Note: Descriptions are shown in the official language in which they were submitted.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
METHODS, SYSTEMS AND DEVICES FOR REDUCING
THE SIZE OF AN INTERNAL TISSUE OPENING
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application
No. 60/821,947, filed August 9, 2006, U.S. Provisional Application No.
60/821,949,
filed August 9, 2006, U.S. Provisional Application No. 60/829,507, filed
October 13,
2006, U.S. Provisional Application No. 60/866,047, filed November 15, 2006,
and
U.S. Provisional Application No. 60/942,625, filed June 7, 2007, the contents
of each
of which are hereby incorporated by reference in their entirety. This
application
relates to U.S. Patent Application Serial No. 11/836,000, filed August 8,
2007, titled
DEVICES FOR REDUCING THE SIZE OF AN INTERNAL TISSUE OPENING
(Attorney Docket No. 16348.27.1), U.S. Patent Application Serial No.
11/836,016,
filed August 8, 2007, titled DEVICES FOR REDUCING THE SIZE OF AN
INTERNAL TISSUE OPENING (Attorney Docket No. 16348.27.2), U.S. Patent
Application Serial No. 11/836,037, filed August 8, 2007, titled DEVICES FOR
REDUCING THE SIZE OF AN INTERNAL TISSUE OPENING (Attorney Docket
No. 16348.27.3), U.S. Patent Application Serial No. 11/836,051, filed August
8, 2007,
titled SYSTEMS AND DEVICES FOR REDUCING THE SIZE OF AN INTERNAL
TISSUE OPENING (Attorney Docket No. 16348.27.4), U.S. Patent Application
Serial No. 11/836,013, filed August 8, 2007, titled SYSTEMS AND DEVICES FOR
REDUCING THE SIZE OF AN INTERNAL TISSUE OPENING (Attorney Docket
No. 16348.27.5), U.S. Patent Application Serial No. 11/836,026, filed August
8, 2007,
titled METHODS FOR DETERMINING CHARACTERISTICS OF AN INTERNAL
TISSUE OPENING (Attorney Docket No. 16348.27.6), and U.S. Patent Application
Serial No. 11/836,123, filed August 8, 2007, titled METHODS, SYSTEMS AND
3o DEVICES FOR REDUCING THE SIZE OF AN INTERNAL TISSUE OPENING
(Attorney Docket No. 16348.27.7) the contents of each of which are hereby
incorporated by reference in their entirety.
BACKGROUND OF THE INVENTION
1. The Field of the Invention
The present invention relates generally to medical devices and methods of use
for treating an internal tissue structure. More particularly, the present
invention
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
2
relates to medical devices, systems, and methods for reducing the size of an
internal
tissue opening.
2. The Relevant Technology
Physical malformations or defects that are present at birth can be detrimental
and even lethal when left uncorrected. A PFO is an example of a cardiac birth
defect
1o that can be problematic and even result in death when combined with other
factors
such as blood clots or other congenital heart defects. A PFO occurs when an
opening
between the upper two chambers of the heart fail to close after birth.
Some of the problems associated with a PFO can occur when a blood clot
travels from the right to the left atria of the heart through the PFO, and
lodges in an
artery that feeds blood to the brain. A blood clot in the left atrium can be
passed
through the aorta and travel to the brain or other organs, and cause
embolization,
stroke, or a heart attack. A PFO can be treated by being closed by a surgical
procedure. Additionally, other similar defects (e.g., septal or otherwise)
where some
tissue needs to be closed in order to function properly can include the
general
categories of atrial-septal defects ("ASDs"), ventricular-septal defects
("VSD's") and
patent ductus arteriosus ("PDA"), and the like.
Figures lA-1C depict various views of a heart having a PFO. The heart 10 is
shown in a cross-section view in Figure IA. In a normal heart 10, the right
atrium 30
receives systemic venous blood from the superior vena cava 15 and the inferior
vena
cava 25, and then delivers the blood via the tricuspid valve 35 to the right
ventricle
60. However, in the depicted heart 10 a septal defect, which is shown as a PFO
50, is
present between right atrium 30 and left atrium 40.
The PFO 50 is depicted as an open flap on the septum between the heart's
right atrium 30 and left atrium 40. In a normal heart 10, the left atrium 40
receives
oxygenated blood from the lungs via pulmonary artery 75, and then delivers the
blood
to the left ventricle 80 via the mitral valve 45. In a heart 10 having a PFO
50 some
systemic venous blood can also pass from the right atrium 30 through the PFO
50 and
mixes with the oxygenated blood in left atrium 40, and then is routed to the
body from
left ventricle 80 via aorta 85.
During fetal development of the heart 10, the interventricular septum 70
divides the right ventricle 60 and left ventricle 80. In contrast, the atrium
is only
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
3
partially partitioned into right and left chambers during normal fetal
development,
which results in a foramen ovale fluidly connecting the right and left atrial
chambers.
As shown in Figure 1B, when the septum primum 52 incompletely fuses with the
septum secundum 54 of the atrial wall, the result can be a tunnel 58 depicted
as a PFO
50.
Figure 1C provides a view of the crescent-shaped, overhanging configuration
of the septum secundum 54 from within the right atrium 30 in a heart 10 having
a
PFO 50. The septum secundum 54 is defined by its inferior aspect 55,
corresponding
with the solid line in Figure 1C, and its superior aspect 53 represented by
the phantom
line, which is its attachment location to the septum primum 52. The septum
secundum 54 and septum primum 52 blend together at the ends of the septum
secundum 54. The anterior end 56a and posterior end 56p are referred to herein
as
"merger points" for the septum secundum 54 and septum primum 52. The length of
the overhang of the septum secundum 54, which is the distance between superior
aspect 53 and inferior aspect 55, increases towards the center portion of the
septum
secundum as shown.
The tunnel 58 between the right atrium 30 and left atrium 40 is defined by
portions of the septum primum 52 and septum secundum 54 between the merger
points 56a and 56p which have failed to fuse. The tunnel 58 is often at the
apex of the
septum secundum 54 as shown. When viewed within right atrium 30, the portion
of
the septum secundum 54 to the left of tunnel 58, which is referred to herein
as the
posterior portion 57p of the septum secundum, is longer than the portion of
the
septum secundum 54 to the right of tunnel 58, which is referred to herein as
the
anterior portion 57a of the septum secundum 54. In addition to being typically
longer,
the posterior portion 57p also typically has a more gradual taper than the
anterior
portion 57a as shown. The anterior pocket 59a is the area defined by the
overhang of
the anterior portion 57a of the septum secundum 54 and the septum primum 52,
and it
extends from the anterior merger point 56a toward the tunnel 58. Similarly,
the
posterior pocket 59p is the area defined by the overhang of the posterior
portion 57p
of septum secundum 54 and the septum primum 52, and it extends from the
posterior
merger point 56p toward the tunnel 58.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
4
Conventional treatments for PFO, and other related conditions have generally
involved invasive surgery, which also presents a risks to a patient. Although
there are
some less invasive treatments for PFO, such treatments have been less
efficient at
closing the PFO opening than techniques involving invasive surgery.
BRIEF SUMMARY OF THE INVENTION
The invention relates to a medical system, devices and methods of use for
reducing the size of an internal tissue opening, such as a Patent Foramen
Ovale
("PFO"). In one embodiment of the invention, the medical system can include a
closure device and an associated delivery device. The medical system can be
configured to enable a practitioner to selectively position and deploy the
closure
device in an internal tissue opening to approximate the tissue of the opening.
According to one embodiment of the invention, the closure device can include
a multi-cellular body portion operatively associated with a first anchor and a
second
anchor. The multi-cellular body portion can be configured to enable the
closure
device to collapse into a relatively narrow non-deployed orientation and
expand into a
2o non-deployed orientation without plastic deformation or failure of the
closure device.
The first and second anchors can be configured to engage at least a portion of
a wall
of the internal tissue opening and/or tissue, such as tunnel tissue, of the
opening.
In one embodiment of the invention the closure device can include an
ingrowth material to facilitate tissue growth. The closure device can also
include one
or more indicators to facilitate the estimation of the position and/or
orientation of the
closure device with respect to the internal tissue opening.
In accordance with the present invention, the delivery device can include a
delivery assembly, an actuating assembly, and a release assembly operatively
associated with a handle body. In one embodiment of the invention, the
delivery
3o assembly facilitates selective delivery of the closure device from the
delivery device,
and is operatively associated with the actuating assembly and the release
assembly.
The actuating assembly interacts with the handle body to selectively deploy
the
closure device from the delivery assembly. In one embodiment of the invention,
the
actuating assembly can be configured to deploy at least a portion of the
closure device
by a first movement and deploy a second portion of the closure device by a
second
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 movement. The release assembly can be linked to the handle body to
facilitate
detachment of the closure device from the delivery device.
In one embodiment, the closure device is linked to the delivery device by one
or more tethers and one or more wires, the tethers being coupled to the handle
body
and the wires being coupled to a biasing member of the release assembly. The
tethers
lo can be configured to receive a portion of the closure device therein to
facilitate
securement of the closure device to the delivery device. The wires can be
detachably
coupled to the closure device to enable selective detachment of the closure
device
from the delivery device by movement of the biasing member.
These and other objects and features of the present invention will become
more fully apparent from the following description and appended claims, or may
be
learned by the practice of the invention as set forth hereinafter.
BRIEF DESCRIPTION OF THE DRAWINGS
To further clarify the above and other advantages and features of the present
invention, a more particular description of the invention will be rendered by
reference
to specific embodiments thereof which are illustrated in the appended
drawings. It is
appreciated that these drawings depict only typical embodiments of the
invention and
are therefore not to be considered limiting of its scope. The invention will
be
described and explained with additional specificity and detail through the use
of the
accompanying drawings in which:
Figs. lA-1C illustrate exemplary views of a heart having a Patent Foramen
Ovale;
Fig. 2 is a flowchart illustrating a method of reducing the size of an
internal
tissue opening according to one example;
Fig. 3A is a schematic diagram illustrating a step for locating a closure
device
with respect to an internal tissue opening using a delivery device according
to one
example;
Fig. 3B is a schematic diagram illustrating a step for deploying a first
portion
of a closure device according to one example;
Fig. 3C is a schematic diagram illustrating a step for deploying a second
portion of a closure device and an internal tissue opening having a reduced
size
according to one example;
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
6
Fig. 3D is a schematic diagram illustrating release of a closure element from
a
delivery device according to one example;
Fig. 4 illustrates a medical system according to one example;
Figs. 5A-5C illustrate a closure device in accordance with the present
invention;
Fig. 6 illustrates a delivery device according to one example;
Figs. 7A-7D illustrate cross-sectional views of a delivery device according to
one example;
Fig. 8 illustrates an exploded view of a delivery device according to one
example;
Fig. 9A illustrates an embodiment of a closure device being partially deployed
in an internal tissue opening;
Fig. 9B illustrates a delivery device in an orientation corresponding to the
partially deployed closure device of Fig. 8A according to one example;
Figs. l0A and lOB illustrate an exploded view of a delivery device according
to one example;
Fig. 11 illustrates the state of the delivery device upon releasing a closure
device according to one example;
Figs. 12A-21B are schematic diagrams of closure devices in accordance with
the present invention;
Figs. 22A-25B illustrate delivery of closure device using distal and/or
proximal locator devices according to the present invention;
Figs. 25C-25G illustrate inflatable closure devices according to the present
invention;
Figs. 26A-27N illustrate release mechanisms according to several examples;
Figs. 28-38B illustrate a delivery device according to the present invention;
and
Figs. 39A-39M illustrate configuration to promote tissue growth according to
several examples.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention extends to medical systems, methods, and apparatus for
reducing the size of an internal tissue opening. By way of explanation, the
devices
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
7
disclosed herein can be used to treat a variety of internal tissue openings,
such as a
left atrial appendage, paravalvular leaks, PDA's, and VSD's, for example.
Although,
for purposes of simplicity, frequent reference is made herein to reducing the
size of or
closing an opening in heart tissue known as Patent Foramen Ovale ("PFO").
Accordingly, it will be understood that references to PFO openings are not
limiting of
the invention.
In at least one example, a closure device is disclosed herein that is
configured
to acutely provide forces to close the opening associated with a PFO and allow
the
natural healing processes to effect a chronic closure. The closure device,
when
deployed, can have a flat aspect having a width and length, but a small
thickness. The
length of the device may correspond to a length of the internal tissue opening
or the
tunnel length of the internal tissue opening. The width of the device may
correspond
to a dimension that is generally transverse to the length.
The closure device may have an expandable, multi-cellular structure that is
configured to exert a lateral force on the walls of the internal tissue
opening. In at
least one example, the lateral force expands the width dimension of the tunnel
a
sufficient amount to reduce the height of the tunnel to thereby reduce the
size of the
tunnel and thereby close the internal tissue opening. The structural
properties of the
device can resist bending or curling out of plane to prevent or substantially
limit the
tendency of the device to prop the PFO open rather than closing it. This
property may
be achieved be utilizing struts with a preferential bending direction that is
oriented
parallel to the plane of the device and a non- preferential bending direction
that is
oriented perpendicular to the plane of the device, as is shown in Fig. IE and
will be
described in more detail hereinafter.
In the following description, numerous specific details are set forth to
assist in
providing an understanding of the present invention. In other instances,
aspects of
delivery and/or closure devices, or medical devices in general have not been
described
in particular detail in order to avoid unnecessarily obscuring the present
invention. In
addition, it is understood that the drawings are diagrammatic and schematic
representations of certain embodiments of the invention, and are not limiting
of the
present invention, nor are they necessarily drawn to scale.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
8
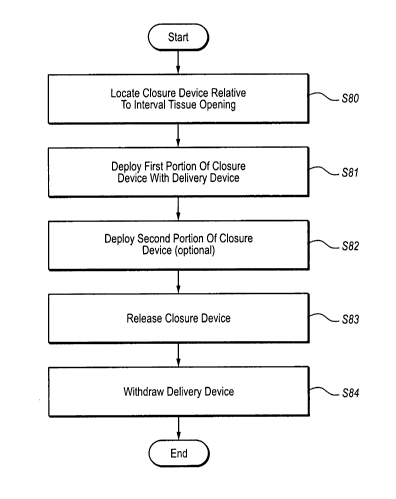
Fig. 2 is a flowchart illustrating a method of reducing the size of an
internal
tissue opening according to one example. Each of the steps will be introduced
generally, followed by a discussion of each step with respect to the schematic
diagrams illustrated in Figs. 3A-3D. The method begins at step S80 by
initially
locating a closure device with respect to the internal tissue opening. In at
least one
lo example, initially locating a closure device with respect to an internal
issue opening
includes using a delivery device that is configured to retain the closure
device in a
distal end while allowing a user to control the deployment of a closure device
at a
proximal end.
The closure devices described herein include collapsible multi-cellular
closure
devices that are configured to be stored in a collapsed state within the
delivery device
while the closure device is located relative to the internal tissue opening.
Further, the
configuration of the closure devices described herein can enable the closure
device to
be movable between a non-deployed or compressed state and a deployed or
decompressed state without causing failure or plastic deformation of the
closure
device.
The method continues at step S81 by deploying a first portion of the closure
device using the delivery device. Deployment of the first portion of the
closure
device may include expanding at least one of the cellular portions from the
collapsed
position within the delivery device to an expanded state. Further, at step S82
the
method may further optionally include the deployment of a second portion of
the
closure device may include expanding additional cellular portions from the
previously
described collapsed position with the delivery device to an expanded state. As
many
cellular portions may be deployed in as many steps as desired.
As will be discussed with reference to Fig. 3A, regardless of the number of
stages in which the closure device is deployed, once deployed the closure
device
exerts a force on the internal tissue opening to close the opening. Once the
closure
device has been deployed to close the internal tissue opening, the closure
device is
released from the delivery device at step S83 and the delivery device is
withdrawn at
step S84. A schematic diagram will now be discussed to illustrate various
steps of the
process illustrated in Fig. 2.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
9
Fig. 3A is a schematic diagram illustrating the step of locating a closure
device
90 with respect to an internal tissue opening 91 using a delivery device 92
(step S80).
The internal tissue opening 91 may be described as an opening having a tunnel
that
extends between a proximal surface and through a distal surface of tissue. For
ease of
reference, the distance between the proximal surface and the distal surface
may be
described as a length of the internal tissue opening 91.
As introduced, the dimension of the closure device 90 that corresponds to the
length of the internal tissue opening 91 is referred to as the length of the
closure
device 90. As the closure device 90 is deployed, the closure device 90 expands
to
apply a lateral force on the wall(s) of the internal tissue opening 91 to
thereby reduce
the size thereof. The direction in which the closure device 90 expands may be
referred to as the width of the closure device 90. In at least one example,
the closure
device 90 may be generally flat across its width both when in the collapsed
state as
well as in the expanded state illustrated and described below.
The delivery device 92 according to the present example includes a distal end
92a and a proximal end 92b. The delivery device 92 further includes delivery
assembly 93 near distal end 92a, and an actuation assembly 94 and a release
assembly
95 near the proximal end 92b. The closure device 90 is a multi-cellular device
that
includes a plurality of collapsible cells that may expand to an expanded state
described above. The closure device 90 is illustrated in a collapsed state
within the
delivery assembly 93. Accordingly, locating the closure device 90 relative to
the
internal tissue opening 91 may include locating a distal end 93a of the
delivery
assembly 93 near the internal tissue opening 31.
While located within the delivery assembly 93, the closure device 90 is
coupled to a push member 96 which in turn is coupled to a control anchor 97.
The
delivery assembly 93 is coupled to control assemblies 98a, b, which may be
part of
the closure device 90. In one example, the control assemblies 98a, b and
delivery
assembly 93 may be held in a fixed relationship relative to each other as the
control
anchor 97 is advanced. As the control anchor 97 advances relative to the
control
assemblies 98a, b and the delivery assembly 93, control anchor 97 drives the
push
member 96 which in turn pushes the closure device 90 distally relative to the
delivery
assembly 93.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 As illustrated in Fig. 3B, the control anchor 97 may be thus advanced until
the
control anchor 97 comes into contact with first control assembly 38a while
driving a
first portion 90a of the closure device 90 from the distal end 93a of the
delivery
assembly 93. As the first portion 90a of the closure device 30 is thus driven
from the
delivery assembly 93, the first portion 90a is deployed by expanding from the
10 compressed state illustrated in Fig. 3A to the expanded state illustrated
in Fig. 3B. In
the example of Fig. 3B, the delivery assembly 93 may extend at least partially
through
the internal tissue opening 91 to deliver the first portion 90a of the closure
device 90
distally of the internal tissue opening 91 (step S81). The first portion 90a
may then be
drawn into contact with the distal opening of the internal tissue opening 91.
Thereafter, the control anchor 97 in contact with the first control assembly
98a, the control anchor 97 and the first control assembly 98a may be moved
together
relative to the second control assembly 98b and the delivery assembly 93 to
drive the
closure device 90 further from the delivery assembly 93. In particular, as
illustrated
in Fig. 3C the control anchor 97 and the first control assembly 98a may be
driven
until the first control assembly 98a comes into contact with the second
control
assembly 98b. In at least one example, this distance may be sufficient for the
push
member 96 to push the closure device 90 clear of the distal end 93a of the
delivery
assembly 93 to thereby fully deploy closure device 90 (step S82).
As the closure device 90 is fully deployed, at least a second portion 90b of
the
closure device 90 expands outwardly within the internal tissue opening 91. As
the
second portion 90b expands outwardly, the width of the second portion 90b
expands
to apply a lateral force on the internal tissue opening 91, the force being
generally
along the width of the internal tissue opening 91. As the second portion 90b
becomes
wider, the portions of the internal tissue opening 91 illustrated as the sides
are drawn
3o apart while the portion of the internal tissue opening illustrated as the
top and bottom
are approximated. The overall result is that the internal tissue opening 91 is
constricted to close down the internal tissue opening 91.
A third portion 90c of the closure device 90 may be deployed proximally of
the internal tissue opening 91 as the closure device 90 is fully deployed. As
previously introduced, the first portion of the closure device 90 may be
deployed
distally of the internal tissue opening 91. Once fully deployed, the third
portion 90c
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
11
may be deployed proximally of the internal tissue opening 91. Such a
configuration
may reduce the likelihood that the closure device 90 will migrate through the
internal
tissue opening 91.
Once the internal tissue opening 91 has been closed, the closure device 90 is
released from the delivery device 92 as in Fig. 3D (step S83). As illustrated
in Figs.
1o 3A-3D, the release portion 95 of the delivery device 92 moves in concert
with the
push member 96 during the deployment of the closure device 90. A release
coupler
99 links the release assembly 95 to the closure device 90. In one example, to
release
the closure device 90 the release assembly 95 is moved proximally relative to
the
actuation assembly 94. As the release assembly 95 moves proximally, the
release
coupler 99 releases the closure device 90 from the delivery device 92 and from
the
delivery assembly 93 in particular. Several release configurations are
discussed in
more detail below.
Accordingly, the system is configured to deploy a closure device to close an
internal tissue opening. One medical system will now be described in more
detail that
includes a detailed discussion of one exemplary delivery device and exemplary
closure device. Additional closure devices will then be discussed, followed by
a
discussion of in-growth material configurations that may be used with closure
devices. Next, additional delivery devices will be discussed as well as
several release
assemblies that may be used with delivery and closure devices.
One configuration of relative movement between several control assemblies
and a control anchor have been described for multi-stage deployment of the
closure
device 90, which includes a plurality of cells. In addition to the movement
described
above, movements may be performed in any order with any number of control
assemblies and/or control anchors to deploy the closure device 90. Several
delivery
devices will be described herein which are configured to fully deploy the
closure
device 90. Each of the components may be combined and as desired and are not
limited to the use with devices or assemblies that may be discussed for
context.
Fig. 4 is a perspective view of a medical system 100 configured to facilitate
closure of an internal tissue opening according to one embodiment of the
present
invention. In the illustrated embodiment, the medical system 100 comprises a
closure
device 200 adapted to reduce the size of the internal tissue opening and a
delivery
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
12
device 300 adapted to facilitate placement and deployment of the closure
device 200
with respect to the internal tissue opening.
The medical system 100 of the present invention can provide benefits. For
example, the medical system 100 can be configured to be used with different
sizes,
shapes and types of internal tissue openings. Furthermore, the medical system
100
lo can provide various safety measures to increase the safety and
effectiveness of
positioning the closure device 200. In addition, the medical system 100 can be
configured to provide distributed lateral force to tissue of the internal
tissue opening.
In the illustrated embodiment, delivery device 300 comprises a handle body
302, an actuating assembly 320 operatively associated with handle body 302, a
release
assembly 340 operatively associated with the handle body 302 and a delivery
assembly 360 operatively associated with the actuating assembly 320, the
release
assembly 340 and the handle body 302. Handle body 302 can be configured to
provide a gripping surface for a user. Handle body 302 can be used to position
closure device 200, as well as facilitate deployment of the closure device 200
from the
delivery assembly 360. Actuating assembly 320 can be moved with respect to
handle
body 302 to selectively deploy portions of the closure device 200 from the
delivery
assembly 360. For example, the actuation assembly 320 is configured to receive
actuation inputs from a user to deploy the closure device 200 in one or more
stages, as
will be discussed more fully herein below.
Delivery assembly 360 can house closure device 200 in a non-deployed
orientation and facilitate deployment of closure device 200. Delivery assembly
360
can include one or more tethers 364 linked to the closure device 200 to
facilitate
selective deployment of the closure device 200 as well as the selective
detachment of
the closure device 200 from the closure device 200. The configuration of the
closure
device 200 will first be discussed in more detail, followed by a discussion of
deploying the closure device 200 with the delivery device 300.
With reference to Fig. 5A, the closure device 200 is illustrated in a fully
deployed, expanded, relaxed or non-constrained orientation. According to one
embodiment of the invention, the closure device 200 can be configured to close
an
internal tissue opening, or to reduce the size of an internal tissue opening
so as to
close the internal tissue opening. In one embodiment, the closure device 200
can
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
13
reduce the size of an internal tissue opening by approximating, or in other
words
bringing together tissue of the internal tissue opening, such as tunnel tissue
in a PFO.
The closure device 200 can approximate tissue by applying lateral force to
tissue of
the internal tissue opening, as will be discussed more fully herein after.
Also, the
closure device 200 can be configured to enable a user to estimate the position
and/or
lo orientation of the closure device 200 with respect to an internal tissue
opening, during
and after positioning of the closure device 200 in the internal tissue
opening.
According to one embodiment of the invention, the closure device 200 can be
a non-tubular stent. The closure device 200 can be configured to assume a
substantially flat configuration, or in other words be configured to be
substantially
planar, such as illustrated in Figures 5A and 39M for example. Furthermore,
the
closure device 200 can be configured to resist movement out of plane, such as
plane
260 of Figure 39M. However, the closure device 200 may bend out of plane when
positioned in a tissue opening.
The closure device 200 according to one embodiment of the invention has
many advantages. For example, the closure device 200 can be configured to be
reliable and compliant. The configuration of the closure device 200 can enable
the
closure device 200 to be movable between a non-deployed orientation and a
deployed
orientation without causing failure or plastic deformation of the closure
device 200.
The closure device 200 can be used to close various types, shapes and sizes of
internal
tissue openings. Furthermore, the closure device 200 can accommodate for a
range of
PFO tunnel lengths, for example. Also, the closure device 200 can be partially
or
fully deployed from or received back into the delivery device 300. Closure
device 200
can be configured to substantially conform to the size and shape of a tissue
opening.
For example, the undulations on the distal and proximal anchors can enable the
3o anchors to substantially, or to a certain degree, conform to the anatomy of
a tissue
opening.
Generally, the closure device 200 can have a substantially flat aspect having
a
length and height greater than its depth or depth thickness. For example, in
one
embodiment, the closure device 200 has an overall length of 22mm, a height of
7.5mm and a depth thickness of 0.4mm. According to one embodiment of the
present
invention, when the closure device 200 is in the relaxed or completely
expanded
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
14
orientation, as illustrated in Figure 5A, the distance between the opposing
ends of the
proximal anchor 218 can be about 22mm, the distance between the most proximal
attachment member 240 of the body portion 202 and the most distal indicator
220 of
the body portion 202 can be about 7.5mm, and the depth thickness, designated
as DT
in Figure 39M, of the closure device 200 can be about 0.4mm.
Furthermore, the majority of segments comprising the closure device 200 can
have a thickness or width that is substantially less than the depth thickness
of the
segments. The closure device 200 can resist out of plane movement due to the
size
and configuration of the segments. For example, the closure device 200 can be
configured to assume a substantially flat configuration in a first plane. The
configuration of the segments, for example the segments having a certain depth
thickness, can facilitate the closure device 200 resisting movement out of the
first
plane in a manner similar to an I beam resisting bending in the direction of
the web of
the beam. The first plane can be plane 260 as illustrated in Figure 39M.
Also, the closure device 200, according to one embodiment of the invention,
can have a unitary construction or may be formed from multiple pieces. If the
closure
device 200 has a unitary construction, the closure device 200 can be cut from
a single
piece of material, such as cut by a laser, thereby removing the need to
assemble or
join different segments together. The closure device may also be formed of
multiple
pieces of material. A unitary construction can provide advantages, such as
ease of
manufacturing and reliability. For example, assembly is not required for a
closure
device having a unitary construction. Also, a closure device having a unitary
construction may not include distinct elements or segments which require
joining by
joints, thereby reducing a likelihood of failure. The closure device 200 can
be made
from a super-elastic material, such as a super-elastic metal or a super-
elastic polymer.
Furthermore, the closure device 200 can be made from NiTiNol, stainless steel
alloys,
magnesium alloys, and polymers including bio-resorbable polymers.
In some embodiments according to the present invention, the closure device
can be formed by utilizing a pressurized stream of water, such as a water jet,
to
remove material from a piece of material to form the closure device.
Furthermore, it
is contemplated that the closure device can be formed by utilizing one or more
of the
following: die casting, chemical etching, photolithography, electrical
discharge
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 machining, or other manufacturing techniques. It is contemplated that the
closure
device can be formed through use of a mill or some other type of device
adapted to
remove material to form a desired shape.
It will be appreciated by one of ordinary skill in the art in view of the
disclosure provided herein that the closure device 200 can comprise multiple
to segments joined together by a known joining process, such as by an
adhesive, by
interference fits, crimping, by fasteners, or a weld, or some combination
thereof. For
example, in one embodiment, the closure device can include multiple segments
joined
together by various welds to form a closure device according to the present
invention.
In other embodiments, the segments can be joined together by a plurality of
means,
15 such as by the combination of welding, fasteners, and/or adhesives. The
segments can
be a wire or multiple joined or rolled wires crimped together or joined by a
joining
process to form the closure device 200.
In the illustrated embodiment, the closure device 200 includes a body portion
202, a first anchor 204 operatively associated with the body portion 202 and a
second
2o anchor 206 operatively associated with the body portion 202. The body
portion 202
can be configured to facilitate application of lateral force against tissue of
an internal
tissue opening. Also, the body portion 202 can be configured to enable the
closure
device 200 be movable between a non-deployed and deployed orientation. For
example, the closure device 200 can be configured to be self-expanding from
the
constrained or non-deployed orientation, as illustrated in Figure 5B for
example, to
the relaxed orientation, as illustrated in Figure 5A. In other words, the
closure device
200 can have a preferential orientation, such that movement of the closure
device 200
from a first orientation to a second orientation can create internal stresses
in the
closure device 200. These internal stresses can serve to bias the closure
device 200 to
the first orientation. For example, in one embodiment, the closure device 200
can
have a preferential orientation of the relaxed or fully deployed orientation
as
illustrated in Figure 5A. In this embodiment, movement of the closure device
200 to
a constrained orientation, such as illustrated in Figure 5B for example, can
create
internal stresses in the closure device 200, thereby creating in the closure
device 200 a
bias to return to the relaxed orientation.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
16
In the illustrated embodiment, body portion 202 includes one or more cells
208 defined by a plurality of segments 210. The body portion 202 can include
one or
more apertures. In one embodiment, an aperture is defined by the cell 208, or
in other
words by the plurality of segments 210. In one embodiment, segment 210 can be
a
strut or a body support segment. Cells 208 can be distinct, or can be at least
partially
to defined by a common segment. For example, cell 208A, as the distal most
cell, and
cell 208C, as the proximal most cell of body portion 202, are distinct and
defined by
distinct segments 210 with respect to each other. However, cell 208B is
partially
defined by a segment 210C which also defines a portion of cell 208A.
Similarly, cell
208B is partially defined by a segment 210G which also partially defines cell
208C.
Likewise, cell 208D shares a segment 210D with cell 208A and shares a segment
210H with cel1208C.
Segments 210 can be shaped and configured to have a substantially uniform
stress at any given point along a certain length, when the segment 210 is
deflected.
For example, segment 210A can include a first portion 230 having a width or
thickness greater than a second portion 232, wherein the width or thickness
decreases
from the first portion 230 to the second portion 232, or in other words is
tapered, in a
manner which provides for substantially uniform stress levels along the
certain length.
In other embodiments, segments can have a substantially constant width along
their
length.
Figure 5C is a cut-out view of a portion of the closure device 200, including
the first portion 230 and the second portion 232 of segment 210A. In the
illustrated
embodiment, the width or thickness of the segment 210A varies along the
portion of
the segment 210A from the location where segment 210A extends from the portion
254 which joins segment 210A to segment 210C to the intermediate portion 234.
As
the closure device 200 moves between an expanded or otherwise related
orientation
and a constrained or otherwise collapsed orientation, the segments 210 are
deflected,
with the highest levels of stress in the segment 210 being concentrated at the
joining
portion 254 and decreasing towards the intermediate portion 234. The segments
210
can be configured in a manner so as to have a substantially equal stress level
along the
length of the segment 210 between the joining portion 254 and the intermediate
portion 234. The uniform stress level can be accomplished by having the width
of the
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
17
segment 210 vary from the first portion 230 to the second portion 232 in a
calculated
manner. In one embodiment, the width of the first portion 230 of the segment
can be
about .1 mm and the taper to a width of about .05mm at the second portion 232
of the
segment.
In other embodiments, the uniform stress level can be accomplished by
utilizing a gradient of material having varying properties. In other
embodiments, the
segment 210 can have varying widths along its length and comprise a gradient
of
material sufficient to achieve a substantially uniform stress level between
the first
portion 230 and the second portion 232 of the segment. In the illustrated
embodiment, the first portion is adjacent the joining portion 254 and the
second
portion is adjacent the intermediate portion 234. In yet additional
embodiments, the
joints of the interconnecting segments can include a biasing member, such as a
spring,
thereby enabling the segments to move relative to each other to collapse or
expand the
closure device 200. Furthermore, the biasing member of the joint can cause the
segments to have a preferential orientation with respect to each other.
With continued reference to Figure 5A, segments 210 can also be configured
to have a rectangular cross-section. In other embodiments, segments 210 can
have an
oval shaped cross section. In yet another embodiment, sections 210 can have a
round
or rounded cross section. Furthermore, in one embodiment, the ratio, or aspect
ratio,
of the thickness or width to the depth thickness of the first and second
portions 230,
232 can range between at least about 1:2 to about 1:20. In one embodiment, the
aspect ratio of the width to the depth thickness of the first portion 230 can
be at least
1:2 and the ratio of the width to the depth thickness of the second portion
232 can be
at least 1:4. In an alternative embodiment, the aspect ratio of the first
portion 230 can
be about 1:4 and the aspect ratio of the second portion 232 can be about 1:8.
In this
manner, the closure device 200 can substantially resist out of plane movement,
while
allowing in-plane movement during reorientation of various portions of the
closure
device 200.
Segments 210 can be configured to be compliant. Compliancy of segments
210 can enable cells 208, and thus the body portion 202, to be oriented in
various
orientations. For example, body portion 202 can be oriented, or in other words
moved, between a non-deployed orientation, such as illustrated in Fig. 513,
and a fully
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
18
deployed orientation, such as illustrated in Fig. 5A. The compliancy of
segments 210
can facilitate the accommodation by the closure device 200 of a variety of
types,
shapes and sizes of internal tissue openings. For example, the size and
configuration
of the first and second anchors 204, 206 and the body portion 202 can enable
the
closure device 200 to accommodate varying sizes, shapes and types of internal
tissue
openings. In one implementation, the first anchor 204 can engage wall tissue
of an
internal tissue opening and the second anchor 206 can engage only the tunnel
tissue of
the internal tissue opening to approximate tissue. In an alternative
implementation
where the internal tissue opening has a shorter tunnel length, the second
anchor 206
can engage the tunnel tissue and an opposing wall of the internal tissue
opening to
approximate tissue.
Segments 210 can include an intermediate portion 234 configured to facilitate
securement of ingrowth materials to the closure device 200, or can be used as
an
indicator 220 to facilitate estimation of the position of the closure device
200 with
respect to an internal tissue opening. Furthermore, intermediate portion 234
can be
configured to facilitate measuring of a characteristic of an internal tissue
opening. In
one embodiment, intermediate portion 234 can include one or more apertures.
The
apertures can be configured to receive a securing element, such as a thread,
therethrough to facilitate securing an ingrowth material to the closure device
200.
Intermediate portion 234 can be configured to be stiffer or more rigid than
first
portion 230, second portion 232, or both. A stiffer intermediate portion 234
can
increase the reliability of segments 210.
In another embodiment, the intermediate portion 234 can include an indicator
220, such as a dense metallic rivet or concentration of dense material, for
use in
estimating the orientation and/or position of the closure device 200.
Understanding of
the orientation and/or position of the closure device 200 can facilitate
estimating a
physical characteristic of an internal tissue opening and/or the relative
position of the
closure device 200 with respect to the internal tissue opening. For example,
if the
distance between the indicators 220 is known, a practitioner can estimate a
physical
characteristic, such as the opening or tunnel width, by determining the new
distance
between the indicators 220 when the closure device 200 is positioned in the
tissue
opening. Similarly, indicators 220 can be positioned on the first and second
anchors
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
19
04, 206. The indicators 220 can be configured and arranged on the closure
device 200
such that when the first anchor 204 is deployed the indicators 220 are
substantially
aligned. In this manner, a practitioner can estimate whether the first anchor
204 has
fully deployed.
In some cases, it may be difficult to view the closure device 200 in the event
1o the closure device 200 is at a skewed angle with respect to the viewing
plane, such as
a fluoroscope. When the closure device 200 is skewed in this manner, it can be
difficult to determine accurately the distance of interest. However, when
various
distances between indicators is known, a user can use the known distances to
calculate
the distances of interest by using geometry.
In one embodiment, segments 210 along a similar or common lateral plane can
have substantially equal lengths. Substantially equal lengths of segments 210
in this
manner can enable body portion 202 to be moved between the non-deployed and
deployed orientation without failure of the segments 210. For example, in one
embodiment, segments 210A and 210B have substantially the same length,
segments
2o 210E, 210C, 210D, and 210K have substantially the same length, segments
210F,
210G, 210H and 210L have substantially the same length, and segments 2101 and
210J have substantially the same length. In this configuration, body portion
202 can
be collapsed or oriented into the non-deployed orientation, as illustrated in
Fig. 5B,
without causing damage to the body portion 202 of closure device.
The closure device 200 can be configured to have a preferential orientation of
the fully deployed orientation as illustrated in Fig. 5A. As the closure
device 200 is
deployed from the delivery device 300, the configuration of closure device 200
can
cause the closure device 200 to preferentially move toward the fully deployed
orientation. Thus, as the closure device 200 is deployed in an internal tissue
opening,
the preferential orientation of the closure device 200 can cause the closure
device 200
to apply lateral force to the tissue of the internal tissue opening. In other
words, the
body portion 202, first anchor 204 and the second anchor 206 are deflected by
an
applied force in order to reorient the closure device 200 from the fully
deployed
orientation to a non-deployed orientation, for example. In this manner, the
closure
device 200, because of the deflection of the body portion 202, first anchor
204 and the
second anchor 206, will have tendency to return to the fully deployed
orientation.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 When the closure device 200 is positioned in an internal tissue opening, the
deflected
body portion 202, first anchor 204 and the second anchor 206 can have a
tendency to
apply a lateral force to tissue of the opening as the closure device 200
attempts to
return to the fully deployed orientation.
Body portion 202 can be operatively associated with the first anchor 204 and
10 the second anchor 206. First and second anchors 204, 206 can be configured
to move
between a deployed and non-deployed orientation. First and second anchors 204,
206
can be configured to apply lateral force to tissue of an internal tissue
opening, and to
engage and/or contact a portion of wall tissue and/or tunnel tissue of an
internal tissue
opening. In one embodiment, the first anchor 204 can be a left atrial anchor,
and the
15 second anchor 206 can be a right atrial anchor.
In the illustrated embodiment, the first anchor 204 can include a first anchor
segment 212 and an opposing second anchor segment 214. Likewise, the second
anchor 206 can include a first anchor member 216 and an opposing second anchor
member 218. The first anchor segment 212 can be configured to move relative to
the
20 second anchor segment 214. Likewise, the first anchor member 216 can be
configured to move relative to the second anchor member 218. In this manner,
the
closure device 200 can accommodate for a variety of types, shapes and sizes of
internal tissue openings. The first anchor segment 212 and the second anchor
segment 214 can be configured to be substantially similar in size, shape and
configuration. As such, reference to the configuration and/or function of one
of the
first or second anchor segments can apply to the other anchor segment. In one
embodiment of the invention, the first anchor 204 and/or the second anchor 206
can
include one or more undulations. The undulations can facilitate reorienting or
movement of the anchors with respect to the body portion 202, for example,
from a
deployed to a non-deployed configuration. Furthermore, the undulations can
facilitate
the anchor substantially conforming to the anatomy of the tissue opening.
The first anchor segment 212 can include a distal end 224 and a proximal end
226. The first anchor segment 212 can be defined by various segments and can
include reinforced segments 228 and one or more engaging members 222. For
example, in the illustrated embodiment, the first anchor segment 212 is at
least
partially defined by segment 210K of cell 208D. The engaging members 222 can
be
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
21
microposts or tines configured to contact and/or engage tissue. The engaging
members 222 can include a sharp tip or can be blunt. The engaging members 222
can
be configured to provide a degree of surface texture in order to increase
engagement
of the first anchor 204 with tissue.
The first anchor segment 212 can be configured to be moved between a non-
1o deployed orientation, as illustrated in Fig. 513, and a fully deployed
orientation, as
illustrated in Fig. 5A. The first anchor segment 212 can be configured such
that the
distance from the proximal end 226 to the distal end 224 of the segment which
includes the engaging members 222 is substantially equal to the distance from
the
proximal end 226 to the distal end 224 of the segment which includes the
reinforced
segments 228 and segment 210K. The second anchor segment 214 can be configured
similar to the first anchor segment 212.
First anchor segment 212 can be configured to define a closed periphery. For
example, first anchor segment 212 can include the reinforced segment 228
extending
from the body portion 202 to the segment having the engaging members 222 which
is
connected to segments 210K, 210L to define a closed periphery with segment
210K.
Furthermore, two reinforced segments 228 can extend from the joining portion
254 of
the body portion 202 and join together near the distal end 224 of the first
anchor 204.
As such, there are multiple anchor portions extending from the body portion
202. In
this manner, anchors of the present invention are reinforced to provide
greater rigidity
and strength to facilitate stabilization and maintenance of the closure device
200
within a tissue structure.
First anchor member 216 can include a distal end 236 and a proximal end 238.
The first anchor member 216 can be defined by various segments and can include
one
or more engaging members 222. For example, in the illustrated embodiment, the
first
anchor member 216 is at least partially defined by segment 210L of cell 208D.
The
engaging members 222 can be microposts or tines configured to contact and/or
engage tissue. The engaging members 222 can include a sharp tip or can be
blunt.
The engaging members 222 can be configured to provide a degree of surface
texture
to increase engagement of the second anchor 206 with tissue.
It will be understood by one of ordinary skill in the art in view of the
disclosure provided herein that the engaging members 222 can vary in size and
shape,
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
22
and can be positioned at various locations on the closure device 200. In
alternative
embodiments, one or more engaging members can extend out of plane of the
closure
device so as to contact tissue which is perpendicular, for example, to the
substantially
flat plane, such as plane 260 of Figure 11 B, of the closure device 200.
The first anchor member 216 can be configured to be moved between a non-
deployed orientation, as illustrated in Fig. 5B, and a fully deployed
orientation, as
illustrated in Fig. 5A. The first anchor member 216 can be configured such
that the
distance from the proximal end 238 to the distal end 236 of the segment which
includes the engaging members 222 is substantially equal to the distance from
the
proximal end 238 to the distal end 236 of the segment which includes segment
210L.
In this manner, first anchor member 216 can be detachably coupled to the
delivery
device 300 when in a non-deployed orientation inside the delivery device 300
as
illustrated in Fig. 5B. The second anchor member 218 can be configured similar
to
the first anchor member 216.
The first anchor segment 212 can also include a first portion 256 and a second
portion 258 configured to facilitate engagement of the internal tissue
opening. For
example, first anchor segment 212 can be configured to include one or more
undulations causing the first portion 256 to be positioned in close proximity
with
second portion 258. In this manner, as tissue is positioned between the first
and
second portions 256, 258, the configuration of the first anchor segment 212
can
engage, or to some degree, pinch the tissue therebetween to facilitate
maintenance of
the position of the closure device 200 with respect to the tissue opening.
The closure device 200 can also include attachment members 240 for use in
detachably linking the closure device 200 to the delivery device 300, as will
be
discussed more fully herein after. The attachment members 240 can include an
aperture 242 for use in facilitating the linking of the closure device 200 to
the delivery
device 300.
Fig. 5B illustrates the closure device 200 in a non-deployed or constrained
orientation. The configuration of the body portion 202, and the first and
second
anchors 204, 206 enables the closure device 200 be reoriented from the fully
deployed
and preferential orientation, as illustrated in Fig. 5A, to the non-deployed
or collapsed
orientation as illustrated. In the collapsed or non-deployed orientation, the
first
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
23
anchor 204 extends distally and the second anchor 206 extends proximally, with
the
attachment members 240 being the proximal most portions of the second anchor
206
and the body portion 202.
In the illustrated embodiment, the closure device 200 is positioned inside of
a
delivery portion 366 of the delivery device 300. The configuration of the
closure
device 200 can cause portions of the closure device to apply force to the wall
of the
delivery portion 366 due to the preferential orientation of the closure device
200. The
closure device 200 is configured to be received into and deployable from the
delivery
portion 366.
Fig. 6 illustrates one embodiment of the delivery device 300. In the
illustrated
embodiment, the delivery assembly 360 includes a catheter 362 having a
delivery
portion 366, and a plurality of tethers 364 at least partially housed by the
catheter 362.
The tethers 364 can be configured to facilitate selective detachment of the
closure
device 200 from the delivery device 300. The delivery portion 366 can be
configured
to receive the closure device 200 therein. The catheter 362 can be coupled to
the
.20 actuating assembly 320, such that movement of the actuating assembly 320
can cause
movement of the catheter 362.
In the illustrated embodiment, the actuating assembly 320 includes a first
member 322 operatively associated with the handle body 302, a second member
324
operatively associated with the first member 322 and the handle body 302, and
a knob
338 linked to the first member 322. The actuating assembly 320 can be utilized
by a
user to selectively deploy the closure device 200 from the catheter 362. As
will be
discussed in more detail below, a practitioner can move the knob 338, which is
coupled to the first member 322, in the proximal direction to deploy first
anchors 204
(Fig. 4). Thereafter, the second member 324 can be rotated in order to
selectively
deploy the remaining portions of the closure device 200 from the delivery
portion 366
of the delivery device 300.
In addition to providing for a two-step deployment process, the exemplary
delivery device 300 illustrated in Fig. 6 is also configured to allow a
practitioner to
estimate the progress of the deployment process. In particular, the handle
body 302
can include indicia 304 to enable a user to estimate the degree of deployment
of the
closure device 200 from the delivery device 300, as well as predict detachment
of the
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
24
closure device 200 from the delivery device 300. For example, indicia 304 can
include deployment indicia 306 and release indicia 308. Deployment indicia 306
can
be utilized to enable a user to estimate the degree of deployment of the
closure device
200 from the catheter 362, and the release indicia 308 can be utilized to
predict the
detachment of the closure device 200 from the delivery device 300. The handle
body
1o 302 can also include a release pin groove 310. The release pin groove 310
can be
operatively associated with the release assembly 340 to facilitate the
selective
detachment of the closure device 200 from the tethers 364.
According to one embodiment of the invention, the release assembly 340 can
include a biasing member 342 operatively associated with the handle body 302
to
facilitate detachment of the closure device 200. A release knob 346 can be
provided
to manipulate the position of biasing member 342 in order to release or detach
the
closure device 200. In one embodiment, the release knob 346 is coupled to the
biasing member 342, such that movement of the release knob 346 can cause
movement of the biasing member 342 relative to the handle body 302 to thereby
cause
separation between the handle body 302 and the release knob 346. In the
present
example, release knob 346 is operatively associated with the tethers 364A-C
such that
as the release knob moves proximally relative to the handle body 302 the
tethers
364A-C are drawn proximally to release closure device 200. Specifics of the
operation of the release assembly 340 and other release assemblies will be
discussed
in more detail below.
Fig. 7A is a cross-sectional view of the distal end of the catheter 362. In
the
illustrated embodiment, the catheter 362 includes a delivery portion 366 for
use in
positioning the catheter 362. The catheter 362 can be made from a resilient
material
having sufficient axial stiffness to allow a practitioner to position the
catheter 362
with respect to an internal tissue opening, and sufficient rotational
stiffness to allow a
practitioner to rotate the catheter 362 by rotating the handle body 302. In
one
embodiment, the catheter 362 comprises a braided polyimide. In other
embodiments,
the catheter 362 can be made from a material having a sufficient axial
stiffness, such
as a braid reinforced polymer, axially reinforced polymer, metal reinforced
polymer,
carbon reinforced polymer, or some other type of axially stiff material. The
delivery
portion 366 can be made from a thermoplastic elastomer, such as PEBAX . In
other
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 embodiments, the delivery portion or tip portion 366 can be made from a
material
having sufficient flexible properties, such as a polymeric material. In other
embodiments, the delivery portion 366 can include a combination of materials,
such
as metallic materials and polymeric materials.
The delivery portion 366 can define a lumen 368 to facilitate placement of the
10 catheter 362. For example, a guidewire can be received in the lumen 368 to
guide the
catheter 362 to a desired location. In this manner, the closure device 200 can
be
located proximate to the internal tissue opening in a quick and efficient
manner.
Furthermore, the delivery portion 366 can be shaped, such as including a bend,
in
order to facilitate placement of the delivery portion 366 through a PFO, for
example.
15 In one embodiment of the invention, the catheter 362 can be considered a
rapid
exchange catheter wherein the delivery or tip portion 366 enables a guidewire
to be
linked to the catheter 362 in a quick and efficient manner for placement of
the
catheter 362.
The catheter 362 and delivery portion 366 can be configured to at least
20 partially house tethers 364 in a lumen which is distinct and separate from
lumen 368.
For example, lumen 368 can be in a spaced apart, non-coaxial arrangement from
the
lumen which houses tethers 364, such that a guidewire can be received through
lumen
368 without being introduced into the lumen or space in which the tethers 364
are
housed. In this manner, a user can introduce a guidewire into the lumen 368 at
the
25 distal end of the catheter 362, rather than the lumen which at least
partially houses the
tethers 364 which would require the guidewire to be introduced into the lumen
at the
proximal end of the catheter 362. In alternative embodiments, the lumen 368
configured to receive the guidewire therein can be positioned inside the lumen
which
houses the tethers 364. In this embodiment, lumen 368 would include an opening
and
3o an exit at the distal end of the catheter 362 in order to facilitate the
quick placement of
a guidewire through the lumen 368.
In one embodiment, catheter 362 can include a rounded cross-section and the
delivery portion 366 can include a rectangular cross-section. The rectangular
cross-
section of the delivery portion 366 can facilitate proper deployment of the
closure
device 200 from the delivery device 300, as well as facilitate the closure
device 200
being reintroduced back into the delivery portion 366. The rectangular cross-
section
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
26
of the delivery portion 366 can be sized to orient the tethers 364 next to
each other in
a linear fashion. In this manner, the likelihood that the tethers 364 cross
each other
upon reintroduction of the closure device 200 into the delivery portion 366
can be
reduced.
In one embodiment of the invention, tethers 364 includes three tethers 364A-
C, each tether 364 being sized and configured to attach to and/or accommodate
therein an attachment member 240 of the closure device 200. One example of a
tether
is a line or hollow tube coupled to the handle body 302. The tether 364 can
comprise
a flexible, hollow shaft having sufficient stiffness such that as actuating
assembly 320
moves the catheter 362 proximally with respect to the handle body 302, the
closure
device 200 is forced out of the delivery portion 366. Likewise, the tether 364
can be
configured to pull the closure device 200 back into the delivery portion 366
as the
actuating assembly 320 is moved distally with respect to the handle body 302.
In one embodiment, the tethers 364 can be a coil of stainless steel covered by
a
heat shrunk tubing to give the coil a degree of tensile strength and rigidity.
In an
alternative embodiment, the tether 364 can be a polymeric tube. In yet an
additional
embodiment, the tether 364 can be a combination of polymeric materials and
metallic
materials. In some embodiments, additional heat shrunk tubing covers a
proximal
segment of the three tethers 364A-C. The heat shrunk covering can increase the
column strength of the tether 364, which can enable the tethers 364 to assist
with
deployment and reintroduction of the closure device 200 from and into the
delivery
portion 366. The tethers 364 can have a distal tip configured to correspond to
the
shape and size of the attachment members 240 of the closure device, such that
the
attachment member 240 can be received into the distal tip of the tether 364,
as
illustrated in Fig. 7B.
Tethers 364 can be made from a material having sufficient flexibility to
substantially prevent distortion or otherwise influence the orientation of the
closure
device 200 when the closure device is deployed from the catheter 362, yet have
sufficient axial strength to facilitate deployment of the closure device 200
when the
catheter 362 is moved proximally with respect to the closure device 200. The
tethers
364 can have a lumen extending therethrough of sufficient size and
configuration to
enable a plurality of wires 378 to be housed and movable therein.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
27
Illustrated in Fig. 7B is a cross-sectional view of attachment member 240 of
the closure device 200 received into a tether 364 and coupled by first and
second
wires 378a, 378b. In the illustrated embodiment, a second wire 378b can extend
through and out of the tether 364 and form a loop. The loop can extend through
an
aperture 242 of the attachment member 240 of the closure device 200. With the
loop
of second wire 378b positioned through the aperture 242 of the attachment
member
240, a first wire 378a, which extends through and out of the tether 364, can
extend
through the loop of the second wire 378b to form a locking feature. When the
first
wire 378a extends sufficiently through the loop of the second wire 378b, the
closure
device 200 can remain coupled to the delivery device 300 until the first wire
378a is
pulled through the loop of the second wire 378b, and the second wire 378b is
pulled
out of the aperture 242 of the attachment member 240.
The first wire 378a and the second wire 378b can be attached at their proximal
ends to the biasing member 342 (Fig. 6). Accordingly, the first and second
wires
378a, b extend from the distal end of the closure device 200 through the
tethers 364a-
c to the biasing member 342 In this manner, movement of the biasing member 342
in
the proximal direction can cause movement of the wires 378a, b also in the
proximal
direction.
Figs. 7C-7D are cross-sectional views illustrating the delivery assembly 360
in
association with the actuating assembly 320. However, for simplicity, Fig. 7C
does
not include the biasing member 342, release wires 378a, b and associated
release knob
346, while Fig. 7D illustrates details about the interaction between the
delivery
assembly 360 and the actuating assembly 320 without illustrating the first
member
322 and details about the handle body 302 and the second member 324. In the
illustrated embodiment, the proximal end of the catheter 362 is coupled to the
distal
end of the second member 324. In this manner, movement of the second member
324
can cause a corresponding movement in the catheter 362. For example, as the
second
member 324 moves proximally with respect to the handle body 302, so also does
the
catheter 362 move proximally with respect to the handle body 302.
According to one embodiment of the invention, the tethers 364 can extend
from the delivery portion 366, through the catheter 362 and the second member
324
and are coupled to the handle body 302. In at least one example, the tethers
364 are
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
28
coupled to the handle body 302 while the first and second members 322, 324 may
be
coupled to the catheter 362 such that movement of the first and second members
322,
324 causes relative movement between the catheter 362 and handle body 302,
which
results in movement between the catheter 362 and the tethers 364a-c. The
tethers
364a-c are secured to the closure device 200, such that movement of the
tethers 364a-
lo c results in deployment of the closure device. As a result, movement of the
first and
second members 322, 324 deploys the closure device 200, as will now be
discussed in
more detail.
The tethers 364 can be secured to the handle body 302 by, for example, an
intermediate member 376. The tethers 364 can be covered with a first and
second
housing 370, 372 to provide a degree of rigidity to the portions of the
tethers 364
located inside of the handle body 302 and the second member 324. For example,
in
one embodiment, the first housing 370 comprises a rigid, hollow, metal rod
configured to house the three tethers 364a-c therein. The first housing 370
can extend
from the intermediate member 376, which facilitates securement of the tethers
364 to
the handle body 302, and terminate at some point beyond the handle body 302.
In the illustrated embodiment, the second housing 372 can extend from the
distal end of the first housing 370 and extend into the catheter 362. The
second
housing 372 can comprise a resilient material configured to resist axial
stretching
while allowing a degree of bending. In one embodiment, the second housing 372
comprises a coil of metal, such as stainless steel, configured to resist axial
stretching,
yet allow a degree of bending. The second housing 372 can allow a practitioner
to
bend a portion of the catheter 362, if needed, in order to manipulate delivery
device
300 for placement of the closure device 200. A seal 374 can be provided
between the
first housing 372 and the second member 324 in order to reduce or
substantially
prevent bodily fluid, which may have entered the catheter 362, from entering
the
handle body 302 or otherwise inappropriately being expelled from the delivery
device
300.
In the illustrated embodiment, the second member 324 can comprise an
elongate shaft defining an axial lumen 348 and a lumen 350 in fluid
communication
therewith. Lumen 350 can be configured to couple to a medical device for
removal of
fluid from the delivery device 300. The axial lumen 348 can be sized to
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
29
accommodate and allow movement of the tethers 362, the first housing 370 and
the
second housing 372 therein. The second member 324 can include a guide 326. The
guide 326 can be configured to cooperate with a first pin 352 and a second pin
354 to
influence movement of the second member 324 with respect to the handle body
302,
as will be discussed more fully herein below.
In the illustrated embodiment, the first member 322 comprises a hollow
elongate tube sized and configured to enable the second member 324 to be
received
into and moveable within the first member 322. The first member 322 can be
operatively associated with the handle body 302 and the second member 324 to
facilitate deployment of the closure device 200. For example, the first member
322 is
linked to the handle body 302 by a third pin 356. The third pin 356 is
received in a
guide 358 of the first member 322. The guide 358 is configured to interact
with the
third pin 356 in order to influence the movement of the first member 322 with
respect
to the handle body 302.
The first pin 352 can link the first member 322 to the second member 324.
When the first pin 352 links the first member 322 to the second member 324,
the
second pin 354 links the handle body 302 to the second member 324, and the
third pin
356 links the handle body 302 to the second member 322, movement of the first
member 322 can selectively deploy the closure device 200 from the delivery
portion
366.
Fig. 8 is an exploded view of the actuating assembly 320 and the release
assembly 340. The second member 324 is received into the first member 322, and
the
first member 322 is received into the knob 338 and the handle body 302. The
second
member 324 can include a guide 326 having a first portion 326a and a second
portion
326b, which guide 326 can be defined by a slot formed on the outer surface of
the
second member 324. In the illustrated embodiment, the first portion 326a is
straight
and extends along at least a portion of the length of the first member 324 and
joins
with the second portion 326b of the guide 326. The second portion 326b can
include
a helical groove or slot that begins with and is contiguous with the first
portion 326a
and extends distally therefrom.
The guide 326 of the second member 324 is configured to interact with the
handle body 302 and the first member 322 to selectively retract the catheter
362 in
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 order to deploy the closure device 200. For example, the first portion 326a
of the
guide 326 is configured to interact with the second pin 354, which is secured
into the
handle body 302 by means of threads and extend into the first portion 326a of
the
guide 326. In this manner, the second member 324 can move laterally with
respect to
the handle body 302. Thus, rotation of the handle body 302 can translate to
rotation
10 of the second member 324, and thus, the catheter 362 and the delivery
portion 366.
The second portion 326b of the guide 326 is configured to interact with the
first pin 352, which is secured to the first member 322 by means of threads
and
extends into the second portion 326b of the guide 326. In this manner, as the
first
member 322 is rotated, the first pin 352 will interact with the second portion
326b to
15 move the second member 324 in the proximal direction. As the second member
324
is moved in the proximal direction with respect to the handle body 302, the
catheter
362 moves proximally with respect to the handle body 302 thereby exposing or
deploying the closure device 200 from the delivery portion 366.
In the illustrated embodiment, the first member 322 can include a guide 358
20 defined by a slot or groove formed in the outer surface of the first member
322. In the
illustrated embodiment, the guide 358 can include a first portion 358a
connected to a
second portion 358b. The first portion 358a of guide 358 can be straight and
extend
along at least a portion of the length of the first member 322, and then join
and be
contiguous with the second portion 358b. The second portion 358b of the guide
358
25 can be a helical groove that wraps around at least a portion of the outer
surface of the
first member 322 and extends along at least a portion of the length of the
first member
322.
As described previously, the third pin 356, which is secured to the handle
body 302 by means of threads, can extend into the guide 358 in order to
influence
30 movement of the first member 322 with respect to the handle body 302. For
example,
as the third pin 356 is positioned in the most proximal portion of the first
portion
358a, the closure device 200 is completely received into and enclosed by the
delivery
portion 366. As the first member 322 is moved in the proximal direction as
illustrated
by the arrow in Fig. 4, the third pin 356 moves in the first portion 358a of
the guide
358 to deploy the first anchor 204 of the closure device 200 from the delivery
portion
366.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
31
The length of the first portion 358a can correspond with the distance that the
first member 322, and thus the catheter 362, must move in order to deploy the
first
anchor 204 of the closure device 200 from the delivery portion 366. For
example, a
practitioner can move the knob 338, which is coupled to the first member 322,
in the
proximal direction. Movement of the knob 338 in the proximal direction can
cause
the third pin 356 to move linearly in the first portion 358a of the guide 358.
In this
manner, the second member 324 can move correspondingly with the first member
322
because of the first pin 352, which links the first member 322 to the second
member
324. As the third pin 356 is positioned in the location of the guide 358 where
the first
portion 358a meets with the second portion 358b, the first member 322 can be
rotated
in order to selectively deploy the remaining portions of the closure device
200 from
the delivery portion 366 of the delivery device 300.
As the first member 322 is rotated, the third pin 356 is positioned in the
second portion 358b to influence movement of the first member 322 with respect
to
the handle body 302, and the first pin 352, which is coupled to the first
member 322,
interacts with the second portion 326b of the guide 326 to move the second
member
324 in the proximal direction with respect to the handle body 302. Movement of
the
second member 324 in the proximal direction in this manner can cause further
deployment of the closure device 200 from the delivery portion 366. As will be
appreciated, the knob 338 can be coupled to the first member 322 to facilitate
and
enable movement of the first member 322 with respect to the handle body 302.
The dual movement required to deploy the closure device 200 can provide
some efficiency and safety advantages. For example, a practitioner can move
the
knob 338 in a first direction (i.e., proximally in a linear fashion) to deploy
the first
anchor 204 from the delivery portion 366. Thereafter, the practitioner can
move the
handle body 302 to position the first anchor 204 against the wall tissue of an
internal
tissue opening, such as against the left atrial wall of a heart, for example.
Once the
first anchor 204 is positioned against the wall, the practitioner can move the
knob 338
in a second direction (i.e., rotate the knob) to further deploy the closure
device 200
from the delivery portion 366. The dual movement enables a user to predict the
deployment of the closure device 200 to reduce the risk of premature
deployment of
the closure device.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
32
It will be understood by one of ordinary skill in the art in view of the
disclosure provided herein that other means of controlling movement of one
member
with respect to the other, such as the first member with respect to the second
member,
can be utilized without departing from the scope and spirit of the invention.
For
example, a structure configured to substantially restrict or control movement
of the
1 o first element with respect to the second element and/or handle body can be
utilized.
In one embodiment, the structure can include a cam and a follower. In an
alternative
embodiment, the structure can include a slider.
The release assembly 340 can be configured to be received in the proximal
end of the handle body 302. The release assembly 340 can be configured to
provide
additional safety features for the practitioner and patient by reducing the
risk of
premature detachment of the closure device 200 before it is positioned
appropriately
in an internal tissue opening. For example, a practitioner using the medical
system
100 of the present invention can manipulate the actuating assembly 320 to
deploy the
closure device 200 for positioning in an internal tissue opening. In order to
deploy a
first portion of the closure device 200, a user can move the knob 338, and
thus the
first member 322, in the proximal direction with a first movement, which is a
linear
movement, and then deploy the remaining portions of the closure device 200 by
a
rotational movement. Once the closure device 200 is deployed, the practitioner
can
be required to move their hands in order to utilize the release assembly 340
to release
the closure device 200 from the delivery device 300.
In the illustrated embodiment the release assembly 340 can include a release
knob 346 coupled to a biasing member 342, which is received into the proximal
end
of the handle body 302. The biasing member 342 can be configured to include a
plurality of slots 318 configured and arranged to act similar to a spring. The
slots 318
can be configured and arranged in the biasing member 342 to enable at least a
portion
of the biasing member 342 to be compressed. Compression of the biasing member
342 can cause the release pin 344 to move toward the distal end of the biasing
member 342.
The biasing member 342 can be configured such that when biasing member
342 is positioned in the handle body 302, the biasing member 342 naturally
tends to
maintain its position with the release pin 344 in the release pin groove 310
as
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
33
illustrated in Fig. 8. As force is applied to the release knob 346 in the
distal direction
(i.e., compress the biasing member 342), the release pin 344 can be moved out
of a
terminating portion of the release pin groove 310 and rotated and moved into a
proximal terminating portion of the release pin groove 310 to release the
closure
device 200 from the delivery device 300.
The closure device 200 is released from the delivery device 300 by moving a
plurality of wires 378 which are housed by a tether 364 and coupled to the
biasing
member 342. Illustrated in Figure 7 is a cross-sectional view of attachment
member
240 of the closure device 200 received into a tether 364 and coupled by first
and
second wires 378a, 378b. In the illustrated embodiment, a second wire 378b can
extend through and out of the tether 364 and form a loop. The loop can extend
through an aperture 242 of the attachment member 240 of the closure device
200.
With the loop of second wire 378b positioned through the aperture 242 of the
attachment member 240, a first wire 378a, which extends through and out of the
tether
364, can extend through the loop of the second wire 378b to form a locking
feature.
When the first wire 378a extends sufficiently through the loop of the second
wire
378b, the closure device 200 can remain coupled to the delivery device 300
until the
first wire 378a is pulled through the loop of the second wire 378b, and the
second
wire 378b is pulled out of the aperture 242 of the attachment member 240.
The first wire 378a and the second wire 378b can be attached at their proximal
ends to the biasing member 342. In this manner, movement of the biasing member
342 in the proximal direction can cause movement of the wires 378 also in the
proximal direction. In one embodiment, the wires 378 can be coupled to the
biasing
member 342 such that movement of the biasing member 342 will cause the first
wire
378a to move a distance sufficient to be removed from the loop of second wire
378b
before the second wire 378b is moved by the biasing member 342. The wire 378
can
comprise a metallic wire such as NiTiNol wire. The wire 378 can also include a
stainless steel wire or some other type of metal or stiff polymer. The wires
378 can be
made from a material having a sufficient tensile strength to secure the
closure device
200 to the tethers 364 without causing the wires 378 to fail or substantially
deform.
In one embodiment of the invention, the wire 378B can include a stainless
steal wire
and wire 378A can include a NiTiNol wire.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
34
Other types and configurations of biasing members can be utilized without
departing from the scope and spirit of the invention. For example, in one
embodiment, the release assembly can include a rotating member coupled to the
securing elements. In this embodiment, rotation of the rotating member can
cause the
securing elements to wind around the rotating member thereby causing the
distal ends
of the securing elements to move proximally with respect to the handle body.
The
closure device 200 is released from the delivery device 300 by moving a
plurality of
wires 378 which are housed by a tether 364 and coupled to the biasing member
342.
The method of use of the medical system 100 will now be described with
reference to a particular internal tissue opening, namely a PFO. Fig. 9A
illustrates the
positioning of the catheter 362 through the tunnel 58 of a PFO with the first
anchor
204 of the closure device 200 deployed. The medical system 100 is utilized to
close
an internal tissue opening by positioning the catheter 362 through an internal
tissue
opening and moving the first member 322 by a first movement (i.e., linearly)
in the
proximal direction to deploy the first anchor 204 of the closure device 200.
After the
first anchor 204 of the closure device 200 is deployed, the delivery device
300 can be
moved in the proximal direction in order to seat the first anchor 204 against
the wall
of the tissue opening or otherwise engage the wall of the internal tissue
opening, as
illustrated in Fig. 9A. This can be done by moving the handle body 302 in the
proximal direction.
After the first anchor 204 has been positioned against the wall of the
internal
tissue opening, the knob 338, and thus the first member 322, can be moved by a
second movement, or in other words, rotated to deploy additional portions of
the
closure device 200 as illustrated in Fig. 9B. After the closure device 200 has
been
fully deployed and conforms to the anatomy of the internal tissue opening, the
release
assembly 340 can be actuated to selectively detach the delivery device 300
from the
closure device 200 as illustrated in Figs. 10 and 11.
The release assembly 340 can be actuated by moving the biasing member 342
distally with respect to the handle body 302, then rotating the biasing member
with
respect to the handle body 302, and then moved proximally with respect to the
handle
body 302. In this manner, closure device 200 substantially conforms to the
anatomy
of the internal tissue opening. As noted previously, the configuration of the
closure
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 device 200 is such that when positioned in the internal tissue opening as
illustrated,
the members of the closure device 200 apply lateral force to the tissue of the
internal
tissue opening, such as the tunnel 58 of the PFO, to approximate tissue of the
PFO for
closure.
The delivery device 300 may be configured to deliver closure devices with
10 additional configurations. In particular, Figs 12A-12N illustrate
additional
configurations for closure devices 200a-n utilizing various patterns and sizes
of cells,
the patterns being selected to conform with typical PFO anatomy. Potential
regularly-
sized cell structures are shown in Fig. 12A-12N. The use of a multiple
cellular
structure may allow the closure elements 12A-12N to exhibit features that will
15 increase the ability of the closure devices 200a-n to conform to typical
PFO anatomy
while being collapsible within a catheter-based delivery system.
Fig. 12A depicts a closure element 200a having a five-cell design which is
narrower in a middle portion 1205 than at the distal and proximal ends 1210a,
1210b
respectively. This basic design exhibits the ability to extend the cells on
the distal end
20 1210a and cells on the proximal end 1210b outside a PFO tunnel having a
relative
narrow waist.
Fig. 12B illustrates a closure device 200b having a five-cell design which
constrains the degree to which the device would extend into the atria of the
heart
while still providing wider anchoring points at the top and bottom-most cells.
Figs
25 12C-E show additional closure devices 200c-e having varied cell
configurations
which include cells of different sizes. The different size cells may provide
stiffness to
selected areas of the structure which in turn may result in the application of
varying
amounts of force to an internal tissue opening. For example, closure device
200c
illustrated in Fig. 12C may provide additional stiffness at the anchoring
points by
30 providing relatively smaller cells located on or near the distal and
proximal ends
1210a, b. The closure device 200d illustrated in Fig. 12D may provide
additional
stiffness at the portion of the closure device 200d configured to be located
in the
tunnel, such as the middle portion 1205; therefore, smaller cells may be
utilized in the
middle portion 1205 as illustrated in Fig. 12D.
35 Fig. 12E illustrates a closure device 200e that includes a combination of
the
structures depicted in Fig. 12B and Fig. 12D. Other combinations of cell-size
and
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
36
placement may be envisioned. Each of the combinations may be tailored to
specific
desired properties of the device. For example, Figs 12F-N illustrate closure
devices
200f-n having additional cell structures that may provide adaptability to the
varying
needs of different PFOs of varying widths and lengths while still providing
for their
typical shape.
Figs. 120-T illustrate closure devices 200o-t having cell structures that have
been adapted to further allow for proximal and distal anchoring and may also
be
adapted for varying-length PFO tunnels. Fig. 120 shows a closure device 200o
having
a single central cell structure with elongated arms 1220a-d at each corner
designed to
proximally and distally anchor the structure within an internal tissue
opening, such as
a PFO tunnel. The elongated arms 1220a-d may be considered as additional cells
that
are collapsible as the closure device 200o is collapsed within a delivery
device.
In cases where the length of the single, central cell of the structure is not
sufficient to completely span the length of the tunnel, the additional length
of the arms
may allow for the structure to be anchored with the central cell being
substantially
completely internal to the tunnel. Figs. 12P and 12Q illustrate closure
devices 200p
and 200q in which additional central cells are added to provide additional
width and
length to the structure respectively. In Figs. 120-Q, the elongate arms 1220a-
d of the
closure devices 200o-q may include serrated edges. When the closure devices
200p, q
are deployed, the serrated edges on the elongate arms 1220a-d may provide
anchoring
to the tissue.
Figs. 12R-T depict single-cell designs with smoother arms. These designs may
provide a less aggressive anchoring to the internal tissue opening and
surrounding
tissues while still utilizing elongate arms 1220a-d, which may provide
adaptability to
accommodate length variations in the internal tissue openings.
Figs. 12U-X illustrates closure devices 200u-x having cell structures that are
similar to the cellular structures illustrated in Figs. 12U-X. These are
examples of
designs that will have adaptability to specific PFO anatomies.
The creation of cell structures that lend themselves to accommodating PFO's
of varying lengths while maintaining anchoring features as well as the desired
lateral
force is possible and shown in Figs. 12Y-Z. A closure device 1200y that makes
use of
the addition of cells with a wider aspect than taller aspect as shown in Fig.
12y. Such
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
37
a configuration may create a structure that exhibits the ability to
foreshorten while
maintaining approximately the same width. Fig. 12Z depicts a cell structure
that also
has the ability to foreshorten while coupling the forces from the top and
bottom of the
structure by a larger amount by moving the attachment points from the middle
of the
structure to the edges of the waist. This provides for varying tunnel lengths
while
limiting the forces applied within the internal tissue opening as well as
allowing the
right and left anchoring features to operate more independently on one side
without
interference from the other side.
The structure of internal tissue openings, such as PFO anatomy, may also
dictate that the right and left sides of a closure device structure have
varying sizes as
shown in Figs. 13A-C. In particular, Fig. 13A shows a closure device 200a'
that
includes a cell structure in which an anchor 1300a on the right (bottom) side
is
relatively smaller than an anchor 1300b on the left side. Figs. 13B-C
illustrate closure
devices 200b', 200c' respectively that include anchors 1300 only on the left
side or
right side respectively. Including anchors on only one side of the internal
tissue
opening will adapt to internal tissue opening with tapered tunnels on which it
may be
desirable to close only the wider side of the tunnel.
Anchoring of the PFO closure device structure within the PFO may also be
done within the PFO tunnel as shown in the Fig 14A-14B. Fig. 14A illustrates a
closure device 200d' that includes small barbs 1400 that are inclusive to the
cell
structure along the sides of the closure device 200d'. When deployed, the
small barbs
1400 may aggressively engage the tissue and prevent migration of the closure
device
200d' through the internal tissue opening. Fig. 14B illustrates separate,
deployable
structures 1410 which may be added the structure of a closure device 200e' and
expand into the width of the internal tissue opening when the device is
deployed. In
3o Figs. 14A-14B it is to be understood that the anchors may be simple wire
ends as
shown or more aggressive, pointed or barbed features to engage the tissues
associated
with a tunnel of an internal tissue opening.
Various methods of construction of the closure device can be used, and as
such, various materials can be used. In one configuration, a closure device
may be
constructed of rectangular cross-section wire that is bent to shape and join
as shown in
Figs 15A-15B. One particular aspect ratio and bending of a single closure
device
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
38
element 1500 is shown Fig. 15A, and two of these components are shown
connected
in Fig. 15B where a row of cells 1510 is shown. Additions to this starting
structure
can be added to provide the desired, complete cellular structure. Starting
wire may be
made from metals, such as stainless steel (SS) and stainless steel alloys,
tantalum, bio-
compatible metals, nickel titanium (NiTi), or polymer extrusions of various
polymers
or bioresorbable polymers. Shape memory materials such as NiTi, and shape-
memory
polymers could be heat-set into the bent configurations as shown in Fig. 15A.
In many cellular structures it may be desirable to keep bending angle within
the elastic limit of the starting material, to allow the structure to be
collapsed within a
catheter or other constraining member and to be deployed to the desire shape
within
the internal tissue opening. Connection of the components, shown in Fig. 15B,
may be
accomplished by small coils of wire held captive at the joint due to the
bending of the
components or with biocompatible solders or adhesives, resistance or laser
welding in
the case of metallic components or simply melting of polymer materials at the
required junctions by application of heat directly or by ultrasonic heating.
Combinations of these connections methods may be used to provide additional
joint
support or properties. For example, coils may be made of radiopaque material
such as
platinum or platinum alloys, which in addition to providing fixation of the
components will provide for visualization by fluoroscope and X-ray.
Closure devices may also be cut from flat sheets of starting materials. By way
of illustration only, cutting of the structure may be accomplished
mechanically for
larger cells and structures and/or by laser or photo-lithography for smaller
structures.
Cutting of these materials may be done with the device in the deployed,
expanded
form for materials without shape-memory. For shape-memory metals, such as
NiTi,
the constrained shape may be cut followed by heat-setting the device to the
expanded
shape, which will provide for more efficient packing of the component when
constrained for delivery.
Another method of creating the desired structure for the closure device is the
utilization of a woven mesh of wires or polymer filaments which create a flat
sheet.
The cellular structure would exist as the voids between the woven wires and
the
structure would be collapsible for delivery through a catheter. Wire ends
which
extend from this construction method may be managed by tucking them back into
the
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
39
woven mesh, or by leaving them exposed and allowing them, when deployed in the
internal tissue opening, to engage the surrounding tissues for fixation. They
may also
be terminated by creating interlocking loops at the edge intersections.
Figs 16A-16C illustrate a closure device that is configured to self-adjust
according to a tunnel length of an internal tissue opening. In particular,
Fig. 16A
illustrates a closure device 200f that includes anchoring features 1600
located on the
distal end 1210a. The distal end 1210a and the proximal end 1210b are both
illustrated as being wider than the central portion 1205, which may be a
relative
narrow waist portion. As previously discussed, as the closure device 200f is
deployed, the closure device 200f expands from a compressed state within the
delivery device to an expanded state in which the closure device 200f becomes
wider
as it is deployed. In the present example, in addition to expanding from the
compressed state, the length of the closure device 200f is reduced to more
closely
approximate the length of the tunnel length of the internal tissue opening. In
Fig.
16A, the width of the closure device 200f is in an expanded state while the
overall
length of the closure device 200fl is in an expanded state. The proximal
portion
1210b may be configured to be reduced in length, such as through rolling.
Fig. 16A illustrates the closure device 200f in a default, unconstrained
state.
In the unconstrained state, the proximal portion 1210b rolls onto itself
toward the
central portion 1205. As a result, the proximal portion 1210b shown in Fig.
16B can
roll up to a PFO tunnel entry point in the right atrium and then provide an
anchor
which may reduce the possibility of migration of the closure device 200f
through the
tunnel. Fig. 16C illustrates a closure device 200fl in which the proximal end
1210b of
the structure comprises a coiled wire rather than coiling the entire
structure. The
closure devices 200f, g' may be formed of metallic materials, including NiTi
with its
shape memory set to the configurations of Fig. 16B and/or Fig. 16C.
Yet another closure device 200h' is shown in Fig 17A. A central portion 1205
of the closure device 200h' includes a mechanical relief point 1700 that may
provide
the distal and proximal ends 1210a, 1210b additional flexibility to adapt to
variable
anatomy of internal tissue openings. This flexibility may allow the distal end
1210a
to rotate with respect to the proximal end 1210b as shown in Fig 17B as well
as
allowing out-of-plane rotation.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 Figs. 18A-18B illustrates closure devices 200i' in which a portion of the
device intended to be deployed in the right atrium of the heart employs a
proximal
anchor 1800 that may be a rigid structural member. The proximal anchor 1800
may be
attached to the rest of the closure device 200i' by a spring member 1810 which
may
allow the deployed position of the proximal anchor 1800 to vary according to
the
1o length of the PFO tunnel. Though the proximal anchor 1800 illustrated may
be solid
in this embodiment, it may be folded length-wise for delivery, as shown in
Fig. 18B.
The solid proximal anchor 1800 may further have configurations not shown which
are
specifically designed to have conformance to the anatomy found on the right
side of
the atrial septum, including the ability to locate itself tucked under the
typical arch of
15 the septum secundum.
To this point, several examples of closure devices have been discussed. The
closure devices have been discussed in the context of closure devices that
expand
from a compressed state to a default or decompressed state in which the
expansion
occurs due to the resiliency of the material used to form the closure device
and/or to
20 the shape of the closure device. Other configurations may be utilized in
which the
closure device is mechanically driven from the compressed to the decompressed
state
by forces apart from and/or in addition to spring forces associated with
compression
of the closure device.
Figs. 19A and 19B are schematic diagrams of a closure element 200j'. The
25 closure element 200j' includes expansion members 1920 with outwardly biased
ends
1921, connecting members 1922, and pinned joints 1923. The connecting members
1922 are coupled the opposing expansion members 1920 by way of the pinned
points
1923. Such a configuration allows the closure element 200j' to expand and
collapse
from a collapsed state as illustrated in Fig. 19A to an expanded state as
illustrated in
30 Fig. 19B. Further, the closure element 200j' may be further collapsed to
fit within a
delivery device.
As the closure element 200j' expands, the expansion members 1920 may exert
a lateral force on the walls of the tunnel of an internal tissue opening in a
similar
manner as described above. As introduced, the expansion members 1920 include
35 outwardly biased ends 1921. The outwardly biased ends 1921 may promote
fixation
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
41
of the expansion members 1920 to the internal tissue opening and/or fit the
desired
anatomy.
The closure element 200j' may be expanded by moving the central portions of
the connecting members 1922 in opposing directions designated by arrows 1924.
The
connecting members 1922 move in opposing directions from the expansion members
1920, which are driving in an outward direction, 1925. The connecting members
1922 may be locked into place by an over-center latching of the connecting
members
1922 when the connecting members 1922 are forced apart from each other in
directions 1924.
Fig. 19C illustrates a partial view of a connecting member 1922 in isolation.
The connecting member 1922 may have engagement features 1927 at the interface
of
the pinned joints 1923 which will interlock at various angles allowing the
separation
between the expansion members 1920 to be varied. In particular, the features
1927
may include ramped tabs. Fig. 19D illustrates the interaction between
connecting
members 1922. The features 1927 may allow adjacent connecting members to be
rotated relative to one another in one direction while prevent rotation in the
opposite
direction. In one example, the features 1927 may interact in a ratcheting
manner.
Fig. 20 illustrates a closure device 200k'. The closure device 200k' includes
expansion members 1920 that are coupled together with struts 2000 positioned
at least
partially within tubes 2010. At the end of the struts 2000, outwardly biased
biasing
elements 2020 are constrained by the tubes 2010. Upon deployment of the
closure
device 200k', such as when the closure device 200k' is freed from a delivery
device,
the biasing elements 2020 allow a sliding fit, but resist compression due to
their
outward bias. The outward bias of the biasing elements 2020 secure the closure
device 200k' in the expanded state to secure the closure device 200k' to the
walls of
3o an internal tissue opening.
Figs. 21.A and 21B illustrate a closure device 2001' that may be mechanically
deployed. The closure device 2001' with pinned joints 1923 and rigid
connecting
members 1923 are initially expanded along the axial direction 2198 of the
structure.
The expansion members 1920 are forced outward by the operation of a tether
2196
that is rigidly connected to a distal connecting member 1922a at point 2191
and
extends through an opening 2192 in a proximal connecting member 1922b. Tension
is
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
42
maintained in the tether 2196 and a clasp 2193 is slid distally along the
tether 2196
until connecting with the proximal connecting member 1922b to force the
proximal
connecting member 1922b proximally. As the proximal connecting member 1922b
moves proximally, the proximal connecting member 1922b moves the expansion
members 1920 outwardly.
Fig. 21B shows a cross section of the clasp 2193 in relation to the opening
2192 of a proximal connecting member 1922b in which a barbed surface 2195 is
configured to secure the clasp 2193 from proximal movement in relation to the
tether,
2196. After the clasp 2193 is cinched against the proximal connecting member
1922b,
the tether 2196 is cut proximally relative to the clasp 2193 thereby releasing
the
closure device 2001' in its expanded position.
Figs. 22A and 22B illustrate a closure device 200m' that can be deformed
from an inline shape (Fig. 22A) to a "T" shape (Fig. 22b). The closure device
200m'
includes anchoring arms 2200 that may be expanded to close the internal issue
opening as described above and/or to locate the system relative to the
internal tissue
opening. The closure device 200m' includes alternating thick portions 2204 and
thin
portions 2208 to form flexural hinges. The closure device 200m' further
includes an
actuation member 2210 that is secured to a distal end 1210a and extends from
the
distal end 1210a through a hole 2220 defined in the proximal end 1210b.
In order to move the closure device 200m' from the in-line position
illustrated
in Fig. 22A to the expanded position illustrated in Fig. 22B, the distal end
1210a is
drawn toward the proximal end 1210b by drawing the actuation member 2210
toward
the proximal end 1210b. As the actuation member 2210 is drawn toward the
proximal
end 1210b, the configuration of the closure device 200m' and of the anchoring
arms
200m' in particular causes the anchoring arms 200m' to expand as illustrated
in Fig.
22B.
The closure device 200m' may be made of NiTiNol, stainless steel or other
material that is capable of elastic recovery from large deformations. The
actuation
member may be made of metal or polymeric materials with one or more strands.
The
flexural structure may be fabricated from tubing or from flat sheet.
Figs. 23A-23D illustrate a closure device 200n' that make use of a
combination of flexural hinges and pivot points. The closure device 200n'
generally
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
43
includes anchoring arms 2200, an actuation member 2210, and a body 2300. The
anchoring arms 2200 are coupled to the actuation member 2210 by pivots 2310.
Such
a configuration may allow the actuation member 2210 to either pull the
anchoring
arms 2200 toward the proximal end 1210b to deploy the anchoring arms 2200, as
illustrated in Fig. 23A, or push the anchoring arms 2200 away from the
proximal end
1210b to deploy the anchoring arms 2200 as illustrated in Fig. 23B.
Accordingly, the
anchoring arms 2200 may be deployed by either pushing or pulling the actuation
member 2210.
The body 2300 includes flexing sections 2320 by which the pivots 2310
couple the anchoring arms 2200 to the body 2300. As illustrated in Fig. 23C,
as the
anchoring arms 2200 are deployed, the flexing sections 2320 flex outwardly to
accommodate the expansion of the anchoring arms 2200 relative to the body
2300.
The body 2300 according to one example may be tubular and may be made of
NiTiNol or stainless steel or other material that is capable of recovering
elastically
from large strains. The anchoring arms 2200 and pivots 2310 may be made from
stainless steel or any other material with sufficient strength and rigidity to
resist the
delivery and stent opening forces. The actuation member 2210 may be made of
metal
or polymeric materials with one or more strands. The flexural structure may be
fabricated from tubing or from flat sheet. Fig. 23C illustrates a closure
device 200o'
which includes all pivots 2310 rather than a combination of pivots and flexing
sections 2320.
Accordingly, closure devices 200 may be opened mechanically using a
combination of actuation members, pivots, and/or flexing sections. Closure
devices
may also be used as part of a system with other closure devices in which one
or more
of the closure devices are deployed as a locator device to locate the system
relative to
an internal tissue opening and while additional closure devices are used to
close the
internal tissue opening. One such configuration will now be discussed in more
detail.
Figs. 24A and 24B illustrate a medical system 100' configured to locate one or
more closure devices relative to an internal tissue opening and to close the
internal
tissue opening. In the illustrated example, the most distal end 1210a of the
system
100' includes a distal locator device 2400a, which may be similar in operation
to
closure device 200m' illustrated in Figs. 22A-22B. The system 100' further
includes
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
44
a closure device 200 as well as a proximal locator device 2400b. The closure
device
200 may be similar to the closure device illustrated in Fig. 5A while the
proximal
locator device 2400b may function in an analogous manner as the distal locator
device
2400b. The distal locator device 2400a includes anchor arms 2200a. The anchor
arms 2200a may be coupled to a first anchor 204. In one example, the anchor
arms
2200a are coupled to the first anchor 204 by way of a distal stent compression
tab
2410a. The closure device 200 is further coupled to the proximal locator
device
2400b. In one example, anchor arms 2200b on the proximal locator device 2400b
are
coupled to a second anchor 206, such as by way of a proximal stent compression
tab
2410b.
In order to deploy the distal locator device 2400a, the distal locator device
2400a may first be located beyond the distal surface of an internal tissue
opening.
The distal locator device 2400a may then be deployed to expand the anchor arms
2200a as well as the first anchor 204. Once the distal locator device 2400a is
deployed, the system 100 may be drawn proximally to bring the closure device
200,
and the first anchor 204 in particular, into contact with the tissue on the
distal side of
the internal tissue opening. In the case of a PFO, the first anchor 204 can be
drawn
into contact with the septum primum.
In one example the proximal locator device 2400b and the second anchor 206
of the closure device 200 are then unsheathed from the delivery catheter 2410
while
maintaining tension on the distal locator device 2400a. In the illustrated
example, as
the second anchor 206 is unsheathed the second anchor 206 expands. While
continuing to maintain tension on distal locator device 2400a, the proximal
locator
device 2400b is pushed toward the distal end 1210a. After the closure device
200 is
deployed, the proximal locator device 2400b may be deployed to expand the
anchoring arms 2200b into position against tissue on the proximal side of the
internal
tissue opening, such as the septum secundum in the left atrium.
The proximal locator device 2400b can then be pulled proximally to release
the second anchor 206 while the proximal locator device 2400b can then be
collapsed
into its linear configuration. The distal locator device 2400a can then be
pushed
distally to release the first anchor 204. The distal locator device 2400a may
then be
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 collapsed and the entire system, less the deployed closure device 200, may
be
withdrawn.
Figs. 25A and 25B illustrate a partial view of a medical system 100" for
delivering a closure device 200p' in which the closure device 200p' itself
does not
have proximal or distal anchoring arms. The closure device 200p' is retained
by
io features 2500, such as lateral extending barbs that embed into the tissue
of the PFO
tunnel due to the lateral force that is applied by the closure device 200p'.
In this case,
the proximal anchor-expander has been omitted and replaced by a tab 2502 that
is
used to compress the closure device 200p' to deploy the closure device 200p'
in the
tunnel of an internal tissue opening, such as in the tunnel of a PFO. In the
case of a
15 PFO, the system 100" can be located against the septum primum at the
entrance to
the PFO tunnel by the distal locator device 2400a. This system 100" may be
configured to reduce or eliminate the presence of anchoring arms in the atria
of the
heart after the closure device 200o' and the rest of the delivery system 100"
has been
removed.
20 Figs. 25C-25F illustrate balloon-type closure devices for closing internal
tissue
openings. In particular Fig. 25C illustrates a balloon-type closure device
200q' that
includes a plurality of interconnected chambers 2505. Fig. 25D illustrates a
view of
the balloon-type closure device 200q' taken along section A-A. As illustrated
in Figs.
25C and 25D, several of the interconnected chambers 2505 can be relatively
long,
25 thin chambers that are arranged in a side-by-side configuration. The
interconnected
chambers 2505 are in fluid communication with a manifold portion 2510. The
manifold portion 2510 receives fluid from a fill port 2515. Fluid entering the
manifold portion 2510 from the fill port is then distributed to the
interconnected
chambers 2505 to fill the interconnected chambers 2505 with fluid.
30 The interconnected chambers 2505 are configured to expand as they are
filled
with fluid in order to close an internal tissue opening. In particular, the
configuration
of the interconnected chambers 2505 allows the balloon-type closure device
200q' to
have a relatively large lateral expansion relative to a thickness expansion as
the
interconnected chambers 2505 are inflated. The relatively large lateral
expansion of
35 the balloon-type closure device 200q' may exert a lateral force on the
tunnel of an
internal tissue opening to close the internal tissue opening as described
above.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
46
Accordingly, the configuration of the interconnected chambers 2505 allows the
closure device 200q' to close an internal tissue opening such as a PFO.
Fig. 25E illustrates a balloon-type closure device 200r' that includes flared
interconnected chambers 2520. At least one of the flared interconnected
chambers
2520 includes a tunnel section 2525 and a flared distal portion 2530. The
flared distal
lo portion 2530 may be configured to be expanded distally of an internal
tissue opening
to thereby provide a distal anchor for the closure device 200r'. Portions
similar to the
flared distal portion 2530 may also be provided on the proximal ends of the
interconnected chambers 2520 to thereby provide proximal anchors for the
closure
device 200r'.
Figs. 25F illustrates a closure device 200s' that includes isolated chambers
2535. The isolated chambers 2535 form a first anchor 2540 and a second anchor
2545. The isolated chambers 2535 may be inflated in a specific sequence in
order to
locate and deploy the closure device 200s' in a desired manner. For example,
an
isolate chamber or chambers corresponding with the first anchor 2540 may be
inflated
first to locate the closure device 200s' in the entrance to the internal
tissue opening.
In the case of a PFO, the first anchor 2540 may be inflated in the left
atrium. A
central chamber may be inflated next to expand the central portion 2550 of the
closure
device 200s' to close the tunnel of the internal tissue opening. A proximal
chamber
may then be inflated to expand the second anchor 2545. Any of the balloon-type
closure device configurations described above and otherwise may be formed of a
resorbable or non-resorbable biocompatible material. Such a configuration may
allow
a practitioner to leave the inflated balloon in the PFO as an occlusive
implant. While
specific configurations are illustrated, balloon-type closure devices may be
utilized
that include any number of chambers that may include any combination of
isolated
and interconnected chambers that may be inflated in any number of stages.
In several examples, after the closure device has been deployed, the closure
device is released from the rest of the system by a release mechanism.
Accordingly,
several release mechanisms may be provided to release the closure devices from
locator devices and/or delivery devices once the closure device has been
deployed to
close an internal tissue opening such as a PFO. For example, the systems 100',
100"
illustrated in Figs. 24A-B and 25A-B respectively may make use a post-in-hole
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
47
configuration of connecting the closure devices 200 and 200o' to the medical
system
100', 100". Other configurations may be utilized to selectively release the
closure
device from the delivery system. Generalized attachment points will be
illustrated and
described for closure devices as well as generalized delivery points for the
delivery
devices. It will be understood that the various configurations illustrated and
described
io below may be adapted to use with any number of delivery devices in
combination
with any number of closure devices.
Figs. 26A-E illustrate several release mechanisms 2600a-e for releasing a
closure device 90 from a delivery device 92. In particular, closure devices 90
may
include an attachment member 240a-e that couples the closure device 90 to a
portion
of the delivery device 92. For ease of reference, push members 96a-e will be
described as the portion of the delivery device to which the attachment member
240 is
selectively secured. For example, Fig. 26A illustrates a release mechanism
2600a in
which the attachment member 240a includes a post 2602 formed on the closure
device
90 that engages a hole 2605 formed in the corresponding portion of the push
member
96a. The attachment member 240a, and thus the associated closure device 90, is
retained by the push member 96a as long as the attachment member 240a remains
under compression by the push member 96a. When the compression is released,
the
post 2602 may slide out of the hole 2605 in the corresponding portion of the
attachment member 240a.
Fig. 26B illustrates release mechanism 2600b that includes an attachment
member 240b and push member 96b for retaining and selectively releasing a
closure
device 90 from a delivery device. The release mechanism 2600b illustrated
includes a
flexible loop 2610 attached to the push member 96. The loop 2610 extends
through a
hole 2615 formed in the attachment member 364b. The loop 2610 is retained in
position by release wire 2620. To release the closure device 90, the release
wire 2620
is pulled out of the tether loop 2610. Releasing the loop 2610 allows the
closure
device 90 to be released by sliding away the loop 2610 from the delivery
device 300.
Fig. 26C shows a release mechanism 2600c that makes use of an attachment
member 240c that includes a tab 2622. The tab 2622 is configured to engage a
push
member 96c. In particular, the tab 2622 may have a dog-leg shape that is
configured
to extend into a slot 2624 formed in the push member 96c. A release wire 2625
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
48
extends into the push member 96c and maintains the tab 2622 in engagement with
the
slot 2624 as illustrated. When the release wire 2625 is pulled proximally, the
release
wire 2625 is withdrawn from engagement with the tab 2622. As tab 2622 is
released
from the tab 2615, the tab 2622 is then freed from the slot 2620 and the push
member
96c may then be withdrawn from the attachment member 240c to thereby release
the
1o closure device 90.
Fig. 26D illustrates a release mechanism 2600d that includes an attachment
member 240d and push member 96d configuration that includes flexible filament
2630. The flexible filament 2630 extends through a cross hole 2635 formed in
the
push member 96d, distally through a hole 2640 in the attachment member 240d,
and
back into the push member 96d as illustrated. A cutting slug 2645 is also
operatively
associated with the push member 96d. The cutting slug 2645 is configured to be
moved distally past the cross hole 2635 to thereby cut flexible filament 2630.
When
the flexible filament 2630 is cut, the attachment member 240d is thereby freed
from
the push member 96d.
Fig. 26E illustrates a release mechanism 2600e that has an attachment member
240e that is retained within a push member 96e by nesting tabs 2650, 2655.
Nesting
tab 2650 may be part of the attachment member 240e while nesting tab 2655 may
be
operative associated with the push member 96e. While the nesting tabs 2650,
2655
the attachment member 240e remains coupled to the push member 96e. In order to
release the attachment member 240e, the nesting tabs 2650, 2655 are moved
distally
relative to the push member 96e. Once the nesting tabs 2650, 2655 are outside
of the
push member 96e the nesting tabs 2650, 2655 are allowed to separate to thereby
release the attachment member 240e from the push member 96e.
In one example depicted in Fig. 27A, a release mechanism 2600f includes a
push member 96f that is formed of a meltable material, such as, but not
limited to, a
polymer filament made from either a single polymer fiber or a bundle or braid
of
multiple fibers. The push member 96f may be secured to an attachment member
240f.
In this illustrated embodiment, the push member 96f can be passed through a
coil
2600 of electrically conductive wire. The coil 2700 can be attached to a
current
source through less resistive leads 2705.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
49
When a current is passed through the coil 2700, the resistivity of the coil
heats
the push member 96f until the push member 96f melts, thereby severing the push
member 96f proximally from the attachment member 240f. As the push member 96f
is severed, the attachment member 240f and thus the closure device associated
with
the push member 96f are released. The current source 2705 may be configured to
provide a direct current to perform the resistive heating of the coil 2700. It
may also
be desirable for the current source 2705 to provide alternating current with
the
alternating current ranging up to radio frequencies. The coil 2700 may also be
covered with an optional insulating layer 2710. The inclusion of an insulating
layer
2710 may aid with transfer of heat from the coil 2700 as it is heated to the
surrounding tissues or fluids.
In another example, a release mechanism 2600f the resistive coi12700 forms a
resistive temperature device (RTD) which provides feedback with respect to the
temperature of the push member 96f while a current is applied to the coil
2700. The
coil 2700 in this case may be made of a metal that exhibits a relatively large
change in
resistance as it is heated such as, but not limited to, nickel, copper, or
platinum. The
actual temperature of the push member 96f may be monitored at intervals during
heating by removal of the heating current, and then applying a known voltage
through
the delivery mechanism 2600f, which may include the coil 2700. The resulting
resistance measurement may then be proportional to the temperature of the coil
as
with a conventional RTD.
Fig. 27B is a schematic diagram of a release mechanism 2600g that includes a
bimetallic actuator 2720. The bimetallic actuator 2720 may be configured to
provide
for mechanical detachment of a closure device (not shown) from a delivery
device at a
specified temperature or temperature range. The bimetallic actuator 2720
includes a
coiled, bimetallic strip 2725 that can be connected to a securing member 2730,
which
couples an attachment member 240g to a push member 96g. As a result, the
securing
member 2730 allows the push member 96g to drive the attachment member 240g
when the securing member 2730 is in place. More specifically, the securing
member
2730 engages both the attachment member 240g and the push member 96g such that
movement of the push member 96g is transferred from the push member 96g to the
securing member 2730 and thence to the attachment member 240g.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 The bimetallic strip 2725 is configured to uncoil at elevated temperatures.
As
the bimetallic strip 2725 uncoils, the securing member 2730 is drawn from its
connection to the push member 96g. After the securing member 2730 is drawn
from
engagement with the attachment member 240g, the attachment member 240g may
move freely and thus be released from the release mechanism 2600g. Further
10 movement of the bimetallic strip 2725 may release the push member 96g,
which may
then be withdrawn proximally as desired.
Fig. 27C is a schematic diagram of a release mechanism 2600h that includes a
bimetallic coil 2725. The bimetallic coil 2725 is coupled to a lever arm 2735
which
in turn is coupled to a securing member 2730. The lever arm 2735 rotates about
a
15 pivot point 2740. As the bimetallic coil 2725 unwinds, such as in response
to an
increase in temperature, the bimetallic coil 2725 drives the lever arm 2735.
The lever
arm 2725 includes a relatively short portion 2745a proximate to the bimetallic
coil
2725 and a relatively long portion 2745b proximate the securing member 2730.
Such
a configuration may amplify the amount of movement that is realized by the
uncoiling
20 of bimetallic coil 2725. As a result, relatively small increases in
temperature may be
used to move the securing member 2730 from engagement with the attachment
member 240g and then the push member 96g. Additional configurations of
bimetallic
release mechanisms may be adapted to be compatible with the geometry of
various
specific closure devices.
25 Release mechanisms and assemblies may also make use of shape memory
actuators to release a closure device. Certain shape memory alloys, such as
NiTiNol,
have the ability to transition from a first shape to a pre-set shape above a
certain
temperature that is dictated by the constituents of the alloy. Figs. 27D-27H
illustrate
release mechanisms 2600i-1 that may make use of shape memory materials. In
Fig.
30 27D, the release mechanism 2600i includes a shape memory actuator 2750. The
shape memory actuator 2750 is secured to a closure device 90. In particular, a
first
portion 2750a of the shape memory actuator 2750 is secured to an attachment
member
240i of the closure device 90. The closure device 90 further has a recess 2755
defined
therein opposite the attachment member 240i. The shape memory actuator 2750
35 extends from the attachment member 240i through a push member 96i and into
engagement with the recess 2755.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
51
In particular, the shape memory actuator 2750 extends through a loop 2757 in
the push member 96i while in the secured position illustrated in Fig. 27D a
second
portion 2750b of the shape memory actuator 2750 extends into the recess 2755
to
retain the push member 96i to the closure device 90. The recess 2755 may also
be
made formed a wire loop or other securing point for the shape memory actuator
2750.
The shape memory actuator 2750 may also have a shape that will adapt to be
secured
within the recess 2755. While a pin and loop configuration is described in
this and
other examples, other pin and receiving member configurations may also be
utilized
to retain a closure device until the closure device is selectively released.
Fig. 27E depicts the release of the push member 96i by heating the shape
memory actuator 2750. More specifically, as the shape memory actuator 2750 is
heated to a temperature above the transition temperature of the shape memory
material, the shape memory actuator 2750 may then recover to the preset shape
illustrated in Fig. 27E. The preset shape of the shape memory actuator 2750
may be a
coiled configuration such that the shape memory actuator 2750 may be drawn
toward
the attachment member 240i to release the push member 96i. As the push member
96i is released, the closure device 90 is free to move relative to the push
member 96i
and is thereby released as well.
Figs. 27F and 27G are schematic views of a release mechanism 2600j that
includes a plurality of shape memory actuators 2750a, 2750b. As illustrated in
Fig.
27F, the shape memory actuators 2750a, 2750b may extend in opposing directions
from opposing portions of a closure device 90 while the shape memory actuators
2750a, 2750b remain below the transition temperatures of the shape memory
materials of which the shape memory actuators 2750a, 2750b are formed. In such
a
configuration, at least a portion of each of the shape memory actuators 2750a,
2750 b
extend through a loop 2757 formed in a push member 96j.
Fig. 27G illustrates the shape memory actuators 2750a, 2750b that have been
heated to a temperature above the transition temperature of the shape memory
material. As the shape memory actuators 2750a, 2750b are heated above the
transition temperature of the shape memory material, the shape memory
actuators
2750a, 2750b return to their preset states, which may be the coiled
configurations
illustrated in Fig. 27G. As the shape memory actuators 2750a, 2750b return to
their
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
52
preset states, the shape memory actuators 2750a, 2750b are removed from their
securing positions relative to a loop 2757 in the push member 96j to release
the push
member 96j as shown in Fig. 27G. The heat input for heating shape memory
actuators 2750, 2750a, 2750b may be provided by external exposure to elevated
temperatures, radio frequency heating or applying an electrical current
through the
actuator member.
In another example (not shown), multiple shape memory securing members
may be made to release the push member. In another embodiment (not shown),
these
multiple shape memory securing members may have actuators that transition at
different temperatures which can allow for one part of the device to be
released at one
temperature and another portion of the device to be released at a second
temperature.
In another embodiment (not shown), the push member or push members may
comprise of a conductive material and an electrical connection to the implant
may
also be severed during the release of the push member. Combinations of the
above
embodiments may be utilized to provide both the desired mechanical detachment
from the implant as well as to cause the actuation to occur at a pre-defined
temperature or temperatures.
In some examples, the push member may include a central portion that
includes one or more filaments, such as a polymer filament, or a bundle of
filaments
or braid. Polymers may include, without limitation nylons, Dacron, polyester,
polyethylene, Teflon, PTFE, Kevlar, Spectra or the like. These materials may
also be
components of a larger push member system which extends to the proximal,
operable
end of the device and consist of a polymer catheter or metallic hypotube of
stainless
steel or other biocompatible alloys.
Figs. 271 and 27J illustrate a release mechanism 2600k that includes a cutting
feature 2760. The cutting feature 2760 is coupled to a shape memory actuator
2750c.
The shape memory actuator 2750c in turn is secured to a first portion 2765 of
a
closure device 90. A push member 96j is secured to a second portion 2770b of
the
closure device 90. The push member 96j extends away from the second portion
2770b and through an opening 2772 formed in the cutting feature 2760 while the
shape memory actuator 2750c remains below the transition temperature and thus
in an
initial position.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
53
As the shape memory actuator 2750c is heated above the transition
temperature, the shape memory actuator 2750c moves to the preset shape
illustrated in
Fig. 27J. Accordingly, the shape memory actuator 2750c is drawn toward the
first
portion 2765 of the closure device 90. Drawing the shape memory actuator 2750c
toward the first portion 2765 of the closure device also draws the cutting
feature 2760
in the same direction, which causes the cutting feature 2760 to sever the push
member
96j. The closure device 90 is thereby released from the push member 96j.
The properties of shape memory alloys may also be used to actuate the release
of a securing member 2730 as illustrated in the release mechanism 2600k' in
Figs.
27K and 27L. In particular, a shape memory actuator 2750d is secured to a
closure
device 90, which is depicted as ground. A linkage 2775 is coupled at a first
end
2775a to the shape memory actuator 2750d and at a second end 2775b to the
securing
member 2730. The linkage 2775 is configured to rotate about a pivot 2735, the
pivot
2735 being secured to the closure device 90. In particular, the linkage 2775
is
configured to rotate in response to movement of the shape memory actuator
2750d
such that movement of the shape memory actuator 2750d is transferred by the
linkage
to the securing member 2730.
While the securing member 2730 engages both the attachment member 240k'
and the push member 96k' such that movement of the push member 96k' is
transferred from the push member 96k' to the securing member 2730 and thence
to
the attachment member 240k'. Accordingly, while the shape memory actuator
2750d
remains at a temperature below the transition temperature of the shape memory
material, the shape memory actuator 2750d remains extended and the securing
member 2730 remains in engagement with the attachment member 240k' and the
push
member 96k'.
Fig. 27L illustrates the shape memory actuator 2750d returning to a preset
state that corresponds to the shape memory material of the shape memory
actuator
2750d being heated to a temperature above the transition temperature. As
illustrated
in Fig. 27L, as the shape memory actuator 2750d returns to its preset state,
the first
end 2775a is drawn toward the closure device 90 thereby drawing the securing
member 2730 first from engagement with the push member 96k' and then
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
54
engagement with the attachment member 240k'. As a result, heating the shape
memory actuator 2750d releases the closure device 90 from the push member
96k'.
A phase change of a material in an enclosed space may also provide for the
actuation of a release mechanism 26501, as shown in 27M and 27N. Fig. 27M
depicts
a cylinder 2780 and piston 2785 attached to a closure device 90. The cylinder
2780
and piston 2785 are operative associated with a linkage 2775 that is coupled
to a
securing member 2730. The securing member 2730 secures the push member 961 to
an attachment member 2401, the attachment member 2401 being part of the
closure
device 90.
Heating the material within the cylinder 2780 generates pressure which drives
the piston 2785 away from the cylinder 2780. As the piston 2785 is driven away
from
the cylinder 2780 the piston 2780 drives the linkage 2775 and thus the
securing
member 2730. As the securing member 2730 is thus driven, the push member 961
is
released from the closure device 90.
Other examples of release mechanisms (not shown) that make use of phase
change include the removal of the linkage 2775 shown in Fig. 27M and 27N and
making a direct connection between the securing member 2730 and the piston
2785.
Another embodiment of the system (not shown) replaces the piston and cylinder
2780,
2785 with a bellows assembly which also provides a sealed container for the
phase-
change material that can expand axially when the phase change occurs. Phase
change
materials that may be utilized in this system for expansion upon heating may
include,
without limitation, various hydro-carbon fluids such as heptane, isopropyl
alcohol and
the like. Formulations of waxes such as those used in the thermostats of
common
automotive engine thermostats may also be utilized.
In some of the examples, portions of the release mechanism, such as the shape
memory actuators, are attached to the closure device. Such attachment may be
switched and/or altered as desired. In other examples (not shown), shape
memory
actuators may be attached to the push member. In still other examples (not
shown),
shape memory actuators may be attached in combination to the closure device
and the
push member. In yet other examples (not shown), shape memory actuators may be
attached to other portions of the medical system.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 Reference to Figures 28-38B will now be made to describe a delivery device
2800 which can be used to deploy a closure device 90 (. Furthermore, the
method of
using delivery device 2800 will also be described. In the illustrated
configuration,
delivery device 2800 can include a main handle 2802, a guide catheter 2818
coupled
to and extending distally from main handle 2802, a pusher handle 2820 coupled
to
1o main handle 2802, a release knob 2826 coupled to pusher handle 2820 and an
end cap
2832. Main handle 2802 can have an elongated cylindrical shape and can be
substantially hollow. Main handle 2802 can be configured to assist a user in
placing
closure device 100.
In fluid communication with a portion of the main handle 2802 can be a flush
15 line 2816, while selectively movable relative to the main handle 2802 can
be first and
second stops 2812, 2814. Flush line 2816 can be configured to be in fluid
communication with guide catheter 2818. First stop 2812 can be configured to
be
received in a first groove 2804 (Fig. 29) and second stop 2814 can be
configured to be
received and at least partially movable in a second groove 2806 (Fig. 29).
First
20 groove 2804 can extend radially along the circumference of main handle
2802. First
groove 2804 can be configured to receive first stop 2812 therein and allow
first stop
2812 to at least partially rotate therein. First groove 2804 can include a
first opening
2808. First opening 2808 can extend through the wall of main handle 2802.
First
opening 2808 can be configured to receive a set screw (not shown) positioned
in a
25 hole disposed through first stop 2812. This hole enables the set screw to
be received
within first opening 2808 can limit rotational movement of first stop 2812
relative to
main handle 2802. It will be understood that a portion of the first stop 2812,
instead
of or in addition to the set screw, can extend into first opening 2808.
Similarly, second groove 2806 can extend radially along the circumference of
30 main handle 2802. Second groove 2806 can be configured to receive second
stop
2814 therein and allow second stop 2814 to at least partially rotate therein.
Second
groove 2806 can include a second opening 2810. Second opening 2810 can extend
through the wall of main handle 2802. Second opening 2810 can be configured to
receive a set screw (not shown) positioned in a hole disposed through second
stop
35 2814. This hole enables the set screw to be received within second opening
2810 can
limit rotational movement of second stop 2814 relative to main handle 2802. It
will
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
56
be understood that a portion of the second stop 2814, instead of or in
addition to the
set screw, can extend into second opening 2810
First stop 2812 can be received in first groove 2804 and can at least
partially
rotate circumferentially in first groove 2804. At least a portion of first
stop 2812, or
the set screw passing through the first stop 2812, can extend in and through
first
opening 2808. First stop 2812 can be configured to enable selective deployment
of
PFO closure device 3000, as will be discussed more fully herein below.
Similarly,
second stop 2814 can be received in second groove 2806 and can at least
partially
rotate circumferentially in second groove 2806. At least a portion of second
stop
2814, or the set screw passing through the second stop 2814, can extend in and
through second opening 2810. Second stop 2814 can be configured to enable
selective deployment of PFO closure device 3000, as will be discussed more
fully
herein below. The distance between first and second stops 2812 and 2814 can
correspond to the distance sufficient to expose and deploy distal anchors 3006
from
guide catheter 2818 as pusher handle 2820 moves with respect to main handle
2802 in
the distal direction while maintaining proximal anchors 3008 within guide
catheter
2818.
PFO closure device 3000 can be inserted into the distal end of a guide
catheter
2818 in a manner such that proximal anchors 3008 extend proximally and distal
anchors 3006 extend distally within guide catheter 2818. PFO closure device
3000
can be attached to delivery device 2800 through use of tabs 3012.
Delivery device 2800 can further include a pusher tube 2834 (Fig. 31). Pusher
tube 2834 can be received and movable in guide catheter 2818 (Fig. 28) and can
substantially extend from the distal end of guide catheter 2818 to pusher
handle 2820.
Pusher tube 2834 can be coupled to pusher handle 2820, such that movement of
pusher handle 2820 relative to main handle 2802 can result in movement of PFO
closure device 3000 out of the distal end of catheter shaft.
In the illustrated embodiment, delivery device 2800 can include one or more
flexible tubes 2836 (Figs. 30A-31). The number of flexible tubes 2836 can
optionally
correspond with the number of tabs 3012 of PFO closure device 100. In one
configuration, flexible tube 2836 can be coupled to pusher tube 2834 such that
movement of pusher tube 2834 can result in movement of flexible tube 2836.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
57
Generally, pusher tube 2834 can receive various wires, tubes, etc. of delivery
device
2800 and the aid with deploying device 3000. For instance, pusher tube 2834
can
receive a thermocouple, a tubular member receiving the thermocouple and
associated
electrically communicating wire(s), RF energy delivery and return wire(s) or
conductor(s), or the like.
As shown in Figure 31, one flexible tube 2836 is spaced apart from two
adjacently positioned flexible tubes 2836. The resultant gap can receive a
portion of
the wires, tubes, etc. received by the pusher tube 2834. For instance, and not
by way
of limitations, the thermocouple, the tubular member receiving the
thermocouple and
associated electrically communicating wire(s), the RF energy delivery and
return
wire(s) or conductor(s), or the like, can exit from the pusher tube 2834 and
pass to the
closure device 3000.
To aid with such movement, each flexible tube 2836 can include a tube cap
2840, as illustrated in Figure 30A-30C. Tube cap 2840 can be sized and
configured to
receive tab 3012 therein. Tube cap 2840 can include a slot 2842 sized and
configured
to receive a foot 3014 of tab 3012 therein. The size and configuration of tube
cap
2840 can be such that with foot 3014 of tab 3012 is in slot 2842, a detachment
wire
2838 can extend through and out of the distal end of tube cap 2840. In this
manner,
detachment wire 2838 can serve to bias and substantially hold tab 3012 in tube
cap
2840 and thus substantially prevent detachment of PFO closure device 3000 from
delivery device 2800 until detachment wire 2838 is moved away from foot 3014,
as
will be described herein below. Fig. 30A-C illustrate a single flexible tube
2836 as
detachment wire 2838 is sequentially moved proximally to enable foot 3014 to
be
removed from tube cap 2840.
Guide catheter 2818 can be coupled to main handle 2802. Guide catheter
2818 can be configured to house at least a portion of pusher tube 2834 and
other
portions of the device 2800 therein. Guide catheter 2818 can further be
configured to
allow pusher tube 2834 to rotate and translate therein. The distal end of
guide catheter
2818 can be configured to receive PFO closure device 3000 therein.
With reference to Figures 28 and 32, pusher handle 2820 can be a substantially
hollow, rigid generally cylindrical member. Pusher tube 2834 can be coupled to
pusher handle 2820. In this manner, movement of pusher handle 2820 relative to
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
58
main handle 2802 in the distal direction can result in the deployment of PFO
closure
device 3000 from guide catheter 2818. In the illustrated embodiment, pusher
handle
2820 can include a first portion 2820a and a second portion 2820b. First
portion
2820a can be sized and configured to be received and movable within at least a
portion of main housing 2802. First portion 2820a can include a track 2822.
Track
2822 can be configured to serve as a guide for stops 2812 and 2814. Track 2822
can
be configured to receive set screws of stops 2812 and 2814 therein, such that
set
screws can translate along track 2822.
Track 2822 can include various points, generally labeled as Point #1, Point
#2,
Point #3 and Point #4. The distance between Points #3 & #1 can correspond to
the
distance sufficient to expose and deploy distal anchors 3006 from guide
catheter 2818
as pusher handle 2820 moves with respect to main handle 2802 in the distal
direction.
Furthermore, this distance can be sufficient to maintain proximal anchors 3008
within
guide catheter 2818. The distance between Point #2 and the most proximal point
in
track 2822 can correspond with a distance sufficient to enable the entire PFO
closure
2o device 3000 to deploy from guide catheter 2818.
First and second stops 2812 and 2814 can be movable between a closed and
open position. For example, first stop 2812 is in a closed position when the
set screw
of first stop 2812 is at Point #1 and in an open position when the set screw
is at either
of Points # 2 or #4. Second stop 2814 is in the closed position when the set
screw of
second stop 2814 is either at Points #3 or #1, and in the open position when
the set
screw of second stop 2814 is at either of Points #2 or #4. As illustrated in
Fig. 28,
first and second stops 2812 & 2814 are in the closed position; as illustrated
in Figures
34A & 35A, first stop 2812 is in the open position and second stop 2814 is in
the
closed position; as illustrated in Figs. 36 & 37A, first and second stops 2812
& 2814
are both in the open position.
Second portion 2820b of pusher handle 2820 can be configured to receive at
least a portion of release knob 2826 therein. Second portion 2820b can include
a pin
hole 2824 through which a pin 2824a can be received. Pin 2824a can be
configured
to be received and movable along a track 2828 of release knob 2826. It will be
understood by one of ordinary skill in the art in view of the disclosure
provided herein
that the various pin and groove configurations serve as a guide and that other
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
59
configurations can perform the same function without departing from the scope
and
spirit of the invention. For example, the pin and groove configurations may be
replaced by various linkages which allow movement sufficient to enable the
various
elements of the invention to function correctly.
Release knob 2826, as illustrated in Fig. 33, can be configured to facilitate
detachment of the closure device 3000 from delivery device 2800. At least a
portion
of release knob 2826 can be sized and configured to be received and movable
within
the proximal end of pusher handle 2820. Detachment wires 2838 can be coupled
to
release knob 2826. In this manner, movement of release knob 2826 relative to
pusher
handle 2820 in the proximal direction can result in detachment of PFO closure
device
3000 from delivery device 2800.
Release knob 2826 can include a track 2828. Track 2828 can be configured to
receive a portion of the pin 2824a (Fig. 32) from second portion 2820b of
pusher
handle 2820 therein and serve as a guide for movement of the pin 2824a. Track
2828
can be configured to allow release knob 2826 rotate and/or translate relative
to pusher
handle 2820 in either clockwise or counterclockwise directions. The
configuration of
track 2828 can constrain the movement of release knob 2826 relative to pusher
handle
2820 such that the path of movement of release knob 2826 relative to pusher
handle
2820 is prescribed by the configuration of track 2828. Track 2828 can include
a
detent 2830. Detent 2830 can be configured to reduce movement of release knob
2826 relative to pusher handle 2820, such that release knob 2826 does not
inadvertently move detachment wires 2836 relative to tube cap 2840 thus
enabling
displacement of foot 3014 of PFO closure device 3000 out of slot 2842 of tube
cap
2840.
Delivery device 2800 can further include an end cap 2832, as illustrated in
Figure 34A. End cap 2832 can be coupled to the proximal end of release knob
2826.
End cap 2832 can be configured to allow various wires and/or tubes to extend
therethrough, such as, but not limited to, a thermocouple, electrode wires, RF
wire(s)
or conductor(s). Guide catheter 2818, main handle 2802, pusher handle 2820 and
release knob 2826 can also be configured to allow various wires to extend
therethrough, such as a thermocouple, electrode wires, and/or RF wire(s) or
conductor(s). Furthermore, guide catheter 2818 and main handle 2802 can
further be
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 configured to allow pusher shaft 2836 to extend therethrough and be moveable
therein.
The guide catheter 2818 is adapted to be positioned through the PFO such that
the distal end of guide catheter 2818 is in the left atrium. The PFO closure
device
3000 can then be deployed from guide catheter 2818 by moving pusher handle
2820
1o relative to main handle 2802, whether such movement includes moving pusher
handle
2820 toward main handle 2802, main handle 2802 toward pusher handle 2820, or a
combination thereof. This can be accomplished by the following procedure.
First,
first stop 2812 can be moved from the closed position to the open position,
thus
moving the set screw of first stop 2812 from Point #1 to Point #2, as
illustrated in
15 Figure 34A. Then, main handle 2802 can be moved proximally with respect to
pusher
handle 2820 such that the set screw of first stop 2812 moves from Point #2 to
Point #4
and the set screw of second stop 2814 moves from Point #3 to Point #1, as
illustrated
in Figure 35A. This movement of main handle 2802 relative to pusher handle
2820
can be sufficient to cause distal anchors 3006 of closure device 3000 to
extend out
20 from the distal end of guide catheter 2818 and deploy into the left atrium.
A user can then manipulate the main handle 2802 until distal anchors 3006 are
positioned against tissue adjacent the PFO in the left atrium. To deploy
proximal
anchors 3004, a user can move second stop 2814 to the open position (set screw
of
second stop 2814 moves from Point #1 to Point #2) as illustrated in Figure 36,
and
25 then further proximally move main handle 2802 relative to pusher handle
2820, as
illustrated in Figure 37A. The length of track 2822 from Point #2 to the
terminating
proximal point can be sufficient so as to allow main handle 2802 to move
enough for
proximal anchors 3008 to fully extend out of the distal end of guide catheter
2818 and
deploy, thus engaging the tissue adjacent the PFO in the right atrium.
30 PFO closure device 3000 can be detached from delivery device 2800 through
use of release knob 2826. Moving release knob 2826 proximally with respect to
pusher handle 2820, as illustrated in Figure 38A, can cause detachment wire
2838 to
be pulled proximally through tube cap 2840. The configuration of track 2828 of
release knob 2826 and pin of pusher handle 2820 enable release knob 2826 to be
35 moved relative to pusher handle 2820 sufficiently to cause detachment wire
2838 to
move proximally past foot 301.4 of tab 3012. As detachment wire 2838 is moved
past
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
61
foot 3014, foot 3014 can be moved out of slot 2842 via the ramp 3014a of foot
3014.
In this manner, tab 3012 can be detached from flexible tube 2836, and thus
detached
from delivery device 2800. At this point, PFO closure device 3000 is
positioned and
delivery device 2800 can be removed from the patient.
Although reference is made hereinto to the delivery device 3000 and
deployment of the same, it will be understood that the present invention can
more
generally apply to the delivery and positioning of a structure disposable
within a body
lumen, where the structure can optionally receive RF or other electromagnetic
energy
to aid with implanting the structure, whether or not the structure is a
generally planar
structure. Additional information regarding the structures, functions, use and
operation of the closure device 3000, delivery device 2800, and the inventions
disclosed herein are disclosed in Exhibits A, B and C attached hereto and by
reference
incorporated herein.
Fig. 39A illustrates a closure device 90 that includes an in-growth media
configuration 3900 attached thereto. The closure device 90 includes a
plurality of
cells 3902. The in-growth configuration 3900a may include filament 3905
secured to
a central portion of the closure device 90. Securing filament 3905 to the
central
portion of the closure device 90 may encourage tissue in-growth into the
closure
device 90 within the center of the closure device 90 rather than the perimeter
portions
of the closure device 90 that may be exposed to flowing blood. As illustrated
in Fig.
39A, the filaments 3905 are coiled around portions of one or more of the cells
3902.
In-grown material in this embodiment can include fibers of bio-compatible
polymers
such as Dacron (polyester),PTFE, or other filaments that provide a relatively
large
amount of surface area into which tissue can grow.
In Fig. 39A, the filament 3905 is depicted as a single fiber. While a single
fiber may be used, the filament 3905 may also represent a fiber bundle which
may
have a braided structure, or a twisted structure. The surface of the fiber
bundle may
have many protruding ends of the individual fibers as a result of a looser
braid or
twist. The additional fiber ends can provide surfaces for in-growth as tissue
forms
around them. The fiber or fiber bundle may be secured to the structure of the
cell
3910 by thermal bonding or adhesives.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
62
An in-growth configuration 3900b that includes filament 3905 is shown in Fig.
39B. As illustrated in Fig. 39B, the closure device 90 may include features
3910,
such as holes, formed into the closure device 90. The features 3915 may
provide
additional anchoring points for the filament 3905 additional anchoring points.
The
filament 3905 may be loosely sewn through these points, or fixed by adhesive
or
1o knots in the filament near each feature 3910 through which the filament
3905 passes.
Though not shown, the filament 3905 may extend over only a single cell or
throughout all of the cells of the structure. Further, the path of the
filament 3905 is
shown being weaved to alternating sides of the structure. In such a
configuration, the
3905 may have the ability to take up space out of the plane of the structure.
Additionally, when the closure device 90 is in a compressed state for
delivery, all of
the fiber material may be kept out from between the struts of the closure
device 90,
which may allow for more efficient packing of the compressed structure for
delivery
through or by way of a catheter.
In at least one example, in-growth media, such as in-growth filaments, may be
2o attached to the closure device by way of loops of wire, suture material or
threads of
in-growth material. In such an example, the in-growth media may be attached to
one
side of the closure device structure. In other examples, the in-growth media
may be
sandwiched between two PFO closure devices that are connected. The use of
multiple
closure devices may provide for the separation of functions of the structure
(e.g.
anchoring and tissue expansion) while also providing a secure place for the
media. In
yet another example, the in-growth media may be woven through the cells of the
closure device and can be secured by the weaving or additional securing
techniques or
members could be provided as well.
In another example illustrated in Fig. 39C, the in-growth configuration 3900c
includes filament 3905 secured to the closure device 90 in a pattern. The
pattern of
the in-growth configuration may increase the relative amount of in-growth
media that
is secured to the closure device 90. Further, in the pattern illustrated the
points at
which the filaments 3905 are attached are maintained at a constant distance
from each
other as the closure device 90 is deployed. Such a configuration may provide
for a
degree of tightness of the filament 3905 remains relatively constant over a
wide range
of deployment.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
63
Fig. 39D illustrates an in-growth configuration 3900d in which the points at
which the filament 3905 is secured to the closure device 90 are not maintained
at a
constant distance from each other as the closure device 90 is deployed. Such a
configuration may result in relative slack in the filament 3905 as the closure
device 90
is deployed.
Fig. 39E illustrates an in-growth configuration 3900e in which strips 3915 in-
growth media are secured to the closure device 90. In particular, the strips
3915 of in-
growth media may be secured to opposite sides of a cellular portion of the
closure
device 90. The strips 3915 may be loosely woven gauze-like material that is
woven
into an interlocking structure. The strips 3915 may be secured to the closure
device
90 in any suitable manner. As illustrated in Fig. 39F, in one configuration
3900f the
strips 3915 may be wrapped around a cellular portion of the closure device 90
and
back onto the strips 3915. The overlapping portions of the strips 3915 may
then be
secured to each other.
Fig. 39G illustrates an in-growth configuration in which one or more
membrane 3920 are secured to a closure device 90. The membrane 3920 may
include
a number of materials, including, without limitation, fibers of Dacron
(polyester),
PTFE, or bio-absorbable polymers which are formed into a membrane through
weaving, knitting, or some other manner. The membrane patterns may be precut
as
desired and secured to the closure device 90 in any suitable manner.
In addition to securing in-growth media to a single closure device, in-growth
media may also be secured to multiple closure devices that are configured to
be
deployed in concert. In particular, Fig. 39H illustrates an in-growth
configuration in
which the membrane 3920 is sandwiched between two closure devices 90a, 90b
that
have been crimped together. The use of multiple closure devices 90a, 90b may
increase the stiffness of the two devices when used together while allowing
secure
attachment of the membrane 3920.
Figs. 391 and 39J also illustrate additional configurations in which multiple
closure devices are used in concert. In Fig. 391, the closure devices 90c, 90d
are
configured differently. Fig. 39K illustrates a similar configuration in which
closure
devices 90e, 90f are different. The second closure device 90f may be biased
out-of-
plane relative to the first closure device 90e. Such a configuration may also
provide
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
64
out-of-plane structure to contact surrounding tissues to thereby secure the
closure
device 90e in the internal tissue opening.
Figure 39K illustrates one embodiment of a closure device 200 that can
include a member 250, such as an ingrowth material. The member 250 can be
configured to induce tissue growth. The member 250 can be fixed to the closure
to device 200 by means of a securing element, such as a thread 252. For
example, the
thread 252 can extend through the member 250 and through the apertures in the
intermediate portions 234 in order to secure the member 250 to the closure
device
200. In other embodiments, the member 250 can be secured to the closure device
220
by a known securing means, such as by an adhesive, a heat weld, or some other
known or hereafter developed means for securement.
The member 250 and the thread 252 can include a bio-resorbable material,
such as polylactide or polyglycolide or collagen. The member 250 can be sized
and
configured to enable the closure device 200 to be deployed from and received
into the
delivery portion 366 of the delivery device 300. Furthermore, the member 250
can be
configured to interact with tissue of the internal tissue opening to stimulate
growth of
tissue for closure of the internal tissue opening. For example, the member 250
can
interact with the tunnel tissue 58 of a PFO in order to stimulate growth of
tissue in the
PFO tunnel 58.
The member 250 can be any suitable material which can or tends to promote
tissue growth. Examples of such material can include a polymeric material, or
a
woven material, such as a woven metallic or biological material. In one
embodiment,
the member 250 can be a piece of foam. In alternative embodiments, the member
250
can be a piece of yarn, fabric or string, or some combination thereof. Other
tissue
growth promoting members can include a coating disposed on the closure device
200.
In other embodiments, the member 250 can be a piece of foam, braided material
such
as a piece of yarn or string, or fabric which has a coating disposed thereon.
The member 250 can include materials such as a piece of polyurethane or
some other biocompatible polymer, including bioresorbable polymers. The member
250 can also include Dacron or polymeric threaded material which have been
woven
or knitted, or formed into compressed, non-woven fabrics. The member 250 can
also
include a metallic material, such as a NiTiNol, stainless steal or some other
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 biocompatible alloy or bioresorbable metal, such as magnesium alloy, or some
combination thereof. In one embodiment, the member 250 comprises a metallic
wire.
Figure 39M illustrates a side view of the closure device 200, and illustrates
one example of the closure device having a substantially flat configuration.
In the
illustrated embodiment, the closure device 200 can include a depth or depth
thickness
1o designated as DT, and a plane 260 extending perpendicular into and out of
the plane
of the page. In this embodiment, the member 250 can extend beyond at least a
first
edge 262 of the closure device 200. Furthermore, the member 250 can extend
beyond
both the first edge 262 and a second edge 264 of the closure device 200. In
this
manner, member 250 can contact tissue adjacent the closure device 200 to
promote
15 tissue growth in the tissue opening.
The member 250 can be sized and configured to extend beyond at least the
first edge 262 of the closure device 200 a sufficient distance to contact
tissue of the
tissue opening. In one embodiment, the member 250 can extend beyond at least
the
first edge 262 a sufficient distance to contact tissue adjacent the first edge
262,
20 thereby causing the end of the member 250 which is in contact with the
tissue to
deflect or bend. In this manner, more surface area of the member 250 can be in
contact with tissue to thereby facilitate an increase in tissue growth. In
other
embodiments, the member 250 can extend beyond both the first edge 262 and the
second edge 264 a sufficient distance to cause both ends of the member 250 to
bend,
25 which can result in more surface area contacting the tissue. In one
embodiment, the
member 250 can extend between at least .5mm and 5mm beyond the first edge 262.
In another embodiment, the member 250 can extend between at least .5mm and 5mm
beyond the first edge 262, and can extend between at least .5mm and 5mm beyond
the
second edge 264. Furthermore, the member 250 can have a thickness of between
at
30 least.25mm and 2mm.
In addition, in some embodiments the member 250 can be configured to
decrease the size of a remaining void in the tissue opening after the closure
device
200 has been positioned in the tissue opening. Member 250 extending beyond the
first edge 262 of the closure device 200 is an example of the member 250
extending
35 substantially out of plane of the substantially flat configuration.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
66
As discussed, cell structures may be variable and/or irregular. A completely
random structure of very small cells can also have properties that provide the
correct
force to the internal tissue opening. These structures may be constructed of
fine wire
that has been shaped into the desired flat form but has sufficient voids to
allow for
compressibility within a delivery system. Other random celled structures may
be
constructed from polymer foams such as but not limited to ePTFE or
polyurethane.
For metallic based structures for implant, the surface finish may be electo-
polished. In this application, all, or specific portions of the device may be
electro-
polished to provide a smooth and trauma-free surface. The edges of the device
may be
specifically designed to contact the inner side walls of the tunnel may be
electro-
polished to prevent sharp edges of the structure from puncturing the tissues
except for
specific locations where it is desired for anchoring. A smooth surface on the
edge can
also be amenable to coating which can add lubricity for ease of delivery.
Rougher
surfaces on surfaces of the device other than the outer edge may be desirable
and can
aid in providing anchoring locations and/or locations for more aggressive
tissue in-
growth after implantation. In portions of the structure where more roughness
is
desired, the roughness may be added by grit-blasting, chemical etching or
other
mechanical means using appropriate abrasives. Polymer structures may be
similarly
smooth or textured as desired for fixation and in-growth.
Closure devices can also be adapted to serve as a drug delivery platform
and/or the placement of other substances that can enhance the closing of
internal
tissue openings. In at least one example, drugs may be delivered by elution,
such as
from a polymer-based coating. Such drugs may include, without limitation,
drugs that
can induce the closing of an internal tissue opening, such as vascular
endothelial
growth factor, synthetic or naturally occurring proteins, and/or refined
proteins such
3o as collagen or bovine serum albumin.
Structures that perform the same functions as the multi-celled structures may
also be constructed from a single member. The single- member structure may
include
anchoring features as shown in where anchor is made to anchor the device from
the
distal side of its deployment. After the waist of the structure, a portion of
the member
is constructed to provide lateral and anchoring force within the opening. The
bottom-
most portion of the device will provide the support for the distal anchors as
well as the
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
67
lateral force exerted through the waist of the device. A relief in the most
proximal
portion of the member may be provided to allow the closure device to be
collapsed
within a catheter for delivery. Other means of providing this relief may be
used such
as a coil spring or a localized material property modification of that section
of the
member. A single member structure may also have integral, more complex anchors
(3) as depicted in Fig. 14B.
In addition to the above, it may be desirable to add additional features to
encourage in-growth to close an internal tissue opening, such as a PFO.
Threads or
fabric of polymeric materials such as Dacron felt, fabrics or filaments, PTFE,
ePTFE
or the like may be wrapped around the struts of the PFO closure device or
woven
through the cells to provide more aggressive tissue in-growth surfaces where
desired.
Fine metallic wires, meshes or braids may also be used. Alternatively, fabric
or thin
membranes may be sewn, welded, or adhered to the struts to cover any desired
part of
the PFO closure device.
In addition to the embodiments and configurations described above, the
present invention can also related to various other medical devices, systems,
and
methods. For instance, in another configuration, disclosed is a medical device
that
has a multi-cellular structure being configured to be moved from a collapsed
state to
an expanded state, the multi-cellular structure including a waist portion and
at least
one anchor portion, wherein the anchor portion is wider in the expanded state
of the
device than the waist portion and the waist portion is configured to engage a
tunnel of
an internal tissue opening in the expanded state to close the internal tissue
opening.
This device can also include one or more distal anchors and/or proximal
anchors.
These anchors can be substantially the same width, or one may be wider than
the
other. These anchors may also include a plurality of elongate arms. One or
more of
the elongate arms may have a serrated edge, such as a serrated edge is
configured to
face toward the center of a tunnel of an internal tissue opening when the
medical
device is deployed. The elongate arms may also have smooth edges. The multi-
cellular structure may include a plurality of cellular portions having
substantially the
same size or the cellular portions may be of different sizes. The medical
device may
also be configured to shorten its overall length dimension upon deployment. If
the
medical device has a distal anchor and a proximal anchor, the proximal anchor
may
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
68
be configured to roll at least partially onto itself upon deployment to
shorten the
overall length of the medical device upon deployment. The medical device may a
spring member secured to the waist and a solid anchor portion. The solid
anchor
portion may be a solid proximal anchor portion. In one example in which the
medical
device includes both proximal and distal anchor arms, the waist portion of the
medical
device may also include a hinged portion. The medical device may be formed of
a
resilient material such that the medical device is configured to expand from
the
compressed state to the expanded state due at least in part to spring forces
associated
with the resilient material. The medical device may also be configured to be
expanded from the compressed state to the expanded state mechanically.
A medical device according to one example includes opposing expansion
members and at least one connecting member coupling the opposing expansion
members. The connecting member may be configured to move the opposing
expansion members from a compressed state to an expanded state to seal an
internal
tissue opening. The medical device may also include a plurality of connecting
members and pinned joints between adjacent connecting members and between the
connecting members and the opposing expansion arms. The pinned joints may
include a ratcheting mechanism configured to allow the connecting members to
move
relative to each other to allow expansion of the expansion members during
deployment but to prevent the expansion members to collapse after deployment
of the
medical device. The medical device may further include an actuation member,
such
as a cable or tether, coupled to at least one connecting member. The expansion
arms
may be configured to be expanded from the compressed to the expanded state by
drawing the actuation member proximally. The medical device may also include a
locking member configured to lock the expansion member in the expanded state.
The
locking member may include a clasp operatively associated actuation member.
Further, the connecting member may have a strut and piston configuration.
In yet another configuration, a medical device includes a plurality of
elongate
arms and an actuation member coupled to the elongate arms, the actuation
member
being configured to mechanically expand the elongate arms from a collapsed
position
to an expanded position. The actuation member may include alternating thinner
portions between relatively thicker portions. Further, the actuation member
may be
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
69
configured to be drawn proximally and/or moved distally to mechanically expand
the
elongate arms. The medical device may also include a body portion operative
associated with the elongate arms. The body portion may include flexing
sections
and/or pivots coupling the elongate arms to the body portion. The medical
device
may be a distal locator device, a proximal locator device, and/or a closure
device.
In yet another configuration, a medical system includes a first medical device
having expandable elongate arms, the expandable elongate arms being configured
to
expand from a collapsed state to an expanded state and to locate an opening of
an
internal tissue opening, and a second medical device operatively associated
with the
first medical device, the second medical device having a multi-cellular
structure being
configured to be moved from a collapsed state to an expanded state, the multi-
cellular
structure including a waist portion configured to engage a tunnel of an
internal tissue
opening in the expanded state to close the internal tissue opening. The first
medical
device may be a distal locator device or a proximal locator device. For
example, the
first medical device may be configured to locate a distal opening of the
internal tissue
opening. The system may further include a third medical device operatively
associated with the second medical device, wherein the third medical device
includes
expandable elongate arms, the expandable elongate arms being to expand from a
collapsed state to an expanded state and to locate a proximal opening of the
internal
tissue opening. The second medical device may include outwardly facing tines,
first
anchor portions, and/or second anchor portions.
A device for releasing an implant within a body lumen includes an attachment
member coupled to the implant, a push member operatively associated with the
attachment member. The device may be configured to secure the implant to a
delivery device before release of the implant and to selectively release the
attachment
member. The attachment member may have a post configuration and the push
member has a hole defined therein to receive the post. The attachment member
may
include a loop of material coupled to the push member and a pin, the loop
extending
through a hole formed in the attachment member and being secured to the
attachment
member by the pin, wherein removing the pin releases the attachment member.
The
attachment member may also include a tab while the push member includes a pin
configured to retain the tab in contact with the push member. The tab may have
a
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 dog-leg shape. The push member may include a slot defined therein configured
to
receive a portion of the tab while the device further includes a release wire
configured
to retain the tab in the slot and to be withdrawn to release the tab from the
slot. The
pin and tab may be interlocking members held in engagement while retained
within
the push member and that are released when moved from the push member. The
push
10 member may be formed from a meltable material. In such an example, the
device
may further include a coil of electrically conductive wire in which a portion
of the
push member extends at least partially through the coil. The device is
configured to
release the attachment member by heating the coil to melt a portion of the
push
member. Insulation may surround at least a portion of the coil. The device may
also
15 include a current source configured to provide a direct current and/or
alternating
current to the coil. The current source may be configured to provide an
alternating
current to the coil at a frequency up to radio frequencies. The coil may also
be
configured a resistive temperature device and may be formed from nickel,
copper,
and/or platinum or any other suitable material.
20 In another example, the device includes bimetallic actuator configured to
release the attachment member from the push member at a specified temperature
range. The bimetallic actuator may include a bimetallic strip and a securing
member
operatively associated with both the attachment member and the push member.
The
securing member may be configured to couple movement of push member to the
25 attachment member when engaged and to be disengaged at a specified
temperature
range to decouple the attachment member from the push member. The push member
and the attachment member may each include a receiving portion, such as a
loop,
defined therein which allows the securing member to pass at least partially
through
each of the receiving portions. The bimetallic actuator may be secured
directly to the
30 securing member and/or a linkage may couple the bimetallic strip and the
securing
member. A pivot may also be coupled to the linkage member. Example 66. A
device
according to example 65, further including a pivot coupled to the linkage
member.
The linkage member may include a first portion proximate the bimetallic strip
relative
to the pivot and a second portion proximate the bimetallic strip relative to
the pivot in
35 which the first portion is shorter than the second portion.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
71
In yet another example, a medical device further includes a shape-memory
actuator configured to move between an initial shape below a transition
temperature
and a preset shape above the transition temperature to secure the attachment
member
in the initial shape and release the attachment member when moved to the
preset
shape. The push member may include a receiving portion. The shape memory
lo actuator may extend through the receiving portion in the initial shape to
secure the
push member to the attachment member and wherein the shape memory actuator is
drawn from engagement with the receiving portion when moved to the initial
state to
release the push member from attachment member. For example, the attachment
member has a recess defined therein and the shape memory actuator engages the
recess when in the initial state and is released from engagement with the
recess when
moved to the preset shape. Multiple shape memory actuators may engage the
receiving portion from opposing sides of the attachment member in the preset
shape.
Further, the device may include a cutting feature secured to the shape memory
actuator, the cutting feature being configured to cut a portion of the push
member
when the shape memory actuator is moved to the preset shape. For example,
cutting
feature may have an opening defined therein and at least a portion of the push
member may extend through the opening. Additionally, the device may include a
linkage and a securing member, the linkage coupling the shape memory actuator
to
the securing member. The securing member may couple the push member to the
attachment member when the shape memory actuator is in the initial shape and
release the push member from the attachment member when the shape memory
actuator is in the preset shape. A pivot may also be operatively associated
with the
linkage. In another example, a device includes a cylinder and piston secured
to the
implant, a linkage, and a securing member. The securing member may be
configured
to release the push member in response to operation of the cylinder and
piston, such
as by expansion of the cylinder and piston. A phase change material may be
expanded within an enclosed space of the cylinder to drive the piston. Phase
change
materials may include a hydro-carbon fluid as well as formulations of waxes
such as
those used in the thermostats of common automotive engine thermostats. In one
example, a cross hole is defined in the push member and a hole is defined in
the
attachment member. The device may further include a flexible filament
extending
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
72
through the cross hole through the hole and into a distal end of the push
member. The
device may include a cutting feature. The cutting feature may engage the
filament at
the cross hole to cut the filament.
A delivery device for delivering a closure device includes a handle body and a
pusher handle operatively associated with the handle body, the pusher handle
having a
1o guide slot defined therein. The guide slot may be configured to allow the
pusher
handle to move linearly with respect to the handle body a first linear
distance to
deploy a first portion of the closure device. The guide slot may be further
configured
to allow the pusher handle to move linearly with respect to the handle body
additional
linear distances to deploy additional portions of the closure device, such as
a second
linear distance to deploy a second portion of the closure device. The guide
slot may
be configured to allow the pusher handle to rotate a rotational distance
between the
first linear distance and the second linear distance. First and second grooves
may be
defined in the handle body while first and second stops may be associated with
the
first and second grooves. The first and second stops may be associated with
the guide
slot. The first and second stops are configured to move between initial
positions and
rotated positions to constrain the movement of pusher handle. For example,
rotating
the first stop to the rotated position allows the pusher handle to move from
the first
position to the second position and rotating the second stop to the rotated
position
after rotating the first stop to the rotated position allows the pusher handle
to move
from the second position to the third position. The linear distance from the
first
position to the second position may correspond to the first linear distance
and the
distance between the second position and the third position may correspond to
the
second linear distance. Accordingly, in one example the guide slot includes a
first
linear portion, a transverse portion transverse to the first linear portion
and in
communication with the first linear portion, and a second linear portion
substantially
parallel to the first linear portion, the second linear portion in
communication with the
transverse portion. The delivery device may also include a release assembly
configured to release the closure device from the delivery device. The release
assembly may include a release cap having a slot defined therein, the slot
having a
linear portion and a transverse portion transverse to the linear portion in
which the
linear portion extends proximally of the linear portion. The slot may also
include a
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
73
detent formed defined therein in communication with the transverse portion. In
one
example, a pin is coupled to the handle portion and is operatively associated
with the
pin. The delivery device may also include a drain lumen in fluid communication
with
the handle body.
A medical device has a multi-cellular structure being configured to be moved
from a collapsed state to an expanded state, the multi-cellular structure
including a
waist portion configured to engage a tunnel of an internal tissue opening in
the
expanded state to close the internal tissue opening, and in-growth media
having an in-
growth configuration secured to the medical device. The in-growth
configuration
may include at least one filament secured to a central portion of the medical
device, a
filament coiled around the central portion of the medial device, and/or
anchoring
points configured to have the in-growth media secured thereto. The anchoring
points
may allow the filament to be secured to the medical device by sewing, fixing
by
adhesive, and/or knots. In another example, points at which the in-growth
media is
attached to the closure device are maintained at a constant distance from each
other as
the closure device is deployed. The in-growth media also include strips of in-
growth
media. The strips may be formed of a loosely woven gauze-like material that is
woven into an interlocking structure. The strips may also be wrapped around a
cellular portion of the closure device. The in-growth media may also include a
membrane. The in-growth media may be located at least partially between the
closure
device and an adjacent closure device. The closure device and the adjacent
closure
device may be different or may be substantially similar.
In yet another configuration, a medical device has multiple chambers
configured to be inflated from a collapsed state to an expanded state, the
multiple
chambers having a waist portion configured to engage a tunnel of an internal
tissue
opening in the expanded state to close the internal tissue opening. The
multiple
chambers may a distal anchor portion and/or a proximal anchor portion. The
distal
anchor portion may be configured to be inflated first and the proximal anchor
portion
may be configured to be inflated subsequent to inflation of the proximal
anchor
portion, such as second. The multiple chambers may be interconnected, isolated
or a
mix of the two. The multiple chambers may also be formed of bioresorbable
materials.
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
74
In another example a method for detaching a tether from an implant within a
body lumen is provided that includes positioning an implant within a body
lumen, a
tether being coupled to the implant to aid with positioning the implant within
the body
lumen, and applying at least one of an electrical input or a thermal input to
the tether
to detach the tether from implant. Applying at least one of an electrical
input or a
lo thermal input may include applying electrical input to the tether to melt
the tether and
detach the tether from the implant, applying thermal input to a bimetallic
actuator to
remove a securing member from engagement with the tether and detach the tether
from the implant, applying thermal input to a shape memory actuator releasably
coupled to the implant to move a portion of the shape memory actuator relative
to the
implant to disengage from the tether and detach the tether from the implant,
applying
thermal input to a shape memory actuator, the shape memory actuator being
mounted
to a cutting structure that at least partially surrounds the tether, wherein
the thermal
input moves the shape memory actuator and the cutting structure to cut the
tether and
detach the tether from the implant, and/ or applying thermal input to a phase
change
2o assembly resulting detaching of the tether from the implant.
The present invention can also include the following methods, systems and
devices.
A medical device comprising: a body portion comprising two or more cells,
said body portion being movable between a deployed and non-deployed
orientation;
and at least one anchor linked to said body portion, said at least one anchor
being
adapted to reduce proximal movement of the medical device when the medical
device
is positioned in an internal tissue opening.
A medical device comprising: a multi-cellular structure adapted to selectively
expand and contract between a deployed and non-deployed orientation; a first
anchor
operatively associated with said multi-cellular structure, said first anchor
being
adapted to selectively engage at least a portion of a wall of an internal
tissue opening;
and a second anchor operatively associated with said multi-cellular structure,
said
second anchor being adapted to engage at least a portion of at least another
portion of
the wall of the tissue opening.
A method for closing a Patent Foramen Ovale, comprising the steps of:
positioning at least a portion of a medical device into a left atrium of a
heart, said
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 medical device comprising a first anchor, a multi-cellular structure linked
to said first
anchor, and a second anchor linked to said multi-cellular structure, said
first anchor,
said multi-cellular structure and said second anchor being adapted to
selectively move
between a non-deployed and deployed orientation; locating at least a portion
of said
first anchor against at least a portion of a left atrial wall of the heart;
and locating at
to least a portion of said second anchor against at least a portion of at
least one of a
tunnel of the Patent Foramen Ovale or a right atrial wall of the heart.
A medical device for approximating tissue of an internal tissue opening
together, the medical device comprising: a body portion comprising two or more
cells,
said body portion being adapted to apply lateral force to tissue of an
internal tissue
15 opening; and at least one anchor operatively associated with said body
portion.
A medical device for approximating tissue of an internal tissue opening
together, the medical device comprising: a multi-cellular structure adapted to
selectively expand and contract between a deployed and non-deployed
orientation,
said multi-cellular structure configured to preferentially expand; and at
least one
20 anchor operatively associated with said multi-cellular structure, said at
least one
anchor being adapted to move between a deployed and non-deployed orientation,
at
least a portion of said at least one anchor being adapted to apply lateral
force to at
least a portion of tissue of an internal tissue opening when said first anchor
is
deployed.
25 A method for reducing the size of an internal tissue opening, comprising
the
steps of: positioning at least a portion of a medical device through an
internal tissue
opening, said medical device comprising a multi-cellular structure and at
least a first
anchor associated with said multi-cellular structure, said at least one anchor
and said
multi-cellular structure being adapted to selectively move between a non-
deployed
30 and deployed orientation; and applying lateral force to tissue of the
internal tissue
opening by at least partially deploying said at least one anchor.
A medical device comprising: two or more cells forming a body portion, said
body portion being adapted to move between a collapsed and expanded
orientation to
apply lateral force to tissue of an internal tissue opening; and at least one
anchor
35 linked to said body portion, said at least one anchor being adapted to
extend distally
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
76
when said at least one anchor is collapsed and extend laterally when said at
least one
anchor is moved from a collapsed to an expanded orientation.
A method for deploying a closure device, the method comprising the steps o
deploying a left anchor of a closure device from a delivery device, said
delivery
device comprising an actuating assembly operatively associated with a handle
body,
said left anchor being adapted to deploy by linearly moving at least a portion
of said
actuating assembly with respect to said handle body; and deploying a second
anchor
of said closure device from said delivery device by rotating at least a
portion of said
actuating assembly with respect to said handle body.
A delivery device for an internal tissue opening closure device, the delivery
device comprising: a handle body including first and second guide members; a
first
member operatively associated with said handle body, at least a portion of
said first
member defining a guide, said first guide member cooperating with said guide
to
influence movement of said first member with respect to said handle body, said
first
member including a guide structure; and a second member operatively associated
with
said first member, at least a portion of said second member defining a second
guide,
said guide structure cooperating with said second guide to influence the
movement of
said second member with respect to said first member, and said second guide
member
cooperating with said second guide to influence the movement of said second
member
with respect to said handle body.
A delivery device for an internal tissue opening closure device, the delivery
device comprising: a handle body; a first pin coupled to said handle body; a
second
pin coupled to said handle body; a first cam adapted to be at least partially
received
into and movable with respect to at least a portion of said handle body, said
first cam
including a slot formed on an external surface of said first cam, said slot
including a
first portion and a second portion, said first portion of said slot extending
along at
least a portion of the length of said first cam, said second portion of said
slot
extending at least partially around said first cam, said first pin received in
said slot; a
third pin coupled to said first cam; and a second cam adapted to be at least
partially
received into and movable with respect to at least a portion of said first
cam, said
second cam including a first and second slot formed on an external surface of
said
second cam, said first slot of said second cam extending at least partially
around said
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
77
second cam and said second slot of said second cam extending along at least a
portion
of the length of said second cam, said third pin received in said first slot
of said
second cam and said second pin received in said second slot of said second
cam.
A medical device for closing an internal tissue opening, the medical device
comprising: a multi-cellular structure configured to assume a substantially
flat
configuration; at least one anchor operatively associated with said multi-
cellular
structure, said at least one anchor comprising a plurality of segments at
least partially
defining a closed periphery.
A medical device for closing an internal tissue opening, the medical device
comprising: a multi-cellular structure adapted to be moveable between a first
orientation and a second orientation; at least one anchor operably associated
with said
multi-cellular structure; and a tissue growth member associated with said
multi-
cellular structure, said tissue growth member being adapted to enhance tissue
growth
in the internal tissue opening.
An expandable medical device deployable at least partially within a tissue
structure, the expandable medical device comprising: a non-tubular multi-
cellular
body portion configured to self expand from a non-deployed orientation, said
body
portion comprising a plurality of interconnecting body support segments
defining at
least two apertures; and at least one anchor linked to said multi-cellular
body portion.
An expandable medical device deployable at least partially within a tissue
structure, the expandable medical device comprising: a frame configured to
assume a
substantially flat configuration, said frame comprising a central portion
adapted to
move between a first orientation and a second orientation, said central
portion
comprising a plurality of struts defining a multi-cellular structure.
A medical device for reducing a size of a Patent Foramen Ovale ("PFO"), the
medical device comprising: a self-expanding frame configured to be constricted
within a catheter and configured to assume a substantially flat configuration,
said
frame comprising a central portion with proximal and distal anchors extending
from
said central portion, said central portion comprising a multi-cellular
structure
configured to self-expand outwardly against a wall of the PFO.
A medical device for reducing the size of an internal tissue opening, the
medical device comprising: a frame including a central portion having a
plurality of
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
78
struts defining a multi-cellular structure, said central portion having at
least one
anchor extending from said central portion, said central portion configured to
assume
a substantially flat configuration; and
a member associated with said frame, said member adapted to induce tissue
growth in
the internal tissue opening.
A medical device for closing an internal tissue opening, the medical device
comprising: a frame including a central portion with at least one anchor
extending
from said central portion, said central portion configured to assume a
substantially flat
configuration; and a tissue growth promoting member attached to said frame,
said
tissue growth promoting member configured to substantially extend out-of-plane
from
the substantially flat configuration and configured to enhance tissue growth
in the
internal tissue opening.
A medical implant delivery system for delivering a medical device in a human
body, the delivery system comprising: a handle; a catheter coupled to the
handle with
lines coupled to the medical device; and a tip portion coupled to a distal end
of the
catheter, the tip portion defining at least a first passageway and a second
passageway
extending at least partially along a length through the tip portion, the first
passageway
configured to move over a guide wire and the second passageway configured to
communicate with the catheter and configured to facilitate delivery of the
medical
device, the first passageway and the second passageway being in a spaced apart
arrangement.
A delivery device configured to be coupled to a catheter for delivering a
medical device in a human body, the delivery device comprising: a tip member
configured to be coupled at a distal end of the catheter, the tip member
defining at
least a first passageway and a second passageway extending at least partially
along a
length through the tip member, the first passageway configured to move over a
guide
wire and the second passageway configured to communicate with the catheter and
configured to facilitate delivery of the medical device, the first passageway
and the
second passageway being in a spaced apart arrangement.
A medical implant delivery system for delivering a medical device in a human
body, the delivery system comprising: a handle; a catheter coupled to the
handle with
lines coupled to the medical device; and a tip portion coupled to a distal end
of the
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
79
catheter, the tip portion defining at least a first passageway and a second
passageway
extending at least partially along a length through the tip portion, the first
passageway
configured to move over a guide wire and the second passageway configured to
communicate with the catheter and configured to deliver the medical device,
the
second passageway and the first passageway being in a non-coaxial arrangement.
A delivery device for delivering a medical device, the delivery device
comprising: a handle body; and an actuating assembly operatively associated
with
said handle body, said actuating assembly being adapted to move linearly with
respect
to said handle body to deploy at least a portion of a medical device, and to
rotate with
respect to said handle body to deploy additional portions of the medical
device.
A delivery device for delivering a Patent Foramen Ovale closure device, the
delivery device comprising: a handle body; a first member operatively
associated with
said handle body, said first member being adapted to move linearly with
respect to
said handle body; and a second member linked to said handle body and said
first
member, said second member being adapted to move linearly with respect to said
2o handle body and adapted to rotate with respect to said handle body and said
first
member.
A method for deploying an internal tissue opening closure device, the method
comprising the steps of: deploying a left anchor of a closure device from a
delivery
device, said delivery device comprising an actuating assembly linked to a
handle
body, said left anchor being adapted to deploy by a first movement of at least
a
portion of said actuating assembly with respect to said handle body; and
deploying a
right anchor of said closure device from said delivery device by a second
movement
of at least a portion of said actuating assembly with respect to said handle
body.
A method for closing an internal tissue opening, wherein the internal tissue
opening includes first and second opposing tissue walls and a tunnel
therethrough
defining the internal tissue opening, the method comprising the steps of:
deploying a
first anchor of a closure device from a delivery device by moving at least a
portion of
an actuating assembly of said delivery device in a linear direction with
respect to a
handle body of said delivery device; positioning said first anchor against a
first tissue
wall of an internal tissue opening; and deploying a second anchor of said
closure
device from said delivery device to engage at least a portion of at least one
of a tunnel
CA 02659365 2009-01-28
WO 2008/021969 PCT/US2007/075608
5 or a second tissue wall of the internal tissue opening by rotating at least
a portion of
said actuating assembly.
A method for closing a Patent Foramen Ovale, the method comprising the
steps of: translating at least a portion of an actuating assembly of a
delivery device
with respect to a handle body of said delivery device to deploy at least a
first portion
10 of a closure device from said delivery device, said closure device
comprising a multi-
cellular structure linked to said first portion and a second portion linked to
said multi-
cellular structure; and rotating at least a portion of said actuating assembly
to deploy
said second portion of said closure device from said delivery device.
A medical system for treating an internal tissue opening, the system
15 comprising: a medical device comprising: a multi-cellular structure, and at
least one
anchor operatively associated with said multi-cellular structure; and a
delivery device
comprising: a handle body, and an actuating assembly operatively associated
with
said handle body, said actuating assembly being adapted to selectively deploy
at least
a first portion of said closure device by a first movement, and said actuating
assembly
2o being adapted to selectively deploy at least a second portion of said
closure device by
a second movement.
A medical system for treating a tissue structure, the system comprising: a
medical device comprising a frame configured to assume a substantially flat
configuration, said frame including a central portion and at least one anchor
extending
25 from the central portion, the central portion including a plurality of
struts defining a
multi-cellular structure; and a delivery device comprising: a handle body, and
an
actuating assembly, said actuating assembly adapted to enable deployment of
said at
least one anchor by movement of at least a portion of said actuating assembly.
The present invention may be embodied in other specific forms without
30 departing from its spirit or essential characteristics. The described
embodiments are
to be considered in all respects only as illustrative and not restrictive. The
scope of
the invention is, therefore, indicated by the appended claims rather than by
the
foregoing description. All changes which come within the meaning and range of
equivalency of the claims are to be embraced within their scope.