Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02581329 2008-04-15
1
WAX EMULSION PRESERVATIVE COMPOSITIONS AND METHOD OF
MANUFACTURE
BACKGROUND OF THE INVENTION
[0002] Certain properties of gypsum (calcium sulfate dihydrate) make it very
popular for use in making industrial and building products; especially gypsum
board and
gypsum wood fiber (GWF) products. It is a plentiful and generally inexpensive
raw
material which, through a process of dehydration and rehydration, can be cast,
molded or
otherwise formed to useful shapes. The base material from which gypsum board
is
manufactured is the hemihydrate form of calcium sulfate (gypsum), commonly
termed
stucco, which is produced by the heat conversion of the dihydrate from which
the water
phase has been removed.
[0003] The manufacture of gypsum products generally comprises preparing a
gypsum-containing slurry that contains gypsum and other components of the
finished
product, and then processing the slurry to remove the water and form and dry
the
remaining solids into the desired form. In the making of gypsum board, the
gypsum
slurry must flow onto a paper substrate. In a continuous process, the
slurry/substrate
combination is then sized by passing this combination between rollers.
Simultaneous
with this sizing step, a paper backing is positioned over the sized gypsum
slurry.
Accordingly, the gypsum slurry must possess sufficient fluidity so that a
properly sized
gypsum board can be made. Fluidity refers to the ability of the gypsum slurry
to flow.
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
2
[0004] It is also important to the manufacture of gypsum board, that the
gypsum sluiTy be capable of being foamed to a limited extent. Foamability
refers to
this ability to be foamed. When the gypsum slurry and paper substrate are
passed
through the sizing rollers, a certain amount of the gypsum slurry must back
flow and
accumulate in the rollers nip so that a steady flow of gypsum is delivered to
the sizing
rollers. Foamability is important to this ability of the gypsum slurry to back
flow at
the rollers nip. Forming plates may be used, eliminating the use of a master
roll, but
foam is important to control density of the finished product. Because of the
continuous nature of a gypsum board manufacturing process wherein the gypsum
slurry flows onto a substrate which then passes througll sizing rollers, the
extent to
which the gypsum slurry flows after it is sized is critical to maintaining the
finished
product dimensions of the gypsum board. The time at which the gypsum slurny
ceases its flow is referred to as the pre-set tine. Therefore, pre-set time is
an
important property of the gypsum slurry. The set time of the gypsum slurry is
also an
important property. The set time refers to the amount of time it takes the
gypsum
slurry to be dried, under heat, to the finished, solid gypsum board. As is
well known
in the art, in a continuous gypsum board manufacturing process, it is
important that
the gypsum slurry possess a consistent set time.
[0005] Unlike the production of gypsum board, the production of gypsum
wood fiber (GWF) products is facilitated through a conventional paper making
process. The process of water felting dilute aqueous dispersions of various
fibrous
materials is a well-lcnown conunercial process for manufacturing many types of
paper
and board products. In this process, an aqueous dispersion of fiber, binder
and other
ingredients, as desired or necessary, is flowed onto a moving foraminous
support
wire, such as that of a Fourdrinier or Oliver mat forming machine, for
dewatering.
The dispersion may be first dewatered by gravity and then dewatered by vacuum
suction means; the wet mat is then pressed to a specified thickness between
rolls and
the support wire to remove additional water. The pressed mat is then dried in
heated
convection or forced air drying ovens, and the dried material is cut to the
desired
dimensions. The manufacture of gypsum wood fiber products may be carried out
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
3
similarly, utilizing a wet end section headbox distribution mechanism
distributing the
gypsum wood fiber slurry onto a vacuum wire for initial mat formation and
dehydration followed by compression through a series of vacuum belt rolls and
into a
kiln for final dehydration. The gypsum wood fiber product does not incorporate
paper
face and back paper but rather is a paperless core that has similar
performance and
uses comparable to conventional sheathing products currently available.
[0006] Gypsum absorbs water, which reduces the strength of the products in
which it is used and enables deleterious biological activity, such as the
growth of
mildew, mold, etc., to occur therein and thereon. Prior art products, like
ordinary
gypsum board, gypsum tile, gypsum block, gypsum casts, and the like have
relatively
little resistance to water. When ordinary gypsum board, for example, is
immersed in
water, the board quickly absorbs a considerable amount of water, and loses a
great
deal of its strength. Tests have demonstrated that when a 2 inch by 4 inch
cylinder of
gypsum board core material was immersed in water at about 70 F. the cylinder
showed a water absorption of 36% after immersion for 40 minutes.
[0007] Attempts to provide water-resistant properties to gypsum board include
incorporation of asphalt, metallic soaps, resins, and wax additives into an
aqueous
gypsum slurry. The resulting materials were difficult to use and the core
properties
difficult to control. Polysiloxane-based systems have also been used in
attempts to
impart water resistance to gypsum board. Finished gypsum products have also
been
coated with water-resistant filins or coatings. One specific example of a past
attempt
to provide a water-resistant gypsum product is the spraying of a molten
paraffin, wax
or asphalt into an aqueous gypsum slurry.
[0008] Another example of a prior art attempt to provide a water-resistant
gypsum product is the addition of an emulsion of wax, such as paraffin wax,
and
asphalt, in the relative proportions of from about 1 part to about 10 parts of
asphalt
per part of wax to the aqueous gypsum slurry. Polyvinyl alcohol has been used
in an
attempt to provide a room temperature system for use in adding water-resistant
properties to gypsum.
[0009] Some emulsions include generic starch species, e.g., from corn, sago,
wheat, rice, etc., with a complexing agent such as sodium borate in
combination with
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
4
other chemical compounds, specifically sodium lignosulfate, C24 and greater
polymerized alkyl phenol and various waxes. While this system shows
significant
advantages over previously available wax emulsions it to suffers from a number
of
deficiencies, including: degradation of the pH due to bacteriological activity
resulting
from the decomposition of the sodium lignosulfate in long-term storage,
viscosity
changes as temperature and age occur manifesting itself as a slight separation
at the
water/wax interface, and less than predictable use rates at the mixer due to
the
changes occurring singularly and in combination.
[0010] The panel board industry, includes, but is not limited to, plywood,
OSB (Oriented Strand Board) (commonly referred to as flake or wafer board),
medium density fiber board, particleboard, and other products, inclusively
refeiTed to
herein as lignocellulosic composite products. In each of these composite
products and
in lumber (the wood of trees cut and prepared for use as building material)
(collectively referred to herein as "lignocellulosic products") it is
desirable to control
the water absorption or "uptake" and swelling, both of which have detrimental
affect
on the utility of the product. For example, in plywood used for floor
underlay,
swelling causes buckling or creep in the final wood or tile overlay. Similar
problems
occur with swelled OSB used as a roofing member applied to areas which will
experience moisture. These coinposite board panels, like wood and other
lignocellulosic products, are also known to deteriorate on the job site due to
open
storage, as a result of water uptake, which leads to biological degradation
resulting
from the growth of, and infestation by, bacteria, fungi, and insects.
[0011] Lignocellulosic composite products are conventionally manufactured
by hot pressing lignocellulosic materials with wax a.nd thermosetting resin.
This is
referred to as a conventional bonding process. The wax is a sizing agent to
improve
the water resistance of the composite. The resin is a bonding agent that holds
the
materials comprising the composite together, thus forming them into a unitary
shape.
Resoles are commonly used as the binding resin for lignocellulosic composite
products.
[0012] In the conventional hot press method of manufacture of lignocellulosic
composite products, a lignocellulosic material is combined with a phenolic
resin and
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
other cornponern.ts in a blender or mixer. The blend or mixture that results
is pressed,
typically under pressures above atmospheric and temperatures greater than room
temperature, to produce the composite. Lignocellulosic materials used in the
production of mats may be selected from the group consisting of wood fiber,
wood
flake, wood strands, wood chips and wood particles, and mixtures thereof. The
lignocellulosic materials listed here are referred to in the art as wood
furnish.
However, it is well known that other wood furnish, such as straw, bagasse,
wood
bark, recycled wood fiber, recycled paper fiber, and mixtures thereof, may
also be
used. The wood furnish, once blended or mixed with the phenolic resin, is then
formed onto a support material to make a pre-fonn in the approximate shape of
the
finished good. The pre-form is then placed on a caul plater in a hot press
where the
finished good is produced by applying pressures above atmospheric and
temperatures
greater than room temperature. The elevated temperatures and pressures cause
the
phenolic resin to polymerize, thus binding the pre-form into a unitary
finished good.
The hot press method is further described in U.S. Pat. No. 4,433,120 to Shui-
Tung
Chiu.
[0013] There remains a need for an additive which is useful in imparting
resistance to biological growth on gypsum products, and which is economical to
employ. When biocides are added to a gypsum product, it is common to over
spray
the face and/or backing paper of the products with mildew resistant chemicals.
There
also remains a need for a useful and effective preservative for
lignocellulosic
composite products.
SUMMARY OF THE INVENTION
[0014] The above described and other features are exemplified by the
following detailed description.
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
6
[0015] An emulsion comprising water as the continuous phase, a wax as the
discontinuous phase, an emulsifier and a preservative has the general
structure:
N
N
, 2
(R)n R
wherein R' can be a heterocycle containing nitrogen and sulfur, such as
thiazolyl,
isothiazolyl, or thiadiazolyl, which can optionally be substituted with C1-C6
alkyl; RZ
can be hydrogen or C1-C6 alkyl, specifically hydrogen; n is 0, 1, 2, or 3;
each instance
of R3 can independently be hydrogen, CI-C6 alkyl, phenoxy, C1-C6 alkoxy, halo,
amino, C1-C6 alkylamino, di C1-C6 alkyl amino, imidazolyl, thiazolyl,
isothiazolyl,
thiadiazolyl, thienyl, furyl, pyrryl, naphthyl, phenyl, halophenyl, C1-C6
alkyl phenyl,
or C1-C6 alkoxyphenyl.
[0016] A method for making a wax emulsion comprises making the emulsion
without a preservative as defined herein, and then adding the preservative
thereto.
[0017] A gypsum product comprises gypsum and a preservative as defined
herein.
[0018] A method for making a gypsum product comprises forming a slurry
from gypsum, water, and a wax-in water emulsion containing a preservative as
described herein, and forming the slurry into a solid product.
[0019] A method for improving the water resistance of a lignocellulosic
composite product prepared by mixing lignocellulosic material with a binder to
form a
mixture and solidifying the mixture in a selected configuration to form the
composite
product, coinprises adding to the mixture an emulsion as defined herein.
[0020] A lignocellulosic composite product made by mixing lignocellulosic
material with a binder to form a mixture, adding to the mixture an emulsion as
defined
herein, and forming the mixture and the emulsion therein into a solid product.
CA 02581329 2008-04-15
7
DETAILED DESCRIPTION
[0021 ] A type of preservative has been found to be particularly advantageous
in
wax emulsions, especially in wax emulsions used in the manufacture of gypsum
products
and wood fiber board and other lignocellulosic composite products.
[0022] The preservatives disclosed herein are useful for inhibiting biological
growth, e.g., the growth of mildew, fungi, etc., on gypsum products. As used
herein
"preservative" includes biocides such as bactericides, fungicides, algaecides,
mildewcides, or a combination thereof. Exemplary preservatives include the
compositions disclosed in U. S. Patent No. 3,370,957 to Wagner et al, and
which
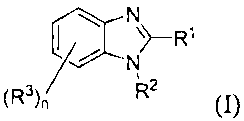
discloses preservatives according to the general structure (I):
N
~-Rl
l ~ N
2
(R) " R (I)
wherein RI can be a heterocycle containing nitrogen and sulfur, such as
thiazolyl,
isothiazolyl, or thiadiazolyl, which can optionally be substituted with C,-C6
alkyl; R2 can
be hydrogen or Ci-C6 alkyl, specifically hydrogen; n is 0, 1, 2, or 3; each
instance of R3
can independently be hydrogen, Ci-C6 alkyl, phenoxy, CI -C6 alkoxy, halo,
amino, Ci-C6
alkylamino, di CI -C6 alkyl amino, imidazolyl, thiazolyl, isothiazolyl,
thiadiazolyl,
thienyl, furyl, pyrryl, naphthyl, phenyl, halophenyl, CI-C6 alkyl phenyl, C1-
C6
alkoxyphenyl, and the like.
[0023] Particular embodiments of the mildewcide include those according to the
general structures (II) and (III):
3
R N \R2 ::>-<NJ
N
3 N
R
R2 R2
(I) (II)
wherein R1, R2, and R3 are as defined previously.
[0024] Exemplary mildewcides according to structure (I) include: 2-(4'-
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
8
thiazolyl) benzimidazole; 2-[3'-(1',2',5'-thiadiazolyl] benzirnidazole; 2-(4'-
thiazolyl)-
5-methoxy benzimidazole; 2-(4'-thiazolyl)-5-phenoxy benzimidazole
hydrochloride;
2-(2'-methyl-4'-thiazolyl) benzimidazole; 2-[4'-(1',2',3'-thiadiazolyl)]
benzimidazole; 1-acetyl-2-(4'-thiazolyl)-5-phenyl benzimidazole; 2-(4'-
isothiazolyl)
benzimidazole; 2-(4'-thiazolyl)-6-fluoro benzimidazole; 2-(4'-thiazolyl)-5-
amino
benzimidazole; 2-(2'-thiazolyl)-5-(1'-imidazolyl) benzimidazole; 2-(4'-
isothiazolyl)-
5-chlorobenzimidazole; 2-(4'-thiazolyl)-5-phenyl benzimidazole; 2-[4'-
(1',2',3'-
thiadazolyl)]-5-(4'-tolyl) benzimidazole; 1-acetyl-2-(2'-thiazolyl)-5-phenyl
benzimidazole; 1-methyl-2-(2'-isothiazolyl)-5-(2'-methoxyphenyl)
benzimidazole; 2-
(4'-isothiazolyl)-5-furyl benzimidazole; 2-(4'-thiazolyl)-5-(4'-fluorophenyl)
benzimidazole hydrochloride; 2-(4'-thiazolyl)-5-bromo benzimidazole; 2-(4'-
thiazolyl)-5-chloro benziinidazole; 2-(2'-thiazolyl)-5-methoxy benzimidazole;
2-(4'-
thiazolyl)-5-(2'-fluorophenyl) benzimidazole hydrochloride; 2-[3'-(1',2',5'-
thiadiazolyl)1-5-methylthio benzimidazole; 2-(4'-thiazolyl)-5,6-difluoro
benzimidazole; 1-benzoyl-2-(4'-thiazolyl) benzimidazole; 2-(2'-thiazolyl)-5-
(2'-
pyrryl) benzimidazole; 1 -methyl-2-(4'-isothiazolyl) benzimidazole
hydrochloride; 2-
(4'-thiazolyl)-5-phenoxy benzimidazole; 2-[3'-(1',2',5'-thiadiazolyl)1-5-
methoxy
benzimidazole; 1-ethyl- 2-(4'-thiazolyf)-5-(2'-thiazolyl) benzimidazole; 1-
acetyl-2-
[3'-(1',2',5'-thiadiazolyl)]-5-(2"-furyl) benzimidazole; 2-(4'-thiazolyl)-4-
fluoro
benzimidazole hydrochloride; 2-(2'-thiazolyl) benzimidazole; 1 -acetyl-2-(4'-
thiazolyl) benzimidazole; and combinations thereof. In a particular embodiment
described below, thiabendazole (2-(4'-thiazolyl) benzimidazole) has been found
to be
effective in inhibiting the growth of biological agents on gypsum board, thus
indicating the advantageous utility of benzimidole compounds generally and the
other
preservatives indicated herein.
[0025] Preferably, the preservative is added as the last ingredient in the wax
emulsion, i.e., the preservative is `post-added' to the already-formed wax
emulsion.
The preservative may be present in the wax emulsion in an amount of 0.01 to
10% by
weight of the wax emulsion (wt.%), optionally 0.1 to 5 wt. %, for example, 0.2
to 4
wt.%. The preservative may be added in any convenient form, including a 100%
solids, as a hydrated paste or in a water diluted system, e.g., 25-50% active
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
9
thiabendazole. The preservative is preferably added with agitation, which is
believed
to thoroughly disperse the preservative into the discontinuous wax phase of
the
einulsion. Optionally, one or more of these biocides or the others mentioned
below
may employed in an amount calculated to be about 0.0025 % to about 0.2 % by
weight of the finished gypsum product. The preservative is post-added to the
wax
emulsion under severe agitation to any desired wax emulsion where the wa.ter
phase is
the continuous phase in amounts ranging from 0.01 to 5% (percent) by volume.
The
resulting wax emulsion can be used in the preparation of gypsum products and
does
not have significant affect on the desired properties of the finished
products, i.e.,
strength, facing bonds, and water resistance where applicable. The emulsions
may be
added to mixtures of gypsum and water without adversely affecting properties
of the
mixture which are necessary to the manufacture of gypsum products such as
gypsum
board and GWF. Such properties include fluidity, formability and set time. In
the
manufacture of gypsuin wallboard products it is important to impart water
resistance
to the finished product, so as to liinit the maximum water absorption realized
by the
wallboard in a defined board soak test. For example, American Standards for
Testing
Materials ASTM 1396 and sub parts thereof describe such a test.
[0026] Generally, the preservative may be used in wax emulsions emulsified
with various wax emulsions useful in the production of gypsum products,
including
emulsions containing stearic/oleic acid amine combinations where the amine
structure
can be described as TEA, DEA, AMP, Morpoline and other fatty acid amine
systems
normally considered known art; emulsions where a lignin sulfate or sulforli-
ate acting
as the surfactant in combination with various waxes; and emulsions comprising
complex combinations consisting of starch compounds and metallic salts and
calcium
phenates, long branched chain calcium alkyl phenols, long straight chain
calcium
alkyl phenols, complex polymers of maleic acid with and without an amirie
attachment, and long chain, C 30 and above alkyl phenols, and wax or a
combination
of waxes. Some non-limiting examples of such emulsions are provided below. The
addition of the preservative provides an emulsion which, when incorporated
into a
gypsum slurry, will provide resistance from mildew and other biological
activity from
occurring to a finished gypsum product while retaining the desired properties
of the
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
finished product, i.e., strength, facing bonds, and water resistance where
applicable.
In addition, the use of these preservatives has been found to result in
increased
product strength relative to the use of other preservatives.
[0027] Wax emulsions containing the preservatives described herein may also
be added to the resin used in making various kinds of panel board that do not
contain
gypsum.
[0028] In one embodiment, the preservative may be used in a wax emulsion
that contains starch, optionally a complexed starch. In one embodiment, such
an
emulsion may include a wax, an alkyl phenol, a polynaphthalenesulfonic acid,
an
alkali metal hydroxide, and a complexed starch. The polynaphthalenesulfonic
acid
and the alkali metal hydroxide react to give a salt of polynaphthalenesulfonic
acid.
Such emulsions may be prepared by (a) mixing the wax and an alkyl phenol to
provide a first pre-mix; (b) mixing polynaphthalenesulfonic acid, an alkali
metal
hydroxide, water, and a complexed starch to provide a second pre-mix; (c)
combining
the first pre-mix and the second pre-mix to provide a mixture; and (d)
homogenizing
the mixture.
[0029] Waxes useful in making the various embodiments of the present
invention may be selected from any of the commercially known waxes which have
a
melting point of from about 120 F to about 150 F, and preferably from about
135 F
to about 145 F. Such waxes are typically of low volatility, exhibiting less
than about a
10% loss in weight during standard thermogravimetric analysis. Also, the oil
content
of these waxes is typically less than about 1% by weight. These waxes are of a
relatively high molecular weight, having an average chain length of C36 (that
is a 36
carbon chain length), or greater.
[0030] In certain embodiments, it is useful to saponify one or more of the
waxes. In this way, the saponified wax functions as an added surfactant. Waxes
useful in this respect are limited to waxes having an acid value or a
saponification
value and a melting point greater than about 180 F. Saponification of such
waxes
may be accomplished by combining the wax with a strongly basic material such
as
ammonium hydroxide or an alkali metal hydroxide such as sodium hydroxide or
potassium hydroxide. Waxes which may be saponified in the preparation of
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
11
emulsions described herein include waxes from the liquefication of coal,
vegetable
waxes and oxidized waxes resulting from the processing and/or refining of
slack wax,
scale wax or crude petroleum. For example, saponifiable waxes include montan
wax,
carnauba wax, beeswax, bayberry- myrtle wax, candelilla wax, caranday wax,
castor
bean wax, esparto grass wax, Japan wax, ouricury wax, retano- (or retamo-)
ceri
mimbi wax, shellac, spermaceti wax, sugar cane wax, wool-lanolin wax, and
others.
The alkali metal hydroxide may be provided in the form of a concentrated
aqueous
solution that may comprise about 45% alkali metal hydroxide, by weight.
Ammonium hydroxide may be provided in solid form. Some or all of the
saponifier
may also react with the dispersant, and/or with other component ingredients of
the
emulsion, in situ. Although ammonium hydroxide is sometimes objected to
because
of the ammonia odor it produces, ammonium hydroxide is believed to be
advantageous because, in addition to saponifying the wax, the ammonia can
serve as a
scavenger for formaldehyde in the resin witli which the emulsion is used, and
may
thus reduce the emission of formaldehyde from the finished composite product.
The
combination of ammonium hydroxide with formaldehyde also ameliorates the
ammonium hydroxide odor, so in some embodiments, formaldehyde may be added to
the emulsion for this purpose, for exainple, in an amount of about 0.02 to
about 0.1%
by weigllt. In addition, ammonium hydroxide is especially advantageous for
when the
emulsion is used with lignocellulosic materials comprising northern wood
species,
i.e., Douglas fir, aspen and the like. The amount of strongly basic material
needed to
saponify a wax may be calculated based on the saponification value of the 5
wax. For
example, the saponification value divided by 1000 equals the grams of
potassium
hydroxide to add per gram of wax.
[0031] Suitable, non-saponifiable, waxes include a wax having a melting point
greater than about 120 F (about 49 C), e.g., about 120 F to about 165 F (about
49 C
to about 74 C), optionally about 120 F to about 150 F (about 49 C to about 66
C),
and preferably about 135 F to about 145 F (about 57 C to about 63 C). Suitable
nonsaponifiable waxes include paraffin waxes, slack waxes and scale waxes.
Such
waxes are commercially known to be of low volatility, exhibiting less than
about a
10% loss in weight during standard thermogravimetric analysis. Also, the oil
content
CA 02581329 2008-07-16
12
of these waxes is typically less than about 5% by weight, preferably less than
about 1%
by weight. Some of these waxes are of a relatively high molecular weight,
having an
average chain length of C36, that is a 36 carbon chain length, or greater.
Paraffin waxes
are typically derived from light lubricating oil distillates and are
predominantly straight
chain hydrocarbons having an average chain length of 20 to 30 carbon atoms.
Suitable
paraffin waxes include Wax 3816* available from Honeywell/Astor of Duluth,
Georgia.
Slack waxes are petroleum waxes having an oil content of 3 wt.% to 50 wt. %.
Suitable
slack waxes include Exxon* 600 Slack Wax and Ashland* 200 Slack Wax, and a
combination of 50 parts Exxon* 600 Slack Wax and 50 parts Ashland* 200 Slack
Wax.
[0032] Starch used in the emulsions of the present invention is complexed
starch.
The starch may be complexed in situ, during manufacture of the emulsion, or
the starch
may be pre complexed prior to being added to the emulsion. Starch is
preferably
complexed by mixing the starch with a complexing agent such as a borate
compound, a
molybdate compound or a molybdenum conipound. For example, a preferred borate
compound is sodium tetraborate decahydrate. For example, a preferred molybdate
compound is ammonia hepta molybdate. For example, a preferred molybdenum
compound is molybdenum disulfide. Other compounds useful in complexing starch
include ammonium biborate, ammonium pentaborate, potassium pentaborate,
potassium
tetraborate, lithium tetraborate, and magnesium borate compounds; ammonium
dimolybdate, ammonium heptamolybbate, barium molybdate, calcium molybdate,
lithium molybdate, magnesium molybdate, sodium molybdate, and potassium
molybdate;
and other molybdenum compounds, and the like.
[0033] The starch useful in making the complexed starch of the present
invention
includes, but is not limited to, corn, rice, wheat, potato, sago and other
starches.
[0034] The ratio of completing agent (a borate compound, a molybdate
compound, or a molybdenum compound) to starch is important to the
functionality of the
complexed starch in the emulsions. It has been found that the ratio may be as
low as
1:20, of complexing agent (a borate compound, a molybdate; compound, or a
* trade-mark
CA 02581329 2008-07-16
13
molybdenum compound) to starch on a weight per weight basis. The ratio may be
as
high as 1:3.5, however it has been found that at this ratio, and higher
ratios, a greater
amount of completed starch is needed in the emulsion to maintain the balance
of
desired properties in the gypsum mixture and final gypsum product. These
desired
properties include fluidity, formability, and water-resistance.
[0035] Borate compounds, molybdate compounds, and molybdenum
compounds are surprisingly effective complexing agents. Examples of useful
complexing agents include, but are not limited to, sodium borate (borax),
magnesium
borate, and other borate compounds, ammonium molybdate, sodium molybdate,
magnesium molybdate, and other molybdate compounds, molybdenum disulfide and
other molybdenum compounds. The ratio of complexing agent (for example, sodium
tetraborate decabydrate, sodium molybdate dehydrate, molybdenum disulfide, or
other compounds) to the modified starches significantly influences the control
of
other necessary properties in the board/slurry process, i.e., foam support and
slurry
additive compatibility.
[0036] Incorporating alkyl phenols into the emulsions has been found useful
in achieving low water absorption in gypsum products. As used herein, "alkyl
phenols" refer to phenolic compounds having a long chain alkyl group. The long
chain alkyl group may be straight or branched. The long chain alkyl group may
be
C24 - C34 (from 24 to 34 carbon chain length), preferably C24 - C28. Such
alkyl
phenols include long chain, C24 - C34 (from 24 to 34 carbon chain length)
polymerized methylene-coupled alkyl phenol, phenate salts, calcium phenates,
long
branched chain calcium alkyl phenols, long straight chain calcium alkyl
phenols and
complex polymers of maleic acid with and without an amine group substitution.
[0037] The alkyl group of the alkyl phenol can be derived from a
corresponding olefin; for example, a C26 alkyl group is derived from a C26
alkene,
preferably a 1-alkene, a C34 alkyl group is derived from a C34 alkene, and
mixed
length groups are derived from the corresponding mixture of olefins. When the
alkyl
group is an alkyl group having at least about 30 carbon atoms, however, it may
be an
aliphatic group (or a mixture of such groups) made from homo- or interpolymers
(e.g.,
copolymers, terpolymers) of mono- and di- olefins having 2 to 10 carbon atoms,
such
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
14
as ethylene, propylene, butene- 1, isobutene, butadiene, isoprene, 1-hexene,
and 1-
octene. Aliphatic hydrocarbyl groups can also be derived from halogenated
(e.g.,
chlorinated or brominated) analogs of such homo- or interpolymers. Such groups
can,
however, be derived from other sources, such as monomeric high molecular
weight
alkenes (e.g., 1-tetracontene) and chlorinated analogs and hydrochlorinated
analogs
thereof, aliphatic petroleum fractions, particularly paraffin waxes and
cracked and
chlorinated analogs and hydrochlorinated analogs thereof, white oils,
synthetic
alkenes such as those produced by the Ziegler-Natta process (e.g.,
poly(ethylene)
greases) and other sources known to those skilled in the art. Unsaturation in
the
hydrocarbyl groups can be reduced or eliminated, if desired, by hydrogenation
according to procedures known in the art. Preparation by methods and materials
that
are substantially free from chlorine or other halogens is sometimes preferred
for
environmental reasons. More than one alkyl group can be present, but usually
no
more than 2 or 3 are present for each aromatic nucleus in the aromatic group.
Most
typically only one hydrocarbyl group is present per aromatic moiety,
particularly
where the hydrocarbyl-substituted phenol is based on a single benzene ring.
[0038] One example of an alkyl phenol useful in the emulsions described
herein is available from Lubrizol Chem. Corp. Wycliffe, Ohio, under the trade
designation 319H, described as a C24 - C34 polymerized methylene- coupled
alkyl
phenol. Various other, commercially available alkyl phenols that may be used
in
these emulsions, include the following (identified by arbitrary identifier
numbers in
the following Table 1:
CA 02581329 2008-07-16
TABLE 1
Identification
No. Description Source
319A Complex polymer= of maleic acid "Flozol 140"*
(no amine group substitution) Lubrizol Chem. Corp.
Wycliffe, Ohio
319B Complex polymer of maleic acid "Flozol 145"*
(with amine group substitution) Lubrizol Chem. Corp.
Wycliffe, Ohio
319C Straight chain, long chain alkyl Lubrizol Chem. Corp.
phenol Wycliffe, Ohio
319D Calcium Phenate Lubrizol Chem. Corp.
Wycliffe, Ohio
319E Branched chain, long chain alkyl Lubrizol Chem. Corp.
phenol Wycliffe, Ohio
319H C 24 -C 34 polymerized methylene- Lubrizol Chem. Corp.
coupled alkyl plienol Wycliffe, Ohio
* trade-mark
[0039] The alkyl phenol and product of the reaction of an alkyl phenol with a
saponifier or with any other component of the emulsion is referred to herein
as the
alkyl phenol component.
[0040] Such emulsions provide an alternative to the use of sodium lignosulfate
or lignosulfonate previously used as both a co-surfactant and a dispersing
aid, further
reducing the need for a biocide to control biological activity. (The
preservatives
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
16
disclosed herein could, however, be used in emulsions comprising lignosulfate
or
lignosulfonate.) The ratios of starch:borate, or starch: molybdate, or starch:
molybdenum compound may be about 4:1 to about 20:1 on a weight/weight basis.
[0041] Emulsions may be prepared by heating the wax and surfactants ("wax
mixture") in one vessel and the water, complexing agent (a borate compound, a
molybdate compound, or a molybdenum compound) and corn starch ("water
mixture") in another vessel. Both mixtures were heated, with mixing, to about
185
(85 C). Next, the wax mixture was poured into the water mixture under mixing.
The
resultant mixture was then placed in a homogenizer.
[0042] With homogenization it is preferred that a distribution of micelle
diarrreters ranging from about 0.6 micron to about 1.8 micron be achieved.
However,
the distribution of micelle diameters may range from about 0.5 micron to about
2.5
micron. This level of homogenization may be attained, for example, by using a
dual
orifice homogenizer operating at from about 2,000 to about 4,000 psi.
[0043] It is preferred that the homogenized mixture be cooled after the
homogenization step.
[0044] It is most preferable that the homogenized mixture be cooled from
approximately 185 F to about 100 F. This may be accomplished by running the
homogenized mixture through a cooling coil immersed in water maintained at
room
temperature.
[0045] More specifically, an emulsion may be prepared by combining water, a
cornplexing agent (that is, a borate compound, a molybdate compound, or a
molybdenum compound) and a starch to make the complexed starch useful in
certain
embodiments. Polynaphthalenesulfonic acid and potassium hydroxide are added to
the aqueous solution of complexed starch. This mixture is brought to a
temperature of
about 185 F to about 205 F and held until the starch reaches its maximum
state of
gelation, which typically occurs in about 20 to about 30 minutes. The wax
cornpounds are incorporated with the polymerized alkyl phenol and brought to a
ternperature of about 185 F to about 205 F. Then, the wax phase is added to
the
water phase and reacted to form an in situ surfactant. A detergent/dispersant
is formed
by the combination and reaction of the polymerized alkyl phenol and the
CA 02581329 2008-04-15
17
polynaphthalenesulfonic acid, which acts to modify the wax crystal and allows
the wax
crystals to resist plating and linking with themselves and instead remain in a
disassociated state until they are transferred due to polarity to the gypsum.
The reacted
system is then passed through a homogenizer at a pressure of about 2,000 to
about 4,000
psi and then cooled at a prescribed rate to control the stability and
viscosity of the
finished wax emulsion. The homogenized composition exits the homogenizer at a
temperature of about 135 F to about 145 F. The mixture is then cooled to
about 80 F
to about 110 F. The cooling rate is controlled to avoid causing the wax to
recrystallize
and breakout of solution.
[0046] By utilizing the modified starch compounds in combination and proper
ratios with other noted compounds, a low viscosity system can be developed
allowing a
broader range of solids, from about 40 % to about 60 % by weight to be
available and
usable.
[0047] In certain embodiments which use a single wax additive, it has been
found
that a dual surfactant system provides a stable emulsion at both room
temperature and
elevated temperatures. Such stable emulsions may be added, for example, to hot
or
boiling water, without the emulsion separating or curdling.
[0048] One example of dual surfactants is a combination of
dodecylisopropanolamine benzene sulfonate and a nonionic ethoxylated aryl
phenol.
[0049] Dodecylisopropanolamine benzene sulfonate may be obtained from
Unichema, Wilmington, Delaware, under the trade name SD 1121. One nonionic
ethoxylated aryl phenol is Ethox* 2938, available from Ethox Corp.,
Greenville, South
Carolina. Alternatively, an alkoxylated fatty acid ester may be combined with
the of
dodecylisopropanolamine benzene sulfonate to form the dual surfactant system.
One
alkoxylated fatty acid ester is Ethox* 2914, also available from Ethox Corp.
It has also
been found that in certain embodiments of the present invention a dispersing
aid, or
fluidity modifier, is useful for the maintenance of the fluidity of the
gypsum/emulsion
mixture. Such dispersing agents are strong lipophiles, which are,
consequently, good
defoamers. One such dispersing agent is poly(oxy-1,2-ethanedyl), alpha phenyl-
omega-
hydroxy styrenate.
[0050] An emulsion can be formed by combining and homogenizing a single
* trade-mark
CA 02581329 2008-07-16
18
wax, a dual surfactant system, an alkyl phenol and a complexed starch. Table 1
below
provides examples.
TABLE 1.
Component/ Emulsion A Emulsion B Emulsion C
Parameter
(amount of component, grams)
Wax 3816 135.0 134.5 134.5
319H 4.0 4.0 4.0
Ethox 2914 14.0 12.0 12.0
SD1121 4.0 4.0 4.0
Water 240.0 240.0 240.0
Borax 0.5 0.5 0.5
Corn Starch 2.5 5.0 5.0
KOH 3.0 3.0 3.0
[0051] Other wax emulsions that may include the preservatives disclosed
herein include those that contain (1) simple stearic/oleic acid amine
combinations
where the amine structure can be described as TEA, DEA, AMP, morpholine and
other fatty acid amines, and (2) a lignin sulfate or sulfonate acting as the
surfactant in
combination with various waxes.
[0052] An emulsion can be formed by combining and homogenizing two or
more waxes, a co surfactant, an alkyl phenol and a complexed starch. Typical
composition ranges for two-wax emulsions are provided in Table 2 below.
CA 02581329 2008-04-15
19
TABLE 2.
Component Typical Amount (% weight basis)
First Wax 25 - 40
Saponifiable Wax 2.5 - 4.5
Alkyl Phenol 0.25 -10.0
Polynaphthalenesulfonic Acid 0.25 - 5.0
Water 55 - 65
Starch + Complexing Agent (4:1 to 20:1) 1.5 - 3.5
Alkali Metal Hydroxide Amount used depends on amount of
saponifiable wax; typically 0.5 - 1.5
[0053] Table 3 below provides examples of emulsions made according to a dual-
wax embodiment.
TABLE 3.
Component/ Emulsion D Emulsion E Emulsion F Emulsion G
Parameter
(amount of Component, % by wt)
Wax 3816D 33.00 33.00 36.00 38.00
Montan Wax 3.30 3.30 3.60 3.80
Alkyl Phenol 0.50 0.50 0.50 0.50
DISAL GPS* 1.00 1.00 1.20 1.50
Water 59.50 59.10 55.58 52.97
Borax 0.37 0.37 0.37 0.37
Acid-modified 1.60 1.60 1.60 1.60
C150 Starch
45% KOH 0.75 0.75 0.818 0.864
METASOL* 0.40 0.40 0.40
D3TA
* trade-mark
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
[0054] The emulsions of Table 3 may be mixed with water, and gypsum may
be added to the water emulsion mixture. The water/emulsion/gypsum mixture may
then be formed into a gypsum product.
[0055] In an alternative embodiment, a wax emulsion useful in making
gypsum products may include lignosulfonate or lignosulfate, as illustrated in
the
following Table 4.
TABLE 4.
Component/ Emulsion H Emulsion I Emulsion J
Parameter
(amount of component , grams)
Wax 3816 134.0 132.0 130.0
Montan Wax 12.0 12.0 12.0
319H 10.0 4.0 6.0
Sodium 4.0 4.0 4.0
lignosulfonate
Water 239.0 237 237
Borax 1.5 1.5 1.5
Corn Starch 6.5 6.5 6.5
KOH 3.0 3.0 3.0
[0056] In still other embodiments, a useful wax emulsion may contain
carboxymethylcellulose. Such emulsions are useful with lignocellulosic
products.
One example of a carboxymethylcellulose-containing wax emulsion for use in a
gypsum slurry useful for the manufacture of gypsum products comprises a
nonsaponifiable wax, a saponified wax, an alkyl phenol component, a
dispersant/surfactant, a carboxymethylcellulose component, and water. In a
particular
embodiment, the nonsaponihable wax may comprise about 33% to about 351,/o of
the
emulsion, by weight, the saponified wax may comprise about 3% to about 51,/o
of the
emulsion, by weight, the alkyl phenol component may comprise about 0.5% to
about
2.5% of the emulsion, by weight, the dispersant may comprise about 0.5% to
about
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
21
2% of the emulsion, by weight, and the carboxymethylcellulose component may
comprise about 0.2% to about 5% of the emulsion, by weight.
[0057] Emulsions described herein comprise a wax component comprising a
nonsaponifiable wax and a saponifiable wax.
[0058] A suitable saponifiable wax has an acid value or a saponification value
and a melting point greater than about 180 F (about 82 C).
[0059] Preferably, the waxes do not contain more than about 5% (by weight)
polar compounds as impurities.
[0060] The wax component may be present in an amount of about 25 percent
by weight (wt. %) to about 50 wt. %, based on the total weight of the
emulsion,
preferably about 30 wt. % to about 40 wt. %. Preferably, the wax component
comprises a combination of a nonsaponiflable wax having a melting poiilt of
greater
than or equal to about 120 F and a saponifiable wax. The nonsaponifiable wax
may
comprise about 25 wt. % to about 44 wt. % of the total weight of the emulsion,
and
the saponifiable wax may coinprise about 0.5 wt. % to about 5 wt. % of the
total
weight of the emulsion. A preferred combination of waxes is a combination of a
paraffin wax such as Honeywell 3816 as the first wax and a saponifiable wax
such as
montan wax. In one embodiment, the wax component comprises paraffin wax in an
amount of about 25 wt. % to about 45 wt. %, preferably about 30 wt. % to about
40
wt. %, and saponifiable wax in an amount of about 2.5 wt. % to about 5 wt. %,
preferably about 3.5 wt. % to about 4.5 wt. %, based on the total weight of
the
emulsion.
[0061] A strongly basic compound as described herein is added to the
emulsion mixture to saponify the saponifiable wax. The saponifier may be
provided
in an amount of about 0.15% to about 4.5%, optionally about 0.5% to about 3%,
of
the emulsion, by weight. Optionally, concentrated aqueous saponifier may be
provided in an amount of about 0.5 to about 3% by weight of the emulsion;
ammonium hydroxide may be added in solid form in an amount of about 0.15 to
about
3% by weight of the emulsion. The amount of saponifier may be varied with the
type
CA 02581329 2008-04-15
22
of saponifiable wax used, or with the type of wood. As a result of the
saponifier, an
emulsion as described herein may have a pH of about 8.5 to about 12.5, for
example, a
pH of about 8.5 to about 9.5.
[0062] Exemplary carboxymethylcellulose materials useful in these emulsions
have molecular carbon chain lengths of about 20 to about 50 carbons. An
example of a
suitable carboxymethylcellulose is carboxymethylcellulose sodium, available
from Penn
Carbose, Somerset, Pennsylvania, under the trade designation LT-30, which is
described
as having carbon chain lengths of about 26 to 30 carbons. Other suitable
carboxymethylcellulose materials include Penn Carbose* LT-20 and LT-42. The
carboxymethylcellulose and the product of its reaction with the saponifier or
with any
other component in the emulsion are referred to herein as the
"carboxymethylcellulose
component".
[0063] A salt of polynaphthalenesulfonic acid is useful in the emulsions
described
herein and, without wishing to be bound by theory, is believed to act as a
dispersant/surfactant. The salt may be the product of an in-situ reaction of
polynaphthalenesulfonic acid and a saponifier, e.g., an alkali metal
hydroxide. One
commercially available polynaphthalenesulfonic acid is DISAL* GPS which may be
obtained from Handy Chemical, Montreal, Quebec, Canada. The acid and acid salt
are
referred to collectively as a polynaphthalenesulfonic acid component or, more
broadly (to
include substitute materials), as the dispersant/surfactant. The
dispersant/surfactant may
comprise about 0.1 % to about 5% of the emulsion, by weight, optionally about
0.25 wt.
% to about 5 wt. %.
[0064] Incorporating an alkyl phenol into emulsions has been found to
facilitate
achieving low water absorption in lignocellulosic composite products.
Preferably, the
alkyl phenol is chosen so that the average carbon chain length of the alkyl
portion
matches, i.e., is approximately the same as or is close to, the average carbon
chain length
of the carboxymethylcellulose. For example, ari alkyl phenol of average chain
length in
the range of about C24 to about C34 may be used in an emulsion comprising
carboxymethylcellulose having an average chain length of about 26 to about 32
carbons,
e.g., Carbose* LT-30 carboxymethylcellulose.
* trade-mark
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
23
[0065] The amount of alkyl phenol component present in the emulsion may be
about 0.25 wt. % to about 10 wt. %, optionally about 0.5 wt. % to about 2.5
wt. %
based on the total weight of the emulsion.
[0066] One method of manufacture for the emulsions described herein results
in time, energy, operator, and production efficiencies. The method involves
mixing
the ingredients of the emulsion in a single vessel and then conveying the
mixture of a
homogenizer under conditions such as the following. An advantage of this
method is
that the emulsion mixture is prepared in a single vessel; it is not necessary
to prepare
and separately store partial mixtures of the ingredients of the emulsion in
separate
vessels before combining thein together.
[0067] In one embodiment of a`single vessel' method, the nonsaponifiable
wax (e.g., 3816 wax, further described below) is melted and stored in molten
form,
e.g., at about 10 F above its melt point temperature, and water is provided at
a
temperature that will not cause the wax to solidify. The vessel is then
charged in the
following illustrative manner:
a. Charge the melted nonsaponifiable wax, e.g., 3816 wax, at a temperature of
about 189 F to about 192 F (about 87 C to about 89 C);
b. Start heat and agitation;
c. Charge molten saponifiable wax and alkyl phenol with continued agitation;
d. Charge a majority of the water, e.g., 95%, and continue agitation;
e. Charge the dispersant/surfactant, (e.g., DISAL polynaphthalenesulfonic
acid, further described elsewhere herein), carboxymethylcellulose and
saponifier;
f. Charge the remaining water - preferably including the water used to rinse
the tubes calculated and subtracted out of the total;
g. Bring the tank up to temperature, e.g., about 190 F to about 210 F (about
88 C to about 100 C);
h. Continue to agitate while maintaining temperature for about 30 to about
150 minutes;
i. Put through homogenizer at about 1500 to about 3500 PSI (about 10
megaPascals (MPa) to about 24 MPa);
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
24
j. Cool, optionally in process that provides two exotherms, including a first
exotherm between the exit temperature from the homogenizer to a temperature
above
ambient, and a second exotherm to ambient (storage) temperature. For example,
the
emulsion composition is passed from the homogenizer to a cooler to achieve a
first
exotherm of, e.g., about 10 F to about 20 F degrees lower than the homogenizer
exit
temperature, and then to a cooling tank to achieve a second exotherm of, e.g.,
about
an additional5 F to about 15 F lower, optionally under agitation. In one
embodiment, the first exotherm may occur by cooling from about 130 F to about
110 F, and the second exotherm may occur by cooling from about 110 F to about
70 F.
[0068] Without wishing to be bound by any particular theory, using a two-
exotherm cooling process allows a phasing process of the formation of the
emulsion
to proceed to completion. As a result, the viscosity of the emulsion is more
stable
over time and the emulsion is more stable when subject to shear agitation than
if a
single exotherm cooling process is used. In an alternative method of preparing
the
emulsion, a batch process may be used in which a first premix comprising the
molten
waxes and alkylphenol may be prepared, and a second premix (an aqueous premix)
comprising the water, carboxymethylcellulose and polynaphthalenesulfonic acid
and
saponifier may be prepared, and the first and second premixes may then be
combined
in a mixing tank for a time sufficient at least for the waxes to become
saponified, e.g.,
for one to three hours, and the resulting mix may then be passed to a
homogenizer and
cooled as described above.
[0069] Illustrative ranges of ingredients in some embodiments of emulsions
described herein are provided in Table 5 below.
CA 02581329 2008-04-15
TABLE 5. ILLUSTRATIVE EMBODIMENTS (CMC)
Component Typical Amount (% weight basis)
Nonsaponifiable Wax 33 - 35
Saponifiable Wax 3-5
Alkyl Phenol 0.5 - 2.5
Polynaphthalenesulfonic 0.5 - 2
Acid
Carboxymethylcellulose 0.2 - 5
Saponifier Amount used depends on amount of
saponifiable wax; typically 0.5 - 3
Water Balance (to 100)
[0070] The following Table 6 provides example proportions of ingredients in a
specific embodiment of an emulsion as described herein.
TABLE 6.
Component- Emulsion K Weight %
Wax 3816 33.00
Saponifiable Wax 3.00
Alkyl Phenol 0.50
Polynaphthalenesulfonic Acid 0.50
(DISAL GPS)*
Carboxymethylcellulose 0.2
45% KOH (saponifier) 0.75
Water Balance (to 100)
[0071 ] Such an emulsion may have a viscosity of about 10 to about 100
centipoise, measured on a Brookfield viscometer. One sample emulsion had a
viscosity
of 9 cps at about 40% solids.
* trade-mark
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
26
[0072] The emulsions described herein, and others, when incorporated into a
gypsum slurry, are useful in the production of gypsum products, and the
preservatives
disclosed herein may be used with all of them.
Example
[0073] A wax emulsion formulation was prepared with the following
components: G Wax (a paraffin wax) 33%, Montan wax 3.3%, alkyl phenol 0.5%,
Disal 0.5%, potassium hydroxide 0.75%, ammonium hepta molybdonate 0.01%,
starch 0.09%, polyfon H (lignosulfonic acid, sodium salt) 0.5%, water 61.35%.
To
this wax emulsion was post-added 0.2% thiabendazole, commercially available
from
Supreme Chemical of Cumming, GA under the trade designation MC-2. The wax
emulsion was used to prepare a gypsum product. The resulting gypsum product
was
tested according to ASTM D3273. During this four week test, a sample of the
subject
material, such as a gypsum board, is placed in a closed chamber along with
potting
soil and mold cultures at a constant temperature of about 90 F (32.2 C) and
relative
humidity of 95% to 98%. The condition of the test sample is monitored weekly
to
determine the extent, if any, of mold growth. The sample prepared as described
above exhibited no biological growth during the first weeks of the test.
[0074] In other tests, test emulsions as described above was used to prepare
sample particle board coupons comprising a urea-formaldehyde binder. Some test
emulsions contained 1% thiabendazole, others 2%, by weight of the wax in the
emulsions. The emulsion was added to the furnish from which the coupons were
formed in an amount of 0.4 % solids, by weight of the furnish. Some of the
coupons
were soaked in water for twenty-four hours before testing. For the test,
various
soaked and non-soaked coupons were placed in various environments including
constant humidity conditions (72 F (22.2 C), 72% relative humidity (RH)),
outdoor
rooftop environmental conditions (exposed to air but shielded from direct
impingement by precipitation); and indoors in an uncontrolled warehouse and in
a
laboratory environment, beside a water bath. Control coupons prepared and
treated
similarly were also place in these environments. After the first week, the
control
CA 02581329 2008-07-16
27
coupons showed visible biological growtli, but after six weeks, none of the
sample
coupons made with thiabendazole had any visible growth thereon.
[0075] Without wishing to be bound by any particular theory, it is believed
that visual comparative evaluation of saniple gypsum products made with wax
emulsions containing thiabendazole to other gypsum products shows that
crystals
believed to be thiabendazole are embedded in the gypsum solids. This is
believed to
indicate that thiabendazole and the related compounds identified above, are
carried by
the wax into the gypsum while the gypsum slurry is wet, and then, as
crystallize as the
gypsum slurry is dehydrated to form the gypsum product and the wax emulsion is
broken, the preservative reverts to its crystalline form and becomes embedded
in the
gypsum crystal. Thus, the preservative is impregnated into, and may be bonded
to,
the gypsum crystal structures, rather than merely residing on the surface of
the
product or being limited to residing in discrete agglomerations of wax that
may
remain after the wax emulsions are broken. The incorporation of the
preservative
into the gypsum extends the effective life of the preservative because the
preservative
is less likely to leech from the internal structure of the gypsum crystals
than from the
surface of the gypsum product or from wax agglomerations therein. It may also
be a
contributing factor to the increased product strength noted in sample gypsum
products
containing this preservative.
[0076] Optionally, any effective amounts of other preservatives may also be
used in the gypsum products. Such preservatives include e.g.,
bactericides/fungicides,
mildewcides, or other biocides, may optionally be included in a gypsum product
by
incorporating the preservative into the emulsion or into the gypsum-containing
slurry.
One example of a preservative suitable for gypsum products is a
bactericide/fungicide
known commercially as METASOL* D3TA, which comprises 3,5-dimethyl-
tetrahydro-1,3,5,2 H thiadiazine-2-thione. METASOL* D3TA may be obtained from
Ondo-Nalco, Houston, Texas. Mildewcide can include any commercially available
mildewcide including formaldehyde. Other suitable biocides include bis-thio-
benzene, propiconazole and bis(tributyltin) oxide.
[0077] Preservatives useful in lignocellulosic products including, e.g., GWF
products and other gypsum products that contain wood fibers or other
lignocellulosic
* trade-mark
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
28
material, and which might optionally be used in addition to thiabendizole
and/or the
preservatives related thereto as disclosed herein, may be inorganic or
organic, and
may include, for example, biocides such as insecticides, fungicides,
bactericides, and
combinations comprising one or more of the foregoing biocides. The biocide may
be
chosen according to (1) the target organism; (2) solubility characteristics;
(3) stability
to the temperature and pH; and other conditions found in the manufacture of
the
composites. Biocides include substances that kill or inhibit the growth of
microorganisms such as molds, slimes, fungi, bacteria, etc. Insecticides,
fungicides
and bactericides are all examples of biocides. Fungicides include substances
that kill
or inhibit the growth of fungi. Bactericides include agents that kill
bacteria.
Insecticides are agents that kill insects. More specific examples of biocides
include,
but are not limited to, chlorinated hydrocarbons, organometallics, halogen-
releasing
compounds, metallic salts, organic sulfur compounds, and phenolics. Preferred
biocides include but are not limited to chromated copper arsenate (CCA); such
as
ammoniacal copper quateinary ammonium (ACQ), ammonial copper zinc arsenate
(ACZA), copper bis(dimethyldithiocarbamate) (CDDC), ammoniacal copper citrate
and copper azole, copper naphthenate, zinc naphthenate, quatemary ammonium
salts,
pentachlorophenol, tebuconazole (TEB), chlorothalonil (CTL), chlorpyrifos,
isothiazolones, propiconazole, other triazoles, pyrethroids, and other
insecticides,
imidichloprid, oxine copper and the like, and combinations comprising one or
more of
the foregoing biocides. In addition to the organic biocides, nanoparticles
with
variable release rates that incorporate such inorganic preservatives as boric
acid,
sodium borate salts, zinc, zinc borate, silicated borate, copper salts and
zinc salts may
be used.
[0078] Suitable general microbicides include, for example, 3-isothiazolones,
3-iodo-2-propynylbutylcarbamate, 1,2-dibromo-2,4-dicyanobutane, methylene-bis-
thio-cyanate (MBT), 2-thiocyano-methylthiobenzothiazole, tetrachloroisophthalo-
nitrile, 5 -bromo- 5 -nitro- 1,3 -dioxane, 2-bromo-2- nitropropane-1,3-diol,
2,2-di-bromo-
3-nitrilopropionamide (DBNPA), N,N'- dimethylhydroxyl-5,5'-dimethyl-hydantoin,
bromochlorodimethylhydantoin, 1, 2-benzisothiazolin-3 -one, 4,5-tri-methylene-
2-
methyl-3-isothiazolone, 5- chloro-2-(2,4-dichlorophenoxy)-phenol, 3,4,4'-
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
29
trichlorocarbanilide, copper naphthenate, copper-8-hydroxy-quinoline, zinc
borate,
boric acid, trimethyl boron, zinc oxide, glutaraldehyde, 1,4-bis(bromo-
acetoxy)-2-
butene, 4,5 -dichloro- 1, 1 -dithiacyclop entene-3 -one, chlorothalonil,
quaternary
ammonium based compounds, and combinations comprising one or more of the
foregoing microbicides.
[0079] Suitable fungicides include, for example, zinc dimethyl
dithiocarbamate, 2-methyl-4-t-butylamino-6-cyclopropylamino-s-triazine, 2,
4,5,6-
tetrachloroisophthalonitrile, N,N-dimethyl dichlorophenyl urea, copper
thiocyanate,
N-(fluorodichloromethylthio)phthalimide, N,N-dimethyl- N'-phenyl-N'-
fluorodichloromethylthiosulfamide; copper, sodium and zinc salts of 2-
pyridinethiol-
1-oxide; tetramethylthiuram disulfide, 2,4,6- trichlorophenyl-maleimide,
2,3,5,6-
tetrachloro-4-(methylsulfonyl)- pyridine, diiodomethyl p-tolyl sulfone, phenyl
(bispyridil) bismuth dichloride, 2-(4-thiazolyl)-benzimidazole, pyridine
triphenyl
borane, phenylamides, halopropargyl compounds, propiconazole, cyproconazole,
tebuconazole and 2-haloalkoxyaxyl-3-isothiazolones (such as 2-(4- trifluoro-
methoxyphenyl)-3-isothiazolone, 2-(4-trifluoromethoxy-phenyl)-5- chloro-3-
isothi-
azolone, 2-(4-trifluoroinethoxyphenyl)-4,5-dichloro-3- isothiazolone), and
combinations comprising one or more of the foregoing fungicides.
[0080] The fungicide may be an agricultural fungicide such as, for example,
dithiocarbamate and derivatives such as ferbam, ziram, maneb (manganese
ethylenebisdithio-carbamate), nzancozeb, zineb (zinc
ethylenebisdithiocarbamate),
propineb, metham, thiram, the complex of zineb and polyethylene thiuram
disulfide,
dazomet, and mixtures of these -with copper salts; nitrophenol derivatives
such as
dinocap, binapacryl and 2-sec-butyl-4,6-dinitrophenyl isopropyl carbonate;
heterocyclic structures such as captan folpet, glyodine, dithianon,
thioquinox,
benomyl, thiabendazole, vinolozolin, iprodione, procymidone, triadimenol,
triadimefon, bitertanol, fluoroimide, triarimol, cycloheximide, ethirimol,
dodemorph,
dimethomorph, thifluzamide and quinomethionate; miscellaneous halogenated
fungicides such as: chloranil, dichlone, chloroneb, tricamba, dichloran and
polychloronitrobenzenes; fungicidal antibiotics such as: griseofulvin,
kasugamycin
and streptomycin; miscellaneous fungicides such as diphenyl sulfone, dodine,
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
methoxyl, 1-thiocyano-2,4-dinitrobenzene, 1- phenyl-thiosemicarbazide,
thiophanate-
methyl and cymoxanil; acylalanines such as furalaxyl, cyprofuram, ofurace,
benalaxyl, and oxadixyl; fluazinam, flumetover, phenylbenzamide derivatives
such as
those disclosed in EP 578,586-A, amino acid derivatives such as valine
derivatives
disclosed in EP 550,788-A, methoxyacrylates such as methyl (E) -2-(2-(6-(2-
cyanophenoxy)pyrimidin-4-yloxy)phenyl)-3-niethoxyacrylate, benzo(1,2,3)thiadia-
zole-7-carbothioic acid S-methyl ester, propamocarb, imazalil, carbendazim,
myclobutanil, fenbu-conazole, tridemorph, pyrazophos, fenarimol, fenpiclonil,
pyrimethanil, and combinations comprising one or more of the foregoing
fungicides.
[0081] Combination bactericides/fungicides can be included in the
preservative compositions. An example of a bactericide/fungicide is METASOL
D3TA, which is 3,5-dimethyl-tetrahydro-1,3,5,2H-thiadiazine-2-thione available
from
Ondo-Nalco, Houston, Texas.
[0082] Suitable insecticides include, foT example, acephate, aldicarb, a -
cypermethrin, azinphos-methyl, bifenthrin, binapacryl, buprofezin, carbaryl,
carbofuran, cartap, chlorpyrifos, chlorpyrifos methyl, clofentezine,
cyfluthrin,
cyhexatin, cypermethrin, cyphenothrin, deltarnethrin, demeton, demeton-S-
methyl,
demeton-O-methyl, demeton-S, demeton-S-methyl sulfoxide, demephion-O,
demephion-S, dialifor, diazinon, dicofol, dicrotophos, diflubenzuron,
dimetlioate,
dinocap, endosulfan, endothion, esfenvalerate, ethiofencarb, ethion, ethoate-
methyl,
ethoprop, etrimfos, fenamiphos, fenazaflor, fenbutatin-oxide, fenitrothion,
fenoxycarb, fensulfothion, fenthion, fenvalerate, flucycloxuron, flufenoxuron,
fluvalinate, fonofos, fosmethilan, furathiocarb, hexythiazox, isazophos,
isofenphos,
isoxathion, methamidophos, methidathion, methiocarb, methomyl, methyl
parathion,
mevinphos, mexacarbate, monocrotophos, nicotine, omethoate, oxamyl, parathion,
permethrin, phorate, phosalone, phosmet, phosphamidon, pirimicarb, pirimiphos-
ethyl, profenofos, promecarb, propargite, pyridaben, resmethrin, rotenone,
tebufenozide, temephos, TEPP, terbufos, thiodicarb, tolclofos-methyl,
triazamate,
triazophos, vamidothion, and combinations coxnprising one or more of the
foregoing
insecticides.
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
31
[0083] Antitermite agents may be used in addition to other insecticides as
long
as they do not detract from the properties of the other insecticides.
Antitermite agents
include Permetorin, lmidachlopride, Etpfenplox, and combinations comprising
one or
more of the foregoing agents.
[0084] Specific examples of suitable preservatives include alkylammonium
compounds such as didecyldimethylammonium chloride (DDAC), BARDAP (N,N-
didecyl-N-methylpolyoxyethylammonium propionate), copper benzalconium chloride
or N-alkylbenzyldimethylammonium chloride (BKC); metal salts of naphthetic
acid
such as copper naphthenate (NCU) or zinc naphthenate (NZN); metal salts of
versatic
acid such as zinc versatate; triazole type compounds such as Cyproconazole
[(2RS,3RS;2RS,3SR)-2-(4-chlorophenyl)-3- (cyclopropyl-l-(1H-1,2,4-triazol-l-
yl)butan-2-01], Tebuconazole [(RS)-l-p- chlorophenyl-4,4-dimethyl-3-(1H-1,2,4-
triazol-l-ylmethyl)pentan-3-01], Propiconazole [1-[2-(2,4-dichlorophenyl)-4-
propyl-
1,3-dioxoran-2-ylmethyl] -1H-1,2,4-triazole],1-[2-(2',4-dichlorophenyl-1,3-
dioxoran-2-
ylmethyl]- 1H-1,2,4-triazol-l-ethanol or 1-[2-(2',4'-dichlorophenyl)-4-propyl-
l,3-
dioxoran-2-ylmethyl]-1H-1,2,4-triazol-l-ethanol; and organic iodine compounds
such
as IF-1000[4-chlorophenyl-3-iodopropargyl formal],1PBC [3-iodo-2-propynyl-N-
butylcarbamate], and combinations comprising one or more of the foregoing
preservatives.
[0085] The lignocellulosic preservatives may be used in coinbination.
Preferred combinations include Cyproconazole and DDAC; Cyproconazole and
BARDAP; Tebuconazole and Propiconazole; and the like.
[0086] A compound which is effective to inhibit or prevent growth of wood
rot soil bacteria and wood soft rot fungi, mainly wood soft rot fungi such as
chaetomium globosum, may also be used as a preservative. Such compounds
include
p-cumylphenol (PCP), and its salts such as the sodium salt of p-cumylphenol,
the
ethylamine salt of p-cumylphenol, and combinations comprising one or more of
the
foregoing wood preservatives. PCP inhibits the growth of wood r t soil
bacteria,
ascomycetes and imperfect fungi, and it is effective as an antimold agent and
antitermite agent. Therefore, PCP is particularly preferable. PCP can exhibit
a
CA 02581329 2007-03-21
WO 2006/036294 PCT/US2005/027495
32
sufficient effect to wood materials in the treatment amount (application
amount) of
about 200-1,000 grams per cubic meter of wood (g/m3).
[0087] All ranges disclosed herein are inclusive and combinable, e.g., the
ranges of "about 120 to about 165 F, optionally from 135 to 145 F", are
inclusive
of the endpoints and all intermediate values of the ranges and combinations
thereof,
including, e.g., about 120 to about 145 F, about 130 to about 150 F, etc.
The terms
"first," "second," and the like, herein do not denote any order, quantity, or
importance, but rather are used to distinguish one element from another, and
the terms
"a" and "an" herein do not denote a limitation of quantity, but rather denote
the
presence of at least one of the referenced item.
[0088] While certain embodiments and best mode of the present invention are
described herein, these embodiments are merely illustrative. It will be
apparent to
those skilled in the art that modifications may be made therein without
departing from
the spirit of the invention and the scope of the appended claims.