Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02498622 2005-03-10
WO 2004/035871 _ 1 _ PCT/IB2003/004649
ALUMINIUM ELECTROWINNING CELLS WITH METAL-BASED ANODES
Field of the Invention
This invention relates to aluminium electrowinning
cells having metal-based anodes which contain at least
one of nickel, iron and copper and which du~'ing use are
inhibited from passivating and dissolving and from
causing unacceptable contamination of the product
aluminium.
Background Art
The technology for the production of aluminium by
the electrolysis of alumina, dissolved in molten
cryolite, at temperatures around 950°C is more than one
hundred years old and still uses carbon anodes and
cathodes.
Using metal anodes in commercial aluminium
electrowinning cells would be new and drastically
improve the aluminium process by reducing pollution and
the cost of aluminium production.
US Patents 4,614,569 (Duruz/Derivaz/Debely/
Adorian), 4,680,094 (Duruz), 4,683,037 (Duruz) and
4,966,674 (Bannochie/Sherriff) describe non-carbon
anodes for aluminium electrowinning coated with a
protective coating of cerium oxyfluoride, formed in-situ
in the cell or pre-applied, this coating being
maintained by the addition of a cerium compound to the
molten cryolite electrolyte. This made it possible to
have a protection of the anode surface from the
electrolyte attack and to a certain extent from the
gaseous oxygen but not from the nascent monoatomic
oxygen.
EP Patent application 0 306 100 (Nguyen/Lazouni/
Doan) describes anodes composed of a chromium, nickel,
cobalt and/or iron based substrate covered with an
oxygen barrier layer and a ceramic coating of nickel,
copper and/or manganese oxide which may be further
covered with an in-situ formed protective cerium
oxyfluoride layer. Likewise, US Patents 5,069,771,
4,960,494 and 4,956,068 (all Nguyen/Lazouni/Doan)
CA 02498622 2005-03-10
WO 2004/035871 - 2 _ PCT/IB2003/004649
disclose aluminium production anodes with an oxidised
copper-nickel surface on an alloy substrate with a
protective oxygen barrier layer. However, full
protection of the alloy substrate was difficult to
achieve.
US Patent 6,248,227 (de Nora/Duruz) discloses an
aluminium electrowinning anode having a metallic anode
body which can be made of various alloys, for example a
nickel-iron-copper alloy. During use, the surface of the
anode body is oxidised by anodically evolved oxygen to
form an integral electrochemically active oxide-based
surface layer. The oxidation rate of the anode body is
equal to the rate of dissolution of the surface layer
into the electrolyte. This oxidation rate is controlled
by the thickness and permeability of the surface layer
which limits the diffusion of anodically evolved oxygen
therethrough to the anode body.
US Patent 6,372,099 (Duruz/de Nora) discloses the
use of transition metal species in an electrolyte below
910°C of an aluminium electrowinning cells to inhibit
dissolution of metal-based anodes of the cell.
W000/06803 (Duruz/de Nora/Crottaz) and WO00/06804
(Crottaz/Duruz) both disclose an anode produced from a
nickel-iron alloy which is surface oxidised to form a
coherent and adherent outer iron oxide-based layer whose
surface is electrochemically active. WO00/06804 also
mentions that the anode may be used in an electrolyte at
a temperature of 820° to 870°C containing 23 to 26.5
weight o AlF3, 3 to 5 weight o A1203, 1 to 2 weight a ZiF
and 1 to 2 weighty MgF2.
US Patents 5,006,209 and 5,284,562 (both
Beck/Brooks), 6,258,247 and 6,379,512 (both Brown/
Brooks/Frizzle/Juric), 6,419,813 (Brown/Brooks/Frizzle)
and 6,436,272 (Brown/Frizzle) all disclose the use of
nickel-copper-iron anodes in an aluminium production
electrolyte at 660°-800°C containing 6-26 weighto NaF,
7-33 weighto KF, 1-6 weighto ZiF and 60-65 weighto A1F3.
The electrolyte may contain A1203 in an amount of up to
30 weighto, in particular 5 to 10 or 15 weight%, most of
which is in the form of suspended particles and some of
which is dissolved in the electrolyte, i.e. typically 1
to 4 weighto dissolved A1203. In US Patents 6,258,247,
6,379,512, 6,419,813 and 6,436,272 such an electrolyte
CA 02498622 2005-03-10
WO 2004/035871 _ 3 _ PCT/IB2003/004649
is said to be useable at temperatures up to 900°C. In US
Patents 6,258,247 and 6,379,512 the electrolyte further
contains 0.004 to 0.2 weighto transition metal additives
to facilitate alumina dissolution and improve cathodic
operation.
US Patent 5,725,744 (de Nora/Duruz) discloses an
aluminium production cell having anodes made of nickel,
iron and/or copper in a electrolyte at a temperature
from 680 ° to 880 °C containing 42-63 weight o A1 F3, up to
48 weight o NaF, up to 48 weight o LiF and 1 to 5 weight o
A1203. MgF~, KF and CaF2 are also mentioned as possible
bath constituents.
Metal or metal-based anodes are highly desirable in
aluminium electrowinning cells instead of carbon-based
anodes. Many attempts were made to use metallic anodes
for aluminium production, however they were never
adopted by the aluminium industry for commercial
aluminium production because their lifetime was too
short and needs to be increased.
Summarv of the Invention
One object of the invention is to provide an
aluminium electrowinning cell incorporating metal-based
anodes which remain substantially insoluble at the cell
operating temperature and which can be operated without
passivation or excessive contamination of the produced
aluminium.
Another object of the invention is to provide an
aluminium electrowinning cell operating with a crustless
and ledgeless electrolyte, which can achieve high
productivity, low contamination of the product
aluminium, and whose components resist corrosion and
wear.
The invention relates to a cell for electrowinning
aluminium from alumina. The cell comprises: a metal-
based anode having an outer part that contains at least
one of nickel, cobalt and iron and that has an
electrochemically active oxide-based surfaced and a
fluoride-containing molten electrolyte at a temperature
below 940°C, in particular in the range from 880° to
920°C, in which the active anode surface is immersed.
The electrolyte consists of : 5 to 14 weight o overall of
CA 02498622 2005-03-10
WO 2004/035871 _ 4 _ PCT/IB2003/004649
dissolved alumina; 35 to 45 weighto aluminium fluoride;
30 to 45 weighto sodium fluoride; 5 to 20 weighto
potassium fluoride; 0 to 5 weighto calcium fluoride; and
0 to 5 weighto in total of one or more further
constituents.
For instance, the electrolyte consists of: 7 to 10
weighto dissolved alumina; 38 to 42 weight% aluminium
fluoride; 34 to 43 weighto sodium fluoride; 8 to 15
weighto potassium fluoride; 2 to 4 weighto calcium
fluoride; and 0 to 3 weighto in total of one or more
further constituents.
Such an electrolyte composition is well adapted for
aluminium electrowinning at reduced temperature, i.e. at
a temperature below the conventional aluminium
electrowinning temperature of about 950°C, using a
metal-based anode containing at least one of nickel,
cobalt and iron, usually in metallic and/or oxide form.
The electrolyte is particularly adapted for anodes
containing at least one of metallic nickel, metallic
cobalt and oxides of iron. Oxides of iron include
ferrous oxide, hematite, magnetite and ferrites (e. g.
nickel ferrite), in stoichiometric and non-
stoichiometric form. For example, the anode has a
metallic alloy body that contains one or more of these
metals - nickel, cobalt and iron - and that is covered
with an integral active oxide layer or film.
The presence in the electrolyte of potassium
fluoride in the given amount has two effects. On the one
hand, it leads to a reduction of the operating
temperature by up to several tens of degrees without
increase of the electrolyte's aluminium fluoride content
or even a reduction thereof compared to standard
electrolytes operating at about 950°C with an aluminium
fluoride content of about 45 weighto. On the other hand,
it maintains a high solubility of alumina, i.e. up to
above about 14 weighto, in the electrolyte even though
the temperature of the electrolyte is reduced by a few
tens of degrees compared to conventional temperature.
Hence, in contrast to prior art low temperature
electrolytes which carry large amounts of undissolved
alumina in particulate form, according to the present
invention a large amount of alumina in the electrolyte
is in a dissolved form.
CA 02498622 2005-03-10
s~:n
- 5 -
~ without being bound to any theory, it is believed
that combining a high concentration of dissolved alumina
in the electrolyte and a limited concentration of
aluminium fluoride leads predominantly to the formation
of (basic) fluorine-poor aluminium vxyfluoride ions
( [A,1202F41 Z-) instead of (acid) fluorine-rich aluminium
vxyfluoride ions ( [A120F6]2-) near the anode. As opposed
to acid fluorine-rich aluminium oxyfluoride ions, basic
fluorine-poor aluminium ~oxyfluoride ions do not
significantly paaeivate the anode's nickel and cobalt,
or dissolve the anode's iron. In particular, basic
fluorine-poor aluminium oxyfluoride ions dv not
significantly passivate metallic nickel and cobalt, or
dissolve iron oxides. The weight ratio of dissolved
alumina/alurninium fluoride in the electrolyte should be
above 1/7, and often above 1/6.5 or even above 1/6, to
vbta3~n a favourable ratio of the fluorine-poor aluminium
oxyfluoride ions and the fluorine-rich aluminium
oxyfluoride ions.
It follows that the use of the above described
electrolyte with metal-based anodes containing at Isast
one of nickel, cobalt and iron inhibits paaaivation and
corrosion thereof.
In order to maintain the alumina concentration
abvv~ the given threshold during normal electrolysis,
the cell is preferably fitted with means tv monitor and
adjust the electrolyte's alumina content.
The abovernentioned one yr more further constituents
of the electrolyte may comprise at least one fluoride
selected from magnesium fluoride, lithium fluoride,
cesium fluoride, rubidium fluoride, strontium fluoride,
barium fluoride and cerium fluoride_
Advantageously, the cell is sufficiently insulated
tv be operated with a substantially crustless and/or
ledgeless electrvlytp_ Suitable cell insulation is
disclosed in U5 Patent 6,402,928 (de Nora/Sekhar),
W002/070784 and US Publicativzl 2003/0102228 (both de
Nara/Herclaz).
The cell can have a cathode that has an aluminium-
4o wettabla surface, in particular a drained horiaontal or
inclined surface. Suitable cathode designs are for
examples disclosed in US Patents 5,683,559, 5,888,360,
CA 02498622 2005-03-10
WO 2004/035871 PCT/IB2003/004649
- 6 -
6,093,304 (all de Nora), 6,258,246 (Duruz/de Nora),
6,358,393 (Berclaz/de Nora) and 6,436,273 (de
Nora/Duruz), and in PCT publications W099/02764 (de
Nora/Duruz), WO00/63463 (de Nora), W001/31086 (de
Nora/Duruz), W001/31088 (de Nora), W002/070785 (de
Nora), W002/097168 (de Nora), W002/097168 (de Nora),
W003/023091 (de Nora) and W003/023092 (de Nora).
The cathode can have an aluminium-wettable coating
that comprises a refractory boride and/or an aluminium-
wetting oxide. Suitable aluminium-wettable materials are
disclosed in W001/42168 (de Nora/Duruz), W001/42531
(Nguyen/Duruz/de Nora), W002/070783 (de Nora),
W002/096831 (Nguyen/de Nora) and W002/096830 (Duruz/
Nguyen/de Nora).
The anode can have a metallic or cermet body and an
oxide layer integral with or applied on the anode body.
Usually, the anode body is made from an iron alloy,
in particular an alloy of iron with nickel and/or
cobalt. Suitable alloys are disclosed in US Patents
6,248,227 (de Nora/Duruz), 6,521,115 (Duruz/de
Nora/Crottaz), 6,562,224 (Crottaz/Duruz), and in PCT
publications W000/40783 (de Nora/Duruz), W001/42534 (de
Nora/Duruz), W001/42536 (Duruz/Nguyen/de Nora),
W002/083991 (Nguyen/de Nora), W003/014420
(Nguyen/Duruz/de Nora) and W003/078695 (Nguyen/de Nora).
For example, the anode body is made from an alloy
consisting of:
- 40 to 80o nickel and/or cobalt, in particular
50 to 60 weighto;
- 9 to 55 weighto iron, in particular 25 to 40
weight%;
- 5 to 15 weight o copper, in particular 6 to 12
weight o ;
- 0 to 4 weighto in total of at least one of
aluminium, niobium and tantalum, in particular 0.5 to
2 weighto; and
- 0 to 2 weighto in total of further
constituents, in particular 0.5 to 1 weighto.
Typically such an alloy is oxidised prior to or
during use. This can lead to diffusion of metals in the
anode, especially at the alloy's surface, which locally
changes the alloy's composition.
CA 02498622 2005-03-10
WO 2004/035871 _ ~ _ PCT/IB2003/004649
The anode body can be covered with an integral iron
oxide-based layer containing less than about 35 weighto
nickel oxide and/or cobalt oxide, in particular from 5
to 10 weighto nickel oxide. Such integral layers are
usually obtained by preoxidation of the body before
and/or during use in the cell.
The anode may also comprise an applied iron oxide-
based coating. Suitable iron oxide-based coatings are
disclosed in US Patents 6,361,681 (de Nora/Duruz),
6,365,018 (de Nora), 6,379,526 (de Nora/Duruz) and
6,413,406 (de Nora), and in PCT applications
PCT/IB03/01479, PCT/IB03/03654 and PCT/IB03/03978 (all
Nguyen/de Nora). For example, the anode coating contains
Fe~03 and optionally: at least one dopant selected from
TiO~, Zn0 and Cu0 and/or at least one inert material
selected from nitrides and carbides.
Especially when used in the upper part of the
abovementioned operating temperature range (e.g. 910°-
940°C), the anode can comprise an applied cerium
oxyfluoride-based outermost coating, for example as
disclosed in the abovementioned US Patents 4,614,569,
4,680,094, 4,683,037 and 4,966,674 or PCT Applications
W002/070786 (Nguyen/de Nora) and W002/083990 (de
Nora/Nguyen). Such a coating may be applied before or
during use and maintained during use by the presence of
cerium species in the electrolyte.
A nickel-containing stem can be used to suspend the
anode in the electrolyte, in particular a stem having a
nickel-containing core covered with an applied oxide
coating, such as a coating containing aluminium oxide
and titanium oxide. The core of the stem can comprise a
copper inner part and a nickel-based outer part. Further
details of anode stems are disclosed in PCT/IB03/02702
(Crottaz/Duruz).
Suitable anode designs are for example disclosed in
W099/02764 (de Nora/Duruz), W000/40781, W000/40782,
W003/023091, W003/023092 and W003/006716 (all de Nora).
Usually, the cell comprises at least one component,
e.g. the cathode, that contains a sodium-active cathodic
material, such as elemental carbon. This sodium-active
cathodic material is preferably shielded from the
electrolyte by a sodium-inert layer to inhibit the
CA 02498622 2005-03-10
WO 2004/035871 _ 8 _ PCT/IB2003/004649
presence in the molten electrolyte of soluble
cathodically-produced sodium metal that constitutes an
agent for dissolving the active oxide-based anode
surface. This mechanism is explained in greater detail
in US Application 2003/0075454 and W003/083176 (both de
Nora/Duruz).
The invention also relates to a cell that
comprises:
- a metal-based anode having an outer part that has an
electrochemically active oxide-based surface and that
is made from an alloy consisting of : 50 to 60 weight o
in total of nickel and/or cobalt; 25 to 40 weight%
iron; ~ to 12 weighto copper; 0.5 to 2 weighty
aluminium and/or niobium; and 0.5 to 1.5 weighto in
total of further constituents, the anode comprising an
applied hematite-based coating and optionally a cerium
oxyfluoride-based outermost coating;
- a nickel-containing anode stem for suspending the
anode in the electrolyte, the stem being covered with
a coating of aluminium oxide and titanium oxide;
- a fluoride-containing molten electrolyte at a
temperature in the range from 880° to 920 or 930°C, in
which the active anode surface is immersed and which
consists of : 7 to 10 weight o dissolved alumina; 38 to
42 weighto aluminium fluoride; 34 to 43 weighto sodium
fluoride; 8 to 15 weighto potassium fluoride; 2 to 4
weighto calcium fluoride; and 0 to 3 weighto in total
of one or more further constituents; and
- a cathode having an aluminium-wettable surface, in
particular a drained horizontal or inclined surface,
formed by an aluminium-wettable coating of refractory
hard material and/or aluminium-wetting oxide.
A further aspect of the invention relates to a
method of electrowinning aluminium in a cell as
described above. The method comprises electrolysing the
dissolved alumina to produce oxygen on the anode and
aluminium cathodically, and supplying alumina to the
electrolyte to maintain therein a concentration of
dissolved alumina of 5 to 14 weight%, in particular 7 to
10 weighto.
Brief Description of Drawings
The invention will be further described with
reference to the accompanying drawings, in which:
CA 02498622 2005-03-10
WO 2004/035871 - 9 _ PCT/IB2003/004649
- Figures 1a and 1b schematically show respectively
a side elevation and a plan view of an anode for use in
a cell according to the invention;
- Figures 2a and 2b show a schematic cross-
sectional view and a plan view, respectively, of an
aluminium production cell for equipment with a potassium
fluoride-containing electrolyte and a metal-based anode
according to the invention; and
- Figure 3 shows a schematic cross-sectional view
of another aluminium production cell for equipment with
a potassium fluoride-containing electrolyte and a metal-
based anode according to the invention.
Detailed Description
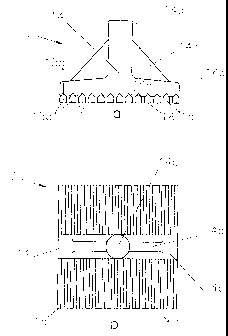
Figures 1a and 1b schematically show an anode 10
which can be used in a cell for the electrowinning of
aluminium according to the invention.
The anode 10 comprises a series of elongated
straight anode members 15 connected to a cast or
profiled support 14 for connection to a positive bus
bar.
The cast or profiled support 14 comprises a lower
horizontally extending foot 14a for electrically and
mechanically connecting the anode members 15, a stem 14b
for connecting the anode 10 to a positive bus bar and a
pair of lateral reinforcement flanges 14c between the
foot 14a and stem 14b.
The anode members 15 may be secured by force-
fitting or welding the foot 14a on flats 15c of the
anode members 15. As an alternative, the connection
between the anode members 15 and the corresponding
receiving slots in the foot 14a may be shaped, for
instance like dovetail joints, to allow only
longitudinal movements of the anode members.
The anode members 15 have a bottom part 15a which
has a substantially rectangular cross-section with a
constant width over its height and which is extended
upwardly by a tapered top part 15b with a generally
triangular cross-section. Each anode member 15 has a
flat lower oxide surface 16 that is electrochemically
CA 02498622 2005-03-10
WO 2004/035871 PCT/IB2003/004649
- zo -
active for the anodic evolution of oxygen during
operation of the cell. Also, the anode may be covered
with a coating of iron oxide-based material, for example
applied from a composition as set out in Table III
below, and/or a coating of one or more cerium compounds
in particular cerium oxyfluoride.
The anode members 15, in particular their bottom
parts 15a, are made of an iron alloy comprising nickel
and/or cobalt as disclosed in Table II below. The
lifetime of the anode may be increased by a protective
coating made of cerium compounds, in particular cerium
oxyfluoride as discussed above.
The anode members 15 are in the form of parallel
rods in a coplanar arrangement, laterally spaced apart
from one another by inter-member gaps 17. The inter-
member gaps 17 constitute flow-through openings for the
circulation of electrolyte and the escape of anodically-
evolved gas released at the electrochemically active
surfaces 16.
Figure 2a and 2b show an aluminium electrowinning
cell having a series of metal-based anodes 10 in a
fluoride-containing cryolite-based molten electrolyte 5
containing dissolved alumina according to the invention.
The electrolyte 5 has a composition that is
selected from Table I below. The metal-based anodes 10
have a composition selected from Table II below,
optionally with a protective coating made of cerium
compounds, in particular cerium oxyfluoride as discussed
above.
The anodes 10 are similar to the anode shown in
Figs. 1a and 1b. Suitable alternative anode designs are
disclosed in W000/40781, W000/40782 and W003/006716 (all
de Nora ) .
The drained cathode surface 20 is formed by tiles
21A which have their upper face coated with an
aluminium-wettable layer. Each anode 10 faces a
corresponding tile 21A. Suitable tiles are disclosed in
greater detail in W002/096830 (Duruz/Nguyen/de Nora).
Tiles 21A are placed on upper aluminium-wettable
faces 22 of a series of carbon cathode blocks 25
CA 02498622 2005-03-10
WO 2004/035871 PCT/IB2003/004649
- 11 -
extending in pairs arranged end-to-end across the cell.
As shown in Figures 2a and 2b, pairs of tiles 21A are
spaced apart to form aluminium collection channels 36
that communicate with a central aluminium collection
groove 30.
The central aluminium collection groove 30 is
located in or between pairs of cathode blocks 25
arranged end-to-end across the cell. The tiles 21A
preferably cover a part of the groove 30 to maximise the
surface area of the aluminium-wettable cathode
surface 20.
As explained hereafter, the cell is thermally
sufficiently insulated to enable ledgeless and crustless
operation.
The cell comprises sidewalk 40 made of an outer
layer of insulating refractory bricks and an inner layer
of carbonaceous material exposed to molten electrolyte 5
and to the environment thereabove. These sidewalls 40
are protected against the molten electrolyte 5 and the
environment thereabove with tiles 21B of the same type
as tiles 21A. The cathode blocks 25 are connected to the
sidewalls 40 by a peripheral wedge 41 which is resistant
to the molten electrolyte 5.
Furthermore, the cell is fitted with an insulating
cover 45 above the electrolyte 5. This cover inhibits
heat loss and maintains the surface of the electrolyte
in a molten state. Further details of suitable covers
are disclosed in the abovementioned references.
In operation of the cell illustrated in Figs. 2a
and 2b, alumina dissolved in the molten electrolyte 5 at
a temperature of 880° to 940°C is electrolysed between
the anodes 10 and the cathode surface 20 to produce gas
on the operative anodes surfaces 16 and molten aluminium
on the aluminium-wettable drained cathode tiles 21A.
The cathodically-produced molten aluminium flows on
the drained cathode surface 20 into the aluminium
collection channels 36 and then into the central
aluminium collection groove 30 for subsequent tapping,
CA 02498622 2005-03-10
WO 2004/035871 _ 12 - PCT/IB2003/004649
The cell shown in Figure 3 comprises a plurality of
metal-based anodes 10 dipping in a molten electrolyte 5
according to the invention.
The anodes 10 are similar to the anode shown in
Figs. la and lb. Suitable alternative anode designs are
disclosed in W000/40781, W000/40782, W003/006716 and
W003/023092 (all de Nora).
The cell bottom comprises a series of pairs of
spaced apart carbon cathode blocks 25 placed across the
cell and having an aluminium-wettable upper surface 22
formed by an aluminium-wettable layer. The upper
surfaces 22 are covered with aluminium-wettable openly
porous plates 21 which are filled with molten aluminium
to form an aluminium-wetted drained active cathode
surface 20 above the upper surfaces 22 of the carbon
cathode blocks 25. Further details of such a cathode
bottom are disclosed in W002/097168 and W002/097169
(both de Nora).
The cathode blocks 25 are made of graphite and have
a reduced height, e.g. 30 cm, and are coated with an
aluminium-wettable layer which forms the upper surface
22 and which protects the graphite from erosion and
wear. Suitable aluminium-wettable layers are disclosed
in US Patent 5,651,874, W098/17842, W001/42168 and
W001/42531. The aluminium-wettable openly porous plates
21 covering the coated cathode blocks 25 can be made of
the material disclosed in W002/070783 (de Nora).
The cell bottom further comprises a centrally-
located recess 35 which extends at a level below the
upper surfaces 22 of the carbon cathode blocks 25 and
which during use collects molten aluminium 60 drained
from the aluminium-wettable drained active cathode
surface 20.
The aluminium collection recess 35 is formed in a
reservoir body 30 which is placed between the blocks 25
of each pair of cathode blocks and spaces them apart
across the cell. As shown in Figure 3, the recess 35
formed in the reservoir body 30 is generally U-shaped
with rounded lower corners and an outwardly curved upper
part.
CA 02498622 2005-03-10
WO 2004/035871 _ 13 _ PCT/IB2003/004649
The reservoir body 30 is made of two generally Z-
shaped sections 31 assembled across the cell. The
reservoir sections 31 are made of anthracite-based
material. The aluminium-wettable layer forming the upper
surfaces 22 extends in the recess 35 to protect the
reservoir body 30 during use against wear and sodium or
potassium intercalation.
As shown in Figure 3, the reservoir body 30 extends
below the cathode blocks 25 into the refractory and
insulating material 26 of the cell bottom permitting
maximisation of the capacity of the aluminium collection
recess 35.
Furthermore, the reservoir body 30 has a solid base
32 which extends from above to below the bottom face of
the cathode blocks 25 and provides sufficient mechanical
resistance to keep the blocks 25 properly spaced apart
across the cell when exposed to thermal expansion during
start-up of the cell and normal operation. As shown in
dotted lines in the upper part of the reservoir body 30,
longitudinally spaced apart spacer bars 33 placed across
the reservoir body 30 may provide additional mechanical
strength to the reservoir body 30. Such spacer bars 33
can be made of carbon material coated with an aluminium-
wettable protective layer.
The openly porous plates 21 placed on the upper
surfaces 22 of the carbon cathode blocks 25 and located
in the central region of the cell bottom extend over
part of the aluminium collection recess 35 so that
during use the protruding part of the aluminium-wetted
drained active cathode surface 20 is located over the
recess 35.
The openly porous plates 21 are spaced apart over
the aluminium collection recess 35 to leave an access
for the tapping of molten aluminium through a
conventional tapping tube. The spacing between the
openly porous plates 21 over the aluminium collection
recess can be much smaller along the remaining parts of
the recess 35, thereby maximising the surface area of
the active cathode surface 20.
The cell shown in Figure 3 comprises a series of
corner pieces 41 made of the same openly porous material
as plates 21 and filled with aluminium and placed at the
CA 02498622 2005-03-10
WO 2004/035871 PCT/IB2003/004649
- 14 -
periphery of the cell bottom against sidewalls 40. The
sidewalls 40 and the surface of the electrolyte 5 are
covered with a ledge and a small crust of frozen
electrolyte 6. The cell is fitted with an insulating
cover 45 above the electrolyte crust 6. Further details
of suitable covers are disclosed in the abovementioned
references.
The cell is also provided with exhaust pipes (not
shown) that extend through the cover 45 for the removal
of gases produced during electrolysis.
The cell comprises alumina feeders 50 with feeding
tubes 51 that extend through the insulating cover 45
between the anodes 10. The alumina feeders 50 are
associated with a crust breaker (not shown) for breaking
the crust 6 underlying the feeding tube 51 prior to
feeding.
In a variation, the insulating material of the
sidewalls 40 and cover 45 may be sufficient to prevent
formation of any ledge and crust of frozen electrolyte.
In such a case, the sidewalls 40 are preferably
completely shielded from the molten electrolyte 5 like
in the cell of Figs. 2a and 2b or by a lining of the
aforesaid openly porous material filled with aluminium.
Enhanced alumina dissolution may be achieved by
utilising an alumina feed device which sprays and
distributes alumina particles over a large area of the
surface of the molten electrolyte 5. Suitable alumina
feed devices are disclosed in US Patent 6,572,757 (de
Nora/Berclaz) and in W003/006717 (Berclaz/Duruz).
Furthermore, the cell may comprise means (not shown) to
promote circulation of the electrolyte 5 from and to the
anode-cathode gap to enhance alumina dissolution in the
electrolyte 5 and to maintain in permanence a high
concentration of dissolved alumina close to the active
surfaces of anodes 10, for example as disclosed in
60000/40701 (de Nora) .
During operation of the cell shown in Figure 3,
alumina dissolved in the electrolyte 5 is electrolysed
to produce oxygen on the anodes 10 and aluminium 60 on
the drained cathode surfaces 20. The product aluminium
60 drains from the cathode surfaces 20 over the openly
CA 02498622 2005-03-10
WO 2004/035871 PCT/IB2003/004649
- 15 -
porous plates 21 that extend over part of the reservoir
30 into the reservoir 30 from where it can be tapped.
Hence, aluminium is produced on the drained active
cathode surface 20 which covers not only the cathode
blocks 25 but also part of the reservoir 30, thereby
maximising the useful aluminium production area (i.e.
the drained cathode surface 22) of the cell.
Figs. 2a, 2b and 3 show specific aluminium
electrowinning cells by way of example. It is evident
that many alternatives, modifications, and variations
will be apparent to those skilled in the art.
For instance, the cell may have a sloping cathode
bottom, as disclosed in W099/02764 (de Nora/Duruz), and
optionally one or more aluminium collection reservoirs
across the cell, each intersecting the collection groove
to divide the drained cathode surface into four
quadrants as described in W000/63463 (de Nora).
Examples of electrolyte compositions according to
the invention are given in Table 1, which shows the
weight percentages of the indicated constituents for
each specimen electrolyte A1-Il at a given temperature.
TABLE l
AlF3 NaF KF CaF~ A1~03 TC
A1 40.4 42.6 6 3 8 935
B1 40.6 41.4 7 3 8 930
C1 40.4 39.6 9 3 8 915
Dl 40.2 37.8 11.5 2.5 8 900
E1 43.5 40 6.5 2 g 8g5
F1 40 3~ 13 3 g 8g0
G1 42 40 8 2 8 890
H1 36 36.5 16 3.5 8 880
I1 38 35 14 4 8 870
CA 02498622 2005-03-10
WO 2004/035871 PCT/IB2003/004649
- 16 -
Examples of alloy compositions of suitable metal-
based anode are given in Table 2, which shows the weight
percentages of the indicated metals for each specimen
alloy A2-K2.
TABLE 2
Ni Co Fe Cu Al Nb Ta other
A2 57 - 30 1fl 2 - - 1
B2 48 - 39 10 2 - - 1
C2 57 - 31 10 1 - - 1
D2 25 43 25 7 - - - _
E2 - 42 50 6 0.5 - 1 0.5
F2 - 45 45 9 - - _ 1
G2 25 25 38 10 - 2 - _
H2 45 - 40 11 - - 2.5 1.5
I2 42 - 42 12 - 3 - 1
J2 21 30 35 13 1 - - -
K2 29 39 22 6 2 - - 1
The "other" elements refer to minor additives such
as manganese, silicon and yttrium which may be present
in individual amounts of 0.2 to 1.5 weighto. Usual
impurities, such.as carbon, have not been listed in
Table 2.
Usually, these alloys will be surface oxidised
before use and further oxidised during use, as described
in the Examples below.
CA 02498622 2005-03-10
WO 2004/035871 PCT/IB2003/004649
- 17 -
Examples of starting compositions of particle
mixtures for producing hematite-based protective anode
coatings are given in Table 3, which shows the weight
percentages of the indicated constituents for each
specimen starting composition of the coating A3-L3.
TABLE 3
Fe203 BN A1N ZrC Ti02 Zr02 Zn0 Ta~05 Cu0
A3 78 10 __ __ 10 __ __ __ 2
B3 78 10 __ __ __ __ 10 _- 2
C3 70 18 -- -- -- -- 10 -- 2
D3 78 10 __ __ __ 10 __ __ 2
E3 80 10 -- -- -- -- -- -- 10
F3 78 10 __ __ __ __ __ 10 2
G3 78 -- 10 -- 10 __ __ __ 2
H3 78 -- 12 __ __ __ 5 3 2
I3 70 10 4 3 -- 2 5.5 3 2.5
J3 75 14 __ __ 5 5 __ __ 1
K3 85 5 4 __ __ __ 6 __ __
L3 75 __ __ 12 5 __ __ 5 3
Comparative Example
A metal-based anode was tested in a potassium
fluoride-free electrolyte at 900°C.
The anode was manufactured from a rod of diameter
mm and total length 20 mm made from a cast nickel-
iron alloy having the composition of sample A2 of Table
15 2. The anode rod was supported by a stem made of an
alloy containing nickel, chromium and iron, such as
Inconel, protected with an alumina sleeve. The anode was
suspended for 16 hours over the molten fluoride-based
CA 02498622 2005-03-10
WO 2004/035871 _ 18 _ PCT/IB2003/004649
electrolyte whereby its surface was oxidised prior to
immersion into the electrolyte.
Electrolysis was carried out by fully immersing the
anode rod in the molten electrolyte. The potassium
fluoride-free electrolyte contained 49 weighto aluminium
fluoride (A1F3), 43 weighto aluminium fluoride (NaF), 4
weight% calcium fluoride (CaF2) and 4 weighto alumina
(A1~03). The saturation concentration of alumina in such
an electrolyte, unattainable in practice, is at 5
weighto.
The current density was about 0.8 A/cm~ and the cell
voltage was at 3.6-3.8 volt for 24 hours. The
concentration of dissolved alumina in the electrolyte
was maintained during the entire electrolysis by
periodically feeding fresh alumina into the cell.
After 32 hours the cell voltage increased to 10
volt and electrolysis was interrupted. The anode was
extracted. Upon cooling the anode was examined
externally and in cross-section.
The anode's outer dimensions had remained
substantially unchanged. The anode's oxide outer part
had grown from an initial thickness of about 70 micron
to a thickness after use of about up to 1000 micron. A
yellow-green layer of nickel fluoride (NiF~) was observed
between the oxide outer part and the metallic inner part
of the anode. Such a nickel fluoride layer is
substantially non-conductive and passivates the anode,
which caused the voltage increase.
Furthermore, a vermicular structure was observed in
the metallic inner part immediately underneath the
nickel fluoride layer over a depth of about 2 to 3 mm.
The vermicular structure had mainly empty pores that had
an average diameter of about 20 to 30 micron.
Example 1
A test was carried out with a cell according to the
invention comprising: a molten potassium fluoride-
containing electrolyte at 900°C having the composition
of sample D1 of Table I, i.e. rich in dissolved alumina,
CA 02498622 2005-03-10
WO 2004/035871 _ 19 _ PCT/IB2003/004649
and an anode made from a nickel-iron alloy having the
composition of sample A2 of Table 2.
The anode was manufactured like in the Comparative
Example and suspended for 16 hours over the molten
electrolyte.
Electrolysis was carried out in the same potassium
fluoride-containing electrolyte: The current density was
about 0.8 A/cm2 and the cell voltage was stable at 3.8
volt during the entire test. The dissolved alumina-
content was maintained around 8 weighto by periodically
feeding fresh alumina into the cell.
After 50 hours electrolysis was interrupted and the
anode extracted. Upon cooling the anode was examined
externally and in cross-section.
The anode's outer dimensions had remained
substantially unchanged. The anode's oxide outer part
had grown from an initial thickness of about 70 micron
to a thickness after use of about up to 500 micron,
instead of the 1000 micron observed in the Comparative
Example. Also, no passivating yellow-green layer of
nickel fluoride (NiF~) was observed.
Immediately underneath the oxide outer part, a
vermicular structure was observed in the metallic inner
part over a depth of about 0.5 to 1 mm, instead of the 2
to 3 mm of the Comparative Example. The vermicular
structure had pores which were partly filled with
oxides, in particular iron oxides, and which had an
average diameter of about 2 to 5 micron.
Example 2
Example 1 was repeated with an anode made form the
nickel-cobalt-iron alloy composition of sample D2 of
Table 2 which was prepared, like in Example 1, over a
potassium fluoride-containing electrolyte having the
composition of sample D1 of Table l, i.e. rich in
dissolved alumina. The anode was then tested in the
electrolyte like in Example 1 and showed similar
results.
CA 02498622 2005-03-10
WO 2004/035871 _ 2 p - PCT/IB2003/004649
Example 3
Example 1 was repeated with an anode made from the
nickel-iron alloy composition of sample H2 of Table 2
prepared, like in Example 1, over a potassium fluoride-
s containing electrolyte having the composition of sample
D1 of Table 1, i.e. rich in dissolved alumina. The anode
was then tested in the electrolyte like in Example 1.
After 50 hours electrolysis was interrupted and the
anode extracted. Upon cooling the anode was examined
externally and in cross-section.
The anode's outer dimensions had remained
substantially unchanged. The anode's oxide outer part
had grown from an initial thickness of about 70 micron
to a thickness after use of about up to 1000 micron like
in the Comparative Example. However, no passivating
yellow-green layer of nickel fluoride (NiF2) was
observed.
A vermicular structure was observed in the metallic
inner part immediately underneath the oxide outer part
over a depth of about 1.5 to 2 mm, instead of the 2 to 3
mm of the Comparative Example. The vermicular structure
had pores which were partly filled with oxides, in
particular iron oxides, and which had an average
diameter of about 2 to 5 micron.
Example 4
Example 1 was repeated with an anode made from the
nickel-iron alloy composition of sample A2 of Table 2
which was prepared, like in Example 1, over a potassium
fluoride-containing electrolyte having the composition
of sample A1 of Table l, i.e. rich in dissolved alumina.
The anode was then tested in the electrolyte like in
Example 1 and showed similar results.
Example 5
Examples 1 to 4 can be repeated using different
combinations of electrolyte compositions (A1-I1)
selected from Table 1 and anode alloy compositions (A2-
K2) selected from Table 2.
CA 02498622 2005-03-10
WO 2004/035871 _ 21 _ PCT/IB2003/004649
Example 6
Another aluminium electrowinning anode was prepared
as follows:
A slurry for coating an anode was prepared by
suspending in 32.5 g of an aqueous solution containing 5
weighto polyvinyl alcohol (PVA) 67.5 g of a particle
mixture made of hematite Fe203 particles, boron nitride
particles, Ti02 particles and Cu0 particles (with.
particle size of -325 mesh, i.e. smaller than 44 micron)
in a weight ratio corresponding to sample A3 of Table 3.
An anode made of the nickel-iron alloy of sample A2
of Table 2 was covered with ten layers of this slurry
that were applied with a brush. The applied layers were
dried for 10 hours at 140°C in air and then consolidated
at 950°C for 16 hours to form a protective hematite
based coating which had a thickness of 0.4 to 0.45 mm.
During consolidation, the Fe~03 particles were
sintered together into a microporous matrix with a
volume contraction. The TiO~ particles and Cu0 particles
were dissolved in the sintered Fe203. The boron nitride
particles remained substantially inert during the
sintering but prevented migration and agglomeration of
the micropores into cracks.
Underneath the coating, an integral oxide scale
mainly of iron oxide had grown from the anode's alloy
during the heat treatment and combined with iron oxide
and titanium oxide from the coating to firmly anchor the
coating to the oxidised alloy. The integral oxide scale
contained titanium oxide in an amount of about 10 metal
weighto. Minor amounts of copper, aluminium and nickel
were also found in the oxide scale (less that 5 metal
weighto in total).
Electrolysis was carried out in a potassium
fluoride-containing electrolyte at 900°C having the
composition of sample D1 of Table 1, i.e. rich in
dissolved alumina. The current density was about 0.8
A/cm2 and the cell voltage was stable at 3.6 volt during
the entire test, instead of the 3.8 volt observed in
Examples 1 to 4. The dissolved alumina-content was
i CA 02498622 2005-03-10
- as -
~ maintained around 8 weight% by periodically feeding
fresh alumina into the ce7.l_
After 50 hours electrolysis was interrupted and the
anode extracted. Upon cooling the anode was examined
externally and in cross-section.
The anode's outer dimensions as well as the anode's
coating had remained substantially unchanged. However,
Ti02 had selectively been dissolved in the electroiyte
from the coating. The anode's structure underneath the
coating was similar to the structure observed in
Examples 1 to
Samples of the used electrolyte and the" product
aluminium were also analysed. It Was found that the
electrolyte contained lees that 7o ppm nickel and the
produced aluminium contained lees than 30D ppm nickel
which is significantly lower than with an uncoated anode
that can cause a typical nickel contamination of 1000
ppm in the product aluminium.
Example 7
Example 6 can be repeated using d~.fferent
combinations of electrolyte compositions (A1-I1)
selected from Table 1, anode alley eompvsitions (A2-KZj
selected from Table 2 and coating compositions (A3-L3)
selected from Table 3.
Further details on the application of such anode
coatings and .suitable compositions are disclosed in
W~03/087435, W02004/016731 and 6102004/024994 (aIl
Nguyen/de Norm) .
In summazy, as can he seen by carnparing Example 1-5
to the Comparative Example, using the potassium-fluoride
electrolyte pf the invention containing about s weight%
dissolved alumina instead of a potassium-fluoride free
electrolyte containing only 4 weight% dissolved alumina,
inhibits fluorination and passivation of the nickel
and/or cobalt of the anode and reduces wear (oxidation
and dis3olution v~ the anode's iron).
CA 02498622 2005-03-10
WO 2004/035871 _ ~3 _ PCT/IB2003/004649
Furthermore, as can be observed from Examples 6-7,
use of a crack-free nickel-free hematite-based
protective coating on a nickel-iron anode alloy reduces
the cell voltage and significantly inhibits
contamination of the product aluminium by nickel from
the anode, compared to an uncoated nickel-iron anode
operated in the same type of electrolyte.