Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02806873 2013-10-22
LIGNIN PRODUCTION FROM LIGNOCELLULOSIC BIOMASS
[0001]
FIELD OF THE INVENTION
[0002] The present invention generally relates to methods of preparing lignin
from
lignocellulosic biomass. More particularly, it relates to methods of preparing
lignin from
lignocellulosic biomass using rapid full or partial pressure reduction to
separate and to pulverize the
lignin without fouling the equipment and with improved energy recovery.
BACKGROUND OF THE INVENTION
[0003] Existing processes delignify lignocellulosic biomass before entering
the cellulose
conversion process using solvents or other chemicals. In such delignification
processes, complex
equipment is typically required and is expensive to operate because of the
solvent or chemical
usage and lack of recovery methods. In other existing processes, the solid
conversion of
lignocellulosic biomass in pre-treatment and hydrolysis requires high
temperatures to fully or
partially solubilize the lignin present. Upon cooling, the lignin precipitates
from solution. The
lignin may be recovered from the process and burned for thermal energy. The
particle size of the
recovered lignin may be variable and too large for efficient burning, thus
requiring a separate
pulverizing step. Furthermore, as the lignin in solution cools, it becomes
sticky (typically in the
glass transition temperature range of lignin, which is about 100 C under
ambient pressure) and
tends to foul the process equipment to the point of making the process
inoperable. It would be
useful to have methods for providing lignin of a substantially uniform, small
particle size for
improving burning efficiency, for enhanced properties for the use of lignin as
a feedstock for the
production of other chemicals, and for avoiding typical equipment fouling
problems. It would
- 1 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
also be useful to maximize energy recovery. The methods and compositions of
the present
invention are directed toward these, as well as other, important ends.
SUMMARY OF THE INVENTION
[0004] In one embodiment, the invention is directed to methods of preparing
lignin from
lignocellulosic biomass, comprising:
providing lignocellulosic biomass at a first pressure and at a first
temperature,
said lignocellulosic biomass comprising:
a first solid fraction comprising:
insoluble lignin; and
a first liquid fraction comprising:
soluble C6 saccharides; and
soluble lignin;
reducing said first temperature of said lignocellulosic biomass to a second
temperature at least about 1 C above the glass transition temperature of
lignin under said
first pressure; and
reducing said first pressure of said lignocellulosic biomass at said second
temperature to a second pressure in a time less than about 1 second to
precipitate said
soluble lignin in said first liquid fraction and form a mixture comprising:
a second solid fraction comprising:
insoluble lignin; and
precipitated lignin; and
a second liquid fraction comprising:
soluble C6 saccharides;
wherein the average particle size of said insoluble lignin and precipitated
lignin is
less than about 500 microns.
[0005] In another embodiment, the invention is directed to lignin products
produced by the
methods of the invention.
- 2 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
[0006] In another embodiment, the invention is directed to compositions,
comprising:
lignin having an average size of no greater than about 500 micron;
wherein said lignin is processed from lignocellulosic biomass using
supercritical
or near critical fluid extraction.
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] The accompanying drawings, which are included to provide a further
understanding of
the invention and are incorporated in and constitute a part of this
specification, illustrate
embodiments of the invention and together with the description serve to
explain the principles
of the invention. In the drawings:
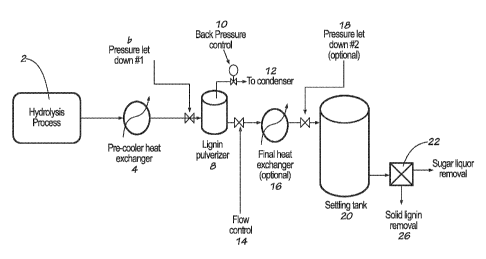
[0008] FIGURE 1 is a schematic diagram of the method of producing lignin from
cellulosic
biomass in one embodiment of the invention.
[0009]
FIGURE 2 is a plot of % moisture content (wet basis) as a function of
cumulative
drying time in hours for lignin.
[0010] FIGURE 3 is a plot of high heating value (HHV) as a function of
moisture content for
extracted lignin and filtered lignin.
DETAILED DESCRIPTION OF THE INVENTION
[0011]
As employed above and throughout the disclosure, the following terms, unless
otherwise indicated, shall be understood to have the following meanings.
[0012]
As used herein, the singular forms "a," "an," and "the" include the plural
reference
unless the context clearly indicates otherwise.
[0013]
While the present invention is capable of being embodied in various forms, the
description below of several embodiments is made with the understanding that
the present
- 3 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
disclosure is to be considered as an exemplification of the invention, and is
not intended to limit
the invention to the specific embodiments illustrated. Headings are provided
for convenience
only and are not to be construed to limit the invention in any manner.
Embodiments illustrated
under any heading may be combined with embodiments illustrated under any other
heading.
[0014]
The use of numerical values in the various quantitative values specified in
this
application, unless expressly indicated otherwise, are stated as
approximations as though the
minimum and maximum values within the stated ranges were both preceded by the
word
"about." In this manner, slight variations from a stated value can be used to
achieve substantially
the same results as the stated value. Also, the disclosure of ranges is
intended as a continuous
range including every value between the minimum and maximum values recited as
well as any
ranges that can be formed by such values. Also disclosed herein are any and
all ratios (and
ranges of any such ratios) that can be formed by dividing a recited numeric
value into any other
recited numeric value. Accordingly, the skilled person will appreciate that
many such ratios,
ranges, and ranges of ratios can be unambiguously derived from the numerical
values presented
herein and in all instances such ratios, ranges, and ranges of ratios
represent various
embodiments of the present invention.
[0015]
As used herein, the phrase "substantially free" means have no more than about
1%,
preferably less than about 0.5%, more preferably, less than about 0.1%, by
weight of a
component, based on the total weight of any composition containing the
component.
[0016] As used herein, the term "saccharification" and "saccharified" refers
to the breakdown
of polysaccharides to smaller polysaccharides, including oligosaccharides, and
monosaccharides,
whether through hydrolysis, the use of enzymes, or other means, generally into
a liquid fraction
and a solid fraction.
[0017] A supercritical fluid is a fluid at a temperature above its critical
temperature and at a
pressure above its critical pressure. A supercritical fluid exists at or above
its "critical point," the
point of highest temperature and pressure at which the liquid and vapor (gas)
phases can exist in
equilibrium with one another. Above critical pressure and critical
temperature, the distinction
- 4 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
between liquid and gas phases disappears. A supercritical fluid possesses
approximately the
penetration properties of a gas simultaneously with the solvent properties of
a liquid.
Accordingly, supercritical fluid extraction has the benefit of high
penetrability and good
solvation.
[0018]
Reported critical temperatures and pressures include: for pure water, a
critical
temperature of about 374.2 C, and a critical pressure of about 221 bar; for
carbon dioxide, a
critical temperature of about 31 C and a critical pressure of about 72.9
atmospheres (about 1072
psig). Near-critical water has a temperature at or above about 300 C and below
the critical
temperature of water (374.2 C), and a pressure high enough to ensure that all
fluid is in the
liquid phase. Sub-critical water has a temperature of less than about 300 C
and a pressure high
enough to ensure that all fluid is in the liquid phase. Sub-critical water
temperature may be
greater than about 250 C and less than about 300 C, and in many instances sub-
critical water has
a temperature between about 250 C and about 280 C. The term "hot compressed
water" is used
interchangeably herein for water that is at or above its critical state, or
defined herein as near-
critical or sub-critical, or any other temperature above about 50 C
(preferably, at least about
100 C) but less than subcritical and at pressures such that water is in a
liquid state.
[0019]
As used herein, a fluid which is "supercritical" (e.g. supercritical water,
supercritical
CO2, etc.) indicates a fluid which would be supercritical if present in pure
form under a given set
of temperature and pressure conditions. For example, "supercritical water"
indicates water
present at a temperature of at least about 374.2 C and a pressure of at least
about 221 bar,
whether the water is pure water, or present as a mixture (e.g. water and
ethanol, water and CO2,
etc.). Thus, for example, "a mixture of sub-critical water and supercritical
carbon dioxide"
indicates a mixture of water and carbon dioxide at a temperature and pressure
above that of the
critical point for carbon dioxide but below the critical point for water,
regardless of whether the
supercritical phase contains water and regardless of whether the water phase
contains any carbon
dioxide. For example, a mixture of sub-critical water and supercritical CO2
may have a
temperature of about 250 C to about 280 C and a pressure of at least about 225
bar.
- 5 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
[0020] As used herein, "continuous" indicates a process which is uninterrupted
for its duration,
or interrupted, paused or suspended only momentarily relative to the duration
of the process.
Treatment of biomass is "continuous" when biomass is fed into the apparatus
without
interruption or without a substantial interruption, or processing of said
biomass is not done in a
batch process.
[0021] As used herein, "resides" indicates the length of time which a given
portion or bolus of
material is within a reaction zone or reactor vessel. The "residence time," as
used herein,
including the examples and data, are reported at ambient conditions and are
not necessarily
actual time elapsed.
[0022] As used herein, the term "substantial free of' refers to a composition
having less than
about 1% by weight, preferably less than about 0.5% by weight, and more
preferably less than
about 0.1% by weight, based on the total weight of the composition, of the
stated material.
[0023] As used herein, the term "glass transition temperature" or "Tg" means
the temperature
at which an amorphous material changes from a brittle, vitreous state to a
plastic state. It is
dependent upon the composition of the material being tested, including
moisture content, the
extent of annealing, and the pressure exerted on the material. Glass
transition temperature may
be measured by differential scanning calorimetry, thermomechanical analysis,
dynamic
mechanical analysis, and the like.
[0024] As used herein, "pulverize" means providing a small particle size,
such as through
spraying or atomizing, or reducing the particle size of a given material,
whether or not through
the use of mechanical means.
[0025] As used herein, "lignocellulosic biomass or a component part thereof'
refers to plant
biomass containing cellulose, hemicellulose, and lignin from a variety of
sources, including,
without limitation (1) agricultural residues (including corn stover and
sugarcane bagasse), (2)
dedicated energy crops, (3) wood residues (including sawmill and paper mill
discards), and (4)
municipal waste, and their constituent parts including without limitation,
lignocellulose biomass
- 6 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
itself, lignin, C6 saccharides (including cellulose, cellobiose, C6
oligosaccharides, C6
monosaccharides, and C5 saccharides (including hemicellulose, C5
oligosaccharides, and C5
monosaccharides).
[0026] Generally, the methods of the invention precipitate out and
pulverize (provide as a
small particle size or reduce the particle size) lignin and avoid fouling of
the process equipment
while maximizing heat recovery. This is accomplished by cooling the stream
containing the
lignin to just above its glass transition temperature (Tg) to prevent sticking
and then rapidly
dropping the pressure so that the lignin is well below its Tg at the new
pressure when it
precipitates out of solution at a small particle size.
[0027] Accordingly, in one embodiment, the invention is directed to methods
of preparing
lignin from lignocellulosic biomass, comprising:
providing a lignocellulosic biomass at a first pressure and at a first
temperature,
said lignocellulosic biomass comprising:
a first solid fraction comprising:
insoluble lignin; and
a first liquid fraction comprising:
soluble C6 saccharides; and
soluble lignin;
reducing said first temperature of said lignocellulosic biomass to a second
temperature at least about 1 C above the glass transition temperature of
lignin under said
first pressure; and
reducing said first pressure of said lignocellulosic biomass at said second
temperature to a second pressure in a time less than about 1 second to
precipitate said
soluble lignin in said first liquid fraction and form a mixture comprising:
a second solid fraction comprising:
insoluble lignin; and
precipitated lignin; and
a second liquid fraction comprising:
soluble C6 saccharides;
- 7 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
wherein the average particle size of said insoluble lignin and precipitated
lignin is
less than about 500 microns.
[0028] A schematic of one embodiment of the invention is shown in FIGURE 1.
The lignin
slurry exits the hydrolysis process 2. It is cooled to just above its glass
transition temperature to
maximize heat recovery, for example, in a pre-cooler heat exchanger 4. The
lignin slurry is then
subjected to a rapid pressure drop, for example, through the pressure letdown
valve 6, and
subsequently the liquid (i.e., water) content in the slurry is flash
evaporated. This results in the
sudden precipitation of the soluble lignin into fine particles inside the
lignin pulverizer 8. In
certain embodiments, the pulverizer is of relatively small volume to keep the
slurry moving and
avoid lignin settling. In other embodiments, it may be of a large volume to
permit settling of the
lignin, which may be recovered by mechanical means, especially when using full
flash. The inlet
pipe to the pulverizer may either be above, below, or to either side of the
pulverizer.
Atmospheric pressure for full pressure reduction, or an intermediate pressure
in the case of a
partial pressure reduction, is maintained in the pulverizer by the back
pressure control valve 10.
In embodiments using full flash to atmospheric pressure, no back pressure
control is needed.
Any recovered steam enters a condenser 12 (not shown) for heat recovery.
Following the
pulverizer, the slurry flows through flow control 14 and then is further
cooled to recover more
heat in a heat exchanger 16, and is reduced to atmospheric pressure, if not
yet at atmospheric
temperature, via a pressure letdown valve 18 in the settling tank 20. In the
tank, the lignin is
permitted to settle to the bottom. Finally, the slurry may be passed through a
solid/liquid
filtration apparatus 22 for final separation of liquor 24 and lignin 26.
[0029] Advantages of the methods of the invention are that the pulverization
(preparation of
small particles and/or reduction in average particle size) of soluble and
insoluble lignin improves
handling, accelerates the drying, and improves combustion of the lignin.
Another advantage of
the methods of the invention is that the glass transition phase of the lignin,
both soluble and
insoluble, is avoided, to avoid fouling of the process equipment and permit
pulverization of the
lignin.
- 8 -
CA 02806873 2013-10-22
[0030] In certain embodiments of the method, lignocellulosic biomass is
fractionated to
remove at least a portion of c, saccharides by any suitable means, including,
but not limited to,
hydrothermal treatment (such as hot compressed water, subcritical, near
critical, or supercritical
water, which may contain other fluids, including alcohol, acid, or base),
enzymatic treatment, and
the like.
[0031] In certain embodiments of the method, the average particle size of
said insoluble lignin and
precipitated lignin is less than about 500 microns.
[0032] The methods of the invention are preferably run continuously, although
they may be run as
batch or semi-batch processes.
[0033] The methods of the invention may be carried out in any suitable
reactor, including, but not
limited to, a tubular reactor, a digester (vertical, horizontal, or inclined),
and the like. Suitable
digesters include the digester system described in US-B-8,057,639, which
include a digester and a
steam explosion unit.
[0034] In certain embodiments, the method further comprises the step of
reducing the temperature
of said mixture. All of the embodiments of the invention involve a temperature
reduction from
the temperature at which the saccharified lignocellulosic biomass is provided,
typically about
280 C to about 375 C (hydrolysis temperature) to eventually ambient or near
ambient
temperatures, typically about 20 C to about 60 C. The key of the temperature
reduction is that
the temperature is reduced instantaneously across the glass transition
temperature range of the
lignin to permit pulverization of the lignin.
[0035] In embodiments where there is a partial pressure reduction in the
method, the second
pressure is greater than atmospheric pressure.
[0036] In embodiments where there is a full pressure reduction in the method,
the second
pressure is about atmospheric pressure.
- 9 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
[0037] In certain embodiments, the method further comprises the step of
reducing the pressure
on said mixture to a third pressure. Pressure control impacts temperature in
the flashing process
where the saccharified lignocellulosic biomass is cooled in a very short
period of time (e.g., less
than one second). The inlet pressure must be equal to or greater than the
saturation pressure at
the given temperature so that the liquid components of fraction remain as
liquids. With respect
to processing of lignocellulosic biomass, it is preferably to avoid the
temperature range of about
180 C and about 240 C, the glass transition temperature range of lignin under
typical processing
conditions. Thus, if the inlet temperature is at least the 240 C +1 C, then
the minimum inlet
pressure needs to be about 34 bar but may be much higher. For example, it is
typical to have the
inlet pressure at 40 bar. The exit temperature is determined and dependent
upon the exit
pressure. If, for example, there is flash cooling of the saccharified
lignocellulosic biomass down
to a temperature of 180 C, then the exit pressure needs to equal to the
saturation pressure at
180 C, which about 10 bar. The exit pressure is controlled by the back
pressure valve, and the
exit temperature is determined by the exit pressure. If the exit pressure is
changed, the exit
temperature will also change. The exit temperature is the saturation
temperature at the selected
pressure.
[0038] In certain embodiments, the method further comprises the step of
permitting said
insoluble lignin and said precipitated lignin, where the lignin has been
pulverized (provided as a
small particle size or reduce the particle size) to separate out by gravity.
[0039] In certain embodiments, the method further comprises the step of
separating said
second solid fraction and said second liquid fraction. Suitable separation
methods including
filtration methods well known to those skilled in the art, such as decanter
filters, filter press,
reverse osmosis and nanofiltration, centrifuge decanters, and the like.
[0040] In certain embodiments, the method further comprises the step of
recovering heat using
at least one heat exchanger, for example, using a pre-cooler heat exchanger 4
or final heat
exchanger 16.
- 10 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
[0041] In another embodiment, the invention is directed to lignin products
produced by the
methods of the invention, including fuels, such as those used in a process
heat boiler. The lignin
product may also be used as a functional replacement for phenol, as a
functional replacement for
polyol, or as a building block for carbon fiber. In other embodiments, the
compositions of the
invention comprising lignin may be utilized in a variety of applications,
including, but not
limited to, fuels, tackifiers, phenol formaldehyde resin extenders in the
manufacture of particle
board and plywood, in the manufacture of molding compounds, urethane and epoxy
resins,
antioxidants, controlled-release agents, flow control agents, cement/concrete
mixing,
plasterboard production, oil drilling, general dispersion, tanning leather,
road covering, vanillin
production, dimethyl sulfide and dimethyl sulfoxide production, phenol
substitute in phenolic
resins incorporation into polyolefin blends, aromatic (phenol) monomers,
additional
miscellaneous monomers, carbon fibers, metal sequestration in solutions, basis
of gel formation,
polyurethane copolymer ¨ as a renewable filler/extender, and the like.
[0042] In another embodiment, the invention is directed to compositions,
comprising:
lignin;
wherein said lignin is processed from lignocellulosic biomass using
supercritical
or near critical fluid extraction.
In preferred embodiments, the composition is substantially free of organic
solvent. In preferred
embodiments, the lignin product has an average particle size less than about
500 microns, more
preferably, less than 300 microns, even more preferably, less than about 250
microns, and yet
even more preferably less than about 50 microns. The particle size of the
lignin may be
measured by standard sieve shaker, microscopy, light scattering, laser
diffraction, and other
standard size analysis techniques.
[0043] In a preferred embodiment, the lignin has a heating value as measured
by ASTM-D240
of at least about 5,000 BTU/lb at 30% moisture content. In a preferred
embodiment, the lignin
has a heating value as measured by ASTM-D240 of at least about 7,500 BTU/lb at
15% moisture
content. In a preferred embodiment, the lignin has a heating value as measured
by ASTM-D240
of at least about 8,000 BTU/lb at 5% moisture content.
- 11 -
CA 02806873 2013-10-22
100441 The
present invention is further defined in the following Examples, in which all
parts
and percentages are by weight, unless otherwise stated. It should be
understood that these
examples, while indicating preferred embodiments of the invention, are given
by way of
illustration only and are not to be construed as limiting in any manner. From
the above
discussion and these examples, one skilled in the art can ascertain the
essential characteristics
of this invention, and without departing from the scope thereof, can make
various changes and
modifications of the invention to adapt it to various usages and conditions.
EXAMPLES
Example 1:
[0045] Pretreatment (fractionation) and cellulose hydrolysis processes
liberate lignin from
lignocellulosic biomass utilized as feedstock. For testing in this example,
lignin samples, which
were generated from the flashing of cellulose effluent, were tested to
determine heating value,
proximate, ultimate, and ash fusion temperature, ash oxide composition,
moisture content, and
particle size.
Drying Rate and Moisture Content
[0046] When the lignin is separated from the flashed cellulose hydrolysis
effluent glucose stream
utilizing gravity and 20 p.m filter paper, it has an average moisture content
between 65% and
75%, by weight. This can be further reduced by using a centrifuge or vacuum
filtration unit to
more effectively separate the solids from the mother liquor. The
representative lignin sample was
obtained from the sludge collected in the bottom of the glucose product tank,
whose product was
generate from multiple runs of 100 mesh wood flour at the cellulose hydrolysis
conditions of
about 225 bar and 375 C. The sample was subsequently allowed to air dry to
measure its drying
rate.
[0047] The results are shown in FIGURE 2. The curve indicates that the lignin
dries to 10%
moisture content, by weight, settling around 5%, by weight, after
approximately 105 hours. This
- 12 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
was drier than expected and may have been due to the location (inside plant)
where the drying
experiments were conducted. Ambient conditions were warmer and drier than
would be
anticipated if the lignin were dried outside where solar insulation, diurnal
temperature changes,
humidity, and precipitation would be expected to keep the final moisture
content between 20%
and 25%, by weight. The first 75 hours of drying follows a typical constant
rate drying period
with moisture moving to the particle surface sufficiently fast to maintain a
saturated condition at
the surface. This indicates that the rate of drying is controlled by the rate
of heat transferred to
the evaporating surface. The lower part of the curve, from 75 to 125 hours, is
typical of a
continuously changing drying rate (usually decreasing) indicating a change in
the controlling
mechanism for drying. The surface area of the particle can no longer remain
fully saturated and
evaporation begins shifting into the particle interior where the internal
particle water diffusion
rate begins to control the drying process.
Heating Value
[0048] The heating values of the lignin at various moisture contents were
analyzed. The
heating value of a fuel is the measure of the heat released during its
complete combustion with
oxygen. Any fuel will contain hydrogen, and water will be formed as a product
of combustion
when hydrogen is burned in air. This generated water may remain the vapor
state or condense to
liquid creating a substantial difference in the measured heat value due to the
latent heat of
vaporization associated with the phase change. When determining the heat given
up by a unit of
fuel, the higher (or gross) heating value (HHV) is usually reported where it
is assumed than any
water generated is all condensed, thus the heating value incorporates the
latent heat of
vaporization. For the lower (or net) heating value (LHV), none of the water is
assumed to have
condensed and all of the products of combustion remain is the gaseous state.
The HHV may be
determined using an oxygen bomb calorimeter and is expressed in terms of heat
related per unit
weight of fuel (Btu/lbf). Determination of LHV may be calculated from the
following equation:
- 13 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
HHV = LHV + nHvp
where: HHV = fuel high heating value (Btu/lbf)
LHV = fuel high heating value (Btu/lbf)
n = stoichiometric mass of water generated per mass of
fuel combusted
(11),/lbf)
Hvp = latent heat of vaporization of water (Btu/lbw)
[0049] The results of testing using an oxygen bomb calorimeter in accordance
with ASTM
Method D240 are shown in FIGURE 3 for extracted lignin and filtered lignin.
The heating
value data decreases with increasing moisture content. The filtered lignin is
the lignin obtained
from flashing the cellulose hydrolysis effluent to atmospheric conditions. The
extracted lignin
was extracted from the fractionation slurry utilizing ethanol. The average
heating value for the
filtered lignin at 25% moisture content is approximately 8,200 Btu/lb.
Particle Size
[0050] Surface area/mass ratio for discrete particles is an important
aspect of the lignin's
usefulness as a fuel because it impacts combustion efficiency, boiler design,
and method of
introduction of combustion air. Improperly sized fuel may not burn completely
and heat energy
can be lost in the form of carbon rich bottom and/or fly ashes. Measuring
particle size may be
done by classification, e.g., sieving, or by observing under a microscope a
representative sample
and comparing to an appropriate scale. The average particle size was
determined using a
Magnaview DC5-153 microscope and a calibrated scaling slide. The average
particle diameter
observed was 10 i_tni to 30 i_tni at 37% moisture content for separated solids
derived from
cellulose hydrolysis, where the solids were determined to be approximately 80%
lignin.
Proximate Analysis
[0051] Proximate analysis of a fuel describes the volatiles, fixed carbon,
moisture content, and
ash present in a fuel as a percentage of dry fuel weight. The percentages of
volatiles and fixed
- 14 -
CA 02806873 2013-01-28
WO 2012/151524
PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
carbon both have a direct impact on the heating value of the fuel, flame
temperature, and
combustion process in general. Other than carbon and metals, all other fuels
burn as a gas. The
percentage of volatiles represents the amount of fuel that would burn in the
gas phase with the
remaining carbon burning as a solid on the grates or as a fine particulate.
The ash content is
important in the design of air pollution control equipment, boiler grates, and
bottom ash handling
equipment.
[0052] The results for a single sample are shown in Table 1.
Table 1
Sample No. % Moisture % Ash Content % Volatile %
Fixed Carbon
Content Matter
1 18.57 0.44 56.75 24.24
Ultimate Analysis
[0053] Ultimate analysis of a fuel describes its elemental composition as a
percentage of the
fuel sample's dry weight. The main elements typically considered are carbon
(C), hydrogen (H),
nitrogen (N), sulfur (S), and oxygen (0), and while not an element, ash.
Sulfur and ash
percentages are particularly important because they are needed to accurately
estimate air
emission rates for sulfur dioxides (S0x) and particulate matter (PM) for use
in effective design of
air pollution control equipment and air permitting.
[0054] The results for a single sample are shown in Table 2.
Table 2
Sample No. % C % H % N % 0 (by % S % Ash
difference)
1 51.00 6.56 0.15 41.74 0.02 0.44
- 15 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
Ash Fusion Temperature
[0055] Ash fusion temperatures are determined by viewing a mounded specimen of
the fuel's
(lignin) ash through an observation window in a high-temperature furnace in
both reducing and
oxidizing atmospheres. The ash, in the form of a cone, pyramid, or cube, is
heated steadily
above 1000 C to as high a temperature as possible, preferably 1600 C (2910 F).
The following
temperatures are then recorded:
= Initial deformation temperature (IT): This is reached when the point of
the mound first
begins to deform and round.
= Softening (spherical) temperature (ST): This is reached when the top of
the mound takes
on a spherical shape, i.e., the base of the cone is equal to its height.
= Hemispherical temperature (HT): This is reached when the entire mound
takes on a
hemispherical shape, i . e. , the base of the cone is twice its height.
= Flow (fluid) temperature (FT): This is reached when the molten ash
collapse to a
flattened button on the furnace floor, i.e., spread to a fused mass.
Generally, a temperature under reducing should be equal to or lower than the
corresponding
temperature under oxidizing conditions. The difference in these temperatures
generally increases
with increasing iron content in the ash. Fusion temperatures should
monotonically increase in
order of IT, ST, HT, and FT.
[0056] The results for a single sample in an oxidizing atmosphere are shown in
Table 3.
Table 3
Initial deformation temperature (IT): 2136 F (1169 C)
Softening (spherical) temperature (ST): 2141 F (1172 C)
Hemispherical temperature (HT): 2143 F (1173 C)
Flow (fluid) temperature (FT): 2144 F (1174 C)
[0057] A spherical temperature, a critical temperature for fuel evaluation,
that is too low will
cause slagging problems in the combustion chamber of a boiler. As the ash
softens and melts, it
subsequently impacts a surface within the combustion chamber where it cools
and forms a glassy
- 16 -
CA 02806873 2013-01-28
WO 2012/151524 PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
substance called clinker, which must be removed. Its removal severely impedes
boiler
operations as the boiler must be shutdown. If the melted ash cools on a heat
transfer surface, the
resultant layer builds up, fouling the heat exchanger decreasing its overall
efficiency and thus the
boiler efficiency as well. It is preferred to have an ST of 35 C to 65 C (100
F to 150 F) above
the actual flue gas temperature peak at the combustion chamber exit to
minimize the impact.
[0058] The ash fusion temperature gives an indication of its softening and
melting behavior.
Ash Mineral Oxide Analysis
[0059] Ash mineral oxide composition is also useful in understanding how the
ash generated
by the combustion of lignin will behave in the combustion chambers of the
biomass boiler.
Composition does affect the ranges of fusion temperatures, particularly the
iron levels and base
to acid oxide rations. Typical analyses determine the weight percentage of the
following mineral
oxides silica (5i02), alumina (A1203), ferric oxide (Fe203), titanium dioxide
(Ti02), phosphorous
pentoxide (P205), calcium oxide (CaO), magnesium oxide (MgO), manganese oxide
(MnO),
sodium oxide (Na20), potassium oxide (K20), and sulfur trioxide (SO3). The
silica, alumina,
and titanium dioxide make up the group of acidic oxides with the remaining
compounds forming
the basic oxides.
[0060] The results for a single sample are shown in Table 4.
Table 4
Sample %Al %Ca %Fe %Mg %Mn %P as %K %Si %Na %Ti %S as %
No. as as as as as P205 as as as as SO3 Sum
A1203 CaO Fe203 MgO MnO K20 SiO2 Na2O TiO2
1 7.52 18.56 22.30 1.57 0.19 1.05 1.20 29.18 1.19 3.67 13.15 99.56
[0061] Table 5 shows a side-by-side comparison for a typical high-rank eastern
Kentucky coal,
a typical hardwood, and the cellulose hydrolysis-derived lignin.
- 17 -
CA 02806873 2013-01-28
WO 2012/151524
PCT/US2012/036591
62630/834317 (SRIINC-015/016/028PCT)
Table 5
Characteristic Eastern Kentucky Coal Typical Hardwood Cellulose
Hydrolysis-
derived Lignin
Heating value (Btu/lb) 13254 @5% MC 8839 (oven dried) 8200
@25% MC
Proximate Analysis
% Moisture content 1.2 45.6 18.57
% Ash 10.15 0.45 0.44
% Volatile matter 36.82 48.58 56.75
% Fixed carbon 53.03 5.52 24.24
Ultimate Analysis
%C 75.0 51.64 51.09
%H 7/0 6.26 6.56
%N 1.0 0 0.15
% S 3.0 0.009 0.02
% 0 (by difference) 6.2 41.45 41.74
% Ash 7.8 0.65 0.44
Ash fusion temperatures
(oxidizing)
IT ( F) 1627 2136
ST ( F) 1647 1652 2141
HT ( F) 1649 2143
FT( F) 1649 2144
Ash mineral oxide analysis
%Al as A1203 30.67 0.03 7.52
%Ca as Ca0 1.16 31.35 18.56
%Fe as Fe203 4.87 0.09 22.30
%Mg as MgO 0.42 7.57 1.57
%P as P205 0.13 0.56 1.09
%K as K20 0.99 10.25 1.20
%Si as 5i02 58.20 0.13 29.18
%Na as Na20 0.17 0.06 1.18
%Ti as TiO2 2.08 - 3.67
%S as 503 1.29 1.21 13.15
[0062] As can be seen, the lignin's HHV is better than typical hardwood
(allowing for moisture
content), but not quite as high as coal. However, the lignin is considered to
be a relatively high
energy density fuel. With better than 55% of the lignin representing volatile
matter and less than
- 18 -
CA 02806873 2013-10-22
=
0.50% ash, most of the lignin is expected to combust and exit the combustion
chamber in the gaseous
phase, minimizing the size of the ash handling equipment needed in the biomass
boiler.
[0063] ST is much greater than the average of the hardwood. This is expected
to help minimize the
impact of slagging on the combustion chamber walls. The elevated ST is likely
related to the
relatively high iron and calcium content in the ash. The ash fusion
temperature is important to boiler
operations and efficiency.
[0064] From an air pollution control standpoint, NO formation (specifically
fuel NOõ) is expected
to be minimal as the nitrogen content of the lignin is very low. The same is
true for particulate matter
(PM).
[0065] Overall, the results indicate that the cellulose hydrolysis-derived
lignin has fuel properties
that will allow it to be effectively combusted in a process boiler. In
particular, the HHV, % volatile
matter, spherical temperature (ST), and ash mineral oxide concentrations are
particularly conducive
for lignin being used as a boiler fuel.
[0066] When ranges are used herein for physical properties, such as molecular
weight, or chemical
properties, such as chemical formulae, all combinations, and subcombinations
of ranges specific
embodiments therein are intended to be included.
[0067]
[0068] Those skilled in the art will appreciate that numerous changes and
modifications can be
made to the preferred embodiments of the invention and that such changes and
modifications can
be made without departing from the scope of the invention. It is, therefore,
intended that the
appended claims cover all such equivalent variations as fall within the scope
of the invention.
- 19 -