Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
HEMOGLOBIN WITH CHEMICALLY INTRODUCED
DISULFIDE CROSS-LINKS AND PREPARATION THEREOF
FIELD OF THE INVENTION
This invention relates to protein modification, and to
processes and reagents useful therein. It also relates to novel, modified
proteins, and to reactions of them to form conjugates with various
biochemically active molecules and groups. More specifically, the
invention relates to reagents for modifying hemoglobin, including cross-
linking of hemoglobin, modified hemoglobins produced using the reagents,
and use of the modified hemoglobin for conjugation of biomolecules
thereto.
BACKGROUND OF THE INVENTION
Modified human hemoglobin is a central component of most
materials that have been developed as potential blood substitutes . Cross-
linking, either by chemical modification or genetic engineering, is
necessary to stabilize the tetrameric protein and counteract its tendency to
dissociate into a~i sub-units. As blood substitutes, cross-linked
hemoglobins have to be tolerated in large quantities . Hemoglobin-based
oxygen carriers (HBOCs) also provide a possible means of delivery of
therapeutically active substances to sites of the body, since HBOCs are a
circulatory, biocompatible medium which perfuse tissues and organs of the
body. Their globin chains have many chemical sites theoretically capable
of bonding to a therapeutic substance to act as a carrier therefor. Any such
chemical bonding will, however, effect modification of the globin chains,
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
2
and care must be taken to ensure that the modification does not have an
adverse effect on other properties of the HBOC.
The functionality introduced by cross-linking hemoglobin also
has the potential for creating sites for bioconjugation.
BRIEF REFERENCE TO THE PRIOR ART
U.S. Patent 5,399.671 Klu~er and Sony, issued March 21,
1995, teaches cross-linking of hemoglobin with a trifunctional reagent in
which only two of the functionalities react with the hemoglobin to effect
cross-linking, leaving one functionality on the cross-linker residue
available for reaction with an exogenous nucleophile.
International Publication W093/08842 (PCTlUS92/097131
Somato~~en Inc. (Anderson et alb describes methods and compositions for
delivering drugs to the body by binding them to hemoglobin, utilizing
cysteine units of the native globin chains of hemoglobin ("internal sites")
as the binding sites.
Many natural proteins contain internal disulfide bonds,
naturally formed and imparting stability to the protein. These are formed
between cysteine units of the peptide chains, and may stabilize the protein
in a naturally folded condition. Albumin, for example, has cysteine
residues which participate in disulfide bonds in naturally folded albumin.
Hemoglobin, although containing one or two cysteine residues in the
CA 02308277 2000-04-26
PGT/CA98/01020
3
globin chains of its sub-units, does not naturally include disulfide cross-
links to confer stability on the tetramer.
SUMMARY OF THE INVENTION
. It is an object of the present invention to provide novel
reagents for modifying proteins such as hemoglobin.
It is a fiuther object to provide novel modified hemoglobin
capable of bioconjugation to biochemically active compounds.
It is a further object of the invention to provide tetrameric
hemoglobin stabilized by disulfide cross-links.
The present invention provides, from one aspect, modifying
reagents for proteins such as hemoglobin, containing disulfide bonds. The
reagents have functionalities which react site-specifically with globin
chains of the hemoglobin either to effect cross-linking, while leaving the
disulfide bonds intact, as part of the cross-link group, or to provide novel
complexes having side chains containing disulfide groups. In either case,
the products can be reacted with reducing agents to cleave the disulfide
bond and to provide modified hemoglobin products with terminal
sulfhydryl groups, available for conjugation to therapeutically active
compounds, e:g. biomolecules. In addition, the products with terminal
sulthydryl groups can be oxidized to re-form the disulfide bonds and hence
produce cross-linked hemoglobin.
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
4
The modified reagents of the present invention are derivatives
of cystine, in which the free amino groups are derivatized with bulky
protectaat groups such as carbobenzoxy, and in which the carboxylic acid
groups are esterified with amino-reactive leaving groups, such as
methylphosphate and electronegatively substituted phenyl groups. The
reagents correspond to the general formula A:
R. O. CO-CH-CH2-S-S-CH2-CH-CO.O-R
CA)
NH.R~ NH.R~
where R represents an amino-reactive leaving group selected from lower
alkyl phosphate and electronegatively substituted phenyl, and R'
represents a bulky, N-protectant group such as carbobenzoxy,
benzenesulfonyl, toluenesulfonyl, t-butyloxycarbonyl, fluorene-
methoxycarbonyl, etc., as commonly used in protein chemistry. The
reagents have a sufficiently short chemical chain length between respective
amino-reactive leaving groups that they do not effect cross-linking
between different hemoglobin tetramers to form 128 kd products
(intermolecular cross-linking), only reaction with different (3-sub-units of
the same tetramer.
The invention also provides a process of modifying
hemoglobin which comprises reacting hemoglobin, in its deoxy state, with
a modifying reagent of formula A give above. The acylation reaction
occurs at amino acid residues in the diphosphoglycerate (DPG) binding
site of the hemoglobin, specifically at the E-amino groups of lys-82 in the
CA 02308277 2000-04-26
WO 99/Z3065 PCT/CA98/01020
S
~3-subunits, and produces some cross-linked product of the general formula
B:
Lys-(38~-NH.co . CH- CAL- S
r
d, a NMR'
Lys_(~'82-NW-Cv.CN ~CN1'S
I
NHft
along with modified products having disulfide linkages in side groups, of
general formula C:
(C)
I ''
NNR' t~NfZ~
In the general formulae, the symbol ~ ~,~ represents
tetrameric hemoglobin. The group -Lys-~3-82-NH represents the lysine
residues at position 82 on the respective ~i-globin chains of hemoglobin
L~S._ ~8~.-IVN.Cp, iH. CNl_$-S-GNz. iN _ CDOH
NHR' NH2~
y,
~yS-(~ ~Z-NI-~.CO.CH.CN . S-S-Cl~ .CI-~- COON
with linkage to the side groups through the E-amino group thereof.
CA 02308277 2000-04-26
wo ~rr~o6s rcric~9aromo
6
Another aspect of the invention is the modified hemoglobins
so produced, which are novel products, represented by formulae B, and C
given above.
A further aspect is the process of preparing modified
hemoglobin having side chains with terminal sulfhydryl groups bonded to
~i-iys-82 and/or Vii'-lys-82, which comprises subjecting modified
hemoglobins of formulae B and/or C, to reduction so as to effect reductive
cleavage of the disulfide bond. The modified hemoglobin so formed, of
general formula D:
NN. ~o . ~ N - t NL- S H
d, ~ a NHR,
rvN. co.cN-c~ly-$H (D)
rt H R
is then reactable with any of a wide variety of biochemically active
compounds, to form a conjugate administrable to a patient as a means for
delivering the biochemically active compound to the patient.
Yet another aspect of the present invention is a process for
preparing cross-linked tetrameric hemoglobin having cross-links between
respective lysine-82 groups of the ~i-globin chains thereof, which
comprises oxidizing the product of formula D given above, to re-form the
disulfide bond. The cross-linked product so formed, which is the product
of formula B given above, also constitutes an aspect of the present
invention. This product B is a hemoglobin tetramer stabilized with
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
7
disulfide cross-links to inhibit dissociation into dimeric sub-units. The
disulfide cross-link resembles the natural disulfide cross-link present in
many other naturally occurring proteins, and effecting a degree of
conformational stability thereon.
The reagents of the present invention do not react with the N-
terminal amino groups (Val-1) as do reagents that are bis methyl
phosphates derived from simple dicarboxylic acids. It is likely that the
bulk of the N-protected amino group in the reagents produces additional
selectivity based on steric effects.
X-ray crystallographic structures of deoxy hemoglobin reveal
that the (3-Lys-82 E-amino groups are outside the protein at the interface
between the DPG-binding site and the surrounding solution . By contrast,
the oc-amino group of the N-terminal (3-Val-1 is further within the DPG-
binding site. Modeling of the reagent into the structure of deoxy
hemoglobin suggests that the reagents bind to the DPG site at the solution
interface, limiting reaction to the lysine residues. The steric bulk of two
Cbz amino-protecting groups in the reagents provides selectivity beyond
that found with acyl phosphate monoesters toward amino groups in
hemoglobin.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is an anionic exchange HPLC chromatogram of the
reaction mixture resulting from Example 2 below, run under conditions
which do not dissociate uncross-linked hemoglobin tetramers;
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
8
Figure 2 is an HPLC chromatogram of the separated globin
chains from the reaction mixture in Example 2 below;
Figure 3 is a chromatogram of separated globin chains from
Example 3 below, taken at an early stage of the reaction; and
Figure 4 is an HPLC chromatogram of the products of
Example 4 below, taken at an early stage of the reaction.
DESCRIPTION OF THE PREFERRED EMBODIIVVIENTS
The preferred cross-linking reagent according to the present
invention is N,N'-bis-carbobenzoxy-cysteinyl-bis-(methylphosphate),
sodium salt of formula I:
O O O O
I l ll ~I
Na+ 'O-l~~ O-C-CH-CH2-S-S-CH2-CH-C-O-P-O- +Na
I I 1
OCH3 NH I~ H OCH3 (I)
C=O C=O
I I
O. CH2. C6H5 O. CH2. C6H5
This new reagent is referred to hereinafter as "ZCMP" . It be
prepared from the reaction of the bis-acid chloride of N-protected cystine
with dimethylphosphate followed by O-demethylation with methyl iodide
in acetone. ZCMP is an example of a compound of general formula A
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
9
above in which R represents methylphosphate and R' represents
carbobenzoxy.
Other preferred reagents according to the invention are those
in which R is an electronegatively substituted phenyl group of general
formula
pZo
where Z represents one or more independently selected electronegative
groups having positive Hamnnet sigma values, and n is an integer from 1-5.
Examples of suitable groups Z are acetanudo (at the ortho or meta
positions), acetoxy, acetyl, carbomethoxy, carboxy, halo, cyano, ethoxy (at
the ortho or meta positions), hydroxy (at the ortho or meta positions),
vitro, phenyl, trifluoromethyl and trimethylammonio. Preferred as
substituent group Z are carboxyl, phosphonate, sulfonate, sulfate,
phosphonate and halogen. Two or three such substituents are preferred,
and especially cases where one of the substituents is carboxyl. When only
one such substituent is present, it is preferred that it is located in the
position orkho to the ester linkage to the aromatic nucleus. Most preferred
for group R according to this embodiment is dibromosalicylate.
The hemoglobin which is used in the process of the present
invention is preferably human hemoglobin, although the invention is
applicable to other types of hemoglobin also, such as bovine hemoglobin
and porcine hemoglobin and other animal hemoglobins. The source of the
CA 02308277 2000-04-26
WO ~~~ PCT/CA98/01020
hemoglobin is normally red blood cells, although hemoglobin obtained by
genetic engineering, recombinant techniques is also useful.
The cross-linked hemoglobin so formed, with the disulfide
bonds intact, can be safely administered to human patients as a
hemoglobin-based oxygen Garner.
Alternatively and preferably, however, the cross-linked
hemoglobin is subjected to reductive cleavage so as to cleave the disulfide
bonds, and form terminal sulfliydryl groups on the side chains bonded to a
specific site of a specific globin chain. The cleaved product is then reacted
with a biochemical molecule, utilizing the terminal sulfhydryl group, and
administered to the body to act as a delivery medium for delivery of the
biochemically active compound to the body.
The attachment of the biochemically active compound to the
modified hemoglobin according to the present invention utilizes sulfhydryl
bonds, located at the terminus of side chains remote from the point of
attachment to the globin chains of the hemoglobin, and hence available for
ready reaction with biochemically active compounds. The preferred
linkage is a disulfide link, formed from the sulfhydryl group of the
hemoglobin side chain and a sulfhydryl group of the biochemically active
compound, or a sulfhydryl group of a linker or spacer molecule to which
the biochemically active compound is in turn linked. A disulfide bond is
slowly reduced by reducing agents present in human serum, so that a
biochemically active compound so linked to the hemoglobin is slowly
released into the blood stream. Reagents and conditions for formation of
CA 02308277 2000-04-26
wo ~n3o6s rcric~9s~omo
m
disulfide bonds are well known in the art. Release of the biochemically
active molecule from the hemoglobin, in order for it to exhibit its
biochemical activity, is not necessary, in many cases. The conjugate with
hemoglobin may exhibit the biochemical activity of the conjugant.
Biochemically active molecules which include sulfhydryl groups in their
native structure include a wide variety of proteinaceous compounds which
incorporate cysteine residues. These include atrial natriurectic factor
(ANF), antithrombotic peptides such as RGDW; antiproliferatives or
antimetastics such as RGD polymers or analogues thereof, and GRDGDS
or analogues thereof; antihypertensives-renin inhibitors such as Boc-
HPFHL-CH(OH)-CH2-VIH or analogues thereof; human growth hormone
releasing factor analogues; anorexigenics; vasoconstrictors such as
arginine vasopressin; vasodilators such as angiotensin converting enzyme
inhibitors; and anti-AIDS drugs such as HIV protease inhibitors.
Other sulthydryl group containing therapeutically active
compounds which can be conjugated to the modified hemoglobin of the
invention include captopril ( 1-(3-mercapto-2-methyl-1-oxopropyl)-L-
proline).
The reactions of ZCNiP with hemoglobin follow the patterns
of other anionic electrophiles. The reagent reacts efficiently only with the
deoxy form. Its acylating reaction occurs at residues in the DPG binding
site at the E-ariiino groups of Lys-82 in the (3 subunits.
The reaction of ZCMP with carbonmonoxy hemoglobin gives
only a very small amount of product under the conditions that extensively
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
12
modify the deoxy form. The product that does form is not cross-linked.
This result is consistent with the conformational change of hemoglobin in
going from the R state to the T state. When deoxy hemoglobin binds to
ligands such as oxygen and carbon monoxide, the extensive quaternary
structural change leads to contraction of the DPG binding site. As a
consequence, the DPG binding site is not large enough to be accessed by
such a bulky reagent.
The disulfides of the two [3-chain-modified hemoglobins,
compounds B and C above, show different reactivity with mild reducing
agents such as (3-mercaptoethanol. With this reagent, the linear disulfide
compound C is reduced to form compounds with terminal sulfhydryl
groups (compound D) whereas the cross-linked disulfide B remains largely
unaffected. The reaction mechanism and accessibility of the S-S bonds of
modified hemoglobins explains this difference. Reduction of a disulfide
by thiol reagents proceeds in two steps. A mixed disulfide of the cleaved
disulfide and mercaptoethanol is an intermediate. In the thiol-disulfide
exchange process, a large excess of the reagent is required to drive the
reaction to completion.
For the cysteinyl hemoglobin with no cross-links, one end of
the reagent is attached to J3Lys-82. The unreacted portion of the large,
hydrophilic reagent is probably out of the DPG binding site. Therefore,
the disulfide is~ accessible to 2-mercaptoethanol. On the other hand, if the
(3 subunits of hemoglobin are cross-linked as the bis cysteinyl derivative,
the S-S bond within the DPG cleft becomes less accessible to the thiol
CA 02308277 2000-04-26
WO 99/Z3065 PCT/CA98/01020
13
reagent. The DPG binding site protects the disulfide from reduction, and
the reaction of that disulfide bond with 2-mercaptoethanol is blocked.
The S-S bond of the cross-linked hemoglobin is cleaved with
more effective reducing reagents such as dithiothreitol (DTT). When
dithiothreitol is used to reduce the S-S bond, the first step is also a thiol-
disulfide exchange as with 2-mercaptoethanol. However, once the
intermediate is formed, a second molecule of reagent does not participate
in the reaction. Instead, an intramolecular reaction can occur, leading to
the formation of the stable cyclic disulfide of DTT. The intramolecular
process has a large entropic advantage in its favor. Since the cross-linked
hemoglobin was reduced without the addition of denaturing reagents, the
S-S bond is near the outside of the DPG cleft of the cross-linked
hemoglobin and is exposed to solvent.
In any event, the end product of reduction of both compound
B and compound C is a modified hemoglobin with terminal sulfhydryl
(thiol) groups, of general formula D above.
Depending upon the choice of protectant group R' in the
modifying reagent, it may be desirable to remove it prior to administering
products of the present invention to a patient. This will depend largely on
toxicity considerations. Removal of the protectant groups can be
accomplished by standard methods employed in peptide chemistry.
The oxidation of thiols to disulfides by oxygen normally
proceeds only in the presence of catalytic quantities of metal ions such as
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
14
iron and copper. The rate of the oxidation of thiols in the same molecule
depends on the distance between the thiol groups. In the present case, it
has been found that the modified hemoglobin with two free sulfhydryl
groups attached at (3-Lys-82 is oxidized spontaneously by atmospheric
oxygen, quantitatively producing the cross-linked tetramer. It is possible
that the iron of the heme facilitates the reaction, especially that which is
present as Fe(III) met-hemoglobin. The sulfliydryl groups produced by
reduction iii the process of the present invention remain in close spatial
proximity in order to form the disulfide bond between subunits by
oxidation. Cleavage of the disulfide at each'site, followed by oxidation
permits spontaneous formation of the cross-link between dimers. This
produces a much higher yield of cross-linked materials and provides an
added benefit of the use of such reagents. Accordingly, a preferred process
for making an HBOC according to the present invention comprises
reacting human deoxy hemoglobin with a disulfide group containing
protein modifying reagent as previously described, to form a mixture of
cross-linked and non-cross-linked modified hemoglobin (products B and
C) above, subjecting the mixture to reductive cleavage of the disulfide
bonds to form a modified hemoglobin of general formula D, and then
oxidizing this modified hemoglobin to form disulfide cross-linked
hemoglobin. Such a process is capable of producing substantially
quantitative yields of cross-linked hemoglobin.
The introduction of specific chemical alterations at defined
sites within a protein is complementary to methods based on genetic
engineering. The design of the modifying reagent ZCNiP of the preferred
embodiment of the invention permits site selection based on charge and
CA 02308277 2000-04-26
WO 99/Z3065 PCT/CA98/01020
steric factors. The fact that ZCMP is an amino acid derivative permits
chemical techniques to be used that are extended from the chemistry of
peptide formation. A wide variety of side chains, as well as longer
peptides for conjugation, can be implemented in combination with the
convenient methods developed for producing the mixed phosphate-amino
acid anhydride.
The use of the disulfide-based reagent in the present study
adds fiuther possibilities for applications of cross-linking by converting
sites that contain lysyl side chains into those connected as disulfides and
also, by reduction, to sites for bioconjugation.
The invention is further described, for illustrative purposes, in
the following specific examples.
EXPERIMENTAL PROCEDURES
General Methods. Water was doubly distilled and deionized. All pH
measurements were standardized against calibrated buffers using a
combination glass electrode. Molecular mechanics was employed to obtain
preferred conformations based on several initially estimated structures of
bis(N-Cbz)-cysteinyl bis(methyl phosphate). The coordinates of deoxy
hemoglobin of Fermi and Perutz (Re f. 1, infra) from the Brookhaven
protein database were used for visualization of the structure of the protein.
Melting points were obtained in a calibrated oil bath apparatus. Proton
NMR spectra were recorded at 200 MHz and 300 MHz with chemical
shifts reported relative to TMS or DSS. '3C NMR spectra were recorded
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/IIlOZO
16
on the same instruments at 50 MHz and 75 MHz. Carbon chemical shims
were measured relative to the chloroform-d with TMS at 0. 31P NMR
spectra were recorded at 120 MHz and chemical shifts are relative to 85%
phosphoric acid in water. Infrared spectra were recorded on a FT-IR
spectrometer in KBr pellets. Mass spectra were recorded by electron-
impact (EI) or fast atom bombardment ionization (FAB). The Mass
Spectroscopy Laboratory, Department of Medical Genetics, University of
Toronto provided electrospray ionization mass spectra. Chemical
modification of hemoglobin and analysis followed general procedures as
reported for related materials. (Refs. 2 and 3, infi-a)
Examule 1- Synthesis of N.N'-bis-Cbz-cystein l~bis ,(,Sodium MethXt
Phosphate) (ZCMP,~
N,N'-bis-Cbz-cysteinyl dichloride (1.0 g, 1.8 mmol) and
sodium dimethyl phosphate (0.54g, 3.6 mmol; from trimethyl phosphate
and one equivalent of sodium iodide in dry acetone) were suspended in 30
mL dry tetrahydrofuran at 0° C under nitrogen and stirred for 1 hr. The
resulting precipitate of sodium chloride was removed by filtration.
The filtrate contained the reaction product N-Cbz-cysteinyl
bis-dimethyl phosphate, 2 Sodium iodide (2.2 g, 14.6 mmol) was added to
the filtrate to replace one methyl group at each phosphate with sodium.
The mixture was stirred at S° C for 48 hr. Solvent was removed by
rotary
evaporation. The resulting solid was crystallized from acetone-ether,
producing an off white powder. This was collected and washed three times
with acetonitrile. The powder was dried in vacuum to give 1.0 g (1.35
CA 02308277 2000-04-26
WO 99113065 PCT/CA98/01020
17
mmol, 74% yield) N-Cbz-cysteinyl bis (sodium methyl phosphate)
(ZCMP) as a white solid, mp > 200 °C. IR (KBr) C~ 1675 cni 1, 1695
crri'; 1H NMR (200 MHz, D20, 87.26 {s, lOH, 2C6H5), 4.88 (s, 4H,
20CH2Ph), 4.44 (m, 4H, NH and NCH), 3.45 (dd, 6H, JP-H = 11.5 Hz,
20CH3), 2.70-3.25 (m, 4H, SCH2); 13C NMR (121 MHz, D20) 8168.3 (d,
JP-C = 9.7 Hz, P-O-C=O), 157.29 (C=O), 135.9, 128.4, 128.3, 127.7,
67.1, 53.9 (d, JP-C = 6.4 Hz, OCH3), 37.8; 31P NMR (121 MHz, D20) 8-
5.4 (q, JP-H = 11.3 Hz); FAB mass (-, glycerol) 717 (38.0, M-Na+).
Example 2 - Preparation of deoxy hemoglobin and its reaction with
ZCMP
A solution of hemoglobin (80 mg/mL in 6 mL O.1M pH 8
MOPS) in a SO-mL rb flask was immersed in an ice-water mixture. This
was connected to a rotating reactor containing tubes for inflow and
outflow of gasses. Humidified oxygen was then passed through the
solution for one hr with the flask illuminated by a tungsten lamp. The
resulting oxy hemoglobin was converted to the deoxy form by passing a
stream of humidified nitrogen over the rotating solution for 3 hr at
37°C.
A solution of ZCMP was added over 15 min to a solution of
hemoglobin in buffer {0.5 mM) so that the final conc. of ZCMP in the
mixture was 0.7 mM and that of hemoglobin was 0.25 mM (0.1 M MOPS,
pH 8.0). Other buffering agents of the kind known as Good buffers may
be used in lieu of MOPS, e.g. sodium borate, but more chemically reactive
conventional buffers such as Tris and bisTris will react adversely with
ZCMP or other protein-modifying agents according to the invention.
CA 02308277 2000-04-26
WO 99/Z3065 PCT/CA98/01020
18
The buffered hemoglobin solution was kept at 37 °C. For
reactions with deoxy hemoglobin, the reagent was degassed and was
introduced under nitrogen. The reaction was carried out under flowing
nitrogen. For reactions with carbonmonoxy hemoglobin, the reaction
solution was saturated initially with carbon monoxide. After the ZCMP
was added to the solution of hemoglobin, the reaction was continued for 2
hr. The flask was then disconnected from the rotating reactor and carbon
monoxide was introduced. The solution was passed through a column of
Sephadex G-25 equilibrated with 0.1 M pH 8 MOPS to remove excess
reagent. The resulting material (ca. 20 mL) was collected in a vial.
Analysis of the resulting modified hemoglobin was done with
a combination of reversed-phase HPLC and ion exchange HPLC following
previously reported procedures. (2)
Product Analysis
Extent of cross-linking. A sample of the reaction product
(1.0 mL) was passed through a gel filtration column of Sephadex-100
(superfine) that had been equilibrated with 1.0 M magnesium chloride.
Under these conditions hemoglobin that is not cross-linked dissociates into
a[3 dimers. The dimers elute more slowly than do cross-linked tetrameric
species. The extent of cross-linking of globin chains was also determined
by polyacrylarnide gel electrophoresis in the presence of sodium dodecyl
sulfate (SDS PAGE ) on Mini-Protean II Ready Gels (12%
polyacrylamide, 0.375 M Tris-HCI, pH 8.8) as reported previously. (Ref.
4, infra) The hemoglobins and globins from HPLC preparations were
CA 02308277 2000-04-26
WO 99!13065 PCT/CA98/01020
19
denatured in 0.065 M Tris-HC1, pH 6.8, 2% SDS, 10-v/v glycerol.
Approximately 20 p,g of protein was applied to each lane of the gel and
processed at 200 V for 1 hr. Protein bands on the gel were stained with
Coomassie brilliant blue R-250.
Analytical and preparative separation of globin chains.
Modified hemoglobins were separated as intact tetramers by anion
exchange HPLC on a SynChropak AX300 column (250 x 4.6 mm) using a
mixture of 0.025 M bis-tris and 0.025 M tris with gradients starting from
pH 8.5 to pH 6.7. The effluent was monitored at 540 nm. Heme and globin
chains were separated by reversed phase HPLC using 330 A pore C4
Vydac columns (250 x 4.6 mm for analytical and 250 x 12 mm for
preparative). Developers contained 0.1 % trifluoroacetic acid and various
gradients of acetonitrile starting at 20% and ending at 60%. The effluent
was monitored at 220 nm. Globin chains were recovered from the effluent
by lyophilization.
Digestion of globin chains. Globin chains were dissolved in
8 M urea to increase susceptibility to hydrolysis and kept at room
temperature for 2-4 hr. The solution was then diluted to 2 M urea with 80
mM ammonium bicarbonate buffer at pH 8.5. Trypsin (2% of total protein)
was added, and the solution was digested for 24 hr at room temperature.
The hydrolysate was heated in boiling water for 2 min, diluted to 1 M urea
with 80 mM ammonium bicarbonate buffer, and further digested with
endoproteinase Glu-C (1% of total protein) for another 72 hr at room
temperature. The hydrolysates were filtered before injection into the
HPLC.
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
Chromatography of peptides. Peptide fragments were
separated by analytical HPLC using reversed phase C 18 columns (3 .9 x
300 nun). Developers consisted of 0.1% trifluoroacetic acid in water with
gradients of acetonitrile from 0 to 100% over ca 2 hr. The HPLC effluent
was monitored at 214 and 280 nm.
Combination of ZCMP with deoxy hemoglobin (3:1 reagent:
protein) in aqueous buffer as described above produced a set of modified
hemoglobins. Under the same conditions, carbonmonoxy hemoglobin
underwent very little reaction. The resulting proteins from reaction of
deoxy hemoglobin and ZCMP were separated by anion-ion exchange
HPLC (AX-300). Enzymic digestion and mass spectral analysis were used
to determine the sites of the modifications resulting from reaction with
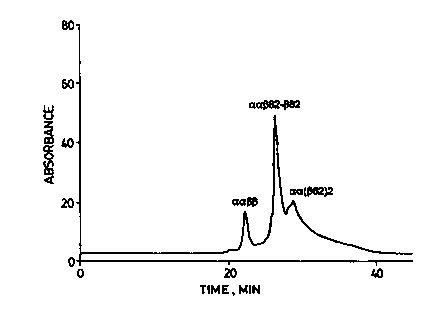
ZCMP. The HPLC chromatogram of the reaction mixture containing
intact tetramers is presented as Figure I of the accompanying drawings
and shows that the solution contained only a small amount of unreacted
hemoglobin (aa(3(3) along with two major peaks corresponding to
modified tetramers.
Two minor products elute after the major constituents.
Further analysis of the components of the modified proteins by reversed-
phase HPLC indicates that the two major products have native a chains
along with modified (3 chains (determined by comparison with unmodified
protein).
Figure 2 shows the results of HPLC analysis of the reaction
mixture under conditions that separate the globin chains. Structural
CA 02308277 2000-04-26
PGT/CA98/01020
21
analysis of each component (from digestion, SDS-PAGE, and peptide
analysis) indicates that there are two (3-modified tetrameric products.
Peaks 1 and 2 correspond to modified (3 subunits. The first peak is material
that is modified by reaction with ZCMP without cross-linking (the
cysteinyl amide, compound corresponding to general formula C above).
The second contains the two subunits cross-linked as the cysteinyl bis
amide (Compound B above). Enzylnic digestion followed by peptide
analysis reveals that in both peaks, modification is at the E-amino group of
lysine-82 of the ~i subunits. Two minor products, peaks 3 and 4, were
shown by a similar analysis to be modified a subunits. Ion-spray mass
spectral analysis (Ref. 5, infra) of material from reversed-phase HPLC
gives the molecular weight of the cross-linked (3 chains as 32208,
consistent with two (3 chains linked as the N,N'-CBZ-cysteinyl bis-amide.
The peaks from the material that is not cross-linked are consistent with a
species in which an amide has formed from reaction of ZCMP with the
lysyl-82 E-amino group while the second acyl group of the reagent has
undergone hydrolysis (Table 1).
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/OlOZO
22
TABLE 1 - Ion-sprays spectral parent peaks of modified g~obin chains
obtained from reaction of deoxy hemoglobin with ZCMP
GLOBIN CHAIN OF FOUND EXPECTED
HEMOGLOBIN (Molecular weight) (Molecular weight)
Unmodified (3- 15868.80 ~ 1.50 15867
subunits
Modified ~i-subunits16359.01 t 1.50 16357
Cross-linked ~3- 32207.78 ~ 1.87 32206
subunits
Unmodified a- 15127.24 t 1.55 15126
subunits
Modified a-subunits15616.19 ~ 1.55 15616
Where a cross-linked hemoglobin of the kind of Compound B
according to the invention is not to be subjected to reductive cleavage of
its disulfide crosslinks but, rather, is to be used as tetrameric (e.g., as an
HOBC), the material may be reacted fiuther with an a cross-linker such as
3,5 DBSF. Since ZCMP reacts with hemoglobin primarily at the ~3 chains
and 3,5 DPSF leads to cross-linking at the a Lys 99 positions, the result is
a doubly cross-linked (or doubly-modified) hemoglobin. As reported
previously by Kluger, et al. (Ref. 3, infra), if the DPG site is blocked (as
it
would be, given a Cbz-cysteinyl cross-link between the (3Lys 82 residues)
then 3,5 DBSF cross-links only at the aLys 99 residues. Unintended
cleavage of the Cbz-cysteinyl link in such a doubly cross-linked
CA 02308277 2000-04-26
WO 99/3065 PGT/CA98/01020
23
hemoglobin would still leave a fumaryl link between the a chains to keep
the tetramer together.
Example 3 - Reduction of the disulfide of bis-c stein3rl deox~r
hemoglobin with 2-mercaotoethanol.
2-Mercaptoethanol (0.018 g) was dissolved in 1 mL 0.1 M
pH 8.0 MOPS. 0.1 mL of the solution was added to one mL of a solution
of the modified hemoglobins from the reaction of deoxy hemoglobin with
ZCMP. The reaction mixture was stirred at 5 °C. Samples were
analyzed
by reversed-phase HPLC.
Progress of the cleavage reaction was followed by reversed-
phase HPLC. When the modified hemoglobins from the reaction of ZCMP
with deoxy hemoglobin were treated with a 20 fold excess of 2-
mercaptoethanol for 1 hr, the modified but not cross-linked hemoglobin
was reduced to yield t-wo products in approximately 1:1 ratio (Figure 3
shows a chromatogram from analysis early in the course of the reaction).
As peak 1 disappeared, two new peaks {peak 5 and 6) appeared with
shorter retention times. The structures of these products were established
by electrospray ionization mass spectral analysis. Peak 5 is the reduced (3
chain corresponding to the desired product that results from the cleavage
of the disulfide bonds. The reaction of 2-mercaptoethanol with modified
(but not cross=linked hemoglobin is summarized in Scheme 1. The
formation of a mixed disulfide intermediate and cleavage.occurs only with
material that is not cross-linked by 1. Peak 6 is the mixed disulfide
intermediate (of mercaptoethanol and modified and hemoglobin), with a
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
24
parent mass of 16178 ~ 2 (calcd 16180). Such a mixed disulfide is
consistent with formation of intermediates by this reagent. (21,22). Thus,
the possible products are reduced of cross-linked hemoglobin and two
hemoglobins with mixed disulfides. However, the reaction of the disulfide
of cross-linked hemoglobin with 2-mercaptoethanol proceeded very slowly
under the same conditions. Peak 2, corresponding to cross-linked
hemoglobin, did not decrease. The calculated mass of the modified ~3 chain
with a cleaved sidulfide, a cysteinyl amide of the ~i subunit of hemoglobin,
is 16,105; found: 16106.71.4.
The modified protein mixture from the reaction of ZCMP
with deoxy hemoglobin followed by treatment with an excess of 2-
mercaptoethanol for a few minutes was analyzed as follows. Mass spectra
reveal that Peak 5 is the (3 chain corresponding to the product that results
from the cleavage of the disulfide bonds derived from the protein modified
by ZCMP.
SCHEME 1
s-s
sH SH
« p « HSCH2CHZOH « p « HSCH2CH20H
P «
s-s P
SSCHiCHzOH SH
« ~ « ~ HSCHZCH20H
- SLOW
a
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
Examule 4 - Reduction of modified hemoglobin with dithiothreitol and
subsequent re-oxidation
Dithiothreitol (DTT) (0.073 g, four equivalents) was
dissolved in 1 mL 0.1 M pH 8.0 MOPS. 0.01 mL of the DTT solution was
added to 1 mL of the solution of modified hemoglobins from the reaction
of deoxy hemoglobin with ZCMP. The reaction mixture was stirred at 5
°C and the sample analyzed by reversed-phase HPLC. After 15 min, the
product was passed through a column of Sephadex G-25 equilibrated with
0.1 M pH 8.0 MOPS to remove excess dithiothreitol. The sample was
collected and analyzed again by reversed-phase HPLC.
Dithiothreitol (DTT) is a more effective reducing agent than
2-mercaptoethanol. In this experiment with DTT, all disulfide bonds were
cleaved within 15 minutes. The reversed phase HPLC showed none of the
original peaks derived from the (3-subunits. There was a single product
peak at the position of the cysteinyl derivative of the ~i subunit. This is
the
same species generated in the reaction with 2-mercaptoethanol and with
the same mass (16,105). The bis cysteinyl amide (aa~i-Lys-82-cys-S-S-
cys-(Lys-82)-(3) is cleaved at the disulfide.
Figure 4 shows the reversed-phase HPLC chromatogram of
the products from an early stage in the reaction with DTT (showing some
remaining unreacted [3 subunits are present). The peaks are numbered as in
Figure 3. The bis sulfide now appears as peak 5 but, as required, the
intermediate from 2-mercaptoethnaol (peak 6) is not present. The reaction
pattern with DTT is summarized in Scheme 2.
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
26
SCHEME 2
S--S SH
DTT
a a a a
s-s p sH
SH
a ~ a S DTT
I ~ a a
S
SH
Gel filtration chromatography was used to separate modified
protein from excess DTT after reduction. The product in the presence of
air and the absence of DTT is rapidly oxidized to produce once again
aa(3-Lys-82(E-NH-cys-S-S-cys-( E-NH-Lys-82)(3 quantitatively. HPLC
reverse phase analysis after 15 minutes reveals the decrease of peak 5
(modified, not cross-linked) and a large increase in peak 2, that of the
cross-linked species. Re-treatment of the protein solution with DTT once
again gives the disulfide-cleaved products exclusively (HPLC analysis).
Therefore, the reduction-oxidation procedure is an efficient way to convert
material that is not cross-linked into cross-linked material (Scheme 3).
CA 02308277 2000-04-26
WO 99/23065 PCT/CA98/01020
27
Scheme 3
SH
a~~
a a
~a
SH
SH
a
CA 02308277 2000-04-26
wo ~n3o6s ~cTicw9sroiozo
28
References
(1) Fermi, G. and Perutz M.F. {1984) The crystal structure of
human deoxyhaemoglobin at 1.74 Angstroms resolution. J. Mol.
Biol., 175, 159-174.
(2) Jones, R.T. (1994) Structural characterization of modified
hemoglobins. Methods in Enzymology, 231, 322-343.
(3) Jones, R.T., Shih D.T., Fujita T.S., Song Y., Xiao H., Head C.
and Kluger R. (1996) A doubly cross-linked human hemoglobin.
Effects of crosslinks between different subunits. J. Biol. Chem.,
271, 675-680.
(4) Kluger, R. and Song Y. (1994) Changing a protein into a
generalized acylating reagent. J. Org. Chem., 59, 733-736.
(5) Fenn, J.B., Mann M., Meng C.K., Wong S.F. and Whitehouse
C.M. (1989) Electrospray ionization for mass spectrometry of large
molecules. Science, 246, 64-71.