Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- 21~9857
St-klu-63 8-PE
A process for the preparation of metal hydroxide
BACKGROUND OF THE INVENTION
The present invention relates to a process for the preparation of metal hydroxides
5 and/or metal oxidesthydroxides from co~ pollding metal ions and hydroxide ions,
in which the metal ions are formed in an electrochemical membrane process by
anodic dissolution of corresponding metals in the anode col,lp~llnent and the
hydroxide ions by cathodic reduction of water in the cathode compartment
bounded by an anion-exchange membrane, and the hydroxide ions are transferred
10 through the anion-exchange membrane into the anode compartment under the
driving force of an electric field.
Metal hydroxides and metal oxides/hydroxides are valuable intermediates for the
plepal~lion of inorganic or organic salts of said metals, for the corresponding
oxides, or the pure metals themselves. For example, a cobalt oxide of defined
15 composition e.g. for use in electronics for the production of varistors or inbatteries, may be plepaled from cobalt hydroxide by calcining or, by reduction, a
cobalt metal powder of defined particle size distribution. Nickel hydroxides areused as pigments or are used with various dopants and particle structures for use
in batteries. Zinc hydroxides may be used as starting materials for pigments and20 the copper compounds can be converted into catalytically active m~t~ri~l.c.
During the prepdl~ion of hydroxides for various applications, the primary aim isto prepare, if possible, compact and free-flowing m~t~ri~l for further processing
Cobalt metal powder, prepared from cobalt hydroxide or cobalt oxide/hydroxide,
yields a particle size distribution and particle structure such that it can be sintered
25 together with t~lng~ten carbide to produce products such as, e.g. special carbide-
tipped structures.
STA 91-Forei~n Countries - 1 -
2149 8ra7
For the newly developed foam anodes which are used particularly in nickel
hydride storage cells, a nickel hydroxide is required the physical properties ofwhich are optimized both in terms of the application and the processing technique
used. Use in high-performance batteries with nickel foam electrodes (based on
5 paste technology) requires a m~t~ri~l with good flow properties, a compact particle
form, narrow particle size distribution and consistent quality. Moreover, the
product should have the ability to mix readily with the conventionally used
additives such as, e.g., cobalt metal powder and cobalt oxide.
A corresponding m~t~riAI and main features of the production process are
described in Japanese patent Hei 4-80513. Nickel hydroxide particles with a
diameter between 1 and 100 ~lm are cryst~lli7ed through a nickel salt solution and
an alkali hydroxide, in solid or liquid form, being introduced contin--ously into a
reaction vessel at a constant pH and a constant temperature. A pH of 11 and a
temperature of 48 C are given as favorable test conditions.
15 It is also known that the pr~p~Lion of a sufficiently compact nickel hydroxide
may be carried out by precipitation in the presence of ammonia or an ammonium
salt. According to Trans. Faraday Soc. 51 (1955) 961, a nickel amine complex
solution is plepared from nickel nitrate and aqueous ammonia solution, from
which a nickel hydroxide is obtained by boiling at cllstom~ry or reduced pressure
20 or by treatment with steam. Such a hydroxide has a much lower specific surface
area (13 to 20 m2/g) compared with the nickel hydroxides that are precipitated in
the absence of ammonia. The prepa~Lion of compact nickel hydroxides in the
presence of ammonia or an ammonium salt is also disclosed by the Japanese
patent applications A 53 6119 and A 61-18107. In the first patent application
25 mentioned, the precipitation of nickel hydroxide by the addition of an alkaline
solution to a corresponding solution with a pH of at least 3.0 is described.
Electrochemical tests on the material prepared in this way revealed particularlyhigh specific charge capacities compared with commercial nickel hydroxides.
STA 91-Forei~n Countries - 2 -
2149857
Such products do not yet, however, fulfill the above-mentioned requirements in
terms of particle form, particle size distribution and flow properties.
Important features of the process for the prepalalion of a compact nickel
hydroxide and the use thereof in alkaline batteries are described in European
patent application A 353 837. A nickel (II)-tetrammine salt solution is preparedby dissolution of nickel nitrate or nickel sulfate in dilute ammonia solution and
decomposed by controlled addition of sodium hydroxide solution in accordance
with the following reaction:
(1) Ni(NH3)4SO4 + 2 NaOH --> Ni(OH)2 + Na2SO4 + 4 NH3
The reaction takes place at tempel~ures between 40 and 50 C and in the pH
range between 11 and 13. The pore volume falls as the pH falls. It has been
ascertained that a pore-free product may be cryst~lli7ed only at sufficiently low
rates of reaction. Moreover, the nickel hydroxide prepared according to said
process has a high cr,vstallinity, a low specific surface area, a low pore volume
and therefore a high physical density. The disadvantages of said product, which
are attributable to the high density, are also described. The low specific surface
area results in a lower proton conductivity and in a higher current density which
promotes the production of ullwanled ~-NiOOH, which leads to swelling of the
electrode. Although the nickel hydroxide crystallised at low pH values has a high
densit,v, it has a greater tendency to form ~-NiOOH. Due to the choice of a meanpH, a compromise may be found between the required high density and the
porosity required to a certain extent. According to said process, a nickel
hydroxide is prepared which contains 3 to 10% of zinc and 1 to 3% of magnesium
in solid solution. Said dopants counteract the production of ~-NiOOH.
A continuous process for the cryst~lli7~tion of a nickel hydroxide with spherical
particle form is implied from the patent JP Hei 4-68249. In said process, a nickel
salt solution (0.5 to 3.5 mole/l), dilute alkaline solution (1.25 to 10 mole/l) and an
ammonia and/or ammonium salt solution are pumped continuously by means of
STA 91-Forei~n Countries - 3 -
- 2149~S7
metering pumps, with intensive stirring, into a heated cylindrical container
provided with an overflow pipe, it also being possible for the ammonia to be
introduced in gaseous form. The ammonia concentration is given as 10 to 28% by
wt. and the ammonium salt concentration as 3 to 7.5 mole/l. In order to complex
the nickel, between 0.1 and 1.5 mole of ammonia per mole of nickel salt solutionare introduced. After about 10 to 30 hours, the system reaches a stationary state,
after which a product with consistent quality may be removed contimlol~ly. The
residence time in the container is between 0.5 and 5 hours.
An important feature of said process is the fact that the reaction is carried out at a
defined pH which is kept constant to + 0.1 pH levels in the region between 9 and12 by the pH-controlled introduction of alkaline solution, and at a constant
temperature in the region between 20 and 80 C, whereby the temperature
deviations should not be more than + 2 K. Under these conditions, the compact
spherical particles are obtained with a particle size of between 2 and 50 ~lm. The
particle size can be adjusted in particular by varying the NH3 inflow, the residence
time and the stirring speed. As the stirring speed decreases and the NH3 inflow
increases, the particle size increases. As the residence time in the container
increases, the product becomes coarser, the particle size distribution nallower.The crystalline product is then filtered, washed with water and dried. The product
prepared according to said process has the properties mentioned at the beginningand does not need to be ground.
A process for the preparation of nickel hydroxide is disclosed in European patent
application A 462 889. The temperature range of cryst~ tion is above 80 C.
Nitrate or sulphate solutions doped with cobalt, cadmium and/or zinc are used.
The cobalt content is between 1 and 8% by wt., and the contents of cadmium
and/or zinc are between 3 and 10% by wt. Complexing takes place with the aid
of an ammonium salt, the molar ratio of NH3/Ni being between 0.3 and 0.6. In
said process, a pH of 9.2 + 0.1 is maintained. Moreover, a three-blade stirrer, the
diameter of which is half as great as the diameter of the container and the rate of
rotation of which is between 300 and 1000 min~l is used.
STA 91-Forei~n Countries - 4 -
214~8~7
As in the processes already described, the product is filtered, washed and dried.
The disadvantages of said processes are, on the one hand, the large quantities of
neutral salts inevitably produced, which are at least twice the stoichiometric
quantity of nickel hydroxide and are released into the effluent. On the other hand,
5 the effluents of these processes contain, in addition to small quantities of nickel
dissolved in the form of complexes, large quantities of ammonia which must be
disposed of.
In the chemical process of precipitation cryst~lli7~tion for the plep~lion of
spherical nickel hydroxide, 2 mole of sodium chloride per mole of nickel
10 hydroxide are inevitably produced. In view of more stringent environmental
guidelines and limits for effluent, on the one hand, and economic considerationsdue to the high consumption of liquor and resulting landfill costs for the salt
produced, on the other hand, closed production circuits must be developed.
In such a process, nickel, for example, is dissolved anodically in a metal salt
15 solution by electrolysis, and precipitated as nickel hydroxide by the cathodically
formed hydroxide ions. After sedimentation and various sl1cces.~ive washing
stages to purify the precipitated product of any salts still present or entrapped
during precipitation, the pure product is obtained.
Processes for the preparation of metal hydroxides are already described in the
20 following patents.
In Japanese patent application A 63/247 385, the electrolytic prep~lion of metalhydroxides is carried out using a perfluorinated anion-exchange membrane of
Toyo Soda and the use of inert electrodes. The metal salt of the metal hydroxideto be prepared is used as the electrolyte on the anode side. In the cathode circuit,
25 an alkaline solution is used.
STA 91-Forei~n Countries - 5 -
~ 2149857
In European patent application A 0 559 590, in a comparable arrangement, the
metal salt is added continuously by anodic dissolution of the electrode. The
requirements regarding the process, particularly the membranes to be used, the
electrolyte solutions and the test conditions are specified only insufficiently.
5 The object of this invention is to provide a process for the prepal~ion of metal
hydroxides and/or metal oxides/hydroxides which does not have the disadvantages
of the prior art described.
SIJMMARY OF THE rNVENTION
This object is achieved by a process for the plep~lion of metal hydroxides and/or
10 metal oxides/hydroxides from corresponding metal ions and hydroxide ions,
whereby the metal ions are formed in an electrochemical membrane process by
anodic dissolution of corresponding metals in the anode compartment and the
hydroxide ions by cathodic reduction of water in the cathode compartment
bounded by an anion-exchange membrane, and the hydroxide ions are transferred
15 through the anion-exchange membrane into the anode compartment by the drivingforce of an electric field, whereby the dissolution of the metals is carried out in
the presence of complexing agent at a pH of >7.
Preferably ammonia and/or organic mono- and/or diamines with a chain length of
1 to 6 C atoms are used as complexing agents within the meaning of this
20 invention. Metals are, in particular, one or more from the group Co, Ni, Cu, Fe,
In, Mn, Sn, Zn, Zr, Ti, Al, Cd and Ni. Co and/or Ni are particularly preferred.
The process according to the invention will be described hereinafter for the case of
the preparation of nickel hydroxide without thereby restricting the invention.
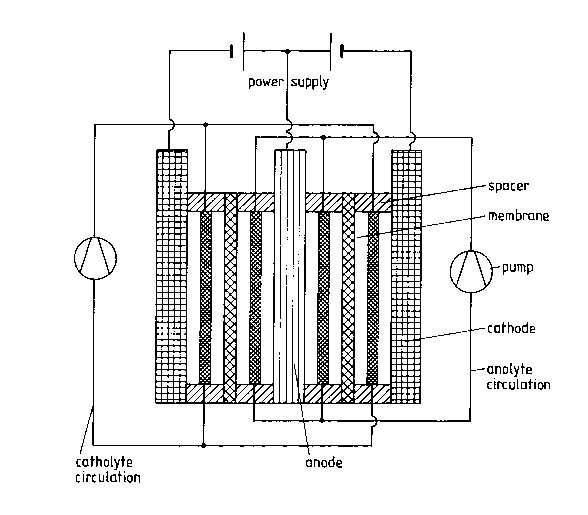
The configuration arising in principle for a membrane electrolytic cell which is25 suitable for carrying out the process according to the invention is presented below.
The cathode compartment and anode compartment of the electrolytic cell are
separated by an anion-exchange membrane such that two separate circuits are
STA 91-Forei~n Countries - 6 -
~ ~498~7
produced. The circuit on the side of the cathode is called the catholyte and that
on the anode side the anolyte. Alkaline solutions such as preferably sodium
hydroxide solution or potassium hydroxide solution are used as catholyte. It is
advantageous for the efficiency of the process if the solution itself has a high5 conductivity and the cation of the alkaline solution used is also used on the anode
side. The cathode itself may be composed of quenched and tempered steel,
pl?~tini7ed titanium, nickel, or a nickel alloy.
The composition of the anolyte is obtained from the starting products for the
prepal~lion of nickel hydroxide, i.e. ammonia, sodium chloride and small
10 quantities of nickel sulfate. The sodium chloride is used primarily to increase the
conductivity of the solution, and the anodic dissolution of the nickel electrode is
improved by the small addition of sulphate. Particularly good results are obtained
when chloride and/or sulfate ions are present in the anolyte. The anode itself is
composed of pure nickel, preferably an electrochemically produced anode.
15 In the preparation of other metal hydroxides and/or metal oxide/hydroxides, the
anode is composed of the corresponding metals. In principle, therefore, a
sacrificial anode is used.
Under active transport conditions in view of the extf~rn~l potential applied, nickel
dissolves as Ni2+ ion, releasing electrons. The presence of ammonia pr~venls the20 spontaneous precipitation of Ni(OH)2 under alkaline conditions and leads via
various intermediate stages to a divalent nickel-amine complex.
Anode: (2) Ni + nNH3 + 2 Cl- ~ Ni (NH3)nC12 + 2e~
(3) Ni(NH3)nCl2 + 20H- ~ Ni(OH)2~l n NH3 1` + 2 Cl-
Cathode: (4) 2 H20 + 2 e~ ~ H2 1` + 2 OH-
(5) Ni + 2H20 ~ H2 1` + NI (OH)
STA 91-Foreign Countries - 7 -
21498~7
The reaction at the cathode yields hydrogen, with electron uptake, which escapesin gaseous form, and hydroxide ions which are transported in accordance with
their charge via the anion-exchange membrane into the anode circuit. The
formation and precipitation of the nickel hydroxide then takes place in the anolyte
5 when the solubility limit is exceeded. Precipitation follows a dynamic
equilibrium, ligand exchange (ammonia for hydroxide) taking place.
The formation of the spherical product is detennined essentially by the
cryst~ tion conditions, i.e. the concentration of the individual components and
the temperature control in the anode circuit. The precipitated product is then
10 separated continuously from the anolyte circuit. Separation may be carried out in
a sedimentation tank that is simple to design from a process technology angle inview of the great difference in density between the product formed and the
solvent. Separation takes place via a filkation stage (microfiltration) in order to
sep6l~le a product of uniform particle size. The essential advantage of said
15 process variant is that additional individual process stages for rec~ ve,ing the
various starting products are not required since they are kept in the anolyte circuit.
In order to put into practice the electrochemical membrane process described, it is
necessary to ensure that the anion-exchange membrane to be used fulfils the
following requirements:
20 It must be resistant to alkalis, in particular, chemically stable in the adjacent
solutions (to NH3 Up to the saturation concentration), resistant to oxidation
(Ni2+/Ni3+; Cl-, Cl03-), temperature-resistant up to 80 C, it must have a high
permselectivity, a low membrane resistance, high mechanical strength and
dimensional stability and sufficient long-term stability.
25 Industrially relevant ion exchange membranes usually have a microheterogeneous
and/or interpolymer morphology. The aim to be achieved thereby is that the
mechanical and electrochemical properties may be membranes adjusted in
isolation. Accordingly, a membrane is constructed from a matrix polymer, a
STA 91-Forei~n Countries - 8 -
2149857
fabric or a binder, and from a polyelectrolyte or an ionomer. A distinction is
made among homogeneous membranes, interpolymer membranes, microhetero-
geneous graft or block copolymer membranes and heterogeneous membranes
according to the degree of heterogeneity of the ion exchange membrane.
5 The polymer network may have a different structure in order to exhlbit sufficiently
good electrical and mechanical properties for most applications. Polyvinyl
chloride and polyacrylate is normally used as the electrically neutral matrix
polymer. Polyethylene, polyl.ropylene or polysulphone may be used as other
matrix polymers, only these having long-term chemical stability under alkaline
10 conditions.
In the process according to the invention, therefore, an anion-exchange membraneis preferably based on polyethylene, poly~lopylene, polyether ketone, poly-
sulphone, polyphenyloxide and/or sulfide.
The ion-conducting polyelectrolytes of an anion-exchange membrane are
15 composed of a network with a positive excess charge and mobile, nega~vely
charged counterions. The fixed ion structure may be built up by weakly basic
amino and imino groups, and from strongly basic ammonium and qu~t~ ry
ammon1um groups:
-NH3+ -RNH2 -R3N+ =R2N
20 It is particularly preferred that the anion-exchange membrane used in the process
of the invention have exchange groups of alkylated polyvinyl imidazole, polyvinyl
pryidine and/or alkylated 1,4-diazabicyclo[2.2.2]octane.
Particularly suitable membranes are described in German patent application A 42
1 1 266.
STA 91-Forei~n Countries - 9 -
21~98~7
The type and concentration of fixed ions determines mainly the permselectivity
and the electrical resistance of the membrane, but it may also influence the
mechanical properties, particularly the swelling of the membrane in view of the
concentration of fixed ions. The strongly basic q~1~t~rn~ry ammonium group is
5 dissociated at all pH values, while the primary ammonium group is dissociated
only with difficulty. For this reason, mostly q l~tern~ry ammonium groups are
incorporated in commercial anion-exchange membranes except if a membrane with
particular properties is to be produced.
Systems based on chloromethylated poly~lylene, styrene/divinylbenzene
10 copolymers and styrene/butadiene copolymers with subsequent qu~tern~ tion with
trimethylamine are used most frequently.
The long-term chemical stability of the anion-exchange membranes may be
influenced only by the following factors:
- destruction of the polymer matrix (inadequate stability of the matrix
polymer or interpolymer in alkaline solution)
- morphological change of the fixed ion structure/polymer matrix system
- chemical degradation of the fixed ion under alkaline or oxidative
conditions.
The electrochemical, mechanical and chemical properties must be optimized in the20 same way for the selection of an anion-exchange membrane for use in the
preparation of spherical nickel hydroxide by membrane electrolysis from
ammoniacal solution. This means that requirements in terms of membrane and
material selection and the physico-chemical properties produced by the
manufacturer must be drawn up and evaluated. Said requirements may be
25 summarized as follows for the membranes used according to the invention:
ST~ 91-Forei~n Countries - 10 -
21498~7
-
As regards the electrochemical properties, the
electrical resistance should be <10 Q. cm2
the perrnselectivity >92%
the swelling <25%, and
S the ion exchange capacity >1.2 mmole.g-l.
As regards the mechanical properties, the fabric should be composed of temperature-, alkali- and oxidation-resistant polymers
(polyplu~ylene, polyethylene, polyether ketone)
and have, as a fixed loading
chemically stable ql1~tprn~ry ammonium salt
(vinyl imidazole, 4.4'-diazabicyclo[2.2.2]octane).
Suitable membranes are described in German patent application 44 21 1266. It is
particularly preferred that the process according to the invention be carried out
continuously, the metal hydroxide and/or metal oxide/hydroxide formed being
15 separated from the anolyte and the complexing agent being returned to the anode
compartment.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The invention is explained by way of example below, without any restriction
being implied therein.
STA 91-Foreign Countries - 11 -
21~9857
Example 1 Preparation of cobalt hydroxides
Structure of the electrolytic cell in principle
The electrolytic cell is shown in attached Fig. 1. It is composed of two nickel
cathodes, two polyethylene spacers, two membranes and the cobalt sacrificial
anode and four compartments of varying thickness. The cell is constructed in
such a way that the nickel cathodes represent the outer sides of the cell with asurface area of 120 x 200 mm2 of effective electrode surface. The electrical
contacting takes place on superimposed electrode surfaces. On the cathode lies apolyethylene frame 5 mm thick on which in turn the membrane lies. The gap
between the membrane and the cobalt anode is m~int~ined by a further frame 10
mm thick, which anode lies above the frame and is provided with the electrical
leads. The cobalt anode is composed of pure cobalt with a thickness of 20 mm.
The entire structure is pressed together in a liquid-tight manner by means of a
support. A polyethylene lattice core mesh is inserted between the cathode and the
membrane, which lattice prevents contact between cathode and membrane. The
frames that separate anode and membrane are provided with holes through which
the anolyte enters and leaves. The cathodes are similarly provided with inlets at
the cathode-membrane separating frames such that a uniform flow with the
catholyte is guaranteed in the entire cathode compartment.
The catholyte and anolyte each contain 100 g/l of NaCl. The catholyte also
contains 40 g/l NaOH.
The catholyte is pumped round (recirculated) at a speed of 100 l/h, which
corresponds to a residence time of the electrolyte of 9 seconds in the cathode
compartment. The anolyte is pumped in a recirculating circuit during electrolysis
- 25 at a rate of 650 l/h which corresponds to an average residence time of 2.7 seconds
in the anode compartment. The temperature of the anolyte is 50 C. The ammonia
concentration in the anolyte is adjusted to 2 mole/l and losses by evaporation are
offset by the addition of ammonia to the anolyte circuit.
STA 91-Forei~n Countries - 12 -
21198~7
-
The stationary solids concentration of cobalt hydroxides formed is 80 g/l with an
average residence time of 4 h.
The electrolysis conditions are chosen such that a current of 12 A correspondingto 500 A/m2 flows, whereby 21 g of cobalt hydroxide in the form of Co(OH)2 are
5 formed each hour, which are expelled from the circuit in a 0.261 suspension and
separated by filtration. After washing with water, a clean cobalt hydroxide is
obtained. The hydrogen formed is expelled from the catholyte storage container.
pH anolyte: 10.5-11.5
Membrane: Neosepta~ AMH, m~mlf~c~lred by Tokuyama Soda
10 Composition of the end product:
Cobalt hydroxide, mixture of Co(OH)2 with CoOOH in a ratio of 80/20 according
to analysis.
Bulk density: 1.6 g/cm3
Cobalt content: 63.5%
15 Color: Dark brown.
Example 2 Plepal~ion of nickel hydroxide
In an electrolytic cell which is constructed in a comparable way (as in Example 1)
as a stack of electrodes and membranes with the electrode compartments in
between, nickel is dissolved electrochemically in the presence of ammonia, and
20 the amine complex formed decomposes to nickel hydroxide.
Electrolyte composition:
Anolyte: 16.5 mole/l NiSO4
220 ml NH3 (25%)/1
STA 91-Forei~n Countries - 13 -
21498~7
2 mole/l NaCl
Catholyte: 1 mole/l NaOH
Anode: Ultra-pure nickel
Cathode: Pl~tini7ed titanium
Temperature: Electrolysis 40 C
Decomposition of the complex 70 C
Current density: 1000 A/m2
Gap between electrode/membrane: 2 mm
Overflow speed: >10 cm/s
pH anolyte: 10.5-11.5
Membrane: Neosepta~) AMH manufactured by Tokuyama Soda
The decomposition of the amine complex formed in electrolysis to nickel
hydroxide is carried out by raising the telllpel~lult; of the electrolyte in a reactor.
a) Preparation of a compact, spherical nickel hydroxide
The amine complex is decomposed in a stirred reactor whereby the
decomposition product agglomerates to compact, spherical particles. The
agglomerated m~tP.ri~l is sepal~ted continuously as a suspension from the
circuit of the anolyte via an overflow.
Nickel hydroxide from the overflow:
Bulk density: 1.35 g/cm3
Mean particle size: 10 ~lm
b) In the presence of substrates such as fibers of nickel or a spherical ion
exchange resin with the mean particle size of 200 ,um, a uniform layer of
nickel hydroxide is deposited on the substrate in the decomposition reactor.
STA 91-Foreign Countries - 14 -