Note: Descriptions are shown in the official language in which they were submitted.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
TITLE OF THE INVENTION
NEEDLELESS SYRINGE FOR THE SUBCUTANEOUS
DELIVERY OF THERAPEUTIC AGENTS.
FIELD OF THE INVENTION
The present invention relates to syringes. More
specifically, the present invention relates to needleless syringes for the
subcutaneous delivery of therapeutic agents.
BACKGROUND OF THE INVENTION
Needleless syringes are known and have previously
been described in the prior art.
In a broad sense, needleless syringes are used for the
subcutaneous injection of therapeutic agents. An obvious advantage of the
use of a needleless syringe, is the avoidance of physically perforating the
epidermis. The therapeutic agents can present themselves in powder form.
The active substances can be vaccines, anesthetics, medicaments,
hormones, and genetic compounds, for example. These agents, while in
the form of particles whose size is of the order of a few microns, are
capable of penetrating the skin of a patient, due to the high velocity
imparted upon them.
In their May 4, 1999 United States Patent no.
5,899,880, Bellhouse et al. describe such a needleless syringe. Bellhouse
et al. propose the use of a needleless syringe having a nozzle presenting a
divergent downstream portion, in order to achieve a pseudo-steady-state
gas expansion to accelerate the particle flow. The problem presented by
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
2
this approach is double: (i) the particle flow is, in reality, not stationary,
that
is, it does not possess a steady state system and, (ii) the particle flow
becomes detached from the inner wall of the divergent downstream nozzle,
which reduces the expansion and hence the acceleration produced.
Additionally, the different needleless syringe
embodiments described by Bellhouse et al., illustrate a membrane that
ruptures when the pressure exerted upon it exceeds its threshold.
Furthermore, a mechanism exploiting the direct rupture of the membrane
as well as an approach without the use of a membrane was not explored.
Finally, Bellhouse et al. do not present concepts linking
the particle dose to the gas reserve, other than the use of a quasi-static
compression piston.
United States Patent no. 5,865,796 issued on February
2, 1999 to McCabe essentially describes a similar device, designed for the
injection of genetic material in a laboratory setting.
Both of the above reported devices are capable of
accelerating an inert gas, and hence the concomitant acceleration of the
particles, through the expansion of the inert gas at high pressures, through
a nozzle that has a convergent upstream section and a divergent
downstream section (commonly referred to as convergent-divergent
nozzle). Hence, one has to appreciate that the above-mentioned
acceleration process functions at a constant or quasi-constant steady state,
that is, once the waves resulting from the rupture of the membrane are no
longer important. It becomes therefore obvious that in the Bellhouse et al.
device, the particles will have been ejected from the device prior to the
establishment of the so-called quasi steady state system.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
3
The consequences of this observation are interesting,
since researchers studying the Bellhouse system, carried out their
optimization calculations based on the quasi steady state system
hypothesis. Indeed, a recent publication (M. A. F. Kendall, N. J. Quinlan,
S. J. Thorpe, R. W. Ainsworth and B. J. Bellhouse: The gas-particle
dynamics of a supersonic drug delivery system. Book of Abstracts, 22Id
International Symposium on Shock Waves, London, 19-23 July, 1999)
illustrates that because of their wrongful interpretation of this phenomenon,
there exists an important discrepancy between the values predicted by the
theoretical model and the observed experimental values, as can be
observed in Figure 1.
The graph of Figure 1, which is labeled "prior art", is
illustrative of a comparison between the calculated particle velocity profile
and the measured particle velocity profile. It can be readily observed that
the theoretical model predicts an exit velocity for the particles in the
proximity of Mach 6, whereas the experimental observations illustrate a
considerably lesser Mach velocity.
In their 25 January, 2001 PCT patent application
published under no. WO 01/05455, Kendall & Brown describe a needless
syringe which incorporates improvements on the Bellhouse system by
correctly accounting for the starting process which thereby increases the
predictability of the device. Kendall & Brown propose the use of a shock
tube, comprised of two chambers of same or similar diameter, with a
divergent nozzle attached to the downstream chamber. In their device, they
explain that the particles are first set in motion via the production of a
shock
wave when the membrane initially separating the two chambers is ruptured
and that further acceleration is then produced by the expansion of the gas
in the divergent nozzle. In reality, this device is quite similar to that of
Bellhouse et al., the only difference being the addition of a constant
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
4
diameter downstream duct section before the divergent nozzle which
increases the time delay between the undesirable nozzle starting
phenomena and the particle acceleration phase.
Furthermore, the method for accelerating a dose of
particles in a needleless device proposed by Kendall & Brown claims to
comprise the production of a primary shock wave. It should be known that it
is possible to carry a dose of particles with a device comprising a driver
chamber and a duct section downstream of said driver chamber without
producing a primary shock wave travelling in downstream direction in said
duct section. Indeed, if the duct were evacuated, i.e., at zero absolute
pressure, the opening of the closure means located between said driver
chamber and said duct section would not, in this case, produce a primary
shock wave travelling in a downstream direction in said duct section since
there would be no pre-existing medium in said duct section for the shock
wave to propagate into, but the dose of particles could still be accelerated
by the expansion of the driver gas. The production of a primary shock
wave, or any number of shock waves thereafter, is therefore not necessary
to achieve particle acceleration.
The correct physical interpretation of these devices is
rather obtained by examining the method of expansion of the gas
contained within said driver section, which causes a gaseous piston to
propagate in the downstream direction in said duct section thereby carrying
particles in the same direction. By optimally producing this expansion, the
performance of the device, as measured by particle velocity and uniformity
of particle velocity achieved, can be predicted and maximized. This
expansion can be produced through steady or quasi-steady means which
usually utilize nozzles of convergent or divergent geometry. This expansion
can also be produced through unsteady means, by sudden or gradual
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
temporal changes in the flow properties, in which case unsteady trains of
rarefaction waves are used.
In the device proposed by Bellhouse et al., the
gaseous expansion mechanism is that of quasi-steady expansion using a
5 converging-diverging nozzle. The shortcomings of using a divergent have
been discussed above and in the patent application of Kendall & Brown
(see Figure 2 and associated discussion lines 28-32 p.4 and lines 1-7 p.3
of the Kendall & Brown document).
In the devices proposed by Kendall & Brown, there are
either one or two driver gas expansion mechanisms. In all devices
proposed by Kendall & Brown, there is an unsteady expansion produced by
the opening of a closure means located between a driver chamber and a
duct section located downstream of the driver chamber. The unsteady
expansion waves can be seen as item 30 (comprising the leading wave 34)
in Figure 3 of the Kendall & Brown document. In most embodiments
proposed by Kendall & Brown, there is also an additional quasi-steady
driver gas expansion mechanism, achieved through the use of a divergent
nozzle positioned downstream of said duct section. The shortcomings of
using a divergent nozzle are in this case similar to those of the Bellhouse
device. Kendall & Brown recognize that their proposed device could
possibly work without having this divergent nozzle, but they fail to mention
that to achieve the desired particle flow velocities without this nozzle, the
required driver gas pressure would be very high thereby increasing the
safety risks associated with the use of the device and also reducing the
utility of the device since a very high pressure source of gas would be
required. This is why they have to resort to a divergent nozzle in all
embodiments of their invention.
CA 02423647 2008-12-22
6
In embodiment 5 of their proposed device, Kendall & Brown
propose to have a driver chamber as having a larger area than the
downstream duct section. However, in that proposal they have failed to
recognize that a quasi-steady expansion would take place within the
contraction and that for a sufficiently large area ratio a second unsteady
expansion would be produced in the driver gas but downstream of the
contraction.
It should be known that there exists other combinations of
quasi-steady and unsteady gaseous expansion means that achieve a
better performance than those in the Bellhouse and the Kendall & Brown
devices.
OBJECTS OF THE INVENTION
An object of the present invention is therefore to provide an
improved needieless syringe for the subcutaneous delivery of therapeutic
agents.
SUMMARY OF THE INVENTION
More specifically, in accordance with the present invention,
there is provided a needieless syringe for delivering therapeutic particles
to a skin surface, comprising:
a gas reservoir for receiving a gas at a first pressure, the gas
reservoir having a first internal diameter and an opening;
an elongate tubular nozzle having an upstream end mounted
to the opening of the gas reservoir, an upstream portion and a
downstream end; and an arrangement.
CA 02423647 2008-12-22
7
The arrangement comprises:
the elongate tubular nozzle having a generally constant
second internal diameter smaller than the first internal diameter and being
at a second internal pressure;
a contraction of the gas reservoir from the first internal
diameter to the second internal diameter;
a source of therapeutic particles positioned in the upstream
end of the elongated tubular nozzle of generally constant second internal
diameter; and
a partition for separating the gas reservoir from the source of
particles, the partition being designed to be opened and to withstand large
pressure differences between the gas reservoir and the elongate tubular
nozzle.
The first and second internal diameters and the first and
second gas pressures define respective, predetermined diameter and
pressure ratios adapted to produce, in response to opening of the
partition, a gaseous expansion generating a) a stationary expansion wave
in the contraction leading to a sonic flow at the upstream end of the
tubular nozzle and b) non-stationary expansion waves traveling
downstream in the elongate tubular nozzle to carry the therapeutic
particles into a supersonic gas flow and deliver the therapeutic particles to
the skin surface through the downstream end of the tubular nozzle.
The present invention also relates to a needieless syringe for
delivering therapeutic particles to a skin surface, comprising:
. ,.
~; ,..
., b ,.
CA 02423647 2008-12-22
8
a gas reservoir having a first internal diameter and an
opening;
an elongate tubular nozzle having an upstream end mounted
to the opening of the gas reservoir and a downstream end;
an arrangement for producing non-stationary expansion
waves traveling downstream in the elongate tubular nozzle and for
carrying by means of the non-stationary expansion waves the therapeutic
particles at a velocity sufficient to deliver the therapeutic particles to the
skin surface.
The arrangement comprises:
the elongate tubular nozzle having a generally constant
second internal diameter;
a contraction of the gas reservoir from the first diameter to
the second diameter;
an upstream portion of the elongated tubular nozzle of
generally constant second internal diameter for accommodating a source
of therapeutic particles;
a rupturable partition for separating the gas reservoir from
the source of particles, the partition being designed to withstand large
pressure differences between the gas reservoir and the elongate tubular
nozzle; artd
a ratio of the first internal diameter to the second internal
diameter which is sufficient to allow the contraction of the gas reservoir to
n.x,.. _ ~=-P.-n , . .~,,._..w,~ , CA 02423647 2008-12-22
9
produce, in response to a rupture of the partition caused by a difference of
gas pressure between the gas reservoir and the elongate tubular nozzle, a
gaseous expansion generating a) a stationary expansion wave in the
contraction leading to a sonic flow at the upstream end of the tubular
nozzle and b) the non-stationary expansion waves traveling downstream
in the elongate tubular nozzle to carry the therapeutic particles into a
supersonic gas flow.
The gas reservoir further comprises:
a first chamber filled with a first pressurized gas;
a second chamber mounted downstream from the first
chamber and including a closed orifice that may be opened to the
atmosphere, the second chamber being filled with a second gas at a lower
pressure relative to the first gas;
a first membrane separating the first chamber from the
second chamber;
a second membrane separating the second chamber from
the tubular nozzle.
Opening the orifice to the atmosphere results in a pressure
drop in the second chamber resulting in a rupture of the first membrane
whereby the first pressurized gas fills the second chamber in turn
rupturing the second membrane, generating a) said stationary expansion
wave in the contraction leading to a sonic flow at the upstream end of the
tubular nozzle and b) the non-stationary expansion waves traveling
downstream in the elongate tubular nozzle, accelerating the particles into
a supersonic gas flow towards the downstream end of the nozzle.
CA 02423647 2008-12-22
The present invention further relates to a needleless syringe
for delivering particles, the syringe comprising: a first reservoir having a
first internal diameter, the reservoir having an opening defining a
contraction; an elongate tubular nozzle having an upstream end and a
5 downstream end; the elongate tubular nozzle having a generally constant
second internal diameter; a second reservoir interposed between the
opening of the first reservoir and the tubular nozzle, the second reservoir
having substantially the second internal diameter; a first partition
separating the first reservoir from the second reservoir; and a second
10 partition separating the second reservoir from the tubular nozzle.
Positively pressurizing with a gas the first reservoir relative to the second
reservoir results in rupturing the first partition whereby the gas fills the
second reservoir in turn rupturing the second partition, generating a) a
stationary expansion wave in the contraction leading to a sonic flow at the
upstream end of the tubular nozzle and b) non-stationary expansion
waves traveling downstream in the elongate tubular nozzle, accelerating
the particles into a supersonic gas flow towards the downstream end of
the nozzle. The first reservoir is filled with a positively pressurized gas
relative to the atmospheric pressure, and the second reservoir further
comprises a closed orifice that may be opened to the atmosphere for
causing the positive pressurizing of the first reservoir relative to the
second reservoir.
The foregoing and other objects, advantages and features
of the present invention will become more apparent upon reading of the
following non-restrictive description of illustrative embodiments thereof,
given by way of example only with reference to the accompanying
drawings.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
11
BRIEF DESCRIPTION OF THE DRAWINGS
In the appended drawings:
Figure 1 which is labeled "Prior Art" is a diagram
comparing the calculated particle velocity profile and the measured particle
velocity profile;
Figure 2A is a schematic outline of a syringe illustrating
the various elements of the present invention in a static mode;
Figure 2B is a schematic outline of the syringe of
Figure 2A, illustrating the various elements of the present invention in a
dynamic mode;
Figure 2C is a schematic outline of the trajectories of
the particle flow and the trajectories of the various waves generated by the
syringe of Figure 2A;
Figure 2D is a graphical representation in pressure-
velocity space of the gas dynamics phenomena taking place in the various
needleless syringe configurations.
Figure 3 is diagram illustrating the observed pressure
in function of the corrected pressure;
Figure 4 is a schematic outline of an experimental
syringe assembly, according to one aspect of the present invention;
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
12
Figure 5A is a schematic outline of a needleless
syringe, according to a first embodiment of the present invention;
Figure 5B is a schematic outline of a particle dose to
be used in the syringe of Figure 5A;
Figure 5C is a schematic outline of an alternative
particle dose to be used in the syringe of Figure 5A;
Figure 6 is a schematic outline of a disposable
needleless syringe, according to a second embodiment of the present
invention;
Figure 7A is a schematic outline of a self-contained
particle dose / pressurized source assembly according to a third
embodiment of the present invention;
Figure 7B is a schematic outline of a reusable device
to be used with the self contained assembly of Figure 7A;
Figure 8 is a schematic outline of a needleless syringe,
comprising an unengaged piston, according to a fourth embodiment of the
present invention; and
Figure 9 is a schematic outline of a needleless syringe,
comprising a spring compressing a piston in a non-stationary manner,
according to a fifth embodiment of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
13
The needieless syringe of the present invention is
essentially designed to deliver particles of a therapeutic agent at velocities
sufficiently high, such that they can penetrate the epidermis or other target
tissue of a patient and can produce the desired medical effect. As
discussed hereinabove, the advantages of using a needleless syringe are
readily apparent, the two principal advantages being a reduced risk of
infection, and the essentially complete elimination of pain and fear for the
case of transdermal delivery.
Generally stated, the present invention aims at
accelerating particles of microscopic size, by means of a non-stationary
expansion of an inert gas at high pressure.
Figure 2A schematically shows the various parts of a
needleless syringe 10, to illustrate the concept of the present invention.
The needleless syringe 10, comprises a generally constant diameter
tubular nozzle 12, near the target 14, e.g. the skin of a patient. The nozzle
12 is filled with an inert gas or air 16, under atmospheric or other
conditions
(it can also be completely evacuated). The upstream end 18 of the nozzle
12 contains particles of a therapeutic agent 20. A triggering assembly,
including a reservoir 22 of an inert gas 24 under high pressure, having a
diameter larger to the tubular nozzle 12 is mounted to the upstream end 18
of the nozzle, the downstream end 26 of the nozzle 12 being designed to
be brought near the target 14. The reservoir 22 can be pre-loaded with
pressurized gas.
The reservoir 22 and the tubular nozzle 12 are linked
through a contraction 28 that is either sudden (as shown in Figure 2A) or
gradual (not shown). The reservoir 22 is separated from the tubular nozzle
by a partition 30 sufficiently resistant to withstand the pressure difference
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
14
existing between the reservoir 22 and the particles 20 in the tubular nozzle
12, which are essentially under atmospheric or other conditions.
To activate the needleless syringe 10, the partition 30
is ruptured or suddenly removed, allowing the pressurized gas 24
contained within the reservoir 22 to expand into the tubular nozzle 12.
As can be seen from Figure 2B, the sudden removal or
rupture of the partition 30 immediately produces i) a shock wave 32 in the
tubular nozzle 12; ii) non-stationary expansion waves 34 in the reservoir
22; and if the diameter of the reservoir is sufficiently large compared to
that
of the tubular nozzle iii) a stationary expansion wave 36 in the contraction
28 and iv) non-stationary expansion waves 38 in the tubular nozzle 12. It
should be noted that if the tubular nozzle 12 were evacuated, the shock
wave 32 would not be produced but the rest of the wave pattern would
essentially be the same.
The rupture of the partition additionally generates non-
stationary waves traveling in the upstream direction of the reservoir 22.
These non-stationary expansion waves reflect from the upstream end of
the reservoir, after which they start to travel in a downstream direction. The
non-stationary expansion waves 38 produce an essentially steady high
velocity flow in the downstream direction 26 of the tubular nozzle 12,
wherein the flow carries the particles of a therapeutic agent in the same
downstream direction.
These waves produce an almost instantaneous
acceleration of the gas contained within the tubular nozzle 12 and of the
gas contained within the reservoir 22. The accelerated gas in the tubular
nozzle 12, moving at high velocity, carries the therapeutic agent 20 towards
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
the exit of the nozzle 40 and hence towards the target 14. The particles 20
are accelerated from an initial position downstream from the partition 30.
A pattern of stationary and non-stationary expansion
waves is generated at the downstream 26 end of the tubular nozzle 12,
5 when the high velocity flow reaches the exit 40 of the tubular nozzle 12.
The essentially steady high velocity flow terminates when the leading front
of the reflected non-stationary expansion waves reaches the downstream
end of the nozzle. Additionally, the high velocity flow exits the downstream
26 end of the tubular nozzle 12, carrying an essentially spatially uniform
10 and parallel particle velocity distribution.
In the present invention, expansions 34, 36 and 38
generated upon rupturing or removal of the partition are primarily
responsible for the observed acceleration of the gases contained within the
device. It is believed possible to obtain a substantial gain in performance,
15 when the internal diameter of the reservoir is largely superior to the
diameter of the tubular nozzle. In this case, the expansion of the driver gas,
and therefore the particle velocity, would be increased while keeping the
pressure ratio between the reservoir and the tubular nozzle the same.
If the velocity of the gas and hence of the particles 20
is sufficiently high, i.e. in the order of 100-2000 m/s, the particles 20 will
be
capable of penetrating the target 14 to a sufficient depth, such that it will
be
ingested by the patient to then produce the desired medical effect.
Figure 2C illustrates a diagram of the trajectories of the
particle flow in the tubular nozzle 12 as well as in the reservoir 22, using
the same notation for the generated waves as in Figure 2B. It can be
readily observed that the particles of the therapeutic agent 20 are carried
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
16
by a quasi steady stream of gas at high velocity produced in the region
between the shock wave 32 and the reflected expansion. Additionally, it
can be observed that the required quantity of inert gas 24 is determined by
the length of the reservoir 22, whose length is itself determined in order to
avoid a possible return of reflection waves 42, prior to the exit of the
therapeutic agent 20 from the tubular nozzle 12.
The fluid dynamics taking place in the device from the
time of partition removal can best be computed and understood with the
help of a pressure-velocity (p-V) diagram. With such a graphical tool, the
specific features of the different possible configurations can clearly be
ascertained. Figure 2D shows the pressure velocity diagram for devices
having various ratios of reservoir diameter versus tubular nozzle diameter
for a given fixed pressure ratio between these two sections.
If the internal diameter of the reservoir is the same as
that of the tubular nozzle, only the non-stationary expansion waves 34 will
be produced. In the pressure-velocity diagram of Figure 2D, this is shown
as "Case A". It is seen that the solution is obtained by the intersection of
the curve representing all possible final states produced by a shock wave
32 in the tubular nozzle with the curve representing all possible final states
produced by unsteady expansion waves 34 in the reservoir gas. It is seen
that Case A (same internal diameter for reservoir and tubular nozzle) yields
a resulting gas velocity, VA thus particle velocity, which is fairly low.
However, as the reservoir internal diameter is
increased with respect to that of the tubular nozzle, a quasi-steady
expansion 36 would appear in the gradual or instantaneous contraction
region, thereby producing higher flow velocities. This is illustrated in
Figure
2D as "Case B" where, the combination of the unsteady expansion 34 and
the quasi-steady expansion 36 produces a final state at higher velocity VB.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
17
Interestingly, as the reservoir internal diameter is
further increased with respect to that of the tubular nozzle, this quasi-
stationary expansion would become stronger and therefore produce higher
flow velocities up to the a point when the flow at the entrance of the tubular
nozzle would become sonic (i.e., Mach number equal to 1). This is
indicated in Figure 2D as "Case C" and, according to the usual convention,
the sonic state is indicated in Figure 2D by an asterisk.
For physical reasons, further increasing the internal
diameter of the reservoir does not produce a higher quasi-steady
expansion past the sonic point within the contraction but instead a new
unsteady train of expansion waves 38 would appear in the tubular nozzle.
Actually, these waves are propagating upstream with respect to the
reservoir gas but since the latter flows downstream at sonic, or higher, flow
velocities, the expansion 38 is being swept downstream into the tubular
nozzle. In the pressure-velocity diagram of Figure 2D, this is shown as
"Case D". Obviously, the highest flow velocities for a given pressure ratio
are produced in this configuration.
The advantages of using a reservoir with a large
internal diameter as compared to that of the tubular nozzle are thus
apparent from Figure 2D, where it can be seen that, for the same pressure
ratio between the reservoir and the tubular nozzle, higher flow and particle
velocities are thus produced. To produce a particle stream at a given
velocity, the present invention therefore requires a lower pressure driver
gas than that of the prior art without the need to use a divergent nozzle.
Generally stated, the prior art failed to recognize that a
quasi-steady expansion would take place within the contraction and that for
a sufficiently large area ratio a second unsteady expansion would be
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
18
produced in the driver gas but downstream of the contraction. This is the
mechanism that is exploited herein.
Based on these flow mechanisms, a theoretical model
to calculate the gas flow in the syringe of the present invention was
established in order to optimize the syringe's parameters and to enhance
its performance. More specifically, the theoretical model suggests that the
velocity produced in the tubular nozzle 12, and therefore the velocity of the
particles 20 at the exit of the nozzle 12, is essentially a function of:
= the pressure ratio between the gas of the
reservoir 24 and the gas 16 of the tubular nozzle 12;
= the thermodynamic properties of the gas of the
reservoir 24, and the gas 16 of the tubular nozzle 12, more specifically the
speed of sound (which is temperature dependent);
= the specific heat ratio of the two gases;
= to a certain extend, the ratio of the length of the
reservoir 22 and the length of the tubular nozzle 12; and
= the diameter ratio of the reservoir 22 and of the
tubular nozzle 12.
It can be seen from Figure 2D that the resulting high
flow velocities produced with the present invention are also accompanied
by a large pressure increases in the tubular nozzle. This is a further
advantage of the present device which produces high particle velocities
without the further pressure reduction caused by divergent nozzles.
Depending on the initial pressure (P16) of the gas 16
within the tubular nozzle 12, the pressure at the exit 40 of the tubular
nozzle during the operation of the device could be lower, equal or larger
than the ambient (e.g., atmospheric) pressure near the target.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
19
If the pressure at the exit 40 is lower than ambient (i.e.,
the so-called overexpanded case), then normal or oblique shock waves
would appear at the exit to bring the pressure up to the required ambient
pressure, at the cost of a reduction in flow velocities. If the pressure at
the
exit would be higher than ambient (underexpanded case), a steady
expansion fan (i.e., Prandtl-Meyer expansion) would be produced outside
the exit 40 to bring the pressure back down, with a resulting potentially
beneficial increase in flow velocity. One can therefore see that it is befter
to
have exit pressures matched with or higher than ambient to avoid the
reduction in flow velocities associated with the shock waves present in the
overexpanded case. Since the particle acceleration method described in
the present invention produces large pressure ratios at the tube exit as
compared to previous ones, this is a further advantage of the present
invention.
Because the present invention produces exit pressures
(PD) that are higher than the initial pressure (P16) of the gas 16 contained
within the tubular nozzle 12, having the gas 16 at the same pressure as the
ambient pressure around the target 14 therefore guarantees that the
undesirable overexpanded case is never produced with the present
invention.
The matched case (i.e., same nozzle exit pressure as
ambient pressure) could be produced by having the gas 16 at a lower initial
pressure than ambient. To achieve this, a thin closure element 31 could
optionally be mounted to the nozzle exit 40 to support the pressure
difference, and this closure means would be removed or break when
impacted by the arrival of the flow at the exit 40.
If the tubular nozzle exit 40 were in direct contact with
the target 14, the arrival of the shock wave 32 at the target 14 would
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
produce a reflected shock wave back into the tubular nozzle thereby
slowing the flow down to a rest. To prevent this from occurring, the exit 40
of the nozzle is to be positioned at a predetermined distance from the
target, preferably by using a nozzle extension having a larger internal
5 diameter than that of the nozzle. This nozzle extension, also called a
spacer, could be shaped in such a way as to also serve as a silencer to
reduce the noise produced by the operation of the device 10.
Figure 3 is illustrative of a comparison between the
theoretical model and the values measured by the experimental assembly
10 illustrated in Figure 4 and described hereinbelow.
This comparison is expressed in terms of the pressure
measured in the high pressure reservoir 22 at the time of rupture of the
membrane 44 (Pobs), and the so-called corrected pressure (Pcorr), that is,
the pressure value required by the theoretical model in order to obtain the
15 observed velocity at the exit 40 of the tubular nozzle 12. One can readily
observe from this comparison that since Pobs < Pcorr, the velocity at the
exit 40 of the tubular nozzle 12 is generally less important than the velocity
predicted by the experimental model (Pobs), but that this discrepancy is
minimal and essentially constant throughout the range of velocities. This
20 illustrates that it is possible to calculate with a high degree of
dependability,
the velocity of the particles at the exit 40 of the tubular nozzle 12 in
function
of the geometric and dynamic parameters of the device.
The theoretical model was validated by experimental
testing of the primary configuration of the device. The experimental
assembly, as illustrated in Figure 4, essentially emulates the geometry
illustrated in Figure 2, the latter being used for the above-mentioned
theoretical calculations.
CA 02423647 2008-12-22
21
The experimental syringe 43 comprises a partition 44 made of a
Mylar membrane, separating the reservoir 22 from the tubular nozzle 12. The
partition 44 punctures when the pressure in the reservoir 22 is sufficiently
increased. The reservoir 22 has an internal diameter of 15.7 mm and is filled
with an inert gas 46, preferably helium, at pressures ranging from 20 to 80
atmospheres.
The tubular nozzle 12 has an internal diameter of 4.9 mm, is
exposed to the atmosphere and is charged with about 2 to 3 grams of an
yttrium oxide powder 48 having a nominal particle size inferior to 10 microns.
The powder 48 is positioned at the upstream end of the tubular nozzle 12, in
direct contact with the Mylar partition 44.
The trajectory of the various waves generated in the tubular
nozzle 12 upon puncturing of the membrane 44, was measured with the help
of two piezoelectric pressure detectors 50. The velocity of the powder exiting
the tubular nozzle 12, was determined with a system composed of diode
lasers 52 and photo-detectors 54, positioned at the exit 40 of the tubular
nozzle 12.
This study therefore illustrates that a viable concept is developed
for the injection of therapeutic agents. Additionally, a theory capable of
predicting the performance of the syringe is developed allowing for further
optimization of the present invention.
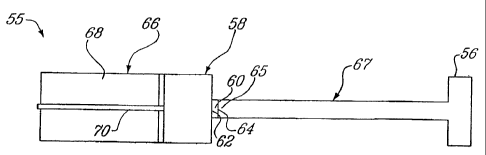
Turning now to Figure 5A, a syringe 55 according to a first
embodiment of the present invention will be described. The syringe 55,
provided with a gas reserve 66 about 25 mm long and 15 mm in diameter; a
reservoir 58 about 10 mm long and 15 mm in diameter; a nozzle 67 about 50
mm long and 4 mm in diamenter; a downstream end 56 about 5 mm long and
15 mm in diameter and a release valve 70 about 1 mm in diameter; includes a
reservoir 58 that is initially at atmospheric pressure, and a particle dose
60.
The particle dose 60 comprises an upstream membrane 62 and a
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
22
downstream membrane 64, containing the particle dose therebetween.
The particle dose 60 is positioned in the upstream end 65 of the tubular
nozzle 67 such that its upstream membrane 62 is directly adjacent to the
reservoir 58. The upstream membrane 62 is designed to support the
pressure differences between the reservoir 58 and the tubular nozzle 67.
The triggering assembly of the syringe 55 additionally comprises a gas
reserve 66 containing a high pressure inert gas 68 positioned upstream
from the reservoir 58, as well as a release valve 70, separating the
reservoir 58 and the gas reserve 66.
It is to be noted that the downstream end 56 of the
nozzle 67 is enlarged to form a spacer and an optional silencer. The
spacer is shaped so as to create a substantially normal wave near the
surface of the target. The substantially normal wave decelerates the
particles such that a radially uniform particle velocity distribution is
obtained. Additionally, the release valve includes a small diameter orifice
controlling the flow rate of the pressurized gas into the reservoir upon
opening of the release valve.
It is also to be noted that the measurements given in
Figure 5A are given as an illustrative example only and could be modified
without departing from the present invention. Additionally, syringe 55 could
also further comprise a silencer mounted to the downstream end 56 of the
tubular nozzle 67.
As can be observed in Figures 5B and 5C, various
shapes of particle doses may be used. More specifically, Figure 5B depicts
an example of a generally cylindrical particle dose 72 that is contained
within an essentially cylindrical envelope 74, confined by an upstream
membrane 76 and by an downstream membrane 78. Furthermore, Figure
5C illustrates a particle dose 80 confined by an upstream hemispheric
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
23
membrane 82, and a downstream hemispheric membrane 84, the two
hemispheric membranes generally defining a sphere. The membranes 76,
78, 82 and 84 can be arranged to rupture in a controlled manner due to
indentations or scoring (not shown) in their surfaces.
Returning to Figure 5A, the syringe 55 is triggered by a
release valve 70, enabling a high pressure inert gas 68 previously
contained in a gas reserve 66 to fill the reservoir 58 until a sufficiently
elevated pressure is reached, causing the upstream membrane 62 to
rupture, followed by the rupture of the downstream membrane 64 (both
being part of the particle dose 60). When the upstream membrane 62 and
the downstream membrane 64 rupture, the particle dose 60 is subjected to
a burst of high pressure gas produced by the various steady and unsteady
waves and is accelerated through the tubular nozzle 67. A non-illustrated
return mechanism closes the release valve 70, in order to avoid any loss
and squandering of inert gas 68 from the reserve 66. The volume and
elevated pressure of the gas contained in the reserve 66, would allow for
several consecutive injections. The gas reserve 66 could have been
previously filled with a gas cylinder or with a disposable high pressure gas
container (not illustrated). The size of the orifice of the release valve 70
would be small in order to decouple the wave phenomenon taking place in
reservoir 58, from the gas reserve 66.
It is to be noted that Figure 5A is schematic.
Syringe 55, according to a first embodiment of the
present invention, can be modified in order to facilitate its ease of
operation
as well as to increase its capabilities. More specifically, the aspects that
warrant particular attention are the membranes, the particle dose, the
source of compressed gas and the sterility.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
24
Control of the velocity of the particles impacting a
surface is achieved by selecting the initial physical properties of the gases
comprised in the reservoir and in the nozzle.
The pressure ratio that exists between the reservoir 58
and the tubular nozzle 67 just before membrane rupture, partially
determines the velocity of the particle dose 60, and for a given upstream
membrane 62, this ratio is constant. The simplicity of operation constitutes
the main advantage of directly introducing compressed gas into the
reservoir 58 until rupture of the upstream membrane 62 once a
predetermined pressure is reached.
For any given dose of particles of a given size
distribution, control of the velocity of the particles is lost and therefore
control is also lost over the penetrating depth of the particles, since the
penetration depth is directly a function of their velocity. It would be
therefore be desirable to better control the rupturing pressure. This is
possible by either replacing the upstream membrane 62 with a quick
release valve or by directly puncturing the membrane. The implementation
of either of these proposed methods, implies a syringe that is triggered by a
previously determined gas pressure in the reservoir 58.
It is to be noted that directly rupturing the upstream
membrane 62 is also envisioned by the present invention. Indeed, it is
possible to use a circularly shaped knife, positioned upstream from the
upstream membrane 62, and having a diameter identical to the inside of
the tubular nozzle 67. Additional concepts, for example involving quick
release valves comprising a radially moving guillotine or an axially moving
door, are also possible. For all the above-mentioned examples, the
reservoir 58 is advantageously equipped with a gas pressure indicator that
is either quantitative or qualitative.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
In the various concepts involving the use of quick
release valves, the particle dose 60 does not require the incorporation of a
membrane of predetermined resistance, in fact, the presence of a
membrane is not necessarily required.
5 As far as the disadvantages of a single dose device
are concerned, several multi-dose concepts are possible by calling upon
cartridges having a linear geometry (like a semi-automatic pistol) or a
circular geometry (like a revolver). With these concepts, time losses
associated with charging the device would be avoided, which could prove
10 very useful in large scale vaccination campaigns for example.
The operation of the needieless syringe 55 requires a
source of compressed inert gas, preferably helium, at pressures that can
reach up to 100 atmospheres. The source of inert gas can potentially be a
commercial gas cylinder, equipped with an appropriate pressure regulator
15 valve. An additional source can be a conventional pressurized disposable
inert gas ampoule.
It is possible to eliminate the need for an external
source of compressed inert gas, by providing for a particle dose wherein a
gas reservoir comprising a compressed inert gas at a desired pressure is
20 already incorporated. Additionally, such a concept could also be extended
to provide for a particle dose comprising an integral tubular nozzle. An
entirely disposable device, with the exception of the spacer/silencer, which
can be sterilized and re-used, would then be provided.
Turning now to Figure 6 a disposable self-contained
25 needieless syringe 87 according to another embodiment of the present
invention will be described. The needleless syringe 87, takes advantage of
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
26
a particle dose that comprises three membranes and two reservoirs of
compressed gas.
The triggering assembly of the disposable needleless
syringe 87 comprises a first large diameter reservoir 88 that is filled with a
high pressure inert gas 90 and that is positioned upstream from a second
small diameter reservoir 92, filled with a gas 94, at a pressure intermediate
between the gas 90 in the first reservoir 88 and the pressure within the
particle dose 100.
A first membrane 96 separates the first reservoir 88
from the second reservoir 92. This first membrane 96 supports the
pressure difference that exists between the gas 90 in the first reservoir 88
and the gas 94 in the second reservoir 92. A second membrane 98
separates the second reservoir 92 from the therapeutic agent 100 and
supports the pressure difference between the gas 94 in the second
reservoir 98 and the pressure within the particle dose 100. The gas 94 in
the second reservoir 92 is at a pressure above atmospheric pressure.
Finally, a third membrane 102, isolates the therapeutic agent 100 from the
gas 105 in the tubular nozzle 106, and therefore depending on the
pressure difference between the dose 100 and the gas 105, may or may
not support any pressure differential.
The disposable needleless syringe 87 is triggered by
temporarily opening the orifice 104 to the atmosphere (see Figure 6),
thereby venting the gas 94 to the atmosphere, which results in a pressure
drop in the second reservoir 92, with a concomitant increase in the
pressure difference across the first membrane 96. This pressure difference
increase on the first membrane 96 inevitably results in its rupture, in turn
exposing the two remaining membranes to a surge in pressure and their
subsequent rupture, thereby establishing the desired flow field which leads
CA 02423647 2008-12-22
27
to the acceleration of the therapeutic agent 100 through the tubular nozzle
106 and the spacer/silencer 108, mounted on the distal end 110 of the
tubular nozzle 106 towards the target 112. The orifice 104 is small so as to
decouple the flow phenomena taking place within the reservoir 88 and the
tubular nozzle 106 from the atmosphere, once the device is triggered.
Turning now to Figures 7A and 7B, another
embodiment of the present invention will be described. Figure 7A
illustrates a disposable particle dose/pressurized gas source assembly 89
similar to the syringe 87 of Figure 6 but lacking the tubular nozzle and the
spacer/silencer. This entire disposable cartridge assembly 89 could then
be inserted into a reusable device 91, illustrated in Figure 7B, comprising
a large diameter housing 93 connected to a tubular nozzle 95 itself
attached to a spacer/silencer 97. By temporarily opening of lateral orifice
104, either using a quick-release valve 101, puncturing the side of the
second reservoir 92 or other suitable means, the gas at intermediate
pressure 94 within the second reservoir 92 of the charged assembly 89 is
vented to the atmosphere, thereby successively triggering the rupture of
membranes 96, 98 and 102 and establishing the desired wave
phenomena within the device and accelerating the particles into the
tubular nozzle 95 towards the target 99.
As discussed hereinabove, the disposable cartridge
assembly 89 could also incorporate the tubular nozzle. In this case, the
reusable device would comprise only the large diameter housing 93
attached via a tubular region of larger size than that of the tubular nozzle
to the spacer/silencer 97.
For a system comprising the disposable charged
assembly 89 or variations thereof and the reusable device 91, or
variations, it is envisioned that the reusable device 91 could be similar to a
revolver or a semi-automatic pistol, with the mechanism 101 for opening
CA 02423647 2008-12-22
28
the orifice 104 incorporated into the revolver/pistol type device and the
"gun barrel" being replaced by the tubular nozzle 95 or the tubular region,
with the silencer spacer at its distal end. For the revolver-type device, the
disposable cartridges would be arranged around the periphery of a
cylindrical barrel and upon firing a spent cartridge would be rotated away
to be replaced with a loaded cartridge. After firing all the cartridges within
the barrel, the spent cartridges would be replaced with loaded ones. For a
pistol-type device, the cartridges would be arranged in a linear array. For
these revolver/pistol type devices, the target and the spacer/silencer
would be sterilized prior to firing. Since this can be done rapidly through
chemical agents, for example, such devices would allow for a minimal
delay between each firing, which could prove useful in large volume
applications such as vaccination campaigns. Another way to ensure
sterility would involve replacing the spacer/silencer after each shot.
Another method, aiming to avoid the use of a high
pressure source of an inert gas, is to directly incorporate into the
needleless syringe, a gas compression mechanism. It is possible to adjoin
a low pressure inert gas reservoir to the particle dose, which leaves the
compression of the inert gas to be accomplished. Two concepts, based on
the acceleration of a so-called free piston, whose inertia is used to
compress the gas until the membrane ruptures, will be described
hereinbelow with reference to Figures 8 and 9.
Figure 8 schematically illustrates the various parts of a
gas compression needieless syringe 113 according to a fourth
embodiment of the present invention. The triggering assembly of the
syringe 113 comprises a reserve 114 filled with compressed air or any
other compressed gas 116, that is mounted upstream from a reservoir
118. The reservoir 118 comprises a first chamber 120 that is filled with air
or an inert
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
29
gas 122 at atmospheric pressure and a second chamber 124, filled with an
inert gas 126 at atmospheric pressure.
A piston 128 is positioned between the first chamber
120 and the second chamber 124. Additionally, a release valve 130 is
positioned between the reserve 114 and the first chamber 120.
The downstream end 132 of the second chamber 124
comprises a membrane 134 against which is placed a particle dose 136. A
second membrane 137 encloses the particle dose 136. The needleless
syringe 113 additionally comprises a tubular nozzle 138 and a
spacer/silencer 140 placed against the target 142.
The advantage of this approach is found in the
potential for compressing the inert gas 126 contained in the second
chamber 124 of the reservoir 118 to a pressure exceeding that of the
compressed air or gas 116 in the reserve 114, by using the inertia of the
moving piston 128. Indeed, it has been found that the compressed air or
gas 116 in reserve 114, at a pressure of 5 atmospheres for example, is
capable of generating a pressure in the second chamber 124 of reservoir
118, reaching about 21 atmospheres. This substantial pressure increase is
accompanied by an increase in temperature of a factor of three, which in
turn results in a beneficial increase of the speed of sound which thereby
amplifies the expansion produced.
The particle dose 136 is positioned in the upstream
end 144 of the tubular nozzle 138. The particle dose 136, in an other
embodiment could potentially also incorporate the upstream membrane
134, the downstream membrane 137, the inert gas 126 at atmospheric
pressure, and possibly the tubular nozzle 138. The reserve 114 comprises
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
compressed air or an inert gas 116 at moderate pressures in the order of a
few atmospheres, coming from a cylinder or an ampoule.
The syringe 113 is activated by opening the release
valve 130, enabling the compressed air or inert gas 116 from the reserve
5 114 to fill the first chamber 120 and hence accelerate the piston 128
towards the second chamber 124 filled with an inert gas 126. The piston
128 compresses the inert gas 126 to a pressure sufficiently high to rupture
the membrane 134, which propels the particle dose 136 toward the target
142.
10 The inertia of the moving piston 128 is capable of
compressing the inert gas 126 to several times the pressure of the
compressed air or gas 116, as initially contained in the reserve 114. The
spacer/silencer 140 of the syringe 113, can be re-used after sterilization.
Figure 9 schematically illustrates the various parts of a
15 gas compression syringe 143 in accordance with a fifth embodiment of the
present invention.
This approach eliminates all sources of compressed
gas. The triggering assembly of the syringe 143 includes a cylindrical
reservoir 144 that comprises a downstream chamber 146, filled with an
20 inert gas 148 at atmospheric pressure or above, and an upstream chamber
150 open to the atmosphere. A piston 152 separates the upstream
chamber 150 from the downstream chamber 146. A compression spring
154 (shown in its compressed state) is positioned in the upstream chamber
150 and is connected to the piston 152.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
31
The syringe 143 additionally comprises a tubular
nozzle 158 mounted to the downstream end of the cylindrical reservoir 144.
A particle dose 160, comprising an upstream membrane 156 and a
downstream membrane 162, is positioned at the upstream end of the
tubular nozzle 158. The tubular nozzle 158 is in contact with the chamber
146.
The exit 164 of the tubular nozzle 158 is mounted to a
spacer / silencer 166, which is placed against the target 168.
In another embodiment, the particle dose 160 could
potentially also incorporate the membranes 156 and 162, the inert gas 148,
either at atmospheric pressure or above, and possibly the tubular nozzle
158.
To activate the syringe 143, the piston 152 is freed
which allows the spring 154 to propel the piston 152 in the downstream
direction resulting in the compression of the inert gas 148 contained in the
downstream chamber 146 of the reservoir 144. The piston 152
compresses the inert gas 148 to a pressure sufficiently high to rupture the
membranes 156 and 162 propelling the particle dose 160 towards the
target 168. Again, using the mass of the piston 152, the potential energy of
the spring is capable of pushing the piston towards the inert gas, resulting
in the compression of the inert gas to several times the quasi-static
pressure achievable by the steady-state extension of the spring. The
spacer/silencer 166 can be re-used after sterilization.
Of course, means must be provided to retain the piston
in its position shown in Figure 8 and to compress it so that this position may
be achieved.
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
32
It is conceivable that the needleless syringes of the
present invention, taking advantage of mechanisms for the direct
compression of an inert gas, could be composed of disposable parts such
as for example a module comprising the tubular nozzle, the particle dose
as well as the inert gas, all contained in a thin and flexible bag at
atmospheric or higher pressures. Additionally, these needleless syringes
could be composed of re-usable parts such as the housing, as well as the
piston and its triggering and accelerating mechanism. The interest of using
these kinds of needleless syringes is essentially found in the sterility that
they provide, since the exposed parts would be disposed of after each
application.
It is obvious that while using the needleless syringe of
the present invention, the propagation of micro-organisms from one patient
to another has to be avoided. The replacement of all the components that
either could or are in contact with the patient, such as for example the
spacer/silencer, the tubular nozzle and the reservoir, should therefore be
replaced. it is therefore advantageous that the primary configuration allows
for a rapid disassembly of the syringe between each use, such that the
above-mentioned components can be replaced. It is apparent that several
of the above-mentioned proposed improvements, strongly encourage the
sterility aspect and ease of operation of the needleless syringe.
The present invention allows for a more efficient and
more predictable acceleration of therapeutic agents, as compared with the
needleless syringes described in the prior art. Essentially, the needleless
syringe of the present invention, produces an acceleration of the
therapeutic agents, that is principally induced by a combination of non-
stationary and steady expansion waves, in a uni-dimensional nozzle.
Additionally, the needleless syringe of the present invention prevents the
CA 02423647 2003-03-25
WO 02/32483 PCT/CA01/01491
33
separation of the particle flow by using an elongate tubular nozzle of
constant cross section.
The terms and descriptions used herein are preferred
embodiments set forth by way of illustration only, and are not intended as
limitations on the many variations which those of skill in the art will
recognize to be possible in practicing the present invention. It is the
intention that all possible variants whether presently known or unknown,
that do not have a material effect upon the way the invention works, are to
be covered by the appended claims. Accordingly, those specialized in the
area covered by the present invention will certainly be in a position to apply
modifications or adaptations to the details described in the preferred
embodiment, while being constrained within the framework of the
appended claims.