Note: Descriptions are shown in the official language in which they were submitted.
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
NANOPARTICULATE IVACAFTOR FORMULATIONS
FIELD OF THE INVENTION
The present invention relates to nanoparticulate compositions of ivacaftor or
a pharmaceutically
acceptable salt thereof, and methods of making and using such compositions.
The compositions
comprise ivacaftor particles having an effective average particle size of less
than about 2000 nm.
BACKGROUND OF THE INVENTION
Ivacaftor is a potent and selective CFTR potentiator approved for the
treatment of cystic fibrosis
with a certain mutations in CFTR gene. It is approved as tablet and granules
for oral
administration under the brand name KALYDECO . Ivacaftor is chemically known
as N-(2,4-
di -tert-b uty1-5-hydroxypheny1)-1,4-dihydro-4-oxoquinoline-3 -carboxami de
and has the
following structural formula:
OH \ ,
0 0
[
-N-'
U.S. Patent No. 7,495,103 discloses modulators of ATP-binding cassette
transporters such as
ivacaftor and also discloses methods of treating CFTR transporter mediated
diseases using such
modulators.
U.S. Patent No. 7,553,855 discloses a pharmaceutical composition comprising: N-
(5-hydroxy-
24-ditert-butyl-phenyl)-4-oxo-1H-quinoline-3-carboxamide (Ivacaftor), PEG 400,
and PVP K30.
U.S. Patent No. 8,410,274 discloses solid dispersion of amorphous N42,4-
bis(1,1-
di methyl ethyl)-5-hydroxyphenyl] -1,4-dihydro-4-oxoquinol i ne-3 -carboxami
de, pharmaceutical
compositions thereof and methods therewith.
U.S. Patent No. 8,163,772 discloses solid forms of ivacaftor in form of co-
forms with 2-methyl
butyric acid, propylene glycol, PEG400.KOAc, lactic acid, isobutyric acid,
propionic acid,
ethanol, 2-propanol, water, besylate, hemibesylate, and besylate monohydrate.
1
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
U.S. Patent No. 8,883,206 discloses a pharmaceutical composition of ivacaftor
in form of solid
dispersion with HPMCAS along with other excipients
U.S. Patent Publication No. 2015/0010628 discloses a pharmaceutical
composition comprising a
solid dispersion of amorphous or substantially amorphous ivacaftor, a filler,
a sweetener, a
disintegrant, a glidant and a lubricant, and optionally a wetting agent.
U.S. Patent No. 8,471,029 discloses crystalline form C of N42,4-bis(1,1-
dimethylethyl)-5-
hydroxypheny1]-1,4-dihy dro-4-oxo quinol ine-3 -carboxami de.
US Patent No. 8,674,108 discloses crystalline solvates of ivacaftor, which are
designated as
Form D, Form E, Form F, Form G, Form H, Form I, Form J, Form K, Form L, Form
M, Form N,
Form 0, Form P, Form Q, Form R, Form S, Form T, Form W and hydrate B and their
preparation.
Ivacaftor is a white to off-white powder. It is freely soluble in methylethyl
ketone/water mixture,
soluble in 2-methyl tetrahydrofuran and PEG 400, slightly soluble in methanol,
acetone and
ethanol and practically insoluble in water (<0.05 microgram/mL) and buffers
with pH 1.0 ¨7ØIvacaftor has been isolated in two physical forms, an
amorphous form and a crystalline form.
Various crystalline polymorphic forms are known and Form C is the most
thermodynamically
stable form. The absorption of ivacaftor in mouse, rats, rabbits and dogs is
rapid, following oral
administration of aqueous suspensions of the amorphous form, and
bioavailability ranged from
30 to 100%. At the same time the crystalline form in suspension had a low oral
bioavailability.
Due to low solubility and low bioavailability of crystalline form of
ivacaftor, the marketed
KALYDECO tablet is prepared in two stages¨ manufacture of spray dispersion
and tabletting.
The spray-dried dispersion (SDD) is manufactured using solvent based spray-
drying followed by
secondary drying to remove residual solvents. In the second stage, the
amorphous SDD is
blended with additional excipients, compressed into a core tablet, film-coated
and printed to
form the final drug product. Due to ivacaftor being insoluble in water, it
needs to be spray-dried
with excipients to produce an amorphous form of the active substance, which
circumvents the
2
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
solubility limited bioavailability issues of the crystalline form and hence is
better absorbed. The
KALYDECO granules are pre-compressed amorphous SDD blended with other
excipients.
The exposure of ivacaftor increased approximately 2.5-to 4-fold when given
with food that
contains fat. Therefore, KALYDECO tablet is administered with fat-containing
food. Examples
of fat-containing foods include eggs, butter, peanut butter, cheese pizza,
whole-milk dairy
products (such as whole milk, cheese, and yogurt), etc. KALYDECO granules (2
x 75 mg) had
similar bioavailability as the 150 mg tablet when given with fat-containing
food in adult subjects.
The effect of food on ivacaftor absorption is similar for KALYDECO granules
and the 150 mg
tablet formulation.
Clinical study during KALYDECO* product development shows clear food affect.
After oral
administration of a single 150 mg dose to healthy volunteers in the fasted
state, the mean (SD)
for AUC0,õ and Cmax were 3620 (1840) ng*hr/mL and 218 (110) ng/mL,
respectively. In the
same study, oral administration of a single 150 mg dose in the fed state led
to a substantial
increase in exposure: AUC0,õ was 10600 (5260) ng*hr/mL and Cmax was 768 (233)
ng/mL. The
exposure of ivacaftor increased approximately 2- to 4-fold when given with
food containing fat.
Therefore, KALYDECO is administered with fat-containing food. There is a
clear effect of
concomitant food intake on the speed and magnitude of absorption of ivacaftor.
In healthy
volunteers high fat breakfast increased Cmax and AUC of 150 mg tablet
formulation by an order
of magnitude around 2- to 4 and delayed Tmax from 3 to 5 hours. A consistent
effect was
observed in patients with CF and pancreatic insufficiency in whom ivacaftor
275 mg oral
solution was given. In this later case a standard CF meal (which contains up
to 200% of the fat
content of a standard meal) was compared with a fasted state. The impact of
food on the PK of
ivacaftor may have implications in clinical practice. Clinical studies were
conducted advising
patients to take ivacaftor with food containing fat. A recommendation that
ivacaftor to be taken
with food or shortly after a meal is included in the labeling information
issued by the FDA and
EMEA in respect of the KALYDECO . If a patient does not take its dose exactly
as indicated,
sub-therapeutic levels of ivacaftor can occur. Further, different fat content
might result in
relevantly different exposure to ivacaftor, there is every chance of
variability of ivacaftor in
blood with different food content.
3
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
The dissolution rate and bioavailability of known ivacaftor formulations are
not optimal. A
similar bioavailability of ivacaftor formulation irrespective of the
polymorphic forms of active
ingredient is the need of the hour. Furthermore, the effectiveness of
ivacaftor may be enhanced if
it could be formulated to be taken with or without food, thus decreasing the
likelihood of patient
compliance problems.
A formulation that enhances the bioavailability of ivacaftor would facilitate
reduction of the
dosage strength with the possibility of achieving a better safety profile.
Nanoparticulate active agent compositions, first described in US5145684, are
particles
comprising a poorly soluble therapeutic or diagnostic agent having adsorbed
onto, or associated
with, the surface thereof a non-cross linked surface stabilizer.
Nanoparticulate active agent compositions and methods of making
nanoparticulate active agent
compositions are disclosed in US5298262, US5302401, US5336507, US5340564,
US5346702,
US5352459, US5429824, US5470583, US5510118, US5518187, US5534270,
US5543133,US5560931, US5560932, US5565188, US5569448, US5571536, US5573783,
US5580579, US5585108, US5587143, US5591456, US5622938, US5662883, US5665331,
US5718388, US5834025, US5862999, US6011068, US6031003, US6211244, US6264922,
US6267989, US6270806, US6313146, US6316029, US6375986, US6406718, US6428814,
US6431478, US6432381, US6582285, US6592903, US6723068, US6742734, US6745962,
US6811767, US6908626, 1JS6969529, US6976647, US6991191, US7198795, US7244451,
US7288267, US7320802, US7390505, US7459283, US7521068, US7575184,
US7695739,US7713551, US7763278, US7780989, US7825087, US7842232, US7850995,
US7879360, 1JS7910577, US7927627, US7931917, US8119163, US8293277,
US8779004,US9012511, USRE41884, US20030087308, US20030215502, US20040015134,
US20040105778,US20040105889, US20040115134, US20040156895, US20040173696,
US20040195413,US20040258757, US20050147664, US20080124393, US20080152585,
US20080171091, US20080213374, US20080213378, US20080220074, US20080226734,
US20080248123, US20080254114, US20080317843, US20090035366, US20090074873,
US20090155331, US20090238884, US20090252807, US20090269400, US20090291142,
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
US20090297596, US20090304801, US20100028439, US20100221327, US20100247636,
US20100260858, US20100260859, US20100316725, US20110008435, US20110027371,
US20110064803, W02009002427.
US4783484 discloses amorphous small particle compositions; US4826689 discloses
method for
making uniformly sized particles from water-insoluble organic compounds;
US4997454
discloses method for making uniformly-sized particles from insoluble
compounds. These are also
specifically incorporated herein by reference.
None of the above references describes nanoparticulate compositions of
ivacaftor or a
pharmaceutically acceptable salt thereof.
The present invention relates to nanoparticulate ivacaftor compositions, such
as nanoparticulate
ivacaftor or a pharmaceutically acceptable salt thereof, which addresses the
needs described
above by providing nanoparticulate ivacaftor compositions which overcome the
shortcomings of
known non-nanoparticulate ivacaftor formulations.
SUMMARY OF THE INVENTION
The present invention relates to stable nanoparticulate compositions
comprising ivacaftor or a
pharmaceutically acceptable salt thereof. The ivacaftor nanoparticles have
good stability and
have an effective average particle size of less than about 2000 nm. One aspect
of the invention
relates to a stable nanoparticulate ivacaftor composition comprising solid
particles of ivacaftor or
a pharmaceutically acceptable salt thereof having an effective average
particle size of less than
about 2000 nm.
Another aspect of the invention relates to a stable nanoparticulate ivacaftor
composition
comprising ivacaftor or a pharmaceutically acceptable salt thereof in a
crystalline form, an
amorphous form, a semi-crystalline form, or mixtures thereof.
One aspect of the invention relates to a stable nanoparticulate composition
comprising ivacaftor
or a pharmaceutically acceptable salt thereof and at least one surface
stabilizer. The
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
nanoparticulate ivacaftor compositions of the invention may further comprise
one or more
pharmaceutically acceptable excipients, carriers and the like.
Another aspect of the invention relates to a stable nanoparticulate ivacaftor
composition
comprising solid particles of ivacaftor or a pharmaceutically acceptable salt
thereof having an
effective average particle size of less than about 2000 nm and at least one
surface stabilizer.
Further, the composition comprises at least one surface stabilizer is selected
from the group
consisting of a non-ionic surface stabilizer, an ionic surface stabilizer, an
anionic surface
stabilizer, a cationic surface stabilizer, and a zwitterionic surface
stabilizer.
Another aspect of the invention relates to a stable nanoparticulate ivacaftor
composition
comprising solid particles of ivacaftor or a pharmaceutically acceptable salt
thereof having an
effective average particle size of less than about 2000 nm and at least one
surface stabilizer,
wherein at least one surface stabilizer is selected from the group consisting
of copolymers of
vinylpyrrolidone and vinyl acetate or copovidone, polyvinyl caprolactam -
polyvinyl acetate -
polyethylene glycol graft copolymer, docusate sodium, hydroxypropylcellulose,
hydroxypropylmethylcellulose, polyoxyethylene sorbitan fatty acid esters,
block copolymers
based on ethylene oxide and propylene oxide, polyvinylpyrrolidone, deoxycholic
acid sodium
salt, sodium lauryl sulphate, benzalkonium chloride, lecithin, distearyl
palmitate glyceryl,
albumin, lysozyme, gelatin, macrogol 15 hydroxystearate, tyloxapol and
polyethoxylated castor
oil, cellulose derivates, dioctylsulfosuccinate, casein, dextran, gum acacia,
cholesterol,
tragacanth, stearic acid, calcium stearate, glycerol monostearate, cetostearyl
alcohol,
cetomacrogol emulsifying wax, sorbitan esters, polyoxyethylene alkyl ethers,
polyoxyethylene
castor oil derivatives, polyethylene glycols, polyoxyethylene stearates,
colloidal silicon dioxide,
phosphates, carboxymethylcellulose calcium, carboxymethylcellulose sodium,
methylcellulose,
hydroxyethylcellulose, hypromellose phthalate, noncrystalline cellulose,
magnesium aluminium
silicate, triethanolamine, polyvinyl alcohol, 4-(1,1,3,3-tetramethylbuty1)-
phenol polymer with
ethylene oxide and formaldehyde, poloxamers, poloxamines, polyethylene oxide-
containing fatty
acid esters like Stearoyl macrogo1-32 glycerides, Lauroyl macrogo1-32
glycerides (e.g.
Gelucire) or a mixture thereof.
6
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
Another aspect of the invention relates to a stable nanoparticulate ivacaftor
composition
comprising solid particles of ivacaftor or a pharmaceutically acceptable salt
thereof, wherein the
composition is formulated: (a) for administration selected from the group
consisting of oral,
pulmonary, intravenous, rectal, ophthalmic, colonic, parenteral,
intracistemal, intravaginal,
intraperitoneal, local, buccal, nasal, and topical administration; (b) into a
dosage form selected
from the group consisting of liquid dispersions, soft gelatin capsules, gels,
aerosols, ointments,
creams, tablets, sachets and capsules; (c) into a dosage form selected from
the group consisting
of lyophilized formulations, fast melt formulations, controlled release
formulations, delayed
release formulations, extended release formulations, pulsatile release
formulations, and mixed
immediate release and controlled release formulations; or (d) any combination
(a) (b), and (c).
One aspect of the invention relates to a stable nanoparticulate ivacaftor
composition comprising
solid crystalline particles of ivacaftor or a pharmaceutically acceptable salt
thereof having an
effective average particle size of less than about 2000 nm, wherein the solid
crystalline particles
of ivacaftor or a pharmaceutically acceptable salt thereof are substantially
free from amorphous
form.
Another aspect of the invention relates a stable nanoparticulate ivacaftor
composition comprising
solid crystalline particles of ivacaftor or a pharmaceutically acceptable salt
thereof having an
effective average particle size of less than about 2000 nm and at least one
surface stabilizer,
wherein the solid crystalline particles of ivacaftor or a pharmaceutically
acceptable salt thereof
are substantially free from amorphous form.
Another aspect of the invention relates a stable nanoparticulate ivacaftor
composition comprising
solid crystalline particles of ivacaftor or a pharmaceutically acceptable salt
thereof and at least
one surface stabilizer.
One aspect of the invention relates to a composition comprising a stable
nanoparticulate of
ivacaftor, or a pharmaceutically acceptable salt thereof exhibits
substantially similar oral
bioavailability whether ivacaftor or a pharmaceutically acceptable salt
thereof is in a crystalline
form or an amorphous form or a semi-crystalline form, or mixtures thereof.
7
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
Another aspect of the invention relates a composition comprising
nanoparticulate composition of
ivacaftor is bioequivalent to the commercially available KALYDECO . This
nanoparticulate
composition of ivacaftor is irrespective of the form of ivacaftor in the
formulation. Preferably a
crystalline form of ivacaftor in form of nanoparticles present in a tablet
formulation or
suspension is bioequivalent to KALYDECO tablet or suspension dosage form.
One aspect of the invention relates to a composition comprising a stable
nanoparticulate of
ivacaftor, or a pharmaceutically acceptable salt thereof shows enhanced
bioavailability. Another
aspect of the invention relates to a composition comprising a stable
nanoparticulate of ivacaftor,
or a pharmaceutically acceptable salt thereof shows a reduced "food effect" as
compared to non-
nanoparticulate ivacaftor compositions. The compositions exhibit substantially
similar oral
bioavailability in fed and fasted subjects.
One aspect of the invention relates to a composition comprising a stable
nanoparticulate of
ivacaftor, or a pharmaceutically acceptable salt thereof exhibits improved
bioavailability as
compared to known non-nanoparticulate ivacaftor compositions having an
effective average
particle size of greater than 2000 nm.
The invention also relates to compositions comprising nanoparticulate
ivacaftor or a
pharmaceutically acceptable salt thereof providing a method of treating or
lessening the severity
of a disease in a patient comprising administering to said patient one of the
compositions as
defined herein, and said disease is selected from cystic fibrosis, asthma,
smoke induced COPD,
chronic bronchitis, rhinosinusitis, constipation, pancreatitis, pancreatic
insufficiency, male
infertility caused by congenital bilateral absence of the vas deferens
(CBAVD), mild pulmonary
disease, idiopathic pancreatitis, allergic bronchopulmonary aspergillosis
(ABPA), liver disease,
hereditary emphysema, hereditary hemochromatosis, coagulation-fibrinolysis
deficiencies, such
as protein C deficiency, Type 1 hereditary angioedema, lipid processing
deficiencies, such as
familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia,
lysosomal
storage diseases, such as I-cell disease/pseudo-Hurler, mucopolysaccharidoses,
Sandhof/Tay-
Sachs, Crigler-Najjar type II, polyendocrinopathy/hyperinsulinemia, Diabetes
mellitus, Laron
dwarfism, myeloperoxidase deficiency, primary hypoparathyroidism, melanoma,
glycanosis
8
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
CDG type 1, congenital hyperthyroidism, osteogenesis imperfecta, hereditary
hypofibrinogenemia, ACT deficiency, Diabetes insipidus (DI), neurohypophyseal
DI,
nephrogenic DI, Charcot-Marie Tooth syndrome, Pelizaeus-Merzbacher disease,
neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease,
amyotrophic lateral
sclerosis, progressive supranuclear palsy, Pick's disease, several
polyglutamine neurological
disorders such as Huntington's, spinocerebellar ataxia type I, spinal and
bulbar muscular atrophy,
dentatorubral pallidoluysian atrophy, and myotonic dystrophy, as well as
spongiform
encephalopathies, such as hereditary Creutzfeldt-Jakob disease (due to prion
protein processing
defect), Fabry disease, Gerstmann-Stra ussler-Scheinker syndrome, COPD, dry-
eye disease,
Sjogren's disease, Osteoporosis, Osteopenia, Gorham's Syndrome, chloride
channelopathies such
as myotonia congenita (Thomson and Becker forms), Bartter's syndrome type III,
Dent's disease,
epilepsy, hyperekplexia, lysosomal storage disease, Angelman syndrome, and
Primary Ciliary
Dyskinesia (PCD), a term for inherited disorders of the structure and/or
function of cilia,
including PCD with situs inversus (also known as Kartagener syndrome), PCD
without situs
inversus and ciliary aplasia. Further, the composition also comprises at least
one surface
stabilizer, and optionally one or more pharmaceutically acceptable excipients,
carriers, and
optionally one or more active agents useful for the treatment of the said
diseases.
The invention also relates to methods of making nanoparticulate ivacaftor
compositions, or salt
thereof. In some embodiments, the methods include contacting ivacaftor
particles with at least
one surface stabilizer for a time and under conditions sufficient to provide a
nanoparticulate
ivacaftor composition having an effective average particle size of less than
about 2000 nm. A
suitable surface stabilizer can be added to a nanoparticulate ivacaftor
composition either before,
during, or after particle size reduction. Any suitable means can be used to
achieve nanoparticles
reduce the particle size of ivacaftor, including, but not limited to, milling,
microfluidization,
precipitation, freeze drying, homogenization and the like.
Both the foregoing summary of the invention and the detailed description of
the invention are
exemplary and explanatory and are intended to provide further details of the
invention as
claimed. Other objects, advantages, and novel features will be readily
apparent to those skilled in
the art from the following detailed description of the invention.
9
CA 03021752 2018-10-22
WO 2016/199085 PCT/I132016/053433
BRIEF DESCRIPTION OF THE DRAWINGS
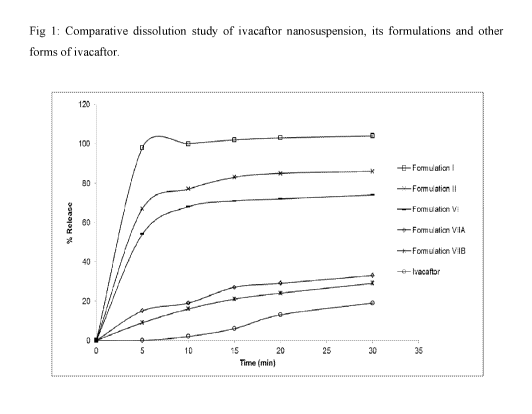
Fig 1: Comparative dissolution study of ivacaftor nanosuspension, its
formulations and other
forms of ivacaftor.
Fig 2: Differential Scanning Calorimetry (DSC) pattern of ivacaftor granulated
nanosuspension
(Formulation II), ivacaftor and MCC
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to a composition comprising nanoparticulate
ivacaftor, or a
pharmaceutically acceptable salt thereof. The nanoparticles of ivacaftor or a
pharmaceutically
acceptable salt thereof have an effective average particle size of less than
about 2000 nm. The
compositions further comprise nanoparticulate ivacaftor, or a pharmaceutically
acceptable salt
thereof, and at least one surface stabilizer, which may be adsorbed onto or
otherwise associated
with the surface of the drug.
As described above, one of the problems present with known non-nanoparticulate
ivacaftor
compositions (KALYDECO ) is that absorption of the drug (AUC) can differ by
almost 2-4 fold
when the drug is given under fed as compared to fasting conditions. This is
highly undesirable,
as it is generally recognized at least about 1/3 of all patients have poor
compliance regarding
consuming drugs per the labeling instructions. This means that for drugs
having a wide
variability in absorption when administered under fed as compared to fasting
conditions, a large
patient population does not receive a therapeutically desirable dosage. One
aspect of the
invention overcomes this problem as nanoparticulate ivacaftor compositions
have minimal, if
any, differences in absorption when the compositions are administered under
fed as compared to
fasting conditions.
Another problem with ivacaftor is low oral bioavailability of crystalline form
as compared to
amorphous form. Nanoparticulate ivacaftor compositions overcomes this problem
as the
bioavailability of nanoparticles of ivacaftor or a pharmaceutically acceptable
salt thereof is
substantially similar irrespective of the form of active ingredient. Ivacaftor
is in crystalline form,
amorphous form, semi-crystalline form, or mixtures thereof.
CA 03021752 2018-10-22
WO 2016/199085 PCT/1B2016/053433
Nanoparticulate ivacaftor compositions result in reduced or eliminated side
effects. However, in
general terms reducing the amount of drug to which patients are exposed, while
at the same time
maintaining efficacious levels, may help to reduce adverse effects and improve
the safety profile
of the product. Because nanoparticulate ivacaftor compositions have a greater
bioavailability, the
nanoparticulate ivacaftor compositions enable the use of a smaller dosage as
compared to known
non-nanoparticulate ivacaftor compositions, thereby facilitating a reduction
in side effects.
The present invention also includes compositions further comprising one or
more non-toxic
physiologically acceptable carriers, adjuvants, or vehicles, collectively
referred to as carriers.
The compositions can be formulated for administration via any pharmaceutically
acceptable
means, including but not limited to, parental injection (e.g., intravenous,
intramuscular, or
subcutaneous), oral administration in solid, liquid, or aerosol form,
bioadhesive, vaginal, nasal,
rectal, ocular, local (powders, ointments, or drops), buccal, intracisternal,
intraperitoneal, or
topical administrations, and the like. The small size of the ivacaftor
particles (i.e. less than 2000
nm) makes the composition of the invention particularly advantageous for oral
and parenteral
formulations.
Oral administration is typically preferred, because of ease of administration
and greater
compliance. Oral dosage forms may be solid or liquid (e.g. syrup). Exemplary
solid oral dosage
forms include, but are not limited to, tablets, capsules (both hard gelatin
and soft gelatin),
sachets, lozenges, powders, pills, or granules, and the solid dosage form can
be, for example, a
fast melt dosage form, controlled release dosage form, lyophilized dosage
form, delayed release
dosage form, extended release dosage form, pulsatile release dosage form,
mixed immediate
release and controlled release dosage form, or a combination thereof.
The present invention is described herein using several definitions, as set
forth below and
throughout the application.
As used herein, "about" will be understood by persons of ordinary skill in the
art and will vary to
some extent on the context in which it is used. Nanoparticulate active agents
or nanoparticles of
active agents as defined herein have an effective average particle size of
less than about 2000
11
CA 03021752 2018-10-22
WO 2016/199085 PCT/1B2016/053433
nm. By way of contrast, the term "non-nanoparticulate active agent" has an
effective average
particle size of greater than 2000 nm.
The term "ivacaftor", as used herein, expressly includes ivacaftor salts and
encompasses different
crystal forms (polymorphs), solvates, co-crystals, and hydrates. It also
includes racemic or
substantially optically pure forms of the foregoing. The terms "drug" or
"active agent," when
used herein, typically refers to ivacaftor but may, if clearly indicated by
its context, refer to
another drug.
The compositions of the invention comprise nanoparticulate ivacaftor, or a
pharmaceutically
acceptable salt thereof. The ivacaftor salt may be an addition salt formed
with a suitable organic
or inorganic acid such as for example HC1, HBr, H2SO4, phosphoric acid,
sulphamic acid, oxalic
acid, lactic acid, malic acid, maleic acid, malonic acid, tartaric acid,
succinic acid, fumaric acid,
acetic acid, citric acid, 4-hydroxy benzoic acid, 2,5-dihydroxy benzoic acid,
adipic acid, glycoli
acid, decanoic acid, un-decanoic acid, cholic acid, dexo-cholic acid, mandelic
acid, d-camphonic
acid, benzoic acid, methansulphonic acid, ethanesulphonic acid, benzesulphonic
acid, p-
toluenesulphonic acid and the like These molecules can also be used as a co-
former for solvates,
and co-crystals of ivacaftor. . Further, U.S. patent no. 7,927,613 can be used
as a reference for
co-formers. The ivacaftor may be present in racemic form or as a substantially
optically pure
enantiomer. Different polymorphic forms, co-crystals, hydrates, solvates of
ivacaftor are
disclosed in various references can be used to prepare nanoparticulate
composition of ivacaftor.
The present invention may be practiced with any single polymorph or a mixture
thereof.
Preferably the composition comprises ivacaftor in the form of a substantially
pure single
polymorph, which may be amorphous form, Form A, Form B or Form C or a mixture
thereof,
preferably Form C. The compositions of the invention comprise nanoparticulate
ivacaftor or a
pharmaceutically acceptable salt thereof, in which the particles can be in a
crystalline form,
semi-crystalline form, amorphous form, or a combination thereof.
The term "effective average particle size," as used herein, means that at
least about 50% of the
nanoparticulate ivacaftor particles have a size of less than about 2000 nm (by
weight or by other
suitable measurement, such as by volume, number, etc.), when measured by, for
example,
12
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
sedimentation flow fractionation, photon correlation spectroscopy, light
scattering, disk
centrifugation, and other techniques known to those of skill in the art.
The term "one," "a," or "an," as used herein, is not limited to singular forms
but also
encompasses the plural equivalent. As used herein, the phrase "therapeutically
effective amount"
shall mean that drug dosage that provides the specific pharmacological
response for which the
drug is administered in a significant number of subjects in need of such
treatment. It is
emphasized that a therapeutically effective amount of a drug that is
administered to a particular
subject in a particular instance will not always be effective in treating the
conditions/diseases
described herein, even though such dosage is deemed to be a therapeutically
effective amount by
those of skill in the art.
The compositions of the invention comprising nanoparticulate ivacaftor can
exhibit increased
bioavailability as compared to the same non-nanoparticulate ivacaftor (in
other words compared
to a composition wherein the ivacaftor component is present at a particle size
greater than 2000
nm). Moreover, the compositions of the invention are expected to require
smaller doses, and
smaller tablet or other solid dosage form size as compared to prior known non-
nanoparticulate
formulations of the same ivacaftor to achieve the same pharmacological effect.
The increased
bioavailability is significant because it means that the nanoparticulate
ivacaftor dosage form will
likely exhibit significantly greater drug absorption compared to the same
amount of ivacaftor
presented in the form of particles greater than about 2000 nm.
The invention also enables production of compositions comprising
nanoparticulate ivacaftor
having a desirable pharmacokinetic (PK) profile when administered to mammalian
subjects.
Standard PK parameters routinely used to assess the behavior of a dosage form
in vivo (in other
words when administered to an animal or human subject) include Cma, (peak
concentration of
drug in blood plasma), Tmax (the time at which peak drug concentration is
achieved) and AUC
(the area under the plasma concentration vs time curve). Methods for
determining and assessing
these parameters are well known in the art. The desirable pharmacokinetic
profile of the
compositions comprising nanoparticulate ivacaftor may comprise but is not
limited to: (I) a Cmax
for a nanoparticulate ivacaftor when assayed in the plasma of a mammalian
subject following
13
CA 03021752 2018-10-22
WO 2016/199085 PCT/1B2016/053433
administration, that is preferably greater than the C. for a non-
nanoparticulate ivacaftor,
administered at the same dosage; and/or (2) an AUC for nanoparticulate
ivacaftor when assayed
in the plasma of a mammalian subject following administration, that is
preferably greater than
the AUC for a non-nanoparticulate ivacaftor, administered at the same dosage;
and/or (3) a Tmax
for nanoparticulate ivacaftor when assayed in the plasma of a mammalian
subject following
administration, that is preferably less than the Tmax for a non-
nanoparticulate formulation of the
same drug administered at the same dosage. Preferably the composition exhibits
a PK profile
having a combination of two or more of the features (I), (2) and (3) in the
preceding sentence.
The desirable pharmacokinetic profile, as used herein, is the pharmacokinetic
profile measured
after the initial dose.
In one embodiment, a composition comprising nanoparticulate ivacaftor
exhibits, in comparative
pharmacokinetic testing with a non-nanoparticulate formulation of the same
drug, administered
at the same dosage, a Tma, not greater than about 90%, not greater than about
80%, not greater
than about 70%, not greater than about 60%, not greater than about 50%, not
greater than about
30%, not greater than about 25%, not greater than about 20%, not greater than
about 15%, not
greater than about 10%, or not greater than about 5% of the Tmax exhibited by
the non-
nanoparticulate ivacaftor formulation.
In another embodiment, the composition comprising nanoparticulate ivacaftor
exhibits in
comparative pharmacokinetic testing with a non-nanoparticulate formulation of
the same drug,
administered at the same dosage, a Cmax which is at least about 50%, at least
about 100%, at least
about 200%, at least about 300%, at least about 400%, at least about 500%, at
least about 600%,
at least about 700%, at least about 800%, at least about 900%, at least about
1000%, at least
about 1100%, at least about 1200%, at least about 1300%, at least about 1400%,
at least about
1500%, at least about 1600%, at least about 1700%, at least about 1800%, or at
least about
1900% greater than the Cmax exhibited by the non-nanoparticulate formulation.
In yet another embodiment, the composition comprising nanoparticulate
ivacaftor exhibits in
comparative pharmacokinetic testing with a non-nanoparticulate formulation of
the same drug,
administered at the same dosage, an AUC which is at least about 25%, at least
about 50%, at
14
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
least about 75%, at least about 100%, at least about 125%, at least about
150%, at least about
175%, at least about 200%, at least about 225%, at least about 250%, at least
about 275%, at
least about 300%, at least about 350%, at least about 400%, at least about
450%, at least about
500%, at least about 550%, at least about 600%, at least about 750%, at least
about 700%, at
least about 750%, at least about 800%, at least about 850%, at least about
900%, at least about
950%, at least about 1000%, at least about 1050%, at least about 1100%, at
least about 1150%,
or at least about 1200% greater than the AUC exhibited by the non-
nanoparticulate formulation.
In one embodiment of the invention, the Tmax of nanoparticulate ivacaftor when
assayed in the
plasma of the mammalian subject is less than about 6 hours. In other
embodiments of the
invention, the Tmax of the ivacaftor is less than about 5.5 hours, less than
about 5 hours, less than
about 4.5 hours, less than about 4 hours, less than about 3.5 hours, less than
about 3 hours, less
than about 2.5 hours, less than about 2 hours, less than about 1.5 hours less
than about 1 hour,
less than about 45 minutes, or less than about 30 minutes after
administration.
In another embodiment of the invention, the compositions when tested in
fasting subjects in
accordance with standard pharmacokinetic practice, are proposed to produces a
maximum blood
plasma concentration profile in less than about 6 hours, less than about 5.5
hours, less than about
hours, less than about 4.5 hours, less than about 4 hours, less than about 3.5
hours, less than
about 3 hours, less than about 2.5 hours, less than about 2 hours, less than
about 1.5 hours, less
than about 1 hour, less than about 45 minutes, or less than about 30 minutes
after the initial dose
of the composition.
The desirable pharmacokinetic profile, as used herein, is the pharmacokinetic
profile measured
after the initial dose of nanoparticulate ivacaftor. The compositions can be
formulated in any
way as described herein and as known to those of skill in the art.
The invention encompasses compositions comprising nanoparticulate ivacaftor
wherein the
pharmacokinetic profile of the drug is not substantially affected by the fed
or fasted state of a
subject ingesting the composition. In other words the composition does not
produce significantly
different absorption levels when administered under fed as compared to fasted
conditions. This
means that there is no substantial difference in the quantity of drug absorbed
(AUC), the rate of
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
drug absorption (Cmax), or the length of time to Cmax(Tmax), when the
nanoparticulate ivacaftor
compositions are administered in the fed versus the fasted state.
The difference in absorption (AUC) or C. of the nanoparticulate ivacaftor
compositions of the
invention, when administered in the fed versus the fasted state, preferably is
less than about
100%, less than about 90%, less than about 80%, less than about 70%, less than
about 65%, less
than about 60%, less than about 55%, less than about 50%, less than about 45%,
less than about
40%, less than about 35%, less than about 30%, less than about 25%, less than
about 20%, less
than about 15%, less than about 10%, less than about 5%, or less than about
3%.
The difference in Trnaõ of the nanoparticulate ivacaftor compositions of the
invention, when
administered in the fed versus the fasted state, preferably is less than about
100%, less than about
90%, less than about 80%, less than about 70%, less than about 65%, less than
about 60%, less
than about 55%, less than about 50%, less than about 45%, less than about 40%,
less than about
35%, less than about 30%, less than about 25%, less than about 20%, less than
about 15%, less
than about 10%, less than about 5%, or less than about 3%.
The invention also encompasses a composition comprising nanoparticulate
ivacaftor in which
administration of the composition to a subject in a fasted state is
bioequivalent to administration
of the composition to a subject in a fed state.
In one of the embodiment of the invention, the nanoparticulate composition of
ivacaftor is
bioequivalent to the commercially available KALYDECO . This nanoparticulate
composition of
ivacaftor is irrespective of the form of ivacaftor in the formulation.
Preferably a crystalline form
of ivacaftor in form of nanoparticles present in a tablet or granules
formulation is bioequivalent
to KALYDECO tablet or granules dosage form.
When formulated into a solid dosage form, the compositions comprising
nanoparticulate
ivacaftor are proposed to have unexpectedly dramatic dissolution profiles.
Rapid dissolution of
an administered active agent is preferable, as faster dissolution generally
leads to greater
bioavailability and faster onset of action. To improve the dissolution profile
and bioavailability
16
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
of ivacaftor it would be useful to increase ivacaftor dissolution so that it
could attain a level close
to 100% dissolution of the drug substance.
The nanoparticulate ivacaftor compositions of the invention, when formulated
into a solid dosage
form, preferably have a dissolution profile in which within about 5 minutes at
least about 5% of
the composition is dissolved. In other embodiments of the invention, at least
about 10%, at least
about 20%, at least about 30% or at least about 40% of the ivacaftor
composition is dissolved
within about 5 minutes. In yet other embodiments of the invention, preferably
at least about 40%,
at least about 50%, at least about 60%, at least about 70%, or at least about
80% of the ivacaftor
composition is dissolved within about 10 minutes. Finally, in another
embodiment of the
invention, preferably at least about 70%, at least about 80%, at least about
90%, or at least about
100% of the ivacaftor composition is dissolved within 20 minutes.
The compositions of the present invention provide a method of treating or
lessening the severity
of a disease in a patient comprising administering to said patient one of the
compositions as
defined herein, and said disease is selected from cystic fibrosis, asthma,
smoke induced COPD,
chronic bronchitis, rhinosinusitis, constipation, pancreatitis, pancreatic
insufficiency, male
infertility caused by congenital bilateral absence of the vas deferens
(CBAVD), mild pulmonary
disease, idiopathic pancreatitis, allergic bronchopulmonary aspergillosis
(ABPA), liver disease,
hereditary emphysema, hereditary hemochromatosis, coagulation-fibrinolysis
deficiencies, such
as protein C deficiency, Type 1 hereditary angioedema, lipid processing
deficiencies, such as
familial hypercholesterolemia, Type 1 chylomicronemia, abetalipoproteinemia,
lysosomal
storage diseases, such as I-cell disease/pseudo-Hurler, mucopolysaccharidoses,
Sandhof/Tay-
Sachs, Crigler-Najjar type II, polyendocrinopathy/hyperinsulinemia, Diabetes
mellitus, Laron
dwarfism, myeloperoxidase deficiency, primary hypoparathyroidism, melanoma,
glycanosis
CDG type 1, congenital hyperthyroidism, osteogenesis imperfecta, hereditary
hypofibrinogenemia, ACT deficiency, Diabetes insipidus (DI), neurohypophyseal
DI,
nephrogenic DI, Charcot-Marie Tooth syndrome, Pelizaeus-Merzbacher disease,
neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease,
amyotrophic lateral
sclerosis, progressive supranuclear palsy, Pick's disease, several
polyglutamine neurological
disorders such as Huntington's, spinocerebellar ataxia type I, spinal and
bulbar muscular atrophy,
17
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
dentatorubral pallidoluysian atrophy, and myotonic dystrophy, as well as
spongiform
encephalopathies, such as hereditary Creutzfeldt-Jakob disease (due to prion
protein processing
defect), Fabry disease, Gerstmann-Stra ussler-Scheinker syndrome, COPD, dry-
eye disease,
Sjogren's disease, Osteoporosis, Osteopenia, Gorham's Syndrome, chloride
channelopathies such
as myotonia congenita (Thomson and Becker forms), Bartter's syndrome type III,
Dent's disease,
epilepsy, hyperekplexia, lysosomal storage disease, Angelman syndrome, and
Primary Ciliary
Dyskinesia (PCD), a term for inherited disorders of the structure and/or
function of cilia,
including PCD with situs inversus (also known as Kartagener syndrome), PCD
without situs
inversus and ciliary aplasia. Further, the composition also comprises at least
one surface
stabilizer, and optionally one or more pharmaceutically acceptable excipients,
carriers, and
optionally one or more active agents useful for the treatment of the said
diseases.
The invention provides compositions comprising nanoparticulate ivacaftor and
at least one
surface stabilizer. The at least one surface stabilizer is preferably adsorbed
on, or otherwise
associated with, the surface of the ivacaftor particles. Surface stabilizers
may physically adhere
on, or associate with, the surface of the ivacaftor particles, but ideally do
not chemically react
with the ivacaftor particles or itself Individually adsorbed molecules of the
surface stabilizer are
essentially free of intermolecular cross-linkages.
The present invention also includes nanoparticulate ivacaftor compositions
together with one or
more non-toxic physiologically acceptable carriers, adjuvants, or vehicles,
collectively referred
to as carriers. The compositions can be formulated for any pharmaceutically
acceptable method
of administration, such as parenteral injection (e.g., intravenous,
intramuscular, or
subcutaneous), oral administration in solid, liquid, or aerosol form, vaginal,
nasal, rectal, ocular,
local (powders, ointments or drops), buccal, intracistemal, intraperitoneal,
or topical
administration, and the like. A preferred route of administration is oral
administration.
Accordingly, compositions of the invention may be formulated: (a) for
administration selected
from the group consisting of oral, pulmonary, intravenous, rectal, ophthalmic,
colonic,
parenteral, intracistemal, intravaginal, intraperitoneal, local, buccal,
nasal, and topical
administration; (b) into a dosage form selected from the group consisting of
liquid dispersions,
18
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
soft gelatin capsules, gels, aerosols, ointments, creams, tablets, sachets and
capsules; (c) into a
dosage form selected from the group consisting of lyophilized formulations,
fast melt
formulations, controlled release formulations, delayed release formulations,
extended release
formulations, pulsatile release formulations, and mixed immediate release and
controlled release
formulations; and (d) combinations of (a), (b), and (c).
The compositions of the invention comprise at least one surface stabilizer.
However,
combinations of more than one surface stabilizer have been found to be useful
and can be used in
the invention. Where a plurality of surface stabilizers is used there may be a
primary surface
stabilizer that is present in greater concentration than the other (secondary)
surface stabilizer(s).
Preferably the composition will comprise a primary surface stabilizer and at
least one secondary
surface stabilizer. Useful surface stabilizers which can be employed in the
invention include, but
are not limited to, known organic and inorganic pharmaceutical excipients.
Such excipients
include various polymers, low molecular weight oligomers, natural products,
and surfactants.
Exemplary surface stabilizers include nonionic and ionic (e.g., anionic,
cationic, and
zwitterionic) stabilizers. Without wishing to be bound by any particular
theory, it is believed that
polymeric materials adhering to a particle surface can present a steric
barrier preventing particle
aggregation, while in the case of ionic stabilizers the stabilizing action may
be attributed to
electrostatic interactions.
Representative examples of surface stabilizers include albumin, including but
not limited to
human serum albumin and bovine albumin, hydroxypropyl methylcellulose (now
known as
hypromellose), hydroxypropylcellulose, polyvinylpyrrolidone, polyvinyl
caprolactam - polyvinyl
acetate - polyethylene glycol graft copolymer, sodium lauryl sulfate,
dioctylsulfosuccinate,
gelatin, casein, lecithin (phosphatides), dextran, gum acacia, cholesterol,
tragacanth, stearic acid,
benzalkonium chloride, calcium stearate, glycerol monostearate, cetostearyl
alcohol,
cetomacrogol emulsifying wax, sorbitan esters, polyoxyethylene alkyl ethers
(e.g., macrogol
ethers such as cetomacrogol 1000), polyoxyethylene castor oil derivatives,
polyoxyethylene
sorbitan fatty acid esters (e.g., Tween 20 and Tween 80'1); polyethylene
glycols (e.g., Carbowax
3550 and 934*), polyoxyethylene stearates, colloidal silicon dioxide,
phosphates,
carboxymethylcellulose calcium, carboxymethylcellulose sodium,
methylcellulose,
19
CA 03021752 2018-10-22
WO 2016/199085 PCT/1132016/053433
hydroxyethylcellulose, hypromellose phthalate, noncrystalline cellulose,
magnesium aluminium
silicate, triethanolamine, polyvinyl alcohol (PVA), polyvinyl caprolactam -
polyvinyl acetate -
polyethylene glycol graft copolymer (Soluplus ), 4-(1,1,3,3-tetramethylbuty1)-
phenol polymer
with ethylene oxide and formaldehyde (also known as tyloxapol, superione, and
triton),
poloxamers (e.g., Pluronics F68 and F108 , which are block copolymers of
ethylene oxide and
propylene oxide); poloxamines (e.g., Tetronic 908 , also known as, Poloxamine
908 , which is a
tetrafunctional block copolymer derived from sequential addition of propylene
oxide and
ethylene oxide to ethylenediamine); Tetronic 1508 (T-1508), Tritons X-200 ,
which is an alkyl
aryl polyether sulfonate; Crodestas F-110 , which is a mixture of sucrose
stearate and sucrose
distearate; p-isononylphenoxypoly-(glycidol), also known as Olin-10G or
Surfactant 10-G ;
Crodestas SL-40 ); and SA9OHCO, which is C18H37CH2(CON(CH3)--
CH2(CHOH)4(CH2OH)2;
decanoyl-N-methylglucamide; n-decyl 13-D-glucopyranoside; n-decyl P-D-
maltopyranoside; n-
dodecyl 13-D-glucopyranoside; n-dodecyl 13-D-maltoside; heptanoyl-N-
methylglucamide; n-
heptyl-f3-D-glucopyranoside; n-heptyl 13-D-thioglucoside; n-hexyl 13-D-
glucopyranoside;
nonanoyl-N-methylglucamide; n-nonyl P-D-glucopyranoside; octanoyl-N-
methylglucamide; n-
octyl-P-D-glucopyranoside; octyl f3-D-thioglucopyranoside; PEG-phospholipid,
PEG-
cholesterol, PEG-cholesterol derivative, PEG-vitamin A, PEG-vitamin E,
lysozyme, random
copolymers of vinyl pyrrolidone and vinyl acetate, and the like.
Examples of useful cationic surface stabilizers include, but are not limited
to, polymers,
biopolymers, polysaccharides, cellulosics, alginates, phospholipids, and
nonpolymeric
compounds, such as zwitterionic stabilizers, poly-n-methylpyridinium, anthryul
pyridinium
chloride, cationic phospholipids, chitosan, polylysine, polyvinylimidazole,
polybrene,
polymethylmethacrylate tri methyl ammonium bromide (PMM'TMAB
r),
hexyldesyltrimethylammonium bromide (HDMAB), and polyvinylpyrrolidone-2-
dimethylaminoethyl methacrylate dimethyl sulfate. Other useful cationic
stabilizers include, but
are not limited to, cationic lipids, sulfonium, phosphonium, and quaternary
ammonium
compounds, such as stearyltrimethylammonium chloride,
benzyl-di(2-
chloroethyl)ethylammonium bromide, coconut trimethyl ammonium chloride or
bromide,
coconut methyl dihydroxyethyl ammonium chloride or bromide, decyl triethyl
ammonium
chloride, decyl dimethyl hydroxyethyl ammonium chloride or bromide, C12-
15dimethyl
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
hydroxyethyl ammonium chloride or bromide, coconut dimethyl hydroxyethyl
ammonium
chloride or bromide, myristyl trimethyl ammonium methyl sulphate, lauryl
dimethyl benzyl
ammonium chloride or bromide, lauryl dimethyl (ethenoxy)4 ammonium chloride or
bromide,
N-alkyl (C12-18)dimethylbenzyl ammonium chloride, N- al kyl (C14-18)dimethyl-
benzyl
ammonium chloride, N-tetradecylidmethylbenzyl ammonium chloride monohydrate,
dimethyl
didecyl ammonium chloride, N-alkyl and (C12-14) dimethyl 1-napthylmethyl
ammonium
chloride, trimethylammonium halide, alkyl-trimethylammonium salts and dialkyl-
dimethylammonium salts, lauryl trimethyl ammonium chloride, ethoxylated
alkyamidoalkyldialkylammonium salt and/or an ethoxylated trialkyl ammonium
salt,
dialkylbenzene dialkylammonium chloride, N-didecyldimethyl ammonium chloride,
N-
tetradecyldimethylbenzyl ammonium, chloride monohydrate, N-alkyl(C12-14)
dimethyl 1-
naphthylmethyl ammonium chloride and dodecyldimethylbenzyl ammonium chloride,
dialkyl
benzenealkyl ammonium chloride, lauryl trimethyl ammonium chloride,
alkylbenzyl methyl
ammonium chloride, alkyl benzyl dimethyl ammonium bromide, C12, C15, C17
trimethyl
ammonium bromides, dodecylbenzyl triethyl ammonium chloride, poly-
diallyldimethylammonium chloride (DADMAC), dimethyl ammonium chlorides,
al kyl dimethyl ammoni um halogenides, tricetyl methyl
ammonium chloride,
de cyltrimethyl ammoni um bromide, dodecyltri ethyl ammoni um
bromide,
tetradecyltrimethylammonium bromide, methyl trioctylammonium chloride (ALIQUAT
336 ),
polyquaternium 10 (POLYQUAT 10 ),
tetrabutyl ammonium bromide, benzyl
trimethylammonium bromide, choline esters (such as choline esters of fatty
acids), benzalkonium
chloride, stearalkonium chloride compounds (such as stearyltrimonium chloride
and Di-
stearyldimonium chloride), cetyl pyridinium bromide or chloride, halide salts
of quatemized
polyoxyethylalkylamines, quaternized ammonium salt polymers (MIRAPOL and
ALKAQUAT ), alkyl pyridinium salts; amines, such as alkylamines,
dialkylamines,
alkanolamines, polyethylenepolyamines, N,N-dialkylaminoalkyl acrylates, and
vinyl pyridine,
amine salts, such as lauryl amine acetate, stearyl amine acetate,
alkylpyridinium salt, and
alkylimidazolium salt, and amine oxides; imide azolinium salts; protonated
quaternary
acrylamides; methylated quaternary polymers, such as poly[dially1
dimethylammonium chloride]
and poly-[N-methyl vinyl pyridinium chloride]; and cationic guar.
21
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
Such exemplary cationic surface stabilizers and other useful cationic surface
stabilizers are
described in J. Cross and E. Singer, Cationic Surfactants: Analytical and
Biological
Evaluation(Marcel Dekker, 1994); P. and D. Rubingh (Editor), Cationic
Surfactants: Physical
Chemistry(Marcel Dekker, 1991); and J. Richmond, Cationic Surfactants. Organic
Chemistry,
(Marcel Dekker, 1990).
Nonpolymeric surface stabilizers are any nonpolymeric compound, such
benzalkonium chloride,
a carbonium compound, a phosphonium compound, an oxonium compound, a halonium
compound, a cationic organometallic compound, a quaternary phosphorous
compound, a
pyridinium compound, an anilinium compound, an ammonium compound, a
hydroxylammonium
compound, a primary ammonium compound, a secondary ammonium compound, a
tertiary
ammonium compound, and quaternary ammonium compounds.
Such compounds include, but are not limited to, behenalkonium chloride,
benzethonium
chloride, cetylpyridinium chloride, behentrimonium chloride, lauralkonium
chloride,
cetalkonium chloride, cetrimonium bromide, cetrimonium chloride, cethylamine
hydrofluoride,
chlorallylmethenamine chloride (Quatemium-15), distearyldimonium chloride
(Quaternium-5),
dodecyl di methyl ethylbenzyl ammonium chlori de(Q uaterni um-14), Q uaterni
um-22,
Quaternium-26, Quatemium-18 hectorite, dimethylaminoethylchloride
hydrochloride, cysteine
hydrochloride, diethanolammonium POE (10) oletyl ether phosphate,
diethanolammonium POE
(3)oley1 ether phosphate, tallow alkonium chloride, dimethyl
dioctadecylammoniumbentonite,
stearalkonium chloride, domiphen bromide, denatonium benzoate, myristalkonium
chloride,
laurtrimonium chloride, ethylenediamine dihydrochloride, guanidine
hydrochloride, pyridoxine
HC1, iofetamine hydrochloride, meglumine hydrochloride, methylbenzethonium
chloride,
myrtrimonium bromide, oleyltrimonium chloride, polyquatemium-1,
procainehydrochloride,
cocobetaine, steara1konium bentonite, stearalkoniumhectonite, stearyl
trihydroxyethyl
propylenedi amine dihydrofluori de, tal lowtrimoni um chloride, and h
exadecyltri methyl
ammonium bromide.
The following surface stabilizers may be particularly useful in the practice
of the present
22
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
invention copolymers of vinylpyrrolidone and vinyl acetate or copovidone
(e.g., Plasdone S630,
which is a random copolymer of vinyl acetate and vinyl pyrrolidone); docusate
sodium (DOSS);
hydroxypropylcellulose (HPC, such as HPC-SL which has a viscosity of 2.0 to
2.9 mPas in
aqueous 2% w/v solution at 20'; hydroxypropylmethylcellulose (HPMC, such as
Pharmacoat
603; polysorbates or polyoxyethylene sorbitan fatty acid esters (e.g. Tween
20
(polyoxyethylene 20 sorbitan monolaurate), Tween 40 (polyoxyethylene 20
sorbitan palmitate)
or Tween 80 (polyoxyethylene 20 sorbitan monooleate)); block copolymers based
on ethylene
oxide and propylene oxide, also known as poloxamers (e.g., poloxamer 407
(*Lutrol F127),
poloxamer 188 (Lutrol F68) or Poloxamer 338 (Lutrol F108); a
polyvinylpyrrolidone (PVP),
e.g. Plasdone C29/32, Plasdone C-30, Plasdone C17 and Plasdone C12;
deoxycholic acid
sodium salt, sodium lauryl sulphate (SLS also known as sodium dodecyl sulphate
or SDS),
benzalkonium chloride (also known as alkyldimethylbenzylammonium chloride),
lecithin,
distearyl palmitate glyceryl or a combination thereof. Other preferred
stabilizers include
albumin, lysozyme, gelatin, macrogol 15 hydroxystearate (e.g. Solutol 15),
tyloxapol and
polyethoxylated castor oil (e.g. Cremophor EL), polyethylene oxide-containing
fatty acid esters
like Stearoyl macrogol-32 glycerides, Lauroyl macrogol-32 glycerides (e.g.
Gelucire ).
The surface stabilizers are commercially available and/or can be prepared by
techniques known
in the art. Most of these surface stabilizers are known pharmaceutical
excipients and are
described in detail in the Handbook of Pharmaceutical Excipients, published
jointly by the
American Pharmaceutical Association and The Pharmaceutical Society of Great
Britain (R. C.
Rowe et al (ed.) 6th Edition, The Pharmaceutical Press, 2009), specifically
incorporated by
reference.
The preferable surface stabilizer is copolymers of vinylpyrrolidone and vinyl
acetate or
copovidone, polyvinyl caprolactam - polyvinyl acetate - polyethylene glycol
graft copolymer,
docusate sodium, hydroxypropylcellulose, hydroxypropylmethylcellulose,
polyoxyethylene
sorbitan fatty acid esters, block copolymers based on ethylene oxide and
propylene oxide,
polyvinylpyrrolidone, deoxycholic acid sodium salt, sodium lauryl sulphate,
benzalkonium
chloride, lecithin, distearyl palmitate glyceryl, albumin, lysozyme, gelatin,
macrogol 15
hydroxystearate, tyloxapol and polyethoxylated castor oil, cellulose
derivates,
23
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
dioctylsulfosuccinate, casein, dextran, gum acacia, cholesterol, tragacanth,
stearic acid, calcium
stearate, glycerol mono stearate, cetostearyl alcohol, cetomacrogol
emulsifying wax, sorbitan
esters, polyoxyethylene alkyl ethers, polyoxyethylene castor oil derivatives,
polyethylene
glycols, polyoxyethylene stearates, colloidal silicon dioxide, phosphates,
carboxymethylcellulose
calcium, carboxymethylcellulose sodium, methylcellulose,
hydroxyethylcellulose, hypromellose
phthalate, noncrystalline cellulose, magnesium aluminium silicate,
triethanolamine, polyvinyl
alcohol, 4-(1,1,3,3-tetramethylbuty1)-phenol polymer with ethylene oxide and
formaldehyde,
poloxamers, poloxamines, polyethylene oxide-containing fatty acid esters like
Stearoyl
macrogo1-32 glycerides, Lauroyl macrogo1-32 glycerides or a mixture thereof.
Pharmaceutical compositions according to the invention may also comprise one
or more binding
agents, filling agents, lubricating agents, suspending agents, sweeteners,
flavoring agents,
preservatives, buffers, wetting agents, disintegrants, effervescent agents,
and other excipients.
Such excipients are known in the art. Non limiting examples of filling agents
include lactose
monohydrate, lactose anhydrous, and various starches; examples of binding
agents include
various celluloses and cross-linked polyvinylpyrrolidone, microcrystalline
cellulose, such as
Avicel PH101 and Avicel , microcrystalline cellulose, and silicified
microcrystalline cellulose;
lubricantsinclude colloidal silicon dioxide, such as Aerosil 200, talc,
stearic acid, magnesium
stearate, calcium stearate, and silica gel; sweeteners include any natural or
artificial sweetener,
such as sucrose, xylitol, sodium saccharin, cyclamate, aspartame, and
acesulfame. Examples of
flavoring agents are Magnasweet , bubble gum flavor, and fruit flavors, and
the like;
preservatives include potassium sorbate, methylparaben, propylparaben, benzoic
acid and its
salts, other esters of parahydroxybenzoic acid such as butylparaben, alcohols
such as ethyl or
benzyl alcohol, phenolic compounds such as phenol, or quaternary compounds
such as
benzalkonium chloride; diluents include pharmaceutically acceptable inert
fillers, such as
microcrystalline cellulose, lactose, dibasic calcium phosphate, saccharides,
and/or mixtures of
any of the foregoing. Examples of diluents include microcrystalline cellulose,
such as Avicel
PH101 and Avicel PH102; lactose such as lactose monohydrate, lactose
anhydrous, and
Pharmatose DCL21; dibasic calcium phosphate such as Emcompress ; mannitol;
starch;
sorbitol; sucrose; and glucose; disintegrants include lightly cross linked
polyvinyl pyrrolidone,
24
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
corn starch, potato starch, maize starch, and modified starches,
croscarmellose sodium, cross-
povidone, sodium starch glycolate, and mixtures thereof.
Examples of effervescent agents include effervescent couples such as an
organic acid and a
carbonate or bicarbonate. Suitable organic acids include, for example, citric,
tartaric, malic,
fumaric, adipic, succinic, and alginic acids and anhydrides and acid salts.
Suitable carbonates
and bicarbonates include, for example, sodium carbonate, sodium bicarbonate,
potassium
carbonate, potassium bicarbonate, magnesium carbonate, sodium glycine
carbonate, L-lysine
carbonate, and arginine carbonate. Alternatively, only the sodium bicarbonate
component of the
effervescent couple may be present.
The compositions of the invention comprise nanoparticulate ivacaftor particles
having an
effective average particle size of less than about 2000 nm (i.e., 2 microns),
less than about 1950
nm, less than about 1900 nm, less than about 1850 nm, less than about 1800 nm,
less than about
1750 nm, less than about 1700 nm, less than about 1650 nm, less than about
1600 nm, less than
about 1550 nm, less than about 1500 nm, less than about 1450 nm, less than
about 1400 nm, less
than about 1350 nm, less than about 1300 nm, less than about 1250 nm, less
than about 1200 nm,
less than about 1150 nm, less than about 1100 nm, less than about 1050 nm,
less than about 1000
nm, less than about 950 nm, less than about 900 nm, less than about 850 nm,
less than about 800
nm, less than about 750 nm, less than about 700 nm, less than about 650 nm,
less than about 600
nm, less than about 550 nm, less than about 500 nm, less than about 450 nm,
less than about 400
nm, less than about 350 nm, less than about 300 nm, less than about 250 nm,
less than about 200
nm, less than about 150 nm less than about 100, less than about 75 nm, or less
than about 50 nm,
as measured by light-scattering methods, microscopy, or other appropriate
methods. Apart from
the methods referred to herein, other methods suitable for measuring effective
average particle
size are known to a person of ordinary skill in the art.
By "an effective average particle size of less than about 2000 nm" it is meant
that at least 50% of
the particles have a particle size less than the effective average, by weight
(or by other suitable
measurement techniques, such as by volume, number, etc.), i.e., less than
about 2000 nm, 1900
nm, 1800 nm, etc., when measured by the above-noted techniques. In other
embodiments of the
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
invention, at least about 60%, at least about 70%, at least about 80% at least
about 90%, at least
about 95%, or at least about 99% of the ivacaftor particles have a particle
size of less than the
effective average, i.e., less than about 2000 nm, 1900 nm, 1800 nm, 1700 nm,
etc.
The amounts of nanoparticulate ivacaftor and one or more surface stabilizers
may vary. The
optimal amount of the individual components can depend, for example, upon the
particular form
of ivacaftor (such as the specific salt) selected, the hydrophilic lipophilic
balance (HLB), melting
point, and the surface tension of water solutions of the stabilizer, etc.
The concentration of the ivacaftor in the nanoparticulate composition may be
from about 99.5%
to about 0.001%, from about 95% to about 0.1%, or from about 90% to about
0.5%, by weight,
based on the total combined dry weight of the ivacaftor and at least one
surface stabilizer, not
including other excipients.
The concentration of the ivacaftor may be present in any amount sufficient to
achieve
therapeutically effective levels upon administration and may vary depending on
the manner in
which the composition is formulated. For example, when considering ivacaftor
particles
dispersed in a liquid medium, the ivacaftor may typically be present in an
amount from about
0.5% to about 90% by weight (salt or free base equivalent) based on the total
combined weight
of the drug substance, stabilizers, any added excipients and the weight of the
dispersion medium.
Or, in the case of a solid dosage form the ivacaftor may typically be present
in an amount from
about 0.1% to about 90% by weight, preferably 0.5% to 60% by weight, and more
preferably
1.0% to 40% by weight (salt or free base equivalent) based on the total
combined weight of the
drug substance, stabilizers, and excipients. (It will be appreciated that the
calculation of a weight
based concentration will depend on whether the concentration is determined as
that of a
particular ivacaftor salt or the free base equivalent.)
Any concentration of surface stabilizer(s) which is sufficient to form stable
nanoparticles of
ivacaftor may be used. For example, the concentration of the at least one
surface stabilizer may
be present from about 0.001% to about 99.99%, from about 5.0% to about 95.0%,
or from about
10% to about 90.0%, by weight, based on the total combined dry weight of the
ivacaftor and at
26
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
least one surface stabilizer, not including other excipients. When considering
dispersed ivacaftor
particles, the at least one stabilizer may typically be present in an amount
from about 0.01% to
about 30% by weight based on the total combined weight of the drug substance,
stabilizers, any
added excipients and the weight of the dispersion medium. Or, in the case of a
solid dosage form
the at least one stabilizer may typically be present in an amount from about
0.001% to about 90%
by weight, preferably 0.5% to 50% by weight, and more preferably 1.0% to 30%
by weight
based on the total combined weight of the drug substance, stabilizers, and
excipients. When more
than one surface stabilizer is utilized the stabilizer present in the greatest
concentration is the
primary stabilizer and the other stabilizers are secondary stabilizers. For
example the
composition may typically comprise a primary surface stabilizer in an amount
from about
0.001% to about 50% w/w (by weight of the total composition (including any
dispersion
medium)) and one or more secondary surface stabilizers each present in an
amount, less than that
of the primary stabilizer, ranging from about 0.01% to about 5% w/w (by weight
of the total
composition (including any dispersion medium). The combination of a primary
stabilizer with
one or more secondary stabilizers can be advantageous over the use of a single
stabilizer. For
example a plurality of stabilizers can be used to combine the steric and
electrostatic stabilization
effects of different types of surface stabilizer molecules.
The present invention further relates to a method of making a nanoparticulate
ivacaftor, or a
pharmaceutically acceptable salt thereof, composition comprising contacting
particles of a
ivacaftor with at least one surface stabilizer for a time and under conditions
sufficient to provide
a composition comprising particles of ivacaftor having an effective average
particle size of less
than about 2000 nm.
The compositions comprising nanoparticulate ivacaftor can be made using, for
example, milling
or attrition (including but not limited to wet milling, dry milling and micro
fluidization),
homogenization, precipitation, crystal engineering, cryogenic spraying,
cyclodextrin
complexation, solid lipid nanoparticles (SLN), solid/liquid self-emulsifying
drug delivery
systems (SEDDS), freeze drying, template emulsion techniques, supercritical
fluid techniques,
nanoelectrospray techniques, combination thereof or and any other techniques
known to those of
27
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
skill in the art. Exemplary methods of making nanoparticulate compositions are
described in
various references mentioned above, all of which are specifically incorporated
by reference.
Milling ivacaftor to obtain nanoparticulate ivacaftor dispersion comprises
dispersing the particles
in a liquid dispersion medium in which the ivacaftor is poorly soluble,
followed by applying
mechanical means in the presence of grinding media to reduce the particle size
of the ivacaftor to
the desired effective average particle size. The dispersion medium can be, for
example, water,
safflower oil, ethanol, t-butanol, glycerin, polyethylene glycol (PEG),
hexane, or glycol or a
mixture thereof, a preferably dispersion medium is water.
The ivacaftor particles can be reduced in size in the presence of at least one
surface stabilizer.
Alternatively, nanoparticulate ivacaftor can be contacted with one or more
surface stabilizers
after attrition. Other compounds, such as a diluent, can be added to the
ivacaftor/surface
stabilizer composition during the size reduction process. Dispersions can be
manufactured
continuously or in a batch mode.
The grinding media can comprise particles that are preferably substantially
spherical in shape,
e.g., beads, consisting essentially of polymeric or copolymeric resin or metal
like stainless steel
etc. Alternatively, the grinding media can comprise a core having a coating of
a polymeric or
copolymeric resin adhered thereon. The grinding media preferably ranges in
size from about 0.01
to about 3 mm. For fine grinding, the grinding media is preferably from about
0.02 to about 2
mm, and more preferably from about 0.03 to about 1 mm in size.
In a preferred grinding process the ivacaftor particles are made continuously.
Such a method
comprises continuously introducing an ivacaftor composition according to the
invention into a
milling chamber, contacting the ivacaftor composition according to the
invention with grinding
media while in the chamber to reduce the ivacaftor particle size of the
composition according to
the invention, and continuously removing the nanoparticulate ivacaftor
composition from the
milling chamber. The grinding media is separated from the milled
nanoparticulate ivacaftor
composition according to the invention using known separation techniques, in a
secondary
28
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
process such as by simple filtration, sieving through a mesh filter or screen,
and the like. Other
separation techniques such as centrifugation may also be employed.
Another method of forming the desired nanoparticulate ivacaftor is by
microprecipitation. This is
a method of preparing stable dispersions of poorly soluble active agents in
the presence of one or
more surface stabilizers and one or more colloid stability enhancing surface
active agents free of
any trace toxic solvents or solubilized heavy metal impurities. Such a method
comprises, for
example: (1) dissolving ivacaftor in a suitable solvent; (2) adding the
formulation from step (1)
to a solution comprising at least one surface stabilizer; and (3)
precipitating the formulation from
step (2) using an appropriate non-solvent step (3) removal of solvent by
various known methods.
The method can be followed by removal of any formed salt, if present, by
dialysis or diafiltrafion
and concentration of the dispersion by known means.
In homogenization method comprises dispersing ivacaftor particles in a liquid
dispersion
medium, followed by subjecting the dispersion to homogenization to reduce the
particle size of
ivacaftor to the desired effective average particle size. The ivacaftor
particles can be reduced in
size in the presence of at least one surface stabilizer. Alternatively, the
ivacaftor particles can be
contacted with one or more surface stabilizers either before or after
attrition. Other compounds,
such as a diluent, can be added to the ivacaftor/surface stabilizer
composition either before,
during, or after the ivacaftor particle size reduction process. Dispersions
can be manufactured
continuously or in a batch mode.
Another method of forming the desired nanoparticulate ivacaftor is by template
emulsion.
Template emulsion creates nanostructured ivacaftor particles with controlled
particle size
distribution and rapid dissolution performance. The method comprises an oil-in-
water emulsion
where ivacaftor is dissolved in oil and dispersed into fine oil globules in
water. The particle size
distribution of ivacaftor is a direct result of the size of the emulsion
droplets which can be
controlled and optimized in this process. Furthermore, through selected use of
solvents and
stabilizers, emulsion stability is achieved with no or suppressed Ostwald
ripening. Subsequently,
the solvent and water are removed, and the stabilized nanostructured ivacaftor
particles are
29
CA 03021752 2018-10-22
WO 2016/199085 PCT/1B2016/053433
recovered. Various ivacaftor particle morphologies can be achieved by
appropriate control of
processing conditions.
Another method of forming the desired nanoparticulate ivacaftor is by spray
freezing into liquid
(SFL). This technology comprises an organic or organoaqueous solution of
ivacaftor with
stabilizers, which is injected into a cryogenic liquid, such as liquid
nitrogen. The droplets of
ivacaftor solution freeze at a rate sufficient to minimize crystallization and
particle growth, thus
formulating nanostructured ivacaftor particles. Depending on the choice of
solvent system and
processing conditions, the nanoparticulate ivacaftor particles can have
varying particle
morphology. In the isolation step, the nitrogen and solvent are removed under
conditions that
avoid agglomeration or ripening of the ivacaftor particles. As a complementary
technology to
SFL, ultra rapid freezing (URF) may also be used to created equivalent
nanostructured ivacaftor
particles with greatly enhanced surface area. URF comprises an organic or
organoaqueous
solution of ivacaftor with stabilizers onto a cryogenic substrate.
In electrospray ionization a liquid is pushed through a very small charged,
usually metal,
capillary. This liquid contains the desired substance, e.g., ivacaftor,
dissolved in a large amount
of solvent, which is usually much more volatile than the analyte. Volatile
acids, bases or buffers
are often added to this solution as well. The analyte exists as an ion in
solution either in a
protonated form or as an anion. As like charges repel, the liquid pushes
itself out of the capillary
and forms a mist or an aerosol of small droplets about 10 um across. This jet
of aerosol droplets
is at least partially produced by a process involving the formation of a
Taylor cone and a jet from
the tip of this cone. A neutral carrier gas, such as nitrogen gas, is
sometimes used to help
nebulize the liquid and to help evaporate the neutral solvent in the small
droplets. As the small
droplets evaporate, suspended in the air, the charged analyte molecules are
forced closer
together. The drops become unstable as the similarly charged molecules come
closer together
and the droplets once again break up. This is referred to as Coulombic fission
because it is the
repulsive Coulombic forces between charged analyte molecules that drive it.
This process repeats
itself until the analyte is free of solvent and is a lone ion. In
nanotechnology the electrospray
method may be employed to deposit single particles on surfaces, e.g.,
ivacaftor particles. This is
accomplished by spraying colloids and ensuring that on average there is not
more than one
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
particle per droplet. Consequent drying of the surrounding solvent results in
an aerosol stream of
single ivacaftor particles. Here the ionizing property of the process is not
crucial for the
application but may be put to use in electrostatic precipitation of the
particles.
Nanoparticulate ivacaftor compositions can also be made in methods utilizing
supercritical
fluids. In such a method ivacaftor is dissolved in a solution or vehicle which
can also contain at
least one surface stabilizer. The solution and a supercritical fluid are then
co-introduced into a
particle formation vessel. If a surface stabilizer was not previously added to
the vehicle, it can be
added to the particle formation vessel. The temperature and pressure are
controlled, such that
dispersion and extraction of the vehicle occur substantially simultaneously by
the action of the
supercritical fluid. Chemicals described as being useful as supercritical
fluids include carbon
dioxide, nitrous oxide, sulphur hexafluoride, xenon, ethylene,
chlorotrifluoromethane, ethane,
and trifl uoromethane.
The resultant nanoparticulate ivacaftor compositions or dispersions can be
utilized in any
pharmaceutically acceptable dosage form, including but not limited to
injectable dosage forms,
liquid dispersions, soft gelatin capsules, gels, aerosols, ointments, creams,
controlled release
formulations, fast melt formulations, lyophilized formulations, tablets,
capsules, delayed release
formulations, extended release formulations, pulsatile release formulations,
mixed immediate
release and controlled release formulations. etc.
The invention also provides a method of treating a mammal in need comprising
administering a
stable nanoparticulate ivacaftor composition comprising: (a) particles of
ivacaftor or a
pharmaceutically acceptable salt thereof having an effective average particle
size of less than
about 2000 nm; and (b) at least one surface stabilizer.
The invention provides a method of increasing bioavailability (e.g.,
increasing the plasma levels)
of ivacaftor in a subject. Such a method comprises administering to a subject,
via any
pharmaceutically acceptable means, an effective amount of a composition
comprising
nanoparticulate ivacaftor. A preferred administration method is oral
administration.
31
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
The compositions of the invention may be useful in the treatment of cystic
fibrosis.
The compositions of the invention comprising nanoparticulate ivacaftor can be
administered to a
subject via any pharmaceutically acceptable means including, but not limited
to, orally, rectally,
ocularly, parenterally (e.g., intravenous, intramuscular, or subcutaneous),
intracistemally,
pulmonary, intravaginally, intraperitoneally, locally (e.g., powders,
ointments or drops), or as a
buccal or nasal spray. In some embodiments, oral administration is preferred.
As used herein, the
term "subject" is used to mean an animal, preferably a mammal, including a
human or non-
human. The terms patient and subject may be used interchangeably.
Compositions suitable for parenteral injection may comprise physiologically
acceptable sterile
aqueous or nonaqueous solutions, dispersions, suspensions or emulsions, and
sterile powders for
reconstitution into sterile injectable solutions or dispersions. Examples of
suitable aqueous and
nonaqueous carriers, diluents, solvents, or vehicles including water, ethanol,
polyols
(propyleneglycol, polyethylene-glycol, glycerol, and the like), suitable
mixtures thereof,
vegetable oils (such as olive oil) and injectable organic esters such as ethyl
oleate. Proper fluidity
can be maintained, for example, by the use of a coating such as lecithin, by
the maintenance of
the required particle size in the case of dispersions, and by the use of
surfactants.
The compositions may also comprise adjuvants such as preserving, wetting,
emulsifying, and
dispensing agents. Prevention of the growth of microorganisms can be ensured
by various
antibacterial and antifungal agents, such as parabens, chlorobutanol, phenol,
sorbic acid, and the
like. It may also be desirable to include isotonic agents, such as sugars,
sodium chloride, and the
like. Prolonged absorption of the injectable pharmaceutical form can be
brought about by the use
of agents delaying absorption, such as aluminum monostearate and gelatin.
Solid dosage forms for oral administration include, but are not limited to,
capsules (Both Hard
Gelatin and Soft Gelatin), tablets, pills, powders, and granules. In such
solid dosage forms, the
active agent is admixed with at least one of the following: inert
excipients,fillers, binders,
humectants, disintegrating agents, solution retarders, absorption
accelerators, wetting agents,
32
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
adsorbents, lubricants, or mixtures thereof. For capsules, tablets, and pills,
the dosage forms may
also comprise buffering agents.
Liquid dosage forms for oral administration include pharmaceutically
acceptable emulsions,
solutions, suspensions, syrups, and elixirs. In addition to the drug, the
liquid dosage forms may
comprise inert diluents commonly used in the art, such as water or other
solvents, solubilizing
agents, and emulsifiers. Exemplary emulsifiers are ethyl alcohol, isopropyl
alcohol, ethyl
carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propyleneglycol,
1,3-butyleneglycol,
dimethylformamide, oils, such as cottonseed oil, groundnut oil, corn germ oil,
olive oil, castor
oil, and sesame oil, glycerol, tetrahydrofurfuryl alcohol,
polyethyleneglycols, fatty acid esters of
sorbitan, or mixtures of these substances, and the like.
Besides such inert diluents, the composition can also include adjuvants, such
as wetting agents,
emulsifying and suspending agents, sweetening, flavoring, and perfuming
agents.
"Therapeutically effective amount" as used herein with respect to, for example
an ivacaftor
dosage shall mean that dosage that provides the specific pharmacological
response for which
ivacaftor administered in a significant number of subjects in need of such
treatment. It is
emphasized that "therapeutically effective amount," administered to a
particular subject in a
particular instance will not always be effective in treating the diseases
described herein, even
though such dosage is deemed a "therapeutically effective amount" by those
skilled in the art. It
is to be further understood that ivacaftor dosages are, in particular
instances, measured as oral
dosages, or with reference to drug levels as measured in blood.
One of ordinary skill will appreciate that effective amounts of ivacaftor can
be determined
empirically and can be employed in pure form or, where such forms exist, in
pharmaceutically
acceptable salt, ester, or prodrug form. Actual dosage levels of ivacaftor in
the nanoparticulate
compositions of the invention may be varied to obtain an amount of a ivacaftor
that is effective
to obtain a desired therapeutic response for a particular composition and
method of
administration. The selected dosage level therefore depends upon the desired
therapeutic effect,
33
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
the route of administration, the potency of ivacaftor, the desired duration of
treatment, and other
factors.
Dosage unit compositions may contain such amounts of such submultiples thereof
as may be
used to make up the daily dose. It will be understood, however, that the
specific dose level for
any particular patient will depend upon a variety of factors: the type and
degree of the cellular or
physiological response to be achieved; activity of the specific agent or
composition employed;
the specific agents or composition employed; the age, body weight, general
health, sex, and diet
of the patient; the time of administration, route of administration, and rate
of excretion of the
agent; the duration of the treatment; drugs used in combination or
coincidental with the specific
agent; and like factors well known in the medical arts.
EXAMPLES
The following examples are provided to illustrate the present invention. It
should be understood,
however, that the invention is not to be limited to the specific conditions or
details described in
these examples.
Example 1:
Nanoparti cul ate Iv acaftor Preparation
Formulations
Ingredients 1 2 3 4 5 6 7
(%w/w) (%w/w) (%w/w) (%w/w) (%w/w) (%w/w) (%w/w)
Iv acaftor 1 1 1 1 1 1 1
Soluplus 0.1 0.1
Mi cro crystall in e 0.2
cellulose
Polaxomer 0.01 0.1 0.1 0.1 0.1
Povi done 0.1 0.1
Lactose 0.2
Water q. s q. s q. s q. s q. s q. s q. s
Formulations
Ingredients 8 9 10 11 12 13
(%w/w) (%w/w) (%w/w) (%w/w) (%w/w) (%w/w)
Ivacaftor 1 1 1 1 1 1
Hydroxypropyl 0.15
34
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
methylcellulose
P1 asdone S-630/C-30 0.15
Docusate sodium 0.05 0.02 0.05
Gelucire 0.1
Tween 80 0.1
Pluronic 0.15
HPC-SL 0.2 0.2
Water q.s q.s q.s q.s q.s q.s
A suspension of ivacaftor and a surface stabilizer and optionally excipients
was prepared in
distilled water and mixed well. The suspension was mixed by a High pressure
Homogenizer at
different speed, pressure and time. The nanoparticulate of ivacaftor can also
be prepared by using
ball milling apparatus. Ivacaftor formulations were processed under varied
milling conditions.
All milling was performed on a ball mill apparatus using a smooth or pegged
agitator. The
chamber was loaded to 40-90% working volume attrition media and the remaining
working
volume of the chamber was filled with the mixture to be milled (the "slurry").
Each formulation
was further processed using either a lyophilizing process or sprayed drying
procedure to remove
the milling media.
The resulting nanoparticulate ivacaftor dispersion (NCD) was examined first by
microscopy and
laser diffraction PSA.
In example 1, a suspension of 1.0% (w/w) of ivacaftor, 0.1% (w/w) of Polaxomer
and deionised
water to 100% (w/w), mixed well using a high speed homogenizer for 15 minutes
and followed
by nanonised with a high pressure homogenizer at 1500-2000 bar. The dispersion
medium was
then removed by lyophilization or spray drying. The same formulation iwa also
prepared using
ball milling apparatus. 1.0% (w/w) of ivacaftor, 0.1% (w/w) of Polaxomer and
deionised water to
100% (w/w), was milled for 30 minutes at vibrational frequency at 3-30 Hz, in
a 50 mL chamber
using a smooth agitator.
The nanoparticulate dispersion of ivacaftor was then combined with various
biorelevant media to
determine the likely dissolution profile of the composition in vivo. Other
nanoparticulate
ivacaftor compositions from the examples were also prepared by using the same
method as
above.
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
Example 2:
Formulations containing nanoparticulate Ivacaftor
Formulations
Ingredients 14 15 16 17 18 19 20
(%w/w) (%w/w) (%w/w) (%w/w) (%w/w) (%w/w) (%w/w)
Nanoparticulate
30-80% 30-80% 30-80% 30-80% 30-80% 30-80% 30-80%
Ivacaftor
Hypromellose 0-20 0-20 0-20 0-20 0-20
Polaxomer 0-25 0-25 0-25 0-25
Povidone 0-30 0-30 0-30 0-30 0-30
Microcrystalline
0-60 0-60 0-60
cellulose
Lactose 0-50 0-50 0-50
Sucrose 0-30 0-30 0-30
Sugar Sphere 0-50
Sodium Lauryl
0-10 0-10 0-10 0-10 0-10
Sulfate
Docusate Sodium 0-15 0-15 0-15
Crospovidone 0-30 0-30 0-30 0-30 0-30 0-30 0-30
Magnesium
0-15 0-15 0-15 0-15 0-15 0-15 0-15
stearate
The excipients were mixed with nanoparticulate of ivacaftor. and was used for
compressing into
tablets or capsules filling directly or granulated using various methods like
dry granulation, wet
granulation, melt granulation either compressed into tablets or filled into
capsules or sachets.
Further, the nanoparticulate solution was sprayed onto sugar spheres in FBP or
used to granulate
a mixture of excipients, dried, mixed with other excipients for tablet
compression or capsule
filling. The nanoparticulate solution was also sprayed onto tablet cores with
a combination of
sugar coating and film coating. The solution was also used for spray
drying/free drying and the
dried nanoparticles formulated further into a solid dosage form. The
nanoparticulate ivacaftor
can also be used in melt extrusion and extrusion spheronization process to
prepare various
dosage forms like tablets, capsules or granules for sachets.
Example 3:
Ivacaftor nanoparticles using PVP K29/32 as surface stabilizer by wet media
milling process:
Formulation I: Nanosuspension
36
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
Ivacaftor 5g
PVP k29/32 2.5g
Water 600m1
To the surface stabilizer solution comprising 2.5g of PVP in 600m1 purified
water, 2.5g Ivacaftor
was added and homogenized forl5mins at 1200rpm for 15mins. The suspension was
charged
into a 0.6L stainless steel chamber of a dyno mill (KDL-A) with Zirconium
beads (0.3mm) at a
temperature of 20-25 C and milled to the desired particle size.
Process parameters used in the milling process are listed below:
Volume of bead: 360m1; Milling speed: 2000-4200rpm
Pump rate: 40-300m1/min; Milling time: 10hrs
The particle size distribution of the nanosuspension was measured by Beckman-
Coulter LS13-
320 as below.
Size (nanometer)
Time d90 d75 (150 d25 d10 Mean
10hrs 1354 670 232 148 110 498
Day 2 1359 759 243 169 139 532
The nanosuspension formulation was further processed into granules using wet
granulation
process.
Formulation II: Granules of nanosuspension of ivacaftor
Formulation II /ovv/w
Nanosuspension
Ivacaftor 10.85
PVP k29/32 5.43
Water
Excipients for granulation
MCC 102 78.14
PVP k2/32 4.99
SLS 0.59
The nanosuspension prepared in formulation I was added to a mixture of MCC
102, PVP and
SLS, mixed well and dried. The dried granules prepared were passed through a
sieve.
37
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
Formulation III: Lyophilized powder
The nanosuspension was lyophilized in a LabconcoFreezone Triad freeze dryer.
Formulation IIIA: Lyophilized Ivacaftor nanosuspension
Formulation IIIB: Lyophilized Ivacaftor nanosuspension with 5% sucrose
The processing parameters for lyophilization of the nanosuspension were as
below.
Temperature Hold Ramp rate
(cr) time ( C/min)
Prefreeze -75 24
Primary drying -55 12 1
-25 12 0.2
-5 8 0.2
Secondary 15 5 0.2
drying 35 10 0.2
After lyophilization, the particle size of the nanosuspension was again
measured by Beckman-
Coulter LS13-320 after re-suspending the dry powder with purified water. The
measured particle
size for the lyophilized batches IIIA and IIIB were as follows:
Size (nanometer)
Nanosuspension d90 c175 dm) d25 d10 Mean
IIIA 1236 230 147 100 78 380
IIIB 1072 220 146 101 79 346
Example 4:
Ivacaftor nanoparticles using HPC-LF and SLS as surface stabilizers by wet
media milling
process:
Formulation IV
Ivacaftor 5g
HPC-LF 0.9g
SLS 0.045g
Water 600m1
To the surface stabilizer solution comprising 0.9g of HPC-LF and 0.045g of SLS
in 600m1
purified water, 5g of Ivacaftor was added and the suspension was homogenized
for 15mins at
38
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
1200rpm. The suspension was then charged into a 0.6L stainless steel chamber
of a dyno mill
(KDL-A) with Zirconium beads (0.3mm) at a temperature of 20-25 C and milled.
The process
parameters for wet media milling in the dyno mill were as below.
Process Parameters:
Volume of bead: 480m1
Milling speed: 2500rpm
Pump rate: 300m1/min
Milling time: 6hrs
The particle size distribution following milling was as below.
Size (nanometer)
Time d90 d75 d50 d25 d10 Mean
6hrs 2416 1858 1128 515 170 1256
Example 5:
Ivacaftor nanoparticles using HPC-LF as surface stabilizer using wet media
milling process:
Formulation V
Ivacaftor lOg
HPC-LF 2.5g
Water 600m1
To the surface stabilizer solution comprising 2.5g of HPC-LF in 600m1 purified
water, 5g of
Ivacaftor was added and the suspension was homogenized for 15mins at 1200rpm.
The
suspension was then charged into a 0.6L stainless steel chamber of a dyno mill
(KDL-A) with
Zirconium beads (0.3mm) at a temperature of 20-25 C and milled. The process
parameters for
wet media milling in the dyno mill were as below.
Process Parameters:
Volume of bead: 480m1
Milling speed: 3200rpm
Pump rate: 300m1/min
Milling time: 2hrs
The particle size distribution following milling was as below.
39
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
Size (nanometer)
Time d90 d75 c150 d25 d10 Mean
2hrs 3037 1866 1132 380 166 1415
Example 6 (Comparative Example):
Solid dispersion of Ivacaftor with HPMCAs:
Formulation VI
Ivacaftor 500g
HPMCAs 500g
Acetone 70m1
Solid dispersion of Ivacaftor with HPMCAs polymer was prepared by rotary
evaporation at bath
temperature 43 C and 200rpm for 30mins using acetone as the solvent. The solid
dispersion was
dried overnight and milled.
Example 7 (Comparative Example):
Drug Excipient mixture:
Formulation VII A (Drug Excipient Slurry)
Ivacaftor 50mg
PVP k29/32 25mg
Water 50m1
Formulation VII B (Drug Excipient Physical mixture)
Ivacaftor 50mg
PVP k29/32 48mg
MCC 102 360mg
SLS 2.7mg
Formulation VII A was prepared by simple dispersion of ivacaftor in PVP
solution. Formulation
VII B was prepared by mixing ivacaftor with PVP, MCC and SLS, passed through
sieve and
mixed well.
Dissolution studies were performed to observe the % release from 50mg drug
equivalent
formulations of Ivacaftor nanoparticulate formulations I and II in comparison
with that of the
CA 03021752 2018-10-22
WO 2016/199085 PCT/IB2016/053433
corresponding microcrystalline ivacaftor (30 microns), microcrystalline
ivacaftor slurry
(Formulation VII A), physical mixture (Formulation VII B) and amorphous
dispersion
(Formulation VI). The dissolution media used was a modified fasted simulated
intestinal fluid
(FaSSIF) wherein Lecithin and Taurocholate were replaced with 0.25% SLS in a
6.5pH
phosphate buffer solution. A USP II method at 65 rpm was used for all the
dissolution studies.
The results are illustrated in Fig 1. The nanoparticulate formulations show
significant
enhancement of drug release compared to the amorphous and microcrystalline
formulations.
The DSC of ivacaftor granulated nanosuspension (formulation II) shows melting
point
corresponding to that of ivacaftor form B indicating the drug is in the
crystalline form in the
granulated nanosuspension formulation II.
These examples are not intended to limit the claims in any respect, but rather
to provide
exemplary tablet formulations of ivacaftor which can be utilized in the
methods of the invention.
Such exemplary tablets may also include a coating agent.
Fed/Fast Pharmacokinetics of oral nanoparticulate ivacaftor tablet and
KALYDECO Tablets is
determined by conducting a bio-study.
It will be apparent to those skilled in the art that various modifications and
variations can be
made in the methods and compositions of the present inventions without
departing from the spirit
or scope of the invention. Thus, it is intended that the present invention
cover the modification
and variations of the invention provided they come within the scope of the
appended claims and
their equivalents.
41