Note: Descriptions are shown in the official language in which they were submitted.
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
ADMINISTRATION OF R-BETA-HYDROXYBUTYRATE AND RELATED
COMPOUNDS IN HUMANS
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application claims the benefit of U.S. Patent Application No.
17/367,206,
entitled "ADMINISTRATION OF R-BETA-HYDROXYBUTYRATE AND RELATED
COMPOUNDS IN HUMANS", filed on July 2, 2021, which is incorporated by
reference for
all purposes.
TECHNICAL FIELD
[002] The present invention relates to administration of butyrate, beta-
hydroxybutyrate, and
related compounds.
BACKGROUND
[003] Currently, beta-hydroxybutyrate salts can be administered orally or
intravenously in
humans to promote weight loss and/or ketosis. However, the excess intake of
salts such as
sodium, magnesium, and potassium may be unwarranted (e.g., high blood
pressure, stroke,
damage to organs, gastrointestinal problems, etc.). Thus, many people may not
be able to
tolerate administration of beta-hydroxybutyrate salts in amounts to promote or
sustain weight
loss and/or ketosis. Polymers of beta-hydroxybutyrate have also been
administered to
humans to promote ketosis. However, since polymers must be processed by the
body to
deliver beta-hydroxybutyrate to the individual, the delivery is slow and/or a
larger amount of
the polymer must be administered to deliver a specified amount of beta-
hydroxybutyrate.
1
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
SUMMARY
[004] In various implementations, a pharmaceutically effective amount of
butyrate, beta-
hydroxybutyrate, related compounds, and/or one or more other compounds may be
administered to an individual. For example, the pharmaceutically effective
amount of the
beta-hydroxybutyrate, related compounds, and/or one or more other compounds
may be
administered to cause weight loss, weight maintenance, elevate blood ketone
levels, maintain
blood ketone levels, reduce blood glucose levels, maintain blood glucose
levels, improve
focus, energy, cognitive function, traumatic brain injury, diabetes,
neurological disorders,
cancer, inflammatory conditions, suppressing appetite, anti-aging, anti-
glycation, epilepsy,
depression, performance, strength, muscle mass, fat loss, body composition,
and/or use as a
medicament etc. The pharmaceutically effective amount of butyrate, beta-
hydroxybutyrate,
related compounds, and/or combinations thereof may be administered to healthy
individuals
and/or not healthy individuals (e.g., with diseases and/or disorders).
[005] Implementations may include one or more of the following features. The
beta-
hydroxybutyrate may include the racemic mixture and/or the individual isomers
of beta-
hydroxybutyrate, such as R-beta-hydroxybutyrate (also known as D-beta-
hydroxybutyrate).
The beta-hydroxybutyrate may include related compounds. The beta-
hydroxybutyrate may
be coupled to a compound such as an amino acid. The beta-hydroxybutyrate may
include
beta-hydroxybutyrate salt and beta-hydroxybutyrate esters, in some
implementations. Other
compounds may include short chain fatty acids, short chain triglycerides,
medium chain fatty
acids, medium chain triglycerides, long chain fatty acids, long chain
triglycerides, berberine,
berberine metabolites, dihydroberberine, tetrahydroberberine and/or
combinations thereof.
One or more of the other compounds may be unencapsulated and/or encapsulated.
[006] In various implementations, a composition may be administered to induce
and/or
maintain ketosis. The composition may include approximately 0.5 g to
approximately 10 g of
R-beta-hydroxybutyrate.
[007] Implementations may include one or more of the following features. The
amount of
the composition administered may include approximately 0.5 to approximately 3
g of R-beta-
hydroxybutyrate. The composition may include additional composition, such as
compositions that are capable of independently increasing ketone levels,
inducing ketosis,
2
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
and/or maintaining ketosis. In some implementations, the composition may
include
additional compositions to provide other health benefits (e.g., increase
mental acuity,
strength, etc.). For example, the composition may include fatty acids and/or
esters of fatty
acids. For example, the composition may include a short chain fatty acid, an
ester of short
chain fatty acid, a medium chain fatty acid, an ester of medium chain fatty
acid, a long chain
fatty acid, or an ester of long chain fatty acid. The composition may include
flavoring(s),
vitamin(s), mineral(s), and/or binder(s). The composition may be administered
up to 5
times daily. The administration of the composition may increase strength,
mental acuity,
metabolism, fat loss, fat oxidation, motor function, muscle mass, and/or
combinations
thereof. In some implementations, the 0.5 to 10 g of R-beta-hydroxybutyrate
administered
includes R-beta-hydroxybutyrate and at least one of a polymer of R-beta-
hydroxybutyrate or
R-beta-hydroxybutyrate-complex.
[008] In various implementations, a composition may include approximately 0.5
g to
approximately 10 g of R-beta-hydroxybutyrate and one or more additional
compounds
capable of maintaining ketosis independently. Administration of the
composition may induce
and/or maintains ketosis in an individual.
[009] Implementations may include one or more of the following features. The R-
beta-
hydroxybutyrate may include R-beta-hydroxybutyrate salt, R-beta-
hydroxybutyrate- amino
acid complex, and/or R-beta-hydroxybutyrate polymer. The additional compounds
may
include fatty acids and/or esters of fatty acids. The fatty acids and/or
esters may include
natural (e.g., cream, coconut oil, macadamia oil, etc.) and/or artificial
fatty acids and/or esters
of fatty acids. For example, the composition may include a short chain fatty
acid, an ester of
short chain fatty acid, a medium chain fatty acid, an ester of medium chain
fatty acid, a long
chain fatty acid, or an ester of long chain fatty acid. In some
implementations, additional
compound(s) may include polymer(s) of beta-hydroxybutyrate, D,L-beta-
hydroxybutyrate,
butyrate, butyric acid, and/or triglyceride tributyrin. The additional
compound(s) may
include berberine, dihydroberberine, and/or tetrahydroberberine.
[010] In various implementations, pharmaceutically effective amounts of R-beta-
hydroxybutyrate and amino acid may be administered for inducing and/or
maintaining
ketosis.
3
CA 03237180 2024-04-30
WO 2023/278881
PCT/US2022/036030
[OM
Implementations may include one or more of the following features. The amount
of
R-beta-hydroxybutyrate to induce and/or maintain ketosis in an individual may
be less than
or equal to half of the amount of D,L-beta-hydroxybutyrate to induce and/or
maintain the
same level of ketosis (e.g., as measured by blood ketone levels). In some
implementations,
the amount of R-beta-hydroxybutyrate to induce and/or maintain ketosis in an
individual may
be less than the amount of D,L-beta-hydroxybutyrate or L-beta-hydroxybutyrate
to induce
and/or maintain the same level of ketosis. The composition may include
approximately 1 g to
approximately 5 grams of R-beta-hydroxybutyrate and approximately 0.5 to 2 g
of amino
acid. The amino acid may include Leucine. The composition may include a
mixture and/or
complex of the R-beta-hydroxybutyrate and amino acid. At least a portion of
the R-beta-
hydroxybutyrate may be complexed with the amino acid, in some implementations.
For
example, a portion of the R-beta-hydroxybutyrate may be administered in the
composition as
a salt and/or polymer and another portion of the R-beta-hydroxybutyrate may be
administered
as a complex with an amino acid (e.g., leucine). In some implementations, the
composition
may include at least one R-beta-hydroxybutyrate salt (e.g., in additional to
the
pharmaceutically effective amounts of R-beta-hydroxybutyrate in the
composition and/or as
the pharmaceutically effective amounts of R-beta-hydroxybutyrate).
[012] In various implementations, a composition for maintaining or increasing
weight loss
may include approximately 0.5 g to approximately 15 g of R-beta-
hydroxybutyrate, one or
more flavorings, one or more vitamins, one or more minerals, one or more
binders, and/or
one or more liquid carriers. The R-beta-hydroxybutyrate comprises one or more
salts of R-
beta-hydroxybutyrate salt. The composition may be orally administered to
maintaining
and/or increasing weight loss in an individual.
[013] Implementations may include one or more of the following features. The
liquid
carrier may include water. The amount of R-beta-hydroxybutyrate salt may
include
approximately 0.5 to approximately 5 g of R-beta-hydroxybutyrate salt. The
composition
may include at least one polymer of beta-hydroxybutyrate and at least one salt
of R-beta-
hydroxybutyrate. The administration of the composition increases mental
acuity. The
administration of the composition increases at least one of metabolism, fat
loss, fat oxidation,
motor function, and/or muscle mass. The composition may be administered up to
5 times
4
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
daily. The R-beta-hydroxybutyrate salt in the composition may include sodium R-
beta-
hydroxybutyrate, potassium R-beta-hydroxybutyrate, magnesium R-beta-
hydroxybutyrate,
and/or calcium R-beta-hydroxybutyrate salt.
[014] In various implementations, a composition for maintaining or inducing
ketosis may
include approximately 0.5 g to approximately 15 g of R-beta-hydroxybutyrate,
one or more
flavorings, one or more vitamins, one or more minerals, one or more binders,
and/or one or
more liquid carriers. The R-beta-hydroxybutyrate comprises one or more salts
of R-beta-
hydroxybutyrate salt. The composition may be orally administered to
maintaining and/or
induce ketosis in an individual.
[015] Implementations may include one or more of the following features. The
amount of
R-beta-hydroxybutyrate salt in the composition may include approximately 0.5
to
approximately 5 g of R-beta-hydroxybutyrate salt. The one or more salts of R-
beta-
hydroxybutyrate may include sodium R-beta-hydroxybutyrate, potassium R-beta-
hydroxybutyrate, calcium R-beta-hydroxybutyrate, and/or magnesium R-beta-
hydroxybutyrate. The liquid carrier may include water, milk, and/or coconut
water. The
administration of the composition may increase metabolism, fat loss, fat
oxidation, motor
function, and/or muscle mass. The administration of the compound may increase
mental
acuity, cognitive functioning, mood, energy, alertness, focus, and/or
performance.
[016] In various implementations, a composition for maintaining or inducing
ketosis may
include approximately 0.5 g to approximately 15 g of R-beta-hydroxybutyrate,
an additional
compound capable of increasing ketone levels independently, one or more
flavorings, one or
more vitamins, one or more minerals, one or more binders, and/or one or more
liquid carriers.
The R-beta-hydroxybutyrate comprises one or more salts of R-beta-
hydroxybutyrate salt.
The additional compound may include less than approximately 500 mg of
caffeine. The
composition may be orally administered to maintaining and/or induce ketosis in
an
individual.
[017] Implementations may include one or more of the following features. The
composition
may include approximately 5 mg to approximately 50 mg of caffeine. The
composition
comprises include approximately 0.5 g to approximately 5 g of R-beta-
hydroxybutyrate and
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
approximately 5 mg to approximately 50 mg of caffeine. The one or more salts
of R-beta-
hydroxybutyrate may include sodium R-beta-hydroxybutyrate, potassium R-beta-
hydroxybutyrate, calcium R-beta-hydroxybutyrate, and/or magnesium R-beta-
hydroxybutyrate. The administration of the composition may increase at least
one of weight
loss, metabolism, fat loss, fat oxidation, motor function, muscle mass, mental
acuity,
cognitive functioning, mood, energy, alertness, focus, and/or performance. The
liquid carrier
may include water, milk, and/or coconut water.
[018] The details of one or more implementations are set forth in the
accompanying
drawings and the description below. Other features, objects, and advantages of
the
implementations will be apparent from the description and drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[019] For a more complete understanding of this disclosure and its features,
reference is
now made to the following description, taken in conjunction with the
accompanying
drawings, in which:
[020] Figure 1 illustrates a table of blood ketone levels over time for 4
subjects for an
implementation of an example administration of D,L-beta-hydroxybutyrate and
R/D-beta-
hydroxybutyrate.
[021] Figure 2 illustrates a table of blood ketone levels over time for an
implementation of
an example administration of the microencapsulated butyrate compared to
traditional sodium
butyrate.
[022] Figure 3 illustrates a chart including lifespan of rats subject to an
implementation of
an administration of R-beta-hydroxybutyrate.
[023] Figure 4 illustrates a chart illustrating the results of motor skill
testing following an
implementation of an example administration protocol.
6
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[024] Figure 5A illustrates a chart illustrating fat loss results following an
implementation
of an example administration protocol.
[025] Figure 5B illustrates a chart illustrating fat mass and lean mass
results following an
implementation of an example administration protocol.
[026] Figure 6 illustrates a chart illustrating LPL levels in rats following
an implementation
of an example administration protocol.
[027] Figure 7 illustrates a chart illustrating blood ketone levels following
an
implementation of an example administration protocol.
[028] Figure 8 illustrates a chart illustrating improvement over a placebo
following an
implementation of an example administration protocol.
[029] Figure 9 illustrates a chart illustrating perceived exertion following
an implementation
of an example administration protocol.
[030] Figure 10 illustrates a chart illustrating blood ketone levels following
an
implementation of an example administration protocol.
[031] Figure 11 illustrates a chart illustrating blood ketone levels following
an
implementation of an example administration protocol.
[032] Figure 12A illustrates a chart illustrating RER levels following an
implementation of
an example administration protocol.
[033] Figure 12B illustrates a chart illustrating RER levels following an
implementation of
an example administration protocol.
[034] Figure 13A illustrates a chart illustrating perceived hunger following
an
implementation of an example administration protocol.
7
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[035] Figure 13B illustrates a chart illustrating perceived satiety following
an
implementation of an example administration protocol.
[036] Figure 13C illustrates a chart illustrating perceived energy following
an
implementation of an example administration protocol.
[037] Figure 14A illustrates a chart illustrating strength test results
following an
implementation of an example administration protocol.
[038] Figure 14B illustrates a chart illustrating strength test results
following an
implementation of an example administration protocol.
[039] Figure 14C illustrates a chart illustrating power test results following
an
implementation of an example administration protocol.
[040] Figure 15 illustrates a chart illustrating blood ketone levels following
an
implementation of an example administration protocol.
[041] Figure 16 illustrates a chart illustrating blood ketone levels following
an
implementation of an example administration protocol.
[042] Like reference symbols in the various drawings indicate like elements.
DETAILED DESCRIPTION
[043] In various implementations, compounds such as butyrate, beta-
hydroxybutyrate
and/or related compounds (e.g., derivatives, esters, polymers, etc.) can be
administered alone
or in combination with one or more other compounds. Administration of a
pharmaceutically
effective amount of these compound(s) may promote and/or maintain weight loss
and/or
ketosis. In some implementations, blood ketone levels and/or blood glucose
levels may be
reduced and/or maintained within a predetermined range when a pharmaceutically
effective
amount of one or more compounds are administered. In some implementations, a
health of
an individual (e.g., strength, symptoms of disease, mental acuity, fasting
glucose levels, etc.)
8
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
may be improved and/or maintained by administration of a compound that
includes butyrate,
beta-hydroxybutyrate and/or related compounds (e.g., derivatives, esters,
polymers, etc.).
[044] In various implementations butyrate, beta-hydroxybutyrate and/or related
compounds
may be administered to a human. Beta-hydroxybutyrate (e.g., R-beta-
hydroxybutyrate, L-
beta-hydroxybutyrate, and/or D,L-beta-hydroxybutyrate) may include beta-
hydroxybutyrate
salts and/or beta-hydroxybutyrate esters. In some implementations, beta-
hydroxybutyrate
may include beta-hydroxybutyrate bound to another compound (e.g., amino acids)
and/or
polymers of beta-hydroxybutyrate. For example, beta-hydroxybutyrate (e.g., R-
beta-
hydroxybutyrate, L-beta-hydroxybutyrate, and/or D,L-beta-hydroxybutyrate) may
include
beta-hydroxybutyrate salts, beta-hydroxybutyrate esters, beta-hydroxybutyrate
sodium salt
(e.g., sodium beta-hydroxybutyrate), beta-hydroxy butyrate potassium salt
(e.g., potassium
beta-hydroxybutyrate), beta-hydroxybutyrate calcium salt (e.g., calcium beta-
hydroxybutyrate), beta-hydroxybutyrate magnesium salt (e.g., magnesium beta-
hydroxybutyrate), beta-hydroxybutyrate lithium salt (e.g., lithium beta-
hydroxybutyrate),
sodium beta-hydroxybutyrate, arginine beta-hydroxybutyrate, lysine beta-
hydroxybutyrate,
histidine beta-hydroxybutyrate, ornithine beta-hydroxybutyrate, creatine beta-
hydroxybutyrate, agmatine beta-hydroxybutyrate, or citrulline beta-
hydroxybutyrate, other
appropriate organic salts that include beta-hydroxybutyrate, and/or
combinations thereof In
some implementations, the beta-hydroxybutyrate may include beta-
hydroxybutyrate salts
including (calcium, sodium, magnesium, potassium, zinc, selenium, chromium,
other
appropriate minerals, and/or combinations thereof. In some implementations,
the beta-
hydroxybutyrate may be complexed and/or coupled to another compound (e.g.,
amino acid
and/or berberine) and a beta-hydroxybutyrate salt may include a complex (e.g.,
chelate) that
includes a mineral (e.g., calcium, zinc, etc.) and the beta-hydroxybutyrate
compound coupled
to another compound. The beta-hydroxybutyrate may include single isomer beta-
hydroxybutyrate and/or polymer beta-hydroxybutyrate. For example, R-beta-
hydroxybutyrate may include single isomer R-beta-hydroxybutyrate and/or
polymer R-beta-
hydroxybutyrate. In some implementations, beta-hydroxybutyrate may be
administered with
1,3-butanediol, ethyl acetoacetate, ethyl beta-hydroxybutyrate.
[045] The beta-hydroxybutyrate may include racemic mixtures and/or individual
isomers of
betahydroxy-butyrate. In some implementations, one or more specific
chiralities of beta-
9
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
hydroxybutyrate may be utilized. For example, R-beta-hydroxybutyrate (also
referred to as
D-beta-hydroxybutyrate), S-beta-hydroxybutyrate (also referred to as L-beta-
hydroxybutyrate), and/or mixtures (e.g., raecemic mixtures) thereof may be
utilized. In
some implementations, R-beta-hydroxybutyrate may be included in the
composition (e.g., a
more purified form of R-beta-hydroxybutyrate rather than D,L-beta-
hydroxybutyrate). For
example, R-beta-hydroxybutyrate may include less than approximately 10
percent, less than
approximately 5 percent, or less than approximately 1 percent L-beta-
hydroxybutyrate. R-
beta-hydroxybutyrate may have a greater bioavailability than other chiralities
of beta-
hydroxybutyrate. R-beta-hydroxybutyrate may have a greater impact on a health
of an
individual (e.g., due to decreased side effects; increase ketone levels,
weight loss, mental
acuity, fat loss, etc.) than L-beta-hydroxybutyrate and/or D,L-beta-
hydroxybutyrate. In some
implementations, R-beta-hydroxybutyrate may cause improvements in health not
capable by
L-beta-hydroxybutyrate and/or D,L-beta-hydroxybutyrate. R-beta-hydroxybutyrate
may have
less impurities due to manufacturing, such as less crotonic acid (e.g., which
can be harmful to
individuals), than other forms of beta-hydroxybutyrate (e.g., L-beta-
hydroxybutyrate and/or
D,L-beta-hydroxybutyrate). In some implementations, R-beta-hydroxybutyrate may
be more
capable of binding with other compounds (e.g., purine, lysine, potassium,
and/or other amino
acids; dihydroberberine; etc.) to deliver the beta-hydroxybutyrate to a human.
Thus, R-beta-
hydroxybutyrate (e.g., greater than 90 percent purity of R-beta-
hydroxybutyrate and less than
percent L-beta-hydroxybutyrate) and/or mixtures with R-beta-hydroxybutyrate
may be
administered to humans. In some implementations, unexpectedly, a smaller
amount of R-
beta-hydroxybutyrate may be as pharmaceutically effective (e.g., in increasing
and/or
maintaining weight loss; in increasing and/or maintaining elevated ketone
levels, etc.) or
more pharmaceutically effective as D,L-beta-hydroxybutyrate (e.g., raecemic
mixture of D-
and L-beta-hydroxybutyrate). For example, approximately half an amount of R-
beta-
hydroxybutyrate may be administered to achieve the approximately the same
efficacy as D,L-
beta-hydroxybutyrate and/or L-beta-hydroxybutyrate. The R-beta-hydroxybutyrate
may be
more bioavailable than other chiralities of beta-hydroxybutyrate and thus
allow a smaller
effective amount than other chiralities. Thus, by utilizing R-beta-
hydroxybutyrate, the
administration amount of beta-hydroxybutyrate to be reduced (e.g., when
compared to the
administration amount of D,L-beta-hydroxybutyrate) while providing a
pharmaceutically
effective amount, such as (e.g., for weight loss and/or maintenance; for
elevating and/or
maintaining blood ketone levels). Reducing the amount of beta-hydroxybutyrate,
when the
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
beta-hydroxybutyrate is provided in salt form, may reduce a user's intake of
the cation of the
salt (e.g., sodium, potassium, etc.). Since intake of some of these cations in
beta-
hydroxybutyrate salts, such as sodium, potassium, magnesium, and calcium, in
amounts
greater than a predetermined recommended amount may cause health problems
(e.g., organ
damage, gastrointestinal problems, etc.), reducing the amount of beta-
hydroxybutyrate salt by
using R-beta-hydroxybutyrate may inhibit side effects and/or health problems
associated salts
combined with beta-hydroxybutyrate administration in users.
[046] In some implementations, a pharmaceutically effective amount of R-beta-
hydroxybutyrate may be administered in an individual to promote and/or
maintain ketosis,
cause weight loss and/or manage weight, and/or increase blood ketone levels.
For example,
approximately .1 g to approximately 50 g of R-beta-hydroxybutyrate may be
administered to
an individual. In some implementations, approximately 0.1 g to approximately
15 g of R-
beta-hydroxybutyrate may be administered to an individual. In some
implementations,
approximately 1 g to approximately 10 g of beta-hydroxybutyrate may be
administered, for
example, once a day to 5 times a day (e.g., to administer up to 50 g of beta-
hydroxybutyrate).
The administration may cause weight loss and/or maintenance; elevated beta-
hydroxybutyrate levels in the blood; elevated, reduced, and/or maintenance of
blood ketone
levels; induction and/or maintenance of ketosis; and/or reduction; improve
mental acuity;
improve focus; improve energy; improve cognitive function; reduce traumatic
brain injury;
improve diabetes; improve gluocose tolerance; decrease blood glucose levels;
reduce
neurological disorders and/or symptoms thereof; improve cancer and/or symptoms
thereof;
improve inflammatory conditions; suppressing appetite; improve symptoms
associated with
aging; provide anti-glycation affects; improve epilepsy and/or symptoms
thereof; improve
depression and/or symptoms thereof; improve performance; improve strength;
increase
muscle mass; increase fat loss; improve body composition; improve energy;
improve focus;
improve cognitive function; improve mood and/or well-being; and/or
combinations thereof
The beta-hydroxybutyrate (e.g., R-beta-hydroxybutyrate) may be administered in
healthy and
not healthy individuals (e.g., individuals with diseases and/or disorders).
[047] In some implementations, the beta-hydroxybutyrate, such as R-beta-
hydroxybutyrate,
may be administered with and/or coupled to a compound such as an amino acid.
For example
beta-hydroxybutyrate may be coupled to (e.g., chemically bonded to) amino
acids, such as
11
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
leucine, lysine, arginine, histidine, ornithine, creatine, agmatine,
citrulline and/or
combinations thereof. In some implementations, R-beta-hydroxybutyrate may be
utilized
rather than other chiralities since R-beta-hydroxybutyrate may be more easily
bound to
leucine, purine, lysine, and/or other amino acids. Administration of beta-
hydroxybutyrate
that is coupled to an amino acid may reduce the intake of cations associated
with beta-
hydroxybutyrate salts (e.g., which may inhibit side effects associated with
administration)
and/or allow administration of another compound that has health benefits
(e.g., administration
of some amino acid may promote smooth muscle growth and/or cell repair). In
some
implementations, approximately 0.5 g to approximately 10 g of amino acid may
be
administered with a beta-hydroxybutyrate. For example, less than approximately
50 g of R-
beta-hydroxybutyrate and less than approximately 60 mg of an amino acid, such
as leucine,
may be administered daily. In some implementations, approximately 0.5 g to
approximately
2 g of an amino acid, such as leucine, may be administered with a beta-
hydroxybutyrate. For
example, approximately the composition administered may include approximately
0.1 to
approximately 7 g R-beta-hydroxybutyrate and approximately 1-3 g of leucine.
The R-beta-
hydroxybutyrate and the leucine may be a mixture; administered separately and
proximate in
timing; a complex, and/or administered in any other appropriate manner.
[048] In some implementations, the composition may include R-beta-
hydroxybutyrate salt
and beta-hydroxybutyrate - amino acid complex (e.g., beta-hydroxybutyrate
bound to amino
acid, such as R-beta-hydroxybutyrate ¨ leucine complex). For example, an
individual may
be administered a first weight amount of sodium beta-hydroxybutyrate and a
second weight
amount of beta-hydroxybutyrate amino-acid complex. The first amount and the
second
amount may be different or the same.
[049] In some implementations, the beta-hydroxybutyrate composition may
include beta-
hydroxybutyrate salt and beta-hydroxybutyrate esters. For example, an
individual may be
administered a first weight amount of sodium beta-hydroxybutyrate and a second
weight
amount of beta-hydroxybutyrate ester. The first amount and the second amount
may be
different or the same. The beta-hydroxybutyrate salt and the beta-
hydroxybutyrate ester may
be a bound complex, a mixture of compounds, and/or separately administered
approximately
concurrently. In some implementations, the beta-hydroxybutyrate ester may be
in powdered
form (e.g., plated beta-hydroxybutyrate ester), liquid and/or gel form. The
combination of
12
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
beta-hydroxybutyrate salt and beta-hydroxybutyrate ester during administration
may allow
less salt to be utilized while producing a result (e.g., weight maintenance
and/or loss;
enhanced and/or maintained ketosis; elevated blood ketone levels; blood
glucose reduction
and/or maintenance; increase in energy; increase in mood; increase in
performance; and/or
increase in cognitive function). In some implementations, elevated ketone
levels (e.g.,
elevated blood ketone levels) may increase energy, mood, performance, and/or
cognitive
function in users. For example, the administration of the first amount of beta-
hydroxybutyrate salt may cause a first level of blood ketone level, which may
be maintained
by processing of the second amount of the beta-hydroxybutyrate ester (e.g., as
the body of the
individual processes the beta-hydroxybutyrate ester the level of beta-
hydroxybutyrate in the
blood, and thus blood ketone level, may also increase over time to enhance
and/or maintain
the initial elevation caused by of the administered beta-hydroxybutyrate
salt.). For example,
a ratio of beta-hydroxybutyrate to beta-hydroxybutyrate ester may be
approximately 1 beta-
hydroxybutyrate salt: approximately 1 beta-hydroxybutyrate ester to
approximately 1 beta-
hydroxybutyrate salt: approximately 20 beta-hydroxybutyrate ester. The ratio
of beta-
hydroxybutyrate to beta-hydroxybutyrate ester may be approximately 20 beta-
hydroxybutyrate salt: approximately 1 beta-hydroxybutyrate ester to
approximately 1 beta-
hydroxybutyrate salt: approximately 20 beta-hydroxybutyrate ester. In some
implementations, a ratio of beta-hydroxybutyrate to beta-hydroxybutyrate ester
may be
approximately 1 beta-hydroxybutyrate salt: approximately 1 beta-
hydroxybutyrate ester to
approximately 1 beta-hydroxybutyrate salt: approximately 5 beta-
hydroxybutyrate ester.
[050] Related compounds that may be included as beta-hydroxybutyrate in the
composition
may include derivatives of beta-hydroxybutyrate, include esters of (R)-3-
hydroxybutyrate and
oligomers of (R)-3-hydroxybutyrate. For example, beta-hydroxybutyrate esters
derived from
alcohols, such as altrose, arabinose, dextrose, erythrose, fructose,
galactose, glucose,
glycerol, gulose, idose, lactose, lyxose, mannose, ribitol, ribose, ribulose,
sucrose, talose,
threose, xylitol, xylose, galactosamine, glucosamine, mannosamine, N-
acetylglucosamine,
mannitol, sorbitol, threitol, (S)-1,2-propanediol and/or (R)-1,3-butanediol.
In some
implementations, a derivative of the beta-hydroxybutyrate may include
structures of (R)-3-
hydroxybutyric acid and an exemplary ester thereof (a glycerol monoester). The
R chirality of
the derivatives may be selected for inclusion in the composition in some
implementations
(e.g., to deliver R-beta-hydroxybutyrate with the administration of the
compound).
13
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[051] In some implementations, butyrate, beta-hydroxybutyrate (e.g., R-beta-
hydroxybutyrate), related compounds, and/or combinations thereof may be
administered
along with one or more additional compounds. The additional compounds may or
may not be
capable of independently increasing ketone levels, maintaining ketone levels,
inducing
ketosis, and/or maintaining ketosis. For example, additional compounds capable
of
independently increasing blood ketone levels may include short chain fatty
acids (e.g., fatty
acid with between 2 carbons than 6 carbons), short chain triglycerides (e.g.,
triglycerides with
less than 6 carbons), medium chain fatty acids (e.g., fatty acid with 6-12
carbons), medium
chain triglycerides (e.g., triglycerides with 7-12 carbons), long chain fatty
acids (e.g., fatty
acids with more than 12 carbons), long chain triglycerides (e.g.,
triglycerides with more than
12 carbons), and/or combinations thereof. In some implementations, short chain
fatty acids
and/or triglycerides may include acetate, propionate, and/or butyrate. Medium
chain fatty
acids and/or triglycerides may include lauric acid and/or coconut oil, coconut
milk powder,
fractionated coconut oil, isolated hexanoic acid, isolated octanoic acid,
isolated decanoic
acid, ethoxylated triglyceride, triglyceride derivatives thereof, aldehyde
triglyceride
derivatives thereof, monoglyceride derivatives thereof, diglyceride
derivatives thereof,
triglyceride derivatives thereof, and/or alkyl esters thereof Long chain fatty
acids and/or
triglycerides may include dairy products and/or palm oil. In some
implementations, a
composition including R-beta-hydroxybutyrate and an additional compound that
is
independently capable of increasing ketone levels may increase ketone levels
greater than
merely the capability of each component individually (e.g., greater than an
additive increase).
For example, the composition may include R-beta-hydroxybutyrate and an
additional
compound independently capable of increasing ketone levels such as caffeine.
In some
implementations, the composition may include approximately 0.5 mg to
approximately 50 g
of R-beta-hydroxybutyrate and caffeine. In some implementations, the
composition may
include approximately 0.5 mg to approximately 15 g of R-beta-hydroxybutyrate
and less than
approximately 500 mg of caffeine. In some implementations, the composition may
include
approximately 0.5 mg to approximately 15 g of R-beta-hydroxybutyrate and
approximately 5
mg to approximately 500 mg of caffeine. In some implementations, the
composition may
include approximately 0.5 mg to approximately 15 g of R-beta-hydroxybutyrate
and
approximately 10 mg to approximately 150 mg of caffeine. In some
implementations, the
composition may include approximately 0.5 mg to approximately 15 g of R-beta-
14
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
hydroxybutyrate and approximately 10 mg to approximately 50 mg of caffeine.
The
composition with R-beta-hydroxybutyrate (e.g., R-beta-hydroxybutyrate
including at least
one R-beta-hydroxybutyrate salt) and caffeine may increase and or maintain
ketosis, weight
loss, fat loss, and/or mental acuity. In some implementations, the composition
with R-beta-
hydroxybutyrate (e.g., R-beta-hydroxybutyrate including at least one R-beta-
hydroxybutyrate
salt) and caffeine may increase mental processes (e.g., acuity including
cognitive functioning,
mood, energy, alertness, focus, performance, effects of aging, etc.); improve
and/or maintain
body composition; function as a therapeutic for one or more of the described
conditions or
disorders (e.g., treat neurological disorders); and/or combinations thereof.
In some
implementations, the composition may include R-beta-hydroxybutyrate and an
additional
compound independently capable of increasing ketone levels, such as 1,3,7,9-
Tetramethyluric
acid (commercially available as theacrine; and/or commerically available as
TeaCrineg from
Compound Solutions, California, USA). In some implementations, the composition
may
include approximately 0.5 mg to approximately 15 g of R-beta-hydroxybutyrate
and less than
approximately 500 mg of 1,3,7,9-Tetramethyluric acid. In some implementations,
the
composition may include approximately 5 mg to approximately 15 g of R-beta-
hydroxybutyrate and less than approximately 500 mg of 1,3,7,9-Tetramethyluric
acid.
[052] For example, a pharmaceutically effective amount of one or more short
chain fatty
acids and/or one or more short chain triglycerides (e.g., butyric acid and/or
butyrate) may be
administered with a pharmaceutically effective amount of beta-hydroxybutyrate.
In some
implementations, approximately 1 g to approximately 10 g of beta-
hydroxybutyrate and
approximately .1 g to approximately 50 g of short chain fatty acid and/or
triglyceride may be
administered from once a day to approximately 5 times a day. In some
implementations,
approximately 1 g to approximately 3 g of beta-hydroxybutyrate and
approximately 1 g of
short chain fatty acid and/or triglyceride may be administered from once a day
to
approximately 5 times a day. In some implementations, the short chain fatty
acid and/or
triglyceride may include butyrate or derivatives of butyrate. Butyrate and/or
derivatives of
butyrate may be administered with and/or without beta-hydroxybutyrate to
manage metabolic
conditions, such as ketosis, and/or for other appropriate therapeutic
purposes. Administered
butyrate may be converted to beta-hydroxybutyrate in humans, and thus may
increase the
amount of beta-hydroxybutyrate delivered to the user. In some implementations,
administration of butyrate and beta-hydroxybutyrate may promote hGH synthesis,
improve
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
basal and GHRH-induced hGH-secretion, increase muscle fiber cross-sectional
area, inhibit
intramuscular fat accumulation; reduce fat mass in a user; improve glucose
metabolism;
increase markers of mitochondrial biogenesis in skeletal muscle and/or whole-
body oxygen
consumption; reduced markers of oxidative stress and apoptosis and altered
antioxidant
enzyme activity; cause butyrate enhanced intracellular free cytosolic calcium
levels (e.g., by
acting through GPR41 and 43); increase beta-hydroxybutyrate levels; and/or
support barrier
function(s) in the gut and/or reduce inflammation associated with ulcerative
colitis. Since
butyrate is processed by the body to provide beta-hydroxybutyrate, the
delivery of beta-
hydroxybutyrate via the butyrate may supplement the directly administered beta-
hydroxybutyrate to maintain a level of beta-hydroxybutyrate in the blood
(e.g., to promote
ketosis, weight loss and/or management, etc.).
[053] However, butyrate and/or butyric acid may not be palatable to
individuals (e.g., since
the odor and taste are often compared to vomit). Thus, in some
implementations, butyrate
and/or beta-hydroxybutyrate (e.g., R-beta-hydroxybutyrate) may be processed to
reduce
organoleptic reactions. For example, the butyrate and/or beta-hydroxybutyrate
(e.g., R-beta-
hydroxybutyrate) may be encapsulated, microemulsion, liposomes, agglomeration,
masking/flavoring technologies, and/or otherwise processed as appropriate to
reduce
organoleptic reactions from individuals administered the described
composition(s). In some
implementations, microencapsulated butyrate, beta-hydroxybutyrate, and/or
butyric acid may
be utilized (e.g., in combination with beta-hydroxybutyrate). Using
microencapsulated
butyrate, beta-hydroxybutyrate, and/or butyric acid (e.g., when compared with
using
unencapsulated forms) may increase individual satisfaction and/or compliance
with an
administration schedule since odor from the butyrate and/or butyric acid may
be reduced
and/or removed. The microencapsulated butyrate, beta-hydroxybutyrate, and/or
butyric acid
may be a free flowing granular powder; dispersible in water; stable in acidic
water solution
for 30 minutes; allow controlled release in stomach and/or small intestines;
inhibit glucose
response (e.g., to any added materials); and/or allow delivery of a high
butyrate content (e.g.,
around 70%).
[054] In some implementations, a pharmaceutically effective amount of butyrate
may be
administered via triglyceride tributyrin (e.g., glyceryl tributyrate or
tributyrin). The butyrate
via triglyceride tributyrin may be administered separately and/or in
conjunction with one or
16
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
more of the other described compounds (e.g., beta-hydroxybutyrate, fatty acids
and/or esters,
etc.). For example, up to approximately 200 mg/kg of the individual may be
administered
(e.g., up to 3 times daily). Administration of the tributyrin may allow a
delayed release of
butyrate to the body as the tributyrin is processed by the body of the
individual. The
tributyrin may be unencapsulated and/or encapsulated (e.g.,
microencapsulated).
[055] In some implementations, administration of beta-hydroxybutyrate and a
short chain
compound (e.g., short chain fatty acid and/or short chain triglyceride) may
unexpectedly
increase beta-hydroxybutyrate concentrations in the blood more than the
administration of
similar amounts of beta-hydroxybutyrate and medium chain compounds (e.g.,
short chain
fatty acid and/or short chain triglyceride) and/or may increase beta-
hydroxybutyrate
concentrations in the blood more than each component individually.
[056] In some implementations, a pharmaceutically effective amount of beta-
hydroxybutyrate may be administered with a pharmaceutically effective amount
of long chain
fatty acid and/or triglyceride. For example, 0.1-50 g of beta-hydroxybutyrate
and 0.1 to 50 g
of long chain fatty acid may be administered to an individual between 1-5
times a day. In
some implementations, approximately 1 g to approximately 3 g of beta-
hydroxybutyrate and
approximately 1 g of long chain fatty acid and/or triglyceride may be
administered from once
a day to approximately 5 times a day.
[057] In some implementations, beta-hydroxybutyrate, short chain compound(s)
(e.g., fatty
acids and/or triglycerides, butyrate), and/or medium chain compound(s) (e.g.,
fatty acids
and/or triglycerides) may be administered approximately simultaneously and/or
sequentially
to an individual. For example, approximately 0.1g to approximately 50 g beta-
hydroxybutyrate, approximately 0.1g to approximately 50 g short chain
triglyceride, and
approximately 0.1g to approximately 50 g medium chain fatty acid such as
lauric acid and/or
coconut oil may be administered between 1-5 times a day. In some
implementations,
approximately 1 g to approximately 3 g of beta-hydroxybutyrate and
approximately 1 g of
short chain fatty acid and/or triglyceride and/or approximately 1 g of medium
chain fatty acid
and/or triglyceride may be administered from once a day to approximately 5
times a day. In
some implementations, approximately 0.1g to approximately 20g beta-
hydroxybutyrate (e.g.,
salts, esters, isomers, and/or other appropriate forms) may be administered in
humans. In
17
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
some implementations, approximately 0.1g to approximately 20g butyrate may be
administered in humans.
[058] In some implementations, other compounds, such as compounds capable of
independently decreasing glucose levels, may be administered with beta-
hydroxybutyrate,
such as berberine and/or associated metabolites (e.g., dihydroberberine and/or
tetrahydroberberine). U.S. Patent Application No. 15/491,933 entitled
"ADMINISTRATION
OF DIHYDROBERBERINE" to Lowery et al, filed April 19, 2017 and U.S.
Provisional
Patent Application No. 62/324,794, entitled "ADMINISTRATION OF
DIHYDROBERBERINE" to Lowery et al, filed April 19, 2016, describe
dihydroberberine
administration with ketone sensitizers such as beta-hydroxybutyrate, and is
hereby fully
incorporated herein. In some implementations, one or more beta-
hydroxybutyrates and/or
other compounds described herein may be utilized as a ketone sensitizer with
the
dihydroberberine.
[059] In some implementations, directly administering beta-hydroxybutyrate
plus another
compound that is processed to deliver beta-hydroxybutyrate (e.g., beta-
hydroxybutyrate ester,
beta-hydroxybutyrate polymer, butyrate, other appropriate compounds, and/or
combinations
thereof) over time may allow a first level of beta-hydroxybutyrate in the
blood to be
maintained over a period of time. For example, since the directly administered
beta-
hydroxybutyrate may elevate blood beta-hydroxybutyrate levels to a first
concentration and
this concentration may be approximately maintained over a period of time by
providing
additional beta-hydroxybutyrate via another compound administered
approximately
concurrently (e.g., short chain fatty acid and/or triglyceride, beta-
hydroxybutyrate ester, beta-
hydroxybutyrate polymer, beta-hydroxybutyrate amino acid complex, etc.).
[060] In some implementations, one or more other compounds may be administered
(e.g.,
included in the composition and/or separately administered) with the butyrate
(e.g.,
microencapsulated butyrate), beta-hydroxybutyrate (e.g., R-beta-
hydroxybutyrate) and/or
fatty acids or esters, such as short chain fatty acids. Other compositions may
include, but are
not limited to amino acids, amino acid metabolites, vitamins, minerals,
coconut milk powder,
flavorings, colorings, binders, electrolytes, tetrahydrobiopeterin, nucleic
acids, alpha-
ketoglutaric acid, alpha lipoic acid, nutritional co-factors, beta-methyl-beta-
hydroxybutyrate,
18
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
arginine alpha-ketoglutarate, R-alpha lipoic acid, thiamine, NAD+, NADH,
riboflavin,
FAD+, FADH, riboflavin-5-phosphate, niacin, nicotinic acid, niacinamide,
inositol
hexanicotinate, pyridoxine, pyridoxal, pyridoxamine, ascorbic acid and
ascorbate salts, citric
acid, malic acid, sodium benzoate, Pyridoxa1-5-Phosphate, methylcobalamin,
cyanocobalamin, adenosylcobalamin, hydroxycobalamin, pantothenic acid,
pantetheine,
potassium sorbate, acesulfame K, aspartame, sucralose, stevia, monk fruit
extract, allulose,
prebiotic fibers, XOS, GOS, MOS, IMO, LOS, xanthan gum and other organic
gums/thickeners/suspension agents, and combinations thereof.
[061] In various implementations, administration of a composition that
includes beta-
hydroxybutyrate may improve the health of an individual. R-beta-
hydroxybutyrate may be
capable of providing a greater impact on the health of an individual than D,L-
beta-
hydroxybutyrate and/or L-beta-hydroxybutyrate. Although previously unknown, L-
beta-
hydroxybutyrate may decrease the effectiveness of R-beta-hydroxybutyrate with
respect to at
least a portion of the impact on health. With respect to some impacts on
health, L-beta-
hydroxybutyrate may have no impact on health. In some implementations, even
double the
amount of D,L-beta-hydroxybutyrate may not achieve some of the same results
(e.g., in
health improvement) as R-beta-hydroxybutyrate. Thus, unexpectedly
administration of D,L-
beta-hydroxybutyrate rather than R-beta-hydroxybutyrate may not have the same
impact on
health and/or have less of an impact on health of an individual. For example,
administration
of a composition that includes R-beta-hydroxybutyrate (e.g., and/or other
compounds) may
improve and/or maintain an individual's health.
[062] Administration of R-beta-hydroxybutyrate as described may increase
lifespan in
individuals following a dietary plan (e.g., standard American low-fat,
ketogenic, Paleo,
Mediterranean, etc.) and/or not following a dietary plan. For example,
approximately 10 g of
R-beta-hydroxybutyrate to approximately 30 g R-beta-hydroxybutyrate may be
administered
to increase lifespan. In some implementations, other appropriate amounts of R-
beta-
hydroxybutyrate may be included in the composition.
[063] In some implementations, administration of R-beta-hydroxybutyrate may
treat and/or
lesson the impact of symptoms of disease(s) and/or disorders, such as diseases
that impact
cognitive function. Administration of R-beta-hydroxybutyrate may increase
motor function
19
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
in individuals with Parkinson's disease. For example, approximately 5 g of R-
beta-
hydroxybutyrate to approximately 15 g R-beta-hydroxybutyrate may be
administered to
increase motor function. In some implementations, other appropriate amounts of
R-beta-
hydroxybutyrate may be included in the composition.
[064] Administration of R-beta-hydroxybutyrate may increase fat loss. Unlike
with
conventional diets, in which weight loss often comes from decreases in water
retention and/or
muscle mass, administration of R-beta-hydroxybutyrate may cause decreases in
fat loss (see
for example, Figure 5B). In addition, administration of R-beta-hydroxybutyrate
may
decrease levels of LPL in the body, and thus reduce or inhibit fat storage
and/or encourage
existing fat storage utilization by the body. For example, approximately 1 g
of R-beta-
hydroxybutyrate to approximately 20 g R-beta-hydroxybutyrate may be
administered to cause
fat loss and/or reduce fat storage. In some implementations, other appropriate
amounts of R-
beta-hydroxybutyrate may be included in the composition. Administration of R-
beta-
hydroxybutyrate may allow fat loss greater than 5 kg while maintaining lean
mass. In some
implementations, the administration of R-beta-hydroxybutyrate increases the
amount of fat
used as fuel.
[065] In some implementations, administration of R-beta-hydroxybutyrate may
improve
and/or maintain health markers such as C-reactive protein and/or fasting
glucose.
Administration of R-beta-hydroxybutyrate may decrease inflammation (e.g., as
shown by C-
reactive protein levels). Administration of R-beta-hydroxybutyrate may
decrease fasting
glucose. For example, approximately 3 g of R-beta-hydroxybutyrate to
approximately 20 g R-
beta-hydroxybutyrate may be administered to cause a reduction in and/or
maintain a low
fasting glucose. In some implementations, other appropriate amounts of R-beta-
hydroxybutyrate may be included in the composition. In some implementations, R-
beta-
hydroxybutyrate may be administered with one or more other compounds to
decrease glucose
levels and/or sensitivity. For example, administration of a composition of R-
beta-
hydroxybutyrate and a berberine, such as dihydroberberine, may cause reduce
and/or
maintain low fasting glucose. Administration of a composition of R-beta-
hydroxybutyrate
and a berberine, such as dihydroberberine, may cause reduce and/or maintain
low glucose
levels. In some implementations, less than approximately 15 g of R-beta-
hydroxybutyrate
may be administered with less than approximately 600 mg of dihydroberberine.
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[066] Administration of R-beta-hydroxybutyrate may decrease ketone levels (see
e.g.,
Figures 11A and 11B). Decreasing blood ketone levels may increase weight loss,
maintain
weight loss, improve performance, increase mental acuity, and/or have other
health
improvement and health maintenance features. For example, even at levels less
than 10 g
(e.g., approximately 5 g), administration of R-beta-hydroxybutyrate may
decrease ketone
levels while L-R-beta-hydroxybutyrate does not, and D,L-beta-hydroxybutyrate
does not to
the same extent. R-beta-hydroxybutyrate may increase blood ketone levels 5
times as much
as similar administration amounts of D,L-beta-hydroxybutyrate. By being able
to decrease an
amount of R-beta-hydroxybutyrate (e.g., when compared with administering D,L-
beta-
hydroxybutyrate) administered and achieve the same results, a decrease in an
amount cation
(e.g., sodium, potassium, etc.) may also be administered. Since some
individuals may prefer
and/or may not tolerate higher dosages of the cations of the R-beta-
hydroxybutyrate salt,
utilizing R-beta-hydroxybutyrate may allow administration to more people,
increase user
satisfaction, and/or decrease side effects (e.g., associated with additional
consumption of
these cations). In some implementations, approximately 0.1 g of R-beta-
hydroxybutyrate to
approximately 10 g R-beta-hydroxybutyrate may be administered to increase
blood ketone
levels. Approximately 0.5 g of R-beta-hydroxybutyrate to approximately 3 g R-
beta-
hydroxybutyrate may be administered to maintain blood ketone levels. In some
implementations, other appropriate amounts of R-beta-hydroxybutyrate may be
included in
the composition.
[067] Administration of R-beta-hydroxybutyrate may increase performance and
decrease
perceived exertion (e.g., as opposed to when administered D,L-beta-
hydroxybutyrate). For
example, approximately 3 g of R-beta-hydroxybutyrate to approximately 15 g R-
beta-
hydroxybutyrate may be administered to increase performance and/or decrease
perceived
exertion. In some implementations, other appropriate amounts of R-beta-
hydroxybutyrate
may be included in the composition.
[068] In various implementations, oral administration of R-beta-
hydroxybutyrate may
increase muscle protein synthesis while D,L-beta-hydroxybutyrate does not
increase muscle
protein synthesis. For example, approximately 10 g of R-beta-hydroxybutyrate
to
approximately 30 g R-beta-hydroxybutyrate may be administered to increase
muscle protein
21
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
synthesis. In some implementations, other appropriate amounts of R-beta-
hydroxybutyrate
may be included in the composition.
[069] In some implementations, the administration of R-beta-hydroxybutyrate,
unlike D,L-
beta-hydroxybutyrate may decrease perceived hunger and/or increase satiety)
which may
inhibit overeating and thus promote weight loss (see e.g., Figures 13A and
13B). In some
implementations, the administration of R-beta-hydroxybutyrate, unlike D,L-beta-
hydroxybutyrate may increased perceived energy (see e.g., Figures 13C).
[070] In some implementations, administration of R-beta-hydroxybutyrate
increased mental
acuity. For example, approximately 0.1 g of R-beta-hydroxybutyrate to
approximately 10 g
R-beta-hydroxybutyrate may be administered to increase mental acuity. In some
implementations, other appropriate amounts of R-beta-hydroxybutyrate may be
included in
the composition.
[071] In some implementations, the administration of R-beta-hydroxybutyrate
may be
supplemented with other forms of beta-hydroxybutyrate, butyric acid, and/or
butyrate.
[072] In some implementations, the composition administered may include R-beta-
hydroxybutyrate. The amount of R-beta-hydroxybutyrate included in the
composition may
be selected to obtain a result (e.g., induce ketosis; maintain ketosis;
increase ketone levels,
mental acuity, strength, etc.) upon administration (e.g., a pharmaceutically
effective amount
may be administered at a dosage and/or over a predetermined time period). In
some
implementations, the dosage and/or frequency of dosage may vary over time
(e.g., initial vs a
lower dosage for maintenance, vary based on time of day, vary based on whether
taken with
or without a meal, etc.).
[073] The R-beta-hydroxybutyrate in the composition may include any
appropriate and/or
appropriate number of forms, such as salts, derivatives (e.g., esters),
polymers, and/or
complexes with other compounds. For example, the composition may include R-
beta-
hydroxybutyrate salt (e.g., sodium R-beta-hydroxybutyrate, magnesium R-beta-
hydroxybutyrate, and/or potassium R-beta-hydroxybutyrate) and/or another form
of R-beta-
hydroxybutyrate (e.g., ester, polymer, complex, etc.). In some
implementations, the
22
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
composition may include an ester of R-beta-hydroxybutyrate. The composition
may include
an amino acid (e.g., separate and/or complexed with R-beta-hydroxybutyrate),
such as
leucine. The use of non-salt base R-beta-hydroxybutyrate may increase user
satisfaction
(e.g., by reducing the cation, such as sodium and/or potassium, load due to
ingestion of the
composition; by decreasing side effects; etc.), increase the applicability of
the administration
(e.g., since users sensitive to the cations of the R-beta-hydroxybutyrate
salts may be less
sensitive to the non-salt and/or lower salt plus non-salt forms of the
composition). The
administration of the composition may increase blood ketone levels, induce
ketosis, maintain
blood ketone levels, maintain ketosis, increase health, increase strength,
increase mental
acuity, etc. In some implementations, a first composition that includes R-beta-
hydroxybutyrate salt may be administered to cause a first impact (e.g., induce
ketosis, quickly
increase mental acuity, quickly increase strength, etc.) and a second
composition that
includes non-salts R-beta-hydroxybutyrate (e.g., esters, polymers, complexes,
etc.) and/or
lower levels of R-beta-hydroxybutyrate salt may be utilized to cause a second
impact (e.g.,
maintain ketosis, maintain mental acuity, maintain increased strength, etc.).
[074] In some implementations, the form(s) of R-beta-hydroxybutyrate included
in the may
be selected based on the delivery form. For example, in some forms of food
products the
composition may include R-beta-hydroxybutyrate polymer (e.g., due to taste
since increased
cations like sodium may decrease palatability; due to nutrition since
increased cations such as
sodium may decrease nutrition; due to mixability, etc.). As another example,
the composition
may include R-beta-hydroxybutyrate salts or other forms (e.g.,
microencapsulated) to provide
quick dissolve powders.
[075] In various implementations, a composition may include R-beta-
hydroxybutyrate. The
R-beta-hydroxybutyrate may be in any appropriate form (e.g., salt, ester,
polymer, complex,
derivatives thereof, and/or combinations thereof). The composition may include
one or more
additional compositions. Additional composition(s) may be capable of
independently
increasing blood ketone levels (e.g., fatty acids or esters, berberine or
berberine metabolites
such as dihydroberberine, etc.). Additional composition(s) may be capable of
independently
decreasing blood glucose levels (e.g., berberine or berberine metabolites such
as
dihydroberberine). In some implementations, additional compounds may not be
capable of
independently increasing blood ketone levels and/or decreasing blood glucose
levels (e.g.,
23
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
additives, flavorings, colorings, minerals, vitamins, binders, anti-caking
agents, etc.). The
composition may be administered in an effective amount to cause a
predetermined health
impact (e.g., predetermined level of ketosis, blood ketone level, mental
acuity, strength
increase, perceived energy, fat loss, weight loss, etc.). The composition may
be administered
to an individual in a predetermined amount and/or different amounts over an
administration
schedule. In some implementations, once a first criteria is satisfied (e.g.,
period of time,
number of doses, predetermined health impact), the dosage amount may be
altered. For
example, first dose(s) of the composition may be administered to cause a
predetermined
health impact and additional lower dose(s) of the composition may be
administered to
maintain the predetermined health impact (e.g., caused in part by the first
doses).
[076] The composition may be administered in any appropriate delivery form
(e.g., tablet;
capsule; food products such as powdered products that can be mixed into food,
mixed into
beverages, and/or consumed directly; beverage product; etc.). The composition
may be
administered according to any appropriate schedule (e.g., periodic dosages,
dosages as user
desires, etc.). The administration schedule may inhibit administration that
elevates blood
ketone levels too high, decreases blood glucose levels too low, and/or causes
an individual to
consume a dosage that substantially elevates the risk of adverse and/or side
effects, in some
implementations.
[077] In some implementations, the composition may include a long acting
component
and/or be long-acting. For example, since the body digests polymers and/or
esters of beta-
hydroxybutyrate (e.g., R-beta-hydroxybutyrate), the delivery of R-beta-
hydroxybutyrate may
be slower than a digestion of a beta-hydroxybutyrate salt (e.g., R-beta-
hydroxybutyrate salt).
In some implementations, the composition may include a R-beta-hydroxybutyrate
and a long
acting R-beta-hydroxybutyrate form (e.g., polymer, ester, coated and/or
processed form to
provide slow release). In some implementations, a first dose(s) may include at
least one non-
long acting form of beta-hydroxybutyrate and a second dose(s) may include at
least one long-
acting form of beta-hydroxybutyrate. The first dose(s) may be administered to
cause a
predetermined health impact and the second dose(s) may be administered to
maintain the
caused predetermined health impact. In some implementations, users may select
the
appropriate dose based on user preference and/or properties (e.g., a user on a
ketogenic diet
may chose the second dose since the user may already be in ketosis).
24
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[078] EXAMPLES
[079] EXAMPLE 1
[080] 4 subjects were administered 10 mg of sodium D,L-beta-hydroxybutyrate
and their
blood ketone level in mmol/dL was tested after administration, 30 minutes, 60
minutes, 90
minutes, 120 minutes, and 180 minutes after administration. Each subject was
also
subsequently studied after administration of 10 g of sodium R-beta-
hydroxybutyrate and 5 g
of sodium R-beta-hydroxybutyrate. As illustrated in Figure 1, on average,
administration of 5
mg of sodium R-beta-hydroxybutyrate produced approximately the same blood
ketone level
in a subject after 30 minutes, 60 minutes, 90 minutes, 120 minutes, and 180
minutes as 10 g
of D,L-b eta-hydroxybutyrate.
[081] EXAMPLE 2
[082] Three subjects were administered 10 grams of medium chain triglycerides
and 8
grams of beta-hydroxybutyrate and blood beta-hydroxybutyrate concentration was
monitored
over time. The same subjects were later administered 10 grams of short chain
triglycerides
and 8 grams of beta-hydroxybutyrate and blood beta-hydroxybutyrate
concentration was
monitored. Figure 2 illustrates an average blood ketone concentration (mmol/L)
for the
subjects after administration, after 30 minutes, after 60 minutes, after 90
minutes, after 120
minutes, and after 180 minutes. As illustrated in Figure 2, administration of
the beta-
hydroxybutyrate with a short chain compound (illustrated in red bars or the
second bar in
each set), such as short chain triglyceride, caused greater elevation of blood
ketone levels
than administration of a similar amount of medium chain compound (illustrated
in the blue
bars or first bar in each set) at least after administration, after 30, 60, 90
minutes, and 180
minutes. Thus, administration of short chain compounds (e.g., fatty acids
and/or
triglycerides) may unexpectedly allow a smaller weight amount, when compared
to medium
chain compounds, to be administered to produce the same result (e.g., blood
ketone level,
weight loss, weight management, etc.) and/or allow greater results (e.g., when
compared with
similar amount of medium chain compounds).
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[083] EXAMPLE 3
[084] Sixteen rats (Fischer 344 rats) were studied for the effect of R-beta-
hydroxybutyrate
on lifespan. A first grouping of eight rats were fed an equivalent to a low-
fat standard
American diet and a second grouping of eight rats were fed the same equivalent
to a low-fat
standard American diet and supplemented with R-beta-hydroxybutyrate salt
(e.g., sodium R-
beta-hydroxybutyrate). The second grouping of rats were supplemented with the
R-beta-
hydroxybutyrate salt at middle age. Figure 3 illustrates the average lifespans
of the
groupings of rats. As illustrated, at 20 months approximately half of the
first grouping of rats
died on the standard diet while only 12.5% of the second grouping of rats had
died at 20
months. Thus, the supplementation of rats diets with R-beta-hydroxybutyrate
increased
lifespan for approximately in at least approximately 38.5% of the rats. Since
the rat study
was performed as an approximation of impact in humans, the addition of R-beta-
hydroxybutyrate to a standard American low-fat diet may increase lifespan.
[085] EXAMPLE 4
[086] An individual with Parkinson's disease was tested for motor function
with and
without administration of approximately 10 g of R-beta-hydroxybutyrate salt.
The testing
included a right-eye visual and motor performance apparatus to track motor
function through
eye movements.
[087] Figure 4 illustrates chart illustrating the results of the motor skill
testing following an
example implementation of administration of R-beta-hydroxybutyrate. Figure 4
illustrates
average results for a similar non-Parkinson's population, the patient pre-
administration of R-
beta-hydroxybutyrate, and the patient post-administration of R-beta-
hydroxybutyrate. As
illustrated, the administration of R-beta-hydroxybutyrate increased motor
function (e.g.,
approximately 30 minutes after administration of the R-beta-hydroxybutyrate).
[088] EXAMPLE 5
[089] An individual was administered 5 g of R-beta-hydroxybutyrate twice daily
for 3
months. Xray absorptiometry was performed to determine the impact of the
administration of
26
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
R-beta-hydroxybutyrate on fat loss. Figure 5A illustrates a chart that shows
the results after 3
months of administration. As illustrated, the individual experienced a greater
than
approximately 10% decrease in fat mass. Figure 5B illustrates that the fat
loss was sustained
while maintaining lean mass. Thus, the R-beta-hydroxybutyrate may cause weight
loss
through fat loss rather than lean mass (e.g., muscle mass).
[090] EXAMPLE 6
[091] A first grouping of 10 rats (labeled SC) were given a standard diet, a
second grouping
of 10 rats (labeled KD) were given a ketogenic diet, a third grouping of 10
rats (labeled SC +
MS) were on the standard diet but given a first dosage of R-beta-
hydroxybutyrate salt (e.g.,
equivalent to 5 g) and a fourth grouping was on the standard iet but given a
second dosage of
R-beta-hydroxybutyrate salt (e.g., equivalent to 10 g). Figure 6 illustrates
the average
Lipoprotien lipase (LPL) in the rats. Since LPL is needed to transport fat
into adipose tissue,
lowering LPL levels would inhibit fat storage and encourage usage of fat
storages. As
illustrated, supplementation of a standard diet with even lower dosages of R-
beta-
hydroxybutyrate decreases LPL levels and thus inhibits fat storage.
[092] EXAMPLE 7
[093] An individual with high C-reactive protein, which is associated with
inflammation,
was administered R-beta-hydroxybutyrate. After administration, the C-reactive
protein levels
were substantially reduced (e.g., 62.5 to 4.4). In addition, fasting glucose
was decreased
(e.g., 104 to 95).
[094] EXAMPLE 8
[095] Five healthy individual were given a 2km time test (e.g., 4 cycles of
low to severely
intense exercise on a wingate cycle ergometer) 30 minutes after administration
of a placebo,
g of R-beta-hydroxybutyrate, and 10 g of R-beta-hydroxybutyrate. Figure 7
illustrates the
average blood ketone levels and Figure 8 illustrates the percentage
improvement over the
administration of the placebo. As illustrated, blood ketone levels
unexpectedly increased
more than double during administration of R-beta-hydroxybutyrate when compared
with
27
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
administration of D,L-beta-hydroxybutyrate. In addition, performance (e.g.,
improvement in
time) increased by more than double during administration of R-beta-
hydroxybutyrate when
compared with D,L-beta-hydroxybutyrate. Figure 9 illustrates the perceived
exertion
experienced by the individuals. As illustrated, the individuals did not feel
an impact in
perceived exertion after administration with D,L-beta-hydroxybutyrate as
compared with the
perceived exertion improvement experienced after administration of R-beta-
hydroxybutyrate.
Thus, the R-beta-hydroxybutyrate has an unexpectedly impact on ketone levels
and
performance.
[096] EXAMPLE 9
[097] Individuals were given a standard diet or ketogenic diet. Some
individuals were
administered R-beta-hydroxybutyrate (e.g., 10 g). R-beta-hydroxybutyrate was
able to
numerically increase superoxide dismutase 2 levels (SOD) in the brain which
indicates
greater antioxidant capacity in the brain.
[098] EXAMPLE 10
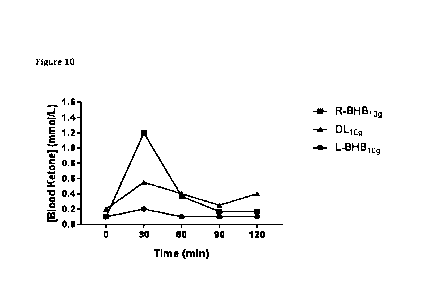
[099] Individuals were 5 g or 10 mg of R-beta-hydroxybutyrate, L-beta-
hydroxybutyrate, or
D,L-beta-hydroxybutyrate and blood ketone levels were measured. Figure 10 and
11
illustrate the measured blood ketone levels. As illustrated, administration of
R-beta-
hydroxybutyrate may decrease ketone levels (see e.g., Figures 11A and 11B).
The reduction
of ketone levels occurs even when R-beta-hydroxybutyrate is administered at a
dosage of
less than 10 g (e.g., approximately 5 g). In addition, unexpectly (e.g., since
it was expected
that both the D and L forms of R-beta-hydroxybutyrate behaved in a similar
manner),
administration of L-beta-hydroxybutyrate does not decrease blood ketones.
Furthermore,
unexpectedly, even D,L-beta-hydroxybutyrate does not lower blood ketone levels
to the same
extent as R-beta-hydroxybutyrate. This indicates that L-beta-hydroxybutyrate
may block
some of the impact of R-beta-hydroxybutyrate, which is unexpected.
28
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[0100] EXAMPLE 11
[0101] 10 subjects were administered approximately 5 g or 10 g of D,L-beta-
hydroxybutyrate
or R-beta-hydroxybutyrate, and Respiratory exchange ratio was examined (RER, a
ratio of
carbon dioxide/oxygen). Generally, a ratio of 1.0 indicates that 100 %
carbohydrate is used
as fuel and at 0.7, 100 % fat is used as fuel. As illustrated in Figure 12A,
at 10 g, R-beta-
hydroxybutyrate administration reduces RER approximately 3 x more than D,L-
beta-
hydroxybutyrate. As illustrated in Figure 12B, 5 g of R-beta-hydroxybutyrate
is capable of
achieving a result that even more D,L-beta-hydroxybutyrate is unable to (e.g.,
D,L-beta-
hydroxybutyrate increases RER by 17 % rather than decreasing RER).
[0102] EXAMPLE 12
[0103] Individuals were administered 5g - 10 g of D,L-beta-hydroxybutyrate or
R-beta-
hydroxybutyrate and tested for perceived hunger, satiety, and perceived
energy. Figures
13A-13C illustrates the results of the testing. Figure 13A illustrates
perceived hunger, Figure
13B illustrates perceived satiety, and Figure 13C illustrates perceived
energy. As illustrated
in Figure 13B, at 30 minutes post consumption R--beta-hydroxybutyrate improved
satiety
levels 2.3x better than DL-beta-hydroxybutyrate relative to baseline levels.
As illustrated in
Figure 13C, R-beta-hydroxybutyrate improved perceived energy from 0 to 30
minutes post
consumption by double that of D,L-beta-hydroxybutyrate. R-beta-hydroxybutyrate
sustained
elevated perceived energy levels from 0 minutes at 60, 90, and 120 minutes
post
consumption, as opposed to D,L-beta-hydroxybutyrate. As illustrated, R-beta-
hydroxybutyrate was able to raise perceived energy by 18 % and sustain it for
2 hours post
ingestion (e.g., more than 2 times greater than the peak value of increase
with the DL-beta-
hydroxybutyrate)
29
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[0104] EXAMPLE 13
[0105] 5 young (20s) resistance trained males lifting 50 % of their 1-RM on
Bench Presses
were tested before and after administration of 5g of R-beta-hydroxybutyrate or
D,L-beta-
hydroxybutyrate. Figures 14A-B illustrate the results of the testing. As
illustrated, R-beta-
hydroxybutyrate administration resulted in an 11 % increase, while DL-beta-
hydroxybutyrate
administration resulted in only a 2% decrease. Thus, R-beta-hydroxybutyrate
experienced a
greater than expected impact when compared with D,L-beta-hydroxybutyrate.
[0106] The individuals were also tested for power. Figure 14C illustrates the
results of the
testing (e.g., averages of power measurements). As illustrated, R-beta-
hydroxybutyrate
administration increased minimum power by 26 %, while the DL-beta-
hydroxybutyrate
administration raised power by 2 %.
[0107] EXAMPLE 14
[0108] Individuals were tested for mental acuity before and after
administration of 5-10g of
R-beta-hydroxybutyrate or D,L-beta-hydroxybutyrate. Circular Tracking testing
(e.g., to
assess their cognitive function) was performed and administration of DL-beta-
hydroxybutyrate (e.g., 10 g) caused no improvement while the R-beta-
hydroxybutyrate (e.g.,
g) administration caused approximately 3 % improvement in tracking accuracy.
Vertical
Tracking testing (e.g., to assess their cognitive function) was performed and
administration of
D,L-beta-hydroxybutyrate (e.g., 10 g) improved performance by 4.6 %, while the
administration of R-beta-hydroxybutyrate (e.g., 10 g) improved performance by
13.8 %,
which is approximately 3 times greater improvement. Horizontal Saccades
testing was
performed (e.g., a saccade is one eye movement and known to become
significantly slower if
cognitive function declines and improve if cognitive function improves). In
the horizontal
saccades testing, performance improvements were 4 times greater with the
administration of
R-beta-hydroxybutyrate (e.g., 5 g) than with administration of D,L-beta-
hydroxybutyrate
(e.g., 13.8% vs. 3.2 %). Processing speed testing was performed (e.g,
processing speed is
considered a true measure of cognitive performance). Administration of R-beta-
hydroxybutyrate (e.g., 5 g) improved processing speed by 27.7 % and only
approximately 18
% with administration of the DL-beta-hydroxybutyrate (e.g., 5g). Response
accuracy was
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
also tested. Administration of R-beta-hydroxybutyrate (e.g., 5 g) increased
accuracy by 37
percentage points when compared to 12.7 % when DL-beta-hydroxybutyrate was
administered.
[0109] Thus, administration of R-beta-hydroxybutyrate increased mental acuity
more than a
similar amount of D,L-beta-hydroxybutyrate. In fact, as the testing revealed,
the
administration of R-beta-hydroxybutyrate increased mental acuity often by than
double when
compared with a similar amount of D,L-beta-hydroxybutyrate.
[0110] EXAMPLE 15
[0111] The compound for administration was prepared to include an R-beta-
hydroxybutyrate
amino acid complex. An R-beta-hydroxybutyrate Agmatine complex was prepared
and an R-
beta-hydroxybutyrate Arginine complex was prepared. Figure 15 illustrates the
average
blood ketone levels achieved with the R-beta-hydroxybutyrate amino acid
complex (e.g., an
average of both complexes) when compared with D,L-beta-hydroxybutyrate. As
illustrated,
blood ketone levels are not only more than double the blood ketone levels
achieved with the
same quanity of D,L-beta-hydroxybutyrate as R-beta-hydroxybutyrate amino acid
complex
(e.g., 10 g), but they are more than an additive result of a similar amount of
R-beta-
hydroxybutyrate and amino acid.
[0112] Use of the R-beta-hydroxybutyrate amino acid complex may reduce the
amount of
cation delivered (e.g. since the complex may deliver the R-beta-
hydroxybutyrate rather than a
R-beta-hydroxybutyrate salt). The reduction of this cation may decrease side
effects (e.g.,
from increased sodium, potassium, and/or magnesium intake), increase user
satisfaction,
and/or increase the population that can tolerate the administration of R-beta-
hydroxybutyrate
(e.g., since some individuals may not be capable of increasing loads of these
cations due to
underlying diseases and/or disorder). The use of the R-beta-hydroxybutyrate
amino acid
complex may also allow a higher yield of R-beta-hydroxybutyrate to be
administered (90.8%
R-beta-hydroxybutyrate, 5% amino acid) when compared with a similar weight of
R-beta-
hydroxybutyrate salt (e.g., average of 83% yield for BHB sodium).
31
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
[0113] EXAMPLE 16
[0114] A composition for administration may include R-beta-hydroxybutyrate and
an amino
acid, such as Leucine. The R-beta-hydroxybutyrate and leucine maybe complexed
and/or
mixed together for administration. The R-beta-hydroxybutyrate and leucine may
be
administered separately but approximately concurrently. Figure 16 illustrates
the blood
ketone levels after administration of R-beta-hydroxybutyrate (5g) and leucine
(2g). As
illustrated, the administration of R-beta-hydroxybutyrate and leucine causes
greater elevation
of blood ketone levels than the administration of R-beta-hydroxybutyrate (5g).
The
administration of R-beta-hydroxybutyrate and leucine causes greater elevation
of blood
ketone levels than merely the additive effect of similar quantities of R-beta-
hydroxybutyrate
and leucine administered separately.
[0115] END OF EXAMPLES
[0116] In some implementations, one or more additives may be included in the
composition,
such as flavorings (e.g., natural and/or artificial), vitamins, minerals,
binders, and/or any
other appropriate additive. The additives may alter flavor, color, and/or
texture. The
additives may increase palatability and/or facilitate inclusion in a delivery
vehicle (e.g.,
tablet, food product, beverage product such as a drink mix, etc.). The
additive may be any
appropriate solid and/or liquid to which the compound is added. For example,
an additive
may include liquid carriers, such as water, milk(s), and/or any other
appropriate drinkable
liquid. In some implementations, the composition may include a
pharmaceutically inert liquid
carrier, such as water (e.g., tap water, filtered water, distilled water,
etc.). The liquid carrier
may include other drinkable liquids such as coconut water, watermelon water,
electrolyte
water, and/or combinations thereof. The liquid carrier may include milks such
as dairy milk,
non-dairy milk, coconut milk, other milks, and/or combinations thereof. The
liquid carrier
may include an electrolyte solution, in some implementations.
[0117] The described compositions may be administered via any appropriate
administration
method. For example, the described compositions may be administered enterally
and/or
parenterally. In some implementations, the described composition may be
administered via a
32
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
tablet and/or capsule. The described composition may be provided in a powdered
form that
allows the described composition to be sprinkled on food, mixed with a liquid
to provide a
beverage, and/or directly administered. The described composition may be
provided in gel
form. The compounds in the composition may be mixed, coupled to each other,
and/or
provided separately. For example, the composition may include beta-
hydroxybutyrate
coupled to another compound (e.g., beta-hydroxybutyrate ester and/or amino
acid). In some
implementations, the beta-hydroxybutyrate and one or more other compounds may
be
provided separately (e.g., in pills). An individual may sequentially and/or
concurrently be
administered (e.g., swallow pills) the beta-hydroxybutyrate and other
compounds.
[0118] The described compositions may be administered on an administration
protocol to
cause weight loss and/or maintain a weight of an individual; elevate and/or
maintain blood
ketone levels; increase and/or maintain ketosis; and/or improve glucose
tolerance (e.g.,
fasting glucose levels may be reduced and/or glucose metabolism may be
improved), in some
implementations. For example, the described compositions may be administered
once a day,
via an extended release preparation, and/or multiple times a day (e.g., 1 to 5
times a day, 2 to
times a day, 3 to 5 times a day, etc.). The described composition may replace
other
pharmaceuticals or dietary supplements taken to promote weight loss, maintain
a weight,
promote ketosis, elevate blood ketone levels and/or be utilized in combination
with one or
more other pharmaceuticals or dietary supplements, as appropriate. The
described
composition may replace other pharmaceuticals or dietary supplements taken for
improving
glucose tolerance, such as metaformin, and/or be utilized in combination with
one or more
other pharmaceuticals or dietary supplements, as appropriate, in some
implementations.
[0119] In various implementations, the described composition(s) (e.g.,
butyrate, beta-
hydroxybutyrate, R-beta-hydroxybutyrate, related compounds, and/or one or more
other
compounds) may include one or more of the described components, equivalent(s)
of the
described component(s), derivatives of the described component(s), complex(es)
of the
described component(s), salt(s) of the described component(s), and/or
combinations thereof
[0120] In various implementations, a pharmaceutically effective amount of one
or more of
the described composition(s) may be administered. Administration of the
pharmaceutically
effective amount may induce and/or maintaining ketosis; maintaining and/or
promoting
33
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
weight loss; increase mental processes (e.g., acuity including cognitive
functioning, mood,
energy, alertness, focus, performance, effects of aging, etc.); improve and/or
maintain body
composition; function as a therapeutic for one or more of the described
conditions or
disorders (e.g., treat neurological disorders); and/or combinations thereof.
[0121] Although various types of increases in mental acuity have been
described, other
features of mental acuity such as memory, focus, concentration, and/or
understanding (e.g.,
speed of processing, accuracy of processing) may be increased by
administration of an
effective amount of the composition that includes R-beta-hydroxybutyrate.
[0122] Although a subject and/or an individual have been described as a human,
a subject
and/or individual may be a person or a group of people. Although various
described systems
and processes have been described as a being administered in humans, the
described systems
and processes may be administered to other mammals, such as rats, dogs, etc.
[0123] In various implementations, beta-hydroxybutyrate may administered
simultaneously
and/or sequentially with one or more other compounds (e.g., short chain,
medium chain,
and/or long chain fatty acids). For example, beta-hydroxybutyrate and/or one
or more other
compounds may be delivered mixed in a powdered, liquid, gel, and/or other
appropriate form.
In some implementations, the beta-hydroxybutyrate and/or one or more other
compounds
may be administered via pills, tablets, capsules, other oral administration
forms,
intravenously, nasal sprays, sublingual tabs/strips, or topical delivery,
rectal, other
appropriate administration forms, and/or combinations thereof.
[0124] Although the term beta-hydroxybutyrate is the terminology used in the
described
implementations, beta-hydroxybutyrate is also referred to as beta-
hydroxybutyrate , (R)-3-
Hydroxybutyric acid, (R)-3-Hydroxybutanoic acid, (3R)-3-hydroxybutanoic acid,
(R)-3-
Hydroxybutanoate, (R)-(-)-3-Hydroxybutyric acid, (R)-(-)-beta-Hydroxybutyric
acid, 3-D-
hydroxybutyrate, BHIB, BHB, 3-delta-hydroxybutyrate, delta-3-hydroxybutyrate,
3-D-
hydroxybutyric acid, D-3-hydroxybutyric acid, 3R-hydroxy-butanoic acid, delta-
beta-
hydroxybutyrate, D-3-hydroxybutyrate, D-(-)-3-hydroxybutyrate, delta-3-
hydroxybutyric
acid, (-)-3-Hydroxybutyric acid, D-beta-hydroxybutyrate, (R)-(-)-b-
Hydroxybutyrate, (R)-
beta-Hydroxybutyric acid, delta-(-)-3-hydroxybutyrate, (R)-3-hydroxybutyrate,
(R)-beta-
34
CA 03237180 2024-04-30
WO 2023/278881 PCT/US2022/036030
Hydroxybutanoic acid, (R)-(-)-beta-hydroxybutyrate, (-)-3-Hydroxy-n-butyric
acid, (R)-(-)-b-
Hydroxybutyric acid, Butanoic acid, 3-hydroxy-, (R)-Butyric acid, 3-hydroxy-,
D-(-)-(R)-3-
82578-46-9, beta-D-Hydroxybutyric acid, D-beta-Hydroxybutyric acid, (3R)-3-
delta-
hydroxybutyric acid, 3-(R)-Hydroxybutyric acid, and/or (-)-beta-
Hydroxybutyrate.
[0125] In various implementations, beta-hydroxybutyrate is described as
included in a
composition; administered in an amount, form, and/or schedule; and/or being in
a particular
form (e.g., complexed and/or coupled). R-beta-hydroxybutyrate may be utilized
in the
various described implementations of beta-hydroxybutyrate in the same or lower
amount as
the described beta-hydroxybutyrate, as appropriate.
[0126] It is to be understood the implementations are not limited to
particular systems or
processes described which may, of course, vary. It is also to be understood
that the
terminology used herein is for the purpose of describing particular
implementations only, and
is not intended to be limiting. As used in this specification, the singular
forms "a", "an" and
"the" include plural referents unless the content clearly indicates otherwise.
Thus, for
example, reference to "a compound" includes a combination of two or more
compounds and
reference to "a beta-hydroxybutyrate" includes different types and/or
combinations of beta-
hydroxybutyrate.
[0127] Although the present disclosure has been described in detail, it should
be understood
that various changes, substitutions and alterations may be made herein without
departing
from the spirit and scope of the disclosure as defined by the appended claims.
Moreover, the
scope of the present application is not intended to be limited to the
particular embodiments of
the process, machine, manufacture, composition of matter, means, methods and
steps
described in the specification. As one of ordinary skill in the art will
readily appreciate from
the disclosure, processes, machines, manufacture, compositions of matter,
means, methods,
or steps, presently existing or later to be developed that perform
substantially the same
function or achieve substantially the same result as the corresponding
embodiments described
herein may be utilized according to the present disclosure. Accordingly, the
appended claims
are intended to include within their scope such processes, machines,
manufacture,
compositions of matter, means, methods, or steps.