Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02770947 2012-02-10
1
Method for reducing the carbon dioxide emissions of a blast furnace, and
associated device
The invention relates to a method for reducing the carbon dioxide emissions
of a blast furnace. The invention also relates to a device implementing said
method.
A blast furnace is a gas-liquid-solid countercurrent chemical reactor whose
main objective is to produce pig iron, which is then converted to steel by
reducing
its carbon content.
The blast furnace is conventionally supplied with solid materials, mainly
sinter, pellets, iron ore and carbonaceous material, generally coke, charged
into its
upper part, called throat of the blast fumace. The liquids consisting of pig
iron and
slag are tapped from the crucible in the bottom of the blast furnace.
The iron-containing burden (sinter, pellets and iron ore) is converted to pig
iron conventionally by reducing the iron oxides with a reducing gas
(containing
CO, H2 and N2 in particular), which is formed by combustion of the
carbonaceous
material in the tuyeres located in the lower part of the blast furnace, where
air,
preheated to a temperature of between '1000 and 1300 C, called hot blast, is
injected.
This process of converting the iron-containing burden takes place in two
distinct zones of the apparatus, separated by an intermediate zone called a
thermal reserve zone. The latter Is characterized by an interruption of the
heat
exchanges associated with the fact that the gas and the solids are practically
at
the same temperature, called the reserve zone temperature. This also causes an
interruption of the chemical reactions between gases and solids, thus defining
a
chemical reserve zone.
The two zones where the iron-containing materials are converted are:
- the lower part of the apparatus, called the production zone, which sets the
energy requirements of the blast furnace and serves to carry out the
conversion of
the iron oxides from the wustite state to iron metal. It also serves to heat
and melt
the materials from the reserve zone temperature to the final pig iron
temperature;
- the upper part of the apparatus, called the preparation zone, which acts as
a recuperator of the thermal and chemical potential of the gas. It serves to
heat
the materials from the ambient temperature to the reserve zone temperature,
and
CA 02770947 2014-10-07
2
to carry out the reduction of the Iron oxides charged (hematite and magnetite)
to
the wustite state.
To Increase productivity and reduce costs, auxiliary fuels are also injected
Into the tuyeres, such as pulverized coal, fuel oil, natural gas or other
fuels,
combined with oxygen which enriches the hot blast.
The gases recovered in the upper part of the blast furnace, called top gases,
mainly consist of CO, CO2, H2 and N2 in proportions of about 22%, 22%, 3% and
53%, respectively. These gases are generally used as fuel In other parts of
the
plant. Blast furnaces are therefore large producers of CO2.
In fact, faced with the considerable increase in the CO2 concentration in the
atmosphere since the onset of the last century, it is essential to reduce the
CO2
emissions where they are produced in large quantities, and therefore in blast
furnaces In particular.
In this context, during the last 50 years, the consumption of reducing agents,
and mainly of the carbonaceous materials used, has been reduced by half, so
that
today, In blast furnaces of a conventional configuration, the carbon
consumption
has reached a low limit, dependent, on the one hand, on the laws of
thermodynamics, and, on the other hand, on the type and intrinsic properties
of the
carbonaceous materials charged into the throat of the installation.
According to various aspects, the present disclosure relates to a method
for reducing carbon dioxide emissions in top gases of a blast furnace, wherein
reducing agents are charged into the throat of said blast furnace and
auxiliary
fuels in pulverized form are injected into tuyeres, characterized in that the
reducing agents charged into the throat comprise charcoal and in that specific
consumption of the charcoal charged into the throat is more than 0% and less
than 20% of the total quantity of the reducing agents charged into the throat.
According to various aspects, the present disclosure relates to a device
implementing the method as defined herein, comprising means for charging the
charcoal into the throat of the blast furnace so that the consumption of the
charcoal charged into the throat is more than 0% and less than 20% of the
total
quantity of the reducing agents charged into the throat.
CA 02770947 2014-10-07
2a
In this context, the invention proposes a method that significantly limits the
carbon dioxide emissions, without entailing any major changes to the
Installations.
For this purpose, the inventive method for reducing the carbon dioxide
emissions of a blast furnace, in which reducing agents are charged into the
throat
and auxiliary fuels in pulverized form are injected into the tuyeres, is
essentially
characterized in that the reducing agents charged into the throat comprise
charcoal.
The inventive method may also comprise the following optional features,
considered separately or in combination:
- the specific consumption of charcoal charged into the throat is less than
20% of the total quantity of reducing agents charged into the throat;
- the specific consumption of charcoal charged Into the throat is less than
10% of the total quantity of reducing agents charged into the throat;
- the remainder of the reducing agents charged into the throat is mineral
carbon;
CA 02770947 2012-02-10
3
- the charcoal charged into the throat is in the form of pieces having a
diameter larger than 20 millimeters;
- the method comprises a screening step which separates the charcoal
pieces charged into the throat from the fine charcoal fraction;
- the fine charcoal fraction is injected in pulverized form into the
tuyeres, in
addition to and/or as replacement of the corresponding quantity of auxiliary
fuel normally injected in pulverized form into the tuyeres;
- the auxiliary fuel is either mineral coal or charcoal.
The invention also relates to a device for implementing the method defined
above. Said device is essentially characterized in that it comprises means for
charging the charcoal into the throat of the blast furnace.
The inventive device may also comprise the following optional features,
considered separately or in combination:
- the device comprises a screen for separating the charcoal pieces intended
to be charged into the throat from the fine charcoal fraction;
- the device comprises a coal mill in which the fine charcoal fraction is
mixed
with carbonaceous material, the combination formed by the charcoal and
the carbonaceous material being intended to be injected into the tuyeres;
- the corresponding carbonaceous material is either mineral coal or
charcoal.
The invention will be better understood from a reading of the description that
follows, provided with reference to the appended figures in which:
- Figure 1 is a schematic representation of the inventive device according to
a
first alternative, in which the fine charcoal fraction obtained from the
screening operation is not re-used in the method, and
- - Figure 2 is a schematic representation of the inventive device
according to a
second alternative, in which the fine charcoal fraction obtained from the
screening operation is re-used in the method.
In the context of the invention, a particular and essential property of these
carbonaceous materials used as reducing agent is their gasification threshold
or
gasification start temperature. This is the temperature at which the carbon
that
they contain starts to react with the CO2 in the gas passing through the stack
of
the blast furnace to produce carbon monoxide by the chemical reaction:
C + CO2 --> 2 CO
CA 02770947 2012-02-10
4
This gasification threshold sets the reserve zone temperature of the blast
furnace. In a conventional blast fumace, this gasification start temperature
is
about 950 C.
The inventive method is based on the fact that, by lowering the temperature
of the blast furnace reserve zone, the specific consumption of reducing agents
decreases, along with the carbon dioxide emissions.
The Applicant has thus estimated that the decrease in coke consumption
would be approximately 20 kilograms per tonne of liquid metal for a 100 C
decrease in the reserve zone temperature.
While it is known to lower the reserve zone temperature by substituting the
reactive coke for the coke conventionally used, this reactive coke requires
time-
consuming and costly preparation. Furthermore, to the knowledge of the
Applicant,
no link has so far been established between the lowering of the reserve zone
temperature and the reduction of the CO2 emissions.
In this context, the Applicant has discovered that the addition of a small
amount of charcoal charged into the throat, instead of the corresponding
quantity
of conventional coke, serves to lower and to control this reserve zone
temperature
to the level of the charcoal gasification threshold.
In fact, charcoal has a reactivity threshold of about, or lower than, 850 C,
which Is therefore considerably lower than that of the metallurgical coke
conventionally used in a blast furnace.
Moreover, charcoal is a source of mineral carbon that is neutral in carbon
dioxide production, or even negative. In fact, in the blast furnace burden, it
can
replace coke which has an impact on the carbon dioxide emissions of about 3
kilograms of CO2 emitted per kilogram of coke used.
These combined effects (decrease in the quantity of coke used associated
with the lowering of the reserve zone temperature and replacement of mineral
carbon (coke) by carbon coming from biomass (charcoal)) give rise to a
substantial decrease in the quantity of coke consumed, and therefore of
mineral
carbon injected or charged into the blast fumace, and subsequently a
significant
reduction of the carbon dioxide emissions.
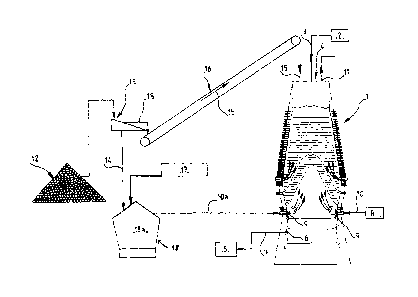
With reference to Figure 1, the blast furnace 1 is supplied with coke, sinter,
pellets, and iron ore 2 via the line 3 into the throat 4. The pig iron and
slag 5 are
recovered at point 6 at the crucible via the line 7. The hot blast and
additional
CA 02770947 2012-02-10
oxygen 8 are introduced into the tuyeres 9 via the line 10. The coal and/or
other
auxiliary reducing agents are also introduced into the tuyeres 9 via the line
10.
The top gases are recovered at point 11 in the upper part of the blast furnace
1.
According to the invention, charcoal as received 12 is sent to a screen 13 in
which the fine fraction 14 is separated from the charcoal pieces 15 charged
into
the throat 4 by means of a charging device 16. These pieces 15 have a diameter
larger than the screen cut-off point, i.e. at least 20 millimeters.
The charcoal pieces 15 can be charged either at the same time as the coke,
or at the same time as the iron ore.
The quantity of charcoal charged into the throat is 20 kilograms per tonne of
pig iron.
With reference to Figure 2, the charcoal pieces 15 are charged into the throat
4 under the same conditions as those described with reference to Figure 1.
According to this alternative, the fine coal fraction 14 obtained from the
screening of the charcoal as received 12 is mixed with coal 17 and pulverized
in
the coal mill 18 to form carbonaceous material 18a intended to be introduced
into
the tuyeres 9 via the line 10a. This coal 17 may either be mineral coal, or
charcoal, as will be described in detail below. Also introduced into the
tuyeres are
the auxiliary reducing agents other than coal schematically shown injected
into the
tuyeres 9 via the line 10a.
The specific consumption of carbonaceous material injected into the tuyeres
is 200 kilograms per tonne of pig iron. This consumption comprises the fine
charcoal fraction 14 from the screening operation, which is estimated to be
equal
to the specific consumption of charcoal charged into the throat, or 20
kilograms
per tonne of pig iron, assuming a screening efficiency of 50%.
For example, Table I below shows the main operating characteristics of a
blast furnace producing 6000 tonnes of pig iron per day, and their variation
when,
according to the second alternative, 20 kilograms of charcoal pieces 15 per
tonne
of molten metal are charged into the throat, and when the fine charcoal
fraction 14
produced during the screening operation is injected in the form of pulverized
charcoal into the tuyeres of the blast furnace, as replacement of an identical
quantity of pulverized auxiliary fuel normally injected into said blast
furnace.
The characteristics mentioned in Table l are as follows:
CA 02770947 2012-02-10
6
- the maximum productivity that can be achieved by this blast furnace under
the conditions considered, expressed in tonnes of molten pig iron per day
(t/d),
- the flow rate of the natural dry blast blown into the tuyeres, expressed in
kilo-Normal cubic meters per hour (kNm3/h),
- the specific consumption, or yield, of coke charged into the throat,
expressed in kilograms per tonne of pig iron (kg/tp),
- the specific consumption of charcoal charged into the throat, expressed in
kilograms per tonne of pig iron (kg/tp),
- the specific consumption of pulverized mineral coal injected into the
tuyeres, expressed in kilograms per tonne of pig iron (kg/tp),
- the specific consumption of pulverized charcoal injected into the
tuyeres,
expressed in kilograms per tonne of pig iron (kg/tp),
- the specific consumption of fine pulverized charcoal fraction injected into
the tuyeres, expressed in kilograms per tonne of pig iron (kg/tp), said fine
fraction
14 being that one, recovered after screening the charcoal 12 during the
production
of the charcoal, charged into the throat 15,
- the flame temperature expressed In degrees Celsius ( C),
- the reserve zone temperature expressed in degrees Celsius ( C),
- the top gas temperature expressed in degrees Celsius ( C), and
- the top gas flow rate expressed in normal cubic meters per hour (Nrn3/h).
Every blast furnace has a given operating range, in which its operation
remains optimal. To make the calculations given in Table I, operational limits
were
therefore set, mainly associated with the temperatures reached in certain
specific
zones of the apparatus, and with the flow rate of gas streams entering and/or
passing through the blast furnace. These limits are as follows:
- the top gas temperature is between 120 and 200 C;
- the flame temperature is between 2000 and 2200 C;
- the top gas flow rate must be lower than or equal to 400 000 Nm3/h
(limitation of the method);
- the natural dry blast flow rate is lower than or equal to 225 kNm3/h
(technological limitation).
Reference 1 corresponds to the charging of coke into the throat and to an
injection of pulverized mineral coal into the tuyeres.
CA 02770947 2012-02-10
7
Reference 2 corresponds to the charging of coke into the throat and to an
injection of pulverized charcoal into the tuyeres. The flame temperature is
controlled at its maximum threshold of 2200 C.
Example 1 corresponds to the charging of 20 kilograms of charcoal pieces 15
per tonne of pig iron into the throat, the remainder of the carbonaceous
material
charged into the throat consisting of coke. Assuming a screening efficiency of
50%, 20 kilograms of fine charcoal fraction 14 obtained from the screening per
tonne of molten metal is pulverized for injection into the tuyeres, as
replacement of
20 kilograms of mineral coal per tonne of pig iron, the remainder of the
pulverized
carbonaceous material injected into the tuyeres being mineral coal. It is
assumed
In this Example 1 that the reserve zone temperature obtained due to the
intrinsic
properties of the charcoal is 850 C.
Example 2 is identical to Example 1, except that the reserve zone
temperature obtained, due to the intrinsic properties of the charcoal, is
assumed to
be 750 C.
Example 3 corresponds to the charging into the throat of 20 kilograms of
charcoal pieces 15 per tonne of pig iron, the remainder of the carbonaceous
material charged into the throat consisting of coke. Assuming a screening
efficiency of 50%, 20 kilograms of fine charcoal fraction 14 from the
screening per
tonne of molten metal is pulverized for injection into the tuyeres, as
replacement of
20 kilograms of injection charcoal obtained independently per tonne of pig
iron.
The remainder of the carbonaceous material pulverized into the tuyeres
consists
of this independently obtained charcoal. In this Example 3, it is assumed that
the
reserve zone temperature obtained due to the intrinsic properties of the
charcoal is
850 C.
Example 4 is identical to Example 3, except that the reserve zone
temperature obtained due to the intrinsic properties of the charcoal is
assumed to
be 750 C. It is found that if the operating conditions of reference 1 are
preserved,
the blast fumace can no longer operate under the conditions selected for this
Example 4. Only a less efficient blast furnace can operate under these
conditions.
So-called less efficient operation may be said to mean a lower efficiency of
reduction of the materials in the upper part of the blast furnace or higher
heat
losses from the apparatus. Reference 3 falls into the latter case, and Example
4
must therefore be compared to this reference 3 and not to references 1 and 2.
In
CA 02770947 2012-02-10
8
other words, the results obtained in Example 4 correspond to the operating
modification of reference 3 when 20 kilograms of charcoal pieces 15 per tonne
of
pig iron are charged into the throat and when the corresponding fine charcoal
fraction 14 is injected in pulverized form into the tuyeres of the blast
furnace.
It is found for Example 1 that all the operating conditions defined above are
satisfied. Moreover, the maximum possible productivity for the blast furnace
under
these conditions is significantly higher than the nominal production of said
blast
furnace. The blast furnace is therefore suitable for operating with a charging
of 20
kilograms of charcoal pieces 15 per tonne of pig iron into the throat and an
injection of 20 kilograms of fine charcoal fraction 14 obtained from the
screening of
the charcoal as received 13 per tonne of pig iron.
For Example 2, assuming that the reserve zone temperature is 750 C, a
number of reaction conditions are no longer satisfied, particularly the flame
temperature, which is substantially lower than 2000 C. The value obtained
nevertheless appears to be sufficiently close to this limit for the blast
furnace to
operate. Moreover, the maximum productivity permitted under these conditions
is
lower than the nominal production of the installation. This type of operation
may
nevertheless be advantageous for low-productivity operation, for example in a
less
favorable economic situation for the steel industry.
It should nevertheless be observed that this result depends on the
assumption concerning the capacities of the installations. In particular, a
blower
having a capacity higher than the limit capacity considered here would help to
maintain the blast furnace productivity at its nominal level of 6000 t/d.
For Example 3, as for Example 1, all the operating conditions are satisfied.
In consequence, the blast fumace can therefore operate with a reserve zone
temperature of 850 C, when 20 kilograms of charcoal pieces 15 per tonne of
molten metal are charged into the throat, 20 kilograms of fine charcoal
fraction 14
obtained from the screening operation are injected in pulverized form into the
tuyeres per tonne of molten metal, the remainder of the coal injected into the
tuyeres possibly being mineral coal such as charcoal.
In Example 4, if all the operating conditions are satisfied, the productivity,
as
for Example 2, is lower than 6000 tonnes/day. In consequence, whether the
remainder of the carbonaceous material injected into the tuyeres is mineral
coal or
charcoal, when the reserve zone temperature is 750 C, the blast fumace does
not
CA 02770947 2012-02-10
9
operate optimally. This type of operation may nevertheless be advantageous for
low-productivity operation, for example in a less favorable economic situation
for
the steel industry.
As for Example 2, this result depends on the assumption concerning the
capacities of the installations.
Table II resumes references 1, 2 and 3 and Examples 1 to 4, and
demonstrates the advantages of the Inventive method according to this second
alternative in terms of reduction of coke consumption, decrease in carbon
dioxide
emissions and beneficiation of charcoal relative to the reduction of the
carbon
dioxide emissions.
Reference 2, which corresponds to a coke injection into the throat and an
injection of pulverized fine charcoal fraction into the tuyeres, is presented
in
comparison with reference 1. In fact, it constitutes an easy-to-implement
solution
for reducing the CO2 emissions. This solution nevertheless has the
disadvantage
of being the least effective of all the solutions presented in terms of
kilograms of
CO2 avoided per kilogram of charcoal used, as shown by the overall results in
said
Table II.
For the configuration of Example 1, a 12% reduction in carbon dioxide
emissions is obtained, for Example 2, a 16% reduction, and for Examples 3 and
4,
reductions of 46 and 48% in the carbon dioxide emissions, respectively.
Coke consumption has been reduced by 13.5% for Example 1, 19.4% for
Example 2, 11.4% for Example 3 and 16.5% for Example 4, whereas it is 2.7%
higher for reference 2.
The ratio of the reduction in carbon dioxide emissions expressed in kilograms
per tonne of pig iron to the charcoal consumption expressed in the same units
illustrates the beneficiation of charcoal for reducing the carbon dioxide
emissions.
It is found that the addition of a small quantity of charcoal into the throat
and
the tuyeres, when the remainder of the carbonaceous materials charged into the
throat and injected into the tuyeres is mineral carbon, provides greater
beneficiation of the charcoal than when the remainder of the carbonaceous
material injected into the tuyeres is charcoal. In fact, this ratio is 4.65
and 6.00 for
Examples 1 and 2, respectively, whereas it is only 3.18 and 3.43 for Examples
3
and 4, and 2.83 for reference 2. In practice, this means that for the same
charcoal
CA 02770947 2012-02-10
availability, the configurations of Examples 1 and 2 serve to maximize the
overall
reduction of CO2 emissions.
According to the first alternative, Table III shows the main operating
characteristics of a blast furnace producing 6000 tonnes of pig iron per day,
and
their variation when 20 kilograms of charcoal pieces 15 per tonne of molten
metal
are charged into the throat without injecting the fine charcoal fraction 14
obtained
from the screening operation into the tuyeres.
The characteristics mentioned in Table III and the operational limits set are
the same as those associated with Table I.
Table III also shows references 1, 2 and 3 already explained for Tables I and
Example 5 corresponds to the charging of 20 kilograms of charcoal pieces 15
into the throat per tonne of pig iron, the remainder of the carbonaceous
material
charged into the throat consisting of coke. 200 kilograms of mineral coal are
injected in pulverized form into the tuyeres per tonne of pig iron. The fine
charcoal
fraction 14 obtained from the screening operation is not injected into the
tuyeres.
Example 6 is identical to Example 5, except that the reserve zone
temperature obtained due to the intrinsic properties of the charcoal is
assumed to
be 750 C.
Example 7 corresponds to the charging of 20 kilograms of charcoal pieces 15
into the throat per tonne of pig iron, the remainder of the carbonaceous
material
charged into the throat consisting of coke. 200 kilograms of charcoal obtained
separately are injected in pulverized form into the tuyeres per tonne of pig
iron.
The fine charcoal fraction 14 obtained from the screening operation is not
injected
into the tuyeres.
Example 8 is identical to Example 7, except that the reserve zone
temperature obtained due to the intrinsic properties of the charcoal is
assumed to
be 750 C. It is found that if the operating conditions of reference 1 are
preserved,
the blast furnace can no longer operate under the conditions selected for this
Example 8. Only a less efficient blast furnace can operate under these
conditions.
So-called less efficient operation can be said to mean a lower efficiency of
the
reduction of the materials in the upper part of the blast furnace or higher
heat
losses from the apparatus. Reference 3 falls into the latter case, and Example
8
must therefore be compared to this reference 3 and not to references 1 and 2.
in
CA 02770947 2012-02-10
11
other words, the results obtained in Example 8 correspond to the operating
modification of reference 3 when 20 kilograms of charcoal pieces 15 are
charged
into the throat per tonne of pig iron without injecting the corresponding fine
charcoal fraction 14 in pulverized form into the tuyeres of the blast furnace.
It is found for Example 5 that all the operating conditions defined above are
satisfied. Moreover, the maximum possible productivity for the blast furnace
under
these conditions is significantly higher than the nominal production of this
blast
furnace. The blast furnace therefore appears suitable for operating with a
charging of 20 kilograms of charcoal pieces into the throat per tonne of
molten
metal, without injecting the fine charcoal fraction 14 obtained from the
screening
into the tuyeres.
For Example 6, assuming that the reserve zone temperature is 750 C, a
number of reaction conditions are no longer satisfied, particularly the flame
temperature, which is slightly lower than 2000 C. The
value obtained
nevertheless appears to be sufficiently close to this limit for the blast
furnace to
operate. Moreover, the maximum productivity permitted under these conditions
is
lower than the nominal production of the installation. This type of operation
may
nevertheless be advantageous for low-productivity operation, for example in a
less
favorable economic situation for the steel industry. As for Examples 2 and 4,
this
result depends on the assumption concerning the capacities of the
installations.
For Example 7, as for Example 5, all the operating conditions are satisfied.
In consequence, the blast furnace can therefore operate with a reserve zone
temperature of 850 C, when 20 kilograms of charcoal pieces 15 per tonne of
molten metal are charged into the throat, without injecting the fine charcoal
fraction
14 obtained from the screening into the tuyeres as a mixture with the
pulverized
charcoal normally injected.
In Example 8, if all the operating conditions are satisfied, the productivity,
as
for Example 6, is lower than 6000 tonnes/day. In consequence, whether the
remainder of the carbonaceous material injected into the tuyeres is mineral
coal or
charcoal, when the reserve zone temperature is 750 C, the blast furnace does
not
operate optimally. This type of operation may nevertheless be advantageous for
low-productivity operation, for example in a less favorable economic situation
for
the steel Industry.
CA 02770947 2012-02-10
12
As for Example 6, this result depends on the assumption concerning the
capacities of the installations.
Table IV resumes references 1, 2 and 3 and Examples 5 to 8, and
demonstrates the advantages of the inventive method according to the first
alternative in terms of reduction in coke consumption, decrease in carbon
dioxide
emissions and beneficiation of the charcoal relative to the reduction in
carbon
dioxide emissions.
For the configuration of Example 5, an 8% reduction in carbon dioxide
emissions is obtained, and for Example 6, a 12% reduction in carbon dioxide
emissions.
For the configuration of Example 7, a 46% reduction in carbon dioxide
emissions is obtained, and for Example 8, a 48% reduction in carbon dioxide
emissions in relation to reference 3.
Coke consumption has been reduced by 13.5% for Example 5 and 19.1% for
Example 6.
it has been reduced by 10.9% for Example 7, and 15.9% for Example 8 in
comparison with reference 3.
The ratio of the reduction in carbon dioxide emissions expressed in kilograms
per tonne of pig iron to the Charcoal consumption expressed in the same units
illustrates the beneficiation of charcoal for reducing the carbon dioxide
emissions.
The impact of not injecting the fine fraction obtained from the screening of
the
charcoal as received into the tuyeres of the blast furnace can be evaluated by
comparing Example 1 to Example 5, Example 2 to Example 6, Example 3 to
Example 7, and Example 4 to Example 8. This shows that the beneficiation of
charcoal relative to the total quantity of charcoal as received is
significantly lower
in Example 5 (3.11) in comparison with Example 1 (4.65), and also in Example 6
(4.37) in comparison with Example 2 (6.00). This is also the case to a lesser
degree when Example 7 (2.87) is compared to Example 3 (3.18) and Example 8
(3.09) to Example 4 (3.43).
On the other hand, if only the charcoal used in the blast furnace is taken
into
account, that is to say the case in which the fine fraction obtained from the
screening can be utilized elsewhere, the beneficiation of charcoal relative to
the
quantity of charcoal actually introduced into the blast furnace is
significantly higher
CA 02770947 2012-02-10
13
in Example 5 (6.21) in comparison with Example 1 (4.65), and also in Example 6
(8.74) in comparison with Example 2 (6.00).
However, this is not true in the case in which pulverized charcoal is injected
into the tuyeres, because the total quantities of charcoal actually used in
the blast
furnace in Examples 7 and 8 are identical to those used in Examples 3 and 4,
respectively. The beneficiation of charcoal relative to the quantity of
charcoal
actually introduced into the blast furnace in Example 7 (3,16) is thus
virtually
identical to that obtained in Example 3 (3.18), and that of Example 8 (3.41)
is very
close to that of Example 4 (3.43). The differenc,es observed are associated
with
the difference in chemical composition between the charcoal as received 13 and
the charcoal normally injected into the tuyeres.
All the results given above reveal that the charging of a small amount of
charcoal into the throat, whether the fine fraction is or is not injected into
the
tuyeres, serves to considerably decrease the coke consumption thanks to the
dual
effect of the replacement of the coke by the charcoal and the lowering of the
reserve zone temperature. Moreover, the carbon dioxide emissions are
significantly reduced, in general with substantial beneficiation of the
charcoal,
particularly in the case in which the carbonaceous material injected into the
tuyeres is mineral coal.
14
Table I
Units Reference 1
Reference 2 Example 1 Example 2 Example 3 Reference 3 Example 4
Maximum possible productivity t/d 6 818 6 131 6 540
5 832 6 098 6 326 5 690
Natural dry blast flow rate kNe/h 204 221 209
225 222 212 225
Specific consumption of dry kg/tp 284 292 246
229 252 302 252
coke charged into throat
Specific consumption of dry kg/tp - - 20
20 20 - 20 (-)
charcoal charged into throat .
0
Specific consumption of
I.)
-I
pulverized mineral coal injected kg/tP 200 - 180
180 - - - -1
0
(wet) into the tuyeres
ko
Specific consumption of
pulverized charcoal injected kgitP - 200 - -
180 200 180 N)
0
H
(wet) into the tuyeres
N)
i
Specific consumption of fine
0
charcoal fraction injected (dry) kgliP - - 20
20 20 - 20 T
,
into the tuyeres
0
Flame temperature oc 2 147 2 200 2 088 1 987
2 139 2 200 2 136
Reserve zone temperature oc 950 950 850 750
850 950 750
Top gas temperature C 150 151 120 120
120 170 120
_
Top gas flow rate Nreih 368 432 370 170 365 306
366 100 365 029 382 461 358 201
15
Table II
= Units Reference 1
Reference 2 Example 1 Example 2 Example 3 Reference 3 Example 4
Reserve zone temperature .0 950 950 850 750 '
850 950 750
'
Maximum possible productivity t/d ' 6 818 6 131 6 540 5
832 6 098 6 326 5 690
Flame temperature C 2 147 - 2 200 2 088 1 987 '
2 139 2 200 2 136
Specific consumption of dry kg/tp 284.5 292.2 246.0
229.4 252.0 301.9 = 252.5
coke charged into throat
n
-
Reduction of ooke consumption % - -2.7 115
19.4 11.4 - 16.5 0
I.),
Decrease in CO2 emissions % - 37 12 16
46 - 48 -1
-1
0
Decrease in CO2 emissions kg/tp - 560 186 240
693 - - 748 ko
a,
-1
_
'
Total charcoal consumption !cop _ 198 40
40 218 198 218 I.)
0
(dry) _
H
I.)
1
Reduction in CO2 emissions/total - - 2.83 4.66
6.00 3.18 - 3.43 0
I.)
charcoal consumption
1
= H
0
16
Table 111
Units Reference 1 Reference 2 Example 5
Example 6 Example 7 Reference 3 Example 8
,
Maximum possible productivity Yid 6 818 6 131 6
569 , 5 854 6 078 6 326 5 677
-'
Natural dry blast flow rate kNr-n3/h 204 221
208 225 223 212 225
_
Specific consumption of dry kgitp 284 292 247
230 254 302 254
coke charged into throat
Specific consumption of dry !cop _ - 20
20 20- 20
charcoal charged into throat ,
Specific consumption of
pulverized mineral coal injected kgitto 200- 200
200 - - - n
(wet) into the tuyeres _
0
Specific consumption of
I.)
pulverized charcoal injected kg/tP - 200 - -
200 200 200 -1
-1
0
(wet) into the tuyeres
ko
-
.1,.
Specific consumption of fine
-1
charcoal fraction in - jected (dry) kgitP - -
- _ - - I.)
0
H
into the tuyeres
N)
.
1
Flame temperature C 2 147 2 200 2 086 1 985 2
142 . 2 200 2 140 0
I.)
I
Reserve zone temperature C 950 950 850 750
850 950 750 H
0
Top gas temperature C 150 151 120 120
120 170 120
Top gas flow rate Neill 368 432 370 170 365 388 367 509
365 080 382 461 357 344
17
Table IV
Units Reference 1 Reference 2 Example 5
Example 6 Example 7 Reference 3 Example 8
Reserve zone temperature C 950 950 850 750
850 950 750
Maximum possible productivity t/d 6 818 ' 6 131 6 569
5 854 6 078 6 326 5 677
'
Flame temperature oc 2 147 2 200 2 086 1 985
2 142 2 200 2 140
_
,
Specific consumption of dry kg/fp 284.5 292.2 246.8
230.3 253.6 301.9 254.0 n
coke charged into throat 1
0
Reduction of coke consumption % - -2.7 13.5 19.1 ,
10.9 - 15.9 ="
-1
-1
,
, Decrease in CO2 emissions % - 37 8 12
46 - 48 ko
.1,.
-1
Decrease in CO2 emissions kg/tp - 560 124 175
688 - 743 I.)
0
H
Consumption of charcoal as kop . 198 40 40
238 198 238 I.)
I
received (dry)
0
I.)
1
Consumption of charcoal in kop _ 198 20 20
218 198 218 H
blast furnace (dry) 0
_
Reduction in CO2 emissions/
consumption of charcoal as - - 2.83 3.11 4.37
2.87 - 3.09
received
Reduction in CO2 emissions/
consumption of charcoal in - - 2.83 821 8/4
3.16 - 3.41
blast furnace