Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
OPTIMIZATION OF GREENHOUSE GAS EMISSIONS IN A SOLVENT-BASED
HEAVY OIL RECOVERY PROCESS
BACKGROUND
Field of Disclosure
[0001] The present disclosure relates to the optimization, including
preferably
minimization, of greenhouse gas (GHG) emissions associated with the production
of heavy
oil from a subterranean reservoir in a solvent-based oil recovery process.
Optimization of
GHG emissions is based on modifications to operating parameters associated
with the process
based on overall GHG emissions modeling based on the analysis of several key
GHG
emissions sources in the overall process as a function of the solvent fraction
of the injected
fluid and/or solvent composition.
Description of Related Art
[0002] This section is intended to introduce various aspects of the art.
This discussion is
believed to facilitate a better understanding of particular aspects of the
present techniques.
Accordingly, it should be understood that this section should be read in this
light, and not
necessarily as admissions of prior art.
[0003] Modern society is greatly dependent on the use of hydrocarbon
resources for fuels
and chemical feedstocks. Subterranean rock formations that can be termed
"reservoirs" may
contain resources such as hydrocarbons that can be recovered. Removing
hydrocarbons from
the subterranean reservoirs depends on numerous physical properties of the
subterranean rock
formations, such as the permeability of the rock containing the hydrocarbons,
the ability of the
hydrocarbons to flow through the subterranean rock formations, and the
proportion of
hydrocarbons present, among other things.
[0004] Easily produced sources of hydrocarbons are dwindling, leaving less
conventional
sources to satisfy future needs. As the costs of hydrocarbons increase, less
conventional
sources become more economical. One example of less conventional sources
becoming more
economical is that of oil sand production. The hydrocarbons produced from less
conventional
sources may have relatively high viscosities, for example, ranging from 1000
centipoise (cP)
- 1 -
CA 3062478 2019-11-22
.1
to 20 million cP with American Petroleum Institute (API) densities ranging
from 8 degree (0)
API, or lower, up to 200 API, or higher. The hydrocarbons recovered from less
conventional
sources may include heavy oil. However, the hydrocarbons produced from the
less
conventional sources may be difficult to recover using conventional
techniques. For example,
the heavy oil may be sufficiently viscous that economical production of the
heavy oil from a
subterranean formation (also referred to as a "subterranean reservoir" herein)
is precluded.
[0005] Several conventional recovery processes, such as but not
limited to thermal
recovery processes, have been utilized to decrease the viscosity of the heavy
oil. Decreasing
the viscosity of the heavy oil may decrease a resistance of the heavy oil to
flow and/or permit
production of the heavy oil from the subterranean reservoir by piping,
flowing, and/or pumping
the heavy oil from the subterranean reservoir. While each of these recovery
processes may be
effective under certain conditions, each possess inherent limitations.
[0006] One of the conventional recovery processes utilizes
steam injection. The steam
injection may be utilized to heat the heavy oil to decrease the viscosity of
the heavy oil. Water
and/or steam may represent an effective heat transfer medium, but the pressure
required to
produce saturated steam at a desired temperature may limit the applicability
of steam injection
to high pressure operation and/or require a large amount of energy to heat the
steam.
Additionally, there are significant greenhouse gas (GHG) emissions
relationships to the amount
of heating required for the steam prior to injection.
[0007] Another of the conventional recovery processes utilizes cold and/or
heated solvents.
Cold and/or heated solvents may be injected into a subterranean reservoir as
liquids and/or
vapors to decrease the viscosity of heavy oil present within the subterranean
reservoir.
Traditionally, low molecular weight hydrocarbons (e.g., propane and/or butane)
are injected
into the subterranean reservoir as the cold and/or heated solvent. The
injected solvent may
dissolve the heavy oil, dilute the heavy oil, and/or transfer thermal energy
to the heavy oil.
Utilizing the cold and/or heated solvents may suffer from limited injection
temperature and/or
pressure operating ranges, and/or an inability to effectively decrease the
viscosity of the heavy
oil. In other recovery processes, the injected fluid may be substantially
comprised of a
hydrocarbon-based solvent and injected in a vaporized form. In hybrid steam-
solvent recovery
processes, the injected fluid is comprised of mixture of steam and solvent, in
which the relative
amount of steam to solvent, or "solvent fraction" of the injection fluid may
be varied. The
- 2 -
CA 3062478 2019-11-22
1
=
WI
composition of the solvent may also be varied in the process.
[0008] However, in these solvent or steam-solvent (collectively
"solvent-based")
processes, a single solvent composition and/or a single solvent fraction is
usually selected and
maintained throughout the majority of the process. This is generally due to
the lack of
knowledge of changes that may be made in the process and the impact of process
changes
and/or operating parameter selections on the overall GHG emissions that are
produced by the
solvent-based hydrocarbon extraction process. Additionally, these operating
parameters
relationship to the overall GHG emissions produced may change over time, due
to either
internal factors, such as changes in the reservoir or well properties or
operating conditions, or
to external factors, such as the compositional selection and solvent fraction
utilized as make-
up solvents from sources separate to the reservoir operation or the impact of
oil product
produced from the reservoir. As such, without a comprehensive GHG emissions
analysis of at
least some of these reservoir variables and key performance indicators of the
process,
preferably performed over multiple periods of time, especially associated with
changes in these
internal and/or external factors, GHG emissions from the steam-solvent
processes cannot be
optimized, or preferably minimized, due use of non-optimal processing
conditions.
[0009] A need therefore exists in the industry for improved
technology, including
technology for methods enabling the optimization of greenhouse gas (GHG)
emissions from
solvent-based (which includes steam-solvent) heavy oil recovery processes and
associated
systems. A need exists for a system of GHG emissions analysis of key emission
sources as a
function of the produced oil and modification of solvent fractions and/or
solvent compositions
in the injection fluids of the solvent-based heavy oil recovery
processes/systems in order to
optimize GHG emissions associated with the overall recovery process.
SUMMARY
[0010] It is an object of the present disclosure to provide
systems and methods for the
optimization of greenhouse gas (GHG) emissions from solvent-based heavy oil
recovery
systems based on the analysis of GHG emissions from individual sources of the
overall solvent-
based heavy oil recovery systems and processes.
[0011] In an embodiment herein is a method for optimizing GHG emissions
from a solvent-
- 3 -
CA 3062478 2019-11-22
,
, =
based heavy oil recovery process for a subterranean reservoir, comprising:
a) determining a solvent having a first solvent composition to be modeled to
be
utilized as an injection fluid in the solvent-based heavy oil recovery
process;
b) determining the GHG emissions for at least one key GHG emissions source of
the
solvent-based oil recovery process over a range of solvent fractions based on
the first solvent
composition;
c) selecting a range of solvent fractions near the solvent fractions
associated with the
GHG minimum emissions point;
d) selecting the solvent fraction of the injection fluid in the solvent-based
heavy oil
extraction process to be within the selected range of solvent fractions;
e) injecting the injection fluid containing the solvent and steam utilizing
the selected
solvent fraction into the subterranean reservoir.
[0012] In another embodiment herein the total GHG emissions intensity
curve is based on
the calculated GHG emissions intensity curve for at least one, more than one
or all of the
following key GHG emissions sources of the solvent-based heavy oil recovery
process:
- the make-up solvent;
- the power demand;
- the solvent recovery unit (SRU); and
- the vaporization unit.
[0013] In another embodiment herein in step c), the selected range of
solvent fractions is
within +1- 20 vol% of the solvent fraction associated with the minimum GHG
emissions
intensity point.
[0014] In another embodiment herein in step c), the selected range of
solvent fractions
corresponds to a point on the total GHG emissions intensity curve that is
within +1- 20% of the
minimum GHG emissions intensity point.
[0015] The foregoing has broadly outlined the features of the present
disclosure so that the
detailed description that follows may be better understood. Additional
features will also be
described herein.
- 4 -
CA 3062478 2019-11-22
DESCRIPTION OF THE DRAWINGS
[0016] These and other features, aspects and advantages of the present
disclosure will
become apparent from the following description and the accompanying drawings,
which are
briefly discussed below.
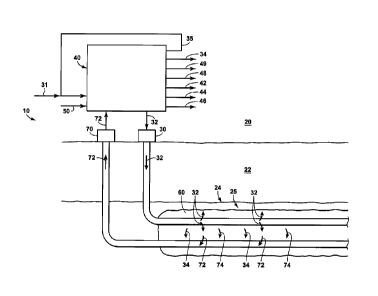
[0017] Figure 1 is a simplified schematic representation of an example of a
solvent-based
heavy oil recovery system.
[0018] Figure 2 illustrates the normalized values of two (2) key
performance indicators for
an example early to mid-life subterranean reservoir operating in a solvent-
based (steam-
solvent) heavy oil recovery process as a function of the solvent fraction of
the injection fluid.
[0019] Figure 3A illustrates a GHG emissions intensity curve of the make-up
solvent
component as a function of the solvent fraction of the injection fluid for an
early to mid-life
subterranean reservoir under exemplary solvent-based heavy oil recovery
process conditions
in accordance with the methods of the current disclosure.
[0020] Figure 3B illustrates a GHG emissions intensity curve of the
power demand
component as a function of the solvent fraction of the injection fluid for an
early to mid-life
subterranean reservoir under exemplary solvent-based heavy oil recovery
process conditions
in accordance with the methods of the current disclosure.
[0021] Figure 3C illustrates a GHG emissions intensity curve of a
vaporization unit
component of a surface facility as a function of the solvent fraction of the
injection fluid for an
early to mid-life subterranean reservoir under exemplary solvent-based heavy
oil recovery
process conditions in accordance with the methods of the current disclosure.
[0022] Figure 3D illustrates a GHG emissions intensity curve of the
solvent recovery unit
(SRU) component of a surface facility as a function of the solvent fraction of
the injection fluid
for an early to mid-life subterranean reservoir under exemplary solvent-based
heavy oil
recovery process conditions in accordance with the methods of the current
disclosure.
[0023] Figure 3E illustrates a total GHG emissions intensity curve
derived from the data in
Figures 3A-3D in accordance with the methods of the current disclosure.
[0024] Figure 4A illustrates a GHG emissions intensity curve of the make-
up solvent
component as a function of the solvent fraction of the injection fluid for a
late-life subterranean
- 5 -
CA 3062478 2019-11-22
õ
reservoir under exemplary solvent-based heavy oil recovery process conditions
in accordance
with the methods of the current disclosure.
[0025] Figure 4B illustrates a GHG emissions intensity curve of the
power demand
component as a function of the solvent fraction of the injection fluid for a
late-life subterranean
reservoir under exemplary solvent-based heavy oil recovery process conditions
in accordance
with the methods of the current disclosure.
[0026] Figure 4C illustrates a GHG emissions intensity curve of a
vaporization unit
component of a surface facility as a function of the solvent fraction of the
injection fluid for a
late-life subterranean reservoir under exemplary solvent-based heavy oil
recovery process
conditions in accordance with the methods of the current disclosure.
[0027] Figure 4D illustrates a GHG emissions intensity curve of the
solvent recovery unit
(SRU) component of a surface facility as a function of the solvent fraction of
the injection fluid
for a late-life subterranean reservoir under exemplary solvent-based heavy oil
recovery process
conditions in accordance with the methods of the current disclosure.
[0028] Figure 4E illustrates a total GHG emissions intensity curve derived
from the data in
Figures 4A-4D in accordance with the methods of the current disclosure.
DETAILED DESCRIPTION
[0029] For the purpose of promoting an understanding of the
principles of the disclosure,
reference will now be made to the features illustrated in the drawings and
specific language
will be used to describe the same. It will nevertheless be understood that no
limitation of the
scope of the disclosure is thereby intended. Any alterations and further
modifications, and any
further applications of the principles of the disclosure as described herein,
are contemplated as
would normally occur to one skilled in the art to which the disclosure
relates. It will be apparent
to those skilled in the relevant art that some features that are not relevant
to the present
disclosure may not be shown in the drawings for the sake of clarity.
[0030] At the outset, for ease of reference, certain terms used in
this application and their
meanings as used in this context are set forth. To the extent a term used
herein is not defined
below, it should be given the broadest definition persons in the pertinent art
have given that
- 6 -
CA 3062478 2019-11-22
term as reflected in at least one printed publication of issued patent.
Further, the present
techniques are not limited by the usage of the terms shown below, as all
equivalents, synonyms,
new developments, and terms or processes that serve the same or a similar
purpose are
considered to be within the scope of the present disclosure.
[0031] A "hydrocarbon" is an organic compound that primarily includes the
elements
hydrogen and carbon, although nitrogen, sulfur, oxygen, metals, or any number
of other
elements may be present in small amounts. Hydrocarbons generally refer to
components found
in heavy oil or in oil sands. However, the techniques described herein are not
limited to heavy
oils, but may also be used with any number of other subterranean reservoirs.
Hydrocarbon
compounds may be aliphatic or aromatic, and may be straight chained, branched,
or partially
or fully cyclic.
[0032] "Bitumen" is a naturally occurring heavy oil material. Generally,
it is the
hydrocarbon component found in oil sands. Bitumen can vary in composition
depending upon
the degree of loss of more volatile components. It can vary from a very
viscous, tar-like, semi-
solid material to solid forms. The hydrocarbon types found in bitumen can
include aliphatics,
aromatics, resins, and asphaltenes. A typical bitumen might be composed of:
19 weight (wt.)% aliphatics (which can range from 5 wt.% - 30 wt.%, or
higher);
19 wt.% asphaltenes (which can range from 5 wt.% - 30 wt.%, or higher);
30 wt.% aromatics (which can range from 15 wt.% - 50 wt.%, or higher);
32 wt.% resins (which can range from 15 wt.% - 50 wt.%, or higher); and
some amount of sulfur (which can range in excess of 7 wt.%).
[0033] The percentage of the hydrocarbon types found in bitumen can
vary. In addition
bitumen can contain some water and nitrogen compounds ranging from less than
0.4 wt.% to
in excess of 0.7 wt.%. The metals content, while small, may be removed to
avoid
contamination of synthetic crude oil. Nickel can vary from less than 75 ppm
(parts per million)
to more than 200 ppm. Vanadium can range from less than 200 ppm to more than
500 ppm.
[0034] The term "heavy oil" includes bitumen, as well as lighter
materials that may be
found in a sand or carbonate reservoir. "Heavy oil" includes oils that are
classified by the
American Petroleum Institute (API), as heavy oils, extra heavy oils, or
bitumens. Thus the
- 7 -
CA 3062478 2019-11-22
=
. ,
term "heavy oil" includes bitumen. Heavy oil may have a viscosity of about
1000 centipoise
(cP) or more, 10,000 cP or more, 100,000 cP or more or 1,000,000 cP or more.
In general, a
heavy oil has an API gravity between 22.3 API (density of 920 kilograms per
meter cubed
(kg/m3) or 0.920 grams per centimeter cubed (g/cm3)) and 10.0 API (density of
1,000 kg/m3
or 1 g/cm3). An extra heavy oil, in general, has an API gravity of less than
10.0 API (density
greater than 1,000 kg/m3 or greater than 1 g/cm3). For example, a source of
heavy oil includes
oil sand or bituminous sand, which is a combination of clay, sand, water, and
bitumen. The
recovery of heavy oils is based on the viscosity decrease of fluids with
increasing temperature
(such as utilizing steam) or with dilution (such as by increasing solvent
concentration). Once
the viscosity of the heavy oils is reduced, the heavy oil is mobilized and can
be recovered via
a production well. When utilized in a gravity drainage based recovery process,
the reduced
viscosity makes the drainage quicker and therefore directly contributes to the
recovery rate. A
heavy oil may include heavy end components and light end components.
[0035] The term "asphaltenes" or "asphaltene content" refers to
pentane insolubles (or the
amount of pentane insoluble in a sample) according to ASTM D3279. Other
examples of
standard ASTM asphaltene tests include ASTM test numbers D4055, D6560, and
D7061.
[0036] "Heavy end components" in heavy oil may comprise a heavy
viscous liquid or solid
made up of heavy hydrocarbon molecules. Examples of heavy hydrocarbon
molecules include,
but are not limited to, molecules having greater than or equal to 30 carbon
atoms (C30+). The
amount of molecules in the heavy hydrocarbon molecules may include any number
within or
bounded by the preceding range. The heavy viscous liquid or solid may be
composed of
molecules that, when separated from the heavy oil, have a higher density and
viscosity than a
density and viscosity of the heavy oil containing both heavy end components
and light end
components. For example, in Athabasca bitumen, about 70 weight (wt.) % of the
bitumen
contains C30+ molecules with about 18 wt. % of the Athabasca bitumen being
classified as
asphaltenes. The heavy end components may include asphaltenes in the form of
solids or
viscous liquids.
[0037] "Light end components" in heavy oil may comprise those
components in the heavy
oil that have a lighter molecular weight than heavy end components. The light
end components
may include what can be considered to be medium end components. Examples of
light end
components and medium end components include, but are not limited to, light
and medium
- 8 -
CA 3062478 2019-11-22
,I
=
hydrocarbon molecules having greater than or equal to 1 carbon atom and less
than 30 carbon
atoms. The amount of molecules in the light and medium end components may
include any
number within or bounded by the preceding range. The light end components and
medium end
components may be composed of molecules that have a lower density and
viscosity than a
density and viscosity of heavy end components from the heavy oil.
[0038] A "fluid" includes a gas or a liquid and may include, for
example, a produced or
native reservoir hydrocarbon, an injected mobilizing fluid, hot or cold water,
or a mixture of
these among other materials. "Vapor" refers to steam, wet steam, and mixtures
of steam and
wet steam, any of which could possibly be used with a solvent and other
substances, and any
material in the vapor phase.
[0039] An "injection fluid" or "injection mixture" as used herein is
a fluid which is injected
into subterranean reservoir through an injection well which is generally
designed to assist in
reducing the viscosity of hydrocarbons (e.g., bitumen) located in the
subterranean reservoir.
The injection fluid may reduce the viscosity of the hydrocarbons located in
the subterranean
reservoir due to heat, dilution (or solvency), or a combination thereof.
Unless otherwise stated,
the injection fluid may be a gas, a liquid, or a combination thereof. In
embodiments, unless
otherwise stated, an "injection fluid" may comprise steam, a solvent, or a
combination thereof,
whereas an "injection mixture" will comprise steam and a solvent.
[0040] "Facility" or "surface facility" is a tangible piece of
physical equipment through
which fluids, including recovered heavy oil, are either produced from a
subterranean reservoir
or injected into a subterranean reservoir, or equipment that can be used to
control production
or completion operations. In its broadest sense, the term facility is applied
to any equipment
that may be present along the flow path between a subterranean reservoir and
its delivery
outlets. Facilities may comprise production wells, injection wells, well
tubulars, wellbore head
equipment, gathering lines, manifolds, pumps, compressors, separators, surface
flow lines,
steam generation plants, processing plants, and delivery outlets. In some
instances, the term
"surface facility" is used to distinguish from those facilities other than
wells.
[0041] "Pressure" is the force exerted per unit area by the gas on
the walls of the volume.
Pressure may be shown in this disclosure as pounds per square inch (psi),
kilopascals (kPa) or
megapascals (MPa). "Atmospheric pressure" refers to the local pressure of the
air. "Absolute
- 9 -
CA 3062478 2019-11-22
pressure" (psia) refers to the sum of the atmospheric pressure (14.7 psia at
standard conditions)
plus the gauge pressure. "Gauge pressure" (psig) refers to the pressure
measured by a gauge,
which indicates only the pressure exceeding the local atmospheric pressure
(i.e., a gauge
pressure of 0 psig corresponds to an absolute pressure of 14.7 psia). The term
"vapor pressure"
has the usual thermodynamic meaning. For a pure component in an enclosed
system at a given
pressure, the component vapor pressure is essentially equal to the total
pressure in the system.
Unless otherwise specified, the pressures in the present disclosure are
absolute pressures.
[0042] A "subterranean reservoir" is a subsurface rock or sand reservoir
from which a
production fluid, or resource, can be harvested. A subterranean reservoir may
interchangeably
be referred to as a subterranean formation. The subterranean formation may
include sand,
granite, silica, carbonates, clays, and organic matter, such as bitumen, heavy
oil (e.g., bitumen),
oil, gas, or coal, among others. Subterranean reservoirs can vary in thickness
from less than
one foot (0.3048 meters (m)) to hundreds of feet (hundreds of meters). The
resource is
generally a hydrocarbon, such as a heavy oil impregnated into a sand bed.
[0043] "Thermal recovery processes" include any type of hydrocarbon
recovery process
that uses a heat source to enhance the recovery, for example, by lowering the
viscosity of a
hydrocarbon. The processes may use injected mobilizing fluids, such as but not
limited to hot
water, wet steam, dry steam, or solvents alone, or in any combination, to
lower the viscosity of
the hydrocarbon. Any of the thermal recovery processes may be used in concert
with solvents.
For example, thermal recovery processes may include cyclic steam stimulation
(CSS), steam
assisted gravity drainage (SAGD), steam flooding, in-situ combustion and other
such
processes.
[0044] "Solvent-based recovery processes", "solvent-based heavy oil
recovery processes",
or the like includes any type of hydrocarbon recovery process that uses a
solvent, at least in
part, to enhance the recovery of heavy oil, for example, by lowering a
viscosity of the in-situ
hydrocarbon through dilution. Solvent-based recovery processes may be used in
combination
with other recovery processes, such as, for example, thermal recovery
processes. In solvent-
based recovery processes, a solvent is injected into a subterranean reservoir.
The solvent may
be heated or unheated prior to injection, may be a vapor, liquid, or a
combination, and may be
injected with or without steam. Solvent-based recovery processes may include,
but are not
limited to, solvent assisted cyclic steam stimulation (SA-CSS), solvent
assisted steam assisted
- 10 -
CA 3062478 2019-11-22
. =
gravity drainage (SA-SAGD), solvent assisted steam flood (SA-SF), vapor
extraction process
(VAPEX), heated vapor extraction process (H-VAPEX), cyclic solvent process
(CSP), heated
cyclic solvent process (H-CSP), solvent flooding, heated solvent flooding,
liquid extraction
process, heated liquid extraction process, solvent-based extraction recovery
process (SEP),
thermal solvent-based extraction recovery processes (TSEP), and any other such
recovery
process employing solvents either alone or in combination with steam. A
solvent-based
recovery process may be a thermal recovery process if the injection mixture is
heated prior to
injection into the subterranean reservoir. The solvent-based recovery process
may employ
gravity drainage.
[0045] "Follow-up process" for the purpose of this specification is a
process that may be
utilized after a "Solvent-based recovery processes" in the later life (or
"late life") stage of a
subterranean reservoir and is defined as a switch to a different process which
is directed to
improving the recovery of the solvent remaining in the subterranean reservoir
(while still also
recovering heavy oil) that has been deposited into the subterranean reservoir
by the solvent-
based recovery process and has not been recovered. Examples of follow-up
processes may
include, but are not limited to, a steam assisted gravity drainage (SAGD)
process, a solvent
assisted cyclic steam stimulation (SA-CSS) process, a steam flood process, a
solvent assisted
steam flood (SA-SF) process, a solvent flood process, a heated vapor solvent
flood process, a
non-condensable gas (NCG) assisted steam process, a non-condensable gas (NCG)
assisted
solvent process, and a non-condensable gas (NCG) flood process.
[0046] "Transition criteria" for the purpose of this specification is
the criteria utilized to
switch from a "Solvent-based recovery process" to a "Follow-up process". The
transition
criteria may include a threshold fraction of the estimated original heavy oil
in the subterranean
reservoir that has been recovered. Examples of the threshold fraction include
at least 10%, at
least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least
70%, and/or at least
80% of the estimated original heavy oil in the subterranean reservoir.
[0047] "Greenhouse gas" or "GHG" for the purpose of this specification
is carbon dioxide
(CO2). "Greenhouse gas emissions" or "GHG emissions" are the quantity of
greenhouse gases
that are emitted into the atmosphere by a process or process component as
denoted herein.
GHG emissions may also be expressed in a normalized value such as a total
weight of GHG
emissions per unit amount of produced oil.
- 11 -
CA 3062478 2019-11-22
[0048] "Solvent fraction" as utilized herein is the fraction by volume %
(vol%) of a solvent
(generally a hydrocarbon) in a mixture. Most notably, as used herein unless
otherwise noted,
the solvent fraction is the fractional amount (by vol%) of a solvent in a
solvent-steam mixture
at standard conditions.
[0049] "Make-up solvent" as used herein is the amount of new, additional
solvent that is
added to an injection fluid. Make-up solvent is generally expressed herein as
the fraction (by
vol%) of the total solvent injected (by injection rate) into the subterranean
reservoir. The total
solvent (or total solvent rate) is comprised of the recycled (or recovered)
solvent and the
makeup solvent. Since the amount of recycled (or recovered) solvent is
generally less than the
amount of total solvent, the make-up solvent is the amount of solvent added
(by fraction of
total solvent) to maintain a certain steam-to-solvent injection ratio in the
injection fluid.
[0050] "Power demand" is the electricity use for the surface facility
units such as pumps
and compressors, which can be estimated based on total fluids in circulation
for a recovery
process.
[0051] "Solvent recovery unit" or "SRU" is the unit in a solvent-based
extraction recovery
processes which recovers solvent from the produced fluids (or a portion
thereof) recovered
from the subterranean reservoir and produces a recycled solvent that is
reutilized in the
injection fluid of the solvent-based extraction recovery process.
[0052] "Vaporization unit" is the portion of a solvent-based extraction
recovery processes
which vaporizes (which also includes partial vaporization) the injection
fluid. In a steam-
solvent process, the steam and solvent components may be vaporized separately
as single
components or after they have been combined in a mixture for use as an
injection fluid.
[0053] "Produced oil" is the amount of oil produced (or "produced oil"
herein) and includes
all hydrocarbons produced from the reservoir, less the amount of recovered
solvent from the
reservoir.
[0054] "Oil Production Rate" (or "OPR") is the amount of oil produced
(i.e., produced oil)
from the reservoir per unit time. Herein the amount of oil produced (or
"produced oil" herein)
includes all hydrocarbons produced from the reservoir, less the amount of
recovered solvent
from the reservoir.
- 12 -
CA 3062478 2019-11-22
I
. =
[0055] "Average Boiling Point", "average boiling point", or "ABP" is
the temperature (in
C) at which 50 vol% of a substance or mixture boils (vaporizes) under standard
atmospheric
conditions. In the case of a solvent mixture as discussed herein, ABP of the
solvent mixture is
the temperature (in C) at which 50 vol% of solvent mixture boils (vaporizes)
under standard
atmospheric conditions.
[0056] "Azeotrope" means the thermodynamic azeotrope as described
further herein.
[0057] A "wellbore" is a hole in the subsurface made by drilling or
inserting a conduit into
the subsurface. A wellbore may have a substantially circular cross section or
any other cross-
sectional shape, such as an oval, a square, a rectangle, a triangle, or other
regular or irregular
shapes. The term "well," when referring to an opening in the formation or
reservoir, may be
used interchangeably with the term "wellbore." Further, multiple pipes may be
inserted into a
single wellbore, for example, as a liner configured to allow flow from an
outer chamber to an
inner chamber.
[0058] A "solvent extraction chamber" is a region of a subterranean
reservoir containing
heavy oil that forms around a well that is injecting solvent, which may
additionally include
other components such as steam or non-condensable gases (also termed herein as
a "solvent
injection mixture"), into the subterranean reservoir. The solvent extraction
chamber has a
temperature and a pressure that is generally at or close to a temperature and
pressure of the
solvent injection mixture injected into the subterranean reservoir. The
solvent extraction
chamber may form when heavy oil has, due to heat from the solvent injection
mixture,
dissolution within the solvent, combination with the solvent injection mixture
components,
and/or the action of gravity, at least partially mobilized through the pore
spaces of the reservoir
matrix. The mobilized heavy oil may be at least partially replaced in the pore
spaces by solvent,
thus forming the solvent chamber. The solvent chamber may contain liquid
solvent, vapor
solvent, condensed solvent, residual heavy oil, water, gas, non-condensable
gas and/or a
combination and/or mixture of them. In practice, layers in the subterranean
reservoir containing
heavy oil may not necessarily have pore spaces that contain 100 percent (%)
heavy oil and may
contain only 70 - 80 volume (vol.) % heavy oil with the remainder possibly
being water. A
water and/or gas containing layer in the subterranean reservoir may comprise
100% water
and/or gas in the pore spaces, but generally contains 5 - 70 vol.% gas and 20 -
30 vol.% water
with any remainder possibly being heavy oil.
- 13 -
CA 3062478 2019-11-22
=
[0059] A "vapor chamber" is a solvent extraction chamber that
includes a vapor, or
vaporous solvent. The vapor chamber may contain other gases including vapor
water, and/or
non-condensable gases. The vapor chamber may also contain vapor mixtures of
water and
solvent. The vapor chamber may also contain near-azeotropic or azeotropic
vapor mixtures of
water and solvent. Thus, when the solvent (or solvent injection mixture) is
injected into the
subterranean reservoir as a vapor, a vapor chamber may be formed around the
well.
[0060] The terms "approximately," "about," "substantially," and
similar terms are intended
to have a broad meaning in harmony with the common and accepted usage by those
of ordinary
skill in the art to which the subject matter of this disclosure pertains. It
should be understood
by those of skill in the art who review this disclosure that these terms are
intended to allow a
description of certain features described and claimed without restricting the
scope of these
features to the precise numeral ranges provided. Accordingly, these terms
should be interpreted
as indicating that insubstantial or inconsequential modifications or
alterations of the subject
matter described and are considered to be within the scope of the disclosure.
These terms when
used in reference to a quantity or amount of a material, or a specific
characteristic of the
material, refer to an amount that is sufficient to provide an effect that the
material or
characteristic was intended to provide. The exact degree of deviation
allowable may in some
cases depend on the specific context.
[0061] The articles "the", "a" and "an" are not necessarily
limited to mean only one, but
rather are inclusive and open ended so as to include, optionally, multiple
such elements.
[0062] As used herein, the phrase "at least one," in reference
to a list of one or more entities
should be understood to mean at least one entity selected from any one or more
of the entity in
the list of entities, but not necessarily including at least one of each and
every entity specifically
listed within the list of entities and not excluding any combinations of
entities in the list of
entities. This definition also allows that entities may optionally be present
other than the
entities specifically identified within the list of entities to which the
phrase "at least one" refers,
whether related or unrelated to those entities specifically identified. Thus,
as a non-limiting
example, "at least one of A and B" (or, equivalently, "at least one of A or
B," or, equivalently
"at least one of A and/or B") may refer, to at least one, optionally including
more than one, A,
with no B present (and optionally including entities other than B); to at
least one, optionally
including more than one, B, with no A present (and optionally including
entities other than A);
- 14 -
CA 3062478 2019-11-22
to at least one, optionally including more than one, A, and at least one,
optionally including
more than one, B (and optionally including other entities). In other words,
the phrases "at least
one," "one or more," and "and/or" are open-ended expressions that are both
conjunctive and
disjunctive in operation. For example, each of the expressions "at least one
of A, B and C,"
"at least one of A, B, or C," "one or more of A, B, and C," "one or more of A,
B, or C" and
"A, B, and/or C" may mean A alone, B alone, C alone, A and B together, A and C
together, B
and C together, A, B and C together, and optionally any of the above in
combination with at
least one other entity.
[0063] As used herein, the term "and/or" placed between a first entity
and a second entity
means one of (1) the first entity, (2) the second entity, and (3) the first
entity and the second
entity. Multiple entities listed with "and/or" should be construed in the same
manner, i.e., "one
or more" of the entities so conjoined. Other entities may optionally be
present other than the
entities specifically identified by the "and/or" clause, whether related or
unrelated to those
entities specifically identified. Thus, as a non-limiting example, a reference
to "A and/or B,"
when used in conjunction with open-ended language such as "comprising" may
refer to A only
(optionally including entities other than B); to B only (optionally including
entities other than
A); to both A and B (optionally including other entities). These entities may
refer to elements,
actions, structures, steps, operations, values, and the like.
[0064] As used herein the terms "adapted" and "configured" mean that the
element,
component, or other subject matter is designed and/or intended to perform a
given function.
Thus, the use of the terms "adapted" and "configured" should not be construed
to mean that a
given element, component, or other subject matter is simply "capable of'
performing a given
function but that the element, component, and/or other subject matter is
specifically selected,
created, implemented, utilized, programmed, and/or designed for the purpose of
performing
the function. It is also within the scope of the present disclosure that
elements, components,
and/or other recited subject matter that is recited as being adapted to
perform a particular
function may additionally or alternatively be described as being configured to
perform that
function, and vice versa.
[0065] As used herein, the phrase, "for example," the phrase, "as an
example," and/or
simply the term "example," when used with reference to one or more components,
features,
details, structures, embodiments, and/or methods according to the present
disclosure, are
- 15 -
CA 3062478 2019-11-22
intended to convey that the described component, feature, detail, structure,
embodiment, and/or
method is an illustrative, non-exclusive example of components, features,
details, structures,
embodiments, and/or methods according to the present disclosure. Thus, the
described
component, feature, detail, structure, embodiment, and/or method is not
intended to be limiting,
required, or exclusive/exhaustive; and other components, features, details,
structures,
embodiments, and/or methods, including structurally and/or functionally
similar and/or
equivalent components, features, details, structures, embodiments, and/or
methods, are also
within the scope of the present disclosure. Any of the ranges disclosed may
include any number
within and/or bounded by the range given.
to [0066] In the illustrative figures herein, in general, elements that
are likely to be included
are illustrated in solid lines, while elements that are optional may be
illustrated in dashed lines.
However, elements that are shown in solid lines may not be essential. Thus, an
element shown
in solid lines may be omitted without departing from the scope of the present
disclosure.
100671 Figures 1 through 4E provide illustrative, non-exclusive examples
of systems
according to the present disclosure, components of systems, data that may be
utilized to select
a composition of a hydrocarbon solvent mixture and or a reservoir injection
mixture that may
be utilized with systems, and/or methods, according to the present disclosure,
of operating
and/or utilizing systems. Elements that serve a similar, or at least
substantially similar, purpose
are labeled with like numbers in each of Figures 1 through 4E, and these
elements may not be
discussed in detail herein with reference to each of Figures 1 through 4E.
Similarly, all
elements may not be labeled in each of Figures 1 through 4E, but associated
reference numerals
may be utilized for consistency. Elements, components, and/or features that
are discussed
herein with reference to one or more of Figures 1 through 4E may be included
in and/or utilized
with any of Figures 1 through 4E without departing from the scope of the
present disclosure.
[0068] Figure 1 is a non-limiting schematic representation of a hydrocarbon
production
system 10 that may be utilized with, may be included in, and/or may include
the systems and
methods according to the present disclosure. Figure 1 is utilized only to
assist in explaining
the details of the present disclosure, and is not meant to be limiting in any
manner, including
any limitations on reservoir or well configurations, solvent or steam usage or
requirements, or
overall recovery system and/or oil processing requirements. For purposes of
illustration, the
hydrocarbon production system 10 may include an injection well 30 and a
production well 70
- 16 -
CA 3062478 2019-11-22
- =
that extend within a subterranean reservoir 24 that is present within a
subsurface region 22
and/or that extend between a surface region 20 and the subterranean reservoir
24. Hydrocarbon
production system 10 may include a surface facility 40. Surface facility 40
may be configured
to receive a reservoir heavy oil product stream 72 from production well 70. A
reservoir heavy
oil product stream 72 may be produced from the subterranean reservoir 24.
Surface facility 40
may be configured to provide a reservoir injection mixture 32 to injection
well 30.
[0069] The reservoir injection mixture (or "injection fluid")
32 may be in liquid form,
vapor form, or both. The reservoir injection mixture preferably is comprised
of a steam and a
solvent mixture. The solvent mixture is comprised of hydrocarbons. In
preferred embodiments,
the solvent mixture is substantially comprised of hydrocarbons, or even
essentially comprised
of hydrocarbons. In the preferred processes herein, the normal boiling point
of the solvent
mixture is selected such as to minimize the greenhouse gas (GHG) emissions of
the oil recovery
process based on one or more process key performance indicators.
[0070] When the reservoir injection mixture 32 comprises a
vaporous hydrocarbon solvent
mixture, the solvent-based recovery process may be referred to as, or maybe, a
vapor extraction
process (VAPEX). Preferably, the reservoir injection mixture 32 includes steam
and a solvent
mixture. Preferably the solvent mixture is comprised essentially of
hydrocarbons. In a
preferred embodiment, the steam and solvent mixture is within 30%+/-, 20%+/-,
or 10%+/- of
the azeotropic solvent molar fraction of the steam and the solvent mixture as
measured at the
reservoir operating pressure. Alternatively, molar fraction of solvent mixture
in the solvent
and steam injection mixture is 70-100%, 80-100%, or 90 to 100% of the
azeotropic solvent
molar fraction of the steam and the solvent mixture as measured at the
reservoir operating
pressure. Alternatively, the molar fraction of solvent mixture in the solvent
and steam injection
mixture is 70-110% of the azeotropic solvent molar fraction of the steam and
the solvent
mixture as measured at the reservoir operating pressure. In another preferred
embodiment, the
reservoir injection mixture is comprised of at least 80% by weight of the
steam and the solvent
mixture. In other preferred embodiments, the reservoir injection mixture is
comprised of at
least 90% or 95% by weight of the steam and the solvent mixture, more
preferably, is comprised
essentially of the steam and the solvent mixture.
[0071] In preferred embodiments, at least 90%, at least 95%, or essentially
all (by weight)
of the reservoir injection mixture is injected into the subterranean reservoir
in vapor form. In
- 17 -
CA 3062478 2019-11-22
=
other embodiments, at least 5 wt%, 10 wt%, 20 wt%, 40 wt%, 60 wt%, 75 wt%, 85
wt%, 90
wt% , 95 wt% or 99 wt% of the reservoir injection mixture is hydrocarbon
compounds.
[0072] When the solvent-based recovery process is performed
using heated solvent, the
solvent-based recovery process may be referred to as a high temperature
solvent (and/or vapor)
solvent-based recovery process. The heated solvent may be injected into the
subterranean
reservoir at an injection temperature and an injection pressure. The injection
temperature may
be at, or near, a saturation temperature for the heated solvent at the
injection pressure. When
more than one solvent is utilized, the extraction process may be referred to
as a multi-solvent-
based recovery process and/or a multi-component solvent-based recovery
process, which, at
elevated temperatures, may be referred to as a high temperature multi-
component solvent-
based recovery process, which may be a high temperature multi-component vapor
extraction
process.
[0073] Once provided to subterranean reservoir 24, the reservoir
injection mixture 32 may
combine with the bituminous hydrocarbon deposit 25 within a solvent extraction
chamber 60,
may dilute the bituminous hydrocarbon deposit 25, may dissolve in the
bituminous
hydrocarbon deposit 25, and/or may dissolve the bituminous hydrocarbon deposit
25, thereby
decreasing the viscosity of the bituminous hydrocarbon deposit. When reservoir
injection
mixture 32 is a vaporous hydrocarbon solvent mixture, solvent extraction
chamber 60 may be
referred to as a vapor chamber 60. The vaporous hydrocarbon solvent mixture
may condense
within vapor chamber 60. When reservoir injection mixture 32 condenses, the
hydrocarbon
solvent mixture may release latent heat (or latent heat of condensation),
transfer thermal energy
to the subterranean reservoir, and/or generate a condensate 34. Condensation
of the reservoir
injection mixture 32 may heat a bituminous hydrocarbon deposit 25 that may be
present within
the subterranean reservoir, thereby decreasing a viscosity of the bituminous
hydrocarbon
deposit. In embodiments, the subterranean reservoir operating temperature may
be 30-250 C
or 80-150 C. In further embodiments, the subterranean reservoir operating
pressure may be 5-
95% of a fracture pressure of the reservoir, or 0.2 to 4 MPa, or 1 to 2.5 MPa.
Conversely, the
subterranean reservoir operating pressure may be equal to the pressure of a
gas cap in the
subterranean reservoir, the pressure of a gas zone within the subterranean
reservoir, the
pressure of a bottom water zone in the subterranean reservoir, or the pressure
of a mobile water
zone within the subterranean reservoir.
- 18 -
CA 3062478 2019-11-22
, = =
[0074] The bituminous hydrocarbon deposit 25 may include
bitumen, gaseous
hydrocarbons, asphaltenes, and/or water. The reservoir injection mixture 32
and/or condensate
34 also may combine with, mix with, be dissolved in, dissolve, and/or dilute
bituminous
hydrocarbon deposit 25, further decreasing the viscosity of the bituminous
hydrocarbon
deposit.
[0075] The energy transfer between the reservoir injection
mixture 32 and bituminous
hydrocarbon deposit 25 and/or the mixing of reservoir injection mixture 32
and/or condensate
34 with bituminous hydrocarbon deposit 25 may generate reduced-viscosity
hydrocarbons 74,
which may flow to production well 70. The reduced-viscosity hydrocarbons 74
may flow to
production well 70 due to gravity. After flowing to production well 70, a
reservoir product
stream 72 containing heavy oil is produced from the subterranean reservoir.
The reduced-
viscosity hydrocarbons 74 may have a lower viscosity than the hydrocarbons
within the
subterranean reservoir 24 had before the reservoir injection mixture 32 was
injected into the
subterranean reservoir 24. The reservoir product stream 72 may comprise
reduced-viscosity
hydrocarbons 74, asphaltenes, gaseous hydrocarbons, water, reservoir injection
mixture 32,
and/or condensate 34 in any suitable ratio and/or relative proportion.
[0076] Surface facility 40 may process the reservoir product
stream 72 and/or may separate
the reservoir product stream 72 into one or more component streams prior to
the product
hydrocarbon stream being conveyed from the surface facility 40. Surface
facility 40 may
separate reservoir product stream 72 into a bitumen product stream 42, a
gaseous hydrocarbon
product stream 44, an asphaltene product stream 48, a solvent mixture 35, a
separated surplus
solvent stream 49, and/or a water product stream 46, which may include water.
The bitumen
product stream 42 may include bitumen and/or asphaltenes. The gaseous
hydrocarbon product
stream 44 may include gaseous hydrocarbons. The asphaltene product stream 48
may include
asphaltenes. The separated surplus solvent stream 49 may include a portion of
hydrocarbon
solvent mixture 32 that was produced with the reservoir heavy oil product
stream 72. The
surplus solvent stream 49 may be referred to as an undesired solvent stream,
an unwanted
solvent stream, and/or an excess solvent stream. Surplus solvent stream 49 may
be generated
as a result of adjustments to the solvent mixture composition. Surplus solvent
stream 49 may
be generated as a result of removing some of the solvents in the reservoir
product stream 72
that are not wanted or desired to be in the solvent mixture 35 or the
reservoir injection mixture
- 19 -
CA 3062478 2019-11-22
, =
32. The surplus solvent stream 49 may be mixed as a diluting agent, blending
agent, and/or
viscosity-reducing agent with the bitumen product stream 42 to facilitate
shipment by pipelines.
[0077] Surface facility 40 may generate a solvent mixture 35
from any suitable source. In
most operations at least a portion of the solvent mixture 35 is recovered from
the subterranean
reservoir in a solvent recovery unit ("SRU"). The SRU (not separately shown)
may be a
subcomponent of the surface facility 40. The solvent recovered from by the SRU
is recycled
to the solvent mixture 35 for reuse as a solvent in the solvent-based
hydrocarbon recovery
processes herein. The solvent mixture 35 may comprise hydrocarbons that have
been produced
by a source separate from the subterranean reservoir. For example, the solvent
mixture may
comprise a natural gas liquid, a natural gas condensate, a liquefied petroleum
gas, or a crude
oil refmery naphtha. Surface facility 40 may receive a supplemental solvent
stream 31 and/or
may supply at least a portion of the solvent mixture 35 recovered from the
reservoir product
stream 72 as a part of the reservoir injection stream 32 to injection well 30.
Surface facility 40
may separate at least a portion of gaseous hydrocarbon product stream 44,
solvent mixture 35,
and/or condensate 34 from the reservoir product stream 72. Surface facility 40
may recycle
and/or re-inject a portion of the gaseous hydrocarbon product stream 44,
separated solvent
mixture 35, and/or separated condensate 34 into injection well 30 as
components of the
reservoir injection mixture 32. Additional steam 50 may be added to the
surface facility 40
and/or injected directly as part of the reservoir injection stream 32. The
solvent mixture 35 may
additionally include a supplemental solvent stream 31. The composition of the
supplemental
solvent stream 31 may be similar in composition to the solvent mixture 35
wherein its main
purpose is to add additional solvent to the solvent mixture 35 for the
reservoir injection mixture
32. Alternatively, the supplemental solvent stream 31 may be tailored to
adjust the composition
of the overall solvent mixture 35 for the reservoir injection mixture 32, as
well as additionally
supply additional solvent to the overall process to make up for losses in the
subterranean
reservoir and/or losses due to the surface facility processing and solvent
recovery.
[0078] In an embodiment, at least a portion of the reservoir
product stream 72 is sent to a
solvent recovery unit (SRU) (not separately shown, but may be a component of
the surface
facility 40) which separates the product stream into the bitumen (or heavy
oil) product stream
42 and the solvent mixture 35, the latter of which may recycled for injection
into the solvent-
based oil recovery process. In other embodiments, the SRU may contain a single
stage flash
- 20 -
CA 3062478 2019-11-22
unit or a multistage flash unit which separates the product stream into the
heavy oil product
stream 42, the recovered solvent mixture 35, and the separated surplus solvent
stream 49. The
flash unit may be a two-stage flash unit. In other embodiments, at least a
portion of the
reservoir product stream 72 may be sent to a separation unit comprising a
multistage distillation
unit which separates the product stream into the heavy oil product stream 42,
the solvent
mixture 35, and the separated surplus solvent stream 49. In any of these
embodiments, the
operational variables of the single stage flash unit, the multistate flash
unit, or the multistage
distillation unit may be regulated to tailor the composition of the solvent
mixture 35 to the
recommend range of the solvent normal boiling point by the this disclosed
method. The
operational variables may include the flash temperature and pressure in each
flash units. In
preferred embodiments, the operational variables of the single stage flash
unit, the multistate
flash unit, or the multistage distillation unit are regulated to match the
composition of the
solvent mixture to an optimized normal boiling point range for the process.
[0079] Conventional recovery processes that utilize an injected vapor
stream to decrease
the viscosity of hydrocarbons may utilize a pure (i.e., single-component), or
at least
substantially pure, injected vapor stream that comprises a light hydrocarbon,
such as propane.
In contrast, the systems and methods according to the present disclosure may
utilize a solvent
mixture 35. The solvent mixture 35 may include a hydrocarbon fraction that
comprises,
consists of, or consists essentially of C4 to C12 hydrocarbons, or C5 to C9
hydrocarbons. The
solvent mixture 35 may include a hydrocarbon fraction that comprises, consists
of, or consists
essentially of at least one of alkanes, iso-alkanes, naphthenic hydrocarbons,
aromatic
hydrocarbons, and olefin hydrocarbons. Supplemental solvent stream 31 may be
tailored to
adjust the composition of the solvent mixture 35 to within the solvent
specification as required
herein for optimizing overall GHG emissions for the associated processes. As
such, the
supplemental solvent stream 31 may include a hydrocarbon fraction that
comprises, consists
of, or consists essentially of C4 to C12 hydrocarbons, or C5 to C9
hydrocarbons. Additionally,
or alternatively, the supplemental solvent stream 31 may include a hydrocarbon
fraction that
comprises, consists of, or consists essentially of at least one of allcanes,
iso-alkanes, naphthenic
hydrocarbons, aromatic hydrocarbons, and olefm hydrocarbons.
[0080] Theoretically, available solvents to use in present solvent-based
oil recovery
processes can range from light hydrocarbon mixtures such as natural gas
liquids (NGLs) and
- 21 -
CA 3062478 2019-11-22
liquefied petroleum gases (LPGs) to heavy fractions such as different refinery
streams. The
concept disclosed herein proposes a new methodology for selecting parameters
of a solvent-
based oil recovery process and determining an optimum range of solvent
fraction to be utilized
in the injection fluid and/or determining an optimum range of the potential
injection solvent
fraction and/or the average boiling point (ABP) in order to optimize (or
minimize) total GHG
emissions from the solvent-based hydrocarbon extraction process. This concept
relies on: 1)
determining at least one solvent composition to be modeled, 2) determining the
GHG emissions
for at least one key GHG emissions source over a range of solvent fractions
based on the at
least one solvent composition; 3) selecting a range of solvent fractions near
the GHG minimum
emissions point based on the at least one key GHG emissions sources; and 4)
tailoring the
solvent fraction of the injected fluid in the solvent-based hydrocarbon
extraction process to be
within a certain envelope from the GHG minimum emissions point. In other
embodiments (as
disclosed later herein), step 4) can be replaced by, or additionally include,
tailoring the
composition of the solvent fraction of the injected fluid in the solvent-based
heavy oil
extraction process to operate within a certain envelope from the GHG minimum
emissions
point.
100811 Figure 2 helps illustrate an underlying concept of the present
disclosure. Figure 2
illustrates the normalized values of two (2) key performance indicators for an
exemplary early
to mid-life subterranean reservoir operating in a solvent-based (steam-
solvent) heavy oil
recovery process plotted as a function of the solvent fraction of the
injection fluid. As shown
in Figure 2, for the modeled steam-solvent recovery processes, energy
intensity ("energy
intensity" = Excess energy delivered to the reservoir (Energy In ¨ Energy Out)
for producing
a volume of oil) and solvent retention ("solvent retention" = retained vol% of
solvent in the
reservoir for producing a volume of oil) operate inversely of one another. The
values of these
two (2) key performance indicators as a function of volume fraction are shown
as "normalized"
values in Figure 2 (i.e., the values shown are actual values divided by their
corresponding
maximum values). The decreasing trend for energy intensity can be explained by
reducing
steam fraction and replacing it with hydrocarbon solvent which takes advantage
of dilution in
addition to heat to decrease the bitumen viscosity. To comprehend the solvent
retention pattern,
one needs to recognize the solvent-steam azeotropic point. Consider
compositions with a
solvent volume fraction less than the azeotropic amount. As the mixture loses
heat at constant
- 22 -
CA 3062478 2019-11-22
pressure, the vapor phase gets richer in solvent while the liquid phase
consists primarily of
water. Thus when tracing this path, little to no solvent condenses and the
solvent remains
primarily in the vapor phase. The majority of solvent retention incurs in the
case and in the
form of the solvent rich liquid phase condensation. So in order to minimize
the solvent retention
within the chamber, compositions with a solvent fraction lower than the
azeotropic condition
are desirable anywhere in the chamber.
[0082] In thermal recovery processes (such as the steam-solvent recovery
processes
discussed herein), the energy intensity for vaporization is proportional to
the required amount
of fuel utilized in the heaters for vaporization of the injection fluid and
consequently
proportional to GHG direct emission for the vaporization unit. As a result,
the reduction in
energy intensity with increased solvent content results in reduction in GHG
direct emission.
While SRU energy intake increases with solvent concentration, it is a minor
contributor to
direct GHG emission. Therefore, direct GHG emission intensity in a steam-
solvent (or solvent-
based) hydrocarbon recovery process is a strong function of the amount of
solvent injected into
the subterranean reservoir during the process.
[0083] In preferred embodiments, the key GHG emissions sources of the
recovery process
for determining the GHG emissions as a function of the solvent fraction in the
processes as
utilized herein are considered as: 1) the make-up solvent, 2) the power
demand, 3) the solvent
recovery unit (SRU), and 4) the vaporization unit. The make-up solvent and the
power demand
are considered parameters of the process which produce "indirect" GHG
emissions. This is
because, while these components are part of the overall GHG emissions for the
process, the
GHG emissions accounted for in these parameters (i.e., emissions sources)
occurs outside of
the direct operation of the surface facility (see element 40 of Figure 1),
such as GHG emissions
associated with the production and transportation of the makeup solvent and
the GHG
.. emissions associated with producing electricity for the power demand of the
surface facilities.
In contrast, the solvent recovery unit (SRU) and the vaporization unit are
considered parameters
of the process which produce "direct" GHG emissions, as most of the energy
(and thus the
associated GHG emissions) are produced in these units that are a part of the
surface facility
unit associated with the solvent-based hydrocarbon recovery processes herein.
[0084] Figures 3A-3D show the analytical modeling analysis and numerical
simulation
results illustrate this concept by plotting the indirect and direct GHG
emissions intensities as a
- 23 -
CA 3062478 2019-11-22
= =
function of solvent fraction for the key GHG emissions sources. In each of
these figures, the
GHG for each source is normalized as a "GHG Emissions Intensity" in tonnes of
CO2
emissions per barrel of produced oil. Figure 3A illustrates the GHG emissions
intensity of
the make-up solvent component as a function of the solvent fraction of the
injection fluid for
an early to mid-life subterranean reservoir under exemplary solvent-based
hydrocarbon
recovery process conditions in accordance with the methods of the current
disclosure. Figure
3B illustrates the GHG emissions intensity of the power demand component as a
function of
the solvent fraction of the injection fluid for an early to mid-life
subterranean reservoir under
exemplary solvent-based hydrocarbon recovery process conditions in accordance
with the
methods of the current disclosure. Figure 3C illustrates the GHG emissions
intensity of a
vaporization unit component of a surface facility as a function of the solvent
fraction of the
injection fluid for an early to mid-life subterranean reservoir under
exemplary solvent-based
hydrocarbon recovery process conditions in accordance with the methods of the
current
disclosure. Figure 3D illustrates the GHG emissions intensity of the solvent
recovery unit
(SRU) component of a surface facility as a function of the solvent fraction of
the injection fluid
for an early to mid-life subterranean reservoir under exemplary solvent-based
hydrocarbon
recovery process conditions in accordance with the methods of the current
disclosure.
[0085] Since all of these GHG emission components are plotted on
a common normalized
basis of GHG emissions intensity as a function of solvent fraction, a total
GHG emissions
intensity curve can be generated which is derived by adding four the GHG
emission sources
over the solvent concentration range. For a set solvent composition, there is
an optimum range
for solvent concentration of a solvent-steam recovery process at which the
total GHG emission
intensity is minimized. Figure 3E illustrates the total GHG emissions
intensity curve derived
from the data in Figures 3A-3D in accordance with the methods of the current
disclosure. The
data shown in these figures was based on a solvent composition of a diluent
with a boiling point
range near that of hexane.
[0086] While in practice, the accuracy of the total GHG
emissions intensity curve is best
derived by looking at a combination of the data from all four (4) key GHG
emissions sources,
it is noted that for rougher calculations/estimations, the total GHG emissions
intensity curve
may be derived from only one or more of the four (4) key GHG emissions
sources. In particular
it has been noted in this example herein that the make-up solvent component
and the
- 24 -
CA 3062478 2019-11-22
=
vaporization component of the calculations appear to be the most significant
source
components to the total GHG emissions intensity curve values. However, such
relationships
may change significantly based on specific well configurations, associated
solvent-based
processes and systems, as well as the "stage" or "life" of the subterranean
reservoir system. As
such, for example, an embodiment herein could include wherein the total GHG
emissions
intensity curve is derived only from the make-up solvent component and the
vaporization
component curves with the understanding that some accuracy in the total GHG
emissions
intensity values may be lacking.
[0087] In a similar manner, that while preferred, it is not
necessary that the GHG emissions
intensity calculations be performed over the entire range of solvent
fractions. It is just
necessary that the minimum GHG emissions intensity point be calculated as well
as some
amount of tolerance above and below this minimum that falls within a desired
tolerance
window be calculated.
[0088] In practice as disclosed herein, in preferred
embodiments, a total GHG emissions
intensity curve is generated for a set solvent composition and the minimum GHG
emissions
intensity point is determined. The solvent fraction is then selected from a
desired tolerance
window with respect to the minimum GHG emissions intensity point. This point
will
correspond to both 1) a minimum GHG emissions intensity point as defined by
the total GHG
emissions intensity curve, and 2) a corresponding solvent fraction. While the
exact minima
may not be practically achievable, in one embodiment, it is desired that the
solvent fraction is
adjusted or chosen for the solvent-based extraction process and such that it
is within +/- 20
vol%, +/- 10 vol%, or +/- 5 vol% of solvent fraction associated with the
minimum GHG
emission intensity point. In other embodiments, it is desired that the solvent
fraction is adjusted
or chosen and such that it corresponds to a point on the total GHG emissions
intensity curve
that is within +/- 20%, +/- 10%, or +/- 5% of the minimum GHG emissions
intensity point.
[0089] It should also be noted that the curves for the four (4)
key GHG emissions sources
may be run for different solvent compositions and multiple Total GHG Emissions
Intensity
curves be generated. The solvent composition with the lowest GHG emission
intensity point
may be chosen and the solvent composition be adjusted or chosen such that it
is within a desired
tolerance window with respect to the solvent composition with the lowest
minimum GHG
emissions intensity point. In this case, the solvent composition would be
defmed by the average
- 25 -
CA 3062478 2019-11-22
boiling point (ABP) of the solvent composition. In embodiments herein, the
average boiling
point range for the optimum solvent composition may fall within 100 C +/-, 75
oc +1_, 50 oc
+/- , 25 C +/-, or 5 C +/- of the average boiling point of the solvent
composition corresponding
to the lowest minimum GHG emissions intensity point calculated from the
multiple total GHG
emissions intensity curves. The solvent fraction can then, optionally, be
adjusted to fall within
the tolerances discussed with respect to the total GHG emissions intensity
curve associated
with the lowest minimum GHG emissions intensity point calculated from the
multiple total
GHG emissions intensity curves as discussed prior.
[0090] The processes described herein can be done at multiple stages in
the life of the well.
.. The following example with respect to Figures 4A-4E illustrates how,
although the analyses
and processes previously described remains the same, due to a changes in the
conditions of the
subterranean reservoir over time, the results of GHG emissions intensity
analysis may change.
Figures 4A-4D show the GHG emissions intensity curves for a subterranean
reservoir in a late-
life stage of operation. In the last phase ("late-life") of a solvent-based
hydrocarbon extraction
process, late-life solvent recovery strategies (e.g. blow down or wind down)
are typically
utilized to minimize solvent retention in the reservoir. Figures 4A-4D
illustrate the GHG
emissions intensity curves derived from the analytical modeling analysis and
numerical
simulation results of the four (4) key GHG emissions sources described prior.
[0091] Figure 4A illustrates the GHG emissions intensity of the make-up
solvent
.. component as a function of the solvent fraction of the injection fluid in
the late-life phase in
accordance with the methods of the current disclosure. Figure 4B illustrates
the GHG
emissions intensity of the power demand component as a function of the solvent
fraction of the
injection fluid in the late-life phase in accordance with the methods of the
current disclosure.
Figure 4C illustrates the GHG emissions intensity of the vaporization unit
component as a
.. function of the solvent fraction of the injection fluid in the late-life
phase in accordance with
the methods of the current disclosure. Figured 4D illustrates the GHG
emissions intensity of
the solvent recovery unit (SRU) component as a function of the solvent
fraction of the injection
fluid in the late-life phase in accordance with the methods of the current
disclosure. Similar to
Figure 3E, Figure 4E illustrates the total GHG emissions intensity curve
derived from the data
in Figures 4A-4D in accordance with the methods of the current disclosure. As
can be seen,
from Figures 4A-4D, the total GHG emissions intensity (Figure 4E) is mainly
driven by direct
- 26 -
CA 3062478 2019-11-22
GHG emissions of vaporization given that indirect GHG emissions of make-up
solvent now
minimized in this late stage operation. It can further be seen how the minima
of the total GHG
emissions intensity has changed in this analysis from about 65% solvent
fraction in early life
(Figure 3E) to about 70% solvent fraction in late life (Figure 4E) of the
subterranean solvent-
based extraction processes. The data shown in Figures 4A-4E was based on the
solvent
composition as modeled in Figures 3A-3E.
[0092] As the examples in Figures 3A-3E and 4A-4E illustrate, the
methods herein can be
used to optimize solvent-based heavy oil recovery process in early-life or mid-
life of a
subterranean reservoir, but also can additionally be utilized to determine
which of possible late-
life "follow-up processes" should be selected and transitioned to optimize or
even minimize
GHG emissions. Examples of follow-up processes may include, but are not
limited to, a steam
assisted gravity drainage (SAGD) process, a solvent assisted cyclic steam
stimulation (SA-
CSS) process, a steam flood process, a solvent assisted steam flood (SA-SF)
process, a solvent
flood process, a heated vapor solvent flood process, a non-condensable gas
(NCG) assisted
steam process, a non-condensable gas (NCG) assisted solvent process, and a non-
condensable
gas (NCG) flood process. The methods herein can be utilized to determine which
of possible
late-life "follow-up processes" that are solvent-based may be selected and
transitioned to from
a solvent-based heavy oil recovery process. Examples of follow-up solvent-
based processes to
which the GHG emissions analyses disclosed here can apply include, but are not
limited to, a
solvent assisted cyclic steam stimulation (SA-CSS) process, a solvent assisted
steam flood (SA-
SF) process, a solvent flood process, a heated vapor solvent flood process,
and a non-
condensable gas (NCG) assisted solvent process. In preferred embodiments
herein, the
transitioning may occur when a transition criteria is reached in the operation
of the subterranean
reservoir. The transition criteria may include a threshold fraction of the
estimated original
heavy oil in the subterranean reservoir that has been recovered. Examples of
the threshold
fraction include at least 10%, at least 20%, at least 30%, at least 40%, at
least 50%, at least
60%, at least 70%, and/or at least 80% of the estimated original heavy oil in
the subterranean
reservoir.
[0093] In the present disclosure, several examples have been discussed
and/or presented in
the context of flow diagrams, or flow charts, in which the methods are shown
and described as
a series of blocks, or steps. Unless specifically set forth in the
accompanying description, the
- 27 -
CA 3062478 2019-11-22
order of the blocks may vary from the illustrated order in the flow diagram,
including with two
or more of the blocks (or steps) occurring in a different order and/or
concurrently.
Industrial Applicability
[0094] The systems and methods disclosed in the present disclosure are
applicable to the
oil and gas industry.
- 28 -
Date Recue/Date Received 2020-11-16