Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
PHARMACEUTICAL SAFETY DOSAGE FORMS
Related Cases
[0001] This application claims priority to U.S. Serial No. 10/339,977, filed
on
January 10, 2003, entitled "Pharmaceutical Safety Dosage Forms".
Field Of The Invention
[0002] The present invention relates to the field of pharmaceutical safety
dosage
forms.
Background Of The Invention
[0003] Many pharmaceuticals are prone or potentially subject to misuse or
abuse
when the intended dosing instructions are ignored and/or the written
instructions are
disregarded. For example, it is well known that sustained-release narcotics,
such as
OXYCONTIN ER tablets (supplied by Perdue Pharma), are prone to abuse and
misuse
when their dosage units are broken, chewed, crushed, dissolved, or otherwise
disrupted,
rather than being taken whole as intended. Other narcotic and analgesic drugs
are liable to
similar misuse. Other types of drugs, including those which are not amenable
to abuse, may,
nonetheless, be inappropriately used. Such inappropriate use can lead to
adverse reactions in
-1-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
persons so using the drugs and can give rise to adverse reactions and even
death. One
example of this is the inappropriate use of metformin sustained-release (e.g.,
GLUCOPHAGE XR, metformin hydrochloride extended-release tablets supplied by
Bristol-
Myers Squibb), a common diabetes drug. If metformin sustained-release is
chewed, or the
tablets otherwise disrupted prior to ingestion, rather than the tablets being
swallowed whole,
dangerous lowering of a person's blood glucose level may result. Very large
numbers of
pharmaceuticals may be inappropriately ingested in this way and a method for
reducing or
eliminating the undesired effects has long been desired.
[0004] Many pharmaceutical products, which have been introduced into the
pharmaceutical market in an immediate-release tablet or capsule form, have
subsequently
been reformulated into a sustained-release form. The sustained-release form
has provided the
advantages of more convenient dosing schedules, increased patient compliance,
more even
blood levels, improved therapeutic activity, or others. Typically, the dosage
of active
pharmaceutical ingredient in these sustained-release formulations is greater
than the dosage
of the corresponding immediate-release formulation. This presents the danger
of "dumping"
in which the sustained-release mechanism fails, either intentionally or
unintentionally, so that
potentially dangerous dosages of the active pharmaceutical ingredient
(agonist) are delivered
to the patient causing dangerously high blood levels of the agonist. For
example, the
Physicians' Desk Reference (PDR), 56th Edition, states for GLUCOTROL XL
Extended
Release Tablets (supplied by Pfizer), under "Information for Patients", that
"Patients should
be informed that GLUCOTROL XL Extended Release Tablets should be swallowed
whole.
Patients should not chew, divide, or crush tablets." For the product RITALIN-
SR (supplied
by Novartis) under "DOSAGE AND ADMINISTRATION", it is written, "Ritalin-SR
tablets
must be swallowed whole and never crushed or chewed." For OXYCONTIN ER
tablets
(supplied by Perdue Pharma) there has been much recent controversy and
numerous
-2-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
published reports of narcotic abuse through mechanical disruption of the
sustained-release
mechanism thereby enabling the abuser to receive a relatively larger immediate
dose of
narcotic.
[0005] Some attempts at providing dosage forms for preventing abuse of
narcotics
have been offered by the prior art. For example, U.S. Patent No. 4,457,933,
Gordon et al.,
discusses both oral and parenteral abuse of strong analgesics, such as
oxycodone,
propoxyphene and pentazocine. Gordon et al. discuss oral administration of
compositions
containing specific ratios of oxycodone to naloxone, a narcotic antagonist.
According to
Gordon et al., the antagonist, naloxone, is supplied in an amount to deter
either oral or
parenteral abuse of an analgesic without substantially affecting the analgesic
activity.
Gordon et al., therefore, contemplates that the antagonist be absorbed into
the blood in
normal use along with the analgesic.
[0006] U.S. Patent No. 5,375,957 in the name of Kaiko et al. recognizes that
oral
and parenteral abuse of oral opioid formulations can occur by self-
administration of more
than the prescribed oral dosage. Kaiko et al. discusses an appropriate ratio
between analgesic
agonist and antagonist in such dosage forms to ensure analgesic efficacy is
maintained. Thus,
Kailco et al. contemplates that the antagonist be absorbed into the blood in
normal use along
with the agonist. Kaiko et al. distinguishes itself over prior art that
teaches inclusion of
antagonists in oral opioid analgesic dosage forms, which are themselves not
orally active, but
which counteract the analgesic effects of the opioid upon parenteral
administration. As an
example, Kaiko et al. describes the commercially available combination of
pentazocine and
naloxone, wherein the amount of naloxone does not interfere with the
pentazocine upon oral
administration. As such, it is understood that the antagonist would still be
absorbed into the
blood in normal use along with the agonist, but would not provide any
pharmacological
activity.
-3-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
[0007] Pharmaceutical dosage forms that permit absorption into the blood of an
antagonist are inefficient because of the resulting potential to hinder the
activity of the
agonist during normal use. This limits the amount of antagonist that can be
used. This also
limits the potential amount of antagonist activity that can be incorporated
into the dosage
form to less than the amount of activity that will substantially inhibit the
agonist activity.
Even if, as in Gordon et al., the antagonist, naloxone, can be present in
amounts that are not
orally active, this may not be possible or desirable with antagonists for
other drugs. Also,
even if the antagonist is not orally active, it may still be absorbed by the
blood and impact the
patient during normal use of the dosage form. Additionally, as in Gordon et
al., if the
antagonist is inactive with oral use then it cannot provide protection against
dumping of the
agonist if the sustained-release tablet is mechanically disrupted.
Furthermore, lack of oral
activity may not deter oral abuse.
[0008] There remains a great need for dosage forms which can minimize or
eliminate the effects of abusive or otherwise inappropriate use of
pharmaceuticals. A need
exists for dosage forms which may be employed for the delivery of a wide range
of drugs and
which do not require the coadministration of a separate second
pharmaceutically active
dosage unit in addition to the desired pharmaceutical. Dosage forms which
ensure the safe
administration of drugs without unnecessarily loading the bloodstream of a
person taking the
drug with additional dosage units are objects of this invention. A further
object is to provide
dosage forms which block an avenue of abusive value to a person in possession
of the dosage
unit. Other objects will become apparent from a review of the present
specification.
Summary Of The Invention
[0009] Pharmaceutical safety dosage forms are provided by the present
invention.
Such pharmaceutical safety dosage forms include a pharmaceutical as well as an
antagonist
for the pharmaceutical. In normal use, that is when the dosage forms are
administered or
-4-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
taken by the person in need of drug treatment, the antagonist has no
significant
bioavailability. The antagonist has significant bioavailability only when the
pharmaceutical
safety dosage form is disrupted. Disruption of the dosage form means in this
context the
mechanical, chemical or other alteration of the dosage form in such a fashion
as to release or
make biologically available the antagonist. Disruption does not mean the
dissolution of the
dosage form or its delivery of the pharmaceutical in accordance with the
intended mechanism
of use of the dosage form.
[0010] The pharmaceutical safety dosage forms of the present invention can be
administered orally, parenterally, rectally, vaginally, transdennally, via
aerosol, via nasal
spray, or otherwise such as via implantation. In connection with each route of
administration,
a normal mechanism of delivery of the pharmaceutical is intended consistent
with good
medical and pharmaceutical practices. Delivery of the pharmaceutical in any of
these
intended ways for the dosage forms of the invention does not deliver a
substantial amount of
the antagonist into the bloodstream. Rather, the antagonist is maintained in
such a way as not
to be substantially bioavailable via such intended method of administration.
In short, it is
intended that the antagonist "pass through" the patient and be substantially
eliminated
thereby. Thus, the antagonist is intended not to become bioavailable to the
patient and not to
require systemic inactivation or excretion therefrom. The overall loading of
active
compounds is, thus, minimized and limited to only the intended pharmaceutical
when the
dosage form is used as intended.
[0011] The present invention generally contemplates placing one or more
antagonist
pharmaceutical products within the dosage formulation of the agonist
pharmaceutical product
so that, under normal conditions, the antagonist is substantially not
bioavailable. However,
disruption of the formulation, through any of a variety of means, will release
the antagonist
thereby diminishing the effects of the agonist. For example, for the case of
OXYCONTIN
-5-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
(supplied by Perdue Pharma) Extended Release tablets abuse, narcotic abusers
are crushing
the tablets to disrupt the sustained-release mechanism thereby gaining a large
immediate dose
of narcotic. This invention contemplates, in one example, placing a narcotic
antagonist in
coated beads in OXYCONTIN (supplied by Perdue Pharma) Extended Release
tablets for
which the coating maintains the beads intact throughout the digestive system,
under normal
use, thereby blocking any significant bioavailability of the antagonist.
However, when the
narcotic abuser crushes the tablets, the abuser will also crush the beads
thereby exposing the
antagonist within the beads to dissolution in the gastrointestinal tract
thereby facilitating
bioavailability of the antagonist. There are numerous such examples including,
but not
limited to, the following: blood glucose lowering drugs such as metformin
(e.g.,
GLUCOPHAGE XR supplied by Bristol-Myers Squibb) or glipizide (e.g., GLUCOTROL
XL Extended Release Tablets supplied by Pfizer) containing beads of a
hyperglycemic
agent such as epinepherine or others; anti-hypertensive drugs such as
propranolol (e.g.,
INDERAL LA Long-Acting capsules supplied by Wyeth-Ayerst), metoprolol (e.g.,
TOPROL-XL supplied by AstraZeneca), nifedipine (e.g., PROCARDIA XL Extended
Release tablets supplied by Pfizer, ADALAT CC supplied by Bayer), diltiazem
(e.g.,
CARDIZEM CD supplied by Biovail), or nisoldipine (e.g., SULAR supplied by
AstraZeneca) containing beads of antagonist sympathomimetic drugs such as
epinepherine or
others; methylphenidate (e.g., RITALIN-SR tablets supplied by Novartis)
containing an
adrenergic beta blocking drug in beads; any antihistamine with a
sympathomimetic
decongestant such as cetirizine HCI/pseudoephedrine HCl (e.g., ZYRTEC-D
l2HourTM
Extended Relief tablets supplied by Pfizer), fexofenadine HC1/pseudoephedrine
HCl
(ALLEGRA-D Extended-Release tablets supplied by Aventis), or others
containing beads
containing one or more adrenergic beta receptor blocker drugs; any sustained-
release drug
-6-
CA 02505661 2005-06-30
63189-628
could contain an emetic agent within the normally non-
bioavailable beads; and numerous other possibilities.
[0012] In all of these cases, the effects of the
dumping of the active ingredient, whether through
intentional abuse, unintentional misuse, or other mechanism
could be offset by the resultant release of the antagonist
thereby undermining the motivation for abuse or protecting
the patient against the harmful effects of dumping of the
intended dose.
According to one aspect of the present invention,
there is provided a pharmaceutical safety dosage form
comprising a pharmaceutical and an antagonist for said
pharmaceutical wherein said antagonist comprises an emetic
agent and has significant bioavailability only when said
pharmaceutical safety dosage form is disrupted.
According to another aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a pharmaceutical contained within a first
microdosage form, said first microdosage form being adapted
for release of said pharmaceutical within a patient,
together with an antagonist for said pharmaceutical, said
antagonist comprising an emetic agent and being contained
within a second microdosage form, said second microdosage
form being substantially insoluble in gastric fluid.
According to still another aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a pharmaceutical contained within a first
plurality of particulated forms, said first particulated
forms being adapted for release of said pharmaceutical
within a patient, together with an antagonist for said
pharmaceutical, said antagonist comprising an emetic agent
-7-
CA 02505661 2005-06-30
63189-628
and being contained within a second plurality of
particulated forms, said second particulated forms being
substantially insoluble in gastric fluid.
According to yet another aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a pharmaceutical contained within a
microdosage form, said microdosage form being adapted for
release of said pharmaceutical within a patient, together
with an antagonist for said pharmaceutical, said antagonist
comprising an emetic agent and being contained within a
plurality of particulated dosage forms, said particulated
dosage forms being substantially insoluble in gastric fluid.
According to a further aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a pharmaceutical contained within a
plurality of particulated forms, said particulated forms
being adapted for release of said pharmaceutical within a
patient, together with an antagonist for said
pharmaceutical, said antagonist comprising an emetic agent
and being contained within a microdosage form, said
microdosage form being substantially insoluble in gastric
fluid.
According to yet a further aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a pharmaceutical in a first form adjacent to
an antagonist for said pharmaceutical in a second form
wherein said antagonist comprises an emetic agent and has
significant bioavailability only when said pharmaceutical
safety dosage form is disrupted.
According to still a further aspect of the present
invention, there is provided a method of administering a
-7a-
CA 02505661 2005-06-30
63189-628
pharmaceutical comprising: providing a pharmaceutical safety
dosage form comprising a pharmaceutical in a first form,
said first form providing a prescribed bioavailability; and
providing an antagonist for said pharmaceutical in a second
form, said antagonist comprising an emetic agent and said
second form providing insignificant bioavailability when
administered; and wherein disruption to said pharmaceutical
safety dosage form may result in significant bioavailability
of said antagonist.
According to another aspect of the present
invention, there is provided a method of delivering a drug
to a patient comprising placing said drug into a
pharmaceutical safety dosage form further comprising an
antagonist for said drug, said antagonist comprising an
emetic agent and being insubstantially bioavailable when
said dosage form is not disrupted; and administering said
safety dosage form to the patient.
According to yet another aspect of the present
invention, there is provided a method of delivering a
narcotic to a patient comprising placing said narcotic into
a pharmaceutical safety dosage form further comprising a
narcotic antagonist and an emetic agent, said narcotic
antagonist and said emetic agent being insubstantially
bioavailable when said dosage form is not disrupted; and
administering said safety dosage form to the patient.
According to another aspect of the present
invention, there is provided a method of delivering a
sympathomimetic to a patient comprising placing said
sympathomimetic into a pharmaceutical safety dosage form
comprising an adrenergic beta blocker, said adrenergic beta
blocker being insubstantailly bioavailable when said dosage
-7b-
CA 02505661 2005-06-30
63189-628
form is not disrupted; and administering said safety dosage
form to the patient.
According to still another aspect of the present
invention, there is provided a method of delivering a blood
pressure-lowering medication to a patient comprising placing
said blood pressure-lowering medication into a
pharmaceutical safety dosage form comprising a
sympathomimetic, said sympathomimetic being insubstantially
bioavailable when said dosage form is not disrupted; and
administering said safety dosage form to the patient.
According to yet another aspect of the present
invention, there is provided a method of delivering a
hypoglycemic agent to a patient comprising placing said
hypoglycemic agent into a pharmaceutical safety dosage form
comprising a hyperglycemic agent, said hyperglycemic agent
being insubstantially bioavailable when said dosage form is
not disrupted; and administering said safety dosage form to
the patient.
According to a further aspect of the present
invention, there is provided a method of making a
pharmaceutical safety dosage form comprising a
pharmaceutical and an antagonist for said pharmaceutical
wherein said antagonist comprises an emetic agent that has
significant bioavailability only when said pharmaceutical
safety dosage form is disrupted.
According to yet a further aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a sympathomimetic pharmaceutical and an
antagonist for said pharmaceutical wherein said antagonist
has significant bioavailability only when said
pharmaceutical safety dosage is disrupted.
-7c-
CA 02505661 2009-10-15
75592-9
According to still a further aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a blood pressure-lowering pharmaceutical and
an antagonist for said pharmaceutical wherein said
antagonist has significant bioavailability only when said
pharmaceutical safety dosage form is disrupted.
According to another aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a hypoglycemic pharmaceutical and an
antagonist for said pharmaceutical wherein said antagonist
has significant bioavailability only when said
pharmaceutical safety dosage form is disrupted.
According to one aspect of the present invention,
there is provided a pharmaceutical safety dosage form
comprising a pharmaceutical and an antagonist for said
pharmaceutical wherein said antagonist comprises an emetic
agent and has bioavailability only when said pharmaceutical
safety dosage form is disrupted, and wherein: i. the
pharmaceutical is a sympathomimetic or a sympathomimetic and
an antihistamine and the antagonist optionally further
comprises an adrenergic beta-blocker, ii. the pharmaceutical
is a blood pressure-lowering agent and the antagonist
optionally further comprises a sympathomimetic, or iii. the
pharmaceutical is a hypoglycemic agent and the antagonist
optionally further comprises a hyperglycemic agent.
According to another aspect of the present
invention, there is provided a pharmaceutical safety dosage
form comprising a pharmaceutical and an antagonist for said
pharmaceutical wherein said antagonist has bioavailability
only when said pharmaceutical safety dosage form is
disrupted, and wherein i. the pharmaceutical is a
-7d-
CA 02505661 2009-10-15
75592-9
sympathomimetic or a sympathomimetic and an antihistamine,
and the antagonist is an adrenergic beta-blocker, ii. the
pharmaceutical is a blood pressure-lowering agent, and the
antagonist is a sympathomimetic, or iii. the pharmaceutical
is a hypoglycemic agent, and the antagonist is a
hyperglycemic agent.
Brief Description Of The Drawings
[0013] Figure 1 shows a dosage form of the
invention containing pluralities of microdosage forms.
[0014] Figure 1A depicts two different types of
bead types of microdosage forms.
[0015] Figure 1B shows a multi-layered bead for
use in one embodiment of the invention.
[0016] Figure 2 depicts an osmotic drug dosage
form in accordance with the invention.
[0017] Figure 3 shows a dosage form of the
invention containing microdosage forms and particulated
forms.
[0018] Figure 4 shows a dosage form of the
invention containing two different types of microdosage
forms.
[0019] In accordance with the invention,
antagonist is delivered from the dosage form, (i.e., becomes
bioavailable), only when the dosage form is physically or
otherwise disrupted through use in a manner not intended by
the drug manufacturer. For example, by reference to only
one type of pharmaceutical, with oxycodone sustained-
release, for which the present dosage forms are applicable,
-7e-
CA 02505661 2009-10-15
75592-9
in normal use, the oxycodone is delivered over a period of
time to a patient ingesting a dosage form so as to provide
extended narcotic effect to such patient. An antagonist for
the narcotic, naloxone, is present in the dosage form, but
is
-7f-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
not released from the dosage form; it does not become bioavailable, in normal
use. However,
if the oxycodone dosage form is disrupted, e.g. physically comminuted, in an
attempt to
release immediately the entire amount of the narcotic in order to abuse the
drug, the
antagonist, naloxone, is also released. The naloxone is preferably present in
an amount
sufficient to interfere with the narcotic effect of the oxycodone, thus
frustrating the attempted
abuse of the drug.
[00201 The present dosage forms are amenable to the delivery of a wide variety
of
narcotic and non-narcotic drugs in a manner having improved safety. The only
requirement
is a practical one. Thus, a drug which is capable of abuse or of significant
adverse effect if
inappropriately ingested during or following disruption of the dosage form
must be one
which has an antagonist. In this context, an antagonist is preferably a
compound or
composition which is capable of interfering or negating all or some of the
effects of the
therapeutic drug. This may be achieved either biochemically, physically,
physiologically, or
otherwise. Thus, while the exemplary narcotic antagonist, naloxone, operates
biochemically,
it is believed, through interfering with a biochemical (receptor) pathway for
narcotics, an
effective antagonist, in the context of this invention, may act otherwise,
e.g. through
stimulation of an excretion or breakdown mechanism for the drug. The mechanism
of action
of antagonists which may be employed herein is not intended to be limiting in
any way. Any
compound, group of compounds or composition which can interfere effectively
with the
action of a drug may be considered to be an antagonist for the drug, providing
the overall
objectives of this invention are met.
[00211 While physical disruption of dosage forms, e.g. comminution or
"grinding
them up," is an important path undertaken for the abuse or inappropriate use
of drugs, non-
physical means may also be employed. Thus, dissolution in solvent systems in
order to
extract drug may be performed. It is preferred that the dosage forms of the
present invention
-8-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
release their antagonist component when subjected to such solvent action if
the particular
intended activity of the antagonist is to counteract the effects of this
solvent disruption.
Conceivably, a dosage form could be melted or sublimed to release a drug. In
such cases, it
may be preferred that dosage forms be available which can release antagonist
under such
conditions if this is the particular activity that is intended to be
counteracted by inclusion of
the antagonist in the dosage form. The antagonist can be formulated to protect
against one or
more such disruption activities.
[0022] Many pharmaceuticals are provided in capsule dosage forms containing
within them microdosage forms. One embodiment of the present invention
provides a
pharmaceutical and an antagonist for the pharmaceutical in a dosage form where
each is
contained within microdosage forms, e.g., coated beads, mini tablets, and
tablets. Thus, for
example, the beads containing the drug are coated or formulated so as to
release the drug on
an intended time profile. The beads containing the antagonist, however, are
either formulated
or coated so as to prevent significant bioavailability of the antagonist when
the dosage form
is consumed as intended. Specific coatings which can attain the foregoing
objectives are
numerous and well-known to pharmaceutical chemists and formulators. Their
identity and
use in achieving coatings or formulations in accordance with the present
requirements are not
a central part of this invention and it is to be understood that all such
coatings and
formulations are comprehended hereby. It is understood, for example, that it
may be
desirable in some formulations to use only a single coating, whereas it may be
desirable in
other formulations to use multiple coatings, and the embodiments of the
present invention are
not intended to be limited thereby.
[0023] An exemplary, but by no means exhaustive, compilation of coatings and
fonnulations for solid dosage forms, and the like is contained within
Remington: The Science
and Practice of Pharmacy by Alfonso R. Gennar, editor, (19th edition 2000)
(Chapters 45-
-9-
CA 02505661 2009-10-15
75592-9
46) and Pharmaceutical Dosage Forms and Drug Delivery Systems by
Howard C. Ansel, et al. (Chapters 6-7).
100241 An exemplary dosage form employing beads in accordance with the
foregoing discussion is presented in Figure 1. Figure I depicts a conventional
capsule dosage
form comprising a shell, 10 and containing pluralities of microdosage fonns,
e.g. beads, 12.
There are preferably at least two different kinds of beads, beads (or other
microdosage forms)
containing the drug and beads (or other microdosage forms) containing
antagonist. The
drug-containing beads are formulated so as to release drug when administered
to a patient in
accordance with conventional practice. The antagonist-containing beads are
formulated so as
not to deliver antagonist to a patient in normal use. Thus, for example, as
shown in Figure
IA, beads 16 can represent a formulated drug 24 within a saccharide, polymer,
or other
matrix designed to deliver the drug, e.g. in the small intestine of a patient.
Another type of
bead 14 comprises antagonist for the drug 20 coated by coating 22 the whole
being
formulated to resist antagonist delivery until all beads are eliminated from
the patient, e.g. in
the stool. As discussed, however, the drug-containing beads may be coated and
the
antagonist not coated as may be desired by the routineer in the art. Moreover,
three or more
different types of beads may be employed, for example, when extended-release
of drug is
desired or there are different active ingredients. As stated, the preparation
of diverse types of
beads and the use of a wide. variety of coatings and formulation components is
an advanced
art and is well known to persons skilled in drug formulation. All such may
find utility herein.
[00251 A further exemplification of the invention is shown in Figure 1B. The
figure
depicts a multi-layer microdosage form or bead 30. Drug 32 is coated by
coating 34 designed
to deliver the drug to a patient at a desired time and in a desired location,
e.g. the stomach.
The whole, irf this embodiment, preferably surrounds a formulation of
antagonist 36 for the
-10-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
drug which is so formulated as not to release antagonist when ingested under
normal
conditions. Thus, the antagonist is designed to pass through the body of the
patient
unabsorbed.
[0026] If any of these exemplary embodiments are subjected to physical
disruption,
e.g. by being ground up, both the drug and the antagonist are likely to be
released. The
dosage forms are, thus, less amenable to abuse if crushed or chewed by a
patient.
Furthermore, such dosage forms provide protection against unexpected dosing
upon crushing
or chewing because the patient will receive both the unexpected dosing and the
antagonist
which will diminish the effect of the unexpected dosage. Increased resistance
to abuse and
overdose result.
[0027] Another exemplary dosage form using different types of microdosage
forms
is shown in Figure 4. Figure 4 presents a conventional capsule dosage form
comprising a
shell 70, and containing two different types of microdosage forms, e.g.,
tablets 74 and beads
78. It is understood that neither the drug nor its antagonist are limited to
certain microdosage
forms. Thus, in one instance, a drug 72 may be contained in tablets 74 which
are formulated
for time-release, and its antagonist 76 may be contained in beads 78
formulated or coated to
not deliver the antagonist during normal use of the dosage form. On the other
hand, it is
possible that the drug may be contained in beads formulated for time-release
and its
antagonist may be contained in tablets coated to prevent significant
bioavailability when the
dosage form is used as intended.
[0028] Many pharmaceuticals are also provided in tabletted or capsule dosage
forms
comprising some particulated forms of ingredients. A further embodiment of the
present
invention comprises a drug in the form of a powder, in an amorphous form or
with one or
more polymorphs, in tabletable form together with antagonist in a microdosage
form such
that it is substantially insoluble in gastric or other fluid. Thus, for
example, a powdered drug,
- 11 -
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
along with excipients, such as fillers, binders, disintegration agents,
lubricants, colorants, or
other conventional adjuvants, is combined with one or more beads, mini
tablets, powder, or
other forms containing antagonist. The antagonist forms are formulated or
coated in such a
way as to render them substantially insoluble in the gastrointestinal tract or
other locus of
administration. An antagonist that is substantially insoluble in the human
body, for example,
is prevented from being released before the antagonist is excreted from the
body. It is
understood in the art that powders can be formulated so as to prevent
dissolution in bodily
fluids and/or prevent significant bioavailability. Solubility of polymorphs or
solvates, for
example, are dependent on the crystallized structure of the molecules, and
thus, have different
solubilities. Hence, certain polymorphs or solvates may be insoluble in the
body, but readily
soluble in specific solvents. The preparation of polymorphs or solvates are
discussed by
numerous patents on numerous molecules, e.g. U.S. Patent Nos. 6,472,563;
6,440,459;
6,337,422; 6,133,289; and 5,900,423. Also contemplated is using one or more
polymorphic
or solvate forms of one or more antagonists wherein the utilized polymorphic
or solvate
forms are not readily bioavailable under normal use.
[0029] In Figure 3, a tabletted dosage form in accordance with one embodiment
of
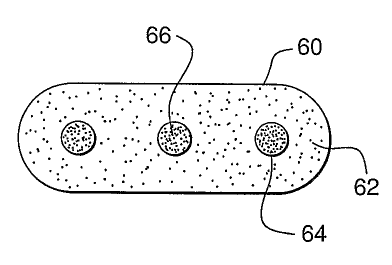
the invention is provided. The tabletted dosage form 60 is formed from
powdered drug 62
together with conventional adjuvants, such as excipients and the like. Beads,
mini tablets, or
the like 64 comprising antagonist for the drug 66 are included within the
tablet. Other
variations of such tabletted dosage forms may also be employed. It is
understood that neither
the drug nor its antagonist are limited to certain particulated forms or
microdosage forms.
Thus, a drug may be present in time-release powder form and its antagonist may
be present as
a polymorph which is insoluble in the body, but readily soluble in specific
solvents.
Furthermore, pharmaceutical safety dosage forms themselves are not limited in
the types of
microdosage forms or particulated forms contained therein. As such, a
conventional capsule
-12-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
dosage form, for example, may contain a drug in time-release powdered form and
an
antagonist to the drug in tabletted form, shellacked to prevent
bioavailability upon normal use
of the dosage form.
[0030] A further dosage form in accordance with this invention is depicted in
Figure
2. This is a type of osmotic pump drug delivery dosage form 40. Thus, a shell
41 contains a
compartment containing drug 42 as well as a compartment containing an osmotic
agent 50.
The two compartments are preferably separated by a piston 48. Preferably, both
the drug-
containing compartment and the osmotic agent-containing compartment have an
orifice
sealed by plugs 46 and 52, respectively. Upon administration, such as oral
administration,
the plugs 46 and 52 dissolve, exposing both the drug-containing compartment
and the
osmotic agent-containing compartment to body fluid. Absorption of water by the
osmotic
agent with concomitant swelling pushes upon the piston 48, expelling the drug
through the
orifice to its compartment. The geometry of the dosage forn, orifice sizes,
identity of the
drug and osmotic agent and other factors are typically designed and optimized
for a desired
delivery location and timing. The art of osmotic drug delivery is relatively
mature and an
extensive patent literature has arisen. Moreover, commercial sources for such
dosage forms
are available, e.g. the Alza Corporation. One exemplary patent showing such
dosage forms is
U.S. patent 6,132,420.
[0031] The present invention may be applied to such osmotic drug dosage forms.
In
one embodiment, shown in Figure 2, a formulation of antagonist, 54 surrounds
the dosage
form. In view of the importance of the orifices to operation of the dosage
form, the
antagonist formulation is generally kept away from those orifices as shown.
Since osmotic
drug delivery vehicles may take a diversity of physical shapes, the shape and
location of
antagonist will reflect such geometry. While, as shown, the antagonist
formulation is one
which does not release antagonist to the body of a person correctly ingesting
the dosage form,
-13-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
it may also be coated if preferred to achieve a similar result. In any event,
physical or other
disruption of the dosage form will release the antagonist as well as the drug.
Another
example could be coated beads of the antagonist that reside in one or more
compartments of
the osmotic drug delivery vehicle.
[0032] Another aspect of the present invention is a method of administering
pharmaceuticals by providing pharmaceutical safety dosage forms that include a
pharmaceutical and an antagonist for the pharmaceutical where the microdosage
forms
provide insignificant bioavailability when the dosage form is administered as
intended.
[0033] Insignificant bioavailability in the context of this invention is
intended to
mean that the antagonist does not interfere with the drug in a meaningful way
and that the
person to whom the dosage form is administered is not burdened with a
significant loading of
antagonist.
[0034] Drug dosage forms ofthis invention are preferably administered through
the
alimentary canal orally or anally. Delivery otherwise to the body from outside
of the
digestive tract, parenteral administration, may also benefit from this
invention and employs,
e.g. subcutaneous, intravenous, intravaginal, intramuscular, transdermal,
nasal, aerosol, or
other routes of administration.
[0035] The use of the present pharmaceutical safety dosage forms is directly
applicable to administration of drugs prone to drug abuse, such as narcotics
and
amphetamines. One combination of agonist and antagonist contemplated by the
present
invention includes narcotics and narcotic antagonists. Examples of narcotics
include, but are
not limited to, codeine, oxycodone, propoxyphene, pentazocine, and derivatives
thereof.
Examples of narcotic antagonists include, but are not limited to, naloxone,
nalmefene, and
derivatives thereof. Another combination of agonist and antagonist is
sympathomimetics,
e.g. amphetamines, and adrenergic beta blockers. Syinpathomimetic agonists
along with
-14-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
antihistamines can also be combined with adrenergic beta blockers. Reference
to derivatives
of chemicals discussed herein include, but are not limited to, chemical
derivatives and salts
and bases thereof.
[0036] Drugs which are not prone to abuse may also be administered using the
safety dosage forms hereof. Thus, drugs intended for sustained-release in the
body can give
rise to unpleasant and undesired reactions if over-administered. Thus, the
disruption of
dosage forms containing, e.g. diabetes drugs, blood pressure lowering drugs
and many other
types of pharmaceuticals can give rise to diabetic shock or shock-inducing low
blood
pressure. Such conditions can be fatal. Examples of diabetes drugs include,
but are not
limited to, hypoglycemic agents, and examples of antagonists to hypoglycemic
agents
include, but are not limited to, hyperglycemic agents. Examples of blood
pressure-lowering
drugs include, but are not limited to, adrenergic beta blockers, calcium
channel blockers, and
ACE inhibitors. Examples of antagonists to blood pressure-lowering drugs
include, but are
not limited to, sympathomimetics. Including antagonists for these drugs as
taught hereby can
guard against accidental overdose, if, for example, sustained-release tablets
are chewed.
[0037] Furthermore, in accordance with the present invention, dosage forms can
include any pharmaceutical combined with an emetic agent, e.g., ipecac, which
is released
upon disruption of the dosage forms.
[0038] While not intended to be limiting, an exemplary list of drugs (as bases
or any
salts thereof) and their antagonists are set forth which may find utility
through delivery via
the safety dosage forms of this invention.
DRUG ANTAGONIST
Codeine, Oxycodone, Propoxyphene, Naloxone, Nalmefene, Naltrexone
Pentazocine, Buprenorphine, Morphine,
Oxymorphone
Methamphetamine, amphetamine, Propranolol, Atenolol, Metoprolol, or
dextroamphetamine, methylphenidate other adrenergic beta blocker
Insulin, Metformin, Glipizide Epinepherine, Glucagon
Propranolol, Metoprolol, Nifedipine, Dopamine, Epinepherine or other
-15-
CA 02505661 2005-05-10
WO 2004/062642 PCT/US2003/040990
Diltiazem, Nisoldipine, Timolol Maleate sympathomimetic
Methylphenidate Adrenergic beta blocker
Cetirizine HCl/pseudoephedrine HCI, Adrenergic beta blocker
fexofenadine HCl/pseudoephedrine HCI
Any drug Ipecac or other emetic agent
[0039] Other aspects of the invention will be apparent from review of the
present
specification and claims and all such falling within the spirit of the
invention are
comprehended hereby.
-16-