Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02561237 2006-09-28
CRD-5224 USNP
STENT DELIVERY SYSTEM USING A STEERABLE GUIDE WIRE
FIELD OF USE
This invention is in the field of devices for percutaneous insertion into a
vessel
of the human body to place a stent at the site of an obstruction.
BACKGROUND OF THE INVENTION
Stents are well known devices for placement in vessels of the human body to
obtain and maintain patency of that vessel. The greatest use for stents has
been for placement within a stenosis in a coronary artery. When a stent is
used for treating a coronary artery stenosis, it has always been necessary to
first place a guide wire through the stenosis. The next step in the stenting
procedure is typically to pre-dilate the stenosis with a balloon angioplasty
catheter that is advanced over that guide wire. The balloon angioplasty
catheter is then removed and a stent delivery system that includes the stent
is
advanced over the guide wire and the stent is then deployed at the site of the
dilated stenosis.
Recent improvements in the design of stent delivery systems have made it
possible to eliminate the step of pre-dilatation for the treatment of many
classes of stenoses. The delivery of a stent to the site of a stenosis without
pre-dilatation has been given the name "direct stenting". However, even with
direct stenting, a guide wire is still required as a precursor to advancing
the
stent delivery system over that guide wire to place the stent at the site of a
stenosis. Placing the guide wire requires additional procedure time and
additional cost for the procedure.
In US Patent Number 6,375,660, Fischell et al. describe a scent delivery
system with a fixed guide wire that is not steerable. This fixed, not
steerable,
guide wire system will not be as capable for rapid delivery of the stent
through
the tortuous coronary arteries as stent delivery systems that are advanced
over a steerable guide wire.
CA 02561237 2006-09-28
CRD-5224 USNP
SUMMARY OF THE INVENTION
The present invention is a stent delivery system that uses a steerable guide
wire that is coaxially enclosed for most of its length in a guide wire tube. A
thin-walled guide wire tube is fixedly and seaiably attached to both a
proximal
section and a distal section of a balloon angioplasty catheter. A stent is co-
axially mounted onto the inflatable balloon of the balloon angiopiasty
catheter.
Because the guide wire tube forms an inner liner for the balloon angioplasty
catheter, the fluid inflation lumen of the catheter is sealed so the inflation
liquid that pressurizes the balloon will not leak as it would be if there were
no
"inner liner" and the balloon angioplasty catheter were attached to the guide
wire itself. By not having a traditional inner shaft through which a
conventional
guide wire slides, the deflated balloon on which the stent is mounted can have
a reduced diameter. Therefore, the outside diameter of the pre-deployed stent
mounted onto that balloon is also minimized. This provides a minimum profile,
i.e., a minimum outside diameter, for the stent.
A minimum profile at the distal section of the stent delivery system is highly
advantageous for improving the percentage of cases that can be treated by
means of direct stenting; i.e., without requiring pre-dilation of a stenosis.
Another advantage of the present invention is that a separate guide wire is
eliminated thus saving the cost of such a guide wire. Additionally, the time
to
perform a stent delivery procedure is reduced because a separate guide wire
does not have to be placed prior to using the stent delivery system to place
the stent at the site of a stenosis.
The present invention uses a steerable guide wire that extends for the entire
length of the stent delivery system. A distal portion of the steerable guide
wire
can have its shaped changed after it is placed in the patient's vascular
system
by manipulation of the proximal portion of the steerable guide wire. Thus the
shape of the guide wire's distal portion can be straight or highly curved or
any
curvature in between. This is a great advantage for getting the distal end of
CA 02561237 2006-09-28
CRD-5224 USNP
the steerable guide wire into the vessel that is to be stented more
efficiently
as compared to the use of a conventional guide wire.
An important feature of the present invention is a thin-walled, guide wire
tube
that extends for essentially the entire length of the balloon angioplasty
catheter. The guide wire tube is fixedly and sealably attached at its proximal
end and its distal end to the balloon angioplasty catheter. Specifically, the
guide wire tube forms a liquid tight seal at its proximal end with the
proximal
fitting of the balloon angioplasty catheter and also a liquid tight seal at
its
IO distal end with the balloon onto which the stent is mounted.
Another important aspect of the present invention is the distal seal that is
attached to the cylindrical distal end of the balloon and also the distal end
of
the guide wire tube. This seal is lubricity coated and also has a taper at a
I S small angle in the distal direction that acts like a wedge to open a tight
stenosis (sometimes called "Dottering") in an artery. The outer diameter of
the
distal seal and the cylindrical distal section of the balloon are optimally
equal
to or slightly larger than the outer diameter of the stent as it is crimped
onto
the balloon prior to deployment of the stent. This diameter of the cylindrical
20 distal section of the balloon and the distal seal, and the lubricity
coating of the
conical front surface of the distal seal, together provide the least
resistance for
pushing through a tight stenosis. Also, the pushability of the combined
steerable guide wire and balloon angioplasty catheter work together to get the
stent to be pushed through a tight stenosis.
It is envisioned that the guide wire tube would be fixedly attached to the
guide
wire at one or more locations. The attachment could be by either the use of
an adhesive and/or by shrinking the guide wire tube down onto the outer
surface of the guide wire to minimize the diameter of the stent delivery
system.
It is also envisioned that instead of using a thin-walled guide wire tube to
form
a sealed inner liner for the inflation lumen of the balloon angioplasty
catheter,
CA 02561237 2006-09-28
CRD-5224 USNP
the guide wire itself could be coated with a polymer to form a water tight
seal,
The polymer coating would then be sealed to the balloon angioplasty catheter
at its proximal and distal ends. This embodiment while more difficult to
produce would have a potentially smaller diameter than the embodiment using
a separate tube shrunk down onto the guide wire's outer surface.
Thus, the present invention provides a means for placing a stent within a
vessel of the human body without requiring a separate guide wire, thus saving
the cost of the guide wire and also saving the time required to place a
separate guide wire through an obstruction such as an arterial stenosis.
The invention reduces the outside diameter (i.e., the profile) of the distal
section of the stent delivery system so as to optimize the capability of the
stent delivery system for direct stenting.
This invention provides a steerable guide wire such that the shape of its
distal
portion can be altered by a means at the guide wire's proximal portion, the
changing shape providing better access to a particular coronary artery into
which the stent is to be guided. The steerable guide wire and the balloon
angioplasty catheter combine in such a manner as to enhance the pushability
of the entire stent delivery system. Further, a highly tapered, lubricity
coated,
distal seal that attaches to the distal end of the balloon of the balloon
angioplasty catheter, which tapered distal seal is designed to open a tight
stenosis to provide easier passage for the stent mounted onto the balloon.
Finally, the outer diameter of the cylindrical distal section of the balloon
and
the distal seal to be equal to or slightly larger than the diameter of the
stent as
crimped onto the balloon so as to provide easier passage for the stent through
a tight stenosis.
BRIEF DESCRIPTION OF THE DRAWING
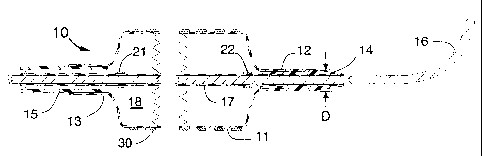
FIG. 1 is a longitudinal cross section of a distal portion of the stent
delivery
system having a balloon angioplasty catheter mounted co-axially over a
steerable guide wire.
4
CA 02561237 2006-09-28
CRD-5224 USNP
FIG. 2 is a longitudinal cross section of the proximal portion of the stent
delivery system that is shown in FIG. 1 utilizing a proximal seal.
FIG. 3 is a longitudinal cross section of the proximal portion of the stent
delivery system that is shown in FIG. 1 utilizing a Luer fitting onto which a
hemostasis valve can be placed to seal pressurized fluid for inflating the
balloon of the balloon angioplasty catheter.
DETAILED DESCRIPTION OF THE INVENTION
FIGS. 1, 2 and 3 illustrate a stent delivery system 10 having a fixed but
steerable guide wire 16 that is placed within a guide wire tube 17. The dismal
end and proximal end of the guide wire tube 17 can be joined by a small
amount of adhesive to fixedly attach the guide wire tube 17 to the steerable
guide wire 16 to prevent the guide wire from moving longitudinally within the
stent delivery system 10. The guide wire 16 would typically have a diameter
that lies between 0.010 and 0.038 inches. The optimum diameter for use in
coronary arteries will be approximately 0.014 inches.
FIG. 1 is a longitudinal cross section of a proximal portion of the stent
delivery
system 10 showing an inflated balloon 11 onto which the stent 30 is mounted,
the balloon having a cylindrical distal section 12 that is fixedly attached to
the
distal seal 14 and the balloon 11 also having a cylindrical proximal section
13
that is fixedly attached to the distal end of the distal shaft 15. The distal
seal
14 is sealed to the distal end of the guide wire tube 17 that surrounds the
guide wire 16. The proximal radiopaque marker band 21 and the distal
radiopaque marker band 22 are used in a conventional manner to indicate to
the operator by fluoroscopy the location of the proximal end and distal end of
the stent 30. These marker bands 21 and 22 assist the operator in accurately
placing the stent 30 at a proper site within a stenosis of a coronary artery.
The distal seal 14 is fixedly and sealably attached to both the distal
cylindrical
section 12 of the balloon 11 and the distal end of the guide wire tube 17. In
J
CA 02561237 2006-09-28
CRD-5224 USNP
this manner, pressurized liquid for inflating the balloon 11 (and thereby
deploying the stent 30) is sealed within the stent delivery system 10. The
distal seal 14 also has other design features to assist in placement of the
stent 30 into a tight stenosis of a coronary (or other) artery. Specifically,
the
tapered front conical surfaces of the distal seal 14 and the guide wire tube
17
are both lubricity coated to assist in having the system pushed through a
tight
stenosis. Also the taper angle is typically less than 30 degrees and optimally
less than 15 degrees. Another feature of this invention is that the outer
diameter "D" of the cylindrical distal section 12 of the balloon 11 and the
distal
seal 14 are optimally designed to be approximately equal to or slightly
greater
in diameter as compared to the outer diameter of the stent 11 as it is crimped
or heat nested onto the balloon 11 in its pre-deployed state. For example, if
the outer diameter of the stent 11 as crimped onto the balloon 11 before it is
inflated is (let us say) 0.7 mm, then the diameter "D" should be approximately
0.7 mm ~ 0.2 mm. Another concept is that the diameter "D" would be optimally
between 0.7 and 0.9 mm; i.e., the diameter "D" should be the same dimension
as the outer diameter of the crimped stent 30 and possibly the diameter "D"
should be as much as 0.2 mm larger than the outer diameter of the crimped
stent 30. This inventive concept of having a lubricity coated distal seal 14
with
a small cone angle that is attached to the cylindrical distal section 12 of
the
balloon 11 and having a diameter at least as large as the diameter of the pre-
deployed stem 30 can enhance the ability of the scent delivery system 10 to
have the pre-deployed stent 30 pushed through even a tight arterial stenosis.
It should be understood that the length of the steerable guide wire 16 that
extends beyond the distal end of the distal seal 14 should optimally be less
than 5 cm. It should also be understood that the wall thickness for the guide
wire tube 17 is less than 0.002 inches and optimally approximately 0.0005
inches.
FIG. 2 is a longitudinal cross section of a proximal portion of the stent
delivery
system 10 which shows the distal shaft 15 being sealably and fixedly joined to
a proximal shaft 23. FIG. 2 also shows the proximal shaft 23 being joined to a
G
CA 02561237 2006-09-28
CRD-5224 USNP
Luer fitting 19 that is used to connect a source of a liquid for inflating and
deflating the balloon 11 of the stent delivery system 10. The liquid used with
such a stem delivery system 10 is typically contrast medium diluted with
normal saline solution. Also shown in FIG. 2 is a proximal seal 20 that is
fixedly and sealably attached to the Luer fitting 19 and the guide wire tube
17
that is placed around the steerable guide wire 16. The length of the distal
shaft 15 would be between approximately 1 cm and 20 cm. The length of the
proximal shaft 23 would be typically more than 100 cm. The reason for having
the smaller diameter distal shaft 15 is to improve the flexibility of the
stent
delivery system 10 near its distal end. The reason why the proximal shaft 23
has a larger diameter is to improve liquid flow for inflating and deflating
the
balloon 11 for deployment of the stent 30. It should be understood that a
shaft
of a single diameter could be used for this invention.
FIG. 3 (like FIG. 2) shows the distal portion of a stent delivery system 40
with
the distal shaft and the proximal shaft formed as a single shaft 35 which is
joined to the Luer fitting 33. The shaft 35 is formed from a single plastic
tube
with most of its length being of a larger diameter and its distal extent of 1
cm
to 20 cm in length being of a smaller diameter. FIG. 3 differs from FIG. 2 in
that there are two Luer fittings 36 and 38. The Luer fitting 36 is used to
inject
and remove the balloon inflation liquid. The Luer fitting 38 is designed to
have
a hemostasis valve (not shown) attached to seal the inflation liquid within
the
balloon angioplasty catheter. Such a hemostasis valve could be tightened
down onto the guide wire tube 17 to farm a liquid-tight seal prior to
inflating
the balloon 11.
The guide wire tube 17 could be solvent swelled for placement over the outer
surface of the steerable guide wire 16. As the solvent leaves the plastic of
the
guide wire tube 17, the final inside diameter of the guide wire tube 17 would
be essentially the same diameter as the outer diameter of the guide wire 16.
Another method for attaching the guide wire tube 17 to the guide wire 16
would be by shrink fitting the guide wire tube 17 onto the outer surface of
the
steerable guide wire 16. For the guide wire 16 to be steerable, the outer coil
of
7
CA 02561237 2006-09-28
CRD-5224 USNP
that guide wire 16 cannot be forced against the inner core of the guide wire
16
but it should exert a gentle pressure so that the steerable guide wire 16
cannot slide easily within the guide wire tube 17. Thus, in its pre-deployed
state, with no liquid pressure within the stent delivery system 10, the inner
surface of the guide wire tube 17 can gently press against the outer surface
of
the steerable guide wire 16 but it cannot exert a large force against the
outer
surface of the guide wire 16. Therefore, in its pre-deployed state, the stent
delivery system 10 can steer the steerabie guide wire 16 into the artery where
the stent 30 is to be placed. When the pre-deployed stent 30 is in place
within
an arterial stenosis, it can be deployed under a high liquid pressure
(typically
8 to 20 atms.) and during that time, the steerable guide wire 16 will not be
steerable because of the high force of guide wire tube 17 against the outer
coil of the steerable guide wire 16. Such a high pressure would create a high
frictional force between the outer coil and the core wire of the steerable
guide
wire 16. Since no steering is necessary at that time, this is not a detriment
to
the operation of the stent delivery system 10.
It is also envisioned that instead of using a thin-walled guide wire tube 17
to
form a sealed inner liner for the inflation lumen of the balloon angioplasty
catheter, the guide wire 16 could be coated with a polymer to form a water
tight seat. The polymer coating would then be sealed to the balloon
angioplasty catheter 10 at its proximal and distal ends. This embodiment,
while more difficult to produce, would have a potentially smaller diameter
than
the embodiment using a separate guide wire tube 17 shrunk down onto the
guide wire 16.
An important goal of this invention is to have an outer diameter of the pre-
deployed stent 30 to be no greater than 0.8 mm. As such, it would present
one of the lowest profiles for any stent that is used to treat an arterial
stenosis.
The diameter of the deployed stent 30 could be in the range from as small as
1.5 mm to as large as 6 mm. The larger diameter stents 30 would have a
larger pre-deployed diameter because of the increased thickness of the pre-
deployed balloon 11. A wall thickness of the stent would optimally be between
CA 02561237 2006-09-28
CRD-5224 USNP
0.0015 and 0.004 inches. Furthermore, the optimal type of stent 30 would be
a drug eluting stent with a drug such as sirolimus or paclitaxel or any other
drug that decreases neointimal hyperplasia subsequent to balloon
deployment. The optimum stent would be formed from a high density (i.e.
radiopaque) metal such as tantalum or a cobalt-chromium alloy such as L605.
Various other modifications, adaptations, and alternative designs are of
course possible in light of the above teachings. Therefore, it should be
understood at this time that within the scope of the appended claims, the
invention might be practiced otherwise than as specifically described herein.
9