Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
ALCOHOL-BASED HEMI-FORMYLS FOR HYDROGEN SULFIDE SCAVENGING
TECHNICAL FIELD
[0001] The present disclosure relates generally to scavengers of sulfur-
based species, such
as hydrogen sulfide and mercaptans.
BACKGROUND
[0002] The removal of sulfur-based species from liquid or gaseous
hydrocarbon streams is
a long-standing problem in many industries. Hydrogen sulfide is a significant
problem in the
oil industry, particularly in the drilling, production, transportation,
storage, and processing of
crude oil, naphtha, fuel, and distillate oils. The same problems exist in the
natural gas
industry.
[0003] The presence of sulfur-containing compounds such as hydrogen sulfide
can result
in the deposition of sulfur containing salts, which cause plugging, and
corrosion of
transmission pipes, valves, regulators and other process equipment. Hydrogen
sulfide is also
toxic and, therefore, desirable to be removed. Even flared natural gas needs
to be treated to
avoid acid rain generation due to SO, formation. Also, in the manufactured gas
or coke
making industries, coal-gas emissions containing unacceptable levels of
hydrogen sulfide are
commonly produced from destructive distillation of bituminous coal.
[0004] Since hydrogen sulfide has an offensive odor, and fluids such as
petroleum
products and natural gas contain it, such fluids are often called "sour."
Treatments to lower
hydrogen sulfide are often referred to as "sweetening" processes. When a
particular
compound is used to remove or lower H25 and mercaptans, it is called
scavenging agent.
[0005] Despite the availability of scavengers for use in the oil and gas
industry, there still
exists a need for improved compounds, compositions and methods for removing
sulfur-based
species from liquid and gas streams. Such improvements include nitrogen-free
scavengers
and scavengers with increased dispersion into the sour hydrocarbon.
SUMMARY
[0006] In one aspect, a method of sweetening a fluid includes treating the
fluid with an
alcohol-based hemi-formyl of formula (I):
1
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
(I) 1V-04-CH2-0-1x-H;
wherein 1Z1 is C1-C3 alkyl; and x is from 1 to 10. In some embodiments, x is
from 1 to 5 or 1
to 3. In certain embodiments, x is 1, 2 or 3.
[0007] In some embodiments, the fluid is treated with the hemi-formyl of
formula (I), and
xis from 1 to 12. In some embodiments, xis from 1 to 5. In some embodiments, x
is 1. In
some embodiments, x is 2.
[0008] In some embodiments, the hemi-formyl is oil-soluble.
[0009] In some embodiments, the hemi-formyl of formula (I) is methanolformyl.
In some
embodiments, the hemi-formyl of formula (I) is ethanolformyl.
[0010] In some embodiments, the fluid is selected from crude oil, naphtha,
fuel, and
distillate oils.
[0011] In some embodiments, the method also includes adding one or more
additional
components, each component independently selected from the group consisting of
asphaltene
inhibitors, paraffin inhibitors, corrosion inhibitors, emulsifiers,
dispersants, emulsion
breakers, hydrogen sulfide scavengers, gas hydrate inhibitors, surfactants,
solvents, and
combinations thereof
[0012] In some embodiments, the surfactant or dispersant is selected from
the group
consisting of alkyl benzyl ammonium chloride, benzyl cocoalkyl(C12-
C18)dimethylammonium chloride, dicocoalkyl (C12-Cis)dimethylammonium chloride,
ditallow dimethylammonium chloride, di(hydrogenated tallow alkyl)dimethyl
quaternary
ammonium methyl chloride, methyl bis(2-hydroxyethyl cocoalkyl(C12-C18)
quaternary
ammonium chloride, dimethyl(2-ethyl) tallow ammonium methyl sulfate, n-
dodecylbenzyldimethylammonium chloride, n-octadecylbenzyldimethyl ammonium
chloride,
n-dodecyltrimethylammonium sulfate, soya alkyltrimethylammonium chloride,
hydrogenated
tallow alkyl (2-ethylhyexyl) dimethyl quaternary ammonium methyl sulfate, and
combinations thereof
[0013] In some embodiments, the method also includes adding an odorant.
[0014] In some embodiments, the fluid is produced or used in a coal-fired
process, a
waste-water process, a farm, a slaughter house, a land-fill, a municipality
waste-water plant, a
coking coal process, or a biofuel process.
[0015] In some embodiments, the method excludes adding any nitrogen-containing
compounds to the fluid.
[0016] The present disclosure also provides for the use of a composition to
sweeten a
fluid, the composition comprising an alcohol-based hemi-formyl of formula (I):
2
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
(I) R1-0-[-CH2-0-1x-H;
wherein Rl is Ci-C3 alkyl; and x is from 1 to 10. In some embodiments, x is
from 1 to 5 or 1
to 3. In certain embodiments, x is 1, 2 or 3. In some embodiments, Rl is Ci or
C2 alkyl.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0017] A detailed description of the invention is hereafter described with
specific
reference being made to the drawings in which:
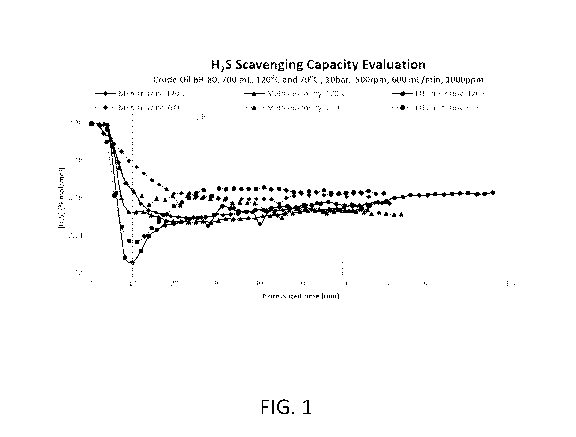
[0018] FIG. 1 shows scavenging capacity data for different scavengers at
different
temperatures; and
[0019] FIG. 2 shows data for various scavengers obtained from a liquid
phase reduction
test.
DETAILED DESCRIPTION
[0020] Disclosed herein are hydrogen sulfide and/or mercaptan scavenging
compounds
and compositions, methods of using said compounds and compositions, and
processes for
their preparation. The compounds and compositions are particularly useful in
the control of
hydrogen sulfide and/or mercaptan emissions from crude oil based, natural gas
based, and
coal based products and processes. The compounds and compositions are
applicable to both
upstream and downstream processes. The scavenging compounds and compositions,
optionally blended with non-aqueous solvents, are useful in a wide range of
climates and
under a wide range of process conditions.
[0021] The processes for preparing the compounds and compositions of the
invention are
economic, waste free, and provide said compounds in quantitative yields. In
certain
embodiments, the compounds and compositions may be obtained in anhydrous form,
thereby
providing use in processes where it is desirable to minimize water content
(e.g., in an oil
production process such as those where the oil temperature is greater than 100
C). Producing
the compounds and compositions in anhydrous form also allows for reduced
transportation
costs. The anhydrous compounds and compositions can optionally be blended with
hydrophilic solvents (e.g., alcohols, glycol, polyols) for non-aqueous
applications.
1. Definition of Terms
[0022] Unless otherwise defined, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art. In
case of
conflict, the present document, including definitions, will control.
3
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
[0023] Various methods and materials are described below, although methods and
materials similar or equivalent to those described herein can be used in
practice or testing in
view of this disclosure. All publications, patent applications, patents and
other references
mentioned herein are incorporated by reference in their entirety. The
materials, methods, and
examples disclosed herein are illustrative only and not intended to be
limiting.
[0024] The terms "comprise(s)," "include(s)," "having," "has," "can,"
"contain(s)," and
variants thereof, as used herein, are intended to be open-ended transitional
phrases, terms, or
words that do not preclude the possibility of additional acts or structures.
The singular forms
"a," "and" and "the" include plural references unless the context clearly
dictates otherwise.
The present disclosure also contemplates other embodiments "comprising,"
"consisting of"
and "consisting essentially of," the embodiments or elements presented herein,
whether
explicitly set forth or not.
[0025] Unless expressly stated to the contrary, use of the term "a" is
intended to include
"at least one" or "one or more." For example, "a compound" is intended to
include "at least
one compound" or "one or more compounds."
[0026] Any ranges given either in absolute terms or in approximate terms
are intended to
encompass both, and any definitions used herein are intended to be clarifying
and not
limiting. Notwithstanding that the numerical ranges and parameters setting
forth the broad
scope of the invention are approximations, the numerical values set forth in
the specific
examples are reported as precisely as possible. Any numerical value, however,
inherently
contains certain errors necessarily resulting from the standard deviation
found in their
respective testing measurements. Moreover, all ranges disclosed herein are to
be understood
to encompass any and all subranges (including all fractional and whole values)
subsumed
therein.
[0027] As used herein, the term "consisting essentially of" means that the
methods and
compositions may include additional steps, components, ingredients or the
like, but only if
the additional steps, components and/or ingredients do not materially alter
the basic and novel
characteristics of the claimed methods and compositions.
[0028] The term "alkyl," as used herein, refers to a linear or branched
hydrocarbon
radical, a defined number of carbon atoms (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 11, 12, 13, 14, 15,
16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, and 30 carbons). Alkyl
groups include,
but are not limited to, methyl, ethyl, propyl, n-butyl, iso-butyl, secondary-
butyl, and tertiary-
butyl.
4
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
[0029] The term "sweetening," as used herein, may refer to a process that
removes sulfur
species from a gas or liquid. The sulfur species may include hydrogen sulfide
and
mercaptans.
[0030] The term "sour gas," as used herein, may refer to a gas that
includes significant
amounts of sulfur species, such as hydrogen sulfide and/or mercaptans.
[0031] The term "sour liquid" or "sour fluid," as used herein, may refer to
a liquid that
includes significant amounts of sulfur species, such as hydrogen sulfide
and/or mercaptans.
2. Compounds
[0032] Compounds disclosed herein include scavengers of sulfur-based
species, such as
hydrogen sulfide and mercaptans. In one aspect, compounds disclosed herein are
of formula
(I):
(I) R1-0-[-CH2-0-lx-H where 1Z1 is C1-C3 alkyl and x is from 1 to 10. In some
embodiments,
x is from 1 to 5 or 1 to 3. In certain embodiments, x is 1, 2 or 3.
[0033] The unit [-CH2-0-1 represents a formaldehye (i.e. when x is 1) and
paraformaldehyde (when x is greater than 1). Thus, the molecular weight of the
compounds
of formula I depends upon both the selection of 1Z1 as well as number of hemi-
formyl units
present.
[0034] Applicant has found that using mono-functionalized, primary alcohols
results in
products that have increased effectiveness in an oil phase. In some
embodiments, 1Z1 is Ci
alkyl, C2 alkyl, or C3 alkyl.
[0035] In some embodiments, the compounds of formula I are not corrosive to
steel or
other iron alloys.
[0036] The compounds of formula I may be prepared by mixing an alkyl alcohol
of the
formula 1V-OH, where 1Z1 is an alkyl group among the options described above,
with
formaldehyde in the presence of a catalyst, for example, an alkaline catalyst
or an acid
catalyst, such as dodecyl benzene sulfonic acid. The resulting hemi-formyl may
have a single
hemi-formyl unit where a single unit of formaldehyde reacts with the alkyl
alcohol or
multiple hemi-formyl units where multiple units of formaldehyde react with the
alkyl alcohol
and resulting hemi-formyls.
3. Compositions
[0037] The compositions disclosed herein include at least one compound as
described
above. In some embodiments, a composition disclosed herein contains a pure
composition of
a compound of formula I. In other embodiments, a composition disclosed herein
contains a
mixture of two or more structurally distinct compounds of formula I.
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
[0038] In some embodiments, a composition comprises from about 20 to about 100
percent by weight of one or more compounds disclosed herein, or from about 20
to about 98
percent by weight of one or more compounds disclosed herein, or from about 50
to 98
percent by weight of one or more compounds disclosed herein, or from about 70
to about 98
percent by weight of one or more compounds disclosed herein.
[0039] The compositions disclosed herein can optionally include one or more
additives.
Suitable additives include, but are not limited to, asphaltene inhibitors,
paraffin inhibitors,
corrosion inhibitors, scale inhibitors, emulsifiers, dispersants, emulsion
breakers, hydrogen
sulfide scavengers, gas hydrate inhibitors, surfactants, solvents, and
combinations thereof
a. Asphaltene Inhibitors
[0040] Suitable asphaltene inhibitors include, but are not limited to,
aliphatic sulphonic
acids; alkyl aryl sulphonic acids; aryl sulfonates; lignosulfonates;
alkylphenol/aldehyde resins
and similar sulfonated resins; polyolefin esters; polyolefin imides;
polyolefin esters with
alkyl, alkylenephenyl or alkylenepyridyl functional groups; polyolefin amides;
polyolefin
amides with alkyl, alkylenephenyl or alkylenepyridyl functional groups;
polyolefin imides
with alkyl, alkylenephenyl or alkylenepyridyl functional groups; alkenyl/vinyl
pyrrolidone
copolymers; graft polymers of polyolefins with maleic anhydride or vinyl
imidazole;
hyperbranched polyester amides; polyalkoxylated asphaltenes, amphoteric fatty
acids, salts of
alkyl succinates, sorbitan monooleate, polyisobutylene succinic anhydride, and
combinations
thereof
[0041] The amount of asphaltene inhibitor present in the composition is not
particularly
limited and may be selected by one of ordinary skill in the art. In some
embodiments, the
asphaltene inhibitor may be present in the composition in an amount of about 0
to about 30%
by weight of the composition.
b. Paraffin Inhibitors
[0042] Suitable paraffin inhibitors include, but are not limited to,
paraffin crystal
modifiers, and dispersant/crystal modifier combinations. Suitable paraffin
crystal modifiers
include, but are not limited to, alkyl acrylate copolymers, alkyl acrylate
vinylpyridine
copolymers, ethylene vinyl acetate copolymers, maleic anhydride ester
copolymers, branched
polyethylenes, naphthalene, anthracene, microcrystalline wax and/or
asphaltenes, and
combinations thereof Suitable paraffin inhibitors also include dodecyl benzene
sulfonate,
oxyalkylated alkylphenols, oxyalkylated alkylphenolic resins, and combinations
thereof
[0043] The amount of paraffin inhibitor present in the composition is not
particularly
limited and may be selected by one of ordinary skill in the art. In some
embodiments, the
6
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
paraffin inhibitor may be present in the composition in an amount of about 0
to about 20% by
weight of the composition.
c. Corrosion Inhibitors
[0044] Suitable corrosion inhibitors include, but are not limited to,
amidoamines,
quaternary amines, amides, phosphate esters, and combinations thereof The
amount of
corrosion inhibitor present in the composition is not particularly limited and
may be selected
by one of ordinary skill in the art. In some embodiments, the corrosion
inhibitor may be
present in the composition in an amount of about 0 to about 10% by weight of
the
composition.
d. Emulsifiers
[0045] Suitable emulsifiers include, but are not limited to, salts of
carboxylic acids,
products of acylation reactions between carboxylic acids or carboxylic
anhydrides and
amines, alkyl, acyl and amide derivatives of saccharides (alkyl-saccharide
emulsifiers), and
combinations thereof The amount of emulsifier present in the composition is
not particularly
limited and may be selected by one of ordinary skill in the art. In some
embodiments, the
emulsifier may be present in the composition in an amount of about 0 to about
10% by
weight of the composition.
e. Dispersants
[0046] Suitable dispersants include, but are not limited to, aliphatic
phosphonic acids with
2-50 carbons, such as hydroxyethyl diphosphonic acid, and aminoalkyl
phosphonic acids, e.g.
polyaminomethylene phosphonates with 2-10 N atoms e.g. each bearing at least
one
methylene phosphonic acid group; examples of the latter are ethylenediamine
tetra(methylene
phosphonate), diethylenetriamine penta(methylene phosphonate) and the triamine-
and
tetramine-polymethylene phosphonates with 2-4 methylene groups between each N
atom, at
least 2 of the numbers of methylene groups in each phosphonate being
different. Other
suitable dispersants include lignin or derivatives of lignin such as
lignosulfonate and
naphthalene sulfonic acid and derivatives, and combinations thereof
[0047] The amount of dispersant present in the composition is not
particularly limited and
may be selected by one of ordinary skill in the art. In some embodiments, the
dispersant may
be present in the composition in an amount of about 0 to about 5% by weight of
the
composition.
f Emulsion Breakers
[0048] Suitable emulsion breakers include, but are not limited to,
dodecylbenzylsulfonic
acid (DDBSA), the sodium salt of xylenesulfonic acid (NAXSA), epoxylated and
7
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
propoxylated compounds, anionic cationic and nonionic surfactants, resins such
as phenolic
and epoxide resins, and combinations thereof The amount of emulsion breaker
present in the
composition is not particularly limited and may be selected by one of ordinary
skill in the art.
In some embodiments, the emulsion breaker may be present in the composition in
an amount
of about 0 to about 10% by weight of the composition.
g. Other Hydrogen Sulfide Scavengers
[0049] Suitable other hydrogen sulfide scavengers include, but are not
limited to, oxidants
(e.g., inorganic peroxides such as sodium peroxide, or chlorine dioxide) and
combinations
thereof The amount of other hydrogen sulfide scavengers present in the
composition is not
particularly limited and may be selected by one of ordinary skill in the art.
In some
embodiments, the other hydrogen sulfide scavengers may be present in the
composition in an
amount of about 0 to about 50% by weight of the composition.
h. Gas Hydrate Inhibitors
[0050] Suitable gas hydrate inhibitors include, but are not limited to,
thermodynamic
hydrate inhibitors (THI), kinetic hydrate inhibitors (KHO, anti-agglomerates
(AA), and
combinations thereof Suitable thermodynamic hydrate inhibitors include, but
are not limited
to, methylethyl benzoate), and combinations thereof Suitable kinetic hydrate
inhibitors and
anti-agglomerates include, but are not limited to, polymers and copolymers,
polysaccharides
(such as hydroxy-ethylcellulose (HEC), carboxymethylcellulose (CMC), starch,
starch
derivatives, and xanthan), lactams (such as polyvinylcaprolactam, polyvinyl
lactam),
pyrrolidones (such as polyvinyl pyrrolidone of various molecular weights),
surfactants (such
as fatty acid salts, ethoxylated alcohols, propoxylated alcohols, sorbitan
esters, ethoxylated
sorbitan esters, polyglycerol esters of fatty acids, alkyl glucosides, alkyl
polyglucosides, alkyl
sulfates, alkyl sulfonates, alkyl ester sulfonates, alkyl aromatic sulfonates,
alkyl betaine, alkyl
amido betaines), hydrocarbon based dispersants (such as lignosulfonates,
iminodisuccinates,
polyaspartates), amino acids, proteins, and combinations thereof
[0051] The amount of gas hydrate inhibitor present in the composition is
not particularly
limited and may be selected by one of ordinary skill in the art. In some
embodiments, the gas
hydrate inhibitor may be present in the composition in an amount of about 0 to
about 5% by
weight of the composition.
i. Surfactants
[0052] Suitable surfactants include, but are not limited to, anionic
surfactants, cationic
surfactants, nonionic surfactants, and combinations thereof Anionic
surfactants include alkyl
aryl sulfonates, olefin sulfonates, paraffin sulfonates, alcohol sulfates,
alcohol ether sulfates,
8
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
alkyl carboxylates and alkyl ether carboxylates, and alkyl and ethoxylated
alkyl phosphate
esters, and mono and dialkyl sulfosuccinates and sulfosuccinamates, and
combinations
thereof Cationic surfactants include alkyl trimethyl quaternary ammonium
salts, alkyl
dimethyl benzyl quaternary ammonium salts, dialkyl dimethyl quaternary
ammonium salts,
imidazolinium salts, and combinations thereof Nonionic surfactants include
alcohol
alkoxylates, alkylphenol alkoxylates, block copolymers of ethylene, propylene
and butylene
oxides, alkyl dimethyl amine oxides, alkyl-bis(2-hydroxyethyl) amine oxides,
alkyl
amidopropyl dimethyl amine oxides, alkylamidopropyl-bis(2-hydroxyethyl) amine
oxides,
alkyl polyglucosides, polyalkoxylated glycerides, sorbitan esters and
polyalkoxylated
sorbitan esters, and alkoyl polyethylene glycol esters and diesters, and
combinations thereof
Also included are betaines and sultanes, amphoteric surfactants such as alkyl
amphoacetates
and amphodiacetates, alkyl amphopropripionates and amphodipropionates,
alkyliminodiproprionate, and combinations thereof
[0053] The amount of surfactant present in the composition is not
particularly limited and
may be selected by one of ordinary skill in the art. In some embodiments, the
surfactant may
be present in the composition in an amount of about 0 to about 10% by weight
of the
composition.
j. Solvents
[0054] Suitable solvents include, but are not limited to, isopropanol,
methanol, ethanol, 2-
ethylhexanol, heavy aromatic naphtha, toluene, ethylene glycol, ethylene
glycol monobutyl
ether (EGMBE), diethylene glycol monoethyl ether, xylene, and combinations
thereof In
some embodiments, the solvent is toluene. In some embodiments, the solvent is
naphtha.
Representative polar solvents suitable for formulation with the composition
include, alcohols
(including straight chain or branched aliphatic such as methanol, ethanol,
propanol,
isopropanol, butanol, 2-ethylhexanol, hexanol, octanol, decanol, 2-
butoxyethanol, etc.),
glycols and derivatives (ethylene glycol, 1,2-propylene glycol, 1,3-propylene
glycol, ethylene
glycol monobutyl ether, etc.), ketones (cyclohexanone, diisobutylketone), N-
methylpyrrolidinone (NMP), N,N-dimethylformamide and the like. Representative
of non-
polar solvents suitable for formulation with the composition include
aliphatics such as
pentane, hexane, cyclohexane, methylcyclohexane, heptane, decane, dodecane,
diesel, and
the like; aromatics such as toluene, xylene, heavy aromatic naphtha, fatty
acid derivatives
(acids, esters, amides), and the like. In some embodiments, the solvent is
monoethyleneglycol, methanol, dimethyl sulfoxide (DMSO), dimethylformamide
(DMF),
tetrahydrofuran (THF), or a combination thereof
9
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
[0055] In some embodiments, a composition disclosed herein comprises from 0
to about
80 percent by weight of one or more solvents, based on the weight of the
composition. In
some embodiments, a composition of the invention comprises from 0 to about 50
percent by
weight of one or more solvents, based on the weight of the composition. In
certain
embodiments, a composition comprises 20%, 25%, 30%, 35%, 40%, 45%, or 50% by
weight
of one or more solvents, based on the weight of the composition.
k. Odorants
[0056] In some embodiments, a composition disclosed herein comprises an
odorant, such
as vanillin. The amount of odorant present in the composition is not
particularly limited and
may be selected by one of ordinary skill in the art. In some embodiments, the
odorant may be
present in the composition in an amount of about 0 to about 50% by weight of
the
composition.
1. Additional Components
[0057] Compositions disclosed herein may further include additional
functional agents or
additives that provide a beneficial property. Additional agents or additives
will vary
according to the particular scavenging composition being manufactured and its
intend use as
one skilled in the art will appreciate. According to one embodiment, the
scavenging
compositions do not contain any of the additional agents or additives. The
amount of an
additional component present in the composition is not particularly limited
and may be
selected by one of ordinary skill in the art. In some embodiments, the
additional component
may be present in the composition in an amount of about 0 to about 90% by
weight of the
composition.
4. Methods of Use
[0058] The compounds and compositions disclosed herein may be used for
sweetening a
gas or liquid, such as a sour gas or a sour liquid. The compounds and
compositions may be
used for scavenging hydrogen sulfide and/or mercaptans from a gas or liquid
stream by
treating the stream with an effective amount of a compound or composition
described herein.
The compounds and compositions can be used in any industry where it is
desirable to capture
hydrogen sulfide and/or mercaptans from a gas or liquid stream. In certain
embodiments, the
compounds and compositions can be used in, condensate/oil systems/gas systems,
or any
combination thereof In certain embodiments, the compounds and compositions can
be
applied to a gas or liquid produced or used in the production, transportation,
storage, and/or
separation of crude oil or natural gas. In some embodiments, the compounds and
compositions can be applied to a gas stream used or produced in a coal-fired
process, such as
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
a coal-fired power plant. In certain embodiments, the compounds and
compositions can be
applied to a gas or liquid produced or used in a waste-water process, a farm,
a slaughter
house, a land-fill, a municipality waste-water plant, a coking coal process,
or a biofuel
process.
[0059] The compounds and compositions may be added to any fluid or gas
containing
hydrogen sulfide and/or a mercaptan, or a fluid or gas that may be exposed to
hydrogen
sulfide and/or a mercaptan. A fluid to which the compounds and compositions
may be
introduced may be an aqueous medium. The aqueous medium may comprise water,
gas, and
optionally liquid hydrocarbon. A fluid to which the compounds and compositions
may be
introduced may be a liquid hydrocarbon. The liquid hydrocarbon may be any type
of liquid
hydrocarbon including, but not limited to, crude oil, heavy oil, processed
residual oil,
bitminous oil, coker oils, coker gas oils, fluid catalytic cracker feeds, gas
oil, naphtha, fluid
catalytic cracking slurry, diesel fuel, fuel oil, jet fuel, gasoline, and
kerosene. In some
embodiments, the gas may be a sour gas. In some embodiments, the fluid or gas
may be a
refined hydrocarbon product.
[0060] A fluid or gas treated with a compound or composition of the invention
may be at
any selected temperature, such as ambient temperature or an elevated
temperature. In some
embodiments, the fluid (e.g., liquid hydrocarbon) or gas may be at a
temperature of from
about 40 C to about 250 C. In some embodiments, the fluid or gas may be at a
temperature
of from
-50 C to 300 C, 0 C to 200 C, 10 C to 100 C, or 20 C to 90 C. In some
embodiments,
the fluid or gas may be at a temperature of 22 C, 23 C, 24 C, 25 C, 26 C,
27 C, 28 C,
29 C, 30 C, 31 C, 32 C, 33 C, 34 C, 35 C, 36 C, 37 C, 38 C, 39 C, or 40 C.
In
some embodiments, the fluid or gas may be at a temperature of 85 C, 86 C, 87
C, 88 C,
89 C, 90 C, 91 C, 92 C, 93 C, 94 C, 95 C, 96 C, 97 C, 98 C, 99 C,
or 100 C.
[0061] The fluid or gas in which the compounds and compositions are introduced
may be
contained in and/or exposed to many different types of apparatuses. For
example, the fluid or
gas may be contained in an apparatus that transports fluid or gas from one
point to another,
such as an oil and/or gas pipeline. In certain embodiments, the apparatus may
be part of an
oil and/or gas refinery, such as a pipeline, a separation vessel, a
dehydration unit, or a gas
line. The fluid may be contained in and/or exposed to an apparatus used in oil
extraction
and/or production, such as a wellhead. The apparatus may be part of a coal-
fired power
plant. The apparatus may be a scrubber (e.g., a wet flue gas desulfurizer, a
spray dry
absorber, a dry sorbent injector, a spray tower, a contact or bubble tower, or
the like). The
11
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
apparatus may be a cargo vessel, a storage vessel, a holding tank, or a
pipeline connecting the
tanks, vessels, or processing units. In certain embodiments, the fluid or gas
may be contained
in water systems, condensate/oil systems/gas systems, or any combination
thereof
[0062] The compounds or compositions may be introduced into a fluid or gas by
any
appropriate method for ensuring dispersal of the scavenger through the fluid
or gas. The
compounds and compositions may be injected using mechanical equipment such as
chemical
injection pumps, piping tees, injection fittings, atomizers, quills, and the
like. The
compounds and compositions of the invention may be introduced with or without
one or
more additional polar or non-polar solvents depending upon the application and
requirements.
In some embodiments, the compounds and compositions may be pumped into an oil
and/or
gas pipeline using an umbilical line. In some embodiments, capillary injection
systems can
be used to deliver the compounds and compositions to a selected fluid. In some
embodiments, the compounds and compositions can be introduced into a liquid
and mixed.
In some embodiments, the compounds and compositions can be injected into a gas
stream as
an aqueous or nonaqueous solution, mixture, or slurry. In some embodiments,
the fluid or
gas may be passed through an absorption tower comprising a compound or
composition.
[0063] The compounds and compositions may be applied to a fluid or gas to
provide a
scavenger concentration of about 1 parts per million (ppm) to about 1,000,000
ppm, about 1
ppm to about 100,000 ppm, about 10 ppm to about 75,000 ppm, about 100 ppm to
about
45,000 ppm, about 500 ppm to about 40,000 ppm, about 1,000 ppm to about 35,000
ppm,
about 3,000 ppm to about 30,000 ppm, about 4,000 ppm to about 25,000 ppm,
about 5,000
ppm to about 20,000 ppm, about 6,000 ppm to about 15,000 ppm, or about 7,000
ppm to
about 10,000 ppm. The compounds and compositions may be applied to a fluid at
a
concentration of about 100 ppm to about 2,000 ppm, about 200 ppm to about
1,500 ppm, or
about 500 ppm to about 1000 ppm. Each system may have its own requirements,
and a more
sour gas (e.g., containing more hydrogen sulfide) may require a higher dose
rate of a
compound or composition. In some embodiments, the compounds and compositions
may be
applied to a fluid or gas in an equimolar amount or greater relative to
hydrogen sulfide and/or
mercaptans present in the fluid or gas. In some embodiments, the compounds and
compositions may be applied to a fluid or gas as a neat composition (e.g., the
compounds and
compositions may be used neat in a contact tower).
[0064] The hydrogen sulfide and/or mercaptan in a fluid or gas may be reduced
by any
amount by treatment with a compound or composition. The actual amount of
residual
hydrogen sulfide and/or mercaptan after treatment may vary depending on the
starting
12
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
amount. In some embodiments, the hydrogen sulfide and/or mercaptan levels may
be
reduced to about 150 ppm by volume or less, as measured in the vapor phase,
based on the
volume of the liquid media. In some embodiments, the hydrogen sulfide levels
and/or
mercaptan may be reduced to 100 ppm by volume or less, as measured in the
vapor phase,
based on the volume of the liquid media. In some embodiments, the hydrogen
sulfide and/or
mercaptan levels may be reduced to 50 ppm by volume or less, as measured in
the vapor
phase, based on the volume of the liquid media. In some embodiments, the
hydrogen sulfide
and/or mercaptan levels may be reduced to 20 ppm by volume or less, as
measured in the
vapor phase, based on the volume of the liquid media. In some embodiments, the
hydrogen
sulfide and/or mercaptan levels may be reduced to 15 ppm by volume or less, as
measured in
the vapor phase, based on the volume of the liquid media. In some embodiments,
the
hydrogen sulfide and/or mercaptan levels may be reduced to 10 ppm by volume or
less, as
measured in the vapor phase, based on the volume of the liquid media. In some
embodiments, the hydrogen sulfide and/or mercaptan levels may be reduced to 5
ppm by
volume or less, as measured in the vapor phase, based on the volume of the
liquid media. In
some embodiments, the hydrogen sulfide and/or mercaptan levels may be reduced
to 1 ppm
by volume, as measured in the vapor phase, based on the volume of the liquid
media. In
some embodiments, the hydrogen sulfide and/or mercaptan levels may be reduced
to 0 ppm
by volume, as measured in the vapor phase, based on the volume of the liquid
media.
[0065] In certain embodiments, a water wash may be added in an amount suitable
for
forming an emulsion with a hydrocarbon. In certain embodiments, the water wash
may be
added in an amount of from about 1 to about 50 percent by volume based on the
volume of
the emulsion. In certain embodiments, the wash water may be added in an amount
of from
about 1 to about 25 percent by volume based on the volume of the emulsion. In
certain
embodiments, the wash water may be added in an amount of from about 1 to about
10 percent
by volume based on the volume of the emulsion. In certain embodiments, the
amount of
hydrocarbon may be present in an amount of from about 50 to about 99 percent
by volume
based on the volume of the emulsion. In some embodiments, the hydrocarbon may
be
present in an amount of from about 75 to about 99 percent by volume based on
the volume of
the emulsion. In some embodiments, the hydrocarbon may be present in an amount
of from
about 90 to about 99 percent by volume based on the volume of the emulsion.
[0066] The water wash and hydrocarbon may be emulsified by any conventional
manner.
In some embodiments, the water wash and hydrocarbon may be heated and
thoroughly mixed
to produce an oil-in-water emulsion. In certain embodiments, the water wash
and
13
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
hydrocarbon may be heated at a temperature in a range of from about 90 C to
about 150 C.
The water wash and hydrocarbon may be mixed in any conventional manner, such
as an in-
line static mixer or an in-line mix valve with a pressure drop of about 0.2 to
about 2 bar
depending on the density of the hydrocarbon. The emulsion may be allowed to
separate, such
as by settling, into an aqueous phase and an oil phase. In certain
embodiments, the aqueous
phase may be removed. In another embodiment, the aqueous phase may be removed
by
draining the aqueous phase.
[0067] Optionally, demulsifiers may be added to aid in separating water
from the
hydrocarbon. In certain embodiments, the demulsifiers include, but are not
limited to,
oxyalkylated organic compounds, anionic surfactants, nonionic surfactants or
mixtures of
these materials. The oxyalkylated organic compounds include, but are not
limited to,
phenolformaldehyde resin ethoxylates and alkoxylated polyols. The anionic
surfactants
include alkyl or aryl sulfonates, such as dodecylbenzenesulfonate. These
demulsifiers may
be added in amounts to contact the water from about 1 to about 1000 ppm by
weight based on
the weight of the hydrocarbon.
[0068] The compounds, compositions, methods, and processes will be better
understood
by reference to the following examples, which are intended as an illustration
of and not a
limitation upon the scope of the invention.
5. Examples
[0069] Example 1.
[0070] Testing of various scavengers was performed using a dynamic reactor.
Figure 1
depicts the scavenging performance of ethanol hemi-formyl and methanol hemi-
formyl
compared to hexahydro-1,3,5-tris(hydroxyethyl)-s-triazine. The triazine
scavenger was
tested at about 60% activity. The pH of the ethanol hemi-formyl and the
methanol hemi-
formyl was adjusted to be near neutral, such as from about 7 to about 7.5,
before testing.
[0071] About 700 mL of crude oil was added to the dynamic reactor for each
test.
Reactions were carried out at about 70 C and about 120 C. The pressure
inside of the
reactor was set to about 10 bar. Stirring was carried out inside of the
reactor using a
magnetic stir bar at about 500 rpm. A mass flow controller was used to control
the flow of
CO2 and H2S at about 600 mL/min. Scavengers were added to the reactor in an
amount of
about 1000 ppm.
[0072] As can be seen in Figure 1, the area under the curves for methanol
hemi-formyl
and ethanol hemi-formyl is bigger than the area observed for hexahydro-1,3,5-
14
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
tris(hydroxyethyl)-s-triazine at all studied time periods. The scavenging
capacity of all
products at different times and at 70 and 120 C was as follows:
HEXAHYDRO-1,3,5-TRIS(HYDROXYETHYL)-S-TRIAZINE @120 C.
Scavenging Capacity:
= 1000 ppm, 10 min, 508,54 L/kg;
= 1000 ppm, 60 min, 29,54 L/kg;
HEXAHYDRO-1,3,5-TRIS(HYDROXYETHYL)-S-TRIAZINE @70 C.
Scavenging Capacity:
= 1000 ppm, 10 min, 837,18 L/kg;
= 1000 ppm, 60 min, 37,81 L/kg;
Methanol hemi-formyl @120 C.
Scavenging Capacity:
= 1000 ppm, 10 min, 400,43 L/kg;
= 1000 ppm, 60 min, 27,80 L/kg;
Methanol hemi-formyl @70 C.
Scavenging Capacity:
= 1000 ppm, 10 min, 520,36 L/kg;
= 1000 ppm, 60 min, 32,53 L/kg;
Ethanol hemi-formyl @120 C
Scavenging Capacity:
= 1000 ppm, 10 min, 247,12 L/kg;
= 1000 ppm, 60 min, 26,85 L/kg;
Ethanol hemi-formyl @70 C
Scavenging Capacity:
= 1000 ppm, 10 min, 251,84 L/kg;
= 1000 ppm, 60 min, 32,09 L/kg;
[0073] Based on the foregoing data, it can be concluded that the alcohol-
based hemi-
formyls outperformed the benchmark hexahydro-1,3,5-tris(hydroxyethyl)-s-
triazine. There
was an increased kinetic reaction with the alcohol-based hemi-formyls as
compared to the
triazine at certain conditions.
[0074] A liquid phase analysis was also performed. A sample of crude oil
was saturated
in a Hastelloy autoclave of nominal volume (about 5 L) using a gas mixture
containing about
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
0.2% in H2S using CO2 as a balanced gas. The equipment was modified with
micrometric
flow control valves and bubble rock in addition to a reactor valve for the
sample collection.
The aliquots were removed by the lower valve of the autoclave where a drain is
located. The
bottle (penicillin, 100 mL) was filled to about 50% (crude oil) of the volume
as it is necessary
to leave a headspace for agitation and homogenization of the microsystem.
[0075] The H2S liquid phase reduction assay was performed by potentiometric
titration
using a silver electrode coated with silver sulfide (Ag/Ag2S), where the
sample containing
H2S was titrated with a solution of about 0.01M silver nitrate in ammoniacal
isopropanol,
previously purged with nitrogen, in order to avoid oxygen as an interfering
agent in the
titration. After weighing the sample, a small amount of the titration solvent
(ammoniacal
isopropanol) was added to aid in electrode equilibration, thus avoiding
possible titration
errors and a small amount of solvent, or solvent mixture, to aid in the
homogenization of
solvent/sample and on the volume for electrode reading. The product dosage
varied greatly
over the various assays. Table 1 shows the various ratios used between H2S and
H2S
scavenger.
[0076] After the aliquot removal, the scavenger was added and, if
necessary, agitation,
stirring, and/or heating may be carried out. Stirring time and temperature
vary according to
the conditions required for each test.
[0077] Table 1
TEST Scavenger dose H25 (mg/kg) Ratio (scavenger: H25)
(PP11)
Control 9.7
Triazine 70% 1000 9.7 50:1
(hexahydro-1,3,5-
tris(hydroxyethyl)-s-
triazine
Ethanol hemi-formyl 1000 9.7 50:1
Ethylene glycol hemi- 1000 9.7 50:1
formyl
[0078] For these tests, the BS&W (base, sediment and water) used was about
0.5%,
meaning that about 99.5% of the system was crude oil.
[0079] Percentage reduction of H25 was calculated from the ratio between
the
concentration obtained at each control point and the concentration obtained at
each point of
16
CA 03127111 2021-07-16
WO 2020/160043
PCT/US2020/015480
the sample. The results obtained are depicted in Figure 2. As can be seen,
ethanol hemi-
formyl showed the best performance among the products tested. At and after
about 5
minutes, there is less than 1 mg/kg of H2S.
[0080] Any ranges given either in absolute terms or in approximate terms
are intended to
encompass both, and any definitions used herein are intended to be clarifying
and not
limiting. Notwithstanding that the numerical ranges and parameters setting
forth the broad
scope of the invention are approximations, the numerical values set forth in
the specific
examples are reported as precisely as possible. Any numerical value, however,
inherently
contains certain errors necessarily resulting from the standard deviation
found in their
respective testing measurements. Moreover, all ranges disclosed herein are to
be understood
to encompass any and all subranges (including all fractional and whole values)
subsumed
therein.
[0081] Furthermore, the invention encompasses any and all possible
combinations of some
or all of the various embodiments described herein. Any and all patents,
patent applications,
scientific papers, and other references cited in this application, as well as
any references cited
therein, are hereby incorporated by reference in their entirety.
[0082] Finally, the compositions of the present disclosure may comprise any
compound(s)
or component(s) disclosed herein and the compositions may also consist of or
consist
essentially of any compound(s) or component(s) disclosed herein.
17