Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-1-
2,3,4,5-TETRAHYDRO-1H-[1,4]-BENZODIAZEPIN&-3-HYDROXAMIC ACIDS AS MATRIX
METALLOPROTEINASE
INHIBITORS
S
FIELD OF INVENTION
This invention relates to 4-(4-substituted-benzenesulfonyl)-2,3,4,5-tetrahydro-
1 H-[ 1,4]benzodiazepine-3-hydroxyamic acids which act as matrix
metalloproteinase
inhibitors. The compounds of the present invention are useful in disease
conditions
mediated by matrix metalloproteinases, such as tumor growth, osteoarthritis,
rheumatoid arthritis and degenerative cartilage loss.
BACKGROUND OF THE INVENTION
Matrix metalloproteinases (MMPs) are a group of enzymes that have been
implicated in the pathological destruction of connective tissue' and basement
membranes. These zinc-containing endopeptidases consist of several subsets of
enzymes, including collagenases, stromelysins and gelatinises. Of these, the
gelatinises have been shown to be the MMPs most intimately involved with the
growth
and spread of tumors.
For example, it is known that the level of expression of gelatinise is
elevated in
malignancies, and that gelatinise can degrade the basement membrane which
leads to
tumor metastasis. Angiogenesis, required for the growth of solid tumors, has
also
recently been shown to have a gelatinise component to its pathology as
reported in
"Matrix Metalloproteinases, Novel Targets for Directed Cancer Therapy", pigs
and
AQinQ, 11:229-244 ( 1997).
Other conditions mediated by MMPs include restenosis, MMP-mediated
osteopenias, inflammatory diseases of the central nervous system, skin aging,
osteoarthritis, rheumatoid arthritis, septic arthritis, corneal ulceration,
abnormal wound
healing, bone disease, proteinuria, aneurysmal aortic disease, degenerative
cartilage
loss following traumatic joint injury, demyelinating diseases of the nervous
system,
cirrhosis of the liver, glomerular disease of the kidney, premature rupture of
fetal
membranes, inflammatory bowel disease, periodontal disease, age-related
macular
degeneration, diabetic retinopathy, proliferative vitreoretinopathy,
retinopathy of
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-2-
prematurity, ocular inflammation, keratoconus, Sjogren's syndrome, myopia,
ocular
tumors. ocular angiogenesis/ neo-vascularization and corneal graft rejection.
Studies
relating to these conditions are set forth, e.g., in "Recent Advances in
Matrix
Metalloproteinase Inhibitor Research", R. P. Beckett et al., Research Focus,
1:16-26,
5 ( 1996); Curr. Opin. Ther. Patents. 4( 1 ): 7-16, ( 1994); Curr. Medicinal
Chem., 2: 743
762, (1995); Ex~. Opin. Ther. Patents, 5(2): 1087-110, (1995); Exp. Opin.
Ther.
n s 5( 12): 1287-1196, ( 1995); "Inhibition of Matrix Metallo-proteinases:
Structure
Based Design", Current Pharmaceutical Design. 2:524-661, (1996). "Matrix
Metalloproteinase Inhibitor Drugs", Emerg ng Drugs, 2:205-230 ( 1997).
TNF-a convening enzyme (TACE) catalyzes the formation of TNF-a from
membrane bound TNF-a precursor protein. TNF-a is a pro-inflammatory cytokine
that
is believed to have a role in rheumatoid arthritis, septic shock, graft
rejection, cachexia,
anorexia, inflammation, congestive heart failure, inflammatory disease of the
central
nervous system, inflammatory bowel disease, insulin resistance and HIV
infection, in
addition to its well-documented antitumor properties. Research with anti-TNF-a
antibodies in transgenic animals has demonstrated that blocking the formation
of
TNF-a inhibits the progression of arthritis. This observation has recently
been
extended to humans as described in "TNF-a in Human Diseases", Current
Pharmaceutical Design, 2:662-667 ( 1996).
It is expected that small molecule inhibitors of MMPs and TACK would have the
potential for treating a variety of disease states. Although a variety of MMP
and TACE
inhibitors are known, many of these molecules are peptidic and peptide-like
which
demonstrate bioavailability and pharmacokinetic problems. Long acting, orally
bioavailable non-peptide inhibitors of MMPs and/or TACE would thus be highly
desirable for the treatment of the disease states discussed above.
U.S. Patent No, 5,455,258 discloses 2-substituted-2-(arylsulfonylamino)
hydroxyamic acids and their use as MMP inhibitors. WO 97/18194, discloses N-
(arylsulfonyl)tetrahydroisoquinolone-hydroxyamic acids and related bicyclic
derivatives
thereof and their use as MMP inhibitors. WO 97/20824 discloses 1-
(arylsulfonyl)-4-
(substituted)piperazine-2-hydroxyamic acids, 4-(arylsulfonyl) morpholine-3-
hydroxyamic acids, 4-(arylsulfonyl)-tetrahydro-2H,1,4-thiazine-3-hydroxyamic
acids,
3-(substituted-1-(arylsulfonyl)hexahydro-2-hydroxyamic acids and related
compounds
as useful MMP inhibitors.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-3-
SUMMARY OF THE INVENTION
This invention relates to novel derivatives of substituted 2.3,4,5-tetrahydro-
1 H-
[1,4]benzodiazepine-3-carboxylic acid hydroxyamide which exhibit inhibitory
activity
against MMPs. The compounds of the present invention are represented by the
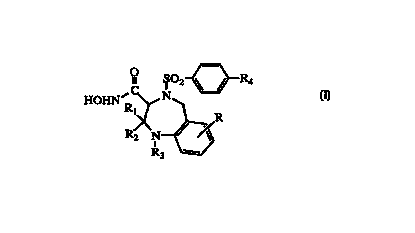
following formula 1
R4
HOHN R N
1 R
R2 N
R3
1
wherein
R is selected from hydrogen, (C1 - C3) alkyl, -CN, -OR', -SR', -CF3, -OCFg,
Cl, F,
NH2, NH(C1 - C3)alkyl, -N(R')CO(C~ - C3)alkyl, -N(R')(R'), N02, -CONH2,
-S02NH2, -S02N(R')(R'), or -N(R')COCH20-(C~ - C3)alkyl, wherein R' is (C1 -
C3)
alkyl or hydrogen;
R4 is (C~ - C6) alkyl-O-, (C~ - C6) alkyl-S-,
R
R> > ~S~ > >
~~R1 ~~RI ~~R~
NON , - D ' , ~ - S fl' ~ '
N N
R~ , -S ~ ~ R~ , or
R, wherein R ~ is hydrogen, halogen, cyano, methyl or -OCH3;
IS
R1 and R2 are each, independently, hydrogen or CH3;
R3 is (C~ - Cg)alkyl, NH2CH2C0-, (C~ - C6)alkylNHCH2C0-, HO(CH2)r,,CO-,
HCO-, Aryl(CH2)"CO-, Heteroaryl(CH2)nC0-, (C 1 - Cg)alkyl-O-(CHZ)"CO-,
(C1 - C3)alkylCO-, (C1 - C3)alkylCO-NHCH2C0-, (C3 - C~)cycloalkylCO-,
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-4-
(C) - C;)alkylSO,-. Aryl(CH2)~SO,-. Heteroaryl(CH,)"S02-, (C~ - C3)alkyl-O-
(CH~)m-SO,-. (C~ - C3)alkyl-O-(CHZ)m, (C~ - C3)alkyl-O-(C~ - C3)alkyl-O-(C~ -
C3)alkyl. HO-(C i - C3)alkyl-O-(C1 - C3)alkyl. Aryl-O-CH,CO-. Heteroaryl-O-
CH~CO-,
AryICH=CHCO-. HeteroarylCH=CNCO-. (C1 - C31a1kylCH=CHCO-,
O / ~ O
CH.,OC- , or ' i~ CH.,OC-NHCH.,CO- ;
S
Aryl(C1 - C3)alkyl, Heteroaryl(C) - C3)alkyl, AryICH=CHCH2-,
HeteroarylCH=CHCH2-. (C ~ - C6)alkylCH=CHCH2-,
Y
~ Co- , Y~~~~
Co- ~ ~ co-
M N
M \~CO- /
Y r~~ CO-
\ ~y ~ W
CO-
/ \ M
~ CO- y ~' '~ CO-
\ / ~ 'J
s
R'OCHZ CH(OR')CO-, (R'OCH2)2C(R')CO-,
CH3- ~N(C~ - C3)alkylCH=CH-CO- , ~N-(Ci - C6)alkylCO-
N-(C~ - C6)alkylCO- , (C~ - C3)alkyICONHCO-, \N-(C~-C~alkylCO- ,
N-(Ci - C6)aIkyICO- , N-(Ci-C6)alkylCO- . ~N-(C~-C6)alkylCO- ,
~ N N= I
~1
CH3-~N(C~ - C6)alkylCO- , t-Boc- ~N-(C~ - C~alkylCO- .
R. CO- p
EtOC~N(C1 - C6)alkylCO- ,
R~ ~,~/O
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-S-
O R'
N- (C ~ - C6)alkylCO-
CH3
R' O CO-
[(C~ - C6)alkyl]Z-N-(C1 - C6)alkyl CO-. or (C~ - C6)alkyl-NH-(C1 - C6)alkylCO-
;
wherein
m=lto3;n=Oto3;
Aryl is
/X
~~, and
R
Heteroaryl is
~~.,~X ~~~X
J X ~ \~ ~ \~ ~ ,
N O
~'r~~\X \r- N ~r,- N
<N~ ° < ~ ~ < ~
R'
~r-.~X ~r-rr X
~N ° ~ ~N ~ °r
O N N
R, R,
wherein X is hydrogen, halogen, (C 1 - C3) alkyl or -OCH3 and R and R' are as
defined
above;
L is hydrogen, (C1-C3)alkyl, -CN, -OR', -SR', -CFg, -OCF3, C1, F, NH2, -NH-(C~
-
C3)alkyl, -N(R')CO(C1 - C3)alkyl, N(R')(R'), -N02, -CONH2, -S02NH2,
-SOZN(R')(R'), -N(R')COCH20-(C~ - C3)alkyl,
CONH- y~~~CONH- y~~/CONH-
<' ,or < ~ ;
S O
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-6-
M is
n ~'-,/
' Y~ J '
N
<N~ N ~O.N ,
R'
\ '~ ~ ~'-,~ Y
< ~ ' <'7 ' « '
N N N
R'' ~ - , ~ ~~ OCO- ~ ~ N-
tBoc - N- . p - , N- ,
U
~N- , or N(R')(R') where R' is as defined above;
W is O, S, NH or N(CI - C3)alkyl;
Y is hydrogen, F, Cl, CF3 or OCHg; and X' is halogen, hydrogen, (CI -
C3)alkyl, O-
(C1 - C3)alkyl, or -CHZOH; and
pharmaceutically acceptable salts thereof.
When used herein the term C1-C3 alkyl covers methyl, ethyl, n-propyl and
i-propyl groups. When used herein the term C1-C( alkyl covers, for example,
methyl,
ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-butyl and pentyl
groups. When
used herein the term C3-C~ cycloalkyl covers saturated and unsaturated
cycloalkyl
groups, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
1-cyclopropenyl, 2-cyclopropenyl, 1-cyclobutenyl and 2-cyclobutenyl.
R is suitably hydrogen, halogen or (C1-C3)alkyl, for example hydrogen, chloro
or methyl. R1 is suitably hydrogen. R2 is suitably hydrogen. R4 is suitably (C
1-C6
alkyl)-O-, for example methoxy. R3 is suitably selected from the group: (C 1-
C3)alkyl-
S02_, aryl(CH2)nS02_, (C1-C3)alkyl-CO-, aryl(CH2)nC0-, heteroaryl(CH2)nC0-,
CA 02317546 2000-07-06
WO 99/37625 PCTNS99/01325
(C1-C3)alkyl-O-(CH2)nC0-, (C3-C~)cycloalkyl-CO-, (Cl-C3)alkyl-O-(CH2)m- or
aryl(C1-C3)alkyl-~ for example methylsulphonyl, n-propylsulphonyl, 4-methyl-
phenylsulphonyl, 4-methoxyphenylsulponyl, methylcarbonyl, 3-
trifluormethylcarbonyl, 2-phenylethylcarbonyl, methoxymethylcarbonyl,
cyclopropylcarbonyl, cyclohexylcarbonyl, 2-methoxyethyl, phenylcarbonyl, 2-
methyl-
5-fluorophenylcarbonyl, 4-biphenylcarbonyl, 2-biphenylcarbonyl, 2,4-
dichlorophenylcarbonyl, phenoxymethylcarbonyl, phenylcarbonyl, 4-
trifluoromethylphenylcarbonyl, 2-imidazol-1-yl-phenylcarbonyl, 2-morpholin-4-
yl-
phenylcarbonyl, 4-ethoxyphenylcarbonyl, 4-(4-methyl-imidazol-1-yl)-2-choloro-
phenylcarbonyl, 2,4-dimethoxyphenylcarbonyl, 4-methyl-piperazin-I-
ylmethylcarbonyl, 3-pyridinylcarbonyl, 2-thienylcarbonyl, 4-pyridinylcarbonyl,
2-
furanylcarbonyl or benzyl.
Preferably, the compounds of the present invention are those of formula 1
wherein R is hydrogen, (C~ - C3) alkyl, -CN, -OR', -SR', -CF3,
-OCF3, CI, F, NH2, NH(C1 - C3)alkyl, -N(R')CO(C1 - C3)alkyl, -N(R')(R'), N02,
-CONHZ, -S02NH2, -S02N(R')(R'), or -N(R')COCHZO-(C~ - C3)alkyl, wherein R'
is (C ~ - C3) alkyl or hydrogen;
R4 is (C1 - C6) alkyl-O-, (C1 - C6) alkyl-S-,
R
O RI , ~S~ > >
~~R' ~~R~ ~~R~
NON ~ -o-': ~ ~ -S f'~
N N
R~ , -S ~ ~ R~ , or
R, wherein R~~ is hydrogen, halogen, cyano, methyl or -OCH3;
R1 and R2 are each, independently, hydrogen or CH3;
R3 is (C ~ - Cg)alkyl, NH2CH2C0-, (C ~ - C6)alkylNHCH2C0-, HO(CH2)I,,CO-,
HCO-, Aryl(CH2)nC0-, Heteroaryl(CH2)nC0-, (C1 - C3)alkyl-O-(CH2)"CO-,
(C~ - C3)alkylCO-, (C~ - C3)alkylCO-NHCH2C0-, (Cg - C~)cycloallcylCO-,
Aryl-O-CH2C0-, HeteroarylOCH2C0-, AryICH=CHCO-, HeteroarylCH=CHCO-,
(C~ - C3)alkyICH=CHCO-,
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
_8_
i ~ i Q
~ CH20C- , ' i~ CH20C-NHCHzCO- ,
wherein
m=lto3;n=Oto3;
Aryl is
X
I ~ and
R'
Heteroaryl is
~r-,~X ~r~~X
J X ~ ~ > ' < ~ '
N S O
X
I j ~'-v
> > ~> >
\N O S
i
R'
'rr ~,~X ~~N
,N , <N,N . or <N J X ,
O
R, R,
wherein X is hydrogen, halogen, (C I - C3) alkyl or -OCHg wherein R and R' are
as
defined above; and pharmaceutically acceptable salts thereof.
More preferably, the compounds of the present invention are those of formula 1
wherein R is hydrogen, (Ct - C3) alkyl, -CN. -OR', -SR', -CFg, -OCF3, Cl, F,
NH2,
NH(C1 - C3)alkyl, -N(R')CO(Ct - C3)alkyl, -N(R')(R'), N02, -CONH2, -S02NH2,
-S02N(R')(R'), -N(R')COCH20-(C~ - C3)alkyl, wherein R' is (C~ - C3) alkyl or
hydrogen;
R4 is (C~ - C6) alkyl-O-, (C~ - C6) alkyl-S-,
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-9-
I ~ ~ I
R~ , ~S~ R~ ,
I ~ ~~R' ~~R~ ~~R~
N~%N ' - D ' ~ ' - S f'' ~ '
N N
I ~ R ~ , - S I ~ R~~ , or
"
R, wherein R~~ is hydrogen, halogen, cyano, methyl or -OCH3;
Rt and R2 are each, independently, hydrogen or CH3;
R3 is
CH3- N(C~ - C3)alkylCH=CH-CO- , N-(C1- C6)alkylCO- ,
N-(C -C alk 1C0-
N-(C~ - C~alkylCO- , (C~ - C~alkylCONHCO-, ' i ~ Y
N-(Ci - C6)alkylCO- , N-(C~-C6)alkylCO- , ~N-(Ci-C6)alkylCO- ,
~N N
CH3- N(C1- C6)alkylCO- , t-Boc-NN~N-(C1 - C~alkylCO-
U
' CO- O ~
EtOC~N(CI - C~alkylCO- ,
R~ ~/O
' O R'
N- (C ~ - C6)alkylCO-
CH3
R' O CO-
[(C1 - C6)alkyl]2-N-(C1 - C6)alkyl CO-,
or (C1 - C6)alkyl-NH-(C1 - C6)alkylCO-, wherein R' is as defined above; and
pharmaceutically acceptable salts thereof.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 10-
It is more preferred that the compounds of the present invention include those
of
formula 1 wherein R is hydrogen, (Ct - C3) alkyl, -CN, -OR', -SR', -CF3, -
OCF3, C1,
F, NH2, NH(C1 - C3)alkyl, -N(R')CO(C~ - C3)alkyl, -N(R')(R'), N02, -CONH2,
-S02NH2, -S02N(R')(R'), or -N(R')COCH20-(Ct - C3)alkyl, wherein R' is (Ct -
C3)
alkyl or hydrogen;
R4 is (Ct - C6) alkyl-O-, (Ct - C6) alkyl-S-,
R
Rt . ~S~ t
~~Rt ~~Rt ~~Rt
N~N ~ - p ' , - S f''
N N
R~ , -S ~ ~ R~ , or
R, wherein R~~ is hydrogen, halogen, cyano, methyl or -OCH3;
R1 and R2 are each, independently hydrogen or CH3;
R3 is (Ct - C3)alky1S02-, Aryl(CH2)nS02-, Heteroaryl(CH2)nS02-, or (Ct -
C3)alkyl-
O-(CH2)m-SO2,
wherein
m=lto3;n=Oto3;
Aryl is
/X
I ~] and
R'
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/013Z5
-11-
Heteroaryl is
~~,/X ~~/X
, ~ ,
X \< '
N O
X
< <~
N O
R'
~r,.--'~/X ~r- N
~N , < ,N , or < J X
O N N
R. R,
wherein X is hydrogen, halogen, (Ct - C3) alkyl or -OCH3 and R and R' are as
defined
above; and pharmaceutically acceptable salts thereof.
A further, more preferred embodiment of the present invention includes
compounds represented by formula 1 wherein
R is selected from hydrogen, (C~ - C3) alkyl, -CN, -OR', -SR', -CF3,
-OCFg, Cl, F, NH2, NH(Ct - Cg)alkyl, -N(R')CO(C~ - C3)alkyl, -N(R')(R'), N02,
-CONH2, -S02NH2, -S02N(R')(R'), or -N(R')COCH20-(Ct - Cg)alkyl, wherein R'
is (C1 - Cg) alkyl or hydrogen;
R4 is (Ct - C6) alkyl-O-, (C~ - C6) alkyl-S-,
R
Rt , ~S~ t ,
~~Rt ~~Rt ~~Rt
NON , - ~ ~ ~ - S f''
N N
R~ , -S ~ ~ R~ , or
R, wherein R~~ is hydrogen, halogen, cyano, methyl or -OCH3;
R~ and R2 are each, independently hydrogen or CH3;
R3 is (C1 - Cg)alkyl, Aryl(C~ - Cg)alkyl, Heteroaryl(Ct - C3)alkyl,
AryICH=CHCH2,
HeteroarylCH=CHCH2-, or (C~ - C6)alkylCH=CHCH2-,
wherein
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 12-
Aryl is
Heteroaryl is
X
./
and
R'
~~,~X ~r,-,~X
J X ' ~/> ° < ~ '
N S O
X
~,-N
<N ' < ~ '
o S
R'
~~ ~~N ~l- N X
<OiN . <N~ ~ or <N~
I I
R, R,
wherein X is hydrogen, halogen, (C~ - C3) alkyl or -OCH3 and R and R' are as
defined
above; and pharmaceutically acceptable salts thereof.
Additionally preferred compounds of the present invention include those of
formula 1 wherein R is hydrogen, (C~ - C3) alkyl, -CN, -OR', -SR', -CF3, -
OCF3, C1,
F, NH2, NH(C1 - C3)alkyl, -N(R')CO(C1 - C3)alkyl, -N(R')(R'), N02, -CONH2, -
S02NH2, -S02N(R')(R'), or -N(R')COCH20-(C1 - C3)alkyl, wherein R' is (C~ - Cg)
alkyl or hydrogen;
R4 is (C~ - C6) alkyl-O-, (C1 - C6) alkyl-S-,
R
R~ , ~S~ 1 ,
- I ~ ~~R1 ~~R1 ~~R~
N~%N ' - ~l ~ . - s f'~ ~ '
N N
R~ , -S ~ ~ R~ , or
R, wherein R ~ is hydrogen, halogen, cyano, methyl or -OCH3;
R~ and R2 are each, independently hydrogen or CH3;
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-13-
R3 is
Y
~ Co- Y~~~~ _
co- , ~ ~ Co ,
L N
M
M NCO- /
Y f"~ ;~ CO- /
J , yv , / w
co-
/ ~ M
~ CO- ~ or y rl~ CO_
w / ~SJ
wherein
m=1 to3;n=Oto3;
L is hydrogen, (C1 - Cg)alkyl, -CN, -OR', -SR', -CF3, -OCP3, C1, F, NH2,
-NH-(C~ - C3)alkyl, -N(R')CO(C1 - C3)alkyl, N(R')(R'), -N02, -CONH2, -S02NH2,
-S02N(R')(R'), -N(R')COCH20-(C1 - Cg)alkyl,
Y r--,, CONH- y ~ CONH-
CONH- ~ ~ ~ , or
S O
M is
w ~''-,/
y ~N J
<O/ ' <N~ <N~ ~O~N
R'
~'-~% Y
< ~ ' <~ ' « .
N N N
R- N- , I \ OCO-N~ N , Ice- ,
/ ~/
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 14-
tB oc - ~ ~ ~N- , N- ,
~l~- , or N(R')(R') where R' is as defined above;
W is O, S, NH or N(C~ - C3)alkyl;
Y is hydrogen, F, Cl, CF3 or OCH3; and X' is halogen, hydrogen, (C~ -
C3)alkyl, O-
(C1 - C3)alkyl, or -CH20H; and pharmaceutically acceptable salts thereof.
The compounds of the invention may be prepared by reacting an appropriate
acid halide such as the acid chloride or bromide with hydroxylamine. The acid
halide
may be prepared by reacting the con esponding acid or a metal salt thereof
with an
activating agent such as oxalyl chloride, oxalyl bromide, thionyl chloride,
thionyl
bromide, (chloromethylene)dimethylammoniumchloride or (bromomethylene)dimethyl
ammonium bromide. The subsequent reaction of the acid halide with
hydroxylamine
may suitably be performed in situ.
Metal salts may be prepared by reacting a ester of formula Ia
Ra
R2~i N
R~
R
R2 N
R3
la
wherein R21 is (C1-6)alkyl, benzyl or arylalkyl and all other groups are as
defined
above, with a base such as lithium hydroxide, potassium hydroxide, sodium
hydroxide
or barium hydroxide. This may suitably be performed in a solvent such as a (C1-
Cg)
alcohol, tetrahydrofuran, N,N-dimethylformide or p-dioxane in the presence or
absence
of water. The resulting metal salt may be directly converted to the desired
product or it
may first be converted to the acid, e.g. by treating it with aqueous
hydrochloric acid or
acetic acid. Alternatively the ester of formula la may be converted to its
acid by
treatment with an aqueous mineral acid such as hydrochloric acid, hydrobromic
acid or
trifluoroacetic acid and the acid converted to the desired product as
described above.
The compounds of formula 1 may be advantageously prepared according to
Reaction Schemes 1 to 7. Variations in these schemes may be made to improve
productivity without negatively impacting the amount and nature of the
product, by
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-15-
means that will be recognized by those skilled in the art. For example,
reactive groups
may be blocked with suitable blocking moieties which may then be deblocked
under
standard conditions (for instance, hydroxy groups may be protected with
trimethylsilyl
or t-butyl-dimethylsilyl moieties which are then removed in a later reaction
step).
In general, the compounds of Formula 1 are synthesized from an alkyl ester
(such as methyl, ethyl, t-butyl and the like) of serine, threonine, or 3,3-
dimethyl-3-
hydroxypropionic acids. One reaction pathway is shown in Reaction Scheme 1. It
is
noted that methyl esters are shown in all of the Reaction Schemes, however, it
is to be
understood that the use of methyl esters is for purposes of illustration only,
and other
suitable alkyl esters or benzyl esters may similarly be used.
In Reaction Scheme 1, serine, threonine, beta-hydroxyvaline and related
derivatives are converted to the corresponding N-(4-substituted-
benzenesulfonyl)
derivatives 3 and alkylated with suitable substituted or unsubstituted 2-
nitrobenzyl
bromides or 2-nitrobenzyl chlorides to provide the corresponding vitro
derivatives 5 .
Reduction under conventional reducing conditions, such as catalytic
hydrogeneration
(with Pd/C) or chemical reduction (e.g., with SnCl2 or FeCl3) results in amino
derivatives 6. Reaction of the N- (2-aminobenzyl) derivatives 6 with alkanoyl
chlorides, alkylsulfonyl chlorides, aroyl chlorides, heteroaroyl chlorides,
aryl sulfonyl
chlorides, heteroarylsulfonyl chlorides and the like, in the presence of
trialkylamines or
pyridene, provides the dihydroalanine derivatives 7. Ring closure to the
[1,4]benzodiazepine compounds 9 is carried out by reaction with a mild base
such as
sodium or potassium bicarbonate in an alcohol solvent such as methanol or
ethanol.
Standard conditions which involve hydrolysis of the ester (NaOH), acid
chloride
formation and reaction of the acid chloride with hydroxylamine are then used
to convert
the ester derivatives 8 to the hydroxamic acids 9. Ester derivatives 8 (where
the ester
function is a t-butyl ester) are converted to the acid with trifluoroacetic
acid under
standard conditions.
As illustrated in Reaction Scheme 2, derivatives 10, which contain a blocked
hydroxyl group, are alkylated with 2-vitro or 2-amino benzyl alcohol
derivatives 11 by
application of the Mitsunobu reaction to give intermediates 12. Reduction of
the 2-vitro
group and removal of the hydroxy blocking group with derivatives 12. where the
R4
group is a protected amino moiety with simultaneous deblocking of the amino
and
hydroxyl functions, gives intermediate compounds 13. The intermediate 13 may
then
be reacted with benzyloxycarbonyl chloride to give the closed ring [1,4J
benzodiazepine
14. Reaction of this compound with acyl chlorides, aroyl chlorides,
heteroaroyl
chlorides, alkysulfonyl chlorides, arylsulfonyl chlorides and
heteroarylsulfonyl
chlorides and the like results in the intermediates 15.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 16-
Scheme 1
O
II
CHgO C NH2 / \ O SO 2 ~ ~ Ra
~R~ C1S02~ Ra --~ CH 30 C NH
HO ~ + Y
R2 2 ~R~ N02
1 HO ~z ~ CH2Br
O S0 2 ~ ~ Ra 3 R I
II
CH30 C N
/ v R O SO -~~ \- Ra
HO ~,, Hz ~ 1I
~z
-- CH30 CY N
6 HO ~~ R ~ / NO 2
Ar(CH 2 ~COCI Heteroaryl(CH 2 ~COCI zR ~J
Ar(CH2)nS02Cl Heteroaryl(CH2)~ 02C1 5
Alkyl-O-CH2~COC1 Alkyl-O-(CH2)m02C1 ~ S02 ~ / Ra
AlkyICOCI AlkylS02C1 CH 30 C
Cycloalkyl( C3to C~)CO Rt~ / ~ R
Rz
R3
O SO 2
I ~ a g
tl
CH30 C
(1) OH-
R~ Rz HN / (2) C1COCOC1
R (3) NH20H
p
II ~02 ~ / Ra
HOHN'C
Rt~ I %R
Rz N
R3
wherein
9
n=Oto3;
m=lto3;
R1 = (Cl - C3)alkyl; R = Hydrogen; halogen; OCH3; NO2; NH2; CF3; NHCOCH3;
NHCOCH20CH3; CONH2; -N(R')(R'), -N(R')CO(C1 - C3)alkyl; (C1 - Cg)alkyl;
R3 = Ar(CH2)"CO-; Heteroaryl(CHZ)"CO-, Ar(CH2)"S02-; Heteroaryl(CH2)nS02-;
Alkyl-O-CH2)nC0-; Alkyl-O-(CH2)mS02-: AlkylCO-; Alky1S02-; AlkylCO-
NHCHZCO-; and cycloalkyl(C3 - C~)CO-; and
R4 is as defined herein.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 17-
Scheme 2
SO ~ ~ g~ O SO 2 ~ ~ Ra
O
I ~ H+ CH3 O C NH
CH30C NH
R5
R~ + ~~O ' ~ \ CH 20H
HO 2 0 + ~
3 10 ' R
11
RS = -NOZ
t-BocNH- ~ 3 P + DEAD
~CH 20CONH-
O SO 2 ~ ~ Ri R g = NO2 O S02 R4
CH3 0 C N ( I ) H 2+ Pd/C CH30 C N
R~ (2.a-) H R~ \
N \ .
HO H2 R 5 = t-BocNH- ~ : I
I J
(1)H+ ;TFA
13 R
R RS= f~CH20CONH- 12
(I) H+
fO CH 20COCI
R6 COCI 2 ~
SO 2 ~ ~ R4 O SO Ra
O or II
CH301C N R~S02C1 CH30C
R, I R~~ . R
R~\NH I % R R6COC1 RZ ~N ~ v
2 _
or R 8
14 R~SO 2CI 15
wherein
n=Oto3;
m=lto3;
~ = phenyl;
DEAD = diethylazodicarboxylate;
R~, = Ar(CH2)~ ; Alkyl-; Heteroaryl(CH2)n ;Alkyl-O-(CH2)n ; Cycloalkyl(Cg -
C~);
R~ = Ar(CH2)"; Alkyl-; Heteroaryl(CH2)n-; Alkyl-O-(CH2)m ;
Rg = Ar(CH2)nC0-; Ar(CH2)"S02-; AIkylCO-; AIkylS02-; Heteroaryl(CH2)nC0-;
Heteroaryl(CH2)"S02-; Alkyl-O-(CH2)"CO-; Alkyl-O-(CH2)t,,S02-.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-18-
1-substituted arylmethyl-2,3,4,5-tetrahydro-1H [1,4)-benzodiazepines may be
prepared in the manner illustrated in Reaction Schemes 3 and 4. In Reaction
Scheme 3,
the methyl 3-hydroxy-2-[4-methoxybenzenesulfonyl)-(2-aminobenzyl)amino]-
propionates 6 are subjected to reductive alkylation with arylcarboxaldehydes
and
heteroarylcarboxaldehydes to provide intermediates 17. Standard reaction
conditions
such as reactions with triphenylphosphine and diethyl azodicarboxylate (DEAD)
or
triplenylphosphine with either carbon tetrachloride or carbon tetrabromide,
results in the
"dehydroalanine" derivatives 18 which are then ring closed to the
[1,4]benzodiazepines
20.
In an alternative route to the 3-hydroxamic acid derivatives 21 (Scheme 4), N-
aroyl derivatives 22 are reduced with reducing agents such as borane or
lithium
aluminum hydride to reduce both the ester and amide functions. The 3-
(hydroxymethyl)-1-(arylmethyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine 23
are
oxidized with stardard reagents known to convert a hydroxymethyl group to a
carboxylic acid:reagents such as NaI04 with catalyst Ru02 (e.g., see J. Org.
Chem.,
46:3936 ( 1981 ); Synlett, p. 143, ( 199b)). Coupling the acids (via the acid
chlorides) to
hydroxylamine then gives products 21. Certain intermediates as exemplified by
formula 25 may be reduced with borane under mild conditions to give
derivatives 25a in
which the amide carbonyl is selectively reduced. These intermediates 25a are
then
converted to hydroxamic acid derivatives via hydrolysis of the ester to the
acid and
coupling the acid chloride with hydroxylamine.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 19-
Scheme 3
SO2~ ~ Ra R S02~ / Ra
R
CH OC
CH 30 C N Ar CHO
_--i R i % R
HO R2 ~H I ~ R OH Ho R2 N
~s II
CHI
6 16 Ar
NaCNBH 3
_ HOAc
R S~ 2 ~ I ~
CH30C ~ N (C6H5 ) P Il S02 ~ ~ Ra
R J ~ / / R ~ 3 CH 30 CAN
t ' H I - DEAD I Rt ~ / R
CH2Ar L.IO~ HN
2 I
i8 ~ _ CH2Ar
17
CH 30C ~ iN~ ~( C6 H 5 )3 P
CCI
C1~R~HN / ~R 4
I
CH2Ar
S02~ ~ R4 ~ 19 _
CH 30C N O SO 2 ~ / Ra
Rt~ I ~ R H0. tt
R~~N
z (
R~ I % R
CHZAr R2
wherein 21 CHZAr
Rto .R9 ~ ~ Rto R9
Ar= ~ / , ~ '/~ ~ ~~~ , ~ 1 , ~~~R9 ~~~R9 ~~~ Rto
CN ,,
/ ~.i C ~ , ~ ~ ~ , or
N R9 S p
9
~~~ R to ; and R 9 and R 1 Q are: C1, Br, F, OCH , OEt , SCH 3 , ~ pCH
p Q O
COEt , CF3 , pCF3 ICNH 2 , -NHCOCH 3 , or Me2N-.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99101325
-20-
Scheme 4
SO 2 ~ / ~ SO 2 ~ / Ra
I
CH 30 C N HOCH N
Rt~ ~ R BHP R~~ / ~~ R
R~ N /_ R2 N
Q ~ ~Ar CH2Ar
22 23
O SO R4 ~ SO 2 ~ / Ra
II I 2 ~ / HO~ N
HOC N N
RtY / '/ R H Rt?'N / ,' R
_ 2
R2 CH CH ZAr
2I
24
wherein Rto ~ Rto R9
Ar= ~ \ I ~~ ~ ~ ~ ~ ~% R9 /~% R~ ~~~Rto
J N /.. S O
R9
9
Rto , and R9 and Rto are hydrogen ,CI, Br, F, OCH3 , OEt ,
q
SCH 3 , COCH 3 , COEt , CF3 , OCF3 , or Me2N-.
I 02 ~ / Ra q S02 ~ /
I
CH HOC N CH30C~ N
Rt]I' / %R B~ Rt?I I %rt
R2 N _.. ~ R2
O'C~ Rs CH2R8
wherein R8 = alkyl, arylalkyl, aryloxyalkyl, heterocyclicalkyl, or
alkyloxyalkyloxyalkyl.
5
CA 02317546 2000-07-06
WO 99/37625 PCT/US99101325
-21 -
Other, preferred compounds of the present invention are those with basic
moieties in the 1-(substituted carbonyl) group which may be prepared in the
manner
shown in Reaction Scheme 5. Reaction of the 2,3,4,5-tetrahydro- I H-[ 1,4]-
benzodiazepines 14 (without a substituent at the I-position) with carbonyl
chloride
derivatives in the manner depicted in Reaction Scheme S, results in
intermediates 25
which are then converted to acid 26 and hydroxamic acids 27. The intermediates
25
may also be synthesized by reaction of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl) amino]-3-hydroxypropionates 6 with acid chlorides to
give
"dehydroalanine" derivatives 28. As previously described, mild bases such as
NaHC03 can be reacted with these derivatives to cause ring closure via a 1,4-
addition
to the double bond in intermediate 28 to provide the 7-membered 2,3,4,5-
tetrahydro-
1 H-[ 1,4] diazepines 25.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-22-
Scheme 5
SO 2 ~ / ~ ~ SO 2 ~ / R4
CH OC ~ CH 30C
R COCI Y
R>>~ I %R 8 Rt]i I ~ R
R N R2 N
2 H Et 3 N or pyridine
O ~ Re
14
Wherein R8 = O N-(CH ) 25
2 n ~-(CH2)~
S~ -(CH2)~ ~ .(CH2)n OH-
N
n
CH3~-(CH2)n ~ ~ N-(CH2)n
CH3
-(CH2) ~ ( CH3( CH2~ 12~(CH2 )n
n
t-B~ -(CH2) n EtOC~ ~ .(CH2 ) n
O
~CH2pC_ CH 3 CONIC
and n = 0 to 3
SO 2 R4 ~ SO 2 ~ / R4
i
N HOC N
HO /~ RY I / R
R~ ~ R i >'
R2 w N I ~ R2 \
O ~' Rs-~ O ~ Ra
27 26
R4 p SO 2 ~ / Ra
CH30C N CH O~~/N
3
HO R2 I ~R R BCOCI R JLR2H / ~ R
--
Et 3 N O ~~ R8
28
NaHC03
CH OH
SO 2 ~ / R4
CH30C
Rt'I I %R
RZ N' _.
O 'R8
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-23-
As illustrated in Reaction Scheme 6, aryl-arylcarbonyl, heteroaryl-
arylcarbonyl,
aryl-heteroarylcarbonyl, heteroaryl-heteroarylcarbonyl derivatives 30 may be
synthesized by standard palladium catalysed coupling of bromoaroyl or
bromheteroaroyl derivatives 29 with appropriate arylstannanes,
heteroarylstannanes,
arylboronic acids, heteroarylboronic acids, aryl triflates, heteroaryl
triflates and the like,
under known conditions. For example, see sis, 563-566 {1997); J.
em , 62:3405-3406, ( 1997); Tetrahedron l..ett., 36:5247-5250, ( 1995);
Heteroc,~, 45:467, ( 1997); Tetrahedron Lett., 38:1118-1182, ( 1997);
Heterocycles,
42:189-I 94, ( 1996); Tetrahedron L_ett., 5005-5006, ( 1993); Synthesis, 843,
( 1987);
Heterocycles, 2711-2716, ( 1987); and Tetrahedron Lett., 4407-4410, ( 1986).
By coupling with such palladium catalysts, aryl-aryl, heteroaryl-aryl, aryl-
heteroaryl and heteroaryl-heteroaryl carboxylic ester derivatives can be
prepared and
these derivatives converted to carboxylic acid intermediates. The acids are
then
converted to acid chlorides which are reacted with esters of 2- [(2-
aminobenzyl)-(4-
substituted-benzenesulfonyl)amino]-3-hydroxypropionate as illustrated for
conversion
of derivatives 6 to intenmediates 31. The following references describe
procedures for
the synthesis of methyl 3-arylpyrrole-4-carboxylates as in J. Ore. Chem.,
62:2649-
2651, (1997); methyl (2-methylphenyl) benzoates as in J. Ors;. Chem.. 62:3405-
3406,
( 1997); and methyl benzoates substitued with heterocycIic moieties such as
furanyl,
thienyl or pyridinyl groups as in Tetrahedron Lett., 27:4407-4410, ( 1986).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-24-
Scheme 6
O SO ~ ~ R4 W
CH O C N 2 y ~~ W W
3 ' R y/ ~ W ; ~, ; Y '~~ ~°ry~\ etc
R~~ ~ / S~ Co~ ;
RZ ~ ~- W = B(OH)2 ; SnMe3 ; Sn(n-butyl)3 ;
O ~ Ar~ O-Triflate; ZnCI ; or ZnBr;
29
Y\~~ B~~~l ~~1 Y\~~~
Ar'= ~~~ ~ CNl , ~S j ~ or <O~ CH OC S02 ~ ~ Ra
Br 3
Pd(PPh3)4 or Pd(PPh3)2C12 H ~ . /R
or Ri R2 H2
equivalent Pd catalyst
_ _ 6 CI
Y
o so ~ ~ R4 o so 2~ R4 of
2~ CH 1
CH30C 3 N
Y;~ R +
R~~N ~ ~ R ~ / % R Et3N L or
R2 I V 2 ... Cl
O~ Ai' ~ p~Ar" ~
30 31 O ~ Ar
M y M M
where Ar" _ ~~~ ~ ~ ~ , ~~~~~ ~ Y ' \ j ~ oY <,r ~ j
,J J N
L
X Y
L is Y ~~ CONH- . ~~ ~ CONH- ~ or y~ O~ CONH- '
S
Y
M is ~ 1'n/ y~~%
y~ ~ ' ~J ' ~Sj , ~OJ ,
N
Y \ N Y\r'_'
'N , N. , < ~ N , or 'N, ;
O I
R'
R'
Y is H, F, Cl, CFg, CHg, or OCH3;
X is halogen, hydrogen, or (C1 - C3)alkyl;
R and R' are as defined herein; and
R4 is as defined herein.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/013Z5
-25-
The intermediates 2.4,5.6-tetrahydro-I H-[ 1.4]benzodiazepines 39 and 38 may
be prepared from glycine esters in the manner e~cemplified in Reaction Scheme
7. In
this synthetic route. N-(4-substituted-benzenesulfonyl) derivatives of glycine
ethyl
ester, glycine t-butyl ester or glycine methyl ester 33 are alkylated with a
substituted (R)
or unsubstituted (R=H) ?-nitrobenzyl bromide in N.N-dimethylformamide or 1-
methyl-
2-pyrrolidinone in the presence of potassium carbonate to give intermediates
34.
Alternatively, the ester of N-(4-substituted-benzenesulfonyl) glycines. such
as the
methyl ester 33. are first reacted with sodium hydride in N. N-
dimethylformamide or 1-
methyl-2-pyrrolidinone and the resulting anion reacted with substituted or
unsubstituted
2-nitrobenzylbromides to provide compounds 34. Reaction of derivates 34 with
N,N-
dimethyl(methylene)ammonium chloride or the iodide salts under standard
reaction
conditions (e.g., as set forth in Fieser~ and Fieser, 10:160-161; 8:194
affords the
dimethylaminomethyl (Mannich type) compounds as intermediates for elimination
to the
"dehydroalanine" derivatives 37 or direct ring closure of 36 to 39 via ..an
elimination-
addition reaction. Ring closure of compounds 37 provides intermediates 38 for
conversion to hydroxamic acids. Variations of the reactions conditions for
conversion
of 36 to 39 involve heating in the presence of Lewis acids, such us BF3, or
heating an
acid salt of 36 to effect the elimination-addition reaction.
CA 02317546 2000-07-06
WO 99/37625 PCTNS99/01325
-26-
Scheme 7
O 10
CH30CCH2NH2 + C1S0 ~ ~ R4 ~ CH30CCH2NHS ~ ~ R4
33 CH2Br
32 2 N02
O
il R
CH30CCH2~ S02 ~ ~ R4
CH2 101
NH2 H2 CH30CCH2 N-S02 ~ ~ R4
I .' R Ps-~---- H
~ 2 N02
35 +
CH2 = N(CH3)2C1- R
or +
CH2 = N(CH3)2I-
O _ S / ~ R
CH30CCI~N-S02 ~ ~ R4 CH~~ ~ 4
(CH3)2I~ CH 2 CH2
H
/,R
NH2 ~ ,~ ~t3 ~
36 R
37
O S02 ~ ~ R4 ~ SO ~ ~ R4
C OC l~t
CH30C 1~T H3
/ /R ~ / %R
R3
39 3g
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-27-
The intermediate carboxylic acids for conversion to the
tetrahydro[ 1,4]benzodiazepine-3-carboxylic acid, hydroxyamides may be
synthesized
via different routes as shown in Schemes 1-8. For the synthesis of some of the
desired
products of Formula 1, alternate routes may be preferred as shown in Scheme 8
.
Under these conditions, intermediate carboxylate esters of Intermediate 41 or
acids of
Intermediate 44 wherein the R,~ substituent is an OH group are prepared.
Intermediates
with R4 an OH group may be prepared from derivatives wherein the OH group is
protected by a group which can be selectively removed. Derivatives 40 wherein
R4 is
an OCH~ moiety are suitable precursors to the desired phenolic compounds 41
and 44
through cleavage of the oxygen methyl bond. As shown in Scheme 8, the anion of
the
phenolic OH group may be prepared in situ and then alkylated. Suitable bases
are
alkaline metal carbonates, hydrides, alkoxides and organic bases. Reaction
with an
alkylating moiety represented by the Formula (C,-C6)alkyl-X wherein X is a
reactive
leaving group such as a chloride, bromide, iodide, O-mesylate of an O-tosylate
gives
the derivatives 42 and 45.
The alkylation reaction may be carried out with caboxylate esters such as 41
or
with the carboxylic acids such as 44. Alternatively, the phenolic compounds 41
and 44
may be reacted under Mitsunobe Reaction conditions to afford the O-alkylated
derivatives 42 and 45. Standard Mitsunobe Reaction conditions, such as those
described in the following literature references, may be used in the coupling
reactions:
J. Heterocyclic Chem. 34:349 ( 1997); Tetrahedron Lett. 37:6439 ( 1996); J.
Ors.
Chem., 56:7173 ( 1991 ); Tetrahedron Lett. 5709 ( 1989); S n he is 1:28 ( 1981
).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-28-
Scheme 8
/ H3 O $Oz \ / H
CH30 R BBr3 N R
R / ~ CH 30
I Rt N
Rz R3 Rz R3
40 (C t-C6)alkyl-X
41
1 ) OH B~ (C t-Ce)alkylOH
2) BBr3 or (C6Hs)~P
DEAD
O ~~ \ / H O ~pz \ / O-alkyl(C,-C6)
N R
HO / / CH30
R, N /
Rz R3 ._ RtRz R
3
44
(C6EIs~P 42
or
Base DEAD (Ct~6~1ky1pH
(C t-C6~1kY1_X
OH
O ~Oz \ / O-alkyl(Ct-Cs) p SOz \ / O-alkyl(Ct-C6)
N '~R OH HO N
(C,-C6)alkYl
/
Rt I Rt N /
Rz R3 - Rz R3
45 43
(C,-C6)alkyl-X
X = halogen, OTs,
OMs
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-29-
The compounds of the present invention which have a basic moiety may be used
in the form of salts derived from pharmaceutically or physiologically
acceptable acids.
These salts include, but are not limited to, salts with inorganic acids (such
as
hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid) or organic
acids (such as
acetic acid, oxalic acid, succinic acid, and malefic acid). Other salts of
compounds with
an acidic moiety include those with alkali metals or alkaline earth metals
(such as
sodium, potassium, calcium, and magnesium) or organic bases.
When the present compounds are utilized in pharmaceutical compositions, they
may be combined with one or more pharmaceutically acceptable Garners, e.g.,
solvents,
diluents and the like. Such compositions containing the present compounds may
be
administered orally, in the form of tablets, capsules, dispersible powders,
granules,
suspensions, syrups or elixirs; parentally, in the form of a sterile
injectable solution or
suspension; or topically, in the form of creams, lotions, ointments, etc. Such
pharmaceutical compositions may contain from about 1 to about 100 mg of active
ingredient in combination with the Garner.
The effective dosage of the present compounds utilized to treat a specific
condition will vary depending upon the particular compound employed, the mode
of
administration and the type and severity of the condition being treated.
However, in
general, satisfactory results are obtained when the present compounds are
administered
at a dosage of about 0.001 to 1000 mg/kg of body weight.
As noted above, the compounds of the present invention may be administered
orally, as well as by intravenous, intramuscular, subcutaneous or topical
routes. Solid
carriers useful for preparing tablets, capsules, etc., include starch,
lactose, dicalcium
phosphate, microcrystalline cellulose, sucrose and kaolin. Liquid carriers
useful for
preparing compositions of the present compounds include sterile water,
polyethylene,
glycols, non-ionic surfactants, and edible oils such as corn, sesame, and
peanut oils.
Adjuvant.c conventionally used in the preparation of pharmaceutical
compositions may
also be included, such as flavoring agents, coloring agents, preservatives and
antioxidants.
The compounds of the present invention were tested for biological activity
according to the following procedures.
CA 02317546 2000-07-06
WO 99137625 PCT/US99101325
-30-
In Vitro Gelatinase Assay
The assay is based on the cleavage of the thiopeptide substrate ((Ac-Pro-Leu-
Gly(2-mercapto-4-methyl-pentanoyl)-Leu-Gly-OEt), available from Bachem
Bioscience) by the enzyme gelatinase, releasing the substrate product which
reacts
colorimetrically with DTNB ((5,5'-dithio-bis(2-nitro-benzoic acid)). This
assay is
disclosed in Weingarten et al., "Spectrophotometric Assay for Vertebrate
Collegenase",
Anal. Biochem., 147:437-440, ( 1985). The enzyme activity is measured by the
rate of
the color increase.
The thiopeptide substrate was made up fresh as a 20 mM stock in 100% DMSO
and the DTNB was dissolved in 100% DMSO as a 100 mM stock and stored in the
dark
at room temperature. The substrate and the DTNB were diluted together to 1 mM
with
substrate buffer (50 mM HEPES, pH 7.5, 5 mM CaCl2) before use. The stock of
human neutrophil gelatinase B was diluted with assay buffer (SO mM HEPES, pH
7.5,
5 mM CaCl2, 0.02% Brij) to a final concentration of 0.15 nM.
The assay buffer, enzyme, DTNB/substrate (500 ErM final concentration) and
vehicle or inhibitor were added to a 96 well plate (total reaction volume of
200p1) and
the increase in color was monitored spectrophotometrically for 5 minutes at
405 nm on
a plate reader.
The increase in OD405 was plotted and the slope of the line was calculated.
The
slope represents the reaction rate. The linearity of the reaction rate was
confirmed (r2
>0.85) and the mean (x t sem) of the control rate was calculated and compared
for
statistical significance (p <0.05 j with drug-treated rates using Dunnett's
multiple
comparison test. Dose-response relationships were generated using multiple
doses of
drug and ICSp values with 95% CI were estimated using linear regression
(IPRED,
HTB).
In Vitro CollaEenase Assay
This assay was based on the cleavage of a peptide substrate ((Dnp-Pro-Cha-
Gly-Cys(Me)-His-Ala-Lys(NMa)-NH2), available from Peptide International, Inc.)
by
collagenase releasing the fluorescent NMa group which was quantitated on the
fluorometer as disclosed in Bickett et al., "A High Throughput Fluorogenic
Substrate
CA 02317546 2000-07-06
WO 99!37625 PCT/US99/01325
-31 -
for Interstitial Collagenase (MMP-1 ) and Gelatinase (MMP-9)", Anal. Biochem.,
212:58-64, ( 1993). Dnp quenches the NMa t7uorescence in the intact substrate.
The assay was inn in HCBC assay buffer (50 n>Ni HEPES, pH 7.0, 5 mM
Ca+2, 0.02% Brij, 0.5% Cysteine), with human recombinant fibroblast
collagenase
(truncated, mw=18,828, from Wyeth-Ayerst Research, Radnor, PA). The substrate
was dissolved in methanol and stored frozen in 1 mM aliquots. Collagenase was
stored frozen in buffer in 25 pNl aliquots. In conducting the assay, the
substrate was
dissolved in HCBC buffer to a final concentration of 10 E.~M and collagenase
to a final
concentration of 5 nM. The compounds being examined were dissolved in
methanol,
DMSO, or HCBC. The methanol and DMSO were diluted in HCBC to < 1.0%. The
compounds were added to a 96 well plate containing enzyme and the reaction was
started by the addition of substrate.
The reaction was read (excitation 340 nm, emission 444 nm) for 10 min. and
the increase in fluorescence over time was plotted as a linear line. The slope
of the line
was calculated representing the reaction rate. The linearity of the reaction
rate was
confirmed (r2 >0.85). The mean (x t sem) of the control rate was calculated
and
compared for statistical significance (p <0.05) with drug-treated rates using
Dunnett's
multiple comparison test. Dose-response relationships were generated using
multiple
doses of drug and IC50 values with 95% CI were estimated using linear
regression.
Procedure for Measuring TACE Inhibition
In a 96-well black microtiter plate, each well received a solution composed of
10
pI. TACE (available from Immunex) at a final concentration of 111g/mL, 701rL.
Tris
buffer, have a pH of 7.4 and containing 10% glycerol (final concentration 10
mM), and
10 pL of test compound solution in DMSO (final concentration 1 pM, DMSO
concentration < 1 %). The plates were incubated for 10 minutes at room
temperature.
The reaction was initiated by addition of a fluorescent peptidyl substrate
(final
concentration 100 ECM) to each well with shaking on a shaker for 5 sec.
The reaction was read (excitation 340 nm, emission 420 nm) for 10 min. and
the increase in fluorescence over time was plotted as a linear line. The slope
of the line
was calculated and this represents the reaction rate. The linearity of the
reaction rate
was confirmed (r2 >0.85). The mean (xtsem) of the control rate was calculated
and
compared for statistical significance (p<0.05) with drug-treated rates using
Dunnett's
CA 02317546 2000-07-06
WO 99/37625 PC'f/US99/01325
-32-
multiple comparison test. Dose-response relationships were generated using
multiple
doses of dmg and ICSp values with 95% CI were estimated using linear
regression.
The results obtained following these standard experimental test procedures are
presented in Table 1.
Table I
O $O~~R4
N
HORN
Rt N / i R
RZ
R3
Compound
of
R3 Example R R~RZ R4 ICs o (nM)
_ - - - - MMP-1 MMP-9MMP- I TALE
3
-SO ~ ~ 2 H H H -OCH3_ 14.1 5.1 391
CH3 12
-SOiCH3 3 H H H -OCH3156.5 7.9 3.0 104
8
-SOZC H2CH2CH H H H -OCH.~183 7.0 2.8 91
3 10 10
-SO~OCH3 4 H H H -OCH~2~.1 12.2 4.3 101
t
3
-COCH 6 H H H -OCH318.4 1.4 10 103
3 7
-CO / ~ 5 H H H -OCH315.8 0.56 95
(23) (1.7) 10
0.4
(1.1)
CA 02317546 2000-07-06
WO 99/37625 PCT/US99I01325
-33-
Compound
of
R3 R R~ RZ R4 ICs
Example (nM)
- - MMP-1 MMP-9 MMP-13 TACE
-CO- 7 H H H -OCH~20.4 0.6 0.4 77.7
I (34) ( ( 1.3) + 7
~ 1.9)
-CO-~ 8 H H H -OCH319.7 1.1 1.1 12.8
1.2
-CO- 13 H H H -OCH354.9 9.8 2.0 154 +_
I , 27
~
~N
COCH20CH3 9 H H H -OCH334.1 1.34 1.19 95,2
14.8
-CO(CH~ 12 H H H -OCH 523 17.9 25
~ 3 7
/
. 207 +
21
-CO 1 H H H -OCH 96.2 5.1 3.7 352
~ ~ 34
/
CF3
CH3
-CO
/ 11 H H H -OCH 55.4 3.9 2.3 271 +
3 20
F
-C 15 H H H -OCH352.7 0.7 0.4 199
19
-CO
/
14 H H H -OCH 542 12.6 3.7 4596
3 ( 1
uM)
55 H H H -OCH3171 4.0 3.3 6g,5
-CO~ 7.2
-CO~ 57 H H H -OCH3465 12.7 7.2 318
27
-CO-~CI 31 H H H -OCH375.5 3.0 2
6
. 367o(luM)
C1
-C 40 H H H -OCH316.6 1.4 1.2 28.5
/~ 6.6
O
-C~H 58 H H H -OCH365.5 4.4 2.9 154
2o- 20
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-34-
Compound of
R~ Example R Ri R, R.~ lCso
(nM)
- - - - MMP-1 MMP-9 MMP-13TACE
-COCH.,OCH~ 59 -CH3 H H -OCH~105 2.6 1.8 12516
7
C / \ 60
7-CH3 H H -OCH~22.7 1.4 1.3 143+4
-C ~ ~ OCF3 61 g-CI H H -OCH~239 1.3 0.4 1248
(265) (3.9) (4.3) t 69
-CHzCH,OCH; 62 H H H -OCH;1000 100 100 51
( 1pM)
CN
\
63 7-CH; H H -OCH3130 5.6 3.1 aa6
t as
-C / \
64 8-Cl H H -OCH3l57 6.1 3.4 384
-CO ~ t 8
-C ~ ~ OCZHS 65 H H H -pCH323.5 1.5 1.5 157
t 13
C1
-C0~1,~CH3 66 H H H -OCH~g3.4 3.4 2.6 148
t 14
-CH2-~ 67 H H H -OCH31323 50.8 73.9 551
f 29
CH30
-C
71 H H H -OCH~41.3 2.4 1.3 136
t 15
OCH
n
-CO-CH~I~N-CH372 H H H -OCH34982 187 317 808
t 90
The present invention will now be illustrated with reference to the following,
non-limiting examples.
Reference Example 1
(L) N-(Benzyloxycarbonyl)-O-benzylserine, t-butyl ester
Into a solution of 25 g (0.076 moI) of N-(benzyloxycarbonyl)-O-benzylserine in
600 ml of CH2C12 cooled to -6°C in an ice-salt bath was bubbled
isobutylene, while
4.1 ml of concentrated sulfuric acid was added dropwise thereto. The mixture
was
stirred for 4 hours and worked up as described in synthetic Commun., 26:2723 (
1996)
to give 29.24 g of product as a yellow oil.
CA 02317546 2000-07-06
WO 99/37625 PC'T/US99/01325
-35-
Reference Example 2
L-Serine, t-butyl ester
A mixture of 29.24 g (0.076 mol) of (L) N-(benzyloxycarbonyl)-O
benzylserine, t-butyl ester from Reference Example 1, 24.1 g (0.38 mol) of
ammonium
formate and 38.3 g of 10% palladium on carbon in 600 ml of methanol was heated
at
65°C for 20 hours and stirred at room temperature overnight. The
mixture was filtered
through diatomaceous earth and the filter pad was washed with methanol. The
filtrate
was concentrated to give 12.18 g (99.6%) of product as described in S, nty
hetic
Commun., 26:2723 ( 1996}.
Reference Example 3
N-(4-Methoxybenzenesulfonyl)-L-serine, t-butyl ester (3-hydroxy-2-(4
methoxybenzenesulfonylamino)propionic acid, tert-butyl ester)
To a solution of 12.18 g (0.0756 mol) of L-serine, t-butyl ester, 26.52 ml of
triethylamine in 160 ml of CH2Cl2 (cooled in an ice bath) was added, in small
portions,
16.1 g (0.0771 mol) of 4-methoxybenzene-sulfonyl chloride. The mixture was
stirred
at 0°C for 0.5 hours and at room temperature overnight. The mixture was
washed with
H20, 2N citric acid, brine and dried with Na2S04. The solvent was removed
under
vacuum to give 25.34 g of solid which was triturated with hexane. The solid
was
recrystallized from 120 ml of toluene to give 12.18 g (48:7%) of product as a
white
solid. The filtrate was concentrated and the residue chromatographed on silica
gel with
hexane-ethyl acetate (7:3) as eluent to give 5.71 g (22.8%) of white solid.
m.p. 70-
75°C. Anal. for C14H21N06S:
Calc'd: C, 50.7; H,6.4; N,4.2;
Found: C, 50.4; H,6.3; N,4.4.
Reference Example 4
3-Hydroxy-2-[(4-methoxybenzenesulfonyl)-(2-nitrobenzyl)amino]
propionic acid, tert-butyl ester
To 6.16 g ( 18.6 mmol} of 3-hydroxy-2-(4-methoxybenzenesulfonylamino)-
propionic acid ~-butyl ester in 50 ml of N,~1-dimethylformamide, cooled in an
ice
bath, was added 0.781 g ( 19.5 mmol) of sodium hydride. After gas evolution
ceased,
a solution of 4.02 g ( 18.6 mmol) of 2-nitrobenzylbromide in 18 ml of N,~1-
dimethylformamide was added dropwise. The mixture was stirred under nitrogen
at
room temperature for 4 hours and 1.0 g of 2-nitrobenzyl bromide was added. The
mixture was stirred at room temperature overnight and the solvent removed
under
vacuum. The residue was diluted with water and extracted with CH2Cl2. The
organic
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-36-
extract was washed with H20, brine and dried with Na2S04. The solvent was
removed to give 11.2 g of solid which was chromatographed on silica gel with
hexane-
ethyl acetate ( 1:1 ) as eluent followed by hexane-ethyl acetate (35:65) as
eluent. The
fractions containing product were combined and the solvent was then removed to
gave
7.7 g (89%) of solid. A sample from a 3 mmol run gave a gum. Anal. for
C21H26N208S:
Calc'd: C,54.1; H,5.6; N,6.0;
Found: C,54.0; H,5.7; N,6Ø
Reference Example 5
2-[(2-Aminobenzyl)-(4-methoxybenzenesulfonyl)amino]-3
hydroxypropionic acid, tert-butyl ester
A mixture of 0.60 g ( 1.28 mmol) of 3-hydroxy-2-[(4-methoxybenzene
sulfonyl)-(2-nitrobenzyl)amino] propionic acid, tit-butyl ester and 1.45 g
(6.45 mmol)
of SnCl2~2H20 in 20 ml of methanol was heated in an oil bath at 90°C
for 2 hours.
The solvent was removed under vacuum and ethyl acetate added to the residue.
The
mixture was neutralized with saturated sodium bicarbonate solution and
filtered through
diatomaceous earth. The ethyl acetate layer was separated and washed with H20,
brine
and dried with Na2S04. The solvent was removed under vacuum to give 0.30 g
(53%)
of a gum. Anal. for C21 H28N206S:
Calc'd: C, 57.8; H,6.5; N,6.4;
Found: C, 57.8; H,7.0; N,6.2.
Reference Example 6
2-[(2-Aminobenzyl)-(4-methoxybenzenesulfonyl)amino]-3-
hydroxypropionic acid
A solution of 0.75 g ( 1.72 mmol) of 2-[(2-aminobenzyl)-(4-methoxybenzene-
sulfonyl)amino]-3-hydroxypropionic acid, tent-butyl ester and 6 ml of
trifluoroacetic
acid in 6 ml of CH2C12 was stirred at room temperature for 3 hours and then
concentrated to dryness under vacuum. To the residue was added H20, CH2Cl2 and
1N NaOH until the aqueous layer reached pH 8. The aqueous layer was then
separated, acidified with 2 N citric acid and extracted with ethyl acetate.
The extract
was washed with H20, brine and dried Na2S04. The solvent was removed under
vacuum to give 0.35 g (54%) of a solid. Anal. for C17H2pN206S:
Calc'd: C, 53.7: H,5.3; N,7.4;
Found: C, 53.0; H,5.3; N,6.9.
CA 02317546 2000-07-06
WO 99/37625 PCTNS99/01325
-37-
Reference Example 7
2-{ (2-[3-(Trifluoromethylbenzoyl)aminobenzyl]-(4
methoxybenzenesulfonyl)amino}acrylic acid, tert-butyl ester
A mixture of 0.431 g (1 mmol) of 2-[(2-amino-benzyl)-(4-methoxybenzene-
S sulfonyl)amino]-3-hydroxy-propionic acid, tert-butyl ester, 0.474 g (2.2
mmol) of 3-
(trifluoromethyl)benzoyl chloride and 1 ml of pyridine in 2 ml of CH2C12 was
stirred at
room temperature for 3.5 hours. The mixture was poured into H20 and extracted
with
CH2C12. The extract was washed with H20, 2 N citric acid, H20, 1 N NaHC03,
brine and dried with Na2S04. The solvent was removed to give 0.72 g of solid.
The
solid was dissolved in 2 ml of tetrahydrofuran and 1.5 ml of triethylamine was
added
thereto. The solution was heated at 65°C overnight and concentrated to
dryness under
vacuum. The residue was extracted with CH2C12 and the extract washed with H20
and dried with Na2S04. The solvent was removed under vacuum to give 0.55 g of
product as a solid. From a similar run the product was chromatographed on
silica gel
with hexane-ethyl acetate to give a solid, m.p. 65-72°C. Anal. for
C29H29F3N2O6S:
Calc'd: C, 59.0; H,5.0; N,4.7;
Found: C, 59.2; H,5.2; N,4.4.
Reference Example 8
20 4-(4-Methoxybenzenesulfonyl)-I-(3-trifluoromethylbenzoyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, tert-butyl ester
A mixture of 0.55 g (0.932 mmol) of 2-{(2-[3-(trifluoromethyl)benzoyl)
aminobenzoyl]-(4-methoxybenzenesulfonyl)amino } acrylic acid, tert-butyl ester
and
O.I02 g (1.21 mmol) of NaHC03 in 4 ml of methanol was stirred at room
temperature
overnight and the solvent removed. The residue was extracted with CH2C12 and
the
extract washed with H20, brine and dried with Na2S04. The solvent was removed
to
give 0.57 g of solid. The solid was chromatographed on thick layer silica gel
plates
with hexane-ethyl acetate (1:1) as solvent to give 0.30 g of a light yellow
solid, m.p.
57-60°C. Anal. for C29H29F3N2O6S:
Calc'd: C,59.0; H,5.0; N,4.7;
Found: C,58.8; N,5.0; N,4.6.
CA 02317546 2000-07-06
WO 9913625 PCT/US99101325
-38-
Reference Example 9
4-(4-Methoxybenzenesulfonyl)-1-(3-trifluoromethylbenzoyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
A mixture of 0.36 g (0.61 mmol) of 4-(4-methoxybenzenesulfonyl)-1-(3
trifluoromethylbenzoyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic
acid,
ten-butyl ester and 3 ml of trifluoroacetic acid in 3 ml of CH2Cl2 was stirred
at room
temperature for 3 hours. The mixture was concentrated to dryness under vacuum
and
the residue extracted with CH2Cl2. The CH2Cl2 was washed with 1 N NaHC03 and
the aqueous layer (pH 8) was acidified with 2 N citric acid and extracted with
ethyl
acetate. The extract was dried (Na2S04}. The original CH2C12 extract was
washed
with 2 N citric acid, H20, brine and dried with Na2S04. The CH2C12 extract and
the
ethyl acetate extract were combined and the solvent removed under vacuum to
give 0.31
g of solid, m.p: 105-110°C. Anal. for C25H21F3N206S:
Calc'd: C,56.2; H,4.0; N,5.2;
Found: C,55.1; H,3.7; N,5Ø
Reference Example 10
Methyl 1-([l,1'-Biphenyl]-2-carbonyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a mixture of 1.5 g (3.8 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate and 2.65 ml of triethylamine
in
12 ml of CH2C12 chilled at 0°C was added a solution of [ 1,1'-biphenyl]-
2-carbonyl
chloride in 6 ml of CH2Cl2. The mixture was stirred at room temperature
overnight
and diluted with CH2Cl2 and H20. The organic layer was separated and washed
with
2 ~1 citric acid, brine and dried with Na2S04, The solvent was removed under
vacuum
to give 2.2 g of a white foam. Anal. for C31 H28N206S:
Calc'd: C,66.9; H,5.1; N,5.0;
Found: C,67.3; H,5.2;N,4.7.
Reference Example 11
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-5-fluorobenzoyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a mixture of 1.5 g (3.80 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate and 2.64 ml ( 18.97 mmol) of
triethylamine in 15 ml of CH2Cl2, chilled to 0°C, was added 1.36 g (
11.4 mmol) of 2
methyl-5-fluorobenzoyl chloride. The mixture was stirred at room temperature
overnight. The solution was then diluted with CH2Cl2 and water and the organic
layer
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-39-
separated. The organic layer was washed with 2 N citric acid. brine and dried
with
Na2S04, The solvent was removed under vacuum to give 2.2 g of a white foam.
Anal. for C~6H25FN~06S:
Calc'd: C,60.9; H,4.9; N,5.5;
Found: C,60.9; H,5.0; N,5.0;
Mass spectrum (ES) 513.4 (M+H).
Reference Example 12
Methyl 4-(4-Methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]
benzodiazepine- 3-carboxylate
To a mixture of 5.0 g (12.68 mmol) of methyl 2-[(2-aminobenzylj-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate and 17.7 ml (26.8 mmol) of
triethylamine in 50 ml of CH2Cl2 chilled to 0°C was added 9.05 ml (63.4
mmol) of
benzyl chloroformate. The mixture was stirred overnight and then cooled to
0°C and .8
ml of triethylamine and 9.05 ml (63.4 mmol) of benzyl chloroformate were added
thereto. The mixture was stirred overnight and then washed with H20, 2 N
citric acid,
brine and dried with Na2S04, The solvent was removed under vacuum to give 6.95
g
of solid. The solid was chromatographed on silica gel with hexane-ethyl
acetate ( 1:1 ) to
give 2.7 g of product as a viscous yellow oil. From a similar 0.5 g run, there
was
obtained 0.178 g of an oil. Anal. for C 1 gH2pN205S:
Calc'd: C,57.4; H,5.4; N,7.4; S,8.5;
Found: C,57.9; H,5.4; N,6.7; S,7.9;
Mass spectrum (ES) 377.2 (M+H).
Reference Example 13
Methyl 3-Hydroxy-2-(4-methoxybenzenesulfonylamino)propionate
To a mixture of 5.0 g (32.14 mmol) of D,L-serine, methyl ester and 15.7 ml
(0.012 mol) of triethylamine in 100 ml of CH2Cl2, cooled to 0°C, was
added
portionwise 6.64 g (32.14 mmol) of 4-methoxybenzenesulfonyl chloride. The
mixture
was then stirred under argon at room temperature for 2 days. The mixture was
diluted
with 100 ml of CH2CI2 and then washed with 60 ml each of H20, 2 N citric acid,
brine and dried with Na2S04, The solvent was removed under vacuum to give a
solid.
Crystallization from ethyl acetate gave 5.0 g (54%) of white crystals, m.p. 92-
94°C.
Anal. for C 11 H 1 SN06S:
Calc'd: C,45.7; H,5.2; N,4.8; S,11.1;
Found: C,45.6; H,5.2; N,4.8; S,11.1.
CA 02317546 2000-07-06
WO 99/3625 PCT/US99/01325
-40-
Reference Example 14
Methyl 3-Hydroxy-2-[(4-methoxybenzenesulfonyl)-(2-nitrobenzyl)
amino]propionate
To a solution of 15.0 g (51.85 mmol) of methyl 3-hydroxy-2-(4-
methoxybenzenesulfonylamino)propionate in 125 ml of N,N-dimethylfolmamide,
cooled in an ice bath, was added portionwise 2.29 g (57.03 mmol) of NaH (60%
in
oil). The mixture was stirred at 0°C for 20 minutes and then a solution
of 12.32 g
(57.03 mmol) of 2-nitrobenzyl bromide in 25 ml of dry N,N-dimethylformamide
was
added dropwise. The solution was stirred at room temperature for 48 hours and
diluted
with 500 ml of ethyl acetate and water. The organic layer was separated and
the
aqueous layer extracted with 250 ml of ethyl acetate. The combined organic
layer and
extract was washed with 200 ml each of H20, 1 N NaHC03, brine and dried with
Na2S04, The solvent was removed and the residual solid was triturated with
ethyl
acetate, cooled and filtered to give 13.5 g (61 %) of white crystals, having a
m.p. 127-
129°C. From a small scale run (3.0 g) there was obtained 2.32 g of
white crystals,
having a m.p. 127-129°C. Anal. for C18H2pN2O8S:
Calc'd: C,50.9; H,4.8; N,6.6;
Found: C,50.9; H,4.8; N,6.5.
Reference Example 15
Methyl 2-[(2-Aminobenzyl)-(4-methoxybenzenesulfonyl)amino]-
3-hydroxypropionate
To a mixture under nitrogen of 1.5 g (3.53 mmol) of methyl 3-hydroxy-2-[(4-
methoxybenzenesulfonyl)-(2-nitrobenzyl)amino]propionate in 5 ml of dry ethanol
was
added 1.12 g ( 17.69 mmol) of ammonium fonnate followed by the addition of
0.50 g
of 10% palladium on carbon. The mixture was stirred overnight at room
temperature
and heated at 80°C for 2 hours. The mixture was filtered through
diatomaceous earth
and the filtrate concentrated to dryness under vacuum to give a semisolid.
Trituration
with ethyl acetate gave 0.65 g (47%) of white crystals, m.p. 138-140°C;
Anal, for
C 18H22N206S:
Calc'd: C,54.8; H,5.6; N,7.1;
Found: C,53.0; H,5.6; N,6.8.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-41 -
Reference Example 16
Methyl 3-Hydroxy-2-{ (4-methoxybenzenesulfonyl)-[2-(2,2,2-
trifluoroacetylamino)benzyl]amino}propionate
To a solution of 0.50 g ( 1.27 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate in 5 ml of CH2Cl2 was added
1.8 ml ( 12.7 mmol) of trifluoroacetic anhydride. The solution was stirred for
1 hour
and concentrated to dryness under vacuum. Methanol was added to the residue
and the
solvent was removed under vacuum. The addition of methanol and concentration
to
dryness was repeated twice. The residue was chromatographed on silica gel
thick layer
plates with hexane-ethyl acetate ( 1:1 ) to give 0.50 g of a colorless glass.
Anal. for
C20H21F3N207S:
Calc'd: C,49.0; H,4.3; N,5.7;
Found: C,49.0; H,4.5; N,5.4.
Reference Example 17
Methyl 2-[(4-Methoxybenzenesulfonyl)-(2-nitrobenzyl)amino]
acrylate
To a solution of 1.0 g (2.356 mmol) of methyl 3-hydroxy-2-[(4-
methoxybenzenesulfonyl)-(2-nitrobenzyl) amino]propionate in 2 ml of pyridine,
cooled
to -10°C was added 0.539 g (2.83 mmol) of 4-methylbenzeuesulfonyl
chloride. The
solution was chilled overnight and 4 ml of pyridine and 0.539 g (2.83 mmol) of
4
methylbenzene-sulfonyl chloride were added. The mixture was stirred and
chilled at
-10°C for 24 hours and diluted with H20. The mixture was extracted with
ethyl acetate
and the extract washed with H20, 2 N citric acid, and brine and then dried
(Na2S04).
The solvent was removed under vacuum to give 1.2 g of an oil. The oil was
dissolved
in 6 ml of pyridine and 1.08 g of 4-methylbenzenesulfonyl chloride was added
thereto.
The mixture was stirred at room temperature overnight and diluted with H20.
The
mixture was extracted with ethyl acetate and the extract was washed with H20,
2 N
citric acid, and brine and then dried with Na2S04, The solvent was removed to
give
1.0 g
of brown oil. The oil was crystallized from ethanol to give white crystals,
m.p. 65-
67°C. Anal. for C18H18N20~7S:
Calc'd: C,53.2; H,4.5; N,6.9;
Found: C,53.7; H,4.5; N,7.2.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-42-
Reference Example 18
Methyl 2-{(4-Methoxybenzenesulfonyl)-[2-(4-pyridinylcarbonyl)
aminobenzyl]amino}acrylate
To a mixture of 1.5 g (3.80 mmol) of methyl 2-[(2-aminobenzyl)-(4
methoxybenzenesulfonyl)amino]-3-hydroxypropionate and 3.0 ml (21.6 mmol) of
triethylamine in 15 ml of CH2C12, cooled to 0°C was added 1.7 g (9.5
mmol) ml of 4
pyridinecarbonyl chloride (isonicotinoyl chloride). The mixture was stirred at
room
temperature overnight and diluted with CH2Cl2. The mixture was washed with
H20,
2 N citric acid, and brine and them dried with Na2S04, The solvent was removed
to
give 1.8 g of a tight tan solid; Anal. for C24H23N306S~
Calc'd: C,59.9; H,4.8; N,8.7; 5,6.6;
Found: C,59.0; H,4.8; N,8.5; S,6.9;
Mass spectrum (ES) 482.6(M+H).
Utilizing the procedure described in Reference Example 18, the following
intermediate compounds can be prepared from the appropriately unsubstituted
methyl 2-
[(2-aminobenzyl)-(4-methoxybenzenesulfonyl)amino]-3-hydroxypropionate or the
appropriately substituted methyl 2-[(substituted-2-aminobenzyl)-(4-
methoxybenzene-
sulfonyl)amino]-3-hydroxypropionate.
Reference Example 19
Methyl 2-{ (4-Methoxybenzenesulfonyl)-[2-(2,2,2-
trifluoroacetylamino)benzyl]amino}acrylate
white crystals, m.p. 120-121 °C. Anal. for C2pH 19F3N206S:
Calc'd: C,50.9; H,4.1; N,5.9;
Found: C,50.8; H,4.2; N,5.6.
Reference Example 20
Methyl 2-[(2-Benzoylamlnobenzyl)-(4-methoxybenzenesulfonyl)
amino]acrylate
yellow oil. Anal. for C25H24N206S:
Calc'd: C,62.5; H,5.0; N,5.8;
Found: C,62.7; H,5.3; N,5Ø
Reference Example 21
Methyl 2-[(2-Acetylaminobenzyl)-(4-methoxybenzenesulfonyl)
amino]acrylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 43 -
Reference Example 22
Methyl 2-((4-Methoxybenzenesulfonyl)-{2-[(3-
pyridinylcarbonyl)amino]benzyl}amino)acrylate
off white solid. Anal. for C24H23N306S:
Calc'd: C,59.9: H,4.8; N,8.7; S,6.6;
Found: C,58.9: H,4.8; N,8.4; S,6.4;
Mass spectrum (ES) 482.8(M+H).
Example 23
Methyl 2-((4-Methoxybenzenesulfonyl)-{ [(2
thienylcarbonyl)amino]benzyl}amino)acrylate
tan solid. Anal. for C23H22N2~6S2:
Calc'd: C,56.8; H,4.6; N,5.8;
Found: C,55.7; H,4.4; N,4.9.
Reference Example 24
Methyl 2-{ [2-(-Methoxyacetylamino)benzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
yellow oil. Anal. for C21 H24N20'7S:
Calc'd: C,56.2; H,5.4; N,6.3;
Found: C,55.3; H,5.6; N,5.8.
Reference Example 25
Methyl 2-{ (4-Methoxybenzenesulfonyl)-[2-(n-
propylsulfonylamino)benzyl]amino}acrylate
light brown oil. Anal. for C21H26N2~7S2:
Calc'd: C,52.3; H,5.4; N,5.8;
Found: C,51.9; H,5.4; N,5.7.
Reference Example 26
Methyl 2-{ [2-(3-Phenylpropionyl)aminobenzyl]-(4
methoxybenzenesulfonyl)amino}acrylate
light brown oil. Anal. for C27H28N206S:
Calc'd: C,63.8; H,5.6; N,5.5;
Found: C,66.7; H,5.8; N,4.1.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
_4ø_
Reference Example 27
tert-Butyl 2-{[2-(3-Trifluoromethylbenzoyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
yellow solid; m.p. 65-72°C.
Reference Example 28
Methyl 2-{ [2-(4~Biphenylcarbonyl)aminobenzyl]-(4
methoxybenzenesulfonyl)amino}acrylate
white solid. Anal for C31H28N206S:
Calc'd: C,66.9; H,S.1; N,5.0;
Found: C,66.1; H;5.0; N,5.1.
Reference Example 29
Methyl 2-{ [2-(Cyclopropylcarbonyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
yellow oil. Anal. for C22H24N2~6s:
Calc'd: C,59.5; H,5.4; N,6.3;
Found: C,60.0; H,5.7; N,6.0;
Mass spectrum (ES) 445.5 (M+H).
Reference Example 30
Methyl 2-{ [2-(Cyclohexylcarbonyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
white foam. Anal. for C25H30N206S:
Calc'd: C,61.7; H,6.2; N,5.8;
Found: C,59.1; H,6.0; N,5.4;
Mass spectrum (ES) 487.5 (M+H).
Reference Example 31
Methyl 2-{ [2-(3-Fluorobenzoyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
Reference Example 32
Methyl 2-{[2-(3-Chlorobenzoyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99101325
-45-
Reference Example 33
Methyl 2-{[2-(2,4-Dichlorobenzoyl)aminobenzyl]-(4
methoxybenzenesulfonyl)amino}acrylate
Reference Example 34
Methyl 2-{ [2-(2,3-Difluorobenzoyl)aminobenzyl]-(4
methoxybenzenesulfonyl)amino}acrylate
Reference Example 35
Methyl 2-{ [2-(2-Chloro-4-fluorobenzoyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
Reference Example 36
Methyl 2-{ [2-(2-Furanylcarbonyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
off-white solid. Anal. for C23H22N2~7S.
Calc'd: C,58.7; H,4.7; N,6.0;
Found: C,58.0; H,4.1; N,3.8;
Mass Spectrum (ES) 470.9 (M+H).
Reference Example 37
Methyl 2-((4-Methoxybenzenesulfonyl)-{2-((3-
thienylcarbonyl)amino]benzyl}amino)acrylate
Reference Example 38
Methyl 2-{ [2-(2-Acetylaminoacetyl)aminobenzyl]-(4
methoxybenzenesulfonyl)amino}acrylate
Reference Example 39
Methyl 2-{ [2-(2-Dimethylacetyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
Reference Example 40
Methyl 2-{ [2-(Cyclobutylcarbonyl)aminobenzyl]-(4-
methoxybenzenesulfonyl)amino}acrylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-46-
Reference Example 41
Methyl 1-Methoxyacetyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a mixture of 0.449g ( 1 mmol) of methyl 2-[[2-(2-methoxyacetamido)-
benzyl]-(4-methoxybenzene-sulfonyl]amino]acrylate in S ml of anhydrous
methanol
was added 0.109 g ( 1.3 mmol) of anhydrous sodium bicar-bonate. The mixture
was
stirred at room temperature overnight and the solvent removed under vacuum. To
the
residue was added ethyl acetate and water. The organic layer was separated and
washed with H20 and brine and then dried with Na2S04, The solvent was removed
to
give 0.41 g of solid. The solid was crystallized from ethyl acetate to give
0.28 g of
white crystals, m.p. 160-163°C. Anal. for C21H24N207S:
Calc'd: C,56.2; H,5.4; N,6.3;
Found: C,56.1; H,5.3; N,6.3; S,6.9;
Mass spectrum (ES) 449.1 (M+H).
Utilizing the procedure in Reference Example 41, the following intermediate
compounds can be prepared from the appropriate methyl 2-{(4-methoxybenzene-
sulfonyl)-[2-(substituted amino)benzyl]amino)acrylates.
Reference Example 42
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(4-methylphenylsulfonyl)
2,3,4,5-tetrahydro-1 H-( 1,4]benzodiazepine-3-carboxylate
white foam. Anal. for C25H26N207S2:
Calc'd: C,56.6; H,4.9; N,5.3
Found: C,56.2; H,5.2; N,5.2.
Reference Example 43
Methyl I,4-Bis-(4-methoxybenzenesu)fonyl)-2,3,4,5-tetrahydro-1H-
(1,4]benzodiazepine-3-carboxylate
white solid. Anal. for C25H26N208S2:
Calc'd: C,54.9; H,4.8; N,5.1;
Found: C,54.8; H,4.9; N,5.1.
CA 02317546 2000-07-06
WO 99137625 PCT/US99/01325
_ 47 _
Reference Example 44
Methyl 1-Methanesulfonyl-4-(4-methoxybenzeuesulfonyl)-2,3,4,5-
tetrahydro-1H-[l,4Jbenzodiazepine-3-carboxylate
white crystals, m.p. 136-137°C. Anal. for C19H22N207S2:
Calc'd: C,50.2; H,4.9; N,6.2;
Found: C,50.1; H,4.9; N,6.4.
Reference Example 45
Methyl 1-Benzoyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylate
tan solid. Anal. for C25H24N2~2S:
Calc'd: C,62.2; H,5.4; N,5.8;
Found: C,62.3; H,5.2; N,5.6.
Reference Example 46
Methyl 1-Acetyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H-
[1,4]benzodiazepine-3-carboxylate
white crystals, m.p. 150-155°C. Anal. for C2pH22N2~6S:
Calc'd: C,57.4; H,5.3; N,6.7;
Found: C,56.6; H,5.2; N,6.5.
Reference Example 47
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(3-pyridinylcarbonyl)-2,3,4,5
tetrahydro-1 H-[ 1,4J benzodiazepine-3-carboxylate
off white solid; Anal. for C24H23N3~6S:
Calc'd: C,59.9; H,4.8; N,8.7;
Found: C,59.2; H,4.8; N,8.3;
Mass spectrum (ES) 482.2 (M+H).
Reference Example 48
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-thienylcarbonyl)-2,3,4,5-
tetrahydro-1H-[l,4Jbenzodiazepine-3-carboxylate
off white solid. Anal. for C23H22N2~6S2:
Calc'd: C,56.8; H,4.6; N,5.8;
Found: C,56.0; H,4.6; N,5.2.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 48 -
Reference Example 49
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(4-pyridinylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
off-white crystals, m.p. 162-164°C. Anal. for C24H23N3~6S:
Calc'd: C.59.9; H,4.8; N,8.7;
Found: C,59.9; H,4.8; N,8.7.
Reference Example 50
Methyl 1-(4-Biphenylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1.H-[1,4]benzodiazepine-3-carboxylate
white solid; Anal, for C31H28N206S:
Calc'd: C,66.9; H,5.1; N,S.O;
Found: C,65.8; H,5.2; N,5.0;
Mass spectrum (ES) 557.6 (M+H).
Reference Example 51
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(propane-1-sulfonyt)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
yellow oil. Anal. for C21H26N2~7S2:
Calc'd: C,52.3; H,5.4; N,5.8;
Found: C,51.8; H,5.4; N,5.6.
Reference Example 52
Methyl 1-([1,1'-Biphenyl]-2-carbonyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
white foam. Anal. for C31H28N206S:
Calc'd: C,66.9; H,5.1; N,5.0;
Found: C,67.3; H,5.2; N,4.7;
Mass spectrum (ES) 557.6 (M+H).
Reference Example 53
Methyl 1-(3-Fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/39625 PCT/US99/01325
- 49 -
Reference Example 54
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-5-fluorobenzoyl)
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
white solid; Anal. for C26H25FN206S:
Calc'd: C,60.9; H,4.9; N,5.5;
Found: C,60.9; H,5.0; N,5Ø
Reference Example 55
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-3-fluorobenzoyl)-
2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 56
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(3-phenylpropionyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
white solid; Anal. for C27H28N206S:
Calc'd: C,63.8; H,5.6; N,5.5;
Found: C,64.0; H,5.7; N,5.3: 5,6.5.
Reference Example 57
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-trifluoromethylbenzoyl)-
2,3,4,5-tetrahydro-1H-[ 1,4] benzodiazepine-3-carboxylate
Reference Example 5$
Methyl 1-(2-Chloro-6-trifluoromethylbenzoyl)-4(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylate
Reference Example 59
Methyl 1-(4-Fluoro-2-trifluoromethylbenzoyl)-4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylate
Reference Example 60
Methyl 1-(2-Fluoro-6-trifluoromethylbenzoyl)-4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-50-
Reference Example 61
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methylbenzoyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 62
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-6-chlorobenzoyl)-
2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 63
Methyl 1-(2,4-Dimethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 64
Methyl 1-(2,5-Dimethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 65
Methyl 1-(2-Chloro-4-fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 66
Methyl 1-(2-Chlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 67
Methyl 1-(2-Fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 68
Methyl 1-(2-Chloro-6-fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 69
Methyl 1-(2,3-Difluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-51 -
Reference Example 70
Methyl 1-(2,4-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Prepared according to the procedure set forth in Reference Example 10; white
solid. Anal. for C25H22C12N206S:
Calc'd: C,54.7; H,4.0; N,5.1;
Found: C,54.4; H,3.8: N,4.9:
Mass spectrum (548.9) (M+H); 550.9 (M+H).
Reference Example 71
Methyl 1-(2,3-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 72
Methyl 1-(2,5-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 73
Methyl 1-(2-Methoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 74
Methyl 1-(4-Chloro-2-methoxybenzoyl)-4-(4-methoxybenzenesulfonyl)
2,3,4,5-tetrahydro-1 H-[ l,4Jbenzodiazepine-3-carboxylate
Reference Example 75
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methylthiobenzoyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 76
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(3-methyl-2-thienylcarbonyl)
2,3,4,5-tetrahydro-1H-[1.4]benzodiazepine-3-carboxylate
Reference Example 77
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(4-methyl-2-thienylcarbonyl)-
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/013Z5
-52-
Reference Example 7$
Methyl 1-(3-Chloro-2-thienylcarbonyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 79
Methyl 1-(2-Furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
off white solid, m.p. 165-167°C. Anal. for C23H22N207S:
Calc'd: C,58.7; H,4.7; N,6.0;
Found: C,58.4; H,4.6; N,5.7;
Mass spectrum (ES) 470.9 (M+H).
Reference Example 80
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(3-methyl-2-furanylcarbonyl)-
2,3,4,5-tetrahydro-1H-[ 1,4)benzodiazepine-3-carboxylate
Reference Example 81
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(4-methyl-2-furanylcarbonyl)
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 82
Methyl 1-(5-Chloro-2-furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)
2,3,4,5-tetrahydro-IH-[1,4]benzodiazepine-3-carboxylate
Reference Example 83
Methyl 1-(5-Chloro-2-thienylcarbonyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 84
Methyl 1-Propionyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylate
Reference Examprle 85
Methyl 1-Hexanoyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCTNS99101325
-53-
Reference Example 86
Methyl 1-(3-Methoxypropionyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[ I,4]benzodiazepine-3-carboxylate
Reference Example 87
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(3-thienylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 88
Methyl 1-(3-Furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 89
Methyl 1-(trans-Crotonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 90
Methyl 1-(Methacryloyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 91
Methyl 1-(Chloroacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Following the method described for Reference Example 18, 3.0 g (7.61 mmol)
of methyl 2-[2-aminobenzyl)-(4-methoxy-benzenesulfonyl)-amino]-3-hydroxy-
propionate was reacted with 1.82 ml (22.8 mmol) of chloroacetylchloride to
give 4.0 g
of solid. Chromatography on silica gel with ethyl acetate-hexane ( 1:1 ) as a
solvent gave
1.5 g of methyl 2-[(2-chloroacetylaminobenzyl)-(4-methoxybenzenesulfonyl)-
amino]-
acrylate. A 1.3 g sample of the preceding compound was reacted with 0.312 g of
anhydrous NaHC03 in 10 ml of anhydrous methanol at room temperature overnight
and the mixture was then heated at 80°C for 5 hours. The solvent was
removed and the
residue partitioned between H20 and ethyl acetate. The ethyl ~etate extract
was
washed with brine, dried with Na2S04 ~d the solvent removed. The residue was
trittlrated with hexane-ethyl acetate, chilled and filtered to give the
product; Mass
sp~~um (ES) 453.1 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-54-
Reference Example 92
Methyl 1-(Acetylaminoacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 93
Methyl 1-(N,N-Dimethylaminoacetyl)-4-(4-methoxybenzenesulfonyl)
2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 94
Methyl 1-(Cyclopropylcarbonyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
white crystals, m.p. 98-100°C. Anal. for C22H24N206S:
Calc'd: C,59.5; H,5.4; N,6.3;
Found: C,59.3; H,5.6; N,6.2;
Mass spectrum (ES) 445.1 (M+H).
Reference Example 95
Methyl 1-(Cyclobutylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahyd ro-1H-[ 1,4] benzodiazepine-3-carboxylate
Reference Example 96
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(trifluoroacetyl)-2,3,4,5-
tetrahydro-1H-[1,4] benzodiazepine-3-carboxylate
To a solution of 1.0 g (2.54 mmol) of methyl 3-hydroxy-2-{ (4-
methoxybenzenesulfonyl)-[2-(2,2,2-trifluoroacetylamino)benzyl]amino}propionate
in
10 ml of CH2C12 was added 1.8 ml ( 12.7 mmol) of trifluoroacetic anhydride.
After 1
hour at room temperature, the solvent was removed. Dichloromethane was added
several times and the solvent removed under vacuum after each addition.
Methanol was
then added 2 times and the solvent removed under vacuum to give methyl 2-( (4-
methoxybenzenesulfonyl)-[2-(2,2,2-trifluoroacetylamino)benzyl]-amino }
acrylate as a
glass. The glass was dissolved in methanol and 0.213 g of anhydrous NaHC03 was
added. The mixture was stirred at room temperature overnight and concentrated
under
vacuum to dryness. To the residue was added ethyl acetate and water. The
organic
layer was separated, washed with H20, brine and dried with Na2S04. The solvent
was removed and the residue ( 1.0 g) was chromatographed on silica gel thick
layer
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-55-
plates with hexane-ethyl acetate ( 1:1 ) as solvent to give 0.365 g of product
as a glass.
Anal. for C2pH 19F3N2O6S:
Calc'd: C,50.9; H,4.1; N,5.9; F,12.1; 5,6.7;
Found: C,50.8; H,4.4; N;5.5; F,11.7; S,6.7;
Mass spectrum (ES) 473.1 (M+H).
Reference Example 97
Methyl 4-(4-Methoxybenzenesulfonyl)-I-(4-methylphenylsulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To 0.50 g ( 1.26 mmol) of 2-[(2-aminobenzyl)-(4-methoxybenzene-
sulfonyl)amino]-3-hydroxypropionate in 5 ml of pyridine cooled to 0°C
was added
0.284 g (2.59 mmol) of tosyl chloride. The mixture was stirred at 0°C
for 2 hours and
then concentrated to remove the solvent. To the residue was added 8 ml of
anhydrous
ethanol and the mixture refluxed for 2 days. The mixture was concentrated to
dryness
and ethyl acetate added. The mixture was washed with H20, 2 N citric acid,
brine and
dried with Na2S04. The filtrate was filtered through a thin pad of hydrous
magnesium
silicate and the filter pad washed with ethyl acetate. The filtrate was
concentrated to
dryness to give 0.60 g of a foam. Anal. for C25H26N207S2:
Calc'd: C,56.6; H,4.9; N,5.3; 5,12.1;
Found: C,56.2; H,5.2; N,5.2; 5,11.4;
Mass spectrum (ES) 531.6 (M+H).
Reference Example 98
Methyl 2-[(4-Methoxybeozenesulfonyl)-(2-
methylsulfonylaminobenzyl)amino]acrylate
To a solution of 1.0 g (2.54 mmol) of methyl [(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate in 10 ml of pyridine cooled
to
-5°C was added 0.432 ml (5.58 mmol) of methanesulfonyl chloride. The
mixture was
stirred at 0°C for 48 hours. To the mixture was added ice and H20 and
the mixture was
extracted with ethyl acetate. The extract was washed with H20, 2 N citric
acid, brine
and dried with Na2S04, The solvent was removed under vacuum and the residue
triturated with ethyl acetate-hexane to give 0.90 g of a solid, 128-
142°C. Anal. for
C 19H22N207S2:
Calc'd: C,50.2; H,4.9; N,6.2; S,14.1;
Found: C,49.6; H,5.0; N,6.9; S,14.0;
Mass spectrum (ES) 455.5 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-56-
Reference Example 99
Methyl 1,4-Bis-(4-Methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H-
[1,4]benzodiazepine-3-carboxylate
To a solution of 1.0 g (2.34 mmot) of methyl ?-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate in 6 ml of pyridine cooled
to
0°C to -5°C was added 1.07 (5.18 mmol) of 4-
methoxybenzenesulfonyl chloride. After
2 hours, the mixture was concentrated to dryness under vacuum. To the residue
was
added 12 ml of ethanol and the mixture refluxed overnight. The solvent was
removed
under vacuum and the residue chromatographed on silica gel thick layer plates
with
ethyl acetate-hexane ( I :1 ) as solvent to give 0.83 g (60%) of product as a
white foam;
Anal. calc'd for C25H2(~N2O8S2: C,54.9; H,4.8; N,5.1; S,11.7. Found: C,54.8;
H,4.9; N,5.0; 5,11.5; Mats spectrum (ES) 547.1 (M+H); and a second component
(0.38 g) methyl 2-{ [2-(4-methoxybenzenesulfonyl)aminobenzyl]-(4-
methoxybenzene-
sulfonyl)amino}-3-hydroxypropionate. Anal. for C25H28N2O9S2:
Calc'd: C,53.2; H,5.0; N,5.0; S,11.4;
Found: C,51.8; H,S.1; N,4.7; S,1 I .3;
Mass spectrum (ES) 565.2 (M+H).
Reference Example 100
Methyl 1-Acetyl-4-(4'-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1 H-[ 1,4] benzodiazepine-3-carboxylate
To a solution of 0.70 g ( 1.52 mmol) of methyl 2-[(2-diacetylaminobenzyl)-(4-
methoxybenzenesulfonyl) amino]acrylate in 5 ml of anhydrous methanol was added
0.332 g (3.95 mmol) of anhydrous sodium bicarbonate. The mixture was stirred
at
room temperature overnight and the solvent removed under vacuum. To the
residue
was added ethyl acetate and H20. The organic layer was separated, washed with
brine
and dried with Na2S04, The solvent was removed and the residue dried under
vacuum
to give 0.59 g of white crystals, m.p. 150-155°C. Anal. for
C2pH22N2O6S:
Calc'd: C,57.4; H,5.3; N,6.7; S,7.7;
Found: C,56.6; H,5.2; N,6.5; S,7.5;
Mass spectrum (ES) 419.9 (M+H).
Reference Example 101
Methyl 3-Acetoxy-2-[(2-diacetylaminobenzyl)-(4-
methoxybenzenesulfonyl)amino]propionate
A mixture of 1.0 g (2.54 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate and 1.3 ml of acetic
anhydride
CA 02317546 2000-07-06
WO 99/37625 PC'T/US99/o1325
-57-
in 8 ml of toluene was heated at 100°C for 2 hours. The mixture was
concentrated and
3 ml of acetic anhydride added thereto. The mixture was heated at 100°C
overnight and
concentrated to dryness under high vacuum to give an oil. The oil was dried at
75°C
under vacuum for 48 hours to give 1.2 g of a yellow oil. Anal. for
C24H28N2O9S:
Calc'd: C,54.5; H,5.2; N,5.5; S,6.2;
Found: C,54.6; H,5.1; N,5.4; S,6.4;
Mass spectrum (ES) 520.8 (M+H).
Reference Example 102
Methyl 2-[(2-Diacetylaminobenzyl)-(4-
methoxybenzenesulfonyl)amino]acrylate
A mixture of 1.0 g ( 1.97 mmol) of methyl 3-acetoxy-2-((2-diacetyl-
aminobenzyl)-(4-methoxybenzenesulfonyl)amino]propionate and 0.826 ml (5.92
mmol) of triethylamine in 5 ml of CH2C12 was stirred at room temperature
overnight.
The solution was diluted with 30 ml of CH2Cl2 and washed with 20 ml each of
H20, 2
~I citric acid, brine and dried with Na2S04, The solvent was removed under
vacuum
to give a brown oil. Anal. for C22H24N207S:
Calc'd: C,57.4; H,5.3; N,6. I ; S,7.0;
Found: C,56.2; H,5:5; N,5.6; S,7.2.
Reference Example 103
Methyl 2-{ (4-Methoxybenzenesulfonyl)-[2-(2,2,2-
trifluoroacetylamino)benzyl]amino}acrylate
To a suspension of 1.0 g (2.54 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate in 10 ml of toluene was
added
1.8 ml (12.7 mmol) of trifluoroacetic anhydride (solid dissolves). The
solution was
stirred for 2 hours at room temperature and heated at 100°C overnight.
The mixture
was then concentrated to dryness under vacuum. To the residue was added 0.9 ml
of
trifluoroacetic anhydride and the solution stirred at room temperature for 1.5
hours and
concentrated to dryness. To the residue was added 10 ml of toluene and the
mixture
refluxed for 2 hours. The solution was cooled to room temperature and 2.5 ml
of
triethylamine added and the mixture stirred at room temperature overnight. The
solution
was concentrated to dryness and the residue dissolved in ethyl acetate. The
ethyl acetate
was washed with H20, brine and dried (Na2S04). The solvent was removed under
vacuum to give 1.0 g of colorless oil. Crystallization from ethyl acetate -
hexane
CA 02317546 2000-07-06
WO 99137625 PCT/US99/01325
-58-
gave 0.625 g of colorless crystals, m.p. 120-121 °C.
Anal. for C2pH 19F3N206S:
Calc'd: C,50.9; H,4.1; N,5.9; S,6.7; F,12.1;
Found: C,50.8; H,4.2; N,5.6; S,6.8; F,11.9;
S Mass spectrum (ES) 473.1 (M+H).
Reference Example 104
4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-5-fluorobenzoyl)-2,3,4,5
tetrahydro-1H-[1,4~]benzodiazepine-3-carboxylic Acid
To a mixture of 1.9 g (3.71 mmol) of methyl 4-(4-methoxybenzenesulfonyl)-1-
(2-methyl-5-fluorobenzoyl)-2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-
carboxylate
in 10 ml of tetrahydrofuran wac added 5 ml (4.82 mmol) of 1 N NaOH. The
mixture
was stirred at room temperature for 1.5 hours and the solvent removed under
vacuum.
To the residue was added ethyl acetate and the mixture neutralized with 1 N
HCI. The
15 organic layer was separated, washed with brine and dried with Na2S04. The
solvent
waa removed under vacuum to give 1.41 g of white solid. Anal. for C25H23~2065:
Calc'd: C,60.2; H,4.7; N,5.6;
Found: C,60.2; H,4.8; N,5.4 S,6.4; F,3.6;
Mass spectrum (ES) 497.5 (M-H).
Utilizing the method described in Reference Example 104, the following
benzodiazepine-3-carboxylic acids can be prepared.
Reference Example 105
4-(4-Methoxybenzenesulfonyl)-1-(4-methylphenylsulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white foam. Anal. for C24H24N20'752:
Calc'd: C,55.8; H,4.7; N,5.4;
Found: C,53.9; H,5.1; N,4.8;
Mass spectrum (ES) 512.2 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-59-
Reference Example 106
1,4-Bis-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H
[1,4]benzodiazepine-3-carboxylic acid
off white solid. Anal. for C24H?4N208S2:
Calc'd: C,54.1; H,4.5; N,5.3;
Found: C,52.4; H,4.8; N.4.7;
Mass spectrum (ES) 533.1 (M+H).
Reference Example 107
1-Methanesulfonyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylic acid
white solid. Anal. for C ~ gH2pN207S2:
Calc'd: C,49.1; H,4.6; N,6.3;
Found: C,47.5; H,5.0; N,5.5;
Mass spectrum (ES) 441.1 (M+H).
Reference Example 108
1-Benzoyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
[1,4]benzodiazepine-3-carboxylic acid
white foam. Anal. for C24H22N206S:
Calc'd C,61.5; H,5.2: N,6.0;
Found: C,60.8; H,5.2; N,5.7;
Mass spectrum (ES) 467.9 (M+H).
Reference Example 109
1-Acetyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H
[1,4]benzodiazepine-3-carboxylic acid
white solid; Anal. for C19H22N206S:
Calc'd: C,56.4; H,5.0; N,6.9;
Found: C,55.2; H,4.9; N,6.6; 5,7.8;
Mass spectrum (ES) 404.9 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCTIUS99/01325
-60-
Reference Example 110
4-(4-Methoxybenzenesulfonyl)-1-(3-pyridinylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white solid; m.p. 250-255. Anal. for C23H? 1 N306S:
S Calc'd: C,59.1; H,4.5; N,9.0;
Found: C,58.3: H,4.7; N,8.3;
Mass spectrum (ES); 468.2 (M+H).
Reference Example 111
4-(4-Methoxybenzenesulfonyl)-1-(2-thienylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white solid; Anal. for C22H20N2~6S2:
Calc'd: C,55.9; H,4.3; N,5.9;
Found: C,54.9; H,4.4; N,5.4;
Mass spectrum (ES) 473.1 (M+H).
Reference Example 112
1-Methoxyacetyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-3-carboxylic acid
white crystals, m.p. 193-194°C. Anal. for C2pH22N2~7S:
Calc'd: C,55.3: H,5.1; N,6.5;
Found: C,55.1; H,4.9; N,6.2;
Mass spectrum (ES) 433.1 (M-H).
Reference Example 113
4-(4-Methoxybenzenesulfonyl)-1-(4-pyridinylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white crystals, m.p. 258-261 °C. Anal. for C23H21 N306S:
Calc'd: C,59.1; H,4.5; N,9.0;
Found: C,58.8: H,4.5; N,8.8;
Mass spectrum (ES) 483.3 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-61 -
Reference Example 114
1-(4-Biphenylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white foam. Anal. for C3pH26N206S:
Calc'd: C,66.4; H,4.8; N,5.2;
Found: C,64.7; H,5.2; N,4.8;
Mass spectrum (ES) 543.6 (M+H).
Reference Example 115
4-(4-Methoxybenzenesulfonyl)-1-(propane-1-sulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white foam. Anal. for C2pH24N2~7S2:
Calc'd: C,51.3; H,5.2; N,6.0;
Found: C,50.3; H,5.3; N,5.7;
Mass spectrum (ES) 467.3 (M-H).
Reference Example 116
1-([1,1'-Biphenyl]-2-carbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-(1,4]benzodiazepine-3-carboxylic acid
white foam; m.p. 106-145°C. Anal. for C3pH26N2~6S:
Calc'd: C,66.4; H,4.8; N,5.2;
Found: C,65.7; H,5.0; N,4.8;
Mass spectrum (ES) 541.1 (M-H).
Reference Example 117
1-(3-Fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 118
4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-3-fluorobenzoyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
CA 02317546 2000-07-06
WO 99/3'I625 PCT/US99/01315
-62-
Reference Example 119
4-(4-Methoxybenzenesulfonyl)-1-(3-phenylpropionyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white solid. Anal. for C26H26N206S:
Calc'd: C,63.1; H,5.3; N,5.7;
Found: C,61.5; H,5.4; N,5.2;
Mass spectrum (ES} 493.2 (M-H).
Reference Example 120
4-(4-Methoxybenzenesulfonyl)-1-(2-trifluoromethylbenzoyl)-2,3,4,5-
tetrahydro-1H-(1,4]benzodiazepine-3-carboxylic acid
Reference Example 121
1-(2-Chloro-6-trifluoromethylbenzoyl)-4-(4-methoxybenzenesulfonyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 122
1-(4-Fluoro-2-trifluoromethylbenzoyl)-4-(4-methoxybenzenesulfonyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 123
1-(2-Fluoro-6-trifluoromethylbenzoyl)-4-(4-methoxybenzenesulfonyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 124
4-(4-Methoxybenzenesulfonyl)-1-(2-methylbenzoyl)-2,3,4,5-tetrahydro
1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 125
4-(4-Methoxybenzenesulfooyl)-1-(2-methyl-6-chlorobenzoyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 126
1-(2,4-Dimethylbenzoyl)-4~(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-63-
Reference Example 127
1-(2,5-Dimethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
S Reference Example 128
1-(2-Chloro-4-fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 129
1-(2-Chlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 130
1-(2-Fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro
1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 131
1-(2-Chloro-6-fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-(1,4]benzodiazepine-3-carboxylic acid
Reference Example 132
1-(2,3-Difluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 133
1-(2,4-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white solid. Anal. for C24H20CI2N2~6S:
Calc'd: C,53.8; H,3.8; N,5.2;
Found: C,52.8; H,3.9; N,4.9;
Mass spectrum (ES) 533 (M-H).
Reference Example 134
1-(2,3-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
CA 02317546 2000-07-06
WO 99/37625 PCT/US99l01325
Reference Example 135
1-(2,5-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-(1,4]benzodiazepine-3-carboxylic acid
Reference Example 136
1-(2-Methoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-IH-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 137
1-(4-Chloro-2-methoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 138
4-(4-Methoxybenzenesulfonyl)-1-(2-methylthiobenzoyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 139
4-(4-Methoxybenzenesulfonyl)-1-(3-methyl-2-thienylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 140
4-(4-Methoxybenzenesulfonyl)-1-(4-methyl-2-thienylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 141
1-(3-Chloro-2-thienylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 142
1-(2-Furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white solid. Anal. for C22H2pN2~7S:
Calc'd: C, 57.9; H, 4.4; N, 6.1;
Found: C, 56.5; H, 4.5; N, 5.7;
Mass spectrum (ES) 455.1 (M-H).
CA 02317546 2000-07-06
WO 99137625 PCT/US99/01325
-65-
Reference Example 143
4-(4-Methoxybenzenesulfonyl)-1-(3-methyl-2-furanylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4)benzodiazepine-3-carboxylic acid
Reference Example 144
4-(4-Methoxybenzenesulfonyl)-1-(4-methyl-2-furanylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 145
1-(5-Chloro-2-furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 146
1-(5-Chloro-2-thienylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 147
1-Propionyl-4-(4-methoxybenzenesulfonyl}-2,3,4,5-tetrahydro-1H
[1,4)benzodiazepine-3-carboxylic acid
Reference Example 148
1-Hexanoyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
[1,4]benzodiazepine-3-carboxylic acid
Reference Example 149
1-(3-Methoxypropionyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 150
4-(4-Methoxybenzenesulfonyl)-1-(3-thienylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 151
4-(3-Furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4)benzodiazepine-3-carboxylic acid
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/Oi325
-66-
Reference Example 152
1-(trans-Crotonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro
1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 153
1-(Methacryloyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
[1,4]benzodiazepine-3-carboxylic acid
Reference Example 154
1-(Pyrrolidinoacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 155
1-(Acetylaminoacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 156
1-(Cyclopropylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white crystals, m.p. 131-135°C. Anal. for C21H22N2~6S:
Calc'd: C,58.6; H,5.2; N,6.5;
Found: C,58.1; H,5.5; N,5.8;
Mass spectrum (ES) 431.5 (M+H).
Reference Example 157
1-(Cyclobutylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
Reference Example 158
1-(Cyclohexylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid
white solid. Anal. for C24H28N206S:
Calc'd: C,61.0; H,6.0; N,5.9;
Found: C,57.0; H,5.7; N,5.4;
Mass spectrum (ES) 471.5 (M-H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-67-
Reference Example 159
(D,L)N-(4-Methoxybenzenesulfonyl)-O-(2-tetrahydropyranyl)serine,
Methyl ester
A mixture of 1.44 g (5 mmol) of N-(4-methoxybenzenesulfonyl)serine, methyl
ester; 1.05 g ( 12.5 mmol) of 3,4-dihydro-2H-pyran and 9.5 mg of 4-methyl
benzenesulfonic acid monohydrate in 5 ml of tetrahydrofuran was refluxed
overnight
and the mixture was concentrated to dryness under vaccum. The residue was
extracted
with CH2Cl2 and the extract washed with 2 N NaHC03, . brine and dried with
Na2S04, The solution was filtered through a thin pad of hydrous magnesium
silicate
and the filter pad washed with CH2C12. The filtrate was concentrated to
dryness and
the residue (2.3 g) was extracted with three SO ml portions of hot hexane to
give 1.92 g
of product as a yellow oil; Mass spectrum (ES) 374.4 (MH+).
Reference Example 160
Methyl 3-Hydroxy-2-{ [4-methoxybenzenesulfonyl]-[2-(4-
morpholinocarbonylamino)benzyl]amino}propionate
To a mixture of 1.0 g (2.54 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate in 8 ml of pyridine chilled
at OP
to -10°C was added 740 pL, (6.34 mmol) of morpholinocarbonyl chloride.
The mixture
was kept at OP to 5°C overnight. The mixture was concentrated under
vacuum and
diluted with ethyl acetate. The solution was washed with H20, 2 N citric acid,
and
brine and dried with Na2S04, The solvent was removed under vacuum to give 1.61
g
of solid (yellow-orange foam). The solid was chromatographed on thick layer
silica gel
plates with hexane-ethyl acetate ( 1:3) as solvent to give 0.86 g of solid.
Anal. for
C23H29N308S:
Calc'd: C,54.4; H,5.8; N,8.3;
Found: C,53.9; H,5.7; N,B.1;
Mass spectrum (ES) 508.4 (M+H).
Reference Example 161
Methyl 2-{ (4-Methoxybenzenesulfonyl)-(2-(4~
morpholinocarbonylamino)benzyl]amino}acrylate
To a solution of 0.70 g ( 1.38 mmol) of methyl 3-hydroxy-2-{ [4-methoxy-
benzenesulfonyl]-[2-(4-morpholinocarbonylamino)benzyl]amino }propionate and
769
lli, (5.54 mmol) of triethylamine in 8 ml of CH2C12, cooled to 0°C, was
added 0.386
g (2.03 mmol) of 4-methylbenzenesulfonyl chloride. The mixture was stirred at
room
temperature for 2 hours, diluted with water and extracted with CH2C12. The
extract
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-68-
was washed with 2 N citric acid, brine and dried with Na2S04, The solvent was
removed to give 0.67 g of a yellow oil. Anal. for C23H27N307S:
Calc'd: C,56.4; H,5.6; N,8.6; S,6.6;
Found: C,56.1; H,5.8; N,8.3; S,6.6.
Reference Example 162
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(4-morpholinocarbonyl)
2,3,4,5-tetrahydro-1 H-[ 1,4]-benzodiazepine-3-carboxylate
A mixture of 0.50 g ( 1.02 mmol) of methyl 2-{ (4-methoxybenzenesulfonyl)-[2
(4-morpholinocarbonyl-amino)benzyl]amino } acrylate and 0.111 g ( 1.32 mmol)
of
anhydrous NaHC03 in 5 ml of anhydrous methanol was stirred at room temperature
for
16 hours. An additional 55 mg of NaHC03 was added and the mixture stirred at
room
temperature for 2 hours. The solvent was removed under vacuum and the residue
diluted with H20 and extracted with ethyl acetate. The extract was washed with
brine
and dried with Na2S04. The solvent was removed and the residue triturated with
hexane-ethyl acetate to give 0.36 g of a yellow solid; Anal. calc'd for
C23H27N30~7S:
C,56.4; H,5.6; N,8.6; 5,6.6. Found: C,56.5; H,5.7; N,8.4; S,6.7; Mass spectrum
(ES) 490.3 (M+H).
Reference Example 163
4-(4-Methoxybenzenesulfonyl)-1-(4-morpholinocarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic Acid
A mixture of 0.36 g (0.735 mmol) of methyl 4-(4-methoxybenzenesulfonyl)-1
(4-morpholinocarbonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
and
1 ml (0.95 mmol) of 1 N NaOH in 5 ml of tetrahydrofuran was stirred at room
temperature for 1 hour. The mixture was concentrated under vacuum and the
acidified
with 1 N HCI and cooled. The mixture was filtered and the solid washed with
water to
give 0.26 g of white solid. Anal. for C22H25N307S:
Calc'd: C,55.6; H,5.3; hI,8.8;
Found: C,53.5; H,5.6; N,8.3;
Mass spectrum (ES) 474.3 (M-H).
CA 02317546 2000-07-06
WO 99/37625 PCT/U599/01325
-69-
Reference Example 164
Methyl 3-[(2-Tetrahydropyranyl)oxy]-2-[(4-methoxybenzenesulfonyl)-
(2-vitro-4-chlorobenzyl)amino]propionate
To a mixture of 1.67 g (4.4 mmol) of (D,L) N-(4-methoxybenzenesulfonyl)-O-
(2-tetrahydropyranyl) serine, methyl ester, 0.825 g (4.4 mol) of 4-chloro-2-
nitrobenzyl
alcohol and 1.16 g (4.4 mmol) of triphenylphosphine in 4.5 ml of
tetrahydrofuran was
added dropwise a solution of 0.766 g (4.4 mmol) of diethyl azodicarboxylate in
1 ml of
tetrahydrofuran. The mixture was stirred at room temperature overnight and the
solvent
removed under vacuum. The residue was triturated with diethyl ether, filtered
and the
filtrate passed through a thin pad of hydrous magnesium silicate. The pad was
washed
with ethyl acetate and the total filtrate concentrated to dryness under vacuum
to give
4.54 g of solid. The solid was chromatographed on silica gel with hexane-ethyl
acetate
(55:45) as solvent. The fractions containing product were combined and the
solvent
removed to give 0.55 g of oily solid; Mass spectrum (ES) 543.1 (M+H).
Reference Example 165
Methyl 2-((2-(4-Pyridinylmethyleneamino)benzyl]-[4-
methoxybenzenesulfonyl]amino}-3-hydroxypropionate
A mixture of 0.50 g ( 1.268 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino]-3-hydroxypropionate and 1.268 mmol of 4-
pyridinecarboxaldehyde in 7 ml of anhydrous ethanol was refluxed for 1.5 hours
and
the mixture concentrated under vacuum to dryness. To the residue was added H20
and
ethyl acetate. The ethyl acetate layer was separated and concentrated to
dryness under
vacuum. The solid was purified by thick layer chromatography on silica gel
with
hexane-ethyl acetate as solvent to give 0.40 g of solid product (plus a small
amount of
starting material). Anal. for C24H25N306S:
Calc'd: C,59.6; H,5.2; N,8.7;
Found: C,57.6; H,5.7; N,7.4;
Mass spectrum (ES) 484 (M+H)-product; 395.1 (M+H)-starting material.
Reference Example 166
Methyl 1-(Cyclohexylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a solution of 0.80 g ( 1.64 mmol) of methyl 2-{ [2-(cyclohexylcarbonyl)
aminobenzyl]-(4-methoxybenzenesulfonyl)amino } acrylate in 10 ml of methanol
was
added 0.207 g (2.46 mmol) of anhydrous sodium bicarbonate. The mixture was
stirred
for 2 days and then an additional 0.207 g of NaHC03 added. The mixture was
stirred
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-70-
overnight and the solvent removed under vacuum. To the residue was added H20
and
ethyl acetate and the organic layer separated. The ethyl acetate extract was
washed with
brine, dried with Na2S04 and the solvent removed under vacuum to give 0.83 g
of the
product as a yellow oil. Anal. for C25H3pN206S: Calc'd: C,61.7; H,6.2; N,5.8;
Found: C,61.0; H,6.4; N,5.3;
Mass spectrum (ES) 487.0 (M+H).
Reference Example 167
Methyl 3-Hydroxy-2-[(4-methoxybenzenesulfonyl)-(4-chloro-2-
nitrobenzyl)amino]propionate
To a solution of 0.289 g ( 1 mmol) of methyl 3-hydroxy-2-(4-
methoxybenzenesulfonylamino)propionate in 4 ml of N,N-dirnethylformamide
cooled
in an ice bath was added 40 mg of NaH (60% in oil) (1 mmol). After the gas
evolution
ceased, 0.165 g ( 1.1 mmol) of sodium iodide was added, followed by the
addition of
0.226 g (1.1 mmol) of 4-chloro-2-nitrobenzyl chloride in 1 ml of
dimethylformamide.
The solution became purple and was stirred at room temperature over the
weekend.
The solvent was removed under vacuum and the residue extracted with CH2Cl2.
The
extract was washed with H20, brine and dried with Na2S04. The solvent was
removed to give 0.53 g of solid which was chromatographed on thick layer
silica gel
plates with hexane-ethyl acetate (2:1) as solvent to give 0.143 g (31 %) of
product, as
crystals, m.p. 112°-114°C. Anal. for C 18H 19C1N2O8S:
Calc'd: C,47.2: H,4.2; N,6.1;
Found: C,47.0; H,4.1; N,6.0;
Mass spectrum (ES) 459.2 (M+H).
Reference Example 168
Methyl 3-Hydroxy-2-[(4-methoxybenzenesulfonyl)-(4-chloro-2-
aminobenzyl)amino]propionate
A mixture of 0.454 g ( 1 mmol) of methyl 3-hydroxy-2-[(4-methoxy-
benzenesulfonyl)-(4-chloro-2-nitrobenzyl)amino]propionate and 0.451 g (2 mmol)
of
SnCl2~2H20 in 12 ml of methanol was refluxed for 2 hours. An additional 0.451
g (2
mmol) of SnCl2~2H20 was added and the mixture refluxed for 2 hours. The
solvent
was removed and ethyl acetate added. The mixture was neutralized with 1 N
NaHC03
and then stirred for 1 hour and filtered. The ethyl acetate layer was
separated and
washed with H20, brine and dried with Na2S04. The solvent was removed to give
0.42 g of solid which was chromatographed on thick layer silica gel plates
with hexane-
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-71 -
ethyl acetate (45:55) as solvent to give 60 mg of product (RF 0.66) as a
glass, m.p.
99°- I I 2°C. Anal. for C I8H21 C1N206S:
Calc'd: C,50.4; H,4.9: N,6.5;
Found: C,49.7; H,4.9; N,6.4:
Mass specwm (ES) 429.1 (M+H).
Reference Example 169
Methyl 3-Hydroxy-2-[(4-methoxybenzenesulfonyl)-(4-chloro-2-
aminobenzyl)amino]propionate
To a solution of 0.458 g ( 1 mmol) of methyl 3-hydroxy-2-[(4-
methoxybenzenesulfonyl)-(4-chloro-2-nitrobenzyl)amino]propionate in 25 ml of
ethanol and 25 ml of ethyl acetate was added 0.045 g of 10% Pd/C (wet - 50%
H20).
The mixture was shaken in a Parr hydrogenator under 35 pounds per square inch
of
hydrogen for 3 hours. The mixture was filtered through diatomaceous earth and
the
filtrate was concentrated to dryness under vacuum to give 0.47 g of the
product as a
solid (approximately 90% pure). Thin layer chromatography on silica gel, NMR
and
Mass spectrum (ES) 429.1 (M+H) 395.1 (M+H) indicated approximately 10% of
deschloro derivative.
A mixture of 4.74 g of methyl 3-hydroxy-2-[(4-methoxybenzenesulfonyl)-(4-
chloro-2-aminobenzyl)amino } propionate, and 0.470 g of 10% Pd/C (wet-50% H20)
in 200 ml of ethyl acetate-ethanol ( I :1 ) was shaken in a Parr hydrogenator
under 35 psi
of hydrogen for 4 hours. The mixture was filtered through diatomaceous earth
and the
solvent removed to give 4.5 g of solid. The solid was chromatographed by HPLC
on a
Waters Prep machine with a 4 x 30 cm silica gel column with a step gradient of
hexane-
ethyl acetate (9:1 to 6:4 to 1:1 to 0:100) to give 1.56 g of a glass, m.p.
110°-123°C.
Anal. for CI8H21CIN206S:
Calc'd: C, 50.4; H, 4.9; N, 6.5; Cl, 8.3;
Found: C, 50.3; H, 4.8; N, 6.5; Cl, 7.8.
Reference Example 170
N-(4-Methoxybenzenesulfonyl)-glycine, Methyl Ester
To a mixture of 12.5 g (0.1 mol) of glycine, methyl ester hydrochloride in 120
ml of CH2Cl2, cooled in an ice bath was added 41.7 ml (0.3 mol) of
triethylamine,
followed by the dropwise addition of a solution of 20.65 g (0.1 mol) of 4
methoxybenzenesulfonyl chloride in 40 ml of CH2Cl2. The mixture was stirred at
room temperature overnight and poured into water. The organic layer was
separated
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-72-
and washed with 2 N citric acid, H20, 1 N NaHC03, brine and dried with Na2S04,
The solvent was removed under vacuum to give 24.6 g of residue which was
triturated
with ethyl acetate to give 19.9 g of crystals, m.p. 59°-61°C.
Anal. for C1pH13NS05:
Calc'd: C,46.3; H,5.1; N,5.4;
Found: C,46.2; H.5.0; N,5.2.
Reference Example 171
Methyl 2-[(4-methoxybenzenesulfonyl)-(2~nitrobenzyl)amino]acetate
To a stirred and cooled mixture of 1.2 g (30 mmol) of NaH (58% in oil) in 50
ml of ~T,N-dimethylformamide was added dropwise a solution of 7.78 g (30 mmol)
of
N-(4-methoxybenzenesulfonyl)glycine, methyl ester in 40 ml of N,N-dimethyl-
formamide. After gas evolution ceased, a solution of 6.80 g (32 mmol) of 2-
nitrobenzyl bromide in 40 ml of N,N-dimethylformamide was added dropwise to
the
mixture. The mixture was then stirred at room temperature overnight under
nitrogen
and the solvent removed under vacuum. The residue was extracted with CH2Cl2
and
the extract washed with H20, 2 N citric acid, H20, 1 N NaHC03, brine and dried
with
Na2S04, The solution was filtered through a thin pad of hydrous magnesium
silicate
and the filter pad washed with CH2Cl2. The filtrate was concentrated under
vacuum to
give 11.79 g of solid. Trituration with ethyl acetate gave 2.64 g (22%) of
crystals,
m.p. 114°C-116°C. Anal. for C17H18N20~7S:
Calc'd: C,51.8; H,4.6; N,7.1;
Found: C,51.7; H,4.6; N,7.1.
From the mother liquors an additional 6.49 g (55%j of product as crystals was
obtained by chilling at 0°C and filtering the mother liquors.
Reference Example 172
Methyl 2-[(2-Aminobenzyl)-(4-methoxybenzenesulfonyl)amino]acetate
(A) To a mixture of 2.15 g (5.45 mmol) of methyl-2-[(4-methoxy-
benzenesulfonyl)-(2-nitrobenzyl)amino]acetate and 1.57 g (25 mmol) of ammonium
formate in 10 ml of anhydrous methanol was added 0.42 g of 10% palladium on
carbon. The mixture was stirred at room temperature for 1.5 hours and then
filtered
through diatomaceous earth. The filtrate was concentrated to dryness under
vacuum
and the residue diluted with H20 (25 ml) and extracted with CH2C12 (75 ml).
The
extract was washed with brine, dried with Na2S04 and the solvent removed to
give
0.45 g of solid. Crystallization from ethyl acetate gave 0.124 g of white
crystals, m.p.
100°-102°C. Anal. for C 17H2pN2O5S:
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/013Z5
-73-
Calc'd: C,56.0: H,5.5; N,7.7;
Found: C,56.1; H,5.6; N,7.6.
(B) To a solution of 4.2 g of methyl 2-[(4-methoxybenzenesulfonyl)-(2-
nitrobenzyl)amino]acetate in 200 ml of ethanol-ethyl acetate ( 1:1 ) was added
0.42 g of
10% Pd on carbon (wet -50% H20) and the mixture shaken in a Purr hydrogenator
under 35 pounds per square inch of hydrogen for 4.5 hours at room temperature.
The
mixture was filtered through diatomaceous earth and the filtrate concentrated
to dryness
under vacuum to give 4.0 g of crystals, m.p. 100°-102°C.
Reference Example 173
2-[(2-Aminobenzyl)-(4-methoxybenzenesulfonyl)amino]acetic Acid
To a solution of 5.14 g ( 14.1 mmol) of methyl 2-[(2-aminobenzyl)-(4-
methoxybenzenesulfonyl)amino] acetate in 50 ml of methanol-tetrahydrofuran (
1:1 ) was
i 5 added 2.86 ml of 10 N NaOH and the mixture refluxed for 2 hours. The
solvent was
removed under vacuum and the residue partitioned between water and ether. The
water
layer was separated and acidified with 2 N citric acid. The solid was
filtered, washed
with H20 and dried in a vacuum oven at room temperature to give 4.45 g (91 %)
of
crystals, m.p. 145°-147°C. Anal. for C16H18N205S:
Calc'd: C,54.9; H,5.2; N,8.0;
Found: C,55.1; H,5.2; N,7.9.
Reference Example 174
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(phenoxyacetyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a cooled (0°C) mixture of 1.5 g (3.8 mmol) of methyl 2-[(2-
aminobenzyl}-
(4-methoxybenzenesulfonyl)amino]-3-hydroxypropionate and 2.7 ml ( 19 mmol) of
triethylamine in 15 ml of CH2Cl2 was added 1.58 g ( 1 l .4 mol) of
phenoxyacetyl
chloride. The mixture was stirred at room temperature overnight and filtered.
The
filtrate was washed with H20, 2 N citric acid, and brine and dried with
Na2S04, The
solvent was removed to give 2.4 g of crude methyl 2-{(4-
methoxybenzenesulfonyl)-[2-
(phenoxyacetylamino)benzyl]amino~acrylate av an oil. Anal. for C26H26N207S:
Calc'd: C,61.2; H,5.1; N,5.5;
Found: C,62.6; H,5.1; N,4.0;
Mass spectrum (ES) S I 1 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-74-
To a 2.0 g (3.92 mmol) sample of the preceding compound in 1 S ml of
methanol was added 0.494 g of anhydrous NaHC03 and the mixture stirred for 5
hours. The mixture was concentrated under vacuum and ethyl acetate and H20
were
added to the residue. The mixture was filtered and the organic layer of the
filtrate
separated, washed with brine and dried with Na2S04, The solvent was removed to
give 0.36 g of product as off white crystals, m.p. I51°-153°C.
Anal. for
C26H26N20?S:
Calc'd: C,61.2; H,5.1; N,5.5;
Found: C,61.1; H,S.1; N,5.4;
Mass spectrum (ES) 511 (M+H).
Reference Example 175
3-hydroxymethyi-4-(4-Methoxybenzenesulfonyl)-1
(3-pyridinylmethyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine
A mixture of 0.100 g (0.208 mmol) of methyl 4-(4-methoxybenzenesulfonyl)-
1-(3-pyridinylcarbonyl)-2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3-
carboxylate and
3 ml of borane-tetrahydroforan complex in tetrahydrofuran ( 1.0 M) was
refluxed
overnight. The solution was cooled to room temperature, diluted with methanol
and the
solvent removed. Methanol was added several times and, after each addition,
the
solvent was removed. To the residue was added 1 N NaHC03. The mixture was
stirred for 45 minutes and then extracted with ethyl acetate. The extract was
concentrated and then washed with H20, brine and dried with Na2S04, The
solvent
was removed under vacuum and the residue chromatographed on thick layer silica
gel
plates with 10% methanol in ethyl acetate as solvent to give 60 mg of solid
(RF 0.26).
Crystallization from ethyl acetate gave 30 mg of white crystals. Anal. for
C23H25N304S:
Calc'd: C,62.8; H,5.7; N,9.6; S,7.3;
Found: C,61.1; H,5.6; N,9.2; S,7.3;
Mass spectrum (ES) 440.2 (M+H).
Reference Example 176
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methoxypyridinyl-3-
carbonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a cooled (0°C) mixture of 1.0 g (2.54 mmol) of methyl 2-[(2-
aminobenzyl}-
(4-methoxybenzenesulfonyl) amino]-3-hydroxypropionate and 1.8 ml ( 12.68 mmol)
of
triethylamine in 10 ml of CH2C12 was added 0.957 g (5.58 mmol) of 2-
methoxypyridine-3-carbonyl chloride in 4 ml of CH2C12. The solution was
stirred at
CA 02317546 2000-07-06
WO 99/37625 PC'f/US99/01325
- 75 -
room temperature overnight. diluted with H20 and CH2Cl2 and the organic layer
separated. The organic Layer was washed with H20, 2 N citric acid, and brine
and
dried with Na2S04, The solvent was removed under vacuum to give 1.2 g of
solid.
The solid was chromatographed on thick layer silica gel plates with ethyl
acetate-hexane
(3:1) as solvent to give 0.27 g of yellow foam. Anal. for C25H25N307S:
Calc'd: C,58.7, H,4.93; N,8.21;
Found: C,57.8; H,4.5; N,8.3; S,6.2.
Reference Example 177
5-Methyl-2-nitrobenzyl Bromide
To a cooled (ice-water bath) mixture of 30% HBr in acetic acid (3 ml) was
added 2.5 g 5-methyl-2-nitrobenzyl alcohol and the chilled solution stirred
for 2 hours.
The mixture was poured into ice-water and extracted with diethyl ether. The
extract
was washed with H20, brine and the solvent removed under vacuum to give a
mixture
of product (50%) and starting material (50%).
Reference Example 178
Methyl 3-Hydroxy-2-[(4-methoxybenzenesulfonyl)-(5-methyl-2-
nitrobenzyl)amino]propionate
A solution of 23.14 g (0.08 mol) of methyl 3-hydroxy-2-(4-
methoxybenzenesulfonylamino)propionate in 120 ml of dry N,N-dimethylformamide
was added dropwise to a stirred suspension of 3.2 g (0.08 mol) of sodium
hydride
(57% in oil) in 120 ml of N,N-dimethylformide. When gas evolution ceased, the
mixture was chilled in an ice bath and a solution of 16.4 g (0.084 mol) of S-
methyl-2-
nitrobenzyl chloride in 100 ml of N,N-dimethylformamide was added. To the
mixture
was added 12.6 g (0.084 mol) of anhydrous sodium iodide and the mixture was
chilled
in an ice bath and stirred for 20 minutes. The mixture was allowed to warm to
room
temperature and was stirred overnight. The solvent was removed under vacuum
and
the residue diluted with 200 ml of H20 and extracted with S00 ml of ethyl
acetate. The
aqueous layer was extracted with an additional 200 ml of ethyl acetate. The
combined
extract was washed with H20, brine and dried with Na2S04, The solvent was
removed to give 41.18 g of crude product. The product was chromatographed on
silica
gel with hexane-ethyl acetate ( 1:1 ) as solvent to give 8.14 g (RF 0.38) of
product as a
yellow semi-solid. From a small scale run ( 1 mmol) the product was
chromatographed
twice on thick silica gel plates with hexane-ethyl acetate ( 1:1 ) to give
0.12 g of a yellow
semi-solid. Anal. for C 19H22N2SOg:
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-76-
Calc'd: C,52.0: H,5.1; N,6.4;
Found: C,51.7; H,S.1; N,6Ø
Reference Example 179
Methyl 3-Hydroxy-2-[(4-methoxybenzenesulfonyl)-(2-amino-5-
methylbenzyl)amino]propionate
To a solution of 3.4 g of methyl 3-hydroxy-2-[(4-methoxybenzenesulfonyl)-(5-
methyl-2-nitrobenzyl)-amino]propionate in 200 ml of ethanol-ethyl acetate (
1:1 ) was
added 0.34 g of 10% palladium on carbon (wet - 50% H20). The mixture was then
shaken in a Parr hydrogenator under 35 psi of hydrogen for 2.5 hours. The
mixture
was filtered through diatomaceous earth and the filtrate concentrated under
vacuum to
give 2.86 g of a brown oil. Anal. for C 19H24N206S:
Calc'd: C,55.9; H,5.9; N,6.9;
Found: C,55.6; H,5.9; N,6.4;
Mass spectrum (ES) 409 (M+H).
Reference Example 180
Methyl 3-[(2-Tetrahydropyranyl)oxy]-2-[(-4-methoxybenzenesulfonyl)-
(5-methyl-2-nitrobenzyl)amino]propionate
To a mixture of 1.75 g (4.68 mmol) of (D,L)~l-(4-methoxybenzenesulfonyl)-O-
(2-tetrahydropyranyl) serine, methyl ester, 0.790 g (4.68 mmol) of 5-methyl-2-
nitrobenzyl alcohol and 1.23 g (4.68 mmol) of triphenylphosphine -in 4.5 ml of
anhydrous tetrahydrofuran was added dropwise (over 15 minutes) a solution of
0.815 g
(4.68 mmol) of diethyl azodicarboxylate (DEAD) in 1 ml of tetrahydrofuran. The
mixture was stirred at room temperature overnight and the solvent removed
under
vacuum. The residue was triturated with diethyl ether and the solid filtered
off. The
filtrate was concentrated to dryness under vacuum to give 4.67 g of solid. The
solid
was chromatographed on silica gel with hexane-ethyl acetate ( 1:1 ) to give
0.56 g of
product (RF 0.48).
Reference Example 181
Methyl 1-Methoxyacetyl-4-(4-methoxybenzenesulfonyl)
7-methyl-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a cooled (0°C) mixture of 1.598 g (3.91 mmol) of methyl 3-
hydroxy-2-[(4
methoxybenzenesulfonyl)-(2-amino-5-methylbenzyl)amino]propionate and 1.97 g
(19.5) mmol) of triethylamine in 15 ml of dichloromethane was added 0.787 ml
(8.60
mmol) of methoxyacetylchloride. The mixture was stirred at room temperature
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
_77_
overnight. The mixture was then diluted with CH2Cl2 and washed with H20. 2 N
citric acid, H20. brine and dried with Na2S04. The solution was filtered
through a
thin pad of hydrous magnesium silicate and the filtrate concentrated to give
1.94 g of
crude methyl 2-{ [2-(methoxyacetylamino)-5-methylbenzyl]-(4-methoxy-benzene-
sulfonyl)-amino}acrylate as a brown oil. Mass spectrum (ES) 463.4 (M+H).
To a solution of 1.62 g (3.5 mmol) of the preceding compound in 15 lnl of
anhydrous methanol was added 0.382 g (4.50 mmol) of anhydrous NaHC03 and the
mixture was stirred overnight at room temperature. The solvent was removed
under
vacuum and the residue partitioned between 100 ml of ethyl acetate and 20 ml
of water.
The ethyl acetate layer was separated and washed with H20, brine and dried
with
Na2S04. The solution was filtered through a thin pad of hydrous magnesium
silicate
and the filtrate concentrated under vacuum to give a yellow oil. Trituration
with ethyl
acetate-hexane gave 1.26 g (78°10) of tan crystals, m. p. 122°-
124°C. Anal. for
C22H26N207S:
Calc'd: C,57.1; H,5.7; N,6.1;
Found: C,57.4; H,5.7; N,6Ø
Reference Example 182
Methyl 1-Benzoyl-4-(4-methoxybenzenesulfonyl)-7-methyl-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazeprine-3-carboxylate
To a cooled (0°C) mixture of 1.465 g (3.586 mmol) of methyl 3-
hydroxy-2-[4-
methoxybenzenesulfonyl)-(2-amino-5-methylbenzyl)amino]propionate and 2.49 ml
(17.93 mmol) of triethylamine in 20 ml of CH2Cl2 was added 0.915 ml (7.89
mmol)
of benzoyl chloride. The mixture was stored at room temperature overnight,
diluted
with CH2C12 and washed with H20, 2 N citric acid, H20, brine and dried with
Na2S04, The solution was filtered through a thin pad of hydrous magnesium
silicate
and the filtrate concentrated under vacuum to give 1.8 g of crude methyl 2-[(2
benzoylamino-5-methylbenzyl)-(4-methoxybenzenesulfonyl)amino]acrylate as a
brown
oil. Anal. for C26H26N206S:
Calc'd: C,63.1; H,5.3; N,5.7;
Found: C,63.9; H,5.2; N,5.2.
As described for Reference Example 181, 1.825 g (3.68 mmol) of the preceding
compound was stirred with 0.402 g (4.78 mmol) of NaHC03 in 1.5 ml of .methanol
to
give an oil. Trituration with hexane (plus several drops of ethyl acetate)
gave crystals,
m. p. 58°-62°C.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
_78_
Reference Example 183
Methyl 1-(traps-Crotonyl)-4-(4-methoxybenzenesulfonyl)-7-methyl
2,3,4,5-tetrahydro-1H-[1,4]benzadiazepine-3-carboxylate
As described for Reference Examples 181 and 182, a mixture of 1.41 g (3.455
mmol) of methyl 3-hydroxy-2-[-(4-methoxybenzenesulfonyl)-(2-amino-5-methyl-
benzyl)amino]propionate, 1.75 g ( 17.3 mmol) of triethylamine and 0.809 ml of
trans-
crotonyl chloride in 15 ml of CH2Cl2 was stirred overnight to give 1.52 g of
methyl 2-
{ [2-(traps-crotonylamino)-5-methylbenzyl]-(4-methoxybenzenesulfonyl)
amino}acrylate as a brown oil; Mass spectrum (ES) 459.4 (M+H).
As described in Reference Example 181, 1.52 g (3.31 mmol) of the preceding
product was stirred with 0.362 g (4.3 mlnol) of NaHC03 in 15 ml of methanol at
room
- temperature overnight. To the mixture was added 0.056 g of NaHC03 and the
mixture
was heated at 80°C for 3 hours and worked up as for Reference Example
181 to give a
I .OS g of a yellow glass, m. p. 75°-84°C. Mass spectrum
(ES) 459.4 (M+H).
Reference Example 184
1-(traps-Crotonyl)-4-(4-methoxybenzenesulfonyl)-7-methyl-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepene-3-carboxylic acid
A mixture of 1.26 g (2.72 mmol) of methyl 1-(traps-crotonyl)-4-(4-
methoxybenzenesulfonyl)-7-methyl-2,3,4,5-tetrahydro-1 H-( 1,4]benzodiazepine-3-
carboxylate and 3.53 ml (3.53 mmol) of 1 N NaOH in 10 ml of tetrahydrofuran
was
stirred at room temperature for 3 hours. The solvent was removed under vacuum
and
the residue dissolved in H20 and the solution extracted with ethyl acetate.
The aqueous
layer was acidified with 1N HCl (pH 2) and extracted with CH2C12. The CH2Cl2
extract was dried with Na2S04 and the solvent removed to give 1.06 g (after
drying
under vacuum) of solid, m. p. 101°-105° C.
Reference Example 185
1-(Benzoyl)-4-(4-methoxybenzenesulfonyl)-7-methyl-2,3-4,5
tetrahydro-1H[1,4]benzodiazepine-3-carboxylic acid
A mixture of 1.18g (2.38 mml) of methyl 1-(benzoyl)-4-(4-methoxy-
benzenesulfonyl)-7-methyl-2,3,4,5-tetrahydro- I H-( 1,4]benzodiazepine-3-
carboxylate
and 3.09 ml (3.09 mmol) of 1N NaOH in 10 ml of tetrahydrofuran was stored at
mom
temperature overnight and the solvent removed under vacuum. The residue was
diluted
with H20, extracted with ethyl acetate and the aqueous layer acidified with 2N
citric
CA 02317546 2000-07-06
WO 99/37625 PCT/US99I01325
-79-
acid. The mixture was extracted with CH2Cl2 and the CH2Cl2 extracts were
washed
with H20, brine and dried with Na2S04, The solvent was removed to give 0.828
of a
light yellow glass, m.p. 95°-100°C; Mass spectrum (ES) 481.4
(M+H).
Reference Example 186
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(2-methoxyethyl)-2,3,4,5
tetrahydro-1 H-[ 1,4]benzodiazepine-3-carboxylate
A mixture of 1.6 g (3.57 mmol) of methyl 1-(methoxyacetyl)-4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-I H-[ 1,4]benzodiazepine-3-
carboxylate
and 32 ml of borane in tetrahydrofuran ( I .0 M) was refluxed under nitrogen
overnight.
Methanol was added and the solvent removed. To the residue was added 25 ml of
CH2Cl2 and 25 ml of 2 N HCl and the mixture stirred at room temperature for I
hour.
The organic layer was separated and washed with H20 and concentrated to
dryness.
The residue was triturated with ethyl acetate-hexane, cooled and filtered to
give 1.2 g of
white crystals, m.p. 86°-90°C; Mass spectrum (ES) 435.4 (M+H).
Anal. for
C21 H26N206S:
Calc'd: C,58.1; H,6.0; N,6.5;
Found: C,58.5; H,6.0; N,6.5.
Reference Example 187
4-(4-Methoxybenzenesulfonyl)-1-(2-methoxyethyl)-2,3,4,5-tetrahydro-
IH[1,4]benzodiazepine-3-carboxylic acid
A mixture of 1.0 g (2.3 mmol) of methyl 4-(4-methoxybenzenesulfonyl)-1-(2
methoxyethyl)-2,3,4,5-tetrahydro-IH-[1,4]benzodiazepine-3-carboxylate and 3.0
ml of
1 N NaOH in 10 ml of tetrahydrofuran was stirred at room temperature for 2
hours and
the solvent removed. To the residue was added water and the mixture acidified
with 1
N HCI. The mixture was extracted with ethyl acetate and the extract was washed
with
brine and dried with Na2S04, The solvent was removed and the residue
triturated with
ethyl acetate-hexane, cooled and filtered to give 0.65 g of white crystals,
m.p. 164°-
165°C; Mass spectrum (ES) 421.4 (M+H). Anal. for C2pH24N2O6S:
Calc'd: C,57.1; H,5.8; N,6.7;
Found: C,57.3; H,5.7; N,6.4.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-80-
Reference Example 188
Methyl 1-(Benzyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro
1H-[ 1,4]benzodiazepine-3-carboxylate
A mixture of 0.20 g (0.416 mmol) of methyl 1-(benzoyl)-4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylate
and 4 ml of borane in tetrahydrofuran ( 1.0 M) was refluxed overnight and the
solvent
removed. To the residue was added 5 ml of CH2C12 and 5 ml of 2N HCI and the
mixture stirred for 1 hour. The organic layer was separated and concentrated
to
dryness. The residue was chromatographed on thick layer silica gel plates with
hexane-
ethyl acetate (2:1 ) as solvent to give 0.140 g of a colorless oil: Mass
spectrum (ES)
467.5 (M+H).
Reference Example 189
4-(4-Methoxybenzenesulfonyl)-1-[4-(trifluoromethoxy)benzoyl]-8
chloro-2,3,4,5-tetrahydro-1H[1,4]benzodiazepine-3-carboxylic acid
As described for Reference Example 18, 1.46 g (3.40 mmol) of methyl 2-[(2-
amino-4-chlorobenzyl)-(4-methoxybenzenesulfonyl)amino]-3-hydroxypropionate was
reacted with 4-(trifluoromethoxy)benzoyl chloride to give 2.59 g of methyl 2-
{ 2-[4-
(trifluoromethoxy) benzoyl]amino-4-chlorobenzyl]amino } acrylate as a yellow
oil: Mass
spectrum (ES) 599.3 (M+H). The preceding compound was stirred with 0.445 g
(5.29
mmol) of anhydrous NaHC03 in 15 ml of methanol at room temperature for 16
hours
and then was heated at 80°C for 2 hours. The solvent was removed and
the residue
extracted with ethyl acetate. The extract was washed with H20, brine, and
dried
(Na2S04). The solvent was removed and the residue crystallized from ethyl
acetate-
hexane to give methyl 4-(4-methoxybenzenesulfonyl)-1-[4-
(trifluoromethoxy)benzoyl}-
8-chloro-2,3,4,5-tetrahydro-1H-(1,4]benzodiazepine-3-carboxylate as yellow
crystals,
m.p. 149°-151°C. Anal. for C26H22C~307S:
Calc'd: C,52.1; H,3.7; N,4.7; C1,6.0; F,9.5;
Found: C,51.8; H,3.6; N,4.7; C1,5.9: F,9.4.
1.58g (2.64 mmol) of the preceding compound was stirred with 3.43 ml of 1 N
NaOH in 10 ml of tetrahydrofuran at room temperature for 2 hours and worked up
as
for Reference Example 104 to give 1.52 g of product. Crystallization from
ethyl
acetate-hexane gave 1.2 g of white crystals, m.p. 184°-186°C.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/OI325
-81 -
Reference Example 190
Methyl 4-(4-Methoxybenzenesulfonyl)-1-(4-morpholinoacetyl)-2,3,4,5
tetrahydro-1H[1,4]benzodiazepine-3-carboxylate
A mixture of 0.10 g (0.22 mmol) of methyl 1-(chloroacetyl.)-4-(4-
methoxybenzenesulfonyl)-2,3,4.5-tetrahydro- 1 H-[ 1,4]benzodiazepine-3-
carboxylate,
21.2 ~1 of morpholine and 125.4 El of N,N-diisopropylethylamine in 3 ml of
CH2Cl2
was stirred overnight at room temperature. An additional 2.2 El of morpholine
was
added and the solution stirred for 2 days at room temperature. The mixture was
diluted
with CH2C12 and washed with H20, brine and dried with Na2S04, The solvent was
removed to give the product as a solid, Mass spectrum (ES) 504.3 (M+H). Anal.
for
C24H29N30'7S:
Calc'd: C,57.2; H,5.8; N,8.3;
Found: C,56.5; H,5.6; N,8.1.
Reference Example 191
Methyl 4-(4-Methoxybenzenesulfonyl)-1-[2-(1
pyrazolyl)phenylcarbonyl]-7-methyl-2,3,4,5-tetrahydro-1H
[l,4Jbenzodiazepine-3-carboxylate
As described for the general reaction of ethyl 2-fluorobenzoate with amines
set
forth in Tetrahedron, 53:7557-7576 ( 1997), ethyl 2-fluorobenzoate was reacted
with
pyrazole by refluxing N,N-dimethylformamide to give ethyl 2-( 1-
pyrazolyl)benzoate,
as a thick yellow oil. Anal. Calc'd: for C,2 H,2 N2O2: C, 66.7; H, 5.6; N
13.0: Found:
C, 66.5: H, 5.4: N, 12.9; Mass spectrum (ES) 217.2 (M+H). A sample (7.02g) of
this
compound and 8.42 ml of SN NaOH in 40 ml of ethanol-tetrahydrofuran (2:1 ) was
refluxed for 2 hrs and the solvent removed. The residue was made acidic (pH6)
with
2N citric acid and the precipated solid was filtered to obtain 3.7g of
product. The pH of
the filtrate was adjusted to 4.5 and extracted with ethyl acetate. The extract
was
concentrated to dryness to give l.Sg of product. The two crops were combined
to give
5.2g of 2-(1-pyrazolyl)benzoic acid, mp 140-142°C. To the preceding
compound
(2.07 g) in 5 ml CH2Cl2 (chilled in an ice bath )was added 11.1 ml of a 2
Molar
solution of oxalyl chloride in CH2CI2 and 0.085 ml of N,N-dimethylformamide.
The
mixture was allowed to warm to room temperature and stirred for 4 hours. The
solvent
was removed and 25 ml of toluene added (twice) and removed under vacuum to
give 2-
(1-pyrazolyl)benzoyl chloride as a yellow solid.
A 2.3 g sample of the preceding compound was reacted with l.Sg of the
compound of Reference Example 179 in 15 ml of CH2CI2 and 5.12 ml of
triethylamine
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-82-
in the manner described for Reference Example 181 to give methyl 2-[(4-
methoxybenzenesulfonyl)-{ 2-[2-( 1-pyrazolyl)phenylcarbonyl]amino-5-
methylbenzyl }-
amino]acrylate. This compound was cyclized with NaHC03 in methanol in the
manner
described in Reference Example 181 to give methyl 4-(4-methoxybenzenesulfonyl)-
1-
[2-(1-pyrazolyl)phenylcarbonyl]-7-methyl-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-
3-carboxylate (m.p. 240-242° C).
A 1.16 g sample of the preceding compound was hydrolysed with 2.69 ml of
1N NaOH in 10 ml of tetrahydrofuran in the manner described for Reference
Example
104 to give 0.71 g of 4-(4-methoxybenzenesulfonyl)-1-[2-(1-pyrazolyl)phenyl-
carbonyl)-7-methyl-2,3,4,5-tetrahydro-1H[1,4]benzodiazepine-3- carboxylic acid
(mp
149-151°C).
Reference Example 192
Methyl 4-(4-Methoxybenzenesulfonyl)-1-[2-(4-
morpholino)phenylcarbonyl}-8-chloro-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-3-carboxylate
Ethyl 2-morpholinobenzoate prepared in the manner described in Tetrahedron,
53:7557, ( 1997) was refluxed with 10 N NaOH in tetrahydrofuran-ethanol (8:2)
for 1.5
hrs to give 2-morpholinobenzoic acid, mp 156-157°C. A 1.8 g sample of
this
compound in 5 ml of CH2Cl2 (chilled) was added to a solution of 7.9 ml of
oxalyl
chloride in CH2C12 (2M) followed by the addition of 0.058 ml of N,N-.
dimethylformamide. The solution was stirred at room temperature for 6 hrs and
the
solvent removed. Toluene was added (2 times) and removed to give 2-(4-
morpholino)benzoyl chloride as a yellow solid.
The preceding 2-(4-morpholino)benzoyl chloride was reacted with methyl 2-[(2-
amino-4-chlorobenzyl)-(4-methoxybenzenesulfonyl)amino]-3-hydroxypropionate in
the
manner described in Reference Examples 181 and 189, and the product was
stirred with
NaHC03 in methanol to give methyl 4-(4-methoxybenzenesulfonyl)-1-[2-(4-
morpholino)phenylcarbonyl]-8-chloro-2,3,4,5-tetrahydro-1 H-[
1,4]benzodiazepine-3-
carboxylate, as a white solid having a mp 100-105°C.
To 0.90g of this compound in 10 ml of tetrahydrofuran was added 1.95 ml of 1
N NaOH and the solution was stirred at room temperature overnight.
Acidification with
2N citric acid gave 0.82 g of 4-(4-methoxybenzenesulfonyl)-1-[2-(4-moiphalino)-
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-83-
phenylcarbonyl]-8-chloro-2,3,4,5-tetrahydro- I H-[ 1,4]benzodiazepine-3-
carboxylic
acid (mp 136-143 °C).
Reference Example 193
Methyl 1-(4-Ethoxybenzoyl)-4-(4-methoxybenzenesulfonyl) -
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
A mixture of 0.270 g of methyl 4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4] benzodiazepine-3-carboxylate of Reference Example 12,
0.291 g
of 4-ethoxybenzoyl chloride and 500 E,~l of triethylamine in 5 ml of CH2Cl2
was stirred
at room temperature overnight. The mixture was diluted with CH=C12 and H20 and
the
CH2CI2 layer was separated and concentrated to dryness. The residue was
triturated
with ethyl acetate to give 0.276g of methyl 1-(4-ethoxybenzoyl)-4-(4-methoxy-
benzenesulfonyl)-2,3,4,5tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate as
white
crystals, (mp 187-190°C).
A 0.47 g sample of this compound was hydrolyzed with 1.2 ml of 1~T NaOH in
4 ml of tetrahydrofuran. Dilution with H20 and acidification with 1 N HCl gave
0.40 g
of the acid as a white solid, mp 144-152°C.
Reference Example 194
Methyl 4-(4-Methoxybenzenesulfonyl) -1-[2-chloro-4-(3-methyl-1-
pyrazolyl)phenylcarbonyl}-2,3,4,5-tetrahydro -1H
[ 1,4]benzodiazepine-3-carboxylate
As described in Example 65, methyl 4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-lH-[1,4]benzodiazepine-3-carboxylate was reacted with 4-{3-methyl-1-
pyrazolyl)-2-chlorobenzoyl chloride to give methyl 4-(4-
methoxybenzenesulfonyl)-I-
[2-chloro-4-(3-methyl- I-pyrazolyl)phenylcarbonyl]-2,3,4,5-tetrahydro- I H-[
1,4]benzo-
diazepine-3-carboxylate as a white solid. Anal. for C29H27CIN4O6S:
Calc'd: C, 58.3; H, 4.6; N, 9.4.
Found: C,58.2; H, 4.9; N, 8.9.
This compound was hydrolysed with 1~1 NaOH in tetrahydrofuran as described
in Reference Example 185 to give the benzodiazepine-3-carboxylic acid
derivative as a
white solid.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-84-
Reference Example 195 .
1-Benzyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
[1,4]benzodiazepine-3-carboxylic acid
A mixture of 1.7 g of the compound of Reference Example 45 and 25 ml of
borane in tetrahydrofuran ( 1.0 Molar) was refluxed under nitrogen overnight.
To the
solution was added 5 ml of CH30H, CH2Cl2 (40 ml) and 30 ml of 2~1 HCl and the
mixture stirred at room temperature for 1.5 hr. The organic layer was
separated,
washed with brine, dried with Na2S04 and the solvent removed. The residue was
crystallized from ethanol-hexane to give 1.15g of methyl 1-benzyl-4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-(1,4]benzodiazepine-3-
carboxylate as
white crystals, mp 120-122°C. A sample (1.0 g) of this compound was
hydrolysed
with 2.8 ml of 1 ~I NaOH in 7 ml of tetrahydrofuran as described in Reference
Example
104 to give 0.64 g of the 2,3,4,5- tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylic acid
derivative as white crystals (mp 183-185°C).
Reference Example 196
Methyl 1-(2,4-Dimethoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a cooled (0°C) solution of 1.0 g (2.66 mmol) of 4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylate
from Reference Example 12 and 1.85 ml ( 13.3 mmol) of triethylamine in 8 ml of
CH2C12 was added 1.17 g (6.65 mmol) of 2,4-dimethoxybenzoyl chloride. The
mixture was stirred at room temperature overnight, diluted with CH2Cl2 and
washed
with 2 ~T citric acid. The organic layer was washed with H20, 1 ~1 Na2C03,
brine and
dried over Na2S04. The solvent was removed and the residue was chromatographed
on thick layer silica gel plates with ethyl acetate-hexane ( 1:1 ) as an
eluent to give 1.0 g
of methyl 1-(2,4-dimethoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate as a white foam. Anal. for
C27H28H208S:
Calc'd: C,60.0; H,5.2; N,5.2;
Found: C,60.0; H,5.2; N,5.1;
Mass Spectrum (ES): 541.0 (M+H).
A 0.80 g ( 1.48 mmol) sample of the preceding compound and 1.92 ml ( 1.92
mmol) of 1 ~l NaOH in 5 ml of tetrahydrofuran was stirred at room temperature
for 1.5
hours. The solvent was removed and the residue diluted with water. The
solution was
acidified with 1 N HCI, chilled and filtered to give 0.70 g of 1-(2,4-
dimethoxy-
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-85-
benzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H-(
1,4]benzodiazepine-
3-carboxylic acid as a white solid. Anal. for C26H26N208S:
Calc'd: C,59.3; H,5.0; N,5.3;
Found: C,56.1; H,4.8; N,S.O;
Mass Spectrum (ES): 527.0 (M+H).
Reference Example 197
Methyl 4-(4-Methoxybenzenesulfonyl)-1-[2-(4-methylpiperazin-1-
yl)acetyl]-2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
To a mixture of 2.5 g (6.64 mmol) of methyl 4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate (Reference Example 12)
and
4.63 ml (33.2 mmol) of triethylamine in 40 ml of CH2Cl2 cooled to 0°C
was added to
1.65 g ( 14.63 mmol) of chloroacetyl chloride. The solution was stirred at
room
temperature for 2 days, chilled to 0°C and 926 lil of triethylamine and
750 mg of
chloroacetyl chloride were added thereto. The mixture was stirred at room
temperature
overnight, diluted with CH2C12 and H20. The insoluble solid was filtered off.
The
organic layer of the filtrate was separated , washed with brine, dried with
Na2S04 and
filtered through diatomaceous earth. The solvent was removed and the residue
triturated with ethyl acetate and a trace of ethanol. Chilling and filtering
gave 0.75 g of
methyl 1-(chloroacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-
[ 1,4]benzo-diazepine-3-carboxylate (Reference Example 91 ). Anal. for
C20H21 C1N206S:
Calc'd: C,53.0; H,4.7; N,6.2;
Found: C,51.6; H,4.6; N,5.7;
Mass Spectrum (ES): 453.0 (M+H).
To a solution of 1.4 g (3.09 mmol) of the preceding compound in 12 ml of
CH2C12 cooled to 0°C was added 1.2 ml (6.79 mmol) of N,N-
diisopropylethylamine
followed by the addition of 753.2 pl (6.79 mmol) of 1-methylpiperazine. The
mixture
was stirred at room temperature overnight, diluted with CH2Cl2, and washed
with 2 ~T
citric acid, H20, 1 M NaHC03, brine and dried (Na2S04). The citric acid wash
was
made basic with saturated NaHC03 and then extracted with CH2Cl2. The extract
was
dried over Na2S04 and the solvent removed under vacuum to give 1.10 g of
methyl 4-
(4-methoxybenzenesulfonyl)-1-[2-(4-methylpiperazin-1-yl)acetyl]-2,3,4,5-
tetrahydro-
IH-[1,4]benzodiazepine-3-carboxylate as a white glass.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/013Z5
-86-
A mixture of 1.0 g ( 1.94 mmol) of the preceding compound and 2.3 ml (2.3
mmol) of 1 N KOH in 5 ml of methanol was stirred at room temperature for 2
hours.
The solvent was removed under vacuum. To the residue was added toluene (2
times)
and the solvent removed under vacuum after each addition. The solid was dried
at 65°C
under vacuum for 6 hours to give 1.1 g of potassium 4-(4-
methoxybenzenesulfonyl)-1
[2-(4-methylpiperazin-1-yl)acetyl]-2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-
3
carboxylate as a white solid.
Reference Example 198
Methyl 1-Acetyl-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4] benzodiazepine-3-carboxylate
To a cooled (0°) solution of 2.Og (4.78 mmol) of methyl 1-acetyl-4-
(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylate in
14 ml of CH2 C12 was added dropwise 143.3m1 ( 14.3mmo1) of a 1.0 molar
solution of
BBr3 in CH2 C12 . The mixture was stirred at room temperature for 1.5 hours.
Ice and
H20 were added to the reaction mixture and the insolubles filtered off. The
filtrate was
diluted with CH2C12 and H20 and the CH2Cl2 layer separated, washed with brine
and
dried (Na2 S04). The solvent was removed under vacuum to give 1.5 g of a white
foam. The solid was chromatographed on silica gel with hexane-ethyl acetate
(1:1 ) as
solvent to give a foam which was dried under vacuum to give 0.52 g of product
as a
white foam; Anal. Calc'd for C,9H2oN2O6S: C, 56 4:H, 5.0; N, 6.9 Found: C
55.1;
H, 4.7: N, 6.5.
Reference Example 199
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(2-thienylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a solution of 4.0 g (8.22 mmol) of methyl 4-(4 methoxybenzenesulfonyl)-1-
(2-thienylcarbonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate in
17 ml
of CH2CI2 chilled to 0°C, was added slowly 16.4 ml ( 16.44 mmol) of 1.0
molar
solution of boron tribromide in CH2C12. The mixture was stirred at room
temperature
overnight and diluted with CH2Cl2. The mixture was filtered and the solid
washed with
CH2Cl2. and H20. The filtrate was diluted with H20 and the organic layer
separated.
The solvent was removed under vacuum and the solid chromatographed on silica
gel
with hexane-ethyl acetate ( 1:1 ) as solvent to give 0.80 g of off white foam;
Mass
Spectrum (ES) 473.5 (M+H); Anal. Calc'd for C22 H2o N206 S2: C, 55.9; H, 4.3;
N,
5.9. Found: C, 54.5; H, 4.4; N, 5.5.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
_87_
Reference Example 200
Methyl 1-Benzoyl-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a solution of 9.8 g (20.39 mmol) of methyl 1-benzoyl-4-(4
methoxybenzenesulfonyl}-2,3,4,5-tetraydro-1H-[1,4]benzodiazepine in 50 ml of
CH2C12 cooled to 0°, was added slowly 40.8 ml (40.8 mmol) of a 1.0
molar solution
of BBr3 in CH2C12, The mixture was stirred under nitrogen at room temperature
overnight. Ice and H20 were added and the mixture diluted with CH2C12. The
organic
layer was separated and the aqueous layer extracted with ethyl acetate. The
combined
organic extracts {CH2CI2+ethyl acetate) were concentrated under vacuum and the
residue dissolved in ethyl acetate. The solution was washed with H20, brine
and dried
(Na2S04). The solution was filtered through a thin pad of hydrous magnesium
silicate
and the filtrate concentrated to dryness. The residue was chromatographed on
silica gel
with hexane-ethyl acetate as solvent to give 8 g of product as an off white
foam; Mass
Spectrum (ES) 467 (M+H); Anal Calc'd for C24H22N206S~ C, 61.8; H, 4.8; N, 6Ø
Found: C, 61.3; H, 4.6; N, 5.8.
Utilizing the method described in Reference Examples 191-193, the following
methyl-1-substituted-4-hydroxybenzenesulfonyl}-2,3,4,5-tetrahydro-1 H-[ 1,4]
benzodiazepine-3-carboxylates can be prepared.
Reference Example 201
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(4-methylphenylsulfonyl)
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate.
Reference Example 202
Methyl 1-Methanesulfonyl-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepinef-3-carboxylate
6.0 g (13.2 mmol) of Reference Example 44 and 22.6 ml (22.6 mmol) of BBr3
in CH2C12 (solution) gave, after chromatography on silica with ethyl acetate-
hexane
(1:1), 0.82g of a white foam; Mass spectrum (ES) 440.9 (M + H).
Reference Example 203
Methyl 4-(4-Hydroxybenzenesu~fonyl)-1-(3-pyridinylcarbonyl) 2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
_88_
Reference Example 204
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(4-pyridinylcarbonyl-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 205
Methyl 1-(4-Biphenylcarbonl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 206
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(propane-1-sulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 207
Methyl 1-([1,1'-Biphenyl]-2-carbonyl)-4-(4-hydroxybenzenesulfonyl}-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 208
Methyl 1-(3-Fluorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 209
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(2-methyl-5-fluorobenzoyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 210
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(2-methyl-3-fiuorobenzoyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 211
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(3-phenylpropionyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/o1325
-89-
Reference Example 212
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(2-trifluoromethylbenzoyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 213
Methyl 1-(2-Chloro-6-trifluoromethylbenzoyl)-4-(4
hydroxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3
carboxylate
Reference Example 214
Methyl 1-(4-Fluoro-2-trifluoromethylbenzoyl)-4-(4
hydroxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3
carboxylate
Reference Example 215
Methyl 1-(2-Fluoro-6-trifluoromethybenzoyl)-4-(4
hydroxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3
carboxylate
Reference Example 216
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(2 methylbenzoyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 217
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(2-methyl-6-chlorobenzoyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylateReference
Example 218
Methyl 1-(2,4-Dimethylbenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 219
Methyl 1-(2,5-Dimethylbenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCTNS99/01325
-90-
Reference Example 220
Methyl 1-(2-Chloro-4-fluorobenzoyl)-4-(4-hydroxybenzenesulfonyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 221
Methyl 1-(2-Chlorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1 H-[ 1,4] benzodiazepine-3-carboxylate
Reference Example 222
Methyl 1-(2-Fluorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 223
Methyl 1-(2-Chloro-6-fluorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 224
Methyl 1-(2,3-Difluorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 225
Methyl 1-(2,4-Dichlorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 226
Methyl I-(2,3-Dichiorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 227
Methyl-1-(2,5-Dichlorobenzoyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 228
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(2-methylthiobenzoyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-91 -
Reference Example 229
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(3-methyl-2-thienylcarbonyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 230
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(4-methyl-2-thienylcarbonyl)
2,3,4,5- tetrahydro-1H-[1,4)benzodiazepine-3-carboxylate
Reference Example 231
Methyl 1-(3-Chloro-2-thienylcarbonyl)-4-( hydroxybenzenesulfonyl)-
2,3,4,5- tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 232
Methyl 1-(2-Furanylcarbonyl)-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
To a solution of 3.Og (6.38 mmol) of methyl 1-(2 furanylcarbonyl)-4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3-
carboxylate
in., 15 ml of CH2C12 (cooled to 0°C ) was added dropwise 12.8m1 (2.8
mmol) of BBr3
in CH2C12 (1.0 M in CH2C12).The mixture was stirred at room temperature for 3
days,
diluted with CH2C12 and then ice was added. The organic layer was separated,
washed
with H20, brine 'and dried (Na~S04). The solvent was removed and the residue
chromatographed on silica gel (flash column) with ethyl acetate-hexane ( 1:1 )
as solvent.
The fractions containing product were combined, the solvent removed and the
residue
triturated with ethyl acetate. Chilling and filtering gave 0.72g of methyl 1-
(2- furanyl-
carbonyl)-4-hydroxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4)benzodiazepine-
3-
carboxylate as a white solid, mp 204-206°C; Anal Cal'd for C22H2oN207S:
C, 57.9; H,
4.2; N, 6.1. Found: C,57.2; H,4.3; N, 6.O.;Mass spectrum (ES) 457.1 (M+H).
Reference Example 233
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(3-methyl-2-
furanylcarbonyl)-2,3,4,5- tetrahydro-1H-[-1,4]benzodiazepine-3-
carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-92-
Reference Example 234
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(4-methyl-2-
furanylcarbonyl)-2,3,4,5-tetrahydro-1H-(1,4]benzodiazepine-3-
carboxylate
Reference Example 235
Methyl 1-(5-Chloro-2-furanylcarbonyl)-4-(4-
hydroxybenzenesulfonyl)-2,3,4,5- tetrahydro-1H-[1,4]benzodiazepine-
3-carboxylate
Reference Example 236
Methyl 1-(5-Chloro-2-thienylcarbonyl)-4-(4-
hydroxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodeiazepine-
3-carboxylate
Reference Example 237
Methyl 1- Propionyl-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodeiazepine-3-carboxylate
Reference Example 238
Methyl 1-Hexanoyl-4-(4-hydroxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 239
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(3-thienylcarbonyl)-
2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-carboxylate
Reference Example 24
Methyl 1-(3-Furanylcarbonyl)-4-(4-hydroxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
Reference Example 241
Methyl 1-(Acetylaminoacetyl)-4-(4-hydroxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-93-
Reference Example 242
Methyl 1-(N,N Dimethylaminoacetyl)-4-(4-
hydroxybenzenesulfonyi)- 2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-
3-carboxylate
Reference Example 243
Methyl 1-(Cyclopropylcarbonyl)-4-(4-hydroxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine
To a solution of 4..44g( 10 mmol) of methyl 1-cyclopropylcarbonyl-4-(4-
methoxybenzenefulfonyl)-2,3,4-tetrahydro-1H-[1,4]benzodiaxepine -3-carboxylate
in
25 ml of CH2C12 chilled to 0~~ C was added dropwise 22 ml (22 mmol) of BBr3 in
CH2Cl2 ( 1.0 molar solution). The mixture was stirred overnight, cooled and
diluted
with ice and H20. Dichloromethane was added and the organic layer separated
and
washed with H20, brine and dried (Na2S04). The solvent was removed under
vacuum
to give a solid which was chromatographed on silica gel with the solvent ethyl
acetate-
hexane (1:1) to give 1.0 g of methyl 1-cyclopropylcarbonyl-4-(4-hydroxybenzene-
sulfonyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate as a foam;
Mass
spectrum (ES) 431.3 (M+H).
Reference Example 244
Methyl 4-(4-Hydroxybenzenesulfonyl)-1-(trifluoroacetyl) -2,3,4,5
tetrahydro-1H-[ 1,4]benzodiazepine
Example 1
4-(4-Methoxybenzenesulfonyl)-1-(3-trifluoromethylbenzoyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
To a solution of 0.297 g (0.556 mmol) of 4-(4-methoxybenzenesulfonyl)-1-(3-
trifluoromethylbenzoyl)-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic
acid
from Reference Example 9 in 5 ml of CH2C12, was added 0.556 ml ( 1. I 1 mmol)
of
2.0 M oxalyl chloride in CH2Cl2 and 0.044 ml of N,N-dimethylformamide. The
mixture was stirred under nitrogen at room temperature for 1.5 hours and
cooled in an
ice bath. To this solution was added a chilled mixture of 0.156 g (2.24 mmol)
of
hydroxylamine hydrochloride and 4.68 ml (3.36 mmol) of triethylamine in 1.39
ml of
tetrahydrofuran and 0.33 ml of H20. The mixture was stirred at room
temperature
overnight and diluted with CH2Cl2. The mixture was washed with 2 N citric
acid,
H20, 1 N NaHC03, brine and dried with Na2S04. The solvent was removed under
vacuum to give 0.29 g of solid. Chromatography on thick layer silica gel
plates with
CA 02317546 2000-07-06
WO 99/3?625 PCT/US99/01325
-94-
ethyl acetate-methanol (9:1) gave 60 mg of solid, m.p. 128-130°C. Anal.
for
C25H22F3N306S:
Calc'd: C,54.6; H,4.0; N,7.7:
Found: C,54.1; H,4.2; N,7.3.
Utilizing the procedure described in Example 1, the following compounds are
prepared from the appropriately 1-substituted-4(4-methoxybenzenesulfonyl)-
2,3,4,5-
tetrahydro- I H-[ 1,4]benzodiazepine-3-carboxylic acids.
Example 2
4-(4-Methoxybenzenesulfonyl)-1-(4-methylphenylsulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Solid. Anal. for C24H2gN307S2:
Calc'd: C,54.2; H,4.7; N,7.9;
Found: C,53.5; H,5.2; N,7.3.
Example 3
1-Methanesulfonyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Solid. Anal. for C 18H21 N307S2:
Calc'd: C,47.5: H,4.7; N,9.2;
Found: C,46.8; H,4.8; N,8.5.
Example 4
1,4-Bis-(4-Methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-
[l,4Jbenzodiazepine-3-carboxylic acid, Hydroxyamide
Solid. Anal. for C24H25N3~8S2:
Calc'd: C,52.6; H,4.6; N,7.7;
Found: C,52.2; H,4.8; N,7.3.
Example 5
1-Benzoyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
(1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
White solid. Anal. for C24H23N3~6S:
Calc'd: C,59.9; H,4.8; N,8.7.;
Found: C,59.2; H,4.6; N,8.6; S, 6.4;
Mass spectrum (ES) 482.3 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-95-
Example 6
1-Acetyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
White crystals. m.p. 195-197°C. Anal. for C19H21N306S:
Calc'd: C,54.4; H,S.1; N,10.0;
Found: C,52.6; H,4.9; N,9.4.
Example 7
4-(4-Methoxybenzenesulfonyl)-1-(3-pyridinylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
White crystals, m.p. 167-169°C. Anal. for C23H22N4O6S:
Calc'd: C,57.3; H,4.6; N,11.6;
Found: 55.3; H,4.6; N,10.6.
Example 8
4-(4-Methoxybenzenesulfonyl)-1-(2-thienylcarbonyl)-2,3,4,5
tetrahydro-lH-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
White solid. Anal. for C22H21 N306S2:
Calc'd: C,54.2; H,4.3; N,8.6;
Found: C,53.7; H,4.4; N,8.1.
Example 9
1-Methoxyacetyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
White crystals, m.p. 143-145°C. Anal. for C2pH23N3O7S:
Calc'd: C,53.4; H,5.2; N,9.4;
Found: C,53.9; H,5.6; N,8.5.
Example 10
4-(4-Methoxybenzenesulfonyl)-1-(propane-1-sulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Off white solid. Anal. for C2pH25N30'752:
Calc'd: C,49.7; H,5.2; N,8.7;
Found: C,48.9; H,5.3; N,8.4.
CA 02317546 2000-07-06
WO 99/37625 PCTNS99/01325
-96-
Example 11
4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-5-fluorobenzoyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Off white solid. Anal. for C25H24FN306S:
Calc'd: C,58.5; H.4.7; N,8.2;
Found: C,57.1; H,4.8; N,7.6.
Example 12
4-(4-Methoxybenzenesulfonyl)-1-(3-phenylpropionyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Solid. Anal. for C26H27N306S:
Calc'd: C,61.3; H,5.3; N,8.3;
Found: C,59.8; H.5.3; N,7.5.
Example 13
4-(4-Methoxybenzenesulfonyl)-1-(4-pyridinylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
White crystals, m.p. 155-165°C. Anal. for C23H22N406S:
Calc'd: C,57.3; H,4.6; N,11.6;
Found: C,56.8; H,4.9; N,10.9.
Example 14
1-([1,1'-Biphenyl]-2-carbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Purified by chromatography on silica gel thick layer plates with hexane-ethyl
acetate as solvent to give a white solid; m.p. 176-178°C. Anal. for
C3pH27N306S:
Calc'd: C,64.6; H,4.9; N,7.5;
Found: C,63.7; H,4.6; N,7.1.
CA 02317546 2000-07-06
WO 99/37625 PC'T/US99/01325
-97-
Example 15
1-(4-Biphenylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Purified by chromatography on silica gel thick layer plates with hexane-ethyl
acetate ( 1:1 ) as solvent to give a white solid, m.p. 160-168°C. Anal.
for
C30H27N306S:
Calc'd: C,64.6; H,4.9; N,7.5;
Found: C,61.2; H,4.9; N,7.0;
Mass spectrum (ES) 558.1 (M+H).
Example 16
1-(3-Fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro
1H-[l,4Jbenzodiazepine-3-carboxylic acid, Hydroxyamide
Example 17
4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-3-fluorobenzoyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 18
4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-3-trifluoromethylbenzoyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid,
Hydroxyamide
Example 19
1-(2-Chloro-6-trifluoromethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid,
Hydroxyamide
Example 20
1-(4-Fluoro-2-trifluoromethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-(1,4]benzodiazepine-3-carboxylic acid,
Hydroxyamide
Example 21
1-(2-Fluoro-6-trifluoromethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid,
Hydroxyamide
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-98-
Example 22
4-(4-Methoxybenzenesulfonyl)-1-(2-methylbenzoyl)-2,3,4,5-tetrahydro
1H-(1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 23
4-(4-Methoxybenzenesulfonyl)-1-(2-methyl-6-chlorobenzoyl)-2,3,4,5-
tetrahydro-tH-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 24
1-(2,4-Dimethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 25
1-(2,5-Dimethylbenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 26
1-(2-Chloro-4-fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 27
1-(2-Chlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro
1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 28
1-(2-Fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro
1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 29
1-(2-Chloro-6-fluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 30
1-(2,3-Difluorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-99-
Example 31
1-(2,4-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
white crystals, m.p. 158-162°C. Anal. for C24H21C12N306S:
Calc'd: C,52.4; H,3.9; N,7.6;
Found: C,52.1; H,3.8; N,7.5;
Mass spectrum (ES) 549.9 (M+H); 552.0 (M+H).
Example 32
IO 1-(2,3-Dichlorobenzoyl)-4-(4-methoxybenzenesulfon~yi)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 33
1-(2,5-Dichlorobenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 34
1-(2-Methoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 35
1-(4-Chloro-2-methoxybenzoyl)-4-(4-methoxybenzenesutfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, hydroxyamide
Example 36
4-(4-Methoxybenzenesulfonyl)-1-(2-methylthiobenzoyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 37
4-(4-Methoxybenzenesulfonyl)-1-(3-methyl-2-thienylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 38
4-(4-(Methoxybenzenesulfonyl)-1-(4-methyl-2-thienylcarbonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 100 -
Example 39
1-(3-Chloro-2-thienylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 40
1-(2-Furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
white solid. Anal. for C22H21N3~7s:
Calc'd: C, 56.0; H, 4.5; N, 8.9;
Found: C, 55.6; H, 4.8; N, 8.3;
Mass spectrum (ES) 472.0 (M+H).
Example 41
4-(4-Methoxybenzenesulfonyl)-1-(3-methyl-2-furanylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 42
4-(4-Methoxybenzenesulfonyl)-1-(4-methyl-2-furanylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 43
1-(5-Chloro-2-furanylcarbonyl)-4-(4-methoxybenzenesulfonyt)-2,3;4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 44
1-(5-Chloro-2-thienylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 45
1-Propionyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 46
1-Hexanoyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 101 -
Example 47
1-(3-Methoxypropionyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 48
4-(4-Methoxybenzenesulfonyl)-1-(3-thienylcarbonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 49
1-(3-Furanylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-(1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 50
1-(traps-Crotonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro
1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 51
1-(Methacryloyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H
[1,4)benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 52
1-(Acetylaminoacetyl)-4-(4-methoxybenzenesulfonyl}-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 53
1-(Aminoacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 54
1-(N,N-Dimethylaminoacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 102 -
Example 55
1-(Cyclopropylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
off-white solid. Anal. for C21 H23N306S:
Calc'd: C,56.6; H,5.2; N,9.4;
Found: C,55.1; H,5.2; N,8.8;
Mass spectrum (ES) 446.5 (M+H).
Example 56
1-(Cyclobutylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Example 57
1-(Cyclohexylcarbonyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
off white solid. Anal. for C24H29N3O6S:
Calc'd: C,59.1; H,6.0; N,8.6;
Found: C,58.0; H,6.0; N,8.1;
Mass spectrum (ES) 488.6 (M+H).
Example 58
4-(4-Methoxybenzenesulfonyl)-1-(phenoxyacetyl)-2,3,4,5-tetrahydro
1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxamide
A mixture of 0.70 g (1.37mmol) of methyl 4-(4-methoxybenzenesulfonyl)-1
(phenoxyacetyl)-2,3,4-S-tetrahydro-1H[1,4]benzodiazepine-3-carboxylate and 1.8
ml
( 1.78 mmol) of 1 N NaOH in 3 ml of tetrahydrofuran was stirred at room
temperature
for 2 hours. The mixture was diluted with 3 ml of H20 and acidified with 1N
HCl to
give a gummy solid. Ethyl acetate was added thereto and the mixture was
chilled
overnight. Filtration gave 4-(4-methoxybenzenesulfonyl)-1-(phenoxyacetyl)-
2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid as crystals, m.p.
188°-191°C.
To a 0.496 g (1 mmol) sample of the preceeding compound in 5 ml of CH2C12
cooled to 0°C, was added 1 ml (2 mmol) of oxalyl chloride followed by
the addition of
77.4 pl ( 1 mmol) of ~1,~T-dimethylformamide. The mixture was stirred at room
temperature under nitrogen for 1 hour (referred to as solution A). In a
separate flask
was added 0.278 g (4 mmol) of hydroxyamine hydrochloride, 0.5 ml of H20 and
836.3 pl (5 mmol) of triethylamine. The mixture was stirred for 20 minutes and
then
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 103 -
cooled to 0°C (referred to as solution B}. The cooled solution B was
added to the
cooled (0°C) and stirred solution A and then this mixture was allowed
to warm to room
temperature and was stirred overnight. The mixture was concentrated under
vacuum,
diluted with CH2C12 and washed with H20, 2N citric acid, 1 N NaHC03, and brine
and dried with Na2S04, The solvent was removed and the residue crystallized
from
hexane-ethyl acetate (3:97) to give 0.396 g of white crystals, m.p.
I59°-163°C. Anal.
for C25H25N30'7S:
Calc'd: C,58.7; H,4.9, N,8.2;
Found: C,58.4; H,5.1: N,7.8;
Mass spectrum (ES} 512 (M+H).
Example 59
1-Methoxyacetyl-4-(4-methoxybenzenesulfonyl)-7-methyl-2,3-4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, hydroxyamine
A mixture of 1.26 g (2.72 mmol) of methyl 1-methoxyacetyl-4-(4-
methoxybenzenesulfonyl)-7-methyl-2,3,4,5-tetrahydro-I H-[1,4]benzodiazepine-3-
carboxylate from Reference Example 181, 3.53 ml of 1N NaOH and 10 ml of
tetrahydrofuran was stirred at room temperature for 3 hours. The solvent was
then
removed under vacuum and the residue dissolved in H20 and extracted with ethyl
acetate. The aqueuous layer was acidified with IN HCl and then extracted with
CH2C12. The CH2C12 layer was dried over Na2S04 and the solvent removed to
provide a solid. This material was dried in a vacuum oven and given 1.06 of
solid,
m.p. 101-105°C.
A 1.02 g (2.27 mmol) sample of 1-methoxyacetyl-4.-(4-methoxy-
benzenesulfonyl)-7-methyl-2,3-4,5-tetrahydro-1H-[ 1,4]benzodiazepine-3-
carboxylic
acid prepared above was dissolved in 2.57 ml of 1N KOH. Toluene was added
several
times and the solvent was removed after each addition. The residue was dried
in a
vacuum oven to give 1.1 g of potassium salt. A mixture of 2.26 ml (4.52 mmol)
of
oxalyl chloride in 20 ml of CH2Cl2 was cooled at 0°C and 0.351 ml (4.52
mmol) of
N,N-dimethylformamide (DMF) was added dropwise. The mixture was stirred for 5
minutes and the potassium salt ( 1.1 g) was added. The mixture was allowed to
warm
to room temperature and was stirred for 2 hours under nitrogen. The mixture
was
cooled (0°C) and this mixture was added to a cooled (0°C)
mixture of 0.628 g (9.04
mmol) of hydroxylamine hydrochloride, 1.89 ml ( 13.56 mmol) of triethylamine
in 1 ml
of tetrahydrofuran-water (8:2). The mixture was stirred and chilled at
0°C for 10
minutes and then stirred at room temperature overnight. The solvent was
removed
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 104 -
under vacuum and the residue diluted with CH2C12-H20 and acidified with 2 N
citric
acid (pH 4). The CH2C12 layer was separated and washed with H20. 1N NaHC03.
H20, and brine and dried with Na2S04, The solution was filtered through a thin
pad
of hydrous magnesium silicate and the solvent removed under vacuum to give
0.73 g of
solid. Crystallization from ethyl acetate gave 0.32 g of crystals, m.p.
146°-148°C.
Example 60
1-Benzoyl-4-(4-methoxybenzenesulfonyl)-7-methyl-2,3,4,5-tetrahydro
1H[1,4]benzodianzepine-3-carboxylic acid, Hydroxyamide
In the manner described in Example 59, 0.83 g ( 1.71 mmol) of 1-benzoyl-4-(4-
methoxybenzene-sulfonyl)-7-methyl-2,3,4,5-tetrahydro-1 H[ 1,4]benzodianzepine-
3-
carboxylic acid from Reference Example 185 was converted to the potassium salt
with
1.87 ml of 1N KOH and the salt reacted with oxalyl chloride-DMF to give the
acid
chloride which was reacted with hydroxylamine. The solid from the reaction
gave from
CH2C12 0.20 g. of yellow solid, m.p. 137°-139°C.
Example 61
4-(4-Methoxybenzenesulfonyl)-1-[4-(trifluoromethoxy)benzoyl]-8
chloro-2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid,
Hydroxyamide
In the manner described for Example 59, the potassium salt was prepared from
1.20 g of 4-(4-methoxybenzenesulfonyl)-1-[4-(trifluoromethoxy)benzoyl)-8-
chloro-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid from Reference
Example
189, m.p. 184°-186°C and reacted with oxalyl chloride-DMF and
the acid chloride
reacted with hydroxylamine to give 1.20 g of solid. Chromatography on thick
layer
silica gel plates with ethyl acetate-methanol (95:5) gave 0.58 g of solid m.p.
134° dec;
Mass spectrum (ES) 601 (M+H).
Example 62
4-(4-Methoxybenzenesulfonyl)-1-(2-methoxyethyl)-2,3,4,5-tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
In the manner described for Example 1, 0.55 g of 4-(4-methoxy-
benzenesulfonyl )-1-(2-methoxyethyl)-2,3,4,5-tetrahydro-1 H-[ 1,4]-
benzodiazepine-3-
carboxylic acid from Reference Example 187 was reacted with oxalyl chloride
and the
resulting acid chloride reacted with hydroxylamine to 0.40 g of solid.
Chromatography
on thick layer silica gel plates with ethyl acetate-methanol (7:3) gave 0.150
g of product
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 105 -
as an off white foam; Mass spectrum (ES) 434.3 (M-H) Anal. for C2pH25N3O6S:
Calc'd: C,55.2; H,5.8; N,9.7;
Found: C,54.0; H,5:8; N,9.3.
Example 63
4-(4-Methoxybenzenesulfonyl)-1-[2- ( 1-
pyrazolyl)phenylcarbonyl]2,3,4,5-tetrahydro-1 H-[ 1,4] benzodiazepine-3
carboxylic acid, Hydroxyamide.
As described for the general reaction of ethyl 2-fluorobenzoate with amines
set
forth in Tetrahedron, 53, 7557-7576 ( 1997), ethyl 2-fluorobenzoate was
reacted with
pyrazole by refluxing N, N-dimethylformamide to give ethyl 2-( 1-
pyrazolyl)benzoate,
as a thick yellow oil. Anal. Calc'd: for C,2 H,2 N2O2: C, 66.7; H, 5.6; N
13:0: Found:
C, 66.5: H, 5.4: N, 12.9; Mass spectrum (ES) 217.2 (M+H). A sample (7.02g) of
this
compound and 8.42 ml of SN NaOH in 40 ml of ethanol-tetrahydrofuran (2:1) was
refluxed for 2 hrs and the solvent removed.The residue was made acidic (pH6)
with 2N
citric acid and the precipated solid was filtered to obtain 3.7g of product.
The pH of the
filtrate was adjusted to 4.5 and extracted with ethyl acetate. The extract was
concentrated to dryness to give l.Sg of product. The two crops were combined
to give
5.2g of 2-(1-pyrazolyl)benzoic acid, mp 140-142°C. To the preceding
compound
(2.07 g) in 5 ml CH2C12 (chilled in an ice bath )was added 11.1 ml of a 2
Molar
solution of oxalyl chloride in CHZCh and 0.085 ml of N,N-dimethylformamide.
The
mixture was allowed to warm to room temperature and stirred for 4 hours. The
solvent
was removed and 25 ml of toluene added {twice) and removed under vacuum to
give 2-
(1-pyrazolyl)benzoyl chloride as a yellow solid.
A 2.3 g sample of the preceding compound was reacted with l.Sg of the
compound of Reference Example 179 in 15 ml of CH2Cl2 and 5.12 ml of
triethylamine
in the manner described for Reference Example 181 to give methyl 2-[ (4-
methoxybenzenesulfonyl)-{2-[2-(1-pyrazolyl)phenylcarbonyl]amino-5-
methylbenzyl}-
amino]acrylate. This compound was cyclized with NaHC03 in methanol in the
manner
described in Reference Example 181 to give methyl 4-(4-methoxybenzenesulfonyl)-
1-
[2-( 1-pyrazolyl)phenylcarbonyl]-7-methyl-2, 3,4,5-tetrahydro-1 H-[
1,4]benzodiazepine-
3-carboxylate, m.p. 240-242° C.
A 1.16 g sample of the preceding compound was hydrolysed with 2.69 ml of
1N NaOH in 10 ml of tetrahydrofuran in the manner described for Reference
Example
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 106 -
104 to give 0.71 g of 4-(4-methoxybenzenesulfonyl)-1-[2-(1-pyrazolyl)phenyl-
carbonyl)-7-methyl-2,3,4,5-tetrahydro-1H(1,4]benzodiazepine-3- carboxylic
acid, mp
149-151 °C.
In the manner described in Example 59, 1.1 g of the preceding compound was
converted to the potassium salt and reacted with oxalyl chloride and then
hydroxylamine
to give the above-identified product as white crystals, mp 194-196°C.
Example 64
4-(4-Methoxybenzenesulfonyl}-1-[2-(4-
morpholino)phenylcarbonyl}-8-chloro-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
Ethyl 2-morpholinobenzoate prepared in the manner described in Tetrahedron,
53:7557, (1997) was refluxed with 10 N NaOH in tetrahydrofuran-ethanol (8:2)
for 1.5
hrs to give 2-morpholinobenzoic acid, mp 156-157°C. A 1.8 g sample of
this
compound in S ml of CH2C12 (chilled) was added a solution of 7.9 ml of oxalyl
chloride
in CH2Cl2 (2M) followed by the addition of 0.058 ml of N, N-dimethylformamide.
The solution was stirred at room temperature for 6 hrs and the solvent
removed.
Toluene was added (2 times) and removed to give 2-(4- morpholino)benzoyl
chloride as
a yellow solid.
In the manner described in Reference Examples 181 and 189, the preceding 2-
(4-morpholino)benzoyl chloride was reacted with methyl 2-[(2-amino-4-
chlorobenzyl)-
(4-methoxybenzenesulfonyl)amino]-3-hydroxypropionate and the product was
stinred
with NaHCOg in methanol to give methyl 4-(4-methoxybenzenesulfonyl)-1-[2-(4-
morpholino)phenylcarbonyl]-8-chloro-2,3,4,5-tetrahydro-1H-[ 1,4]benzodiazepine-
3-
carboxylate, as a white solid having a mp 100-105°C.
To 0.90g of this compound in 10 ml of tetrahydrofuran was added 1.95 ml of 1
~1 NaOH and the solution was stirred at room temperature overnight.
Acidification with
2~T citric acid gave 0.82 g of solid, mp 136-143°C. This compound, 4-(4-
methoxybenzenesulfonyl)-1-[2-(4-morpholino)phenylcarbonyl]-8-chloro-2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid (0.78g) was converted to
the
potassium salt and reacted first with oxalyl chloride and then with
hydroxylamine as
described in Example 63 to give 0.276g of product as a light yellow solid, mp
132° C.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 107 -
Example 65
1-(4-Ethoxybenzoyl)-4-(4-methoxybenzenesulfonyl) -2,3,4,5-
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
A mixture of 0.270 g of methyl 4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro- I H-( 1,4] benzodiazepine-3-carboxylate of Reference Example 12,
0.291 g
of 4-ethoxybenzoyl chloride and 500 E.11 of triethylamine in 5 ml of CH2Ch was
stirred
at room temperature overnight. The mixture was diluted with CH~Ch and H20 and
the
CH2Cl2 layer was separated and concentrated to dryness. The residue was
triturated
with ethyl acetate to give 0.2768 of methyl 1-(4-ethoxybenzoyl)-4-(4-
methoxybenzene-
sulfonyl)-2,3,4,Stetrahydro-1H-[1,4]benzodiazepine-3-carboxylate as white
crystals,
mp 187-190°C.
A 0.47 g sample of this compound was hydrolyzed with 1.2 ml of IN NaOH in
4 ml of tetrahydrofuran. Dilution with H20 and acidification with 1N HCl gave
0.40 g
of the acid as a white solid, mp 144-152°C. The preceding compound
(0.358) was
converted to the above-titled compound in the manner described in Example 1 to
provide 0.1958 of solid, mp 136-142°C.
Example 66
4-(4-Methoxybenzenesulfonyl) -1-[2-chloro-4-(3-methyl-1-
pyrazolyl)phenylcarbonyl}-2,3,4,5-tetrahydro -1H-
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
As described in Example 65, methyl 4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-lH-[1,4]benzodiazepine-3-carboxylate was reacted with 4-(3-methyl-1
pyrazolyl)-2-chlorobenzoyl chloride to give methyl 4-(4-
methoxybenzenesulfonyl)-1
[2-chloro-4-( 3-methyl-1-pyrazolyl)phenylcarbonyl]-2,3,4,5-tetrahydro-1 H-[
1,4Jbenzo-
diazepine-3-carboxylate as a white solid. Anal. for C29H27C1N4O6S:
Calc'd: C, 58.3; H, 4.6; N, 9.4.
Found: C,58.2; H, 4.9; N, 8.9.
This compound was hydrolysed with 1 N NaOH in tetrahydrofuran as described
in Reference Example 185 to give the benzodiazepine-3-carboxylic acid
derivative as a
white solid. This compound was reacted with oxalyl chloride and then reacted
with
hydroxylamine as described in Example I to give the product as white crystals,
mp
189-191°C.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 108 -
Example 67
1-Benzyl-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
[1,4]benzodiazepine-3-carboxylic acid, Hydroxylamide
A mixture of 1.7 g of the compound of Reference Example 45 and 25 ml of
S borane in tetrahydrofuran ( 1.0 Molar) was refluxed under nitrogen
overnight. To the
solution was added 5 ml of CH30H, CH2Cl2 (40 ml) and 30 ml of 2N HCl and the
mixture stirred at room temperature for 1.5 hr. The organic layer was
separated,
washed with brine, dried with Na2S04 and the solvent removed. The residue was
crystallized from ethanol-hexane to give 1.15g of methyl 1-benzyl-4-(4
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro- 1 H-[ 1,4]benzodiazepine-3-
carboxylate as
white crystals, mp 120-122°C. A sample ( 1.0 g) of this compound was
hydrolysed
with 2.8 ml of 1 N NaOH in 7 ml of tetrahydrofuran as described in Reference
Example
104 to give 0.64 g of the 2,3,4,5- tetrahydro-1H-[1,4]benzodiazepine-3-
carboxylic acid
derivative as white crystals, mp 183-185°C.
A 0.55 g sample of this compound was converted to the acid chloride which
was reacted with hydroxylamine as described in Example 1 to give the product
as a light
brown foam; Mass spectrum (ES) 468.1 (M +H).
Utilizing the procedure described in Example 65 above, the following
compounds may be prepared.
Example 68
4-(4-Methoxybenzenesulfonyl)-1-(4-(2-thienyl)phenyl-carbonyl)
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid,
Hydroxyamide
Example 69
4-(4-Methoxybenzenesulfonyl)-1-(4-(3-thienyl)phenyl-carbonyl)
2,3,4,5-tetrahydro-1H-[1,4)benzodiazepine-3-carboxylic acid,
Hydroxyamide
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 109 -
Example 70
4-(4-Methoxybenzenesulfonyl)-1-[2-(3-pyrazol)phenyl-carbonylJ
2,3,4,5-tetrahydro-1H-[l,4Jbenzodiazepine-3-carboxylic acid,
Hydroxyamide
Example 71
1-(2,4-Dimethoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-
tetrahydro-1H-[l,4Jbenzodiazepine-3-carboxylic acid, Hydroxyamide
To a cooled (0°C) solution of 1.0 g (2.66 mmol) of 4-(4-
methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H-[ 1,4]benzodiazepine-3-
carboxylate
from Reference Example 12 and 1.85 ml ( 13.3 mmol) of triethylamine in 8 ml of
CH2Cl2 was added 1.17 g (6.65 mmol) of 2,4-dimethoxybenzoyl chloride. The
mixture was stirred at room temperature overnight, diluted with CH2CI2 and
washed
with 2 N citric acid. The organic layer was washed with H20, 1 N Na2C03, brine
and
dried over Na2S04. The solvent was removed and the residue was chromatographed
on thick layer silica gel plates with ethyl acetate-hexane ( 1:1 ) as an
eluent to give 1.0 g
of methyl 1-(2,4-dimethoxybenzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[l,4Jbenzodiazepine-3-carboxylate as a white foam. Anal. for
C2~H2gH20gS:
Calc'd: C,60.0; H,5.2; N,5.2;
Found: C,60.0; H,5.2; N,5.1;
Mass Spectrum (ES): 541.0 (M+H).
A 0.80 g ( 1.48 mmol) sample of the preceding compound and 1.92 ml ( 1.92
mmol) of 1 N NaOH in 5 ml of tetrahydrofuran was stirred at room temperature
for 1.5
hours. The solvent was removed and the residue diluted with water. The
solution was
acidified with 1 N HCI, chilled and filtered to give 0.70 g of 1-(2,4-
dimethoxy-
benzoyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro-1 H-[
1,4]benzodiazepine-
3-carboxylic acid as a white solid. Anal. for C26H26N208S:
Calc'd: C,59.3; H,5.0; N,5.3;
Found: C,56.1; H,4.8; N,5.0;
Mass Spectrum (ES): 527.0 (M+H).
A 0.80 g ( 1.52 mmol) sample of the preceding compound in 10 ml of CH2Cl2
(chilled to 0°C) was added to 1.52 ml (3.04 mmol) of oxalyl cholride
(2.0 M solution in
CH2Cl2). To the solution was added 118 pl ( 1.52 mmol) of N,N-
dimethylformamide
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 110-
and the solution stirred at 0°C for 1.5 hours (Mixture A). A mixture of
0.422 g (6.08
mmol) of hydroxylamine hydrochloride, 1.27 ml (9.14 mmol) of triethylamine, 5
ml of
N,N-dimethyformamide and 0.5 ml of water was prepared in a separate flask,
stirred
for 20 minutes at room temperature and then cooled to 0°C in an ice
bath (Mixture B).
S The cooled solution of Mixture A was added to the cooled Mixture B and then
stirred at
room temperature overnight. The mixture was diluted with CH2C12 and 2 N citric
acid
added. The organic layer was separated, washed with H20, brine and dried with
NaZS04. The solvent was removed and the residue crystalized from ethanol to
give
0.40 g of product as white crystals, mp 189-191°C. Anal. for
C26H2~N30gS:
Calc'd: C,57.7; H,5.0; N,7.7;
Found: C,57.6; H,4.9; N,7.7;
Mass Spectrum (ES): 542.2 (M+H).
Example 72
4-(4-Methoxybenzenesulfonyl)-1-[2-(4-methylpiperazin-1-yl)acetyl]-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid,
Hydroxyamide
To a mixture of 2.5 g (6.64 mmol) of methyl 4-(4-methoxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate (Reference Example 12)
and
4.63 ml (33.2 mmol) of triethylamine in 40 ml of CH2C12 cooled to 0°C
was added to
1.65 g ( 14.63 mmol) of chloroacetyl chloride. The solution was stirred at
room
temperature for 2 days, chilled to 0°C and 926 ~.~1 of triethylamine
and 750 mg of
chloroacetyl chloride were added thereto. The mixture was stirred at room
temperature
overnight, diluted with CH2C12 and H20. The insoluble solid was filtered off.
The
organic layer of the filtrate was separated , washed with brine, dried with
Na2S04 and
filtered through diatomaceous earth. The solvent was removed and the residue
triturated with ethyl acetate and a trace of ethanol. Chilling and filtering
gave 0.75 g of
methyl 1-(chloroacetyl)-4-(4-methoxybenzenesulfonyl)-2,3,4,5-tetrahydro- 1 H-[
1,4J-
benzodiazepine-3-carboxylate (Reference Example 91 ). Anal. for CZpH2 i
CIN206S:
Calc'd: C,53.0; H,4.7; N,6.2;
Found: C,51.6; H,4.6; N,5.7;
Mass Spectrum (ES): 453.0 (M+H).
To a solution of 1.4 g (3.09 mmol) of the preceding compound in 12 ml of
CH2C12 cooled to 0°C was added 1.2 ml (6.79 mmol) of N,N-
diisopropylethylamine
followed by the addition of 753.2 Irl (6.79 mmol) of 1-methylpiperazine. The
mixture
was stinted at room temperature overnight, diluted with CH2Cl2, and washed
with 2 N
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
-111-
citric acid, H20, 1 M NaHC03, brine and dried (Na2S04). The citric acid wash
was
made basic with saturated NaHC03 and then extracted with CH2C12. The extract
was
dried over Na2S04 and the solvent removed under vacuum to give 1.10 g of
methyl 4-
(4-methoxybenzenesulfonyl)-I-[2-(4-methylpiperazin-1-yl)acetyl]-2,3,4,5-
tetrahydro-
1H-[1,4]benzodiazepine-3-carboxylate as a white glass.
A mixture of 1.0 g ( 1.94 mmol) of the preceding compound and 2.3 ml (2.3
mmol) of 1 N KOH in 5 ml of methanol was stirred at room temperature for 2
hours.
The solvent was removed under vacuum. To the residue was added toluene (2
times)
and the solvent removed under vacuum after each addition. The solid was dried
at 65°C
under vacuum for 6 hours to give 1.1 g of potassium 4-(4-
methoxybenzenesulfonyl)-1-
[2-(4-methylpiperazin- I -yl)acetyl]-2,3,4, 5-tetrahydro-1 H-[
1,4]benzodiazepine-3-
carboxylate as a white solid.
To 1.85 mI (3.69 mmol) of a 2.0 molar solution of oxalyl chloride in CH2C12,
cooled to 0°C, was added slowly 286 Erl (3.69 mmol) of N,N-
dimethylformamide
(precipitate formed). To this stirred mixture was 1.0 g ( 1.85 mmol) of the
preceding
compound in 5 ml of CH2C12. The mixture was stirred under nitrogen for two
hours
(Mixture A).
In a separate flask, a mixture of 0.514 g (7.4 mmol) of hydroxylamine
hydrochloride, 1.55 ml ( 11.1 mmol) of triethylamine in tetrahydrofuran-water
(4:1 )
was stirred at room temperature and then cooled to 0°C and stirred for
5 minutes. To
this mixture was added the cooled (0°C) Mixture A and then the
resulting solution
stirred at room temperature overnight. The mixture was concentrated under
vacuum
and CH2Cl2 added. The organic layer was separated and concentrated to dryness
to
give 1.4 g of product. The product was chromatographed on thick layer silica
gel plates
with CH2C12-CH30H-NH40H(45:6:1 ) as a solvent to give 65 mg of brown solid:
Mass Spectrum (ES): 518.3 (M + H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 112 -
Example 73
4-[4-(4-Chlorophenyloxy)benzenesulfonyl]-1-(methoxyacetyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
A. N-f4-(4-Chlorophenyloxvbenzenesulfonvl)serine. methyl ester (methyl-
3-hydroxy-2-f4-(4-chloro hp enoxv)benzenesulonylaminolpro in onate~. To a
mixture of
3.42 g (22 mmol) of serine, methyl ester, hydrochloride and 10.7 ml (77.0
mmol) of
triethylamine in 60 ml of CH2C12, chilled to 0°C, was added 6.063 g (20
mmol) of 4-
(4-chlorophenyloxy)benzenesulfonyl chloride. The mixture was stirred at room
temperature overnight, diluted with CH2C12 and washed with 2 N citric acid,
H20, 1 N
NaHC03, brine and dried with Na2S04. The solvent was removed to give an oil
which
was dried under vacuum at 68°C to give a solid. Trituration with hexane-
ethyl acetate
gave 5.85 g of off white crystals, mp 90-94°C. Anal. for C16H16C1N06S:
Calc'd: C,49.8; H,4.2; N,3.6;
Found: C,50.1; H,4.1; N,3.8;
Mass Spectrum (ES): 385.9 (M+H).
B. Met yl 3-hyd, roxy-2-f f4-(4-chloronhen~y)benzene-sulfony1112-
nitrobenzrLl)amino}rro nn 'ovate. To a cooled (0°C) solution of 5.5 g
(14.76 mmol) of
compound from part A in 60 ml of dry N,N-dimethylformamide was added
(portionwise), 0.682 g ( 17 mmol) of sodium hydride (60% in oil). After gas
evolution
ceased, 3.7 g ( I 7 mmol) of 2-nitrobenzylbromide in 15 ml of N,N-
dimethylformamide
was added slowly. The mixture was stirred at room temperature overnight and
diluted
with 200 ml of ethyl acetate and 150 ml of water. The organic layer was
separated and
washed with H20, brine and dried with Na2S04. The solvent was removed under
vacuum and the residue chromatographed on a silica gel column with hexane-
ethyl
acetate (2:1) as an eluent to give 4.7 g of a brown oil. Anal. for
C23H21C1NZOgS:
Calc'd: C,53.0; H,4.1; N,5.4;
Found: C,53.2; H,4.2; N,5.1;
Mass Spectrum (ES): 521.2 (M+H).
C. Mme'~,Y12-{,(2-aminobenzyly-[4-(4-chlorQphenyloxy)benzene-
~ulfon lv laming-3-~,ydro~ynropionate. A mixture of 3.0 g (5.77 mmol) of the
compound from part B and 0.300 g of 10% wet palladium on carbon (50% in H20)
in
300 ml of ethyl acetate-ethanol ( 1:1 ) was shaken in a Parr hydrogenator
under 35 psi of
hydrogen for 4 hours. The mixture was filtered through diatomaceous earth and
the
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 113 -
solvent removed under vacuum. The residue was dried at 65°C under
vacuum to give
2.63 g of an off white solid. Anal. for C23H23C1N206S:
Calc'd: C,56.3; H,4.7; N,5.7;
Found: C,56.6; H,4.6; N,5.6;
Mass Spectrum (ES): 491.1 (M+H).
D. yl 2-(f4-(4-chloropheny c~ylbenzenesulfon
y.~[2-(metho~~
ace lamino)benzyllamino )~ acrvlate. To a mixture of 0.80 g ( 1.63 mmol) of
the
compound from Part C and 1.14 ml (8.15 mmol) of triethylamine in 8 ml of
CH2C12,
cooled to 0°C, was added 328 lrl (3.58 mmol) of methoxyacetyl chloride.
The mixture
was stirred at room temperature overnight, diluted with CH2CI2 and washed with
H20,
2 N citric acid, brine and dried (Na2S04). The solvent was removed under
vacuum and
the residue chromatographed on thick layer silica gel plates with hexane-
ethylacetate
(2:1 ) as a solvent to give 0.48 g of a white foam. Anal. for C26H25C1N20~S:
Calc'd: C,57.3; H,4.6; N,5.1;
Found: C,56.?; H,4.7; N,5.0;
Mass Spectrum (ES): 545.2 (M+H).
E. Methyl 4-f4-(4-chlorophen loxy)benzenesulfonvll-1-(methox3r~ce~rl)-
2.3.4.5-tetrah~rdro-1H-[1.41benzodiazenine-3-carboxylate. A mixture of 0.45 g
(0.827
mmol) of the compound from part D and 0.09 g of anhydrous NaHC03 in 5 ml of
dry
methanol was stirred at room temperature overnight. The solvent was removed,
ethyl
acetate added and the mixture washed with H20, brine and dried with Na2S04.
The
solvent was removed to give 0.43 g of an off white solid. Anal. for
C26H25C1N20~S:
Calc'd: C,57.3; H,4.6; N,5.1;
Found: C,57.6; H,4.6; N,5.0;
Mass Spectrum (ES): 545.2 (M+H).
F. 4-f(4-Chlorophenyloxy)benzenesulfon l~methoxvacetyl)-2 3 4 5-
~ydro-1H-f 1.4]benzodiaz~ine-3-carboxylic acid. A mixture of 0.52g (0.956
mmol) of the compound from Part F and 1.2 ml ( 1.2 mmol) of 1 N KOH in 8 ml of
methanol was stirred at room temperature for 2 hours. An additional 0.6 ml of
1N KOH was added and the mixture was stirred at room temperature overnight.
The
mixture was concentrated, diluted with H20 and extracted with ethyl acetate.
The
extract was washed with brine and dried over Na2S04. The solvent was removed
and
the product dried at 65°C under vacuum to give 0.49 g of off white
foam. Anal. for
C25H23C1N20~S:
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 114 -
Calc'd: C,56.6; H,4.4; N,5.3;
Found: C,56.6; H,4.3; N,5.0;
Mass Spectrum (ES): 531.2 (M+H).
To a solution of 0.45 g (0.848 mmol) of the compound from Part F in 4 ml of
CH2C12 cooled to 0°C was added 850 Nl ( 1.69 mmol) of oxalyl chloride
(2.0 molar
solution in CH2Cl2) and then 50.2 pl (0.848 mmol) of N,N-dimethylflormamide.
This
mixture was stirred under nitrogen for 2 hours (Solution A). In a separate
flask a
mixture of 2.12 g (5.0 mmol) of hydroxylamine hydrochloride, 1.07 ml (7.65
mmol)
of triethylamine, 4 ml of tetrahydroforan and 0.4 ml of H20 was stirred for 15
minutes
and cooled to 0°C. To this mixture was added the cooled (0°C)
Solution A and the
mixture was stirred at room temperature overnight. The mixture was
concentrated
under vacuum, diluted with ethyl acetate and washed with H20, 1N NaHC03 2 N
citric
acid, brine and dried over Na2S04. The solvent was removed under vacuum and
the
residue chromatographed on thick layer silica gel plates with 2% methanol in
ethyl
acetate to give 0.20 g of the product of the Example as a brown solid. Anal.
for
C25H24C1N30~S:
Calc'd: C,55.0; H,4.4; N,7.7;
Found: C,53.1; H,5.0; N,6.7;
Mass Spectrum (ES): 546.3 (M+H).
Example 74
4-[4-(4-Chlorophenyloxy)benzenesulfonylJ-1-(2-thienylcarbonyl)
2,3,4,5-tetrahydro-1H[l,4Jbenzodiazepine-3-carboxylic acid,
Hydroxyamide
The following reactions were carried out in the manner described for Example
73, Parts D, E, and F. A 1.4 g (2.85 mmol) sample of methyl 2-{(2-aminobenzyl)-
[4-
(4-chlorophenyloxy)benzenesulfonyl]amino}-3-hydroxypropionate (the compound of
Part C of Example 73) was reacted with 1.25 g (8.55 mmol) of 2-
thiophenecarbonyl
chloride to give 1.7 g of methyl 2-{ [4-(4-chlorophenyloxy)benzyenesulfonyl]-
[2-(2-
thienylcarbonyl-amino)benzyl]amino } acrylate as a yellow oil.
Mass Spectrum (ES): 583.1 (M+H).
The reaction of 1.5 g of the preceding compound with 0.251 g of NaHC03 in 8
ml methanol gave 1.6 g of methyl 4-[4-(4-chlorophenyloxy)benzenesulfonyl)-1-(2-
thienylcarbonyl)-2,3,4,5-tetrahydro-lH-[1,4]benzodiazepine-3-carboxylate as a
yellow
oil.
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 115 -
Mass Spectrum (ES): 583.1 (M+H).
The hydrolysis of 1.5 g of the preceding compound with 3.3 ml of 1 N NaOH
in 6 ml of tetrahydroforan gave 1.2 g of 4-[4-(4-
chlorophenyloxy)benzenesulfonyl]-I-
(2-thienylcarbonyl)-2,3,4,5-tetrahydro-IH-[1,4]benzodiazepine-3-carboxylic
acid as an
off white foam. As described for Example 73, 1.0 g of the preceding
benzodiazepine-
3-carboxylic acid was reacted with oxalyl chloride and then with hydroxylamine
to give
the product of the Example as a solid (off white foam).
Mass Spectrum (ES): 584.2 (M+H)
Example 75
4-[4-(4-Chlorophenyloxy)benzenesulfonyl]-1-(benzoyl)-2,3,4,5
tetrahydro-1H[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
The following reactions were carried out in the manner described for Example
73, Parts D, E,.and F. A 1.0 g (2.04 mmol) sample compound C of Example 73 was
reacted with 710 Nl (6.10 mmol) of benzoyl chloride to give I.25 g of methyl 2-
{[4-(4-
chlorophenyloxy)benzenesulfonyl]-[2-(benzoylamino)benzyl]amino}acrylate as a
brown oil.
Mass Spectrum (ES): 577.2 (M+H).
The reaction of 1. I g ( 1.9 mmol) of the preceding compound with 0.208 g
(2.48 mmol) of NaHC03 in 8 ml of methanol gave 1.1 g of methyl 4-[4-(4-
chlorophenyloxy)benzenesulfonyl]-1-(benzoyl)-2,3,4,5-tetrahydro-1 H-
[I,4]benzodiazepine-3-carboxylate as a brown oil.
Mass Spectrum (ES): 577.1 (M+H).
A 1.0 g ( 1.73 mmol) sample of the preceding compound was hydrolysed with
2.3 ml (2.75 mmol) of 1 N NaOH in 5 ml of tetrahydrofuran to give 0.50 g of 4-
[4-(4-
chlorophenyloxy)benzenesulfonyl]-1-(benzoyl)-2,3,4,5-tetrahydro-IH-
[1,4]benzodiazepine-3-carboxylic acid as a white foam. As described for
Example 73,
0.460 g (0.817 mural) of the preceding benzodiazepine-3-carboxylic acid was
reacted
with oxalyl chloride and then with hydroxylamine to give 0.04 g of the product
of the
Example as a light brown solid.
Mass Spectrum (ES): 578.2 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 116-
Example 76
4-[4-(4-Pyridinyloxy)benzenesulfonyl]-1-(methoxyacetyl)-2,3,4,5-
tetrahydro-1H[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
A. To a cooled mixture of 6.84 g (44 mmol) of D, L-serine, methyl ester
hydrochloride and 21.4 ( 144 mmol) of triethylamine in 90 ml of CH2C12 was
added a
solution of 10.78 g (40 mmol) of 4-(4-pyridinyloxy)benzenesulfonyl chloride in
50 ml
of CH2C12. The mixture was stirred at room temperature overnight, diluted with
50 ml
of CH2CI2 and the solution washed with H20, 1N NaHC03, 2 N citric acid, brine
and
dried (with Na2S04). The solvent was removed under vacuum to give a solid. The
aqueous 2 N citric acid wash was made basic with saturated NaHC03 and then
extracted with CH2Cl2. The solvent was removed to give a solid. The two crops
of
solid were combined, washed with H20 and then hexane. The solid was dried at
80°C
to give 10.95 g of methyl 3-hydroxy-2-[4-(4-pyridinyloxy)-
benzenesulfonylamino]
propionate as white crystals, mp. 137-139°C.
B . To a solution of 4.5 g ( 12,.78 mmol) of the product from Part A in 35
ml of dry N,N-dimethylformanude cooled to 0°C was added (portionwise)
0.662 g
(16.61 mmol) of NaHCOg (60% in oil). The mixture was stirred 15 minutes and
3.59
g ( 16.61 mmol) of 2-nitrobenzylbromide in 1 S ml of N,N-dimethylfonmamide was
added hereto. The mixture was stirred at room temperature overnight, diluted
with
ethyl acetate (200 ml) and H20 (100 ml). The organic layer was separated and
washed
with H20, brine and dried (with Na2S04). The solvent was removed to give 5.9 g
of
solid. Column chromatography on silica gel with ethyl acetate-hexane ( 10:1 )
as an
eluant gave 1.4 g of methyl 2-{(2-nitrobenzyl)-[4-(4-pyridinyloxy)
benzenesulfonyl]-
amino}-3-hydroxypropionate as an off white solid.
Mass Spectrum (ES): 488.1 (M+H).
The compound from Part B was converted to the product of the Example in the
manner described for Example 73 in Parts D, E, and F.
CA 02317546 2000-07-06
WO 99/37625 PCTIUS99/Ot3Z5
- 117 -
Example 77
1-(Benzoyl)-4-(4-pentyloxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H
[1,4]-benzodiazepine-3-carboxylic Acid, Hydroxyamide
To a stirred solution of 1.24 g (4.82 mmol) of triphenylphosphine in 12 ml of
toluene and 3 ml of N,N-dimethylformamide was added 524 ~tL (4.82 mmol) of I-
pentanol and 1.5 g (3.22 mmol) of methyl I-(benzoyl)-4-(4-
hydroxybenzenesulfonyl)-
2,3,4,5-tetrahydro-1H-[1,4]benzodiazepine-3-carboxylate. To this stinred
mixture was
added 259 l,tL (4.82 mmol) of diethyl azodicarboxylate and the mixture was
stirred
overnight. The solvent was removed and the residue chromatographed on silica
gel
with ethyl acetate-hexane ( 1:3) as solvent. Concentration of the fractions
containing
product gave 1.59 g of a white solid; mp 170-172°C; Anal. Calcd for
C29H32N2O6: C,
64.9; H, 6.0; N, 5.2. Found: C, 64.7; H, 6.0; H, 5.4.
A mixture of a 1.4 g (2.61 mmol) sample of the preceding compound and 3.4
ml (3.4 mmol) of 1N KOH in 7 ml of tetrahydrofuran was stirred at room
temperature
for 2 hrs and the solvent removed under vacuum. To the residue was added
toluene
and the solvent removed (repeated two times). The residue was dried at
85°C under
vacuum overnight to give 1.5 g of 1-(benzoyl)-4-(4-pentyloxybenzenesulfonyl)-
2,3,4,5- tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid as the potassium
salt.
To 10 ml of CH2Cl2 was added 4.8 ml (9.6 mmol) of oxalyl chloride in CH2CI2
(2.0
molar) and the solution chilled to 0°C. To the chilled solution was
added 740 N.L, (9.56
mmol) of N,N-dimethylformamide and 1.34g (2.39 mmol) of the preceding
potassuim
salt in 5 ml of dry N,N-dimethylformamide. The mixture was stirred at room
temperature for 1.5 hr, cooled to 0°C ,and added to a chilled
(0°C) solution of 2.2 ml
(35.9 mmol) of 50% aqueous hydroxylamine in 10 ml of tetrahydrofuran. The
mixture
was stirred at room temperature overnight and diluted with CH2Cl2. The CH2Cl2
layer
was separated, washed with H20 and concentrated to dryness under vacuum. The
residue was chromatographed on silica gel with ethyl acetate-hexane ( 1:1 ) as
solvent.
Fraction containing product was concentrated to dryness and the residue
dissolved in
ethyl acetate. The solution was washed with three times with H20 and once with
brine
and dried (Na2S04). The solvent was removed and the residue dried at
85°C under
vacuum overnight to give 0.96 g of product as a white foam; Mass spectrum (ES)
538.0 (M+H).
CA 02317546 2000-07-06
WO 99/37625 PCT/US99/01325
- 118 -
Example 78
1-Acetyl-4-(4-Hydroxybenzenesulfonyl)-2,3,4,5-tetrahydro-1H-
[1,4]benzodiazepine-3-carboxylic acid, Hydroxyamide
To a crude mixture of 1-acetyl-4-(4-hydroxybenzenesulfonyl)-2,3,4,5
tetrahydro-1H-[1,4]benzodiazepine-3-carboxylic acid (O.SSg) ~ and N-hydroxy
benzotriazole (0.414g) in 5 ml of N,N-dimethylformamide was added 0.684 g of 1-
[3
(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride. The mixture was
stirred at
room temperature for 1 hr and then 750 ~,L of hydroxylamine in water (50%) was
added and the mixture stirred at room temperature overnight. The mixture was
diluted
with ethyl acetate and then washed with H20, 2N citric acid, brine and dried
(Na~S04).
The solvent was removed under vacuum to give a solid. Chromatography on silica
gel
with 10% methanol in ethyl acetate as solvent gave a solid which was dried at
78°C
under vacuum overnight to give an off white foam; Mass spectrum (ES) 406.1
(M+H);
Anal. Calcd. For C,gH,9N306S; C, 53.3; H, 4.7; N, 10.4. Found: C, 52.6; H,
5.2; N,
10.4.
The present invention may be embodied in other specific forms without
departing from the spirit or essential attributes thereof and, accordingly,
reference
should be made to the appended claims, rather than to the foregoing
specification, as
indicating the scope of the invention.