Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-1-
Recarburisation Method
Technical Field
A method for recarburising ferro-alloys (such as steel) is disclosed. The
method
finds particular application in recarburising ferro-alloys in tapping ladles
and ladle
furnaces that are employed subsequent to both integrated mill steelmaking
(that
typically comprises a blast furnace and a basic oxygen furnace) and mini-mill
steelmaking (that typically comprises an electric arc furnace (EAF)). Whilst
the method
will primarily be described in the context of recarburising in tapping ladles
and ladle
furnaces it should be appreciated that it is not limited to such types of
recarburising.
Background Art
There are increasing problems with plastics and tyre disposal. Recycling of
both
plastics and tyres accounts for a small proportion of material recovery, with
the bulk
still being disposed of either through landfill or burning in incinerators. In
landfill
neither material degrades readily, and either material may also leach toxic
elements to
soils and groundwater, whilst conventional burning often generates hazardous
emissions such as dioxins and can also increase greenhouse gas emissions.
Worldwide the steel industry is facing pressure to minimise its impact on the
environment by improving the efficiency of energy and resource utilisation,
and
especially to reduce CO2 emissions.
Waste plastics addition to electric arc furnaces is known. Examples are shown
in US 5,554,207 and JP 2004-052002.
WO 2006/024069 to the present applicant also discloses the addition of waste
plastics to electric arc furnaces and further discloses the possible use of
waste plastics
as a recarburiser, but only in the context of an induction furnace and without
disclosing
how this method may be practiced.
A reference herein to a prior art document is not an admission that the
document
forms a part of the common general knowledge of a person of ordinary skill in
the art in
Australia or elsewhere.
Summary of the Disclosure
In a first aspect there is provided a method for recarburising a molten ferro-
alloy
in a ladle or ladle furnace. The method comprises the step of adding a carbon-
containing polymer to the ladle or ladle furnace, wherein the polymer is
adapted to
function as a recarburiser of the ferro-alloy.
It has not previously been investigated how a carbon-containing polymer could
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-2-
best function as a recarburiser in the production of a ferro-alloy (i.e. where
the polymer
is used to substitute traditional recarburisers such as coal, coke and
graphite that are in
turn used to increase the amount of carbon present in the final ferro-alloy
produced). A
carbon-containing polymer can be selected and adapted such that it can replace
or
reduce the use of expensive recarburisers such as anthracite coal and
graphite.
In this regard, whilst WO 2006/024069 discloses the potential use of waste
plastics as a recarburiser, it does not teach how this may be practised, nor
does it
disclose how a waste plastic can be used in recarburising ferro-alloys in
tapping ladles
and ladle furnaces.
When the term "ferro-alloy" is used herein it is intended to include a broad
range
of iron-carbon alloys (including steels) and other iron-carbon and/or iron-
based alloys,
including ferrochromium, ferrochromium silicon, ferromanganese,
ferrosilicomanganese, ferrosilicon, magnesium ferrosilicon, ferromolybdenum,
ferronickel, ferrotitanium, ferrophosphorous, ferrotungsten, ferrovanadium,
ferrozirconium etc.
In one form of the method the carbon-containing polymer can be specifically
adapted to suit the ladle or ladle furnace prior to being added so that carbon
in the
polymer preferentially dissolves into the ferro-alloy and does not combust to
any
substantial or detrimental extent.
For example, one way in which the polymer can best be adapted to function as a
recarburiser can comprise the step of optimising the size (e.g. its shape
and/or
dimension) of polymer to the given ladle or ladle furnace prior to addition
thereto. This
size optimisation has been observed to promote carbon dissolution and minimise
polymer combustion when contacted by the molten ferro-alloy.
In one embodiment the size optimisation can comprise the binding together of
polymer layers to form a block. For example, in the case of a polymer
comprising waste
rubber, layers of tyre tread/wall or conveyor belt can be tied together into a
bundle by a
suitable ferro-alloy wire.
In the case of ladle addition, the carbon-containing polymer can be added into
the ladle prior to the tapping of molten ferro-alloy thereinto.
In the case of ladle furnace addition, the carbon-containing polymer can be
added into the furnace with or onto the molten ferro-alloy from the ladle. For
example,
the carbon-containing polymer may be injected into the ladle furnace (e.g.
into an
uppermost layer such as a slag layer).
In one form the carbon-containing polymer is a waste plastic or rubber. In
this
form the waste plastic can comprise polyethylene (e.g. HDPE), and other
plastics such
as polypropylene, polystyrene, poly butadiene styrene, ABS, etc, as well as
difficult to
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-3-
re-process plastics such as Bakelite, etc. Also, in this form the rubber can
be derived
from a used tyre or belt. The belt can be a used/discarded rubber conveyor
belt.
The addition of a waste plastic or waste rubber into the ladle or ladle
furnace
provides another effective means of disposal of the waste, which wastes
otherwise pose
environmental challenges.
Whilst usually the carbon-containing polymer will comprise the atoms C, H and
optionally 0 only, other elements may be present in the polymer (e.g. N, S, P,
Si,
halogens etc). Where these elements interfere with ferro-alloy production
and/or
produce contaminants, pollutants, noxious or harmful gases (e.g. hydrogen gas)
etc, the
carbon-containing polymer can be judiciously selected and judiciously added,
and/or
certain flux additives can be introduced to the ladle/ladle furnace, to avoid
or mitigate
the formation of noxious/harmful gases and other detrimental or harmful by-
products.
In one form the ferro-alloy produced is a steel or steel alloy.
In one variation of the method, in addition to the carbon-containing polymer,
another source of carbon can be added to the ladle or ladle furnace, with the
other
source of carbon being one or more of coal, coke, carbon char, charcoal and/or
graphite.
In one form the ladle or ladle furnace forms part of an electric arc
steelmaking
process, with the ladle receiving molten ferro-alloy from the electric arc
furnace, and
with the ladle furnace receiving molten ferro-alloy from the ladle.
In a second aspect there is provided the use of a carbon-containing polymer as
a
recarburiser of a ferro-alloy in a ladle or ladle furnace.
In the second aspect the carbon-containing polymer can be as defined in the
first
aspect.
In a third aspect there is provided a method for recarburising a molten ferro-
alloy, the method comprising the step of contacting the alloy with a carbon-
containing
polymer that can function as a recarburiser, whereby the polymer has a format
such
that, when it contacts the molten ferro-alloy, it promotes dissolution of
carbon from the
polymer into the molten ferro-alloy.
It has been observed that polymer format (e.g. its shape and/or dimension) can
be optimised so that, when it contacts the molten ferro-alloy, a bulk of
carbon in the
polymer dissolves rather than combusts or gasifies. This, in turn, can enhance
the
recarburisation function of the polymer.
In the method of the third aspect the polymer format can comprise a unit that
is
dimensioned so as to minimise its exposed surface area relative to its mass.
Further, the
dimension of the polymer can be optimised to the given ladle or ladle furnace.
This
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-4-
allows for maximum carbon dissolution to occur, and can minimise combustion or
gasification of carbon in the polymer. One or more such units (e.g. one or
more 10 kg
blocks of waste polymer) can be employed as a recarburiser of a ferro-alloy.
In the method of the third aspect the polymer can be added into the molten
alloy,
or the molten alloy can be added onto the polymer, or the polymer can be added
together with the molten alloy into e.g. a ladle or ladle furnace.
The method of the third aspect can otherwise be as defined in the first
aspect.
Brief Description of the Drawings
Notwithstanding other embodiments which may fall within the method for
recarburising a ferro-alloy as defined in the Summary, specific embodiments of
the
method will now be described, by way of example only, with reference to the
accompanying drawings in which:
Figure 1 shows an X-ray Diffraction plot for each of a) raw metallurgical coke
(as a current recarburiser); and b) raw high density polyethylene (as a waste
plastic
recarburiser); as described in Example 1;
Figure 2 shows X-ray Diffraction plots for raw high density polyethylene and
metallurgical coke; and high density polyethylene and metallurgical coke after
combustion; as described in Example 1;
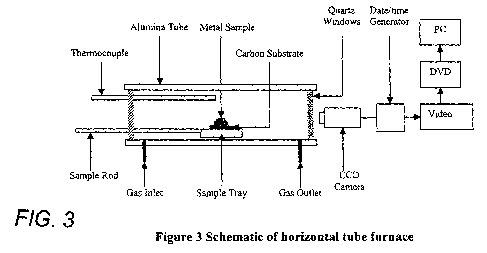
Figure 3 shows a first schematic diagram of a horizontal tube resistance
furnace
set up for a sessile drop approach, as described in Example 1;
Figure 4 shows plots of carbon pick-up (% carbon content) over time, for two
experimental runs as described in Example 2, for a 100% metallurgical coke as
well as
a mixture of 30% high density polyethylene and 70% metallurgical coke;
Figure 5 shows a schematic diagram of a drop tube furnace, as described in
Example 3;
Figure 6 shows a second schematic diagram of a horizontal tube resistance
furnace set up for a sessile drop approach, as described in Example 3; and
Figure 7 shows a plot of carbon pick-up (% carbon content) over time, for an
experimental run as described in Example 3, for a 100% metallurgical coke as
well as a
mixture of 30% Bakelite and 70% metallurgical coke;
Figure 8 shows a schematic diagram of an electric arc process for the
production
of a ferroalloy such as steel;
Figure 9 shows a schematic detail of an electric arc furnace being tapped into
a
ladle;
Figure 10 shows a schematic detail of the ladle of Figure 9;
Figures 11A and 11B respectively show side and top perspective views of a
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-5-
bundle of tyre tread suitable for addition to a tapping ladle; and
Figures 12A to 12C respectively plot the pick-up (in % per l0kg sample) of
plastic (waste rubber) and standard carbon recarburiser:
Fig 12A - in a transfer ladle;
Fig 12B - in a ladle furnace; and
Fig 12C - standardised data.
Detailed Description of Specific Embodiments
It was postulated that a carbon-containing polymer (e.g. a waste plastic or a
waste rubber) could be introduced into ferro-alloy (e.g. steel) production to
function as
a recarburiser (i.e. to "trim" carbon content in the alloy) in stages
occurring subsequent
to basic ferro-alloy formation (e.g. subsequent to steel formation in a blast
furnace and
basic oxygen furnace, or subsequent to steel formation in an EAF furnace). In
this
regard, it was postulated that the carbon-containing polymer can function as a
recarburiser in either or both of the transfer ladle and the ladle metallurgy
furnace.
Currently worldwide, there are two major process routes for steelmaking: the
"Integrated Mill" route, which produces iron from ore and coke and then
converts the
iron into steel, and the "Mini-Mill" route, which produces steel from scrap
steel. The
major differences between the two routes are the type of furnaces used to
produce steel.
However, common to both processes are the transferring of the molten steel
into ladles,
the trimming of the steel temperature and composition in the ladles using a
Ladle
Metallurgy Furnace (LMF), and the casting of the steel (e.g. using a
Continuous Casting
Machine (CCM)).
An integrated mill produces high-carbon molten iron in a blast furnace charged
with iron ore, coke, fluxes and fed with a hot air blast. The iron from the
blast furnace is
transferred in its molten state to one or more Basic Oxygen Furnaces (BOFs).
Oxygen
is used to remove most of the carbon to convert the iron into low-carbon
steel. Up to
25% of the BOF charge can be solid scrap heavy steel. Steel trimming for
carbon
content is then subsequently performed.
A mini-mill uses one or more Electric Arc Furnaces (EAFs) to melt solid scrap
steel, which can consist of heavy scrap, light scrap, and pig iron (from blast
furnaces).
Oxygen is used to remove carbon and other impurities from the molten steel,
such as
silicon, aluminium and manganese, which react with the oxygen to form silicon
oxide
(Si02) aluminium oxide (A1203) and manganese oxide (MnO). A large amount of
iron
also reacts with the injected oxygen to form iron oxide (FeO or Fe203 ).
Calcium
oxide (CaO) and magnesium oxide (MgO) are added to the furnace in order to
build a
slag layer on top of the steel. This slag layer traps the various oxides of
impurities that
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-6-
have been burnt out of the steel, along with a percentage of iron oxide, and
protects the
refractory material that lines the furnace from chemical attack by the
impurity oxides,
and also lowers the heat loss from the arcs to the furnace roof and sidewalls.
Once the composition and the temperature of the steel are correct, the
electric
furnace is tapped. This involves transferring the steel from the furnace to a
ladle, where
the steel can be moved in its molten state to the LMF. A schematic of the EAF
production process is shown in Figure 5. Figure 6 shows a detail of the steel
being
tapped into a ladle, and Figure 7 shows a detail of the ladle in which a first
stage of steel
trimming can take place.
During tapping, carbon ('recarburiser') in a relatively pure form (typically
metallurgical grade carbon) is added to the steel (known as `recarburisation')
to bring it
into a desired specification. The metallurgical grade carbon is granulated and
forms a
comparatively expensive part of the process. Various ferro-alloys are also
added to the
steel to enhance the physical properties of the metal. Thus, investigations
were
conducted into alternative carbon substitutes not heretofore considered as
suitable.
Examples
Non-limiting examples of methods for producing a ferro-alloy will now be
provided. Examples 1, 2 and 3 provide laboratory derived experimental data
that
supports that the carbon in a carbon-containing polymer (waste plastic) is
able to
dissolve into molten metal and thus function as a recarburiser. Example 4
provides
actual on-site trial data for a carbon-containing polymer (waste rubber) as a
recarburiser
in a transfer ladle and in a ladle furnace.
The methodology of Examples 1 to 3 involves the removal of volatile matter
(VM) prior to testing for carbon dissolution, whereas the method of Example 4
(being
an on-site trial) involves no such prior removal. Thus, the data of Examples 1
to 3 is not
directly comparable with the data of Example 4.
It should also be noted that the type and quality of metallurgical coke that
was
employed varied between Examples 1 and 2 and Example 3, and it was this
variation
that was noted to contribute to different outcomes on the carbon dissolution
into liquid
steel. Thus, a direct comparison does not apply between the results of
Examples 1 and 2
and Example 3.
It was further noted that experimentation into the effects of the coke
characteristics, and also the waste plastic characteristics, could be
performed, whereby
those characteristics could be optimised to enhance the carbon dissolution
into liquid
steel.
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-7-
Example 1 - Carbon Dissolution/Recarburisation of Waste Plastics
Experiments were conducted to investigate the dissolution of carbon from a
mixture of 30% HDPE and 70% metallurgical coke into liquid steel at 1550 *C to
check
for suitability for use in ladles and ladle furnaces. The experiments provided
data
representing sample characterization, including proximate analyses and X-ray
patterns,
as well as the details and results of carbon dissolution experiments.
Sample Characterization
Carbonaceous residues of waste plastics and metallurgical coke mixtures to be
used for a carbon dissolution study were prepared by combustion in a drop tube
furnace
(DTF). The collected residues from the DTF were found to contain a level of
volatile
matter. Therefore, these residues were further devolatilised using a
horizontal tube
furnace (HF) - Figure 3. Raw samples and their carbonaceous residues collected
from
the drop tube furnace and the horizontal tube furnace respectively were
analysed for
percentages of fixed carbon, ash, volatile matter (VM) and moisture, and their
structures were characterized using X-Ray diffraction.
Proximate Analysis
The proximate analysis data of samples was obtained and is shown in Table 1.
For the reference material - metallurgical coke (Met Coke) - the fixed carbon
content of
raw samples and samples after combustion in the drop tube furnace and the
horizontal
tube furnace was almost constant at 64.5%. It was therefore understood that
the
combustion of Met Coke in the drop tube furnace and the horizontal tube
furnace did
not change its carbon content under the experimental conditions. When Met Coke
was
mixed with plastics, the fixed carbon content increased after combustion in
the drop
tube furnace and the horizontal tube furnace, whereas volatile matter
decreased
significantly.
Table 1 - Proximate Analysis of HDPE and Met Coke samples
Proximate Analysis
Samples %Moisture %Ash %VM %Fixed
Carbon
Raw 1.30 31.80 2.40 64.50
After 1.30 33.50 0.70 64.50
Met Coke DTF
After 0.60 33.70 0.90 64.80
HF
Raw 0.70 22.60 36.00 40.70
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-8-
30% After 1.20 28.10 6.00 64.70
HDPE+ 70% DTF
Met Coke After 1.00 29.60 1.40 68.00
HF
X-Ray Diffraction
X-ray diffraction patterns of carbonaceous residues from HDPE and coke
mixtures were obtained using a Siemens D5000 X-ray diffractometer. Raw Met
Coke
and raw plastics were firstly analysed, followed by their mixtures. Then,
their residues
after combustion in the drop tube furnace and further devolatilisation in the
horizontal
tube furnace were characterised. Metallurgical coke was considered as the
reference
coke, and all X-ray patterns of the mixtures were compared with it. X-ray
patterns for
all the carbonaceous samples are shown in Figures 1 and 2. From these Figures,
it was
clear that the raw mixtures show high intensity peaks of hydrocarbons
(plastics). After
combustion in the drop tube furnace and the horizontal tube furnace, the X-ray
pattern
of the residue samples still shows a hydrocarbon peak of plastics having a low
intensity.
This indicated that the plastics would be suitable for use as a recarburiser.
Example 2 - Experimental Details for Waste Plastics Dissolution
Carbon dissolution from 100% metallurgical coke and the mixture of 30%
HDPE and 70% metallurgical coke was investigated using the sessile drop
technique.
Firstly, material to be investigated was ground and sieved to obtain particles
of size less
than 1 mm and then combusted in the drop tube furnace at 1200 C in 80%
nitrogen and
20% oxygen atmosphere. The residue collected from the drop tube furnace was
found to
contain high volatile matter content. Thus, it was devolatilised again in the
horizontal
tube furnace at 1200 C in an argon atmosphere for 15 minutes. The collected
residue
was again ground into powder using a grinding machine and then used for the
carbon
dissolution experiment.
To make the substrate, approximately 1.6 g of residue sample was used. The
residue was compacted in a steel die under 7 KN load applied using a hydraulic
press.
The substrate obtained from the die had a top surface area of 3.14 cm2. The
substrate
was placed on a graphite sample holder, and then approximately 0.5 g of
electrolytic
pure iron (99.98% Fe) was placed on the centre of the substrate. The carbon
dissolution
experiment was run under an inert argon atmosphere at 1550 C. The sessile drop
assembly was firstly put in the cold zone of the horizontal tube furnace where
the
temperature was approximately 1200 C to protect the sample holder from thermal
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-9-
shock and to allow volatile matter from the substrate to escape. After
approximately 15
minutes it was pushed into the hot zone where the temperature was 1550 C. The
time
generator started counting once the metal melted and formed a liquid drop. The
sample
was quenched after 1, 2, 4, 8, 15, 20, 30 and 60 minutes. During the
experiment, the
reaction inside the furnace was observed using a CCD camera. After the sessile
drop
experiment, the carbon content contained in the droplet was measured using a
carbon-
sulphur LECO analyser (model CS 230). The horizontal tube furnace schematic is
presented in Figure 3.
Experimental Results
Carbon pick-up from carbonaceous substrates by liquid iron was obtained and is
shown in Figure 4. It was clearly observed that carbon pick-up by the iron
which
reacted with the 30% HDPE + 70% Met Coke substrate was higher than the iron
which
reacted with metallurgical coke.
Example 3 Carbon Dissolution using Bakelite/Coke blend
Material Selection and Preparation
In this example electrolytic pure iron (99.98 wt% Fe) was employed The
carbonaceous materials investigated include pure metallurgical coke, and
blends of
coke with Bakelite. Bakelite (Phenol Formaldehyde) is a high cross-linking
thermosetting material, produced by condensation polymerization of phenol and
formaldehyde. Bakelite consists of C, H and 0 atoms. The chemical composition
depends on the relative phenol to formaldehyde ratio used (1:1 or 1:2).
However,
CaCO3 is commonly added into commercially grade bakelite as a filler.
To prepare the samples, Bakelite and coke were blended in a ratio of 30% and
70% respectively. The mixture was crushed in a jaw crusher, was sieved to a
size of less
than 1 mm, and was then mixed homogeneously in a ball mill. The mixture was
fed into
a drop tube furnace (DTF) at the rate of 0.52 g/min and combusted at 1200 C in
an
atmosphere of 20% 02 and 80% N2. A schematic diagram of the drop tube furnace
is
shown in Figure 5.
Carbonaceous residues were analysed for proximate analysis and ash analysis.
The proximate analysis values of all residual chars are shown in Table 2, and
include
the fixed carbon, ash, volatile matter and sulphur contents. The chemical
composition
of ash in the residue samples was also analyzed and is reported in Table 3.
Table 2 Proximate analysis
Composition (wt%) Coke Bakelite/Coke
Fixed Carbon 75.5 68.1
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-10-
Volatiles 2.1 3.4
Ash 22.4 28.5
Sulphur 0.3 0.25
Table 3 Ash composition
Ash
Composition Si02 A1203 Fe203 CaO P205 Ti02 MgO K20 Na20 SO3 Mn304
(wt %)
Coke 61.10 32.10 1.60 0.71 0.68 1.00 0.17 0.29 0.19 0.13 0.05
Bakelite/Coke 47.30 22.80 2.20 18.30 0.52 0.77 1.70 0.35 0.18 3.50 0.13.
Carbon Dissolution
Carbon dissolution experiments were carried out using the sessile drop method.
The sessile drop method was employed to study carbon transfer into liquid
iron, as well
as interfacial phenomena during wetting of graphite/Fe and coke/Fe. To make a
substrate, approximately 1.6 g of the powder residue collected from the DTF
was put in
a die and compacted by applying 75 KN of force using a hydraulic press. The
substrate,
with a top surface area of 3.14 cm2, was placed on a graphite sample holder.
Approximately 0.5 g of electrolytic pure iron (99.98% Fe) was placed on the
centre of
the substrate. This assembly was first placed at the cold zone of a horizontal
tube
furnace where the temperature was 1200 C and sealed while Ar gas flowed
through the
furnace at the rate of 1.0 L/min. After approximately 15 minutes, the assembly
was
inserted into the hot zone where the temperature was 1550 C. The reaction time
was
noted to start when the metal completely melted and formed the droplet.
Samples were
quenched after 1, 2, 4, 8, 15, 20, 30, 60 and 180 minutes by sliding the
assembly into
the cold zone thus terminating the reactions occurring on the metal/carbon
interface.
The schematic of the horizontal tube furnace is presented in Figure 6.
After the experiment, the carbon content of the metal droplet was measured
using a Carbon-Sulphur analyzer (LECO CS 230). Meta/carbon interface and the
reaction products were investigated using a Scanning Electron Microscope (SEM
Hitachi 3400X) coupled with Energy dispersive Spectroscopy (EDS).
Results
Carbon pick up from Bakelite/Coke blend by liquid iron as compared to coke
were
plotted with time and are shown in Fig. 7. The data was tabulated and is
presented in Table
4.
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-11-
Table 4 Carbon pick up from Bakelite/Coke blend with time compared to coke
Time(min) %Carbon picked up
Coke Bakelite/Coke
1 0.07812 0.1323
2 0.08280 0.1265
4 0.08333 0.1362
8 0.06800 0.1318
15 0.08702 0.1469
20 0.10690 0.1405
30 0.07326 0.1719
60 0.10170 0.1927
180 0.26540 0.34840
Example 4 - Recarburisation in EAF Ladle and EAF Ladle Furnace using Waste
Plastic and Waste Tyres
Experimental trials were conducted in an EAF steel production process to
investigate the use of polymer material as a recarburiser in steelmaking
operations. The
aim of the trials was to replace a percentage of the relatively expensive
recarburising
material (metallurgical grade carbon costing at around $650/tonne) in current
use with
waste polymer (obtainable at significantly lesser cost). It was thus
understood that
replacement of the carbon material would have benefits in terms of cost but
also
environmental impact.
The first polymer trialled was a high density polyethylene (HDPE) which was
observed to contain about 85% bonded carbon and 15% bonded hydrogen compared
to
the existing recarburiser containing about 95% carbon. The first trial was
conducted
using virgin plastic rather than recycled material to optimise the conditions
and provide
feasibility of converting to recycled material.
The majority of trials were conducted with polymer charging into the EAF ladle
just prior to tapping, however further trials were also conducted at the ladle
furnace.
Polymer was added to the ladle while it was in the isle before being moved
into the
tapping position. 10kg of polymer was weighed out into buckets ready to be
added to
the ladle. Once the electric arc furnace sample was taken, the amount of
recarburiser
required was taken from a recipe. Polymer was then added to the ladle (10kg)
and the
volatiles were allowed to bum off, leaving a residue high in carbon. Normal
(known)
recarburiser was optionally added on top of this according to the recipe. The
ladle was
then moved into the tapping position and tapped.
Trials at the ladle furnace were performed upon arrival where polymer material
was added on top of the steel over the porous plug and allowed to dissolve.
The data
taken for the EAF ladle was initial carbon content, plastic added,
recarburiser added,
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-12-
ladle arrival carbon. The data taken for the ladle furnace was arrival carbon,
polymer
added, recarburiser added, and ladle furnace departure carbon. This data was
compared
to normal heats and the Experimental Results are discussed below.
Tyre and belt-derived polymer additives were found to be optimally added as
bundles of mats, typically having a weight or around 10kg and an approximate
300x300x300 mm3 volume, as shown in Figures 11A&B. The bundles were added to
the ladle by hand. Ferro-alloys were also added in the form of lumps of metal
approximately 50mm across, and were batched into hoppers before being gravity-
fed
into the ladle. These alloys were added midway through the tapping process. A
proportion of carbon was optionally added just before the EAF was tapped, or
just after
the tapping process began.
The bundles of mats were shaped and dimensioned so as to minimise the surface
area of the bundle relative to its mass (e.g. an optimum shape may approximate
a
generally spherically-shaped bundle). This was observed to provide for maximum
dissolution into molten metal of the carbon in the polymer, and to minimise
the amount
of carbon in the polymer bundle that combusted or gasified. It also allowed
the molten
metal to quickly cover the bundle, thus restricting oxygen flow to the bundle,
thereby
further reducing combustion and gasification of carbon in the bundle.
The process steps were as follows:
1. The ladle was taken off the pre-heater and placed in the ladle car.
2. The ladle furnace operator inspected the ladle brickwork for possible
damage and
sanded the slidegate nozzle.
3. The ladle was transferred to the EAF for tapping.
4. Prior to being moved under the taphole, carbon additions in the form of
10kg bags of
coke were added to the ladle, according to the percentage of carbon in the
steel. Where
polymer recarburiser was solely being used, this step was omitted. Once the
required
number of bags had been added, the ladle was moved under the taphole.
5. Aluminium bars (30-80kg) were added to the ladle base to reduce alloy
oxidation
during tapping. The polymer recarburiser was then placed on top of these bars.
6. The taphole was opened and mats/bundles (Figure 11) of polymer recarburiser
were
continued to be added to the ladle in 10kg batches, according to the
percentage of
carbon required in the steel/alloy.
7. Alloying additions, such as ferro-alloys, were added to the ladle once a
quarter of the
ladle was full.
8. Flux additions were added to the ladle shortly after the alloying
additions. Depending
on the grade of steel being made, which stipulated the amount of recarburiser
to be
added to the ladle, some of the carbon additions were also batched into
hoppers, prior to
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
- 13-
gravity-feeding into the molten alloy in the ladle. It was noted that polymer
recarburiser
could be added during tapping of the furnace and also at the ladle furnace.
Experimental Results
Trials were conducted during a so-called "ES35 Green Shift" and were
compared to normal recarburiser uptake results that usually occurred during
the ES35
Green Shift. The results of % carbon picked-up are plotted Figure 12A. These
results
indicate an acceptable level of carbon pick-up from the use of polymer
recarburiser.
The carbon values were all taken from Celox measurements (for consistency) and
were
compared to arrival LF samples. The results for the column labeled "PLASTIC"
are for
20kg of plastic and the remainder comprising normal recarburiser. The chart
shows
pickup per 10kg of material added. Figure 12A shows that plastic as a
recarburiser is
less efficient by weight than pure recarburiser, however this difference was
in part
attributed to the difference in percentage of bonded carbon in the two
materials (i.e. less
carbon in the plastic). Similar trends were observed in Ladle Furnace trial as
shown in
Figure 12B.
To estimate the contribution of the different components in the mixture, the
weight of plastic was multiplied by 0.85 and the recarburiser by 0.95,
assuming that all
bonded carbon was dissolved into the steel. The result of this calculation was
plotted
and is shown in Figure 12C. There was only a slight increase in the disparity
between
the "PLASTIC" and "RECARB".
From this analysis it was noted that the particular form of the metallurgical
grade
carbon recarburiser resulted in a more efficient uptake than that arising from
the form of
polymer recarburiser. The difference was attributed to carbon losses from
gaseous
emissions (i.e. in the form of CO/CO2) whereby a portion of the polymer
combusted
upon contact with the molten alloy.
From this, the format (e.g. shape and dimension) of the polymer was optimised
to ameliorate and minimise such combustion. In this regard, reducing the
amount of
surface area of polymer recarburiser whilst increasing its mass, in each
addition, was to
be further optimised.
In general, the experiments demonstrated that waste plastics and waste
rubber can provide an effective alternative to coke and graphite for the
recarburisation
of ferro-alloys. Thus, an effective means for using and consuming the vast
quantities of
waste plastics and rubbers in society is provided.
Whilst a number of specific embodiments have been described, it should
be appreciated that the method can be embodied in many other forms.
For example, whilst specific waste plastics and waste rubbers have been
CA 02733085 2011-02-04
WO 2010/015041 PCT/AU2009/001018
-14-
described, it will be appreciated that the carbon-containing polymer may come
from a
wide variety of sources including (but not limited to) waste polymer from
white goods,
waste carpet (especially underlay), automotive scrap residue, textiles,
building waste
material and other forms of industrial and domestic waste. Sources that
currently
represent a disposal or environmental issue are preferred.
In the claims which follow, as well as in the preceding description, the
word "comprising" (and its grammatical variants "comprise" and "comprises") is
used
in an inclusive sense and not an exclusive or "consisting only of' sense,
whereby further
features can be associated with the features as recited.