Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02533433 2006-O1-23
WO 20051014542 PCTIEP20041007573
1
NOVEL N-SUBSTITUTED INDOLYL-3-GLYOXYLIC ACID AMIDES, USE
THEREOF AS A MEDICAMENT, AND METHOD FOR THE PRODUCTION
THEREOF
Description of the invention:
For the next few years, a dramatic increase in oncoses and tumor-related cases
of
death is expected worldwide. In 2001, worldwide approximately 10 million
people
were suffering from cancer and over 6 million people died from this disease.
The
development of tumors is a fundamental disease of higher organisms in flora
and
fauna. The generally recognized multistep model of carcinogenesis assumes that
as
a result of accumulation of a number of mutations in an individual cell this
is so
modified in its proliferation and differentiation behavior that finally, via
benign
intermediate stages, a malignant state with metastasis is reached. Behind the
term
cancer or tumor, a clinical picture with more than 200 various individual
diseases
hides itself. Oncoses can proceed in a benign or malignant manner. The most
important tumors are those of the lung, the breast, the stomach, the neck of
the
uterus, the prostate, the head and neck, the large and small intestine, the
liver and
the blood system. There are great differences with respect to course,
prognosis and
therapy behavior. More than the 90% of the cases recognized relate to solid
tumors,
which in particular in the advanced stage or on metastasis are treatable with
difficulty
or untreatable. The three pillars of cancer control are still surgical
removal, irradiation
and chemotherapy. In spite of great advances it has still not been possible to
develop
medicaments which bring about a marked prolongation of the survival time or
even a
complete cure in the widespread solid tumors.
It is therefore meaningful to invent novel medicaments for the control of
cancer. In
particular, the disadvantageous formation of resistance, as is known of many
antitumor agents, should be circumvented.
Indolyl-3-glyoxylamides are frequently used as pharmacologically active
compounds
and as synthesis components in pharmaceutical chemistry.
CA 02533433 2006-O1-23
2
In W003/022280, N-substituted alkyl- and aryl 3-glyoxylamide indoles having
antitumoral action are described. Actual exemplary embodiments of this
substitution
pattern on the glyoxylamide nitrogen atom are, however, not given.
In the documents W099/51224 A1 and W001/22954 A1, N-substituted indol-3-yl
derivatives having antitumor action are described. Actual exemplary
embodiments of
this substitution pattern are, however, not given.
In W099/55696 A1, substituted hydroxyindoles are described as
phosphodiesterase
4 inhibitors. An antitumoral activity of the compounds according to the
invention is
neither described nor suggested.
In WO 02/08225 A1, 2-(1H-indol-3-yl)-2-oxoacetamide derivatives having
antitumor
action against solid tumors are described. However, the invention does not
relate to
actual exemplary embodiments with substitution on the glyoxylamide nitrogen
atom.
In patent specification WO 00/67802, indole-3-glyoxylamides which are
substituted
by higher chain fatty acids are described as potential antitumor agents.
Actual
exemplary embodiments are, however, not given or confirmed by biological data.
In the publication of W.-T. Li et al. (J. Med. Chem. 2003, 46, 1706 ff.), N-
heterocyclic
indolylglyoxylamides are described as orally active compounds having
antitumoral
activity.
WO 02/10152 A2 of the applicant already describes another class of indole
derivatives for the treatment of tumors. Inter alia, the active compound N-(2-
methyl-6-
quinolyl)-[1-(4-chlorobenzyl)-indol-3-yl]glyoxylamide was tested here on
various
tumor cell lines for its antiproliferative action.
The present invention relates to novel N-substituted indolyl-3-glyoxylamides,
their
preparation and use as medicaments for the treatment of benign and malignant
tumors in mammals, including humans.
CA 02533433 2006-O1-23
3
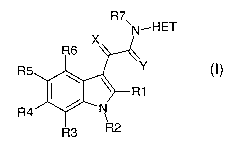
In the present application, N-substituted indolyl-3-glyoxylamides as in the
general
formula I
R7
'N..HET
X
R6
v,
R5
-R1
R4 ~ N
1
R3 R2
are described, in which
R't, R3-R6:
-are hydrogen,
-unsubstituted or substituted alkyl,
-unsubstituted or substituted cycloalkyl,
-unsubstituted or substituted aryl,
-unsubstituted or substituted heteroaryl,
-unsubstituted or substituted alkylaryl,
-unsubstituted or substituted alkylheteroaryl,
-amino, monoalkylamino, dialkylamino,
-halogen,
-alkyl substituted by one or more fluorine atoms, preferably a
trifluoromethyl group,
-cyano, straight-chain or branched cyanoalkyl,
-alkylcarbonyl,
-carboxyl, alkoxycarbonyl, carboxyalkyt or alkoxycarbonylalkyl,
-alkoxy,
-arylalkoxy, preferably benzyloxy,
CA 02533433 2006-O1-23
4
-alkoxycarbonylamino, alkoxycarbonylaminoalkyl,
R2:
-is unsubstituted or substituted alkyl,
-unsubstituted or substituted alkylaryl,
-unsubstituted or substituted alkylheteroaryl,
R7: -is a sulfone of the formula -S02-X1, where X1 is N(alk)2, hydroxyl,
unsubstituted or substituted alkyl, unsubstituted or substituted
cycloalkyl, unsubstituted or substituted aryl, unsubstituted or substituted
heteroaryl, unsubstituted or substituted alkyfcycloalkyl, unsubstituted or
substituted alkylheterocyclyl, unsubstituted or substituted alkylaryl,
unsubstituted or substituted alkylheteroaryl;
-C(O)-X2, where X2 is unsubstituted or substituted aryl, unsubstituted or
substituted heteroaryl, unsubstituted or substituted alkylaryl and
unsubstituted or substituted alkylheteroaryl,
-C(O)O-X3, where X3 is unsubstituted or substituted cycloalkyl,
unsubstituted or substituted heterocycly(, unsubstituted or substituted
aryl, unsubstituted or substituted heteroaryl, unsubstituted or substituted
alkylcycloalkyl, unsubstituted or substituted alkylheterocyclyl, and
unsubstituted or substituted alkylheteroaryl,
-C(~)NX4X5, where X4 and X5 independently of one another are
hydrogen, unsubstituted or substituted alkyl, unsubstituted or
substituted cycloalkyl, unsubstituted or substituted heterocyclyl,
unsubstituted or substituted aryl, unsubstituted or substituted heteroaryl,
unsubstituted or substituted alkylcycloalkyl, unsubstituted or substituted
alkylheterocyclyl, unsubstituted or substituted alkylaryl, unsubstituted or
substituted alkylheteroaryl,
or X4 and X5 together are cycloalkyl or cycloheteroalkyl,
CA 02533433 2006-O1-23
-C(S)NX6X7, where X6 and X7 independently of one another are
hydrogen, unsubstituted or substituted alkyl, unsubstituted or
substituted cycloalkyl, unsubstituted or substituted heterocyclyl,
unsubstituted or substituted aryl, unsubstituted or substituted heteroaryl,
unsubstituted or substituted alkylcycloalkyl, unsubstituted or substituted
alkylheterocyclyl, unsubstituted or substituted alkylaryl, unsubstituted or
substituted alkylheteroaryl,
or X6 and X7 together are cycloalkyl or cycloheteroalkyl,
X: is O, S or geminally linked hydrogen and hydroxyl,
Y: is O or S
and
HET: is a saturated, unsaturated or aromatic (C2-C14)-heterocycle
comprising one or more heteroatoms selected from the group consising of N, O
and
S, which can be bonded to the amide nitrogen directly or via a (C1-C6)-alkyl
bridge
and the alkyl radical can be substituted or unsubstituted and optionally one
or two
aryl or cycloalkyl groups can be fused to the heterocycle, and
the heterocyclyl, aryl or cycloalkyl groups can be unsubstituted or
substituted and the
alkyl radical can in all cases be branched or unbranched and saturated or
unsaturated,
and their pharmaceutically tolerable salts.
The substituent HET can in particular be pyrrole, furan, thiophene, pyrazole,
thiazole,
indoie, oxazole, imidazole, isothiazole, isoxazole, 1,2,3-triazole, 1,2,4-
triazole, 1,2,4,
oxadiazole, 1,3,4-oxadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, tetrazole,
pyridine,
pyrimidine, pyridazine, pyrazine, benzofuran, indazole, carbazole,
benzoxazole,
CA 02533433 2006-O1-23
6
benzimidazole, benzothiazole, benzotriazole, quinoline, isoquinoline,
cinnoline,
quinoxaline, quinazoline, phthalazine, pyridopyrazine, 1,2,3-triazine, 1,2,4-
triazine,
1,3,5-triazine, purine, pteridine, acridine and phenanthridine.
The expression "alkyl" within the meaning of this invention comprises acyclic
saturated or unsaturated hydrocarbons having 1 to 20 C atoms, which can be
branched or straight-chain and unsubstituted or mono- or polysubstituted.
The expression "cycloalkyl" denotes cyclic hydrocarbons having 3 - 12
hydrocarbons, which can be saturated or unsaturated, unsubstituted or mono- or
poly-substituted.
The expression "aryl" denotes aromatic hydrocarbons having 6 - 14 C atoms,
which
can be unsubstituted or mono- or polysubstituted, where the aryl substituents
are
identical or different and can be present in any desired and possible position
of the
aryl.
The expression °heteroaryl° stands for a 5-, 6- or 7-membered
cyclic aromatic radical,
which contains at least 1, optionally also 2, 3, 4 or 5 heteroatoms, where the
heteroatoms are identical or different and the heterocycle can be
unsubstituted or
mono- or polysubstituted: In the case of the substitution on the heteroaryl
moiety the
heteroaryl substituents can be identical or different and can be in any
desired and
possible position of the heteroaryl. Preferred heteroatoms are nitrogen,
oxygen and
sulfur.
The expression "heterocyclyl" stands for a 3-, 4-, 5-, 6-, 7- or 8-membered
cyclic
organic radical, which contains at least 1, optionally 2, 3, 4 or 5
heteroatoms, where
the heteroatoms are identical or different and the cyclic radical is saturated
or
unsaturated, but not aromatic and can be unsubstituted or mono- or
polysubstituted.
Preferred heteroatoms are nitrogen, oxygen and sulfur.
The expressions "alkylcycloalkyl", "alkylheterocyclyl", "alkylaryl or
"alkylheteroaryl"
mean that alkyl and cycloalkyl, heterocyclyl, aryl and heteroaryl have the
meanings
CA 02533433 2006-O1-23
7
mentioned and the cycloalkyl, heterocyclyl, aryl or heteroaryl radical is
bonded to the
compound of the general formula I via a C1-C8-alkyl group.
In connection with "alkyl", the term substituted is to be understood within
the meaning
of this invention as meaning the substitution of a hydrogen radical by F, CI,
Br, l, CN,
NH2, NH-alkyl, NH-cycloalkyl, NH-aryl, NH-heteroaryl, NH-alkylaryl, NH-
alkylheteroaryl, NH-heterocyclyl, NH-alkyl-OH, N(alkyl)2, N(alkylaryl)2,
N(alkylheteroaryl)2, N(heterocyclyl)2, N(alkyl-OH)2, NO, N02, SH, S-alkyl, S-
cycloalkyl, S-aryl, S-heteroaryl, S-alkylaryl, S-alkylheteroaryl, S-
heterocyclyl, S-alkyl-
OH, S-alkyl-SH, S-alkyl, S-S-cycloalkyl, S-S-aryl, S-S-heteroaryl, S-S-
alkylaryl, S-S-
alkylheteroaryl, S-S-heterocyclyl, S-S-alkyl-OH, S-S-alkyl-SH, S-S-alkyl-C(O)-
NH-
heterocyclyl, OH, O-alkyl, O-cycloalkyl, O-aryl, O-heteroaryl, O-alkylaryl, O-
alkylheteroaryl, O-heterocyclyl, O-alkyl-OH, CHO, C(O)-alkyl, C(S)-alkyl, C(O)-
aryl,
C(S)-aryl, C(O)-alkylaryl, C(S)-alkylaryl, C(O)-heterocyc(yl, C(O)-heteroaryl,
C(O)-
alkylheteroaryl, C(S)-heterocyclyl, C02H, C02-alkyl, C02-cyclyl, C02-
heterocyclyl,
C02-aryl, C02-heteroaryl, C02-alkylaryl, C(O)-NH2, C(O)NH-alkyl, C(O)NH-aryl,
C(O)NH-heterocyclyl, C(O)NH-alkylheterocyclyl, C(O)N(alkyl)2,
C(O)N(alkylaryl)2,
C(O)N(alkylheteroaryl)z, C(O)N(heterocyclyl)2, SO-alkyl, S02-alkyl, S02NH2,
S03H,
alkyl, cycloalkyl, aryl, heteroaryl, or heterocyclyl, where polysubstituted
radicals are to
be understood as meaning those which are either polysubstituted, e.g. di- or
trisubstituted, on different or on identical atoms, for example trisubstituted
on the
same C atom as in the case of CF3, -CH2CF3 or in different positions as in the
case of
-CH(OH)-CH=CH-CHCI2. Polysubstitution can take place with the same or
different
substituents.
With respect to aryl, heterocyclyl, heteroaryl, alkylaryl and cycloalkyl, mono-
or
polysubstituted is understood within the meaning of this invention as meaning
the
mono- or polysubstitution, e.g. di-, tri- or tetrasubstitution, of one or more
hydrogen
atoms of the ring system by F, CI, Br, I, CN, NH2, NH-alkyl, NH-aryl, NH-
heteroaryl,
NH-alkylaryl, NH-alkylheteroaryl, NH-heterocyclyl, NH-alkyl-OH, N(alkyl)2,
NC(O)alkyl, N(alkylaryl)2, N(alkylheteroaryl)2, N(heterocyclyl)2, N(alkyl-
OH)2, NO,
N02, SH, S-alkyl; S-aryl, S-heteroaryl, S-alkylaryl, S-alkylheteroaryl, S-
heterocyclyl,
S-alkyl-OH, S-alkyl-SH, OH, O-alkyl, O-aryl, O-heteroaryl, O-alkylaryl, O-
alkylheteroaryl, O-heterocyclyl, O-alkyl-OH, O-C(O)-alkyl, CHO, C(O)-alkyl,
C(S)-
CA 02533433 2006-O1-23
8
alkyl, C(O)-aryl, C(S)-aryl, C(O)-alkylaryl, C(S)-alkylaryl, C(O)-
heterocyclyl, C(S)-
heterocyclyl, C02H, C02-alkyl, C02-alkylaryl, C(O)-NH2, C(O)NH-alkyl, C(O)NH-
aryl,
C(O)NH-heterocyclyl, C(O)N(alkyl)2, C(O)N(alkylaryl)2,
C(O)N(alkylheteroaryl)2,
C(O)N(heterocyclyl)2, SO-alkyl, S02-alkyl, S02-aryl, S02-heteroaryl, S02NH2,
S03H,
CF3, CHO, CHS, alkyl, cycloalkyl, aryl, heteroaryl, and/or heterocyclyl, on
one or
optionally different atoms (where one substituent can optionally for its part
be
substituted). Polysubstitution in this case takes place with the same or with
different
substituents.
If the compounds of the general formula I according to the invention have at
least one
asymmetric center, they can be present in the form of their racemates, in the
form of
the pure enantiomers and/or diastereomers or in form of mixtures of these
enantiomers and/or diastereomers, namely both in substance and as
pharmaceutically acceptable salts of these compounds. The mixtures can be
present
in any desired mixing ratio of the stereoisomers.
If possible, the compounds according to the invention can be present in the
form of
the tautomers.
The compounds of the general formula I according to the invention can be
converted,
if they have a sufficiently basic group, such as, for example, a secondary or
tertiary
amine, into salts with inorganic and organic acids. Preferably, the
pharmaceutically
acceptable salts of the compounds according to the invention as in the general
formula I are formed with hydrochloric acid, hydrobromic acid, sulfuric acid,
phosphoric acid, methanesulfonic acid, p-toluenesulfonic acid, carbonic acid,
formic
acid, acetic acid, trifluoroacetic acid, oxalic acid, malonic acid, malefic
acid, succinic
acid, tartaric acid, racemic acid, malic acid, embonic acid, mandelic acid,
fumaric
acid, lactic acid, citric acid, glutamic acid or aspartic acid. The salts
formed are, inter
alia, hydrochlorides, hydrobromides, sulfates, phosphates, methanesulfonates,
sulfoacetates, tosylates, carbonates, hydrogencarbonates, formates, acetates,
triflates, oxalates, malonates, maleates, succinates, tartrates, malates,
embonates,
mandelates, fumarates, lactates, citrates and glutamates. The stoichiometry of
the
salts of the compounds according to the invention formed can in this case be
integral
or nonintegral multiples of 1.
CA 02533433 2006-O1-23
9
The compounds of the general formula I according to the invention can, if they
contain a sufficiently acidic group, such as, for example, the carboxyl group,
be
converted into their physiologically tolerable salts with inorganic and
organic bases.
Possible inorganic bases are, for example, sodium hydroxide, potassium
hydroxide,
calcium hydroxide, organic bases are ethanolamine, diethanolamine,
triethanolamine,
cyclohexylamine, dibenzylethylenediamine and lysine. The stoichiometry of the
salts
of the compounds according to the invention formed can in this case be
integral or
nonintegral multiples of 1.
Likewise preferred are solvates and in particular hydrates of the compounds
according to the invention, which can be obtained, for example, by
crystallization
from a solvent or from aqueous solution. It is possible here to combine one,
two,
three or as many solvate or water molecules as desired with the compounds
according to the invention to give solvates and hydrates.
Most preference is given to compounds as per the general formula 1 which have
been
included in the following selection:
N-{2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxoacetyl}-N-quinolin-6-yl-benzamide
(1)
N-{2-[1-(4-chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}-2-phenyl-N-quinolin-6-yl-
acetamide (2)
phenyl {2-[1-(4-chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}quinolin-6-
ylcarbamate (3)
(1 R,2S,5R)-2-isopropyl-5-methylcyclohexyl {2-1-(4-chlorobenzyl)-1 H-indol-3-
yl]-
2-oxoacetyl}quinolin-6-ylcarbamate (4)
N-{2-[1-(4-chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}-4-methyl-N-quinolin-6-yl-
benzenesulfonamide (5)
The present invention's indolyl-3-glyoxylamides as per the general formula I
are
useful for treating mammals, including humans. Mammals other than humans can
be
domestic animals such as horses, cows, dogs, cats, hares, sheep and the like.
A further aspect of the invention is a process for the treatment of tumors in
a
mammal, including a human being, which comprises administering at least one
indolyl-3-glyoxylamide of the general formula I to the mammal in a dose which
is
CA 02533433 2006-O1-23
efficacious for tumor treatment. The therapeutically effective dose to be
administered
for the treatment of the respective indolyl-3-glyoxylamide according to the
invention
depends, inter alia, on the nature and the stage of the tumor disease, the age
and
sex of the patient, the manner of administration and the duration of the
treatment.
The medicaments according to the invention can be administered as liquid,
semisolid
and solid pharmaceutical forms. This is carried out in the manner suitable in
each
case in the form of aerosols, powders and dusting powders, tablets, coated
tablets,
emulsions, foams, solutions, suspensions, gels, ointments, pastes, pills,
pastilles,
capsules or suppositories.
The pharmaceutical forms, in addition to at least one constituent according to
the
invention, contain, depending on the pharmaceutical form employed, excipients
where appropriate, such as, infer alia, solvents, solution accelerants,
solubilizers,
emulsifiers, wetting agents, antifoams, gel-formers, thickeners, film-formers,
binders,
buffers, salt-formers, driers, flow regulators, fillers, preservatives,
antioxidants,
colorants, mold release agents, lubricants, disintegrants, taste and odor
coregents.
The selection of the excipients and also the amounts which are to be used
thereof
depends on the pharmaceutical form chosen and is guided by the formulations
known to one skilled in the art.
The medicaments according to the invention can be administered in a suitable
administration form to the skin, epicutaneously as a solution, suspension,
emulsion,
foam, ointment, paste or patch; via the oral and buccal mucosa, buccally,
lingually or
sublingually as a tablet, pastille, coated tablets, linctus or gargle; via the
gastric and
intestinal mucosa, enterally as a tablet, coated tablets, capsule, solution,
suspension
or emulsion; via the rectal mucosa, rectally as a suppository, rectal capsule
or
ointment; via the nasal mucosa, nasally as drops, ointments or spray; via the
bronchial and alveolar epithelium, pulmonarily or by inhalation as an aerosol
or
inhalant; via the conjunctiva, conjunctivally as eyedrops, eye ointment, eye
tablets,
lamellae or eye lotion; via the mucosae of the genital organs, intravaginally
as vaginal
suppositories, ointments and flush, intrauterinely as uterine pessaries; via
the efferent
ureters, intraurethrally as a flush, ointment or medicated probe; into an
artery,
intraarterially as an injection; into a vein, intravenously as an injection or
infusion,
parverously as an injection or infusion; into the skin, intracutaneously as an
injection
CA 02533433 2006-O1-23
11
or implant; under the skin, subcutaneously as an injection or implant; into
the muscle,
intramuscularly as an injection or implant; into the abdominal cavity,
intraperitoneally
as an injection or infusion.
The compounds of the general structure I according to the invention can be
retarded
in their pharmaceutical action with respect to practical therapeutic
requirements by
means of suitable measures. This aim can be achieved in a chemical andlor
pharmaceutical way. Examples of the achievement of a prolongation of action
are the
use of implants, liposomes, sustained release forms, nanoparticle suspensions
and
"prodrugs" of the compounds according to the invention, the formation of
poorly
soluble salts and complexes or the use of crystal suspensions. .
The compounds according to the invention can be employed as an individual
substance or in combination with further cytotoxic substances, such as, for
example,
cisplatin, carboplatin, doxorubicin, ifosfamide, cyclophosphamide, 5-FU,
methotrexate and in particular in combination with inhibitors of signal
transduction,
such as, for example, Herceptin, Glivec or Iressa.
Particular preference is given to medicaments which comprise at least one
compound from the following group of indolyl-3-glyoxyl derivatives:
N-{2-[1-(4-chlorobenzyl)-1H-indol-3-yl]-2-oxoacetyl}-N-quinolin-6-yl-benzamide
(1)
N-{2-[1-(4-chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}-2-phenyl-N-quinolin-6-yl-
acetamide (2)
phenyl {2-[1-(4-chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}quinolin-6-
ylcarbamate (3)
(1 R,2S,5R)-2-isopropyl-5-methylcyclohexyl {2-1-(4-chlorobenzyl)-1 H-indol-3-
yl]-
2-oxoacetyl}quinolin-6-ylcarbamate (4)
N-{2-[1-(4-chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}-4-methyl-N-quinolin-6-yl-
benzenesulfonamide (5)
These compounds can be present not only as a free base but also as salts of
physiologically tolerable acids.
CA 02533433 2006-O1-23
12
The invention additionally comprises processes for the preparation of the
compounds
of the structure I according to the invention.
The compounds (I) according to the invention can be synthesized as in scheme 1
below:
St~a a 1 H ET
1) base, R2-Hal
aprotic solverit
R J
N 2) (COCI)2 R
3) HET-NH2, base
aprotic solvent
H- R7,
ET
Stage 2
"R7", b ase
R aprotic solvent
R2
Scheme 1
According to this general procedure for stages 1 and 2, on which synthesis
scheme 1
is based, the following compounds were 'synthesized which follow from the list
below
with statement of the respective chemical name. The analytical
characterization of
I the compounds according to the invention was carried out by means of their
melting
I
points or by'H-NMR spectroscopy and/or mass spectrometry.
The chemicals and solvents employed were obtained commercially from the
conventional suppliers (Acros, Avocado, Aldrich, Fluka, Lancaster, Maybridge,
Merck, Sigma, TCI etc.) or synthesized.
CA 02533433 2006-O1-23
13
The invention will now be illustrated in more detail with the aid of the
examples
below, without being limited thereto.
The present invention's compounds 1-5 were synthesized as per the following
General Prescription:
General Prescription
To a mixture of 1.2 mmol of sodium hydride (60% mineral oil suspension) in 50
ml of
dimethylformamide or THF are added 1.2 mmol of 2-[1-(4-chlorobenzyl)-1 H-indol-
3-yl]-2-oxo-N-quinolin-6-ylacetamide with ice cooling. After 15 min of
stirring at room
temperature, 1.3 mmol of the corresponding acyl chloride are added and the.
ice
cooling is removed. After 3 h, the reaction solution is poured onto ice-water
and
extracted three times with 100 ml of ethyl acetate each time. The combined
organic
phases are dried over sodium sulfate and concentrated to dryness. The crude
product thus obtained is subsequently purified by recrystallization or by
column
chromatography.
Example 1:
N-{2-[1-{4-Chlorobenzylj-1 H-indol-3-yl]-2-oxoacetyl}-N-quinolin-6-ylbenzamide
(1)
CA 02533433 2006-O1-23
14
N
O
Melting point: 213 °C
1 H-NMR (DMSO-ds} 8 = 8.94-8.95 (m, 2H),. 8.70 (s, 1 H), 8.35 (d, 1 H), 8.08-
8.09 (m,
2H}, 8.03 (d, 1 H), 7.85 (dd, 1 H), 7.73 (d, 2H), 7.62 (d, 1 H), 7.55-7.58 (m,
1 H), 7.41-
7.46 (m, 3H), 7.27-7.36 (m, 6H), 5.60 (s, 2H) ppm.
Example 2:
N-{2-[1-(4-Chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyly-2-phenyl-N-quinolin-6-yl-
acetamide (2)
Example 3:
Phenyl {2-[1-(4-chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}quinolin-6-
ylcarbamate
(3)
MS: m/e = 558.0 (M+H+)
CA 02533433 2006-O1-23
Example 4:
(1 R,2S,5R)-2-Isopropyl-5-methylcyclohexyl f 2-[1-(4-chlorobenzyl)-1 H-indol-3-
yl]-
2-oxoacetyl}quinolin-6-ylcarbamate (4)
Example 5:
N-~2-[1-(4-Chlorobenzyl)-1 H-indol-3-yl]-2-oxoacetyl}-4-methyl-N-quinolin-6-yl-
benzenesulfonamide (5)
MS: mle = 560.0 (M+H+)
MS: m/e = 622.0 (M+H+)
CA 02533433 2006-O1-23
16
\ /
00 ~ N
O=ff.
o N ~ ~ /
0
N
CI
MS: m/e = 594 (M+H*)
Biological data:
Example 6:
-Inhibition of selected tumor cell lines:
Substance 1 was investigated for its antiproliferative action in a
proliferation test
using established tumor cell (D.A. Scuderio et al. Cancer Res. 1988, 48, 4827-
4833).
The test used determines the cellular dehydrogenase activity and permits a
determination of the cell vitality and, indirectly, of the cell count. The
cell lines used
are the human cervical carcinoma cell line KB/HeLa (ATCC CCL17), the ovarial
adeno carcinoma cell line SKOV-3 (ATCC HTB77), the human glioblastoma cell
line
SF-268 (NCI 503138) and the pulmonary carcinoma cell line NCI-H460
(NCI 503473).
Compound KB/HeLa NCI-H460 SF-268 SK-OV-3
IC50[Nglml]IC50(Nglml]IC50[Nglm!]IC50[Nglml]
1 0.170 0.222 0.261 0.139
Table 1: Proliferation inhibition of the present invention's substances in XTT
cytotoxicity test on human tumor cell lines
Example 7:
CA 02533433 2006-O1-23
17
- Antiproliferative action on MDR tumor cell lines:
Substance 1 was further characterized by testing with regard to multidrug-
resistant
cell lines as compared with the nonresistant wild-type cell fines.
The cell lines tested are the murine cell line L1210, the acute myeloid
leukemia cell
line LT12 and the resistant lines L1210/mdr and LT121mdr. Further test systems
used
are the murine P388 cell line (methylcholanthrene-induced lymphoid neoplasm)
and
the doxorubicin-resistant P388 cell line.
Table 2 summarises the results:
Com- LT12 LT12mdr L1210 L1210VCR P388 P388ADR
poundIC50[Irg/ml]IC50[Nglml]IC50[Irglml]IC50[Nglml]IC50[Nglml]IC50[Nglmt]
1 0.226 0.277 0.255 0.577 0.219 0.252
Table 2: Inhibitory effect of substance (1) in XTT proliferation test on human
tumor cell lines.