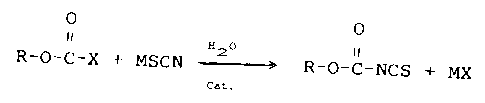Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2101 193
31,716
NOVEL PROCESS OF ALKOXY AND ARYLOXY
ISOTHIOCYANATE PREPARATION
iBACKGROUND OF THE INVENTION
Alkoxy and aryloxy isothiocyanates are well-known
versatile, organic intermediates which, by virtue of highly
reactive multifunct:ionalities, can undergo a variety of
condensation and cyclization reactions. For instance,
Heilbron et al, J. C:hem. Soc. 1948, 1340, made use of the
ready addition of alkoxycarbonyl isothiocyanate to alpha
7_0 aminonitrites in. their synthesis related to penicillin and
purines. Other useful organic syntheses based on alkoxy
and aryloxy iso~~yanates are set forth by Esmail et al.,
Synthesis, 301, (1975).
Ethoxycarbonyl isothiocyanate was first prepared by R.
L5 E. Doran in 1896 by reacting lead thiocyanate with
ethylchloroformate in boiling toluene with relatively poor
yields, J. Chem. Soc. 69, 324 (1896). In 1908, Dixon and
Taylor reported, J. Chem. Soc. 93, 684 (1908), that
methylchloroformate, ethylchloroformate,
20 benzylchloroformate, phenyl chloroformate and o- and p-
tolylchloroformate, were reacted with potassium thiocyanate
in boiling acetone to form the corresponding
isothiocyanate.
One of the reasons for low yields in Dixon et al's
25 method was confirmed by Takamizawa et al to be the
simultaneous formation of an unreactive thiocyanate isomer.
With ethylchloroformate, two isomers were obtained in about
30~ yields whils: with butylchloroformate the yields were
34~ for the isothiocyanate and 21$ for the thiocyanate
30 isomer. Yields of 31$ for the isothiocyanate and 3.5$ for
the thiocyanate isomer were obtained with
allylchloroformate, it being postulated that the formation
of the two isomers was due to the existence of the
mesomeric thiocyanate ion and isothiocyanate ion both of
35 which can effect: thE~ nucleophilic attack on the carbonyl
carbon and cause the thiocyanate and isothiocyanate isomer
product. Later workers improved reaction conditions to
-2-
favor the isot:hi.ocyanate formation by adding catalytic
amounts of triethy:lamine and longer reaction times;
Goerdeler et a)_, Chem Ber. 104, 1606 (1971). The use of
acetonitrile and ethyl acetate as solvents was reported by
Lamon; J. Heterocyclic Chem., 5, 837 (1968); Goerdeler et
al, Chem. Ber. 96, 526, (1963). Yields, however, were
only up to about 60~. Anders et al, Ger. Pat. No. 1215144,
1956, reported th<~ preparation of alkoxy and aryloxy
isothiocyanates using silyl isothiocyanate while Goerdeler
et al, Chem. Ber. 98, 2954 (1965) disclosed the pyrolysis
of thiozol:inediones as a means for producing them, see also
Schenk, Chem. Ber. 99, 1258 (1966).
In a later publication, Chem. Ber. 116, 2044, (1983),
Goerdeler et a:l reported the use of an aromatic hetero
cyclic nitrogen catalyst such as pyridine in carbon tetra
chloride for the preparation of alkoxythiocarbonyl iso-
thiocyanate, however, the yield was only 52$. Using the
propoxy analog, Goerdeler et al obtained only a 13~ yield
of the corresponding isothiocyanate.
The production. of phenoxycarbonyl isothiocyanate is
also taught b~~ Babu; J. Heterocyclic Chem. 20, 1127,
(1983). Thus, the prior art methods for preparing alkoxy
and aryloxy isothiocyanates are ineffective in terms of
yields and the formation of the undesirable thiocyanate
isomer. Additionally, the use of organic solvents creates
costly removal problems and poses serious air emission
hazards.
In Lewellyn et al., commonly-assigned U.S. Patent No.
4,778,921, is described a procedure for the formation of
alkoxy and aryloxy isothiocyanates whereby the desired
products are obl~ainE~d in higher yields and purity than when
the aforementioned prior art techniques are employed. The
process of the I:ewel_lyn et al. invention eliminates the use
of organic solvents, and furthermore, since the by-product
is alkali metal halide, waste disposal is facilitated.
The Lewellyn et al. process depends on the synthesis
of the alkoxy and a:ryloxyisothiocyanates in an all aqueous
2~.~~1~~
-3-
medium; it being completely unexpected that such a medium
could be employed since both the starting material, i.e.,
the alkyl or aryl hal.oformate and product isothiocyanate are
normally water-reactive. The use of the aqueous medium was
made possible because of the conjoint use therewith of a
catalyst comprising a six membered mononuclear or a ten
membered fused :polynuclear aromatic, heterocyclic compound
having 1 or 2 nitrogen atoms as the only hetero atoms in
the ring. The unique combination of catalyst and the
aqueous medium unexpectedly allowed isothiocyanate
formation in high yields and purity. Furthermore, the
product isothiocyanate, a strong lachrymator, although
isolatable, was not required to be isolated, i.e. it could
be reacted in :situ with other compounds, e.g., alcohols,
amines, mercaptans, etc., to produce many useful
derivatives, su~~h as promoters, as is well known to those
skilled in this art.,
It has now been discovered that the Lewellyn et al.
process can be rendered even more useful if an effective
amount of a co-~~ata:lyst is added to the reaction mixture.
Such co-catalysts, comprising salts of alkali metals and
alkaline earth metals of weak acids, such as, by way of
typical example, sodium acetate, sodium borate, sodium
phosphate, sodium carbonate, and the like, have been found
:25 to accelerate ithe rate of formation to the respective
alkoxy or arylo};y carbonyl isothiocyanates and to overcome
the deleterious effects of impurities, such as thiourea,
often encounterE~d in commercial alkali metal and alkaline
earth metal thiocyanate solutions. As will be shown
:30 hereinafter, judicious selection of the co-catalysts is
required because, unexpectedly, compounds such as ammonium
salts and quaternary ammonium salts have a decidedly
adverse effect.
2~.0~11~~
DESCRIPTION OF THE INVENTION INCLUDING PREFERRED
EMBODIMENTS
The present irm ention relates to a process for the
production of an alkoxy or aryloxy isothiocyanate which
comprises contacting a haloformate having the formula
O
R-O-('-X
wherein R is an alkyl radical., preferably a C1-C8 alkyl
radical, an alkene radical, preferably a C2-C4 radical, or
an aryl radical, preferably a C6-Clo aryl radical, and X is
a halogen, with an alkali or alkaline earth metal
thiocyanate under an appropriate rate of addition of
haloformate such as to prevent a run-away reaction, in the
presence of water,
(i) from about 0.1~ to about 10.0 by weight, based
on the weight of haloformate, of a catalyst comprising a
six membered mononuclear or ten membered fused polynuclear
aromatic, hete:~ocyclic compound having 1 or 2 nitrogen
atoms as the only hetero atoms in the ring; and
,(ii) from about 0.1$ to about 15.0 by weight, based
on~the weight of thiocyanate of a co-catalyst comprising
an alkali meta:L or alkaline earth metal salt of an acid
having a pKa of about 10 3 or below,
and at a temperature ranging from about -10~C. to about 40~C
for up to about 16 hours.
In preferred embodiments, the invention contemplates
such a process wherein R is an ethyl or a phenyl radical;
the haloformate is ethyl chloroformate; the alkali metal
thiocyanate is sodium thiocyanate; the catalyst comprises
a pyridine or a quinoline compound unsubstituted in the 2-
position; part9_cularly, pyridine or quinoline. Preferred
processes are those wherein the co-catalyst is a sodium or
potassium salt of a substituted or unsubstituted saturated
or unsaturated carboxylic acid, a substituted or
unsubstituted aromatic acid, carbonic acid, boric acid,
_5 2~~~~93
phosphoric acid, or a mixture of any of the foregoing;
especially proc<~sses wherein the co-catalyst is selected
from sodium ac~atate, sodium phosphate, sodium borate,
sodium carbonate, or a mixture of any of the foregoing.
Special mention is made of the process as above-defined
wherein R is ethyl, X is chlorine, the alkali metal
thiocyanate is sod'.ium thiocyanate, the catalyst is
quinoline, and t~ze co-catalyst is sodium acetate.
The reaction proceeds according to the equation:
ly O
O
R-O-C-X ~- MSCN H2-----~ R-O-C-NCS +
Cat.
wherein M is an alkali or alkaline earth metal and R and X
are as described above.
Both starting materials used in the process of the
present invention are well known and any method for their
preparation can be used. The alkali or alkaline earth
metal thiocyanate may be formed by the reaction of alkali
20 or alkaline earth metal cyanide with sulfur in the presence
of a phase transfer catalyst such as a quaternary ammonium
salt, see U.S. Pat. No. 4,482,500. It has been found that
commercial alkali metal isothiocyanate solutions commonly
contain impurities, such as thiourea and ammonium
25 isothiocyanates. Such .impurities have been found to have an
uncommonly adverse effE~ct on the reaction rate and to cause
deposition of heav~t precipitates during the reaction. Such
problems are readily overcome with the use of the co-
catalysts disclosed herein.
30 In following the process of the present invention, an
equimolar equivalent o:E the alkoxy or aryloxy haloformate
is carefully added to an aqueous solution of the alkali or
alkaline earth metal t.h:iocyanate in the presence of the
catalyst and co-catalyst under the above temperature
35 conditions. A preferred temperature range is 5~-15~C. A
preferred catalyst concentration is 0.5 to 5.0$, by weight,
-6-
based on the weight of haloformate used. A preferred co-
catalyst concentration is 0.1 to 5.0%, same basis.
Useful ha:loformates include the methoxy, ethoxy,
isopropoxy, n-butox:y,, isobutoxy, amyloxy, hexyloxy, 2
ethylhexyloxy, benzoxy, phenoxy, o- or p-tolyloxy, allyloxy
etc, chloro, bromo, iodo, etc., formates.
The catalysts employed in the novel process of the
present invention include pyridine, or quinoline,
pyrimidine, py.razine, quinoxaline and the like and
substituted derivatives thereof such as their alkyl, halo,
nitrite, a.lkox~~, etc . , substituted derivatp.ves . Any
derivative may be u:>ed except those substituted in the 2-
position.
The co-cata.lysts employed in the novel process of the
.L5 present invention include sodium, potassium, lithium,
calcium, magne~;ium, barium, and the like, salts of
saturated and unsaturated carboxylic acids, such as formic,
acetic, acrylic, met.hacrylic, butyric, valeric, hexanoic,
heptanoic, octanoic, palmitic, stearic, oleic, linoleic,
~~0 halo- or cyano-substituted carboxylic acids such as
chloroacetic acid or cyanoacetic acid, and aromatic acids
such as benzoic acid or naphthoic acids and the
corresponding halo or cyano or nitro substituted aromatic
acids may be used. Also useful are salts of carbonic acid,
25 phosphoricacid, boric: acid, and the like. The catalyst
and the co-catalyst can each comprise mixtures of any of
the compounds described.
The reaction is conveniently monitored by gas chro
matography, whereby samples of the organic layer are
30 periodically withdrawn from the reaction flask and injected
into the GC instrument for the disappearance of the
haloformate and the appearance of the corresponding
isothiocyanate. The reaction time can proceed for up to 16
hours and can vary from one to two hours or eight to ten
35 hours depending on the reaction temperatures, the amount of
the catalyst and the concentration of the aqueous alkali or
alkaline earth metal thiocyanate. The resultant
2~O~.i~3
_7_
isothiocyanates can be isolated from the reaction mixture
by the addition of sufficient amount of water to dissolve
the alkali or alkaline earth metal halide salt and the
organic layer :separated from the aqueous salt layer by
virtue of lower density. Besides GC, the individual
isothiocyanate rnay be characterized by IR, NMR and boiling
point. The infrared spectrum of the isothiocyanate includes
absorption bands at 1960-J_990 cm 1 for the N=C=S group,
together with pE~aks at 1750 cm 1 and at 1220-1260 cm 1 for
:LO the C-0 group in i~he alkoxy or aryloxy moiety of the
molecule. Boiling points of recovered exemplary
isothiocyanates are essentially the same as those reported
in the literature. They are given below:
PRODUCT b.p./torr
7.5 Methoxycarbonyl isothiocyanate 30~C/12
Ethoxycarbonyl isothiocyanate 26~C./1.8
Butoxycarbonyl isothiocyanate 60~C/6
Phenoxycarbonyl isothiocyanate 87~C/27
The following examples are set forth for purposes of
20 illustration only and are not to be construed as limita-
tions on the present invention except as set forth in the
appended claims. All parts and percentages are by weight
unless otherwise specified.
FYBMDT L' 1
25 To 162 pari~s of a 50~ aqueous solution of sodium
thiocyanate are added 4.37 parts of refined grade
quinoline. The reaction mixture is cooled to 10~C under
constant agitati~~n, and ethyl chloroformate 110 parts, is
carefully introduced to the reaction mixture through a
30 calibrated addition funnel in 10 minutes. The reaction
temperature is kept at 10~C throughout the procedure with
ice water cooling. Samples of organic layer are
withdrawn hourly from the reaction flask and inspected
with the GC instrument for the disappearance of the ethyl
35 chloroformate and the appearance of the ethoxycarbonyl
isothiocyanate. For the purpose of comparison, the
reaction time is defined as the time required for the
2~ 02~~~
_8_
ethyl chloroFormate content to decrease to 2~. Using
this procedure <~nd an impurity-free reagent grade of
sodium thioc~~anat.e and adding sodium acetate as a co-
catalyst in an amount of 2.98 parts/162 parts of 500
sodium thiocyanate, the reaction time is 5.0 hours and
no precipitate is formed. If no sodium acetate is added
as co-catalyst, the reaction time increases to 6.0 hours
and a slight precipitate is formed.
To test the utility of a given additive, the
foregoing procedure is repeated, but a weighed amount of
the given additive is added as part of the 50~ sodium
thiocyanate solution. The procedure is conducted under
the identical conditions using the same lot of quinoline
and ethyl chloroformate. In addition to the reaction
time, the amount of formation of a precipitate which as
been identified as the quinolinium thiocyanate complex
salt, an undEairable impurity, is monitored at the end
of each reacl~ion., The additives used and the results
obtained are set forth in Table I:
25
35
~~.~119~
C1
1) J ~ yJ
O
~ ro ro
7 .~ ~ 1~ ~nT ~ ro >~>, >. ~ >, 1
b '.r ~
- U U' 47 v .C iD> p~ T > > > ~ >
> C
x ~ '~ G tT ~:)ro Q7 > roro ro b
p~ o o - o
H a -~ ~ ..~n>a~ o o ~ ~ a~ u~ ~
p
W G.G.. ~ ._L F E .c .~~ L c ~ F
a
z
w
~.
z
c . . - ~ . . . .
_ m . m m m m
m m -
U ~ m ~ ~ is m sJ y 1J y a m is ~ s .c
, .
O U ~ ~ ~ ~ '_a.t _C .~ , .Cs_'.G y n
~
~ ~:' "' u, p "'
x cd ~ o O m ~ rn ,n O .
~
E-~O - . . CV C N . . . ~ '-1 py
. ) CO
r~ r <P ~'~1 Oo
~ Cnn
:7
v-
~
q
z
U
c7
z ro
H z
H
U ~
da
+~
o
W Ul
V1
a
Hw
0
m v
>m
W .a
+~
H ~
a
z .
ro
t0 CV
YW . CO tD M C- <V V (p O O ~
N
U ~ N
O O N N O C:) O O O ~ ~ N
\ roro
x u~ _ , ~ '
O ~~ to1 4l U U
z z i " ' ' J
U ~
~ m m m o 0 0 0
ro o o ~n ' z
~ e ~ ~ ~ -.J..Jz ~a z
H a '~ ~ x :~o m m m .c~ z
a
z z z ,._H H E H H N
z
z z
U
U U
m ~
Vi V]
v v
'~ 'a
x x
U JJ I I I , H I H
W -..1 . z z
V7
x a ~ o o p p I , I 1 I I dry o ~~ I
ro o 0 0 0 0 o
o a ~ v 'o .d .d.~ ~o 'o 'a w o N 'd N o
z ~ o
C-x-rE Z I I I I I I I I I 1 c.~ t C,~ 'o
~ N o
H W p p
'a O O
U
z ,~ ,--m , ,--a
o
~ ~a ,~ m
H
..-I--1 ..J --I
H
~ ~ ~ ~ +~ ~I s-~ ~, ~
~ ~ m m
Z C C ~ t~t~ 1~ +~ 1' +~
I ~ U m U m
p ~
I U ~ N N N m ' ~
'
41 I t ~ tP O~ L1~aTCP ~ b ~ ~
LL T1 O O b ~ t3 b
W ' '
p, p O o ro ro ro c0of 'p t7 rt1 p
H V7 'O 'O - ~ ~0 ~ ro
td
~ p ~ x ~ ~ N a7~ ~ C Sr C
I f 1.J S-I iJ
(1az QJ I I Z !x I~ CL R:R: H H H H
U ~ U U C~ U
P
.
H O
[x,
U
W
(x
ao
ma
" " " ' ' ' ' ' " '
~ x ~ rs m U o w ~. c>x H
x
H W .-i.--~N c~ r'7M r1 M M M M c~ M
U
2~~11~3
-10-
a~
+~ v a~
b ,~
a
>, ~ ~' ~ +~
~ c o
b . ,
'
' ~ ~; -
~ E C E '~
N ' . m m
O m
P~
C4
m m m
m
m
O
S-,
-~ ~ -C
U ~n o G
O
N , ~ O O O
O
c~ n w
H
a, ,-~
z
U
ro
z
~ O
O
N m
m ~
~n
a' co - ~ o .-,
N
m p
O U O N
-'i U ~ P
~
~ ~
a z p o b ~ roN roN
ro
z z z z
aN
m
ro
a.
z z
z U z U
U cn U v7
w
U ~ Z H
a
~ E x
x .,
+~ H Z H
V) z
-.1 ~ dv eP
ro dP
~Iz ~~ ~,o~ N~
,n I ~ I o~ N I I
a0 I ~ I N
N ~ N ~ N N
O ~ O ~, O O
.
~ T3
H O I O I G O 1 I
I O I O I O O
N
- -.i
E
O
la
-a .~ .-~ .-~ .-, E
ro ro ro ro m ro ro
-'' -a
zap ,,U s~U ~,a uo >am ~m s~m
'bU 'O i, +~ ~ a~ a~ ~ a.~ E O
v V7 a7 m N m m m m v
b iv a) U v
m m - - ~
a ro ab a~ a~ a a av a
s.~ o v o
c z c~ .~ 'o .n ~ a v v ro w ro
ro ro ro ro ro ro
'-1 ~ C C C C C C SJ
S-, N 1-1 1-1 S-I 1J
J W --~ H N H ~ H H U
C~ C~ (7 C7 a C7
C~
ro
~ ~ Q)
U
ro ro a
,, ~ o
~,a~ -a
ro i,
.-a a al H
,
W >r H
o
U
7C ~ r~
H W m w p ~ r m a . .
2I0~1~~
_,I_
The data in Table I show the following:
Comparative E};ample No. lA*: This is the control
experiment using Reagent grade NaSCN which contains no
impurities.. The re;_~ction time observed is 6 hours.
Example Nos . 1 and _2 : These two runs ( in comparison
with lA*) demonstrate the rate acceleratin ef
g fects of
sodium acei~ate. In ~:~cample 1 a 16.7 reduction in cycle
time is achieved with the use of NaOAc. In Example 2 there
is shown a levelinc_~ effect when the amount of NaOAc is
7.0 doubled.
Comparative Exam le Nos. 3A*, 3B*, and 3C* These
three procedure; show the adverse effects of NH4SCN on
reaction rate. Even in small concentrations, the rate is
decreased and the rEaaction time is increased to 8.25 and
9.0 hours at the 0.3E~ and 0.72 part levels. Furthermore,
when NaSCN is cornplet:ely replaced with NH4SCN, the reaction
is extremely slow (>.24 hrs).
Comparative Example Nos . 3D*, 3E*, 3F* ~ These three
procedures demonstrate the adverse effects of a phase
transfer catalyst such as tetrabutylammonium bromide
(TBA$). The reaction time increases and moderate to heavy
precipitate formation is observed.
Comparative Example Nos. 3G* and 3H*~ These two
procedures show the adverse effects of thiourea (TU) on
2!i both reaction rare and the precipitate formation.
Comparative Exa~le No. 3I* and Example No 3,
Comparative Exam~~le No. 4A* and Example No. 4, Com arative
Example No. 5A* a.nd Example No. 5, and Comparative Exam le
No. 6A* and Exam~le N_o. 6: These eight procedures or four
pairs of experiments are conducted using four different
lots of commerc~La115r available, industrial grade NaSCN
solution in which impurities such NH4SCN and thiourea are
known to be present in varying amounts. The reaction times
for Comparative hxample Nos. 3I*, 4A*, 5A*, and 6A* vary
from 9 1/2 to 10 hrs. In addition, heavy precipitate
~~~~11~
-12-
formation is observed. However, when sodium acetate is
added to the reaction, in accordance with the present
invention, in Example Nos. 3, 4, 5, and 6, the reaction
time is reduced to 7.25 to 8.0 hrs. with a resultant
reduction in manufacturing vessel usage of 15.8, Example
No . 3 , to 2 3 . 7 '~ , E;~cample No . 5 . At the s ame t. i me only a
moderate preci~~itate formation is observed with use of
sodium acetate co-cat:alyst.
Examples tJos. 7, 8, and 9: Using the same lot of
industrial grade NaSCN as Example No. 4), the co-catalytic
effects of a trisodium phosphate salt, Example No. 7, of a
sodium tetraborate, Example No. 8, and of a sodium salt of
carbonic acid, Example No. 9, are evaluated. All three
procedures show that the adverse effects of thiourea and
NH4SCN on rea~ctio~n rate are essentially eliminated.
Furthermore, the observed precipitation formation is only
slight.
EXAMPLE 10
The procedure of Example 1 is again followed except
that the quinoline is replaced by pyridine. Substantially
the same results are obtained.
EXAMPLE 11
Commercial grade quinoline which contains about 5~
isoquinoline is used in place of the pure quinoline of
Example 1. Similar results are obtained.
EXAMPLES 12-14
The procedure of Example 1 is again followed except
that the catal~~sts are varied. Instead of quinoline, the
following are used: 6-methoxyguinoline, 6-chloroquinoline,
and 4-chloropyridine. Substantially the same results are
obtained.
EXAMPLE 15
The procedure of Example 1 is again followed except
that potassium thi.ocyanate solution (50g) is used.
Substantially t:he game results are obtained.
~~~~~~3
- 13
EXAMPLE 16
Again following the procedure of Example 1, sodium
thiocyanate solution (50~) is reacted with 2-ethylhexyl-
chloroformate. The resultant 2-ethylhexylcarbonyl
isothiocyanate is recovered with no substantial precipitate
formation.
EXAMPLE 17
Following the procedure of Example 16, n-octyl
chloroformate is used in place of the 2-ethylhexyl
chloroformate, all else remaining equal. An excellent yield
of n-octylcarbonyl isothiocyanate is recovered, with no
precipitate formation.
EXAMPLE 18
Again following the procedure of Example 16 except
that ethyl bromoformate is employed, similar results are
achieved.
EXAMPLE 19
To 162 Parts of a 50~ aqueous sodium thiocyanate
solution are added 2.0 parts of quinoline and 2.8 parts
of sodium acetate. With stirring, the mixture is cooled
to 8°C with ice/water. Phenylchloroformate (78.3 parts)
is introduced dropwise in 1 1/2 hours. The reaction is
carried out <~t l.OoC, and monitored for completeness by
GC. The p:roduca, phenoxycarbonyl isothiocyanate is
recovered in good yield and with only a slight
precipitate formation.
EXAMPLE 20
The procedure of Example 19 is repeated substituting
for the phenylchloroformate 60.3 parts of allychloro
formate. All:yloxycarbonyl isothiocyanate is produced in
good yield. Similar results are achieved.
The abovEa-mentioned patents, any application(s), and
publications are incorporated herein by reference.
Many variations in the present invention will sugg-
est themselves to those skilled on this are in light of the
-14-
above, detailed description. All such obvious variations
are within the full intended scope of the appended claims.