Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
--1--
CARBON GASIFICATION
The present invention relates to a process for generating
a hot gas consisting primarily of CO and H2 from a
carbonaceous starting material which, in pulverulent form,
with the aid of a carrier gas, together with oxidizing
agent and optionally also a slag-former, is introduced
into a gasification chamber, said starting material in
said chamber being partially combusted and at least
partially gasified.
Reduction or combustion gas based on a content of carbon
monoxide and hydrogen is currently generated using several
types of processes/ which operate in accordance with a
variety of entirely different principles, and all of which
have certain drawbacks. The energy required Is usually
generated by combustion of a carbonaceous starting
material injected into an empty chamber with oxidizing
agent. To cover the energy requirement in the process a
certain amount of the CO and H2 formed must be combusted
~ C2 and H20, and this results in the gas having higher
contents of carbon dioxide and water than desired.
20~ Consequently,~the gas generated in known carbon
gasification~processes must be relieved of a part of its
carbon dioxide content and this means that the gas must
:~:: :
...
: , , .. , ~: . .. . .
.,
. : : - . . .; : , : ~
,, ,.,~ ::
2~172-19
first be cooled before being heated again. In other processes
such a low temperature is used that tar and phenols are formed.
The gas must therefore be washed and then reheated if lt is to be
used in subsequent process steps.
Against the background of the above, the prime object of
the present invention is to effect a process for generating a hot
reduction or combustion gas consisting primarily of carbon
monoxide and hydrogen, said process permitting control of the
temperature and composition of the emerging gas, as well as the
process offering optimal utilization of energy.
According to the present invention there is provided a
process for generating a gas consisting primarily o~ C0 and H2
from a carbonaceous starting material, which process comprises
heating a carrier gas stream comprising water vapour as oxidizing
agent in a plasma generator, conducting the hot carri~r ~as
emerging from the plasma generator to a gasification chamber,
injecting, with the aid of a transport gas, a carbonaceous
starting material in finely distributed form the carrier gas and
starting material having rotary movement in the gasification
chamber where the startin~ material is partially combusted and at
least partially gaslfied to form reaction gas containing C0, H2,
H20 and C~2, the rotary movement causing a layer of slag to be
; formed on the inner wall of the gasification chamber; introducing
the reaction gas which emerges from the gasification chamber into
a shaft furnace containing a bed o solid carbonaceous lump
material, wherein the physical thermal content of the reaction gas
is used in the hed of lump material to reduce the content of
-- 2 --
;
~ .
.~
3~L~
27172-19
carbon dioxide and water in the reaction gas and thereby to form a
gas consisting primarily of carbon monoxide and hydrogen; and
re~oving this product gas from the shaft furnace.
: : ~
:
2a -
: ,
i: , , ~ . :. ; .
~2~5~
--3--
Preferably the solid carbonaceous lump material is coke.
The oxidizing agent may consist of 2~ CO2, H20~ air or
various arbitrary mixtures thereof.
According to one embodiment of the invention the energy
required is generated in the gasification chamber by
supplying an excess of air and/or oxygen gas with a
maximum of ca.20% H20 which reacts exothermically or
autothermically with a part of the carbonaceous starting
material,
According to a second embodiment of the invention external
thermal energy is supplied to the gasiication chamber.
This external thermal energy may be supplied by means of a
gas heated in plasma generators, the gas being selected
from a group consisting of 2~ H20r air and recycled gas
containing C0 + H2 + C2 + H20, or mixtures of two or more
components from the group.
According to another embodiment Oe the invention the
transport gas cons1sts of the oxidizing agent.
According to a further embodiment of the invention water
; ~ 20 vapour for introducing material and/or for use as carrier
gas is partially or wholly generated with the aid of
.
., ~ .: ,. : ..
..
:, : '
~Ç;5~
- 4 ~ 27172-19
coolant heated in a pressurized cooling system in water-cooled
parts of the installation and/or by making use of the physical
hea-t in the gas generated.
According to a further embodiment of the invention the
physical thermal content of the gas mixture emerging from the
gasification chamber is used to convert gas injected into the coke
bed, and containing carbon dioxide and/or water, to carbon
monoxide and hydrogen.
According to a further embodiment of the invention the
entire gas genera-ting process is controlled by analyzing the
o~ygen potential in the gas mixture before and/or after the
coke-filled shaft.
According to a further embodiment of the invention the
slag is fed separately out of the gasification chamber and the
coke-filled shaft. Alternatively all the slag may be removed from
the shaft.
According to a further embodiment of -the invention
`, sulphur acceptors are injected in pulverulent form into the
~ gasi~i~ation chamber and/or in lump form into the coke shaft.
.
.
.~ ,
.
:~
.,
;.
~5;3~
- 5 - 27172~19
Addi-tional features, advantages and embodiments of the
invention will be revealed in the following detailed description.
The process is of course not limited to only one
gasification chamber per shaft. On the contrary, several
gasification chambers are preferably arranged in connection with
one coke-filled shaft.
There are considerable advantages in performing the
majority of the gasification reactions in a yasification chamber
and then completing the reactions in a coke bed. One advantage is
that gasification can be performed at a high temperature which
shall always be above the melting point of the slag, and generally
above ca 1400C, after which the physical thermal content of the
gas can be utilized in the subsequent shaft to perform the
carburizing reactions which are discontinued at about 1000C.
~ s intimated above, the excess heat in the gas can be
used in sevaral ways. For instance, a relatively high content of
c-rbon dioxide 1S permitt~d in thc gas qenerated in the
,
:,
. ~ ~-, ., . :
.;, ~
..
. : . , :
--6--
gasification chamber, and this is then converted to carbon
monoxide in the coke bed, utilizing the physical thermal
content of the gas.
The advantage of working with increased CO2 content in the
gasification stage is that the resultant higher oxygen
potential gives higher reaction speed between the fuel and
the oxidizing agent and at the same time, the process is
caused to operate further from the limit where soot
deposits occur.
Another, or supplementary method of exploiting the surplus
heat is to inject carbon dioxide and/or water into the
coke bed. This can be achieved, for instance, by
utilizing a partially spent recycled gas with a high
~`~ content of carbon dioxide and water.
The coke bed fulfils a number of important functions, as
indicated above, and as will be described in more detail
below.
The coke bed collects any coke particles and slag drops
accompanying the gas mixture from the gasification
chamber. These particles and drops will then be returned
:
~-~ to the process as the coke in the coke hed is spent. The
fuel is thus used extremely efficiently,
~,
,
.
.
.. . .. .
,
..
". :.; :. ~'
:. ~ ,: :.. ,:~ ,
' . '' ~ ' - -~ :
.. ~:; : ,
. ;, . :
-
--7--
The coke bed also functions as a heat store, equalizing
any variations in the heat supplied in the gasification
chamber. It also acts as carbon store, equalizing any
variations in the quantity of carbonaceous material
supplied in relation to the quantity of oxidizing agent
supplied. This in turn gives reduced risk of explosion,
in practice entirely eliminating this risk, even should
pure oxygen be added without an equivalent quantity of
carbonaceous material being supplied to the gasification
chamber. The risk of explosion is otherwise àn extremely
serious problem.
The fuel or the carbonaceoous starting material is
supplied in finely distributed form. If it consists of
pulverulent, solid material this may be injected with the
aid of water vapour, for instance, according to the
preferred embodiment of the invention.
The carbonaceous starting material may be selected from a
group consisting of oil, coal, coke, charcoal, gas, peat,
saw-dust and various mixtures thereof. This offers
considerable~economic advantages~over the known
gasification processes, all of which are limited wlth
respect to the choice o starting material. To a great
.
extent the coke consumption is kept~low since the
oxidizing agent is forced to react with the carbonaceous
material in the gasification chamber before it reaches the
.~:
, hot, and thus reactive, coke.
"~ ~
. ~
::
: ` .: ' `
. `
i. :
. .,,`: :-. `:.: ` . ..
' '`'` ' :` '~ ',.. : ~ :,
` " ` " ` : ' `; ` ' '; ' '
" ' " :' '- ': " ` :
~2~3~iD
--8~
If only combustion is utilized to generate the heat
required for the reactions, a limitation is caused by the
thermal balance of the reactions, which results in a
relatively high carbon dioxide content. This has
previously meant that carbon dioxide must be subsequently
removed from the gas in a separate process stage.
However, in the process according to the invention, this
involves no problem. When oxygen is used as oxidizing
agent with the addition of H20, autothermic conditions
will occur if ca.20% water is added, i.e. the reactions
continue but no excess energy is generated. If thermal
energy is supplied externally, excess energy is obtained
which, according to the above, can be used in the
following coke bed. Electric energy can thus be used,
preferably by the use of plasma generators in which a
carrier gas is heated to a considerable temperature upon
passage through an electric arc generated between
electrodes in the plasma generated. In the preferred
embodiment, the carrier gas used is oxygen and water
vapour. However, water vapour, a mixture of H20 and
oxygen, pure oxygen or even air may be used as carrier
gas~
The supply of external energy enables the use of a spent
reducing gas containing high contents of carbon dioxide
and water as oxidizing agent.
,, ~'
: . . , . , ,: ,
- . .:
.
,.' ~ :
3~
_9_
According to a preferred embodiment, the process can be
controlled by recording the content of C02 or 2 partial
pressure in the gas generated, immediately after the
gasification chamber, prior to entering the coke bed
and/or in the gas outlet after the coke bed. The analysis
immediately after the gasification chamber is preferably
utilized to control the ratio between carbonaceous
material fed in and oxidizing agent, and possibly also the
temperature of the gas leaving, whereas the analysis of
the gas after the coke bed is used to control the quantity
f C2 and/or H20 fed into the coke bed. A final gas can
thus be produced having the composition and temperature
desired for the process following, which may be a
sponge-iron proces, for instance, while at the same time
almost optimal energy consumption is achieved.
In view of the high temperatures used in the process, at
least parts of the shaEt, and the entire gasification
chamber with its burners, must be water-cooled. Arranging
the cooling system to operate under pressure, preEerably
in the vicinlty of 5-6 bar over-pressure, enables the
heated coolant to be used to generate steam which can then
be used as carrier and/or transport gas. Heat losses from
:
-~ the cooling system can thus be utilized.
':
The gasification chamber shall preferably have
substantially circular inner walls. According to the
:
~,
" , .
;
' :. : ~ , . ~;
.~,........... . .
, ,: ~ - i .: .
~2~5~
--10--
invention the flows of gas and material introduced into
the gasification chamber can then be given a rotatory
motion. ~lag particles will then be separated off and
form a protective layer on the inner walls of the
gasification
chamber. The thickness of this slag deposit is determined
by the thermal balance between thermal energy removed to
the cooling casing and thermal energy supplied to the slag
surface by convection and radiation. Excess slag runs out
through a slag overflow which may be arranged separately
for the gasification chamber, or may be combined with the
slag overflow for the sha~t.
Slag-formers and/or sulphur acceptors in finely divided
form, may be injected into the gasification chamber
together with the carbonaceous material or separately from
it and/or they may be introduced together with the solid
carbonaceous material in lump form in the shaft, thus
i
forming a part of the coke bed.
To achieve rapid and efficient mixing between the hot gas
- generated in the plasma generator and the pulverulent,
carbon-carrying fuel supplied, the hot gas is introduced
through the orifice of the plasma generator into the
gasification chamber. This is achieved by the carrier gas
being given a rotary motion during its passage through the
plasma generator, the pulverulent fuel having been
-i 25 given a rotary motion prior to entering the gasification
~ . .
. ,
,
.
' :
~S3~
chamber, and/or by the plasma generator and~or supply
means for carbonaceous fuel being arranged to open
tangentially into the gasification chamber. This will
cause the hot gas to expand upon entering the gasification
chamber and this turbulence will provide an extremely
short mixing space.
The invention will now be described more fully with
reference to the accompanying drawing showing one
embodiment of an installation for performing the process
according to the invention.
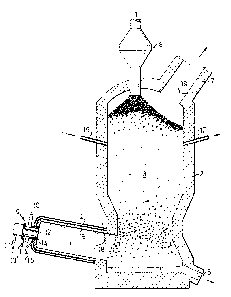
The figure shows a gasiEication plant having a
gasification chamber 1, and a shaft ~ filled with coke 3.
The gasification chamber 1 has an outer, water-cooled
`:
casing 4 and a refractory lining;5 and is preferabiy
essentially cylindrical. Several gasification chambers
are preferably arranged in connection with one shaft.
~ ~The shaft~2 has a lower slag outlet 6 and an upper gas
; outlet 7. Coke in lump form is supplied to the shaft
; ~ through a gas-tight supply means 8 at the top of the
shaft~ The gasification~chamber 1 leads into the lower
~; ;part of the shaft, from whence the gas passes up through
the coke bed and out through the gas outlet. In the
~, .
embodiment shown the slag outlet 6 ls common for both
gasification~;chamber and shaft. ;
`: :~ : : : : :
: i
.
,., . : . .: : ~ : ::
.. , : . ,
' , , ' ~,,': ~ '
.,-~.. :: -
-12-
Associated with the gasification chamber is at least one
burner consisting, in the embodiment shown, of a plasma
generator 9. The plasma generator is connected to the
gasification chamber via a valve means 10. Oxidizing
agent is introduced into ~he plasma generator through a
supply pipe 11. The oxidizing agent may comprise a
carrier gas which is led through the plasma generator or a
separate carrier gas may be used. The hot, turbulent gas
generated in the plasma generator, is introduced into the
gasification chamber through the opening 12 of the plasma
generator. The carbonaceous fuel, preferably in
pulverulent form, is introduced with the aid of a,
transport gas through a supply pipe 13 into an annular gap
14 arranged concentrically around the plasma generator,
and/or lance 15 which may also be used with advantage for
the supply of any additive, such as slag-former.
Lances 16, 17 are also arranged in the shaft for the
supply, if necessary, of additional oxidizlng agent, such
as H20 or C02, to exploit the physical surplus heat in the
gas. This also allows for control of the temperature and
composition of the gas.
; : '
At the end of the gasification chamber close to the coke
bed, a first sensing device 18 is arranged and a second
-~ sensing device 19 is arranged in the gas outlet 7 from the
! 25 shaft for measuring temperature and/or analyzin~ the gas.
`~ :
', ~'''-'
..
:- , ... . .: ,~,
.
'' ' ~
~2~i3~
-13-
These two sensing devices enable control of the process by
regulation of the external energy supplied and/or by
variation in the flows of material supplied.
The figure shows only one embodiment of a plant for
performing the process according to the invention and many
other solutions are feasible. For example, the plasma
generators or burners may be arranged tangentially on the
periphery of the gasification chamber so as to produce a
circulating flow in the gasification chamber.
Furthermore, to facilitate slag separation, the
gasification chamber may be vertical, or the gasification
chamber and shaft may have separate slag outlets.
.
:. :
:
, .~.,
..
.
:` ` ,
..
~. , .