Note: Descriptions are shown in the official language in which they were submitted.
CA 02240738 2012-08-01
30041-356
- 1 -
Pesticidal Composition Containing Thiamethoxam and Fungicides
The present invention relates to a composition that
comprises one or more insecticidal or acaricidal compounds and
one or more fungicidal compounds and that is suitable for
controlling simultaneously insects and/or representatives of
the order Acarina and microorganisms, especially
phytopathogenic fungi, for example on plants, to a method of
controlling those pests, to a process for the preparation of
the appropriate composition and to the use thereof.
According to one aspect of the present invention,
there is provided a composition for controlling insects or
representatives of the order Acarina and phytopathogenic fungi,
which composition comprises a compound of formula Ia
N~NO2
S N"k N,- C H3
C
0 Ia
at least one adjuvant and fludioxonil, wherein the compound of
formula Ia and the fludioxonil are present in a pesticidally
synergistic amount.
According to another aspect of the present invention,
there is provided a method of controlling pests and
microorganisms, which comprises applying a composition
CA 02240738 2007-02-14
30584-117
- la -
described herein to the pests and microorganisms or to the
habitat thereof, wherein the pests are insects or
representatives of the order Acarina and the microorganisms
are phytopathogenic fungi.
According to yet another aspect of the present
invention, there is provided a method of controlling insects
or representatives of the order Acarina and phytopathogenic
fungi in plant propagation material which comprises treating
the plant propagation material or a site where the plant
propagation material has been planted or is to be planted
with the composition described herein.
Certain mixtures of active ingredients for
controlling pests are described in the literature. The
biological properties of those known mixtures are not
entirely satisfactory in the area of pest control and there
is therefore a need to make available other mixtures,
especially those having synergistic properties, for example
synergistic pesticidal properties, especially for
controlling insects and representatives of the order Acarina
and microorganisms. That problem is solved according to the
invention by the provision of the present composition.
The invention accordingly relates to a composition
for controlling insects or representatives of the order
Acarina and microorganisms, which composition comprises at
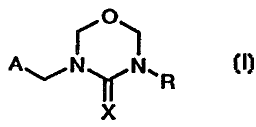
least one compound of formula
ro~
ANyN~'R
x (I) ,
wherein
CA 02240738 2007-02-14
30584-117
- lb -
A is an unsubstituted or mono- to tetra-
substituted aromatic or non-aromatic, monocyclic or
bicyclic, heterocyclic radical, one or two of the
substituents of A being selected from the group consisting
of: halo-C1-C3-alkyl, cyclopropyl, halocyclopropyl,
C2-C3alkenyl, C2-C3alkynyl, halo-C2-C3alkenyl,
halo-C2-C3alkynyl, halo-C1-C3alkoxy, C1-C3alkylthio,
halo-C1-C3alkylthio, allyloxy, propargyloxy, allylthio,
propargylthio, haloallyloxy, haloallylthio, cyano and nitro,
and from one to four of the substituents of A being selected
from the group consisting of: C1-C3alkyl, C1-C3alkoxy and
halogen;
R is hydrogen, C1-C6alkyl, phenyl-Cl-C4alkyl,
C3-C6cycloalkyl, C2-C6alkenyl or C2-C6alkynyl; and
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-2-
X is N-NO2 or N-CN,
or, where appropriate, a tautomer thereof, in each case in free from or in
salt form,
at least one microbicidal compound, and at least one adjuvant, and, where
appropriate, at
least one further insecticidal compound.
Preference is given especially to a composition that comprises the compound of
formula
ClS 0
N~NYN`CH3 (la),
~N,N.:0
I-
0
and one or more compounds selected from the substance classes consisting of
azoles,
cyanopyrroles and acylalanines.
Preference is given likewise to compositions that comprise in addition to at
least one
compound of formula (I) at least one further compound selected from the active
ingredient
class of the pyrethroids, and to compositions that comprise one or more
compounds
selected from hymexazole, triazoxide, acylalanines, anilinopyrimidines, azoles
and cyano-
pyrroles.
There are understood by cyanopyrroles, for example, compounds of formula
Ri
CN
(II),
N
I
R3
wherein R, and R2 are each independently of the other hydrogen, fluorine,
chlorine,
bromine or trifluoromethyl, or two radicals R, and R2 in the ortho-position
relative to one
another are -O-CF2-O- , and R3 is hydrogen or a group -C(=O)-C,-C4alkyl;
by acylalanines, for example, compounds of formula
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-3-
R4 R5
ON/\CH3
CH3 CH3 (Ill),
wherein R4 and R5 are organic radicals; R4 is preferably C1-C4alkoxy-C1-
C4alkyl, phenyl,
benzyl or furyl and R5 is preferably -C(=O)-C1-C4alkyl;
by azoles, for example, compounds of formula
R8
i
N~N'O ,
N R R (IV),
R6 7
wherein R6 and R7 are each independently of the other hydrogen, hydroxy,
cyano, C1-C4-
alkyl, C1-C4alkoxy, C2-C4alkenyloxy, C1-C4alkoxy-Cl-C4alkyl, halo-C1-C4alkoxy-
C1-C4alkyl,
4-chlorophenyl-substituted C1-C4alkyl, cyclopropyl-C1-C4alkyl, phenyl that is
unsubstituted or
mono- or di-substituted by halogen, or R6 and R7, together with the carbon
atom to which
they are bonded, form a three- to six-membered ring containing one or two
hetero atoms,
preferably nitrogen or oxygen, especially a 5-membered ring containing two
oxygen atoms,
that is unsubstituted or mono- or poly-substituted by halogen, C1-C4alkyl,
halo-C1-C4alkyl,
C1-C4alkoxy or by halo-C1-C4alkyl,
R8 and R9 are each independently of the other hydrogen, fluorine, chlorine,
bromine, cyano,
nitro or 4-chlorophenoxy,
Q is [C(R1o)2]n,
the two radicals Rio are each independently of the other hydrogen, phenoxy or
4-chloro-
phenoxy and
n is 1 or 2,
and bitertanol, diniconazole, epoxiconazole (BAS 480F), fluquiconazole,
flusilazole, imazalil,
imibenconazole, ipconazole, metconazole, pefurazoate, prochloraz, SSF-109,
tebuconazole, triticonazole, triadimefon, triadimenol, triflumizole and
uniconazole; and,
by anilinopyrimidines, compounds of formula
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-4-
NH N Rõ
R,3
N (V)
R12
wherein Rõ and R12 are each independently of the other hydroxy, halogen,
cyano, C,-C8-
alkyl, C3-C6cycloalkyl, C3-C6cycloalkenyl, C2-C8alkenyl, C2-Cealkynyl, phenyl-
substituted
C1-C4alkyl, cyclopropyl-C1-C4alkyl, C1-C4alkoxy or C2-C4alkenyloxy; and
R13 is hydrogen, cyano, halogen, Ct-C8alkyl, halo-C1-C8alkyl, C,-C8alkoxy,
halo-C1-C8alkoxy,
C3-C6cycloalkyl or C2-C8alkenyl.
Pyrethroids are to be understood to be, preferably, those listed in The
Pesticide Manual,
10'h Ed. (1994), The British Crop Protection Council, London. There may be
mentioned by
way of example: Tefluthrin, mentioned on page 953 of the said Pesticide
Manual, Cyper-
methrin, mentioned on page 259, beta-Cyfluthrin, mentioned on page 250,
Deltamethrin,
mentioned on page 287 and tau-Fluvalinate, mentioned on page 515. Tefluthrin
is
preferred.
Some of the compounds of formula (I) may be in the form of tautomers. When,
for example,
R is hydrogen, corresponding compounds of formula (1), i.e. those having a 3-H-
4-imino-
perhydro-1,3,5-oxadiazine partial structure, are in equilibrium with the
respective tautomers
having a 4-amino-1,2,5,6-tetrahydro-1,3,5-oxadiazine partial structure.
Accordingly, herein-
before and hereinafter, where appropriate the compounds of formula (I) are to
be
understood to include corresponding tautomers, even if the latter are not
specifically
mentioned in each case.
Compounds of formula (I) having at least one basic centre are capable, for
example, of
forming acid addition salts. Those salts are formed, for example, with strong
inorganic
acids, such as mineral acids, for example perchloric acid, sulfuric acid,
nitric acid, nitrous
acid, a phosphoric acid or a hydrohalic acid, with strong organic carboxylic
acids, such as
unsubstituted or substituted, for example halo-substituted, C1-
C4alkanecarboxylic acids, for
example acetic acid, saturated or unsaturated dicarboxylic acids, for example
oxalic,
malonic, succinic, malefic, fumaric or phthalic acid, hydroxycarboxylic acids,
for example
ascorbic, lactic, malic, tartaric or citric acid, or benzoic acid, or with
organic sulfonic acids,
such as unsubstituted or substituted, for example halo-substituted, C,-
C4alkane- or aryl-
sulfonic acids, for example methane- or p-toluene-sulfonic acid. Furthermore,
compounds of
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-5-
formula (I) having at least one acid group are capable of forming salts with
bases. Suitable
salts with bases are, for example, metal salts, such as alkali metal or
alkaline earth metal
salts, for example sodium, potassium or magnesium salts, or salts with ammonia
or an
organic amine, such as morpholine, piperidine, pyrrolidine, a mono-, di- or
tri-lower alkyl-
amine, for example ethyl-, diethyl-, triethyl- or dimethyl-propyi-amine, or a
mono-, di- or tri-
hydroxy-lower alkylamine, for example mono-, di- or tri-ethanolamine. In
addition, corres-
ponding internal salts may also be formed. Preference is given within the
scope of the
invention to agrochemically advantageous salts. In view of the close
relationship between
the compounds of formula (I) in free form and in the form of their salts, any
reference
hereinbefore or hereinafter to the free compounds of formula (1) or to their
salts Is to be
understood as including also the corresponding salts or the free compounds of
formula (I),
where appropriate and expedient. The same applies in the case of tautomers of
compounds
of formula (l) and the salts thereof. The free form is generally preferred in
each case.
Unless otherwise defined, the general terms used hereinbefore and hereinafter
have the
following meanings:
Suitable as heteroatoms in the basic ring structure of the heterocyclic
radical A of
compounds of formula (1) are any elements of the Periodic Table that are
capable of
forming at least two covalent bonds.
Halogen - as a group perse and as a structural element of other groups and
compounds,
such as haloalkyl, haloalkylthio, haloalkoxy, halocyclopropyl, haloalkenyl,
haloalkynyl, halo-
allyloxy and haloallylthio, - is fluorine, chlorine, bromine or iodine,
especially fluorine,
chlorine or bromine, more especially fluorine or chlorine, especially
chlorine.
Unless otherwise defined, carbon-containing groups and compounds each contain
from 1
up to and including 6, preferably from 1 up to and including 3, especially 1
or 2, carbon
atoms.
Cycloalkyl is cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl, especially
cyclopropyl. Cyclo-
alkenyl is cyclopropenyl, cyclobutenyl, cyclopentenyl or cyclohexenyl,
especially cyclo-
propenyl.
Alkyl - as a group per se and as a structural element of other groups and
compounds, such
as phenylalkyl, haloalkyl, alkoxy, haloalkoxy, alkylthio and haloatkylthio, -
is, in each case
taking due account of the number of carbon atoms contained in the group or
compound in
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-6-
question, either straight-chained, i.e. methyl, ethyl, propyl, butyl, pentyl
or hexyl, or
branched, e.g. isopropyl, isobutyl, sec-butyl, tert-butyl, isopentyl,
neopentyl or isohexyl.
Alkenyl, haloalkenyl, alkenyloxy, alkynyl and haloalkynyl are straight-chained
or branched
and each contain two or, preferably, one unsaturated carbon-carbon bond(s).
The double or
triple bonds of those substituents are separated from the remainder of the
compound I
preferably by at least one saturated carbon atom. There may be mentioned by
way of
example allyl, methallyl, but-2-enyl, but-3-enyl, propargyl, but-2-ynyl and
but-3-ynyl.
Halo-substituted carbon-containing groups and compounds, such as haloalkyl,
haloalkylthio,
haloalkoxy, halocyclopropyl, haloalkenyl, haloalkynyl, haloallyloxy and
haloallylthio, may be
partially halogenated or perhalogenated, the halogen substituents in the case
of multiple
halogenation being identical or different. Examples of haloalkyl - as a group
per se and as a
structural element of other groups and compounds, such as haloalkylthio and
haloalkoxy, -
are methyl mono- to tri-substituted by fluorine, chlorine and/or by bromine,
such as CHF2 or
CF3; ethyl mono- to penta-substituted by fluorine, chlorine and/or by bromine,
such as
CH2CF3, CF2CF3, CF2CCI3, CF2CHC12i CF2CHF2, CF2CFCI2, CF2CHBr2, CF2CHCIF,
CF2CHBrF or CCIFCHCIF; propyl or isopropyl mono- to hepta-substituted by
fluorine,
chlorine and/or by bromine, such as CH2CHBrCH2Br, CF2CHFCF3, CH2CF2CF3,
CF2CF2CF3
or CH(CF3)2; and butyl, or one of the isomers of butyl, mono- to nona-
substituted by
fluorine, chlorine and/or by bromine, such as CF(CF3)CHFCF3i CF2(CF2)2CF3 or
CH2(CF2)2CF3. Examples of haloalkenyl are 2,2-difluoroethen-1 -yl, 2,2-
dichloroethen-1 -yl, 2-
chloroprop-1 -en-3-yl, 2,3-dichloroprop-l-en-3-yi and 2,3-dibromoprop-l-en-3-
yl. Examples
of haloalkynyl are 2-chioroprop-1-yn-3-yl, 2,3-dichloroprop-1-yn-3-yl and 2,3-
dibromoprop-1-
yn-3-yl. Examples of halocyclopropyl are 2-chlorocyclopropyl, 2,2-
difluorocyclopropyl and 2-
chloro-2-fiuoro-cyclopropyl. Examples of haloallyloxy are 2-chioroprop-l-en-3-
yloxy, 2,3-
dichloroprop-l-en-3-yloxy and 2,3-dibromoprop-1 -en-3-yloxy. Examples of
haloallylthio are
2-chloroprop-1 -en-3-ylthio, 2,3-dichloroprop-1 -en-3-ylthio and 2,3-
dibromoprop-1-en-3-
ylthio.
In phenylalkyl, an alkyl group bonded to the remainder of the compound in
question is
substituted by a phenyl group, the alkyl group preferably being straight-
chained and the =
phenyl group preferably being bonded in a position higher than the a-position,
especially in
the w -position, of the alkyl group; examples are benzyl, 2-phenylethyl and 4-
phenylbutyl.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-7-
Preferred forms within the scope of the invention are compositions that
comprise a
compound of formula (I) wherein:
= (1) A is an unsubstituted or mono- to tetra-substituted, aromatic or non-
aromatic,
monocyclic or bicyclic, heterocyclic radical, one or two of the substituents
of A being
selected from the group consisting of: halo-Cl-C3alkyl, cyclopropyl,
halocyclopropyl, C2-
C3alkenyl, C2-C3alkynyl, halo-C2-C3alkenyl, halo-C2-C3alkynyl, halo-Cl-
C3alkoxy, C1-
C3alkylthio, halo-C1-C3alkylthio, allyloxy, propargyloxy, allylthio,
propargylthio, haloallyloxy,
haloallylthio, cyano and nitro, and from one to four of the substituents of A
being selected
from the group consisting of: C1-C3aikyl, C1-C3alkoxy and halogen;
R is hydrogen, C1-C6alkyl, C3-C6cycioalkyl, C2-C6alkenyl or C2-C6alkynyl; and
X is N-NO2 or N-ON;
(2) the basic ring structure of A consists of a ring having 5 or 6 ring
members to which a
further ring having 5 or 6 ring members may be fused,
especially a ring having 5 or, preferably, 6 ring members;
(3) the basic ring structure of A is unsaturated,
more especially contains one double bond or, preferably, from 2 to 4 double
bonds, which
are preferably conjugated,
preferably 2 double bonds, which are preferably conjugated,
and is especially aromatic;
(4) the basic ring structure of A contains from 1 up to and including 4,
especially from 1 up
to and including 3, more especially 1 or 2, hetero atoms, preferably 1 hetero
atom;
(5) the basic ring structure of A contains 1, 2 or 3 hetero atoms selected
from the group
consisting of oxygen, sulfur and nitrogen, not more than one of the hetero
atoms present in
the basic ring structure being an oxygen atom and not more than one of the
hetero atoms
present in the basic ring structure being a sulfur atom,
especially 1, 2 or 3 hetero atoms selected from the group consisting of
oxygen, sulfur and
nitrogen, not more than one of the hetero atoms present in the basic ring
structure being an
oxygen or a sulfur atom,
preferably at least one. nitrogen atom;
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-8-
(6) A is bonded to the remainder of the compound I via a carbon atom of its
basic ring
structure;
(7) A is unsubstituted or mono- or di-substituted by substituents selected
from the group
consisting of halogen, C,-C3alkyl, halo-C,-C3alkyl, C,-C3alkoxy and halo-C,-
C3alkoxy,
preferably unsubstituted or mono- or di-substituted by substituents selected
from the group
consisting of halogen and C,-C3alkyl;
(8) the basic ring structure of A is a pyridyl, 1-oxidopyridinio or thiazolyl
group,
the basic ring structure of A is preferably a pyrid-3-yl, 1-oxido-3-pyridinio
or thiazol-5-yl
group,
A is especially a pyrid-3-yl, 2-halopyrid-5-yl, 2,3-dihalopyrid-5-yi, 2-C,-
C3alkylpyrid-5-yl,
1 -oxido-3-pyridinio, 2-halo-1 -oxido-5-pyridinio, 2,3-dihalo-1-oxido-5-
pyridinio or 2-halo-
thiazol-5-yl group,
A is more especially a pyrid-3-yl, 2-halopyrid-5-yl, 2-halo-1 -oxido-5-
pyridinio or 2-
halothiazol-5-yl group,
A is preferably a 2-chloropyrid-5-yi, 2-methylpyrid-5-yi, 1-oxido-3-pyridinio,
2-chloro-1 -oxido-
5-pyridinio, 2,3-dichloro-1-oxido-5-pyridinio or 2-chlorothiazol-5-yi group,
A is more especially a pyrid-3-yl, 2-chloropyrid-5-yi, 2-chloro-1-oxido-5-
pyridinio or 2-
chlorothiazol-5-yl group,
A is especially a 2-chloropyrid-5-yl group or preferably a 2-chlorothiazol-5-
yl group;
(9) R is C,-C6alkyl, phenyl-C,-C4alkyl, C3-C6cycloalkyl, C3-C4alkenyl or C3-
C4alkynyl,
especially C,-C6alkyl, C3-C6cycloalkyl, C3-C4alkenyl or C3-C4alkynyl,
preferably C,-C6alkyl, phenyl-C,-C4alkyl, C3-C4alkenyl or C3-C4alkynyl,
especially C,-C4alkyl, preferably methyl;
(10) X is N-NO2;
(11) A is a pyridyl, 1-oxidopyridinio or thiazolyl group that is unsubstituted
or mono- or di-
substituted by substituents selected from the group consisting of halogen and
C,-C3alkyl
and is bonded to the remainder of compound I via a carbon atom of its basic
ring structure;
R Is C,-C6alkyl, phenyl-C,-C4alkyl, C3-C6cycloalkyl, C3-C4alkenyl or C3-
C4alkynyl and
X is N-NO2 or N-CN;
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-9-
(12) A is a 2-chloropyrid-5-yl, 2-methylpyrid-5-yl, 1-oxido-3-pyridinio, 2-
chloro-1-oxido-5-
pyridinio, 2,3-dichloro-1 -oxido-5-pyridinio or 2-chlorothiazol-5-yi group,
R is C1-C4alkyl and
X is N-NO2; and
(13) A is a 2-chlorothiazol-5-yi or 2-chloropyrid-5-yl group,
R is C1-C4alkyl and
X is N-N02.
Among the compounds of formula (I), within the scope of the invention
preference is given
specifically to
(a) 5-(2-chloropyrid-5-ylmethyl)-3-methyl-4-nitroimino-perhydro-1,3,5-
oxadiazine,
(b) 5-(2-chlorothiazol-5-ylmethyl)-3-methyl-4-nitroimino-perhydro-1,3,5-
oxadiazine,
(c) 3-methyl-4-nitroimino-5-(1-oxido-3-pyridiniomethyl)-perhydro-1,3,5-
oxadiazine,
(d) 5-(2-chloro-1 -oxido-5-pyridiniomethyl)-3-methyl-4-nitroimino-perhydro-
1,3,5-oxadiazine
and
(e) 3-methyl-5-(2-methylpyrid-5-ylmethyl)-4-nitroimino-perhydro-1,3,5-
oxadiazine.
The invention relates preferably to a composition that comprises a compound of
formula (I)
and either one or two of the following compounds:
cyproconazole, cyprodinil, difenoconazole, epoxiconazole, fenpiclonil,
fludioxonil, flutriafol,
furalaxyl, hymexazol, imazalil, metalaxyl (enantiomeric mixture), R-metalaxyl
(D-form),
penconazole, propiconazole, tebuconazole, tefluthrin, triazoxide or
triticonazole.
Preference is given especially to a composition that comprises in addition to
the compound
of formula (I) only one microbicidal compound,
especially to a composition that comprises a compound of formula (I) and
fludioxonil, a
compound of formula (1) and difenoconazol or a compound of formula (1) and R-
metalaxyl;
above all to a composition that comprises the compound formula (la) and
fludioxonil, the
compound formula (la) and difenoconazol or the compound formula (la) and R-
metalaxyl.
The compounds of formula (I) are known from EP-A-580 553.
Bitertanol is known from The Pesticide Manual, 10tt' Ed. (1994), The British
Crop Protection
Council, London, page 106;
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-10-
cyproconazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 268;
cyprodinil (CGA 219417) is known from The Pesticide Manual, 10th Ed. (1994),
The British
Crop Protection Council, London, page 161;
difenoconazole is known from The Pesticide Manual, 10th Ed. (1994), The
British Crop
Protection Council, London, page 328;
diniconazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 356;
epoxiconazole (BAS 480F) is known from The Pesticide Manual, 10"' Ed. (1994),
The British
Crop Protection Council, London, page 67;
fenpiclonil is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 444;
fludioxonil is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 482;
fluquiconazole is known from The Pesticide Manual, 10th Ed. (1994), The
British Crop
Protection Council, London, page 498;
flusilazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 510;
flutriafol is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 514;
furalaxyl is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 534;
hymexazole is The Pesticide Manual, 10th Ed. (1994), The British Crop
Protection Council,
London, page 576;
Imazalil is known from The Pesticide Manual, 10th Ed. (1994), The British Crop
Protection
Council, London, page 580;
imibenconazole is known from The Pesticide Manual, 10th Ed. (1994), The
British Crop
Protection Council, London, page 590;
ipconazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 600;
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-11-
metalaxyl is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 660; R-metalaxyl (D-form) is known from DE-P-25 15 091;
metconazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 669;
pefurazoate is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 774;
penconazole is known from The Pesticide Manual, loth Ed. (1994), The British
Crop
Protection Council, London, page 776;
prochloraz is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 832;
propiconazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 855;
SSF-1 09 is known from The Pesticide Manual, 10th Ed. (1994), The British Crop
Protection
Council, London, page 919;
tebuconazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 942;
triazoxide is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop Protection
Council, London, page 1009;
triadimefon is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 1000;
triadimenol is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 1001;
trifiumizole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 1022;
triticonazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 1033; and
uniconazole is known from The Pesticide Manual, 10th Ed. (1994), The British
Crop
Protection Council, London, page 1034.
It has now been found, surprisingly, that the compositions according to the
invention not
only bring about the additive enhancement of the biocidal and physical
properties of the
individual active ingredients they contain that was in principle to be
expected, but achieve a
CA 02240738 1998-06-17
WO 97/22254 PCT/PP96/05489
-12-
synergistic effect which, inter alia, extends the boundaries of the pesticidal
activity of the
compounds.
In particular, it has now been found, surprisingly, that, for example, the
pesticidal activity of
the compositions according to the invention, compared with the pesticidal
activity of the
individual components, is not merely additive, as may essentially be expected,
but that a
synergistic effect exists. The term "synergistic" is not, however, in any way
limited in this
context to the pesticidal activity, but refers equally to other advantageous
properties of the
compositions according to the invention as compared with the individual
components.
Examples of such advantageous properties that may be mentioned are: a
broadening of the
spectrum of pesticidal activity to other pests, for example to resistant
strains, a reduction in
the rate of application of the compounds of the formulae, adequate control of
the pests with
the aid of the compositions according to the invention, even at a rate of
application at which
the individual compounds are totally ineffective; advantageous behaviour
during formulating
and/or upon application, for example upon grinding, sieving, emulsifying,
dissolving or
dispersing; increased storage stability; improved stability to light; more
advantageous
degradability; improved toxicological and/or ecotoxicological behaviour, or
other advantages
familiar to a person skilled in the art.
In the area of pest control, the compositions according to the invention are
valuable preven-
tive and/or curative active ingredients having a very advantageous biocidal
spectrum even
at low rates of concentration, while being well tolerated by warm-blooded
animals, fish and
plants. The compositions of the invention are effective against all or
individual development
stages of normally sensitive animal pests, but also of resistant animal pests,
such as insects
and representatives of the order Acarina, and phytopathogenic fungi. The
insecticidal
and/or acaricidal action of the compositions of the invention may manifest
itself directly, i.e.
in the mortality of the pests, which occurs immediately or only after some
time, for example
during moulting, or indirectly, for example in reduced oviposition and/or a
reduced hatching
rate, the good activity corresponding to a mortality of at least 50 to 60 %.
The mentioned animal pests include, for example:
of the order Lepidoptera, for example,
Acleris spp., Adoxophyes spp., Aegeria spp., Agrotis spp., Alabama
argillaceae, Amylois
spp., Anticarsia gemmatalis, Archips spp., Argyrotaenia spp., Autographa spp.,
Busseola
fusca, Cadra cautella, Carposina nipponensis, Chilo spp., Choristoneura spp.,
Clysia
ambiguella, Cnaphalocrocis spp., Cnephasia spp., Cochylis spp., Coleophora
spp.,
Crocidolomia binotalis, Cryptophlebia leucotreta, Cydia spp., Diatraea spp.,
Diparopsis
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-13-
castanea, Earias spp., Ephestia spp., Eucosma spp., Eupoecilia ambiguella,
Euproctis spp.,
Euxoa spp., Grapholita spp., Hedya nubiferana, Heliothis spp., Hellula
undalis, Hyphantria
cunea, Keiferia lycopersicella, Leucoptera scitella, Lithocollethis spp.,
Lobesia botrana,
Lymantria spp., Lyonetia spp., Malacosoma spp., Mamestra brassicae, Manduca
sexta,
Operophtera spp., Ostrinia nubilalis, Pammene spp., Pandemis spp., Panolis
flammea,
Pectinophora gossypiella, Phthorimaea operculella, Pieris rapae, Pieris spp.,
Plutella
xylostella, Prays spp., Scirpophaga spp., Sesamia spp., Sparganothis spp.,
Spodoptera
spp., Synanthedon spp., Thaumetopoea spp., Tortrix spp., Trichoplusia ni and
Yponomeuta
spp=;
of the order Coleoptera, for example,
Agriotes spp., Anthonomus spp., Atomaria linearis, Chaetocnema tibialis,
Cosmopolites
spp., Curculio spp., Dermestes spp., Diabrotica spp., Epilachna spp., Eremnus
spp.,
Leptinotarsa decemlineata, Lissorhoptrus spp., Melolontha spp., Orycaephilus
spp.,
Otiorhynchus spp., Phlyctinus spp., Popillia spp., Psylliodes spp.,
Rhizopertha spp.,
Scarabeidae, Sitophilus spp., Sitotroga spp., Tenebrio spp., Tribolium spp.
and Trogoderma
spp.;
of the order Orthoptera, for example,
Blatta spp., Blattella spp., Gryllotalpa spp., Leucophaea maderae, Locusta
spp., Periplaneta
spp. and Schistocerca spp.;
of the order Isoptera, for example,
Reticulitermes spp.;
of the order Psocoptera, for example,
Liposcelis spp.;
of the order Anoplura, for example,
Haematopinus spp., Linognathus spp., Pediculus spp., Pemphigus spp. and
Phylloxera
spp=;
of the order Maltophaga, for example,
Damalinea spp. and Trichodectes spp.;
of the order Thysanoptera, for example,
Franklinietta spp., Hercinothrips spp., Taeniothrips spp., Thrips palmi,
Thrips tabaci and
Scirtothrips aurantii;
CA 02240738 1998-06-17
WO 97/22254 PCP/EP96/05489
-14-
of the order Heteroptera, for example,
Cimex spp., Distantiella theobroma, Dysdercus spp., Euchistus spp., Eurygaster
spp.,
Leptocorisa spp., Nezara spp., Piesma spp., Rhodnius spp., Sahibergella
singularis,
Scotinophara spp. and Triatoma spp.;
of the order Homoptera, for example,
Aleurothrixus floccosus, Aleyrodes brassicae, Aonidiella spp., Aphididae,
Aphis spp.,
Aspidiotus spp., Bemisia tabaci, Ceroplaster spp., Chrysomphalus aonidium,
Chrysomphalus dictyospermi, Coccus hesperidum, Empoasca spp., Eriosoma
larigerum,
Erythroneura spp., Gascardia spp., Laodelphax spp., Lecanium comi,
Lepidosaphes spp.,
Macrosiphus spp., Myzus spp., Nephotettix spp., Nilaparvata spp., Paratoria
spp.,
Pemphigus spp., Planococcus spp., Pseudaulacaspis spp., Pseudococcus spp.,
Psylla
spp., Pulvinaria aethiopica, Quadraspidiotus spp., Rhopalosiphum spp.,
Saissetia spp.,
Scaphoideus spp., Schizaphis spp., Sitobion spp., Trialeurodes vaporariorum,
Trioza
erytreae and Unaspis citri;
of the order Hymenoptera, for example,
Acromyrmex, Atta spp., Cephus spp., Diprion spp., Diprionidae, Gilpinia
polytoma,
Hoplocampa spp., Lasius spp., Monomorium pharaonis, Neodiprion spp.,
Solenopsis spp.
and Vespa spp.;
of the order Diptera, for example,
Aedes spp., Antherigona soccata, Bibio hortulanus, Calliphora erythrocephala,
Ceratitis
spp., Chrysomyla spp., Culex spp., Cuterebra spp., Dacus spp., Drosophila
melanogaster,
Fannia spp., Gastrophilus spp., Glossina spp., Hypodemia spp., Hyppobosca
spp.,
Lirlomyza spp., Lucilia spp., Melanagromyza spp., Musca spp., Oestrus spp.,
Orseolia spp.,
Oscinella frit, Pegomyia hyoscyami, Phorbia spp., Rhagoletis pomonella, Sciara
spp.,
Stomoxys spp., Tabanus spp., Tannia spp. and Tipula spp.;
of the order Siphonaptera, for example,
Ceratophyllus spp. and Xenopsylla cheopis;
of the order Thysanura, for example,
Lepisma saccharina;
of the order Acarina, for example,
Acarus siro, Aceria sheldoni, Aculus schlechtendali, Amblyomma spp., Argas
spp.,
Boophilus spp., Brevipalpus spp., Bryobia praetiosa, Calipitrimerus spp.,
Chorioptes spp.,
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-15-
Dermanyssus gallinae, Eotetranychus carpini, Eriophyes spp., Hyalomma spp.,
Nodes spp.,
Olygonychus pratensis, Omithodoros spp., Panonychus spp., Phyliocoptruta
oleivora,
Polyphagotarsonemus latus, Psoroptes spp., Rhipicephalus spp., Rhizoglyphus
spp.,
Sarcoptes spp., Tarsonemus spp. and Tetranychus spp..
The mentioned phytopathogenic fungi include, for example:
of the class of the Fungi imperfecti, for example,
Botrytis spp., Pyricularia spp., Helminthosporium spp., Fusarium spp.,
Septoria spp.,
Cercospora spp. and Altemaria spp.;
of the class of the Basidiomycetes, for example,
Rhizoctonia spp., Hemilela spp. and Puccinia spp.;
of the class of the Ascomycetes, for example,
Venturia spp., Erysiphe spp., Podosphaera spp., Monilinia spp. and Uncinula
spp.; and
of the class of the Oomycetes, for example,
Phytophthora spp., Pythium spp. and Plasmopara spp..
With the compositions according to the invention it is possible especially to
control, i.e. to
inhibit or destroy, pests of the mentioned type occurring on plants,
especially on useful
plants and ornamentals in agriculture, in horticulture and in forestry, or on
parts of such
plants, such as the fruit, blossom, leaves, stems, tubers or roots, while in
some cases the
parts of the plants which grow later are also still protected against those
pests.
Target crops are especially cereals, such as wheat, barley, rye, oats, rice,
maize and
sorghum; beet, such as sugar beet and fodder beet; fruit, such as pomes, stone
fruit and
soft fruit, such as apples, pears, plums, peaches, almonds, cherries, or
berries, for example
strawberries, raspberries or blackberries; leguminous plants, such as beans,
lentils, peas
and soybeans; oil plants, such as rape, mustard, poppy, olives, sunflowers,
coconut, castor
oil plants, cocoa beans and groundnuts; cucumber plants, such as marrows,
cucumbers
and melons; fibre plants, such as cotton, flax, hemp and jute; citrus fruit,
such as oranges,
lemons, grapefruit and mandarins; vegetables, such as spinach, lettuce,
asparagus,
cabbages, carrots, onions, tomatoes, potatoes and paprika; lauraceae, such as
avocados,
cinnamon and camphor; and tobacco, nuts, coffee, aubergines, sugar cane, tea,
pepper,
vines, hops, bananas and natural rubber plants, as well as ornamentals.
Preference is
given especially to the-control of pests and microorganisms on wheat, barley,
rape, maize
and sugar beet.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-16-
The compositions of the invention are suitable especially for controlling
insects and
representatives of the order Acarina, especially plant-destructive feeding
insects, such as
Anthonomus grandis, Diabrotica balteata, Heliothis virescens larvae, Piutella
xylostella and
Spodoptera littoralis larvae, and spider mites, such as Tetranychus spp., in
cotton, fruit,
maize, soybean, rape and vegetable crops.
Further areas of use of the compounds according to the invention are the
protection of
stored goods and stocks and materials, and also In the hygiene sector,
especially the
protection of domestic animals and productive livestock against pests of the
mentioned
type.
The composition according to the invention is therefore an emulsifiable
concentrate, a
suspension concentrate, a directly sprayable or dilutable solution, a coatable
paste, a dilute
emulsion, a wettable powder, a soluble powder, a dispersible powder, a
wettable powder, a
dust, granules or an encapsulation in polymer substances, comprising in
addition to the
compound of formula (1), and where appropriate at least one further
insecticidally active
compound, at least one fungicidally active compound, the type of formulation
being
chosen in accordance with the intended objectives and prevailing
circumstances.
The active ingredients are used in those compositions in pure form, a solid
active
ingredient, for example, in a specific particle size, or preferably together
with - at least - one
of the adjuvants customary in formulation technology, such as extenders, for
example
solvents or solid carriers, or surface-active compounds (surfactants).
Suitable solvents are, for example: optionally partially hydrogenated aromatic
hydrocarbons,
preferably the fractions of alkylbenzenes containing 8 to 12 carbon atoms,
such as xylene
mixtures, alkylated naphthalenes or tetrahydronaphthalene, aliphatic or
cycloaliphatic
hydrocarbons, such as paraffins or cyclohexane, alcohols, such as ethanol,
propanol or
butanol, glycols and their ethers and esters, such as propylene glycol,
dipropylene glycol
ether, ethylene glycol or ethylene glycol monomethyl or monoethyl ether,
ketones, such as
cyclohexanone, isophorone or diacetone alcohol, strongly polar solvents, such
as N-
methylpyrrolid-2-one, dimethyl sulfoxide or N,N-dimethylformamide, water,
vegetable oils or
epoxidised vegetable oils, such as rape oil, castor oil, coconut oil or
soybean oil or
epoxidised rape oil, castor oil, coconut oil or soybean oil, and silicone
oils.
The solid carriers used, e.g. for dusts and dispersible powders, are normally
natural mineral
fillers such as calcite, talcum, kaolin, montmorillonite or attapulgite. In
order to improve the
physical properties it is also possible to add highly dispersed silicic acids
or highly dispersed
absorbent polymers. Suitable granulated adsorptive carriers are porous types,
such as
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-17-
pumice, broken brick, sepiolite or bentonite; and suitable nonsorbent carriers
are calcite or
sand. In addition, a great number of granulated materials of inorganic or
organic nature can
be used, especially dolomite or pulverised plant residues.
Depending on the nature of the compound to be formulated, suitable surface-
active
compounds are non-Ionic, cationic and/or anionic surfactants or mixtures of
surfactants
having good emulsifying, dispersing and wetting properties. The surfactants
listed below are
to be regarded merely as examples; many more surfactants customarily employed
in
formulation technology and suitable for use according to the invention are
described in the
relevant literature.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic
or cycloaliphatic
alcohols, saturated or unsaturated fatty acids and alkylphenols, said
derivatives containing
3 to 30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic)
hydrocarbon moiety
and 6 to 18 carbon atoms in the alkyl moiety of the alkylphenols. Further
suitable non-ionic
surfactants are water-soluble adducts of polyethylene oxide with polypropylene
glycol, ethyl-
enediaminopolypropylene glycol and alkylpolypropylene glycol containing 1 to
10 carbon
atoms in the alkyl chain, which adducts contain 20 to 250 ethylene glycol
ether groups and
to 100 propylene glycol ether groups. These compounds usually contain 1 to 5
ethylene
glycol units per propylene glycol unit. Representative examples of non-ionic
surfactants are
nonylphenol polyethoxyethanols, castor oil polyglycol ethers,
polypropylene/polyethylene
oxide adducts, tributylphenoxypolyethoxyethanol, polyethylene glycol and
octylphenoxypolyethoxyethanol. Fatty acid esters of polyoxyethylene sorbitan,
e.g.
polyoxyethylene sorbitan trioleate, are also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which contain,
as substi-
tuent, at least one C8-C22alkyl radical and, as further substituents,
unsubstituted or halo-
genated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are
preferably in the
form of halides, methyl sulfates or ethyl sulfates. Examples are
stearyltrimethylammonium
chloride and benzyidi(2-chloroethyl)ethylammonium bromide.
Both water-soluble soaps and water-soluble synthetic surface-active compounds
are
suitable anionic surfactants. Suitable soaps are the alkali metal salts,
alkaline earth metal
salts or unsubstituted or substituted ammonium salts of higher fatty acids
(C,o-C22), e.g. the
sodium or potassium salts of oleic or stearic acid, or of natural fatty acid
mixtures which can
be obtained e.g. from coconut oil or tall oil; mention may also be made of
fatty acid methyl-
taurine salts. More frequently, however, so-called synthetic surfactants are
used, especially
fatty sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or
alkylarylsulfonates.
The fatty sulfonates or sulfates are usually in the form of alkali metal
salts, alkaline earth
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-18-
metal salts or unsubstituted or substituted ammonium salts and generally
contain a C8-C22-
alkyl radical, which also includes the alkyl moiety of acyl radicals; there
may be mentioned
by way of example the sodium or calcium salt of lignosulfonic acid, of dodecyl
sulfate or of a
mixture of fatty alcohol sulfates obtained from natural fatty acids. These
compounds also
comprise the salts of sulfated and sulfonated fatty alcohol/ethylene oxide
adducts. The
sulfonated benzimidazole derivatives preferably contain 2 sulfonic acid groups
and one fatty
acid radical containing approximately 8 to 22 carbon atoms. Examples of
alkylarylsulfonates are the sodium, calcium or triethanolammonium salts of
dodecylbenzenesulfonic acid, dibutyinaphthalenesulfonic acid or of a
condensate of
naphthalenesulfonic acid and formaldehyde. Also suitable are corresponding
phosphates,
e.g. salts of the phosphoric acid ester of an adduct of p-nonylphenol with 4
to 14 mol of
ethylene oxide, or phospholipids.
The compositions usually comprise 0.1 to 99%, preferably 0.1 to 95%, of active
ingredient
mixture, and 1 to 99.9%, preferably 5 to 99.9%, of - at least - one solid or
liquid adjuvant, It
generally being possible for 0 to 25%, preferably 0.1 to 20%, of the
composition to be
surfactants (in each case percentages are by weight). Whereas commercial
products will
preferably be formulated as concentrates, the end user will normally employ
dilute
formulations which have considerably lower active ingredient concentrations.
Preferred
compositions are especially the following (throughout, percentages are by
weight):
The active ingredient combinations according to the invention preferably
comprise a
compound of formula (I) and two compounds selected from the compounds of
formulae (Cl)
to (V) in a mixing ratio of from 1 (compound of formula I) : 1 (compound of
formulae (II)
to (V)) : 100 (compound of formulae (II) to (V)), 1:100:1 to 100:1:1,
especially from 1:1:20,
1:20:1 to 20:1:1, more especially from 1:1:10, 1:10:1 to 10:1:1, especially
from 1:1:5, 1:5:1
to 5:1:1, very especially from 1:1:2, 1:2:1 to 2:1:1, and above all 1:1:1;
1:1:2; 1:2:1; 2:1:1;
1:1:3; 1:3:1; 3:1:1; 1:1:4; 1:4:1; 4:1:1; 7:1:1; 35:5:1; 35:5:3; 35:2.5:1;
52.5:3.75:1.5; 35:5:1;
52.5:5:2; 52.5:5:3; 52.5:5:7.5; 52.5:5:1; 52.5:5:3; 52.5:2:2; 420:50:12;
315:1:2.5; 15:4:18 to
7:4:1.
On the other hand, preference is given also to active ingredient combinations
comprising a
compound I, a pyrethroid and two compounds of formulae (II) to (V), especially
in the ratio
of 7:4:1:1.
Preference is given likewise to an active ingredient combination comprising a
compound of
formula (1) and only one compound selected from the compounds of formulae (II)
to (V) in a
mixing ratio of from 1:50 to 50:1, especially in a ratio of from 1:20 to 20:1,
especially from
10:1 to 1:10, very especially from 5:1 to 1:5, preferably from 2:1 to 1:2,
above all in the ratio
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-19
of 1:1; or else in the ratio of 7:1, 5:1, or 5:2, or 5:3, or 5:4, or 4:1, or
4:3, or 3:1, or 3:2, or
2:1, or 1:5, or 2:5, or 3:5, or 4:5, or 1:4, or 3:4, or 1:3, or 2:3, or 1:2.
Preference is given furthermore to an active ingredient combination comprising
1. a compound of formula (I), especially of formula (la), fludioxonil and
difenoconazole;
2. a compound of formula (I), especially of formula (la), fludioxonil and
tebuconazole;
3. a compound of formula (I), especially of formula (la), fludioxonil and
triticonazole;
4. a compound of formula (1), especially of formula (la), fludioxonil and
penconazole;
5. a compound of formula (1), especially of formula (la), fludioxonil and
epoxiconazole;
6. a compound of formula (I), especially of formula (la), fludioxonil and
cyproconazole;
7. a compound of formula (1), especially of formula (la), and fludioxonil;
8. a compound of formula (I), especially of formula (la), cyprodinil and
tebuconazole;
9. a compound of formula (I), especially of formula (la), cyprodinil and
epoxiconazole;
10. a compound of formula (I), especially of formula (la), cyprodinil and
flutriafol;
11. a compound of formula (1), especially of formula (Ia), cyprodinil and
cyproconazole;
12. a compound of formula (I), especially of formula (la), cyprodinil and
triticonazole;
13. a compound of formula (I), especially of formula (la), triazoxide and
tebuconazole;
14. a compound of formula (1), especially of formula (la), R-metalaxyl and
fludioxonil;
15. a compound of formula (I), especially of formula (la), R-metalaxyl and
fludioxonil;
16. a compound of formula (I), especially of formula (la), tefluthrin and
hymexazol;
17. a compound of formula (I), especially of formula (la), tefluthrin and
fludioxonil; and
18. a compound of formula (1), especially of formula (la), tefluthrin,
fludioxonil and
difenoconazole.
Preference is given especially to an active ingredient combination comprising
Al) the compound of formula (la), fludioxonil and difenoconazole in a mixing
ratio of
7:1 :1;
A2) the compound of formula (la), fludioxonil and tebuconazole in a mixing
ratio of
35:5:1;
A3) the compound of formula (la), fludioxonil and triticonazole in a mixing
ratio of 35 : 5: 3;
A4) the compound of formula (la), fludioxonil and epoxiconazole in a mixing
ratio of
35:2.5:1;
A5) the compound of formula (la), fludioxonil and epoxiconazole in a mixing
ratio of
52.5: 3.75:1.5;
A6) the compound of formula (la), fludioxonil and cyproconazole in a mixing
ratio of
35:5:1;
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-20-
A7) the compound of formula (la) and fludioxonil in a mixing ratio of 7 : 1;
A8) the compound of formula (la), cyprodinil and tebuconazole in a mixing
ratio of
52.5:5:2;
A9) the compound of formula (la), cyprodinil and epoxiconazole in a mixing
ratio of
52.5 : 5 : 3;
Al0) the compound of formula (la), cyprodinil and flutriafol in a mixing ratio
of 52.5 : 5 : 7.5;
All) the compound of formula (la), cyprodinil and cyproconazole in a mixing
ratio of
52.5:5:1;
A12) the compound of formula (la), cyprodinil and triticonazole in a mixing
ratio of
52.5 : 5: 3;
A13) the compound of formula (la), triazoxide and tebuconazole in a mixing
ratio of
52.5:2:2;
A14) the compound of formula (la), R-metalaxyl and fludioxonil in a mixing
ratio of
420:50:12;
A15) the compound of formula (la), R-metalaxyl and fludioxonil in a mixing
ratio of
315:1: 2.5;
A16) the compound of formula (la), tefluthrin and hymexazol in a mixing ratio
of 15 : 4:18;
A17) the compound of formula (la), tefluthrin and fludioxonil in a mixing
ratio of 7: 4 : 1; and
A18) the compound of formula (la), tefluthrin, fludioxonil and difenoconazole
in a mixing ratio
of 7:4:1 :1.
The above-mentioned mixing ratios relate on the one hand to parts by weight of
the individual
components, but on the other hand also to the mixing ratios in moles. Thus,
for example, the
ratio of 4: 1 : 1 denotes four parts by weight of a compound of formula (I) to
one part by
weight of a fungicidal compound to one part by weight of a further
fungicidally or insecticidally
active compound, but also four moles of a compound of formula (I) to one mole
of a
fungicidal compound to one mole of a further fungicidally or insecticidally
active compound.
Finally, the figures relate also to mixtures in the ratio of the LD50 values
of the individual pests
to be controlled.
Emulsifiable concentrates:
insecticidally active compounds 1 to 90%, preferably 5 to 20%
compounds (11) to (V) 1 to 90%, preferably 5 to 20%
surfactant: 1 to 30%, preferably 15 to 20%
solvent: to 98%, preferably 70 to 85%
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-21-
Dusts:
insecticidally active compounds 0.1 to 10%, preferably 0.1 to 1 %
compounds (II) to (V) 0.1 to 10%, preferably 0.1 to 1 %
solid carrier: 99.9 to 90%, preferably 99.9 to 99%
Suspension-c-oncentrates:
insecticidally active compounds 5 to 75%, preferably 10 to 50%
compounds (II) to (V) 5 to 75%, preferably 10 to 50%
water: 94 to 24%, preferably 88 to 30%
surfactant: I to 40%, preferably 2 to 30%
Wettable powders:
insecticidally active compounds 0.5 to 90%, preferably 1 to 80%
compounds (II) to (V) 0.5 to 90%, preferably 1 to 80%
surfactant: 0.5 to 20%, preferably 1 to 15%
solid carrier. 5 to 99%, preferably 15 to 98%
Granules:
insecticidally active compounds 0.5 to 30%, preferably 3 to 15%
solid carrier 99.5 to 70%, preferably 97 to 85%
The compositions according to the invention may also comprise further solid or
liquid
adjuvants, such as stabilisers, for example vegetable oils or epoxidised
vegetable oils (e.g.
epoxidised coconut oil, rape oil or soybean oil), antifoams, for example
silicone oil,
preservatives, viscosity regulators, binders and/or tackifiers, as well as
fertilisers or other
active ingredients for obtaining special effects, for example bactericides,
nematicides,
molluscicides or selective herbicides.
The compositions according to the invention are prepared in known manner, in
the absence
of adjuvants, for example by grinding and/or sieving a solid active ingredient
or mixture of
active ingredients, for example to a specific particle size, and in the
presence of at least one
adjuvant, for example by intimately mixing and/or grinding the active
ingredient or mixture of
active ingredients with the adjuvant(s). The invention relates also to those
processes for
the preparation of the compositions according to the invention and to the use
of the
compounds of formula (1) in the preparation of those compositions.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-22-
The invention relates also to the methods of application of the compositions,
i.e. the
methods of controlling pests and microorganisms of the mentioned type, such as
spraying,
atomising, dusting, coating, dressing, scattering or pouring, which are
selected in
accordance with the intended objectives and prevailing circumstances, and to
the use of the
compositions for controlling pests of the mentioned type. Typical rates of
concentration are
from 0.1 to 1000 ppm, preferably from 0.1 to 500 ppm, of active ingredient.
The rates of
application per hectare are generally from 1 to 2000 g of active ingredient
per hectare,
especially from 10 to 1000 g/ha, preferably from 20 to 600 g/ha.
A preferred method of application in the area of plant protection is
application to the foliage
of the plants (foliar application), the number of applications and the rate of
application
depending on the risk of infestation by the pest in question. However, the
active ingredient
can also penetrate the plants through the roots (systemic action) if the locus
of the plants is
impregnated with a liquid formulation or if the active ingredient is
incorporated in solid form
Into the locus of the plants, for example into the soil, e.g. in granular form
(soil application).
In paddy rice crops, such granules may be applied in metered amounts to the
flooded rice
field.
The compositions according to the invention are also suitable for protecting
plant
propagation material, e.g. seed material, such as fruit, tubers or grains, or
plant cuttings,
from fungal infections and animal pests. The propagation material can be
treated with the
composition before planting: seed, for example, can be dressed before being
sown. The
compounds of the invention can also be applied to grains (coating), either by
impregnating
the grains with a liquid formulation or by coating them with a solid
formulation. The
composition can also be applied to the planting site when the propagation
material is being
planted, for example to the seed furrow during sowing. The invention relates
also to that
method of treating plant propagation material and to the plant propagation
material thus
treated.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-23-
Example Fi : Emulsifiable concentrates a) b) c)
pesiticidaily active compounds 25% 40% 50%
calcium dodecylbenzenesulfonate 5% 8% 6%
castor oil polyethylene glycol ether (36 mol EO) 5% - -
tributylphenol polyethylene glycol ether (30 mol EO) - 12% 4%
cyclohexanone - 15% 20%
xylene mixture 65% 25% 20%
Such concentrates can be diluted with water to give emulsions of any desired
concentration.
Example F2: Solutions a) b) C) d)
pesiticidally active compounds 80% 10% 5% 95%
ethylene glycol monomethyl ether 20% - - -
polyethylene glycol (mol. wt. 400) - 70% - -
N-methylpyrrolid-2-one - 20% - -
epoxidised coconut oil - - 1% 5%
petroleum fraction
(boiling range: 160-190 C) - - 94% -
The solutions are suitable for use in the form of microdrops.
Example F3: Granules a) b) c) d)
pesiticidally active compounds 5% 10% 8% 21%
kaolin 94% - 79% 54%
highly dispersed silicic acid 1 % - 13% 7%
attapulgite - 90% - 18%
The active ingredients are dissolved in dichloromethane and the solution is
sprayed onto
the carrier and the solvent is then evaporated off in vacuo.
Examole F4: Dusts a) b)
pesiticidally active compounds 2% 5%
highly dispersed silicic-acid 1% 5%
talcum 97% -
kaolin - 90%
The active ingredient is homogeneously mixed with the carriers, giving dusts
that are ready
for use.
CA 02240738 1998-06-17
WO 97/22254 PC /EP96/05489
-24-
Example F5: Wettable powders a) b) c)
pesiticidally active compounds 25% 50% 75%
sodium lignosulfonate 5% 5% -
sodium lauryisulfate 3% - 5%
sodium diisobutylnaphthalenesulfonate - 6% 10%
octylphenol polyethylene glycol ether (7-8 mol EO) - 2% -
highly dispersed silicic acid 5% 10% 10%
kaolin 62% 27% -
The active ingredient is mixed with the adjuvants and the mixture is
thoroughly ground in a
suitable mill, affording wettable powders which can be diluted with water to
give
suspensions of the desired concentration.
Example F6: Emulsifiable concentrate
pesiticidally active compounds 10%
octylphenol polyethylene glycol ether (4-5 mol EO) 3%
calcium dodecylbenzenesulfonate 3%
castor oil polyethylene glycol ether (36 mot EO) 4%
cyclohexanone 30%
xylene mixture 50%
This concentrate can be diluted with water to give emulsions of any desired
concentration.
Example F7: Dusts a) b)
pesiticidally active compounds 5% 8%
talcum 95% -
kaolin - 92%
Ready-for-use dusts are obtained by mixing the active ingredient with the
carrier and
grinding the mixture in a suitable mill.
Example F8: Extruder granules
pesiticidally active compounds 10%
sodium lignosulfonate 2%
carboxymethylcellulose 1%
kaolin 87%
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-25-
The active ingredient is mixed with the adjuvants and the mixture is ground
and moistened
with water. The mixture is extruded and granulated and the granules are dried
in a stream
of air.
Example F9: Coated granules
pesiticidally active compounds 3%
polyethylene glycol (mol. wt. 200) 3%
kaolin 94%
The finely ground active ingredient is uniformly applied, in a mixer, to the
kaolin moistened
with polyethylene glycol. Non-dusty coated granules are obtained in this
manner.
Example F10: Suspension concentrate
pesiticidally active compounds 40%
ethylene glycol 10%
nonylphenol polyethylene glycol ether (15 mol EO) 6%
sodium lignosulfonate 10%
carboxymethyl cellulose 1%
aqueous formaldehyde solution (37%) 0.2%
aqueous silicone oil emulsion (75%) 0.8%
water 32%
The finely ground active ingredient is homogeneously mixed with the adjuvants,
giving a
suspension concentrate from which suspensions of any desired concentration can
be
obtained by dilution with water.
Biological Examples (throughout, percentages are by weight, unless otherwise
indicated)
Example : Action against Boophilus microplus
Adult female ticks which are replete with blood are aff ixed to a PVC plate
and covered with
a cotton wool swab. For treatment, 10 ml of an aqueous test solution
containing 125 ppm of
the test compound mixture are poured over the test insects. The cotton wool
swab is then
removed and the ticks are incubated for 4 weeks until oviposition has taken
place. The
action against Boophilus microplus manifests itself either as mortality or
sterility of the
females or as ovicidal action in the eggs.
The compositions according to the invention exhibit good activity in this
test.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-26-
Example B2: Action against Nilaparvata lugens
Rice plants are sprayed with an aqueous emulsion comprising 400 ppm of the
test
compound mixture. After the spray coating has dried, the rice plants are
populated with
cicada larvae in the 2nd and 3rd stages. Evaluation is made 21 days later. The
percentage
reduction in the population (% activity) is determined by comparing the number
of surviving
cicadas on the treated plants with that on untreated plants.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (la), fludioxonil and
difenoconazole in a
mixing ratio of 7: 1 : 1 is more than 80 % effective.
Example 63: Action against Diabrotica balteata larvae
Maize seedlings are sprayed with an aqueous emulsion comprising 400 ppm of the
test
compound mixture. After the spray coating has dried, the maize seedlings are
populated
with 10 Diabrotica balteata larvae in the second stage and placed in a
plastics container.
Evaluation is made 6 days later. The percentage reduction in the population (%
activity) is
determined by comparing the number of dead larvae on the treated plants with
that on
untreated plants.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (1a), R-metalaxyl and
fludioxonil in a
mixing ratio of 315:1: 2.5 is more than 80 % effective.
Example B4: Action against Anthonomus grandis adults
Young cotton plants are sprayed with an aqueous emulsion comprising 400 ppm of
the test
compound mixture. After the spray coating has dried, the cotton plants are
populated with
Anthonomus grandis adults and placed In a plastics container. Evaluation is
made
3 days later. The percentage reduction in the population or the percentage
reduction in
feeding damage (% activity) is determined by comparing the number of dead
beetles and
the feeding damage on the treated plants with that on untreated plants.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (1a), fludioxonil and
tebuconazole in a
mixing ratio of 35 : 5: 1 is more than 80 % effective.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-27-
Example B5: Action against Heliothis virescens caterpillars
Young soybean plants are sprayed with an aqueous emulsion comprising 400 ppm
of the
test compound mixture. After the spray coating has dried, the soybean plants
are
populated with 10 Heliothis virescens caterpillars in the first stage and
placed in a plastics
container. Evaluation is made 6 days later. The percentage reduction in the
population or
the percentage reduction in feeding damage (% activity) is determined by
comparing the
number of dead caterpillars and the feeding damage on the treated plants with
that on
untreated plants.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (Ia), fludioxonil and
tebuconazole,
especially in a mixing ratio of 35 : 5 : 1; is more than 80 % effective.
Example B6: Action against Spodoptera littoralis caterpillars
Young soybean plants are sprayed with an aqueous emulsion comprising 400 ppm
of the
test compound mixture. After the spray coating has dried, the soybean plants
are
populated with 10 Spodoptera littoralis caterpillars in the third stage and
placed in a plastics
container. Evaluation is made 3 days later. The percentage reduction in the
population or
the percentage reduction in feeding damage (% activity) is determined by
comparing the
number of dead caterpillars and the feeding damage on the treated plants with
that on
untreated plants.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (Ia), fludioxonil and
tebuconazole in a
mixing ratio of 35 : 5: 1 is more than 80 % effective.
Example B7: Action against Crocidolomia binotalis cate illars
Young cabbage plants are sprayed with an aqueous emulsion comprising 400 ppm
of the
test compound mixture. After the spray coating has dried, the cabbage plants
are
populated with 10 Crocidolomia binotalis caterpillars in the third stage and
placed in a
plastics container. Evaluation is made 3 days later. The percentage reduction
in the
population or the percentage reduction in feeding damage (% activity) is
determined by
comparing the number of dead caterpillars and the feeding damage on the
treated plants
with that on untreated plants.
The compositions according to the invention exhibit good activity in this
test.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-28-
Exam lp a B8: Systemic action against Nilaparvata lugens
Pots containing rice plants are placed in an aqueous emulsion solution
containing 400 ppm
of the test compound mixture. The rice plants are then populated with larvae
in the 2nd and
3rd stages. Evaluation is made 6 days later. The percentage reduction in the
population
(% activity) is determined by comparing the number of cicadas on the treated
plants with
that on untreated plants.
The compositions according to the invention exhibit good activity in this
test.
Exam lQ a 139: Ovicidal action against Tetranychus urticae
Young bean plants are populated with Tetranychus urticae females which are
removed
24 hours later. The plants populated with eggs are sprayed with an aqueous
emulsion
comprising 400 ppm of the test compound mixture. The plants are then incubated
for
6 days at 25 C and then evaluated. The percentage reduction in the population
(% activity)
is determined by comparing the number of dead eggs, larvae and adults on the
treated
plants with that on untreated plants.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (la), cyprodinil and flutriafol
in a mixing
ratio of 52.5: 5: 7.5 is more than 80 % effective.
Example B10: Action against Dermanyssus gallinae
2 to 3 ml of a solution comprising 10 ppm of the test compound mixture, and
approximately
200 mites at various stages of development, are placed in a glass container
that is open at
the top. The containeris then closed with a cotton wool plug, shaken for 10
minutes until
the mites are completely wetted, and then inverted for a short time so that
the remaining
test solution can be absorbed by the cotton wool. After 3 days, the percentage
mortality of
the mites is determined by counting the dead individuals.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (Ia), tefluthrin, fludioxonil
and difenocon-
azole in a mixing ratio of 7: 4 : 1 : 1 is more than 80 % effective.
Example B11: Action against Blattella germanica
An amount of 0.1 % solution of the test compound mixture in acetone
corresponding to a
rate of concentration of 2 g/m2 is introduced into a petri dish. When the
solvent has
evaporated, 20 Blattella germanica nymphs (final nymph stage) are introduced
into the
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-29-
prepared dish and exposed to the action of the test compound for 2 hours. The
nymphs are
then anaesthetised with C02, placed in a fresh petri dish and kept in the dark
at 25 C and
50 to 70 % humidity. After 48 hours the insecticidal activity is determined by
establishing the
mortality.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al to Al 8 are more than 80 % effective.
Example B12: Action against Lucilia cuprina blowflies
Freshly laid eggs of the blowfly variety Lucilia cuprina are introduced in
small portions (30 to
50 eggs) into test tubes in which 4 ml of nutrient medium and 1 ml of test
solution compris-
ing 16 ppm of the test compound mixture have already been mixed. After
inoculation of the
culture medium, the test tubes are closed with a cotton wool plug and
incubated in an
incubating cabinet for 4 days at 30 C. By that time, larvae approximately 1 cm
long
(stage 3) have developed in the untreated medium. If the substance is active,
the larvae are
either dead or severely retarded by that time. Evaluation is carried out after
96 hours.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (Ia), fludioxonil and
difenoconazole in a
mixing ratio of 7 : 1 : 1 is more than 80 % effective.
Example B13: Action against Musca domestica
A sugar cube is treated with a solution of the test compound mixture in such a
manner that
after drying overnight the concentration of test compound in the sugar is 250
ppm. The
treated sugar cube, together with a wet cotton wool swab and 10 Musca
domestica adults
of an OP-resistant strain, is placed in an aluminium dish, covered with a
glass beaker and
incubated at 25 C. After 24 hours the mortality is determined.
The compositions according to the invention exhibit good activity in this
test. In particular, a
composition comprising the compound of formula (la), triazoxide and
tebuconazole in a
mixing ratio of 52.5: 2 2 is more than 80 % effectve.
Example B14: Action against Plutella xylostella caterpillars
Young cabbage plants are sprayed with an aqueous emulsion comprising 400 ppm
of the
test compound mixture. After the spray coating has dried, the cabbage plants
are
populated with 10 Plutella xylostella caterpillars in the third stage and
placed in a plastics
container. Evaluation is made 3 days later. The percentage reduction in the
population or
CA 02240738 1998-06-17
WO 97/22254 PCd'/EP96/05489
-30-
the percentage reduction in feeding damage (% activity) is determined by
comparing the
number of dead caterpillars and the feeding damage on the treated plants with
that on
untreated plants.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al, A3, A7 and Ala effect almost complete inhibition of
infestation.
Example BI 5: Action Against Phytophthora infestans on tomatoes
a) Curative action
After a cultivation period of 3 weeks, tomato plants of the "Red Gnome"
variety are sprayed
with a zoospore suspension of the fungus and incubated in a cabinet at 18 to
20 C and
100 % humidity. Humidification is stopped after 24 hours. When the plants have
dried, they
are sprayed with a mixture comprising the test compound mixture formulated as
a wettable
powder in a concentration of 200 ppm. After the spray coating has dried, the
plants are
again placed in the humidity cabinet for 4 days. The activity of the test
compounds is
evaluated on the basis of the number and size of the typical leaf specks that
have occurred
after that time.
b) Preventive-systemic action
The test compound mixture formulated as a wettable powder is applied in a
concentration of
60 ppm (based on the volume of the soil) to the soil surface of three-week-old
tomato plants
of the "Red Gnome" variety planted in pots. After a 3-day waiting period, the
undersides of
the leaves of the plants are sprayed with a zoospore suspension of
Phytophthora infestans.
The plants are then kept in a spray cabinet for 5 days at 18 to 20 C and 100 %
humidity.
After that time, typical leaf specks form, the number and size of which are
used to evaluate
the activity of the test compounds.
Whereas untreated but infected control plants exhibit 100 % infestation, good
activity is
achieved with the compositions according to the invention. In particular,
compound mixtures
Al to Al 8 inhibit infestation in both tests to 20 % or less.
Example B16: Action against Plasmopara viticola (Bert. et Curt.) (Berl. et
DeToni) on vines
a) Residual-preventive action
Vine seedlings of the "Chasselas" variety are grown in a greenhouse. 3 plants
are sprayed
at the 10-leaf stage with a mixture (200 ppm active ingredient mixture). After
the spray
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-31-
coating has dried, the undersides of the leaves of the plants are infected
uniformly with a
spore suspension of the fungus. The plants are then kept in a humidity chamber
for 8 days.
After that time, the control plants exhibit marked symptoms of disease. The
activity of the
test compounds is evaluated on the basis of the number and size of the sites
of infection on
i=
the treated plants.
h) Curative action
Vine seedlings of the "Chasselas" variety are grown in a greenhouse and the
undersides of
the leaves are infected at the 1 0-leaf stage with a spore suspension of
Plasmopara viticola.
After 24 hours in a humidity cabinet, the plants are sprayed with a mixture of
the test
compounds (200 ppm,-60 ppm, 20 ppm active ingredient mixture). Then the plants
are kept
in the humidity cabinet for a further 7 days. After that time, the control
plants exhibit
symptoms of disease. The activity of the test compounds is evaluated on the
basis of the
number and size of the sites of infection on the treated plants.
The compositions according to the invention exhibit good activity in this
test. In comparison
with the control plants, infestation is 20 % or less, especially on the plants
treated with
compound mixtures Al to A18.
Example B17: Action against Pythium debaryanum on sugar beet (Beta vuloaris)
a) Action following soil application
The fungus is cultivated on sterile oat grains and added to a soil/sand
mixture. The soil so
infected is introduced into plant pots and sown with sugar beet seeds.
Immediately after
sowing, the test compounds, formulated as wettable powders, are poured in the
form of an
aqueous suspension over the soil (20 ppm active ingredient mixture, based on
the volume
of the soil). The pots are then placed in a greenhouse at 20-24 C for 2-3
weeks. The soil is
kept uniformly moist by light spraying with water. The test is evaluated by
determining the
emergence of the sugar beet plants and the proportion of healthy and diseased
plants.
b) Action following application by dressing
The fungus is cultivated on sterile oat grains and added to a soil/sand
mixture. The soil so
infected is introduced into plant pots and sown with sugar beet seeds which
have been
dressed with the test compounds formulated as dressing powders (1000 ppm
active
ingredient mixture, based on the weight of the seeds). The pots containing the
seeds are
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-32-
then placed in a greenhouse at 20-24 C for 2-3 weeks. The soil is kept
uniformly moist by
light spraying with water.
The test is evaluated by determining the emergence of the sugar beet plants
and the
proportion of healthy and diseased plants.
The compositions according to the invention exhibit good activity in this
test. In particular,
after treatment with compound mixtures Al to Al 8 more than 80 % of the plants
emerge
and have a healthy appearance. In the control pots, only the occasional
emerged plant, with
a diseased appearance, is observed.
ample 1318: Residual-protective action against Cercospora arachidicola on
groundnut
lets
Groundnut plants 10-15 cm in height are sprayed to drip point with an aqueous
spray
mixture (0.02% active ingredient mixture) and infected 48 hours later with a
conidia
suspension of the fungus. The plants are incubated for 72 hours at 21 C and
high humidity
and then placed in a greenhouse until the typical leaf specks appear.
Evaluation of the
activity of the active ingredient is made 12 days after infection and is based
on the number
and size of the leaf specks.
The compositions according to the invention exhibit good activity in this
test. In particuar,
compound mixtures A2 to AS and A7 to Al 2 bring about a reduction in leaf
specks to less
than approx. 10 % of the leaf surface. In some cases, the disease is inhibited
completely
(0-5% infestation).
Example B19: Action against Puccinia graminis on wheat
a) Residual protective action:
6 days after sowing, wheat plants are sprayed to drip point with an aqueous
spray mixture
(0.02 % active ingredient mixture) and infected 24 hours later with a
uredospore suspension
of the fungus. The plants are then incubated for 48 hours (conditions: 95-100
% relative
humidity and 20 C) and then placed in a greenhouse at 22 C. Evaluation of rust
pustule
development is made 12 days after infection.
by Systemic action:
Wheat plants are watered 5 days after sowing with an aqueous spray mixture
(0.006 %
active ingredient mixture, based on the volume of the soil). Care is taken
that the spray
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-33-
mixture does not come into contact with the parts of the plants above the
soil. 48 hours later
the plants are infected with a uredospore suspension of the fungus. The plants
are then
incubated for 48 hours (conditions: 95-100 % relative humidity and 20 C) and
then placed in
a greenhouse at 22 C. Evaluation of rust pustule development is made 12 days
after
infection.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al to A18 bring about a marked reduction in fungus
infestation, in
some cases to 10-0 %.
Example B20: Action against Pyricularia oryzae on rice
a) Residual-protective action:
After a cultivation period of 2 weeks, rice plants are sprayed to drip point
with an aqueous
spray mixture (0.02 % active ingredient mixture) and infected 48 hours later
with a conidia
suspension of the fungus. Evaluation of fungus infestation is made 5 days
after infection,
during which time a relative humidity of 95-100 % and a temperature of 22 C
are
maintained.
b) Systemic action:
2-week-old rice plants are watered with an aqueous spray mixture (0.006%
active ingredient
mixture, based on the volume of the soil). Care is taken that the spray
mixture does not
come into contact with-the parts of the plants that are above the soil. The
pots are then
filled with water so that the lowermost parts of the stems of the rice plants
stand in water.
After 96 hours, the plants are infected with a conidia suspension of the
fungus and then
kept for 5 days at 95-100% relative humidity and a temperature of 24 C.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures A7 to A9 and Al 4 to A18 largely prevent the disease from
breaking out
on the infected plants.
Example B21: Residual protective action against Venturia inaequalis on apples
Apple cuttings with 10-20 cm long fresh shoots are sprayed to drip point with
a spray
mixture (0.02 % active ingredient mixture) and infected 24 hours later with a
conidia
suspension of the fungus. The plants are then incubated for 5 days at 90-100 %
relative
humidity and placed in a greenhouse for a further 10 days at 20-24 C. Scab
infestation is
evaluated 15 days after infection.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-34-
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al to Al 8 mainly exhibit sustained activity against scab
diseases.
Example B22: Action against Erysiphe graminis on barley
a) Residual-protective action:
Barley plants about 8 cm in height are sprayed to drip point with an aqueous
spray mixture
(0.02 % active ingredient mixture) and dusted 3 to 4 hours later with conidia
of the fungus.
The infected plants are placed in a greenhouse at 22 C. The fungus infestation
Is
evaluated 10 days after infection.
b) Systemic action:
An aqueous spray mixture (0.002% active ingredient mixture, based on the
volume of the
soil) is used to water barley plants about 8 cm in height. Care is taken that
the spray
mixture does not come into contact with the parts of the plants above the
soil. The plants
are dusted 48 hours later with conidia of the fungus. The infected plants are
then placed in
a greenhouse at 22 C. The fungus infestation is evaluated 10 days after
infection.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al to Al 8 are generally able to reduce disease infestation
to less than
20 %, and in some cases even completely.
Example B23: Action against Podosphaera leucotricha on apple shoots
Residual-protective action
Apple cuttings with about 15 cm long fresh shoots are sprayed with a spray
mixture (0.06 %
active ingredient mixture). After 24 hours, the treated plants are infected
with a conidia
suspension of the fungus and are placed in a climatic chamber at 70 % relative
humidity
and 20 C. Fungus infestation is evaluated 12 days after infection.
The compositions according to the invention exhibit good activity in this
test. In particular,
following treatment with compound mixtures Al to Al 8 disease infestation is
less than 20
%. Control plants exhibit 100 % infestation.
Example B24: Action against Botrytis cinerea on apple fruits
Residual-protective action
Artificially damaged apples are treated by dropping a spray mixture (0.02 %
active
ingredient mixture) onto the damaged sites. The treated fruits are then
inoculated with a
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-35-
spore suspension of the fungus and are incubated for one week at high humidity
and about
20 C. The fungicidal activity of the test compound is derived from the number
of rotting
damaged sites.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al to Al 8 are able to prevent the rot from spreading almost
completely.
Example B25: Action against Helminthos orium gramineum
Wheat grains are contaminated with a spore suspension of the fungus and are
left to dry.
The contaminated grains are dressed with a suspension of the test compound
(600 ppm
active ingredient mixture, based on the weight of the seeds). Two days later,
the grains are
placed on suitable agar dishes and, after a further four days, the development
of the fungus
colonies around the grains is assessed. The evaluation of the test compound is
based on
the number and size of the fungus colonies.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al to Al 8 exhibit good activity, i.e. inhibition of the
fungus colonies.
Example B26: Action against Colletotrichum lagenarium on cucumbers
After a cultivation period of 2 weeks, cucumber plants are sprayed with a
spray mixture
(concentration of active ingredient mixture: 0.002%). Two days later, the
plants are infected
with a spore suspension (1.5x105 spores/ml) of the fungus and are incubated
for 36 hours
at 23 C and high humidity. Incubation is then continued at normal humidity and
about 22-
23 C. The fungus infestation that has occurred is evaluated 8 days after
infection. Fungus
infestation is 100% on untreated and infected control plants.
The compositions according to the invention exhibit good activity in this
test. In particular,
compound mixtures Al to A6 and Al 7 inhibit infestation with the disease
almost completely.
Example B27: Action against Fusarium nivale on rye
Rye of the Tetrahell variety which is naturally infected with Fusarium nivale
is dressed in a
roller mixer with the test compound mixture, the following concentrations
being used: 20 or
6 ppm active ingredient mixture (based on the weight of the seed).
The infected and treated rye is sown in October in the open with a seeder in
plots 3 metres
long and in 6 rows. Three replicates are carried out with each concentration.
CA 02240738 1998-06-17
WO 97/22254 PCT/EP96/05489
-36-
Until evaluation of the infestation is made, the test crop is cultivated under
normal field
conditions (preferably in a region with unbroken snow cover during the winter
months).
In order to evaluate the phytotoxicity, the emergence is assessed in the
autumn and the
crop density/number of plants per unit area is assessed in the spring.
To determine the activity of the test compounds, the percentage of plants
attacked by
Fusarium is assessed in the spring directly after the snow has melted. The
number of
Infested plants is less than 5% in the present case. The plants that have
emerged have a
healthy appearance.
The compositions according to the invention exhibit good activity in this
test. In particular,
following treatment with compound mixtures Al and Al 1 to Al 8 infestation is
completely
suppressed.
Example B28: Action against Septoria nodorum on wheat
Wheat plants are sprayed at the 3-leaf stage with a spray mixture (60 ppm
active ingredient
mixture) prepared from a wettable powder formulation of the test compounds
(2.8:1).
24 hours later, the treated plants are infected with a conidia suspension of
the fungus. The
plants are then incubated for 2 days at 90-100% relative humidity and are
placed in a
greenhouse for a further 10 days at 20-24 C. Fungus infestation is evaluated
13 days after
infection. Less than I % of the wheat plants are infested.
The compositions according to the invention exhibit good activity in this
test. In particular,
following treatment with compound mixtures Al, A5 to A8 and A14 infestation is
completely
suppressed.