Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02274815 1999-06-11
PCT/US96/19949
- 1 -
OftIS~RY I~TC0~1'fIS~C~ D89ICL
Backcround o~he Invent~ton
This invention relates to the field of devices or
' appliances used to relieve or mitigate the problems
associated with human urinary incontinence. More
specifically, the present invention relates to a
removable external closure for the human urethra.
Urinary incontinence, due to disease, injury, or
other causes, is a troublesome problem for many
i0 individuals. Surgical intervention is often required to
treat severe cases of incontinence, but in tho:e cases
where the patient.suffers from only a partial loss of
bladder control, or where the patient is otherwise a poor
candidate for surgery, nonsurgical treatment is called
i5 for. While both male and female patients may be good
candidates for nonsurgical treatment, such nonsurgical
approaches are particularly appropriate for f~male
patients who suffer from the partial, sporadic loss of
bladder control sometimes referred to as "stress
2o incontinence" or "urge incontinence". Such stress or
urge incontinence, in fact, is the most common cause of
urine loss in adult women.
Nonsurgical management of urinary incontinence
includes non-therapeutic management, wherein the patient
25 wears an appliance or device proximate the urethral
orifice ("meatus") that collects or captures urinary
discharge. Such devices fall generally into two
categories: (1) urine collection devices, and (2)
absorbent pads.
30 Urine collection devices typically comprise a
receiving orifice or receptacle for capturing urine
flowing from the urethra; retention means, associated
with the receptacle or orifice, for holding the
receptacle or orifice in the proximity of the urethral
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98125524 PGTlUS96/19949
- 2 -
meatus; and means for directing urine from the receptacle
or orifice to a reservoir or a container or the like for
disposal. Devices of this general description, for use
by female patients, are disclosed in the following U.S.
Patents: 3,512,185 - Ellis; 3,661,155 - Lindan; 4,412,511
- Steer et al.; 4,457,314 - Knowles; 4,484,917 -
Blackmon; 4,690,677 - Erb; 4,822,347 - MacDougall; and
4,846,819 - Welch. A variation on the urinary
collection device theme is the "female external
catheter", disclosed in U.S. Patent No. 4,563,183 -
Barrodale et al., which includes a catheter tube having
one end inserted into the urethra. In many of these
devices, the retention means are configured so as to be
inserted into the interlabial space, being retained
therein by the anatomical structure of the external
female genitalia. The Blackmon and MacDougall devices
also use an adhesive to assist in retention.
The category of absorbent pads includes a wide
variety of devices, primarily for use by female patients,
which generally comprise a body of absorbent material
configured so as to be insertable into the interlabial
space, and retained therein by the anatomical structure
of the external female genitalia. Such devices typically
resemble (and, indeed, can function as) catamenial
sanitary napkins. The following U.S. Patents disclose
devices that may generally be considered within this
category: 3,983,873 - Iiirschman; 4,595,392 - Johnson et
al.; 4,627,848 - Lassen et al.; 4,673,403 - Lassen et
al.; 4,743,245 - Lassen et al.; 4,804,380 - Lassen et
al.; and 4,846,824 Lassen et al. A sanitary napkin that
is configured for interlabial retention, and that could
be used to capture and absorb urine flow, is disclosed in
British Patent No: 754,481.
While the above-described devices are useful in
certain applications, they are subject to a number of
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98/25524 PCT/US96/19949
- 3 -
disadvantages. For example, the urine collection devices
r~quire the user to wear a reservoir or container that
may be prone to overflow or spillage. Also, such devices
are better suited to users who suffer from chronic or
severe loss of bladder function, rather than those who
suffer only from moderate stress or urge incontinence.
The absorbent pads tend to be bulky, and may be
uncomfortable for.some users, especially when wet. Odor
associated with urine collection devices is often
l0 noticeable by others, and is therefore undesirable.
Use of the prior art devices described above is
based upon the assumption that the flow of urine out of
the urethra cannot or should not be stopped. This
assumption may not be true in many cases of stress or
i5 urge incontinence, which are transient in nature. In
such cases, external occlusion of the urethral meatus may
provide an adequate degree of continence for many
patients, but this approach has been overlooked, at least
for the most part, by the prior art.
20 There is, therefore, a need for a device that
provides for the effective management of urinary
incontinence by means of the external occlusion of the
urethral meatus; that is easy to use and comfortable to
wear; and that provides for secure retention with good
25 sealing qualities.
Summary of the Invention
Broadly, the present invention is a urethral
meatus occlusion device, comprising a resilient body,
configured to engage and seal against the urethral
30 meatus, and to be retained in place by engagement with
the anatomical structure of the external genitalia. More
specifically, in one preferred female embodiment, the
body is a pad that includes a base, having a
substantially triangular or arrowhead-shaped outline,
35 that is adapted to seat against the vestibule of the
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
wo ~ss~,a rc~r~s9sn~a9
vulva, anteriorly of the vaginal orifice, thereby
occluding the urethral meatus. The lateral edges of the
pad are configured to fit inside the labia minors, the
engagement between the pad and the labia thereby
retaining the pad firmly against the vestibule, in
sealing engagement against the meatus. The side of the
pad opposite the base is configured with a central
longitudinal ridge that, when the pad is installed in the
vestibule, extends into the interlabial space. A loop of
thread may be inserted through the ridge to facilitate
removal of the device, or a finger hole may be provided
into the posterior of the ridge for the same purpose.
In a second preferred female embodiment of the
invention, the pad has a substantially tubular
l5 configuration, and thus lacks the lateral edges or
"wings" of the first preferred female embodiment. This
"wingless" embodiment is adapted for use where the floor
of the vestibule is narrower than what may be considered
"normal". As with first preferred female embodiment, the
pad seats against the floor of the vestibule, anteriorly
of the vaginal orifice, thereby occluding the urethral
meatus. The tubular portion of the pad is configured to
fit inside the labia minors, the engagement between the
pad and the labia thereby retaining the pad firmly
against the vestibule, in sealing engagement against the
meatus. The side of the pad opposite the base is
configured with a central longitudinal ridge that, when
the pad is installed in the vestibule, extends into the
interlabial space, thereby facilitating insertion and
removal.
In both of the aforementioned embodiments, at
least that portion of the pad that lies in sealing
engagement against the meatus is coated with a
pressure-sensitive, hydrophilic hydrogel adhesive for
retention against the vestibule. The adhesive, in
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO '95/25524 PCT/US96/19949
. 5 -
concert with the resilient pad, spreads to fill the
interlabial space proximate the vestibule, thereby
' providing a confoimal fit with the anatomical structure,
which enhances the retention of the device. The pad
' S itself can be coated or impregnated with a suitable
anti-bacterial or germicidal agent to inhibit infection.
In a third preferred female embodiment of the
invention, the body comprises an elastomeric bladder or
sac, filled with a soft, compliant, biocompatible gel or
i0 liquid, and coated with a pressure-sensitive hydrophilic
hydrogel adhesive, to enhance retention. The gel-filled
sac spreads within the interlabial space to conform
closely to the anatomic structure of the external female
genitalia, and thereby seals against the urethral meatus,
15 with the aid of the adhesive.
In several embodiments suitable for use by a male
patient, the invention comprises a thin, resilient,
absorbent pad, the inner surface of which is provided
with a pressure-sensitive hydrophilic hydrogel adhesive
Z0 layer. The pad conforms to the glares of the penis, and
it is removably attached to the penis by means of the
adhesive, whereby the adhesive also seals against and
occludes the urethral meatus. In one such male
embodiment, the pad has a substantially elliptical
25 central portion, with a pair of laterally-extending tabs
at each end that are wrapped around the glares for
securing the pad in place. In another male embodiment,
the pad has a central sealing portion with a plurality of
radially-extending tabs for securing the device. In
30 still another male embodiment, the pad is in the
configuration of a generally hemispherical cap that
covers a substantial portion of the glens. As with the
female embodiments, the pad of the male embodiments can
be coated or impregnated with a medicinal compound, such
35 as an anti-bacterial or germicidal agent.
SUBSTITUTE SHEET (RULE 28)
CA 02274815 1999-06-11
wo ~ssza rcTrt~s~n~a9
- 6 -
It will be appreciated that the present invention
offers a new and advantageous approach to the management
of incontinence. For example, the device is small,
unobtrusive, easy to uee, and comfortable to wear. By
allowing the user effectively to retain urine, the device
avoids the problems associated with prior art devices,
enumerated above, that allow the discharge of urine. The
device can be made in a variety of sizes and shapes for
optimal fit for each individual user. The device is
i0 economical to manufacture, and can, therefore, be a
disposable item.
These and other advantages will be better
appreciated from the detailed description that follows.
Brief Descri~otion of the Drawinc~s_
Figure 1 is a perspective view of a female urinary
incontinence device, in accordance with a first preferred
female embodiment of the invention;
Figure 2 is a bottom plan view of the device of
Figure 1;
Figur~ 3 is a side elevational view of the device
of Figure 1;
Figur~ 4 is an anterior elevational view of the
device of Figure 1;
Figure 5 is plan view of the device of Figure 1,
showing the device installed in the external genitalia of
a human female;
Figuro 6 is a cross-sectional view taken along
line 6 - 6 of Figure 5;
Figure 7 is an anterior elevational view of a
first modified form of the first preferred female
embodiment of the device;
Figure 8 is a perspective view of a second
modified form of the first preferred female embodiment;
Figure 9 is cross-sectional view taken along line
9-9 of Figure 8;
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98/25524 PCT/US96J19949
-
Figure i0 is a cross-sectional view, similar to
that of Figure 9, showing the flexing of the lateral
edges of the pad;
Bigots ii is a cross-sectional view of a third
modified form of the first preferred female embodiment;
Bigure i2 is a cross-sectional view, similar to
that of Figure 11, showing the flexing of the lateral
edges of the pad;
BiQur~ 13 is a perspective view of a second
preferred female embodiment of the invention;
Bigots 14 is a cross-sectional view taken along
line 14-14 of Figure 13;
Bigur~ i5 is a cross-sectional view, similar to
that of Figure 14, showing a modified form of the second
i5 preferred female embodiment;
Bigur~ i6 is a cross-sectional view of the
external female genitalia, showing a vestibule of the
configuration for which the second preferred female
embodiment is adapted;
Bigots i~ is a cross-sectional view of a fourth
modification of the first preferred female embodiment,
wherein the pad includes a layer of super-absorbent
material;
Bigur. ie is a cross-sectional view, similar to
that of Figure 17, showing the invention as installed
in
the external genitalia of a human female;
Bigots i9 is a cross-sectional view, similar to
that of Figure 18, showing the super-absorbent material
after it has absorbed moisture;
Bigots 20 is a perspective view of a fifth
modified form of the first preferred female embodiment,
which includes a finger hole:
Bigots 21 is a cross-sectional view, taken along
line 21-21 of Figure 20;
SWBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98/25524 PCT/U596I19949
g -
Figure 22 is a perspective view, similar to that
of Figure 20, showing the device with a human finger
inserted into the finger hole;
Figure 23 is a cross-sectional view, similar to
that of Figure 21, showing a sixth modification of the
first preferred female embodiment;
Figure 24 is a perspective view of a third
preferred female embodiment of the invention;
Figure 25 is a cross-sectional view taken along
line 25-25 of Figure 24;
Figure 2b is a perspective view of a first
preferred male embodiment of the invention;
Figure 27 is a perspective view, showing the
embodiment of Figure 26 attached to the glens of a penis;
Figure 28 is a perspective view of a second
preferred male embodiment of the invention;
Figure 29 is a perspective view, showing the
embodiment of Figure 28 attached to the glens of a penis;
and
Figure 30 is a perspective view of a third
preferred male embodiment of the invention.
Detailed Description of the Invention
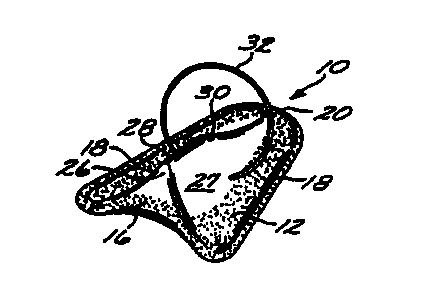
Referring first to Figures Z through 4 of the
drawings, a female urinary incontinence device 10, in
accordance with a first preferred female embodiment of
the present invention, is shown. The device comprises a
body or pad 12, formed of a resilient foam material that
is biocompatible. One suitable class of materials is
that of foams formed from the water actuation of
prepolymers based on either toluene diisocyanate (TDI) or
methylene diphenyl diisocyanate (MDI). Such prepolymers
are marketed by W: R. Grace & Co.-Conn., Organic
Chemicals Division, Lexington, Massachusetts, under the
trademarks "HYPOL" (TDI) and "HYPOL PLUS" (MDI).
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98/25524 PCTIITS96/19949
g -
Alternatively, the pad 12 can be made of a
biodegradable material, such as a cellulose or cotton
fiber. A polyurethane foam can also be used, being
rendered biodegradable by hydrolysis of a weak backbone
link, such as an amine group. Other foam materials, such
as polyolefins, can be used and made hydrolytically
biodegradable by using weak links such as starches in the
polymer backbones.
The pad 12 includes a base 14 that has the general
outline of a blunt arrowhead, as shown in Figure 2. In
the first preferred embodiment of the invention, the base
may be slightly concave, as shown in Figure 4.
Alternatively, the base 14 can be made slightly convex,
as shown in Figure 7, for those users who might find such
a configuration more comfortable to wear. The base 14
has a concave posterior end 16, with lateral edges 18
that taper slightly toward each other as they extend
toward a rounded anterior end 20. The anterior end 20 is
thus somewhat narrower than the posterior end 16.
Z0 The pad is provided with an adhesive surface for
retention against the floor of the vestibule. In this
embodiment of the~invention, the base is coated with an
adhesive layer 22, comprising a pressure-sensitive,
hydrophilic hydrogel adhesive material. Such hydrogel
adhesives are marketed by Promeon Division of Medtronic,
Inc., of Minneapolis, Minnesota, under the trademark
"PROMEON". A detailed description of such a hydrogel
composition is contained in U.S. Patent No. 4,593,053 -
Jevne et al., the disclosure of which is incorporated
3o herein by reference.
Another type of adhesive that has shown good
results is a mixture of poly 2-hydroxyethyl methacrylate
(PHEMA) and polyethylene glycol (PEG) as a plasticizes.
The percentage of PHEMA may range from about 45% to about
75%, with a corresponding range of PEG of about 55% to
SUBSTITUTE SHEET (RULE 28)
CA 02274815 1999-06-11
WO 98125524 PCT/US96/I9949
- 10 -
about 25%. The preferred composition is about 53% to 54%
PHEMA and about 47% to 46% PEG. Lower percentages of
PHEMA yield greater adhesiveness, while higher
percentages of PHEMA yield greater durability. The PEG
has a molecular weight between about 400 and about 1000,
with 400 preferred. The PHEMA is preferably a mixture of
low molecular weight PHEMA (Mw between about 10,000 and
about 100,000) and high molecular weight PHEMA (Mw~
greater than about 100,000). The low Mw PHEMA provides
i0 adhesive properties, while the high Mw PHEMA improves
adhesive structural integrity. The PHEMA mixture is
between about 10%~- 50% low Mw PHEMA and between about
90% and 50% high Mw PHEMA, with the precise mixture being
determined by the particular adhesive properties desired.
While the preferred plasticizer is PEG, as
described above, other plasticizers can be used, such as
propylene glycol, polypropylene glycol (PPG), or
glycerin.
If the pad 12 is made of TDI or MDI, the material
of the pad itself can be rendered adhesive by combining
the TDI or MDI one-to-one by weight with about 0.25 to
0.50 molar ammonium hydroxide during the water actuation
of the foam. The resulting material has a surface that
is positively charged, so that it will adhere to a
negatively-charged mucoid surface (such as the surface of
the vestibule and the inner portions of the labia
minors ) .
Alternatively, the entire pad can be formed of an
adhesive, such as the PHEMA/PEG mixture described above.
The side of the pad 12 opposite the base 14
includes a central longitudinal stiffening ridge 26 which
forms the thickest part of the pad 12. If one adopts the
convention that the base is the "bottom" of the pad 12,
then the pad can be defined as having a surface 27
opposite the base that slopes "downwardly" from either
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98/25524 PCT/US96/19949
li -
side of the ridge 26 toward the edges i8, so that there
is a gradual reduction in pad thickness from the ridge to
the edges. Viewed another way, the pad can be defined as
having a cross-sectional shape that narrows from the base
14 to the "top" or apex 28 of the ridge 26. The
resulting configuration is such that a lateral cross
section of the pad, taken through the ridge 26, produces
a shape resembling a triangle with rounded corners and
slightly concave sides, as shown in Figure 6. Similarly,
the ridge 26 has an anterior edge 30 that tapers
"downwardly" from the apex 28 toward anterior end 20 of
the pad 12, as shown in Figure 3, so that the anterior
end 20 of the pad 12 is substantially reduced in
thickness as compared to the posterior end 16.
The device 10 is advantageously provided with a
handle or tab that is either integrally molded with the
pad 12, or subsequently attached to it. In the first
preferred embodiment, handle is a ring or loop 32,
preferably of thread, that is inserted laterally through
Z0 the pad 12. The loop is preferably located near the
anterior edge 28 of the ridge 26, although the precise
location of the loop 32 is not critical to its function,
as will be described below.
Figures 5 and 6 show the incontinence device 10
installed in the external genitalia of a human female.
The device 10 is installed so that the base 14 is seated
against the vestibule 34 of the vulva 36, anteriorly of
the vaginal orifice 37, thereby occluding the urethral
meatus 38. The adhesive surface seals the meatus
sufficiently to prevent the escape of urine. The lateral
. edges 18 and the anterior end 20 of the pad are tucked
under the labia minora 40. The engagement between the
labia minora and the sloping surface 27 enhances the
retention of the pad 12 in engagement with the vestibule
34. The concavity in the posterior end 16 of the pad 12
SUBSTITUTE SHEET (RULE 2S)
CA 02274815 1999-06-11
WO 98125524 PCT/US96119949
- 12 -
allows for somewhat greater surface area for engagement
by the labia minors, while leaving a clearance for the
vaginal opening 37. The ridge 26 extends into the
interlabial space, and the loop 32 protrudes from between
the labia majors (not shown), so as to be exposed to
facilitate manual grasping, for removal of the device.
The pad 12 can be provided in a number of sizes to
fit a large variety of individuals. The length of the
pad should be approximately the same as the distance
between the anterior lip of the vaginal orifice and the
juncture of the labia minors. The width of the pad
should optimally conform substantially to the width of
the vestibule. Predetermined sizes can be trimmed
individually for optimum fit. ~In some cases, a mold of
i5 the relevant portions of the vulva may be taken prior to
sizing the pad.
The adhesive layer 22 not only provides a
fluid-tight seal for the urethral meatus, but it also
minimizes slippage of the device. The central ridge 26
lends rigidity that resists deformation of the pad and
rupture of the adhesive layer under fluid pressure from
the urethra, thereby enhancing the fluid-tight seal
provided by the pad against the urethral meatus. It may
be advantageous to extend the adhesive layer onto the
labia-engaging surface 27, thereby further enhancing the
stability of the device.
An incontinence device constructed in accordance
with the first preferred female embodiment of the
invention, as described above, can be made to withstand
short-term fluid pressures from the urethra in the range
of up to at least about 100, and preferably to about 170,
centimeters of water without significant leakage.
Pressures in this range are those that would typically
result in involuntary urine voiding in cases of stress
and urge incontinence, with 170 centimeters of water
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
wo s~.a rcT~crs~m49
- 13 -
being the approximate maximum bear-down pressure for a
typical adult human female.
As an option, the foam material of the pad, and/or
the adhesive surface, can be provided with a
' S medically-active composition. An antibacterial or
germicidal agent, such as silver oxide or silver azide
may be used, for example.
The first preferred embodiment lends itself to
several modifications that may provide better comfort for
certain individuals. For example, figures 8, 9, and 10
show a modified device 50, which includes a pad 52 of
substantially uniform thickness, except for a
longitudinal ridge 54. This modification provides
lateral edges 56 that flex more easily than those of the
embodiment of Figures 1-7. Still greater flexibility may
be provided by forming a longitudinal groove 58 on either
side of the ridge 54, as shown in Figures 11 and 12.
As still another option, a short protuberance 59
may be provided on the base, as shown in Figures 9 and
10. The protuberance 59 serves as a locator for the
urethral meatus, facilitating proper placement of the
device. The protuberance 59 may also enhance the
occlusion of the meatus.
Another modification of the first preferred female
embodiment is shown in Figures 17, 18, and 19. As shown
in these figures a modified device 60 includes a layer 62
of super-absorbent hydrophilic material adjacent the
adhesive layer 64 on the base of the pad. The
hydrophilic layer 62 is preferably a mixture of the
PHEMA/PEG adhesive and a microsponge material, such as
carboxymethylcellulose (CMC). The hydrophilic layer 62
draws moisture from the adhesive layer 64 and absorbs the
moisture, thereby prolonging the useful lifetime of the
adhesive by delaying saturation. Absorption of moisture
causes the hydrophilic layer 62 to swell, as shown in
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98125524 PCT/US96119949
- 14 -
Figure 19, which may enhance the sealing properties of
the device.
Still another modification of the first preferred
female embodiment is shown in Figures 20, 21 and 22. In
these figures, a modified device 70 has a pad 72 having
an integral longitudinal ridge 74. The ridge 74 a finger
hole 76 in its posterior edge. The finger hole 76 is
normally in a collapsed state, as shown in Figure 20. It
expands to receive the user's finger 78, as shown in
Figure 22, to facilitate installation and removal.
In Figure 21, the device 70 is shown as having an
adhesive layer 80 applied directly to the base of the pad
72, as previously described. Figure 23 shows still
another feature that can be incorporated, as a further
modification, into any of the previously-described
variations of the -first preferred female embodiment. In
this variation or modification, a scrim layer 90 is
enclosed within the adhesive 92 applied to the base of
the pad. The scrim layer 90 is preferably a thin,
non-woven sheet of polyester that can reinforce an
elastomeric material. In the present invention, the
scrim layer 90 adds structural integrity to the adhesive
material, thereby enhancing the durability of the device.
As shown in Figure 23, the scrim layer 90 is placed in
the adhesive before the adhesive is cured to a
semi-solid. Alternatively, the scrim layer 90 can be
applied to the base of the pad before the adhesive is
applied, in which case the scrim layer would be
sandwiched between the adhesive and the base of the pad.
It has been noted that some potential users of the
present invention'have a relatively narrow vestibule
floor. This type of anatomical structure is shown in
Figure 16, which shows a simplified cross-sectional view
of external female genitalia, wherein the vestibule floor
94 and the labia minors 96 define a relatively narrow
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98/235?.~t PCT/USQ6/19949
- 15 -
space proximate the urethral meatus 98. For tho:e with
this type of anatomical structure, the above described
first preferred female embodiment may be uncomfortable,
or altogether unsuitable. Consequently, a second
' 5 preferred female embodiment, illustrated in Figures 13,
14, and 15, is contemplated for such users.
In accordance with this second preferred female
embodiment, a female urinary incontinence device 100
includes substantially tubular pad 102, substantially the
i0 entire exterior surface of which is coated with an
adhesive 104, of a type described above. The pad 102 has
a longitudinal ridge 106, preferably not coated with the
adhesive, that is used as a gripping element to
facilitate installation and removal. As shown in Figures
15 13 and 14, the tubular pad may have a substantially
elliptical cross section. Alternatively, as shown in
Figure 15, a pad 102a, having a cross-sectional shape
similar to a rounded triangle, may be more suitable for
some users.
20 Figures 24 and 25 illustrate a third preferred
female embodiment~of the invention. A urinary
incontinence device 110, in accordance with this
embodiment, includes a thin, elastomeric shell or bladder
112, formed of polyurethane or a similar thin, resilient,
25 elastomeric material. The bladder 112 is filled with a
suitable biocompatible liquid or gel 114 by means of a
needle, and the needle hole is then sealed, thereby
forming a compliant sac. A preferred material for
filling the sac is a hydrogel, similar the hydrogel
30 adhesives described above. Substantially the entire
exterior surface of the sac is coated with an adhesive
116, of a type described above.
In use, the device 110 is inserted under the labia
minors so as to be seated against the floor of the
35 vestibule, occluding the urethral meatus. The sac
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98125524 PCTIUS96/19949
- 16 -
conforms to the anatomical structure of the external
female genitalia, filling the interlabial space, and
sealing against the urethral meatus with the aid of the
adhesive. Because the sac is so compliant, it can be
used for a wide variety of anatomical structures,
providing high levels of comfort. The device may
advantageously be provided with a raised tab 118, not
coated with the adhesive, to be gripped by the user, to
facilitate the installation and removal of the device
l0 110.
Figures 26 through 30 illustrate several
embodiments of the invention suitable for use by male
patients. A male urinary incontinence device, in
accordance with the embodiment of Figures 26 and 27
comprises a thin, flexible, resilient pad 122, which may
be formed from any of the above-described materials used
for the pads of the female embodiments of Figures 1
through 23. The pad 122 has a generally elliptical
central portion 124, with a pair of laterally extending
tabs 126 at each end. The pad has an inner surface which
is provided with a pressure-sensitive hydrophilic
hydrogel adhesive layer 128, which may be formed in any
of the manners described above. The pad 122 conforms to
the glans 130 of a patient's penis 132, and it is
removably retained thereon by means of the adhesive layer
128, whereby the adhesive also seals against and occludes
the urethral meatus (not shown). The generally
elliptical central portion 124 engages against the
urethral meatus, and provides the sealing and occlusion
functions. The laterally-extending tabs 126 are wrapped
around the distal portion of the shaft of the penis 132
for adhesively securing the pad 122 in place.
In the embodiment of Figures 27 and 28, a male
urinary incontinence device comprises a pad 142 that has
a central sealing and occluding portion 144 with a
SUBSTITUTE SHEET (RULE 26)
CA 02274815 1999-06-11
WO 98/25524 PCTlUS96/19949
~ 17 -
plurality of radially-extending tabs 146 for adhesively
securing the device to the glass 130. Again, the inner
surface of the pad 142 is provided with a layer 148 of
the above-described adhesive.
S In accordance with the embodiment of Figure 30, a
male urinary incontinence device comprises a pad 152 that
is in the configuration of a generally hemispherical cap
that conforms to and covers a substantial portion of the
glass 130. Again, the inner surface of the pad 152 is
provided with a layer 154 of the above-described
adhesive. In this embodiment, the pad 152 has a central
portion 154 that engages against the urethral meatus, and
a peripheral portion, integral with and extending from
the central portion 156, that is removably attached by
the adhesive layer 154 to the plans 130.
As with the female embodiments, the pad of the
male embodiments can be coated or impregnated with a
medically active compound, such as an anti-bacterial or
germicidal agent. Alternatively, the compound can be
incorporated into the adhesive.
From the foregoing, the advantages of the present
invention will be readily appreciated. The incontinence
device in accordance with the present invention provides
effective management of urinary incontinence, especially
stress and urge incontinence, without the inconvenience
and discomfort associated with prior art urine collection
devices and absorbent pads. The present invention is
easy to use and comfortable to wear. It is easily shaped
and sized to fit each individual user's anatomy with
optimum effectiveness and comfort. Easily and
inexpensively manufactured, it can be made as a
disposable item.
While several preferred embodiments and
modifications thereof have been described above, it
should be understood that still further modifications and
SUBSTITUTE SHEET (RULE Z6)
CA 02274815 1999-06-11
WO 98/~Z4 PCT/U596/19949
- 18 -
variations will suggest themselves to those skilled in
the pertinent arts. Such variations and modifications
should be considered within the spirit and scope of the
invention, as defined in the claims that follow.
SUBSTITUTE SHEET (RULE 26)