Note: Descriptions are shown in the official language in which they were submitted.
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
CRYSTALLINE FORMS AND PROCESSES FOR THE PREPARATION OF PYRIMIDINE
DERIVATIVES USEFUL AS MODULATORS OF THE 5-HT2A SEROTONIN RECEPTOR
The present disclosure relates to crystalline forms and salts of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la) and pharmaceutical compositions thereof that modulate the
activity of the 5-HT2A
serotonin receptor. The present invention further relates to processes useful
in the preparation
of crystalline forms and salts of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide and pharmaceutical compositions
thereof.
BACKGROUND
Receptors for serotonin (5-hydroxytryptamine, 5-HT) are an important class of
G protein
coupled receptors. Serotonin receptors are divided into seven subfamilies,
referred to as 5-HT1
through 5-H-17, inclusive. These subfamilies are further divided into
subtypes. For example, the
5-HT2 subfamily is divided into three receptor subtypes: 5-HT2A, 5-HT2B, and 5-
HT2c. Certain
modulators of 5-HT2A serotonin receptor activity are useful in the treatment
of platelet
aggregation, coronary artery disease, myocardial infarction, transient
ischemic attack, angina,
stroke, atrial fibrillation, blood clot formation, or symptoms thereof.
There is a need for compounds that can be used to treat disorders related to
the 5-HT2A
serotonin receptor, including disorders of the cardiovascular system. In
particular, there is a need
for compounds that possess physical and chemical stability and favorable
pharmacokinetic
properties.
SUMMARY
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(10:
N)
0
0
v)Lil
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide comprising the steps of:
1
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
a) contacting said N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) with an acid, in the
presence of a
contacting-step solvent;
b) crystallizing N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) to obtain a
crystalline salt of N-(3-
(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Formula (la)) in a crystallizing mixture; and
c) isolating said crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-
(2-(pyrrolidin-
1-yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) from said
crystallizing mixture to
obtain said crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)).
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide, wherein the process further
comprises stirring after
the contacting step.
One aspect of the present disclosure relates to processes wherein the the
contacting-step
solvent is selected from a group consisting of acetone, acetonitrile, 1-
butanol, 2-butanol, butyl
acetate, 1,2-dimethoxyethane, N,N-dimethylacetamide, 1,4-dioxane, ethanol, 2-
ethoxyethanol,
ethyl acetate, isopropyl acetate, heptane, methyl isobutyl ketone (MIBK), 2-
methyl-1-propanol,
N-methyl pyrrolidone, 1-propanol, 2-propanol, n-propyl acetate, tert-butyl
methyl ether (TBME),
tetrahydrofuran (THF), water, and dimethyl sulfoxide (DMSO) and combinations
thereof.
One aspect of the present disclosure relates to processes wherein the acid is
a mineral
acid or an organic acid.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide comprising the step of:
isolating said crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) from said
crystallizing mixture to
obtain said crystalline salt of
N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)).
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)) prepared by a process as described herein.
One aspect of the present disclosure relates to compositions comprising a
crystalline
form of N-(3-(4 ,6-d imethylpyrimid in-5-yI)-4-(2-(pyrrolid in-1 -
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) as described herein.
One aspect of the present disclosure relates to compositions comprising a
crystalline
form of N-(3-(4 ,6-d imethylpyrimid in-5-yI)-4-(2-(pyrrolid in-1 -
2
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) as described herein,
and a
pharmaceutically acceptable carrier.
One aspect of the present disclosure relates to processes of making a
composition
comprising mixing a crystalline form of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) as described herein,
with a
phamaceutically acceptable carrier.
One aspect of the present disclosure relates to methods of treating a 5HT2A-
related
disorder in an individual, comprising administering to an individual in need
thereof, a
therapeutically effective amount of a crystalline form of N-(3-(4 ,6-
dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) as
described herein or a
pharmaceutical composition thereof.
One aspect of the present disclosure relates to the use of a crystalline form
of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)) as described herein, in the manufacture of a medicament for the
treatment of a 5HT2A-related
disorder.
One aspect of the present disclosure relates to a crystalline form of 5HT2A-
related disorder
as described herein, for use in a method of treatment of the human or animal
body by therapy.
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)) as described herein, for use in a method of treatment of a 5HT2A-related
disorder.
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)) as described herein, for use in a method of treatment of a 5HT2A-related
disorder.
One aspect of the present disclosure relates to crystalline salts of N-(3-(4,6-
dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline besylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline besylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline citrate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)).
One aspect of the present disclosure relates to a crystalline fumarate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
3
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
One aspect of the present disclosure relates to a crystalline hydrochloride
salt of N-(3-
(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Formula (la)).
One aspect of the present disclosure relates to a crystalline mesylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline phosphate salt
of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline succinate salt
of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline tosylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)).
These and other aspects of the invention disclosed herein will be set forth in
greater detail
as the patent disclosure proceeds.
BRIEF DESCRIPTION OF THE DRAWINGS
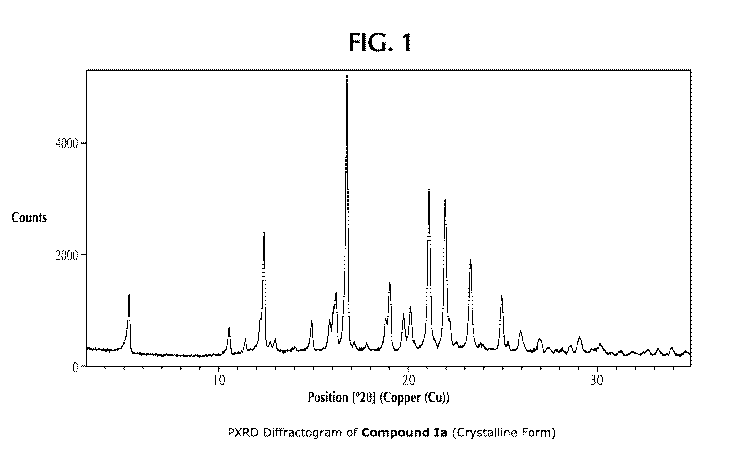
Figure 1 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline form of Compound of Formula (la).
Figure 2 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline form of Compound of Formula (la).
Figure 3 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline form of Compound of Formula (la).
Figure 4 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Besylate Salt).
Figure 5 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Besylate Salt).
Figure 6 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Besylate Salt).
Figure 7 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Citrate Salt).
Figure 8 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Citrate Salt).
Figure 9 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Citrate Salt).
4
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Figure 10 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Fumarate Salt).
Figure 11 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Fumarate Salt).
Figure 12 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Fumarate Salt).
Figure 13 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Hydrochloride Salt).
Figure 14 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Hydrochloride Salt).
Figure 15 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Hydrochloride Salt).
Figure 16 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Mesylate Salt).
Figure 17 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Mesylate Salt).
Figure 18 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Mesylate Salt).
Figure 19 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Phosphate Salt).
Figure 20 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Phosphate Salt).
Figure 21 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Phosphate Salt).
Figure 22 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Succinate Salt).
Figure 23 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Succinate Salt).
Figure 24 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Succinate Salt).
Figure 25 shows a powder X-ray diffraction (PXRD) pattern for a sample
containing a
crystalline salt of Compound la (Tosylate Salt).
Figure 26 shows a differential scanning calorimetry (DSC) thermogram for a
sample
containing a crystalline salt of Compound la (Tosylate Salt).
Figure 27 shows a thermogravimetric analysis (TGA) thermogram of a sample
containing
a crystalline salt of Compound la (Tosylate Salt).
5
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
DETAILED DESCRIPTION
DEFINITIONS
For clarity and consistency, the following definitions will be used throughout
this patent
document.
The term "agonists" is intended to mean moieties that interact and activate
the receptor,
such as the 5-HT2A serotonin receptor, and initiate a physiological or
pharmacological response
characteristic of that receptor. For example, when moieties activate the
intracellular response
upon binding to the receptor, or enhance GTP binding to membranes.
The term "antagonists" is intended to mean moieties that competitively bind to
the receptor
at the same site as agonists (for example, the endogenous ligand), but which
do not activate the
intracellular response initiated by the active form of the receptor, and can
thereby inhibit the
intracellular responses by agonists or partial agonists. Antagonists do not
diminish the baseline
intracellular response in the absence of an agonist or partial agonist.
The term "contact or contacting" is intended to mean bringing the indicated
moieties
together, whether in an in vitro system or an in vivo system. Thus,
"contacting" a 5-HT2A serotonin
receptor with a compound of the invention includes the administration of a
compound of the
present invention to an individual, preferably a human, having a 5-HT2A
serotonin receptor, as
well as, for example, introducing a compound of the invention into a sample
containing a cellular
or more purified preparation containing a 5-HT2A serotonin receptor.
The term "inverse agonists" is intended to mean moieties that bind to the
endogenous
form of the receptor or to the constitutively activated form of the receptor,
and which inhibit the
baseline intracellular response initiated by the active form of the receptor
below the normal base
level of activity which is observed in the absence of agonists or partial
agonists, or decrease GTP
binding to membranes. Preferably, the baseline intracellular response is
inhibited in the presence
of the inverse agonist by at least 30%, more preferably by at least 50%, and
most preferably by
at least 75%, as compared with the baseline response in the absence of the
inverse agonist.
The term "modulate or modulating" is intended to mean an increase or decrease
in the
amount, quality, response or effect of a particular activity, function or
molecule.
As used herein, "administering" means to provide a compound or other therapy,
remedy,
.. or treatment such that an individual internalizes a compound.
The term "prescribing" refers to order, authorize, or recommend the use of a
drug or other
therapy, remedy, or treatment. In some embodiments, a health care provider
orally advises,
recommends, or authorizes the use of a compound, dosage regimen, or other
treatment to an
individual. The health care provider may or may not provide a written
prescription for the
compound, dosage regimen, or treatment. Further, the health care provider may
or may not
provide the compound or treatment to the individual. For example, the health
care provider can
advise the individual where to obtain the compound without providing the
compound. In some
embodiments, a health care provider can provide a written prescription for the
compound, dosage
6
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
regimen, or treatment to the individual. A prescription can be written on
paper or recorded on
electronic media. In addition, a prescription can be called in (oral) or faxed
in (written) to a
pharmacy or a dispensary. In some embodiments, a sample of the compound or
treatment is
given to the individual. As used herein, giving a sample of a compound
constitutes an implicit
prescription for the compound. Different health care systems around the world
use different
methods for prescribing and administering compounds or treatments, and these
methods are
encompassed by the disclosure herein.
A health care provider can include, for example, a physician, nurse, nurse
practitioner, or
other health care professional who can prescribe or administer compounds
(drugs) for the
disorders disclosed herein. In addition, a health care provider can include
anyone who can
recommend, prescribe, administer, or prevent an individual from receiving a
compound or drug,
including, for example, an insurance provider.
The terms "in need of treatment" and "in need thereof' when referring to
treatment, are
used interchangeably to mean a judgment made by a caregiver (e.g., physician,
nurse, or nurse
practitioner in the case of humans; veterinarian in the case of animals,
including non-human
mammals) that an individual or animal requires or will benefit from treatment.
This judgment is
made based on a variety of factors that are in the realm of a caregiver's
expertise, but that
includes the knowledge that the individual or animal is ill, or will become
ill, as the result of a
disease, condition or disorder that is treatable by the compounds of the
disclosure. Accordingly,
the compounds of the disclosure can be used in a protective or preventive
manner; or compounds
of the disclosure can be used to alleviate, inhibit, or ameliorate the
disease, condition, or disorder.
The term "individual" refers to any animal, including mammals such as mice,
rats, and
other rodents, rabbits, dogs, cats, swine, cattle, sheep, horses, primates,
and humans. In some
embodiments, "individual" refers to humans.
The term "composition" refers to a compound or crystalline form thereof,
including but not
limited to, salts, solvates, and hydrates of a compound of the present
invention, in combination
with at least one additional component, such as, a composition
obtained/prepared during
synthesis, preformulation, in-process testing (i.e., TLC, HPLC, NMR samples),
and the like.
The term "hydrate" as used herein means a compound of the invention or a salt
thereof
that further includes a stoichiometric or non-stoichiometric amount of water
bound by non-
covalent intermolecular forces.
The term "pharmaceutical composition" refers to a specific composition
comprising at
least one active ingredient, including but not limited to, salts, solvates,
and hydrates of
compounds of the present disclosure, whereby the composition is amenable to
investigation for
a specified, efficacious outcome in a mammal (for example, without limitation,
a human). Those
of ordinary skill in the art will understand and appreciate the techniques
appropriate for
determining whether an active ingredient has a desired efficacious outcome
based upon the
needs of the artisan.
7
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
The phrase "pharmaceutically acceptable salts, solvates, and hydrates" when
referring to
a compound/compounds as described herein embraces pharmaceutically acceptable
solvates
and/or hydrates of the compound/compounds, pharmaceutically acceptable salts
of the
compound/compounds, as well as pharmaceutically acceptable solvates and/or
hydrates of
pharmaceutically acceptable salts of the compound/compounds. It is also
understood that when
the phrase "pharmaceutically acceptable solvates and hydrates" or the phrase
"pharmaceutically
acceptable solvate or hydrate" is used when referring to a compound/compounds
as described
herein that are salts, it embraces pharmaceutically acceptable solvates and/or
hydrates of such
salts. It is also understood by a person of ordinary skill in the art that
hydrates are a subgenus of
solvates.
The term "compound," as used herein, is meant to include all stereoisomers,
geometric
isomers, tautomers, and isotopes of the structures depicted, unless otherwise
specified. All
compounds, and pharmaceutically acceptable salts thereof, can be found
together with other
substances such as water and solvents (e.g., in the form of hydrates and
solvates) or can be
.. isolated. For example, the term "solvate," as used herein, means a compound
or a salt thereof
that further includes a stoichiometric or non-stoichiometric amount of a
solvent bound by non-
covalent intermolecular forces. Exemplary solvents are volatile, non-toxic,
and/or acceptable for
administration to humans in trace amounts. The term "hydrate" as used herein
means a
compound or a salt thereof that further includes a stoichiometric or non-
stoichiometric amount of
water bound by non-covalent intermolecular forces. The term "compound" is also
meant to be
agnostic as to how the compound is formed, be it synthetically or
biologically. For example, a
compound of the present disclosure can be produced in the body through
metabolism.
The terms "prevent," "preventing," and "prevention" refer to the elimination
or reduction of
the occurrence or onset of one or more symptoms associated with a particular
disorder. For
example, the terms "prevent," "preventing," and "prevention" can refer to the
administration of
therapy on a prophylactic or preventative basis to an individual who may
ultimately manifest at
least one symptom of a disorder but who has not yet done so. Such individuals
can be identified
on the basis of risk factors that are known to correlate with the subsequent
occurrence of the
disease, such as the presence of a biomarker. Alternatively, prevention
therapy can be
administered as a prophylactic measure without prior identification of a risk
factor. Delaying the
onset of the at least one episode and/or symptom of a disorder can also be
considered prevention
or prophylaxis.
The terms "treat," "treating," and "treatment" refer to the administration of
therapy to an
individual who already manifests, or who has previously manifested, at least
one symptom of a
disease, disorder, condition, dependence, or behavior. For example, "treating"
can include any
of the following with respect to a disease, disorder, condition, dependence,
or behavior:
alleviating, abating, ameliorating, improving, inhibiting (e.g., arresting the
development), relieving,
or causing regression. "Treating" can also include treating the symptoms,
preventing additional
8
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
symptoms, preventing the underlying physiological causes of the symptoms, or
stopping the
symptoms (either prophylactically and/or therapeutically) of a disease,
disorder, condition,
dependence, or behavior. For example, the term "treating" in reference to a
disorder means a
reduction in severity of one or more symptoms associated with a particular
disorder. Therefore,
treating a disorder does not necessarily mean a reduction in severity of all
symptoms associated
with a disorder and does not necessarily mean a complete reduction in the
severity of one or
more symptoms associated with a disorder.
The term "therapeutically effective amount" refers to the amount of active
compound or
pharmaceutical agent that elicits the biological or medicinal response in a
tissue, system, animal,
or human that is being sought by an individual, researcher, veterinarian,
medical doctor, or other
clinician or caregiver, which can include one or more of the following:
(1) preventing the disorder, for example, preventing a disease, condition, or
disorder in
an individual who may be predisposed to the disease, condition, or disorder
but does not yet
experience or display the relevant pathology or symptomatology;
(2) inhibiting the disorder, for example, inhibiting a disease, condition, or
disorder in an
individual who is experiencing or displaying the relevant pathology or
symptomatology (i.e.,
arresting further development of the pathology and/or symptomatology); and
(3) ameliorating the disorder, for example, ameliorating a disease, condition,
or disorder
in an individual who is experiencing or displaying the relevant pathology or
symptomatology (i.e.,
reversing the pathology and/or symptomatology).
In some embodiments, the term "therapeutically effective amount" refers to the
amount of
active compound or pharmaceutical agent that elicits the biological or
medicinal response in a
tissue, system, animal, or human that is being sought by an individual,
researcher, veterinarian,
medical doctor, or other clinician or caregiver, which includes preventing the
disorder, for
example, preventing a disease, condition, or disorder in an individual who may
be predisposed
to the disease, condition, or disorder but does not yet experience or display
the relevant pathology
or symptomatology.
In some embodiments, the term "therapeutically effective amount" refers to the
amount of
active compound or pharmaceutical agent that elicits the biological or
medicinal response in a
tissue, system, animal, or human that is being sought by an individual,
researcher, veterinarian,
medical doctor, or other clinician or caregiver, which includes inhibiting the
disorder, for example,
inhibiting a disease, condition, or disorder in an individual who is
experiencing or displaying the
relevant pathology or symptomatology (i.e., arresting further development of
the pathology and/or
symptomatology).
In some embodiments, the term "therapeutically effective amount" refers to the
amount of
active compound or pharmaceutical agent that elicits the biological or
medicinal response in a
tissue, system, animal, or human that is being sought by an individual,
researcher, veterinarian,
medical doctor, or other clinician or caregiver, which includes ameliorating
the disorder, for
9
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
example, ameliorating a disease, condition, or disorder in an individual who
is experiencing or
displaying the relevant pathology or symptomatology (i.e., reversing the
pathology and/or
symptomatology).
As used herein, "alkyl" means a branched, or straight chain chemical group
containing
only carbon and hydrogen, such as methyl, ethyl, n-propyl, iso-propyl, n-
butyl, iso-butyl, sec-butyl,
tert-butyl, n-pentyl, iso-pentyl, sec-pentyl and neo-pentyl. Alkyl groups can
either be unsubstituted
or substituted with one or more substituents. In some embodiments, alkyl
groups include 1 to 9
carbon atoms (for example, 1 to 6 carbon atoms, 1 to 4 carbon atoms, or 1 to 2
carbon atoms).
As used herein, "alkylene" means a bivalent branched, or straight chain
chemical group
containing only carbon and hydrogen, such as methylene, ethylene, n-propylene,
iso-propylene,
n-butylene, iso-butylene, sec-butylene, tert-butylene, n-pentylene, iso-
pentylene, sec-pentylene
and neo-pentylene. Alkylene groups can either be unsubstituted or substituted
with one or more
substituents. In some embodiments, alkylene groups include 1 to 9 carbon atoms
(for example,
1 to 6 carbon atoms, 1 to 4 carbon atoms, or 1 to 2 carbon atoms).
As used herein, "cycloalkyl" means a non-aromatic cyclic ring system
containing only
carbon atoms in the ring system backbone, such as cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl, and cyclohexenyl. Cycloalkyl may include multiple fused rings.
Cycloalkyl may have
any degree of saturation provided that none of the rings in the ring system
are aromatic.
Cycloalkyl groups can either be unsubstituted or substituted with one or more
substituents. In
some embodiments, cycloalkyl groups include 3 to 10 carbon atoms, for example,
3 to 6 carbon
atoms.
As used herein, "aryl" means a mono-, bi-, tri- or polycyclic group with only
carbon atoms
present in the ring backbone having 5 to 14 ring atoms, alternatively 5, 6, 9,
or 10 ring atoms;
and having 6, 10, or 14 pi electrons shared in a cyclic array; wherein at
least one ring in the
system is aromatic. Aryl groups can either be unsubstituted or substituted
with one or more
substituents. Examples of aryl include phenyl, naphthyl, tetrahydronaphthyl,
and 2,3-dihydro-IH-
indenyl. In some embodiments, the aryl is phenyl.
As used herein, "halo," "halide," or "halogen" refers to a chloro, bromo,
fluoro, or iodo atom
radical. In some embodiments, a halo is a chloro, bromo or fluoro. For
example, a halide can be
fluoro.
As used herein, "haloalkyl" means a hydrocarbon substituent, which is a linear
or
branched alkyl substituted with one or more chloro, bromo, fluoro, and/or iodo
atom(s). In some
embodiments, a haloalkyl is a fluoroalkyl, wherein one or more of the hydrogen
atoms have been
substituted by fluoro. In some embodiments, haloalkyls are of 1 to 3 carbons
in length (e.g., 1 to
2 carbons in length or 1 carbon in length).
As used herein, "haloalkylene" means a bivalent branched, or straight chain
alkylene
substituted with one or more chloro, bromo, fluoro, and/or iodo atom(s), such
as chloromethylene,
dichloromethylene, 1,1-dichloroethylene, and 1,2-dichloroehtylene. Alkylene
groups can either
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
be unsubstituted or substituted with one or more substituents. In some
embodiments, alkylene
groups include 1 to 9 carbon atoms (for example, 1 to 6 carbon atoms, 1 to 4
carbon atoms, or 1
to 2 carbon atoms).
As used herein, "oxo" means =0, wherein the double bond is to a carbon atom.
As used herein, the term "heteroaryl" means a mono- or bicyclic group having 5
to 10 ring
atoms, such as 5, 6, 8, 9, or 10 ring atoms, such as 5, 6, 9, or 10 ring
atoms; wherein at least one
ring in the system is aromatic, and at least one ring in the system contains
one or more
heteroatoms independently selected from the group consisting of N, 0, and S.
Heteroaryl groups
can either be unsubstituted or substituted with one or more substituents.
Examples of heteroaryl
include thienyl, pyridinyl, fury!, oxazolyl, oxadiazolyl, pyrrolyl,
imidazolyl, triazolyl, thiodiazolyl,
pyrazolyl, isoxazolyl, thiadiazolyl, pyranyl, pyrazinyl, pyrimidinyl,
pyridazinyl, triazinyl, thiazolyl
benzothienyl, benzoxadiazolyl, benzofuranyl, benzimidazolyl, benzotriazolyl,
cinnolinyl, indazolyl,
indolyl, isoquinolinyl, isothiazolyl, naphthyridinyl, purinyl,
thienopyridinyl, pyrrolo[2,3-6]pyridinyl,
quinazolinyl, quinolinyl, thieno[2,3-c]pyridinyl, pyrazolo[3,4-6]pyridinyl,
pyrazolo[3,4-c]pyridinyl,
pyrazolo[4,3-c]pyridine, pyrazolo[4,3-6]pyridinyl, tetrazolyl, chromane, 2,3-
dihydrobenzofuran,
tetrahydroquinoline, and isoindoline. In some embodiments, the heteroaryl is
selected from
thienyl, pyridinyl, fury!, pyrazolyl, imidazolyl, isoindolinyl, pyranyl,
pyrazinyl, and pyrimidinyl.
As used herein, "heterocyclyl" or "heterocycloalkyl" means a 3-14 membered,
such as 3-
11 membered, such as 3-8 membered nonaromatic mono-, bi- or tricyclic group
comprising at
least one heteroatom in the ring system backbone. Bicyclic and tricyclic
heterocyclyl groups may
include fused ring systems, spirocyclic ring systems, and bridged ring systems
and may include
multiple fused rings. In some embodiments, heterocyclyls have one to four
heteroatom(s)
independently selected from N, 0, and S. In some embodiments, heterocyclyls
have one to three
heteroatom(s) independently selected from N, 0, and S. In some embodiments,
heterocyclyls
have one to two heteroatom(s) independently selected from N, 0, and S. In some
embodiments,
monocyclic heterocyclyls are 3-membered rings. In some embodiments, monocyclic
heterocyclyls are 4-membered rings. In some embodiments, monocyclic
heterocyclyls are 5-
membered rings. In some embodiments, monocyclic heterocyclyls are 6-membered
rings. In
some embodiments, monocyclic heterocyclyls are 7-membered rings. As used
herein,
"monocyclic heterocyclyl" means a single nonaromatic cyclic ring comprising at
least one
heteroatom in the ring system backbone. Examples of heterocyclyls include
azirinyl, aziridinyl,
azetidinyl, oxetanyl, thietanyl, 1,4,2-dithiazolyl, dihydropyridinyl, 1,3-
dioxanyl, 1,4-dioxanyl, 1,3-
dioxolanyl, morpholinyl, thiomorpholinyl, piperazinyl, pyranyl, pyrrolidinyl,
tetrahydrofuryl,
tetrahydropyridinyl, oxazinyl, thiazinyl, thiinyl, thiazolidinyl,
isothiazolidinyl, oxazolidinyl,
isoxazolidinyl, piperidinyl, pyrazolidinyl imidazolidinyl, and
thiomorpholinyl. In some
embodiments, the heterocyclyl is selected from azetidinyl, morpholinyl,
piperazinyl, pyrrolidinyl,
and tetrahydropyridinyl. As used herein, "bicyclic heterocyclyl" means a
nonaromatic bicyclic ring
system comprising at least one heteroatom in the ring system backbone.
Examples of bicyclic
11
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
heterocyclyls include 2-azabicyclo[1.1.0] butane, 2-
azabicyclo[2.1.0]pentane, 2-
azabicyclo[1.1.I]pentane, 3-azabicyclo[3.1.0]hexane, 5-
azabicyclo[2.1.1]hexane, 3-
azabicyclo[3.2.0]heptane, 2-oxa-5-azabicyclo[2.2.1]heptane,
octahydrocyclopenta[c]pyrrole, 3-
azabicyclo[4.1.0]heptane, 7-azabicyclo[2.2.1]heptane, 6-
azabicyclo[3.1.1]heptane, 7-
azabicyclo[4.2.0]octane, and 2-azabicyclo[2.2.2]octane. As used herein,
"spirocyclic
heterocycly1" means a nonaromatic bicyclic ring system comprising at least one
heteroatom in
the ring system backbone and with the rings connected through just one atom.
Examples of
spirocyclic heterocyclyls include 2-azaspiro[2.2]pentane, 2-oxa-6-
azaspiro[3.3]heptane, 4-
azaspiro[2.5]octane, 1-azaspiro[3.5]nonane, 2-azaspiro[3.5]nonane, 7-
azaspiro[3.5]nonane, 2-
azaspiro[4.4]nonane, 6-azaspiro[2.6]nonane, 1,7-
diazaspiro[3.5]nonane, 2,7-
diazaspiro[3.5]nonane, 1,7-diazaspiro[4.5]decane, 2,5-
diazaspiro[3.6]decane, 1-oxa-8-
azaspiro[4.5]decane, 2-oxa-8-azaspiro[4.5]decane.
Examples of heterocycloalkyls include azetidinyl, azetidiny1-3-ol, 3-
fluoroazetidinyl,
pyrrolidinyl, pyrrolidiny1-3-ol, 3-methoxypyrrolidinyl, 2-(pyrrolidin-3-
yl)acetic acid, piperidinyl,
piperidiny1-4-ol, 2-(piperidin-4-yl)acetic acid, 4-methoxypiperidinyl,
piperazinyl, piperaziny1-1-
carbaldehyde, 1-methylpiperaziny1-2-one, 1-(piperazin-1-yl)ethan-1 -
one, 1-
(methylsulfonyl)piperazinyl, 2-
hydroxy-1 -(piperazin-1-yl)etha n-1-one, 2-oxo-2-(piperazin-1-
yl)acetic acid, morpholinyl, 3-azabicyclo[3.1.0]hexanyl, 2-oxa-5-
azabicyclo[2.2.1]heptanyl, 3-oxa-
6-azabicyclo[3.1.1]heptanyl, octahydropyrrolo[1,2-a]pyrazinyl,
hexahydropyrrolo[1,2-a]pyrazinyl-
6(2H)-one, hexahydro-3H-oxazolo[3,4-a]pyrazinyl, hexahydro-3H-oxazolo[3,4-
a]pyraziny1-3-one,
1,4-oxazepany1-7-one, 2-oxa-6-azaspiro[3.3]heptanyl, 6-oxa-1-
azaspiro[3.3]heptanyl, 1,7-
diazaspiro[3.5]nonanyl, 1,7-diazaspiro[3.5]nonany1-2-one, 2,7-
diazaspiro[3.5]nonanyl, 2,7-
diazaspiro[3.5]nonany1-1-one, 1-oxa-8-azaspiro[4.5]decanyl, 1-oxa-8-
azaspiro[4.5]decany1-2-
one, 2-oxa-8-azaspiro[4.5]decanyl, and 2-oxa-8-azaspiro[4.5]decany1-1-one.
It is appreciated that certain features of the invention, which are, for
clarity, described in
the context of separate embodiments, may also be provided in combination in a
single
embodiment. Conversely, various features of the invention, which are, for
brevity, described in
the context of a single embodiment, may also be provided separately or in any
suitable
subcombination. In addition, subcombinations of uses and medical indications
listed in the
embodiments describing such uses and medical indications described herein, are
also
specifically embraced by the present invention just as if each and every
subcombination of uses
and medical indications was individually and explicitly recited herein.
PROCESSES
The present disclosure is directed to, inter alia, processes useful in the
preparation of a
crystalline form of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide, a modulator of the 5-HT2A serotonin
receptor.
12
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
The present invention provides, inter alia, processes for preparing compounds
of
Formula (Ile):
R2
0
R3
R1 1(N
I -3
He R4 N
comprising the step of:
coupling the compound of Formula (lid) or a salt thereof:
R2
0
R3
H2N I )\I
IId R4 N
wherein R2 is selected from 4-6 membered heterocycloalkyl, (Ci-C3 alkylene)-(4-
10
membered heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein R2A and R2B,
taken together
with the nitrogen to which they are attached, form a 3-9 membered
heterocycloalkyl ring; and;
R3 and R4 are each independently selected from H, C1-C6 alkyl, and C1-C6
haloalkyl;
with an acyl chloride of Formula (lid-1);
0
R1)L'CI
lid-1
wherein R1 is selected from R1 is selected from C1-C6 alkyl, C3-C6 cycloalkyl,
phenyl, 5-10
membered heteroaryl, and 5-9 membered heterocycloalkyl;
in the presence of a base and a solvent to form said compound of Formula
(Ile).
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)):
N)
0
0
la
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide comprising the steps of:
13
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
a) contacting
said N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) with an acid, in the
presence of a
contacting-step solvent;
b) crystallizing N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) to obtain a
crystalline salt of N-(3-
(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Formula (la)) in a crystallizing mixture; and
c) isolating said crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-
(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) from said
crystallizing mixture to
obtain said crystalline salt of
N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)).
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the process
further
comprises stirring after the contacting step.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the stirring
is conducted at
a temperature of about 20 C to about 75 C.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the stirring
is conducted at
a temperature of about 30 C to about 65 C.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the stirring
is conducted at
a temperature of about 30 C to about 55 C.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the stirring
is conducted at
a temperature of about 30 C to about 45 C.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the
contacting-step solvent
is selected from a group consisting of acetone, acetonitrile, 1-butanol, 2-
butanol, butyl acetate,
1,2-dimethoxyethane, N,N-dimethylacetamide, 1,4-dioxane, ethanol, 2-
ethoxyethanol, ethyl
acetate, isopropyl acetate, heptane, methyl isobutyl ketone (MIBK), 2-methyl-1-
propanol, N-
14
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
methyl pyrrolidone, 1-propanol, 2-propanol, n-propyl acetate, tert-butyl
methyl ether (TBME),
tetrahydrofuran (THF), water, and dimethyl sulfoxide (DMSO) and combinations
thereof.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the acid is a
mineral acid or
an organic acid.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the mineral
acid is selected
from a group consisting of hydrochloric acid, phosphoric acid, and sulfuric
acid.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein the organic
acid is selected
from a group consisting of benzenesulfonic acid, benzoic acid, citric acid,
fumaric acid, maleic
acid, methanesulfonic acid, succinic acid, and p-toluenesulfonic acid.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein isolating
comprises filtering
said crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide from said crystallizing mixture.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein isolating
comprises
removing said crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide from said crystallizing mixture.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein isolating
comprises drying
said crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide from said crystallizing mixture.
In some embodiments, drying is conducted at a temperature of about 25 C to
about 90
C. In some embodiments, drying is conducted at a temperature of about 25 C to
about 85 C.
In some embodiments, drying is conducted at a temperature of about 35 C to
about 75 C. In
some embodiments, drying is conducted at a temperature of about 35 C to about
45 C.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein after
isolating, said
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide has a chemical purity of about 95% or
greater.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein after
isolating, said
crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide has a chemical purity of about 98% or
greater.
One aspect of the present disclosure relates to processes for preparing a
crystalline salt
of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolid in-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)), wherein after
isolating, said
crystalline salt of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide has a chemical purity of about 99% or
greater.
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)) prepared by a process described herein.
One aspect of the present disclosure relates to processes of making a
composition
comprising mixing a crystalline form of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) as described herein
with a
phamaceutically acceptable carrier.
One aspect of the present disclosure relates to processes of making a
composition further
comprising forming the composition into drug product, such as, a tablet, a
pill, a powder, a
lozenge, a sachet, a cachet, an elixir, a suspension, an emulsion, a solution,
a syrup, a soft gelatin
capsule, a hard gelatin capsule, a suppository, a sterile injectable solution,
or a sterile packaged
powder.
PROCESSES OF THE INVENTION
The present invention is directed, inter alia, to processes and intermediates
useful in the
preparation of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide of Formula (la) and/or salts related
thereto.
Representative coupling, alkylation, and reduction steps, and intermediates of
Formulae
(11b), (11c), and (11d) of the present invention are provided below in Schemes
1-111.
16
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Scheme I
SF
,OH
R3 02N R3
LG1 OH
I
__________________________ 02N I )1 Coupling Step
R-A N
Ha lib
Scheme 11
R2
o
R2¨OH
R3 R3
1113-1
02N N _______ - 02N
Hb
Alkylation Step Ilc A %
R4 N R- N
Scheme III
R2 R2
o o
R3 R3
Reduction Step
02N N H2N N
I I
He R4 N lid R4 N
Representative amide formation step and intermediate of Formulae (11d) of the
present
invention are provided below in Scheme IV.
Scheme IV
R2 R2
0 0
R3 0 0 R3
R1J-N
Cl
H2N I NY ____________________________ N'Y
R4 Amide Formation Step R4 N
lid Ile
One aspect of the present invention pertains to processes, such as those
exemplified by
Schemes 1, II, III, and IV (supra), that involve Compounds (11b), (11c),
(11d), and (Ile).
One aspect of the present invention pertains to intermediates, Compounds
(11b), (11c),
(11d), and (Ile), as exemplified in Schemes 1, II, III, and IV (supra), useful
in the preparation of
Compounds of Formula (Ile), (la) and/or a salt related thereto, a salt of
compound of Formula
(la).
One aspect of the present invention pertains to intermediates as exemplified
in Schemes
1, II, III, and IV (supra), that involve Compounds of formulae (11b), (11c),
(11d), and (Ile), wherein:
17
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
R1 is selected from R1 is selected from C1-C6 alkyl, C3-C6 cycloalkyl, phenyl,
5-10
membered heteroaryl, and 5-9 membered heterocycloalkyl;
wherein R2 is selected from 4-6 membered heterocycloalkyl, (Ci-C3 alkylene)-(4-
10
membered heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein the alkylene
and
heterocycloalkyl are each optionally substituted with one or more substituents
independently
selected from halogen, oxo, -OH, (Ci-C3 alkyl), -0-(Ci-C3 alkyl), (Ci-C3
alkylene)-C(0)0H, -
C(0)H, -C(0)0H, -C(0)(Ci-C3 alkyl), -C(0)(Ci-C3 alkylene)-0H, -C(0)C(0)0H, and
-S02(Ci-C3
alkyl);
R2A and R2B are each independently selected from H, C1-C3 alkyl, C1-C3
haloalkyl, and C3-
C6 cycloalkyl;
or wherein R2A and R2B, taken together with the nitrogen to which they are
attached, form
a 3-9 membered heterocycloalkyl ring optionally substituted with one or more
substituents
independently selected from halogen, oxo, -OH, Cl-C3 alkyl, (Ci-C3 haloalkyl),
-0-(Ci-C3 alkyl),
(Ci-C3 alkylene)-C(0)0H, -C(0)H, -C(0)(Ci-C3 alkyl), -C(0)(Ci-C3 alkylene)-0H,
-C(0)C(0)0H,
and -S02(Ci-C3 alkyl), and optionally containing one additional heteroatom
selected from the
group of N, 0, and S; and;
R3 and R4 are each independently selected from H, C1-C6 alkyl, and C1-C6
haloalkyl; and
LG1 comprises the groups Cl, Br, I, TfO, or Ts0.
In some embodiments, R1 is selected from C1-C6 alkyl, C3-C6 cycloalkyl, and 5-
10
membered heteroaryl.
In some embodiments, R1 is selected from C1-C6 alkyl, and C3-C6 cycloalkyl.
In some embodiments, R1 is selected C3-C6 cycloalkyl and 5-10 membered
heteroaryl.
In some embodiments, R1 is C3-C6 cycloalkyl.
In some embodiments, R2 is selected from (Ci-C3 alkylene)-(4-10 membered
heterocycloalkyl) and (Ci-C3 alkylene)-NR2AR2B, wherein the alkylene and
heterocycloalkyl are
each optionally substituted with one or more substituents independently
selected from halogen,
oxo, -OH, (Ci-C3 alkyl), and -0-(Ci-C3 alkyl);
R2A and R2B are each independently selected from H, C1-C3 alkyl, C1-C3
haloalkyl, and C3-
C6 cycloalkyl; or wherein R2A and R2B, taken together with the nitrogen to
which they are attached,
form a 3-7 membered heterocycloalkyl ring optionally substituted with one or
more substituents
independently selected from halogen, oxo, -OH, C1-C3 alkyl, (Ci-C3 haloalkyl),
and -0-(Ci-C3
alkyl).
In some embodiments, R2 is selected from azetidinyl, pyrrolidinyl, and
piperidinyl, each of
which is optionally substituted with one or more substituents independently
selected from
halogen, oxo, -OH, (Ci-C3 alkyl), and -0-(Ci-C3 alkyl).
In some embodiments, R2 is selected from azetidinyl, pyrrolidinyl, and
piperidinyl, each of
which is optionally substituted with one or more C1-C3 alkyl.
18
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, R2 is (Ci-C3 alkylene)-(4-10 membered heterocycloalkyl)
and the
4-10 membered heterocycloalkyl is selected from azetidinyl, pyrrolidinyl,
piperidinyl, piperazinyl,
morpholinyl, 3-azabicyclo[3.1.0]hexanyl, 2-
oxa-5-azabicyclo[2.2.1]heptanyl, 3-oxa-6-
azabicyclo[3.1.1]heptanyl, octahydropyrrolo[1,2-a]pyrazinyl,
hexahydro-3H-oxazolo[3,4-
a]pyrazinyl, 1,4-oxazepanyl, 2-oxa-6-azaspiro[3.3]heptanyl, 6-oxa-1-
azaspiro[3.3]heptanyl, 1,7-
diazaspiro[3.5]nonanyl, 2,7-diazaspiro[3.5]nonanyl, 1-oxa-8-
azaspiro[4.5]decanyl, and 2-oxa-8-
azaspiro[4.5]decanyl, each of which is optionally substituted with one or more
substituents
independently selected from halogen, oxo, -OH, (Ci-C3 alkyl), and -0-(Ci-C3
alkyl).
In some embodiments, R2 is an optionally substituted (Ci-C3 alkyl)-(4-10
membered
heterocycloalkyl), where the 4-10 membered heterocycloalkyl is selected from
azetidinyl,
azetidiny1-3-ol, 3-fluoroazetidinyl, pyrrolidinyl, pyrrolidiny1-3-ol, 3-
methoxypyrrolidinyl, 2-
(pyrrolidin-3-yl)acetic acid, piperidinyl, piperidinyl-4-ol, 2-(piperidin-4-
yl)acetic acid, 4-
methoxypiperidinyl, piperazinyl, piperazinyl-1-carbaldehyde, 1-
methylpiperaziny1-2-one, 1-
(piperazin-1-yl)ethan-1-one, 1-(methylsulfonyl)piperazinyl, 2-hydroxy-1-
(piperazin-1-yl)ethan-1-
one, 2-oxo-2-(piperazin-1-yl)acetic acid, morpholinyl, 3-
azabicyclo[3.1.0]hexanyl, 2-oxa-5-
azabicyclo[2.2.1]heptanyl, 3-oxa-6-azabicyclo[3.1.1]heptanyl,
octahydropyrrolo[1,2-a]pyrazinyl,
hexahydropyrrolo[1,2-a]pyraziny1-6(2H)-one, hexahydro-3H-oxazolo[3,4-
a]pyrazinyl, hexahydro-
3H-oxazolo[3,4-a]pyraziny1-3-one, 1,4-oxazepany1-7-one, 2-oxa-6-
azaspiro[3.3]heptanyl, 6-oxa-
1-azaspiro[3.3]heptanyl, 1,7-diazaspiro[3.5]nonanyl, 1,7-
diazaspiro[3.5]nonany1-2-one, 2,7-
.. diazaspiro[3.5]nonanyl, 2,7-diazaspiro[3.5]nonany1-1-one, 1-oxa-8-
azaspiro[4.5]decanyl, 1-oxa-
8-azaspiro[4.5]decany1-2-one, 2-oxa-8-azaspiro[4.5]decanyl, and 2-oxa-8-
azaspiro[4.5]decanyl-
1-one.
In some embodiments, R2 is (Ci-C3 alkylene)-(4-10 membered heterocycloalkyl)
and the
4-10 membered heterocycloalkyl is pyrrolidinyl optionally substituted with one
or more
substituents independently selected from halogen, oxo, -OH, (Ci-C3 alkyl), and
-0-(Ci-C3 alkyl).
In some embodiments, R2 is (Ci-C3 alkylene)-NR2AR2B, wherein R2A and R2B,
taken
together with the nitrogen to which they are attached, form a 3-7 membered
heterocycloalkyl ring.
In some embodiments, R2A and R2B, taken together with the nitrogen to which
they are
attached, form a pyrrolidinyl ring optionally substituted with one or more
substituents
independently selected from ¨OH, C1-C3 alkyl, and -0-(Ci-C3 alkyl).
In some embodiments, R2 is (Ci-C3 alkylene)-NR2AR2B.
In some embodiments, R2 is CH2-NR2AR2B.
In some embodiments, R2 is (CH2)2-NR2AR2B.
In some embodiments, R2 is (CH2)3-NR2AR2B.
In some embodiments, R3 and R4 are each independently selected from H and Cl-
C6 alkyl.
In some embodiments, R3 and R4 are each C1-C6 alkyl.
In some embodiments, R3 and R4 are each C1-C3 alkyl.
In some embodiments, R3 and R4 are each methyl.
19
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, LG1 is selected from the group consisting Cl, Br, I, and
Tf0.
In some embodiments, LG1 is selected from the group consisting Cl, Br, and I.
In some embodiments, LG1 is Br or I.
In some embodiments, LG1 is Br.
One aspect of the present invention pertains to a compound of Formula (Ile):
R2
0
0 R3
R11( N
H II 'y
He R4 N
wherein: R1 is C3-C6 cycloalkyl; R2 is (Ci-C3 alkylene)-NR2AR213, wherein R2A
and R2B,
taken together with the nitrogen to which they are attached, form a 3-7
membered
heterocycloalkyl ring; and
R3 and R4 are each C1-C6 alkyl.
In some embodiments, R1 is cyclopropyl.
In some embodiments, R2 is
In some embodiments, R3 and R4 are each methyl.
It is appreciated that certain features of the invention, which are, for
clarity, described in
the context of separate embodiments, may also be provided in combination in a
single
embodiment. Conversely, various features of the invention, which are, for
brevity, described in
the context of a single embodiment, may also be provided separately or in any
suitable
subcombination. All combinations of the embodiments pertaining to the chemical
groups
represented by the variables (e.g., LG1, R17
R3, and R4) contained within the generic chemical
formulae described herein are specifically embraced by the present invention
just as if each and
every combination was individually explicitly recited, to the extent that such
combinations
embrace stable compounds (i.e., compounds that can be isolated, characterized
and tested for
biological activity). In addition, all subcombinations of the chemical groups
listed in the
embodiments describing such variables, as well as all subcombinations of uses
and medical
indications described herein, are also specifically embraced by the present
invention just as if
each and every subcombination of chemical groups and subcombination of uses
and medical
indications was individually and explicitly recited herein.
I. Coupling Step
One aspect of the present invention pertains to processes for preparing the
intermediate
compound of Formula (lib):
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
R3
02N
lib R4I =
comprising reacting (2-fluoro-5-nitrophenyl)boronic acid with a compound of
Formula (11a):
R3
LG1
RL1-
IIa
wherein R3 and R4 are each independently selected from H, C1-C6 alkyl, and C1-
C6
haloalkyl; and LG1 comprises the groups Cl, Br, I, TfO, or Ts0;
in the presence of:
i) a palladium catalyst;
ii) a base; and
iii) a solvent;
to form compound of Formula (11b).
In some embodiments, R3 and R4 are each independently selected from H and C1-
C6 alkyl.
In some embodiments, R3 and R4 are each C1-C6 alkyl.
In some embodiments, R3 and R4 are each C1-C3 alkyl.
In some embodiments, R3 and R4 are each methyl.
In some embodiments, LG1 is selected from the group consisting Cl, Br, and I.
In some embodiments, LG1 is Br or I.
In some embodiments, LG1 is Br.
In some embodiments, the palladium-based catalyst comprises (2-
Dicyclohexylphosphino-2',6'-dimethoxybiphenyl) [2-
(2'-amino-1,1'-biphenyl)]palladium(11)
methanesulfonate (SPhos Pd G3), 2-Dicyclohexylphosphino-2',4',6'-triisopropy1-
1,1'-bipheny1)[2-
(2'-amino-1,1'-biphenyl)]palladium(11) methanesulfonate (XPhos Pd G3),
Methanesulfonato(2-
dicyclohexylphosphino-2',4',6'-tri-i-propy1-1,1'-biphenyl)(2-methylamino-1,1'-
biphenyl-2-
yl)palladium(l I)
(XPhos Pd G4), Chloro(2-dicyclohexylphosphino-2',4',6'-triisopropy1-1,1'-
bipheny1)[2-(2'-amino-1,1'-biphenyl)]palladium(l I) (XPhos Pd
G2), Chloro(2-
dicyclohexylphosphino-2',6'-dimethoxy-1,1'-bipheny1)[2-(2'-amino-1,1'-
biphenyl)]palladium(11)
(SPhos Pd G2), or [(2-Di-tert-butylphosphino-2',4',6'-triisopropy1-1,1'-
biphenyl)-2-(2'-amino-1,1'-
biphenyl)] palladium(II) methanesulfonate (tBuXPhos Pd G3).
In some embodiments, the base comprises dipotassium hydrogenphosphate
(K2HPO4),
potassium bicarbonate (KHCO3), potassium carbonate (K2CO3), potassium
dihydrogen
phosphate (KH2PO4), potassium phosphate (K3PO4), potassium tert-butoxide
(KOtBu), sodium
bicarbonate (NaHCO3), or sodium carbonate (Na2CO3).
21
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, the solvent comprises acetonitrile; 2-
methyltetrahydrofuran;
tetrahydrofuran (THF); THF-water; THF-water with ethanol; THF-water with 2-
propanol; or
to
In some embodiments, the solvent is 2-methyltetrahydrofuran; tetrahydrofuran
(THF); or
THF-water.
In some embodiments, the solvent is THF-water; THF-water with 10% ethanol; or
THF-
water with 10% 2-propanol.
In some embodiments, said reacting step is conducted at a temperature of about
40 C
to about 100 C.
In some embodiments, said reacting step is conducted at a temperature of about
50 C
to about 100 C.
In some embodiments, said reacting step is conducted at a temperature of about
60 C
to about 100 C.
In one embodiment, a compound of Formula (11b-1):
02N I 'y
(11b-1)
is prepared by process according to the coupling step.
Alkylation Step
One aspect of the present invention pertains to processes for preparing the
intermediate
compound of Formula (11c) or salt thereof:
R2
0
R3
02N lic
R4 N
comprising the step of alkylating a compound of Formula (11b-1):
R2-0H
IIb-1
with the compound of Formula (11b):
R3
02N
I
lib Ra N
wherein R2 is selected from 4-6 membered heterocycloalkyl, (Ci-C3 alkylene)-(4-
10
membered heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein the alkylene
and
heterocycloalkyl are each optionally substituted with one or more substituents
independently
selected from halogen, oxo, -OH, (Ci-C3 alkyl), and -0-(Ci-C3 alkyl);
22
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
R2A and R2B are each independently selected from H, C1-C3 alkyl, C1-C3
haloalkyl, and C3-
C6 cycloalkyl; or wherein R2A and R2B, taken together with the nitrogen to
which they are attached,
form a 3-7 membered heterocycloalkyl ring optionally substituted with one or
more substituents
independently selected from halogen, oxo, -OH, C1-C3 alkyl, (Ci-C3 haloalkyl),
and -0-(Ci-C3
alkyl); and;
R3 and R4 are each independently selected from H, C1-C6 alkyl, and C1-C6
haloalkyl; and
optionally followed by treatment with a mineral acid in presence of an
alcoholic solvent to
form the salt of the compound of Formula (11c).
In some embodiments, R2 is (Ci-C3 alkylene)-NR2AR2B, wherein R2A and R2B,
taken
together with the nitrogen to which they are attached, form a 3-5 membered
heterocycloalkyl ring.
In some embodiments, R2A and R2B, taken together with the nitrogen to which
they are
attached, form a pyrrolidinyl ring optionally substituted with one or more
substituents
independently selected from ¨OH, Cl-C3 alkyl, and -0-(Ci-C3 alkyl).
In some embodiments, R2 is (Ci-C3 alkylene)-NR2AR2B.
In some embodiments, R2 is CH2-NR2AR2B.
In some embodiments, R2 is (CH2)2-NR2AR2B.
In some embodiments, R2 is (CH2)3-NR2AR2B.
In some embodiments, R3 and R4 are each independently selected from H and C1-
C6 alkyl.
In some embodiments, R3 and R4 are each C1-C3 alkyl.
In some embodiments, R3 and R4 are each methyl.
In some embodiments, the alkylating step is done in the presence of an
alkylating-step
base and an alkylating-step solvent.
In some embodiments, the alkylating step comprises a alkylating-step base
comprises
calcium carbonate (CaCO3), cesium carbonate (Cs2CO3), N,N-
diisopropylethylamine (DIPEA),
dipotassium hydrogenphosphate (K2HPO4), potassium bicarbonate (KHCO3),
potassium
carbonate (K2CO3), potassium dihydrogen phosphate (KH2PO4), potassium
phosphate (K3PO4),
potassium tert-butoxide (KOtBu), sodium bicarbonate (NaHCO3), sodium carbonate
(Na2CO3), or
triethylamine.
In some embodiments, the alkylating-step solvent comprises acetonitrile, N,N-
dimethylacetamide, N ,N-d imethylformamide (DMF),
dimethylsulfoxide (DMSO),
hexamethylphosphoric triamide (HMPA), tetrahydrofuran (THF) or mixtures
thereof.
In some embodiments, said alkylating step is conducted at a temperature of
about 25 C
to about 100 C.
In some embodiments, said alkylating step is conducted at a temperature of
about 45 C
to about 100 C.
In some embodiments, said alkylating step is conducted at a temperature of
about 55 C
to about 100 C.
23
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, said alkylating step is conducted at a temperature of
about 65 C
to about 100 C.
In some embodiments, alkylating atep is followed by treatment with a mineral
acid in
presence of an alcoholic solvent to form the salt of the compound of Formula
(11c).
In some embodiments, alkylating atep is followed by treatment with HCI in
presence of
methanol to form the hydrochloride salt of the compound of Formula (11c).
In some embodiments, alkylating atep is followed by treatment with HCI in
presence of 2-
propanol to form the hydrochloride salt of the compound of Formula (11c).
In one embodiment, a compound of Formula (11c-1) or a hydrochloride salt
thereof:
0
02N I )\I
IIc-1
is prepared by process according to the alkylation step.
Reduction Step
One aspect of the present invention pertains to processes for preparing the
intermediate
compound of Formula (11d) or salt thereof:
R2
0
R3
H2N
I
IId Ra N
wherein R2 is selected from 4-6 membered heterocycloalkyl, (Ci-C3 alkylene)-(4-
10 membered heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein the
alkylene and
heterocycloalkyl are each optionally substituted with one or more substituents
independently
selected from halogen, oxo, -OH, (Ci-C3 alkyl), and -0-(Ci-C3 alkyl);
R2A and R2B are each independently selected from H, Cl-C3 alkyl, Cl-C3
haloalkyl, and
C3-C6 cycloalkyl; or wherein R2A and R2B, taken together with the nitrogen to
which they
are attached, form a 3-7 membered heterocycloalkyl ring optionally substituted
with one
or more substituents independently selected from halogen, oxo, -OH, Cl-C3
alkyl, (Ci-C3
haloalkyl), and -0-(Ci-C3 alkyl); and; R3 and R4 are each independently
selected from H,
C1-C6 alkyl, and C1-C6 haloalkyl;
is prepared by process comprising the step of reducing a compound of Formula
(11c) or a
salt thereof:
24
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
R2
0
R'
02N I )\I
11cR
wherein R2 is selected from 4-6 membered heterocycloalkyl, (Ci-C3 alkylene)-(4-
10
membered heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein R2A and R2B,
taken together
with the nitrogen to which they are attached, form a 3-7 membered
heterocycloalkyl ring; and;
R3 and R4 are each independently selected from H, C1-C6 alkyl, and C1-C6
haloalkyl.
In some embodiments, R2 is selected from (Ci-C3 alkylene)-(4-10 membered
heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein R2A and R2B, taken
together with the
nitrogen to which they are attached, form a 3-5 membered heterocycloalkyl
ring.
In some embodiments, R2A and R2B, taken together with the nitrogen to which
they are
attached, form a pyrrolidinyl ring optionally substituted with one or more
substituents
independently selected from ¨OH, C1-C3 alkyl, and -0-(Ci-C3 alkyl).
In some embodiments, R2 is (Ci-C3 alkylene)-NR2AR2B.
In some embodiments, R2 is CH2-NR2AR2B.
In some embodiments, R2 is (CH2)2-NR2AR2B.
In some embodiments, R2 is (CH2)3-NR2AR2B.
In some embodiments, R3 and R4 are each independently selected from H and C1-
C6 alkyl.
In some embodiments, R3 and R4 are each C1-C3 alkyl.
In some embodiments, R3 and R4 are each methyl.
In some embodiments, the reducing step is done in the presence of a reducing-
step agent
and a reducing-step solvent.
In some embodiments, the reducing step comprises a reducing-step agent is
selected
from the group consisting of:
a) iron (Fe) and ammonium chloride (NI-14C1); or
b) hydrogen and palladium/carbon (Pd/C); or
c) sodium dithionite.
In some embodiments, the reducing step comprises a reducing-step solvent
comprises
dimethylsulfoxide (DMSO), ethanol, 2-propanol, water, or mixtures thereof.
In some embodiments, the said reducing is conducted at a temperature of about
15 C to
about 90 C
In some embodiments, the said compound of Formula (11c) or salt thereof is:
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
N)
0
R3
02N
I
R4 N =
wherein R3 and R4 are each independently selected from H and C1-C6 alkyl;
the reducing-step agent comprises hydrogen and palladium/carbon (Pd/C); and
the reducing-step solvent is ethanol and water.
In one embodiment, a compound of Formula (lid-1) or a hydrochloride salt
thereof:
0
H2N I
is prepared by process according to the reduction step.
IV. Amide Formation Step
One aspect of the present invention pertains to processes for preparing the
intermediate
compound of Formula (Ile) or salt thereof:
R2
0
0 R'
R1J(N
I 'y
He R4 N
comprising the step of:
coupling the compound of Formula (lid) or a salt thereof:
R2
0
R3
H2N I )\I
lid R4 N
wherein R2 is selected from 4-6 membered heterocycloalkyl, (Ci-C3 alkylene)-(4-
10
membered heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein the alkylene
and
heterocycloalkyl are each optionally substituted with one or more substituents
independently
selected from halogen, oxo, -OH, (Ci-C3 alkyl), and -0-(Ci-C3 alkyl);
26
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
R2A and R2B are each independently selected from H, C1-C3 alkyl, C1-C3
haloalkyl, and C3-
C6 cycloalkyl; or wherein R2A and R2B, taken together with the nitrogen to
which they are attached,
form a 3-9 membered heterocycloalkyl ring optionally substituted with one or
more substituents
independently selected from halogen, oxo, -OH, C1-C3 alkyl, (Ci-C3 haloalkyl),
and -0-(Ci-C3
alkyl); and;
R3 and R4 are each independently selected from H, C1-C6 alkyl, and C1-C6
haloalkyl;
with an acyl chloride of Formula (lid-1)
0
R1)CCI
lid-1
wherein R1 is selected from R1 is selected from C1-C6 alkyl, C3-C6 cycloalkyl,
phenyl, 5-10
membered heteroaryl, and 5-9 membered heterocycloalkyl;
in the presence of a base and a solvent to form said compound of Formula
(Ile).
In some embodiments, R2 is selected from (Ci-C3 alkylene)-(4-10 membered
heterocycloalkyl), and (Ci-C3 alkylene)-NR2AR2B, wherein the alkylene and
heterocycloalkyl are
each optionally substituted with one or more substituents independently
selected from halogen,
oxo, -OH, (Ci-C3 alkyl), and -0-(Ci-C3 alkyl);
R2A and R2B are each independently selected from H, C1-C3 alkyl, C1-C3
haloalkyl, and C3-
C6 cycloalkyl; or wherein R2A and R2B, taken together with the nitrogen to
which they are attached,
form a 3-7 membered heterocycloalkyl ring optionally substituted with one or
more substituents
independently selected from halogen, oxo, -OH, C1-C3 alkyl, (Ci-C3 haloalkyl),
and -0-(Ci-C3
alkyl); and; R3 and R4 are each independently selected from H and Cl-C6 alkyl.
In some embodiments, R2 is (Ci-C3 alkylene)-NR2AR2B, wherein R2A and R2B,
taken
together with the nitrogen to which they are attached, form a 3-5 membered
heterocycloalkyl ring.
In some embodiments, R2A and R2B, taken together with the nitrogen to which
they are
attached, form a pyrrolidinyl ring optionally substituted with one or more
substituents
independently selected from ¨OH, C1-C3 alkyl, and -0-(Ci-C3 alkyl).
In some embodiments, R2 is (Ci-C3 alkylene)-NR2AR2B.
In some embodiments, R2 is CH2-NR2AR2B.
In some embodiments, R2 is (CH2)2-NR2AR2B.
In some embodiments, R2 is (CH2)3-NR2AR2B.
In some embodiments, R3 and R4 are each independently selected from H and C1-
C6
alkyl.
In some embodiments, R3 and R4 are each C1-C3 alkyl.
In some embodiments, R3 and R4 are each methyl.
In some embodiments, R1 is selected from R1 is selected from C1-C6 alkyl and
C3-C6
cycloalkyl.
27
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, the base is selected from the group consisting of N,N-
dimethylpyridin-4-amine, 2,6-Dimethylpyridine, N-ethyl-N-isopropylpropan-2-
amine, potassium
carbonate (K2CO3), potassium phosphate (K3PO4), potassium hydrogen phosphate
(K2HPO4),
pyridine, and triethylamine.
In some embodiments, the base is selected from the group consisting of
potassium
carbonate (K2CO3), potassium phosphate (K3PO4), potassium hydrogen phosphate
(K2HPO4),
and triethylamine.
In some embodiments, the solvent is selected from the group consisting of
acetonitrile,
dichloromethane (DCM), dimethylformamide (DMF), dimethylacetamide (DMA),
dimethylsulfoxide (DMSO), 1 ,4-d ioxane, 2-methyltetrahydrofuran , tetrahyd
rofu ran and
dichloromethane-water mixture.
In some embodiments, the said compound of Formula (lid) or salt thereof is:
N)
0
R3
H2N N
I
R4 N =
wherein R3 and R4 are each independently selected from H and C1-C6 alkyl;
the base comprises potassium carbonate, potassium phosphate, potassium
hydrogen
phosphate, or triethylamine; and
the solvent comprises dichloromethane, 2-methyltetrahydrofuran,
tetrahydrofuran or
dichloromethane-water mixture.
In some embodiments, R3 and R4 are each methyl.
In some embodiments, R1 is cyclopropyl.
In some embodiments, the base is potassium carbonate.
In some embodiments, the solvent is dichloromethane and water mixture.
In one embodiment, a compound of Formula (la) or a hydrochloride salt thereof:
0
0
V)L11 I ,J11
(Ia)
is prepared by process according to the amide formation step.
In one embodiment, the compound of Formula (la) is crystalline.
28
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
The processes described herein can be monitored according to any suitable
method
known in the art. For example, product formation can be monitored by
spectroscopic means, such
as nuclear magnetic resonance spectroscopy (e.g., 1H or 13C), infrared
spectroscopy,
spectrophotometry (e.g., UV-visible), or mass spectrometry, or by
chromatography such as high
performance liquid chromatography (HPLC) or thin layer chromatography.
In some embodiments, preparation of compounds can involve the protection and
deprotection of various chemical groups. The need for protection and
deprotection, and the
selection of appropriate protecting groups can be readily determined by one
skilled in the art. The
chemistry of protecting groups can be found, for example, in Greene and Wuts,
Protective Groups
in Organic Synthesis, 3rd Ed., Wiley & Sons, 1999.
The reactions of the processes described herein can be carried out in suitable
solvents
which can be readily selected by one of skill in the art of organic synthesis.
Suitable solvents can
be substantially nonreactive with the starting materials (reactants), the
intermediates, or products
at the temperatures at which the reactions are carried out, e.g., temperatures
which can range
from the solvent's freezing temperature to the solvent's boiling temperature.
A given reaction can
be carried out in one solvent or a mixture of more than one solvent. Depending
on the particular
reaction step, suitable solvents for a particular reaction step can be
selected. In some
embodiments, reactions can be carried out in the absence of solvent, such as
when at least one
of the reagents is a liquid or gas.
Suitable solvents can include halogenated solvents such as: carbon
tetrachloride,
bromodichloromethane, dibromochloromethane, bromoform, chloroform,
bromochloromethane,
dibromomethane, butyl chloride, dichloromethane, tetrachloroethylene,
trichloroethylene, 1,1,1-
trichloroethane, 1,1,2-trichloroethane, 1,1-dichloroethane, 2-chloropropane,
hexafluorobenzene,
1,2,4-trichlorobenzene, 1,2-dichlorobenzene, 1,3-
dichlorobenzene, 1,4-dichlorobenzene,
chlorobenzene, fluorobenzene, fluorotrichloromethane, chlorotrifluoromethane,
bromotrifluoromethane, carbon tetrafluoride, dichlorofluoromethane,
chlorodifluoromethane,
trifluoromethane, 1,2-dichlorotetrafluorethane and hexafluoroethane.
Suitable solvents can include ether solvents, such as: dimethoxymethane,
tetrahydrofuran, 2-mthyltetrahydrofuran, 1,3-dioxane, 1,4-dioxane, furan,
diethyl ether, ethylene
glycol dimethyl ether, ethylene glycol diethyl ether, diethylene glycol
dimethyl ether, diethylene
glycol diethyl ether, triethylene glycol dimethyl ether, anisole, or t-butyl
methyl ether.
Suitable solvents can include protic solvents, such as: water, methanol,
ethanol, 2-
nitroethanol, 2-fluoroethanol, 2,2,2-trifluoroethanol, ethylene glycol, 1-
propanol, 2-propanol, 2-
methoxyethanol, 1-butanol, 2-butanol, isobutyl alcohol, t-butyl alcohol, 2-
ethoxyethanol,
diethylene glycol, 1-, 2-, or 3- pentanol, neo-pentyl alcohol, t-pentyl
alcohol, diethylene glycol
monomethyl ether, diethylene glycol monoethyl ether, cyclohexanol, benzyl
alcohol, phenol, or
glycerol.
29
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Suitable solvents can include aprotic solvents, such as: benzene, cyclohexane,
pentane,
hexane, toluene, cycloheptane, methylcyclohexane, heptane, ethylbenzene, o, m-
, or p-xylene,
octane, indane, nonane, naphthalene, tetrahydrofuran, acetonitrile, dimethyl
sulfoxide,
propionitrile, ethyl formate, methyl acetate, hexachloroacetone, acetone,
ethyl methyl ketone,
ethyl acetate, isopropyl acetate, sulfolane, 1,3-dimethy1-3,4,5,6-tetrahydro-
2(1
1,3-dimethy1-2-imidazolidinone, N-methylpyrrolidinone, tetramethylurea,
nitromethane, and
nitrobenzene, and amides, including but not limited to, N,N-dimethylformamide,
N,N-
dimethylacetamide, formamide, N-methylacetamide, N-
methylformamide, N,N-
dimethylpropionamide, and hexamethylphosphoramide. It is understood by a
person of ordinary
skill in the art that that the term amide refers to the following formula:
0
RAN,R'
R"
wherein R, R', and R" may be the same or different. In some embodiments, R,
R', and R"
are each independently selected from H and C1-C6 alkyl. In some embodiments,
R, R', and R"
are each independently selected from H and C1-C4 alkyl. In some embodiments,
R, R', and R"
.. are each independently selected from H and C1-C2 alkyl.
Supercritical carbon dioxide can also be used as a solvent.
The reactions of the processes described herein can be carried out at
appropriate
temperatures which can be readily determined by one skilled in the art.
Reaction temperatures
will depend on, for example, the melting and boiling points of the reagents
and solvent, if present;
the thermodynamics of the reaction (e.g., vigorously exothermic reactions may
need to be carried
out at reduced temperatures); and the kinetics of the reaction (e.g., a high
activation energy
barrier may need elevated temperatures).
The reactions of the processes described herein can be carried out in air or
under an inert
atmosphere. Typically, reactions containing reagents or products that are
substantially reactive
with air can be carried out using air-sensitive synthetic techniques that are
well known to one
skilled in the art.
In some embodiments, preparation of compounds can involve the addition of
acids or
bases to effect, for example, catalysis of a desired reaction or formation of
salt forms such as
acid addition salts.
Example acids can be inorganic or organic acids. Inorganic acids include
hydrochloric
acid, hydrobromic acid, sulfuric acid, phosphoric acid, and nitric acid.
Organic acids include formic
acid, acetic acid, trifluoroacetic acid, propionic acid, butanoic acid,
methanesulfonic acid, p-
toluene sulfonic acid, benzenesulfonic acid, propiolic acid, butyric acid, 2-
butynoic acid, vinyl
acetic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid,
nonanoic acid and
decanoic acid.
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Example bases include lithium hydroxide, sodium hydroxide, potassium
hydroxide, lithium
carbonate, sodium carbonate, and potassium carbonate. Some example strong
bases include,
but are not limited to, hydroxide, alkoxides, metal amides, metal hydrides,
metal dialkylamides
and arylamines, wherein; alkoxides include lithium, sodium and potassium salts
of methyl, ethyl
and t-butyl oxides; metal amides include sodium amide, potassium amide and
lithium amide;
metal hydrides include sodium hydride, potassium hydride and lithium hydride;
and metal
dialkylamides include sodium and potassium salts of methyl, ethyl, n-propyl,
isopropyl, n-butyl, t-
butyl, trimethylsilyl and cyclohexyl substituted amides.
The compounds described herein can be asymmetric (e.g., having one or more
stereocenters). All stereoisomers, such as enantiomers and diastereomers, are
intended unless
otherwise indicated. Salts of the present invention that contain
asymmetrically substituted carbon
atoms can be isolated in optically active or racemic forms. Methods on how to
prepare optically
active forms from optically active starting materials are known in the art,
such as by resolution of
racemic mixtures or by stereoselective synthesis.
The processes described herein can be stereoselective such that any given
reaction
starting with one or more chiral reagents enriched in one stereoisomer forms a
product that is
also enriched in one stereoisomer. The reaction can be conducted such that the
product of the
reaction substantially retains one or more chiral centers present in the
starting materials. The
reaction can also be conducted such that the product of the reaction contains
a chiral center that
is substantially inverted relative to a corresponding chiral center present in
the starting materials.
Resolution of racemic mixtures of compounds can be carried out by any of
numerous
methods known in the art. An example method includes fractional
recrystallization (for example,
diastereomeric salt resolution) using a "chiral resolving acid" which is an
optically active, salt-
forming organic acid. Suitable resolving agents for fractional
recrystallization methods are, for
example, optically active acids, such as the D and L forms of tartaric acid,
diacetyltartaric acid,
dibenzoyltartaric acid, mandelic acid, malic acid, lactic acid or the various
optically active
camphorsulfonic acids such as p-camphorsulfonic acid. Other resolving agents
suitable for
fractional crystallization methods include stereoisomerically pure forms of p-
methylbenzylamine
(e.g., S and R forms, or diastereomerically pure forms), 2-phenylglycinol,
norephedrine,
ephedrine, N-methylephedrine, cyclohexylethylamine, 1,2-diaminocyclohexane,
and the like.
Resolution of racemic mixtures can also be carried out by elution on a column
packed
with an optically active resolving agent (e.g., dinitrobenzoylphenylglycine).
Suitable elution
solvent composition can be determined by one skilled in the art.
The compounds described herein and salts thereof can also include all isotopes
of atoms
occurring in the intermediates or final compounds or salts thereof. Isotopes
include those atoms
having the same atomic number but different mass numbers. For example,
isotopes of hydrogen
include tritium and deuterium.
31
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
The compounds described herein and salts thereof can also include tautomeric
forms,
such as keto-enol tautomers. Tautomeric forms can be in equilibrium or
sterically locked into one
form by appropriate substitution.
Upon carrying out preparation of compounds according to the processes
described
herein, the usual isolation and purification operations such as concentration,
filtration, extraction,
solid-phase extraction, recrystallization, chromatography, and the like may be
used, to isolate the
desired products.
Crystalline Salts of Compound of Formula (la)
One aspect of the present disclosure relates to crystalline salts of N-(3-(4,6-
dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline besylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline besylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline citrate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)).
One aspect of the present disclosure relates to a crystalline fumarate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline hydrochloride
salt of N-(3-
(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Formula (la)).
One aspect of the present disclosure relates to a crystalline mesylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline phosphate salt
of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
One aspect of the present disclosure relates to a crystalline succinate salt
of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)).
One aspect of the present disclosure relates to a crystalline tosylate salt of
N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
32
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Crystalline salts described herein can be identified by their unique solid
state signature
with respect to, for example, differential scanning calorimetry (DSC), X-ray
powder diffraction
(PXRD), and other solid state methods.
Further characterization with respect to water or solvent content of
crystalline forms can
be gauged by any of the following methods for example, thermogravimetric
analysis (TGA), DSC
and the like.
For DSC, it is known that the temperatures observed will depend upon sample
purity, the
rate of temperature change, as well as sample preparation technique and the
particular
instrument employed. Thus, the values reported herein relating to DSC
thermograms can vary by
plus or minus about 4 C. The values reported herein relating to DSC
thermograms can also vary
by plus or minus about 20 joules per gram.
In some embodiments, the DSC thermogram values reported herein relate to
desolvation
events. When DSC thermogram values reported herein relate to desolvation
events, the values
reported herein are estimates. Scan rate and pan closure can influence DSC
values for
desolvation events, which can vary by plus or minus about 25 C. DSC values
for desolvation
events reported herein were recorded using a sample in an aluminum pan with an
uncrimped lid
and a scan rate of 10 C/min.
For PXRD, the relative intensities of the peaks can vary, depending upon the
sample
preparation technique, the sample mounting procedure and the particular
instrument employed.
Moreover, instrument variation and other factors can often affect the 28
values. Therefore, the
peak assignments of diffraction patterns can vary by plus or minus 0.2 28
(i.e., 0.2).
For TGA, the features reported herein can vary by plus or minus about 5 C.
The TGA
features reported herein can also vary by plus or minus about 2% weight change
due to, for
example, sample variation.
1. Compound la (Crystalline Form).
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The physical properties of the crystalline form of Compound la
are summarized
in Table 1 below.
33
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Table 1
Compound la (Crystalline Form, Example 4)
Figure 1: Peaks of about E 9.9% relative intensity at 5.3 0.2 ,
10.6 0.2 , 12.4 0.2 , 14.9 0.2 , 15.9 0.2 , 16.2 0.2 ,
PXRD
16.8 0.2 , 18.8 0.2 , 19.0 0.2 , 19.8 0.2 , 20.1 0.2 ,
21.1 0.2 , 22.0 0.2 , 23.3 0.2 , and 24.9 0.2"2E
TGA Figure 3: Decrease in weight
of about 3.6% out to about 240 C
DSC Figure 2: Endotherm
extrapolated onset temperature: about
140.1 C
Certain X-ray powder diffraction peaks for the crystalline form of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la) are shown in Table 2 below.
Table 2
d-spacing d-spacing Rel. Int.
[A]
Pos. [ 2 O.] Rel. Int. ro] Pos. [ 2 O.]
5.3 16.8 21.1 22.5 3.9 4.3
10.6 8.4 9.9 23. 3.8 35.1
11.4 7.8 6.2 23.9 3.7 3.8
12.4 7.1 42.4 24.9 3.6 21.9
12.7 7.0 5.3 25.3 3.5 4.6
13.0 6.8 6.0 25.9 3.4 8.9
14.0 6.3 2.9 26.9 3.3 5.
14.9 5.9 12.3 27.4 3.2 3.0
15.9 5.6 13.3 27.8 3.2 1.9
16.2 5.5 23.4 28.1 3.2 2.4
16.8 5.3 100 28.6 3.1 3.6
17.2 5.2 4.6 29.0 3.1 5.7
17.8 5.0 4.5 30.2 3.0 4.1
18.8 4.7 13.3 31.2 2.9 1.7
19.0 4.7 26.5 31.8 2.8 1.2
19.8 4.5 15.3 32.7 2.7 2.3
20.1 4.4 17.8 33.2 2.7 2.7
21.1 4.2 60.5 33.9 2.6 3.1
22.0 4.0 56.9 34.7 2.6 1.4
22.2 4.0 12.1
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide.
34
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide, wherein
the crystalline form has an X-ray owder diffraction pattern comprising a peak,
in terms of 28, at
5.3 0.2 , and 10.6 0.2 . In some embodiments, the crystalline form has
an X-ray owder
diffraction pattern comprising a peak, in terms of 28, at 5.3 0.2 , 10.6
0.2 , and 12.4 0.2 .
In some embodiments, the crystalline form has an X-ray powder diffraction
pattern comprising
peaks, in terms of 28, at 5.3 0.2 , 10.6 0.2 , 12.4 0.2 , 14.9 0.2
, and 15.9 0.2 . In
some embodiments, the crystalline form has an X-ray powder diffraction pattern
comprising
peaks, in terms of 28 at 5.3 0.2 , 10.6 0.2 , 12.4 0.2 , 14.9 0.2
, 15.9 0.2 , 16.2
0.2 , 16.8 0.2 , 18.8 0.2 , and 19.0 0.2 . In some embodiments, the
crystalline form has
an X-ray powder diffraction pattern comprising peaks, in terms of 28, at 5.3
0.2 , 10.6 0.2 ,
12.4 0.2 , 14.9 0.2 , 15.9 0.2 , 16.2 0.2 , 16.8 0.2 , 18.8
0.2 , and 19.0 0.2 ,
19.8 0.2 , 20.1 0.2 , and 21.1 0.2 . In some embodiments, the
crystalline form has an X-
ray powder diffraction pattern comprising peaks, in terms of 28, at 5.3 0.2
, 10.6 0.2 , 12.4
0.2 , 14.9 0.2 , 15.9 0.2 , 16.2 0.2 , 16.8 0.2 , 18.8 0.2 ,
and 19.0 0.2 , 19.8
0.2 , 20.1 0.2 , and 21.1 0.2 , 22.0 0.2 , 23.3 0.2 , and 24.9
0.2 .
In some embodiments, the crystalline form has an X-ray powder diffraction
pattern
substantially as shown in Figure 1, wherein by "substantially" is meant that
the reported peaks
can vary by about 0.2 28.
In some embodiments, the crystalline form has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
138.1 C and about 153.5 C. In some embodiments, the crystalline form has a
differential
scanning calorimetry thermogram comprising an endotherm with an extrapolated
onset
temperature between about 138.1 C and about 152.5 C. In some embodiments,
the crystalline
form has a differential scanning calorimetry thermogram comprising an
endotherm with an
extrapolated onset temperature between about 139.1 C and about 152.5 C. In
some
embodiments, the crystalline form has a differential scanning calorimetry
thermogram comprising
an endotherm with an extrapolated onset temperature at about 140.1 C. In some
embodiments,
the crystalline form has a differential scanning calorimetry thermogram
substantially as shown in
Figure 2, wherein by "substantially" is meant that the reported DSC features
can vary by about
4 C and that the reported DSC features can vary by about 20 joules per
gram.
In some embodiments, the crystalline form has a thermogravimetric analysis
profile
showing about 4.0% weight loss below about 240 C. In some embodiments, the
heptane solvate
has a thermogravimetric analysis profile showing about 3.8% weight loss below
about 240 C. In
some embodiments, the heptane solvate has a thermogravimetric analysis profile
showing about
3.6% weight loss below about 240 C. In some embodiments, the crystalline form
has a
thermogravimetric analysis profile substantially as shown in Figure 3, wherein
by "substantially"
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
is meant that the reported TGA features can vary by about 5 C, and that
that the reported TGA
features can vary by about 2% weight change.
One aspect of the present disclosure relates to the crystalline form having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 20, at
.3 0.2 ,
10.6 0.2 , 12.4 0.2 , 14.9 0.2 , 15.9 0.2 , 16.2 0.2 , 16.8
0.2 , 18.8 0.2 , and
19.00 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 138.1 C and about 153.5 C;
and/or
c) a thermogravimetric analysis profile showing about 4.0% weight loss
below about
240 C.
One aspect of the present disclosure relates to the crystalline form having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 20 at 5.3
0.2 ,
10.6 0.2 , 12.4 0.2 , 14.9 0.2 , 15.9 0.2 , 16.2 0.2 , 16.8
0.2 , 18.8 0.2 , and
19.00 0.2 , 19.8 0.2 , 20.10 0.2 , and 21.10 0.2";
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 138.1 C and about 152.5 C;
and/or
c) a thermogravimetric analysis profile showing about 3.8% weight loss
below about
240 C.
One aspect of the present disclosure relates to the crystalline form having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 20 at 5.3 0.2 ,
10.6 0.2 , 12.4 0.2 , 14.9 0.2 , 15.9 0.2 , 16.2 0.2 , 16.8
0.2 , 18.8 0.2 , and
19.00 0.2 , 19.8 0.2 , 20.10 0.2 , and 21.10 0.2 , 22.0 0.2 , 23.3
0.2 , and 24.9
0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 139.1 C and about 152.5 C;
and/or
c) a
thermogravimetric analysis profile showing about 3.6% weight loss below about
240 C.
One aspect of the present disclosure relates to the crystalline form having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 20 at
5.3 0.2 ,
10.6 0.2 , 12.4 0.2 , 14.9 0.2 , 15.9 0.2 , 16.2 0.2 , 16.8
0.2 , 18.8 0.2 , and
19.00 0.2 , 19.8 0.2 , 20.10 0.2 , and 21.10 0.2 , 22.0 0.2 , 23.3
0.2 , and 24.9
0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature at about 140.1 C; and/or
c) a
thermogravimetric analysis profile showing about 3.6% weight loss below about
240 C.
One aspect of the present disclosure relates to the crystalline form having:
a) an X-ray powder diffraction pattern substantially as shown in
Figure 1;
36
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
2; and/or
c) a thermogravimetric analysis profile substantially as shown in Figure 3.
Compound la (Crystalline Salts).
One aspect of the present disclosure relates to crystalline salts of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(1a)).
The crystalline salts of Compound la are characterized by PXRD. The physical
properties
for the crystalline salts as determined by PXRD are summarized below.
2. Compound la (Besylate Salt)
One aspect of the present disclosure relates to besylate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The besylate salt is characterized by PXRD. The physical
properties for the
besylate salt as determined by PXRD are summarized in Table 3 below.
Table 3
Compound la (Besylate Salt, Example 5)
Figure 4: Peaks of about 10.7 `)/0 relative intensity at 8.8 0.2 ,
10.3 0.2 , 10.8 0.2 , 11.30 0.2 , 11.6 0.2 , 11.90 0.2 ,
PXRD 14.0 0.2 , 14.3 0.2 , 14.7 0.2 , 16.8 0.2 ,
18.0 0.2 ,
18.5 0.2 , 20.8 0.2 , 21.70 0.2 , 23.3 0.2 , 24.0 0.2 ,
24.3 0.2 , 25.2 0.2 , and 26.4 0.2"28
The physical properties for a besylate salt (Example 5) prepared using
procedure from
Example 5 are summarized in Table 4 below.
Table 4
Compound la (Besylate Salt, Example 5)
TGA Figure 6: Decrease in weight of about 2.1% out to about 220
C
DSC Figure 5: Endotherms extrapolated onset temperature at
about
145 C and at about 172 C
Certain X-ray powder diffraction peaks for the besylate salt of N-(3-(4,6-
dimethylpyrimidin-
5-y1)-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound
la) are shown in
Table 5 below.
37
CA 03235907 2024-04-17
WO 2023/067520 PCT/IB2022/060059
Table 5
d-spacing Rel. Int. Pos. d-spacing
Pos. [ 20.] Rel. Int. [0/
[A] [0/] [0201 [A] 0]
0
6.9 12.8 1.9 21.7 4.1 15.2
8.8 10.1 10.9 22.2 4.0 8.2
10.3 8.6 28.0 22.7 3.9 40.0
10.8 8.2 13.3 23.3 3.8 13.5
11.3 7.9 42.4 23.8 3.7 11.6
11.6 7.6 17.9 24.0 3.7 13.2
11.9 7.4 13.1 24.3 3.7 15.6
13.0 6.8 7.7 24.7 3.6 12.3
13.6 6.5 10.2 25.2 3.5 13.6
14.0 6.3 20.1 26.4 3.4 10.7
14.3 6.2 20.3 27.1 3.3 6.5
14.7 6.0 45.1 27.4 3.3 5.4
15.9 5.6 4.8 28.4 3.1 7.1
16.5 5.4 11.4 29.4 3.0 2.1
16.8 5.3 37.3 31.0 2.9 1.4
17.6 5.0 3.7 31.4 2.8 2.5
18.0 4.9 12.5 31.7 2.8 3.7
18.5 4.8 70.1 32.0 2.8 3.1
18.7 4.7 11.1 32.6 2.7 2.5
20.2 4.4 4.5 33.3 2.7 1.8
20.8 4.3 95.5 34.0 2.6 2.3
21.3 4.2 8.9
One aspect of the present disclosure relates to besylate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The besylate salt of Compound la was prepared by procedure from
Example 5.
One aspect of the present disclosure relates to a besylate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide, having a
powder X-ray diffraction pattern comprising a peak, in terms of 20, at 8.8
0.2 , 10.3 0.2 ,
10.8 0.2 , 11.3 0.2 , and 11.6 0.2 . In some embodiments, the
besylate salt has an X-
ray powder diffraction pattern comprising peaks, in terms of 20, at 8.8 0.2
, 10.3 0.2 , 10.8
0.2 , 11.3 0.2 , 11.6 0.2 , 11.9 0.2 , 14.0 0.2 , 14.3 0.2 ,
14.7 0.2 , 16.8
0.2 , and 18.0 0.2 .
In some embodiments, the besylate salt has an X-ray powder diffraction pattern
comprising peaks, in terms 0f28, at 8.8 0.2 , 10.3 0.2 , 10.8 0.2 ,
11.3 0.2 , 11.6
0.2 , 11.9 0.2 , 14.0 0.2 , 14.3 0.2 , 14.7 0.2 , 16.8 0.2 ,
18.0 0.2 , 18.5 0.2 ,
38
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
20.8 0.2 , and 21.7 0.2 . In some embodiments, the besylate salt has an
X-ray powder
diffraction pattern comprising peaks, in terms of 28, at 8.8 0.2 , 10.3
0.2 , 10.8 0.2 , 11.3
0.2 , 11.6 0.2 , 11.9 0.2 , 14.0 0.2 , 14.3 0.2 , 14.7 0.2 ,
16.8 0.2 , 18.0
0.2 , 18.5 0.2 , 20.8 0.2 , 21.7 0.2 , 23.3 0.2 , and 24.0 0.2
. In some embodiments,
the besylate salt has an X-ray powder diffraction pattern comprising peaks, in
terms of 28, at 8.8
0.2 , 10.3 0.2 , 10.8 0.2 , 11.3 0.2 , 11.6 0.2 , 11.9 0.2 ,
14.0 0.2 , 14.3
0.2 , 14.7 0.2 , 16.8 0.2 , 18.0 0.2 , 18.5 0.2 , 20.8 0.2 ,
21.7 0.2 , 23.3 0.2 ,
24.0 0.2 , 24.3 0.2 , 25.2 0.2 , and 26.4 0.2 . In some
embodiments, the besylate salt
has an X-ray powder diffraction pattern substantially as shown in Figure 4,
wherein by
"substantially" is meant that the reported peaks can vary by about 0.2 28.
In some embodiments, the besylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
217.4 C and about 228.7 C. In some embodiments, the besylate salt has a
differential scanning
calorimetry thermogram comprising an endotherm with an extrapolated onset
temperature
between about 217.4 C and about 227.7 C. In some embodiments, the besylate
salt has a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated
onset temperature between about 218.4 C and about 227.7 C. In some
embodiments, the
besylate salt has a differential scanning calorimetry thermogram comprising an
endotherm with
an extrapolated onset temperature between about 218.4 C and about 226.7 C.
In some
embodiments, the besylate salt has a differential scanning calorimetry
thermogram comprising
endotherms with an extrapolated onset temperature at about 219.4 C. In some
embodiments,
the besylate salt has a differential scanning calorimetry thermogram
substantially as shown in
Figure 5, wherein by "substantially" is meant that the reported DSC features
can vary by about
4 C and that the reported DSC features can vary by about 20 joules per
gram.
In some embodiments, the besylate salt has a thermogravimetric analysis
profile showing
about 2.6% weight loss below about 220 C. In some embodiments, the besylate
salt has a
thermogravimetric analysis profile showing about 2.4% weight loss below about
220 C. In some
embodiments, the besylate salt has a thermogravimetric analysis profile
showing about 2.2%
weight loss below about 220 C. In some embodiments, the besylate salt has a
thermogravimetric
analysis profile showing about 2.1% weight loss below about 220 C. In some
embodiments, the
besylate salt has a thermogravimetric analysis profile substantially as shown
in Figure 6, wherein
by "substantially" is meant that the reported TGA features can vary by about
5 C, and that that
the reported TGA features can vary by about 2% weight change.
One aspect of the present disclosure relates to the besylate salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 8.8 0.2 ,
10.3 0.2 , 10.8 0.2 , 11.3 0.2 , 11.6 0.2 , 11.9 0.2 , 14.0
0.2 , 14.3 0.2 , 14.7
0.2 , 16.8 0.2 , and 18.0 0.2 ;
39
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 217.4 C and about 227.7 C.
c) a thermogravimetric analysis profile showing about 2.6% weight loss
below about
220 C.
One aspect of the present disclosure relates to the besylate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
8.8 0.2 ,
10.3 0.2 , 10.8 0.2 , 11.3 0.2 , 11.6 0.2 , 11.90 0.2 , 14.0
0.2 , 14.3 0.2 , 14.7
0.2 , 16.8 0.2 , 18.0 0.2 , 18.5 0.2 , 20.8 0.2 , and 21.7
0.2";
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 218.4 C and about 227.7 C;
and/or
c) a thermogravimetric analysis profile showing about 2.4% weight loss
below about
220 C.
One aspect of the present disclosure relates to the besylate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
8.8 0.2 ,
10.3 0.2 , 10.8 0.2 , 11.3 0.2 , 11.6 0.2 , 11.9 0.2 , 14.0
0.2 , 14.3 0.2 , 14.7
0.2 , 16.8 0.2 , 18.0 0.2 , 18.5 0.2 , 20.8 0.2 , 21.7 0.2 ,
23.3 0.2 , 24.0
0.2 , and 25.2 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 218.4 C and about 226.7 C;
and/or
c) a thermogravimetric analysis profile showing about 2.2% weight loss
below about
220 C.
One aspect of the present disclosure relates to the besylate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
8.8 0.2 ,
10.3 0.2 , 10.8 0.2 , 11.3 0.2 , 11.6 0.2 , 11.9 0.2 , 14.0
0.2 , 14.3 0.2 , 14.7
0.2 , 16.8 0.2 , 18.0 0.2 , 18.5 0.2 , 20.8 0.2 , 21.7 0.2 ,
23.3 0.2 , 24.0
0.2 , 24.3 0.2 , 25.2 0.2 , and 26.4 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 218.4 C and about 226.7 C;
and/or
c) a thermogravimetric analysis profile showing about 2.1% weight loss
below about
220 C.
One aspect of the present disclosure relates to the besylate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
8.8 0.2 ,
10.3 0.2 , 10.8 0.2 , 11.3 0.2 , 11.6 0.2 , 11.9 0.2 , 14.0
0.2 , 14.3 0.2 , 14.7
0.2 , 16.8 0.2 , 18.0 0.2 , 18.5 0.2 , 20.8 0.2 , 21.7 0.2 ,
23.3 0.2 , 24.0
0.2 , 24.3 0.2 , 25.2 0.2 , and 26.4 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature at about 219.4 C; and/or
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
c) a thermogravimetric analysis profile showing about 2.1% weight
loss below about
220 C.
One aspect of the present disclosure relates to the besylate salt having:
a) an X-ray powder diffraction pattern substantially as shown in
Figure 4;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
5; and/or
c) a thermogravimetric analysis profile substantially as shown in
Figure 6.
3. Compound la (Citrate Salt).
One aspect of the present disclosure relates to a citrate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The citrate salt of Compound la are characterized by PXRD. The
physical
properties for the citrate salt as determined by PXRD are summarized in Table
6 below.
Table 6
Compound la (Citrate Salt, Example 6)
Figure 7: Peaks of about 6.2% relative intensity at 9.6
0.2 , 12.1 0.2 , 12.6 0.2 , 13.5 0.2 , 13.8 0.2 ,
15.8 0.2 , 16.2 0.2 , 16.6 0.2 , 17.5 0.2 , 18.1
PXRD 0.2 , 18.6 0.2 , 19.1 0.2 , 19.3 0.2 , 20.1
0.2 ,
20.7 0.2 , 21.1 0.2 , 22.3 0.2 , 22.6 0.2 , 23.0
0.2 , 24.0 0.2 , 25.1 0.2 , 25.9 0.2 , 26.1 0.2 , 27.5
0.2 , and 28.4 0.2"28
The physical properties for a citrate salt (Example 6) prepared using
procedure from
Example 6 are summarized in Table 7 below.
Table 7
Compound la (Citrate Salt, Example 6)
TGA Figure 9: No observable weight loss below about 175 C
DSC Figure 8: Endotherms extrapolated onset temperature at
about
172.2 C
Certain X-ray powder diffraction peaks for the citrate salt of N-(3-(4,6-
dimethylpyrimidin-
5-y1)-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound
la) are shown in
Table 8 below.
41
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Table 8
Rel.
Pos. Rel. Int. Pos.
d-spacing [A] d-spacing [A] Int.
[020.] [0/0] [020.] [0/0]
6.7 13.3 1.7 23.4 3.8 3.8
9.6 9.2 38.4 24.0 3.7 26.8
12.1 7.3 6.6 24.4 3.6 8.5
12.6 7.0 48.0 24.6 3.6 6.0
13.5 6.6 100.0 25.1 3.6 16.4
13.8 6.4 6.2 25.9 3.4 20.5
15.8 5.6 29.5 25.9 3.4 26.1
16.2 5.5 7.8 26.1 3.4 16.6
16.6 5.4 29.1 26.5 3.4 5.0
17.5 5.1 17.1 27.5 3.2 20.1
18.1 4.9 28.2 27.9 3.2 7.2
18.6 4.8 23.8 28.4 3.1 11.1
19.1 4.7 50.8 29.4 3.0 3.6
19.3 4.6 24.4 30.4 2.9 5.4
19.8 4.5 4.8 31.0 2.9 5.6
20.1 4.4 20.7 31.6 2.8 6.1
20.7 4.3 18.0 32.2 2.8 2.0
21.1 4.2 44.7 32.9 2.7 7.3
22.3 4.0 41.4 33.5 2.7 6.2
22.6 3.9 21.4 34.3 2.6 4.5
23.0 3.9 74.9 34.7 2.6 2.6
One aspect of the present disclosure relates to a citrate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide.
One aspect of the present disclosure relates to a citrate salt having an X-ray
powder
diffraction pattern comprising a peak, in terms of 20, at 9.6 0.2 , 12.1
0.2 , 12.6 0.2 ,
13.5 0.2 , 13.8 0.2 , and 15.8 0.2 . In some embodiments, the citrate
salt has an X-ray
powder diffraction pattern comprising peaks, in terms of 20, at 9.6 0.2 ,
12.1 0.2 , 12.6
0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2 0.2 , 16.6 0.2 ,
17.5 0.2 , 18.1 0.2 ,
18.6 0.2 , 19.1 0.2 , and 19.3 0.2 . In some embodiments, the citrate
salt has an X-ray
powder diffraction pattern comprising peaks, in terms of 20, at 9.6 0.2 ,
12.1 0.2 , 12.6
0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2 0.2 , 16.6 0.2 ,
17.5 0.2 , 18.1 0.2 ,
18.6 0.2 , 19.1 0.2 , 19.3 0.2 , 20.1 0.2 , 20.7 0.2 , 21.1
0.2 , 22.3 0.2 , 22.6
0.2 , 23.0 0.2 , and 24.0 0.2 . In some embodiments, the citrate salt
has an X-ray powder
diffraction pattern comprising peaks, in terms of 20, at 9.6 0.2 , 12.1
0.2 , 12.6 0.2 , 13.5
0.2 , 13.8 0.2 , 15.8 0.2 , 16.2 0.2 , 16.6 0.2 , 17.5 0.2 ,
18.1 0.2 , 18.6
42
CA 03235907 2024-04-17
WO 2023/067520 PCT/IB2022/060059
0.2 , 19.1 0.2 , 19.3 0.2 , 20.1 0.2 , 20.7 0.2 , 21.1 0.2 ,
22.3 0.2 , 22.6 0.2 ,
23.0 0.2 , 24.0 0.2 , 25.1 0.2 , and 25.9 0.2 . In some embodiments,
the citrate salt has
an X-ray powder diffraction pattern comprising peaks, in terms of 20, at 9.6
0.2 , 12.1 0.2 ,
12.6 0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2 0.2 , 16.6
0.2 , 17.5 0.2 , 18.1
0.2 , 18.6 0.2 , 19.1 0.2 , 19.3 0.2 , 20.1 0.2 , 20.7 0.2 ,
21.1 0.2 , 22.3
0.2 , 22.6 0.2 , 23.0 0.2 , 24.0 0.2 , 25.1 0.2 , 25.9 0.2 , 26.1
0.2 , 27.5 0.2 , and
28.4 0.2 . In some embodiments, the citrate salt has an X-ray powder
diffraction pattern
substantially as shown in Figure 7, wherein by "substantially" is meant that
the reported peaks
can vary by about 0.2 '26
In some embodiments, the citrate salt has a differential scanning calorimetry
thermogram
comprising an endotherm with an extrapolated onset temperature between about
169.2 C and
about 176.5 C.
In some embodiments, the citrate salt has a differential scanning calorimetry
thermogram
comprising an endotherm with an extrapolated onset temperature between about
170.2 C and
about 176.5 C.
In some embodiments, the citrate salt has a differential scanning calorimetry
thermogram
comprising an endotherm with an extrapolated onset temperature between about
171.2 C and
about 175.5 C.
In some embodiments, the citrate salt has a differential scanning calorimetry
thermogram
comprising an endotherm with an extrapolated onset temperature between about
171.2 C and
about 174.5 C.
In some embodiments, the citrate salt has a differential scanning calorimetry
thermogram
comprising an endotherm with an extrapolated onset temperature at about 172.2
C.
In some embodiments, the citrate salt has a differential scanning calorimetry
thermogram
substantially as shown in Figure 8, wherein by "substantially" is meant that
the reported DSC
features can vary by about 4 C and that the reported DSC features can vary
by about 20
joules per gram.
In some embodiments, the citrate salt has a thermogravimetric analysis profile
showing
no observable weight loss below about 175 C.
In some embodiments, the citrate salt has a thermogravimetric analysis profile
substantially as shown in Figure 9, wherein by "substantially" is meant that
the reported TGA
features can vary by about 5 C, and that that the reported TGA features can
vary by about
2% weight change.
One aspect of the present disclosure relates to the citrate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 9.6
0.2 ,
12.1 0.2 , 12.6 0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2
0.2 , 16.6 0.2 , 17.5
0.2 , 18.1 0.2 , 18.6 0.2 , 19.1 0.2 , and 19.3 0.2 ;
43
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 169.2 C and about 176.5 C;
and/or
c) a thermogravimetric analysis profile showing no observable weight loss
below
about 175 C; and/or
One aspect of the present disclosure relates to the citrate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 9.6
0.2 ,
12.1 0.2 , 12.6 0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2
0.2 , 16.6 0.2 , 17.5
0.2 , 18.1 0.2 , 18.6 0.2 , 19.1 0.2 , 19.3 0.2 , 20.1 0.2 ,
20.7 0.2 , 21.1
0.2 , 22.3 0.2 , 22.6 0.2 , 23.0 0.2 , and 24.0 0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 170.2 C and about 176.5 C;
and/or
c) a
thermogravimetric analysis profile showing no observable weight loss below
about 175 C; and/or
One aspect of the present disclosure relates to the citrate salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 9.6 0.2 ,
12.1 0.2 , 12.6 0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2
0.2 , 17.5 0.2 , 18.1
0.2 , 18.6 0.2 , 19.1 0.2 , 19.3 0.2 , 20.1 0.2 , 20.7 0.2 ,
21.1 0.2 , 22.3
0.2 , 22.6 0.2 , 23.0 0.2 , 24.0 0.2 , 25.1 0.2 , and 25.9 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 171.2 C and about 175.5 C;
and/or
c) a thermogravimetric analysis profile showing no observable weight loss
below
about 175 C; and/or
One aspect of the present disclosure relates to the citrate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 9.6
0.2 ,
12.1 0.2 , 12.6 0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2
0.2 , 16.6 0.2 , 17.5
0.2 , 18.1 0.2 , 18.6 0.2 , 19.1 0.2 , 19.3 0.2 , 20.1 0.2 ,
20.7 0.2 , 21.1
0.2 , 22.3 0.2 , 22.6 0.2 , 23.0 0.2 , 24.0 0.2 , 25.1 0.2 ,
25.9 0.2 , 26.1 0.2 ,
27.5 0.2 , and 28.4 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 171.2 C and about 174.5 C;
and/or
c) a thermogravimetric analysis profile showing no observable weight loss
below
about 175 C.
One aspect of the present disclosure relates to the citrate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 9.6
0.2 ,
12.1 0.2 , 12.6 0.2 , 13.5 0.2 , 13.8 0.2 , 15.8 0.2 , 16.2
0.2 , 16.6 0.2 , 17.5
0.2 , 18.1 0.2 , 18.6 0.2 , 19.1 0.2 , 19.3 0.2 , 20.1 0.2 ,
20.7 0.2 , 21.1
0.2 , 22.3 0.2 , 22.6 0.2 , 23.0 0.2 , 24.0 0.2 , 25.1 0.2 ,
25.9 0.2 , 26.1 0.2 ,
27.5 0.2 , and 28.4 0.2';
44
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature at about 172.2 C; and/or
c) a thermogravimetric analysis profile showing no observable weight loss
below
about 175 C
One aspect of the present disclosure relates to the citrate salt having:
a) an X-ray powder diffraction pattern substantially as shown in Figure 7;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
8; and/or
c) a thermogravimetric analysis profile substantially as shown in Figure 9.
4. Compound la (Fumarate Salt).
One aspect of the present disclosure relates to fumarate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The fumarate salt of Compound la are characterized by PXRD. The
physical
properties for the fumarate salt as determined by PXRD are summarized in Table
9 below.
Table 9
Compound 1 (Fumarate Salt, Example 7)
Figure 10: Peaks of about 10.0% relative intensity at 6.9
0.2 , 9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 ,
13.4 0.2 , 13.9 0.2 , 14.7 0.2 , 15.3 0.2 , 15.8
PXRD 0.2 , 16.6 0.2 , 17.0 0.2 , 18.8 0.2 , 19.5
0.2 ,
20.9 0.2 , 21.6 0.2 , 22.6 0.2 , 22.9 0.2 , 23.1
0.2 , 23.5 0.2 , 23.9 0.2 , 26.1 0.2 , and 26.9 0.2
'28
The physical properties for a fumarate salt (Example 7) prepared using
procedure from
Example 7 are summarized in Table 10 below.
Table 10
Compound 1 (Fumarate Salt, Example 7)
TGA Figure 12: Decrease in weight of about 4.9% out to about
200 C
Figure 11: Endotherm extrapolated onset temperature at about
DSC
156.1 C
Certain X-ray powder diffraction peaks for the fumarate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yDethoxy)phenyl)cyclopropanecarboxamide
(Compound la) are shown in Table 11 below.
45
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Table 11
Pos. Rel. Int. Pos. Rel.
d-spacing [A] d-spacing [A] Int.
NO.] [0/0] [ 02O.] [0/0]
6.4 13.7 100.0 15.7 5.6 7.6
7.3 12.0 38.4 18.8 4.7 8.4
7.8 11.3 67.0 19.4 4.6 5.2
10.6 8.4 25.3 21.1 4.2 4.2
11.1 7.9 11.3 21.6 4.1 4.6
12.9 6.9 8.5 22.2 4.0 3.7
13.7 6.5 8.2 23.6 3.8 8.8
14.3 6.2 18.1 26.0 3.4 5.9
14.7 6.0 21.1
One aspect of the present disclosure relates to an fumarate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide.
One aspect of the present disclosure relates to an fumarate salt having an X-
ray powder
diffraction pattern comprising a peak, in terms of 28, at 6.9 0.2 , 9.2
0.2 , 9.3 0.2 , and
12.2 0.2 . In some embodiments, the fumarate salt has an X-ray powder
diffraction pattern
comprising peaks, in terms 0f28, at 6.9 0.2 , 9.2 0.2 , 9.3 0.2 ,
12.2 0.2 , 12.5 0.2 ,
13.4 0.2 , 13.9 0.2 , 14.7 0.2 , 15.3 0.2 , and 15.8 0.2 . In
some embodiments, the
fumarate salt has an X-ray powder diffraction pattern comprising peaks, in
terms of 28, at 6.9
0.2 , 9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 , 13.4 0.2 , 13.9
0.2 , 14.7 0.2 ,
15.3 0.2 , 15.8 0.2 , 16.6 0.2 , 17.0 0.2 , 18.8 0.2 , and 19.5
0.2 . In some
embodiments, the fumarate salt has an X-ray powder diffraction pattern
comprising peaks, in
terms of 28, at 6.9 0.2 , 9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5
0.2 , 13.4 0.2 , 13.9
0.2 , 14.7 0.2 , 15.3 0.2 , 15.8 0.2 , 16.6 0.2 , 17.0 0.2 ,
18.8 0.2 , 19.5
0.2 , 20.9 0.2 , 21.6 0.2 , 22.6 0.2 , and 22.9 0.2 . In some
embodiments, the fumarate
salt has an X-ray powder diffraction pattern comprising peaks, in terms of 28,
at 6.9 0.2 , 9.2
0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 , 13.4 0.2 , 13.9 0.2 ,
14.7 0.2 , 15.3 0.2 ,
15.8 0.2 , 16.6 0.2 , 17.0 0.2 , 18.8 0.2 , 19.5 0.2 , 20.9
0.2 , 21.6 0.2 , 22.6
0.2 , 22.9 0.2 , 23.1 0.2 , 23.5 0.2 , 23.9 0.2 , 26.1 0.2 ,
and 26.9 0.2 In some
embodiments, the fumarate salt has an X-ray powder diffraction pattern
substantially as shown
in Figure 10, wherein by "substantially" is meant that the reported peaks can
vary by about 0.2
2 O.
In some embodiments, the fumarate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
148.3 C and about 159.1 C.
46
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, the fumarate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
149.3 C and about 158.1 C.
In some embodiments, the fumarate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
149.3 C and about 157.1 C.
In some embodiments, the fumarate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
150.3 C and about 157.1 C.
In some embodiments, the fumarate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature at
about 156.1 C.
In some embodiments, the fumarate salt has a differential scanning calorimetry
thermogram substantially as shown in Figure 11, wherein by "substantially" is
meant that the
reported DSC features can vary by about 4 C and that the reported DSC
features can vary by
about 20 joules per gram.
In some embodiments, the fumarate salt has a thermogravimetric analysis
profile showing
about 5.5% weight loss below about 200 C.
In some embodiments, the fumarate has a thermogravimetric analysis profile
showing
about 5.3% weight loss below about 200 C.
In some embodiments, the fumarate salt has a thermogravimetric analysis
profile showing
about 5.1% weight loss below about 200 C.
In some embodiments, the fumarate salt has a thermogravimetric analysis
profile showing
about 4.9% weight loss below about 200 C.
In some embodiments, the fumarate salt has a thermogravimetric analysis
profile
.. substantially as shown in Figure 12, wherein by "substantially" is meant
that the reported TGA
features can vary by about 5 C, and that that the reported TGA features can
vary by about
2% weight change.
One aspect of the present disclosure relates to the fumarate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
6.9 0.2 ,
9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 , 13.4 0.2 , 13.9
0.2 , 14.7 0.2 , 15.3
0.2 , and 15.8 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 148.3 C and about 159.1 C;
and/or
c) a thermogravimetric analysis profile showing about 5.5% weight loss
below about
200 C.
One aspect of the present disclosure relates to the fumarate salt having:
47
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
6.9 0.2 ,
9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 , 13.4 0.2 , 13.9
0.2 , 14.7 0.2 , 15.3
0.2 , 15.8 0.2 , 16.6 0.2 , 17.0 0.2 , 18.8 0.2 , and 19.5
0.2";
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 149.3 C and about 158.1 C;
and/or
c) a thermogravimetric analysis profile showing about 5.3% weight loss
below about
200 C.
One aspect of the present disclosure relates to the fumarate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
6.9 0.2 ,
9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 , 13.4 0.2 , 13.9
0.2 , 15.3 0.2 , 15.8
0.2 , 16.6 0.2 , 17.0 0.2 , 18.8 0.2 , 19.5 0.2 , 20.9 0.2 ,
21.6 0.2 , 22.6 0.2 ,
and 22.9 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 149.3 C and about 157.1 C;
and/or
c) a thermogravimetric analysis profile showing about 5.1% weight loss or
less below
about 200 C.
One aspect of the present disclosure relates to the fumarate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
6.9 0.2 ,
9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 , 13.4 0.2 , 13.9
0.2 , 14.7 0.2 , 15.3
0.2 , 15.8 0.2 , 16.6 0.2 , 17.0 0.2 , 18.8 0.2 , 19.5 0.2 ,
20.9 0.2 , 21.6 0.2 ,
22.6 0.2 , and 22.9 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 150.3 C and about 157.1 C;
and/or
c) a thermogravimetric analysis profile showing about 4.9% weight loss or
less below
about 200 C.
One aspect of the present disclosure relates to the fumarate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
6.9 0.2 ,
9.2 0.2 , 9.3 0.2 , 12.2 0.2 , 12.5 0.2 , 13.4 0.2 , 13.9
0.2 , 14.7 0.2 , 15.3
0.2 , 15.8 0.2 , 16.6 0.2 , 17.0 0.2 , 18.8 0.2 , 19.5 0.2 ,
20.9 0.2 , 21.6 0.2 ,
22.6 0.2 , 22.9 0.2 , 23.1 0.2 , 23.5 0.2 , 23.9 0.2 , 26.1
0.2 , and 26.9 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature at about 156.1 C; and/or
c) a thermogravimetric analysis profile showing about 4.9% weight loss or
less below
about 200 C.
One aspect of the present disclosure relates to the fumarate salt having:
a) an X-ray powder diffraction pattern substantially as shown in Figure 10;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
11; and/or
48
CA 03235907 2024-04-17
WO 2023/067520 PCT/IB2022/060059
c) a
thermogravimetric analysis profile substantially as shown in Figure 12.
5. Compound la (Hydrochloride Salt).
One aspect of the present disclosure relates to a hydrochloride salt of N-(3-
(4,6-
dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The hydrochloride salt of Compound la are characterized by
PXRD. The physical
properties for the fumarate salt as determined by PXRD are summarized in Table
12 below.
Table 12
Compound la (Hydrochloride Salt, Example 8)
Figure 10: Peaks of about 8.3% relative intensity at 12.4
PXRD + 0.2 , 12.8
+ 0.2 , 13.8 + 0.2 , 15.9 + 0.2 , 16.2 + 0.2 ,
18.4 0.2 , 20.8 0.2 , 23.2 0.2 , 23.5 0.2 , 24.8
0.2 , 27.7 0.2 , 27.8 0.2 , and 32.7 0.2"28
The physical properties for a hydrochloride salt (Example 8) prepared using
procedure
from Example 8 are summarized in Table 13 below.
Table 13
Compound la (Hydrochloride Salt, Example 8)
TGA Figure
12: Decrease in weight of about 4.3% out to about 100 C
DSC Figure
11: Endotherms extrapolated onset temperature at about
23.8 C and at about 188.5 C
Certain X-ray powder diffraction peaks for the hydrochloride salt of N-(3-(4,6-
dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la) are shown in Table 14 below.
Table 14
Pos. Rel. Int. Pos. Rel. Int.
d-spacing [A] d-spacing [A]
[ 28.] [0/0] [ 28.] [0/0]
6.4 13.8 9.2 21.4 4.1 3.0
6.9 12.8 7.0 21.9 4.1 3.1
9.0 9.9 6.7 22.2 4.0 5.7
9.6 9.2 1.8 22.9 3.9 3.1
12.0 7.3 6.0 23.2 3.8 18.0
12.4 7.2 61.0 23.5 3.8 16.2
12.5 7.1 7.5 23.9 3.7 4.7
12.8 6.9 28.3 24.8 3.6 27.8
13.8 6.4 10.3 24.8 3.6 26.6
14.1 6.3 2.1 26.6 3.3 3.0
15.9 5.6 55.8 27.7 3.2 100.0
16.2 5.5 65.4 27.8 3.2 51.2
49
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Pos. Rel. Int. Pos. Rel. Int.
d-spacing [A] d-spacing [A]
[020.] [0/0] NO] [0/0]
16.7 5.3 5.7 29.4 3.0 2.3
17.8 5.0 5.2 30.4 2.9 9.9
18.4 4.8 34.0 31.4 2.8 2.6
18.8 4.7 3.7 32.2 2.8 3.3
19.3 4.6 2.0 32.7 2.7 19.6
20.1 4.4 1.8 32.8 2.7 10.7
20.8 4.3 8.3 34.2 2.6 3.8
One aspect of the present disclosure relates to an hydrochloride salt of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide.
One aspect of the present disclosure relates to an hydrochloride salt having
an X-ray
powder diffraction pattern comprising a peak, in terms of 28, at 12.4 0.2 ,
12.8 0.2 , and
13.8 0.2 . In some embodiments, the hydrochloride salt has an X-ray powder
diffraction pattern
comprising peaks, in terms of 28, at 12.4 0.2 , 12.8 0.2 , 13.8 0.2 ,
15.9 0.2 , and
16.2 0.2 .
In some embodiments, the hydrochloride salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 28 at 12.4 0.2 , 12.8 0.2 , 13.8 0.2 ,
15.9 0.2 , 16.2
0.2 , 18.4 0.2 , 20.8 0.2 , 23.2 0.2 , and 23.5 0.2 .
In some embodiments, the hydrochloride salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 28 at 12.4 0.2 , 12.8 0.2 , 13.8 0.2 ,
15.9 0.2 , 16.2
0.2 , 18.4 0.2 , 20.8 0.2 , 23.2 0.2 , 23.5 0.2 , and 24.8 0.2
.
In some embodiments, the hydrochloride salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 28 at 12.4 0.2 , 12.8 0.2 , 13.8 0.2 ,
15.9 0.2 , 16.2
0.2 , 18.4 0.2 , 20.8 0.2 , 23.2 0.2 , 23.5 0.2 , 24.8 0.2 ,
27.7 0.2 , 27.8 0.2 ,
and 32.7 0.2 ..
In some embodiments, the hydrochloride salt has an X-ray powder diffraction
pattern
substantially as shown in Figure 13, wherein by "substantially" is meant that
the reported peaks
can vary by about 0.2 28.
In some embodiments, the hydrochloride salt has a differential scanning
calorimetry
thermogram comprising endotherms with an extrapolated onset temperature
between about 20.8
C and about 77.6 C and between about 185.5 C and about 196.9 C..
In some embodiments, the hydrochloride salt has a differential scanning
calorimetry
thermogram comprising endotherms with an extrapolated onset temperature
between about 21.8
C and about 76.6 C and between about 186.5 C and about 195.9 C.
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, the hydrochloride salt has a differential scanning
calorimetry
thermogram comprising endotherms with an extrapolated onset temperature
between about 21.8
C and about 74.6 C and between about 186.5 C and about 194.9 C.
In some embodiments, the hydrochloride salt has a differential scanning
calorimetry
thermogram comprising endotherms with an extrapolated onset temperature
between about 22.8
C and about 75.6 C and between about 187.5 C and about 194.9 C.
In some embodiments, the hydrochloride salt has a differential scanning
calorimetry
thermogram comprising endotherms with an extrapolated onset temperature at
about 23.8 C
and at about 188.5 C.
In some embodiments, the hydrochloride salt has a differential scanning
calorimetry
thermogram substantially as shown in Figure 14, wherein by "substantially" is
meant that the
reported DSC features can vary by about 4 C and that the reported DSC
features can vary by
about 20 joules per gram.
In some embodiments, the hydrochloride salt has a thermogravimetric analysis
profile
showing about 5.0% weight loss below about 100 C.
In some embodiments, the hydrochloride salt has a thermogravimetric analysis
profile
showing about 4.8% weight loss below about 100 C.
In some embodiments, the hydrochloride salt has a thermogravimetric analysis
profile
showing about 4.6% weight loss below about 100 C.
In some embodiments, the hydrochloride salt has a thermogravimetric analysis
profile
showing about 4.3% weight loss below about 100 C.
In some embodiments, the hydrochloride salt has a thermogravimetric analysis
profile
substantially as shown in Figure 15, wherein by "substantially" is meant that
the reported TGA
features can vary by about 5 C, and that that the reported TGA features can
vary by about
2% weight change.
One aspect of the present disclosure relates to the hydrochloride salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
12.4
0.2 , 12.8 0.2 , 13.8 0.2 , 15.9 0.2 , and 16.2 0.2';
b) a differential scanning calorimetry thermogram comprising endotherms
with an
extrapolated onset temperature between about 20.8 C and about 77.6 C and
between about
185.5 C and about 196.9 C; and/or
c) a thermogravimetric analysis profile showing about 5.0% weight loss
below about
200 C.
One aspect of the present disclosure relates to the hydrochloride salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 12.4
0.2 , 12.8 0.2 , 13.8 0.2 , 15.9 0.2 , 16.2 0.2 , 18.4 0.2 ,
20.8 0.2 , 23.2 0.2 ,
and 23.5 0.2';
51
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
b) a differential scanning calorimetry thermogram comprising endotherms
with an
extrapolated onset temperature between about 21.8 C and about 76.6 C and
between about
186.5 C and about 195.9 C; and/or
c) a thermogravimetric analysis profile showing about 4.8% weight loss
below about
200 C.
One aspect of the present disclosure relates to the hydrochloride salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 20, at 12.4
0.2 , 12.8 0.2 , 13.8 0.2 , 15.9 0.2 , 16.2 0.2 , 18.4 0.2 ,
20.8 0.2 , 23.2 0.2 ,
23.5 0.2 , and 24.8 0.2';
b) a
differential scanning calorimetry thermogram comprising endotherms with an
extrapolated onset temperature between about 21.8 C and about 75.6 C and
between about
186.5 C and about 194.9 C; and/or
c) a
thermogravimetric analysis profile showing about 4.6% weight loss below about
100 C.
One aspect of the present disclosure relates to the hydrochloride salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 20, at
12.4
0.2 , 12.8 0.2 , 13.8 0.2 , 15.9 0.2 , 16.2 0.2 , 18.4 0.2 ,
20.8 0.2 , 23.2 0.2 ,
23.5 0.2 , and 24.8 0.2';
b) a differential scanning calorimetry thermogram comprising endotherms
with an
extrapolated onset temperature between about 22.8 C and about 75.6 C and
between about
187.5 C and about 194.9 C; and/or
c) a thermogravimetric analysis profile showing about 4.3% weight loss or
less below
about 100 C.
One aspect of the present disclosure relates to the hydrochloride salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 12.4
0.2 , 12.8 0.2 , 13.8 0.2 , 15.9 0.2 , 16.2 0.2 , 18.4 0.2 ,
20.8 0.2 , 23.2 0.2 ,
23.5 0.2 , 24.8 0.2 , 27.7 0.2 , 27.8 0.2 , and 32.7 0.2';
b) a
differential scanning calorimetry thermogram comprising endotherms with an
extrapolated onset temperature at about 23.8 C and at about 188.5 C; and/or
c) a
thermogravimetric analysis profile showing about 4.3% weight loss or less
below
about 100 C.
One aspect of the present disclosure relates to the hydrochloride salt having:
a) an X-ray powder diffraction pattern substantially as shown in Figure 13;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
14; and/or
c) a thermogravimetric analysis profile substantially as shown in Figure
15.
52
CA 03235907 2024-04-17
WO 2023/067520 PCT/IB2022/060059
6. Compound 1 (Mesylate Salt).
One aspect of the present disclosure relates to mesylate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The mesylate salt of Compound la are characterized by PXRD. The
physical
properties for the mesylate salt as determined by PXRD are summarized in Table
15 below.
Table 15
Compound la (Mesylate Salt, Example 9)
Figure 16: Peaks of about 9.8% relative intensity at 6.3
0.2 , 9.9 0.2 , 10.5 0.2 , 12.3 0.2 , 12.6 0.2 , 13.6
0.2 , 14.0 0.2 , 14.3 0.2 , 14.9 0.2 , 15.9 0.2 ,
PXRD 16.4 0.2 , 17.6 0.2 , 18.5 0.2 , 18.9 0.2 ,
19.7
0.2 , 20.8 0.2 , 21.1 0.2 , 21.4 0.2 , 22.3 0.2 ,
23.0 0.2 , 23.3 0.2 , 23.5 0.2 , 24.3 0.2 , 24.6
0.2 , 25.3 0.2 , and 27.5 0.2"28
The physical properties for a mesylate salt (Example 9) prepared using
procedure from
Example 9 are summarized in Table 16 below.
Table 16
Compound la (Mesylate Salt, Example 9)
TGA Figure 18: Decrease in weight of about 2.2% out to about
180 C
Figure 17: Endotherm extrapolated onset temperature at about
DSC
181.4 C
Certain X-ray powder diffraction peaks for the methanol solvates of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la) are shown in Table 17 below.
Table 17
Rel.
Pos. Rel. Int. Pos.
d-spacing [A] d-spacing [A] Int.
NO.] [0/0] NO.] [0/0]
6.3 14.1 9.8 21.4 4.1 100.0
9.9 8.9 26.3 22.3 4.0 67.4
10.5 8.4 20.0 23.0 3.9 18.2
12.3 7.2 51.3 23.3 3.8 34.1
12.6 7.0 23.5 23.5 3.8 18.7
13.6 6.5 59.4 24.3 3.7 47.0
14.0 6.3 13.1 24.6 3.6 16.2
14.3 6.2 12.0 25.3 3.5 20.4
14.9 6.0 16.7 26.1 3.4 8.6
16.0 5.5 14.0 26.9 3.3 5.6
53
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Pos. Rel. Int. Pos. Rel.
d-spacing [A] d-spacing [A] Int.
NO.] [0k] [ 02O.] [0/0]
16.4 5.4 41.9 27.5 3.2 13.8
16.9 5.2 5.3 28.1 3.2 6.5
17.6 5.0 43.7 29.4 3.0 6.1
18.5 4.8 21.0 30.8 2.9 11.1
18.9 4.7 46.2 31.4 2.9 5.7
19.2 4.6 8.2 32.3 2.8 4.1
19.7 4.5 14.8 33.1 2.7 6.2
20.8 4.3 42.4 33.6 2.7 1.8
21.1 4.2 26.6
One aspect of the present disclosure relates to an mesylate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide.
One aspect of the present disclosure relates to a mesylate salt having an X-
ray powder
diffraction pattern comprising a peak, in terms of 2 8, at 6.3 0.2 , 9.9
0.2 , 10.5 0.2 , 12.3
0.2 , and 12.6 0.2 . In some embodiments, the mesylate salt has an X-ray
powder diffraction
pattern comprising peaks, in terms of 28, at 6.3 0.2 , 9.9 0.2 , 10.5
0.2 , 12.3 0.2 ,
12.6 0.2 , 13.6 0.2 , 14.0 0.2 , 14.3 0.2 , 14.9 0.2 , 15.9
0.2 , and 16.4 0.2 .
In some embodiments, the mesylate salt has an X-ray powder diffraction pattern
comprising peaks, in terms of 28, at 6.3 0.2 , 9.9 0.2 , 10.5 0.2 ,
12.3 0.2 , 12.6
0.2 , 13.6 0.2 , 14.0 0.2 , 14.3 0.2 , 14.9 0.2 , 15.9 0.2 ,
16.4 0.2 , 17.6 0.2 ,
18.5 0.2 , 18.9 0.2 , 19.7 0.2 , 20.8 0.2 , 21.1 0.2 , and 21.4
0.2 .
In some embodiments, the mesylate salt has an X-ray powder diffraction pattern
comprising peaks, in terms of 28, at 6.3 0.2 , 9.9 0.2 , 10.5 0.2 ,
12.3 0.2 , 12.6
0.2 , 13.6 0.2 , 14.0 0.2 , 14.3 0.2 , 14.9 0.2 , 15.9 0.2 ,
16.4 0.2 , 17.6 0.2 ,
18.5 0.2 , 18.9 0.2 , 19.7 0.2 , 20.8 0.2 , 21.1 0.2 , 21.4
0.2 , 22.3 0.2 , 23.0
0.2 , 23.3 0.2 , and 23.5 0.2 .
In some embodiments, the mesylate salt has an X-ray powder diffraction pattern
comprising peaks, in terms of 28, at 6.3 0.2 , 9.9 0.2 , 10.5 0.2 ,
12.3 0.2 , 12.6
0.2 , 13.6 0.2 , 14.0 0.2 , 14.3 0.2 , 14.9 0.2 , 15.9 0.2 ,
16.4 0.2 , 17.6 0.2 ,
18.5 0.2 , 18.9 0.2 , 19.7 0.2 , 20.8 0.2 , 21.1 0.2 , 21.4
0.2 , 22.3 0.2 , 23.0
0.2 , 23.3 0.2 , 23.5 0.2 , 24.3 0.2 , 24.6 0.2 , 25.3 0.2 ,
and 27.5 0.2 .
In some embodiments, the mesylate salt has an X-ray powder diffraction pattern
substantially as shown in Figure 16, wherein by "substantially" is meant that
the reported peaks
can vary by about 0.2 28.
54
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, the mesylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
178.4 C and about 192.7 C.
In some embodiments, the mesylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
179.4 C and about 192.7 C.
In some embodiments, the mesylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
179.4 C and about 191.7 C.
In some embodiments, the mesylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
180.4 C and about 190.7 C.
In some embodiments, the mesylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature at
about 181.4 C.
In some embodiments, the mesylate salt has a differential scanning calorimetry
thermogram substantially as shown in Figure 17, wherein by "substantially" is
meant that the
reported DSC features can vary by about 4 C and that the reported DSC
features can vary by
about 20 joules per gram.
In some embodiments, the mesylate salt has a thermogravimetric analysis
profile showing
about 2.8% weight loss below about 180 C.
In some embodiments, the mesylate salt has a thermogravimetric analysis
profile showing
about 2.6% weight loss below about 180 C.
In some embodiments, the mesylate salt has a thermogravimetric analysis
profile showing
about 2.4% weight loss below about 180 C.
In some embodiments, the mesylate salt has a thermogravimetric analysis
profile showing
about 2.2% weight loss below about 180 C.
In some embodiments, the mesylate salt has a thermogravimetric analysis
profile
substantially as shown in Figure 18, wherein by "substantially" is meant that
the reported TGA
features can vary by about 5 C, and that that the reported TGA features can
vary by about
2% weight change.
One aspect of the present disclosure relates to the mesylate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 6.3
0.2 ,
9.9 0.2 , 10.5 0.2 , 12.3 0.2 , 12.6 0.2 , 13.6 0.2 , 14.0
0.2 , 14.3 0.2 , 14.9
0.2 , 15.9 0.2 , and 16.4 0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 178.4 C and about 192.7 C;
and/or
c) a
thermogravimetric analysis profile showing about 2.8% weight loss below about
180 C.
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
One aspect of the present disclosure relates to the mesylate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 6.3
0.2 ,
9.9 0.2 , 10.5 0.2 , 12.3 0.2 , 12.6 0.2 , 13.6 0.2 , 14.0
0.2 , 14.3 0.2 , 14.9
0.2 , 15.9 0.2 , 16.4 0.2 , 17.6 0.2 , 18.5 0.2 , 18.9 0.2 ,
19.7 0.2 , 20.8
0.2 , 21.10 0.2 , and 21.40 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 179.4 C and about 192.7 C;
and/or
c) a thermogravimetric analysis profile showing about 2.6% weight loss
below about
180 C.
One aspect of the present disclosure relates to the mesylate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 6.3
0.2 ,
9.9 0.2 , 10.5 0.2 , 12.3 0.2 , 12.6 0.2 , 13.6 0.2 , 14.0
0.2 , 14.3 0.2 , 14.9
0.2 , 15.9 0.2 , 16.4 0.2 , 17.6 0.2 , 18.5 0.2 , 18.9 0.2 ,
19.7 0.2 , 20.8
0.2 , 21.1 0.2 , 21.4 0.2 , 22.3 0.2 , 23.0 0.2 , 23.3 0.2 ,
and 23.5 0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 179.4 C and about 191.7 C;
and/or
c) a
thermogravimetric analysis profile showing about 2.4% weight loss or less
below
about 180 C.
One aspect of the present disclosure relates to the mesylate salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 6.3 0.2 ,
9.9 0.2 , 10.5 0.2 , 12.3 0.2 , 12.6 0.2 , 13.6 0.2 , 14.0
0.2 , 14.3 0.2 , 14.9
0.2 , 15.9 0.2 , 16.4 0.2 , 17.6 0.2 , 18.5 0.2 , 18.9 0.2 ,
19.7 0.2 , 20.8
0.2 , 21.1 0.2 , 21.4 0.2 , 22.3 0.2 , 23.0 0.2 , 23.3 0.2 ,
and 23.5 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 180.4 C and about 190.7 C;
and/or
c) a thermogravimetric analysis profile showing about 2.4% weight loss or
less below
about 180 C.
One aspect of the present disclosure relates to the mesylate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 6.3
0.2 ,
9.9 0.2 , 10.5 0.2 , 12.3 0.2 , 12.6 0.2 , 13.6 0.2 , 14.0
0.2 , 14.3 0.2 , 14.9
0.2 , 15.9 0.2 , 16.4 0.2 , 17.6 0.2 , 18.5 0.2 , 18.9 0.2 ,
19.7 0.2 , 20.8
0.2 , 21.1 0.2 , 21.4 0.2 , 22.3 0.2 , 23.0 0.2 , 23.3 0.2 ,
23.5 0.2 , 24.3 0.2 ,
24.6 0.2 , 25.3 0.2 , and 27.5 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature at about 181.4 C; and/or
c) a thermogravimetric analysis profile showing about 2.2% weight loss or
less below
about 180 C.
One aspect of the present disclosure relates to the mesylate salt having:
56
CA 03235907 2024-04-17
WO 2023/067520 PCT/IB2022/060059
a) an X-ray powder diffraction pattern substantially as shown in Figure 16;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
17; and/or
c) a thermogravimetric analysis profile substantially as shown in Figure
18.
7. Compound la (Phosphate Salt).
One aspect of the present disclosure relates to phosphate salt of N-(3-(4,6-
dimethylpyrimidin-5-
y1)-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la).
The
phosphate salt of Compound la are characterized by PXRD. The physical
properties for the
phosphate salt as determined by PXRD are summarized in Table 18 below.
Table 18
Compound la (Phosphate Salt, Example 10)
Figure 19: Peaks of about 11.4% relative intensity at 6.2
0.2 , 9.0 0.2 , 9.2 0.2 , 9.9 0.2 , 11.30 0.2 , 11.70
0.2 , 12.2 0.2 , 12.4 0.2 , 12.7 0.2 , 13.7 0.2 ,
PXRD 16.9 0.2 , 17.2 0.2 , 17.8 0.2 , 18.1 0.2 ,
18.5
0.2 , 18.8 0.2 , 19.6 0.2 , 20.3 0.2 , 20.9 0.2 ,
22.3 0.2 , 22.7 0.2 , 23.6 0.2 , 25.0 0.2 , 25.5
0.2 , 27.3 0.2 , and 27.8 0.2"28
The physical properties fora phosphate salt (Example 10) prepared using
procedure from
Example 10 are summarized in Table 19 below.
Table 19
Compound la (Phosphate Salt, Example 10)
TGA Figure 21: No observable weight loss below about 212.5 C
DSC Figure 20:
Endotherms extrapolated onset temperature at about
145 C and at about 173 C
Certain X-ray powder diffraction peaks for the phosphate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la) are shown in Table 20 below.
Table 20
Rel.
Pos. Rel. Int. Pos.
[ 2q.][0/0] [[ 2q.]d-spacing [A] d-spacing [A] Int.
[0/0]
6.2 14.3 29.8 20.3 4.4 100.0
9.0 9.8 23.3 20.9 4.2 51.0
9.2 9.6 38.7 21.4 4.2 12.2
9.9 8.9 11.4 22.3 4.0 27.9
11.3 7.8 20.2 22.7 3.9 78.2
11.7 7.5 38.8 23.6 3.8 21.0
57
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Rel.
Pos. Rel. Int. Pos.
d-spacing [A] d-spacing [A] Int.
[ 2 q 1 [% 2 q.] [cyo]
12.2 7.3 17.6 24.1 3.7 12.5
12.4 7.1 26.3 24.6 3.6 9.8
12.7 7.0 33.1 25.0 3.6 28.7
13.7 6.5 14.8 25.5 3.5 18.5
14.9 6.0 10.0 26.0 3.4 8.8
16.9 5.3 52.0 26.5 3.4 8.1
17.2 5.2 50.0 27.3 3.3 23.1
17.5 5.1 13.0 27.8 3.2 18.2
17.8 5.0 24.0 29.9 3.0 3.5
18.1 4.9 33.6 30.2 3.0 7.0
18.3 4.9 9.6 31.8 2.8 3.9
18.5 4.8 29.6 33.0 2.7 1.0
18.8 4.7 24.4 34.4 2.6 9.4
19.6 4.5 26.9 34.7 2.6 6.4
19.8 4.5 13.6
One aspect of the present disclosure relates to an phosphate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yDethoxy)phenyl)cyclopropanecarboxamide.
One aspect of the present disclosure relates to an phosphate salt having an X-
ray powder
diffraction pattern comprising a peak, in terms of 28, at 6.2 0.2 , 9.0
0.2 , 9.2 0.2 , 9.9
0.2 and 11.3 0.2 . In some embodiments, the phosphate salt has an X-ray
powder diffraction
pattern comprising peaks, in terms of 28 at 6.2 0.2 , 9.0 0.2 , 9.2
0.2 , 9.9 0.2 , 11.30
0.2 , 11.7 0.2 , 12.2 0.2 , 12.4 0.2 , 12.7 0.2 , 13.7 0.2 ,
16.9 0.2 , 17.2
0.2 , 17.8 0.2 , 18.10 0.2 , 18.5 0.2 , and 18.8 0.2 .
In some embodiments, the phosphate salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 28 at 6.2 0.2 , 9.0 0.2 , 9.2 0.2 , 9.9
0.2 , 11.3 0.2 ,
11.7 0.2 , 12.2 0.2 , 12.4 0.2 , 12.7 0.2 , 13.7 0.2 , 16.9
0.2 , 17.2 0.2 , 17.8
0.2 , 18.1 0.2 , 18.5 0.2 , 18.8 0.2 , 19.6 0.2 , 20.3 0.2 ,
and 20.9 0.2 .
In some embodiments, the phosphate salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 28 at 6.2 0.2 , 9.0 0.2 , 9.2 0.2 , 9.9
0.2 , 11.3 0.2 ,
11.7 0.2 , 12.2 0.2 , 12.4 0.2 , 12.7 0.2 , 13.7 0.2 , 16.9
0.2 , 17.2 0.2 , 17.8
0.2 , 18.1 0.2 , 18.5 0.2 , 18.8 0.2 , 19.6 0.2 , 20.3 0.2 ,
20.9 0.2 , 22.3
0.2 , 22.7 0.2 , and 23.6 0.2 .
In some embodiments, the phosphate salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 28 at 6.2 0.2 , 9.0 0.2 , 9.2 0.2 , 9.9
0.2 , 11.3 0.2 ,
11.7 0.2 , 12.2 0.2 , 12.4 0.2 , 12.7 0.2 , 13.7 0.2 , 16.9
0.2 , 17.2 0.2 , 17.8
58
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
0.2 , 18.1 0.2 , 18.5 0.2 , 18.8 0.2 , 19.6 0.2 , 20.3 0.2 ,
20.9 0.2 , 22.3
0.2 , 22.7 0.2 , 23.6 0.2 , 25.0 0.2 , 25.5 0.2 , 27.3 0.2 ,
and 27.8 0.2 .
In some embodiments, the phosphate salt has an X-ray powder diffraction
pattern
substantially as shown in Figure 19, wherein by "substantially" is meant that
the reported peaks
can vary by about 0.2 '26
In some embodiments, the phosphate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
198.7 C and about 220.9 C.
In some embodiments, the phosphate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
199.7 C and about 220.9 C.
In some embodiments, the phosphate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
199.7 C and about 219.9 C.
In some embodiments, the phosphate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
200.7 C and about 218.9 C.
In some embodiments, the phosphate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature at
about 201.7 C.
In some embodiments, the phosphate salt has a differential scanning
calorimetry
thermogram substantially as shown in Figure 20, wherein by "substantially" is
meant that the
reported DSC features can vary by about 4 C and that the reported DSC
features can vary by
about 20 joules per gram.
In some embodiments, the phosphate salt has a thermogravimetric analysis
profile
showing about 4.0% weight loss below about 190 C.
In some embodiments, the phosphate salt has a thermogravimetric analysis
profile
showing about 3.8% weight loss below about 190 C.
In some embodiments, the phosphate salt has a thermogravimetric analysis
profile
showing about 3.6% weight loss below about 190 C.
In some embodiments, the phosphate salt has a thermogravimetric analysis
profile
showing about 3.4% weight loss below about 190 C.
In some embodiments, the phosphate salt has a thermogravimetric analysis
profile
substantially as shown in Figure 21, wherein by "substantially" is meant that
the reported TGA
features can vary by about 5 C, and that that the reported TGA features can
vary by about
2% weight change.
One aspect of the present disclosure relates to the phosphate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 6.2
0.2 ,
9.0 0.2 , 9.2 0.2 , 9.9 0.2 , 11.3 0.2 , 11.7 0.2 , 12.2 0.2
, 12.4 0.2 , 12.7
59
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
0.2 , 13.7 0.2 , 16.9 0.2 , 17.2 0.2 , 17.8 0.2 , 18.1 0.2 ,
18.5 0.2 , and 18.8
0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 198.7 C and about 220.9 C;
and/or
c) a
thermogravimetric analysis profile showing about 4.0% weight loss below about
190 C.
One aspect of the present disclosure relates to the phosphate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
6.2 0.2 ,
9.0 0.2 , 9.2 0.2 , 9.9 0.2 , 11.3 0.2 , 11.7 0.2 , 12.2 0.2
, 12.4 0.2 , 12.7
0.2 , 13.7 0.2 , 16.9 0.2 , 17.2 0.2 , 17.8 0.2 , 18.1 0.2 ,
18.5 0.2 , 18.8 0.2 ,
19.6 0.2 , 20.3 0.2 , and 20.9 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 199.7 C and about 220.9 C;
and/or
c) a thermogravimetric analysis profile showing about 3.8% weight loss
below about
190 C.
One aspect of the present disclosure relates to the phosphate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
6.2 0.2 ,
9.0 0.2 , 9.2 0.2 , 9.9 0.2 , 11.3 0.2 , 11.7 0.2 , 12.2 0.2
, 12.4 0.2 , 12.7
0.2 , 13.7 0.2 , 16.9 0.2 , 17.2 0.2 , 17.8 0.2 , 18.1 0.2 ,
18.5 0.2 , 18.8 0.2 ,
19.6 0.2 , 20.3 0.2 , 20.9 0.2 , 22.3 0.2 , 22.7 0.2 , and 23.6
0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 199.7 C and about 219.9 C;
and/or
c) a thermogravimetric analysis profile showing about 3.6% weight loss or
less below
about 190 C.
One aspect of the present disclosure relates to the phosphate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 6.2
0.2 ,
9.0 0.2 , 9.2 0.2 , 9.9 0.2 , 11.3 0.2 , 11.7 0.2 , 12.2 0.2
, 12.4 0.2 , 12.7
0.2 , 13.7 0.2 , 16.9 0.2 , 17.2 0.2 , 17.8 0.2 , 18.1 0.2 ,
18.5 0.2 , 18.8 0.2 ,
19.6 0.2 , 20.3 0.2 , 20.9 0.2 , 22.3 0.2 , 22.7 0.2 , and 23.6
0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 200.7 C and about 218.9 C;
and/or
c) a
thermogravimetric analysis profile showing about 3.6% weight loss or less
below
about 190 C.
One aspect of the present disclosure relates to the phosphate salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 6.2 0.2 ,
9.0 0.2 , 9.2 0.2 , 9.9 0.2 , 11.3 0.2 , 11.7 0.2 , 12.2 0.2
, 12.4 0.2 , 12.7
0.2 , 13.7 0.2 , 16.9 0.2 , 17.2 0.2 , 17.8 0.2 , 18.1 0.2 ,
18.5 0.2 , 18.8 0.2 ,
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
19.6 0.2 , 20.3 0.2 , 20.9 0.2 , 22.3 0.2 , 22.7 0.2 , 23.6
0.2 , 25.0 0.2 , 25.5
0.2 , 27.3 0.2 , and 27.8 0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature at about 201.7 C; and/or
c) a
thermogravimetric analysis profile showing about 3.4% weight loss or less
below
about 190 C.
One aspect of the present disclosure relates to the phosphate salt having:
a) an X-ray powder diffraction pattern substantially as shown in Figure 19;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
20; and/or
c) a thermogravimetric analysis profile substantially as shown in Figure
21.
8. Compound la (Succinate Salt).
One aspect of the present disclosure relates to a succinate salt of N-(3-(4,6-
dimethylpyrimidin-5-
yI)-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la).
The succinate
salt of Compound la are characterized by PXRD. The physical properties for the
succinate salt
as determined by PXRD are summarized in Table 21 below.
Table 21
Compound la (Succinate Salt, Example 11)
Figure 22: Peaks of about 9.5% relative intensity at 7.3
0.2 , 12.1 0.2 , 12.8 0.2 , 13.1 0.2 , 15.4 0.2 ,
15.6 0.2 , 16.1 0.2 , 17.0 0.2 , 17.5 0.2 , 18.2
PXRD
0.2 , 20.2 0.2 , 20.9 0.2 , 21.4 0.2 , 22.2 0.2 ,
22.4 0.2 , 23.4 0.2 , 23.5 0.2 , 23.8 0.2 , 26.8
0.2 , and 27.4 0.2"28
The physical properties for a succinate salt (Example 11) prepared using
procedure from
Example 11 are summarized in Table 22 below.
Table 22
Compound la (Succinate Salt, Example 11)
Figure 24: Decrease in weight of about 24.8% out to about 2750
TGA
C
Figure 23: Endotherm extrapolated onset temperature at about
DSC
116.3 C
Certain X-ray powder diffraction peaks for the succinate salt of N-(3-(4,6-
dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la) are shown in Table 23 below.
61
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Table 23
Rel.
Pos. Rel. Int. Pos.
d-spacing [A] d-spacing [A] Int.
[ 2 q.] [% 2 1J [cyo]
7.3 12.1 79.9 22.2 4.0 36.6
7.6 11.7 6.3 22.4 4.0 22.7
10.5 8.4 5.9 22.9 3.9 4.0
12.1 7.3 19.3 23.4 3.8 13.1
12.8 6.9 30.4 23.5 3.8 22.7
13.1 6.8 18.1 23.8 3.7 23.9
13.5 6.5 3.8 24.7 3.6 5.3
14.7 6.0 7.1 25.0 3.6 6.1
15.4 5.7 41.3 26.8 3.3 12.6
15.6 5.7 15.9 27.4 3.3 28.3
16.1 5.5 100.0 28.0 3.2 4.0
17.0 5.2 45.3 28.4 3.1 8.3
17.5 5.1 9.5 28.5 3.1 6.4
18.2 4.9 21.7 29.1 3.1 3.4
18.9 4.7 6.1 29.5 3.0 4.9
19.3 4.6 2.4 30.1 3.0 2.3
19.7 4.5 4.6 30.7 2.9 3.1
20.2 4.4 18.2 31.9 2.8 5.9
20.9 4.3 33.6 32.4 2.8 3.1
21.4 4.2 81.8 34.3 2.6 5.6
One aspect of the present disclosure relates to a succinate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide.
One aspect of the present disclosure relates to a succinate salt having an X-
ray powder
diffraction pattern comprising a peak, in terms 0f28, at 7.3 0.2 , 12.1
0.2 , 12.8 0.2 , and
13.1 0.2 . In some embodiments, the succinate salt has an X-ray powder
diffraction pattern
comprising peaks, in terms of 20 at 7.3 0.2 , 12.1 0.2 , 12.8 0.2 ,
13.1 0.2 , 15.4
0.2 , 15.6 0.2 , 16.1 0.2 , 17.0 0.2 , and 17.5 0.2 .
In some embodiments, the succinate salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 20 at 7.3 0.2 , 12.1 0.2 , 12.8 0.2 ,
13.1 0.2 , 15.4
0.2 , 15.6 0.2 , 16.1 0.2 , 17.0 0.2 , 17.5 0.2 , 18.2 0.2 ,
20.2 0.2 , 20.9 0.2 ,
and 21.4 0.2 .
In some embodiments, the succinate salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 20 at 7.3 0.2 , 12.1 0.2 , 12.8 0.2 ,
13.1 0.2 , 15.4
0.2 , 15.6 0.2 , 16.1 0.2 , 17.0 0.2 , 17.5 0.2 , 18.2 0.2 ,
20.2 0.2 , 20.9 0.2 ,
21.4 0.2 , 22.2 0.2 , 22.4 0.2 , 23.4 0.2 , and 23.5 0.2 .
62
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, the succinate salt has an X-ray powder diffraction
pattern
comprising peaks, in terms of 28, at 7.3 0.2 , 12.1 0.2 , 12.8 0.2 ,
13.1 0.2 , 15.4
0.2 , 15.6 0.2 , 16.1 0.2 , 17.0 0.2 , 17.5 0.2 , 18.2 0.2 ,
20.2 0.2 , 20.9 0.2 ,
21.40 0.2 , 22.2 0.2 , 22.4 0.2 , 23.4 0.2 , 23.5 0.2 , 23.8
0.2 , 26.8 0.2 , and
27.4 0.2 .
In some embodiments, the succinate salt has an X-ray powder diffraction
pattern
substantially as shown in Figure 22, wherein by "substantially" is meant that
the reported peaks
can vary by about 0.2 '26
In some embodiments, the succinate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
113.3 C and about 122.8 C.
In some embodiments, the succinate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
114.3 C and about 122.8 C.
In some embodiments, the succinate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
114.3 C and about 121.8 C.
In some embodiments, the succinate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
115.3 C and about 120.8 C.
In some embodiments, the succinate salt has a differential scanning
calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature at
about 116.3 C.
In some embodiments, the succinate salt has a differential scanning
calorimetry
thermogram substantially as shown in Figure 23, wherein by "substantially" is
meant that the
reported DSC features can vary by about 4 C and that the reported DSC
features can vary by
about 20 joules per gram.
In some embodiments, the succinate salt has a thermogravimetric analysis
profile
showing about 25.4% weight loss below about 275 C.
In some embodiments, the succinate salt has a thermogravimetric analysis
profile
showing about 25.2% weight loss below about 275 C.
In some embodiments, the succinate salt has a thermogravimetric analysis
profile
showing about 25.0% weight loss or less below about 275 C.
In some embodiments, the succinate salt has a thermogravimetric analysis
profile
showing about 24.8% weight loss or less below about 275 C.
In some embodiments, the succinate salt has a thermogravimetric analysis
profile
substantially as shown in Figure 24, wherein by "substantially" is meant that
the reported TGA
features can vary by about 5 C, and that that the reported TGA features can
vary by about
2% weight change.
63
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
One aspect of the present disclosure relates to the succinate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 7.3
0.2 ,
12.1 0.2 , 12.8 0.2 , 13.1 0.2 , 15.4 0.2 , 15.6 0.2 , 16.1
0.2 , 17.0 0.2 , and
17.5 0.2";
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 113.3 C and about 122.8 C;
and/or
c) a
thermogravimetric analysis profile showing about 25.4% weight loss below about
275 C.
One aspect of the present disclosure relates to the succinate salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 7.3 0.2 ,
12.1 0.2 , 12.8 0.2 , 13.1 0.2 , 15.4 0.2 , 15.6 0.2 , 16.1
0.2 , 17.0 0.2 , 17.5
0.2 , 18.2 0.2 , 20.2 0.2 , 20.9 0.2 , and 21.4 0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 114.3 C and about 122.8 C;
and/or
c) a
thermogravimetric analysis profile showing about 25.2% weight loss below about
275 C.
One aspect of the present disclosure relates to the succinate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
7.3 0.2 ,
12.1 0.2 , 12.8 0.2 , 13.1 0.2 , 15.4 0.2 , 15.6 0.2 , 16.1
0.2 , 17.0 0.2 , 17.5
0.2 , 18.2 0.2 , 20.2 0.2 , 20.9 0.2 , 21.4 0.2 , 22.2 0.2 ,
22.4 0.2 , 23.4
0.2 , and 23.5 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 114.3 C and about 121.8 C;
and/or
c) a thermogravimetric analysis profile showing about 25.0% weight loss or
less
below about 275 C.
One aspect of the present disclosure relates to the succinate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
7.3 0.2 ,
12.1 0.2 , 12.8 0.2 , 13.1 0.2 , 15.4 0.2 , 15.6 0.2 , 16.1
0.2 , 17.0 0.2 , 17.5
0.2 , 18.2 0.2 , 20.2 0.2 , 20.9 0.2 , 21.4 0.2 , 22.2 0.2 ,
22.4 0.2 , 23.4
0.2 , and 23.5 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 115.3 C and about 120.8 C;
and/or
c) a thermogravimetric analysis profile showing about 25.0% weight loss or
less
below about 275 C.
One aspect of the present disclosure relates to the succinate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 7.3
0.2 ,
12.1 0.2 , 12.8 0.2 , 13.1 0.2 , 15.4 0.2 , 15.6 0.2 , 16.1
0.2 , 17.0 0.2 , 17.5
64
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
0.2 , 18.2 0.2 , 20.2 0.2 , 20.9 0.2 , 21.4 0.2 , 22.2 0.2 ,
22.4 0.2 , 23.4
0.2 , 23.5 0.2 , 23.8 0.2 , 26.8 0.2 , and 27.4 0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature at about 116.3 C; and/or
c) a
thermogravimetric analysis profile showing about 24.8% weight loss or less
below about 275 C.
One aspect of the present disclosure relates to the succinate salt having:
a) an X-ray powder diffraction pattern substantially as shown in Figure 22;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
23; and/or
c) a thermogravimetric analysis profile substantially as shown in Figure
24.
9. Compound la (Tosylate Salt).
One aspect of the present disclosure relates to a tosylate salt of N-(3-(4,6-
dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide
(Compound la). The tosylate salt of Compound la are characterized by PXRD. The
physical
properties for the tosylate salt as determined by PXRD are summarized in Table
30 below.
Table 30
Compound la (Tosylate Salt, Example 12)
Figure 25: Peaks of about 17.9% relative intensity at 5.2
0.2 , 10.0 0.2 , 10.4 0.2 , 12.0 0.2 , 13.0 0.2 ,
13.4 0.2 , 15.7 0.2 , 16.4 0.2 , 17.4 0.2 , 17.9
PXRD 0.2 , 18.4 0.2 , 18.9 0.2 , 19.6 0.2 , 20.0
0.2 ,
20.6 0.2 , 20.9 0.2 , 21.3 0.2 , 21.5 0.2 , 22.08
0.2 , 23.1 0.2 , 23.5 0.2 , 24.0 0.2 , 24.2 0.2 , and
26.4 0.2"28
The physical properties for a tosylate salt (Example 12) prepared using
procedure from
Example 12, Method 2, are summarized in Table 31 below.
Table 31
Compound 1 (Tosylate Salt, Example 12)
TGA Figure 27: Decrease in weight of about 1.5% out to about
180 C
DSC Figure 26: Endotherm extrapolated onset temperature at
about
151.3 C
Certain X-ray powder diffraction peaks for the tosylate salt of N-(3-(4,6-
dimethylpyrimidin-
5-yI)-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound
la) are shown in
Table 32 below.
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Table 32
Rel.
Pos. Rel. Int. Pos.
d-spacing [A] d-spacing [A] Int.
[ 2 q.] [% 2 1J [cyo]
5.2 16.9 51.4 20.0 4.4 40.6
10.0 8.9 67.6 20.6 4.3 26.1
10.4 8.5 36.3 20.9 4.2 30.7
10.8 8.2 15.7 21.3 4.2 24.5
11.4 7.7 100.0 21.5 4.1 23.6
12.0 7.4 25.0 22.0 4.0 63.7
13.0 6.8 50.3 23.1 3.9 70.7
13.4 6.6 25.1 23.5 3.8 31.1
15.2 5.8 13.9 24.0 3.7 48.7
15.7 5.7 22.0 24.2 3.7 39.9
16.4 5.4 21.2 25.6 3.5 9.8
17.1 5.2 14.5 26.4 3.4 20.7
17.4 5.1 19.0 26.9 3.3 11.4
17.9 5.0 17.9 28.7 3.1 4.3
18.4 4.8 33.4 29.9 3.0 10.2
18.9 4.7 99.4 30.3 3.0 16.5
19.6 4.5 19.7
One aspect of the present disclosure relates to a tosylate salt of N-(3-(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide.
One aspect of the present disclosure relates to a tosylate salt having an X-
ray powder
diffraction pattern comprising a peak, in terms of 20, at 5.2 0.2 , 10.0
0.2 , 10.4 0.2 ,
12.0 0.2 , and 13.0 0.2 . In some embodiments, the tosylate salt has an
X-ray powder
diffraction pattern comprising peaks, in terms of 20 at 5.2 0.2 , 10.0
0.2 , 10.4 0.2 , 12.0
0.2 , 13.0 0.2 , 13.4 0.2 , 15.7 0.2 , 16.4 0.2 , 17.4 0.2 ,
17.9 0.2 , 18.4
0.2 , and 18.9 0.2 .
In some embodiments, the tosylate salt has an X-ray powder diffraction pattern
comprising peaks, in terms 0f28, at 5.2 0.2 , 10.0 0.2 , 10.4 0.2 ,
12.0 0.2 , 13.0
0.2 , 13.4 0.2 , 15.7 0.2 , 16.4 0.2 , 17.4 0.2 , 17.9 0.2 ,
18.4 0.2 , 18.9 0.2 ,
19.6 0.2 , 20.0 0.2 , 20.6 0.2 , and 20.9 0.2 .
In some embodiments, the tosylate salt has an X-ray powder diffraction pattern
comprising peaks, in terms 0f28, at 5.2 0.2 , 10.0 0.2 , 10.4 0.2 ,
12.0 0.2 , 13.0
0.2 , 13.4 0.2 , 15.7 0.2 , 16.4 0.2 , 17.4 0.2 , 17.9 0.2 ,
18.4 0.2 , 18.9 0.2 ,
19.6 0.2 , 20.0 0.2 , 20.6 0.2 , 20.9 0.2 , 21.3 0.2 , 21.5
0.2 , 22.08 0.2 , and
23.1 0.2 .
66
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
In some embodiments, the tosylate salt has an X-ray powder diffraction pattern
comprising peaks, in terms 0f28, at 5.2 0.2 , 10.0 0.2 , 10.4 0.2 ,
12.0 0.2 , 13.0
0.2 , 13.4 0.2 , 15.7 0.2 , 16.4 0.2 , 17.4 0.2 , 17.9 0.2 ,
18.4 0.2 , 18.9 0.2 ,
19.6 0.2 , 20.0 0.2 , 20.6 0.2 , 20.9 0.2 , 21.3 0.2 , 21.5
0.2 , 22.08 0.2 ,
23.1 0.2 , 23.5 0.2 , 24.0 0.2 , 24.2 0.2 , and 26.4 0.2 .
In some embodiments, the tosylate salt has an X-ray powder diffraction pattern
substantially as shown in Figure 25, wherein by "substantially" is meant that
the reported peaks
can vary by about 0.2 '26
In some embodiments, the tosylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
148.3 C and about 173.1 C.
In some embodiments, the tosylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
149.3 C and about 173.1 C.
In some embodiments, the tosylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
149.3 C and about 172.1 C.
In some embodiments, the tosylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature
between about
150.3 C and about 171.1 C.
In some embodiments, the tosylate salt has a differential scanning calorimetry
thermogram comprising an endotherm with an extrapolated onset temperature at
about 151.3 C.
In some embodiments, the tosylate salt has a differential scanning calorimetry
thermogram substantially as shown in Figure 26, wherein by "substantially" is
meant that the
reported DSC features can vary by about 4 C and that the reported DSC
features can vary by
about 20 joules per gram.
In some embodiments, the tosylate salt has a thermogravimetric analysis
profile showing
about 2.1% weight loss below about 180 C.
In some embodiments, the tosylate salt has a thermogravimetric analysis
profile showing
about 1.9% weight loss below about 180 C.
In some embodiments, the tosylate salt has a thermogravimetric analysis
profile showing
about 1.7% weight loss below about 180 C.
In some embodiments, the tosylate salt has a thermogravimetric analysis
profile showing
about 1.5% weight loss below about 180 C.
In some embodiments, the tosylate salt has a thermogravimetric analysis
profile
substantially as shown in Figure 27, wherein by "substantially" is meant that
the reported TGA
features can vary by about 5 C, and that that the reported TGA features can
vary by about
2% weight change.
67
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
One aspect of the present disclosure relates to the tosylate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 5.2
0.2 ,
10.00 0.2 , 10.4 0.2 , 12.0 0.2 , 13.0 0.2 , 13.4 0.2 , 15.7
0.2 , 16.4 0.2 , 17.4
0.2 , 17.9 0.2 , 18.4 0.2 , and 18.9 0.2";
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 148.3 C and about 173.1 C;
and/or
c) a
thermogravimetric analysis profile showing about 2.1% weight loss below about
180 C.
One aspect of the present disclosure relates to the tosylate salt having:
a) an X-ray
powder diffraction pattern comprising peaks, in terms of 28, at 5.2 0.2 ,
10.0 0.2 , 10.4 0.2 , 12.0 0.2 , 13.0 0.2 , 13.4 0.2 , 15.7
0.2 , 16.4 0.2 , 17.4
0.2 , 17.9 0.2 , 18.4 0.2 , 18.9 0.2 , 19.6 0.2 , 20.0 0.2 ,
20.6 0.2 , and 20.9
0.2";
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 149.3 C and about 173.1 C;
and/or
c) a
thermogravimetric analysis profile showing about 1.9% weight loss below about
180 C.
One aspect of the present disclosure relates to the tosylate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 5.2
0.2 ,
10.0 0.2 , 10.4 0.2 , 12.0 0.2 , 13.0 0.2 , 13.4 0.2 , 15.7
0.2 , 16.4 0.2 , 17.4
0.2 , 17.9 0.2 , 18.4 0.2 , 18.9 0.2 , 19.6 0.2 , 20.0 0.2 ,
20.6 0.2 , 20.9
0.2 , 21.3 0.2 , 21.5 0.2 , 22.08 0.2 , and 23.1 0.2';
b) a
differential scanning calorimetry thermogram comprising an endotherm with an
extrapolated onset temperature between about 149.3 C and about 172.1 C;
and/or
c) a
thermogravimetric analysis profile showing about 1.7% weight loss or less
below
about 180 C.
One aspect of the present disclosure relates to the tosylate salt having:
a) an X-ray powder diffraction pattern comprising peaks, in terms of 28, at
5.2 0.2 ,
10.0 0.2 , 10.4 0.2 , 12.0 0.2 , 13.0 0.2 , 13.4 0.2 , 15.7
0.2 , 16.4 0.2 , 17.4
0.2 , 17.9 0.2 , 18.4 0.2 , 18.9 0.2 , 19.6 0.2 , 20.0 0.2 ,
20.6 0.2 , 20.9
0.2 , 21.3 0.2 , 21.5 0.2 , 22.08 0.2 , and 23.1 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature between about 150.3 C and about 171.1 C;
and/or
c) a thermogravimetric analysis profile showing about 1.7% weight loss or
less below
about 180 C.
One aspect of the present disclosure relates to the tosylate salt having:
a) an
X-ray powder diffraction pattern comprising peaks, in terms of 28, at 5.2
0.2 ,
10.0 0.2 , 10.4 0.2 , 12.0 0.2 , 13.0 0.2 , 13.4 0.2 , 15.7
0.2 , 16.4 0.2 , 17.4
68
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
0.2 , 17.9 0.2 , 18.4 0.2 , 18.9 0.2 , 19.6 0.2 , 20.0 0.2 ,
20.6 0.2 , 20.9
0.2 , 21.30 0.2 , 21.50 0.2 , 22.08 0.2 , 23.10 0.2 , 23.5 0.2 ,
24.0 0.2 , 24.2 0.2 ,
and 26.4 0.2';
b) a differential scanning calorimetry thermogram comprising an endotherm
with an
extrapolated onset temperature at about 151.3 C; and/or
c) a thermogravimetric analysis profile showing about 1.5% weight loss or
less below
about 180 C.
One aspect of the present disclosure relates to the tosylate salt having:
a) an X-ray powder diffraction pattern substantially as shown in
Figure 25;
b) a differential scanning calorimetry thermogram substantially as shown in
Figure
26; and/or
c) a thermogravimetric analysis profile substantially as shown in
Figure 27.
The crystalline forms described herein can be prepared by any of the suitable
procedures
known in the art for preparing crystalline polymorphs. In some embodiments the
crystalline forms
described herein are prepared according to the Examples. In some embodiments,
the crystalline
forms described herein can be prepared by heating crystalline forms other than
the crystalline
forms described herein. In some embodiments, the crystalline forms described
herein can be
prepared by recrystallizing crystalline forms other than the crystalline forms
described herein.
Compounds of Formula (Ile) of the present disclosure may be prepared according
to
relevant published literature procedures that are used by one skilled in the
art. Exemplary
reagents and procedures for these reactions appear hereinafter in the working
Examples.
Protection and deprotection may be carried out by procedures generally known
in the art (see,
for example, Greene, T. W. and Wuts, P. G. M., Protecting Groups in Organic
Synthesis, 3rd
Edition, 1999 [Wiley]).
It is understood that the present disclosure embraces each enantiomer and
mixtures
thereof. Separation of the individual isomers (such as, by chiral HPLC,
recrystallization of
diastereoisomeric mixtures and the like) or selective synthesis (such as, by
enantiomeric
selective syntheses and the like) of the individual isomers is accomplished by
application of
various methods which are well known to practitioners in the art.
INDICATIONS AND METHODS OF PROPHYLAXIS AND/OR TREATMENT
In addition to the foregoing beneficial uses for the modulators of 5-HT2A
serotonin receptor
activity disclosed herein, the compounds disclosed herein are useful in the
treatment of several
additional diseases and disorders, and in the amelioration of symptoms
thereof. Without limitation,
these include the following:
The compounds of Formula (Ile), and pharmaceutically acceptable salts thereof,
are
useful as 5-HT2A serotonin receptor modulators for the treatment of disorders
associated with 5-
HT2A serotonin receptor expression and/or activity, such as cardiovascular
disorders (for
69
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
example, coronary artery disease, myocardial infarction, transient ischemic
attack, angina,
stroke, atrial fibrillation, platelet aggregation, and blood clot formation or
symptoms thereof.
The modulators of 5-HT2A receptor activity disclosed herein are believed to be
useful in
the treatment of several diseases and disorders, and in the amelioration of
symptoms thereof.
VVithout limitation, some of them include the following:Antiplatelet agents
(antiplatelets) are
prescribed for a variety of conditions. For example, in coronary artery
disease they are used to
help prevent myocardial infarction or stroke in patients who are at risk of
developing obstructive
blood clots (e.g., coronary thrombosis).
In a myocardial infarction (heart attack), the heart muscle does not receive
enough
oxygen-rich blood as a result of a blockage in the coronary blood vessels. If
taken while an attack
is in progress or immediately afterward (preferably within 30 minutes),
antiplatelets can reduce
the damage to the heart.
A transient ischemic attack ("TIA" or "mini-stroke") is a brief interruption
of oxygen flow to
the brain due to decreased blood flow through arteries, usually due to an
obstructing blood clot.
Antiplatelet drugs have been found to be effective in preventing TIAs.
Angina is a temporary and often recurring chest pain, pressure or discomfort
caused by
inadequate oxygen-rich blood flow (ischemia) to some parts of the heart. In
patients with angina,
antiplatelet therapy can reduce the effects of angina and the risk of
myocardial infarction.
Stroke is an event in which the brain does not receive enough oxygen-rich
blood, usually
due to blockage of a cerebral blood vessel by a blood clot. In high-risk
patients, taking
antiplatelets regularly has been found to prevent the formation blood clots
that cause first or
second strokes.
Angioplasty is a catheter-based technique used to open arteries obstructed by
a blood
clot. Whether or not stenting is performed immediately after this procedure to
keep the artery
open, antiplatelets can reduce the risk of forming additional blood clots
following the procedure(s).
Coronary bypass surgery is a surgical procedure in which an artery or vein is
taken from
elsewhere in the body and grafted to a blocked coronary artery, rerouting
blood around the
blockage and through the newly attached vessel. After the procedure,
antiplatelets can reduce
the risk of secondary blood clots.
Atrial fibrillation is the most common type of sustained irregular heart
rhythm (arrythmia).
Atrial fibrillation affects about two million Americans every year. In atrial
fibrillation, the atria (the
heart's upper chambers) rapidly fire electrical signals that cause them to
quiver rather than
contract normally. The result is an abnormally fast and highly irregular
heartbeat. When given
after an episode of atrial fibrillation, antiplatelets can reduce the risk of
blood clots forming in the
heart and traveling to the brain (embolism).
5-HT2A receptors are expressed on smooth muscle of blood vessels and 5-HT
secreted
by activated platelets causes vasoconstriction as well as activation of
additional platelets during
clotting. There is evidence that a 5-HT2A inverse agonist will inhibit
platelet aggregation and thus
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
be a potential treatment as an antiplatelet therapy (see Satimura, K, etal.,
Clin Cardiol 2002 Jan.
25 (1):28-32; and Wilson, H.0 etal., Thromb Haemost 1991 Sep 2;66(3):355-60).
The 5-HT2A inverse agonists disclosed herein provide beneficial improvement in
microcirculation to patients in need of antiplatelet therapy by antagonizing
the vasoconstrictive
products of the aggregating platelets in, for example and not limitation, the
indications described
above. Accordingly, in some embodiments, the present invention provides
methods for reducing
platelet aggregation in a patient in need thereof comprising administering to
said patient a
composition comprising a 5-HT2A inverse agonist disclosed herein. In further
embodiments, the
present invention provides methods for treating coronary artery disease,
myocardial infarction,
transient ischemic attack, angina, stroke, atrial fibrillation, or a symptom
of any of the foregoing
in a patient in need of said treatment, comprising administering to said
patient a composition
comprising a 5-HT2A inverse agonist disclosed herein.
In further embodiments, the present invention provides methods for reducing
risk of blood
clot formation in an angioplasty or coronary bypass surgery patient, or a
patient suffering from
atrial fibrillation, comprising administering to a said patient a composition
comprising a 5-HT2A
inverse agonist disclosed herein at a time where such risk exists.
In further embodiments, the present invention provides methods for reducing
risk of, or
treating the effects of, PCI, comprising administering to a patient a
composition comprising a 5-
HT2A inverse agonist disclosed herein at a time where such risk exists.
In further embodiments, the present invention provides methods for the
prevention or
treatment of Raynaud's, comprising administering to a patient a composition
comprising a 5-HT2A
inverse agonist disclosed herein.
Synthetic Methods:
Example processes and intermediates of the present disclosure are provided
below in
Scheme A. As will be appreciated by those skilled in the art, the compounds
provided herein,
including salts thereof, can be prepared using known organic synthesis
techniques and can be
synthesized according to any of numerous possible synthetic routes, such as
that provided in
Scheme A.
The reactions for preparing compounds described herein can be carried out in
suitable
solvents which can be readily selected by one of skill in the art of organic
synthesis. Suitable
solvents can be substantially non-reactive with the starting materials
(reactants), the
intermediates or products at the temperatures at which the reactions are
carried out, e.g.,
temperatures which can range from the solvent's freezing temperature to the
solvent's boiling
temperature. A given reaction can be carried out in one solvent or a mixture
of more than one
solvent. Depending on the particular reaction step, suitable solvents for a
particular reaction step
can be selected by the skilled artisan.
71
CA 03235907 2024-04-17
WO 2023/067520 PCT/IB2022/060059
Reactions can be monitored according to any suitable method known in the art.
For
example, product formation can be monitored by spectroscopic means, such as
nuclear magnetic
resonance spectroscopy (e.g., 1H or 13C), infrared spectroscopy,
spectrophotometry (e.g., UV-
visible), mass spectrometry or by chromatographic methods such as high
performance liquid
chromatography (HPLC), liquid chromatography-mass spectroscopy (LC-MS), or
thin layer
chromatography (TLC). Compounds can be purified by those skilled in the art by
a variety of
methods, including high performance liquid chromatography (HPLC) and normal
phase silica
chromatography.
Scheme A provides general guidance in connection with preparing the compounds
of the
invention. For instance, the compound of Formula (Ile) can be prepared as
shown in Scheme
A.
Scheme A
F
B4OH R2-0H
R3 02N R3
LGO OH IIb-1
N _________________________________ "- 02N N _________
I Coupling Step I _I Alkylation Step
Fet"N- R-A N
Ha Hb
R2
0 0
R3 R3
Reduction Step
02N H2N
I I )\I
IIc R4 N Hd R4 N
R2
0
0 0 R3
R1 CI
H ii
)\I
Amide Formation Step R4 N
He
Polymorphs and Pseudopolymorphs
The present disclosure includes polymorphs and pseudopolymorphs of the
compound of
Formula (la) of the present disclosure. Polymorphism is the ability of a
substance to exist as two
or more crystalline phases that have different arrangements and/or
conformations of the
molecules in the crystal lattice. Polymorphs show the same properties in the
liquid or gaseous
state but they behave differently in the solid state.
Besides single-component polymorphs, drugs can also exist as salts and other
multicomponent crystalline phases. For example, crystalline phases can contain
an API host and
either solvent or water molecules, respectively, as guests. Crystalline phases
that share the same
API host, but differ with respect to the guests, can be referred to as
pseudopolymorphs of one
another.
72
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Solvates contain molecules of the solvent of crystallization in a definite
crystal lattice.
Solvates, in which the solvent of crystallization is water, are termed
hydrates. Because water is
a constituent of the atmosphere, hydrates of drugs may be formed rather
easily.
By way of example, Stahly published a polymorph screen of 245 compounds
consisting
of a "wide variety of structural types" that revealed about 90% of them
exhibited multiple solid
forms. Overall, approximately half of the compounds were polymorphic, often
having one to three
forms. About one-third of the compounds formed hydrates, and about one-third
formed solvates.
Data from cocrystal screens of 64 compounds showed that 60% formed cocrystals
other than
hydrates or solvates. (G. P. Stahly, Crystal Growth & Design (2007), 7(6),
1007-1026).
CERTAIN EMBODIMENTS
One aspect of the present disclosure relates to methods for the treatment of a
5HT2A-
related disorder disorder in an individual, comprising administering to the
individual in need
thereof, a therapeutically effective amount of a crystalline form as described
herein or a
pharmaceutical composition thereof.
One aspect of the present disclosure relates to a crystalline form of 5HT2A-
related disorder
as described herein, for use in a method of treatment of the human or animal
body by therapy.
One aspect of the present disclosure relates to a crystalline form of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)) as described herein, for use in a method of treatment of a 5HT2A-related
disorder.
One aspect of the present disclosure relates to methods for the treatment of
5HT2A-related
disorder in an individual,wherein the 5HT2A-related disorder is selected from
a condition
associated with platelet aggregation, coronary artery disease, myocardial
infarction, transient
ischemic attack, angina, stroke, atrial fibrillation, blood clot formation, or
symptoms thereof,
comprising administering to the individual in need thereof, a therapeutically
effective amount of a
crystalline form as described herein or a pharmaceutical composition thereof.
One aspect of the present disclosure relates to methods for the treatment of
5HT2A-related
disorder in an individual, wherein the 5HT2A-related disorder is an effect of
PCI selected from
microvascular obstruction (MVO), myocardial injury, reduced cardiac function,
or a major adverse
cardiac event (MACE), comprising administering to the individual in need
thereof, a
therapeutically effective amount of a crystalline form as described herein or
a pharmaceutical
composition thereof.
One aspect of the present disclosure relates to methods for the treatment of
5HT2A-related
disorder in an individual, wherein the 5HT2A-related disorder is Raynaud's,
comprising
administering to the individual in need thereof, a therapeutically effective
amount of a crystalline
form as described herein or a pharmaceutical composition thereof.
One aspect of the present disclosure relates to a method for treating a
condition
associated with platelet aggregation in an individual, comprising
administering to said individual
73
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
in need thereof, a therapeutically effective amount of a crystalline form as
described herein or a
pharmaceutical composition thereof.
One aspect of the present disclosure relates to a method for reducing the risk
of blood
clot formation in an individual, comprising administering to said individual
in need thereof, a
therapeutically effective amount of a crystalline form as described herein or
a pharmaceutical
composition thereof.
One aspect of the present disclosure relates to a method for reducing the risk
of blood
clot formation in an angioplasty or coronary bypass surgery individual,
comprising administering
to said individual in need thereof, a therapeutically effective amount of a
crystalline form as
described herein or a pharmaceutical composition thereof.
One aspect of the present disclosure relates to a method for reducing the risk
of blood
clot formation in an individual suffering from atrial fibrillation, comprising
administering to said
individual in need thereof, a therapeutically effective amount of a
crystalline form as described
herein or a pharmaceutical composition thereof.
One aspect of the present disclosure relates to methods for the treatment of a
5HT2A-
related disorder disorder in an individual, comprising administering to the
individual in need
thereof, a therapeutically effective amount of a crystalline salt as described
herein or a
pharmaceutical composition thereof.
One aspect of the present disclosure relates to a crystalline salt of 5HT2A-
related disorder
as described herein, for use in a method of treatment of the human or animal
body by therapy.
One aspect of the present disclosure relates to a crystalline salt of N-(3-
(4,6-
dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula
(la)) as described herein, for use in a method of treatment of a 5HT2A-related
disorder.
One aspect of the present disclosure relates to methods for the treatment of
5HT2A-related
.. disorder in an individual,wherein the 5HT2A-related disorder is selected
from a condition
associated with platelet aggregation, coronary artery disease, myocardial
infarction, transient
ischemic attack, angina, stroke, atrial fibrillation, blood clot formation, or
symptoms thereof,
comprising administering to the individual in need thereof, a therapeutically
effective amount of a
crystalline salt as described herein or a pharmaceutical composition thereof.
One aspect of the present disclosure relates to methods for the treatment of
5HT2A-related
disorder in an individual, wherein the 5HT2A-related disorder is an effect of
PC1 selected from
microvascular obstruction (MVO), myocardial injury, reduced cardiac function,
or a major adverse
cardiac event (MACE), comprising administering to the individual in need
thereof, a
therapeutically effective amount of a crystalline salt as described herein or
a pharmaceutical
.. composition thereof.
One aspect of the present disclosure relates to methods for the treatment of
5HT2A-related
disorder in an individual, wherein the 5HT2A-related disorder is Raynaud's,
comprising
74
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
administering to the individual in need thereof, a therapeutically effective
amount of a crystalline
salt as described herein or a pharmaceutical composition thereof.
One aspect of the present disclosure relates to a method for treating a
condition
associated with platelet aggregation in an individual, comprising
administering to said individual
in need thereof, a therapeutically effective amount of a crystalline salt as
described herein or a
pharmaceutical composition thereof.
One aspect of the present disclosure relates to a method for reducing the risk
of blood
clot formation in an individual, comprising administering to said individual
in need thereof, a
therapeutically effective amount of a crystalline salt as described herein or
a pharmaceutical
composition thereof.
One aspect of the present disclosure relates to a method for reducing the risk
of blood
clot formation in an angioplasty or coronary bypass surgery individual,
comprising administering
to said individual in need thereof, a therapeutically effective amount of a
crystalline salt as
described herein or a pharmaceutical composition thereof.
One aspect of the present disclosure relates to a method for reducing the risk
of blood
clot formation in an individual suffering from atrial fibrillation, comprising
administering to said
individual in need thereof, a therapeutically effective amount of a
crystalline salt as described
herein or a pharmaceutical composition thereof.
PHARMACEUTICAL COMPOSITIONS
A further aspect of the present disclosure pertains to pharmaceutical
compositions
comprising the compounds of Formula (Ile), or pharmaceutically acceptable salt
thereof as
described herein and one or more pharmaceutically acceptable carriers. In some
embodiments,
the pharmaceutical composition comprises a compound of Formula (Ile), and a
pharmaceutically
acceptable carrier. In some embodiments, the pharmaceutical composition
comprises a salt of
the compound of Formula (Ile), and a pharmaceutically acceptable carrier.
Some embodiments of the present disclosure include a method of producing a
pharmaceutical composition comprising admixing the compound of Formula (Ile),
or
pharmaceutically acceptable salt thereof and a pharmaceutically acceptable
carrier.
Some embodiments of the present disclosure include a method of producing a
pharmaceutical composition comprising admixing a salt of the compound of
Formula (Ile), as
disclosed herein and a pharmaceutically acceptable carrier.
One aspect of the present disclosure relates to compositions comprising a
crystalline
form of N-(3-(4 ,6-d imethylpyrimid in-5-yI)-4-(2-(pyrrolid in-1 -
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) as described herein.
One aspect of the present disclosure relates to compositions comprising a
crystalline
form of N-(3-(4 ,6-d imethylpyrimid in-5-yI)-4-(2-(pyrrolid in-1 -
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
yl)ethoxy)phenyl)cyclopropanecarboxamide (Formula (la)) as described herein,
and a
pharmaceutically acceptable carrier.
Some embodiments of the present disclosure include a method of producing a
pharmaceutical composition comprising admixing at least one compound according
to any of the
compound embodiments disclosed herein and a pharmaceutically acceptable
carrier.
One aspect of the present disclosure relates to process of making a
composition
comprising mixing a crystalline form of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide, with a phamaceutically acceptable
carrier.
Formulations may be prepared by any suitable method, typically by uniformly
mixing the
active compound(s) with liquids or finely divided solid carriers, or both, in
the required proportions
and then, if necessary, forming the resulting mixture into a desired shape.
Conventional excipients, such as binding agents, fillers, acceptable wetting
agents,
tabletting lubricants and disintegrants may be used in tablets and capsules
for oral administration.
Liquid preparations for oral administration may be in the form of solutions,
emulsions, aqueous
or oily suspensions and syrups. Alternatively, the oral preparations may be in
the form of dry
powder that can be reconstituted with water or another suitable liquid vehicle
before use.
Additional additives such as suspending or emulsifying agents, non-aqueous
vehicles (including
edible oils), preservatives and flavorings and colorants may be added to the
liquid preparations.
Parenteral dosage forms may be prepared by dissolving the compound of the
disclosure in a
suitable liquid vehicle and filter sterilizing the solution before filling and
sealing an appropriate vial
or ampule. These are just a few examples of the many appropriate methods well
known in the art
for preparing dosage forms.
A compound of the present disclosure can be formulated into pharmaceutical
compositions using techniques well known to those in the art. Suitable
pharmaceutically-
acceptable carriers, outside those mentioned herein, are known in the art; for
example, see
Remington, The Science and Practice of Pharmacy, 20th Edition, 2000,
Lippincott Williams &
Wilkins, (Editors: Gennaro etal.)
While it is possible that, for use in the prophylaxis or treatment, a compound
of the
disclosure may, in an alternative use, be administered as a raw or pure
chemical, it is preferable
however to present the compound or active ingredient as a pharmaceutical
formulation or
composition further comprising a pharmaceutically acceptable carrier.
Pharmaceutical formulations include those suitable for oral, rectal, nasal,
topical
(including buccal and sub-lingual), vaginal or parenteral (including
intramuscular, sub-cutaneous
and intravenous) administration or in a form suitable for administration by
inhalation, insufflation
or by a transdermal patch. Transdermal patches dispense a drug at a controlled
rate by
presenting the drug for absorption in an efficient manner with minimal
degradation of the drug.
Typically, transdermal patches comprise an impermeable backing layer, a single
pressure
sensitive adhesive and a removable protective layer with a release liner. One
of ordinary skill in
76
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
the art will understand and appreciate the techniques appropriate for
manufacturing a desired
efficacious transdermal patch based upon the needs of the artisan.
The compounds of the disclosure, together with a conventional adjuvant,
carrier, or
diluent, may thus be placed into the form of pharmaceutical formulations and
unit dosages thereof
and in such form may be employed as solids, such as tablets or filled
capsules, or liquids such
as solutions, suspensions, emulsions, elixirs, gels or capsules filled with
the same, all for oral
use, in the form of suppositories for rectal administration; or in the form of
sterile injectable
solutions for parenteral (including subcutaneous) use. Such pharmaceutical
compositions and
unit dosage forms thereof may comprise conventional ingredients in
conventional proportions,
with or without additional active compounds or principles and such unit dosage
forms may contain
any suitable effective amount of the active ingredient commensurate with the
intended daily
dosage range to be employed.
For oral administration, the pharmaceutical composition may be in the form of,
for
example, a tablet, capsule, suspension or liquid. The pharmaceutical
composition is preferably
made in the form of a dosage unit containing a particular amount of the active
ingredient.
Examples of such dosage units are capsules, tablets, powders, granules or a
suspension, with
conventional additives such as lactose, mannitol, corn starch or potato
starch; with binders such
as crystalline cellulose, cellulose derivatives, acacia, corn starch or
gelatins; with disintegrators
such as corn starch, potato starch or sodium carboxymethyl-cellulose; and with
lubricants such
as talc or magnesium stearate. The active ingredient may also be administered
by injection as a
composition wherein, for example, saline, dextrose or water may be used as a
suitable
pharmaceutically acceptable carrier.
Compounds of the present disclosure or a solvate, hydrate or physiologically
functional
derivative thereof can be used as active ingredients in pharmaceutical
compositions, specifically
as 5-HT2A serotonin receptor modulators. By the term "active ingredient" is
defined in the context
of a "pharmaceutical composition" and refers to a component of a
pharmaceutical composition
that provides the primary pharmacological effect, as opposed to an "inactive
ingredient" which
would generally be recognized as providing no pharmaceutical benefit.
The dose when using the compounds of the present disclosure can vary within
wide limits
and as is customary and is known to the physician, it is to be tailored to the
individual conditions
in each individual case. It depends, for example, on the nature and severity
of the illness to be
treated, on the condition of the patient, on the compound employed or on
whether an acute or
chronic disease state is treated or prophylaxis conducted or on whether
further active compounds
are administered in addition to the compounds of the present disclosure.
Representative doses
of the present disclosure include, but not limited to, about 0.001 mg to about
5000 mg, about
0.001 mg to about 2500 mg, about 0.001 mg to about 1000 mg, 0.001 mg to about
500 mg, 0.001
mg to about 250 mg, about 0.001 mg to 100 mg, about 0.001 mg to about 50 mg
and about 0.001
mg to about 25 mg. Multiple doses may be administered during the day,
especially when relatively
77
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
large amounts are deemed to be needed, for example 2, 3 or 4 doses. Depending
on the
individual and as deemed appropriate from the patient's physician or caregiver
it may be
necessary to deviate upward or downward from the doses described herein.
The amount of active ingredient, or an active salt or derivative thereof,
required for use in
treatment will vary not only with the particular salt selected but also with
the route of
administration, the nature of the condition being treated and the age and
condition of the patient
and will ultimately be at the discretion of the attendant physician or
clinician. In general, one
skilled in the art understands how to extrapolate in vivo data obtained in a
model system, typically
an animal model, to another, such as a human. In some circumstances, these
extrapolations may
merely be based on the weight of the animal model in comparison to another,
such as a mammal,
preferably a human, however, more often, these extrapolations are not simply
based on weights,
but rather incorporate a variety of factors. Representative factors include
the type, age, weight,
sex, diet and medical condition of the patient, the severity of the disease,
the route of
administration, pharmacological considerations such as the activity, efficacy,
pharmacokinetic
and toxicology profiles of the particular compound employed, whether a drug
delivery system is
utilized, on whether an acute or chronic disease state is being treated or
prophylaxis conducted
or on whether further active compounds are administered in addition to the
compounds of the
present disclosure and as part of a drug combination. The dosage regimen for
treating a disease
condition with the compounds and/or compositions of this disclosure is
selected in accordance
with a variety factors as cited above. Thus, the actual dosage regimen
employed may vary widely
and therefore may deviate from a preferred dosage regimen and one skilled in
the art will
recognize that dosage and dosage regimen outside these typical ranges can be
tested and,
where appropriate, may be used in the methods of this disclosure.
The desired dose may conveniently be presented in a single dose or as divided
doses
administered at appropriate intervals, for example, as two, three, four or
more sub-doses per day.
The sub-dose itself may be further divided, e.g., into a number of discrete
loosely spaced
administrations. The daily dose can be divided, especially when relatively
large amounts are
administered as deemed appropriate, into several, for example 2, 3 or 4 part
administrations. If
appropriate, depending on individual behavior, it may be necessary to deviate
upward or
downward from the daily dose indicated.
The compounds of the present disclosure can be administrated in a wide variety
of oral
and parenteral dosage forms. It will be obvious to those skilled in the art
that the following dosage
forms may comprise, as the active component, either a compound of the
disclosure or a
pharmaceutically acceptable salt, solvate or hydrate of a compound of the
disclosure.
For preparing pharmaceutical compositions from the compounds of the present
disclosure, the selection of a suitable pharmaceutically acceptable carrier
can be either solid,
liquid or a mixture of both. Solid form preparations include powders, tablets,
pills, capsules,
cachets, suppositories and dispersible granules. A solid carrier can be one or
more substances
78
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
which may also act as diluents, flavoring agents, solubilizers, lubricants,
suspending agents,
binders, preservatives, tablet disintegrating agents, or an encapsulating
material.
In powders, the carrier is a finely divided solid which is in a mixture with
the finely divided
active component.
In tablets, the active component is mixed with the carrier having the
necessary binding
capacity in suitable proportions and compacted to the desire shape and size.
The powders and tablets may contain varying percentage amounts of the active
compound. A
representative amount in a powder or tablet may contain from 0.5 to about 90
percent of the
active compound; however, an artisan would know when amounts outside of this
range are
necessary. Suitable carriers for powders and tablets are magnesium carbonate,
magnesium
stearate, talc, sugar, lactose, pectin, dextrin, starch, gelatin, tragacanth,
methylcellulose, sodium
carboxymethylcellulose, a low melting wax, cocoa butter and the like. The term
"preparation"
includes the formulation of the active compound with encapsulating material as
carrier providing
a capsule in which the active component, with or without carriers, is
surrounded by a carrier,
which is thus in association with it. Similarly, cachets and lozenges are
included. Tablets,
powders, capsules, pills, cachets and lozenges can be used as solid forms
suitable for oral
administration.
For preparing suppositories, a low melting wax, such as an admixture of fatty
acid
glycerides or cocoa butter, is first melted and the active component is
dispersed homogeneously
therein, as by stirring. The molten homogenous mixture is then poured into
convenient sized
molds, allowed to cool and thereby to solidify.
Liquid form preparations include solutions, suspensions and emulsions, for
example,
water or water-propylene glycol solutions. For example, parenteral injection
liquid preparations
can be formulated as solutions in aqueous polyethylene glycol solution.
Injectable preparations,
for example, sterile injectable aqueous or oleaginous suspensions may be
formulated according
to the known art using suitable dispersing or wetting agents and suspending
agents. The sterile
injectable preparation may also be a sterile injectable solution or suspension
in a nontoxic
parenterally acceptable diluent or solvent, for example, as a solution in 1,3-
butanediol. Among
the acceptable vehicles and solvents that may be employed are water, Ringer's
solution and
isotonic sodium chloride solution. In addition, sterile, fixed oils are
conventionally employed as a
solvent or suspending medium. For this purpose any bland fixed oil may be
employed including
synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid
find use in the
preparation of injectables.
The compounds according to the present disclosure may thus be formulated for
parenteral
administration (e.g., by injection, for example bolus injection or continuous
infusion) and may be
presented in unit dose form in ampoules, pre-filled syringes, small volume
infusion or in multi-
dose containers with an added preservative. The pharmaceutical compositions
may take such
forms as suspensions, solutions, or emulsions in oily or aqueous vehicles and
may contain
79
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
formulatory agents such as suspending, stabilizing and/or dispersing agents.
Alternatively, the
active ingredient may be in powder form, obtained by aseptic isolation of
sterile solid or by
lyophilization from solution, for constitution with a suitable vehicle, e.g.,
sterile, pyrogen-free
water, before use.
Aqueous formulations suitable for oral use can be prepared by dissolving or
suspending
the active component in water and adding suitable colorants, flavors,
stabilizing and thickening
agents, as desired.
Aqueous suspensions suitable for oral use can be made by dispersing the finely
divided
active component in water with viscous material, such as natural or synthetic
gums, resins,
methylcellulose, sodium carboxymethylcellulose, or other well-known suspending
agents.
Also included are solid form preparations which can be converted, shortly
before use, to
liquid form preparations for oral administration. Such liquid forms include
solutions, suspensions
and emulsions. These preparations may contain, in addition to the active
component, colorants,
flavors, stabilizers, buffers, artificial and natural sweeteners, dispersants,
thickeners, solubilizing
agents and the like.
For topical administration to the epidermis the compounds according to the
disclosure
may be formulated as ointments, creams or lotions, or as a transdermal patch.
Ointments and creams may, for example, be formulated with an aqueous or oily
base with
the addition of suitable thickening and/or gelling agents. Lotions may be
formulated with an
aqueous or oily base and will in general also contain one or more emulsifying
agents, stabilizing
agents, dispersing agents, suspending agents, thickening agents, or coloring
agents.
Formulations suitable for topical administration in the mouth include lozenges
comprising
active agent in a flavored base, usually sucrose and acacia or tragacanth;
pastilles comprising
the active ingredient in an inert base such as gelatin and glycerin or sucrose
and acacia; and
mouthwashes comprising the active ingredient in a suitable liquid carrier.
Solutions or suspensions are applied directly to the nasal cavity by
conventional means,
for example with a dropper, pipette or spray. The formulations may be provided
in single or multi-
dose form. In the latter case of a dropper or pipette, this may be achieved by
the patient
administering an appropriate, predetermined volume of the solution or
suspension. In the case of
a spray, this may be achieved for example by means of a metering atomizing
spray pump.
Administration to the respiratory tract may also be achieved by means of an
aerosol
formulation in which the active ingredient is provided in a pressurized pack
with a suitable
propellant. If the compounds of the present disclosure or pharmaceutical
compositions
comprising them are administered as aerosols, for example as nasal aerosols or
by inhalation,
this can be carried out, for example, using a spray, a nebulizer, a pump
nebulizer, an inhalation
apparatus, a metered inhaler or a dry powder inhaler. Pharmaceutical forms for
administration of
the compounds of the present disclosure as an aerosol can be prepared by
processes well known
to the person skilled in the art. For their preparation, for example,
solutions or dispersions of the
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
compounds of the present disclosure in water, water/alcohol mixtures or
suitable saline solutions
can be employed using customary additives, for example benzyl alcohol or other
suitable
preservatives, absorption enhancers for increasing the bioavailability,
solubilizers, dispersants
and others and, if appropriate, customary propellants, for example include
carbon dioxide, CFCs,
such as, dichlorodifluoromethane, trichlorofluoromethane, or
dichlorotetrafluoroethane; and the
like. The aerosol may conveniently also contain a surfactant such as lecithin.
The dose of drug
may be controlled by provision of a metered valve.
In formulations for administration to the respiratory tract, including
intranasal formulations,
the compound will generally have a small particle size for example of the
order of 10 microns or
less. Such a particle size may be obtained by means known in the art, for
example by
micronization. When desired, formulations adapted to give sustained release of
the active
ingredient may be employed.
Alternatively, the active ingredients may be provided in the form of a dry
powder, for
example, a powder mix of the compound in a suitable powder base such as
lactose, starch, starch
derivatives such as hydroxypropylmethyl cellulose and polyvinylpyrrolidone
(PVP). Conveniently
the powder carrier will form a gel in the nasal cavity. The powder composition
may be presented
in unit dose form for example in capsules or cartridges of, e.g., gelatin, or
blister packs from which
the powder may be administered by means of an inhaler.
The pharmaceutical preparations are preferably in unit dosage forms. In such
form, the
preparation is subdivided into unit doses containing appropriate quantities of
the active
component. The unit dosage form can be a packaged preparation, the package
containing
discrete quantities of preparation, such as packeted tablets, capsules and
powders in vials or
ampoules. Also, the unit dosage form can be a capsule, tablet, cachet, or
lozenge itself, or it can
be the appropriate number of any of these in packaged form.
Tablets or capsules for oral administration and liquids for intravenous
administration are
preferred compositions.
The compounds according to the disclosure may optionally exist as
pharmaceutically
acceptable salts including pharmaceutically acceptable acid addition salts
prepared from
pharmaceutically acceptable non-toxic acids including inorganic and organic
acids.
Representative acids include, but are not limited to, acetic, benzenesulfonic,
benzoic,
camphorsulfonic, citric, ethenesulfonic, dichloroacetic, formic, fumaric,
gluconic, glutamic,
hippuric, hydrobromic, hydrochloric, isethionic, lactic, maleic, malic,
mandelic, methanesulfonic,
mucic, nitric, oxalic, pamoic, pantothenic, phosphoric, succinic, sulfiric,
tartaric, oxalic, p-
toluenesulfonic and the like. Certain compounds of the present disclosure
which contain a
carboxylic acid functional group may optionally exist as pharmaceutically
acceptable salts
containing non-toxic, pharmaceutically acceptable metal cations and cations
derived from organic
bases. Representative metals include, but are not limited to, aluminium,
calcium, lithium,
magnesium, potassium, sodium, zinc and the like. In some embodiments the
pharmaceutically
81
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
acceptable metal is sodium. Representative organic bases include, but are not
limited to,
benzathine (N1,N2-dibenzylethane-1,2-diamine), chloroprocaine (2-
(diethylamino)ethyl 4-
(chloroamino)benzoate), choline, diethanolamine, ethylenediamine, meglumine
((2R,3R,4R,5S)-
6-(methylamino)hexane-1,2,3,4,5-pentaol), procaine (2-(diethylamino)ethyl 4-
aminobenzoate),
and the like. Certain pharmaceutically acceptable salts are listed in Berge,
et al., Journal of
Pharmaceutical Sciences, 66:1 -1 9 (1977).
The acid addition salts may be obtained as the direct products of compound
synthesis. In
the alternative, the free base may be dissolved in a suitable solvent
containing the appropriate
acid and the salt isolated by evaporating the solvent or otherwise separating
the salt and solvent.
The compounds of this disclosure may form solvates with standard low molecular
weight solvents
using methods known to the skilled artisan.
Compounds of the present disclosure can be converted to "pro-drugs." The term
"pro-
drugs" refers to compounds that have been modified with specific chemical
groups known in the
art and when administered into an individual these groups undergo
biotransformation to give the
parent compound. Pro-drugs can thus be viewed as compounds of the disclosure
containing one
or more specialized non-toxic protective groups used in a transient manner to
alter or to eliminate
a property of the compound.
Some embodiments of the present disclosure include a method of producing a
pharmaceutical composition for "combination-therapy" comprising admixing at
least one
compound according to any of the compound embodiments disclosed herein,
together with at
least one known pharmaceutical agent as described herein and a
pharmaceutically acceptable
carrier.
OTHER UTILITIES
Another object of the present disclosure relates to radio-labeled compounds of
the present
disclosure that would be useful not only in radio-imaging but also in assays,
both in vitro and in
vivo, for localizing and quantitating 5-HT2A receptors in tissue samples,
including human, and for
identifying 5-HT2A receptor ligands by inhibition binding of a radio-labeled
compound. It is a further
object of this disclosure to develop novel 5-HT2A receptor assays of which
comprise such radio-
labeled compounds.
The present disclosure embraces isotopically-labeled crystalline forms of the
present
disclosure. Isotopically or radio-labeled compounds are those which are
identical to compounds
disclosed herein, but for the fact that one or more atoms are replaced or
substituted by an atom
having an atomic mass or mass number different from the atomic mass or mass
number most
commonly found in nature. Suitable radionuclides that may be incorporated in
compounds of the
present disclosure include but are not limited to 2H (also written as D for
deuterium), 3H (also
written as T for tritium), 11C, 13C, 14C, 13N, 15N, 150, 170, 180, 18F, 35S,
36C1, 75Br, 76Br, 77Br, 82Br,
1231, 1241, 1251, and 1311. The radionuclide that is incorporated in the
instant radio-labeled compounds
will depend on the specific application of that radio-labeled compound. For
example, for in vitro
82
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
5-HT2A serotonin receptor labeling and competition assays, compounds that
incorporate 3H, 14C,
82gr, 1251, 1311 or 35S will generally be most useful. For radio-imaging
applications 11C, 18F, 1251, 1231,
1241, 1311, 75Br, 76Br or 77Br will generally be most useful.
It is understood that a "radio-labeled " or "labeled compound" is a
crystalline form of
Compound la that has incorporated at least one radionuclide; in some
embodiments the
radionuclide is selected from the group consisting of 3H, 14C, 1251 ,35S and
82Br.
Certain isotopically-labeled crystalline forms of the present disclosure are
useful in
compound and/or substrate tissue distribution assays. In some embodiments the
radionuclide 3H
and/or 14C isotopes are useful in these studies. Further, substitution with
heavier isotopes such
as deuterium (i.e., 2H) may afford certain therapeutic advantages resulting
from greater metabolic
stability (e.g., increased in vivo half-life or reduced dosage requirements)
and hence may be
preferred in some circumstances. Isotopically labeled crystalline forms of the
present disclosure
can generally be prepared by following procedures analogous to those disclosed
in the and
Examples infra, by substituting an isotopically labeled reagent for a non-
isotopically labeled
reagent. Other synthetic methods that are useful are discussed infra.
Moreover, it should be
understood that all of the atoms represented in the compounds of the
disclosure can be either
the most commonly occurring isotope of such atoms or the scarcer radio-isotope
or
nonradioactive isotope.
Synthetic methods for incorporating radio-isotopes into organic compounds are
applicable
to compounds of the disclosure and are well known in the art. These synthetic
methods, for
example, incorporating activity levels of tritium into target molecules, are
as follows:
A. Catalytic Reduction with Tritium Gas: This procedure normally yields high
specific
activity products and requires halogenated or unsaturated precursors.
B. Reduction with Sodium Borohydride [31-I]: This procedure is rather
inexpensive and
requires precursors containing reducible functional groups such as aldehydes,
ketones, lactones,
esters and the like.
C. Reduction with Lithium Aluminum Hydride [31-I]: This procedure offers
products at
almost theoretical specific activities. It also requires precursors containing
reducible functional
groups such as aldehydes, ketones, lactones, esters and the like.
D. Tritium Gas Exposure Labeling: This procedure involves exposing precursors
containing exchangeable protons to tritium gas in the presence of a suitable
catalyst.
E. N-Methylation using Methyl Iodide [31-I]: This procedure is usually
employed to prepare
0-methyl or N-methyl (3/-1) products by treating appropriate precursors with
high specific activity
methyl iodide (3I-I). This method in general allows for higher specific
activity, such as for example,
about 70-90 Ci/mmol.
Synthetic methods for incorporating activity levels of 1251 into target
molecules include:
A. Sandmeyer and like reactions: This procedure transforms an aryl amine or a
heteroaryl
amine into a diazonium salt, such as a diazonium tetrafluoroborate salt and
subsequently to 1251
83
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
labeled compound using Na1251. A represented procedure was reported by Zhu, G-
D. and co-
workers in J. Org. Chem., 2002, 67, 943-948.
B. Ortho 125I0dinati0n of phenols: This procedure allows for the incorporation
of 1251 at the
ortho position of a phenol as reported by Collier, T. L. and co-workers in J.
Labelled Compd.
Radiopharm., 1999, 42, S264-S266.
C. Aryl and heteroaryl bromide exchange with 1251: This method is generally a
two step
process. The first step is the conversion of the aryl or heteroaryl bromide to
the corresponding
tri-alkyltin intermediate using for example, a Pd catalyzed reaction [i.e.,
Pd(Ph3P).4] or through an
aryl or heteroaryl lithium, in the presence of a tri-alkyltinhalide or
hexaalkylditin [e.g.,
(CH3)3SnSn(CH3)3].
Some embodiments of the present disclosure include a method of producing a
pharmaceutical composition for "combination therapy" comprising admixing the
compound of
Formula (Ile) or any other Formula herein, such as Formula (la), or
pharmaceutically acceptable
salt thereof together with at least one known pharmaceutical agent as
described herein and a
pharmaceutically acceptable carrier.
It is noted that when the 5-HT2A receptor modulators are utilized as active
ingredients in
a pharmaceutical composition, these are not intended for use only in humans,
but in other non-
human mammals as well. Indeed, advances in the area of animal health-care
mandate that
consideration be given for the use of active agents, such as 5-HT2A receptor
modulators, for the
treatment of a 5-HT2A mediated diseases or disorders in domestic animals
(e.g., cats and dogs)
and in other domestic animals (e.g., such as cows, chickens, and fish). Those
of ordinary skill in
the art will understand the utility of such compounds in such settings.
Other uses of the disclosed receptors and methods will become apparent to
those skilled
in the art based upon, inter alia, a review of this disclosure.
As will be recognized, the steps of the methods of the present invention need
not be
performed any particular number of times or in any particular sequence.
Additional objects,
advantages and novel features of this invention will become apparent to those
skilled in the art
upon examination of the following examples thereof, which are illustrative and
not limiting.
EXAMPLES
Example 1: Preparation of 5-(2-fluoro-5-nitrophenyI)-4,6-dimethylpyrimidine
(Compound Ilb-1).
02N I ,r;'
,
IIb-1 N
Tetrahydrofuran (THF) (37.20 kg) was added to a solution of potassium
phosphate tribasic
(10.24 kg) in purified water (24.32 kg), and the mixture was heated to 20-30
C. 5-Bromo-4,6-
84
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
dimethylpyrimidine (3.00 kg, 16.0 mol) and 2-Fluoro-5-nitrophenylboronic acid
(3.26, 17.6 mol)
were added, and the reactor interior was rinsed down with purified water (6.00
kg). The mixture
was sparged with nitrogen, and a nitrogen atmosphere was maintained for the
duration of the
reaction. SPhos Pd G3 (0.50 kg) was added, and the reaction mixture was heated
to 40-45 C
for 1 h. The mixture was heated to 55-65 C and stirred at this temperature
for 4 h, when HPLC
analysis showed 1% of 5-bromo-4,6-dimethylpyrimidine remained.
The reaction was cooled to 25-35 C, celite (0.46 kg) was added, and the
mixture was
stirred for 1 h. Solids were filtered and washed with THF (3.80 kg), and the
washing was
combined with the filtrate. The filtrate was heated to 25-35 C, stirring was
stopped, and the
mixture was allowed to settle. The bottom aqueous layer was back-extracted
with methyl tett-
butyl ether (MTBE) (11.18 kg), and the organic layers were combined. Celite
(0.3 kg) was added,
and the mixture was stirred for 1 h. Solids were filtered and washed with MTBE
(3.00 kg), and
the washing was combined with the filtrate. The filtrate was concentrated at
atmospheric
pressure and temperature 65 C to a target volume of 15.00 L. Heptane (21.00
kg) was added,
and the mixture was concentrated at atmospheric pressure and temperature 65 C
to a target
volume of 18.00 L. The mixture was cooled to < 50 C, Isopropyl Alcohol (IPA)
(0.6 kg) was
added, and the resulting mixture was cooled to 20-30 C and stirred for 2 h.
The mixture was
filtered, and the filter cake was washed with Heptane/IPA (95:5 v/v, 10.35
kg). The solids were
dried under vacuum 26 inches Hg) and nitrogen purge at 30-40 C for 12 h to
give 3.90 kg (15.8
mol, 98% yield) of 5-(2-fluoro-5-nitrophenyI)-4,6-dimethylpyrimidine (11b-1).
1H NMR (400 MHz,
methanol-d4) 6 = 8.96 (s, 1H), 8.50 - 8.44 (m, 1H), 8.36 (dd, J = 2.9, 6.1 Hz,
1H), 7.58 (t, J = 8.8
Hz, 1H), 2.32 (s, 6H).
Example 2: Preparation of 4,6-dimethy1-5-(5-nitro-2-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)pyrimidine (Compound 11c-1, Hydrochloride Salt).
N)
HCI
0
02N N
1
IIc-1
Dimethylformamide (DMF) (31.65 kg), cesium carbonate (milled) (11.18 kg), 5-(2-
fluoro-
5-nitropheny1)-4,6-dimethylpyrimidine (3.36 kg, 13.6 mol), and 1-(2-
hydroxyethyl)pyrrolidine (1.88
kg, 16.3 mol) were combined under nitrogen, and the mixture was heated to 80-
90 C and stirred
at this temperature for 22 h, when HPLC analysis showed <3% of 5-(2-fluoro-5-
nitrophenyI)-4,6-
dimethylpyrimidine remained.
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
The mixture was cooled to 15-25 C, and purified water (50.55 kg) was added
over 1 h
while maintaining temperature 30 C. MTBE (50.55 kg) was added, and the mixture
was stirred
for 30 min at 20-30 C. Layers were allowed to settle, and the bottom aqueous
layer was back-
extracted with MTBE (50.65 kg). The organic layers were combined, washed with
5% sodium
chloride aqueous solution (2 x 16.80 kg), and concentrated under vacuum at 55
C to a target
volume of 16.80 L. MTBE (80.30 kg) was added, and the mixture was filtered to
remove solids.
The solids were washed with MTBE (6.8 kg), and the wash was combined with the
filtrate.
Hydrogen chloride (3M in methanol) (3.94 kg) was added to the filtrate while
maintaining
temperature 30 C over 30 min, during which, the product began to precipitate.
The resulting
slurry was stirred for 1 h at 15-30 C, filtered, washed with MTBE (2 x 10
kg), and dried under
vacuum 26 inches Hg) and nitrogen purge at 15-25 C for 14 h to give 3.00 kg of
product.
The product was dissolved in ethanol (45.50 kg) at 50-60 C and recrystallized
by addition
of MTBE (22.20 kg), cooling to 15-25 C, and stirring at this temperature for
at least 8 h. The
recrystallized product was filtered and washed with MTBE/Ethanol (3:1 v/v, 11
kg) and MTBE
(8.60 kg), and dried under vacuum 26 inches Hg) and nitrogen purge at 15-25
C for at least
12 h to give 2.25 kg (5.94 mol, 44% yield) of 4,6-dimethy1-5-(5-nitro-2-(2-
(pyrrolidin-1-
yl)ethoxy)phenyl)pyrimidine hydrochloride (11c-1).
Example 3: Preparation of 3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-
yl)ethoxy)aniline (Compound Ild-1, Hydrochloride Salt).
N)
HCI
0
H2N N
lid-1 I )
4,6-Dimethy1-5-(5-nitro-2-(2-(pyrrolidin-1-yl)ethoxy)phenyl)pyrimidine
hydrochloride (2.25
kg, 5.94 mol) was dissolved in purified water (25.14 kg) under nitrogen. In a
separate reactor,
palladium (Pd) (10% on Carbon, 0.24 kg) and ethanol (26.80 kg) were combined,
and the mixture
was purged with nitrogen/vacuum three times. To this Pd/C in ethanol mixture,
the 4,6-Dimethy1-
5-(5-nitro-2-(2-(pyrrolidin-1-yl)ethoxy)phenyl)pyrimidine hydrochloride
solution was added under
vacuum, and the mixture was purged with nitrogen/vacuum three times. The
reactor was
pressurized to 10-20 psig with hydrogen and maintained at this pressure until
reaction completion.
The reaction mixture was stirred at 15-25 C for 27 h, when HPLC analysis
showed 1% 4,6-
Dimethy1-5-(5-nitro-2-(2-(pyrrolidin-1-yl)ethoxy)phenyl)pyrimidine
hydrochloride remained.
The hydrogen pressure was released through a scrubber, and the reactor was
purged
with nitrogen three times and maintained under nitrogen for the duration of
the workup. The
86
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
mixture was filtered, washed with purified water (5.62 kg), and the wash was
combined with the
filtrate. The filtrate was stirred with SiliaMetS Thiourea (0.30 kg) at 15-25
C for 24 h to scavenge
residual Pd. The solids were filtered, washed with purified water (5.50 kg),
and the wash was
combined with the filtrate. The filtrate was concentrated under vacuum at
temperature 45 C to
a volume of 15 L. Residual water was removed through azeotropic distillation
with Ethanol (10 x
18 kg) to a final volume of -20 L until Karl Fischer analysis showed 1% water
remained. The
mixture was cooled to 0-10 C and stirred at this temperature for 5 h. The
product was filtered,
washed with cold Ethanol (11.44 kg, precooled to -5 C), cold MTBE (11.48 kg,
precooled to -
5 C), and dried under vacuum 26 inches Hg) and nitrogen purge at 15-25 C for
17 h until LOD
5% to give 1.71 kg (4.90 mol, 83% yield) of 3-(4,6-dimethylpyrimidin-5-yI)-4-
(2-(pyrrolidin-1-
yl)ethoxy)aniline hydrochloride (11d-1). 1H NMR (400 MHz, DMSO-d6) 6 = 8.81
(s, 1H), 6.88 (d, J
= 8.3 Hz, 1H), 6.62 (dd, J = 2.9, 8.8 Hz, 1H), 6.35 (d, J = 2.9 Hz, 1H), 4.78
(br s, 2H), 3.86 (t, J =
5.9 Hz, 2H), 2.51 (d, J = 2.0 Hz, 2H), 2.26 - 2.20 (m, 4H), 2.17 (s, 6H), 1.54
(td, J = 3.1, 6.6 Hz,
4H).
Example 4: Preparation of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la).
0
0
I
la
To a solution of potassium carbonate (1.84 kg) in purified water (29.70 kg)
was added
methylene chloride (DCM) (13.60 kg) and 3-(4,6-dimethylpyrimidin-5-yI)-4-(2-
(pyrrolidin-1-
yl)ethoxy)aniline hydrochloride (1.55 kg, 4.44 mol) at 15-25 C. A solution of
cyclopropane
carbonyl chloride (0.96 kg) in DCM (31.15 kg) was added over 45 min,
maintaining temperature
at 15-25 C. The biphasic reaction mixture was stirred at 15-25 C for 90 min,
when HPLC
analysis showed 1%
of 3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-1-yl)ethoxy)aniline
hydrochloride remained.
Stirring was stopped, the layers were allowed to settle, and the bottom
product organic
layer was separated. The aqueous layer was back-extracted with DCM (20.65 kg),
and the
organic layers were combined. The organic layer was washed with potassium
carbonate
aqueous solution (5%, 15.52 kg), purified water (15.50 kg), and polish-
filtered through a 0.2-
micron cartridge filter, rinsing with DCM (6.20 kg) and combining the rinse
with the filtrate.
MTBE (23.30 kg) was added to the filtrate, and the mixture was concentrated to
a residue by
distillation at atmospheric pressure and temperature of 50-55 C. The residue
was cooled to 45
87
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
C, MTBE (20.55 kg) was added, and the mixture was cooled to 15-25 C over 1 h,
during
which, a slurry formed. The slurry was cooled to 0-10 C and stirred at this
temperature for 1 h.
The solids were filtered, washed with cold MTBE (12.00 kg, precooled to 0 C),
and dried under
vacuum 26 inches Hg) and nitrogen purge at 15-25 C for at least 12 h to give
1.13 kg (2.97
mol, 67% yield) of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (la) in a crystalline form. 1H NMR
(400 MHz,
methanol-d4) 6 = 8.83 (s, 1H), 7.58 (dd, J= 2.6, 8.9 Hz, 1H), 7.39 (d, J= 2.6
Hz, 1H), 7.12 (d, J
= 8.9 Hz, 1H), 4.11 (t, J = 5.4 Hz, 2H), 2.73 (t, J = 5.4 Hz, 2H), 2.41 - 2.33
(m, 4H), 2.27 (s, 6H),
1.78 - 1.72 (m, 1H), 1.69 (td, J = 3.3, 6.9 Hz, 4H), 0.97 - 0.90 (m, 2H), 0.88
- 0.80 (m, 2H).
LC/MS m/z = 380.9 [M+H].
The material was characterized by PXRD (Figure 1), and DSC/TGA (Figures 2 and
3
respectively). DSC analysis showed a broad endothermic melt with onset at
140.1 C and peak
at 151.5 C. TGA analysis displayed a 3.6 wt. `)/0 loss up to 240 C
(theoretically 0.8 eq. water).
Example 5: Preparation of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Besylate Salt).
N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (1.0 g, 2.13 mmol) was dissolved in
20 mL ethyl
acetate. Benzenesulfonic acid (0.416 g, 2.63 mmol) was dissolved in 4 mL ethyl
acetate and this
solution was added dropwise over 5 minutes to the N-(3-(4,6-dimethylpyrimidin-
5-y1)-4-(2-
(pyrrolidin-1-yl)ethoxy)phenyl)cyclopropanecarboxamide solution at 40 C and
was stirred at this
temperature for a further two hours. The benzenesulfonate (besylate) salt
precipitated and was
collected by filtration using a Buchner funnel and dried at 40 C for 16
hours.
The resulting crystalline material, besylate salt characterized by PXRD
(Figure 4), and
DSC/TGA (Figures 5 and 6 respectively). DSC analysis showed a endothermic melt
with onset
at 219.4 C and peak at 225.7 C. TGA analysis displayed a 2.1 wt. % loss up
to 220 C
(theoretically 0.64 eq. water).
Example 6: Preparation of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Citrate Salt).
N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (2.00 g, 5.26 mmol) was added to a
250 mL round
bottom flask and dissolved in ethanol (40 mL). Citric acid monohydrate (1.105
g, 5.26 mmol)
was added to a 100 mL Erlenmeyer flask and dissolved in ethanol (10 mL). The
citric acid
solution was added to the N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide solution in 0.5 mL portions over 10
minutes while
stirring the reaction. The citrate salt began to slowly precipitate form
solution after a few
88
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
minutes and the reaction was stirred for 24 h. The salt was isolated by
Buchner filtration and
subsequently dried in the oven at 40 C for 5 hours.
The resulting crystalline material, citrate salt characterized by PXRD (Figure
7), and
DSC/TGA (Figures 8 and 9 respectively). DSC analysis showed a endothermic melt
with onset
at 172.2 C and peak at 173.5 C. TGA analysis displayed no significant mass
loss until the
melt/dissociation point.
Example 7: Preparation of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Fumarate Salt).
N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (20 mg) was dissolved in 0.2 ml ethyl
acetate and an
equimolar amount of fumaric acid was also dissolved in 0.2 mL ethyl acetate.
N-(3-(4 ,6-dimethylpyrimid in-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopro pan ecarboxamid e solution was added to the fumaric
acid solution while
stirring at 40 C and then stirred at 40 C40 C for one hour. Then it was
cooled to 20 C with
stirring for additional 72 hours. The precipitated salt was isolated by
centrifugation.
The resulting crystalline material, fumarate salt, was characterized by PXRD
(Figure 10),
and DSC/TGA (Figures 11 and 12 respectively). DSC analysis showed a
endothermic melt with
onset at 151.3 C and peak at 156.1 C. TGA analysis displayed a 4.9 wt. `)/0
loss up to 200 C
(theoretically 2.5 eq. water).
Example 8: Preparation of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Hydrochloride Salt).
N-(3-(4 ,6-dimethylpyrimid in-5-y1)-4-(2-(pyrrolidin-1 -
yl)ethoxy)phenyl)cyclopropanecarboxamide (100 mg) was dissolved in 2 mL
isopropyl acetate
and heated to 40 C and an equimolar amount of aqueous 3.3M HC1 solution (79.6
mL) was
added and stirred for one hour at 40 C. The reaction was stirred overnight
during which it cooled
down to 20 C. The reaction mixture was evaporated at room temperature and
then the resulting
solid was dried in a vacuum oven at 40 C for 48 hours.
The resulting crystalline material, hydrochloride salt, was characterized by
PXRD (Figure
13), and DSC/TGA (Figures 14 and 15 respectively). DSC analysis showed a broad
endothermic
event (onset: 23.8 C; peak: 74.6 C) associated with solvent loss and a
second endothermic
event (onset: 188.5 C; peak: 193.9 C) associated with melting. TGA analysis
displayed a 4.3
wt. % loss up to 200 C (theoretically 1.03 eq. water).
Example 9: Preparation of N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Mesylate Salt).
89
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (20 mg) was dissolved in 0.4 mL ethyl
acetate and
heated to 40 C with rapid stirring and 1.0 equivalent of methanesulfonic acid
(3.3M in ethyl
acetate) solution was added and continued stirring at 40 C for one hour and
then stirred overnight
during which it cooled down to 20 C. The reaction mixture was evaporated at
room temperature
and then the resulting solid was dried in a vacuum oven at 40 C for 24 hours.
The resulting crystalline material, mesylate salt, was characterized by PXRD
(Figure 16),
and DSC/TGA (Figures 17 and 18 respectively). DSC analysis showed one
endothermic event
(onset: 181.4 C; peak: 189.7 C) associated with melting. TGA analysis
displayed a 2.2 wt. `)/0
loss up to 180 C (theoretically 0.6 eq. water).
Example 10: Preparation of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-
1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Phosphate Salt).
N-(3-(4 ,6-dimethylpyrimid in-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (100 mg) was dissolved in 2.0 mL
acetonitrile and
heated to 40 C with rapid stirring and 1.0 equivalent of phosphoric acid (79
mL, 3.3M) solution
was added and continued stirring at 40 C for one hour and then it was cooled
down to 20 C.
The reaction mixture was decanted and the collected solids dried in a vacuum
oven at 40 C for
3 hours.
The resulting crystalline material, phosphate salt, was characterized by PXRD
(Figure
19), and DSC/TGA (Figures 20 and 21 respectively). DSC analysis showed one
endothermic
event (onset: 201.7 C; peak: 217.9 C) associated with melting. TGA analysis
displayed a 3.4
wt. % loss up to 180 C (theoretically 0.9 eq. water).
Example 11: Preparation of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-
1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Succinate Salt).
N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (175 mg) was dissolved in 3.5 mL
ethyl acetate and
heated to 40 C with rapid stirring and 1.0 equivalent of succinic acid
solution (in ethyl acetate)
was added and continued stirring at 40 C for one hour and then it was cooled
down to 20 C
overnight. The precipitated solids were collected by filtration and the
collected solids dried in a
vacuum oven at 40 C.
The resulting crystalline material, succinate salt, was characterized by PXRD
(Figure 22),
and DSC/TGA (Figures 23 and 24 respectively). DSC analysis showed one
endothermic event
(onset: 116.3 C; peak: 119.8 C) associated with melting. TGA analysis
displayed a 24.8 wt. %
loss up to 150 C (theoretically 1.05 eq succinic acid).
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Example 12: Preparation of N-(3-(4,6-dimethylpyrimidin-5-yI)-4-(2-(pyrrolidin-
1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (Compound la; Tosylate Salt).
N-(3-(4,6-dimethylpyrimidin-5-y1)-4-(2-(pyrrolidin-1-
yl)ethoxy)phenyl)cyclopropanecarboxamide (175 mg) was dissolved in 3.5 mL
ethyl acetate and
heated to 40 C with rapid stirring and 1.0 equivalent of p-toluenesulfonic
acid solution (in ethyl
acetate) was added and continued stirring at 40 C for one hour and then it
was cooled down to
20 C overnight. The precipitated solids were collected by filtration and the
collected solids dried
in a vacuum oven at 40 C.
The resulting crystalline material, tosylate salt, was characterized by PXRD
(Figure 25),
and DSC/TGA (Figures 26 and 27 respectively). DSC analysis showed one
endothermic event
(onset: 151.3 C; peak: 170.1 C) associated with melting. TGA analysis
displayed a 1.5 wt. `)/0
loss up to 168 C (theoretically 0.47 eq. water).
Example 12: Powder X-ray Diffraction.
PXRD analysis was carried out on a PANalytical X'pert pro with PIXcel detector
(128
channels), scanning the samples between 3 and 35 20. The material was gently
ground (where
required) to release any agglomerates and loaded onto a multi-well plate with
Kapton or Mylar
polymer film to support the sample. The multi-well plate was then placed into
the diffractometer
and analysed using Cu K radiation (al A = 1.54060 A; a2 = 1.54443 A; 3 =
1.39225 A; al : az ratio
= 0.5) running in transmission mode (step size 0.0130 20, step time 18.87s)
using 40 kV / 40
mA generator settings. Data were visualized and images generatedusing the
HighScore Plus 4.7
desktop application (PANalytical, 2017).
Example 13: Differential Scanning Calorimetry (DSC).
Approximately, 1-5 mg of material was weighed into an aluminium DSC pan and
sealed
non-hermeticallywith an aluminium lid. The sample pan was then loaded into a
TA Instruments
Discovery DSC 2500 differential scanning calorimeter equipped with a RC90
cooler. The sample
and reference were heated to 200 C, 250 C, or 300 C at a scan rate of 10
C/min and the
resulting heat flow response monitored. The sample was re-cooled to 20 C and
then reheated
again to the specified temperature all at 10 C/min. Nitrogen was used as the
purge gas, at a flow
rate of 50 cm3/min.
Example 14: Thermal Gravimetric Analysis.
Approximately, 5-10 mg of material was added into a pre-tared open aluminium
pan and
loaded into a TA Instruments Discovery SDT 650 Auto - Simultaneous DSC and
held at room
temperature. The sample was then heated at a rate of 10 C/min from 30 C to
400 C during
which time the change in sample weight was recorded along with the heat flow
response (DSC).
Nitrogen was used as the sample purge gas, at a flow rate of 200 cm3/min.
91
CA 03235907 2024-04-17
WO 2023/067520
PCT/IB2022/060059
Those skilled in the art will recognize that various modifications, additions,
substitutions,
and variations to the illustrative examples set forth herein can be made
without departing from
the spirit of the invention and are, therefore, considered within the scope of
the invention.
92