Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
METHOD FOR DECONTAMINATING OXIDE LAYER
[0001] Intentionally left blank.
BACKGROUND
1. FIELD
[0002] The present disclosure relates to a method for
decontaminating an oxide layer, capable of efficiently
decontaminating the oxide layer, and more particularly, to a
method for effectively decontaminating a relatively thick oxide
layer formed in a carbon steel material system.
2. DESCRIPTION OF RELATED ART
[0003] Decontamination for a system is essential for
maintenance of a nuclear power plant in step, and dismantling
of an aged nuclear power plant. Decontamination for a primary
system of a light-water nuclear power plant maybe carried out
in a number of decontamination cycles, and accordingly, a
considerable amount of decontamination waste liquid containing
radionuclides may be generated. Currently, although chemical
Page 1
Date Recue/Date Received 2022-02-23
decontamination processes using organic acids may be widely
used for decontamination of the primary system of a light-water
nuclear power plant, there are problems that waste liquid
generated from such processes is not easy to treat, and
radioactive waste, such as waste ion exchange resins, is
generated as waste in the decontamination process.
[0004] In order to solve these problems, the Korea Atomic
Energy Research Institute (KAERI) has developed a
hydrazine-based reductive metal ion decontamination (HyBRID)
process using inorganic acids, as disclosed in Korean Patent
No. 1601201. Since decontamination waste liquid generated in
this process may be effectively treated by precipitation and
decomposition reactions, this process has an advantage of
greatly reducing radioactive waste, as compared to the
conventional commercial decontamination process.
[0005] Meanwhile, a thickness of an oxide layer formed in a
system of a heavy-water reactor made of a carbon steel material,
in a heavy-water nuclear power plant, may be tens of times
greater than a thickness of an oxide layer formed in a system
of a light-water reactor (the light-water reactor: 1 to 3pm,
the heavy-water reactor: 75pm or more) . In this case, when the
existing HyBRID process is used as is to decontaminate a
relatively thick oxide layer, a pH of a decontamination agent
may greatly increase, and a function of the decontamination
agent may be deteriorated. Therefore, a situation in which a
Page 2
Date Recue/Date Received 2020-11-16
considerable amount of sulfuric acid should be used may be
accompanied. In this case, a concentration range of ions in the
decontamination process water may increase to reach a limit of
an amount of an oxide layer, capable of being decontaminated.
Therefore, it is difficult to effectively perform
decontamination.
[0006] For this reason, when a relatively thick oxide layer
is decontaminated, since used decontamination process water
should be discharged and new decontamination process water
should be supplied, a process for decontamination becomes
inefficient. Furthermore, since a significant amount of
decontamination waste liquid may be generated, waste to be
disposed may also significantly increase. In addition, in order
to reuse used decontamination process water without discharging
the used decontamination process water, it is possible to
consider a method of removing ionic components by using an ion
exchange resin, but in this case, there is a problem that a
considerable amount of waste ion exchange resin may be generated
as radioactive waste.
[0007] Therefore, there is demand for a technology capable of
effectively decontaminating a relatively large amount of oxide
layer while minimizing the number of times of discharging used
decontamination process water and supplying new
decontamination process water to be developed.
Page 3
Date Recue/Date Received 2020-11-16
SUMMARY
[0008] An aspect of the present disclosure is to provide a
method for decontaminating an oxide layer, capable of
effectively decontaminating a relatively large amount of
corrosive oxides formed in a system of a heavy-water reactor
made of a carbon steel material while significantly reducing
the occurrence of radioactive waste.
[0009] According to another aspect of the present disclosure,
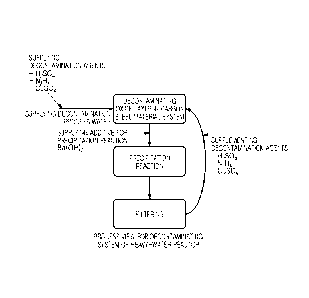
a method for decontaminating an oxide layer, includes (a)
supplying decontamination process water including sulfuric
acid (H2SO4), hydrazine (N2B4), and copper sulfate (CuSO4) to
a pipeline system, to decontaminate an oxide layer in the
pipeline system; (b) mixing Ba(OH)2 with the decontamination
process water, formed after step (a) decontaminating the oxide
layer, to adjust a pH of the decontamination process water to
have a range of 8.2 to 9.4, to perform a precipitation
reaction; (c) filtering the decontamination process water,
formed after step (b) in which the precipitation reaction is
performed, to remove an insoluble substance from the
decontamination process water; and (d) reusing the
decontamination process water, formed after step (c) from which
the insoluble substance has been removed, as the
decontamination process water of step (a).
BRIEF DESCRIPTION OF DRAWINGS
Page 4
Date Recue/Date Received 2020-11-16
[0010] The above and other aspects, features, and advantages
of the present disclosure will be more clearly understood from
the following detailed description, taken in conjunction with
the accompanying drawings, in which:
[0011] FIG. 1 is a schematic flowchart illustrating a method
for decontaminating an oxide layer according to an embodiment
of the present disclosure.
DE TAILED DESCRIPTION
[0012] Hereinafter, preferred embodiments of the present
disclosure will be described with reference to the accompanying
drawings. However, embodiments of the present disclosure may
be modified in various other forms, and the scope of the present
disclosure is not limited to embodiments described below.
[0013] A relatively thick oxide layer in a carbon steel
material system of a heavy-water reactor may be mostly composed
of iron oxide. For example, 99 wt% or more of the oxide layer
may be composed of iron oxide. Therefore, it is possible to
decontaminate the oxide layer using only a reduction process,
among chemical decontamination processes.
[0014] The present disclosure provides a method for
efficiently decontaminating the oxide layer.
[0015] In detail, the present disclosure provides a
method for decontaminating an oxide layer, including (a)
supplying decontamination process water including sulfuric
Page 5
Date Recue/Date Received 2020-11-16
acid (H2SO4) , hydrazine (N2H4) , and copper sulfate (CuSO4) to
a pipeline system, to decontaminate an oxide layer in the
pipeline system; (b) mixing Ba (OH)2 with the decontamination
process water, formed after step (a) decontaminating the oxide
layer, to adjust a pH of the decontamination process water to
have a range of 8.2 to 9.4, to perform a precipitation reaction;
(c) filtering the decontamination process water, formed after
step (b) in which the precipitation reaction is performed, to
remove an insoluble substance from the decontamination process
water; and (d) reusing the decontamination process water,
formed after step (c) from which the insoluble substance has
been removed, as the decontamination process water of step (a) .
In the present disclosure "the decontamination process water
of step (b) " means the decontamination process water formed
after step (a) and supplied to the step (b) , and "the
decontamination process water of step (c) " means the
decontamination process water formed after step (b) and
supplied to the step (c) .
[0016] The above method may be a method capable of efficiently
decontaminating a relatively thick oxide layer in a carbon steel
material system of a heavy-water reactor. In this case,
decontamination process water containing sulfuric acid (H2SO4)
hydrazine (N2H4) copper sulfate (CuSO4), and the like in
predetermined concentrations may be used to perform a reductive
decontamination step in an existing HyBRID decontamination
Page 6
Date Recue/Date Received 2020-11-16
process, significant amounts of sulfuric acid (SO4) ions and
iron (Fe) ions (< 1ppm) , which cause deterioration of
decontamination performance, may be almost removed, and further,
a decontamination agent in a reduction step may be added in a
relatively small amount, to reuse this decontamination process
water in a manner similar to the decontamination process water
used in the initial step. For this reason, since the
decontamination process water used in the reductive
decontamination step may be used again under conditions of the
decontamination process water of the initial reductive
decontamination step without separately discharging the
decontamination process water, it is possible to effectively
decontaminate the relatively thick oxide layer in the system
made of a carbon steel material.
[0017] In the present disclosure, the decontamination process
water may refer to a solution in which a decontamination agent
is included in process water flowing through a pipeline system.
[0018] Therefore, the pipeline system may be a cooling system
of a nuclear power plant. In this case, the cooling system may
be a system made of a carbon steel material, and further, the
system made of a carbon steel material may be a cooling system
of a heavy-water reactor. An oxide layer having a thickness of
several tens of times more than a thickness of a primary system
of a light-water reactor may be formed in the cooling system
of the heavy-water reactor. For example, a thickness of an oxide
Page 7
Date Recue/Date Received 2020-11-16
layer formed on the primary system of the light-water reactor
may be 1 to 3pm, and a thickness of an oxide layer formed on
the cooling system of the heavy-water reactor may be 50 to 75pm.
[0019] NiFe204, Fe304, Fe2O3, or the like may be formed in an
oxide layer formed on a surface of a cooling system of a nuclear
power plant, due to relatively high dissolved oxygen in a
solution in the cooling system. Therefore, the oxide layer as
a decontamination target of the present disclosure may contain
iron oxide, and the iron oxide may be decontaminated by being
dissolved in the decontamination process water of the present
disclosure.
[0020] To this end, initial decontamination process water of
the present disclosure supplied to the pipeline system may
contain the sulfuric acid (H2SO4) in a concentration range of
25 to 32mM, the hydrazine (N2H4) in a concentration range of
30 to 70mM, and the copper sulfate (CuSO4) in a concentration
range of 0.4 to 0.6mM. Preferably, the initial decontamination
process water may contain the sulfuric acid in a concentration
range of 27 to 30mM, the hydrazine in a concentration range of
45 to 55mM, and the copper sulfate in a concentration range of
0.45 to 0.55mM.
[0021] In this case, when a concentration range of sulfuric
acid exceeds 32mM, a pH of the decontamination process water
may be lowered to increase corrosion of a base material of the
pipeline system, and when a concentration range of sulfuric acid
Page 8
Date Recue/Date Received 2020-11-16
is less than 25mM, a decontamination effect may decrease. In
addition, when a concentration range of hydrazine exceeds 70mM
or is less than 30mM, a dissolution performance of the oxide
layer may decrease to reduce a decontamination effect.
Furthermore, when a concentration range of copper sulfate
exceeds O. 6mM, a precipitate of copper may be formed, and
excessive ionic components may be formed. In this case, an
additional process may be required to remove ionic components.
When a concentration range of copper sulfate is less than 0.4mM,
an decontamination effect may be reduced.
[0022] A process of supplying the decontamination process
water to the pipeline system to decontaminate the oxide layer
in step (a) may be performed at a temperature range of 93 to
97 C for 6 to 10 hours, and preferably at a temperature of 95 C
for 6 to 10 hours. A time period for performing the above process
may be changed, depending on a scale of decontamination.
[0023] Furthermore, in step (b) of the present disclosure, when
the Ba (OH) 2 is mixed with decontamination process water of step
(b) formed after decontaminating the oxide layer, the mixing
step may be configured that a pH of the decontamination process
water of step (b) may be 8.2 to 9.4, preferably 8.35 to 8.65.
In the case that the Ba (OH) 2 is mixed, when a pH of the
decontamination process water is less than 8.2, iron ions in
the decontamination process water may not decrease to less than
1 ppm, and further, a relatively large amount of fine iron
Page 9
Date Recue/Date Received 2020-11-16
hydroxide particles in the decontamination process water may
be generated to reduce filtration efficiency. Therefore, there
is a problem that iron components may not be effectively removed.
When a pH of the decontamination process water exceeds 9.4, a
significant amount or a majority of a decontamination agent,
capable of being reused, in the decontamination process water
may be removed, to have problems resulting in an increase in
amounts of waste and a decontamination agent to be used, and
the like.
[0024] To this end, in step (b) , the Ba (OH)2 may be mixed to
have a molar number of 50 to 70% of sulfate ions in the
decontamination process water of step (b) , and preferably, a
mole number of 55 to 60% of sulfate ions in the decontamination
process water. When the Ba (OH) 2 is mixed to have a molar number
of less than 50% of sulfate ions in the decontamination process
water of step (b) , iron ions in the decontamination process
water may not decrease to less than 1 ppm, and further, a
relatively large amount of fine iron hydroxide particles in the
decontamination process water may be generated to reduce
filtration efficiency. Therefore, there is a problem that iron
components may not be effectively removed. When the molar number
of sulfate ions in the decontamination process water exceeds
70%, a significant amount or most of a decontamination agent,
capable of being reused, in the decontamination process water
may be removed, to have problems resulting in an increase in
Page 10
Date Recue/Date Received 2020-11-16
amounts of waste and a decontamination agent to be used, and
the like.
[0025] Various insoluble substances may be generated in the
decontamination process water formed after step (b) , due to the
precipitation reaction. The insoluble substances may be, for
example, BaSO4, Fe (OH) 2, Cu (OH) 2, or the like. When the insoluble
substances remain in the decontamination process water formed
after step (b) , it may interfere with reuse of the
decontamination process water. Therefore, it is necessary to
remove the insoluble substance.
[0026] To this end, the present disclosure may perform a step
(c) of filtering the decontamination process water formed after
step (b) in which the precipitation reaction is performed. The
above step (c) may be performed to remove the insoluble
substance formed by the precipitation reaction, and may be
performed using a filtration device used in the art. For example,
as the filtration device, a filter having pores having a
diameter range of 0.2 to 0 . 45pm may be used. Most of the insoluble
substances may be removed from the decontamination process
water filtered as described above.
[0027] Since most of the components (hydrazine, sulfate ions,
copper ions, and the like) present in the initial
decontamination process water remain in the decontamination
process water from which most of the insoluble substances are
removed, the decontamination process water may be used again
Page 11
Date Recue/Date Received 2022-02-23
as the initial decontamination process water of step (a) .
Therefore, hydrazine, sulfuric acid, and copper sulfate may be
mixed with the decontamination process water formed after step
(c) , to prepare decontamination process water in a manner
similar to the decontamination process water used in step (a) .
Therefore, the reusing step may be performed by mixing at least
one of sulfuric acid (H2SO4) , hydrazine (N2H4) , or copper sulfate
(CuSO4) with the decontamination process water from which the
insoluble substances is removed, to regenerate the
decontamination process water.
[0028] In this case, by comparing concentrations of hydrazine,
sulfuric acid, and copper sulfate contained in the
decontamination process water used in step (a) , to
concentrations of hydrazine, sulfuric acid, and copper sulfate
contained in the decontamination process water formed after
step (c) , hydrazine, sulfuric acid, and/or copper sulfate, as
a component lacking therein, may supplement and be mixed with
the decontamination process water formed after step (c) .
[0029] For example, hydrazine, sulfuric acid, and copper
sulfate may be mixed with the decontamination process water
regenerated in step (d) , to include the sulfuric acid (H2SO4)
in a concentration range of 25 to 32mM, the hydrazine (N2H4)
in a concentration range of 30 to 70mM, and the copper sulfate
(CuSO4) in a concentration range of 0.4 to 0.6mM.
[0030] The regenerated decontamination process water may be
Page 12
Date Recue/Date Received 2020-11-16
supplied to the pipeline system again, to decontaminate an oxide
layer. Therefore, the present disclosure may include a step of
using the decontamination process water recycled in step (d)
as the initial decontamination process water of step (a). In
this case, steps (a) to (d) may be repeated several to tens of
times without discharging process water. For efficiency of the
method, the repetition may be performed, for example, about 2
to 20 times. Therefore, steps (a) to (d) may be repeated 2 to
20 times without discharging process water.
[0031] For example, the present disclosure may repeatedly
perform a step of regenerating initially used decontamination
process water, a step of decontaminating an oxide layer reusing
the regenerated decontamination process water, and an step of
regenerating the reused decontamination process water, to
significantly reduce an amount of a decontamination agent, as
compared to the existing decontamination process.
[0032] Hereinafter, the present disclosure will be described
in more detail by specific examples. The following examples are
only examples to assist in an understanding of the present
disclosure, and the scope of the present disclosure is not
limited thereto.
Example
1) Preparation of Decontamination Process Water
[0033] Sulfuric acid, hydrazine, and copper sulfate were
supplied to and mixed with 1 L of distilled water, to have the
Page 13
Date Recue/Date Received 2022-02-23
sulfuric acid at a concentration of 20mM, the hydrazine at a
concentration of 31.2mM, and the copper sulfate at a
concentration of 0.5mM, as conditions of decontamination
process water after a reduction step, in a HyBRID
decontamination process.
[0034] Thereafter, assuming that an oxide layer in a carbon
steel material of a primary system made of iron oxide was
decontaminated, iron sulfate was dissolved in the
decontamination process water such that a concentration of iron
ions was 350 ppm. In this case, 350 ppm of iron ions were adjusted
using the iron sulfate, and as a result, it was confirmed that
a final concentration of sulfuric acid was 27mM.
[0035] This was used as decontamination process water in which
an decontamination step was performed in a cooling system of
a heavy-water reactor, to prepare used decontamination process
water in which step (a) of the present disclosure was performed.
2) Removal of Sulfate Ion and Iron Ion
[0036] Ba(OH)2 was used to remove sulfate ions and iron ions
in the prepared and used decontamination process water.
[0037] Based on the number of moles of sulfate ions, an amount
of Ba(OH)2 was changed, and Table 1 illustrated the results in
which a pH of the used decontamination process water and a
concentration of each of the components were measured.
[Table 1]
Molar Ratio pH Concentration (ppm)
Page 14
Date Recue/Date Received 2020-11-16
(Ba(OH)2/E042) Hydrazine Sulfate Ion Iron Ion Copper Ion
CE1 0 2.61 1,010 2,600 350 31
CE2 0.45 8.17 1,000 1,460 1.843 0.282
TEl 0.5 8.27 1,020 1,310 0.714 0.322
1E2 0.55 8.36 1,000 1,180 0.536 0.216
1E3 0.6 8.63 1,030 1,020 0.332 0.150
1E4 0.7 9.31 1,010 790 0.150 0.046
CE3 0.75 9.62 1,010 640 0.121 0.038
*IE: Inventive Example, CE: Comparative Example
[0038] As a result, as illustrated in Table 1, it was confirmed
that iron ions were removed to less than 1 ppm, even when only
50% of a total amount of Ba(OH)2 required to precipitate all
sulfate ions was used. In this case, it was confirmed that
sulfate ions were removed in a concentration range half of that
as theoretically expected, and most of copper ions were removed
in the form of precipitates under all conditions.
[0039] Since it was confirmed that a concentration range of
hydrazine was almost unchanged, hydrazine may be easily
prepared as decontamination process water for reduction process
decontamination by using purified decontamination process
water, when supplementing only an amount thereof consumed
during the process thereinto.
[0040] 3) Regeneration of Decontamination Process Water
[0041] In order to regenerate the purified decontamination
process water as the decontamination process water for
reduction process decontamination, hydrazine, sulfate ions,
and copper ions were added to the purified decontamination
Page 15
Date Recue/Date Received 2020-11-16
process water. In this case, only an insufficient amount of
hydrazine, consumed during the decontamination process, as
compared to 1,600 ppm, the concentration of the initial
decontamination process water, was added.
[0042] Each of the added components was summarized in Table
2. In this case, when each of the components was added, a pH
of the regenerated decontamination process water was set to be
2.75.
[Table 2]
Molar Ratio Concentration to be supplied (ppm)
(Ba(OH)2/S0421 Hydrazine Sulfate Ion Copper Ion
TEl 0.5 600 1,290 31
1E2 0.55 600 1,420 31
1E3 0.6 600 1,580 31
1E4 0.7 600 1,810 31
*IE: Inventive Example
[0043] As a result, as illustrated in Table 2, amounts of the
components to be supplied were almost the same as amounts
removed by precipitation/filtration during
the
decontamination process, respectively. By this, it can be seen
that, when the decontamination process water used after
performing the reduction process decontamination was purified
and then regenerated to produce decontamination process water,
it was possible to regenerate the decontamination process water
having a composition, almost similar to the initial
decontamination process water.
[0044] When a system of a heavy-water reactor made of a carbon
Page 16
Date Recue/Date Received 2020-11-16
steel material is decontaminated by a method for
decontaminating an oxide layer according to an aspect of the
present disclosure, since decontamination process water may be
reused without discharging the decontamination process water,
the occurrence of decontamination waste liquid may be greatly
reduced. In addition, since an amount of a decontamination agent
to be used may be reduced by 50 to 70%, an amount of
decontamination waste liquid finally generated may be reduced
by at least 30%, as compared to the existing process. In addition,
the method according to an aspect of the present disclosure may
be relatively simple and may be thus easily applied to the
existing process. In particular, since the method according to
an aspect of the present disclosure may be easily applied to
the decontamination technology for the primary system of the
light-water reactor, it is judged that commercial use as a
process for decontaminating the system of the heavy-water
reactor may be relatively high.
[0045] In addition, since the method according to an aspect
of the present disclosure does not use an ion exchange resin
at all, in a different manner to the conventional
decontamination technology proposed for decontaminating a
system of a heavy-water reactor made of a carbon steel material,
it is expected that an amount of decontamination waste generated
may be reduced by more than 60%, as compared to the conventional
decontamination technology using the ion exchange resin. In
Page 17
Date Recue/Date Received 2020-11-16
addition, since decontamination waste finally generated is a
solid BaSO4 and metal hydroxide, having high stability, not a
waste ion exchange resin that may be difficult to treat,
handling and stabilization may be very easy. Considering these
advantages and applicability of a relatively simple process,
it is judged that a demand for decontamination technology of
decontaminating a system of a heavy-water reactor made of a
carbon steel material may be satisfied.
[0046] While example embodiments have been shown and described
above, it will be apparent to those skilled in the art that
modifications and variations could be made without departing
from the scope of the present disclosure as defined by the
appended claims.
***
[0047] In some aspects, embodiments of the present invention
as described herein include the following items:
Item 1. A method for decontaminating an oxide layer,
comprising:
(a) supplying decontamination process water including
sulfuric acid (H2SO4), hydrazine (N2H4), and copper sulfate
(CuSO4) to a pipeline system, to decontaminate the oxide layer
in the pipeline system;
(b) mixing Ba (OH) 2 with the decontamination process water,
formed after step (a), to adjust a pH of the decontamination
process water to have a range of 8.2 to 9.4, to perform a
Page 18
Date Recue/Date Received 2022-02-23
precipitation reaction;
(c) filtering the decontamination process water, formed
after step (b) in which the precipitation reaction is performed,
to remove an insoluble substance from the decontamination
process water; and
(d) reusing the decontamination process water, formed
after step (c) from which the insoluble substance has been
removed, as the decontamination process water of step (a),
wherein the Ba(OH)2 of step (b) is mixed to have a molar
number of 50 to 70% of sulfate ions in the decontamination
process water formed after step (a).
Item 2. The method of item 1, wherein the pipeline system
is a carbon steel material system.
Item 3. The method of item 1 or 2, wherein the pipeline
system is a cooling system of a heavy-water reactor.
Item 4. The method of any one of items 1 to 3, wherein
the oxide layer comprises iron oxide.
Item 5. The method of any one of items 1 to 4, wherein
the oxide layer has 50 to 75 pm thickness.
Item 6. The method of any one of items 1 to 5, wherein
the decontamination process water of step (a) contains the
sulfuric acid (H2SO4) in a concentration range of 25 to 32 mM,
the hydrazine (N2H4) in a concentration range of 30 to 70 mM,
and the copper sulfate (CuSO4) in a concentration range of 0.4
to 0.6 mM.
Page 19
Date Recue/Date Received 2022-02-23
Item 7. The method of any one of items 1 to 6, wherein
step (a) is performed at a temperature range of 93 to 97 C for
6 to 10 hours.
Item 8. The method of any one of items 1 to 7, wherein
step (d) is performed by mixing at least one of sulfuric acid
(H2SO4), hydrazine (N2H4), and copper sulfate (CuSO4) with the
decontamination process water formed after step (b) from which
the insoluble substance has been removed, to regenerate the
decontamination process water.
Item 9. The method of any one of items 1 to 8, wherein
the steps (a) to (d) are repeated 2 to 20 times without
discharging the decontamination process water.
Page 20
Date Recue/Date Received 2022-02-23