Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1
SYSTEM AND METHOD FOR RECOVERING SOLVENTS USED DURING BITUMEN
PRODUCTION
TECHNICAL FIELD
[0001] The following relates to systems and methods for recovering solvents
used during
bitumen production.
BACKGROUND
[0002] Bitumen is known to be considerably viscous, does not flow like
conventional crude
oil, and can be present in an oil sand reservoir. As such, bitumen is
recovered using what are
considered non-conventional methods. For example, bitumen reserves are
typically extracted
from a geographical area using either surface mining techniques, wherein
overburden is
removed to access the underlying pay (e.g., oil sand ore-containing bitumen)
and transported to
an extraction facility; or using in situ techniques, wherein subsurface
formations (containing the
pay) are heated such that the bitumen is caused to flow into one or more wells
drilled into the
pay while leaving formation rock in the reservoir in place. Both surface
mining and in situ
processes produce a bitumen product that is subsequently sent to an upgrading
and refining
facility, to be refined into one or more petroleum products, such as gasoline
and jet fuel.
[0003] Bitumen reserves that are too deep to feasibly permit bitumen
recovery by mining
techniques are typically accessed by drilling wellbores into the hydrocarbon
bearing formation
(i.e. the pay) and implementing an in situ technology. There are various in
situ technologies
available, such as steam driven-based techniques (e.g., Steam Assisted Gravity
Drainage
(SAGD) and Cyclic Steam Stimulation (CSS)), steam-solvent co-injection
techniques (e.g.,
expanding solvent-SAGD (ES-SAGD)) and waterless solvent-based techniques
(e.g., N-Solv,
Enhanced Solvent Extraction Incorporating Electromagnetic Heating (ESEIEH)
also known as
"EASE".
[0004] In a typical implementation of the SAGD method, a pair of
horizontally oriented wells
are drilled into the bitumen reserve, such that the pair of horizontal wells
are vertically aligned
with respect to each other and separated by a relatively small distance,
typically in the order of
several meters. The well installed closer to the surface and above the other
well is generally
referred to as an injector well, and the well positioned below the injector
well is referred to as a
producer well. The injector well and the producer well are then connected to
various equipment
installed at a surface site. The injector well facilitates steam injection
into the reservoir. The
injected steam propagates vertically and laterally into the reservoir in a
formation referred to as
a steam chamber. Latent heat released by the injected steam mobilizes the
bitumen, which
23177848.1
CA 2974752 2017-07-28
2
drains due to gravity and is produced continuously along with condensed water
in the producer
well (i.e., continuous bitumen recovery process). SAGD can be used to achieve
high production
rates, but requires a continuous water supply and generates CO2 emissions due
to the natural
gas combusted for steam generation.
[0005] Solvent-steam co-injection methods involve co-injecting with steam a
hydrocarbon
solvent, which propagates into the reservoir along with steam in the vapour
phase and
condenses at the vapour liquid interface. Dilution of oil by the condensed
solvent further
reduces the viscosity of bitumen and the co-injected solvent may then be
partially recovered
with the production fluids and reused. For example, ES-SAGD involves co-
injection with
steam solvents having a close saturation temperature to that of steam (e.g.,
hexanes and
diluents).
[0006] Waterless or "non-steam" solvent-based techniques rely on one or
more solvents to
mobilize the bitumen in the absence of steam. The solvent is typically
injected as a vapour into
the reservoir at a predetermined pressure. The solvent is typically, but not
always, selected to
be at the vapour boiling point at the bitumen chamber's temperature and
pressure. As the
solvent condenses it releases its latent heat, thereby both warming and
dissolving the bitumen.
The combination of warming and liquid solvent dilution mobilizes the bitumen
permitting it to
flow.
[0007] For example, in heated solvent-based techniques, bitumen mobilized
by a heated
solvent (e.g., propane or butane) flows under the influence of gravity, to a
production well. In
ESEIEH/EASE techniques, radio frequency is used to heat an in situ bitumen
reservoir and a
solvent is provided to facilitate mobilization of the bitumen.
[0008] Hydrocarbon recovery techniques that utilize solvent at least in
part facilitate
mobilization of hydrocarbons at lower temperatures than are required for SAGD,
at least
because the viscosity of the bitumen is reduced by being dissolved by the
solvent. However,
the solvents used for dissolving bitumen can be considered expensive.
[0009] Partial recovery of solvents is possible. For example, the portion
of the solvent
present in the mobilized bitumen which is produced may be collected. However,
a portion of the
solvent is typically expected to not be recoverable, because it will have
condensed into a liquid
form and remain in the chamber, e.g., in the unrecovered bitumen reservoir
and/or in the
reservoir pore space, thereby reducing cost-efficiency of the bitumen recovery
operation.
23177848.1
CA 2974752 2017-07-28
3
SUMMARY
[0010] In one aspect, there is provided a method for recovering solvent
used during bitumen
production, the method comprising: subsequent to injecting a first solvent
into a bitumen
reservoir to reduce the viscosity of the bitumen in the bitumen reservoir
during a continuous
bitumen production process, injecting a second solvent into the bitumen
reservoir, wherein the
second solvent is lighter than the first solvent, wherein the second solvent
remains in the vapour
phase when injected in a vapour chamber in the bitumen reservoir, wherein the
second solvent
maintains pressure in the bitumen reservoir, and wherein a bitumen containing
fluid produced
using the second solvent at least in part comprises a portion of the first
solvent.
[0011] An advantage of the solvent recovery method described herein stems
from the
injection of a relatively lighter solvent after the injection of a relatively
heavier solvent during a
bitumen recovery process, to enable greater recovery of the previously
injected solvent. As
the chamber pressure decreases, either during a continued production process
or after
commencing wind-down or blowdown, at least one relatively lighter solvent is
injected, allowing
a greater proportion of the heavier and more valuable solvent to be recovered.
In an
implementation of the method, a plurality of solvents, each being
progressively lighter than one
previously injected can be injected.
[0012] This transition from heavier to lighter solvents during a continuous
production
process allows for a greater recovery of previously used heavier solvent(s),
at least because
these heavier solvents can be recovered during the bitumen recovery process
rather than at the
end of the recovery process. .
[0013] The lightest solvent can be disposed at the end of the process
using, for example, a
non-condensable gas such as carbon dioxide (CO2). This can contribute both to
additional
recovery of solvent, and to sequestering CO2 in the formation.
[0014] The subsequent injection of relatively lighter solvents can be
combined with other
heat sources such as conductive heating, e.g., electrical, radio frequency
antennae, hot oil
closed loop, etc. It can also be appreciated that in other implementations,
steam can be used in
place of, or in conjunction with such a relatively lighter solvent to recover
a previously injected
solvent.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] Embodiments will now be described by way of example only with
reference to the
appended drawings wherein:
23177848.1
CA 2974752 2017-07-28
4
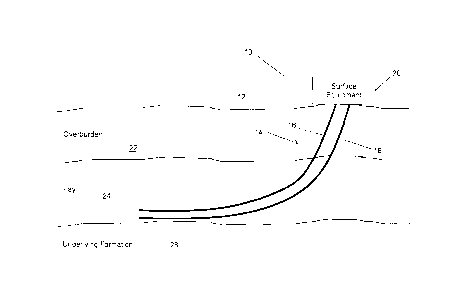
[0016] FIG. 1 is a cross-sectional elevation view of a well pair used for a
continuous solvent
based oil recovery process;
[0017] FIG. 2 is a cross-sectional elevation view of a set of three well
pair in a bitumen
reservoir;
[0018] FIG. 3(a) is a cross-sectional elevation view of a well pair and
surrounding region of
solvent in a first solvent injection phase;
[0019] FIG. 3(b) is a is a cross-sectional elevation view of a well pair
and surrounding region
of solvent in a second solvent injection phase;
[0020] FIG. 3(c) is a is a cross-sectional elevation view of a well pair
and surrounding region
of solvent in a third solvent injection phase; and
[0021] FIG. 4 is a flow chart illustrating operations performed in a non-
steam solvent-based
continuous bitumen production process using a progression from heavier to
lighter solvents.
DETAILED DESCRIPTION
[0022] A solvent recovery process is described herein, which can be used to
recover
relatively heavier, more valuable solvents used during an in-progress or
recently completed
bitumen production process. The solvent recovery process operates by using a
solvent that is
relatively lighter than the previously used solvent to increase recovery of
one or more of the
heavier, more expensive solvents stranded or otherwise retained in the
reservoir. The relatively
lighter solvent remains in the vapour phase when injected in the chamber and
maintains
pressure in the reservoir, which acts to i) lower the partial pressure of the
heavier solvent that is
being targeted for recovery, resulting in vaporization of that solvent, and
ii) displace the heavier
solvent that is in the vapour phase. This can improve cost-effectiveness of
various in situ
bitumen recovery processes that use solvent at least in part, such as, for
example, processes
that use solvent with steam and/or electromagnetic heating.
[0023] Turning now to the figures, FIG. 1 illustrates an example of a
bitumen production site
at a surface location 12 in a particular geographical region. The production
site 10 is
positioned to allow one or more well-pairs 14 to be drilled from the surface
location 12 towards a
bitumen reservoir (i.e., the pay 24). The one or more well-pairs 14 include an
injector well 16
configured to inject solvent into the pay 24, positioned above a producer well
18 configured to
recover a bitumen-containing fluid that has been mobilized by the injected
solvent. The injector
well 16 is typically located about 4 to 6 meters above the producer well 18,
although a shorter or
23177848.1
CA 2974752 2017-07-28
5
longer distance is possible, as is a lateral offset. The one or more well-
pairs 14 are drilled
vertically into the overburden 22 towards and into the underlying pay 24, and
as they are drilled
become oriented substantially horizontally, such that the producer well 18 is
above but near the
formation 26 underlying the pay 24 (hereinafter the "underlying formation
26"). The one or more
well pairs 14 are operated using surface production equipment 20. After
determining the
surface location 12 and production site 10, and determining where the one or
more well-pairs 14
will be located at the production site 10 (e.g., by conducting typical
computer simulations using
geological and reservoir data), the corresponding locations of the production
site 10 are drilled,
as is known in the art.
[0024] After drilling the wells 16, 18, the surface production equipment 20
is installed in one
or more production facilities for operating the one or more well pairs 14.
Completing a
particular well for production can involve several steps, as is known in the
art, and includes the
installation of solvent injection apparatus for injecting solvent via the
injector well 16, and can
include the installation of other equipment for introducing an additional heat
source into the
injector well 16, e.g., steam and/or an electrical based heat source.
[0025] Typically, multiple well pairs 14 are drilled from the surface
location 12 into the
subsurface hydrocarbon-bearing formation to recover bitumen within the
particular geographical
area. FIG. 2 illustrates multiple well pairs 14 used to extract a targeted
region of the pay 24, the
view in FIG. 2 being towards and along the ends of the horizontal portions of
the injector and
producer wells 16, 18. It will be appreciated that three well pairs 14 are
shown for illustrative
purposes only and more or fewer well pairs 14 can be employed in different
implementations.
as shown in FIG. 2.
[0026] Generally, the solvent recovery process described herein is applied
after the injection
of a first solvent into a bitumen reservoir during a bitumen production
process. The first solvent
can have been applied in a solvent-only process or a process that utilizes the
first solvent with
another source of heat, such as steam or an electrical heat source. The
solvent recovery
process described herein can be applied during the production process that
utilizes the first
solvent, or can be applied thereafter, e.g., after commencing wind-down or
blowdown, in order
to increase recovery of the first solvent. In one implementation, the solvent
recovery process
is combined with another in situ bitumen recovery process such as ESEIEH/EASE
that uses
electromagnetic heating. It can be appreciated that in other implementations,
the solvent
recovery process can be combined with other heat sources such as conductive
heating, e.g.,
electrical, radio frequency antennae, hot oil closed loop, etc. It can also be
appreciated that in
23177848.1
CA 2974752 2017-07-28
6
other implementations, steam can be used in place of, or in conjunction with a
relatively lighter
hydrocarbon solvent to recover a previously injected solvent.
[0027] In an implementation, the method can be applied to a system
comprising a well pair
as shown in FIG. 1, with the first solvent having been injected as a vapour or
liquid into the
pay 24 from an injector well 16, preferably as a vapour. The first solvent is
typically, but not
always, selected to be at the vapour boiling point at the operating
temperature and pressure
of the chamber. The first solvent propagates vertically and laterally into the
bitumen
reservoir and condenses at the vapour liquid interface. Bitumen is mobilized
by the
condensation of, and latent heat released by, the first solvent, which
dissolves a portion of
the bitumen in the reservoir, thereby forming a first fraction comprising the
first solvent and
the mobilized bitumen. The mobilized bitumen then drains under the effect of
gravity and is
produced along with the first solvent from a producer well 18.
[0028] In the method disclosed herein, at a later time during the well
life, e.g., during
continued production or therefore, such as after commencing wind-down or
blowdown,
solvent injection is transitioned from the first solvent to a second solvent,
or restarted using
the second solvent. The second solvent is lighter than the first solvent, and
remains in the
vapour phase when injected in the chamber. This allows for the increased
recovery of the
first relatively heavier solvent, by maintaining pressure in the reservoir,
acting to lower the
partial pressure of the heavier first solvent being targeted for recovery
resulting in
vaporization of the relatively heavier solvent, and to displace the first
relatively heavier
solvent out of the chamber enabling its recovery. Over time, the second
solvent can
substantially replace the first solvent in the bitumen reservoir as the first
solvent is
displaced.
[0029] Optionally, at a later time, when the concentration of the first
solvent in the
recovered fluid has further decreased, the second solvent can be replaced with
a third
solvent, the third solvent being lighter than the second solvent, to allow for
the increased
recovery of the second solvent according to the mechanism discussed above.
Over time,
the third solvent can substantially replace the second solvent in the bitumen
reservoir as the
second solvent is displaced.
[0030] An example of the presently described solvent recovery process would
be with or
following a thermal solvent recovery process that uses butane as the first
solvent. During
23177848.1
CA 2974752 2017-07-28
7
the start-up and primary production phases, butane is injected, and a certain
percentage of
the injected butane would remain in the reservoir, either in liquid or gaseous
form. Later
on in the life of the well, some or all of the injected butane would be
replaced by a lighter
solvent, such as ethane, methane or CO2, allowing for the stranded butane to
be produced
to surface over a period of time, reducing the amount of this more valuable
solvent being
stranded in the reservoir. The application of this solvent recovery process
would also allow
for more bitumen to be produced by maintaining pressure in the reservoir that
would have
otherwise dropped, once butane injection ceases.
[0031] Optionally, at one or more later times, additional and subsequently
lighter
solvents may replace the heavier solvents previously injected into the bitumen
reservoir,
thereby facilitating continuous mobilization of bitumen in the pay 24,
continuous production
of bitumen and recovery of the heavier solvent(s) during the recovery process.
Transitioning to, or restarting injection with, progressively lighter
solvent(s) may progress in
succession or it may skip one or more compounds in the succession. In an
implementation,
transition to lighter solvents ends with a non-condensable gas such as, for
example,
nitrogen, methane or carbon dioxide; which can be left behind or "sequestered"
in the
reservoir upon completion of the process.
[0032] The presently described method of solvent recovery can utilize a set
of multiple
solvents having different "weights" (i.e. being relatively lighter and
relatively heavier), each of
the solvents having a corresponding boiling point. An example of such a set of
solvents is a set
of alkane-based solvents. The method of solvent recovery can contribute to
various bitumen
recovery processes, while minimizing loss of heavier and more costly solvents
by progressing
from heavier to lighter solvents as the pressure in the vapour chamber
decreases. The method
of solvent recovery can achieve this by utilizing solvents from the set
according to the relative
weight of the solvent compared to a previously injected solvent. For example,
with a relatively
heavier alkane such as octane (i.e. relatively higher carbon solvent) that was
injected into the
bitumen reservoir at an early stage, at subsequent stages in the well life,
progressively lighter
alkanes such as butane, etc. (i.e. progressively lower carbon solvent) can be
injected to
maintain the pressure in the bitumen reservoir and act to vaporize the
previous solvent and
displace that previous solvent that is in the vapour phase.
[0033] An example of a set of solvents is a set of alkanes ranging from a
relatively lighter
alkane such as methane to a relatively heavier alkane such as octane, e.g., a
set of solvents
23177848.1
CA 2974752 2017-07-28
8
including methane (CH4), ethane (02H8), propane (C3H8), butane (C4I-110),
pentane (C51-112),
hexane (C6-114), heptane (07H16), octane (C81118) etc. Other examples of sets
of solvents can be
selected from n (normal) and iso-alkanes according to the boiling points of
such solvents.
Similarly, other sets of solvents can be chosen from the following solvents,
based on the boiling
points and, in some cases the costs, of the respective solvents, for example:
naphtha, toluene,
xylene, benzene, diesel, natural gas, etc.
[0034] The solvent recovery process can occur with or without an additional
heat source,
such as steam injected into the reservoir The solvent can be injected in a
liquid state and
penetrates the pay 24 surrounding the injector wells 16 before vapourizing at
deeper zones,
thereby creating a region of solvent 40a around the injector well 16 as shown
in FIG. 3(a).
FIGS. 3(a)-3(c) illustrate regions of solvent 40a, 40b, 40c that develop
during the solvent
recovery process described herein, as lighter and lighter solvents are
successively injected (with
the region of solvent 40 being larger, the lighter the solvent). The region of
solvent 40a in FIG.
3(a) has a diffusion boundary 42a at the periphery of the region 40a. The
diffusion boundary
42a represents substantially the outermost boundary of the region of solvent
40a, within which
diffusion of the solvent occurs. The diffusion boundary 42a can be used to
determine a relative
volume that would be consumed, for different solvents being injected (as
described in greater
detail below). The region of solvent 40b in FIG. 3(b) has a diffusion boundary
42b at the
periphery of the region 40b. The region of solvent 40c in FIG. 3(c) has a
diffusion boundary 42c
at the periphery of the region 40c. The diffusion boundaries 42a, 42b, 42c are
progressively
deeper into the pay 24 as the progressively heavier solvents are injected. The
larger the region
of solvent 40 defined by the diffusion boundary 42, the larger the volume of
solvent that
penetrates the pay 24, and the higher volume of solvent that would be
consumed. As such,
injected solvents having a closer diffusion boundary 42 and smaller region of
solvent 40
consume less injected solvent.
[0035] As illustrated in FIG. 4, the solvent recovery process described
herein can include
determining a transition point during a continuous production process or a
restart point for
injecting the second solvent at step 104. It can be appreciated that in an
implementation,
commencement of the production process can correspond to such a transition
point, wherein a
first, relatively heavy solvent is selected. The relatively lighter solvent to
be used for a period of
time following the transition or restart point is selected at step 106 and is
injected at the injector
well 16 at step 108. A bitumen containing fluid can then be recovered at step
110 via the
23177848.1
CA 2974752 2017-07-28
9
producer well 18, and if applicable (i.e. during injection of the second or a
subsequent solvent),
previously injected solvent can be recovered from the bitumen containing fluid
at step 112.
[0036] The solvent is injected in the liquid state, e.g., from a truck at
surface 12. It is
expected that near the end of the injection stage for a particular solvent,
due to an increase in
temperature surrounding the injector wells 16, the solvent will be in a
gaseous state where the
temperature is higher than the condensation temperature of the solvent under a
given pressure.
As such, the liquid solvent initially diffuses into the formation at a larger
flux due a larger
concentration that is exposed to the bitumen. Once the solvent is vapourized,
the flux of solvent
diffusion decreases due to less solvent being available at the interface with
the bitumen. The
mobilized bitumen, due to solvent dissolution in the surrounding bitumen is
produced in a
mixture of liquid solvent and bitumen.
[0037] For constant boundary conditions, the diffusion boundary (45Diff )
can be defined as
opiff = V4Dt , where 6Diff is the diffusion length, boundary or front
(referred to herein as
"diffusion boundary"), and D is a diffusion coefficient or diffusivity in
dimensions of [length2
time-1], for example m2/sec . The diffusion boundary provides a measure of how
far the
concentration has propagated in the x-direction by diffusion in time t (Bird,
R.B., Stewart, W.E.,
Lightfoot, E.N., "Transport Phenomena", John Wiley & Sons, 1976).
[0038] The Wilke-Chang equation (Wilke C.R., Chang, P., "Correlation Of
Diffusion
Coefficients in Dilute Solutions", A.I.Ch.E. Journal, 1:264-270, 1955) can be
used for estimating
the diffusivity of nonelectrolytes (i.e., hydrocarbon solvent) in an
infinitely dilute solution (i.e.,
bitumen):
VITIMB,õ _________________ (T + 273.15)
[0039] D = 7.4 x =
P. vSolv
[0040] where D is diffusion coefficient (cm2/sec), (1) is association
factor of bitumen,
MBitu is molecular weight of bitumen (i.e., 500-550), 1.4.8 is viscosity of
the bitumen (cP) and
VSolv is molal volume of solvent at normal boiling point (cc/g.mole). Based on
current available
data, the temperature dependence of the diffusion coefficient can be assumed
to be linear.
Linear correlation is proposed in other correlations such as Stokes-Einstein
(Einstein, Albert,
Ann. Phys. 17, 549, 1905 and Miller C.C., "The Stokes-Einstein Law for
Diffusion in Solution",
Proceedings of the Royal Society of London. Series A, Containing Papers of a
Mathematical
and Physical Character, Vol. 106, No. 740, pp. 724-749, 1924) or close to
linear in other
23177848.1
CA 2974752 2017-07-28
10
correlations such as in Sitaraman (Sitaraman R., Ibrahim S. H., Kuloor N. R.
"A Generalized
Equation for Diffusion in Liquids" J. Chem. Eng. Data, 1963,8 (2), pp 198-201
doi:10.1021/je60017a017). The formulae suggested above for calculating the
diffusion
coefficient are expected to hold true for low-viscosity liquids but to
introduce error for a high-
viscosity solvent. However, the solute (i.e., bitumen) the solvents use in the
present diffusion
controlled mobilization process are light and less than 10 cP viscosity.
[0041] The Wilke-Chang equation shows that by increasing the temperature
surrounding
the infill well, the diffusion increases linearly. It is noted that since the
diffusion is increasing
inversely by viscosity of the bitumen ( ,,,u ), the diffusion reduces for
higher temperatures.
[0042] An increase in diffusion means that more solvent is diffusing and
6Diff (diffusion
length) can increase by heating up the area surrounding the injector wells 16,
e.g., by combining
the solvent injection described herein with other non-steam-based recovery
processes.
[0043] It can be appreciated that the relative volume of solvent that
penetrates the pay 24
can also be modeled according to dispersion of the solvent, which is a
combination of diffusion
and convection and has a linear relationship with diffusion. That is, the
relative volume of each
type of solvent can also be modeled by way of a dispersion boundary. Such a
dispersion
boundary can be estimated using a constant multiplier applied to the above-
described diffusion
coefficient D, as would be understood by those skilled in the art.
[0044] For simplicity and clarity of illustration, where considered
appropriate, reference
numerals can be repeated among the figures to indicate corresponding or
analogous elements.
In addition, numerous specific details are set forth in order to provide a
thorough understanding
of the examples described herein. However, it will be understood by those of
ordinary skill in the
art that the examples described herein can be practiced without these specific
details. In other
instances, well-known methods, procedures and components have not been
described in detail
so as not to obscure the examples described herein. Also, the description is
not to be
considered as limiting the scope of the examples described herein.
[0045] The examples and corresponding diagrams used herein are for
illustrative purposes
only. Different configurations and terminology can be used without departing
from the principles
expressed herein. For instance, components and modules can be added, deleted,
modified, or
arranged with differing connections without departing from these principles.
23177848.1
CA 2974752 2017-07-28
11
[0046] The steps or operations in the flow charts and diagrams described
herein are just for
example. There can be many variations to these steps or operations without
departing from the
principles discussed above. For instance, the steps can be performed in a
differing order, or
steps can be added, deleted, or modified.
[0047] Although the above principles have been described with reference to
certain specific
examples, various modifications thereof will be apparent to those skilled in
the art as outlined in
the appended claims.
23177848.1
CA 2974752 2017-07-28