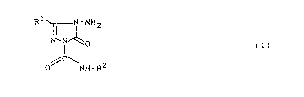Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- 2 0 0 3~2 0 8
The lnventlon relates to new substituted
trlazollnones, to several processes for their preparatlon,
and to thelr use as herblcldes.
It ls known that certaln substltuted trlazollnones,
such as, for example, the compound 4-amlno-1-(N-
lsopropylcarbamoyl)-3-methylthlo(lH,4H)-1,2,4-trlazolln-5-one
or the compound 4-amino-l-(N-propylcarbamoyl)-3-methylthlo-
tlH,4H)-1,2,4-trlazolln-5-one or the compound 4-amlno-1-(N-
butylcarbamoyl)-3-methylthio-(lH,4H)-1,2,4-triazolin-5-one or
the compound 4-amlno-1-lN-cyclohexylcarbamoyl)-3-methylthlo-
(lH,4H)-1,2,4-trlazolln-5-one (cf., for example, JP
52/125,168), have herblcldal propertles.
However, the herblcldal actlvlty of these
prevlously known compounds towards problem weeds, as well as
thelr tolerance by important crop plants, ls not entirely
satlsfactory ln all flelds of appllcatlon.
Furthermore, certaln substltuted trlazollnones,
such as, for example l-(N,N-dlmethylcarbamoyl)-3-phenyl-4-
amlno-1,2,4-trlazolln-5-one, are known (cf. J. Heterocycl.
Chem. 17, 1691 - 1696 [1980]; Org. Mass, Spectrom. 14, 369 -
378 [1979~).
Nothlng was known hltherto about an actlvlty of
these prevlously known trlazollnones as herblcldes.
Accordlng to one aspect of the present lnventlon
there ls provlded a substltuted trlazollnone of the general
formula (I)
~-~ 23189-7020
20Q3208
.
R ~
N-NH2
i ~
// \NH R2
ln whlch
Rl repreæents alkyl havlng 3 to 4 carbon atoms, or
represents cyclopropyl, and
R represent 8 hydrogen, or represents ln each case
stralght-chaln or branched alkyl havlng 1 to 18 carbon atoms,
alkenyl havlng 2 to 8 carbon atoms, alklnyl havlng 2 to 8
carbon atoms, halogenoalkyl havlng 1 to 8 carbon atoms and 1
to 17 ldentlcal or dlfferent halogen atoms, halogenoalkenyl
or halogenoalklnyl, each of whlch has 2 to 8 carbon atoms and
1 to 15 or 13 ldentlcal or different halogen atoms,
cyanoalkyl havlng 1 to 8 carbon atoms, hydroxyalkyl havlng 1
to 8 carbon atoms and 1 to 6 hydroxyl groups, alkoxyalkyl,
alkoxycarbonylalkyl or alkoxycarbonylalkenyl, each havlng up
to 6 carbon atoms ln the lndlvldual alkyl or alkenyl
moletles, or alkylamlnoalkyl or dlalkylamlnoalkyl each havlng
1 to 6 carbon atoms ln the lndlvldual alkyl moletles, or
represents cycloalkyl havlng 11 carbon atoms, or represents
cycloalkyl, cycloalkylalkyl, cycloalkenyl or
cycloalkenylalkyl, each of whlch has 3 to 8 carbon atoms ln
the cycloalkyl or cycloalkenyl molety and lf approprlate 1 to
6 carbon atoms ln the alkyl molety, and each of whlch ls
-- 2
A~ 23l89-7o2o
2~3208
optionally monosubstituted or polysubstituted by identical or
different substituents selected from the group consisting of
halogen, cyano as well as ln each case straight-chain or
branched alkyl or halogenoalkyl, each of which has 1 to 4
carbon atoms and if appropriate 1 to 9 identical or different
halogen atoms, stralght-chain or branched halogenoalkenyl
having up to 4 carbon atoms and 1 to 5 identical or different
halogen atoms, and in each case double-linked alkanediyl or
alkenediyl, in each case having up to 4 carbon atoms; R2
furthermore represents heterocycloalkyl which has 1 to 6
carbon atoms in the straight-chain or branched alkyl moiety
and 1 to 9 carbon atoms and 1 to 3 hetero atoms in the
heterocyclo moiety and which is optionally monosubstituted or
polysubstituted in the heterocyclyl moiety by identical or
different substltuents selected from the group consisting of
halogen, cyano, nltro, and in each case straight-chain or
branched alkyl, alkoxy, alkylthio, halogenoalkyl,
halogenoalkoxy, halogenoalkylthio and alkoxycarbonyl, each of
which has 1 to 5 carbon atoms and if appropriate 1 to 9
identical or different halogen atoms; R2 furthermore
represents in each case straight-chain or branched alkoxy
having 1 to 8 carbon atoms, alkenyloxy having 2 to 8 carbon
atoms or alkinyloxy having 2 to 8 carbon atoms, and finally
represents aralkyl, aroyl, aryl, aralkyloxy or aryloxy, each
of which has 6 to 10 carbon atoms in the aryl moiety and if
appropriate 1 to 8 carbon atoms in the straight-chain or
branched alkyl moiety and each of which is optionally
monosubstituted or polysubstituted by identical or different
-- 3
23189-7020
20n~2~8
substltuents, the alkyl substituents when present selected
from the group conslstlng of halogen and cyano, and the aryl
substltuents when present being selected from the group
conslstlng of halogen, cyano, nitro, hydroxyl, ln each case
stralght-chaln or branched alkyl, alkoxy, alkylthlo,
halogenoalkyl, halogenoalkoxy, halogenoalkylthlo,
alkylsùlphlnyl, alkylsulphonyl, halogenoalkylsulphlnyl,
halogenoalkylsulphonyl, alkanoyl and alkoxycarbonyl, each of
which has 1 to 6 carbon atoms ln the alkyl molety and lf
approprlate 1 to g ldentlcal or different halogen atoms, and
cycloalkyl havlng 3 to 6 carbon atoms and phenoxy; or R
represents benzyl wlth an O-~H2-O- group fused to the phenyl
molety.
Where approprlate, the compounds of the formula (I)
can be ln the form of geometrlc and~or optlcal lsomers or
mlxtures of lsomers of varlous composltlons, dependlng on the
type of the substltuents Rl and R2. The lnventlon clalms
both the pure lsomers and the mlxtures of lsomers.
Furthermore, lt has been found that the new
substltuted trlazollnones of the general formula (I) as
deflned above are obtalned when
(a) hydrazones of the formula (II)
-- 4
23189-7020
2~n~208
.
Rl R3
N-N-C ~ 4
IN~o (~)
\NH R2
in whlch
Rl and R2 have the abovementioned meaning and
R3 and R4 lndependently of one another each
represent hydrogen, or represent stralght-chaln or branched
alkyl having 1 to 4 carbon atoms, or represent phenyl or
benzyl, are reacted with an acid, lf approprlate ln the
presence of a diluent, or when
(b) lH-triazolinones of the formula (III)
R ~N-NH2
lN~o
20
in which
Rl has the abovementioned meaning are reacted with
isocyanates of the formula (IV)
R2-N=C=O (IV)
in whlch
R2 has the abovementloned meaning, if appropriate
ln the presence of a dlluent and lf approprlate ln the
-- 5
23189-7020
A
2~Q3~2 ~8
presence of a reactlon auxlllary, or ln that
(c) trlazollnones of the formula (V)
N-~ 2
I ~o
o// \o RS
ln whlch
Rl has the abovementloned meaning and
R5 represents stralght-chain or branched alkyl
havlng 1 to 4 carbon atoms, or represents phenyl or benzyl,
each of whlch ls optlonally monosubstltuted to trlsubstituted
by halogen, cyano, nltro, ln each case stralght-chaln or
branched alkyl, alkoxy or alkylthio, each of whlch has 1 to 4
carbon atoms, or ln each case a stralght-chaln or branched
halogenoalkyl, halogenoalkoxy or halogenoalkylthlo, each of
whlch has 1 to 4 carbon atoms and 1 to 9 ldentlcal or
dlfferent halogen atoms are reacted wlth amlnes of the
formula (VI)
R2-NH2 (VI)
ln whlch
R has the abovementloned meanlng, lf approprlate
ln the presence of a dlluent and lf approprlate ln the
presence of a reactlon auxlllary, or when
(d) lH-trlazollnones of the formula (III)
- 5a -
A 23l89-7020
~03~8
Rl
~"~NH2
ln whlch
Rl has the abovementioned meaning are reacted wlth
urethanes of the formula (VII)
o
RS
ln which
R2 and R5 have the abovementloned meanlng and if
approprlate ln the presence of a dlluent and lf approprlate
ln the presence of a reactlon auxlllary.
- 5b -
23189-7020
Z0032~)8
- Finally, it has been found that the new sub-
stituted triazolinones of the general formula (I) have
herbicidal properties.
Surprisingly, the substituted triazolinones of
the general formula (I) according to the invention show
a considerably higher herbicidal potency towards problem
weeds than the substituted triazolinones which are known
from the prior art, such as, for example, 4-amino-1-(N-
isopropylcarbamoyl)-3-methylthio-(lH,4H)-1,2,4-triazolin-
5-one or the compound 4-amino-1-(N-propylcarbamoyl)-3-
methylthio-(lH,4H)-1,2,4-triazolin-5-one or the compound
4-amino-1-(N-butylcarbamoyl)-3-methylthio-(lH,4H)-1,2,4-
triazolin-5-one or the compound 4-amino-1-(N-cyclohexyl-
carbamoyl)-3-methylthio-(lH,4H)-1,2,4-triazolin-5-one,
which are compounds of a similar chemical structure and
a similar type of action.
Formula (I) provides a general definition of the
substituted triazolinones according to the invention.
Preferred compounds of the formula (I) are those in which
Rl- represents n~propyl, i~propyl, n-butyl, i-butyl, s-
butyl, t-butyl or cyclopropyl and
R2 represents hydrogen, or represents in each case
straight-chain or branched alkyl having 1 to 18
carbon atoms, alkenyl having 2 to 8 carbon atoms,
alkinyl having 2 to 8 carbon atoms, halogenoalkyl
having 1 to 8 carbon atoms and 1 to 17 identical or
different halogen atoms, halogenoalkenyl or halo-
genoalkinyl, each of which has 2 to 8 carbon atoms
and 1 to 15 or 13 identical or different halogen
atoms, cyanoalkyl having 1 to 8 carbon atoms,
Le A 26 538 - 6 -
2003Z08
~ hydroxyalkyl having 1 to 8 carbon atoms and 1 to 6
hydroxyl groups, alkoxyalkyl, alkoxycarbonylalkyl or
alkoxycarbonylalkenyl, each having up to 6 carbon
atoms in the individual alkyl or alkenyl moieties,
or alkylaminoalkyl or dialkylaminoalkyl, each having
1 to 6 carbon atoms in the individual alkyl moie-
ties, or represents cycloalkyl having 11 carbon
atoms, or represents cycloalkyl, cycloalkylalkyl,
cycloalkenyl or cycloalkenylalkyl, each of which has
3 to 8 carbon atoms in the cycloalkyl or cyclo-
alkenyl moiety and if appropriate 1 to 6 carbon
atoms in the alkyl moiety, and each of which is
optionally monosubstituted or polysubstituted by
identical or different substituents, suitable
substituents in each case being: halogen, cyano as
well as in each case straight-chain or branched
alkyl or halogenoalkyl, each of which has 1 to 4
carbon atoms and if appropriate 1 to 9 identical or
different halogen atoms, or straight-chain or
2C branched halogenoalkenyl having up to 4 car~on atoms
and 1 to 5 identical or different halogen atoms, or
in each case double-linked alkanediyl or alkene-
diyl, in each case having up to 4 carbon atoms; R2
furthermore represents heterocyclylalkyl which has
1 to 6 carbon atoms in the straight-chain or
branched alkyl moiety and 1 to 9 carbon atoms and 1
to 3 hetero atoms - in particular nitrogen, oxygen
and/or sulphur - in the heterocyclyl moiety and
which is optionally monosubstituted or polysub-
stituted in the heterocyclyl moiety by identical or
Le A 26 538 - 7 -
v - -
ZQ0320~3
~ different substituents, suitable substituents being:
halogen, cyano, nitro, and in each case straight-
chain or branched alkyl, alkoxy, alkylthio,halogeno-
alkyl, halogenoalkoxy, halogenoalkylthio or alkoxy-
carbonyl, each of which has 1 to 5 carbon atoms and
if appropriate 1 to 9 identical or different halogen
atoms; R2 furthermore represenls in each case
straight-chain or branched alkoxy having 1 to 8
carbon atoms, alkenyloxy having 2 to 8 carbon atoms
or alkinyloxy having 2 to 8 carbon atoms, and
finally represents aralkyl, aroyl, aryl, aralkyloxy
or aryloxy, each of which has 6 to 10 carbon atoms
in the aryl moiety and if appropriate 1 to 8 carbon
atoms in the straight-chain or branched alkyl moiety
and each of which is optionally monosubstituted or
polysubstituted by identical or different substi-
tuents, suitable alkyl substituents, where appro-
priate, being halogen and cyano, and suitable aryl
substituents in each case being: halogen, cyano,
~itro; hydroxyl, in each Gase straight-ch~in or
branched alkyl, alkoxy, alkylthio, halogenoalkyl,
halogenoalkoxy, halogenoalkylthio, alkylsulphinyl,
alkylsulphonyl, halogenoalkylsulphinyl, halogeno-
alkylsulphonyl, alkanoyl or alkoxycarbonyl, each of
which has 1 to 6 carbon atoms in the alkyl moiety
and if appropriate 1 to 9 identical or different
halogen atoms, or cycloalkyl having 3 to 6 carbon
atoms and phenoxy; or R2 represents benzyl with an -
O-CH2-O- group fused to the phenyl moiety.
Particularly preferred compounds of the formula
Le A 26 538 - 8 -
2003208
~- (I) are those in which
Rl represents n-propyl, i-propyl, i-butyl, s-butyl, t-
butyl or cyclopropyl and
R2 represents hydrogen, methyl, ethyl, n- or i-propyl,
n-, i-, s- or t-butyl, in each case straight-chain
or branched pentyl, hexyl, heptyl, octyl, nonyl,
decyi or doaecyl, or represents allyl, in each case
straight-chain or branched butenyl, pentenyl or
hexenyl, propargyl, in each case straight-chain or
branched butinyl, pentinyl or hexinyl, or represents
straight-chain or branched halogenoalkyl having 1 to
8 carbon atoms and 1 to 9 identical or different
halogen atoms, in particular fluorine, chlorine or
bromine, or represents in each case straight-chain
or branched halogenoalkenyl or halogenoalkinyl, each
of which has 3 to 8 carbon atoms and 1 to 3 halogen
atoms, in particular fluorine or chlorine, or
represents in each case straight-chain or branched
cyanoalkyl having 1 to 6 carbon atoms in the alkyl
moiety, hydroxyalkyl having 1 to 6 carbon atGms ~nd
1 to 3 hydroxyl groups, or alkoxyalkyl, alkoxy-
carbonylalkyl or alkoxycarbonylalkenyl, alkylamino-
alkyl or dialkylaminoalkyl, each of which has up to
4 carbon atoms in the individual alkyl or alkenyl
moieties, or represents cyclopropyl, cyclopropyl-
methyl, cyclopropylethyl, cyclopentyl, cyclohexyl,
cycloheptyl, cyclooctyl, cyclohexylmethyl, cyclo-
hexylethyl, cyclohexenyl or cyclohexenylmethyl, each
of which is optionally monosubstituted to trisubsti-
tuted by identical or different substituents,
Le A 26 538 - 9 -
2003Z08
suitable substituents in each case being: fluorine,
chlorine, bromine, methyl, ethyl, n- or i-propyl,
n-,i-, s- or t-butyl, cyano, methanediyl, ethane-
diyl, butanediyl or butadienediyl or dichloroallyl;
R2 furthermore represents heterocyclylmethyl, hetero-
cyclylpropyl or heterocyclylethyl, each of which is
optionally monosubstituted to trisubstituted in the
heterocyclyl moiety by identical or different
substituents, suitable heterocycles in each case
being:
~Z~l; N~Z~; N~l --N~>
~ --N O or --N~ N}~ '
Z in each case representing oxygen or sulphur and suit-
able substituents in each case being:
fluorine, chlorine, bromine, cyano, nitro, methyl, ethyl,
n- or i-propyl, n-, i-, s- or t-butyl, methoxy, ethoxy,
methylthio, trifluoromethyl, trifluoromethoxy or
trifluoromethylthio;
R2 additionally represents in each case straight-chain
or branched alkoxy having 1 to 6 carbon atoms,
Le A 26 538 - 10 -
.
2003;~08
-- alkenyloxy having 3 to 6 carbon atoms or alkinyloxy
having 3 to 6 carbon atoms, or represents benzyl,
phenylethyl, phenylpropyl, phenylbutyl, phenyl-
pentyl, phenylhexyl, phenylheptyl, phenylcyano-
methyl, phenylcyanoethyl, phenylcyanopropyl, benzyl-
oxy, phenylethyloxy, phenoxy, benzoyl, phenyl or
naphthyl, where approprlate straight-chain or
branched, each of which is optionally monosubsti-
tuted to trisubstituted by identical or different
substituents, suitable phenyl substituents in each
case being: fluorine, chlorine, bromine, hydroxyl,
cyano, nitro, methyl, ethyl, n- or i-propyl, n-, i-,
s- or t-butyl, methoxy, ethoxy, methylthio, tri-
fluoromethyl,trifluoromethoxy,trifluoromethylthio,
trifluoromethylsulphinyl, trifluoromethylsulphonyl,
methylsulphinyl, methylsulphonyl, acetyl, propionyl,
methoxycarbonyl, ethoxycarbonyl, cyclohexyl or
phenoxy.
Very particularly preferred compounds of the
formula (I) arç those in which
Rl represents isopropyl, cyclopropyl or s-butyl and
R2 represents methyl, ethyl, n- or i-propyl, n-, i-, s-
or t-butyl, allyl, propargyl, in each case straight-
chain or branched pentyl, hexyl, heptyl, octyl,
butenyl, pentenyl, hexenyl, butinyl, pentinyl or
hexinyl, each of which is optionally monosubstituted
to trisubstituted by fluorine and/or chlorine;
additionally represents cyclopropyl, cyclopentyl,
cyclohexyl, cyclohexenyl, cyclopropylmethyl, cyclo-
propylethyl, cyclohexylmethyl, cyclohexylethyl or
Le A 26 538 - 11 -
2003;~08
-
- cycloheptyl, each of which is optionally monosubsti-
tuted to trisubstituted by identical or different
substituents from the series comprising fluorine,
chlorine, methyl, ethyl and/or cyano, and finally
represents benzyl or phenylethyl.
The following substituted triazolinones of the
general formula (I) may be mentioned individually:
Rl~N-NH2
N~NI ~ O (I)
O NH-R2
Le A 26 538 - 12 -
-
200;~208
- Rl R2
(CH3~2CH-
(CH3)2CH- ~
1 0 /CH2
(CH3~2CH- \ CH2
(CH3)2CH- H3C
(CH~)2CH- C2H5
(CH3)2CH- H3C
(CH3)2CH- C2H5 ~
(CH3)2CH- -CH2 ~ l
(CH3)2CH- -CH2-CH2 ~ l
~CH2F
30 (CH3)2CH ~CH2F
fH3
(CH3)2CH- -f-CH=CH2
CH3
Le A 26 538 - 13 -
~003Z(~8
Rl R2
.
fH3
(CH3)2CH- -f -CH2 CH cH2
CH3
C!H3
10 (CH3)2CH- -f-CH2F
CH3
fH3
(CH3)2CH- -f-CH2Cl
CH3
fH2F
(CH3)2CH- -f-CH~
CH2F
(CH3)2CH- -fH-CH2-Cl
CH3
fH2Cl
25 (CH~)2CH- -f-CH3
CH2C 1
ICH3
(CH3)2CH- -IC-CHCl2
CH3
(CH3)2CH- -CH-CH2-CH(cH3)2
CH3
Le A 26 538 - 14 -
!~'' ' '' ' '
200320~3
Rl R2
(CH3)2CH- -fH-(CH2)2-CH(cH3)2
CH3
(CH3)2CH- -CH-(CH2)3-CH(cH3)2
CH3
CH3
(CH3)2CH- -C-CF3
CH3
(CH3)2CH- -(CH2)2-CH3
(CH3~2CH- -(CH2)3-CH3
(CH3)2CH- -CH(CH3)2
(CH3)2CH- -CH-C2H5
CH3
(CH3)2CH- -fH-CH(CH3)2
CH3
(CH3)2CH- -CH
CH3
(CH3)2CH- -CH
C2H5
CH3
(CH3)2CH- - 1~3
CH~
Le A 26 538 - 15 -
e
2003208
Rl R2
CH3
(CH3)2CH- -C-C-CH
CH3
IH3
10 (CH3)2CH- -C-C2H5
CH3
CIH3
15 (CH3)2CH- f ( CH2~2 CH3
CH3
IH3
(CH3)2CH- -C-(CH2)3-CH3
CH3
(CH3)2CH- -C(CH3)3
¦ CH-
H2C~'
H21C~
H2C~
H2C~ /CH2
¦ CH- -CH
H2C~ \CH2 . .
H21~ H3C ~
H2C~ C 2H5~0
Le A 26 538 - 16 -
- 2003208
- R2
5 H2f~ H3C
H2C
H2 IC~ C 2H5
H2C~
H2C~ - CH2{~C 1
H2C
H2 IC~ _CH2_cH2{~C
H2C~
H2C~ ~cH2F
CH - - CH
H2C~ ~CH2F
H2C~
20 H2C~ - f CH=CH2
CH3
CH3
H2C~
H2C~ -C-CH2-CH=CH2
CH3
CH3
H2C~
¦ CH- -C-CH2F
H2C~
CH3
CH3
H2C~
¦ CH- -C-CH2Cl
H2C~
CH3
Le A 26 538 - 17 -
.. . .
Z00~20~3
Rl R2
S CH2F
H2C~
¦ CH- -C-CH3
H2C~
CH2F
H2C~ -CH;CH2-Cl
CH2C 1
H2
¦ CH- -C-CH~
H2C~
CH2Cl
CH3
H2C~
¦ CH- -C-CHCl2
H2C ~ CH3
H21C~ -cH-cH2-cHtcH3)2
H2C~ rH3
H2CI~ -CH-(CH2)2-CH(cH3)2
25 H2C~ CH~
H2C~
¦ CH- -CH-(CH2)3-~H(CH3)2
H2C~
CH3
CH3
H2C~
¦ CH- -C-CF3
H2C~ CH3
Le A 26 538 - 18 -
s - ~
Z00;~208
Rl R2
H2C~
¦ CH- -(CH2)2-CH3
H2C~
H2C`
¦ CH- -(CH2)3-CH3
H2C~
H2C`
¦ CH- -CH(CH3)2
H2
H2C~
¦ CH- -CH-C2H5
H2C~
CH3
H2C`
¦ CH- -CH-CH(CH3)2
H2C~
CH3
2¦ CH- -CH
CH3
H2C~ -CH
C2H5
CH3
H2CI ~ - C~3
H2C~
CH3
CH3
H2C~
¦ CH- -C-C-CH
H2C~
CH3
Le A 26 538 - 19 -
- ~003208
- -- Rl R2
CH3
H2C~
CH - - C - C 2H5
H2C~
CH3
CH3
10 H2CI~ C (CH2)2 CH3
H C~
2 CH3
CH3
H2f~ -C-(CH2)3-CH3
15 H2C ~ CH3
H21C~ -C(CH3)3
H2
C2H5 fH
CH3
C2H5-CH-
CH3
/CH2
C2H5-CH- \ CH2
CH3
~ C2H5-CH- H3C
CH3
Le A 26 538 - 20 -
2003~i0~3
- Rl R2
_
C2H5 fH- C2H5
CH3
C~H~-fH- H3C
10CH3
C2H5 fH C2H5
CH3
C2H5-fH; -CH2 ~ 1
C2H5-CH- -CH2-CH2 ~ 1
20CH3
~CH2F
C2H5 fH ~CH2F
CH3
fH3
C2H5 fH- -f-CH=CH2
CH3 CH3
fH3
30 C2H5-fH- -f-CH2-CH=cH2
CH3 CH3
Le A 26 538 - 21 -
Z003208
Rl R2
fH3
C2Hs-fH- -f-CH2F
CH3 CH3
~H3
10C2H5-fH- -f-CH2Cl
CH3 CH3
fH2F
C2Hs-fH- -f-CH3
CH3 CH2F
C2Hs-fH -fH-CH2-Cl
CH3 CH3
fH2Cl
C2H5-CH- f -CH3
CH3 CH2Cl
fH3
25 C2Hs-fH- -f-CHC12
CH3 CH3
C2H5 fH-fH-CH2-CH(cH3~2
CH3 CH3
C2H5 CIH-fH-(CH2)2-CH(CH3)2
CH3 CH3
C2H5 fH-fH-(CH2)3-CH(cH3)2
CH3 CH3
Le A 26 538 - 22 -
2003;208
Rl R2
fH3
C2H5 fH -C-CF3
CH3 CH3
C2H5 fH -(CH2)2-CH3
CH3
C2H5 fH -(CH2)3-CH3
CH3
C2H5-fH- -CH(CH3)2
c~3
C2H5 fH -fH-C2H5
CH3 CH3
20 C2H5-fH- -fH-CH(CH3)2
CH3 CH3
C2H5-fH-
CH3 CH3
C2H5 fH
CH3 C2H5
CH3
C2H5 ICH f
CH3 CH3
Le A 26 538 - 23 -
. ......
~003Z08
- Rl R2
fH3
C2H5-CH- -C-C--CH
CH3 CH3
IH3
10 C2H5-CH- -C-C2H5
CH3 CH3
CIH3
15 C2H5-CH- f (CH2)2 CH3
CH3 CH3
ICH3
C2H5-CH- -C-(CH2)3-CH3
20CH3 CH3
C2H5-CH- -C(CH3)3
CH~
Le A 26 538 - 24 -
i, . . .
20032013
If, for example, l-(N-isobutylcarbamoyl)-4-
isopropylideneimino-3-isopropyl-1,2,4-triazolin-5-one is
used as the starting compound, the course of the reaction
of process (a) according to the invention may be repre-
sented by the following equation:
~CH3
CH3 H201H
N`N~o >
I -(CH3)2CO
~C~
o NH-CH2-CH(cH3)2
~CH3)2-CH~ NH2
N`N o
o NH-CH2-CH~CH3)2
If, for example, 4-amino-3-cyclopropyl-1,2,4-(lH)-tri-
azolin-5-one and t-butyl isocyanate are used as the
starting substances, the course of the reaction of
process (b) according to the invention may be represented
by the following equation:
H2C \
H2C/ ~Nl -NH2 t ~ CH3)3C-N=C=O
N`N~o
H2C\
I f ~N - NH 2
> N`IN o
O=C-NH-C(CH3)3
Le A 26 538 - 25 -
~003~)8
If, for example, l-phenoxycarbonyl-4-amino-3-s-butyl-
1,2,4-triazolin-5-one and N,N-diethylpropane-1,3-diamine
are used as starting substances the course of the reac-
tion of process (c) according to the invention may be
represented by the following equation:
CH
j 3
C2H5-CH~N-NH2 ~52H5
ll ~ H2N-CH2-CH2-cH2 N
N~N~ ~0 ~C2H5
O=C-OC6H5
Cl H3
C2H5 - CH~_N- NH2
- C6H5-OH ll l
> N`N~o
~C2H5
O=C-NH-CH2 CH2 CH2 N
C 2H5
If, for example, 4-amino-3-isopropyl-1,2,4-(lH)-tri-
azolin-5-one and N-isopropyl phenylcarbamate are used as
the starting substances, the course of the reactiGn of
process (d) according to the invention may be represented
- by the following equation:
o
H3C~ 11 ~ ( CH3 ) 2CH~r--N-NH2
CH-NH-C-O~ ~ 1 1
H3C~ N`NI ~o
H
( CH3 ~ 2C~_N-NH2
- C6H50H
N`N~o
~CH3
O=C-NH-CH
Le A 26 538 - 26 -
,
200;~08
- ~~ Formula (II) provides a general definition of the hydra-
zones required as starting substances for carrying out
process (a) according to the invention. In this formula
(II), Rl and R2 preferably represent those radicals which
have already been mentioned in connection with the
description of the substances of the formula (I) accord-
ing to the invention as being preferrea ~or these sub-
stituents. R3 and R4 in each case independently of one
another represent hydrogen, or represent straight-chain
or branched alkyl having 1 to 4 carbon atoms, or repre-
sent phenyl or benzyl.
The hydrazones of the formula (II) were hitherto
unknown. They are likewise the subject-matter of the
present invention. However, they are obt~ine~ in analogy
to known processes (cf., for example, Acta Pol. Pharm.
38, 153 - 162 tl981] or C.A. 95: 203841j), for example by
reacting l-unsubstituted 4-aminotriazolinones of the
formula (III)
R 1 ~T--N'NH 2
I l (III)
N`NI ~0
in which
R1 has the abovementioned meaning,
with aldehydes or ketones of the formula (VIII)
c = o (VIII)
R4~
Le A 26 538 - 27 -
ZQ03~08
in which
R3 and R4 have the abovementioned meaning,
if appropriate in the presence of a diluent, such as, for
example, dichloromethane or toluene, and if appropriate
in the presence of a catalyst, such as, for example, p-
toluenesulphonic acid, at temperatures between 40C and
120C, an~ reacting the resulting l-unsubstituted tri-
azolinone hydrazones of the formula (IX)
R~ N-N=C
¦ ~R4 (IX)
H
in which --
R1, R3 and R4 have the abovementioned meaning
either in a subsequent 2nd step with isocyanates of the
formula (IV)
R2 - N = C =
in which
R2 has the abovementioned meaning,
if appropriate in the presence of a diluent, such as, for
example, dichloromethane or dioxane, and if appropriate
in the presence of a reaction auxiliary, such as, for
example, triethylamine, at temperatures between 50C and
150C;
or alternatively in a subsequent 2nd step with chloro-
formic acid esters of the formula (X)
Le A 26 538 - 28 -
2003208
o
R5 - o - C - C~ (X)
in which
R5 represents alkyl, aryl or arylalkyl,
if appropriate in the presence of a diluent, sucn as, for
example, tetrahydrofuran, and if appropriate in the
presence of a reaction auxiliary, such as, for example,
sodium hydride or potassium t-butoxide, at temperatures
between -20C and +40C, and reacting the resulting
triazolinones of the formula (XI)
R1 N-N=C (XI3
¦ ~ R4
N~IN~o
o=c -o-R5
in which
ln Rlo R30 R4 ~nd R5 hav~ the abovementioned ~eaning
in a subsequent 3rd step with amines of the formula (VI)
R2 - N~2 (~I)
in which
R2 has the abovementioned meaning,
if appropriate in the presence of a diluent, such as, for
example, tetrahydrofuran, and if appropriate in the
presence of a base, such as, for example, sodium hydrox-
ide, potassium hydroxide or diazabicycloundecene (DBU),
Le A 26 538 - 29 -
2003208
at temperatures between 20C and 50C.
In this context, it is also possible and may be
advantageous to carry out the reaction of the 1-unsubsti-
tuted triazolinone hydrazones of the formula (IX) with
chloroformic acid esters of the formula (x) and the sub-
sequent reaction of the resulting triazolinones (XI) with
amines of the formula (VI) in one reaction step in a
so-called one-pot process.
Some of the l-unsubstituted 4-amino-triazolinones
of the formula (III) are known and can be obtAine~ in
analogy to known processes (cf., for example, J. Hetero-
cycl. Chem. 16, 403 tlg79]; J. Heterocycl. Chem. 17, 1691
[1980]; Europ. J. Med. Chem. 18, 215 [1983]; Chem. Ber.
98, 3025 [1965]; Liebigs Ann. Chem. 637, 135 [1960]; J.
Heterocycl. Chem. 21, 1769 - 1774 [1984]; Chim. Acta
Turc. 7, 269 - 290 [1979] or CA 106 (17): 138338e
[1986]).
The aldehydes or ketones of the formula (VIII)
are generally known compounds of organic chemistry.
Some of the 1-unsubstituted triazolinone hydra-
zones of the formula (IX) are known (cf., for example,
Chim. Acta. Turc. 7, 269 - 290 [1979]).
The chloroformic acid esters of the formula (X)
are generally known compounds of organic chemistry.
The triazolinones of the formula (XI), which are
mentioned as intermediates, were hitherto unknown and are
also the subject-matter of the invention.
Rl in this context preferably represents those radicals
which have already been mentioned in connection with
the description of the substances of the formula (I)
Le A 26 538 - 30 -
- - - . .. . .
200321D8
according to the invention as being preferred for
this substituent.
R3 and R4 in each case independently of one another
preferably represent hydrogen, or representstraight-
chain or branched alkyl having 1 to 4 carbon atoms,
or represent phenyl or benzyl, and
R' preferably represents straight-chain or branched
alkyl having 1 to 4 carbon atoms, or represents
phenyl or benzyl, each of which is optionally
monosubstituted to trisubstituted by identical or
different substituents, suitable substituents in
each case being: halogen, cyano, nitro, in each case
straight-chain or branched alkyl, alkoxy or alkyl-
thio, each of which has 1 to 4 carbon atoms, or in
each case a straight-chain or branched halogeno-
alkyl, halogenoalkoxy or halogenoalkylthio, each of
which has 1 to 4 carbon atoms and 1 to 9 identical
or different halogen atoms;
R5 in particular represents methyl, ethyl, n- or i-
2G propyl, n-, i-, s- or t-butyl, or represen~s phenyl
or benzyl, each of which is optionally monosubsti-
tuted to disubstituted by identical or different
substituents, suitable substituents in each case
being: fluorine, chlorine, bromine, cyano, nitro,
2S methyl, ethyl, n- or i-propyl, n-~ i-, s- or t-
butyl, methoxy, ethoxy, n- or i-propoxy, methylthio,
trifluoromethyl, trifluoromethoxy or
trifluoromethylthio.
Formula (III) provides a general definition of
the lH-triazolinones required as starting substances for
Le A 26 538 - 31 -
f ` ` - . .
- ZQ03208
~~ carrying out processes (b) and (d) according to the
invention. In this formula (III), R1preferably represents
those radicals which have already been mentioned in
connection with the description of the substances of the
formula (I) according to the invention as being preferred
for this substituent.
The lH-triazolinones of the formula (III) are
either known, or they can be obtained in analogy to known
processes (cf., for example, B.J. Heterocycl. Chem. 16,
10403 [1979]. J. Heterocycl. Chem. 17, 1691 [1980]; Europ.
J. med. Chem. 18, 215 [1983]; Chem. Ber. 98, 3025 [1965];
Liebigs Ann. Chem. 637, 135 [1960]; J. Heterocycl. Chem.
21, 1769 - 1774 [1984]; Chim. Acta. Turc. 7, 269 - 270
[1979]; CA 106 (17): 138338e [1986]).
15l-Unsubstituted 4-aminotriazolinones of the
formula (IIIa)
Rl_ l~N-NH2
1 (IIIa)
H
in which
R1-1 represents n-butyl, i-butyl, s-butyl or cyclopropyl,
in particular represents s-butyl or cyclopropyl,
were hitherto unknown and are also the subject-matter of
the invention.
They are obtained in analogy to known processes
(cf., for example, J. Heterocycl. Chem. 21, 1769 [1984],
and Chem. Ber. 98, 3025 [1965]), for example when
Le A 26 538 - 32 -
Z003Z08
- hydrazine hydrate is reacted with diphenyl carbonate in
a customary manner, and the resulting carbonic acid
hydrazide is cyclized, likewise in a customary manner,
with carboxylic acid derivatives of the formula (XII)
R1-1 - COOR6 (~II)
in which
R1-l has the abovementioned meaning and
R6 represents hydrogen, or represents straight-chain or
branched alkyl having 1 to 4 carbon atoms,
at temperatures between 150C and 250C (cf. also the
preparation examples).
Carboxylic acid derivatives of the formula (XII)
are generally known compounds of organic chemistry.
Formula (IV) provides a general definition of the
isocyanates fur~hermore required as starting substances
for carrying out process (b) according to the invention.
In this formula (IV), R2 preferably represents those
radicals which have already-been mentioned in connectiDn
with the description of the substances of the formula (I)
according to the invention as being preferred for this
substituent.
The isocyanates of the formula (IV) are generally
known compounds of organic chemistry. The compounds
2,2,2-trifluoroisopropylcyanate and 2,2,2-trifluoro-1,1-
dimethylethyl cyanate were hitherto unknown, but they can
be prepared by known methods.
Formula (V) provides a general definition of the
triazolinones required as starting substances for
Le A 26 538 - 33 -
Z003208
carrying out process (c) according to the invention. In
this formula (V), R1 preferably represents those radicals
which have already been mentioned in connection with the
description of the substances of the formula (I) accord-
ing to the invention as being preferred for this
substituent.
R5 preferably represents straight-chain or
branched alkyl having 1 to 4 carbon atoms, or represents
phenyl or benzyl, each of which is optionally monosubsti-
tuted to trisubstituted by identical or different sub-
stituents, suitable substituents in each case being:
halogen, cyano, nitro, in each case straight-chain or
branched alkyl, alkoxy or alkylthio, in each case having
1 to 4 carbon atoms, or in each case straight-chain or
branched halogenoalkyl, halogenoalkoxy or halogenoalkyl-
thio, in each case having 1 to 4 carbon atoms and 1 to 9
identical or different halogen atoms;
R5 in particular represents methyl, ethyl, n- or i-
propyl, n-, i-, s- or t-butyl, or represents phenyl
or benzyl, each of which is optionally monosubstl-
tuted to disubstituted by identical or different
substituents, suitable substituents in each case
being: fluorine, chlorine, bromine, cyano, nitro,
methyl, ethyl, n- or i-propyl, n-, i-, s- or t-
butyl, methoxy, ethoxy, n- or i-propoxy, methylthio,
trifluoromethyl, trifluoromethoxy or
trifluoromethylthio.
The triazolinones of the formula (V) were hither-
to unknown.
They are obt~ine~ in analogy to known processes,
Le A 26 538 - 34 -
r-
200320~3
~ by reacting lH-triazolinones of the formula (III)
R1 ~ N-NH2 (III)
N~N O
H
in which
Rl has the abovementioned meaning
with chloroformic acid esters of the formula (X)
R5 - O - C - Cl (x)
in which
R5 has the abovementioned meaning,
if appropriate in the presence of a diluent, such as, for
example, tetrahydrofuran, and if appropriate in the
presence of a reaction auxiliary, such as; for example,
potassium t-butoxide or sodium hydride, at temperatures
between -20C and +40C (cf. also the preparation
examples).
Formula (VII) provides a general definition of
the ureth~nes furthermore required as starting substances
for carrying out process (d) according to the invention.
In this formula (VII), R2 preferably represents those
radicals which have already been mentioned in connection
with the description of the substances of the formula (I)
according to the invention as being preferred for these
Le A 26 538 - 35 -
Z003208
- ~ substituents. R5 preferably represents those radicals
which have already been mentioned in connection with the
description of the precursors of the formula (V) as being
preferred, or particularly preferred, for this
substituent.
The urethanes of the formula (VII) are generally
known compounds of organic chemistry, or they can be
obt~i ne~ with the aid of generally known processes.
Suitable acids for carrying out process (a)
according to the invention are all inorganic and organic
acids which can customarily be used for hydrazone cleav-
ages. Inorganic mineral acids, such as hydrochloric acid,
sulphuric acid or phosphoric acid, are preferably used.
Suitable diluents for carrying out process (a)
according to the invention are all customary organic or
inorganic solvents. Polar, water-miscible organic sol-
vents, in particular alcohols, such as methanol, ethanol,
propanol or butanol, or their mixtures with water, or
pure water, are preferably used as the diluents.
~hen carrying out process (a) according to the
invention, the reaction temperatures can be varied within
a substantial range. In general, the process is carried
out at temperatures between 20C and 150C, preferably at
temperatures between 50C and 120C.
Process (a) according to the invention is conven-
tionally carried out under atmospheric pressure or under
reduced pressure. If the process is carried out under
reduced pressure, suitable pressure ranges are those
between 20 and 400 mbar, preferably between 100 and
200 mbar.
Le A 26 538 - 36 -
200;~20~3
- ~~ For carrying out process (a) according to the
invention, 1 to 50 moles, preferably 1 to 20 moles, of
acid are generally employed per mole of hydrazone of the
formula (II). For this purpose, the hydrazone of the
formula (II) is dissolved in a suitable amount of dilu-
ent, the required amount of acid is then added, and the
mixture is slowly concentrated under reduced pressure in
the course of several hours.
In a special embodiment of the procedure, it is
also possible to carry out process (a) according to the
invention and the preparation of the precursors of the
formula (II) required for this, in one reaction step in
a so-called one-pot process.
One possibility in this process is to choose the
triazolinones of the formula (XI) as starting compounds,
and to react these in succession in a one-pot process
with amines of the formula (VI) and then with acid, in
accordance with process (a) according to the invention
(cf. in this context, also the preparation examples), or,
alternatively, to choose the trlaæoline hydr~zones of the
formula (IX) as the starting compounds and to react these
in succession in a one-pot process with chloroformic acid
esters of the formula (X), then with amines of the
formula (VI) and subsequently with acid, in accordance
with process (a) according to the invention.
Suitable diluents for carrying out process (b)
according to the invention are inert organic solvents.
These particularly include aliphatic, alicyclic or aro-
matic, optionally halogenated hydrocarbons, such as, for
example, benzine, benzene, toluene, xylene,chlorobenzene,
Le A 26 538 - 37 -
2003X08
.
petroleum ether, hexane, cyclohexane, dichloromethane,
chloroform and carbon tetrachloride, ethers, such as
diethyl ether, dioxane, tetrahydrofuran or ethylene
glycol dimethyl ether or ethylene glycol diethyl ether,
nitriles, such as acetonitrile or propionitrile, amides,
such as dimethylformamide, dimethylacetamide, N-
methylformanilide, N-methylpyrrolidone or hexamethyl-
phosphoric triamide, or esters, such as ethyl acetate.
If appropriate, process (b) according to the
invention is carried out in the presence of a suitable
reaction auxiliary. Suitable reaction auxiliaries are all
customary inorganic or organic bases. These include, for
example, tertiary amines, such as triethylamine, N,N-
dimethylaniline, N,N-diethylbenzylamine, N,N-dimethyl-
cyclohexylamine or dibutyltin dilaureate, pyridine, N,N-
dimethylaminopyridine, diazabicyclooctane (DABCO),
diazabicyclononene (DBN) or diazabicycloundecene (DBU).
When carrying out process (b) according to the
invention, the reaction temperatures can be varied within
a substantial range. In general, the process is carried
out at temperatures between 0C and 150C, preferably at
temperatures between 20C and 100C.
For carrying out process (b) according to the
invention, 1.0 to 2.0 moles, preferably 1.0 to 1.5 moles,
of isocyanate of the formula (IV) and, if appropr~ate,
0.001 to 2.0 moles, preferably 0.001 to 1.0 mole, of
reaction auxiliary are generally employed per mole of lH-
triazolinone of the formula (III). The reaction is
carried out and the reaction products are worked up and
isolated by generally customary methods.
Le A 26 538 - 38 -
2003208
- Suitable diluents for carrying out processes (c)
and (d) according to the invention are inert organic
solvents. These in particular include aliphatic, ali-
cyclic or aromatic, optionally halogenated hydrocarbons,
S such as, for example, benzine, benzene, toluene, xylene,
chlorobenzene, petroleum ether, hexane, cyclohexane,
dichloromethane, chloroform and carbon tetrachloride,
ethers, such as diethyl ether, dioxane, tetrahydrofuran
or ethylene glycol dimethyl ether or ethylene glycol
diethyl ether, nitriles, such as acetonitrile or propio-
nitrile, amides, such as dimethylformamide, dimethyl-
acetamide, N-methylformanilide, N-methylpyrrolidone or
hexamethylphosphoric triamide, or esters, such as ethyl
acetate, or sulphoxides, such as dimethyl sulphoxide.
If appropriate, processes (c) and (d) according
to the invention can be carried out in the presence of a
suitable reaction auxiliary. Suitable reaction aux-
iliaries are all customary inorganic or organic bases.
These preferably include alkali metal hydroxides, such as
2~ sodium hydroxide or potassium hydoxide, alhali me~di
carbonates, such as sodium carbonate, potassium carbonate
or sodium hydrogen carbonate, and also tertiary amines,
such as triethylamine, N,N-dimethylaniline, pyridine,
N,N-dimethylaminopyridine, diazabicyclooctane (DABCO),
diazabicyclononene (DBN) or diazabicycloundecene (DBU).
When carrying out processes (c) and (d) according
to the invention, the reaction temperatures can be varied
within a substantial range. In general, the processes are
carried out at temperatures between 0C and 120C, pre-
ferably at temperatures between 20C and 50C.
Le A 26 538 - 39 -
- 2003~08
For carrying out process (c) according to the
invention, 1.0 to 5.0 moles, preferably 1.0 to 2.5 moles,
of amine of the formula (VI) and if appropriate 1.0 to
2.0 moles, preferably 1.0 to 1.2 moles, of reaction
auxiliary are generally employed per mole of triazolinone
of the formula (V).
The reaction is carried out and the reaction products are
worked up and isolated by generally customary methods.
In a special embodiment of the procedure, it is
also possible to carry out process (c) according to the
invention and to prepare the precursors of the formula
(V) required for this, in one reaction step in a so-
called one-pot process.
In this way of carrying out the reaction, the
starting materials are lH-triazolinones of the formula
(III), which are reacted in succession in a one-pot
process, initially with chloroformic acid esters of the
formula (X) and subsequently with amines of the formula
(VI), in accordance with process (c) according to the
invention.
For carrying out process (d) according to the
invention, 1.0 to 5.0 moles, preferably 1.0 to 2.5 moles,
of urethane of the formula (VII) and, if appropriate, 1.0
to 5.0 moles, preferably 1.0 to 2.5 moles, of reaction
auxiliary are generally employed per mole of lH-triazoli-
none of the formula (III).
The reaction is carried out and the reaction products are
worked up and isolated by generally customary methods.
Another method to obtain compounds of the formula
(I) according to the invention comprises reacting
Le A 26 538 - 40 -
Z003Z08
- ~ oxadiazolinones of the formula (XIII)
Rl o
~1 ~ (XIII)
0~ NH-R2
in which
R1 and R2 have the abovementioned meaning,
with hydrazine hydrate in the presence of a suitable
diluent, such as, for example, methanol or ethanol, at
temperatures between 20C and 100C, and thermally cycliz-
ing the resulting carbacic acid derivatives of the
formula (XIV)
O O
Il 11
Rl -C-NH-N-C-NH-NH2
I (XIV)
O=C-NH-R2
in which
Rl and R2 have the abovementioned meaning
in the presence of a suitable diluent, such as, for
example, toluene, chlorobenzene or dichlorobenzene, at
temperatures between 80C and 200C.
Oxadiazolinones of the formula (XIII) are known
(cf., for example, FR 1,415,605 or C.A. 64: P5105g, and
NL 6,510,645 or C.A. 65: P2274d-f), or they can be
Le A 26 538 - 41 -
Z003208
- - obtained by generally known processes, for example by
reacting the corresponding 4H-oxadiazolinones with
isocyanates of the formula (IV) in analogy to the proce-
dure of process (b) according to the invention or to the
synthesis of the precursors of the formula (II).
The end products of the formula (I) are purified
with the aid of customary processes, for example by means
of column chromatography or by recrystallization.
They are characterized with the aid of the melting point
or, in the case of non-crystallizing compounds, with the
aid of the proton nuclear magnetic resonance spectrum.
The active compounds according to the invention
can be used as defoliants, desiccants, agents for des-
troying broad-leaved plants and, especially, as weed-
killers. By weeds, in the broadest sense, there are to beunderstood all plants which grow in locations where they
are undesired. Whether the substances according to the
invention act as total or selective herbicides depends
essentially on the amount used.
The active compounds according to the invention
can be used, for example, in connection with the follow-
ing plants:
Dicotyledon weeds of the genera: Sinapis,
Lepidium, Galium, Stellaria, Matricaria, Anthemis,
Galinsoga, Chenopodium, Urtica, Senecio, Amaranthus,
Portulaca, Xanthium, Convolvulus, Ipomoea, Polygonum,
Sesbania, Ambrosia, Cirsium, Carduus, Sonchus, Solanum,
Rorippa, Rotala, Lindernia, Lamium, Veronica, Abutilon,
Emex, Datura, Viola, Galeopsis, Papaver, Centaurea and
Mercurialis.
Le A 26 538 - 42 -
2003208
- Dicotyledon cultures of the genera: Gossypium,
Glycine, Beta, Daucus, Phaseolus, Pisum, Solanum, Linum,
Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis,
Brassica, Lactuca, Cucumis and Cucurbita.
Monocotyledon weeds of the genera: Echinochloa,
Setaria, Panicum, Digitaria, Phleum, Poa, Festuca,
Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus,
Sorghum, Agropyron, Cynodon, Monochoria, Fimbristylis,
Sagittaria, Eleocharis, Scirpus, Paspalum, Ischaemum,
Sphenoclea, Dactyloctenium, Agrostis, Alopecurus and
Apera.
Monocotyledon cultures of the genera: Oryza,
Zea, Triticum, Hordeum, Avena, Secale, Sorghum, Panicum,
Saccharum, AnAn~, Asparagus and Allium.
However, the use of the active compounds accord-
ing to the invention is in no way restricted to these
genera, but also extends in the same manner to other
plants.
The compounds are suitable, depending on the
concentration, for the total combating of weeds, for
example on industrial terrain and rail tracks, and on
paths and squares with or without tree plantings. Equal-
ly, the compounds can be employed for combating weeds in
perennial cultures, for example afforestations, decor-
ative tree plantings, orchards, vineyards, citrus groves,
nut orchards, h~n~n~ plantations, coffee plantations, tea
plantations, rubber plantations, oil palm plantations,
cocoa plantations, soft fruit plantings and hopfields,
and for the selective combating of weeds in annual
cultures.
Le A 26 538 - 43 -
r ` ` `
2003Z0~
- ~~ In this context, the active compounds according
to the invention can be employed with particularly good
success for combating monocotyledon and dicotyledon weeds
in monocotyledon and dicotyledon crops, such as, for
example, sugar beet, maize, wheat and barley.
The active compounds can be converted into the
customary formulations, such as solutions, emulsions,
wettable powders, suspensions, powders, dusting agents,
pastes, soluble powders, granules, suspension-emulsion
concentrates, natural and synthetic materials impregnated
with active compound, and very fine capsules in polymeric
substances.
These formulations are produced in a known
manner, for example by ~;xing the active compounds with
extenders, that is liquid solvents and/or solid carriers,
optionally with the use of surface-active agents, that is
emulsifying agents and/or dispersing agents and/or foam-
forming agents.
In the case of the use of water as an exten~er,
organic solvents can, for example, alsG be used as
auxiliary solvents. As liquid solvents,there are suitable
in the main: aromatics, such as xylene, toluene or alkyl-
naphthalenes, chlorinated aromatics and chlorinated ali-
phatic hydrocarbons, such as chlorobenzenes, chloroethy-
lenes or methylene chloride, aliphatic hydrocarbons, suchas cyclohexAne or paraffins, for example petroleum
fractions, mineral and vegetable oils, alcohols, such as
butanol or glycol as well as their ethers and esters,
ketones, such as acetone, methyl ethyl ketone, methyl
isobutyl ketone or cyclohexanone,strongly polar solvents,
Le A 26 538 - 44 -
2003208
such as dimethylformamide and dimethyl sulphoxide, as
well as water.
As solid carriers there are suitable: for example
ammonium salts and ground natural minerals, such as
S kaolins, clays, talc, chalk, quartz, attapulgite, montmo-
rillonite or diatomaceous earth, and ground synthetic
minerals, such as highly disperse silica, alumina and
silicates, as solid carriers for granules there are
suitable: for example crushed and fractionated natural
rocks such as calcite, marble, pumice, sepiolite and
dolomite, as well as synthetic granules of inorganic and
organic meals, and granules of organic material such as
sawdust, coconut shells, maize cobs and tobacco stalks;
as emulsifying and/or foam-forming agents there are
suitable: for example non-ionic and anionic emulsifiers,
such as polyoxyethylene fatty acid esters,polyoxyethylene
fatty alcohol ethers, for example alkylaryl polyglycol
ethers, alkylsulphonates, alkyl sulphates, arylsulphonat-
es as well as albumen hydrolysis products; as dispersing
agents there are suitable: for example lignin-sulphite
waste liquors and methylcellulose.
Adhesives such as carboxymethylcellulose and
natural and synthetic polymers in the form of powders,
granules or latexes, such as gum arabic, polyvinyl
alcohol and polyvinyl aGe~ate,as well as natural phospho-
lipids, such as cephalins and lecithins, and synthetic
phospholipids, can be used in the formulations. Further
additives can be mineral and vegetable oi~ls.
It is possible to use colorants such as inorganic
pigments, for example iron oxide, titanium oxide and
Le A 26 538 - 45 -
2003208
- - Prussian Blue, and organic dyestuffs, such as alizarin
dyestuffs, azo dyestuffs and metal phthalocyanine dye-
stuffs, and trace nutrients such as salts of iron, man-
ganese, boron, copper, cobalt, molybdenum and zinc.
The formulations in general contain between 0.1
and 95 per cent by weight of active compound, preferably
between 0.5 and 90%.
For controlling weeds, the active compounds
according to the invention, as such or in the form of
their formulations, can also be used as mixtures with
known herbicides, finished formulations or tank mixes
being possible.
Possible components for the mixtures are known
herbicides, such as, for example, 1-amino-6-ethylthio-3-
(2,2-dimethylpropyl)-1,3,5-triazine-2,4(lH,3H)-dione
(Ahh~YvIONE) or N-(2-benzothiazolyl)-N,N'-dimethyl-urea
(ME~AR~N~THIAZURON) for combating weeds in cereals; 4-
amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one
(METAMITRON) for combating weeds in sugar beet and 4-
2Q amino-6-(1,1-dimethylethyl)-3-methylthio-1,2,4-triazin-
5(4H)-one (METRIBUZIN) for combating weeds in soya beans.
Other possible mixtures are those with 2,4-dichloro-
phenoxyacetic acid (2,4-D); 4-(2,4-dichlorophenoxy)-
butyric acid (2,4-DB); 2,4-dichlorophenoxypropionic acid
(2,4-DP); N-(methoxymethyl)-2~6-diethyl-chloroacet~nilide
~C~T.OR); 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-
triazine (ATRAZINE); methyl 5-(2,4-dichlorophenoxy)-2-
nitrobenzoate (BIFENOX); 3,5-dibromo-4-hydroxy-benzo-
nitrile (BROMOXYNIL); 2-chloro-N-{~(4-methoxy-6-methyl-
1,3,5-triazin-2-yl)-amino]-carbonyl}-benzenesulphonamide
Le A 26 538 - 46 -
,
2003208
- (CHLORSULFURON); N,N-dimethyl-N'-(3-chloro-4-methyl-
phenyl)-urea (CHLORTOLURON); 2-chloro-4-ethylamino-6-(3-
cyanopropylamino)-1,3,5-triazine (CYANAZINE); 2-[4-(2,4-
dichlorophenoxy)-phenoxy]-propionic acid, its methyl
ester or its ethyl ester (DICLOFOP); 3,6-dichloro-2-
pyridinecarboxylic acid (CLOPYRALID); S-ethyl N,N-di-n-
propyl-thiocarbamate (EPTAME); 4-amino-6-t-butyl-3-
ethylthio-1,2,4-triazin-5(4H)-one (ETHIOZIN); 2-{4-[(6-
chloro-2-benzoxazolyl)-oxy]-phenoxy}-propanoic acid, its
methyl ester or its ethyl ester (FENOXAPROP); [(4-amino-
3,5-dichloro-6-fluoro-2-pyridinyl)-oxy]-acetic acid or
its l-methylheptyl ester (FLUROXYPYR); methyl 2-[4,5-
dihydro-4-methyl-4-(1-methylethyl)-5-oxo-lH-imidazol-2-
yl]-4(5)-methylbenzoate ( TMA~AM~T~AR~NZ ); 3,5-diiodo-4-
hydroxybenzonitrile (IOXYNIL); N,N-dimethyl-N'-(4-iso-
propylphenyl)-urea (ISOPROTURON); (2-methyl-4-chloro-
phenoxy)-acetic acid (MCPA); (4-chloro-2-methylphenoxy)-
propionic acid (MCPP); N-methyl-2-(1,3-benzothiazol-2-
yloxy)-acetanilide (MEFENACET); 2-ethyl-6-methyl-N-(1-
methyl 2-methoxyethyl)-chloroacetanilide (METor~A~FToR)~
2-{[((4-methoxy-6-methyl-1,3,5-triazin-2-yl)-amino)-
carbonyl]-amino]-sulphonyl}-benzoic acid or its methyl
ester (METSULFURON); 2-chloro-4-trifluoromethylphenyl 3-
ethoxy-4-nitro-phenyl ether (OXYFLUORFEN); N-(1-ethyl-
propyl)-3,4-dimethyl-2,6-dinitroaniline (PENDIMETHALIN);
2-chloro-N-isopropylacetanilide (PROPACHLOR); 0-(6-
chloro-3-phenyl-pyridazin-4-yl) S-octyl thiocarbonate
(PYRIDATE); 2-chloro-4,6-bis-(ethylamino)-1,3,5-triazine
(SIMAZINE); 4-ethylamino-2-t-butylamino-6-methylthio-s-
triazine (TER~UlK~N~); methyl 3-[[[[(4-methoxy-6-methyl-
Le A 26 538 - 47 -
Z003~0~3
-1,3,5-triazin-2-yl)-amino]-carbonyl]-amino]-sulphonyl]-
thiophene-2-carboxylate (THIAMETURON); S-(2,3,3-tri-
chloroallyl) N,N-diisopropylthiolcarbamate (TRI-ALLATE)
and 2,6-dinitro-4-trifluoromethyl-N,N-dipropylaniline
(TRIFLURALIN). Surprisingly, some mixtures also show a
synergistic action.
Mixtures with other known active compounds, such
as fungicides, insecticides, acaricides, nematicides,
bird repellants, plant nutrients and agents which improve
soil structure, are also possible.
The active compounds can be used as such, in the
form of their formulations or in the use forms prepared
therefrom by further dilution, such as ready-to-use
solutions, suspensions, emulsions, powders, pastes and
granules. They are used in the customary manner, for
example by watering, spraying, atomizing or scattering.
The active compounds according to the invention
can be applied either before or after emergence of the
plants.
20They can also be incorporated into the ~oil
before sowing.
The amount of active compound used can varywithin
a substantial range. It depends essentially on the nature
of the desired effect. In general, the amounts used are
25between 0.01 and 10 kg of active compound per hectare of
soil surface, preferably between 0.05 and 5 kg per ha.
The preparation and use of the active compounds
according to the invention can be seen from the following
examples.
Le A 26 538 - 48 -
200;~2~)~
Preparation Examples
Example 1
(CH3)2C ~ N-NH2
N`N~:O
O=C-NI~
(Process (b))
6.1 g (0.055 mol) of cyclopentyl isocyanate and a small
amount of diazabicycloundecene (DBU) are added to 7.1 g
(0.05 mol) of 4-amino-3-isopropyl-(lH)-1,2,4-triazolin-
5-one in 100 ml of absolute acetonitrile, the mixture is
stirred for 12 hours at room temperature and then concen-
trated in vacuo, the residue is taken up in dichloro-
methane, the solution is washed with water to neutrality,dried over magnesium sulphate and concentrated, and the
residue is crystallized by trituration with diethyl
ether.
10.0 g (78 % of theory) of 4-amino-1-(N-cyclo-
pentylcarbamoyl)-3-isopropyl-1,2,4-triazolin-5-one of
melting point 109C are obtained.
Le A 26 538 - 49 -
" .,. ,., . ., .. , :
Z003208
Example 2
H2C`
H2C~ H~
N`N~
¦ CH3
~C~ I
~H-f-C--CH
CH3
(Process (a) - one-pot variant)
5.5 g (0.05 mol) of 3-methyl-3-butinyl isocyanate and a
small amount of diazabicycloundecene (DBU) are added to
11.1 g (0.05 mol) of 4-(4-methylpent-2-ylideneimino)-3-
cyclopropyl-1,2,4-(lH)-triazolin-5-one in 150 ml of
absolute acetonitrile, the mixture is stirred for 12
hours at 20C and subsequently concentrated in vacuo, the
residue is taken up in aqueous ethanol, 2 ml of concen-
trated hydrochloric acid are added, the mixture is slowly
concentrated at 60C under reduced pressure, the residue
is taken up in dichloromethane, the solution is washed
using saturated sodium hydrogen carbonate solution, dried
over magnesium sulphate and concentrated, and the residue
is crystallized by trituration with diethyl ether.
10.4 g (84 % of theory) of 4-amino-3-cyclopropyl-
l-[N-(3-methyl-3-butinyl)-carbamoyl]-1,2,4-triazolin-5-
one of melting point 160C are obtained.
The following substituted triazolinones of the
general formula (I) are obtAine~ in a corresponding
manner and following the general preparation
instructions:
Le A 26 538 - 50 -
. ~ 2003208
- , Rl~N'NH2
1 ~ 0 (I)
0~ NH-R2
Ex. ~1 R2 Physica~
No. constant
3 (CH3)3c -(CH2~5-CH3 n20: 1,4980
CIH3
CH3-(CH2)2 -C-C-CH m.p 106 C
CH3
fH3
(CH3)2CH- -C-C--CH m.p. 90 C
CH3
6 (CH3)2CH ~ I m.p. 133 C
~2
~CH2
CH3-(CH2)2 ~CH2 m.p. 98 C
8 (CH3)3C ~ICH2 m.p. 158 C
~CH2
9 CH3-(CH2)2 {~3 m.p. 100 C
(CH3)2CH ~ nD: 1,5168
Le A 26 538 - 51 -
r . - .
2003208
- ~ Ex. R1 RZ Physical
No. constant
fH3
11 CH3-(CH2)2 -C-CH2Cl nD: 1,5890
CH3
12 (CH3)2CH- IH3 n20: 1.5650
CH3
1H-NMR*):
13 (CH3)3C- ~ 4 48 (2H)
1H-NMR*):
14 (CH3)3C- ~ 1,44 (9H),
(CH3)3C- -C(CH3)3 m.p. 142 C
16 (CH3)3C- {~Cl m.p. 203 C
17 CH3-(CH2)2 -C~CH3~? m.p. 112 C
18 (CH3)2CH- -C(CH~)3 m.p. 108 C
19 ~ -CtCH3)3 m.p. 151 C
H2C CH3
(CH3)3C- -CH ~ n20: 1~5171
(S-) config,
CH3
21 (CH3)3C- -CH ~ n20: 1,5411
(R~ ) conf i 9 .
Le A 26 538 - 52 -
2003~08
Ex. Rl R2 Physical
No. constant
fH3
22 (CH3)3C- -f-CH2-Cl m.p. 133 C
CH3
CH3
23 (CH3~3c- -IC-C--CH m.p. 148 C
CH3
fH3
24 (CH3)3C- f (CH2)3 CH3 n20: 1,4844
CH3
(CH3)3C- -fH-CH(CH3)2 m . P 135 C
CH3
ICH3
26 (CH3)3C- -C-CH2F m . p . 141 C
CH3
27 (CH3)3C- -CH2 ~ l m.p- 208 C
CH3
28 (CH3)3C- f ~ nD: 1,5520
CH3
29 (CH3)3C- (CH2)2 ~ 1 m.p. 172 C
(CH3)2CH -fH-C2H5 m.p. 83 C
CH3
Le A 26 538 - 53 -
2003208
Ex. Rl R2 Physical
No. constant
CH3
3~ (CH3)2CH -f-C2H5 n20 1,4801
CH3
32 H2f` ~ m.p. 177 C
H2
H2C
33 ¦ CH- n m.p. 107 C
H2C~
fH3
34 ~CH3~2CH -c-cH2F m.p. 124 C
CH3
CH2Cl lH-NMR*):
¦ 1,34 (6H),
20 35 (CH )2CH- -C-CH3 3,12 (lH),
3 1 . 4,40 (2H)
CH2C 1
CH3
(CH3)2CH- -f-CHCl2 m.p. 121 C
CH3
37 (CH3)2CH -CH(CH3)2 m.p. 124 C
CH3
38 (CH3)2CH- -f (CH2)3 CH3 nD: 1~4874
CH3
CH3
39 (CH3)2CH -f-(CH2)3 CH3 nD: 1,4847
CH3
Le A 26 538 - 54 -
2003Z08
Ex. R1 R2 Physical
No. constant
(CH3)2CH- -cH-cH(cH3)2 nD: 1~4860
CH3
41 (CH3)2CH- -CH~3 nD: 1.5308
CH3 (S-) conf jg.
CH3
42 (CH3)2CH ¢{~ n20: 1,5355
CH3
43 (CH3)2CH- H3C~1 n20: 1,5011
H2C~ fH3
44 ¦ CH- -C-CH2Cl m.p. 94 C
H2C~
CH3
CH3
H2C~
H2C~ -C-CH2F m.p. 138 C
CH3
~--~ amorphous
46 (CH3)2CH- ~ H > ~ = 1.34 Duplett (2CH3)
~~ 3,13 Multiplett (lH)
Cl 3.90 Multiplett (2H)
4.48 Singulett (NH2)
8.04 Duplett (NH)
Le A 26 538
- 55 -
. .
2003Z08
Ex.Rl R2 Physical
No. constant
amorphous
H C r--~ S = 1.05 Multiplett ~2H,
21 ~ ~ 1.16 Multiplett (2H,
Cl cyclopropyl)
2,06 Multiplett (lH,
cyclopropyl)
3.89 Multiplett (2H)
4.55 Singulett (NH2)
8,03 Duplett (NH)
48(CH3)2CH -C4H9-n n~: 1,4969
49¦ ~CH -C4H9-n mp: 84C
The lH-NMR spectra were recorded in deuterochloroform
(CDCl3) with tetramethylsilane (TMS) as the internai
st~n~rd. The chemical shift is indicated as a
value in ppm.
Le A 26 538
- 56 -
~ . . ... . ... . . . .
ZOO;~Z08
- Preparation of the starting compounds
_____________________________________
Example III-l
H2C`
H2C~ ~ ~ N~2
N~N O
H
1892 g (8.84 mol) of diphenyl carbonate are added in
portions to 884 g (17.68 mol) of hydrazine hydrate with
stirring and ice-cooling, so that the temperature of the
reaction mixture does not exceed 30C. When the addition
is complete, the mixture is stirred for about 3 hours at
80C, any reaction water formed during this process is
removed in vacuo, 760 g (8.84 mol) of cyclopropane-
carboxylic acid are then added, the mixture is subse-
quently heated to 200C in the course of 6 hours and under
an atmosphere of inert gas, and any reaction water
liberated is simultaneously distilled off. When the
reaction is complete, the mixture is evaporated in vacuo
to dryness, the residue is extracted using 3000 ml of
boiling ethanol, the extract is filtered and cooled, and
the crystalline precipitate which has formed is filtered
off with suction and dried.
420 g (34 % of theory) of 4-amino-3-cyclopropyl-
1,2,4-(lH)-triazolin-5-one of melting point 181C are
obt~ine~.
The following are obt~ineA in a corresponding
manner:
Le A 26 538
- 57 -
2003208
Example III-2
fH3
C 2H5 - C~ IN- NH2
N~N~o
melting point 168C
Example III-3
(CH3~2C ~ _I-NH2
N`NI ~0
melting point 168C
5 Example III-4
CH3~
CH ~CH CH2~ N-NH2
N~N~o
H
ExamPle III-5
CH3-(CH2)3 ~ N-NH2
N~H~O
Le A 26 538 r
- 58 -
2003208
ExamPle (III-6)
(CH3)3C~ r NI-NH2
i I -
melting point 261C
Example VIII-1
H2C~ ~H ~CH~
H2C~ ~ ~CH2-CH(CH3)2
N` I O
142 g (1.0 mol) of 4-amino-3-cyclopropyl-1,2,4-
(lH)-triazolin-5-one in 1000 ml of methyl isobutyl ketone
with the addition of 100 mg of p-toluenesulphonic acid
are refluxed in a water sep~rator until the liberation of
further reaction water has ceased. For working up, the
mixture is concentrated in vacuo, and the residue is
crystallized by trituration with petroleum ether.
66 g (30 % of theory) of 4-(4-methylpent-2-
ylideneimino)-3-cyclopropyl-1,2,4-(lH)-triazolin-5-one of
melting point 64C are obtained.
Use Examples
In the use examples which follow, the compounds
listed below were employed as comparison substances:
Le A 26 538
- 59 -
,. . .. . .
- - 2003Z08
_ CH3S~N-NH2
N~NI ~ O (A)
o=c-NH-cH(cH3)2
4-amino-1-(N-isopropylcarbamoyl)-3-methylthio(lH,4H)-
1,2,4-triazolin-5-one
CH3S~I ~ NH2 (B)
N~7 o
O=C-NH-(CH2)2-cH3
4-amino-1-(N-propylcarbamoyl)-3-methylthio-(lH,4H)-1,2,4-
triazolin-5-one
CH35 ~ ~ NH2
N`IN o (C)
O=C-NH-(CH2)~-CH3
4-amino-1-(N-butylcarbamoyl)-3-methylthio-(lH,4H)-1,2,4-
triazolin-5-one and
CH35~r_lN- NH2
N~IN~o (D)
~C~ \
o NH ~
4-amino-1-(N-cyclohexylcarbamoyl)-3-methylthio-(lH,4H)-
1,2,4-triazolin-5-one;
all disclosed in JP 52/125,168.
Le A 26 538
- 60 -
2003208
- Example A
Pre-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active
compound, 1 part by weight of active compound is mixed
with the stated amount of solvent, the stated amount of
emulsifier is added and the concentrate is diluted with
water to the desired concentration.
Seeds of the test plants are sown in normal soil
and, after 24 hours, watered with the preparation of the
active compound. It is expedient to keep constant the
amount of water per unit area. The concentration of the
active compound in the preparation is of no importance,
only the amount of active compound applied per unit area
being decisive. After three weeks, the degree of damage
to the plants is rated in % damage in comparison to t~e
development of the untreated control. The figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, a clearly superior activity, combined with
a comparably good selectivity towards crop plants,
compared with the prior art, is shown in this test, for
example, by the compounds of the following preparation
examples: (1), (10), (18), (30), (31), (34), (37) and
(40).
Le A 26 538
- 61 -
- - 2003208
Example B
Post-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: 1 part by weight of alkylaryl polyglycol
ether
To produce a suitable preparation of active
compound, 1 part by weight of active compound is mixed
with the stated amount of solvent, the stated amount of
emulsifier is added and the concentrate is diluted with
water to the desired concentration.
Test plants which have a height of 5 - 15 cm are
sprayed with the preparation of the active compound in
such a way as to apply the particular amounts of active
compound desired per unit area. The concentration of the
spray liquor is chosen so that the particular amounts of
active compound desired are applied in 2,000 l of
water/ha. After three weeks, the degrea of ddmaga to the
plants is rated in % damage in comparison with the
development of the untreated control. The figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, a clearly superior astivity, combined with
a comparably good selectivity towards crop plants,
compared with the prior art, is shown in this test, for
example, by the compounds of the following preparation
examples: (12), (18), (19), (30), (34), (36), (37), (38),
(39) and (40).
Le A 26 538
- 62 -