Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02495623 1992-02-27
1
REGULATORY CONTROL FOR AN
OPHTHALMOLOGICAL SURGERY SYSTEM
This is a divisional patent application of co-pending Canadian
Patent Application No. 2,061,976 filed February 27, 1992.
BACKGROUND OF THE INVENTION
This invention relates to ophthalmological surgery techniques
which employ an ultraviolet laser used to provide ablative photodecomposition
of the surface of the cornea in order to correct vision defects.
Ultraviolet laser based systems and methods are known for
enabling ophthalmological surgery on the external surface of the cornea in
order to correct vision defects by the technique known as ablative
photodecomposition of the cornea. In such systems and methods, the
irradiated flux density and exposure time of the cornea to the ultraviolet
laser
radiation are so controlled as to provide a surface sculpting of the cornea to
achieve a desired ultimate surface change in the cornea, all in order to
correct
an optical defect. Such systems and methods are disclosed in the following
U.S. patents. U.S. Patent No. 4,665,913 issued May 19, 1987 for "Method for
Ophthalmological Surgery"; U.S. Patent No. 4,669,466 issued June 2, 1987
for "Method and Apparatus for Analysis and Correction of Abnormal
Refractive Errors of the Eye"; U.S. Patent No. 4,732,148, issued
March 22, 1988 for "Method for Performing Ophthalmic Laser Surgery";
U.S. Patent No. 4,770,172 issued September 13, 1988 for "Method of
Laser-Sculpture of the Optically Used Portion of the Cornea"; and U.S. Patent
No. 4,773,414 issued September 27, 1988 for "Method of Laser-Sculpture of
the Optically Used Portion of the Cornea".
The art has now advanced to the stage at which self-contained
laser based systems are sold as stand alone units to be installed in a
surgeon's
CA 02495623 1992-02-27
2
operatory or a hospital, as desired. Thus, hospitalization is not necessarily
required in order to perform such ophthalmological surgery. Such systems
typically include a p.c. (personal computer) type work station, having the
usual
elements (i.e., keyboard, video display terminal and microprocessor based
computer with floppy and hard disk drives and internal memory), and a
dedicated
microprocessor based computer which interfaces with the p.c. work station and
appropriate optical power sensors, motor drivers and control elements of the
ultraviolet laser, whose output is delivered through an optical system to the
eye of
the patient. In use, after the patient has been accommodated on a surgery
table
or chair, the system is controlled by the operator (either the surgeon or the
surgeon and an assistant) in order to prepare the system for the delivery of
the
radiation to the patient's eye at the appropriate power level and spatial
location on
the corneal surface. Patient data is typically entered, either manually via
the p.c.
work station keyboard or from a memory storage element (e.g., a floppy disk),
and
the system automatically calculates the beam delivery parameters and displays
the
resulting calculations on the video display terminal, with an optional hard-
copy
printout via a suitable printer. The laser is also prepared to deliver the
appropriate
radiation in accordance with the calculated beam delivery parameters, and the
delivery system optics are likewise preconditioned. In some systems, a
provision
is made for permanently recording on a plastic card made of PMMA
(polymethylmethacrylate) a spot image of the laser beam used in the surgical
operation. This spot is recorded prior to the operation to ensure that the
beam
power is properly adjusted and to provide a permanent record of the beam used.
PMMA is typically used due to the characteristic of this material of having a
closely
similar ablative photodecomposition response to that of the human corneal
tissue.
After the surgery has been performed, the resultant data is typically made
part of a
permanent record, which becomes part of the patient's file.
Such systems and methods are presently emerging as the technique
of choice for ophthalmological surgery to correct various vision defects in
humans.
However, as a relatively recent development this technique in general is still
subject
to close scruutiny and careful evaluation by the medical community as welt as
by
certain regulatory agencies (e.g., the Food and Drug Administration in the
United
CA 02495623 1992-02-27
3
States of America). Although the p.c. work station provides some ability to
collect
pertinent information for the evaluation of system performance and to aid in
tracking the efficacy of the surgical technique, as well as to provide quality
control
assistance to the manufacturer of the system, existing laser systems lack a
simple
effective control mechanism for this purpose.
SUMMARY OF THE INVENTION
The invention provides a simple control mechanism for monitoring the
actual usage of ophthalmological laser surgery systems, which is relatively
inexpensive to implement and highly reliable in tracking information relating
to
machine usage and patients' data relating to surgeries performed.
In a first aspect of the invention, an ophthalmological laser surgery
system is provided with a patient data card read/write device for controlling
and
monitoring the operation of the laser surgical system in conjunction with a
pre-
coded patient data card. The data card and read/write device interact in such
a- w
manner that the laser surgical system cannot be operated unless an authorized
patient data card is inserted into the read/write device. Once the patient
data card
is recognized by the system as a legitimate and authorized card, the system is
unlocked for normal operation. Preferably, during normal operation the beam
delivery parameters calculated by the system, as well as other actual surgical
operation data (such as the configuration of the delivery system optics, the
duration and power of the laser irradiation of the patient's cornea, the
coordinates
of the projected laser beam, and the like) are recorded on the patient data
card to
form a permanent record independently of or parallel to the information stored
in
the p.c. work station. Also, a test spot of the actual laser beam can be
permanently recorded onto the patient data card by directing the beam onto a
preselected region of the data card to perform an ablation of that region.
In another aspect, the invention comprises a patient data card having
encoded therein several kinds of information for use in evaluating and
controlling a
laser based ophthalmological surgery system and surgeries performed therewith.
A first type of information comprises an authorization code required by the
surgery
system for enablement to an operative state. Preferably, this first type of
CA 02495623 1992-02-27
4
information includes a code unique to a specific laser surgery system so that
a
given patient data card can be used on one and only one machine. Further
information stored on the card identifies all authorized surgeons, the
patient, the
patient's past history, the desired prescription or other identifying
information
regarding the permissible surgery to be performed on that patient, and
preoperative diagnostic information for checking the laser system settings.
The
card may also contain downloadable software for~controlling or altering the
operation of the laser system. The card may also contain a photograph of the
patient, one or more fingerprints of the patient, or a combination of this or
other
identifier information. In addition, the card preferably contains an ablation
region
capable of forming and retaining a physical laser ablation imprint of the
intended
laser treatment for future analysis and comparison.
!n use, the card is pre-coded by the system manufacturer or some
other control agency, and issued for use with a specific system. If desired,
the
patient information may be intentionally left blank and provided by the
surgeon--or~
some other authorized person prior to the surgical operation. After the
surgery
has been performed, the actual data pertaining to the surgery is encoded onto
the
. card for future use. Preferably, the data card is issued for a single
surgery and is
invalidated immediately thereafter, e.g., by permanently recording an
invalidation
character onto the card.
The data stored on the card can be transferred from the card to any
one of a number of interested parties. The surgeon, for example, may transfer
the
information from the card to a patient data file or some other master file
maintained by the surgeon. This can be done at the data card read/write device
and the p.c. work station at the site of the laser system. In addition, the
information recorded in the patient data card can be transferred to the system
manufacturer's files either from the surgeon's office using the p.c. work
station and
a modem, or directly from the patient data card. In the latter case, the card
can
be physically transferred to the manufacturer's office by either the surgeon
or the
patient, or the patient may visit one of a number of convenient sites having a
compatible card reader device.
CA 02495623 1992-02-27
In accordance with one aspect of the present invention there is
provided a regulatory control method for use with a laser eye surgery system,
the method comprising: issuing a control token from a manufacturer or other
control agency, wherein the issued control token has enabling information
5 including a pre-coded authorization code; entering patient data for a
particular
patient into a system control unit of the laser eye surgery system; reading
the
enabling information of the control token into the system control unit,
wherein
the system control unit renders the laser eye surgery system operative in
response to said enabling information; executing the laser eye surgery system
on the patient with the operative system; and preventing operation of the
laser
eye surgery system after execution of the laser eye surgery system enabled
by said issue control token and until enabling information from another
control
token is read into the system control unit.
In accordance with another aspect of the present invention there
is provided a regulatory control method for use with a laser eye surgery
system, the method comprising: issuing a control token from manufacturer or
other control agency, wherein the issued control token has enabling
information including a pre-coded authorization code; entering patient data
for
a particular patient into a system control unit of the laser eye surgery
system;
reading the enabling information of the control token into the system control
unit, wherein the system control unit renders the laser eye surgery system
operative in response to said enabling information; executing the laser eye
surgery system on the patient with the operative system; and transferring the
patient data to the manufacturer, wherein the transferring step is performed
from the system control unit using a modem.
In accordance with yet another aspect of the present invention
there is provided a regulatory control method for use with a laser eye surgery
system, the method comprising: issuing enabling information including an
CA 02495623 1992-02-27
5a
authorization code; entering patient data for a particular patient into a
system
control unit of the laser eye surgery system; reading the enabling information
issued by a manufacturer or other control agency into the system control unit,
wherein the system control unit renders the laser eye surgery system
operative in response to the enabling information; executing the laser eye
surgery system on the patient with the operative system; and transferring the
patient data to the manufacturer.
For a fuller understanding of the nature and advantages of the
invention, reference should be had to the ensuing detailed description taken
in
conjunction with the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention will now be described in detail
hereinbelow in conjunction with the parent invention described in co-pending
Canadian Patent Application No. 2,061,976.
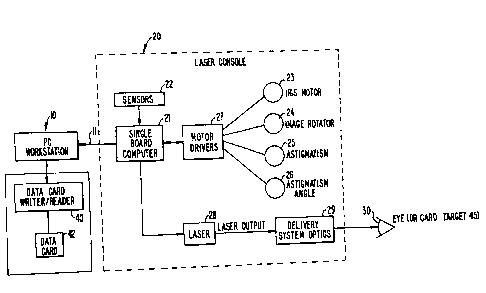
Fig. 1 is a block diagram of an ophthalmological laser surgery
system incorporating the invention; and
Fig. 2 is a plan view of a patient data card according to the
invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Turning now to the drawings, Fig. 1 illustrates a block diagram of
an ophthalmological surgery system incorporating the invention. As seen in
this figure, a p.c. work station 10 is coupled to the single board computer 21
of
a laser surgery unit 20 by means of a first bus connection 11. P.C. work
station 10 and the subcomponents of laser surgery unit 20 are known
components and preferably comprise the elements of the VISX
TWENTY/TWENTYT""
CA 02495623 1992-02-27
5b
excimer laser system available from Visx, Incorporated of Sunnyvale,
California. Thus, the laser surgery system 20 includes a plurality of sensors
generally designated with reference numeral 22 which produce feedback
signals from the moveable mechanical and optical components in the laser
optical system, such as the elements driven by an iris motor 23, an image
rotator 24, an astigmatism motor 25 and an astigmatism angle motor 26. The
feedback signals from sensors 22 are provided via appropriate signal
conductors to the single board computer 21, which is preferably an STD bus
compatible single board computer using a type 8031 microprocessor. The
single board computer 21 controls the operation of the motor drivers generally
designated with reference numeral 27 for operating the elements 23-26. In
addition, single board computer 21 controls the operation of the excimer laser
28, which is preferably an argon-fluorine laser with a 193 nanometer
wavelength output designed to provide feedback stabilized fluence of
160 mJoules per cm2 at the cornea of the patient's eye 30 via the delivery
system optics generally designated with reference numeral 29. Other
ancillary components of the laser surgery system 20 which are not necessary
to an understanding of the invention, such as a high
CA 02495623 1992-02-27
6
resolution microscope, a video monitor for the microscope, a patient eye
retention
system, and ablation effluent evacuator/filter, and the gas delivery system,
have
been omitted to avoid prolixity. Similarly, the keyboard, display, and
conventional
p.c. subsystem components (e.g., flexible and hard disk drives, memory boards
and the like) have been omitted from the depiction of the p.c. work station
10.
P.C. work station 10 is actively intercoupled with a patient data card
writer/reader 40 designed to interact with an individual patient data card 42
schematically illustrated in Fig. 2. As seen in Fig. 2, the patient data card
42 is
similar to a credit card and has a first surface region 43 for carrying
visually
readable Information, such as the name of the patient, the card supplier
(e.g., laser
surgery system manufacturer, health care provider or the like), the patient's
name
and any other information which is deemed desirable for visual presentation.
Another region 44 is reserved for information identifying the authorized
bearer or
user of the card, such as a fingerprint or a photograph of the patient. An
ablation
region or target area 45 is provided for permanently recording the laser beam -
---
operating characteristics just prior to or after performance of a surgery. For
this
purpose, ablaflon region 45 may comprise an insert of a
poiymethylmethacrylate,
which as noted above has close matching ablative photodecomposition
characteristics to that of human corneal tissue. Alternatively, the entire
card 42
may be fabricated of PMMA, or some other substance such as polycarbonate
which has similar ablation characteristics to PMMA. The purpose of the
ablation
region 45 is to provide a permanent ablative photodecomposition record
produced
by the actual laser beam used in the surgery.
Patient data card 42 is preferably an optical memory card of the type
manufactured and marketed by Drexler Technology Corporation under the
trademark LaserCard, which is a credit card sized optical data storage device
capable of holding more than four megabytes of write once/read many (WORM)
data. Similarly, the data card writer/reader 40 may be a known unit compatible
with the Drexler optical memory card. !f desired, a suitable magnetic memory
card
may be employed along with a compatible card writer/reader device 40.
The pa#ent data card 42 is initially provided with read only information
optically encoded into the subsurface recording layers (not visible in Fig.
2). This
CA 02495623 1992-02-27
7
information includes the serial number or other identifying characteristic of
a
speck laser surgery system 20 so that the data card 42 can only be used with a
specific system 20. The purpose for this limitation is to provide controlled
information relating to the amount of use of the system 20 and a match between
the identity of the system 20 and the actual beam used during the eye surgery
(the ablation record permanently formed in ablation region 45 of the data card
42).
tn addition, other qualifying data may be permanently recorded by the card
producer, such as the personal identification number of the surgeon or
surgeons
(or other personnel) qualified to operate the specific system 20, the
prescription of
the patient to control the amount and type of laser surgery on a particular
patient,
the eye upon which surgery will be allowed (e.g., right eye only, left eye
only or
both, including any differences in prescription between the two eyes), and any
other relevant and pertinent information deemed desirable for monitoring the
specific patient and the specfic system 20.
In order to render the system 20 operative, an authorized data card ~~
42 must be read by the writer/reader 40, and this information must then be
presented to the p.c. work station 10, which functions as the master control
for the
system 20. Once an authorized card has been inserted and identified, the
operation of the system 20 proceeds in a somewhat conventional fashion in that
the beam delivery parameters are calculated in the p.c. work station 10 and
transferred to the single board computer 21 for control of the various motors
23-
26, the laser 28 and the delivery system optics 29. At some time during the
surgery procedure, preferably just prior to the actual irradiation of the eye
30, the
data card 42 may be installed in a fixture (not shown) in the output beam path
of
the laser 28 (i.e., within the delivery system optics 29 or at the output side
thereof)
and the laser 28 is pulsed at the surgical rate and power to form the
permanent
record of the laser beam in the ablation region 45. Thereafter, the surgery is
performed and the post operation data is measured, calculated and stored in an
appropriate memory location within the p.c. work station i 0. Certain
information
may then be recorded onto the patient data card 42 by means of the data card
writer/reader 40 so that the data card 42 obtains post operative information
useful
for monitoring purposes. For example, the date of the operation, the total
length
CA 02495623 1992-02-27
8
of the eXposure of the corneal surface of the eye 30 to the laser beam 28, the
pulse duration, the time between pulses, the exact coordinate settings of the
laser
beam radiation throughout the operation may all be recorded on the patient
data
card 42. This information is then available until destruction of the card for
any
informational purposes the surgeon, the patient, the health insurance company,
the
regulatory agency and the system manufacturer may require. In addition, if
desired the card 42 may be permanently altered to prevent repeated use with
specific surgery system 20 or any other system 20 as an added check on the
operational use of a specific system 20.
The patient data card 42 may contain program instructions required
for the operation of the system 20. In such an embodiment, p.c. workstation 10
receives the necessary program instructions from the card 42 using a
conventional
software downloading operation at the beginning of system operation. At the
conclusion of system operation, the program instructions resident in the p.c.
workstation 10 are erased to prevent subsequent operation of system 20
withoufiwa
fresh data card 42.
As will now be apparent, laser surgery systems provided with the
personal data card functioning as a control token offer an unparalleled degree
of
control over the use of the surgery system and afford a rigorous information
gathering capability for quality control and monitoring studies. In
particular, every
single use of a given surgery system 20 can be accurately monitored by use of
the patient data card 42, and the actual operating characteristics and optical
parameters can be permanently stored in an independently verifiable manner for
future study. Such a capability is particularly important for laser surgery
systems
still subject to regulatory control, as well as to fully approved laser
surgery systems
for which cumulative historical data is highly desirable. The added cost of
the data
card reader/writer 40 is nominal compared to the overall system, and the
patient
data card is no more inconvenient to carry and use than any conventional
credit
card.
While the above provides a ful( and complete disclosure of the
preferred embodiments of the invention, various modifications, ahemate
constructions and equivalents may be employed as desired. For example, while
CA 02495623 1992-02-27
9
the invention has been described with specific reference to an optically
encoded
data card 42, data cards having read/write storage capability and using
magnetic
or semiconductor technology may be employed, as desired. In addition, other
laser surgery systems than the VISX system noted above can be used to
implement the invention. Therefore, the above description. and illustrations
should
not be construed as limiting the invention, which is defined by the appended
daims.