Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-- 1 --
4-17468/+
Peptide lactone and derivatives thereof, a process for the preParation
thereof and means for carrYin~ out the process
The invention relates to a fermentation product obtained from Strepto-
myces griseoflavus, which has the structure of a peptide lactone, to a
fermentation process for the preparation thereof, to derivatives ob-
tainable from the peptite lactone by lactone cleavage and optionally
subsequent esterification with a lower alkanol, to the use of the peptide
lactone and the said derivatives as medicaments and for the stimulation
of aerial mycelium formation and antibiotic production in Streptomycetes,
to pharmaceutical preparations which contain the peptide lactone or one
of the said derivatives, to novel decomposition (cleavage) products and
to the novel producer strain.
Bacteria of the genus Streptomyces are characterised by a complex de- ,
velopment cycle in which an aerial mycelium forms from a substrate
mycelium on a solid nutrient substrste. In a sporulation process,
terminal aerial mycelium hyphae can then fragment into spore chains.
Experimental findings allow the supposition that the cell differentiation
into aerial mycelia and spores takes place during the secondary
metabolism, that is to say simultaneously with the synthesis of anti-
biotics, pigments, storage substances and geosmins.
Little has been known hitherto about the regulation of the secondary
metabolism and of differentiation in Streptomycetes, but both processes
appear to be controlled by two groups of signal substances which mediate
either in the cell or between the cells.
'
:. . : . .:: .:. . . : ,: . . ., ~ .
' :. ; ,, ,' . :,
- 2 - 2 0 ~ O m
Recently it has been shown in the case of Streptomycetes that both the
development of aerial mycelia and the synthesis of antibiotics are
stringently controlled~ The stringent control is initiated by two intra-
cellular effectors: guanosine tetraphosphate and guanosine pentaphos-
phate.
In addition to those effectors, which are generally widespread in
bacteria, intercellular, strain-specific signal molecules have been
found. In Actinomycetes they induce the formation of aerial mycelia
and/or antibiotics, such as, for example, the A factor (2-isooctanoyl-
3(~)-hydroxymethyl-r-butyrolactone) and the B factor (3'-(1-butylphos-
phoryl)-adenosine).
In the search for new intercellular signal substances there has now been
found with the aid of the agar diffusion test a strain which produces a
substance having the desired activity. The substance, in a low concentra-
tion, stimulates the formation of aerial mycelia and antibiotics in the
producer strain and in several other Streptomycetes strains and is there-
fore designated "hormaomycin" (hormao = "I set in motion" in Greek).
The invention relates to the substance hormaomycin which is obtainable by
fermentàtion with the strain Streptomyces griseoflavus DSM 5195 and
exhibits with respect to the strains Arthrobacter crystallopoietes
ATCC 15481 and Arthrobacter oxydans ATCC 14358 à minimum inhibiting
concentration of les~ than 0.01 ~g/ml, and indeed even less than
0.001 mg/ml.
.
~:
:::
. .. ~. . : . . : ~
_ 3 _ 2010~
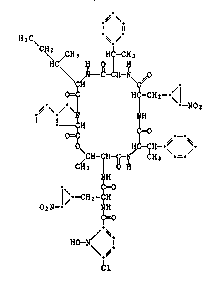
According to previous findings, hormaomycin probably has the structure of
formula I
~'\
T ~.~
H 3 C~
C~ ~_C-CH-~ ~0
H ~ ( I)
C~CH-- H--~ H \ --~-
~H
j\---CH2--
02N-- IIH
~=o
/ ~. - .
H0--h~
Cl
The invention relates especially to hormaomycin having the features
indicated in the Examples section inso$ar as these features can be
regarded as certain, a selection of these features being sufficient
for clear characterisation, that is to say, for example, hormaomycin
having, within measuring accuracy, at least one or preferably all of the
following physical characteristics: a) melting point 166-168C,
b) optical rotation [~]D ~ +20.8 (c - 0.5; methanol), c) molecular
weight 1129+2, d) in CDCl3 as solvent ~H-NMR signals having a chemical
shift ~ with respect to tetramethylsilane as internal standard of from
+0.5 to -0.7 ppm, especially signals for one proton each at 0.48, 0.29,
0.15 and -0.63 ppm.
It is also especially characteristic of hormaomycin that it has two
radicals of the amino acid 2-amino-3-(2-nitrocyclopropyl)-propionic acid,
which is also referred to as 3-(2-nitrocyclopropyl)-alanine (abbreviated
to NCP-Ala) below, it being possible for these two radicals to differ
from one another as regards the configuration at the asymmetric centres.
~ :
-: . . . ........................... ; .
. , , . ;
_ 4 _ Z0107~7
The following text and the Examples section describe the findings from
which the structure of formula I can be deduced:
The optically active hormaomycin exhibits typical UV maxima at 278 and
206 nm (methanol) which shift only slightly with changes in pH. In the IR
spectrum, bands for CO-lactone (1741 cm 1) and CO-amide (around 1640 and
1545 cm 1) can be observed. The molar mass of 1128.4 is inferred from the
~A~ mass spectrum. According to elemental analysis, hormaomycin contains
a chlorine atom in addition to C, H, N and O, corresponding to the
empirical formula CssH69Nlool4cl.
The 1H-NMR spectrum in CDCl3 exhibits six clearly distinguisbable NH
groups to some extent slowly interchanging with CD30D, and an OH group at
11.1 ppm. Ten closely adjacent aromatic H and an AB system
(6.75/6.08 ppm, J = 4.5) can be identified. In the range 2 to 5.5 ppm
there are numerous lH signals well separated from one another. A cluster
of slgnals occurs between 1-2 ppm, it being possible to identify five
doublets and one triplet for methyl groups. Remarkable and typical of
hormaomycin are two lH multiplets at 0.48 and 0.29 ppm and a further two
beyond tetramethylsilane (TMS) at -0.15 ant -0.63 ppm.
In the 13C-NMR spectrum it is possible to identify a total of 55 carbonatoms of the following type: 6 CH3, 7 CHz, 16 sp3-CH, 14 sp2-CH, 4 sp2-C
and 8 CO. In the region of the sp3-C atoms two methyl groups (13.4 ppm)
occur superimposed, and in the region of the sp2-CH four signals (127.4,
127.7, 128.5, 128.6 ppm) and in the region of the CO groups one CO group
(168.7 ppm) have to be counted twice. The spectrum allows the supposition
that hormaomycin is a peptide containing two aromatic and other un-
saturated amino acids.
Amino acid buildin~ blocks
The a-ino acids present in hormaomycin are deduced by identification of
the components obtained on hydrolysis and by allocation in the intact
antibiotic with the aid of lH/lH- and lHIl3C-CoSY-NMR spectra.
. , . ., , : :,. ,. .~ . .
. . : .. ,, '. ': : ,
_ 5 ~ 2010~
In the total hydrolysate of hormaomycin (6M HCl/acetic acid, 1:1, 110C,
48 hours) threonine (Thr) and isoleucine (Ile) can be detected using
automatic amino acid analysis and by microdansylation [H. Laatsch, J.
Chromatogr. 173 (1979) 398]. Unusual, neutral amino acids are observed in
the region of proline, valine (two bands) and phenylalanine (band having
double intensity). For the purpose of testing in GC-MS coupling (gas
chromatograph connected to a mass spectrometer), the amino acids are
converted into the trifluoroacetylated methyl esters or isopropyl esters,
or trimethylsilyl derivatives are produced. From the mass spectra of the
substances having the smallest retention time it is possible to allocate
a-Thr and Ile. Purthermore, the amino acid occurring twice can be allo-
cated as ~-methyl-phenylalanine (~-Me-Phe) by the characteristic frag-
ments at m/z 230 (M -COOCH3) and 105 ([C6H5CHCH3] ) in the EI-MS (elec-
tron impact ionisation mass spectrum) of the trifluoroacetylated methyl
ester and by the molecular ion at m/z 318 ([M ~ H] ) in the CI-MS (chemi-
cal ionisation mass spectrum) of the trifluoroacetylated isopropyl ester.
The data agree with that for synthetic ~-Me-Phe [Y. Kataoka et al., Bull.
Chem. Soc. Jap. 49 (1976) 1081]. For the previously mentioned amino
acids, starting from the methyl groups or from the NH and ~-CH groupsj
the associated coupling partners in the 1H/1H-COSY spectrum can be dis-
covered and linked by means of the 1H/13C-CoSY spectrum with the asso-
ciated C atoms. The signals of the two ~-Ms-Phe building blocks differ
markedly in some cases. It is alsc striking that the H/H~-coupling in
one instance is J ~ 4.5 Hz as in the free amino acid and in another is
J ~ 10.5 Hz, which points to different conformations of the side chains.
One of three new amino acids is identified as (Z)-4-propenyl-proline
(4-P~-Pro). An indication of the molar mass or the proline structure is
provided by the ion at m/z 265 (M~) of the trifluoroacetylated methyl
ester in conjunction with the ions at m/z 206 (M~-COOCH3) and 164. The
structure of the side chaln and its position on the proline structure is
obtained from the correlation spectra. H-C(4) and H2-C(5) (see Table 1)
overlap in CDC13 and cannot be allocated beyond all doubt in the
H/1H-COSY spectrum (3.21 and 3.21/3.91 ppm). In CDCl3/(CD3)2CO/C6D6
(1:1:1), however, it is possible to obtain an accurate allocation (2.98
and 3.07/3.85 ppm). The olefinic AB system (~H 5.20/5.58; ~C 127.5/128.2
in CDCl3) has an AB coupling of J ~ 10.5 Hz (Z configuration) and on one
~, . . .. . . . .
~, ~ . . . .
;' .' ~ : ~ '- ~'
.
.
.
~010777
-- 6 --
side is further split by an olefinic methyl group (~H 1.61) and on the
other side by a CH group. Starting from ~-CH (4.20 ppm), via a CH2 group
(1.72/2.30 ppm) and a CH group (3.21 ppm) a further CH2 group
(3.21l3.91 ppm) is reachQd. The latter corresponds in its ~C value
(52.8 ppm) to the C(5) of proline, which demonstrates the 4-position of
the propenyl radical on ths proline structure. In automatic amino acid
analysis, the proportion of 4-PE-Pro is always clearly less than 1. In
the GC-MS analysis, in addition to the N-trifluoroacetyl-4-propenyl-
proline methyl ester it is also possible to detect corresponding 4-(1- or
2-trifluoroacetoxypropyl) or 4-(1- or or 2-chloropropyl) derivatives with
the aid of the mass spectra. This is explained by the supposition that in-
the hydrolysis of hormaomycin, 4-PE-Pro adds water or HCl to the double
bond.
In GC-MS coupling, the two as yet unallocated amino acids of the total
hydrolysate give, as trifluoroacetylated methyl esters, identical EI mass
spectra which cannot be allocated to any known compound. In order to be
able to investigate these amino acids more closely, the total hydrolysate
of 10 mg of hormaomycin is subjected to HPLC separation on a ~-Bondapak-
NH2 column (silica gel charged with NH2 groups) in the system aceto-
nitrile/water (7:3), The separation is not complete but in one fraction,
according to automatic amino acid analysis, the unknown amino acids and
isoleucine (Ile) are present in approximately equal amounts. After deri-
vatisation the former again produce identical EI mass spectra, and in the
CI-MS yield the molecular ion at m/z 285 [M + H] The even-numbered molar
mass indicates two N atoms in the molecule, which is demonstrated by high
resolution of the fragment ions in the EI-MS and of the molecular ion of
the trimethylsilyl derivatives and gives the empirical formula C6H1oN
for the free amino acids. The trifluoroacetylated methyl ester loses NO2
(mlz 238) or COOCH3 (mlz 225) first and exhibits further fragment ions
typical of a nitroamino acid. In the search for the associated 1H-NMR
signals in the correlation spectra of hormaomycin, starting from the
NHI~-CH groups, two identical correlation series are found of which one
can be clearly distinguished and the other, because of overlapping
signals, is revealed only by combination of 1H/1H-COSY and 1H/13C-CoSY
spectra at 400 MHz (Table 1). MS and NMR data demonstrate the presence of
3-(2-nitrocyclopropyl)-alanine (NCP-Ala) which is present twice in
.... , ~ . . : , . ,
-: . . '
: . ~ ' '` , : :
:: . . . ` ,, :' . :
: ~ ` . ,
: ' :; ' ` '
20~07~7
-- 7 --
hormaomycin. While the corresponding 1H-NMR signals of the two building
blocks in the intact peptide are clearly different (~ = 0.82 to
1.67 ppm), the comparable 13C-NMR signals are very similar (~ < 2 ppm,
Table 1). The high field-shifted 1H-NMR signals typical of hormaomycin
belong to a NCP-Ala. The phenyl radicals of the ~-Me-Phe building blocks
could be responsible for the shielding.
Having the same constitution, the separability of the free amino acid in
automatic amino acid analysis and, after derivatisation, in GC-MS
analysis can be accounted for only by stereochemistry. H-C(5) (2.90 and
3.99 ppm) is not masked in the two building bloc~s and exhibits an
identical coupling pattern (ddd, 5 lines) from which the coupling
constants (J = 6.6, 3.3 and 3.3) can be derived. It follows from the
magnitude of the coupling constants of H atoms on the cyclopropane ring
[H. Fritschi et al., Helv. Chim. Acta 71 (1988) 1553] that on H-C(5)
there is only a cis-coupling to one of the two protons at C-(6), and the
nitro group and the alanyl radical are thus in the trans-configuration
relative to one another on the cyclopropane ring. The H-C(5) coupling
pattern recurs in the same manner in the 1H-NMR spectrum of the free,
HPLC-enriched amino acid NCP-Ala on the signal at 4.40 ppm, which does
not occur twice despite the presence of stereoisomers. Presumably the two
amino acids differ in the configuration at the -C atom.
If the amino acids detected in the total hydrolysate of hormaomycin arecombined with condensation, then on the peptide chain an amino end, a
carboxy end and the a-threonine-OH group (allo-threonine-OH group) are
free, and a CsN radical, which must contain the detected Cl atom in
addition to H and O atoms, is still missing. The amino end of the peptide
chain is acylated, as only then is a total of six amide NH groups
obtained in the lH-NMR spectrum. Accordingly, end group analyses with
2,4-dinitrofluorobenzsne and dansyl chloride sre negative. The
a-threonine-OH group is esterified, as is detectable from the low-field
position of H-C(3) (5.35 ppm). The fact that the C5N radical is bonded
not as an ester but amidically to the peptide chain can be inferred from
the lactone cleavage of hormaomycin with methanol/K2CO3 [W.A. Ayer and
L.M. Pena-Rodriguez, J. Nat. Prod. 50 (1987) 400]. With the absorption of
methanol there is formed a hormaomycin methyl ester having the molar
.: .. , :
: .
,
20~0777
-- 8 --
mass 1160 (Fast atom bombardment mass spectrum, FA~-MS): 1161 [M + H] ,
1183 [M + Na] , in the 1H-NMR spectrum of which H-C(3) of the threonine
is shifted to ~H < 5 ppm and an ester-OCH3 appears at 3.74 ppm. The as
yet unallocated part of the molecule therefore consists of a CO group (~C
159.3 ppm) and a radical C4H3NOCl. The C atoms are sp2-hybridised with ~C
values of from 103.5 to 121.4 ppm. Two of the H atoms form the AB system
at ~H 6.08/6.75 ppm, the third is at 11.10 (Table 1). These data can
plausibly be combined to produce amidically bonded 5-chloro-N-hydroxy-
pyrrole-2-carboxylic acid (CHPCA), which is unknown in the literature and
which could not hitherto be detected in the hydrolysates of hormaomycin.
The NMR data (Table 1) agree satisfactorily with those for pyrrole-2-
carboxylic acids substituted in the 5-position and with those for
comparable radicals in the antibiotics chromoxymycin [Y. Kawai et al.,
Tetrahedron Lett.-26 (1985) 3273] and 55185 RP [M. Dechamps et al.,
European Patent Application 246 975].
In hormaomycin, the amino acids detected and the postulated pyrrole-2-
carboxylic acid form, by means of amide bonds, a linear peptide chain of
which the carboxy end closes with the a-threonine-OH group to form a
lactone ring. The lactone ring carries an amidically bonded side chain on
a-threonine.
,
The postulated sequence of amino acid building blocks in the lactone ring
is teduced from the following experiments: ~
As described above, a-threonine participates with its hydroxy function in
the lactone bond, which can be seen from lH-NMR data.
It should be possible to determine the amino acid that provides its
carboxy function for the lactone bond by reduction of the ester function
to an alcohol. In the reaction of hormaomycin with LiAlH4 in tetrahydro-
furan at 0C, after total hydrolysis of the reduction product and sub-
sequent derivatisation to the trifluoroacetylated methyl esters, the
corresponding 4-propenyl-prolinol derivative can be detected in GC-MS
analysis.
:
, . . . . .. ~, ,, : . , :. ~ ...
-~:, ~ ; . - : . . ,
.'',' '';' ' ' ' ' '`.: ' '` .''`~' . .' - :': ' :
. .
20~07~7
g
N,O-bis-trifluoroacetyl-4-propenyl-prolinol: EI-MS: m/z - 333 (10 %, M ),
206 (100 %, M - [CH2-0C0CF~]), 164 tlO %, M - [CH2-OC0CF3 +
CH=CH-CH 3 + H]).
By opening the lactone ring of hormaomycin and forming the methyl ester
there is obtained the compound of formula II, designated hormaomycin
methyl ester.
c~ C-NCP-Ala-a-Thr-B-Me-Phe-NCP-Ala-B-Me-Phe-Ile-4-PE-Pro-OCH 3
~ (II)
The invention relates also to the substances obtainable from hormaomycin
by lactone cleavage and esterification with a lower alkanol, especially
hormaomycln methyl ester.
A comparative analysis of the FAB mass spectra of hormaomycin and the
open-chain methyl ester of formula II yields the following data:
Pos. FAB-MS of hormaomYcin: m/z = 1129.4/1131.5 (11 %, [M+H] ), 938 (1 %,
[M+H] -(CHPCA + HNO2)), 830 (2 %, [M+2H] - (NCP-Ala-CHPCA)), 700/702
(2 %, [M+2H] -(NCP-Ala-~-Me-Phe-Ile)), 538/540 (1 %, [M+H] -(B-Me-
Phe-NCP-Ala-~-Me-Phe-Ile)), 516/518 (2 %, [M+H] -tNCP-Ala-~-Me-
Phe-Ile-4-PE-Pro + N02)), 401/403 (2 %, [M+H] -(~-Me-Phe-NCP-Ala-~-Me-
Phe-Ile-4-PE-Pro)), 300/302 (2 %, NCP-Ala-CHPCA), 144/146 (22 %, CHPCA,
134 (100 %)).
~ ,
POS. FAB-MS of the compound of formula II: m/z s 1183/1185 (3.5 ~,
[M+Na] ), 1161/1163 (2 %, [M+H] ), 992/994 (3.6 %, [M+H~
(4-PE-Pro-OMe + H)), 879/881 (1 %,[M+H] -(Ile-4-PE-Pro-OMe + H)),
718/720 (3 %, [M+H3 - (~-Me-Phe-Ile-4-PE-Pro-OMe + H)), 562/564 (0.9 %,
[M+H] -(NCP-Ala-3-Me-Phe-Ile-4-PE-Pro-OMe + H)), 534t536 (0.7 %, ~M+H]
- (OC-NCP-Ala-~-Me-Phe-Ile-4-PE-Pro-Ome + H)), 419 (3 %, a-Thr-~-Me-
Phe-NCP-Ala + H), 401/403 (0.9 %, a-Thr-~-Me-Phe-NCP-Ala + H).
Mass numbers of the smino acid building blocks CO-CHR-NH:
a-Thr: 100 or 101 4-PE-Pro: 137 Ile: 113 NCP-Ala: 156 ~-Me-Phe: 161
CHPCA: 144
:, ., . , . :
. -: :.
Z0~07~
-- 10 -
The FAB-MS of the compound of formula II also shows the splitting off of
the 4-PE-Pro-OCH3 radical from the carboxy end of the open-chain peptide,
which confirms its participation in the lactone bond in hormaomycin.
In the case of partial hydrolysis of hormaomycin (12N HCl/acetic acid
1:1, room temperature, 48 hours), after derivatisation to the trifluoro-
acetylated methyl esters, two dipeptides and one tripeptide are dis-
covered in GC-MS analysis.
- N-trifluoroacetyl-~-methyl-phenylalanine-isoleucine methyl ester:
EI-MS: m/z = 402 (5 %, M ), 343 (2 %, M -COOCH3), 230 (5 %,
M -(OC-Ile-OCH3)), 105 (100 %, C6Hs-CH-CH3).
- N,O-bis-trifluoroacetyl-allo-threonine-~-methYl-phenylalanine-methyl
ester: EI-MS: m/z = 427 (0.3 %, M -COOCH3), 266 (0.2 %, M -(OC-~-Me-Phe-
OCH3)), 192 (1.5 %, B-Me-Phe-OCH3), 176 (16 %, C6Hs-C(CHj)=CH-COOCH3),
134 (9 %, NH2-CH-CH(CH3)-C6Hs), 105 (100 %, C6Hs-CH-CH3).
- N-trifluoroacetYl-C~-methyl-phenylalanine-isoleucine-4-propenyl-proline .
methyl ester: EI-MS: m/z ~ 539 (1 %, M ), 483 (3 %, M
- (C(CH3)=CH(CH3)), 343 (5 %, M - (OC-4-PE-Pro-OCH3)), 230 (1 %, M
-(OC-Ile-4-PE-Pro-OCH3), 105 (96 %, C6Hs-CH-CH3).
These results confirm parts of the sequence analysis from the FAB-MS
spectra.
The stereochemistry of the amino acids and the conformation of the
lactone ring is still unresolved to some extent.
In vitro in an agar dilution series test, hormaomycin exhibits an anti-
microbial action against a number of bacteria, for example Arthrobacter
crystallopoietes, Arthrobacter oxydans, Corynebacterium species, Brevi- -
bacterium linens and Salmonella typhimurium. The minimum inhibiting con-
centration is in the range of from 0.0001 to 50 ~g/ml. The following Table
gives some specific examples:
.
: :. - . -, ... .;. ,.-. . ~ ~
' . .
- 11 - 210777
Strain minimum inhibiting concentration (~g/ml)
Arthrobacter crystallopoietes ATCC 15481* 0.0001
Arthrobacter oxydans ATCC 1~358 0.0005
Corynebacterium sp. ATCC 23830 0.1
Brevibacterium linens ATCC 9172 10
Salmonella typhimurium ATCC 13311 50
*ATCC: American Type Culture Collection, Rockville, MD, USA
Hormaomycin can therefore be used as an anti-microbial agent. When usedas an antibiotic in the case of an infection by Salmonella typhimurium,
the daily dose to be administered to a warm-blooded animal of about 70 kg
body weight is approximately 0.5 to 5 g, preferably 1 to 2 g.
In the case of some Streptomycetes strains, hormaomycin brings about anincrease in the formation of antibiotics in a concentration as low as
approximately 0.05 ~g/ml and above. For example, the addition of
hormaomycin in a concentration of 0.05 ~g/ml to a fermentation broth
containing Streptomyces griseoflavus (Tu 1306) brings about a doubling of
the production of the antibiotic tirandamycin, while a concentration of
1 ~g/ml brings about approximately a four-fold increase in production.
Hormaomycin can therefore also be used for increasing the yield of
valuable fermentation products.
Hormaomycin induces the formation of aerial mycelia of Streptomyces
griseoflavus (DSM 5195) and of other Streptomyces strains. If these
strains are used as indicator bacteria for test plates and if there is
used, in addition, a nutrient agar on which aerial mycelia (identifiable
by the white colouring) do not develop, then 1 ~g of hormaomycin applied
in a paper disc to the surface of the agar is able to initiate copious
formation of aerial mycelia around the disc. In addition, the substance
induces the production of antibiotics in these strains.
The method according to the invention for the preparation of hormaomycin
is as follows: the strain Streptomyces griseoflavus DSM 5195 or a culture
derivable from that strain is cultured in a suitable nutrient`medium, and
hormaomycin is isolated from the culture broth.
', ' '` . ~
- 12 - 201~
The above process is explained in more detail below: hormaomycin is
obtained with the aid of a novel microbiological process, namely by
fermentation with the novel strain Streptomyces griseoflavus DSM 5195,
which was deposited on number DSM 5195 at the DSM Deutsche Sammlung von
Mikroorganismen und Zellkulturen, Mascheroder Weg 1~, D-3300 ~raun-
schweig, Federal Republic of Germany, on 31st January, 1989. - -
The producer strain of hormaomycin DSM 5195 was isolated from an earth
sample collected at Anuradhapura (Sri Lanka). It belongs to the genus
Streptomyces, conforms in all type-determining features to the type
strain of Streptomyces griseoflavus and is therefore to be assigned to
that type.
Strain DSM 5195 is characterised by the following features (nomenclature
and taxonomy according to R. Hutter, "Systematik der Streptomyceten",
Verlag Karger, Basle 1967): The spores are spiky. The aerial mycelium is
initially pale white and becomes ash-grey (cinereous) in the ripened ~ `
state. The aerial mycelium is monopodially branched and has side limbs
having more or less regular helices of generally 4 to 6 windings (spira-
type B). On peptone/iron/agar the strain does not form melanin. The
strain utilises the following sugars or alcohols as a single C source:
D-fructose, D-glucose, myo-inositol, D-mannose, L-rhamnose and especially
well D-xylose. No growth is observed on L-arabinose, raffinose or
saccharose.
A derivable culture in the above sense is any culture which still has the
features of the deposited culture that are essential for carrying out the
process of the invention, that is to say chiefly a derived culture, es-
pecislly a culture of which the microorganisms contain the same struc-
tural genes as the structural genes of strain DSM 5195 that are causative
of the formation of hormaomycin. A derivable culture is also any culture
of which the microorganisms contain an equivalent, possible because of
the degeneracy of the genetic code, of the structural genes of strain
DSM 5195 that are causative of the formation of hormaomycin. A derivable
culture is especially any culture which contains microorganisms of the
genus Streptomyces, preferably the species Streptomyces griseoflavus,
' ' ' ' ' ' : ., , '' ', '. . . . .
- 13 - 2010~
that are capable of producing hormaoMycin. The term "derivable culture of
strain DSM 5195" also includes all mutants of that strain that are
capable of producing hormaomycin.
The nutrient medium used is a solution or suspension, preferably an
aqueous solution or suspension, that contains at least one carbon source
and at least one nitrogen source, and preferably also minerals. Examples
of suitable carbon sources are: glycerine, assimilable carbohydrates,
such as cyclitols, for example mannitol, polysaccharides, for example
starch, disaccharides, for example lactose and saccharose, and mono-
saccharides, especially glucose and fructose, and also corresponding
carbohydrate-containing industrial raw materials, such as sugar beet or
sugar cane molasses. Suitable nitrogen sources are: amino acids, es-
pecially the naturally occurring ~-amino acids, peptides and proteins and
the decomposition products thereof, such as peptones and tryptones, and
also ammonium salts and nitrates, and corresponding industrial nitrogen-
containing raw materials, such as meat extracts, casein hydrolysate and
yeast autolysate and yeast extract. Also suitable are mixed industrial C
and N sources, such as various plant seeds that are used in the form of
aqueous extracts, meal or mash, for example of beans, for example soy- -~
beans, cereal grains, for example wheat, and especially maize ("corn-
steep liquor"); also cotton seeds and malt extract. In addition to
ammonium salts, such as, especially, ammonium chloride, sulfate or
nitrate, and other nitrates, the nutrient medium may contain as inorganic
salts chlorides, carbonates, sulfates and especially phosphates of alkali
metals and alkaline earth metals, such as, especially, of sodium,
potassium, magnesium and calcium, and also of trace elements, such as
iron, ~inc and manganese.
Preferably the nutrient medium describet in Example 1 is used.
The nutrient medium is stsrilised and inoculated with a culture of the
producer strain while following the customary procedures.
.
Culturing takes place aerobically, that is to say in a static surface
culture or preferably submarsed with shaking or stirring with air or
oxygen in a shaking flask or in fermenters. A suitable temperature is
,,
20107~7
approx:Lmately from 15C to 40C, especially approximately from 20C to
30C. The fermentation period is preferably from 1 to 3 days. In a
preferred form of the process according to the invention, the culture is
incubated firstly for 8 hours at 27C and then the temperature is reduced
to 20C. The change in temperature brings about an increase in the yield
of hormaomycin. Under these conditions, the formation of hormaomycin
reaches a maximum after 45 hours, as can be seen with the aid of the agar
diffusion test and by high-pressure liquid chromatography. Culturing is
preferably carried out in several stages, that is to say first of all one
or more precultures in a liquid nutrient medium are prepared, and the
precultures are then inoculated into the actual production medium, for
example in a ratio of 1:20.
Isolation is effected by physico-chemical methods using separation
methods known per se, especially by centrifugation, filtration, solvent
extraction, precipitation, crystallisation and chromatography, more
especially adsorption and partition chromatography.
Hormaomycin is found in the cells in a proportion of 98 %. It is first of
all extracted therefrom using a suitable solvent, for example an alcohol,
such as methanol. The resulting crude product is purified by extraction
using a suitable solvent, such as ethyl acetate, and chromatography in
succession on silica gel and a modified dextran gel, for example
Sephadex0LH-20, in various solvents.
The invention relates especially to a process for the preparation of
hormaomycin, which process comprises aerobically culturing the strain
Streptomyces griseoflavus DSM 5195 or a microorganism containing the same
structural genes as the structural genes of said strain that are
causative of the formation of hormaomycin, in an agueous nutrient medium
containing a carbon and nitrogen source and inorganic salts, and
isolating hormaomycin.
The invention relates especially to a form of the above-mentioned process
which comprises culturing a hormaomycin-forming microorganism of the
species Streptomyces griseoflavus.
.. i ., , , ,, . " , , " ~ . .", , , ,, .. .. ", ,. ., , ., ~ , .. . .
:`,: , ::. . . - . : , , . , .: ' ' ,', :, .. , : , :.:, " : ... ~ . . . ~ :,
. ::: :,.: ' :~ ' . '.; ':,, ' ': , : .. ' . ` ' ' ,' ` ' ' . " ' ' , .
. ,.. : : ., :. . . ..
.:: . . ... . . . .
.
20~0~77
- 15 -
A preferred form of the above-mentioned process comprises culturing the
strain Streptomyces griseoflavus DSM 5195 or a hormaomycin-forming mutant
of that strain.
An especially preferred form of the above-mentioned process comprises
culturing the strain Streptomyces griseoflavus DSM 5195.
The fermentation is preferably carried out under the conditions described
in the Examples section.
Microorganisms that contain the same structural genes causative of the
formation of hormaomycin as does the strain DSM 5195 can be produced
artificially, for example by means of gene manipulation, by in a manner
known ~ se isolating the corresponding structural genes from the strain
DSH 5195 and incorporating them, at a suitable site, into the genetic
material of a suitable different microorganism. Suitable microorganisms
are those not only into which the structural genes in question can be
incorporated but also in which those genes can be expressed and in which
the hormaomycin formed is not decomposed but, preferably, is secreted
into the fermentation broth. Such suitable microorganisms are especially
other strains of the genus Streptomyces, especially those of the spscies
Streptomyces griseoflavus, insofar as they do not already possess the
above-mentioned structural genes.
Once the nuclsotids sequence of the above-mentioned structural genes has
been clarified, they too can be produced synthetically, in which case it
is possible, as a result of the tegeneration of the genetic code, to
replace certain nucleotide sequences consisting of three successive
nucleotides, so-called codons, by other codons that code for the same
amino acid.
Hormaomycin-forming mutants can be produced, for example, under the
action of ultraviolet rays or X-rays or chemical mutagens, for example
N-methyl-N'-nitro-N-nitroso-guanidine, and isolated in a manner known
per se by selection according to their specific properties.
.
.. .. .. .
.. .. . . . .
.
: ... .:
' : . .
' . : .~ . , , ', : : , . :
- , ~ . , :
. ': ' . ,, '
- 16 - 2010777
The invention relates also to a process for the preparation of a
fermentation material containing hormaomycin in a detectable amount,
which process comprises culturing the strain Streptomyces griseoflavus
DSM 5195 or a culture derivable from that strain in a nutrient medium and
interrupting the process at a suitable point in order to isolate or
purify hormaomycin.
The invention relates also to novel cleavage or decomposition products of
hormaomycin, especially novel amino acids, for example 2-amino-3-(2-
nitrocyclopropyl)-propionic acid, (Z)-4-propenyl-proline and 2-amino-3-
phenylbutyric acid, and also 5-chloro-N-hydroxypyrrole-2-carboxylic acid
and peptide fragments preformed in hormaomycin, containing at least one
of the afore-mentioned novel acids.
These cleavage products can be used for the chemical synthesis of
hormaomycin.
The invention relates especially to the compounds of formula III
obtainable by lactone cleavage of hormaomycin and optionally subsequent
esterification with a lower alkanol `
.
, . ~ .. . . . . . ............... . . .
.
.. . .. : . .
- 17 - 2
H3C~
~2 ~CH3 H~-CH3
-CH-~ ~O
--\.~ o2 (III)
R- ~OH ~H--~
C~3 f ~ ~
H
j~-CH2-~ -
02N-- ~H
~:=0
HO- ~ ¦
1~1 ' . .
wherein R is hydrogen or lower alkyl, and salts of the compound of
formula III wherein R is hydrogen.
Salts of a compound of formula III are preferably pharmaceutically
acceptable and non-toxic, for example alkali metal or alkaline earth
metal salts, for example sodium, potassium, magnesium or calcium salts,
or salts with ammonia or suitable organic amines, there being especially
suitable for the salt formation aliphatic, cycloaliphatic, cycloali-
phatic-aliphatic or araliphatic primary, secondary or tertiary mono-, di-
or poly-amines, and also heterocyclic bases, such as lower alkylamines,
for example triethylamine, hydroxy-lower alkylamines, for example
2-hydroxyethylamine, bis-(2-hydroxyethyl)-amine, 2-hydroxyethyldiethyl-
amine or tri-(2-hydroxyethyl)-amine, basic aliphatic esters of carboxylic
scids, for example 4-aminobenzoic acid 2-diethylaminoethyl ester, lower
alkyleneamines, for example l-ethylpiperidine, cycloalkylamines, for
example ticyclohexylamine, or benzylamines, for example N,N'-dibenzyl-
ethylenediamine, and also bases of the pyridine type, for example
pyridine, collidine or quinoline.
~ '
.
.. : . , ~ , . ,: .. . ..
, ,. ~
.
20107~7
- 18 -
For isolation or purification purposes it is also possible to use pharma-
ceutically unsuitable salts. Only the pharmaceutically acceptable, non-
toxic salts are used therapeutically, however, and these are therefore
preferred.
The invention relates also to a process for the preparation of a compound
of formula III or a salt of the compound of formula III wherein R is
hydrogen, in which process hormaomycin of formula I is subjected to
lactone cleavage, thus yielding a compound of the formula III wherein R
is hydrogen, or a salt thereof, and for the preparation of a compound of
formula III wherein R is lower alkyl, optionally the compound of
formula III wherein R is hydrogen is esterified, if necessary after
protecting the free hydroxy groups by readily removable protecting `
groups, and any protecting groups are removed, or for the preparation of
a salt the compound of formula III wherein R is hydrogen is converted
into a salt, or a resulting salt is converted into the free acid.
The lactone cleavage is carried out in a manner known per se. Preferably,
hormaomycin is treated in a suitable solvent with a suitable basic agent,
especially a weakly basic agent, such as the salt of a strong base and a
weak acit, for example potassium carbonate, alkali metal cyanide or
sodium hydrogen sulfite. If desired, the basic agent can be used in
catalytic amounts. If th0 lactone cleavage is carried out in a suitable
lower alkanol, such as methanol or ethanol, there is obtained an ester of
formula III wherein R is the lower alkyl radical corresponding to that
lower alkanol.
If the lactone cleavage is not carried out in such a lower alkanol, the
resulting compound of formula III wherein R is hydrogen can be esterified
in a manner known ~er se.
The esterification can be carried out, for example, by reacting a
reactive carboxylic acid derivative of a compound of formula III wherein
R is hydrogen with the corresponding alcohol. Alternatively, the compound
of formula III wherein R is hydrogen can be esterified by reaction with a
reactive derivative of the alcohol desired as esterification component.
~ .
, , ~ . .~. . .. .. . . . . . .. . .
. :. , , ,'. '., ,';., '`, , ' ; ; .
,. . :. ., . ,:
,, . , .. ,, .. ~ . :.
- l9- `~ Z010777
Suitable agents for esterification are, for example, corresponding diazo-
lower alkanes, for example diazomethane, diazoethane or diazo-n-butane.
These reagents are employed in the presence of a suitable inert solvent,
such as an aliphatic, cycloaliphatic or aromatic hydrocarbon, such as
hexane, cyclohexane, benzene or toluene, a halogenated aliphatic hydro-
carbon, for example methylene chloride, or an ether, such as a di-lower
alkyl ether, for example diethyl ether, or a cyclic ether, for example
tetrahydrofuran or dioxane, or a solvent mixture, and, depending upon the
diazo reagent, with cooling, at room temperature or with gentle heating,
and also, if necessary, in a closed vessel and/or under an inert gas
atmosphere, for example a nitrogen atmosphere.
Other suitable esterification agents are esters of corresponding
alcohols, especially those with strong inorganic or organic acids, such
as mineral acids, for example hydrohalic acids, such as hydrochloric,
hydrobromic or hydriodic acid, also sulfuric acid, or halosulfuric acid,
for example fluorosulfuric acid, or strong organic sulfonic acids, such
as lower alkanesulfonic acids that are unsubstituted or substituted, for
example, by halogen, such as fluorine, or aromatic sulfonic acids, for
example benzenesulonic acids that are unsubstituted or substituted, for
example, by lower alkyl, such as methyl, halogen, such as bromine, and/or
by nitro, for example methanesulfonic, trifluoromethanesulfonic or
p-toluenesulfonic acid. Such esters are inter alia lower alkyl halides,
ti-lower alkyl sulfates, such as dimethyl sulfate, also fluorosulfonic
acit lower alkyl esters, for example fluorosulfonic acid methyl ester, or
unsubstituted or halo-substituted methanesulfonic acid lower alkyl
esters, for example trifluoromethanesulfonic acid methyl ester. They are
customarily used in the presence of an inert solvent, such as an unsub-
stituted or halogenated, such as chlorinated, aliphatic, cycloaliphatic
or aromatic hydrocarbon, for example methylene chloride, an ether, such
as dioxane or tetrahydrofuran, or a mixture thereof. It is preferable to
use suitable condensation agents, such as alkali metal carbonates ~r
hydrogen carbonates, for example sodium or potassium carbonate or hydro-
gen carbonate (usually together with a sulfate), or organic bases, such
as usually sterically hindered tri-lower alkylamines, for example N,N-di-
isopropyl-N-ethylamine (preferably together with halosulfonic acid lower
alkyl esters or unsubstituted or halo-substituted methanesulfonic acid
2107~7
- 20 -
lower alkyl esters), the operation being carried out with cooling, at
room temperature or with heating, for example at temperatures of from
approximately -20C to approximately +50C, and, if necessary, in a
closed vessel and/or in an inert gas atmosphere, for example a nitrogen
atmosphere.
Other esterification agents are corresponding trisubstituted oxonium
salts (so-called Meerwein salts), or disubstituted carbenium or halonium
salts, wherein the substituents are the etherifying radicals, for example
tri-lower alkyloxonium salts, and also di-lower alkoxycarbenium or di-
lower alkylhalonium salts, especially the corresponding salts with - -
complex, fluorine-containing acids, such as the corresponding tetra-
fluoroborates, hexafluorophosphates, hexafluoroantimonates, or hexa-
chloroantimonates. Such reagents are, for example, trimethyloxonium or
triethyloxonium hexafluoroantimonate, hexachloroantimonate, hexafluoro-
phosphate or tetrafluoroborate, dimethoxycarbenium hexafluorophosphate or
dimethylbromonium hexafluoroantimonate. These agents are preferably used
in an inert solvent, such as an ether or a halogenated hydrocarbon, for
example diethyl ether, tetrahydrofuran or methylene chloride, or in a
mixture thereof, if necessary in the presence of a base, such as an ;
organic base, for example a tri-lower alkylamine, preferably a sterically
hindered tri-lower alkylamine, for example N,N-diisopropyl-N-ethylamine,
and with cooling, at room temperature or wlth gentle heating, for example
a~ from approximately -20C to approximately 50C, if necessary in a
closed vessel and/or in an inert gas atmosphere, for example a nitrogen
atmosphere.
A preferred form of the esterification reaction is the reaction of a
caesium salt of the compound of formula III wherein R is hydrogen with
the alcohol desired as esterification component wherein the hydroxy group
is in reactive esterified form, for example in halide form.
:`
In order to avoid lactone formation, either the operation is carried out
with a large excess of the lower alkanol desired as esterification
component, which is used, for example, simultaneously as solvent, or the
free hydroxy groups present in the compound of formula III are protected
prior to tbe esterification.
.
.
:. ~, . . .. , ,.......... . , - . : .
. . . . . . :: . . ~ . -:: .
20~0777
Hydroxy-protecting groups and the methods by which they are introduced
and removed are described, for example, in "Protective Groups in Organic
Chemistry", Plenum Press, London, New York, 1973, and in "Methoden der
Organischen Chemie", Houben-Weyl, 4th Edition, Vol. 15il, Georg-Thieme-
Verlag, Stuttgart 1974, and in Theodora W. Greene, "Protective Groups in
Organic Synthesis", John Wiley & Sons, New York, 1981. It is charac-
teristic of protecting groups that they can readily be removed, that is
to say without undesirable secondary reactions taking place, for example
by solvolysis, reduction, photolysis or alternatively under physiological
conditions.
Hydroxy-protecting groups are, for example, acyl radicals, such as lower
alkanoyl that is unsubstituted or substituted, for example, by halogen,
such as 2,2-dichloroacetyl, or acyl radicals of carbonic acid semiesters,
especially tert.-butoxycarbonyl, unsubstituted or substituted benzyloxy-
carbonyl, for example 4-nitrobenzyloxycarbonyl, or diphenylmethoxy-
carbonyl, or 2-halo-lower alkoxycarbonyl, such as 2,2,2-trichloroethoxy-
carbonyl, also trityl or formyl, or organic silyl or stannyl radicals,
and also readily removable etherifying groups, such as tert.-lower alkyl,
for example tert.-butyl, 2-oxa- or 2-thia-aliphatic or 2-oxa- or 2-thia-
cycloaliphatic hydrocarbon radicals, especially 1-lower alkoxy-lower
alkyl or 1-lower alkylthio-lower alkyl, for example methoxymethyl,
1-methoxyethyl, 1-ethoxyethyl, methylthiomethyl, 1-methylthioethyl or
1-ethylthioethyl, or 2-oxa- or 2-thia-cycloalkyl having 5 or 6 ring
atoms, for example tetrahydrofuryl or 2-tetrahydropyranyl or
corresponding thia analogues, and also unsubstituted or substituted
1-phenyl-lower alkyl, such as unsubstituted or substituted benzyl or
diphenylmethyl, suitable substituents of the phenyl radicals being, for
example, halogen, such as chlorine, lower alkoxy, such as methoxy, and/or
nitro.
,
The above-mentioned organic silyl or stannyl radlcals preferably contain
lower alkyl, especially methyl, as substituent of the silicon or tin
atoms. Corresponding silyl or stannyl groups are especially tri-lower
alkylsilyl, especially trimethylsilyl, also dimethyl-tert.-butyl-silyl,
or correspondingly substituted stannyl, for example tri-n-butylstannyl.
.
-~: , . , , :. . . .. :
.. ~ . . .
20l0m
The removal of the hydroxy-protecting groups is effected in a manner
known per se, for example by means of solvolysis, especially hydrolysis,
alcoholysis or acidolysis, or by means of reduction, especially hydro-
genolysis or chemical reduction, optionally in stages or simultaneously.
Tri-lower alkylsilyl can also be removed by treatment with a salt of
hydrofluoric acid that yields the fluoride anion, such as an alkali metal
fluoride, for example sodium or potassium fluoride, in the presence of a
macrocyclic polyether ("crown ether"), or with a fluoride of an organic
quaternary base, such as tetra-lower alkylammonium fluoride or tri-lower :
alkyl-arylammonium fluoride, for example tetraethylammonium fluoride or
tetrabutylammonium fluoride, in the presence of an aprotic polar solvent,
such as dimethyl sulfoxide or N,N-dimethylacetamide.
Salts of the compound of formula III wherein R is hydrogen can be
prepared in a manner known per se, for example by reaction with a
suitable base, for example by treatment with suitable metal compounds,
such as alkali metal salts of suitable organic carboxylic acids, for
example the sodium salt of ~-ethylcaproic acid, or with suitable in-
organic alkali metal or alkaline earth metal salts, especially those
which are derived from a weak and preferably volatile acid, for example
sodium hydrogen carbonate, or with ammonia or a suitable organic amine,
prefersbly stoichiometric amounts or only a small excess of the salt-
forming agent being used.
The salts can be converted into the free compound in customary manner,
for example by treatment with a suitable acid.
Unless otherwise indicated, the processes described above, including the
processes for removing protecting groups and the additional process
steps, are carried out in a manner known per se, for example in the
presencs of solvents and diluents, if necessary, in the presence of
condensation agents or catalysts, at reduced or elevated temperature, for
example in a temperature range of from approximately -20C to approxi-
mately +100~C, especially from approximately +1C to approximately +70C,
~; .
- - ~
:' :'' `'. : : ' .
'' . ' ~ ' '' .
- 23 - 2 0 1 0 ~ 7
chiefly from room temperature (approximately +20C) to +45C, at normal
pressure or elevated pressure in a suitable vessel and, if necessary, in
an inert gas atmosphere, for example a nitrogen atmosphere.
In consideration of all the substituents present in the molecule, if
necessary, for example when readily hydrolysable radicals are present,
especially mild reaction conditions should be used, such as short re-
action times, the use of mild acidic or basic agents in low concentra-
tions, stoichiometric quantity ratios, and the selection of suitable
catalysts, solvents, temperature and/or pressure conditions.
The invention relates also to those forms of the process in which a
compound obtainable as intermediate at any stage of the process is used
as starting material and the remaining process steps are carried out or
in which the process is interrupted at any stage or a starting material
is formed under the reaction conditions or used in the form of a reactive
derivative or salt. In view of the close relationship between a free acid
and its salts, a reference to the free acid can also include the
corresponding salt, and vice versa, wherever possible or appropriate
under the circumstances. It ls preferable to use those starting materials
which in accordance with the process result in the compounds described
above as being especially valuable.
The present invention relates also to novel starting materials and/or
intermediates and processes for their preparation. Preferably the
starting materials used and the reaction conditions employed are so
chosen that the compounds mentioned as being especially preferred in this
Application are obtained.
In addition to the biological and pharmacological activities described
above for hormaomycin, hormaomycin and the compounds of formula III and
pharmaceutically acceptable salts thereof exhibit anti-proliferative,
immunosuppressive and inflammation-inhibiting properties, as can be seen,
for example, from the followlng experiments:
: - ,
... . . . : . .
Z010~777
- 24 -
In order to demonstrate the anti-proliferative properties, the inhibitory
action of the compounds of formula I on the growth of human T24 bladder
carcinoma cells is determined. These cells are incubated in "Eagle's
minimal essential medium" to which 5 % (v/v) foetal calf serum have been
added, in a humidified incubator at 37C and 5 % by volume CO2 in the
air. The carcinoma cells (1000-1500) are inoculated into 96-well micro-
titre plates and incubated overnight under the above-mentioned condi-
tions. The test substance is added in serial dilutions on day 1. The
plates are incubated under the above-mentioned conditions for 5 days.
During this period, the control cultures undergo at least four cell
divisions. After incubation the cells are fixed in 3.3 % (w/v) aqueous
glutaraldehyde solution, washed with water and stained with 0.05 %
(weight/volume) aqueous methylene blue solution. After washing, the dye
is eluted with 3 % (w/v) aqueous hydrochloric acid. The optical density
(OD) per well, which is directly proportional to the number of cells, is
then measured with a photometer (Titertek multiskan) at 665 nm. The IC50
values are calculated with a computer system using the formula
OD66s (test) - OD66s (start)
x 100
D66s (control) - OD66s (start)
The ICso values are defined as that active ingredient concentration at
which the number of cells per well at the end of the incubation period
constitutes only 50 % of the number of cells in the control cultures. The
resulting ICso values for the compounds of formulae I and III are
approximately from 0.5 to 5 ~mol/litre, for example 1 ~mol/litre.
.
The compounds of formulae I and III are therefore also suitable for the
treatment of tumours, for example of the bladder.
The immunosuppressive properties can be inferred, for example, from the
following tests:
.,,,. ~ ,, . :
- .
',
- 2~ - 2010777
Inhibition of T-cell proliferation in human blood induced bY anti-CD 3
monoclonal antibodies
10 units of heparin per ml are added to venous human blood which is then
diluted in a ratio of 1:6 (v:v) with RPMI 1640 medium (Gibco). After the
addition of 50 ng/ml of anti-CD 3 monoclonal antibodies (TR 66, IgGlK),
the diluted blood is introduced into a 96-well titre plate in portions of
150 ~1 per well. A compound of formula I or III in the above-mentioned
RPMI medium is then added in portions of 50 ~1 per well. Cyclosporin A
(CSA, Sandoz, Basle, Switzerland) or prednisolone is used as reference
compound.
After 48 hours at 37C and a content of 7 % by volume COz in the air,
1.0 ~Ci of 3H-thymidine in 10 ~1 of RPMI medium (Amersham: specific
activity 5 Ci/mmol) is added per well.
After a further 16 hours the cells are harvested on glass fibre filters
(LKB, Bromma, Sweden) using a 96-well harvesting machine (LKB). After the
filters have dried, their radioactivity is measured (Optiscint [LKB], -
Betaplate-scintillation-counter [LKB]). The results are expressed as mean
values of the cpm (counts per minute) determined for each well at three
different concentrations in the presence and absence of the test
substances and the action of the substances is given as percentage
inhibition, which is defined as follows:
% inhibition ~ 100 _ ~ Exp- cpm x 100 ¦ -
cpm of control
Exp. cpm ~ cpm of the T-cells and the anti-CD 3 monoclonal antibodies and
the test substance.
'
cpm of control = cpm of the T-cells and the anti-CD 3 monoclonal anti- -
boties (100 % incorporation).
: ;
'
.. . . .. ..
. : ., .
20 ;L0777
- 26 -
An inhibition curve is drawn and the ICso values are given in ~mol/litre.
In the above-mentioned test, ICso values of from 1.5 to 15, for example
7.5 ~mol/litre, are determined for hormaomycin and the compounds of for-
mula III, and an ICso value of 0.3 ~mol/litre is determined for cyclo-
sporin A.
Inhibition of T-cell proliferation in human blood induced by inter-
leukin-2
The test is carried out analogously to that described above, but with
100 units/ml of recombinant interleukin-2 (Hoffmann-La Roche, Basle,
Switzerland) being added instead of the 50 ng/ml of anti-CD 3 antibodies.
In this test, ICso values of from 4 to 40, for example 17, ~mol/litre are
tetermined for hormaomycin and the compounds of formula III.
The compounds of formulae I and III can therefore also be used as immuno-
suppressive agents for suppressing undesirable immune reactions, for
example in transplantation or allergies.
The inflammation-inhibiting properties can be demonstrated, for example,
in vitro by the inhibition of the activity of phospholipase Az (obtained
from human leucocytes).
In vitro test for teterminin~ the inhibition of phospholipase A2 obtained
from human leucocytes
Neutrophilic polymorphonuclear h~man leucocytes are isolated from "buffy
coats" by multistage fractional sedimentation and are deep-frozen. The
phospholipase Az is extracted from the cell suspension by homogenisation
with the addition of ice-cold 0.36N H2S04 in 2N NaCl and the supernatant
obtained after centrifugation at 10,000 g is dialysed against sodium
acetate buffer pH 4.5.
:~ :
For determining the enzyme activity, enzyme (10-30 ~g of protein) is
incubatsd at 37 for 1 hour in O.lM tris/HCl buffer pH 7 with the
~-~ addition of 1 mmol of CaClz and substrate consisting of phospholipids
(2 ~mol) of Escherichia coli biosynthetically radioactively labelled with
4C-oleic acid. The reaction is stopped by the addition of Dole reagent
-. .: ::. , .;
. ~ , ~ . .` . - .
: ~' . , ' . ` ' :. ' ~
" . . , . ,, ` . .
- 27 - 2010777
(isopropanol/heptane/lN H2S04 40:10:1, v/v) and the 14 C-oleic acid freed
selectively by phospholipase A2 is extracted. Substrate extracted there-
with is removed completely by filtration of the extract through a column
of silica gel. The determination of 14C-oleic acid in the eluate is
effected by radiometry.
In order to determine the inhibitory action of the compounds of
formulae I and III on phospholipase A2, the compounds are added to the
incubation batch in the form of solutions in water, dimethyl sulfoxide
(final concentration in the batch up to 5 % by volume) or ethanol (final
concentration in the batch up to 2.5 % by volume). The strength of action
of the test compounds is expressed by the IC50, that is to say the
concentration that effects inhibition of 50 % of the control activity.
The ICso is determined graphically by plotting the percentage inhibition
on the ordinate against the logarithm of the concentration (~mol) on the
a'~scissa.
In this model, ICso values of from 2 to 18, for example 7, in ~mol/litre
are obtainet for the compounds of formulae I and III.
The invention relates also to the use of hormaomycin, a compound of
formula III or a pharmaceutically acceptable salt of a compou~d of
formula III,
. . .
,_ 2~107~7
- 28 -
H3C\ !~
H~-CH3
-CH-~ O
R- OH H, ÇH--~ ~-
~-fH-G-~ ~H3 \-=-/
H
j/--CHz-~ H
02N-- H
,=0 :: .
Cl '
wherein R is hytrogen, for the metical treatment of diseases in warm-
blooded animals, including humans, especially by the administration of
therapeutically effective amounts of the above-mentioned compounds. The
dosage when administered to warm-blooded animals of approximately 70 kg
body welght, for example a human of such a weight, depends inter alia on
the disease to be treatet ant is from 0.1 to 5 g, for example 0.5 to 5 g,
preferably 0.5 to 2 g, for example 1 to 2 g, per day. The administration
,
is effected, preferably in the form of pharmaceutical-preparations,
parenterally, for example intravenously, intramuscularly or intra-
peritoneally, divided lnto several doses per day.
The invention relates also to pharmaceutical preparations that contain
hormaomycin, especially an effective dose, that is to say a dose having,
for~example, antibiotic or tumour-inhibiting effect or suppress~ng un-
desirable immune reactions, of hormaomycin, a compound of formula III or
a~pharmaceutically acceptable salt of a compound of formula III wherein R
is hydrogen~, together with a pharmaceutical carrier material, preferably
more than~10 % carrier material.
20107~
- 29 -
The pharmacologically active compounds of the present invention can be
used in the form of parenterally administrable preparations or in the
form of infusion solutions. Such solutions are preferably isotonic
aqueous solutions or suspensions which, for example in the case of
lyophilised preparations which contain the active ingredient alone or
together with a carrier material, for example mannitol, may be prepared
before use. The pharmaceutical preparations can be sterilised andtor
contain adjuncts, for example preservatives, stabilisers, wetting agents
and/or emulsifiers, solubilisers, salts for regulating the osmotic
pressure and/or buffers. The present pharmaceutical preparations which,
if desired, may contain other pharmacologically active substances, such
as antibiotics, are manufactured in a manner known E~ se, for example by
means of conventional dissolving or lyophilising processes, and contain
approximately from 0.1 % to 90 %, especially from approximately 1 % to
approximately 30 %, active ingredient(s).
',
The following Examples illustrate the i~vention but do not limit the
invention in any form. Unless indicated otherwise, the Rf values are
determined on silica gel thin-layer plates (Sil G/UV2s 4 ) . The ratio to
one another of the eluants in the eluant mixtures used is given in parts
by volume (v/v), and temperatures are given in degrees Celsius. The
concentration, c, of substance in a solvent or solvent mixture in the
case of optical rotation is given in percent (weight/volume~,
The following abbreviations and combinations are used to indicate nuclear
resonance spectra (chemical shlft ~ in ppm with respect to tetramethyl-
silane):
;~ b - broad
d = doublet
Hz - Hertz
J ~ coupling constant
m ~ multiplet
q - quartet
s - singlet
t - triplet
~ ; .
. ~
, . ;. . . . . .
, . . ;
:. . ,: . , - . . ... ,: .
. . . . .
.
2010~7
- 30 -
Example 1: A 10 litre fermenter is charged with 9 litres of nutrient
solution (20 g of full-fat soya flour, 20 g of meat flour, 10 g of
mannitol, 0.2 g of NaCl, 0.5 g of ZnSO4 x 7H20, 1 litre of tap water,
pH 7.0) and sterilised, inoculated with 1 litre of a preculture of the
strain Streptomyces griseoflavus DSM 5195 (same nutrient solution,
32 hours old, 27C) and run for 45 hours under the following conditions:
temperature 27C (after 8 hours reduced to 20C), agitator speed: 300
revolutions per minute, air supply 0.5 vvm (vvm = volume air/~olume
nutrient medium/minute).
The mycelium obtained by centrifugation of the culture broth is extracted
twice with methanol. The extract is concentrated to the aqueous phase and
extracted by shaking twice with ethyl acetate. After evaporation of the
solvent, the organic phase yields a brownish product. This is separated
on a column of silica gel (CHCl3 as eluant). The active fractions are
then purified with methanol on a Sephadex~LH-20 column (motified dextran
gel). The enrichment of hormaomycin is effected by high-pressure liquid
chromatography (HPLC) (RP hypersil-5 ~m, H2O/CN3CN (0.1 % H3P04)
-gradient: 30 % CH3CN to 100 % CH3CN, detection: 254 nm, retention time =
6.7 minutes (77 % CH3CN)). For the purpose of final purification, 140 mg
of this substance charge are chromatographed on silica gel (< 0.08 mm
pore diameter; column: 2.5 x 50 cm) with n-hexane/acetone (2:1), there
being obtained 18 mg of a colourless impurity that can be stained with
molybtatophosphoric acid (TLC). Hormaomycin is eluted with CHCl3~MeOH
(95:5). The substance is dlssolved and reprecipitated by the dropwise
addition of a concentrated chloroform solution in 500 ml of n-hexane.
Hormaomycin is obtained in the form of a colourless, amorphous powder
with a melting point of 166-168; Rf ~ 0.39 (CHCl3:methanol - 100:4);
Rf - 0.28 (n-hexane:acetone ~ 1:1) [~]D ' +20.8 (c = 0.5; methanol);
UV (methanol): 278 (9150), 206 (42950) nm; IR (KBr): 3390, 3100-2800,
1741, 1645-1620 (broad), 1612, 1545, 1450, 1365 cm 1; Past atom bombard-
ment mass spectrum (glycerine as matrix): 1129.4 ([M~H] ), 938, 830, 700,
516, 401, 373, 300, 264, 218; lN-NMR snd 13C-NMR see Table 1;
CssH69Nlool4cl (1129.68):
calc. C 58.48 H 6.16 N 12.40 0 19.82 Cl 3.14
found C 58.35 H 6.31 N 11.66 O 19.30 Cl 2.94
.
.
-
:. ~ ~ ` ~ ' ` :
:
20~7~7
- 31 -
Hormaomycin is neutral, readily soluble in methanol, chloroform, acetone
and ethyl acetate and insoluble in water and hexane. On thin-layer
chromatography plates it extinguishes UV light (254 nm), slowly turns
yellow on standing in air and can be stained according to Barrollier
(blue-green) and with Ehrlich's reagent (pale violet) but not with
ninhydrin.
:. . , : . , . ; .
', .' ' "' ,. . , .~ ' ' . , .' .', ~ ' ' ' . , '
" ' , ' , ' ` ' ' ' . ' . ' '
' ' ' ,. , ' ' .
' ` ' '" .'. ` ' ' ' : ' ' ' . ' ' ,
20107~
rn~ ~ ~ ~ ô ~^ ~
~r~ ~ ~ ~ _~ ~ ~O
t~ l ~ u l l l l l o t~
O ~I IC ~ r~ ~ ,~ I 1 5 r~
_1 ~3 2Z 2 2 22 2 2 Z 2 22 2 2 55
U~ U~
I~ . U~
C~
.~.1 c7~ ~ . a~ 1`
~ ~_ 1111 cr~ 1` 11'~ ~ 11 ~ 2
_l ~ ~ ~ ).
~ ~ ~ ^ o~
v~ ~ ~ ~ ~ v ~ ~ ~ ~ ~ a .
N ~ CO ~U'~ 0~N r~ ~C~ O O _~U~ O ~I tO u~
2 2 ~t U~~CJ~ O;~ O~ N~t 00~D t~Cl~ ~D ~ O _~ CO
~ ~ . .. . . .. . . . . . .. . . I
O o ~ oo O O ~ ~ D
.
,a~
~1 . ~ ooCJ~
.J ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ O ~ ~ ~ ^ ^
O~a ~ ~ ~_, u~ ~o ~ ~ ~ ~'~ ~ ~ ~ ,
O ~ ~ ~ ,~ ) V O ~ ~~
~1 '¢ .'
,
. .'
' X X .
~1 la ~ ~
`_ _
E--l C~ :C 2 ~ 2~ t2~ ~ 5~ t)
O
~ 'Co
~ ~ O O ~ ~ O CJ~ ~ ~O ~ ~ O ~ o _~
V ~ . . . ~ . . . . . ~ . . . , . . .
~ _~ r~ ~ c~ 3C O U~ ~ ~ ~ ~ CJ~ O 1~ 0~ CO
~ <o ~ D ~ t)
:
: ~ 2 1-
.. C ~ ~1
a o ~1 ~ ~ 2
~3 ~ ~ C ~ N ~--
~ 8 ¦ 1 1~I N ~Z ~ 2
O r ~ I ~ I ~ ~ ~)
_ _I c: I ~ 2 2
t~ '~1 ~ 2 ~ ~ ~) ¦ s.
I~ ~ N C~ ~
r~ . ~ O . I ~ N
1 ~ ,C ~ _~ ~g .S ~
~ ~ ) 0 ~ X ~
: : . E-~ u~ ~ 2 ~ 2
: ~ . .
. ~, . . .: . , ~ `; . : . . :, . . .
: . ': i ~' ' . . `'', ' ' ' ': . . .
:: , . . . ` :
" ",~ ' ''' ' .' ' ' , .,' ~., ',' ' '~
`: ~ , ' ' .
:' - . . . .
20107~7
o~
~ô oô
~ O ~ ~D ~ CO ~
V '_ ~I N 1~) _ `~ N
t~ t~ )
t I ~ I I
~1 _1 3~ P~
_~ U~ ~O 1~ . ;',".
t~ I~ U`) , -~ . .
~ O O _ _~ O
v ~_ 11 11 ~11 ~ a ~ 11 11
~ ~ ~ ~ 5 ,y ~
.~ ^ . ' .:
~ ~ 1 ~ tl~ tO 10
v~ ~ ~ ~ i3 E3 Ei ~ Ei 6 E ~ o
.,
~ ~ ~ I~ U~ _ ~ o ~ ~ _ o o ~
~_ N J~ ~ ~ O r~ V~ I~ ~ N N ~ N N U~ .:
_'I N ~S 'D~ 'D _ _I N ~ t~ ~ `;t U-) 1
V O ~ ~ ~ ~ ^~ ~ ~ ~ ~ ~ N ~D
~1 ~ ~
Z ~ ~
. .,
~ X X
~ N N
1'1 N N
,'
.
. .'
O~ 0 ~~ ~ ~ ~ ~) N
0 ~` ~ ~ ~ t~ `D N ~
~O ~4 ~t 1~) NN N ~ ~ 0 N N ~:
'`~`'
. .-.
J ~ . . ,.'~
U _I ~ r~ ~ ~ I
~ t~ 1 1
VU ~ ~ I ~ il
.~ I tl~ t~ `~ .
',''":: '' . ' ~,' ', ' ,', ' , ' ' ;' ,: .~'" ,' . ': . ,.. , . ' .. ' , : . ` :' :' ,, ' ,.:. '.. , . : ., .
20~0777
,
g ~ ~
.,, ~ ~ ~ ~ ~ ~ ~ ~ ~o ~ ~ ~ ~ ~ ~ ,
V _ _ ~ _ _ ~ u ) _ _ _ ~ _ _ N
~ I I C ) I I C~ t~ I
O ~ Z ~
~i ~
l_ ~ ~
, ~D ~D
.,1~ ~ ~ cr~ ~ ~ ~ 'O ~ ~ ~
v a~ 11
.~1_ ,!~: ^ ~ ~ ~ ~ ~ ~_ t~
_I~ q ~ ~ O . ..
~n~ E3 ~3 E ~13 ~ ~ 6 ~ ~ ~
. ':'
<~ Oo ~ u) Oo oo O o~ O~ o ~ u~
:~ ~ o cr~ o cr~ o
_ ooooo~o o~
.
o
.,, .
~ ~ ~ .,
o ~o ~ ~ ~ ~ _ o ~ _ _ _ _
_l ~ ~
~ . .
~ N N N N . '
~ ~ ~ ~~O O~ O _~
:' t.)I~ O ~ _~ CO * I~
:~ ~O~ ~ U')
V~ ~ O ~11
~ I1-~ P~
O O ~ ,I ~ .
O ~~rl N ~L ~ 1.1
a ~ ' ~ ~ ~
~ ~ O ~ 3 O ~
:~1~ ~ ~
. ~ . . V Z ~ ~ ~ :Z ~
~ ' ~i: t ~ '
- ~ _ o Z _ _,
~ I 1.1 N
~ ~ R O , c~ 04
2010777
g
O ~ ,_ ~
.,, ~ ~ ~ .,,
U~ o C~ Z
e~ ,~ .
V~
E
. -.
~ U~ U7
'v
V~ ~ ~ ~ P~
~ 'd ~ ,n ~ '
. g
~o ~ O V~
O r~ , O
_ ~ ~ _. ~o .
~o _. . '.
a~ ~ , ":,
~g ,~
V ~ ~ D ~ V^
~ ~ ~_ _ _ _ ~a ~ .
_l ~ ~O E-~
~1 _- g
_o '=
,~ I~ a1 ..
~a ~
0
o~
Cr~ - ~, ' .
u~ ~ ~ ~
_. ..... ~ o
~ ~o~ Vt~
_~ O O ~ ~ ~ ~ ,~ I
~o ~ . X
. ~
v ~
c x ~ O æ
O O X ~ ~
: C e~ ~ c: ~ I .
O ~ ~ a ~ c ~
~ O I ~ ~ ~
_, ~ ~ Ql ~a I ;~0 ~ E 1~ E3
V ~ / ~ 14 14 ',
~- I
V~ '~
'
~ .. : ., ,, i . . . .
. `' , . ~ , , , ~, .
. . . : ~ . . ,, ' , ,.: ' '
i ' ' : ` ;. - . , , : , . -
: ;' ' . ' . - ' . , ~ ' . .
20107~'7
- 36 -
Example 2: 7 mg of hormaomycin are dissolved in 2 ml of methanol. After
the addition of catalytic amounts of solid K2C03, the solution is stirred
at room temperature for 14 hours, then poured onto 20 ml of ice-water and
extracted with C}~2Cl2 (4 x 6 ml). The evaporation residue of the combined
organic phases is chromatographed on silica gel (column 1.0 x 3.0 cm,
CHCl3:methanol = 9:1). From the main zone there is obtained the open-
chain hormaomycin methyl ester of formula II; m.p. 172C, Rf = 0.05
(CHCl3:methanol = 100:4), Rf = 0.46 (CHCl3:methanol = 9:1; staining
behaviour is the same as hormaomycin) f UV (methanol): 278 (11200), 214
(31300) nm, IR (KBr): 3400, 3300, 3100-2850, 1740, 1635 (broad), 1545,
1450, 1370, 1030 cm 1; fast atom bombardment mass spectrum (2-nitrobenzyl
alcohol as matrix): 1183/1185 ([M+Na] ), 1161/1163 ([M+H? ), 992, 718,
419; 1159/1161 ([M-H] ), lH-NMR (CDCl3, 200 MHz): 3.74 (s, 3H, OCH3), the
remaining signal pattern is similar to hormaomycin but the signals are in
some cases broad and overlap more strongly, and the signals beyond tetra-
methylsilane are absent.
Example 3: Pharmaceutical preparation for intramuscular iniection 250 mg
of hormaomycin are dissolved in polyethylene glycol 300. The solution is
sterile-filtered and introduced into a 3 ml ampoule under aseptic
conditions.
-, :, - ~. .
: ,. ~ , - .. , ; .. .
:: ' `. ', ' : ,
.