Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE I)E CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE _2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
COMPOSITIONS AND METHODS FOR REMOVING HEAVY METALS FROM CONTAMINATED
SAMPLES USING MEMBRANES PROVIDED WITH PURIFIED METALLOTHIONEIN (MT)
PROTEINS
FIELD OF THE INVENTION
[0001] The present invention relates to compositions and methods for removing
heavy
metals from contaminated samples. More specifically the present invention
relates to
removing heavy metals from contaminated samples using solid supports having
metallothionein proteins bound thereto.
BACKGROUND OF THE INVENTION
[0002] Metal recovery and metal remediation and the associated need for
efficient and
safe methods for clean up of metal waste is a continuing environmental and
business
concern due to the toxicity and potential risk to human health posed by metal
contaminants,
as well as the economic value of precious heavy metals. Indeed, as the
discharge of toxic
wastes from agricultural, industrial and other commercial operations
continues, the need for
effective, safe and low-cost metal remediation methods increases. In a recent
report by the
United States Environmental Protection Agency (US EPA), metal contamination
remains and
historically has been a key concern at many contaminated sites (US EPA Work
Assignment
#011059, Mar. 5, 1997, Contract #68-W5-0055). In addition, there are numerous
published
reports of damage to wildlife, livestock, plant life as well as danger to
human health as a
result of metal poisoning from contaminated soil or waste matter (Impact of
Lead-
Contaminated Soil on Public Health by Xintaras, C. May 1992 at
http://www.atsdr.cdc.gov/cxlead.html). For example, a primary concern to
humans is the
health hazard created by lead (Pb) contamination. Exposure to lead can occur
through a
variety of methods such as by ingestion of lead from food, water, soil, or
even inhalation of
dust. Lead poisoning is extremely dangerous and potentially fatal, with
symptoms including
seizures, mental retardation and behavioral disorders. Therefore, methods for
metal
remediation are extremely valuable both for their protection of our
environment as well as for
protection from diseases.
[0003] Recovered metals from various waste, discard or recycling efforts
provide
immense economic value as well as augmenting environmental pollution control.
Metal
recovery can be from innumerable and varied sources such as from waste
electronic devices
(transistors, chips, transformers, bus bars, cathodes, and microprocessors,
populated
computer circuit boards PCBs, motherboards). Costs associated with hazardous
disposal of
industrial waste in the absence of metal reclamation are enormous. Therefore,
metal
recycling or reuse of metal extracted from scrap or discarded metal-containing
items not only
1
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
reduces the volume and cost of metal waste requiring specialized disposal and
handling
efforts, but the reclaimed metal can also be resold or reused to provide
additional economic
value.
[0004] Prior art attempts at treating metal contamination have traditionally
employed
cleanup technologies which consist primarily of physically removing and then
disposing of
contaminated matter. These methodologies are not only labor intensive and less
efficient,
but also carry a high expense associated with removal and disposal of large or
bulk
quantities of contaminated waste. Metal contamination is especially difficult
to remediate
because unlike other types of waste such as chemical or organic matter, metals
cannot be
directly destroyed or converted. For example, current technologies for
remediating metal
contaminated soils consist primarily of landfilling or soil excavation with
physical or chemical
separation of the metal contaminants. Treatment of contaminated ground water
usually
involves flushing, filtration or chemical extraction to remove the
contaminating metals. As a
result, the cost of soil or ground water remediation is high, ranging in the
hundreds to
thousands of millions of dollars in projected five-year costs per site (U.S.
EPA, 1993).
[0005] In addition, the risk to humans and the environment from heavy metal
contamination is not limited to soil or ground water, but also includes other
sources such as
industrial waste, sludge waste, wastewater, radioactive waste (such as
radionuclides from
research and medical waste) and mining waste. Depending on the physical and
chemical
form of the metal contaminant to be removed, as well as the cost-benefit
analysis for a
particular remediation approach, which of the existing technologies is better
suited for a
particular site will vary. However, due to the high cost of traditional
cleanup technologies,
there still remains a great need for a less-expensive, safe and effective
heavy metal
recovery and cleanup technology.
[0006] There are some technologies currently available for the recovery or
remediation
of heavy metal contaminated waste. In general, these technologies combine one
or more of
the following general approaches: isolation, immobilization, toxicity
reduction, physical
separation or extraction of metal contamination from a waste product.
Isolation technologies
utilize a containment strategy in an attempt to confine a contaminated site or
area so as to
prevent further spread of the toxic metal waste. Immobilization technologies
reduce the
mobility of metal contaminants and include systems which provide an
impermeable barrier to
separate underlying layers of soil (containing the metal contaminants) from
the topsoil layer.
Also used are physical barriers which restrict the flow of uncontaminated
groundwater
through a contaminated site. Additionally, there are toxicity reduction
processes which
generally use chemical or biological techniques to decrease the toxicity or
mobility of metal
2
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
contaminants. Included in toxicity reduction processes are biological
treatment technologies,
which apply newer biotechnical approaches.
[0007] Metal remediation is a relatively new application of biological
treatment
technologies and includes processes such as bioaccumulation, phytoremediation,
phyotextraction, and rhizofiltration. All of these biological treatments use
certain plants and
microorganisms to remediate metals through either adsorption, absorption, or
concentration
of contaminating metal ions. For example, in bioaccumulation, plants or
microorganisms
actively take up and accumulate metals from contaminated surroundings.
[0008] In phytoremediation, specific plants that have developed the ability to
selectively
remove metal ions from soil are used. Such plants include certain
"hyperaccumulator"
species such as the alpine pennycrass plant, which is capable of accumulating
metals at
levels of 260 times greater than most plants before showing toxicity symptoms.
Most
hyperaccumulator plants, however, are very slow growing and have specific
growth
requirements. Some of these growth requirements are not conducive to the use
of these
plants at sites or in situations where metal recovery or remediation is
needed. Furthermore,
there are very few plant species known or available for recovery or
remediation use.
Therefore, given the persistent and high incidence of metal contamination at
environmental
and waste sites (about 75% of Superfund Sites contain metal ions as a form of
contamination, U.S. EPA, 1996), more efficient methods and approaches for
removing heavy
metals from contaminated sources are still needed.
[0009] More recently, in an attempt to meet these needs, biotechnological
approaches
have been employed as an alternative strategy to metal recovery and
remediation. Included
in these biotechnology approaches are the use of tobacco plants that have been
manipulated to express metallothionein genes (Maiti et al. Seed-transmissible
expression of
mammalian metallothionein in transgenic tobacco, Biochem Biophys Res Commun.
150(2):640-7,1988). Metallothioneins (MTs) are small metal binding proteins
ubiquitously
distributed throughout the animal kingdom. They have high metal binding
affinities and are
believed to be important in controlling the intracellular levels of free metal
ions. However,
little else is known about their function or biological purpose.
Metallothioneins were first
discovered in 1957 in horse tissue. Since then, they have been identified in
species ranging
from fungi and shellfish to mice and humans.
[0010] The structural features of MTs include a high cysteine composition and
lack of
aromatic amino acids. The cysteine residues are responsible for the protein's
high affinity
metal ion binding capabilities. In general, MTs have a high degree of amino
acid sequence
similarity. However, the proteins or known gene sequences encoding the
proteins have been
used primarily in either the research setting. or in disease treatment
methodologies.
3
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0011] Accordingly, one of the objects of the present invention is to provide
novel metal
binding proteins for the removal of metals from a variety of substrates. This
technology
would allow for the efficient, cost effective, safe and simple removal of
heavy metals from
environmental waste or other materials contaminated with heavy metal.
SUMMARY OF THE INVENTION
[0012] Metallothionein (MT) proteins are generally about 60-68 amino acid
residues in
size and have a high degree of sequence conservation among the different
species. In
contrast, MTs from brine shrimp (Artemia) are much smaller in size (about 48
amino acid
residues) and have distinctly unique amino acid and DNA sequences. The metal
binding
proteins of the present invention are capable of high capacity and high
affinity metal binding.
This makes them particularly suitable for use in pollution control, metal
recycling, metal
mining and other metal recovery and metal remediation technologies.
[0013] These and other objects are achieved by the compositions and methods of
the
present invention which provide for the efficient and reliable sequestration
of heavy metals
from a variety of sources using a regenerative metal binding support comprised
of at least
one metal binding protein immobilized on a solid support. The metal binding
proteins can be
expressed and produced easily for purposes such as metal remediation, metal
recycling,
metal mining or other types of processes where binding of one or more heavy
metals is
desired.
[0014] In accordance with the teachings of the present invention, at least one
substantially purified metal binding protein is provided. In one embodiment of
the present
invention the metal binding protein is from the brine shrimp (Artemia) and has
the amino acid
sequence of SEQ ID NO. 2 or SEQ ID NO. 4.
SEQ ID NO. 2
MET ASP CYS CYS LYS ASN GLY CYS THR CYS ALA PRO ASN CYS LYS 15
.CYS ALA LYS ASP CYS LYS CYS CYS LYS GLY CYS GLU CYS LYS SER 30
ASN PRO GLU CYS LYS CYS GLU LYS ASN CYS SER CYS ASN SER CYS 45
GLY CYS HIS 48
SEQ ID NO. 4
MET ASP CYS CYS LYS ASN GLY CYS THR CYS ALA PRO ASN CYS LYS 15
CYS ALA LYS ASP CYS LYS CYS 22
In another embodiment of the present invention, the metal binding protein
sequences
incorporate one or more conservative amino acid substitutions of SEQ ID NO. 2
or SEQ ID
NO. 4. It should be noted that while the metal binding proteins will be
discussed in the
context of metal recovery and metal remediation, the proteins are readily
applicable to many
4
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
other uses where removal, recovery or simply binding of heavy metals or heavy
metal
complexes is desired.
[0015] In further accordance with the teachings of the present invention, the
novel metal
binding proteins can be utilized as a naked composition or can be provided in
association
with a support, or other delivery system to aid in either the dispersal,
handling, packaging or
function of the metal binding proteins in metal recovery, metal remediation or
metal binding
processes as disclosed herein. Therefore, any of the metal binding proteins of
the present
invention can be coupled to a support such as a membrane filter, to form a
regenerative
metal binding support, through which metal containing fluids are brought into
contact.
[0016] The present invention is particularly well suited for use in metal
recovery, metal
remediation or metal recycling processes and methods. These methods include
contacting a
metal binding protein of the present invention having an amino acid sequence
analogous to
at least one metal binding protein sequence from brine shrimp (Artemia) with a
substrate or
material having a concentration of at least one heavy metal in order to bind
the metal to the
metal binding protein; and then separating the bound metal from the substrate
or material.
[0017] For example, the metal binding proteins, and devices comprising them,
disclosed herein are useful in connection with the treatment of any substrate
having a
concentration of at least one metal, such as a heavy metal. As will be
appreciated by those
skilled in the art, such heavy metal containing substrates can be any
environmental or
industrial material such as ground water, drinking water, contaminated soil,
waste, or the
like, containing a concentration of metal. Similarly, the methods of the
present invention are
equally useful in treating industrial or municipal wastes containing metals
that are desirable
to remove. This broad utility makes the compositions and associated methods of
the present
invention particularly useful in a wide variety of circumstances.
[0018] The metal binding proteins of the present invention retain high binding
affinity for
heavy metals in a variety of conditions, making them particularly useful in
situations where
removal or recovery of heavy metals from a substrate or any metal containing
or metal
contaminated source is desired. The metal binding proteins and the associated
methods of
the present invention provide for the efficient, cost effective, and safe
removal and recovery
of heavy metals from a wide variety of substrates.
[0019] In one embodiment of the device of the present invention, a device for
removing
heavy metals from a substrate comprising: a regenerative metal binding support
comprising
a polymer membrane having associated therewith and at least one substantially
purified
metallothionein (MT) protein, or a portion thereof, from an organism selected
from the group
consisting of mammals, fish, mollusks, echinoderms, crustaceans, reptiles,
nematodes,
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
grains and yeast; wherein the regenerative metal binding support binds heavy
metals
thereby removing the heavy metals from the substrate; and the binding of heavy
metal to the
regenerative metal binding support is reversible and wherein the regenerative
metal binding
support is reusable.
[0020] In additional embodiments of the device of the present invention, the
mammal is
a human, a monkey or a rabbit; the fish is a catfish; the mollusk is mussel;
the echinoderm is
a sea urchin; the reptile is a frog, the grain is rice or wheat and the
crustacean is a brine
shrimp (Artemia).
[0021] In another embodiment of the device of the present invention, the MT
protein
has an amino acid sequence selected from the group consisting of SEQ ID NO. 2,
SEQ ID
NO. 4, SEQ ID NO. 11, SEQ ID NO. 12, SEQ ID NO. 13, SEQ ID NO. 14, SEQ ID NO.
15,
SEQ ID NO. 16, SEQ ID NO. 17, SEQ ID NO. 18, SEQ ID NO. 19, SEQ ID NO. 20, SEQ
ID
NO. 21, SEQ ID NO. 21 and SEQ ID NO. 23.
[0022] In yet another embodiment of the device of the present invention, the
polymer
membrane is nylon.
[0023] In still another embodiment of the device of the present invention, the
substrate
is a liquid.
[0024] In another embodiment of the device of the present invention, the heavy
metal is
a heavy metal complex.
[0025] In one embodiment of the method of the present invention, a method is
provided
for removing metals from a substrate comprising contacting a substrate having
heavy metals
therein with a regenerative metal binding support comprising a polymer
membrane having
associated therewith at least one substantially purified metallothionein (MT)
protein, or a
portion thereof, from an organism selected from the group consisting of
mammals, fish,
mollusks, echinoderms, crustaceans, reptiles, nematodes, grains and yeast;
binding the
heavy metal to the regenerative metal binding support thereby producing a
substrate having
less heavy metal contained therein.
[0026] In another embodiment of the method of the present invention, the
polymer
membrane is nylon.
[0027] In yet another embodiment of the method of the present invention, the
heavy
metal is a heavy metal complex.
[0028] In still another embodiment of the method of the present invention, the
substrate
is a liquid.
6
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0029] In another embodiment of the method of the present invention, the
method
further comprises releasing the bound heavy metal from the regenerative metal
binding
support; and regenerating the metal-binding capacity of the regenerative metal
binding
support.
BRIEF DESCRIPTION OF THE DRAWINGS
[0030] FIG. 1 is an elution profile of exemplary metal binding proteins of the
present
invention illustrating co-elution of metal binding proteins with the heavy
metal zinc.
[0031] FIG. 2 is a map of an exemplary cloning cassette containing the gene
sequence
of the metal binding protein gene, in accordance with the teachings of the
present invention.
[0032] FIG. 3 illustrates metallothionein (MT) protein selectively binding
heavy metals in
solution in accordance with the teachings of the present invention.
[0033] FIG. 4 illustrates MT proteins coupled to a solid support in accordance
with the
teachings of the present invention.
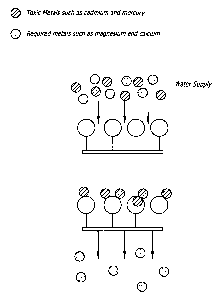
[0034] FIG. 5 illustrates the removal of heavy metals from water in accordance
with the
teachings of the present invention.
[0035] FIG. 6 illustrates the recovery of removed metals from a membrane
coated with
MT proteins in accordance with the teachings of the present invention.
[0036] FIG. 7 illustrates the selectivity and affinity of the present
invention for binding
heavy metals.
[0037] FIG. 8 depicts the sequence homology in the cysteine metal binding
motifs
between metallothionein proteins isolated from divergent species.
DETAILED DESCRIPTION OF THE INVENTION
[0038] Metal binding proteins such as metallothioneins (MTs) that have been
isolated
from various species such as humans, mice, bacteria species, crabs, fish,
yeast and
chickens, are known to have very similar structural characteristics such as
similar size
(about 6.0-6.8 kDa), high amino acid sequence conservation, and a high
percentage of
cysteine residues in the proteins' total amino acid compositions. It is the
cysteine
composition of these MTs that accounts for the protein's binding affinity for
heavy metals
including, but not limited to, arsenic, zinc, copper, cadmium, mercury,
cobalt, lead, nickel,
chromium, uranium, platinum, silver and gold. Unless otherwise stated, the
term protein
refers to proteins, polypeptides and peptides. The metal binding proteins of
the present
invention also bind heavy metals complexes in which the heavy metals are
associated with a
protein or other molecule.
7
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0039] For example, the metal binding proteins, and devices comprising them,
called
regenerative metal binding supports, disclosed herein are useful in connection
with the
treatment of any substrate having a concentration of at least one metal, such
as a heavy
metal. As will be appreciated by those skilled in the art, such heavy metal
containing
substrates can be any environmental or industrial material such as ground
water, drinking
water, contaminated soil, waste, or the like, containing a concentration of
metal. Similarly,
the methods of the present invention are equally useful in treating industrial
or municipal
wastes containing metals that are desirable to remove. This broad utility
makes the
compositions and associated methods of the present invention particularly
useful in a wide
variety of circumstances.
[0040] The metal binding proteins and regenerative metal binding supports of
the
present invention are useful in the recovery of metals, particularly precious
metals from
metal-containing substrates. For example, the metal binding proteins of the
present invention
can be used in metal mining processes for the isolation and removal of
precious metals such
as gold, platinum and silver. Doing so eliminates the need to use other toxic
materials such
as cyanide in the final stages of metal purification from ore. These same
novel techniques
can be utilized to recover such metals from industrial or municipal waste.
With the ever-
increasing use of disposable and other electronic devices, such waste sources
are
increasingly full of such metals, making recovery a worthwhile endeavor.
[0041] The metal binding proteins of the present invention can be isolated
easily and
efficiently from natural sources or synthetically produced as disclosed herein
for use in metal
recovery, metal mining, metal recycling, metal remediation, pollution control
or any process
including metal sequestering. Therefore, the metal binding proteins and
associated methods
of the present invention provide a versatile, easily produced, efficient and
reliable resource
for use in any process having a metal binding aspect.
[0042] In one embodiment of the present invention, metal binding proteins were
isolated
from brine shrimp (Artemia). Artemia MT are a family of metal binding proteins
that are
referred to as "isomers". Analysis of these proteins' unique amino acid
compositions showed
each isoform to be essentially equivalent. At least five individual Artemia MT
isoforms have
been identified in accordance with the teachings of the present invention.
Unlike MTs from
other organisms which share a high degree of sequence homology or similarity,
the Artemia
metal binding proteins have unexpectedly different structural characteristics
but possess a
high degree of sequence homology to one another.
[0043] The following techniques were utilized to provide nucleic acid sequence
encoding a Artemia metal binding protein. First, metal binding proteins from
brine shrimp
(Artemia) were isolated and purified. N-terminal amino acid sequence analysis
was
8
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
performed on the isolated metal binding protein. Amino acid sequence analysis
indicated
that the metal binding motif of the first six cysteine residues of the Artemia
metal binding
protein was conserved when compared to rabbit and human MTs, indicating the
importance
of these amino acid residues in the protein's metal binding function (Hamer
DH,
Metallothionein. Ann. Rev. Biochem. 55:813-51, 1986). This conservation of the
cysteine-
rich metal binding motif is seen across a wide variety of divergent species
(FIG. 8).
[0044] Using this N-terminal amino acid sequence information, oligonucleotide
primers
corresponding to the N-terminal amino acid sequence were constructed as known
in the art.
These oligonucleotide primers were used to amplify, by polymerase chain
reaction (PCR)
potential candidates for a MT gene sequence encoding at least one of the
target metal
binding proteins from brine shrimp (Artemia). The PCR product was purified
using QiaPrep
spin columns (Qiagen, Inc.) and cloned into the TA cloning vector CR2.1
(Invitrogen) using
the manufacturer's protocol. Electrocompetent Escherichia coli (Sure Shot
cells from
Invitrogen) were transformed with the recombinant vector and plated onto LB
agar plates
containing ampicillin (100 Ng/ml) and 1% glucose. The plates were placed at 37
C overnight.
Individual colonies were picked and used to inoculate 5 mL of LB broth
supplemented with
ampicillin and 1% glucose. The cultures were incubated overnight in a rotary
incubator at
37 C. Plasmid was isolate from 2 mL of the cell suspension using QiaPrep spin
columns as
per the manufacturer's protocol (Qiagen). The plasmid was then sequenced on a
Li Cor
4200L using the M13 universal forward and reverse primers. Once verified and
determined
to be a sequence encoding a metal binding protein, the brine shrimp MT gene
was
subcloned into the bacterial expression vector pTMZ. Based upon the identified
MT
encoding sequence, the amino acid sequence of the first novel metal binding
proteins of the
present invention was determined.
[0045] FIG. 1 details an exemplary elution profile utilizing an exemplary
metal binding
protein of the present invention. This profile was obtained utilizing the
following exemplary
protocol. E. coli (Strain ER 2566) were transformed with a plasmid expression
vector
containing the MT gene sequence of SEQ ID NO. 1 in pTMZ. Bacteria were grown
in LB
broth containing 1% glucose at 37 C to an Asoo of 0.60. The bacterial cells
were collected
and resuspended in LB broth containing 0.1 % glucose and incubated for 45
minutes at the
same temperature. Isopropyl b-D-thiogalactopyranoside (IPTG) was added to a
final
concentration of 0.1 mM. The bacterial cells were incubated for about 16
hours. Non-
transformed bacteria were used as controls. The cells were collected by
centrifugation and
sonicated in 10 mM Tris, pH 8.0, 5 mM dithiothreitol. (DTT) and 0.5 mM
phenylmethylsulfonylfluoride (PMSF). The homogenate was centrifuged at 150,000
x g for 1
hour at 4 C. The supernatant was collected and incubated with 2 pCi of 109Cd
at room
9
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
temperature. The radiolabeled supernatant was then applied to a G-50 molecular
exclusion
column and eluted with 50 mM Tris, pH 8Ø Five milliliter fractions were
collected and
assayed for radioactivity (CPM) and zinc (Zn), the zinc being an endogenous
metal that
associates with the exogenous metal binding protein expressed by the
transformed bacteria.
Each fraction eluting from the column was assayed for Zn by ICPMS (Inductively
Coupled
Plasma Mass Spectroscopy). Other nucleotide sequence that encode a functional
metal
binding protein, including, but not limited to SEQ ID NO. 3, may also be
utilized, as provided
and disclosed by the teachings of the present invention.
[0046] Therefore, the present invention provides substantially purified metal
binding
proteins for use in removal of metals from metal-containing substrates by
reversibly binding
the metal to the metal binding proteins immobilized on a solid support. The
term
"substantially purified", as used herein, refers to nucleic acids, amino acids
or proteins that
have been removed from their natural environment, isolated or separated and
are at least
60% free, preferably 75% free, to 90% or more free from other components with
which they
are naturally associated.
[0047] A substantially purified metal binding protein in accordance with the
teachings of
the present invention has an amino acid sequence analogous to:
SEQ ID NO. 2
MET ASP CYS CYS LYS ASN GLY CYS THR CYS ALA PRO ASN CYS LYS 15
CYS ALA LYS ASP CYS LYS CYS CYS LYS GLY CYS GLU CYS LYS SER 30
ASN PRO GLU CYS LYS CYS GLU LYS ASN CYS SER CYS ASN SER CYS 45
GLY CYS HIS 48
[0048] Also within the scope of the present invention are substantially
purified metal
binding proteins that are variants of the sequence of the above SEQ ID NO. 2
that preserve
the protein's metal binding affinity. In particular, conservative amino acid
substitutions within
the scope of the present can include any of the following: (1) any
substitution of isoleucine
for leucine or valine, leucine for isoleucine, and valine for leucine or
isoleucine; (2) any
substitution of aspartic acid for glutamic acid and of glutamic acid for
aspartic acid; (3) any
substitution of glutamine for asparagine and of asparagine for glutamine; and
(4) any
substitution of serine for threonine and of threonine for serine.
[0049] A "conservative amino acid substitution" as used herein, refers to
alteration of an
amino acid sequence by substituting an amino acid having similar structural or
chemical
properties. Those skilled in the art can determine which amino acid residues
may be
substituted, inserted or altered without the metal binding properties of the
proteins of the
present invention.
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0050] Other substitutions can also be considered conservative, depending upon
the
environment of the particular amino acid. For example, glycine and alanine can
be
interchangeable, as can be alanine and valine. Methionine, which is relatively
hydrophobic,
can be interchanged frequently with leucine and isoleucine, and sometimes with
valine.
Lysine and arginine are interchangeable in locations in which the significant
feature of the
amino acid residue is its charge and the different pKs of these two amino acid
residues and
where their different sizes are not significant. Still. other changes can be
considered
"conservative" in particular environments, as known in the art.
[0051] For example, if an amino acid on the surface of a protein is not
involved in a
hydrogen bond or salt bridge interaction with another molecule, such as
another protein
subunit or a ligand bound by the protein, negatively charged amino acids such
as glutamic
acid and aspartic acid can be substituted with positively charged amino acids
such as lysine
or arginine and vice versa. Histidine, which is more weakly basic than
arginine or lysine, and
is partially charged at neutral pH, can sometimes be substituted for these
more basic amino
acids as well. Additionally, the amides glutamine and asparagine can sometimes
be
substituted for their carboxylic acid homologues, glutamic acid and aspartic
acid.
[0052] The Artemia metal binding proteins of the present invention, and their
associated
methods of production and use, are a family of metal binding proteins having
multiple
isomeric forms. As a result, the present invention includes at least five
isomeric forms of
Artemia metal binding proteins suitable for use in removal or recovery of
heavy metals. An
isomer is one of two or more compounds that have the same chemical composition
but differ
in structural form. The "isomers" of the present invention have the requisite
structural
features that classify them as metal binding proteins. These features include
their high
cysteine content, which confers their metal binding capacity. The isomers
differ by two or
more amino acid residues, resulting in different pl's for the individual
isomer. This pl
difference allows easy separation and characterization of the isoforms.
Therefore, the metal
binding proteins of the present invention can be expressed and produced
efficiently and with
ease.
[0053] In addition to their metal binding properties, these metal binding
proteins also
exhibit features which render them particularly useful in a wide variety of
metal recovery and
metal remediation settings. For example, these metal binding proteins are
capable of heavy
metal binding under a range of conditions such as under moderate to high
temperature
conditions. The metal binding proteins are capable of heavy metal binding at
room
temperature and therefore particularly ideal for many applications. The metal
binding
proteins are also capable of heavy metal binding within a wide temperature
range such as,
for example, a temperature range of about 4 C to about 100 C. Those skilled in
the art will
11
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
appreciate that depending on a particular application or operation in which
the metal binding
proteins are to be utilized, a particular temperature range may be preferred
for practical or
economic reasons. For example, it may be more practical to use the metal
binding proteins
"on-site" or at the location of an environmental contamination (which would
dictate that
particular temperature range that can be obtained within available costs). On
the other hand,
more effective metal extraction on certain substrates may be achieved by use
of the metal
binding proteins of the present invention under relatively high temperature
conditions.
Therefore, in accordance with the teachings of the present invention, a
suitable range of
temperatures for practicing the present invention includes a range of about 4
C to about
100 C. This range of temperature conditions makes the metal binding proteins
of the present
invention more versatile and useful.
[0054] In further accordance with the teachings of the present invention, the
metal
binding proteins can be utilized as a naked composition or in association with
a support or
dispersal means to aid in either the dispersal, handling, packaging or
function of the metal
binding protein in metal recovery, metal remediation or metal binding
processes. Such metal
binding proteins are particularly useful in metal recovery, metal remediation
and metal
binding processes because they can be more easily and safely used as compared
to other
methodologies, such as chemical extraction, which exposes the user to toxic or
other
potentially dangerous types of chemicals.
[0055] A variety of solid supports to aid in the handling or dispersal of the
novel metal
binding proteins can be used and include a hydrophilic membrane, partially
hydrophilic
membrane, composite membrane, porous organic solid support, nonporous organic
solid
support, porous inorganic solid support, nonporous inorganic solid supports
and
combinations thereof. If the solid support is a membrane, membranes such as
those
described in U.S. Pat. Nos. 5,618,433 and 5,547,760, both of which are herein
incorporated
by reference in their entirety, are exemplary. If the solid support is an
inorganic or organic
particulate solid support, preferred solid supports include sand, silicas,
silicates, silica gel,
glass, glass beads, glass fibers, alumina, zirconia, titania, nickel oxide
polyacrylate,
polystyrene, polyphenol and others as described in U.S. Pat. Nos. 4,943,375,
4,952,321,
4,959,153, 4,960,882, 5,039,419, 5,071,819, 5,078,978, 5,084,430, 5,173,470,
5,179,213,
5,182,251, 5,190,661, 5,244,856, 5,273,660 and 5,393,892 which are herein
incorporated by
reference. Specific examples include flexible membranes, beads or
particulates, filters, or
any other solid supports known in the art that are useful for separations.
[0056] In one illustrative embodiment, the solid support is in the form of a
membrane.
Preferably, the membrane is a polymer, and more preferably is a member
selected from the
group consisting of fluorinated polymers, polyolefins, polystyrene,
substituted polystyrenes,
12
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
polysulfones, polyesters, polyacrylates, polycarbonates; vinyl polymers,
copolymers of
butadiene and styrene, fluorinated ethylene-propylene copolymers,
ethylenechlorotrifluoroethylene copolymers, nylon and mixtures thereof.
[0057] The metal binding proteins of the present invention are associated with
a
support, such as a polymer membrane, by covalent bonding of the metal binding
protein to
the polymer to by non-covalent binding such as, but not limited to,
electrostatic attractions,
dispersion forces and solvent-mediated forces.
[0058] In one embodiment of the present invention, at least one metal binding
protein is
associated with a solid support such that a regenerative metal binding support
is provided,
wherein the regenerative metal binding support can bind heavy metals from a
substrate, the
heavy metals can be released from the regenerative metal binding support and
the
regenerative metal binding support can be reused to bind heavy metalss.
[0059] In general, a substrate from which one or more heavy metal species are
to be
removed is contacted with a metal binding protein bound to a solid support,
where the metal
binding protein has an affinity for the heavy metal. The solid support forms a
support for the
metal binding protein and can be in the form of a membrane, beads or solid
support
particulates, or any other form commonly used in biochemical or chemical
separations. If a
membrane is used as the solid support, the metal binding protein- solid
support composition
can be incorporated into a contacting device comprising a housing, - e.g.,
cartridge,
containing the composition of matter of the invention by causing solution
containing desired
ions to flow through the cartridge and thus come in contact with the
composition of the
invention. In one embodiment, the membrane configuration is a pleated
membrane, although
other membrane configurations, such as flat sheet, stacked disk or hollow
fibers may be
used. However, various contact apparatus may be used instead of a cartridge
such as but
not limited to a cassette, syringe, unit, canister, multi-well plate or filter
holder. If a solid
support is used, separation columns can be used as are known in the art.
[0060] It should be noted, that an additional characteristic feature of the
metal binding
proteins are that they are also capable of reversible heavy metal binding. For
example,
bound metals can be eluted off or away from the metal binding proteins using
acidic
conditions or by instantaneous exchange reactions or inorganic chelators. For
example,
during incubation of a metal binding protein with radioactive Cd, the 109Co
metal exchanges
for endogenous metal bound to the metal binding protein. At about pH 1.0, the
metal is
released from the protein. Bringing the pH of the solution up to about pH 8.0
regenerates the
metal binding activity of the protein. Therefore, due to the reversible
binding characteristics
of the novel metal binding proteins, the present invention also provides
compositions,
13
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
formulations, powders, liquids, devices or apparatuses comprising the
substantially purified
metal binding proteins which can be utilized more than once.
[0061] Turning now to an exemplary discussion of the genetic engineering of
the novel
metal binding proteins of the present invention, a nucleotide sequence for one
of the
isoforms of a metal binding protein from a brine shrimp (Artemia) was
identified, as
discussed above. Generally, the isolation process comprises: (1) preparation
of one or more
sample(s) containing nucleic acids from brine shrimp (Artemia); (2) isolation
of total RNA
from Artemia; (3) preparation of cDNA from the total RNA; (4) amplification of
metal binding
protein gene sequences; and (5).cloning, sequencing and verification of an
isolated nucleic
acid sequence as a metal binding protein gene (MT) from brine shrimp
(Artemia).
[0062] The above procedure yielded the entire coding sequence for one of the
metal
binding protein genes, metallothionein (MT). This sequence is:
SEQ ID NO. 1
5'-ATG GAC TGC TGC AAG AAC GGT TGC ACC TGT GCC CCA AAT TGC AAA
45 TGT GCC AAA GAC TGC AAA TGC TGC AAA GGT TGT GAG TGC AAA
AGC 90 AAC CCA GAA TGC AAA TGT GAG AAG AAC TGT TCA TGC AAC
TCA TGT 135 GGT TGT CAC TGA-3' 147
[0063] Species as divergent as humans and wheat express metallothionein
proteins
with similar binding affinities for heavy metals. These MT proteins contain
from 12 to 22
cysteine residues, which are conserved across divergent species. These
cysteine residues
form metal binding motifs responsible for the metal binding function of the
proteins (Hamer
DH, Metallothionein. Ann. Rev. Biochem. 55:813-51, 1986). Therefore, one
embodiment of
the present invention provides MT proteins immobilized on solid supports, such
as
membranes, wherein the MT are isolated from organisms including, but not
limited to,
mammals, fish, mollusks, echinoderms, crustaceans, reptiles, nematodes, grains
and yeast.
Non-limiting examples of these organisms include, but are not limited to,
brine shrimp
(Artemia), rabbit (Oryctolagus cuniculus), green monkey (Cercopithecus
aethiops), human
(Homo sapiens), channel catfish (Ictalurus punctatus), African clawed frog
(Xenopus laevis),
blue mussel (Mytilus edulis), painted sea urchin (Lytechinus pictus), fruit
fly (Drosophila
melanogaster), roundworm (Caenorhabditis elegans), rice (Oryza sativa), wheat
(Triticum
aestivum) and yeast (Candida glabrata).
[0064] One embodiment of the present invention provides one or more nucleic
acid
sequences encoding a substantially purified metal binding protein having amino
acid
sequence analogous to at least one metallothionein protein from an organism
including, but
not limited to, Artemia, mammals and marine species, or other species having a
14
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
metallothionein protein with conserved amino acid sequence homology in the
cysteine
residues, e.g. the metal binding motifs, as compared to Artemia MT (FIG. 8).
[0065] Another embodiment of the present invention provides one or more amino
acid
sequences encoding a substantially purified metal binding protein analogous to
at least one
metallothionein protein from an organism including, but not limited to,
Artemia, mammals
and marine species, or other species having a metallothionein protein with
conserved amino
acid sequence homology in the cysteine residues, e.g. the metal binding
motifs, as
compared to Artemia MT (FIG. 8). Exemplary amino acid sequences include the
sequences
of SEQ ID NO. 2 and SEQ ID NOs. 11-23 (FIG. 8).
[0066] Alternatively, an isolated nucleic acid can comprise the minimal DNA
sequences
sufficient to allow translation of a functional metal binding protein. A
functional metal binding
protein need not be the entire native metal binding protein but can be just
those portions or
regions of SEQ ID NO. 1 that encodes a protein capable of binding to heavy
metals.
Therefore, the invention also includes isolated nucleic acids including DNA
having at least
80% sequence identity to a DNA molecule having the sequence of nucleotide
residues 1 to
66 of SEQ ID NO 1.
[0067] Also within the present invention is a nucleic acid sequence encoding
any one of
the novel metal binding proteins of the present invention. Such novel metal
binding proteins
can have molecular weight of about 5,800 daltons and are able to bind with
high affinity to
heavy metal ions such as arsenic, zinc, copper, cadmium, mercury, cobalt,
lead, nickel,
platinum, silver and gold. The novel metal binding proteins include therein an
amino acid
sequence selected from the group consisting of: SEQ ID NO: 2 and sequences
incorporating
one or more conservative amino acid substitutions thereof wherein the
conservative amino
acid substitutions are any of the following: (1) any of isoleucine, leucine
and valine for any
other of these amino acids; (2) aspartic acid for glutamic acid and vice
versa; (3) glutamine
for asparagine and vice versa; and (4) serine for threonine and vice versa.
Alternative
nucleic acid sequences can be determined using the standard genetic code; the
alternative
codons are readily determinable for each amino acid in this sequence.
[0068] It should be noted, that while the isolated nucleic acids provided
herein can be
used to produce or express novel metal binding proteins, they are also
particularly useful for
isolation and identification of additional metal binding protein genes
encoding the novel
metal binding proteins of the present invention. For example, using the
strategy, exemplary
methods and nucleic acid sequences provided herein, DNA sequences encoding any
of the
metal binding protein isomers can be obtained. Therefore, the present
invention includes
nucleic acids encoding any and all of the isomeric or alternative forms of the
metal binding
proteins of the present invention. Additionally, isolated nucleic acids need
not comprise
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
entire coding sequences of an MT isomer, but include nucleic acid sequences
encoding
domains or portions of a coding sequence encoding an MT isomer, such as the
functional or
metal binding regions of the metal binding protein isomers of the present
invention.
[0069] Another aspect of the invention is a vector comprising a nucleic acid
sequence
according to the present invention operatively linked to at least one control
sequence that
controls the expression or regulation of the nucleic acid sequence. Such
control sequences
are well known in the art and include operators, promoters, enhancers,
promoter-proximal
elements and replication origins. The techniques of vector construction,
including cloning,
ligation, gap-filling, the use of the polymerase chain reaction (PCR), solid-
state
oligonucleotide synthesis, and other techniques, are all well known in the art
and need not
be described further here. The vectors of the present invention are
particularly useful in
producing the novel metal binding proteins either by modified organisms, host
cells or other
types of expression systems. The metal binding proteins of the present
invention can be
produced in bacterial cells, insect cells, plant cells or mammalian cells.
Appropriate vectors
and cells for production of metal binding proteins in each of these species
are well known to
persons skilled in the art.
[0070] Turning now to uses for the metal binding proteins of the present
invention.
Exemplary uses of these proteins include pollution control applications such
as metal
remediation, pollution control, metal recycling or metal mining. For example,
the metal
binding proteins can be used to reduce the concentration of heavy metals in an
environmental substance. The substance can be a fluid, such as ground water,
sludge,
waste-water and the like. Additionally, the metal binding proteins can be
incorporated into
one or more compositions or devices used for pollution control. For example,
the metal
binding proteins can be applied on site in the form of a flocculent or powder,
or can be used
in treatment plants as part of a membrane filtration or other type of solid
support device used
for removal of heavy metal from a contaminated substrate.
[0071] The metal binding proteins used in these metal binding processes can be
provided as a product purified from its natural source or can be produced by
bioengineering
techniques. For example, the metal binding proteins can be produced by
transgenic or
modified organisms. Modified organisms include transgenic animals, bacteria or
plants. For
example, a modified plant can be a transgenic tobacco plant whose genome has
been
genetically altered to express one or more metal binding protein of the
present invention. A
modified organism can also include a plant or biomass that is capable of
growing at or within
contaminated sites where metal remediation is desired. Extraction of metal
contaminants by
the modified organisms also concentrates the toxic metals from the
contaminated site. This
provides the additional advantage of converting the heavy metals to a smaller
quantity as
16
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
well as providing final product that is more easily and safely handled for
disposal or further
processing.
[0072] Methods for reducing the concentration of heavy metals in a substrate
include
contacting a metal binding protein of the present invention with a substrate
having heavy
metals. In a non-limiting example, a metal binding protein having an amino
acid sequence
analogous to at least one metal binding protein sequence from brine shrimp
(Artemia) can
be contacted with a substance having a concentration of at least one heavy
metal to bind the
heavy metal to the metal binding protein. Subsequently, the bound heavy metal
can be
separated from the substrate, reducing the concentration of heavy metals in
the original
substrate.
[0073] As mentioned previously, an additional advantageous feature of the
metal
binding proteins of the present invention include their ability to release
bound heavy metals
using acid extraction, inorganic chelators, and/or exchange reaction
technologies. This
allows the user, if desired, to elute bound heavy metals off the metal binding
proteins. Once
the heavy metals are eluted off the metal binding proteins of the present
invention, the metal
binding proteins can be regenerated (or recycled) for additional uses in metal
extraction.
Therefore, the invention also provides methods for reducing the concentration
of heavy
metals in a substrate using reusable compositions, devices and apparatuses
comprising the
metal binding.proteins.
[0074] Metal binding proteins of the present invention, when used in methods
for
reducing the concentration of a metal in a substrate can be provided in such a
way as is
appropriate for the particular use, situation, mode of administration or
environment in which
the metal binding proteins are to be used. For example, when used in metal
remediation, or
in pollution control, the metal binding proteins can be coupled to a support,
such as a
powder and used, for example, as a flocculent to provide a convenient and
efficient means
of dispersing the metal binding proteins.
[0075] Alternatively, the metal binding proteins can be provided coupled to a
membrane, a semi-permeable membrane, a filter, or any other means appropriate
for
allowing sufficient exposure of the metal binding proteins to the heavy metal
containing
substrate so as to bind or sequester the heavy metals from the substrate. A
membrane or
filter comprising the metal binding proteins provides a particularly efficient
means of treating
ground water or waste water, as contaminated water can be purified by passage
through the
membrane or filter without further clean up as is required in chemical
extraction processes.
Coupling the metal binding proteins to a support or supporting matrix also
affords easier
handling of the metal binding proteins especially when used in large scale or
industrial
applications.
17
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0076] Use of the metal binding proteins of the present invention are not
limited only to
those methods where removal of heavy metals is desired, but can also include
methods
where recovery or concentration of heavy metals in a substance is to be
achieved. For
example, the metal binding proteins can be used for metal mining, such as in
the recovery of
precious metals including gold, platinum and silver, or can be used to
concentrate metals in
hazardous conditions, such as hazardous waste containing radioactive metals.
Such
hazardous metal waste can result either from numerous research, commercial or
industrial
uses.
[0077] Use of the metal binding proteins in concentrating radioactive metals
from waste
also reduces the amount or quantity of hazardous waste to be disposed of.
Reducing the
quantity of hazardous metal waste also reduces the level of radioactivity to
which certain
individuals are exposed.
[0078] Methods for reducing the concentration of heavy metals in a substance
include
producing the metal binding proteins in a modified organism. Modified
organisms include, for
example, transgenic organisms or transgenic hosts. For example, hosts or
organisms such
as shrimp, plants, bacteria, yeast or algae can be modified using molecular
and genetic
engineering techniques well known in the art. Using these techniques, which
are described
for example, in Sambrook et al., Molecular Cloning: A Laboratory Manual (New
York: Cold
Spring Harbor Press, 2001); Ausubel et al. Current Protocols in Molecular
Biology (Wiley
Interscience Publishers, 1995); US Dept Commerce/NOAA/NMFS/NWFSC Molecular
Biology Protocols (URL:http://research.nwfsc.noaa.gov/protocols.html); or
Protocols Online
(URL:www.protocol-online.net/molbio/index.htm), organisms whose genomes are
modified
so as to result in expression of a metal binding protein are provided. Metal
binding proteins
of the present invention include metal binding proteins having an amino acid
sequence
analogous to at least one metal binding protein sequence from a brine shrimp
(Artemia).
Modified organisms can be made and used to produce these metal binding
proteins, and the
metal binding proteins useful in the methods provided herein.
[0079] A modified organism producing a metal binding protein of the present
invention
includes a modified organism producing at least one metal binding protein
having an amino
acid sequence substantially similar to a metal binding protein from a brine
shrimp (Artemia).
A modified organism also includes an organism producing a metal binding
protein having an
amino acid sequence substantially similar to SEQ ID NO. 2 or conservative
amino acid
substitutions thereof.
[0080] Alternatively, production or expression of the metal binding proteins
of the
present invention from modified organisms is not limited to genomic expression
of the metal
binding proteins, but also includes epigenetic expression of the metal binding
proteins from
18
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
the modified organisms. Methods and techniques for obtaining epigenetic
expression from a
modified organism include, for example, adenoviral, adeno-associated viral,
plasmid and
transient expression techniques which are known in the art.
[0081] The present invention includes methods for producing the metal binding
proteins
of the present invention. For example, a method for producing a metal binding
protein having
an amino acid sequence analogous to at least one metal binding protein from a
brine shrimp
(Artemia) includes providing an expression system, producing a metal binding
protein using
the expression system and purifying or isolating the metal binding proteins to
obtain a metal
binding protein of the present invention.
[0082] Expression systems can be systems such as traditional manufacturing
plants.
For example, organisms such. as brine shrimp can be grown and the metal
binding proteins
of the present invention purified or extracted from the tissues of the brine
shrimp.
Alternatively, biomanufacturing systems using genetically engineered organisms
(produced
as described herein) capable of producing the metal binding proteins can be
used to
produce the metal binding proteins. For example, bacteria containing a metal
binding protein
expression vector can be cultured on large or small scale (depending on the
particular
need). The metal binding proteins can then be purified from the bacterial
broth and used in
metal binding processes.
[0083] Therefore, a metal binding protein of the present invention can be
produced by
expression of a nucleic acid sequence encoding a metal binding protein in a
modified
organism or host cell. Such a nucleic acid sequence includes, for example, a
MT gene such
as SEQ ID NO. 1 or a sequence encoding a fragment or functional metal binding
domain of
a MT gene.
[0084] The expressed metal binding proteins are purified using standard
techniques.
Techniques for purification of cloned proteins are well known in the art and
need not be
detailed further here. One particularly suitable method of purification is
affinity
chromatography employing an immobilized antibody to a metal binding protein.
Other protein
purification methods include chromatography on ion-exchange resins, gel
electrophoresis,
isoelectric focusing, and gel filtration, among others. Alternatively, the
metal binding proteins
of the present invention can be purified following their expression from
modified organisms
by methods such as precipitation with reagents (e.g. ammonium sulfate, acetone
or
protamine sulfate as well as other methods known in the art).
[0085] A further understanding of the present invention will be accorded to
those skilled
in the art from a consideration of the following non-limiting Examples.
19
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0086] It is emphasized that these examples are illustrative of the principles
and
teachings of the present invention and are not intended to limit the scope of
the invention to
exemplary brine shrimp (Artemia) metal binding proteins alone.
EXAMPLE 1
[0087] In accordance to the teachings of the present invention, the following
exemplary
protocols illustrate methods useful in the production, purification and
analysis of the metal
binding proteins of the present invention.
Sample Preparation
[0088] As a preliminary step in the isolation of the metal binding proteins,
Artemia brine
shrimp were grown in artificial seawater (AS) (422.7 mM NaCI, 7.24 mM KCL,
22.58 mM
MgCI2=6H20, 25.52 mM MgSO4=7H2O, 1.33 mM CaC12=2H20 and 0.476 mM NaHCO3).
Artemia cysts (2.5 g) were incubated for 48 hours in 250 mL of AS supplemented
with
antibiotics at 30 C and rotation at 125 rpm. After 24 hrs, phototropic Artemia
were collected,
cultured for an additional 24 hrs and then collected by cloth filtration. The
shrimp were
weighed and if not used immediately, stored at -80 C.
[0089] The Artemia were then homogenized in homogenization buffer (HB) (10 mM
Tris-HCI (pH 8.0), 0.1 mM DTT, 0.5 mM PMSF and 10 Ng/mi Soybean Trypsin
Inhibitor) and
resuspended in HB at 4 mL/gm wet weight of shrimp. The homogenate was passed
through
a Yamato LH-21 homogenizer three times at a setting of 800 rpm, filtered
through Miracloth
(Calbiochem) and the filtrate centrifuged in a Sorvall SA-600 rotor at 14,300
rpm, 4 C for 30
min. The lipid layer on top of the supernatant was removed by vacuum
aspiration and the
lower supernatant layer collected and centrifuged in a Beckman 50.2TI rotor at
40K rpm, 4 C
for 90 min. Again, the upper lipid layer was removed and the lower supernatant
recentrifuged
at 150K (150K sup). The 150K sup was then used immediately or stored at -80 C.
If used
immediately, this product was then subjected to gel filtration as follows. The
gel filtration
studies verified the metal binding proteins' ability to bind to heavy metals.
Gel Filtration Studies
[0090] The 150K sup was centrifuged in a Sorvall SA-600 rotor at 8,500 rpm and
4 C
for 30 min. The resulting supernatant was then filtered through a HPLC
certified 0.45 micron
LC13 acrodisc filter (Gelman Sciences). A 20 mL aliquot of filtered 150K
supernatant was
incubated at 4 C for 20 min with 2 pL of 109Cd (0.066 pCi) to radiolabel the
metal binding
proteins. The sample was then applied to a Sephadex G-50 molecular weight
exclusion
column (2.6 cm x 94 cm) previously equilibrated with 50 mM Tris-HCI (pH 8.0)
saturated with
Nz. One molar DTT (2 pL) was added to fractions 60-100 prior to sample loading
in order to
maintain reducing conditions in the fractions containing the low molecular
weight metal
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
binding proteins. The column.was eluted with 50 mM Tris (pH 8.0) at a flow
rate of 20 mL/hr
while monitoring the eluate at 280 nm. During the elution period, the buffer
reservoir was
continually purged with N2. Samples used for amino acid analysis were not
radiolabeled.
[0091] The109Cd content (CPM) of the column fractions was determined with an
Auto-
Logic gamma counter (ABBOTT Laboratories). Zinc content was measured by Flame
or
Furnace Atomic Absorption Spectroscopy and expressed as PPB zinc/fraction.
Prior studies
indicated that two classes of metal binding proteins were present, one class
being a high
molecular weight fraction. However, the majority of 109Cd eluted with a low
molecular weight
class of zinc-containing metal binding protein. As shown in FIG. 1,
radioactive metal binding
protein had a elution peak corresponding to that for Zinc (roughly, fraction
#50). The protein
concentration of the Sephadex G-50 fractions was determined with a BCA Total
protein
assay kit (Pierce) according to manufacturers protocol. The distinct
structural features of the
metal binding proteins of the present invention were then identified in the
following studies.
Metal Binding Protein Characterization Studies
[0092] Chromatographic and molecular weight studies were performed to
ascertain
structural features of the metal binding proteins. All protocols used were as
described
previously in B. Harpham, "Isolation of Metal Binding Proteins From Artemia",
Master's
Thesis, California State University, Long Beach Library, 1998. Using anion
exchange and
reverse phase chromatography techniques well known in the art and described,
for example,
in B. Harpham "Isolation of Metal Binding Proteins From Artemia", supra, metal
binding
proteins from Artemia were purified and determined to have molecular weights
and amino
acid sequence length unexpectedly lower than other known metal binding
proteins. Under
SDS-PAGE conditions, Artemia metal binding proteins have molecular weight of
about 5.8
kDa as compared to 6-7 kDa for metal binding proteins from other mammalian
species.
Protein analysis of Artemia metal binding proteins indicate a sequence length
of 48 amino
acids. The Artemia MT amino acid sequence was unexpectedly and significantly
shorter in
length than other known metal binding proteins, which range in length from 60
to 68 amino
acid residues.
EXAMPLE 2
Cloning and Sequencing of a Gene Encoding Artemia Metal Binding Protein
[0093] Total RNA was isolated from 48 hour nauplii (the larval stage of
Artemia) using
the RNAzoI method. Forty-eight hour nauplii samples were prepared as described
above in
Example 1. The PolyTract Procedure (Promega, WI) was then used to isolate mRNA
from
the total RNA samples. cDNA was generated from the mRNA using SuperScript and
3'
21
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
RACE Kit procedures (Cat #18373, Gibco/BRL, WI) and then subjected to the
following
synthesis reaction.
cDNA synthesis reaction:
Artemia mRNA 25 pl (500 ng)
DEPC H2O 30 pl
pM AP 5 N1
[0094] The above mixture was incubated for 10 min at 70 C, then placed on ice
for 1-2
min. Volatilized liquid was collected by centrifugation for 10 sec at 10,000
rpm. The following
were then added to the above RNA cocktail to produce a cDNA solution:
10x PCR Buffer 10 pI
25 mM MgCl2 10 NI
10 mM dNTP 5N1
0.1mMDTT 10pI
[0095] The above resulting cDNA solution was then mixed and incubated at 42 C
for 5
min. Five (5) pL of Superscript II RT was added and the mixture incubated at
42 C for 50
min for cDNA synthesis. The reverse transcription reaction was terminated by
incubating the
solution for 15 min. at 70 C, 5 pL of RNase was then added and the solution
incubated for 20
min at 37 C. The final solution containing Artemia cDNA was then stored at -20
C until used
for PCR amplification as described below.
[0096] The initial PCR Primer Sequences used were as follows: the 5' primer (N-
terminal side) was designated "MT-Not I" (SEQ ID NO. 5) and the 3' primer (C-
terminal side)
was designated "dT-Spe I" (SEQ ID NO. 6, SEQ ID NO. 7, SEQ ID NO. 8, or SEQ ID
NO. 9)
SEQ ID NO. 5 5'-ACC TAT GCG GCC GCA AAT GGA CTG CTG CAA GAA C-3'
SEQ ID NO. 6 5'-GCA CCA ACT AGT GCC TTT TTT TTT TTT TTT A-3'
SEQ ID NO. 7 5'-GCA CCA ACT AGT GCC TTT TTT TTT TTT TTT C-3'
SEQ ID NO. 8 5'-GCA CCA ACT AGT GCC TTT TTT TTT TTT TTT G-3'.
[0097] The above 5' and 3' primers were then used in the following
amplification
cocktail.
PCR Reaction Cocktail:
10X PCR Buffer 5 pi
25 mM MgCI2 3 pi
10 mM dNTP 1 pi
10 pM dT-Spel 1 pi
10 pM MT-Not I 1 NI
22
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0098] To the above PCR Reaction Cocktail, a Gem 50 wax bead was added to the
tube and the tube incubated at 80 C for 2-3 minutes. Upon hardening of the wax
at room
temperature for 10-15 min, the following were layered on top of the hardened
wax:
Sterile H20 36.5 NI
Artemia cDNA mixture 2 pl
Taq Polymerase 0.5 pl
[0100] This final mixture was then subjected to the following PCR
amplification
program.
PCR Program:
Initial denaturation for 3 min at 95 C, followed by 29 cycles of:
94 C for 1 min
49 C for 1 min
72 C for 1 min
72 C for 10 min
Hold at 4 C
[0101] Once amplified, the PCR product was verified for successful
amplification on a
1.2% agarose gel. The PCR product was then purified for subsequent cloning
using Qiagen
QiAquick Gel Extraction (Qiagen, CA). The following primers which contain
modifying
restriction sites incorporated into their sequence were used to amplify and
subclone the
purified PCR product containing brine shrimp Artemia metal binding protein
gene
sequences.
MT Nco I(5' primer containing an Nde I site):
SEQ ID NO. 9 5' -GCT ACA CAT ATG TCC ATG GAC TGC TGC AAG AAC-3'
MT Sal I(3' primer containing Sal I site):
SEQID NO.10 5'-ACG AAC GTC GAC GCC TTT TTT TTT TTT TTT A-3'
[0102] Using the MT Nco I and MT Sal I primers, with an annealing temperature
of
72 C for 1 min, the Artemia MT nucleotide sequence was amplified and then
subsequently
subcloned into the pGEM3 vector's Eco RI site. Once subcloned, the cloned
metal binding
protein gene can then be easily modified or further processed for use in
expression,
production or other methods requiring use of an isolated nucleic acid encoding
a metal
binding protein.
[0103] The entire coding sequence for MT gene was then determined using a
LiCor
4200L DNA sequencer. Sequence comparison studies of the MT gene from Artemia
indicate
it to have unexpectedly different sequence as compared to other known metal
binding
protein genes. When the Artemia MT gene sequence was aligned with that of
equine and
23
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
human MT, homology was observed at the locations of the metal-binding cysteine
residues.
The ability of the exemplary metal binding protein of the present invention to
bind heavy
metals was then confirmed in the following studies.
EXAMPLE 3
Transgenic Tobacco Expression of Artemia MT
[0104] The following provides an exemplary study which can be performed on any
of
the novel metal binding proteins of the present invention to aid in the
verification of a protein
as a metal binding protein. For example, the metal binding proteins of the
present invention
are capable of binding heavy metals such as zinc, cadmium and copper. The
ability of an
isolated protein to bind heavy metals was described and detailed in the
disclosed
transformation of E. coli with an exemplary MT of the present invention and
shown, as
indicated in FIG. 1.
[0105] As described previously, modified organisms useful for producing the
novel
metal binding proteins of the present invention can be made following the
teachings provided
herein. An exemplary modified organism includes a transgenic tobacco plant
which is
particularly useful in the methods described herein.
[0106] The cDNA for MT cloned into TOPO.CR2 vector is referred to as pARTmt.
The
coding sequence for the MT was cloned into a pUC18 based plasmid containing
the omega
5' untranslated region of the TMV coat protein in frame with the multiple
cloning site. (See
FIG. 2). This was accomplished by amplification of the MT coding sequence from
pARTmõ
using PCR primers containing an Nco I restriction site on the 5' primer and a
Sal I site on the
3' primer. The PCR product and vector were each restricted with Nco I and Sal
I and
purified. The PCR product was then ligated into the vector using T4 DNA
ligase. The ligation
mixture was used to transform DH5a cells by electroporation. LB media was
inoculated with
individual colonies and grown overnight. Plasmid was isolated and sequenced to
verify the
presence and integrity of the MT coding sequence.
[0107] The Eco RI/Xba I cassette was removed and cloned into the corresponding
sites
on the plant expression vector pSS. The pSS vector contains the constitutive
CMV promotor
and transcription terminator sequence in frame with the multiple cloning site.
The resultant
pSSmt construct was propagated in DH5a cells, isolated and sequenced to verify
the
presence and integrity of the MT gene as described above.
MT Expression in Tobacco Leaves
[0108] A. tumefaciens were transformed with the cytosolic pSSmt construct by
electroporation and grown overnight at 27 C in YEB medium, pH 7.4, containing
antibiotics.
The cells were collected and resuspended in induction medium (YEB, pH 5.8,
antibiotics and
24
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
20 pM Acetosyringone) and grown overnight at 27 C. The next morning the cells
were
collected by centrifugation and resuspended in infiltration medium (MMA buffer
containing
antibiotics and 200 pM Acetosyringone) to an Asoo of 1.5 and incubated at room
temperature
for 2 hrs. Tobacco (Nicotiana tabacum) leaves were submerged in the bacterial
suspension
and placed in a vacuum dessicator. The leaves were infiltrated under a vacuum
of 30-40
mbar. The leaves were placed at room temperature for 72 hours then ground to a
fine
powder in liquid nitrogen and extracted with 10 mM Tris pH 8.0, 0.05 mM DTT, 1
mM PMSF.
The solution was clarified by centrifugation at 30,000 x g and the supernatant
assayed for
MT using a 109Co metal binding assay. Metal binding activity is evident in the
leaves
containing the gene for Artemia MT (Table 1).
Table 1
Treatment Bound Cd (CPM)
Buffer 747
Untreated Leaves 5052
Infiltrated Leaves I 12874
Infiltrated Leaves II 12763
Stable Transformation of Tobacco
[0109] A suspension of A. tumefaciens transformed with pSSmt were grown as
described above. Tobacco leaves were cut into small pieces (without the
central vein) and
transferred into sterile weck glasses containing 50-100 mL of bacterial
suspension (A600
about.1.0) and incubated at room temperature for 30 minutes. The leaf pieces
were then
transferred onto sterile Whatman 3MM filterpaper pre-wetted with sterile water
in plastic petri
dishes. The dishes were sealed with saran wrap and incubated at 26-28 C in the
dark for
two days. The leaf pieces were then washed with sterile water containing
antibiotics and
transferred onto MS II agar plates. The pieces were incubated at 25 C for 3-4
weeks with a
16 hr photoperiod. When shoots began to form, the shoots were removed and
transferred
onto MS III agar plates and incubated at 25 C with a 16 hr photoperiod until
roots began to
form. The small plants were transferred into weck glasses containing MS III
medium and
incubated at 25 C with a 16 hr photoperiod for about two weeks. The young
plants were then
planted into soil. Young leaves from the plants were collected and assayed for
MT activity as
described above to determine the transgenic plants.
EXAMPLE 4
Polymer Membranes for Toxic Metal Removal From Water
[0110] Metallothionein was extracted from Artemia embryos as described above.
The
protein extract (80 mL) was placed in a boiling water bath for 15 minutes. The
solution was
centrifuged at 30,000 x g (16,000 rpm in a SA600 rotor) for 30 minutes at 4 C.
The
supernatant containing the metallothionein was transferred to a clean tube
containing 60 pL
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
of109Cd (Amersham Biosciences). The solution was mixed well and allowed to
stand at room
temperature for five minutes. This allows for exchange of the radioactive
cadmium onto the
metallothionein and provides us with a method for detecting the protein during
its
purification. The solution was then applied to a 100 x 4.8 cm G-50 molecular
exclusion
column and eluted with nitrogen saturated 50 mM Tris, pH 8Ø Fifteen
milliliter fractions
were collected into tubes containing 25 pL of 1 M DTT. The peak metal binding
activity were
pooled and stored at 4 C. The solution is referred to as MT. (See FIG. 3
through FIG 7)
Metal Binding at Neutral pH
[0111] Pall Biodyne membranes (Biodyne A and Biodyne B, 0.45 pm, Lot numbers
002245 and 035241, respectively) were used as a solid support for these
experiments. A 1
cm 2 piece of membrane was placed in a 10 ml Millipore glass frit filtering
unit. Ten milliliters
of MT was passed through the membrane under vacuum at a flow rate of
approximately 100
mL/minute (See FIG. 4). The flow through was collected for protein analysis.
Next, 10 mL of
a solution of cadmium (0.1 pg/mL of CdC12 and 10 NL109Cd in 50 mL of water)
was passed
through the membrane under vacuum (See FIG. 5). The membrane was then washed
twice,
each with 10 mL of PBS. Five milliliters of the pooled eluate was analyzed of
radioactivity.
The membrane was removed from the filtering unit, place in a 12 x 75 mm
centrifuge tube
and analyzed for radioactivity in an LKB gamma counter. As a control, the
procedure was
repeated with a second membrane that had not been treated with MT. This
membrane is
referred to as the "blank." The results are shown below in Table 2.
Table 2
Sample MT Membrane Blank
Biodyne A 152,876 3768
Biodyne B 158,762 1774
[0112] The results demonstrate that membrane-bound MT is capable of removing
cadmium(as 109Cd) from a solution of the metal passed through the membrane.
Membranes
without MT remove little, if any, metal from the solution.
Metal Binding at Varying pH
[0113] The next series of experiments were to determine the effect of extremes
of pH
on the metal binding activity of the protein on the membrane. A fresh sample
of MT was
prepared for these studies. The solution of cadmium used for these experiments
was
prepared as follows: 2 pL of 109Cd was added to 1 mL of an aqueous solution of
CdCI2
(lppm). Then 100 pL of this radioactive cadmium solution was added to 10 mL of
each of
the following solution: PBS, 10 mM glycine, 150 mM NaCI, pH 3Ø, and 10 mM
HZC03/HCO3, 150 mM NaCI, pH 10.1. Only the Biodyne A membrane was used for
this
26
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
study. Membranes not treated with MT washed with PBS containing radioactive
cadmium
served as the controls. Membranes were placed in the Millipore filtering unit
and processed
as follows:
Membrane #1 (blank) was washed with 5 mL of PBS containing radioactive
cadmium. The
membrane was then washed twice with 10 mL of non-radioactive, metal-free
PBS.
Membrane #2 was washed first with 10 mL of MT solution and then 5 mL of PBS
containing
radioactive cadmium. The membrane was then washed twice with 10 mL of
non-radioactive, metal-free PBS.
Membrane #3 was washed with 10 mL of MT solution and then 5 mL of 10 mM
H2CO3/HCO3, 150 mM NaCI, pH 10.1, containing radioactive cadmium. The
membrane was then washed twice with 10 mL of non-radioactive, metal-free
mM H2C03/HCO3, 150 mM NaCI, pH 10.1.
Membrane #4 was washed with 10 mL of MT solution and then 5 mL of 10 mM
glycine, 150
mM NaCI, pH 2.0, containing radioactive cadmium. The membrane was then
washed twice with 10 mL of non-radioactive, metal-free 10 mM glycine, 150
mM NaCI, pH 2Ø
[0114] Each membrane was analyzed for radioactivity as described above, The
results
are shown below in Table 3.
Table 3
Sample CPM
Membrane 1 (blank) 174
Membrane 2 pH7.5 33380
Membrane 3 pH 10.1 6890
Membrane 4 pH 2.0 651
[0115] This experiment demonstrates that the membrane-bound MT is capable of
binding metal at pHs ranging from 7.5 to 10.1 but does not occur at a pH of 2.
Once metal is
bound to the MT, it can be recovered by exposing the membrane to acid (pH=2)
(See FIG.
6). These experiments were conducted by adding all the solutions directly to
the membrane.
To evaluate effects of pre-equilibrating the membranes with buffer prior to
addition of MT,
i.e., is the efficiency of metal binding effected, membranes (Biodyne B) were
processed as
follows:
27
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
Membrane #1 (blank) was washed with 5 mL of PBS containing radioactive
cadmium. The
membrane was then washed twice with 10 mL of non-radioactive, metal-free
PBS.
Membrane #2 was washed first with 10 mL of MT solution and then 5 mL of PBS
containing
radioactive cadmium. The membrane was then washed twice with 10 mL of
non-radioactive, metal-free PBS.
Membrane #3 was pre-washed with 10 mL of metal-free 10 mM H2CO3/HCO3, 150 mM
NaCI, pH 10.1, then washed with 10 mL of MT solution and then 5 mL of 10
mM H2CO3/HCO3, 150 mM NaCI, pH 10.1, containing radioactive cadmium.
Finally, the membrane was washed twice with 10 mL of non-radioactive,
metal-free 10 mM H2CO3/HCO3, 150 mM NaCI, pH 10.1.
[0116] The results are shown below in Table 4
Table 4
Sample CPM
Membrane #1 190
Membrane #2 4218
Membrane #3 7431
[0117] Equilibrating the membrane at pH 10.1 results in better efficiency of
protein
binding to the membrane.
Specificity of MT metal binding
[0118] Binding affinity/specificity was measured against bovine serum albumin,
a
protein containing several cysteine residues and known to bind heavy metals.
The Biodyne
A membrane was used for this experiment. The concentration of MT solution was
found to
be approximately 7 pg/mL. The concentration of the flow through is equivalent
to the starting
material indicating that the amount bound to the membrane is in ng
(nanograms), thus
indicating that the metal binding capacity of the protein is significant.
Therefore, 7 pg/mL and
100 pg/mL solutions of BSA were made in D-PBS using the 2 mg/mL BSA standard
from
Pierce Chemical, Inc. The cadmium binding solution was prepared as follows:
1.5 mL of
aqueous 1 ppm CdCI2 was mixed with 3 pL of 109Cd. The solution is stored at 4
C. The
assay was run as follows:
Membrane #1 (blank) was washed with 5 mL of PBS containing radioactive
cadmium. The
membrane was then washed twice with 10 mL of non-radioactive, metal free
PBS.
28
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
Membrane #2 was washed first with 5 mL of MT solution and then 5 mL of PBS
containing
radioactive cadmium. The membrane was then washed twice with 10 mL of non-
radioactive, metal free PBS.
Membrane #3 was washed with 5 mL of BSA solution (7 pg/mL)and then 5 mL of PBS
containing radioactive cadmium. The membrane was then washed twice with
mL of non-radioactive, metal-free PBS.
Membrane #4 was washed with 10 mL of BSA solution (100 Ng/mL) and then 5 mL of
PBS
containing radioactive cadmium. The membrane was then washed twice with
10 mL of non-radioactive, metal-free PBS.
[0119] The results of these experiments are shown below in Table 5.
Table 5
Sample CPM
Membrane 1 No MT 174
Membrane 2 MT (5 mL) 1171
Membrane 3 BSA (5 mL of 7 pg/mL) 77
Membrane 4 BSA (10 mL of 100 pg/mL) 151*
* this membrane was tested a different day where the MT binding activity was
greater than 3000 CPM.
[0120] Under these experimental conditions, BSA does not remove metal from
aqueous solutions, even when using a 10-fold higher concentration of BSA than
MT to
prepare the membrane. This experiment demonstrates the utility of membrane
bound MT
for remediation of metal from water or other aqueous substrates (see FIG. 7).
Effect of Temperature on Metal Binding Activity.
[0121] These binding experiments were performed with Biodyne A membranes.
Membrane #1 (blank) was washed with 5 mL of PBS containing radioactive
cadmium. The
membrane was then washed twice with 10 mL of non-radioactive, metal-free
PBS.
Membrane #2 was washed with 10 mL of MT solution and then 5 mLof PBS
containing
radioactive cadmium pre-warmed to 60 C. The membrane was then washed
twice with 10 mL of non-radioactive, metal-free PBS pre-warmed to 60 C.
Membrane #3 was washed with 10 mL of MT solution and then 5 mL of PBS
containing
radioactive cadmium cooled to 4 C. The membrane was then washed twice
with 10 mL of non-radioactive, metal free PBS cooled to 4 C.
[0122] The results of these experiments are shown below in Table 6.
Table 6
29
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
Sample CPM
Membrane #1 139
Membrane #2 3886
Membrane #3 2672
EXAMPLE 5
Comparison of Rabbit and Artemia MT
[0123] Metal remediation with the metal binding proteins of the present
invention can
be accomplished using metallothionein proteins from a variety of sources.
Rabbit liver MT
was obtained as a lyophilized protein (Sigma) and solubilized in 400 pL of 50
mM Tris, pH
8.0, 0.001 M DTT to a final concentration of 2.5 mg/mL (rabbit MT stock
solution). Artemia
MT was purified as described supra in Example 4.
[0124] Membranes were prepared having bound Artemia MT or rabbit liver MT by
passing an MT-containing solution through the membrane, as described supra in
Example 4.
Three membranes, a blank, a membrane bound with Artemia MT and a membrane
bound
with rabbit liver MT, were then placed in a 13 mm scintered glass filtering
unit and 10 mL of
a metal binding solution (a stock solution of 9000 cpm of 109Cd/25 pL of
solution diluted to 75
NL/10mL PBS to form the metal binding solution) was passed through the
membrane under
vacuum. The membrane was then washed three times in PBS, and the membrane-
bound
radioactivity was measured in a Packard gamma counter. In a second experiment,
a larger
quantity of Artemia MT was bound to the membrane. The results of these two
experiments
are found in Tables 7 and 8.
Table 7
Sample CPM
Membrane 1 Blank 351
Membrane 2 Artemia (20mL bound to the 685
membrane
Membrane 3 Rabbit (25 pL of a 2.5 mg/mL 985
solution
Table 8
Sample CPM
Membrane 1 Blank 231
Membrane 2 Artemia (25mL bound to the 980
membrane
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
[0125] Membrane-bound metallothionein, regardless of source, provides removal
of
metals from aqueous solutions. In addition, the metal binding activity is a
function of the
amount of protein applied to the membrane and increasing the amount of MT
protein on the
membrane results in increased metal binding activity by the membrane.
[0126] In closing it is to be understood that the embodiments of the invention
disclosed
herein are illustrative of the principals of the invention. Other
modifications may be employed
which are within the scope of the invention and accordingly, the present
invention is not
limited to that precisely as shown and described in the present specification.
[0127] Unless otherwise indicated, all numbers expressing quantities of
ingredients,
properties such as molecular weight, reaction conditions, and so forth used in
the
specification and claims are to be understood as being modified in all
instances by the term
"about." Accordingly, unless indicated to the contrary, the numerical
parameters set forth in
the following specification and attached claims are approximations that may
vary depending
upon the desired properties sought to be obtained by the present invention. At
the very
least, and not as an attempt to limit the application of the doctrine of
equivalents to the scope
of the claims, each numerical parameter should at least be construed in light
of the number
of reported significant digits and by applying ordinary rounding techniques.
Notwithstanding
that the numerical ranges and parameters setting forth the broad scope of the
invention are
approximations, the numerical values set forth in the specific examples are
reported as
precisely as possible. Any numerical value, however, inherently contains
certain errors
necessarily resulting from the standard deviation found in their respective
testing
measurements.
[0128] The terms "a" and "an" and "the" and similar referents used in the
context of
describing the invention (especially in the context of the following claims)
are to be construed
to cover both the singular and the plural, unless otherwise indicated herein
or clearly
contradicted by context. Recitation of ranges of values herein is merely
intended to serve as
a shorthand method of referring individually to each separate value falling
within the range.
Unless otherwise indicated herein, each individual value is incorporated into
the specification
as if it were individually recited herein. All methods described herein can be
performed in
any suitable order unless otherwise indicated herein or otherwise clearly
contradicted by
context. The use of any and all examples, or exemplary language (e.g. "such
as") provided
herein is intended merely to better illuminate the invention and does not pose
a limitation on
the scope of the invention otherwise claimed. No language in the specification
should be
construed as indicating any non-claimed element essential to the practice of
the invention.
[0129] Groupings of alternative elements or embodiments of the invention
disclosed
herein are not to be construed as limitations. Each group member may be
referred to and
31
CA 02584518 2007-04-17
WO 2006/045103 PCT/US2005/038136
claimed individually or in any combination with other members of the group or
other
elements found herein. It is anticipated that one or more members of a group
may be
included in, or deleted from, a group for reasons of convenience and/or
patentability. When
any such inclusion or deletion occurs, the specification is herein deemed to
contain the
group as modified thus fulfilling the written description of all Markush
groups used in the
appended claims.
[0130] Preferred embodiments of this invention are described herein, including
the best
mode known to the inventors for carrying out the invention. Of course,
variations on those
preferred embodiments will become apparent to those of ordinary skill in the
art upon
reading the foregoing description. The inventor expects skilled artisans to
employ such
variations as appropriate, and the inventors intend for the invention to be
practiced otherwise
than specifically described herein. Accordingly, this invention includes all
modifications and
equivalents of the subject matter recited in the claims appended hereto as
permitted by
applicable law. Moreover, any combination of the above-described elements in
all possible
variations thereof is encompassed by the invention unless otherwise indicated
herein or
otherwise clearly contradicted by context.
.[0131] Furthermore, numerous references have been made to patents and printed
publications throughout this specification. Each of the above cited references
and printed
publications are herein individually incorporated by reference in their
entirety.
[0132] In closing, it is to be understood that the embodiments of the
invention disclosed
herein are illustrative of the principles of the present invention. Other
modifications that may
be employed are within the scope of the invention. Thus, by way of example,
but not of
limitation, alternative configurations of the present invention may be
utilized in accordance
with the teachings herein. Accordingly, the present invention is not limited
to that precisely
as shown and described.
32
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.