Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
SILVER ION WATER GENERATING APPARATUS
Technical Field
The present invention relates to a silver ion water
generating apparatus, particularly to a silver ion water
generating apparatus which can remarkably improve sterilizing
and purifying forces even in a large water tank, can be
easily maintained, and can prevent silver ions from not being
properly generated due to inferiority of current flow between
a silver foam and an electrode terminal.
Background Art
Conventionally, a silver ion generating apparatus
which produces silver ion water by disposing electrode rod
formed of silver in the water stored in a water tank and by
applying a voltage to the electrode rod and electrolyzing
silver has been circulated in the market. The silver ion
generating apparatus has been widely used in facilities for
purifying the water stored in a water tank or flowing water
or in sterilizirig facilities. FIG. 1 schematically shows the
conventional silver ion water generating apparatus. The
conventional silver ion water generating apparatus includes a
water tank (110) for storing water, a pair of silver rods
(115) installed in the interior of the water tank (110), and
a positive electrode terminal (120) and a negative electrode
terminal (125) which are electrically connected to the silver
1
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
rods (115) respectively. In the apparatus, if positive and
negative electric potentials are applied to the silver rods
(115) by the electrode terminals (120) and (125)
respectively, silver ions are generated from the silver rods
(115) by the electrolysis reaction. Then, the generated
silver ions are discharged into the water in the water tank
and sterilizing germs in the water.
While the silver ion generating apparatus is effective
in a small sized purifying system such as a domestic water
purifier, the cost of silver is relatively high. Further,
there is a limit in the surface area of the silver rod, it is
not effective in a swimming pool or in an apparatus such as
an industrial facility for purifying water of high capacity.
Disclosure of the Invention
Accordirigly, it is the object of the present invention
to provide a silver ion water generating apparatus which can
impr_ove sterilizing and purifying forces even in a large
water tank by widening the contact surface of silver as same
weight and increasing the amount of the generated silver ions
and can be easily maintained by facilitating the exchange of
a silver foam, and can prevent the silver iori water from not
being properly generated due to the inferiority of current
flow between the silver foam and the electrode terminal in
use.
The foregoing and/or other objects of the present
2
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
inverition are achieved by providing a silver ion water
generating apparatus comprising: a water tank (10) in which a
hollow portion is formed in the interior thereof, an
introducing opening through which water is introduced is
formed on the one side surface thereof, and a discharging
opening through which the introduced water is discharged is
formed on the other side surface thereof; and a positive
electrode and a negative electrode which are positioned in
the hollow portion of the water tank (10), wherein the
positive electrode includes a silver foam (20) in which
silver forms open cells, one end of the negative electrode is
electrically connected to a negative electrode terminal (7),
and the silver foam formed of silver is electrolyzed to
generate silver ions if a current is applied to the positive
and negative electrodes positiori in the water tank (10).
The silver foam (20) positioned ir1 the hollow portion
of the water tank is positioned in an interior hollow portion
of a conductive net body in which a conductive material forms
a net-like shape, and the conductive net body is detachably
mounted to the positive electrode terminal.
According to the present invention, an insertion hole
penetrating through both ends of the silver foam is formed in
the interior of the silver foam, a conductive electrode rod
irl which an expanding portion extends radially to the outside
thereof is inserted into the circumferential portion of the
insertion hole of the silver foam, the electrode rod is
3
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
connected to the exterior positive electrode terminal, and a
conductive spring resiliently supporting one end of the
silver foam and the expanding portion of the electrode rod so
that a current can flow between the silver foam and the
electrode rod is interposed between the end of the silver
foam and the expanding portion of the electrode rod.
Further, in the negative electrode, an insertion hole
penetrating through both upper and lower ends of the silver
foam is formed at an central portion the silver foam in which
silver forms the open cells, a conductive electrode rod in
which an expanding portion extends radially to the outside
thereof is inserted into the circumferential portion of the
insertion hole of the silver foam, the electrode rod is
corlriected to the exterior negative electrode terminal, and a
conductive spring resiliently supporting one end of the
silver foam and the expanding portion of the electrode rod so
that a current can flow between the silver foam and the
electrode rod is interposed between the end of the silver
foam and the expanding portion of the electrode rod.
Brief Description of the Drawings
These and/or other aspects and advantages of the
invention will become apparent and more readily appreciated
from the following description of the preferred embodiments,
taken in conjunction with the accompanying drawings of which:
FIG. 1 is a view for schematically showing the
4
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
constitution of a conventional silver ion water generating
apparatus;
FIG. 2 is a side cross-sectional view for showing a
preferred embodiment of the present invention;
FIG. 3 is a front cross-sectional view of FIG. 2;
FIG. 4 is a side cross-sectional view for showing
another preferred embodiment of the present invention; and
FIG. 5 is an exploded view for showing main parts of
the preferred embodiment of FIG. 4.
Best Mode for Carrying Out the Invention
Hereinafter, preferable embodiments accordirig to the
present invention will be described with reference to the
accompanying drawings. It should be noted that, in the
dr_awirigs, the same elements are endowed with the same
reference numerals.
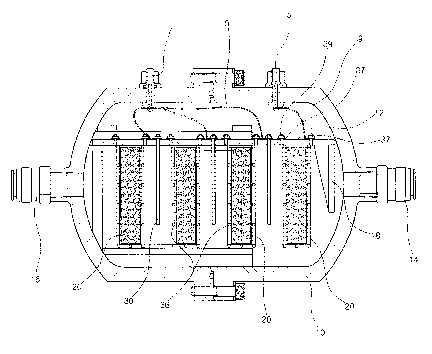
FIG. 2 is a side cross-sectional view for showing a
preferred embodiment of the present invention, and FIG. 3 is
a front cross-sectional view of FIG. 2. As shown in FIGs. 2
and 3, a positive electrode and a negative electr_ode are
iristalled iri the interior of a water tank (10) for storing
water, and the positive electrode includes a silver foam (20)
in which open cells are formed and silver ion water is
generated by silver ions generated from the silver foam (20).
The water tarik (10) has a box-like shape and is
insulated. An insulated cover plate (12) is horizontally
5
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
installed on the upper side of the interior of the water tank
(10). An introducing opening (14) through which the original
water is introduced and a discharging opening (16) through
which the silver ion water is discharged are formed in both
side walls of the water tank (10). A separation plate (18) is
installed on the side of the introducing opening (14) in the
interior of the water tank (10). The water introduced through
the introducing opening (14) passes round the separation
plate (18).
The silver foam (20) has a block-like shape in which
open cells are formed, and is made of pure silver. The silver
foam (20) is connected to the exterior positive electrode
terminal (5) by the medium of a conductive net body (36) in
order to constitute a positive electrode.
The cover plate (12) is horizontally installed on the
upper side of the interior of the water tank (10) . A
plurality of rectangular conductive net bodies (36) are
installed in the cover plate (12), and the silver foams (20)
are accommodated in the conductive net bodies (36) . Each
conductive net body has a block-like shape, and one end
thereof is opened. Preferably, the conductive net body (36)
is formed of titanium dioxide. A bolt member (37) is provided
at the opened end of each conductive net body (36). The bolt
member (37) penetrates through the cover plate (12) and is
erlgaged with a nut member (39). In this way, the plurality of
conductive net bodies (36) are mounted to the cover plate
6
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
(12).
Namely, the silver foam can independently connected to
the positive electrode terminal to form a positive electrode,
but it is preferable that the silver foam is detachably
mounted into the interior of the conductive net body (36) to
form a positive electrode.
Then, since the nut member (39) is engaged with the
bolt member (37), with a wire (9) connected to the positive
electrode terminal (5) being inserted between the nut member
(39) and the cover plate (12), the conductive net body (36)
carl be connected to the positive electrode terminal (5).
Further, since the silver foam (20) is accommodated in the
interior of the conductive net body (36) so that currents can
flow through the silver foam (20), the silver foam (20) forms
the positive electrode. The silver foam (20) is disposed on
the right and left sides of the water tank (10) so that the
wider surface thereof faces the introducing openirig (14) and
the discharging opening (16) . Therefore, when the water
passes through the water tank (10), the contact area between
the water and the silver foam (20) can become wider. Further,
a plurality of electrode plates (30) are disposed in the
water tank (10) so as to be located between the silver foams
(20) . Since the electrode plates (30) are connected to the
wires (9) connected to the positive electrode terminal (7),
they form the negative electrodes.
According to the preferred embodiment of the present
7
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
invention, silver ions can be generated in the silver foams
(20), which are positive electrodes and the water in the
water tank can be changed to silver ion water by the
principle of electrolysis. Then, since the water in the water
tank makes contact not only with the surface of the silver
foam (20) of sponge-type but also with through-holes in the
silver foam (20) while passing through the through-holes, the
contact area between the water and the silver is remarkably
increases when compared with a conventional silver rod.
Accordingly, since the generated amount of the silver ions
increases due to the silver foam, the sterilizing and
purifying forces in the water in a large purifyirlg facility
or a large water tank can remarkably increase.
Further, the sterilizing and purifying forces can
1.5 remarkably iricreased even with silver of a same or lower
weight, when compared with a silver rod. Furthermore, since
the water passes round the separation plate (18) installed at
the introducing opening (14) of the water tank (10), the stay
time of the water becomes increasing. Therefore, since the
water makes contact with the silver foam (20) more longer,
the silver ion generating effect can increase.
Further, since the silver foam (20) is accommodated in
the conductive net body (36) made of titanium dioxide and the
conductive net body (36) is detachably mounted to the cover
plate (12) in the water tank (10), when the silver foam (20)
is exhausted due to the long time use, the conductive net
8
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
body (36) can be separated and only the silver foam (20) can
be promptly and easily exchanged.
FIGs. 4 and 5 show another preferred embodiment of the
present invention. Referring to FIGs. 4 and 5, a water tank
(10) has a box-like shape and is insulated and the upper and
lower portions of the water tank (10) are closed. An
insulated cover plate (12) is horizontally installed on the
upper side of the interior of the water tank (10). A pair of
electrode rods (40) connected to a positive electrode
terminal (5) and a negative electrode terminal (7) are
disposed in the cover plate (12) . A silver foam (20) is
mourited onto a circumferential portion of each electrode rod
(40) and a conductive spring (50) is interposed between the
electrode rod (40) and one end of the silver foam (20).
The one end of the electrode rod (40) penetrates
through the cover plate (12) and protrudes from the interior
of the water tank (10) outside the cover plate (12) . The
positive electrode terminal (5) and the negative electrode
terminal (7) are connected to upper end portions of the
electrode rods (40) by bolts (44), respectively. A nut (46)
is engaged with the circumferential portion of each electrode
rod (40) protruding to the upper side of the cover plate
(12), and the silver foams (20) and the electrode rods (40)
are fixed to the cover plate (12) by the nuts (46) . Further,
ari exparidirig portion (48) of flange-type is formed at a lower
end portion of each electrode rod (40) . The electrode rod
9
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
(40) is formed of titanium dioxide, which is preferable since
it is not rusted well when compared with a stainless steel
and its electrical conductivity is good next to that of
copper.
The silver foam (20) has a cylindrical shape of
sponge-Lype, in which open cells are formed. An insertion
hole (22) through which the upper and lower ends of the
silver foam (20) is communicated is formed at a central
portion of the silver foam (20). The circumferential portion
of the electrode rod (40) is inserted into the insertion hole
(22) of the central portion of the silver foam (20) so that
the inner wall surface of the insertion hole (22) formed in
the silver foam (20) makes contact with the circumferential
portion of the electrode rod (40).
Further, washer members (49a) and (49b) are mounted to
the upper erid portions of each silver foam (20),
respectively. The washer members (49a) and (49b) are ring-
like shape, and preferably are formed of silver. A conductive
spring (50) is interposed between the lower side washer
member (49a) and an expanding portion (48) of the electrode
rod (40). An upper end portion of the conductive spring (50)
resiliently makes contact with the lower side washer member
(49a) and a lower end portion thereof resilieritly makes
contact with the expanding portion (48) of the electrode rod
(40), so that the lower end portion of the silver foam (20)
and the lower side washer member (49a) and the expanding
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
portion (48) of the electrode rod (40) resiliently make
contact with each other. Namely, since the silver foam (20)
and the electrode rod (40) always make contact with each
other due to the resilient force of the spring (50), currents
can constantly flow through the silver foam (20) and the
electrode rod (40).
According to the preferred embodiment of the present
invention, since the silver foam (20) is engaged with the
circumferential portions of the electrode rod (40) and the
conductive washer members (49a), (49b) and the conductive
spr_irlg (50) are interposed between the silver foam (20) and
the expanding portion (48) of the electrode rod (40) for the
contact of the silver foam (20) and the electrode rod (40),
the silver foams (20) are electrolyzed by positive and
negative voltages applied from the electrode rods (40), with
the silver foam (20) and the conductive electrode rods (40)
always making contact with each other, thereby generating
silver ions and sterilizing and purifying the water in the
water tank (10).
Acco.rdirigly, since the water in the water tank (10)
makes coritact with the surfaces of the silver foams of
sporige-type and also makes contact with the pores in the
silver foams (20), the amount of silver ions generated by the
silver foams increases. Further, since currents can
constaritly flow through the silver foams (20) and the
electrode rods (40) due to the conductive springs (50)
11
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
interposed between the expanding portions (48) of the
electrode rods (40) and the ends of the silver foams (20),
the electrical connections between the silver foams (20) and
the electrode rods (40) can be effectively secured, even when
the silver foams (20) are exhausted by a long time use.
On the other hand, since sealing members (13a) are
provided at portions where the electrode rods (40) are
engaged with the water tank (10), the water is prevented from
being leaked between the water tank (10) and the electrode
rods (40). Further, although FIGs. 4 and 5 show a
coristitution in which the positive and negative terminals are
formed by inserting the silver foams (20) are inserted into
the circumferential portions of the electrode rods (40) and
the conductive springs (50) are interposed between the
electrodes (40) and the one end portion of each silver foam
(20), the riegative terminal can employ a lead electrode,
whi_ch is generally used in electrolysis.
According to the present invention, since the water in
the water tank not only makes contact with the surfaces of
the silver foams of sponge-type but also makes contact with
the pores in the silver foams while passing through the
pores, the contact area of the water and the silver
rernarkably increases, when compared with the conventional
silver rod. Accordingly, the amount of silver ions generated
by the silver foams increases and the sterilizing and
purifying forces of the water in a large facility or water
12
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
tarik remarkably increase. Further, the sterilizing and
purifying forces can remarkably increase even with silver of
a same or lower weight, when compared with a silver rod.
Further, since the silver foams are accommodated in the
conductive net bodies formed of titanium dioxide, when the
silver foams are exhausted due to the long time uses, the
conductive net bodies can be separated and only the silver
foams can be promptly and easily exchanged.
Further, since the silver foams are engaged with the
circumferential portions of the electrode rods and the
conductive water members and the conductive springs are
iriterposed for the contacts of the silver foams and the
electrode rods, silver ions can be generated by electrolyzing
the silver foams, with the silver foams always making contact
with the electrode rods, thereby sterilizing and purifying
the water irl the water tank. Accordingly, since currents can
always flow through the silver foams and the electrode rods
due to the conductive springs due to the electrical
connections between the silver foams and the electrode rods,
the silver ions are prevented from not being generated from
the silver foams due to the inferiority of current flow in
use.
Although a few embodiments of the present invention
have been shown and described, it would be appreciated by
those skilled in the art that changes might be made in this
embodiment without departing from the pririciples and spirit
13
CA 02603089 2007-09-28
WO 2006/115333 PCT/KR2006/001193
of the inventiori, the scope of which is defined in the claims
and their equivalents.
14