Note: Descriptions are shown in the official language in which they were submitted.
F2023
The Swedish Patent Office PST/ S E 9 9 / 0 1 6 0 0
P ,T International Appllcatton
1
NOVEL COMPOUNDS
This invention relates to novel pyridyl derivatives, their use as medicaments,
pharmaceutical formulations including them and methods for their preparation.
s
16 -10- 2000
PCT Patent Applications PCT/SE98/00423, PCT/SE98/00505 and PCT/SE98/00575
disclose pyridine alkanol derivatives and their use as mast cell inhibitors.
A series of structurally distinct compounds have now been found to be useful
for the
~o modulation of inflammatory conditions. In a first aspect the present
invention therefore
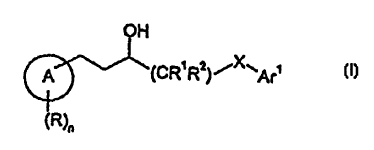
provides a compound of formula I:
OH
A (CR' R2) ~X~Ar'
(R)n
~ s (I)
wherein;
A is a phenyl or a S- or 6-membered heterocyclic ring containing one or more
heteroatoms
selected from nitrogen, oxygen and sulfur;
R is halogen, nitro, C,~ alkyl, C,~ alkoxy, (CHZ)PR3 where p is 0 to 3 and R3
is hydroxy, or
zo NR4R5 where R~ and RS are independently hydrogen or C,~ alkyl or R is
CONR4R5;
nis0, l,2or3;
X is O, S or CHz;
R' and R' are independently hydrogen, C,~ alkyl or C3_6 cycloalkyl or R' and
RZ together
with the carbon atom to which they are attached form a C3_f; cycloalkyl group;
zs Ar' is biphenyl, naphthyl or tetrahydronaphthyl, each Ar' group being
optionally
substituted by halo, nitro, C,~ alkyl (optionally substituted by one or more
fluorine atoms),
CN, -Y-NRgC(O)NR9R'°, -O-Y-C(O)NR9R'°, -O-Y-
C(S)NR9R'°, -Y-C(O) NR9R'°,
-Y-C(S)NR9R'°, -Y-SOzNR9R'°, -Y-NR9R'°, SOzNR9R'°,
C(,O)NR9R'°, C(S)NR9R'°,
C(O)R", - OC(O)R" , -Y-OR", -Y-COZR", SOZR'3, N(R'Z)SOZR'3, N(R'2)C(O)R'3 or
3o N(R'')COzR'3 where:
Y is a bond, C,_6 alkylene or CZ~ alkenylene;
CA 02341440 2001-02-21
Amended Sheet
F2023
F'C~/St99/01600
16 -10- 2000
R9 and R'° are independently hydrogen or C,_6 alkyl or together with
the nitrogen atom to
which they are attached form an optionally substituted 5- to 7-membered
heterocyclic ring
optionally containing a further heteroatom selected from nitrogen, oxygen or
sulfur;
R8, R", R'z and R'3 are independently hydrogen or C,_,° alkyl
(optionally substituted by one
or more fluorine atoms);
or a salt or solvate thereof,
~ provided that when n is zero then A is not pyridyl or pyrimidinyl.
Alkyl, alkylene and alkenylene groups, whether alone or part of another group,
can be
~ o straight chained or branched and can be optionally substituted by one or
more fluorine
atoms and optionally interrupted by one or more oxygen atoms.
Suitably A is a phenyl or a 5- or 6-membered heterocyclic ring containing one
or more
heteroatoms selected from nitrogen, oxygen and sulfur. Examples of such rings,
which can
is be saturated or aromatic, include pyridine, pyrimidine, thiazole,
pyrrolidone, pyridazine
and pyridone. Preferably A is phenyl, pyridine, pyrimidine, thiazole or
pyrrolidone.
Suitably n is 0, 1, 2 or 3 such that multiple substituents can be present.
Preferably n is 0, or
when A is pyridine n is preferably 1.
Suitably X is O, S or CHZ. Preferably X is O.
Suitably R is halogen, nitro, C,~ alkyl, C,~ alkoxy, (CHZ)pR3 where p is 0 to
3 and R3 is
hydroxy, or NR°R5 where R4 and R5 are independently hydrogen or C,~
alkyl or R is
zs CONR'R5. Substituents can be present on carbon or appropriate nitrogen
atoms of the ring
A. Preferably R is fluoro, nitro, methyl, methoxy, dimethylamino, (CHZ)PR3
where p is 1
and R3 is hydroxy or NR4R5 where R4 is hydrogen and RS is methyl or C,~ alkyl,
or R is or
R is CONR4R5 where R4 is hydrogen and RS is methyl.
3o Suitably R' and RZ are independently hydrogen, C,~ alkyl or C3_6 cycloalkyl
or R' and RZ
together with the carbon atom to which they are attached form a C3_6
cycloalkyl group.
Preferably R' and RZ are both hydrogen.
Suitably Ar' is biphenyl, naphthyl or tetrahydronaphthyl, each Ar' group being
optionally
3s substituted by halo, nitro, C,~ alkyl (optionally substituted by one or
more fluorine atoms),
CN, -Y-NRBC(O)NR9R'°, -O-Y-C(O)NR9R'°, -O-Y-
C(S)NR9R'°, -Y-C(O) NR9R'°,
CA 02341440 2001-02-21
Amended Sheet
F2023
('CT/SE99/01600
1 6 -10- 2000
-Y-C(S)NR9R'°, -Y-SOzNR9R'°, -Y-NR9R'°, SOzNR9R'°,
C(O)NR9R'°, C(S)NR9R'°,
C(O)R", - OC(O)R" , -Y-OR", -Y-COZR", SOzR'3, N(R'z)SOzR'3, N(R'z)C(O)R'3 or
N(R'z)COZR'3 where Y is a bond, C,~ alkylene or Cz_6 alkenylene. More than one
substituent can be present, preferably one or two substituents are present.
Particularly preferred compounds of the invention include:
(~)-1-(Biphenyl-4-yloxy)-4-(3-(2-fluoro)pyridyl)-2-butanol,
(~)-1-(Biphenyl-4-yloxy)-4-(3-(2-methoxy)pyridyl)-2-butanol,
(~)-1-(Biphenyl-4-yloxy)-4-(3-(6-fluoro)pyridyl)-2-butanol,
'o (t)-1-(Biphenyl-4-yloxy)-4-(3-(6-methoxy)pyridyl)-2-butanol,
(~)-1-(Biphenyl-4-yloxy)-4-(6( 1 H)-pyridone-3-yl)-2-butanol,
1-(Biphenyl-4-yloxy)-4-[4-(3-fluoro)pyridyl]-2-butanol,
1-(Biphenyl-4-yloxy)-4-[(3-methoxy)-4-pyridyl]-2-butanol,
1-(Biphenyl-4-yloxy)-4-(4-pyridin-2-one)-2-butanol,
's 1-[(Biphenyl-4-yloxy)]-4-(4-pyridazin-3-yl)-2-butanol,
1-[(Biphenyl-4-yloxy)]-4-(pyridazin-4-yl)-2-butanol,
1-[(Biphenyl-4-yloxy)]-4-(pyrimidin-4-yl)-2-butanol,
(t)-4-(2-(Hydroxymethyl)phenyl)-1-(2-thionaphthyl)-2-butanol,
(t)-N-Methyl-2-(4-(2-thionaphthyl)-3-hydroxybutyl)benzamide,
zo (~)-N-Methyl-2-(4-(2-thionaphthyl)-3-hydroxybutyl)benzylamine,
(t)-N-Methyl-2-(4-(2-(5,6,7,8-tetrahydronaphthyloxy))-3-
hydroxybutyl)benzamide,
(~)-N-Methyl-2-(4-(2-(5,6,7,8-tetrahydronaphthyloxy))-3-
hydroxybutyl)benzylamine,
(~)-2-(4-(2-(5,6,7,8-Tetrahydronaphthyloxy))-3-hydroxybutyl)benzyl alcohol,
( 1 S.2RS)-4'-(2-Hydroxy-1-methyl-4-thiazol-2-yl-butoxy)-biphenyl-3-
carbonitrile,
4-[4-(Biphenyl-4-yloxy)-3-hydroxy-butyl]-2-methoxy-phenol,
4-[4-(Biphenyl-4-yloxy)-3-hydroxy-butyl]-1-methyl-pyrrolidin-2-one,
(t)-4-[4-(Biphenyl-4-yloxy)-3-hydroxybutyl]-1-methyl-11~ pyridin-2-one,
or salts or solvates thereof.
3o Compounds of the invention can form pharmaceutically acceptable solvates
and salts. The
compounds of the formula (I) can form acid addition salts with acids, such as
conventional
pharmaceutically acceptable acids, for example malefic, hydrochloric,
hydrobromic,
phosphoric, acetic, fumaric, salicylic, citric, lactic, mandelic, tartaric,
trifluoroacetic and
methanesulphonic acids. Compounds of the invention may also form alkali metal
salts
3a such as magnesium, sodium, potassium and calcium salts.
CA °2341440 2001-02-21 Amended Sheet
F2023
PCT/St99/01600
4
16 -10- 2000
Certain compounds of formula (I) are capable of existing in stereoisomeric
forms including
enantiomers and the invention extends to each of these stereoisomeric forms
and to
mixtures thereof including racemates. The different stereoisomeric forms may
be
separated one from the other by the usual methods, or any given isomer may be
obtained
s by stereospecific or asymmetric synthesis. The invention also extends to any
tautomeric
forms and mixtures thereof, particularly in the case of compounds where A is
pyridyl and
R is hydroxy.
According to the invention there is also provided a process for the
preparation of
io compounds of formula (I) as hereinbefore defined which comprises:
(a) reduction of a compound of formula (II):
O
A (CR' R2) ~X ~Ar'
(R)n
(II)
~s wherein A, R, n, R', R2, X and Ar' is as defined in formula (I) or are
protected derivatives
thereof;
(b) for compounds of formula (I), wherein Ar' is a group R6-R', forming the R6-
R' bond by
reaction of a compound of formula (III):
OR'6
(CR~R2 /X~Rs~R
A v )
(R)n
(III)
with a compound of formula (IV):
RoR~s
(IV)
where A, R, n, R', RZ, R6, R' and are as defined in formula (II), R'6 is a
hydroxy protecting
group, and one of R"/R'g is triflate or halo and the other is B(OH)z or ZnHal;
or
CA 02341440 2001-02-21
Amended Sheet
F2023
~'CT/ F 9 9 / 0 1 6 0 0
16 -10- 2000
(c) for compounds of formula (I) where R' and Rz are both hydrogen, reaction
of a
compound of formula (V):
O
A
(V)
where A, R and n are as defined in formula (II), with a compound of formula
(VI):
MYAr' (VI)
io where Y is O, S or CHz, M is Li, Na, K, Cs or MgHal where Hal is halogen
and Ar' is as
defined in formula (I);
or with a compound of formula (VII):
HYAr' (VII)
's
where Y is as defined in formula (VI) in the presence of a base; or
(d) for compounds of formula (I), where R' and Rz are both hydrogen, and X
represents O
or S, reaction of a compound of formula (V) or (VI), as hereinbefore defined,
with a
suitably protected and activated derivative of a diol of formula (VIII):
zo
OH
OH
A
(R)" (VIII)
where R, n and A are as defined in formula (II); or
(e) preparation of compounds of formula (I) wherein X represents O, from a
compound of
z, formula (IX):
CA 02341440 2001-02-21 Amended Sheet
F2023
PCT/ S ~ 9 9 / 0 1 6 0 0
16 -10- 2000
R' R2)~OH
(R)n
(Ix)
in which R, n, A, R', R2, and R'6 are as defined in process (b) by reaction
with a compound
of formula (VII) wherein Y represents O; or
s (f) reaction of a compound of formula (X):
Hal
A
(R)n (x)
in which A, R and n are as defined in formula (II) and Hal is halogen with a
compound of
io formula (XI):
OH
CR' R2 ~X~Ar'
( )
(XI)
in which R', RZ, X and Ar' are as defined in formula (II) in the presence of a
suitable
is catalyst, or
(g) preparation of compounds of formula (I) wherein X represents O, from a
compound of
formula (XII):
O
~~OAr'
(XII)
Zo
in which Ar' is as defined in formula (II) with a compound of formula (XIII):
A jCH2M
R)n (XIII)
R~s
'(C
CA 02341440 2001-02-21
Amended Sheet
F2023
Pt;T/SE 99 / 0 1 600
7
1 6 -10- 2000
in which R and n are as defined in formula (II) and M is a metal, or
(h) preparation of compounds of formula (I) wherein X represents O from a
compound of
formula (XIV):
O
OAr~
s Ph3P (XIV)
in which Ar' is as defined in formula (II) with a compound of formula (XV):
jCHO
A
R)n
(XV)
io in which R and n are as defined in formula (II), followed by hydrogenation
of the resulting
product using a suitable catalyst
and optionally thereafter process (a) to (h):
~ removing any protecting groups
converting a compound of formula (I) into a further compound of formula (I)
~ s ~ forming a pharmaceutically acceptable salt or solvate.
Reduction of a compound of formula (II) can be carried out with a suitable
reducing agent
(e.g. sodium borohydride) for example at room temperature in the presence of a
suitable
organic solvent (e.g. ethanol).
Reaction of compounds of formulae (III) and (IV) can be carried out under the
conditions
of the Suzuki reaction (Synthetic Communications 11 (7), 51. 3-519, 1981 ) for
example at
100 °C in the presence of a suitable catalyst and base (e.g.
tetrakis(triphenylphosphine)-
palladium(0) and aqueous sodium carbonate) in a suitable solvent (e.g.
ethanol/toluene).
Reaction of a compound of formula (V), examples of which are (~)-3-(2-
oxiranylethyl)pyridine or a-(chloromethyl)-3-pyridinepropanol, with a compound
of
formula (VI) can be carried out in the presence of a base
such as sodium hydroxide in a suitable solvent such as aqueous ethanol.
CA 02341440 2001-02-21
Amended Sheet
F2023
PCT; S E 9 9 / 0 1 6 0 0
~ s -~o- zooo
Reaction of a compound of formula (V) with a compound of formula (VII) carried
out at
ambient or reduced temperature in a suitable solvent such as dimethylformamide
or
tetrahydrofuran.
s Process (d) is carried out at elevated temperature, for example at about
60°C, in the
presence of suitable base (e.g. sodium hydride) and an appropriate organic
solvent (e.g.
dimethylformamide). Suitably protected and activated derivatives include the
carbonate
compounds of formula (VIIIa):
O
A
(R)n
(VIIIa)
Processes (e) is carried out under the conditions of the Mitsonobu reaction,
for example at
approximately 0-25°C in the presence of diethyl azodicarboxylate and
triphenylphosphine
in an appropriate solvent (e.g. toluene).
~s
Process (f) is carried out in the presence of a suitable catalyst such as a
palladium catalyst,
for example palladium acetate.
In process (g) M is a metal such as lithium or magnesium.
zo
Compounds of formula (III) wherein R" is B(OH)2, can be prepared from a
compound in
which R" is bromine or iodine by, for example, treatment with n-butyllithium
and tri-
isopropylborate in an appropriate solvent (e.g. tetrahydrofuran), at low
temperature (e.g. -
78°C).
zs
An alternative preparation of compounds of formula (III) wherein X represents
O is from
compounds of formula (VII) as defined above by reaction with a compound of
formula
(XVI), in which R" is triflate, or halogen:
CA 02341440 2001-02-21
Amended Sheet
F2023
PCT/ St 99 / 0 'i 600
9
16 -10- 2000
R' ~
I \
(XVI)
under the conditions of the Mitsonobu reaction as described above.
s Compounds of formula (IX) can be prepared by reduction of a compound of
formula
(XVII)
OR'6 '
A \ (CR'R2)~O \
(R)n
(XVII)
~o in which R', R' and R'6 are as defined above using conventional procedures,
for example
hydrogenation using a palladium catalyst in an inert solvent such as ethyl
acetate, followed
by debenzylation using conventional methods such as those described in
'Protective Groups
in Organic Synthesis', 2"d Edition, T.W. Greene & P.G.M. Wuts, Wiley-
Interscience
(1991).
~s
Compounds of formula (XVII) can be prepared by the reaction of compound
(XVIII):
O
H~(CR'R2)~O \
(XVIII)
2o reported by Reetz et. al. Angew. Chem. Suppl., ( 1983), 1511.) in which R'
and Rz are as
defined above with a compound of formula (XIX):
M
A
(R)n (XIX)
CA 02341440 2001-02-21
Amended Sheet
F2023
PC./St=99/01600
l0 1 6 -10- 2000
in which A, R and n are as defined above and M is lithium, sodium, potassium,
MgX or
ZnX, where X is halogen, optionally in the presence of additives such as boron
trifluoride.
It will be appreciated by those skilled in the art that in the process
described above the
s functional groups of intermediate compounds may need to be protected by
protecting
groups.
Functional groups which it is desirable to protect include hydroxy, amino and
carboxylic
acid. Suitable protecting groups for hydroxy include organosilyl groups (e.g.
to tert-butyldimethylsilyl, tert-butyldiphenylsilyl or trimethylsilyl), benzyl
and tetrahydro-
pyranyl. Suitable protecting groups for amino include tert-butoxycarbonyl or
benzyloxy
carbonyl. Suitable protecting groups for carboxylic acid include C,~ alkyl or
benzyl esters.
The protection and deprotection of functional groups may take place before or
after a
reaction step.
The use of protecting groups is fully described in 'Protective Groups in
Organic
Chemistry', edited by J. W. F. McOmie, Plenum Press (1973), and 'Protective
Groups in
Organic Synthesis', 2nd edition, T. W. Greene & P. G. M. Wutz, Wiley-
Interscience
(1991).
Compounds of formula (I) can be converted into further compounds of formula
(I) using
standard procedures known in the art such as reduction, alkylation,
esterification etc.
Novel intermediates form a further aspect of the invention.
2s
Diastereoisomers may be separated using conventional techniques, e.g.
chromatography or
fractional crystallisation. The various optical isomers may be isolated by
separation of a
racemic or other mixture of the compounds using conventional, e.g. fractional
crystallisation or HPLC, techniques.
The compounds of the invention are useful because they possess pharmacological
activity
and more particularly activity in the modulation of inflammatory and allergic
conditions,
for example as shown in the test described below. The compounds of the
invention inhibit
the activation of a range of cell types from haematopoetic lineage, including
mast cells,
3s neutrophils and eosinophils.
CA 02341440 2001-02-21
Amended Sheet
F2023
PCT/ ~E 9 9 / 0 1 600
11 16 -10- 2000
In a further aspect the invention therefore provides a compound of formula
(IA)
OH
A (CR' RZ)~X~Ar'
(R)n
(I)
wherein;
A is a phenyl or a S- or 6-membered heterocyclic ring containing one or more
heteroatoms
selected from nitrogen, oxygen and sulfur;
R is halogen, nitro, C,~ alkyl, C,~ alkoxy, (CHZ)PR~ where p is 0 to 3 and R3
is hydroxy, or
~o NR4R5 where R4 and RS are independently hydrogen or C,~ alkyl or R is
CONR4R5;
nis0, l,2or3;
X is O, S or CH2;
R' and RZ are independently hydrogen, C,~ alkyl or C3_6 cycloalkyl or R' and
RZ together
with the carbon atom to which they are attached form a C3_6 cycloalkyl group;
~s Ar' is a fused bicyclic ring system optionally containing one or more
heteroatoms, a fused
tricyclic ring system optionally containing an oxygen atom, or Ar' is a group
R6-R' where
one of R6/R' is a phenyl ring and the other is phenyl or a 5- or 6-membered
heterocyclic
ring containing one or more heteroatoms, each Ar' group being optionally
substituted by
halo, nitro, C,~ alkyl (optionally substituted by one or more fluorine atoms),
CN,
20 -Y-NRgC(O)NR9R'°, -O-Y-C(O)NR9R'°, -O-Y-C(S)NR9R'°, -Y-
C(O) NR9R'°,
-Y-C(S)NR9R'°, -Y-SOzNR9R'°, -Y-NR9R'°, SOZNR9R'°,
C(O)NR9R'°, C(S)NR9R'°,
C(O)R", - OC(O)R" ,-Y-OR", -Y-COzR", SOZR'3, N(R'z)SOzR'3, N(R'Z)C(O)R'3 or
N(R'Z)COZR'3 where:
Y is a bond, C,_6 alkylene or CZ_6 alkenylene;
2s R9 and R'° are independently hydrogen or C,~ alkyl or together with
the nitrogen atom to
which they are attached form an optionally substituted 5- to 7-membered
heterocyclic ring
optionally containing a further heteroatom selected from nitrogen, oxygen or
sulfur;
R8, R", R'z and R'3 are independently hydrogen or C,_,° alkyl
(optionally substituted by one
or more fluorine atoms);
30 or a pharmaceutically acceptable salt or solvate thereof for use as a
therapeutic agent,
~ provided that when n is zero then A is not pyridyl.
CA °234144° 2001-02-21 Amended Sheet
F2023
PCT/ S ~ 9 9 / 0 1 6 0 0
12
The compounds of the invention are indicated for use in the treatment or
prevention of
allergic, inflammatory, auto-immune, proliferative and hyper-proliferative
diseases.
The compounds of the invention are also indicated in the treatment and
prevention of
s allergic, inflammatory or auto-immune conditions of the lung, including
reversible
obstructive airways diseases which includes asthma (e.g. bronchial, allergic,
intrinsic
asthma, extrinsic and chronic asthma), and associated manifestations of the
disease (late
responses, hyper-responsiveness), also farmer's lung and related diseases,
fibrosis,
ideopathic interstitial pneumonia, chronic obstructive airways disease (COPD),
io bronchiectasis, cystic fibrosis, eosinophilic pneumonias, adult respiratory
distress
syndrome CARDS), emphysema and alveolitis, for example cryptogenic fibrosing
alveolitis.
Further, the compounds of the invention are indicated in the treatment or
prevention of
is allergic, inflammatory or auto-immune conditions in the nose including all
conditions
characterised by inflammation of the nasal mucous membrane such as acute
rhinitis,
allergic rhinitis, atrophic rhinitis, chronic rhinitis including rhinitis
caseosa, hypertrophic
rhinitis, rhinitis purulenta and rhinitis sicca, rhinitis medicamentosa,
membranous rhinitis
including croupous, fibrinous and pseudomembranous rhinitis, scrofulous
rhinitis, seasonal
2o rhinitis including rhinitis nervosa (hay fever) and vasomotor rhinitis. Of
particular interest
are allergic rhinitis and seasonal rhinitis including rhinitis nervosa (hay
fever). The
compounds are also indicated for the treatment of nasal polyps and allergic
menifestations
of the nasopharynx other than those described hereintofore.
zs The compounds of the invention are also indicated the treatment or
prevention of allergic,
inflammatory or auto-immune conditions of the eye such as conjunctivitis
(allergic, acute,
vernal, of hay fever, chronic), inflammation disorders of the eyelids, cornea,
uveal tract and
retina.
3o The compounds of the invention are also indicated in the treatment and
prevention of
allergic, inflammatory and auto-immune conditions of the gastrointestinal
tract such as
food allergy and food intolerance, ulcerative colitis, Crohn's disease,
irritable bowel
disease, gastric ulcers, and food related allergic diseases which have
symptomatic
manifestations remote from the gastrointestinal tract, for example migraine,
rhinitis and
3s eczema.
CA 02341440 2001-02-21
Amended Sheet
F2023
13
PCT/ ~ E 9 9 / 0 I 6 7 0
1 6 -10- 200
The compounds of the invention are indicated for use in the treatment or
prevention of
allergic, inflammatory or auto-immune conditions of the skin such as
psoriasis, atopical
dermatitis, contact dermatitis/dermatitis herpetiformis, erythema nodosum,
urticaria,
cutaneous eosinophilias, acne, Alopecia areata, eosinophilic fascitis
dermatomyositis,
photoallergic sensitivity and periodontal disease.
The compounds of the invention are therefore indicated for use in the
treatment or
prevention of allergic, inflammatory or auto-immune conditions of the joints
and
connective tissue, including osteoarthritis, rheumatoid arthritis, systemic
lupus
io erythematosis, vasculitis, Wegener's granulomatosis, polyarthritis nodosa,
bursitis,
tendonitis, gout, Behcet's syndrome, ankylosing sponditis, Reiter's syndrome
and psoriatic
arthritis.
The compounds of the invention are indicated in the treatment and prevention
of allergic,
i s inflammatory, and auto-immune conditions of the circulatory system
including atheroma,
reperfusion injury (e.g. on angioplasty), myocardial infarction, thrombosis
and vascular
and tissue damage caused by ischaemic disease or injury.
The compounds of the invention are indicated in the treatment and prevention
of allergic,
zo inflammatory or auto-immune conditions of the CNS including Parkinson's
disease,
Alzheimers and other dementias, stroke and subarachnoid haemorrhage. The
compounds of
the invention are indicated in the treatment and prevention of inflammatory
conditions of
the liver for example hepatitis, cirrhosis and glomerulonephritis.
zs The compounds of the invention are indicated in the treatment and
prevention of allergic,
inflammatory or auto-immune conditions of the bladder and uro-genital tract
including
cystitis.
The compounds of the invention are indicated in the treatment and prevention
of tumours
so and other proliferative diseases.
Of particular interest amongst the above indications is use of the compounds
of the
invention in a reversible obstructive airways disease, most particularly
asthma and
especially the treatment and prophylaxis of asthma and rhinitis.
CA 02341440 2001-02-21
Amended Sheet
F2023
PCT/SE 99/ 0 1 600
14 1 6 -10- 20~U
According to a further aspect of the invention there is thus provided the use
of a compound
of formula I, as hereinbefore defined, or a pharmaceutically acceptable salt
or solvate
thereof in the manufacture of a medicament for the treatment of the above
diseases, in
particular reversible obstructive airways disease.
Administration of the compounds of the invention may be topical (for example
by
inhalation to the lung). The compounds of the invention may be inhaled as a
dry powder
which may be pressurised or non-pressurised.
io In non-pressurised powder compositions, the active ingredient in finely
divided form may
be used in admixture with a larger sized pharmaceutically acceptable inert
carrier.
The composition may alternatively be pressurised and contain a compressed gas,
e.g.
nitrogen, or a liquefied gas propellant. In such pressurised compositions, the
active
is ingredient is preferably finely divided. The pressurised composition may
also contain a
surface active agent. The pressurised compositions may be made by conventional
methods.
The compounds of the invention may be administered systemically (for example
by oral
administration to the gastrointestinal tract). The active ingredient may be
formulated
together with known adjuvants, diluents or carriers using conventional
techniques to
2o produce tablets or capsules for oral administration to the gastrointestinal
tract.
Examples of suitable adjuvants, diluents or carriers for oral administration
in the form of
tablets, capsules and dragees include microcrystalline cellulose, calcium
phosphate,
diatomaceous earth, a sugar such as lactose, dextrose or mannitol, talc,
stearic acid, starch,
2s sodium bicarbonate and/or gelatin.
According to a further aspect of the invention there is provided a
pharmaceutical
composition including a compound of formula I or a salt or solvate thereof as
hereinbefore
defined in assuciation with a pharmaceutically acceptable adjuvant, diluent or
carrier.
Suitable doses for such oral administration are in the range from 0.3 to 30 mg
kg' day-', for
example 3 mg kg-' day-'.
According to a further aspect of the present invention, there is provided a
method of
3s treatment or prophylaxis of a reversible obstructive airways disease, in
particular asthma,
which method comprises administration of a therapeutically effective amount of
a
CA 02341440 2001-02-21 Amended Sheet
F2023
PCT/ S E 9 9 / 0 1 6 0 0
Is 1 6 -10- 2000
compound of formula I as hereinbefore defined, or a pharmaceutically
acceptable
derivative thereof, to a person suffering from, or susceptible to, the
disease.
The invention is illustrated by the following Examples.
CA 02341440 2001-02-21 Amended Sheet
WO 00/15614
16
Example 1
(t)-1-(Biphenyl-4-yloxyr4-(3-(2-fluoro)pyridyl)-2-butanol
PCT/SE99/01600
a) (t)-I-(Biphenyl-4-yloxy)-but-3-en-2-of
s A solution of 1-allyloxybiphenyl (15 g) in dry dichloromethane (500 ml), at -
78°C, under
nitrogen, was treated with an ozone rich stream of oxygen over a period of 1.5
hours.
Excess ozone was removed, and the reaction quenched by the addition of
triphenyl
phosphine ( I 8.7 g), and allowed to warm to room temperature with stirring
overnight.
The solution was recooled to -78°C and a solution of vinylmagnesium
chloride (142m1, IM
io in tetrahydrofuran) was added dropwise. The resulting mixture was allowed
to warm to
room temperature over 2 hours, and the reaction quenched by the addition of
saturated
ammonium chloride solution (250 ml). The organic phase was separated, and the
aqueous
extracted with dichloromethane (2x200 ml). The combined extracts were washed
with
brine, dried over magnesium sulfate, filtered, and the filtrate concentrated
under reduced
~s pressure. The residue was purified by column chromatography over silica,
eluting with
isohexane/diethyl ether (1:1) to give the sub-title compound as a pale yellow
solid (10 g).
MS (ESI) 240(M)+
'H NMR (CDCl3) 7.6-7.5(4H, m); 7.41(2H, t); 7.3(1H, t); 7.0(2H, dd); 6.05-
5.95(1H,
m); 5.45( 1 H, d); 5.3( I H, d); 4.63-4.55( 1 H, m); 4.07( 1 H, d), 3.93( 1 H,
t); 2.41 ( I H, d).
Zo
b) (t)-1-(Biphenyl-4-yloxy)-4-(3-(2-fluoro)pyridyl)-2-butanol
A mixture of (~)-1-(biphenyl-4-yloxy)-but-3-en-2-of (1.4183 g), acetonitrile
(12 ml),
triethylamine (4 ml), 2-fluoro-3-iodopyridine (1.32 g,,LOrg.Chem., 1988,
53{12), 2740),
tri-o-tolylphosphine (0.37 g) and palladium (II) acetate (0.13 g) were
combined in that
is order then heated in a sealed tube at 100°C for 90 minutes. The
reaction mixture was
concentrated under reduced pressure and the residue purified by column
chromatography
over silica eluting with ethyl acetate : hexane (1:4) to give a solid which
was a mixture of
(~)-1-(biphenyl-4-yloxy)-4-(3-(2-fluoro)pyridyl)-but-3-en-2-of aad (t)-1-
(biphenyl-4-
yloxy)-but-3-en-2-of and an oil which was 1-(biphenyl-4-yloxy)-4-(3-(2-
fluoro)pyridyl)-2-
3o butanone. The latter ketone was dissolved in methanol (30 ml) and
dichloromethane (IO
ml) and treated with solid sodium borohydride (0.18 g). After 5 minutes the
reaction was
concentrated under reduced pressure and the residue purified by column
chromatography
over silica eluting with ethyl acetate : hexane (3:7) to give a product (0.35
g) which was
crystallised from ether : hexane to give the title compound.
ss mp 91-93°C
MS (APCI) 338 (M+H)+
CA 02341440 2001-02-21
WO 00/15614
PCT/SE99/01600
17
'H NMR (DMSO-d6) 8.15(1H, d); 7.96(1H, t}; 7.68(4H, t); 7.51(2H, t); 7.45-
7.35(2H,
m); 7.10(2H, d); 5.19( I H, d); 4.01 (2H, d); 3.95-3.8( i H, m); 3.0-2.85( 1
H, m); 2.85-2. 7( 1 H,
m); 2.0-1.85(1H, m); 1.85-1.75(1H, m).
s Example 2
(t)-1-(Biphenyl-4-yloxy)-4-(3-(2-methoxy)pyridyl)-2-butanol
A mixture of (t)-1-(biphenyl-4-yloxy)-but-3-en-2-of (1.44 g, Example la),
acetonitrile (12
ml), triethylamine (4 ml), 3-iodo-2-methoxypyridine (0.44 g, J.Org.Chem.,
1988, 53(12),
io 2740), tri-o-tolylphosphine (0.37 g) and palladium (II) acetate (0.18 g)
were heated in a
sealed tube at 100°C for 2 hours. The reaction mixture was concentrated
under reduced
pressure and the residue purified by column chromatography over silica to give
a mixture
of (+)-1-(biphenyl-4-yloxy)-4-(3-(2-methoxy)pyridyl)-but-3-en-2-of and i-
(biphenyl-4-
yloxy)-4-(3-(2-methoxy)pyridyl)-2-butanone. This mixture of compounds was
dissolved in
is methanol (100 m1) and sodium borohydride (0.25 g) was added. When the
reaction was
complete it was concentrated under reduced pressure and the residue
partitioned between
dichIoromethane and water. The organic extract was dried over anhydrous
magnesium
sulphate, filtered and concentrated under reduced pressure. The residue was
dissolved in
ethyl acetate (100 ml) and was hydrogenated at 1.5 atmospheres pressure for 3
hours using
zo palladium on carbon (10%, I70 m g) as catalyst. The reaction was filtered
and concentrated
under reduced pressure to give a solid ( 1.75 g). This was purified by column
chromatography over silica eluting with dichloromethane : ethyl acetate (9:1)
to give a
colourless solid (1.70 g). A sample was crystallised from aqueous methanol.
mp 115°C
is MS (APCI) 350 (M+H)+
'H NMR (DMSO-d6) 8.01(1H, dd); 7.65-7.5(5H, m); 7.43(2H, t); 7.30(1H, t);
7.02(2H, t); 6.91(1H, dd); 5.03(1H, d); 3.92(2H, d); 3.87(3H, s); 3.85-
3.75(1H, m); 2.85-
2.70(1H, m); 2.65-2.50(IH, m); 1.9-1.75(1H, m); 1.75-1.60(IH, m).
so Example 3
(t)-1-(Biphenyl-4-yloxy)-4-(3-(6-fluoro)pyridyl)-2-butanol
a) 3-Bromo-6-fluoropyridine
A solution of 2-amino-5-bromopyridine (1 g) in chloroform (20 ml) was added to
a slurry
3s of nitrosonium fluoroborate (0.745 g) in chloroform (10 ml) at 0°C.
The mixture was
stirred at this temperature for 30 minutes, followed by the addition of 1,2-
dichlorobenzene
CA 02341440 2001-02-21
w0 00/15614 PCTlSE99/01600
18
s
io
(10 ml). The mixture was heated allowing the chloroform to distil. Heating at
150°C was
continued for 2 hours. The reaction was cooled, poured onto water and made
basic by the
addition of 2N sodium hydroxide. The aqueous phase was extracted into ethyl
acetate
(3x100 ml), the extracts combined and dried over magnesium sulfate. The dried
organics
were filtered, and the filtrate concentrated under reduced pressure to give an
orange oil,
which was further purified by column chromatography over silica, eluting with
40-60
petrol then 5% ethyl acetate in 40-60 petrol to give the sub-title compound as
a yellow oil
(0.6 g).
MS (ESI) 175/177 (M)+
b) 1-(Biphenyl-4-yloxy)-4-(3-(6-fluoro)pyridyl)-2-butanone
A mixture of (t)-1-(biphenyl-4-yloxy)-but-3-en-2-of (0.68 g, Example la),
acetonitrile
(10 ml), triethylamine (4 ml), 3-bromo-6-fluoropyridine (0.5 g), tri-o-
tolylphosphine
(0.173 g) and palladium (II) acetate (0.064 g) were combined in that order
then heated in
~s a sealed tube at 120°C for 8 hours. The reaction mixture was
filtered through cotton wool
and then concentrated under reduced pressure. The residue was purified by
column
chromatography over silica eluting with 50:50 ethyl acetate/isohexane to give
the sub-title
compound as a yellow gum, which crystallised on standing (0.266 g).
MS (APCI) 336 (M+H)+
zo 'H NMR (DMSO-d6) 8.10(1H, s); 7.86(1H, td); 7.62-7.52(4H, m); 7.42(2H, t);
7.31 ( 1 H, t); 7.11 ( 1 H, dd); 6.95 (2H, d); 4.88(2H, s); 2.95-2.82(4H; m).
c) (t)-1-(Biphenyl-4-yloxy)-4-(3-(6-fluoro)pyridyl)-2-butanol
Sodium borohydride (0.03 g) was added to a stirred solution of 1-(Biphenyl-4-
yloxy)-
zs 4-(3-(6-fluoro)pyridyl)-2-butanone (0.26 g) in ethanol (i0 ml) at
0°C. Allowed to warm up
to room temperature, with stirring, and then concentrated under reduced
pressure. The
residue was partitioned between water and dichloromethane (SOmI of each). The
organic
phase was separated, and the aqueous extracted with dichloromethane (2x50 ml).
The
combined organics were washed with saturated brine, dried over magnesium
sulfate and
3o filtered. The filtrate was concentrated under reduced pressure, and the
resudue further
purified by column chromatography eluting with 3:1 ethyl acetate/isohexane to
give the
title compound, after trituration under diethyl ether, as a colourless solid
(0.14 g).
m.p. 80-81 °C
MS (APCl7 338 (M+H)+
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
19
'NMR (DMSO-d6) 8.1(1H, s); 7.87(1H, td); 7.65-7.55(4H, m); 7.43(2H, t);
7.30(1H,
t); 7.10( 1 H; dd); 7.01 (2H, d); 5.09( 1 H, d); 3.92(2H, d); 3.85-3.72( 1 H,
m); 2.90-2.72(2H,
m); 1.90-1.63(2H, m).
Example 4
s (t)-Z-(Biphenyl-4-yloxy)-4-(3-(6-methoxy)pyridyl)-2-butanol
a) 3-Iodo-6-methoxypyridine
5-Amino-2-methoxypyridine (5 g) was dissolved in a 1:1 mixture of concentrated
hydrochloric acid and water, and cooled to 0°C. To this was added a
solution of sodium
nitrite (2.9 g) in water (5 ml), keeping the temperature below 5°C.
Stirred between 0 and
~0 5°C for 15 minutes. The diazonium mixture was then treated with a
solution ofpotassium
iodide (7.3 g) in water (10 ml), again keeping the temperature below
5°C. Stirred for 2
hours, whilst allowing to warm up to room temperature. Extracted into ethyl
acetate
(3x 150 ml), and the combined organics were washed with saturated sodium
metabisulfite
solution (3x 100 ml), dried over magneasium sulfate and filtered. The filtrate
was
is concentrated under reduced pressure, and the residue distilled with
Kugekohr apparatus at
35-45°C (1.5 ton) to give the sub-title compound as a light brown oil
(3.87 g).
MS (ESI) 234 (M-H)+
'NMR (DMSO-d6) 8.38(1H, d); 7.98(1H, dd); 6.75(1H, d).
b) 1-(Biphenyl-4-yloxy)-4-(3-(6-methoxy)pyridyl)-2-butanone
zo A mixture of (t)-1-(biphenyl-4-yloxy)-but-3-en-2-of (1.0 g, Example la),
acetonitrile (10
ml), triethylamine (4 ml), 3-iodo-6-methoxypyridine ( 1.1 g), tri-o-
tolylphosphine (0.286
g) and palladium (II) acetate (0.105 g) were combined and heated in a sealed
tube at
100°C for 2.5 hours. The reaction mixture was filtered through cotton
wool and then
concentrated under reduced pressure. The residue was purified by column
chromatography
zs over silica eluting with 1:3 ethyl acetate/isohexane to give the sub-title
compound as a
yellow gum, which crystallised on standing (0.550 g).
MS (APCI) 348 (M+H)+
'NMR (DMSO-d6) 8.01(1H, d); 7.62-7.55(SH, m); 7.43(2H, t); 7.30(1H, t);
6.95(2H,
d); 6.75(1H, d); 4.87(2H, s); 3.80(3H, s); 2.90-2.82(2H, m); 2.81-2.75(2H, m).
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99101600
c) (t}-1-(Biphenyl-4-yloxy)-4-(3-(6-methoxy)pyridyl)-2-butanol
Sodium borohydride (0.06 g) was added to a stirred solution of I-(Biphenyl-4-
yIoxy)-4-(3-
(6-methoxy)pyridyl)-2-butanone (O.S3S g) in ethanol (20 ml) at 0°C.
Allowed to warm up
to room temperature, and stirred for 2 hours. Concentrated under reduced
pressure. The
s residue was partitioned between water and dichloromethane (SOmI of each).
The organic
phase was separated, and the aqueous extracted with dichloromethane (2xS0 ml).
The
combined organics were washed with saturated brine, dried over magnesium
sulfate and
filtered. The filtrate was concentrated under reduced pressure, and the
resudue further
purified by column chromatography eluting initially with 3:1 ethyl
acetate/isohexane, then
io 1:1, to give the title compound, after trituration under diethyl ether, as
a colourless solid
(0.230 g).
m.p. 101-I04°C
MS(APCI} 3S0 (M+H)+
'NMR (DMSO-d6) 8.01(1H, d); 7.63-7.SS(SH, m); 7.43(2H, t); 7.30(IH, t);
7.02(2H,
is d); 6.75(1H, d); S.OS(1H, d}; 3.91(2H, d); 3.83-3.70(4H, m); 2.78-2.SS{2H,
m); 1.88-
1.7{2H, m).
Example 5
(~)-1-(Biphenyl-4-yloxy)-4-(6(1 H)-pyridone-3-yl)-2-butanol
A solution of (t)-1-(Biphenyl-4-yloxy)-4-(3-(6-methoxy)pyridyl)-2-butanol
(0.130g,
zo Example 4) in ethanol (10 ml), was treated with concentrated hydrochloric
acid (10 ml} for
6 hours. Cooled, and basified by the addition of sodium hydrogen carbonate.
The basic
solution was extracted into ethyl acetate (3xS0 ml), combined, dried over
magnesium
sulfate, filtered, and the filtrate concentrated under reduced pressure. The
residue was
further purified by column chromatography over silica eluting with ethyl
acetate, to give
zs the title compound as a colourless solid (0.08 g).
m.p. I S7-I60
MS (APCI) 336(M+H)+
'NMR (DMSO-d6) 11.37(1H, br s); 7.63-7.SS(4H, m); 7.47-7.25(4H, m); 7.16(1H,
d);
7.03(2H, d); 6.28(1H, d); 5.01(1H, d); 3.90(2H, d); 3.81-3.70(1H, m); 2.58-
2.32(2H, m);
1.8-1.53(2H, m).
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
21
Example 6
1-(Biphenyl-4-yloxy)-4-[4-(3-fluoro)pyridylJ-2-butanol
Prepared according to the method described in Example lb) from (t)-1-(biphenyl-
4-
s yloxy)but-3-en-2-of (2.15g, Example la), acetonitrile (12m1), triethylamine
(4ml), 2-
fluoro-4-iodopyridine (2g, J.Org.Chem., 1993, 58, 7832-7838), tri-o-
tolylphosphine
(O.SSg), and palladium (II) acetate (0.28g). After cooling the reaction
mixture was filtered
through Celite~ then concentrated under reduced pressure. The residue, which
contained a
mixture of (t)-1-(biphenyl-4-yloxy)-4-(4-(2-fluoro)pyridyl)but-3-en-2-of and 1-
(biphenyl-
,0 4-yloxy)-4-(4-(2-fluoro)pyridyl)-2-butanone, was purified by column
chromatography over
silica eluting with ethyl acetate : hexane (2:3) to give 1-(biphenyl-4-yloxy)-
4-(4-(2-
fluoro)pyridyl)-2-butanone as an oil (0.78g). This was dissolved in ethanol
(lOm1) and
sodium borohydride (0.1 g) was added. The reaction mixture was stirred
overnight then
concentrated under reduced pressure. The residue was purified by column
chromatography
is over silica eluting with ethyl acetate : hexane (1:4) to give the title
compound as a white
solid (0.27g).
mp 104-106°C
MS (APCI) 338 (M+H)+
'H NMR (DMSO-D6) 8.13(1H, d); 7.63-7.56(4H, m); 7.43(2H, t}; 7.33-7.24(2H, m);
zo 7.07-7.00(3H, m); 5.11 ( 1 H, d); 3.93(2H, d); 3.83-3.78( 1 H, m); 2.95-
2.68(2H, m); 1.95-
1.68(2H, m).
Example 7
1-(Biphenyl-4-yloxy)-4-[(3-methoxy}-4-pyridyl]-2-butanol
zs
Sodium methoxide in methanol (Sml, 25% w/v} was added to (t)-1-(biphenyl-4-
yloxy)-4-
(4-(2-fluoro)pyridyl)-2-butanol (0.22g, Example 6) and the mixture was heated
under
reflux for 6 hours. After cooling, the reaction mixture was concentrated under
reduced
pressure and the residue was partitioned between water and dichloromethane.
The organic
3o extract was washed with brine, dried over anhydrous magnesium sulfate,
filtered and
concentrated under reduced pressure to give a solid. This was purified by
column
chromatography over silica eluting with ethyl acetate : hexane {1:3) then
recrystallised
from ether to give the title compound as a white solid (0.16g).
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99101600
22
mp 96-97°C
MS (APCI) 350 (M+H)+
'H NMR (DMSO-D6) 8.05(1H, d); 7.62-7.57(4H, m); 7.43(2H, t); 7.30(1H, t);
7.02(2H, dt); 6.87(1H, dd); 6.67(lH,s); 5.07(1H, d); 3.92(2H, d); 3.82-
3.75(4H, m); 2.82-
2.60(2H, m); 1.90-1.68(2H, m).
Example 8
1-(Biphenyl-4-yloxy)-4-(4-pyridin-2-one)-2-butanol
(t)-1-(Biphenyl-4-yloxy)-4-(4-(2-methoxy)pyridyl}-2-butanol (0.138 Example 7)
was
to added to ethanol (1 Oml) and concentrated hydrochloric acid (2m1) then
heated under reflex
overnight. After cooling the reaction mixture was partitioned between water
and ethyl
acetate. The organic extract was washed with brine, dried over anhydrous
magnesium
sulfate, filtered and concentrated under reduced pressure. The residue
obtained was
purified by column chromatography eluting with dichloromethane : methanol
(95:5) then
is recrystallised from methanol to give the title compound as a solid
(0.043g).
mp 178-180°C
MS (APCI) 336 (M+H)*
'H NMR (DMSO-D6) 11.33(1H, br.s); 7.58(4H, dd); 7.43(2H, t); 7.32-7.25(2H, m);
7.02(2H, d); 6.14( 1 H, s); 6.07( 1 H,dd); 5.04( 1 H, d); 3.91 (2H, d); 3 .82-
3.77( 1 H, m); 2.69
20 2.42(2H, m); 1.86-1.60(2H, m).
Example 9
1-[(Biphenyl-4-yloxy)]-4-(4-pyridazin-3-yl)-2-butanol
n-Butyllithium (4.25m1, 2.SM solution in hexanes) was slowly added to a
solution of
is diisopropylamine (1.08g) in tetrahydrofuran (30m1) and stirred for 0.5 hour
at 0°C. The
reaction mixture was then cooled to -78°C (dry ice / acetone bath)
before the slow addition
of 3-methylpyridazine. A deep red colour was immediately produced. This was
stirred for
0.5 hour then treated with a solution of 2-(biphenyl-4-ylmethyl)oxirane (2.Sg,
J.MedChem., 1968, 11(6), 1139-44) in tetrahydrofuran (15m1). The reaction
mixture was
3o allowed to reach room temperature overnight and poured into a saturated
solution of
ammonium chloride in water (100m1). The organic layer was removed and the
aqueous
layer was extracted with ethyl acetate. The organic extracts were combined,
washed with
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
23
brine, dried over magnesium sulfate, filtered and concentrated under reduced
pressure. The
residue obtained was purified by column chromatography over silica eluting
with
dichloromethane : methanol (95:5) and reverse phase HPLC eluting with a
mixture of
0.1% w/v ammonium acetate and acetonitrile to give the title compound as a
solid
(0.114g).
mp I 11 °C
MS (APCI) 321 (M+H)+
'H NMR (DMSO-D6) 9.07(1H, t); 7.63-7.57(6H, m); 7.43(2H, dd); 7.33-7.29(1H,
m);
7.05-7.01(2H, m); 5.10(1H, d); 3.96-3.80(3H, m); 3.20-2.92(2H, m); 2.12-
1.80(2H, m).
~o
Example 10
1-[(Biphenyl-4-yloxy)]-4-(pyridazin-4-yl)-2-butanol
Prepared according to the method described in Example 9 from diisopropylamine
(O.Sg),
tetrahydofuran (35m1), n-butyllithium (2m1, 2.SM solution in hexanes), 4-
methylpyridazine
,5 (O.Sg) and 2-(biphenyl-4-ylmethyl)oxirane (1.13g,.LMedChem., 1968, 11(6),
1139-44).
After work up, the crude material was purified by column chromatography over
silica
eluting with dichloromethane : ethanol (95:5) and reverse phase HPLC eluting
with a
mixture of 0.1 % w/v ammonium acetate and acetonitrile to give the title
compound as a
cream solid (0.25g).
2o mp 119-121 °C
MS (APCI) 321 (M+H)+
'H NMR (DMSO-D6) 9.17(1H, s); 9.08(1H, dd); 7.62-7.56(SH, m); 7.42(2H, t);
7.30(1H, t); 7.02(2H, d); S.I2(1H, d); 3.93(2H, d); 3.83-3.77(1H, m); 2.90-
2.65(2H, m);
1.95-1.69(2H, m).
Example 11
1-((Biphenyl-4-yloxy)]-4-(pyrimidin-4-yl)-2-butanol
Prepared according to the method described in Example 9 from diisopropylamine
(1.08g),
tetrahydofuran (60m1), n-butyllithium (4m1, 2.SM solution in hexanes), 4-
so methylpyrimidine (I .Og) and 2-(biphenyl-4-ylmethyl)oxirane (2.3g), J.Med
Chem., 1968,
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
24
11(6), 1139-44). After work up, the crude material was purified by column
chromatography over silica eluting with dichloromethane : ethanol (95:5) and
reverse
phase HPLC eluting with a mixture of 0.1 % w/v ammonium acetate and
acetonitrile to
give the title compound as a white solid (O.IOg).
s mp 105-107°C
MS (APCI) 321 (M+H)+
'H NMR (DMSO-D6) 9.07(1H, s); 8.66(IH, d); 7.63-7.56(4H, m); 7.45-7.40(3H, m);
7.33-7.28(IH, m); 7.05-7.00(2H, m); 5.07(1H, d); 3.94(2H, d); 3.88-3.82(IH,
m); 3.00-
2.75(2H, m); 2.08-1.75(2H, m).
io
Example 12
(~)-4-(2-(Hydroxymethyl)phenyl)-1-(2-thionaphthyl)-2-butanol
a) (t)-2-(3,4-Epoxybutyl)benzyl alcohol
is Solid m-chloroperoxybenzoic acid (50-60%, 2.1 g) and then potassium
carbonate (1.5 g)
were added to a solution of 2-(3-butenyl)benzyl alcohol (3.Sg; Tetrahedron
Lett, 88), 29,
4799) in dichloromethane (100 ml) and the mixture stirred at room temperature
for 5 days.
Water was added and the organic layer separated. The latter was then washed
with
saturated aqueous sodium sulphite, dried over anhydrous magnesium sulphate,
filtered and
Zo concentrated under reduced pressure. The residue was purified by column
chromatography
over silica eluting with ether/hexane ( 1:1 ) to give the title compound as an
oil (0.82 g).
IH NMR (CDCl3) 7.37(IH, d); 7.3-7.I5(3H, m); 4.73(2H, s); 3.0-2.7(4H, m); 2.55-
2.45(1H, m); 2.05-1.6(3H, m).
is b) (t)-4-(2-(Hydroxymethyl)phenyl)-1-(2-naphthalenylthio)-2-butanol
Solid 2-thionaphthalene (1.12 g) was added to a stirring mixture of sodium
hydride (60%
dispersion in oil, 0.27 g) in tetrahydrofiwan (20 ml) at room temperature.
After 15 minutes
this solution was added to a solution of (t)-2-(3,4-epoxybutyl}benzyl alcohol
(0.82 g) in
tetrahydrofuran (20 ml) followed by stirring for 15 minutes. Water (50 ml) was
added and
3o the mixture extracted with ethyl acetate. The combined organic extracts
were dried over
anhydrous magnesium sulphate, filtered and concentrated under reduced
pressure. The
residue was purified by column chromatography over silica eluting with
ether/hexane (I:1)
then ether/hexane (9:1) to give the title compound as a white solid (1.14 g).
mp 109-1 I 1 °C
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
MS (FAB) 338 (M)+
IH NMR (CDCl3) 7.85-7.65(4H, m); 7.55-7.4(3H, m); 7.3-7.1(4H, m); 4.73(1H, d);
4.63(1H, d); 3.75-3.65(1H, m); 3.2-2.75(SH,m); 2.39(1H, bs); 2.0-1.8(2H, m).
s Example 13
(t)-N-Methyl-Z-(4-(2-thionaphthyl)-3-hydroxybutyl)benzamide
a) (~)-N-Methyl 2-(3,4-epoxybutyl)benzamide
A solution of n-butyllithium (2.SM in hexanes, 27 ml) was added over I0
minutes with
~o stirnng to a solution of N-methyl 2-methylbenzamide (5.03 g) in
tetrahydrofuran (100 mI)
at 0°C. After a further 2 minutes allyl bromide (3.5 ml) was rapidly
added and the
temperature rose to 30°C. After 2 minutes saturated aqueous ammonium
chloride (100 mI)
was added and the mixture extracted with ethyl acetate. The combined organic
extracts
were dried over anhydrous magnesium sulphate, filtered and concentrated under
reduced
is pressure. The residue was purified by column chromatography over silica
eluting with
acetone/dichloromethane (1:19) to give N-methyl 2-(3-hutenyl)benzamide, as a
liquid
(4.34 g).
Solid m-chloroperoxybenzoic acid (50-60%, 14.25 g) and then potassium
carbonate
(7.03 g) were added to a solution of N-methyl 2-(3-butenyl)benzamide (4.34 g)
in
Zo dichloromethane (200 ml) at 0°C. The mixture was then stirred at
room temperature for 8
days. Water was added and the organic phase separated and washed with
saturated aqueous
sodium sulphite. The organic layer was dried over anhydrous magnesium
sulphate, filtered
and concentrated under reduced pressure. The residue was purified by column
chromatography over silica eluting with acetone/ dichloromethane (1:9) then
zs acetone/dichloromethane (3:7) to give recovered N-methyl 2-(3-
butenyl)benzamide
(1.30 g) and the sub-title compound as a liquid (1.68 g).
IH NMR (CDCl3) 7.4-7.15(4H, m); 6.03(1H, bs); 3.0-2.95(6H, m); 2.73(1H, t);
2.5-
2.4(1H, m); 2.0-I.7(2H, m).
3o b) (~)-N-Methyl-2-(4-(2-thionaphthyl)-3-hydroxybutyl)benzamide
Prepared according to the method described in Example 12b) from 2-
naphthalenethiol
(2.01 g), sodium hydride (O.SOg, 60% dispersion in oil) and (t)-N-methyl 2-
(3,4-
epoxybutyl)benzamide (1.66 g) in tetrahydrofuran (40 ml) to give the title
compound as an
oil ( 1.43 g).
ss MS (EI) 365 (M)+
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
26
1H NMR (CDC13) 7.8-7.6(4H, m); 7.5-7.35(3H, m); 7.~5-7.2(3H, m); 7.14(1H, t);
5.97(1H, bs); 4.43(1H, d); 3.7-3.55(1H, m); 3.15-2.9(3H, m); 2.95(3H, d); 2.85-
2.75(1H,
m); 2.15-2.0(1H, m); 1.95-1.8(1H, m).
Example 14
(t)-N-Methyl-2-(4-(2-thionaphthyl)-3-hydroxybutyl)benzyiamine hydrochloride
A solution of lithium aluminium hydride. (0.26 g) and (t)-N-methyl-2-(4-(2-
thionaphthyl)-
3-hydroxybutyl)benzamide (0.50 g) in tetrahydrofuran (50 ml) was boiled for 24
hours and
~o then stirred at room temperature for 4 days. The reaction was quenched by
careful, slow
addition of water (0.5 ml) then aqueous sodium hydroxide (50%, 0.5 mI) then
water
(1.5 ml). The mixture was filtered and the residue washed with ethyl acetate.
The filtrate
and the washings were combined, dried over anhydrous magnesium sulphate,
filtered and
concentrated under reduced pressure. The residue was purified by column
chromatography
is over silica eluting with ethyl acetate then triethylamine/methanol (1:9) to
give an oil
(0.32 g). This material was converted to the hydrochloride salt by treatment
with ethereal
hydrogen chloride. The salt so produced was purified by precipitating from
ethanol by
addition of ether to give a cream solid (0.17 g).
mp 158-160°C
zo MS {ESI) 352 (M+H-HCl)+
1H NMR (d6-DMSO) 9.35-9.15(2H, bs); 7.9-7.8(4H, m); 7.55-7.4(4H, m); 7.35-
7.2(3H, m); 5.35-5.25(1H, m); 4.14(2H, s); 3.69(1H, s); 3.25-3.1(2H, m); 2.9-
2.7(2H, m);
2.57(3H, s); 1.95-1.8(1H, m); 1.75-1.6(1H, m).
is Example 15
(~)-N-Methyl-2-(4-(2-(5,6,7,8-tetrahydronaphthyloxy))-3-hydroxybutyl)benzamide
a) (t)-(2-(5,6,7,8-Tetrahydronaphthalenyl)oxymethyl)oxirane
5,6,7,8-Tetrahydro-2-naphthol (7.0 g), acetonitrile (300 ml), epichlorohydrin
(20 ml) and
3o cesium carbonate ( 15.4 g) were combined in that order and then the mixture
was boiled for
4 hours. After cooling to room temperature the mixture was concentrated under
reduced
pressure. The residue was partitioned between ether (300 ml) and water (I00
ml), the
organic extract dried over anhydrous magnesium sulphate, filtered and
concentrated under
reduced pressure. The residue was purified by column chromatography over
silica eluting
3s with hexane/ether (4:I), to give the sub-title compound as a clear liquid
(8.52 g).
MS (EI) 204 (M)+
CA 02341440 2001-02-21
WO 00/15614 PCTlSE99/01600
27
1H NMR (CDC13) 6.95(1H, d); 6.67(1H, d); 6.6(1H, d); 4.15(1H, dd); 3.95(1H,
dd);
3.4-3.3(1H, m); 2.9(1H, t); 2.8-2.65(SH, m); 1.85-1.7(4H, m).
b) A solution of n-butyllithium (1.6M in hexanes, 17.5 ml) was added with
stirring to a
s solution ofN-methyl 2-methylbenzamide (2.04 g) in tetrahydrofuran (100 ml)
at 0°C. After
2 minutes a solution of (t)-(2-(5,6,7,8-
tetrahydronaphthalenyl)oxymethyl)oxirane (2.80 g)
in tetrahydrofuran (15 ml) was rapidly added and the solution was allowed to
room
temperature. After 15 minutes saturated aqueous ammonium chloride ( 100 ml)
was added.
The organic phase was separated and the aqueous phase extracted with ethyl
acetate
i o ( 100 ml}. The combined organic extracts were washed with brine ( 100 ml),
dried over
anhydrous magnesium sulphate, filtered and concentrated under reduced
pressure. The
residue was purified by column chromatography over silica eluting with
dichloromethane
then ether to give the title compound as an oil (4.17 g).
MS (ESI) 354 (M+H}+
is IH NMR (CDC13) 7.4-7.15(4H, m); 6.93(1H, d); 6.63(IH, dd); 6.57(IH, d);
6.13(1H,
bs); 4.01(1H, d); 3.84(3H, d); 3.05-2.8(SH, m); 2.75-2.6(4H, m); 2.05-1.85(2H,
m); I.85-
1.75(4H, m).
Example 16
zo (f}-N-Methyl-2-(4-(2-(5,6,7,8-tetrahydronaphthyioxy))-3-
hydroxybutyl)benzylamine
Prepared according to the method described in Example 14 from (t)-2-(3-hydroxy-
4-(2-
(5,6,7,8-tetrahydronaphthalenyl)oxy)butyl)-N-methyl benzamide (2.50 g) and
lithium
aluminium hydride (1.25 g) in tetrahydrofuran (200 ml) to give the title
compound as an oil
zs (2.17 g).
MS (ESI) 340 (M+H)+
1H NMR (CDCl3) 7.3-7.25(2H, m); 7.25-7.1(2H, m}; 6.92(1H, d); 6.63(1H, dd);
6.75
(1H, d); 3.97(1H, d); 3.9-3.75(2H, ABX); 3.6-3.5(2H, m); 3.1-3.0(1H, m); 2.85-
2.75(1H,
m); 2.75-2.6(4H, m); 2.52(3H, s); 2.1-I.85(2H, m); 1.85-1.7(4H, m).
Example 17
(t}-2-(4-(2-(5,6,7,8-Tetrahydronaphthyloxy)}-3-hydroxybutyl)benzyl alcohol
a) (~)-2-(4-(2-(5,6,7,8-Tetrahydronaphthyloxy)}-3-hydroxybutyl)benzoic acid
3s A solution ofn-butyllithium (1.6M in hexanes, 31 ml) was added to a
solution of
diisopropylamine (7 ml) in tetrahydrofuran (200 ml) at 0°C. After 5
minutes a solution of
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
28
o-toluic acid (3.33 g) in tetrahydrofixran (200 ml) was slowly added. After a
further 5
minutes a solution of (t)-(2-(5,6,7,8-tetrahydronaphthalenyl)oxymethyl)oxirane
(5.00 g,
Example 15a) in tetrahydrofuran (20 ml) was added rapidly. The ice bath
cooling was
removed and the reaction stirred at room temperature for 90 minutes. Dilute
aqueous
s hydrochloric acid (1M, 200 ml) was added and the layers separated. The
aqueous phase
was washed with ether (100 ml) and the combined organic phases concentrated
under
reduced pressure. The residue was dissolved in aqueous sodium hydroxide (1M,
200 ml)
and the solution extracted with ether (2 x 100 ml). The aqueous phase was then
neutralised
with aqueous hydrochloric acid (2M, 100 ml), and extracted with ether (3 x 100
ml). The
~o latter extracts were dried over anhydrous magnesium sulphate, filtered and
concentrated
under reduced pressure. The residue was purified by column chromatography over
silica
eluting with dichloromethane then dichloromethane : ether (4:1 ) then
dichloromethane
ether (1:1) then ether to give the subtitle compound as a white solid (4.64
g).
mp 75-78°C
is MS (EI) 340 (1VI)+
1 H NMR (CDCl3 ) 8.04( 1 H, dd); 7.49( 1 H, td); 7.3 5-7.25 (2H, m); 6.94( 1
H, d);
6.66(IH, dd); 6.61(1H, d); 4.1-4.0(1H, m); 4.0-3.9(1H, m); 3.9-3.8(1H, m); 3.3-
3.1(2H,
m); 2.75-2.6(4H, m); 2.0-1.85(2H, m); 1.8-1.7(4H, m).
Zo b) (~)-2-(4-(2-(5,6,7,8-Tetrahydronaphthyloxy))-3-hydroxybutyl)benzyl
alcohol
Solid (f)-2-(4-(2-(5,6,7,8-Tetrahydronaphthyloxy))-3-hydroxybutyl)benzoic acid
(1.01 g)
was added to a solution of diborane (1.OM in tetrahydrofuran, 20 ml) and the
solution
stirred at room temperature for 3 hours. Water ( 10 ml) was added followed by
brine ( 100
ml). The mixture was extracted with ethyl acetate (2 x I00 ml), the combined
organic
is extracts dried over anhydrous magnesium sulphate, filtered and concentrated
under reduced
pressure. The residue was purified twice by column chromatography over silica
eluting
with ether : hexane (4:1 ) (first column) and ether (second column) to give
the title
compound as a colourless solid (0.39 g).
mp 98-99°C
3o MS (EI) 326 (M)+
1H NMR (CDCl3) 7.35(1H, d); 7.3-7.15(3H, m); 6.95(1H, d); 6.64(1H, dd);
6.58(1H,
d); 4.78(1H, ABX); 4.65(1H, ABX); 3.95-3.85(2H, m); 3.80(1H, dd); 3.0-2.85(3H,
m);
2.75-2.65(4H, m); 2.62(1H, t); 1.90(2H, q); 1.80-1.75(4H, m).
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
29
Example 18
(1 S,2RS)-4'-(2-Hydroxy-1-methyl-4-thiazol-2-yI-butoxy)-biphenyl-3-
carbonitrile.
a) (3S)- [3-(4-Bromo-phenoxy)-2-oxo-butyl]-phosphoric acid dimethyl ester
s Diethylazodicarboxylate (8.54 ml) in dry tetrahydrofuran (2S ml) was added
dropwise over
30 minutes to a stirred solution of triphenylphosphine (13.1 g), (R)-(+)-
methyl lactate (S.2
g) and 4-bromophenol (8.65 g) in dry tetrahydrofuran (12S ml). The resulting
solution was
stirred at room temperature for I8 hours then concentrated under reduced
pressure. A
mixture of isohexane : ether (9 : 1) (200 ml) was added to the residue and the
mixture was
~o stirred at room temperature for 30 minutes. The solution was filtered and
the filtrate
concentrated under reduced pressure. The residue was purified by column
chromatography
over silica eluting with isohexane : dichloromethane ( 1:1 ) to give the sub-
title compound
as an oil (13.5 g) that was immediately taken onto the next step without
analysis. A
solution of butyl lithium (2.S molar in hexanes, 35.2 ml) was added dropwise
to a solution
is of dimethylinethylphosphonate (12.4 g) in tetrahydrofuran (200 mI) at -
70°C. The
resulting solution was stirred for 10 minutes and then the compound from above
(10.92 g)
it tetrahydrofuran (20 ml) was added dropwise. The reaction was stirred for 30
minutes
and was then poured into water (200 ml) and extracted into ethyl acetate
(3x100 ml), the
combined extracts were dried over anhydrous magnesium sulfate, filtered and
zo concentrated. The residue was purified by chromatography on silica gel
eluting with ethyl
acetate to afford the title compound as an oil.
MS (APCI) 351, 3S3 (M+H)+
'H NMR (CDCI,) 7.38 (2H, d); 6.80 (2H, d); 4.78 (1H, c~; 3.77 (3H, d); 3.73
(3H, d);
3.38-3.16 (2H, m); 1.52 (3H, d).
zs b) (4S)-4-(4-Bromo-phenoxy)-1-thiazol-2-yl-pent-1-en-3-one.
2-Formylthiazole (0.38 ml) in dry acetonitrile (15 ml) was added dropwise to a
mixture of
dried lithium chloride (0.85 g), diisopropylethylamine (1.OS ml) and (3S)-[3-
(4-
Bromophenoxy)-2-oxo-butyl)phosphonic acid dimethyl ester (1.41g)) in dry
acetonitrile
(SO ml) which had been stirred at room temperature fox 20 hours. The resulting
mixture
3o was stirred at room temperature for 90 minutes. The reaction mixture was
poured into
water and extracted with ethyl acetate. The combined extracts were dried over
magnesium
sulfate, filtered and concentrated under reduced pressure to give the sub-
title compound as
a yellow oil (1.35 g).
MS(APCI)337.9(M+H]+
ss c) (42S,3RS)-4-(4-Bromophenoxy)-1-thiazol-2-yl-pent-I-en-3-ol.
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
Sodium borohydride (0.15 g) was added to a solution of cerium trichloride
heptahydrate
(1.49 g) and (4S)-4-(4-Bromophenoxy)-1-thiazol-2-yl-pent-1-en-3-one (1.35 g)
in
methanol at -20 °C. The solution was stirred at -20 °C for 30
minutes then allowed to
warm to room temperature. The solution was concentrated in vacuo. The residue
was
s partitioned between ethyl acetate and water. The organic layer was dried
over magnesium
sulfate, filtered and concentrated in vacuo. The residue was purified by
column
chromatography over silica eluting with ethyl acetate:isohexane (1:1) to give
the sub-title
compound as a yellow oil and as a 6:1 mixture of diastereomers (0.91 g).
MS(APCI) 342[M+H]+
io 'H1VMR(CDC13, majordiastereomer) 1.33(3H,d); 3.01(lH,d); 4.36-4..28(lH,m);
4.44-
4.39(lH,m); 6.70-6.61(lH,m); 6.83(2H,d); 7.04-6.96(lH,m); 7.25(lH,t);
7.36(2H,d);
7.77( 1 H,d).
d) (4S,3RS)-4-(4-Bromophenoxy)-1-thiazol-2-yl-pentan-3-ol.
(4S,3RS)-4-(4-Bromophenoxy)-1-thiazol-2-yl-pent-1-en-3-of (0.66 g) was
dissolved in
,s ethyl acetate (20 ml) and hydrogenated at 2 atmospheres using 5% rhodium on
carbon
(0.02 g) as catalyst. The mixture was filtered through celite and the filtrate
concentrated
under reduced pressure to give a grey liquid. The residue was purified by
column
chromatography over silica eluting with isohexane:ethyl acetate (2:1) to give
the sub-title
compound as a colourless oil and as a 6:1 mixture of diasteromers (0.35 g).
zo MS(APCI) 344 [M+H]+
'H1VMR (CDCI,, major diastereomer) 1.28(3H,d); 2.14-1.95(2H,m); 3.28-
3.22(2H,m);
3.37(lH,d); 3.78-3.74(lH,m); 4.30-4.24(lH,m); 6.82-6.76(2H,m); 7.21(lH,d);
7.39-
7.34(2H,m); 7.68(lH,d).
e) (1S,2RS)-4'-(2-Hydroxy-1-methyl-4-thiazol-2-yl-butoxy-biphenyl-3-
carbonitrile
zs A solution of (4S,3RS)-4-(4-Bromophenoxy)-1-thiazol-2-yl-pentan-3-of (0.35
g), 3-
cyanobenzene boronic acid (0.30 g), aqueous sodium carbonate solution (2M,
1.18 ml) and
tetrakis(triphenylphosphine)palladium (0) (0.02 g) in toluene (5 ml) and
ethanol (2 ml) was
refluxed for 4 hours. The reaction mixture was cooled and concentrated under
reduced
pressure. The residue was purified by column chromatography over silica
eluting with
3o dichloromethane:methanol (19:1) to give the title compound as a yellow oil
and as a 6:1
mixture of diastereomers (0.27 g).
MS(APCI) 365[M+H)+
'HrTMR(CDCI" major diasteromer) 1.36(3H,d); 2.18-2.01(2H,m); 3.30-3.25(2H,m);
3.42(lH,d); 3.83-3.79(lH,m); 4.42-4.36(lH,m); 7.00(2H,d); 7.22(lH,d); 7.68-
ss 7.47(4H,m); 7.69( 1 H,d); 7.78-7.75( 1 H,m); 7.8I ( 1 H,s).
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
31
Example 19
4-[4-(Biphenyl-4-yloxy)-3-hydroxy-butylj-2-methoxy-phenol
a) 1-(Biphenyl-4-yloxy)-3-chloro-propan-2-one
To a stirred suspension of 4-phenylphenol (9.5 g) and caesium carbonate (21.25
g) in acetonitrile
(80 ml) was added epichlorohydrin (20 ml} and the resulting mixture was
stirred at reflux for 3
hours, cooled, filtered and concentrated. The residue was dissolved in
tetrahydrofuran (200 ml)
and treated with hydrochloric acid (~ molar, 1 ~ ml). The reaction was stirred
for 15 minutes,
poured into water (200 ml) and extracted into ethyl acetate (3 x 100 mI) and
the combined organic
~o extracts were dried over anhydrous magnesium sulfate, filtered and
concentrated under reduced
pressure. To the residue in acetone (200 ml) was added, dropwise a solution of
sodium dichromate
in water in sulfuric acid [made by dissolving sodium dichromate ( 18.34 g} in
water (53 ml) and
cautiously adding concentrated sulfuric acid (20 ml)]. The reaction was
stirred for 20 hours and
then propan-2-of (20 ml) was added. The reaction was stirred for 1 hour and
was then poured into
water (1000 ml) and extracted into diethyl ether (3x300 ml) and the combined
organic extracts
were dried over anhydrous magnesium sulfate, filtered and concentrated under
reduced pressure.
The residue was purif ed by chromatography on silica gel eluting with
dichloromethane/hexane
( 1:1 ) to afford the sub-title compound (7.34 g).
MS (EI) 260/262 (M)'
Zo 'H NMR (CDCI,) 7.56-7.52 (4H, m); 7.43 (2H, t); 7.32 (1H, t); 6.97 (2H, d);
4.82 (2H,
s); 4.44 (2H, s).
b) [3-(Biphenyl-4-yloxy}-2-oxo-propyl]-triphenyl-phosphonium chloride
1-(Biphenyl-4-yloxy)-3-chloro-propan-2-one (7.34 g) was dissolved in
acetonitrile (150 ml) and
triphenylphosphine (8.88 g) added. The resulting mixture was stirred at reflux
for 15 hours, cooled
zs and the precipitate isolated by filtration to afford the sub-title compound
as a solid that was used in
the next reaction without fiuther purification ( 11.28 g).
MS (APCI) 497 (M-Cl)'
c) 1-(Biphenyl-4-yloxy)-4-(4-hydroxy-3-methoxy-phenyl)-but-3-en-2-one
(3-(Biphenyl-4-yloxy)-2-oxo-propyl]-triphenyl-phosphonium chloride (3.14 g)
was partitioned
so between dichloromethane (20 ml) and sodium hydroxide solution ( 1 molar, 10
ml). The organic
layer was separated, dried over anhydrous magnesium sulfate, filtered and
concentrated. The
residue was dissolved in anhydrous tetrahydrofitran (30 ml) and vanillin (0.76
g) added. The
resulting solution was stirred at reflux for 20 hours and then a second
aliquot of vanillin (1.57 g)
was added and the reaction refluxed for a further 100 hours. The mixture was
cooled, concentrated
3s under reduced pressure and the residue purified by chromatography on silica
gel eluting with
diethyl ether/hexane ( 1: i ) to afford the sub-title compound as a solid (
1.9 g)
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99101600
32
m.p. 142-143°C
MS (APCI) 361.3 (M+H)'
'H NMR (CDC13) 7.73 (1H, d); 7.56-7.52 (4H, m); 7.41 (2H, t); 7.31 (1H, t);
7.17 (1H,
dd); 7.08 ( I H, d); 7.01 (2H, d); 6.96 ( 1 H, d); 6.93 ( I H, d); 5.94 ( 1 H,
s); 4.82 (2H, s);
s 3.94 (3H, s).
d) 4-[4-(Biphenyl-4-yloxy)-3-hydroxy-butyl]-2-methoxy-phenol
1-(Biphenyl-4-yloxy)-4-(4-hydroxy-3-methoxy-phenyl)-but-3-en-2-one (0.65 g)
was dissolved in
ethyl acetate (20 ml) and 10% palladium on carbon (0.050 g) added. The mixture
was
hydrogenated at 3 atmospheres until no further uptake of hydrogen was
observed. The mixture was
~o filtered and concentrated and the residue was purified by chromatography on
silica geI eluting with
diethyl ether/hexane (3:2) to afford the title compound as a solid (0.056 g).
m.p. 74-76°C
MS (APCI, -ve) 363.4 (MH)'
'H NMR (CDCI,) 7.57-7.54 (4H, m); 7.53 (2H, t); 7.39 (1H, t); 6.98 (2H, d);
6.85 (1H, d);
~s 6.75 (1H, s), 6.75 (1H, d); 5.47 (1H, s); 4.08-4.00 (2H, m), 3.90-3.87 (1H,
m); 3.88 (3H, s);
2.59-2.52 (2H, m); 2.32 (1H, d); 1.93-1.80 (2H, m).
Example 20
4-(4-(Biphenyl-4-yloxy)-3-hydroxy-butyl)-1-methyl-pyrrolidin-2-one
a). 4-Hydroxymethyl-1-methyl-pyrolidin-2-one
A solution of dimethyl itaconate (10 g) and methylamine (7.2 ml, 30% w/v in
methanol) in
methanol (100 ml) was stirred at room temperature for 3 days. The mixture was
concentrated and the residue was dissolved in ethanol (200 ml), cooled to OoC
and sodium
zs borohydride (4.8 g) was portionwise added. The reaction mixture was stirred
at room
temperature for 2 days and concentrated under reduced pressure. The residue
was
dissolved in chloroform (100 ml) and heated at reflux for 30 minutes, cooled
and dried over
anhydrous magnesium sulfate, filtered and concentrated to afford the sub-title
compound
that was used without further purification (8.12 g)
3o MS (EI) 129 (M)"
'H NMR (CDCI,) 3.68-3.58 (2H, m); 3.49 (1H, dd); 3.25 (1H, dd); 2.84 (3H, s);
2.61-
2.45 (2H, m); 2.40 ( 1 H, br), 2.20 ( 1 H, dd).
b) 4-[4-(Biphenyl-4-yloxy)-3-oxo-but-1-enyl]-1-methyl-pyrrolidin-2-one
3s 4-Hydroxymethyl-1-methyl-pyrolidin-2-one (0.516 g) was dissolved in
dichloromethane
(50 ml) and was added to a stirred suspension of Dess-Martin periodinane (2.65
g) and
CA 02341440 2001-02-21
WO 00115614 PCTlSE99/01600
33
pyridine (0.3 ml) in dichloromethane (15 m1). The reaction was stirred at room
temperature
for 2 hours and concentrated under reduced pressure. The filtrate was
dissolved in
tetrahydrofuran (20 ml) and filtered. To the filtrate was added the compound
from example
19b (1.045 g) and the resulting solution was stirred at reflux for 20 hours,
cooled and
s concentrated under reduced pressure. The residue was purified by
chromatography on
silica gel eluting with ethyl acetate to afford the sub-title compound (0.305
g) as an oil.
MS (APCI) 336 (M+H)+
'H NMR (CDCI,) 7.55 (4H, d); 7.40 (2H, d); 7.31 (1H, dd); 7.01 (1H, dd); 6.95
(2H, d);
6.52 (1H, d); 4.73 (2H, s); 3.60-3.54 (1H, m); 3.25-3.20 (2H, m); 2.83 (3H,
s); 2.63 (1H,
a o dd); 2.34 ( 1 H, dd)
c) 4-[4-(Biphenyl-4-yloxy)-3-hydroxy-but-I-enylJ-1-methyl-pyrrolidin-2-one
4-[4-(Biphenyl-4-yloxy)-3-oxo-but-1-enylJ-1-methyl-pyrrolidin-2-one (0.30 g)
was
dissolved in methanol (5 ml) and cerium trichloride heptahydrate (0.33g) was
added. The
is suspension was cooled to 5°C and sodium borohydride (0.034 g) was
added. The mixture
was stirred for 15 minutes and was poured into water (20 ml) and was extracted
into ethyl
acetate, dried over anhydrous magnesium sulfate, filtered and concentrated
under reduced
pressure. The residue was columned on silica gel eluting with ethyl acetate to
afford the
sub-title compound (0.209 g) as a solid.
zo m.p.113-115°C
MS (APCI,) 338 (MH)+
'H NMR (CDCI,) 7.55-7.51 (4H, m); 7.41 (2H, t); 7.30 (1H, dd); 6.96 (2H, d);
5.90 (1H,
dd); 5.67 ( 1 H, dd); 4.6-4.52 ( 1 H, m); 4.05 ( 1 H, dd); 3.90 ( 1 H, dd); 3
.52 ( 1 H, dt); 3.21
(1H, dt); 3.07 (1H, c~; 2.80 (3H, s); 2.60 (1H, dd); 2.45 (1H, t); 2.35-2.25
(1H, m).
2s
d) 4-[4-(Biphenyl-4-yloxy)-3-hydroxy-butyl]-1-methyl-pyrrolidin-2-one
4-[4-(Biphenyl-4-yloxy)-3-hydroxy-but-1-enylJ-I-methyl-pyrrolidin-2-one (0.15
g) was
dissolved in ethyl acetate and was hydrogenated at 3 atmospheres pressure
using 10%
palladium on carbon (0.020 g) as catalyst. The mixture was filtered and
concentrated under
3o reduced pressure. The residue was purified by normal phase HPLC eluting
with ethyl
acetate to afford the title compound (0.082 g) as an oil.
MS (A.PCI,) 340.3 (MH)+
'H NMR (CDCI,) 7.57-7.52 (4H, m); 7.41 (2H, t); 7.30 (1H, dd); 6.97 (2H, d);
4.05-3.98
(2H, m); 3.88 (IH, t); 3.51 (1H, t); 3.06 (1H, dd); 2.71 (3H, s); 2.61-2.50
(1H, m); 2.49
3s 2.35 (IH, m); 2.35 (1H, t); 2.16-2.05 (IH, m); 1.80-1,67 {1H, m); 1.60-1.50
(3H, m).
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
34
Example 21
(t)-4-[4-(Biphenyl-4-yloxy~3-hydroxybutyl]-I-methyl-1H pyridin-Z-one
(f)-1-(Biphenyl-4-yloxy)-4-(4-(2-fluoro)pyridyl)-2-butanol (0.25g, Example 6)
was
dissolved in dimethylformamide (3.Om1) and treated with methyl iodide (l.Om1).
The
reaction mixture was heated under nitrogen at 100°C overnight. After
cooling, the reaction
mixture was partitioned between ether and water then 2M sodium hydroxide (Sml)
was
added. The organic extract was washed with brine, dried over anhydrous
magnesium
sulfate, filtered and concentrated under reduced pressure. The residue
obtained was purified
by column chromatography over silica eluting with dichloromethane : methanol
(97:3) to
o give the title compound as a solid (0.12g).
mp 129°C
MS (APCI) 350 (M+H)+
'H NMR (DMSO-D6) 7.62-7.56(SH, m); 7.43(2H, t); 7.30(1H, t); 7.02(2H, d);
6.21(1H, s); 6.11(1H, dd); 5.04(1H, d); 3.91(2H, d); 3.84-3.76(1H, m);
3.36(3H, s); 2.65-
,5 2.42(2H, m); 1.90-1.60(2H, m).
Pharmacological activity
Superoxide assay
zo
96-well plates contained 50 ml hIgGl in HBSS in each well from overnight
incubation at 4
°C. To this 50 ml cytochrome c solution was added per well and 20 m1
compound or 1
(v/v) DMSO in HBSSg. The plate with additions was pre-warmed with the lid on
at 37 °C
for 15 min by placing in a water-bath ensuring that no air-bubbles were
trapped under the
zs plate. The assay was started by addition of 200 000 neutrophils (37
°C) in 80 ml with a
Biohit 8-channel pipettor and the plate incubated in a waterbath with Iid on
at 37 °C for 60
min. At this point the reduction of cytochrome c was determined (A550- A468)
in a
spectramax plate reader. In each individual experiment incubations were in
triplicate.
3o Hank's Balanced Salt Solution (HBSS)
Hank's Balanced Salt Solution with gelatine 1 mg.ml-' (HBSSg)
Cytochrome c solution: cytochrome c 400 ~,M , NaN3 8 mM in HBSSg
CA 02341440 2001-02-21
WO 00/15614 PCT/SE99/01600
Compounds exemplified herein inhibit superoxide production from neutrophils in
the range
1 E-6 to 1 E-1 OM
CA 02341440 2001-02-21