Note: Descriptions are shown in the official language in which they were submitted.
6~3763
3-ACY ~ ~I~10-2-OXO-l-AZETIDINYL ESTERS
OF PHOSPHONIC ACIDS, PHOSPHO~IC PCID A~D
PHOSPHORIC ACID ESTERS
This invention is directed to a novel
family of ~-lactam antibiotics, and to the use
of such compounds as antibacterial agents.
It has been discovered that the B-lactam nucleus
can be biologically activated by a substituent
y
having the formula -O-P-R5, and pharmaceutically
OH
acceptable salts thereof, attached to the nitrogen
atom in the nucleus.
3-Lactams having a -O-P-R5 substituent
OH
(or a pharmaceutically acceptable salt thereof)
in the l-position and an acylamino substituent
in the 3-position exhibit activity against a
range of gram-negative and gram-positive bacteria.
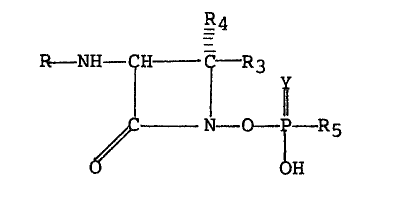
Illustrative members of the novel family
of ~-lactam antibiotics of this invention are
those encompassed by the formula
-2 R4
Rl-NH-C f R3 y
l 11
~C N--O - P - R5
O OH
or a pharmaceutically acceptable salt ~lereof.
This application is a divisional application from
patent application No. 435,268 filed August 24, l983.
The invention as described and claimed herein, is for those
compounds or formu]a I above wherein Rl and R2 both stand
for hydrogen, such compounds being useful as intermediates
in the preparation of the ~-lac-tam antibiotics of
formula I.
As used in formula I and throughout the
specification, the symbols are as defined below.
Rl is acyl:
R2 is hydrogen or methoxy;
R3 and R4 are the same or different and
each is hydrogen, alkyl, alkenyl, alkynyl,
cycloalkyl, phenyl, substituted phenyl or a
4,5,6 or 7-membered heterocycle (referred to
hereinafter as ~6) or one of R3 and R4 is hydrogen
and the o~her is azido, halomethyl, dihalomethyl,
trihalomethyl, alkoxycarbonyl, 2-phenylethenyl,
2-phenylethynyl, carboxyl, -CH2Xl, -S-X2,
X~3 Xl3 l
-O-X2, -O-C-X4, -S-C-X4, or -A-C-NX6X7 ;
X5 X5
X1 is azido, amino (-NH2), hydroxy, alkanoyl-
amino, alkylsulfonyloxy, phenylsulfonyloxy,
(substituted phenyl)sulfonyloxy, phenyl, substituted
phenyl, cyano, -S-X2 or -O-X2 ;
X2 is alkyl, substituted alkyl, phenyl,
substituted phenyl, phenylalkyl, (substituted
phenyl)alkyl, alkanoyl, substituted alkanoyl,
phenylcarbonyl, (substituted phenyl)carbonyl or
heteroarylcarbonyl;
one of X3 and X4 is hydrogen and the other
is hydrogen or alkyl, or X3 and X4 when taken
together with the carbon atom to which they are
attached form a cycloalkyl group;
X5 is formyl, alkanoyl, phenyicarbonyl,
(substituted phenyl)carbonyl, phenylalkylcarbonyl,
(substituted phenyl)alkylcarbonyl, carboxyl,
o
alkoxycarbonyl, aminocarbonyl (NH2-C-), (substituted
amino)carbonyl, or cyano (-C--N);
-3- 1 2l6 8 7 6 3
A is -CH=CH-, -CH2-CH=CH-, -(CH2)n,
(C~ ) -O-, -(CH2)n,NH-, or (CH2)n, 2
n is 0, 1, 2 or 3;
n' is 1 or 2;
X6 and X7 are the s.ame or different and each
is hydrogen or alkyl, or X6 is hydrogen and X7
is amino, substituted amino, acylamino or alkoxy;
R5 is hydroxyl, alkyl, substituted alkyl,
phenyl, substituted phenyl, alkoxy, alkylthio,
(substituted alkyl)oxy, (substituted alkyl)thio,
phenyloxy, phenylthio, (substituted phenyl)oxy or
(substituted phenyl)thio; and
Y is oxygen or sulfur.
Listed below are definitions of various
terms used to describe the B-lactams of this
invention. These definitions apply to the
terms as they are used throughout the specification
(unless they are otherwise limited in specific
instances) either individually or as part of a
larger group.
The terms "alkyl" and "alkoxy" refer to
both straight and branched chain groups. Those
groups having 1 to 10 carbon atoms are preferred.
The terms "cycloalkyl" and "cycloalkenyl"
refer to cycloalkyl and cycloalkenyl groups
having 3,4,5,6 or 7 carbon atoms.
The term "substituted alkyl" refers to
alkyl groups substituted with one, or more, azido,
amino (-NH2), halogen, hydroxy, carboxy, cyano,
-4- ~26~763
alkoxycarbonyl, aminocarbonyl, alkanoyloxy, alkoxy,
phenyloxy, (substituted phenyl)oxy, P6-oxy,
mercapto, alkylthio, phenylthio, (substituted phenyl)-
thio, alkylsulfinyl, or alkylsulfonyl groups.
The terms "alkanoyl", "alkenyl", "alkenyl"
and "alkynyl" refer to both stra-ght and branched
chain groups. Those groups ha~ing 2 to 10 carbon
atoms are preferred.
The terms "halogen" and "halo" refer to
fluorine, chlorine, bromine and iodine.
The term "protected carboxyl" refers to
a carboxyl group which has been esterified
with a conventional acid protecting group.
These groups are well known in the art; see,
for example, United States patent 4,144,333,
issued March 13, 1979. The preferred protected
carboxyl groups are benzyl, benzhydryl, t-butyl,
E-methoxybenzyl, and ~-nitrobenzyl esters.
~he term "substituted phenyl" refers to
a phenyl group substituted with 1, 2 or 3
amino(-NH2), halogen, hydroxyl, trifluoromethyl,
alkyl (of 1 to 4 ca~bon atoms), alkoxy (of 1
to 4 carbon atoms), or carboxyl groups.
The expression "a 4,5,6 or 7-membered
heterocycle" (referred to as "R6") refers to
substituted and unsubstituted, aromatic and
non-aromatic groups containing one or more
nitrogen, oxygen or sulfur atoms. Exemplary
substituents are oxo(=O), halogen, hydroxy, nitro,
amino, cyano, trifluoromethyl, alkyl of 1 to 4
-5- ~2G8~63
carbons, alkoxy of 1 to 4 carbons, alkylsulfonyl,
phenyl, substituted phenyl, 2-Eurylimino
O~_CH=N-
( ~ ), benzylimino and substituted alkyl
groups (wherein the alkyl group has 1 to 4 car~ons).
One type of "4,5,6 or 7-membered heterocycle" is
the "heteroaryl" group. The term "heteroaryl"
refers to those 4,5,6 or 7-membered heterocycles
which are aromatic. Exemplary heteroaryl groups
are substituted and unsubstituted pyridinyl,
furanyl, pyrrolyl, thienyl, 1,2,3-triazolyl,
1,2,4-triazolyl, imidazolyl, thiazolyl,
thiadiazolyl, pyrimidinyl, oxazolyl, triazinyl,
and tetrazolyl. Exemplary nonaromatic heterocycles
lS (i.e., fully or partially saturated heterocyclic
groups)are substituted and unsubstituted azetinyl,
oxetanyl, thietanyl, piperidinyl, piperazinyl,
imidazolidinyl, oxazolidinyl, pyrrolidinyl,
tetrahydropyrimidinyl, dihydrothiazolyl and
hexahydroazepinyl. Exemplary of the substituted
4,5,6 or 7-membered heterocycles are 1-alkyl-3-
azetinyl, 2-oxo-1-imidazolidinyl, 3-alkylsulfonyl-2-
oxo-l-imidazolidinyl, 3-benzylimino-2-oxo-1-
imidazolidinyl, 3-alkyl-2-oxo-1-imidazolidinyl,
3-phenyl (or substituted phenyl)-2-oxo-1-
imidazolidinyl, 3-benzyl-2-oxo-1-imidazolidinyl,
- 6- 126~763
3-(2-aminoethyl)-2-oxo-1-imidaæolidinyl, 3-amino-
2-oxo-1-imidazolidinyl, 3-I(alkoxycarbonyl)amino~-
2-oxo-1-imidazolidinyl, 3-[2-l~alkoxycarbonyl)-
amino]ethyl]-2-oxo-1-imidazolidinyl, 2-oxo-1-
pyrrolidinyl, 2-oxo-3-oxazolidinyl, 4-hydroxy-6-
methyl-2-pyrimidinyl, 2-oxo-1-hexahydroazepinyl,
2-oxo-3-pyrrolidinyl, 2-oxo-3-furanyl, 2,3-dioxo-
l-piperazinyl, 2,5-dioxo-1-piperazinyl, 4-alkyl-
2,3-dioxo-1-piperazinyl, and 4-phenyl-2,3-dioxo-
l-piperazinyl.
The term "substituted amino" refers to a
group having the formula -NYlYz wherein Yl is
hydrogen, alkyl, phenyl, substitùted phenyl,
phenylalkyl or (substituted phenyl)alkyl, and
Y2 is alkyl, phenyl, substituted phenyl,
phenylalkyl, (substituted phenyl)alkyl, hydroxy,
cyano, alkoxy, phenylalkoxy, or amino (-NH2).
The term "substituted alkanoyl" includes
within its scope compounds having the formula
O
(substituted alkyl) -C- (wherein "substituted alkyl"
is defined above) and phenylalkanoyl.
-7_ ~Z68~63
The term "acyl" refers to all organic radi-
cals derived from an organic acid (i.e., a car-
boxylic acid) by removal of the hydroxyl group.
Certain acyl groups are, of course, preferred but
this preference should not be viewed as a limita-
tion of the scope of this invention. Exemplary
acyl groups are those acyl groups which have been
used in the past to acylate ~-lactam antibiotics
including 6-aminopenicillanic acid and derivatives
and 7-aminocephalosporanic acid and derivatives;
see, for example, Cephalosporins and Penicillins,
edited by Flynn, Academic Press (1972), German Of-
fenlegungsschrift 2,716,677, published October 10,
1978, Belgian patent 867,994, published December
11, 1978, United States patent 4,152,432, issued
May 1, 1979, United States patent 3,971,778, is-
sued July 27, 1976, United States patent 4,172,199,
issued October 23, 1979, and British patent 1,348,
894, published March 27, 1974. The following list
of acyl groups is presented to further exemplify
the term "acyl"; it should not be regarded as lim-
iting that term. Exemplary acyl groups are:
(a) Aliphatic groups having the formula
R -C-
wherein Ra is alkyl; cycloalkyl; alkoxy; alkenyl;
GCl95
-8- ~268763
cycloalkenyl; cyclohexadienyl; or alkyl or alkenyl
substituted with one or more halogen, cyano,
nitro, amino, mercapto, alkylthio, or cyanometh~l-
thio groups.
(b) Carbocyclic aromatic groups having the
formula
Rb~(CH2 ) n~C~,
Rb ~ CIH-C- ,
Rb~ CH2-o-c-,
b ~ O-CH2-C- ,
- 9- ~,61 3763
b ~ d 1l
S C H 2 C
b~Rd
~ -CH -S-C-
wherein n is 0, 1, 2 or 3; Rb, Rc, and Rd each
is independently hydrogen, halogen, hydroxyl,
nitro, amino, cyano, trifluoromethyl, alkyl
of 1 to 4 carbon atoms, alkoxy of 1 to 4 carbon
atoms or aminomethyl; and Re is amino, hydroxyl,
a carboxyl salt, protected carboxyl, formyloxy,
a sulfo salt, a sulfoamino salt, azido, halogen,
hydrazino, alkylhydrazino, phenylhydrazino, or
l(alkylthio)thioxomethyl]thio.
20Preferred carbocyclic aromatic acyl groups
include those having the formula
.
HO ~ CH -C-,
~ H -C-,
CH2NH2
GC195
-10- ~,2~763
O
HO ~ H-C- (Re is preferably
Re
s
a carboxyl salt or sulfo salt) and
~ CH-C- (Re is preferably
Re
a carboxyl salt or sulfo salt).
(c) Heteroaromatic groups having the
formula
Rf (CH2)n
o
Rf-CH-C-
Re Ol
Rf-O--CH2-C- ~
O
Rf-S-CH2-C-
O O
ll ll
Rf-C -C-
wherein n is 0, 1, 2 or 3; Re is as defined
above; and Rf is a substituted or unsubstituted
5-, 6- or 7-membered heterocyclic ring (heteroaryl
group) containing 1,2,3 or 4 tpref~ably 1 or 2)
ni~gen, oxygen and ~fur aboms. Exem~lary heb~lic
Z68763
rings are thienyl, furyl, pyrrolyl, pyridinyl,
pyrazolyl, pyrazinyl, thiazolyl, pyrimidinyl,
thiadiazolyl and tetrazolyl. Exemplary substituents
are halogen, hydroxyl, nitro, amino, protected amino,
cyano, trifluoromethyl, alkyl of 1 to 4 carbon atoms,
alkoxy of 1 to 4 carbon atoms, or
R
HOOC-CH-CH2-O-C-NH
NH2
Preferred heteroaromatic acyl groups
include those groups of the above formulas
wherein Rf is 2-amino-4-thiazolyl, 2-amino-5-
halo-4-thiazolyl, 4-aminopyrimidin-2-yl,
5-amino-1,2,4-thiadiazol-3-yl, 2-thienyl;
2-furanyl, or 6-aminopyridin-2-yl.
(d) [~(4-Substituted-2,3-dio~o-1-piper-
azinyl)carbonyl]amino]arylacetyl groups having
the formula
O
-c-cH-NH-c-N~ _~N Rh
g O O
wherein Rg is an aromatic group (including
carbocyclic aromatics such as those of the
formula Rc
and heteroaromatics as included within the
definition of Rf); and Rh is alkyl, substituted
. GC195
-12- ~26~763
alkyl (wherein the alkyl group is substituted
with one or more halogen, cyano, nitro, amino
or mercapto groups), arylmethyleneamino (i e.,
~N=CH~Rg wherein Rg is as defined above),
O
arylcarbonylamino (i e., ~NH~C~Rg wherein R
is as defined above) or alkylcarbonylamino.
Preferred [[(4-substituted-2,3-dioxo-l-
piperazinyl)carbonyl]amino]arylacetyl groups
include those wnerein Rh is ethyl, phenylmethylene-
amino or 2-furylmethyleneamlno.
(e) (Substituted oxyimino)arylacetyl groups
having the formula
-C-C=N-O-
Rg
wherein Rg is as defined above and Ri is hydrogen, R6,
alkyl, cycloalkyl, alkylaminocarbonyl, arylamino-
carbonyl (i.e., ~C~NH~Rg wherein Rg is as defined
above) or substituted alkyl (wherein the alkyl
group is substituted with l or more halogen,
cyano, nitro, amino, mercapto, alkylthio,
aromatic group (as defined by Rg), carboxyl
(including salts thereof), amido, alkoxycarbonyl,
phenylmethoxycarbonyl, diphenylmethoxycarbonyl,
hydroxyalkoxyphosphinyl, dihydroxyphosphinyl,
hydroxy(phenylmethoxy)phosphinyl, or dialkoxy-
phosphinyl substituents).
-13- ~ ~ 6 ~ 7 6 3
Preferred (substituted oxyimino)arylacetyl
groups include those wherein Rg is 2-amino-4-
thiazolyl. Also preferred are those groups
wherein Ri is methyl, ethyl, carboxymethyl,
l-carboxy-l-methylethyl, 2,2,2-trifluoroethyl or
l-carboxycyclopropyl.
~ f) (Acylamino)arylacetyl groups having
the formula O O
Il 11
-C-CH-NH-C-R
Rg
wherein Rg is as defined above and Rj is
Rc
Rb ~ (CH2)n-O-, amino, alkylamino, (cyanoalkyl)-
amino, amido, alkylamido, (cyanoalkyl)amido,
NH NH2
-CH -NH-C ~ N -CH-CH2-C-NH-CH3,
HO
~N(CH2~CH2 OH~2' ~ CH3,
OH
QH OH
N ~_J
~ f ~1
HO ~ C-
O O
GC195
-14- ~ 76~
Preferred (acylamino)arylacetyl groups of
the above formula include those groups wherein
Rj is amino or amido. Also preferred are
those groups wherein R is phenyl or 2-thienyl.
(g) [[[3-Substituted-2-oxo-1-imidazoli-
dinyl]carbo~yl]amino]arylacetyl groups having
~ the formula
O O C
Il 11 ~ ~
-C-CH-NH-C-N N-Rk
Rg CH --CH2
wherein Rg is as defined above and Rk is
hydrogen, alkylsulfonyl, arylmethyleneamino
(i.e., ~N=CH~Rg wherein Rg is as defined
above), -C-Rm (wherein Rm is hydrogen, alkyl
or halogen substituted alkyl), aromatic group
(as defined by Rg above), alkyl or substituted
alkyl (wherein the alkyl group is substituted
with one or more halogen, cyano, nitro, amino
or mercapto groupsl.
Preferred [[3-substituted-2-oxo-1-imidazoli-
dinyl]carbonyl]amino]arylacetyl groups of the
above formula include those wherein Rg is phenyl
or 2-thienyl. Also preferred are those groups
wherein Rk is hydrogen, methylsulfonyl, phenyl-
methyleneamino or 2-furylmethyleneamino.
-15- ~2~8763
The terms "salt" and "salts" refer to
basic salts formed with inorganic and organic
bases. Such salts include ammonium salts, alkali
metal salts like sodium and potassium salts
5 (which are preferred), alkaline earth metal
salts like the calcium and magnesium salts, salts
wlth organic bases, e.g., dicyclohexylamine salt,
benzathine, N-methyl-D-glucamine, hydrabamine
salts, salts with amino acids like arginine,
lysine and the like. The nontoxic, pharmaceutically
acceptable salts are preferred, although other
salts are also useful, e.g., in isolating or
purifying the product.
The salts are formed in conventional manner
by reacting the free acid form of the product
with one or more equivalents of the appropriate
base providing the desired cation is in a solvent
or medium in which the salt is insoluble, or in
water and removing the water by freeze drying.
By neutralizing the salt with an insolùble acid
- like a cation exchange resin in the hydrogen
form (e.g., polystyrene sulfonic acid resin llke
Dowex 50) or with an aqueous acid and extraction
with an organic solvent, e.g., ethyl acetate,
dicloromethane or the like, the free acid form
can be obtained, and, if desired, another salt
formed.
GCl9~
-16- ~2~763
y
~-Lactams having a -O-P-R5 substituent
OH
(or a pharmaceutically acceptable salt thereof)
in the l-position and an amino or acylamino
substituent in the 3-position contain at least
o~e &hiral center - the carbon atom (in the
3-position of the B-lactam nucleus) to which
the amino or acylamino substituent is attached.
This invention is directed to those ~-lactams
which have been described above, wherein the
stereochemistry at the chiral center in the
3-position of the 3-lactam nucleus is the
same as the configuration at the carbon atom
in the 6-position of naturally occurring .
penicillins (e.g., penicillin G) and as the
configuration at the carbon atom in the
7-position of naturally occurring cephamycins,
(e.q., cephamycin C).
With respect to the preferred 3-lactams
of formula I, the structural formulas have
been drawn to show the stereochemistry at
the chiral center in the 3-position. Because
of the nomenclature convention, those compounds
of formula I wherein R2 is hydrogen have the
S-configuration and those compounds of formula I
wherein R2 is methoxy have the R-configuration.
Also included within the scope of this
invention are racemic mixtures which contain
the above-described ~-lactams.
-
GC195
-17- ~26~763
~-Lactams having a -O-P-R5 substituent
OH
(or a pharmaceutically acceptable salt thereof)
in the 1-position of the ~-lactam nucleus and
an acylamino substituent in the 3-position of
the 3-~actam nucleus have activity against a
range of gram-negative and gram-positive organisms.
1 0 Y
The -O-P-R5 substituent (or a pharmaceuticall~
0~
acceptable salt thereof) is essential to the
activity of the compounds of this invention.
The compounds of this invention can be
used as agents to combat bacterial infections
(including urinary tract infections and
respiratory infections) in mammalian species,
such as domesticated animals (e.g., dogs, cats,
cows, horses, and the like) and humans.
For combating bacterial infections in
mammals a compound of this invention can be
administered to a mammal in need thereof in
an amount of about 1.4 mg/kg/day to about
350 mg/kg/day, preferably about 14 mg/kg/day
to about 100 mg/kg/day. All modes of adminis-
tration which have been used in the past to
deliver penicillins and cephalosporins to the
site of the infection are also contemplated
for use with the novel family of ~-lactams
of this invention. Such methods of administration
include oral, intravenous, intramuscular, and
as a suppository.
-18- 1Z68763
The B-lactams of this invention can be
prepared from hydroxamic acids of formula VIII
(infra.), which are obtainable from an amino
acid having the formula
II OH~R4
NH2-1H C R~
~C OH
o
utilizing the methodology disclosed in U. S.
patent 4,337,197. As disclosed therein, the
amino group is first protected with a classical
protecting group (e.g., t-butoxycarbonyl,
benzyloxycarbonyl, _-nitrophenylsulfenyl, etc.),
yielding a compound having the formula
III IOH~R4
Al-NH-CH C - R3
~C OH .
O~
In formula III, and throughout the specification,
the symbol "Al" refers to a nitrogen protecting
group.
The carboxyl group of a protected amino
acid of formula III is then reacted with an amine
salt having the formula
IV
Y~O-NH3Cle,
In formula IV, and throughout the specification,
the symbol "Y3' refers to benzyl, pivaloyl,
-CH2(NHA)CO2alkyl, t-butyl, ~-nitrobenzyl, benzhydryl,
2-cyanoethyl, 2-trimethylsilylethyl, trichloroethyl,
inter alia. The reaction proceeds in the
presence of a coupling agent such as l-ethyl-3-
(3-dimethylaminopropyl)carbodiimide or dicyclo-
hexylcarbodiimide, and yields a compound having
the formula
~68763
-19-
A1NH-fH C - R3
~ NH-O-Y3 .
o
The hydroxyl group of a compound of formula V
is converted to a leaving group, using, for
example, a classical reagent such as methane-
sulfonyl chloride (methanesulfonyl is referred
to hereinafter as "Ms").
The fully protected compound having the
formula
VI OM~s~R4
AlNH-IH - C - R3
~ - NH-O-Y3
O
is cyclized by treatment with base, e g.,
potassium carbonate. The reaction is preferably
carried out in an organic solvent such as acetone,
under reflux conditions, and yields a compound
having the formula
VII R4
Al-NH-CH Cl-R3
~C N-O-Y3 .
Alternatively, cyclization of a compound
of formula V can be accomplished without first
converting the hydroxyl group to a leaving group.
Treatment of a compound of formula V with
triphenylphosphine and diethylazodicarboxylate
or carbon tetrachloride, triphenylphosphine and
a base such as triethylamine, yields a compound
of formula VII.
~6876~
-20-
Both of the methods disclosed above for
ring closure of a compound of formula V result
in the inversion of the stereochemlstry at the
carbon bonded to the R3 and R4 substituents.
Selective reduction of a compound of
formula VII (using catalytic hydrogenation if
Y3is benzyl or by treatment with a base such
as sodium sulfide or sodium hydroxide if Y3is
pivaloyl or with DBU if Y3is -CH2CH(NHA)CO2alkyl)
yields the corresponding compound having the
formula
VIII -4
Al-NH-CH C-R3
O~ N-OH
Phosphorylation of a hydroxamic acid of
formula VIII can be accomplished by first
treating the compound with base (e.g.,
2,6-lutidine or trie.thylamine) to generate the
corresponding anion and then reacting the salt
with a phosphorous derivative having the formula
IX IYI
(Act) -P-R
wherein the activating group "Act" is, most
preferably, chlorine to yield the corresponding
compound having the formula
X
Al-NH~-2 C4~R3
l l ll~ Rs
,C N -O - P~
o~ Act
1,2~,8763
-21-
. Hydrolysis of a compound of formula X under
neutral or mildly acidic conditions yields
the corresponding compound having the formula
XI R4
NH CH C R
~C - N -O -P\
Alternatively, phosphorylation of a hydroxamic
acid of formula VIII can be accomplished by
first treating the compound with a base
(e g., 2,6-lutidine) and then reacting it
with a phosphorous derivative having the .
formula
XII 1l R'
Act-P ~ S
O-alkyl ,
wherein R5 is alkyl or alkoxy, to obtain the
corresponding compound having ~he formula
XIII _4
Al-NH-CH C-R3 y
C N -O -P ~ 5
O~ \ O-alkyl .
~Z~7~3
-22-
Treatment of a compound of formula XIII with
an acid-scavenger and drying agent such as
bis-trimethylsilylacetamide,followed by
treatment with trLmethylsilyl bromide,
yields an intermediate silyl ester having
the formula
XIV -4
Al-NH-CH f-R3
0 l I Il ~ R5
, C--N--O--P
~' ~o-si (CH3)3
wherein R5 is alkyl or -O-Si(CH3)3.
A compound of formula XI~ is readily converted
to a salt of the corresponding compound of
formula XI by treatment with aqueous buffer in
the range of pH 2.5 to pH 6, with or without an
alcohol.
Deprotection of the 3-amino substituent
of a compound of formula XI can be accomplished
using art-recognized techniques. If, for example,
the protecting group is t-butoxycarbonyl,
trifluoroacetic acid-anisole can be used to deprotect
the amino group. If the protecting group is
benzyloxycarbonyl, catalytic (e.g., palladium
on charcoal) hydrogenation can be used. If
the protecting group is o-nitrophenylsulfenyl,
~-toluenesulfonic acid can be used in combination
with ~-thiocresol. The deprotected compound
has the formula
~26~3763
-23-
=4
NH -CH --C-R
~C N -O -P\
O OH
and is a key intermediate for preparing the
compounds of this invention.
Well known acylation techniques can be
used to convert a compound of formula XV to
the corresponding compound having the formula
XVI =R4
Rl-NH-CH C-R
I l ll ~ R5
~C N -O - P~
O~ OH
Exemplary techniques include reaction with a
carboxylic acid (Rl-OH) or corresponding carboxylic
acid halide or carboxylic acid anhydride. The
reactions with a carboxylic acid proceed most
readily in the presence of a carbodiimide such
as dicyclohexylcarbodiimide and a substance
capable of forming a reactlve intermediate in situ
such as ~-hydroxybenzotriazole or 4-dimethyl-
aminopyridine. In those instances wherein the
acyl group (Rl) contains reactive functionality
(such as amino or carboxyl groups) it may be
necessary to first protect these functional
groups, then carry out the acylation reaction,
and finally deprotect the resulting product.
~6S763
--2g--
The products of formula I wherein R2
is methoxy can be prepared from the corresponding
compound of formula XI wherein Al is benzyloxy-
carbonyl. Halogenating (~referably chlorinating)
the amide nitrogen of a compound of formula XI
yields a compound having the formula
XVII
~C - I_O _ p ~ 5
O OH .
Reagents and procedures of N-chlorinating amides
are known in the art. Exemplary reagents are
tert. -butyl hypochlorite, sodium hypochl.orite,
and chlorine. The reaction can be run in an
organic solvent (e.g., a lower alkanol such
as methanol) or in a two phase solvent system
(e.q., water/methylene chloride) in the presence
of a base such as sodium borate decahydrate.
The reaction is preferably run at a reduced
temperature.
Reaction of a compound of formula XVII
with a methoxylating agent, e.~., an alkali
metal methoxide, yields a compound (in combination
with its enantiomer if R3 and R4 are the same
or if XVII is a racemic mixture) having the
formula
XVIII
oCH3 R4
~ CH2-O-C-NH-C C-R3 y
~,C N-O -P~
~Z6~3~63
The reaction can be run in an organic solvent,
e.g., a polar organic solvent such as tetra-
hydrofuran, at a reduced temperature.
Aiternatively, a compound of formula XI,
wherein ~1 is benzyloxycarbonyl, can be
converted to a compound of formula XVIII
using a single step procedure. The methoxylating
agent can first be mixed with a compound of
formula XI and the N-chlorinating reagent then
added to the reaction mixture.
Conversion of a compound of formula XVIII
to the desired products of formula I can be
accomplished using the procedures described
above for the conversion of an intermediate
of formula XI to a product of this invention.
The following examples are specific
embodiments of this invention.
~6~763
-26-
Example 1
.
Me~hylphosphonic acid,[3S-[3~(Z),4B]]-3-[[t2-
amino-4-thiazolyl)(methoxyimino)acetyl]amino]-4-
methyl-2-oxo-l-azetidinyl ester, potassium salt
A) O-Benzyl--N-t-butoxycarbonyl-L-threonine
hydroxamate
To a stirred solution of 10.95 g (50 mmol)
of N-t-butoxycarbonyl-L-threonine in 50 ml of
water was added a solution of 8.75 g tS5 mmol)
of O-benzylhydroxylamine hydrochloride and
50 ml of water, which had been adjusted to
pH 4.0 using 2N KOH. After the addition,
the pH was adjusted to 4.0, and a solution
of 10.55 g (55 mmol) of l-ethyl-3-[(3-dimethyl-
amino)propyl]carbodiimide hydrochloride (water
soluble carbodiimide, in 50 ml of water was
added over 10 minutes while maintaining the pH
at 4.0 - 4.5 using lN HCl. The reaction was
continued for 20 minutes in this pH range,
and then extracted with ethyl acetate. The
ethyl acetate extract was washed at pH 8.5
(aqueous NaHCO3) and then at pH 3.0 (lN HCl),
dried (Na2SO4), and evaporated to a crystalline
residue. Treatment with ethyl acetate-hexane
gave 9.60 g of crystalline product.
~;~6~763
B) O-Benzyl-a-N-t-butoxycarbonyl-L-(O-mesyl-
threonine)hydroxamate
To a stirred solution of O-benzyl-~-N-
t-butoxycarbonyl-L-threonine hydroxamate
(0.60 g, 29.6 mmol) in 24 ml of dry pyridine
at 0-5C under nitrogen was added dropwise
2.63 ml (34 mmol) of methylsulfonyl chloride.
The reaction was stirred at this temperature
for 4 hours, poured into 250 ml of water,
adjusted to pH 3.5 (3 N HC1), treated with
saturated NaCl solution, and extracted
repeatedly with ethyl acetate. The combined
ethyl acetate extract was washed with water,
then water at pH 7, dried !Na2SO4), and
evaporated to give 11.68 g of desired product
as a crystalline mass.
C) [3S- ( 3a, 4 B) ] -3- [ [ (1, l-Dimethylethoxy)carbonyl]-
amino]-4-methyl-2-oxo-1-(phenylmethoxy)azetidine
Potassium carbonate (12 g, 0.087 mol)
wa added to a stirred solution of 11.65 g
(0.029 mol) of O-benzyl-~-N-t-butoxycarbonyl-
L-(O-mesylthreonine)hydroxamate in 490 ml of
acetone under nitrogen and the reaction was
refluxed. After 6 hours, the reaction mixture
was cooled and filtered through ~elite.
Evaporation of the filtrate gave a crystalline
residue, which was recrystallized from ethyl
acetate-hexane to give 4.65 g of crystalline
product.
* Trade-mark
,ci~.
~Z6~3763
-28-
D) ~3S-(3~,4~)]-3-[~(1,1-Dimethylethoxy)carbonyl]-
amino]-4-methyl-2-oxo-1-hydroxyazetidine
To a solution of [3S-(3~,4~)]-3-[[(1,1-
dimethylethoxy)carbonyl]amino~-4-methyl-2-
oxo-l-(phenylmethoxy)azetidine (1.22 g, 4 mmole)
in 40 ml of methanol was added 10~ palladium on
charcoal (0.8 g), and the reaction mixture was
reduced at atmospheric pressure for 15 minutes
(until hydrogen uptake stopped). ~he reaction
mixture was filtered through Celite and
concentrated in vacuo. The solid that was
obtained, was dried over P2OS at 45C to yield
0.75 g of product.
E) Methylphosphonic acid, [3S-(3a,4~)]-3{[(1,1-
dimethvlethoxy)carbonyl]aminq~4-methyl-2-oxo-1-
azetidinyl ester, potassium salt
[3S-(3a,43]-3{[(1,1-Dimethylethoxy)carbonyl]-
amino]-4-methyl-2-oxo-1-hydroxyazetidine (1.02 g,
4.7 mmole) was partially dissolved in 14 ml
of dry dichloromethane and cooled to -10C under
nitrogen. 2,6-Lutidine (0.6 ml, 4.9 mmole) was
then added followed by the dropwise addition
of methylphosphonic dichloride (O.62 g, 4.6 ~mole)
in 5 ml of dichloromethane. After addition,
the reaction was stirred at -10C for 1 hours.
The termperature was allowed to rise to 0C,
and then 20 ml of 0.5 ~ KH2PO4 containing 2 ml
of 2 N KOH and 15 ml of tetrahydrofuran was
added (pH 6.6). This solution was stirred at
0-15C for 5 hours, and the pH was maintained
lX 61~7 63
-29-
at 4.2 by the addition of 1 N KOH. The reaction
mixture was concentrated _ vacuo to remove
solvent and the remainder was lyophilized. The
lyophilate was washed with two 200 ml portions
of dichloromethane, and the dichloromethane was
concentxated in vacuo to yield 1.63 g of crude
material. This was dissolved in 5 ml of water
(pH 4.5) and passed through 60 ml of ~owex 50
resin (K~, 0.7 meq/ml) to yield 1.03 g of crude
product. The product was further purified by
chromatography through 80 ml of ~P-20 resin
using water as eluent. The product which was
found to elute in a wide band ~500 ml) gave,
after lyophilization, 0.4 g of hygroscopic
material.
F) Methylpho~phonic acid, t3S-(3~,4B~]-3-amino-2-
methyl-4-oxo-1-azetidinyl ester, trifluoroacetic
acid salt
Methylphosphonic acid, [3S-(3,4B)]-3-
[~(l,l-dimethylethoxy)carbonyl]amino~4-methyl-2-
oxo-l-azetidinyl ester, potassium salt (0.35 g,
1 mmole) was suspended in 1.5 ml of dichloromethane
and 1.25 ml of anisole. The reaction mixture
was cooled to -1~C, and trifluoroacetic acid
(0.95 ml) was added. This was stirred under
nitrogen at -10C for 1 hour. The reaction
mixture was evaporated in vacuo to a residue,
which was evaporated from toluene (twice) to
give a viscous oil. Ether was added, and the
oil solidified. The ether was decanted and
the product was dried _ vacuo.
7 * Trade-mark
l~'
. lZ687~3 ~195
-30-
G) Methylphosphonic acid, [3S-[3(Z),4B~]-3-
[~(2-amino-4-thiazolyl)tmeth ~ zetyl]-
amino]-4-methyl-2-oxo-1-azetidinyl ester,
potassium salt
l-Hydroxybenzotriazole (.169 g, 1.1 mmole)
and (Z)-(2-amino-4-thiazolyl)(methoxyimino)
acetic acid (.223 g, 1.1 mmole) were dissolved -
in 3 ml of dry dimethylformamide under nitrogen.
This was cooled to 0C, and N,N'-dicyclohexyl-
carbodiimide (.228 g, 1.1 mmole) was added
portionwise. After addition, the reaction was
stirred at 0C for 1 hour. To this was added
a solution of methylphosphonic acid, ~3S-(3~,4B)]-
- 3-amino-2-methyl-4-oxo-1-azetidinyl ester.,
trifluoroacetic acid salt in 2 ml of dimethyl-
formamide and 1.1 ml of N,N-diisopropylethylamine
at 0C. The reaction was stirred at 0C for
1 hour and then at room temperature overnight.
The solution was filtered, and the filtrate
was concentrated in vacuo. The residue was
dissolved in water (pH 4.5), and the solution
was washed with dichloromethane. The aqueous
solution was passed through 80 ml of Dowex 50
(K~ .7 meq/ml). Partial purification of
product was obtained by taking 8 ml fractions.
Those fractions that contained product (4-8,
40 ml) were pooled and lyophilized to yield
0.4 g of material which was purified further
by chromatography through 150 ml of HP-20
resin using water as eluent. Lyophilization
~2~;8763 ~,
-31-
gave 32 mg of desired product containing
ca. 0.1 - 0.2 equivalents of l-hydroxy-
benzotriazole; melting point 160 - 180C, dec.
~nalysiS Calc'd- for CllH15N56SPK C~ 31-81;
H, 3.64; N, 16.86; S, 7.72; P, 7.46
Found: C, 30.24; H, 3.71; N, 16.22; S, 7.23; P, 5.6
Example 2
[35-[3~(Z),43]]-2-[[[1-(2-Amino-4-thiazolyl)-2-
r [1- t (hydroxymethylphosphinyl)oxy]-4-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidine]amino]-
oxy]-2-methylpropanoic acid, dipotassium salt
A) Methylphosphonic acid, ~3S-(3,4~)]-3.amino-4-
methyl-2-oxo-1-azetidinyl ester, trifluoroacetic
acid salt
Methylphosphonic acid, [3S-(3~,43)]-3-
[Ul,l-dimethylethoxy)carbonyl]amino}4-methyl-2-
oxo-l-azetidinyl ester, potassium salt (0.223 g,
0.7 mmole; see example lE) was suspended in
0.53 ml of anisole and 0.53 ml of dry dichloro-
methane under nitrogen. Trifluoroacetic acid
(1.0 ml) was added dropwise at 0C, and the
reaction mixture was stirred at 0C to 5C
for 2 hours. This was concentrated in vacuo
to a residue, which was dried by concentration
two times from 30 ml portions of toluene. The
crude reaction product was triturated twice
with ether to give, upon drying, a solid.
~26~3763
-32-
B) [3S-[3~(Z),43]]-2-[[[1-(2-Amino-4-thiazolyl)-
2-[[1-[(hydroxvmethYlphosphinyl)oxy]-4-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidine~amino]-
ox~}2-methylpro ~noic acid, diphenylmethyl ester,
potassium salt
(z)-(2-Amino-4-thiazolyl)[~2-(diphenyl-
methoxy)-l,l-dimethyl-2-oxoethoxy]imino]acetic
acid (0.310 g, 0.7 mmole) and l-hydroxybenzotriazole
(0.108 g, 0.7 mmole) were dissolved in 4 ml of
L0 dry dimethylformamide under nitrogen. This
was cooled to 0C, and N,N'-dicyclohexylcarbodiimide
(0.145 g, 0.7 mmole) was added portionwise.
After addition, the reaction was stirred at
0C for 1 hour. To this was added a solution
lS of methylphosphonic acid, L3S-(3~,4~)]-3-amino-
4-methyl-2-oxo-l-azetidinyl ester, trifluoro-
acetic acid salt (ca. 0.7 mmole) in 2 ml of
dimethylformamide and 0.5 ml of N,N-diisopropyl-
ethylamine at 0C. The reaction was stirred
at 0C for l hour and then at room temperature
overnight. The solution was filtered and the
filtrate was concentrated ln vacuo. The residue
was dissolved in 50 ml of dichloromethane and
washed with 2 ml of water (pH 4.5). The
dichloromethane was concentrated in vacuo to
yield 0.581 g of crude product. This was
purified partially by dissolving in 20 ml of
ethyl acetate and washing with 5 ml portions
of KH2PO4 buffer a~ pH 4.5 (four times). The
GC195
~26~3763
-33-
aqueous washes were lyophilized overnight to
give 0.261 g of a residue which was passed
through 10 ml of Dowex 50 (K~ 0.7 meq/ml)
using water, and lyophilized to give 0.233 g
of crude product contaminated with hydroxy-
benzotriazole.
C) [3S-[3~(Z),4B]]-2-[[[1-(2-Amino-4-thiazolyl)-
2-[[l-[(hydroxYmethylphosphinyl)oxv]-4-methvl-
2-oxo-3-azetidinyl]amino]-2-oxoethylidine]amino]-
oxy]-2-methyl~ropanoic acid, dipotassium salt
[3S-[3~(Z),43]]-2-[[[l-(2-Amino-4-thiazolyl)-
2-[[1-(hydroxymethylphosphinyl)oxy]-2-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidine~aminol-
oxy-2-methylpropanoic acid, diphenylmethyl ester,
potassium salt (0.223 g) was dissolved in 1.8 ml
of dichloromethane, 0.5 ml of anisole, and 1.5 ml
of trifluoroacetic acid, and stirred under
nitrogen at 0C for 2 hours. The reaction
mixture was concentrated in vacuo and evaporated
from toluene twice. The residue was washed with
ether:ethyl acetate (l:l) (three times) to give
the trifluoroacetic acid salt as a white solid.
This was dissolved in 1.5 ml of pH 4.5 0.5 M KH2PO4,
and the pH was adjusted to 6.5 with lN KOH.
This aqueous fraction was chromatographed through
80 ml of HP-20 resin with water to give 94 mg
of desired product, melting point 60-70C.
Analysis Calc'd for C14H18N5O8PS 2K-3.75 H2O:
C, 28.35; H, 4.33; N, 11.80; P, 5.22
Found: C, 28.61; H, 3.76; N, 11.45; P, 4.9
~Z6~763 ~C195
-34-
Exam~le 3
Methylphosphonic acid,[3S-[3~(R),43~]-3-[ f [ ~ (4-
ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino~-
phenyIacetyl]amino]-4-methyl-2-oxo-1-azetidinyl
ester potassium salt
(3S-trans)-3-[[[[(4-Ethyl-2,3-dioxo-1-
piperazinyl)carbonyl]amino~phenylacetyl]amino]-
l-hydroxy-4-methyl-2-azetidinone (0.22 g,
.53 mmol; see U. S. patent 4,337,197) was
suspended in 8 ml of dry dichloromethane at
-10C under nitrogen. 2,6-Lutidine (.07 ml,
.6 mmol) was added followed by the dropwise
addition of methylphosphonic dichloride in 0.5 ml
of dichloromethane. After addition,the
reaction was stirred at -10C for 2 hours. The
temperature was allowed to rise to 0C, 8 ml
of 0.5 M KH2PO4 containing 0.6 ml of 2N KOH
(pH 6.6) was added and the reaction was stirred
at room temperature for 2 hours. The organic
layer was separated and the aqueou~ layer was
lyophilized. The lyophilate was washed
(3 times) with 100 ml portions of dichloromethane.
These washes were concentrated in vacuo,
dissolved in 2 ml of water (pH 4.5) and passed
through 10 ml of Dowex 50 resin (K~, 0.7 meq/ml)
to yield 120 mg of crude product. This was
chromatographed through 50 ml of HP-20 resin
packed in water; product was eluted with 20
acetone:water. After lyophilization, 62 mg
of analytical product was obtained, melting
point 175-180C, dec.
Analysis Calc'd for C20H25N5O8P 2
C, 41.85; H, 5.18; N, 12.20; P, 5.40
Found: C, 42.05; H, 5.01; N, 12.08; P, 5.0
126~3763 ~ J~
-35-
Example 4
Methylphosphonic acid, (S)-2-oxo-3-[(phenylacetyl)-
amino]-l-azetidinyl ester, potassium salt
(S)-N-(l-Hydroxy-2-oxo-3-azetidinyl)-2-
phenylacetamide (0.119 g, 0.55 mmole; see
U. S. patent 4,337,197) was dissolved in 3 ml
of dry dichloromethane and the solution was
cooled to -10C under nitrogen. 2,6-Lutidine
(0.065 ml, 0.56 mmole) was added, followed by
the dropwise addition of a solution of methyl-
phosphonic dichloride in 1 ml of dichloromethane.
After addition, the reaction was stirred at
-10 C for 2 hours. The remaining chloro group
was hydrolyzed at room temperature with 8.ml
of 0.5 M KH2PO4 containing 0.6 ml of lN KOH
(pH 6.0). The solution was stirred vigorously
for 2 hours. The dichloromethane layer was
separated and the aqueous layer was lyophilized.
The lyophilate was washed 3 times with 100 ml
portions of dichloromethane and with 100 ml
of ethanol. These washes were concentrated
in vacuo, combined and dissolved in 2 ml of
water. The pH of this solution was adjusted
to 4.5 with lN KOH from pH 2.5. This material
was passed through 8 ml of Dowex resin (K~,
0.7 meq/ml) to yield 67 mg of crude product.
This was placed on 15 ml of HP-20 resin and
product was eluted with water. After lyophili-
zation, 20 mg of analytical product was obtained,
melting point 135-140C, dec.
Analysis Calc'd for C12H14N2O5PK H2
H, 4.56; N, 7.90; P, 8.73
Found: C, 40.64; H, 4.47; N, 1.89; P, 8.4
~6~3763
-36-
Example 5
~3S-[3a(Z),4s~]-2-[[[1-(2-Amino-4-thiazolyl)-2-
[[l-~(hydroxymethoxyphosphinyl)oxy]-4-methyl-2-
oxo-3-a~etidinyl]amino]-2-oxoethylidine]amino]-
oxy]-2-methylpropanoic acid, dipotassium salt
A) Methylphosphoric acid,[3S-(3~,4~)]-3-U(l,l-
dimethylethoxy)carbonyl]amino}4-methyl-2-oxo-1-
azetidinyl ester, potassium salt
[3S-(3,43)]-3-[[(1,l-Dimethylethoxy)carbonyl]-
amino]-4-methyl-2-oxo-1-hydroxyazetidine
(1.18 g, 5.46 mmole, see example lD) was partially
dissolved in 14 ml of dry dichloromethane and
cooled to -70C under nitrogen. TriethylaMine
(.78 ml, 5.46 mmole) was added followed by the
dropwise addition of methyl phosphonic dichloride
(0.79 g, S.46 mmole) in 6 ml of dichloromethane.
The reaction mixture was stirred for 1.2 hours
while warming from -60 to -30 C. A solution
of 0.5 M KH2PO~ pH 5.5 buffer (55 ml) was added,
and the reaction was stirred vigorously. The
reaction flask was removed from the cooling bath
and the solution was stirred at ambient temperature
for 45 minutes. The pH during this time was
maintained at 3.5 to 4.0 by occasional addition
of 2 N KOH. The aqueous layer was lyophilized.
The lyophilate was washed with three 150 ml
portions of dichloromethane, and the dichloro-
methane was concentrated in vacuo to yield the
crude triethyl ammonium salt (1.8 g). This was
126~3763
dissolved in water (pH 4.2) and passed through
90 ml of Dowex 50 resin (K , 0.7 meq/ml) to
yield 0.87 g of crude material, which was
purified further by chromatography through
100 ml of HP-20 resin packed in water. The
product eluted with 20% acetone-water (170 ml)
to yield, after lyophilization, 0.22 g of
analytically pure material, melting point 143,dec.
B) Methylphosphoric acid, [3s-(3~,43)]-3-amino-
4-methyl-2-oxo-1-azetidinyl ester, trifluoro-
acetic acid salt
Methylphosphoric acid, ~3S-(3~,4B)]-3-
[[(l,l-dimethylethoxy)carbonyl]amino}4-methyl-2-
lS oxo-l-azetidinyl ester, potassium salt (0.20 g,
.57 mmole) was suspended in 0.65 ml of dichloro-
methane and 0.65 ml of anisole. The reaction
mixture was cooled to -10C, and trifluoroacetic
acid (1.3 ml) was added. This was stirred at
0C for 1 hoùr. The solution was concentrated
in vacuo to a residue, which was evaporated
from benzene (twice) to give a viscous oil.
This was triturated with ether to give a white
solid, which was dried ln vacuo.
~z6a~763 '~
-38-
C) ~3S-[3(Z),4B]]-2-[[[l-(2=Amino-4-thiazolyl)-2-
[[l-[(hydroxymethoxy~hosphinyl)oxy]-4-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidine]amino]-
oxy]-2-methylpropanoic acid, diphenylmethyl
ester, potassium salt
(z)-(2-Amino-4-thiazolyl)[[2-diphenylmethoxy)-
1,1-dimethyl-2-oxoethoxy]imino]acetic acid
(.29 g, 0.66 mmole) and l-hydroxybenzotriazole
(0.10 g, 0.66 mmole) were dissolved in 8 ml of
dry dimethylformamide (DMF) nitrogen. This was
cooled to 0C, and N,N-dicyclohexylcarbodiimide
(0.14 g, 0.66 mmole) was added portionwise.
After addition, the reaction was stirred at
0C for 1 hour~ Methylphosphoric acid, L3S-(3,4~)]-
3-amino-4-methyl-2-oxo-1-azetidinyl ester,
trifluoroacetic acid salt (0~57 mmol) in 2 ml
of DMF and 0~5 ml of N,N-diisopropylethylamine
were added to the activated acid side chain,
and the reaction was stirred overnight at room
temperature. The solution was filtered, and the
filtrate was concentrated in vacuo~ The residue
was dissolved in 8 ml of water, and the pH was
adjusted to 4~5 with 1 N KOH~ This solution
was passed through 100 ml of Dowex 50 (K~,
0~7 meq/ml) using water, and lyophilized to
give 0~202 g of crude material contaminated
with hydroxybenzotriazole~
~26~7~;3
--39--
D) f 3s- [ 3 ( z), 4~]]-2-~[[1-(2-Amino-4-thiazolyl)-2-
.
[[l-[(hydroxymethoxyphosphinyl)oxy]-4-methyl-2-
oxo-3-azetidinyl]amino~-2-oxoethylidine]amino]-
oxy]-2-methylpropanoic acid, dipotassium salt
s [3s-[3(Z) ,4B]]-2-[[[1-(2-Amino-4-thiazolyl)-2-
[~1-[(hydroxymethoxyphosphinyl)oxy]-4-methyl-2-
oxo-3-azetidinyl]amino~-2-oxoethylidine]amino]-
oxy]-2-methylpropanoic acid, diphenylmet'nyl ester,
potassium salt was dissolved in 1.8 ml of
dichloromethane, 0.5 ml of anisole, and 1.5 ml
of trifluoroacetic acid, and stirred under N2 at
-10C for 1 hour. The reaction mixture was
concentrated in vacuo, and the residue was
evaporated from benzene (three times). T4e
residue was washed with ether: ethyl acetate
(1:1) and ether:acetonitrile (1:1) to give a
white solid. This material was dissolved in
2 ml of pH 5.5, 0.5 M KH2PO4 and the pH was
adjusted to 6.5 with 1 N KOH. This was
chromatographed through 100 ml of HP-20 resin
with water to give 77 mg of desired product,
melting point 178-185C, dec.
Calc'd- for C14H18N5SPOgK2 2 4H20
H, 3.92; N, 11.96; S, 5.47; P, 5.29
Found: C, 28.72; H, 3.73; N, 11.86;
- S, 5.51; P, 5.0
~Z6~ ~
-40-
Addi~ional embodiments of compounds
following within the scope of this invention
are: -
[ 3S- [ 3a ( Z), 4]~-2-[[[1-(2-amino-4-thiazolyl)-
2-[[1-[(hydroxymethylphosphinyl)oxy]-4-
methyl-2-oxo-3-azetidinyl~amino~-2-
oxoethylidene]amino]oxy]-2-methylpropanoic
acid, dipotassilLm salt
[3S-~3a(Z),4]~-2-~[1-(2-amino-4-thiazolyl)-
2-[[1-(hydroxymethoxyphosphinyl)oxy]-4-
methyl-2-oxo-3-azetidinyl]amino]-2-
oxoethylidene]amino]oxy]-2-methylpropanoic
acld, dipotassium salt
methylphosphonic acid, [3S-[3~(Z),4B]]-3-
[[(2-aminothiazolyl)(2,2,2-trifluoroethoxy-
imino)acetyl]amino]-4-methyl-2-oxo-1-
azetidinyl ester
[3S-~3~(Z),4B]]-[[[1-(2-amino-4-thiazolyl)-2-
~[l-[(hydroxymethylphosphinyl)oxy]-4-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidene]-
amino]oxy]acetic acid
methylphosphonic acid,[3S-[3~(Z),4B]]-3-
[[(2-amino-4-thiazolyl)[(2-amino-2-oxoethoxy)-
imino]acetyl]amino]-4-methyl-2-oxo-1-
azetidinyl ester
~ 26~3763
-41-
methylphosphonic acid,[3S-(3~,4B)]-3-
~[(S)-~-[(aminocarbonyl)amino]-2-
thiopheneacetyl]amino]-4-methyl-2-oxo-1-
azetidinyl ester
s
methylphosphonic acid,[3S-(3~,4B)]-3-
[(aminophenyacetyl)amino]-4-methyl-2-
oxo-l-azetidinyl ester
methylphosphonic acid,[2S-(2~,3B)]-3-
[[(phenylsulfo)acetyl]amino]-2-methyl-4-
oxo-l-azetidinyl ester
methylphosphonic acid,[3S-[3(Z),4~]]-3-
[[(2-amino-4-thiazolyl)(methoxyimino)-
acetyl]amino]-4-methyl-2-oxo-1-azetidinyl ester
[3S-[3(Z),4]]-[t[1-(2-amino-4-thiazolyl)-
2-~[1-[(hydroxymethylphosphinyl)oxy]-4-
methyl-2-oxo-3-azetidinyl]amino]-2-
oxoethylidene]amino]oxy]acetic acid
methylphosphoric acid,[3S-[3~(Z), 4a ] ] -3-
[[(2-amino-4-thiazolyl)(methoxyimino)-
acetyl]amino]-4-methyl-2-oxo-1-azetidinyl ester
methylphosphoric acid,[3S-~3~(Z) ,4~] ]-3-
[~(2-amino-4-thiazolyl)~(2-amino-2-
oxoethoxy)imino]acetyl]amino]-4-methyl-2-
oxo-l-azetidinyl ester
~z~763 ~C195
-42-
[35-[3(z),4g]]-2-[[[1-(2-amino-4-~hiazolyl)-2-[[1-
[(hydroxyphenylphosphinyl)cxy]-4-me~hyl-2-oxo-3-
azetidinyl]amino]-2-oxoethylidene]amino]oxy-2-
me~hylp!opanoic acid
[3S-[3~(Z),4~]]-2-[~[1-(2-amino-4-~hiazolyl)-2-[[1-
[[hydroxy(4-methoxyphenyl)phosphinyl]oxy]-4-methyl-2-
oxo-3-azecidinyl]amino]-2-oxoethylidene]amino]oxy-2-
methylpropanoic acid
[3S-[3~(Z),4~]]-2-~[[1-(2-amino-4-thiazolyl)-2-[[1-
[[hydroxy(4-dimethylaminophenyl)phosphinyl]oxy]-4-
methyl-2-oxo-3-aze~idinyl]amino]-2-oxoethylidene]-
amino]oxy-2-methylpropanoic acid
[3S-[3~(Z),4~]]-2-~[[1-(2-amino-4-thiazolyl)-2-
~[[hydroxy(phenylmethyl)phosphinyl]oxy]-4-methyl-
2-oxo-3-azetidinyl]amino]-2-oxoe~hylidene]amino]oxy-
2-methylpropanoic acid
[35-[3(Z),4~]~-2-[[[1-(2-amino-4-thiazolyl)-2-[[1-
[[[(azidomethyl)hydroxy]phosphinyl]oxy]-4-methyl-
2-oxo-3-aze~idinyl]amino]-2-oxoe~hylidene]amino]oxy-
2-methylpropanoic acid
[3S-[3(z),4~]]-2-[[[1-(2-amino-4-~hiazolyl)-2-[[1-
[[hydroxy(methoxymethyl)phosphinyl]cxy]-4-methyl-
2-Gxo-3-azetidinyl]aminG]-2-oxoethylidene]amino]ox~-
2-methylpropanoic acid
l~Z68763
Gclgs
-43-
[3S-[3~(Z),4B]]-2-[[[1-(2-amino-4-thiazolyl)-2-[[1-
[(hydroxyethylphosphinyl)oxy]-4-methyl-2-oxo-3-
aze~idinyl]amino]-2-oxoethylidene]aminojoxy-2-me~hyl-
propanoic acid
[3s-[3 a( Z ) ,4B]]-2-[[[1-(2-amino-4-thiazolyl) 2-[[1-
[[hydroxy(2-propenyl)phosphinyl]oxy]-4-methyl-2-oxo-3-
azetidir.yl]amino]-2-oxoethylidene]aminG]oxy-2-r,le~hyl-
propanoic acid
[3S-[3~(Z),4g]]-2-[[[1-(2-amino-4-~hiazolyl)-2-[[1-
[(hydroxyphenoxyphosphinyl)oxy]-4-me~hyl-2-oxo-3-
azetidinil]amino]-2-oxoethylidene]amino]oxy-2-me~hyl-
propanoic acid
~3S-[3(Z),4g]~-2-~[1-(2-amino-4-thiazolyl)-2-[[1-
~hydroxy(4-methylphenoxy)phosphinyl]oxy]-4-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidene]amino,oxy-2-
methylpropanoic acid
[3S-[3(2),4B]]-2-[~[1-(2-amino-4-thiazolyl)-2-[[1-
[[hydroxy(phenylmethoxy)phosphinyl]oxy]-4-methy1-2-
oxo-3-azetidinyl]ar,lino]-2-Gxoethylidene]amino]oxy-2-
methylpropanoic acid
[3S-[3~(Z),4B]]-2-~[[1-(2-amino-4-thiazolyl)-2-[[1-
[(hydroxyethoxy)phosphinyl]oxy]-4-methyl-2-oxo-3-
azetidinyl]amino]-2-oxoethylidene]amino]oxy-2-
methylpropanoic acid
[3S-[3~(Z),4B]]-2-[[[1-(2-amino-4-~hiazolyl)-2-[[1-
[[(2-fluoroethoxy)hydroxyphosphinyl]oxy]-4-me~hyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidene~amino]oxy-2-
me~hylpcopanoic acid
6 3 GC195
_4~
[3S-[3(2),4~]]-2-[[[1-(2-amino-4-thiazolyl)-2-[[1-
[[hydroxy(methylchio)phosphinyl]oxy]-4-me~hyl-2-
oxo-3-aze~idinyl]amino]-2-oxoethylidene]amino]oxy-2-
me~hylpcopanoic acid
[3S-[3~(2),4~]]-2-[[[1-(2-amino-4-~hiazolyl)-2-[[1-
~[(hydroxyme~hyl)~hiophosphinyl]oxy]-4-me~hyl-2-
oxo-3-aze~idinyl]amino]-2-oxoe~hylidene]aminG~ox~-2-
methylpropanoic acid
[3S-[3(Z),4B]]-2-[[[1-(2-amino-4-thiazolyl)-2-[[1-
[[(hydroxyphenyl)thiophosphinyl]oxy]-4-methyl-2-
oxo-~-azetidinyl]amino]-2-oxoe~hylidene]aminG]oxy-2-
methylpropanoic acid
[3S-[3 a( Z ) ,4B]]-2-[[[l-~2-amino-4-thiazolyl)-2-[[
[[(hydroxymethoxy)chiophosphinyl~oxy]-4-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy-2-
methylpropanoic acid
[3s-[3~(z)~4B]]-2-[[[l-(2-amino-4-thiaol~l)-2-[[
[[(hydroxyphenoxy)thiophosphinyl]oxy]-4-methyl-2-
oxo-3-azetidinyl]amino]-2-oxoe~hylidene]amino]oxy-2-
me~hylpropanoic acid