Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02769851 2016-08-23
73185-36
METHODS, SYSTEMS AND APPARATUSES FOR MEDICATION MANAGEMENT
AND STORAGE
RELATED APPLICATIONS
This application is related to US Provisional Application No. 61/233,426,
filed
August 12, 2009, and US Provisional Application No. 61/233,781, filed August
13, 2009.
FIELD OF THE INVENTION
The present invention relates generally to medication management and storage.
BACKGROUND OF THE INVENTION
Studies consistently show that patients have limited "health literacy." In
other
words, patients, generally speaking, have limited understanding of the role
they should play in
their own health care, and more specifically, how to take and manage their
medications.
Limited patient health literacy results in limited use of preventive services,
delayed diagnoses,
lack of understanding of medical conditions, failure to adhere to medical
instructions, poor
self-management skills, increased risk of hospitalization, poor physical and
mental health,
increased mortality risk, and greater healthcare costs. Some studies have
shown these costs to
range between $106 and $223 billion per year. What is needed is a
comprehensive system
enabling patients, physicians and pharmacists to more effectively communicate,
and for
patients to gain a greater understanding of their own health care and
medication management.
The present invention solves these and other problems involved in the current
state of the art, as will be explained below.
BRIEF SUMMARY OF THE INVENTION
The present disclosure is best understood with reference to the claims, the
entire specification, and the drawings submitted herewith, which describe the
systems,
methods, and apparatuses of the present disclosure in greater detail. The
summary is merely
intended to convey aspects of illustrative embodiments.
1
81700877
The present disclosure relates to systems, methods, and apparatuses for the
management, dispensing, and administration of over-the-counter and
prescription medications
through use of a pharmaceutical container labeling system and an optional
integrated
computer-based or online management system.
In certain aspects, the invention relates to a pharmaceutical container
comprising a hollow body, a child-resistant cap, and a specialized information
label affixed to
the hollow body. In certain embodiments, the hollow body may have a squared
form that has
a rectangular longitudinal shape and a rectangular or square cross-section,
with one corner cut
across.
In certain embodiments, the specialized information label may be configured
such that, upon being affixed to the hollow body, one side of the hollow body
is visible, such
that that medication is easily visible though the label. In addition, in
certain embodiments, the
label may provide extra space for relevant information by providing fold-out
panels.
In certain aspects, information printed on the specialized label may be
correlated or linked with a computerized or online management system, such as
an interactive
website to keep patients, pharmacists, physicians and other interested parties
organized and
informed with respect to all of a patient's prescribed and over-the-counter
medications.
In certain embodiments, there is provided a pharmaceutical container
comprising: a hollow body having a top portion, a bottom portion and side
portions extending
between the top and bottom portions to enclose the hollow body; wherein the
top portion of
the hollow body further includes a neck having threads; wherein the side
portions of the
hollow body are configured such that one or more corners, edges, or select
portions of the
cross-sectional form are cut-off at an angle to create one or more
longitudinal flat portions; a
cap having a top portion, a bottom portion and side portions extending between
the top and
.. bottom portions, and configured so as to correspond in cross-sectional
shape with the hollow
body such that the cap includes one or more longitudinal flat portions that
align with the one
or more longitudinal flat portions of the hollow body when the cap is secured
to the hollow
body; wherein the cap includes a socket having threads generally corresponding
to the threads
2
CA 2769851 2019-04-24
81700877
of the neck of the hollow body so as to engage and secure closure of the cap
to the neck of the
hollow body; wherein the hollow body and the cap further include interlocking
tab portions to
provide a child resistant closure mechanism; a specialized information label
affixed to the
hollow body; wherein the specialized information label comprises multiple
information panels
including a panel having visual, pictorial administration instructions and a
fold-out
information panel, and wherein the information panel having visual, pictorial
administration
instructions comprises: two or more divided areas separated into multiple
corresponding
subareas, one of the divided areas including a pictorial representation in
each of the
corresponding subareas indicating the timeframe in which a medication may be
administered,
and a second divided area including, in the corresponding subareas, a
pictorial representation
of the amount of medication to be taken during that particular timeframe; and
wherein the two
or more divided areas are configured to display visual alignment of the amount
of the
medication taken in each timeframe across a next pharmaceutical container also
having an
affixed specialized information label, when the pharmaceutical container and
the next
pharmaceutical container are arranged with the two or more divided areas
facing outwards in
the same direction, and wherein each timeframe is aligned horizontally or
vertically.
Additional objects, advantages and novel features of this invention will be
set
forth in part in the detailed description that follows, and in part will
become apparent to those
skilled in the art upon examination of the following description, or may be
learned by
practicing the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
In the accompanying drawings that form a part of the specification and are to
be read in conjunction therewith, the present invention is illustrated by way
of example and
not limitation, with like reference numerals referring to like elements,
wherein:
FIG. 1 is a top right perspective view of a squared pharmaceutical container
with the cap attached according to one embodiment.
FIG. 2 is a top right perspective view of a prescription container with the
cap
detached according to one embodiment.
2a
CA 2769851 2019-04-24
81700877
FIGS. 3A-3B illustrate bottom front and right perspective views of a cap
according to embodiments.
FIG. 4 is a rear and left side view of a prescription container with a
specialized
label attached and folded out according to one embodiment.
FIG. 5A-5B illustrates front and rear views of a label detached from a
prescription container and folded out according to embodiments.
FIG. 6 illustrates a front view of an alternative label detached from a
prescription container and folded out according to an embodiment.
FIG 7A-7I describes and illustrates an exemplary interactive medication
management system according to an embodiment.
2b
CA 2769851 2017-10-04
CA 02769851 2012-02-01
WO 2011/019967
PCT/US2010/045388
DETAILED DESCRIPTION
In the following detailed description, numerous specific details are set forth
in order
to provide a thorough understanding of embodiments of the disclosure. In other
instances,
well known structures, interfaces, and processes have not been shown in
detail, as they are
understood by those of skill in the art. It is intended that no part of this
specification be
construed to effect a disavowal of any part of the full scope of the
disclosure.
The present invention relates to systems, methods, and apparatuses for the
management, dispensing, and administration of over-the-counter and
prescription
medications through use of a pharmaceutical container labeling system and an
optional
integrated computer-based or online management system.
In certain aspects, the invention relates to a pharmaceutical container
comprising a
hollow body, a child-resistant cap, and a specialized information label
affixed to the hollow
body. In certain embodiments, the hollow body may have a squared form that has
a
rectangular longitudinal shape and a rectangular or square cross-section, with
one corner cut
.. across.
More particularly, the cap and the neck of the hollow body provide a unique
child-
resistant mechanism. In certain embodiments, the mechanism is tabbed,
requiring a user to
press the corners of the hollow body instead of the sides. This closure
provides the spring
mechanism required to reposition the tab after it has been reassembled onto
the container.
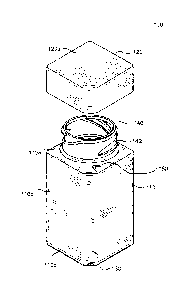
In one embodiment, as illustrated in Figurel, the hollow body 110 of the
pharmaceutical container 100 has a squared form. In one particular embodiment,
the squared
form may have a rectangular longitudinal shape. In an alternative embodiment,
the squared
form may have a square cross-section shape. However, the disclosure is not so
limited and
alternative forms of the hollow body are within the scope of the present
disclosure, e.g.,
round, triangular, etc.
With reference to Figure 1, hollow body 110 and cap 120 including one or more
flat
surfaces 130 may be preferred, as opposed to hollow forms including solely
curved surface
(e.g., a round bottle). However, any suitable hollow form may be used. In
certain
embodiments, the hollow body may have a square form, triangular form, round
form, etc.,
except that one or more corners, edges, or select portions of the cross-
sectional form is cut off
at an angle to create a flat side(s) 130 (the "Flat", see., e.g., Fig. 1). In
certain aspects, the
Flat 130 may be used to accommodate a unique label feature, printed
information, organizing
information, etc.
3
CA 02769851 2012-02-01
WO 2011/019967
PCT/ES2010/045388
In certain embodiments, the hollow body may be round in form with one or more
portions of the cross-sectional form cut off at an angle to create one or more
flat side(s). In
other embodiments, the hollow body may be triangular in form with one or more
corners of
the cross-sectional form cut off at an angle to create one or more flat
side(s). In yet other
embodiments, the hollow body may be square or rectangular in form with one or
more edges
or corners cut off at an angle to create one or more flat side(s).
Figure 2 illustrates pharmaceutical container 100 with cap 120 in a detached
position
from hollow body 110, exposing a container neck 140 and threads or locking
protrusions 142
(which generally correspond to threads or locking grooves 144 of cap socket
146, see Fig.
3A-3B).
In one embodiment, multi-lead threads 142 of various sizes and configuration
on the
neck 140 of the hollow body 110, for which the bottle cap 120 is
correspondingly grooved
144, ensure that the closure can be applied in only one position. Again, in
certain
embodiments, a locking mechanism may be including, such as tab 150 on hollow
body 110
and corresponding locking tab 152 on cap 120, requiring a user to press the
corners of the
hollow body 110 to disengage locking tab 152. This closure provides the spring
mechanism
required to reposition the tab after it has been reassembled onto the
container.
In certain embodiments, non-uniform spacing of the thread configuration may
prevent
the closure from being cross-threaded, preventing the threads from engaging in
the wrong
location or position. By way of example, as shown in Figures 3A-3B, three non-
uniformly
spaced threads may be incorporated to ensure proper functioning. The shape of
the thread
"bottoms" at the thread lead-in may be flat so that the thread, while
disengaged, will ride
around the ledge on the bottle finish until the proper thread engagement is
aligned. The size
and spacing may be used to ensure that the closure is stable prior to
engagement and to
prevent improper engagement and cross-threading.
Generally, with reference to Figures 1 and 3, the hollow body 110 includes a
top
portion 110a, a bottom portion 110b and side portions 110c extending between
the top and
bottom portion to enclose the hollow body. The top portion 110a of the hollow
body further
includes a neck 140 having threads 142, and the side portions 110c of the
hollow body 110
are configured such that one or more corners, edges, or select portions of the
cross-sectional
form is cut-off at an angle to create one or more longitudinal flat portion(s)
130. The cap 120
generally includes a top portion 120a, a bottom portion 120b and side portions
120c
extending between the top and bottom portions, and configured so as to
correspond in shape
with the hollow body. The cap 120 includes a socket 146 having threads144
generally
4
CA 02769851 2012-02-01
WO 2011/019967 PCT/US2010/045388
corresponding to the threads142 of the neck 140 of the hollow body 110 so as
to engage and
secure closure of the cap 120 to the neck 140. The hollow body 110 and the cap
120 further
include interlocking tab porti0ns150, 152 to provide a child resistant closure
mechanism.
In another aspect, a specialized label is provided, which may be adhered to
the hollow
body. The specialized label is generally designed to make it easier for a
consumer to
understand medicament administration instructions and information. The
specialized label
may be configured in any suitable manner so as to accommodate the hollow body.
More
particularly, in certain embodiments, the specialized label may be configured
to include
multiple panels sufficient in number to align with surfaces of the hollow
body. In certain
embodiments, the label may wrap around three sides of the hollow body (plus
the Flat(s)) or a
portion of the body, leaving the remaining side or portion unlabeled, to
thereby leave a side
or portion of the hollow body available for viewing of the contents of the
hollow body (e.g.,
pills or liquid remaining in the pharmaceutical container).
As depicted in Figure 4 and explained in further detail herein, in one
embodiment, the
label may have multiple panels secured to the hollow body of the
pharmaceutical container,
e.g., three and one quarter outer panels (not shown, see FIG. 5A), two inner
panels 410a and
an expandable feature 410b to store additional information on the reverse, or
inside, side.
As shown in Figures 5A-5B, in one embodiment of the invention, a specialized
label
(not secured to a hollow body) is illustrated, wherein the first outer panel
510a may list drug,
pharmacy and patient information. A second outer panel 510b may list dosing
instructions
and primary warnings associated with taking the medication. A third outer
panel 510c may
show visual, pictorial administration instructions, e.g., a MedChart. The
third outer panel
510c may also include the first name of the consumer/patient in large, bold
type. This feature
allows the consumer to immediately recognize which medications belong to him
or her.
In certain embodiments, the MedChart may have two or more vertical columns or
multiple divided areas (e.g., horizontal divided areas, diagonal, etc.),
separated into multiple
rows or subareas. In the first column or divided area, an icon or pictorial
representation may
listed in each corresponding row or subarea for a medication administration
timeframe, such
as morning, noon, evening, bed time, meal time (breakfast, lunch, dinner,
snack time), etc.
The second column or divided area may call out (e.g., through pictorial
representation), in the
corresponding row or subarea, the amount of medication to be taken during that
particular
time frame. This MedChart provides several benefits to the consumer. First, it
is a quick
iconic reference for someone to see their exact dosage during the correct time
frame. It is
also beneficial for people who do not read English well. Third, if the
consumer arranges
5
CA 02769851 2012-02-01
WO 2011/019967
PCT/US2010/045388
multiple bottles with the MedChart facing outwards, it is possible to see at a
glance when
multiple medications must be taken. In certain aspects, prescriber pads could
be integrated
with the MedChart, wherein the prescriber provides clear information to the
pharmacist/dispenser in a format similar to the MedChart regarding dosage
administration in
the appropriate time frame, e.g., AM, Noon, Eve, Bed, meal time, etc.
A fourth outer panel 510d may include the name of the medication in large,
bold type,
as well as measurement markers for liquid medications. This feature allows an
individual to
immediately determine the contents of the bottle. The fourth out panel, in
certain
embodiments, may be configured to be aligned with the Flat 130 of the hollow
body upon
securing to the pharmaceutical container.
An expandable feature of the label 510e, configured to fold out from the
hollow body
upon securing to the pharmaceutical container (see, e.g., Fig. 4, 410b)
provides for one or
more inner panels, 510e-1, 510e-2, that can include useful information, e.g.,
for user
administration, medicament usage and warnings, etc..
With reference to Figure 5B, in one embodiment, the specialized label may
optionally
be printed in a double sided manner. By way of example, first 512a and second
512b inner
panels may display relevant advice and secondary precautions for the
prescribed medication.
If desired (depending on the size of the expandable feature), third and fourth
inner panels
may display patient information, drug information, common uses of the
medication,
pharmacy information and provider information. The patient and drug
information can be
repeated on inner and outer panels so it is always visible.
In an alternative embodiment, e.g., as illustrated in Figure 6, a single sided
specialized
label may be desired. As shown, a first outer panel 610a may list drug,
pharmacy and patient
information. A second outer panel 610b may list dosing instructions and
primary warnings
associated with taking the medication. A third outer panel 610c may show
visual, pictorial
administration instructions, e.g., a MedChart as described above. The third
outer panel 610c
may also include the first name of the consumer/patient in large, bold type.
This feature
allows the consumer to immediately recognize which medications belong to him
or her.
A fourth outer panel 610d may include the name of the medication in large,
bold type,
as well as measurement markers for liquid medications. This feature allows an
individual to
immediately determine the contents of the bottle. The fourth out panel, in
certain
embodiments, may be configured to be aligned with the Flat 130 of the hollow
body upon
securing to the pharmaceutical container.
6
CA 02769851 2012-02-01
WO 2011/019967 PCT/US2010/045388
An expandable feature of the label 610e, configured to fold out along 610e-3
from the
hollow body upon securing to the pharmaceutical container (see, e.g., Fig. 4,
410b) provides
for one or more inner panels, 610e-1, 510e-2, that can include useful
information, e.g., for
user administration, medicament usage and warnings, etc.
An optional feature may be included which allows for a clear view window,
610h. In
certain embodiment, panel 610h may be configured with a die-cut window 610h-1
that may
be removed prior to, upon application, or following application to a
pharmaceutical container,
to thereby allow visual access to the container.
The pharmaceutical containers described herein provide a variety of benefits.
Without intending to be limited, in certain embodiments, storing the bottles
is simple: all
bottles can be organized with the "front" facing outwards, so that relevant
information is
easily legible. This is convenient both for pharmacists and consumers. The
configuration
and sizing allows for more information to be conveyed on the specialized
label. Further, the
configuration and shape may improve ease of use. Child resistant caps are
usually
troublesome to push and twist even for the most skilled set of hands. The
unique shape of the
container, combined with the novel form of child resistant cap, provides an
additional point
of leverage making the container easy to grip and open. In certain
embodiments, the Flat
may provide a convenient surface on the closure for a thumb or finger while
pressing inward
to release the child resistant tab from the latch on the bottle.
In yet another aspect, the specialized label described herein may link users
(e.g.,
pharmacists, prescribers, patients, etc.) to an interactive medication
management system
where users can get assistance in monitoring medications, dosing regimens, as
well as
accurately track current and past medications. In certain embodiments, the
interactive
medication management system may be a stand-alone computer system, a networked
computer system, an on-line computer system, a website, etc.
In certain embodiments, the MedChart of the specialized label may be
replicated on
and linked to the interactive medication management system. By way of example,
the
specialized label may include information which directs patients to a website,
where patients
may view information concerning medication related to the MedChart, input
additional
medication information, organize and keep track of their medications, as well
as share their
information with other authorized users, including authorized family members,
authorized
prescribers, authorized pharmacists, etc. Further, the website may connect the
patient with
his or her prescribers, dispensers, and other health professionals to improve
communication
7
CA 02769851 2012-02-01
WO 2011/019967
PCT/US2010/045388
across these lines of health care, assuring proper understanding around
medication regimens
and promoting adherence.
In one embodiment of the invention, the interactive medication management
system
may include one or more of the following types of interactive online content:
a "home" page providing an overview of the medication management system (see
Figure 6A);
a "tour" page providing a more comprehensive explanation of how the
interactive
management system works (not shown);
an encrypted registration page and portal for consumers to enter their 1)
personal
health history, 2) daily/weekly/monthly medication regimen, and 3) provider
profiles
(pharmacy, physicians). "Sign up" may, in some embodiments, be driven by the
pharmacy or
the physician (see Figures 6B-6D);
a resources page to direct patients to useful web links and local community
resources
(not shown); a "medical organizer" page which pulls together all pertinent
information and
displays any necessary alerts, such as "Your medication X has been recalled,"
or "You have a
prescription ready at the pharmacy." This page may also provide options for
adding and
organizing medications (both over-the-counter and prescription) and user
preferences, such as
alert notifications (see Figures 6E-6F);
a "medical reminder" page, which provides adherence support to consumers by
providing options for consumers to receive electronic reminders to take
medicine, order a
refill, or go to a medical appointment via social networking sites (such as
Facebook,
MySpace, Twitter, etc.) or via email, SMS, text, etc. (see Figure 6G); and
a "medical share" page, which provides an option to share personal medication
information and communicate with pharmacists, health care providers,
caregivers, and/or
family members (see Figure 6H).
By way of non-limiting example, an illustrative map of online content of an
interactive medication management system is shown in Figure 61. As will be
generally
recognized by those of skill in the art, information for use in connection
with the interactive
medication management system may be stored in one or more database(s),
displayed via one
or more user interfaces, and communicated over any suitable technology (e.g.,
intranet,
extranet, intemet, cellular networks, etc). Data and information may be
uploaded, stored,
input, etc. into databases in any known manner, and interactive online content
may be
displayed in any known manner (websites, VPN networks, intranets, etc.).
8
CA 02769851 2012-02-01
WO 2011/019967
PCT/US2010/045388
The interactive medication management system may also provide an option for an
on-
line viewable and/or computer printable pictorial management service. By way
of example,
the on-line viewable and/or computer printable pictorial management service
may provide an
option to view and/or print dosing and/or appointment calendars, e.g., pocket
calendars, desk
calendars, etc., including daily, weekly, monthly, etc. dosing instructions
(e.g., MedChart
information), refill reminders, appointment reminders, etc.
The interactive medication management system provides numerous benefits for
users,
such as reducing the need for in-person or telephone interactions with medical
providers,
assisting the patient in adhering to a schedule, organizing medications,
providing reminders,
sharing information, and tracking side effects. The interactive system can
also consolidate all
of the patient's medications, whether over-the-counter or prescribed. It may
generate a
complete MedChart which gives a visual indicator of medication schedules and
may be
linked to the MedChart shown on each label. The interactive system may also
provide for
comprehensive information sharing; by patient invitation, the system may allow
healthcare
providers access to medical record and to be notified when the patient fills
or refills a
medication. It may also allow a patient to share information with family
members or friends.
The system may further provide links for medication information, such as
medical references,
the FDA website, or insurance education websites. It may also include a
forums, where users
can discuss health issues or consult with physicians or pharmacists about
concerns.
The interactive system also provides benefits for physicians. The website
provides a
medication reconciliation tool, which serves as a master list of all
medications taken by each
patient, including over-the-counter medications and medications prescribed by
other
providers. This may prevent harmful interactions not caught at the pharmacy.
The system
also provides adherence follow-up information, such as a confirmation of
whether the patient
filled or refilled medications at the right times. It may assist in early
intervention, by
increasing communication and counseling with patients through a chat room or
emailed
questions.
The system further provides benefits for pharmacists and dispensers. It may
serve as
the "Medication Therapy Management" component under Medicare Part D, which
allows
pharmacies to include counseling over medications as part of the dispensing
fee. The system
may direct patient communication; if a patient is late on refills or if
important updates are
issues for a medication precaution, these issues are easy to address. The
system may serve a
bridging function for medical and pharmacy technology. Further, the system may
encourage
efficiency, by allowing for easier and faster refill orders, and ensuring that
these occur at the
9
CA 02769851 2012-02-01
WO 2011/019967
PCT/US2010/045388
proper time by the ability to notify patients that they need to call in
refill, or even allow for
'opt out' function.
What has been described and illustrated herein is a preferred embodiment of
the
invention along with some of its variations. The terms, descriptions and
figures used herein
are set forth by way of illustration only and are not meant as limitations.
Those skilled in the
art will recognize that many variations are possible within the spirit and
scope of the
invention, which is intended to be defined by the following claims, in which
all terms are
meant in their broadest reasonable sense unless otherwise indicated therein.