Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
BASIC ALUMINUM NITRATE
BACKGROUND OF THE INVENTION
[0001]The present invention relates to a basic aluminum nitrate composition
and
a method of making such basic aluminum nitrate.
[0002] Basic aluminum nitrate compositions are suitable for use in a variety
of
applications, such as a surface modifier, a binder, a ceramic, an
antiperspirant,
etc. Aqueous solutions of basic aluminum nitrate (aluminum oxynitrate) may be
produced by dissolving a compound of aluminum, for example aluminum
hydroxide or alumina, in nitric acid or aqueous aluminum nitrate solutions.
[0003] U.S. Patent No. 5,202,115, provides for the production of basic
aluminum
materials that are utilized in antiperspirant formulations. The reaction
scheme for
forming the basic aluminum material is described as follows:
58H20+28AI+16AI(N03)3 ->22AI2 (OH)5NO3 + 26N0+3H2,
where the production of nitric oxide represents a reduction of the nitrate
oxoanion
from a formal +5 to +2 oxidation state on the nitrogen atom. This patent also
discloses that the aluminum salt of the univalent complex oxoanion can be
formed in situ, by reacting aluminum metal with, e.g., an inorganic acid of
the
univalent complex oxoanion. Consistent with techniques in connection with
conventional basic aluminum halide materials, this patent describes forming
the
described basic aluminum material having the univalent complex oxoanion by
adding small turnings of aluminum metal in the form of oblong pieces 1/16 inch
to
1/8 inch long and 1/100 inch to 3/100 inch thick, in excess, to a solution of
monomeric aluminum ion and univalent complex oxoanion.
[0004] U.K. Patent Application No. 2,048,229 describes a group of complexes
(AI`') within the aluminum chlorhydroxides, which are more efficacious as an
antiperspirant. Such group AI`' complexes with a ferron reagent at a reaction
rate
1
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
characteristic of AI (of Ala, AIb and AIc, AI is the group that exhibits the
slowest
complexing reaction ratio with ferron), and has a permeation rate in gel
permeation chromatography which is within that range generally found for AIb
(of
Ala, AIb and AI , Alb has an intermediate retention time, indicating it
includes
complexes of intermediate molecular size). This U.K. patent application
describes that the AI`' group of complexes was present in amounts of 10%-30%
by weight in then-available aluminum chlorhydroxides, and that these then-
available aluminum chlorhydroxides can be modified to contain substantially
lager amounts of the AI i group. This patent application discloses a technique
to
increase the amount of the AI`' group, by aging aluminum chlorhydroxide.
[0005]European Patent Application No. 191,628 discloses a direct process of
making a basic aluminum halide in powder form having an aluminum:halogen
molar ratio of from 1.7 to 2.2:1. This process includes steps of: (a)
dissolving
metallic aluminum, in an aqueous starting solution of an aluminum compound
selected from aluminum chloride and aluminum bromide, the starting solution
being held at a temperature of about 50 C. to about 105 C., for a time just
long
enough to dissolve sufficient aluminum to produce an aqueous solution of a
final
basic aluminum halide having an aluminum:halide molar ratio in the range 1.7:1
to 2.2:1, the concentration of the aluminum in the starting solution and the
amount of aluminum dissolved being such that the aluminum concentration in the
solution of the final basic aluminum halide is from 0.8% to 6.75% by weight;
and
(b) drying the solution of the final basic aluminum halide.
[0006] European Patent Application No. 191,628 describes a direct preparative
procedure for forming the described basic aluminum halide material containing
a
high proportion of the aluminum in the form of a polymer having a
characteristic
line in the 27AI NMR (nuclear magnetic resonance) spectrum. This patent
application discloses this characteristic line is 62.5 ppm downfield from the
resonance of AI3+(6H2O), and has been attributed to a complex aluminum ion
referred to as the AI13040 ion. In one embodiment of the disclosed process, at
least 20% of the aluminum of the final basic aluminum compound is in the form
of
the AI13040 ion.
2
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
[0007] European Patent Application No. 285,282 discloses antiperspirant
materials, including partially neutralized aluminum salts, the salts having at
least
25% of the total aluminum present in a form having a 27AI NMR spectrum
wherein 8% to 25% of the total area under the spectrum from 140 ppm to -80
ppm is contained in a peak at approximately 63 ppm (corresponding to
tetrahedrally coordinated aluminum ions). This European patent document
describes a technique for forming the described aluminum salt by partially
neutralizing an aqueous acid (such as a mineral acid) using a source of
aluminate ion (the mineral acid optionally being an aluminum salt), with no
subsequent heating step required. Specifically embodied in this patent
document
are aluminum halohydrate materials, such as aluminum chlorhydrate.
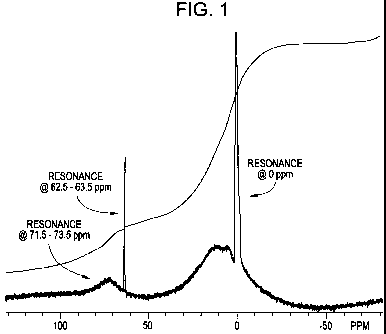
[0008] U.S. Patent No. 5,626,827 describes basic aluminum materials (polymeric
aluminum materials) having certain size exclusion high performance liquid
chromatography peaks produced from a high performance liquid chromatography
(HPLC) with less than 25% of the aluminum being in the form of APe
polyhydroxyaquoaluminum; and an 27AI NMR (nuclear magnetic resonance)
spectrum wherein 5%-30%, preferably 8%-18%, of the total area under the
spectrum from 140 ppm to -80 ppm is contained in a resonance line at 71.5-73.5
ppm; and an 27AI NMR spectrum in which the area of the 71.5-73.5 ppm
resonance line includes more than 50% of the combined areas of the 62.5-63.5
ppm and 71.5-73.5 ppm resonance lines.
[0009]U.K. Patent Application No. 2,053,172 A describes a process for the
preparation of a stable, liquid aqueous solution of basic aluminum nitrate
(aluminum oxynitrate) which is suitable for spinning into fibres, if desired
after
incorporation of a polymeric spinning aid. The process comprises (i) reacting
an
aluminum oxide with nitric acid solution at a temperature below 25 C, to form
aluminum hydroxide, (ii) separating the resulting aluminum hydroxide
precipitate,
(iii) if necessary removing alkali metal ions and other undesirable ions from
the
precipitate, and (iv) digesting the aluminum hydroxide precipitate in nitric
acid or
aluminium nitrate.
3
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
[0010] However, it has not been possible hitherto to produce satisfactory
stable
solutions having an acceptable aluminum to nitrate molar ratio and also
maintain
high purity.
SUMMARY OF THE INVENTION
[0011]ln one embodiment, the present invention relates to a basic aluminum
composition having an empirical formula:
AI2(OH)6-aXa
where 0.55 a:55.0, and X is an anion of nitrogen; and wherein said composition
possesses an NMR spectrum in which a -40 to +40 ppm resonance line
comprises at least 60% of the total area of the NMR spectrum.
[0012] In another embodiment, the present invention relates to a basic
aluminum
composition having an empirical formula:
AI2(OH)6-aXa
where 0.55 a<_ 5.0, and X is an anion of nitrogen; and wherein the composition
comprises less than 3% by weight metal oxide impurities based on the total
alumina weight of the composition.
[0013] In an even further embodiment, the present invention relates to a basic
aluminum composition having an empirical formula:
AI2(OH)6-aXa
where 0.5:5 a_5.0, and X is an anion of nitrogen; and wherein the composition
possesses an NMR spectrum in which substantially no other resonance line
other than at least one resonance line within -40 to +40 ppm is present in the
NMR spectrum.
4
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
[0014] In a further embodiment, the present invention relates to a method of
preparing a basic aluminum nitrate composition comprising, reacting aluminum
oxide metal salt with nitric acid at a pH of less than or equal to about 6 to
form
alumina precipitate and metal nitrate; removing said metal nitrate from the
precipitate; adding nitric acid to the precipitate to form a slurry and to
adjust
aluminum to nitrate ratio of the precipitate; and heating the slurry to form a
solution of the basic aluminum nitrate.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] FIG. 1 is a NMR spectrum for basic aluminum nitrate composition of the
prior art.
[0016] FIG. 2 is a NMR spectrum for basic aluminum nitrate composition of the
prior art.
[0017] FIG. 3 is a NMR spectrum for basic aluminum nitrate composition
according to the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0018] The present invention relates to a basic aluminum nitrate composition
and
a method of making such basic aluminum nitrate.
[0019] It must be noted that as used herein and in the appended claims, the
singular forms "a", "and", and "the" include plural referents unless the
context
clearly dictates otherwise. Thus, for example, reference to "a basic aluminum
nitrate composition" includes a plurality of such compositions and reference
to
"basic aluminum nitrate composition" includes reference to one or more such
compositions and equivalents thereof known to those skilled in the art, and so
forth.
[0020] "About" modifying, for example, the quantity of an ingredient in a
composition, concentrations, volumes, process temperatures, process times,
recoveries or yields, flow rates, and like values, and ranges thereof,
employed in
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
describing the embodiments of the disclosure, refers to variation in the
numerical
quantity that can occur, for example, through typical measuring and handling
procedures; through inadvertent error in these procedures; through differences
in
the ingredients used to carry out the methods; and like proximate
considerations.
The term "about" also encompasses amounts that differ due to aging of a
formulation with a particular initial concentration or mixture, and amounts
that
differ due to mixing or processing a formulation with a particular initial
concentration or mixture. Whether modified by the term "about" the claims
appended hereto include equivalents to these quantities.
[0021]As described herein, the term "basic" means a compound that is more
alkaline than other compounds of the same name. For example, in the chemical
formula mentioned herein, if a<6 then the compound is "basic". For a=6 then
the
compound may be represented by AI2(N03)6 or AI(N03)3 which is neutral
aluminum nitrate with mole ratio AI/N03=0.333 .
[0022]As utilized herein, the term "impurities" means anything other than, H,
0,
N, Al (in element form) or H20, NO3 and the AI2(OH)6_aXa. Impurities include,
for
example, metals and non-metals and any derivatives thereof, such as metal
oxides (e.g., Na20, Fe203, MgO, Ti02, Zr02, CaO, etc.), halides, sulfates and
other oxoanions, and mixtures thereof.
[0023]As used herein, the term "metal oxides" means a compound that contains
a metal cation and an oxide anion that typically reacts with water to form
bases
or with acids to form salts. Metals typically fall into the following
classifications,
but are not mutually exclusive and not rigidly defined: alkali metals,
alkaline earth
metals, transition elements, noble (precious) metals, platinum metals,
lanthanide
(rare earth) metals, actinide metals, light metals and heavy metals, and
mixtures
thereof.
6
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
[0024] In one embodiment, the present invention relates to a basic aluminum
composition comprising an empirical formula:
AI2(OH)6-aXa
where 0.5:5a55.0, and X is an anion of nitrogen; and wherein the composition
possesses an NMR spectrum in which a -40 to +40 ppm resonance line
comprises at least about 60% of the total area of the NMR spectrum, or the -40
to +40 ppm resonance line comprises at least about 70% of the total area of
the
NMR spectrum, or the -40 to +40 ppm resonance line comprises at least about
80% of the total area of the NMR spectrum, or the -40 to +40 ppm resonance
line comprises at least about 90% of the total area of the NMR spectrum, or
the -
40 to +40 ppm resonance line comprises at least about 95% of the total area of
the NMR spectrum. In another embodiment, the NMR spectrum comprises
substantially no resonance line other than at least one resonance line
appearing
within -40 to +40 ppm of the spectrum.
[0025] In an embodiment, the composition may comprise less than about 3% by
weight metal oxide impurities based on the total alumina (expressed as AI203)
weight of said composition, or less than about 1% by weight metal oxide
impurities based on the total alumina weight of said composition, or less than
about 0.5% by weight metal oxide impurities based on the total alumina weight
of
said composition, or less than about 0.1 % by weight metal oxide impurities
based
on the total alumina weight of said composition, or less than about 0.07% by
weight metal oxide impurities based on the total alumina weight of said
composition. The metal oxide impurities may include, but are not limited to,
oxides of sodium, iron, magnesium, titanium, zirconium, calcium or mixtures
thereof. The composition may comprise less than about 0.3% by weight sodium
oxide impurities based on the total alumina weight of said composition, or
less
than about 0.07% by weight sodium oxide impurities based on the total alumina
weight of said composition.
7
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
[0026] In another embodiment, present invention relates to a basic aluminum
composition comprising an empirical formula:
AI2(OH)6-aXa
where 0.55 a<_ 5.0, and X is an anion of nitrogen; and wherein the composition
comprises less than about 3% by weight metal oxide impurities based on the
total alumina weight of the composition, or less than about 1% by weight metal
oxide impurities based on the total alumina weight of said composition, or
less
than about 0.1% by weight metal oxide impurities based on the total alumina
weight of said composition, or less than about 0.07% by weight metal oxide
impurities based on the total alumina weight of said composition. The metal
oxide impurities may include, but are not limited to, oxides of sodium, iron,
magnesium, titanium, zirconium, calcium or mixtures thereof. The composition
may comprise less than about 0.3% by weight sodium oxide impurities based on
the total alumina weight of said composition, or less than about 0.07% by
weight
sodium oxide impurities based on the total alumina weight of said composition.
The composition possesses an NMR spectrum in which a -40 to +40 ppm
resonance line comprises at least about 60% of the total area of the NMR
spectrum, or the -40 to +40 ppm resonance line comprises at least about 70% of
the total area of the NMR spectrum, or the -40 to +40 ppm resonance line
comprises at least about 80% of the total area of the NMR spectrum, or the -40
to +40 ppm resonance line comprises at least about 90% of the total area of
the
NMR spectrum, or the -40 to +40 ppm resonance line comprises at least about
95% of the total area of the NMR spectrum. In another embodiment, the NMR
spectrum comprises substantially no resonance line other than at least one
resonance line within -40 to +40 ppm of the spectrum.
[0027] In a further embodiment, the present invention relates to a method of
preparing a basic aluminum nitrate composition including, reacting aluminum
oxide metal salt with nitric acid at a pH of less than or equal to about 6 to
form
alumina precipitate and metal nitrate; removing the metal nitrate from the
8
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
precipitate; adding nitric acid to the precipitate to form a slurry and to
adjust
aluminum to nitrate ratio of the precipitate; and heating the slurry to form a
solution of the basic aluminum nitrate. The metal may include sodium,
potassium, or mixtures thereof. The aluminum oxide metal salt may include
sodium aluminate, potassium aluminate or mixtures thereof. The metal nitrate
may include sodium nitrate, potassium nitrate, or mixtures thereof. The
aluminum oxide metal salt may be reacted with the nitric acid at a pH of less
than
or equal to about 5.5, or less than or equal to about 5.0, or less than or
equal to
about 4.5, or less than or equal to about 4. The nitric acid is added in an
amount
that is necessary to maintain the desired pH. The metal nitrate may be removed
from the precipitate by washing with deionized water using ultrafiltration, or
by
other suitable means. After washing, nitric acid is added to the precipitate
to
form a solution such that the aluminum to nitrate mole ratio of the
precipitate may
be equal to or greater than about 1.0, equal to or greater than about 1.2, or
equal
to or greater than 1.4. The solution may be concentrated by removal of water
using conventional techniques such as by distillation, evaporation, centrifuge
or
similar technique. The solution may also be filtered any time after formation
to
remove impurities such as by depth filtration or the like. If desired, the
solution
may further be dried to form a powder of basic aluminum nitrate such as, for
example, by spray drying, tray drying or similar method.
EXAMPLES
[0028]The following Examples are given as specific illustrations of the
claimed
invention. It should be understood, however, that the invention is not limited
to
the specific details set forth in the Examples.
EXAMPLE I
[0029] 3200g of room temperature deionized water are charged in an 18 liter
reactor, equipped with baffles and a stirrer. Sodium aluminate (4186g; 11.5%
9
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
A1203) is added simultaneously with a 20% nitric acid solution (4928g) in
order to
maintain a pH of -4 for 20 minutes to form precipitated alumina and sodium
nitrate. The final temperature is 41.6 C. 3078g of the resulting alumina is
filtered
and washed using a filter funnel with 9.2Kg of deionized water to remove the
sodium nitrate byproduct. This filtration is repeated three more times, in
order to
wash the entire contents of the reactor. The resulting filter cake, 3390g, has
an
AI/N03 mole ratio of 5.6. The filter cake is then liquefied with the
application of
shear, using a dispersion blade. To that, 656g of 40% nitric acid is added in
order to adjust the mole ratio of Al/N03 to -1.5. The resulting slurry is
heated to
95 C for approximately 2 hours in order to obtain the desired NMR spectrum.
The resulting solution is analyzed and found to contain 17.7% A1203 and less
than 20ppm of Na20. The solution is ready to use and is remains stable for at
least six months. The empirical formula of the basic aluminum composition is
AI2(OH)4.7(N03)1.3=
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
EXAMPLE 2
[0030]2100g of room temperature deionized water are charged in an 18 liter
reactor, equipped with baffles and a stirrer. Aluminum sulfate (8050g; 8.3%
AI203) is added simultaneously with a 25% sodium carbonate solution (9289g) in
order to maintain a pH of -5.3 for 20 minutes to form precipitated alumina and
sodium sulfate. The alumina slurry is filtered and washed using a filter
funnel
with six volumes of deionized water to remove the sodium sulfate byproduct.
The resulting filter cake, 4980g, is dispersed in a minimum amount of
deionized
water using a dispersion blade. To that 1362g of 40% nitric acid is added in
order to adjust the mole ratio of AI/NO3 to -1.5. The resulting slurry is
heated to
95 C for approximately 2 hours in order to obtain the desired NMR spectrum.
The resulting solution is analyzed and found to contain 16.3% A1203, 104ppm of
Na20, 39ppm of Fe203 and 12ppm of MgO. The solution is ready to use and is
remains stable for at least six months. The empirical formula of the basic
aluminum composition is AI2(OH)4.7(N03)1.3.
[0031]While the invention has been described with a limited number of
embodiments, these specific embodiments are not intended to limit the scope of
the invention as otherwise described and claimed herein. It may be evident to
those of ordinary skill in the art upon review of the exemplary embodiments
herein that further modifications, equivalents, and variations are possible.
All
parts and percentages in the examples, as well as in the remainder of the
specification, are by weight unless otherwise specified. Further, any range of
numbers recited in the specification or claims, such as that representing a
particular set of properties, units of measure, conditions, physical states or
percentages, is intended to literally incorporate expressly herein by
reference or
otherwise, any number falling within such range, including any subset of
numbers
within any range so recited. For example, whenever a numerical range with a
lower limit, RL, and an upper limit Ru, is disclosed, any number R falling
within
the range is specifically disclosed. In particular, the following numbers R
within
the range are specifically disclosed: R RL + k(Ru -RL), where k is a variable
11
CA 02693434 2010-01-15
WO 2009/011900 PCT/US2008/008770
ranging from 1% to 100% with a 1% increment, e.g., k is 1%, 2%, 3%, 4%, 5%.
... 50%, 51%, 52%. ... 95%, 96%, 97%, 98%, 99%, or 100%. Moreover, any
numerical range represented by any two values of R, as calculated above is
also
specifically disclosed. Any modifications of the invention, in addition to
those
shown and described herein, will become apparent to those skilled in the art
from
the foregoing description and accompanying drawings. Such modifications are
intended to fall within the scope of the appended claims. All publications
cited
herein are incorporated by reference in their entirety.
12