Note: Descriptions are shown in the official language in which they were submitted.
CA 02926384 2016-04-07
LOW ACIDIC SPECIES COMPOSITIONS AND METHODS FOR PRODUCING
AND USING THE SAME
RELATED APPLICATIONS
This application claims priority to International Patent Application Serial
No.
PCT/US2013/031485, filed on March 14, 2013 and International Patent
Application Serial
No. PCT/US2013/031681, filed on March 14, 2013.
BACKGROUND OF THE INVENTION
The production of compositions comprising proteins for biopharmaceutical
applications involves the use of upstream process technologies (e.g., cell
culture) and
downstream process technologies (e.g., protein purification) that are known to
produce
proteins exhibiting varying levels of protein variants and impurities within
the composition.
Such protein variants include, but are not limited to, the presence of acidic
species, including
process-related impurities. For example, in monoclonal antibody (mAb)
preparations, acidic
species can be detected by various methods, such as ion exchange
chromatography, for
example, WCX-10 HPLC (a weak cation exchange chromatography) or IEF
(isoelectric
focusing). Because of
their similar chemical characteristics to the antibody product
molecules of interest, reduction of acidic species is a challenge in
monoclonal antibody
production.
Reduction of acidic species is particularly advantageous in the context of
commercially produced recombinant biotherapeutics, as they have the potential
to impact
numerous product characteristics, including, but not limited to, product
stability, product
safety and product efficacy. Accordingly, there remains a need in the art for
low acidic
species compositions and high-efficiency methods of producing protein
compositions, e.g.,
antibodies, having low levels of acidic species.
SUMMARY OF THE INVENTION
The present invention is based on the identification and optimization of
upstrearn and
downstream process technologies for protein production, e.g., production of
antibodies or
antigen-binding portions thereof, resulting in the production of compositions
comprising
proteins that comprise low percentages of acidic species. As demonstrated
herein, these low
acidic species compositions have improved therapeutic efficacy and improved
biological
1
CA 02926384 2016-04-07
properties, for example, increased cartilage tissue penetration, reduced
cartilage destruction,
reduced synovial proliferation, reduced bone erosion, increased protection
against the
development of arthritis as measured by arthritic scores and/or histopathology
scores, reduced
cell infiltration, reduced proteoglycan loss, reduced chondrocyte death,
and/or increased
TNFa affinity, as compared to a non-low acidic species composition.
Accordingly, in one embodiment, the present invention provides a low acidic
species
(low AR) composition comprising an antibody, or antigen-binding portion
thereof, where the
composition comprises about 15% or less AR. In one aspect of this embodiment,
the low AR
composition comprises about 14% or less AR, 13% or less AR, 12% or less AR,
11% or less
AR, 10% or less AR, 9% or less AR, 8% or less AR, 7% or less AR, 6% or less
AR, 5% or
less AR, 4.5% or less AR, 4% or less AR, 3.5% or less AR, 3% or less AR, 2.5%
or less AR,
2% or less AR, 1.9% or less AR, 1.8% or less AR, 1.7% or less AR, 1.6% or less
AR, 1.5%
or less AR, 1.4% or less AR, L3% or less AR, L2% or less AR, 1.1% or less AR,
1% or less
AR, 0.9% or less AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less AR, 0.5%
or less AR,
0.4% or less AR, 0.3% or less AR, 0.2% or less AR, 0.1% or less AR, or 0.0%
AR, and
ranges within one or more of the preceding. In one aspect of this embodiment,
the present
invention provides a low AR composition comprising an antibody, or antigen-
binding portion
thereof, where the composition comprises about 0.0% to about 10% AR, about
0.0% to about
5% AR, about 0.0% to about 4% AR, about 0.0% to about 3% AR, about 0.0% to
about 2%
AR, about 3% to about 5% AR, about 5% to about 8% AR, or about 8% to about 10%
AR, or
about 10% to about 15% AR, and ranges within one or more of the preceding.
In one embodiment, the low AR composition comprises a first acidic species
region
(ARI) and a second acidic species region (AR2). In one aspect of this
embodiment, the low
AR composition comprises about 0.1% or less AR1 and about 3% or less AR2, or
about 0.0%
ARI and about 1.4% or less AR2. In a related embodiment, the low AR
composition
comprises about 15% or less AR1, 14% or less AR1, 13% or less AR1, 12% or less
AR1,
11% or less AR1, 10% or less AR1, 9% or less AR1, 8% or less AR1, 7% or less
AR1, 6% or
less AR1, 5% or less AR1, 4.5% or less AR1, 4% or less AR1, 3.5% or less AR1,
3% or less
AR1, 2.5% or less AR1, 2% or less ARI, 1.9% or less AR1. 1.8% or less AR1,
1.7% or less
ARI. 1.6% or less ARI, 1.5% or less ARI, 1.47c or less AR1, 1.3% or less ARI,
1.2% or less
AR1, 1.17c or less ARI, 17c or less AR1, 0.9% or less AR1. 0.87c or less ARI,
0.7c7c or less
AR!. 0.6% or less AR1. 0.5% or less AR1, 0.4% or less AR1. 0.3% or less AR 1.
0.2% or less
ARI. 0.1% or less AR1. or 0.0c/c ARI, and ranges within one or more of the
preceding. In
CA 02926384 2016-04-07
one aspect of this embodiment, the present invention provides a low AR
composition
comprising an antibody, or antigen-binding portion thereof, where the
composition comprises
about 0.0% to about 10% AR1, about 0.0% to about 5% AR1, about 0.0% to about
4% AR1,
about 0.0% to about 3% AR1, about 0.0% to about 2% AR1, about 3% to about 5%
AR1,
about 5% to about 8% AR1, or about 8% to about 10% AR I, or about 10% to about
15%
AR1, and ranges within one or more of the preceding.
In one aspect of this embodiment, the low AR composition comprises about 15%
or
less AR2, 14% or less AR2, 13% or less AR2, 12% or less AR2, 11% or less AR2,
10% or
less AR2, 9% or less AR2, 8% or less AR2, 7% or less AR2, 6% or less AR2, 5%
or less
AR2, 4.5% or less AR2, 4% or less AR2, 3.5% or less AR2, 3% or less AR2, 2.5%
or less
AR2, 2% or less AR2, 1.9% or less AR2, 1.8% or less AR2, 1.7% or less AR2,
1.6% or less
AR2, 1.5% or less AR2, 1.4% or less AR2, 1.3% or less AR2, 1.2% or less AR2,
1.1% or less
AR2, 1% or less AR2, 0.9% or less AR2, 0.8% or less AR2, 0.7% or less AR2,
0.6% or less
AR2, 0.5% or less AR2, 0.4% or less AR2, 0.3% or less AR2, 0.2% or less AR2,
0.1% or less
AR2, or 0.0% AR2, and ranges within one or more of the preceding. In one
aspect of this
embodiment, the present invention provides a low AR composition comprising an
antibody,
or antigen-binding portion thereof, where the composition comprises about 0.0%
to about
10% AR2, about 0.0% to about 5% AR2, about 0.0% to about 4% AR2, about 0.0% to
about
3% AR2, about 0.0% to about 2% AR2, about 3% to about 5% AR2, about 5% to
about 8%
AR2, or about 8% to about 10% AR2, or about 10% to about 15% AR2, and ranges
within
one or more of the preceding.
In another embodiment, the low AR composition, e.g., a low AR composition of
adalimumab. comprises about 1.4% or less AR. For example, in one aspect of
this
embodiment, the low AR composition, e.g., a low AR composition of adalimumab
comprising about 1.4% or less AR can comprise about 0.0% AR1 and about 1.4% or
less
AR2.
In another aspect, the present invention provides compositions comprising an
antibody, or antigen-binding portion thereof. wherein the composition is
substantially free of
acidic species and other process-related impurities, including, for example,
host cell proteins
(FICPs), host nucleic acids, chromatographic materials, and/or media
components, as well as
product related impurities such as aggregates.
3
CA 02926384 2016-04-07
In one embodiment, the antibody, or antigen-binding portion thereof, of the
compositions disclosed herein is an anti-TNFa antibody, or antigen-binding
portion thereof.
For example, in one aspect of this embodiment, the anti-TNFa antibody, or
antigen-binding
portion thereof, dissociates from human TNFa with a Kd of about 1 x 10-8 M or
less and a
Koff rate constant of lx iO3 S.' or less. In another aspect of this
embodiment, the anti-TNFa
antibody, or antigen-binding portion thereof, comprises a light chain variable
region (LCVR)
having a CDR1 domain comprising the amino acid sequence of SEQ ID NO: 7, a
CDR2
domain comprising the amino acid sequence of SEQ ID NO: 5, and a CDR3 domain
comprising the amino acid sequence of SEQ ID NO: 3; and a heavy chain variable
region
(HCVR) having a CDR] domain comprising the amino acid sequence of SEQ ID NO:
8, a
CDR2 domain comprising the amino acid sequence of SEQ ID NO: 6, and a CDR3
domain
comprising the amino acid sequence of SEQ ID NO: 4.
In still another aspect of this embodiment, the anti-TNFa antibody, or antigen-
binding
portion thereof, comprises a light chain variable region comprising the amino
acid sequence
set forth in SEQ ID NO: 1 and a heavy chain variable region comprising the
amino acid
sequence set forth in SEQ ID NO: 2. In yet another aspect of this embodiment,
the anti-
TNFoc antibody, or antigen-binding portion thereof, is adalimumab, or an
antigen binding-
portion thereof.
In one embodiment, the low AR composition of the invention comprises
adalimumab,
and has a percentage of AR that is not the same as the percentage of AR
present in
adalimumab formulated as HUMIRA as currently approved and described in the
"Highlights
of Prescribing Infort-nation" for HUMIRA (adalimumab) Injection (Revised Jan.
2008).
In another embodiment, the low AR composition of the invention comprises
adalirnumab, and has a percentage of AR that is lower than the percentage of
AR present in
adalimumab formulated as HUMIRe as currently approved and desciibed in the
"Highlights
of Prescribing Information" for HUMIRA (adalimumab) Injection (Revised Jan.
2008).
In another embodiment, the present invention provides low AR compositions
comprising an anti-TNFa antibody, or antigen-binding portion thereof,
comprising a light
chain variable region (LCVR) having a CDR1 domain comprising the amino acid
sequence
of SEQ ID NO: 7, a CDR2 domain comprising the amino acid sequence of SEQ ID
NO: 5,
4
CA 02926384 2016-04-07
and a CDR3 domain comprising the amino acid sequence of SEQ ID NO: 3; and a
heavy
chain variable region (HCVR) having a CDRI domain comprising, the amino acid
sequence
of SEQ ID NO: 8, a CDR2 domain comprising the amino acid sequence of SEQ ID
NO: 6,
and a CDR3 domain comprising the amino acid sequence of SEQ ID NO: 4, wherein
the
composition comprises less than about 10% AR. In one aspect of this
embodiment, the anti-
TNFa antibody, or antigen-binding portion thereof, comprises a light chain
variable region
comprising the amino acid sequence set forth in SEQ ID NO: 1 and a heavy chain
variable
region comprising the amino acid sequence set forth in SEQ ID NO: 2, wherein
the
composition comprises less than about 10% AR. In another aspect of this
embodiment, the
anti-TNFa antibody, or antigen-binding portion thereof, is adalimumab, or an
antigen
binding-portion thereof, and the composition comprises less than about 10% AR.
In one
aspect of this embodiment, the low AR composition comprising an anti-TNFa
antibody, or
antigen-binding portion thereof, comprises about 0.1% or less AR1 and about 3%
or less
AR2, or about 0.0% AR1 and about 1.4% or less AR2.
In one embodiment, the acidic species in the low AR composition comprising an
antibody, or antigen-binding portion thereof (e.g., an anti-TNFa antibody, or
antigen binding
portion thereof, such as adalimumab) comprise one or more variants selected
from the group
consisting of charge variants, structure variants and fragmentation variants
(see, for example,
Figure 188).
For example, in one aspect of this embodiment, the charge variants in the low
AR
composition are AR1 species and comprise, for example, deamidation variants,
glycation
variants, afucosylation variants, methylglyoxal (MGO) variants or citric acid
variants. For
example, when the low AR composition comprises adalimumab, the deamidation
variants can
result from deamidation occuiTing at asparagine residues comprising Asn393 and
Asn329 of
adalimumab and at glutamine residues comprising Gln3 and G1n6. In another
aspect of this
embodiment, when the low AR composition comprises adalimumab, the glycation
variants
can result from glycation occurring at Lys98 and Lys151 of adalimumab.
In another aspect of this embodiment, the structure variants in the low AR
composition comprising an antibody, or antigen-binding portion thereof (e.g.,
an anti-TNFa
antibody, or antigen binding portion thereof, such as adalimumab) are AR1
species and
comprise, for example, glycosylation variants or acetonation variants.
CA 02926384 2016-04-07
In still another aspect of this embodiment, the fragmentation variants in the
low AR
composition comprising an antibody, or antigen-binding portion thereof (e.g.,
an anti-TNFa
antibody, or antigen binding portion thereof, such as adalimumab), are AR1
species and
comprise, for example, Fab fragment variants, C-terminal truncation variants
or variants
missing a heavy chain variable domain.
In another embodiment, the acidic species in the low AR composition comprising
an
antibody, or antigen-binding portion thereof (e.g., an anti-TNFcc antibody, or
antigen binding
portion thereof, such as adalimumab), are AR2 species, and comprise charge
variants, such as
deamidation variants or glycation variants. For example, when the low AR
composition
comprises adalimumab, the deamidation variants can result from deamidation
occurring at
asparagine residues comprising Asn393 and Asn329 of adalimumab and at
glutamine
residues comprising G1n3 and G1n6. In another aspect of this embodiment, when
the low AR
composition comprises adalimumab, the glycation variants result from glycation
occurring at
Lys98 and Lys151 of adalimumab.
In one embodiment, the percent of acidic species in a low AR composition is
determined using ion exchange chromatography, for example WCX-10 HPLC. In
another
aspect of this embodiment, the percent acidic species in a low AR composition
is determined
using isoelectric focusing (IEF).
In one embodiment, the low AR compositions of the invention comprise product
preparation-derived acidic species. For example, in one aspect of this
embodiment, the acidic
species are cell culture-derived acidic species. In another aspect of this
embodiment, the
acidic species of the low AR compositions are storage-derived acidic species
which are
primarily generated when stored under process, intermediate or shelf storage
conditions prior
to use..
In still another embodiment, the invention provides low AR compositions that
further
comprise a pharmaceutically acceptable carrier.
In another aspect, the present invention provides methods for treating a
subject having
a disorder in which TNFla is detrimental, by administering to the subject a
low AR
composition of the invention, e.g., a lovv AR adalimumab composition, thereby
treating the
subject having a disorder in which TNFoc is detrimental. In one aspect of this
embodiment,
the disorder in which TNFa is detrimental is selected from the group
consisting of
6
CA 02926384 2016-04-07
rheumatoid arthritis (RA), psoriasis, psoriatic arthritis, ankylosing
spondylitis, juvenile
idiopathic arthritis (JIA), ulcerative colitis, and Crohn's Disease.
In one aspect, upstream methods for producing the low AR compositions of the
invention are included. In one embodiment, a method for producing a low acidic
species
composition comprising an antibody, or antigen binding portion thereof,
comprises culturing
cells expressing the antibody, or antigen binding portion thereof, in a cell
culture media
comprising an increased concentration of an amino acid selected from the group
consisting of
arginine, lysine, ornithine and histidine, or a combination thereof, as
compared to the amino
acid concentration in cell culture media used to produce a non-low acidic
species
composition comprising the antibody, or antigen binding portion thereof. In
another aspect
of this embodiment, the amino acid concentration in the culture media is
between about 0.025
and 20 g/L.
In another embodiment, a method for producing a low acidic species composition
comprising an antibody, or antigen binding portion thereof, comprises
culturing cells
expressing the antibody, or antigen binding portion thereof, in a cell culture
media
comprising an increased concentration of calcium as compared to the calcium
concentration
in cell culture media used to produce a non-low acidic species composition
comprising the
antibody, or antigen binding portion thereof. In one aspect of this
embodiment, the calcium
concentration is between about 0.005 and 5 mM. In another aspect of this
embodiment, the
cell culture media further comprises an increased concentration of an amino
acid selected
from the group consisting of arginine, lysine, omithine and histidine, or a
combination
thereof, as compared to the amino acid concentration in cell culture media
used to produce a
non-low acidic species composition comprising the antibody, or antigen binding
portion
thereof.
In still another embodiment, a method for producing a low acidic species
composition
comprising an antibody, or antigen binding portion thereof, comprises
culturing cells
expressing the antibody, or antigen binding portion thereof, in a cell culture
media
comprising an increased concentration of niacinamide, calcium, and at least
one amino acid,
as compared to the concentration of niacinamide, calcium, and amino acid in
the cell culture
media used to produce a non-low acidic species composition comprising the
antibody, or
antigen binding portion thereof. In one aspect of the embodiment, the at least
one amino acid
is selected from the group consisting of arginine, lysine, omithine and
histidine, and
combinations thereof.
7
CA 02926384 2016-04-07
In yet another embodiment, a method for producing a low acidic species
composition
comprising an antibody, or antigen binding portion thereof, comprises
culturing cells
expressing the antibody, or antigen binding portion thereof, in a cell culture
media having a
pH of between about 7.1-6.8.
In still another embodiment, a method for producing a low acidic species
composition
comprising an antibody, or antigen binding portion thereof, comprises
culturing cells
expressing the antibody, or antigen binding portion thereof, in a cell culture
media having an
altered exchange rate as compared to the exchange rate of cell culture media
used to produce
a non-low acidic species composition comprising the antibody, or antigen
binding portion
thereof.
In another embodiment, a method for producing a low acidic species composition
comprising an antibody, or antigen binding portion thereof, comprises
cultufing cells
expressing the antibody, or antigen binding portion thereof, extracting a
clarified harvest
from the cell culture, and adding one or more amino acids to the clafified
harvest. In one
aspect of this embodiment, the one or more amino acids are selected from the
group
consisting of arginine, histidine, lysine, aspartic acid, glutamic acid and
leucine, and
combinations thereof.
In yet another embodiment, a method for producing a low acidic species
composition
compfising an antibody, or antigen binding portion thereof, comprises
culturing cells
expressing the antibody, or antigen binding portion thereof, extracting a
clafified harvest
from the cell culture, and adjusting the pH of the clarified harvest to
between about 4.5 and
6.5.
In another aspect of the invention, upstream methods for producing the low AR
compositions of the invention are included. In one embodiment, the invention
includes a
method for producing a low acidic species composition comprising an antibody,
or antigen
binding portion thereof, comprising contacting a first sample comprising the
antibody, or
antigen binding portion thereof, to a chromatography media, wherein the
contact occurs in
the context of a loading buffer; washing the chromatography media with a wash
buffer that is
substantially the same as the loading buffer; and collecting a chromatography
sample,
wherein the chromatography sample comprises a composition of the antibody, or
antigen
binding portion thereof, which contains less than about 10% acidic species,
thereby
producing a low acidic species composition comprising an antibody, or antigen
binding
portion thereof. In one aspect of this embodiment, the bound antibody material
is eluted with
8
CA 02926384 2016-04-07
a buffer having a different composition than the wash buffer. In another
aspect of this
embodiment, the chromatography media is selected from the group consisting of
anion
exchange adsorbent material, cation exchange adsorbent material, mixed mode
media, cation
exchange mixed mode media, and anion exchange mixed mode media. In one
embodiment,
the chromatography media is a mixed mode media comprising cation exchange
(CEX) and
hydrophobic interaction functional groups. In another embodiment, the
chromatography
media is a mixed mode media comprising anion exchange (AEX) and hydrophobic
interaction functional groups. For example, the mixed mode media may be Capto
MMC
resin, the CEX resin may be the Poros XS resin, and the AEX resin may be the
Poros 50HQ
resin.
In one embodiment, the chromatography media is a CEX adsorbent material or a
mixed mode media, and the pH of the loading and wash buffers is lower than the
isoelectric
point of the antibody. In another embodiment, the chromatography sample
contains a
reduced level of antibody fragments as compared to the first sample. In still
another
embodiment, the chromatography sample contains a reduced level of host cell
proteins as
compared to the first sample. In yet another embodiment, the chromatography
sample
contains a reduced level of one or more of charge variants (e.g., deamidation
variants,
glycation variants, afucosylation variants, MGO variants or citric acid
variants), structure
variants (e.g., glycosylation variants or acetonation variants), or
fragmentation variants (e.g.,
Fab fragment variants, C-terminal truncation variants or variants missing a
heavy chain
variable domain) as compared to the first sample.
In one embodiment, a method for producing a low acidic species composition
comprising an antibody. or antigen binding portion thereof, comprises
contacting a first
sample comprising the antibody, or antigen binding portion thereof, to an
affinity
chromatography media (e.g., a Protein A resin) in a load buffer, and eluting
said sample from
the affinity chromatography media as a first eluted sample; contacting the
first eluted sample
to an anion exchange (AEX) chromatography adsorbent material (e.g., a Poros
50HQ resin)
in a load buffer, and eluting said sample from the AEX chromatography
adsorbent material as
a second eluted sample; and contacting the second eluted sample to a cation
exchange (CEX)
chromatography adsorbent material (e.g., a Poros XS resin) in a load buffer,
and eluting said
sample from the CEX chromatography adsorbent material as a third eluted
sample, wherein
the third eluted sample comprises a composition of the antibody, or antigen
binding portion
thereof. which contains less than about 3% acidic species, thereby producing a
low acidic
9
CA 02926384 2016-04-07
species composition comprising an antibody, or antigen binding portion
thereof. In one
embodiment, the second eluted sample is contacted to a CEX chromatography at
least one
additional time. In one embodiment, the method further comprises performing
viral filtration
on the third eluted sample resulting in a filtered sample. In another
embodiment, the method
further comprises filtering the filtered sample using
ultrafiltration/diafilteration (UF/DF).
In another aspect, the invention provides a method for producing a low acidic
species
composition comprising an antibody, or antigen binding portion thereof, the
method
comprising contacting a sample comprising an antibody, or antigen binding
portion thereof to
one or more of the group consisting of: an anion exchange (AEX) chromatography
adsorbent
material, a cation exchange (CEX) clu-omatography adsorbent material, a mixed
mode media,
a cation exchange mixed mode media, and an anion exchange mixed mode media, in
a load
buffer, and eluting the sample from the AEX chromatography adsorbent material,
the CEX
chromatography adsorbent material, the mixed mode media, the cation exchange
mixed mode
media, or the anion exchange mixed mode media, wherein the eluted sample
comprises a
composition of the antibody, or antigen binding portion thereof, which
contains less than
about 3% acidic species, thereby producing a low acidic species composition
comprising an
antibody, or antigen binding portion thereof. In one aspect of this
embodiment, the method
further comprises contacting the eluted sample to a hydrophobic interaction
chromatography
(HIC) media.
The present invention is further illustrated by the following detailed
description and
claims.
BRIEF DESCRIPTION OF THE DRAWINGS
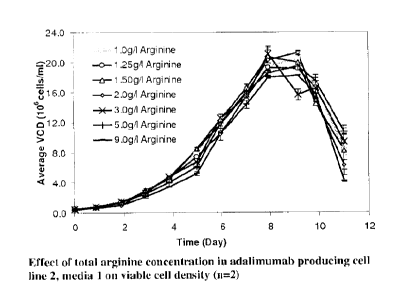
Figure 1 depicts the effect of total arginine concentration in adalimumab
producing
cell line 2, media 1 on viable cell density (n=2).
Figure 2 depicts the effect of total arginine concentration in adalimumab
producing
cell line 1 media 1 on viability (n=2).
Figure 3 depicts the effect of total arginine concentration in adalimumab
producing
cell line 2. media 1 on harvest titer (n=2).
Figure 4 depicts the effect of total arginine concentration in adalimumab
producing
cell line 2. media 1 on day 10 WCX- 10 profile total acidic regions (n=2).
CA 02926384 2016-04-07
Figure 5 depicts the effect of total arginine concentration in adalimumab
producing
cell line 2, media 1 on day 12 WCX-10 profile total acidic regions (n=2).
Figure 6 depicts the effect of total arginine concentration in adalimumab
producing
cell line 3, media 1 on viable cell density (n=2).
Figure 7 depicts the effect of total arginine concentration in adalimumab
producing
cell line 3, media 1 on viability (n=2).
Figure 8 depicts the effect of total arginine concentration in adalimumab
producing
cell line 3, media 1 on harvest titer (n=2).
Figure 9 depicts the effect of total arginine concentration in adalimumab
producing
cell line 3, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 10 depicts the effect of total arginine concentration in adalimumab
producing
cell line 1, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 11 depicts the effect of arginine addition to adalimumab producing cell
line 1,
media 2 on day 11 on WCX-10 profile total acidic regions (n=2).
Figure 12 depicts the effect of arginine addition to adalimumab producing cell
line 2,
media 3 on WCX-10 profile total acidic regions (n=2).
Figure 13 depicts the effect of total arginine concentration in mAbl producing
cell
line on WCX-10 profile total acidic regions (n=1).
Figure 14 depicts the effect of total arginine concentration in mAb2 producing
cell
line on WCX-10 profile total acidic regions (n=2)
Figure 15 depicts the effect of carboxypeptidase digestion of product from
adalimumab producing cell line 3, media 1 experiment on WCX-10 profile total
acidic
regions (n=1).
Figure 16 depicts the effect of carboxypeptidase digestions of product from
mAb2
producing cell line on WCX-10 profile total acidic regions (n=2).
11
CA 02926384 2016-04-07
Figure 17 depicts the effect of total lysine concentration in adalimumab
producing
cell line 2, media 1 on viable cell density (n=2).
Figure 18 depicts the effect of total lysine concentration in adalimumab
producing
cell line 2, media 1 on viability (n=2).
Figure 19 depicts the effect of total lysine concentration in adalimumab
producing
cell line 2, media 1 on harvest titer (n=2).
Figure 20 depicts the effect of total lysine concentration in adalimumab
producing
cell line 2, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 21 depicts the effect of total lysine concentration in adalimumab
producing
cell line 3, media 1 on viable cell density (n=2).
Figure 22 depicts the effect of total lysine concentration in adalimumab
producing
cell line 3, media 1 on viability (n=2).
Figure 23 depicts the effect of total lysine concentration in adalimumab
producing
cell line 3, media 1 on harvest titer (n=2).
Figure 24 depicts the effect of total lysine concentration in adalimumab
producing
cell line 3, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 25 depicts the effect of total lysine concentration in adalimumab
producing
cell line 1, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 26 depicts the effect of lysine addition to adalimumab producing cell
line 1,
media 2 on WCX- 10 profile total acidic regions (n=2).
Figure 27 depicts the effect of lysine addition to adalimumab producing cell
line 2,
media 3 on WCX-1O profile total acidic regions (n=2).
Figure 28 depicts the effect of total lysine concentration in mAbl producing
cell line
on WCX-1O profile total acidic regions (n=1).
Figure 29 depicts the effect of total lysine concentration in mAb2 producing
cell line
on WCX- 10 profile total acidic regions (n=2).
12
CA 02926384 2016-04-07
Figure 30 depicts the effect of carboxypeptidase digestion of product from
cell line 3,
media 1 experiment on WCX-10 profile total acidic regions (n=1).
Figure 31 depicts the effect of carboxypeptidase digestions of product from
mAb2
producing cell line on WCX-10 profile total acidic regions (n=2).
Figure 32 depicts the effect of total histidine concentration in adalimumab
producing
cell line 2, media 1 on viable cell density (n=2).
Figure 33 depicts the effect of total histidine concentration in adalimumab
producing
cell line 2, media 1 on viability (n=2).
Figure 34 depicts the effect of total histidine concentration in adalimumab
producing
cell line 2, media 1 on harvest titer (n=2).
Figure 35 depicts the effect of total histidine concentration in adalimumab
producing
cell line 2, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 36 depicts the effect of total histidine concentration in adalimumab
producing
cell line 3, media 1 on viable cell density (n=2).
Figure 37 depicts the effect of total histidine concentration in adalimumab
producing
cell line 3, media 1 on viability (n=2).
Figure 38 depicts the effect of total histidine concentration in adalimumab
producing
cell line 3, media 1 on harvest titer (n=2).
Figure 39 depicts the effect of total histidine concentration in adalimumab
producing
cell line 3, media I on WCX-I 0 profile total acidic regions (n=2).
Figure 40 depicts the effect of total histidine concentration in adalimumab
producing
cell line 1, media _1 on WCX-10 profile total acidic regions (n=2).
Figure 41 depicts the effect of histidine addition to adalimumab producing
cell line 1,
media 2 on WCX- 10 profile total acidic regions (n=2).
Figure 42 depicts the effect of histidine addition to adalimumab producing
cell line 2.
media 3 on WCX-10 profile total acidic regions (n=2).
13
CA 02926384 2016-04-07
Figure 43 depicts the effect of total histidine concentration in mAb 1
producing cell
line on WCX-10 profile total acidic regions (n=1).
Figure 44 depicts the effect of total histidine concentration in mAb2
producing cell
line on WCX-10 profile total acidic regions (n=2).
Figure 45 depicts the effect of carboxypeptidase digestion of product from
cell line 3,
media 1 experiment on WCX-10 profile total acidic regions (n=1).
Figure 46 depicts the effect of carboxypeptidase digestions of product from
mAb2
producing cell line on WCX-10 profile total acidic regions (n=2).
Figure 47 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 2, media 1 on viable cell density (n=2).
Figure 48 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 2, media 1 on viability (n=2).
Figure 49 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 2, media 1 on harvest titer (n=2).
Figure 50 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 2, media 1 on WCX-10 profile total acidic regions.
Figure 51 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 3, media 1 on viable cell density (n=2).
Figure 52 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 3, media l on viability (n=2).
Figure 53 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 3, media I on harvest titer (n=2).
Figure 54 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 3, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 55 depicts the effect of total ornithine concentration in adalimumab
producing
cell line 1, media 1 on WCX-10 profile total acidic regions (n=2).
14
CA 02926384 2016-04-07
Figure 56 depicts the effect of omithine addition to adalimumab producing cell
line 1,
media 2 on WCX-10 profile total acidic regions (n=2).
Figure 57 depicts the effect of omithine addition to adalimumab producing cell
line 2,
media 3 on WCX-10 profile total acidic regions (n=2).
Figure 58 depicts the effect of total omithine concentration in mAb 1
producing cell
line on WCX-10 profile total acidic regions (n=1).
Figure 59 depicts the effect of total omithine concentration in mAb2 producing
cell
line on WCX-10 profile total acidic regions (n=2).
Figure 60 depicts the effect of carboxypeptidase digestion of product from
cell line 3,
media 1 experiment on WCX-10 profile total acidic regions (n=1).
Figure 61 depicts the effect of carboxypeptidase digestions of product from
mAb2
producing cell line on WCX-10 profile total acidic regions (n=2).
Figure 62 depicts the effect of multiple amino acid additions to adalimumab
producing cell line 2, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 63 depicts the effect of increased arginine and lysine concentration in
adalimumab producing cell line 1, media 1 on viable cell density (n=3).
Figure 64 depicts the effect of increased arginine and lysine concentration in
adalimumab producing cell line 1, media 1 on viability (n=3).
Figure 65 depicts the effect of increased arginine and lysine concentration in
adalimumab producing cell line 1, media 1 on culture titer (n=3).
Figure 66 depicts the effect of increased arginine and lysine concentration in
adalimumab producing cell line 1, media 1 on WCX-10 profile total acidic
regions (n=2).
Figure 67 depicts the effect of arginine, lysine and pH modulation to
adalimumab
producing cell line 1, media 1 on viable cell density (n=2).
Figure 68 depicts the effect of arginine, lysine and pH modulation to
adalimumab
producing cell line 1, media 1 on viability (n=2).
CA 02926384 2016-04-07
Figure 69 depicts the effect of arginine, lysine and pH modulation to
adalimumab
producing cell line 1, media 1 on culture titer (n=2).
Figure 70 depicts the effect of arginine, lysine and pH modulation to
adalimumab
producing cell line 1, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 71 depicts the effect of total calcium concentration in adalimumab
producing
cell line 2, media 1 on viable cell density (n=2).
Figure 72 depicts the effect of total calcium concentration in adalimumab
producing
cell line 2, media 1 on viability (n=2).
Figure 73 depicts the effect of total calcium concentration in adalimumab
producing
cell line 2, media 1 on harvest titer (n=2).
Figure 74 depicts the effect of total calcium concentration in adalimumab
producing
cell line 2, media I on WCX-10 profile total acidic regions (n=2).
Figure 75 depicts the effect of total calcium concentration in adalimumab
producing
cell line 3, media 1 on viable cell density (n=2).
Figure 76 depicts the effect of total calcium concentration in adalimumab
producing
cell line 3, media 1 on viability (n=2).
Figure 77 depicts the effect of total calcium concentration in adalimumab
producing
cell line 3, media 1 on harvest titer (n=2)
Figure 78 depicts the effect of total calcium concentration in adalimumab
producing
cell line 3, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 79 depicts the effect of total calcium concentration in adalimumab
producing
cell line 1, media 1 on WCX-10 profile total acidic regions (n=2).
Figure 80 depicts the effect of calcium addition to adalimumab producing__
cell line 1,
media 2 on WCX-10 profile total acidic regions (n=2).
Figure 81 depicts the effect of calcium addition to adalinriumab producing
cell line 2,
media 3 on WCX-10 profile total acidic regions (n=2).
16
CA 02926384 2016-04-07
Figure 82 depicts the effect of total calcium concentration in mAb 1 producing
cell
line on WCX-10 profile total acidic regions (n=2).
Figure 83 depicts the effect of total calcium concentration in mAb2 producing
cell
line on WCX-10 profile total acidic regions (n=2).
Figures 84A-B depict the effect of multiple amino acid additions to cell line
1, media
1 on WCX-10 profile total acidic regions a) overall prediction plot, b)
prediction plots for
each additive (n=2).
Figure 85 depicts the effect of niacinamide addition to adalimumab producing
cell
line 1, media 1 on viable cell density (n=2).
Figure 86 depicts the effect of niacinamide addition to adalimumab producing
cell
line 1, media 1 on viability (n=2).
Figure 87 depicts the effect of niacinamide addition to adalimumab producing
cell
line 1, media 1 on harvest titer (n=2).
Figure 88 depicts the effect of niacinamide addition to adalimumab producing
cell
line 1, media 1 on Day 11 WCX-10 profile total acidic regions (n=2).
Figure 89 depicts the effect of niacinamide addition to adalimumab producing
cell
line 1, media 1 on Day 12 WCX-10 profile total acidic regions (n=2).
Figure 90 depicts the effect of niacinamide addition to mAb2 producing cell
line,
media 1 on viable cell density (n=2).
Figure 91 depicts the effect of niacinamide addition to mAb2 producing cell
line,
media 1 on viability (n=2).
Figure 92 depicts the effect of niacinamide addition to mAb2 producing cell
line,
media 1 on harvest titer (n=2).
Figure 93 depicts the effect of niacinamide addition to mAb2 producing cell
line,
media 1 on WCX- 10 profile total acidic regions (n=2).
17
CA 02926384 2016-04-07
Figures 94A-D depict the effect of amino acid supplementation to CD media GIA-
1
in adalimumab-producing CHO cell line #1 on (A) culture growth, (B) culture
viability, (C)
acidic species, and (D) MGO modification.
Figure 95 depicts the effect of pH modulation of adalimumab producing cell
line 1,
media 1 on viable cell density.
Figure 96 depicts the effect of pH modulation of adalimumab producing cell
line 1,
media 1 on viability.
Figure 97 depicts the effect of pH modulation of adalimumab producing cell
line 1,
media 1 on harvest titer.
Figure 98 depicts the effect of pH modulation of adalimumab producing cell
line 1,
media 1 on WCX-10 profile total acidic regions.
Figure 99 depicts the effect of pH modulation of adalimumab producing cell
line 1,
media 2 on viable cell density.
Figure 100 depicts the effect of pH modulation addition of adalimumab
producing
adalimumab producing cell line 1, media 2 on viability.
Figure 101 depicts the effect of pH modulation of adalimumab producing cell
line 1,
media 2 on harvest titer.
Figure 102 depicts the effect of pH modulation of adalimumab producing cell
line I,
media 2 on WCX-10 profile total acidic regions.
Figure 103 depicts the effect of pH modulation of adalimumab producing cell
line 3,
media 1 on viable cell density.
Figure 104 depicts the effect of pH modulation adalimumab producing cell line
3,
media 1 on viability..
Figure 105 depicts the effect of pH modulation of adalimumab producing cell
line 3,
media 1 on harvest titer.
18
CA 02926384 2016-04-07
Figure 106 depicts the effect of pH modulation of adalimumab producing cell
line 3,
media 1 on WCX-10 profile total acidic regions.
Figure 107 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 2 at 35 C on viable cell density.
Figure 108 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 2 at 35 C on viability.
Figure 109 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 2 at 35 C on harvest titer.
Figure 110 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 2 at 35 C on WCX-10 profile total acidic regions.
Figure 111 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 2 at 33 C on viable cell density.
Figure 112 depicts the effect of dissolved oxygen modulation to adalimumab
producing cell line 1, media 2 at 33 C on viability.
Figure 113 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line I, media 2 at 33 C on harvest titer.
Figure 114 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 2 at 33 C on WCX-10 profile total acidic regions.
Figure 115 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 1 at 35 C on viable cell density.
Figure 116 depicts the effect of dissolved oxygen modulation to adalimumab
producing cell line I, media 1 at 35 C on viability.
Figure 117 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 1 at 35 C on harvest titer.
Figure 118 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 1, media 1 on WCX-10 profile total acidic regions.
19
CA 02926384 2016-04-07
Figure 119 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 3, media 1 on viable cell density.
Figure 120 depicts the effect of dissolved oxygen modulation to adalimumab
producing cell line 3, media 1 on viability.
Figure 121 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 3, media 1 on harvest titer.
Figure 122 depicts the effect of dissolved oxygen modulation of adalimumab
producing cell line 3, media 1 on WCX-10 profile total acidic regions.
Figure 123 depicts the effect of dissolved oxygen modulation to mAb2 producing
cell
line, media 1 on viable cell density.
Figure 124 depicts the effect of dissolved oxygen modulation addition to mAb2
producing cell line, media 1 on viability.
Figure 125 depicts the effect of dissolved oxygen modulation to mAb2 producing
cell
line, media 1 on harvest titer.
Figure 126 depicts the effect of dissolved oxygen modulation to mAb2 producing
cell
line, media 1 on WCX-10 profile total acidic regions.
Figure 127 depicts an acidification sample preparation scheme.
Figure 128 depicts an arginine sample preparation scheme.
Figure 129 depicts a histidine sample preparation scheme.
Figure 130 depicts a lysine sample preparation scheme.
Figure 131 depicts a methionine sample preparation scheme.
Figure 132 depicts an amino acid sample preparation scheme.
Figure 133 depicts a CDM clarified harvest sample preparation scheme.
Figure 134 depicts an acid-type pH study sample preparation scheme.
CA 02926384 2016-04-07
Figure 135 depicts the effect of low pH treatment with subsequent
neutralization on
initial acidic variant content.
Figure 136 depicts the effect of low pH treatment with subsequent
neutralization on
acidic variant formation rate.
Figure 137 depicts the effect of sample preparation method on initial acidic
variant
content.
Figure 138 depicts the effect of sample preparation method on initial acidic
variant
content.
Figure 139 depicts the dose dependent effect of arginine on reduction of
acidic
variant formation rate.
Figure 140 depicts the effect of histidine concentration on initial acidic
variant
content.
Figure 141 depicts the effect of histidine concentration on acidic variant
formation
rate.
Figure 142 depicts the effect of lysine on initial acid variant content.
Figure 143 depicts the effect of lysine on acidic variant formation rate.
Figure 144 depicts the effect of methionine on initial acid variant content.
Figure 145 depicts the effect of methionine on acidic variant formation rate.
Figure 146 depicts the effect of amino acids on initial acid variant content.
Figure 147 depicts the effect of amino acids on acidic variant formation rate.
Figure 148 depicts the effect of alternative additives on initial acid variant
content.
Figure 149 depicts the effect of alternative additives on acidic variant
formation rate.
Figure 150 depicts the effect of low pH/arginine treatment on adalimumab CDM
initial acid variant content.
21
CA 02926384 2016-04-07
Figure 151 depicts the effect of low pH/arginine treatment on adalimumab CDM
acidic variant formation rate.
Figure 152 depicts the effect of low pH/arginine treatment on mAb B
hydrolysate
initial acid variant content.
Figure 153 depicts the effect of low pH/arginine treatment on mAb B
hydrolysate
acidic variant formation rate.
Figure 154 depicts the effect of low pH/arginine treatment on mAb C
hydrolysate
initial acid variant content.
Figure 155 depicts the effect of low pH/arginine treatment on mAb C
hydrolysate
acidic variant formation rate.
Figure 156 depicts the effect of acid type/pH on acid variant content.
Figure 157 depicts the effect of acid concentration on acid variant content.
Figure 158 depicts the effect of acid concentration on acid variant content.
Figure 159 depicts the effect of neutralization on acid variant content.
Figure 160 depicts the effect of neutralization on acid variant content.
Figure 161 depicts the effect of medium exchange rate and the supplementation
of
amino acids arginine and lysine on total acidic species reduction.
Figure 162 depicts LC/MS peptide mapping analysis of exemplary antibodies
expressed in the context of the cell culture conditions of the instant
invention, including
preparation of specific mass traces for both modified and non-modified
peptides in order to
accurately quantify the total amount of MGO modification. Mass spectra are
also analyzed
for the specific region of the chromatogram to confirm the peptide identity.
Figure 163 depicts a chromatogram wherein the total acidic species associated
with
the expression of adalimumab is divided into a first acidic species region
(ARO and a second
acidic species region (AR2).
Figure 164 depicts the AR growth at 25cC of low and high AR containing
samples.
CA 02926384 2016-04-07
Figure 165 depicts a process chromatogram of pH gradient elution in the
context of
AEX chromatography.
Figure 166 depicts a process chromatogram of a linear gradient elution by
increasing
anion concentration in the context of AEX chromatography.
Figure 167 depicts a process chromatogram of fractionation of 300 g/L load and
wash
in the context of AEX chromatography.
Figure 168 depicts the effect of pH on AR reduction in the context of AEX
chromatography.
Figure 169 depicts a process chromatogram at different salt (cation)
concentrations in
the context of CEX chromatography.
Figure 170 depicts recovery versus AR reduction in the context of CEX
purification
of adalimumab.
Figure 171 depicts the WCX-10 profile of glycated load material and CEX
eluate.
Figure 172 depicts the WCX-10 profile of MGO modified load material and eluate
from CEX column employing Toyo Pearl MX TRP 650M resin.
Figure 173 depicts the change in lysine distribution during CEX
chromatography,
highlighting the effect of Tris concentration.
Figure 174 depicts the effect of pH and conductivity on adalimumab AR
reduction
and recovery yield in the context of MM chromatography.
Figure 175 depicts the AR reduction achieved with the corresponding protein
recovery in the context of MM chromatography.
Figure 176 depicts the total adalimumab Protein concentration levels and AR
levels
during Flow Through and Wash.
Figure 177 depicts the total mAb B Protein concentration levels and AR levels
during
Flow Through and Wash in the context of MM chromatography.
Figure 178 depicts the total mAb C Protein concentration levels and AR levels
during
Flow Through and Wash in the context of MM chromatography.
Figure 179 depicts the Cumulative % AR breakthrough of mAb C on different MM
resins.
23
CA 02926384 2016-04-07
Figure 180 depicts the impact of pH-pl and conductivity on adalimumab AR
reduction in the context of MM chromatography.
Figure 181 depicts the impact of pH-pI and conductivity on mAb B AR reduction
in
the context of MM chromatography.
Figure 182 depicts the impact and trend of pH-pI on mAb C AR reduction with
multiple resins in the context of MM chromatography.
Figure 183 depicts the effect of pH and conductivity on AR reduction and Yield
in
the context of MM chromatography.
Figure 184 depicts AR reduction and protein recovery vs. pH in the context of
MM
chromatography.
Figure 185 depicts the effect of pH, conductivity and protein load amount on
AR
reduction and yield.
Figure 186 depicts the effect of pH, conductivity and protein load amount on
AR
reduction and yield.
Figure 187 depicts the effect of AEX adsorbent pKa for mAb B with several
different
AEX adsorbents, with different pKa values, run at with an acetate/Tris buffer
at pH 9.1.
Figure 188 is a schematic depiction of exemplary AR1 and AR2 present in a
composition comprising an exemplary antibody. Preparation-derived ARs and
storage-
derived ARs are depicted.
Figure 189 depicts cumulative AR reduction as a function of yield for various
formic
acid concentrations.
Figure 190 depicts an exemplary flow path for the production of a low AR
composition. Figure 191 depicts an experimental scheme for a -Continuous
Chromatography' process of producing a low AR composition.
Figure 192 depicts the percent AR in each of the cycles of the continuous MM
process.
Figure 193 depicts a chromatogram wherein acidic and basic species are
identified in
adalimumab and various fractions are delineated.
24
CA 02926384 2016-04-07
Figures 194A-B depict (A) the average arthritic scores and (B) growth related
weight
gain of mice administered low AR composition, AR1 composition, .Lys-1/2
composition, and
control AR composition.
Figure 195 depicts the average arthritic scores (area under the curve) of mice
administered low AR composition, AR1 composition, Lys-1/2 composition, and
control AR
composition.
Figures 196A-B depict (A) the average trough serum drug levels and (B) the
average
trough serum ADA levels for mice administered low AR composition, AR1
composition,
Lys-1/2 composition, and control AR composition.
Figure 197 depicts the average PK and ADA profiles (area under the curve) for
mice
administered low AR composition, AR1 composition, Lys-1/2 composition, and
control AR
composi ti on .
Figure 198 depicts complexed TNF levels (area under the curve) and shows that
the
cumulative serum concentration values of adalimumab for mice administered low
AR
composition, AR1 composition, Lys-I/2 composition, and control AR composition
during the
ten week treatment period was highest for the low AR and the control AR
compositions and
lowest for the AR I fraction.
Figure 199 depicts the chondrocyte death, synovial proliferation, proteoglycan
loss,
cartilage destruction, and bone erosion of mice administered low AR
composition, AR1
composition, Lys-1/2 composition, and control AR composition.
Figures 200A-D illustrate the average drug levels for various tissues (paw,
lymph
node, spleen, skin, knee and serum) for mice administered (A) low AR
composition; (B)
control AR composition; (C) AR 1 composition; and (D) Lys-1/2 composition.
Figures 201A-D illustrate the average ADA levels for various tissues (paw,
lymph
node, spleen, skin, knee and serum) for mice administered (A) low AR
composition; (B)
control AR composition; (C) AR1 composition; and (D) Lys-I/2 composition.
Figures 202A-D show the results of a micro CT analysis of spines and femurs
obtained from TNF-Tg197 transgenic mice which were administered placebo, low
AR
composition. control (normal) AR composition, AR 1 composition, and Lys-1/2
composition.
The graphs depict the effect of the administered compositions on (A) vertebra
bone volume;
CA 02926384 2016-04-07
(B) vertebra trabecular number; (C) vertebra trabecular thickness; and (D)
vertebra trabecular
space.
Figures 203A-D show the results of a micro CT analysis of spines and femurs
obtained from TNF-Tg197 transgenic mice which were administered placebo, low
AR
composition, control (normal) AR composition, AR1 composition, and Lys-1/2
composition.
The graphs depict the effect of the administered compositions on (A) vertebra
bone loss; (B)
vertebra trabecular number; (C) vertebra trabecular thickness; and (D)
vertebra trabecular
space.
Figures 204A-D show results of a micro CT analysis of spines and femurs
obtained
from TNF-Tg197 transgenic mice which were administered placebo, low AR
composition,
control (normal) AR composition, AR1 composition, and Lys-1/2 composition. The
graphs
depict the effect of the administered compositions on (A) trabecular bone
volume/total
volume at the femoral metaphysis; (B) trabecular number at the femoral
metaphysis; (C)
trabecular thickness at the femoral metaphysis; and (D) trabecular separation
at the femoral
metaphysis.
Figure 205 depicts micro CT images of the spine from each of six groups of
mice
administered the following compositions: naïve, vehicle (control), low AR
composition
(group 5), low host cell protein (HCP) composition (group 7), AR1 composition
(containing
only AR1 acidic variants) (group 8), and Lys-1/2 composition (containing only
Lys 1 and Lys
2 variants) (group 9).
Figure 206 depicts micro CT images of the femur from each of six groups of
mice
administered the following compositions: naïve, vehicle (control), low AR
composition
(group 5), low host cell protein (HCP) composition (group 7), AR1 composition
(containing
only AR 1 acidic variants) (uoup 8), and Lys-1/2 composition (containing only
Lys 1 and Lys
2 variants) (group 9).
DETAILED DESCRIPTION OF THE INVENTION
The present invention is based on the identification and optimization of
upstream and
downstream process technologies for protein production, e.g., production of
antibodies or
antigen-binding portions thereof, resulting in the production of protein
compositions that
26
CA 02926384 2016-04-07
comprise low percentages of acidic species (AR) and/or low levels of process-
related
impurities (e.g., host cell proteins and media components).
As demonstrated herein, the compositions of the present invention exhibit
increased
therapeutic efficacy when administered to a subject. For example, compositions
comprising
anti-TNFa antibodies, or antigen binding portions thereof, comprising low AR
are capable of
increased therapeutic efficacy in the treatment and prevention of a disorder
in which TNFot is
detrimental, e.g., rheumatoid arthritis (RA), juvenile idiopathic arthritis
(BA), psoriasis,
psoriatic arthritis, ankylosing spondylitis, Crohn's disease, and ulcerative
colitis.
Accordingly, the instant invention provides compositions comprising proteins
that comprise
low AR and/or low levels of process-related impurities, and methods for
producing and using
the same.
In one embodiment, the low AR compositions of the invention comprise about 15%
or
less AR, 14% or less AR, 13% or less AR, 12% or less AR, 11% or less AR, 10%
or less AR,
9% or less AR, 8% or less AR, 7% or less AR, 6% or less AR, 5% or less AR,
4.5% or less
AR, 4% or less AR, 3.5% or less AR, 3% or less AR, 2.5% or less AR, 2% or less
AR, 1.9%
or less AR, 1.8% or less AR, 1.7% or less AR, 1.6% or less AR, 1.5% or less
AR, 1.4% or
less AR, 1.3% or less AR, 1.2% or less AR, 1.1% or less AR, 1% or less AR,
0.9% or less
AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less AR, 0.5% or less AR, 0.4%
or less AR,
0.3% or less AR, 0.2% or less AR, 0.1% or less AR, or 0.0% AR, and ranges
within one or
more of the preceding. In one aspect of this embodiment, the low AR
compositions of the
invention comprise about 0.0% to about 1.0% AR, about 0.0% to about 5% AR,
about 0.0% to
about 4% AR, about 0.0% to about 3% AR, about 0.0% to about 2% AR, about 3% to
about
5% AR, about 5% to about 8% AR, or about 8% to about 10% AR, or about 10% to
about
15% AR, and ranges within one or more of the preceding. In one embodiment, the
composition of the invention is not a composition, e.g., an adalimumab
composition,
comprising 2.4% or 2.57c AR.
In another embodiment, the low AR composition comprises a first acidic species
(AR1) and a second acidic species (AR2). In one aspect of this embodiment, the
low AR
composition comprises about 0.1% or less AR I and about 3% or less AR2. In
another aspect
of this embodiment, the low AR composition comprises about 0.0% AR1 and about
1.4% or
less AR2.
27
CA 02926384 2016-04-07
In another aspect of this embodiment, the low AR composition comprises about
15%
or less AR1, 14% or less AR1, 13% or less AR1, 12% or less AR1, 11% or less
AR1, 10% or
less AR1, 9% or less AR1, 8% or less AR1, 7% or less AR1, 6% or less AR1, 5%
or less
AR1, 4.5% or less AR1, 4% or less AR1, 3.5% or less AR1, 3% or less AR1, 2.5%
or less
AR1, 2% or less AR1, 1.9% or less AR1, 1.8% or less AR1, 1.7% or less AR1,
1.6% or less
AR I, 1.5% or less AR1, 1.4% or less AR1, 1.3% or less AR1, 1.2% or less AR I,
1.1% or less
AR1, 1% or less AR1, 0.9% or less AR I, 0.8% or less AR1, 0.7% or less AR1:
0.6% or less
AR I, 0.5% or less AR-1, 0.4% or less AR1 or less, 0.3% or less AR1 or less,
0.2% or less
AR1 or less, 0.1% or less AR1, or 0.0% AR1, and ranges within one or more of
the
preceding. In one aspect of this embodiment, the low AR compositions of the
invention
comprise about 0.0% to about 10% AR1, about 0.0% to about 5% AR1, about 0.0%
to about
4% AR1, about 0.0% to about 3% AR1, about 0.0% to about 2% AR1, about 3% to
about 5%
AR1, about 5% to about 8% AR1, or about 8% to about 10% AR1, or about 10% to
about
15% AR1, and ranges within one or more of the preceding. In one embodiment,
the
composition of the invention is not a composition, e.g., an adalimumab
composition,
comprising 0.2% AR1.
In yet another aspect of this embodiment, the low AR composition comprises
about
15% or less AR2, 14% or less AR2, 13% or less AR2, 12% or less AR2, 11% or
less AR2,
10% or less AR2, 9% or less AR2, 8% or less AR2, 7% or less AR2, 6% or less
AR2, 5% or
less AR2, 4.5% or less AR2, 4% or less AR2, 3.5% or less AR2, 3% or less AR2,
2.5% or
less AR2, 2% or less AR2, 1.9% or less AR2, 1.8% or less AR2, 1.7% or less
AR2, 1.6% or
less AR2, 1.5% or less AR2, 1.4% or less AR2, 1.3% or less AR2, 1.2% or less
AR2, 1.1% or
less AR2, 1% or less AR2, 0.9% or less AR2, 0.8% or less AR2, 0.7% or less
AR2, 0.6% or
less AR2. 0.5% or less AR2, 0.4% or less AR2, 0.3% or less AR2, 0.2% or less
AR2, 0.1% or
less AR2, or 0.0% AR2, and ranges within one or more of the preceding. In one
aspect of
this embodiment, the low AR compositions of the invention comprise about 0.0%
to about
10% AR2, about 0.0% to about 5% AR2, about 0.0% to about 4% AR2, about 0.0% to
about
3% AR2, about 0.0% to about 2% AR2, about 3% to about 5% AR2. about 5% to
about 8%
AR2, or about 8% to about 10% AR2, or about 10% to about 15% AR2, and ranges
within
one or more of the preceding. In one embodiment, the composition of the
invention is not a
composition, e.g., an adalimumab composition, comprising 2.2% AR2.
In another embodiment, the low AR composition. e.g., a low AR composition of
adalimumab, comprises about 1.4% or less AR. For example, in one aspect of
this
28
CA 02926384 2016-04-07
embodiment, the low AR composition, e.g., a low AR composition of adalimumab
comprising about 1.4% or less AR comprises about 0.0% AR I and about 1.4% or
less AR2.
In one embodiment, the protein is an antibody or antigen binding portion
thereof, such
as adalimumab, or an antigen binding portion thereof.
1. Definitions
In order that the present invention may be more readily understood, certain
terms are
first defined.
As used herein, the terms "acidic species," "acidic region," and "AR," refer
to the
variants of a protein, e.g., an antibody or antigen-binding portion thereof,
which are
characterized by an overall acidic charge. For example, in monoclonal antibody
(mAb)
preparations, such acidic species can be detected by various methods, such as
ion exchange,
for example, WCX-10 HPLC (a weak cation exchange chromatography), or IEF
(isoelectric
focusing). As depicted in Figure 188, acidic species of an antibody may
include charge
variants, structure variants, and/or fragmentation variants.
Exemplary charge variants
include, but are not limited to, deamidation variants, afucosylation variants,
methylglyoxal
(MGO) variants, glycation variants, and citric acid variants. Exemplary
structure variants
include, but are not limited to, glycosylation variants and acetonation
variants. Exemplary
fragmentation variants include any truncated protein species from the target
molecule due to
dissociation of peptide chain, enzymatic and/or chemical modifications,
including, but not
limited to, Fc and Fab fragments, fragments missing a Fab, fragments missing a
heavy chain
variable domain, C-terminal truncation variants, variants with excision of N-
terminal Asp in
the light chain, and variants having N-terminal truncation of the light chain.
Other acidic
species variants include variants containing unpaired disulfides, host cell
proteins, and host
nucleic acids, chromatographic materials, and media components.
In certain embodiments, a protein composition can comprise more than one type
of
acidic species variant. For example, but not by way of limitation, the total
acidic species can
be divided based on chromatographic retention time of the peaks appearing, for
example, in a
WCX-10 Weak Cation Exchange HPLC of the protein preparation. Figure 163
depicts a non-
limiting example of such a division wherein the total acidic species
associated with the
expression of adalimumab is divided into a first acidic species region (AR1)
and a second
acidic species region (AR2).
29
CA 02926384 2016-04-07
As depicted schematically in Figure 188, AR1 can comprise, for example, charge
variants such as deamidation variants. MGO modified species, glycation
variants, and citric
acid variants, structural variants such as glycosylation variants and
acetonation variants,
and/or fragmentation variants. In another embodiment, AR2 can comprise, for
example,
charge variants such as glycation variants and deamidation variants.
With respect, in particular, to adalimumab (and antibodies sharing certain
structural
characteristics of adalimumab, e.g., one or more CDR and/or heavy and light
chain variable
regions of adalimumab), AR1 charge variants can comprise, but are not limited
to,
deamidation variants, glycation variants, afucosylation variants, MGO (e.g.,
MGO variants at
the residues shown in Table 5, below) variants or citric acid variants. In one
embodiment,
deamidation variants result from deamidation occurring at asparagine residues
comprising
Asn393 and Asn329 and at glutamine residues comprising G1n3 and G1n6. In
another
embodiment, the glycation variants result from glycation occurring at Lys98
and Lys151.
AR1 structure variants can comprise, but are not limited to, glycosylation
variants or
acetonation variants.
AR1 fragmentation variants can comprise Fc and Fab fragments, fragments
missing a
Fab, fragments missing a heavy chain variable domain, C-terminal truncation
variants,
variants with excision of N-terminal Asp in the light chain, and variants
having N-terminal
truncation of the light chain.
AR2 charge variants can comprise, but are not limited to, deamidation variants
or
glycation variants, wherein the deamidation variants can result from
deamidation occurring at
asparagine residues comprising Asn393 and Asn329 and at glutamine residues
comprising
CI1113 and G1n6, and the glycation variants can result from glycation occun-
ing at Lys98 and
Lys151.
The term "acidic species" does not include process-related impurities. The
term
"process-related impurity," as used herein, refers to impurities that are
present in a
composition comprising a protein but are not derived from the protein itself.
Process-related
impurities include, but are not limited to, host cell proteins (HCPs), host
cell nucleic acids,
chromatographic materials, and media components. A "low process-related
impurity
composition," as used herein, refers to a composition comprising reduced
levels of process-
related impurities as compared to a composition wherein the impurities were
not reduced.
CA 02926384 2016-04-07
For example, a low process-related impurity composition may contain about 10%,
9%, 8%,
'7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.5%, or less of process-related impurities.
In one
embodiment, a low process-related impurity composition is free of process-
related impurities
or is substantially free of process-related impurities.
The acidic species may be the result of product preparation (referred to
herein as
"preparation-derived acidic species"), or the result of storage (refen-ed to
herein as "storage-
derived acidic species"). Preparation-derived acidic species are acidic
species that are formed
during the preparation (upstream and/or downstream processing) of the protein,
e.g., the
antibody or antigen-binding portion thereof. For example, preparation-derived
acidic species
can be formed during cell culture ("cell culture-derived acidic species").
Storage-derived
acidic species are acidic species that may or may not be present in the
population of proteins
directly after preparation, but are formed or generated while the sample is
being stored. The
type and amount of storage-derived acidic species can vary based on the
formulation of the
sample. Formation of storage-derived acidic species can be partially or
completely inhibited
when the preparation is stored under particular conditions. For example, an
aqueous
formulation can be stored at a particular temperature to partially or
completely inhibit AR
formation. For example, formation or storage-derived AR can be partially
inhibited in an
aqueous formulation stored at between about 2 C and 8 C, and completely
inhibited when
stored at -80 C. In addition, a low- AR composition can be lyophilized or
freeze-dried to
partially or completely inhibit the formation of storage-derived AR.
The term "low acidic species composition," or "low AR composition," as used
herein, refers to a composition comprising an antibody or antigen-binding
portion thereof,
wherein the composition contains less than about 15% acidic species. As used
herein, the
percent AR in the low AR composition refers to the weight of the acidic
species in a sample
in relation to the weight of the total antibodies contained in the sample. For
example, the
percent AR can be calculated using weak cation exchange chromatography such as
WCX-10,
as described in, for example, Example 1 below.
In one embodiment, a low AR composition of the invention may comprise about
15%
or less AR, 14% or less AR, 13% or less AR, 12% or less AR, 11% or less AR,
10% or less
AR, 9% or less AR, 8% or less AR, 7% or less AR, 6% or less AR, 5% or less AR,
4.5% or
less AR, 4% or less AR, 3.5% or less AR, 3% or less AR, 2.5% or less AR, 2% or
less AR,
1.9% or less AR, 1.8% or less AR, 1.7% or less AR. 1.6% or less AR, 1.5% or
less AR, 1.4%
or less AR, 1.3% or less AR. 1.2% or less AR. 1.1% or less AR, 1% or less AR,
0.9% or less
31
CA 02926384 2016-04-07
AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less AR, 0.5% or less AR, 0.4%
or less AR,
0.3% or less AR, 0.2% or less AR, 0.1% or less AR, or 0.0% AR, and ranges
within one or
more of the preceding. A low AR composition of the invention may also comprise
about
0.0% to about 10% AR, about 0.0% to about 5% AR, about 0.0% to about 4% AR,
about
0.0% to about 3% AR, about 0.0% to about 2% AR, about 3% to about 5% AR, about
5% to
about 8% AR, or about 8% to about 10% AR, or about 10% to about 15% AR, and
ranges
within one or more of the preceding.
A low AR composition of the invention may comprise about 15% or less AR1, 14%
or less AR1, 13% or less AR1, 12% or less AR1, 11% or less AR1, 10% or less
AR1, 9% or
less AR1, 8% or less AR1, 7% or less AR1, 6% or less AR1, 5% or less AR1, 4.5%
or less
AR1, 4% or less AR1, 3.5% or less AR1, 3% or less AR1, 2.5% or less AR1, 2% or
less
AR1, 1.9% or less AR1, 1.8% or less AR1, 1.7% or less AR1, 1.6% or less AR1,
1.5% or less
AR1, 1.4% or less AR1, 1.3% or less AR1, 1.2% or less AR1, 1.1% or less AR1,
1% or less
AR1, 0.9% or less AR1, 0.8% or less AR I, 0.7% or less AR I, 0.6% or less AR1,
0.5% or less
AR1, 0.4% or less AR1, 0.3% or less AR1, 0.2% or less AR1, 0.1% or less AR1,
or 0.0%
AR1, and ranges within one or more of the preceding. A low AR composition of
the
invention may also comprise about 0.0% to about 10% AR1, about 0.0% to about
5% AR1,
about 0.0% to about 4% AR1, about 0.0% to about 3% AR1, about 0.0% to about 2%
AR1,
about 3% to about 5% AR1, about 5% to about 8% AR1, or about 8% to about 10%
AR1, or
about 10% to about 15% AR1, and ranges within one or more of the preceding.
A low AR composition of the invention may also comprise about 15% or less AR2,
14% or less AR2, 13% or less AR2, 12% or less AR2, 11% or less AR2, 10% or
less AR2,
9% or less AR2, 8% or less AR2, 7% or less AR2, 6% or less AR2, 5% or less
AR2, 4.5% or
less AR2, 4% or less AR2, 3.5% or less AR2, 3% or less AR2, 2.5% or less AR2,
2% or less
AR2, 1.9% or less AR2, 1.8% or less AR2, 1.7% or less AR2, 1.6% or less AR2,
1.5% or less
AR2, 1.4% or less AR2, 1.3% or less AR2, 1.2% or less AR2, 1.1% or less AR2,
1% or less
AR2, 0.9% or less AR2, 0.8% or less AR2, 0.7% or less AR2, 0.6% or less AR2,
0.5% or less
AR2, 0.4% or less AR2, 0.3% or less AR2, 0.2% or less AR2, 0.1% or less AR2,
or 0.0%
AR2, and ranges within one or more of the preceding. A low AR composition of
the
invention may also comprise about 0.0% to about 10% AR2, about 0.0% to about
5% AR2,
about 0.0% to about 4% AR2, about 0.0% to about 3% AR2, about 0.0% to about 2%
AR2,
about 3% to about 5% AR2, about 5% to about 8% AR2. or about 8% to about 10%
AR2, or
about 10% to about 15% AR2, and ranges within one or more of the preceding.
CA 02926384 2016-04-07
In one embodiment, a low AR composition comprises between about 0.0% and about
3% AR1. In another embodiment, a low AR composition comprises about between
about
0.0% and about 3% AR2. In still another embodiment, a low acidic species
composition
comprises about 3% or less AR2.
In another embodiment, the low AR composition comprises about 1.4% or less AR.
For example, in one embodiment, the composition comprises about 1.4% AR2 and
about
0.0% AR1.
In one embodiment, a low AR composition of the invention may comprise about
15%
or less, 14% or less, 13% or less, 12% or less, 11% or less, 10% or less, 9%
or less, 8% or
less, 7% or less, 6% or less, 5% or less, 4.5% or less, 4% or less, 3.5% or
less, 3% or less,
2.5% or less, 2% or less, 1.9% or less, 1.8% or less, 1.7% or less, 1.6% or
less, 1.5% or less,
1.4% or less, 1.3% or less, 1.2% or less, 1.1% or less, 1% or less, 0.9% or
less, 0.8% or less,
0.7% or less, 0.6% or less, 0.5% or less, 0.4% or less, 0.3% or less, 0.2% or
less, 0.1% or
less, or 0.0% of one or more of a deamidation variant, an afucosylation
variant, an MGO
variant, a glycation variant, a citric acid variant, a glycosylation variant,
an acetonation
variant, or a fragmentation variant, and ranges within one or more of the
preceding. In one
aspect of this embodiment, a low AR composition of the invention may also
comprise about
0.0% to about 10%, about 0.0% to about 5%, about 0.0% to about 4%, about 0.0%
to about
3%, about 0.0% to about 2% about 3% to about 5%, about 5% to about 8%, or
about 8% to
about 10%, or about 10% to about 15%, of one or more of a deamidation variant,
an
afucosylation variant, an MGO variant, a glycation variant, a citric acid
variant, a
glycosylation variant, an acetonation variant, or a fragmentation variant, and
ranges within
one or more of the preceding. For example, a low AR composition of the
invention may
comprise less than 15% of a deamidation variant, while each of the other
acidic variants,
alone or in combination, are at a percentage that is greater than 15%.
The term "non-low acidic species composition," as used herein, refers to a
composition comprising an antibody or antigen-binding portion thereof, which
contains more
than about 16% acidic species. For example, a non-low acidic species
composition may
contain about 16% or more, 177c or more, 18 (7c. or more, 19% or more, 20% or
more, 21% or
more, 22% or more, 23% or more, 24% or more, or 25% or more acidic species. In
one
embodiment, a non-low acidic species composition can comprise about 16% or
more, 17% or
more, 18% or more, 19% or more. 20% or more, 21% or more, 22% or more, 23% or
more,
24% or more. or 25% or more of AR1. In another embodiment, a non-low acidic
species
33
CA 02926384 2016-04-07
composition can comprise about 16% or more, 17% or more, 18% or more, 19% or
more,
20% or more, 21% or more, 22% Or more, 23% or niore, 24% or more, or 25% or
more of
AR2, and ranges within one or more of the preceding.
In one embodiment, a low AR composition has improved biological and functional
properties, including increased efficacy in the treatment or prevention of a
disorder in a
subject, e.g., a disorder in which TNFa activity is detrimental, as compared
to a non-low
acidic species composition. In one embodiment, the low AR composition
comprises an anti-
TNFa antibody, or antigen-binding portion thereof, such as adalimumab or a
fragment
thereof. For example, in one embodiment, a low AR composition comprising an
antibody, or
antigen-binding portion thereof, exhibits increased cartilage penetration,
decreased bone
erosion, and/or reduced cartilage destruction, as compared to a non-low acidic
species
composition cotnprising the same antibody or antigen binding portion thereof,
when
administered to a subject suffering from a disorder in which TNFa activity is
detrimental.
As used herein, the term "increased cartilage penetration" refers to increased
penetration of cartilage in vivo by a low AR composition as compared to a non-
low AR
composition comprising the same antibody or antigen binding portion thereof.
As used herein, the term "reduced cartilage destruction" refers to measurable
decrease
in destruction of cartilage tissue in vivo by a low AR composition as compared
to a non-low
AR composition comprising the same antibody or antigen binding portion
thereof. As used
herein, the term "decreased bone erosion" refers to measurable decrease, in
vivo, of the
erosion of bone tissue by a low AR composition as compared to a non-low acidic
species
composition comprising the same antibody or antigen binding portion thereof.
For example,
an in vivo model of a disease or disorder in which TNFa activity is
detrimental, e.g., a mouse
model of arthritis, can be used to measure cartilage penetration, bone
erosion, and/or cartilage
destruction by a composition comprising an anti-TNFa antibody or antigen
binding portion
thereof. One non-limiting example of an art-recognized mouse model of
arthiitis is the
human TNF transgenic 197 mouse model of arthritis (TNF-Tg197) (see Keffer, J.
et al.,
EMBO J (1991) 10:4025-4031,
for further description of the TNF-Tg197 model of arthritis).
In another embodiment, a low AR composition comprising an antibody, or antigen-
binding portion thereof, exhibits increased protection against the development
of arthritis, as
measured by arthritic scores, and/or histopathology scores as compared to a
non-low acidic
species composition when administered to an animal model of arthritis, e.g.,
the TNF-Tg197
34
CA 02926384 2016-04-07
model of arthritis. As used herein, "arthiitic scores" refer to signs and
symptoms of arthritis
in an animal model of arthritis. As used herein, "histopathology scores" refer
to radiologic
damage involving cartilage and bone as well as local inflammation.
In another embodiment, a low AR composition comprising an antibody, or antigen-
binding portion thereof, exhibits reduced synovial proliferation, reduced cell
infiltration,
reduced chondrocyte death, and/or reduced proteoglycan loss as compared to a
non-low
acidic species composition. In another embodiment, a low AR composition
compiising an
anti-TNFa antibody, or antigen-binding portion thereof, exhibits increased
TNFa affinity as
compared to a non-low acidic species composition.
As used herein, the term "a disorder in which TNFa activity is detrimental" is
intended to include diseases and other disorders in which the presence of TNFa
in a subject
suffering from the disorder has been shown to be or is suspected of being
either responsible
for the pathophysiology of the disorder or a factor that contributes to a
worsening of the
disorder. Accordingly, a disorder in which TNFa activity is detrimental is a
disorder in
which inhibition of TNFa activity is expected to alleviate the symptoms and/or
progression of
the disorder. Such disorders may be evidenced, for example, by an increase in
the
concentration of TNFa in a biological fluid of a subject suffering from the
disorder (e.g., an
increase in the concentration of TNFa in serum, plasma, or synovial fluid of
the subject),
which can be detected, for example, using an anti-TNFa antibody as described
above. There
are numerous examples of disorders in which TNFa activity is detrimental. In
one
embodiment, the disorder in which TNFa activity is detrimental is an
autoimmune disorder.
In one embodiment, the autoimmune disorder is selected from the group
consisting of
rheumatoid arthritis, juvenile idiopathic arthritis, rheumatoid spondylitis,
ankylosing
spondylitis, psoriasis, osteoarthritis, gouty arthritis, an allergy, multiple
sclerosis, psoriatic
arthritis, autoimmune diabetes, autoimmune uveitis, nephrotic syndrome,
juvenile rheumatoid
arthritis, Crohn's disease, ulcerative colitis, active axial spondyloarthritis
(active axSpA) and
non-radiographic axial spondyloarthritis (nr-axSpA). Disorders in which TNFa
activity is
detrimental are set forth in U.S. Patent No. 6,090,382 and also in the
"Highlights of
Prescribing Information" for FIUMIRA (adalimumab) Injection (Revised Jan.
2008).
The use of TNFa antibodies
and antibody portions obtained using methods of the invention for the
treatment of specific
disorders is discussed in further detail below.
CA 02926384 2016-04-07
The term "antibody" includes an immunoglobulin molecule comprised of four
polypeptide chains, two heavy (H) chains and two light (L) chains inter-
connected by
disulfide bonds. Each heavy chain is comprised of a heavy chain variable
region (abbreviated
herein as HCVR or VH) and a heavy chain constant region (CH). The heavy chain
constant
region is comprised of three domains, CH1, CH2 and CH3. Each light chain is
comprised of a
light chain variable region (abbreviated herein as LCVR or VL) and a light
chain constant
region. The light chain constant region is comprised of one domain, CL. The VH
and VL
regions can be further subdivided into regions of hypervariability, termed
complementarity
determining regions (CDRs), interspersed with regions that are more conserved,
termed
framework regions (FR). Each VH and VL is composed of three CDRs and four FRs,
arranged from amino-terminus to carboxy-terminus in the following order: FR1,
CDR1, FR2,
CDR2, FR3, CDR3, FR4.
The term "antigen-binding portion" of an antibody (or "antibody portion")
includes
fragments of an antibody that retain the ability to specifically bind to an
antigen (e.g., in the
case of adalimumab, hTNFa). It has been shown that the antigen-binding
function of an
antibody can be performed by fragments of a full-length antibody. Examples of
binding
fragments encompassed within the term "antigen-binding portion" of an antibody
include (i) a
Fab fragment, a monovalent fragment comprising the VL, VH, CL and CH1 domains;
(ii) a
F(ab')2 fragment, a bivalent fragment comprising two Fab fragments linked by a
disulfide
bridge at the hinge region; (iii) a Fd fragment comprising the VH and CHI
domains; (iv) a Fv
fragment comprising the VL and VH domains of a single arm of an antibody, (v)
a dAb
fragment (Ward et al., (1989) Nature 341:544-546),
which comprises a VH domain; and (vi) an isolated
comptementarity determining region (CDR). Furthermore, although the two
domains of the
Fv fragment, VL and VH, are coded for by separate genes, they can be joined,
using
recombinant methods, by a synthetic linker that enables them to be made as a
single protein
chain in which the VL and VH regions pair to form monovalent molecules (known
as single
chain Fv (scFv); see, e.g., Bird et al. (1988) Science 242:423-426; and Huston
et al. (1988)
Proc. Natl. Acad. Sci. USA 85:5879-5883).
Such single chain antibodies are also intended to be encompassed within
the term "antigen-binding portion" of an antibody. Other forms of single chain
antibodies,
such as diabodies are also encompassed. Diabodies are bivalent, bispecific
antibodies in
which VH and VL domains are expressed on a single polypeptide chain, but using
a linker
36
CA 02926384 2016-04-07
that is too short to allow for pairing between the two domains on the same
chain, thereby
forcing the domains to pair with complementary domains of another chain and
creating two
antigen binding sites (see, e.g., Holliger, P,, et al. (1993) Proc. Natl.
Acad. Sci. USA
90:6444-6448; Poljak, R. J., et al. (1994) Structure 2:1121-1123).
Still further, an antibody or antigen-binding
portion thereof may be part of a larger immunoadhesion molecule, formed by
covalent or
non-covalent association of the antibody or antibody portion with one or more
other proteins
or peptides. Examples of such itru-nunoadhesion molecules include use of the
streptavidin
core region to make a tetrameric scFv molecule (Kipriyanov, S. M., et al.
(1995) Human
Antibodies and Hybridomas 6:93-101),
and use of a cysteine residue, a marker peptide and a C-terminal polyhistidine
tag
to make bivalent and biotinylated scFv molecules (Kipriyanov, S. M., et al.
(1994) Mol.
Immunol. 31:1047-1058 ).
Antibody portions, such as Fab and F(ab')2 fragments, can be prepared from
whole antibodies
using conventional techniques, such as papain or pepsin digestion,
respectively, of whole
antibodies. Moreover, antibodies, antibody portions and imrnunoadhesion
molecules can be
obtained using standard recombinant DNA techniques, as described herein. In
one aspect, the
antigen binding portions are complete domains or pairs of complete domains.
The terms "Kabat numbering" "Kabat definitions" and "Kabat labeling" are used
interchangeably herein. These terms, which are recognized in the art, refer to
a system of
numbering amino acid residues which are more variable (i.e., hypervariable)
than other
amino acid residues in the heavy and light chain variable regions of an
antibody, or an
antigen binding portion thereof (Kabat et al. (1971) Ann. NY Acad, Sci.
190:382-391 and,
Kabat, E. A., et al. (1991) Sequences of Proteins of Immunological Interest,
Fifth Edition,
U.S. Department of Health and Human Services, NIH Publication No. 91-3242).
For the heavy chain variable
region, the hypervariable region ranges from amino acid positions 31 to 35 for
CDR1, amino
acid positions 50 to 65 for CDR2, and amino acid positions 95 to 102 for CDR3.
For the
light chain variable region, the hypervariable region ranges from amino acid
positions 24 to
34 for CDR], amino acid positions 50 to 56 for CDR2, and amino acid positions
89 to 97 for
CDR 3.
37
CA 02926384 2016-04-07
The term "human antibody" includes antibodies having variable and constant
regions
corresponding to human gen-nline immunoglobulin sequences as described by
Kabat et al.
(See Kabat, et al. (1991) Sequences of proteins of Immunological Interest,
Fifth Edition, U.S.
Department of Health and Human Services, N1H Publication No. 91-3242). The
human
antibodies of the invention may include amino acid residues not encoded by
human germline
immunoglobulin sequences (e.g., mutations introduced by random or site-
specific
mutagenesis in vitro or by somatic mutation in vivo), e.g., in the CDRs and in
particular
CDR3. The mutations can be introduced using the "selective mutagenesis
approach." The
human antibody can have at least one position replaced with an amino acid
residue, e.g., an
activity enhancing amino acid residue which is not encoded by the human
germline
immunoglobulin sequence. The human antibody can have up to twenty positions
replaced
with amino acid residues which are not part of the human germline
immunoglobulin
sequence. In other embodiments, up to ten, up to five, up to three or up to
two positions are
replaced. in one embodiment, these replacements are within the CDR regions.
However, the
term "human antibody", as used herein, is not intended to include antibodies
in which CDR
sequences derived from the gertnline of another mammalian species, such as a
mouse, have
been grafted onto human framework sequences.
The phrase ''recombinant human antibody" includes human antibodies that are
prepared, expressed, created or isolated by recombinant means, such as
antibodies expressed
using a recombinant expression vector transfected into a host cell, antibodies
isolated from a
recombinant, combinatorial human antibody library, antibodies isolated from an
animal (e.g.,
a mouse) that is transgenic for human immunoglobulin genes (see, e.g., Taylor,
L. D., et al.
(1992) Nucl. Acids Res. 20:6287-6295),
or antibodies prepared, expressed, created or isolated by any other means that
involves splicing of human immunoglobulin gene sequences to other DNA
sequences. Such
recombinant human antibodies have variable and constant regions derived from
human
gennline immunoglobulin sequences (see, Kabat, E. A., et al. (1991) Sequences
of Proteins
of Immunological Interest, Fifth Edition, U.S. Department of Health and Human
Services,
NIH Publication No. 91-3242). In certain embodiments, however, such
recombinant human
antibodies are subjected to in vitro mutagenesis (or, when an animal
transgenic for human Ig
sequences is used, in vivo somatic mutagenesis) and thus the amino acid
sequences of the VH
and VL regions of the recombinant antibodies are sequences that, while derived
from and
related to human germline VH and VL sequences, may not naturally exist within
the human
38
CA 02926384 2016-04-07
antibody germline repertoire in vivo. In certain embodiments, however, such
recombinant
antibodies are the result of selective mutagenesis approach or back-mutation
or both.
An "isolated antibody" includes an antibody that is substantially tree of
other
antibodies having different antigenic specificities (e.g., an isolated
antibody that specifically
binds hTNFa is substantially free of antibodies that specifically bind
antigens other than
hTNFa). An isolated antibody that specifically binds hTNFa may bind TNFa
molecules from
other species. Moreover, an isolated antibody may be substantially free of
other cellular
material and/or chemicals. A suitable anti-TNFa antibody is adalimumab.
As used herein, the term "adalimumab," also known by its trade name HUMIRA
(AbbVie) refers to a human Ig01 antibody that binds human tumor necrosis
factor a (TNFa).
In general, the heavy chain constant domain 2 (CH2) of the adalimumab IgG-Fc
region is
glycosylated through covalent attachment of oligosaccharide at asparagine 297
(Asn-297).
The light chain variable region of adalimumab is provided herein as SEQ ID
NO:1, and the
heavy chain variable region of adalimumab is provided herein as SEQ ID NO:2.
Adalimurnab comprises a light chain variable region comprising a CDR1 of SEQ
ID NO:7, a
CDR2 of SEQ ID NO:5, and a CDR3 of SEQ ID NO:3. Adalimumab comprises a heavy
chain variable region comprising a CDR1 of SEQ ID NO:8, a CDR2 of SEQ ID NO:6
and
CDR3 of SEQ ID NO:4. The nucleic acid sequence of the light chain variable
region is set
forth in SEQ ID NO:9. The nucleic acid sequence of the heavy chain variable
region is set
forth in SEQ ID NO:10. The full length amino acid sequence of the light chain
is set forth as
SEQ ID NO:11 and the full length atnino acid sequence of the heavy chain is
set forth as SEQ
ID NO:12. Adalimumab is described in U.S. Patent Nos. 6,090,382; 6,258,562;
6,509,015;
7,223,394; 7,541,031; 7,588,761; 7,863,426; 7,919,264; 8,197,813; 8,206,714;
8,216,583;
8,420,081; 8,092,998; 8,093,045; 8,187,836; 8,3'72,400; 8,034,906; 8,436,149;
8,231,876;
8,414,894; 8,372,401.
AcIalimumab is also described in the "Highlights of Prescribing
Information" for HUMIRA (adalimumab) Injection (Revised Jan. 2008) .
In one embodiment, adalimumab dissociates from human TNFa with a Kd of lx 10 8
M or less and a Koff rate constant of 1x10-3 .s.1 or less, both determined by
surface plasmon
resonance, and neutralizes human TNFa cytotoxicity in a standard in vitro L929
assay with
an IC50 of lx10- M or less. In another embodiment, adalimumab dissociates from
human
39
CA 02926384 2016-04-07
TNFa with a Kõff of 5x10-4 s-1 or less, or with a Kõff of 1x10-4 s-1 or less.
In still another
embodiment, adalimumab neutralizes human TNFa cytotoxicity in a standard in
vitro L929
assay with an IC50 of 1x10-8 M or less, an IC50 of 1x109 M or less or an IC50
of 1x10-1 M
or less.
In general, the heavy chain constant domain 2 (CH2) of the adalimumab IgG-Fc
region is glycosylated through covalent attachment of oligosaccharide at
asparagine 297
(Asn-297). Analysis of adalimumab has shown that it has three main basic
variants (i.e., Lys
0, Lys 1, and Lys 2), referred to herein as "lysine variant species." As used
herein, the term
"lysine variant species" refers to an antibody, or antigen-binding portion
thereof, comprising
heavy chains with either zero, one or two C-terminal lysines. For example, the
"Lys 0"
variant comprises an antibody, or antigen-binding portion thereof, with heavy
chains that do
not comprise a C-terminal lysine. The "Lys 1" variant comprises an antibody,
or antigen-
binding portion thereof, with one heavy chain that comprises a C-terminal
lysine. The "Lys
2" variant comprises an antibody with both heavy chains comprising a C-ten-
ninal lysine.
Lysine variants can be detected, for example, by weak cation exchange
chromatography
(such as WCX-10) of the expression product of a host cell expressing the
antibody, or
antigen-binding portion thereof. For example, but not by way of limitation,
Figures 163 and
193 depict WCX-10 analysis of adalimumab wherein the three lysine variants, as
well as the
two acidic species regions, are resolved from each other.
A composition of the invention may comprise more than one lysine variant
species of
an antibody, or antigen-binding portion thereof. For example, in one
embodiment, the
composition may comprise a Lys 2 variant of an antibody, or antigen-binding
portion thereof.
The composition may comprise a Lys 1 variant of an antibody, or antigen-
binding portion
thereof. The composition may comprise a Lys 0 variant of an antibody, or
antigen-binding
portion thereof. In another embodiment, the composition may comprise both Lys
1 and Lys 2
or Lys 1 and Lys 0 or Lys 2 and Lys 0 variants of an antibody, or antigen-
binding portion
thereof. In another embodiment, the composition may comprise all three lysine
variant
species, i.e.. Lys O. Lys 1 and Lys 2, of an antibody, or antigen-binding
portion thereof.
As used herein, the term "upstream process technology." in the context of
protein,
e.g., antibody. preparation, refers to activities involving the production and
collection of
proteins (e.g. antibodies) from cells (e.g.. during cell culture of a protein
of interest). As used
herein. the term "cell culture" refers to methods for generating and
maintaining a population
of host cells capable of producing a recombinant protein of interest, as well
as the methods
CA 02926384 2016-04-07
and techniques for optimizing the production and collection of the protein of
interest. For
example, once an expression vector has been incorporated into an appropriate
host, the host
can be maintained under conditions suitable for expression of the relevant
nucleotide coding
sequences, and the collection and purification of the desired recombinant
protein.
When using the cell culture techniques of the instant invention, the protein
of interest
can be produced intracellularly, in the periplasmic space, or directly
secreted into the
medium. In embodiments where the protein of interest is produced
intracellularly, the
particulate debris, either host cells or lysed cells (e.g., resulting from
homogenization) can be
removed by a variety of means, including but not limited to, centrifugation or
ultrafiltration.
Where the protein of interest is secreted into the medium, supernatants from
such expression
systems can be first concentrated using a commercially available protein
concentration filter,
e.g., an ArniconTM or Millipore PelliconTm ultrafiltration unit.
As used herein, the term "downstream process technology" refers to one or more
techniques used after the upstream process technologies to purify the protein,
e.g., antibody,
of interest. For example, downstream process technology includes purification
of the protein
product, using, for example, affinity chromatography, including Protein A
affinity
chromatography, ion exchange chromatography, such as anion or cation exchange
chromatography, hydrophobic interaction chromatography, or displacement
chromatography.
The phrase "isolated nucleic acid molecule," as used herein in reference to
nucleic
acids encoding antibodies or antibody portions (e.g., VH, VL, CDR3), e.g..
those that bind
hTNFa, includes a nucleic acid molecule in which the nucleotide sequences
encoding the
antibody or antibody portion are free of other nucleotide sequences encoding
antibodies or
antibody portions that bind antigens other than hTNFa, which other sequences
may naturally
flank the nucleic acid in human genomic DNA. Thus, e.g., an isolated nucleic
acid of the
invention encoding a VH region of an anti-TNFa antibody contains no other
sequences
encoding other VH regions that bind antigens other than, for example, hTNFa.
The phrase
"isolated nucleic acid molecule" is also intended to include sequences
encoding bivalent,
bispecifie antibodies, such as diabodies in which Vfl and VL regions contain
no other
sequences other than the sequences of the diabody.
The phrase "recombinant host cell" (or simply "host cell") includes a cell
into which a
recombinant expression vector has been introduced. It should be understood
that such terms
are intended to refer not only to the particular subject cell but to the
progeny of such a cell.
Because certain modifications may occur in succeeding generations due to
either mutation or
41
CA 02926384 2016-04-07
environmental influences, such progeny may not, in fact, be identical to the
parent cell, but
are still included within the scope of the term "host cell" as used herein.
As used herein, the term "recombinant protein" refers to a protein produced as
the
result of the transcription and translation of a gene carried on a recombinant
expression
vector that has been introduced into a host cell. In certain embodiments the
recombinant
protein is an antibody, e.g., a chimeric, humanized, or fully human antibody.
In certain
embodiments the recombinant protein is an antibody of an isotype selected from
group
consisting of: IgG (e.g., IgGl, IgG2, IgG3, IgG4), IgM, IgAl, IgA2, IgD, or
IgE. In certain
embodiments the antibody molecule is a full-length antibody (e.g., an IgG1 or
IgG4
immunoglobulin) or alternatively the antibody can be a fragment (e.g., an Fc
fragment or a
Fab fragment).
The phrase "clarified harvest" refers to a liquid material containing a
protein of
interest, for example, an antibody of interest such as a monoclonal antibody
of interest, that
has been extracted from cell culture, for example, a fermentation bioreactor,
after undergoing
centrifugation to remove large solid particles and subsequent filtration to
remove finer solid
particles and impurities from the material.
The term "preparative scale," as used herein, refers to a scale of
purification operation
that can be readily scaled-up and implemented at large scale manufacturing
while still
providing desired separation. For instance, one skilled in the field may
develop a process
using, e.g., a 0.5 cm (i.d.) x 20 cm (L) column in the lab, and transfer it to
large scale
production using, e.g., a 30 cm (i.d.) x 20 cm (L) column packed with the same
resin and
operated with the same set of buffers, same linear flow rates (or residence
times.) and buffer
volumes. In preparative scale separation, column bed height is typically <
about 30 cm and
column pressure drop < about 5 bar.
11. Low Acidic Species Compositions of the Invention
The present invention provides low AR compositions comprising a protein, e.g.,
an
antibody, or antigen-binding portion thereof, such as adalimumab. where the
composition
comprises about 15% or less AR, 14% or less AR, 13% or less AR, 12% Or less
AR. 11% Or
less AR, 10% or less AR, 9% Of less AR, 8% or less AR, 7% or less AR. 6% or
less AR. 5%
or less AR, 4.5% or less AR, 4% or less AR, 3.5% or less AR. 3% or less AR.
2.5% or less
AR, 2% or less AR, 1.9% or less AR, 1.8% or less AR, 1.7% or less AR, 1.6% or
less AR.
1.5% or less AR, 1.4% or less AR, 1.3% or less AR, 1.2% or less AR, 1.1% or
less AR. 1%-
47
CA 02926384 2016-04-07
or less AR, 0.9% or less AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less
AR, 0.5% or
less AR, 0.4% or less AR, 0.3% or less AR, 0.2% or less AR, 0.1% or less AR,
or 0.0% AR,
and ranges within one or more of the preceding. A low AR composition of the
invention may
also comprise about 0.0% to about 10% AR, about 0.0% to about 5% AR, about
0.0% to
about 4% AR, about 0.0% to about 3% AR, about 0.0% to about 2% AR, about 3% to
about
5% AR, about 5% to about 8% AR, or about 8% to about 10% AR, or about 10% to
about
15% AR, and ranges within one or more of the preceding.
In one embodiment, a low AR composition of the invention may comprise about
15%
or less AR1, 14% or less AR1, 13% or less AR1, 12% or less ARI, 11% or less
AR1, 10% or
less AR1, 9% or less AR1, 8% or less AR1, 7% or less AR1, 6% or less ARI, 5%
or less
AR1, 4.5% or less ARI, 4% or less AR1, 3.5% or less AR1, 3% or less AR1, 2.5%
or less
AR1, 2% or less AR1, 1.9% or less AR1, 1.8% or less AR1, 1.7% or less AR1,
1.6% or less
AR1, 1.5% or less AR1, 1.4% or less AR1, 1.3% or less AR1, 1.2% or less AR1,
1.1% or less
ARI, 1% or less ARI, 0.9% or less AR1, 0.8% or less AR1, 0.7% or less AR1,
0.6% or less
AR1, 0.5% or less AR1, 0.4% or less AR1, 0.3% or less AR1, 0.2% or less AR I
0.1% or less
AR1, or 0.0% AR1, and ranges within one or more of the preceding. A low AR
composition
of the invention may also comprise about 0.0% to about 10% AR1, about 0.0% to
about 5%
AR1, about 0.0% to about 4% AR1, about 0.0% to about 3% AR-I, about 0.0% to
about 2%
AR1, about 3% to about 5% AR1, about 5% to about 8% ARI, or about 8% to about
10%
AR1, or about 10% to about 15% ARI, and ranges within one or more of the
preceding.
In another embodiment, a low AR composition of the invention may also comprise
about 15% or less AR2, 14% or less AR2, 13% or less AR2, 12% or less AR2, 11%
or less
AR2, 10% or less AR2, 9% or less AR2, 8% or less AR2, 7% or less AR2, 6% or
less AR2,
5% or less AR2, 4.5% or less AR2, 4% or less AR2, 3.5% or less AR2, 3% or less
AR2, 2.5%
or less AR2, 2% or less AR2, 1.9% or less AR2. 1.8% or less AR2, l.7% or less
AR2, 1.6%
or less AR2, 1.5% or less AR2, 1.4% or less AR2, 1.3% or less AR2, 1 .2% or
less AR2, 1.1%
or less AR2, 1 c7c. or less AR2, 0.9% or less AR2, 0.8% or less AR2. 0.7% or
less AR2, 0.6%
or less AR2, 0.5% or less AR2, 0.4% or less AR2, 0.3% or less AR2. 0.2% or
less AR2. 0.1%
or less AR2, or 0.0% AR2, and ranges within one or more of the preceding. A
low AR
composition of the invention may also comprise about 0.0% to about 10% AR2.
about 0.0%
to about 5% AR2, about 0.0% to about 4% AR2, about 0.0% to about 3% AR2, about
0.0% to
about 2% AR2, about 3% to about 5% AR2, about 5% to about 8% AR2. or about 8%
to
43
CA 02926384 2016-04-07
about 10% AR2, or about 10% to about 15% AR2. and ranges within one or more of
the
preceding.
As demonstrated herein, these low AR compositions have improved biological
properties (see Example 13). For example, the low AR compositions of the
invention are
characterized by increased cartilage tissue penetration, reduced cartilage
destruction, reduced
synovial proliferation, reduced bone erosion, increased protection against the
development of
arthritic scores and/or histopathology scores, reduced cell infiltration,
reduced proteoglycan
loss, reduced chondrocyte death, and/or increased TNF affinity, as compared to
non-low
acidic species compositions. In addition, the compositions of the present
invention exhibit
increased therapeutic efficacy when administered to a subject.
In one embodiment, the protein in the low AR composition of the invention is
an
antibody or antigen binding portion thereof. For example, the antibody, or
antigen binding
portion thereof may be an anti-TNFa antibody, or antigen binding portion
thereof, such as
adalimumab, or an antigen binding portion thereof. In one aspect of this
embodiment, the
antibody, or antigen binding portion thereof, can comprise a light chain
variable region
comprising the sequence set forth as SEQ ID NO:1, and a heavy chain variable
region
comprising the sequence set forth as SEQ ID NO:2. In another aspect of this
embodiment,
the antibody can comprise a light chain variable region comprising a CDR1
having the
sequence set forth as SEQ ID NO:7, a CDR2 having the sequence set forth as SEQ
ID NO:5,
and a CDR3 having the sequence set forth as SEQ ID NO:3. In another aspect of
this
embodiment, the antibody can comprise a heavy chain variable region comprising
a CDR1
having the sequence set forth as SEQ ID NO:8, a CDR2 having the sequence set
forth as SEQ
ID NO:6 and a CDR3 having the sequence set forth as SEQ ID NO:4.
The antibody, or antigen binding portion thereof, used in the low AR
compositions of
the invention, may be a human, humanized, or chimeric antibody.
The antibodies that can be used in the low AR compositions of the present
disclosure
can be generated by a variety of techniques, including immunization of an
animal with the
antigen of interest followed by conventional monoclonal antibody methodologies
e.g., the
standard somatic cell hybridization technique of Kohler and Milstein (1975)
Nature 256: 495.
Somatic cell hybridization procedures can be used. ln principle. other
techniques for
producing monoclonal antibody can be employed as well. including viral or
oncogenic
transformation of B lymphocytes.
44
CA 02926384 2016-04-07
One exemplary animal system for preparing hybridomas is the murine systetn.
Hybiidoma production is a very well-established procedure. Immunization
protocols and
techniques for isolation of immunized splenocytes for fusion are known in the
art. Fusion
partners (e.g., murine myeloma cells) and fusion procedures are also known.
An antibody used in the low AR compositions of the invention can be a human, a
chimeric, or a humanized antibody. Chimeric or humanized antibodies used in
the low AR
compositions of the invention can be prepared based on the sequence of a non-
human
monoclonal antibody prepared as described above. DNA encoding the heavy and
light chain
immunoglobulins can be obtained from the non-human hybridoma of interest and
engineered
to contain non-rnurine (e.g., human) immunoglobulin sequences using standard
molecular
biology techniques. For example, to create a chimeric antibody, murine
variable regions can
be linked to human constant regions using methods known in the art (see e.g.,
U.S. Patent No.
4,816,567 to Cabilly et al.). To create a humanized antibody, murine CDR
regions can be
inserted into a human framework using methods known in the art (see e.g., U.S.
Patent No.
5,225,539 to Winter, and U.S. Patent Nos. 5,530,101; 5,585,089; 5,693,762 and
6,180,370 to
Queen et al.).
In one non-limiting embodiment, the antibodies to be used in the low AR
compositions of the invention are human monoclonal antibodies. Such human
monoclonal
antibodies can be generated using transgenic or transchromosomic mice carrying
parts of the
human immune system rather than the mouse system. These transgenic and
transchromosomic mice include mice referred to herein as the HuMAb Mouse
(Medarex,
Inc.), KM Mouse (Medarex, Inc.), and XenoMouse0 (Amgen). The antibodies, or
antigen-
binding portions thereof, used in the low AR compositions of the invention can
also be
produced using the methods described in U.S. Patent No. 6,090,382 .
Moreover, alternative transchromosomic animal systems expressing human
immunoglobulin genes are available in the art and can be used to raise
antibodies of the
disclosure. For example, mice carrying both a human heavy chain
transchromosome and a
human light chain transchromosome, referred to as "TC mice" can be used; such
mice are
described in Tomizuka et al. (2000) Proc. Natl. Acad. Sci. USA 97:722-727,
Fuithennore,
cows carrying human heavy and light chain transchromosomes have been described
in the art
(e.g., Kuroiwa et al. (2002) Nature Biotechnology 20:889-894 and PCT
application No. WO
2002/092812) and can be used to raise antibodies of this disclosure.
CA 02926384 2016-04-07
Recombinant human antibodies to be used in the low AR compositions of the
invention can be isolated by screening of a recombinant combinatorial antibody
library, e.g.,
a scFv phage display library, prepared using human VL and VH cDNAs prepared
from
mRNA derived from human lymphocytes. Methodologies for preparing and screening
such
libraries are known in the art. In addition to commercially available kits for
generating phage
display libraries (e.g., the Pharmacia Recombinant Phage Antibody System,
catalog no, 27-
9400-01; and the Stratagene SurIZAPim phage display kit, catalog no. 240612),
examples of methods and reagents particularly
amenable for use in generating and screening antibody display libraries can be
found in, e.g.,
Ladner et al. U.S. Patent No. 5,223,409; Kang et al. PCT Publication No. WO
92/18619;
Dower et al. PCT Publication No. WO 91/17271; Winter et al. PCT Publication
No. WO
92/20791; Markland et al. PCT Publication No. WO 92/15679; Breitling et al.
PCT
Publication No. WO 93/01288; McCafferty et al. PCT Publication No. WO
92/01047;
Garrard et al. PCT Publication No. WO 92/09690; Fuchs et al. (1991)
Bio/Technology
9:1370-1372; Hay et al. (1992) Hum Antibody Hybridomas 3:81-85; Huse et al.
(1989)
Science 246:1275-1281; McCafferty eí al., Nature (1990) 348:552-554; Griffiths
et al. (1993)
EMBO J 12:725-734; Hawkins et al. (1992) J Mol Biol 226:889-896; Clackson et
al. (1991)
Nature 352:624-628; Gram et al. (1992) PNAS 89:3576-3580; Garrard et al.
(1991)
Bio/Technology 9:1373-1377; Hoogenboom et al. (1991) Nue Acid Res 19:4133-
4137; and
Barbas et al. (1991) PNAS 88:7978-7982.
Human monoclonal antibodies to be used in the low AR compositions of the
invention can also be prepared using SOD mice into which human immune cells
have been
reconstituted such that a human antibody response can be generated upon
immunization.
Such mice are described in, for example, U.S. Patent Nos. 5,476,996 and
5,698,767 to Wilson
et al.
In certain embodiments, the human antibodies to be used in the low AR
compositions
of the invention are anti-TNFa antibodies and antibody portions thereof, anti-
TNFa-related
antibodies and antibody portions, and human antibodies and antibody portions
with
equivalent properties to anti-TNFa antibodies, such as high affinity binding
to hTNFct with
low dissociation kinetics and high neutralizing capacity. In one aspect, the
invention provides
low AR compositions containing an isolated human antibody, or an antigen-
binding portion
thereof, that dissociates from hTNFa with a Kd of about 1 x 10-8 M or less and
a Koff rate
46
CA 02926384 2016-04-07
constant of 1 x 10-3 s-1 or less, both determined by surface plasmon
resonance. In specific
non-limiting embodiments, an anti-TNFa antibody to be used in the low AR
compositions of
the invention competitively inhibits binding of adalimumab to TNFa under
physiological
conditions. In one embodiment, the low AR compositions of the invention
comprise
adalimumab, or an antigen binding fragment thereof.
Antibodies or fragments thereof to be used in the low AR compositions of the
invention can be altered wherein the constant region of the antibody is
modified to reduce at
least one constant region-mediated biological effector function relative to an
unmodified
antibody. To modify an antibody of the invention such that it exhibits reduced
binding to the
Fe receptor, the immunoglobulin constant region segment of the antibody can be
mutated at
particular regions necessary for Fc receptor (FcR) interactions (see, e.g.,
Canfield and
Morrison (1991) J. Exp. Med. 173:1483-1491; and Lund et al. (1991) J. of
Immunol.
147:2657-2662). Reduction in
FcR
binding ability of the antibody may also reduce other effector functions which
rely on FcR
interactions, such as opsonization and phagocytosis and antigen-dependent
cellular
cytotoxicity.
=
III. Preparation of Low AR Compositions Using Upstream Process Technologies
The low AR compositions comprising a protein, e.g., an antibody, or antigen
binding
portion thereof, such as adalimumab, of the invention can be produced by
modulating
conditions during upstream protein production, such as cell culture. In one
embodiment, the
methods of the invention comprise lowering the amount of acidic species
variants or process-
related impurities expressed by host cells producing a protein of interest
including an
antibody or antigen-binding portion thereof during an upstream process
technology (e.g.,
duiing cell culture).
The upstream process technologies may be used alone or in combination with the
downstream process technologies described in Section IV, below, and Example
10.
In one embodiment, one or more of the upstream process technologies described
herein produce a low AR composition comprising an antibody, or antigen binding
portion
thereof, which comprises 1 5% or less AR, 14% or less AR, 1 3% or less AR, 12%
or less AR,
1 I% or less AR, 10% or less AR, 9% or less AR, 8% or less AR, 7% or less AR,
6% or less
AR, 5% or less AR, 4.5% or less AR, 4% or less AR, 3.5% or less AR, 3% or less
AR, 2.5%
47
CA 02926384 2016-04-07
or less AR, 2% or less AR, 1.9% or less AR, 1.8% or less AR, 1.7% or less AR,
1.6% or less
AR, 1.5% or less AR, 1.4% or less AR, 1.3% or less AR, 1.2% or less AR, 1.1%
or less AR,
1% or less AR, 0.9% or less AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less
AR, 0.5%
or less AR, 0.4% AR, 0.3% or less AR, 0.2% or less AR, 0.1% or less AR, or
0.0% AR, and
ranges within one or more of the preceding. In one aspect of this embodiment,
the low AR
composition of the invention comprises about 0.0% to about 10% AR, about 0.0%
to about
5% AR, about 0.0% to about 4% AR, about 0.0% to about 3% AR, about 0.0% to
about 2%
AR, about 3% to about 5% AR, about 5% to about 8% AR, or about 8% to about 10%
AR, or
about 10% to about 15% AR, and ranges within one or more of the preceding.
In another embodiment, one or more of the upstream process technologies
described
herein produce a low AR composition comprising an antibody, or antigen binding
portion
thereof, which comprises 15% or less AR1, 14% or less AR1, 13% or less AR1,
12% or less
AR1, 11% or less AR1, 10% or less AR1, 9% or less AR1, 8% or less AR1, 7% or
less AR1,
6% or less AR I , 5% or less AR1, 4.5% or less AR 1 , 4% or less AR I, 3.5% or
less AR1, 3%
or less AR1, 2.5% or less AR1, 2% or less AR I , 1.9% or less AR1, 1.8% or
less AR1, 1.7%
or less AR1, 1.6% or less AR1, 1.5% or less AR1, 1.4% or less AR1, 1.3% or
less AR1, 1.2%
or less AR1, 1.1% or less AR I, 1% or less AR1, 0.9% or less AR1, 0.8% or less
AR1, 0.7%
or less AR1, 0.6% or less AR1, 0.5% or less AR I, 0.4% or less AR1, 0.3% or
less AR1, 0.2%
or less AR1, 0.1% or less AR1, or 0.0% AR1, and ranges within one or more of
the
preceding. In one aspect of this embodiment, the low AR composition of the
invention
comprises about 0.0% to about 10% AR1, about 0.0% to about 5% AR1, about 0.0%
to about
4% AR I, about 0.0% to about 3% AR1, about 0.0% to about 2% AR1, about 3% to
about 5%
AR1, about 5% to about 8% AR I, or about 8% to about 10% AR1, or about 10% to
about
15% AR1, and ranges within one or more of the preceding.
In still another embodiment, one or more of the upstream process technologies
described herein produce a low AR composition comprising an antibody, or
antigen binding
portion thereof, which comprises 15% or less AR2, 14% or less AR2, 13% or less
AR2, 12%
or less AR2, 1 1 % or less AR2, 10% or less AR2. 9% or less AR2, 8% or less
AR2, 7% or less
AR2, 6% or less AR2, 5% or less AR2, 4.5% or less AR2, 4% or less AR2. 3.5% or
less
AR2, 3% or less AR2, 2.5% or less AR2. 2% or less AR2. 1.9% or less AR2, 1.8%
or less
AR2, 1.7% or less AR2, 1.6% or less AR2, 1.5% or less AR2, 1.4% or less AR2,
1.3% or less
AR2, 1.2% or less AR2, 1.1% or less AR2. 1% or less AR2. 0.9% or less AR2,
0.8% or less
AR2, 0.7% or less AR2, 0.6% or less AR2. 0.5% or less AR2, 0.4% or less AR2,
0.3% or less
48
CA 02926384 2016-04-07
AR2, 0.2% or less AR2, 0.1% or less AR2, or 0.0% AR2, and ranges within one or
more of
the preceding. In one aspect of this embodiment, the low AR composition of the
invention
comprises about 0.0% to about 10% AR2, about 0.0% to about 5% AR2, about 0.0%
to about
4% AR2, about 0.0% to about 3% AR2, about 0.0% to about 2% AR2, about 3% to
about 5%
AR2, about 5% to about 8% AR2, or about 8% to about 10% AR2, or about 10% to
about
15% AR2, and ranges within one or more of the preceding.
Some embodiments of the invention comprise culturing host cells to express a
protein
of interest under conditions that limit the amount of acidic species that are
expressed by the
cells. Some embodiments of the invention comprise culturing host cells under
conditions that
limit the conversion of the product to acidic species variants.
The cell culture conditions can be modified as compared to conditions during
production of a non-low acidic species composition comprising the same
protein. In one
embodiment, the low acidic species composition is produced by culturing cells
expressing the
antibody, or antigen binding portion thereof, in a cell culture media
comprising an increased
concentration of one or more amino acids. In another embodiment, the low
acidic species
composition is produced by culturing cells expressing the antibody, or antigen
binding
portion thereof, in a cell culture media comprising an increased concentration
of calcium
(e.g., as calcium chloride dihydrate). ln still another embodiment, the low
acidic species
composition is produced by culturing cells expressing the antibody, or antigen
binding
portion thereof, in a cell culture media comprising an increased concentration
of niacinamide.
In certain embodiments, the methods described herein comprise culturing cells
in media
supplemented with one or more amino acids, calcium (e.g., as calcium chloride
dihydrate)
and/or niacinamide, and combinations thereof.
In certain embodiments, the low acidic species composition is produced by
culturing
host cells in a culture wherein process parameters, such as pH or dissolved
oxygen (DO), are
modulated, e.g., lowered to decrease the amount of acidic species produced by
the host cells
and/or reduce the conversion of the product to the acidic species variants.
Furtherrnore, a continuous or perfusion technology can utilized to obtain low
AR. In
certain embodiments, reduction of acidic species is obtained by modulating the
medium
exchange rate during cell culture.
49
CA 02926384 2016-04-07
In another embodiment, one or more of the above supplements and modifications
can
be combined and used during cell culture of one protein, e.g., antibody,
composition.
To express an antibody or antigen-binding fragment thereof to be used in the
low AR
compositions of the invention, DNAs encoding the protein, such as DNAs
encoding partial or
full-length light and heavy chains in the case of antibodies, are inserted
into one or more
expression vector such that the genes are operatively linked to
transcriptional and
translational control sequences. (See, e.g., U.S. Pat. No. 6,090,382).
In this context, the term "operatively linked'' is
intended to mean that a gene encoding the protein of interest is ligated into
a vector such that
transcriptional and translational control sequences within the vector serve
their intended
function of regulating the transcription and translation of the gene. The
expression vector and
expression control sequences are chosen to be compatible with the expression
host cell used.
In certain embodiments, the protein of interest will comprising multiple
polypeptides, such as
the heavy and light chains of an antibody. Thus, in certain embodiments, genes
encoding
multiple polypeptides, such as antibody light chain genes and antibody heavy
chain genes,
can be inserted into a separate vector or, more typically, the genes are
inserted into the same
expression vector. Genes are inserted into expression vectors by standard
methods (e.g.,
ligation of complementary restriction sites on the gene -fragment and vector,
or blunt end
ligation if no restriction sites are present). Prior to insertion of the gene
or genes, the
expression vector may already carry additional polypeptide sequences, such as,
but not
limited to, antibody constant region sequences. For example, one approach to
converting the
anti-TNFa antibody or anti-TNFa antibody-related VH and VL sequences to full-
length
antibody genes is to insert them into expression vectors already encoding
heavy chain
constant and light chain constant regions, respectively, such that the VH
segment is
operatively linked to the CH segment(s) within the vector and the VL segment
is operatively
linked to the CL segment within the vector. Additionally or alternatively, the
recombinant
expression vector can encode a signal peptide that facilitates secretion of
the protein from a
host cell. The gene can be cloned into the vector such that the signal peptide
is linked in-
frame to the amino terminus of the gene. The signal peptide can be an
immunoglobulin signal
peptide or a heterologous signal peptide (i.e., a signal peptide from a non-
immunoglobulin
protein).
CA 02926384 2016-04-07
In addition to protein coding genes, a recombinant expression vector can cany
one or
more regulatory sequence that controls the expression of the protein coding
genes in a host
cell. The term "regulatory sequence" is intended to include promoters,
enhancers and other
expression control elements (e.g., polyadenylation signals) that control the
transcription or
translation of the protein coding genes. Such regulatory sequences are
described, e.g., in
Goeddel; Gene Expression Technology: Methods in Enzymology 185, Academic
Press, San
Diego, CA (1990). It will be
appreciated by those skilled in the art that the design of the expression
vector, including the
selection of regulatory sequences may depend on such factors as the choice of
the host cell to
be transformed, the level of expression of protein desired, etc. Suitable
regulatory sequences
for mammalian host cell expression include viral elements that direct high
levels of protein
expression in mammalian cells, such as promoters and/or enhancers derived from
cytornegalovirus (CMV) (such as the CMV promoter/enhancer), Simian Virus 40
(SV40)
(such as the SV40 promoter/enhancer), adenovirus, (e.g., the adenovirus major
late promoter
(AdMLP)) and polyoma. For further description of viral regulatory elements,
and sequences
thereof, see, e.g., U.S. Patent No. 5,168,062 by Stinski, U.S. Patent No.
4,510,245 by Bell et
al. and U.S. Patent No. 4,968,615 by Schaffner et al.
A recombinant expression vector may also carry one or more additional
sequences,
such as a sequence that regulates replication of the vector in host cells
(e.g., origins of
replication) and/or a selectable marker gene. The selectable marker gene
facilitates selection
of host cells into which the vector has been introduced (see e.g., U.S.
Patents Nos. 4,399,216,
4,634,665 and 5,179,017, all by Axel et al.).
For example, typically the selectable marker gene confers resistance to
drugs, such as G418, hygromycin or methotrexate, on a host cell into which the
vector has
been introduced. Suitable selectable marker genes include the dihydrofolate
reductase
(DHFR) gene (for use in dhfr- host cells with methotrexate
selection/amplification) and the
neo gene (for G418 selection).
An antibody, or antibody portion, to be used in the low AR compositions of the
invention can be prepared by recombinant expression of immunoglobulin light
and heavy
chain genes in a host cell. To express an antibody recombinantly, a host cell
is transfected
with one or more recombinant expression vectors carrying DNA fragments
encoding the
51
CA 02926384 2016-04-07
immunoglobulin light and heavy chains of the antibody such that the light and
heavy chains
are expressed in the host cell and secreted into the medium in which the host
cells are
cultured, from which medium the antibodies can be recovered. Standard
recombinant DNA
methodologies are used to obtain antibody heavy and light chain genes,
incorporate these
genes into recombinant expression vectors and introduce the vectors into host
cells, such as
those described in Sambrook, Fritsch and Maniatis (eds), Molecular Cloning; A
Laboratory
Manual, Second Edition, Cold Spring Harbor, N.Y., (1989), Ausubel et al.
(eds.) Current
Protocols in Molecular Biology, Greene Publishing Associates, (1989) and in
U.S. Patent
Nos. 4,816,397 & 6,914,128.
For expression of protein, for example, the light and heavy chains of an
antibody, the
expression vector(s) encoding the protein is (are) transfected into a host
cell by standard
techniques. The various forms of the term "transfection" are intended to
encompass a wide
variety of techniques commonly used for the introduction of exogenous DNA into
a
prokaryotic or eukaryotic host cell, e.g., electroporation, calcium-phosphate
precipitation,
DEAE-dextran transfection and the like. Although it is theoretically possible
to express the
proteins of the invention in either prokaryotic or eukaryotic host cells,
expression of
antibodies in eukaryotic cells, such as mammalian host cells, is suitable
because such
eukaryotic cells, and in particular mammalian cells, are more likely than
prokaryotic cells to
asserrible and secrete a properly folded and immunologically active protein.
Prokaryotic
expression of protein genes has been reported to be ineffective for production
of high yields
of active protein (Boss and Wood (1985) Immunology Today 6:12-13 ).
Suitable host cells for cloning or expressing the DNA in the vectors herein
are the
prokaryote, yeast, or higher eukaryote cells described above. Suitable
prokaryotes for this
purpose include eubacteria, such as Gram-negative or Gram-positive organisms,
e.g.,
Enterobacteriaceae such as Escherichia, e.g., E. coli, Enterobacter, Erwinia,
Klebsiella,
Proteus, Salmonella, e,g., Salmonella typhimurium, Serratia, e.g., Serratia
marcescans, and
Shigella, as well as Bacilli such as B. subtilis and B. licheniformis (e.g.,
B. licheniformis 41P
disclosed in DD 266,710 published Apr, 12, 1989), Pseudomonas such as P.
aeruginosa, and
Streptomyces. One suitable E. coli cloning host is E. coli 294 (ATCC 31,446),
although other
strains such as E. coli B. E. coli X1776 (ATCC 31,537), and E. coli W3110
(ATCC 27,325)
are suitable. These examples are illustrative rather than limiting.
52
CA 02926384 2016-04-07
In addition to prokaryotes, eukaryotic microbes such as filamentous fungi or
yeast are
suitable cloning or expression hosts for polypeptide encoding vectors.
Saccharomyces
cerevisiae, or common baker's yeast, is the most commonly used among lower
eukaryotic
host microorganisms. However, a number of other genera, species, and strains
are commonly
available and useful herein, such as Schizosaccharornyces pombe; Kluyveromyces
hosts such
as, e.g., K. lactis, K. fragilis (ATCC 12,424), K. bulgaticus (ATCC 16,045),
K. wickeramii
(ATCC 24,178), K. waltii (ATCC 56,500), K. drosophilarum (ATCC 36,906), K.
therrnotolerans, and K. marxianus; yarrowia (EP 402,226); Pichia pastoris (EP
183,070);
Candida; Trichoderma reesia (EP 244,234); Neurospora crassa; Schwanniomyces
such as
Schwanniomyces occidentalis; and filamentous fungi such as, e.g., Neurospora,
Penicillium,
Tolypocladium, and Aspergillus hosts such as A. nidulans and A. niger.
Suitable host cells for the expression of glycosylated proteins, for example,
glycosylated antibodies, are derived from multicellular organisms. Examples of
invertebrate
cells include plant and insect cells. Numerous baculoviral strains and
variants and
corresponding permissive insect host cells from hosts such as Spodoptera
frugiperda
(caterpillar), Aedes aegypti (mosquito), Aedes albopictus (mosquito),
Drosophila
melanogaster (fruitfly), and Bombyx mori have been identified. A variety of
viral strains for
transfection are publicly available, e.g., the L-1 variant of Autographa
californica NPV and
the Bm-5 strain of Bombyx mori NPV, and such viruses may be used as the virus
herein
according to the present invention, particularly for transfection of
Spodoptera frugiperda
cells. Plant cell cultures of cotton, corn, potato, soybean, petunia, tomato,
and tobacco can
also be utilized as hosts.
Mammalian cells can be used for expression and production of the recotnbinant
protein used in the low AR compositions of the invention, however other
eukaryotic cell
types can also be employed in the context of the instant invention. See, e.g.,
Winnacker,
From Genes to Clones, VCH Publishers, N.Y., N.Y. (1987). Suitable mammalian
host cells
for expressing recombinant proteins according to the invention include Chinese
Hamster
Ovary (CHO cells) (including dhfr- CHO cells, described in Urlaub and Chasin,
(1980)
PNAS USA 77:4216-4220, used with a DHFR selectable marker, e.g., as desciibed
in
Kaufman and Sharp (1982) Mol. Biol. 159:601-621),
NSO myeloma cells, COS cells and SP2 cells. When
recombinant expression vectors encoding protein genes are introduced into
mammalian host
53
CA 02926384 2016-04-07
cells, the antibodies are produced by culturing the host cells for a period of
time sufficient to
allow for expression of the antibody in the host cells or secretion of the
antibody into the
culture medium in which the host cells are grown. Other examples of useful
mammalian host
cell lines are monkey kidney CV1 line transformed by SV40 (COS-7, ATCC CRL
1651);
human embryonic kidney line (293 or 293 cells subcloned for growth in
suspension culture,
Graham et al., J. Gen Virol. 36:59 (1977)); baby hamster kidney cells (BHK,
ATCC CCL
10); Chinese hamster ovary cells/-DHER (CHO, Urlaub et al., Proc. Natl. Acad.
Sci. USA
77:4216 (1980)); mouse sertoli cells (TM4, Mather, Biol. Reprod. 23:243-251
(1980));
monkey kidney cells (CV1 ATCC CCL 70); African green monkey kidney cells (VERO-
76,
ATCC CRL-1587); human cervical carcinoma cells (HELA, ATCC CCL 2); canine
kidney
cells (MDCK, ATCC CCL 34); buffalo rat liver cells (BRL 3A, ATCC CRL 1442);
human
lung cells (W138, ATCC CCL 75); human liver cells (Hep G2, H13 8065); mouse
mammary
tumor (MMT 060562, ATCC CCL51); TRI cells (Mather et al., Annals N.Y. Acad.
Sci.
383:44-68 (1982)); MRC 5 cells; FS4 cells; and a human hepatoma line (Hep G2).
Host cells are transformed with the above-described expression or cloning
vectors for
protein production and cultured in conventional nutrient media modified as
appropriate for
inducing promoters, selecting transformants, or amplifying the genes encoding
the desired
sequences.
The host cells used to produce a protein may be cultured in a variety of
media.
Commercially available media such as Ham's Elam (Sigma), Minimal Essential
Mediumml
(MEM), (Sigma), RPMI-1640 (Sigma), and Dulbecco's Modified Eagle's Mediummi
(DMEM), (Sigma) are suitable for culturing the host cells. In addition, any of
the media
described in Ham et al., Meth. Enz. 58:44 (1979), Barnes et al., Anal.
Biochem. 102:255
(1980), U.S. Pat. Nos. 4,767,704; 4,657,866; 4,927,762; 4,560,655; or
5,122,469; WO
90/03430; WO 87/00195; or U.S. Pat. No. Re. 30,985 may be used as culture
media for the
host cells . Any of these
media may he supplemented as necessary with hormones and/or other growth
factors (such as
insulin, transferrin, or epidermal growth factor), salts (such as sodium
chloride, calcium,
magnesium, and phosphate), buffers (such as HEPES), nucleotides (such as
adenosine and
thyrnidine), antibiotics (such as gentamycin drug), trace elements (defined as
inorganic
compounds usually present at final concentrations in the micrornolar range),
and glucose or
54
CA 02926384 2016-04-07
an equivalent energy source. Any other necessary supplements may also be
included at
appropriate concentrations that would be known to those skilled in the art.
The culture
conditions, such as temperature, pH. and the like, are those previously used
with the host cell
selected for expression, and will be apparent to the ordinarily skilled
artisan.
Host cells can also be used to produce portions of intact proteins, for
example,
antibodies, including Fab fragments or scFv molecules. It is understood that
variations on the
above procedure are within the scope of the present invention. For example, in
certain
embodiments it may be desirable to transfect a host cell with DNA encoding
either the light
chain or the heavy chain (but not both) of an antibody. Recombinant DNA
technology may
also be used to remove some or all of the DNA encoding either or both of the
light and heavy
chains that is not necessary for binding to an antigen. The molecules
expressed from such
truncated DNA molecules are also encompassed by the antibodies of the
invention. In
addition, bifunctional antibodies may be produced in which one heavy and one
light chain are
an antibody of the invention and the other heavy and light chain are specific
for an antigen
other than the target antibody, depending on the specificity of the antibody
of the invention,
by crosslinking an antibody of the invention to a second antibody by standard
chemical
crosslinking methods.
In a suitable system for recombinant expression of a protein, for example, an
antibody, or antigen-binding portion thereof, a recombinant expression vector
encoding the
protein. for example, both an antibody heavy chain and an antibody light
chain, is introduced
into dhfr-CHO cells by calcium phosphate-mediated transfection. Within the
recombinant
expression vector, the protein gene(s) are each operatively linked to CMV
enhancer/AdMLP
promoter regulatory elements to drive high levels of transcription of the
gene(s). The
recombinant expression vector also carries a DHFR gene, which allows for
selection of CHO
cells that have been transfected with the vector using methotrexate
selection/amplification.
The selected transformant host cells are cultured to allow for expression of
the protein, for
example, the antibody heavy and light chains, and intact protein, for example,
an antibody. is
recovered from the culture medium. Standard molecular biology techniques are
used to
prepare the recombinant expression vector, transfect the host cells. select
for transformants,
culture the host cells and recover the protein from the culture medium.
When using recombinant techniques. the protein, for example. antibodies or
antigen
binding fragments thereof, can be produced intracellularly. in the periplasmic
space, or
CA 02926384 2016-04-07
directly secreted into the medium. In one aspect, if the protein is produced
intracellularly, as a
first step, the particulate debris, either host cells or lysed cells (e.g.,
resulting from
homogenization), can be removed, e.g., by centrifugation or ultrafiltration.
Where the protein
is secreted into the medium, supernatants from such expression systems can be
first
concentrated using a commercially available protein concentration filter,
e.g., an ArniconTM
or Millipore PelliconTM ultrafiltration unit.
Some antibodies can be secreted directly from the cell into the surrounding
growth
media; others are made intracellularly. For antibodies made intracellularly,
the first step of a
purification process typically involves: lysis of the cell, which can be done
by a variety of
methods, including mechanical shear, osmotic shock, or enzymatic treatments.
Such
disruption releases the entire contents of the cell into the homogenate, and
in addition
produces subcellular fragments that are difficult to remove due to their small
size. These are
generally removed by differential centrifugation or by filtration. Where the
antibody is
secreted, supernatants from such expression systems are generally first
concentrated using a
commercially available protein concentration filter, e.g., an ArniconTM or
Millipore
PelliconTm ultrafiltration unit. Where the antibody is secreted into the
medium, the
recombinant host cells can also be separated from the cell culture medium,
e.g., by tangential
flow filtration. Antibodies can be further recovered from the culture medium
using the
antibody purification methods of the invention.
Adjusting Amino Acid Concentration to Modulate Acidic Species (AR)
In certain embodiments, the amount of one or more amino acids in the media is
modulated (e.g., increased or decreased) in order to produce a low acidic
species composition
of the invention (see the Examples Section, below). Such increases or
decreases in the
amount of the one or more amino acids can be of about 1%, 5%, 10%, 15%, 20%,
25%, 30%,
35%. 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%. 85%, 90%, 95%, 100%, and
ranges
within one or more of the preceding, of the original amount used during cell
culture where a
non-low acidic species composition of the same protein is produced.
In certain embodiments, a cell culture media will include one or more of the
amino
acids, or other compositions described herein as lowering acidic species.
Accordingly, the
amount of the amino acid, or other composition, that is supplemented may be
adjusted to
account for the amount present in the media prior to supplementation.
56
CA 02926384 2016-04-07
In certain embodiments, the cell culture media is supplemented with one or
more
amino acids in an amount of between about 0.025 and 20 g/L, or between about
0.05 and 15
g/L, or between about 0.1 and 14 g/L, or between about 0.2 and 13 g/L, or
between about
0.25 and 12 g/L, or between about 0.5 and 11 g/L, or between about 1 and 10
g/L, or between
about 1.5 and 9.5 g/L, or between about 2 and 9 g/L, or between about 2.5 and
8.5 g/L, or
between about 3 and 8 g/L, or between about 3.5 and 7.5 g/L, or between about
4 and 7 g/L,
or between about 4.5 and 6.5 g/L, or between about 5 and 6 g/L. In certain
embodiments, the
cell culture media is supplemented with one or more amino acids in an amount
of about 0.25
g/L, or about 0.5 g/L, or about 1 g/L, or about 2 g/L, or about 4 g/L, or
about 8 g/L.
In certain embodiments, the cell culture media is supplemented with one or
more
amino acids in an amount effective to produce a low AR composition comprising
about 15%
or less AR, 14% or less AR, 13% or less AR, 12% or less AR, 11% or less AR,
10% or less
AR, 9% or less AR, 8% or less AR, 7% or less AR, 6% or less AR, 5% or less AR,
4.5% or
less AR, 4% or less AR, 3.5% or less AR, 3% or less AR, 2.5% or less AR, 2% or
less AR,
1.9% or less AR, 1.8% or less AR, 1.7% or less AR, 1.6% or less AR, 1.5% or
less AR, 1.4%
or less AR, 1.3% or less AR, 1.2% or less AR, 1.1% or less AR, 1% or less AR,
0.9% or less
AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less AR, 0.5% or less AR, 0.4%
or less AR,
0.3% or less AR, 0.2% or less AR, 0.1% or less AR, or 0.0% AR, and ranges
within one or
more of the preceding.
In another embodiment, the cell culture media is supplemented with one or more
amino acids in an amount effective to produce a low AR composition comprising
about 15%
or less AR1, 14% or less AR1, 13% or less AR1, 12% or less AR1, 11% or less
AR1, 10% or
less AR1, 9% or less AR1, 8% or less AR1, 7% or less AR1, 6% or less AR1, 5%
or less
AR1, 4.5% or less AR1, 4% or less AR1. 3.5% or less AR1, 3% or less AR1, 2.5%
or less
ARI, 2% or less AR1, 1.9% or less AR1, 1.8% or less AR1, 1.7% or less AR1,
1.6% or less
AR1, 1.5% or less AR1, 1.4% or less ARI, 1.3% or less AR1, 1.2% or less ARI,
1.1% or less
AR1. 1% or less AR1, 0.9% or less AR1, 0.8% or less ARI. 0.7% or less AR!,
0.6% or less
AR I. 0.5% or less AR1, 0.4% or less AR], 0.3% or less AR I, 0.2% or less AR
I, 0.1% or less
AR1, or 0.0% AR1, and ranges within one or more of the preceding.
In yet another embodiment, the cell culture media is supplemented with one or
more
amino acids in an amount effective to produce a low AR composition comprising
about 15%
or less AR2, 14% or less AR2, 13% or less AR2. 12% or less AR2, 11% or less
AR2, 10% or
less AR2, 9c7c or less AR2, 8% or less AR2. 7% or less AR2. 6% or less AR2, 5%
or less
57
CA 02926384 2016-04-07
AR2, 4.5% or less AR2, 4% or less AR2, 3.5% or less AR2, 3% or less AR2, 2.5%
or less
AR2, 2% or less AR2, 1.9% or less AR2, 1.8% or less AR2, 1.7% or less AR2,
1.6% or less
AR2, 1.5% or less AR2, 1.4% or less AR2, 1.3% or less AR2, 1.2% or less AR2,
1.1% or less
AR2, 1% or less AR2, 0.9% or less AR2, 0.8% or less AR2, 0.-7% or less AR2,
0.6% or less
AR2, 0.5% or less AR2, 0.4% or less AR2, 0.3% or less AR2, 0.2% or less AR2,
0.1% or less
AR2, or 0.0% AR2, and ranges within one or more of the preceding.
In another embodiment, the cell culture media is supplemented with one or more
amino acids in an amount effective to reduce the percentage of acidic species
in a protein or
antibody composition by about 1%, L2%, 1.5%, 2%, 2.2%, 2.5%, 3%, 3.2%, 3.5%,
4%,
4.2%, 4.5%, 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%,
70%,
75%, 80%, 85%, 90%, 95%, 100%, and ranges within one or more of the preceding.
In some embodiments, the one or more amino acids used to supplement the cell
culture media is a basic amino acid. In certain embodiments the one or more
amino acids is
arginine, lysine, histidine, ornithine, or certain combinations of arginine or
lysine with
ornithine or of all four amino acids. In certain embodiments, the amino acids
are single
peptides, as dipeptides, as tripeptides or as longer oligopeptides. In certain
embodiments, the
di-, tri-, and/or oligopeptides are individually composed of a single amino
acid, while in
alternative embodiments, the di-, tri-, and/or oligopeptides are individually
composed of two
or more particular amino acids. In certain embodiments, the amount of amino
acid
supplemented to the cell culture to achieve concentrations of about 0 to about
9 g/L for
arginine, about 0 to about 11 g/L for lysine, about 0 to about 11 g/L
histidine, and about 0 to
about 11 g/L ornithine. Wider ranges are also within the scope of the instant
invention,
including, but not limited to: about 0 to about 30 g/L for arginine, about 0
to about 30 g/L for
lysine, about 0 to about 30 g/L histidine, and about 0 to about 30 g/L
omithine.
For example, and not by way of limitation, as detailed in Example 1, below,
when the
production medium employed in the example was supplemented with arginine to
achieve a
total concentration of 9 g/L arginine, the total amount of acidic species of
adalimumab
present in a cell culture sample after purification was reduced from 19.7% of
a control sample
to 12.2% of the sample purified from the cells cultured with the arginine
supplemented
media. Similarly, when the production medium employed in the example was
supplemented
with lysine, or histidine, or ornithine to achieve total concentrations of 11
g/L lysine, 10 g/L
ornithine or 10 g/L histidine, respectively, the total amount of acidic
species of adalimumab
58
CA 02926384 2016-04-07
present in a cell culture sample after purification was reduced by 11.5%,
10.4% and 10.9%,
respectively, compared to a control sample.
In certain embodiments, the cell culture media is supplemented, for example,
at the
start of culture, or in a fed-batch or in a continuous manner. The feed
amounts may be
calculated to achieve a certain concentration based on offline or online
measurements. The
supplements may be added as multimers, e.g., arg-arg, his-his, arg-his-orn,
etc., and/or as
chemical variants, e.g., of amino acids or analogs of amino acids, salt forms
of amino acids,
controlled release of amino acids by immobilizing in gels, etc, and/or in
fully or partially
dissolved form. The addition of one or more supplements may be based on
measured amount
of acidic species. The resulting media can be used in various cultivation
methods including,
but not limited to, batch, fed-batch, chemostat and perfusion, and with
various cell culture
equipment including, but not limited to, shake flasks with or without suitable
agitation,
spinner flasks, stin-ed bioreactors, airlift bioreactors, membrane
bioreactors, reactors with
cells retained on a solid support or immobilized/entrapped as in microporous
beads, and any
other configuration appropriate for optimal growth and productivity of the
desired cell line. In
addition, the harvest criterion for these cultures may be chosen, for example,
based on choice
of harvest viability or culture duration, to further optimize a certain
targeted acidic species
profile.
Adjusting CaC12 and/or Niacinamide Concentration to Modulate Acidic Species
(AR)
In certain embodiments, the cell culture media is supplemented with calcium
(e.g., as
calcium chloride dihydrate) to achieve a calcium concentration of between
about 0.05 and 2.5
mM, or between about 0.05 and 1 mM, or between about 0.1 and 0.8 mM, or
between about
0.15 and 0.7 mM, or between about 0.2 and 0.6 mM, or between about 0.25 and
0.5 mM, or
between about 0.3 and 0.4 mM calcium.
In certain embodiments. the cell culture media is supplemented with calcium
(e.g., as
calcium chloride dihydrate) in an amount effective to produce a low AR
composition
comprising- about 15% or less AR, 14% or less AR, 13% or less AR, 12% or less
AR, 11% or
less AR, 10% or less AR, 9% or less AR, 8% or less AR, 7% or less AR, 6% or
less AR, 5%
or less AR, 4.5% or less AR, 4% or less AR, 3.5% or less AR, 3% or less AR,
2.5% or less
AR. 2% or less AR, 1.9% or less AR, 1.8% or less AR. 1.7% or less AR, 1.6% or
less AR,
1.5% or less AR, 1.4% or less AR, 1.3% or less AR, 1.2% or less AR, 1.1% or
less AR, 1%
59
CA 02926384 2016-04-07
or less AR, 0.9% or less AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less
AR, 0.5% or
less AR, 0.4% or less AR, 0.3% or less AR, 0.2% or less AR, 0.1% or less AR,
or 0.0% AR,
and ranges within one or more of the preceding.
In another embodiment, the cell culture media is supplemented with calcium
(e.g., as
calcium chloride dihydrate) in an amount effective to produce a low AR
composition
comprising about 15% or less AR 1, 14% or less AR1, 13% or less AR1õ 12% or
less AR1,
11% or less AR1, 10% or less AR1, 9% or less ARE 8% or less AR1, 7% or less
AR1, 6% or
less AR1, 5% or less AR1, 4.5% or less AR1, 4% or less AR1, 3.5% or less AR1,
3% or less
AR1, 2.5% or less AR1, 2% or less AR1, 1.9% or less AR1, 1.8% or less AR1,
1.7% or less
AR1, 1.6% or less AR1, 1.5% or less AR1, 1.4% or less AR I, 1.3% or less AR1,
1.2% or less
AR1, 1.1% or less AR1, 1% or less AR1, 0.9% or less AR1, 0.8% or less ARI,
0.7% or less
AR I, 0.6% or less AR1, 0.5% or less AR I, 0.4% or less AR1, 0.3% or less AR1,
0.2% or less
AR1, 0.1% or less AR1, or 0.0% AR1, and ranges within one or more of the
preceding.
In yet another embodiment, the cell culture media is supplemented with calcium
(e.g.,
as calcium chloride dihydrate) in an amount effective to produce a low AR
composition
comprising about 15% or less AR2, 14% or less AR2, 13% or less AR2, 12% or
less AR2,
11% or less AR2, 10% or less AR2, 9% or less AR2, 8% or less AR2, 7% or less
AR2, 6% or
less AR2, 5% or less AR2, 4.5% or less AR2, 4% or less AR2, 3.5% or less AR2,
3% or less
AR2, 2.5% or less AR2, 2% or less AR2, 1.9% or less AR2, 1.8% or less AR2,
1.7% or less
AR2, 1.6% or less AR2, 1.5% or less AR2, 1.4% or less AR2, 1.3% or less AR2,
1.2% or less
AR2, 1.1% or less AR2, 1% or less AR2, 0.9% or less AR2, 0.8% or less AR2,
0.7% or less
AR2, 0.6% or less AR2, 0.5% or less AR2, 0.4% or less AR2, 0.3% or less AR2,
0.2% or less
AR2, 0.1% or less AR2, or 0.0% AR2, and ranges within one or more of the
preceding.
In another embodiment, the cell culture media is supplemented with calcium
(e.g., as
calcium chloride dihydrate) in an amount effective to reduce the amount of
acidic species in a
protein or antibody sample by about 1%, 1.2%, 1.5%, 2%, 2.2%, 2.5%, 3%, 3.2%,
3.5%, 4%,
4.2%, 4.5%, 5%, 10%, 15%, 20%, 25%. 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%.
70%,
75%, 80%, 85%, 90%, 95%, 100%. and ranges within one or more of the preceding.
For example, and not by way of limitation, as detailed in Example 1, below,
when the
production medium employed in the example was supplemented with calcium (e.g.,
as
calcium chloride dihydrate) at a concentration of 1.05 mM, the total amount of
acidic species
of adalimumab present in a cell culture sample after purification was reduced
front 23.2% of
CA 02926384 2016-04-07
a control sample to 16.5% of the sample purified from the cells cultured with
the calcium
supplemented media.
In certain embodiments, the cell culture can be supplemented with a
combination of
calcium, e.g., CaCb, and one or more a basic amino acids, as described above.
In certain
embodiments, the amount of basic amino acid concentrations in combination with
calcium in
the cell culture is between about 0 to about 9 g/L for arginine, about 0 to
about 11 g/L for
lysine, about 0 to about 11 g/L hi stidine, and about 0 to about 11 g/L
ornithine. Wider ranges
are also within the scope of the instant invention, including, but not limited
to: about 0 to
about 30 g/L for arginine, about 0 to about 30 g/L for lysine, about 0 to
about 30 g/L
histidine, and about 0 to about 30 g/L ornithine.
In certain embodiments, the cell culture media is supplemented with
niacinamide to
achieve a niacinamide concentration of between about 0.2 and 3.0 mM, or
between about 0.4
and 3.0 mM, or between about 0.8 and 3.0 mM.
In some embodiments, the cell culture media is supplemented with niacinamide
in an
amount effective to reduce the amount of acidic species heterogeneity in a
protein or antibody
sample by about 15% or less AR, 14% or less AR, 13% or less AR, 12% or less
AR, 11% or
less AR, 10% or less AR, 9% or less AR, 8% or less AR, 7% or less AR, 6% or
less AR, 5%
or less AR, 4.5% or less AR, 4% or less AR, 3.5% or less AR, 3% or less AR,
2.5% or less
AR, 2% or less AR, 1.9% or less AR, 1.8% or less AR, 1.7% or less AR, 1.6% or
less AR,
1.5% or less AR, 1.4% or less AR, 1.3% or less AR, 1.2% or less AR, 1.1% or
less AR, 1%
or less AR, 0.9% or less AR, 0.8% or less AR, 0.7% or less AR, 0.6% or less
AR, 0.5% or
less AR, 0.4% or less AR, 0.3% or less AR, 0.2% or less AR, 0.1% or less AR,
or 0.0% AR,
and ranges within one or more of the preceding.
In another embodiment. the cell culture media is supplemented with niacinamide
in an
amount effective to produce a low AR composition comprising about 15% or less
AR1, 14%
or less AR1, 13% or less AR1, 12% or less AR1, 11% or less AR1, 10% or less
ARI, 9% or
less AR], 8% or less AR1, 7% or less AR I, 6% or less AR1, 5% or less AR1,
4.5% or less
AR1, 4% or less AR1, 3.5% or less AR I, 3% or less AR1, 2.5% or less AR1, 2%
or less
AR I, 1.9% or less AR1, 1.8% or less AR1, 1.7% or less AR1, 1.6% or less AR1,
1.5% or less
AR I, 1.4% or less AR1, 1.3% or less AR1, 1.2% or less AR1, 1.1% or less AR1,
1% or less
AR1, 0.9% or less AR1. 0.8% or less AR1, 0.7% or less AR I, 0.6% or less AR!,
0.5% or less
61
CA 02926384 2016-04-07
AR1, 0.4% or less AR1, 0.3% or less AR1, 0.2% or less AR I, 0.1% or less AR1,
or 0.0%
AR1, and ranges within one or more of the preceding.
In yet another embodiment, the cell culture media is supplemented with
niacinamide
in an amount effective to produce a low AR composition comprising about 15% or
less AR2,
14% or less AR2, 13% or less AR2, 12% or less AR2, 11% or less AR2, 10% or
less AR2,
9% or less AR2, 8% or less AR2, 7% or less AR2, 6% or less AR2, 5% or less
AR2, 4.5% or
less AR2, 4% or less AR2, 3.5% or less AR2, 3% or less AR2, 2.5% or less AR2,
2% or less
AR2, 1.9% or less AR2, 1.8% or less AR2, 1.7% or less AR2, 1.6% or less AR2,
1.5% or less
AR2, 1.4% or less AR2, 1.3% or less AR2, 1.2% or less AR2, 1.1% or less AR2,
1% or less
AR2, 0.9% or less AR2, 0.8% or less AR2, 0.7% or less AR2, 0.6% or less AR2,
0.5% or less
AR2, 0.4% or less AR2, 0.3% or less AR2, 0.2% or less AR2, 0.1% or less AR2,
or 0.0%
AR2, and ranges within one or more of the preceding.
For example, and not by way of limitation, as detailed in Example 1, below,
when the
production medium employed in the example was supplemented with niacinamide at
a
concentration of 1.6 mM, the total amount of acidic species of adalimumab
present in a cell
culture sample after purification was reduced from 19.9% of a control sample
to 15.9% of the
sample purified from the cells cultured with the niacinamide supplemented
media. In a
separate example, where the media was supplemented with 3 mM niacinamide, the
total
amount of acidic species of adalimumab present in a cell culture sample after
purification was
reduced from 27.0% of a control sample to 19.8% of the sample purified from
the cells
cultured with the niacinamide supplemented media.
In certain embodiments, the cell culture can be supplemented with a
combination of
niacinamide, calcium, e.g., CaCI,, and/or one or more basic amino acids. In
certain
embodiments, the amount of basic amino acid concentrations (after
supplementation) in
combination with calcium in the cell culture is between about 0 to about 9 g/L
for arginine,
about 0 to about 11 ga, for lysine, about 0 to about 11 g/L histidine, and
about 0 to about 11
g/L ornithine. Although wider ranges are also within the scope of the instant
invention,
including, but not limited to: about 0 to about 30 g/L for arginine, about 0
to about 30 g/L for
lysine, about 0 to about 30 g/L histidine, and about 0 to about 30 g/L
ornithine.
In certain embodiments, the one or more amino acids, calcium, and/or
niacinamide
can be included in the medium at the start of culture, or can be added in a
fed-batch or in a
continuous manner. The feed amounts may be calculated to achieve a certain
concentration
62
CA 02926384 2016-04-07
based on offline or online measurements. The addition of the supplement may be
based on
measured amount of acidic species. Other salts of particular supplements,
e.g., calcium, may
also be used, for example calcium nitrate. The resulting media can be used in
various
cultivation methods including, but not limited to, batch, fed-batch, chemostat
and perfusion,
and with various cell culture equipment including, but not limited to, shake
flasks with or
without suitable agitation, spinner flasks, stirred bioreactors, airlift
bioreactors, membrane
bioreactors, reactors with cells retained on a solid support or
immobilized/entrapped as in
microporous beads, and any other configuration appropriate for optimal growth
and
productivity of the desired cell line.
In certain embodiments, a low AR composition is produced by supplementing a
clarified harvest. For example, but not by way of limitation, such clarified
harvests can be
supplemented as described above (e.g., with calcium, niacinamide, and/or basic
amino acids
or combinations thereof) to reduce AR formation (see Example 3).
Adjusting Process Parameters to Modulate Acidic Species (AR)
In certain embodiments, a low AR composition is produced by adjustment of the
dissolved oxygen (DO) concentration, and/or pH of the cell culture run. In
certain
embodiments, such adjustment includes increasing the DO concentration of the
cell culture,
or decreasing the pH of the cell culture. Such increases in the DO
concentration or decreases
in the pH can be of a magnitude of about 1%, 5%, 10%, 15%, 20%, 25%, 30%, 35%,
40%,
45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 100%, and ranges within
one
or more of the preceding, of the original amount.
In certain embodiments, cell cultures are run in DO concentrations maintained
above
about 15%, above about 20%, above about 30%, or between about 15% and about
80%,
between about 30% and about 50%, or at about 80%, and ranges within one or
more of the
preceding, to achieve the desired reduction in acidic species.
63
CA 02926384 2016-04-07
In certain embodiments, pH is either increased or decreased in order to
increase or
decrease the amount of acidic species and/or the rate at which such acidic
species for-n. For
example, but not by way of limitation, a reduction in pH to about 6.7 from a
control pH of
about 7.1 can be employed to decrease the acidic species during cell culture
and the rate of
acidic species formation in the context of a clarified harvest.
In certain embodiments, the DO concentration, and/or pH is maintained in such
a
manner as to produce a low AR composition comprising about 15% or less AR, 14%
or less
AR, 13% or less AR, 12% or less AR, 11% or less AR, 10% or less AR, 9% or less
AR, 8%
or less AR, 7% or less AR, 6% or less AR, 5% or less AR, 4.5% or less AR, 4%
or less AR,
3.5% or less AR, 3% or less AR, 2.5% or less AR, 2% or less AR, 1.9% or less
AR, 1.8% or
less AR, 1.7% or less AR, 1.6% or less AR, 1.5% or less AR, 1.4% or less AR,
1.3% or less
AR, 1.2% or less AR, 1.1% or less AR, 1% or less AR, 0.9% or less AR, 0.8% or
less AR,
0.7% or less AR, 0.6% or less AR, 0.5% or less AR, 0.4% or less AR, 0.3% or
less AR, 0.2%
or less AR, 0.1% or less AR, or 0.0% AR, and ranges within one or more of the
preceding.
In another embodiment, the DO concentration, and/or pH is maintained in such a
manner as to produce a low AR composition comprising about 15% or less AR I,
14% or less
AR1, 13% or less AR1, 12% or less AR1, 11% or less AR1, 10% or less AR1, 9% or
less
AR I, 8% or less AR1, 7% or less AR1, 6% or less AR1, 5% or less AR1, 4.5% or
less AR1,
4% or less AR I, 3.5% or less AR1, 3% or less AR1, 2.5% or less AR1, 2% or
less AR1, 1.9%
or less AR1, 1.8% or less AR1, 1.7% or less AR1, 1.6% or less AR1, 1.5% or
less AR1, 1.4%
or less AR1, 1.3% or less AR I, 1.2% or less AR1, 1.1% or less AR1, 1% or less
AR I, 0.9%
or less AR1. 0.8% or less AR1, 0.7% or less ARI, 0.6% or less AR1, 0.5% or
less AR I , 0.4%
or less AR1, 0.3% or less AR1, 0.2% or less AR1, 0.1% or less AR1, or 0.0%
AR1, and
ranges within one or more of the preceding.
In yet another embodiment, the DO concentration, and/or pH is maintained in
such a
manlier as to produce a low AR composition comprising about 15% or less AR2,
14% or less
AR2, 13% or less AR2, 12% or less AR2, 11% or less AR2, 10% or less AR2, 9% or
less
AR2, 8% or less AR2, 7% or less AR2, 6% or less AR2, 5% or less AR2, 4.5% or
less AR2.
4% or less AR?, 3.5% or less AR2, 3% or less AR2, 2.5% or less AR2, 2% or less
AR2, 1.9%
or less AR?. 1.8% or less AR2. 1.7% or less AR2, 1.6% or less AR2, 1.5% or
less AR2, 1.4%
or less AR2, 1.3% or less AR2, 1.2% or less AR2, 1.1% or less AR2, 1% or less
AR2, 0.9%
or less AR?, 0.8% or less AR2. 0.7% or less AR2, 0.6% or less AR2, 0.5% or
less AR2, 0.4%
64
CA 02926384 2016-04-07
or less AR2, 0.3% or less AR2, 0.2% or less AR2, 0.1% or less AR2, or 0.0%
AR2, and
ranges within one or more of the preceding.
In certain embodiments, the pH and/or DO is maintained in such a manner as to
reduce the amount of acidic species in a protein or antibody sample by about
1%, 1.2%,
1.5%, 2%, 2.2%, 2.5%, 3%, 3.2%, 3.5%, 4%, 4.2%, 4.5%, 5%, 10%, 15%, 20%, 25%,
30%,
35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 100%, and
ranges
within one or more of the preceding.
For example, and not by way of limitation, as detailed in Example 2, below,
when
five different pH conditions were assessed during cell culture: 7.1 7.0, 6.9,
6.8, and 6.7, the
percent acidic species decreased with a decrease in pH from 29.7% in the pH
7.1 condition to
21.5% in the pH 6.7 condition, for a total reduction of 8.2%.
In addition, as detailed in Example 2, below (and not by way of limitation),
three
different DO concentrations were assessed during cell culture: 20% DO
concentration, 30%
DO concentration and 50% DO concentration, at 35 C. The percentage of acidic
species was
overall lower at higher DO concentrations. In particular, the percentage of
acidic species
decreased with an increase in DO concentration from 23.9% in the 20% DO
concentration
sample to 20.3% in the 50% DO concentration sample, for a total reduction of
3.6%.
In certain embodiments, a low AR composition is produced by cell culture can
be
exerted by maintaining the DO concentration, and/or pH of the cell culture
expressing the
protein of interest as described herein along with choice of suitable
temperature or
temperature shift strategies, for example, but not limited to, lower process
temperature of
operation, temperature shift to a lower temperature or a temperature shift at
an earlier culture
time point. These culture conditions can be used in various cultivation
methods including,
but not limited to, batch, fed-batch, chemostat and Peifusion, and with
various cell culture
equipment includin2, but not limited to, shake flasks with or without suitable
agitation,
spinner flasks, stirred bioreactors, airlift bioreactors, membrane
bioreactors, reactors with
cells retained on a solid support or immobilized/entrapped as in microporous
beads, and any
other configuration appropriate for optimal growth and productivity of the
desired cell line.
These methods of modulating pH and/or DO and/or temperature may also be used
in
combination with supplementation of culture media with additives such as one
or more amino
acids, niacinamide, and/or calcium, or combinations thereof, as described
above to maintain
or achieve a target level of AR or to reduce the formation of AR during cell
culture.
CA 02926384 2016-04-07
Continuous/Perfusion Cell Culture Technology to Modulate Acidic Species (AR)
In certain embodiments, a low AR composition is produced by the choice of cell
culture methodology. In certain embodiments, use of a continuous or perfusion
technology
may be utilized to achieve the desired lowering of acidic species in
combination. In certain
embodiments, this may be attained by modulation of medium exchange rate (where
the
exchange rate is the rate of exchange of medium in/out of a reactor).
In certain, non-limiting, embodiments, maintenance of the medium exchange
rates
(-working volumes/day) of a cell culture run between about 0 and about 20, or
between about
0.5 and about 12 or between about 1 and about 8 or between about 1.5 and about
6 can be
used to achieve the desired reduction in acidic species.
For example, and not by way of limitation, as detailed in Example 4, below,
when the
medium exchange rate was chosen to be 1.5, the acidic species was 8.1%. With
further
increase in exchange rates to 6, a further reduction in acidic species to 6%
was obtained.
In certain embodiments, continuous or perfusion technology (e.g., modulation
of
exchange rate) may result in a low AR composition comprising about 15% or less
AR, 14%
or less AR, 13% or less AR, 12% or less AR, 11% or less AR, 10% or less AR, 9%
or less
AR, 8% or less AR, 7% or less AR, 6% or less AR, 5% or less AR, 4.5% or less
AR, 4% or
less AR, 3.5% or less AR, 3% or less AR, 2.5% or less AR, 2% or less AR, 1.9%
or less AR,
1.8% or less AR, 1.7% or less AR, 1.6% or less AR, 1.5% or less AR, 1.4% or
less AR, 1.3%
or less AR, 1.2% or less AR, 1.1% or less AR, 1% or less AR, 0.9% or less AR,
0.8% or less
AR, 0.7% or less AR, 0.6% or less AR, 0.5% or less AR, 0.4% or less AR, 0.3%
or less AR,
0.2% or less AR, 0.1% or less AR, or 0.0% AR, and ranges within one or more of
the
preceding.
In another embodiment, continuous or perfusion technology (e.g., modulation of
exchange rate) may result in a low AR composition comprising about 15% or less
AR1, 14%
or less AR1, 13% or less AR1, 12% or less AR1, 11% or less AR1, 10% or less AR
I. 9% or
less AR1. 8% or less AR1, 7% or less AR1, 6% or less AR1, 5% or less AR1, 4.5%
or less
AR1. 4% or less AR1, 3.5% or less AR1, 3% or less AR1, 2.5% or less AR1, 2% or
less
AR1, 1.9% or less AR1, 1.8% or less AR1, 1.7% or less AR I, 1.6% or less AR1,
1.5% or less
AR1, 1.4% or less AR1, 1.3% or less AR1, 1.2% or less AR I, 1.1% or less AR',
1% or less
AR1, 0.9% or less AR1, 0.8% or less AR1, 0.7% or less AR I , 0.6% or less AR1,
0.5% or less
66
CA 02926384 2016-04-07
AR1, 0.4% or less AR I, 0.3% or less AR1, 0.2% or less AR I, 0.1% or less AR1,
or 0.0%
AR I, and ranges within one or more of the preceding.
In yet another embodiment, continuous or perfusion technology (e.g.,
modulation of
exchange rate) may result in a low AR composition comprising about 15% or less
AR2, 14%
or less AR2, 13% or less AR2, 12% or less AR2, 11% or less AR2, 10% or less
AR2, 9% or
less AR2, 8% or less AR2, 7% or less AR2, 6% or less AR2, 5% or less AR2, 4.5%
or less
AR2, 4% or less AR2, 3.5% or less AR2, 3% or less AR2, 2.5% or less AR2, 2% or
less
AR2, 1.9% or less AR2, 1.8% or less AR2, 1.7% or less AR2, 1.6% or less AR2,
1.5% or less
AR2, 1.4% or less AR2, 1.3% or less AR2, 1.2% or less AR2, 1.1% or less AR2,
1% or less
AR2, 0.9% or less AR2, 0.8% or less AR2, 0.7% or less AR2, 0.6% or less AR2,
0.5% or less
AR2, 0.4% or less AR2, 0.3% or less AR2, 0.2% or less AR2, 0.1% or less AR2,
or 0.0%
AR2, and ranges within one or more of the preceding.
In certain embodiments, continuous or perfusion technology (e.g., modulation
of
exchange rate) may result in a low AR composition comprising about 1%, 1.2%,
1.5%, 2%,
2.2%, 2.5%, 3%, 3.2%, 3.5%, 4%, 4.2%, 4.5%, 5%, 10%, 15%, 20%, 25%, 30%, 35%,
40%,
45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 100%, and ranges within
one
or more of the preceding.
In one embodiment, media containing additives, such as, for example, one or
more
amino acids, calcium, and/or niacinamide, or combinations thereof, as
described above, may
be used as the perfusion media to maintain or achieve a target level of AR or
to reduce the
formation of AR during cell culture.
In certain embodiments, a low AR composition is produced by, for example,
employment of an intermittent harvest strategy or through use of cell
retention device
technology.
IV. Preparation of Low AR Compositions Using Downstream Process
Technologies
In certain embodiments, the low AR compositions of the present invention may
be
produced using downstream process technologies (e.g., purification), following
cell culture of
a protein. The downstream process technologies may be used alone or in
combination with
the upstream process technologies described in Section III, above, and Example
10.
The methods described herein for the production of compositions comprising low
AR
and/or low process-related impurities comprise the purification of a protein,
such as an
antibody or antigen-binding portion thereof, by, for example, chromatography.
such as
67
CA 02926384 2016-04-07
multimodal (MM) chromatography, wherein the MM media comprises both ion
exchange and
hydrophobic interaction functional groups, and an aqueous salt solution. In
one embodiment,
the same or substantially the same aqueous salt solution is used as a loading
buffer and a
wash buffer.
In further embodiments, the methods described herein for the production of
compositions comprising low AR and/or low process-related impurities comprise
the
purification of a protein, such as an antibody or antigen-binding portion
thereof, by
chromatography comprising an anion exchange (AEX) resin and an aqueous salt
solution. In
one embodiment, the same or substantially the same aqueous salt solution is
used as a loading
buffer and a wash buffer.
In yet further embodiments, the methods described herein for the production of
compositions comprising low AR and/or low process-related impurities comprise
the
purification of a protein, such as an antibody or antigen-binding portion
thereof, by
chromatography comprising a cation exchange (CEX) adsorbent resin and an
aqueous salt
solution. In one embodiment, the same or substantially the same aqueous salt
solution is used
as a loading buffer and a wash buffer, and the target protein bound to the CEX
adsorbent
resin is eluted with a buffer having a higher conductivity and/or pH than the
loading/wash
buffer.
In still further embodiments, the methods described herein for production of
compositions comprising low AR and/or low process-related impurities comprise
the
purification of a protein, such as an antibody or antigen-binding portion
thereof, by a
combination of several media, for example by using an anion exchange (AEX)
resin, and
chromatography using a cation exchange (CEX) adsorbent resin, in a suitable
buffer, such as,
for example, a Tris/Formate buffer system. In one embodiment, the sample is
purified
affinity chromatography media, e.g., Protein A, prior to the ion
chromatography resins. For
example, in one embodiment, the methods described herein for production of
compositions
comprising low AR comprise the exemplary process reflected in Figure 190.
In one embodiment, the method for producing a low AR composition comprising an
antibody, or antigen binding portion thereof, comprises contacting a first
sample comprising
the antibody, or antigen binding portion thereof, to affinity chromatography
media in a load
buffer (for example a low concentration Tris/Forinate buffer), and eluting the
sample from
the affinity chromatography media as a first eluted sample, contacting the
first eluted sample
to a first chromatography media, such as an AEX chromatography resin, in a
load buffer. and
68
CA 02926384 2016-04-07
eluting the sample from the AEX chromatography resin as a second eluted
sample. The
second eluted sample is then contacted with a second chromatography media,
such as a CEX
chromatography resin, in a load buffer, and the sample is eluted from the CEX
chromatography resin as a third eluted sample. In one
embodiment, the CEX
chromatography resin is eluted one, two, three or more times. In one
embodiment, the
process optionally includes one or more intermediate filtration steps, pH
adjustment steps and
inactivation steps.
In one embodiment, the downstream process technologies described herein, alone
or
in combination with other downstream process technologies or with one or more
upstream
process technology, produce a low AR composition comprising an antibody, or
antigen
binding portion thereof, which contains 15% or less AR, 14% or less AR, 13% or
less AR,
12% or less AR, 11% or less AR, 10% or less AR, 9% or less AR, 8% or less AR,
7% or less
AR, 6% or less AR, 5% or less AR, 4.5% or less AR, 4% or less AR, 3.5% or less
AR, 3% or
less AR, 2.5% or less AR, 2% or less AR, 1.9% or less AR, 1.8% or less AR,
1.7% or less
AR, 1.6% or less AR, 1.5% or less AR, 1.4% or less AR, 1.3% or less AR, 1.2%
or less AR,
1.1% or less AR, 1% or less AR, 0.9% or less AR, 0.8% or less AR, 0.7% or less
AR, 0.6%
or less AR, 0.5% or less AR, 0.4% AR, 0.3% or less AR, 0.2% or less AR, 0.1%
or less AR,
or 0.0% AR, and ranges within one or more of the preceding. In one aspect of
this
embodiment, the low AR composition of the invention comprises about 0.0% to
about 10%
AR, about 0.0% to about 5% AR, about 0.0% to about 4% AR, about 0.0% to about
3% AR,
about 0.0% to about 2% AR, about 3% to about 5% AR, about 5% to about 8% AR,
or about
8% to about 10% AR, or about 10% to about 15% AR, and ranges within one or
more of the
preceding.
In one embodiment, the downstream process technologies described herein, alone
or
in combination with other downstream process technologies or with one or more
upstream
process technology, produce a low AR composition comprising an antibody, or
antigen
binding portion thereof, which contains 15% or less AR1, 14% or less AR1, 13%
or less
AR1, 12% or less AR1, 11% or less AR1, 10% or less AR1, 9% or less AR1, 8% or
less
AR1, 7% or less AR1, 6% or less AR1, 5% or less AR1, 4.5% or less AR I, 4% or
less AR1,
3.5% or less AR1, 3% or less AR1, 2.5% or less AR I, 2% or less AR1, 1.9% or
less AR1,
1.8% Of less AR1, 1.7% or less AR1, 1.6% or less AR1, 1.5% or less AR1. 1.4%
or less AR1,
1.3% or less AR1, 1.2% or less AR1, 1.1% or less AR1, 1% or less AR1, 0.9% or
less AR1,
0.8% or less AR1, 0.7% or less AR1, 0.6% or less AR1, 0.5% or less AR1, 0.4%
or less AR1,
69
CA 02926384 2016-04-07
0.3% or less AR1, 0.2% or less AR1, 0.1% or less AR1 , or 0.0% AR1, and ranges
within one
or more of the preceding. In one aspect of this embodiment, the low AR
composition of the
invention comprises about 0.0% to about 10% AR1, about 0.0c7c to about 5% AR1,
about
0.0% to about 4% AR1, about 0.0% to about 3% AR1, about 0.0% to about 2% AR1.,
about
3% to about 5% AR1, about 5% to about 8% AR1, or about 8% to about 10% AR1, or
about
10% to about 15% AR1, and ranges within one or more of the preceding.
In one embodiment, the downstream process technologies described herein, alone
or
in combination with other downstream process technologies or with one or more
upstream
process technology, produce a low AR composition comprising an antibody, or
antigen
binding portion thereof, which contains 15% or less AR2, 14% or less AR2, 13%
or less
AR2, 12% or less AR2, 11% or less AR2, 10% or less AR2, 9% or less AR2, 8% or
less
AR2, 7% or less AR2, 6% or less AR2, 5% or less AR2, 4.5% or less AR2, 4% or
less AR2,
3.5% or less AR2, 3% or less AR2, 2.5% or less AR2, 2% or less AR2, 1.9% or
less AR2,
1.8% or less AR2, 1.7% or less AR2, 1.6% or less AR2, 1.5% or less AR2, 1.4%
or less AR2,
1.3% or less AR2, 1.2% or less AR2, 1.1% or less AR2, 1% or less AR2, 0.9% or
less AR2,
0.8% or less AR2, 0.7% or less AR2, 0.6% or less AR2, 0.5% or less AR2, 0.4%
or less AR2,
0.3% or less AR2, 0.2% or less AR2, 0.1% or less AR2, or 0.0% AR2, and ranges
within one
or more of the preceding. In one aspect of this embodiment, the low AR
composition of the
invention comprises about 0.0% to about 10% AR2, about 0.0% to about 5% AR2,
about
0.0% to about 4% AR2, about 0.0% to about 3% AR2, about 0.0% to about 2% AR2,
about
3% to about 5% AR2, about 5% to about 8% AR2, or about 8% to about 10% AR2, or
about
10% to about 15% AR2, and ranges within one or more of the preceding.
Protein Purification Generally
Following upstream processing of a protein of interest, downstream process
technologies can be used to purify the protein. For example, but not by way of
limitation,
once a clarified solution or mixture comprising the protein of interest, for
example, an
antibody or antigen binding fragment thereof, has been obtained, separation of
the protein of
interest from the acidic species can be effected using a combination of
different purification
techniques, including, but not limited to, affinity separation steps, ion
exchange separation
steps, mixed mode separation steps, and hydrophobic interaction separation
steps singularly
or in combination. The separation steps separate mixtures of proteins on the
basis of their
charge, degree of hydrophobicity, or size, or any combination thereof,
depending on the
CA 02926384 2016-04-07
particular form of separation, including chromatographic separation. In one
aspect of the
invention, separation is performed using chromatography, including cationic,
anionic, and
hydrophobic interaction. Several different chromatography resins are available
for each of
these techniques, allowing accurate tailoring of the purification scheme to
the particular
protein involved. Each of the separation methods result in the protein
traversing at different
rates through a column, to achieve a physical separation that increases as
they pass further
through the column, or adhere selectively to the separation medium. The
proteins are then
differentially eluted by different elution buffers. In some cases, the
antibody is separated
from impurities when the impurities preferentially adhere to the column and
the antibody less
so, i.e., the desired antibody variant is present in the Flow Through.
In certain embodiments, a low AR composition is produced using chromatographic
separation to identify the particular conditions, e.g., salt concentration,
pH, DO concentration,
temperature, load amount and conditions, and washing conditions, sufficient to
elicit the
desired fractionation profile, e.g., fractionation of acidic species and
lysine variants, of a
sample comprising the protein of interest and at least one process-related
impurity. In certain
embodiments, the method further comprises pooling the resulting fractions
comprising the
desired low AR composition compositions.
The purification process may begin at the separation step after the antibody
has been
produced using upstream production methods described above and/or by
alternative
production methods conventional in the art. Once a clarified solution or
mixture comprising
the protein of interest, e.g., an antibody, has been obtained, separation of
the protein of
interest from process-related impurities, such as the other proteins produced
by the cell, as
well as product-related substances, such acidic or basic variants, is
perforrned. In certain
non-limiting embodiments, such separation is performed using CEX, AEX, and/or
MM
chromatography. In certain embodiments, a combination of one or more different
purification
techniques, including affinity separation step(s), ion exchange separation
step(s), mixed-
mode step(s), and/or hydrophobic interaction separation step(s) can also be
employed. Such
additional purification steps separate mixtures of proteins on the basis of
their charge, degree
of hydrophobicity, and/or size. In one aspect of the invention, such
additional separation
steps are performed using chromatography, including hydrophobic, anionic or
cationic
interaction (or a combination thereof). Numerous chromatography resins are
commercially
available for each of these techniques, allowing accurate tailoring of the
purification scheme
to the particular protein involved. Each of the separation methods allow
proteins to either
71
CA 02926384 2016-04-07
traverse at different rates through a column, achieving a physical separation
that increases as
they pass further through the column, or to adhere selectively to a separation
resin (or
medium). The proteins are then differentially eluted using different eluents.
In some cases,
the protein of interest is separated from impurities when the impurities
specifically adhere to
the column's resin and the protein of interest does not, i.e., the protein of
interest is contained
in the effluent, while in other cases the protein of interest will adhere to
the column's resin,
while the impurities and/or product-related substances are extruded from the
column's resin
during a wash cycle.
Primary Recovery and Virus Inactivation
In certain embodiments, the initial steps of the purification methods of the
present
invention involve the clarification and primary recovery of antibody from a
sample matrix.
In certain embodiments, the primary recovery will include one or more
centrifugation steps to
separate the antibody product from the cells and cell debris. Centrifugation
of the sample can
be performed at, for example, but not by way of limitation, 7,000 x g to
approximately
12,750 x g. In the context of large scale purification, such centrifugation
can occur on-line
with a flow rate set to achieve, for example, but not by way of limitation, a
turbidity level of
150 NTU in the resulting supernatant. Such supernatant can then be collected
for further
purification, or in-line filtered through one or more depth filters for
further clarification of the
sample.
In certain embodiments, the primary recovery will include the use of one or
more
depth filtration steps to clarify the sample matrix and thereby aid in
purifying the antibodies
of interest in the present invention. In other embodiments, the primary
recovery will include
the use of one or more depth filtration steps post centrifugation to further
clarify the sample
matrix. Non-limiting examples of depth filters that can be used in the context
of the instant
invention include the Millistak+ XOHC, FOHC, DOHC, A1HC, B I HC depth filters
(EMD
Millipore), CunoTM model 30/60ZA, 60/90 ZA, VR05. VR07, delipid depth filters
(3M
Corp.). A 0.2 i_tm filter such as Sartorius's 0.45/0.2!_trn Sartoporem bi-
layer or Millipore's
Express SHR or SHC filter cartridges typically follows the depth filters.
In certain embodiments, the primary recovery process can also be a point at
which to
reduce or inactivate viruses that can be present in the sample matrix. For
example, any one
or more of a variety of methods of viral reduction/inactivation can be used
during the primary
recovery phase of purification including heat inactivation (pasteurization),
pH inactivation,
72
CA 02926384 2016-04-07
buffer/detergent treatment, UV and 7-ray irradiation and the addition of
certain chemical
inactivating agents such as 13-propiolactone or e.g., copper phenanthroline as
described in
U.S. Pat. No. 4,534,972. In certain embodiments of the present invention, the
sample matrix
is exposed to detergent viral inactivation during the primary recovery phase.
In other
embodiments, the sample matrix may be exposed to low pH inactivation during
the primary
recovery phase.
In those embodiments where viral reduction/inactivation is employed, the
sample
mixture can be adjusted, as needed, for further purification steps. For
example, following
low pH viral inactivation, the pH of the sample mixture is typically adjusted
to a more neutral
pH, e.g., from about 4.5 to about 8.5, prior to continuing the purification
process.
Additionally, the mixture may be diluted with water for injection (WFI) to
obtain a desired
conductivity.
Additives to the Clarified Harvest
In certain embodiments, a low AR composition is produced by supplementing a
clarified harvest containing antibodies or antigen binding portions thereof.
A clarified
harvest can be extracted from a cell culture, for example, a fermentation
bioreactor, after
undergoing centrifugation to remove large solid particles and subsequent
filtration to remove
finer solid particles and impurities from the material. Such clarified
harvests can be
supplemented as described above (e.g., with calcium, niacinamide, and/or basic
amino acids,
or combinations thereof) or modulation, e.g., lowering, of pH, to reduce AR
formation (see
Example 3).
Affinity Chromatography
In certain embodiments, it will be advantageous to subject a sample produced
by the
techniques of the instant invention to affinity chromatography to further
purify the protein of
interest away from acidic species. In certain embodiments the chromatographic
material is
capable of selectively or specifically binding to the protein of interest
("capture"). Non-
limiting examples of such chromatographic material include: Protein A, Protein
G,
chromatographic material comprising, for example, an antigen bound by an
antibody of
interest, and chromatographic material comprising an Fc binding protein. In
specific
embodiments, the affinity chromatography step involves subjecting the primary
recovery
sample to a column comprising a suitable Protein A resin. In certain
embodiments, Protein A
73
CA 02926384 2016-04-07
resin is useful for affinity purification and isolation of a variety of
antibody isotypes,
particularly IgGI, 1gG2, and IgG4. Protein A is a bacterial cell wall protein
that binds to
mammalian IgGs primarily through their Fc regions. In its native state,
Protein A has five
IgG binding domains as well as other domains of unknown function.
There are several commercial sources for Protein A resin. One suitable resin
is
MabSelectIm from GE Healthcare. Suitable resins include, but not limited to,
MabSelect
SUReTM, MabSelect SuRe LX, MabSelect, MabSelect Xtra, rProtein A Sepharose
from GE
Healthcare, ProSep HC, ProSep Ultra, and ProSep Ultra Plus from EMD Millipore,
MapCapture from Life Technologies. A non-limiting example of a suitable column
packed
with MabSelectTM is an about 1.0 cm diameter x about 21.6 cm long column (-17
mL bed
volume). This size column can be used for small scale purifications and can be
compared
with other columns used for scale ups. For example, a 20 cm x 21 cm column
whose bed
volume is about 6.6 L can be used for larger purifications. Regardless of the
column, the
column can be packed using a suitable resin such as MabSelectTM.
The Protein A column can be equilibrated with a suitable buffer prior to
sample
loading. Following the loading of the column, the column can be washed one or
multiple
times using a suitable set of buffers. The Protein A column can then be eluted
using an
appropriate elution buffer. For example, glycine-HCL or citric acid can be
used as an elution
buffer. The eluate can be monitored using techniques well known to those
skilled in the art.
The eluate fractions of interest can be collected and then prepared for
further processing.
The Protein A eluate may subject to a viral inactivation step either by
detergent or low
pH, provided this step is not performed prior to the Protein A capture
operation. A proper
detergent concentration or pH and time can be selected to obtain desired viral
inactivation
results. After viral inactivation, the Protein A eluate is usually pH and/or
conductivity
adjusted for subsequent purification steps.
The Protein A eluate may be subjected to filtration through a depth filter to
remove
turbidity and/or various impurities from the antibody of interest prior to
additional
chromatographic polishing steps. Examples of depth filters include, but not
limited to,
Millistak+ XOHC, FOHC, DOHC, A I HC. and B I FIC Pod filters (EMD Millipore),
or Zeta
Plus 30ZA/60ZA, 60ZA/90ZA, delipid, VR07. and VRO5 filters (3M). The Protein A
eluate
pool may need to be conditioned to proper pH and conductivity to obtain
desired impurity
removal and product recovery from the depth filtration step.
74
CA 02926384 2016-04-07
The invention is not limited to capture of the protein of interest using
Protein A
chromatography. A non-Protein A chromatography capture step can also be
carried out. For
example, cation exchange capture and non-chromatographic methods, such as
aqueous two
phase extraction or precipitation, or other methods known in the art, can be
used.
Anion Exchange Chromatography
In certain embodiments, the low AR compositions are produced by subjecting the
primary recovery sample to at least one anion exchange separation step. In
certain
embodiments, the anion exchange step will occur after the above-described
affinity
chromatography, e.g., Protein A affinity, step.
The use of an anionic exchange material versus a cationic exchange material,
such as
those cation exchange materials discussed in detail below, is based on the
local charges of the
protein of interest in a given solution. Therefore, it is within the scope of
this invention to
employ an anionic exchange step prior to the use of a cationic exchange step,
or a cationic
exchange step prior to the use of an anionic exchange step. Furthermore, it is
within the
scope of this invention to employ only an anionic exchange step, only an
cationic exchange
step, or any serial combination of the two (including serial combinations of
one or both ion
exchange steps with the other chromatographic separation technologies
described herein).
In peifonning the separation, the initial protein composition can be contacted
with the
anion exchange material by using any of a variety of techniques, e.g., using a
batch
purification technique or a chromatographic technique.
For example, in the context of batch purification, anion exchange material is
prepared
in, or equilibrated to, the desired starting buffer. Upon preparation, or
equilibration, a slurry
of the anion exchange material is obtained. The protein of interest, e.g.,
antibody, solution is
contacted with the slurry to allow for protein adsorption to the anion
exchange material. The
solution comprising the acidic species that do not bind to the AEX material is
separated from
the slurry, e.g., by allowing the slurry to settle and removing the
supernatant. The slurry can
be subjected to one Or more washing steps and/or elution steps.
In the context of chromatographic separation, a chromatographic apparatus,
commonly cylindrical in shape, is employed to contain the chromatographic
support material
(e.g., AEX material) prepared in an appropriate buffer solution. The
chromatographic
apparatus, if cylindrical, can have a diameter of about 5 mm to about 2
meters, and a height
of 5 cm to 50 cm, and in certain embodiments, particularly for large scale
processing, a
CA 02926384 2016-04-07
height of < 30 cm is employed. Once the chromatographic material is added to
the
chromatographic apparatus, a sample containing the protein of interest, e.g.,
an antibody, is
contacted to the chromatographic material to induce the separation. Any
portion of the
solution that does not bind to the chromatographic material, e.g., which may
comprise,
depending on the AEX material being employed, the protein of interest, acidic
species, is
separated from the chromatographic material by washing the material and
collecting fractions
from column. The chromatographic material can be subjected to one or more wash
steps. If
desired, the chromatographic material can then be contacted with a solution
designed to
desorb any components of the solution that have bound to the chromatographic
material.
In certain embodiments, a wash step can be performed in the context of AEX
chromatography using conditions similar to the load conditions or
alternatively by decreasing
the pH and/or increasing the ionic strength/conductivity of the wash in a step
wise or linear
gradient manner. The resulting Flow Through and wash fractions can be analyzed
and
appropriate fractions pooled to achieve the desired reduction in charged
variant species. In
certain embodiments, the aqueous salt solution used as both the loading and
wash buffer has a
pH that at or near the isoelectric point (pI) of the protein of interest. In
certain embodiments
the pH is about 0 to 2 units higher or lower than the pI of the protein of
interest. In certain
embodiments, it will be in the range of 0 to 0.5 units higher or lower. In
certain
embodiments, it will be at the pI of the antibody.
In certain non-limiting embodiments, the anionic agent is selected from the
group
consisting of acetate, formate, or combinations thereof. In certain non-
limiting embodiments,
the cationic agent is selected from the group consisting of Tris, arginine, or
combinations
thereof. In one embodiment, the buffer solution is a Tris/formate buffer. In
another
embodiment, the buffer is selected from the group consisting of pyridine,
piperazine, L-
histidine, Bis-tiis, Bis-tris propane, imidazole, N-Ethylmorpholine, TEA
(triethanolamine),
Tris, Morpholine, N-Methyldiethanolamine, AMPD (2-amino-2-methyl- L3-
propanediol),
diethanolamine, ethanolamine, AMP (2- am i no-2-methyl- I -pro paol),
piperazine, 1,3-
Diaminopropane, piperidine
A packed anion-exchange chromatography column, anion-exchange membrane
device, anion-exchange monolithic device, or depth filter media can be
operated either in
bind-elute mode, flow-through mode, or a hybrid mode wherein the product
exhibits binding
to the chromatographic material, yet can be washed from the column using a
buffer that is the
same or substantially similar to the loading buffer. In the bind-elute mode,
the column or the
76
CA 02926384 2016-04-07
membrane device is first conditioned with a buffer with appropriate ionic
strength and pH
under conditions where certain proteins will be immobilized on the resin based
matrix. For
example, in certain embodiments, during the feed load, the protein of interest
will be
adsorbed to the resin due to electrostatic attraction. After washing the
column or the
membrane device with the equilibration buffer or another buffer with different
pH and/or
conductivity, the product recovery is achieved by increasing the ionic
strength (i.e.,
conductivity) of the elution buffer to compete with the solute for the charged
sites of the
anion exchange matrix. Changing the pH and thereby altering the charge of the
solute is
another way to achieve elution of the solute. The change in conductivity or pH
may be
gradual (gradient elution) or stepwise (step elution). In the flow-through
mode, the column
or the membrane device is operated at selected pH and conductivity such that
the protein of
interest does not bind to the resin or the membrane while the acidic species
will either be
retained on the column or will have a distinct elution profile as compared to
the protein of
interest. In the
context of this hybrid strategy, acidic species will bind to the
chromatographic material (or Flow Through) in a manner distinct from the
protein of interest,
e.g., while the protein of interest and certain aggregates and/or fragments of
the protein of
interest may bind the chromatographic material, washes that preferentially
remove the protein
of interest can be applied. The column is then regenerated before next use.
Non-limiting examples of anionic exchange substituents include
diethylaminoethyl
(DEAE), quaternary aminoethyl (QAE) and quaternary amine (Q) groups.
Additional non-
limiting examples include: Poros 50PI and Poros 50HQ, which are a rigid
polymeric bead
with a backbone consisting of cross-linked poly[styrene-divinylbenzene]; Capto
Q Impres
and Capto DEAE, which are a high flow agarose bead; Toyopearl QAE-550,
Toyopearl
DEAE-650, and Toyopearl GigaCap Q-650, which are a polymeric base bead;
Fractogel(')
EMD TMAE Hicap, which is a synthetic polymeric resin with a 'tentacle ion
exchanger;
Sartobind STIC PA nano, which is a salt-tolerant chromatographic membrane
with a
primary amine ligand; Sartobind Q nano; which is a strong anion exchange
chromatographic
membrane; CUNO BioCap; which is a zeta-plus depth filter media constructed
from
inorganic filter aids, refined cellulose, and an ion exchange resin; and XOHC,
which is a
depth-filter media constructed from inorganic filter aid, cellulose, and mixed
cellulose esters.
The detailed information is listed in Table 1.
77
CA 02926384 2016-04-07
,
Table 1: List of AEX Adsorbent Properties
Media Particle/
AEX Adsorbent Vendor Ligand Type Catalog Number
Type Pore Size
Poros PI Weak -50 m 1-2459-11
Applied
Biosystems
Poros HQ Strong -50 m 1-2559-11
Capto DEAE Weak -90 m 17-5443-10
GE
CaptoQ Impres Strong -90 m 17-5316-10
Resin
QAE-550 Strong -100 m 43271
DEAE-650 Tosoh Weak -65 m 43201
GigaCap Q-650 Strong -75 m 21854
TMAE HiCap EMD/Millipore Strong -40-90 m 1.16881.0013
Sartobind 92STPA42DN-
Weak 3-5 m
ST1C PA Nano 11¨A
Sartorius Membrane
Sartobind Q
Strong 3-5 m 92IEXQ42DN-11
Nano
CUNO BioCap
3M NA NA BC0025L6OZA05A
25 Depth
Filter
XOHC Millipore NA NA MX0HC23CL3
In certain embodiments. the protein load of the mixture comprising protein of
interest
is adjusted to a total protein load to the column of between about 50 and 500
g/L, or between
78
CA 02926384 2016-04-07
about 75 and 350 g/L, or between about 200 and 300 g/L. In certain
embodiments, the
protein concentration of the load protein mixture is adjusted to a protein
concentration of the
material loaded to the column of about 0.5 and 50 g/L, between about 1 and 20
g/L, or
between 3 and 10 g/L. In certain embodiments, the protein concentration of the
load protein
mixture is adjusted to a protein centration of the material to the column of
about 37 g/L.
In certain embodiments, additives such as poly ethylene glycol, detergents,
amino
acids, sugars, chaotropic agents can be added to enhance the performance of
the separation,
so as to achieve better recovery or product quality.
In certain embodiments, including, but not limited to those relating to
adalimumab,
the methods of the instant invention can be used to selectively remove,
significantly reduce,
or essentially remove all of AR in the Flow Through and wash fractions while
enriching for
the same in the flow elution &action, thereby producing protein compositions
that have
reduced AR or are free of AR. In certain embodiments relating to the
purification of
adalimumab, the methods of the instant invention can be used to selectively
remove,
significantly reduce, or essentially remove all of AR1 charge variants in the
Flow Through
and wash fractions while enriching for the same in the flow elution fraction,
thereby
producing protein compositions that have reduced AR1 or are free of AR1
variants. In certain
embodiments relating to adalimumab, the methods of the instant invention can
be used to
selectively remove, significantly reduce, or essentially remove all of AR2
charge variants in
the flow-through and wash fractions while enriching for the same in the flow
elution fraction,
thereby producing protein compositions that have reduced AR2 or are free of
AR2 variants.
In certain embodiments, including but not limited to those relating to
adalimumab, the
methods of the instant invention can be used to selectively remove,
significantly reduce, or
essentially remove all of the MGO variants in the Flow Through and wash
fractions while
enriching for the same in the elution fraction, thereby producing protein
compositions that.
have reduced MGO or are free of MGO variants (for example, see U.S. Patent
Application
Serial No. 61/777.883, tiled on March 12, 2013). In certain embodiments,
including, but not
limited to those relating to acialimurnab, the methods of the instant
invention can be used to
selectively remove, significantly reduce, or essentially remove all of the
glycated variants
(SchitT's base and permanently glycated forms) in the Flow Through and wash
fractions
while enriching for the same in the elution fraction, thereby producing
protein preparations
with reduced or free of glycated variants.
79
=
CA 02926384 2016-04-07
In certain embodiments, the loading, pH, conductivity of the AEX
chromatography
step, as well as elution pH conductivity, can be modified to achieve a desired
distribution of
product-relates substances (AR or lysine variants) For example, but not by way
of limitation,
certain embodiments are directed to the modulation of the lysine distribution
of purified
sample of a protein of interest, e.g., increasing Lys 0 and decreasing Lys 1
and Lys 2. In
certain embodiments, the methods of the present invention allow for the
preparation of
samples wherein the amount of Lys 0 is decreased, while the amount of Lys 1
and/or Lys 2 is
increased.
In certain embodiments, an AEX chromatographic separation can be performed and
combinations of fractions can be pooled to achieve a combination of desired
process-related
impurity and/or product-relates substance levels, in addition to, or in place
of merely
modulating charge variant concentration.
Spectroscopy methods such as UV, NIR, FTIR, Fluorescence, and Raman may be
used to monitor levels of AR species in an on-line, at-line or in-line mode,
which can then be
used to control the level of charge variants, e.g., acidic species, in the
pooled material
collected from the AEX effluent.
In certain embodiments, specific signals arising from the chemical
modification of the
proteins such as glycation, MGO modification, deamidation, glycosylation may
be
specifically measurable by spectroscopic methods through such in-line, on-line
or at-line
methods, enabling realtime or near-real time control of product quality of the
resulting
product. In certain embodiments, on-line, at-line or in-line monitoring
methods can be used
either on the effluent line of the chromatography step or in the collection
vessel, to enable
achievement of the desired product quality/recovery. In certain embodiments,
the UV signal
can be used as a surrogate to achieve an appropriate product quality/recovery,
wherein the
UV signal can be processed appropriately, including, but not limited to, such
processing
techniques as integration, differentiation, moving average, such that normal
process
variability can be addressed and the target product quality can be achieved.
In certain
embodiments. such measurements can be combined with in-line dilution methods
such that
ion concentration/conductivity of the load/wash can be controlled by feedback
and hence
facilitate product quality control.
CA 02926384 2016-04-07
In certain embodiments, a combination of AEX and CEX and MM methods can be
used to prepare product-related substance-modulated materials, including
certain
embodiments where one technology is used in a complementary/supplementary
manner with
another technology. In certain embodiments, such a combination can be
performed such that
certain sub-species are removed predominantly by one technology, such that the
combination
provides the desired final composition/product quality. In certain
embodiments, such
combinations include the use of additional intervening chromatography,
filtration, pH
adjustment, and/or UF/DF steps so as to achieve the desired AR, product
quality, ion
concentration, and/or viral reduction.
As described below and in Example 11, AEX chromatography can be used in
conjunction with recycle chromatography modes and continuous chromatography
modes.
Cation Exchange Chromatography
The low AR compositions of the present invention can be produced by subjecting
the
composition, e.g., a primary recovery sample, to at least one cation exchange
separation step.
In certain embodiments, the CEX step will occur after the above-described
affinity
chromatography, e.g., Protein A affinity, step.
The use of a cationic exchange material versus an anionic exchange material,
such as
those anionic exchange materials discussed in detail above, is based on the
local charges of
the protein of interest in a given solution. Therefore, it is within the scope
of this invention to
employ a cationic exchange step prior to the use of an anionic exchange step,
or an anionic
exchange step prior to the use of a cationic exchange step. Furthermore, it is
within the scope
of this invention to employ only a cationic exchange step, only an anionic
exchange step, or
any serial combination of the two (including serial combinations of one or
both ion exchange
steps with the other chromatographic separation technologies described
herein).
In performing the separation, the initial protein mixture can be contacted
with the
cation exchange material by using any of a variety of techniques, e.g., using
a batch
purification technique or a chromatographic technique, as described above in
connection with
Protein A or AEX.
In certain embodiments, the aqueous salt solution used as both the loading and
wash
buffer has a pH that is lower than the isoelectric point (pl) of the protein
of interest. In certain
81
CA 02926384 2016-04-07
embodiments, the pH is about 0 to 5 units lower than the pI of the protein. In
certain
embodiments, it is in the range of 1 to 2 units lower. In certain embodiments,
it is in the
range of 1 to 1.5 units lower.
In certain embodiments, the concentration of the anionic agent in aqueous salt
solution is increased or decreased to achieve a pH of between about 3.5 and
10.5, or between
about 4 and 10, or between about 4.5 and 9.5, or between about 5 and 9, or
between about 5.5
and 8.5, or between about 6 and 8, or between about 6.5 and 7.5. In certain
embodiments, the
concentration of anionic agent is increased or decreased in the aqueous salt
solution to
achieve a pH of 5, or 5.5, or 6, or 6.5, or 6.8, or 7.5. Buffer systems
suitable for use in the
CEX methods include, but are not limited to tris formate, tris acetate,
ammonium sulfate,
sodium chloride, and sodium sulfate.
In certain embodiments, the conductivity and pH of the aqueous salt solution
is
adjusted by increasing or decreasing the concentration of a cationic agent. In
certain
embodiments, the cationic agent is maintained at a concentration of between
about range of
20mM to 500mM, or between about 50 to 350mM or between about 100 to 300mM or
between about 100 to 200mM. In
certain non-limiting embodiments, the cationic agent
is selected from the group consisting of sodium, Tris, tromethalmine,
ammonium, arginine, or
combinations thereof. In certain non-limiting embodiments, the anionic agent
is selected from
the group consisting of formate, acetate, citrate, chloride anion, sulphate,
phosphate or
combinations thereof.
A packed cation-exchange chromatography column or a cation-exchange membrane
device can be operated either in bind-elute mode, flow-through mode, or a
hybrid mode
wherein the product exhibits binding to the chromatographic material, yet can
be washed
from the column using a buffer that is the same or substantially similar to
the loading buffer.
The details of these modes are outlined above.
Cationic substituents include carboxymethyl (CM), sulfoethyl (SE), sulfopropyl
(SP),
phosphate (P) and sulfonate (S). Additional cationic materials include, but
are not limited to:
Canto SP ImpRes. which is a high flow agarose bead; CM Hyper D grade F; which
is a
ceramic bead coated and permeated with a functionalized hydrogel. 250 ¨ 400
ionic groups
peq/mL; Eshmuno S. which is a hydrophilic polyvinyl ether base matrix with 50-
100 peq/mL
ionic capacity: Nuvia C Prime. which is a hydrophobic cation exchange media
composed of a
CA 02926384 2016-04-07
macroporous highly crosslinked hydrophilic polymer matrix 55-75 neq/mL; Nuvia
S, which
has a UNOsphere base matrix with 90 -150 eq/mL ionic groups; Poros HS; which
is a rigid
polymetic bead with a backbone consisting of cross-linked poly[styrene-
divinylbenzene];
Poros XS; which is a rigid polymetic bead with a backbone consisting of cross-
linked
poly[styrene-divinylbenzene]; Toyo Pearl Giga Cap CM 650M, which is a
polymeric base
bead with 0.225meq/mL ionic capacity; Toyo Pearl Giga Cap S 650M which is a
polymeric
base bead; Toyo Pearl MX TRP, which is a polymeric base bead. Detailed
information
concerning the aforementioned materials is listed in Table 2. It is noted that
CEX
chromatography can be used with MM resins, described herein.
Table 2: Cationic Materials
Catalog
Resin Vendor type particle size Number
Capto SP ImpRes GE Strong ¨40 m
17-5468-10
CM Hyper D Pall Weak ¨50 m 20050-027
Eshmuno S Millipore Strong ¨85pm
1.20078
Mix
Nuvia C Prime Biorad Mode ¨70pm 156-3401
Nuvia S Biorad Strong ¨85 m
156-0315
Applied
Poros HS Biosystems Weak ¨50pm
13359-06
Applied
Poros XS Biosystems Strong ¨50 m
4404337
Toyo Pearl Giga Cap CM
650M Tosoh Weak -75pm 21946
Toyo Pearl Giga Cap S
650M Tosoh Strong ¨75pm 21833
Mix
Toyo Pearl MX Trp 650M Tosoh Mode ¨75 m 22817
83
CA 02926384 2016-04-07
In certain embodiments, the protein load of the mixture comprising protein of
interest
is adjusted to a total protein load to the column of between about 5 and 150
g/L, or between
about 10 and 100 g/L, between about 20 and 80 g/L, between about 30 and 50
g/L, or
between about 40 and 50 g/L. In certain embodiments, the protein concentration
of the load
protein mixture is adjusted to a protein concentration of the material loaded
to the column of
about 0.5 and 50 g/L, or between about 1 and 20 g/L.
In certain embodiments, additives such as poly ethylene glycol, detergents,
amino
acids, sugars, chaotropic agents can be added to enhance the performance of
the separation,
so as to achieve better recovery or product quality.
In certain embodiments, including, but not limited to those relating to
adalimumab,
the methods of the instant invention can be used to selectively remove,
significantly reduce,
or essentially remove all of AR in the Flow Through and wash fractions while
enriching for
the same in the elution fraction, thereby producing protein compositions that
have reduced
AR or are free of AR. In certain embodiments relating to the purification of
adalimumab, the
methods of the instant invention can be used to selectively remove,
significantly reduce, or
essentially remove all of AR I charge variants in the Flow Through and wash
fractions while
enriching for the same in the flow elution fraction, thereby producing protein
compositions
that have reduced AR I or are free of AR1 variants. In certain embodiments
relating to
adalimumab, the methods of the instant invention can be used to selectively
remove,
significantly reduce, or essentially remove all of AR2 charge variants in the
flow-through and
wash fractions while enriching for the same in the flow elution fraction,
thereby producing
protein compositions that have reduced AR2 or are free of AR2 variants.
In certain embodiments, including, but not limited to those relating to
adalimumab,
the methods of the instant invention can be used to selectively remove,
significantly reduce,
or essentially remove all of the MGO variants in the elution fractions while
enriching for the
same in the Flow Through and wash fractions, thereby producing protein
preparations with
reduced or free of MGO variants. In certain embodiments, including, but not
limited to those
relating to adalimumab, the methods of the instant invention can be used to
selectively
remove. significantly reduce, or essentially remove all of the glycated
variants (Schiff's base
and permanently glycated forms) in the elution fractions while enriching for
the same in the
Flow Through and wash fractions, thereby producing protein preparations with
reduced or
free of glycated variants.
84
CA 02926384 2016-04-07
In certain embodiments, the loading, pH, conductivity of the CEX
chromatography
step, as well as elution pH conductivity, can be modified to achieve a desired
distribution of
acidic species. For example, but not by way of limitation, certain embodiments
are directed
to the modulation of the lysine distribution of a purified sample of a protein
of interest, e.g.,
increasing Lys 0 and decreasing Lys 1 and Lys 2. In certain embodiments, the
methods of
the present invention allow for the preparation of samples wherein the amount
of Lys 0 is
decreased, while the amount of Lys 1 and/or Lys 2 is increased.
In certain embodiments, a CEX chromatographic separation can be performed and
combinations of fractions can be pooled to achieve a combination of desired
process-related
impurity and/or product-relates substance levels, in addition to, or in place
of merely
modulating charge variant concentration.
In certain embodiments, spectroscopy methods such as UV, NIR, FTIR,
Fluorescence,
Raman may be used to monitor levels of product-related charge variants,
aggregates, low
molecular weight variants (e.g., fragments of the protein of interest) in an
on-line, at-line or
in-line mode, which can then be used to control the level of charge variants,
e.g., acidic
species, in the pooled material collected from the CEX effluent. In certain
embodiments,
specific signals arising from the chemical modification of the proteins such
as glycation,
MGO modification, deamidation. glycosylation may be specifically measurable by
spectroscopic methods through such in-line, on-line or at-line methods,
enabling realtime or
near-real time control of product quality of the resulting product. In certain
embodiments,
on-line. at-line or in-line monitoring methods can be used either on the
effluent line of the
chromatography step or in the collection vessel, to enable achievement of the
desired product
quality/recovery. In certain embodiments, the UV signal can be used as a
surrogate to
achieve an appropriate product quality/recovery, wherein the UV signal can be
processed
appropriately, including, but not limited to, such processing techniques as
integration,
differentiation, moving average, such that normal process variability can be
addressed and the
target product quality can be achieved. In certain embodiments, such
measurements can be
combined with in-line dilution methods such that ion
concentration/conductivity of the
load/wash can be controlled by feedback and hence facilitate product quality
control.
In certain embodiments, a combination of CEX and AEX and/or MM methods can be
used to prepare product-related substance-modulated materials, including
certain
embodiments where one technology is used in a complementary/supplementary
manner with
CA 02926384 2016-04-07
another technology. In certain embodiments, such a combination can be
performed such that
certain sub-species are removed predominantly by one technology, such that the
combination
provides the desired final composition/product quality. In certain
embodiments, such
combinations include the use of additional chromatography, filtration, pH
adjustment, UF/DF
steps so as to achieve the desired product quality, AR, ion concentration,
and/or viral
reduction.
As described below and in Example 11, CEX chromatography can be used in
conjunction with recycle chromatography and continuous chromatography modes.
Mixed Mode Chromatography
Mixed mode ("MM") chromatography may also be used to prepare the low AR
compositions of the invention. MM chromatography, also referred to herein as
"multimodal
chromatography", is a chromatographic strategy that utilizes a support
comprising a ligand
that is capable of providing at least two different, and in certain
embodiments co-operative,
sites that interact with the substance to be bound. In certain embodiments,
one of these sites
gives an attractive type of charge-charge interaction between the ligand and
the substance of
interest and the other site provides for electron acceptor-donor interaction
and/or hydrophobic
and/or hydrophilic interactions. Electron donor-acceptor interactions include
interactions
such as hydrogen-bonding, 7C-7E, cation- it, charge transfer, dipole-dipole,
induced dipole etc.
In certain embodiments, the resin employed for a mixed mode separation is
Capto
Adhere. Capto Adhere is a strong anion exchanger with multimodal
functionality. Its base
matrix is a highly cross-linked agarose with a ligand (N-Benzyl-N-methyl
ethanol amine) that
exhibits many functionalities for interaction, such as ionic interaction,
hydrogen bonding and
hydrophobic interaction. In certain embodiments. the resin employed for a
mixed mode
separation is selected from PPA-HyperCel and HEA-HyperCel. The base matrices
of PPA-
HyperCel and HEA-HyperCel are high porosity cross-linked cellulose. Their
ligands are
Phenylpropylamine and Hexylamine, respectively. Phenylpropylamine and
Hexylamine offer
different selectivity and hydrophobicity options for protein separations.
Additional mixed
mode chromatographic supports include. but are not limited to, Nuvia C Prime.
Toyo Pearl
MX Trp 650M, and Eshmuno HCX.
86
CA 02926384 2016-04-07
In certain embodiments, the mixed mode chromatography resin is comprised of
ligands coupled to an organic or inorganic support, sometimes denoted a base
matrix, directly
or via a spacer. The support may be in the fori-n of particles, such as
essentially spherical
particles, a monolith, filter, membrane, surface, capillaries, etc. In certain
embodiments, the
support is prepared from a native polymer, such as cross-linked carbohydrate
material, such
as agarose, agar, cellulose, dextran, chitosan, konjac, carrageenan, gellan,
alginate etc. To
obtain high adsorption capacities, the support can be porous, and ligands are
then coupled to
the external surfaces as well as to the pore surfaces. Such native polymer
supports can be
prepared according to standard methods, such as inverse suspension gelation (S
Hjerten:
Biochim Biophys Acta 79(2), 393-398 (1964). Alternatively, the support can be
prepared
from a synthetic polymer, such as cross-linked synthetic polymers, e.g.
styrene or styrene
derivatives, divinylbenzene, acrylamides, acrylate esters, methacrylate
esters, vinyl esters,
vinyl amides etc. Such synthetic polymers can be produced according to
standard methods,
see e.g. "Styrene based polymer supports developed by suspension
polymerization" (R
Ai-shady: Chimica e L'Industria 70(9), 70-75 (1988)). Porous native or
synthetic polymer
supports are also available from commercial sources, such as Amersham
Biosciences,
Uppsala, Sweden.
In certain embodiments, the protein load of the mixture comprising protein of
interest
is adjusted to a total protein load to the column of between about 50 and 750
g/L, or between
about 75 and 500 g/L, or between about 100 and 300 g/L. In certain
embodiments, the
protein concentration of the load protein mixture is adjusted to a protein
concentration of the
material loaded to the column of about 1 and 50 g/L, or between about 9 and 25
g/L.
In certain embodiments, additives such as poly ethylene glycol, detergents,
amino
acids, sugars, chaotropic agents can be added to enhance the performance of
the separation,
so as to achieve better recovery or product quality.
In certain embodiments, including. but not limited to those relating to
adalimumab,
the MM methods of the instant invention can be used to selectively remove,
significantly
reduce, or essentially remove all of AR in the Flow Through and wash fractions
while
enriching for the same in the flow elution fraction, thereby producing protein
compositions
that have reduced AR or are free of AR. In certain embodiments relating to the
purification
of adalimumab, the methods of the instant invention can be used to selectively
remove,
significantly reduce. or essentially remove all of AR1 charge variants in the
Flow Through
87
CA 02926384 2016-04-07
and wash fractions while enriching for the same in the flow elution fraction,
thereby
producing protein compositions that have reduced AR I or are free of AR I
variants. In certain
embodiments relating to adalimumab, the methods of the instant invention can
be used to
selectively remove, significantly reduce, or essentially remove all of AR2
charge variants in
the flow-through and wash fractions while enriching for the same in the flow
elution fraction,
thereby producing protein compositions that have reduced AR2 or are free of
AR2 variants.
In certain embodiments, including, but not limited to those relating to
adalimumab,
the MM methods of the instant invention can be used to selectively remove,
significantly
reduce, or essentially remove all of the MGO variants in the Flow Through and
wash
fractions while enriching for the same in the elution fraction, thereby
producing protein
preparations with reduced or free of MGO variants. In certain embodiments,
including, but
not limited to those relating to adalimumab, the methods of the instant
invention can be used
to selectively remove, significantly reduce, or essentially remove all of the
glycated variants
(Schiff's base and permanently glycated forms) in the Flow Through and wash
fractions
while enriching for the same in the elution fraction, thereby producing
protein preparations
with reduced or free of glycated variants.
In certain embodiments, the loading, pH, conductivity of the MM chromatography
step, wash pH and conductivity, as well as elution pH conductivity, can be
modified to
achieve a desired distribution of acidic species. For example, but not by way
of limitation,
certain embodiments are directed to the modulation of the lysine distribution
of a purified
sample of a protein of interest, e.g., increasing Lys 0 and decreasing Lys I
and Lys 2. In
certain embodiments. the methods of the present invention allow for the
preparation of
samples wherein the amount of Lys0 is decreased, while the amount of Lys I
and/or Lys 2 is
increased.
In certain embodiments, a MM chromatographic separation can be performed and
combinations of fractions can be pooled to achieve a combination of desired
process-related
impurity and/or product-relates substance levels, in addition to. or in place
of merely
modulating change variant concentration.
In certain embodiments. spectroscopy methods such as UV, NIR, FTIR,
Fluorescence,
Raman may be used to monitor levels of AR species in an on-line, at-line or in-
line mode,
which can then be used to control the level of charge variants. e.g., acidic
species, in the
88
CA 02926384 2016-04-07
pooled material collected from the MM effluent. In certain embodiments,
specific signals
arising from the chemical modification of the proteins such as glycation, MGO
modification,
deamidation. glycosylation may be specifically measurable by spectroscopic
methods through
such in-line, on-line or at-line methods, enabling realtime or near-real time
control of product
quality of the resulting product. In certain embodiments, on-line, at-line or
in-line monitoring
methods can be used either on the effluent line of the chromatography step or
in the
collection vessel, to enable achievement of the desired product
quality/recovery. In certain
embodiments, the UV signal can be used as a surrogate to achieve an
appropriate product
quality/recovery, wherein the UV signal can be processed appropriately,
including, but not
limited to, such processing techniques as integration, differentiation, moving
average, such
that normal process variability can be addressed and the target product
quality can be
achieved. In certain embodiments, such measurements can be combined with in-
line dilution
methods such that ion concentration/conductivity of the load/wash can be
controlled by
feedback and hence facilitate product quality control.
In certain embodiments, a combination of mixed mode and AEX and CEX methods
can be used to prepare the low AR compositions of the invention, including
certain
embodiments where one technology is used in a complementary/supplementary
manner with
another technology. In certain embodiments, such a combination can be
performed such that
certain sub-species are removed predominantly by one technology, such that the
combination
provides the desired final composition/product quality. In certain
embodiments, such
combinations include the use of additional intervening chromatography,
filtration, pH
adjustment, UF/DF steps so as to achieve the desired product quality, AR, ion
concentration,
and/or viral reduction.
As described below and in Example 11, MM chromatography can be used in
conjunction with recycle chromatography and continuous chromatography modes.
Continuous and Recycle Chromatography
Continuous and recycle chromatography modes can be used to produce the low AR
compositions of the invention, and are described below. These methods result
in significant
improvements in recovery of the protein, e.g., antibody. of interest while
maintaininE., the AR
reduction levels. These continuous and recycle chromatography modes are
applicable to
chromatography methods where (a) the low acidic species component of interest
is collected
89
CA 02926384 2016-04-07
in the unbound fraction during the chromatography (Flow Through/wash
chromatography) or
(b) where the low acidic species component of interest is first bound to the
media and
subsequently recovered by washing the media with conditions that elute the
bound
component.
Continuous and Recycle Chromatography -- Flow Through/Wash
Chromatography
In the case where the low acidic species component of interest is collected in
the
unbound fraction, the following approach is employed which prevents loss of
the material
loaded on the column.
In one embodiment, a recycle chromatography mode is used wherein the column is
loaded and the unbound fractions that results in the target AR level are
collected.
Subsequently, instead of regenerating the column and losing the product, the
column is
washed under conditions that result in recovery of the product remaining bound
to the
column. This product recovered under these conditions contains significantly
higher AR
levels than the original feed material. This wash fraction is adjusted to the
appropriate
conditions to achieve the separation desired on subsequent processing
(typically similar
conditions to the initial preparation) and combined with the original feed
material and loaded
on the column again (after preparing the column appropriately for the next
cycle). The
amount of material prepared for the next cycle, combining the wash fraction
from the first
cycle and the fresh material is adjusted to the target loading capacity for
the column to
achieve the desired separation (typically similar to the capacity targeted for
the first cycle).
In performing the second cycle, a similar strategy is employed, collecting the
unbound
fraction so as to achieve the target AR level and then subsequently washing
the column under
conditions to recover the product remaining on the column.
In one embodiment, this recycle chromatography mode is continued until all the
load
materials are used. The number of cycles can be controlled by designing the
column size
appropriately.
In employing the recycle chromatography mode, the recovery of the product
loaded
on the column is significantly improved while achieving the target AR levels.
Several variations of the recycle chromatography mode can be employed. In one
embodiment, the fractions that are collected targeting a certain AR level can
be deterniined
CA 02926384 2016-04-07
based on predetermined criteria or based on at-line, off-line or on-line
analysis of the effluent
of the column or the collected pool.
In another embodiment, the wash conditions used for the first cycle can be
adjusted to
recover the desired amount of product at the desired product quality, only
limited by the
feasibility of preparing an appropriate load mixture for the subsequent step.
In one aspect of
this embodiment, the wash condition may be similar to the load condition. In
another aspect
of this embodiment, the wash condition can be stringent to recover all of the
product species
(desired and undesired) remaining on the column.
In still another embodiment, the loading amount, the loading conditions and
the
washing conditions used for the subsequent cycles can be modified to achieve
the desired
purity, given that that loading material for the subsequent cycles are likely
to contain higher
levels of AR.
In another embodiment, the last cycle of the operation can be performed under
different conditions such that the target purity and target recovery can be
achieved to
optimize overall recovery and purity.
The methods for producing the low AR composition of the invention can also be
implemented in a continuous chromatography mode. In this mode, at least two
columns are
employed (refeiTed to as a -first" column and a "second" column). In one
embodiment, the
feed material is loaded onto the first column, and the unbound fraction from
the first column
is collected such that the pool material contains the target AR level. The
column is then
washed under conditions that recover the remaining product. This material is
then
dynamically diluted with appropriate solutions to achieve the desired loading
conditions,
mixed with fresh feed material and directed to the second column. The unbound
fraction
from the second column is collected to achieve the target AR level. The second
column is
then washed under conditions to recover the product and diluted with
appropriate solutions,
mixed with fresh materials dynamically and directed to the first column (which
is prepared to
receive the load after regeneration/cleaning). In one embodiment, this cycling
is continued
until all the load material is used. The last cycle can be operated in a
"typical" mode, with
appropriate adjustments to the load and wash conditions as necessary.
In certain embodiments this continuous chromatography mode can be caiTied out
such
that the wash material containing the higher AR levels can be directed back
into the load tank
after appropriate dilution. This material can then be loaded subsequently or
concurrently
91
CA 02926384 2016-04-07
onto the second column, such that the operation of the two columns are not in
tandem,
reducing complexity of the operation.
This continuous chromatography mode, while similar to the recycle
chromatography
mode, can be carried out more efficiently, and therefore has a reduced
processing time.
For this continuous chromatography mode, several variations can be employed.
In one
embodiment, the fractions that are collected targeting a certain AR level can
be determined
based on predetermined criteria or based on at-line, off-line or on-line
analysis of the effluent
of the column or the collected pool.
In another embodiment, the wash conditions used for the first cycle can be
adjusted to
recover the desired amount of product at the desired product quality, only
limited by the
feasibility of preparing an appropriate load mixture for the subsequent step.
In one aspect of
this embodiment, the wash conditions may be similar to the load conditions. In
another
aspect of the embodiment, the wash conditions can be stringent to recover all
of the product
species (desired and undesired) remaining on the column.
In still another embodiment, the loading amount, the loading conditions and
the
washing conditions used for the subsequent cycles can be modified to achieve
the desired
purity, given that that loading material for the subsequent cycles are likely
to contain higher
levels of AR.
In another embodiment, the last cycle of the operation can be performed under
different conditions such that the target purity/recovery can be achieved to
optimize overall
recovery and and/or purity.
In one embodiment, the media choice for the recycle or continuous modes can be
one
of many chromatographic resins with pendant hydrophobic and anion exchange
functional
groups, monolithic media, membrane adsorbent media or depth filtration media.
In certain embodiments, membrane or depth filter based media ("convective
media")
can be used in the recycle or continuous chromatography modes because
selectivity of
separation is not required to be high given the fact that the less enriched
portions of the
product are "recycled" while the pure fractions are selectively pooled.
Continuous and Recycle Chromatography -- Elution Chromatography
In the elution mode of chromatography or separation, as exemplified by the CEX
technology for AR reduction, the conditions are chosen for the load and wash
steps such that
92
CA 02926384 2016-04-07
the AR enriched material is collected in the Flow Through and/or wash
fractions. while the
AR reduced material is collected in the elution fraction. In the typical
implementation of the
CEX technology, about 10 to 40% of the product (the desired charge variant)
may be lost in
the Flow Through/Wash fractions. Two
modes of operation, namely the recycle
chromatography mode and the continuous chromatography mode provide improved
recovery,
while maintaining the target AR levels.
In the recycle chromatography mode, the load material is, in general,
processed over
multiple cycles. In implementing the recycle chromatography mode, the load
material is
prepared such that the eluate contains the target product purity or AR level.
Under these
conditions, the AR enriched material is collected in the Flow Through/wash
fractions. This
material is pooled and additional fresh load material is added to achieve the
appropriate
loading capacity for the next cycle of chromatography on the same column. In
particular, in
one embodiment, the column is eluted under conditions where the bound product
(having low
AR levels) is recovered, and subsequently regenerated and equilibrated to
prepare for the next
cycle.
In the next cycle, the combined load (Flow Through/wash from cycle 1 above, as
well
as fresh material) is loaded to the target capacity. The Flow Through/wash
fractions are
collected and pooled. The column is eluted to obtain the second eluate, again
containing the
target low AR composition. In one embodiment, this sequence is continued until
all the load
materials are processed.
In another embodiment, by implementing the recycle chromatography mode, the
material that would otherwise be discarded as AR enriched material is further
purified to
-recover" pure protein product, thereby improving the overall recovery of the
protein. In one
embodiment, the level of recovery depends on the number of cycles employed.
For the recycle chromatography mode, several variations can be employed. In
one
embodiment, the entire pool of the Flow Through/wash fractions are typically
combined with
fresh materials to maximize recovery of the entire operation. However, a
portion of the flow
through wash can be discarded to achieve higher purity or efficiency. For
example. in one
embodiment, certain fractions containing very high levels of AR species can be
discarded.
To enable such selective pooling, off-line, in-line or at line methods can be
used to directly or
indirectly measure the levels of AR.
93
CA 02926384 2016-04-07
In another embodiment, the loading amount and the conditions for loading,
washing
and eluting can be modified for the second and subsequent cycles to
accommodate the higher
levels of AR that will be present in the loading pool.
In still another embodiment, the last cycle of the method can be performed
under
conditions such that the target purity and recovery can be achieved to
optimize overall
recovery and purity.
A continuous chromatography mode provides additional advantages in terms of
time
efficiency. In this mode of operation, two or more columns are used.
Specifically, as with
the recycle mode, an appropriate condition for the load capacity, load, wash
and elution
conditions are chosen for the operation. The Flow Through and wash fractions
(or a portion
thereof) is directed to the load tank containing the fresh material. After
completion of the
load and wash steps, the first column is eluted and subsequently regenerated.
Meanwhile, the
second column is loaded with the material that is a mix of fresh material and
the wash and
Flow Through from the previous cycle. The wash and Flow Through from the
second
column is again directed back to the load tank. The second column is then
eluted and
regenerated. The first column is then ready to be loaded and the cycle
continues. This
continuous chromatography mode is efficient as the product is processed
continuously and
the purified product is obtained in a semi-continuous manner.
Several variations of the continuous chromatography mode can be employed. In
one
embodiment, the entire pool of the Flow Through/wash fractions is combined
with fresh
materials to maximize recovery of the entire operation. However, a portion of
the Flow
Through wash can be discarded to achieve higher purity or efficiency. For
example, certain
fractions containing very high levels of AR species can be discarded. To
enable such
selective pooling, off-line, in-line or at line methods can be used to measure
directly or
indirectly the levels of acidic species.
In another embodiment, the loading amount. conditions for loading, washing and
eluting can be modified for the second and subsequent cycles to accommodate
the higher
levels of AR that will be present in the loading pool.
In still another embodiment, the last cycle of the operation can be perforrned
under
different conditions to optimize overall recover and purity.
The recycle chromatography mode and the continuous chromatography mode are not
limited to use with any particular chromatography resin. The media used for
the recycle or
94
CA 02926384 2016-04-07
continuous modes can be one of many chromatographic resins with pendant
hydrophobic and
anion exchange functional groups, monolithic media, membrane adsorber media or
depth
filtration media.
In certain embodiments, membrane depth filter-based media ("convective media")
can
be used with the recycle or continuous modes as the selectivity of separation
is not required
to be high given the fact that the less enriched portions of the product are
"recycled" while
the pure fractions are selectively pooled.
Recycle chromatography mode and the continuous chromatography mode can be used
inconjunction with AEX, CEX, or MM chromatography methods, as described
herein, to
produce the low AR compositions of the invention. For example, Example 1 1,
below,
describes the recycle mode of chromatography for AR reduction using AEX, CEX,
and MM
technologies.
Hydrophobic Interaction Chromatography
The low AR compositions of the invention may also be prepared using a
hydrophobic
interaction chromatography (HIC) step in addition to the displacement
chromatography step.
In performing the separation, the sample mixture is contacted with the HIC
material,
e.g., using a batch purification technique or using a column or membrane
chromatography.
Prior to HIC purification it may be desirable to adjust the concentration of
the salt bufTer to
achieve desired protein binding to the resin or the membrane.
Whereas ion exchange chromatography relies on the local charge of the protein
of
interest for selective separation, hydrophobic interaction chronnatography
employs the
hydrophobic properties of the proteins to achieve selective separation.
Hydrophobic groups
on the protein interact with hydrophobic groups of the resin or the membrane.
The more
hydrophobic a protein is the stronger it will interact with the column or the
membrane. Thus
the H1C step removes process-related impurities (e.g., HCPs) as well as
product-related
substances (e.g., aggregates and fragments).
Like ion exchange chromatography, a HIC column or membrane device can also be
operated
in product a bind-elute mode, a flow-through, or a hybrid mode wherein the
product exhibits
binding to the chromatographic material, yet can be washed from the column
using a buffer
that is the same or substantially similar to the loading buffer. The details
of these modes are
outlined above in connection with AEX purification.
CA 02926384 2016-04-07
As hydrophobic interactions are strongest at high ionic strength, this form of
separation is
conveniently performed following salt elution step, such as those that are
typically used in
connection with ion exchange chromatography. Alternatively, salts can be added
into a low
salt level feed stream before this step. Adsorption of the antibody to a HIC
column is favored
by high salt concentrations, but the actual concentrations can vary over a
wide range
depending on the nature of the protein of interest, salt type and the
particular HIC ligand
chosen. Various ions can be arranged in a so-called soluphobic series
depending on whether
they promote hydrophobic interactions (salting-out effects) or disrupt the
structure of water
(chaotropic effect) and lead to the weakening of the hydrophobic interaction.
Cations are
ranked in terms of increasing salting out effect as Ba2+; Ca2+; Mg2+; Li+ ;
Cs+ ; Na+ K+;
Rb+ ; NH4+, while anions may be ranked in terms of increasing chaotropic
effect as P043-;
S042-; CH3CO3- ; Cl- ; Br- ; NO3- ; C104- ; I- ; SCN-.
In general, Na+, K+ or NH4+ sulfates effectively promote ligand-protein
interaction
in HIC. Salts may be formulated that influence the strength of the interaction
as given by the
following relationship: (NH4)2SO4 > Na2SO4 > NaC1 > NH4C I > NaBr > NaSCN. In
general, salt concentrations of between about 0.75 M and about 2 M ammonium
sulfate or
between about 1 and 4 M NaC1 are useful.
HIC media normally comprise a base matrix (e.g., cross-linked agarose or
synthetic
copolymer material) to which hydrophobic ligands (e.g., alkyl or aryl groups)
are coupled. A
suitable HIC media comprises an agarose resin or a membrane functionalized
with phenyl
groups (e.g.. a Phenyl Sepharose" from GE Healthcare or a Phenyl Membrane from
Sartorius). Many HIC resins are available commercially. Examples include. but
are not
limited to, Capto Phenyl, Phenyl Sepharosem 6 Fast Flow with low or high
substitution.
Phenyl Sepharosem High Peiformance, Octyl SepharoseTM High Performance (GE
Healthcare); Fractogel EMD Propyl or Fractogel" EMD Phenyl (E. Merck.
Germany);
Macro-Prep' m Methyl or Macro-Prep" t-Butyl columns (Bio-Rad. California): WP
HI-
Propyl (C3)'" (J. T. Baker, New Jersey); and Toyopearl" ether, phenyl or butyl
(TosoHaas,
PA).
Viral Filtration
Viral filtration is a dedicated viral reduction step in the entire
purification process.
This step is usually performed post chromatographic polishing steps. Viral
reduction can be
96
CA 02926384 2016-04-07
achieved via the use of suitable filters including, but not limited to,
Planova 20NTm, 50 N or
BioEx from Asahi Kasei Pharma, ViresolveTM filters from EMD Millipore,
ViroSart CPV
from Sartorius, or Ultipor DV20 or DV50Tm filter from Pall Corporation. It
will be apparent
to one of ordinary skill in the art to select a suitable filter to obtain
desired filtration
performance.
Ultrafiltration/Diafiltration
Certain embodiments of the present invention employ ultrafiltration and
diafiltration
steps to further concentrate and formulate the protein of interest, e.g., an
antibody product.
Ultrafiltration is described in detail in: Microfiltration and
Ultrafiltration: Principles and
Applications, L. Zeman and A. Zydney (Marcel Dekker, Inc., New York, N.Y.,
1996); and in:
Ultrafiltration Handbook, Munir Cheryan (Technomic Publishing, 1986; ISBN No.
87762-
456-9). One filtration process is Tangential Flow Filtration as described in
the Millipore
catalogue entitled "Pharmaceutical Process Filtration Catalogue" pp. 177-202
(Bedford,
Mass., 1995/96). Ultrafiltration is generally considered to mean filtration
using filters with a
pore size of smaller than 0.1 1.tm. By employing filters having such small
pore size, the
volume of the sample can be reduced through permeation of the sample buffer
through the
filter membrane pores while proteins, such as antibodies, are retained above
the membrane
surface.
Diafiltration is a method of using membrane filters to remove and exchange
salts,
sugars, and non-aqueous solvents, to separate free from bound species, to
remove low
molecular-weight species, and/or to cause the rapid change of ionic and/or pH
environments.
Microsolutes are removed most efficiently by adding solvent to the solution
being diafiltered
at a rate approximately equal to the permeate flow rate. This washes away
microspecies from
the solution at a constant volume, effectively purifying the retained protein
of interest. In
certain embodiments of the present invention, a diafiltration step is employed
to exchange the
various buffers used in connection with the instant invention, optionally
prior to further
chromatography or other purification steps, as well as to remove impurities
from the protein
preparations.
One of ordinary skill in the art can select appropriate membrane filter device
for the
UF/DF operation. Examples of membrane cassettes suitable for the present
invention
include, but not limited to. Pellicon 2 or Pellicon 3 cassettes with 10 MD,
30kD or 50 kD
membranes from EMD Millipore, Kvick 10 kD, 30 kD or 50 kD membrane cassettes
from
97
CA 02926384 2016-04-07
GE Healthcare, and Centramate or Centrasette 10 kD, 30 kD or 50 kD cassettes
from Pall
Corporation.
Exemplary Purification Strategies
In certain embodiments, primary recovery can proceed by sequentially employing
pH
reduction, centrifugation, and filtration steps to remove cells and cell
debris (including HCPs)
from the production bioreactor harvest. In certain embodiments, the present
invention is
directed to subjecting a sample mixture from said primary recovery to one or
more AEX,
CEX, and/or MM purification steps. Certain embodiments of the present
invention will
include further purification steps. Examples of additional purification
procedures which can
be performed prior to, during, or following the ion exchange chromatography
method include
ethanol precipitation, isoelectric focusing, reverse phase HPLC,
chromatography on silica,
chromatography on heparin Sepharoseim, further anion exchange chromatography
and/or
further cation exchange chromatography, chromatofocusing, SDS-PAGE, ammonium
sulfate
precipitation, hydroxylapatite chromatography, gel electrophoresis, dialysis,
and affinity
chromatography (e.g., using protein G, an antibody, a specific substrate,
ligand or antigen as
the capture reagent).
Specific examples of such combinations of strategies is presented below, with
specific
data relating to particular combinations useful in the context of the instant
invention included
in Tables 80-87 and 76-78.
In certain embodiments the unbound Flow Through and wash fractions can be
further
fractionated and a combination of fractions providing a target product purity
can be pooled.
In certain embodiments the protein concentration can be adjusted to achieve a
differential partitioning behavior between the antibody product and the
product-related
substances such that the purity and/or yield can be further improved. In
certain embodiments
the loading can be performed at different protein concentrations during the
loading operation
to improve the product quality/yield of any particular purification step.
In certain embodiments the column temperature can be independently varied to
improve the separation efficiency and/or yield of any particular purification
step.
In certain embodiments, the loading and washing buffer matrices can be
different or
composed of mixtures of chemicals, while achieving similar "resin interaction"
behavior such
that the above novel separation can be effected. For example, but not by way
of limitation,
98
CA 02926384 2016-04-07
the loading and washing buffers can be different, in terms of ionic strength
or pH, while
remaining substantially similar in function in terms of the washout of the
product achieved
during the wash step. In certain embodiments, additives such as amino acids,
sugars, PEG,
etc can be added to the load or wash steps to modulate the partitioning
behavior to achieve
the separation efficiency and/or yield.
In certain embodiments, the loading & washing steps can be controlled by in-
line, at-
line or off-line measurement of the product related impurity/substance levels,
either in the
column effluent, or the collected pool or both, so as to achieve the target
product quality
and/or yield. In certain embodiments, the loading concentration can be
dynamically
controlled by in-line or batch or continuous dilutions with buffers or other
solutions to
achieve the partitioning necessary to improve the separation efficiency and/or
yield.
V. Methods of Assaying Sample Purity
Assaying Host Cell Protein
The present invention also provides methods for determining the residual
levels of
host cell protein (HCP) concentration in the low AR compositions of the
invention. As
described above, HCPs are desirably excluded from the final target substance
product.
Exemplary HCPs include proteins originating from the source of the antibody
production.
Failure to identify and sufficiently remove HCPs from the target antibody may
lead to
reduced efficacy and/or adverse reactions in a subject.
As used herein, the term "HCP ELISA" refers to an ELISA where the second
antibody used in the assay is specific to the HCPs produced from cells, e.g.,
CHO cells, used
to generate the antibody of interest. The second antibody may be produced
according to
conventional methods known to those of skill in the art. For example, the
second antibody
may be produced using HCPs obtained by sham production and purification runs,
i.e., the
same cell line used to produce the antibody of interest is used, but the cell
line is not
transfected with antibody DNA. In an exemplary embodiment, the second antibody
is
produced using HCPs similar to those expressed in the cell expression system
of choice. i.e.,
the cell expression system used to produce the target antibody.
Generally, HCP ELISA comprises sandwiching a liquid sample comprising HCPs
between two layers of antibodies, i.e., a first antibody and a second
antibody. The sample is
99
CA 02926384 2016-04-07
incubated during which time the HCPs in the sample are captured by the first
antibody, for
example, but not limited to goat anti-CHO, affinity purified (Cygnus). A
labeled second
antibody, or blend of antibodies, specific to the HCPs produced from the cells
used to
generate the antibody, e.g., anti-CHO HCP Biotinylated, is added, and binds to
the HCPs
within the sample. In certain embodiments the first and second antibodies are
polyclonal
antibodies. In certain aspects the first and second antibodies are blends of
polyclonal
antibodies raised against HCPs. The amount of HCP contained in the sample is
determined
using the appropriate test based on the label of the second antibody.
HCP ELISA may be used for deten-nining the level of HCPs in an antibody
composition, such as an eluate or flow-through obtained using the process
described above.
The present invention also provides a composition comprising an antibody,
wherein the
composition has no detectable level of HCPs as determined by an HCP Enzyme
Linked
Immunosorbent Assay ("ELISA").
Assaying Acidic Species (AR)
The levels of acidic species in the chromatographic samples produced using the
techniques described herein may be analyzed as described in the Examples
section. In certain
embodiments a CEX-HPLC method is employed. For example, but not by way of
limitation,
cation exchange chromatography can be performed on a Dionex ProPac WCX-10,
Analytical
column 4 mm x 250 mm (Dionex, CA). An Agilent 1200 HPLC system can then be
used as
the HPLC. In certain embodiments, mobile phases such as 10mM Sodium Phosphate
dibasic
pH 7.5 (Mobile phase A) and 10mM Sodium Phosphate dibasic, 500 mM Sodium
Chloride
pH 5.5 (Mobile phase B) can be used. In certain embodiments, a binary gradient
(94% A, 6%
B: 0-20 min; 84% A, 16% B: 20-22 min; 0% A. 100%B: 22-28 min; 94% A, 6% B: 28-
34
min) can be used with detection at 280 nm. In certain embodiments,
quantitation is based on
the relative area percent of detected peaks. In certain embodiments, the peaks
that elute at
relative residence time less than a certain time are together represented as
the acidic peaks.
Assaying Size Variants
In certain embodiments, the levels of aggregates, monomer, and fragments in
the
chromatographic samples produced using the techniques described herein are
analyzed. In
certain embodiments, the aggregates, monomer, and fragments are measured using
a size
100
CA 02926384 2016-04-07
exclusion chromatographic (SEC) method for each molecule. For example, but not
by way of
limitation, a TSK-gel G3000SWxL, 5 um, 125 A, 7.8 X 300 mm column (Tosoh
Bioscience)
can be used in connection with certain embodiments, while a TSK-gel Super
SW3000, 4 jun,
250 A, 4.6 X 300 mm column (Tosoh Bioscience) can be used in alternative
embodiments.
In certain embodiments, the aforementioned columns are used along with an
Agilent or a
Shimazhu HPLC system. In certain embodiments, sample injections are made under
isocratic
elution conditions using a mobile phase consisting of, for example, 100 mM
sodium sulfate
and 100 mM sodium phosphate at pH 6.8, and detected with UV absorbance at 214
nm. In
certain embodiments, the mobile phase will consist of 1X PBS at pH 7.4, and
elution profile
detected with UV absorbance at 280 nm. In certain embodiments, quantification
is based on
the relative area of detected peaks.
Any additional technique, such as mass spectroscopy, can be used for assaying
size
variants.
vI. Methods of Treatment Using the Low AR Compositions of the Invention
The low AR compositions of the invention may be used to treat any disorder in
a
subject for which the therapeutic protein comprised in the composition is
appropriate for
treating.
A "disorder" is any condition that would benefit from treatment with the
protein. This
includes chronic and acute disorders or diseases including those pathological
conditions
which predispose the subject to the disorder in question. In the case of an
anti-TNFa
antibody, or antigen binding portion thereof, such as adalimumab, a
therapeutically effective
amount of the low AR composition may be administered to treat a disorder in
which TNFa
activity is detrimental.
A disorder in which TNFa activity is detrimental includes a disorder in which
inhibition of TNFa activity is expected to alleviate the symptoms and/or
progression of the
disorder. Such disorders may be evidenced, for example, by an increase in the
concentration
of TNFa in a biological fluid of a subject suffering from the disorder (e.g.,
an increase in the
concentration of TNFa in serum, plasma, synovial fluid, etc. of the subject),
which can be
detected, for example, using an anti-TNFa antibody.
TNFa has been implicated in the pathophysiology of a wide variety of a TNFa-
related
disorders including sepsis, infections, autoimmune diseases, transplant
rejection and graft-
versus-host disease (see e.g., Moeller, A.. ei al. (1990) Ovakine 2:162-169;
U.S. Patent No.
101
CA 02926384 2016-04-07
5,231,024 to Moeller et al.; European Patent Publication No. 260 610 B1 by
Moeller, A., et
at.Vasilli, P. (1992) A111114. Rev. Immunol. 10:411-452; Tracey, K.J. and
Cerami, A. (1994)
Amur. Rev. Med. 45:491-503). Accordingly, the low AR compositions or a low
process-
related impurity compositions of the invention may be used to treat an
autoimmune disease,
such as rheumatoid arthritis, juvenile idiopathic arthritis, or psoriatic
arthritis, an intestinal
disorder, such as Crohn's disease or ulcerative colitis, a
spondyloarthropathy, such as
ankylosing spondylitis, or a skin disorder, such as psoriasis.
Disorders in which TNFo. activity is detrimental are well known in the art and
described in detail in U.S. Patent No. 8,231,876 and U.S. Patent No.
6,090,382.
In one
embodiment, "a disorder in which TNFt activity is detrimental" includes sepsis
(including
septic shock, endotoxic shock, grain negative sepsis and toxic shock
syndrome), autoimmune
diseases (including rheumatoid arthritis, rheumatoid spondylitis,
osteoarthritis and gouty
arthritis, allergy, multiple sclerosis, autoimmune diabetes, autoimmune
uveitis, nephrotic
syndrome, multisystem autoimmune diseases, lupus (including systemic lupus,
lupus
nephiitis and lupus cerebritis), Crohn's disease and autoimmune hearing loss),
active axial
spondyloarthritis (active axSpA) and non-radiographic axial spondyloarthtitis
(nr-axSpA),
infectious diseases (including malaria, meningitis, acquired immune deficiency
syndrome
(AIDS), influenza and cachexia secondary to infection), allograft rejection
and graft versus
host disease, malignancy, pulmonary disorders (including adult respiratory
distress syndrome
(ARDS), shock lung, chronic pulmonary inflan-unatory disease, pulmonary
sarcoidosis,
pulmonary fibrosis, silicosis, idiopathic interstitial lung disease and
chronic obstructive
airway disorders (COPD), such as asthma), intestinal disorders (including
inflammatory
bowel disorders, idiopathic inflammatory bowel disease, Crohn's disease and
Crohn's
disease-related disorders (including fistulas in the bladder, vagina, and
skin; bowel
obstructions; abscesses; nutritional deficiencies; complications from
corticosteroid use;
inflammation of the joints; erythem nodosurn; pyodertna gangrenosum; lesions
of the eye,
Crohn's related arthralgias, fistulizing Crohn's indeteiminant colitis and
pouchitis), cardiac
disorders (including ischemia of the heart, heart insufficiency, restenosis,
congestive heart
failure, coronary artery disease, angina pectoris, myocardial infarction,
cardiovascular tissue
damage caused by cardiac arrest, cardiovascular tissue damage caused by
cardiac bypass,
cardiogenic shock, and hypertension, atherosclerosis, cardiomyopathy, coronary
artery
spasm, coronary artery disease, valvular disease, arrhythmias, and
cardiomyopathies),
102
CA 02926384 2016-04-07
spondyloarthropathies (including ankylosing spondylitis, psoriatic
arthritis/spondylitis,
enteropathic arthritis, reactive arthritis or Reiter's syndrome, and
undifferentiated
spondyloarthropathies), metabolic disorders (including obesity and diabetes,
including type I
diabetes mellitus, type 2 diabetes mellitus, diabetic neuropathy, peripheral
neuropathy,
diabetic retinopathy, diabetic ulcerations, retinopathy ulcerations and
diabetic
macrovasculopathy), anemia, pain (including acute and chronic pains, such as
neuropathic
pain and post-operative pain, chronic lower back pain, cluster headaches,
herpes neuralgia,
phantom limb pain, central pain, dental pain, opioid-resistant pain, visceral
pain, surgical
pain, bone injury pain, pain during labor and delivery, pain resulting from
burns, including
sunburn, post partum pain, migraine, angina pain, and genitourinary tract-
related pain
including cystitis), hepatic disorders (including hepatitis, alcoholic
hepatitis, viral hepatitis,
alcoholic cin-hosis, al antitypsin deficiency, autoimmune cirrhosis,
cryptogenic cirrhosis,
fulminant hepatitis, hepatitis B and C, and steatohepatitis, cystic fibrosis,
primary biliary
cin-hosis, sclerosing cholangitis and biliary obstruction), skin and nail
disorders (including
psoriasis (including chronic plaque psoriasis, guttate psoriasis, inverse
psoriasis, pustular
psoriasis and other psoriasis disorders), pemphigus vulgaris, scleroderma,
atopic dermatitis
(eczema), sarcoidosis, erythema nodosum, hidradenitis suppurative, lichen
planus, Sweet's
syndrome, sclerodenna and vitiligo), vasculitides (including Behcet's
disease), and other
disorders, such as juvenile rheumatoid arthritis (jRA), endometriosis,
prostatitis, choroidal
neovascularization, sciatica, Sjogren's syndrome, uveitis, wet macular
degeneration,
osteoporosis and osteoarthritis.
As used herein, the term "subject" is intended to include living organisms,
e.g.,
prokaryotes and eukaryotes. Examples of subjects include mammals, e.g.,
humans, dogs,
cows, horses, pigs, sheep, goats, cats, mice, rabbits, rats, and transgenic
non-human animals.
In specific embodiments of the invention, the subject is a human.
As used herein, the terrn "treatment" or "treat" refers to both therapeutic
treatment and
prophylactic or preventative measures. Those in need of treatment include
those already with
the disorder, as well as those in which the disorder is to be prevented.
In one embodiment, the invention provides a method of administering a low AR
composition comprising an anti-TNFu antibody, or antigen binding portion
thereof, to a
subject such that TNFu activity is inhibited or a disorder in which TNFu,
activity is
detrimental is treated. In one embodiment, the TNFR is human TNFa and the
subject is a
1 03
CA 02926384 2016-04-07
human subject. In one embodiment, the anti-TNFa antibody is adalimumab, also
referred to
as HUMIRA .
The low AR compositions can be administered by a variety of methods known in
the
art. Exemplary routes/modes of administration include subcutaneous injection,
intravenous
injection or infusion. In certain aspects, a low AR compositions may be orally
administered.
As will be appreciated by the skilled artisan, the route and/or mode of
administration will
vary depending upon the desired results.
Dosage regimens may be adjusted to provide the optimum desired response (e.g.,
a
therapeutic or prophylactic response). For example, a single bolus may be
administered,
several divided doses may be administered over time or the dose may be
proportionally
reduced or increased as indicated by the exigencies of the therapeutic
situation. In certain
embodiments it is especially advantageous to formulate parenteral compositions
in dosage
unit forrn for ease of administration and uniformity of dosage. Dosage unit
form as used
herein refers to physically discrete units suited as unitary dosages for the
mammalian subjects
to be treated; each unit comprising a predetemined quantity of active compound
calculated
to produce the desired therapeutic effect in association with the required
pharmaceutical
carrier. The specification for the dosage unit forms of the invention are
dictated by and
directly dependent on (a) the unique characteristics of the active compound
and the particular
therapeutic or prophylactic effect to be achieved, and (b) the limitations
inherent in the art of
compounding such an active compound for the treatment of sensitivity in
individuals.
An exemplary, non-limiting range for a therapeutically or prophylactically
effective
amount of a low AR composition of the invention is 0.01-20 mg/kg, or 1-10
mg/kg, or 0.3-1
mg/kg. With respect to low AR compositions comprising an anti-TNFa antibody,
or antigen-
binding portion thereof, such as adalimumab, an exemplary dose is 40 mg every
other week.
In some embodiments, in particular for treatment of ulcerative colitis or
Crohn's disease, an
exemplary dose includes an initial dose (Day 1) of 160 mg (e.g., four 40 mg
injections in one
day or two 40 mg injections per day for two consecutive days), a second dose
two weeks later
of 80 mg, and a maintenance dose of 40 mg every other week beginning two weeks
later.
Alternatively, for psoriasis for example, a dosage can include an 80 mg
initial dose followed
by 40 mg every other week starting one week after the initial dose.
It is to be noted that dosage values may vary with the type and severity of
the
condition to be alleviated. It is to be further understood that for any
particular subject,
104
CA 02926384 2016-04-07
specific dosage regimens should be adjusted over time according to the
individual need and
the professional judgment of the person administering or supervising the
administration of the
compositions, and that dosage ranges set forth herein are exemplary only and
are not intended
to limit the scope or practice of the claimed composition.
VII. Pharmaceutical Formulations Containing the Low AR Compositions of the
Invention
The present invention further provides preparations and formulations
comprising the
low AR compositions of the invention. It should be understood that any of the
antibodies and
antibody fragments described herein, including antibodies and antibody
fragments having any
one or more of the structural and functional features described in detail
throughout the
application, may be formulated or prepared as described below. When various
formulations
are described in this section as including an antibody, it is understood that
such an antibody
may be an antibody or an antibody fragment having any one or more of the
characteristics of
the antibodies and antibody fragments described herein. In one embodiment, the
antibody is
an anti-TNFu antibody, or antigen-binding portion thereof.
In certain embodiments, the low AR compositions of the invention may be
formulated
with a pharmaceutically acceptable carrier as pharmaceutical (therapeutic)
compositions, and
may be administered by a variety of methods known in the art. As will be
appreciated by the
skilled artisan, the route and/or mode of administration will vary depending
upon the desired
results. The term "pharmaceutically acceptable carrier" means one or more non-
toxic
materials that do not interfere with the effectiveness of the biological
activity of the active
ingredients. Such preparations may routinely contain salts, buffering agents,
preservatives,
compatible carriers, and optionally other therapeutic agents. Such
pharmaceutically
acceptable preparations may also routinely contain compatible solid or liquid
fillers, diluents
or encapsulating substances which are suitable for administration into a
human. The terrn
"carrier" denotes an organic or inorganic ingredient, natural or synthetic,
with which the
active ingredient is combined to facilitate the application. The
components of the
pharmaceutical compositions also are capable of being co-mingled with the
antibodies of the
present invention, and with each other, in a manner such that there is no
interaction which
would substantially impair the desired pharmaceutical efficacy.
The low AR compositions of the invention are present in a form known in the
art and
acceptable for therapeutic uses. In one
embodiment, a formulation of the low AR
105
CA 02926384 2016-04-07
compositions of the invention is a liquid formulation. In another embodiment,
a formulation
of the low AR compositions of the invention is a lyophilized formulation. In a
further
embodiment, a formulation of the low AR compositions of the invention is a
reconstituted
liquid formulation. In one embodiment, a formulation of the low AR
compositions of the
invention is a stable liquid formulation. In one embodiment, a liquid
formulation of the low
AR compositions of the invention is an aqueous formulation. hi another
embodiment, the
liquid formulation is non-aqueous. In a specific embodiment, a liquid
formulation of the low
AR compositions of the invention is an aqueous formulation wherein the aqueous
can-ier is
distilled water.
The formulations of the low AR compositions of the invention comprise an
antibody
in a concentration resulting in a w/v appropriate for a desired dose. The
antibody may be
present in the fon-nulation at a concentration of about img/m1 to about
500mg/ml, e.g., at a
concentration of at least 1 mg/ml, at least 5 ing/rnl, at least 10 mg/ml, at
least 15 mg/ml, at
least 20 mg/ml, at least 25 mg/ml, at least 30 mg/ml, at least 35 mg/ml, at
least 40 mg/ml, at
least 45 mg/ml, at least 50 mg/ml, at least 55 mg/ml, at least 60 mg/ml, at
least 65 mg/ml, at
least 70 mg/ml, at least 75 mg/ml, at least 80 mg/ml, at least 85 mg/ml, at
least 90 mg/ml, at
least 95 mg/ml, at least 100 mg/ml, at least 105 mg/ml, at least 110 mg/ml, at
least 115
mg/ml, at least 120 mg/ml, at least 125 mg/ml, at least 130 mg/ml, at least
135 mg/ml, at least
140 mg/ml, at least 150 mg/ml, at least 200 mg/ml, at least 250 mg/ml, or at
least 300 mg/ml.
In a specific embodiment, a formulation of the low AR compositions of the
invention
comprises at least about 100 ing/ml, at least about 125 mg/ml, at least 130
mg/ml, or at least
about 150 mg/ml of an antibody of the invention.
In one embodiment, the concentration of antibody, which is included in the
formulation of the invention, is between about 1 mg/ml and about 25 mg/ml,
between about 1
mg/m1 and about 200 in2/ml. between about 25 rng/ml and about 200 mg/ml,
between about
50 mg/ml and about 200 ing/ml, between about 75 mg/ml and about 200 mg/ml,
between
about 100 mg/ml and about 200 mg/ml, between about 125 mg/ml and about 200
mg/ml,
between about 150 mg/ml and about 200 mg/ml, between about 25 mg/ml and about
150
mg/ml, between about 50 mg/ml and about 150 mg/ml, between about 75 mg/ml and
about
150 mg/ml, between about 100 mg/ml and about 150 mg/ml, between about 125
mg/m1 and
about 150 mg/ml, between about 25 mg/ml and about 125 mg/ml, between about 50
mg/ml
and about 125 mg/ml. between about 75 mg/ml and about 125 mg/ml, between about
100
mg/ml and about 125 mg/ml, between about 25 mg/ml and about 100 mg/ml, between
about
106
CA 02926384 2016-04-07
50 mg/ml and about 100 mg/ml, between about 75 mg/ml and about 100 mg/rird,
between
about 25 mg/ml and about 75 mg/ml, between about 50 mg/ml and about 75 mg/ml,
or
between about 25 mg/ml and about 50 mg/mi.
In a specific embodiment, a formulation of the low AR compositions of the
invention
comprises between about 90 mg/ml and about 110 mg/ml or between about 100
mg/ml and
about 210 mg/ml of an antibody.
The formulations of the low AR compositions of the invention comprising an
antibody may further comprise one or more active compounds as necessary for
the particular
indication being treated, typically those with complementary activities that
do not adversely
affect each other. Such additional active compounds are suitably present in
combination in
amounts that are effective for the purpose intended.
The formulations of the low AR compositions of the invention may be prepared
for
storage by mixing the antibody having the desired degree of purity with
optional
physiologically acceptable carriers, excipients or stabilizers, including, but
not limited to
buffering agents, saccharides, salts, surfactants, solubilizers, polyols,
diluents, binders,
stabilizers, salts, lipophilic solutions, amino acids, chelators,
preservatives, or the like
(Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12th edition,
L.
Brunton, et al. and Remington 's Pharmaceutical Sciences, 16th edition, Osol,
A. Ed. (1999)),
in the form of lyophilized formulations or aqueous solutions at a desired
final concentration.
Acceptable carriers, excipients, or stabilizers are nontoxic to recipients at
the dosages and
concentrations employed, and include buffers such as histidine, phosphate,
citrate, glycine,
acetate and other organic acids; antioxidants including ascorbic acid and
methionine;
preservatives (such as octadecyldimethylbenzyl ammonium chloride;
hexamethonium
chloride; benzalkonium chloride, benzethonium chloride; phenol, butyl or
benzyl alcohol;
alkyl parabens such as methyl or propyl paraben; catechol; resorcinol;
cyclohexanol;
3-pentanol; and m-cresol); low molecular weight (less than about 10 residues)
polypeptide;
proteins, such as serum albumin, gelatin, or immunoglobulins; hydrophilic
polymers such as
polyvinylpyrolidone; amino acids such as glycine, glutamine, asparagine,
histidine, arginine,
or lysine; monosaccharides, disaccharides, and other carbohydrates including
trehalose,
glucose. mannose, or dextrins; chelating agents such as EDTA; sugars such as
sucrose,
mannitol. trehalose or sorbitol; salt-forming counter-ions such as sodium;
metal complexes
Zn-protein complexes); and/or non-ionic surfactants such as TWEEN, polysorbate
80.
PLURONICSIN' or polyethylene glycol (PEG).
107
CA 02926384 2016-04-07
The buffering agent may be histidine, citrate, phosphate, glycine, or acetate.
The
saccharide excipient may be trehalose, sucrose, mannitol, maltose or
raffinose. The
surfactant may be polysorbate 20, polysorbate 40, polysorbate 80. or Pluronic
F68. The salt
may be NaC1, KC1, MgC1), or CaCI,
The formulations of the low AR compositions of the invention may include a
buffering or pH adjusting agent to provide improved pH control. A formulation
of the
invention may have a pH of between about 3.0 and about 9.0, between about 4.0
and about
8.0, between about 5.0 and about 8.0, between about 5.0 and about 7.0, between
about 5.0
and about 6.5, between about 5.5 and about 8.0, between about 5.5 and about
7.0, or between
about 5.5 and about 6.5. In a further embodiment, a formulation of the
invention has a pH of
about 3.0, about 3.5, about 4.0, about 4.5, about 5.0, about 5.1, about 5.2,
about 5.3, about
5.4, about 5.5, about 5.6, about 5.7, about 5.8, about 5.9, about 6.0, about
6.1, about 6.2,
about 6.3, about 6.4, about 6.5, about 6.6, about 6.7, about 6.8, about 6.9,
about 7.0, about
7.5, about 8.0, about 8.5, or about 9Ø In a specific embodiment, a
formulation of the
invention has a pH of about 6Ø One of skill in the art understands that the
pH of a
formulation generally should not be equal to the isoelectric point of the
particular antibody to
be used in the formulation.
Typically, the buffering agent is a salt prepared from an organic or inorganic
acid or
base. Representative buffering agents include, but are not limited to, organic
acid salts such
as salts of citric acid, ascorbic acid, gluconic acid, carbonic acid, tartaric
acid, succinic acid,
acetic acid, or phthalic acid; Tris, tromethamine hydrochloride, or phosphate
buffers. In
addition, amino acid components can also function in a buffering capacity.
Representative
amino acid components which may be utilized in the formulations of the
invention as
buffering agents include, but are not limited to, glycine and histidine. In
certain
embodiments. the buffering agent is chosen from histidine, citrate, phosphate,
glycine. and
acetate. In a specific embodiment, the buffering agent is histidine. In
another specific
embodiment, the buffering agent is citrate. In yet another specific
embodiment, the buffering
agent is glycine. The purity of the buffering agent should be at least 98%, or
at least 99%, or
at least 99.5%. As used herein, the term "purity" in the context of hi stidine
and glycine refers
to chemical purity of histidine or glycine as understood in the art, e.g., as
described in The
Merck Index. 131h ed.. O'Neil et al. ed. (Merck & Co.. 2001).
Buffering agents are typically used at concentrations between about 1 mM and
about
200 mM or any range or value therein. depending on the desired ionic strength
and the
108
CA 02926384 2016-04-07
buffering capacity required. The usual concentrations of conventional
buffering agents
employed in parenteral formulations can be found in: Pharmaceutical Dosage
Form:
Parenteral Medications, Volume 1, 2nd Edition, Chapter 5, p. 194, De Luca and
Boylan,
"Fon-nulation of Srnall Volume Parenterals", Table 5: Commonly used additives
in Parenteral
Products. In one embodiment, the buffering agent is at a concentration of
about 1 mM, or of
about 5 mM, or of about 10 mM, or of about 15 mM, or of about 20 mM, or of
about 25 mM,
or of about 30 mM, or of about 35 mM, or of about 40 mM, or of about 45 mM, or
of about
50 mM, or of about 60 mM, or of about 70 mM, or of about 80 mM, or of about 90
mM, or of
about 100 mM. In one embodiment, the buffering agent is at a concentration of
1 mM, or of
mM, or of 10 mM, or of 15 mM, or of 20 mM, or of 25 mM, or of 30 mM, or of 35
mM, or
of 40 mM, or of 45 mM, or of 50 mM, or of 60 mM, or of 70 mM, or of 80 mM, or
of 90
mM, or of 100 mM. In a specific embodiment, the buffering agent is at a
concentration of
between about 5 mM and about 50 mM. In another specific embodiment, the
buffering agent
is at a concentration of between 5 mM and 20 mM.
In certain embodiments, the formulation of the low AR compositions of the
invention
comprises histidine as a buffering agent. In one embodiment the histidine is
present in the
formulation of the invention at a concentration of at least about 1 mM, at
least about 5 mM, at
least about 10 mM, at least about 20 mM, at least about 30 mM, at least about
40 mM, at least
about 50 mM, at least about 75 mM, at least about 100 mM, at least about 150
mM, or at least
about 200 mM histidine. In another embodiment, a formulation of the invention
comprises
between about 1 mM and about 200 mM, between about 1 mM and about 150 mM,
between
about 1 mM and about 100 mM, between about 1 mM and about 75 mM, between about
10
mM and about 200 mM, between about 10 mM and about 150 mM. between about 10 mM
and about 100 mM, between about 10 mM and about 75 mM, between about 10 mM and
about 50 mM. between about 10 mM and about 40 mM, between about 10 mM and
about 30
mM. between about 20 mM and about 75 mM, between about 20 mM and about 50 mM,
between about 20 mM and about 40 mM, Of between about 20 mM and about 30 mM
histidine. In a further embodiment, the formulation comprises about 1 mM,
about 5 mM,
about 10 mM. about 20 mM, about 25 mM, about 30 mM, about 35 mM, about 40 mM,
about
45 mM. about 50 mM, about 60 mM, about 70 mM, about 80 mM, about 90 mM, about
100
mM, about 150 mM. or about 200 mM histidine. In a specific embodiment. a
formulation
may comprise about 10 mM, about 25 mM, or no histidine.
109
CA 02926384 2016-04-07
The formulations of the low AR compositions of the invention may comprise a
carbohydrate excipient. Carbohydrate excipients can act, e.g., as viscosity
enhancing agents,
stabilizers. bulking agents, solubilizing agents, and/or the like.
Carbohydrate excipients are
generally present at between about 1% to about 99% by weight or volume, e.g.,
between
about 0.1% to about 20%, between about 0.1% to about 15%, between about 0.1%
to about
5%õ between about 1% to about 20%, between about 5% to about 15%. between
about 8%
to about 10%, between about 10% and about 15%, between about 15% and about
20%,
between 0.1% to 20%, between 5% to 15%, between 8% to 10%, between 10% and
15%,
between 15% and 20%, between about 0.1% to about 5%, between about 5% to about
10%,
or between about 15% to about 20%. In still other specific embodiments, the
carbohydrate
excipient is present at 1%, or at 1.5%, or at 2%, or at 2.5%, or at 3%, or at
4%, or at 5%, or at
10%, or at 15%, or at 20%.
Carbohydrate excipients suitable for use in the formulations of the invention
include,
but are not limited to, monosaccharides such as fructose, maltose, galactose,
glucose, D-
mannose, sorbose, and the like; disaccharides, such as lactose, sucrose,
trehalose, cellobiose,
and the like; polysaccharides, such as raffinose, melezitose, maltodextrins.
dextrans, starches,
and the like; and alditols, such as mannitol, xylitol, maltitol, lactitol,
xylitol sorbitol (glucitol)
and the like. In one embodiment, the carbohydrate excipients for use in the
present invention
are chosen from, sucrose, trehalose, lactose, mannitol, and raffinose. In a
specific
embodiment, the carbohydrate excipient is trehalose. In another specific
embodiment, the
carbohydrate excipient is mannitol. In yet another specific embodiment, the
carbohydrate
excipient is sucrose. In still another specific embodiment, the carbohydrate
excipient is
raffinose. The purity of the carbohydrate excipient should be at least 98%, or
at least 99%, or
at least 99.5%.
In a specific embodiment, the formulations of the low AR compositions of the
invention may comprise trehalose. In one embodiment, a formulation of the
invention
comprises at least about 1%, at least about 2%, at least about 4%, at least
about 8%, at least
about 20%. at least about 30%. or at least about 40% trehalose. In another
embodiment, a
formulation of the invention comprises between about 1% and about 40%, between
about 1%
and about 30%. between about 1% and about 20%, between about 2% and about 40%,
between about 2% and about 30%. between about 2% and about 20%. between about
4% and
about 40%. between about 4% and about 30%, or between about 4% and about 20%
trehalose. In a further embodiment, a formulation of the invention comprises
about 1%,
110
CA 02926384 2016-04-07
about 2%, about 4%, about 6%, about 8%, about 15%, about 20%, about 30%, or
about 40%
trehalose. In a specific embodiment, a formulation of the invention comprises
about 4%,
about 6% or about 15% trehalose.
In certain embodiments, a formulation of the low AR compositions of the
invention
comprises an excipient. In a specific embodiment, a formulation of the
invention comprises
at least one excipient chosen from: sugar, salt, surfactant, amino acid,
polyol, chelating agent,
emulsifier and preservative. In one embodiment, a formulation of the invention
comprises a
salt, e.g., a salt selected from: NaC1, KC], CaC12, and MgC12. In a specific
embodiment, the
formulation comprises NaCl.
A formulation of the low AR compositions of the invention may comprise at
least
about 10 mM, at least about 25 mM, at least about 50 mM, at least about 75 mM,
at least
about 80 mM, at least about 100 mM, at least about 125 mM, at least about 150
mM, at least
about 175 mM, at least about 200 mM, or at least about 300 mM sodium chloride
(NaC1). In
a further embodiment, the formulation may comprise between about 10 mM and
about 300
mM, between about 10 mM and about 200 mM, between about 10 mM and about 175
mM,
between about 10 mM and about 150 mM, between about 25 mM and about 300 mM,
between about 25 mM and about 200 mM, between about 25 mM and about 175 mM,
between about 25 mM and about 150 mM, between about 50 mM and about 300 mM,
between about 50 mM and about 200 mM, between about 50 mM and about 175 mM,
between about 50 mM and about 150 mM, between about 75 mM and about 300 mM,
between about 75 mM and about 200 mM, between about 75 mM and about 175 mM,
between about 75 mM and about 150 mM, between about 100 mM and about 300 mM,
between about 100 mM and about 200 mM, between about 100 mM and about 175 mM,
or
between about 100 mM and about 150 mM sodium chloride. In a further
embodiment, the
formulation may comprise about 10 mM, about 25 mM, about 50 mM, about 75 mM,
about
80 mM. about 100 mM, about 125 mM, about 150 mM, about 175 mM, about 200 mM,
or
about 300 mM sodium chloride.
A formulation of the low AR compositions of the invention may also comprise an
amino acid, e.g., lysine, arginine, glycine, histidine or an amino acid salt.
The formulation
may comprise at least about 1mM, at least about 10mM, at least about 25 mM, at
least about
50 mM. at least about 100 mM, at least about 150 mM, at least about 200 mM, at
least about
250 mM, at least about 300 mM, at least about 350 mM, or at least about 400 mM
of an
amino acid. In another embodiment, the formulation may comprise between about
1 mM and
111
CA 02926384 2016-04-07
about 100 mM. between about 10 mM and about 150 mM, between about 25 mM and
about
250 mM, between about 25 mM and about 300 mM, between about 25 mM and about
350
mM, between about 25 mM and about 400 mM, between about 50 mM and about 250
mM,
between about 50 mM and about 300 mM, between about 50 mM and about 350 mM,
between about 50 mM and about 400 mM, between about 100 mM and about 250 mM,
between about 100 mM and about 300 mM, between about 100 mM and about 400 mM,
between about 150 mM and about 250 mM, between about 150 mM and about 300 mM,
or
between about 150 mM and about 400 mM of an amino acid. In a further
embodiment, a
formulation of the invention comprises about 1 mM, 1.6 mM, 25 mM, about 50 mM,
about
100 mM, about 150 mM, about 200 mM, about 250 mM, about 300 mM, about 350 mM,
or
about 400 mM of an amino acid.
The formulations of the low AR compositions of the invention may further
comprise a
surfactant. The term "surfactant" as used herein refers to organic substances
having
amphipathic structures; namely, they are composed of groups of opposing
solubility
tendencies, typically an oil-soluble hydrocarbon chain and a water-soluble
ionic group.
Surfactants can be classified, depending on the charge of the surface-active
moiety, into
anionic, cationic, and nonionic surfactants.
Surfactants are often used as wetting,
emulsifying, solubilizing, and dispersing agents for various pharmaceutical
compositions and
preparations of biological materials.
Pharmaceutically acceptable surfactants like
polysorbates (e.g., polysorbates 20 or 80); polyoxamers (e.g., poloxamer 188);
Triton;
sodium octyl glycoside; lauryl-, myristyl-, linoleyl-, or stearyl-
sulfobetaine; lauryl-, myristyl-,
linoleyl- or stearyl-sarcosine; linoleyl-, myristyl-, or cetyl-betaine;
lauroamidopropyl-,
cocamidopropyl-, linoleamidopropyl-. myristamidopropyl-,
palmidopropyl-, or
isostearamidopropyl-betaine (e.g., lauroamidopropyl); myristamidopropyl-,
palmidopropyl-,
or isostearamidopropyl-dimethylamine; sodium methyl cocoyl-, or disodium
methyl oleyl-
taurate; and the MONAQUATm series (Mona Industries, Inc.. Paterson, N.J.),
polyethyl
polypropyl glycol, and copolymers of ethylene and propylene glycol (e.g.,
PLURONICSIm. PF68, etc.). can optionally be added to the formulations of the
invention to
reduce aggregation. In one
embodiment, a formulation of the invention comprises
Polysorbate 20, Polysorbate 40, Polysorbate 60. or Polysorbate 80.
Surfactants are
particularly useful if a pump or plastic container is used to administer the
formulation. The
presence of a pharmaceutically acceptable surfactant mitigates the propensity
for the protein
to aggregate. The formulations may comprise a polysorbate which is at a
concentration
112
CA 02926384 2016-04-07
ranging from between about 0.001% to about 1%, or about 0.001% to about 0.1%,
or about
0.01% to about 0.1%. In other specific embodiments, the formulations of the
invention
comprise a polysorbate which is at a concentration of 0.001%, or 0.002%, or
0.003%, or
0.004%, or 0.005%, or 0.006%, or 0.007%, or 0.008%, or 0.009%, or 0.01%, or
0.015%, or
0.02%.
The formulations of the low AR compositions of the invention may optionally
further
comprise other common excipients and/or additives including, but not limited
to, diluents,
binders, stabilizers, lipophilic solutions, preservatives, adjuvants, or the
like.
Pharmaceutically acceptable excipients and/or additives may be used in the
formulations of
the invention. Commonly used excipients/additives, such as phamaceutically
acceptable
chelators (for example, but not limited to, EDTA, DTPA or EGTA) can optionally
be added
to the formulations of the invention to reduce aggregation. These additives
are particularly
useful if a pump or plastic container is used to administer the formulation.
Preservatives, such as phenol, m-cresol, p-cresol, o-cresol, chlorocresol,
benzyl
alcohol, phenylmercuric nitrite, phenoxyethanol, formaldehyde, chlorobutanol,
magnesium
chloride (for example, but not limited to, hexahydrate), alkylparaben (methyl,
ethyl, propyl,
butyl and the like), benzalkonium chloride, benzethonium chloride, sodium
dehydroacetate
and thimerosal, or mixtures thereof can optionally be added to the
formulations of the
invention at any suitable concentration such as between about 0.001% to about
5%, or any
range or value therein. The concentration of preservative used in the
formulations of the
invention is a concentration sufficient to yield a microbial effect. Such
concentrations are
dependent on the preservative selected and are readily determined by the
skilled artisan.
Other contemplated excipients/additives, which may be utilized in the
formulations of
the invention include, for example, flavoring agents, antimicrobial agents,
sweeteners,
antioxidants, antistatic agents, lipids such as phospholipids or fatty acids,
steroids such as
cholesterol, protein excipients such as serum albumin (human serum albumin
(HSA),
recombinant human albumin (rHA), gelatin, casein, salt-forming counterions
such as sodium
and the like. These and additional known pharmaceutical excipients and/or
additives suitable
for use in the formulations of the invention are known in the art, e.g., as
listed in "Remington:
The Science (.4. Practice of Pharmacy", 21st ed., Lippincott Williams &
Wilkins, (2005), and
in the "Physician's Desk Reference-, 60th ed., Medical Economics, Montvale,
N.J. (2005).
Pharmaceutically acceptable carriers can be routinely selected that are
suitable for the mode
l 13
CA 02926384 2016-04-07
of administration, solubility and/or stability of an antibody, as well known
those in the art or
as described herein.
In one embodiment, the low AR compositions of the invention are formulated
with
the same or similar excipients and buffers as are present in the commercial
adalimumab
(HUMIRA ) formulation, as described in the "Highlights of Prescribing
Information" for
HUMIRA (adalimumab) Injection (Revised Jan. 2008).
For example, each prefilled syringe of HUMIRA , which
is administered subcutaneously, delivers 0.8 mL (40 mg) of drug product to the
subject. Each
0.8 mL of HUMIRA contains 40 mg adalimumab, 4.93 mg sodium chloride, 0.69 mg
monobasic sodium phosphate dihydrate, 1.22 mg dibasic sodium phosphate
dihydrate, 0.24
mg sodium citrate, 1.04 mg citric acid monohydrate, 9.6 mg mannitol, 0.8 mg
polysorbate 80,
and water for Injection, USP. Sodium hydroxide is added as necessary to adjust
pH.
It will be understood by one skilled in the art that the formulations of the
low AR
compositions of the invention may be isotonic with human blood, wherein the
formulations
of the invention have essentially the same osmotic pressure as human blood.
Such isotonic
formulations will generally have an osmotic pressure from about 250 mOSm to
about 350
mOSm. Isotonicity can be measured by, for example, using a vapor pressure or
ice-freezing
type osmometer. Tonicity of a formulation is adjusted by the use of tonicity
modifiers.
"Tonicity modifiers" are those pharmaceutically acceptable inert substances
that can be added
to the formulation to provide an isotonity of the formulation. Tonicity
modifiers suitable for
this invention include, but are not limited to, saccharides, salts and amino
acids.
In certain embodiments, the formulations of the low AR compositions of the
invention have an osmotic pressure from about 100 in0Sin to about 1200 mOSm,
or from
about 200 mOSm to about 1000 mOSm, or from about 200 mOSm to about 800 mOSm,
or
from about 200 mOSm to about 600 mOSm, or from about 250 mOSm to about 500
in0Sm,
or from about 250 mOSm to about 400 mOSm, or from about 250 na0Sm to about 350
mOSm.
The concentration of any one component or any combination of various
components,
of the formulations of the low AR compositions of the invention is adjusted to
achieve the
desired tonicity of the final formulation. For example, the ratio of the
carbohydrate excipient
to antibody may be adjusted according to methods known in the art (e.g., U.S.
Patent No.
6,685,940). In certain embodiments, the molar ratio of the carbohydrate
excipient to
antibody inay be from about 100 moles to about 1000 moles of carbohydrate
excipient to
1 14
CA 02926384 2016-04-07
about 1 mole of antibody, or from about 200 moles to about 6000 moles of
carbohydrate
excipient to about 1 mole of antibody, or from about 100 moles to about 510
moles of
carbohydrate excipient to about 1 mole of antibody, or from about 100 moles to
about 600
moles of carbohydrate excipient to about 1 mole of antibody.
The desired isotonicity of the final formulation may also be achieved by
adjusting the
salt concentration of the formulations. Pharmaceutically acceptable salts and
those suitable
for this invention as tonicity modifiers include, but are not limited to,
sodium chloride,
sodium succinate, sodium sulfate, potassuim chloride, magnesium chloride,
magnesium
sulfate, and calcium chloride. In specific embodiments, formulations of the
invention
comprise NaC1, MgC12, and/or CaC12. In one embodiment, concentration of NaC1
is between
about 75 mM and about 150 mM. In another embodiment, concentration of MgC12 is
between about 1 mM and about 100 mM. Pharmaceutically acceptable amino acids
including
those suitable for this invention as tonicity modifiers include, but are not
limited to, proline,
alanine, L-arginine, asparagine, L-aspartic acid, glycine, serine, lysine, and
histidine.
In one embodiment the fori-nulations of the low AR compositions of the
invention are
pyrogen-free formulations which are substantially free of endotoxins and/or
related pyrogenic
substances. Endotoxins include toxins that are confined inside a microorganism
and are
released only when the microorganisms are broken down or die. Pyrogenic
substances also
include fever-inducing, therrnostable substances (glycoproteins) from the
outer membrane of
bacteria and other microorganisms. Both of these substances can cause fever,
hypotension
and shock if administered to humans. Due to the potential harmful effects,
even low amounts
of endotoxins must be removed from intravenously administered pharmaceutical
drug
solutions. The Food & Drug Administration ("FDA") has set an upper limit of 5
endotoxin
units (EU) per dose per kilogram body weight in a single one hour period for
intravenous
drug applications (The United States Pharmacopeial Convention, Pharmacopeial
Forum 26
(1):223 (2000)). When therapeutic proteins are administered in amounts of
several hundred or
thousand milligrams per kilogram body weight, as can be the case with
antibodies, even trace
amounts of harmful and dangerous endotoxin must be removed. In certain
specific
embodiments, the endotoxin and pyrogen levels in the composition are less then
10 EU/mg,
or less then 5 EU/mg, or less then 1 EU/mg. or less then 0.1 EU/mg, or less
then 0.01 EU/mg,
or less then 0.001 EU/mg.
When used for in vivo administration, the formulations of the low AR
compositions of
the invention should be sterile. The formulations of the invention may be
sterilized by
115
CA 02926384 2016-04-07
various sterilization methods, including sterile filtration, radiation, etc.
In one embodiment,
the antibody formulation is filter-sterilized with a presterilized 0.22-micron
filter. Sterile
compositions for injection can be formulated according to conventional
pharmaceutical
practice as described in "Remington: The Science & Practice of Pharmacy", 2Ist
ed.,
Lippincott Williams & Wilkins, (2005). Formulations comprising antibodies,
such as those
disclosed herein, ordinarily will be stored in lyophilized form or in
solution. It is
contemplated that sterile compositions comprising antibodies are placed into a
container
having a sterile access port, for example, an intravenous solution bag or vial
having an
adapter that allows retrieval of the formulation, such as a stopper pierceable
by a hypodermic
injection needle. In one embodiment, a composition of the invention is
provided as a pre-
filled syringe.
In one embodiment, a formulation of the low AR compositions of the invention
is a
lyophilized formulation. The term "lyophilized" or "freeze-dried" includes a
state of a
substance that has been subjected to a drying procedure such as
lyophilization, where at least
50% of moisture has been removed.
The phrase "bulking agent" includes a compound that is pharmaceutically
acceptable
and that adds bulk to a lyo cake. Bulking agents known to the art include, for
example,
carbohydrates, including simple sugars such as dextrose, ribose, fructose and
the like, alcohol
sugars such as mannitol, inositol and sorbitol, disaccharides including
trehalose, sucrose and
lactose, naturally occuning polymers such as starch, dextrans, chitosan,
hyaluronate, proteins
(e.g., gelatin and serum albumin), glycogen, and synthetic monomers and
polymers.
A "lyoprotectant" is a molecule which, when combined with a protein of
interest
(such as an antibody of the invention), significantly prevents or reduces
chemical and/or
physical instability of the protein upon lyophilization and subsequent
storage. Lyoprotectants
include, but are not limited to, sugars and their corresponding sugar
alcohols; an amino acid
such as monosodium glutamate or histidine; a methylamine such as betaine; a
lyotropic salt
such as magnesi-um sulfate; a polyol such as trihydric or higher molecular
weight sugar
alcohols, e.g., glycerin, dextran, erythritol, glycerol, arabitol, xylitol,
sorbitol, and mannitol;
propylene glycol; polyethylene glycol; PLURONICSmi; and combinations thereof.
Additional examples of lyoprotectants include. but are not limited to,
glycerin and gelatin,
and the sugars mellibiose. melezitose, raffinose, mannotriose and stachyose.
Examples of
reducing sugars include, but arc not limited to, glucose, maltose, lactose,
maltulose, iso-
maltulose and lactulose. Examples of non-reducing sugars include, but are .not
limited to,
116
CA 02926384 2016-04-07
non-reducing glycosides of polyhydroxy compounds selected from sugar alcohols
and other
straight chain polyalcohols. Examples of sugar alcohols include, but are not
limited to,
monoglycosides, compounds obtained by reduction of disaccharides such as
lactose, maltose,
lactulose and maltulose. The glycosidic side group can be either glucosidic or
galactosidic.
Additional examples of sugar alcohols include, but are not limited to,
glucitol, maltitol,
lactitol and iso-maltulose. In specific embodiments, trehalose or sucrose is
used as a
lyoprotectant.
The lyoprotectant is added to the pre-lyophilized formulation in a
"lyoprotecting
amount" which means that, following lyophilization of the protein in the
presence of the
lyoprotecting amount of the lyoprotectant, the protein essentially retains its
physical and
chemical stability and integrity upon lyophilization and storage.
In one embodiment, the molar ratio of a lyoprotectant (e.g., trehalose) and
antibody
molecules of a formulation of the invention is at least about 10, at least
about 50, at least
about 100, at least about 200, or at least about 300. In another embodiment,
the molar ratio
of a lyoprotectant (e.g., trehalose) and antibody molecules of a formulation
of the invention is
about 1, is about 2, is about 5, is about 10, about 50, about 100, about 200,
or about 300.
A "reconstituted" formulation is one which has been prepared by dissolving a
lyophilized antibody formulation in a diluent such that the antibody is
dispersed in the
reconstituted formulation. The reconstituted formulation is suitable for
administration (e.g.,
parenteral administration) to a patient to be treated with the antibody and,
in certain
embodiments of the invention, may be one which is suitable for intravenous
administration.
The "diluent" of interest herein is one which is pharrnaceutically acceptable
(safe and
non-toxic for administration to a human) and is useful for the preparation of
a liquid
formulation, such as a formulation reconstituted after lyophilization.. In
some embodiments,
diluents include, but are not limited to, sterile water, bacteriostatic water
for injection
(BWFI), a pH buffered solution (e.g., phosphate-buffered saline), sterile
saline solution,
Ringer's solution or dextrose solution. In an alternative embodiment, diluents
can include
aqueous solutions of salts and/or buffers.
In certain embodiments, a formulation of the low AR compositions of the
invention is
a lyophilized formulation comprising an antibody of the invention, wherein at
least about
90%. at least about 957c. at least about 97%, at least about 98%, or at least
about 99% of said
antibody may be recovered from a vial upon shaking said vial for 4 hours at a
speed of 400
shakes per minute wherein the vial is filled to half of its volume with the
formulation. In
117
CA 02926384 2016-04-07
another embodiment, a formulation of the invention is a lyophilized
formulation comprising
an antibody of the invention, wherein at least about 90%, at least about 95%,
at least about
97%, at least about 98%, or at least about 99% of the antibody may be
recovered from a vial
upon subjecting the formulation to three freeze/thaw cycles wherein the vial
is filled to half
of its volume with said formulation. In a further embodiment, a formulation of
the invention
is a lyophilized formulation comprising an antibody of the invention, wherein
at least about
90%, at least about 95%, at least about 97%, at least about 98%, or at least
about 99% of the
antibody may be recovered by reconstituting a lyophilized cake generated from
said
formulation.
In one embodiment, a reconstituted liquid formulation may comprise an antibody
at
the same concentration as the pre-lyophilized liquid formulation.
In another embodiment, a reconstituted liquid formulation may comprise an
antibody
at a higher concentration than the pre-lyophilized liquid formulation, e.g.,
.about 2 fold, about
3 fold, about 4 fold, about 5 fold, about 6 fold, about 7 fold, about 8 fold,
about 9 fold, or
about 10 fold higher concentration of an antibody than the pre-lyophilized
liquid formulation.
In yet another embodiment, a reconstituted liquid formulation may comprise an
antibody of the invention at a lower concentration than the pre-lyophilized
liquid formulation,
e.g., about 2 fold, about 3 fold, about 4 fold, about 5 fold, about 6 fold,
about 7 fold, about 8
fold, about 9 fold or about 10 fold lower concentration of an antibody than
the pre-
lyophilized liquid formulation.
The pharmaceutical formulations of the low AR compositions of the invention
are
typically stable fon-nulations, e.g., stable at room temperature.
The terms -stability" and "stable" as used herein in the context of a
formulation
comprising an antibody of the invention refer to the resistance of the
antibody in the
formulation to aggregation. degradation or fragmentation under given
manufacture,
preparation, transportation and storage conditions. The -stable" formulations
of the invention
retain biological activity under given manufacture. preparation,
transportation and storage
conditions. The stability of the antibody can be assessed by degrees of
aggregation.
degradation or fragmentation, as measured by HPSEC, static light scattering
(SLS), Fourier
Transfon-n Infrared Spectroscopy (FTIR), circular dichroism (CD), urea
unfolding
techniques. intrinsic tryptophan fluorescence, differential scanning
calorimetry, and/or ANS
binding techniques, compared to a reference formulation. For example, a
reference
118
CA 02926384 2016-04-07
formulation may be a reference standard frozen at -70 C consisting of 10 mg/ml
of an
antibody of the invention in PBS.
Therapeutic formulations of the low AR compositions of the invention may be
formulated for a particular dosage. Dosage regimens may be adjusted to provide
the
optimum desired response (e.g., a therapeutic response). For example, a single
bolus may be
administered, several divided doses may be administered over time or the dose
may be
proportionally reduced or increased as indicated by the exigencies of the
therapeutic
situation. It is especially advantageous to formulate parenteral compositions
in dosage unit
form for ease of administration and uniformity of dosage. Dosage unit forin as
used herein
refers to physically discrete units suited as unitary dosages for the subjects
to be treated; each
unit contains a predetermined quantity of active compound calculated to
produce the desired
therapeutic effect in association with the required pharmaceutical carrier.
The specification
for the dosage unit forms of the invention are dictated by and directly
dependent on (a) the
unique characteristics of the antibody and the particular therapeutic effect
to be achieved, and
(b) the limitations inherent in the art of compounding such an antibody for
the treatment of
sensitivity in individuals.
Therapeutic compositions of the low AR compositions of the invention can be
formulated for particular routes of administration, such as oral, nasal,
pulmonary, topical
(including buccal and sublingual), rectal, vaginal and/or parenteral
administration. The
formulations may conveniently be presented in unit dosage form and may be
prepared by any
methods known in the art of pharmacy. The amount of active ingredient which
can be
combined with a carrier material to produce a single dosage form will vary
depending upon
the subject being treated, and the particular mode of administration. The
amount of active
ingredient which can be combined with a carrier material to produce a single
dosage form
will generally be that amount of the composition which produces a therapeutic
effect. By
way of example, in certain embodiments, the antibodies (including antibody
fragments) are
formulated for intravenous administration. In certain other embodiments, the
antibodies
(including antibody fragments) are formulated for local delivery to the
cardiovascular system.
for example, via catheter, stent, wire, intramyocardial delivery,
intrapericardial delivery, or
intraendocardial delivery.
Formulations of the low AR compositions of the invention which are suitable
for
topical or transdermal administration include powders, sprays, ointments,
pastes, creams,
lotions, gels, solutions, patches and inhalants. The active compound rnay be
mixed under
1 19
CA 02926384 2016-04-07
sterile conditions with a pharmaceutically acceptable carrier, and with any
preservatives,
buffers, or propellants which may be required (US Patent No. 7,378,110;
7,258,873;
7,135,180; 7,923,029; and US Publication No. 20040042972).
The phrases "parenteral administration" and "administered parenterally" as
used
herein means modes of administration other than enteral and topical
administration, usually
by injection, and includes, without limitation, intravenous, intramuscular,
intraarterial,
intrathecal, intracapsular, intraorbital, intracardiac, intradermal,
intraperitoneal, transtracheal,
subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid,
intraspinal, epidural
and intrasternal injection and infusion.
Actual dosage levels of the active ingredients in the pharmaceutical
compositions of
the low AR compositions of the invention may be varied so as to obtain an
amount of the
active ingredient which is effective to achieve the desired therapeutic
response for a
particular patient, composition, and mode of administration, without being
toxic to the
patient. The selected dosage level will depend upon a variety of
pharmacokinetic factors
including the activity of the particular compositions of the present invention
employed, or the
ester, salt or amide thereof, the route of administration, the time of
administration, the rate of
excretion of the particular compound being employed, the duration of the
treatment, other
drugs, compounds and/or materials used in combination with the particular
compositions
employed, the age, sex, weight, condition, general health and prior medical
history of the
patient being treated, and like factors well known in the medical arts.
In certain embodiments, antibodies of the invention can be formulated to
ensure
proper distribution in vivo. For example, the blood-brain barrier (BBB)
excludes many
highly hydrophilic compounds. To ensure that the therapeutic compounds of the
invention
can cross the BBB (if desired), they can be formulated, for example, in
liposomes. For
methods of manufacturing liposomes, see, e.g., U.S. Pat. Nos. 4,522,811;
5,374,548;
5,399,331. The liposomes may comprise one or more moieties which are
selectively
transported into specific cells or organs, thus enhance targeted drug delivery
(see, e.g., V. V.
Ranade (1989) J. (Jlin. Pharmacy'. 29:685). Exemplary targeting moieties
include folate or
biotin (see, U.S.
Pat. No. 5,416,016); mannosides (Umezawa et al., (1988) Biochem.
Biophys. Res. C0171/711111. 153:1038); antibodies (P. G. Bloeman et al. (1995)
FEBS Lea.
357:140; M. Owais et (1l. (1995) Antimicrob. Agents Chemother. 39:180);
surfactant Protein
A receptor (Briscoe et (1l. (1995) Am. J. Physiol. 1233:134), different
species of which may
comprise the formulations of the invention, as well as components of the
invented molecules;
120
CA 02926384 2016-04-07
p120 (Schreier et al. (1994) .7. Biol. Chem. 269:9090); see also K. Keinanen;
M. L.
Laukkanen (1994) FEBS Lett. 346:123; J. J. Killion; I. J. Fidler (1994)
hniminomethods
4:273. In one embodiment of the invention, the therapeutic compounds of the
invention are
formulated in liposomes; in another embodiment, the liposomes include a
targeting moiety.
In another embodiment, the therapeutic compounds in the liposomes are
delivered by bolus
injection to a site proximal to the desired area. When administered in this
manner, the
composition must be fluid to the extent that easy syringability exists. It
must be stable under
the conditions of manufacture and storage and may be preserved against the
contaminating
action of microorganisms such as bacteria and fungi. Additionally or
alternatively, the
antibodies of the invention may be delivered locally to the brain to mitigate
the risk that the
blood brain barrier slows effective delivery.
In certain embodiments, the low AR compositions of the invention may be
administered with medical devices known in the art. For example, in certain
embodiments an
antibody or antibody fragment is administered locally via a catheter, stent,
wire, or the like.
For example, in one embodiment, a therapeutic composition of the invention can
be
administered with a needleless hypodermic injection device, such as the
devices disclosed in
U.S. Pat. Nos. 5,399,163; 5,383,851; 5,312,335; 5,064,413; 4,941,880;
4,790,824; 4,596,556.
Examples of well-known implants and modules useful in the present invention
include: U.S.
Pat. No. 4,487,603, which discloses an implantable micro-infusion pump for
dispensing
medication at a controlled rate; U.S. Pat. No. 4,486,194, which discloses a
therapeutic device
for administering medicants through the skin; U.S. Pat. No. 4,447,233, which
discloses a
medication infusion pump for delivering medication at a precise infusion rate;
U.S. Pat. No.
4,447,224, which discloses a variable flow implantable infusion apparatus for
continuous
drug delivery; U.S. Pat. No. 4,439,196, which discloses an osmotic drug
delivery system
having multi-chamber compartments; and U.S. Pat. No. 4,475,196. which
discloses an
osmotic drug delivery system. Many other such implants, delivery systems, and
modules are
known to those skilled in the art.
The efficient dosages and the dosage regimens for the low AR compositions of
the
invention depend on the disease or condition to be treated and can be
determined by the
persons skilled in the art. One of ordinary skill in the art would be able to
determine such
amounts based on such factors as the subject's size, the severity of the
subject's symptoms.
and the particular composition or route of administration selected.
121
CA 02926384 2016-04-07
VIM Alternative Formulations Containing the Low AR Compositions of the
Invention
Alternative Aqueous Formulations
The invention also provides a low AR composition formulated as an aqueous
formulation comprising a protein and water, as described in U.S. Patent No.
8,420,081 and
W02012/065072,
In these aqueous formulations,
the protein is stable without the need for additional agents. This aqueous
formulation has a
number of advantages over conventional formulations in the art, including
stability of the
protein in water without the requirement for additional excipients, increased
concentrations of
protein without the need for additional excipients to maintain solubility of
the protein, and
low osmolality. These also have advantageous storage properties, as the
proteins in the
formulation remain stable during storage, e.g., stored as a liquid form for
more than 3 months
at 7 C or freeze/thaw conditions, even at high protein concentrations and
repeated
freeze/thaw processing steps. In one embodiment, formulations described herein
include high
concentrations of proteins such that the aqueous formulation does not show
significant
opalescence, aggregation, or precipitation.
In one embodiment, an aqueous low AR composition comprising a protein, e.g.,
an
antibody, e.g., an anti-TNFa antibody or antigen biding portion thereof, and
water is
provided, wherein the foimulation has certain characteristics, such as, but
not limited to, low
conductivity, e.g., a conductivity of less than about 2.5 mS/cm, a protein
concentration of at
least about 10 ug/mL, an osmolality of no more than about 30 mOsmolfkg, and/or
the protein
has a molecular weight (Mw) greater than about 47 kDa. In one embodiment, the
formulation
has improved stability, such as, but not limited to, stability in a liquid
form for an extended
time (e.g., at least about 3 months or at least about 12 months) or stability
through at least one
freeze/thaw cycle (if not more freeze/thaw cycles). In one embodiment, the
formulation is
stable for at least about 3 months in a form selected from the group
consisting of frozen,
lyophilized, or spray-dried.
In one embodiment, the formulation has a low conductivity, including, for
example, a
conductivity of less than about 2.5 rnS/cm, a conductivity of less than about
2 mS/cm, a
122
CA 02926384 2016-04-07
conductivity of less than about 1.5 mS/cm, a conductivity of less than about 1
mS/cm, or a
conductivity of less than about 0.5 mS/cm.
In another embodiment, low AR compositions included in the formulation have a
given concentration, including, for example, a concentration of at least about
1 mg/mL, at
least about 10 mg/mL, at least about 50 mg/mL, at least about 100 mg/mL, at
least about 150
mg/mL, at least about 200 mg/mL, or greater than about 200 rng/mL. In another
embodiment,
the formulation of the invention has an osmolality of no more than about 15
mOsmol/kg.
The aqueous formulations described herein do not rely on standard excipients,
e.g.. a
tonicity modifier, a stabilizing agent, a surfactant, an anti-oxidant, a
cryoprotectant, a bulking
agent, a lyroprotectant, a basic component, and an acidic component. In other
embodiments
of the invention, the formulation contains water, one or more proteins, and no
ionic
excipients (e.g., salts, free amino acids).
In certain embodiments, the aqueous formulation as described herein comprise a
low
AR composition comprising a protein concentration of at least 50 mg/mL and
water, wherein
the formulation has an osmolality of no more than 30 mOsmol/kg. Lower limits
of osmolality
of the aqueous formulation are also encompassed by the invention. In one
embodiment the
osmolality of the aqueous formulation is no more than 15 mOsmol/kg. The
aqueous
formulation of the invention may have an osmolality of less than 30 mOsmol/kg,
and also
have a high protein concentration, e.g., the concentration of the protein is
at least 100 mg/mL,
and may be as much as 200 mg/mL or greater. Ranges intermediate to the above
recited
concentrations and osmolality units are also intended to be part of this
invention. In addition,
ranges of values using a combination of any of the above recited values as
upper and/or lower
limits are intended to be included.
The concentration of the aqueous formulation as described herein is not
limited by the
protein size and the formulation may include any size range of proteins.
Included within the
scope of the invention is an aqueous formulation comprising at least 40 mg/mL
and as much
as 200 ing/mL or more of a protein, for example, 40 mg/mL, 65 mg/mL. 130
mg/mL. or 195
mg/ml, which may range in size from 5 kDa to 150 kDa or more. In one
embodiment. the
protein in the formulation of the invention is at least about 15 kD in size,
at least about 20 kD
in size; at least about 47 kD in size; at least about 60 kD in size; at least
about 80 kD in size:
at least about 100 kD in size; at least about 120 kD in size; at least about
140 kD in size; at
123
CA 02926384 2016-04-07
least about 160 kD in size; or greater than about 160 kD in size. Ranges
intermediate to the
above recited sizes are also intended to be part of this invention. In
addition, ranges of values
using a combination of any of the above recited values as upper and/or lower
limits are
intended to be included.
The aqueous formulation as described herein may be characterized by the
hydrodynamic diameter (Ph) of the proteins in solution. The hydrodynamic
diameter of the
protein in solution may be measured using dynamic light scattering (DLS),
which is an
established analytical method for determining the Dh of proteins. Typical
values for
monoclonal antibodies, e.g., IgG, are about 10 nm. Low-ionic formulations may
be
characterized in that the Dh of the proteins are notably lower than protein
formulations
comprising ionic excipients. It has been discovered that the Dh values of
antibodies in
aqueous formulations made using the disfiltration/ultrafilteration (DF/UF)
process, as
described in U.S. Patent No. 8,420,081, using pure water as an exchange
medium, are notably
lower than the Dh of antibodies in conventional fonnulations independent of
protein
concentration. In one embodiment, antibodies in the aqueous formulation as
described herein
have a Dh of less than 4 nm, or less than 3 nm.
In one embodiment, the Ph of the protein in the aqueous formulation is smaller
relative to the Dh of the same protein in a buffered solution, irrespective of
protein
concentration. Thus, in certain embodiments, protein in an aqueous formulation
made in
accordance with the methods described herein, will have a Dh which is at least
25% less than
the Dh of the protein in a buffered solution at the same given concentration.
Examples of
buffered solutions include, but are not limited to phosphate buffered saline
(PBS). In certain
embodiments, proteins in the aqueous formulation of the invention have a Dh
that is at least
50% less than the Ph of the protein in PBS in at the Elven concentration; at
least 60% less
than the Dh of the protein in PBS at the given concentration; at least 70%
less than the Dh of
the protein in PBS at the given concentration; or more than 70% less than the
Dh of the
protein in PBS at the given concentration. Ranges intermediate to the above
recited
percentages are also intended to be part of this invention, e.g., about 55%.
56%. 57%. 64%.
68%, and so forth. In addition, ranges of values using a combination of any of
the above
recited values as upper and/or lower limits are intended to be included. e.g..
about 50% to
about 80%.
124
CA 02926384 2016-04-07
In one aspect, the aqueous formulation includes the protein at a dosage of
about 0.01
mg/kg-10 mg/kg. In another aspect, the dosages of the protein include
approximately 1 mg/kg
administered every other week, or approximately 0.3 mg/kg administered weekly.
A skilled
practitioner can ascertain the proper dosage and regime for administering to a
subject.
Alternative Solid Unit Formulations
The invention also provides a low AR composition of the invention formulated
asa
stable solid composition of a protein (preferably a therapeutic protein) and a
stabilizer,
referred to herein as solid units, as described in Attorney Docket No. 117813-
31001.
Specifically, it has been
discovered that despite having a high proportion of sugar relative to the
protein, the solid
units of the invention maintain structural rigidity and resist changes in
shape and/or volume
when stored under ambient conditions, e.g., room temperature and humidity, for
extended
periods of time. The solid units of the invention remain free-flowing and are
able to maintain
long-term physical and chemical stability of the protein without significant
degradation
and/or aggregate formation. The solid units of the invention have many
advantages over the
art, including that they can be formulated for oral delivery and are easily
reconstituted in a
diluent, such as water. Because the solid units are readily dissolved, they
may be used in .
dual chamber delivery devices and may be prepared directly in a device for
patient use.
As used herein, the term "solid unit," refers to a composition which is
suitable for
pharmaceutical administration and comprises a protein, e.g., an antibody or
peptide, and a
stabilizer, e.g., a sugar. The solid unit has a structural rigidity and
resistance to changes in
shape and/or volume. In a preferred embodiment, the solid unit is obtained by
lyophilizing a
pharmaceutical formulation of a therapeutic protein. The solid unit may be any
shape, e.g.,
geometric shape, including, but not limited to, a sphere, a cube, a pyramid, a
hemisphere, a
cylinder, a teardrop, and so forth, including irregularly shaped units. In one
embodiment, the
solid unit has a volume ranging from about 1 I to about 20 1. In one
embodiment, the solid
unit is not obtained using spray drying techniques, e.g., the solid unit is
not a powder or
granule.
As used herein, the phrase "a plurality of solid units" refers to a collection
or population of
solid units, wherein the collection comprises two or more solid units having a
substantially
uniform shape, e.g., sphere, and/or volume distribution. In one embodiment,
the plurality of
solid units is free-flowing.
125
CA 02926384 2016-04-07
IX. Kits
and Articles of Manufacture Comprising the Low AR Compositions
of the Invention
Also within the scope of the present invention are kits comprising the low AR
compositions of the invention and instructions for use. The term "kit" as used
herein refers to
a packaged product comprising components with which to administer the
antibody, or
antigen-binding portion thereof, of the invention for treatment of a disease
or disorder. The
kit may comprise a box or container that holds the components of the kit. The
box or
container is affixed with a label or a Food and Drug Administration approved
protocol. The
box or container holds components of the invention which may be contained
within plastic,
polyethylene, polypropylene, ethylene, or propylene vessels. The vessels can
be capped-tubes
or bottles. The kit can also include instructions for administering an
antibody of the
invention.
The kit can further contain one more additional reagents, such as an
immunosuppressive reagent, a cytotoxic agent or a radiotoxic agent or one or
more additional
antibodies of the invention (e.g., an antibody having a complementary activity
which binds to
an epitope in the TNFa antigen distinct from a first anti-TNFa antibody). Kits
typically
include a label indicating the intended use of the contents of the kit. The
term label includes
any writing, or recorded material supplied on or with the kit, or which
otherwise accompanies
the kit.
The invention also provides a pharmaceutical pack or kit comprising one or
more
containers filled with a liquid fon-nulation or lyophilized formulation of an
antibody or
antibody fragment thereof of the invention. In one embodiment, a container
filled with a
liquid formulation of the invention is a pre-filled syringe. In a specific
embodiment, the
formulations of the invention are formulated in single dose vials as a sterile
liquid. For
example, the formulations may be supplied in 3 cc USP Type I borosilicate
amber vials (West
Pharmaceutical Services - Part No. 6800-0675) with a target volume of 1.2 mL.
Optionally
associated with such container(s) can be a notice in the form prescribed by a
governmental
agency regulating the manufacture, use or sale of pharmaceuticals or
biological products,
which notice reflects approval by the agency of manufacture, use or sale for
human
administration.
In one embodiment, a container filled with a liquid formulation of the
invention is a
pre-filled syringe. Any pre-filled syringe known to one of skill in the art
may be used in
126
CA 02926384 2016-04-07
combination with a liquid formulation of the invention. Pre-filled syringes
that may be used
are described in, for example, but not limited to, PCT Publications
W005032627,
W008094984, W09945985, W003077976, US Patents US6792743, US5607400,
US5893842, US7081107, US7041087, US5989227, US6807797, US6142976, US5899889,
US7699811, US7540382, US7998120, US7645267, and US Patent Publication No.
US20050075611. Pre-filled syringes may be made of various materials. In one
embodiment
a pre-filled syringe is a glass syringe. In another embodiment a pre-filled
syringe is a plastic
syringe. One of skill in the art understands that the nature and/or quality of
the materials used
for manufacturing the syringe may influence the stability of a protein
formulation stored in
the syringe. For example, it is understood that silicon based lubricants
deposited on the
inside suiface of the syringe chamber may affect particle formation in the
protein
formulation. In one embodiment, a pre-filled syringe comprises a silicone
based lubricant.
In one embodiment, a pre-filled syringe comprises baked on silicone. In
another
embodiment, a pre-filled syringe is free from silicone based lubricants. One
of skill in the art
also understands that small amounts of contaminating elements leaching into
the formulation
from the syringe barrel, syringe tip cap, plunger or stopper may also
influence stability of the
formulation. For example, it is understood that tungsten introduced during the
manufacturing
process may adversely affect formulation stability. In one embodiment, a pre-
filled syringe
may comprise tungsten at a level above 500 ppb. In another embodiment, a pre-
filled syringe
is a low tungsten syringe. In another embodiment, a pre-filled syringe may
comprise
tungsten at a level between about 500 ppb and about 10 ppb, between about 400
ppb and
about 10 ppb, between about 300 ppb and about 10 ppb, between about 200 ppb
and about 10
ppb, between about 100 ppb and about 10 ppb. between about 50 ppb and about 10
ppb,
between about 25 ppb and-about 10 ppb.
In certain embodiments. kits comprising antibodies of the invention are also
provided
that are useful for various purposes, e.g., research and diagnostic including
for purification or
immunoprecipitation of protein of interest from cells. detection of the
protein of interest in
vitro or ill vivo. For isolation and purification of a protein of interest,
the kit may contain an
antibody coupled to beads (e.g., sepharose beads). Kits may be provided which
contain the
antibodies for detection and quantitation of a protein of interest in vitro,
e.g., in an ELISA or
a Western blot. As with the article of manufacture, the kit comprises a
container and a label
or package insert on or associated with the container. The container holds a
composition
comprising at least one antibody of the invention. Additional containers may
be included that
127
CA 02926384 2016-04-07
contain, e.g., diluents and buffers, control antibodies. The label or package
insert may
provide a description of the composition as well as instructions for the
intended in vitro or
diagnostic use.
The present invention also encompasses a finished packaged and labeled
pharrnaceutical product. This article of manufacture includes the appropriate
unit dosage
form in an appropriate vessel or container such as a glass vial, pre-filled
syringe or other
container that is hermetically sealed. In one embodiment, the unit dosage form
is provided as
a sterile particulate free solution comprising an antibody that is suitable
for parenteral
administration. In another embodiment, the unit dosage form is provided as a
sterile
lyophilized powder comprising an antibody that is suitable for reconstitution.
In one embodiment, the unit dosage forrn is suitable for intravenous,
intramuscular,
intranasal, oral, topical or subcutaneous delivery. Thus, the invention
encompasses sterile
solutions suitable for each delivery route. The invention further encompasses
sterile
lyophilized powders that are suitable for reconstitution.
As with any pharmaceutical product, the packaging material and container are
designed to protect the stability of the product during storage and shipment.
Further, the
products of the invention include instructions for use or other informational
material that
advise the physician, technician or patient on how to appropriately prevent or
treat the disease
or disorder in question, as well as how and how frequently to administer the
pharmaceutical.
In other words, the article of manufacture includes instruction means
indicating or suggesting
a dosing regimen including, but not limited to, actual doses, monitoring
procedures, and other
monitoring information.
Specifically, the invention provides an article of manufacture comprising
packaging
material, such as a box, bottle, tube, vial, container, pre-filled syringe,
sprayer, insufflator,
intravenous (i.v.) bag, envelope and the like; and at least one unit dosage
form of a
pharmaceutical agent contained within said packaging material, wherein said
pharmaceutical
agent comprises a liquid formulation containing an antibody. The packaging
material
includes instruction means which indicate how that said antibody can be used
to prevent, treat
and/or manage one or more symptoms associated with a disease or disorder.
The present invention is further illustrated by the following examples which
should
not be construed as limiting in any way.
1 28
CA 02926384 2016-04-07
X. EXAMPLES
Example 1: Method for reducing the extent of acidic species in cell culture by
the addition of medium components
Production of recombinant proteins by host cells can result in product-related
charge
heterogeneities present in the population of proteins produced by the cells.
The presence of
acidic species in the population of proteins is an example of a product-
related charge
heterogeneity. Control of the amount of acidic species present in the
population of proteins
produced by the host cells can be accomplished by modifying the culture
conditions of the
host cells.
The experiments in this Example demonstrate that supplementation of cell
culture
medium with supplemental amounts of amino acids, calcium chloride and
niacinamide
enhances product quality by decreasing the amount of acidic species in the
culture harvest.
The amino acids included in the study were arginine, lysine, ornithine and
histidine, which
belong to the group of amino acids that are basic. The study includes examples
from multiple
cell lines and antibodies, in shake flasks and bioreactors and in batch and
fed-batch culture
formats. A dose dependent effect in the extent of reduction of acidic species
with increasing
concentrations of the supplements was observed. In addition, the possibility
to supplement
these medium additives individually or in suitable combinations for acidic
species reduction
was also demonstrated.
Materials and Methods
Cell source and adaptation cultures
Three adalimumab producing cell lines (cell line 1. cell line 2, and cell line
3), one
mAbl producing cell line and one mAb2 producing cell line were employed in the
studies
covered below. For adalimumab producing cell lines. cells were cultured in
their respective
growth media (chemically defined media (media 1) or a hydrolysate based media
(media 2 or
media 3)) in a combination of vented non-baffled shake flasks (Corning) on a
shaker platform
at 110 RPM (cell line 1),180 RPM (cell line 2). 140 RPM (cell line 3) and 10L
20L wave
bags (GE). For experiments with cells in the hydrolysate based media (media
3). cells were
120
CA 02926384 2016-04-07
thawed in media 1 and then adapted to media 3 over a few passages. Cultures
were
propagated in a 35 C, 5% CO, incubator for cell line 1 and 2 and in a 36 C, 5%
CO,
incubator for cell line 3 in order to obtain the required number of cells to
be able to initiate
production stage cultures.
For the mAb 1 producing cell line, cells were cultured in chemically defined
growth
media (media 1) in a combination of vented non-baffled shake flasks (Corning)
on a shaker
platform at 130 RPM and 20L wave bags (GE). Cultures were propagated in a 36
C, 5% CO)
incubator to obtain the required number of cells to be able to initiate
production stage
cultures.
For the mAb2 producing cell line, cells were cultured in chemically defined
growth
media (media 1) in a combination of vented non-baffled shake flasks (Corning)
on a shaker
platform at 140 RPM and 20L wave bags (GE). Cultures were propagated in a 35
C, 5% CO)
incubator to obtain the required number of cells to be able to initiate
production stage
cultures.
Cell culture media
Growth and production media were prepared from either a chemically defined
media
formulation (media 1) or hydrolysate-based medium formulations (media 2 and
media 3). For
preparation of the media 1, the media (IVGN GIA-1, a proprietary basal media
fon-nulation
from Invitrogen) was supplemented with L-glutamine. sodium bicarbonate, sodium
chloride,
and methotrexate solution. Production media consisted of all the components in
the growth
medium, excluding methotrexate. For cell line 1, both growth and production
medium were
also supplemented with insulin. For mAb 1 and mAb2 producing cell lines, the
growth
medium were also supplemented with insulin.
130
CA 02926384 2016-04-07
For the hydrolysate-based formulation (media 2), the growth media was composed
of
PFCHO (proprietary chemically defined formulation from SAFC), Dextrose, L-
Glutamine, L-
Asparagine, HEPES, Poloxamer 188. Ferric Citrate, Recombinant Human Insulin,
Yeastolate
(BD), Phytone Peptone (BD), Mono- and Di-basic Sodium Phosphate, Sodium
Bicarbonate,
Sodium Chloride and methotrexate. Production media consisted of all the
components listed
in the growth medium, excluding methotrexate.
For the hydrolysate-based formulation (media 3), the growth media was composed
of
OptiCHO (Invitrogen), L-Glutamine, Yeastolate (BD), Phytone Peptone (BD) and
methotrexate. Production media consisted of all the components listed in the
growth medium,
excluding methotrexate.
Amino acids used for the experiments were reconstituted in Milli-Q water to
make a
100g/L stock solution, which was subsequently supplemented to both growth and
production
basal media. After addition of amino acids, media was brought to a pH similar
to
unsupplemented (control) media using 5N hydrochloric acid/5N NaOH, and it was
brought to
an osmolality similar to unsupplemented (control) media by adjusting the
concentration of
sodium chloride.
Calcium Chloride Dihydrate (Sigma or Fluka) used for the experiments were
reconstituted in Milli-Q water to make a stock solution, which was
subsequently
supplemented to the production basal media. After addition of calcium
chloride, media was
brought to a pH similar to non-supplemented (control) media using 6N
hydrochloric acid/5N
NaOH, and it was brought to an osmolality similar to non-supplemented
(control) media by
adjusting the concentration of sodium chloride.
Niacinamide (Sigma or Calbiochem) used for the experiments were reconstituted
in
Milli-Q water to make a stock solution. which was subsequently supplemented to
the
production basal media. After addition of niacinamide, media was brought to a
pH similar to
non-supplemented (control) media using 6N hydrochloric acid/5N NaOH, and it
was brought
to an osmolality similar to non-supplemented (control) media by adjusting the
concentration
of sodium chloride.
All media was filtered through Corning IL filter systems (0.22 pm PES) and
stored at
4 C until usage.
131
CA 02926384 2016-04-07
Table 3: List of medium additives supplemented to culture media
Medium Catalog No./Source
additive of medium
supplements
Arginine Sigma, A8094
Lysine Calbiochem, 4400
Histidine Sigma, H5659
Ornithine Sigma, 06503
Calcium Fulka, 21097
Chloride Sigma, C8106
Niacinamide Calbiochem, 481907
Sigma, N0636
Production cultures
Production cultures were initiated either in 500 ml shake flasks (Corning) or
in 3L
Bioreactors (Applikon). For shake flask experiments, duplicate 500 mL Corning
vented non-
baffled shake flasks (200 mL working volume) were used for each condition. The
shake
flasks were kept in incubators either maintained at 35 C or 36 C and 5% CO,
on shaker
platforms that were either set at 110 rpm for adalimumab producing cell line
1, 180 rpm for
adalimumab producing cell line 2, 140 rpm for adalimumab producing cell line
3, for 130
rpm for mAb I producing cell line, or 140 rpm for mAb2 producing cell line.
For the
bioreactor experiments, 3L bioreactors (1.5L working volume) were run at 35
C, 30%
dissolved oxygen (DO), 200 rpm, pH profile from 7.1 to 6.9 in three days and
pH 6.9
thereafter. In all experiments, the cells were transferred from the seed train
to the production
stage at a split ratio of 1:5.
Cultures were run in either batch or fed-batch mode. In the batch mode, cells
were
cultured in the respective production medium. 1.25% (v/v) of 40% glucose stock
solution was
fed when the media glucose concentration reduced to less than 3 g/L. In the
fed-batch mode,
cultures were run with either the IVGN feed (proprietary chemically defined
feed formulation
from Invitrogen) as per the following feed schedule - (4% (v/v) - day 6, day
7, and day 8,
respectively) along with 10X Ex-Cell PFCHO feed (proprietary chemically
defined
132
CA 02926384 2016-04-07
formulation) ¨ 3% (v/v) on day 3. The cultures were also fed with 1.25% (v/v)
of 40%
glucose stock solution when the glucose concentration was below 3.0 2/L.
Retention samples for titer analysis, of 2 x 1.5 mL, were collected daily for
the
bioreactor experiments beginning on Day 8, and frozen at -80 C. The samples
taken from
each were later submitted for titer analysis.
The harvest procedure of the shake flasks and reactors involved centrifugation
of the
culture sample at 3,000 RPM for 30 min and storage of supernatant in PETG
bottles at -80 C
before submission for Protein A purification and WCX-10 analysis.
WCX-10 Assay
This method is employed towards the quantification of the acidic species and
other
variants present in cell culture harvest samples. Cation exchange
chromatography was
performed on a Dionex ProPac WCX-10, Analytical column (Dionex, CA).
For adalimumab and mAb 1 samples, the mobile phases used were 10mM Sodium
Phosphate dibasic pH 7.5 (Mobile phase A) and 10mM Sodium Phosphate dibasic,
500 mM
Sodium Chloride pH 5.5 (Mobile phase B). A binary gradient (94% A, 6% B: 0-20
min; 84%
A, 16% B: 20-22 min; 0% A. 100%B: 22-28 min; 94% A, 6% B: 28-34 min) was used
with
detection at 280 nm.
For mAb2 samples, the mobile phases used were 20 mM (4-
Morpholino)ethanesulfonic Acid Monohydrate (MES) pH 6.5 (Mobile phase A) and
20 mM
MES, 500 mM Sodium Chloride pH 6.5 (Mobile phase B). An optimized gradient
(minute/%B): 0/3. 1/3. 46/21. 47/100, 52/100, 53/3, 58/3 was used with
detection at 280 nm.
Quantitation is based on the relative area percent of detected peaks. The
peaks that
elute at relative residence time earlier than the main peak corresponding to
the drug product
are together represented as the acidic peaks (Figure 1).
Lysine-C peptide mapping for methylglyoxal (MGO) quantification
Typical trypsin digestion employed almost universally for peptide mapping
cleaves a
denatured, reduced and alkylated protein at the carboxyl side of the two basic
amino acids,
lysine and arginine. Methylglyoxal (MGO) is a small molecule metabolite
derived as a
133
CA 02926384 2016-04-07
glycolysis byproduct which can modify arginine residues. A modification of an
arginine
prevents trypsin from cutting this site and results in a mis-cleavage. The
challenge of
quantifying the amount of MGO modified peptide is that it is not compared to
an equivalent
non-modified peptide but rather two parental cleaved peptides which will
likely have
different ionization potential than the modified peptide. In order to
determine a truly accurate
direct measurement of an MGO-modified peptide, it must be compared to its non-
modified
counterpart and expressed as a percent. Using endoproteinase lysine-C as an
alternative
enzyme, cleavages only occur at lysine residues. The result is a direct
comparison of the
same peptide with and without an MGO modification which provides a high degree
of
accuracy in quantifying even trace levels of the modified species.
Procedure: Samples were diluted to a nominal concentration of 4 mg/mL. 8 M
guanidine-HC1 was added to the sample in a 3:1 ratio resulting in a 1 mg/mL
concentration in
6M guanidine-HC1. The samples were reduced with 10 mM final conc. DTT for 30
minutes
at 37 C followed by an alkylation with 25mM final concentration iodoacetic
acid for 30
minutes at 37 C. in the dark. The samples were then buffer exchanged into 10
mM Tris pH
8.0 using NAP-5 columns. The samples were then digested for 4 hours at 37 C
using
endoproteinase Lys-C at an enzyme to protein ratio of 1:20. The digest was
quenched by
adding 5 !,,t1_, of formic acid to each sample. Samples ere analyzed by LC/MS
peptide
mapping. Briefly, 50 dlL of sample was loaded onto a Waters BEH C18 1.7 1.0 x
150 mm
UPLC column with 98% 0.08% formic acid, 0.02% TFA in water and 2% 0.08% formic
acid,
0.02% TFA in acetonitrile. The composition was changed to 65% 0.08% formic
acid, 0.02%
TFA in water and 35% 0.08% formic acid, 0.02% TFA in acetonitrile in 135
minutes using a
Waters Acquity UPLC system. Eluting peaks were monitored using a Thermo
Scientific
LTQ-Orbitrap Mass Spectrometer. Specific mass traces were extracted for both
modified and
non-modified peptides in order to accurately quantify the total amount of MGO
modification
at each site. Mass spectra were also analyzed for the specific region of the
chromatogram to
confirm the peptide identity. An example data set is shown in Figure 162.
Results
Effect of arginine supplementation to cell culture media
The addition of arginine was tested in several experimental systems covering
multiple
cell lines, media and monoclonal antibodies. The following is a detailed
description of two
134
CA 02926384 2016-04-07
representative experiments where two different adalimumab producing cell lines
(cell line 2
and cell line 3) were cultured in a chemically defined media (media I).
Cell line 2 was cultured in media 1 with different total amounts of arginine
(I
(control), 1.25, 1.5, 2, 3, 5, 9 g/L). The cultures were performed in shake
flasks in batch
format with only glucose feed as described in the materials and methods. The
cells grew to
maximum viable cell densities (VCD) in the range of 18-22 x 106 cells/ml for
the different
conditions tested. The growth and viability profiles were comparable between
the different
test conditions, although a slight decrease in viable cell density profile was
observed in
samples with the 9 g/L arginine test condition (Figures 1 and 2). The harvest
titers were
comparable between the conditions (Figure 3). On Day 10 and Day 12 of culture,
duplicate
shake flasks for each of the conditions were harvested and then subsequently
analyzed using
WCX-10 post Protein A purification and the percentages of total peak(s) area
con-esponding
to the acidic species were quantified (Figures 4 and 5). The percentage of
acidic species in
the control sample was as high as 19.7% on day 10. In the sample with the
highest total
concentration of arginine in this experiment (9 g/L), the percentage of acidic
species was
reduced to 12.2%. A dose dependent decrease in acidic species was observed in
test
conditions with arginine concentrations beyond 2 g/L (Figure 4). A similar
trend in reduction
of acidic species with arginine increase was also observed in the day 12
harvest samples
(Figure 5). Further, while the extent of acidic species in the 1g/L arginine
samples increased
from 19.7 % (day 10 harvest) to 25.5% (day 12 harvest), this increase in the
9g/L arginine
test condition was significantly smaller from 12.2% (day 10 harvest) to 13.9%
(day 12
harvest). Thus, the increase of total arginine led to a reduction in the
extent of total acidic
species at a particular time point in culture as well the rate of increase of
acidic species with
time of culture.
Cell line 3 was cultured in media 1 with different total amounts of arginine
(I
(control), 3, 5, 7, 9 g/L). The cultures were performed in shake flasks in
batch format with
only glucose feed as described in the materials and methods. The cells grew to
maximum
VCD in the range of 7-10 x 106 cells/ml for the different conditions tested.
The growth and
viability profiles were comparable between the different test conditions,
although a slight
decrease in viable cell density and viability profiles was observed in samples
with the 9 g/L
arginine condition (Figures 6 and 7). The product titer was also comparable
between all
conditions (Figure 8). On Day 10 of culture, duplicate shake flasks for each
of the conditions
135
CA 02926384 2016-04-07
were harvested and then subsequently analyzed using WCX-10 post Protein A
purification
and the percentages of total peak(s) area corresponding to the acidic species
were quantified
(Figure 9). The percentage of acidic species in the control sample was as high
as 23.3% on
day 10. In the sample with the highest total concentration of arginine in this
experiment (9
g/L), the percentage of acidic species was reduced to 17.0%. A dose dependent
decrease in
acidic species was observed in conditions with higher concentrations of
arginine.
Additional experiments were performed with multiple cell lines in chemically
defined
or hydrolysate based media to demonstrate the wide range of applicability of
this method.
The experimental setup for each of these experiments was similar to that
described above.
The summaries of results of the different experiments performed for adalimumab
are
summarized in Figures 10, 11, and 12. A reduction in acidic species with
increased arginine
concentration was also observed in each case.
In addition to adalitnumab, the utility of this method for acidic species
reduction was
also demonstrated for processes involving two other mAb producing cell lines
(cell lines
producing mAb I and mAb2). The experimental setup for each of these
experiments was
similar to that described in section above and in the materials and methods.
The reduction of
acidic species with increased arginine concentration for experiments
corresponding to each
inAb is summarized in Figures 13 and 14. For mAb2, a significant reduction in
acidic species
was observed at arginine concentration of 9 g/L.
In Attorney Docket No. 117813-74101 ,
we describe the utility of arginine supplementation to culture media towards
modulation of the lysine variant distribution. It is possible that a fraction
of acidic species
also shifted along with shift in lysine variants (from Lys 0 to Lysl and
Lys2), in addition to
the fraction of acidic species that is completely removed from the entire
protein population.
To estimate the acidic species reduction that is independent of this
redistribution of lysine
variants, Protein A eluate samples from a representative set of arginine
supplementation
experiments were pre-treated with the enzyme carboxypeptidase before WCX-10.
One set of
samples from adalimuinab experiment and another set of samples from a mAb2
experiment
were used for this analysis. The carboxypeptidase treatment of the samples
resulted in the
cleavage of the C-terminal lysine residues as demonstrated by the complete
conversion of
Lys 1/Lys2 to Lys 0 in each of these samples (data not shown here). As a
result of this
conversion, the acidic species quantified in these samples corresponded to an
aggregate sum
136
CA 02926384 2016-04-07
of acidic species that would be expected to also include those species that
may have
previously shifted corresponding to the lysine variant shift and perhaps gone
unaccounted for
in the samples that were not treated with carboxypeptidase prior to WCX-10. A
dose
dependent reduction in acidic species was observed in the carboxypeptidase
treated samples
with increasing concentration arginine (Figures 15 and 16). This suggests that
the acidic
species reduction described here is not completely attributed to a probable
shift of the acidic
species corresponding to the lysine variant redistribution.
Effect of lysine supplementation to cell culture media
The addition of lysine was tested in several experimental systems covering
multiple
cell lines, media and monoclonal antibodies. The following is a detailed
description of two
representative experiments where two different cell lines (cell line 2 and
cell line 3) were
cultured in a chemically defined media (media 1) for the production of
adalimumab.
Cell line 2 was cultured in media 1 with different total concentrations of
lysine (1
(control), 5, 7, 9, 11 g/L). The cultures were performed in shake flasks in
batch fon-nat with
only glucose feed as described in the materials and methods. The cells grew to
maximum
viable cell densities (VCD) in the range of 17-23 x 106 cells/ml for the
different conditions
tested. A slight dose dependent decrease in viable cell density profile was
observed in all
samples with respect to the control sample (Figure 17). The viability profiles
were
comparable between the conditions (Figure 18). On Days 10 and 11 of culture
samples were
collected for titer analysis (Figure 19). The titers for all conditions were
comparable. On Day
11 of culture, duplicate shake flasks for each of the conditions were
harvested and then
subsequently analyzed using WCX-10 post Protein A purification and the
percentages of total
peak(s) area corresponding to the acidic species were quantified (Figure 20).
The percentage
of acidic species in the control was as high as 26.5%. In the sample with the
highest tested
concentration of lysine in this experiment (11 g/L), the percentage of acidic
species was
reduced to 15.0%. A dose dependent decrease in acidic species was observed in
test
conditions with higher total concentrations of lysine.
Cell line 3 was cultured in media 1 with different total concentrations of
lysine (1
(control), 3, 5, 7, 9, l 1 g/L). The cultures were performed in shake flasks
in batch fonnat with
only glucose feed as described in the materials and methods. The cells grew to
maximum
VCD in the range of 9.5-11.5 x 106 cells/ml for the different conditions
tested. The growth
137
CA 02926384 2016-04-07
and viability profiles were comparable between the different test conditions,
although a slight
decrease in viable cell density and viability profiles was observed in samples
with higher
lysine concentrations than that in the control sample (Figures 21 and 22). On
Days 10, 11 and
12 of culture samples were collected for titer analysis (Figure 23). The
titers for all conditions
were comparable. On Day 12 of culture, duplicate shake flasks for each of the
conditions
were harvested and then subsequently analyzed using WCX-10 post Protein A
purification
and the percentages of total peak(s) area comsponding to the acidic species
were quantified
(Figure 24). The percentage of acidic species in the control sample was as
high as 26.6%. In
the sample with the highest tested concentration of lysine in this experiment
(11 g/L) the
percentage of acidic species was reduced to 18.1%. A dose dependent decrease
in acidic
species was observed in test conditions with higher total concentrations of
lysine.
Additional experiments were performed with multiple cell lines in chemically
defined
or hydrolysate based media to demonstrate the wide range of applicability of
this method.
The experimental setup for each of these experiments was similar to that
described above and
in materials and methods section. The summaries of results of the different
experiments
performed for adalimumab are summarized in Figures 25, 26, and 27. A reduction
in acidic
species with increased lysine concentration was also observed in each case.
In addition to adalimumab, the utility of this method for acidic species
reduction was
also demonstrated for processes involving two other mAbs. The experimental
setup for each
of these experiments was similar to that described above and in the materials
and methods
section. The reduction of acidic species with lysine addition for experitnents
corresponding to
each triAb is suinmarized in Figures 28, 29. For mAb2, a significant reduction
in acidic
species was observed at lysine concentration of 11 g/L.
In Attorney Docket No. 117813-74101 ,
the utility of lysine supplementation to culture media for the modulation of
the
lysine variant distribution is described. To estimate the acidic species
reduction that is
independent of this redistribution of lysine variants, Protein A eluate
samples from a
representative set of lysine supplementation experiments were pre-treated with
the enzyme
carboxypeptidase before WCX-10. One set of samples from an adalimumab
experiment and
another set of samples from a mAb2 experiment were used for this analysis. The
carboxypeptidase treatment of the samples resulted in the cleavage of the C-
terminal lysine
residues as demonstrated by the conversion of Lysl/Lys2 to Lys 0 in each of
these samples.
138
CA 02926384 2016-04-07
As a result of this conversion, the acidic species quantified in these samples
corresponded to
an aggregate sum of acidic species that would be expected to also include
those species that
may have previously shifted corresponding to the lysine variant shift and
perhaps gone
unaccounted for in the samples that were not treated with carboxypeptidase
prior to WCX-10.
A dose dependent reduction in acidic species was observed in the
carboxypeptidase treated
samples with increasing concentration of lysine for the adalimumab samples
from 26.8% in
the non-supplemented sample to 21.1% in the 10 g/L lysine supplemented sample,
a
reduction of 5.7% in total acidic species (Figure 30). Similar results were
also observed for
the mA2 samples (Figure 31). This suggests that the acidic species reduction
described here
is not completely attributed to a probable shift of the acidic species
corresponding to the
lysine redistribution.
Effect of histidine supplementation to cell culture media
The addition of histidine was tested in several experimental systems covering
multiple
cell lines, media and monoclonal antibodies. The following is a detailed
description of two
representative experiments where two different cell lines (cell line 2 and
cell line 3) were
cultured in a chemically defined media (media 1) for the production of
adalimumab.
Cell line 2 was cultured in media 1 with different total concentrations of
histidine (0
(control), 4, 6, 8, 10 g/L). The cultures were performed in shake flasks in
batch format with
only glucose feed as described in the materials and methods. The cells grew to
maximum
VCD in the range of 12-22 x 106 cells/int for the different conditions tested.
A dose
dependent decrease in viable cell density profile was observed with the 10g/L
histidine
condition having significant reduction in growth (Figure 32). A corresponding
effect on
viability was also observed (Figure 33). On Days 10, 11 and 12 of culture
samples were
collected for titer analysis and reported for the harvest day for each sample
(Figure 34). There
was a small dose dependent decrease in titers for conditions with histidine
supplementation.
On Days 11-12, duplicate shake flasks were harvested and then subsequently
analyzed using
WCX-10 post Protein A purification and the percentages of total peak(s) area
corresponding
to the acidic species were quantified (Figure 35). The percentage of acidic
species in the
control sample was as high as 26.5%. In the sample with the highest tested
concentration of
histidine in this experiment (10 g/L), the percentage of acidic species was
reduced to 15.6%.
A dose dependent decrease in acidic species was observed in test conditions
with increased
histidine concentrations.
I 39
CA 02926384 2016-04-07
Cell line 3 was cultured in media 1 with different total concentrations of
histidine (0
(control), 2, 4, 6, 8 g/L). The cultures were performed in shake flasks in
batch format with
only glucose feed as described in the materials and methods. The cells grew to
maximum
viable cell densities (VCD) in the range of 6-10 x 106 cells/ml for the
different conditions
tested. A dose dependent decrease in viable cell density profile was observed
in all conditions
with histidine concentrations higher than that in the control (Figure 36). The
viability profiles
were more comparable between conditions with this cell line (Figure 37). On
Day 12 of
culture, samples were collected for titer analysis (Figure 38). The titers for
all conditions
were comparable. On Day 12 of culture, duplicate shake flasks for each of the
conditions
were harvested and then subsequently analyzed using WCX-10 post Protein A
purification
and the percentages of total peak(s) area corresponding to the acidic species
were quantified
(Figure 39). The percentage of acidic species in the control sample was 26.2%.
In the sample
with the highest tested concentration of histidine in this experiment (8 g/L),
the percentage of
acidic species was reduced to 20.0%. A dose dependent decrease in acidic
species was
observed in test conditions with increased histidine concentration.
Additional experiments were performed with multiple cell lines in chemically
defined
or hydrolysate based media to evaluate the wide range of applicability of this
method. The
experimental setup for each of these experiments was similar to that described
above and in
the materials and methods section. The summaries of results of the different
experiments
performed for adalimumab are set forth in Figures 40, 41, and 42. A reduction
in acidic
species with increased histidine concentration was observed with cell line 1
in media 1
(Figure 40) and with cell line 2 in media 3 (Figure 42). For cell line 2 in
media 3, a dose
dependent reduction in acidic species was observed up to 4 g/L histidine, with
no further
significant reduction at higher concentrations of histidine (Figure 42). For
cell line 1, media
2, no significant reduction of acidic species was observed within the
histidine concentration
range (0-4 g/L) (Figure 41). In
addition to adalimumab, the utility of this
method for acidic species reduction was also demonstrated for processes
involving two other
mAbs. The experimental setup for each of these experiments was similar to that
described
above and in the materials and methods section. The reduction of acidic
species with
increased histidine concentration for experiments corresponding to each mAb is
summarized
in Figures 43 and 44. For mAb2, in contrast with the results reported with
arginine and lysine
supplementation shown previously, a clear significant dose dependent reduction
in total
acidic species from 28.1% in the control to 21.5% in 4 g/L histidine sample
was observed.
140
CA 02926384 2016-04-07
In Attorney Docket No. 117813-74101 ,
the utility of increased histidine to culture media towards modulation of the
lysine variant distribution is described. To estimate the acidic species
reduction that is
independent of this redistribution of lysine variants, Protein A eluate
samples from a
representative set of histidine supplementation experiments were also pre-
treated with the
enzyme carboxypeptidase before WCX-10. One set of samples from adalimumab
experiment
and another set of samples from a mAb2 experiment were used for this analysis.
The
carboxypeptidase treatment of the samples resulted in the cleavage of the C-
terminal lysine
residues as demonstrated by the complete conversion of Lysl/Lys2 to Lys 0 in
each of these
samples (data not shown here). A dose dependent reduction in acidic species
was observed in
the carboxypeptidase treated samples with increasing concentration of
histidine (Figures 45
and 46). This indicates that the acidic species reduction described here is
not completely
attributed to a probable shift of the acidic species corresponding to the
lysine redistribution.
Effect of ornithine supplementation to cell culture media
The addition of ornithine was tested in several experimental systems covering
multiple cell lines, media and monoclonal antibodies. The following is a
detailed description
of two representative experiments where two different cell lines (cell line 2
and cell line 3)
were employed in a chemically defined media (media 1) for the production of
adalimurnab.
Cell line 2 was cultured in media 1 with different total concentrations of
ornithine (0
(control), 4, 6, 8, 10 g/L). The cultures were performed in shake flasks in
batch format with
only glucose feed as described in the materials and methods. The cells grew to
maximum
VCD in the range of 15-22 x 106 cells/ml for the different conditions tested.
A slight decrease
in viable cell density with ornithine supplementation was observed (Figure
47).
Corresponding differences in the viability profiles were also observed (Figure
48). On Day 11
of culture, samples were collected for titer analysis (Figure 49). The titers
for all conditions
were comparable. On Day 11, duplicate shake flasks were harvested for each
condition and
then subsequently analyzed using WCX-10 post Protein A purification and the
percentages of
total peak(s) area corresponding to the acidic species were quantified (Figure
50). The
percentage of acidic species in the control sample was 26.5%. In the sample
with the highest
tested concentration of ornithine in this experiment (10 g/L), the percentage
of acidic species
was reduced to 16.1%. A dose dependent decrease in acidic species was observed
in test
conditions with increased ornithine concentration.
141
CA 02926384 2016-04-07
Cell line 3 was cultured in media 1 supplemented with different total
concentrations
of ornithine (0 (control), 2, 4, 6, 8 g/L). The cultures were performed in
shake flasks in batch
format with only glucose feed as described in the materials and methods. The
cells grew to
maximum viable cell densities (VCD) in the range of 9.5-11.5 x 106 cells/ml
for the different
conditions tested. The viable cell density and viability profiles were
comparable (Figures 51
and 52). On Day 12 of culture, samples were collected for titer analysis
(Figure 53). The
titers for all conditions were comparable. On Day 12 of culture, duplicate
shake flasks for
each of the conditions were harvested and then subsequently analyzed using WCX-
10 post
Protein A purification and the percentages of total peak(s) area corresponding
to the acidic
species were quantified (Figure 54). The percentage of acidic species in the
control sample
was 24.8%. In the sample with the highest tested concentration of ornithine in
this experiment
(8 g/L), the percentage of acidic species was reduced to 20.5%. A dose
dependent decrease in
acidic species was observed in test conditions with increased ornithine
concentration.
Additional experiments were performed with multiple cell lines in chemically
defined
or hydrolysate based media to evaluate the wide range of applicability of this
method. The
experimental setup for each of these experiments was similar to that described
above and in
the materials and methods section. The summaries of results of the different
experiments
performed for adalimumab are summarized in Figures 55, 56 and 57. For cell
line 1 in media
1, a dose dependent reduction was observed (Figure 55). However, for cell line
1 in media 2,
a hydrolysate media, no significant reduction in acidic species was observed
across the
conditions (Figure 56). For cell line 2 in media 3, a reduction in acidic
species from 22.1% in
the control sample to 18.7% in the 2 g/L ornithine sample with no further
reduction at higher
ornithine concentrations was observed (Figure 57).
In addition to adalimumab, the utility of this method for acidic species
reduction was
also demonstrated for processes involving two other mAbs. The experimental
setup for each
of these experiments was similar to that described in the section above and in
the materials
and method section. The reduction of acidic species with ornithine addition
for experiments
corresponding to each mAb is summarized in Figures 58 and 59. In the case of
mAbl, a 7.3%
dose dependent reduction in total acidic species was observed within the
concentration range
tested. For mAb2, about 2% reduction was observed in the 1 g/L ornithine
concentration
sample with minimum further reduction at higher ornithine concentrations.
142
CA 02926384 2016-04-07
Similar to the analysis conducted with the other amino acids, Protein A eluate
samples from a representative set of ornithine experiments were also pre-
treated with the
enzyme carboxypeptidase before WCX-10. One set of samples from adalimumab
experiment
and another set of samples from a mAb2 experiment were used for this analysis.
A dose
dependent reduction in acidic species was observed in the carboxypeptidase
treated samples
with increasing concentration of omithine (Figures 60 and 61). The percentage
of acidic
species was also comparable between an untreated and a carboxypeptidase
treated sample for
a particular concentration of ornithine. This indicates that the acidic
species reduction is
independent of any probable shift of the acidic species that may be
corresponding to any
lysine redistribution.
Effect of increasing a combination of arginine, lysine, histidine, ornithine
to cell
culture media
In this experiment, the combined use of the four amino acids arginine, lysine,
histidine and ornithine for acidic species reduction is demonstrated. The
experiment
described here was performed using adalimumab producing cell line 2 in
chemically defined
media (media 1). The concentration range for arginine and lysine in this
experiment was 1-
3g/L while the concentration range for histidine and ornithine in this
experiment was between
0-2 g/L. In comparison to the lower concentrations, or conditions where a
single amino acid
concentration was increased, a further reduction in total acidic species was
observed in
conditions where combinations of amino acids were increased in the media
(Figure 62). A
progressive decrease was observed in total acidic species when more amino
acids were
increased in combination. The percentage of acidic species was reduced from
21.9% in the
lowest concentration sample to 12.3% in the sample with high concentrations of
all four
amino acids.
Control of acidic species through cell culture with increased arginine and
lysine
and choice of harvest criterion and/or modulation of pH
The increase of the amino acid (arginine, lysine) concentration in basal media
may
also be combined with choice of when to harvest a culture to achieve optimal
reduction in
total acidic species. In this example, a study was carried out in 3L
bioreactors with cell line 1
(producing adalimumab) in media I. Two sets of conditions were tested: control
condition
(arginine 1g/L, lysine 1g/L); Test condition 1 (arginine 3g/L, lysine 5g/L).
Cell growth,
143
CA 02926384 2016-04-07
viability and titer profiles were comparable between the conditions (Figures
63, 64, and 65).
A small amount of cell culture harvests were collected every day from day 4 to
day 10 from
each of the reactors and submitted for Protein A purification and WCX-10
analysis. The
percentage of acidic species in the control condition increased from 12.1% (on
day 4) to
24.6% (on day10) (Figure 66). The percentage of acidic species in the test
condition 1 was
lower than that observed in the control condition at each corresponding
culture day. The
percentage of acidic species in the test condition also increased from 8.7%
(day 4) to 18.8%
(day 10). The rate of increase in acidic species with culture duration also
correlated with the
drop in viability for both conditions, with a sharp increase on day 8. Thus,
along with
increasing arginine and lysine concentrations in culture media, choice of
harvest day/harvest
viability can be used in combination to achieve a desired acidic species
reduction.
The increase of the amino acid (arginine, lysine) concentration in basal media
may be
combined with process pH modulation to achieve further reduction in total
acidic species. In
this example, a study was carried out in 3L bioreactors with cell line 1
(producing
adalimumab) in media 1. Three sets of conditions were tested in duplicates:
Control condition
(arginine (lg/L), lysine (1g/L), pH 7.1->6.9 in 3 days, pH 6.9 thereafter);
Test condition 1
(arginine (3g/L), lysine (3g/L), pH 7.1->6.9 in 3 days, pH 6.9 thereafter);
Test condition 2
(arginine (3g/L), lysine (3g/L), pH 7.1->6.8 in 3 days, pH 6.8 thereafter). In
comparison to
the control, a slight decrease in VCD profile and harvest titer was observed
for condition 2
(Figures 67, 68, and 69). The cultures were harvested when the viability was
less than 50%
and the culture harvests were submitted for Protein A and WCX-10 analysis. The
percentage
of acidic species in the control sample was 19.1%. The percentage of acidic
species was
reduced to 14.3% in test condition 1 and to 12.8% in test condition 2 (Figure
70). Thus, this
demonstrates that the increase of amino acid concentration along with choice
of lower final
process pH can be used in combination for further reducing the extent of
acidic species.
Effect of supplementation of CaC12 to cell culture media
The addition of calcium chloride was tested in several experimental systems
covering
multiple cell lines, media and monoclonal antibodies. Following is a detailed
description of
two representative experiments where two different cell lines (cell line 2 and
cell line 3) were
cultured in a chemically defined media (media 1) for the production of
adalimurnab.
144
CA 02926384 2016-04-07
Cell line 2 was cultured in media 1 with different concentrations of calcium
(0.14,
0.84 and 1.54 mM). The cultures were peiforrned in shake flasks in batch
format with only
glucose feed as described in the materials and methods. The cells grew to
maximum viable
cell densities (VCD) in the range of 22-24.5 x 106 cells/ml for the different
conditions tested.
The viable cell density and viability profiles for all test conditions were
comparable (Figures
71 and 72). On Day 10 of culture samples were collected for titer analysis
(Figure 73). The
titers for all conditions were comparable. On Day 10 duplicate shake flasks
were harvested
for each condition and then subsequently analyzed using WCX-10 post Protein A
purification
and the percentages of total peak(s) area corresponding to the acidic species
were quantified
(Figure 74). The percentage of acidic species in the 0.14mM calcium condition
was 23.8%. In
the sample with the highest tested concentration of calcium in this experiment
(1.54mM), the
percentage of acidic species was reduced to 21.6%. A dose dependent decrease
in acidic
species was observed in test conditions with increased calcium concentration.
Cell line 3 was cultured in media 1 with different total concentrations of
calcium
(0.14, 0.49, 0.84, 1.19, 1.54, 1.89 g/L). The cultures were performed in shake
flasks in batch
format with only glucose feed as described in the materials and methods. The
cells grew to
maximum viable cell densities (VCD) in the range of 9.5-10.5 x 106 cells/nil
for the different
conditions tested. The viable cell density and viability profiles for all test
conditions were
comparable (Figures 75 and 76). On Day 11 of culture, samples were collected
for titer
analysis. The harvest titers for all conditions were comparable (Figure 77).
On Day 11 of
culture, duplicate shake flasks for each of the conditions were harvested and
then
subsequently analyzed using WCX-10 post Protein A purification and the
percentages of total
peak(s) area corresponding to the acidic species were quantified (Figure 78).
The percentage
of acidic species in the 0.14mM calcium condition was 23.7%. In the sample
with the highest
tested concentration of calcium in this experiment (1.89mM), the percentage of
acidic species
was reduced to 20.7%. A dose dependent decrease in acidic species was observed
in test
conditions with increased calcium concentration.
Additional experiments were performed with multiple cell lines in chemically
defined
or hydrolvsate based media to evaluate the wide range of applicability of this
method. The
experimental setup for each of these experiments was similar to that described
in the section
above and in the materials and methods section. The summaries of results of
the different
experiments performed for adalimumab are summarized in Figures 79, 80 and 81.
A
1 45
CA 02926384 2016-04-07
reduction in acidic species with increased calcium concentration was also
observed in each
case.
In addition to adalimumab, the utility of this method for acidic species
reduction was
also demonstrated for processes involving two other mAbs. The experimental
setup for each
of these experiments was similar to that described above. The dose dependent
reduction of
acidic species with ornithine addition for experiments corresponding to each
mAb is
summarized in Figures 82 and 83. For mAbl, a small yet significant acidic
species reduction
from 15.4% (0.14mM calcium sample) to 11.8% (1.54 mM calcium chloride
supplemented
sample) was observed. For mAb2, a larger dose dependent reduction from 28.9%
(0.14mM
calcium sample) to 23.1% (1.40 mM calcium chloride supplemented sample) was
observed.
Effect of increased concentration of arginine, lysine, calcium chloride,
niacinamide in combination
In this experiment, the effect of the combined use of the amino acids
arginine, lysine,
inorganic salt calcium chloride and vitamin niacinamide for acidic species
reduction was
evaluated. The experiment described here was performed using cell line 2
(producing
adalimumab) in chemically defined media (media 1) supplemented with 3% (v/v)
PFCHO
(proprietary chemically defined medium formulation from SAFC). A central
composite DOE
experimental design was used in this experiment. The basal media for each
condition was
supplemented with different concentrations of the four supplements. Cell
cultures were
carried out in duplicates for each condition. Upon harvest, WCX-10 analysis
was perfomied
post Protein A purification. In Table 4, below, the experimental conditions
from DOE design,
including the concentration of each component supplemented, and the % total
acidic species
(or AR) obtained for each condition is summarized. Reduction of acidic species
through the
increased concentration of these components in combination was observed. For
instance,
condition (#24), where all four components were at their maximum
concentration, the % total
AR was reported to be reduced to 9.7%. Using the data from the experiment, a
model
predicting the effects of addition of these components to media for AR
reduction (R2: 0.92,
P<0.0001 ) is described in Figure 84. The model predicted a contribution from
each of the
four components towards acidic species reduction. It may be also possible to
utilize this
model to predict the choice of concentrations of these different components to
the media, in
order to achieve a target reduction in total AR.
146
CA 02926384 2016-04-07
Table 4: Experimental design and summary for the combined addition of
arginine,
lysine, calcium chloride and niacinamide
Conditions Arginine Lysine Calcium Niacinamide %Total
(WI) (g/I) Chloride (mM) AR
(mM)
1 0.0 4.0 0.7 0.8 13.0
2 0.0 6.0 1.4 0.0 12.6
3 4.0 2.0 0 1.6 12.3
4 4.0 6.0 0 1.6 11.6
5 2.0 4.0 0.7 0.8 11.2
6 0.0 6.0 0 0.0 15.0
7 0.0 6.0 1.4 1.6 10.7
8 0.0 2.0 0 0.0 16.7
9 2.0 4.0 0.7 0.8 11.0
10 4.0 6.0 1.4 1.6 11.0
11 2.0 2.0 0.7 0.8 12.9
12 2.0 4.0 1.4 0.8 11.1
13 0.0 6.0 , 0 1.6 13.2
..
214 -4.0 _. -.0 o 0.0 12.3.
15 2.0 4.0 0.7 0.0 13.0
16 2.0 4.0 0.7 1.6 11.4
17 0.0 2.0 1.4 1.6 12.0
18 2.0 4.0 0 0.8 12.0
19 4.0 4.0 0.7 0.8 12.0
20 0.0 2.0 1.4 0.0 14.0
21 4.0 6.0 1.4 0.0 11.0
22 0.0 2.0 0 1.6 13.6
23 2.0 6.0 0.7 0.8 11.0
24 4.0 2.0 1.4 1.6 9.7
25 4.0 6.0 0 0.0 11.8
26 4.0 2.0 1.4 0.0 10.4
27 2.0 4.0 0 0.0 12.7
Use of niacinamide supplementation to cell culture media for acidic species
reduction
In addition to the use of niacinamide in combination with other supplements
described
in the previous section, niacinamide addition may also be used independent of
the other
supplements as demonstrated in the experiments below for two mAbs: adalimumab
and
mAbl.
For the experiment corresponding to adalimumab, cell line I was cultured in
media 1
supplemented with different 'amounts of niacinamide (0, 0.2, 0.4, 0.8 and 1.6
mN4). The
cultures were perforined in shake flasks in batch format with only glucose
feed as described
147
CA 02926384 2016-04-07
in the materials and methods. The cells grew to maximum VCD in the range of
8.5-11 x 106
cells/ml for the different conditions tested. A slight decrease in the viable
cell density profile
was observed with the maximum niacinamide supplementation (1.6mM for this
experiment)
(Figure 85). The viability profile for the test conditions were comparable
(Figure 86). On Day
12 of culture, samples were collected for titer analysis. The titers for all
conditions were
comparable (Figure 87). On Day 11 and day 12, duplicate shake flasks were
harvested for
each condition and then subsequently analyzed using WCX-10 post Protein A
purification
and the percentages of total peak(s) area corresponding to the acidic species
were quantified
(Figures 88 and 89). The percentage of acidic species in the day 10 control
sample (without
niacinamide supplementation) was 19.6%. In the day 10 sample with the highest
tested
concentration of niacinamide in this experiment (1.6mM), the percentage of
acidic species
was reduced to 15.9%. Similar acidic species reduction with niacinamide
supplementation
was also observed in the day 12 samples.
For the experiment corresponding to mAb2, a mAb2 producing cell line was
cultured
in media 1 supplemented with different amounts of niacinamide (0, 0.1, 0.5,
1.0, 3.0 and 6.0
mM). The cultures were performed in shake flasks in batch format with only
glucose feed as
described in the materials and methods. The cells grew to maximum viable cell
densities
(VCD) in the range of 14-21.5 x 106 cells/ml for the different conditions
tested. A slight
decrease in the viable cell density profile was observed for the conditions
with 3.0 mM and
6.0 mM niacinamide concentrations (Figure 90). The viability profiles for all
test conditions
were comparable (Figure 91). On Day 12 of culture samples were collected for
titer analysis
(Figure 92). The titers for all conditions were comparable. On Day 12
duplicate shake flasks
were harvested for each condition and then subsequently analyzed using WCX-10
post
Protein A purification and the percentages of total peak(s) area corresponding
to the acidic
species were quantified (Figure 93). The percentage of acidic species in the
control sample
(without niacinamide supplementation) was 27.0%. In the sample with the
highest tested
concentration of niacinamide in this experiment (6.0mM), the percentage of
acidic species
was reduced to 19.8%. A dose dependent decrease in acidic species was observed
in test
conditions with niacinamide supplementation.
Supplementation of basic amino acids arginine and lysine to cell culture media
for reduction of metbylglyoxal (MGO) modification of antibody
148
CA 02926384 2016-04-07
In this experiment, the effect of MGO modification on acidic species reduction
was
examined. Adalimumab producing cell line 1 was cultured in a chemically
defined media
(media 1) which was supplemented with amino acids, as described below.
Materials and methods
Cell source and adaptation cultures
Cells were cultured in their respective growth media (chemically defined media
(media 1)) in a combination of vented non-baffled shake flasks (Corning) on a
shaker
platform at 110 RPM (cell line 1), and IOL or 20L wave bags (GE). Cultures
were propagated
in a 35 C, 5% CO2 incubator in order to obtain the required number of cells to
initiate
production stage cultures.
Cell culture media
For preparation of media 1, the media (IVGN GIA-1, a proprietary basal media
formulation from Invitrogen) was supplemented with L-glutamine, sodium
bicarbonate,
sodium chloride, and methotrexate solution. Production media consisted of all
the
components in the growth medium, excluding methotrexate. Both growth and
production
medium were also supplemented with insulin.
Amino acids used for the experiments (arginine (Sigma, A8094) and lysine
(Calbiochem, 4400)) were reconstituted in Milli-Q water to make a 100g/L stock
solution,
which was subsequently supplemented to both growth and production basal media.
After
addition of amino acids, media was brought to a pH similar to unsupplemented
(control)
media using 6N hydrochloric acid/5N NaOH, and it was brought to an osmolality
similar to
unsupplemented (control) media by adjusting the concentration of sodium
chloride.
149
CA 02926384 2016-04-07
All media was filtered through Corning IL filter systems (0.22 t.tm PES) and
stored at
4 C until usage.
Production cultures were initiated in 3L Bioreactors (Applikon). For the
bioreactor
experiments, 3L bioreactors (1.5L working volume) were run at 35 C, 30% DO,
200 rpm,
pH set-point of 7.1. The cells were transferred from the seed train to the
production stage at a
split ratio of 1:5.
Cultures were run in either batch mode and were cultured in the respective
production
medium (media I supplemented with arginine (4 g/L) or lysine (4 g/L)). 1.25%
(v/v) of 40%
glucose stock solution was fed when the media glucose concentration reduced to
less than 3
g/L.
Retention samples for titer analysis, of 2 x 1.5 rnL, were collected daily
beginning on
Day 8, and frozen at -80 C. The samples taken from each were later submitted
for titer
analysis.
The harvest procedure of the shake flasks and reactors involved centrifugation
of the
culture sample at 3,000 RPM for 30 min and storage of supernatant in PETG
bottles at -80 C
before submission for Protein A purification and WCX-10 analysis.
WCX-10 Assay
The WCX-10 assay method was employed as described above in the Materials and
Methods section.
Lysine-C peptide mapping for MGO quantification
The procedure for lysine-C peptide mapping for MGO quantification was carried
out
as described above in the Materials and Methods section.
Results and Discussion
The majority of cultures grew to a similar peak VCD in the range of 9-10 x 106
cells/mL (Figure 94A). The viability profiles of the cultures were also
comparable with
harvest viabilities between 10-25%. The culture duration (10 days) was similar
between the
conditions (Figure 94B).
150
CA 02926384 2016-04-07
Using WCX-10 analysis on harvest samples post Protein A purification, the
percentages of total peak(s) area corresponding to the acidic species were
quantified. The
percentage of acidic species in the control sample was 36.5%. In the samples
from cultures
supplemented with arginine and lysine, the percentage of total acidic species
was reduced to
20.1% and 28.0%, respectively (Figure 94C). Significant reduction in % AR1 was
also
observed in these cultures: from 16.8% in the control samples to 7.3%
(arginine
supplemented cultures) and 12.8% (lysine supplemented cultures) (Figure 94C).
The extent
of MGO modification was also quantified using the Lys-C peptide mapping and
reported as
the percentage of MGO modified peptides among those that are more susceptible
to MGO
modification. From these results, it is apparent that % MGO modification was
also
significantly reduced in the cultures supplemented with the amino acids
(Figure 94D).
Example 2: Method for reducing the extent of acidic species in cell culture by
adjusting process parameters
The experiments described below in the instant Example demonstrate that
altering cell
culture process parameters on-line can be used to modulate and/or reduce the
acidic species
of a protein of interest, e.g., the antibody adalimumab or mAb2. For example,
an increased
dissolved oxygen concentration and/or a decrease in final pH can lead to
reductions in AR.
Materials and Methods
Cell source and adaptation cultures
Two adalimumab producing CHO cell lines (cell line 1 and cell line 3) and a
mAb2
producing cell line were employed in the studies covered in this Example. Upon
thaw,
adalimumab producing cell line 3 was cultured in chemically defined growth
media (media 1)
in a combination of vented shake flasks on a shaker platform at 140 rpm and
20L wave bags.
Cultures were propagated in a 36 C, 5% CO) incubator to obtain the required
number of cells
to be able to initiate production stage cultures.
Upon thaw, adalimumab producing cell line 1 was cultured in a hydrolysate
based
growth media (media 2) in a combination of vented shake flasks on a shaker
platform at 110
rpm and 20L wavebags in a 35 C, 5% CO, incubator. In some cases, the culture
might be
151
CA 02926384 2016-04-07
transferred into a seed reactor with pH 7.1, 35 C and 30% DO. The culture
would be adapted
to either media 1 or media 2 by propagated in a IOL or 20L wavebag for 7 - 13
days with one
or two passages before initiating production stage cultures.
Upon thaw, mAb2 producing cells were cultured in media 1 in a combination of
vented non-baffled shake flasks (Corning) on a shaker platform at 140 RPM and
20L wave
bags (GE). Cultures were propagated in a 35 C, 5% CO, incubator to obtain the
required
number of cells to be able to initiate production stage cultures.
Cell culture media
Media 1, the chemical defined growth or production media, was prepared from
basal
IVGN CD media (proprietary formulation). For preparation of the IVGN CD media
formulation, the proprietary media was supplemented with L-glutamine, sodium
bicarbonate,
sodium chloride, and methotrexate solution. Production media consisted of all
the
components in the growth medium, excluding methotrexate. For cell line 1 and
mAb2, the
medium was also supplemented with insulin. In addition, 10mM or 5mM of
Galactose
(Sigma, G5388) and 0.21.IM or 10p.M of Manganese (Sigma, M1787) were
supplemented into
production medium for cell line 3 or 1, respectively. Osmolality was adjusted
by the
concentration of sodium chloride. All media was filtered through filter
systems (0.22 p.m
PES) and stored at 4 C until usage.
Media 2 is the hydrolysate based media, which contains basal proprietary
media,
Bacto TC Yeastolate and Phytone Peptone.
Production cultures
Production cultures were initiated in 3L Bioreactors (Applikon). The
bioreactors (1.5-
2.0L working volume) were run at the following conditions (except for the
different
experimental conditions): 35 C, 30% DO (dissolved oxygen), 200 rpm, pH profile
from 7.1 to
6.9 in three days and pH 6.9 thereafter. In all experiments, the cells were
transferred from the
wavebag to the production stage at a split ratio of 1:5.6 (except mAb2 with a
ratio of 1:5).
When the media glucose concentration reduced to less than 3 g/L, approximately
1.25% (y/v)
of 40% glucose stock solution was fed.
152
CA 02926384 2016-04-07
The harvest procedure of reactors involved centrifugation of the culture
sample at
3,000 RPM for 30 min and storage of supernatant in PETG bottles at -80 C
before
submission for Protein A purification and WCX-10 analysis.
WCX-10 Assay
The acidic species and other variants present in cell culture harvest samples
were
quantified. Cation exchange chromatography was performed on a Dionex ProPac
WCX-10,
Analytical column (Dionex, CA). For adalimumab producing cell lines, a
Shimadzu LC10A
HPLC system was used as the HPLC. The mobile phases used were 10mM Sodium
Phosphate dibasic pH 7.5 (Mobile phase A) and 10mM Sodium Phosphate dibasic,
500 mM
Sodium Chloride pH 5.5 (Mobile phase B). A binary gradient (94% A, 6% B: 0-20
min; 84%
A, 16% B: 20-22 min; 0% A, 100%B: 22-28 min; 94% A, 6% B: 28-34 min) was used
with
detection at 280 nm. The WCX-10 method used for mAb B used different buffers.
The
mobile phases used were 20 mM (4-Morpholino) ethanesulfonic Acid Monohydrate
(MES)
pH 6.5 (Mobile phase A) and 20 mM MES, 500 mM Sodium Chloride pH 6.5 (Mobile
phase
B). An optimized gradient (minute/%B): 0/3, 1/3, 46/21, 47/100, 52/100, 53/3,
58/3 was used
with detection at 280 nm.
Quantitation is based on the relative area percent of detected peaks. The
peaks that
elute at relative residence time earlier than the main peak corresponding to
the drug product
are together represented as the acidic peaks.
Results
Effect of process pH in media 1 with cell line 1
Five different pH conditions were assessed in this study: 7.1, 7.0, 6.9, 6.8
and 6.7. The
cultures were started at pH set point of 7.1; then were ramped down to the
target pH set
points within 4 days. All cultures reached the same maximum viable cell
density on day 8,
except for the culture at pH 6.7 condition, in which the maximum cell density
was much
lower than the other cultures (Figure 95). In addition, the viability of the
culture at pH 7.1 and
pH 7.0 dropped much earlier than the other cultures. The viability of cultures
at pH 7.1 and
pH 7.0 were 38% and 54% on day 10, respectively; while the viability of the
cultures at lower
pH (including pH 6.9. 6.8 and 6.7) was above 70% on the same day (Figure 96).
Samples
taken on the last day of the cultures were measured for IgG concentration. The
titer of each
153
CA 02926384 2016-04-07
tested condition increased corresponding to the decrease in pH, from 1.2 g/L
in the pH 7.1
condition to 1.8 g/L in the pH 6.8 condition; however, product titer was not
continued to
increase at pH 6.7 (1.6g/L) (Figure 97). The cultures were harvested either on
day 10 or on
day 12. The harvest was Protein A purified, then analyzed using WCX-10. The
resulting peak
areas from WCX-10 analysis were quantified (Figure 98). The percentage of
acidic species
decreased corresponding to the decrease in pH, from 56.0% in the pH 7.1
condition to 14.0%
in the pH 6.7 condition. Since the cultures at pH 6.9, 6.8 and 6.7 were at 70%
viability on
day10, additional samples were taken on day 12 for these cultures, when
viability reached
¨50%. WCX-10 analysis was also peifon-ned for these samples. The percentage of
acidic
species on day 12 was increased for these three conditions (i.e., pH 6.9, 6.8
and 6.7)
comparing to day 10; however, the increase in the percentage of acidic species
was smaller at
lower pH. The percentage of acidic species increased 18.8% (pH 6.9), 8.1% (pH
6.8) and
3.5% (pH 6.7), respectively from day 10 (70% viability) to day 12 (50%
viability). Therefore,
the percentage of acidic species was lower at lower pH on day 12 too. The
percent acidic
species decreased with decrease in pH from 39.1% in the pH 6.9 condition to
17.5% in the
pH6.7 condition, for a total reduction of 21.6% on day 12.
The effect of process pH to specifically reduce particular acidic variants
within the
larger fraction of total acidic species was also evaluated. In Table 5, a
summary of the extent
of some of the sub-species of the acidic species fraction have been presented.
Along with the
reduction in total acidic species, the methods presented in this section may
also be used for
reduction of sub-species that include, but not limited to, AR1, AR2 and MGO
modified
product variants.
1 54
CA 02926384 2016-04-07
Table 5: Effect of process pH on reduction of sub-species of acidic variants
Sample % AR A,AR1 %AR2 %MGO modified species
Final pH LIGHT CHAIN HEAVY CHAIN TOTAL
Arg 30 Arq 93 Arg 108 Arg 16 (19) Arq 259 Arq
359 Arq 420
7.1 56.0 32.8 23.3 26.1 10.6 0.2 6.1 2.7 3.5
0.5 49.7
6.9 39.1 18.9 20.2 9.5 3.8 0.0 2.2 0.9 1.2 0.2
18.8
6.7 17.5 5.2 12.2 1.2 0.5 0.0 0.2 0.1 0.1 0.0
2.0
Effect of process pH in media 2 with cell line 1
Three different pH conditions were assessed in this study: 7.0, 6.9, and 6.8.
The
cultures were started at pH of 7.1; then were ramped down to the target pH set
points within 3
days of culture. The viable cell density and viability were comparable across
the different pH
set points until day 8. After day 8, the viable cell density and viability
were slightly higher
with lower pH set points (Figure 99 and 100). The cultures were harvested on -
50% viability.
The product titer was slightly higher at pH 6.8 comparing to pH 6.9 and 7.0
(Figure 101). The
resulting peak areas from WCX-10 analysis were quantified (Figure 102). The
percentage of
acidic species decreased with decrease in pH from 20.7% in the pH 7.0
condition to 18.1% in
the pH6.8 condition, for a total reduction of 2.6%.
Effect of process pH in media 1 with cell line 3
Five different pH conditions were assessed in this study: 7.1 7.0, 6.9, 6.8,
and 6.7. The
cultures were started at pH set point of 7.1; then were ramped down to the
target pH set
points within 4 days of culture. The pH set points showed significant effect
on the cell growth
and viability with this cell line and media. Cell density was lower at higher
pH and viability
also dropped earlier at higher pH (Figure 103 and 104). The cells were
harvested either on
day 10 or when viability dropped to equal or less than 50%. The titer was
slightly increased
as the pH was reduced, reached the highest titer at pH 6.8 condition (Figure
105). The
resulting peak areas from WCX-10 analysis were quantified (Figure 106). The
percent acidic
species decreased with decrease in pH from 29.7% in the pH 7.1 condition to
21.5% in the
pH6.7 condition, for a total reduction of 8.2%.
Effect of dissolved oxygen (DO) in media 2 with cell line 1 at 35 C
Three different dissolved oxygen (DO) conditions were assessed in this study:
20%,
30% and 50%. The cultures were set at 35 C. The cell density and viability
were very
155
CA 02926384 2016-04-07
comparable at different DO conditions (Figure 107 and 108). The cultures were
harvested at
the target viability of 50% for each condition. The harvest titer was higher
at 50% DO
compared to 20% DO (Figure 109). The harvest was also taken through Protein A
purification before WCX-10 analysis. The percentage of acidic species in each
of the test
conditions was 20.6% (20% DO), 19.0% (30% DO), and 17.7% (50% DO),
respectively
(Figure 110). The percentage of acidic species was in general lower at higher
dissolved
oxygen concentrations. The percentage of acidic species decreased with
increase in DO from
20.6% in the 20% DO condition to 17.7% in the 50% DO condition, for a total
reduction of
2.9%.
Effect of dissolved oxygen (DO) in media 2 with cell line 1 at 33 C
Three different DO conditions were assessed in this study: 20%, 30% and 60%.
The
cell density, viability and product titer were very comparable at different DO
condition
(Figures 111, 112 and 113). The percentage of acidic species in each of the
test conditions
was 20.1% (20% DO), 17.8% (30% DO), and 17.7% (60% DO), respectively (Figure
114).
The percentage of acidic species was in general lower at higher dissolved
oxygen
concentrations. The percentage of acidic species decreased with increase in DO
from 20.1%
in the 20% DO condition to 17.7% in the 60% DO condition, for a total
reduction of 2.4%.
Effect of dissolved oxygen (DO) in media 1 with cell line 1 at 35 C
Three different DO conditions were assessed in this study: 20%, 30% and 50%.
The
cultures were set at 35 C. The cell density and viability were very comparable
at different DO
conditions (Figure 115 and 116). The cultures were harvested at the target
viability of 40%
for each condition. The harvest titer was higher at 30% and 50% DO comparing
to 20% DO
(Figure 117). The harvest was also taken through Protein A purification before
WCX-10
analysis. The percentage of acidic species in each of the test conditions was
23.9% (20%
DO), 22.4% (30% DO), and 20.3% (50% DO), respectively (Figure 118). The
percentage of
acidic species was in general lower at higher dissolved oxygen concentrations.
The
percentage of acidic species decreased with increase in DO from 23.9% in the
20% DO
condition to 20.3% in the 50% DO condition, for a total reduction of 3.6%.
Effect of dissolved oxygen (DO) in media 1 with cell line 3
156
CA 02926384 2016-04-07
The study was performed at four different temperature levels (33 C, 34 C, 35 C
and
36 C) with two different DO conditions (20% DO and 50% DO). In general, the
cell growth
at different dissolved oxygen levels was similar except at 35 C, in which the
cell density was
lower at 50% DO (Figure 119). The cultures were harvested either on day 10 or
at -50%
viability (Figure 120). The titer at -50% viability is comparable at different
DO conditions
(Figure 121). The percentage of acidic species was in general lower at higher
dissolved
oxygen at each tested temperature condition (Figure 122). On day 10, the
percentage of
acidic species decreased with increase in DO at 36 C from 25.2% in the 20% DO
condition to
22.7% in the 50% DO condition, which is 2.5% of decrease; the percentage of
acidic species
decreased with increase in DO at 35 C from 23.2% in the 20% DO condition to
19.4% in the
50% DO condition, for a total reduction of 3.8%; the percentage of acidic
species decreased
with increase in DO at 34 C from 18.2% in the 20% DO condition to 17.1% in the
50% DO
condition, for a total reduction of 1.1% and the percentage of acidic species
decreased with
increase in DO at 33 C from 14.3% in the 20% DO condition to 12.9% in the 50%
DO
condition, for a total reduction of 1.4% . On day 12, when the viability was
at -50% for the
34 C test conditions, the percentage of acidic species decreased with increase
in DO from
21.5% in the 20% DO condition to 20.6% in the 50% DO condition, for a total
reduction of
0.9%. Lastly, on day 14, when the viability was at -50% for the 33 C test
conditions, the
percentage of acidic species decreased with increase in DO from 19.7% in the
20% DO
condition to 17.9% in the 50% DO condition, for a total reduction of 1.8%. In
summary, at
all tested temperature conditions on different harvest days, the percentage of
acidic species
was lower at higher dissolved oxygen concentrations.
Effect of dissolved oxygen (DO) in media 1 with mAb2
Six different DO conditions were assessed: 10%, 20%, 30%, 50%, 60% and 80%.
The
cultures were set at 35 C. In general, the cell density, viability and titer
at different dissolved
oxygen levels were comparable (Figure 123, 124 and 125). The percentage of
acidic species
in each of the test conditions was estimated to be 26.5% (10% DO), 27.3% (20%
DO), 27.3%
(30% DO), 25.8% (50% DO), 24.4% (60% DO) and 24.5% (80% DO), respectively
(Figure
126). The percentage of acidic species was in general lower at higher
dissolved oxygen. The
percentage of acidic species decreased with increase in DO from 27.3% in the
20% DO
condition to 24.5% in the 80% DO condition. for a total reduction of 2.8%.
157
CA 02926384 2016-04-07
Example 3: Method for reducing acidic species by the addition of amino acids
to
clarified cell culture harvest and by modifying the pH of the clarified
harvest.
The present Example describes processes for reducing and controlling levels of
acidic
species in antibody preparations. Specifically, this Example provides a method
for reducing
the acidic variant content in clarified harvest, as well as a method for
reducing the formation
rate of acidic species in clarified harvest. The method involves adding
additives like various
amino acids to clarified harvest or adjusting the pH of the clarified harvest
using acidic
substances.
As shown below, antibody acidic species in clarified harvest can be reduced by
adding additives such as arginine or histidine to clarified harvest at
concentrations of more
than 100mM and 50mM, respectively. AR reduction can also be achieved by pH
adjustment
of the clarified harvest to pH 6 or pH 5. In addition, the rate of acidic
variant formation can
be reduced through the use of arginine or histidine in a concentration
dependent manner, or
by low pH treatment of the clarified harvest.
Materials and methods
Clarified Harvest Material
Different batches of adalimumab clarified harvest material were employed in
the
following experiments described below. Clarified harvest is liquid material
containing a
composition of interest, e.g., a monoclonal antibody of interest that has been
extracted from a
fermentation bioreactor after undergoing centrifugation to remove large solid
particles and
subsequent filtration to remove finer solid particles and impurities from the
material.
Clarified harvest was used for low pH treatment studies described herein.
Clarified harvest
was also used for the experiments to study the effect of amino acid
concentration on the
presence of acidic species in clarified harvest, and for acid type-pH
treatment studies
described herein. Different batches of mAb-B and mAb-C clarified harvest
material were
employed for experiments to study the effect of amino acid and low pH
treatment studies on
the presence of acidic species described herein.
1 58
CA 02926384 2016-04-07
Preparation of materials
The clarified harvest material was first adjusted to pH 4 using 3M citric
acid. The
material at pH 4 was then agitated for 60 minutes before adjusting the pH to a
target pH of 5,
6 or 7 with 3M sodium hydroxide. The material was then agitated for a further
60 minutes.
The samples were then subjected to centrifugation at 7300 x g for 15 minutes
in a Sorvall
Evolution RC with an SLA-3000 centrifuge bowl. The supernatants obtained from
the
centrifuged material were then depth filtered using BIHC depth filters
(Millipore) followed
by 0.22m sterile filters. The filtrates of different pH were then subjected to
holding for
different period of time for evaluating the formation rate of acidic variants.
After the holding,
the material was purified with Protein A affinity column and the eluate was
sampled and
analyzed using the WCX-10 method. The preparation scheme is shown below in
Figure 127.
The material to study the effect of arginine on acidic species was prepared in
two
ways. For lower target arginine concentrations of 5mM, 10mM, 30mM and 100mM,
they
were made by adding the appropriate amount of 0.5M arginine stock buffer at pH
7 (pH
adjusted with acetic acid) to attain the target arginine concentrations
needed. For higher target
arginine concentrations of 50mM, 100mM, 300mM, 500mM, 760mM, 1M and 2M, they
were made by adding the appropriate amount of arginine (solid) to the samples
to attain the
target arginine concentrations, with subsequent titration to a final pH of 7
using glacial acetic
acid. Arginine was adjusted to a final concentration of 100mM using the two
methods to
determine if the method of preparation would result in different effects. For
all the
experiments, following the arginine addition, treated clarified harvests were
held at room
temperature for the indicated duration followed by purification with Protein A
column and
analysis of acidic variants. This study provided two results; (1) data of
samples from Day 0
gave the effects of arginine on reducing acidic species in clarified harvest,
(2) data of samples
with different holding days gave effect of arginine on reducing the formation
rate of acidic
species. The preparation scheme is shown in Figure 128.
The material to study the effect of histidine was prepared with target
concentrations of
5mM, 10mM, 30mM 50mM, 100mM, 200mM and 250mM. The samples were prepared by
adding the appropriate amount of histidine (solid) to the samples to attain
the target histidine
concentrations, with subsequent titration to a final pH of 7 using glacial
acetic acid. The
sample preparation scheme is shown in Figure 129.
159
CA 02926384 2016-04-07
The material to study the effect of lysine was prepared with target
concentrations of
5mM, 10mM, 30mM 50mM, 100mM, 200mM, 300mM, 500mM and 1000mM. The samples
were prepared by adding the appropriate amount of lysine hydrochloride (solid)
to the
samples to attain the target lysine concentrations, with subsequent titration
to a final pH of 7
using hydrochloric acid. The sample preparation scheme is shown below in
Figure 130.
The material to study the effect of methionine was prepared with target
concentrations
of 5mM, 10mM, 30mM 50mM, 100mM, 200mM and 300mM. The samples were prepared
by adding the appropriate amount of methionine (solid) to the samples to
attain the target
methionine concentrations, with subsequent titration to a final pH of 7 using
glacial acetic
acid. The sample preparation scheme is shown in Figure 131.
The material to study the effect of different amino acids was prepared with
different
target concentrations for each of the 20 amino acids evaluated as well as two
controls using
sodium acetate in place of an amino acid, and the other simply bringing the pH
of the
clarified harvest down to pH 7 using glacial acetic acid. The target
concentrations for the
amino acids are shown below in Table 6.
Table 6: Amino Acid Target Concentrations
Concentration
Amino Acid (mM)
Alanine 100
Arginine 100
Asparagine 100
Aspartic Acid 30
Cysteine 100
Glutamic
Acid 30
Glutamine 100
Glycine 100
Histidine 100
Isoleucine 100
Leucine 100
Lysine 100
Methionine 100
Phenylalanine 100
Proline 100
Serine 100
Threonine 100
160
CA 02926384 2016-04-07
Tryptophan 30
Tyrosine 2
Valine 100
NaAc 100
The samples were prepared by adding the appropriate amount of amino acid
(solid) to
the samples to attain the target amino acid concentrations as shown in Table
6, with
subsequent titration to a final pH of 7 using glacial acetic acid. The sample
preparation
scheme is shown in Figure 132.
The material to study the effect of additives other than amino acids was
prepared with
different target concentrations for each of the additives evaluated as well as
a control in
which sodium hydroxide was used in place of arginine to bring the pH of the
material to pH
before neutralizing it back to pH 7 with glacial acetic acid. The target
concentrations for
the additives are shown below in Table 7.
Table 7: Alternative Additive Target Concentrations
Additive Low Conc High Conc
Sucrose 0.1M 1M
Trehalose 0.1M 1M
Mannitol 4% w/v 10% w/v
Glycerol 1% v/v 10% v/v
PEG 1% w/v 2% w/v
Tween80 0.5% v/v 2% v/v
The samples were prepared by adding the appropriate amount of additive to the
samples to attain the target amino acid concentrations as shown in Tables 6 or
7, with
subsequent titration to a final pH of 7 using glacial acetic acid.
The material to study the effect of the aforementioned methods on CDM
clarified
harvest was prepared using the following scheme shown in Figure 133.
The mAb B hydrolysate clarified harvest was used to study the effect of the
aforementioned methods.
The mAb C hydrolysate clarified harvest was used to study the effect of the
aforementioned methods.
161
CA 02926384 2016-04-07
Hold Studies for treated clarified harvest
After the aforementioned sample preparations, the samples were placed in
separate
sterile stainless steel containers for the purpose of holding at either 4 C or
at room
temperature. For each material, different containers were used for each day of
holding
evaluated. For the acidified samples, the acidic variant compositions of the
samples were
evaluated on days 0, 3, 7 and 14 of holding at either temperature. For the
arginine containing
materials, the acidic variant compositions of the samples were evaluated on
days 0, 5 and 8 of
holding at room temperature. For the histidine containing materials, the
acidic variant
compositions of the samples were evaluated on days 0, 3 and 7 of holding at
room
temperature.
Acid Type and pH effects on clarified harvest
The effects of acid type, clarified harvest pH and arginine content on acidic
variant
reduction were evaluated in this study. The samples were prepared in
triplicates on 3
consecutive days to target arginine concentrations of either OmM (no arginine
added) or
500mM, then titrated with either glacial acetic acid, phosphoric acid, 3M
citric acid or 6M
hydrochloric acid to target pH values of either 5, 6 or 7. One other sample
was prepared by
adding a 2M arginine acetate pH 7 stock buffer to clarified harvest to attain
a target arginine
concentration of 500mM. The sample preparation scheme is shown in Figure 134.
Protein A Purification
Protein A purification of the samples was performed using a 5mL rProtein A FF
Hitrap column (GE Healthcare) at lOg adalimumab/L resin loading and a
operating flow rate
of 3.4mL/min. Five column volumes (CVs) of equilibration (IX PBS pH 7.4) is
followed by
loading of the sample, then washing of the column with equilibration buffer to
remove non-
specifically bound impurities, followed by elution of the protein with 0.1M
Acetic acid,
0.15M sodium chloride.
The eluate samples were collected and neutralized to pH 6.9-7.2 with IM Ti-is
pH 9.5
at 45-75 minutes after collection. The samples were then frozen at -80 C for
at least one day
before thawing and subjecting to WCX-10 analysis.
162
CA 02926384 2016-04-07
Effects of purification method, acid concentration and neutralization on
clarified
harvest
The effects of purification methods with different types of chromatography
resins,
acid concentration and pH neutralization on acidic variant reduction were
evaluated in this
study. The following samples were prepared as shown below in Table 8.
Table 8: Acid Concentration Sample Treatments
Sample Treatment
Control None
3M Citric Acid pH 6 Titrate to pH 6 with 3M Citric Acid
1M Citric Acid pH 6 Titrate to pH 6 with 1M Citric Acid
Glacial Acetic Acid pH 6 Titrate to pH 6 with Glacial Acetic Acid
3M Acetic Acid pH 6 Titrate to pH 6 with 3M Acetic Acid
3M Citric Acid pH 5 Titrate to pH 5 with 3M Citric Acid
3M Acetic Acid pH 5 Titrate to pH 5 with 3M Acetic Acid
3M Citric Acid pH 5 to 7 Titrate to pH 5 with 3M Citric Acid, then 3M Ti-is
to pH 7
3M Acetic Acid pH 5 to 7 Titrate to pH 5 with 3M Acetic Acid, then 3M Ti-is
to pH 7
Each of the material made was then subjected to either Mabselect Sure or
Fractogel S
capture in duplicate. The eluate samples are collected and neutralized to pH
6.9-7.2 with 1M
Tris pH 9.5 at 45-75 minutes after collection. The samples are then frozen at -
80 C for at
least one day before thawing and subjecting to WCX-10 analysis.
Acidic Variant Analysis (WCX-10 Assay)
Cation exchange chromatography was performed on a 4 mm x 250 mm Dionex
ProPac WCX-10 Analytical column (Dionex, CA). A Shimadzu LC1OA I-IPLC system
was
used to perform the HPLC assay. The mobile phases used were 10mM Sodium
Phosphate
dibasic pH 7.5 (Mobile phase A) and 10mM Sodium Phosphate dibasic. 500 mM
Sodium
Chloride pH 5.5 (Mobile phase B). A binary gradient (94% A, 6% B: 0-20 min:
84% A, 16%
B: 20-22 min; 0% A, 100%B: 22-28 min; 94% A, 6% B: 28-34 min) was used with
detection
at 280 nm.
163
CA 02926384 2016-04-07
Quantitation is based on the relative area percent of detected peaks. The
peaks that
elute at relative residence time less than that of the dominant Lysine 0 peak
are together
represented as the acidic variant peaks (AR).
Results
Effect of low pH treatment with subsequent neutralization
The results of the low pH treatment with subsequent neutralization are shown
below
in Figures 135 and 136. Figure 136 shows that the low pH treatment with
subsequent
neutralization to pH 5 or 6 reduces the rate of acidic variant formation over
time. However,
there is no significant reduction in initial acidic valiant content, as shown
in Figure 135.
Effect of arginine treatment
The results of the arginine treatment are shown in Figure 137 and Figure 138.
Figures
137 and 138 show that the sample preparation method resulted in different
levels of acidic
species in clarified harvest. Adding a 0.5M arginine pH 7 stock buffer tends
to increase acidic
species, while adding pure arginine with subsequent acetic acid titration to
pH 7 reduced
acidic variants at arginine concentrations of greater than 100mM. Moreover,
the effect due to
treatment method is demonstrated when comparing the two 100mM arginine
samples, which
show an absolute difference of 1% in acidic variants between the two methods.
Figure 139 shows that the rate of acidic variant formation decreases with
increasing
arginine concentration in clarified harvest, plateauing at around
concentrations of SOOrnM
arginine and higher. However, the two methods of sample preparation do not
result in
significantly different formation rate of acidic variants.
Effect of histidine treatment
The results of the histidine treatment are shown in Figure 140 and Figure 141.
Similar
to arginine treatment effect, as shown in Figure 149, when histidine was added
to clarified
harvest with subsequent pH neutralization with acetic acid, acidic variants
were reduced at
histidine concentrations higher than 50mM. Figure 141 shows that the rate of
acidic variants
formation decreases with increasing histidine concentration in clarified
harvest, plateauing at
around concentrations of 200mM histidine and higher.
164
CA 02926384 2016-04-07
Effect of lysine treatment
The results of the lysine treatment are summarized in Figure 142 and Figure
143.
Similar to arginine treatment effect, as shown in Figure 149, when lysine was
added to
clarified harvest with subsequent pH neutralization with acetic acid, acidic
variants were
significantly reduced by -1% or more. Figure 153 shows that the rate of acidic
variants
formation decreases with increasing lysine concentration in clarified harvest.
Effect of methionine treatment
The results of the methionine treatment are summarized below in Figure 154 and
165.
Similar to arginine treatment effect, as shown in Figure 149, when methionine
was added to
clarified harvest with subsequent pH neutralization with acetic acid, acidic
variants were
significantly reduced by -1% or more at concentrations of >10mM. Figure 145
shows that
the rate of acidic variants formation is not affected significantly by
methionine presence in
clarified harvest.
Effect of other amino acid treatment
The results of the treatments with the various amino acids are summarized
below in
Figures 146 and 147. As shown in Figure 146, the addition of 14 amino acids
including
arginine, histidine, lysine and methionine resulted in lower amounts of acidic
variant content
in clarified harvest. The addition of sodium acetate or the use of acetic acid
also caused a
reduction in acidic variant content as well. Figure 147 shows that the rate of
acidic variants
formation is reduced by several amino acids including arginine, histidine,
lysine, aspartic
acid, glutamic acid, and leucine.
Effect of alternative additive treatment
The results of the treatments with the other additives are summarized below in
Figures 148 and 149. As shown in Figure 148, the addition of any of the
additives did not
result in lower acidic variant content in adalimumab hydrolysate clarified
harvest. However,
165
CA 02926384 2016-04-07
Figure 149 shows that the rate of acidic variants formation is reduced by most
of the
additives.
Effect of low pH/arginine treatment on adalimumab CDM clarified harvest
The results of CDM clarified harvest study are summarized below in Figures l
50 and
151. As shown in Figure 150, low pH/arginine treatment did not result in lower
acidic
variant content in adalimumab CDM clarified harvest. However, Figure 151 shows
that the
rate of acidic variants formation is reduced significantly by all the
treatments.
Effect of low pH/arginine treatment on mAb B hydrolysate clarified harvest
The results of mAb B hydrolysate clarified harvest study are summarized below
in
Figures 152 and 153. As shown in Figures 152 and 153, low pH/arginine
treatment results in
both lower acidic variant content and slower rates of acidic variants
formation in mAb B
hydrolysate clarified harvest.
Effect of low pH/arginine treatment on mAb C hydrolysate clarified harvest
The results of mAb C hydrolysate clarified harvest study are summarized below
in
Figures 154 and 155. As shown in Figures 154 and 155, low pH/arginine
treatment results in
both lower acidic variant content and slower rates of acidic variants
formation in mAb C
hydrolysate clarified harvest.
Effect of acid type and pH
The results obtained from the acid type-pH study are summarized in Figure 156.
Greater acidic species reduction is obtained at lower pH. Arginine addition
also reduces
acidic species content further, but not to a significant extent when taking
the high
concentrations (500mM) used into consideration. The results also show that
acidic species
reduction of ¨1% can be achieved with the usage of an arginine acetate stock
buffer, although
using pure arginine powder with subsequent acid titration performs slightly
better. With
regard to acid type used for pH adjustment, there were no significant
differences between
different acids observed.
1 66
CA 02926384 2016-04-07
Effect of purification method, acid concentration and neutralization
The results obtained from the study are summarized in Figures 157, 158, 159,
and
160. Figures 157 and 158 indicate that when the acid used is of higher
concentration, there is
an decrease in acidic variant content in hydrolysate clarified harvest as
compared to a lower
concentration acid being used. Figures 159 and _160 show that when the
clarified harvest is
subjected to base neutralization to pH 7 after being treated with low pH,
there is an increase
in acidic variant content. The figures also show that the Fractogel resin is
better able to clear
acidic variants than Mabselect Sure.
Example 4: Method for reducing AR in cell culture using a continuous media
perfusion technology
As demonstrated in Example 3, above, generation or formation of acidic species
in a
population of proteins may occur during the hold of the antibody in clarified
harvest or spent
media. Thus, the possibility of enhanced stability of the product antibody or
a reduction in
acidic species generation was explored using a continuous/perfusion based cell
culture
technology. Control or reduction in the amount of acidic species present in
the population of
proteins obtained at the end of cell culture can be accomplished by modifying
the exchange
rate of fresh medium into the bioreactor (or removal of spent medium with
product antibody
out of the bioreactor).
Materials and Methods
Cell source
One adalimumab producing CHO cell line (cell line 1) was employed in the study
covered here. Upon thaw, the vial was cultured in a chernically defined growth
media (media
1) in a series of vented shake flasks on a shaker platfoim at 110 rpm in a 35
C, 5% CO,
incubator. Cultures were propagated to obtain a sufficient number of cells for
inoculation of
the perfusion cultibag.
Cell culture media
A chemically defined growth or production media was used in this study. For
preparation of the media formulation, the proprietary media (Invitrogen) was
supplemented
with L-glutamine, sodium bicarbonate. sodium chloride. recombinant human
insulin and
167
CA 02926384 2016-04-07
methotrexate solution. Perfusion stage media consisted of all the components
in the growth
medium, with the exception of a higher concentration of recombinant human
insulin and the
exclusion of methotrexate solution.
Perfusion culture
The perfusion culture was carried out with the Sartorius BIOSTAT RM 20 optical
perfusion system (SN# 00582112) in a Sartorius Cultibag RM 10L perfusion pro
1.2my (lot
1205-014) perfusion bag. The perfusion bag was run with a working culture
volume of 1.5L
and operation conditions of; pH: 7.00, dissolved oxygen 30%, 25 rpm, 35 C, an
air overlay of
0.3 slpm and a CO2 overlay of 15sccm. pH control was initiated on day three of
the culture.
pH was controlled with 0.5M sodium hydroxide and CO, additions.
Perfusion was carried out by 'harvesting' spent culture through an integrated
1.2 tm
filter integrated into the perfusion cultibag. Fresh media was added to the
culture through a
feed line at the same rate as the harvest. Perfusion began on day four of the
process at a rate
of 1.0 exchanges per day (ex/day). The perfusion rate was adjusted throughout
the run to
acconunodate glucose needs, lactate accumulation and sampling plans. Perfusion
cell-free
harvest samples were collected at perfusion rates of 1.5, 3.0 and 6.0 exchange
volumes/day
on day 5-6 of perfusion. A fresh harvest bag was used for each harvest sample.
The samples
were then purified using Protein A and analyzed using WCX-10 assay.
The perfusion culture was ended on day 8 of the process.
WCX-10 Assay
The acidic species and other charge variants present in cell culture harvest
samples
were quantified. Cation exchange chromatography was performed on a Dionex
ProPac WCX-
10, Analytical column (Dionex, CA).
The mobile phases used were 10mM Sodium Phosphate dibasic pH 7.5 (Mobile phase
A) and 10mM Sodium Phosphate dibasic, 500 mM Sodium Chloride pH 5.5 (Mobile
phase
B). A binary gradient (94% A, 6% B: 0-20 min; 84% A. 16% B: 20-22 min: 0% A,
100%B:
22-28 min; 94% A. 6% B: 28-34 min) was used with detection at 280 nm. The WCX-
10
method used for mAb2 samples used different buffers. The mobile phases used
were 20 mM
(4-Morpholino) ethanesulfonic Acid Monohydrate (MES) pH 6.5 (Mobile phase A)
and 20
168
CA 02926384 2016-04-07
mM MES, 500 mM Sodium Chloride pH 6.5 (Mobile phase B). An optimized gradient
(minute/%B): 0/3, 1/3, 46/21, 47/100, 52/100, 53/3, 58/3 was used with
detection at 280 nm.
Quantitation is based on the relative area percent of detected peaks, as
described above.
Results
Effect of use of perfusion technology and choice of medium exchange rates on
acidic species
Adalimumab producing cell line I was cultured in media 1 and the cultures were
carried out as described in the materials and methods. As described in Table
8, the exchange
rates were modified over a period of 24 hrs between day 5 and day 6 to explore
the influence
of medium exchange rates on the extent of acidic species. At a continuous
medium exchange
rate of 1.5 volumes/day, the product antibody in spent medium was collected in
a harvest bag
over a period of 17 hrs. The harvest bag was then exchanged with a new bag and
the old bag
was transferred to 4 C. Subsequently and in succession, the medium exchange
rates were
increased to 3 and 6 volumes/day and the product harvest was collected over a
time period of
and 2 hrs, respectively. After an overnight hold at 4 C, the three harvest
samples were
processed through Protein A and analyzed for acidic species using WCX-10. The
percentage
of acidic species in the sample with a medium exchange rate of 1.5 volumes/day
was 8.1%.
In the sample with the highest tested exchange rate in this experiment (6
volumes/day), the
percentage of acidic species was reduced to 6%. An exchange rate dependent
reduction in
acidic species was observed in the three samples (Table 9). Reductions in
different sub-
species within the acidic variants (AR1 and AR2) were also noted. An increase
in volumetric
productivity, with exchange rate, was also observed.
Table 9: Effect
of medium exchange rates in a perfusion bioreactor on acidic
species
Harvest bag
Exchange rate Exchange time Volumetric
Start Time (day, (no. of working (for collection in Productivity
hrs:min) volumes/day) harvest bag) (hrs) (mg/I-hr) %
Total AR % AR1 %AR2
Day 5, 16:00 1.5 17 10.94 8.1 2.0 6.1
Day 6, 10:25 3 5 39.80 6.9 1.7 5.2
Day 6, 15:25 6 2 69.50 6.0 1.3 4.7
169
CA 02926384 2016-04-07
Example 5: Method For Acidic Species Reduction Through The Use Of
Continuous Perfusion Technology and Addition of Amino Acids to Culture Medium
As set forth above in Example 4, reduction in the amount of acidic species
present in
the population of proteins obtained at the end of cell culture can be
accomplished by
modifying the exchange rate of fresh medium into the bioreactor (or removal of
spent
medium with product antibody out of the bioreactor). In this Example, the
ability to further
reduce acidic species through the use of high medium exchange rates in
combination with
supplementation of basic amino acids (arginine and lysine) to the culture
medium is
described.
Materials and Methods
Cell source
An adalimumab producing CHO cell line (cell line 1) was employed. Upon thaw,
the
vial was cultured in a chemically defined growth media (media 1) in a series
of vented shake
flasks on a shaker platform at 110 rpm in a 35 C, 5% CO2 incubator. Cultures
were
propagated to obtain a sufficient number of cells for inoculation of the
perfusion cultibag.
Cell culture media
A chemically defined growth or production media was used in this study. For
preparation of the media formulation, the proprietary media (Invitrogen) was
supplemented
with L-glutamine, sodium bicarbonate, sodium chloride, recombinant human
insulin and
methotrexate solution. Perfusion stage media consisted of all the components
in the growth
medium, with the exception of a higher concentration of recombinant human
insulin and the
exclusion of methotrexate solution. Arginine and lysine were added as powders
directly to
the media solution. After the amino acid addition the pH was adjusted to that
of the
unsupplemented media using 5N NaOH and 5N HCL as necessary, and the osmolality
was
adjusted to that of the unsupplemented media by varying the concentration of
sodium
chloride.
Perfusion culture
The perfusion culture was carried out with the Sartorius BIOSTAT RM 20 optical
perfusion system (SN# 00582112) in a Sartorius Cultibag RM 10L perfusion pro
1.2my (lot
170
CA 02926384 2016-04-07
1205-014) perfusion bag. The perfusion bag was run with a working culture
volume of 1.5L
and operation conditions of; pH: 7.00, dissolved oxygen 30%, 25 rpm, 35 C, an
air overlay of
0.3 slpm and a CO, overlay of 15sccm. pH control was initiated on day three of
the culture.
pH was controlled with 0.5M sodium hydroxide and CO, additions.
Perfusion was carried out by 'harvesting' spent culture through an integrated
1.2 [tin
filter integrated into the perfusion cultibag. Fresh media was added to the
culture through a
feed line at the same rate as the harvest. The perfusion rate was adjusted
throughout the run
to accommodate glucose needs, lactate accumulation and sampling plans.
Perfusion cell-free
harvest samples were collected at perfusion rates of 1.5, 3.0, 4.0, 6.0 and
8.0 exchange
volumes/day on day 6-8 of perfusion. A fresh harvest bag was used for each
harvest sample.
The samples were then purified using Protein A and analyzed using WCX-10
assay.
WCX-10 Assay
The acidic species and other charge variants present in cell culture harvest
samples
were quantified. Cation exchange chromatography was performed on a Dionex
ProPac WCX-
10, Analytical column (Dionex, CA).
The mobile phases used were 10mM Sodium Phosphate dibasic pH 7.5 (Mobile phase
A) and 10mM Sodium Phosphate dibasic. 500 mM Sodium Chloride pH 5.5 (Mobile
phase
B). A binary gradient (94% A, 6% B: 0-20 min; 84% A, 16% B: 20-22 min; 0% A,
100%B:
22-28 min; 94% A. 6% B: 28-34 min) was used with detection at 280 nm. The WCX-
10
method used for mAb2 samples used different buffers. The mobile phases used
were 20 mM
(4-Morpholino) ethanesulfonic Acid Monohydrate (MES) pH 6.5 (Mobile phase A)
and 20
mM MES, 500 mM Sodium Chloride pH 6.5 (Mobile phase B). An optimized gradient
(minute/%B): 0/3, 1/3, 46/21, 47/100, 52/100, 53/3, 58/3 was used with
detection at 280 nm.
Quantitation is based on the relative area percent of detected peaks, as
described above.
Results: Effect of use of perfusion technology and choice of medium exchange
rates on acidic species
Adalimumab producing cell line 1 was cultured in media 1 and the cultures were
carried out as described in the materials and methods. The exchange rates were
modified over
a period of 2 days between day 6 and day 8 to explore the influence of medium
exchange
rates on the extent of acidic species. At a continuous medium exchange rate of
1.5
I 7 1
CA 02926384 2016-04-07
volumes/day, the product antibody in spent medium was collected in a harvest
bag over a
period of 22 hrs. The harvest bag was then exchanged with a new bag and the
old bag was
transferred to 4 C. Subsequently and in succession, the medium exchange rates
were
increased to 3 and 6 volumes/day on day 7 and to 4 and 8 volumes/day on day 8
and the
product harvests were collected and transferred to 4 C. The harvest samples
were processed
through Protein A and analyzed for acidic species using WCX-10. The percentage
of acidic
species in the control sample with a medium exchange rate of 1.5 volumes/day
was 7.7%.
The percentage of acidic species in the arginine and lysine supplemented cell
culture with a
medium exchange rate of 1.5 volumes/day was 4.3%. In the sample with the
highest tested
exchange rate in this experiment (8 volumes/day), the percentage of acidic
species was
reduced to 5.4% in the control sample, and reduced to 3.0% in the arginine and
lysine
supplemented cell culture sample. An exchange rate dependent reduction in
acidic species
was observed in both the cultures (Figure 161). Thus, the combination of
arginine/lysine
supplementation to culture media along with exchange rate modulation can be
used to further
reduce AR.
Example 6: Upstream and Downstream process combinations to achieve target
%AR or AR Reductions
Upstream and downstream process technologies, e.g., cell culture and
chromatographic separations, of the inventions disclosed herein can be
combined together or
combined with methods in the art to provide a final target AR value or achieve
a % AR
reduction. Upstream methods for AR reduction include, but are not limited to,
those
described in the instant application. Downstream methods for AR reduction are
also
described herein. Exemplary upstream and downstream process technologies
include, but are
not limited to: cell culture additives and conditions; clarified harvest
additives and pH/salt
conditions; mixed mode media separations; anion exchange media separations;
and cation
exchange media separations.
The instant Example demonstrates the combined effect of one or more of these
technologies in achieving a target AR value or AR reduction, thereby
facilitating the
preparation of an antibody material having a specific charge heterogeneity.
Additional
examples of combinations of downstream technologies and upstream technologies
are
provided herein.
172
CA 02926384 2016-04-07
In this Example, the combination of upstream and downstream methods involves
the
reduction of acidic species in 3L bioreactor cell cultures supplemented with
arginine (2 g/L)
and lysine (4g/L) as has been previously demonstrated in the instant
application. The results
of that strategy are summarized in Table 10. The total acidic species was
reduced from 20.5%
in the control sample to 10.2% in sample from cultures that were supplemented
with the
additives. In this study, adalimumab producing cell line 1 was cultured in
media I
(chemically defined media) supplemented with amino acid arginine (2g/L) and
lysine (4 g/L)
in a 300L bioreactor. On Day 12 of culture, the culture was harvested and then
subsequently
analyzed using WCX-10 post Protein A purification and the percentages of total
peak(s) area
con-esponding to the acidic species were quantified. The percentage of acidic
species was
estimated to be 9.1% in the 300L harvest sample.
Table 10: AR levels achieved with use of upstream technologies
3L Bioreactor 300L Rioreactor
Control Arginine (2g/L) + Lysine (4
g/L) Arginine (2g/L) + Lysine (4 g/L)
Total Total
Total AR AR AR
AR1(%) AR2(%) (%) AR1(%) AR2(%) (%) AR1(%) AR2(%) (%)
6.3 14.2 20.5 2.6 7.6 10.2 2.4 6.7 9.1
The material produced by the 300 L Bioreactor employing arginine and lysine
additions, that effectively reduced the AR levels to 9.1% was purified using a
downstream
process employing Mixed Mode chromatography as the primary AR reduction
method.
Adalimumab was purified by a Protein A chromatography step followed with a low
pH viral inactivation step. The filtered viral inactivated material was buffer
exchanged and
loaded onto a Capto Adhere column. The Flow Through of Capto Adhere material
was then
purified with a HIC coluinn with bind/elute mode as well as Flow Through mode.
As shown
in Table 11, AR reduction was achieved primarily with MM step, with some
contribution
from other steps. The table also shows that additional product related
substances such as
aggregates and process related impurities such as HCP can be effectively
reduced employing
these combined technologies.
173
CA 02926384 2016-04-07
Table 11: Complete Downstream Process Train with Protein A Capture ¨ AR, HMW
and H CP reduction
Yield %AR %HMW
Process HCP LRF
(%) reduction reduction
Clarified Harvest 97.0% n/a n/a n/a
Prt-A Eluate Pool 89.6% 0.06 1.87
Viral Inactivated
99.7% No reduction 0.07 0.39
Filtrate
MM FT pool 91.9% 2.26 0.83 1.63
H1C (B/E) Eluate 90.1% 0.40 0.22 1.41
Nanofiltrate Filtrate 90.7% No reduction No reduction
0.15
BDS (B/E) 102.0% No reduction No reduction 0.22
HIC FT-pool 98.5% 0.16 0.23 0.46
VF(FT) Filtrate 96.1% No reduction No reduction 0.10
BDS (FT) 103.8% No reduction No reduction No
reduction
As is evident from the above example, the MM method further reduced the AR
levels,
by 2.26%. Therefore upstream technologies for reduction can be combined with
downstream
technologies to achieve AR levels/AR reduction.
Example 7: Anion Exchange (AEX) Chromatography Examples
Materials & Methods
Chromatography Method
Except where noted, the Materials and Methods described in connection with the
instant example were also employed in Examples 8 and 9, below.
Pre-packed resin columns were used in the following experiments. except where
specified. The column was equilibrated in a buffer system with appropriate pH
and
conductivity. The column load was prepared from Protein A affinity
chromatography eluates
or concentrated CEX chromatography elutes by buffer exchange (if the eluates
were with
different buffer components from the mixed mode target buffer system) or
addition of the
stock solutions and/or water to obtain the target pH and conductivity as
specified (if the
eluates were with the same buffer components as the mixed mode target buffer
system). The
prepared load material was filtered and loaded on the column according to the
target load
174
CA 02926384 2016-04-07
amount (g protein/L resin) as specified followed by washing with the
equilibration buffer or
buffer similar to equilibration buffer with volumes as specified. The column
Flow
Through/Wash were collected as fractions or as a pool. Mixed mode column was
regenerated
with 0.1M acetic acid, 0.15M NaCl pH3, or 0.1M Acetic acid solution, pH 3, or
as specified.
1M NaOH solution was used for column cleaning.
Buffer Preparation Method
Buffers for AEX were prepared targeting specific ion concentration for the
anion by
fixing the anion concentration (acid) to the target value, and adjusting the
solution with the
cationic component (base) to achieve the appropriate pH. For example, to
prepare a 10 mM
Acetate-Tris buffer solution, pH 8.7, glacial acetic acid was dissolved in
water to a target
concentration of 10 mM and adjusted with concentrated Tris-base to pH 8.7.
Also for
example, to prepare a 10 mM Formate-Tiis buffer solution, pH 8.7, formic acid
was dissolved
in water to a target concentration of 10 mM and adjusted with concentrated
Tris-base to pH
8.7.
Buffers for CEX were prepared targeting specific ion concentration for the
cation by
fixing the cation concentration (base) to the target value, and adjusting the
solution with the
anionic component (base) to achieve the appropriate pH. For example, to
prepare a 140 mM
Tris-Formate buffer solution, pH 7.5, Tris base was dissolved in water to a
target
concentration of 140 mM and adjusted with formic acid to pH 7.5.
AR Reduction and Recovery Calculations
In general, the Flow Through/wash fractions were collected and analyzed with
WCX-
method for AR levels. By actual or calculated pooling of the fractions the
recovery and
the corresponding AR levels were calculated.
WCX-10 for Adalimumab
The acidic species and other charge variants present in the adalimumab process
samples were quantified according to the following methods. Cation exchange
chromatography was performed on a Dionex ProPac WCX-10, Analytical column 4 mm
x
250 mm (Dionex, CA). An Agilent 1200 HPLC system was used as the HPLC. The
mobile
phases used were 10mM Sodium Phosphate dibasic pH 7.5 (Mobile phase A) and
10mM
Sodium Phosphate dibasic, 500 mM Sodium Chloride pH 5.5 (Mobile phase B). A
binary
175
CA 02926384 2016-04-07
gradient (94% A, 6% B: 0-20 min; 84% A, 16% B: 20-22 min; 0% A, 100%B: 22-28
min;
94% A. 6% B: 28-34 min) was used with detection at 280 nm.
Quantitation was based on the relative area percent of detected peaks. The
peaks that
elute at relative residence time less than a certain time are together
represented as the acidic
peaks.
WCX-10 for mAb-B
The acidic species and other charge variants present in the mAb-B process
samples
were quantified according to the following methods. Cation exchange
chromatography was
performed on a Dionex ProPac WCX-10, Analytical column 4 mm x 250 mm (Dionex,
CA).
An Agilent 1200 HPLC system was used as the HPLC. The mobile phases used were
20mM
4-Morpholineethanesulfonic acid (MES), pH 6.5 (Mobile phase A) and 20mM 4-
Morpholineethanesulfonic acid (MES), 500mM Sodium Chloride pH 6.5 (Mobile
phase B).
A binary gradient (87% A, 13% B: 0-5 min; 87% A, 13% B: 5-35 min; 75% A, 25%B:
35-40
min; 0% A, 100% B: 40-43 min; 87%A, 13% B: 43-46 min; 87%A, 13% B: 46-55 min)
was
used with detection at 280 nm, bw 8nm; ref 360nm, bw 100nm.
Quantitation was based on the relative area percent of detected peaks. All
peaks
eluting prior to the Main Isoform peak were summed as the acidic region, and
all peaks
eluting after the LYS-2 peaks will be summed as the basic region.
WCX-10 for mAb-C
The mAb-C method was employed towards the quantification of the acidic species
and other charge variants present mAb-C process samples. Cation exchange
chromatography
was performed on a Dionex ProPac WCX-10, Analytical column 4 mm x 250 mm
(Dionex,
CA). An Agilent 1200 HPLC system was used as the HPLC. The mobile phases used
were
20mM 4-Morpholineethanesulfonic acid (MES), pH 6.0 (Mobile phase A) and 20mM 4-
Morpholineethanesulfonic acid (MES), 250mM Sodium Chloride pH 6.0 (Mobile
phase B).
A binary gradient (97% A, 3% B: 0-1 min; 79% A, 21% B: 1-46 min; 0% A, 100%B:
46-47
min; 0% A. 100% B: 47-52 min; 97%A, 3% B: 52-53 min; 97%A, 3% B: 53-60 min)
was
used with detection at 280 nm, bw 8nm; ref 360nm, bw 100nm.
176
CA 02926384 2016-04-07
Quantitation was based on the relative area percent of detected peaks. All
peaks
eluting prior to the Main Isoform peak will be summed as the acidic region,
and all peaks
eluting after the Main Isoform peak will be summed as the basic region.
Size Exclusion Chromatography
The molecular weight distribution of collected samples were quantified
according to
the following methods. Size exclusion chromatography (SEC) was performed using
a TSK-
gel G3000SWxL, 51.tm, 125 A, 7.8 X 300mm column (Tosoh Bioscience) on an HP
Agilent
HPLC system. Injections were made under isocratic elution conditions using a
mobile phase
of 200 mM sodium sulfate, 100 mM sodium phosphate, pH 6.8, and detected with
absorbance
at 214 nm. Quantification is based on the relative area of detected peaks.
Host Cell Protein (HCP) ELISA
HCP assay is based on process specific antigen based ELISA. Sample dilutions
were
applied to achieve readings within the calibration range. The limit of
quantitation of the assay
is 0.625 ng/mL.
UV spectroscopy A280
UV A280 was used to determine protein concentrations for the samples post
Protein
A elution. The assay was performed on an Agilent UV Spectrophotometer. The
protein
concentration was determined using Beer-Lambert's Law, A = cic, where A is
Absorbance, c
is the extinction coefficient, 1 is the path length, and c is the
concentration. The absorbance
was taken at 280 nm, the path length was 1 cm, and the extinction coefficients
were 1.39 for
adalimumab, 1.38 for mAb B, and 1.43 for mAb C.
Example AEX 7.1: Determining Operating Conditions Appropriate For A Mab:
Media: Buffer Combination
The demonstration of the current invention for a specific antibody & resin is
provided
in this example, and consists of
1. Choosing an anion concentration that allows product and impurities to bind
at a
given pH above the pI of the product.
177
CA 02926384 2016-04-07
2. Performing a pH gradient elution covering a range above, at, and below the
pI of
the product.
3. Determining pH range in which the protein elutes from the anion exchange
media
In this example, adalimumab and Poros 50PI were chosen. The experiment was
performed at acetate (anion) concentration of 5 mM. The column was
equilibrated with 5
mM acetate/Tris at a pH of 9Ø Adalimumab was prepared at 5 mM acetate/Tris
pH 9.0 and
loaded to the column at 20 g-protein/L of resin. The column was washed with 10
CVs of the
equilibration buffer. A pH gradient from 9.0 to 7.0 at an anion concentration
of 5 mM
acetate/Tris was then performed. The process chromatograms are shown in Figure
165.
The demonstration of the current invention for a specific antibody & resin is
provided
in this example, and consists of
1. For a given pH, choosing a starting anion concentration that allows product
and impurities
to bind to the AEX adsorbent.
2. Loading a small amount of protein to the column and then performing a
linear gradient
elution by increasing the anion concentration keeping pH constant.
3. Determining anion concentration range in which the protein elutes from the
anion
exchange media.
In this example, adalimumab and Poros 50HQ were chosen. The experiment was
performed at a pH 8.7. The column was equilibrated with 10 mM acetate/Tris at
pH 8.7.
Adalimumab was prepared at 10 mM acetate/Tris pH 8.7 and loaded to the column
at 20 g-
protein/L of resin. The column was washed with 10 CVs of the equilibration
buffer. A linear
gradient from 10-100 mM Acetate/Tris at pH 8.7 was performed. The process
chromatograms are shown in Figure 166.
178
CA 02926384 2016-04-07
This general approach is used to determine the appropriate operating
condition,
example shown in Table 12, for any resin/mAb combination, to implement the
invention.
Table 12: Example Experimental Design Scope determined from pH and anion
gradient elution
Poros 50HQ - 300 g/L Loading - 30 g/L Fractionation
pH Range 8.2 - 9.0
Anion Concentration (acetate) 10 - 20 mM
In practicing the current invention, the acidic species reduction desired can
be
achieved by appropriate pooling of the load and wash fractions. By collecting
and
subsequently determining the product quality of each fraction throughout the
load and wash,
the accumulative AR reduction and accumulative yield can be calculated using
the weighted
averages up to a given fraction. Additionally, the instantaneous yield can be
estimated by
comparing the protein recovered against the total protein loaded to the column
at a given
fraction. Sample calculations are shown below:
Sample Calculation A: Accumulative Yield up to a given fraction
Accumulated Protein Mass Recovered up to Fraction
Accumulative Yield = ___________________________________
Total Mass Protein Load
Sample Calculation B: Accumulative AR reduction up to a given fraction
Accumulated Acidic Species Mass Recovered up to Fraction
Accumulative AR Reduction = Load AR%
Accumulated Total Protein Mass Recovered up to Fraction
Sample Calculation C: Instantaneous Yield up to a given fraction
Accumulated Protein Mass Recovered up to Fraction
Instantaneous Yield = __________________________________
Total Protein Mass Loaded to Column at lraction
179
CA 02926384 2016-04-07
The demonstration of the current invention for a specific antibody & resin is
provided
in this example, and consists of
1. For a given pH and anion concentration and anion exchange media.
2. Loading the anion exchange media in excess of the dynamic binding capacity
for the
product for the given condition.
3. Washing the column with a buffer containing a similar pH and anion
concentration used
for the equilibration and loading steps.
4. Collecting fractions throughout the loading and wash steps and subsequently
determining
the product quality profile (e.g. AR, aggregate, etc.).
In this example, adalimumab and Poros 50PI were chosen. The experiment was
performed at 5 mM acetate/arginine pH 8.8. The column was equilibrated with 5
mM
acetate/arginine at pH 8.8. Adalimumab was prepared at 5 mM acetate/arginine
pH 8.8 and
loaded to the column at 300 g-protein/L-resin. The column was washed with 20
CVs of the
equilibration buffer. Fractions were collected in volumes representing 30 g-
protein/L-resin,
shown in Figure 167. Each fraction was then analyzed for product quality and
the
accumulative yield and AR reduction calculated, shown in Table 13. From this
example, it is
clear to one skilled in the art to determine a run condition which delivers a
targeted product
quality and/or step yield.
1 80
CA 02926384 2016-04-07
This general approach is used to evaluate the performance for a given
operating
condition for any resin/mAb/buffer combination.
Table 13: Cumulative Yield and AR Reduction from Figure 167
Cumulative
Fraction Load Yield AAR
A2 7 g/L 0.0% 10.8%
A3* 37 g/L 0.5% 1O.8%
A4 67 g/L 6.7% 9.7%
A5 97 g/L 16.7% 8.9%
A6 127 g/L 26.9% 8.4%
B1 157 g/L 37.0% 7.7%
B2 187 g/L 47.1% 7.1%
B3 217 g/L 57.4% 6.4%
B4 247 g/L 67.8% 5.8%
B5 277 g/L 78.0% 5.3%
B6 300 g/L 84.4% 5.0%
B7 Wash 87.0% 4.8%
CI Wash 88.5% 4.7%
C2 Wash 89.6% 4.6%
*Dynamic Binding Capacity (DBC) = 39 g/L
Example AEX 7.2: Demonstration of AR reduction with AEX adsorbents
This data set demonstrates the AR reduction achieved with three different AEX
adsorbents. Each resin was evaluated using adalimumab at an acetate
concentration
determined from the process outlined in Example 7.1 and at pH values below,
near, and
above the pl (e.g. pH 8.5 to 9.0). Table 14 outlines the results from these
experiments.
181
CA 02926384 2016-04-07
Table 14: Effect of AEX Resins on AR reduction of Adalimumab
Resin Buffer Condition Load Yield AAR
mM Acetate / Tris pH 8.5 150 g/L 90% 2.4%
5 mM Acetate / Tris pH 8.5 300 g/L 94%
0.9%
5 mM Acetate / Tris pH 8.7 150 g/L 87% 3.6%
Poros 50P1
5 mM Acetate / Tris pH 8.7 300 g/L 94% 1.2%
5 mM Acetate / Tris pH 9.0 150 g/L 83% 3.9%
5 mM Acetate / Tris pH 9.0 300 g/L 92%
1.5%
18 mM Acetate / Tris pH 8.5 250 g/L 91% 3.8%
18 mM Acetate / Tris pH 8.5 350 g/L 88% 2.2%
18 mM Acetate / Tris pH 8.7 250 g/L 85% 6.0%
Poros 50HQ
18 mM Acetate / Tris pH 8.7 350 g/L 84% 3.1%
18 mM Acetate / Tris pH 8.9 250 g/L 67% 5.9%
18 mM Acetate / Tris pH 8.9 350 g/L 75% 3.6%
mM Acetate / Tris pH 8.5 150 g/L 98% 0.7%
10 mM Acetate / Tris pH 8.5 300 g/L 97% 0.1%
10 mM Acetate / Tris pH 8.7 150 g/L 78% 7.1%
CaptoDEAE
10 mM Acetate / Tris pH 8.7 300 g/L 95%
2.5%
10 mM Acetate / Tris pH 9.0 150 g/L 29% 9.2%
10 mM Acetate / Tris pH 9.0 300 g/L 82% 5.0%
This data set is compiled to demonstrate the AR reduction achieved with eight
different AEX adsorbents. Each resin was tested using an advanced screening
method using
the process outlined in Example 7.1, and subjected to four runs using
adalimumab at two
different pH (e.g., pH 8.7 and 9.0) and two different acetate concentrations
(e.g. lOmM and
20mM). In these experiments, the instantaneous (e.g. not accumulative) AR
reduction was
measured by analyzing the load fraction at 150 g/L and subsequently compared
across all
resins. Table 15 outlines the results from these experiments.
182
CA 02926384 2016-04-07
Table 15: Advanced Screen of AEX Resins for AR reduction of adalimumab
Instantaneous AR
Resin pH Acetate
Reduction @ 150 g/L
10 mM 15.O%
8.7
20 mM 10.7%
Poros 50HQ
10 mM 8.6%
9.0
20 mM 13.4%
10 mM 6.2%
8.7
20 mM -0.1%
Poros 50PI
10 mM 6.5%
9.0
20 mM 3.O%
10 mM 9.3%
8.7
20 mM -O.2%
Capto DEAE
10 mM 8.6%
9.0
20 mM 7.8%
10 mM 12.3 %
8.7
20 mM 4.2%
Capto Q Impres
10 mM 12.3%
9.0
20 mM 6.5%
10 mM 1O.1%
8.7
20 mM 3.5%
QAE-550C
10 mM 7.8%
9.0
20 mM 4.5 %
10 mM 5.2%
8.7
20 mM O.1%
DEAE 650M
10 mM 6.9%
9.0
20 mM -2.7 %
10 mM 8.1%
8.7
GigaCap Q 20 mM 5.8 %
650M 10 mM 1.8%
9.0
20 mM O.4%
10 mM 4.1%
8.7
20 mM 2.8 %
TMAE HiCap
10 mM 1.2%
9.0
20 rnM -0.1%
This data set is compiled to demonstrate the AR reduction achieved with two
different
AEX chromatographic membranes. Each membrane was tested using conditions
outlined in
Table 15. The results from these experiments are presented in Table 16.
183
CA 02926384 2016-04-07
Table 16: Effect of AEX Chromatographic Membrane on AR reduction of
Adalimumab
Chromatographic
Equil/Wash Buffer Load Yield AAR
Membrane
500
mM Acetate/Tris pH 8.7 94% 1.7 %
Sartobind STIC
500
mM Acetate/Tris pH 9.0 grL 100% 0.7 %
500
Sartobind Q 20 mM Acetate/Ms pH 9.0 100% 0.3 %
This data set is compiled to demonstrate the AR reduction achieved with two
different
charged depth filters. The results from these experiments are presented in
Table 17.
Table 17: Effect of Charged Depth Filters on AR reduction of adalimumab
Depth Filter Media Equil/Wash Buffer Load Yield AAR
CUNO BioCap 25 18 mM Acetate/Tris pH 8.7 500 g/m2 92 % 1.9 %
XOHC 18 mM Acetate/Tris pH 8.7 500 g/m2 84 % 1.1 %
Example AEX 7.3: Demonstration of AR Reduction with other antibodies, Mab
B And Mab C
AR reduction technology of the current invention has been demonstrated with
multiple antibodies using AEX adsorbents. Antibodies have different amount
charged
residues and at different positions. leading to a charge interaction behavior
on an AEX
column that differs from one antibody to another. Therefore the impact of
anion type, anion
concentration is different for each antibody.
Table 18 and Table 19 below show the data for mAb B and mAb C. The data
clearly
demonstrates that the AR reduction technology works very effectively for other
antibodies.
184
CA 02926384 2016-04-07
Table 18: AR reduction for mAb B, pl - 9.1
Resin Buffer Condition pH Load Yield AAR
300
9.5 83% 1.1%
g/L
300
Poros 50P1 5 mM Acetate/Tris 9.1 94% 1.6%
g/L
300
8.5 98% <0.5%
g/L
300
9.5 69% <0.5%
g/L
300
Poros 50HQ 10 mM Acetate/Tris 9.1 78% 5.7%
g/L
300
8.5 81% 3.4%
g/L
300
9.5 69% 4.2%
g/L
300
Capto DEAE 10 mM Acetate/Tris 9.1 82% 4.9%
g/L
300
8.5 96% <0.5%
g/L
185
CA 02926384 2016-04-07
Table 19: AR reduction for mAb C, pI ¨ 7.0
Resin Buffer Condition PH Load Yield AAR
300
7.5 90% 2.6%
g/L
Poros 50PI 12 mM Acetate/Ms 7 300.0 89% 2.2%
g/L
300
6.5 87% 4.0%
g/L
300
7.5 86% 1.2%
g/L
300
Poros 50HQ 45 mM Acetate/Ms 7.0 88% 1.2%
g/L
300
6.5 91% 0.7%
g/L
300
7.5 79% 1.8%
g/L
Capto DEAE 25 mM Acetate/Ms300 7.0 80% 1.9%
g/L
300
6.5 89% 1.8%
g/L
Example AEX 7.4: Demonstration of AR reduction with different pH conditions
¨ adalimumab
The AR species in the cunent invention is bound during the loading step;
therefore
the binding pH is a key variable. The anion concentration that provides the
desired
performance will vary with the operational pH.
In this example, data compiled from different experiments is shown to
demonstrate
the impact of the pH choice, relative to the pI of the protein on AR
reduction. This data set
provides the basis for one skilled in the art to determine a pH range to
perform the
experiments to implement the current invention. Furthermore, this reiterates
the fact that the
pH choice depends on several factors and the relationship between pH and AR
reduction is
also mAb dependent
In this example, adalimumab and Poros 50PI were chosen. The experiments were
petformed at a concentration of 5 mM acetate/arginine at each pH specified.
Adalimumab
was prepared at 5 mM acetate/arginine at each pH specified and loaded to the
column at 300
g-protein/L of resin. The column was washed with 20 CVs of the equilibration
buffer. The
results showing the pH effect on AR reduction is shown in Figure 168.
186
CA 02926384 2016-04-07
It is also clear that the AR reduction can be achieved with the present
invention with a
range of pH choices in the range of +0.5 pH units from the pI of multiple
mAbs, which are
listed in Table 20. Each of these experiments was peiformed with Poros5OHQ
resin at a 300
g/L load with an acetate/Tris buffer system.
Table 20: AR reduction at pH above, at, and below protein pI
Range pH - PI Antibody Yield AAR
0.2 adalimumab 71% 7.0%
pH > pI 0.5 mAb B 69% 3.4%
0.5 mAb C 86% 1.2%
0 adalimumab 86%
5.9%
pH¨pI 0 mAb B 78% 5.7%
0 mAb C 88% 1.2%
-0.2 adalimumab 93% 4.1%
pH < pI -0.5 mAb B 81% <0.5%
-0.5 mAb C 91% 0.7%
Example AEX 7.5: Demonstration of AR reduction with different ion
concentrations ¨ adalimumab
Anion concentration is a key variable in the performance of anion exchange
chromatography. For every combination of antibody/resin/pH there is a range of
anion
concentrations that provides AR reduction; the strategy outlined in Example
7.1 can be
followed to determine the AR reduction and the corresponding recovery for each
anion
concentration.
Table 21 below shows the effect of anion concentration on AR reduction. The
table
also includes the effect of anion concentration for different pH values. The
data demonstrates
that the AR reduction can be effectively achieved over a range of anion
concentrations at
each pH and that the concentration ranges depend on the pH.
187
CA 02926384 2016-04-07
Table 21: Effect of Anion Concentration and pH on AR reduction
Resin pH Buffer Condition Load Yield AAR
5 mM Acetate/Arginine 300 g/L 81% 4.8%
9 10 mM Acetate/Arginine 227 g/L
80% 2.4%
18.5 mM Acetate/Arginine 107 g/L 88% 1.0%
Poros 50PI
5 mM Acetate/Arginine 300 g/L 93% 4.5%
8.8 10 mM Acetate/Arginine 227 g/L
88% 2.5%
18.5 mM Acetate/Arginine 108 g/L 96% 1.2%
Example AEX 7.6: Demonstration of AR reduction with different buffer systems
with adalimumab
The anion type and concentration are key variables in Anion Exchange
Chromatography. The invention has been demonstrated using Acetate and Formate
as the
anion type and Tris and arginine as the counter cation type. The optimal pH
and cation
concentration is different for each cation type/mixture and was derived by
using the strategy
outlined above in Example 7.1. Table 22 shows the data of AR reduction and
corresponding
recovery for the different anion/cation types.
Table 22: Effect of Anion/Cation Type AR reduction
Resin Buffer Condition Load Yield AAR
mM Acetate/Tris, pH 8.7 300 g/L 94% 1.2 %
Poros 50P1 2.5 mM Formate/Tris, pH 8.7 300 g/L
92% 1.3 %
5mM Acetate/Arginine, pH 8.8 300 g/L 93% 4.5 %
Poros 50HQ 15 mM Acetate/Arginine, pH 8.7 300 g/L 89% 3.2 %
1 88
CA 02926384 2016-04-07
mM Formate/Tris, pH 8.7 300 g/L 83% 4.9 %
18mM Acetate/Tris, pH 8.7 300 g/L 86% 5.9 %
10 mM Acetate/Tris, pH 8.7 300 g/L 95% 2.5 %
Capto DEAE 10 mM Formate/Tris, pH 8.7 300 g/L 94% 1.0 %
5 mM Acetate/Arginine, pH 9.0 200 g/L 41% 7.5 %
Example AEX 7.7: Demonstration of AR Reduction With Different Loading
Furthermore, the strategy outlined in Example 7.1 to reduce acidic species
through
careful control of buffer anion type, anion concentration, AEX adsorbent, and
pH can be
applied to any range of protein loading. A range of relevant protein loadings
(e.g. 100-350
g/L) for Poros 50HQ at pH 8.7 using Acetate as the anion is shown in Table 23,
displaying a
robust AR reduction across the loading range investigated.
Table 23: Impact of Column loading
Yield
Load AAR
(100-100mAU)
100 g/L 78% 9.7%
200 g/L 78% 4.7%
250 g/L 85% 6.0%
300 g/L 89% 3.9%
350 g/L 84% 3.1%
Example AEX 7.8: Demonstration of AR reduction with different load
concentration
Furthermore, the strategy outlined in Example 7.1 to reduce acidic species
through
careful control of buffer anion type, anion concentration, AEX adsorbent. and
pH can be
applied to any range of column feed streams of varying protein concentration.
A range of
varying protein load concentration for a 300 g/L load of adalimumab to Poros
5011Q at 15
mM acetate/Tris pH 8.7 is shown in Table 24.
189
CA 02926384 2016-04-07
Table 24: Effect of Protein Load concentration
Load Yield
AAR
Concentration (100-100mAU)
mg/mL 90% 4.7%
mg/mL 86% 4.5%
mg/mL 85% 6.3%
mg/mL 84% 6.2%
Example AEX 7.9: Alternative Wash Modalities
In this example, adalimumab and Poros5OHQ resin were selected. In each
experiment, variations were made in the equilibration, loading, and washing pH
values at a
given acetate concentration (as specified). Table 25 and Table 26 show the
effect of the pH
variation in the step yield and AR reduction.
Table 25: Differences in pH in Equil/Wash/Load
Poros 50HQ - 15mM Acetate/Tris - pH 8.7 - 200 g/L
Equilibration
Load pH Wash pH Yield
pH (100-100mAU) AAR
8.7 8.7 8.5 83% 8.7%
9 8.5 8.5 89% 5.1%
100 g/L at pH
9.0
9 8.5 94% 4.5%
100 g/L at pH
8.5
Table 26: Differences in pH in Load/Wash
Poros 50HQ - 18mM Acetate/Tris pH 8.7
Load pH Wash pH Load Yield AAR
8.6 8.4 75 g/L 88.8% 4.1%
8.6 8.5 125 g/L 89.5% 4.2%
190
CA 02926384 2016-04-07
8.6 8.6 100 g/L 75.5% 5.3%
8.7 8.4 100 g/L 93.8% 4.1%
8.7 8.5 100 g/L 81.7% 3.5%
8.7 8.5 75 g/L 94.5% 4.0%
8.7 8.6 125 g/L 81.1% 5.4%
8.7 8.6 75 g/L 65.8% 6.5%
8.8 8.4 125 g/L 93.5% 3.8%
8.8 8.5 100 g/L 83.7% 5.8%
8.8 8.6 100 g/L 78.4% 6.4%
8.8 8.6 75 g/L 72.7% 7.0%
As discussed in the previous sections, the operational pH and its relation to
the
product pi is important in the reduction of AR species in AEX. Similarly, the
operational pH
relative to the pKa of the AEX adsorbent is also important as many mAbs have
pI similar to
the pKa of the AEX adsorbent. This effect is shown in Figure 187 for mAb B
with several
different AEX adsorbents, with different pKa values, run at with an
acetate/Tris buffer at pH
9.1.
As described in previous sections, the AR for adalimumab is further grouped
into two
regions termed AR1 and AR2, based on a certain retention time of the peaks
seen on the
WCX-10 method. The characteristics of the variants in these two regions are
expected to be
different and hence the methods that reduce variants belonging to these groups
can be
specifically delineated.
Further, in addition to achieving a certain AR reduction, it may be desirable
to
achieve a certain absolute level of AR levels, in consideration of reducing or
removing
certain variants. The capability of the current invention in achieving a
certain absolute level
of AR, AR1 and AR2 is demonstrated in Table 27. The method of the cuiTent
invention can
effectively reduce AR2 levels, as an overall decrease in AR levels is
achieved. The method
can be used to achieve a target absolute level, as exemplified by the data
presented in Table
27. Multiple species are present under the group of AR2 and that the current
method of
191
CA 02926384 2016-04-07
invention can be used to reduce such sub-species. The method of the current
invention can
effectively achieve AR reduction as well as achieve a target absolute level of
acidic species
as exemplified by the data presented in Table 27.
Table 27: AR1, AR2, and AR removal
Buffer Final Final
Resin tion pH Load Yield AAR I AAR2 AAR
Condi AR I AR2
150
90% 0.7% 1.5% 1.7% 9.4% 2.4%
g/L
8.5
300
94% 0.3% 1.9% 0.6% 10.5% 0.9%
g/L
150
87% 0.9% 1.2% 2.7% 8.2% 3.6%
g/L
Poros 50P1 5 mM 8.7
Acetate/Tris
300
94% 0.4% 1.7% 0.8% 10.1% 1.2%
g/L
150
83% 1.1% 1.4% 2.8% 8.4% 3.9%
g/L
8.9
300
92% 0.7% 1.8% 0.7% 10.5% 1.5%
g/L
250
91% 2.9% 1.1% 0.9% 10.8% 3.8%
g/L
8.5
350
88% 2.7% 1.3% -0.5% 12.2% 2.2%
g/L
250
88% 3.1% 0.9% 2.9% 9.0% 6.0%
g/L
Poros 50HQ 18 mM 8.7
Acetate/Tris
350
84% 2.8% 1.2% 0.3% 11.6% 3.1%
g/L
250
67% 2.6% 1.4% 3.2% 8.6% 5.9%
g/L
8.9
350
75% 2.3% 1.7% 1.3% 10.5% 3.6%
g/L
CaptoDEAE
mM c 150
Acetate/Tris g/L 98% -0.1% 2.1% 0.8% 10.0% 0.7%
192
CA 02926384 2016-04-07
300
97% 0.0% 2.0% 0.1ck 10.8% 0.1%
g/L
150
78% 2.4% 0.8% 4.7% 6.4% 7.1%
g/L
8.7 _____________________________________________
300
95% 1.5% 1.7% 1.0% 10.1% 2.5%
g/L
150
29% 2.1% 0.8% 8.0% 3.0% 10.2%
g/L
8.9 _____________________________________________
300
82% 1.7% 1.2% 3.3% 7.7% 5.0%
g/L
Example AEX 7.10: Demonstration of HCP and aggregate reduction in
addition to AR reduction
AEX chromatography is effective in reducing aggregate and HCP levels. In the
present invention, it has been demonstrated that HCP and aggregate levels can
be effectively
reduced under operating conditions selected for AR reduction. Table 28 and
Table 29 shows
the aggregate and HCP removal achieved along with AR reduction. The data
clearly shows
that other process related and product related substances/impurities can be
achieved using the
current invention on the AEX adsorbents, and hence functions as an effective
polishing step
in the large scale purification of monoclonal antibodies.
Table 28: Aggregate removal during AEX Chromatography
AAggregate
Buffer Condition Load Yield AAR
Absolute Relative
mM Acetate/Tris, pH 9.0 300 g/L 81% 0.92% 93% 4.5%
mM Acetate/Tris, pH 9.0 227 g/L 80% 0.81% 88% 2.4%
18.5 mM Acetate/Tris, pH 9.0 107 g/L 88% 0.37% 41%
1.0%
5 mM Acetate/Tris, pH 8.8 300 g/L 93% 0.91% 91%
4.5%
193
CA 02926384 2016-04-07
mM Acetate/Arginine, pH
227 g/L 88% 0.67% 77% 2.5%
8.8
18.5 mM Acetate/Arginine, pH
108 g/L 96% 0.34% 40% 1.2%
8.8
Table 29: HCP Removal during AEX Chromatography
Poros 50P1 - adalimumab - 300 g/L
Load Pool
HCP
Buffer Condition Yield HCP HCP AAR
(LRF)
(ng/mL) (ng/mL)
5 mM Acetate/Tris, pH 9.0 81% 69 2.2 4.8%
11,617
10 mM Acetate/Tris, pH 9.0 95% 83 2.1 0.8%
5 mM Acetate/Tris, pH 8.8 93% 51 2.4 4.5%
13,507
10mM Acetate/Arginine, pH 8.8 97% 84 2.2 1.5%
Example AEX 7.11: Demonstration of means of controlling AR reduction
Controlling the final product quality by modifying the process based on the
quality of
the intermediate material is an approach that has been proposed as an
effective way of
ensuring product quality, with the view of ensuring safety and efficacy.
Considering that the AR levels generated during cell culture and other
upstream steps
can be variable, it is desirable to design a downstream process step that
implements a means
of controlling the product quality; and to further have a specific means of
controlling a
process parameter to influence the quality of the product.
In the current invention. such a control is possible. as the pH and load
(i.e.. g/L) are
parameters that can be modified to achieve a desired separation of the AR
species. For
l 94
CA 02926384 2016-04-07
example, to achieve a higher level of AR reduction at a given anion
concentration and pH, the
load to the column can be reduced. Additionally, for a given anion
concentration and
loading, the pH can be increased in order to achieve a higher reduction in AR
species.
As an example, and not to be restrictive in any manner, it has been
demonstrated in
this example that the AR levels can be controlled by changing the pH of the
load and wash
solutions as well as the total load to the column. A pilot scale Poros HQ
column (10 cm
diameter x 22.5 cm height, 1.8L), was used for this study.
The load material and the stock buffer are both prepared at 18mM Acetate/Tris
the
specified pH by titrating the affinity captured material with a stock Tris
solution. The AR
level of the load material was the same for both runs. This experiment
demonstrates how the
final AR level can be modulated, while maintaining acceptable yields, by
adjusting the pH
and protein load to the column, shown in Table 30.
Table 30: Modulating AR Reduction using Process Analytical Technology approach
Final
Buffer Condition Load Yield AAR
AR
18 mM Acetate/Tris, pH 8.7 200 g/L 77% 5.6% 5.5%
18 mM Acetate/Tris, pH 8.5 300 g/L 89% 3.1% 8.2%
Example AEX 7.12: AEX with Tris/Formate Buffer System: Acidic Species
Reduction For Adalimumab On Poros 50HQ In A Formic Acid Buffer System
This Example provides demonstration of the use of a Tris/Formate buffer system
for
AR reduction using AEX. In practicing the current Example, the acidic species
reduction
desired can be achieved by appropriate pooling of the load and wash fractions.
By
collecting and subsequently determining the product quality of each fraction
throughout the
load and wash, the accumulative AR reduction and accumulative yield can be
calculated
using the weighted averages up to a given fraction. Additionally, the
instantaneous yield
can be estimated by comparing the protein recovered against the total protein
loaded to the
column at a given fraction.
195
CA 02926384 2016-04-07
AEX Adsorbent
Poros 50HQ (Applied Biosciences, part# 1-2459-11), a rigid 50 um polymeric
bead
with a backbone consisting of cross-linked poly[styrene-divinylbenzenel, was
used in this
experiment.
AEX Chromatography Method
Poros 50HQ was packed in 1.0 cm x 10.0 cm (OmniFit) columns. The column was
equilibrated in a buffer system with appropriate pH and conductivity. The load
was prepared
in the equilibration buffer by addition of the stock solutions to obtain the
target ion
concentrations and loaded on the column, followed by washing with the
equilibration buffer
for 20 CV. The antibody product was collected in the flow-through and wash
fractions during
the load and washing steps. The columns/housings were then regenerated with
100 mM
formate and 1M of NaOH solution was used for column cleaning.
Sample calculations are shown below:
Sample Calculation A: Accumulative Yield up to a given fraction
Accumulated Protein Mass Recovered up to Fraction
Accumulative Yield = ___________________________________
Total Mass Protein Load
Sample Calculation B: Accumulative AR Reduction up to a given fraction
Accumulated Acidic Species Mass Recovered up to Fraction
Accumulative AR Reduction =1,oad AR% -
Accumulated Total Protein Mass Recovered up to Fraction
Sample Calculation C: Instantaneous Yield up to a given fraction
Accumulated Protein Mass Recovered up to Fraction
Instantaneous Yield =
Total Protein Mass Loaded to Column at Fraction
In this Example, adalimumab and POMS 50HQ were chosen. The experiment was
peifonned at 10 mM, 15 mM, 20 rnM, 30 mM. and 40 mM forrnate/Tris pH 8.8. The
column was equilibrated with the respective formate/Tris at pH 8.8 for each
run.
196
CA 02926384 2016-04-07
Adalimumab was prepared at 10 mM, 15 mM, 20 mM, 30 mM, and 40 mM forrnate/Tris
pH
8.8 and loaded to the column at 300 - 500 g-protein/L-resin. Fractions were
collected in
volumes representing ¨25 g-protein/L-resin. These fractions were analyzed for
product
quality, accumulative yield, and accumulative AR reduction throughout the run
(shown in
Figure 189). The instantaneous yield and AR reduction at 100, 200, 300, 400,
and 500 g/L
load are tabulated in Table 31. This example demonstrates the effectiveness of
the
Tris/Fori-nate buffer system in general and specifically the effectiveness of
the Formate anion
on the AEX column for AR reduction. Further it confirms that the AEX AR
reduction
method applies to a variety of buffer systems.
Table 31: Accumulative Yield and AR Reduction for a range of Formic Acid
concentrations from Figure 189
mM 15 mM 20 mM 30 mM 40 mM
Load
giL Yield AAR Yield AAR Yield AAR Yield AAR Yield AAR
100 32% 9.2 % 54% 8.7% 62% 8.4% 69% 5.5% 75% 4.5%
200 64% 7.4% 74% 6.8% 78% 6.0% 82% 3.2% 85% 2.6%
300 75% 6.1% 82% 5.3% 85% 4.4% 84% 2.2% 86% 1.8%
400 81% 5.1% 86% 4.2% 88% 3.3%
500 83% 4.5% 87% 3.6% 89% 2.8%
Example 8: Cation Exchange Chromatography Examples
Example CEX 8.1: Determining operating conditions appropriate for A Mab:
Resin: Buffer Combination
The demonstration of the current invention for a specific antibody Sz resin is
provided
in this example, and consists of
1. Choosing a pH that is below the pI of the protein.
197
CA 02926384 2016-04-07
2. Choosing a NaCI concentration in the range of 100 to 150 mM and performing
the
experiments at, for example, 115, 125, 135 concentrations.
3. Determining the acidic species distribution in the Flow Through/wash
fraction vs. the
elution.
4. Choosing a NaC1 concentration that provides the desired acidic species
levels and recovery
In this example, adalimumab was chosen and Poros XS was chosen. The
experiments
were performed at pH 6Ø The process chromatograms are shown in Figure 169.
The
recovery vs. AR reduction curves for each of the experiments is shown in
Figure 170 and
Table 32. From this set of experiments, a sodium concentration of 125 mM can
be chosen
and such that the recovery of the eluate is 74%, which provides an AR
reduction of 5.4%.
Alternately, an AR reduction value of 5.4% can be chosen which will provide a
recovery of
¨75%.
This general approach is used to determine the appropriate operating condition
for any
resin/mAb combination, to implement the invention.
In practicing certain embodiments of the current invention, the acidic species
reduction desired can be achieved by appropriate pooling of the elution
fraction with the
wash fractions. In the example described in the previous section the elution
fractions can be
pooled with wash fractions as shown in Table 32 to achieve AR reductions from
about I
percent to about 7 percent depending on the fractions pooled. This approach
can be
implemented to achieve a target yield and AR reduction as exemplified in
Figure 170.
Table 32: Wash fractions and eluate combination versus AR reduction
Recovery %AR
Wash Fractions
(%) reduction
Eluate 74 5.4
Eluate + Fraction 1 82 4.3
Eluate + Fraction 1+ Fraction 2 88 3.0
Eluate + Fraction 1+ Fraction 2 + Fraction 3 95 0.9
Eluate + Fraction 1+ Fraction 2 + Fraction 3 + Fraction 4 96 0.1
198
CA 02926384 2016-04-07
Example CEX 8.2: Demonstration of AR reduction with CEX adsorbents
This data set is compiled to demonstrate the AR reduction achieved with 8
different
CEX adsorbents. Conditions were derived for each resin based on the strategy
outlined in
Example 8.1, above. Table 33 outlines the conditions used and the AR reduction
achieved
and the corresponding recovery achieved.
The data clearly shows that the technology is robust in delivering AR
reduction in all
the 10 resins. As described in Example 8.1, above, the AR reduction can be
balanced with
recovery and an optimal condition can be chosen. Experiments were perfon-ned
at pH 7.5. 29
mM Tris-acetate was used for pH control.
Table 33: Effect of CEX adsorbents on AR reduction
Tris concentration %AR
Resin (mM) Yield (%) Reduction
135 103.3 0.7
Poros XS 140 78.6 6.8
145 72.6 7.3
100 70.0 6.7
Poros HS 105 68.7 7.1
110 60.6 7.6
50 71.5 5.7
Capto SP ImpRes 55 61.0 6.3
60 46.2 6.8
75 67.6 10.0
Nuvia S 80 54.3 10.8
85 41.0 12.2
55 70.3 6.0
Giga Cap CM 650 57.5 62.7 7.0
60 55.6 8.6
65 52.7 9.0
Eshmuno S 70 35.4 11.2
75 21.7 12.1
199
CA 02926384 2016-04-07
65 66.3 8.4
Giga Cap S 650 70 43.6 11.1
75 31.4 12.1
45 72.2 8.9
CM Hyper D 47.5 63.2 9.9
50 51.5 10.3
Example CEX 8.3: Demonstration Of AR reduction with other antibodies: mAb B
and
mAb C
AR reduction technology of the cinTent invention has been demonstrated with
multiple antibodies using CEX Adsorbents. Antibodies have different amounts of
charged
residues and at different positions, leading to a charge interaction behavior
on a CEX column
that differs from one antibody to another. Therefore the impact of cation
type, cation
concentration is different for each antibody.
For each antibody/resin combination, the experimental strategy outlined in
Example
8.1, above, was employed to determine the cation concentration for each cation
type that
provided AR reduction.
Table 34 and Table 35 below shows the data for mAb B and mAb C. The data
clearly
demonstrates that the AR reduction technology works very effectively for other
antibodies. It
is also clear that the concentration ranges are different between different
antibodies. The pH
range chosen was related to the isoelecnic point of the antibody and was
chosen to be
approximately 1 to 2 units less than the pI of the molecule.
200
CA 02926384 2016-04-07
Table 34: AR reduction for mAb B
Buffer Concentration Yield %AR
Resin pH
System (mM) (%) Reduction
120 57.2 8.4
Poros XS 125 46.5 9.3
130 37.1 10.3
85 72.5 16.6
Nuvia S Tris 90 7.5 56.1 16.9
Acetate
95 44.2 17
50 73 8.2
CM Hyper
55 62 9.2
D
60 52.6 9.2
Table 35: AR reduction for mAb C
Buffer Concentration Yield Load %AR
Resin pH
System (mM) (%) %AR Reduction
40 87.4 15.6 8.5
Poros XS 45 56.8 15.7 12.8
50 31.3 15.7 14.3
35 45.1 11.5 11.2
Nuvia S Tris 37 6.0 28.5 15.4 15.2
Acetate
40 15.3 15.2 15.2
18 83.6 16.3 6.3
CM
20 64.9 16.3 11.2
Hyper D
22 50.7 16.4 12.3
Example CEX 8.4: Demonstration of AR reduction with different pH conditions
- adalimumab
The AR species in the current invention is removed in the Flow through/Wash
fraction. Therefore the binding pH is a key variable. The cation concentration
that provides
the desired peiformance will vary with the binding pH. Therefore for each
binding pH, the
experimental strategy outlined in Example 8.1, above, is carried out to
determine the range of
ion concentration that results in AR reduction.
The results of the experiments with different pHs for adalimumab is shown in
Table
36. As can be seen. at lower pH, the cation concentration required to achieve
AR removal in
the wash fraction is higher. It is unexpected that the AR reduction is
significantly more
robust and optimal at higher pHs (closer to pI) than at lower pHs. It is not
obvious to one
skilled in the art to operate a cation exchange chromatography at pH closer to
pI as shown in
201
CA 02926384 2016-04-07
Table 37. Literature data suggests an optimal pH of at least 3 units less than
the pI of the
molecule.
Table 36: Effect of pH on AR reduction
Buffer
%AR
PH Resin Buffer System Concentration Yield
(%)
Reduction
(mM)
5.5 350 58.2 5.9
6.5 225 61.4 6.4
7 Poros XS Tris Acetate 170 75.3 5.6
7.5 140 78.6 6.8
8 125 75.8 5.7
Ammonium
7.5 4 77.9 7.4
Sulfate
6 CM Hyper Sodium 45 86.1 4
6.8 D 30 71.5 7
Chloride
7.5 10 71.3 6.8
7.5 Tris Acetate 45 72.2 8.9
Table 37: Effect of delta pH and pI on AR reduction
[Catio
Yield %AR
pI-pH Antibody Resin Buffer system n]
(%) Reduction
(mM)
Arginine/Tris
1.1 60/29 58.9 7.8
Acetate
2.2 125 73.5 5.4
Sodium
1.8 75 90 1.5
Chloride
1.1 50 72.1 7.2
adalimumab Poros XS
3.1 350 58.2 5.9
2.1 225 61.4 6.4
1.6 Tris Acetate 170 75.3 5.6
1.1 145 72.6 7.3
0.6 125 75.8 5.7
1.6 Poros XS Tris Acetate 120 57.2 8.4
mAb B CM Hyper
Tris Acetate
1.6 D 50 73 8.2
1.6 Nuvia S Tris Acetate 85 72.5 8.4
1.0 Poros XS Tris Acetate 40 87.4 8.5
,
mAb C CM Hyper
Tris Acetate
1.0 D 18 83.6 6.3
1.0 Nuvia S Tris Acetate 35 45.1 11.2
Example CEX 8.5: Demonstration of AR reduction with different ion
concentrations - adalimumab
202
CA 02926384 2016-04-07
Cation concentration is a key variable in the performance of cation exchange
chromatography. For every combination of antibody/resin/pH there is a range of
cation
concentrations that provides AR reduction; the strategy outlined in Example
8.1, above, can
be followed to determine the AR reduction and the con-esponding recovery for
each cation
concentration.
Table 38 below shows the effect of cation concentration on AR reduction. The
table
also includes the effect of cation concentration for different pH values. The
data
demonstrates that the AR reduction can be effectively achieved over a range of
cation
concentrations at each pH and that the concentration ranges depend on the pH.
The table also
includes an example of the concentration range for a different cation type.
Table 38: Effect of cation concentration and pH on AR reduction
Cation
Yield %AR
concentration Buffer system pH Resin
(mM) (%) Reduction
60/29 58.9 7.8
65/2947.4 8.7
Arginine/Tris
23 7.5 80.5 5.8
Acetae
25 72.9 7.3
27 52.2 9.5
115 85.4 4.2
125 6 73.5 5.4
13048.7 7.1
Sodium
75 90 1.5
Chloride 6.8
90 _________________________________________________ 53.7 2.1
45 7 .5 60.7 7.9
50 72.1 7.2
350 58.2 5.9
Poros XS
375 5.5 38.4 7.4
400 29.9 6.2
225 6L4 6.4
250 6 59.5 6.6
.5
275 37.6 7.8
30021.6 8.8
rfris Acetate
165 83.8 4.3
170 7 75.3 5.6
175 70.3 5.7
140 78.6 6.8
145 72.6 7.3
7.5
150 69.2 7.8
175 29.8 10.3
203
CA 02926384 2016-04-07
125 75.8 5.7
130 8 67.7 6.5
135 57.4 7.5
Example CEX 8.6: Demonstration of AR reduction with different buffer systems
with adalimumab
The cation type and concentration are key variables in Cation Exchange
Chromatography. The invention has been demonstrated with Tris, Sodium/Tris,
Ammonium/Tris and Arginine/Tris as cation types/mixtures with effective
reduction of AR in
each case. As one skilled in the art would appreciate the optimal pH and
cation concentration
is different for each cation type/mixture and was derived by using the
strategy outlined in
Example 8.1, above. Experiment were performed at pH 7.5. 29 mM Tris-acetate
was used for
pH control. Table 39 shows the data of AR reduction and corresponding recovery
for the
different cation types/mixtures.
Table 39: Effect of cation types/mixtures on AR reduction
Cation
Yield c/o AR
Buffer System Resin concentration pH
(%) Reduction
(mM)
Arginine/Tris
60 58.9 7.8
acetate
Ammonium
25 72.9 7.3
Sulfate Poros XS ______________
Sodium
50 72.1 7.2
Chloride
Tris Acetate 140 78.6 6.8
Ammonium
4 77.9 7.4
Sulfate CM 7.5
Sodium Hyper D 10 71.3 6.8
Chloride
Tris Acetate 45 72.2 8.9
Ammonium
11 66.6 12.6
Sulfate
Nuvia S
Sodium
75.9 10.5
Chloride
Tris Acetate 75 67.6 10
Example CEX 8.7: Demonstration of AR Reduction With Different Loading
204
CA 02926384 2016-04-07
Furthermore, the strategy outlined in Example 8.1, above, to reduce acidic
species
through careful control of buffer cation type, concentration and pH can be
applied to any
range of protein loading which represents an operational mode of binding
followed by
elution, i.e. not overloaded or a column load factor below that of the
adsorbents binding
capacity. A range of relevant protein loadings for Poros XS at pH 7.5 using
Tris as the cation
is shown in Table 40 showing robust AR reduction.
Table 40: Impact of Column loading
Column
Loading
Buffer Concentration Yield % AR
(gpH
product/L System (mM) (%) Reduction
resin)
25 160 83.6 6.4
30 155 79.4 6.0
35 140 87.4 4.8
38 Tris 140 7.5 83.5 5.0
40 140 76.4 6.0
42 140 74.5 5.7
45 140 67.0 6.6
Example CEX 8.8: Demonstration of AR reduction with different load
concentration
Furthermore, the strategy outlined in Example 8.1, above, to reduce acidic
species
through careful control of buffer cation type, concentration and pH can be
applied to any
range of column feed streams of varying protein concentration. A range of
varying protein
load concentration for Poros XS at pH 7.5 using Tris as the cation is shown in
Table 41
showing robust AR reduction.
Table 41: Effect of Protein Load concentration
Load
Buffer Concentration % AR
Concentration Resin pH Yield (%)
System (mM)
Reduction
(mg/mL)
____ 3 140 _____________________________________________ 77.3 7
4 ____________________________________ 145 60.7 7
5140 78.7 6.7
Poros XS Tris Acetate __________ 7.5
145 ____________________________________________________ 64.1 7
6 145 59.5 6.9
7 140 77.6 6.5
205
CA 02926384 2016-04-07
As described above, the AR for adalimumab is further grouped into two regions
termed AR1 and AR2, based on a certain retention time of the peaks seen on the
WCX-10
method. The characteristics of the variants in these two regions are expected
to be different
and hence the methods that reduce variants belonging to these groups can be
specifically
delineated.
Further, in addition to achieving a certain AR reduction, it may be desirable
to
achieve a certain absolute level of AR levels, in consideration of reducing or
removing
certain variants. The capability of the current invention in achieving a
certain absolute level
of AR, AR1 and AR2 is demonstrated in Table 42.
The specific species comprising the AR1 species can be identified and
quantitated, to
demonstrate reduction of such species by methods of the current invention. Two
of such
species, glycated mAb, and MGO modified mAb have been identified and shown to
be
reduced by the methods of this invention. While these species are among the
acidic species
part of the charge variants, the acidic species typically described in the
literature is the
deamidated mAb, which is distinctly different.
Table 42: The final impurity level
Cation Conc. Yield %Final %Final
Buffer System (mM) pH (%) AR1 AR
Arginine/Tris 60 7.5 58.9 0.3 5.8
Acetate 65 7.5 47.4 0.3 4.7
23 7.5 80.5 0.6 8.3
Ammonium
25 7.5 72.9 0 6.4
Sulfate
27 7.5 52.1 0.4 5.0
115 6 85.4 1.3 10.2
125 6 73.5 0 8.1
135 6 48.7 0 6.1
Sodium Chloride 75 6.8 90 1.4 10.9
90 6.8 53.7 0.7 11.2
45 7.5 60.7 0 6.2
50 7.5 72.1 0 7.8
206
CA 02926384 2016-04-07
350 5.5 58.2 0 7.7
375 5.5 38.4 0.1 6.2
400 5.5 29.9 1.5 7.3
Tris Acetate 225 6.5 61.4 0.8 7.2
250 6.5 59.5 0 6.8
275 6.5 37.6 0 5.6
300 6.5 21.6 0 4.7
The method of the current invention can effectively reduce AR2 levels, as an
overall
decrease in AR levels is achieved. The method can be used to achieve a target
absolute level,
as exemplified by the data presented in Table 42.
The method of the cuiTent invention can effectively achieve AR reduction as
well as
achieve a target absolute level of acidic species as exemplified by the data
presented in Table
42.
Example CEX 8.9 Demonstration of glycated and methylglyoxylated species
reduction
The strategy outlined in Example 8.1, above, to reduce acidic species through
careful
control of buffer cation type, concentration and pH can be further extended to
specific post-
translational modifications. While acidic species are defined in the
application as impurities
that are less retained than the main peak on an analytical weak cation
exchange (WCX)
HPLC column, specific known product related substances derived from cellular
metabolism
modification such as glycation and methylglyoxal (MGO) can be specifically
identified as
being part of the acidic species. Figure 171 and Figure 172 shows the outcome
of in-vitro
labeling experiments which demonstrate that glycation and MGO modified
antibody are
unique species that are resolved by the WCX method in the AR1 region of the
chromatogram
and can be enriched in vitro. Furthermore, the invention described here shows
that glycated
and MGO modified antibody can be effectively removed through the careful
control of buffer
cation type, concentration and pH using the CEX as described in Example 8.1,
above.
Quantitative reduction of AR I and hence the glycated and MGO species by CEX
and CEX-
Mixed Mode resins is show in Table 43 and Table 44.
207
CA 02926384 2016-04-07
Table 43: Glycated species removal
Buffer Conc. Yield Load % Load % % AR1 % AR
Resin pH
System (mM) (%) AR1 AR
Reduction Reduction
Poros
Tris 135 7.5 54.0 40.8 58.6 30.8 34.8
XS
Table 44: MGO peak removal
Buffer Concentration %AR1 %AR
Resin pH Yield (%)
System (mM) Reduction Reduction
Toyo Pearl
MX TRP 80 66.7 2.8 7.2
650M Tris 7.5
Poros XS 145 64.1 2.7 7
Nuvia S 90 48.5 3.1 9.6
Example CEX 8.10: Demonstration of lysine distribution modification
The strategy outlined in Example 8.1, above, to reduce acidic species also can
be used
to modulate the distribution of C-terminal Lys variants of monoclonal
antibodies, a known
post-translational modification leading to charge heterogeneity. Some minor
changes in the
distribution of Lys isoforms is expected through the reduction of acidic
species as the WCX
analysis is a compositional analysis. However, through careful control of
buffer cation type,
concentration and pH care, in addition to reducing acidic species, the elution
pool can be
enriched for the more basic isoforms (Lys 1 and Lys2). Table 45 and Figure 173
depicts a
non-limited example of the impact of pH and cation (Tris) concentration on
basic isoform
enrichment.
208
CA 02926384 2016-04-07
Table 45: Change in Lysine distribution during CEX Chromatography - impact of
Tris
concentration
% LYSO % LYS1 % LYS2 Buffer Buffer
Concentration pH
decrease Increase Increase System
(mM)
1.6 4.4 350
6.5 5.5 375 5.5
9.7 7.5 11.9 400
1.9 5 2.9 225
1.9 5.3 3 250
6.5
6.1 7.4 6 Tris 275
Acetate
11.8 8.6 10.8 300
0.2 5.2 1.6 140
0.6 5.7 1.8 145
7.5
1.8 6.8 2.4 150
16.4 14.9 10.3 175
Example CEX 8.11: Demonstration of HCP and aggregate reduction in addition
to AR reduction
In the present invention, it has been demonstrated that HCP and aggregate
levels can
be effectively reduced by appropriate adjustment of the elution conditions,
after washing off
the AR enriched species in the Flow Through/wash fractions.
Table 46 and Table 47 shows the HCP and aggregate removal achieved along with
AR reduction. The data clearly shows that other process related impurities and
product
related substances can be achieved using the current invention on the CEX
adsorbents, and
hence functions as an effective polishing step in the large scale purification
of monoclonal
antibodies.
209
CA 02926384 2016-04-07
Table 46: Aggregate removal during CEX Chromatography
%
%Aggregate %Fragment
Monomer
Resin Antibody Buffer system pH
Reduction Reduction
Increase
mM
Ammonium 0.04 0.17 0.2
CM Hyper
Sulfate
D
45 mM Tris
0.01 0.18 0.19
Acetate
11.5 mM
Ammonium 0.16 0.17 0.33
Sulfate
75 mM Tris
Nuvia S 0.09 0.11 0.2
Acetate
adalimumab
22.5mM Sodium
0.08 0.19 0.27
Chloride 7.5
27 mM
Ammonium 0.75 0.27 1.02
Sulfate
Poros XS 140mM Tris
0.51 0.41 0.92
Acetate
145mM Tris
0.58 0.41 0.98
Acetate
85mM Tris
Nuvia S 0.19 0.27 0.47
Acetate
mAb B
130mM Tris
Poros XS 0.36 0.04 0.39
Acetate
35mM Tris
Nuvia S 0.07 0.01 0.07
Acetate
mAb C 6.0
50mM Tris
Poros XS 0.27 0 0.28
Acetate
210
CA 02926384 2016-04-07
Table 47: HCP Removal during CEX Chromatography
Load Eluate
Buffer Reduction
Resin Antibody pH HCP Pool HCP
system fold
(ng/mg) (ng/mg)
mM
CM Ammonium
Hyper D Sulfate 8105 3844 2.1
45 mM Tris _ 8628 5615 1.5
11.5 mM
Ammonium
Sulfate 5314 2405 2.2
75 mM Tris
Nuvia S
Acetate 17317 12845 1.4
22.5mM
adalimumab Sodium
Chloride 9091 4115 2.2
¨ 27 mM
7.5
Ammonium 21857 12574 1.0
Sulfate
140mM
Poros
Tris
XS
Acetate 14732 9181 1.7
145mM
Tris
Acetate 15359 10113 1.6
85mM Tris
Nuvia S
Acetate 735 319 2.3
mAb B 130mM
Poros
Tris
XS
Acetate 2183 404 5.4
35mM Tris
Nuvia S
Acetate 27 31 0.9
¨Poros mAb C 6.0
50mM Ti-is
XS Acetate 25 15 1.7
Example CEX 8.12: Demonstration of means of controlling AR reduction
Controlling the final product quality by modifying the process based on the
quality of
the intermediate material is an approach that has been proposed as an
effective way of
ensuring product quality, with the view of ensuring safety and efficacy.
Considering that the AR levels generated during cell culture and other
upstream steps
can be variable, it is desirable to design a downstream process step that
implements a means
211
CA 02926384 2016-04-07
of controlling the product quality and to further have a specific means of
controlling a
process parameter to influence the quality of the product.
In the current invention, such a control is possible, as the cation
concentration is a
single parameter that can be modified to achieve a desired separation of the
AR species. For
example, to achieve a higher level of AR reduction, the Tris concentration of
the loading
material and the wash buffer can be decreased, such that the AR enriched
species is collected
in the Flow Through fraction.
As an example, and not to be restrictive in any manner, it has been
demonstrated in
this example that the AR levels can be controlled by changing the Tris
concentration of the
load and wash solutions. A pilot scale Poros XS column (10 cm diameter x 22 cm
height,
I .7L), was used for this study.
The load material and the stock buffer are both prepared at 300mM Tris
concentration
at the same pH. The AR level of the load material was measured to be X%. The
load
material and equilibration/wash buffer are in-line diluted to the target Tris
concentration
based on predetermined correlation between the AR levels and Tris
concentration. As
demonstrated in the example, when the Tris concentration was adjusted to 156
mM, a final
AR reduction of 4.1% was achieved, whereas when the Tris concentration was
adjusted to
150 mM, a final AR level of 3.1 was achieved (Table 48). This allows very
predictable
control of the AR levels ensuring achievement of the desired product quality.
Table 48: Controlling AR Reduction using Process Analytical Technology
approach
%AR
Tris conc (mM) Yield (%)
Reduction
156 51.9 4.1
150 70.5 3.1
131 95.3 1.3
In addition to the acidic species reduction demonstrated in Example CEX 8.1
through
careful control of the pH cation type and concentration in the load (process
stream) and
equilibration/wash buffers, the composition of the elution buffer can also be
used to further
improve the product quality profiles. The impact of various cation types,
concentration and
212
CA 02926384 2016-04-07
pH were tested for eluting the product. There is a wide selection for elution
buffer as shown
in Table 49. The experiments were performed using Poros XS resin.
Table 49: Elution buffer types on aggregates removal
Buffer System pH Yield (%) Aggregate
Reduction
200mM Sodium Sulfate/29mM Tris
5.2 76.1 0.36
Acetate
160mM Sodium Sulfate/29mM Tris
5.2 82.3 0.82
Acetate
150M Sodium Sulfate/29mM Tris
5.2 78.8 0.90
Acetate
140M Sodium Sulfate/29mM Tris
5.2 78.2 1.00
Acetate
400mM Sodium Sulfate/29mM Tris
4.0 78.5 0.98
Acetate
100mM Sodium Sulfate/140mM Tris
5.2 70.9 1.25
Acetate
150mM Sodium Sulfate/140mM Tris
5.2 79.6 1.05
Acetate
140M Sodium Sulfate/140mM Tris
5.2 75.4 1.07
Acetate
130mM Sodium Sulfate/140mM Tris
5.2 78.2 1.07
Acetate
300mM Sodium Sulfate/30mM Tris
4.6 80.3 0.57
Acetate
150mM Sodium Sulfate/29mM Tris
Acetate 7.5 75.0 0.92
Example CEX 8.13: Demonstration of AR reduction with cation-HIC Mixed
Mode resin
The strategy outlined in Example 8.1, above, to reduce acidic species through
careful
control of buffer cation type, concentration and pH can be expanded to include
other
chromatography adsorbents such as mixed mode or multi-modal absorbents which
include a
cation exchange mechanism. Table 50 outlines the conditions used and the AR
reduction
achieved for two cation-hydrophobic interaction mixed mode resins. The data
clearly shows
that the technology outlined in Example 8.1 is robust in delivering AR
reduction for these
types of resins across in addition to traditional cation exchange adsorbents.
As described in
Example 8.1, the AR reduction can be balanced with recovery and an optimal
condition can
be chosen. As a further demonstration, mAb 2 was also evaluated (Table 51)
with the same
213
CA 02926384 2016-04-07
outcome showing the same relationship between cation concentration, recovery
and AR
reduction. As previously shown in Example 8.1, the optimal condition for
different
molecules varies. Furthermore, this technology when applied to CEX-HIC mixed
mode
resins also shows reduction of impurities as previously described.
Table 50: Adalimumab AR Reduction by Cation Exchange Mixed Mode
Chromatography
Buffer Tris Yield %AR
Resin Concentration pH
System (mM) (%) Reduction
70 7.5 63.8 6.5
Nuvia C Prime 72.5 7.5 61.1 6.0
75 7.5 57.1 6.7
Tris Acetate
75 7.5 80 5.7
Toyo Pearl MX Trp
80 7.5 66.7 7.2
650M
85 7.5 51.8 8.6
Table 51: mAb B AR Reduction by Cation Exchange Mixed Mode Chromatography
Buffer Concentration Yield %AR
Resin pH
System (mM) (%)
Reduction
75 7.5 86.0 2.0
Nuvia C Prime 85 7.5 74.6 5.9
95 7.5 61.3 6.8
Tris Acetate
90 7.5 81.1 6.4
Toyo Pearl MX Tip
95 7.5 68.8 8.8
650M
100 7.5 53.5 10.7
As described in previous sections, the AR for adalimumab is further grouped
into two
regions termed AR1 and AR2, based on a certain retention time of the peaks
seen on the
WCX-10 method. The characteristics of the variants in these two regions are
expected to be
different and hence the methods that reduce variants belonging to these groups
can be
specifically delineated.
Further, in addition to achieving a certain AR reduction, it may be desirable
to
achieve a certain absolute level of AR levels, in consideration of reducing or
removing
certain variants. The capability of the current invention in achieving a
certain absolute level
214
CA 02926384 2016-04-07
of AR, AR1 and AR2 is demonstrated in Table 52A with Tables 52B and 52C
indicating the
levels of additional process-related impurities or acidic species.
The specific species comprising the AR1 species can be identified and
quantitated, to
demonstrate reduction of such species by methods of the current invention.
While these
species are among the acidic species part of the charge variants, the acidic
species typically
described in the literature is the deamidated mAb, which is distinctly
different. These results
show that the Cation Exchange Resin with additional pendant hydrophobic
interaction
functionality. is able to provide AR reduction effectively, similar to the CEX
Adsorbents.
Table 52A: Final acidic species level for adalimumab
Tris
Buffer Yield
Final Final Final
Resin Concentration pH
System (%) %AR1 %AR2 %AR
(mM)
70 7.5 63.8 0.39 4.64 5.03
Nuvia C Prime 72.5 7.5 61.1 0.36 4.4 4.75
Tris 75 7.5 63.8 0.39 4.06 4.45
Acetate 75 7.5 80 0.6 4.7 4.8
Toyo Pearl
80 7.5 66.7 0.5 3.2 3.7
MX Trp 650M
85 7.5 51.8 0.2 2.2 2.4
Table 52B: Aggregates/Fragments Reduction by Cation Exchange Mixed Mode
Chromatography
%Fragmen %
Buffer %Aggregat
Resin Antibody pH t Monomer
System e Reduction
Reduction Increase
70mM
Nuvia C prime 0.3 0.34 0.63
adalimuma Tris
Toyo Pearl MX b 75mM
0.08 0.56 0.65
Trp 650M Tris
7.5
85mM
Nuvia C prime Tris 0.87 1.18 2.04
mAb B
Toyo Pearl MX 95mM
0.0 1.8 1.8
Trp 650M Tris
215
CA 02926384 2016-04-07
Table 52C: HCP Reduction by Cation Exchange Mixed Mode Chromatography
Load Eluate Fold
Resin Antibody Buffer pH HCP pool HCP
Reductio
(ng/mg) (ngimg)
Toyo Pearl MX Trp 70mM
202.6 38.9 5.2
650M adali muma Tris
Nuvia C prime 75mM 205.5 72.8 2.8
Tris
7.5
Toyo Pearl MX Trp 95mM
983.3 137.1 7.2
650M Tris
mAb B
85mM
Nuvia C prime1011.3 88.2 11.5
Tris
Example 8.14: CEX with Tris/Formate Buffer System: AR Reduction With
Different Tris/Formate Concentrations ¨ Adalimumab
This Example provides a demonstration of AR reduction using a Tris/Formate
buffer
system and CEX. Cation (e.g. Tris) concentration is a key variable in the
performance of
cation exchange chromatography.
CEX Adsorbent:
Poros XS (Applied Biosciences, part# 4404338), a rigid 50 i_tm polymeric bead
with a
backbone consisting of cross-linked poly[styrene-divinylbenzenel, was used in
this
experiment.
CEX Chromatography Method
Poros XS was packed in 1.0 cm x 10.0 cm (OmniFit) columns. The column was
equilibrated in a buffer system with appropriate pH and conductivity. The
column load was
prepared in the equilibration buffer by buffer exchange or addition of the
stock solutions to
obtain the target ion concentrations as specified and loaded on the column at
approximately
40 g protein/L resin (or as specified) followed by washing with the
equilibration buffer for 20
CV (or as specified). The antibody product was then eluted, and the column
regenerated.
In this Example, adalimumab and Poros XS were chosen. The experiment was
perfomed at Tris concentrations of 120 ¨ 150 mM buffered to pH 7.5 with formic
acid. The
column was equilibrated with the respective Tris/Formate at pH 7.5 for each
run.
216
CA 02926384 2016-04-07
Adalimumab was prepared at the respective Tris/Formate pH 7.5 and loaded to
the column at
35 - 45 g-protein/L-resin. The column was then washed with 20 CVs with the
equilibration
buffer, and then eluted with a 140 mM Tris/Formate + 140 mM Sodium Sulfate
buffer at pH
5.2. The eluate was analyzed for product quality and yield.
Table 53, below, shows the effect of Tris concentration on AR reduction and
aggregate reduction for adalimumab on Poros XS in a Tris/Formate buffer system
at a pH of
7.5. The data demonstrates that AR and aggregate reduction can be effectively
achieved over
a range of Tris concentrations and column loadings. This example demonstrates
the
effectiveness of the Tris/Formate buffer system in general and specifically
the effectiveness
of the Tris cation in the context of the Formate on the CEX column for AR
reduction.
Further, it confirms that the CEX AR reduction method applies to a variety of
buffer systems.
Table 53: Effect of Tris concentration at pH 7.5 on AR and aggregate reduction
TrisAR2
Loading . AR AR1 Final Final Aggregate
Concen- - Yield Reduc-
g/L Reduction Reduction AR I .
AR2 Reduction
tration ton
120 mM 96% 0.5% 0.4% 0.1% 0.1% 4.2%
0.1%
88% 1.8% 0.6% 0.0% 1.3% 2.9% 0.2%
125 mM 90% 2.4% 0.5% 0.1% 1.8% 3.0%
0.2%
78% 3.0% 0.6% 0.0% 2.4% 2.4% 0.2%
35 76% 3.1% 2.3% 0.8% 1.0% 8.5% 1.3%
130 mM 40 64% 4.0% 2.5% 0.4% 2.7% 6.9%
1.4%
70% 4.0% 2.4% 0.3% 3.6% 6.0% 1.5%
135 mM 78% 5.6% 0.8% 0.0% 4.8% 2.8%
0.3%
58% 5.3% 0.8% 0.0% 4.4% 3.1% 0.2%
35 63% 6.1% 2.5% 0.3% 3.5% 6.4% l.3%
140 mM 40 55% 6.0% 2.4% 0.3% 4.3% 5.3%
1.3%
55% 5.8% 2.4% 0.3% 4.8% 4.9% 1.0%
14 mM 35 55% 4.1% 0.6% 0.0% 3.5% 1.7%
0.3%
5
40 44% 4.2% 0.6% 0.0% 3.6% 1.6% 0.3%
35 50% 7.4% 2.4% 0.2% 5.0% 4.5% 1.1%
150 mM 40 44% 7.4% 2.6% 0.3% 5.5% 4.3%
0.7%
45 40% 6.9% 2.5% 0.2% 5.7% 4.1% 0.5%
Wash Volumes for CEX Chromatography
The experiments were performed using Protein A eluate as CEX loading material.
Run I was performed under the load/wash buffer conditions of 128 mM Ms-formate
buffer
system, pH 7.5. 40 g/L loading. Wash was performed with 20CV of the wash
buffer. Run 2
217
CA 02926384 2016-04-07
was performed under the loacUvvash buffer conditions of 160 mM Tris-formate
buffer
system, pH 7.5, 40 g/L loading. Wash was performed with 6CV of the wash
buffer.
As shown in Table 54, Run 1 and Run 2 gave similar yield and AR reduction.
Therefore, these results demonstrate that process performance can be achieved
by varying
the wash volume with corresponding loading conditions.
Table 54: Wash volume effect on AR reduction and yield
Product quality Run 1 (20 CV Wash Run 2 (6 CV Wash)
%Yield 92.1 89.6
%Load AR1 3.44 3.43
%Eluate AR1 1.43 1.21
% AR1 Reduction 2.01 2.22
%Load AR2 10.44 9.87
%Eluate AR2 9.25 8.86
% AR2 reduction 1.19 1.01
% Total load AR 13.88 13.3
% Total eluate AR 10.68 10.07
% total AR reduction 3.20 3.23
Example 9: Mixed Mode Chromatography Examples
Example MM 9.1: Resin and pH Combination
In this Example one of the approaches outlined in the general description was
employed to deterinine the operating conditions to implement the invention.
Specifically. a
218
CA 02926384 2016-04-07
response surface design DOE was applied to evaluate mAb AR reductions and
recovery
yields.
The demonstration of the current invention for a specific antibody & resin is
provided
in this Example, and consists of
1. Choosing a pH in the range of 6.8 to 8.4.
2. Choosing a conductivity in the range of 2.3 to 13.7 mS/cm.
3. Determining the acidic species distribution in the Flow Through/wash
fractions.
4. Choosing an optimal pH and conductivity that provides the desired acidic
species
levels and recovery
In this example, adalimumab and resin Capto Adhere were chosen. The
experiments
were performed with Tris/Acetate buffer system at target pH and conductivity
listed in Table
55. The load material was from Protein A affinity capture and pH adjusted.
This study
demonstrated the effect of loading pH and conductivity on acidic species
reduction. The
acidic species reduction can be significantly affected by operating pH. AR
reduction
increased with increasing pH and/or decreasing conductivity (Table 55, Table
56 and Figure
174)
Table 55: DOE study condition
Tris Acetate Range Edge points for Response
Buffer Surface
pH 7.0 - 8.2 6.8. 8.4
Conductivity 4.0- 12.0 2.3, 13.7
Table 56: DOE Study Operating Conditions and Results
DOE exp pH Conductivity (mS/cm) AAR (%) Yield (%)
1 7.0 4.0 0.4 83
2 7.6 8.0 0.4 73
3 7.6 2.3 1.3 82
4 7.6 8.0 0.6 68
7.6 8.0 0.2 70
6 7.6 8.0 -0.2 69
7 8.2 4.0 2.1 67
8 7.6 8.0 1.3 69
219
CA 02926384 2016-04-07
9 7.0 12.0 -0.2 70
7.6 8.0 1.2 71
11 8.2 12.0 1.4 74
12 6.8 8.0 1.2 76
13 8.4 8.0 1.8 67
14 7.6 8.0 1.4 71
7.6 13.7 1.0 74
16 7.6 8.0 1.6 70
Note: AR reductions and protein recovery yields were calculated based on the
Flow Through
fractions at about loading 200 g protein per L of resin.
Example MM 9.2: Fraction Pooling
In this example, adalimumab and resin Capto Adhere were chosen. The
experiments
were performed with Tris/Acetate buffer system at pH 7.85 and conductivity of
2.5 mS/cm.
The load material was from Protein A affinity capture and pH adjusted. Column
Flow
Through was fractionated throughout the entire load and wash phases. Each
fraction was
analyzed for acidic species and protein recovery. Figure 175, Figure 176 and
Table 57
demonstrate AR reduction achieved with the corresponding recovery. These AR
reductions
and recoveries correspond to the cumulative pools of the fractions from the
start to the
various points during the load/wash. This is depicted in Table 57 where the AR
reductions
corresponding to each of these pools. This data is plotted in Figure 175.
220
CA 02926384 2016-04-07
Table 57: Cumulative AR reduction in Flow Through/wash fractions
Flow Through Fraction Yield A AR1 A AR2 A AR ALys(%)
(Load & wash) (%) (%) (%) (%)
A2 23 2.56 3.13 5.69 5.61
A2+A3 45 2.31 2.19 4.49 4.37
A2+A3+A4 58 1.83 1.89 3.72 3.63
A2+A3+A4+A5 65 1.57 1.58 3.15 3.06
A2+A3+A4+A5+A6 73 1.38 1.32 2.70 2.61
A2+A3+A4+A5+A6+B7 86 1.26 1.12 2.38 2.30
A2+A3+A4+A5+A6+B7+B6 89 1.19 0.91 2.09 2.02
A2+A3+A4+A5+A6+B7+B6 90 1.14 0.82 1.96 1.89
+B5
Note: "A" Fractions are load fractions and 13" Fractions are wash fractions
Example MM 9.3: Demonstration of AR Reduction with Mixed Mode
Adsorbents In this Example, adalimumab was chosen. The experiments were
performed
with Tris/Acetate buffer system at pH 7.85 and conductivity of 2.5, 3.5, and
4.5 mS/cm. The
same load material was applied to different mixed mode resin columns. The load
material
was from Protein A affinity capture and pH adjusted. Table 58 shows that all
three mixed
mode resins could reduce mAb acidic species. Due to the differences of resin
ligands, the AR
reduction level may slightly vary under certain conditions.
Table 58: Adalimumab AR Reduction and Protein Recovery Yields Processed with
Different Mixed Mode media
Tris/Ac Buffer
Capto Adhere HEA PPA
Operating pH 7.85 pH 7.85 pH 7.85
Condition 4.5 3.5 2.5 4.5 3.5 2.5 4.5 3.5 2.5
s mS/c mS/c mS/c mS/c mS/c mS/c mS/c mS/c mS/c
m m m m m m m m m
Yield (%) 50 52 58 49 52 56 40 43 47
_
AR
Reduction 1.8 3.8 3.7 1.1 2.7 3.2 1.4 2.2 3.5
(%)
Yield (%) 68 71 73 65 75 69 61 64 63
AR
Reduction 1.1 2.7 2.7 0.5 1.8 2.1 0.4 1.9 2.6
(%)
221
CA 02926384 2016-04-07
Example MM 9.4: Demonstration of AR reduction with other antibodies: mAb B
and mAb C
In this Example, another two different monoclonal antibodies besides
adalimumab
(mAb B and mAb C) and resin Capto Adhere was chosen. The experiments were
performed
with Tris/Acetate buffer system at multiple pH and conductivity condition. The
load
materials of all mAbs were from Protein A affinity capture and pH adjusted.
mAb C was also
applied to another two MM resins besides Capto Adhere under the same operating
conditions.
Table 59 outlines the operating conditions and the AR reduction achieved and
the
corresponding recovery achieved. The results demonstrate that the technology
can also
reduce acidic species for other monoclonal antibodies with optimal pH and
conductivity
conditions. Experiments were performed with Tris-acetate buffer system.
Table 59: AR Reductions and Protein Recovery for different mAb with Capto
Adhere
columns
mAb pH conductivity AAR (%) Yield (%)
(mS/cm)
adalimumab 7.85 3.5 3.8 52 ,
7.85 2.5 3.7 58
mAb B 6.8 3.0 6.3 51
6.8 4.5 4.2 53
7.0 3.0 5.1 77
8.0 3.0 3.4 60
mAb C 9.0 3.0 5.3 73
8.5 3.0 3.5 54
8.0 3.0 3.7 50
11-)
CA 02926384 2016-04-07
Figure 177 displays the mAb B cumulative pool AR broke through the column of
Capto Adhere operated at pH 7.0 and conductivity of 3.0mS/cm with Tris-Acetate
buffer.
Figure 178 shows the rriAb C cumulative pool AR broke through the column of
Capto
Adhere operated at pH 8.5 and conductivity of 3.0mS/cm with Tris-Acetate
buffer. Both of
graphs demonstrate similar AR breakthrough curves with different AR values
comparing to
adalimumab (Figure 176). Figure 179 presents the AR breakthrough curves of Mab
C with
three different mixed mode resins with Tris-acetate buffer operated at pH 8.5
and
conductivity of 3.0mS/cm. The data clearly demonstrates that the AR reduction
technology
using mixed mode resins works very effectively for other antibodies.
Example MM 9.5: Demonstration of relative pH on AR reduction with different
resins using adalimumab antibody material
In this Example, data compiled from different experiments is shown to
demonstrate
the impact of the pH choice, relative to the pI of the protein on AR
reduction. This data set
provides the basis for one skilled in the art to determine a pH range to
implement the current
invention. Further, this reiterates the fact that the pH choice depends on
several factors and
the relationship between pH and AR reduction is also mAb dependent. Figure 180
demonstrates the impact of pH-pI and conductivity on AR reduction which
compiled data
from the experiments performed with Capto Adhere under conditions listed in
Table 60.
Figure 181 shows the impact of pH-pI and conductivity on mAb B AR reduction
including
the experiments operated with Tfis/Acetate buffer system and multiple mixed
mode resins
under the conditions listed in Table 61. Figure 182 shows the impact of pH-pI
and
conductivity on mAb C AR reduction including the experiments operated with
Tris/Acetate
buffer system and multiple mixed mode resins under the conditions listed in
Table 62. All the
load materials were from Protein A affinity capture and pH adjusted. It is
also clear that the
AR reduction can be achieved with the present invention with a range of pH
choices, in the
range of + 0.5 to -2.5 pH units from pI for adalimumab. One skilled in the art
can choose an
appropriate pH to achieve a target AR reduction.
223
CA 02926384 2016-04-07
Table 60: Operating conditions and AR reductions for adalimumab
Buffer system pH pH-pI Conductivity (mS/cm) AR reduction
Tris/Ac 7 -2.02 4 0.4
7.6 -1.42 8 0.4
7.6 -1.42 2.3 1.3
7.6 -1.42 8 0.6
7.6 -1.42 8 0.2
7.6 -1.42 8 -0.2
8.2 -0.82 4 2.1
7.6 -1.42 8 1.3
7 -2.02 12 -0.2
7.6 -1.42 8 1.2
8.2 -0.82 12 1.4
6.8 -2.27 8 1.2
8.4 -0.57 8 1.8
7.6 -1.42 8 1.4
7.6 -1.42 13.7 1.0
7.6 -1.42 8 1.6
7.5 -1.52 3.75 1.7
7.6 -1.42 2.5 2.7
7.6 -1.42 2.5 2.0
7.6 -1.42 5 1.3
7.6 -1.42 5 1.1
7.85 -1.17 2 3.5
7.85 -1.17 3.75 3.2
7.85 -1.17 3.75 2.1
7.85 -1.17 3.75 2.8
7.85 -1.17 3.75 2.2
7.85 -1.17 5.5 2.1
8.1 -0.92 2.5 5.0
8.1 -0.92 2.5 , 2.6
8.1 -0.92 5 -0.2
8.1 -0.92 5 -1.1
8.2 -0.82 3.75 2.9
_
Arg/Ac 8.5 -0.52 1 6.8
9.0 -0.02 1 6.5
9.5 0.48 1 1.9
Trol/Ac 7.85 -1.17 1 5.7
8.0 -1.02 1 8.0
I 8.5 -0.52 1 6.0
224
CA 02926384 2016-04-07
Table 61: Operating conditions and AR reductions for mAb B
pH pH-pI Conductivity (mS/cm) AR reduction
Capto Adhere 6.8 -0.45 3 6.3
7 -0.25 3 6.2
7.5 0.25 3 4.0
8 0.75 3 3.2
6.8 -0.45 4.5 4.1
7.5 0.25 4.5 3.3
PPA 6.8 -0.45 3 1.1
7 -0.25 3 0.9
7.5 0.25 3 1.3
8 0.75 3 0.5
6.8 -0.45 4.5 1.6
7.5 0.25 4.5 3M
HEA 6.8 -0.45 3 1.8
7 -0.25 3 1.4
7.5 0.25 3 3.6
8 0.75 3 0.7
6.8 -0.45 4.5 2.2
7.5 0.25 4.5 0.9
Table 62: Operating conditions and AR reductions for mAb C
pH pH-pl Conductivity (mS/cm) A%AR
Capto Adhere 8.0 -1.11 , 1 1.5
8.5 -0.61 1 3.5
, 9.0 -0.11 1 5.4
PPA 8.0 -1.11 1 -0.4
8.5 , -0.61 1 1.1
9.0 -0.11 1 2.1
HEA 8.0 -1.11 1 -1.6
8.5 -0.61 1 1.9
9.0 -0.11 1 2.8
Example MM 9.6: Effect of pH on AR reduction
Response surface design DOE was applied to evaluate the impact of pH and
conductivity on mAb AR reductions. In this example, adalimumab and Capto
Adhere were
chosen. The experiments were performed with Tris/Acetate buffer system. The
load material
was from Protein A affinity capture and pH adjusted. Besides the pH and
conductivity ranged
tested and demonstrated in Table 63 and Table 64, higher pH ranges were also
studied
(Figure 183).
225
CA 02926384 2016-04-07
The results in Figure 183 and Figure 184 demonstrated that mAb acidic species
can be
reduced at wide pH range from 6.8 to 9.5.
Table 63: DOE study condition
Tris Acetate Range Edge points for Response
Buffer Surface
pH 7.0 - 8.2 6.8,8.4
Conductivity 4.0- 12.0 2.3, 13.7
Table 64: AR reduction and Yield in DOE study
Experiment # pH Conductivity AAR Yield
1 7.0 4.0 0.4 83
2 7.6 8.0 0.4 73
3 7.6 2.3 1.3 82
4 7.6 8.0 0.6 68
5 7.6 8.0 0.2 70
6 7.6 8.0 -0.2 69
7 8.2 4.0 2.1 67
8 7.6 8.0 1.3 69
9 7.0 12.0 -0.2 70 ,
10 7.6 8.0 1.2 71
11 8.2 12.0 1.4 74
12 6.8 8.0 1.2 76
13 8.4 8.0 1.8 67
14 7.6 8.0 1.4 71 ,
15 7.6 13.7 1.0 74
16 7.6 8.0 1.6 70
Note: AR reductions and protein recovery yields were calculated based on the
Flow Through
fractions at about loading 200 g protein per L of resin
Example MM 9.7: Demonstration of AR reduction with different ion
concentrations/ion strength - adalimumab
In this Example. adalimumab was chosen. Besides the conductivity range tested
presented before, lower conductivity and higher conductivity ranges were also
studied with
the Capto Adhere. Table 65 and Table 66 display the DOE study conditions using
Capto
Adhere columns with Tris/Acetate buffer system. The load material was from
Protein A
affinity capture and pH adjusted. Column Flow Through pool was collected in
each run from
50 mAU of UV A280 on the ascending and 150 mAU on the descending side of the
peak.
Figure 185 demonstrates the effect of pH (6.8 to 8.4). conductivity (2.3 to
13.7 mS/cm), and
226
CA 02926384 2016-04-07
protein load amount (116 to 354 g/L). Figure 186 demonstrates the AR reduction
at
conductivity as low as - lmS/cm. Table 67 demonstrates the AR reduction at
conductivity 86
mS/cm with Ammonia Sulfate-Tris-Acetate buffer system.
The results demonstrated that mAb acidic species can be reduced at wide
conductivity
ranges from 1 to 86 mS/crn.
Table 65: DOE study condition
Tris Acetate Range Edge points for Response
Buffer Suiface
PH 7.6 - 8.1 7.5, 8.2
Conductivity 2.5 - 5.0 2.0, 5.5
Protein load 150 - 320 116, 354
amount (g/L)
Table 66: DOE operating condition and results
pH Conductivity (mS/cm) Load amount
(g/L) AAR (%) Yield (%)
7.5 3.75 235 1.7 89
7.6 2.5 150 2.7 94
7.6 2.5 320 2.0 95
7.6 , 5 150 1.3 97
7.6 5 320 1.1 103
7.85 2 235 3.5 94
7.85 , 3.75 116 3.2 86
7.85 3.75 235 2.1 90
7.85 3.75 235 2.8 90
7.85 3.75 354 2.2 91
7.85 5.5 235 2.1 92
8.1 2.5 150 5.0 80
8.1 2.5 320 2.6 87
8.1 5 150 -0.2 95
8.1 5 320 -1.1 98
8.2 3.75 235 2.9 90
227
CA 02926384 2016-04-07
Table 67: AR reduction and protein recovery at conductivity of 86 mS/cm and pH
7.9
Conductivity
pH Yield (%) AAR (%)
(mS/cm)
62 2.7
86 7.9 87 2.0
91 1.8
59 1.4
86 7.9 81 1.1
94 0.7
Note: adalimumab in Protein A eluate containing 25 mM acetate and 18 mM Ti-is
or
0.89 mM Tris were pH adjusted to pH 3.5 with 3M Acetic acid solution and
neutralized to pH
7.9 with 3M Ti-is solution. One part of this viral inactivated material was
then diluted by
adding 0.3 part of a stock buffer containing 2.2 M (NH4)2S0.4/90 mM Tris/60 mM
Acetic pH
7.9 to reach conductivity of 86 mS/cm.
Example MM 9.8: Demonstration of AR reduction with different buffer systems
with adalimumab
In this Example, adalimumab and resin Capto Adhere were chosen. The
experiments
were performed with different buffer systems listed in the tables below at
multiple pH and
conductivity condition. The load material pH was adjusted from Protein A
eluate or CEX
eluate. The results in Table 68 and Table 69 demonstrates that mAb acidic
species can be
reduced using various buffer systems.
228
CA 02926384 2016-04-07
Table 68: Effect of Cation type on mAb acidic species reduction and recovery
yield
Tris/Acetae Operating Capto Adhere HEA PPA
Condition
pH 7.85 pH 7.85 pH 7.85
4.5 3.5 2.5 4.5 3.5 2.5 4.5 3.5
2.5
mS/cm mS mS/c mS/c mS/c mS/c mS/cm mS mS/c
/cm m m m m /cm m
%Yiel 50 52 58 49 52 56 40 64 63
A%A 1.8 3.8 3.7 1.1 2.7 3.2 1.4 1.9 2.6
Arginine/Acet Operating -1mS/cm -1mS/cm -1mS/cm
ate Condition
pH8.5 pH pH pH8.5 pH pH pH8.5 pH pH
9.0 9.5 9.0 9.5 9.0 9.5
%Yiel 65 62 49 77 71 66 69 70 71
A%A 8.6 6.5 1.9 4.9 3.5 N/R 4.5 1.9 0.6
Trolamine/Ac Operating - 1 mS/cm - 1 mS/cm - 1
mS/cm
etate Condition
pH7.85 pH pH pH7.8 pH pH pH7.85 pH pH
8.0 8.5 5 8.0 8.5 8.0 8.5
%Yiel 62 54 49 69 64 58 64 64 590
A%A 4.1 6.0 4.6 1.7 2.9 3.0 1.4 2.1 2.1
Note: Load material was adalimumab from Protein A affinity capture and pH
adjusted
Table 69: Effect of Anion type on mAb acidic species reduction and recovery
yield
load amt conductivity Yield
Buffer pH A%AR
(g/L) (mS/cm) (%)
Ms/Acetate' 200 4.00 7.80 90 1.6
NaPhosphaste/Citrate/Trolamine/NaC12 200 3.53 7.87 87 1.5
Tri s/Formatel 300 0.92 8.50 69 3.7
1. Load material was adalimumab from Protein A affinity capture and pH
adjusted
2. The load material was adalimumab from CEX capture and pH adjusted
Example MM 9.9: Demonstration of AR reduction with different loading
The experiments were performed with Tris/Acetate buffer system under the
conditions in Table 66. The load material was adalimumab from Protein A
affinity capture
and pH adjusted. Column Flow Through pool was collected in each run from 50
mAU of UV
229
CA 02926384 2016-04-07
A280 on the ascending and 150 mAU on the descending side of the peak. As seen
from the
profile (Figure 186), the loading capacity has an impact on AR reduction but
the AR
reduction can be achieved over a wide range of loading capacities, and is
merely a trade-off
between AR reduction and recovery.
Example MM 9.10: Demonstration of AR reduction with different load
concentration
In this example, Capto Adhere was chosen. The experiment was performed with
Tris/Acetate buffer system at pH 7.8 0.1 and conductivity 3.0 0.05 mS/em.
The load
material was adalimumab from concentrated CEX capture and pH adjusted. The
prepared
load material was then split to be two parts. One was directly loaded on to a
Capto adhere
column; the other part was diluted 2 folds with equilibration buffer to make
different protein
concentration. Table 70 demonstrates that the load protein concentration did
not have
significant impact on mAb acidic species reduction.
Table 70: Adalimumab AR Reduction and Yield with Different Load Protein
Concentration
Capture Buffer Load Conducti pH Load Yiel A%A
step amount vity protein
(g/L) (mS/cm) conc. (%)
(g/L)
CEX Tri s/Ace 200 2.9 7.8 22.0 87 2.4
tate
CEX Ti-is/Ace 200 3.0 7.7 11.0 89 2.1
tate
CEX NaPhios 200 3.5 7.9 4.9 87 1.5
phaste/C
itrate/Tr
olamine/
NaC1
Protein Ti-is/Ace 200 3.1 7.8 9.0 89
2.5
A tate
Protein Tri s/Ace 200 4.0 7.8 11.8 90 1.6
A tate
Protein Ti-is/Ace 200 3.0 7.8 9.9 93
2.4
A tate
Protein Tri s/Ace 208 3.0 7.8 8.4 95 3.2
A tate
Protein Tris/Ace 222 3.0 7.9 12.9
89 3.4
A tate
230
CA 02926384 2016-04-07
Example MM 9.11: Alternative wash modalities
In this example, mAb adalimumab and resin Capto Adhere were chosen. The
experiments were performed with Tris/acetate buffer system and the load
material pH was
adjusted from Protein A eluates. The equilibration buffer for both run was
Tris/Acetic acid
pH 7.8 0.1 and conductivity of 3.0 0.1 mS/cm. In the gradient conductivity
wash study,
second buffer was Tris/Acetic acid pH 7.8 0.1 and conductivity 6.0 mS/cm.
The results demonstrated that post load pH and conductivity can be varied with
minimal AR reduction impacted (see Table 71).
Table 71: Comparison of AR reduction and yield under different wash conditions
Experiment Wash Load load Load Yield Wash A%
conductiv pH conc (%) CV AR
ity (mg/m
(mS/cm) L)
Equilibration Equilibration buffer 3.09 7.85 9.04 89
16.4 2.5
buffer wash (Tris/Ac pIl 7.8 and
3.0 mS/cm) wash only
Gradient conductivity 1CV Equilibration 3.04 7.78 7.17 91 .. 8.0
.. 2.2
wash buffer
10CV gradient
conductivity wash
from 100% "fris/Ac
pH 7.8, 3.0mS/cm to
100% Tris/Ac pH 7.8,
6mS/cm,
Example MM 9.12: Demonstration of achievement of absolute value of AR levels
in antibody
preparations using Mixed Mode Chromatography
In this example, mAb adalimumab was chosen. The experiments were performed
with
multiple buffer systems and multiple MM absorbents under conditions listed in
Table 72. The
load materials pH was adjusted from Protein A eluates.
As described above, the AR for adalimumab is further grouped into two regions
termed AR1 and AR2, based on a certain retention time of the peaks seen on the
WCX-10
method. The characteristics of the variants in these two regions are expected
to be different
and hence the methods that reduce variants belonging to these groups can be
specifically
delineated. Further, in addition to achieving a certain AR reduction, it may
be desirable to
achieve a certain absolute level of AR levels. in consideration of reducing or
removing
certain variants. The capability of the current invention in achieving a
certain absolute level
of AR. AR I and AR2 is demonstrated in Table 72.
231
CA 02926384 2016-04-07
Table 72: Acidic species level in MM resin Flow Through
Resin Buffer pH Conductivity (mS/cm) Yield (%)
ET %AR1 Fl %AR2
.
Capto Adhere Tris/Acetate 7.85 4.5 50 2.8 9.7
7.85 4.5 68 3.0 10.3,
7.85- 3.5 52 1.6 10.0'
7.85 3.5 71 2.2 10.5
7.85 3.0 93 3.2 9.7
7.85 2.5 58 1.7 9.4'
7.85 2.5 72 2.2 10.0
Arginine/Acetate 8.5 1 65 1.2 6.1
9.0 1 62 1.6 7.2,
9.5 1 49 0.8 11.8
Trolamine/Acetate 7.9 1 44 1.5 6.6
7.9 1 62 1.8 8.0
8.0 1 37 1.1 5.8
8.0 1 54 1.2 7.7
8.5 1 32 1.7 9.0
8.5 1 49 1.9 10.1
Tris/Formate 8.5- 1 69 0.6 6.4
HEA Arginine/Acetate 8.5 1 77 1.6 8.
9.0 1 71 0.8 12.0
PPA Arginine/Acetate , 8.5 1 69 2.2 8.7
9.0 1 70 1.0 13.5
9.5 1 71 0.7 13.1
232
CA 02926384 2016-04-07
Example MM 9.13: Demonstration of HCP and aggregate reduction in addition
To AR reduction
Besides the acidic species reduction, the MM adsorbent is able to reduce other
product/process related substances/impurities effectively. In the
implementation of the
current invention the fact that AR reduction is effected, other
impurities/substances are
expected to be cleared significantly as they should bind stronger than the
acidic species. The
data shown in Table 73 and Table 74 demonstrates significant HCP and aggregate
reductions
with different resins, buffer systems, pH, conductivities and molecules
Table 73: Aggregate reduction
Conductivity (mS/cm) pH Buffer medium A%HMW
adalimumab 3.75 7.5 , Tris/Acetate Capto
Adhere _0.7
2.5 7.6 0.9
2 7.85 0.9
3.75 7.85 1.0
5.5 7.85 0.7
2.5 8.1 1.0
3.75 8.2 0.8
4.0 8.2 1.0
8.0 6.8 0.2
8.0 8.4 1.0
1.0 8.5 Arginine/Acetate Capto Adhere
0.5
1.0 9.0 0.8
1.0 9.5 0.9
1.0 8.5 HEA 0.4
1.0 9.0 2.5
1.0 9.5 0.7
1.0 8.5 PPA =0.5
1.0 9.0 =2.8
1.0 9.5 0.4
mAb C 3.0 8 Ti-is/Acetate Capto
Adhere 1.0
3.0 8.5 Capto Adhere 1.1
3.0 9 Capto Adhere 0.6
3.0 8 PPA 0.7 ,
3.0 8.5 PPA , 0.5
3.0 8 HEA 0.7
3.0 8.5 HEA 0.6
233
CA 02926384 2016-04-07
Table 74: HCP Log reduction
Conductivity (mS/cm) pH Buffer medium HCP LRF
adalimumab 3.75 7.5 Tris/Acetate Capto
Adhere 1.5
2.5 7.6 1.7
2.0 7.85 2.2
3.75 7.85 1.9
5.5 7.85 1.4
2.5 8.1 2.3
3.75 8.2 2.1
4.0 8.2 1.7
8.0 6.8 0.3
8.0 8.4 0.7
mAb B 3 6.8 Capto
Adhere 2.0
4.5 6.8 1.3
3 6.8 PPA 1.2
4.5 6.8 1.2
3 6.8 HEA 1.3
4.5 6.8 1.1
Example MM 9.14: Combinations of MM With Alternative Separation Strategies
Acidic species reduction by MM adsorbents is expected to be performed after
capture
of the antibody by other means, or after one or more intermediate steps
following the capture
step. In the Examples below the MM adsorbent steps were performed either
following a
Cation Exchange Capture step or Protein A affinity capture step. As shown in
Table 75, AR
reduction was achieved at two different conductivities following Protein A
Chromatography
and CEX Chromatography.
Table 75: AR Reduction with different source materials
Capture Buffer conductivity (mS/cm) pH
Yield A%AR
(%)
Protein A Tris/Acetate 3.1 7.8 89 2.5
Protein A 4.0 7.8 90 1.6
CEX 2.9 7.8 87 2.4
CEX 3.0 7.7 89 2.1
Adalimumab was purified by a CEX chromatography step followed with a low pH
viral inactivation step. The filtered viral inactivated material was buffer
exchanged and
loaded onto a Capto Adhere column. The Flow Through of Capto Adhere material
was then
purified with a HIC column with bind/elute mode. As shown in Table 76. AR
reduction was
achieved primarily with MM step, with some contribution from other steps.
234
CA 02926384 2016-04-07
Table 76: Complete Process train with CEX Chromatography Capture- AR Reduction
A%AR A%Lys Yield (%)
CEX eluate n/a n/a n/a
MM Load 0.29 0.34 90%
MM Flow Through 2.57 2.57 93%
HIC eluate 0.95 0.94 97%
Adalimumab was purified using a Protein A chromatography step followed with a
low
pH viral inactivation step. The filtered viral inactivated material was buffer
exchanged and
loaded onto a Capto Adhere column. The Flow Through of Capto Adhere material
was then
purified with a HIC column with bind/elute mode as well as Flow Through mode.
As shown
in Table 77, AR reduction was achieved primarily with MM step, with some
contribution
from other steps.
Table 77: Complete Process Train with Protein A Capture - AR, HMW and HCP
reduction
Yield %AR %HMW
ProcessHCP LRF
(%) reduction reduction
Clarified Harvest 97.0% n/a n/a n/a
Prt-A Eluate Pool 89.6% 0.06 1.87
Viral Inactivated
99.7% No reduction 0.07 0.39
Filtrate
MM FT pool 91.9% 2.26 0.83 1.63
HIC (B/E) Eluate 90.1% 0.40 0.22 1.41
Nanofiltrate Filtrate 90.7% No reduction No
reduction 0.15
BDS (B/E) 102.0% No reduction No
reduction 0.22
HIC FT-pool 98.5% 0.16 0.23 0.46
VF(FT) Filtrate 96.1% No reduction No
reduction 0.10
BDS (FT) 103.8% No reduction No
reduction No
reduction
Example 10: Upstream and Downstream Process Combinations to Achieve
Target %AR or AR Reductions
The instant example demonstrates the combined effect of one or more upstream
and
downstream process technology in achieving a target AR value or AR reduction,
thereby
facilitating the preparation of an antibody composition having a specific
charge
heterogeneity.
235
CA 02926384 2016-04-07
Example 10.1: Combination of upstream and downstream technologies using
MM
In this Example, the combination of upstream and downstream methods involves
the
reduction of acidic species in 3L bioreactor cell cultures supplemented with
arginine (2 g/L)
and lysine (4e/L). The results of that strategy are summarized in Table 78.
The total acidic
species was reduced from 20.5% in the control sample to 10.2% in sample from
cultures that
were supplemented with the additives.
In this study, adalimumab producing cell line I was cultured in media I
(chemically
defined media) supplemented with amino acid areinine (2g/L) and lysine (4 g/L)
in a 300L
bioreactor. On Day 12 of culture, the culture was harvested and then
subsequently analyzed
using WCX-10 post Protein A purification and the percentages of total peak(s)
area
corresponding to the acidic species were quantified. The percentage of acidic
species was
estimated to be 9.1% in the 300L harvest sample.
Table 78: AR levels achieved with use of upstream technologies
3L Bioreactor 300L Bioreactor
Arginine (2g/L) + Lysine (4 Arginine (2g/L) + Lysine
Control g/L) (4 g/L)
Total
Total AR Total AR
AR1(%) AR2(%) (%) AR1(%) AR2(%) AR (%) AR1(%) AR2(%) (%)
6.3 14.2 20.5 2.6 7.6 10.2 2.4 6.7 9.1
236
CA 02926384 2016-04-07
The material produced by the 300 L Bioreactor employing arginine and lysine
additions, that effectively reduced the AR levels to 9.1% was purified using a
downstream
process employing Mixed Mode chromatography as the primary AR reduction
method.
Adalimumab was purified by a Protein A chromatography step followed with a low
pH viral inactivation step. The filtered viral inactivated material was buffer
exchanged and
loaded onto a Capto Adhere column. The Flow Through of Capto Adhere material
was then
purified with a HIC column with bind/elute mode as well as Flow Through mode.
As shown
in Table 79, AR reduction was achieved primarily with MM step, with some
contribution
from other steps. The table also shows that additional product related
substances such as
aggregates and process related impurities such as HCP can be effectively
reduced employing
these combined technologies.
Table 79: Complete Downstream Process Train with Protein A Capture ¨ AR, HMW
and HCP reduction
Yield %AR %HMW
ProcessHCP LRF
(%) reduction reduction
Clarified Harvest 97.0% n/a n/a n/a
Prt-A Eluate Pool 89.6% 0.06 1.87
Viral Inactivated
99.7% No reduction 0.07 0.39
Filtrate
MM FT pool 91.9% 2.26 0.83 1.63
HIC (B/E) Eluate 90.1% 0.40 0.22 1.41
Nanofiltrate Filtrate 90.7% No reduction No reduction 0.15
BDS (B/E) 102.0% No reduction No reduction 0.22
HIC FT-pool 98.5% 0.16 0.23 0.46
VF(FT) Filtrate 96.1% No reduction No reduction 0.10
BDS (FT) 103.8% No reduction No reduction No
reduction
As is evident from the above example, the MM method further reduced the AR
levels
by 2.26%. Therefore upstream technologies for reduction can be combined with
downstream
technologies to achieve desired AR levels/AR reduction.
237
CA 02926384 2016-04-07
Example CEX 10.2: Demonstration of AR reduction in process combinations
The methods described above for reducing acidic species using cation exchange
can
be used as an independent operation or in combination with other process steps
that provide
additional acidic species reduction or those providing additional
complementary and
supplementary purification (See Tables 80-87). The following process
combinations are
provided here as non-limiting examples
I. Affinity MM CEX
2. Affinity AEX CEX
3. Affinity --> CEX
4. CEX Capture CEX
Table 80 : AR Reduction by Capto Adhere(mixed mode) followed by Poros XS (CEX)
Yield % % AR1 % AR
Step qo AR1 % AR Reduction Reduction
MabSure Eluate 2.90
10.08 .
............................................................
Viral Inact 89 2.89 10.42 .........
...............
Mixed Mode FTW 94 2.26 8.52 0.64 1.90 =
CEX Load 1.!.!.]::%:::Iga 2.29 8.97
CEX Eluate 91 0.25 4.88 2.04 4.10
Overall 76 2.65 5.20
Table 81: Aggregate reduction by combination of Capto Adhere (mix mode) Poros
XS
(CEX)
Yield Mono % Agg. % Frag
Step %= Monomer Aggregate Fragment increase decrease decrease
MabSure : = =,i¶ .
Eluate 99.08 0.85 0.08 ... . ... . . . .
. .
Viral Inact 89 99.14 0.73 0 13
Mixed Mode
FTW 96 99.64 0.26 0.10 0.50 = 0.47 0.03
CEX Load K-=-= = = =-= 99.64
0.26 0.10 .
.............................................................................
CEX Eluate 89 99.74 0.18 0.08 0.10 0.08 0.02
overall 76 0.66 0.67 0.00
238
CA 02926384 2016-04-07
Table 82: AR Reduction by Poros PI (AEX) followed by Poros XS (CEX)
AEX CEX Cycle C
Yield % % AR1 % AR
Step % AR1 % AR Reduction Reduction
MabSure Eluate i:O.N:.!..i!!ii.::A 2.90 10.08
AEX Load iiL::::.t:g,i,ilii 2.73 10.16
AEX FTW 90 1.64 6.7 1.09 3.46
Viral Inact 100 1.39 6.03 ,
-.,,.,.....:...:..:.: .. . . :::...:õ,.
CEX Load gii:!:N,:.-::'..].,:. iiii 2.76 6.18
...,..::=.:õ.õ=::=::.=::.:.::,,,
CEX Eluate 91 0.15 3.222.61 2.96
=============================,.
Overall 82:itaa]:::.]...E1]..a.;1'....M.::kPaM._ 2.75
6.86
Table 83: Aggregate reduction Poros PI (AEX) Poros XS (CEX)
AEX CEX Cycle C
%
Yield % % % Mono
% Agg. % Frag
Step %
Monomer Aggregate Fragment increase decrease decrease ,
MabSure 114141111
:.:.:õ....:=-
=::::====:õ.:=:.:=:.õ..:=:=õ:õ.,õõõ::::::=:=:õõõ,:,,,,,,,:,,,::,:::,::,=:,,,,,:
,.......)
...õ:õõõ,õ:õ,õ,õ,.õ:õ:õ,:õ,õõ,=õõ,õ.....,.,.õ,,.õ,.,.,.õ............,.,...:.,.,
...:,.....:....õ..........................
=====================--- ===== ========== == ===== ==
=================== .............
::i:imi':=,.:gi.::'ni]-: :-.i'::.::].i:=ii:;='-:=',:;in=]=.:
:::.:==::.::::=====:=::=:
Eluate ilil:::=!.!.: . :!!:!:45
99.08 0.85 0.08 M:::ii::-:::mi.;.V;::Mi """'"=''.:',:!=-:.:::::::Wft
:::::::.!:;:q:m!:.!='..:::::.:
, .. ,:=:=:,=:=,=õ=:=:=:=:=:=::::.,=õ=::=:=, == . = .....
. .. . .. . . ,= . ..... ....
AEX Load:.6::..::::':.:::;.:.:;;;,1:N_ 98.67 1.25 0.03
=::.;:i...i.M:i..:R::.i...:i.::.',..g.'.õ'.''..Rq!..::.:!...:...]:.:I.'..:.g:..
!'"'....:g.:=':...:::-..:f
AEX FTW 90 99.88 0.05 0.07 1.21 1.2 -
0.04
================== =========
===============.======.v.v.õ.======.=======.========.=.======.========.=======,
,
Viral Inact 100 99.94 0.05
0.02 '0,'"-E,!=':::::'.;=:.m-...::=",:'.:.='n::ff:::::::::::,=:::-
.::.:!.:::.:;.=:.]::=:]=:-.r.
==:::::.,.....=:.:õ..,.......: . : . ..t..=:::::::::õ=:.....................
,..
...............................................................................
....................õ.,..:.õ...:.............õ..
CEX Load 99 99.64 0.26
0.10
.:14.!';',::::1=:::';=:='.;.=:='=::='.:::.q.ni!:='i=:='::;:n:::.]...:::=:='ij::
:::=:.]:='.::::::='!=':='.=':::='=='='=='=.;::='.:::..:.:=;.:::;=''':::::::i
CEX Eluate 91 99.79 0.13 0.08 0.14 0.13 0.02
Overall 82 IligniiiiiiiiiligAllitigagj. l'..1:14.11.11ii]a....--
0.71 0.72 0.00
Table 84: AR reduction from an Affinity capture pool followed by Poros XS
(CEX)
Yield % % AR1 % AR
Step % AR1 % AR Reduction Reduction ,
MabSure Eluate=== ======= =========-==
=====================================::=====:=====:= . =:=::::.=:==:,-
iel.'::;:'=!..:'='::::::3=0 3.0 10.5 Iiin12:1!!Pl..;
..... .......... Ar: .::::...]=:!=::::!!.!=zi=.,=.,=iiii;i
CEX Eluate 82.7 0.3 4.9 2.8 5.6
Table 85: Aggregate reduction: Affinity capture pool followed by Poros XS
(CEX)
670,
Yield % % % Mono
% Agg. % Frag
Step%
Monomer Aggregate Fragment increase decrease decrease...
.........=........................õ : . ........... ::..... :....
...::.. ..: .. .... .......,.
MabSure ..= : - =="..= , .
=-===:=====:===========::::====== ==:-,=: . :. ::=.::.: .. = . .:..:=:=,=:
: ..:===:::..,::::,:::::::.: . ...,
....:::.....::: ' ..:::::::::-............,=:==
===============,,,,,,,,,,:=:===:.,: :. ====,,,:::::::,,,,,, ...:. = = ,
01
Eluate i.:__. : = = i..,...,..... 98.5 1.4
239
CA 02926384 2016-04-07
I CEX Eluate 82.7 99.7 0.2 1 0.1 I 1.2 I 1.2 I 0.0
Table 86: AR reduction CEX Capture (Fractogel S03 ) followed by Poros XS (CEX)
145mM TA Poros XS adalimumab
Yield % % AR1 % AR
Step % AR1 % AR Reduction _ Reduction
Concentrated Fractogel
Eluate VI 3.3
CEX Eluate 72.6 0/1/1 6.7 2.8 7:3
Table 87: Aggregate reduction: CEX Capture (Fractogel) followed by Poros XS
(CEX)
145mM TA Poros XS adalimumab
Yield Mono %
Agg. % Frag
Step
Monomer Aggregate Fragment increase. ... decrease . decrease..
Concentrated NOTTI
Fractogel 11=11:
Eluate VI 97.9 1.5 07
CEX Eluate 72.6 98.7 1.1 0.2 0.9 0.4 0.5
Example 10.3: Process Combination: Protein A, AEX, CEX Combination
With Tris/Formate Buffer System
In Example 10.3, AR reduction through a process combination of Protein A
affinity
capture followed by fine purification with AEX and CEX chromatography in a
Tris/Forrnate
buffer system was examined.
Materials and Methods
Materials
Antibody
Adalimumab monoclonal antibody preparation was obtained after affinity capture
of
the clarified harvest. The eluate from the capture step was buffer exchanged
as required.
240
CA 02926384 2016-04-07
AEX Adsorbent:
Poros 50HQ (Applied Biosciences, part# 1-2459-11), a rigid 50 pm polymeric
bead
with a backbone consisting of cross-linked poly[styrene-divinylbenzene] was
used in this
experiment.
CEX Adsorbent:
Poros XS (Applied Biosciences, part# 4404338), a rigid 50 pm polymeric bead
with a
backbone consisting of cross-linked poly[styrene-divinylbenzene], was used in
this
experiment.
Methods
AEX Chromatography Method
Poros 50HQ was packed in 1.0 cm x 10.0 cm (OmniFit) columns. The column was
equilibrated in a buffer system with appropriate pH and conductivity. The load
was prepared
in the equilibration buffer by addition of the stock solutions to obtain the
target ion
concentrations, as specified, and loaded on the column, as specified, followed
by washing
with the equilibration buffer for 20 CV. The antibody product was collected in
the flow-
through and wash fractions during the load and washing steps. The
columns/housings were
then regenerated with 100 mM formate and 1M of NaOH solution was used for
column
cleaning.
CEX Chromatography Method
Poros XS was packed in 1.0 cm x 10.0 cm (OmniFit) columns. The column was
equilibrated in a buffer system with appropriate pH and conductivity. The
column load was
prepared in the equilibration buffer by buffer exchange or addition of the
stock solutions to
obtain the target ion concentrations as specified and loaded on the column at
approximately
40 g protein/L resin (or as specified) followed by washing with the
equilibration buffer for 20
CV (or as specified). The antibody product was then eluted, and the column
regenerated.
Buffer Preparation Method
Buffers for AEX were prepared targeting a specific ion concentration for the
anion by
fixing the anion concentration (acid) to the target value, and adjusting the
solution with the
cationic component (base) to achieve the appropriate pH. For example, to
prepare a 10 mM
241
CA 02926384 2016-04-07
Formate-Tris buffer solution, pH 8.7, formic acid was dissolved in water to a
target
concentration of 10 mM and adjusted with concentrated Tris-base to pH 8.7.
Buffers for CEX were prepared targeting a specific ion concentration for the
cation by
fixing the cation concentration (base) to the target value, and adjusting the
solution with the
anionic component (base) to achieve the appropriate pH. For example to prepare
a 140 mM
Tris-Formate buffer solution, pH 7.5, Tris base was dissolved in water to a
target
concentration of 140 mM and adjusted with Formic Acid to pH 7.5.
AR Reduction and Recovery Calculations
In general, eluate fractions and Flow Through (FT)/Wash fractions were
collected and
analyzed with a WCX-10 method for AR levels. By actual or calculated pooling
of the
fractions the recovery and the corresponding AR levels were calculated.
Analytical Methods
WCX-10 for Adalimumab
The acidic species and other charge variants present in the adalimumab process
samples were quantified according to the following methods. Cation exchange
chromatography was performed on a Dionex ProPac WCX-10, Analytical column 4 mm
x
250 mm (Dionex, CA). An Agilent 1200 HPLC system was used as the HPLC. The
mobile
phases used were 10mM Sodium Phosphate dibasic pH 7.5 (Mobile phase A) and
10mM
Sodium Phosphate dibasic, 500 mM Sodium Chloride pH 5.5 (Mobile phase B). A
binary
gradient (94% A, 6% B: 0-20 min; 84% A, 16% B: 20-22 min; 0% A, 100%B: 22-28
min;
94% A. 6% B: 28-34 min) was used with detection at 280 nm.
Quantitation was based on the relative area percent of detected peaks. The
peaks that
elute at relative residence time less than a certain time are together
represented as the acidic
peaks.
Size Exclusion Chromatography
The molecular-weight distribution of collected samples was quantified
according to
the following methods. Size exclusion chromatography (SEC) was perfon-ned
using a TSK-
gel G3000SWx1., .5[tm, 125 A, 7.8 X 300mm column (Tosoh Bioscience) on an HP
Agilent
HPLC system. Injections were made under isocratic elution conditions using a
mobile phase
242
CA 02926384 2016-04-07
of 200 mM sodium sulfate, 100 mM sodium phosphate, pH 6.8, and detected with
absorbance
at 214 nm. Quantification was based on the relative area of detected peaks.
UV spectroscopy A280
UV A280 was used to determine protein concentrations for the samples post
protein A
elution. The assay was performed on an Agilent UV Spectrophotometer. The
protein
concentration was determined using Beer-Lambert' s Law, A = cic, where A is
Absorbance, c
is the extinction coefficient, 1 is the path length, and c is the
concentration. The absorbance
was taken at 280 nm, the path length was 1 cm, and the extinction coefficients
were 1.39 for
Adalimumab, 1.38 for mAb B, and 1.43 for mAb C.
Results
AR reduction through a process combination of Protein A affinity capture
followed by
fine purification with Poros 50HQ and Poros XS in a Tris/Formate buffer system
was carried
out as follows, resulting in a final AR of 1.4%. This exemplary low AR process
followed the
flow path set forth in Figure 190.
Protein A
For Protein A affinity capture, a 2.2 x 20cm MabSelect SuRe (GE Healthcare)
column
was packed and qualified by HETP/Asymmetry analysis. The chromatography was
run in
bind-elute mode with a 4-minute residence time. Columns were loaded with 37g
mAb protein
per liter of resin.
The column was washed with a high concentration Tris/Formate buffer, rinsed
with a
low concentration Tris/Formate buffer and subsequently eluted with a low pH
Tris/Formate
buffer. The column was then regenerated and cleaned with hydroxide solutions
appropriate
for the resin.
The MabSelect SUReTM eluate pool was titrated to pH 3.7 with formic acid and
held
for an hour. The acidified materials were mixed for 1 hour at ambient
temperature. The VI
pool was neutralized with to a pH of 8.7 (i.e., AEX Load). The AEX load was
filtered prior
to loading.
AEX Chromatography
243
CA 02926384 2016-04-07
All AEX chromatography experiments were carried out on an AKTAavant25 system
using a 1.0 cm diameter x 9.5 cm length column packed with Poros 50HQ resin,
and qualified
by HETP/Asymmetry analysis. Each experiment was performed at ambient
temperature. The
AEX step was performed at 225g/L of resin loading. Equilibration and loading
was
performed with a low concentration Formate/Tris buffer, e.g., a 15mM
Formate/Tris buffer at
a pH of 8.7. Wash was performed with Acetate and Ti-is at the same pH. Each
run was
performed at ambient temperature with a load concentration of ¨10g/L at a
residence time of
3 minutes. The column was regenerated and cleaned with solutions appropriate
for the resin.
The Flow Through was collected in the following fractions: 100mAu-175g/L,
175g/L-
200g/L, 200g/L-225g/L +1CV of wash. The fractions were then measured by A280
mass
spectroscopy and analyzed by WCX-10 and SEC assays.
Poros 50HQ FTW pool was adjusted to 135 mM Tris/Formate pH 7.5 using stock
solutions of Tris and Formic acid.
CEX Chromatography
All CEX chromatography experiments were carried out on an AKTAavant150 system
using a 1.0 cm diameter x 11 cm length column packed with Poros XS resin, and
qualified by
HETP/Asymmetry analysis. Each experiment was performed at ambient temperature.
with a
5.8mg/mL load, 40g\L resin loading, and a residence time of 6 minutes.
Equilibration,
loading, and wash was performed with a high concentration Tris/Formate buffer
at a pH of
7.5. Elution was with sodium sulfate and Tris/Formate buffers. The eluate was
collected in
one fraction from 400mAU to 100mAU. Three cycles were performed. The column
was
regenerated and cleaned with solutions appropriate for the resin.
Viral filtration was performed on the Poros XS Eluate before the UFDF
processing,
using a Virosart CPV Viral Filter.
Ultracel 3 Biomax 30-kDa filters were used for diafiltration (into water) and
concentration of the CEX Eluate.
The cumulative AR of the Poros5OHQ fractions was below 6% allowing them to be
pooled together, and adjusted to CEX Load conditions. Three cycles of CEX were
244
CA 02926384 2016-04-07
performed. All three CEX eluate cycles had an AR below 3% and a HMW below 0.2%
and
were pooled together.
The step yield for each unit operation is listed in Table 88 with a final
overall yield of
38% being achieved. The process was able to achieve an adalimumab composition
with a
final AR of 1.4% (an AR1 of 0.0% and an AR2 of 1.4%) and final HMW of 0.10%.
Table 88: Step Yields for Low AR Material Generation
Step Yield AR % AR1 %
AR2 % HMW
MabSuRe 86% 10.0% 1.6% 8.4% NA
Poros 50HQ - FTW 80% 5.4% 0.8% 4.6% NA
Poros 50XS 55% 1.4% 0.0% 1.4% 0.13%
Overall 38% 1.4% 0.0% 1.4% 0.10%
Example 11: AR Reduction Using "Recycled" AEX and CEX Technologies
This Example describes the "recycle" mode of chromatography for AR reduction
using AEX, CEX, and MM technologies.
Materials and Methods
Materials
Antibody
Adalimumab monoclonal antibody preparation was material obtained after
affinity
capture of a clarified harvest. The eluate from the capture step was buffer
exchanged as
required.
AEX Adsorbent:
Poros 50HQ (Applied Biosciences, part# 1-2459-11), a rigid 50 pm polymeric
bead
with a backbone consisting of cross-linked poly[styrene-divinylbenzene], was
used in this
experiment.
245
CA 02926384 2016-04-07
CEX Adsorbent:
Poros XS (Applied Biosciences, part# 4404338), a rigid 50 pm polymeric bead
with a
backbone consisting of cross-linked poly[styrene-divinylbenzene], was used in
this
experiment.
Methods
AEX Chromatography Method
Poros 50HQ was packed in 1.0 cm x 10.0 cm (OmniFit) columns. The column was
equilibrated in a buffer system with appropriate pH and conductivity. The load
was prepared
in the equilibration buffer by addition of the stock solutions to obtain the
target ion
concentrations and loaded on the column, followed by washing with the
equilibration buffer
for 20 CV. The antibody product was collected in the flow-through and wash
fractions during
the load and washing steps. The columns/housings were then regenerated with
100 mM
formate and 1M of NaOH solution was used for column cleaning.
CEX Chromatography Method
Poros XS was packed in 1.0 cm x 10.0 cm (OmniFit) columns. The column was
equilibrated in a buffer system with appropriate pH and conductivity. The
column load was
prepared in the equilibration buffer by buffer exchange or addition of the
stock solutions to
obtain the target ion concentrations as specified and loaded on the column at
approximately
40 g protein/L resin (or as specified) followed by washing with the
equilibration buffer for 20
CV (or as specified). The antibody product was then eluted, and the column
regenerated.
Buffer Preparation Method
Butlers for AEX were prepared targeting specific ion concentration for the
anion by
fixing the anion concentration (acid) to the target value. and adjusting the
solution with the
cationic component (base) to achieve the appropriate pH. For example, to
prepare a 10 mM
Formate-Tris buffer solution, pH 8.7, formic acid was dissolved in water to a
target
concentration of 10 mM and adjusted with concentrated Ti-is-base to pH 8.7.
Buffers for CEX were prepared targeting specific ion concentration for the
cation by
fixing the cation concentration (base) to the target value, and adjusting the
solution with the
anionic component (base) to achieve the appropriate pH. For example to prepare
a 140 mM
246
CA 02926384 2016-04-07
Tris-Formate buffer solution, pH 7.5, Tris base was dissolved in water to a
target
concentration of 140 mM and adjusted with Formic Acid to pH 7.5.
AR Reduction and Recovery Calculations
In general, eluate fractions and Flow Through/wash fractions were collected
and
analyzed with WCX-10 method for AR levels. By actual or calculated pooling of
the
fractions the recovery and the corresponding AR levels were calculated.
Analytical Methods
WCX-10 for Adalimumab
The acidic species and other charge variants present in the adalimumab process
samples were quantified according to the following methods. Cation exchange
chromatography was performed on a Dionex ProPac WCX-10, Analytical column 4 mm
x
250 mm (Dionex, CA). An Agilent 1200 HPLC system was used as the HPLC. The
mobile
phases used were 10mM Sodium Phosphate dibasic pH 7.5 (Mobile phase A) and
10mM
Sodium Phosphate dibasic, 500 mM Sodium Chloride pH 5.5 (Mobile phase B). A
binary
gradient (94% A, 6% B: 0-20 min; 84% A, 16% B: 20-22 min; 0% A, 100%B: 22-28
min;
94% A, 6% B: 28-34 min) was used with detection at 280 nm.
Quantitation was based on the relative area percent of detected peaks. The
peaks that
elute at relative residence time less than a certain time are represented
together as the acidic
peaks.
Size Exclusion Chromatography
The molecular-weight distribution of collected samples was quantified
according to
the following methods. Size exclusion chromatography (SEC) was perfon-ned
using a TSK-
gel G3000SWxL, 51.1m, 125 A, 7.8 X 300mm column (Tosoh Bioscience) on an HP
Agilent
HPLC system. Injections were made under isocratic elution conditions using a
mobile phase
of 200 mM sodium sulfate, 100 mM sodium phosphate. pH 6.8, and detected with
absorbance
at 214 nm. Quantification is based on the relative area of detected peaks.
UV spectroscopy A280
UV A280 spectroscopy was used to determine protein concentrations for the
samples
post Protein A elution. The assay was perfori-ned on an Agilent UV
Spectrophotometer. The
247
CA 02926384 2016-04-07
protein concentration was determined using Beer-Lambert's Law, A = Elc, where
A is
Absorbance, c is the extinction coefficient, 1 is the path length. and c is
the concentration. The
absorbance was taken at 280 nm, the path length was 1 cm, and the extinction
coefficients
were 1.39 for adalimumab, 1.38 for mAb B, and 1.43 for mAb C.
Example 11.1 : Recycled AEX Chromatography
In this Example, a cycling strategy was employed to increase the recovery
yield for a
given target product quality attribute. The AR reduction for a given Formate
concentration
and pH can be modulated by adjusting the load. Also, the recovery yield is
fixed for a given
loading. In this strategy, the load is chosen to achieve a target AR level in
the Flow Through
fraction. The column is then eluted with a Formate concentration that is
slightly higher than
the load. The elutate is collected, and then diluted with water to match the
load Formate
concentration, and added back into the load tank. This column cycle is
repeated several times
(and is referred to as "recycled" chromatography).
For this experiment, the target AR level in the Flow Through (FT) pool was set
at 5%.
The Poros5OHQ column was first loaded to 200g/L of resin and the Flow Through
was
collected at 20g/L of protein loaded on the resin with the equilibration/wash
buffer and load
condition 15mM Acetate/Tris pH8.7. The Flow Through fractions were run on WCX-
10
assay and the cumulative AR breakthrough was calculated. The cumulative AR
breakthrough
of 5% was observed to occur at 150g/L of protein loaded onto the resin and all
the subsequent
experiments were run at 150g/L loading.
The cycling phase involved the scheme detailed in Table 89. The AEX load was
prepared by adjusting the MabSelect SuRe eluate with 3M Tris to the
appropriate pH and
diluted to 15mM Acetate and then filtered. The Flow Through of the load and
wash were
collected in two separate vessels. The wash was spiked with enough MabSelect
SuRe Eluate
to perform another cycle at 150g/L and the condition was adjusted to 15mM
Acetate/Ti-is
pH8.7. A total of 4 cycles were performed using the sequence of steps
described above.
Each run was performed at ambient temperature with a residence time of 3
minutes following
the chromatographic conditions listed in Table 89. The flow-through was
collected in one
fraction from 100mAu until the end of step, and the wash was collected from
the beginning
of the step to 50mAU. The FT Wash was then measured by A280 and analyzed by
the WCX-
10, and SEC assays.
248
CA 02926384 2016-04-07
Table 89: AEX Chromatography Conditions
Column
Step Solution
Volumes
Equilibration* 15mM Acetate/Tris pH8.7 30
adalimumab -15mM Acetate/Tris
Load* 150/L of resin
pH8.7
Wash* 30mM Acetate/Tris pH8.5 Wash down to
50mAu
100mM Acetate/Tris + 500mM
Regeneration 5
NaC1 pH3.5
Cycling the wash fraction on the AEX column as a means of controlling the
level of
process impurities was implemented in this study. The wash fraction was
collected at each
cycle(C) and adjusted to proper loading conditions and loaded at the
subsequent cycle (Cn+1).
The loading amount was dialed in to provide an AR breakthrough of 5%. A total
of four
cycles were performed.
Table 90: AEX Cycling Product Quality
ld
Load FT Wash Regen Load FT L Wash Regen
Yieys
Cycle AR AR AR AR Lys Lys Lys
(%) (%) (%) (%) (%) (%) (%)
1 64.8 13.2 5.1 24.8 89.1 85.8 93.7
74.3 10.3
2 = 65.3 16.5 6.1 33.9 90.1 82.6 92.9 65.2 9.0
3 58.5 18.6 5.9 36.1 90.1 80.6 93.0
63.0 9.1
4 58.2 18.4 5.9 38.4 85.1 80.8 93.1
60.8 8.8
The step yield and product quality is listed in Table 90. The % lysine (sum
lysine
variants, i.e., Lys 0, Lys 1 and Lys 2 which are mAbs containing 0, 1 or 2
terminal lysines) is
the quantitation of the desired (non-AR containing) fraction of the product
and is provided
here to show that the recycle method is able to recover over 93% of the
desired product.
Product containing higher levels of AR is recovered in the Wash fraction of
each cycle,
which is then recycled back onto the subsequent AEX cycle. The recycling of
the wash
fraction improves the cumulative yield and while maintaining the product
quality as shown in
Table 91. The Cumulative Yield increased from 65% to 81% in the four cycles,
while
maintaining the AR level at -6% and the monomer level at -99.4%.
249
CA 02926384 2016-04-07
Table 91: Cumulative Product Quality for AEX Cycling
Cumulative Cumulative Cumulative Cumulative Cumulative Cumulative
Cycle Monomer
Yield (%) AR (%) AR1 (%) AR2 (%) Lys (%)
1 65 5.1 0.6 4.5 93.7 99.4
2 77 5.6 0.6 5.6 93.3 , 99.5
3 79 5.7 0.7 5.1 93.2 99.4
4 81 5.8 0.7 5.1 93.2 99.3
Example 11.2: Recycled CEX Chromatography
These experiments were performed using Protein A eluate as CEX loading
material.
Cycle 1 (control) was performed under load/wash buffer conditions of 160 mM
tris-acetatet,
pH 7.5, 40g protein/L resin. Cycle 2 was perfon-ned by combining part of the
wash from
Cycle 1 and fresh Protein A eluate as loading material. The earlier wash
(prior to reaching the
peak) which contained higher AR was discarded and the rest of the wash was
included in the
load. The loading and wash conditions were the same as Cycle 1. Cycle 3 and
Cycle 4 were
performed the same way as Cycle 2.
The results shown in Table 92 indicate that the recycle chromatography with
four runs
increase the yield from 53.4% to 65.1%. AR reduction for Cycle 1 is 8.84% and
whereas with
the 4 cycle Recycle Chromatography is 7.79%. While achieving similar product
quality, the
recycle chromatography approach can significantly improve the yield.
Table: 92: Recycle Chromatography impact on AR reduction and yield
Cycle Cycle Cycle Cycle Recycle
Product Quality
1 2 3 4 Chrom.
Yield (%) 53.4 52.2 52.1 51.6 65.1
%AR I in load 3.75 3.71 3.31 3.40 n/a
%AR I in eluate 0.02 0.08 0.05 0.02 0.03
%AR 1 reduction 3.72 3.64 3.26 3.38 3.72
%AR2 in load 9.6 11.5 12.0 12.0 n/a
%AR2 in eluate 4.48 5.61 6.00 6.06 5.52
%AR 2 reduction 5.11 5.88 5.96 5.98 4.07
Total AR (%) in
13.3 15.2 15.3 15.4 n/a
load
Total AR (%) in 4.51 5.69 6.05 6.08 5.55
250
CA 02926384 2016-04-07
eluate
%Total AR
8.84 9.51 9.22 9.36 7.79
reduction
Example 11.3: AR Reduction Using MM Recycled Chromatography
The following Materials and Methods were used for Example 11.3.
Materials and Methods
Material captured by Protein A affinity chromatography
Adalimumab clarified harvest material obtained from 300L bioreactor
(SUL101912)
was loaded on a Protein A affinity column chromatography (such as MabSelect
SuRe) and
eluted with designed buffer system containing only buffer components used in
downstream
processes product trains. In the case of this study, adalimumab bound on
MabSelect SuRe
resin was eluted with 20 mM acetic acid.
Resin
Multimodal media have ligands and/or base matrix with multiple functional
groups
giving a different selectivity compared to traditional ion exchange media. In
these examples,
the multimodal media having anion exchange and hydrophobic interaction
functional groups
are shown to remove acidic species as well as other impurities from antibody
preparations.
Capto adhere (GE Healthcare, HiScreenli prepacked column, Cat# 28-9269-81), a
strong anion exchanger with multimodal functionality, was evaluated in this
study. Its base
matrix is a highly cross-linked agarose with a ligand (N-Benzyl-N-methyl
ethanol amine) that
exhibits many functionalities for interaction, such as ionic interaction,
hydrogen bonding and
hydrophobic interaction. Those ligands offer different selectivity and
hydrophobicity options
for protein separations.
251
CA 02926384 2016-04-07
Capto Adhere Ligand Structure
oH oFi
o
I ,
Methods
Chromatography Method
Pre-packed resin column was used in the following experiments. The column was
equilibrated in a buffer system with appropriate pH and conductivity. The
process is
illustrated as Figure 191. The column load was prepared from Protein A
affinity
chromatography. The prepared load material was filtered and loaded on the
column according
to the target load amount (g protein/L resin) as specified followed by washing
with the
equilibration buffer and wash buffer similar to equilibration buffer with
volumes as specified.
The column Flow Through during load was collected as a pool and the column
Flow Through
during wash was collected separately. The column was then regenerated with
0.1M Acetic
acid (pH 3) solution for next cycle use. The cycle A wash pool was mixed with
Protein A
eluate to make e antibody material to load at the target capacity for the
following cycle. pH
and conductivity of the combined pool (Wash pool + Protein A Eluate) was
adjusted with 2M
Tris and Milli Q water to achieve designed pH and conductivity. This material
was then
filtered through a 0.45 pm filter (Corning polystyrene).
Buffer Preparation Method
Buffers were prepared targeting specific pH and conductivity by starting with
an
anionic component solution (acid) to a target value, and adjusting the
solution with the
cationic component (base) to achieve the appropriate pH and subsequently
adding water to
achieve the target conductivity. For example to prepare a Tris-Acetate buffer
solution with
pH 7.85 and conductivity of 2.5 mS/cm. a 250 mM acetic acid solution was
adjusted pH to
7.85 0.05 with 3 M Ti-is solution, the solution conductivity was then
adjusted to 2.5 0.5
mS/cm with addition of water. final solution pH was then confirmed or adjusted
to
7.85 0.05 by addition of 3 M acetic acid solution or 3 M or 2M Ti-is
solution as needed.
252
CA 02926384 2016-04-07
In this study. Tris/Acetate buffer with pH 7.9 and conductivity 2.5 mS/cm was
used
for column equilibration; Tris/Acetate buffer with pH 7.9 and conductivity 5.0
mS/cm was
used for post load wash buffer.
Capto Adhere load material preparation
Cycle A:
The Protein A eluate was titrated to pH 7.9 with 2M Tris and diluted to
conductivity
of 2.5 mS/cm with Milli Q water. The prepared material was then filtered with
0.22 trn filter
before load to column.
Cycle B:
The entire cycle A wash pool was mixed with Protein A eluate to make enough
load
for the following cycle. pH and conductivity was adjusted after mixing with 2M
Tris and
Milli Q water to achieve pH 7.9 and conductivity of 2.5 mS/cm. This material
was then
filtered through a 0.45 pm filter (Corning polystyrene filter) before load to
column.
Cycle C:
The entire cycle B wash pool was mixed with Protein A eluate to make enough
load
for the following cycle. pH and conductivity was adjusted after mixing with 2M
Tris and
Milli Q water to achieve pH 7.9 and conductivity of 2.5 mS/cm. This material
was then
filtered through a 0.45 pm filter (Corning polystyrene filter) before load to
column.
Cycle D:
The entire cycle C wash pool was mixed with Protein A eluate to make enough
load
for the following cycle. pH and conductivity was adjusted after mixing with 2M
Tris and
Milli Q water to achieve pH 7.9 and conductivity of 2.5 mS/cm. This material
was then
filtered through a 0.45 pm filter (Corning polystyrene filter) before load to
column.
AR Reduction and Recovery Calculations
In general, the Flow Through/wash fractions were collected and analyzed with
WCX-
method for AR levels. By actual or calculated pooling of the fractions the
recovery and
the corresponding AR levels were calculated.
Analytical Methods
WCX-10 for Adalimumab
The acidic species and other charge variants present in the adalimumab process
samples were quantified according to the following methods. Cation exchange
253
CA 02926384 2016-04-07
chromatography was performed on a Dionex ProPac WCX-10, Analytical column 4 mm
x
250 mm (Dionex, CA). An Agilent 1200 HPLC system was used as the HPLC. The
mobile
phases used were 10mM Sodium Phosphate dibasic pH 7.5 (Mobile phase A) and
10mM
Sodium Phosphate dibasic, 500 mM Sodium Chloride pH 5.5 (Mobile phase B). A
binary
gradient (94% A, 6% B: 0-20 min; 84% A, 16% B: 20-22 min; 0% A, 100%B: 22-28
min;
94% A. 6% B: 28-34 min) was used with detection at 280 nm.
Quantitation was based on the relative area percent of detected peaks. The
peaks that
elute at relative residence time less than a certain time are together
represented as the acidic
peaks.
UV spectroscopy A280
UV A980 was used to determine protein concentrations for the samples post
Protein A
elution. The assay was performed on an Agilent UV Spectrophotometer. The
protein
concentration was determined using Beer-Lambert's Law, A = Elc, where A is
Absorbance, c
is the extinction coefficient, 1 is the path length, and c is the
concentration. The absorbance
was taken at 280 nm, the path length was 1 cm, and the extinction coefficients
were 1.39 for
adalimumab.
Demonstration of Recycle and Continuous Chromatography
In this Example, adalimumab and resin Capto Adhere were chosen. 75 grams of
adalimumab per liter of resin was loaded on a Capto Adhere column in each
cycle and a total
four cycles were performed. A single run with 100g/L of load material loaded
on the Capto
Adhere column was run as a reference to compare the AR reduction and mAb
recovery.
Figure 192 illustrates percent AR in load, flow-through pool (FT), wash pool
(wash) of each
cycle of the MM process, and the cumulative % AR in overall FT. As shown in
Figure 192,
the load %AR in each cycle increased due to higher % AR in wash pool obtained
from
previous cycle was re-processed, which led to slight increase of % AR in flow-
through pool.
It is clear from Figure 192 that the AR levels are maintained in the collected
pools in
all four cycles, achieving an overall reduction of approximately 5%. Thus, it
is evident that
the recycle mode can maintain the product AR levels. Table 93 shows the
recovery obtained
for each step and the overall recovery. It is evident that the recycle mode
results in
significant improvement in recovery (a 10% increase) when the four cycles are
run, as
compared to a single run achieving similar product quality. As seen in Table
93, cumulative
254
CA 02926384 2016-04-07
recovery increases with each additional cycle. Therefore, additional
improvement can be
achieved by increasing the number of cycles. Moreover, when comparing the
performance in
cycle 1 vs. the performance in cycles 1 to 4 (cumulative), it is clear that a
20% increase in
recovery can be achieved by using mode of chromatography.
Table 93: Acidic Species Reduction and mAb recovery in a Proof-of-Concept
continuous MM chromatography
Load amount per Cumulative Cumulative step C umultive
Cycle
cycle (g/L) yield (%) %AR A%AR A%AR
single run 100 62 7.7 4.1 4.1
1 75 52 6.3 5.5 5.5
2 75 62 6.5 6.6 5.3
3 75 67 6.7 7.1 5.1
4 75 72 6.8 7.1 5.0
Example 12: Storage of AR reduction
The cun-ent invention provides a method for reducing acidic species for a
given
protein of interest. In this Example, adalimumab was prepared using a
combination of
supplementation of arginine and lysine to cell culture as shown in this
invention along with
AEX and CEX purification technologies, as described herein, to produce a Low-
AR and
High-AR sample with a final AR of 2.5% and 6.9%, respectively. Both samples
were
incubated in a controlled environment at 25 C and 65% relative humidity for 10
weeks, and
the AR measured every two weeks. Figure 164 shows the growth of AR for each
sample
over the 10 week incubation. It is evident from Figure 164 the growth rate of
AR is linear
and similar between both the Low-AR and High-AR samples. Based on these
results the
reduced AR material can be stored 3 fold longer before reaching the same AR
level as the
High¨AR sample. This is a significant utility as this can be very beneficial
in storage
handling and use of the antibody or other proteins for therapeutic use.
Moreover. as indicated
above, the formation of storage-derived AR can be inhibited when the
preparation is stored
under particular conditions. For example, an aqueous formulation can be stored
at a particular
temperature to partially or completely inhibit AR formation. In addition,
formation or
storage-derived AR can be partially inhibited in an aqueous formulation stored
at between
about 2 C and 8 C. and completely inhibited when stored at -80 C. Moreover. a
low AR
255
CA 02926384 2016-04-07
composition can be lyophilized to partially or completely inhibit the
formation of storage-
derived AR.
Example 13: Increased Biological Activity of Low AR Compositions
This Example describes the increased efficacy of an exemplaiy low AR
composition
comprising adalimumab in vivo. The low AR composition used in this Example was
produced as described in Example 8.14, above, using a CEX reduction method. In
particular,
the low AR composition used in this example was produced using a Poros XS
column in a
Tris/Fonnate buffer system at a pH of 7,5. The low AR composition has an AR of
3.1%,
wherein the composition comprises 0.1% AR1 and 3.0% AR2. In this example, this
composition is referred to as the "low AR composition."
Animal Model for Arthritis
In order to study the efficacy of this low AR adalimumab composition,
experiments
were carried out in vivo using human TNF-Tg197 mice. The TNF-Tg197 mouse model
is a
well recognized mouse model of arthritis used to test anti-human 1NFa
treatment modalities.
The TNF-Tg197 mouse model is described in Keifer, J. et al., (1991) EMBO J
10:4025-
4031. The
transgenic mice
carrying human TNF gene were developed to study the effects of excess TNF
production in
vivo.
Tg197 mice develop swelling in the ankle joints of both hind paws and impaired
movement, which is very similar to human rheumatoid arthritis. Clinical signs
of disease in
Tg197 mice start at 4 weeks of age and include slower weight gain, joint
distortion and
swelling, joint deformation and ankylosis and impaired movement.
Histopathological
analysis reveals hyperplasia of synovial membrane, leukocyte infiltration at
around 3 weeks
of age, and then pannus formation, articular cartilage destruction and massive
production of
fibrous tissue at advanced stage of disease at 9-11 weeks of age. This model
has been used in
the development of anti-TNFi biologics, including adalimumab.
Methods
Groups of mice (6 males and 6 females), were administered one of the following
adalimumab formulations: low AR composition (group 5), low host cell protein
(HCP)
256
CA 02926384 2016-04-07
composition (group 7), AR1 composition (containing only AR1 acidic variants)
(group 8),
and Lys-1/2 composition (containing only Lys 1 and Lys 2 variants) (group 9).
These
compositions (fractions) are shown in the chromatograph in Figure 193. Another
group of
mice was administered a control composition, also refen-ed to as the -control
AR
composition," or "normal" composition, which contains adalimumab with
unmodified AR
levels and unmodified Lys variants. A placebo group, comprising 6 mice, was
also included.
Each composition, including the control AR composition, was administered to
the
mice in each group beginning with a tolerizing dose of adalimumab at age 1
week, and
followed by additional weekly dosages of 1 mg/kg for 10 weeks. From weeks 2.5
through
weeks 13.5, weekly measurements of weight and arthritic scores were taken and
weekly
serum collection was made. In addition, at the end of the study, tissue
samples from perfused
mice were obtained and analyzed. The following tissues were harvested for
testing drug
levels, anti-drug antibodies (ADA), and complexed and free TNF levels: front
paws, inguinal,
popliteal and mesenteric lymph nodes, spleen, tail (for skin sample), knees.
The femur and
spine tissues were harvested for micro-CT scanning.
Results
As shown in Figure 194A, the mice receiving the low AR composition had the
lowest
arthritic scores of all of the compositions tested, including the control AR
composition,
indicating increased efficacy in the treatment of arthritis. Furthermore, as
shown in Figure
194B, the rnice administered the low AR composition exhibited an average
weight gain that
was comparable to the control composition, indicating safety of the low AR
composition and
a lack of adverse effects of the low AR composition that impact weight gain
and growth of
the mice.
As shown in Figure 195, during the 12-13 week treatment period of the mice,
the low
AR composition provided the best protection against development of arthritis
in the mice. as
measured by arthritic scores, as compared to the other compositions tested.
The Lys-1/2
composition was the next most effective. The AR1 composition offered the least
protection
against development of arthritic scores, and it was less protective than the
control AR
composition.
Serum levels of ADA and drug levels were measured from 3 to 14 weeks of age.
As
shown in Figure 196B. animals administered the low AR composition exhibited
low average
257
CA 02926384 2016-04-07
levels of ADA across the time frame measured. In addition, animals
administered the low AR
composition exhibited drug serum levels comparable to the control (Figure
196A), indicating
that a lack of presence of the drug in the serum was not responsible for the
low levels of
serum ADA.
As set forth in Figure 197, cumulative serum concentration values (PK) during
the ten
week treatment period was highest for the animals administered the low AR
composition and
lowest for the animals administered the AR1 composition. The Lys-1/2
composition was the
next best following the low AR composition, and was higher than the AR control
composition. As also shown in Figure 197, the highest ADA titers were observed
for animals
administered the AR1 composition and the lowest for animals administered the
low AR
composition.
Furthen-nore, complexed TNF levels show that cumulative serum concentration
values
during the ten week treatment period were highest for animals administered the
control AR
composition and lowest for the animals administered the AR1 composition
(Figure 198).
Cumulative serum concentration values for the low AR composition were slightly
less than
the levels of the control AR composition.
A histopathology evaluation of the joints of the mice indicated that the best
protection
was afforded by the low AR composition and the Lys-1/2 composition, indicating
that the low
AR composition and the Lys-1/2 composition protect against the formation of
arthritis in the
joints in vivo. As shown in Figure 199, the low AR composition protected
against cell
infiltration, synovial proliferation, proteoglycan loss, cartilage
destruction, and bone erosion
more effectively than the other compositions, including the control AR
composition.
Protection by the AR1 composition was lower than the control AR composition,
indicating a
detrimental effect by AR1 with respect to joint damage.
Figures 200A-D illustrate the average drug (PK) levels for various tissues
(paw,
lymph node. spleen, skin, knee and serum) for the low AR composition, the
control AR
composition. the AR1 composition, and the Lys-1/2 composition. As shown
therein. animals
administered the low AR composition had drug levels as high or higher than
animals
administered the other compositions tested.
Figure 201A-D illustrates average ADA levels in the same tissues for the same
compositions (the low AR composition, the control AR composition, the AR1
composition,
258
CA 02926384 2016-04-07
and the Lys-1/2 composition). As shown in Figure 201A-D, for the low AR
composition, the
highest ADA concentrations are present in the paws (which corresponds to the
location of the
highest levels of inflammation in the animals), and the serum.
Figures 202A-D and 203A-D show the results of a micro CT analysis of spines
and
femurs obtained from the transgenic mice at the end of the study that were
administered low
AR composition, control AR composition, ARI composition, Lys-1/2 composition,
as well as
naïve, (control) and placebo. Samples were analyzed for L5 vertebra bone
volume, L5
vertebra trabecular number, L5 vertebra trabecular thickness, and L5 vertebra
trabecular
space. As shown in Figures 202A-D and 203A-D, the low AR composition and the
Lys-1/2
composition resulted in greater bone volume, trabecular number, trabecular
thickness and
trabecular space, as compared to the control (normal) AR composition.
Figures 204A-D show additional results of a micro CT analysis of spines and
femurs
obtained from the transgenic mice at the end of the study that were
administered low AR
composition, control AR composition, AR1 composition, Lys-1/2 composition, as
well as
naïve (control), and placebo. Samples were analyzed for trabecula bone volume
at the
femoral metaphysis, trabecular number at the femoral metaphysis, trabecular
thickness at the
femoral metaphysis, and trabecular separation at the femoral metaphysis. As
shown in
Figures 204A-D, the low AR composition resulted in greater trabecula bone
volume at the
femoral metaphysis, trabecular number at the femoral metaphysis, and
trabecular thickness at
the femoral metaphysis, as compared to the control (normal) AR composition.
Furthen-nore, Figures 205 and 206 show actual micro CT images of the spine and
femur, respectively, from each of six groups of mice administered the
following
compositions: naïve, vehicle (control), low AR composition (group 5), low host
cell protein
(HCP) composition (group 7), ARI composition (containing only ARI acidic
variants) (group
8), and Lys-1/2 composition (containing only Lys 1 and Lys 2 variants) (group
9). As seen in
both the spine and the femur, the low AR composition (group 5), provided
protection from
bone erosion, as compared to the vehicle, as there is less bone erosion
visible in the group 5
image as compared to the vehicle.
The results of these experiments demonstrate that a weekly dose of 1 mg/kg
adalimumab in TNF-Tg197 mice provides protection from arthritis development as
measured
by arthritic scores and histopathology scores (radiologic damage involving
cartilage and bone
259
CA 02926384 2016-04-07
as well as local inflammation) in the TNF-Tg197 mouse model. Thus, the control
AR
composition, with norrnal level of AR variants, was efficacious at a certain
level.
Formulations containing either the low AR formulation or the Lys-1/2
composition
provided greatest protection, as compared to the control AR group, from
development of
arthritis as measured by arthritic scores and histopathology scores, and
showed increased
efficacy, as compared to the control AR group, in all parameters tested
including cell
infiltration, synovial proliferation, proteoglycan loss, cartilage
destruction, and bone erosion.
Accordingly, the low AR composition and the Lys-1/2 composition have increased
efficacy in
the treatment and prevention of arthritis as compared to the control AR
composition.
The adalimumab AR1 composition was less efficacious than the normal AR
containing adalimumab control group in all aspects interrogated in the current
study: less
weight gain, higher arthritic scores, and higher histopathology scores in the
joints, indicating
a detrimental effect exerted by ARI.
Notewoithy differences were observed in serum levels of the various
formulations
include the following: the animals treated with the AR1 composition had the
lowest
concentration of adalimumab as compared to the other groups, and the animals
treated with
the low AR composition had the highest concentration of adalimumab as compared
to the
other groups. The AR I composition also had the highest titers of ADA in
serum.
The present invention is not to be limited in scope by the specific
embodiments
described herein. Indeed, various modifications of the invention in addition
to those described
herein will become apparent to those skilled in the art from the foregoing
description and the
accompanying figures. Such modifications are intended to fall within the scope
of the
appended claims.
Reference is made to U.S. Provisional Patent Application 61 XXX,XXX, "STABLE
260
CA 02926384 2016-04-07
SOLID PROTEIN COMPOSITIONS AND METHODS OF MAKING SAME", Attorney
Docket Number 117813-31001; U.S. Provisional Patent Application 61/XXX,XXX,
entitled
-LOW ACIDIC SPECIES COMPOSITIONS AND METHODS FOR PRODUCING THE
SAME USING DISPLACEMENT CHROMATOGRAPHY", Attorney Docket Number
117813-73602, filed on even date herewith; U.S. Provisional Patent Application
61/XXX,XXX, entitled "MUTATED ANTI-TNFoc ANTIBODIES AND METHODS OF
THEIR USE", Attorney Docket Number 117813-73802, filed on even date herewith;
U.S.
Provisional Patent Application 61/XXX,XXX, entitled "MODULATED LYSINE VARIANT
SPECIES AND METHODS FOR PRODUCING AND USING THE SAME", Attorney
Docket Number 117813-74101, filed on even date herewith; and U.S. Provisional
Patent
Application 61/XXX,XXX. entitled "PURIFICATION OF PROTEINS USING
HYDROPHOBIC INTERACTION CHROMATOGRAPHY", Attorney Docket Number
117813-74301, filed on even date herewith.
261