Note: Descriptions are shown in the official language in which they were submitted.
CA 02790763 2012-09-21
APTAMER BIOCONJUGATE DRUG DELIVERY DEVICE
CROSS-REFERENCES TO RELATED APPLICATIONS
[0001] This patent claims the benefit of US provisional patent application
numbers
61/653,636 filed on May 31, 2012 and 61/656,313 filed on June 6, 2012 which
are
incorporated by reference.
FIELD
[0002] This specification relates to a delivery device for drugs or other
agents, to
methods of making and using the delivery device, and to the treatment of
cancer.
BACKGROUND
[0003] The following discussion is not an admission that anything
described below is
common general knowledge.
[0004] US Patent Number 6,340,527 to Van Soest et al. describes
microparticles
having a particle size of 50 nm to 1 mm consisting of a chemically crosslinked
starch shell
containing an active ingredient. The particles are obtained by first preparing
an oil in water
emulsion of the active ingredient in a hydrophobic phase and starch, or a
dispersion of a
solid active ingredient and starch in water. The active ingredient may be a
medicament
which is released in the digestive tract when the starch degrades.
[0005] US Patent Application Publication US 2008/0241257 to Popescu et
al.
describes a nanoparticle of a biodegradable polymer containing a hydrophilic
cationic drug
such as streptomycin. The biodegradable polymer may be chitosan. A
pharmaceutical
preparation containing the nanoparticles is administered to a patient orally
and the
nanoparticles release the drug in vivo. The drug can be complexed with a
naturally occurring
polymer, such as dextran sulfate. The drug, optionally complexed, is mixed
with the
biodegradable polymer followed by an inorganic polyanion to form the
nanoparticle. In one
example, the nanoparticles were about 560 nm in average size, had a zeta
potential of about
+54 mV and were used to treat tuberculosis in mice.
[0006] US Patent Number 7,550,441 to Farokhzad et al. describes a
conjugate that
includes a nucleic acid ligand bound to a controlled release polymer system
contained within
a pharmaceutical compound. Some examples of the polymer system are based on
poly(lactic) acid (PLA) and have mean particle sizes ranging from 137 to 2805
nm. The
1
CA 02790763 2012-09-21
ligands have an affinity for a target and are prepared through the Systemic
Evolution of
Ligands by Exponential Enrichment (SELEX) process.
[0007] US Patent Publication 2009/0312402 to Contag et al. describes
nanoparticles
with encapsulated nucleic acid. The polymer may be PLA, PLG or PLGA and PEG.
The
particles may have ligands or antibodies attached to them for targeting the
nanoparticles to a
site of interest. The nanoparticles may have a polymer coating to provide
controlled release.
The particles are in the size range of about 50 nm to about 500 nm, with most
of them in the
sub-200 nm range.
[0008] US Patent Publication 2011/0244048 to Amiji et al. describes a
method of
making a nanoparticle comprising combining an aqueous solution of a
solubilized therapeutic
agent with a water-soluble polymer comprising polyethylene glycol (PEG) and a
fatty acid.
These components self assemble into a nanoparticle. Various dextran based
particles have
means sizes ranging from 14 nm to 430 nm. The therapeutic agent may be
doxorubicin.
[0009] US Patent 8,048,453 to Sung et al. describes nanoparticles of
chitosan, poly-
glutamic acid, and an active agent. The particles have a mean particle size
between about
50 nm and 400 nm. The active agent may be insulin for the treatment of
diabetes or an
active for treating Alzheimer's disease. The nanoparticles may be freeze-dried
and loaded
into a capsule for oral administration.
INTRODUCTION TO THE INVENTION
[0010] The following introduction is intended to introduce the reader to
the invention
and the detailed description to follow and not to limit or define the claims.
[0011] This specification describes a nanoparticle based delivery device.
The device
may be used for the treatment of various indications or for other purposes.
However, this
specification will primarily describe the use of the device to deliver
chemotherapeutic drugs,
for example, for the treatment of cancer.
[0012] The delivery device described in this specification includes a
nanoparticle that
is made predominantly from a biopolymer, for example a starch comprising
annylose,
amylopectin or both. The biopolymer may have its crystal structure broken, for
example by
shear forces and intensive mixing in the presence of a hydroxilic solvent, or
by other
methods. After the crystal structures have been broken, a crosslinking agent
is added to
stabilize the resulting biopolymer nanoparticles. The resulting nanoparticles
comprise, for
2
CA 02790763 2012-09-21
example, crosslinked high molecular weight starch polymer that can be handled
as dry
agglomerated particles. The dry particles can be dispersed in an aqueous
medium to
produce a stable latex dispersion of crosslinked hydrogel nanoparticles.
[0013] The inventors believe that these crosslinked biopolymer
nanoparticles have
attributes that make them useful as a drug delivery device. In an aqueous
medium, the
crosslinked biopolymer nanoparticles form a stable dispersion of swollen
crosslinked
biopolymer hydro-colloid particles. The crosslinked biopolymer nanoparticles
swell by taking
water into the core of the particle. This mechanism may be used to load an
active agent, into
the core of the crosslinked biopolymer nanoparticles. Loading of the active
agent into the
core of the crosslinked biopolymer nanoparticles also allows unloading, or
release, of the
drug, for example at, near or within target cells. In one example, the active
agent is a
chemotherapy drug that is released at, near or within cancer cells.
Optionally, the
crosslinked biopolymer nanoparticles that are loaded with the drug can be
administered as a
liquid suspension or dried to produce a powder.
[0014] One useful attribute of the crosslinked biopolymer nanoparticles
is that they
can be broken down by chemical and enzymatic elements, but they may persist in
the body
long enough to provide a sustained release of the active agent. While native
starch particles
would typically survive for less than 30 minutes in the body, starch-based
crosslinked
biopolymer nanoparticles have a considerably longer half-life. In a related
attribute, the
nanoparticles may provide two mechanisms for releasing a loaded active agent.
According
to a first mechanism, the active agent is released from a generally intact
crosslinked
biopolymer nanoparticle. According to a second mechanism, the crosslinked
biopolymer
nanoparticle can degrade and release more of the drug. This second mechanism
provides a
sustained release of the drug, which is useful for active agents that require
several hours or
more of residence time for an optimal effect.
[0015] Another attribute of the crosslinked biopolymer nanoparticles is
that the
biopolymers are compatible with the body and ultimately can be reabsorbed. The
biopolymers and their metabolites are nontoxic. In contrast, some synthetic
polymers can
cause side effects when used as a drug delivery device. For example,
polyanhydride
copolymers used for drug delivery have been associated with tissue
inflammation and an
enhanced rate of infections. These side effects may be due to the synthetic
copolymers
degrading via hydrolysis and yielding acidic functionalities. Starch, however,
is ordinarily a
3
CA 02790763 2012-09-21
food source and can be taken up by the body and degraded essentially without
complications.
[0016]
Another useful attribute of the crosslinked biopolymer nanoparticles is their
size, and the narrow particle size distribution range within a given sample.
In particular, the
crosslinked biopolymer nanoparticles are predominantly in the range of 50-150
nm. Particles
outside of this size range may be removed from the body through passive
processes, such
as through capillary wall passage, or more active processes, such as by the
reticuloendothelial system (RES).
[0017] Yet
another useful attribute of the nanoparticles is that the biopolymers may
be functionalized. For example, amylose and amylopectin molecules may be
oxidized and
provided with carboxyl functionalities. In
this example, the functionalized, crosslinked
biopolymer nanoparticles have a more negative zeta potential which aids in the
loading of
some active agents. Optionally, the functionalizing reactions may allow
attaching of targeting
molecules, such as antibodies or ligands, to the crosslinked biopolymer
nanoparticles. For
example, the targeting molecule may be an aptamer that attaches, for example
via a
carbodiimide linkage, directly to the surface of a crosslinked biopolymer
nanoparticle. The
selection of specific aptamers, for example nucleotide or peptide aptamers,
may direct, or
facilitate, interactions between the crosslinked biopolymer nanoparticles and
target cells.
Other forms of functionalization may influence the release profile of the
active agent.
[0018] The
inventors have further observed that the degree of crosslinking of the
starch nanoparticle influences the release profile of the active agent from a
crosslinked
biopolymer nanoparticles drug delivery system. In one example, a drug delivery
system
comprises biopolymer nanoparticles that are crosslinked. The crosslinked
biopolymer
nanoparticles may be functionalized to facilitate loading of an active agent
and to attach a
targeting molecule, such as an aptamer. The conjugated crosslinked biopolymer
nanoparticles are loaded with an active agent. In this example, the size of
the crosslinked
starch nanoparticle may provide a longer systemic viability. The longer
systemic viability
may increase the likelihood of an interaction between the targeting molecule
and a target
cell. Upon a successful interaction, the drug delivery system may cross the
phospholipid
bilayer and enter the target cell, through receptor-mediated transport or
otherwise. The
degree of crosslinking of the biopolymer nanoparticles may provide a desired
release profile
of the loaded drug into the target cell. Optionally, the amount of attached
targeting molecule
may be varied to increase or decrease the rate of target cell uptake of the
drug delivery
4
CA 02790763 2012-09-21
system. Further optionally, the crosslinked biopolymer nanoparticle may be
used without a
targeting molecule.
[0019] The inventors have further observed that the amount of targeting
molecule
that is attached to the crosslinked biopolymer nanoparticle influences the
uptake by the
target cell.
[0020] The drug delivery system provides the ability to specifically
tailor the targeting
molecule to a specific target cell, for example a specific type of cancer
cell. The rate of
uptake of the drug delivery system can also be tailored based upon the amount
of the
targeting molecule that is attached. The drug delivery system provides the
ability to tailor the
active agent that is delivered directly into the target cell, for example an
anti-cancer,
chemotherapy drug. The drug delivery system further provides the ability to
tailor the release
profile of a tailored drug to optimize the effect of the active agent, for
example, by varying the
degree of crosslinking to prolong or shorten the release profile.
[0021] An example drug delivery device may have: 1) a nanoparticle
comprising
crosslinked biocompatible or resorbable polymers, the polymers modified after
the particle
was formed by chemical or enzymatic modification, 2) an encapsulated
therapeutically active
agent within the colloidal hydrogel, and, optionally, 3) an aptamer attached
to the crosslinked
polymers. The nanoparticles may be colloidal hydrogel starch particles.
[0022] A medicament described in this specification comprises a plurality
of
crosslinked nanoparticles, the nanoparticles are made up mostly of high
molecular weight
starch with an active agent conjugated to at least some of the nanoparticles.
Optionally, the
nanoparticles may include a targeting molecule. The medicament may be useful
in the
treatment of cancer. A method of making a medicament comprises the steps of
forming a
plurality of high molecular weight, starch-based nanoparticles, wherein the
nanoparticles
having a size predominantly in the range of 50 to 150 nm and are crosslinked
to a degree
selected to provide a desired active agent release profile and loading an
active agent within
the nanoparticles. Optionally, the nanoparticles can be functionalized to
increase loading of
the active agent or attach a targeting molecule or both.
[0023] A compound described in this specification comprises a high
molecular weight
starch based nanoparticle core having a size in the range of 50 to 150 nm, a
drug and,
optionally, an aptamer targeting molecule. The compound may be used for the
treatment of
cancer.
CA 02790763 2012-09-21
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] Figure 1 is a chart showing a target particle size relative to
particle removal
mechanisms.
[0025] Figure 2A shows an electron microscopy image of native corn starch
granules.
[0026] Figure 2B shows an electron microscopy image of example
crosslinked
biopolymer nanoparticles, referred to as EcoSphere 2202.
[0027] Figure 3 shows an analysis of particle size for an aqueous
dispersion of the
crosslinked biopolymer nanoparticles of Figure 2 by Dynamic Laser Light
Scattering (DLS).
[0028] Figure 4 shows an analysis of particle size for an aqueous
dispersion of the
crosslinked starch nanoparticles of Figure 2 by Nanoparticle Tracking Analysis
(NTA).
[0029] Figure 5 is a schematic graph comparing particle sizes for the
crosslinked
biopolymer nanoparticles of Figure 2, synthetic polymer colloids and native
corn starch.
[0030] Figure 6 is a schematic representation of minor swelling in an SB
latex particle
and significant swelling of the crosslinked biopolymer nanoparticles of Figure
2 in an
aqueous dispersion, illustrating the hydrocolloid structure of the starch
based nanoparticles.
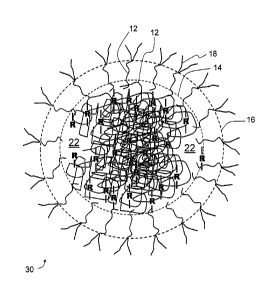
[0031] Figure 7 is a schematic model of an example crosslinked biopolymer
nanoparticle.
[0032] Figure 8 is a chart showing the fluorescence spectrum of free
doxorubicin and
doxorubicin entrapped in the example crosslinked biopolymer nanoparticles of
Figure 2B.
[0033] Figure 9 is a chart showing a release profile of doxorubicin from
the example
crosslinked starch nanoparticles of Figure 2B.
[0034] Figure 10 is a chart showing the fluorescence spectrum of Calcein
and a
release profile of Calcein from the crosslinked biopolymer nanoparticles of
Figure 2B.
[0035] Figure 11 is a schematic model of a crosslinked biopolymer
nanoparticle of
Figure 2 conjugated with a drug and an aptamer.
[0036] Figure 12 is a chart showing the release profile of doxorubicin
from biopolymer
nanoparticles with different degrees of crosslinking.
[0037] Figure 13 is a chart that shows the uptake of crosslinked
biopolymer
nanoparticles with varying levels of attached aptamer, where the captions mean
as follows:
"Free aptamer" is the fluorescence result for cellular binding or uptake of
the unconjugated
AS1411 aptamer; "100:1", "500:1", "1000:1" and "5000:1" is the fluorescence
result for
cellular binding or uptake of crosslinked bioconjugates formulated with
relative ratios of 100,
500, 1000, and 5000 parts of glucose repeating units in the biopolymer to one
part AS1411
6
CA 02790763 2012-09-21
aptamer; and "Control 500:1" is the fluorescence result for cellular binding
or uptake of a
bioconjugate formulated with 500 parts of glucose repeating units in the
biopolymer to one
part of a control aptamer sequence which is untargeted to cancer cells.
[0038] Figures 14A and 14B are charts that show the viability of cells
treated with
crosslinked biopolymer nanoparticles with and without a loaded active agent.
DETAILED DESCRIPTION
Target Particle Size
[0039] Referring to Figure 1, particle size plays a role in determining
the fate of a
drug or a drug delivery mechanism after administration. Without intending to
be bound by
any particular theory of operation, particles having a size in the range of
about 50 to 150 nm
may enjoy longer systemic circulation as a result of being within this size
range, independent
of other properties of the particle such as surface density or hydrophilicity
which may also
affect uptake by the reticuloendothelial system (RES).
[0040] The term biopolymer nanoparticle will be used in this
specification to refer to a
form of a biopolymer in which the native structure of the biopolymer source
material has
been substantially removed but multiple molecules of the bio-polymer are
complexed to form
discrete particles, for example by way of cross-links between molecules within
the particles.
Crosslinked biopolymer nanoparticles 10 can be made by various processes.
[0041] The presence of biopolymer nanoparticles can be determined by
observation
under a scanning electron microscope (SEM); detecting particle sizes larger
than individual
molecules by DLS or NTA measurements; or, observing a maximum swelling value
(alternatively called a volume factor or swell ratio) in a very dilute
dispersion of the
biopolymer nanoparticles that is less than the swell ratio of the native or
dissolved form of the
biopolymer. Regarding the last technique, the swell ratio of native starch
granules is about
32 and the swell ratio of cooked (dissolved) starch is about 44. In
comparison, the swell ratio
of starch nanoparticles may be between about 2 and 20 with lower swell ratios
corresponding
to more tightly cross-linked particles. A method of determining swell ratio is
described in the
examples section herein and in International Application No. PCT/CA2012/050375
which is
incorporated herein by this reference to it. Biopolymer nanoparticles useful
as a drug
delivery device may have a swell ration between about 2 and 20, between about
6 and 18 or
between about 6 and 16.
7
CA 02790763 2012-09-21
[0042] Waxy corn starch is a preferred bio-based material due to its
resistance to
retrograding after it has been processed relative to other starches. Waxy corn
starch also
produces nanoparticles with less cross-linker or without added cross-linker.
[0043] In one example, the biopolymer nanoparticles 10 are made according
to a
process described in US Patent Number 6,677,386 (which corresponds to
International
Publication WO 00/69916), which is incorporated herein by reference. In this
example
process, a biopolymer feed stock, such as starch comprising amylose or
amylopectin or both,
is combined with a plasticizer. This combination is mixed under high shear
forces, preferably
in a twin screw fully intermeshing co-rotating extruder, to plasticize the
biopolymer and create
a thermoplastic melt phase in which the crystalline structure of the
biopolymer is removed. A
crosslinking agent is then added, while mixing continues, to form the
crosslinked biopolymer
nanoparticles 10. The crosslinked biopolymer nanoparticles 10 exit the
extruder as a strand
of extrudate, which is ground to a fine dry powder. The crosslinked biopolymer
nanoparticles
are present in the powder in an agglomerated form, and can be dispersed in an
aqueous
medium. One example of crosslinked biopolymer nanoparticles 10 made by this
process is
the commercially available EcoSphere 2202 from EcoSynthetix Inc. of
Burlington, Ontario,
Canada.
[0044] The biopolymer feed stock may be starch or other polysaccharides
such as
cellulose and gums, as well as proteins (e.g. gelatin, whey protein). The
biopolymers may
also be previously modified, e.g. with cationic groups, carboxy-methyl groups,
by acylation,
phosphorylation, hydroxyalkylation, oxidation and the like. Starch and
mixtures of at least
50% starch with other polymers are preferred. The starch, whether used alone
or in a
mixture, is preferably a high molecular weight starch, for example a molecular
weight of at
least 10,000, and not dextran or dextrin. For example, the starch can contain
amylose,
amylopectin, or both. Waxy starches, such as waxy cornstarch, are particularly
preferred.
[0045] The following paragraphs are repeated or summarized from US Patent
Number
6,677,386 to further describe a process of making the nanoparticles.
[0046] The biopolymer preferably has a dry substance content of at least 50%
by weight at
the time when processing starts. Processing is preferably done at a
temperature of at least
40 degrees C, but below the degradation temperature of the polymer, for
example 200
degrees C. The shear can be affected by applying at least 100 J of specific
mechanical
energy (SME) per g of biopolymer. Depending on the processing apparatus used
the
8
CA 02790763 2012-09-21
minimum energy may be higher; also when non-pregelatinised material is used,
the minimum
SME may be higher, e.g. at least 250 J/g, especially at least 500 J/g.
[0047] The plasticiser may water or a polyol (ethyleneglycol, propyleneglycol,
polyglycols,
glycerol, sugar alcohols, urea, citric acid esters, etc.). The total amount of
plasticisers (i.e.
water and others such as glycerol) is preferably between 15 and 50%. A
lubricant, such as
lecithin, other phospholipids or monoglycerides, may also be present, e.g. at
a level of 0.5-
2.5% by weight. An acid, preferably a solid or semi-solid organic acid, such
as maleic acid,
citric acid, oxalic, lactic, gluconic acid, or a carbohydrate-degrading
enzyme, such as
amylase, may be present at a level of 0.01-5% by weight of biopolymer. The
acid or enzyme
assists in slight depolymerisation, which is assumed to be advantageous in the
process of
producing nanoparticles.
[0048] The
crosslinking is preferably at least in part reversible, i.e. the crosslinks
are
partly or wholly cleaved during the mechanical treatment step. Examples of
reversible
crosslinkers are a) dialdehydes and polyaldehydes, which form more stable full
acetals and
reversibly form hemiacetals, and b) anhydrides and mixed anhydrides, which
form ester
linkages (e.g. succinic and acetic anhydride) and the like. Suitable
dialdehydes and
polyaldehydes are glutaraldehyde, glyoxal, periodate-oxidised carbohydrates,
and the like.
[0049]
Such crosslinkers may be used alone or as a mixture of reversible
crosslinkers, or as a mixture of reversible and non-reversible crosslinkers.
Thus,
conventional crosslinkers such as epichlorohydrin and other epoxides,
triphosphates, divinyl
sulphone, can be used as non-reversible crosslinkers for polysaccharide
biopolymers, while
dialdehydes, thiol reagents and the like may be used for proteinaceous
biopolymers. The
crosslinking reaction may be acid- or base-catalyzed. The level of
crosslinking agent can
conveniently be between 0.1 and 10 weight % with respect to the biopolymer.
The
crosslinking agent may be present at the start of the mechanical treatment,
but in case of a
non-pre-gelatinised biopolymer such as a starch with native starch granules,
it is preferred
that the crosslinking agent is added later on, i.e. during the mechanical
treatment.
[0050] The
mechanically treated, crosslinked biopolymer is then formed into a latex
by dispersion in a suitable medium, usually water and/or another hydroxylic
solvent such as
an alcohol), to a concentration of between 4 and 50 weight % especially
between 10 and 40
wt. %. Prior to the dispersion a cryogenic grinding step may be performed, but
stirring with
mild heating may work equally well.
This treatment results in a gel which either
spontaneously or after induction by water adsorption, is broken into a latex.
This viscosity
9
CA 02790763 2012-09-21
behavior can be utilised for applications of the particles, such as improved
mixing, etc. If
desired, the dispersed biopolymer may be further crosslinked, using the same
or other
crosslinking agents as describe above. The extrudate is characterised by
swelling in an
aqueous solvent, e.g. water or a mixture of at least 50% water with a water-
miscible solvent
such as an alcohol, and by exhibiting a viscosity drop afterwards to produce a
dispersion of
nanoparticles.
[0051] International Patent Application Publication No. WO 2008/022127 A2
and its
equivalent US Patent Application Publication Number 2011/0042841 Al describe a
process
for producing biopolymer nanoparticles in large quantities. US Patent
Application Publication
Number 2010/0143738 Al describes a process for producing biopolymer
nanoparticles
conjugative with additives during the extrusion process. US Patent Application
Publication
Numbers 2010/0143738 Al describes a process for producing biopolymer
nanoparticles
conjugated with additives during the extrusion process. These publications are
incorporated
herein by reference.
[0052] The production of biopolymer nanoparticles similarly formed by
reactive
extrusion and comprising starch essentially without crystalline structures is
described in
Starch nanoparticle formation via reactive extrusion and related mechanism
study, Delong
Song et al., Carbohydrate Polymers 85 (2011) 208-214. The contents of this
publication are
incorporated herein by reference. This publication is incorporated herein by
reference.
Using various materials and reaction conditions, dispersions having particles
with number
average particle sizes up to about 2000 nm were produced. Various other
methods of
making biopolymer nanoparticles are also summarized in this paper.
[0053] Another method reported to produce biopolymer nanoparticles by
reactive
extrusion process from waxy corn starch is described in International
Publication Number
WO 2011/071742 A2, Process for Preparing Stable Starch Dispersions, by Welsch
et al.,
published on June 16, 2011. This publication is incorporated herein by
reference. This
process comprises introducing a feed starch and an hydroxylc liquid to an
extruder. Shear
forces are applied in the extruder to the starch and the liquid in the
substantial absence of a
crosslinker under conditions sufficient to prepare a stable dispersion of
starch particles in the
hydroxylic liquid.
[0054] Another method reported to produce biopolymer nanoparticles is
described in
International Publication Number WO 2011/155979 A2, Process for Preparing
Stable
Dispersions of Starch Particles, by Welsch et al., published on December 15,
2011. The
CA 02790763 2012-09-21
contents of this publication are incorporated herein by reference. In this
process, a feed
starch and an aqueous liquid are introduced into a rotor stator mixer. The
feed starch and
aqueous liquid are maintained in the rotor stator mixer at a temperature
ranging from a
gelation temperature to less than a solubilization temperature. The feed
starch is sheared
into starch particles with the rotor stator mixer to form the dispersion of
starch particles in the
aqueous liquid.
[0055] Another method of producing a starch nanoparticle is described in
U.S.
6,755,915 to Van Soest et al. (June 29, 2004) which teaches a method of
preparing starch
particles with a size range of 50 nanometers to 100 microns. The disclosure of
this patent
document is incorporated herein by reference. The method includes the steps
of: dispersing
starch in a first water phase; dispersing a second hydrophobic phase in the
first phase to
form an oil-in-water emulsion; inverting the oil-in-water emulsion to a water-
in-oil emulsion;
crosslinking the starch in the first phase; and separating the formed starch
particles. The
phase inversion can occur by including a surfactant that stabilizes a water-in-
oil emulsion or
the surfactant can be temperature sensitive and increasing the reaction
temperature. The
inversion can also occur by the addition of further hydrophobic liquids or
various suitable
salts. In this process the starch molecules can remain partially granular
during both the
crosslinking reaction and complete gelatinisation of the granular starch can
be effected
before, during or after the phase inversion. Gelatinization occurs by
increased temperature,
salts or combinations thereof.
[0056] Another method reported to produce biopolymer nanoparticles is
described in
WO 2010/084088 to Santander Ortegea et al. (international publication July 29,
2010). The
contents of this publication are incorporated herein by reference. The method
includes the
steps of preparing starch derivatives by a first disintegration step, with
solvent and increased
temperatures, followed by common substitution methods, such as esterification,
etherification. The starch derivatives are added to an organic solvent and an
oil/water
emulsion is prepared with a high shear mixer. Sonication may be used to
improve the oil
droplet distribution. The organic phase is then removed through a membrane,
which results
in an aqueous dispersion of starch-based nanoparticles.
[0057] Another method of making biopolymer nanoparticles is described in
WO
2010/065750 to Bloembergen et al. which teaches that Brabender static high
shear mixers
and Sigma Blade mixers may be used in place of an extruder to produce
nanoparticles by
way of shearing starch granules in the presence of a crosslinker. The contents
of this
11
CA 02790763 2012-09-21
publication are incorporated herein by reference.
[0058] Alternatively, fragmented particles may be used. British patent GB
1420392, for
example, describes a method of producing fragmented starch particles that are
partially
cross-linked and partially crystalline or soluble that may be used as an
alternative to
nanoparticles. Nanoparticles are preferred, however, since they are likely to
be less prone to
retrogradation.
[0059] The process can be operated to produce particles that have a
number
average particle size in the range of 50 to 150 nm and which, considering a
distribution of
their particle sizes, are also predominantly in the range of 50 to 150 nm in
size. Such
particles include, for example, EcoSphere 2202 particles commercially
available from
Ecosynthetix Inc. of Burlington, Ontario, Canada and EcoSynthetix Ltd. of
Lansing, Michigan,
USA. These products are made primarily from starch including amylose and
amylopectin.
The product is normally sold to replace petroleum based latex binders in
industrial
applications, such as coated paper and paperboard. The product is provided in
the form of a
dry powder of agglomerated nanoparticles with a volume mean diameter of about
300
microns. When mixed in water and stirred, the agglomerates break apart and
form a stable
dispersion of the nanoparticles.
[0060] Comparing Figure 2A to Figure 2B, the EcoSphere 2202, as an
example of
crosslinked biopolymer nanoparticles 10, are about 100 to 300 times smaller
than native
starch granules 20. Whereas a starch granule 20 may be 15 microns in size, the
nanoparticles 10 are clearly well under 200 nm in size. Accordingly, the
effective surface
area of the nanoparticles 10 is much greater, for example 200 m2/g or more.
[0061] Figures 3 and 4 illustrate particle size measurements of an
aqueous
dispersion of the EcoSphere 2202, as an example of crosslinked biopolymer
nanoparticles
10, by Dynamic Laser Light Scattering (DLS) and by Nanoparticle Tracking
Analysis (NTA),
respectively. These two techniques are complementary, given that the NTA
technique is a
direct measurement of the diffusion coefficient for individual particles
tracked via video
tracking software (and relates that to particle diameter via the Stokes-
Einstein equation), and
can measure particles in the range of 50-1000 nm, while DLS can measure to
smaller
particle sizes below 50 nm. Other techniques, including oscillating probe
Atomic Force
Microscopy (AFM), Scanning Electron Microscopy (SEM), Environmental SEM
(ESEM),
Transmission Electron Microscopy (TEM) and Scanning/Transmission Electron
Microscopy
(STEM), all provided similar particle size images consistent with the data in
Figures 3 and 4.
12
CA 02790763 2012-09-21
[0062]
Referring to Figure 3, most of the EcoSphere 2202 particles have a size in
the range of about 50 to 100 nm. As indicated in the NTA measurements, most of
the
particles (D50) are under 120 nm in size and there are virtually no particles
larger than 400
nm. Any particles larger than 1000 nm would be removed quickly from the body
causing no
harm but wasting some of an intended dosage of the drug. Accordingly, if a
sample includes
material amounts of particles over 1000 nm in size, these may be removed by
filtration, or
otherwise, before an active agent 22 is loaded into the nanoparticles.
[0063]
Referring to Figure 5, the crosslinked biopolymer nanoparticles 10 are
generally smaller than particles found in synthetic latex emulsions such as
styrene-butadiene
(SB) emulsions, acrylic emulsions and polyvinyl acetate (PVAc) emulsions. The
crosslinked
biopolymer nanoparticles 10 have a narrow size-distribution, with a
polydispersity index of
about 30%, and properties characteristic of polymer colloids.
Since the crosslinked
biopolymer nanoparticles 10 are predominantly in the size range of about 50 to
150 nm (for
example 50% or more of the nanoparticles by number or mass may be in this
range) the
crosslinked biopolymer nanoparticles 10 may be cleared more slowly from the
systemic
circulation (liver, spleen) than is the case of larger particles. The
crosslinked biopolymer
nanoparticles 10 may have hydrophilic properties, which may further inhibit
removal by the
RES. The degradation products of the starch nanoparticles (D-glucose and
maltodextrans)
are non-toxic. The additional natural materials and chemicals that are used to
make the
starch crosslinked biopolymer nanoparticles are also relatively non-toxic.
[0064] The
crosslinked biopolymer nanoparticles 10 are not water soluble, but
instead form a stable latex dispersion of swollen hydrogel colloidal
crosslinked particles in
water.
[0065]
Figures 6A and 6B depict the latex dispersion consisting of water-swollen
crosslinked biopolymer nanoparticles 10, which can de-swell with increasing
solids. This
permits dispersions that can be made at higher solids. In contrast, the
particles in synthetic
latex emulsions do not swell nor contain a substantial portion of water inside
the colloid
particles. The swelling characteristics of typical SB latex and colloids of
biopolymer
nanoparticles have been compared and reported in a number of articles (see Do
lk Lee,
Steven Bloembergen, and John van Leeuwen, "Development of New Biobased
Emulsion
Binders", PaperCon2010, "Talent, Technology and Transformation", Atlanta, GA,
May 2-5,
2010; and, Steven Bloembergen, Edward VanEgdom, Robert Wildi, Ian. J.
McLennan, Do lk
Lee, Charles P. Klass, and John van Leeuwen, "Biolatex Binders for Paper and
Paperboard
13
CA 02790763 2012-09-21
Applications", Journal of Pulp and Paper Science, 36, No 3-4, p. 151-161,
2011; J. Y. Shin,
N. Jones, D. I. Lee, P. D. Fleming, M. K. Joyce, R. DeJong, and S.
Bloembergen,
"Rheological Properties of Starch Latex Dispersions and Starch Latex-
Containing Coating
Colors", TAPPI, PaperCon 2012, "Growing the Future", New Orleans, LA, April 21-
25, 2012).
The contents of these publications are incorporated herein by reference.
[0066] Figure 7 illustrates a schematic model for the crosslinked
biopolymer
nanoparticles 10. The crosslinked biopolymer nanoparticles 10 can be thought
of as one
crosslinked macromolecular unit, with ¨R¨ representing an intermolecular
crosslink
between individual biopolymers 12. Other types of crosslinked structures may
exist, such as
intramolecular crosslinks.
[0067] The crosslinked biopolymer nanoparticles 10 comprise a core 14 and
a shell
16. The core 14 receives and releases water as it swells and de-swells and the
shell 16
provides a steric stabilization mechanism for the dispersed colloid particles.
Water is
released, bound and adsorbed from the core 14 through the shell 16. The
structure of the
crosslinked biopolymer nanoparticles 10 is further described in Steven
Bloembergen, Ian. J.
McLennan, John van Leeuwen and Do lk Lee, "Specialty Biobased Monomers and
Emulsion
Polymers Derived from Starch", 2010 PTS Advanced Coating Fundamentals
Symposium,
Munich, Germany, Oct. 11-13, 2010.
[0068] Aqueous dispersions of the crosslinked biopolymer nanoparticles 10
are
stable for up to 12 months or longer. Because typical native starches contain
very high
molecular weight amylopectin polymer (millions of daltons) and high molecular
weight
amylose polymer (hundreds of thousands of daltons), solutions up to 5 or 10%
solids can
have very high gel-like viscosities. Commercial dispersions of corn starch
granules typically
reach up to about 30% solids or higher, because these products have been
chemically,
thermally or enzymatically treated to reduce their molecular weight in order
to attain higher
solids contents. This is the typical molecular weight/solids trade off that
one faces to
maintain a reasonably low viscosity for polymer solutions. Purer dispersions
with higher
solids content (up to about 40% solids), and ultra-high solids formulations
(up to 72% solids)
have been developed using the crosslinked biopolymer nanoparticles 10. This
may be
beneficial for drug delivery applications, where a high solids concentration
facilitates loading
of greater amounts of the active agent 22 into the crosslinked biopolymer
nanoparticles 10.
14
CA 02790763 2012-09-21
[0069] The crosslinked biopolymer nanoparticles 10 may be loaded with an
active
agent 22, for example a drug, or other agent, and used as a delivery device.
Loading of the
active agent 22 may also be referred to as conjugating or encapsulating.
[0070] As discussed above, the core 14 of the nanoparticles takes in water
as it
swells. Similarly, small molecules, such as some drugs, or other agents can be
taken up,
adsorbed, or otherwise loaded into the core of the nanoparticles. An example
presented
further below will describe loading of the drug doxorubicin in the crosslinked
biopolymer
nanoparticles 10 by a phase separation method (Example 1) and by ethanol
precipitation
(Example 4). By itself, doxorubicin has been linked to acute cardiotoxicity
which limits its
use. In other experiments, Carmustine and BCNU (bis(chloroethylnitrosourea))
have been
also been loaded into the crosslinked biopolymer nanoparticles 10.
[0071] It can be expected that other methods of loading the drug may also
be used,
and that other drugs and agents can similarly be loaded. For example, other
active agents
22 may include cyclophosphoramide and camptothecins that may be loaded into
the
crosslinked biopolymer nanoparticle 10 and, like doxorubicin, make the
crosslinked
biopolymer nanoparticles 10 useful in the treatment of cancer. The crosslinked
biopolymer
nanoparticles 10 may also encapsulate non-chemoactive agents, such as
antisense
oligonucleotides, peptides, and cytokines for other therapeutic applications.
[0072] After the active agent 22 is loaded, the crosslinked biopolymer
nanoparticles
can be recovered by lyophilization, which results in a powder of the
nanoparticles loaded
with the encapsulated active agent 22. The powder can be mixed with water, or
another
hydroxylic solution, to disperse the crosslinked biopolymer nanoparticles 10
into a stable
colloidal dispersion. The dispersion can be administered to treat a patient in
the liquid form,
for example orally, by intra-venous infusion or injection. The powder can be
mixed with a
pharmaceutical carrier and made into a solid or gelled drug product, such as a
tablet or
capsule. The drug product may be administered in any known manner used for
pharmaceutical products, such as orally, rectally, transdermally and the like.
[0073] The biopolymers 12 may be modified, also referred to as
functionalized,
through chemical or enzymatic modifications before, during or after forming
the crosslinked
biopolymer nanoparticle 10. In principle, any chemical or enzymatic
modification known for
polysaccharides can be employed. For example, a summary of various chemical
and
enzymatic oxidation processes is provided in column 1, line 66 to column 3,
line 50 in R. A.
Jewel et al., US Patent 6,379,494, "Method of Making Carboxylated Cellulose
Fibers and
CA 02790763 2012-09-21
Products of The Method", April 30, 2002, the disclosure of which is
incorporated herein by
reference. Although these methods are discussed in relation to cellulose, many
if not all are
adaptable to starch polymers.
[0074] In
Example 4, a biopolymer 12 of starch is functionalized after the crosslinked
biopolymer nanoparticles 10 are formed. In particular, the biopolymers 12 were
oxidized to
add carboxyl functional groups. While this is described in Example 4 as
relating primarily to
the attachment of a targeting molecule 18, to be discussed further below,
functionalizing the
crosslinked biopolymer nanoparticle 10 may also facilitate loading of the
active agent 22.
[0075]
Functionalizing the crosslinked biopolymer nanoparticle 10 by chemical or
enzymatic methods may also attach other types of functional groups to the
biopolymers to
provide binding sites for the targeting molecule 18, the active agent 22 or
both. The surface
of the crosslinked biopolymer nanoparticles 10 may also be modified,
chemically or
otherwise to alter systemic clearance rates to provide a better control of the
delivered active
agent 22 and a targeted delivery, if any.
[0076] For
example, the oxidation resulted in a change in the zeta potential of the
crosslinked biopolymer nanoparticle 10. The
zeta potential of a non-functionalized
crosslinked biopolymer nanoparticle 10 is in the range of 0 to negative 6 mV.
An oxidized
crosslinked biopolymer nanoparticle 10 demonstrates a zeta potential of about
negative 25
mV. The oxidation reaction may also be controlled to provide functionalized
crosslinked
biopolymer nanoparticles 10 that have an intermediate zeta potential value,
for example
between about negative 6 to about negative 25 mV. Tuning the zeta potential of
the
crosslinked biopolymer nanoparticles 10 may allow selective loading of the
active agent 22
and, additionally, this tuning may provide control of the release profile of
the active agent 22.
Many small molecules being developed for cancer treatment are hydrophobic and
lipophilic
and, hence, they are difficult to dissolve.
Functionalizing, for example by oxidative
modification, or otherwise, of the crosslinked biopolymer nanoparticle 10 may
enhance the
ability of the small molecule, hydrophobic/lipophilic drugs to be loaded onto
the crosslinked
biopolymer nanoparticle 10.
[0077]
While the water soluble TEMPO catalyst (2, 2, 6, 6-tetramethylpiperidine-1-
oxyl radical) used in Example 4 provided starch functionalities throughout the
crosslinked
biopolymer nanoparticle 10, an immobilized TEMPO catalyst causes only
biopolymers 12, or
portions of biopolymers 12, located at or near the shell 16 to be
functionalized. This
approach could be used, for example, to attach the targeting molecule 18 to
the surface of
16
CA 02790763 2012-09-21
the crosslinked biopolymer nanoparticle 10 with less modification of the zeta
potential of the
core 14. Optionally, a soluble catalyst can be used if a greater change in
zeta potential is
desired.
[0078] While any form of oxidation may be used, the TEMPO oxidation is
preferred.
The TEMPO catalyst is used to specifically modify the C6 hydroxyl of the
glucopyranoside
position to a carboxyl functionality. This process prevents the molecular
weight reduction of
the polysaccharide polymer that is common with many other oxidative processes.
[0079] Many functionalizing techniques are known to add aldehyde groups
to
polysaccharide polymers. Without intending to exclude the possibility that one
of these
functionalization techniques might be useful, they are not currently
preferred. The aldehyde
groups are reactive and tend to cause the crosslinked biopolymer nanoparticles
10 to
agglomerate and stick together. This interferes with creating a colloidal
dispersion, and so
may also interfere with distribution of the crosslinked biopolymer
nanoparticles 10 in the
body.
[0080] As described above, the zeta potential of unmodified crosslinked
biopolymer
nanoparticles 10 is low, hence the observed colloidal stability is attributed
mainly to steric
stabilization. Without being bound by theory, the shell 16 contains short
polysaccharide
chains which project into the aqueous environment. These chains may function
as a
colloidal stabilizer for the crosslinked biopolymer nanoparticle 10 in water
and as a partial
hydrophilic shell for bound water. This in turn may prevent or slow the
release, efflux or
diffusion of hydrophobic active agents 22 from the crosslinked biopolymer
nanoparticle 10.
[0081] In some of the examples provided below, doxorubicin was used as
the active
agent 22. The doxorubicin was loaded into the crosslinked biopolymer
nanoparticles 10 so
that the release profile could be followed using a fluorescence technique.
This work has
demonstrated a biphasic release profile with suitable release kinetics
spanning multiple
hours of sustained release of the doxorubicin. The fluorescence of the
doxorubicin-loaded
crosslinked biopolymer nanoparticles 10 declines over time but some
fluorescence remains
even after 12 hours. This indicates that not all of the doxorubicin is
released from an intact
particle. The remainder of the loaded doxorubicin, however, will be released
in the body as
the crosslinked biopolymer nanoparticle 10 degrades, for example due to alpha-
amylase
enzymes. The complete release time may be 24, 48, 72 hours or more. Other
drugs or
compounds that are used as the active agent 22 may demonstrate a similar
sustained and
biphasic release profile.
17
CA 02790763 2012-09-21
[0082] Referring to Figure 12, the inventors have observed a relationship
between
the degree of crosslinking of the biopolymer 12 and the release profile of the
active agent 22.
As described in Example 5, three different batches of crosslinked biopolymer
nanoparticles
were produced, with relatively low, medium and high degrees of crosslinking.
The greater
the degree of crosslinking of the crosslinked biopolymer nanoparticle 10 the
slower the rate
of release of the active agent 22. In contrast, the batch of crosslinked
biopolymer
nanoparticles 10 that had a relatively lower degree of crosslinking
demonstrated a faster rate
of active agent 22 release.
[0083] In animal studies described in Example 3, doxorubicin loaded
crosslinked
biopolymer nanoparticles were used to treat glioblastoma muitiforme, a primary
brain tumor in athymic
mice. These studies demonstrated a 30% increase in survival for the mice
treated with
doxorubicin-loaded crosslinked biopolymer nanoparticles 10 relative to the
appropriate
controls. Without intending to limit the invention to any particular theory,
this success is
attributed to one or more of several factors including the size, the surface
properties, and the
sustained release kinetics of the crosslinked biopolymer nanoparticles 10. The
encapsulated
doxorubicin is believed to enter the cell via endocytosis due to the
relatively small size of the
nanoparticle, while the free drug is metabolized and excreted.
[0084] Figure 11 is a schematic of an example bioconjugate device 30
comprising a
crosslinked biopolymer nanoparticle 10, a functionalized biopolymer 12, an
optional targeting
molecule 18 and a loaded active agent 22, that is shown within the core 14.
Figure 11 is
merely a schematic and is not a representation or limitation of how the active
agent 22 may
be loaded in the bioconjugate device 30.
[0085] The bioconjugate device 30 may be used for the delivery of
therapeutically
effective doses of the active agent 22 to targeted cells for the treatment of
specific disorders.
The bioconjugate device 30 can be made by functionalizing crosslinked
biopolymer
nanoparticles 10, loading an active agent 22 within the colloidal polymer
hydrogel.
Optionally, the surface of the crosslinked biopolymer nanoparticles 10 can
also be
functionalized by attaching the targeting molecule 18. The targeting molecule
18 can be any
antibody, ligand, signal sequence or molecule that attaches to a
functionalized biopolymer
12, for example within the shell 16, and that is capable of increasing the
bioconjugate device
30 interaction with specified target cells. The term interaction refers to
target cell surface
receptor - targeting molecule 18 recognition and bonding, or other indirect
mechanisms,
whereby the presence of the targeting molecule 18 increases the likelihood of
a bioconguate
18
CA 02790763 2012-09-21
device 30 in the systemic circulation to target, interact with and ultimately
transport into a
target cell. Fluorescence studies indicate that the crosslinked biopolymer
nanoparticles 10
can be taken into the cell nucleus. Without intending to be limited by theory,
the transport
mechanism is believed to be endocytosis, which may be receptor mediated, or
not. Optionally,
the degree of crosslinking of the crosslinked biopolymer nanoparticle 10 can
be varied to
provide a desired release rate of the active agent 22 from the core 14.
[0086] In one example, the targeting molecule 18 is an aptamer that
typically has a
size of less than about 10 nm and increases the diameter of the bioconjugate
device 30 by
only about 20 nm or less. The aptamer is capable of binding to a target
molecule that is
located in a specific site which may include cancer cells. For example, AS1411
is an
aptamer that has been shown to bind to nucleolin (Soundararajan et al.,
"Plasma Membrane
Nucleolin Is a Receptor for the Anticancer Aptamer AS1411 in MV4-11 Leukemia
Cells",
Molecular Pharmacology, Vol. 76, No. 5, 2009). Binding to nucleolin receptors
is useful in
the treatment of a wide array of cancers such as renal cell carcinoma, breast
cancer,
prostate cancer and others. AS1411 may also be tagged with, for example, a Cy3
fluorescent tag for imaging purposes.
[0087] Another potentially useful targeting molecule 18 is the aptamer
sgc4. This
aptamer was developed by way of the SELEX process from T-cell leukemia cell
lines and is
able to recognize leukemia cells (Shannguan et al., "Aptamers Evolved from
Cultured Cancer
Cells Reveal Molecular Differences of Cancer Cells in Patient Samples",
Clinical Chemistry
53, No.6, 2007). However, sgc4 has a short biological life if it is not
conjugated. Its
sequence is described in US Patent Publication 2009/0117549. Shorter variants
of the
sequence may also be effective. Sgc8c aptamers have also been reported to be
useful for
targeting leukemia cells (Ozalp et al., Pharmaceuticals 2011, 4, 1137-1157)
[0088] Targeting molecules, such as aptamers, with an amine modification
on the 3'
end of the DNA can be linked or attached, for example by one or more covalent
bonds, to the
carboxyl groups of the functionalized biopolymers 12. The linkage may be made,
for
example, using EDC chemistry, or by another linkage between the carboxyl and
the amine.
An example of such a linking using an amine modified test strand of DNA is
described in
Example 4. Similarly, aptamers such as AS1411 and sgc4 can also be provided
with an
amine modification and are expected to also attach to functionalized
biopolymers 12. When
also loaded with an active agent 22, such as doxorubicin, the resulting 22
bioconjugate
19
CA 02790763 2012-09-21
device 30 may deliver therapeutically effective amounts of the active agent 22
to targeted
leukemia cells, or other cancer cells.
[0089] By using an immobilized TEMPO as the catalyst to oxidize the
biopolymer 12
forms carboxyl groups in the shell 14. These carboxyl groups may be activated
by NHS and
EDC to attach an amine-modified targeting molecule 18, such as an aptamer, to
the surface
of the polymer colloid, thereby forming a covalent linkage. The number of
functional groups
on the surface of the nanoparticle may determine the aptamer surface density
and,
ultimately, the rate of target cell uptake.
[0090] TEMPO reacts with the hydroxyl groups on the starch polymers in an
aqueous
medium to create the desired carboxyl groups (-COOH) by the process known as
TEMPO-
mediated carboxylation. NaBr is used to stabilize this reaction. Hypochlorite
(NaC10)
initiates the reaction by keeping the pH at 10.2-10.5. Then HCI can be used to
lower the pH
and reprotonate the carboxyl groups. 1-Ethy1-3-(3-dimethylaminopropyl)
carbodimide
hydrochloride (EDC) and N-hydroxysuccinimide (NHS) are chemicals which can act
as
coupling agents to form carboxyl-amino covalent linkages, which link the
functionalized
biopolymers 12 to the 3'-amine-modified ssDNA aptamer. In this manner, a
bioconguate
device 30 can be loaded with an active agent 22 and provided with a targeting
molecule 18
to increase the interaction of the bioconjugate device 30 with target cells.
[0091] Optionally, other molecules can be attached to the crosslinked
biopolymer
nanoparticle 10, through functional groups or other modifications. For
example, PEG or
other passivating polymer molecules can be attached to improve the half-life
and possibly the
bioavailability of the crosslinked biopolymer nanoparticles 10 and the
bioconjugate devices
30 based thereupon.
[0092] The following examples serve to illustrate one or more parts of
one or more
inventions and are not intended to limit any claim. Reference is made in the
examples to
EcoSphere 2202 from EcoSynthetix Inc. as a non-limiting example of a
crosslinked
biopolymer nanoparticles 10. However, other nanoparticles with similar
properties of drug
loading, functionalization, target molecule attachment and variable degrees of
crosslinking
are also contemplated.
Example 1
[0093] Incorporation of fluorescent agents into starch based
nanoparticles
CA 02790763 2012-09-21
[0094] Incorporation of two compounds, in particular the fluorescent
model
compound Calcein and the fluorescent anticancer agent doxorubicin OUPAC Name:
(7S,9S)-
7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-
(2-
hydroxyacety1)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; commercial
products
include AdriamycinTM and DoxilTm), into crosslinked biopolymer nanoparticles
10
(EcoSphere 2202 from EcoSynthetix Inc.) was accomplished by a phase
separation
technique. This technique involves the formation of a water-in-oil emulsion.
In a 250 mL
round bottom flask, the starch based nanoparticles were dispersed at <5%
solids (w/w) in
water under mechanical agitation at a pH of about 10 using dilute caustic. The
resultant
dispersion was titrated to a pH of 7 using dilute hydrochloric acid. The
substance to be
incorporated in the crosslinked biopolymer nanoparticle colloid matrix
(calcein or doxorubicin)
was dissolved in the dispersion containing the biopolymer nanoparticles. The
amount of
encapsulated active agent 22 prepared ranged from 0.04%-0.4% (w/w). The flask
was
placed inside an insulated container and secured properly. The solution was
then stirred for
several minutes. Hexane was added drop wise under continuous agitation until
an emulsion
was formed. The emulsion was immediately frozen using liquid nitrogen. The
flask was
connected to a vacuum system and lyophilization was carried out at -85 C.
After 24 hours,
when the vacuum gauge indicated no further vapor removal, the dried sample was
removed
from the vacuum system and stored at -10 C.
Example 2
[0095] Drug release studies
[0096] The use of fluorescent dyes as spectral probes to investigate
inclusion
complexation is known (see Saenger, W. Angew. Chem. 1980, 92, 343-61 and Wenz,
G.
Angew. Chem. 1994, 106, 851-70). This approach was adopted in studying the
efficacy of
starch based nanoparticles (in this example we used EcoSphere 2202 from
EcoSynthetix
Inc.) to encapsulate selected drugs and the ability of this material to
release the drug over
time. Fluorescent compounds such as calcein and doxorubicin are very sensitive
to
environmental changes. The fluorescent signal of the molecules was enhanced
when it was
incorporated into the matrix of starch based nanoparticles. As shown in Figure
8, the signal
intensity of free doxorubicin is much lower than that of the encapsulated
doxorubicin. In
addition, a significant hypochromic shift (change of spectral band position in
the emission
spectrum of a molecule to a shorter wavelength) is observed when doxorubicin
is
21
CA 02790763 2012-09-21
encapsulated. Figure 9A shows a series of fluorescence spectra of doxorubicin
obtained as
a function of time. It can be seen that there is a significant decrease in
signal intensity with
time, indicating sustained release of the active agent 22. In addition, there
was a relatively
small bathochromic shift (change of spectral band position in the emission
spectrum of a
molecule to a shorter wavelength) observed. Without intending to be limited to
any theory of
operation, it appears that the reduced shift indicates a biphasic release
mechanism given
that not all of the active agent 22 was released over the course of the 12
hour experiment.
Figure 10A shows a series of fluorescence spectra of calcein obtained as a
function of time.
It can be seen that there is a decrease in signal intensity with time,
indicating sustained
release of the active agent 22.
[0097] The data shown in Figures 9A and 10A illustrate that enhancement
in signal
intensity for calcein and doxorubicin due to inclusion complexation with the
starch based
nanoparticles can be used to monitor the release of the active agent . Figures
9B and 10B
are plots of signal intensity as a function of time at the maximum signal
intensity of the
fluorescence emission spectra for doxorubicin and calcein, respectively. These
data show
that the concentration of fluorophore molecules inside the supramolecular
cavity is changing
with time. The release of the molecules appears to be proportional to the
concentration
gradient of the active agent . The sustained release of active agent from the
biopolymer
nanoparticles extended to more than 10 hours. The results demonstrate that the
biopolymer
nanoparticles provide a stable matrix for the steady release of active agent
over an
extended time period. The release mechanism appears to be predominantly
diffusion
controlled.
Example 3
[0098] In vivo studies of human xenographs implanted in athymic mice
[0099] In order to demonstrate the efficacy of the crosslinked biopolymer
nanoparticles 10 as a drug delivery device, they were loaded with the
anticancer drug
doxorubicin as described in Example 1. The doxorubicin loaded in crosslinked
biopolymer
nanoparticles 10 was administered to athymic mice which had a human xenograph
of a
primary brain tumor (D 245 glioblastoma multiform) previously grown at a
subcutaneous site.
Athymic mice were chosen for these studies because normal mice are capable of
immunologically rejecting implanted foreign xenographs, specifically human
tumors. The
22
CA 02790763 2012-09-21
animals (both control and treated) were monitored for tumor regression and
survival. The
results of the study are presented in Table 1.
[00100] The procedure consisted of inoculation of the tumor xenograph
into a
subcutaneous site in athymic mice. The subcutaneous tumors were grown to
approximately
200 cubic millimeters in size (6-8 mm in diameter). Subsequently, either the
free drug or the
drug loaded nanoparticles were injected at the tumor site or i.p. (intra
peritoneal). Typically it
took approximately 20 days for the animals to test out. The animals were
treated in groups
of 8 to 10 individuals. The highest survival rates (highest T-C values or
increased life span in
days) occurred in individuals in which several doses of doxorubicin loaded
nanoparticles
were administered. Table 1 demonstrates the efficacy as well as the safety of
the
doxorubicin loaded biopolymer nanoparticles in treating a primary human brain
tumor in
athymic mice.
Table 1: In vivo studies of human xenographs implanted in athymic mice
1
Dose x 0.03% x 2 x 4 x
1 x 0.5% 1 x 0.5% 4 x 0.5% 1 x 2%
Free
(Dox- (Dox- (Dox- (Dox- Dox
Conc. (w/w) (Dox- (Dox- (Dox-
nano) nano) nano) nano)
Control
nano) nano) nano)
0.075 1.25 1.25 2.5 5
Drug dose 5 mg/kg 5 mg/kg 5 mg/kg
mg/kg mg/kg mg/kg mg/kg
mg/kg
T-C(days) 0.99 4.8 5.31 8.09 7.56 6.57 2.44
1.53
P value 0.14 0.14 0.14 0.04 0.001 0.012 0.03
0.32
Regressions 2/8 2/8 0/10 0/10 2/9 0/9 2/8
0/10
Toxic
IS 0/8 0/8 0/10 0/10 2/9 0/9 0/8
0/10
deat
[00101] The following abbreviations are used in Table 1: "Dox" =
doxorubicin; "Dox-
nano" = doxorubicin loaded crosslinked biopolymer nanoparticles 10.
[00102] Dispersions were 100 mg crosslinked biopolymer nanoparticles /5
mL saline
(1 x 0.5% Dox-nano), 200 mg crosslinked biopolymer nanoparticles/5 mL saline
(2 x 0.5%
Dox-nano) or 400 mg crosslinked biopolymer nanoparticles/5 mL saline (4 x 0.5%
Dox-
nano).
[00103] Injections were each 0.25 mL/20 gram mouse (all single
injections).
[00104] T-C is defined as: Average of (days lived by drug-treated animal
minus days
lived by control animals); i.e., increased life span in days.
23
CA 02790763 2012-09-21
[00105] The Control was provided as follows: Varied - Untreated mice
injected with
saline; Untreated mice; Mice treated with drug-free crosslinked biopolymer
nanoparticles
(100 mgs/5 mL saline).
[00106] P value: Test run for significance. P values are calculated using
non-
parametric statistical method by Hollander and Wolfe.
[00107] Regressions: Indicate that tumors drop below previous measurement
and stay
below for 2 consecutive measurements.
Example 4
[00108] Attaching a targeting molecule
[00109] Targeting molecules can be attached to the crosslinked biopolymer
nanoparticle bioconjugate delivery system to facilitate the interaction of the
delivery system
with a tumor, metastatic cancer cell, or other targeting tissue or organ. This
capability was
demonstrated by the following procedures and tests.
[00110] Oxidation of crosslinked biopolymer nanoparticles
[00111] Various different types of functionalities may be introduced onto
the
crosslinked biopolymer nanoparticles 10 to provide binding sites for the
aptamer as well as
the active agent 22. As described above, various chemical modification
techniques can be
employed. A particularly useful chemical modification is oxidation of starch
biopolymers 12
to produce carboxyl functionalities. To illustrate this, TEMPO-mediated
oxidation was carried
out for both crosslinked biopolymer nanoparticles 10 (EcoSphere 2202 from
EcoSynthetix
Inc.) as well as for regular native (unmodified) corn starch. In this method,
the starch
biopolymer 12 was oxidized with sodium hypochlorite (NaC10) and 2,2,6,6-
tetramethylpiperidin-1-oxyl (TEMPO) radicals, at temperatures between 0 and 4
C and pH of
10.8. The degree of oxidation was controlled by amount of NaCIO added. As
noted, two
types of starch were used. The first was EcoSphere starch based nanoparticles
and the
second one was regular corn starch purchased from Sigma-Aldrich. The
procedures were as
described below.
[00112] In a glass jar, 4 g of EcoSphere and 80 mL MilliQ water were
added and
mixed thoroughly to create a ¨5% dispersion. In a second jar 4 g of Corn
Starch and 80mL
of MilliQ water were added to create a ¨5% solution. The second jar was heated
up to above
80 C (max 95 C) under agitation and allowed to fully dissolve. Subsequently
it was cooled
to room temperature. Separately, in two 45 mL tubes 40 mL of water, 38 mg
TEMPO, and
24
CA 02790763 2012-09-21
508 mg NaBr were added into each tube (0.01 mol TEMPO per anhydroglucose unit
of
starch; 0.2 mol NaBr per anhydroglucose unit of starch), stirred until fully
dissolved, and
cooled for 30 minutes in an ice batch. Next the content of one tube was mixed
into each jar.
A pH measurement was taken, which initially was 3.8 for the EcoSphere jar and
7.4 for the
Corn Starch jar. Next 450 pL of 0.5 M NaOH was added to the EcoSphere jar to
reach pH
10.75, and 200 pL of 0.5 M NaOH was added to the Corn Starch jar to reach pH
10.75.
Subsequently, 10 mL of NaCIO was added when the pH dropped to around 6-7, and
pH
measurements were taken every 10-15min. As the mixtures continued to stir and
the pH
dropped, the color became darker (yellow/orange). A total of 60 mL NaCIO was
added and
the pH was finally adjusted to 8.0 before the oxidized starch was diluted 1:1
with ethanol.
Ethanol precipitated the modified EcoSphere nanoparticles and modified starch
and they
were harvested by centrifugation and washed by water and ethanol and finally
dried by
lyophilization (freeze-drying).
[00113] The oxidized EcoSphere was characterized by zeta-potential
measure and
dynamic light scattering. Zeta measurement showed that the modified particles
carried a
negative charge with zeta-potential of -25.5 mV, while unmodified particles
were essentially
neutral. The size of the particles appeared to be slightly smaller compared to
the non-
oxidized ones (i.e. the NTA Mode was 113 versus 141 nm).
[00114] The color of the final product depended on the pH of the solution
after
oxidization. If the pH was too high (higher than 10), a yellow colored product
was obtained. It
was found that this color can be removed by lowering the pH.
[00115] DNA attachment
[00116] Subsequently, amino-modified and fluorescently labeled DNA was
attached to
the starch nanoparticles using N-(3-DimethylaminopropyI)-N'-ethylcarbodiimide
hydrochloride
(EDC) as a coupling agent. The reaction mixture contained 5 1AM FAM (6-
carboxyfluorescein) and amino dual labeled DNA, 1-5% COOH-modified starch, 20
mM 2-(N-
morpholino)ethanesulfonic acid (MES) buffer, pH 6.0 and 20 mM freshly prepared
EDC was
added the last. Agarose gel electrophoresis was carried out for DNA and DNA-
conjugated to
TEMPO-oxidized EcoSphere nanoparticles. It was found that the gel
fluorescence intensity
was more evenly distributed and some of the DNA migrated more slowly,
indicating
conjugation to the starch nanoparticles. In some of the alternative DNA
attachment protocols
the carboxyl groups on starch were first activated using N-Hydroxylsuccinimide
(NHS) at
5mM (1/4 of the amount of EDC) for 15 minutes before adding the DNA. Next this
mixture
CA 02790763 2012-09-21
was allowed to react for several hours. Without intending to be bound by any
particular
theory of operation, NHS may help to facilitate the EDC linking reaction by
activating
carboxyl groups so it can react with an amine to form an amide, rather than a
salt with an
amine.
[00117] Thus the DNA used in this example, which served as a model
compound for
ligand attachment, was successfully attached. The DNA sequence was 5'-FAM- ACG
CAT
CTG TGA AGA GM CCT GGG-NH2-31.
[00118] Attachment of an Aptamer
[00119] An aptamer was attached to EcoSphere 2202 particles using the
procedure
described above. Attachment of the aptamer was confirmed by laboratory
observations of
nanoparticle fluorescence. The aptamer was, AS1411, which is believed to have
(as
modified) the sequence: 5-Cy3-TTGGTGGTGGTGGTTGTGGTGGTGGTGG-NH2-3' (i.e.
AS1411 aptamer with Cy3 fluorescent tag and amine group). The fluorescent tag,
used for
imaging purposes in the diagnostic gel electrophoresis test, can of course be
omitted if
needed. However, an additional purpose for the fluorescent tagging is to
facilitate monitoring
of the binding and uptake of the modified nanoparticles by a cell. As for the
DNA described
above, the aptamers had an amine modification on the 3' end of the DNA so that
it could be
linked using EDC chemistry to carboxyl functionalities on the nanoparticle.
[00120] Four 200 microliter wells were prepared with cells of a cervical
cancer cell line
(HeLa) and given time to culture and grow. Well 1 was left with only the HeLa
cells. Well 2
had unconjugated AS1411 added to it. Well 3 had EcoSphere 2202 nanoparticles
with
conjugated AS1411 added to it. Well 4 had nanoparticles conjugated with a
control
sequence added to it. The control sequence has no known affinity for HeLa
cells. The wells
were then allowed to culture for a further 48 hours.
[00121] After the 48 hours had elapsed, cells from the wells were washed
to remove
any fluorescent marks on any unbound particles external top the cells. The
cells were then
observed under a fluorescence microscope. Fluorescent marks were observed
within the
cells of well 3 confirming that the nanoparticle/aptamer conjugate had been
taken into the
cells.
[00122] Drug adsorption and release studies
[00123] In a dilute aqueous dispersion (e.g. 1-5%) the EcoSphere
nanoparticles are
highly swollen and their density is close to that of water. As a result,
centrifugation and even
26
CA 02790763 2012-09-21
ultracentrifugation were ineffective methods to separate the particles from
the aqueous
dispersion media. Instead, drug loading was evaluated by way of fluorescence
change. It
was found that the adsorption of the anticancer drug doxorubicin (Dox) was
very much
improved after modification of the EcoSphere nanoparticles with carboxylate
groups. Upon
adsorption, the fluorescence of doxorubicin was also quenched by the
carboxylated
EcoSphere . This was clearly visible under the 245 nm excitation in a dark
room using a
handheld UV lamp. The fluorescence quenching provides an analytical method to
monitor
doxorubicin adsorption.
[00124] To
ensure that the observed quenching was not due to a pH effect, the
fluorescence was subsequently compared for the following: doxorubicin was
dissolved at a
final concentration of 0.01 mg/mL in unmodified EcoSphere , COOH-modified
EcoSphere
and buffer (no EcoSphere). For each condition, two pH conditions were tested
to contain
either 20 mM sodium acetate buffer (pH 5.0) or 20 mM 4-(2-hydroxyethyl)-1-
piperazineethanesulfonic acid (HEPES) buffer, pH 7.6. The final pH was
confirmed to be at
the intended values.
[00125]
Free doxorubicin fluorescence was strong in both pH 5 and 7.6 in water.
Mixing with 1% unmodified EcoSphere nanoparticle dispersion induced about 50%
fluorescence quenching but mixing with a COOH-modified EcoSphere nanoparticle
dispersion completely quenched the fluorescence. This confirmed that COOH-
modified
EcoSphere is better at adsorbing doxorubicin. Without intending to be bound
by any
particular theory of operation, this is likely due to electrostatic
interactions with the positively
charged doxorubicin.
Therefore, tuning the EcoSphere charge will allow selective
adsorption of various drugs and in addition provide a way of controlling the
release profile.
[00126] Electrokinetic Measurements
[00127] To
evaluate the presence of electrostatic charges on the surface of the
particles, the zeta potential of the biopolymer nanoparticles and TEMPO
oxidized biopolymer
nanoparticles was determined from the analysis of the electrokinetic
measurements using a
Brookhaven ZetaPlus instrument. The crosslinked starch particles were
suspended in a
solution of NaCI ranging from 0.001 M to 0.1 M concentration, and their
electrophoretic
mobilities were determined. The electrophoretic mobilities were converted to
zeta ( )
potentials using the Smoluchowski expression, which assumes small particles
and dilute ion
concentration. The zeta potential of the un-modified starch based
nanoparticles was
determined to be close to zero, whereas the zeta potential of the TEMPO
modified
27
CA 02790763 2012-09-21
biopolymer nanoparticles was determined to be -25 mV which indicates
negatively charged
nanoparticles.
[00128] Particle size analysis
[00129] The particle size of dispersed starch based nanoparticles and the
TEMPO
modified nanoparticles was determined by Nanoparticle Tracking Analysis (NTA)
using an
LM 20 tracking analysis device (NanoSight Ltd.) equipped with a blue laser
(405 nm). The
device uses a 50 mW laser operating in the CW mode to illuminate the
particles. The light
scattered by the particles is captured using a digital camera and the motion
of each particle
is tracked from frame to frame using NanoSight software. A high speed video is
obtained (30
frames per second, average video about 30 s). The trajectories of individual
particles are
generated from the video sequence and the mean squared displacement determined
for
each particle. Typically at least 20 trajectories are acquired and 250 to 500
sets of
trajectories (each set corresponding to an individual particle) are
accumulated in a video
sequence. The analysis of the mean squared displacement is used to calculate
the diffusion
coefficient and the hydrodynamic radius (rh) is determined using the Stokes-
Einstein
equation. Thus, the diameter of each particle in the sample can be determined
and a true
particle size distribution derived. Because a diffusion coefficient is
obtained for each particle
in the field of view, a particle size distribution can be obtained which does
not assume a
particular mathematical model as in dynamic laser light scattering (DLS)
analysis.
[00130] Dispersions of biopolymer nanoparticles were prepared using the
following
procedure: 1) dry agglomerate EcoSphere powder was mixed in water containing
0.4 wt%
sodium carbonate ("lite soda ash") based on dry weight in a SiIverson high
shear mixer for 15
minutes; the final concentration of the dispersed biolatex ranged from 0.015
to 0.030% (w/w);
2) this dispersion was heated to 45 C for 15 minutes in a water bath prior to
measurement to
ensure the agglomerate particles were fully dispersed into nanoparticles.
Example 5
[00131] Three batches of crosslinked biopolymer nanoparticles 10 with
different
degrees of crosslinking were made. A first batch 100 had the lowest amount of
crosslinking,
a second batch 102 had a moderate amount of crosslinking and a third batch 104
had the
highest degree of crosslinking. One manner to assess the degree of
crosslinking is to
determine the maximum volume swell ratios, also referred to as the effective
volume factor,
as described in Do lk Lee, Steven Bloembergen, and John van Leeuwen,
"Development of
28
CA 02790763 2012-09-21
New Biobased Emulsion Binders", PaperCon2010, "Talent, Technology and
Transformation",
Atlanta, GA, May 2-5, 2010, the disclosure of which is incorporated herein by
reference.
Briefly, crosslinked biopolymer nanoparticles swell under conditions of
extreme dilution with
water to achieve the maximum swelling value that is balanced between their
elastic
constraint due to their crosslinked network and the osmotic pressure (see
Bloembergen, S.,
McLennan, I., Lee, D. I., and van Leeuwen, J., "Paper Binder Performance with
Nanoparticle
BiolatexTM: EcoSynthetix develops EcoSphere biolatex for replacement of
petroleum based
latex binders", ACFS, Montreal, June 11-13, 2008).
[00132] By measuring the relative viscosity, lir, at low concentrations
(i.e. low volume
fraction) for a latex (a polymer colloid), one can gather relevant information
about the
viscosity and swelling behavior of that colloid. The relative viscosity (rir=1-
1/11.) of a biobased
latex binder is obtained by simply measuring the flow times between two
demarcations of a
glass Ubbelohde viscometer for the biobased latex dispersion (n) and for its
dispersion
medium (io), which is water. Using the Einstein equation, Tir = 1 + 2.5 f 4) ,
where f is the
effective volume factor and 4) is the volume fraction, one can obtain the
effective volume
factor (f) that is equal to the maximum volume swelling of biobased latex
nanoparticles at
very low concentrations. The first batch 100 had a maximum volume swell ratio
of 16.0, the
second batch 102 had a maximum volume swell ratio of 9.33 and the third batch
104 had a
maximum volume swell ratio of 6.67.
[00133] The three batches 100, 102, 104 were loaded with 5% doxorubicin by
mass,
as described above, and dispersed in 5mL of Milli-Q water. The released
doxorubicin was
separated from the crosslinked biopolymer nanoparticles 10 using dialysis with
a molecular
weight cut off of 25 kDa. Samples were drawn off at various times over 72
hours and the
fluorescence was measured using a fluorescence plate reader.
[00134] As shown in Figure 12, the initial background was close to zero,
suggesting
that little free doxorubicin was present and the loading capacity of the three
batches 100,
102, 104 was high. The first batch 100 demonstrated the fastest rate of
doxorubicin release.
The third batch 104 released approximately 20% of the drug after 3 days while
the first batch
100 released more than 50% of the doxorubicin over the same period. Without
being bound
by theory, these results indicate that the level of crosslinking may be used
to control the
release profile of the active agent 22 from the core 14 of the bioconjugate
device 30.
29
CA 02790763 2012-09-21
Example 6
[00135] The degree, or effect, of multivalent binding between the
targeting molecule
18 and the target cell surface receptors may modulate the transport of the
bioconjugate
device 30 into the phospholipid membrane of the target cell. Samples were
prepared with
different ratios of functionalized, crosslinked biopolymer nanoparticles 10
relative to the
targeting molecule 18, in this example the AS1411 aptamer was attached similar
to the
approach described above. The concentration of functionalized crosslinked
biopolymer
nanoparticles 10 is represented by (glycosidic) repeating units of glucose.
The inventors
prepared samples with the following molar ratios of glucose repeating units to
aptamer:
100:1; 500:1; 1000:1; and 5000:1. These samples, free (unattached) aptamer and
a control
DNA aptamer were incubated with HeLa cells for 2 hrs. The HeLa cells were
seeded and
cultured in a CO2 incubator with 5% oxygen in an eight-well slide for two days
to achieve a
confluence of -70%. The culture media was DMEM/F-K12 1:1 (Hyclone) with 10%
fetal
bovine serum and 1% penicillin and streptomycin. In pairs of wells, we added
to each 20 pL
of 10 1AM unconjugated A51411 aptamer, 10 Al unconjugated control DNA aptamer
or 20 pL
of 3 mg/mL conjugated SNPs. These were incubated for a further 2 hours at 37
C and 5%
CO2. The unbound materials were washed away with phosphate buffered saline
(PBS, from
Cellgro).
[00136] The control DNA aptamer was attached in a ratio of 500:1
(glucose:control
aptamer) and the control DNA aptamer is not targeted to recognize or interact
with surface
features of cancer cells. After washing the cells to remove unbound and free
material,
uptake was quantified using a fluorescence microplate reader. The fluorescence
was
normalized to the equivalent dose of free aptamer. As depicted in Figure 13,
the 500:1
sample demonstrated the highest uptake. It is likely that at ratios at or
beyond 1000:1, or
possibly beyond about 750:1, the distance between aptamers was too far to
impart a
significant multivalent binding effect. If the aptamers are packed too
closely, proper folding
and interaction, for example binding between the bioconjugate device 30 and
the cells, may
have been hindered.
Example 7
[00137] HeLa cells, cultured as described above, were exposed for 24 hours
to
different bioconjugate devices 30 to assess cell viability. Cell viability was
measured with a
CA 02790763 2012-09-21
lactose dehydrogenase (LDH) assay and the fluorescence was measured at 490 nm
using a
microplate reader. Unmodified, crosslinked biopolymer nanoparticles 10 without
any loaded
active agent 22 were non-toxic to cells over a range of doses (Figure 14A).
Bioconjugate
devices 30, with the AS1411 attached aptamer, were loaded 5% by mass with
doxorubicin.
Different doses of these bioconjugate devices were given to the cells, and
there was
increased cell killing with doxorubicin levels greater than 0.625 jig.
Under similar
experimental conditions, 0.625 vtg of free doxorubicin (not shown) was less
effective at killing
the cells (83% viability, p=9.0 E-4) than 12.5 mg of loaded bioconjugate
device 30 that
delivered a total of 0.625 lAg of doxorubicin. Further, the bioconjuguate
device 30, with the
AS1411 aptamer attached, and no doxorubicin was non-toxic to the cells (not
shown).
Without being bound by theory, the inventors attribute these results to the
ability of the
bioconjugate device 30 to penetrate the cell membrane and release the
doxorubicin inside of
the cells, which is compared to the passive penetration of the cell membrane
by free
doxorubicin.
[00138] The
above description and attached figures are intended to illustrate at least
one embodiment of each claim and not to limit any invention. The invention is
defined by the
following claims.
31