Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
1
Wastewater Treatment
Field of the Invention
The present invention relates to biological processes for at least partial
removal of
nitrogen, phosphorus and BOD from wastewaters which have very high levels of
nitrogen
as well as significant phosphorous levels, such as abattoir wastewaters.
Background to the Invention
The meat processing industry requires large quantities of water, much of which
is
discharged as high biochemical oxygen demand (BOD) wastewater containing high
levels
of nutrients such as nitrogen (N) and phosphorus (P).
Similar to high BOD and nitrogen levels in waterways, the presence of excess
phosphorus in waterways is also of major concern as it has the potential to
promote
eutrophication. As major sources of phosphorus include agricultural runoff and
a variety
of domestic, commercial and industrial processes, the removal of phosphorus
during
wastewater treatment is of significant environmental importance.
Over the past two decades, BOD and N removal from abattoir wastewater has
received much attention, and reliable BOD and N removal systems have been
successfully developed and applied to abattoir wastewater treatment using
continuous
activated sludge systems. However, phosphorous removal has received less
attention.
A common means for removing phosphorus from wastewaters is chemical
precipitation. Typically this involves the addition of metal salts, such as
aluminium
sulphate, ferric sulphate and ferric chloride, to react with soluble
phosphorus and form
solid precipitates that can be removed by solid separation processes.
However, chemical precipitation suffers from a number of disadvantages.
Typically, chemical precipitation increases the volume of sludge produced and
can
produce sludge with poor settling qualities. Further there are environmental
concerns
associated with the use and release of chemicals.
Nonetheless, phosphorous removal continues to be achieved primarily through
chemical precipitation, despite biological P removal being potentially a much
cheaper and
more environmentally sustainable option.
Enhanced biological phosphorus removal (EBPR) provides a means of removing
phosphorus from wastewater through a biological process as an alternative to
chemical
precipitation. EBPR relies on the ability of polyphosphate-accumulating
microorganisms
(PAOs) to take up phosphorus in excess of their metabolic requirements.
However, complete nitrification of wastewaters containing high levels of
ammonium and other nitrogenous sources produces a high level of nitrate, which
interferes with the development of a stable and reliable EBPR process.
Another difficulty in developing an effective biological process for
simultaneous
removal of nitrogen and phosphorous from abattoir wastewater is that such
wastewaters
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
2
contain substantial amounts of fat, oil and grease (FOG), which deteriorate
the sludge
settling properties when directly fed to activate sludge systems. Such
wastewaters are
therefore typically pre-treated before being treated in biological nutrient
removal systems.
In Australia, the raw abattoir wastewater is typically pre-treated in
anaerobic ponds with a
hydraulic retention time ranging between 7 - 14 days. While reducing the FOG
content,
this anaerobic treatment process also removes a large fraction of the BOD from
the
wastewater, often resulting in limiting concentrations of available carbon
sources
(particularly Volatile Fatty Acids - VFAs) required for N removal, and P
removal by
PAOs.
Thus, an objective of the present invention is to provide an effective
biological
process for the simultaneous removal of nitrogen and phosphorous from
wastewaters
comprising elevated levels of nitrogen and phosphorous sources which overcomes
or
ameliorates one or more of the above identified difficulties.
Summary of the Invention
Through the present studies, it was found that nitrogen and phosphorous in
wastewaters comprising elevated levels of nitrogen can be efficiently removed
using a
biological process employing a combination of biological nitrogen removal and
enhanced
biological phosphorus removal (EBPR) employing activated sludge comprising
nitrifying
and denitrifying organisms and polyphosphate accumulating organisms (PAOs), by
feeding the wastewater into a reaction vessel in steps and manipulating the
aeration
conditions in such a manner that significant nitrate and nitrite levels do not
accumulate,
thereby ensuring anaerobic conditions appropriate for efficient polyphosphate
accumulation by the PAOs.
Thus, according to an aspect of the invention, there is provided a biological
process for reducing the levels of nitrogen and phosphorous in wastewater,
wherein said
wastewater comprises at least 100mg/L total nitrogen, wherein said process
comprises
feeding said wastewater into a reaction vessel in at least two steps, wherein
said reaction
vessel comprises an active biomass comprising nitrifying and denitrifying
organisms and
polyphosphate accumulating organisms (PAOs), wherein at least the first feed
step is
followed by a non-aerated period of sufficient duration to result in
sufficiently low
concentrations of NO,, species in the wastewater to allow for accumulation of
polyhydroxyalkanoates inside the PAO cells, and at least the first non-aerated
period is
followed by an aerated period of sufficient duration to allow for ainrnonium
oxidation by
the nitrifying organisms and assimilation by the PAOs of at least a portion of
the
phosphorous in the wastewater.
According to an embodiment, the first aerated period is followed by at least
one
cycle of a feed step, a non-aerated period and a subsequent aerated period.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
3
According to another embodiment, the wastewater is fed into said reaction
vessel
in at least three steps, wherein each feeding step is followed by a non-
aerated period and a
subsequent aerated period.
At least the first aerated period may be of sufficient duration to ensure
substantially complete ammonium oxidation by the nitrifying organisms and, in
a specific
embodiment, each aerated period is of sufficient duration to ensure
substantially complete
aininonium oxidation by the nitrifying organisms.
In such an embodiment, about 50% of the volume of wastewater to be treated may
be fed into the reaction vessel in the first feeding step, about 30% of the
volume of
wastewater to be treated may be fed into the reaction vessel in the second
feeding step,
and about 20% of the volume of wastewater to be treated may be fed into the
reaction
vessel in the third feeding step.
The reaction vessel contents may be allowed to settle after the third aerated
period, and treated wastewater and settled sludge separated.
A source of volatile fatty acids may also be fed into the reaction vessel or
added to
said wastewater before feeding into said reaction vessel. The source of
volatile fatty acids
may be co-fed into the reaction vessel with the wastewater.
The source of volatile fatty acids may comprise a pre-fermented high BOD waste
coinprising elevated levels of acetic and propionic acids, such as at least
100mg/L of each
of acetic and propionic acids.
The source of volatile fatty acids may be fed into said reaction vessel or
added to
said wastewater in an amount such that the overall soluble COD per litre of
influent into
said reaction vessel is from about 500 mg COD/L to about 600 mg COD/L.
Alternatively, the source of volatile fatty acids may be fed into said
reaction vessel
or added to said wastewater in an amount such that the overall ratio of total
COD to total
nitrogen in the influent to said reaction vessel is from about 5 to about 10,
and the overall
ratio of soluble COD to phosphorous in said influent is about 15.
In an embodiment, each feeding step may comprise distributing wastewater into
settled sludge at the bottom of the reaction vessel. In such an embodiment,
although the
reaction vessel contents may be mixed during the feeding, it may be
advantageous if the
contents of the reaction vessel are not mixed during at least a portion of the
feeding step.
Similarly, although the reaction vessel contents may be mixed during the non-
aerated
period following feeding, it may be advantageous if they are not mixed during
at least a
portion of the non-aerated period following the feeding step. If the feeding
step is carried
out slowly, then there may be no need for a non-mixed, non-aerated period
after the
feeding step.
The concentration of NOX species in wastewater undergoing treatment may be
measured with an on-line NOX sensor or the NOX depletion point may be
estimated by
measuring the oxidation reduction potential of the reaction vessel contents.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
4
The dissolved oxygen concentration of the reaction vessel contents may be
monitored during an aeration period, and maintained within predetermined
concentrations, for example between about 4mg/L and about 0.3mg/L, by
adjustment of
the rate of aeration. For example, air may be provided intermittently during
an aeration
period using an on/off control system in communication with a dissolved oxygen
probe in
contact with the reaction vessel contents.
According to another embodiment of the present invention, the first aerated
period
may be followed by at least one cycle of a feed period and an aerated period
during which
the level of dissolved oxygen is controlled to allow for simultaneous
nitrification and
denitrification in the contents of said reaction vessel. In such an
embodiment, the
dissolved oxygen level may be maintained between about 0.5mg/L to about
0.3mg/L.
According to another embodiment of the invention, the duration of an aerated
period in a process of the invention is determined based on the rate of change
of the pH
(for example, change in pH over a window of 5 minutes) of the reaction vessel
contents
due to ammonium oxidation. For example, an aerated period may be stopped when
the
rate of decrease of the pH of the reaction vessel contents decreases to at
least a
predetermined value, for example to about 10% or less of the maximum rate of
decrease
of the pH of the reaction vessel contents due to ammonium oxidation, or when
the rate of
decrease of the pH of the reaction vessel contents decreases to about 0.01 pH
units per
five minutes or less. Alternatively, or as well, the duration of an aerated
period may be
determined by the oxygen uptake rate of the reaction vessel contents. For
example, an
aerated period may be stopped when the oxygen uptake rate of the reaction
vessel
contents drops below a predetermined value, for example when the oxygen uptake
rate of
the reaction vessel contents drops below 80% or less of the maximum oxygen
uptake rate
of the reaction vessel after introduction of said aerated period, or when the
oxygen uptake
rate of the reaction vessel contents drops below about lmg/min/L of the
reaction vessel
contents. The duration of the aerated period may be controlled to promote
nitrite
production rather than nitrate production during an aerated period, such that
nitrogen
removal from the wastewater during a non-aerated period occurs predominantly
through
direct denitritation of nitrite, with reduced need for nitrate reduction and
requirement of
BOD.
In an embodiment in which the treated waste may be used for land irrigation, a
process according to the invention may comprise two feed steps only. Such a
process
may result in a treated wastewater suitable for land application, which may
comprise less
than about 50mg/L total nitrogen and less than about 15 mg/L total
phosphorous.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
Brief Description of the Drawings
Figure 1 shows a schematic diagram of a sequencing batch reactor set up to
carry
out a process according to the invention.
Figures 2a to 2d show characteristics of the influent (2a and 2b), effluent
nutrient
levels and the Accumulibacter-PAOs population (2c), and MLSS and the VSS/MLSS
ratio
in a reactor (2d) in which a process according to the invention is carried
out.
Figure 3 shows nitrogen and phosphorus profiles during a study of a cycle of a
process according to the invention.
Figure 4 shows concentration of the main VFAs, P043- and NH4+ in raw abattoir
wastewater before and after pre-fermentation, and after one week storage at 4
C. Other
VFAs include iso-butyric, butyric and iso-valeric acids.
Figure 5 shows the concept of a process according to the invention for
treatment
of abattoir wastewater for land irrigation (two feed steps)
Figures 6A and 6B show influent and effluent concentrations of N and P species
over 7 months operation of a two step feed sequencing batch reactor (SBR)
process
according to the invention: A.- Nitrogen species: = N-NH4 influent, = NH4
effluent, 0
nitrite effluent, ^ nitrate effluent. B.- Phosphorus: = P-P04 influent;. = P-
P04 effluent.
The solids lines represent the upper and lower discharge limits.
Figures 7A and 7B show carbon evolution in pond wastewater used in the
process for which the influent and effluent concentrations of N and P species
are shown in
Figures 6A and 6B. A.- = Total COD; = Soluble COD. B.- Volatile Fatty Acids: =
acetate; = propionate.
Figure 8 shows experimental profiles obtained for the different compounds
analysed along a cycle of the SBR process for which the influent and effluent
concentrations of N and P species are shown in Figures 6A and 6B: o N-NH4, V N-
NO3,
= N-NO2, = P-P043"
Figure 9A and 9B shows experimental profiles obtained for the different
compounds analysed for a later, more optimised cycle of the SBR process for
which the
influent and effluent concentrations of N and P species are shown in Figures
6A and 6B:
=N-NH4, 0 N-NO3, ^ N-NO2, = P-PO43-
Figure 10A shows a schematic representation of a normal denitrification
process
via nitrate reduction. Figure lOB shows a schematic of denitrification via
nitrite
reduction
Figure 11 illustrates a control strategy employed to cease aeration when NH4}
is
fully oxidised in an SBR process according to the invention. The 1st condition
to be met
is based on the pH slope (1), the 2nd is based on the length of time the valve
is in an "off'
state which is directly related to the OUR in the case that aeration is
controlled in an on-
off manner (2) and the 3 `d condition is based on minimum duration of aeration
(3).
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
6
Figure 12A shows the degree of nitrite accumulation in the three stages and
the
abundance of NOB Nitrospira (FISH probe Nitspa-662). The NOB quantification
shown
is an average (error bars=S.E., n=3). Figure 12B shows the ammonium, oxidised
nitrogen
and phosphate in the effluent and VFAs in the influent.
Figure 13 shows an example of pH, DO, OUR, nitrogen and phosphorous profiles
during a SBR cycle when aeration was manually extended to destabilise nitrite
pathway
(black arrow). White arrows indicate feeding times.
Figure 14 shows an exainple of pH, DO, OUR, nitrogen and phosphorous profiles
during a SBR cycle after the automatic aeration control was implemented.
Vertical dot
lines indicate when the aeration was automatically stopped and black arrows
represent the
anoxic time gained by stopping the aeration. White arrows indicate feeding
times.
Figure 15 is a photograph of a lab-scale SBR unit as used in the experiments
as
described in the examples.
Figures 16A to 16C show nitrogen and phosphorous removal from abbattoir
wastewater over almost 7 months operation of a three step pilot scale feed
sequencing
batch reactor (SBR) process using a gentle, uniformly distributed non-stirred
bottom
feeding system according to the invention: A. - Treated wastewater effluent
nitrogen (as
ammonium species -=- and NOx species - ^) and phosphorous (as P04 - =). B.-
Nitrogen removal: = soluble inorganic nitrogen removal, as a percentage; and o
total
nitrogen removal , as a percentage. C.- Phosphorus: = removal of phosphorous
(as
phosphate), as a percentage; and o total phosphorous removal , as a
percentage.
Figures 17A to 17C show nitrogen and phosphorous removal from abbattoir
wastewater over almost 7 months operation of a three step pilot scale feed
sequencing
batch reactor (SBR) process using a bottom feeding system according to the
invention
(employing mixing and aeration): A. - Treated wastewater effluent nitrogen (as
ammonium species -=- and NOx species - ^) and phosphorous (as P04 - =). B.-
Nitrogen removal: = soluble inorganic nitrogen removal, as a percentage; and o
total
nitrogen removal , as a percentage. C.- Phosphorus: = removal of phosphorous
(as
phosphate), as a percentage; and o total phosphorous removal , as a
percentage.
Figure 18 shows NOx species concentrations and phosphorous (as phosphate)
concentrations) and pH profile for a representative cycle of the SBR process
represented
in Figures 16A to C, carried out on 3 September 2007.
Figure 19 shows NOx species concentrations and phosphorous (as phosphate)
concentrations) and pH profile for a representative cycle of the SBR process
represented
in Figures 17A to C, carried out on 3 September 2007.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
7
Abbreviations and Definitions
The following abbreviations are used herein:
AOB ammonia oxidising bacteria
BOD biochemical oxygen demand
COD chemical oxygen demand
DO dissolved oxygen
EBPR enhanced biological phosphorous removal
FOG fat, oil and grease
GAO glycogen accumulating organism
HRT hydraulic residence time
MLSS mixed liquor suspended solids
N nitrogen
NH4 ammonium
NO2 nitrite
NO3 nitrate
NO, sum of nitrate and nitrite
NOB nitrite oxidising bacteria
OUR oxygen uptake rate
P phosphorous
P04 phosphate
PAO polyphosphate accumulating organism
PHA polyhydroxyalkanoate
SBR sequencing batch reactor
SRT sludge retention time
TKN total Kjedahl nitrogen
TP total phosphorous
TSS total suspended solids
VFA volatile fatty acid
VSS volatile suspended solids
As used herein, the term "comprising" means "including principally, but not
necessarily solely". Variations of the word "comprising", such as "comprise"
and
"comprises", have correspondingly similar meanings.
As used herein, the term "phosphate accumulating organism" means any organism
capable of taking up phosphorus in excess of its metabolic requirements and
accumulating it intracellularly as a phosphate rich species.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
8
Detailed Description of the Invention
Efficient simultaneous removal of phosphorous and nitrogen from wastewaters
containing elevated amounts of nitrogen, such as abattoir wastewaters, which
contain at
least 100mg/L and often more than 150mg/L total nitrogen, and at least 20 mg/L
total
phosphorous in the form of phosphate and organic phosphorous is complicated by
the fact
that the high levels of NO,, resulting from nitrification of the biologically
available
nitrogen thwart phosphate uptake by polyphosphate accumulating organisms
(PAOs).
Another problem that faces treatment of such wastewaters is lack of BOD, and
particularly volatile fatty acids (VFAs) in the wastewater to be treated. PAOs
require
VFAs during an anaerobic period to store polyhydroxyalkanoates to provide
energy for
phosphate uptake during an aerobic period. Although raw abattoir wastewater
has a high
BOD due to elevated levels of fats oils and grease (FOG), these wastewaters
are typically
pre-treated to improve the settling properties of these wastes, resulting in
significant
depletion of biologically available carbon sources.
The present invention provides processes for simultaneous removal of BOD,
nitrogen and phosphorous from wastewaters having high N levels using a
sequencing
batch reactor (SBR) system employing an active biomass comprising nitrifying
and
denitrifying organisms as well as polyphosphate accumulating organisms.
In the current studies it has been found that a step-feed SBR scheme,
characterised
by alternating aerobic and anoxic phases in a SBR cycle allows timely removal
of nitrate
or nitrite so that, when an adequate amount of COD is available, nitrate or
nitrite build-up
is avoided.
A process of the present invention for at least significantly reducing the
levels of
nitrogen, phosphorous and BOD in a wastewater containing elevated total
nitrogen, such
as abattoir wastewater, involves feeding said wastewater into a reaction
vessel in at least
two separate steps. The wastewater may contain at least 100mg/L total
nitrogen, such as
at least about 150mg/L total nitrogen, at least about 200mg/L total nitrogen,
at least about
250mg/L total nitrogen, at least about 275mg/L total nitrogen, at least about
300mg/L
total nitrogen, at least about 325mg/L total nitrogen, or even at least about
350mg/L total
nitrogen. The total nitrogen content of the wastewater may be significantly
higher than
350mg/L - this may require feeding the wastewater into the SBR system in more
than
three feeds, allowing for longer process steps (such as nitrification and/or
denitrification),
reducing the volume of wastewater fed into the SBR system each cycle, or any
combination thereof.
The reaction vessel contains an active biomass including nitrifying and
denitrifying microorganisms and polyphosphate accumulating organisms (PAOs).
At
least the first feed step is followed by a rion-aerated period of sufficient
duration to result
in sufficiently low concentrations of NOX species in the wastewater to allow
for
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
9
accumulation of polyhydroxyalkanoates in the PAOs, thereby allowing for
phosphate
accumulation by PAOs in a subsequent aerated/aerobic period.
At least the first non-aerated period is followed by an aerated period of
sufficient
duration to allow for ammonium oxidation by the nitrifying organisms and
assimilation
by the PAOs of at least a portion of the phosphorous in the wastewater.
Depending on the
quality of effluent desired from the process, at least the first aerated
period may be of
sufficient duration so as to allow for substantially complete oxidation of
ammonium
introduced into the SBR system by the feed step. Subsequent aerated periods
may also be
of sufficient duration to allow for substantially complete ammonium oxidation
by the
nitrifying organisms after each feeding step.
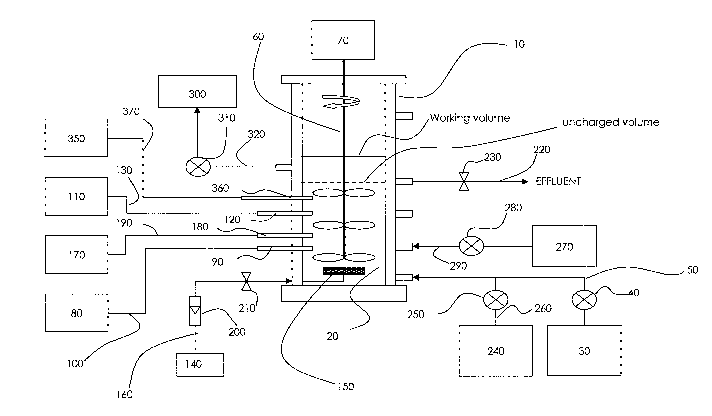
Referring to Figure 1, an einbodiment of a process according to the invention
may
be carried out in a sequencing batch reactor system comprising a reaction
vessel 10
containing a biologically active sludge 20.
In a first feeding step, a portion of wastewater to be treated is fed into
reaction
vessel 10 from wastewater reservoir 30 by pump 40 via conduit 50. The amount
of
wastewater to be fed may depend on the extent of P and N removal to be
achieved - if the
treated wastewater is to meet standards for discharge into waterways, the
wastewater may
be fed into the reaction vessel in three steps, or more. Although the amounts
of
wastewater fed at each stage may be the same, they may also be of increasingly
smaller
volume, increasingly larger volume, alternating larger and smaller volumes, or
any
permutation tliereof. However, a large final feed step may result in
significant NOX levels
in the reactor discharge, and therefore according to an embodiment feed steps
of
progressively smaller size are employed. In a specific embodiment, about 50%
of the
wastewater to be treated may be fed into the reaction vessel in a first feed
step, about 30%
in a second feed step, and about 20% in a third feed step.
Although the wastewater may be introduced into the reaction vessel in any
appropriate manner, feeding using the UniFEDTM process, as described in
international
patent publication WO 95/24361, or an adaptation thereof may be used. Briefly,
the
sludge in reaction vessel 10 may be allowed to settle before any or each
feeding step, and
feeding may comprise distributing the wastewater into the bottom of the
reaction vessel,
into the settled sludge, without aeration or stirring. This allows for
intensive contacting
of all biomass with the fresh feed stream entering the reactor, avoidance of
mixing of the
biomass with supernatant water from a previous process cycle, which often
contains
nitrates which can be detrimental to the performance of the phosphorous
removal
processes, and quickly established anaerobic conditions favourable to VFA
uptake by
PAOs.
The feeding step may be followed by a non-mixed, non-aerated period or, if the
feeding step (which is non-mixed, non-aerated) is carried out slowly, a
subsequent non-
mixed non-aerated period might not be necessary: due to efficient contact
between the
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
wastewater and settled sludge when feed is distributed into settled sludge, if
the feed rate
is sufficiently slow, all NOx species present in the settled sludge may be
denitrified, and
volatile fatty acids taken up by PAOs soon after the feeding step is
completed. Slower
feed rates also result in less disturbance of the settled sludge, and
therefore better contact
of the feed with the sludge.
`Sufficiently slow' feed rates may comprise inflow rates into reaction vessel
10 of
from about 20% to about 1% of the original, uncharged volume per hour, such as
from
about 15% to about 2% of the uncharged volume per hour, from about 12% to
about 4%
of the uncharged volume per hour, from about 10% to about 5% of the uncharged
volume
per hour, about 10% of the uncharged volume per hour, about 9% of the
uncharged
volume per hour, about 8% of the uncharged volume per hour, about 7% of the
uncharged
voluine per hour, about 6% of the uncharged volume per hour, or about 5% of
the
uncharged volume per hour.
After a sufficient non-mixed, non-aerated period or, if the feed step is
carried at a
sufficiently slow inflow rate, once the feeding step is over, the contents of
reaction vessel
10 may optionally be mixed by any appropriate means, without aeration. For
example,
mixing may be by an impeller 60 driven by motor 70.
During or after the feeding step, the concentration of NOX species in the
reaction
vessel contents may be monitored by monitoring the oxidation/reduction
potential (ORP)
and/or pH of the reaction vessel contents, by using an online NOX sensor, or
any
combination thereof. ORP may also be monitored to assess uptake of volatile
fatty acids
from the contents of reaction vessel 10 - as VFAs are taken up by organisms
from the
extracellular contents of reaction vessel 10, the ORP signal decreases, and as
the VFAs
are depleted from the extracellular contents of reaction vessel 10, the rate
of decrease of
the ORP signal slows and may plateau or even rise depending on the complexity
of the
contents of reaction vessel 10.
Oxidation/reduction potential may be assessed using an ORP meter 80
communicating by any appropriate means with an ORP probe 90 which is in
contact with
the contents of reaction vessel 10. ORP meter 80 may be connected by
conductive lines
100 to ORP probe 90.
pH may be determined using a pH meter 110 comiuunicating by any appropriate
means with a pH probe 120 which is in contact with the contents of reaction
vessel 10.
pH meter 110 may be connected by conductive lines 130 to pH probe 120.
The concentration of NO, (and oxygen) in the reaction vessel contents after at
least the first non-aerated period needs to be sufficiently low before uptake
of VFAs from
the extracellular medium and intracellular accumulation of
polyhydroxyalkanoates by the
PAOs (to provide energy for phosphate uptake during the subsequent aerobic
phase) will
occur. Once at least most of the VFAs have been depleted from the
extracellular contents
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
11
of reaction vessel 10, which may be determined by a break in declining ORP
slope
observed at ORP meter 80, a period of aeration may be started.
Alternatively, for example for a SBR process operating industrially for the
treatment of wastewaters, each cycle of wastewater treatment (that is, from
first feed to
treated effluent discharge) may be of a substantially fixed timing, for
scheduling
purposes. In such a case, at least the first non-aerated period, and possibly
other non-
aerated periods may be of a fixed length of time, which may be of sufficient
time to
ensure sufficiently low NOX concentrations and depletion of the VFAs (based on
the
ongoing performance of the SBR) before commencing an aerated period. For
example,
for a three feed step process having a cycle time of approximately 6 hours,
the first non-
aerated period may be fixed at, say, about 20 minutes to about 1.5 hours
duration
(depending on the ongoing performance of the SBR system), such as about 20
minutes,
about 25 minutes, about 30 minutes, about 35 ininutes, about 40 minutes, about
45
minutes, about 50 minutes, about 55 minutes, about 60 minutes, about 65
minutes, about
70 ininutes, about 75 mfnutes, about 80 minutes, about 85 minutes or about 90
minutes.
During an aerated period, air is pumped into reaction vessel 10 from blower
140
through aerator device 150 (such as, for example, an air diffuser), via
conduit 160. Other
possible aeration means/configurations, as are known in the art, such as a
surface aerator
(which does not require the use of a blower), may be used.
Although aeration may be uncontrolled, control of aeration may be required to
avoid excessive dissolved oxygen in the contents of reaction vessel 10, which
could
require a longer subsequent non-aerated period to provide sufficiently anoxic
conditions
for subsequent PHA storage by PAOs in the active sludge. In addition,
excessive DO
during, and particularly towards the end of an aerated period may promote
nitrate
accumulation, rather than nitrite accumulation. Removal of nitrogen using
complete
nitrification (to nitrate) consumes 33% more oxygen than oxidation to nitrite
alone, and
overall carbon consumption for N removal via the nitritation/denitritation
process is about
40% lower than for the nitrification/denitrification process (see Figures 10A
and lOB).
Thus significant savings on aeration and BOD can be made by promoting N
removal by
nitritation/denitritation rather than nitrification/denitrification.
Thus, the amount of dissolved oxygen in the contents of reaction vessel 10 may
be
controlled during an aerated step. In order to do so, the dissolved oxygen
content of the
wastewater may be monitored through a DO meter 170 communicating by any
appropriate means with a DO probe 180 which is in contact with the contents of
reaction
vessel 10. DO meter 170 may be connected to DO probe 180 by conductive lines
190. A
flow meter 200 and/or a valve 210 may be used to monitor and/or regulate
aeration, and
may be positioned in line with conduit 160 to monitor and/or control the air
flow
respectively so as to maintain the DO level in the contents of reaction vessel
10 within
desired ranges. The valve 210 may be any appropriate type of valve capable of
providing
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
12
the type of air flow control desired, such as an on/off valve, or a mass flow
controller, and
may be in communication with a suitable controlling module, such as a
programmable
logic controller (PLC) unit, which may also be in communication with DO meter
170.
The controlling module may also be in communication with flow meter 200 for
feedback
control of air flow rate via valve 210. Alternatively, air flow rate may be
controlled by
other means, such as by appropriate control of blower 140 and monitoring of
air flow by
flow meter 200. In such an arrangement, DO meter 170, blower 140 and flow
meter 200
may be in comnlunication with a controlling module.
The contents of reaction vessel 10 may be mixed during the aerated step. This
may be achieved by any appropriate means known in the art. For example, mixing
may
be achieved by the aeration itself, or as well as by an impeller 60 driven by
motor 70.
The DO level in the contents of reaction vessel 10 may be maintained at any
desired level during the aerated period. However, to facilitate rapid
achievement of
anoxic/anaerobic conditions before or during a subsequent feeding step and/or
to promote
nitritation/denitritation rather than nitrification/denitrification, dissolved
oxygen levels
may be maintained at limiting levels throughout an aerated step. Thus,
according to an
embodiment the DO levels in the contents of reaction vessel 10 are maintained
at a level
between about 5mgO2/L and about 0.lmgO2/L, such as between about 4mgO2/L and
about 0.1mgOa/L, between about 4mgO2/L and about 0.3mg02/L, between about
3mgOa/L and about 0.5mg02/L, between about 3mgOa/L and about lmgO2/L, between
about 3mgO2/L and about 1.5mg02/L, or between about 2mgO2/L and about
1.5mg02/L
In an alternative aeration regime, the first aerated period may be followed by
at
least one cycle of a feed period and an aerated period during which the level
of dissolved
oxygen is controlled to allow for simultaneous nitrification and
denitrification in the
contents of said reaction vessel. This is possible as, if the dissolved oxygen
level (DO) is
kept low enough, anoxic zones may develop within the reaction vessel 10, such
as within
flocs or other aggregates that may form in the contents of reaction vessel 10,
allowing for
NO,t reduction within those zones, and ammonium oxidation within oxic zones.
Suitable
DO levels at which this may be achieved, if suitable aeration monitoring and
control is
available, may be from about 1mgO2/L to about 0.1mgO2/L, about 0.8mg02/L to
about
0.2mgO2/L about 0.8mgO2/L to about 0.3mg02/L about 0.7mg02/L to about
0.3mgO2/L
or about 0.5mgO2/L to about 0.3mgO2/L.
The duration of an aerated period may be determined based on the average rate
of
change of the pH in a moving window of the mixed liquor. The pH of the
contents of
reaction vessel 10 typically increases quickly as soon as aeration is
introduced but then
decreases due to ammonium oxidation until nitritation is complete, after which
the pH
starts to rise again or decrease more slowly. This turning point is referred
to as the
ammonia valley (the point at which substantially all ammonium has been
oxidised),
characterised by a reduction in rate of pH decrease, possibly followed by a pH
increase.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
13
Thus an aerated period may be completed when the ammonia valley for the
contents of
reaction vessel 10 is approached or has passed.
If aeration is allowed to continue beyond the ammonia valley, accumulation of
nitrate at the expense of nitrite may occur in reaction vessel 10. Thus, if N
removal by
nitritation/denitritation is to be promoted rather than
nitrification/denitrification, the
aerated period may be ended once the ammonia valley is being approached or has
just
passed, and therefore may be ended when the rate of change of pH of the
contents of
reaction vessel 10 has reached to a predeterinined value. The predetermined
value may
be, for example, a rate of decrease of pH which is about 20% or less of the
maximum rate
of decrease observed earlier in the same aerated period (not having regard to
any pH
changes observed immediately after introduction of aeration, such as within 5-
10 minutes
after introduction of aeration, such as about 20% or less, about 15% or less,
about 10 / or
less, about 8% or less, about 6% or less, about 4% or less, about 2% or less,
or about 0%
of the maximum rate of decrease observed. Alternatively, the predetermined
value may
be an absolute value for the rate of change of pH of the contents of reaction
vessel 10,
such as a rate of pH decrease of about 0.05pH units or less per five minutes
(not having
regard to any pH changes observed immediately after introduction of aeration,
such as
within 5-10 minutes after introduction of aeration), such as a rate of pH
decrease of about
0.04pH units or less per five minutes, 0.03pH units or less per five minutes,
0.02pH units
or less per five minutes, 0.01pH units or less per five minutes, or OpH units
per five
minutes, but this value may differ widely for a given active sludge
composition. The
predeterinined value may also comprise a positive rate of change of pH, such
as the first
sign of a positive rate of change of pH of the contents of reaction vessel 10,
or soon
thereafter (again, not having regard to any pH changes observed immediately
after
introduction of aeration, such as within 5-10 minutes after introduction of
aeration).
Alternatively, or as a complimentary mechanism for detection of the end-point
of
an aerated period, the duration of the aerated period may be determined based
on the
oxygen uptake rate (OUR) of the contents of reaction vessel 10 - when
nitrification is
complete, oxygen demand by the active sludge decreases markedly - a point also
known
as the `DO elbow'. The oxygen uptake rate may be estimated by any appropriate
method
as is known in the art. For example, OUR may be estimated by the amount of
aeration
required to maintain the DO level at a given value, or within a given range of
values.
Alternatively, if valve 210 is an on/off valve, the OUR may be indirectly
estimated by the
amount of time valve 210 is in an "off' state (this time is inversely
proportional to the
OUR). End of nitrification may also be detected by a sudden rise in DO in the
contents of
reaction vessel 10, especially if constant aeration is employed using a
variable throughput
valve 210.
In addition, oxygen demand during oxidation of nitrite to nitrate is lower
than
oxygen demand during oxidation of ammonium to nitrite, and this can be
detected as a
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
14
drop in OUR as well. Thus, an aerated period may be stopped when the oxygen
uptake
rate of the contents of reaction vessel 10 drops to or below a predetermined
value. The
predetermined value may be, for example, an OUR which is about 80% or less of
the
maximum OUR observed earlier in the same aerated period (not having regard to
any
OUR values observed immediately after introduction of aeration, such as within
5-10
minutes after introduction of aeration, such as about 70% or less, about 65%
or less, about
60% or less, about 55% or less, or about 50% or less of the maximum OUR
observed.
Alternatively, the predetermined value may be an absolute value for the OUR,
such as
about 1.5 mg02/min/L, about 1.2 mgOa/min/L, about lmgO2/min/L, about 0.9
mg02/min/L, about 0.8 mg02/min/L, about 0.7 mg02/min/L, about 0.6 mg02/min/L,
or
about 0.5 mg02/min per litre of the contents of reaction vessel 10, but this
value may
differ widely for a given active sludge composition.
As the nitritation and/or nitrification endpoint is approached, reached or
passed,
aeration may be stopped, and the contents of reaction vessel 10 optionally
mixed without
aeration prior to carrying out the second step of wastewater feed into the
reaction vessel
10. If nitrogen removal by the nitritation/denitritation pathway is to be
promoted,
aeration may be stopped once the nitritation endpoint is approached or
reached.
Without wishing to be bound by theory, it is believed that by turning off
aeration
as soon as nitritation is complete, or nearing completion, nitrite oxidising
bacteria (NOBs)
are limited for nitrite, and therefore being disadvantaged compared to
ammonium
oxidising bacteria (AOBs). Over many cycles, this may lead to washing out of
the NOB
population within an active sludge, which in turn is believed will
strengthen/further
promote the nitritationldenitritation pathway (that is, reduce the amount of
nitrate
produced, and subsequent need for denitratation) from within the sludge. This
in turn will
return reduced aeration and COD requirements/costs, as described previously.
Second and third, and optionally further cycles of feeding, non-aerated
periods
and aerated periods may be carried out substantially as described above for
the first feed
step, although the feed may be introduced while the contents of reaction
vessel 10 are
being mixed.
After the final aerated period is completed, the biomass and at least a
portion of
the treated wastewater may be separated by any appropriate means known in the
art, such
as by filtration, centrifugation or settling and decanting/discharging the
supernatant.
Where the active sludge is to be re-employed for several consecutive processes
according
to the invention, the contents of reaction vessel 10 may be allowed to settle
after the fmal
aerated period, and supernatant, treated wastewater decanted via conduit 220,
controlled
by valve 230.
In order to control the level of solids/sludge in the SBR over a number of
cycles or
processes according to the invention, at least a portion of the contents of
reaction vessel
may also be removed as waste during each cycle, or between cycles by any
appropriate
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
means, such as by pump 310 via conduit 320 to waste receiver 300. Control of
the solids
in the SBR is necessary as excessive solids accumulation, as a result of, for
example,
multiplication of organisms within the contents of reaction vessel 10, may
result in
excessive settling times before decanting treated waste and/or a first feed
step of a
subsequent treatment cycle. In addition, solids removal from the system is
also necessary
for removal of phosphorous accumulated in the PAO population. Insufficient
solids
retention may result in washout/depletion of the organisms required for the
treatment
process. The amount of wastage during or between cycles may depend on the
temperature at which the process is carried out, and may be determined so as
to allow a
sludge retention time (SRT) of from about 5 days to about 30 days. A lower SRT
may be
applicable when organisms have higher specific growth rates (shorter doubling
times) due
to for example a high temperature, while a longer SRT may be required when the
specific
growth rates of the microorganisms required have lower specific growth rates
caused by,
for example, a lower temperature. Under normal operating conditions (such as a
temperature of about 20 C), the SRT may be from about 10 to about 20 days,
such as
about 15 days.
For a given SRT, which is determined by the specific growtli rates of
microorganisms, the hydraulic retention time (HRT - the average time that a
soluble
compound remains in the reaction vessel 10, or reactor volume/influent flow
rate) may be
designed such that the resulting sludge concentration in the reactor would
have a
reasonable settling rate, for example, so as to allow decanting of treated
wastewater to
start after 30min - 1 hour settling. Typically, the higher the sludge
concentration is, the
longer the settling time required would be. For a given SRT, the sludge
concentration in
a reactor is determined by two factors, namely HRT and the solids and COD
concentrations in the wastewater. The shorter the HRT is, the higher the
sludge
concentration in the reactor will be. The higher the COD and solids
concentrations in the
wastewater are, the higher the sludge concentration in the reactor will be.
Nitrogen
supports the growth of nitrifiers and therefore has some impact on the sludge
concentration as well. However, nitrifiers typically represent a small
percentage of the
bacterial population in treatment systems receiving wastewaters containing
high levels of
COD and solids such as domestic and abattoir wastewaters.
For treatment of wastes with high nitrogen loads (such as from about 200mg/L
nitrogen or higher, the HRT may vary from about 24 hours to about 72 hours,
such as
about 24 hours, about 30 hours, about 36 hours, about 42 hours, about 48
hours, about 54
hours, about 60 hours, about 66 hours or about 72 hours. According to a
specific
embodiment, the HRT is about 42 hours or more, especially if the nitrogen
levels are
250mg/L or higher. The HRT also may need to be balanced against a target SBR
cycle
schedule - when using an SBR process, the HRT is directly related to the
length of each
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
16
cycle. Increasing the SBR cycle time will increase the HRT which means that
less
wastewater is treated per day.
The treated wastewater resulting from a process as described above may
comprise
less than about 2mg/L total phosphorous and less than about 20mg/L total
nitrogen and,
with proper tuning of the system, may produce effluent comprising less than
about lmg/L
total phosphorous and less than about 10-15mg/L total nitrogen, which would
meet most
Australian standards for discharge into waterways. Total phosphorous in
effluent
obtained from such processes of the present invention may be expected to even
be lower
than about 0.8mgP/L, such as less than about 0.6mg/L, less than about 0.5mg/L,
less than
about 0.4mg/L, less than about 0.3mg/L, or less than about 0.2mg/L. Total
nitrogen in
effluent obtained from processes of the present invention may be expected to
even be
lower than about 10mgN/L, such as less than about 9mg/L, less than about
8mg/L, less
than about 7mg/L, less than about 6mg/L, or less than about 5mg/L.
In contrast to waterway discharge, although disposal of wastewaters by land
irrigation requires a high level of biological oxygen demand (BOD) removal
(>95%),
only medium levels of nitrogen and phosphorus removal are required. The
presence of
total phosphorus at a level of up to about 10-20mgP/L and total nitrogen,
preferably
mostly in the form of ammonium, at up to about 50-100mgN/L in the treated
effluent is
considered appropriate for this purpose. To meet these objectives, a process
according to
the invention may produce effluent with some presence of nitrogen (primarily
ammonia
nitrogen) and phosphorus. Such a process may be effectively similar to that
described
above, although only two feed steps are required, and an aerated period after
the second
non-aerated period is only optional, as phosphorous removal is not as
important. If total
nitrogen in the process effluent is to be predominantly ammonium, any aeration
after the
second feed step may be kept to a minimum, although a brief aeration step may
be
desirable to strip the effluent of any nitrogen gas formed by denitrification,
and thereby
improve the settling properties of the sludge in reaction vessel 10. The
treated wastewater
resulting from such a process will typically comprise up to about 20mgP/L and
total
nitrogen at up to about 100mgN/L, such as less than about 50mg/L total
nitrogen and less
than about 15 mg/L total phosphorous. Total phosphorous in effluent obtained
from such
a process may be between about 10mgP/L and about 15mgP/L, although values
below
10mgP/L may occur. For example, total phosphorous in the resulting effluent
may be less
than about 12mgP/L, such as less than about lOmg/L, less than about 8mglL,
less than
about 7mg/L, less than about 6mg/L, or less than about 5mg/L. Total nitrogen
in effluent
obtained from such a process may be expected to be between about 20mgN/L and
about
50mgN/L, although values below 20mgP/L may occur. For example, total nitrogen
in the
resulting effluent may be less than about 40mg/L, less than about 35mg/L, less
than about
30mg/L, less than about 25mg/L, or less than about 20mg/L.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
17
BOD supplementation
Due to pre-treatment of raw wastewaters containing high FOG levels, there are
often insufficient carbon resources in the pre-treated wastewater for
efficient or complete
phosphorous uptake by PAOs or denitritation and/or denitrification by
denitrifiers.
To address this, a process according to the invention may comprise
supplementation of the wastewater to be treated, or being treated with a
source of COD,
such as VFAs (which are most readily used by PAOs for intracellular PHA
storage) when
the wastewater to be treated does not contain a sufficient amount of these for
biological
phosphorus and nitrogen removal.
For wastewaters comprising from about 200-300mg/L total nitrogen, if
necessary,
the wastewater being fed into reaction vessel 10 may be supplemented with
extra COD, or
a source of COD may also be added to reaction vessel 10, to provide a total
influent COD
(CODt) concentration of from about 1,000mg/L to about 3,000mg/L. This value
will also
depend on whether the process is using nitrification and denitrification
predominantly via
nitrate, or via the nitritation/denitritation pathway, which uses
approximately 40% less
carbon sources. In addition, if the PAOs utilised are capable of
denitrification as well as
phosphate accumulation (as appears to be the case for, for example, Candidatus
Accumulibacterphosphatis), further COD economies may be achieved.
The ratio of CODt to total influent nitrogen may be from about 5 to about 15,
such
as from about 5 to about 12, from about 5 to about 10, from about 6 to about
10, from
about 7 to about 10, from about 8 to about 10, from about 5 to about 9, from
about 5 to
about 8, or from about 5 to about 7.
For phosphorous removal from wastewater, VFAs are important, being a preferred
substrate for intracellular storage of PHAs by PAOs. For wastewaters
comprising from
about 30-50mg/L total phosphorous, if necessary, the wastewater being fed into
reaction
vessel 10 may be supplemented with extra VFAs, or a source of VFAs may also be
added
to reaction vessel 10, to provide a total influent VFA concentration of from
about
300mg/L to about 1,000mg/L, such as from about 350mg/L to about 900mg/L VFAs,
from about 350mg/L to about 800mg/L VFAs, from about 350mg/L to about 700mg/L
VFAs, from about 400mg/L to about 650mg/L VFAs, from about 400mg/L to about
600mg/L VFAs, from about 450mg/L to about 600mg/L VFAs, from about 450mg/L to
about 550mg/L VFAs, about 250mg/L VFAs, about 300mg/L VFAs, about 350mg/L
VFAs, about 400mg/L VFAs, about 450mg/L VFAs, about 500mg/L VFAs, about
550mg/L VFAs, about 600mg/L VFAs, about 650mg/L VFAs, or about 700mg/L VFAs.
VFAs typically make up the majority, but not all of soluble COD, and
therefore, if
considering soluble COD levels instead of VFA concentrations, the amount of
soluble
COD will be to be fed in an SBR process of the invention will be
commensurately higher
than the values provided above for VFAs.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
18
The ratio of total influent VFAs to total influent phosphorous may be from
about 5
to about 30, such as from about 10 to about 25, from about 12 to about 25,
from about 13
to about 20, from about 14 to about 18, from about 14 to about 17, from about
14 to about
16, about 14, about 15, about 16, about 17, about 18, about 19 or about 20.
A convenient source of VFAs may comprise pre-fermented raw wastewater.
Although the additional source(s) of COD/VFAs may be added to reaction vessel
in any appropriate manner and at any appropriate time, for ease of operation
and
timing of the various steps/periods during the process, including feeding
steps, non-
aerated periods and aerated periods, the additional COD/VFAs may be co-fed
into
reaction vessel 10, or may be added to the wastewater to be treated before
feeding into
reaction vessel 10.
Having reference to Figure 1, raw wastewater with a high BOD (such as raw
abattoir wastewater, with a high FOG level) may be pre-fermented and then held
in a
reservoir 240. The pre-fermented raw wastewater reservoir 240 may be linked to
wastewater conduit 50 via conduit 260 and co-fed into reaction vessel 10 by
pump 250
with the wastewater during a feed step. VFAs may be further supplemented
during a
process of the invention, if necessary, by pumping VFAs into reaction vessel
10 from a
VFA reservoir 270 via conduit 290 by pump 280, independently of wastewater
feeding.
The source(s) of volatile fatty acids may comprise elevated levels of acetic
and
propionic acids, such as at least 100mg/L of each of acetic and propionic
acids, and may
be co-fed into said reaction vessel with said wastewater at the desired ratio
to provide the
desired CODt: total nitrogen ration and VFA: total phosphorous ratio. For
example,
where a pre-fermented raw abattoir wastewater is used to supplement the
CODt/VFA of
anaerobic abattoir pond wastewater (which is typically low in CDOt and VFAs),
the ratio
of pre-fermented waste to abattoir pond wastewater may be from about 1:20 to
about 1:1,
such as about 1:15, about 1:10, about 1:8, about 1:7, about 1:6, about 1:5,
about 1:4, about
1:3, about 1:2 or about 1:1.
Excess use of pre-fermented high FOG waste should be avoided due to the
possibility of impaired settling ability of the resulting sludge.
Other process parameters
a) Organisms
i) PAOs
Polyphosphate accumulating organisms for use in active sludges in processes
according to the invention may be any appropriate known PAO, or combination of
PAOs.
The PAO(s) may be obtained from purified/isolated cultures, or may be part of
a
consortium of organisms enriched from naturally occurring sources, such as
wastes.
A non-exhaustive list of PAOs considered to be useful for the purposes of the
invention includes Actinobacteria and the Rhodocyclus group of organisms,
including
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
19
Candidatus Accuinulibacter phosphatis. The latter bacterium has also been
shown to be
capable of denitrification, and may be beneficial in further reducing carbon
requirements
in processes of the invention.
ii) Nitrifying and denitrifying organisms
Many nitrifying, nitriting, denitrifying and denitriting organisms are known
in the
art, and are typically present in wastewaters naturally. Any suitable
combination of such
microorganisms which will provide at least nitritation and denitritation in a
process
according to the invention may be used. Such microorganisms may be obtained
from
purified/isolated cultures, or may be part of a consortium of organisms
enriched from
naturally occurring sources, such as wastes.
A non-exhaustive list of nitrifying and denitrifying microorganisms considered
to
be useful for the purposes of the invention includes:
Nitriting organisms (ammonia oxidisers)
Nitrosomonas spp.
Nitrosococcus spp.
Nitrosospira spp.
Nitrosolobus spp.
Nitrifying organisms (nitrite oxidisers)
Nitrobacter spp.
Nitrospina spp.
Nitrococcus spp.
Nitrospira spp.
Denitrifying organisms (nitrate and nitrite reducers): a wide range of
facultative
anaerobes, including:
Achroynobacter spp.
Alcaligenes spp.
Conzomonas denitrificans
Eschericia spp.
Micrococcus denitf ificans
Pseudomonas spp. (such as P. aeruginosa)
Paracoccus spp. (such as P. denitrificans)
Serratia spp.
Thiobacillus spp. (such as T. denitrificans)
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
b) Temperature (see components 350, 360 and 370 in Figure 1)
The operating temperature for processes of the invention is not crucial, but
may be
kept below 40 C, as many of the bacteria important to the process may perish
at such
temperatures. The temperature may also be maintained above at least 5 C. For
practical
process turnover times, the temperature at which the process is carried out
may be at least
10 C, such as at least 15 C, at least 18 C, at least 20 C, at least 22'C at
least 24 C, at least
26 C, at least 28 C, at least 30 C, about 20 C, about 22 C, about 24 C, about
25, about
26 C, about 28'C, or about 30 C.
The temperature of the contents of reaction vessel 10 may be monitored at
temperature meter 350, communicating with temperature probe 350 by any
appropriate
means, such as conductive line 360. If necessary, reaction vessel 10 and its
contents may
be heated or cooled by any appropriate means known in the art.
c) pH Control
The optimum pH for Nitrosomonas and Nitrobacter is between 7.5 and 8.5 and
nitrification by these organisms has been reported to stop at a pH at or below
6Ø
However, there has also been a recent report of nitrification at pH 4Ø pH of
the contents
of reaction vessel 10 may be monitored as described previously. Although in
most cases
the pH of the reaction vessel contents will self-regulate to within pH values
at which the
biological processes necessary for processes of the present invention will
take place, if
necessary the pH of the reaction vessel contents may be adjusted by any
appropriate
means. For example, an alkaline agent, such as a carbonate or bicarbonate
salt, or even a
hydroxide, such as sodium hydroxide may be added to the reaction vessel
contents to
raise the pH if necessary, or an acid such as hydrochloric or sulphuric acids
may be added
to the reaction vessel contents to reduce pH. Such additions may be controlled
automatically by a controlling module, such as a PLC, in communication with pH
meter
110 and a pump controlling flow of acid or alkali from suitable reservoirs.
Preferred forms of the present invention will now be described, by way of
example only, with reference to the following examples, which are not to be
taken to be
limiting to the scope or spirit of the invention in any way.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
21
Examples
Example 1- Treatment of abattoir wastewater for discharge into
waterways
Reactor set-up and operation
A lab-scale SBR (set up as per Figure 1 and as described above. See also
Figure
15) with a working volume of 7L was used in these studies. The SBR was seeded
with
non-EBPR (enhanced biological phosphorus removal) sludge from a full-scale SBR
treating abattoir wastewater in Queensland, Australia. 1L of EBPR sludge (MLSS
around
4g/L) enriched in a lab reactor was added on Day 60 to initiate the EBPR
process in the
reactor as there seemed to be no EBPR organisms present in the initial seed
sludge used.
The SBR was operated with a cycle time of 6h in a temperature-controlled room
(18-
22 C). In each cycle, 1L of abattoir wastewater (more details given below) was
pumped
into the reactor over the three filling periods with a volume distribution of
0.5 L, 0.3 L
and 0.2 L respectively. Each filling period was followed by non-aerated
(either anoxic or
anaerobic depending on when the oxidized nitrogen was completely consumed) and
aerated periods (Table 1). During aerated periods, air was provided
intermittently using
an on/off control system to keep the DO level between 1.5 and 2mg/L. After the
settling
period, 1 L supematant was removed from the reactor resulting in a HRT of 42
h. 11 5mL
of mixed liquor was wasted every cycle resulting in a SRT of 15 days. The pH
in the
system was recorded, ranging between 7.1-7.9, but not controlled. The ORP
signal was
also recorded to give indications of the nitrate levels in the reactor during
the anoxic
periods. The reactor was mixed with an overhead mixer except during the
settling,
decanting and first filling periods.
Table 1: Operating conditions of lab-scale SBR (6h cycle)
HRT 1.75 days
=
SBR sequences
duration (min) DO levels (mg Q2/L)
Fill no-mix 1 5 -0
No-aerated mix 1 (anoxic or anaerobic*) 30 -0
Aerated mix 1 (no aeration in the last 5 min) 55 1.5-2
Fill mix 2 3 -0
No-aerated mix 2 (anoxic or anaerobic*) 70 -0
Aerated mix 2 (no aeration in the last 5 min) 35 1.5-2
Fill mix 3 2 -0
No-aerated mix 3 (anoxic or anaerobic*) 60 -0
Aerated mix 3 (sludge wasted at the end) 20 1.5-2
Settle 70 -0
Decant 10 -0
* when nitrate and nitrite depleted
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
22
Wastewater
The wastewater used in this study was from a local abattoir in Queensland,
Australia. At this site, the raw effluent passes through four parallel
anaerobic ponds
before being treated in a SBR for biological nitrogen and COD removal. The
anaerobic
ponds serve to reduce FOG and COD, and also to produce easily biodegradable
COD,
particularly VFAs, to facilitate the down-streain biological nitrogen removal.
The pond
from which wastewater was sourced for these studies (pond A) was under-loaded,
leading
to much lower COD and VFAs concentrations in comparison to other ponds (see
Table
2). Therefore, extra VFAs were added to this wastewater to simulate the higher
VFA
levels present in other ponds, as will be detailed in Table 3.
Raw wastewater and anaerobic pond effluent from the abattoir were collected on
a
weekly basis and stored at 4 C.
Raw wastewater was subjected to one-day pre-fermentation before being pumped
into the SBR with anaerobic pond effluent. The pre-fermentation was performed
in a
50L tank continuously mixed with a submersible pump. No inoculum was
introduced in
the pre-fermenter, and hence the microbial population present in the raw
abattoir
wastewater was used to carry out the fermentation. The temperature inside the
tank was
kept at 37 C by a heating probe, but would not require a special heating
system in a full-
scale installation due to the temperature of the abattoir raw wastewater
(typically around
40 C). The aim of this pre-fermentation step was to increase the level of easy
biodegradable COD, in particular VFAs, which is critical for bio-P removal.
The
characteristics of the pre-fermented raw wastewater and the anaerobic pond
effluent are
compared in Table 2.
Table 2. Characteristics of the different types of wastewater used in this
study. The
intervals represent the mid-95% range.
Parameter Pre-fermented raw Anaerobic pond A Anaerobic pond B
wastewater effluent b effluent
CODtotal (mg/L) 7460-9300 430-720 740-950
CODsoluble (mg/L) 2360-2840 205-245 440-531
VFAa (mg COD/L) 703-869 24-32 234-285
TN (mg/L) 271-317 218-262 240-262
NH4-N (mg/L) 139-160 207-224 220-226
TP (mg/L) 44-53 33-37 37-40
PO4-P (mg/L) 38-43 32-34 33-36
$ Acetate and propionate only
b Pond effluent used in these studies; additional acetate and propionate was
added (see Table 3) to simulate
Pond B effluent, which was non-accessible for wastewater collection on site.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
23
Table 3. Characteristics of the SBR influent during its nine-month operation.
Influent Parameters Day 0-30 Day 30-80 After day 80
Ratio VFA:TP 3.7 12.2 15.1
Ratio CODt:TN 5.5 8.7 12
% pre-fermented raw 15% 15% 25%
wastewater in influent
VFAs added to Pond A No Yes Yes
to simulate other ponds (250 mgCOD/L acetate, (250 mgCOD/L acetate,
and 100 mgCOD/L and 100 mgCOD/L
propionate) propionate)
Analyses
The ammonia (NH4+), nitrate (N03'), nitrite (NOZ ) and orthophosphate (P043"-
P) were
analysed using a Lachat QuikChem8000 Flow Injection Analyser (Lachat
Instrument,
Milwaukee). Total and soluble chemical oxygen demand (CODt and CODs,
respectively), total Kjeldahl nitrogen (TKN), total phosphorus, mixed liquor
suspended
solids (MLSS) and volatile MLSS (MLVSS) were analysed according to standard
methods (APHA (1995). Standard methods for the examination of water and
wastewater.
Washington, DC, American Public Health Association). VFAs were measured by
Perkin-
Elmer gas chromatography with column DB-FFAP 15m x 0.53mm x 1.0 m (length x ID
x film) at 140 C, while the injector and FID detector were operated at 220 C
and 250 C,
respectively. High purity helium was used as carrier gas at a flow rate of
17mL/min.
0.9mL of the filtered sample was transferred into a GC vial to which 0.1 mL of
formic
acid was added.
Fluorescence in situ hybridisation (FISH) was performed as specified in Amann
R. I. (1995) ("In situ identification of microorganisms by whole cell
hybridization with
rRNA-targeted nucleic acid probes" Molecular Microbial Ecology Manual.
Dordrecht,
Holland, Kluwer Academic Publications. 3.3.6: 1-15).
Oligonucleotide probes used in this study were: EUBmix for the detection of
all
Bacteria (Daims H., Bruhl A., Amann R., Schleifer K. H. and Wagner M. (1999),
"The
domain-specific probe EUB338 is insufficient for the detection of all
Bacteria:
Development and evaluation of a more comprehensive probe set", Systematic and
Applied
Microbiology. 22(3): 434-444); PAOmix for detection of Accumulibacter
(Crocetti G. R.,
Hugenholtz P., Bond P. L., Schuler A., Keller J., Jenkins D. and Blackall L.
L. (2000),
"Identification of polyphosphate-accumulating organisms and design of 16S rRNA-
directed probes for their detection and quantitation", Applied and
Environmental
Microbiology. 66(3): 1175-1182); GAOQ989 and GB_G2 for detection of
Coinpetibacter
(Crocetti G. R., Banfield J. F., Keller J., Bond P. L. and Blackall L. L.
(2002), "Glycogen
accumulating organisms in laboratory-scale and full-scale activated sludge
processes",
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
24
Microbiology. 148(11): 3353-3364; Kong Y., Ong S. L., Ng W. J. and Liu W.-T.
(2002),
"Diversity and distribution of a deeply branched novel proteobacterial group
found in
anaerobic-aerobic activated sludge processes", Environmental Microbiology.
4(11): 753-
757); DFlmix (TFO DF218 plus TFO_DF618) for detection of Cluster 1
Defluvicoccus
vanus-related Alphapyoteobacter=ia (Wong M. T., Tan F. M., Ng W. J. and Liu W.
T.
(2004), "Identification and occurrence of tetrad-forming Alphaproteobacteria
in
anaerobic-aerobic activated sludge processes", Microbiolog.y-Sgm. 150: 3741-
3748); and
DF2mix (DF988, DF1020 plus helper probes H966 and H1038) for detection of
Cluster 2
Defluvicoccus vanus-related Alphaproteobactef ia (Meyer R. L., Saunders A. M.
and
Blackall L. L. (2006), "Putative glycogen-accumulating organisms belonging to
the
Alphaproteobacteria identified through rRNA-based stable isotope probing",
Microbiology-Sgm. 152: 419-429). FISH quantification was performed as
described in
Crocetti G. R., Banfield J. F., Keller J., Bond P. L. and Blackall L. L.
(2002) ("Glycogen
accumulating organisms in laboratory-scale and full-scale activated sludge
processes",
Microbiology. 148(11): 3353-3364.
RESULTS
Figure 2 presents the influent and effluent COD, N and P concentrations, along
with the MLSS concentration in the reactor and its volatile fraction, during
the nine
months operation of the SBR. Also presented in Figure 2 is the fraction of
Accumulibacter-PAO in the system. Potentially competing glycogen accumulating
organisms (GAOs) Competibacter-GAO and the putative Defluvicoccus vanus-
related
GAO (Cluster 1 and 2) were negligible in this reactor (<1 % of the total
microbial
population at all time). According to the effluent and MLSS data (Figs. 2c and
2d), the
SBR reached a steady state around day 100. The study can be divided into two
periods:
the start-up period from day 0 to 100 and the steady state period from day 100
to 27,5.
Start up period (day 0 to 100)
Complete nitrification was achieved in the SBR after less than one week of
operation as shown by the absence of NH4+ in the effluent (Fig. 2c). However,
denitrification was incomplete and NOX accumulated in the reactor reaching 60
mgN/L in
the effluent during the first 30 days of operation (Fig. 2c). In order to
improve the
denitrification, more COD was needed during anoxic periods. Therefore, extra
VFAs (i.e.
acetate and propionate) were added to pond A effluent on day 30 in order to
simulate the
concentration in the other ponds (typically 250 mg COD/L acetate and 100 mg
COD/L
propionate). These additional VFAs improved the denitrification and the level
of NOX in
the effluent dropped to 15 mgN/L (Fig. 2c). The similar levels of P043'
measured in the
influent (Fig. 2a) and effluent (Fig. 2c) indicate that phosphorus removal was
negligible
during the first 60 days. P removal was likely limited by the slow development
of PAOs,
which was possibly inhibited by the level of nitrate present during most of
the time over a
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
cycle. The fact that non-EBPR sludge was used to seed the reactor could have
also
contributed to the slow development of PAOs.
After the introduction of 1 L lab-scale EBPR sludge enriched in Accumulibacter-
PAO on Day 60 (details of the culture can be found in Lemaire R., Meyer R.,
Taske A.,
Crocetti G. R., Keller J. and Yuan Z. G. (2006) "Identifying causes for N20
accumulation
in a lab-scale sequencing batch reactor performing simultaneous nitrification,
denitrification and phosphorus removal", Journal of Biotechnology. 122(1): 62-
72), P
removal improved dramatically, and consistent high-level of P removal was
achieved and
maintained since then. The process data suggests that the seeded PAOs managed
to
survive and develop in a very different environmental setting. This is
confirmed by the
FISH quantitation results (Fig. 2c) and the decrease of the organic fraction
in the biomass
due to poly-P storage in the PAO cells (Fig. 2d).
However, Figure 2c also shows that while P removal was improving, NO,,-
started
to accumulate again in the system. This was believed to be due to a shortage
of easily
biodegradable COD in the reactor as the PAOs and denitrifiers were now
competing for
the saine carbon sources. In order to further increase the amount of VFA
available for P
and N removal, the amount of pre-fermented raw wastewater in the influent was
increased
from 15% to 25% on day 80 resulting in a higher VFA:TP ratio and CODt:TN ratio
in the
influent (Table 3). Denitrification improved immediately and from day 100
onwards, less
than 10 N-mg/L was present in the effluent.
Steady state period (from day 100 to 275)
Following the addition of extra VFAs in the pond effluent on day 30, the
introduction of EBPR sludge in the reactor on day 60 and the increase of the
pre-
fermented raw wastewater proportion in the influent on day 80, the reactor
reached a
steady state around day 100 with excellent removal of COD, nitrogen and
phosphorus.
There was one interruption to the reactor operation between day 125 and 160,
when the
abattoir closed down and no wastewater could be supplied to the SBR (Fig. 2).
The
reactor performance quickly recovered (within four days) after the starvation
period, as
indicated by the low nutrient level in the effluent shortly after the normal
SBR operation
was resumed (Fig. 2c). The reactor biomass concentration decreased by 30%
during this
long starvation period but returned to its previous level after only 2 weeks
and then
remained relatively constant around 5g/L with an organic fraction fluctuating
between 0.7
and 0.75 (Fig 2d).
During the starvation period, the reactor was put into a`sleeping mode' in a
five-
week period when the abattoir, where the wastewater was sourced, was closed
down for
annual maintenance. The `sleeping mode' operation consisted of 15 minutes
aeration in a
6 hour SBR cycle, which has been shown to provide significantly lower decay of
nitrifying bacteria populations than anaerobic conditions only. The sludge was
allowed to
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
26
settle in the remaining time of the cycle. The decay rates for ammonia
oxidising bacteria
(AOB) and nitrite oxidising bacteria (NOB) were deterinined to be 0.0 17
day"1, and 0.004
day 1, respectively, through monitoring the ammonia- and nitrite-oxidation
rates over the
starvation period on a weekly basis. These decay rates were supported by the
FISH
(Fluorescence In-Situ Hybridization) quantification data. The decay rate of
the poly-
phosphate accumulating organisms (PAOs) could not be determined, but phosphate
release was observed throughout the five-week period. Three different
phosphate release
rates were clearly visible on the measured phosphate profile, suggesting
different
processes were dominating in the three periods. A resuscitation period with a
gradual
increase of the wastewater load was applied during the re-startup of the
reactor. The
performance of the reactor in terms of nitrogen and phosphorus removal fully
recovered
in just four days after the re-startup.
Table 4 details the SBR effluent quality after the starvation period, between
day
170 and 275. For comparison, the COD and nutrient levels in the influent are
also
presented in the table. The SBR process consistently achieved 95, 97 and 98%
of COD,
TN and TP removal, respectively. The remaining COD in the effluent could be
regarded
as non-biodegradable and represented about 5% of the total COD initially
present in the
influent. It was observed that the sludge velocity index (SVI) was relatively
high
throughout the study period, between 180 and 250 mL/gMLSS. This could have
partially
been caused by the remaining high fat/oil/grease content of the pre-fermented
raw,
.
wastewater. However, the suspended solids concentration in the effluent was
lower than
25mg/L at all times (data not shown).
Table 4. Influent and effluent characteristics between day 170 and 275 (n
represents the
number of samples analysed between day 170 and 275)
Parameter Influent (N=13) Effluent (N=32) Removal of
(mg/L) mid-95% range mean mid-95% range mean COD, N & P
CODt 2600-3120 2870 129-151 140
95 %
CODs 1150-1320 1240 114-127 121
TKN 236-277 256 5.3-7.7 6.5
N-NH4 196-215 206 0.2-0.8 0.5 97 %
N-NOx not detected 1.9-2.8 2.3
TP 38-41 40 0.7-1.4 1.0
98%
P-P04 35-38 37 0.04-0.09 0.06
Figure 3 shows the nitrogen and phosphorus transformations in a typical
process
according to the invention during the steady state period. At the end of each
aerated
period, NH4+ was fully oxidised, and the low level of NO,t accumulated was
then
removed in the following non-aerated period. It can be seen that a very low
level of NOX
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
27
was carried over to the next cycle, and was denitrified very quickly at the
beginning of
the first non-aerated period. P043- levels increased during each non-aerated
period due to
both anaerobic P release and wastewater feeding (containing approximately
40mgP/L),
but most P release occurred in the first non-aerated period. P043" was then
fully taken up
during the subsequent aerated periods.
Performance of the pre-fermenter
The impact of the one-day pre-fermentation perfonned on raw wastewater is
depicted in Figure 4. The overall VFA concentration more than doubled as a
direct result
of this pre-ferinentation. Acetate and propionate were the most abundant VFAs
in the
raw abattoir wastewater before and after pre-fermentation, with propionate
having a
slightly higher production rate than acetate. Also shown in Figure 4 is the
impact of pre-
fermentation on the NH4+ and P043" concentrations. While P043" concentration
stayed
constant, NH4+ concentration doubled due to partial mineralisation of the
organic
nitrogen, which represents around 75% of the raw wastewater total nitrogen.
One week
storage of the pre-fermented raw wastewater in the cold room at a temperature
of 4 C
affected VFAs levels more than nutrient levels, with a 20% reduction of
acetate and
propionate concentration.
DISCUSSION
Multi-feed strategy to promote biological P removal
Biological phosphorus removal from wastewaters containing a high level of
nitrogen, such as abattoir wastewater, is challenging. Large accumulation of
nitrate or
nitrite must be avoided in order to secure anaerobic conditions required by
the PAOs.
The use of a multi-feed strategy in this study aimed to limit the level of NOX
recycled to the anaerobic period. Figure 2 shows that the strategy was very
successful.
The NOX level was limited to below 8mgN/L throughout the cycle, despite the
high level
of NH4+ and organic nitrogen in the wastewater (over 250 mgN/L, see Table 4).
P release
occurred in all three non-aerated periods. According to the amount of
wastewater fed
over the 3 feeding steps in the SBR cycle (i.e. 0.5 L, 0.3 L and 0.2 L,
respectively), the
increases in PO¾3- concentration in the reactor directly attributable to the
wastewater
feeding were estimated to be 2.7, 1.5 and 1.OmgP/L, respectively. The P
release following
the three feeding periods was thus estimated to be 25.3, 4.5 and 1.0mgP/L,
respectively.
The considerably higher P release observed after the first feeding step
compared to the
second and third feeding steps indicates that the first non-aerated period is
crucially
important for P removal. This would not have been achieved if a high level of
NOX was
allowed to accumulate in the reactor.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
28
Pre-fermentation of raw wastewater
The performance of a biological nutrient removal system depends greatly on the
availability of easily biodegradable carbon sources in the wastewater,
particularly VFAs.
Considering the fact that it is difficult to control the VFAs content in large
anaerobic
ponds, a more controllable VFA source may be necessary for reliable biological
nutrient
removal from abattoir wastewater. In this study, a high-rate pre-fermentation
step was
demonstrated to be a cheap and effective option for providing sufficient VFAs
for N and
P removal where the wastewater to be treated has insufficient levels of these
carbon
sources. Table 3 shows that the VFA:TP ratio increased from 12.2 to 15.1 when
the pre-
fermented wastewater fraction in the SBR influent increased from 15% to 25% on
day 80.
This caused an immediate reduction in the nitrate level, with a drastic
improvement to the
reliability of P removal (Figs 2b and 2c). The results show that it is both
necessary and
practically feasible to include a high-rate pre-fermenter to generate VFAs
that may be
supplemented to the nutrient removal SBR when an inadequate amount of VFAs is
present in the pond effluent.
However, it should be highlighted that the use of raw wastewater should be
minimised. There is evidence suggesting that a high fraction of raw feed
deteriorates the
sludge settling properties (data not shown) likely due to its higher FOG
content compared
to pond effluent. An oversupply of carbon sources through this stream would
also
increase aeration costs and sludge production in the SBR system. Controlled
addition of
this stream using an on-line control system would be highly beneficial.
However, the
control of VFAs supplement to biological phosphorus removal systems in
accordance to
the actual demand for VFAs (varying with time) is still unresolved.
An alternative solution that is being investigated is to reduce the demand for
carbon sources by achieving nitrogen removal via nitrite instead of nitrate.
This strategy,
if successf-ul, would reduce the carbon demand for denitrification by 40%.
This would
therefore reduce the amount of additional carbon supply, which in turn will
also reduce
the overall oxygen requirement. Such an improvement would have significant
benefits
for the operation of large-scale wastewater treatment facilities. On-line
control systems
based on simple pH and DO signals are being developed to achieve this nitrite
pathway in
our lab-scale SBR and this is described in Example 3.
A further opportunity to reduce the demand for carbon sources is to enhance
denitrification by PAOs. It has been found that Accumulibacter-PAOs are
capable of
taking up phosphorous under anoxic conditions. This is particularly attractive
as the same
carbon could be used for both denitrification and P removal. However, the
exact
conditions necessary to promote this type of denitrification are still unclear
and further
investigations are needed.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
29
The low abundance of GAOs in the sludge
Competibacter-GAOs have been widely reported to be abundant in both lab-scale
EBPR reactors and full-scale EBPR plants. Surprisingly, in this study,
Coinpetibacter-
GAOs were scarcely present in the reactor, representing always less than 1% of
the total
microbial population. The Defluvicoccus vanus-related Alphaproteobacter=ia
organisms, a
new putative GAO recently reported in literature, was also found to be in very
low
abundance in the reactor.
Several factors may influence competition between PAOs and GAOs. For
example, pH has a significant impact on PAO and GAO competition with
Accumulibacter-PAOs possessing advantages over Competibacter-GAOs for
anaerobic
carbon uptake at relatively high pH (>8). The pH in the study fluctuated
between 7.1 and
7.9 during a cycle (uncontrolled), which is unlikely to have provided any
selective
advantages to PAOs over GAOs. The presence of nitrite in the anaerobic or
aerobic
periods may inhibit PAO activity, and could therefore enhance the presence of
GAOs in
the system. However, from the results, the presence of nitrite in the reactor
during all
three aerated periods and during the second and third non-aerated periods
apparently did
not promote the growth of Competibacter-GAOs. Some studies have also shown
that
better EBPR performance may be achieved at relatively low temperature (5-15 C)
due to
a shift in the microbial community from GAOs to PAOs. The temperature used in
this
study, controlled between 18-22 C, is very similar to many reactor studies
where GAOs
appeared to be a problem, and is therefore not believed to be a significant
contributor to
the low abundance of GAOs. A more likely reason for the limited growth of GAOs
in
this reactor could be the large fraction of propionate present in the influent
(propionate to
acetate COD ratio was 0.8) - propionate as a carbon source may provide
selective
advantage to PAOs. The pre-fermenter used in this study largely contributed to
the
increase of the propionate fraction. If this hypothesis is true, the operation
of the pre-
fermenter should be optimised to not only maximise the total amount of VFAs
produced
but also to control the VFAs composition and particularly the acetate to
propionate ratio.
CONCLUSION
A sequencing batch reactor system was demonstrated to effectively remove
nitrogen, phosphorus and COD from abattoir wastewater. This provides a more
cost-
effective and environmentally friendly alternative to chemical phosphorus
removal, which
is a common practice at present. Each 6h cycle contained three
anoxic/anaerobic and
aerobic sub-cycles with wastewater fed at the beginning of each
anoxic/anaerobic period.
The following conclusions are drawn:
= It is possible to achieve a high degree (>98%) of biological phosphorus
removal from
abattoir wastewater in the presence of high levels of nitrogen (200 -
300mgN/L)
while simultaneously substantially removing total nitrogen (>97%) and total
COD
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
(>95%). The concentrations of phosphate and inorganic nitrogen in the effluent
were
consistently lower than 0.2 P-mg/L and 8 N-mg/L respectively.
= The multi-step feeding strategy prevents high-level accumulation of nitrate
or nitrite,
and hence facilitates the creation of anaerobic conditions. The strategy is
strongly
reconnnended for practical use in the biological treatment of abattoir
wastewater.
= It is important to incorporate a high-rate pre-ferinenter as an integrated
component of
the nutrient removal system if the wastewater to be treated is deficient in
VFAs. This
stream, which contains a high-level of VFAs, is effective in providing
supplementary
carbon sources for both phosphorus and nitrogen removal.
Example 2- Treatment of abattoir wastewater for land irrigation
This project was designed to develop a strategy for SBR operation to produce
effluent with a quality suitable for land irrigation. The same multi-feed
concept as used
for producing effluent suitable for river discharge is used. The sequence of
operation is
however different.
Land irrigation requires a high level of biological oxygen demand (BOD)
removal
(>95%), and medium levels of nitrogen and phosphorus removal. The presence of
total
phosphorus at a level of 10-20 mgP/L and total nitrogen at a level of 50-
100mgN/L in the
treated effluent is considered appropriate for this purpose. Ammonia/ammonium
as
opposed to nitrate is the preferred form of nitrogen in the final process
effluent for land
application.
The SBR, inoculated in June 2005 with sludge from a local abattoir in
Queensland
and set up similarly to that described in Example 1, was operated over a 12
month period
from the beginning of June 2005 to August 2006, with a 2-step feed in
accordance to the
concept shown in Figure 5, and in 6h cycles (from June to mid September), 8h
cycles
(from mid September to mid March) and 4h cycles (from mid March to August),
with
operational conditions as detailed in Table 5.
The hydraulic residence time (HRT) was kept at 28 hours and the sludge
retention
time (SRT) at 15 days. Each cycle consisted of a non-aerated feeding period
allowing
phosphate release, an aerated period allowing nitrification and phosphorus
uptake, a non-
aerated feeding period for denitrification, a short aerated period, and a
settling and
decanting period. An aim of the design is complete removal of nitrate at the
end of the
second feeding period so that phosphorous release will not be inhibited in the
first feeding
period in the next cycle. The design was expected to produce effluent with
some presence
of nitrogen (primarily ammonia nitrogen) and phosphorus, with the levels
determined by
the volume exchange ratio for the second feeding period.
Figure 6A shows the influent and effluent concentrations of nitrogen, and
Figure
6B shows the same data for phosphorus removal, during the operation of the
SBR.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
31
Figure 7A illustrates the total and soluble COD (TCOD and SCOD) and Figure 7B
illustrates VFA concentrations (acetate and propionate) over the same period.
Table 5 Irrigation-SBR cycle confi urations.
Process stage SBR-Irrigation 6 h cycle 8 h cycle 4 h cycle
min min min
Feed 1 5 5 5
Anaerobic 40 50 30
Aerobic 130 100 65
Idle 3 3 3
Feed 2 5 5 5
Anoxic 160 267 112
Aerobic 0 0 3
Wastage 2 2 2
Settle 10 40 10
Decant 5 5 5
Total 360 480 240
As can be seen in Figures 6B and 7B, biological phosphorus removal in the
reactor was not very stable at the beginning because P removal was strongly
related with
the amount of COD in particular volatile fatty acids (VFAs) fed to the
reactor. Moreover,
the presence of nitrite in the aerobic phase of the reactor was also affecting
the P-uptake.
As can be seen from the results, good nitrogen removal was achieved soon after
the start up of the reactor but no phosphorus removal was observed. In the
early stages
nitrate and nitrite were present in the effluent of the system and also in the
anaerobic
period during the first few weeks of operation. As such, denitrifiers were
able to take up
the substrate, competing with the PAOs. In order to improve the nitrogen
removal and
also with the aim of removing the nitrate and nitrite from the effluent, the
anoxic part of
the cycle was extended and the feed pattern was changed, increasing the volume
of
wastewater added in the second feed (1.25L in the 6 and 8h cycle) and reducing
the
volume of wastewater in the first feed (0.75 L in the 6 and 8h cycle). This
was done to
have higher COD in the anoxic period to be used for denitrification. The
reactor
performance improved in terins of nitrogen removal achieving 87% of
efficiency. On the
other hand, phosphorous removal was achieved since the nitrate and nitrite
were not
present in the effluent. Nevertheless, biological phosphorus removal is a very
sensitive
process and when minor changes were implemented to improve the nitrogen
performance,
P-removal from the system was affected. After about two months, the system was
achieving 38% of P-removal.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
32
In the three months from July, efforts were focused on improving biological
phosphorus removal from the system, first of all trying to avoid the presence
of NO, at
the beginning of the non-aerated periods. When the wastewater presents a low
COD
(achieving values of 400ppm of COD and 120ppm of acetate) the reduction of
NO,, to
nitrogen gas could not be achieved completely, remaining at the end of the
cycle. Then,
in the subsequent cycle, most of the COD is consumed by the denitrifying
bacteria and
PAOs can not compete for the substrate, decreasing its ability to uptake
phosphate. In
October, the reactor achieved 90% of nitrogen removal and 85% of phosphorus
removal
with effluent concentrations of 18ppm NH4-N and 5ppm of P04-P, and was
typically
achieving the nutrient levels required for land irrigation (between 20 and
50ppm for
nitrogen, and between 10 and 15ppm for phosphorus) thereafter. This
achievement was
possible by extending the cycle time from 6h to 8h in order to have a longer
anoxic period
at the end of the cycle, so the slowly biodegradable COD could also be
consumed by the
denitrifying organisms.
Results of a cycle performed in the SBR reactor on 11 October 2005 are
illustrated
in Figure 8. In the three months to late December 2005, efforts were focused
on
improving the enhanced biological phosphorus removal (EBPR) performance in the
SBR
reactor, without affecting the nitrogen removal ability achieved since the
first month of
operation. Carbon was the limiting compound to achieve good P removal in this
reactor.
The ainount of COD, particularly the amount of VFAs, present in the pond
wastewater
was crucial in achieving nutrient standard levels required for land
irrigation, particularly
as the reactor was being operated with 100% pond wastewater, and no raw
wastewater
was being added to the influent.
As can be seen from Figures 7A and 7B, the continuous oscillations in the
amount
of COD and VFAs available in the pond wastewater were probably the reason for
the
varying P removal efficiency. Nitrogen removal seemed not to be significantly
affected
by these changes, possibly because the microorganisms involved in the
denitrification
process are more competitive for the substrate than the PAOs and the anoxic
period in the
reactor is long enough to allow these bacteria to degrade slowly biodegradable
COD.
Denitrifier diversity is also greater than PAO diversity. On the other hand,
PAO activity
is very dependent on the amount of VFAs available during anaerobic conditions.
So, if
the level of VFAs in the pond wastewater is low, the EBPR performance of the
system
may be directly affected.
Figure 9A illustrates cycle study data from the 7th of March, when the SBR was
working with an 8h cycle. An interesting observation is that all the ammonia
from the
system was converted to nitrite in the first aerobic period and no nitrate was
produced.
This process is known as nitritation. In the subsequent anoxic period, most of
this nitrite
was reduced to nitrogen gas during denitritation. Compared with full
nitrification, air
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
33
requirements for nitritation are 25% less while carbon requirements for
denitritation are
approximately 40% lower than for denitrification from nitrate.
Moreover, almost no P-uptake was observed. This was due to the concentration
of
nitrite present during the aerobic period inhibiting P-uptake by PAOs. To
overcome the
inhibitory concentrations of nitrite in the SBR, the cycle was reduced to 4
hours, and the
P-removal performance immediately recovered (Figure 9B).
Example 3 - Establishment and maintenance of nitritation/denitritation
by automatic aeration control
This study focussed on control of aeration to achieve nitrogen removal via
nitrite,
rather than nitrate (see Figures 10A and lOB). The control strategy was based
on the
slope of the pH signal and on the oxygen uptake rate (OUR). During an aerated
period,
the pH slope is calculated once the maximum pH has been reached and starts
decreasing.
The first condition to automatically stop aeration is met when the slope of
the pH
signal is lower than a minimum value entered by the operator.
The second condition is on the OUR. Due to the on-off aeration control system
employed for control DO in the reactor, the OUR is directly proportional to
the time the
02 valve is in an "off' state. The second condition is met when the valve is
off for longer
than the maximum time entered by the operator.
A third "safety" condition assuring a minimum aeration time of 15 min is
applied.
Figure 11 illustrates these 3 conditions during a typical aeration period. It
should
be noted that the condition on the pH slope is important, due to its
flexibility and its
practicability, and the 2"d and 3rd conditions were not conservative, playing
more of a
back-up security role to ensure that enough aeration was provided if the 1 St
condition was
met too early.
Reactor set-up and operation
A lab-scale SBR as described in Example 1 was operated with a cycle time of 6
h
in a temperature-controlled room (18-22 C). In each cycle, 1 L of abattoir
wastewater
(more details given below) was pumped into the reactor over the three filling
periods with
a volume distribution of 0.5 L, 0.3 L and 0.2 L respectively. Each filling
period was
followed by non-aerated (either anoxic or anaerobic depending on when the
oxidized
nitrogen was completely consumed) and aerated periods. During aerated periods,
air was
provided intermittently using an on/off control system to keep the DO level
between 1.5
and 2 mgO2/L. After the settling period, 1 L supematant was removed from the
reactor
resulting in a HRT of 42h. 11 5mL of mixed liquor was wasted every cycle to
keep a
constant SRT of 15 days. The pH in the system was recorded, ranging between
7.0 and
8.0, but not controlled. The ORP signal was also recorded to give indications
of the
nitrate levels in the reactor during the anoxic periods. The reactor was mixed
with an
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
34
overhead mixer except during the settling, decanting and first filling
periods. The SBR
cycle operation was controlled by a programmable logic controller (PLC - Opto
Control).
Wastewater
The wastewater used in this study was from a local abattoir in Queensland,
Australia. At this site, the raw effluent passes through four parallel
anaerobic ponds
before being treated in a SBR for biological N and COD reinoval. The anaerobic
ponds
serve to reduce FOG and COD, and also to produce some easily biodegradable
COD,
particularly VFAs, to facilitate the down-stream biological nitrogen removal.
Raw wastewater and anaerobic pond effluent from the abattoir were collected on
a
weekly basis and stored at 4 C. The raw wastewater was subjected to one-day
pre-
ferinentation before being pumped into the SBR (as per Example 1) to further
increase the
level of easy biodegradable COD, in particular VFAs, which is critical for bio-
P removal.
The characteristics of the pre-fermented raw wastewater and the anaerobic pond
effluent
are compared in Table 6.
The wastewater fed to the lab-scale SBR consisted of a mixture of anaerobic
pond
effluent and pre-fermented raw wastewater as shown in Table 7 further below.
The
modification of the fraction of raw pre-fermented wastewater used in the
influent did not
modify the overall N and P content of the influent due to the identical level
of N and P
present in both type of wastewater.
Table 6 - Characteristics of the different types of wastewater used in this
study. The
intervals represent the mid-95% range.
Parameter Pre-fermented raw Anaerobic pond
(mid-95% range) wastewater effluent b
CODtotal (mg/L) 7,460-9,300 930-1,220
CODsoluble (mg/L) 2,360-2,840 705-745
VFAa (mg COD/L) 699-797 548-601
TN (mg/L) 260-306 235-254
NH4-N (mg/L) 141-157 223-229
TP (mg/L) 44-50 36-39
PO4-P (mg/L) 37-42 34-35
a Acetate and propionate only
b Additional acetate and propionate were added to the anaerobic pond effluent
to mimic
the effluent of better operated, but physically inaccessible pond
Aerobic phase length control to promote nitrite pathway
The SBR was operated for approximately 18 months. Aerobic phase length
control for achieving N removal via nitrite was trialled in the last 13
months. The control
strategy employed was based on the slope of the pH signal and on the oxygen
uptake rate
(OUR). Figure 1 shows that the exact time of complete NH4+ oxidation in each
aeration
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
periods could be detected through the pH bending point and the sharp OUR drop.
During
each aeration period, the pH slope was calculated once the maximum pH has been
reached and started to decrease. The slope of the pH was detennined based on
pH values
in a 2 minute moving window. Due to the on-off control system of the DO in the
reactor,
the OUR was calculated during the time the oxygen valve was in an "off' state.
The
aeration length control strategy was based on three different criteria that
had to be met to
automatically stop the aeration:
= when the slope of the pH signal became less than a minimum value entered by
the operator. This was the main criterion and therefore the set-point was
relatively
aggressive;
= when the OUR became less than a minimum value entered by the operator
(typically 1.2 mgO2/L.min). This was only a safety condition and the set-point
were
not conservative giving more weight to the pH criteria; and
= when the aeration period length became greater than a minimum aeration time
entered by the operator (typically 15 min). This criteria was the less
conservative and
its role was to secure some aeration periods in the SBR if the pH and OUR
criteria
failed and were met too early.
The control of the length of each aeration period in the SBR to promote the
nitrite
pathway was implemented in 3 stages. In the first stage, from Day 160 to 340,
the
aeration control was performed manually by the operator. Based on the
observation of the
on-line pH and real-time OUR calculation, the length of each three aeration
period was
adjusted on a daily basis to ensure that the aeration was stopped immediately
after
complete oxidation of NH4+ in the SBR. Then, from Day 340 to 410, the manual
aeration
control was stopped and fixed aeration lengths that were longer than the time
required for
coinplete NH4+ oxidation were applied. The purpose of Stage II was to
deteriorate the
nitrite pathway previously established under Stage I by promoting the growth
of NOBs.
In Stage III (from Day 410 onwards), the automatic aeration length control was
implemented to re-establish the nitrite pathway. The abattoir closed down from
Day 480
to 525 due to annual maintenance. During this period, the SBR cycle operation
was
modified in order to preserve the reactor biomass as no wastewater was
available. As
also described in Example 1, to place the reactor into `sleep mode', the
sludge was
aerated and mixed for 15 min in each 6h cycle and was allowed to settle for
the rest of the
cycle.
RESULTS and DISCUSSION
Analyses were carried out as described in Exainple 1.
Effect of the aeration control strategy on the nitrite pathway
Figure 12a presents the level of nitrite pathway achieved in the SBR, measured
as
the average amount of NOZ produced (mgN/L) per NOX produced (mgN/L) during the
3
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
36
aeration periods, and the relative abundance of NOBs in the SBR throughout
Stage I,
Stage II and Stage III. Tests to identify the main NOB species present in the
SBR using
common FISH probes (i.e. NIT3 for Nitrobacter and NITSPA662 for Nitrospira)
showed
that only Nitrospira was present in the system (data not shown). Therefore,
the
quantification of the Nitrospira population was considered to be
representative of the total
NOB population present in the SBR. The reactor used in this study had been
already
running for 5 months performing high level of COD, N and P removal (Lemaire et
al.
submitted) before the aeration lengtll control was implemented. In that time,
no nitrite
accumulation was observed during the aerobic periods as depicted in Figure
12a.
During Stage I, the manual control of the length of each aeration periods
resulted
in a rapid accumulation of nitrite reaching 95% of the total amount of NOX
produced on
Day 280 (Figure 12a). This high level of nitrite pathway was maintained until
the start of
Stage II. During that second stage, the implementation of fixed aeration
periods
deteriorated the nitrite pathway previously established but not completely as
20% of NOa
accumulation was still observed 50 days after the start of Stage II (Figure
12a). The
implementation of the automatic aeration lengths control strategy during Stage
III resulted
in the recovery of the nitrite pathway in the SBR. However the level of
nitrite
accumulation increased at a slower rate than when the aeration length control
was
performed manually and only reached 85% after 150 days (including 50 days of
starvation period). The strategy consisting of stopping the aeration in the
SBR
immediately after NH4+ was oxidised was therefore successful in controlling
the level of
the nitrite pathway.
When comparing the level of nitrite pathway in the SBR and the Nitrospira
population dynamic it clearly appears that the nitrite pathway was achieve
through the
elimination or the reduction of the NOB population in the system. However,
some delay
could be observed between the level of nitrite pathway measured and the
abundance of
NOB. While the NOa accumulation decreased from 98% to 20% during Stage II, the
Nitrospira population only increased from 0.3% to 0.5%, but later increased to
1.2% of
the total bacterial population 40 days into Stage III (Figure 12a). The
presence of this lag
phase could be due to the complex dynamics involved in the NOB growth
processes when
the availability of their main energy source (i.e. N02 ) is modified.
Effect of nitrite pathway on the overall SBR performance
In order to demonstrate the benefit of the nitrite pathway in COD savings, the
COD concentration in the SBR influent was adjusted several times during the
experimental period through changing the fraction of fermented raw feed and/or
the VFA
content in the pond effluent. The resulting VFA concentration profile in the
SBR influent
along with nutrient levels in the effluent are shown in Figure 12b.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
37
From Day 160 to 250, the level of nitrite pathway increased from 0 to 95%
after
the length of the aeration periods were manually controlled. At the same time,
high levels
of COD, N and P removal were consistently achieved, respectively 95%, 97% and
98%.
Figure 12b shows that, as the level of nitrite pathway increased the amount of
NO,t in the
effluent decreased. This was likely the consequence of the amount of COD saved
via the
nitrite pathway that enhances the subsequent denitrification process where
influent was
step-fed into the SBR instead of adding an external carbon side-stream. The
period
between Day 250 and 280 with stable high level of nitrite pathway is referred
as "Period
A" and is later described in Table 7.
From Day 280 to 310, the VFA concentration in the SBR feed was gradually
decreased by first, reducing the fraction of pre-fermented raw wastewater in
the influent
from 25% to 15% and then, gradually reducing the VFA concentration in pond
effluent by
40%. The reduction of the raw wastewater fraction lowered the amount of FOG
and
colloidal matter, which are detrimental to good sludge settling properties.
The bio-P
removal was immediately affected due to this sudden VFA shortage but soon
recovered
(Figure 12b). As the amount of VFA was further reduced, NOX started to
accumulate in
the effluent on Day 300 due to incomplete denitrification. The accumulation of
NOX is
very detrimental to bio-P removal as it prevents anaerobic periods to occur in
the SBR.
As a result, PO¾3- started to accumulate soon after and the amount of VFA had
to be
adjusted to provide sufficient amount for both N and P removal (Figure 12b).
The stable
period from Day 310 and 340 is referred as "Period B" and is later described
in Table 7.
With the implementation of fixed length aeration control on Day 340, the
effluent
NOX and P levels deteriorated considerably, likely due to the gradual
conversion from the
nitrite to nitrate pathway. Therefore, more VFAs had to be supplied to ensure
that NOX
and P in the effluent were kept at sufficient low concentration to avoid any
long term
damage of the SBR nutrient removal performance. It took about two weeks for
the level
of nitrite pathway to start decreasing following the end of the manual
aeration control and
the introduction of fixed aeration periods. On Day 370, the amount of VFA
added to the
pond effluent was further increased by 15% to compensate for the rapid
deterioration of
the nitrite pathway from 95% to 45% which trigger the accumulation of NOX in
the
effluent (Figure 12b). The stable period from Day 380 to 420 is referred as
"Period C"
and is later described in Table 7.
On Day 420, the fraction of pre-fermented raw wastewater and the amount of
extra VFA were both reduced (by 5% and 30% respectively) following the
implementation of the automatic aeration control strategy and the increase of
the degree
of nitrite pathway. Once again, NOX and P043" immediately accumulated in the
effluent
due to the sudden VFA and COD shortage but promptly recovered (Figure 12b).
"Period
D" ranges from Day 540 to 600, after the normal wastewater load was resumed in
the
SBR following the long starvation period.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
38
Table 7 - Comparison of the degree of nitrite pathway, the influent
composition and the
effluent quality in four distinctive periods during the 15-month study.
Parameter Period A Period B Period C Period D
(mid-95% range) Day 230-280 Day 310-340 Day 380-420 Day 540-600
NOZ accumulation (%) 81-92 87-99 29-47 78-84
NOX effluent (mgN/L) 0.9-1.6 2.9-6.5 5.4-8.3 1.5-2.6
P04 " effluent (mgP/L) 0.06-0.20 0.01-0.07 0.1-3.6 0.05-0.13
% pre-fermented raw in 25% 15% 15% 10%
SBR influent
Total VFAs in SBR 579-632 450-531 710-813 540-571
influenta (mgCOD/L)
a Includes additional acetate and propionate
The four distinctive stable periods defined earlier are compared in Table 7 in
terms of degree of nitrite pathway, influent composition and effluent quality.
The overall
amounts of COD and VFAs in the influent were considerably reduced during
"Period B"
while the average NOX and PO43- levels in the effluent were kept at reasonable
low levels
even if they were higher than in "Period A" (Table 7). In addition, Table 7
shows that
during "Period C", while the level of nitrite pathway was low, the N removal
deteriorated
compare to that in "Period B" even if the amount of VFA in the influent was at
its
highest. This demonstrates the importance of the nitrite pathway in saving COD
and also
in enhancing the nutrient removal performance. At the same time, it is
important to
consider both N and P removal when assessing the possible COD and/or VFA saved
via
the nitrite pathway in a BNR system as P removal depends strongly on the level
of N
removal achieved. The reduction of the COD and VFAs amounts in the influent in
"Period D" did not forfeit the overall N and P removal which indeed improved '
considerably as shown by the low levels of NOX and PO43" in the effluent
reported in
Table 7.
The aeration control strategy was successful in achieving stable N removal via
the
nitrite pathway which clearly benefited the nutrient removal performance of
the SBR by
efficiency in use of COD and VFAs. As a direct result, the fraction of pre-
fermented raw
wastewater in the influent was reduced from an initial 25% to 10% without
affecting the
performance of the SBR. The steep-feed strategy employed in this SBR ensured
that no
external carbon addition was needed to carry out the post denitrification or
denitritation
making the overall BNR process more attractive.
Aeration length control strategy based on pH and DO signals
SBRs usually operate with fixed time lengths for the different phases of
filling,
mixing (anaerobic, aerobic or anoxic), settling and decanting. Due to influent
fluctuation
and system state variations, it is beneficial to operate a SBR process with
varying phase
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
39
lengths. Therefore, higher levels of process control and automation may be
necessary to
optimise the SBR operation. ORP, DO and pH provide means to detect the end of
the
nitrification process, via the "ammonia valley" (pH), the "DO elbow" (DO) or
the
"nitrogen break point" (ORP) and the denitrification process, via the "nitrate
knee" (ORP)
or the "nitrate apex" (pH). In particular, it is possible to achieve nitrite
pathway in a SBR
by stopping the aeration as soon as NH4+ was oxidised and start the addition
of external
carbon (glucose) for the denitritation. In this study, we integrated the
control strategy to a
more complex system (i.e. COD, N and P removal process) where denitrification
was
performed through a step-feed strategy suppressing the need of external carbon
dosage.
Figure 11 clearly demonstrates the simultaneity between the depletion of NH4+,
the "ammonia valley" and the OUR drop symbolising the "DO elbow" during a SBR
cycle where the aeration lengths were not controlled. The aeration length
control strategy
~ previously described could therefore be implemented in this complex BNR
system.
Figure 14 presents the pH, DO, OUR, nitrogen and phosphorus profiles in a SBR
cycle
after this automatic aeration length control strategy was implemented. This
strategy was
reliable in detecting the end of the nitritation process and stopping the
aeration as
indicated by the dot lines on Figure 14. The success of this control strategy
was further
confirmed by the good long-term performances of the SBR presented previously.
However, some technical issues and possible improvement of the control
strategy
were identified. The pH and OUR profiles in each of the three aeration periods
were
quite different (Figure 14) making it difficult to design a universal
algorithm for all three
aerobic periods based on pre-determined absolute values. The pH profile also
changed
over time as shown by the difference observed between that depicted in Figure
11 and in
Figure 14. This was mainly due to the large difference of the initial pH value
observed at
the start of each aeration period but also to the unbalanced PAOs activity
existing
between each aeration period, with most of the activity occurring in the first
aeration
period as indicated by the high P release and subsequent P uptake in Figure 5.
This
evolution over time of the pH and OUR profiles in the SBR suggests that it may
be
preferable to design an algorithm where the different control set-points (i.e.
pH slope
minimum value and OUR minimum value) are determined based on relative
threshold
values instead of absolute values pre-determined by the operator. These set-
points could
have been determined as a percentage (e.g. 20%, 10% or 5%) of the maximum pH
slope
and OUR values calculated in real-time once the aeration started. Such an
algorithm
would be straightforward to implement.
Example 4- Pilot plant studies
Pilot plant studies were undertaken to validate the laboratory-scale studies
described
above. Two sequencing batch reactors were set up at a local (Queensland,
Australia)
abattoir, in the wastewater treatment section. The main wastewater source, an
anaerobic
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
pond (AP) effluent, is typically variable in COD and an often insufficient
amount of
volatile fatty acids (VFAs). Accordingly, a pre-fermenter was employed to
provide a feed
stream supplement so as to increase the influent VFAs, especially for the
purpose of
phosphorous removal by PAOs.
Significant difficulties were encountered with the reliability and control of
an old
prefermenter existing on the site. Accordingly, a new prefermenter was
installed in
February 2007. This is a 7500 L polypropylene tank, with a mixer installed.
The mixer
runs for 3 min following feed batch preparation (off at all other times) and
serves to stir
the tank sufficiently to prevent accumulation of a significant crust of fat/
grease. DAF
effluent (pre-treated raw abattoir wastewater) was pumped through the
prefermenter at a
rate of 11 L/min in a cycle of 19 min on/ 41 min off in every hour, which gave
a nominal
HRT of 1.5 days on the days when fed. The prefermenter was not fed on
weekends,
when the abattoir does not slaughter cattle and cleaning takes place. This
avoided
diluting/ washing out the prefermenter aiid gave a true HRT of 2.2 days on a 7-
day basis.
Wastewater from the AP effluent overflow tank and from the prefermenter were
pumped
into a mixing tank periodically. This feed mixture was then pumped into
asynchronously
operated SBRs during feed periods in the cycles. The operating volume of each
SBR is
6000L.
The SBRs were connected to blowers connected to two variable speed drives
(Toshiba
VSF9 3.7kW), each having a maximum capacity of 164m3/hr at 50Hz. Sampling
valves
at varying heights on the tank sides are also located within the hut.
pH Probes (Burkett #8205) and differential pressure transducers (WIKA SL-1; 0-
60mbar)
were inserted into the tank sides. DO transmitters (Danfoss #OXY3000)
connected to
DO sensors (Danfoss OXY1100) were also inserted into the tanks, through the
top. A
PLC (Opto22 with Mistic Controller board), for controlling the whole pilot-
plant, was
also connected to the SBRs.
The two SBRs were operated identically except for the mode of feed delivery
during the
first feed period of each cycle. Both SBRs have a UniFed feed delivery system
(see Table
8 below). However, for SBR 2, the contents of the reactor were mixed (by 30
second air
burst) just prior to feed entering the reactor. This mixing did not occur for
SBR 1 on the
first feed addition (subsequent feed additions followed the same mixing as for
SBR 2).
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
41
Table 8- SBR feeding/mixing regimes
SBR 1 (UniFed) SBR 2
Distributed Feed Yes Yes
Mix during Feed 1 No Yes (air burst)
Mix during Feed 2 Yes (air burst) Yes (air burst)
Mix during Feed 3 Yes (air burst) Yes (air burst)
Note: Air burst is 30sec aerator on in every 15 min.
UniFed Feeding System SBR 1)
The Unifed system involves evenly distributing the feed through the sludge
blanket on the
horizontal plane. This was usually performed near the base of the tank. No
additional
mixing was employed during the feeding period and the total feeding period was
extended
for some time (i.e. a gradual flow) to achieve an anaerobic zone within the
sludge blanket.
Only the first feed period of the cycle for SBR 1 was operated as a
traditional UniFed
system witllout mixing. During subsequent feed periods the tank was mixed by a
short air
burst prior to feeding). This was employed because laboratory scale
experiments have
shown that only one of the feed periods for each cycle needs to reach
anaerobic
conditions to support the phosphorus release and uptake from polyphosphate
accumulating organisms. While mixing of the SBR contents will occur during the
2 d and
3rd feeding periods, an evenly distributed bottom feed delivery was still be
used for these
periods as the pipework was already present.
For both SBRs, the feed line divided into 4 vertical pipes with horizontal
sections
attached. Each horizontal length of pipe had two 0.9mm diameter holes drilled
into them
at 45 to the bottom.
Mixed Feeding S st~(SBR 2)
For SBR 2 the feed is delivered through a series of pipes as for the UniFed
feeding
system. However, the tank has an initial air burst mix during all feeds.
A mixing tank was used to mix the two feed sources, the prefermenter effluent
and the
anaerobic pond effluent. Initially the two feeds were mixed in the ratio of
90% AP
effluent: 10% PF effluent. This was subsequently been changed to 84% AP
effluent: 16%
PF effluent.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
42
Each SBR cycle had 3 feed periods to give a total feed volume of 857L per
reactor per
cycle (to give a HRT of 1.75 days). 50% of a cycle's feed occurs in the lst
feed period,
30% in the 2"a feed period and 20% in the final feed period. The respective
volumes
required for each feed stream and feed period are given in Table 9. The feed
for each
feed period for both SBRs will be prepared in single batches and the SBRs
operated out
of sync with each other by 20 minutes, minimizing the length of time the feed
mixture is
retained in the mixing tank. The asynchronous nature of the SBRs is due to the
decant
lines combining into 1 decant line and the flow needs to be sufficiently low
to handle the
flow.
Table 9: Volumes of Prefermenter feed and AP effluent feed required for feed
batches.
Prefermenter Feed AP Effluent Combined Feed
(16%) (84%)
Single Both Single Both Single Both
SBR SBRs SBRs SBRs SBR SBRs
Feed 1 69 138 361 722 430 860
Feed 2 41 84 216 432 257 514
Feed 3 27 54 143 286 170 340
Total 137 274 720 1440 857 1714
The mixing tank was a polyethylene tank with dimensions of 1.07m diameter and
1.51 m
wall height, located on a lm high platform due to possible flooding of the DAF
bunded
area. The mixing tank operating volumes for the 3 feed batches were under PLC
control
from specified volumes input from Table 8 (or similar).
A pump was assigned to each feed source (PF or APO/F tank). The volumes pumped
were critical and control of these were be achieved using an on-line pressure
transducer
(WIKA S-10; 0-0.lbar) attached to the mixing tank.
A Davey DC 10A (single phase) submersible pump was used to transfer the AP
effluent to
the mixing tank. The on-period of this pump needs to be low enough to ensure
complete
drainage of the anaerobic pond overflow does not occur (this is particularly
important for
the first feed period when the maximum volume of anaerobic pond effluent is
required)
and is dependent upon the flowrate from the pond due to the small size of the
anaerobic
pond overflow.
A Davey DC10A (single phase) submersible pump was located in the prefermenter
approximately 0.5m below the surface (supported on a steel frame).
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
43
A small on-line controlled, submersible pump (Davey DC10A, single phase) is
located in
the mixing tank and operated intermittently (during batch feeds preparation)
to mix the
two feed streams and ensure no settling of solids occurs. This is particularly
important for
the feeding of the second SBR which is delayed by 20 minutes, relative to the
first SBR.
Cycle Periods
Table 10 shows representative cycle periods and associated times used for
running the
SBRs, at least during early phases of the pilot plant trials. These periods
were loosely
based on the results from the laboratory-scale reactor with alterations of the
times set for
the settle, decant and feed periods. Following start-up of the pilot plant,
the cycle times
were adjusted to suit the observed nutrient removal patterns.
Table 10: Cycle periods for pilot-plant.
Period Length Purpose of Period
Settle 70 min Settle sludge
Decant 20 min Remove supernatant
Add feed to reactor. For SBR 1, provide
Feed 1 20 min anaerobic conditions for P release by
PAOs
Mixed, `Anoxic' period 1 15 min Denitrification/Anaerobic P release
Aerated period 1(max.) 64 min Nitritation
Idle 1 (mixed) 0 min Depletion of maximum aeration time
Feed 2 15 min Add feed to reactor.
Mixed, `Anoxic' period 2 30 min Denitrification/Anaerobic P release
(partial)
Aerated period 2 (max.) 45 min Nitritation
Idle 2 (mixed) 0 min Depletion of maximum aeration time
Feed 3 15 min Add feed to reactor.
Mixed, `Anoxic' period 3 30 min Denitrification/Anaerobic P release
(partial)
Aerated period 3 (max.) 36 min Nitritation/Sludge wastage (1 cycle/day)
Idle 3 (mixed) 0 min Depletion of maximum aeration time
Total 360 min 6h cycle period; 4 no. cycles per day
Settle
During the settling period, feed, WAS, decant and mixing pumps and the blowers
were
switched off to allow the suspended solids to settle, in preparation for
decanting. The
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
44
settle period was assigned a length of 70 min, which was found to be
sufficient, given the
relatively good sludge settling rates observed to date.
Decant
During decanting, the decant pump (located alongside the pilot-plant control
room)
pumped out the supernatant to the Effluent Holding tank. The pump suction line
in the
SBRs is attached to a float (25L sealed drum) via a flexible pipe, such that
the pipe
entrance is approx. 150mm below the surface level.
The maximum decant time allowed was 20 min, during which time, the SBR water
level
is registered by the PLC via a pressure sensor located side of the tank. If
the pressure/
water level signal reaches the operator-adjusted setpoint, the decant pumps
switch off and
the solenoid valves on the decant lines close. The SBR then idles for any
remaining
minutes available in the decant phase.
Feed Periods
Each SBR had three feed periods per cycle. A fresh batch of feed (to be used
for both
SBRs) was made for each feed period. The feed was pumped into the tanks by the
Mono
pumps at a rate of approximately 25L/min. The pumps could be operated as
on/off to
extend the length of the feed periods if necessary. The total volumes fed into
the SBRs
were controlled by the on-line pressure transducer located in the mixing tank.
Mixed, Non-Aerated Periods
During the non-aerated periods, the SBRs were mixed. These periods were used
for
denitrification and also phosphorous release from PAO bacteria, after
anaerobic
conditions are achieved.
Aerated Periods and Sludge Wastage
During the aerated periods, the SBRs were aerated by the blowers through fined
bubble
diffusers. The blowers were controlled by a PID loop coupled with the DO
sensors for
continuous operation. Additional mixing was also be employed by the
submersible
pumps located in the SBRs.
The aim of the aerated periods was to convert the NH4-N to N02-N
(nitritation), not NO3-
N. Since reduction of NO2 to N2 gas requires less carbon substrates than
reduction of
N03-N to N2 gas. As the system is carbon-limited, this is the preferred
option. In the
laboratory-scale reactor, oxidation of the NH4-N to N02-N (and not N03-N) was
controlled by both the OUR and pH profiles such that the rate of change of the
pH drop
(and OUR) indicates when complete nitritation has been achieved. However, the
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
laboratory-scale reactor uses an on/off aeration system as compared to the
variable air
flowrate to be used on the pilot-plant. Thus, although pH levels were
monitored, aeration
control was by DO probe, maintaining the dissolved oxygen, ideally, between
1.5 and
1.75mg OaIL. pH profiles of recent runs have shown that this was sufficient.
Aeration phases were stopped once the maximum allocated aeration time had
lapsed (see
Table 10) and the cycle moved into the next Feed phase.
Waste collected in the waste tank (from AP effluent overflow tank and sludge
storage
overflow), was pumped periodically (by submersible pump controlled by a float
switch)
to the existing on-site DAF effluent holding tank. The full-scale wastewater
stream in
this tank was pumped to the AP. The flow from the pilot-plant was very low
compared to
flow from the full-scale DAF during normal operating days.
As shown in Figures 16A-C and 17A-C, in both SBRs, early trials provided
highly
variable results. This was predominantly due to equipment issues (including
blockage of
lines, unreliable flows) and, especially, inconsistent and insufficient
VFA/soluble COD
levels which are assumed to have disadvantaged the PAOs in the SBRs. A major
source
of unreliable VFA levels in the feed was the old prefermenter. Once this was
replaced,
and the ration of prefermenter to anaerobic pond feed adjusted, the results
achieved for
nutrient removal stabilised (as apparent from mid to late August 2007), and
better than
90% nitrogen and phosphorous were reliably achieved. In addition, as apparent
from the
low nitrate levels observed (see, for example, Figures 18 and 19), nitrogen
removal by the
nitrite pathway (rather than via nitrate) was also achieved.
Figures 18 and 19 provide data for representative recent SBR cycles for SBR1
and SBR2
(run on 3 September 2007), including ammonium, nitrate, nitrite, phosphorous
and pH
profiles for the wastewater undergoing treatment. The two pilot-scale SBRs
were
operated identically except for the mode of feed delivery during the first
feed period of
each cycle. SBRl has a UniFed feed delivery system but not SBR2.
Both SBRs were operated with a cycle time of 6h (see Table 12). In each cycle,
857L of
influent was pumped into each SBR over the three filling periods with a volume
distribution of 50%, 30% and 20% respectively. Each filling period was
followed by
non-aerated and aerated periods. During aerated periods, DO level was kept at
1.5
mgOz/L. The SBRs hydraulic retention time was 42h and the sludge retention
time was
10 days.
CA 02666331 2009-04-15
WO 2008/046139 PCT/AU2007/001570
46
Table 11 - Characteristics of the two types of wastewater and the combined
influent as on
03/09/2007. The combined SBR feed was constituted of 30% of pre-fermented raw
wastewater and 70% anaerobic pond effluent (volumetric):
Parameter Pre-fermented Anaerobic pond Combined feed
raw wastewater effluent for SBRs
TCOD (mg.l" ) 5540 1015 2373
SCOD (mg.1"1) 1276 170 502
VFA (mgCOD.I"1) 1157 38 373
TN (mg.1"1) 199 221 214
NH4-N (mg.1"1) 139 183 170
TP (mg.1'1) 25 36 33
P04-P (mg.l"1) 20 33 28
TCOD/TN 28 4.6 11.1
SCOD/TP 51 4.7 15.2
Table 12 - Cycle periods for SBRs 1 and 2 on 3 September 2007
Period Length Purpose of Period
Settle 30 min Settle sludge
Decant 20 min Remove supernatant
Feed 1 20 min Add feed to reactor. For SBR 1,
Mixed, `Anoxic' period 1 30 min Denitrification/Anaerobic P release
Aerated period 1(DO=1.5mgO2/L) 70 min Nitritation
Idle 1(mixed) 15 min Depletion of dissolved oxygen
Feed 2 15 min Add feed to reactor
Mixed, `Anoxic' period 2 30 min Denitrification/Anaerobic P release
Aerated period 2(D0=1.5mg02/L) .43 min Nitrification/Sludge wastage
Idle 2 (mixed) 15 min Depletion of dissolved oxygen
Feed 3 15 min Add feed to reactor
Mixed, `Anoxic' period 3 26 min Denitrification/Anaerobic P release
Aerated period 3(D0=1.5mg02/L) 31 min Nitrification
Total 360 min 6h cycle period; 4 cycles per day
As can be seen from Figures 18 and 19, almost complete nitrogen removal was
achieved
for both of the SBRs, and that phosphorous levels in the effluent for the
reactors were less
than 2-2.5mg/L.
It will be appreciated that, although a specific embodiment of the invention
has
been described herein for the purpose of illustration, various modifications
may be made
without deviating from the spirit and scope of the invention as defined in the
following
claims.