Note: Descriptions are shown in the official language in which they were submitted.
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 1 -
BIOAHSORBABLE HEMO8TATIC SEALING A88EMBLY
FIELD OF THE INVENTION
The present invention relates generally to closure
devices and more particularly to an improved sealing
assembly and closure device which are insertable into an
incision or puncture formed in the body of a patient to
seal the incision or puncture from the flow of body fluids
therethrough.
BACKGROUND OF THE INVENTION
During catheterization or other medical procedures, a
physician will create an opening into an artery, vessel or
organ of a patient with a conventional needle, catheter,
introducer or dilator. The size of the opening will vary
depending on the type of procedure and the size of the
catheter which is to be used. For example, the diameter of
the catheter and catheter sheath used in standard
angiography procedures is typically between 5 to 8 French
(1.67 mm and 2.67 mm, respectively). The diameter of the
catheter and catheter sheath used in angioplasty procedures
and an increasing number of stmt placement procedures may
be about 8 (2.67 mm) or 9 (3.33 mm) French. The diameter
of the catheter and catheter sheath used in intro-aortic
balloon pump procedures is typically between 14 to 16
French (4.67 mm and 5.33 mm, respectively) and the diameter
of the catheter and catheter sheath used with
cardiopulmonary support systems is typically between 18 and
20 French (6.0 mm and 6.67 mm, respectively).
Additionally, the catheter may often be twisted or
otherwise manipulated as it is advanced to the treatment
site, thereby causing a further enlargement of the incision
or puncture in the body of the patient.
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 2 -
When the medical procedure is completed and the
catheter is removed from the artery or other blood vessel,
the conventional practice has been to apply external
pressure to the entry site until clotting occurs. Because
many of the patients undergoing these procedures have been
medicated with an anticoagulant such as heparin, the nurse
may be required to apply external pressure to the incision
site for an extended period of time. The time required to
stop bleeding at the incision is not an efficient use of
the nurses time and a painful hematoma or unsightly bruise
may still occur at the incision site because the artery
will continue to bleed internally until clotting blocks the
opening in the artery.
U.S. Patent No. 4,829,994 granted to Kurth on May 16,
1989, attempts to resolve the above-described problem by
providing an apron-like device consisting of a pelvic apron
and a groin strap to apply a compressive force to the
femoral vessel of the patient. Although this device
effectively eliminates the need to have a nurse apply
direct pressure to the incision site, a decrease in blood
flow through the femoral artery may be caused by the use of
this device and may increase the likelihood of clot
formation in the compromised patient.
Another approach to resolving the above-identified
problem is disclosed in U.S. Patent No. 4,929,246 granted
to Sinofsky on May 29, 1990. The method of using the
device disclosed in this patent includes the steps of
advancing a semi-rigid tube having an inf latable balloon at
its distal end through the overlying tissue to a location
3 0 adj acent to the outer wal l of the punctured artery . The
balloon is then inflated to apply pressure directly to the
outer wall of the artery. Laser energy is then directed to
the outer wall of the artery via an optical fiber centrally
located in the semi-rigid tube such that the laser energy
passes through the optical fiber and balloon of the semi-
rigid tube to thermally weld the artery and seal the
incision.
r,
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 3 -
A further approach to resolving the above-identified
problem is disclosed in U.S. Patent No. 4,744,364 granted
to Kensey on May 17, 1988, and related U.S. Patent
Nos. 4,852,568 and 4,890,612 granted to Kensey on August 1,
1989, and January 2, 1990, respectively. The first two
Kensey patents disclose a device for sealing an opening in
the wall of a blood vessel which consists of an elongate
tubular body having an anchor member removably disposed
therein. The tubular body also includes an ejecting device
disposed within the tubular body for forcing the anchor
member from the tubular body into the interior of the blood
vessel. A retraction suture is secured to the anchor
member so that the engagement surface of the anchor member
hemostatically engages the inner surface of the blood
vessel contiguous with the puncture. The '612 Kensey
patent discloses a device which includes an elongate member
having a portion thereof which is adapted to engage
portions of the tissue adjacent to the punctured vessel and
a sealing portion which extends through the incision to
engage the tissue contiguous therewith to seal the
puncture. Subsequent patents granted to Kensey et al. are
illustrative of improvements to the basic approach
described above and generally include an anchor member
which is~used in combination with a suture and a collagen
member to seal an incision and blood vessel.
U.S. Patent No. 5,411,520 granted to Nash et. al. is
illustrative of an improvement to the basic approach
described above and generally includes a spacer which is
movable along a suture to position the spacer between the
anchor member and the collagen member to seal the puncture
and blood vessel. The spacer is positioned between the
collagen member and the anchor to prevent the collagen
member from being entering the blood vessel of the patient.
U.S. Patent No. 5,108,421 granted to Fowler and
assigned to the assignee of the present invention discloses
the use of a "vessel plug" type approach wherein the
hemostatic closure device is inserted into the incision of
CA 02278243 1999-07-20
WO 98/31287 PCT/fJS98/01189
- 4 -
the patient and may be positioned in the incision using a
locating member such as an elongate balloon type member or
a syringe type device. U.S. Patent No. 5,391,183 granted
to Janzen et al. discloses another vessel plug type
approach wherein one or more oversized vessel plugs are
inserted into the incision using a device with a plunger
member.
From the foregoing, it is apparent that while
significant efforts have recently been directed to the
development of a simple and relatively inexpensive means
for reliably effecting the closure of a puncture or
incision in the wall of a blood vessel, duct or organ to
significantly reduce the time to ambulation of a patient as
well as to reduce the risk of hematoma or clot formation
further efforts are needed to satisfy these goals.
SUN~IARY OF THE INVENTION
Accordingly, it is an object of the present invention
to provide a device and method of use which overcomes the
disadvantages of the prior art relating to simplicity of
construction, ease of deployment, operation and/or safety
of a vessel closure device.
It is another object of the present invention to
reduce the time required for sealing an incision in an
artery and to decrease the likelihood that a hematoma will
form after the catheter is removed from the incision.
These and other objects of the present invention are
achieved by providing a device and method for sealing an
incision in a blood vessel, duct or organ using the device
as described hereinafter.
One form of the present invention preferably includes
a sealing assembly consisting of a vessel closure device
including an elongate bioabsorbable rod member having an
anchor member on the distal end thereof. The anchor member
is preferably prestressed to extend laterally from the
~r
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 5 -
distal end of the rod member after insertion while being
sufficiently flexible to be aligned with the lengthwise
dimension of the rod member during insertion of the closure
device into the incision. The rod member also preferably
includes a collagen tube extending along a portion thereof,
locking groove and a retainment ring which is slidable
lengthwise along the rod member.
In use, the sealing assembly includes a delivery which
includes an introducer or procedure sheath, an extension
rod and a push rod and a closure device which includes a
bioabsorbable rod member, an anchor member and a retainment
ring. The delivery device is initially positioned in the
puncture so that the distal end of the introducer is
positioned in the blood vessel of the patient and the
closure device is positioned in and through the puncture.
The distal end of the closure device is then pushed beyond
the distal end of the introducer and the anchor member is
allowed to move from a first position which is generally
parallel to the lengthwise dimension of the rod member to
a second position which is generally perpendicular to the
lengthwise dimension of the rod member. The introducer and
the closure device are then withdrawn together until the
anchor member contacts and engages the wall of the blood
vessel adjacent to the incision. Withdrawal of the
introducer is then continued to expose the rod member and
collagen member of the sealing device to the fluids in the
incision. Next, the cylindrical extension rod of the
delivery device is moved distally along the rod member
sealing device to push a retainment ring distally along the
rod member until the retainment ring reaches a locking
point or locking groove on the rod member. The distal
movement of the retainment ring along the rod member causes
the compression and distal movement of the collagen tube
along the rod member so that the collagen tube expands
radially and is moved to a location generally adjacent to
and proximally of the wall of the blood vessel.
CA 02278243 2005-06-22
- 6 -
The collagen tube of the closure device includes a
distal end which is preferably sized and slightly blunt
shaped so that the distal end of the collagen tube is
reliably positioned proximally of the outer surface of the
blood vessel and extends generally along the distal portion
of the incision or puncture so that the collagen tube will
not enter into the blood vessel. Additionally, the wall of
the blood vessel is sandwiched between the anchor member and
collagen tube. One function of the anchor member is to
l0 allow for the convenient and reliable positioning of the rod
member and collagen tube in the incision or puncture and
therefore, the size and shape of the anchor member is chosen
to minimize any disruption in the flow of fluid past the
incision and to be absorbed in the body of the patient
within a relatively short period of time.
An advantage of the present invention is that the
closure device does not include a suture member and the
closure device functions essentially as a one piece sealing
device.
Another advantage of the present invention is that
anchor member and introducer of the present invention may be
used to reliably position closure device generally along the
wall of the blood vessel without significantly obstructing
the blood vessel, duct or organ of the patient.
Another advantage of the present invention is that
closure device of the present invention may be used to
reliably position closure device generally along the wall of
the blood vessel without being significantly affected by the
depth of the puncture in the patient.
Yet another advantage of the present invention is that
sealing device of the present invention may be readily
modified to reliably position closure device generally along
the wall of the blood vessel in nearly any diameter puncture
without adversely affecting the operation of the device.
In one aspect, the present invention resides in an
assembly for sealing an incision or puncture in the body of
CA 02278243 2005-06-22
- 6a -
a patient wherein the incision or puncture extends through
the tissue of the patient into a blood vessel, duct or lumen
of the patient, the assembly comprising; an anchor member
formed of a first bioabsorbable material and sized to be
positioned in the blood vessel, duct or lumen of the
patient; an elongate member formed of said first
bioabsorbable material; a compressible member formed of a
bioabsorbable material and said compressible member being
slidably positioned along said elongate member and said
l0 compressible member cooperatively sealing the incision or
puncture from the flow of fluids therethrough in combination
with said anchor member.
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
BRIEF DESCRIPTION OF THE DRAWINGS
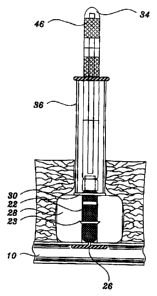
Figure 1 is a side view, partially in cross section,
showing the closure device and extension rod of the present
invention;
Figure 2 is a partial side view showing the access
sheath and bypass member of the present invention;
Figure 3 is a partial side view, partially in cross
section, showing a front view of the anchor member of the
closure device positioned in the access sheath;
Figure 4 is an enlarged partial side view of the
folded anchor member in the access sheath;
Figure 5 is a side view, partially in cross section,
showing the closing device and access sheath in the
puncture tract with the anchor member of the closure device
extended from the access sheath;
Figure 6 is a side view, partially in cross section,
showing the sealing assembly of the present invention with
the anchor member extended and positioned along the inner
wall of the patient s blood vessel;
Figure 7 is a side view, partially in cross section,
showing the closure device and extension rod of Figure 1
after the access sheath has been removed from the patient
and having the closure device initially positioned in the
incision;
Figure 8 is a side view, partially in cross section,
showing the push rod installed on the extension rod and
closure device;
Figure 9 is a side view, partially in cross section,
showing the closure device and extension rod of Figure 1 in
the incision after the push rod and retainment ring have
been moved distally along the rod member to cause the
radial expansion and distal movement of the collagen tube
along the rod member;
Figure 10 is a side view, partially in cross section,
showing the closure device of Figure 1 finally positioned
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
_ g _
in the puncture tract after the proximal portion of the rod
member has been removed;
Figure 11 is a side view showing the extension rod of
Figure 1;
Figure 12 is a side view showing the push rod of the
present invention;
Figure 13 is a side view showing the closure device of
the present invention;
Figures 14A, 14B and 14C are front, side and bottom
views respectively showing an alternate form of the
retainment ring of Figure 1; and
Figure 15 is a side elevational view, partially in
cross section, showing the alternate form of the retainment
ring of Figure 14 showing the closure device finally
positioned in the puncture tract.
DETAILED DESCRIPTION OF THE PRESENT INVENTION
The present invention is described hereinafter with
specific reference to the use of the present invention for
sealing an incision or puncture in a blood vessel such as
the femoral artery 10 of a patient. The sealing assembly
16 has particular utility when used in connection with
intravascular procedures, such as angiographic dye
injection, stems, cardiac catheterization, balloon
angioplasty and other types of recanalizing of
atherosclerotic arteries, etc. because the closure device
18 is designed to cause immediate hemostasis of the tissue
and blood vessel of the patient. It is contemplated that
the sealing assembly 16 of the present invention may be
used with nearly any catheterization or other medical
procedure wherein it is desirable to seal an incision or
puncture to prevent the loss of the patient's body fluid
therethrough, including laparoscopic or similar procedures.
Additionally, the closure device 18 of the present
invention may be used with nearly any catheterization or
?.
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
_ g _
other medical procedure or introduction device wherein it
is desirable to reliably locate the lumen of a blood
vessel, duct or target organ of a patient s body to prevent
the loss of the patient s body fluid therethrough,
including laparoscopic, cardioscopic, endoscopic,
intracardiac or similar procedures.
Referring now in greater detail to the various figures
of the drawings wherein like reference characters refer to
like parts, there is shown at 16 a combination of
components forming the sealing assembly, a portion of which
includes various non-bioabsorbable devices for deploying
the bioabsorbable closure device 18 to seal a percutaneous
puncture tract 14. The puncture tract 14 includes not only
the opening in the wall of the vessel but also the
passageway in the tissue located between the vessel and the
skin surface 12 of the patient which is formed when the
procedure is performed.
As used herein, the distal end of an element is
referred to as the end of the element nearest to the
patient and the proximal end of an element is referred to
as the element furthest from the patient.
In order to more fully understand and appreciate the
present invention, a brief description of a conventional
angiographic catheterization procedure through the femoral
artery of the patient is set forth herein. In such a
procedure, an angiographic needle (not shown) is inserted
percutaneously through the epidermal and dermal layer of
the skin 12 of the patient at a preferred angle of
approximately 25 to 45 degrees. The needle is inserted
between about 6 mm and 70 mm percutaneously into the skin
of the patient until the needle pierces the femoral artery.
The puncture of the artery 10 by the needle is then
confirmed by the physician and a small diameter guide wire
(not shown) is inserted through the needle for a distance
of approximately 15 to 20 cm. The needle is then withdrawn
over the guidewire while pressure is applied to the artery
ZO to limit the bleeding and prevent the formation of a
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 10 -
hematoma at the puncture site. A dilator (not shown) and
an outer introducer or catheter procedure sheath are
inserted over the guidewire and the guidewire is then
removed from the inside of the dilator. Next, the catheter
is advanced through the procedure sheath to the final
desired location and the procedure is performed. Once the
procedure has been completed, the catheter is removed and
only the procedure sheath remains in the puncture to allow
the user to perform a sheath exchange or insert the
procedure sheath of the present invention into the puncture
over a guide wire or using another conventional procedure
to insert the sealing assembly 16. Therefore, the
procedure sheath which is used to perform the initial
procedure may be the same as or different from the access
sheath 2 0 which forms part of the seal ing assembly 16 as
described hereinafter
As shown in Figures 1-11, a preferred form of the
present invention consists of the sealing assembly 16 which
generally includes the access or procedure sheath 20, the
closure device 18 and related components such as the push
rod 36, extension rod 34 and bypass member 42 as described
below. As shown in the drawings, the closure device 18 of
the present invention includes a generally elongate and
preferably cylindrical rod-shaped member 22 which is
constructed of a porous, biodegradable material such as a
polymerized polylactic acid, or polyglycolic acid matrix or
similar bioabsorbable materials may also be used. The
distal end 24 of the rod member includes the anchor member
26 as described in further detail below. The present
invention also preferably includes a tubular collagen
member 28 which surrounds a portion of the rod member 22
proximally of the anchor member 26 and a retainment ring 30
which is located proximally thereof as also described
below. Additionally, one or more of the bioabsorbable
rod member 22, collagen member 28 and retainment ring 30 of
the closure device 18 may be formulated to include a
conventional clotting agent, such as a tissue
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 11 -
thromboplastin, which is incorporated in at least a portion
of the desired material to accelerate local hemostasis and
which will allow the physician to maintain the patient on
an anticlotting agent such as heparin after the procedure
has been performed. It is further anticipated that at
least a portion of the closure device 18 may be formulated
to include a radiopaque material therein to allow the
placement of the closure device 18 to be observed using
conventional visualization methods. The use of radiopaque
materials also allows the physician to identify the
location of the closure device at a later time if a further
procedure is necessary.
The closure device 18 preferably has three basic
bioabsorbable components; namely, a rod member 22 with a
preferably integral or substantially integral intraarterial
anchor member 26, a tubular collagen member 28 and a
retainment ring 30. The proximal end of the rod member 22
is preferably threadedly or otherwise releasably connected
to an elongate extension rod 34. The extension rod 34
forms part of the initial sealing assembly that is inserted
through the access sheath 20. Alternately, the push rod 36
may be included as part of the initial sealing assembly
although the push rod 36 is preferably used once the access
sheath 20 is removed from the puncture tract 14 as
described below. The extension rod 34 is a relatively
small diameter memk~er which extends through the access
sheath 20 to enable the user to manipulate the closure
device 18 while it is in the access sheath 20. The push
rod 36 is a larger diameter member which encircles the
extension rod 34 and is used to push the retainment ring 30
distally along the rod member 22 as described in more
detail below. Both the extension rod 34 and push rod 36
are removed after the closure device 18 is positioned in
the puncture tract 14 and may therefore be constructed of
conventional catheter type materials such a PVC or similar
polymeric materials.
CA 02278243 1999-07-20
WO 98/31287 PCTIUS98/01189
- 12 -
The rod member 22 is in the form of a preferably
molded and generally elongated cylindrical-shaped member;
e.g., a hemostatic and resorbable material such as a
lactide and glycolide polymer or similar material which may
further include a hemostasis promoting and/or radiopaque
material in at least a portion thereof. In one form of the
rod member 22, a lip member 23 may be positioned proximally
of the anchor member 26 to provide resistance to the
passage of the collagen member 28 thereover. The length
and diameter of the rod member 22 is chosen to facilitate
the insertion of the closure device 18 into and through the
puncture tract 14 for the prompt sealing of the puncture
tract 14 and wall of the blood vessel.
The anchor member 26 is preferably an elongated,
relatively stiff, low-profile, resorbable member which is
arranged to be seated inside the artery against the artery
wall generally adjacent to or contiguous with the puncture
11. The anchor member 26 is preferably molded as part of
the distal end 24 of the rod member 22 and is preferably
made of a non-hemostatic resorbable polymer which is
similar to or compatible with the resorbable rod member 22.
The collagen member 28 is preferably shaped as an
elongate tubular member which is formed of a non-allergenic
hemostatic resorbable material such as a collagen sponge or
foam material. The retainment ring 30 is preferably a
relatively small member which is slidably positioned along
the rod member 22 proximally of the collagen member 28 and
is preferably formed of a molded resorbable polymeric
material such as a lactide/glactide copolymer. The outer
diameter of the retainment ring 30 is preferably greater
than the outer diameter of the extension rod 34 to enable
the push rod 36 to move the retainment ring 30 distally
along the rod member 22 as described below. A reinforcing
suture 32 or other material may be molded into the distal
end portion 24 of the rod member 22 to reinforce and
connect the anchor member 26 to the rod member 22. The
interconnection between the anchor member 26 and the rod
r~
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 13 -
member 22 serves to provide a pivot point for the relative
movement of the anchor member 26. This interconnection is
preferably formed to enable the movement of the anchor
member 26 from an insertion position wherein the anchor
member 26 is aligned with the lengthwise dimension of the
rod member 22 and a laterally oriented position wherein the
anchor member 26 extends laterally from the distal end 24
of the rod member 22 to engage the wall of the blood vessel
of the patient. The collagen member 28 is slidably
positioned along the rod member 22 so that distal movement
of the push rod 36 and retainment ring 30 along the rod
member 22 causes compression of the collagen member 28 as
well as radial expansion and distal movement of the
collagen member 28 along the rod member 22. Moreover, as
will be appreciated from the description to follow, the
entire closure device 18 is designed to reduce post-
procedure puncture complications, provide secure puncture
sealing, enable faster patient ambulation, cause minimal
inflammatory reaction and resorb completely within a
relatively short period of time; e.g., sixty to ninety
days.
In accordance with the following general description
of the method of use of the closure device 18 of the
present invention, the closure device 18 is used after the
interventional procedure is finished. In accordance with
the method of this invention, the access sheath 20 is
exchanged with the procedure sheath using conventional
catheter exchange procedures or is left in the artery and
the procedure sheath is used as the access sheath 20. The
location of the distal end of the access sheath 20 is then
confirmed as being within the blood vessel and extending
slightly beyond the puncture tract 14. The closure
device 18 is either previously inserted into the access
sheath 20 or is next inserted into the access sheath 20 so
that the extension rod 34 extends from the proximal end of
the access sheath 20. The closure device 18 is then moved
distally in the access sheath and partially deployed
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 14 -
(ejected) from the distal end of the access sheath 20 by
moving the extension rod 34 distally with respect to the
access sheath 20 while holding the access sheath 20 fixed
relative to the skin surface 12 of the patient. On further
distal movement of the extension rod 34 relative to the
access sheath 20, the anchor member 26 and a portion of the
rod member 22 are passed out of the distal end of the
access sheath 20 and deployed into the artery lumen. As
the anchor member 26 passes from the distal end of the
access sheath 20, the orientation of the anchor member 26
is changed with respect to the lengthwise dimension of the
rod member 22 as the proximal end of the anchor member 26
passes beyond the distal end of the access sheath. The
anchor member 26 pivots to extend laterally from the distal
end 24 of the rod member 22 as a result of the anchor
member's preferred bias towards the lateral orientation.
The closure device 18 is then withdrawn with respect
to the access sheath 20 by withdrawing the extension rod 34
until resistance is felt. The resistance is caused when
the anchor member 26 catches on the distal end of the
access sheath 20. Once this occurs (and assuming that the
anchor member 26 is in the correct orientation when it
catches on the end of the access sheath 20), the access
sheath 20 and the extension rod 34 are then withdrawn
together a short distance relative to the skin 12 of the
patient. This withdrawal or proximal movement with respect
to the puncture causes the anchor member 26 to engage
(catch) on the artery wall contiguous with the puncture
tract 14. The access sheath 20 is then withdrawn relative
to the skin of the patient and the extension rod 34. The
proximal movement of the access sheath relative to the
skin 12 of the patient and the closure device causes the
rod member 22, collagen member 28 and the retainment
ring 30 to be exposed to the tissue in the puncture
tract 14.
The push rod 36 may then be positioned on the sealing
assembly to surround a portion of the extension rod 34 and
r*
CA 02278243 1999-07-20
WO 98/31287 PCT/LTS98/01189
- 1.5 -
rod member 22. The push rod 36 is then moved distally
relative to the extension rod 34 and rod member 22 to push
the retainment ring 30 distally along the rod member 22 and
bring the collagen member 28 into engagement with the wall
of the artery adjacent to puncture site. The distal
movement of the retainment ring 30 along the rod member 22
also has the effect of deforming the collagen member 28
into a collagenous mass which preferably has a larger
diameter than the diameter of the puncture in the wall of
the blood vessel. This expansion in the diameter of the
collagen member aids in holding the closure device 18 in
place in the blood vessel and puncture without requiring a
separate procedure or step to expand the puncture tract 14.
As shown in the drawings, the installed closure device
sandwiches the wall of the blood vessel between the anchor
member 26 and the collagen member 28. Moreover, since the
collagen member 28 is preferably formed of a compressed
collagen, it also preferably expands in the presence of
fluid or blood within the puncture tract 14 when deployed,
thereby further contributing to the collagen member s 28
radial enlargement. The retainment ring 30 is moved along
the rod member 22 by pushing the push rod 36 distally with
respect to the extension rod 34 and rod member 22 until the
retainment ring 30 encounters a locking member 38 which may
be a groove or other change in the diameter or
circumference on the distal portion of the rod member 22.
The push rod 36, retainment ring 30 and locking member 38
serve as an immediate tamper of the collagen member 28 to
provide rapid sealing and hemostasis in the puncture
tract 14. Once the retainment ring 30 reaches the locking
member 38, the extension rod 34 may be disconnected from
the proximal end of the rod member 22 and the extension rod
34 and push rod 36 may be removed from the puncture
tract 14.
The closure device 18 is now maintained in the desired
position wherein the wall of the blood vessel is clamped
between the collagen member 28 and the anchor member 26 by
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 16 -
the natural contraction of the tissue of the patient around
the closure device 18, the initial clotting action of the
patient and the expansion of the hemostatic collagen
member 28. Thus the artery wall is sandwiched between the
collagen member 28 and the anchor member 26 and the flow of
fluids through the puncture tract and puncture is stopped
by physical and physiological actions. Within a few hours
after deployment, the anchor member 26 will be coated with
fibrin and thus attached firmly to the arterial wall,
thereby minimizing the possibility of distal embolization.
Furthermore, the preferred use of a reinforcing suture 32
or similar member in the rod member 22 and anchor member 26
further reduces the already unlikely possibility that the
anchor member 26 may somehow separate from the distal
end 24 of the rod member 22.
During prior clinical testing of a similar anchor
member, only a small deposit of the anchor material will
remain along the wall of the blood vessel after
approximately thirty days. In fact, resorption of all
components of the closure device 18 is believed to occur
after approximately sixty days. Thus, the resorbable
anchor has an insignificant hemodynamic effect on blood
flow and functions to assist in the reliable positioning of
the remaining components of the closure device as described
above while being completely absorbed in a relatively short
period of time.
As will be appreciated by the more detailed
description of the components of the present invention to
follow, deployment of the preferred form of the closure
device 18 using the access sheath 20 is easy, quick and
reliable. Anchoring and deployment of the collagen member
28 is repeatable, safe and effective without the need for
external pressure to enable more rapid ambulation of the
patient. Hemostasis is believed to occur almost
instantaneously; e.g., in 15 seconds or less, when the
closure device 18 is deployed properly.
r,
CA 02278243 1999-07-20
WO 98/31287 PCT/ITS98/01189
- 17 -
The collagen member 28 comprises a cylindrical and
tubular member formed of a preferably compressible,
resorbable, collagen foam, such as that sold by Colla-Tec,
Inc. of Plainsboro, New Jersey. The collagen member 28 is
arranged to be compressed from a larger diameter
configuration to a smaller diameter, elongated
configuration which is positioned along the rod member 22
proximally of the anchor member 26 and inserted into the
access sheath 20. In the configuration wherein the
collagen member 28 is compressed and inserted into the
access sheath 20, the additional diameter of the collagen
member 28 along the rod member 22 is very small and,
therefore, suitable for disposition within the access
sheath 20. The length of the collagen member 28 is
preferably sufficient to seal a substantial portion of the
lengthwise dimension of the puncture proximally of the wall
of the blood vessel and is also sufficient to prevent
puncture tract bleeding which results from capillary
bleeding in the puncture tract.
The anchor member 26 basically comprises a thin,
narrow strip or bar of resorbable material such as a
resorbable lactide/glycolide polymer sold by Medisorb
Technologies International L.P. under the trade designation
MEDISORB. The strip is sufficiently rigid such that once
it is in position with the artery it is resistant to
deformation to preclude it from bending to pass back
through the puncture tract 14 through which it was f first
introduced. The anchor member 26 preferably has a
generally planar top surface, a generally planar bottom
surface and a centrally located surface which is pivotally
connected the distal end 24 of the rod member 22. Each end
of the anchor member 26 is preferably rounded. The
centrally located surface of the anchor member 26
preferably includes a hemispherical projection which is
located at the center of the top surface. The
hemispherical projection preferably includes one or more
reinforcing sutures 32 extending therethrough and a
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 18 -
longitudinally extending slot disposed perpendicularly to
the top surface of the anchor member 26 having the sutures
molded therein. The overall shape of the anchor member 26
is chosen so as to allow the anchor member 26 to initially
pass through the wall of the blood vessel and into the
blood vessel while preventing the retraction therethrough
and without significantly obstructing the flow of blood
through the blood vessel. In this regard the biased and
laterally extending connection between the anchor member 26
and the rod member 22 is preferably effected during the
molding of the rod member 22 and anchor member 26 and is
preferably further reinforced by the use of the relatively
stiff suture 32.
The details of the preferred form of the access sheath
20 will now be described. As can be seen, the access
sheath 20 basically comprises a conventional catheter
introducer which includes an elongated tube 40 formed of a
somewhat flexible material, such as polyethylene or
polyvinyl chloride, so that the closure device 18 may be
freely passed through the access sheath 20 into an
operative position within the patient's artery,
notwithstanding any curvature of the elongated tube 40
which may exist.
In accordance with a preferred embodiment of this
invention, the outside diameter of the access sheath 20 is
approximately 8-French because the majority of relevant
procedures utilize an 8 french procedure sheath. It should
be understood that the choice of an 8 french access sheath
is for illustration purposes in describing the preferred
form of the present invention and it is contemplated that
other sizes are readily within the scope of the present
invention. The proximal end of the access sheath 20 may
include a rigid funnel shaped bypass member 42 inserted or
mounted thereon to enable the closure device 18 and
extension rod 34 to be inserted through a conventional
hemostasis valve (not shown) which is formed on the distal
portion of the access sheath 20. The distal end of the
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 19 -
elongated tube 40 preferably necks down into a conventional
tapered configuration and may include a longitudinally
extending slot 44 thereon to facilitate the pivoting of the
anchor member 26 and to indicate the desired alignment of
the anchor member 26 relative to the distal end of the
access sheath 20 as described below.
As shown in the drawings, the extension rod 34, bypass
member 42 and push rod 36 of the present invention include
various markings and projections thereon to provide the
l0 user with visual and tactile indications of when certain
steps in the insertion of the closure device I8 have been
accomplished. As described above, the extension rod 34 is
an elongate member which is releasably attached to the
proximal end of the rod member 22. The length of the
extension rod 34 is chosen so that when the anchor
member 26 of the closure device 18 extends beyond the
distal end of the access sheath 20, the finger grip area 46
on the proximal end of the extension rod 34 will extend
proximally of the proximal end of the access sheath 20.
Additionally, a pair of circumferential bands 48 are
located on the extension rod 34 distally of the finger grip
area 46. The proximal circumferential band is used to
signal to the user that the anchor member 26 of the closure
device 18 is extended beyond the distal end of the access
sheath 20 when the proximal circumferential band 48 reaches
the bypass member 40 that is positioned in the proximal end
of the access sheath 20. The extension rod 34 also
includes a further circumferential colored band 50 located
distally of the pair of circumferential bands 48 on the
proximal portion of the extension rod 34 to signal to the
user when the retainment ring 30 has reached the locking
member 38 on the rod member 22. Finally, the bypass member
42 preferably includes the slot or alignment markings 44
thereon which may be aligned with the slot 52 on the distal
end of the access sheath to allow the user to readily align
the anchor member 26 with the slot 52 on the distal end of
the access sheath 20 by aligning the anchor member 26 with
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 20 -
the slot or markings 44 on the bypass member 42 as the
closure device is inserted into the proximal end of the
access sheath 20. The bypass member 42 may also include a
further reference detent (not shown) in its periphery
located diametrically opposite to the desired position of
the anchor member 26 to serve as a visual guide to help the
user orient the closure device 18 to a proper yaw angle
with respect to the central longitudinal axis of the
closure device 18 for insertion within the access sheath 20
as will be described later.
In the preferred method of use of the present
invention, the closure device 18 is inserted through the
distal end of the access sheath 20 prior to deployment. In
particular, the anchor member 26 is passed through the
bypass member 42 prior to use to temporarily change the
orientation of the anchor member 26 from lateral to
longitudinal with respect to the lengthwise dimension of
the rod member 22. Therefore, during the initial insertion
of the closure device 18 into the tract and puncture, the
2o anchor member 26 is disposed longitudinally within the
elongated tube portion 40 of the access sheath 20. The
collagen member 28 is located within the elongated tube
portion 40 of the access sheath 20 proximally of the
longitudinally oriented anchor member 26. Additionally,
the distal end 24 of the rod member 22 preferably overlies
the proximal end of the anchor member 26 so that the
overall diameter of the distal end 24 of the rod member 22
and the longitudinally disposed anchor member 26 is
approximately equal to the diameter of the rod member 22
proximally thereof and less than the diameter of the
portion of the rod member 22 having the collagen member
disposed therearound.
As can be seen in Figure 2, the access sheath 20
preferably includes a conventional luer fitting side port
on the proximal end thereof. The bypass member 42 is
preferably a funnel shaped member which is sized to extend
into the opening in the luer fitting and is secured in
CA 02278243 1999-07-20
WO 98/31287
- 21 -
PCT/US98/01189
place therein by any suitable means, including frictional
contact, to maintain the hemostatic valve on the proximal
end of the access sheath 20 open during the procedure.
The positioning of the access sheath 20 in the artery
may be accomplished utilizing an artery locator device or
an obturator which identify the location of the artery wall
by the flow of blood or by physical contact with the wall
of the blood vessel. Alternately, and significantly less
preferable, the depth of the puncture may be physically
measured and then the access sheath 20 may be moved to the
appropriate measured depth in the puncture using various
markings on the access sheath and/or by using selected
access sheaths having specified lengths. After the access
sheath 20 is positioned in the artery, a stopcock may be
opened to observe the flow of blood therefrom (thereby
indicating that the inlet port or window is within the
artery). The access sheath 20 is then retracted (moved
proximally) until the blood flow through the stopcock just
stops, thereby indicating that the distal end of the access
sheath has just left the artery lumen. The access sheath
20 is then reinserted approximately 10 mm into the puncture
to ensure that the distal end of the access sheath 20 is at
the desired position within the artery. Blood flow should
be reestablished through the stopcock at this time to
verify that the elongated tube 40 is not kinked or
otherwise obstructed. Then the stopcock is closed. From
this point on, the access sheath 20 must be kept fixed with
respect to the skin 12 of the patient to ensure that the
access sheath 20 does not move distally or proximally in
the puncture tract 14. The bypass tube 42 is then inserted
into the proximal end of the access sheath 20 to open the
hemostatic valve. The closure device is then inserted into
and aligned with the bypass member 42 to cause the anchor
member 26 to be deflected from the laterally extending
position to the longitudinal position. The closure device
18 is then moved distally past the bypass member 42 and
into the access sheath 20.
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 22 -
The slot or markings 44 on the bypass tube 42 are
identified by the user and the bypass tube 42 is grasped by
the user and oriented so that the slot or markings face up
and away from the patient. This alignment ensures that the
anchor member 26 is located towards the patient. The rigid
nature of the bypass tube 42 facilitates the passage of the
closure device 18 through the hemostasis valve and also
orients the anchor member 26 longitudinally with respect to
the rod member 22 to protect the closure device 18 from
damage. The closure device 18 and extension rod 34 are
then pushed fully down the access sheath 20 so that
proximal circumferential band 48 on the extension rod 34 is
aligned with the bypass member 42 on the proximal end of
the access sheath 20 to indicate to the user that the
distal end of the closure device 18 is aligned with the end
of the access sheath and the anchor member 26 is located in
the artery 10 and extends beyond the distal end of the
access sheath 20.
The sealing assembly 16 is then operated to determine
if the anchor member 26 has been properly deployed. To
that end the access sheath 20 is continued to be held by
the user to prevent axial and rotational movement, and the
closure device 18 is carefully withdrawn with respect to
the access sheath 20 and the skin 12 of the patient. This
action causes the anchor member 26 to engage or catch onto
the distal end of the access sheath 20. As the anchor
member 26 catches on the distal end of the access
sheath 20, resistance will be felt by the user. This
resistance must be noted by the time the distal
circumferential band 48 on the extension rod 34 is visible
along the bypass member 42. If resistance is felt, then
the anchor member 26 will have caught on the distal end of
the access sheath 20 at the location of the hemispherical
projection on the anchor member 26. If resistance is not
felt, the anchor member 26 has not deployed and the above
insertion sequence must be repeated by turning the closure
r.
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 23 -
device 18 about its axis by one-quarter turns to either
side before it is again withdrawn.
If the resistance is felt before the distal
circumferential band 48 is visible above the bypass member
42, this will indicate that only one of the ends of the
anchor member 26 has caught on the end of the access sheath
20, an undesired occurrence. Accordingly, the closure
device 18 must be reinserted within the access sheath 20
and the foregoing procedure retried, this time by turning
the closure device 18 about its axis by one-quarter turns
to either side before it is again withdrawn relative to the
access sheath 20.
Once the anchor member 26 has been properly deployed
to the laterally extending orientation (Figure 5), the
access sheath 20 and the closure device 18 are held
together and withdrawn as a unit from the puncture, whilst
swinging the unit toward the vertical. This action causes
the anchor 26 to engage or catch onto the inner surface of
the artery 10 contiguous with the puncture tract 14. The
access sheath 20 is then removed. Inasmuch as the anchor
member 26 is trapped against the interior of the artery
wall, the removal of the access sheath 20 causes the rod
member 22 and the collagen member 28 to be exposed to the
fluids and blood present in the puncture tract 14. As the
access sheath 20 comes out of the puncture tract,
continuous steady resistance must be felt on the rod member
to ensure that the anchor member 26 is properly deployed.
At this point the anchor member 26 has been deployed
along the wall of the blood vessel 10 and the collagen
member 28 is positioned in the puncture tract 14 proximally
of the wall of the artery. At this time, the push rod 36
is installed over the extension rod and the collagen
member 28 is moved distally in the puncture tract 14 by the
distal movement of the push rod 36. In particular, the
user compacts the collagen member 28 by gently moving the
push rod 36 distally to cause the retainment ring 30 to
move distally along the rod member 22. The push rod 36 and
CA 02278243 1999-07-20
WO 98/31287 PCTlUS98/01189
- 24 -
retainment ring 30 are manually slid down the rod member 22
by the user so that the retainment ring 30 is moved
distally in the puncture tract 14 until the retainment
ring 30 engages the locking member 38 as indicated to the
user by the colored band 50 reaching the proximal end of
the push rod 36. This movement of the retainment member 30
moves the collagen member 28 distally to the desired
location in the puncture tract 14. A gentle distal
movement is adequate to achieve the desired result; i.e.,
to assist the distal end portion of the collagen member 28
to conform to the outside of the blood vessel contiguous
with the puncture and to assist to lock the collagen member
28 and anchor member 26 in place until hemostasis occurs
(which happens very quickly, thereby further locking the
closure device in place). It should be noted that during
the distal movement of the retainment ring 30 and collagen
member 28, care must be taken to maintain tension on the
rod member 22 at a load greater than that used on the push
rod 36 to ensure that the action doesn't propel the
collagen member 28 into the interior of the blood vessel.
After the retainment ring 30 reaches and is engaged by
the locking ring 38, the extension rod 34 may be rotated or
otherwise manipulated relative to the rod member 22 to
separate the extension rod 34 from the rod member 22.
Alternately, the length of the rod member 22 may be chosen
so that the proximal portion of the rod member 22 extends
beyond the skin level of the patient and the rod member 22
may then be cut to separate the rod member 22 from the
extension rod 34. The extension rod 34 and push rod 36 are
then removed and any portion of the rod member 22 extending
above the retainment ring 30 may be excised. The closure
device 18 is then preferably left in this condition without
being disturbed for a few minutes to allow complete
hemostasis. After that time the condition of the patient
may be evaluated and the patient may be ambulated or
discharged as determined by the physician.
CA 02278243 1999-07-20
WO 98/31287
- 25 -
PCT/US98/01189
With the closure device 18 in the final sealing
position, the anchor member 26 (the only portion of the
closure device within the blood vessel) does not take up a
substantial portion of the interior of the blood vessel
and, thus, does not block off or otherwise impede the flow
of blood therethrough. Since the components of the closure
device 18 are all formed of resorbable materials, the
closure device 18 can be left in place within the body
until it is absorbed.
As shown in Figures 14 and 15, the retainment ring of
the present invention may be formed of various shapes. In
this embodiment, the alternate form of the retainment
ring 60 preferably includes a circular base section 62 with
an opening 64 for the rod member 22 to pass therethrough
and a pair of leg members 66 which are biased to press
against the rod member 22 as the retainment ring is moved
distally therealong. As shown in Figure 15, the leg
members 66 preferably assist in expanding the proximal
portion of the collagen member 28 to provide yet another
mechanism to securely retain the sealing device in the
puncture tract.
As should also be appreciated from the foregoing, the
closure device of the present invention, the instrument for
deploying it and the method of use enables the ready,
effective and efficient sealing of a percutaneous puncture
in an blood vessel. The closure device 18 may allow for
the continuance of anticoagulation post-procedure, more
aggressive use of thrombolytic agents and safer use of
large bore catheters. It should also reduce discomfort and
complication rates for patients, allow many in-patient
procedures to be performed safely on an out-patient basis,
decrease the time and cost of interventional procedures and
reduce exposure of hospital personnel to human blood.
While the preferred forms of the present invention are
described and illustrated herein, it will be obvious to
those skilled in the art that various changes and
modifications may be made thereto without departing from
CA 02278243 1999-07-20
WO 98/31287 PCT/US98/01189
- 26 -
the scope of the present invention as defined by the
following claims. For example, it is anticipated that
various modifications may be made to the anchor member and
access sheath to facilitate the deployment and retention of
the closure device in the desired position in the puncture.