Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/039287
PCT/US2022/043350
LENS-FREE HOLOGRAPHIC OPTICAL SYSTEM FOR HIGH SENSITIVITY LABEL-
FREE CELL AND MICROBIAL GROWTH DETECTION AND QUANTIFICATION
FOR SCREENING, IDENTIFICATION, AND SUSCEPTIBILITY TESTING
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Patent
Application Serial No. 17/474,005,
filed September 13, 2021, which is continuation-in-part of U.S. Patent
Application Serial No.
17/353,943, filed June 22, 2021, which is a continuation of U.S. Patent
Application Serial No.
16/028,287, now U.S. Patent no. 11,079,719, filed July 5, 2018, which claims
the benefit of
U.S. Provisional Patent Application No. 62/528,825, filed July 5, 2017, the
disclosures of which
are hereby incorporated by reference in their entireties.
FIELD
[0002] The present disclosure relates to cell and microorganism
detection.
BACKGROUND
[0003] Microbial infections are best treated as early as
possible to confer the greatest
opportunity for patient recovery and to limit morbidity and mortality. For
example, roughly 85%
of patients demonstrating symptoms of infection will not have sufficient
microorganism
concentrations in their blood at initial presentation to enable detection of
the causative agent.
Corresponding blood samples may appear negative for microorganisms until many
doubling
events occur, at which point sufficient numbers of microbial cells will be
present and reach the
lower threshold of standard detection testing.
[0004] Automated microscopy systems traditionally used to detect
host and microbial cells
(hereinto referred to as cells, microbes, organisms and microorganisms) in
patient samples
comprise various configurations of sample containers, reaction reservoirs,
reagents, and optical
detection systems. Such optical detection systems are generally configured to
obtain images via,
for example, dark field and fluorescence photomicrographs of microorganisms
contained in
reaction reservoirs, such as flowcel I s (e.g., microfl ui di c channel s/ch
am ber, perfusion chambers,
and the like). Such optical detection systems also comprise a controller
configured to direct
operation of the system and process microorganism information derived from
photomicrographs. These systems generally are not capable of detecting
extremely low
concentrations of cells and microorganisms in direct from patient samples, and
require a
culturing period to ensure that if present, viable cells reach a detectable
level to statistically
ensure that a negative detection reading is truly negative.
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
1100051 A phenotypical approach to detection of a viable cell or
microbial population in a
sample involves in vivo monitoring of cell growth. While many approaches have
been
proposed to achieve this (impedance, weight, growth by-product concentration
monitoring,
etc.), solutions based on direct optical interrogation remain elusive as an
alternative. Optical
approaches are typically constrained by factors such as optical resolution as
well as the need
for timely acquisition of cell division (growth) over time (time-lapse
microscopy). For
example, detection of small concentrations of viable bacteria (typically <<
105 cfu/mL)
presents additional challenges as it requires large volumes of direct from
patient sample to be
interrogated (on the order of milliliters) to ensure a high probability of
detection. Moreover,
achieving better sensitivity in time-to-detection, such large volumes need to
be scanned at rates
higher than cellular division rates.
[0006] Usually, optical interrogation at high resolution relies
on lengthy multiple pass
scanning methodologies employing high precision 3-D stages, high quality
objectives, and fine
focusing techniques. Moreover, label-free (unstained) cells require the
employment of less
common imaging modes - such as phase contrast or differential contrast
interference
microscopy - due to a very small difference in refractive index with
suspension media. As a
result, hardware and software requirements for such applications scale poorly
with sample
volume under examination.
SUMMARY
1100071 To address this problem of detecting the presence of
cells and organisms in low
concentrations in patient samples, an imaging system was devised, which uses a
three-
dimensional ("3D-) or four-dimensional ("4D-) holographic approach. For
example, images of
a patient sample in a volume (3D) can be obtained over time (4D), for example
using video
frame rates. Unlike conventional imaging techniques, the instant holographic
imaging system
does not rely on multiple focal planes of cells growing in a location
requiring repeated image
capturing over time. Instead, a matrix array of optoelectronic sensors is
employed to obtain a
plethora of single images captured per time point from a 3D or 4D suspension
of cells and
microorganisms in a medium whose properties physically retain cells and
microorganisms in a
volume of sample and in some cases more substantially immobilized in a single
location. As
those cells divide, their offspring remain in the proximity of each other
affording rapid detection
of localized growth or tracking of individual cell movement with observation
frequent enough to
track individual cells. Furthermore, in the case of immobilization, the
offspring remain proximal
to the location of their mother cells, eliminating the need to track
individual cell movement
across a large volume of sample. In all embodiments, the focal point is
numerically determined
- 2 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
after the holographic image is captured affording significant advantages, one
of which being the
elimination of motion in the z direction to capture images at multiple focal
points. The process
permits simultaneous imaging of a large volume of patient sample to improve
the chances of
detecting viable cells present in low concentrations. Furthermore, because
rate of acquisition of
an entire 3D/4D volume of information across the matrix array of
optoelectronic sensors is
limited by electronic readout time and essentially real-time or near real time
on the timescale of
typical cell life cycle (on the order of milliseconds compared to average
doubling rates of 2 to 3
divisions per hour with bacteria) the constraint on physical retention of
cells in a single location
is significantly reduced affording flexibility to support a diverse set of
requirements associated
with a number of conventional tests. The embodied holographic imaging system
enables
sufficiently instant microscopic examination of these methods conferring
advantages including
but not limited to the near real time or instantaneous monitoring of cells and
microorganisms
and the response to the environment during testing or characterization.
[0008] In one embodiment, the media can be essentially water-
like liquid broth growth
media, or a desired minimal media, or a suspended biological sample (such as a
blood, urine,
respiratory, or saliva sample). Liquid growth media conditions are relevant to
diagnostic testing
including but not limited to traditional culturing methods to detect cell
presence in diagnostic
samples and susceptibility testing like Broth Microbiology Dilution (BMD)
testing (CLSI.
Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow
Aerobically,
11th edition. CLSI guideline M07-ED11. Wayne, PA: Clinical and Laboratory
Standards
Institute; 2018. The entire contents of this reference incorporated within
this application).
[0009] In another embodiment involving bacteria, the retention
of the microorganisms can
be achieved using conventional manual or automated processes that deposit of
organisms on agar
plates (including but not limed to manual streaking of plates using swabs or
automated means to
accomplish the same) in a substantially uniform layer, as required with
conventional Kirby Bauer
or Disk Diffusion methods (CLSI. Performance Standards for Antimicrobial Disk
Susceptibility
Tests, 13th edition. CLSI guideline M02-ED11. Wayne, PA: Clinical and
Laboratory Standards
Institute; 2018. The entire contents of this reference incorporated within
this application).
[00010] In yet another embodiment, droplet deposition of liquid suspensions of
organisms on
agar plates as described in traditional plating and overnight culture of micro-
organisms (L. S.
Garcia (ed.), 2007 Update: Clinical Microbiology Procedures Handbook, 2nd ed.,
2007 Chapter
3. Section 11.2 Lower Respiratory Tract Cultures) and the agar dilution method
of susceptibility
testing (CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for
Bacteria that Grow
Aerobically, 11th edition. CLSI guideline M07-ED11. Wayne, PA: Clinical and
Laboratory
- 3 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
Standards Institute; 2018. The entire contents of this reference incorporated
within this
application).
[00011] Further embodiments, include but are not limited to, use of agar
overlay or similar
methodologies that enhance immobiliLation of organisms depending on the
requirements of the
testing performed. Provided herein is a system that includes a holographic
optical apparatus
situated to determine the presence of a cell or microorganism in a sample
volume based on a
detected variation over time of a hologram of the sample volume, such as a
detected variation
corresponding to four or fewer cell doubling events, or three or fewer cell
doubling events. In
some examples, the holographic apparatus is an in-line holographic apparatus,
and the hologram
is an in-line hologram. In some examples, the in-line holographic optical
apparatus includes a
reference beam source situated to direct a reference beam to the sample
volume; a sample
receptacle situated to hold the sample volume in view of the reference beam;
an optical sensor
(such as a complementary metal oxide semiconductor (CMOS or CCD) sensor having
a pixel
pitch of 1.5 pm or smaller) situated to detect the in-line hologram formed by
the reference beam
and the sample volume; and a controller coupled to the optical sensor and
configured to
determine the variation over time of the in-line hologram. In some examples,
the optical sensor
has a pixel pitch of 1 pm/pixel or smaller and the controller is configured to
determine, based
on the detected in-line hologram, morphological characteristics of the
microorganism
determined to be present. The reference beam source can include a pinhole
aperture situated to
receive multi-wavelength illumination from an illumination source and the
reference beam is
directed lens-free from the pinhole aperture to the sample volume and optical
sensor. In some
examples, the reference beam source is situated to direct a plurality of
reference beams to the
sample volume and to adjacent portions of the optical sensor so as to mosaic
the field of view
of the in-line holographic apparatus, for example, wherein the adjacent
portions of the optical
sensor portions correspond to separate CMOS sensors. In some examples, the
multi-
wavelength illumination received by the illumination source is incoherent and
the reference
beam comprises incoherent illumination. In some examples, the controller is
configured to
reconstruct the spatial characteristics of the sample volume based on the
detected in-line
hologram, diffraction propagation approximation, and a phase retrieval
algorithm. In some
examples, the controller is configured to determine a focal plane of the cell
or microorganism
in the sample volume. In some examples, the sample volume includes at least
one sample
reaction chamber situated as a growth control with a first sample portion
situated in the
absence of an antimicrobial agent, and at least one sample reaction chamber
situated as an
antimicrobial susceptibility test with a second sample portion situated in the
presence of an
antimicrobial agent. The sample volume can include a plurality of growth
channels having
- 4 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
selective media. In some examples, the holographic apparatus is situated to
determine the
presence based on the detected variation with the sample volume having a
microorganism
concentration of 100 CFU /mL or less, such as 10 CFU/mL or less. In some
examples, the
holographic apparatus is situated to display a time-lapse image associated
with the sample
volume at a time-resolution that is faster than a microorganism division rate.
In some examples,
the time-lapse image corresponds to one or more of the hologram and one or
more planes of the
sample volume.
[00012] Also provided are methods for detecting a microorganism in a sample
(and in some
examples also identifying the microorganism, determining the antimicrobial
susceptibility of the
microorganism, or both), which can use the disclosed systems (such as those
that utilize
holography). In some examples, the sample is a polymicrobial sample. In some
examples, the
biological sample comprises 100 CFU/mL or less, such as 10 CFU/mL or less, of
the
microorganism. In some examples, the microorganism comprises bacteria,
protozoa, fungi, or
combinations thereof. In some examples, the method includes detecting an in-
line hologram of a
suspended biological sample (such as a blood, urine, respiratory, or saliva
sample); and for at
least one object in the suspended biological sample, determining a variation
over time of the in-
line hologram that is associated with an indication that the at least one
object is a cell or
microorganism in the biological sample. In some examples, determining a
variation over time
includes determining a spatial difference over time associated with the at
least one object and
corresponding to a cell's growth or decline. In some examples, the suspended
biological sample
is suspended in a porous medium, and the method further includes incubating
the suspended
biological sample in an environment conducive to cell replication (e.g.,
growth, division, or
both). In some examples, the method further includes interrogating the
suspended biological
sample in an optical interrogation system; wherein the optical interrogation
system includes at
least one optical sensor situated to perform the detecting of the in-line
hologram. In some
examples, the method further includes determining a focal plane corresponding
to a plane of
highest variance in the suspended biological sample that is associated with
the at least one
object. In some examples, the method further includes reconstructing spatial
characteristics of
the suspended biological sample based on the detected in-line hologram and a
numerical
reconstruction algorithm. In some examples, the optical sensor has a pixel
pitch of 1 um/pixel or
smaller and the method further includes determining, based on the detected in-
line hologram,
morphological characteristics of the at least one object corresponding to a
microorganism. In
some examples, the method further includes directing a plurality of reference
beams to the
suspended biological sample and to adjacent portions of the optical sensor so
as to mosaic the
field of view of the optical interrogation system. In some examples, the
suspended biological
- 5 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
sample includes at least one sample reaction chamber situated as a growth
control with a first
sample portion situated in the absence of an antimicrobial agent, and at least
one sample
reaction chamber situated as an antimicrobial susceptibility test (AST) with a
second sample
portion situated in the presence of at least one antimicrobial agent. An
exemplary growth control
includes Mueller-Hinton media in broth formulation (MHB) or agar formulation
(MHA) that
enables immobilization or entombment for immobilization purposes. Exemplary
antimicrobial
agents include one or more of amikacin, ampicillin, ampicillin-sulbactam,
aztreonam, cefazolin,
cefepime, ceftaroline, ceftazidime, ceftriaxone, ciprofloxacin, colistin,
daptomycin,
doxycycline, erythromycin, ertapenem, gentamicin, imipenem, linezolid,
meropenem,
minocycline, piperacillin-tazobactam, tobramycin, trimethoprim-
sulfamethoxazole, and
vancomycin. The suspended biological sample can be present in a plurality of
flow cells or
chambers, each comprising selective and differential media, such as blood
containing broth or
agar, Eosin Methylene Blue (EMB) broth or EMB agar, mannitol salt) broth or
mannitol salt
agar, MacConkey ) broth or MacConkey agar, phenylethyl alcohol (PEA) ) broth
or PEA agar,
and YM ) broth or YM agar, by way of example and not limitation. In some
examples, the
method further includes displaying a time-lapse image associated with the
suspended
biological sample at a time-resolution that is faster than cell division or
multiplication rate
(e.g., the rate at which a bacterium or yeast divides into two daughter cells,
the rate at which
a protist divides itself into two or more daughter cells). In some examples,
the time-lapse
image corresponds to one or more of the detected in-line hologram and one or
more planes of
the suspended biological sample.
[00013] In some examples, the methods include detecting a variation of an in-
line hologram over
time of a biological sample; and determining the presence of a cell or
microorganism in the
biological sample based on the detected variation.
[00014] In some examples, the methods include detecting an in-line hologram of
a biological
sample at a first time and a second time; comparing the in-line holograms to
determine a
hologram variation associated with the cell or microorganism; and determining
whether a
microorganism is present in the biological sample based on the variation.
[00015] In some examples, the system includes at least one processor, and one
or more
computer-readable storage media including stored instructions that, responsive
to execution by
the at least one processor, cause the system to compare a first in-line
hologram of a sample
volume at a first time and a second in-line hologram of the sample volume at a
second time
and to determine a hologram variation between the first in-line hologram and
second in-line
hologram that is associated with an indication as to the presence of a cell or
microorganism in
the sample volume.
- 6 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[00016] In particular sequences of using holographic optical apparatus and
methods examples
herein, screening can be performed to determine the presence of a
microorganism, AST can be
performed, and then identification.
[00017] Also provided are optical interrogation platform systems. In some
examples, such a
system includes an in-line holographic setup comprising a single-aperture
multi-wavelength
illumination; and a complementary metal oxide semiconductor (CMOS) sensor
having a pixel
pitch selected so as to detect a holographic variation over time associated
with the presence of a
cell or microorganism in a sample volume.
[00018] Also provided are automated methods of lens-free microscopy for
detecting one or
naore cells or microorganisms in a sample (and in some examples also
identifying the
microorganism, determining the antimicrobial susceptibility of the
microorganism, or both). In
some examples, the methods include suspending a biological sample in a porous
medium;
introducing the suspended biological sample to a sample reaction chamber;
subjecting the
porous medium to a phase change to immobilize microorganism cells in the
suspended
biological sample in three-dimensional space; incubating the suspended
biological sample in an
environment conducive to microorganism replication; interrogating the
suspended biological
sample in an automated optical interrogation system using one or more
optoelectronic sensors
to locate the optimal focal plane for each microorganism in the sample;
tracking spatial
differences to detect changes in growth of microorganisms over time; and
acquiring
holographic images of replicating microorganisms, thereby detecting their
presence in the
biological sample. In some examples, the phase change produces a gelled
medium. In some
examples, the microorganisms are present in the biological sample at a
concentration of
approximately 102 bacteria per 1 mL or 102 bacteria per 300 L of sample.
[00019] According to another aspect of the disclosed technology, systems can
be automated and
include an automated holographic optical apparatus situated to determine at
least the
antimicrobial susceptibility of a microorganism corresponding to an object in
a sample volume
based on a detected variation over time of a hologram of the sample volume, an
output of at least
one deeply supervised convolutional neural network, and a phenotypical
behavior of the
microorganism, wherein the phenotypical behavior of the microorganism is
classified based on
the detected variation and the output of the at least one deeply supervised
convolutional neural
network. In representative systems, the holographic apparatus is an in-line
holographic apparatus
and the hologram is an in-line hologram, and the in-line holographic optical
apparatus includes a
reference beam source situated to direct a reference beam to the sample
volume, a sample
receptacle situated to hold the sample volume in view of the reference beam,
an optical sensor
situated to detect the in-line hologram formed by the reference beam and the
sample volume, and
- 7 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
a controller coupled to the optical sensor and that includes at least one
processor and one or more
computer-readable storage media including stored instructions that, responsive
to execution by
the at least one processor, cause the controller to determine the variation
over time of the in-line
hologram. In some examples, the controller is configured to reconstruct the
spatial characteristics
of the sample volume based on the detected in-line hologram, diffraction
propagation
approximation, and a phase retrieval algorithm. In further examples, the
controller is configured
to determine a focal plane of the microorganism in the sample volume based on
the reconstructed
spatial characteristics. In particular examples, the at least one deeply
supervised convolutional
neural network includes a spatial reconstruction deeply supervised
convolutional neural network
configured to produce an output corresponding to a reconstruction of the
spatial characteristics of
the sample volume based on a trained set of network layers, and wherein the
controller is
configured to reconstruct the spatial characteristics of the sample volume
using the
reconstruction deeply supervised convolutional neural network. In selected
examples, the at
least one deeply supervised convolutional neural network includes a
microorganism
identification deeply supervised convolutional neural network configured to
produce an output
corresponding to a microorganism identification, microorganism morphology
identification,
microorganism movement identification, and/or microorganism phenotypic
classification for
the microorganism in the sample volume based on a trained set of network
layers, and wherein
the controller is configured to identify the microorganism, microorganism
morphology,
microorganism movement, and/or classify the microorganism phenotypical
behavior using the
microorganism identification deeply supervised convolutional neural network.
In some
examples, the controller is configured to determine a 3D position and/or
morphological
characteristics of the microorganism based on the in-line hologram. In further
embodiments,
the controller is configured to associate the object detected in a later
hologram with the object
detected in an earlier hologram, based on proximity or morphological
characteristics of the
objects detected from the variation over time of the in-line hologram. In
particular examples,
the controller is configured to form an object track for the object in the
sample volume based
on the detected variation over time of the in-line hologram. In some
embodiments, the
controller is configured to identify the object as the microorganism in the
sample volume based
on the detected variation over time of the in-line hologram. In some
embodiments, the
controller is configured to classify a phenotypical behavior of the
microorganism in the sample
volume based on the detected in-line hologram. In some examples classifying a
phenotypical
behavior, the controller is configured to determine a correspondence between
the phenotypic
behavior of the microorganism and presence, concentration, and taxon of the
microorganism in
the sample volume. In further examples classifying a phenotypical behavior,
the sample volume
- 8 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
includes a plurality of sample volume portions situated in a respective at
least one growth
control, at least one selective media, and at least one antimicrobial flow
cell that are held by the
sample receptacle, and the controller is configured to determine the presence,
taxon, and an
antibiogram of the microorganism or multiple microorganisms based on the at
least one growth
control, the at least one selective media, and the at least one anti-microbial
flow cell. In some
embodiments with an optical sensor, the optical sensor is a complementary
metal oxide
semiconductor (CMOS) sensor having a pixel pitch of 1.5 gm or smaller. In
further
embodiments with an optical sensor, the optical sensor has a pixel pitch of 1
gm/pixel or smaller
and the controller is configured to determine, based on the detected in-line
hologram,
morphological characteristics of the microorganism. In some embodiments, the
reference beam
source includes a plurality of pinhole apertures spaced apart from each other
by 1 mm or less
with each of the pinhole apertures configured to emit respective reference
subbeams at different
respective wavelengths. In further embodiments, the reference beam source
includes a pinhole
aperture situated to receive illumination from an illumination source and the
reference beam
source is configured to direct the reference beam lens-free from the pinhole
aperture to the
sample volume and optical sensor. In some pinhole aperture examples, the
illumination source is
configured to generate illumination at multiple wavelengths. In further
pinhole aperture
examples, the illumination received from the illumination source by the
pinhole aperture is
incoherent and the reference beam comprises incoherent illumination. In some
embodiments,
the reference beam source is situated to direct a plurality of reference beams
to the sample
volume and to adjacent portions of the optical sensor so as to mosaic the
field of view of the in-
line holographic apparatus. In some mosaic examples, the adjacent portions of
the optical sensor
correspond to separate CMOS sensors. In further examples, the sample volume
includes a
plurality of sample volume portions, including a first sample volume portion
situated in a first
sample reaction chamber that is held by the sample receptacle, wherein the
first sample volume
portion is situated as a growth control volume by having an absence of an
antimicrobial agent,
and including a second sample volume portion situated in a second sample
reaction chamber,
wherein the second volume portion is situated as an antimicrobial
susceptibility test volume in
the presence of a predetermined antimicrobial agent. In particular examples,
the sample reaction
chambers include a plurality of growth channels having selective media. In
some embodiments,
the holographic apparatus is situated to determine a presence of the
microorganism based on the
detected variation with the sample volume having a microorganism concentration
of 10 cfu/mL
or less. In further embodiments, the holographic apparatus is situated to
display a time-lapse
image associated with the sample volume at a time-resolution that is faster
than a
microorganism division rate. In some time-lapse examples, the time-lapse image
corresponds to
- 9 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
one or more of the hologram and one or more planes of the sample volume. In
some
embodiments, a time period of the detected variation corresponds to four or
fewer
microorganism doubling events. In further embodiments, a time period of the
detected variation
corresponds to three or fewer microorganism doubling events. In representative
systems, the
microorganism is in the sample volume.
1000201 According to a further aspect of the disclosed technology, methods
includes
detecting an in-line hologram of a suspended biological sample, measuring for
at least one
microorganism in the suspended biological sample, a variation over time of the
in-line
hologram, and determining the presence or absence of antimicrobial
susceptibility for the at
least one microorganism in the suspended biological sample based on the
measured variation
over time of the in-line hologram of the suspended biological sample, an
output of at least one
deeply supervised convolutional neural network associated with the measured
hologram, and a
phenotypical behavior of the at least one microorganism, wherein the phenotypi
cal behavior is
classified based on the detected variation and the output of the at least one
deeply supervised
convolutional neural network. In some examples, the at least one microorganism
is in the
suspended biological sample. Some embodiments can include, before determining
presence or
absence of antimicrobial susceptibility, determining whether a microorganism
is present in the
suspended biological sample based on the measured variation over time of the
in-line hologram.
Particular examples include suspending a biological sample in a porous medium
to form the
suspended biological sample, introducing the suspended biological sample to a
sample reaction
chamber, subjecting the porous medium to a phase change to immobilize the at
least one
microorganism in the suspended biological sample in three-dimensional space,
incubating the
suspended biological sample in an environment conducive to microorganism
replication, wherein
detecting the in-line hologram and determining the variation over time
includes interrogating the
suspended biological sample in an automated optical interrogation system using
one or more
optoelectronic sensors to locate an optimal focal plane for each of the
microorganisms in the
biological sample, tracking spatial differences to detect changes in growth of
the at least one
microorganism over time, and acquiring holographic images of the replicating
at least one
microorganism, thereby detecting its presence in the biological sample. In
some examples, the
phase change produces a gelled medium. In further examples, the at least one
microorganism is
present in the biological sample at a concentration of approximately 102
bacteria per 1 mi., of
sample. In some examples, the at least one microorganism is, and the
determining a variation
over time includes determining a spatial difference over time associated with
the at least one
microorganism and corresponding to a microorganism growth or decline. In
selected examples,
the at least one deeply supervised convolutional neural network includes a
spatial reconstruction
- 10 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
deeply supervised convolutional neural network configured to produce an output
corresponding
to a reconstruction of the spatial characteristics of the suspended biological
volume based on a
trained set of network layers. In additional examples, the at least one deeply
supervised
convolutional neural network includes a microorganism identification deeply
supervised
convolutional neural network configured to produce an output corresponding to
a
microorganism identification, microorganism morphology identification,
microorganism
movement identification, and/or microorganism phenotypic classification for
the at least one
microorganism in the suspended biological sample based on a trained set of
network layers. In
examples, the sample material of the suspended biological sample is suspended
in a porous
medium, and the suspended biological sample is incubated in an environment
conducive to
microorganism replication. In representative embodiments, the in-line hologram
is detected with
an optical sensor comprising one or more sensor portions. In selected
examples, each of the
optical sensor portions includes a plurality of pixels with a pixel pitch of 1
pm/pixel or smaller.
Some examples can include directing a plurality of reference beams to the
suspended biological
sample and to adjacent portions of the optical sensor corresponding to the
respective optical
sensor portions to produce a mosaicked field of view of the in-line hologram.
Some
embodiments include determining a focal plane corresponding to a plane of
highest variance in
the suspended biological sample that is associated with the at least one
object. Additional
examples include reconstructing spatial characteristics of the suspended
biological sample based
on the detected in-line hologram and a numerical reconstruction algorithm. In
some
embodiments, the suspended biological sample is supported by a sample
receptacle of an in-line
holography apparatus situated to perform the detecting, measuring, and
determining, and
wherein a first sample portion of the suspended biological sample is located
in a first sample
reaction chamber in the absence of an antimicrobial agent so as to correspond
to a growth
control, and wherein a second sample portion of the suspended biological
sample is located in a
second sample reaction chamber in the presence of at least one antimicrobial
agent. In some
examples, growth control comprises Mueller-Hinton broth (MHB) or agar (MHA).
In selected
examples, the at least one antimicrobial agent comprises amikacin, ampicillin,
ampicillin-
sulbactam, aztreonam, cefazolin, cefepime, ceftaroline, ceftazidime,
ceftriaxone, ciprofloxacin,
colistin, daptomycin, doxycycline, erythromycin, ertapenem, gentamicin,
imipenem, linezolid,
meropenem, minocycline, piperacillin-tazobactam, tobramycin, trimethoprim-
sulfamethoxazole,
vancomycin, or combinations thereof. In some embodiments, suspended biological
sample
includes sample volume portions that are present in a plurality of respective
flowcells
comprising selective and differential media. In particular examples, the
selective and differential
media comprise blood broth or agar, Eosin Methylene Blue (EMB) broth or EMB
agar,
- 11 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
mannitol salt broth or mannitol salt agar, MacConkey broth or MacConkey agar,
phenylethyl
alcohol (PEA) broth or PEA agar, or YM broth or YM agar. Some embodiments can
include
displaying a time-lapse image associated with the suspended biological sample
at a time-
resolution that is faster than a microorganism division rate. In some time-
lapse examples, the
time-lapse image corresponds to one or more of the detected in-line hologram
and one or more
planes of the suspended biological sample. In some examples, the suspended
biological sample
is obtained from blood, urine, respiratory sample, or saliva. In further
examples, the suspended
biological sample is a polymicrobial sample. In some examples, the suspended
biological
sample comprises 10 CFU/ml or less of the at least one microorganism. In
further examples, the
microorganism comprises one or more bacteria, protozoa, fungi, or combinations
thereof.
[00021] The foregoing and other objects and features of the disclosure will
become more
apparent from the following detailed description, which proceeds with
reference to the
accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
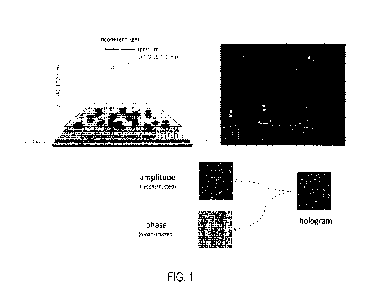
[00022] FIG. 1 depicts a lens free imaging using an optoelectronic sensor
array to generate a
holographic image of sample objects.
[00023] FIG. 2A is a view of a perfusion chamber mounted on a glass microscope
slide.
[00024] FIG. 2B is a view of alternative perfusion chambers with multiple
individual
chambers, which can be mounted on a glass microscope slide, for example to
analyze
multiple samples contemporaneously, a single sample under multiple different
media, or
combinations thereof.
1000251 FIG. 3 shows images obtained showing proof of concept of the optical
interrogation
platform using transparent silicone beads.
11000261 FIG. 4 shows images obtained by the optical interrogation platform
imaging E. coli
growth over a period of 0 to 180 minutes.
[00027] FIG. 5 shows images obtained by the optical interrogation platform
imaging E. coil
growth during a period from 240 to 540 minutes.
[00028] FIG_ 6 is a perspective schematic of an example in-line holographic
apparatus.
[00029] FIG. 7 is a perspective schematic of an example mosaicked in-line
holographic
apparatus.
[00030] FIGS. 8-12 are flowcharts of example holography methods.
[00031] FIG. 13 is a schematic of an example computing environment.
[00032] FIGS. 14A-14C are perspective schematics of example sample volumes
undergoing
growth and detection with holography methods herein.
- 12 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[00033] FIG. 15 is a flowchart of another example holography method.
[00034] FIGS. 16-17 are flowcharts of example convolutional neural network
training and
trained testing.
DETAILED DESCRIPTION
11000351 The following explanations of terms and methods are provided to
better describe the
present disclosure and to guide those of ordinary skill in the art in the
practice of the present
disclosure. The singular forms "a,- "an,- and "the- refer to one or more than
one, unless the
context clearly dictates otherwise. For example, the term "comprising a
bacterium" includes
single or plural bacteria and is considered equivalent to the phrase
"comprising at least one
bacterium." The term -or" refers to a single element of stated alternative
elements or a
combination of two or more elements, unless the context clearly indicates
otherwise. As used
herein, "comprises- means "includes.- Thus, "comprising A or
means "including A, B, or A
and B," without excluding additional elements. All references cited herein are
incorporated by
reference.
[00036] Unless explained otherwise, all technical and scientific terms used
herein have the same
meaning as commonly understood to one of ordinary skill in the art to which
this disclosure
belongs. Although methods and materials similar or equivalent to those
described herein can be
used in the practice or testing of the present disclosure, suitable methods
and materials are
described below. The materials, methods, and examples are illustrative only
and not intended to
be limiting. For example, the steps recited in any of the method or process
descriptions may be
executed in any order and are not necessarily limited to the order presented.
Also, any reference
to attached, fixed, connected or the like may include permanent, removable,
temporary, partial,
full and/or any other possible attachment option. Additionally, any reference
to without contact
(or similar phrases) may also include reduced contact or minimal contact.
Furthermore, the
connecting lines shown in the various figures contained herein are intended to
represent
exemplary functional relationships and/or physical couplings between the
various elements. It
should be noted that alternative or additional functional relationships or
physical connections
may be present in a practical system. However, the benefits, advantages,
solutions to problems,
and any elements that may cause any benefit, advantage, or solution to occur
or become more
pronounced are not to be construed as critical, required, or essential
features or elements of the
inventions.
[00037] The disclosed methods, apparatus, and systems should not be construed
as limiting.
Instead, the present disclosure is directed toward all novel and nonobvious
features and aspects
of the various disclosed embodiments, alone and in various combinations and
subcombinations
- 13 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
with one another. The disclosed methods, apparatus, and systems are not
limited to any specific
aspect or feature or combination thereof, nor do the disclosed embodiments
require that any one
or more specific advantages be present or problems be solved.
[00038] In the detailed description herein, references to "one embodiment,"
"an embodiment,"
"an example embodiment," etc., indicate that the embodiment described may
include a
particular feature, structure, or characteristic, but every embodiment may not
necessarily include
the particular feature, structure, or characteristic. Moreover, such phrases
are not necessarily
referring to the same embodiment. Further, when a particular feature,
structure, or characteristic
is described in connection with an embodiment, it is submitted that it is
within the knowledge of
one skilled in the art to affect such feature, structure, or characteristic in
connection with other
embodiments whether or not explicitly described. After reading the
description, it will be
apparent to one skilled in the relevant art(s) how to implement the disclosure
in alternative
embodiments.
[00039] Furthermore, no element, component, or method step in the present
disclosure is
intended to be dedicated to the public regardless of whether the element,
component, or method
step is explicitly recited in the claims. No claim element herein is to be
construed under the
provisions of 35 U.S.C. 112(f), unless the element is expressly recited using
the phrase "means
for." As used herein, the terms "comprises," "comprising," or any other
variation thereof, are
intended to cover a non-exclusive inclusion, such that a process, method,
article, or apparatus
that comprises a list of elements does not include only those elements but may
include other
elements not expressly listed or inherent to such process, method, article, or
apparatus.
[00040] In some examples herein, optical beam cross-sectional areas,
diameters, or other beam
dimensions can be described using boundaries that generally correspond to a
zero intensity
value, a 1/e value, a 1/e2 value, a full-width half-maximum (FWHM) value, or
other suitable
metric. As used herein, optical illumination refers to electromagnetic
radiation at wavelengths of
between about 100 nm and 10 urn, and typically between about 200 nm and 2
1.t.m. Optical
illumination can be provided at particular wavelengths (typically narrow
wavelength bands) or
ranges of wavelengths.
[00041] As used herein, "AST" is antimicrobial susceptibility testing,
antimicrobial agent
susceptibility testing, or antibiotic susceptibility testing, and can include
MIC (minimum
inhibitory concentration) and/or SIR (susceptible, intermediate, resistant).
[00042] As used herein, "ID" is identification, such as a process of
determining the species
identity of a microorganism, such as determining or identifying the genus,
species, Gram status,
and/or strain of a microorganism. This is distinct from detecting the presence
of an unknown
microorganism in that it is more specific.
- 14 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[00043] As used herein, "MHB" is Mueller Hinton Broth and MHA is Mueller
Hinton Agar.
[00044] As used herein, "3D- refers to three-dimensional space.
[00045] As used herein, "4D" refers to four-dimensional space.
[00046] In order to facilitate review of the various embodiments of the
disclosure, the following
explanations of specific terms are provided:
11000471 Administration: To provide or give a subject an agent, such as an
antimicrobial
agent (such as an antibiotic or antifungal), by any effective route. Exemplary
routes of
administration include, but are not limited to, oral, injection (such as
subcutaneous,
intramuscular, intradermal, intraperitoneal, intravenous, intra-articular, and
intrathecal),
sublingual, rectal. transdermal, intranasal, vaginal and inhalation routes.
[00048] Cell, microorganism or microbe: A microscopic cell or organism that in
some
examples causes disease, for example in a mammal, bird, or fish. Examples of
cells include host
cells in a host clinical specimen or microorganisms including, but not limited
to bacteria, fungi
(including mold and yeast morphologies), and protozoa.
[00049] Sample or specimen: A biological sample or biological specimen, such
as those
obtained from a subject (such as a human or other mammalian subject, such as a
veterinary
subjects, for example a subject known or suspected of having a disease or
infection). The
sample can be collected or obtained using methods well known to those skilled
in the art.
Samples can contain nucleic acid molecules (such as DNA, cDNA, and RNA),
proteins, cells,
cell membranes, or combinations thereof. In some examples, the disclosed
methods include
obtaining the sample from a subject prior to analysis of the sample using the
disclosed methods
and devices. In some examples, a sample to be analyzed is lysed, extracted,
concentrated,
diluted, or combinations thereof, prior analysis with the disclosed methods
and devices.
[00050] Exemplary samples include, without limitation, cells, cell lysates,
blood smears,
cytocentrifuge preparations, flow-sorted or otherwise selected cell
populations, cytology smears,
bodily fluids (e.g., blood and fractions thereof such as white blood cells,
serum or plasma;
saliva; respiratory samples, such as sputum or lavages; urine; cerebrospinal
fluid; gastric fluid;
sweat; semen; puss; etc.), buccal cells; extracts of tissues, cells or organs,
tissue biopsies (e.g.,
tumor or lymph node biopsies); liquid biopsies; fine-needle aspirates;
brocoscopic lavage; punch
biopsies; bone marrow; amniocentesis samples; autopsy material; fresh tissue;
vaginal swabs;
rectal swabs; and the like. The biological sample may also be a laboratory
research sample such
as a cell culture sample or supernatant. As used herein, samples can include a
sample volume
and can be introduced to a container or receptacle that houses or supports the
sample volume.
Sample volumes can include liquid or particulates of the sample (e.g.,
microorganisms, if
present) obtained from a sampled subject. In typical examples, the liquid
and/or particulate
- 15 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
portions of the sample can include a mixture with supporting media, such as
growth media. The
container or receptacle housing the sample can include sample reaction
chambers, which can
include solid supports (e.g., polycarbonate, silicone, glass, etc.) into which
patient sample
material is loaded and which can define separations between portions of the
sample volume. In
typical examples, "sample" can refer to the material of a biological sample,
such as when the
material is transferred between supporting structures (e.g., introducing a
sample to a flow cell).
Sample receptacles can also refer to structures that receive and support
samples and also
structures that receive and support sample containers that house samples.
[00051] Subject: Any mammal, such as humans and veterinary subjects, such as,
non-human
primates, pigs, sheep, cows, dogs, cats, rodents and the like. In one example,
a subject is a
human subject. In some examples, the subject is known or suspected of having a
disease or an
infection. In some examples, the subject is septic.
Overview
[00052] Patient samples, such as blood, respiratory, and other biological
samples, are the
primary biological starting point for assessing the etiology of a patient's
disease and
determining the appropriate therapy course for treating that disease. Key to
reducing morbidity
and mortality is initiating the proper therapeutic treatment of a critically
ill patient at the
appropriate dosage regimen as soon as possible. The historically weak link in
this process is
sufficient cultivation of a cell or microbial population in the patient sample
to enable
identification of pathogen(s) present and to determine which compounds the
pathogen(s) will
respond to in therapy. Reducing the assay time required to properly identify
cells and
microorganism(s) in a patient sample and assess their drug sensitivity is
crucial to improving
patient survival odds.
[00053] In many instances, patient samples contain only a single type of
microorganism. In
other instances, patient samples contain multiple types of cells
microorganisms, such as
mixtures of host cells and bacteria from differing genera, species, and even
strains (also known
as "polymicrobial" samples). Diagnostic accuracy is traditionally expressed in
terms of
sensitivity and specificity. Sensitivity refers to the probability of
assigning a diagnostic test as
positive when it is in fact, positive (the fraction of true positives), which
confound the
identification and antimicrobial sensitivity processes. The counter to
sensitivity is specificity,
which is the rate of obtaining false negative test results. Current methods of
identifying
unknown cells or microorganisms are prone to failure in both false positive
and false negative
modes. These difficulties with sensitivity and specificity are typically
fostered by factors that
impede sample detection, such as noise, crosstalk, borderline resistance, and
the like. Traditional
analysis methods often trade sensitivity of detection for the specificity of
cell or microorganism
- 16 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
identification. In other applications, the reverse is true, prioritizing
sensitivity over accurate
identification. But to maximize efficiency, and thus improve the odds of
achieving a better
treatment outcome for the patient, improving sensitivity for detecting the
presence of cells as
early as possible is desirable. In doing so, clinicians and laboratory
personnel can determine
which samples may be eliminated from the microbial identification and
antimicrobial sensitivity
workflow stream due to a true negative reading at the earliest possible time.
[00054] Traditional methods for identification (ID) and antimicrobial
susceptibility testing
(AST) of organisms from clinical specimens typically require overnight
subculturing to isolate
individual species (e.g., determine if the sample is positive for the presence
of pathogenic
bacteria, protozoa, and/or fungi) prior to biochemical assay-based
identification, followed by
growing isolated organisms in the presence of various antimicrobials to
determine
susceptibilities. Although molecular identification methods can provide
organism identification
in a few hours directly from clinical specimens as well as resistance marker
detection, these
methods do not provide the antimicrobial susceptibility information required
by clinicians to
inform treatment decisions. Studies demonstrating the feasibility of using
various sample types
including whole blood and respiratory samples have been reported, but sample
preparation
techniques require further refinement. Current rapid molecular-based
diagnostic methods only
report identification and genotypic resistance marker results. While available
in a couple of
hours, these results only provide a partial answer. This leaves the clinician
to prescribe overly-
broad spectrum empiric therapy while waiting two to four days for conventional
antibiotic
susceptibility test results before adjusting therapy. The availability of an
antimicrobial
susceptibility test result in as few as 5 hours or less, as opposed to a few
days, potentially
decreases morbidity and mortality in critically ill patients due to delays in
administration of
appropriate therapy. In addition, rapid de-escalation from broad-spectrum
empiric therapies to
targeted specific antimicrobials could assist antimicrobial stewardship
efforts to decrease the
emergence and spread of multi-drug resistant organisms (MDR0s). By using the
disclosed
holographic approach to determine which patient samples actually have
microorganisms
present therein (e.g., as an alternative to overnight culturing), patients who
can truly benefit
from identification and antimicrobial susceptibility testing can be
pinpointed. Only those
patients samples deemed positive for the presence of microorganisms would then
be subjected
to ID and AST evaluation, saving resources and time. Furthermore, in some
examples,
microorganisms can be precisely quantified, movements tracked, morphological
characteristics identified, and/or phenotypic behavior classified.
[00055] To address these problems, the disclosed system provides an automated
microscopy
system designed to provide rapid microorganism detection prior to typical
identification and
- 17 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
antibiotic susceptibility testing results. An aspect of this system is an
optical interrogation
platform capable of detecting bacterial and/or fungal growth in a sample
obtained directly
from a patient without prior overnight culturing. Exemplary samples include
blood,
respiratory material, urine, CSF, spinal fluid, and other bodily fluids and
tissue (such as soft
tissue samples and wound material). Samples can contain a very low
concentration of cells
and microorganisms, so low that direct from patient samples would typically be
deemed
negative for the presence of microorganisms, despite a patient demonstrating
symptoms
consistent with an infection. For example, a sample may have a target
bacterial concentration of
as low as about 10 cfu/mL or even 1 cfu/mL. The optical interrogation platform
can be
integrated into a small (portable) incubator or contain a temperature
controlled environmental
chamber to ensure normal bacterial growth during the interrogation process.
System
[00056] FIG. 1 depicts a lens free imaging system using an optoelectronic
sensor array to
generate a holographic image of sample objects. Large scale optical
inferometry targets objects
in a sample reaction chamber (e.g., a flowcell, such as a microfluidic flow
cell or perfusion
chamber), with incident light. When light waves encounter an object ¨ such as
a microbial cell
or debris ¨ the light waves are distorted from their original path and the
interference or light
scatter is recorded by the novel optical system as a hologram. When an
interference wave spot
changes over a period of time, the system records that perturbation as a
growing object. Thus, in
a phenotypic assessment of whether a viable cell or microorganism exists in a
sample, having
multiple sensors to screen a relatively large volume of sample in a short
period of time may
permit the detection of cells in as little as 1.5 to 2 hours by capturing
images 15-30 minutes
apart (or faster in some examples) over that period. In principle, microbial
cells can be detected
within 2-3 doubling times using this process.
[00057] An embodiment of the optical interrogation platform includes an in-
line holographic
setup that includes a single-aperture multi-wavelength illumination and a
complementary metal
oxide semiconductor (CMOS) sensor having a pixel pitch of 1.12 micrometers.
Holograms
obtained using the optical interrogation platform are reconstructed
¨propagated via diffraction
theory, then intensity and phase retrieved, for example using the iterative
phase retrieval
algorithm (such as Gerchberg¨Saxton (GS)). A reference wave (illumination) can
interact with
sample as propagating thru sample and at any point along reference wave, and
every point
becomes another point source (Huygens), and sensor records interference
pattern of all of these
waves (e.g., hologram). Because a sensor records only the intensity component
of the complex
wave function (hologram), phase component needs to be extracted. To gain back
phase, it can be
reconstructed numerically. Step 1 goes back to complex diffraction pattern in
a particular focal
- 18 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
plane via diffraction theory - solving Fresnel-Kirchoff integral (using
Fresnel approximation or
convolutional methods). Step 2 then reconstructs phase via iterative phase
retrieval algorithm
(such as GS). This platform can be paired with or contain a subsystem which,
for certain types of
samples (such as whole blood and respiratory samples) performs necessary
preparatory steps,
including but not limited to dilution, centrifugation, application of an
electrical field, and spin-
and-resuspension, to reduce amounts of non-bacterial debris load. The optical
interrogation
platform is scalable in space by "mosaicking" illumination-sensor "pairs"
(e.g., multiplexing),
thereby providing extensible spatial configurations.
[00058] The in-line holographic configuration of a lens-free setup includes
multi-wavelength
illumination to remove twin-distortion during the phase retrieval stage as
well as to improve
resolution, but it could be any in-line holographic setup such as multiple
illumination apertures,
single-wavelength or multi-wavelength illumination, and the like that provides
effective
resolution of -1 micro-meter/pixel. Although one embodiment of the optical
interrogation
platform utilized a perfusion chamber mounted on top of a standard glass
microscopy slide, the
platform may be designed to support imaging of other sample reaction chambers
(e.g., flowcells,
such as microfluidic channels or perfusion chambers) of a different
configuration. FIG. 2A is a
view of exemplary sample reaction chamber (e.g., perfusion chamber) mounted on
glass
microscope slides. FIG. 2B shows other exemplary perfusion chambers that can
be used.
[00059] As previously noted, the optical interrogation platform automates
growth detection of
microorganisms present in very low concentrations. In general, given the
system's acquisition
setup, each object suspended in a 3-dimensional (3D) volume has an optimal
focal plane
(plane of highest variance) at any given time. The optimal focal plane can be
found
automatically for each object in the imaged volume for every time of the time-
lapse sequence.
Then, tracking or equivalent spatial differencing techniques can be employed
to detect
changes. Various optical transforms are possible to return to object. Because
optical
transforms underlying hologram construction are linear operators, multiple
holograms can be
obtained and manipulate without loss of information, for example to determine
the presence or
identification of a microbe, or measure growth of a microbe over time. FIG. 3
shows images
obtained showing proof of concept of the optical interrogation platform using
heads. The
beads mimicking bacteria, protozoa, or fungi in a patient sample can be "seen"
using
holograms, but are not visible by standard bright field microscopy. One
hologram can be
stored per 3D stack. The holographic image of the volume may be stored, and
then later the
focal plane can be reconstructed numerically. The holograms may be stored as
TIFF, JPEG, or
other files routinely used in imaging.
- 19 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[00060] In a "mosaicked" embodiment, the optical interrogation platform can be
extended to
conduct simple antimicrobial susceptibility testing. This is accomplished by
dedicating at least
one microfluidic channel to serving as a growth control channel containing a
sample in the
absence of antimicrobial agent. One or more other microfluidic channels
containing samples
with antimicrobial agents added at appropriate concentrations may be utilized
to assess
antimicrobial susceptibility. For example, antibiotic susceptibility may be
assessed by pre-
mixing antibiotics with sample before introducing the mixture to one or more
sample reaction
chambers (e.g., flowcell, such as a microfluidic flowcell or perfusion
chamber). Alternatively,
antibiotics may be added after a sample has been deposited into the sample
reaction chamber, or
antibiotics may diffuse into contact with the sample from a dried-down state
in the sample
reaction chamber. During growth supporting conditions, microbial replication
in the "growth
control" channel is compared to replication in one or more antimicrobial
channels over time can
yield first-order susceptibility/resistance information.
[00061] Another embodiment of the optical interrogation platform supports a
multi-channel
scanning configuration (a tiled or "mosaicked" arrangement) call be extended
with "growth
control" channels that use selective media. Growth information from these
channels, in
conjunction with a standard "growth control" channel, can be used to infer
bacterial families and
even species. In another embodiment, the optical interrogation platform
permits microbial
differentiation based on organism morphology. Under certain optical resolution
(-0.51.tm/pixel),
the platform can be used to conduct morphological analysis to differentiate
morphology of
individual bacterial cells within each micro-colony. Such information can be
reported to a
clinician.
[00062] In some embodiments, microorganism detection is achieved by
simultaneously
scanning a sample volume as large as -300 microliters (IL) in a single optical
field-of-view of
up to 30 tirnin2 of surface area and up to linm depth. The system can perform
time-lapse imaging
of the same volume without mechanical motion at acquisition rates that are
much higher than
microbial division rates. Thus, the system enables the imaging of bacteria
faster than a small
number of their doubling events, such as fewer than 4 doubling events, fewer
than 3 doubling
events, or fewer than two doubling events. Some bacteria have a doubling time
of about 15-30
minutes, meaning that detection of the presence of such bacteria in a
patient's sample could be
achieved by the system in about 30 to 45 minutes.
11000631 The detection of a microorganism in a sample in such a short period
of time permits
clinicians to rapidly determine which patient samples should be further
subjected to multiplexed
automated single cell digital microscopy. One such digital microscopy system
is the fully
automated, microscopy-based system disclosed in U.S. patent publication no. US
2017/0023599
- 20 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
(herein incorporated by reference), which can perform bacterial or yeast
identification in about
one (I) hour and AST in about five (5) or fewer hours.
Methods of Identifying Microorganisms
[00064] The disclosed systems and devices can be used in methods to aid in the
diagnosis of
bacteremia and fungemia. They can also be used for susceptibility testing of
specific pathogenic
bacteria commonly associated with or causing bacteremia. Results can be used
in conjunction
with other clinical and laboratory findings.
[00065] The disclosed methods can be used to quickly determine if the patient
has a microbial
infection, and in sonic examples also identify the microbes infecting the
patient and identify
which antimicrobial agents are likely to be effective in treating the
infection. Such methods are
faster than currently available assays. In currently available assays, a
patient sample is incubated
overnight in the presence of a culture medium (such as at least 8 hours, at
least 10 hours, at least
12 hours, or at least 18 hours, such as 8 to 24 hours or 8 to 12 hours), to
allow for microbes
present in the sample to grow and multiply. If this results in a positive
result (i.e., microbes are
present), then additional assays are used to identify the microbe, identify an
effective
antimicrobial agent to administer to the patient to treat their infection, and
determine a minimal
inhibitory concentration (MIC) of antimicrobial agent to use. In contrast, in
representative
examples, the disclosed methods and systems do not require overnight
incubation of the patient
sample (e.g., in a culture medium) to determine whether the patient sample is
positive (i.e.,
microbes are present). In some embodiments, the disclosed methods identify the
microbe(s) in
the patient sample (e.g., the genus, species, Gram status and/or strain of the
microbe(s)) and
identify an effective antimicrobial agent to administer to the patient to
treat their infection. In
some examples, the disclosed methods take less than 3 hours to complete, such
as less than 2
hours, less than 1.5 hours or about 1.5 hours, such as 1 to 3 hours, or L5 to
2 hours. For
example, using the disclosed methods, it can take less than 3 hours, or less
than 2 hours, such
as 1.5 to 3 hours, or 1.5 to 2 hours to determine if the sample is positive
for bacteria, protozoa
and/or fungi. For example, using the disclosed methods, it can take less than
3 hours, such as
less than 2 hours, such as 2 to 3 hours, or 1.5 to 2 hours to identify the
bacteria, protozoa,
and/or fungi in the sample. For example, using the disclosed methods, it can
take less than 6
hours, less than 5 hours, or less than 4 hours, such as 3 to 6 hours, or 4 to
5 hours to identify
the antimicrobial that the bacteria, protozoa, and/or fungi in the sample are
sensitive to (e.g.,
will kill the bacteria, protozoa, and/or fungi).
[00066] Patients can include human and veterinary subjects, such as cats,
dogs, cows, pigs,
horses, sheep, goats, chickens, turkeys, and other birds, fish, and the like.
In some examples, a
- 21 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
patient is one who is known to have or is suspected of having an infection
(such as a bacterial or
fungal infection). In one example, the patient is septic. Patient samples
include but are not
limited to blood (e.g., whole blood, plasma, or serum), respiratory samples
(such as
bronchoalveolar lavage, oropharyngeal swab, nasopharyngeal swab, or sputum),
saliva, urine,
rectal swab, vaginal swab, tissue samples, or other biological specimens (such
as those
described herein).
[00067] In some examples, the patient sample contains only a single type of
microorganism. In
other instances, the patient sample contains multiple types of cells and
microorganisms, such as
mixtures of host cells, bacteria, protozoa, and/or fungi from differing
genera, species, and even
strains (also known as "polymicrobial- samples), such as at least 2, at least
3, at least 4 or at
least 5 different types of bacteria, protozoa, and/or fungi. In some examples,
the patient sample
contains bacteria that are about 0.2 to 5 microns in width or diameter, such
as 0_5 to 5 microns
in width or diameter, I to 2 microns in width or diameter, or 0.5 to 1 microns
in width or
diameter. In some examples, a patient sample has a bacterial, protozoal,
and/or fungal
concentration of less than 100 CFU/mL, less than 50 CFU/mL, Or less than 10
CFU/mL, such as
1 to 20 cfu/ML, 1 to 100 CFU/mL, or 10 to 200 CFU/mL, such as about 5 CFU/mL,
10
CFU/mL, about 20 CFU/mL, about 30 CFU/mL, about 40 CFU/mL, about 50 CFU/mL,
about
60 CFU/mL, about 70 CFU/mL, about 80 CFU/mL, about CFU/mL, or about 100
CFU/mL.
Thus, in some examples, the method is capable of detecting bacteria, protozoa,
and/or fungi at
less than 100 CFU/mL, less than 50 CFU/mL, or less than 10 CFU/mL, such as 1
to 20 cfu/ML,
1 to 100 CFU/mL, or 10 to 200 CFU/mL, such as about 5 CFU/mL, 10 CFU/mL, about
20
CFU/mL, about 30 CFU/mL, about 40 CFU/mL, about 50 CFU/mL, about 60 CFU/mL,
about
70 CFU/mL, about 80 CFU/mL, about CFU/mL, or about 100 CFU/mL.
[00068] In some examples, the patient sample is used directly. In other
examples, the patient
sample is subjected to one or more pre-processing steps prior to imaging the
sample. For
example, the patient sample can be concentrated, diluted, filtered,
centrifuged, and/or separated
before analysis. In one example, the patient sample is lysed prior to
analysis, for example to
remove or reduce the number of non-bacterial or non-fungal cells in the sample
(e.g., to lyse
blood cells). In some examples, the patient sample is concentrated prior to
analysis, for example
by centrifugation, which can also remove debris from the sample. In one
example, the patient
sample is subjected gel electrofiltration (GEF) (for example, to remove or
reduce lysed cells and
debris in the sample). GEF is a process of sample preparation that relies on
application of an
electrical field to cause sample debris present in a sample to be separated
from microorganism
cells. Likewise, membrane assisted purification may be used in some
embodiments, such that in
response to an electrical potential, sample contaminants enter a porous filter
medium through
- 22 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
one or more walls of a well disposed in the filter medium, thereby separating
them from cells of
interest in the sample.
[00069] The patient sample (or portion thereof) is loaded in or introduced
into one or more solid
supports (e.g., sample reaction chamber, such as a flowcell, microfluidic
chancel, or perfusion
chamber) of a sample container that allows microbes to be visualized using the
disclosed
holographic methods. In one example, the support includes one or more
flowcells, microfluidic
channels, perfusion chambers, or combinations thereof, such as one on a
microscope slide (or
other solid support that is optically transparent (e.g., glass or plastic)
and, non-toxic to
microorganisms). In some example, the perfusion chamber is a CoverWellTm
perfusion chamber
(see FIGS. 2A and 2B). An exemplary perfusion chamber mounted on a microscope
slide is
shown in FIG. 2A. The perfusion chamber has a 20 mm diameter, with two ports
(which allow
for introduction of the sample, for example in a MHA gel suspension, as well
as removal of
materials). In this example, the volume of the perfusion channel is about 300
vi_Lõ with an
effective imaging area of about 16 mm2 at 0.9 um/pixel. One skilled in the art
will appreciate
that other perfusion chambers can be used, such as other shapes (e.g., square,
rectangular, oval,
etc.). In addition, a single slide can include multiple individual sample
reaction chambers, for
example to allow multiple samples to be analyzed contemporaneously, to allow a
single sample
to be analyzed in the presence of different reagents (e.g., different growth
media and/or
antimicrobial agents), or combinations thereof (FIG. 2B).
[00070] After introducing the sample into a micro-fluidic channel, a perfusion
chamber, or both
(for example using a manual or automated pipettor), the cells in the sample
can be immobilized,
for example by entombing them in three-dimensional space in a growth medium
containing a
gelling or solidification agent, such as agar or agarose. In some embodiments,
the entombing
creates a microenvironment around the immobilized microorganism, the
characteristics of which
are not influenced by neighboring microorganisms during the identification
and/or susceptibility
testing periods. In some examples, the method includes retaining the
microorganism on a
detection surface of the support, thereby producing a retained microorganism,
and subsequently
introducing a gel medium (such as one containing agar) into the micro-fluidic
channel, perfusion
chamber, or both, wherein the gel medium is in contact with the retained
microorganism
following introduction into the micro-fluidic channel, perfusion chamber, or
both; immobilizing
the retained microorganism in the micro-fluidic channel, perfusion chamber, or
both at the same
location where the microorganism is retained, to produce an immobilized
microorganism,
wherein offspring of the immobilized microorganism remain over time at a
location with the
- 23 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
immobilized microorganism; and incubating the immobilized microorganism for a
period of
time to allow for growth of the microorganism.
[00071] The sample and microorganisms therein can be incubated and immobilized
in the
growth media in the sample reaction chamber at various temperatures, which in
some examples
is selected based on the microorganism thought to be present in the sample. In
some examples,
the immobilized microorganism are incubated at a temperature of at least 15 C,
at least 20 C, at
least 25 C, at least 30 C, or at least 37 C, such as 20 C to 40 C, or 25 C to
37 C.
[00072] The gel medium in which the microorganisms (and their offspring) are
immobilized in
some examples does not include antimicrobial agents. In one example, the gel
medium in
which the microorganisms (and their offspring) are immobilized is MHA,
trypticase soy agar,
or any other non-selective culturing media, which permits growth of most
microorganisms.
This can be referred to as the "growth control" channel or chamber. Thus, if
microorganisms
are present in the sample, they should grow and be detectable in this medium.
[00073] In some examples, the cells of the sample are present in liquid media
containing no
gelling, porous or semi-solid agents and are not immobilized. The system is
used to identify
and track specific cells and microorganisms.
[00074] For some samples, clinical decisions may require an estimate of the
concentration of a
pathogen (or multiple pathogens) in the sample, which is usually reported on
log-scale. For
example, a clinician may determine that a urine sample is negative if the
concentration of a
particular pathogen is less than 104 cfu/mL. Therefore, treatment decisions
may be made based
on such information. In some examples herein, reporting of such information is
allowed via
direct optically resolved observation of the sample with accuracy that is
better than half-log for
each target species in the sample (including polymicrobial samples). For
example, urines are
not typically pathogenic if less than 104. Similarly, respiratory samples are
not typically
pathogenic if less than 103. But, normal methods of quantifying such samples
is very poor, with
samples plated and colonies counted, leading to highly error prone results,
such as merely
obtaining second derivative of what was actually in the sample. In examples
herein, time-
evolved holographic results can be used to directly discern particles from
bacteria, which can
produce cost-effective and accurate quantity estimates.
[00075] In some examples, a sample is introduced into multiple sample reaction
chambers (e.g.,
flowcell or perfusion chamber) of a sample container, such that at least one
sample reaction
chamber does not include antimicrobial agents, and the others can include
different
antimicrobial agents, for example to assess antimicrobial susceptibility. In
some examples, the
antimicrobial agents selected are based on the identification of the
microorganism(s) present in
the sample. For example, antibiotic susceptibility may be assessed by pre-
mixing antimicrobial
- 24 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
agents with the patient sample before introducing the mixture to one or more
sample reaction
chambers. Alternatively, antimicrobial agents may be added after a patient
sample has been
introduced into the sample reaction chambers, or antibiotics and/or antifungal
agents may
diffuse into contact with the patient sample in the sample reaction chambers.
During growth
supporting conditions, microbial replication in the "growth control" channel
is compared to
replication in one or more "antimicrobial channels" over time can yield first-
order
susceptibility/resistance information. In some examples, different amounts of
the same
antimicrobial agent are used (e.g., serial dilution). In some examples, the
media containing the
sample includes one or more of the following antimicrobial agents: amikacin,
ampicillin,
ampicillin-sulbactam, aztreonam, ceftazidime, ceftaroline, cefazolin,
cefepime, ceftriaxone,
ciprofloxacin, colistin, daptomycin, oxycycline, erythromycin, ertapenem,
gentamicin,
imipenem, linezol id, meropenem, minocycline, piperacillin-tazobactam,
trimethoprim-
sulfamethoxazole, tobramycin, vancomycin, or combinations of two or more
thereof. Other
antimicrobial agents that can be used also include aminoglycosides (including
but not limited to
kanamycin, neomycin, netilmicin, paromomycin, streptomycin, and
spectinomycin), ansamycins
(including but not limited to rifaximin), carbapenems (including but not
limited to doripenem),
cephalosporins (including but not limited to cefadroxil, cefalotin,
cephalexin, cefaclor,
cefprozil, fecluroxime, cefixime, cefdinir, cefditoren, cefotaxime,
cefpodoxime, ceftibuten,
and ceftobiprole), glycopeptides (including but not limited to teicoplanin,
telavancin,
dalbavancin, and oritavancin), lincosamides (including but not limited to
clindamycin and
lincomycin), macrolides (including but not limited to azithromycin,
clarithromycin,
dirithromycin, roxithromycin, telithromycin, and spiramycin), nitrofurans
(including but not
limited to furazolidone and nitrofurantoin), oxazolidinones (including but not
limited to
posizolid, radezolid, and torezolid), penicillins (including but not limited
to amoxicillin,
flucloxacillin, penicillin, amoxicillin/clavulanate, and
ticarcillin/clavulanate), polypeptides
(including but not limited to bacitracin and polymyxin B), quinolones
(including but not
limited to enoxacin, gatifloxacin, gemifloxacin, levofloxacin, lomefloxacin,
moxifloxacin,
naldixic acid, norfloxacin, trovafloxacin, grepafloxacin, sparfloxacin, and
temafloxacin),
suflonamides (including but not limited to mafenide, sulfacetamide,
sulfadiazine,
sul fadi methoxi ne, sul famethi zol e, sulfamethox azol e, sulfasal azi ne,
and sul fi sox azol e),
tetracyclines (including but not limited to demeclocycline, doxycycline,
oxytetracycline, and
tetracycline), and others (including but not limited to clofazimine,
ethambutol, isoniazid,
rifampicin, arsphenamine, chloramphenicol, fosfomycin, metronidazole,
tigecycline, and
trimethoprim), or any combination of two or more thereof. Further
antimicrobial agents
include amphoteri cm n B, ketoconazole, fl uconazol e, itracon azol e, posacon
azole, vo ri con azol e,
- 25 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
anidulafungin, caspofungin, micafungin, flucytosine, or any combination of two
or more
thereof.
[00076] In some examples, a sample is introduced into multiple sample reaction
chambers of a
sample container, such that at least one sample reaction chamber does not
include antimicrobial
agents, and the others can include different selective and differential growth
media, for
example to identify the microorganisms present in the sample. Some growth
media only
supports growth and replication of particular microorganisms or types of
microorganisms.
Examples of selective and differential media include blood agar, Eosin
Methylene Blue (EMB)
broth or EMB agar, mannitol salt broth or mannitol salt agar, MacConkey broth
or MacConkey
agar, phenylethyl alcohol (PEA) broth or PEA agar, and YM broth or YM agar.
For example,
EMB broth or agar inhibits Gram-positive organisms, and is thus selective for
Gram-negative
species. MacConkey broth or agar is also selective for Gram-negative species
and differential
with respect to lactose fermentation. Mannitol salt broth or agar (7.5% NaCl)
is selective for
staphylococci and differential with respect to mannitol fermentation, wherein
fermentation of
mannitol is only seen in the pathogenic species of Staphylococcus. PEA broth
or agar is a
selective medium which inhibits the growth of most Gram-negative organisms.
For example,
MacConkey broth or agar can be used to select for Gram-negative bacteria
(e.g., permits
growth of Gram-negative bacteria), mannitol salt broth or agar can be used to
select for Gram-
positive bacteria (such as Staphylococcus), and YM broth or agar can be used
to select for
yeast. Thus, detection of growth in a particular media, and in some examples
not in other
media, can allow for the identification of the microorganism. For example, the
identification of
a microorganism, for example determining its genus, species, Gram status
and/or strain can be
assessed by pre-mixing a particular growth media with the patient sample
before introducing
the mixture to one or more sample reaction chambers. Alternatively, particular
growth media
may be added after a patient sample has been introduced into the sample
reaction chamber, or
selective agents may diffuse into contact with the patient sample in the
sample reaction
chamber. During growth supporting conditions, microbial replication in the
"growth control"
channel is compared to replication in one or more "selective media" channels
over time can
yield microorganism identification information. In some examples,
alternatively or in addition
to the use of "selective media" channels, the microorganisms are identified by
morphology
(e.g., shape, size) information obtained using the disclosed methods.
[00077] In some examples, the method includes determining the number of
minimum number
of microbes needed in the "growth control" channel to ensure that all the
channels containing
the patient sample will have detectable microbes, if present in the sample.
For example, serial
dilutions can he performed.
- 26 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[00078] After the microorganisms are immobilized, they (and their offspring)
are imaged using
the disclosed holographic imaging methods. Images of one or more (such as 1-
100, for
example, 2-25, 10-40, 30-80, or 50-100) fields of view (scaled depending on
the volume of the
channel to be interrogated) of one or more microorganisms are captured.
Multiple images of
the same field of view may be captured, for example under one or more
different imaging
modalities. 1-or example, images can be obtained over a period of seconds, to
minutes, to hours,
such as every 5 minutes, 10 minutes, 15 minutes, 20 minutes, 30 minutes, or 60
minutes. In
some examples, images (such as images of the "growth control" channel) are
obtained for at
least 1 hour, at least 1.5 hours, at least 2 hours, at least 3 hours, at least
4 hours, at least 5
hours, at least 6 hours, at least 7 hours, at least 8 hours, at least 9 hours,
or at least 10 hours,
such as 1 to 4 hours, 1 to 2 hours, 1.5 to 2 hours, or 2 to 4 hours. The
results from the "growth
control" channel allow for the determination as to whether the patient sample
contains bacteria,
protozoa, and/or fungi, that is, whether the sample is "positive-.
[00079] in some examples, during a microbial identification assay period,
images are obtained
about every 5-30 minutes (such as about every 5 minutes, 10 minutes, 15
minutes, 20 minutes,
25 minutes, or 30 minutes) for about 1 to 8 hours, such as up to about 1.5
hours, 2 hours, 3
hours, 4 hours, 4.5 hours, 5 hours, 6 hours, 7 hours, or 8 hours. In some
examples during this
stage, the images are subjected to morphological or other analysis (such as
morphokinetic
analysis) to identify characteristics of the imaged microorganisms, including
one or more of
noise, cross-talk, and microorganism morphology. The results from the
"selective media"
channel allow for the identification of the microorganisms (e.g., Gram status,
genus, species,
and/or strain) present in the patient sample.
[00080] In some examples, during an AST assay period, images are obtained
about every 5-30
minutes (such as about every 5 minutes, 10 minutes, 15 minutes, 20 minutes, 25
minutes, or 30
minutes) for about 1 to 6 hours, such as about 1.5 hours, 2 hours, 3 hours, 4
hours, 4.5 hours, 5
hours, or 6 hours, creating a time-lapse record of microorganism growth.
During the AST
process, various microorganism clone features can be measured, such as
morphology and
division rates and used for analysis. In some examples, the growth of the
microorganisms is
measured qualitatively or quantitatively, for example by measuring the growth
(or amount of
growth), lack of growth, or lysis of the microorganisms. Based on the behavior
of the
microorganisms over time in the presence of the one or more antimicrobials
(for example,
compared to a control that is not exposed to the antimicrobial(s)), a
determination of
susceptibility (or indeterminate susceptibility) or resistance of the
identified microorganisms to
each antimicrobial is made. The results from the "antimicrobial channel" allow
for the
determination as to which antibiotic(s) the microorganism in the sample is
susceptible to. Thus,
- 27 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
in some embodiments, the system reports susceptibility, intermediate, or
resistance to one or
more antimicrobials. In some embodiments, the following resistance phenotypes
are reported
by the system in response to AST data analysis: Methicillin-resistant
Staphylococcus aureus
(MRSA), methicillin-resistant staphylococci (MRS), vancomycin-resistant S.
aureus (VRSA),
vancomycin- resistant Enterococcus species (VRE), high-level aminoglycoside
resistance
(HLAR) and macrolide-lincosamide-streptogramin 13 resistance (MLS b). Upon
this
determination, the subject from whom the sample was obtained can be
administered a
therapeutically effective amount of the identified antibiotic(s).
Exemplary microbes detected
[00081] The disclosed methods and systems can be used to detect various Gram-
positive and
Gram-negative bacteria, protozoa, and fungi (e.g., yeasts), including but not
limited to:
Staphylococcus aureus, Staphylococcus lugdunensis, coagulase-negative
Staphylococcus
species (Staphylococcus epidermidisõStaphylococcus haemolyticusõStaphylococcus
hominis,
Staphylococcus capitis, not differentiated), Enterococcus faecalis,
Enterococcus faecium
(Enterococcus faecium and other Enterococcus spp., not differentiated,
excluding Enterococcus
faecalis), Streptococcus pneumoniae, Streptococcus pyo genes, Streptococcus
agalactiae,
Streptococcus spp., (Streptococcus mitis, Streptococcus pyogenes,
Streptococcus gallolyticus,
Streptococcus agalactiae, Streptococcus pneumoniae, not differentiated),
Pseudomonas
aeruginosa, Acinetobacter baumannii, Klebsiella spp. (Klebsiella pneumoniae,
Klebsiella
oxytoca, not differentiated), Escherichia coli, Enterobacter spp.
(Enterobacter cloacae,
Enterobacter aero genes, not differentiated), Proteus spp. (Proteus mirabilis,
Proteus vulgaris,
not differentiated), Citrobacter spp. (Citrobacter freundii, Citrobacter
koseri, not
differentiated), Serratia rnarcescens, Candida albicans, and Candida glabrata.
[00082] Other specific bacteria that can be detected with the disclosed
systems and methods,
include without limitation: Acinetobacter baumannii, Actinobacillus spp.,
Actinomycetes,
Actinomyces spp. (such as Actinomyces israelii and Actinomyces naeslundii),
Aeromonas spp.
(such as Aeromonas hydrophila, Aeromonas veronii hiovar sobria (Aerornonas
sobria), and
Aerornonas caviae), Anaplasma phagocytophilum, Alcaligenes xylosoxidans,
Actinobacillus
actinomycetemcomitans, Bacillus spp. (such as Bacillus anthracis, Bacillus
cereus, Bacillus
subtilis, Bacillus thuringiensis, and Bacillus stearothermophilus),
Bacteroides spp. (such as
Bacteroides .fragilis), Bartonella spp. (such as Bartonella bacilliformis and
Bartonella hen selae,
Bifidobacterium spp., Bordetella spp. (such as Bordetella pertussis,
Bordetella parapertu,ssis,
and Bordetella bronchiseptica), Borrelia spp. (such as Borrelia recurrentis,
and Borrelia
burgdorferi), Brucella sp. (such as Brucella abortus, Brucella canis, Brucella
melintensis and
Brucella suis), Burkholderia spp. (such as Burkholderia pseudomallei and
Burkholderia
- 28 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
cepacia), Campylobacter spp. (such as Campylobacter jejuni, Campylobacter
coli,
Campylobacter lari and Campylobacter fetus), Capnocytophaga spp.,
Cardiobacteriwn hominis,
Chlatnydia trachomatis, Chlamydophila pnewnoniae, Chlarnydophila psittaci,
Citrobacter spp.
Coxiella burn etii, Cory nebacterium spp. (such as, Corynebacterium
diphtheriae,
Coiynebacterium jeikeum and Corynebacterium), Clostridium spp. (such as
Clostridium
perfringens, Clostridium difficile, Clostridium botulinum and Clostridium
tetani), Eikenella
corrodens, Enterobacter spp. (such as Enterobacter aero genes, Enterobacter
agglomerans,
Enterobacter cloacae and Escherichia coli, including opportunistic Escherichia
coli, such as
enterotoxigenic E. coli, enteroinvasive E. coli, enteroputhogenic E. coli,
enterohemorrhagic E.
coli, enterouggregutive E. coli and uroputhogenic E. coli) Enterococcus spp.
(such as
Enterococcus faecalis and Enterococcus faecium) Ehrlichia spp. (such as
Ehrlichia chafeensia
and Ehrlichia canis), Erysipelothrix rhusiopathiae, Eubacterium spp.,
Francisella tularensis,
Fusobacterium nucleatum, Gardnerella vaginalis, Gemella morbillorum,
Haemophilus spp.
(such as Haemophilus influenzae, Haemophilus ducreyi, Haemophilus aegyptius,
Haemophilus
parainfluenzae, Haemophilus haemolyticus and Haemophilus parahaemolyticus,
Helicobacter
spp. (such as Helicobacter pylori, Helicobacter cinaedi and Helicobacter
fennelliae), Kingella
kingii, Klebsiella spp. ( such as Klebsiella pneumoniae, Klebsiella
granulomatis and Klebsiella
oxytoca). Lactobacillus spp., Listeria monocytogenes, Leptospira interrogans,
Legionella
pneumophila, Leptospira interrogans, Peptostreptococcus spp., Moraxella
catarrhalis,
Morganella spp., Mobiluncus spp., Micrococcus spp., Mycobacterium spp. (such
as
Mycobacterium leprae, Mycobacterium tuberculosis, Mycobacterium
intracellulare,
Mycobacterium avium, Mycobacterium bovis, and Mycobacterium marinum),
Mycoplasm spp.
(such as Mycoplasma pneumoniae, Mycoplasma hominis, and Mycoplasma
genitalium),
Nocardia spp. (such as Nocardia asteroides, Nocardia cyriacigeorgica and
Nocardia
brasiliensis). Neisseria spp. (such as Neisseria gonorrhoeae and Neisseria
meningitidis),
Pasteurella multocida, Plesiomonas shigelloides. Prevotella spp.,
Porphyromonas spp.,
Prevotella melaninogenica, Proteus spp. (such as Proteus vulgaris and Proteus
mirabilis),
Providencia spp. (such as Providencia alcalifaciens, Providencia rettgeri and
Providencia
stuartii), Pseudomonas aeruginosa, Propionibacterium acnes, Rhodococcus equi,
Rickettsia spp.
(such as Rickettsia rickettsii, Rickettsia akari and Rickettsia prowazekii,
Orientia tsutsugamttshi
(formerly: Rickettsia tsutsugamushi) and Rickettsia typhi), Rhodococcus spp.,
Serratia
marcescens, Stenotrophomonas maltophilia, Salmonella spp. (such as Salmonella
enterica,
Salmonella typhi, Salmonella paratyphi, Salmonella enteritidis, Salmonella
cholerasttis and
Salmonella typhimurium), Serratia spp. (such as Serratia marcesans and
Serratia liquifaciens),
S'higella spp. (such as S'higella dysenteriae, Shigella flexneri, Shigella
boydii and Shigella
- 29 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
sonnei), Staphylococcus spp. (such as Staphylococcus aureus, Staphylococcus
epidermidis,
Staphylococcus hemolyticus, Staphylococcus saprophyticus), Streptococcus spp.
(such as
Streptococcus pneumoniae (for example chloramphenicol-resistant serotype 4
Streptococcus
pneumoniae, spectinomycin-resistant serotype 6B Streptococcus pneumoniae,
streptomycin-
resistant serotype 9V Streptococcus pneumoniae, erythromycin-resistant
serotype 14
Streptococcus pneumoniae, optochin-resistant serotype 14 Streptococcus
pneumoniae,
rifampicin-resistant serotype 18C Streptococcus pneumoniae, tetracycline-
resistant serotype
19F Streptococcus pneumoniae, penicillin-resistant serotype 19F Streptococcus
pneumoniae,
and trimethoprim-resistant serotype 23F Streptococcus pneumonicte,
chloramphenicol-
resistant serotype 4 Streptococcus pneumoniae, spec tinomycin-resistant
serotype 6B
Streptococcus pneumoniae, streptomycin-resistant serotype 9V Streptococcus
pneumoniae,
optochin-resistant serotype 14 Streptococcus pneumoniae, rifampicin -resistant
serotype 18C
Streptococcus pneumoniae, penicillin-resistant serotype 19F Streptococcus
pneumoniae, or
trimethoprim-resistant serotype 23F Streptococcus pneumoniae)õ Streptococcus
agalactiae,
Streptococcus mutans, Streptococcus pyo genes, Group A streptococci,
Streptococcus
pyo genes, Group B streptococci, Streptococcus agalactiae, Group C
streptococci,
Streptococcus anginosus, Streptococcus equismilis, Group D streptococci,
Streptococcus
bovis, Group F streptococci, and Streptococcus anginosus Group G
streptococci), Spirillum
minus, Streptobacillus moniliformi, Treponema spp. (such as Treponema curate
urn,
Treponema petenue, Treponema pallidum and Treponema endemicum, Tropheryma
whippelii,
Ureaplasma urealyticum, Veillonella sp., Vibrio spp. (such as Vibrio cholerae,
Vibrio
parahemolyticus, Vibrio vulnificus, Vibrio parahctemolyticus, Vibrio
vulnificus, Vibrio
alginolyticus, Vibrio rnimicus, Vibrio hollisae, Vibrio fluvialis, Vibrio
rnetchnikovii, Vibrio
damsela and Vibrio furnisii), Yersinia spp. (such as Yersinia enterocolitica,
Yersinia pestis,
and Yersinia pseudotuberculosis) and Xanthomonas maltophilia among others.
[00083] Exemplary fungi that can be detected with the disclosed systems and
methods, include
without limitation: Candida spp. (such as Candida albicans, Candida glabrata,
Candida
tropicalis, Candida parapsilosis, and Candida krusei), Aspergillus spp. (such
as Aspergillus
litmigatous, Aspergillus flavus, Aspergillus clavatus), Cryptococcous spp.
(such as
Cryptococcus neoformans, Cryptococcus gattii, Cryptococcus laurentii, and
Cryptococcus
albidus), Fusarium spp. (such as Fusarium oxysporum, Fusarium solani, Fusarium
verticillioide.s, and Fusarium proliferatum), Rhizopus oryzae, Penicillium
marneffei,
Coccidiodes immitis, and Blastotnyces dermatitidis.
[00084] Exemplary protozoa include, that can be detected with the disclosed
systems and
methods, include without limitation: Plasmodium (e.g., Plasmodium falciparum),
Leishmania,
- 30 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
Acanthamoeba, Giardia, Entamoeba, Cryptosporidium, Isospora, Balantidium,
Trichomonas,
Trypanosoma (e.g., Trypanosoma brucei), Naegleria, and Toxoplasma.
EXAMPLE
[00085] A micro-fluidic channel was constructed by placing a cover well on top
of a glass
microscopy slide measuring about 20mm in diameter and lmm in height. A patient
sample was
simulated by diluting an E. coli 25922 isolate into Mueller-Hinton agar
suspension. The
concentration of bacterial isolate was chosen such that there were
approximately 102 bacteria
per mL. The bacterial-agar suspension was premixed and pipetted into an inlet
opening on top
of the cover well. Thereafter, the bacterial-agar suspension was subjected to
a phase change to
solidify the agar and suspend the bacteria in three-dimensional space. Prior
to the beginning of
image acquisition, the micro-fluidic channel was placed inside an incubator
for lhr to promote
the growth phase of the suspended bacteria.
[00086] Time-lapse imaging was conducted on a laboratory benchtop at ambient
temperature
(approximately 20 C). Holograms of the full field-of-view (approximately 16m
m2) were
acquired automatically every 30 minutes. Visible division of bacterial micro
colonies were
detected as early as 60 minutes after the start of image acquisition. Reliably
detectable division
across most micro colonies in the suspension is achieved approximately 2-3
hours after the start
of acquisition for these bacteria. Because detection is based on change over
time, presence of
debris is not expected to have a significant impact on time-to-detection
sensitivity. FIG. 4 shows
images obtained by the optical interrogation platform imaging E. coli growth
over a period of 0
to 180 minutes. FIG. 5 shows images obtained by the optical interrogation
platform imaging E.
coli growth during a period from 240 to 540 minutes.
[00087] Time-to-detection highly depends on the optical resolution supported
by the system. It
is also related to growth media as the experiment used agar phase changed to a
gel to contain
growth to a particular three-dimensional location in the volume. Hence,
tracking of individual
micro colonies was not necessary. Thus, the optical interrogation system can
be used to detect
the presence of growing microorganisms in a biological sample long before
traditional methods
are capable of doing so. Upon detection of a microorganism present at a very
low concentration
in a biological sample, the sample may be further tested to determine the
identity of the
microorganism and its susceptibility to antimicrobial agents.
Additional System Examples
11000881 In FIG. 6, an in-line holographic apparatus 600 is situated to
determine the presence of
microorganisms 602, 604 immobilized or that are free to move in a sample
volume 606 of a
biological sample container 607. In representative examples, the apparatus 600
can detect a
variation over time of an in-line hologram 608 of the sample volume 606,
including in an
- 31 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
automated fashion, through detection of the sample volume at predetermined
times, e.g.,
during incubation. For example, growth of the microorganisms 602, 604 can
produce variation
of respective holographic interference patterns 610, 612 of the in-line
hologram 608.
Variations indicative of the presence of microorganisms 602, 604 can be
detected based on
time durations associated with microorganism growth rates, such as doubling
events, negative
growth rates (e.g., rates associated with antimicrobial activity), etc. In
typical examples, time
durations of doubling events of the immobilized microorganisms 602, 604 are
longer than
temporal resolutions typically associated with in-line holography, allowing
some example
systems to provide improved detection, detection over time, imaging, and
imaging over time
capabilities and lower costs with simpler components and reduced storage
and/or processing
requirements. In some examples, the in-line hologram 608 can be detected and
recorded at
rates suitable for detecting doubling events, such as at least twice the
doubling rate, or faster,
with substantially faster rates possible depending on the detection
requirements, such as
morphological detection, etc.
[00089] In some examples, the in-line holographic apparatus 600 includes a
reference beam
source 614 situated to direct a reference beam 616 to the sample volume 606, a
sample
receptacle 618 situated to hold the sample volume 606 in view of the reference
beam 616, an
optical sensor 620 situated to detect the in-line hologram 608 formed by the
reference beam 616
and the sample volume 606, and a holography controller 622 coupled to the
optical sensor 620
and configured to determine the variation over time of the in-line hologram
608. The sample
volume 606 can include one or more (e.g., a plurality of) sample volume
portions corresponding
to volumes in microfluidic channels, flow channels, perfusion chambers, etc.,
of the biological
sample container 607 and that can contain biological samples, such as
suspended biological
samples with microorganisms to be detected, including immobilized
microorganisms. In typical
examples, the sample receptacle 618 can include a tray or other holding
support that receives the
biological sample container 607 such that the biological sample can be
removable inserted into
the in-line holographic apparatus and held by the sample receptacle 618 so
that the sample
volume 606 can be imaged by the in-line holography apparatus 600. In some
examples, the
microfluidic channels, flow channels, perfusion chambers, or other parts of
the biological
sample container 607, can form at least part of the sample receptacle 618.
11000901 In representative embodiments, the reference beam source 614 includes
a pinhole
aperture 624 situated to receive an illumination 628 from an illumination
source 626 and the
reference beam 616 is directed lens-free from the pinhole aperture 624 to the
sample volume 606
and the optical sensor 620. In some examples, the illumination source 626
includes one or more
light emitting diodes, laser, or other light source that is situated to
produce the illumination 628
- 32 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
with multiple wavelengths that can be used to reduce a twin-image in the
hologram 608. In some
examples the illumination 628 and the reference beam 616 are incoherent, the
illumination 628
and the reference beam 616 are coherent, or the illumination 628 is incoherent
and the reference
beam 616 is coherent. The shape, diameter, and shape quality (e.g., roughness,
ellipticity, etc.) of
the pinhole aperture 624 can vary in different embodiments. In typical
examples, the pinhole
aperture is circular and has a diameter selected in range of 1 lam or smaller,
1 to 10 i_tm, 10 to 50
lam, 50 to 100 p_tm, or larger, and together with the wavelength or
wavelengths of the illumination
628 determines the numerical aperture of the reference beam 606.
[00091] In typical examples, the reference beam 616 diverges to define an
imaging area 630 and
field of view of the sample volume 606 based on the divergence angle of the
reference beam 616
and the distance between a position ZAPERTURE of the pinhole aperture 624 and
top and bottom
plane positions Zvoti , ZVOT2 of the sample volume 606. In representative
embodiments, the
positions ZvoLi , ZVOL2 are sufficiently proximate each other, i.e., the
sample volume 606 is
sufficiently thin, in relation to the distance between ZvoLi and ZAPERTURE
that the positions ZVOL1,
ZVOL2 can be considered effectively one position for purposes of the imaging
area 630. In
representative examples, the distance ZAPERTURE-ZVOLlis selected to be in the
range of 40 mm to
100 mm, though distances smaller than 40 mm or greater than 100 mm are also
possible. In some
examples, the thickness of the sample volume 606 corresponding to the
difference ZvoL1-ZvoL2is
2 mm or smaller, 1 mm or smaller, 0.5 mm smaller, etc. Representative imaging
areas of the
sample volume 606 for a single aperture and reference beam can vary, and can
include 50 mm2
or larger, 40 mm2 or larger, 30 mm2 or larger, 20 mm2 or larger, 10 mm2 or
larger, 5 mm2 or
larger, or smaller than 5 mm2, by way of example. In some examples, areas are
increased with
additional apertures and/or optical sensors. Imaging areas for a single field
of view can typically
correspond to large volumes, including greater than 2 FtL, 5 L, 10 L, 20 L,
50 vtL, or greater.
[00092] Representative examples of the optical sensor 620 include CMOS or CCD
type sensors,
that include a plurality of pixels 632 (shown in an expanded cutout) arranged
with one or more
pixel pitches A to form a sensor surface 634 situated to detect the in-line
hologram 608. In
representative examples, the pitch A corresponds to a detector resolution that
is sufficiently small
to detect the spatial intensity variation of the holographic interference
patterns 610, 612 of the in-
line hologram 608 or to detect a variation over time of the spatial intensity
variation, such as a
pitch A of 10 lim/pixel, 5 nm/pixel, 2 pm/pixel, 1 nm/pixel, or smaller. In a
particular
embodiment the pitch A is 1.12 vim/pixel. In some examples, the pixel pitch A
is selected to be
sufficiently small to detect spatial intensity characteristics of the in-line
hologram 608 that are
associated with morphological characteristics of the microorganisms 602, 604.
In some
examples, characteristics of the reference beam 616 or other components of the
in-line
- 33 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
holographic apparatus 600 are varied to enhance detection resolution,
including varying
reference beam wavelength to sample different portions of the pixels 632,
varying aperture
characteristics such as aperture angles, super-resolution techniques employing
relative
superposition of sample and illumination, and numerical techniques such as
super-resolution
via compressed sensing.
11000931 In representative examples, the distance between the bottom plane
position ZVOL2 of the
sample volume 606 and the plane position ZnoLo of the sensor surface 634 is 10
mm or smaller,
mm or smaller, 2 mm or smaller, etc. In a particular example, the distance
ZvoL2-ZnoLo is 4
mm or smaller. In some examples, the distance ZvoL2-ZnoLo is selected so as to
provide suitable
spatial characteristics for the interference patterns 610, 612 or other
interference characteristics
of the in-line hologram 608, such as a sufficient propagation distance to
produce a
corresponding holographic interference between the reference beam 616 and
object scattered
beams 611, 613. While some examples of the sample volume 606 are generally
depicted with a
cuboid shape, other shapes can be used, including cylindrical, frustum,
elliptoid, etc.
[00094] The holography controller 622 includes a detector control 640 and an
illumination
control 642 respectively in communication with the optical sensor 620 and the
illumination
source 626 or other light modulation device, such as an optical chopper, light
modulator, etc., so
that the illumination 628 is provided to form the reference beam 616 and
associated hologram
608 that is detected by the optical sensor 620 and so that the optical sensor
620 is ready (e.g.,
gated, reset, etc.) to detect the hologram 608.
[00095] In some examples, the holography controller 622 is a computing device
that includes a
memory 636 that can include one or more computer readable instructions, such
as program
modules, that can be executed by at least one processor 638, such as one or
more of a
microcontroller unit, complex programmable logic device, field programmable
gate array,
application-specific integrated circuit, programmable logic controller,
computer system, etc.,
arranged singularly or in distributed fashion. Generally, program modules
include routines,
programs, objects, components, data structures, etc., that perform particular
tasks or implement
particular abstract data types. Moreover, the disclosed technology may be
implemented with
other computer system configurations, including hand-held devices,
multiprocessor systems,
microprocessor-based or programmable consumer electronics, network PCs,
minicomputers,
mainframe computers, etc.
[00096] The memory 636 can includes read only memory (ROM) and random access
memory
(RAM), one or more storage devices, such as a hard disk drive for reading from
and writing to a
hard disk, a magnetic disk drive for reading from or writing to a removable
magnetic disk, and
an optical disk drive for reading from or writing to a removable optical disk
(such as a CD-
- 34 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
ROM or other optical media). The drives and their associated computer-readable
media provide
nonvolatile storage of computer-readable instructions, data structures,
program modules, and
other data for the holography controller 622. Other types of computer-readable
media which can
store data that is accessible by a PC, such as magnetic cassettes, flash
memory cards, digital
video disks, CDs. DVDs, RAMs, ROMs, etc., may also be used in the example
holographic
control environment.
[00097] In some examples, a number of program modules can be stored in the
memory 636,
including an operating system, one or more application programs, other program
modules, and
program data. In further examples, a user can enter commands and information
into the
holography controller 622 through one or more input devices, such as a
keyboard, and a pointing
device, such as a mouse. Other input devices can be included. Thus, in
representative examples,
the various routines, programs, and program modules can be automated so that
biological
samples may be received by the in-line holography apparatus 600 so that tests
can be performed
on the biological samples with little intervention from a user. In some
examples, a display
device 648 is situated to display images of the hologram 608 or holographic
reconstructions of
one or more planes of the sample volume 606, including time-lapse images or
video recordings
associated with microorganism growth or size variation.
[00098] An image timer 644 can be used in different examples to synchronize
detection and
recording of the hologram 608 or associated hologram information in the memory
636 for
subsequent comparison or imaging. In some examples, the holography controller
622 includes a
spatial difference comparison routine 646 that determines spatial differences
associated with
holograms recorded at different times, such as by comparing variations of
holographic fringes
and other spatial frequency encoding features. In some examples, spatial
differences can be
determined between hologram reconstructions of one or more planes of the
sample volume 606
associated with holograms recorded at different times, including area and
texture variations of
one or more objects, such as the microorganisms 602, 604. Other approaches may
include
"learning" holographic representation of growth over time with higher-
dimensional techniques
such as Convolutional Neural Networks and conducting direct inference on
observed pixels at
each time point of the time-lapse. In representative examples, improved
microorganism
detectability is achieved for the sample volume 606 based on the immobilized
but growing (or
declining) microorganisms and background immobilized objects that have spatial
characteristics
that do not vary over time. For example, in comparing spatial differences, a
substantial set of the
background objects and associated signal characteristics can be eliminated
through image
subtraction so as to improve a signal to noise ratio for the spatial
difference comparison routine
646.
- 35 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[00099] In some examples, the holography controller 622 includes a numerical
reconstruction
routine 652 that is configured to reconstruct one or more planes of the sample
volume 606
associated with the microorganisms 602, 604 based on the hologram 608 detected
at the plane
ZHOLO of the optical sensor 620. In general, such routines approximate
solutions to Fresnel-
Kirchoff diffraction integral by employing a Fresnel approximation (Fresnel
integral) or a
convolutional approach at any focal plane between ZvoLt and ZVOL2, followed by
intensity and
phase extraction. In some examples, the numerical reconstruction routine 652
includes a
Gerchberg-Saxton algorithm. Objects, such as the microorganisms 602, 604, that
are identified
can be tracked in an object growth table 654 for comparison with holograms
detected at later
times to determine if a spatial variation occurs that is associated with the
presence of the
microorganisms 602, 604.
[000100] The disclosed technology may also be practiced in distributed
computing environments
where tasks are performed by remote processing devices that are linked through
a
communications network_ In a distributed computing environment, program
modules may be
located in both local and remote memory storage devices. For example,
holographic comparison
by the holography controller 622 associated with an indication as to the
presence of the
microorganisms 602, 604, immobilized or motile, in the sample volume 606 can
be performed
locally upon receiving a plurality of holograms for comparison or can also be
performed
remotely in space and/or time from the detection of holograms by the optical
sensor 620. In some
examples, the holography controller includes a network communication
connections 650 to
communicate with external device or other computers, e.g., through a local
area network (LAN)
or wide area network (WAN). The in-line holographic apparatus 600 can further
include a
movement stage 652 coupled to the sample volume 606, such as through a side of
the sample
receptacle 618, though it will be appreciated that various couplings can be
used to provide
translational and/or rotational movement of the sample volume 606. The
controller 622 can
include a stage control 654 that can command and cause movement of the sample
receptacle 618
to different positions, e.g., based on a flow cell map 656, so that different
flow cells or portions
of a flow cell can be aligned in view of the reference beam 616 and
interrogated, e.g., between
the reference beam source 614 and the optical sensor 620. In representative
examples, a
movement stage 652 can be omitted.
[000101] FIG. 7 shows an example in-line holographic apparatus 700 that has a
mosaicked
field of view 702 of a sample volume 704 with a plurality of reference beams
706a-706d
emitted from respective pinhole apertures 708a-708d based on respective
illuminations 710a-
710d (typically multi-wavelength) received from respective illumination
sources 712a-712d. In
some examples, a single illumination source can be used to illuminate the
pinhole apertures
- 36 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
708a-708d, and in other examples other quantities of illumination sources can
be used. In
particular examples, a pinhole aperture 708a can include a plurality of spaced
apart pinhole
apertures, typically at a small distance (e.g., less than about 1 mm), and the
illumination source
708a can include separate illumination sub-sources emitting at separate
respective wavelengths
and coupled to the respective spaced apart pinhole apertures. Alternatively,
each spaced apart
pinhole aperture can be coupled to a respective wavelength filter so that
reference subbeams at
different wavelengths are emitted from the respective spaced apart pinhole
apertures. The other
pinhole apertures 708b-708d and illumination sources 712b-712d can be
similarly configured
and detected holograms can be registered with respect to each other based on
subsampling of the
respective optical sensor portions 718a-718d.
[000102] The sample volume 704 typically includes a suspended biological
sample having
immobilized or motile microorganisms 714a-714d in respective sample volume
portions 715a-
715d. Sample containers and sample receptacles are omitted for clarity and
convenience of
illustration though it will be appreciated that various containers and
receptacles for supporting
and manipulating biological samples can be used. Respective in-line holograms
716a-716d are
formed and detected, including holographic pattern features 720a-720d that are
generated
based on the immobilized or mobile microorganisms 714a-714d, with the
different optical
sensor portions 718a-718d. In some examples, the optical sensor portions 718a-
718d can form
a single sensor or multiple sensors. In some mosaic embodiments, at least one
of the sample
volume portions 715a-715d is used as a growth control and one or more others
of the sample
volume portions 715a-715d include selective media or antimicrobial agents. In
further mosaic
embodiments, the sample volume portions 715a-715d are not isolated from each
other and the
multiple reference beams 706a-706d effectively increase the imaging area of
the in-line
holographic apparatus 700. In some examples, the reference beams 706a-706d
have respective
imaging areas that can overlap at the sample volume 704 so that the mosaicked
field of view
702 can have continuous coverage over at least a portion of the sample volume
704 including
all of the sample volume 704 in selected examples. The sample volume can be
relatively large,
with some examples have a volume of 0.01 mL or greater, 0.05 mL or greater,
0.1 niL or
greater, 0.5 mL or greater, or 1 mL or greater, etc.
[000103] The in-line holographic apparatus 700 can include a holography
controller 722 that
can control holographic imaging and holographic imaging over time of the
sample volume 704.
The holography controller typically includes at least one processor 724, and a
memory 726 that
includes stored instructions associated with the detection of the holograms
716a-716d. In
representative examples, the holography controller 722 includes an
illumination control 728 that
can cause the illumination sources 712a-712d to generate the illuminations
710a-710d at
- 37 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
respective times or periods that can be the same or different from each other,
and can be
controlled based on an image timer 732. In representative examples, the
hologram controller
722 includes a detector control 730 in communication with the optical sensor
portions 718a-
718d so as to receive one or more hologram signals associated with the
holograms 716a-716d.
In some examples, objects are detected, such as the microorganisms 714a-714d,
and monitored
over time, e.g., in an object growth table 734, so as to determine the
presence of the
microorganisms 714a-714d, or other characteristics, such as morphological
characteristics,
microorganism quantity or concentration, growth control characteristics,
antimicrobial
responsiveness, selective media based species determination, etc., depending
on the particular
application. In some examples, objects and object variations (e.g., growth),
can be determined
with a spatial differences comparison routine 738 that compares spatial
variations within the
holograms 716a-716d, within reconstructions of the sample volume portions 715a-
715d based
on the holograms 716a-716d and one or more reconstruction algorithms 736, or
spatial
variations over time of holograms or reconstructions. In some examples, one or
more displays
are included to show holographic information, amplitude and/or phase features,
reconstructed
sample volume features, sample volume feature variation over time (e.g.,
microorganism growth
or decline), etc. Some examples can include one or more communications modules
for remote
communication. Selected examples can include a movement stage (not shown) to
move the
sample volume.
1000104] FIG. 8 depicts an example method SOO for detecting the presence of a
microorganism.
At 804, a first in-line hologram of a sample volume is detected at a first
time, and at 812, a
second in-line hologram of the sample volume is detected at a second time. At
818, a variation
over time associated with the in-line holograms is determined (e.g., between
the first and second
in-line holograms) that is associated with an indication that one or more
objects immobilized in
the sample volume is a microorganism. In some examples, at 802, the sample
volume includes a
biological sample that includes microorganisms suspended in a porous medium so
as to
immobilize the microorganisms to be detected. In some examples, at 806, the
spatial
characteristics of objects in the sample volume are reconstructed from the
first in-line hologram,
forming a first reconstruction of the sample volume.
10001051 Reconstructions can be performed according to various methods, such
as with various
diffraction propagation approximations (e.g., Fresnel approximation) and
iterative phase
retrieval approaches, such as Gerchberg-Saxton algorithms. The in-line
holograms are generated
as a reference beam interacts with the sample volume and produces a complex
interference
pattern based on object beams that are formed from optical interaction between
the reference
beam and the immobilized objects and resulting interference between the
reference beam and
- 38 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
object beams. Phase components associated with the immobilized objects is
extracted from the
intensity characteristics of the hologram. In typical examples, a Fresnel
integral is applied to the
hologram intensity to determine a plane associated with an immobilized object
and an iterative
Gerchberg-Saxton algorithm is used to reconstruct intensity and phase of the
immobilized
object.
[000106] In some examples. at 808, the reconstructions allow a determination
of the position
(e.g., a z-position, an x-y position, an x-y-z position, etc.) of one or more
of the objects
immobilized in the sample volume based on spatial differences of the first in-
line hologram or
the first reconstruction. In typical examples, at 810, the suspended
biological sample is
incubated in an environment conducive to microorganism replication. At 814, in
some
examples, spatial characteristics of the sample volume are reconstructed from
the second in-line
hologram detected at a later time, sometimes selected in relation to a
suitable microorganism
division rate or other biological rate. In representative examples, at 816,
the first in-line
hologram and the second in-line hologram, and/or the reconstructions of the
first in-line
hologram and the second in-line hologram, are compared so as to identify
holographic and/or
reconstructed spatial differences, so that the variations over time can be
determined at 818. In
some examples, growth detection is performed without reconstructing the
precise position
and/or plane of the immobilized object, or without performing reconstruction
at every
holographic detection event. Various examples herein can use the linearity of
optical transforms
associated with reconstruction and manipulate holographic information (e.g.,
add, subtract, etc.)
without loss of information. Additionally, intensity variation of the
holograms over time (e.g.,
spatial intensity variation) can be used to determine microorganism presence.
In typical
examples, at 820, multiple holograms can be obtained so that numerical
features of the detected
objects can be accumulated over time. The accumulated features can be
associated with an
indication that one or more of the immobilized objects corresponds to a
microorganism in the
suspended biological sample. In some examples, at 822, the phenotypic behavior
of an object
can be classified based on the accumulated numerical features, such as growth,
death, lysis,
filamentation, debris, etc.
[000107] FIG. 9 shows an example method 900 that includes, at 902, suspending
a biological
sample in a sample volume having a plurality of flow-cell volumes isolated
from each other such
that at least one cell corresponds to a growth control and one or more other
cells correspond to
anti-microbial or selective media cells. At 904, a variation over time of an
in-line hologram of at
least the growth control cell is detected. At 906, a correspondence between
the detected variation
and a presence and/or concentration of a microorganism in the growth control
cell is determined.
- 39 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
At 908, a microorganism concentration sufficient to indicate a presence in the
one or more other
anti-microbial or selective media cells is determined.
[000108] FIG. 10 is an example method 1000 that includes, at 1002, suspending
a biological
sample in a sample volume containing a growth medium supporting a
microorganism of the
biological sample therein. In representative examples, the supporting growth
medium allows the
microorganism to move within the sample volume, though the microorganism can
also be
immobilized by the supporting growth medium. At 1004, an automated in-line
holographic
apparatus that typically directs an illumination beam lenslessly from a pin-
hole aperture through
the sample volume to an optical detector, detects a first in-line hologram of
the sample volume
at an initial time (e.g., at a beginning of a test or at a selected time or
sequence point during the
test). At 1006, the 3D spatial characteristics of the sample volume are
reconstructed from the
first in-line hologram, so as to form a first hologram reconstruction. Various
techniques can be
used for hologram reconstruction, diffraction theory (e.g., iterative
Gerchberg¨Saxton), and/or
deep learning (e.g., convolutional neural networks). For example, in deep
learning approaches,
such as convolutional neural networks, the network layers can be supervised
and the network
activations can be trained to map raw hologram (interferometric) space into in-
focus image
plane at a specified focal distance.
[000109] In selected examples, hologram reconstruction processes can include
pre-processing of
detected hologram data. For example, multi-wavelength hologram registration
can be used where
multiple pinhole apertures are physically separated by less than about 1 mm to
form fixed
predetermined offsets, such as with multiple wavelengths directed to a common
sample volume
or sample volume portion through the respective proximate apertures. The pixel
grid of the
optical detector subsampled with the multiple wavelengths, and the acquired
image data is
shifted relative to each other on an upsampled grid such that the relative
offsets are
eliminated. De-noising of the detected hologram data can be provided with deep
learning
approaches (such as convolutional neural networks) or deconvolution of an
estimated/theoretical Point Spread Function (PSF) in 2D or volumetric PSF in
3D. For
example, de-noising with convolutional neural networks can remove or suppress
imaging
sensor non-uniformities (e.g. pixel response non-uniformity or striping), an
image degradation
of the optical system (e.g. PSF), and diffraction ring cross-talk
interference, including without
formulating analytical models for corresponding sources of noise. In
convolutional neural
network examples, the network layers are typically trained using one or more
suboptimally
acquired holograms (single-wavelength/single-aperture or numerically degraded)
where target
data is a higher fidelity hologram (multi-wavelength/multi-aperture, multi-
sampled/averaged).
The corresponding trained network can then be applied to both lower-fidelity
holograms as well
- 40 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
as higher-fidelity holograms to suppress various noise contributions, such as
those described
above. In some examples, in a manner similar to PSF deconvolution in optical
(e.g., confocal)
microscopy, PSF of a lens-free holographic system can be established either
empirically (e.g.,
by recording a signal associated with particles below a resolution limit) or
analytically.
Techniques such as Richardson-Lucy algorithm can then he employed to
deconvolve (or "take
out-) the PSI' from the image data. The principal difference is that in lens-
free imaging the
above procedure can be applied directly to a raw hologram (i.e., before
reconstruction). By pre-
processing the detected hologram data, the de-noising and/or deconvolution can
improve
volumetric position estimation accuracy as well as amplitude and phase
representation of small
spherical objects that approximate point sources. Such approximations can be
particularly
applicable and valid for individual and/or clustered bacteria.
[000110] At 1008, from the sample volume reconstruction, a 3D position of one
or more objects
in the sample volume (typically many objects in biological sample volumes)
and/or
morphological characteristics of the one or more objects in the sample volume
can be
determined, based on amplitude and phase characteristics associated with the
first in-line
hologram and/or first reconstruction. The suspended biological sample is
typically incubated in
an environment conducive to microorganism replication, at 1010, for a
predetermined time
period. In representative examples, multiple holograms are detected in a test
run at different
points in time, and the time intervals need not be identical. Time resolution
and time interval
variation can be selected based on incubation characteristics, growth media,
microorganism
growth stages, etc. At 1012, a second in-line hologram is detected at a second
time. The second
in-line hologram is reconstructed at 1014, and can use one or more techniques
that were used in
the reconstructions of the first in-line hologram.
[000111] At 1016, a 3D position of one or more objects in the sample volume
and/or
morphological characteristics of the one or more objects in the sample volume
can be
determined, based on amplitude and phase characteristics associated with the
second in-line
hologram and/or second reconstruction. In some examples, detected hologram
data or respective
reconstructions can be compared over time to determine object locations and
characteristics by
analyzing differential variations on a spatial (or per-pixel) basis. Deep
learning approaches based
on Bayesian statistical inference, including convolutional neural networks,
can also be employed
to recognize and quantify variation patterns arising from differential
holograms or differential
reconstructed images. In convolutional neural network examples, the network is
trained in a
supervised fashion to recognize variational spatial patterns due to, by way of
example, multiple
species of bacteria and fungus versus other biological or non-biological
particles.
- 41 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[000112] Because the objects detected with the first hologram may grow, die,
move, or provide
other microorganism signatures that vary over the time interval between the
first and second
hologram detections, objects detected at 1016 may be closely related to
objects detected in the
first in-line hologram. In some examples, at 1018, an object of the one or
more objects detected
from the second in-line hologram and/or second reconstruction is associated
with an object of
the one or more objects detected from the first in-line hologram and/or the
first reconstruction,
based on proximity and/or morphological amplitude/phase characteristics. In
some immobilized
sample volume examples, object associations can be omitted or relaxed as the
object does not
change position (though growth, death, and/or other morphological
characteristics may change)
between first and second hologram detections due to the immobilizing growth
medium. At
1020, an object track for the associated objects can be created over time to
accumulate position,
morphological, and amplitude/phase characteristics for the tracked object.
Object tracks can be
1D, 2D, and/or 3D in some examples_ In selected immobilized examples, object
tracks can be
omitted. In some examples (and particularly convenient in immobilized
examples), numerical
features of a detected object can be accumulated over time that are associated
with an indication
that the immobilized object is a microorganism suspended in the biological
sample volume. At
1022, phenotypical behavior of the tracked object can be classified, such as
object motility,
growth, death, lysis, filamentation, debris, etc. At 1024, additional objects
can be associated,
object tracks formed for the additional tracked objects, and phenotypical
behavior of the tracked
objects classified, individually or as a population. In selected immobilized
examples, motility
can be omitted. In either mobilized or immobilized examples, based on the
resolution of the
apparatus (e.g., resolving 10 um, 5 p_tm, 1 Inn, or 0.5 um dimension of the
sample volume, for
relative large fields of view) and the ability to identify individual objects,
actual object
quantities and corresponding volumetric concentrations in the sample volume
can be
determined, including individual cells or populations of cells.
[000113] FIG. 11 is an example iterative object association method 1100 in the
testing a sample
volume that can contain a microorganism to be detected. At 1102, an in-line
hologram is
provided of time t, a corresponding hologram reconstruction can be provided,
and position
and/or morphology of objects at time ti in the sample volume are provided that
are determined
based on the ti in-line hologram and/or ti reconstruction. For example, if ti
corresponds to a first
in-line hologram of a series of holograms for a test of the sample volume,
then the in-line
hologram can be produced and detected at the time t, the sample volume
reconstructed, and/or
object positions determined rather than, e.g., being provided in another way,
such as through
access from a local or remote data storage. At 1104, a selected time interval
ti+i-t] is provided
after the time t1. At 1106, an in-line hologram is detected at time ti+1, a
corresponding hologram
- 42 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
reconstruction is produced, and position and/or morphology of objects at time
ti+] in the sample
volume are determined based on the t,+] in-line hologram and/or t,+] hologram
reconstruction.
The t,+] objects with t, objects are compared and associated at 1108 based on
proximity to and/or
morphological characteristics to identify object types. At 1110, a check is
performed as to
whether the in-line hologram imaging test of the sample is complete for the
sample volume. If
the test is not yet complete, the time t]+] can be set to a time t, and the
process of providing an in-
line hologram at 1102 (which can correspond to the in-line hologram provided
in the previous
step 1106) can be repeated. In representative examples, object associations
can be updated,
revised, including with new object associations, as subsequent holograms are
obtained,
analyzed, and compared with previous hologram, sequences of holograms, and/or
object
association histories. Multiple objects in a sample volume can be associated
and identified and
thereby quantified. Based on the size of the sample volume and the ability to
quantify the
multiple objects within the sample volume at different growth stages, precise
object
concentrations (including for different object types or taxa) can be
determined.
[000114] FIG. 12 is an example of an iterative phenotype classification method
1200 that can be
used in testing a biological sample in a sample volume with an automated in-
line holography
apparatus. At 1202, an in-line hologram is provided of time ti, a
corresponding hologram
reconstruction can be provided, and position and/or morphology of objects at
time ti in the
sample volume are provided that are determined based on the t, in-line
hologram and/or t,
reconstruction. For example, if ti corresponds to a first in-line hologram of
a series of holograms
for a test of the sample volume, then the in-line hologram can be produced and
detected at the
time ti, the sample volume reconstructed, and/or object positions determined
rather than, e.g.,
being provided in another way, such as through access from a local or remote
data storage. At
1204, a selected time interval 4+14, is provided after the time t1. At 1206,
an in-line hologram is
detected at the time t+1, a corresponding hologram reconstruction is produced,
and position
and/or morphology of objects at time t,+] in the sample volume, including
previously identified
and associated objects in the sample volume or previously associated objects
(e.g., that move,
grow, die, etc.), are determined based on the t,+] in-line hologram and/or
t,+] hologram
reconstruction. In typical examples, objects are identified and object
associations are formed
after a plurality of holographic samples in a time sequence of the test. At
1208, an object history
of an associated object (e.g., an object that changes position in the sample
volume through
flagellation, or a bacterial growth, splitting, including individual or
populations, etc.) can be
updated, e.g., in computer memory, based on changes of detected or computed
object
parameters, such as an object track (e.g., a movement path, a centroid
position change of a
population, filamentation direction, etc.) or morphological characteristics
(e.g., shape,
- 43 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
microorganism features, patterns, colors, size, etc.). In some examples,
objects can be compared
between times time ti+i and object associations produced or updated, similar
to as shown in the
example method 1100. At 1210, a check is performed as to whether the set of
hologram events
is sufficient (e.g., sufficient number of events and/or a sufficient duration
for incubation, etc.) to
support a phenotypic classification of the identified objects based on the
object histories. If a
sufficient set of in-line hologram events has not yet been collected, the time
ti-Ft can be set to a
time ti and the process of providing an in-line hologram at 1202 (which can
correspond to the in-
line hologram provided in the previous step 1206) can be repeated. If the set
of events is
sufficient, at 1214, phenotypic behavior of one or more of the identified
objects, individually or
as a population, is classified based on the accumulated object history for the
object. In some
examples, such classifications can be updated, revised (including being
replaced), as additional
holograms in a test sequence are detected. Classification of numerical
features that represent
phenotypic behavior over time for an identified object can be accomplished
with various
techniques, such as, but not limited to, regression, discriminant analysis,
decision trees, and/or
neural networks (e.g. convolutional neural networks). Classification
categories can include (but
are not limited to) object motility, growth, death, lysis, filamentation, and
debris. Detected
objects that exhibit response that can be representative of bacterial
phenotypic response can be
selected for further analysis along with their respective measured features.
Such analyses can be
performed on an individual object basis as well as population basis.
j000115] FIG. 13 and the following discussion are intended to provide a brief,
general
description of an exemplary computing environment in which the disclosed
technology may be
implemented. Although not required, the disclosed technology is described in
the general
context of computer-executable instructions, such as program modules, being
executed by a
computing unit, dedicated processor, or other digital processing system or
programmable logic
device. Generally, program modules include routines, programs, objects,
components, data
structures, etc., that perform particular tasks or implement particular
abstract data types.
Moreover, the disclosed technology may be implemented with other computer
system
configurations, including hand-held devices, multiprocessor systems,
microprocessor-based or
programmable consumer electronics, network PCs, minicomputers, mainframe
computers,
dedicated processors, MCUs, PLCs, ASICs, FPGAs, CPLDs, systems on a chip, and
the like.
The disclosed technology may also be practiced in distributed computing
environments where
tasks are performed by remote processing devices that are linked through a
communications
network. In a distributed computing environment, program modules may be
located in both
local and remote memory storage devices.
- 44 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[000116] With reference to FIG. 13, an exemplary system for implementing the
disclosed
technology includes a computing device 1300 that includes one or more
processing units 1302, a
memory 1304, and a system bus 1306 that couples various system components
including the
system memory 1304 to the one or more processing units 1302. The system bus
1306 may be any
of several types of bus structures including a memory bus or memory
controller, a peripheral bus,
and a local bus using any of a variety of bus architectures. The memory 1304
can include various
types, including volatile memory (e.g., registers, cache, RAM), non-volatile
memory (e.g.. ROM,
EEPROM, flash memory, etc.), or a combination of volatile and non-volatile
memory. The
memory 1304 is generally accessible by the processing unit 1302 and can store
software in the
form computer-executable instructions that can be executed by the one or more
processing units
1302 coupled to the memory 1304. In some examples, processing units can be
configured based
on RISC or CSIC architectures, and can include one or more general purpose
central processing
units, application specific integrated circuits, graphics or co-processing
units or other
processors. In some examples, multiple core groupings of computing components
can be
distributed among system modules, and various modules of software can be
implemented
separately.
[000117] The exemplary computing device 1300 further includes one or more
storage devices
1330 such as a hard disk drive for reading from and writing to a hard disk, a
magnetic disk drive
for reading from or writing to a removable magnetic disk, and an optical disk
drive for reading
from or writing to a removable optical disk (such as a CD-ROM or other optical
media). Such
storage devices can be connected to the system bus 1306 by a hard disk drive
interface, a
magnetic disk drive interface, and an optical drive interface, respectively.
The drives and their
associated computer-readable media provide nonvolatile storage of computer-
readable
instructions, data structures, program modules, and other data for the
computing device 1300.
Other types of non-transitory computer-readable media which can store data
that is accessible
by a PC, such as magnetic cassettes, flash memory cards, digital video disks,
CDs, DVDs,
RAMs, ROMs, and the like, may also be used in the exemplary computing
environment. The
storage 1330 can be removable or non-removable and can be used to store
information in a non-
transitory way and which can be accessed within the computing environment.
[000118] As shown in FIG. 13, the computing device 1300 is coupled to an
output device 1/0
1332 so that suitable output signals (e.g., digital control voltage and/or
current signals) are
provided to imaging devices 1340 of an in-line holography generator 1342. The
imaging devices
1340 typically include illumination sources generating light at one or more
wavelengths and
pinhole apertures to receive the illumination and lensles sly direct the
illumination to a sample
volume 1346. A hologram is formed at a hologram detector 1344. Input device
1/0 1334 is
- 45 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
coupled to the bus 1306 so that data signals and/or values corresponding to in-
line holograms
detected with the detector 1344 can be stored in the memory 1304 and/or
storage 1330 and/or
processed with the processing unit 1302. In some examples, a control stage
1348, such as a
translation and/or rotation stage, can be coupled to the sample volume (and/or
the detector 1344
and imaging device 1340) so that relative movement between the sample volume
1346 and
illumination/detection beams can be produced. The control stage 1348 can
provide a translation
so that different cells for the sample volume 1346, e.g., for large sample
volumes, can be
illuminated and detected at different times.
[000119] In representative examples, the detected holograms are used to
reconstruct the 3D
physical characteristics of the sanaple volume 1346, so that immobilized or
mobile objects in the
sample volume 1346 (such as microorganisms) can be detected. Imaging and/or
detection
intervals, gating, synchronization, etc., can be stored in a memory 1310A
along with various data
tables for storing detected hologram data, manipulated data (e.g., holographic
reconstructions),
and algorithms for analyzing data. For example, identified/associated objects
(e.g., a moving
microorganism, a growing bacterial colony, etc.) and unassociated or static
objects can be stored
in objects tables 1310B. Hologram reconstruction algorithms, such as
Gerchberg¨Saxton (GS)
and/or Bayesian deep learning methods, can be stored in a memory 1310C. Object
identification,
object tracking, and/or morphological identification algorithms, such as
convolutional neural
networks, can be stored in a memory 1310D. As objects are tracked and
associated
morphological characteristics detected, histories of object characteristics
can be stored in a
memory 1310E. Phenotype classifications that can be determined based on the
object tracks and
morphological characteristics can be stored in a memory 1310F.
[000120] A number of program modules (or data) may be stored in the storage
devices 1330
including an operating system, one or more application programs, other program
modules, and
program data. A user may enter commands and information into the computing
device 1300
through one or more input devices such as a keyboard and a pointing device
such as a mouse.
Various other input devices can be used as well. These and other input devices
are often
connected to the one or more processing units 1302 through a serial port
interface that is
coupled to the system bus 1306, but may be connected by other interfaces such
as a parallel
port, game port, or universal serial bus (USB). In representative examples,
the various routines,
programs, and program modules can be automated so that biological samples may
be received
by the in-line holography generator 1342. The in-line holography generator
1342 can include
or be coupled to the computing device 1300 so that tests can be performed on
the biological
samples with little intervention from a user. A monitor 1350 or other type of
display device is
also connected to the system bus 1306 via an interface, such as a video
adapter. The monitor
- 46 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
1350 can be used to display hologram images, reconstructed sample volume
images in 2D or
3D (e.g., perspective images, focal planes, z-planes, etc.), time lapse images
of growth, images
with static objects and/or debris subtracted, etc. Some or all data and
instructions can be
communicated with a remote computer 1360 through communication connections
1355 (e.g.,
wired, wireless, etc.) if desired.
[000121] FIGS. 14A-14C are sample volumes 1400A-1400C with contents that can
be detected
over time through generation and detection of in-line holograms with a
holographic apparatus.
In sample volume 1400A, an object 1402A is detected at a time to, which can
correspond to an
initiation of an incubation and test of the sample volume 1400A or a time at a
selected point
during the test. At a time ti, an object is detected at a different position
and the holographic
apparatus can determine that the object is associated with the object 1402A,
such as through a
movement to the new position. Different detected characteristics can be
associated with the
movement, such as the lack of, or change in the characteristics of (e.g.,
image variation
corresponding to a flagellation or movement wake), the object at the position
detected at time to.
The object 1402A can be detected at subsequent times t2 and t3 and an object
track 1404A can be
formed. As shown in FIGS. 14A-14C, the various object tracks and morphological
characteristics can be detected in one, two, and/or three spatial dimensions.
[000122] The sample volume 1400B shows a growth of an object 1402B in a motile
or
immobilizing support media. For example, at a time to the object 1402B can be
detected. At
subsequent times t143, a growth is detected such as through the change in
position of an object
boundary that corresponds to an area enlargement associated with the object
1402B. An object
track 1404B can also be identified, e.g., based on centroid calculations or
morphological
characteristics of the object 1402B (e.g., color, opacity, shape, size, etc.).
In the sample volume
1400C, an object 1402C is detected at a time to with no other objects detected
in the surrounding
volume, or with some objects detected that can be later subtracted as not
corresponding to
growing microorganisms. At a time ti, multiple objects are detected
surrounding the object
1402C defining a growing object boundary 1404C (e.g., with no objects detected
outside the
object boundary 1404C). In some examples, each individual object can be
detected and
movement can be tracked. At later times t243, additional objects are detected
indicative of
growth of the initial object 1402C and defining respective growing population
boundaries
1406C, 1408C. An object 1410C detected at time ti can be associated with a
movement along a
track 1412C to a new position at time t2. Another object 1414C detected at
time t2 can be
associated with a movement along a track 1416C to a new position at time t3.
Track
characteristics, including directional changes, can be determined based on
additional holograms
- 47 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
between selected time intervals, debris and/or wake detection, and
morphological characteristics
including associations between size or shape and movement speed/distance.
[000123] FIG. 15 is an example multiplexed method 1500 of testing biological
samples. At
1502, a biological sample is suspended in a sample volume having a plurality
of flow-cells or
chambers isolated from each other such that at least one cell corresponds to a
growth control
and one or more other chambers correspond to anti-microbial or selective media
cells. At 1504,
a variation over time is detected in multiple in-line holograms of at least
the growth control cell
and phenotypic behavior of individual objects and/or populations of objects in
at least the
growth control cell is classified based on the detected hologram variation.
The hologram
variation can be derived from several natthods including, but not limited to
image reconstruction
or by using pixel intensity to calculate a statistical variability metric
including, but not limited to
standard deviation, range, interquartile range and coefficient of variation.
At 1506, a
correspondence is determined between the detected phenotypic behavior and a
presence,
concentration, and taxon of a microorganism or multiple microorganisms in at
least the growth
control flow cell. At 1508, a presence, taxon, and antibiogram of a
microorganism or multiple
microorganisms is determined based on at least one growth control, at least
one selective media,
and at least one anti-microbial flow cell.
[000124] FIG. 16 is an example hologram reconstruction framework 1600 with a
convolutional
neural network. The hologram reconstruction framework 1600 typically includes
a training
phase 1602-1608 that refines the parameters of the convolutional neural
network. At 1602, a set
of hologram training data is provided to a deeply supervised multi-layer
convolutional neural
network. Training data typically includes a set of holographic data having a
known ground truth
amplitude/phase spatial reconstruction for a sample volume. At 1604, the
training hologram data
is processed through the deeply supervised convolutional neural network to
produce a
reconstruction of the sample volume based on the input training hologram data.
At 1606, the
output hologram-based reconstruction is compared with the ground truth
representation of the
sample volume, and at 1608, based on the detected errors, the non-linear
activations (e.g.,
softplus, ReLU, etc.) of one or more network layers of the convolutional
neural network are
updated by back-propagating comparison error through the convolutional neural
network, e.g.,
via gradient descent. A testing phase 1610-1614 is used on field samples after
the convolutional
neural network is sufficiently trained. At 1610, data corresponding to an in-
line holographic
image of a biological test sample volume is provided from an imaging detector
and/or
memory/storage. At 1612, the data is processed through the trained deeply
supervised
convolutional neural network, and at 1614 a reconstruction of the sample
volume based on the
hologram data is produced.
- 48 -
CA 03231753 2024- 3- 13
WO 2023/039287
PCT/US2022/043350
[000125] FIG. 17 is an example micro-object identification/classification
framework 1700 with
a convolutional neural network. The micro-object identification/classification
framework 1700
typically includes a training phase 1702-1708 that refines the parameters of
the convolutional
neural network to converge on an improved output accuracy as additional
training data sets are
processed. At 1702, a set of hologram training data is provided to a deeply
supervised multi-
layer convolutional neural network. '[raining data typically includes a set of
hologram data
and/or hologram reconstruction data having a known ground truth object
identification and/or
object classification correspondence for a sample volume that includes various
objects. At 1704,
the training data is processed through the deeply supervised multi-layer
convolutional neural
network to produce an output object identification and/or object
classification, such as an
identification of objects, object morphologies, object movements, and
phenotypic
classifications. At 1706, output identification and/or classification is
compared to the ground
truth associated with the training data. At 1708, activations of one or more
network layers are
updated by back-propagation (e.g., through gradient descent) of the comparison
error through
the convolutional neural network. A testing phase 1710-1714 can be used on
field samples after
the convolutional neural network is sufficiently trained. At 1710, data
corresponding to an in-
line holographic image or reconstructed 3D spatial image of a biological test
sample volume is
provided. At 1712, the data are processed through the trained deeply
supervised convolutional
neural network, and at 1714 an object identification and/or classification is
produced based on
the hologram or reconstruction data.
[000126] In view of the many possible embodiments to which the principles of
the disclosure
may be applied including, but not limited to growth and phenotypical
discrimination of cells,
cell units, microbes and microorganisms, it should be recognized that the
illustrated
embodiments are only examples and should not be taken as limiting the scope of
the disclosed
technology. Rather, the scope of the disclosed technology is defined by the
following claims.
We therefore claim all that comes within the scope of these claims.
- 49 -
CA 03231753 2024- 3- 13