Note: Descriptions are shown in the official language in which they were submitted.
C:HS 03 1 011-Fore~n Countries WK/li/XP
CA 02548352 2006-06-06
-1-
Means for nrotectin~ technical materials
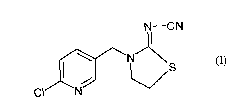
The application relates to the use of the compound 1-(2-chloro-5-
pyridylmethyl)-2-
cyanoiminothiazolidine (thiacloprid) as a microbicide for protecting
industrial materials
against attack and destruction by insects. The compound thiacloprid is known
from EP-
A 235 725, where it is described as being suitable for protecting plants.
The present application provides the use of thiacloprid of the formula (I)
N-CN
I \ ~N S (I)
CI N
its metal salts or acid addition compounds as microbicide for protecting
industrial materials
against attack and destruction by insects.
The pyridine derivative may not only be present in the form of the free base
but also in the
form of a metal salt complex or as acid addition salt. Preferred metal salts
are salts of
metals of the II. to IV. main group and the I. and II. and the IV. to VII.
transition group of
the Periodic Table of the Elements, examples which may be mentioned being
copper, zinc,
manganese, magnesium, tin, iron, calcium, aluminium, lead, chromium, cobalt
and nickel.
Suitable anions of the salts are those which are preferably derived from the
following acids:
hydrohalic acids, such as, for example, hydrochloric acid and hydrobromic
acid,
furthermore phosphoric acid, nitric acid and sulphuric acid.
The metal salt complexes of the pyridine derivative can be obtained in a
simple manner by
customary processes, for example by dissolving the metal salt in an alcohol,
for example
ethanol, and adding it to the thiacloprid. Metal salt complexes can be
isolated in a known
manner, for example by filtration, and, if appropriate, be purified by
recrystallization.
To prepare acid addition salts of the pyridine derivative, preference is given
to using the
following acids: the hydrohalic acids, such as, for example, hydrochloric acid
and
hydrobromic acid, in particular hydrochloric acid, furthermore phosphoric
acid, nitric acid,
sulphuric acid, mono- and bifunctional carboxylic acids and hydroxycarboxylic
acids, such
as, for example, acetic acid, propionic acid, 2-ethylhexanoic acid, butyric
acid, mandelic
CHS 03 1 011-Foreign Countries
CA 02548352 2006-06-06
-2-
acid, oxalic acid, succinic acid, 2-hydroxyethanedicarboxylic acid, malefic
acid, fumaric
acid, tartaric acid, citric acid, salicylic acid, sorbic acid, lactic acid,
and also sulphonic
acids, such as, for example, p-toluenesulphonic acid, p-decylphenylsulphonic
acid,
p-dodecylphenylsulphonic acid, 1,4-naphthalenedisulphonic acid,
alkanesulphonic acids,
benzoic acid and optionally substituted benzoic acids.
The acid addition salts of the compounds can be obtained in a simple manner by
customary
methods of forming salts, for example by dissolving a compound in a suitable
inert solvent
and adding the acid, for example hydrochloric acid, and be isolated in a known
manner, for
example by filtration, and, if appropriate, be purified by washing with an
inert organic
solvent.
Surprisingly, the compound of the formula (I) has a particularly high
insecticidal activity
against wood- and plastic-destroying insects, such as, for example,
A: Hymenoptera:
Sirex juvencus
Urocerus augur
Urocerus gigas
Urucerus gigas taignus
B: Beetles:
Anobium punctatum
Apate monachus
Bostrychus capucins
Chlorophores pilosus
Dendrobium pertinex
Dinoderus minutus
Ernobius mollis
Heterobostrychus brunneus
Hylotrupes bajulus
Lyctus africanus
Lyctus Brunneus
Lyctus linearis
Lyctus planicollis
CHS 03 1 011-Foreign Countries
CA 02548352 2006-06-06
-3-
Lyctus pubescens
Minthea rugicollis
Priobium carpini
Ptilinur pecticornis
Sinoxylon spec.
Trogoxylon aequale
Trypto dendron spec.
Xestobium rufovillosum
Xyleborus spec.
C: Termites:
Coptotermes formosanus
Cryptotermes brevis
Heterotermes indicola
Kalotermes flavicollis
Mastotermes darwiniensis
Reticulitermes flavipes
Reticulitermes lucifugus
Reticulitermes santonensis
Zootermopsis nevadensis
The amount of active compound (I) to be employed depends on the nature and the
occurrence of the insects and the material to be protected. During
application, the optimum
amount employed can in each case be determined by test series. However, it is
generally
sufficient to employ from 0.00005 to 1% by weight, preferably from 0.0005 to
0.1% by
weight, of the active compound (I), based on the material to be protected.
The insecticides employed in the protection of wood to date - organophosphorus
esters (for
example phoxim, chlorpyrifos), synthetic pyrethroids (for example permethrin,
cyfluthrin,
bifenthrin), IGRs (insect growth inhibitors; for example flufenoxuron,
fenoxycarb),
nitroimines (for example clothianidin, imidacloprid) - have at least one of
the following
disadvantages:
a) generally weak activity
CHS 03 1 O11-Foreign Countries '
CA 02548352 2006-06-06
- c~
b) activity gaps
c) high acute toxicity
d) poor weather persistence, for example against leaching
e) unbalanced activity spectrum
Surprisingly, it has now been found that the active compound of the formula
(I), having
relatively low acute toxicity, has a particularly high insecticidal activity
both against wood-
destroying beetles and against wood- and plastic-destroying termites.
Furthermore, it has
been found, unexpectedly, that, after highly intensive leaching tests
according to the
European standard test method EN 84, the high activity is not reduced.
The active compound of the formula (I) can be used as such, in the form of
concentrates or
in the form of generally customary formulations, such as powders, granules,
solutions,
suspensions, emulsions or pastes.
The formulations mentioned can be prepared in a manner known per se, for
example by
mixing the active compound of the formula (I) with at least one solvent or
diluent,
emulsifier, dispersant and/or binder or fixative, water repellent, if
appropriate, desiccants
and UV stabilizers and, if appropriate, dyes and pigments and other processing
auxiliaries.
Suitable solvents or diluents are organochemical solvents or solvent mixtures
and/or a
polar organic solvent or solvent mixtures and/or an oily or oil-like
organochemical solvent
or solvent mixture and/or water comprising, if appropriate, an emulsifier
and/or wetting
agents. Preferred for use as customary water-insoluble oily or oil-like
solvents with low
volatility are the respective mineral oils/mineral oil-containing solvent
mixtures or their
aromatic fractions. White spirit, petroleum and alkylbenzenes may be mentioned
as being
preferred, and additionally spindle oil and monochloronaphthalene. The boiling
ranges of
these slowly evaporating solvents (solvent mixtures) are in the range of from
more than
about 170°C to at most 350°C.
To some extent, the slowly evaporating oily or oil-like solvents described
above may be
replaced by more volatile organochemical solvents.
To prepare a composition for protecting wood, part of the solvent or solvent
mixture
C:HS U3 1 U11-Foreign Countries
CA 02548352 2006-06-06
_j_
described above is preferably replaced by a polar organochemical solvent or
solvent
mixture. Here, preference is given to using solvents which contain hydroxyl
groups, ester
groups, ether groups or mixtures of these functionalities. Esters and glycol
ethers may be
mentioned by way of example. According to the invention, binders are to be
understood as
being: synthetic resins which can be diluted in water and/or dissolved,
dispersed or
emulsified in organochemical solvents, binding drying oils, for example those
based on
acrylate resins, vinyl resins, polyester resins, polyurethane resins, alkyd
resins, phenol
resins, hydrocarbon resins, silicone resins. The binder used can be employed
as a solution,
an emulsion or a dispersion. Preference is given to using mixtures of alkyd
resins and
drying vegetable oil. Particularly preferred are alkyd resins having an oil
content between
45 and 70%.
Some or all of the binder mentioned may be replaced by a fixative (mixture) or
a plasticizer
(mixture). The purpose of these additives is to prevent evaporation of the
active
compounds and crystallization and/or precipitation. Preferably, they replace
from 0.01 to
30% of the binder (based on 100% of the binder employed).
The plasticizers are from the chemical classes of the phthalic esters, such as
dibutyl
phthalate, dioctyl phthalate or benzyl butyl phthalate, phosphoric esters,
such as tributyl
phosphate, adipic esters, such as di-(2-ethylhexyl) adipate, stearates, such
as butyl stearate
and amyl stearate, oleates, such as butyl oleate, glycerol ethers or higher-
molecular-weight
glycol ethers, glycerol esters and p-toluenesulphonic esters.
Fixatives are based chemically on polyvinyl alkyl ethers such as, for example,
polyvinyl
methyl ether, or ketones such as benzophenone and ethylenebenzophenone.
A preferred solvent or diluent is water, if appropriate in a mixture with one
or more of the
abovementioned solvents or diluents, emulsifiers and dispersants.
The active compound of the formula (I) or the compositions or concentrates
comprising it
are preferably employed for protecting wood and timber products and also
plastics against
attack and destruction by insects, in particular in the protection of tropical
wood.
In the context of the present invention, the term "wood" is meant to include
solid wood,
wood products and wood composites, such as, for example, round timber, cut
timber,
construction timber, wooden beams, railway sleepers, bridge components,
jetties, wooden
CHS 03 1 011-Foreign Countries
CA 02548352 2006-06-06
-6-
vehicles, boxes, pallets, containers, telephone poles, wooden fences, wood
cladding,
windows and doors made of wood, plywood, particle board, joiners' articles, or
wood
products which, quite generally, are used in the construction of houses or in
joinery.
"Plastics" are to be understood as meaning, in particular, polyvinyl chloride
(PVC),
polystyrene, polyurethane, polyethylene, polypropylene and polyesters.
Particularly effective protection of wood can be achieved by employing
industrial
impregnation processes, for example vacuum, double vacuum or pressure
processes.
If appropriate, the active compound of the formula (I) can be employed in
combination
with at least one other active compound from the group of the insecticides or
the
fungicides, to widen .the activity spectrum or to achieve particular effects,
such as, for
example, additional protection against wood-destroying fungi. Mixing partners
preferred
for this purpose are, for example, the following compounds from the group of
the
fungicides:
sulphenamides, such as dichlofluanid, tolylfluanid, folpet, fluorfolpet;
benzimidazoles, such as carbendazim, benomyl, fuberidazole, thiabendazole or
salts
thereof;
thiocyanates, such as thiocyanatomethylthiobenzothiazole, methylene
bisthiocyanate;
quaternary ammonium compounds and guanidines, such as benzalkonium chloride,
benzyldimethyltetradecylammonium chloride, benzyldimethyldodecylammonium
chloride,
dichlorobenzyldimethylalkylammonium chloride, didecyldimethylammonium
chloride,
dioctyldimethylammonium chloride, N-hexadecyltrimethylammonium chloride,
didecylmethylpoly(oxyethyl)ammonium propionate;
morpholine derivatives, such as tridemorph, fenpropimorph, azoles, such as
cyproconazole,
ipconazole, epoxyconazole, fluquinconazole, triadimefon, triadimenol,
bitertanol,
tebuconazole, propiconazole, azaconazole, hexaconazole, prochloraz,
bromuconazole,
metconazole, penconazole, clotimazole, climbazole, imizalil, iodine
derivatives, such as
diiodomethyl-o-tolylsulphone, 3-iodo-2-propynyl-n-butyl carbamate, 3-iodo-2-
propynyl-n-
hexyl carbamate, 3-iodo-2-propynylcyclohexyl carbamate, 3-iodo-2-
propynylphenyl
carbamate, phenol derivatives, such as tribromophenol, tetrachlorophenol, 3-
methyl-4-
CHS 03 1 011-Foreign Countries
CA 02548352 2006-06-06
-
chlorophenol, dichlorophene, o-phenylphenol, 2-benzyl-4-chlorophenol;
isothiazolinones, such as N-methylisothiazolin-3-one, 5-chloro-N-
methylisothiazolin-3-
one, 4,5-dichloro-N-octylisothiazolin-3-one, N-octylisothiazolin-3-one,
benzisothiazolinones, 4,5-trimethylene-N-methylisothiazol-3-one;
methoxyacrylates, such as azoxystrobin, trifloxystrobin;
pyridines, such as 1-hydroxy-2-pyridinthione (and their Na, Fe, Mn, Zn salts),
tetrachloro-
4-methylsulphonylpyridine;
metal soaps, such as tin naphthenate, copper naphthenate, zinc naphthenate,
tin octoate,
copper octoate, zinc octoate, tin 2-ethylhexanoate, copper 2-ethylhexanoate,
zinc
2-ethylhexanoate, tin oleate, copper oleate, zinc oleate, tin phosphate,
copper phosphate,
zinc phosphate, tin benzoate, copper benzoate, zinc benzoate;
Metal salts and oxides, such as tributyltin oxide, Cu20, CuO, ZnO, CuS04,
CuCIZ, copper
borates, copper fluorosilicates, sodium dichromate, potassium dichromate,
copper
hydroxycarbonate; tris-N-(cyclohexyldiazeniumdioxy)aluminium,
N-(cyclohexyldiazeniumdioxy)tributyltin and potassium salts, bis-N-
(cyclohexyldiazeniumdioxy)copper;
dialkyl dithiocarbamates, such as Na and Zn salts of dialkyl dithiocarbamates,
tetramethylthiuram disulphide;
nitrites, such as 2,4,5,6-tetrachloroisophthalidinitrile;
benzothiazoles, such as 2-mercaptobenzothiazole;
benzothiophenes, such as bethoxazin;
quinolines, such as quinoxyfen, 8-hydroxyquinoline and their Cu salts;
boron compounds, such as boric acid, boric esters, borax.
Possible insecticides which may be mentioned are;
acetamiprid, allethrin, alpha-cypermethrin, beta-cyfluthrin, bifenthrin,
bioallethrin, 4-
chloro-2-(2-chloro-2-methylpropyl)-5-[(6-iodo-3-pyridinyl)methoxy]-3 (2H)-
pyridazinone
CHS 03 1 011-Foreign Countries
CA 02548352 2006-06-06
(CAS-RN: 120955-77-3), chlorfenapyr, chlorpyrifos, clothianidin, cyfluthrin,
cyhalothrin,
cypermethrin, deltamethrin, ethofenprox, fenoxycarb, fipronil, flufenoxuron,
hexaflumuron, imidacloprid, nitenpyram, permethrin, pyriproxifen, silafluofen,
tebufenozide, thiamethoxam, tralomethrin, triflumuron.
Preference is given to active compound combinations with the following
insecticides:
alpha-cypermethrin, bifenthrin, chlorfenapyr, clothianidin, cyfluthrin,
cypermethrin,
deltamethrin, fipronil, imidacloprid, permethrin, thiamethoxam.
Particular preference is given to active compound combinations with the
following
insecticides:
alpha-cypermethrin, bifenthrin, chlorfenapyr, cypermethrin, fipronil,
imidacloprid,
permethrin, thiamethoxam.
Particularly preferred mixing partners are:
azaconazole, cyproconazole, fluquinconazole, hexaconazole, propiconazole,
tebuconazole,
triadimenol, triadimefon, imazalil, prochloraz, dichlofluanid, tolylfluanid,
thiabendazole,
fenpropimorph, tridemorph, bethoxazin, thiocyanatomethylthiobenzothiazole,
benzalkonium chloride, didecyldimethylammonium chloride,
didecylmethylpoly(oxyethyl)ammonium propionate, 3-iodo-2-propynylbutyl
carbamate,
trifloxystrobin.
Especially preferred mixing partners are:
cyproconazole, fluquinconazole, tebuconazole, triadimefon, prochloraz,
tolylfluanid,
bethoxazin, benzalkonium chloride, didecyldimethylammonium chloride,
didecylmethylpoly(oxyethyl)ammonium propionate, 3-iodo-2-propynylbutyl
carbamate.
The insecticidal compositions or concentrates used according to the invention
for
protecting industrial materials, in particular wood and plastics, comprise
from 0.00001 to
20% by weight, preferably from 0.0001 to 5% by weight, particularly preferably
from 0.001
to 1 % by weight, of at least one insecticidally active compound, 50 to 100%
by weight,
preferably 80 to 100% by weight, particularly preferably 90 to 100% by weight
and very
particularly preferably 98 to 100% by weight of the insecticidally active
compound being
CHS U3 1 U11-Foreign Countries
CA 02548352 2006-06-06
the active compound of the formula (I).
The compositions according to the invention may comprise at least one further
active
compound from the group of the abovementioned fungicides in an amount of from
0.01 to
40% by weight, preferably from 0.05 to 25% by weight.
Using the compositions according to the invention, it is possible, in an
advantageous
manner, to replace the insecticidal compositions available to date by more
effective
compositions. They have good stability and, in an advantageous manner, a broad
activity
spectrum.
CHS U3 1 U11-Fore~n Countries
CA 02548352 2006-06-06
- 1~ -
Examples
Example 1 (composition for impregnation)
0.025% of thiacloprid, 0.6% of tebuconazole, 2.67% of alkyd resin, 96.705% of
toluene
Example 2 (primer)
0.01% of thiacloprid, 0.45% of dichlofluanid, 10% of alkyd resin, 6% of
Dowanol DPM,
83.54% of white spirit
Example 3 (emulsifiable concentrate)
0.5% of thiacloprid, 5% of tebuconazole, 35% of Texanol, 32% of emulsifier,
27.5% of
cyclohexanone
CHS 03 1 011-Foreign COllntrleS CA 02548352 2006-06-06
- 11-
O
O
'b c~a
i.
T
O
b
v~
U C
~cC N N _ U
;, ~ ~ ~
w > ~ ~n o ~ o
~e ~ ~
0 0 ~ O
o ~ ~.
o ,
.~
o '~ x ~.~
. w
M N N O c~
b ~ ~ ~ ~ V U > U
o ,~ . o~ o~ a~ ~ .o ~ .o
3 ~ ~ o
,~ O O o ~ o
v O '
O O ~ _O ~ _O
'-' V V
~ s..~ s..
b
z .~ ~. ~ ~.
O U O
.
3 v 3
,~ ~ >,
'> a
..r ~~ M M N N o
U w w
~i.~ ~ ~ c~ C1
~ \b~A~ O N O
~s p ~ ~ ~ b-0~ b-0
V ,-r .-i c,- ~ w C
~ V V O O O '~ O 'aj
o s o o G
~ V V .~ ,~ .o
c_ v
v '
'> -d ~ -v ~ b
'''''~ o ai ai c~ ai
> > > >
0
ar C~ ~ ~ ~~ i>..iU i.>,nN
s.~. ~ ~ N GJ N
C~ ~ 5 ~ ~ 5 0~0 CO l/~V~ cn
Q, D. ~ ~ N
C~ L _s..~ O ~ '~ b b '~
O ~ p 'n ~~-- w
v w ~ ~~ o w ~ ~ 3 3 3 3
.o
~_ 3 ~3 ~_
_I~ ~ ~ _~ ~ O~O'~f'l~
H H x l_~ ~ M ~~
w ~ ~ '~ ~' w w w w
w ~''.~ w w
N t~1~