing electromagnetic radiation,
such as the devices
described in US Patent No. 10,099,064, or curing lights used in dental
offices, or devices similar to
that described in the clinical protocol section specified herein.
The compositions of this invention are useful for both human and other animals
(e.g. pets, zoo,
or domestic animals) applications.
Packaging Materials for the Toothpaste Compositions
The jammed oil-in-water emulsion toothpaste composition can include primary
packaging,
such as a tube, bottle, and/or tub. The primary package can be placed within
secondary package, such
as a carton, shrink wrap, or the like, The oral care product can include a
primary package, but be free
of a secondary package to reduce materials used.
Instructions for use of the oral care composition can be printed on the
piitnary package and/or
the secondary package. The user can be instructed to dispense the toothpaste
from the toothpaste tube.
The primary and/or secondary packaging can be made from material that are
sustainable,
recyclable, compostable, and/or disintegrable. The toothpaste tube can be made
entirely from
materials that can be recyclable in commercial and/or municipal recycling
streams. The user can be
instructed to place the primary packaging, such as a toothpaste tube, and/or
the secondary packaging,
such as a carton, directly into a home recycling container to be picked up by
a recycling service, and/or
into a store-hosted collected receptacle. Suitable materials that can be
recyclable include paper,
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
57
cardboard paper, corrugated cardboard, polyethene, such as low density
polyethylene, medium density
polyethylene, and/or high density polyethylene, polyethylene terephthal ate,
polyvinyl chloride,
aluminum, glass, polypropylene, polystyrene, and/or combinations thereof. The
primary and/or
secondary packaging can be made from a single material, such as high density
polyethylene, so that
commercial and/or municipal recycling streams are not poisoned with another
material that can be
difficult to remove.
CLINICAL PROTOCOL
The bleaching efficacies of compositions are measured according to the
following clinical
protocol. Per treatment group, 17 to 25 participants are recruited to complete
the clinical study when
testing compositions with less than about 1% bleaching agent, and 8 to 25
participants when testing
compositions with at least about 1% bleaching agent. Recruited participants
must have four natural
maxillary incisors with all measurable facial sites. The mean baseline L* of
the group of participants
must be from 71 to 76, and the mean baseline b* of the group of participants
must be from 13 to 18.
In addition, participants with malocclusion on maxillary anterior teeth,
severe or atypical intrinsic
staining, such as that caused by tetracycline, fluorosis or hypo-
calcification, dental crowns or
restorations on the facial surfaces of maxillary anterior teeth, self-reported
medical history of
melanoma, current smoking or tobacco use, light-sensitivity or a pigmentation
skin disorder, self-
reported tooth sensitivity, or previous tooth whitening using a professional
treatment, over-the-counter
kit, or investigational product, are excluded from the study. Participants are
provided with take-home
kits with Crest Cavity Protection toothpaste and Oral-B Indicator soft manual
toothbrush (both from
Procter & Gamble, Cincinnati, OH, USA) to be used twice a day in the customary
manner.
The participants use a toothbrush to brush their teeth with the composition
for a specified
period of time for a specified number of times per day for a specified number
of days.
The change in tooth color due to the treatment with the composition is
measured using the
procedure described below.
Tooth color is measured using a digital camera having a lens equipped with a
polarizer filter
(Camera model no. CANON EOS 70D from Canon Inc., Melville, NY with NIKON 55 mm
micro-
NIKKOR lens with adapter). The light system is provided by Dedo lights (model
number DLH2)
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
58
equipped with 150 watt, 24V bulbs model number (Xenophot model number HL
X64640), positioned
about 30 cm apart (measured from the center of the external circular surface
of one of the glass lens
through which the light exits to the other) and aimed at a 45 degree angle,
such that the light paths
intersect at the vertical plane of the chin rest about 36 cm in front of the
focal plane of the camera.
Each light has a polarizing filter (Lee 201 filter), and a cutoff filter
(Rosco 7 mil Thermashield filter
from Rosco, Stamford, CT, USA).
At the intersection of the light paths, a fixed chin rest is mounted for
reproducible repositioning
in the light field. The camera is placed between the two lights such that its
focal plane is about 36 cm
from the vertical plane of the chin rest. Prior to beginning the measurement
of tooth color, color
standards are imaged to establish calibration set-points. A Munsell N8 grey
standard is imaged first.
The white balance of the camera is adjusted, such that the RGB values of grey
are 200. Color standards
are imaged to get standard RGB values of the color chips. The color standards
and grey standard are
listed below (from Munsell Color, Division of X-rite, Grand Rapids, MI, USA).
Each color standard
is labeled with the Munsell nomenclature. To create a grid of color standards
they can be arranged in
the following manner. This enables multiple color standards to be contained in
a single image captured
of the grid of color standards.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
59
Color standard grid 1
7.5R 6 8 2.5R 6 10 10YR 6.5 3 POLARIZATION 5R 7 8 N 3.5
0
CHECK
7.5RP 6 6 lOR 5 8 5YR 7 3 2.5Y 8.5 2 2.2YR 6.47 7.5YR
7 4
4.1
5YR 8 2 N 8 0 10R 7 4 N 8 0 5YR 7.5 2.5Y
8 4
2.5
5YR 7 3.5 5YR 7 2.5 5YR 5 2 5YR 7.5 2 N 6.5 0 N 9.5
0
Color standard grid 2
5YR 7.5 2.5Y 6 4 10YR 7.5 2.5R 7 8 7.5R 7 8 10YR 7.5 2
3.5 3.5
10YR 7.5 N 5 0 2.5R 6 8 10YR 7 2 5R 7 4 10YR 7 2.5
2.5
N 6.5 0 7.5RP 6 8 7.5R 8 4 5Y 8 1 7.5YR 8 2 2.2YR 6.47
4.1
N 5 0 2.5Y 8 4 10YR 7 3 N 9.5 0 lORP 7 4 2.5Y 7 2
Color standard grid 3
5R 6 10 N 8.5 0 10YR 6.5 lORP 6 10 N 8 0 7.5YR 7 3
3.5
2.5Y 3.5 0 10YR 7 3.5 5Y 8.5 1 5YR 8 2.5 5YR 7.5 3 5R 5 6
10YR 7.5 3 5YR 6.5 2.5YR 5 4 2.5Y 8 2 10YR 8 2 2.5Y 7 2
3.5
2.5R 6 6 5R 7 6 10YR 8 2.5 lOR 5 6 N 6.5 0 7.5YR 8 3
For baseline tooth color, participants use a toothbrush ("Anchor 41 tuft white
toothbrush" from
Team Technologies, Inc. Morristown, TN, USA) to brush their teeth with water
to remove debris from
their teeth. Each participant then uses cheek retractors (from Washington
Scientific Camera Company,
Sumner, WA, USA; treated with at frosted matte finish at A&B Deburring
Company, Cincinnati, OH,
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
USA) to pull the cheeks back and allow the facial surfaces of their teeth to
be illuminated. Each
participant is instructed to bite their teeth together such that the incisal
edges of the maxillary incisors
contact the incisal edges of the mandibular incisors. The participants are
then positioned on the chin
rest at the intersection of the light paths in the center of the camera view
and the tooth images are
5 captured. After all participants are imaged, the images are processed
using image analysis software
(Optimas manufactured by Media Cybernetics, Inc. of Silver Spring, MD). The
central four incisors
are isolated and the average RGB values of the teeth are extracted.
After the participants have used a whitening product, but prior to capturing
participant's tooth
images, the system is set to the baseline configuration and calibrated as
previously discussed. After
10 calibration, each participant is imaged a second time using the same
procedure as before making sure
the participant is in the same physical position as the pre-treatment image
including orientation of the
teeth. The images are processed using the image analysis software to obtain
the average RGB values
of the central four maxillary incisors. The RGB values of all of the images
are then mapped into CIE
L*a*b* color space using the RGB values and the L*a*b* values of the color
chips on the color
15 standard. The L*a*b* values of the color chips on the color standard are
measured using a Photo
Research SpectraScan PR650 from Photo Research Inc., LA using the same
lighting conditions
described for capturing digital images of the facial dentition. The PR650 is
positioned the same
distance from the color standards as the camera. Each chip is individually
measured for L*a*b* after
calibration according to the manufacturer's instructions. The RGB values are
then transformed into
20 L*a*b* values using regression equations such as:
L* = 25.16 + 12.02*(R/100) + 11.75*(G/100) ¨ 2.75*(B/100) + 1.95*(G/100)3
a* = -2.65 + 59.22*(R/100) -50.52*(G/100) + 0.20*(B/100) ¨ 29.87*(R/100)2
+ 20.73 *(G/100)2 + 8. 14*(R/100)3 - 9.17(G/100)3 + 3 .64* [(B/100)2] *
[R/100]
b* = -0.70 + 37.04*(R/100) + 12.65*(G/100) - 53.81*(B/100) -18.14*(R/100)2
25 + 23.16*(G/100)*(B/100) + 4.70*(R/100)3 ¨ 6.45*(B/100)3
The R2 for L*, a*, and b* should be > 0.95. Each study should have its own
equations.
These equations are generally valid transformations in the area of tooth color
(60 <L* < 95,
0 <a* < 14, 6 <b* <25). The data from each participant's set of images is then
used to calculate
product whitening performance in terms of changes in L*, a* and b* -a standard
method used for
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
61
assessing whitening benefits. Changes in L* is defined as AL* = L* after
treatments ¨ L *baseline where a
positive change indicates improvement in brightness; Changes in a* (red-green
balance) is defined as
Aa* = a* after treatments ¨ a*baseline where a negative change indicates teeth
which are less red; Changes in
b* (yellow-blue balance) is defined as Ab* = b* after treatments ¨ b*baseline
where a negative change
indicates teeth are becoming less yellow. -Ab* is used as the primary measure
of bleaching efficacy.
The overall color change is calculated by the equation AF = (AT *2 Aa*2
Ab*2)1/2.
After using the whitening products, color changes in CIE Lab color space can
be calculated for
each participant based on the equations given.
Preparation of the present Multi-phase oral care compositions
Preparation of emulsions is well known in the art and any suitable
manufacturing process can
be used to make the multi-phase oral care compositions which may be in the
form of an emulsion; see
for example, Remington: the Science and Practice of Pharmacy, 19th ed., Vol.
II, Chapters 20, 80, 86,
etc. Generally, the components are separated into those that are oil-soluble
and those that are water-
soluble. These are dissolved in their respective solvents by heating if
necessary. The two phases are
then mixed and the product is stirred and cooled. After combining the phases,
the present multi-phase
oral care compositions, which may be in the form of emulsions may be agitated
or sheared by various
methods, including shaking, intermittent shaking, high shear mixing, or by
using high speed mixers,
blenders, colloid mills, homogenizers, or ultrasonic techniques. Depending on
the specific ingredients,
it may be recognized by one of skill in the art that certain modifications may
need to be made to the
manufacturing process to accommodate the specific properties of the
ingredients. The type of multi-
phase oral care composition prepared may be observed using a microscope.
Further description of test
methods are disclosed in Remington: The Science and Practice of Pharmacy, 19th
ed., volume 1, 1995,
pp. 282-283.
In certain aspects, multi-phase oral care compositions, which may be in the
form of a jammed
oil-in-water emulsion, as disclosed herein may be made as follows:
1) The water-soluble ingredients are dissolved in the aqueous phase, and the
oil-soluble
components in the hydrophobic phase.
2) The hydrophobic phase is added to the aqueous phase in portions in a
SpeedMixer container
with thorough mixing (2 minutes at 800RPM in a Speedmixer for example) between
portions.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
62
Ideally, 1) the size of the initial portion is less than 20% of the amount
aqueous phase, 2) the
size of subsequent portions may be increased gradually toward the amount of
aqueous phase,
and 3) the size of each portion is less than the amount of aqueous phase. As
the jamming
concentration is approached, an oil-in-water emulsion forms during this step,
and the
composition develops a lotion-like semisolid consistency - this is evidence
that the droplets of
the hydrophobic phase are jammed against each other and deform each other
(note, they are
still separated by a region of aqueous phase). This jamming is evidenced by
the development
of a lotion-like consistency of the composition.
3) Once all the hydrophobic phase has been incorporated, the contents of the
Speedmixer
container are mixed 3 times at 800 RPM for 2 minutes each time in a
Speedmixer.
Note, in certain aspects, 1) it may be possible to add the hydrophobic phase
to the aqueous
phase at a suitably slow but continuous or pulsed rate with concurrent mixing
in step-2 above, and 2)
the mixing in step-3 above may be accomplished with other types of mixers over
various lengths of
time, such as a recirculation loop through static mixers, rotor-stator mixers,
or other mixing devices,
such as those described in the Handbook of Industrial Mixing.
The mixing procedure of the SpeedMixerTm series is based on the double
rotation of the mixing
cup using a dual asymmetric centrifugal mixing. This combination of
centrifugal forces acting on
different levels enables very rapid mixing of the entire cup. Optionally the
composition may be heated,
if necessary, to facilitate mixing. When the active is included in solid
particulate form, the addition of
an optional viscosity modifier, may be appropriate to keep the solid
particulate dispersed and
suspended within the composition. Flavorants or sweeteners may also be added
to one of the phases
of the composition, as desired. Thereafter the composition may be added to the
delivery carrier, as
desired.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
63
METHODS
Method To Measure The Dv 50, D[4,3], and D[3,2] of Droplets or Regions of
Hydrophobic Phase Of
A Multi-phase oral care composition
1. Weigh 0.20g (+/- 0.02g) of the sample to be tested into a 20m1 HDPE
scintillation vial (VWR
66021-690).
2. Add water (for example WFI Quality OmniPur Sterile Filtered CAS#7732-18-5)
19.80g (+/-
0.02g) to the vial and secure cap.
3. Roll the vial on a countertop gently until the sample to be tested is
dispersed throughout the
water. Avoid shaking or mixing vigorously. Agitation beyond that specified may
alter the
droplet size thereby leading to a result that is not representative of the
starting sample.
4. Set up the Mastersizer 3000 (Malvern Panalytical Inc., Westborough, MA) and
the Hydro unit
(Model #MAZ3210), and ensure the hoses are securely attached.
5. Add water (for example MilliporeSigma Ultrapure Lab water system) to the
lowest edge of
silver rim and initialize the system (this measures the background).
6. When the system is ready, roll the vial gently about 4 or 5 times to mix
the contents, and then
slowly pipet contents of the vial (generally from about 0.1 gram to about 5
grams) using a
1.7m1 pipet (VWR #414004-031) into the Hydro unit until Obscuration is in
range to be
measured (1-10%). If the obscuration % is >10%, remove some of the sample
solution from
the vessel and add water (for example MilliporeSigma Ultrapure Lab water
system) until
Obscuration is less than 10%.
7. Start testing. Testing is done for 10 measurements and the sample is
flushed upon completion.
Stirrer speed is set at 500rpm.
8. Add water when indicated for rinsing the system between samples (water is
added generally
about 5 to 6 times)
9. Repeat testing 2 more times with rinses in between.
10. Record the average Dv 50, D[4,3], and D[3,2] for each set of data (10
measurements x 3
replications).
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
64
Additional information on the use of the Mastersizer 3000 can be found in the
user manual
(MAN0474 MRK1953-0 on the website malvernpanalytical.com).
Owing to the delicate nature of the emulsion and possibility of changing the
droplet size during
sample preparation, it is recommended to validate the method. To validate the
above method, the
D[4,3] of Validation Composition A made according to the procedure specified
herein must be
measured and demonstrated to be from 15 microns to 30 microns.
VALIDATION COMPOSITION A
Weight%*
35% aqueous solution of H2021 8.5714
PEG-20 Sorbitan monolaurate
1
(Tween 20)2
Mineral oil3 90.4286
%H202 3
% Aqueous phase 9.5714
% Hydrophobic phase 90.4286
% Aqueous phase by volume 7.57190
% Hydrophobic phase by volume 92.4290
'Ultra cosmetic grade 35% from Solvay, Houston, TX
2Tween20-LQ-(AP) from Croda Inc. Edison, NJ
3Kaydol grade from Sonneborn LLC., Parsippany, NJ
* %wt of total multi-phase composition unless otherwise indicated
Batches of Validation Composition A are made according to the following
procedure:
1. The Tween 20 and aqueous solution of H202 were weighed into a Speedmixer
container (Max
300 Long Cup Translucent item number 501 218t or Max 300 X Long Cup
Translucent item
number 501 217t, for the 150-gram and 250-gram batches, all containers from
Flacktek Inc.,
Landrum, SC) and mixed by manually swirling the container until dissolved.
2. The mineral oil was added in portions (see table below, generally starting
with small portions
and increasing to larger portions) and mixed for about 1 to 2 minutes between
portions with a
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
rubber spatula. An oil-in-water emulsion formed during this step, and the
composition
developed a lotion-like semisolid consistency.
3. Once all the mineral oil was added, the contents of the Speedmixer
container were mixed 3
times at 800 RPM for 2 minutes each time in a Speedmixer.
5
VALIDATION Batch Approximate
COMPOSITION size (g) portion size (g)
A
Batch-1 150 25
Batch-2 150 15 to 25
Batch-3 250 23 to Si
Batch-4 150 20 to 25
Batch-5 150 20 to 25
Batch-6 150 20 to 25
Batch-7 250 5 to 20
Batch-8 250 5 to 20
Method To Measure The Water-Dispersibility of a Multi-phase oral care
composition
1. Allow the multi-phase oral care composition and sterile filtered water
(Calbiochem catalog
number 4.86505.1000 from EMD Millipore Corporation, Billerica, Massachusetts)
to
10 equilibrate at 23C +/-2C for at least 12 hours.
2. Record the tare weight of the bottom portion of a petri dish (VWR,
Polystyrene, 100 mm x 15
mm, catalog number 25384-342, purchased from VWR, Batavia, IL).
3. Weigh 0.30 to 0.35 gram of the multi-phase oral care composition into
the center of the petri
dish in one single blob. Record the initial weight of the sample.
15 4. Add 30 ml of sterile filtered water to the petri dish without
disturbing the sample - with a
syringe (30m1 BD Syringe with Luer Lok tip, item number 302832), taking care
to go around
the edges of the petri dish and directing the stream away from the sample.
5. After 10 minutes, decant the contents of the petri dish, dry it in
an oven set at 60C for at least
60 minutes, allow it to cool, and record the weight of petri dish + residual
sample.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
66
6. Calculate:
Weight of residual sample = (Weight of petri dish + residual sample from step-
5) MINUS
(Tare weight of petri dish from step-2)
7. Calculate:
% Water-dispersibility = 100 MINUS [100x(Weight of residual sample from step-
6) /
(Initial weight of sample from step-3)]
8. Repeat steps-1-7 for a total of at least 3 measurements. Calculate the
average. This is the
water-dispersibility of the multi-phase oral care composition.
To validate the above method, the water-dispersibility of Validation
Composition A made according
to the procedure specified herein must be measured and demonstrated to be from
60 to 100%.
Method To Measure The Brookfield Viscosity Of A Multi-phase oral care
composition or
Hydrophobic Phase
1. Transfer 40 to 50 ml of the multi-phase oral care composition or
hydrophobic phase into a 50
ml polypropylene conical tube (Falcon brand catalog number REF 352098, Corning
Science,
Tamaulipas, Mexico). If the multi-phase oral care composition or hydrophobic
phase exhibits
macroscopic separation of one or more components prior to transferring into
the conical tube,
mix the multi-phase oral care composition or hydrophobic phase in a Speedmixer
(for example
at 800 RPM for 2 minutes) and transfer into the conical tube before it
exhibits macroscopic
separation of one or more components. If the multi-phase oral care composition
or
hydrophobic phase has macroscopic air-bubbles or voids: 1) Tap the conical
tube on a hard
surface or mix the conical tube on a vortex mixer (for example Vortex Genie 2
from Scientific
Industries Inc. Bohemia, NY, or Mini Vortexer from VWR Scientific Products)
until it is
substantially free of macroscopic air-bubbles or voids or 2) Use a different
method to transfer
the multi-phase oral care composition into the conical tube such that it is
substantially free of
macroscopic air-bubbles or voids.
2. Allow the multi-phase oral care composition or hydrophobic phase to
equilibrate in the conical
tube for at least 12 hours at the desired temperature (e.g. -7 C, 4 C, 23 C,
25 C, 30 C, 40 C,
50 C, or 60 C).
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
67
3. Confirm the viscometer (Brookfield 1/2RV DVII+Pro Viscometer) is level,
turn it on, and
autozero it according to the instruction manual.
4. Attach the appropriate spindle (for example Spindle D, E, or F, depending
on the viscosity
range of interest) and set the appropriate speed (for example 0.5, 1.0, 2.0,
2.5, 4.0, 5.0, 10, 20,
50 and 100 RPM) for the Brookfield Viscosity anticipated to be measured.
5. Place the conical tube under the spindle, lower the spindle until the t-bar
is a few mm above
the surface of the multi-phase oral care composition, and center the conical
tube under the
spindle.
6. Turn on the viscometer allow it to spin 3 to 5 rotations to confirm the
spindle spins freely
without grazing the walls of the conical tube. Turn on the helipath stand.
When helipath
lowers the t-bar completely under the multi-phase oral care composition or
hydrophobic phase,
turn on a timer set to 60 seconds. At 60 seconds record the Brookfield
Viscosity in cPs.
7. Tap the conical tube on a hard surface or mix the conical tube on a vortex
mixer (for example
Vortex Genie 2 from Scientific Industries Inc. Bohemia, NY, or Mini Vortexer
from VWR
Scientific Products) until it is substantially free of macroscopic air-bubbles
or voids, repeat
steps-5-6 for a minimum of 3 measurements, with about 10 minutes between
measurements.
8. Tap the conical tube on a hard surface or mix the conical tube on a vortex
mixer (for example
Vortex Genie 2 from Scientific Industries Inc. Bohemia, NY, or Mini Vortexer
from VWR
Scientific Products) until it is substantially free of macroscopic air-bubbles
or voids, and repeat
steps 2-7 for a second set of 3 measurments. Calculate the average of all 6
measurements. This
is the Brookfield Viscosity of the multi-phase oral compositon or hydrophobic
phase.
To validate the above method, the Brookfield Viscosity of Validation
Composition A made according
to the procedure specified herein must be measured at 2.5 RPM with Spindle D
at 23 C and
demonstrated to be from 15,000 to 45,000 cPs.
Method To Measure The Yield Stress Of A Multi-phase oral care composition or
Hydrophobic Phase
1. Transfer 40 to 50 ml of the multi-phase oral care composition or
hydrophobic phase into a 50
ml polypropylene conical tube (Falcon brand catalog number REF 352098, Corning
Science,
Tamaulipas, Mexico). If the multi-phase oral care composition or hydrophobic
phase exhibits
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
68
macroscopic separation of one or more components prior to transferring into
the conical tube,
mix the multi-phase oral care composition or hydrophobic phase in a Speedmixer
(for example
at 800 RPM for 2 minutes) and transfer into the conical tube before it
exhibits macroscopic
separation of one or more components. If the multi-phase oral care composition
or
hydrophobic phase has macroscopic air-bubbles or voids: 1) Tap the conical
tube on a hard
surface or mix the conical tube on a vortex mixer (for example Vortex Genie 2
from Scientific
Industries Inc. Bohemia, NY, or Mini Vortexer from VWR Scientific Products)
until it is
substantially free of macroscopic air-bubbles or voids or 2) Use a different
method to transfer
the multi-phase oral care composition into the conical tube such that it is
substantially free of
macroscopic air-bubbles or voids.
2. Allow the multi-phase oral care composition or hydrophobic phase to
equilibrate in the conical
tube for at least 12 hours at the desired temperature (e.g. -7 C, 4 C, 23 C,
25 C, 30 C, 40 C,
50 C, or 60 C).
3. Confirm the rheometer (Brookfield HAYR-1 Rheometer) is level, turn it on,
and autozero it
according to the instruction manual.
4. Attach the appropriate spindle-vane (for example V72, V73, or V75,
depending on the
viscosity range of interest) and set to program for the specific spindle-vane
being used. The
program parameters are specified below:
Spindle>> V-72 V-73 V-7
Yield Stress Range(Pa) 4-40 20-200 80-800
Immersion Primary Primary Primary
Pre-Sheer rpm 0 0 0
Pre-Sheer time 0 0
Zero Speed t,rpm) 0.1 0,1 0.1
Wait Time (sec) 30 30 30
Run Speed (rpm) 0.1 ft1 0.3
5. Place the conical tube under the spindle-vane, and lower the spindle-vane
slowly into the
sample, taking care to minimize any disturbance to the sample this may cause.
Continue
lowering the spindle-vane until the top surface of the sample is at the
primary immersion mark
(bulge on the shaft) or secondary immersion mark (notch on the spindle-vane).
If the spindle-
vane is immersed to the secondary immersion mark, the value generated by this
method will
need to be multiplied by two.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
69
6. Run the program selected in step-4. Without removing the spindle-vane run
the program a
total of 3 times. Record the 3 measurements. If the spindle-vane was immersed
to the
secondary immersion mark, multiply each measurement by 2; and if the spindle-
vane was
immersed to the primary immersion mark, multiply each measurement by 1. Record
the 3
calculated values.
7. Tap the conical tube on a hard surface or mix the conical tube on a vortex
mixer (for example
Vortex Genie 2 from Scientific Industries Inc. Bohemia, NY, or Mini Vortexer
from VWR
Scientific Products) until it is substantially free of macroscopic air-bubbles
or voids, and repeat
steps 2-6 for a second set of 3 values. Calculate the average of all 6 values.
This is the Yield
Stress of the multi-phase oral composition or hydrophobic phase.
To validate the above method, the Yield Stress of Validation Composition A
made according
to the procedure specified herein must be measured with spindle-vane V72
immersed to the secondary
immersion mark at 23 C and demonstrated to be from 5 to 20 Pa.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
METHOD TO MEASURE THE PERCENT MACROSCOPIC SEPARATION OF ONE OR MORE
COMPONENTS OF A MULTI-PHASE ORAL CARE COMPOSITION
1.
Transfer 50 mL of the multi-phase oral composition into a 50 ml
polypropylene conical tube
(Falcon brand catalog number REF 352098, Corning Science, Tamaulipas, Mexico).
If the
5
multi-phase oral composition exhibits macroscopic separation of one or more
components
prior to transferring into the conical tube, mix the multi-phase oral
composition in a
Speedmixer (in a "Max 300 Long Cup Translucent", item number 501 218t from
Flacktek Inc.,
Landrum, SC) (for example at 800 RPM for 2 minutes) and transfer into the
conical tube before
it exhibits macroscopic separation of one or more components. If the multi-
phase oral
10
composition has macroscopic air-bubbles or voids: 1) Tap the conical tube on
a hard surface
until it is free of macroscopic air-bubbles or voids, or 2) Use a different
method to transfer the
multi-phase oral composition into the conical tube such that it is
substantially free of
macroscopic air-bubbles or voids. Screw the cap onto the conical tube. Repeat
for a total of
three conical tubes.
15
2. Position all three conical tubes in a vertical orientation (for example
in a test tube rack) with
the conical end on the bottom and the cap on top.
3. Allow all three conical tubes to stay undisturbed in the vertical position
in a room or chamber
in which the air is maintained at the temperature (e.g. -7 C, 4 C, 23 C, 25 C,
30 C, 40 C,
50 C, or 60 C) for the period of time after which the macroscopic separation
is to be measured.
20
4. At the end of period of time after which the macroscopic separation is to
be measured (for
example 1 day, 2 days, 1 week, 2 weeks, 1 month, 2 months, 3 months, 6 months,
12 months,
18 months, or 24 months) in the vertical position, measure the volume of
material that has
macroscopically separated on the bottom of the conical tube (aided by the
graduations on the
conical tube). If the volume of material that has macroscopically separated on
the bottom of
25
the conical tube is greater than 25 ml, measure the volume of material that
has macroscopically
separated to the top of the conical tube.
= Calculate the average volume of material that has macroscopically
separated in all three
tubes.
= Assess the tube to tube variability of the volume of material that has
macroscopically
30
separated as follows: The volume of material that has separated in each and
every tube
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
71
must be within the range of +/-2.5 ml of the average. If the volume of
material that has
separated in any one or more of the tubes is outside the range of +/-2.5 ml of
the average:
This is an indication of sample to sample variability potentially due to
macroscopic
separation of one or more components prior to transferring into the conical
tubes, and the
method needs to be repeated starting at step-1 to minimize sample to sample
variability.
5. Calculate the percent macroscopic separation as: 100 x (average volume of
material that has
macroscopically separated measured and calculated in step-4 DIVIDED by 50 m1).
To validate the above method, the percent macroscopic separation of one or
more components of
Validation Composition B specified below must be measured and demonstrated to
be from 6% to 10%.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
72
VALIDATION COMPOSITION B - FOR METHOD TO
MEASURE PERCENT MACROSCOPIC SEPARATION (Wt %)
35% aqueous solution H2021 1.43
Sterile Filtered Water2 4.24
Aerosol 0T3 1.00
Mineral Oil4 93.33
'ultra Cosmetic Grade from Solvay, Houston, Texas
2Calbiochem catalog number 4.86505.1000 from EMD Millipore Corporation,
Billerica,
Massachusetts
.. 3Aerosol OT-100 from Cytec Industries, Princeton, NJ
4Kaydol grade from Sonneborn LLC, Petrolia, Pennsylvania
PROCEDURE TO MAKE VALIDATION COMPOSITION B - FOR METHOD TO MEASURE
PERCENT MACROSCOPIC SEPARATION
Three 50-gram batches of the validation composition are made according to the
following procedure:
a) The Aerosol OT and mineral oil are weighed into a Speedmixer container
("Max 40 Long Cup
Translucent", item number 501 223Lt from Flacktek Inc., Landrum, SC). The
mixture is heated
in a convection oven at 60C and swirled to dissolve the Aerosol OT in the
mineral oil.
b) In a separate plastic container, 42.4 grams of sterile filtered water and
14.3 grams of 35%
aqueous solution of H202 are weighed and swirled to dissolve the H202 into the
water. This
diluted solution of H202 is heated in a convection oven at 60C for about 10
minutes. 2.84
grams of this diluted solution of H202 in water is weighed into the Speedmixer
container.
c) The contents of the Speedmixer container are mixed at 800RPM for 5 seconds,
1200 RPM for
5 seconds, and 1950 RPM for 2 minutes. The walls of the container are then
scraped down
with a rubber spatula, and the contents are mixed a second time at 800RPM for
5 seconds, 1200
RPM for 5 seconds, and 1950 RPM for 2 minutes. The walls of the container are
then scraped
down with a rubber spatula, and the contents are mixed a third time at 800RPM
for 5 seconds,
1200 RPM for 5 seconds, and 1950 RPM for 2 minutes.
METHOD TO DETERMINE IF A COMPOSITION IS EASY TO MANUALLY DISPENSE FROM
A TUBE
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
73
1. Select a foil laminate tube with the following dimensions:
a. Total length from tip of nozzle to bottom of barrel: About 112 mm
b. Internal diameter of barrel: About 28mm
c. Length of nozzle: About 21 mm
d. Internal diameter of nozzle: About 9.7 mm for half the length of the nozzle
attached to
the barrel, and about 4.2 mm for the other half the of the nozzle leading to
the exit
orifice of the nozzle.
2. Fill from about 35 to about 40 grams of the composition through the bottom
of the barrel into
the tube from step-1. Seal the bottom of the barrel using an ultrasonic
sealer.
3. Allow the tube to stay undisturbed in a room or chamber in which the air is
maintained at the
temperature (e.g. -7 C, 4 C, 23 C, 25 C, 30 C, 40 C, 50 C, or 60 C) for the
period of time
after which the ease of dispensing is to be measured.
4. Allow the tube to equilibrate at about 23 C for at least a day.
5. Pick up the tube between the thumb and fingers of one hand. While holding
the tube in the air,
squeeze the tube firmly between the thumb and fingers for about 10 seconds.
Measure the
length of the bead of the composition dispensed out of the nozzle of the tube.
6. The composition is considered easy to dispense manually from a tube after
the specified period of
time at the specified temperature if at least 1 inch of product is dispensed
in step-5.
METHOD TO MEASURE THE L* ¨ a* ¨ b* OF A SUBSTANCE OR COMPOSITION
1. The substance or composition is loaded into a clear disposable petri-dish
(60mm diameter x
15mm high, made from virgin crystal grade polystyrene, VWR catalog number
25384-092,
purchased from VWR, Batavia, IL). Tap the petri-dish on a hard surface until
it is substantially
free of macroscopic air-bubbles or voids. The amount loaded needs to be enough
to establish
a circular area of contact that is at least about 45 mm in diameter on the
bottom of the petri-
dish and at least about lOmm deep.
2. The L* (brightness), a* (red-green balance), and b* (yellow-blue
balance) of the substance or
composition is measured using a hand-held spectrophotometer Konica Minolta
700d. The
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
74
spectrophotometer is used with an aperture of about 6.3mm diameter, the
observer angle is set
at 2 degrees, the illuminant is set at daylight color temperature of 5003K,
and specular
reflection is excluded. The spectrophotometer is positioned such that the
aperture is pointing
upward, and the digital read-out is on the counter. The loaded petri-dish is
now carefully
centered and placed on the aperture so that it completely covers the aperture.
The L*, a*, and
b* are then measured with the spectrophotometer. Record these values.
3. Repeat step-2 for a total of three measurements. Calculate the average of
the three
measurements ¨ this is the L*, a*, and b* of the substance or composition.
METHOD TO MEASURE ACTIVE RELEASE RATE FROM COMPOSITIONS
The Active Release Rate Method employs a dialysis cell containing a membrane
on which
composition is applied and through which the active diffuses depending on the
rate of its release from
the composition. The dialysis cell serves as a proxy for a tooth and this
method can be used to measure
the release of any water soluble active for example hydrogen peroxide or
fluoride.
A 15 mL dialysis cell (2K MWCO, Slide-A-Lyzer G2 Dialysis Cassette) is filled
with WFI
MilliQ Water (16 grams) and the cap affixed. To one side of the cassette a
test product is applied
covering the entire cell membrane surface at a depth defined by the cell
plastic housing by leveling
the applied product with a spatula. A piece of parafilm is applied over the
product composition to
protect it during cassette mixing during sampling. The cell is placed either
vertically or horizontally
product facing down on a tared balanced. A timer is started post product
application and samples of
the WFI MilliQ water within the cassette are taken at the defined time points
and assayed for mg/L or
ppm of active. The results are presented as: 1) A chart of the mg/L or ppm of
active released Vs. time
¨ this is the "Active Release Rate Profile", and 2) The mg/L or ppm active
released after 60 and 120
seconds are also reported ¨ this is the "mg/L or ppm Active Released in 60 and
120 Seconds".
At each defined sample point, the cell is taken and inverted 180 degrees
twice, the lid removed
and a sample (0.3 ¨ 0.50g) pulled via a pipet. Following sampling, the
dialysis cell is returned to the
balance until the next sample is required. Each sample is assayed for active
using any suitable assay
procedure. The procedure to assay for peroxide in mg/L peroxide is outlined
below.
Procedure To Assay Contents of Dialysis Cell for Peroxide
A Reflectoquant RQ Flex peroxide test strip reader (Millipore Sigma) is
calibrated using both
0.2 ¨ 0.20 mg/L (604) and 100 ¨ 1000 mg/L (609) test strips (Supelco) with
peroxide standard
solutions as follows:
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
0.2 ¨ 20.0 mg/L strips:
1. 5 grams of 35% Hydrogen Peroxide is diluted to 500 grams total with WFI
MilliQ water.
2. 1 gram from (1) is diluted to 500 grams total weight using WFI MilliQ
water
3. 2 drops from (2) are applied to a 0.2 ¨ 20.0 mg/L test strip for 5
seconds and the excess solution
5 dabbed on a paper towel.
4. The test strip is inserted into a RQ Flex 10 with the 0.2 ¨ 20.0 mg/L
test strip program loaded
and the measurement recorded. Note total strip development and program is 15
seconds in duration.
5. 2 grams from solution (1) are diluted to 500 grams with WFI MilliQ water
and steps (3) and (4) are
repeated.
10 100 ¨ 1000 mg/L strips:
1. 0.5 grams of 35% Hydrogen Peroxide is diluted to 500 grams total with
USP water.
2. 2 drops from (1) are applied to a 0.2 ¨ 20.0 mg/L test strip for 10
seconds and the excess
solution dabbed on a paper towel. The strip is developed by sitting for an
additional 50 seconds.
3. The test strip is inserted into a RQ Flex 10 with 10 seconds remaining
on the 100 - 1000 mg/L
15 test strip program and the measurement recorded. Note total strip
development and program is
approximately 60 seconds in duration for this test strip.
4. 1 gram of 35% Hydrogen Peroxide is diluted to 500 grams total with WFI
MilliQ water
5. The test strip is inserted into a RQ Flex 10 with the 100 - 1000 mg/L
test strip program loaded
and the measurement recorded.
Peroxide Sample analysis
1. 2 drops from a given test sample time point are applied to a either
a 0.2 ¨ 20.0 mg/L or 100 -
1000 mg/L test strip following the development time period and analysis steps
defined above (a3-a4
& b2-b3).
2. The diffused peroxide concentration defines the appropriate strip to
use. If concentrations
exceed the respective strip concentration ranges, serial dilutions are
performed to bring the
concentration into range.
Procedure To Assay Contents of Dialysis Cell for Fluoride
1. A Fluoride ion selective electrode was calibrated with 5 ppm, 50 ppm, and
500 ppm Sodium
Fluoride standards prepared in 50% water and 50% TSIABII with CDTA Buffer.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
76
2. Samples (-0.5g) were pulled from the dialysis cell at the defined
timepoints and diluted with
50% TSIABII with CDTA Buffer
3. The Fluoride ion selective probe was inserted into each defined sample and
the reading
(mg/L) was recorded.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
77
EXAMPLES
The invention is further illustrated by the following examples, which are not
to be construed
in any way as imposing limitations to the scope of this invention. Various
other aspects, modifications,
and equivalents thereof which, after reading the description herein, may
suggest themselves to one of
ordinary skill in the art without departing from the spirit of the present
invention or the scope of the
appended claims.
EXAMPLES I
TABLE 1. Example I-A and Example I-B
Weight% A
Mineral Oil' 81.357 81.3570
35% aqueous solution of H2022 8.571 17.1410
Purified Water, USP 8.571
5ucra1ose3 0.100 0.1000
PEG-20 Sorbitan monolaurate (Tween 20)4 1.000 1.0000
Peppermint5 0.400 0.4000
Yield Stress (Pa) 14.75 64.67
Appearance Opaque
Color White
Consistency Lotion-like
Dv 50 11.0 3.39
D[4,3] 11.6
[3,2] 9.87
(microns) mean equivalent-diameter of
regions or droplets of the hydrophobic phase
measured according to the method specified
herein (average of 10 x 1 measurements)
1Drakeol 35, USP grade from Calumet, Indianapolis, IN
2Ultra cosmetic grade 35% from Solvay, Houston, TX diluted down to 17.5%
35ucra1ose Micronized Powder USP/NF/FCC grade from Newtrend Technology Co.
Ltd. Jiangxi,
China
4Tween20-LQ-(AP) from Croda Inc. Edison, NJ
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
78
5Peppermint flavor TAK-121065 from Takasago International Corporation,
Rockleigh, NJ
TABLE 1 shows the formulation for Example A-1 and Example A-2 (same
ingredients
processed differently), both of which are jammed oil-in-water emulsions.
Example I-A (350
kilograms) was produced by combining polysorbate 20, water, sucralose and the
aqueous solution of
H202 in a 20-gallon premix tank. The premix mixture was transferred to a 400L
vessel and agitated
(35 RPM) while mineral oil was slowly added over 12 minutes. A jammed oil-in-
water emulsion was
formed during this step. Flavor was added and the batch homogenized at 1000
RPM for seven
turnovers, and 2000 rpm for one turnover. This was Example I-A.
Example I-B (350 kilograms) was produced by combining polysorbate 20, water,
sucralose
and the aqueous solution of H202 in a 20-gallon premix tank. The premix
mixture was transferred to
a 400L vessel and agitated (35 RPM) while mineral oil was slowly added over 27
minutes. A jammed
oil-in-water emulsion was formed during this step. Flavor was added and the
batch homogenized at
2000 rpm for three turnovers, 3000 RPM for three turnovers, and 3900 RPM for
three turnovers. This
was Example I-B.
These data show that, surprisingly, shearing the composition at a high rate of
shear using
homogenizer energy and time thickened the composition, as evidenced by the
500% increase in its
yield stress for the same ingredients.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
79
COMPARATIVE COMPOSITION I-A, COMPARATIVE COMPOSITION I-B, and
COMPARATIVE COMPOSITION I-C
TABLE 2. Comparative Composition I-A, Comparative Composition I-B, and
Comparative
Composition I-C
Comparative
Ingredients Number of
Ingredients
Composition
Comparative Propylene Glycol, Calcium
Composition I-A Pyrophosphate, PVP, PEG/PPG-116/66
Colgate Optic White Copolymer, Hydrogen Peroxide, Flavor,
Renewal Toothpaste Sodium Lauryl Sulfate, Tetrasodium 14
(3% H202) Pyrophosphate, MFP, Sodium
Saccharin, Di sodium Pyrophosphate,
Silica, Sucralose, BHT
Comparative Alcohol, Acryl ates/Octyl
acryl ami de
Composition I-B Copolymer, Water, Hydrogen Peroxide
Colgate Optic White -
Overnight Pen (4%
H202)
Comparative Water, S orb itol , Hydrated Silica,
Composition I-C Glycerin, Potassium Nitrate, PEG-8,
Sensodyne Pronamel Cocoamidopropyl betaine, Sodium
13
(1100 ppm Fluoride) Fluoride, Flavor, Titanium dioxide,
xanthan gum, sodium saccharin, sodium
hydroxide
Comparative Glycerin, PEG-8, Hydrated Silica,
Composition I-D Pentasodium triphosphate, flavor,
Paradontax Stannous fluoride, Sodium lauryl sulfate,
11
(1100 ppm Fluoride) Titanium dioxide, Polyacrylic acid,
Cocoamidopropyl betaine, sodium
saccharin
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
The peroxide (active) release rate was determined according to the method
specified herein for
four compositions: 1) Example I-A, 2) Example I-B, 3) Comparative Composition
I-A, 4)
Comparative Composition I-B. Example I-A and Example I-B are inventive
compositions.
Comparative Composition I-A and Comparative Composition I-B are commercial
products marketed
5 by the Colgate-Palmolive Company. Comparative Composition I-A is Colgate
Optic White Renewal
Toothpaste, which is marketed to include hydrogen peroxide at 3% wt%.
Comparative Composition
I-B is Colgate Optic White Overnight, which is also marketed to include
hydrogen peroxide at 4 wt%.
The Peroxide Release Rate Profiles [mg/L peroxide released over time] of these
four compositions are
shown in FIG. 1. Surprisingly, both the inventive compositions (Example I-A
and Example I-B) have
10 a much higher peroxide release rate profile Vs. Comparative Composition
I-A and Comparative
Composition I-B. The mg/L peroxide released in 60 and 120 Seconds by these
four compositions are
presented in TABLE 3. While the recommended brushing time by the American
Dental Association
is 120 seconds, many people brush for 60 seconds or less. Surprisingly, both
the inventive
compositions (Example I-A and Example I-B) released a much higher amount of
peroxide in both 60
15 and 120 seconds Vs. Comparative Composition I-A and Comparative
Composition I-B. It is worth
noting that the inventive compositions (Example I-A and Example I-B) released
about 300% more
mg/L peroxide after 60 seconds and 120 seconds Vs. Comparative Composition I-B
even though the
inventive compositions had 25% less peroxide (4% Vs. 3% peroxide).
CA 03231717 2024-03-07
WO 2023/044470 PCT/US2022/076631
81
TABLE 3. mg/L Peroxide Released In 60 and 120 Seconds
Example I-A Example I-B Comparative Comparative
(Jammed Oil-in- (Jammed Oil-in- Composition I-A Composition I-B
Water Emulsion Water Emulsion (Traditional (Traditional
Toothpaste) Toothpaste) Toothpaste) Toothpaste)
mg/L peroxide
released in 60
seconds
measured 390 332 0 0
according to the
method specified
herein
mg/L peroxide
released in 120
seconds
measured 717 627 0 2
according to the
method specified
herein
CA 03231717 2024-03-07
WO 2023/044470 PCT/US2022/076631
82
EXAMPLES II
TABLE 4. Example II-A, Example II-B, Example II-C, Example II-D, Example II-E,
Example II-F,
Example II-G, Example II-H, Example II-I
Weight % A
35%
aqueous
8.5147 8.5147 8.5147 8.5147 8.5147 8.5147 8.5147 8.5147 8.5147
solution of
H2021-
PEG-20
Sorbitan
monolaurat 1.0000 1.0000 3.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000
e (Tween
20)2
2.0000
(pre-
mix) +
Mineral
86.385 -
oil3
3
(main
batch)
2.0000
(pre-
mix) +
Mineral
- 86.385
oil4
3
(main
batch)
2.0000 2.0000 2.0000 2.0000 2.0000 2.0000
2.0000 (pre- (pre- (pre- (pre-
(pre- (pre-
Mineral
- (pre-mix) mix) + mix) + mix) + mix) + mix) + mix) +
oil
86.385 86.142 84.385 84.385 82.142 86.263
3 3 3 3 3 8
CA 03231717 2024-03-07
WO 2023/044470 PCT/US2022/076631
83
84.3853 (main (main (main (main (main (main
(main batch) batch) batch) batch) batch) batch)
batch)
Sucralose6 0.1000 0.1000 0.1000 0.1000 0.1000 0.1000 0.1000 0.1000 0.1000
Flavor
menthol' 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000
Peppermint8
1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000
Sodium
- 0.2430 - - 0.2430 0.1215
fluoride9
Sodium
lauryl - 2.0000 - 2.0000
-
sulfatel
Calcium
pyrophosp -
2.0000 2.0000 -
hate"
%H202 3 3 3 3 3 3 3 3 3
ppm
1100 1100
550
Fluoride
Free of
YES YES YES YES YES YES No No Yes
opacifier?
Number of
ingredients 6 6 6 6 7 7 7 9 7
Yield
Stress
measured
Measure
according
168 205 ment 178 156 596 190 191 148
to the
limit of
method
800
specified
herein (Pa)
CA 03231717 2024-03-07
WO 2023/044470 PCT/US2022/076631
84
Appearanc Opaqu Opaqu Opaqu Opaqu Opaqu Opaqu Opaqu
Opaqu
Opaque
e e
Color White White White White White White White White White
Consistenc Semis Semis Semisoli Semis Semis Semis Semis Semis Semis
y olid olid d olid olid olid olid olid
olid
L* 63.51 80.66 66.21
a* -3.10 -0.39 -3.89
b* -9.71 -1.25 -9.69
of the
compositio
n measured -
according
to the
method
specified
herein
Dv 50 0.56 2.36 0.44
D[4,3] 0.58 2.42 0.45
[3,2] 0.51 1.97 0.40
(microns)
mean
equivalent-
diameter of
regions or -
droplets of
the
hydrophobi
c phase
measured
according
to the
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
method
specified
herein
'Ultra cosmetic grade 35% from Solvay, Houston, TX
2Tween20-LQ-(AP) from Croda Inc. Edison, NJ
3Hydrobrite HV grade from Sonneborn LLC., Parsippany, NJ
5 4Drakeol 35, USP grade from Calumet, Indianapolis, IN
5Hydrobrite 1000 grade from Sonneborn LLC., Parsippany, NJ
6Sucralose Micronized Powder USP/NF/FCC grade from Newtrend Technology Co.
Ltd. Jiangxi,
China
'Menthol USP grade from Boody Menthol International Inc., Paramus, NJ
10 8Peppermint flavor TAK-121065 from Takasago International Corporation,
Rockleigh, NJ
9Sodium Fluoride USP from Sunlit Fluo Chemical, Taiwan, R.O.C.
mSodium lauryl sulfate, Stepanol WA-100 NF/USP grade from Stepan Company,
Northfield, IL
"Calcium pyrophosphate, Prayphos SCPP 0000 Dental grade from Prayon, Augusta,
GA
12This counts flavor as one ingredient comprising a mixture of peppermint and
menthol, and counts
15 35% aqueous solution of H202 as two ingredients comprising water and
H202.
700-gram batches of Example II-A, Example II-B, Example II-C, Example II-D,
Example II-E,
Example II-F, and Example II-I were made according to the following procedure:
1. The Peppermint, Mineral Oil for Pre-mix, and Menthol were weighed into a
Speedmixer
container ("Max 40 Long Cup Translucent", item number 501 223Lt from Flacktek
Inc.,
20 Landrum, SC), heated in a convection oven set at 33C to 35C for about 30
to 60 minutes, and
Speedmixed at 800RPM for 2 minutes and visually checked to make sure the
Menthol was
dissolved.
2. The Sucralose, Tween 20, and Aqueous solution of H202 were weighed (along
with Sodium
lauryl sulfate, and Sodium fluoride if listed above) into a Speedmixer
container (Max 200 Long
25 Cup Translucent item number 501 220t from Flacktek Inc., Landrum, SC)
and mixed in
Speedmixer at 800 RPM for 2 minutes and visually checked to make sure all the
ingredients
were dissolved. The contents of this Speedmixer container were then
transferred into a High
Shear Mixer (KFP0711 Food Processor from KitchenAid, Benton Harbor, MI).
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
86
3. The Mineral Oil for Main Batch was weighed into a separate container and
slowly added over
3 to 5 minutes into the High Shear Mixer while it was running at LOW-speed
setting.
4. The Pre-mix (of Peppermint, Mineral Oil for Pre-mix, and Menthol) from step-
1 was added
into the High Shear Mixer while it was running at LOW-speed setting.
5. The contents of the High Shear Mixer were mixed at HIGH-speed setting for
about 5 minutes.
The High Shear Mixer was stopped, opened and allowed to cool for about 5
minutes. The
contents of the High Shear Mixer were again mixed at HIGH-speed setting for
about 5 minutes.
The above procedure resulted in jammed oil-in-water emulsion.
500-gram batches of Example II-G and Example II-H were made according to the
following
procedure:
A. A 700-gram base-batch was prepared using the above steps 1 to 5.
B. 10 grams of Calcium pyrophosphate was weighed into a separate Speedmixer
container (Max 300 X Long Cup Translucent item number 501 217t, from Flacktek
Inc., Landrum, SC), 490 grams of the base-batch from step-A was transferred
into
this Speedmixer container, and the contents of this Speedmixer container were
mixed at 800 RPM for 2 minutes.
The above procedure resulted in jammed oil-in-water emulsion comprising
Calcium
pyrophosphate.
TABLE 4 shows that:
1. Surprisingly, Examples II-A, II-B, II-C, II-D, II-E, II-F, and II-I have a
yield stress much
greater than 20 Pa even though they do not contain polymeric binders,
polymeric rheology
modifiers, or particulate thickeners such as silica.
2. Surprisingly, Examples II-A, II-B, II-C, II-D, II-E, II-F, and II-I are
opaque even though they
do not contain an opacifier.
3. Surprisingly, while the L* of all the Examples whose L* was measured
(Examples II-C, II-D,
and II-F) have a L* value greater than 20, only Example II-D has a L* greater
than 70 ¨ even
though it does not have any added opacifiers or brighteners.
4. Surprisingly, while the D[4,3] of all the Examples whose D[4,3] was
measured (Examples II-
C, II-D, and II-F) have a D[4,3] greater than 0.4 micron, only Example II-D
has a D[4.3] greater
than 0.7 micron.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
87
5. Without wishing to be bound by theory, it is believed that:
a. The higher L* of Example II-D (80.66 units) may be due to the larger
droplet size
D[4,3] (2.42 microns, which is much larger than the longest wavelength of
visible light
which is about 0.7 microns).
b. The lower L* of Example IT-C and II-F (63.51 units and 66.21 units) may be
due to the
smaller droplet size D[4,3] (0.58 microns and 0.45 microns, which are both
smaller
than the longest wavelength of visible light which is about 0.7 microns.
6. Despite the droplet size D[4,3] of Example II-C (0.58 microns) and II-F
(0.45 microns) being
smaller than the longest wavelength of visible light, both Examples IT-C and
II-F are opaque.
Without wishing to be bound by theory, it is believed that this may due to the
fact that their
D[4,3] is still larger than the shortest wavelength of visible light which is
about 0.4 micron.
7. It is also worth noting that Examples IT-C and II-F have a yield stress
substantially higher than
the rest, and specifically higher than Example II-D (> 800 Pa and 596 Pa Vs. a
maximum of
205 Pa for the rest, and 178 Pa for Example II-D). Without wishing to be bound
by theory, it
is believed that this may due to the fact that the droplet size D[4,3] of
Examples IT-C and II-F
are lower than that of II-D (0.58 micron and 0.45 micron Vs. 2.42 micron).
8. It is worth noting that the brochure titled "Manufacture of Toothpastes"
(Issue No. 14TA4,
Silverson Machines, East Longmeadow, MA) teaches that "Toothpastes are
generally either
white abrasive pastes or clear gels. Although the formulations differ, they
share many common
ingredients; these may vary from country to country according to legislation
on use of
ingredients, etc. Typical ingredients and their function are shown in TABLE 5.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
88
TABLE 5. Typical toothpaste ingredients
Ingredient type Typical % Function
White - Polyols, most commonly sorbitol (glycerin is also used) act as a
humectant, preventing
Liquid Base 30 the product from drying out and preserving the
texture and flavor.Polyol solutions can
Gel - Up contain up to 30% water; additional water (10-25%) completes the
liquid base.
to 80
Fillers and White - Various ingredients provide the polishing action in
white toothpastes; these
include calcium carbonate, hydrated silica, sodium bicarbonate, dicalcium
Abrasives 20-50phosphate and sodium metaphosphate. In clear gel type
products, hydratedsilica is
Gel - 15- used to provide polishing and "body."
Used to obtain several properties: the toothpaste must flow easily but not too
rapidly
Rh
from the tube; it must "break" easily without being "stringy"; it must siton
the eology 0.5-2 toothbrush without sinking in; these ingredients are
also used to keep fillers/abrasives
in suspension. Various ingredients are used, including CMC, carrageenan,
xanthan
Modifiers gum and cellulose gum.
Added to make the product foam when brushing. This helps dispersion and
retention
Detergent 0.5-2.5 of the product in the mouth. SLS (Sodium Lauryl
Sulphate) is most commonly used.
Active Fluoride can be added to help prevent tooth decay.
Sodium fluoride, sodium
Ingredient 0.3 monofluorophosphate and stannous fluoride are used,
subject to legislation, etc.
Flavoring is added to disguise the unpleasant taste of the detergent. It also
provides
Flavor 0.5-2 "freshness." Typically mint (and sometimes menthol
and cinnamon)flavoring oils are
used.
Sweetener 0.2 Sweeteners include sodium saccharinate.
Titanium dioxide can be added to white toothpaste as a coloring; gel
toothpastes may
Coloring 0.1 be manufactured in a number of colors using food
gradeproducts.
Preservative 0.2 Sodium benzoate, ethyl paraben, methyl paraben.
Surprisingly, in contrast to the above teaching from SiIverson, Examples II-A
to II-I are
5 toothpaste compositions containing very few and very simple ingredients
(with just 6 to 9 ingredients)
¨ see TABLE 4 above and TABLE 6 below.
CA 03231717 2024-03-07
WO 2023/044470 PCT/US2022/076631
89
TABLE 6
Example II-A II-B IT-C II-D TI-E II-F II-G II-H II-I
Free of
Liquid No No No No No No No No No
Base?
Free of
Fillers and YES YES YES YES YES YES No No YES
Abrasives?
Free of
Rheology
YES YES YES YES YES YES YES YES YES
Modifiers
such as
gums?
Free of
YES YES YES YES YES No YES No YES
Detergents
such as SLS?
Free of
Active
Ingredients YES YES YES YES No YES YES No No
such as
Fluoride?
Free of
No No No No No No No No No
Flavor?
Free of
No No No No No No No No No
Sweetener?
Free of YES YES YES YES YES YES No No YES
Coloring?
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
Free of
Preservatives
YES YES YES YES YES YES YES YES YES
such as
parabens?
The Fluoride (active) release rate was determined according to the method
specified herein for
four compositions: 1) Example II-E, 2) Example II-I, 3) Comparative
Composition I-C (Sensodyne
Pronamel, 1100 ppm fluoride) and 4) Comparative Composition I-D (Paradontax,
1100 ppm fluoride).
5 Example II-E and Example II-I are inventive compositions containing 1100 ppm
and 550 ppm
Fluoride respectively. The Fluoride Release Rate Profiles [ppm Fluoride
released over time] of these
four compositions are shown in FIG. 5. FIG. 5 shows that both the inventive
compositions (Example
II-E and Example II-I) have a much higher Fluoride release rate profile Vs.
Comparative Composition
I-D. The ppm Fluoride released in 60 and 120 Seconds by these four
compositions are additionally
10 presented in TABLE 7.
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
91
TABLE 7. ppm Fluoride Released In 60 and 120 Seconds
Example II- Example II-I Comparative Comparative
(Jammed Composition I- Composition I-D
(Jammed Oil-in-Water C (Traditional
Oil-in-Water Emulsion (Traditional Toothpaste)
Emulsion Toothpaste) Toothpaste)
Toothpaste)
1100 ppm F 550 ppm F 1100 ppm F 1100 ppm
ppm
Fluoride
released in
60 seconds
measured
12.0 4.7 4.9 1.5
according
to the
method
specified
herein
ppm
Fluoride
released in
120
seconds
measured 14.4 6.0 6.6 2.2
according
to the
method
specified
herein
TABLE 7 shows that surprisingly in 60 seconds:
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
92
a) Example II-E released a much higher amount (over 300%) of Fluoride Vs.
Comparative
Composition I-C and Comparative Composition I-D (12 ppm Vs. 4.9 ppm and 1.5
ppm)
b) Example II-I released almost the same amount of Fluoride as Comparative
Composition I-
C (4.7 ppm Vs. 4.9 ppm) even though it was formulated with half the Fluoride
(550 ppm
II-I vs 1100 ppm)
c) Example II-E released a much higher amount (over 300%) of Fluoride Vs.
Comparative
Composition I-D (4.7 ppm Vs. 1.5 ppm) even though it was formulated with half
the
amount of Fluoride (550 ppm Vs. 1100 ppm)
Similar results are observed after 120 seconds.
These results clearly demonstrate that a 550 ppm composition of the present
invention
(Example II-I) performs like a 1100 ppm traditional toothpaste (Comparative
Composition II-C) or
better than a 1100 ppm traditional toothpaste (Comparative Composition II-D).
EXAMPLE III
TABLE 8. Example III
Weight% A
35% aqueous solution
8.5714
of H2021-
PEG-20 Sorbitan
monolaurate (Tween 1.0000
20)2
Fractionated coconut
90.4286
oil3
Appearance Opaque
Color White
Consistency Lotion-like
'Ultra cosmetic grade 35% from Solvay, Houston, TX
2Tween20-LQ-(AP) from Croda Inc. Edison, NJ
3Fractionated Coconut Oil (or MCT - Medium Chain Triglycerides), Supplier
website
bulkapothecary.com (August 8 2021) states it to be "Nearly clear. Typically,
colorless to light
yellow", "MCT can also be derived from Palm Oil through the esterification
process.", "Also known
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
93
as MCT Oil or Medium Chain Triglycerides is a fraction of the whole coconut
oil.", and
" ...fractionated coconut oil has the long chain triglycerides like lauric
acid removed retaining the
capric and caprylic acids.", Item number J-007-bna-123 from Bulk Apothecary,
Aurora, OH.
A 100-gram batch of Example III-A was made according to the following
procedure:
4. The Tween 20, and aqueous solution of H202 were weighed into a Speedmixer
container
("Max 200 Long Cup Translucent", item number 501 220t from Flacktek, Landrum,
SC) and
mixed by manually swirling the container until dissolved.
5. The oil was added in portions starting at about 2g first, about 3g next,
and about 5g thereafter
and mixed at 800RPM for 2 minutes between portions in a Speedmixer. An oil-in-
water
emulsion formed during this step, and the composition developed a lotion-like
semisolid
consistency.
6. Once all the oil was added, the contents of the Speedmixer container
were mixed 3 times at
800 RPM for 2 minutes each time in a Speedmixer.
TABLE 9. Yield Stress of Hydrophobic Phase (without flavor) and Aqueous Phase
(without
sweetener) of Example II-B
Yield Stress (Pa)
Hydrophobic Phase (Mineral 0i1)1 < Detection Limit of 4
Aqueous Phase (Plus H202 and Tween < Detection Limit of 4
20)2
1Kaydol grade from Sonneborn LLC., Parsippany, NJ
2Ultra cosmetic grade 35% from Solvay, Houston, TX (8.5714 parts) + Tween20-LQ-
(AP) from Croda
Inc. Edison, NJ (1.0000 part)
TABLE 10. Appearance, Color, Consistency, and L*, a*, b* of Hydrophobic Phase
(without flavors)
and Aqueous Phase (without sweetener) of Example II-D
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
94
Hydrophobic Phase (Mineral Aqueous Phase (Plus
H202
0i1)1 and Tween 20)2
Appearance Clear Translucent
Color Colorless Pale yellow
Consistency Free flowing liquid Free flowing
liquid
L* 1.24 0.93
a*
-0.04 -0.24
b*
-0.54 -0.73
measured according to the
method specified herein
1 Hydrobrite 1000 grade from Sonneborn LLC., Parsippany, NJ
2Ultra cosmetic grade 35% from Solvay, Houston, TX (8.5147 parts) + Tween20-LQ-
(AP) from Croda
Inc. Edison, NJ (1.0000 part)
TABLE 10 compared with TABLE 4 shows that Hydrophobic Phase and Aqueous Phase
are
not opaque, but surprisingly, when they are combined, as in Example II-D the
final composition is
opaque even though it does not contain an opacifier. TABLE 9 compared with
TABLE 4 also shows
that Hydrophobic Phase and Aqueous Phase have a L* much less than 25, but
surprisingly, when they
are combined as in Example II-D, the final composition has a L* greater than
25 even though it does
not contain an opacifier. It is worth noting that the L* of the final
composition of Example II-D has a
L* value 6500% more than the L* of the Hydrophobic Phase and 8700% more than
the L* of the
Aqueous Phase (80.66 Vs. 1.24 and 0.93) even though it does not have an
opacifier.
FIG. 3 shows that Hydrophobic Phase and Aqueous Phase are not opaque, but
surprisingly,
when they are combined as in Examples II-C, II-D, and II-F the final
compositions are opaque even
though these Examples do not contain an opacifier. FIG. 3 also shows that
Example II-D is brighter
than Examples II-C and II-F.
FIG. 4A-D shows a nurdle of Examples II-C, II-D, II-F, and II-G dispensed onto
a toothbrush.
FIG. 4A-D shows that these Example compositions have a yield stress that
allows them to stand-up
on the bristles without sinking into the bristles or flow down the sides of
the bristles ¨ surprisingly
even though they do not contain polymeric binders, polymeric rheology
modifiers, or particulate
CA 03231717 2024-03-07
WO 2023/044470
PCT/US2022/076631
thickeners such as silica. FIG. 4A-D also shows that Examples II-C, II-D, and
II-F are surprisingly
opaque even though they do not contain an opacifier.
FIG. 5 shows a nurdle of Example I-A dispensed onto a toothbrush. FIG. 5 shows
that this
Example, in contrast to the Examples in FIG. 4, does not have a yield stress
that allows it to stand-up
5 on the bristles.
FIG. 6A-D shows microscopic images of jammed emulsions of Example I-A, II-C,
II-D, and
II-F with varying droplet sizes of the hydrophobic phase.
The dimensions and values disclosed herein are not to be understood as being
strictly limited
to the exact numerical values recited. Instead, unless otherwise specified,
each such dimension is
10 intended to mean both the recited value and a functionally equivalent
range surrounding that value.
For example, a dimension disclosed as "40 mm" is intended to mean "about 40
mm."
Every document cited herein, including any cross referenced or related patent
or application
and any patent application or patent to which this application claims priority
or benefit thereof, is
hereby incorporated herein by reference in its entirety unless expressly
excluded or otherwise limited.
15 The citation of any document is not an admission that it is prior art
with respect to any invention
disclosed or claimed herein or that it alone, or in any combination with any
other reference or
references, teaches, suggests or discloses any such invention. Further, to the
extent that any meaning
or definition of a term in this document conflicts with any meaning or
definition of the same term in a
document incorporated by reference, the meaning or definition assigned to that
term in this document
20 shall govern.
While particular embodiments of the present invention have been illustrated
and described, it
would be obvious to those skilled in the art that various other changes and
modifications can be made
without departing from the spirit and scope of the invention. It is therefore
intended to cover in the
appended claims all such changes and modifications that are within the scope
of this invention.