Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02386069 2008-03-04
Indolyl-3-Glyoxylic Acid Derivatives as Antitumor Agents
Technical Field
The invention relates to indole-3-glyoxylamide acid
derivatives and uses thereof.
Background of the Invention
In connection with chemotherapy, in oncoses the
greatest problems result due to the occurrence of
pharmaceutical resistance on the one hand and due to the
serious side effects of these agents on the other hand.
It is furthermore known that many primary tumors,
after reaching a certain size, have a premature tendency
to form metastases via the bloodstream and lymph tracts.
The continuing process of tumor invasion and the formation
of metastases is the most frequent cause of death of
cancer patients.
There are various starting points to explain this
spread, inter alia intensified angiogenesis, increased
extracellular matrix degradation, tumor cell migration and
modulation of cell adhesion. These factors can also act in
combination, but until now have been only partially
explained.
The metastasis of a tumor is usually accompanied by
poor prognoses in the tumor treatment. The prerequisite
for metastasis is the detachment of cells from the primary
tumor, the migration of the cells to the blood vessels,
the invasion into the blood vessels and the invasion of
the cells from the blood vessels into other tissue.
An inhibitory action of certain antitumor agents such
as tamoxifen on the migration and invasion of cancer cells
is known [J Clin Endocrinol Metab 1995 Jan;80(1) :308-13].
The inhibition of tumor cell invasion by verapamil
has been reported [Pigment Cell Res 1991 Dec; 4 (5-6)
:225-33.]
CA 02386069 2008-03-04
2 -
The influence of melatonin on invasive and metastatic
properties of MCF-7 human breast cancer cells has been
reported [Cancer res 1998 Oct 1; 58 (19) :4383-901.
The published PCT Application WO 96/23506 demonstrated
the overcoming of pharmaceutical resistance with certain
tumor pharmaceuticals as a result of the gene amplification
of the multi-drug resistance gene (MDR gene) brought about by
such antitumor agents. Antitumor agents such as vincristine
and taxol furthermore have a not inconsiderable
neurotoxicity, which proves disadvantageous in chemotherapy.
Summary of the Invention
The object of the invention is now to widen the area of
use of N-substituted indole-3-glyoxylamides and thus to
enrich the available pharmaceutical wealth. The possibility
of a lower, longer-lasting and more tolerable medication for
the class of substance having antitumor action described in
German Patent Application 19814 838.0, published October 14,
1999, should thereby be opened up. In particular, the
disadvantageous development of resistance, such as is known
of many antitumor agents, should be circumvented.
Development and spread of the tumor by metastases should
moreover be counteracted.
Since, according to more recent knowledge, angiogenesis
is obviously also responsible for tumor growth and the
development of metastases, the property of inhibition of
angiogenesis is a further advantageous pharmaceutical
potential, for example in cancertherapy.
The intensification of action achieved with the N-
substituted indole-3-glyoxylamides should make pharmaceutical
use in tumor therapy more effective. Moreover, it should be
possible to shorten the treatment time and to extend it to
therapy-resistant cases.
Recurrences and metastases should furthermore be
restricted or prevented and thus the survival time of the
patients should additionally be increased. The aim is to
develop medicaments which can intervene in the
CA 02386069 2011-01-07
- 3 -
metastatic process.
According to an aspect of the present invention
there is provided use of a N-substituted indol-3-
glyoxylamide of formula I or a physiologically tolerable
acid-addition salt thereof, or any combination thereof, in
the manufacture of a medicament for treating a multi-drug
resistant tumor or inhibiting metastasis:
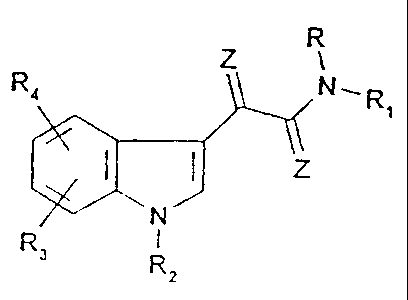
R4 Z R
N,R
1
N Z
R3 i
R2 (I)
wherein radicals R, R1, R2, R3, R4, and Z have the
following meanings:
R is hydrogen;
R1 is a pyridine structure of formula II:
R5 4
3
N 2
R6 (II)
where the pyridine structure is bonded at either the 2, 3,
or 4 position of the ring and is optionally substituted by
substituents R5 or R6 or both R5 and R6, wherein R5 and R6
can be identical or different and are independently a(C1-
C6) -alkyl, (C3-C7) -cycloalkyl, (C1-C6) -alkoxy, nitro, amino,
hydroxyl, halogen, trifluoromethyl, ethoxycarbonylamino
radical or a carboxyalkyloxy group in which the alkyl group
has 1-4 C atoms;
R2 is a (C1-C6)-alkyl group, the alkyl group of which is
mono- or polysubstituted by phenyl, which is optionally
substituted by one or more substituents, wherein at each
occurence the substituent is a halogen, (C1-C6)-alkyl, (C3-
CA 02386069 2011-01-07
- 3a -
C7)-cycloalkyl, a carboxyl group, a carboxyl group
esterified with a C1-C6-alkanol, a trifluoromethyl group, a
hydroxyl group, a methoxy group, an ethoxy group, a
benzyloxy group, a 2-quinolyl group or a 2-, 3- or 4-
pyridyl group, wherein the 2-quinolyl and 2-, 3-, or 4-
pyridyl groups can both in each case be mono- or
polysubstituted by halogen, (C1-C4) -alkyl group or (C1-C4) -
alkoxy,
R3 and R4 can be identical or different and are
independently a hydrogen, (C1-C6) -alkyl, (C3-C7) -cycloalkyl,
(C1-C6) -alkanoyl, (C1-C6) -alkoxy, halogen, benzyloxy, a nitro
group, an amino group, a (C1-C4)-mono or dialkyl-substituted
amino group, a (C1-C6) alkoxycarbonylamino group or a (C1-
C6)-alkoxycarbonylamino-(C1-C6)-alkyl group; and
Z is 0 or S.
According to an embodiment of the present
invention, there is provided a pharmaceutical composition
comprising at least one N-substituted indol-3-glyoxylamide
of formula I or a physiologically tolerable acid-addition
salt thereof:
R4 Z R
N,R
1
Z
R3 R
2 (I)
wherein radicals R, R1r R2, R3, R4, and Z have the
following meanings:
R is hydrogen;
R1 is a pyridine structure of formula II:
CA 02386069 2011-10-06
- 3b -
R5 4
3
>N)2
R6 (II)
where the pyridine structure is bonded at either the 2, 3,
or 4 position of the ring and is optionally substituted by
substituents R5 or R6 or both R5 and R6, wherein R5 and R6
can be identical or different and are independently a(C1-
C6) -alkyl, (C3-C7) -cycloalkyl, (C1-C6) -alkoxy, nitro, amino,
hydroxyl, halogen, trifluoromethyl, ethoxycarbonylamino
radical or a carboxyalkyloxy group in which the alkyl group
has 1-4 C atoms;
R2 is a (C1-C6) -alkyl group, the alkyl group of which is
mono- or polysubstituted by phenyl, which is optionally
substituted by one or more substituents, wherein at each
occurence the substituent is a halogen, (C1-C6) -alkyl, (C3-
C7)-cycloalkyl, a carboxyl group, a carboxyl group
esterified with a C1-C6-alkanol, a trifluoromethyl group, a
hydroxyl group, a methoxy group, an ethoxy group, a
benzyloxy group, a 2-quinolyl group or a 2-, 3- or 4-
pyridyl group, wherein the 2-quinolyl and 2-, 3-, or 4-
pyridyl groups can both in each case be mono- or
polysubstituted by halogen, (C1-C4) -alkyl group or (C1-C4) -
alkoxy,
R3 and R4 can be identical or different and are
independently a hydrogen, (C1-C6) -alkyl, (C3-C7) -cycloalkyl,
(C1-C6)-alkanoyl, (C1-C6)-alkoxy, halogen, benzyloxy, a nitro
group, an amino group, a (C1-C4)-mono or dialkyl-substituted
amino group, a (C1-C6) alkoxycarbonylamino group or a (C1-
C6)-alkoxycarbonylamino-(C1-C6)-alkyl group; and
Z is 0 or S; and
a pharmaceutically acceptable carrier,
CA 02386069 2011-10-06
- 3c -
wherein the composition is for treating a multi-drug
resistant tumor or inhibiting metastasis.
Brief Description of the Drawings
FIG. 1 shows the cytotoxic action of compound D-24851
against MDR murine leukemic subline L1210/VCR.
FIG. 2 demonstrates the action of compound D-24851 on
a multidrug-resistant tumor.
FIG. 3 shows the influence on the multi-drug-resistant
murine leukemia L1210 (dose 10% of the LD50)=
FIG. 4 compares the effect compound D-24851 on human
leukemia cells with the effect of other neoplastic agents
on the same leukemia cells.
FIG. 5 shows the inhibition of migration of M04 cells
by compound D-24851.
FIG. 6 shows a comparison of neurotoxicity induced by
compound D-24851 versus other neoplastic agents.
FIG. 7 shows the influence of compound D-24851 on
nerve conduction velocity in rat.
FIG. 8 compares angiogenesis in human endothelial
cells in compound D-24851-treated cells versus DMSO (44
hours after induction of angiogenesis).
FIG. 9 compares angiogenesis in human endothelial
cells in compound D-24851-treated cells versus DMSO (22
hours after induction of angiogenesis).
Detailed Description of Preferred Embodiments
It has surprisingly been found that the N-substituted
indole-3-glyoxylamides described in German Patent Application
19814 838.0, which was published on October 14, 1999, and of the
general formula 1 indicated below, which are suitable
for the treatment of oncoses, further have advantageous
properties for tumor treatment of the type which can
widen their area of use.
CA 02386069 2011-01-07
- 3d -
The invention relates to the use of N-
substituted indole-3-glyoxylamides according to a
general formula 1 for tumor treatment in particular
in the case of pharmaceutical resistance and
metastatic carcinoma and for the suppression of
metastasis formation, as well as angiogenesis
inhibitors
R
Z
R. N-R,
Z
N
R
i R
formula 1
where the radicals R, R1, R2, R3, R4 and Z have the
following meaning:
R = hydrogen, (C1-C6) -alkyl, where the alkyl group can
be mono- or polysubstituted by the phenyl ring and
this phenyl ring, for its part, can be mono- or
polysubstituted by halogen, (C1-C6)-alkyl,
(C3-C7)-cycloalkyl, by carboxyl groups, carboxyl
groups esterified with C1-C6-alkanols, trifluoro-
methyl groups, hydroxyl groups, methoxy groups,
ethoxy groups, benzyloxy groups, and by a benzyl
group which is mono- or polysubstituted in the
phenyl moiety by (C1-C6)-alkyl groups, halogen
atoms or trifluoromethyl groups,
R is furthermore the benzyloxycarbonyl group
(Z group) or the tertiary butoxycarbonyl radical
CA 02386069 2008-03-04
4 -
(Boc-radical), furthermore the acetyl group.
R1 can be the phenyl ring which is mono- or poly-
substituted by (C1-C6) -alkyl, (C1-C6) -alkoxy,
cyano, halogen, trifluoromethyl, hydroxyl,
benzyloxy, nitro, amino, (C1-C6)-alkylamino,
(C1-C6)-alkoxycarbonylamino and by the carboxyl
group or by the carboxyl group esterified with
Cl-C6-alkanols, or is a pyridine structure of the
formula 2 and its N-oxide
s 4
3
formula 2
22
PIS
and its N-oxide, where the pyridine structure is
alternatively bonded to the ring carbon atoms 2, 3
and 4 and can be substituted by the substituents
R5 and R6. The radicals R5 and R6 can be identical
or different and have the meaning (Cl-C6) -alkyl,
and the meaning (C3-C7) -cycloalkyl, (C1-C6) -alkoxy,
nitro, amino, hydroxyl, halogen and trifluoro-
methyl and are furthermore the ethoxycarbonylamino
radical and the group carboxyalkyloxy in which the
alkyl group can have 1-4 C atoms.
R1 can furthermore be a 2- or 4-pyrimidinyl
heterocycle, where the 2-pyrimidinyl ring can be
mono- or polysubstituted by the methyl group,
furthermore the 2-, 3-, 4- and 8-quinolyl
structure substituted by (C1-C6)-alkyl, halogen,
the nitro group, the amino group and the
(C1-C6) -alkylamino radical, a 2-, 3- or
4-quinolylmethyl group, where the ring carbons. of
the pyridylmethyl radical of the quinolyl group
and of the quinolylmethyl radical can be
substituted by (C1-C6) -alkyl, (C1-C6) -alkoxy,
nitro, amino and (C1-C6)-alkoxycarbonylamino.
R1 can furthermore be, in the case where
CA 02386069 2002-03-27
-
R = hydrogen, the methyl or benzyl group and the
benzyloxycarbonyl radical (Z radical), the tert-
butoxycarbonyl radical (BOC radical) and the
acetyl group, the following radicals:
5 -CH2COOH; -CH (CH3) -OOOH; - (CH3) 2-CH- (CH2) 2-CH-COO;
H3C-H2C-CH (CH3) -CH (COOH) -; HO-H2C-CH (COON) -;
phenyl-CH2-CH(COOH)-; (4-imidazolyl)-CH2-CH-COOH)-;
HN=C (NH2) -NH- (CH2) 3 -CH (COOH) - ; H2N- (CH2) 4 -CH (COOH) - ;
H2N-CO-CH2-CH- (COOH) -; HOOC (CH2) 2-CH (COON) -;
R1 can furthermore be, in the case where R is
hydrogen, the Z group, the BOC radical, the acetyl
or the benzyl group, the acid radical of a natural
or unnatural amino acid, e.g. the a-glycyl, the
a-sarcosyl, the a-alanyl, the a-leucyl, the
a-isoleucyl, the a-seryl, the a-phenylalanyl, the
a-histidyl, the a-prolyl, the a-arginyl, the
a-lysyl, the a-asparagyl and the a-glutamyl
radicals, where the amino groups of the respective
amino acids can be present in unprotected or
protected form. Possible protective groups for the
amino function are the carbobenzoxy radical
(Z radical) and the tert-butoxycarbonyl radical
(BOC radical) as well as the acetyl group. In the
case of the asparagyl and glutamyl radical claimed
for R1, the second, unbonded carboxyl group is
present as a free carboxyl group or in the form of
an ester with C1-C6-alkanols, e.g. as a methyl,
ethyl or as a tert-butyl ester.
R1 can furthermore be the allylaminocarbonyl-
2-methylprop-1-yl group.
R and R1 can furthermore, together with the
nitrogen atom to which they are bonded, form a
piperazine ring of the formula 3 or a
homopiperazine ring, if R1 is an aminoalkylene
group in which
CA 02386069 2002-03-27
6 -
-N N-R7
formula 3
R7 is an alkyl radical, a phenyl ring which can be
mono- or polysubstituted by (C1-C6) -alkyl, (C1-C6) -
alkoxy, halogen, the nitro group, the amino
function and by the (C1-C6)-alkylamino group. R7 is
furthermore the benzhydryl group and the bis-p-
fluorobenzylhydryl group.
R2 can be hydrogen or the (C1-C6)-alkyl group, where
the alkyl group is mono- or polysubstituted by
halogen and phenyl, which for its part can be
mono- or polysubstituted by halogen, (C1-C6)-alkyl,
(C3-C7)-cycloalkyl, carboxyl groups, carboxyl
groups esterified with C1-C6-alkanols,
trifluoromethyl groups, hydroxyl groups, methoxy
groups, ethoxy groups or benzyloxy groups. The
(C1-C6)-alkyl group applying for R2 can furthermore
be substituted by the 2-quinolyl group and the 2-,
3- or 4-pyridyl structure, which can both each be
mono- or polysubstituted by halogen, (C1-C4)-alkyl
groups or (C1-C4)-alkoxy groups. R2 is furthermore
the aroyl radical, where the aryl moiety on which
this radical is based is the phenyl ring, which
can be mono- or polysubstituted by halogen,
(C1-C6)-alkyl, (C3-C7)-cycloalkyl, carboxyl groups,
carboxyl groups esterified with C1-C6-alkanols,
trifluoromethyl groups, hydroxyl groups, methoxy
groups, ethoxy groups or benzyloxy groups.
R3 and R4 can be identical or different and are hydrogen
(C1-C6) -alkyl, (C3-C7) -cycloalkyl, (C1-C6) -alkanoyl,
(C1-C6)-alkoxy, halogen and benzyloxy. R3 and R4
can furthermore be the nitro group, the amino
group, the (C1-C4)-mono- or dialkyl-substituted
amino group, and the (C1-C6)-alkoxycarbonylamino
CA 02386069 2008-03-04
7 -
function or (C1-C6)-alkoxycarbonylamino-(C1-C6)-
alkyl function.
Z is O or S.
The designation alkyl, alkanol, alkoxy or alkylamino
group for the radicals R, R1, R2, R3, R4, R5, R6, R7 is
normally to be understood as meaning either "straight-
chain" or "branched" alkyl groups, where "straight-
chain alkyl groups" can be, for example, radicals such
as methyl, ethyl, n-propyl, n-butyl, n-pentyl, n-hexyl
and "branched alkyl groups" designates, for example,
radicals such as isopropyl or tert-butyl. "Cycloalkyl"
is understood as meaning radicals such as, for example,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or
cycloheptyl.
The designation "halogen" stands for fluorine,
chlorine, bromine or iodine. The designation "alkoxy
group" represents radicals such as, for example,
methoxy, ethoxy, propoxy, butoxy, isopropoxy, isobutoxy
or pentoxy.
The compounds can also be employed as acid
addition salts, for example as salts of mineral acids,
such as, for example, hydrochloric acid, sulfuric acid,
phosphoric acid, salts of organic acids, such as, for
example, acetic acid, lactic acid, malonic acid, maleic
acid, fumaric acid, gluconic acid, glucuronic acid,
citric acid, embonic acid, methanesulfoni.c acid,
trifluoroacetic acid, succinic acid and 2-
hydroxyethanesulfonic acid.
Both the compounds of the formula 1 and their
salts are biologically active.
The compounds of the formula 1 can be administered in
free form or as salts with physiologically tolerable
acids.
Administration can be carried out perorally,
parenterally, intravenously, transdermally or by
inhalation.
The invention furthermore relates to
CA 02386069 2008-03-04
8
pharmaceutical preparations containing at least one of
the compounds of the formula 1 or their salts with
physiologically tolerable inorganic or organic acids
and, if appropriate, pharmaceutically utilizable
vehicles and/or diluents or excipients.
Suitable administration forms are, for example,
tablets, coated tablets, capsules, solutions for
infusion or ampoules, suppositories, patches, powder
preparations which can be employed by inhalation,
suspensions, creams and ointments.
The preparation processes for the substances
can be taken from the examples of the German Patent
DE 196 36 150 Al.
The therapeutically valuable properties found
relate specifically to the following advantages:
no development of resistance was detected
parameters were detected which are characteristic
of the inhibition of metastasis formation
(migration)
- parameters were found which confirm the inhibition
of angiogenesis
in various models, no neurotoxicity was found with
the N-substituted indole-3-glyoxylamides of
the general formula 1, in contrast to most
antitumor preparations.
The absent development of resistance is
confirmed in the following pharmacological models and
cell cultures:
1. The cytotoxic activity of D-24851 on the MDR
(multidrug-resistant) leukemia cell line of the mouse L
1210/VCR is not influenced in vivo and in vitro (Figure 1,
2 and 3). As illustrated in Figure 1, in contrast to
taxol, doxorubicin, vincristine and Epothilone B., D-24851
has the same cytotoxic activity against the MDR mouse
leukemic subline L1210/VCR as against the normal L1210.
D-24851 has an unchanged cytoxic activity against the
multigrug-resistant mouse leukemia cell subline L1210/VCR in
contrast to taxol, doxorubicin, vincristine or epothilone B.
CA 02386069 2008-03-04
- 9 -
Experimental procedure:
The mouse leukemia cell lines L 1210 was
adapted to vincristine. The unadapted (L 1210) and the
adapted (L 1210/VCR) cells were exposed to cytostatic
agents and the cell growth, which was determined by the
metabolic activity, was determined (XTT test).
The curves which connect the XTT data points were
calculated using a nonlinear regression program.
These experimental results are also confirmed in vitro
on the human resistant LT12/MDR. cell line, see Figure
4.
2. The proof of lacking metastasis formation was
furnished by means of the inhibition of migration of
M04 cells. As illustrated in Figure 5 D-24851
inhibits the migration of M04 cells in a dose-
dependent manner.
From this, an anti-invasive and an antimetastic
action can be derived for D-24851.
D-24 851 inhibits the migration of M04 cells in a
dose-dependent manner. An antiinvasive and an
antimetastatic action for D-24851 can be derived
therefrom.
In vitro, the migration ability of M04 cells can be
measured by inoculating the cells in the center of a
cell culture dish and determining the migration by
means of radius or the covered area of the cells after
different numbers of days with and without D-24851.
Figure 4 shows that the migration of the cells
decreases with increasing D-24851 concentration.
In order to test whether D-24851 also acts
antiinvasively, the invasion of M04 fibrosarcoma cells
was investigated in chicken heart. It is also seen here
that at a concentration of 260 and 1000 nM the
invasion is completely inhibited while at lower
concentrations the invasiveness of the M04 cells
CA 02386069 2008-03-04
- 9a -
increases. On the basis of these findings, it is seen
that D-24851 inhibits both the migration and the
invasion of tumor cells and thereby has a strong
antimetastatic potential.
3. From comparison experiments of the compound
CA 02386069 2008-03-04
- 10 -
according to the invention D-24851 with vincristine and
taxol on rats, in which ataxia, traction and reaction
were assessed (see Figure 6), it emerges that this
compound has no neurotoxic effect, in contrast to taxol
and vincristine. As illustrated in Figure 6, in
contrast to taxol and vincristine, D-24851 shows no
neurotoxicity in maximally antitumor-active doses.
In comparison to taxol and vincristine, D-24851
furthermore has no adverse effect on the nerve
conduction velocity, see Figure 7.
This confirms that D-24851, on account of the lacking
neurotoxicity.; has markedly lower side effects than
other chemotherapeutics.
4. From further investigations, according to Figure 8
and 9 it is evident that the compound D-24851 has a
potential as an angiogenesis inhibitor.
As a result of the physiological relationship to tumor
growth, angiogenesis inhibitors are at the same time
2"0 also agents for the inhibition of tumor growth, in that
the formation of new blood vessels, which should feed
the tumor, is inhibited.
In an antiangiogenesis model on endothelial cells, D-
24851 causes a complete inhibition of the formation of
blood vessels, which is not based on a cytotoxic
effect.
In Figure 8, it can be seen that 0.1 pMol/L D 24851
almost completely breaks up existing cell-cell contact
(see vital staining). Normally, the cells maintain at
least partial contact. Cell migration is markedly
reduced and many cells are rounded.
Lethal staining in the monolayer before angiogenesis
induction did not show any increased cell mortality
with D-24851. Increased cell mortality was also still
not detectable in the first 22 hours after induction in
comparison to the control.
Lethal staining is evident in Figure 9, by white dots.
CA 02386069 2008-03-04
- l0a -
The cells originated from human umbilical cord
vein (arterial function)_ They were employed for the
investigation in the third and fourth passage.
Angiogenesis is induced by a natural stimulus. The
CA 02386069 2008-03-04
- 11 -
primary inducer of the endothelial migration is a
protein which is expressed to an increased extent in
vascularizing tissue. The substances are added to the
culture medium shortly before the induction of
angiogenesis.
The concentration for the antiangiogenetic
action of D-24851 is markedly below the concentration
for the cytotoxic activity. It is thereby possible to
separate the two qualities of action (cytotoxic
activity and antiangiogenetic action) from one another.
Without wanting to restrict the scope of the
invention with the following statement, it can be said
that doses from approximately 20 mg up to 500 mg daily
orally are possible. -
In the case of intravenous administration as an
injection or as an infusion, up to 250 mg/day or more
can be administered depending on the body weight of the
patient and individual tolerability.
As a result of the lacking development of resistance
and suppression of metastasis, a high effectiveness and
wide use of the agents is to be expected to even
in patients who are refractory to tumors.
The antiangiogenesis effect is additionally suitable
for suppressing the spread of the tumor.
The invention, however, also comprises the use
of the N-substituted indole-3-glyoxylamides according
to general formula 1 in further diseases in
which an angiogenesis-inhibitory effect is functionally
desirable. (e.g. wound healing).
The invention furthermore also relates to the
fixed or free combination of the N-substituted indole-
3-glyoxylamides according to general formula 1
with antitumor agents known per se, and to the
replacement of antitumor agents which have become
inactive as a result of development of resistance by N-
substituted indole-3-glyoxylamides according to
general formula 1.