Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
Novel compounds and their use as glycine transport inhibitors
The present invention provides novel compounds of the general formula I, and
their use as
glycine transport inhibitors.
Background of the invention
Glutamic acid is the major excitatory amino acid in the mammalian central
nervous system
(CNS), and acts through two classes of receptors, the ionotropic and
metabotrobic receptors,
io respectively. The ionotropic glutamate receptors are divided into three
subtypes based on the
affinities of agonists for these receptors, namely N methyl-D-aspartate
(NMDA), (R,~-2-
amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propanoic acid (AMPA) and kainic acid
(or
kainate) receptors.
The NMDA receptor contains binding sites for modulatory compounds such as
glycine and
is polyamines. Binding of glycine to its receptor enhances the NMDA receptor
activation. Such
NMDA receptor activation may be a potential target for the treatment of
schizophrenia and
other diseases linked to NMDA receptor dysfunction. An activation can be
achieved by am
inhibitor of the glycine transporter.
Molecular cloning has revealed the existence of two types of glycine
receptors, GIyT-1 and
zo GIyT-2, wherein GIyT-1 can be further subdivided into GIyT-la, GlyT-lb and
GIyT-lc.
The NMDA receptor is blocked by compounds such as phencyclidine which induce a
psychotic state which resembles schizophrenia. Likewise, the NMDA antagonists,
such as
ketamine, induce negative and cognitive symptoms similar to schizophrenia.
This indicates
that NMDA receptor dysfunction is involved in the pathophysiology of
schizophrenia.
zs The NMDA receptor has been associated with a number of diseases, such as
pain (Yaksh Paisz
1989, 37, 111-123), spasticity, myuoclonus and epilepsy (Truong et. al.
Movef~zerzt Disorders
1988, 3, 77-87), learning and memory (Bison et. al. NeuYOSCi. Biobehav. Rev.
1995, 19, 533-
552,).
Thus, glycine transporter antagonistists or inhibitors are believed to be
highly beneficial in the
so treatment of schizophrenia, including both the positive and the negative
symptoms of
schizophrenia, other psychoses, dementia, and improving cognition in
conditions where the
cognitive processes are diminished, i.e. Alzheimer's disease, multi-infarct
dementia, AIDS
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
2
dementia, Huntington's disease, Parkinson's disease, amyotrophic lateral
sclerosis or diseases
wherein the brain is damaged by inner or outer influence, such as trauma to
the head or stroke.
Clinical trials with glycine have been reported (Javitt et. al. Am. J.
Psychiatry 1994, 151,
s 1234-1236), (Leiderman et. al. Biol. Psychiatry 1996, 39, 213-215). The
treatment with high-
dose glycine is reported to improve the symptoms of schizophrenia. There is a
need for more
efficient compounds as ligands for the glycin transporter for the treatment of
NMDA
associated diseases.
The compounds of the present invention are potent ligands for the glycine
transporter.
io
Summary of the invention
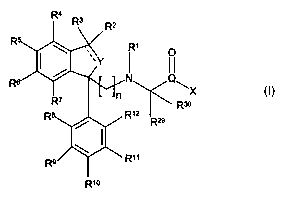
The invention provides novel compounds of the formula I below:
R1
N Q~
X
Rso
R12
R29
R11
is R1o I
wherein
R' represents hydrogen, Cl_6 alkyl, cycloalkyl or cycloalkylalkyl;
RZ and R3 independently represent hydrogen, halogen, C,_6 alkyl, C3_8
cycloalkyl or C3_$
cycloalkyl-Cl_6 alkyl or Rz and R3 together form a C3_8-cycloall~yl;
zo R4, R5, R6 and R' independently represent hydrogen, halogen, CF3, NO2, CN,
C,_6 alkyl, Cz_s
alkenyl, CZ_6 allcynyl, C3_$ cycloalkyl, Cl_6 alkoxy, C1_6 alkylthio, OH, SH,
NRI4RIS, wherein R14
and Rls independently represent hydrogen or C1_6 alkyl; -COR'6 wherein R16
represents OH,
C,_6 alkyl, Cl_6 alkoxy, NRl'R18, wherein Rl' and Rl$ independently represent
hydrogen or Cl_
6 alkyl; aryl or heteroaryl, wherein aryl and heteroaryl are optionally
substituted one or more
R4 ~z
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
3
times with halogen, CF3, OCF3, CN, NOz, Cl_6 alkyl, Cz_6 alkenyl, Cz_6
alk5myl, C3_8 cycloalkyl,
Cl_6 alkoxy, C1_6 thioalkyl, OH, SH or NRz4Rz5, wherein Rz4 and Rzs
independently represent
hydrogen or Cl_6 alkyl;
or R4 and R5, or RS and R6, or Rg, and R'together form a fused, aromatic,
saturated or partly
s saturated ring which optionally contains one or more heteroatoms such as O,
N or S;
R8, R9, Rl°, R'1 and Rlz independently represent hydrogen, halogen,
CF3, OCF3, CN, NOz, Cl_6
all~yl, Cz_6 alkenyl, Cz_6 alkk~mmyl, C3_8 cycloalkyl, Cl_6 alkoxy, CI_6-
alkylthio, OH, SH, NR'9Rzo
wherein R19 and Rz° independently represent hydrogen or C,_6 alkyl; or
R8, R9, R'°, Rl' and Rlz
io independently represent -CORz', wherein Rz' represents OH, C,_6 allcoxy,
NRzzRz3, wherein
Rzz and Rz3 independently represent hydrogen or C,_6 alkyl; or R8, R9,
Rl°, R", and Rlz
independently represent aryl or heteroaryl, wherein aryl and heteroaryl are
optionally
substituted one or more times with halogen, CF3, OCF3, CN, NOz, C,_6 all~yl,
Cz_6 alkenyl, Cz_6
alkynyl, C3_$ cycloalkyl, Cl_6 alkoxy, Cl_6 alkythio, OH, SH, CORz6, wherein
Rz6 represents
is OH, C,_6 alkoxy or Cl_6 alkyl; or NR3°R3', wherein R3° and
R3i independently represent
hydrogen or Cl_6 alkyl;
R8 and R9, or R9 and R'°, or R'° and Rl', or R'1 and Rlz
together form a fused, axomatic,
saturated or partly saturated ring which optionally contains one or more
heteroatoms such as
O, N, or S;
zo Y is O, S, CHz or CH, and when Y is CH then the dotted line is a bond;
n is 2, 3, 4, 5 or 6;
Q represents C, P-ORz9, or S=O, wherein Rz9 represents hydrogen or Cl_6
allcyl;
X is OR'3 or NRz'RzB, wherein R13, Rz', and Rz8 independently represent
hydrogen, C,_6 alkyl,
aryl or aryl-Cl_6 alkyl, wherein aryl may be substituted with halogen, CF3,
OCF3, CN, NOz, or
zs C,_6 alkyl; optionally Rz' and Rz8 together form a ring which may contain
further nitrogen,
oxygen or sulfur atoms and the ring may optionally be partly saturated;
Rz9 and R3° represent hydrogen, Cl_6 alkyl, cycloalkyl or
cycloalkylalkyl
so or a pharmaceutically acceptable addition salt thereof;
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
4
The compounds are useful as inhibitors of the glycine transporter and useful
in treatment of
diseases responsive to the inhibition of the glycine transporter.
Detailed description of the invention
In a preferred embodiment of the invention, Q is C;
Other preferred embodiments are wherein n is 2 or 3;
Another preferred embodiment is wherein R' is CH3;
Yet another preferred embodiment is wherein X is OH or C,_6 alkoxy; more
preferred is
io wherein X is OH, OCH3 or OCZHS
Other preferred embodiments are wherein R' represent hydrogen, and R~, RS or
R6 represent
hydrogen, CN, halogen, C,_6 alkyl, CF3 or phenyl optionally substituted one or
more times
with halogen, C1_6 alkyl, C,_6 alkoxy, CF3, or R', RS or R6 represent
heteroaryl optionally
substituted one or more times with halogen, or wherein R4 and RS or RS and R6
together form
is a fused aryl;
Another preferred embodiment of the invention is wherein R8, R9, Rl°,
R" or R'z
independently represent hydrogen, halogen, C1_6 alkyl, C,_6 alkoxy, or R$ and
R9 or R9 and R'o
together form a fused aryl;
In an more preferred embodiment, one or two of Rg, R9, Ri°, R11 or R'z
represent halogen, Cl_6
alkyl, CF3 or C,_6-alkoxy;
In a more preferred embodiment of the invention R4, R6, R', R8, R9 and Rlz are
all hydrogen
2s and RS represents halogen, CF3, CN, C,_6 alkyl, C1_6 alkoxy or -COR16,
wherein R16 represents
Cl_6 alkyl; and R'° and Rl' represent hydrogen, halogen, CF3, or CN,
provided that at least one
of RI° and Rl' is not hydrogen;
Rz9 and R3° independently represent hydrogen or Cl_6 alkyl or Rz and R3
together form a C3_$-
cycloalkyl;
Another preferred embodiment of the invention is wherein the compounds are the
following
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
N-~3-[5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}glycine ethyl
ester,
N- f 3-[5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
xr~ethylglycine ethyl ester,
s N- f 3-[5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}glycine,
N- f 3-[5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
N- f 3-[1-(3-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
N-~3-[1-(3-trifluoromethylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
io methylglycine,
N- f 3-[1-(3-trifluoromethylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methyl (1-
ethyl)glycine,
N- f 3-[1-(4-methylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
N- f 3-[1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
is N- f 3-[1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylalanine,
N- f 3-[1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-methyl
(1-
ethyl)glycine,
N- f 3-[4-chloro-1-(3-methyl-4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylglycine,
ao N-(3-[4-chloro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-
N-
methylglycine,
N- f 3-[5-chloro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylalanine,
N- f 3-[6-chloro-1-(3-methyl-4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
as methylglycine,
N- { 3-[6-chloro-1-(4-chlorophenyl)-1, 3-dihydroisobenzofuran-1-yl]-1-propyl} -
N-
methylglycine,
N-{3-[6-chloro-1-(4-methylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
3o N-~3-[6-chloro-1-(4-methoxyphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-
N-
methylglycine,
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
N-{3-[5-fluoro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
N-{3-[5-fluoro-1-(4-methoxyphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
s N-{3-[5-trifluoromethyl-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylglycine,
N-{3-[5-trifluoromethyl-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylalanine,
N-{3-[5-cyano-1-(3-methyl-4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
io methylglycine,
N-{3-[5-cyano-1-(4-cyanophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylalanine,
N-{3-[5-cyano-1-(4-methoxyphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
is N-{3-[5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine, N-{2-[5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-
yl]ethyl}-N-
methylglycine,
N- { 3-[ 5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl} -N-methylglycine,
N-{3-[5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl}-N-methylalanine,
ao N-{3-[3-cyclo-1-(4-methylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N
methylglycine,
N-[3-(3,3-Dimethyl-1-phenyl-1, 3-dihydro-b enzo [c] thiophen-1-yl)-propyl]-N-
methylglycine,
N-[3-(3,3-Dimethyl-1-phenyl-1,3-dihydro-benzo[c]thiophen-1-yl)-propyl]-N-
methylalanine,
N-{3-[1-(4-Fluoro-phenyl)-3,3-dimethyl-1,3-dihydro-isobenzofuran-1-yl]-propyl}-
N-
as methylglycine,
N-{3-[5-Bromo-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine,
N-{2-[ 1-(4-Chloro-phenyl)-3,3-dimethyl-1,3-dihydro-isobenzofuran-1-yl]-ethyl}-
N-
methylglycine,
so N-[3-(3-methyl-1-phenyl-1H inden-1-yl)-propyl]-N-methylglycine,
N-[3-(5-Chloro-1-thiophen-2-yl-1,3-dihydro-isobenzofuran-1-yl)-propyl]-N-
methylglycine,
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
N-[3-(5-Chloro-1-thiophen-2-yl-1,3-dihydro-isobenzofuran-1-yl)-propyl]-N-
methyl (1-ethyl)-
glycine,
N-[3-(3-methyl-1-phenyl-1,3-dihydro-isobenzo~uran-1-yl)-propyl]-N-
methylalanine,
N-[3-(3-methyl-1-phenyl-1,3-dihydro-isobenzofuran-1-yI)-propyl]-N-methyl (I-
ethyl)-
s glycine,
N-[3-(3,3-Dimethyl-1-phenyl-1,3-dihydro-isobenzofuran-1-yl)-ethyl]-N-
methylalanine,
N-[3-(3,3-Dimethyl-1-(4-fluoro-phenyl)-1,3-dihydro-isobenzofuran-1-yl)-ethyl]-
N-
methylalanine,
N-[3-(3,3-Dimethyl-1-phenyl-1,3-dihydro-isobenzofuran-1-yl)-ethyl]-N-methyl-(1-
io ethyl)glycine,
N-[3-(3,3-Dimethyl-1-(4-fluoro-phenyl)-1,3-dihydro-isobenzofuran-1-yl)-ethyl]-
N-methyl-(1-
ethyl)glycine,
N-[3-(3,3-Diethyl-1-phenyl-1,3-dihydro-isobenzofuran-1-yl)-propyl]-N-
methylalanine,
N-[3-(3,3-Diethyl-1-(4-chloro-phenyl)-1,3-dihydro-isobenzofuran-1-yl)-propyl]-
N-
is methylalanine,
N-[3-(3,3-Diethyl-1-(4-chloro-phenyl)-1,3-dihydro-isobenzofuran-1-yl)-propyl]-
N-
methylglycine,
N-[3-(1-phenyl-1,3-dihydro-benzo[c]thiophen-1-yl)-propyl]-N-methylalanine,
N-~3-[1-(4-Chloro-phenyl)-3,3-dimethyl-indan-1-yl]-propyl}-N-methylglycine,
ao N-{3-[1-(4-Chloro-phenyl)-3,3-diethyl-1,3-dihydro-isobenzofuran-1-yl]-
propyl}-N-methyl-
alanine,
N-[2-(3-methyl-1-phenyl-indan-1-yl)-ethyl]-amino}-N-methyl alanine,
N-[3-(1-phenyl-(lI~-inden-1-yl)-propyl]-N-methyl-alanine,
N- f 3-[I-(4-Fluoro-phenyl)-5-(4-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
zs propyl}-N-methyl-glycine,
N- f 3-[5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl}-N-methyl-glycine,
N-{3-[5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl}-N-methyl-alanine,
N-~3-[1-(4-chloro-phenyl)-5-(4-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
ethyl}-N-methyl-glycine,
3o N- f 3-[1-(4-Chloro-phenyl)-5-(4-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl}-N-
methyl-glycine,
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
N-{3-[1-(4-Chloro-phenyl)-5-(4-methoxy-phenyl)-1,3-dihydro-isobenzofuran-1-yl]-
ethyl-N-
methyl-glycine,
N-~3-[1-(4-Chloro-phenyl)-5-(2-thiophenyl)-1,3-dihydro-isobenzofuran-1-yl]-
ethylj -N-
methyl-glycine,
s N- f 3-[1-(4-Chloro-phenyl)-5-(4-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-propyl~-N-
methyl-glycine,
N-{3-[1-(4-Chloro-phenyl)-5-(4-methoxy-phenyl)-1,3-dihydro-isobenzofuran-1-yl]-
propyl~-
N-methyl-glycine,
N-~3-[1-(4-chloro-phenyl)-5-(4-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
io propyl]-N-methyl-glycine,
N- f 3-[1-(4-Chloro-phenyl)-5-(4-chloro-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl}-N-
methyl-glycine,
N- {2-[ 1-(4-Chloro-phenyl)-5-(5-chloro-thiophen-2-yl)-1,3-dihydro-
isobenzofuran-1-yl]-
ethyl]-N-methyl-glycine,
is N- f 3-[1-(4-Chloro-phenyl)-5-(3-methyl-phenyl)-1,3-dihydro-isobenzofuxan-1-
yl]-ethyl]-N-
methyl-glycine,
N- f 3-[1-(4-Chloro-phenyl)-5-(2-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl-N-
methyl-glycine,
N-{3-[1-(4-Chloro-phenyl)-5-(2,5-dichloro-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl]-
zo N-methyl-glycine,
N-{3-[1-(4-chloro-phenyl)-5-(3-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
ethyl]-N-methyl-glycine,
N-{3-[1-(4-chloro-phenyl)-5-(3-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
propyl]-N-methyl-glycine ,
as N-{3-[1-(4-Chloro-phenyl)-5-(3,4-dichloro-phenyl)-1,3-dihydro-isobenzofuran-
1-yl]-ethyl}-
N-methyl-glycine,
N-(3-[1-(4-Chloro-phenyl)-5-(4-chloro-phenyl)-1,3-dihydro-isobenzofuran-1-yl]-
propyl)-N-
methyl-glycine,
N-~3-[1-(4-Chloro-phenyl)-5-(3-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-yl]-
propyl}-N-
so methyl-glycine,
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
9
N- f 3-[1-(4-Chloro-phenyl)-5-(2-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-propyl}-N-
methyl-glycine,
N-~3-[I-(4-Chloro-phenyl)-5-(2,5-dichloro-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-propyl}-
N-methyl-glycine,
s N- f 3-[1-(4-Chloro-phenyl)-5-(3,4-dichloro-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-propyl~-
N-methyl-glycine,
N- f 3-[1-(4-chloro-phenyl)-5-(2-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
propyl}-N-methyl-glycine ,
or a pharmaceutically acceptable addition salt thereof.
io
The invention provides a pharmaceutical composition comprising at least one
compound of
Formula I as defined above or a pharmaceutically acceptable acid addition salt
thereof in a
therapeutically effective amount and in combination with one or more
pharmaceutically
acceptable Garners or diluents.
is
The invention also provides the use of compounds as above for the manufacture
of
medicaments for treatment of diseases responsive to ligands of the glycine
transporter.
The invention provides a method for treatment of diseases responsive to
ligands of the glycine
ao transporter.
In preferred embodiments of the invention, the ligands are antagonists of the
glycine
transporter.
as Pharmaceutically acceptable addition salts are those which form
pharmcological acceptable
anions such as malefic, fumaric, benzoic, ascorbic, succinic, oxalic, bis-
methylenesalicylic,
methanesulfonic, ethanedisulfonic, acetic, propionic, tartaric, salicylic,
citric, gluconic, lactic,
malic, mandelic, cinnamic, citraconic, aspartic, stearic, palmitic, itaconic,
glycolic, p-
aminobenzoic, glutamic, benzenesulfonic and theophylline acetic acids, as well
as the 8-
3o halotheophyllines, for example 8-bromotheophylline. Exemplary of such
inorganic salts are
those with hydrochloric, hydrobromic, sulfuric, sulfamic, phosphoric and
nitric acids.
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
The compound of the invention may be administered in any suitable way such as
orally or
parenterally, and it may be presented in any suitable form for such
administration, for
example in the form of tablets, capsules, powders, syrups or solutions or
dispersions for
injection. Preferably, and in accordance with the purpose of the present
invention, the
s compound of the invention is administered in the form of a solid
pharmaceutical entity,
suitably as a tablet or a capsule or in the form of a suspension, solution or
dispersion for
inj ection.
Methods for the preparation of solid pharmaceutical preparations are well
lfflown in the art.
io Tablets may thus be prepared by mixing the active ingredients with ordinary
adjuvants and/or
diluents and subsequently compressing the mixture in a convenient tabletting
machine.
Examples of adjuvants or diluents comprise: corn starch, lactose, talcum,
magnesium stearate,
gelatine, lactose, gums, and the lilce. Any other adjuvant or additive such as
colourings,
aroma, preservatives, etc. may also be used provided that they are compatible
with the active
is ingredients.
Furthermore, the compounds of this invention may exist in unsolvated as well
as in solvated
forms with pharmaceutically acceptable solvents such as water, ethanol, and
the like. In
general, the solvated forms are considered equivalent to the unsolvated forms
for the purposes
zo of this invention.
Some of the compounds of the present invention contain chiral centres and such
compounds
exist in the form of isomers (e.g. enantiomers). The invention includes all
such isomers and
any mixtures thereof including racemic mixtures.
Racemic forms can be resolved into the optical antipodes by known methods, for
example by
separation of diastereomeric salts thereof with an optically active acid and
liberating the
optically active amine compound by treatment with a base. Another method for
resolving
racemates into the optical antipodes is based upon chromatography on an
optically active
3o matrix. Racemic compounds of the present invention can thus be resolved
into their optical
antipodes, e.g. by fractional crystallisation of d- or 1- (tartrates,
mandelates or
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
11
camphorsulphonate) salts for example. The compounds of the present invention
may also be
resolved by the formation of diastereomeric derivatives.
Additional methods for the resolution of optical isomers, known to those
skilled in the art,
may be used. Such methods include those discussed by J. Jaques, A. Collet, and
S. Wilen in
"Enantiomers, Racemates, and Resolutions", John Wiley and Sons, New York
(1981).
Optically active compounds can also be prepared from optically active starting
materials.
io
Definition of substituents
Halogen means fluoro, chloro, bromo or iodo. Preferred halogens are F and Cl.
is The term C,_6 alkyl refers to a branched or unbranched alkyl group having
from one to six
carbon atoms inclusive, such as methyl, ethyl, 1-propyl, 2-propyl, 1-butyl, 2-
butyl, 2-methyl-
2-propyl and 2-methyl-1-propyl. Preferred alkyls are methyl and ethyl.
Similarly, CZ_6 alkenyl and CZ_6 alk5myl, respectively, designate such groups
having from two
2o to six carbon atoms, including one double bond and triple bond
respectively, such as ethenyl,
propenyl, butenyl, ethynyl, propynyl, and butynyl.
The term C3_8 cycloalkyl designates a monocyclic or bicyclic carbocycle having
three to eight
C-atoms, such as cyclopropyl, cyclopentyl, cyclohexyl, etc.
The term C3_$-cycloalkylalkyl designates a cycloalkyl as defined above and an
alkyl as above.
The terms Cl_6 alkoxy and Cr_6 alkylthio designate such groups in which the
alkyl group is
Cl_6 alkyl as defined above.
The term aryl designates an aromatic hydrocarbon such as phenyl or naphtyl.
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
12
The term heteroaryl refers to a mono- or bicyclic heterocyclic aromatic group
containing at
least one N, S or O atom, such as furyl, pyrrolyl, thienyl, oxazolyl,
isoxazolyl, thiazolyl,
isothiazolyl, imidazolyl, pyridyl, pyrimidyl, tetrazolyl, benzofuranyl,
benzothienyl,
benzimidazolyl, indolyl. Preferred heteroaryls are monocyclic heteroaryls.
Especially
s preferred is thienyl.
Preparatory examples
The compounds of the invention may be prepared as follows:
io
1) alkylating an amine of formula II with an alkylating agent of formula I
G is a suitable leaving group such as e.g. halogen or mesylate.
O
N
X
is
the substituents Rl-R12, n, Y and X are as defined above;
2) alkylating an amine of formula III with an alkylating agent of formula IV
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
13
R1
O
N
Br~
X
'- (IV)
wherein the substituents Rl-Rlz , n, Y and X are as defined above;
s
3) Coupling of an aryl subsitituent of formula VI to the aryl bromide
derivative of
formula V wherein the substituents R4-R' are halogens, Rl-R3 and R8-R12 , n, Y
and X are as
defined above
R5
1
OH \ ~
ARYL- ~ ~X
B~ R
OH
(VI)
(V)
to
4) hydrolysing the ester group of a compound of formula VII to obtain the
corresponding
carboxylic acid derivative
is
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
14
R' O
X
(V11)
Rio
the substituents Rl-R'Z, n, and Y are as defined above and X is OH in the
final product.
The alkylations according to methods 1 and 2 are conveniently carned out in an
inert solvent
s such as a suitably boiling alcohol or ketone or in tetrahydrofuran,
preferably in the presence of
an organic or inorganic base (potassium carbonate, diisopropylethylamine or
triethylamine) at
reflux temperature. Alternatively, the alkylation can be performed at a fixed
temperature
which is different from the boiling point in one of the above-mentioned
solvents or in
dimethylformamide, dimethylsulfoxide or N-methylpyrrolidin-2-one, preferably
in the
io presence of a base.
Reagents of formula I are prepared by methods described in the literature,
see. e.g. US
3,549,656 , GB 1166711 and Dykstra et al. J. Med. Chem. 1967, 10(3), 418-28.
Glycine derivatives of formula II are well described in the literature.
is Amines of formula III are prepared as described by Bigler et. al. Eu~. J.
Med. Chem. 1977, 12,
289.
Biaryl derivatives of formula IV are prepared by suzuki type coupling of an
aryl boronic acid
with the desired halide in dimethoxyethane,tetrahydrofuran or toluene
containing an
inorganic base such as sodium carbonate and a palladium catalyst at a
temperature between
zo room temperature and the boiling point of the solvent.
The hydrolysis according to method 4 is conveniently performed in a suitably
boiling alcohol
in the presence of an aqueous base such as e.g. sodium hydroxide at ambient
temperature. The
starting materials of formula V are prepared by methods 1 or 2.
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
1S
Experimental
Melting points were determined on a Biichi SMP-20 apparatus and are
uncorrected. Analytical
LC-MS data were obtained on a PE Sciex API 1SOEX instrument equipped with
IonSpray
s source and Shimadzu LC-8A/SLC-10A LC system. The LC conditions (SO X 4.6 rmn
YMC
ODS-A with S ~m particle size) were linear gradient elution with
water/acetonitrile/trifluoroacetic acid (90:10:0.0S) to
water/acetonitrile/trifluoroacetic acid
(10:90:0.03) in 7 min at 2 mL/min. Purity was determined by integration of the
UV trace (2S4
nm). The retention times, Rt, are expressed in minutes.
io Mass spectra were obtained by an alternating scan method to give molecular
weight
information. The molecular ion, MH+, was obtained at low orifice voltage (S-
20V) and
fragmentation at high orifice voltage (100V).
Preparative LC-MS-separation was performed on the same instrument. The LC
conditions (SO
X 20 mm YMC ODS-A with S ~m particle size) were linear gradient elution with
is water/acetonitrile/trifluoroacetic acid (80:20:0.0S) to
water/acetonitrile/trifluoroacetic acid
(10:90:0.03) in 7 min at 22.7 mL/min. Fraction collection was performed by
split-flow MS
detection.
'H NMR spectra were recorded at 500.13 MHz on a Bruker Avance DRXS00
instrument or at
250.13 MHz on a Bruker AC 2S0 instrument. Deuterated chloroform (99.8%D) or
dimethyl
ao sulfoxide (99.9%D) were used as solvents. TMS was used as internal
reference standard.
Chemical shift values are expressed in ppm-values. The following abbreviations
are used for
multiplicity of NMR signals: s=singlet, d=doublet, t=triplet, q=quartet,
qui=quintet, h=heptet,
dd=double doublet, dt=double triplet, dq=double quartet, tt=triplet of
triplets, m~nultiplet,
b=broad singlet. NMR signals corresponding to acidic protons are generally
omitted. Content
zs of water in crystalline compounds was determined by Karl Fischer titration.
Standard workup
procedures refer to extraction with the indicated organic solvent from proper
aqueous
solutions, drying of combined organic extracts (anhydrous MgS04 or Na2S04),
filtering and
evaporation of the solvent in vacuo. For column chromatography, silica gel of
type Kieselgel
60, 230-400 mesh ASTM was used. For ion-exchange chromatography, SCX, 1 g,
Varian
3o Mega Bond Elut~, Chrompack cat. No. 220776 was used. Prior use of the SCX-
columns was
pre-conditioned with 10 % solution of acetic acid in methanol (3 mL).
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
16
The following examples will illustrate the invention further. They are,
however, not to be
construed as limiting.Example 1
la, N-(3-(5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzoft~ran-1-yl)-1-
propyl)glycine.ethyl
s ester.
A stirred mixture of 3-(5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuxan-1-
yl)-1-propyl
amine (1.5 g), potassium carbonate (1.3 g) and ethanol (15 mL) was treated
dropwise with a
solution of ethyl bromoacetate (0.75 g) in ethanol (15 mL) at room
temperature. After reflux
for 1.5 h, the mixture was cooled and concentrated ih vacuo. Standard work-up
with ethyl
io acetate gave an oil which was purified by flash chromatography (eluent
heptane/ethyl
acetate/triethylamine 26:70:4). The title compound was obtained as a clear oil
(0.77 g).
LC/MS (m/z) 383 (MH+), purity (UV): >99%.
Example 2
is
2a, N-(3-(5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-1-propyl)-
N-
methylglycine.ethyl ester.
A stirred mixture of 3-(5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzoftiran-1-
yl)-1-propyl
iodide (3.1 g), ethyl N-methylglycinate (4.4 g) and diethylisopropylamine (4.4
g) in
ao tetrahydrofuran (50 mL) was refluxed for 16 h. Standard worlc-up with ethyl
acetate gave an
oil which was purified by flash chromatography (eluent heptanelethyl
acetate/triethylamine
64:32:4) giving the title compound as a clear oil (1.4 g).
LC/MS (m/z) 397 (MFi+), purity (UV): >99%
zs Example 3
3a, N-(3-(5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-1-
propyl)glycine
hydrochloride
A mixture of N-(3-(5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-1-
3o propyl)glycineethyl ester (0.7 g), methanol (6 mL) and 6 M sodium hydroxide
(2 mL) was
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
17
stirred at room temperature for 2 h. Adjustment of pH to < 6.5 with dilute
hydrochloric acid
followed by standard work-up with ethyl acetate gave the title compound as an
oil (0.2 g).
LC/MS (rn/z) 355 (MH+), purity (UV): >90%
s In a similar manner, the following compound were prepared:
3b, N-(3-(5-Cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl)-1-propyl)-
N-
methylglycine hydrochloride_
LC/MS (mlz) 369 (MH+), purity (W): >90%
io 3c, Example 4:
N-{3-[1-(3-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl~-N-
methylglycine
hydrochloride
LC/MS (m/z) 360 (MH+), purity (UV 90%)
3d,Example 5:
is N- f 3-[1-(3-trifluoromethylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylglycine hydrochloride.
LC/MS (m/z) 394 (MH+), purity (UV 79%)
3e,Example 6:
N- f 3-[1-(3-trifluoromethylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methyl (1-
zo ethyl)glycine hydrochloride
LC/MS (m/z) 422 (MH+), purity (UV 79%)
3f, Example 7:
N- f 3-[1-(4-methylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl~-N-
methylglycine.
hydrochloride
zs LC/MS (m/z) 378 (MH+), purity (LTV 91%)
3g, Example 8:
N- f 3-[1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl)-N-
methylglycine
hydrochloride
LC/MS (m/z) 344 (MFi+), purity (UV 81%)
30 3h, Example 9
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
18
N-{3-[1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl~-N-
methylalanine
hydrochloride
LC/MS (m/z) 358 (MH+), purity (UV 81%)
3i, Example 10
s N-~3-[1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl~-N-methyl
(1-
ethyl)glycine hydrochloride
LC/MS (m/z) 372 (MH+), purity (UV 86%)
3j, Example 11
N- ~3-[4-chloro-1-(3-methyl-4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl)-N-
io methylglycine hydrochloride.
LC/MS (mlz) 392 (MH+), purity (W 86%)
3k, Example 12
N- f 3-[4-chloro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine hydrochloride.
is LC/MS (m/z) 394 (MH+), purity (UV 98%)
31, Example 13
N- f 3-[5-chloro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylalanine hydrochloride
LC/MS (mlz) 408 (MH+), purity (LTV 85%)
zo 3m, Example 14
N-{3-[6-chloro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl~-N-
methylglycine hydrochloride.
LC/1VIS (mlz) 394 (MFi+), purity (UV 99%)
3n, Example 15
as N- f 3-[6-chloro-1-(4-methylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl)-N-
methylglycine hydrochloride.
LC/MS (m/z) 374 (MFi+), purity (UV 76%)
30, Example 16
N-~3-[6-chloro-1-(4-rnethoxyphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl~-N-
so methylglycine hydrochloride
LC/MS (m/z) 390 (NCI+), purity (UV 98%).
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
19
3p, Example 17
N- f 3-[5-fluoro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine hydrochloride.
LCrn~S (miz) 378 (lvlH+), purity (uv 8s°~°).
s 3q, Example 18
N-{3-[5-fluoro-1-(4-methoxyphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
methylglycine hydrochloride.
LC/MS (m/z) 378 (MMl +), purity (UV 99%).
3r, Example 19
io N-{3-[5-trifluoromethyl-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylglycine hydrochloride
LC/MS (m/z) 412 (MH+), purity (UV 81 %)
3s, Example 20.
N- {3-[5-trifluoromethyl-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl} -N-
is methylalanine hydrochloride
LC/MS (m/z) 426 (MH+), purity (UV 98%).
3t, Example 21
N- f 3-[5-cyano-1-(3-methyl-4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylglycine hydrochloride.
ao LC/MS (mlz) 383 (MH+), purity (UV 83%).
3u, Example 22
N-{3-[5-cyano-1-(4-cyanophenyl)-indan-1-yl]-1-propyl}-N-methylalanine
hydrochloride.
LCrn~s (miz) 388 (lvlH+), purity (w 8o°i°).
3v, Example 23
zs N- f 3-[5-cyano-1-(4-methoxyphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylglycine hydrochloride
Lcrn~s (miz) 3 81 (lvlH+), purity (w s 1 °i°)..
3x, Example 24
N-{3-[5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl}-N-
3o methylglycine hydrochloride.
LC/MS (m/z) 369 (MH+), purity (UV 98%)
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
3y, Example 25
N- f 2-[5-cyano-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-1-yl]ethyl}-N-
methylglycine
hydrochloride.
LC/MS (m/z) 355 (MH+), purity (UV 94%)
s 3z, Example 26
N-~3-[5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl~-N-methylglycine
hydrochloride
LC/MS (m/z) 392 (MH+), purity (UV 98%)
3aa, Example 27
N- f 3-[5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl]-N-methylalanine
hydrochloride
io LC/MS (m/z) 406 (MMFi+), purity (UV 95%)
3ab, Example 28
N- f 3-[3-spirocyclopentyl-1-(4-methylphenyl)-1,3-dihydroisobenzofuran-1-yl]-1-
propyl}-N-
methylglycine hydrochloride
Sac, Example 29
is N-[3-(3,3-Dimethyl-1-phenyl-1,3-dihydro-benzo[c]thiophen-1-yl)-propyl]-N-
methylglycine
hydrochloride
Sad, Example 30
N-[3-(3,3-Dimethyl-1-phenyl-1,3-dihydro-benzo[c]thiophen-1-yl)-propyl]-N-
methylalanine
LC/MS (m/z) 370 purity (UV 96%)
zo 3ae, Example 32
N- f 3-[5-Bromo-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-yl]-1-propyl]-N-
methylglycine
LC/MS (m/z) 440 (MH+), purity (ELSD 93%)
3af, Example 33
zs N-~2-[1-(4-Chloro-phenyl)-3,3-dimethyl-1,3-dihydro-isobenzofuran-1-yl]-
ethyl}-N-
methylglycine
LC/MS (m/z) 374 (MH+), purity (LTV 72%)
Sag, Example 34
N-[3-(3-methyl-1-phenyl-1H inden-1-yl)-propyl]-N-methylglycine
so LCIMS (mlz) 336 (MH+), purity (UV 85%)
3ah, Example 35
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
21
N-[3-(5-Chloro-1-thiophen-2-yl-1,3-dihydro-isobenzofuran-1-yl)-propyl]-N-
methylalanine
LC/MS (m/z) 380 (M1-i+), purity (UV 85%)
3ai, Example 36
N-[3-(5-Chloro-1-thiophen-2-yl-1,3-dihydro-isobenzofuran-1-yl)-propyl]-N-
methyl (1-ethyl)-
s glycine
LC/MS (xn/z) 394 (MFi+), purity (UV 80%)
3aj, Example 37
N-[3-(3-methyl-1-phenyl-1,3-dihydro-isobenzofuran-1-yl)-propyl]-N-
methylalanine
LC/MS (m/z) 354 (MH+), purity (UV 78%)
io 3ak, Example 38
N-[2-(3-methyl-1-phenyl-indan-1-yl)-ethyl]-amino}-N-methyl alanine
LC/MS (m/z) 451, purity (UV 92%)
3a1, Example 39
N- f 3-[1-(4-Chloro-phenyl)-S-(4-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl-N-
is methyl-glycine
Example 4
zo 4a, N- f 3-[5-Bromo-chloro-1-(4-chlorophenyl)-1,3-dihydroisobenzofuran-1-
yl]-1-ethyl}-N-
methylglycine ethyl ester (226mg, O.Smmol) was dissolved in a 1:1 mixture of
tetrahydrofuran and dimethoxyethane (3 mL) containing tetrakis
(triphenylphosphine)palladium under nitrogen. To the reaction was added 4-
chlorophenyl
boronic acid (102mg, 0.75 mmol) and O.SM aqueous sodium carbonate solution (2
mL,
as lmmol) and the reaction was heated to 65 °C for 18 hours. The
solution was diluted with
water (5 mL) and ethyl acetate (7 mL). The organic layer was separated and the
aqueous layer
was re-extracted with ethyl acetate (5 mL). The organic extractions were
combined and
washed with saturated brine solution (7 mL) before being evaporated in the
presence of 1g of
silica gel. The crude product absorbed on silica gel was poured on top of a
20g silica gel
3o cartridge and eluted with a gradient solvent system eluting from heptane to
heptane/ethyl
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
22
acatet (1:1) over 37 minutes. The product was isolated as a light oil (135 mg,
64%). LC/MS
479.
The compound was hydrolysed as described for Experimental 3a to give the N-
methyglycine
hydrochloride derivative.
s LC/MS (m/z) 436, purity (UV 92%)
In an analogous fashion, the following compounds were prepared:
4b, Example 40
N- f 3-[1-(4-Chloro-phenyl)-5-(4-methoxy-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl}-N-
methyl-glycine
io LC/MS (xn/z) 452, purity (UV 94%)
4c, Example 41
N-~3-[1-(4-Chloro-phenyl)-5-(2-thiophenyl)-1,3-dihydro-isobenzofuran-1-yl]-
ethyl}-N-
methyl-glycine
LC/MS (m/z) 428, purity (UV 96%)
is 4d, Example 42
N- f 3-[1-(4-Chloro-phenyl)-5-(4-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-propyl}-N-
methyl-glycine
LC/MS (mlz) 450, purity 91%
4e, Example 43
zo N- f 3-[1-(4-Chloro-phenyl)-5-(4-methoxy-phenyl)-1,3-dihydro-isobenzofuran-
1-yl]-propyl}-
N-methyl-glycine
LC/MS (m/z) 466, purity (UV 95%)
4f, Example 44
N- f 3-[1-(4-chloro-phenyl)-5-(4-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
as propyl}-N-methyl-glycine
LC/MS (mlz) 504, purity (UV 89%)
4g, Example 45
N-~3-[1-(4-Chloro-phenyl)-5-(4-chloro-phenyl)-1,3-dihydro-isobenzofuran-1-yl]-
ethyl}-N-
methyl-glycine
so LC/MS (m/z) 456, purity 96%
4h, Example 46
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
23
N- {2-[ 1-(4-Chloro-phenyl)-5-(5-chloro-thiophen-2-yl)-1,3-dihydro-
isobenzofuran-1-yl]-
ethyl~-N-methyl-glycine
LC/MS (m/z) 462, purity 74%
4i, Example 47
s N-{3-[1-(4-Chloro-phenyl)-5-(3-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl-N-
methyl-glycine
LC/MS (mlz) 436, purity UV 94
4j, Example 48
N-{3-[1-(4-Chloro-phenyl)-5-(2-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-yl]-
ethyl-N-
io methyl-glycine
LC/MS (mlz) 436, purity (UV 91%)
4k, Example 49
N-{3-[1-(4-Chloro-phenyl)-5-(2,5-dichloro-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-ethyl~-
N-methyl-glycine
is LC/MS (m/z) 490, purity 94%
41, Example 50
N-{3-[1-(4-chloro-phenyl)-5-(3-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
ethyl}-N-methyl-glycine
LC/MS (m/z) 490, purity 89%
ao 4m, Example 51
N-{3-[1-(4-chloro-phenyl)-5-(3-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
propyl~-N-methyl-glycine
LC/MS (m/z) 506, purity 91
4n, Example 52
as N-{3-[1-(4-Chloro-phenyl)-5-(3,4-dichloro-phenyl)-1,3-dihydro-isobenzofuran-
1-yl]-ethyl}-
N-methyl-glycine
LC/MS (m/z) 490, purity 89%
40, Example 53
N- {3-[ 1-(4-Chloro-phenyl)-5-(4-chloro-phenyl)- l, 3-dihydro-isob enzofuran-1-
yl]-propyl } -N-
3o methyl-glycine
LC/MS (m/z) 470, purity (LTV 94%)
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
24
4p, Example 54
N-~3-[1-(4-Chloro-phenyl)-5-(3-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-yl]-
propyl}-N-
methyl-glycine
LClMS (m/z) 450, purity 96%
s 4q, Example 55
N- f 3-[1-(4-Chloro-phenyl)-5-(2-methyl-phenyl)-1,3-dihydro-isobenzofuran-1-
yl]-propyl}-N-
methyl-glycine
LC/MS (m/z) 450, purity 93%
4r, Example 56
io N-{3-[1-(4-Chloro-phenyl)-5-(2,S-dichloro-phenyl)-1,3-dihydro-isobenzofuran-
1-yl]-propyl}-
N-methyl-glycine
LC/MS (m/z) 506, purity 91%
4s, Example 57
N- f 3-[1-(4-Chloro-phenyl)-5-(3,4-dichloro-phenyl)-1,3-dihydro-isobenzofuxan-
1-yl]-propyl}-
is N-methyl-glycine
LC/MS (m/z) 504, purity 95%
4t, Example 58
N-~3-[1-(4-chloro-phenyl)-5-(2-trifluoromethyl-phenyl)-1,3-dihydro-
isobenzofuran-1-yl]-
propyl}-N-methyl-glycine
ao LC/MS (m/z) 504, purity 78%
Pharmacological Testing
The compounds of the invention were tested in a well-recognised and reliable
test measuring
as glycine uptake:
[3H]-Glycine uptake
Cells transfected with the human GlyT-lb were seeded in 96 well plates. Prior
to the
3o experiment the cells were washed twice in HBS (10 mM Hepes-tris (pH 7,4),
2,5 mM ICI, 1
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
mM CaCl2, 2,5 mM MgS04,) and pre-incubated with test compound for 6 minutes.
Afterwards, 10 nM 3H-glycine was added to each well and the incubation was
continued for
15 minutes. The cells were washed twice in HBS. Scintillation fluid was added
and the Plates
were counted on a Trilux (Wallac) scintillation counter.
The test results were as follows:
Inhibition of Glycine Transport by hGlyT-
Compound Compound Name ICSO GIyT-lb
3f N-~3-[1-(4-methylphenyl)-1,3-dihydroisobenzoftiran-1-yl]-5400
1-propyl~ -N-methylglycine.
3k N-{3-[4-chloro-1-(4-chlorophenyl)-1,3- 4100
dihydroisobenzofuran-1-yl]-1-propyl~ -N-methylglycine.
31 N- ~3-[5-chloro-1-(4-chlorophenyl)-1,3- 5500
dihydroisobenzofuran-1-yl]-1-propyl}-N-methylalanine
3m N- f 3-[6-chloro-1-(4-chlorophenyl)-1,3- 7200
dihydroisob enzofuran-1-yl]-1-propyl~
-N-methylglycine.
3n N- ~3-[6-chloro-1-(4-methylphenyl)-1,3- 9600
dihydroisobenzofuran-1-yl]-1-propyl~-N-methylglycine.
3t N-~3-[5-cyano-1-(3-methyl-4-fluorophenyl)-1,3-5700
dihydroisobenzofuran-1-yl]-1-propyl}-N-methylglycine.
3v N- f 3-[S-cyano-1-(4-methoxyphenyl)-1,3- 8600
dihydroisobenzofuran-1-yl]-1-propyl~-N-methylglycine
3z N-{3-[5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl~-1100
N-methylglycine
3aa N-{3-[5-Chloro-1-(4-chloro-phenyl)-indan-1-yl]-propyl}-470
N-methylalanine
Sae N- f 3-[5-Bromo-1-(4-chlorophenyl)-1,3- 4000
dihydroisobenzofuran-1-yl]-1-propyl)-N-methylglycine
CA 02416447 2003-O1-17
WO 02/08216 PCT/DKO1/00510
26
3af N- f 2-[1-(4-Chloro-phenyl)-3,3-dimethyl-1,3-dihydro-3500
isobenzofuran-1-yl]-ethyl' -N-methylglycine
3ak N-[2-(3-methyl-1-phenyl-indan-1-yl)-ethyl]-amino-N-2200
methyl alanine
3a1 N-{3-[1-(4-Chloro-phenyl)-5-(4-methyl-phenyl)-1,3-2200
dihydro-isob enzo fuxan-1-yl]-ethyl -N-methyl-glycine
4c N-~3-[1-(4-Chloro-phenyl)-5-(2-thiophenyl)-1,3-dihydro-1200
isobenzofuran-1-yl]-ethyl-N-methyl-glycine
4d N-{3-[1-(4-Chloro-phenyl)-5-(4-methyl-phenyl)-1,3-1500
dihydro-isobenzofuran-1-yl]-propyl}-N-methyl-glycine
4j N- f 3-[1-(4-Chloro-phenyl)-5-(2-methyl-phenyl)-1,3-710
dihydro-isobenzofuran-1-yl]-ethyl-N-methyl-glycine
4k N-~3-[1-(4-Chloro-phenyl)-5-(2,5-dichloro-phenyl)-1,3-950
dihydro-isobenzofuran-1-yl]-ethyl}-N-methyl-glycine
The above results demonstrate that the compounds of the invention are able to
inhibit glycine
uptake into synaptosomes in micromolar concentrations