Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 93/20862 . PCr/US93/03366 ~.
~ 'r't ~ 0 9~
I ~
LIQUID DELIVERY APPARATU~
5 P E C I F I C A T I O N
Background of the Inv~ntion
Field of the Invention
The present invention relates generally to liquid delivery
systems. More particularly, the invention concerns an appara-
tus for enteral feeding applications.
Di~cu~sion of the Invention
When patients are comatose, or for some reason are unable
to take nourishment by mouth, enteral feeding becomes ne¢es-
sary. Enteral nutrition, or tube feeding, is typically accom-
plished by nasogastric administration or by direct delivery of
~iquids to the stomach via a surgically implanted feeding tube.
Parastalic pumps are currently used for nasogastric feediny
wh~n gravity flow from an elevated container is insufficient to
instill flow or when an exact amount of regulated feeding is
necessary. Such devices are cumbersome to use and at times
have proven unreliable.
The apparatus of the present invention overcomes the draw-
backs of prior art enteral feeding systems by providing a self-
contained apparatus which includes an internal energy source
that automatically expels prepackaged nutritional liquids from
a sealed aseptic container at a desired uniform rate.
Aseptic packaging is, of Gourse, not new. Such packaging
is being used more and more in the food industry for packaging
uit juice, milk products and the like. ~dditionally, some
use of aseptic packaging has been made in the medical field for
packaginy medical~solutions~
When the packaged, aseptically filled liquid i5 a food
rl
'VBS7~1TUTE ~H~T
W093/20862 PCT/US93~03366
.;~. ~ .
O 4 O
product, such as fruit juice, the sealed package is typically
punctured at a specific site location and the juice is~with-
drawn through a straw. When the packaged liquid is a medical
solution, the package is typically opened, mixed with other
components when required and emptied into a traditional, wide~
mouth flexible hag solution container for enteral delivery by
conventional gravity means and parastalic pump. However, in
U~S. Patent No. 4,826,500 issued to Rantsola, a system for the
enteral delivery of a medical solution directly from an aseptic
container is there described. In accordance with the methods
of the Rantsola patent, the solution is passed from a container
through an elongated giving set and metering system into a
nasal tube. The. container is an aseptic carton having penetra-
ble side walls, with the giving set being provided with a
fitting having a fluid passage extending therethrough. The
fitting terminates at a carton cooperating portion which in-
cludes a first portion for penetrating tne carton side walls to
form an orifice therein, the orifice establishing fluld commu-
nication between the carton interior and~ the fitting fluid
passage, and a second portion for engaging the carton side wall
to maintain cooperation between the ca~rton and f1tting.
;~ ~In U. S. Patent No. 4,688,595, issued~t~ Srebnik, et~a1,
; ~here is described an ente~al nutrition delivery system which
;,, .
comprises an integral molded plastic base which 1ncIudes a
firslt Ipl~tform to which is secured ~an~infusion pump alnd~iai`
second platform ha~ing a recess in which~is secured a~specially~
designed bott1e cont~ain~ing nutri;tional f1uid to be ~f~ed to a
patient. A tubing net-work is included for interconnec~ing the
pump~r~bottle~ànd~the p~tient.
Neither Rant~sola nor SrebniX, et;al disclose or remotely
suyg~sb the novel apparatus o~ ehe p~resent 1nventlon, which
UBSTITUTE SHEET
W093/20862 PCT/US93/03366 ~
( Q ,~
comprises a prefilled, self~contained system, including a
unique stored energy source disposed within an aseptic package
for delivering the nutritional liquid at a controlled, uniform
rate.
Through use of the novel apparatus of the present in~en-
tion, the disadvantageous current practice of preparing the dry
nutrient composition and mixing it wi~h sterile water at the
point of use is avoided. The curren~ practice of preparing the
dry nutrient composition and mixing with sterile water at point
of use has many obvious disadvantages. Historically, the use
of this t~o-step method of txeatment preparation has, in part,
been driven by the problems resulting from combined solution
sterilization, including chemical reactivity of certain nutri-
ent materials under au~oclave conditions because of the flexi-
ble bag. Prior art practices also typically employ intermittent
feeding of the patient. Recent clinical practice now favors
continuous feeding rather than intermittent feeding in most
cases. In accordance with the present invention, certain
drugs, minerals, n~trients and the like are aseptically sealed
in a multi-barrier layer, oxygen impermeable, moisture proof,
microorganism-impermeable aseiptic dispenser for automatic, on-
demand continuous delivery to the patient without the required
use of a parastalic pump or external energy sources of any
kind.
' ! I ; I ~ Summary of th~ I~vention ~ i
It is an object of the present invention to provide a l-
self-contained ~ystem for the enteral deli~ery of a nutrient
solution from an aseptic package without the intermediate step
of emptying the package into a traditional flexible bag solu-
tion container ~or del.iv~ry by parastalic pump, gravity ~eans
or the like.
~ ~ SUBSTITUTE SHEE~T
W0~3/2086~ ~X ~ 0l10 PCT/US93/033~6
Mora particularly, it is an object of the invention to
provide a system of the aforementioned character in which an
integral, inherently sterile, flat film energy source is con-
tained within the aseptic package for automatically delivering
on demand the premixed solution contained within the package to
the patient at a precisely controlled rate.
Another object of the invention is to provide an aseptic
carton having a non-permeable oxygen barrier with a penetrating
portion for sealable penetration by a fitting having a fluid
passageway therethrough in communication with a giving set.
Another object of the invention is to provide a carton as
described in the preceding paragraph in which provision is made
; for ingress of make-up air so that an even outflow of solution
to the patient is precisely maintained.
Another object of the invention is to provide a carton
which utilizes a paper-board barrier laminated structure that
maintains an isolated gas environment within the container.
Still another object of the invention is to pxovide a
s~stem of the class described in which the aseptic container
and flat film integral energy source, or elastomeric membrane,
can be economically mass produced at low cost to permlt the
discard of the assembly after use.
; Brie~ D~scription of the Drawings
Fi~ure 1 is a generally perspective exploded view ofjth~e
nutrient delivery apparatus of the present invention.
` Figure 2 is a perspective view of the~apparatus partly
' ~ ~ J
; ~ broken away to show internal construction and exploded to show ~,
;~ the manner of interconnection of the liquid delivery spike.
; Figure 3 is a top-plan view of the~device partly broken
away to show internal construction.~
` ` ~ SUBSTITUTE SH~EET
W0~3/20862 ~ i'b ~ ~ O PCT/US93/03366
- . , .
. I `;' '
Figure ~ is a cross-sectional view taken along lines ~-4
of Figure 3.
Figure 5 is a cross-sectional view taken along lines 5-S
of Figure 4.
Figure 6 is a fragmentary side-elevational, cross-section-
al view of the fill port of the device.
Figure 7 is a cross-section~l view similar to Figure 4 but
illustrating the appearance of the apparatus when filled with
fluid.
Figure 8 is a fragmentary view taken along lines 8-8 of
Figure 7.
Figure 9 is a fragmentary cross-sectional view illustrat-
ing one form of the multi-film barrier construction of the
device.
Figure 10 is a generally perspective exploded view of an
alternate form of the apparatus of the invention.
Figure lOA is a cross-sectional view taken along lines
lOA-lOA of Figure 10.
Figure 11 is a cross-sectional view of the check valve
assembly of Fig~re 10.
Figure 12 is a generally perspective view of the apparatus
of Figure 10 partly broken away to show internal construction
and exploded to show the manner of intarconnection of the
delivery spike.
F`ilgùre 13;is a generally perspective exploded view of
another embodiment of the nutrient delivery apparatus of the
invention. ~
~: Figure 14 is a plan view of the~ device partly broken away
to show internal construction. ~ :
Fiqure 15 is a cross-sectional view taken along lines 15-
15 of Figure 14.
SU~STlTlJT~ Slt EET .
W0~3/20862 4~ PCT/US93/0336S
,. ,
,
Figure 16 is a cross-sectional view taken along lines 16-
16 of Figure 15.
Figure 17 is a cross-sectional view taken along lines 17-
17 of Figure 15.
Figure 18 is an enlarged fragmentary view of the outlet
portion of the device.
Figure 19 is an enlarged fragmentary view o~ the delivery
spike of this latest embodiment.
Figure 20 is a fragmentary cross-sectional view of area
20-20 of Figure 15.
Figure 21 is an enlarged fragmentary cross-sectional view
of the delivery spike mated with the outlet port assembly of
the device.
Description of the In~ention
Referring to the drawings and particularly to Figures 1, 2
and 3, the liquid delivery apparatus of the present invention
comprises a body made up of cooperating first and second por-
t.ions 12a and 12b respectively. As can best be seen by refer-
ring to Figure 5, each body portion 12a and 12b includes inter-
nal walls defining cavitles 14a and 14b respectively with each
cavity being circumscribed by an edge 16a and 16b respectively.
Each body portion is also provided at either end with semi-
circular shaped, indexable openings generally designated by the
numerals 18 and 20.
A firs~ distendable membrane 22 is provided with an edge
portion 22a which is disposed in engagement with edge portion
16a of first body portion 12a. A second distendable membrane
24 having an edge portion 24a is disposed in engagement with
edge portion 15h of second body portion 12b. Each of the
~ distendable membranes 22 and 2~, the unique character of which
J~
SUBSTITIJ~E ~
.i;~ . :
W0~3/20862 1 ~ 0'~ PC~`/U593/~3366
... . .
.
will presently be described, includes a central portion 22b and
24b respectively which spans the cavity of the body portion
with which the membrane is associated (Figure 5).
Disposed between distendable membranes 22 and 24 is a
rigid support, or ullage member 26. Support member 26, which
can be constructed ~rom any suitable plastic such as polypropy-
lene, polystyrene, polyethylene, or polycarbonate, is provided
with a longitudinally extending fluid passageway 30 which is in
communication at one end with a fluid inlet port 32 of an inlet
port assembly 33 and is in communication at its opposite end
with a fluid outlet port 34 of an outlet port assembly 35.
Fluid inlet port assembly 33 includes an inlet adapter 36
having a flange portion 36a and a neck portion 36b which is
closely recei.ved within apertures 18 provided in body portions
12a and 12b. Similarly, outlet port 34 includes a flange
portion 34a and a neck portion 34b which portion ls closely
received within apertures 20 provided in body portions 12a and
12b. It is to be noted that body portions 12a and 12b are also
provided with semicircular shaped recessed portions 39 which
are adapted to closely receive flange portion 38a of outlet
port adapter 38 which in this form of the invention comprises a
port of the vent means for permitting the flow of gases between
atmosphere and the interior of the li.quid delivery apparatus.
Also comprising a part of the liquid dellvery apparatus of
the form of the invention shown in the drawings is a fluid
delivery means which is Ln communication with the fluid outlet
port of the apparatus. :In a manner presently to be described,
the fluid delivery means functions to deliver ~luid to the pa-
tient. This fluid delivery means is shown in Figure 2 as com-
prising a delivery spike~ assembly 40, which is adapted to
cooperate with the fluid outlet port assembly 35.
SUBSTITUTE SHEET
WOg3/20~62 ~ ~CT/US93/~3366
h.l ~040 ! '"``` '~
As best seen by referring to Figures 2 and 5, body~por-
tions 12a and 12b are encapsulated by oxygen non-permeable
encapsulating barrier means shown here as thin layers of mate-
rial 41 and 42 sealably surrounding body portions 12a and 12b
(Figure 9). The character of this sealing material and the
manner in which it is applied will be discussed hereinafter.
~ eceivable within fluid filling inlet adapter 36 is a
check valve assembly comprising a d~ckbill-type check valve 44
of conventional construction which is held in position within
neck portion 36b by an internally threaded retainer ring 46
which is received over flange portion 36a. A threaded closure
plug 4a is threadably received within retainer ring 46 in the
manner best seen in Figure 4. Duckbill valve 44 includes a
yieldably deformable "bill" 44a which functions in the tradi~
tional manner illustrated in Figures 6 and 7, permitting fluid
to flow inwardly in the direction of the arrows designated by
the numerals 50 in Figure 6, but blocking fluid flow in the
opposite direction in the manner shown in Figure 7. It is to
be understood that varicus types of check valves of a character
well known to those skilled in the art can be used in place of
the duckbill valve 44.
Body portions, or structural support members 12a and 12b
can be constructed of any suitable gas permeable, porous mate-
rialls~ch as Polypropylene (PP), Ultra~High Molecular We,ight
Polyethylene (UHMWPE) t High Density Polyethylene (HDPE), Poly~
vinylidene Fluoride (PVDF), Ethyle-vinyl Acetate (EV~), Styrene
Acrylonitrile (SAN), Polytetrafluroethylene (PTFE) a~d porous
cPllulose acetate. A suitable source of these materials is
Porex Technologies of Fairburn, Georgia. Howe~er, practic~
has shown that any porous plastic material including an open
~ Sl3B~;TITUTE SHEET
:'
W093~t086~ 4 ~ PCT/U593~03366 !:
~, I
, 9 .
cell, porous sponge material which permits the free passage of
gases therethrough is suitable. As described in the following
paragraphs, to enable venting of gases from the fluid chamber, ~-
membranes 22 and 24 can also be constructed from a suitable gas
permeable material.
In practice membranes 22 and 2~ can be single layers or
laminates and can be manufactured from several alternate mate-
rials including rubber, plasti~s and other thermo-plastic
elastomers. These include latex, rubber polyisoprene, butyl
rubber, nytrial rubber~ other homopolymer, copolymers, mechani-
cal poly blends and interpenetrating polymer networks. Exam-
1~ .
ples of materials found particularly well suited for the con-
struction of the high gas permeable membranes include silicon
polymers which are castable into thin film membranes having
high gas permeability. Depending upon the fluid to be dis-
pen~sed from the apparatus, other materials of choice for fabri-
cating the membranes include polyurethane-polysiloxane, copo-
lymers, blends and IPNs (interpenetrating polymer network
materials). In certain applications, low gas permeable mem-
branes such as floro-silicons and floro-elastomers may be
desirable. Manufacturers of materials;suitable for use in the
construction of the distendable membranes 22 and 24 include Dow
~ :
Chemical, 3M Company, General Electric, Mobay Chemical, Shell
Oil Corporation, DuPont, and Union Carbide Corporation.
Th~e previou~ly mentioned vent means of the lnvention lS
adapted to provide for make-up aLr during Liquid delivery so
that an even outflow of solution from the apparatus is ob-
tained. To permit the flow of gases between atmospheré and the
. ~ ;,
interior of the apparatus, flange 38a of outlet adapter 3& is
j~ provided with circumferential~ly ~spaced~apertures 38c which
permit free flow of air toward~fluid chambers 14a and 14b in
SUB~TITUTE S;HEFT
W O 93/20~62 `~ 4 ~ PC~r/US93/03366
the manner shown by the arrows 51 in Figure 7. In practice an
oxygen impermeable sterile barrier patch 53 is removably af-
fixed to flange 34 so as to cover apertures 38c.
By referring to Figure 8, it can be seen that the encapsu-
lating means, shown here as an outer barrier which surrounds
the body portions 12a and 12b is perforated in the area of the
apertures 38c provided in the flange 38. These apertures in
the outer barrier permit air from atmosphere to flow into the
porous body portions 12a and 12b in the manner shown by the
arrows in Figure ~ and designated by the numeral 51.
Turning to Figure 9, the encapsulating means or outer
barrier in the embodiment of the invention there shown compris-
es an outer paper wrap 42 covering an inner metalized wrap or
encapsulation material ~1. The outer harrier or encapsulating
means can take several forms so long as it produces an oxygen
impermeable, anti-microbial leak-free aseptic container. For
example, the encapsulation means can comprise a barrier lami-
nate structure which is made up of a plurality of specific
high-strength polymer resin layers which effectively prevent
formation of pin holes or cracking of oxygen barrier layers
during package formation. One type of oxygen impermeable,
leak-free container material is disclosed in U.S. Patent No.
4,983,431 issued to Gibbons et al. Disclosures in this patent
r~lating to leak-free packaging are also applicable to the
! I ~ encapsulation means of the aseptic package of the present
~ invention. U.S. Patent No. 3,998,378 issued to Vetten de-
I scribes methods of fabricating a folding box having a liquid
tight cemented bottom and improved stability. Techniques
discussed in the Vetten patent can also be used in the con-
struction of the outer barrier or encapsulating means of the
present invention. To patents cited in Giboons, et al. and
SUBSTlTlJTE SHEET
W0~3/2086~ PCT/US93/03366 .Ii.;;
. r~ 3 0
Vetten are also pertinent ~o the construction of the encapsula-
tion barrier of the present inven~ion. Other pertinent prior
art United States patents include 4,239,150 issued to Schadows- ``
ki, et al., 4,~54,693 issued to Shadowsski, et al., and 4,287,
2.47 issued to Reil, et al. The teachings of these prior art
patents and the patents cited therein are more than adei~uate to
inform those skilled in the art of the various techniques and
materials that can be used in fabricating the encapsulating
means, including oxygen impermeable aseptic containers, of the
invention.
Turning now to Figures 4 and 7, it is to be observed that
outlet port 34 is initially closed by a frangible diaphragm 56
which form an integral part of the outlet adapter 38. It is
also to be noted that neck 38b o~ outlet adapter 38 is inter-
nally threaded with threads 58 which are adapted to threadably
receive external threads 60 provided on the delivery spike
assembly 40. Delivery spike assembly 40 includes an outwardly
extending, generally cylindrically shaped portion 40a that
termiinates in a sharp spike or point 40~. Point 40b i5 adapted
to pi~rce frangible membrane 56 when the delivery spike is
threadably connected with the outlet port assembly 34 in the
manner illustrated in Figure 7. An elastomeric O ring 64 is
received within a groove 65 provided in cylindrical po~tion 40a i:
and sealably engages the internal walls o~ neck 3~b in the
mannerishown in Figure 7. This prevents leakage of fluid from
;
the pressurized container past the deliv~ery spike and to the ~ .
:` outside of the container.
Also formin~ a part of the deli~ery spike asse~ily of this
form of the invention is a tubular conduit C that communicate
with an external flow rate control means shown here as a cylin-
drical housing 65 having contained therewithin a porous mass of
SU13STITUTE SHEET
~ : .
: ~
W093/20862 PCT/US93/~3366 .~
l O
12
material 6-/ such as porous teflon, through which the discharg-
ing liquid must flow. Flow rate can be precisely controlled by
proper selection of the material 67 in a manner well known to ~ ;
those skilled in the art.
In using the apparatus of the present invention, plug 48
is first removed and threadably inserted in its place is a fill
tube 66 having a threaded fitting 68 which is receivable within
retaining ring 46 ~Figure 6). In the aseptic filling process,
the nutrient solution to be delivered to the patient is intro-
duced through the check valve 4~. The fluid being introduced
will impinge upon membranes 22 and ~4 causing them to distend
from a first at rest position shown in Figures 4 and 5, whèrein
the central portions of the membranes are in proximity with
support member 26, to a second distended position shown in
Figures 6 and 7, wherein the central portions of the membranes
are in proximity with the internal walls deflning cavities 14a
and 14b. Where permeable elastomeric membranes are used, gas
in the solution being introduced into the carton can pass
through the membranes 22 and 24 and mlgrate to porous foam
blocks 12a and 12b with subsequent venting at time of use to
atmosphere through orifices 38c. It is to be understood that
distention of the membranes from the first to the second posi-
tion creates internal stressas of predetermined direction and
magnitude in the speciflcally tailored thin films of elastomer-
ic memb~anes which tend to uniformly return them to their
original, non-distended position shown in Figures 4 and 5. ~;
So long as frangible diaphragm 56 is in tact, the benefi-
cial agent or solut.ion to be delivered to the patient will
remain within the device. However, as soon as the diaphragm is
ruptured by the delivery spike 40, the controllably stressed
elastomeric membranes 22 and 24 will attempt to return to their
Sl1B5Tl~UTE SHEET
W093/20862 PCT/US93/03366 i~
;... 1``.`.
~J118i~0 ' I
13
original, non-distended configuration and will controllably and
uniformly force the fluid outwardly through the delivery passa-
yeway 62 of the delivery spike into -tube 63 and toward the
patient in the direction of the arrow 64 shown in Figure 7.
In certain applications, the retaining ring 46 and check
val~e assembly 44 can be recessed into body portions 12a and
12b so that after aseptic filling of the carton is complete,
the outer most barrier of the encapsulation means can be folded
over the check valve assembly in a manner to effectively seal
it relative to atmosphere.
Referring now to Figures lOa, 11 and 12, another form of
the liquid delivery apparatus of the present invention is there
illustrated. This form of the invention is imilar in most
respects to the form of the invention described in the preced-
ing paragraphs. Accordingly like numbers have been used to
identify like components. The principal differ~nces between
this latter embodiment of the invention and the former embodi-
ment resides in the provision of a differently configured check
valve assembly 80 as well as differently configured distendable
membrane assemblies 84.
The check valve assembly of this latter embodiment of the
invention comprises an outer sIeeve 86 which is receivable
within fluid inlet 32. Provided proximate the outboard end of
sleeve 86 is a flange 88 which is bondably interconnected with
flanqei36a of thé inIèt adapter 36. The check ~alve member of
this alternate form of the invention comprises a generally
cylindrically shaped member so havi~g a body portion 92 and a
reduced diameter neck portion 94. ~A shoulder 96 is formed at
the junctiQn of neck portlon 94 and body~portlon 92~. Check
valve member 90 is reciprocally movable within sleeve 86 from
n outward sealing posit~ion wherein shoulder 96 sealably en-
SUB~IT~TE SHE~T
W093/20~6~ PCT/US93/03366
4 0 , ~ : `
14
gages an internal shoulder provided in member 88 to a retractedposition wherein liquid will be permitted to flow through inlet
port 32 and into pressural communication with the distendable
membrane assemblies 84 of this form of the invention. It
should be understood that once the device is pressurized by the
filling of the nutritional fluids, the check valve member 90
will be urged into a sealing forward position blocking liquid
flow outwardly through the inlet port 32. However, during the
filling op~ration, the check valve member is movable rearwardly
of sleeve 86 so as to pe~mit fluid flow through a plurality of
circumferentially spaced fluid flow passageways 97 provided in
check valve member 90 (Figure 11).
Turning now to Figure lOa, it is to be noted that each of
the distendable membranes of this later form of the invention
comprises a laminate structure made up of a plurality of layers
of elastomeric material 84a, 84b and 84c. This assemblage
functions in much the same way as earlier described distendable
membranes 22 and 24. However, by constructing each of the
~tored energy members from a composite of several distinct thin
films or layers, the elastic characterlstic of the stored
energy means can be precisely tailor~d and can be uniquely
constructed to function not only as a fluid driving medium but
also as a gas permeability valvP. The selective arrangement of
the di~ferent films that make up the stored energy means, each
j
with its~own asc`ending permeability constant, will dictate the
direction of flow ~f various gases and vapors. Vapors con-
tained within the solution introduced into the device can pass ~ i.
through the stored~energy means in one direction while external
gases will be precluded from negative migration into the reser- '
.
voir .
The solution conta1ned within the device is delivered to
: :
~ SUBSTITUTE $HEET
W093/20862 P~/US93/03366
. 1`;
i
the patient through the delivery spike assembly 40 in the same
manner as was described in the discussion of the previous
embodiment. Similarlyl make-up air is supplied by the vent
means throu~h apertures 38(c) in the manner discussed in the
preceding paragraphs.
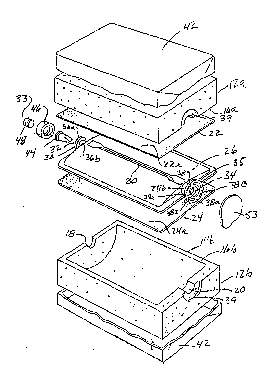
Referring now to Figures 13, 14, 15 and 16~, still another
form of the liquid delivery apparatus of the present invention
is there illustrated. This latest form of the invention is
also similar in many respects to the invention described in the
preceding paragraphs. Accordingly, like numbers are used in
these figures to identify like components.
As best seen by referring to Figure 13, the liquid deliv-
ery apparatus of this latest form of the lnvention comprises a
base assembly 100, a carton-like body 102 within which the base
assembly is encapsulated and a distendable membrane assembly
104 which overlays base assembly 100. Base assembly 100 has a
fluid inlet and a fluid outlet 106 and 108 respectively and
includes a cenkral, convex portion ll0 which~is circumscribed
by an edge portion 112. Distendable membrane~assembly 104 also
includes a central portion 114 which is circumscribed by upper
and lower edge portions 116 and 118 respectively. As shown in
ure 20, distendable membrane assembly 104 can be made up of
at least two, but ~referably a plurality of~thin film distend-
able membranes 104a, 104b and 104c. For example, layer 104a
which isidis~al to,the!reservoir comprises a thin film elastom-
er of a first thickness and a first permeabillty. On the other
hand, layer~104c which is proximal to the~reservoir, comprises
a thin elastomer film~of a second thickness and a second permie-
;ability. This film~is uniquely selected to~be compatible~in
all~ respects with~the ~luid continued w~ithln the reserv~oir.
L~ay~104b~can be of yet another thiokness and permeability and,
Sll88TITUTE S5~E~E T :: ~ ~
". ~
, ~
W093/20~62 . ~ U ~ PCT/US93/~3366
16
if desired can also have different perm-select characteristics.
As previously described, the selective arrangement of the
different films, each with its own individual permeability
constants in ascending order, will dictate the direction of
flow of selected gases and vapors through the stored energy
means.
Turning now to Figures 15, 16, and 20, it can be seen that
body assembly 102 includes an inner barrier member 119 having
internal surfaces 120 which define a cavity or fluid reservoir
122. Barrier member 119 is provided with end flaps (not shown)
whic~h can be folded over into the position shown in the draw-
ings after the reservoir 122 is filled in the manner presently
to be described. Distendable membrane assembly 104 is distend-
able from a ~irst position wherein the central portion 114
thereof is in close proximity with the central portion 110 of
base assembly 100 to a second distended position wherein cen-
tral portion 114 is in close proximity with the upper internal
walls 120a of body assembly or carton 102. The membrane assem-
bly also moves into close proximity with the internal surfaces
120b of walls 120 of the carton (Figure 16~ and with the inter-
nal surfaces of end walls 120c (Figure 15.) As before, when
the distendable membrane assembly lQ4 is distended from the
first to the second position, internal stresses are developed
within the membrane which tend to uniformly return it toward
its fi.rs~ position in close proximity with the cen~ral portion
110 of base assembly 100.
Referring to Figures 13, 14, and 16 it can be seen that
base assembly 100 is provided with a longitudinally extending
~low channel 130 which communicates with an internal longitudi-
nally extending flow passageway 132 that extends between and
interconnects tugether inlet 106 and ou~let 108. As best seen
S~BSTITUTE SH~T
W093/20862 PCT/US93/03366
` t` ~l80~ i
17
in Figure 16, flow passageway 132 is formed internally of a
semitubular shaped, longitudinally extending protuberan~e 133
formed integrally with base assembly 100.
Disposed proximate the inlet portion of fluid passageway
132 is a check valve assembly generally designated by the
numeral 134. A similar checX valve 136 is disposed proximate
the outlet portion of fluid passageway 132. Referring to
Figure 15, it can be seen that inlet check valve 134 is receiv-
able within a cylindrically shaped ret~ining member 140 which
is receivably within an enlarged diameter portion 133a of
protuberance 133. Member 140 has an lnternal shoulder 142
adapted to engage an external shoulder 144 formed on check
valve 134. When the check valve 134 is in the closed position
shown i Figure 15 wherein shoulder 144 is in enyagement with
internal shoulder 142 of member 140, the flow of fluid inwardly
into fluid passageway 132 is blocked.
Check valve 136 which is positioned proximate the outiet
of ~luid passageway 132 is held in position within the fluid
passageway by a retainer member 146 which is disposed within
passageway 132 in engagement with a~ internally threaded outlet
receptacle 147. Outlet receptacle 147 which comprises a part
of the vent means of the invention, includes a tubular body
portion 147a and flange portio~ 148 having a~plurality of
cir~umferentially spaced apertures 150 (Figure 13). As best
, ~ j seen by referring ~o Figure 15, the reduced diameter po~tion
136a of check valve 136 is movably receivable within a bore
provided in retainer member 146. With the~shoulder 136b of the
che~k valve 136 in sealable engagement member 146 fluid flow
through passageway 132 in a directlon toward outlet 108 is
ef~ectively blocked.
Also disposed~ within f luid passageway 13 2, intermediate
S~BSTITUTE S~EET
~`.;''`:: :~ :
W0~3/2~862 , PC~/US93~03366
0 4
18
chack valves 134 and 136, is a flow rate control means shown
here as an elongated, generally cylindrically shaped porous
fil~er member 160. Member 160 can be constructed of any inert
porous material such as a ceramic or porous plactic, fluid
permeable material and can be tailored to provide a precise
rate of fluid flow through passageway 132 in a manner well
known to those skilled in the art.
As best seen in Figure 18, at the outlet portion of the
apparatus tuhular body portion 147a of receptacle 147 is posi-
tioned within an enlarged diameter portion 132a of flow passa-
geway 132 with flange 148 of member 147 positioned against base
assembly 100. A hydrophobic filter vent means for venting air
but not moisture is here shown as disk shaped member 166 which
is appropriately bonded to the interior surfaces of flange 148
I of member 147 in the manner shown in Figure 18. A material
such as hydrophobic PTFE, polytetrafluoroethylene incorporating
laminated polypropylene or hydrophobic acrylic copolymer sup-
ported on nylon nonwoven substrates is suitable for t.he con-
struction of member 166.
With outlet check valve 136 in a closed position, chamber
or reservoir 122 is filled with the selected eeding solution
by inserting an appropriate filling conduit into the inlet
portion of the device (not shown). The filling conduit is
adapted to move check valve 134 inwardly permitting the feeding
solution to flow into passageway 132 and then outwardly of
channel 130 where it impinges on membrane assembly 104 with
sufficient pressure to distend it in to the position shown in
Figures 15 and 16. ~
:
After xeservoir 122 has been filled, the pressure of the
solution within the xeservoir will~maintain both the inlet and
outle.t check valves 134 and 136 in the closed position shown in
~` ~
~: : SUE3STITUTE SHEE~T
!l ;
''i
i'`t~ :
W093/20862 ,~l`i,~Q~ P~T/~S93/03366 ~ `
' . , ':
19
Figure 15~ Following filling of the reservoir, a retainer disk
162 is positioned over the inlet or filling port 106 and the
end .flaps of barrier member 119 and folded over to hold disk
162 as well as outlet receptacle 147 in position. This done,
an outer barri,er layer 167 (Figures 13 and 20) is emplaced over
the entire assemblage so as to completely encapsulate it within
a sealed oxygen impe~meable, antimicrobial, leak-free aseptic
container of the character previously descr.ibed herein. Final-
ly a disk shaped metalized seal 168 is positioned over the
outer barrier in the proximity of the vent means or flange 148
of outlet receptacle 147. The apparatus of the invention is
now ready for shipment storage and subsequent use in the field.
The ~eeding solution contained within reservoir 122 is
accessed by a delivery spike 170 of a construction similar to
delivery spike 140 of the earlier dascribed embodiments. As
shown in Figure 19, delivery spike 170 comprises a finger grip
portion 172, a flange portion 174 and an externally threaded
neck portion 176. For a purpose presently to be described, a
plurality of circumferentially spaced, outwardly extending
poinked protuberances 177`are provided on flange portion 174.
Turnin~ now to Figure 21, upon piercing the metalized seal
168 and the barrier layers 119 and 167, threads provided on
: neck portion 176 of the delivery spike can ~e moved into
threadable engagement with the internal threads 181 provided on
~,outlet'receptacle 147 in the manner shown in Figure 21. As the
neck portion of the delivery spike advances into receptacle
147, the end of the neck portion will engage outlet check valve
136 moving it into an open position which will permit liquid
within reservoir 122 to flow through channel 130, into passaye-
¦ way 132 and then into central passageway 180 provided in the
¦ d~eilivery spike. Ais elastomeric o ring 182 is carried ~y neck
1 ~ . SU8STITVTE SHEET
W093/~0862 ~ Q ~ u PCr/U593/03366 `''~
portion 176 for engagement with the internal wall of the outlet
receptacle to prevent leakage of the feeding solution past the
delivery spike.
As illustrated in Figure 21, as the check valve 136 is
moved into the open position, protuberances 177 will pierce
metalized seal 168 and will extend into vent apertures lS0
I thereby creating openings which permit make-up air to flow into
I the device as the feeding solution is introduced into the¦ patient.
Having now described the invention in detail in accordance
with the requirements of the patent statutes, those skilled in
this art will have no difficulty in making changes and
modifications in the individual parts or their relative assem-
bly in order to meet specific requirements or conditions. Such
changes and modifications may be made without departing from
the scope and spirit of the invention, as set forth in the
following claims.
~ .
~ :
: : ''
~ i
' 1 ~ ?
, ~ !
' ' I~
'i: .
i :
SUBSTITUTE SH~ET
~?
'!I