Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- 1 - ~ ~ ~ ~ ~ ~ ~ ND-A852/PCT
DESCRIPTION
Phosphate Chemical Treatment Method
TECHNICAL FIELD
The present invention relates to a phosphate
chemical treatment method by which a phosphate chemical
film is formed on a metal surface, and more specifically,
it relates to a treatment method by which a phosphate
chemical film is formed on an electroconductive metal
surface.
BACKGROUND ART
Methods of phosphate chemical treatment have been
used in the past in various fields including surface
preparation treatment before point-coating, pretreatment
prior to cold working, and the like.
For example, in Japanese Unexamined Patent
Publication (Kokai) No. 60-208479 there is disclosed a
method for acid phosphate chemical treatment of iron,
steel, zinc and/or aluminum surfaces.
Also, Japanese Unexamined Patent Publication (Kokai)
No. 64-68481 discloses a method for the phosphate
chemical treatment of steel and/or galvanized steel, or
of metals consisting of aluminum and steel and/or
galvanized steel.
Also, Japanese Unexamined Patent Publication (Kokai)
No. 2-190478 discloses a chemical treatment bath
containing Fe+' ion in a method of forming a phosphate
film onto aluminum surfaces.
Also, in Japanese Unexamined Patent Publication
(Kokai) No. 4-120294 there is disclosed a method of
forming a phosphate chemical treatment coating as a
surface preparation treatment before point-coating for
stainless steel, in which the phosphate coating is formed
by applying a PR (periodic reverse) pulse electric
current to the stainless steel for electrolysis in a
phosphate chemical treatment bath.
- 2 _ ~1~.2~92
However, regarding methods for phosphate chemical
treatment according to the prior art, there are many
known methods of forming phosphate chemical coatings onto
materials to be treated other than iron, as described in
Japanese Unexamined Patent Publication (Kokai) No. 60-
208479, Japanese Unexamined Patent Publication (Kokai)
No. 64-68481 and Japanese Unexamined Patent Publication
(Kokai) 2-190478, etc., but problems have been caused by
the need to change the components of tl:e phosphate
chemical treatment bath and the conditions at the time of
treatment, depending on the type of the material to be
treated. In addition, the components and conditions for
the phosphate chemical treatment bath are extremely
critical, and not at all practical.
Furthermore, as in Japanese Unexamined Patent
Publication (Kokai) No. 4-120294, the possibility has
been known of forming phosphate chemical treatment
coatings even onto materials to be treated other than
steel, such as stainless steel, by subjecting the
material to electrolysis in a phosphate chemical
treatment bath, but such coatings are still limited to
the formation of very thin films, such as surface
preparation treatments before paint coating.
The present invention was accomplished with the
object of overcoming the above mentioned problems, and
its purpose is to provide a method for phosphate chemical
treatment which makes it possible to produce a phosphate
chemical coating of adequate film thickness onto any
metal surfaces regardless of the degree of electric
conductivity thereof.
DISCLOSURE OF THE INVENTION
The inventors of the present invention have
conducted diligent research regarding the question of why
the complicated conditions described above are necessary
for the treatment of surfaces other than iron in the
methods for phosphate chemical treatment according to the
prior art, and further regarding why a method of
- 3 -~112~9~
treatment capable of providing an adequate thickness is
not possible, and as a result we have pinpointed the
cause thereof, and have also discovered a means of
overcoming that cause.
In other words, according to the methods of
phosphate chemical treatment of the prior art, those
methods in which the material to be treated was steel
have been simply applied in the same manner for other
materials to be treated, and thus it was thought that the
treatment conditions for materials other than steel are
extremely critical, and that phosphate chemical treatment
coatings could only be formed onto composite materials
which include steel.
Here, according to the present invention, first the
process of forming phosphate chemical treatment coatings
was investigated in detail, considering the phosphate
chemical treatment reaction from the following two points
of view.
Since the chemical reaction by which the phosphate
chemical treatment coating is formed may be understood to
be an electrochemical reaction, the first analysis was
made from the standpoint of the "chemical reaction".
Also, a second analysis was made regarding the
phenomenon of the "phase transition". This refers to the
phenomenon occurring in the phosphate chemical treatment
reaction by which the soluble component (liquid)
undergoes a chemical reaction to become a film (solid ).
Regarding both of the investigations (analyses)
mentioned above, it should be noted that the First and
Second Laws of Thermodynamics play an important role in
the phenomenon.
A detailed description of the results of the
investigation are provided below.
First we will give the analysis from the point of
view of the chemical reaction.
Phosphate chemical treatment is a kind of so-called
chemical coating treatment method by which a coating is
- 4 _ ~11~~~~
formed onto a metal surface using a chemical reaction
between the metal surface and a chemical solution. Also,
the chemical treatment solutions used are aqueous
phosphate solutions containing coat-forming metal ions
such as iron, manganese, nickel, calcium, zinc, etc.
Phosphate chemical treatment methods may be
considered as comprising a step of an etching reaction on
a steel material and a step of a coat-forming reaction to
form a coating. These are electrochemical. reactions,
consisting of a cathode reaction involving the reduction
of nitrate ion, etc., for example:
[Chemical Equation 1]
N03 + 3H+ + 2e -- HNOZ + HZO
[Chemical Equation 2]
HNOZ + H+ + a ~ NO + HZO
and an anode reaction involving the dissolution of the
metal (etching) (Chemical Equation 3) and the forming of
the coating (Chemical Equation 4):
[Chemical Equation 3)
Fe -. Fez+ + 2e -. nH (exothermic reaction)
[Chemical Equation 4)
3 ( Zn2+, Fe2+) + 2HZP0~
(Zn, Fe)3(P04) + 4H+ (endothermic reaction)
In addition to the Chemical Equations 1-4, the
balance-maintaining reactions in the chemical treatment
bath include:
[Chemical Equation 5)
H3P04 ~ HZP04 + H+
[Chemical Equation 6)
40H- ~ OZ + 2H20 + 4e
[Chemical Equation 7)
N03 + 3H+ + 2e ~ HNOZ + Hz0
It is thought that the reaction in Chemical Equation
3 acts as the main reaction in most non-electrolytic
chemical treatment reactions of steel materials, and the
coating is formed when the reactions in Chemical
Equations l, 2 and 4 utilizing the internal energy (nH)
- z~~.z~~z
released into the solution by the reaction in Chemical
Equation 3, occur on the surface of the metal material
(solid). Therefore, if additional energy such as heat,
etc., cannot be input into the reaction system (i.e., the
5 chemical treatment bath), then the forming of the
chemical coating is accomplished by the reduction
reaction on nitrogen-containing oxoacid ion such as
nitrate ion, etc., represented by Chemical Equations 1
and 2, and the oxidation reaction consisting of the
dissolution of iron and the oxidation of phosphate ion
represented by Chemical Equations 3 and 4.
Thus, the non-electrolytic forming of chemical
coatings according to the prior art in which no
additional energy is supplied is carried out using only
the energy (eH) released by the dissolution of the metal
material, and no chemical coating is formed beyond the
energy (eH) released by dissolution.
In contrast, the dissolution reaction in cases where
the metal material used is a non-iron metal such as
aluminum, copper, or the like is as follows.
[Chemical Equation 8]
M ~ Mn+ + ne
However, if the aluminum, for example, is immersed
into a phosphate chemical treatment bath for steel
materials, a pasivation film is formed on the surface of
the aluminum, and therefore the aluminum does not
dissolve in the phosphate chemical treatment bath, thus
prohibiting the reaction in Chemical Equation 8. As a
result, the energy expected to be generated by the
dissolution of the aluminum surface is not produced.
In the past, when aluminum has been used as the
metal material, it has been considered preferable to
introduce fluoride ion (F-) into the chemical treatment
bath in order to promote the dissolution reaction in
Chemical Equation 8.
Furthermore, when copper (Cu) has been used as the
metal material in the same manner, it has been considered
~~12~9~
- 6 -
best to introduce a halide ion other than a fluoride ion,
for example, chloride ion (C1 ), into the chemical
treatment bath.
Nevertheless, as described above, even if the metal
material is dissolved, it has not been possible to form a
favorable phosphate chemical treatment coating onto these
base metal. materials.
The reason for this is that, as described earlier,
when employing the conventional non-electrolytic methods
and electrolytic methods in treatment baths containing
sludge, no technical thought has been given regarding the
use of energy for the effective promotion of the entire
system of phosphate chemical treatment reactions in
Chemical Equations 1-8 described above, for common metal
materials other than steel (such as stainless steel,
copper, etc.). Consequently, no concrete measures have
been undertaken for the control of the entire reaction
system.
In other words, in the case of aluminum materials,
the dissolution reaction
[Chemical Equation 9J
A1 ~ Al+3 + 3e
replaces Chemical Equation 3 for steel, but in such cases
it has been discovered that sufficient energy cannot be
supplied to form the coating, for the reasons given
below.
(1) Chemical Equation 9 proceeds at an extremely
low rate if F- is not added, and the energy produced
thereby is also extremely low, and therefore the entire
reaction system is not established.
(2) If F- is added then Chemical Equation 9
proceeds at a sufficient rate, but a complex (A1F4-)
forms between the resulting A13+ and F- ions and becomes
stable in the solution, thus prohibiting the coat-forming
reaction with aluminum which replaces Chemical
Equation 4.
As described above, it has been discovered that, by
~112~~2
considering the chemical react_Lon of the forming of
phosphate chemical treatment coatings as an
electrochemical reaction, and simply attempting to
promote the reaction of Chemical Equation 8 by the
addition of some chemical component, as according to the
prior art, it is impossible to form phosphate chemical
treatment coatings onto metal materials or
electroconductive materials other than steel.
The following is an analysis from the point of view
of the phenomenon of the phase transition occurring in
the phosphate chemical treatment reaction.
That is, the present inventors have considered the
phosphate chemical treatment reaction to be basically a
"liquid phase-solid phase" reaction in which the soluble
component ion (liquid) in the solution undergoes a
chemical reaction to become a film (solid), believing
that it may be understood in terms of a phase transition
phenomenon.
However, the inventors were unable to explain the
phosphate chemical treatment reactions according to the
prior art in this manner, as a type of phase transition
phenomenon.
This is because, in the treatment baths according to
the prior art, the chemical treatment reaction is not
adequately controlled, and therefore a plurality of
different chemical reactions occur simultaneously in the
phosphate chemical treatment bath, including a portion
other than on the surface of the material to be treated.
When a plurality of different chemical reactions occur in
this manner, not merely a single "liquid phase-solid
phase" reaction, but additional multiple "liquid phase-
solid phase" reactions and "liquid phase-liquid phase"
reactions also occur in the bath. As a result, sludge is
included in the treatment bath. Consequently, the energy
transfer between the reactions becomes complicated, and
thus it is impossible to explain the forming of the film
on the metal surface in terms of a phase transition
?~.~.~aJ2
phenomenon.
In other words, a thermodynamic analysis of the
phase transition phenomenon is easily understood with a
single-component system, such as water, but with multiple
components in a complicated chemical reaction such as the
reaction in a phosphate chemical treatment bath, it is
very difficult to understand.
Here, the present inventors have discovered that the
reaction in a phosphate chemical treatment bath may be
considered in terms of a phase transition phenomenon by
simplifying it to a physical phenomenon. That is, the
bath is controlled to maintain a state comprising only
liquid, so that the only reaction occurring in the
phosphate chemical treatment bath is that of formation of
the film (solid) from the components in the solution
(liquid). Also, since the chemical reaction in the
phosphate chemical treatment bath occurs in only a single
phase (liquid) and a film (solid) is produced thereby,
the phosphate chemical treatment reaction may be
considered to be a phase transition phenomenon. Further,
it was thought that by utilizing this in a concrete
manner, it might be possible to discover a means for
chemical film formation which is fundamentally different
and more effective than the conventional ones.
A concrete explanation will now be provided
regarding the contents of the analysis in terms of a
phase transition phenomenon.
To begin with, phosphate chemical treatment entails
contacting a metal material (solid) which is to be
treated, with a solution (liquid) containing the
components which form the film. Therefore, the reactions
involved in the chemical treatment may be largely
classified as:
(1) A reaction (solid phase-liquid phase reaction)
between the metal material (solid phase) and the solution
(liquid phase).
(2) A reaction between the components in the
:l.l~a~~
_ g _
solution (liquid phase-liquid phase reaction).
Also, upon examination from the standpoint of
thermodynamics, it is found that the phase transition
phenomenon (liquid ~ solid) more easily occurs by the
action (reaction) between the solid phase-liquid phase,
than by the action (reaction) between the liquid phase-
liquid phase. Likewise, for example, the condensation of
moisture in the air occurs more easily onto solid
surfaces (solid phase-gaseous phase) than onto the same
phase (gaseous phase-gaseous phase), and this will be
easily understood by considering two examples thereof,
dew and frost.
In other words, the deposition of a solid by a
"liquid phase-liquid phase" reaction in the solution can
only occur by adding a larger amount of energy to the
reaction system than is required by the "solid phase-
liquid phase" reaction on the surface of the substance to
be treated.
Therefore, based on the above facts, the present
inventors, considering the reaction in a phosphate
chemical treatment bath in terms of a phase transition
reaction, restricted the energy applied to the chemical
treatment reaction system to a range in which no reaction
(phase transition) could occur between the liquid phase-
liquid phase, while controlling it in a range in which a
reaction (phase transition) could occur between the solid
phase-liquid phase, and have thus first discovered the
fact that it is possible to limit a chemical treatment
reaction to the "solid phase-liquid phase" transition
phenomenon (film formation).
Further, considering the conventional method (method
of heating the treatment bath) from the standpoint of the
phase transition phenomenon, when energy is applied to
the treatment bath for the formation of a phosphate
chemical treatment coating onto the material to be
treated, since the chemical reaction in the bath is not
adequately controlled, reactions (phase transitions)
~1~.259~
- 10 -
other than the one on the surface of the material to be
treated occur due to the excess energy, and therefore
sludge is formed in the bath. As a result, a plurality
of solid phase-liquid phase transitions occur in the
treatment bath. Consequently, the externally supplied
energy cannot be used in any way to control the film
thickness of the phosphate chemical treatment coating, as
it simply accelerates the production of more sludge, and
thus it is difficult to form a favorable phosphate
chemical treatment coating onto the surface of the
material being treated.
Thus, by analyzing the reaction in phosphate
chemical treatment baths from 2 points of view, that is,
from the point of view of both the chemical reaction and
the phase transition phenomenon, it became possible for
the first time to understand why favorable phosphate
chemical treatment coatings with adequately controlled
film thicknesses have not been able to be formed onto
metal materials and electroconductive materials other
than steel, using the methods according to the prior art.
Furthermore, based on the analyses described above,
the present inventors have discovered how it is possible
to form phosphate chemical treatment coatings with
adequately controlled film thicknesses onto
electroconductive metal materials.
Based on this background, the present inventors
determined that the phosphate chemical treatment reaction
is essentially an electrochemical reaction system and the
control of the reaction should be considered with this
idea as the basis.
In other words, we have succeeded in discovering a
completely novel method of forming a phosphate chemical
film onto an electroconductive metal surface by
contacting the metal material with a phosphate chemical
treatment solution containing at least phosphate ion, a
nitrogen-containing oxoacid ion and a chemical film-
forming metal ion, wherein the phosphate chemical
_ 11 - ;~~12~9~
treatment method is carried out in a phosphate chemical
treatment bath which contains no solid matter other than
the unavoidable components, and involves electrolytically
treating the above mentioned metal material in the above
mentioned phosphate chemical treatment bath.
As a concrete means, the method used (1) the removal
of solid matter (sludge) from a chemical treatment bath
and (2) an external electric power source for the
reaction.
Here, the statement that the phosphate chemical
treatment bath contains no solid matter other than the
unavoidable components is used to mean that the bath is
free of any sludge which might cause energy instability,
that is, the bath is free of suspended particles which
are reactive and could interfere with the reaction.
The reaction of the electrolytic treatment according
to the present invention accelerates the reactions in
Chemical Equations 1-8 by supplying electrical energy
from the above mentioned external electric power source,
and in this point it differs greatly from conventional
electroplating and anodic oxidation.
The anodizing, which is one of the reactions
accompanying the supplying of energy from the external
power source according to the present invention, promotes
the dissolution reaction of the material to be treated
(Chemical Equations 3 and 8), in cases where it does not
proceed naturally or adequately under the thermodynamic
conditions of the solution, by applying electrical energy
to the system, and thus the entire reaction system
including Chemical Equations 1-8 is promoted to form the
film. The anodizing accelerates the dissolution reaction
of the material to be treated, and therefore it is
effective for guaranteeing the adherence of the resulting
chemical film.
The cathodizing, which is the other reaction which
accompanies the supplying of energy from the external
power source according to the present invention,
12 _ ~1~~~~~
guarantees the thickness of the chemical film formed, by
acting on the component ions in the solution phase and
depositing them onto the cathode. Consequently, since
the dissolution reaction of the metal material to be
treated does not occur by cathodizing alone, the
cathodizing is preferably performed after the anodizing.
In cathodizing, the film-forming metal material such as
zinc, etc., which is used at the anode is dissolved and
reacted with the phosphate ion or nitrate ion in the
solution phase to form a film on the surface of the
cathode (the material to be treated).
As a result, if the material to be treated which is
connected to the cathode is an electroconductive
material, then a phosphate chemical film may be formed on
the desired metal material to be treated, by cathodizing
using the specified metal material and chemical products
which contain the chemical components relative to
phosphate, etc., for the anode and the solution phase.
Also, the cathodizing is preferably carried out after the
anodizing, and thus a phosphate chemical film with
excellent adherence may be formed onto common materials
other than steel, such as stainless steel, magnetic
materials, aluminum, copper, and the like.
Here, the anodizing definitely causes the
dissolution reaction for materials capable of forming
films, and thus it is effective for accelerating the
formation of films. Also, application of the anodizing
alone increases the adherence of the film, but since it
does not create a large film thickness, it is effective
for surface preparation treatment for paint-coating, etc.
of steel materials. Further, by the combined use of
anodizing and cathodizing (anodizing -- cathodizing), the
technique according to the present invention allows the
formation of phosphate chemical films of adequate
thickness with guaranteed adherence onto all kinds of
metal materials.
For example, it may be used to produce thick
phosphate films as inorganic insulation films, insulation
films onto magnetic materials, lubricating foundations,
rust prevention, surface preparation for painting,
adhesion and plasticizing, etc. of aluminum, and the cold
forging lubricating foundation, surface preparation for
painting of stainless steel, etc.
The present invention is limited only to soluble
components (H3P04, N03 , HNO2, metal ions such as Zn2+,
etc.) with no sludge, in chemical treatment baths, and
the substance to be treated and the electrode are placed
in the treatment bath and an external power source
connected between them, thus applying an electrical
current between the substance to be treated (work-piece)
and the electrode.
Further, the phosphate chemical treatment bath is
controlled so that sludge is not produced therein.
Here, the control of the phosphate chemical
treatment bath may be accomplished by, for example, the
following method.
That is, the phosphate chemical treatment is
preferably carried out by employing a means for
controlling the input of energy into the chemical
treatment bath (temperature control, control of the
pressure to the liquid by controlling revolving speed of
the circulation pump, stabilization of the state of
energy in the solution by alternating between a state of
reaction in the treatment bath and a state of no reaction
therein) and filtration, etc., to create and maintain a
condition in which no sludge is formed in the chemical
treatment bath, and thus limit the phase transition
phenomenon in the treatment bath to only the formation of
the coating onto the surface of the metal being treated.
Also, according to the present invention, it is
preferable to equip the phosphate chemical treatment bath
cell with a filtering circulation pump and a filter.
The first purpose of the filtering circulation pump
and the filter is the stabilization of the thermodynamic
- 14 -~I~.~~i~~
energy state of the solution phase of the reactive
solution. If the reactive-chemical-components of the
treatment bath remains in a location which allows a
constant reaction site (if there is no circulation
alternating between the "non-reaction site" and the
"reaction site"), then the thermodynamic energy will
accumulate in the solution phase as the chemical
treatment reaction proceeds. As a result, the stability
of the treatment bath solution phase as a liquid will be
lost, and solid matter (sludge) will be produced in the
solution phase. The filtering circulation pump and the
filter are provided to prevent a loss of the
thermodynamic stability of the solution as a liquid.
Therefore, the filter itself has a specific volume, and
more than simply functioning as a filter, it maintains
the non-reacting state of the treatment bath for a
specific period of time, and thus contributes to the
thermodynamic stability of the solution phase of the
entire reaction system.
The circulation of the treatment bath to alternate
between the "non-reaction site" and the "reaction site"
for maintenance of the thermodynamic stability of the
solution phase should be considered for the entire
reaction system of the phosphate chemical treatment bath
(Chemical Equations 1-8), but as a representative
example, an explanation is provided below regarding the
equilibrium state of phosphoric acid.
A phosphate chemical treatment bath is a solution of
pH (hydrogen ion concentration) of 2-4 which contains a
large amount of phosphoric acid. At pH of 2-4, the
phosphoric acid exists in the solution in a state of
equilibrium of Chemical Equation 5.
Also, as the chemical treatment (film forming)
reaction progresses, Chemical Equation 5 proceeds to the
right. This is because the formation of the film occurs
by the bonding of the phosphate ion which is
dehydrogenated by H3P04 -~ HZP04- -a PO43- with metal ions
15 - ;?~12~9~
such as Zn+2 and the like, forming Zn3 ( P04 ) 2 . I f the
solution simply remains in the treatment cell without
being circulated, then the components in the solution
change such that Chemical Equation 5 shifts to the right.
As a result, the chemical treatment reaction system in
the solution phase (Chemical Equations 1-7) tends to
produce sludge.
On the other hand, if the treatment bath is
circulated, the phosphate ion in the solution, separated
from the treatment cell, acts in a direction to restore
the state of equilibrium (shifting Chemical Equation to
the left), which is the direction stabilizing the
thermodynamic energy state in the solution.
Thus, the deposition of sludge in the solution phase
is suppressed.
The filtering circulation pump is preferably
operated while controlling the revolving speed thereof.
Operating the circulation pump at a high revolving speed
applies a high pressure to the solution phase. As a
result, the energy of the solution phase increases to a
point where the solution phase can no longer be
maintained in a liquid state, and finally solid matter
(sludge) is deposited. Conversely, if the revolving
speed is too low, then a large-capacity pump must be
provided, thus raising the cost. Therefore, if the
circulation pump is a conventional centrifugal pump, an
inverter is preferably used to appropriately control the
revolving speed, in order to suppress the pressure of the
solution phase while ensuring the proper circulation
volume.
The second purpose of the filtering circulation pump
and the filter is the removal of sludge which is produced
in the treatment bath. If the produced sludge,
particularly energy-unstable sludge, is allowed to
remain, then the treatment bath tends to produce even
more sludge. It is thus preferable to rapidly remove
sludge which is produced.
- 16 -
Also, the temperature regulation of the chemical
treatment reaction system is preferably effected slowly.
The temperature of the chemical treatment bath
according to the present invention is in a range of about
20-35°C. This temperature range is roughly in the range
of normal room temperature, and of normal aqueous
solutions. However, heating will be required during the
winter to maintain the prescribed temperature. What is
important according to the present invention is not the
use of heating to accelerate the reaction. That is, the
temperature range of 20-35°C for the chemical treatment
reaction system is a necessary condition for the control
of the chemical treatment reaction, and it is not
directly used as thermal energy for the chemical
treatment reaction. Presently, the method of heating the
phosphate chemical treatment bath to a temperature of
40°C or greater involves placing a heat exchanger into
the chemical treatment bath to provide steam as a heat
source for direct heating of the chemical treatment bath.
In this method, since the vicinity of the heat exchanger
rises to a very high temperature, the decomposition of
the components in the chemical treatment bath is
accelerated by the heat in that area, and sludge is
produced. In this point, the significance of the heating
clearly differs.
In the method according to the present invention,
the suppression of sludge production is the first
consideration. Therefore, the introduction of a direct
heat source into the chemical treatment bath is not
preferred. Also, the chemical treatment bath should be
warmed as slowly as possible, and indirectly.
Specifically, the preferred method is to provide a heat
exchanger in the treatment bath circulation cycle of the
electrolytic chemical treatment reaction system, and to
effect warming while the circulation pump is in
operation. Also preferable is a method by which the
entire treatment cell is surrounded by water at about 30-
- 17 -
40°C.
In the method according to the present invention,
the hydrogen ion concentration (PH), the oxidation-
reduction potential (ORP), the electric conductivity (EC)
and the temperature, etc., of the chemical treatment bath
are preferably measured, and the chemical solution added
in response to changes therein, to constantly maintain
each of the component ions in the chemical treatment bath
within the prescribed concentration ranges. Also, the
sensors for the pH, ORP, EC and temperature are
preferably located away from the treatment cell.
According to the present invention, an electrolytic
reaction occurs in the treatment cell using an external
power source. Therefore, the electric current flowing in
the treatment cell influences nearby sensors, making the
display of accurate values impossible.
It is most preferable to control the bath in the
manner described above so that absolutely no sludge
accumulates in the phosphate chemical treatment bath, but
even if reactive substances have accumulated at the
bottom of the treatment cell after the reaction has
reached an energy-stable state, as the unavoidable
components of the solid matter in the chemical treatment
bath, the bath itself may simply be kept free of
impurities. This is because such stably accumulated,
energy-stable sludge exerts practically no influence on
the ion components in the solution in which the reaction
takes place.
In the case of the present invention, since an
electric current is applied to the treatment bath, the
treatment bath exists in an electric field which is in a
saturated state due to the constant application of
electrical energy, and therefore the solid matter
produced therein continues to solidify until it becomes
energy-stable, without floating in the treatment bath in
an intermediate state. In other words, each of the
components in the treatment bath becomes either energy-
~~.1~~9~
- 18 -
stable solid matter (sludge or film), or an energy-stable
soluble component in solution, and even if sludge is
produced, it is stable and remains at the bottom of the
cell.
As a result, by the method of electrolysis of a
clear treatment bath according to the present invention,
the treatment bath may be maintained in a constantly
stable state containing no unstable (energy-intermediate)
sludge.
A more detailed explanation will now be given
regarding the method of electrolysis by which the present
invention is characterized.
The electrolysis according to the present invention
is preferably direct current electrolysis.
The electrolysis is divided into the following
types, depending on the site (electrode) connected to the
substance to be treated (work).
(1) Anode electrolysis: Electrolysis with the
work-piece as the anode.
(2) Cathode electrolysis: Electrolysis with the
work-piece as the cathode.
(3) Anode electrolysis + cathode electrolysis
Also, a combination of any of the above methods of
electrolysis with a non-electrolytic method of forming a
film may be used.
The electrolytic chemical treatment system according
to the present invention will now be described with
reference to Figs. 1-4.
According to the present invention, the electrolytic
system in Figs. 1-4 may be considered.
Here, each of the electrolytic chemical treatment
systems in the figures comprises a treatment cell 1, a
circulation pump 2, a filter 3, a sensor 4, a power
source 5, an electrode 6, a substance to be treated 7 and
a temperature controlling system 8. The electrolytic
reaction system consists of one or more subsystems, and
if it consists of 2 or more subsystems, then it is
- 19 -
divided into a main electrolysis (reaction) system A and
a secondary electrolysis (reaction) system B. Also, the
secondary electrolysis (reaction) system B is sometimes
in the same cell and sometimes in a separate cell.
Fig. 1 is a normal electrolytic treatment system.
In this case, the electrode and the substance to be
treated are sometimes exchanged.
Fig. 2 is a system comprising a main electrolysis
system A and a secondary electrolysis system B. Also,
Fig. 2 is an electrolytic treatment system in which
cathodizing is performed.
The system is constructed so that a voltage/current
is applied to the main electrolysis system A, but no
voltage or current is directly applied to the secondary
electrolysis system. The secondary electrolysis system B
is constructed so that the current from the external
circuit does not flow directly via the wire from the
substance to be treated 7 to the electrode 10 and the
electrode 11, etc.
The electrical current which is applied to the main
electrolysis system A flows through the solution to the
substance to be treated 7 and to the electrodes 10, 11
which are the opposite electrodes of the secondary
electrolysis system. Also, the current flowing to the
opposite electrodes of the secondary electrolysis system
B (electrodes 10 and 11) reaches the substance to be
treated 7 again via the solution. Further, part of the
current which flows to the opposite electrodes of the
secondary electrolysis system B reaches the substance to
be treated 7 via a diode D. The main electrolysis system
A functions as the electrolytic reaction which is
directly connected with the formation of the chemical
film, while the secondary electrolysis system B functions
to favorably promote the main reaction.
The reason for this is as follows. In the
electrolysis system which has been connected as shown in
Fig. 2, the electric potential in the treatment bath
- 20 -
during the electrolytic treatment (application of the
electric current) is such that "the anode of the main
electrolysis system A" > "the opposite electrodes of the
secondary electrolysis system B" > "substance to be
treated 7". Also, by operating the main electrolysis
system A, not only the metal ions in the main
electrolysis system A, but also the metal ions in the
secondary electrolysis system B, being linked to the main
electrolysis system A, can be deposited onto the surface
of the substance to be treated.
The main electrolysis system A is constructed with
the main metal used to form the phosphate coating, such
as zinc, as the electrode 6 at the anode end, and the
substance to be treated 7 as the cathode. The secondary
electrolysis system B is constructed with metal materials
such as iron and nickel, etc., which are to form
secondary components of the phosphate chemical coating,
immersed in the treatment bath as the electrodes.
Consequently, the iron and nickel also dissolve in the
treatment bath by the action of the main electrolysis
system A, and the dissolved ions will be deposited along
with zinc as phosphate salts on the surface of the
substance to be treated, forming a film.
Furthermore, if the metal materials such as iron,
nickel, etc., are simply immersed in the bath without
being connected in the manner shown in Fig. 2, then the
iron will remain immersed in the electrolysis system, and
as a result the amount of iron dissolving and being
deposited will increase, thus producing a rough film with
inferior properties. That is, in such a case the
dissolution and deposition of the iron will be less
linked to the dissolution and deposition of the zinc,
than in the case shown in Fig. 2.
zt is well known that iron ion plays an important
role in the formation of phosphate films, but an overly
large amount thereof is also inconvenient.
As shown in Fig. 2, due to the connecting wire, the
_ 21 _ ~1~.~~92
electric current applied to the main electrolysis system
(between the Zn electrode and the substance to be
treated) A is also applied to the electrodes l0 and 11 in
the same treatment bath, and the current consists of a
portion which is released into the treatment bath and a
portion of the current which flows from the iron and
nickel to the substance to be treated 7 via the external
wire. As a result, the dissolution of the iron due to
electrolysis in the chemical treatment bath is reduced
compared with the case where a direct current flows to
the bath from the iron electrode. Consequently, the
resulting chemical film has its iron component minimized,
and is thus more dense.
For the electrodes 10, 11 of the secondary
electrolysis system B may be used iron and nickel in
combination, or either one alone, or another metal.
Also, the diode D in Fig. 2 may be arranged in the
opposite direction.
Fig. 3 shows a case in which the main electrolysis
system A and the secondary electrolysis system B are
prepared in separate cells.
In this case, if anodizing is carried out with a
constant voltage of 0.5 V or less applied to the
substance to be treated (iron) 7 in the main electrolytic
cell 13, then the excess ferrous ion (Fe2+) dissolves in
the reaction system, but when the anodizing voltage is
too low then the dissolved Fez+ is not oxidized to ferric
ion (Fe3+). Consequently, the oxidation-reduction
potential (ORP) of the treatment bath is lowered. If it
is attempted to control the ORP of the treatment bath to
560 mV or greater, then it will be necessary to oxidize
the Fe2+ to Fe3+, as described in detail later.
The secondary electrolytic cell 14 in Fig. 3 is
provided for this purpose. That is, the excess Fez+
which is eluted into the reaction system by the
electrolytic reaction in the main electrolytic cell 13 is
converted from Fe2+ ~ Fe3+ in the secondary electrolytic
~1.1~~~~
- 22 -
cell 14 by electrolysis at a greater voltage and current,
and thus the ORP of the treatment bath may be controlled
within a prescribed range of 560 mV or greater.
Fig. 4 shows a case in which a plurality of main
electrolysis systems A are provided. The anodes are an
electrode 7 using zinc and an electrode 15 using another
metal (iron, etc.), and the substance to be treated 6 is
connected as the cathode. Also, this case allows the
simultaneous electrolytic treatment of a plurality of
metals for the formation of a chemical films thereon.
An explanation will now be given regarding the
method of applying the electric current and voltage. The
following methods may be mentioned for the application of
the electric current and voltage to the bath from the
power source 5.
A summary thereof is shown in Figs. 5 (a) - 5 (d).
(a) Constant current electrolysis: method wherein a
constant current is applied (including pulse
electrolysis).
(b) Constant voltage electrolysis: method wherein a
constant voltage is applied (including pulse
electrolysis).
(c) Current scanning electrolysis: method of
electrolysis wherein the applied current is
controlled (scanned) using a function generator or
the like, to produce a specified current after a
specified period of time. Sometimes repeated n
number of times.
(d) Voltage scanning electrolysis: method of
electrolysis wherein the applied voltage is
controlled (scanned) using a function generator or
the like, to produce a specified voltage after a
specified period of time. Sometimes repeated n
number of times.
Electrolytic methods (a), (b), (c) and (d) may be
carried out at the anode or the cathode, and thus there
are actually 8 possible methods, as shown in Table 1.
- 23 r=112x92
In actual practice, any one of the 8 methods may be
used alone, or any number of the 8 methods may be used in
combination as a series of steps.
Also, a non-electrolytic method may be used in
combination with one of the electrolytic methods
mentioned above.
Table 1 - Combination of Electrolytic Methods
Anode Cathode
electrolysis electrolysis
Constant (1) (2)
current
electrolysis
Constant (3) (4)
voltage
electrolysis
Current (5) (6)
scanning
electrolysis
Voltage (7) (8)
scanning
electrolysis
The electrolytic treatment according to the present
invention results in the production of less sludge than
in the case of non-electrolytic baths. This is due to
the fact that the electrical energy supplied to the bath
raises the electrochemical energy level of the bath as a
whole, and greater stability of the individual component
ions in liquid state is made possible. That is, in a
clear electrolytic bath, the supply of electrons (e) to
the solution phase contributes to the stabilization of
the various ions in the solution phase. Consequently,
since the various ions are stable in this clear
electrolytic bath, the solution is also thermodynamically
stable. As a result, in order to cause a phase
transition (corresponding in this case to a "liquid-
solid" reaction) such as the formation of a coating,
etc., a larger amount of electrochemical energy is
required than fox a clear non-electrolytic bath.
- 24 -
Therefore, in comparison with non-electrolytic baths, the
electrolytic treatment according to the present invention
provides greater stability for the solution and is less
likely to produce sludge.
The voltage and current applied during the
electrolytic treatment are preferably about 0.1 V - 10 V
and 10 mA/dm2 - 4 A/dm2, respectively. Also, the
preferred electrolysis is carried out by insuring the
maximum amount of current with as low a voltage as
possible.
The oxidation-reduction potential of the phosphate
chemical treatment bath according to the present
invention (expressed as the AgCl electrode potential) is
preferably 250-650 mV. Also, the 250-650 mV in the
present invention preferably corresponds to 460-860 mV of
a hydrogen standard electrode potential.
If the treatment is limited to steel materials, then
the oxidation-reduction potential of the chemical
treatment bath reflects the entirety of the various
equilibrium systems in the treatment bath, but is
reflects Chemical Equation 4 as regards the Fe2+ ion.
That is, if the amount of a soluble metal ion,
particularly Fe2+, is increased, then the oxidation-
reduction potential will be reduced, while conversely if
the amount of soluble metal ion, particularly Fe2+, is
decreased, then the oxidation-reduction potential will be
increased. Also, if during non-electrolysis there is no
supply of energy such as heating, etc., then an
oxidation-reduction potential will not reach 560 mV or
greater. This is because the AgCl electrode potential
according to the present invention is about 210 mV less
than the hydrogen standard electrode potential, and an
ORP (AgCl electrode potential) of 560 mV corresponds to
770 mV in terms of the hydrogen standard electrode
potential, and that potential reflects the equilibrium:
[Chemical Equation lOJ
Fe2+ ~ Fe3+ + a + 0 . 7 7 V
25
In other words, for an ORP of 560 mV or greater, it
is necessary to further oxidize the ferrous ion (Fe2+)
dissolved from the iron material. However, if thermal
energy is not directly used to form the coating in the
non-electrolytic bath, then the only energy supplied to
the treatment bath is the energy which accompanies the
dissolution of the iron (Chemical Equation 3). With that
energy alone, the equilibrium of Chemical Equation 10
cannot be shifted towards the right.
However, since according to the present invention
electrical energy is supplied by the electrolytic
treatment, the iron is dissolved and oxidized by Chemical
Equations 3 and 10, causing the treatment bath to contain
both Fez+ and Fe3+, and so the ORP may be 560 mV or
greater. In addition, the reaction of the formation of
the film (Chemical Equation 4) is also promoted, and thus
the formation of the chemical film takes place. Since
Fe3+ is stably present in the bath with an ORP of 560 mV
or greater, the chemical treatment coating which is
formed is assumed to be a phosphate chemical coating
including iron in the form of both Fe2+ and Fe'+.
Furthermore, at 250 mV or less, the amount of the
soluble metal ion becomes too large causing sludge to be
easily produced in the treatment bath, and thus making it
difficult to maintain the clarity of the chemical
treatment bath. As a result, a strong chemical film
cannot be formed.
Even if metal materials other than steel are to be
treated, the oxidation-reduction potential of the
chemical treatment bath is generally in the range of 250-
650 mV. This is because the oxidation-reduction
potential reflects the balance of oxidation-reduction of
Chemical. Equations 1, 2, 4 and 8 in the treatment bath,
and even if Chemical Equation 8 is generalized to
Chemical Equation 3, the balance of the oxidation-
reduction of Chemical Equations 1, 2 and 4 does not
change very greatly.
- 26 -
The chemical film treatment bath according to the
present invention preferably contains phosphate ion at
about 4 g/1 (grams/liter) or more, the film-forming metal
ion at about 1.5 g/1 or more, and nitrate ion at about 3
g/1 or more. On the other hand, preferably the maximum
limit of phosphate ion is usually about 150 g/1, the
maximum limit of the film-forming metal ion is usually
about 40 g/1, and the maximum limit of nitrate ion is
usually about 150 g/1. Furthermore, the most preferred
ion concentrations are usually about 5-80 g/1 of
phosphate ion, 2-30 g/1 of the film-forming metal ion,
and 10-60 g/1 of nitrate ion.
The management of the chemical treatment bath
basically involves the control of the oxidation-reduction
potential. Hence, it is preferable to add main reagents
(an acidic chemical containing phosphoric acid, nitric
acid, zinc, etc.) in response to the change in the
oxidation-reduction potential; however, for a stricter
management of the chemical treatment bath, it is
preferable to additionally utilize the other
electrochemical parameters of the chemical treatment
bath, such as the hydrogen ion concentration (PH) and the
electric conductivity (EC).
The hydrogen ion concentration (PH) is preferably in
a range of about 2.5 - 4Ø
Raising of the PH is accomplished by introducing a
chemical such as caustic soda which will shift the
treatment bath towards the alkaline end. Conversely,
lowering of the PH is accomplished by introducing more of
the main reagents, i.e., the acidic chemical containing
phosphoric acid, nitric acid, zinc, etc.
The suitable range of the electric conductivity
varies depending on the type of chemical treatment bath.
It is preferably set higher for baths containing large
amounts of active ion such as nitrate ion, and set lower
for baths containing small amounts of nitrate ion or the
like but large amounts of phosphate ion. It is generally
~9.~.~~92
- 27 -
preferable to add the main reagents at a minimum set
value of conductivity so as to adjust the conductivity of
the chemical treatment bath within a specific range. The
electric conductivity also varies depending on the
structure of the ions in the chemical treatment bath, and
the conduct~.vity will decrease as the ions in the
solution become more structured, even if the composition
does not change. In light of the above, the conductivity
of the chemical treatment bath is preferably controlled
to about 10-200 ms~cml.
According to the present invention, there is
provided a method for phosphate chemical treatment which
makes it possible to produce a phosphate chemical coating
of adequate film thickness onto metal surfaces regardless
of the degree of electric conductivity thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic drawing of an electrolytic
treatment system for phosphate chemical treatment; Fig. 2
is a schematic drawing of an electrolytic treatment
system for phosphate chemical treatment; Fig. 3 is a
schematic drawing of an electrolytic treatment system for
phosphate chemical treatment; Fig. 4 is a schematic
drawing of an electrolytic treatment system for phosphate
chemical treatment; Fig. 5 (a), (b), (c) and (d) are
characteristic graphs showing the states of application
of electric current and voltage; Fig. 6 is an SEM
photograph of the crystalline structure of a phosphate
film obtained by the method in Example 1; Fig. 7 is a
fluorescent X-ray analysis chart for a phosphate film
obtained by the method in Example 1; Fig. 8 is an X-ray
diffraction chart for a phosphate film obtained by the
method in Example 1; Fig. 9 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in Example 2; Fig. 10 is a fluorescent X-ray
analysis chart for a phosphate film obtained by the
method in Example 2; Fig. 11 is an X-ray diffraction
chart for a phosphate film obtained by the method in
~1125~2
- 28 -
Example 2; Fig. 12 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in Example 3; Fig. 13 is a fluorescent X-ray
analysis chart for a phosphate film obtained by the
method in Example 3; Fig. 14 is an X-ray diffraction
chart for a phosphate film obtained by the method in
Example 3; Fig. 15 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in Example 4; Fig. 16 is a fluorescent X-ray
analysis chart for a phosphate film obtained by the
method in Example 4; Fig. 17 is an X-ray diffraction
chart for a phosphate film obtained by the method in
Example 4; Fig. 18 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in Example 5; Fig. 19 is an X-ray diffraction
chart for a phosphate film obtained by the method in
Example 5; Fig. 20 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in Example 6; Fig. 21 is an X-ray diffraction
chart for a phosphate film obtained by the method in
Example 6; Fig. 22 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in the Comparison; Fig. 23 is a rough drawing of a
part used in Example 7; Fig. 24 is an SEM photograph of
the crystalline structure of a phosphate film obtained by
the method in Example 8; Fig. 25 is an X-ray diffraction
chart for a phosphate film obtained by the method in
Example 8; Fig. 26 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in Example 9; Fig. 27 is an X-ray diffraction
chart for a phosphate film obtained by the method in
Example 9; Fig. 28 is an SEM photograph of the
crystalline structure of a phosphate film obtained by the
method in Example 10; Fig. 29 is an X-ray diffraction
chart for a phosphate film obtained by the method in
Example 10; Fig. 30 is a rough drawing of a segment used
in Example 11; Fig. 31 is a rough drawing showing the
~~12~9~
- 29 -
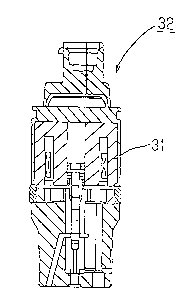
core in Example 11; Fig. 32 is a cross-sectional view of
a bulb comprising the core in Example 11; Fig. 33 is a
rough drawing showing a core according to the prior art;
Fig. 34 is a cross-sectional view of a bulb comprising a
core according to the prior art; Fig. 35 is a
characteristic graph showing the properties for Example
11; Fig. 36 is a diagram of explanation for Example 12;
Fig. 37 is a characteristic graph showing the properties
for Example 12; Fig. 38 (a) and (b) are frontal and side
views, respectively, of the core in Example 13; Fig. 39
is an enlarged view of a part of the core in Example 13;
Fig. 40 is an enlarged view of a part of a core according
to the prior art; and Fig. 41 is a characteristic graph
showing the current and voltage characteristics for
Example 14.
Best Mode for Carrying Out the Invention
In Examples 1-6 and 8-10 according to the present
invention, the materials to be treated were a flat test
piece (A) with a length, width and thickness of 15 cm, 7
cm and 1 mm, respectively, and a test piece (B) of 7.5
cm, 3.5 cm and 1 mm, respectively, and the opposite
electrodes were flat having a length, width and thickness
of 20 cm, 5 cm and 1-2 mm, respectively.
Also, in Example 7 a clutch from an automobile air
conditioner compressor was used.
In Example 11 a part (core segment) was used made of
a magnetic material (ILSS), which is used to form a
solenoid stator core for controlling an automobile fuel
injection pump.
In Example 12 a magnetic material (ILSS) was used
from the same type of solenoid core segment used in
Example 11, of length 500 mm, width 28 mm and thickness
2 mm prior to cold-forging.
In Example 13 the stator core of an automobile
alternator was used. The amount of the treatment bath
used for the treatment was about 20 liters in all cases.
The treating time of the test pieces in each of the
'~12~92
- 30 -
Examples was 2 minutes for each step, except for the
phosphate chemical treatment, and the process is the
following: degreasing -- water washing -- water washing --
acid washing (1-2~ HN03, normal temperature, 1-2 minutes)
-- water washing -- water washing -- surface preparation
(0.1 - 0.2~ PL-ZT, product of Nihon Parkerizing)
phosphate chemical treatment -~ water washing ~ water
washing. The times for the phosphate chemical treatment
differed between each of the Examples and the Comparison.
The water washing after the degreasing was followed by
spraying with fresh water for industrial use, to ensure
thorough washing.
Also, in Examples 5, 6, 7-13 and the Comparison,
there was no acid washing or water washing following it.
The Examples and the Comparison are summarized in
Tables 2 and 3.
Also, the ORPs (oxidation-reduction potentials)
referred to in the Examples are all AgCl electrode
potentials. Further, in cases where the AgCl electrode
potential is substituted by the hydrogen standard
electrode potential, approximately 210 mV is added
thereto.
Furthermore, Figs. 6, 9, 12, 15, 18, 20, 22, 24, 26
and 28, which are the SEM photographs of the phosphate
chemical treatment films obtained by each of the
Examples, are all at 1,000-fold magnification.
- 31 - ~~12~92
N
.-I + + N
la N
Cd .--1 C
L'.U y U U
E a C 7D
O I I I I O I O I I COO .-/I I
.Z,1 I t I M I Z I I N M W I I
y + + o rl
r-I ri rl N N
CL ~1 ~I
E N N U C ' U
N N a~m CD O ;n ;-0 cD .~.0
y( t~ .NN ri V1O L O r1 It1O r1I -1
W cn v7~-IW c~1N M H >. C:.W N M W I W
M I
d + + OJ O~
r-i ri r1 rl
C..ri .-1 O c O E
E N N M U G M U
fd N N N ~ I O 1 CD CD
fe s~ ~ a .-m n o a o t m n o rlI
W Cl7 U1'N ILClaN M H Z I I N M (x.,I Gr
s
d + + M ~D r
r1 l-I rl rl riri r1
rl O G O E
E 41 'y M U C M U
a1 ~'-r N a7 oD I O 'n .0 1 CDCD eD
7< O ~rv i V7O l..Iv .i It1O .1'i .-I
W U cAYI GTb N M H 7 f:.,ctlN M Ix,Lt,Cs.
M N
N O t + N M T
.t
.-iri ri ri rlri ri
O
f3.G. .-i G E O E7
.-1
M
E .i v M U C M U
O
fn
c0 cC N N 60 I O ;n CO t c70DD n0
O
~
y~ i~ i.JO .i V1O 1..14) .I n1O rl.i .1
L
V1
W V1 V7y CLf=lN M H ? L.,W N M Cc,(s.,Cc,
c/~
~
N
d + + O ri
~ F ~ ri ri
C1.~ .-I p C O E
G tv M J G' M U
m ~ y v~ no I o ~ on I caco ac
X ~-I ~ v ~I Ino sav .a Ino .~.~ .rr
w 6 cn>. w w N M f-,y. w as N M w w w
r,
a~ + +
ra rd I r1 iDn CO
a .~ * I 6 m E
E N U I U U U U
~p p~ C N o C of eDN c0c-000
'p,'.a.~ .iO I I O O r1y rlI b O rlri r1
W N N Z I * M M N T * N M Cs,Cx,Cc,
'C :7
O O
yJ 1J
O v
E E _ c
v y
y C ~ C
cno Ino
T .I T -I
v~~r U ;na~ U
o n1 0
-d y.lU J U 1.iS..I
a~ C -.iv C .I y m cu
y a~.-as :n y r-aa .>~
I
m 8 C C ~ a a a
~
v a~~n ~ c ~ u~v~ 1 c. ~t
_ _
t-t 'CSi cGcritE O T N tC c0 T C
a~ O w O 4 E O .-I:J 4 E 10 O
1-iT N 4J N U 1-IO 4 v v U 4 riT
!U 1~r-1l~CD L'.~ a.l1-ni.1CO ~ " ,C.'I L rl
.17 U O Ic1E U ~ c0 ~ CL7C U rl
N S-IU L N T d U U t~ y T IC N
O rl1~ .-i.-11~U riN ~i.-i~ U 4 L l.n
a~ d U t~O G N .-Ia~O C 50C w
W T > a.lp~ y T > ~ QI o d w i'
.-I y ,-I-I C s.ry rl G a ~ a ..~T
~n ~ a~ o ~ y m .~a o ~ ;rm o m -o-I
v ..i .i 1.1C E C. ri'O fJC ~ G .CN
.-If-I V1a) ~ '.U~ N :n'J L CJ iJN C 7-IT
C. y O O U S. mOC O '' U 1-It:C O c0
E ~ ~ o v >a v ce a :~ y ~I y m ~ ~ a a
In
m ~a a C .-ra ~ sa a ~ ~ ~ >a>.~W .-a
V1 ~"" O d' W U E~F O U W U c E V7W >C"I
y
Go
CO I
rt
C o
w
on .I o
C N C
C
N 'C7 'O
-l O N
C
2s .>r 6
3
o ~ a
o
o
.~
~ U w
tn
L
ri N N
:n
CL
d'
I C-~'
O d ri
N
L tJ I
I
tn v7
- 32 - ~~ 1 2~
M 1r
o a
0
+ a +
a a -i =
.-I
-.
~ _ o E
c. a o o
d
u~
N t~ C M U U M U
N
1~
a1 ~ c0 O ~n:-0 I C tnC-0 I
~
S..n
k .-I a a~ w n o -I a~.~ Ino r I
~
~n
m
W a, H ~.fr c',N M N ~.C~ U N M I I
~n
J
C
N
r-I
U r-I
y i cc7 t t
ri11 H H
.i
r.
~ COG
C.O 4 O
M
G N G N U G N U
(n
m co o m co I o ~n:n I
~
a
k c~ a o~ w n o a a~-I Ino I I
w
H
W ~"" H ~-IC:r C, N M H ~ Czax7 N M I I
~
~
rl G
U .-i O
N ~ It1 t S-t +
.--Iy~ rf .i ~T
.i
~
CLN a ~ E ~ O G
V7
N U N U
ofOD O ofo0 C of~p
a.l
a
k m ca a d .~ ~ o a n~.~ ~ o I I
H
W ',~, F-i'ylW Q',N M N ~ W U N M I I N
~
~
O
H bD
O t t COU~[c,
H N N N
GL.-I 8 C E C
~ U ~ U ~ 'I
c0O O ~nCO C cE tnCD ~
k t~ S-tv ri t~O r1 ~ v .i 1~O ri.i
W cn H Y.W C N M N ..- ?IW U N M L4L.
H N
T
m .:l H U
N -I t ~ N t 10n
-I ,-~ ~ r-1 N N N .i
a U
.P.N C V1U U (.' In
, .x
U
fflN O N CD C O tf1CD ' b0OU
U N H
k y f.-IpI.~i d O .i 4 N ri d O 'i.-IO
rl
fn
M W fn H ~ Lti Q',N M N H 7-IW U N M LLfznN
Z ~
y C ~ U
ri c0 .-1 O
U
p ~ N
N t .~., t Y'V1O
N
E- ,..,.i H ~ N N N
T
a N G U V G: U U
c0v O N CO G O N CO o 00OD
k ~ a d a r~o ~ a a~~I n o ~ ~rT7
W V7 H 'yW C, N M N H T W U N M Ix,(s,C
O
U
N
d t
-1 M
P..-i E
v~
a~ C U v I
a-~
_
s0y a o a CO o I I
k yW 7.tO rl n O I 1 I 1 I I U
b
W tn H ~.W C N M 1 I N 1 I f d
G
N
b
..i -I f
'
a~ U ~ U
0 of o t~
I U ~ U S-a
v ~.,'.i N C 'i O It7
tJ N '-iLI ~; N
G CL C U
Q O. O ~ ri
O d tnt~ C3.y a~ cn~ C. L T
a 'bria1 N co b T co cc cC C
a o cna~ a E o -Iv a ~ o
a T a a~ v a a o a a~ v a .I
a ~ .-a~ :.oa --~ a ~ on c --.o~ w
.n a o ~a E a a~ ~a E a a
N 7-tU a.~O T d U U i~ d T cU~ T
O -1s.~.I rl 1~U r-i N .i rl t~U 1-tSrH
a.i N U a~ O C N r1~ O C oD4-IO
a~T 1 ~ v d T = ~ y o w a
N r-i.-i ~" ('..S.~a) r1 ~~ C tla.~"1
~
t0 tlN O aW N i0v N O ~ N IdO 'OU
.x
a)ri .~i i.l C 8 C .I b 1.1C c P.,C d
~ 'cJ c 'd
.-IW n N a~ N s.W v~ O a.~N W n C.T H
d O p~ O
N O 'OU a c0C O .CU t-t cCC cCCD
a.l ,C aJ ~ N
J-1 CLO d S-1N IbC3 YI!L S-1 v tU~,1-1"I
N 1~ fA JJ
a1of C1.C .-I ~ 1..1saC1. a1.-IC W a W I Cc~
T N T a C
v7~ O 6 W U H H O U W U :-~E-~cnX 'i
N E ~ E C
Iri
I
N I 00
a
C ,C
O a~ N O
CD
C c~ -I .C
C C
6 U d cn
.i ri
rf N m
E
C4
d O N
M
t~ a.l t
t
N In .k
.k
- 33 -
Example 1
A steel material (SPCC) was used as the material to
be treated. The phosphate chemical treatment began with
non-electrolytic treatment for 2 minutes as the first
step.
The phosphate chemical treatment bath used contained
3.0 g/1 of Zn2+, 8 g/1 of H3P04, 32 g/1 of N03-, 0.8 g/1 of
Ni2+ and 0.1 g/1 of F-. The PH, ORP and temperature of
the treatment bath were 3.20, 400-500 mV and 30°C,
respectively, and the total acidity, free acidity and
accelerator concentration were 16 pt, 0-0.12 pt and 6 pt,
respectively. Also, the transparency of the treatment
bath was 30 cm or greater, and the chemical treatment
bath contained no sludge.
Next, electrolytic treatment was carried out with
the material to be treated as the cathode, and a zinc
plate as the anode. The phosphate chemical treatment
bath used contained 3.0 g/1 of Znz+, 16 g/1 of H3P04, 17
g/1 of N03-, 2.4 g/1 of Ni2+, 0.1 g/1 of F- and 4.0 g/1 of
Mn2+. The PH, ORP and temperature of the treatment bath
were 3.20, 400-500 mV and 28°C, respectively, and the
total acidity, free acidity and accelerator concentration
were 16 pt, 0-0.01 pt and 6 pt, respectively. Also, the
transparency of the treatment bath was 30 cm or greater.
The electrolytic treatment was carried out under
conditions of a voltage of 0.5 - 1.5 V, a current of 0.2
A/dm2, and a time of 40 minutes. The method of
electrolysis (electrolysis treatment system and method of
application of current and voltage) is shown in Table 2.
The methods of electrolysis of the following Examples are
also shown in Tables 2 and 3.
As a result of this treatment a phosphate chemical
film was obtained with a film thickness 27 ~m and a
dielectric breakdown voltage of 250 V or greater, based
on JIS-K6911. The film thickness was measured using an
electromagnetic film thickness meter Model 1~E-300,
product of Ketto Kaaaku. The film thicknesses of the
- 34 - ~112~92
following steel materials were all measured by the same
method as in Example 1. The SEM photograph and
fluorescent X-ray analysis chart for the obtained
phosphate chemical film are shown in Figs. 6 and 7,
respectively. In addition, the X-ray diffraction chart
is shown in Fig. 8. In Fig. 8, the symbol o indicates
the peaks f or Zn3 ( P04 ) 2 ~ 4Hz0 and Zn3 ( P04 ) .
The film obtained in Example 1 may be described as a
thick-film containing nickel, manganese and zinc, with an
excellent withstand voltage.
Example 2
An aluminum plate (A1100) was used as the material
to be treated, and a steel plate was used as the opposite
electrode. The phosphate chemical treatment bath used
was identical to the one used for electrolytic treatment
in Example 1, containing 3.0 g/1 of Zn2+, 16 g/1 of H3P04,
17 g/1 of N03-, 2.4 g/1 of Niz+, 0.1 g/1 of F- and 4.0 g/1
of Mnz+. The PH, ORP and temperature of the treatment
bath were 3.00-3.40, 560-570 mV and 25-30°C,
respectively, and the total acidity, free acidity and
accelerator concentration were 18 pt, 0.1 pt and 6 pt,
respectively. Also, the transparency of the treatment
bath was 30 cm or greater, and the treatment bath
contained no sludge.
The electrolytic treatment was carried out first
with the aluminum plate to be treated as the anode and
the steel plate as the cathode, at a voltage of 1-3 V, a
current of 0.3-0.6 A/dmz for 0.5-1 minutes, and then
using the same treatment bath, with the aluminum plate to
be treated as the cathode and the steel plate as the
anode, at a voltage of 1-3 V, a current of 0.3-0.6 A/dm2
for 5 minutes.
As a result of this treatment, a phosphate film was
formed on the surface of the aluminum plate with a
coating weight of 6.12 g/dmz.
The SEM photograph and fluorescent X-ray analysis
chart for the obtained phosphate chemical film are shown
- 35 - ~11~~92
in Figs. 9 and 10, respectively. In addition, the X-ray
diffraction chart for the coating is shown in Fig. 11.
In Fig. 11, as in Fig. 8, the symbol o indicates the
peaks for Zn3 ( P04 ) 2 ~ 4H20 and Zn3 ( P04 ) , and the symbol a
indicates the peaks for aluminum.
The coating obtained in Example 2 may be described
as a phosphate chemical thick film containing manganese,
nickel and zinc, with an excellent withstand voltage.
Example 3
A stainless steel plate (SUS304) was used as the
material to be treated, and a steel plate was used as the
opposite electrode. The phosphate chemical treatment
bath used was the same as in Example 2, containing 3.0
g/1 of Znz+, 16 g/1 of H3P04, 17 g/1 of N03-, 2.4 g/1 of
NiZ+, 0.1 g/1 of F- and 4.0 g/1 of Mn2~~. The PH, ORP and
temperature of the treatment bath were 3.00-3.40, 560-570
mV and 25-30°C, respectively, and the total acidity, free
acidity and accelerator concentration were 18 pt, 0.1 pt
and 6 pt, respectively. Also, the transparency of the
treatment bath was 30 cm or greater, and the treatment
bath contained no sludge.
The electrolytic treatment was carried out first
with the stainless steel plate to be treated as the anode
and the steel plate as the cathode, at a voltage of 1-3
V, a current of 0.3-0.6 A/dm2 for 1 minute, and then
using the same treatment bath, with the stainless steel
plate to be treated as the cathode, at a voltage of 1-3
V, a current of 0.3-0.6 A/dm2 for 10 minutes.
As a result of this treatment, a phosphate chemical
film was formed on the surface of the stainless steel
plate with a coating weight 13.27 g/dm2.
The SEM photograph and fluorescent X-ray analysis
chart for the obtained phosphate chemical coating are
shown in Figs. 12 and 13, respectively. In addition, the
X-ray diffraction chart for the film is shown in Fig. 14.
In Fig. 14, as in Fig. 8, the symbol o indicates the
peaks f or Zn3 ( P04 ) 2 ~ 4H20 and Zn3 ( P0~ ) .
- 36 - ~1~.~~~2
The film obtained in Example 3 was a phosphate
chemical film containing zinc.
Example 4
An oxygen-free copper plate (C1020) was used as the
material to be treated, and a steel plate was used as the
opposite electrode. The phosphate chemical treatment
bath used was the same as in Example 2, containing 3.0
g/1 of Zn2+, 16 g/1 of H3P04, 17 g/1 of N03-, 2.4 g/1 of
Niz+, 0.1 g/1 of F- and 4.0 g/1 of Mn2+. The PH, ORP and
temperature of the treatment bath were 3.00-3.40, 560-570
mV and 25-30°C, respectively, and the total acidity, free
acidity and accelerator concentration were 18 pt, 0.1 pt
and 6 pt, respectively. Also, the transparency of the
treatment bath was 30 cm or greater, and the treatment
bath contained no sludge.
The electrolytic treatment was carried out first
with the copper plate to be treated as the anode, at a
voltage of 1-3 V, a current of 0.3-0.6 A/dm2 for 30
seconds, and then using the same treatment bath, with the
copper plate to be treated as the cathode, at a voltage
of 1-3 V, a current of 0.3-0.6 A/dmz for 10 minutes.
As a result of this treatment, a phosphate chemical
film was obtained on the copper plate with a coating
weight 6.67 g/m2.
The SEM photograph and fluorescent X-ray analysis
chart for the obtained phosphate chemical coating are
shown in Figs. 15 and 16, respectively. In addition, the
X-ray diffraction chart for the coating is shown in Fig.
17. In Fig. 17, as in Fig. 8, the symbol o indicates the
peaks for Zn3 ( P04 ) 2 ~ 4H20 and Zn3 ( P04 ) .
The film obtained in Example 4 may be described as a
phosphate chemical film containing manganese and zinc.
Example 5
A steel plate (SPCC) was used as the material to be
treated, and a steel plate was used as the opposite
electrode. The phosphate chemical treatment bath used
contained 4.0 g/1 of ZnZ+, 12 g/1 of H3P04, 40 g/1 of N03 ,
37 ~1~2~~~
6 g/1 of Niz*, 0.2 g/1 of F- and 5 g/1 of Mn2*. The PH,
ORP and temperature of the_treatment bath were 2.70, 300-
400 mV and 22°C, respectively, and the total.acidity and
accelerator concentration were 15.8 pt and 1.6 pt,
respectively. Also, the transparency of the treatment
bath was 30 cm or greater, and the treatment bath
contained no sludge.
The electrolytic treatment was carried out first
with the steel plate to be treated as the anode, at a
voltage of 2.5-3.5 V and a current of 0.5-1.0 A/dmz
applied for 30 seconds, after which the treatment was
repeated 12 times cutting off the current for 10 seconds
between each time, for a total treatment time of 8
minutes. No cathodizing of the material to be treated
was carried out thereafter.
As a result of this treatment; a dense phosphate
chemical coating with a film thickness of 2-3 um was
obtained. The SEM photograph and X-ray diffraction chart
for the obtained phosphate chemical coating are shown in
Figs. 18 and 19, respectively.
The film obtained in Example 5 was a dense phosphate
film.
Example 6
A steel plate (SPCC) was used as the material to be
treated, and the same type of steel plate was used as the
opposite electrode. The phosphate chemical treatment
bath used was the same as in Example 5, containing 4.0
g/1 of Zn2*, 12 g/1 of H3P04, 40 g/1 of N03 , 6 g/1 of Ni2*,
0.2 g/1 of F- and 5 g/1 of Mn2*. The PH, ORP and
temperature of the treatment bath were 2.70, 300-400 mV
and 23°C, respectively, and the total acidity and
accelerator concentration were 16 pt and 1.6 pt,
respectively. Also, the transparency of the treatment
bath was 30 cm or greater, and the treatment bath
contained no sludge.
The electrolytic treatment was carried out first
with the steel plate to be treated as the anode, at a
_ 38 _ ~a_a.2~sz
voltage of 1.5-2.5 V and a current of 0.5 A/dm2 applied
for 30 seconds, after which the treatment was repeated 12
times cutting off the current for 10 seconds.between each
time, for a total treatment time of 8 minutes. Next,
using the same treatment bath with the material to be
treated as the cathode, a voltage of 1.5-2.5 V and a
current of 0.5 A/dm2 were applied for 30 seconds, after
which the treatment was repeated 12 times cutting off the
current for 10 seconds between each time, for a total
treatment time of 8 minutes.
As a result of this treatment was obtained a
phosphate chemical film with a film thickness of 7 um and
a dielectric breakdown voltage of 250 V or greater, based
on JISK6911.
The SEM photograph and X-ray diffraction chart for
the obtained phosphate chemical coating are shown in
Figs. 20 and 21, respectively.
The film obtained in Example 6 was an insulating
phosphate chemical coating.
Comparison
An example wherein electrolysis treatment was not
effected is provided for comparison.
A steel plate (SPCC) was used as the material to be
treated. The phosphate chemical treatment bath used
contained 3.2 g/1 of Zn2+, 8 g/1 of H3P04, 32 g/1 of N03 ,
0.8 g/1 of Niz+ and 0.2 g/1 of F-. The PH, ORP and
temperature of the treatment bath were 3.20, 510-540 mV
and 28°C, respectively, and the total acidity, free
acidity and accelerator concentration were 16 pt, 0-0.1
pt and 6 pt, respectively. Also, the transparency of the
treatment bath was 30 cm or greater, and the treatment
bath contained no sludge.
The material to be treated was immersed in the
treatment bath for 8 minutes.
As a result of this treatment was obtained a
phosphate chemical coating with a film thickness of 1 um
and a dielectric breakdown voltage of 50 V, based on
- 39 -
JISK6911.
An SEM photograph of the obtained phosphate chemical
coating is shown in Fig. 22.
The phosphate chemical coating obtained in the
Comparison was obtained in a conventional manner using a
non-electrolytic method, and it is not expected that the
thickness of the film would be increased or that the
withstand voltage would be improved even if the immersion
time were extended.
Example 7
As shown in Fig. 23, steel parts usually used as a
clutch for an automobile air conditioner compressor were
used as the material to be treated, and a steel plate was
used as the opposite electrode.
The steel part had simple hollow shape with a
diameter of 96 mm and a thickness of 27 mm.
The phosphate chemical treatment bath used contained
4.2 g/1 of Znz+, 8 g/1 of H3P04, 24.1 g/1 of N03-, 2.6 g/1
of Niz+ and 0.1 g/1 of F~. The PH, ORP and temperature of
the treatment bath were 2.93, 530-590 mV and 27°C,
respectively, and the total acidity and accelerator
concentration were 20 pt and 6.0 pt, respectively. Also,
the transparency of the treatment bath was 30 cm or
greater, and it contained no sludge.
The electrolytic treatment was carried out following
the method shown in Fig. 3, with the parts to be treated
as the anode and the steel plate as the cathode in the
main electrolysis system, at a voltage of 0.3-1.0 V and
at a current of 0.01 A - 0.14 A/treated material
according to the method in Fig. 5 (a) for 2 minutes.
In the secondary electrolysis system B, when the ORP
of the treatment bath fell to about 560 mV, current
scanning electrolysis was performed according to the
method in Fig. 5 (c) to remove the Fe2+ which had
dissolved in the treatment bath and raise the ORP. Then,
Cation electrodeposit painting (POWER TOP U56, product of
Nihon Paint) was performed, followed by baking at 190°C
_ 40 - r~1_'~_2 i~2
for about 25 minutes. The painted material was allowed
to stand for 24 hours or more, after which the flat
section 20 and edge side 21 of the part were.sliced to
the base using a cutter knife, and then the part was
immersed in 5~ saline at 55°C for 240 hours for a salt
immersion test. After 240 hours had passed the material
was washed with water and held in the air for about 2
hours, after which adhesive tape was pasted over the
paint film surface which had been sliced with the cutter
knife, and then peeled off forcefully. The width of the
paint film which was peeled off by the adhesive tape was
measured and found to be 5 mm or less for both the flat
section 20 and the edge side 21.
A similar bath (but with an ORP value of 560 mV or
less) was used for non-electrolytic treatment, and when
the part was immersed for 2 minutes far chemical
treatment and painted in the same manner and then
subjected to the same test for evaluation of the paint
film, the peeled films produced were found to be 5 mm or
less for the flat section 20, but about 8-12 mm for the
edge side 21.
From the above evaluation, it may be said that the
method according to the present invention provides a
favorable corrosion resistivity for the edge side 21
after painting. The edge side 21 is the section which
displays the greatest degree of deformity when this part
is formed by prossing and thus its chemical treatment has
been troublesome by the non-electrolytic method according
to the prior art. Therefore, by non-electrolytic
chemical treatment the corrosion resistivity of the paint
is inferior, but by carrying out anode electrolysis as in
Example 7, the dissolution of materials and their
chemical treatment are made possible even for sections
with materials whose dissolution has been troublesome
according to the prior art, and thus the corrosion
resistivity of the paint is improved.
In addition, the method in Fig. 5 (c) was carried
- 41 -
out in electrolytic chemical treatment, using the same
type of part in the same type of treatment bath, in the
same electrolytic treatment system, as above, for a 2
minute electrolytic treatment by a method in which the
current was raised from 0 A ~ 0.01 A over a 30 second
period, maintained for 30 seconds, and then lowered from
0.01 A ~ 0 A over a 60 second period. The part was then
painted, and a salt immersion test such as described
above was conducted. As a result, the flat section 20
and the edge side 21 both had a tape peeled width of 5 mm
or less, and the corrosion resistivity of the paint was
superior to the product of non-electrolytic treatment.
In Example 7 above, a secondary electrolysis system
was used for dissolution of the material, but this is
sometimes unnecessary depending on the conditions
(current, voltage, etc.) used for the anodizing.
Example 8
A steel plate (SPCC) was used as the material to be
treated, and for the opposite electrodes were used iron
for the anodizing, and for the cathodizing iron in the
secondary electrolysis system and zinc in the main
electrolysis system.
The phosphate chemical treatment bath used contained
7.6 g/1 of Znz+, 28.3 g/1 of H3P04, 27.1 g/1 of N03 , 1.44
g/1 of Ni2+ and 0.1 g/1 of F-. The PH, ORP and
temperature of the treatment bath were 3.03, 573 mV and
27°C, respectively, and the total acidity, free acidity
and accelerator concentration were 38.4 pt, 1.6 pt and
5.0 pt, respectively. Also, the transparency of the
treatment bath was 30 cm or greater, and the treatment
bath contained no sludge.
The electrolytic treatment was carried out first
with the material to be treated as the anode and iron as
the cathode, by constant current electrolysis as in Fig.
5 (a) in the system shown in Fig. 1, for 1 minute at a
current of 0.05 A/dm2 (voltage: 0.3 V). Next, using the
same treatment bath, a main electrolysis system was
- 42 - v'11_2~9~
formed using the material to be treated as the cathode
and zinc as the anode.
In addition, wiring was connected between the
material to be treated and the iron electrode, but the
wiring was arranged so as to allow the current to flow
only in the direction from the iron electrode to the
material to be treated. The path comprising the material
to be treated and the iron became the secondary
electrolysis system.
The cathodizing in the main electrolysis system A in
Fig. 2 was carried out by current scanning electrolysis,
slowly raising the current applied between two electrodes
of the main electrolysis system A from 0 A/dmZ ~ 1.5
A/dm2 over a period of 5 minutes. The maximum applied
voltage at this time was 4.5 V. The same procedure was
then repeated for 6 cycles, for a total of 30 minutes of
cathodizing.
As a result of this treatment, a phosphate chemical
film with a film thickness of 15-30 ~m was formed on the
surface of the steel. (The film thickness was measured
using an electromagnetic film thickness meter Model LE-
300, product of Ketto Kaaaku). The insulation resistance
of this film was measured using a superinsulation meter
MODEL SM-8210, product of Toa Denpa KK. The measurement
was performed by lightly contacting the cylindrical
probes (positive electrode, negative electrode) of the
superinsulation meter onto the surface. As a result, the
flat section and edge section of the steel plate both
exhibited an insulation resistance of 500 V DC or
greater.
The SEM photograph and X-ray diffraction chart for
the obtained phosphate chemical film are shown in Figs.
24 and 25, respectively. In Fig. 25, as in Fig. 8, the
symbol o indicates the peaks for Zn3 ( P04 ) 2 ~ 4H20 and
Zn3 ( P04 ) .
Example 9
A steel plate (SPCC) was used as the material to be
43
treated, and for the opposite electrodes iron was used
for the anodizing, and for the cathodizing zinc was used
in the main electrolysis system A and iron and nickel
were used in the secondary electrolysis system B.
The phosphate chemical treatment bath used contained
7.0 g/1 of Znz+, 45.0 g/1 of H~POu, 26.0 g/1 of N03-, 1.4
g/1 of Ni2+ and 0.1 g/1 of F-. The PH, ORP and
temperature of the treatment bath were 3.02, 565 mV and
24.5°C, respectively, and the total acidity, free acidity
and accelerator concentration were 51.8 pt, 2.4 pt and
5.6 pt, respectively. Also, the transparency of the
treatment bath was 30 cm or greater, and the treatment
bath contained no sludge.
The electrolytic treatment was carried out first
with the material to be treated as the anode and iron as
the cathode, by constant current electrolysis as in Fig.
5 (a) in the apparatus shown in Fig. 1, for 1 minute at a
current of 0.05 A/dm2 (voltage: 0.3 V).
Next, using the same treatment bath, the apparatus
in Fig. 2 was used. That is, a main electrolysis system
A was formed using the material to be treated 7 as the
cathode and zinc as the anode. In addition, wiring was
connected between the material to be treated 7 and the
iron and nickel electrodes 10, 11, but the wiring was
arranged so as to allow the current to flow only in the
direction from the iron and nickel electrodes to the
material to be treated. The path comprising the material
to be treated 7 and the iron and nickel electrodes 10, 11
became the secondary electrolysis system B.
The cathodizing in the main electrolysis system A
was carried out by current scanning electrolysis, slowly
raising the current applied between the electrodes of the
main electrolysis system A from 0 A/dm2 ~ 2.0 A/dm2 over
a period of 5 minutes. The maximum applied voltage at
this time was 4.9 V. The same procedure was then
repeated for 6 cycles, for a total of 30 minutes of
cathodizing.
44 -
As a result of this treatment, a phosphate chemical
film with a film thickness of 15-30 ~m was formed on the
surface of the steel plate. (The film thickness was
measured using an electromagnetic film thickness meter
Model LE-300, product of Ketto Kagaku). The insulation
resistance of this film was measured using a
superinsulation meter MODEL SM-8210, product of Toa Denpa
KK.
The measurement was performed by lightly contacting
the probes (positive electrode, negative electrode) of
the superinsulation meter onto the surface.
As a result, the flat section and edge section of
the steel plate both exhibited an insulation resistance
of 500 V DC or greater.
The SEM photograph and X-ray diffraction chart for
the obtained phosphate chemical film are shown in Figs.
26 and 27, respectively. In Fig. 27, as in Fig. 8, the
symbol o indicates the peaks f or Zn3 ( P04 ) 2 ~ 4H20 and
Zn3 ( P04 ) .
Example 10
A steel plate (SPCC) was used as the material to be
treated, and for the opposite electrodes iron was used
for the anodizing, and zinc was used for the cathodizing.
Also, the iron electrode plate was disconnected from
the power source and immersed in the bath. The phosphate
chemical treatment bath used contained 7.0 g/1 of Znz+,
45.0 g/1 of H3P04, 26.0 g/1 of N03-, 1.4 g/1 of Niz+ and
0.1 g/1 of F-. The PH, ORP and temperature of the
treatment bath were 3.02, 569 mV and 27.5°C,
respectively, and the total acidity, free acidity and
accelerator concentration were 51.8 pt, 2.4 pt and 5.6
pt, respectively. Also, the transparency of the
treatment bath was 30 cm or greater, and the treatment
bath contained no sludge.
The electrolytic treatment was carried out first
with the material to be treated as the anode and iron as
the cathode, by constant current electrolysis as in Fig.
(a) in the apparatus shown in Fig. 1, for 1 minute at a
current of 0.05 A/dmz (voltage: 0.8 V).
Next, using the same treatment bath, an,electrolysis
system was formed using the material to be treated 7 as
5 the cathode and zinc as the anode. Here, the steel plate
was immersed in the bath. When a steel plate is immersed
in a treatment bath, it exists as a component in the
electrolytic reaction system. That is, the iron is
easily dissolved from the steel plate, and the dissolved
FeZ+ adheres to the surface of the material being treated
as a chemical film. As a result, the film thickness of
the chemical film is much greater in comparison with
Examples 8 and 9. The cathodizing in the main
electrolysis system A was carried out by current scanning
electrolysis, slowly raising the current applied between
the electrodes of the main electrolysis system A from 0
A/dm2 -- 2.0 A/dm2 over a period of 5 minutes. The
maximum applied voltage at this time was 5.8 V. The same
procedure was then repeated for 6 cycles, for a total of
30 minutes of cathodizing.
As a result of this treatment, a phosphate chemical
film with a film thickness of 50-60 ~m was formed on the
surface of the steel plate. (The film thickness was
measured using an electromagnetic film thickness meter
Model LE-300, product of Ketto Kagaku). The insulation
resistance of this film was measured using a
superinsulation meter MODEL SM-8210, product of Toa Denpa
KK. The measurement was performed by lightly contacting
the probes (positive electrode, negative electrode) of
the superinsulation meter onto the surface. As a result,
the flat section of the steel plate exhibited an
insulation resistance of 500 V DC or greater. However,
the withstand voltage of the edge section was about 250
V. Also, its adherence to the foundation of the film was
also inferior with respect to the above Examples 8 and 9.
From the above results it may be said that the control of
the iron ion in the chemical treatment bath is necessary
to form a thick-film type i sula~~ g~~~m~cal film.
The SEM photograph and X-ray diffraction chart for
the obtained phosphate chemical film are shown in Figs.
28 and 29, respectively. In Fig. 29, as in Fig. 8, the
symbol o indicates the peaks for Zn3 ( P04 ) z ~ 4Hz0 and
Zn3 ( P04 ) .
Example 11
As the material to be treated was used a solenoid
stator core segment 30, shown in Fig. 30, used in
automobile fuel injection pumps, which is made of a
magnetic material (1LSS, containing 1~ Si).
For the opposite electrodes iron was used for the
anodizing, and iron and zinc were used for the
cathodizing. The phosphate chemical treatment bath used
contained 12 g/1 of Znz' and 1 . 6 g/1 of Ni2+. ( In
addition, N03, H3P04 and F were also used, but they were
not measured). The PH, ORP and temperature of the
treatment bath were 2.96-3.02, 577-581 mV and 26-28°C,
respectively, and the total acidity and accelerator
concentration were 40 pt and 3.0 pt, respectively. (The
free acidity was not measured). Also, the transparency
of the treatment bath was 30 cm or greater, and the
treatment bath contained no sludge.
The chemical treatment was carried out by a method
in which 200 segments identical to the segment 30 in Fig.
were placed in a small acrylic resin barrel for
electrolytic treatment.
A total of 4 barrels, or 800 parts, were used for
the treatment. The barrels were rotated at 2 rpm, and a
30 number of 5 m/m holes were made in the side to allow
greater fluidity of the bath.
The electrolytic treatment was carried out first
with the material to be treated as the anode and iron as
the cathode, by constant current electrolysis as in Fig.
5 (a) in the connected system shown in Fig. 1.
Here, the current was 0.06 A/barrel, and the voltage
was between 1.2 V and 3.5 V. The surface area per barrel
47
corresponded to 6.2 dm . The aiio izing was carried out
for 5 minutes, after which_the power source was cut off
for 2.5 minutes.
The cathodizing was carried out with iron and zinc
as the anodes and a barrel containing the material to be
treated as the cathode to form an electrolysis system
such as shown in Fig. 4, by the method of current
scanning electrolysis shown in Fig. 5 (c).
Here, the current applied at the iron electrode was
successively raised from 0 A (amperes)/barrel -- 0.06 A-
0.1 A/barrel over a period of 90 seconds, while that at
the zinc electrode was successively raised from 0
A/barrel -~ 0.5-1.0 A/barrel also over a period of 90
seconds, and the same procedure was then repeated for 15
cycles.
As a result of this treatment, a chemical film with
a film thickness of 3-10 um was formed on the surface of
the magnetic material, i.e., the surface of the segment
30. (The film thickness was measured using an
electromagnetic film thickness meter, product of Ketto
Kagaku).
The insulation resistance of this film was measured
using a superinsulation meter, product of Toa Denpa KK.
The method of measurement was the same as the one used in
Examples 8-10. As a result, the flat section exhibited
an insulation resistance of 100 V (DC) or greater.
The solenoid stator core segments 30 in Fig. 30
which were used in Example 11 were stacked to prepare a
stator core 31 such as shown in Fig. 31.
Also, as shown in Fig. 32, the stator core 31 was
coiled and set in place to produce a bulb 32 for
controlling the injection amount of an automobile fuel
(gas oil) injection pump.
A conventional solenoid stator core segment 35 and a
stator core 36 using it are shown in Fig. 33.
The conventional segment 35 was an F-shaped segment
(Matexial G09) which had already undergone insulation
- 48 - 'M11~~~~
treatment.
Forging (deformation) is not possible by the
insulation treatment of magnetic materials according to
the prior art, and therefore the conventional stator core
36 is in the form of a stack of punched plates. Using
this stator core 36, a fuel injection pump bulb 37 was
produced as shown in Fig. 34.
Here, the size (measurements) of the bulb 32 in Fig.
32 relating to Example 11 and that of the conventional
bulb 37 in Fig. 34 are identical.
A comparison of the properties of each of the bulbs
32, 34 is shown in Fig. 35.
As a result of the evaluation of the static suction
strength against a driving current (A), the bulb 32
(solid curve in Fig. 35) was confirmed to have a more
excellent suction (actuation) capability for a solenoid
in comparison with the bulb 37 (dotted curve in Fig. 35),
though their structures were identical.
Example 12
As the material to be treated was used a magnetic
material (ILSS) from the same type of solenoid core
segment used in Example 11, of length 500 mm, width 28 mm
and thickness 2 mm prior to forging.
Iron was used for the opposite electrodes, and
anodizing was followed by cathodizing. The phosphate
chemical treatment bath used contained 6 g/1 of Zn2+ and
6 g/1 of Ni2r. The treatment bath had a PH of 3.03, an
ORP of 576 mV and a temperature of 25-30°C, with a total
acidity of 44 pt and an accelerator concentration of 5.2
pt. (The free acidity was not measured). Also, the
transparency of the treatment bath was 30 cm or greater,
and the treatment bath contained no sludge.
The electrolytic treatment was carried out first
with the material to be treated as the anode and iron as
the cathode, by constant current electrolysis as in Fig.
5 (a) in the electrolysis system shown in Fig. 1, for 1
minute. Here, the current was 0.4 A/material and the
~1~2~~~
- 49 -
voltage was 2.4 V.
The cathodizing was carried out in the same bath
with the material to be treated as the cathode and iron
as the anode, by a method of current application in the
same electrolysis system as the one used for the
anodizing, for 3 minutes. Here, the current was 0.4
A/material and the voltage was 2.4 V. The coated
material was subjected to water washing and then drying,
after which it was immersed for 10 minutes in an 80°C
solution of 5~ sodium stearate, to obtain a zinc stearate
metal soap film on the surface thereof.
This material was rolled in a direction which
reduced the plate thickness at the center, as shown in
Fig. 36.
The rolling was performed using a 200-ton press,
applying a load of 60 tons and 70 tons each time with a
10 mm shift each time, for a total of 6 rolls, and the
resulting thin-plate thickness (tl) was measured.
The results are shown in Fig. 37.
Curve (A) in Fig. 37 shows the results for the
chemical film according to the present invention. For a
rolling comparison, curve (B) in Fig. 37 shows the
results for a case in which no chemical film was formed,
arid only processed oil (D200-A, product of Sugimura
Kagaku) was used.
From Fig. 37 it is clear that, for the rolling of
magnetic materials, the chemical film according to the
present invention is more excellent than the materials
according to the prior art rolled using only processed
oil.
Example 13
As the material to be treated was used an automobile
alternator stator core 40, shown in Figs. 38(a) and (b).
This core 40 contained multiple layers of segments
41 each with a plate thickness of 0.5 mm.
The phosphate chemical treatment bath used for
treatment of the core 40 contained 5 g/1 of ZnZ~, 25 g/1
- 5~ - ~~12~92
of H3P04 , 0.8 g/1 of Ni2+, 16 g/1 of N03 and 0.1 g/1 of
F .
The treatment bath had a PH of 3.30, an,ORP of 540-
550 mV and a temperature of 28°C, with a total acidity of
35 pt, a free acidity of 0.2 pt and an accelerator
concentration of 4-6 pt. Also, the transparency of the
treatment bath was 30 cm or greater, and the treatment
bath contained no sludge.
The electrolytic treatment was carried out first
with the material to be treated as the anode and iron as
the cathode, by constant current electrolysis as in Fig.
5 (a) in the system shown in Fig. 1, with a current of
0.4 A/material (voltage: 1.8 V), for 5 minutes. Then,
using the same treatment bath, a main electrolysis system
was formed using the material to be treated as the
cathode and zinc and iron as the anodes.
Also, an electrolytic treatment system such as the
apparatus shown in Fig. 4 was formed for cathodizing.
The cathodizing was carried out by current scanning
electrolysis, slowly raising the current applied between
the electrodes of the zinc electrolysis system from 0 A --
1.25 A/material over a period of 40 seconds. Also, the
current applied between the electrodes of the iron
electrolysis system was slowly raised from 0 A -» 0.4
A/material over a period of 40 seconds. Further, the
electrolysis of the zinc and the iron was carried out
simultaneously. The same procedure was then repeated for
20-30 cycles, for a total of 13-20 minutes of
cathodizing.
As a result of this treatment, a phosphate chemical
film with a film thickness of 20-25 um was formed on the
surface of the material. (The film thickness was
measured using an electromagnetic film thickness meter
Model LE-300, product of Ketto Kagaku, KK). The
insulation resistance of this film was measured using a
superinsulation meter MODEL SM-8219, product of Toa Denpa
KK. The measurement was performed by lightly contacting
51 - ~1'~~~92
the probes (positive electrode, negative electrode) of
the superinsulation meter onto 'the surface. As a result,
the flat section of the material exhibited an insulation
resistance of 500 V DC or greater.
The material was then subjected to Cation
electrodeposition painting using a POWER TOP U-600E,
product of Nihon Paint, to form an organic film with a
thickness of 40-50 Vim. The baking was performed at 180°C
for 30 minutes.
In this manner an alternator stator core 40 having
an insulation layer with a thickness of 50-70 ~m was
obtained.
Using the stator core 40 in Example 13, mechanical
coiling was performed in the slot sections 44.
The coils 42 having a wire diameter of 1.4 mm were
automatically placed with 12 coils per slot.
The condition inside the slot sections 44 after the
coils were completed is shown in Fig. 39.
After the coils were completed, a wedge 43 was
placed inside to prevent the coils 42 from slipping out.
Then, to check for an earth (tearing of the
insulation) in the coils 42 and body of the stator core
40, 600 V AC was applied thereto, and the treated product
withstood mechanical coil processing, having a withstand
voltage of 600 V (AC) or greater.
Conventional non-electrolytic chemical treatment was
then carried out instead of the chemical treatment in
Example 13, followed by Cation electrodeposite painting
as in Example 13, and the insulation layer thereof tore
under the above mentioned mechanical coil processing, and
could not support 600 V AC. Thus, it may be said that
the inorganic insulation film according to the present
invention is effective for alternator insulation
treatment.
Furthermore, for insulation treatment of this type
of conventional alternator stator core 45, a paper
insulator (organic insulation paper) 47 is used between
- 52 - '~11~~~2
the core 45 and the coils 46, as shown in Fig. 40, and
then a wedge 48 is used to seal in the coils 46.
However, the film thickness of the paper insulator is 200
Vim, and this portion complicates the miniaturization of
the core 40. Also, with paper insulators of 200 ~m or
less problems arise such as tearing during the mechanical
coil processing.
Therefore, by the insulation treatment in Example
13, a film may be produced with a thickness of 50-70 um,
which is thinner than according to the method of the
prior art, and with an adequate insulating effect.
Thus, by employing the phosphate chemical treatment
method according to the present invention to the
necessary sections of an insulation, as in the core 40,
it is possible to eliminate the conventional insulating
materials, and this method may be applied in a variety of
ways.
Finally, Table 4 lists the electrochemical
differences between the electrolytic chemical treatment
method in the transparent treatment bath according to the
present invention and the non-electrolytic chemical
treatment method according to the prior art.
53 - z~~ ~~~~
Table 4
Electrolytic Non-
treatment
method electrolytic
treatment
method
Electrochemical High Low
energy level in Supply of Supply of
electrons
from
treatment bath external electrons only
power source
from
dissolution
of
iron
Iron ion state Fe3+ presentFe3+ absentFe'+ absent
Fe2+ presentFe2+ presentFe2+ present
Oxidation- 560 mV or 560 mV or 560 mV or less
reduction greater less
potential of
treatment bath
(AgCl electrode
potential)
As shown in Table 4 above, the electrolytic method
(clear bath) was performed with an ORP of either 560 mV
or greater, or 560 mV or less.
Since at an ORP of 560 mV or greater the treatment
bath contains paramagnetic ion (Fe3+), the following
points must be considered regarding the circulation
cycle, in order to maintain the treatment bath at an ORP
of 560 mV or greater.
That is, the magnetic field must not be allowed to
influence the circulation cycle. If the magnetic field
acts on the treatment bath, then it will affect the
paramagnetic components (Fe3+), and as a result the Fe3+
will dissolve in the treatment baths) and disappear,
leaving no Fe3+ in the treatment bath(s). Consequently,
the ORP will by necessity fall below 560 mV.
A bath with an ORP of 560 mV or greater contains
Fe3+, and therefore its electrolytic tendency is stronger
compared with a conventional non-electrolytic bath (which
contains no Fe3+). Also, its properties are thought to
render it easy to form a chemical film onto metal
materials having a passivation film on the surface of
- ~~~z~~~
aluminum, stainless steel, and the like. In other words,
since its electrolytic tendency is stronger, the
electrolytic treatment is thought to be capable of acting
on a passivation film on the surface and dissolving it to
form a film. Furthermore, a film which is formed from a
bath at 560 mV or less contains no Fe3', and thus it has
the same properties as a conventional non-electrolytic
chemical film. Nevertheless, by the method according to
the present invention it is possible to control the film
thickness thereof.
An additional explanation is provided below of the
main points relating to the electrolytic treatment
constituting the present invention. The main points
regarding the electrolysis according to the present
invention are:
(1) The electrolytic reaction system is separated
into a "main electrolysis system" and a "secondary
electrolysis system", to control the iron component
contributing to the formation of the coating; and
(2) Current scanning electrolysis is performed;
and the reasons therefor are described again below.
Reasons for (1)
The iron ion contributing to the electrolysis
reaction must be controlled, and the "secondary
electrolysis system" performs this role. Particularly,
during the cathodizing, since the material to be treated
is used as the cathode, the manner in which the iron ion
is dissolved and deposited onto the surface of the
material to be treated is important. Also, if the iron
is used as the electrode material, the concrete method of
applying the current and voltage to the iron electrode is
important. The secondary electrolysis system mainly
controls the dissolution and deposition of the iron ion,
and combined with the main electrolysis system it is
effective for the formation of a favorable coating.
Reasons for (2)
This is a necessary condition for the production of
- 55 - ~1'~2~~'~
a thick coating.
An embodiment of the current scanning electrolysis
is shown in Fig. 41 as Example 14.
Fig. 41 relates to the current application in Fig. 5
(c) in the apparatus shown in Fig. 2, and shows the
voltage change I in the "main electrolysis system"
between the material to be treated 7 and the electrode 6
(with positive being the direction from the electrode 6
to the material to be treated 7) and the voltage change
II in the "secondary electrolysis system" between the
material to be treated 7 and the electrodes 10, 11 (with
positive being the direction from the material to be
treated 7 to the electrodes 10, 11).
Here, in Fig. 41, the current applied to the main
electrolysis system from an external power source as in
Fig. 5 (c), was successively raised over a period of 300
seconds from 0 A ~ 4.0 A/cm2.
Under such conditions, as shown in Fig. 41, although
during the initial 90-100 seconds of application of the
current for 300 seconds the current is applied
externally, the voltage change I is a negative value, and
the voltage change II is approximately zero.
This indicates that the potential between the
electrodes in the chemical treatment bath when no current
is applied, or when only an extremely small current is
applied, is:
[Material to be treated] . [opposite electrodes of
secondary electrolysis system (Fe Ni)] > [opposite
electrode of main electrolysis system (Zn)].
In other words, since the chemical treatment bath is
itself an electrolytic bath, an electric potential
difference arises between the electrodes (materials)
immersed therein. Furthermore, the state of the bath
reflecting the potential difference when no current is
applied may be said to be the most stable state of the
chemical treatment bath.
During the period in which the voltage change I
- ~,~:~6z7~~z
produces a minus potential, no current flows between the
anode (zn) and the cathode (material to be treated) in
the main electrolysis system A, despite the current being
input from the external power source in Fig. 41.
However, the current here may be seen as acting upon the
components in the solution. Also, this action on the
components in the solution is very important for the
formation of a dense film. The voltage change I in Fig.
41 indicates that the current flows in the main
electrolysis system by this process to form a film.
Furthermore, while the current flows for the voltage
change I, the voltage of the voltage change II in Fig. 41
becomes a minus value, and this indicates that the
current from the positive electrode 6 in the main
electrolysis system in Fig. 2 is acting on the opposite
electrodes 10, 11 in the secondary electrolysis system B
in Fig. 2.
In other words, the current from the positive
electrode 6 in Fig. 2 produces a minus potential as it
flows through the electrodes 11, 12 via the diode D to
the material to be treated in Fig. 2. Thus, the voltage
changes I and II are related.
This fact shows that the electrolysis of the zinc in
the main electrolysis system A is the controlling factor
over the electrolysis of the iron and nickel, etc., in
the secondary electrolysis system B. By repetition of
the processes, a film is formed.
Thus, by carrying out current scanning electrolysis
as shown in Fig. 5 (c) for cathodizing in the main
electrolysis system of the apparatus shown in Fig. 2, it
is possible to constantly restore the bath to an energy-
stable state for the formation of the film from that
state, while it is also possible to control the excess
dissolution of the electrodes 10, 11 in the secondary
electrolysis system B by controlling the electrolysis at
the electrode 6 in the main electrolysis system A. As a
result, a dense film may be formed onto the material
- 57 - c
~1~~:~~~
being treated.
As a comparison of the electrolytic methods will be
clearly seen by comparison with the constant current
electrolysis in Fig. 5 (a).
In the method in Fig. 5 (a), the current immediately
flows at a prescribed voltage. Also, an electrolytic
reaction occurs, but it is similar to that which occurs
for the forming of good conductive coatings, such as
electroplatings, etc., and it is clearly different from
the method in Fig. 5 (c). In the method in Fig. 5 (c),
the energy state during the electrolysis constantly
displays the maximum voltage of the voltage change I in
Fig. 4. Thus, the solution always has a strong current
applied to it. In addition, the majority of the current
constantly flows through a given section of the material
being treated (for example, the edge section), and
consequently the adhesion at such sections is poor.
The current scanning electrolysis according to the
present invention differs greatly from constant current
electrolysis in that during the forming of the coating,
the electrolytic coat-forming reaction of the components
in the solution is constantly repeated beginning from the
initial state in which the solution is not electrolyzed.
This design contributes greatly to the adhesion of the
coating.
[Industrial Applicability]
As mentioned above, the phosphate chemical treatment
method according to the present invention may be used as
a method of pretreatment prior to the cold forging of a
metal material such as a stator.