Note: Descriptions are shown in the official language in which they were submitted.
Z~
1 61051-2000
AN IMPROVED METHOD AND APPARATUS FOR RECYCLING ENERGY
IN COUNTERFLOW HEAT EXC~ANGE A~D DISTILLATION
DESCRIPTIC)N
BACKGROVND OF THE INVENTION
This invention is a method and apparatus for heat
exchange. Its main applications are in distillation and in
counterflow heat exchange. Various models of the invention
distill water, distill fuel alcohol, concentrate juices and
brines, separate toxic chemicals from industrial wastewater,
remove moisture from grains or clothing, exchange haat between
liquids~ and generate electrical power. The fundamental design
strategy of the invention is to move heat over a large surface
area relative to the flow to the flow rate through the system.
This background section identifies some of the problems addressed
by the invention and describes the conventional solutions.
Water Supply
Global Water Supply
Our planet's ecosystem includes about 326 billion cubic
miles ~1 trillion, 336 billion cubic kilometers~ of water. Only a
2b tiny ~raction of this water is available for drinking or
irrigation. Ninety-seven lies in the oceans, too salty for human
life support. Another two and a half percent is frozen in the
polar ice caps. For drinking and irrigation we rely almost
entirely on fresh water - less than half of 1% of the global water
supply. A billion people, or one-fourth of us, lack clean
drinking water. Contaminated drinking water is involved in 80% of
all human illness and disease, according to the World Health
Organization. Gastroen-teritis, dysentery, cholera, and other
L08
2 61051-2000
waterborne diseases claim ten mlllion lives each year. The United
Nations has declared the 1980's the International Drinking Water
and Sanitation Decade and set an ambitious goal: to supply clean
drinking water Eor all people by 1990. To accomplish this goal
the United Nations will have to bring new sources of clean
drinking water to half a million people each day (on the average)
until the end of the decade. Dr. Peter Bourne, U. N. coordinator,
has predicted that abundant clean water will "totally
revolutionize the life style of rural people in every country of
the world."
W~ter Supply in the United States
The United States draws on groundwater resources and
surface water resources in roughly equal measure. Most Americans
grew up with plenty of safe, clean water available at the turn of
a tap and learned to take it for granted. But our water supply
systems show increasing signs of strain. Rivers, lakes, poundsr
streams, and other sources of surface water depend on rainfall,
and regions involving twenty-eight states were parched by drought
in the spring of 1981. New York City declared a drought emergency
on January 19, 1981, enacting water restrictions that lasted
several months. The city of West Palm Beach rationed drinking
water in May of 1981.
Many regions of our country have enjoyed a steady supply
of water from vast underground reservoirs called aquifers. The
Ogallala a~uiEer, spanning 800 miles (1280 kilometers), supplies
drinking water to communities from West Texas to South Dakota and
provides irrigation water Eor a major agricultural region.
AquiEers accumulate over eons - only a tiny fraction o our
X
3 61051-2000
rainEall seeps into them. In some parts of the American southwest
people are drinking groundwater that fell as rain ten thousand
years a~o.
We're pumping water out of our aquifers a billion
gallons a day faster than rainwater is seeping into them. We're
mining a precious resource, and in many places the lode is
running dry. In California's San Joaquin Valley an area the size
of Connecticut has sunk as much as 30 eet (9.1 meters) in the
last twenty-five years because of excessive groundwater removal.
Overuse of groundwater threatens the existence of midwestern
agriculture. "It varies, depending on where you are, but there
are some people projecting that as early as the year 2000 there
will be parts of Nebraska with their water supplies so depleted
that farming may never return" according to Michael Jess, Nebraska
wa-ter planner (Newsweek, 2/23/81). In May 1981 in Winter Park,
Florida the earth caved in, swallowing part of the city and
creating a circular chasm 400 feet (121 meters) wide and 100 ~eet
(30 meters) deep~ Underground limestone caverns collapsed to
create this huge sinkhole. When too much water was removed the
limestone became brittle and crumbled~
~ater Supply in American Coas~al Cities
Our major coastal cities, like inland areas, rely on
fresh water. Purification of ocean wa-ter is rarely at-tempted New
York City draws water from reservoirs in the Catskill Mountains
125 miles (200 kilometers) -to the northwest, then delivers this
water to citizens with a highly centralized distribution system~
Within the city all the water for eight million people rushes
through two immense underground waterways known as City Tunnel #l
~2~21(~8
4 61051-2000
and City ~unnel ~2. Built in 1917 and 1936, these tunnels have
operated continuously ever since. They lie as far as 800 feet
(2~3 meters) below the yround and provide no access Eor
maintenance or inspection. Maurice Feldman, a former New York
City water commissioner, has predicted that the tunnels will
collapse within ten to forty years. In 1970 the city began
digging a third tunnel 60 miles (96 km) long and 24 feet (7
meters) in diameter, blasted out of solid rock and lined with
concrete. New York City's financial crisis interrupted
construction in 1975. At that time the Army Corps of Engineers
estimated that the city water pipes were leaking 100,000,000
gallons (4,4~0,000,000 liters) a day, the rate of water
consumption in San Francisco. Construction resumed in 1977.
Current estimates oE the total cost of building City Tunnel ~3
range from five to ten billion dollars.
Los Angeles, too, imports fresh water by mammoth feats
of engineering rather than purifying ocean water. Residents of
Los Angeles rely on the Owens Valley 340 miles (544 km) to the
north and Colorado River 240 miles (384 km) to the east. Removal
of water from the Owens Valley caused Mono Lake to drop 44 feet
(13 4 m) since Los Angeles began drawing water from its tributary
streams in the 1920's. Until 1981 tens of thousands of migratory
birds nes-ted at Mono Lake and fed on brine shrimp from the lake 1 5
salty waters. But as the lake drained its salinity increased, and
it became too salty Eor brine shrimp to survive. Without an
ade~uate food source, virtually all the CaliEornia ~ull chicks
hakched a-t Mono Lake in 1981 died. A recent congressional bill
that would make Mono Lake a national park has jeopardized the Elow
X
o~
61051-2000
of water from the Owens Valley to souther CaliEornia~
Three fourths oE the water used in Los Angeles and San
Diego comes from the Colorado ~i~er. Nearly a third of the power
generated by Hoover Dam is used to pump Colorado River water
through enormous canals, tunnels, and aqueducts to the deserts and
coastlines of southern California. The Colorado is the only major
source oE surface water in the southwest - residents of Colorado,
Utah, and Arizona also rely on it. In 1985 the Central Arizona
Project will begin transporting Colorado River water eastward
across three hundred miles of desert to Phoenix and Tucson.
CaliEornia's allotment will diminish by about 20%, or 325 billion
gallons (1.4 trillion liters) per year. Southern Californians may
face stringent water conservation measures unless they can find
another source of water.
Water Pollution
Problems of water scarcity are intensified by pollution
of our fresh water supplies. Trihalomethane gases, known to cause
cancer in laboratory animals, contaminate virtually all our
drinking water as a result of the chlorination process city water
systems use to prevent the spread of waterborne diseases.
Trihalomethanes form when chlorine interacts with algae, micro-
organisms or other organic materials in the wa-ter. Other
contaminants originate in the delivery system - lead and asbestos
from water pipes leach into our tapwater.
Pollutants are also contaminating groundwater~ Sal-t
thrown on icy roadways has worked its way into a~uiEers in New
England - residents oE several Massachusetts communities receive
notes wlth their water bills warning them that the sodium content
~ ~2~)8
6 61051-2000
of their tapwa~er is dangerously high and advising them to drink
bottled water. More ~han 600 wells ln the New York City area have
been closed during the past three years due to chemical
contamination. Wells providing half the drinking water for
residents of Atlantic City, New Jersey are in imminent danger of
contamination by a huge plume of toxic chemicals dumped over a
decade ago. In California's San Joaquin Valley, where 80% o~ the
people rely on wells ~or drinking water, many wells are being shut
down because they contain a highly toxic pesticide known as DBCP
(dibromochloropropane). Agricultural pesticides and industrial
solvents such as trichloroathylene~ dioxane/ and benzene had
entered groundwater in at least 250 sites as of September 1980,
according to a report by the House Government Operations
subcommittee. There are more than 50,000 hazardous waste dumps in
the United States - no one knows how many of them are leaking
toxic chemicals into our water supplies. Once groundwater is
contaminated it's likely to stay contaminated for hundreds, even
thousands of years.
Water Distillation
For thousands of years people have respected
distillation as a perfect separation technique for purifying
water. Distillation involves boiling the water, moving its vapors
to a different location, and condensing the vapors to obtain pure
water. Aristotle (384 B.C~) mentions the evaporation of salt
water to obtain fresh water. Alexander oE Aphrodisias (circa 100
B.C.) tells of sailors boiling seawater and hanging sponges in the
steam to collect pure water for drinking. The individual water
vapor molecules rising Erom the boiling seawater have no way to
,~,
~t~62~L08
7 61051-2000
carry off the salt - the steam is pure water vapor. The main
problem with distillation is the extremely high amount o~ energy
it takes to boil water.
~om~ Water Stills
Some people purify their tapwater with a home
distillation apparatus known as a still. Conventional tapwater
stills consist of a boiling chamber, a condensing chamber, and an
electric heater. The heater boils the impure water. Steam
travels to the condensing chamber and condenses, becoming
distilled water. These stills remove any solid pollutants that
contaminate our drinking watar: asbestos or lead from
decomposing water pipes, salt thrown on icy roadways, arsenic or
cadmium from industrial wastewater. But most tapwater stills
won't remove toxic gases or liquids - these bubble off with the
steam and contaminate the produck water.
The cost of operating tapwater stills limits their
usefulness. They don't recycle energy. The electric heater has
to supply all the energy for heating and boiling the water - 2~8
kilowatt hours per gallon. At ll cents per kilowatt hour/ energy
for distillation costs thirty-one cents per gallon (seven cents
per liter). Most home stills can purify ten gallons (44 liters)
of water a day, enough for drinking and cooking ~or a small
Eamily~ The energy to distiIl that much water costs $92.00 per
month. It would be advantageous to puri~y 200 gallons (880
liters) of water a day for bathing, showering, washing clothes,
and washing dishes. But conventional stills with such a large
output are huge and expensive, and the energy to run them costs
over $1800 a month.
~ ~6~
8 61051-~000
Conventional Seawater Stills
The abandoned desalting plant in Fountain Valley,
CaliEornia e~emplifies the American experience with seawater
purlfication. Built in 1975 at a cost oE $14 millionr the plant
was designed to distill fifteen million gallons (66 million
liters) of pure water per day, operating around the clock. But
neighbors complained that the noise from its boilers was
intolerable and succeeded in shutting the plant down at night.
The plant produced only three million gallons (13 million liters)
per day but consumed all the energy its designers expected it
would need for the full fifteen million. After nine months of
operation the government decided the plant was too expensive to
operate and shut it down. Today pigeons roost there, using its
insulation to build nests along the catwalks. Similar stills in
Chula Vista, California and Freeport, Texas were sold for scrap.
Vapor ~o~pression ni~tillation
Some large-scale seawater stills reduce the cost of
distillation by recycling energy. One energy-recycling process is
known as vapor compression distillation (Holdenl U.S. Patent
3,423,293). The goal of this process is -to boil the seawater with
heat given by the steam when it condenses. (When the steam
condenses it returns all the heat that went into boiling it off.)
To recycle this heat~ vapor compression stills compress the steam
so it will condanse in metal tubes which contact the boiling
seawater. The heat released by the condensing steam flows
through the tube walls into the boiling seawater to generate more
steam. The condensed steam, now distilled liquid water, passes
through a counter~low heat e~changer to heat up the incoming
~:,
1262~L08
9 61051-2000
seawater.
By recycling energy this way vapor compression stills
reduce the energy requirements for distilling a thousand gallons
(4.4 thousand liters) of seawater from 2800 kilowatt hours to 75
kilowatt hours, lowering the cost from $196 to $5.25 (assuming
industrial rates of 7 cents per kwh). From an engineering
standpoint that's a remarkable accomplishment. But from a
practical standpoint it's still too expensive. If Los Angeles
used conventional vapor compression stills to purify enough
Pacific Ocean water to replace the Colorado River water it will
lose to Arizona in 1985, the cost of energy alone would come to $5
billion in just three years.
People resort to seawater distillation only in extreme
circumstances. Kuwait, an Arab nation rich in oil revenues but
virtually devoid of fresh water, relies almost entirely on
desalted water. All the desalting plants in the world produce a
total of a billion gallons (4~4 billion liters) of pure water a
day, approximately the rate of water use by people in the Los
Angeles basin.
Reasons for the Inefficiency of ~apor Compression Stills
Vapour compression stills are inefficient because the
tubes which transfer heat from the condensing steam into the
boiling seawater don't have enough surface area. Many factors
llmit the available surface area. Tubes are expensive to build
and expensive to buy. They usually must be welded along one seam
and at both ends. It's impractical to pack the tubes too densely
hecause all the tubes must be accessible for periodic cleaning,
and tubes welded in the center of complicated tube bundles are
X
61051-2000
di~ficuLt to clean. With a limited are for condensing, the steam
has tended to condense slowly.
The only way to condense the steam faster with limited
surface area has been to compress it substantially - and high
compression creates a multitude of problems. Most important, high
compression requires substantial energy. High compression demands
bigger, more e~pensive, less e~ficient compressors and thicker,
more heat resistant tubes. Compressing the steam substantially
makes the tubes much hotter than the seawater, creating a violent
boiling action that wastes energy in turbulent motion of the
water. A vapor barrier forms on the hot tubes and interferes with
their ability to transfer heat into the water. All these factors
reduce efficiency in conventional vapor compression stills.
Similar problems plaque the counterflow heat exchange
process which these systems use to heat up the cold seawater. To
prevent heat exchangers from being massive and expensive the
universal design strategy has been to move heat through a small
area by forcing the liquids through their channels under high
velocities and high pressures and by agitating the liquids to
create a turbulent flow pattern. This approach presents many
problems. High velocities and high pressures require a lot of
energy. High pressures demand that the metal walls transferring
heat be relatively thick -to withstand the stress~ The thicker the
walls, the more they resist the flow of heat. The turbulence of
the water dissipates energy. These factors reduce e~iciency and
increase the cost oE the process.
The high operating cost oE conventional seawater stills
reflects their inefficiency. Scientists have long known oE the
~62~0Eil
11 61051-2000
theoretical possibility oE a more efEicient process. Weinberg
wrote in the Bulletin oE the Atomic Scientists (26~ 6, 69, 1970):
"Theoretically, about 3 kilowatt hours of work are required to
separate the 300 pounds of salt which are contained in 1000
gallons of seawater. If energy is available at, say, five mills
per kilowatt hour, then the thermodynamic sninimum cost of
desalinating seawater would be 1.5 cents per thousand gallons. OE
course this minimUTn can never be attained." In other words, the
work which costs $5.25 with conventional vapor compression stills
can theoretically be done for twenty-one cents at today's
industrial rates of seven cents per kilowatt hour. The other
$S.04 derives mainly from the ineEficiency of the stills.
The inefficiency of conventional seawater stills is the
main reason why ocean water is essentially unavailable for human
life support. In the drought year of 1981 not one si~able
American metropolitan area purifîed seawater for drinking. Not
one acre of ~merican farmland is irrigated with purified seawater.
Distilling seawater requires too much eneryy.
Energy ~upplies
Global Energy Supplies
Every day the sun showers the earth with 400 trillion
kilowatt hours of solar heat, 25,000 times more energy than people
consume as fuel and electricity. Instead of deriving fuel and
electricity from this daily income of solar energy, we rely on our
planetary savings account of fossil fuels - petroleum, coal, and
natural gas. These fuels formed as plants collected solar energy,
stored it as chemical ene~gy, then lay inside the earth under high
pressures for millions oE years. Much oE the petroleum has
12 61051~2000
already been burned. The remalnder can last only a Eew more
decades at currellt rates of consumption.
Baslng our world energy system on fossil fuels creates
many immediate problems. The price of oil has skyrocketed from
$1.50 a baxrel in the 1960's to $32.00 today. The United States
pays a billion dollars a week for imported oil. Countries such as
Chad, Ethiopia, Nepal, Burma, Burundi, Upper Volta, and India,
having a per capita gross national product of less than $200,
can't afEord oil and are blocked in their efforts to achieve a
higher standard of living by a shortage of energy. Mining the
fossil fuels is hazardous - deep mining of coal impairs human
health, strip mining of coal disfigures the environment, oil
spills destroy marine life. Burning Eossil fuels causes
contamination oE the earth's atmosphere.
Power Generatio~
In the United States we produce 90% of our electricity
in power stations which burn fossil fuels. These power stations
consist of three main elements: a boiler, a condenser, and a
turbine. The burning fuel boils water to generate a head of
steam. As the steam expands ~rom the boiler -to the condenser it
spins turbine blades. Electrical generators convert the rotary
motion of the turbines in-to electricity.
These power stations are fraught with many di~Eiculties
apart from their dependence on expensive fuel supplies. They are
enormously inefEicient - they discharge more energy into the
environment as waste heat than they convert into electricity. A
conventional coal-Eired plant burns 500,000 pounds (226,500
kilograms) of coal per hour to produce 1,000 megawatts of
~<1
~l~fi~1~8
13 61051-2000
electricity (enough to supply a million people) and 1,300
megawatts of waste heat. Furthermore, a coal-fired plant this
size discharges 28,000 pounds (12,684 kilograms) o~ pollutants per
hour into the atmosphere. Acid rain caused by pollutan-ts Erom
midwestern power plants has killed all the fish in more than two
hundred lakes in the Adirondack Mountains of New York, once a
favorite resort area.
~uclear Power Stations
Nuclear power plants now supply 4-5~ of our nation's
electricity. Like power plants fired by coal or petroleum,
nuclear power stations boil water to generate steam for spinning
the blades steam of a turbine. Several factors limit the
viability oE nuclear power~ One by-product of nuclear power
plants is plutonium, a man-made element 20,000 times more
poisonous than cobra venom. Plutonium must be stored for hundreds
of thousands of years before humans can handle it safely. Someone
with 20 pounds (9 kilograms) of plutonium could make an atomic
bomb with in~ormation from unclassified publications. Another
factor weighing against nuclear power plants is the phenomenal
cost of building them. In February 1982 two nuclear plants under
construction near Satsop, Washington were scrapped. They were
going to cost at least $23 billion - four times the cost their
buyers expected when construction began. In 1980 sixty-nine
nuclear construction projects were delayed and sixteen other
cancelled in the United States~ most oE them victimized by high
costs and steep interest rates.
Fuel Alcohol
Alcohol is an ideal universal Euel, appropriate for
14a 61051-2000
powering automobiles, heating buildings, or any other process now
burning petroleum, coal or natural gas. ~lcohol fuel i5 solar
energy, collected and transformed into chemical energy by plants.
Plants use the energy of sunlight to build large sugar molecules
from carbon dioxide and water. When the plants ferment under
proper conditions the sugar breaks down into carbon dloxide and
alcohol. When the alcohol burns, energy is released.
Alcohol has received widespread appreciation as a high-
grade motor fuel. When Otto invented internal combustion engines
in Germany in -the nineteenth century his prototypes burned
alcohol. Henry Ford advocated the use of "power alcohol'l to
stimulate agriculture and capitalize and renewable resource - he
equipped his model T's and some later cars with adjustable
carburettors to accommodate either alcohol or gasoline. Before
World War II forty countries were blending alcohol into gasoline.
Detroit built cars for the Phillipines and New Zealand that ran on
straight alcohol.
Gasoline became the conventional motor fuel because it
was readily available at low cost. But as petroleum becomes
scarce and expensive more countries are turning to alcohol
distilled from farm commodities as a fuel Eor motor vehicles.
Brazil has begun a major program to eliminate oil imports entirely
by running all i-ts cars and industries on alcohol distilled from
home-grown sugar cane and manioc. (Manioc is a starchy plant
whose roots are a valuable food product. It is the source of
tapioca.) ~overnments of New Zealand, Australia, South Africa,
Thailand~ ~enya, the Sudan, the Dominican Republic, Guyana and
~i2108
l~b 61051-2000
Jamaica are consLdering large-scale alcohol fuel production.
Science magaæine (l979, Volume 195) and many other
sources indicate that alcohol delivers more power and better
mileage than gasoline while burning cooler and quieter. Most cars
can run on alcohol with only minor adjustments to their
carburetors. A professional race car driverl Bobby Unser,
testified beEore Congress recently that he has burned alcohol in
his race cars for years and that he finds it "a lot safer, a lot
nicer to work with than gasoline." Alcohol is saEer because it
doesn't burn explosively at normai atmospheric pressure. I~
spilled in the ocean alcohol has no toxic effectsO Alcohol burns
clean - converting all American automobiles to alcohol would
reduce air pollution in this country by 90%, according to StanEord
Research Institute (now S~I International). The waste products
oE engines burning alcohol are carbon dioxide and water vapor.
These are also waste products o~ human beings. Engines burning
alcohol in a closed room won't asphyxiate people.
Some writers have expressed concern that emissions from
engines burning alcohol will cause a dangerous buildup oE carbon
dioxide in the atmosphere~ Actually, alcohol-burning engines
release the same amount of carbon dioxide the plants absorbed form
the atmosphere ~o build the sugar molecules that produced the
alcohol. In this sense, automobiles burning alcohol maintain a
harmonious balance wi-th nature.
Alcohol is as renewable as sunlight, and people can
produce it almost anywhere. In a September 1980 report entitled
'IAlcohol Erom Biomass in the Developing Countries" the World Bank
2~()8
l~c 61051-2000
offered the opinion that alcohol ls the main renewable energy
source developing countries can produce Erom their own resources.
The World Bank expressed interest in helping to design national
alcohol programs.
Alcohol Distillation
Fuel alcohol must be distilled because when plants
Eerment they yield only 5% to 15% alcohol - the rest is water.
Fuel mixtures require at least 75% alcohol. Since alcohol
evaporates more readily than water, the two can be separated by
distillation. Alcohol producers have borrowed intact the stills
of the beverage industry, consisting of metal columns 30 to 60
feet (9 to 18 meters) high filled with packing material. Alcohol
and water vapors condense and re-evaporate many -times on the
packing material as they rise through the column. The vapours
become richer in alcohol as they reach the top.
The widespread use oE alcohol fuel is restricted by the
energy requirements of distillation. Column stills require
approximately 45,000 British Thermal Units (12.6 kwh) of energy to
distill one gallon (4.4 liters) of 100~ alcohol fuel. A gallon of
alcohol has a fuel value of 84,000 BTU (24.6 kwh). Burned in a
15% efficient internal combustion engine, it can accomplish only
14,000 BTU (4.1 kwh) of useful work. It takes more energy to
distill alcohol than you can get back when you burn it~ That's
why alcohol is known as an "anergy loser."
Column stills were designed for distilling alcoholic
beverages in the early nineteenth century when energy was cheap
and whiskey was expensive. They require too much energy to
~!
~62~1~8
14d 61051-2000
produce fuel economically today. The main source of their
ineEEiciency i5 this: aEter the distilled alcohol vapors leave
the top of the columns, the heat they yield in condensation
doesn't get recycled. This heat is lost to the process. All the
heat for boiling comes instead from steam produced in boilers
fired by petroleum, coal, or natural gas.
Fuel Alcohol U.S.A., a monthly maga~ine devoted to the
alcohol fuel industry, reported in February 1982 that 160 proof
alcohol (80~ alcohol, 20% water) is an excellent automotive fuel,
superior in performance and economy to straight alcohol.
Distilling 160 proof alcohol fuel requires only half as much
energy as distilling straight alcohol. Even so, it7S not clear
that alcohol fuel production can be economical.
Distillation has earned a reputation as a problematic
technology, capable of making many contributions to human society
but too energy-intensive to realize its potential value.
126~
~ lS 61051-2000
SUMMAR~ 0~ THE INVENTION
The invention provides a method of producing a
concentra~e and a distilla~e from a glven feed material whlch is
at least ln part llquid, ~aid method comprising the steps of,
(a) providing means for defining a vertically extending
hoiling chamber and a vertically ex~ending condensing chamber on
opposite sides of a vertically extending common plate mamber which
includes on one side thereof, a specific boillng surface within
and forming part of said boiling chamber and, on the opposite side
thereof, condensing surPace within and forming part of said
condenslng chamber and aligned with said boiling sur~ace, said
piate member being sufficiently thermally conductive and
suf~iciently thin in the area of said boiling and condensing
surfaces to conduat heat across the two surfaces relatlvely
efficiently;
(b) direating a con~inuously replenlshed supply of said
feed material into said boiling chamber so as to maintain said
boiling chamber filled with said feed material to a level which
entirely covers said bolling surface when said ~eed material is
cause~ to boil and cauæing the feed material therein to boil,
whereby it does so evenly over substantially the entire boiling
s~rface o~ æaid plate member sufflclen~ to produce vapor from some
of the liquid of said feed material and to form a concentxate of
the rest of the material;
(c) continuouæly directlng said concentrate, as i~ is
formed, out of said boiling chamber and ultimately into a
collection chamber and, at the same time, continuously dlrecting
said vapor, a~ it is formed, out o~ said boiling chamber and into
~, .
i2~LI)8
~ l5a 61051~2000
said condensing chamber through a compression chamber where, by
means of compresslon, the vapor ls elevated in pressure by an
amount not ~o exceed about two psi h~yher than the minimum
required for condensation at a~mospheric pre~sure;
~ d) a~ vapor con~inuously enter~ sald condensing
chambex from said compressiny chamber, direc~ing at least a
portion theraof vertlcally downward uniformly and evenly across
tha antire condensing surface of said plate member so as to cause
it to condense and form a diætillate thereon and at the same time,
transfer its heat of condensatlon across the plate member to said
bol1ing suxface for aiding in boiling the ~eed materlal at said
bolling surface;
(e) as said distilla~e forms on said condensing surface
continuou~ly directing it out of said condensin~ chambar; and
(f) wherein said means deining said boiling chamber
lncludes a second, vertically extending plate member deflning a
surface spaced a small distance from and in confronting
relationship with said common pla~e member such that the two plate
member~ define a vertlcally extendingr laterally narrow boiling
chamber, and wherein said step of continllously replenishing said
boiling chamber wlth feed material includes the step of
maintaining the li~uid within said boiling chamber at a partlcular
level from its bottom end to allow the li~uid as ik boils ~o boil
rom the bottom of the bolling chamber to it~ top end and
therefore over the entire boiling sur~ace.
The invention also provides energy-recycling
distillation apparatus comprising a boiler-condensor unit and a
compressor for flowlng vapor, aharact~rized in that said boiler-
"~ :
,. . .
~26;~10~3
,~ l5~ 61051-2000
condensor uni~ includes at least one heat transfer sheet, sald
heat transfer ~heet de~ining at least one pair of alternatlng
chambers for hotter and cooler fluids flowing through them, and
~hat in the boiler-condensor unlt the or each chamber for the
cooler fluid is ~illed with the feed from the bottom to a level
which entirely covers the bolling surfa~es of the heat ~ransfer
sheets when ~aid feed ig caused to boil.
The invention distills li~uids wlth llttle energy
because it recycles energy efficiently. In essence this invention
is a heat transfer technology. It recycles energy to reduce the
costs of many domestic and industrial processes. The key to lts
e~flc~ency is trans~erring heat over an extensive surface area.
Thls section br:Lefly describes ~he maln embodimen~ o~ the
inventlon, discusses various appli~ations, and explalns how the
invention solves the problems discussed in the background.
Brlef Description of tha Main ~mbodlment
The DistilIation Process
Distillation is a proces~ of evaporation and
condensation. The ~eed liquid enter6 a boiling chamber, where
part of it boils o~f. Vapors travel to a condensing chamber and
condense~ becoming the product, or the distilled liquid. The park
of the feed liquid that doesn't boil off becomes con~entrated.
This concentrated liquid, known as the blowdown, carrles
lmpurities out o~ the ~oiler. The inYention reaycles anergy at
two point~ ~irst to heat the cold feed liquid to its boiling
point, then to boil it.
In water dlstillatlon about 1~00 BTU per gallon ~1.5 kwh
per liter) are required to heat the ~eed water from 60F (16 C) to
,~
~6~
l5c 61051-2000
21~F (lOO~C), lt~ boillng point. The produc~ and the blowdown
give of~ the same amount o~ energy, 1200 BTU per gallon (1.5 kwh
per liter), when they cool from 212 F to 60 F (from 100 C to 16
C). The invention txansfers heat from the product and the
blowdown lnto the feed water.
After the ~eed water reache~ its boiling point, about
8000 BTU (2.3 kwh) of heat energy are required to convert a gallon
of it to steam. The steam gives back 8000 BTU (2.3 kwh) when it
condenses. The invention txansfers heat from the condensing steam
into the boiling wa~er.
. ~
2~
16 61051-2000
The Hardware
A counterflow heat exchanger transfers eneryy from the
hot product and the hot blowdown into the cold feed liquid. The
counterflow heat exchanger is built by stacking thin sheets of
stainless steel with gaskets to Eorm channels for the liquids.
Every sheet transEers heat from hot liquid 1Owing in one
direction on one side into cold liquid flowing in the opposite
direction on the other side.
A boiler-condenser unit called a core transfers energy
rom the condensing vapors into the boiling liquid. The core is
built by stacking thin sheets of stainless steel with gaskets to
form an alternati~g sequence of boiling chambers and condensing
chambers. Heat from condensing vapors flows through the sheets to
boil the Liquid on the other side.
A compressor raises the pressure of the vapors so they
will condense and give up their energy. The work of the
compressor yields a multiplier - the input energy for compressing
the vapors allows you to recycle more than 100 times that much
energy from the condensing vapors back into the boiling liguid.
Th~ Desi~n Stra~egy
The design stra-tegy of this invention is to transfer
heat over an extensive surface area - at least two square feet
~or every gallon per hour of fluid passing through the system (0.8
square meters per liter per hour). This strategy makes low-energy
distillation possible for the first time. In water distillation
the invention recycles more than ~9~ of the energy for heating and
boiling the ~ater. ~ess -than 1% of the total energy for
distLllation must be added continuously from an external source.
~,,?~\
08
17 61051-2000
The next two paragraphs explain how extensive surface area leads
to efficiency in the counterflow heat exchanger and the core.
Extensive S~rface Area and ~entle Liquid Flow in the Counterflow
~eat Exchanger
When the liquids communicate over an extensive area,
they exchange heat readily even ~hen they flow gently ~ with low
velocities, low pressures, and a laminar or regular flow pattern.
Passing the liquids through their channels gently is a novel
procedure offering many advantages. First, the liquids require
little input energy to move through their channels. Since the
liquids place little stress on the heat transEer sheets, the
sheets may be very thin. Thinner sheets conduct heat better,
contain less material, weigh less, and cost less, and fewer of
them are needed for a given rate of heat transEer. The gentle
movemant of the liquids dissipates little energy in turbulence,
and also allows the heat transfer sheets to be stacked very close
together. Close spacing of the sheets places the hot liquids and
the cold liquid in intimate contact for optimal heat transfer.
This gentle approach results in a compact, inexpensive, efficient
counterflow heat exchanger. The energy transferred from the hot
liquids to the cold liquid may be 800 times greater than the input
energy required to move the liquids through the heat exchanger.
Extensive Surface Area and Low Compression in the Core
When the condensing vapors are placed in heat exchange
relationship with the boiling liquid over a large area, the vapors
condçnse readily with a very small compression step. Compressing
the vapors a minimal amount is a novel approach to distillation
offering many advantages. Little energy is required to compress
~6~1013
18 61051-2000
the vapors. Since the compressed vapors exert little Eorce on
the heat transEer sheets, the sheets may be very thin. Low
compression also makes it possible to s~ack the sheets very close
together for efficient heat transfer. In addition, standard
inexpensive compressors of simple construction may be used. The
cumulative effect of this strategy is a compact, inexpensive,
efficient boiler/condenser unit. The energy transferred from the
condensing vapors to the boiling liquid may be 100 times greater
than the input energy required to compress the vapors.
~anufacturing Techniques
This invention is relatively easy to manufactureO The
basic construction technique is simply stacking sheets of metal
with gaskets and then bolting them together. The modular design
oE the invention makes it appropriate for any scale - many sheets
form a large unit, a -few sheets form a small one. The heat
transfer sheets are so thin they may be constructed from materials
which are not particularly heat conductive such as glass or
plastic, both of which are plentiful and inexpensive.
Mai~tenance
The owner can perform all routine maintenance of the
inven-tion with common tools. To gain access to the heat transfer
sheets for periodic cleaning one simply removes the bolts which
hold them together.
Appllcations of -the Invention
In some applications of this technology the object of
the boiling and condensin~ process is to collect the substance
that boils off. This is true, for example, in purifying water and
distilling alcohol. In some other applications the object is to
~2~0~3
19 ~1051-2000
collect the part that doesn't boLI off. This is true in drying
grains, drying clothing, and concentrating or dehydrating
solutions. In a third ~ype of application, the invention uses
steam to generate electrical power, a novel capability for a
distillation apparatus. In all these situations the invention
recycles heat e~ficiently. The remainder of this section will
discuss some of its applications.
Wat~r Purification
A device slightly larger than a microwave oven will
purify 15 gallons (66 liters) of tapwater per hour while drawing
less than l kilowatt o~ power. It removes all pollutants -
solids, li~uids, or gases. Its energy costs for distilling ten
gallons (4~ liters) per day come to less than $3 a month (at 11
cents per kilowatt hour). Wi-th this invention the energy to
purify two hundred gallons (880 liters) o~ water a day would cost
only $44 a month. The invention also makes it economically
~easible to purify and recycle household wastewater during
droughts or in areas o~ Eresh water scarcity.
Larger units will enable municipal water districts to
remove to~ic chemicals from drinking water supplies. This
invention will purify a thousand gallons (4.4 thousand liters) of
fresh water while drawing only two kilowatt hours oE energy, at a
cost oE 14 cents (assuming industrial rates oE 7 cents per ~wh).
The invention will also prevent the formation oE trihalomethane
gases by removing the organic materials in the water before
chlorine is added. AEter the water has been distilled, only trace
amounts of chlorine will be needed to keep it pure.
Many industries will value this invention because it can
, .~,
61051-2000
extract toxic ch~micals inexpensively from thei~ wastewater, so
both the chemicals and the water can be recycled. In industri-
ali~ed countries water recycling will significantly reduce
environmental pollution. In developlng countries the ability to
recycle water inexpensively will facilitate industrial develop-
ment, since industries will not be dependent on vast water
resources.
The invention also gives inexpensive access to pure
water from the oceans. In large-scale settings this invention
will purify a thousand gallons (4.~ thousand liters) of ocean
water for 6 kilowatt hours, the amount of energy it takes to pump
that much water from the Colorado River to Los Angeles. The
invention has the potential to make ocean water available for
drinking and irrigation on a large scale for the first time in
human history. In the ~nited States the pipelines which carry
fresh water to our coastal cities could carry water in the
opposite directionr from the oceans to the deserts and the Great
Plains. Abundant pure water from the oceans will enable people to
reclaim deserts along 50,000 miles (80,000 kilometers) of
coastline in the Americas, Africa, Australia, and the Middle East.
~lcohol Distillati~n
By rec~cling heat efficiently this invention reduces the
energy requirements of alcohol dis-tillation by more than 90~. To
distill a gallon (4.~ liters) of 160 proof alcohol the invention
requires only 2000 BTU (0.6 kwh), about 5 cents' worth of energy~
This new energy-recycling distillation technology will make it
possible Eor almost any state or nation to become energy
independent. The invention is an ideal village-scale technology
.~ .
2l 61051-2000
for the underdeveloped countries: ecologically sound, consistent
with human dignity, easy to understand and repair, capable of
operating with any source of rotary motion as an energy input, and
above al] capable o producing premium liquid fuel from local
materials at low cost. In coastal areas where feed stocks for
alcohol are scarce, the ability of the invention to purify
seawater for irri~atlon will make it easier to grow crops for
energy production. The invention is much smaller than a
conventional still - a unit the size of a small refrigerator will
enable many farmers -to produce enough alcohol from their waste
crops to run their machinery.
Dehydration and ~oncentr~tion
Many domestic and industrial processes are designed to
remove water. The invention can reduce the cost of these
processes up to 99% by recycling energy. For homeowners the
invention will dry clothing, fruits, or vegetables. For the food
processing industry it will concentrate fruit juices, dehydrate
food products, and dehydrate watery waste materials. Oil
companies, who draw up huge ~uantities of brine when drilling for
oil and pay to dispose of it, will value the invention's ability
to separate the salts from the water at low cost. The geothermal
energy industry will also welcome the ability -to dehydrate brines
inexpensively for safe and easy disposal of salts and other
minerals. Alcohol uel producers can use the invention to
concentrate their fruit juices before fermentation to obtain a
higher yield o alcohol, and also to dry their grains and liquid
residues to sell them as animal feed.
.~
22 61051-2000
Po~r Goneratio~
Like power plants Eired by fossil Euels or nuclear
energy, the invention generates electricity by boiling water to
create a head of steam4 The invention is novel in that it
recycles heat to sustain the boiling action. The power generation
process requires a flow of fresh water into the boiling chambers
and a ~low oE concentrated brine into the condensing chambers.
Energy becomes available because of the difference in steam
pressure between the fresh water and the brine. The fresh water,
having a higher steam pressure, evaporates easily. The brine,
having a lower steam pressure, evaporates with more difficulty.
Steam naturally ~lows from the high pressure area (the boiling
fresh water) to the low pressure area (the hot brine). The
Elowing steam spins the blades of a -turbine placed in its path,
and an electrical generator linked to the turbine transforms the
rotary motion into electricity~ When the steam reaches the hot
brine it condenses and gives up its heat. This heat flows
through stainless steel sheets back into the ~resh water on the
other side -to sustain the boiling.
Any fresh water mixed into saturated brine by a power
still can theoretically generate as much power as it would from a
dam nearly three miles (4.8 kilometers) high. Hoover Dam, by
comparison, is 726 feet (221 meters) high. In practical
applications every gallon of fresh water a power still mixes into
saturated brine can generate 50 watt hours of electrical power (11
watt hours per llter). Wherever ~resh water and brine exist
naturally this process may prove very valuable. The river water
and household wastewater running into the Great Salt Lake
X
~L262108
23 61051-2000
represent a substantial untapped energy source. The I.sraelis
already have plans to pump ocean water from the Mediterranean Sea
to the Dead Sea just to keep the Dead Sea from drying up. Since
the Dead Sea water is twenty times saltier than the Mediterranean
Sea Water, power stills could generate electrical power by mixing
the two. In this process no fuel is burned, virtually no waste
heat is released/ and there is no environmental pollution.
The table on the following page compares the energy
requirements of this invention with those of conventional
equipment. Comparative costs are also listed. The cost figures
assume a rate of 11 cents per kilowatt nour for every process
except large-scale seawater distillation. The costs of large-
scale seawater distillation are figured at industrial rates of 7
cents per kilowatt hour. The remainder of this document describes
in detail the new heat exchange technology which makes these cost
breakthroughs possible.
.~
~2~;~flO~3
2~ 61051-2000
TABLE OF COMPARATIVE ENERGY REQUIREMENTS AND COSTS
Conventional This
Stills Invention
Tapwater 2800 watt hours 50 watt hours
Distillation ($.31) per gal. ($.006) per galO
636 wat-t hours 11 watt hours
($.08) per liter ($.001) per liter
Small-Scale 2800 watt hours 60 watt hours
Seawater Distil~ ($.31) per gal. ($.007) per gal.
lation
636 watt hours 13 watt hours
($nO8) per liter ($~001) per liter
Large-Scale 75 kwh ($5.25) 6 kwh ($0.42)
Seawater Distil- per 1000 gal. per 1000 gal.
lation
19 kwh ($1~33) 1~4 kwh ($0.10)
per 1000 liters per 1000 liters
Alcohol Fuel 25~000-451000 BTU 2,000 BTU
Dî~tillation ($~81-$1~45) per gal. ($.06) per gal.
1.7 - 3.0 kwh 0.13 kwh
($~18-$o33) ($~01) per liter
per liter
.~
. .
~26;~
61051-2000
8RIEF D~SCRIPTION OF THE DRAWINGS
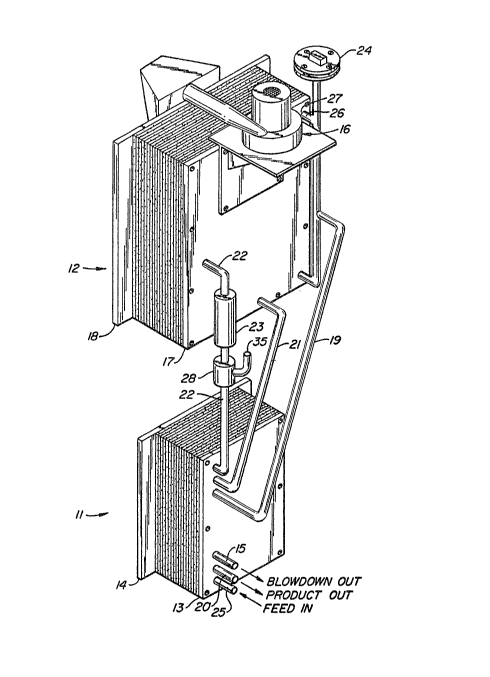
FIGURE 1 is an isometric view oE a stacked-sheet core
and heat exchanger.
FIGURE 2 is an exploded drawing of the core of Figure 1.
FIGURE 3 is an exploded drawing of a variation of the
core of Figure 1.
FIGURE 4 is an exploded drawing of the heat exchanger o~
Figure 1.
FIGURE 5 is an isometric view, partially exploded and
partially in section, oE a spiral core and heat exchanger.
FIGURE 6 is an isometric view, partially in section, of
a batch dehydrator-concentrator.
FIGURE 7 is a cross-section oE the boiling chamber of
Figure 6 along lines 7-7.
FIGURE 8 is a cross-section of the condensing chamber of
Figure 6 along lines 8-8.
FIGURE 9 is an isometric view, partially exploded, of a
batch dehydrator.
FIGURE 10 is a cross-sectional end view oE a batch
dehydrator of Figure 9.
FIGURE 11 is a cross-sectional side view of a batch
dehydrator of Figure 9.
FIGURE 12 is an exploded drawing of a power generator.
::
-` ~2~i2~08
26 61051 2000
DETAILED DESCRIPTION OF ~E PREFERRED EMBODIMENTS
This section describes the various embodiments of the
invention and their applications: water purification, counterflow
heat exchange, alcohol distillation, dehydration and concentration
of liquids, drying of sollds, and generation of electrical po~er.
In each application the invention recycles energy efficiently. In
essence, every embodiment of the invention is a heat exchange
apparatus, and every process described is a method of heat
exchange.
Water Purification
The method is distillation: boiling water to convert it
to pure steam. When the steam condenses it becomes pure liquid
water. The invention recycles energy in two ways. First a
counterflow heat exchanger heats the feed liquid nearly to its
boiling point with energy given by the product and the blowdown.
Then a boiler-condenser unit called a core boils the feed liquid
with energy given by the condensing steam.
The Stacked-Sheet Still
Figure 1 shows a stacked-sheet heat exchanger (11) and
core tl2) for purifying or concentrating liquids. Both the heat
exchanger (11) and the core (12) are built by stacking sheets of
metal, or some othar material, with gaskets.
The counterflow heat exchanger.
The counter flow heat exchanger (11) is built from 100
sheets of stainless steel type 316~ known for its ability to
resist corrosion from seawater. Fach sheet measures 9" x 12l' by
0.01'i (23 cm x 30 cm x 0.25 mm). Silicon rubber gaskets maintain
a separation of 0.032" (0.81 mm) between the sheets. Fiberglass
,
~,
~62~
27 61051-2000
ends plates stabilize the heat exchanger. The Eront end plate
(13) measures 9" x 12" x 0.25" (64 cm x 30 cm x 6.35 mm), and the
rear end plate (14) measures 11" x 12" x 0.25" (27.9 cm x 30 cm x
6.35 mm).
In the heat exchanger (11) the areas between the sheets
form chambers for liquid flow. Chambers for hot liquid (the
product or the blowdown) alternate with chambers for cold liquid
(the feed water)~ Every sheet transfers heat from hot liquid
flowing in one direction on one side into cold liquid flowing in
the other direction on the other side.
The ~ore.
The core (12) is built from 51 sheets of stainless steel
type 316. Each sheet is 16" tall, 12" wide, and 0.01" thick (41
cm x 30 cm x 0.25 mm). One side oE each sheet serves as a boiling
surface, and the other side serves as a condensing surface.
Silicone rubber gaskets keep the sheets 0.06" (1.52 mm) apart.
Two fiberglass end plates stabilize the core - the front end plate
(17) measures 16~ x 12~ x O ~ 251~ ( 41 cm x 30 cm x 6n 35 mm), and the
rear end plate (18) measures 16" x 14" x 0.25" (41 cm x 36 cm x
6.35 mm).
In the core (12) the areas between the sheets serve as
chambers for boiling and condensing. A11 the boiling chambers
interconnect and form the boiler; all the condensing chambers
interconnect and form the condenser. Boiling chambers and
condensing chambers alternate. Every sheet transEers heat Erom
steam condensing on one side into a liquid boiling on the other.
' ' ''`I
~l~62~1L0a
28 61051-2000
The peripheral eq~ipment.
A compressor (16) bolted onto the core (12) blows vapors
from the boiling chambers to the condensing chambers. (One
e~ample of a suitable compressor is Lamb compressor #115962.)
Three hoses in Figure 1 connect the core (12) to the
heat exchanger (11). The hose on the right (19) carries the
blowdown rom the core (12) to the heat exchanger (11). The hose
in the center (21) carries the product from the core (12) to the
heat exchanger (11). The hose on the left (22) carries feed
liquid from the heat exchanger (11) to the core (12). This hose
(22) contains a gas-liquid separator (28) for removing dissolved
gases and liquids from the feed liquid. Dissolved gases and
liquids come out oE solution and leave -through the gas-vapor
outlet (35). (This process will be explained in more detail in a
later section.)
The same hose (22) also contains a supplemental heater
(23) which provides energy for start-up, then operates
intermittently to maintain the desired operating conditions. (One
example of a suitable heater is the "Immersion Heater"
manufactured by A. o. Smith Co.) The heater (23) is controlled by
a pressure switch (24) sensitive to steam pressure in ths boiler.
This pressure switch (24) connects to the boiler through a hose
(26) which penetrates an op0ning ~27) in the end plate (17),
When the pressure in the boiler raises more -than 1 p.s.i (0.07
kilograms per square meter) above ambient ,pressure the switch
(24) opens and shuts o~f power to the heater (23). When the
pressure in the boiler becomes less than 0.5 p.s.i (0.035) kg/sq
m) above ambient pressure the switch (24) closes, and the flow o~
~z~
29 61051--2000
power ~o the heater resumes. The switch manuEactured by Henry G.
Dietz Co. (#17lD8WC) gives acceptable results.
An alternative way to control the heater would be to
monitor the temperature of the water in the boiler. In this event
a thermistor would replace the pressure switch. Thermistor
APlH10~-6 manufactured by Midwest Components Inc. would serve
adequately. The thermistor would shut off power to the heater
(23) when the water temperature reached 213F (101C) and restore
power to the heater (23) when the water temperature dropped to
212F (100C).
The three hoses near the lower left corner of the heat
exchanger (11) convey liquids to and from the system - hose (25)
is an inlet for the feed liquid, hose (20) is an outlet for the
product, and hose (15) is an outlet for the blowdown.
The Relative I~portance of the Specifi~ations.
Of the structural dimensions specified for the core tl2)
and the heat exchanger (11) only two are critical: the thickness
of the sheets and the distance be~ween them. The thickness of the
sheets should be within the range of 0.001" to 0.02" (0.025 mm to
0.51 mm). It would be possible to use sheets outside this range,
but thinner sheets would easily tear or puncture, and thicker
sheets would reduce efficiency, besides being prohibitively
expensive. Sheets with a thickness of 0.01" (0.25 mm) are sturdy,
inexpensive, and extremely heat conductive. The distance between
the sheets must be within the range of 0.005" to 1.0" (0.13 mm to
2.5~ cm) in the core (12) and within the range oE 0.005" to 0.25"
(0.13 mm to 6.4 mm) in the counkerflow heat exchanger (11). The
counterflow heat exchanger (11) achieves optimal efficiency when
~fiZ~0~3
~1051-2000
the spacing between the sheets is 0.1" (2.54 mm) or less, a
spacing of 0.032" (0.~1 mm) being ideal for most purposes.
Other aspects of the hardware offer a broad range of
choices. The sheets may be fabricated from stainless steel,
aluminum, brass, copper-nickel 90/10, glass, or polyester - any
material which can be formed into a thin sheet and maintain its
structural integrity at the operating temperatures. Ihe height
and width of the sheets are not particularly important, except in
subtle ways to be mentioned later. Many common gasket materials
will serve for the gaskets. The dimensions and materials of the
end plates are unimportant, so long as they provide enough
stability. Virtually any vapor compressor will serve adequately
if its Elow rate matches the flow rates of the core and the heat
exchanger.
The number of sheets in the core and in the counterflow
heat exchanger depends on the desired flow rate and efficiency.
For a minimal flow rate, one heat transfer sheet is sufficient to
create either a core or a counterflow heat exchanger. Combined
with two end plates, one sheet will create the basic structure: a
chamber containing rela-tively warm 1uid on one side of the sheet
and a chamber containing relatively cool fluid on the other side.
The stacked-sheet core ordinarily comprises at least five sheets.
The core (12) of Figure 1, built from 51 sheets, purifies 15
gallons (66 liters) of water per hour. The counterElow heat
exchanger (11) of Figure 1, containing 100 sheets, heats up 30
gallons (132 liters) of cold water per hour, drawing heat from 15
gallons ~66 liters) of product and 15 gallons (66 liters) of
blowdownO
~262108
31 61051-2000
An exploded Drawing of the Core.
Figure 2 is an exploded drawing of the core (12) of
Figure 1 exposing two heat transEer sheets (29 and 31). Both
sheets have a gasket af~ixed to one surface. The sheet (29) on
the left has a gasket (32) for a boiling chamber (33). The
boiling chamber (33) lies between the two sheets, its boundaries
defined by reference numbers (51) at the top, (30) at the bottom,
(41) on the left, and (60) on the right.
The sheet (31) on the right has a gasket (34) or a
condensing chamber (36). The condensing chamber (36) lies between
the heat transfer sheet (31) and the front end plate (17).
(Within the stack, of course, the condensing chambers lie between
two adjacent heat transfer sheets - not between a sheet and an end
plate.) The boundaries of the condensing chamber are indicated by
reerence numbers (52) at the top, (40) at the bottom, (39) on the
left, and (80) on the right.
The Paths ~f Water and ~te~m through the Core of Figure 2: The
feed water.
A hose (22) carries the heated feed water into the core
(12) through an opening (37) in the end plate tl7). Then the feed
water passes through holes (38) 1" (2.54 cm) in diameter in all
the sheets. It can't enter the condensing chambers (36) because
the gaskets form a barrier (39). But it passes into all the
boiling chambers l33) through openings (41) in the gaskets (32).
A seal (42) holds the water and steam inside the system. When the
water touches the heat transfer sheets (29 and 31) it receives
heat rom steam condensing in the adjacent chambers. The water
boils and steam rises.
1~2~)8
32 61051 2000
Th~ Steam.
The compressor (16) draws steam out of the boiling
chambers ~33) and blows it into the condenslng chambers (36). The
steam leaves the boiling chambers through holes (43) 1" (2.54 cm)
in diameter near the top of all the sheets. It flows through an
opening (44) in the end plate (17) and an opening (46) in the
compressor manifold (47). Then the compressor (16) compresses the
steam and ejects it through a second compressor maniold (48) and
through a hole (not shown) in the rear end plate (18). The
compressed steam flows through holes (49) 1/2" (1.27 cm) in
diameter in each sheet. It can't enter the boiling chambers (33)
because the gasket (32) forms a barrier (51). But it enters every
condensing chamber ~36) through an intake manifold (52).
When the steam touches the heat transfer sheets it
condenses and gives up its heat. The steam condenses at a
temperature hotter than the boiling liquid as a result of its
being compressed. The heat given by the condensing steam flows
from hot to cold - from the condensing surface, through the sheet,
into the boiling water.
2a The product.
The condensed steam, now distilled liquid water, drips
down the sides of the sheets and flows out of the core. It leaves
the condensing chambers through an outlet mani~old ~40). Then it
flows through holes (53) 1/2 inch (1.27 cm) in diameter at the
bottom of each sheet. As the product leaves the core (12) a
barrier (30) keeps it from entering the boiling chambers. The
product e~lts through an opening (54) in the front end plate (17).
A hose (21) carries it to the heat exchanger.
~,~
, .....
33 61051~2000
The blowdownt
The blowdown leaves the boilLng chambers through
openings (~0) in the gasket. I'hen it passes through holes (56) 1'
(2.54 cm) in diameter at the side of every sheet. A barrier (80)
prevents the blowdown from entering the condensing chambers as it
leaves the core. The blowdown passes through an opening (57) in
the front end plate (17), then enters an outlet hose (19). A
spill tube (58) in this hose regulates the level of water in the
boiling chambers.
Other liquids may be distilled with this procedure. The
operating conditions will vary, depending on the boiling
temperature of the liquid.
Other ~etails of Figure 2.
A few details of the drawing remain to be men-tioned~
Small assembly holes (59) are punched on the perimeter of all the
sheets. There are also four assembly holes ~61~ in the center o~
each sheet. In addition, the drawing shows assembly holes (50) in
the end plates and assembly holes (55) in the compressor manifold
(47). Bolts (not shown) pass through all these assembly holes to
hold the system together. The small sections of gasket on the
boiling and condensing surfaces are spacers (62). They hold the
sheets apart at any pressure from a complete vacuum to two
atmospheres. The core (12) and heat exchanger (11) are built by
cutting stainless steel sheets to size, punching holes in them,
stacking them with gaskets, and bolting them together.
Perfo~mance Characteristics of the Stacked-Sheet Core.
The stacked-sheet core ~12) of Figures 1 and 2 purifies
fiEteen gallons of water per hour (66 liters/hour) while
~L~62~
34 61051-2000
compressing the steam by 1 p.s.i. (0.07 kg/sq cm). Li~e the other
embodiments oE the invention, it yields a high energy multiplier -
in other words, it recycles large quantities of energy in return
for a small energy input. This core recycles 120,000 BTU (35.2
kwh) of heat energy per hour (enough to boil oEE fifteen gallons
of 66 liters of water). The energy required to compress the steam
by 1 p.s.i. (0.07 kg/sq cm) is 163 watt hours" or 556 BTU. The
ratio of the recycled energy to the input energy is 215 to 1.
The 163 watt hours of energy required for compressing
the steam do not include losses due to inefficiency of the
compressor and compressor motor. The Lamb compressor is
approximately 50~ efficient, and its motor is approximately 50~
efficient. With this peripheral equipment 652 actual watt hours
are required to compress ~he steam. The ratio of recycled energy
to input energy is slightly greater than 50 to 1.
There are several ways to increase the efficiency o~ the
process~ First, one could capture the waste heat from the
compr,essor motor. Second, one could ~Ise more eFficient peripheral
equipment: an 80% efficient compressor driven by a 90% efficient
electric motor. Third, one could use more sheets - doubling the
heat transfer surface area will reduce by half the compression
step required for a ~iven output of distilled water. Incorpor-
ating all these steps would reduce the total energy requirements
to 6.8 watt hours per gallon (1.5 watt hours/liter) - raising the
ratio of transferred energy to input energy to 347 to 1.
Design Strategy in the Stacked-Sheet Still.
The basic design strategy for all embodiments of the
invention is to provide extensive heat transfer surface area for a
~L2~ 0~
61051-2000
given flow rate - at least 2 square Eeet of heat transfer surface
for every gallon per hour (0.8 sq m/liter/hour) oE fluid passing
through. (Tlle "Eluid passing through" refers to the distilled
llquid in the coLes and to the cooler liquid in the counterflow
heat exchangers.) The core (12) of Figures 1 and 2 establishes
heat exchange relationship between the condensing steam and the
boiling water over approximately 45 square feet (4.2 sq m) to boil
off 15 gallons (66 liters) of water per hour - a ratio of 3 square
feet per gallon per hour (1.2 sq m/liter/hour).
Sheet material is an ideal medium for transferring heat
because it offers extensive surface area at low cost. Compared to
the metal tubes used in previous heat-recycling stills, sheet
material provides at least twenty times the surace area for the
same price.
Low Compression
Extensive heat transfer surface area in the core makes
it possible to condense the steam readily while compressing it
only a small amount, less than 2 p.s.i. (0.14 kg/sq cm). Several
important advantages of a low compression step have already been
mentioned: the low energy requirements for compressing the steam,
the opportunity to use thin sheets, the opportunity to stack the
sheets close together for improved heat transfer, and the
opportunity to use simple, inexpensive, long-lasting, quiet,
efficient compressors.
Another advantage o~ low compression is a low
temperature difEerence between the sheets and the boiling liquid.
Wh~n the steam is compressed by less than 2 p.s.i. (0.14 kg/sq cm)
~;
~2~ 08
35a 61051-2000
the boiling surEace of the heat transfer sheets will remain less
than 8F (4 ~C) hotter than the boiling water. With less than 1
p.s.i. (0.07 kg/sq cm) of compression this temperature difference
will not exceed 4F (2~2C)o A small temperature difference is
valuable Eor three reasons. First, the water doesn't dissipate
energy in random, turbulent motion - it boils gently, staying
mostly at rest. Second, the water stays in close contact with
the sheets. Virtually no steam barrier forms on the boiling
surface to insulate the water from its heat source. Third, the
relatively low temperature of the sheets reduces the problem of
scale. Scale, a crust formed by the impurities left behind when
seawater evaporates, reduces heat flow and must be removed
periodically. This invention has
~i2~
36 61051-2000
the boiling surface oE the heat transfer sheets will remain less
than 8F (~.~C) hotter than the boiling water. With less than 1
p.s.i. (0.07 kg/sq cm) of compression this temperature diEference
will not exceed 4F (2.2C). A small -temperature difference is
valuable for three reasons. First, the water doesn't dissipate
energy in random, turbulent motion - it boils gently, staying
mostly at rest. Second, the water stays in close contact with
the sheets. Virtually no steam barrier forms on the boiling
surface to insulate the water from its heat source~ Third, the
relatively low temperature of the sheets reduces the problem of
scale. Scale, a crust formed by the impurities left behind when
seawater evaporates, reduces heat flow and must be removed
periodically~ This invention has less problem with scale than any
previous distillation technology because the sheets aren't hot
enough to bake the impurities onto the metal. Most of the
impurities simply wash out with the blowdown. All these
advantages derive directly from the extensive surface area-low
compression approach.
Some minimum of compression is always necessary
~ because steam must be pushed slightly "uphill," from a low
pressure area in the boiler to a high pressure area in the
condenser. The condenser has a higher pressure because the pure
water there evaporates even more readily than the impure water in
the boiler~ and so exerts a higher steam pressure. To move steam
uphill from the boiler to the condenser and cause it to condense,
the compressor must supply a pressure step equal to the difference
in steam pressures, plus a little more. As the compression step
becomes lower and approaches the difference in steam pressures,
~L~62~
37 61051-2000
the energy requirements oE the process may approach the
theoretical minimum.
In seawater distillation the difEerence in steam
pressure between the distilled water in the condenser and the
salty water in the boiler is equal to seventeen inches (43 cm) of
water pressure, or about 0.6 p~s.i. (0.042 kg/sq cm). The minimum
compression step to cause condensation in seawater distillation,
then, is some amount slightly greater than 0.6 p.s.i. (0.042 kg/sq
cm). The energy to provide this pressure difference comes to
approximately 6 kilowatt hours per thousand gallons - very near 3
kilowatt hours, the theoretical minimum of energy required to
distill seawater according to classical physics~ This invention
demonstrates an operational method of distilling seawater with
energy requirements approaching the theoretical minimum.
Fresh water distillation requires even less energy than
seawater distillation. In fresh water distillation less
compression is required to move steam uphill into the condenser
because the difference in steam pressure between the distilled
water and the impure water is very slight.
Other Considerations for High Efficiency in Dis~illation
In order to recycle heat in distillation with efficiency
approaching the theoretical limits, it is also necessary to do the
following things:
1. Distribute impurities evenly throughout the boiler~
Local concentrations of impurities will raise the
boiling temperature of the wat0r and may prevent it from
boiling.The core of Figures 1 and 2 removes impurities ~rom all
38 61051-2000
parts of the boiler continuously by malntaining an even ~low o~
water across the sheets in every boiling chamber.
2. Distribute heat evenly throughout the boiler.
For high efficiency the water mus-t boil throughout its
volume r not just at hot spots. The core of Figures 1 and 2
distributes heat evenly by spreading water evenly across the
boiling surfaces and by spreading steam evenly across the
condensing surfaces. It spreads water across the boiling surfaces
by partially filling each boiling chamber with water, regulating
the level so that when the water boils it covers the entire
boiling surface. This method has one disadvantage: the pressure
on the water at the bottom of the boiler increases due to the
weight of the water above it. This pressure increase raises the
boiling temperature of the water and can keep it from boiling.
The effects of the pressure increase are minimized by having a
boiler only 1 foot (0.3 meters) deep. Even in large-scale
applications the depth of the boiler in this embodiment would not
exGeed 3 feet (0.9 meters). This core spreads vapors evenly
across the condensing surfaces with a rubber manifold containing
many small holes, the pressure drop across each hole being grea-t
enough to ensure that steam enters each hole at an equal rate.
3. Minimi~e th~ pressure drop across the condenser from entrance
to exit.
Any pressure drop across the condenser means that the
compressor must do extra work just to push the steam through the
condensing chambers. In the core of Figures 1 and 2 the
condensing chambers are only 1 to 3 feet (0.3 -to 0.9 meters) long,
and so the pressure drop across them is minimal - less than 0.25
39 61051-2000
p.s.i. (0.01~ k~/sq cm). Very little o the compressor's energy
is wasted in pushin~ the steam through the chambers. Most of the
compressor's energy is used productively, for compressing and
condensing the steam.
4. Remove non-condensible gases.
One example of a non-condensible gas is the air
dissolved in seawater. When the seawater boils, the air comes out
of solution and enters the condenser along with the steam. But
the air won't condense. If the air is allowed to accumulate in
the condenser it will slow down the condensation of the steam and
reduce efficiency. The core of Figures 1 and 2 exhausts non-
condensible gases by maintaining a continuous flow of steam
throughout the condenser and ensuring that some excess steam exits
the condenser at all times to entrain the gases.
5. ~emove the distilled water fro~ the condensiny chambers
quickly and easily.
This is important for two reasons. First, the distilled
water inter~eres with the ~low of heat by insulatin~ steam from
the condensing surfaces. Second, the compressor's energy
re~uirements increase if it has to blow the distilled water out of
the condenser. The core of Figures 1 and 2 has vertical
condensing surfaces only 1 foot (0.3 meters) high. Distilled
water drips down quickly and easily. The compressor does very
little work to push it out of the condensing chambers.
6. Reep the ~ystem in thermal balance.
This means adding or removing small amounts o~ heat to
maintain the desired operating conditions. The core of Figures 1
and 2 keeps itself in thermal balance by adding make-up heat
~,
~0 61051-2000
intermittently, only when necessary. To determine when make~up
heat is needed one could monitor either water temperature or steam
pressure ln the boiler. The core in Figures 1 and 2 monitors
steam pressure and controls the heater with a pressure switch in
the manner described earlier.
7. ~eat the feed liquid nearly to its ~oiling point before
putting it into the boiler.
If -the feed li~uid hasn't reached its boiling point when
it enters the boiler, some of the energy given by the condensing
steam must heat up the feed liquid, and heat from an outside
source must be added to sustain the boiling rate. The high
efficiency of this invention depends heavily on the ability of the
counterflow heat exchanger to heat the feed liquid to a
temperature near its boiling point~ The heat exchanger will be
examined in detail after a variation of the stacked-sheet core is
discussed.
stacked-~heet Core with a Vertical-Flow Boiler
The core (65~ of Figure 3 is identical to the core of
Figures 1 and 2 in most respects. It is built from 51 sheets of
stainless steel 0.01" (0.25 mm) thick, spaced 0.06" (1.52 mm)
apart. It purifies 15 gallons of water per hour (66 liters/hr)
with a compression step of 0.9 p.s.i. (0.06 kg/sq cm). It
provides approximately 3 square feet (0.28 square meters~ of heat
transfer surface area for each gallon of distilled water produced
per hour. It differs from the core of E'igure 2 only in the way it
spreads liquids across the boiling surfaces. This core (65)
introduces the feed liquid at the top of each boiling chamber and
allows it to drip down the sides oE the heat transfer sheets in a
~i
~,"
~ ~2108
41 61051-2000
thin film. The structural changes required for the vertical-flow
boiler are minor - one extra hole (63) is punched in the sheets,
and the gaskets (70 and 75) have a slightly different
configuration.
The Paths o~ Fl~ids in the ~ertical-Flow Boiler.
Fluids move through the core (65) of Figure 3 just as
they move through the core (12) of Figure 2, except or the
passage of feed liquid into the boiling chambers, which occurs in
the following manner: First a hose (64) brings the feed liquid to
the core (65). The feed liquid enters the core (65) through an
opening (66) in the front end plate. Then it flows through a hole
(63) 1/2" (1.27 cm) in diameter in all the sheets. The feed
liquid can't enter the condensing chambers (67) because the gasket
forms a barrier (68). But in each boiling chamber (69) the feed
liquid enters an intake manifold (71) near the top of the sheet.
It flows through openings (72) in the intake manifold and cascades
down the sides of the sheets. The rest of the process occurs
exactly as described in the discussion of Figure 2.
Advantages and Disadvantages of the Vertical-Flow Boiler.
The stacked sheet core (65) with the vertical-flow
boiler offers two main advantages. First, it recycles heat with
slightly greater efficiency than the horizontal-flow boiler shown
in Figures 1 and 2 because pressure remains e~ual throughout the
boiling chambers - there is no column of water to bear its own
weight. ~s a result the water bolls consistently in all parts of
the chambers. Slightly less compression is required for a 15
gallon-per-hour (66 liters/hr) flow rate, and less make-up heat is
needed. The second advantage is closely related to the first:
~L2~)Zi~0~3
~ 2 61051-2000
no matter how deep the boiling chambers are, the water at the
bottom of the chambers will boil readily. For this reason the
vertical-flow boiler is more suitable for large-scale units.
The main disadvantage of the vertical-flow boiler is the
difEiculty in getting the water to flow evenly over the boiling
surfaces The water tends to flow in rivulets rather than an even
film.
Having examined the stacked-sheet boiler condenser units
in detail, this discussion will now explain how the same design
principles are embodied in the stacked-sheet coun-terflow heat
exchanger.
The Stacked~Sheet ~ea~ ~xchanger
Flgure 4 is an exploded drawing of the heat exchanger
~11) of Figure 1 exposing four sheets (73, 74, 76, and 77) of
stainless steel. Each sheet has a gasket (78) attached to one
surface. The gaskets (78) keep the sheets separated by 0.03
inches (0.76 mm) and also guide the paths of the liquids. The
areas between the sheets serve as chambers (79, 81, 82 and 83) for
liquid flow. Chambers for the incoming cold liquid alternate with
chambers for the outgoing hot liquids. The sheets transfer heat
from the hot llquids Elowing in one direction on one side into the
cold liquid Elowing in the opposite direction on the other side.
The six holes (84, 86, 87, 88, 89, and 91) at the left
edge of the sheets, measuring 0.5 inches (1.27 cm) in diameter,
allow liquids to enter and leave the heat exchanger. The smaller
holes around the periphery of the sheets are assembly holes (92)
for bolts to hold the heat exchanger together.
`~'6?-
~ ~2~L~3
~ 3 61051-2000
Th~ Paths of Liqui~ through the Stacked-~heet Heat Exch~nger:
The Eeed liquid.
The feed liquid arrives at the heat exchanger throuyh a
hose (25). It enters the heat exchanger through an opening ~94)
at the bottom of the end plate (13). Then it flows through the
lowest hole (84) in every sheet. It can't get into the first
chamber (79) because the gasket (78) orms a barrier (96). But
the feed liquid enters the second chamber ~81) and the fourth
chamber (83) through openings (97 and 93) in the gaskets (78).
Within its chambers (81 and 83) the feed liquid flows
back and forth across the sheets along a path outlined by the
gaskets, absorbing heat from the hot liquids in the adjacent
chambers. After being heated the ~eed liquid lea~es its chambers
through the uppermost hole (91) in the sheet. Then it flows
through holes (91) in every sheet and through an opening (98) in
the end plate (13). A hose (22~ carries it to the boiler.
Th~ product.
The product arrives at the heat exchanger from the
condenser through a hose (21). It enters the heat exchanger
through an opening (102) in the end plate (13). Then it flows
through the second hole (89) ~rom the top in every sheet. The
product can't get into the first chamber (79) or the second
chamber (81) because the gaskets (78) form barriers (101 and 103)~
But the product enters the third chamber (82) and other chambers
through openings (104) in the gaskets (78).
As the product flows through its chambers (82 and
others) it gives heat to the feed liquid in the adjacent chambers
(such as 81 and 83). The product leaves its chambers thxough the
~62 ~0~
4~ 61051-2000
second hole (86) ~rom the bottom in every sheet. Then it passes
through an opening (106) in the end plate (13). A hose (20)
carries the product to a point of use or storage.
The blowdown.
The blowdown arrives at the heat exchanger from the
boiler through a third hose (19). It enters the heat exchanger
through an openis~g (109) in the end plate (13). Then it flows
through a ho]e (88) in all the sheets. The blowdown enters the
firs~ chamber (79) and other chambers (not shown) through openings
(111) in the gasket (78).
As the blowdown streams through its chambers it gives
heat to the feed liquid in the adjacent chambers. The blowdown
leaves the heat exchanger through the third hole (87) ~rom the
bottom in every sheet, then flows through an opening (112) in the
end plate (13). A hose (15) carries it out of the system.
Performance Characteristics of the Stacked-Sheet Heat Exchanger.
The counterflow heat exchanger (11) shown in Figure 4
heats thirty gallon (132 liters) of water per hour, raising i~s
temperature from 60F to 207F (from 16C to 97C). Like the core
of Figure 2, the stacked-sheet heat exchanger achieves a high
energy multiplier. It transfers 3~,603 sTu (10.7 kwh~ of heat
energy from the product and the blowdown into the feed liquid
every hour. The input energy required to move the liquids through
the heat exchanger is 3.3 watt hours, or 11.2 BTU. The ratio of
transferred energy to input energy is 3268 to 1.
Since the pressure drop across the heat exchanger is
only one poS~i~ (0.07 kg/sq cm), the force of gravity is
sufficient to cause liquids to pass through it. Alternatively one
0~
61051-2000
could use a simple pump. If the pump were 25% eEEicient, the
total energy required to move the liquids through the heat
exchanger would come to ~5 BTU (13.2 watt hours). In this case
the ratio of transferred energy to energy input would be 813 to
1.
Another way of measuring the effectiveness of the
counterflow heat exchanger tll) is to compare its actual
performance with the theoretical limit. The heat exchanger (11)
of Figure 4 raises the temperature of the feed liquid by 147F,
from 60F to 207F (by 81C, from 16C to 97C). The highest
temperature the feed liquid could possibly reach is 212~F,
(100C), which is the temperature of the product and the blowdown
as they enter the heat exchanger. This heat exchanger, then,
heats the cooler liquid to withi~ 5F (3C) of the theoretical
limit. This 5F difference between the actual and the theoretical
is known as the "approach temperatureO" The ratio of the total
temperature shift of the cooler liquid to the approach temperature
is a measure of the heat exchanger's performance. In this heat
exchanger the ratio between the actual temperature shift of the
feed liquid and the approach temperature is 147 to 5, or 29 to 1.
(Using the degrees centigrade, the ratio is 27 to 1~.
An even higher standard of performance can be achieved
by adding more surface area for the same flow rate. If the number
of sheets were doubled, the approach temperature would be reduced
by half. In this case the ratio between the actual temperature
shift and the approach temperature would be twice as great, or 4
to 1~
~X6~
46 ~1051-2000
Desi~n Critexia for the Stacked-Sheet Heat ~xchanger.
Extensive surface area is the ~ey to eEficiency. Like
the ot~ler embodiments of this lnvention, the stacked-sheet heat
exchangers provide a-t least 2 square feet of hea-t transfer surface
area for each gallon per hour (0.8 sq m/liter/hr) of cooler fluid
passing throughO The heat exchanger of Figure 4 uses
approximately 90 square feet (8.37 sq m) of surface area to heat
30 gallons (132 liters) of liquid per hour, a ratio of 3 square
feet per gallon per hour (1.2 sq m/liter/hr).
With so much surface area available, the liquids can
exchange heat readily even though they flow with low velocities
(less ~han 1 foot per second, or 0.3 meters per second), low
pressure heads (less than 1 p.s.i., or 0.07 kg/sq cm), and a
laminar flow pattern. (Laminar flow is smooth and orderly, with
individual molecules tending to follow the same paths.) The
advantages of this gentle approach have already been mentioned:
little energy is required to move the liquids -through their
chambers, the smoothly flowing liquids dissipate little energy in
turbulence, the sheets transferring heat may be very thin, and the
2~ sheets may be placed very close together for optimal heat
transfer.
In distillation the counterflow heat exchanger (11)
should raise the temperature of the feed liquid high enough so
available waste heat ~rom the compressor motor can bring its
temperature all the way to the boiling point. The counterflow
heat exchangers of the invention meet -this requirement with
simpLe, compact, inexpensive hardware.
.~
", .
2~
~ 7 6105l-2000
Removal of ~issolved Liquids and Gases.
The abllity of the heat exchanger (11) to heat the feed
liquid nearly to its boiling point offers another important
advantage: a convenient way to remove toxic gasex and liquids
dissolved in the impure water. If these gases and liquids are
allowed to enter the boiler they go off with the steam and con-
taminate the distilled water. The heat exchanger helps to remove
dissolved gases and liquids because the water can't hold them in
solution as it approaches its boiling point. The liquids
vaporize, and both the gases and liquids form bubbles. A gas-
liquid separator in the feed line between the heat exchanger ~11)
and the core (12) will allow the bubbles to raise from the water,
thereby removing the toxic substances. In its simplest form the
separator is an exit pipe to atmosphere with a valve to allow the
exhaustion of gases except when the liquid level nears the outlet,
at which time the valve closes. A gas-liquid separator is
valuable for removing trihalomethanes or other poisonous gases
from tapwater, for removing carbon dioxide from seawater to help
reduce scale formation, and for removing any non-condensible gases
to keep them from slowing down the condensation process.
Other Applications for the ~eat Exchanger.
Although the counterflow heat exchangers shown in the
drawings are all three-fluid heat exchangers appropriate for
distillation, other embodiments of the invention could easily
accommodate any number of liquids for different applications.
Many applications exist for two-fluid counterflow heat exchangers.
In homes, for example, heat exchangers can capture heat from hot
water leaving the shower, the clothes washer, or the dish washer,
'~
~i2~
48 61051-2000
and transEer that heat into the cold water from the city water
pipes. The energy re~uirements of ~lOt water heaters will drop
drastically if people feed heated water instead of cold water into
them. There are also many industrial applications for heat
exchangers, and still more applications will be found as energy
efficiency becomes more important.
This discussion will now turn to another still, similar
to the stacked-sheet model in its structure and function but
different in its appearance and construction techniques.
The Spiral Still
Figure 5 shows two more embodiments of the invention
suitable for purifying liquids: a spiral core (114) and a spiral
counterflow heat exchanger (116).
~he spiral core.
Ihe core (114) of Figure 5 is cons-tructed from two
sheets of stainless steel (type 316) wrapped around each other to
form two spiral-shaped chambers: a boiler (100) and a condenser
(10~) in heat exchange relationship. The sheets are 25 feet long,
1 foot wide, and 0.01" thick (5.1 m x 0.3 m x 0.25 mm). Spacers
keep the sheets 0.06" (1.52 mm) apart. Gaskets (115) seal the
chambers~ The sheets transfer heat from vapors condensing on one
side into a liquid boiling on the other.
The spiral heat ex~hanger.
The heat exchanger (116) of Figure 5 is built from three
sheets of stainless steel. Each sheet measures 32 feet by 1 foot
by 0.01" (9.8 m x 0.3 m x 0.25 mm). They wrap around each other
to form three spiral-shaped chambers or the feed liquid, the
product, and the blowdown. Spacers keep the sheets 0.032" (0.81
1~6~
49 61051-2000
mm) apart. Gaskets (110) seal the chambers. The sheets transfer
heat from hot liquid on one side into the cold liquid on the
other.
The ~elative Imp~rtance of the Specification~.
In the spiral core (114) and heat exchanger (116) the
most significant structural dimensions are the thickness of the
sheets and the distance between them. The thickness of the sheets
must be within the range of 0.001" to 0.02" (0.025 mm to 0.51 mm).
The separation between the sheets must be within the range of
0.005" to 1.0" (0.13 mm to 2~54 cm) in the core, and within the
range oE 0O005" to 0.25" (0.13 mm to 6.4 mm) in the counterflow
heat exchanger. The sheets may be constructed from stainless
steelr aluminum, glass, polyester, or any other material that can
be formed into a thin sheet and maintain its structural integrity
at operating temperatures. The length and height of the sheets
are not critical, except that the arnount of surface area
determines flow rate and efficiency. Any standard gasket material
w~uld be suitable.
Design Stra-tegy and Performance Characteristics of the Spiral
Still.
The design strategy and performance characteristics are
nearly identical to those of the stacked-sheet stills. Both the
spiral core (114) and the spiral counterflow heat exchanger (116)
supply extensive surface area for heat transfer, slightly more
than three square Eeet per gallon per hour (1.~ sq m/liter/hr).
With extensive surface area in the core (114), the vapors can
condense r~adily with only a small compression step. The spiral
core (114) purifies fifteen gallons per hour t66 liters/hr) with a
X~
~L2~2~
S0 61051~2000
compression step of 1 p.s.i. (0.07 kg/sq cm)~ With extensive
surface area in the counterflow heat exchanger (116), the liqulds
can exchange heat readily even though they flow gen-tly. The
spiral heat exchanger (116) h0ats thirty gallons (132 liters) of
water per hour from 60E' to 206F with a pressure drop of 1 p.s.i.
across the chambers (from 16C to 97C with 0.07 kg/sq cm).
T~e Paths of Liqllids and Vapors through th~ Spiral Still: The
feed liquid.
The feed liquid enters the central chamber of the heat
exchanger (116) through the opening (117) at the lower right of
the drawing. It receives heat from the product and the blowdown
as it winds through the turns of the spiral. Then the feed liquid
flows -through a hose (118) from the heat exchanger to the core
(114)o This hose (118) connects with metal tubes (119) which
spill the eed liquid near the top of the boiler (100). Inside
the boiler (100) the feed liquid receives heat from vapors
condensing on the opposite sides of the sheets and boils.
The vapors.
A compressor (121) driven ~y a motor (122) draws vapors
out at the top of the boiler (100). First the vapors enter a
demister (123). The demister (123) consists of a series of
baffles - it leads the vapors in a tortuous path to prevent them
from carrying any entrained droplets of liquid into the condenser
(105). (In most applications a demister ~ill not be necessary.
In seawater distillation, for example, the spiral still reduces
the total dissolved solids from 35,000 parts per million to fewer
than 3 parts per mill;on w1thout a demister. A demister would be
important only when extremely pure water is needed r Eor e~ample in
~i2~0E~
51 61051-2000
manufacturing computer parts.) The compressor (121) compresses
the vapors, then blows them through the compressor manifold (124)
into the condenser (105). tThe compressor mani~old (124) rests on
the core and the compressoc when the core is in operation - the
drawing elevates the manifold to reveal the compressor and the
openings in the seals.) When the compressed vapors enter the
condenser (105) and touch the heat transfer sheets, they condense
and give up their heat. This heat flows through the sheets to
boil the liquid on the other side.
The product and the blowdo~n.
The distilled liquid drips down the walls of the
condenser (105). A hose (126) carries it from the condenser (105)
to the heat e~changer. Another hose (127) carries blowdown from
the boiler (100) to the heat exchanger.
Advantages and Disadvanta~es of the Spiral Configurations.
The spiral configurations are easier to build in some
respects - t~ere is no need to cut the sheets into small sections,
to punch holes, or to make complicated gaskets. But the spirals
have two main disadvantages: it's difficult to seal them, and
it's difficult to disassemble them for cleaning.
Vacuum Distillation
By drawing a vacuum on any spiral or stacked-sheet core
one can boil the feed liquid at a lower temperature. Vacuum
distillation o~fers the three main advantages:
1) Vacuum distillation of seawater can reduce scale
formation. Hard scale forms only at temperatures above 185F
(85C). At lower temperatures only soft scale forms. Soft scale
is much easier to remove. By drawing enough of a vacuum to boil
52 61051-2000
the seawater at 185F (35C) or a lower temperature one can
eliminate hard scale entirely.
2) Vacuum distillation sometimes makes it possible to
capture the waste heat from the compressor motor. This waste heat
can be captured by passing the heated feed liquid through a metal
coil wrapped around the compressor mo-tor. But the heated feed
liquid must be cooler than the motor because heat will only flow
from hot to cold. In some circumstances a vacuum will lower the
boiling temperature of the liquid below the operating temperature
of the motor.
3) By drawing a vacuum one can distill liquids at
ambient temperature, in which case a heat exchanger would not be
necessary. In large~scale situations it might be valuable to
eliminate the capital costs of the heat exchanger.
To this point the discussion has focused on water
distillationr a process in which only one liquid will vaporize
(unless traces o~ a second liquid are dissolved in the water).
Next it will examine how the invention performs in a distillation
process in which two liquids will vaporize.
Alcohol Distillation
The goal of this process is to separate alcohol from
water. Nature gives a mixture o~ alcohol and water when plants
ferment. Since alcohol evaporates more easily than water, it is
possible to concentrate the alcohol by distilling the mixture.
The Hardware.
The cores and heat exchangers shown in Figures 1, 2, 3,
4, and 5 will distill alcohol with no adaptation or adjustment.
~...
~2~8
53 61051~2000
The P-roc0ss.
The invention distills alcohol with the salne energy-
recycling process it uses to puriEy water. rrhe feed liquid ls a
mixture of alcohol and water. In the alconol Euel industry this
mixture is commonly called "beer." The beer enters a heat
exchanger, where it receives heat Erom the product and the
blowdown. Then it enters a boller, where it receives heat from
condensing vapors and boils. Both alcohol and water evaporates,
but alcohol evaporates preEerentially.
A compressor blows alcohol and water vapors from the
boiler to the condenser. The vapors condense on a thin sheet and
give up their heat.
This heat ~lows through the sheet to boil the beer on
the other side. The condensed liquid has a higher concentration
of alcohol than the original beer. This product liquid, the
distilled alcohol~ leaves the condenser and Elows through a hea-t
exchanger to give heat to incoming beer.
The blowdown, the liquid remaining in the boiler, is a
mixture of alcohol and water with a lower concentration of alcohol
than the beer. The blowdown also enters the heat exchanger to
give heat to the incoming beer.
Sp~ ications Unique to Alcohol Distillation.
This method of distilling alcohol differs from the
method of purifying water described earlier in two important
respects - first, alcohol distillation requires a slightly higher
pressure step, within the range of 1 to 8 p.s.i. (0.07 to 0.56
kg/sq cm~. The higher pressure step is needed because there are
two vapors (alcohol and water) in the condenser~ Until 1 to 8
08
54 61051-~000
p.s.i. (0.07 to 0.56 kg/sq cm) oE compression is added, neither
alcohol vapors nor water vapors may have enough pressure to
condense. Second, the temperature difference between the boiling
surfaces of the heat transEer sheets and the boiling liquid is
greater in alcohol distillation, up to ~5F (8C). This higher
temperature diEference is a result of the higher compression step.
Compressing the vapors and recycling their heat is a
novel approach to alcohol distillation. It reduces the energy
requirements of the process more than tenfold. The design of the
heat exchanger and the core leads to high efficiency for all the
same reasons explained earlier with reference to water
puriEication.
Operating Conditions.
Three passes are usually required to distill uel-grade
alcohol from a fermentation product. Assuming the fermentation
product contains 15% alcohol, the first distillation may employ a
compression step of 7 p.s.i. (0.~9 kg/sq cm) and increase the
alcohol to 35%. The second distillation may compress the vapors
by 5 p.s.i. ~0.35 kg/sq cm) and raise the alcohol content to 60~.
The third pass may involve 7 p.5.i. (0.49 kg/sq cm~ of compression
and bring the mixture to 75% alcohol. Alcohol distillation may
also be performed at a vacuumr either to eliminate the need for a
heat exchanger or to capture the waste heat from the compressor
motor.
Separation o~ Alcohol and ~ter in the ~ondenser.
A second method of alcohol distillation separates
alcohol Erom water in the condenser as well as the boiler. By
compressing the vapors slightly, within the range of 1 to ~ p.s.i.
~26~
61051-2000
(0.07 to 0.28 kg/sq cm), one can condense water vapors selectively
in the first distillation. (Water vapors condense more easily
than alcohol vapors in the Eirst distillation because they have a
much higher percentage of the total volume of vapors. They have a
higher percentage of the volume because the beer is mostly water
and because water expands two-and-a-half times as much as alcohol
when the two evaporate.) With a compression step in the range of
1 to 4 p.s.i. (0.07 to 0.28 kg/sq cm) the water vapors condense
readily, but the alcohol vapors still don't have a high enough
pressure to condense. By collecting the uncondensed vapors
leaving the condenser and condensing them separatelyr one san
obtain an alcohol-water mixture with a higher concentration o
alcohol than the vapors which rose from the boiling beer. This
method requires slightly more energy than the method described
earlier because the energy o the uncondensed vapors cannot be
recycled. But this method separates alcohol from water more
effectively in the Eirst distillation.
The next process differs from alcohol distillation and
water purification in that the main product is the substance that
won't boil off.
Dehydrating and Concentrating Liquids
The goal of these distillation processes is to boil of
a liquid to collect what remains. In most cases the liquid to be
removed is water. Dehydrating means removing all the water,
concentrating means removing some of -the water. In alcohol fuel
production these processes are valuable for concentrating fruit
juices before fermentation and for dehydrating residues in the
blowdown. The invention will also dehydrate the watery residues
,/, ...
~i2~l~)8
56 61051-2000
of food processing plants, industrial wastewater, and raw sewaye.
I~ the liquid to be concentrated is not too viscous, the invention
will concentrate it in a continuous Elow. The cores and heat
exchangers shown in Figures 1, 2, 3, 4, and 5 concentrate liquids
with the same procedure they use to purify water - the only
difference is that the "blowdown" from the boiler becomes the main
product, and the distilled water from the condenser becomes a
valuable by-product. No changes are required in the hardware.
The method and the performance characteristics are also the same
as in water purification/ except that compression requirements
will increase if a high concentration of the liquid is desired.
By recycling heat eficiently this invention reduces the energy
requirements for concentrating liquids up to 99%.
Whenever the liquid is too viscous for a continuous flow
process it is possible to concentrate or dehydrate one batch of it
at a time. Figure 6 shows a core for a batch process.
Th~ Batch Dehyd~ator/Concentrat~r
Figure 6 is a cutaway drawing of a batch dehydrator/-
concentrator. A folded core (128) lies inside a vat (129). The
vat (129), serving as a boiler, contains a mixture of solids and
liquids to be separated. A lid (131 rests atop the vat (129). A
compressor (125) and compressor motor (132) Easten to the lid
(131).
The core (128) is built by folding a single sheet of
stainless steel many times to create an alternating sequence o
boiling and condensing chambers in heat exchange relationship.
The sheet is EiEty feet long, twelve inches wide, and 0.01 inches
thick (15.2 m x 0.3 m x 0.25~ mm). Gaskets maintain a separation
,
.. ..
~6~
57 61051-2000
of 0.06" (1.52 mm) between the folds. F'ach vertical section of
the sheet transfers heat from condensing vapors on one side into
the boiling liquid on the other.
The thickness of the sheet and the spacing between the
folds are the most important structural dimensions. The thickness
of the sheet may range from 0~001" to 0.02" (0.025 mm to 0.51 mm).
Tha distance between the folds may range from 0.005" to 1.0" (0.13
mm to 2.54 cm). The sheet may be constructed from the same broad
range of materials described with reference to the other cores.
The specific gasket material is unimportant.
The Process.
This section explains briefly how the batch dehydrator/-
concentrator of Figure 6 can dehydrate watery wastes of food
processing plants. The first step is to bring the wastewater to a
temperature near its boiling point. This may be accomplished
either by heating the wastewater or by drawing a vacuum on the vat
(129). (In either cases it will be necessary to add or remove
small amounts of heat to maintain the desired operating
conditions.) Once the wastewater is in the vat (129), the next
steps are lowering the core (12~) into the vat (129) and fastening
the lid. The wastewater can't get into the condensing chambers
because they're sealed on the sides and at the bottom. But it
enters the boiling chambers, which are open at the bottom.
The compressor compresses the steam from the boiling
chambers by l p.s.io (0.07 kg/sq cm), then blows it into the
condensing chambers. When the compressed steam touches the
condensing surfaces of the heat transfer sheet it condenses at a
temperature hotter than the boiling liquid. Its heat flows
~6~
5~ 61051-2000
through the sheet to boil the wastewater on the other side.
The condensed liquid is pure distilled water. It flows
through a hole at the bottom of the sheets and into a tube (133)
which carries it out of the system. (The distilled water will
flow upward for a few inches because of the slight pressure
increase in the condenser.)
After the wastewater has boiled off, the solids remain
in the vat (129). They may be collected easily after the lid
(131) has been lifted and the core (128) has been removed. Any
solids remaining in the boiling chambers can be blown out with
compressed air.
Cros~-Sectional Views of ~he Batch Deh~drator/Concent~ator.
The next two drawings offer detailed views of the inside
of this core: Figure 7, a cross-sectional drawing of a boiling
chamber, and Figure 8, a cross-sectional drawing of a condensing
chamber. Both drawings show the vat (129), the lid (131), the
compressor (125), and the compressor motor (13~). The gaskets are
the only parts of Figures 6 and 7 that differ. The gaskets (130)
in the boiling chamber are open on the top and bottom; the gaske~s
(135) in the condensing chamber are open on the sides.
Arrows in Figure 7 show how steam flows out the top of
the boiling chambers into the compressor. The narrow strip of
gasket material at the top of the boiling chambers is a demister
(134). The demister (134) forces the steam to flow to either side
a short distance before it can reach the compressor (125). This
short diversion makes it more difficult for the steam to carry
away any droplets of liquid.
Arrows in Figure ~ show the path of the steam from the
~2fi2~ 8
59 61051-2000
compressor (125) into the condensing chamber. The steam condenses
on the heat transfer sheets. The distilled water flows out of the
condensing chambers through a hole (136) in the bottom of the
sheets. In the boiling chambers (Figure 7) the gasket forms a
barrier (137) around this hole to ~eep the distilled water out of
the boiling chambers as it leaves ~he core.
The small pieces oE gasket on the sheet are spacers
(140) to keep the folds apart at any pressure from a complete
vacuum to two atmospheres.
Performance Character;stics o~ the Batch Dehydrator~Con~entrator.
The Batch Dehydrator/Concentrator of Figure 6 removes
liquid from a concentrated solution at the rate of fifteen gallons
(66 liters) per hour. The pressure step required depends on how
concentrated the solution is. The more concentrated the solution
in the boiler, the lower its vapor pressure, and the more work the
compressor has to do to move steam uphill into the high-pressure
area of the condenser. Assuming a compression step of 2 p.s.i.
(0.14 kg/sq cm) with a 50~ efficient compressor driven by a 50%
efficient motor, the ratio of recycled energy to actual input
energy is approximately 25 to 1. Higher energy multipliers can be
achieved by increasing the heat transfer surface area or by using
a more efEicient compressor and compressor motor.
Although the process described above is applicable only
to liquids, a similar dehydration process will remove moisture
from solids.
Dr~ing Soli~s
The goal of this distillation process is to remove a
liquid from a wet solid - for example, to dry clothing, fruits,
'`'~;
1262~L0 !3
59a 61051-2000
vegetabl~s, or distillers' grains. The invention recycles energy
by evaporating the liquid, compressing its vapors, condensing the
vapors on a thin sheet of material, and recycling heat from the
condensing vapors back into the evaporator to dry the solids.
The Batch Deh~rator
Figure 9 is a cutaway drawing of a batch dehydrator. It
consists of a curved stainless steel heat transfer sheet ~13~)
inside a cylindrical case (139). The sheet measures ten feet
long, three feet wide, and 0.01" thick (3.04 m x 0.91 m x 0.254
mm). The area inside the curved sheet serves as a boiler or
evaporator (147) - a receptacle for the solids to be dried. The
area between the sheet (138) and the case (139) serves as a
condenser. A compressor (141~ and compressor mo-tor ~145) are
mounted on the end.
The heat transfer sheet (138) may be constructed from
any material which can be formed into a thin sheet and maintain
its structural integrity at the operating temperatures. For best
results the thickness of the sheet should be within the range of
0.001" to 0.02" (0.025 mm to 0.51 mm). The length and the width
of the sheet may vary depending on the quantity of grains or
clothing to be dried.
The Process.
This section explains how the batch dehydrator dries
clothing (or any other wet solids). The first steps are inserting
a load of wet clothing through the front opening (142) and closing
the door (143). The nex-t step is adding heat or drawing a vacuum
to begin evaporating the water. The compressor (141) draws the
59b 61051-2000
water vapor (steam) through a small hole (144) at the end of the
evaporator. Then it compresses the vapor by 1 p.s.i. (0.07 kg/sq
cm) and blows it around to the other side of the sheet.
When the compressed water vapor strikes the condensing
surface of the heat transfer sheet it condenses at a temperature
hotter than the clothing. Its heat ~lows through the heat
transfer sheet into the evaporator to dry the clothing. The
condensed liquid leaves the condenser throu~h a hole (146) at the
bottom of the case.
Other Views of the Batch Dehydrator.
Figure 10 shows the batch dehydrator of Figure 9 in a
cross-sectional view from the end. The heat transfer sheet (138)
and the case (139) are shown, as are the evaporator (147), the
condenser (148), and the hole (146) where distilled water drains
from the condenser.
Figure 11 shows the same batch dehydrator in a cross-
sectional view from the side. This drawing shows the heat
transfer sheet (138), the case (139), the front opening (142)
where the solids are insertedj the hole (144) where the compressor
draws out the steam, and the hole (146) at the bottom of the case
where distilled water leaves the condenser.
Other eonsiderations for ~igh Efficiency.
For optimal efficiency in drying solids, it's necessary
to do three more things:
1. ~emove non-condensible gases.
If air or other non-condensible gases are allowed to
accumulate in the condenser they will slow down the condensation
, ., .~ . ~
59c 61051-2000
process. The easiest way to remove non-condenslble gases is to
draw a vacuum on the core from the outlet to the condenser.
2. ~aintain thermal balance~
It will be necessary to add or remove small amounts of
heat to maintain the desired operating conditions.
3. ~eep the wet solids in close contact with the heat transf~r
sheet.
For this reason it's advisable to rotate the batch
dehydrator like a conventional clothes dryer. The solids dry by
tumbling against the evaporation surface~
Perfor~ance Characteristics o~ the Batch Dehydra~or.
The dehydrator of Figure 9 will accept a ten-pound (4.5
kg) load of wet clothing. The fabric makes up six pounds (2.7 kg)
of the load, and moisture makes up the other four pounds (1.8 kg).
The dehydrator dries the clothing in thirty minutes. It recycles
3840 BTU (1~13 kwh) of heat energy to evaporate the four pounds
~1.8 kg) of water. The theoretical energy input required to
compress the water vapor by 1 p.s.i. is 18 BTU (5.3 watt hours).
The ratio of the recycled energy to the theoretical input energy
is 3840 to 18, or 213 to 1. Assuming the compressor is 50%
efficient and the compressor motor is 50% efficient,
approximately 72 BTU (21.2 watt hours) of energy will be required
to compress the steam. Under these circumstances, the ratio of
recycled energy to actual input energy will be slightly greater
than 50 to 1.
Design Criteria fo~ the Batch Dehydra~orO
The most important design consideration for the batch
21~3
59d 61051-2000
dehydrator i5 extensive heat transfer surEace area Eor the flow
rate of distilled water - at least two square feet each for gallon
o fluid evaporated per hour (0.8 sq m/liter/hr). The dehydrator
of Figure 9 supplies thirty square feet per gallon per hour ~12 sq
m/liter/hr).
The final application of thls invention, unlike the
others, is not a separation process at all. It might be called a
mixing process - it mixes liquids of different salinities to
capture the energy that is released.
Power Generation
The goal of this distillation process is to generate a
head of steam for doing useful work. The invention recycles
energy to sustain the boiling action.
The Process.
The apparatus boils fresh water to generate a head of
steam. The steam spins the blades oE a turbine. An electrical
generator linked to the turbine converts the rotary motion into
electricity. Then the steam condenses in hot concentrated brine
and gives up its heat. This heat Elows through sheets of material
to boil the fresh water on the other side. The diluted brine
flows from the condenser to a heat exchanger, where it gives heat
to incoming fresh water and salt water.
This process requires that the boiler be a high pressure
area relative to the condenser, so steam will naturally flow from
the boiler to the condenser. But it also requires that the
condenser be a high temperature area relative to the boiler, so
heat will naturally Elow from the condenser to the boiler. Both
.~
~V~6~
S9e 61051-2000
these conditions are met by introducing fresh water into the
boiler and concentrated brine into the condenser. The dissolved
salts lower the steam pressure of the brine. Its steam pressure
remains lower than that of the fresh water, even when the brine
becomes slightly hotter.
The Power Generator
Figure 12 is an exploded drawing of a stacked-sheet core
for generating power. This core was built by stacking together 51
heat transfer sheets made of stainless steel type 316. The sheets
measure 12" x 16" x 0.01" (0.3 m x 0.4 m x 0.25 mm). Gaskets
maintain a separation of 0.06" (1.52 mm) between the sheets. The
areas between the sheets serve as chambers for boiling the fresh
water and for condensing i-ts vapors in hot salt water. Boiling
chambers and condensing chambers alternate. Each sheet transfers
heat from the ho-t salt water on one side into the boiling fresh
water on the other.
The Relative importance of the Specifications.
As in the other cores, the important structural
dimensions which define the invention are the thickness of the
sheets and the distance between them. The thickness of the sheets
must be within the range of 0.001" to 0.02" (0.025 mm to 0.51 mm).
The distance between the sheets must be within the range of 0.005"
to 1.0" (0.13 mm to 2.54 cm). The sheets may be constructed from
the same range of materials described with reference to Figure 1.
The height and width of the sheets are not critical. The number
of sheets depends on the desired Elow rate. The turbine may be
replaced by a posltive displacement piston or any other means of
59f 61051-2000
convertlng vapor flow into mechanical motion.
The Sheets and the Cham~ers.
Figure 12 exposes two heat transfer sheets (149 and
151). Both sheets have a gasket affixed to one surface. The
sheet (149) on the right has a gasket (.l52) to form a boiling
chamber (153). The boiling chamber (153) lies between the sheet
(152) and the front end plate (154). (Within the stack the
boi].ing chambers lie between successive heat transfer sheets.)
The boundaries of the boiling chamber (153) are defined by
reference numbers (182) a-t the top, (193) at the bottom, (168) at
the left, and (202) at the right. The sheet (151) on the let has
a gasket (156) to form a condensing chamber (157). The condensing
chamber (157) lies between the two heat transfer sheets (14g and
151). Its boundaries are indicated by reference numbers (184) at
the top~ (194) at the bottom, (169) at the left, and (203) at the
right.
The other prominent elements of the drawing are a
turbine (153), a pressure switch (159), and the rear end plate
(161).
Paths of Liquids and Vapors through the Core of Figure 12.
This section will explain how salt water enters the
condensin~ chambers, fresh water enters the boiling chambers,
steam flows from the boiling chambers to the condensing chambers,
and diluted salt water leaves the condensing chambers.
The salt water.
The salt water arrives at the core in a hose (162) at
the left of the front end plate (154). (IE a heat exchanger is in
~2~X~
59g 61051-2000
use, the salt water will have already been heated by diluted water
l~aving the process.) A heater (163) connected to this hose (162)
adds small amounts of heat intermittently to maintain the desired
operating conditions.
The heater (163) is controlled by a pressure switch
(159) sensitive to steam pressure in the condenser. The pressure
switch (159) communicates with the condenser through a hose (150),
an opening (155) in the end plate, and holes (160) 1" (2.54 cm) in
diameter in the sheets. When the steam pressure drops too low the
switch (159) closes, completing a circuit so power can flow to the
heater (163). When the steam pressure becomes high enoughl the
switch (159) opensr breaking the circuit and shutting off power to
the heater (163~.
The salt water enters the core through an opening (164)
in the end plate, then flows through holes (166) 1" (2.54 cm) in
diameter in all the sheets. Gaskets form a seal (167) to keep the
liquids and vapors inside the system. The salt water canlt enter
tha boiling chambers (153) because the gaskets form barriers
(168). But it enters each condensing chamber (157) through
openings (169) in the gaskets (156). The salt water partially
-Eills each condensing chamber (157). A spill tube (171) regulates
the height of the salt water so it covers the entire surEace of
the sheets.
The fresh water~
Fresh water approaches the core in a hose (172) to the
right of the end plate (15~). (IE a heat exchanger is being used,
the Eresh water has also been heated to a temperature near its
'~`~
- ~621(~8
S9h 61051-2000
boiling point by the outgoing diluted salt water.) The fresh
water streams into the core through an opening ( 173~ in the end
plate (154)~ then flows through holes (174) 1~ (2~54 cm) in
diameter in the lower corners of each sheet. The fresh water
can't enter the condensing chambers ( 157) because the gaskets
(156) form a barrier ~176)o But it enters each boiling chamber
(153) through an opening (177) in the gaskets (152) and spreads
evenly across the boiling surfaces of the heat transfer sheets.
The fresh water partially fills each boiling chamber (153) ~ its
height regulated by a spill tube (178) SO it covers the entire
boiling surface when it boils.
Fresh water from the boiling chambers (153) seeks its
own level in the spill tube ( 178) assembly. The fresh water flows
through an opening (170) in the gaskets and through holes (165) in
the sheets. The gasket forms a barrier (190) to keep the fresh
water out of the condensing chambers. Then the fresh water flows
through an opening ( 180) in the end plate and into a hose ( 185)
cosltaining the spill tube (178)~ A second hose (175) connects the
spill tube with the top of the boiling chambers to provide a
pressure reference. Steam from the boiling chambers enters this
hose ( 175) through holes ( 195) in the sheets and an opening ( 179)
in the end plate. Any fresh water overflow falls through the
spill tube (178~ and into a hose (181)~ This hose (181) carries
the fresh water overflow to the heat exchanger, where it joins the
diluted salt water to give heat to the incoming fresh water and
salt water.
;~ The s~eam.
59i 61051-2000
Inside each boiling chamber (153) the fresh water
receives heat fro~ hot salt water on the opposite sides of the
sheets. The fresh water boils and steam rises. rrhe steam expands
toward the low-pressure area in the condensing chambers (157). It
rushes out of each boiling chamber (153) through an outlet
manifold (182) at the top. Then the steam flows through holes
(183) half an inch in diameter at the top oE each sheet on its
way out of the core. As it exits, a barrier (184) keeps it out o
each condensing chamber.
The steam rushes through an opening (186) in the end
plate (154) and enters an expander (201). The expander consists
of a small maniold (187), two hoses (188 and 189), and a turbine
(158). The expanding steam spins the turbine blades, just like a
wind spins a windmill. The spining motion of the turbine can
generate electrical power, pump water, or do other useful work.
The second hose (189) carries steam from the turbine
back into the core. The steam flows through an opening (191) in
the end plate (154) and through holes (192) 1/2" (1.27 cm) in
diameter at the bottom of every sheet. The steam can't enter the
boiling chambers (153) because the gasket (152) forms a barrier
(193~. Bu-t the steam enters each condensing chamber (157) through
an inlet manifold (194) formed by the gasket (156). This inlet
manifold (194) contains many small openings, the pressure drop
across each opening being great enough so steam enters each
opening a-~ an equal rate. As a result steam spreads evenly
throughout the salt water in the condensing chambers (157).
When the steam contacts the hot salt water it condenses
~L2~
ssj 61051-2000
and gives up its heat. This heat raises the temperature of the
salt water in the condensing chambers (157), making it hotter than
the ~resh water in the boiling chambers (153). Heat from the salt
water flows through the sheets to boil the Eresh water on the
other side. The bolling fresh water generates more steam, and the
cycle continues.
The diluted salt water.
The diluted salt water leaves the condensing chambers
(157) through openings (203) in the gasket. It flows through
holes (196) 1" (2.54 cm) in diameter in the sheets on its way out
o~ the core. Barriers (202) prevent the diluted salt water from
entering the boiling chambers. It passes through an opening (197)
in the end plate (154), then enters a hose (198) which carries it
to the heat exchanger, if a heat exchanger is in use.
Other Details of Figure 12.
The tiny sections of gasket in the central area of the
sheets are spacers (204) to hold the sheets apart at any pressure
from a complete vacuum to two atmospheres. ~ssembly holes (199)
in the sheets and assembly holes (200) in the end pl~tes are
intended for bolts (not shown) to hold the core together.
Counter Flow ~eat Exchange.
The power generation process may use the heat exchanger
shown in Figure 4. In this case the heat exchanger heats cold
fresh water and cold salt water to temperatures near their boiling
points by capturing heat from hot diluted salt water leaving the
core. The liquids flow through their chambers with low velocities
(less than 1 foot per second, or 0.3 meters per second), low
59k 61051-2000
pressure heads (less than 1 p.s.i. or 0.07 kg/sq cm), and laminar
flow patterns. Instead of using a heat exchanger it would be
possible to draw a vacuum on the core to boil the fresh water at
ambient temperature.
Design Criteria for the Power Gen~rator.
Like all the other embodiments of the invention, the
power yenerator is a form of heat exchanger. The most important
aspect of its design is extensive surface area for heat exchanye.
The power generation embodiments supply at least two square feet
of heat transfer surface area for every gallon of fresh water
evaporated per hour (0.8 sq m/liter/hr). The embodiment shown in
Figure 12 supplies approximately three square eet of surface area
per gallon per hour ~1.2 sq m/liter/hr)l
Performance Characteristics of the Power Generator.
This power generator evaporates fifteen gallons (66
liters) of fresh water per hour. When distilled water feeds into
the boiling chambers and concentrated brine feeds into the
condensing chambers, a pressure difference of 4O5 p.s.i. (0.32
kg/sq cm) between the boiler and the condenser is created. It
generates 750 watts of power, enough to supply electricity for the
average residence.
Like the other embodiments of the invention, the power
generator recycles energy eEficiently with simple, easily
manufactured hardware7 By reducing the energy requirements of
distillation and broadening the scope oE its application, this
invention will allow this ancient process to solve some of the
most critical problems of contemporary human societies.
..~
GUIDE TO RE~E~ENCE NUMEIEE~S
11. heat exchanger
1 2. core
13. front end plate for the heat exchan~er
14. rear end plate for the heat exchanger
15. -outlet hose for the blowdown
16. compressor
17. fror~t end plate for ~he core
18~ rear end plate for the core
19, outlet hose for the blowdown
20. outiet hose for the product
21. outlet hose for the product
22. inlet hose :Eor the feed liquid
23. heater
24. pressure switch
2S, inlet hose for the feed liquid
26. hose connecting pressure switch to i~oiler
27. opening in the front end pla~e for hose (26)
28. ga~liquid separator
29. heat transfer sheet
30. i~arrier to prevent the product from entering the boiling chambers
31. heat tr3ns~er sheet
32. gasket to form a boiling chamber
33~ boiling chamber
34. gasket to orm a condensing chamber
35. gas-vapor outlet from the gas-liquid separator
36. condensing chamber
37. opèning in the front end plate for the feed l~quid
38. holes in the sheets to allow feed liquid to enter the core
39. barrier to prevent the feed liquid from entering the condensing
chambers
40. outlet manifold to allow the product to ieave the condensing chami~ers
41. openin~s in the gasket to allow thç feed liquid to enter the boiling
chambers
42. seal to hold liquid and vapors inside the system
.
~3
.....
Ei2~0~3
61
43. holes in the sheets to allow steam to leave boiling chambers
44. opening in the front end plate allowing steam to leaYe boiling chambers
.~ 46. opening in the compressor manifold
47. front compressor manifold
, 48. rear compressor manifold
49. holes in the sheets to allow steam to re-enter the core
50. assembly holes in the front end plates
51. barrier to prevent the compressed steam from entering the boiling
chambers
52. intake manifold for spreading the compressed steam in the condensing
chambers
S3. holes in the sheets to allow the product to leave the condensing
chambers
54. opening in the front end plate for the product
55. assembly holes in the compressor manifold
56. holes in the sheets to allow the blowdown to leave the core
57. opening in the front end plate for the blowdown
58, spill tube to regulate liquid level in the boiling chambers
59. assembly holes at the periphery of the sheets
60. openings in the gasket to allow the blowdown to leave the boiling
chambers
61. assembly holes in the center of the sheets
62. ~pacers to hold the sheets apart
63. holes in all the sheets to allow the feed liquid to enter the core
64. inlet hose for the feed liquid
6S. core
66. opening in the front end plate for the feed liquid
67. condensing chamber
68. barrier to prevent the feed liquid frorn entering the condensing
chambers
6~. boiling chamber
70. gasket to form a boiling chamber
71. intake manifold to allow the feed liquid to enter boiling chambers
72. openings in the intake manifold
73. heat transfer sheet
74. heat transfer sheet
75. gasket to form. a condensing chamber
;,'~`~
2~
62
76. heat transfer sheet
77. heat transfer sheet
7S. ~askets
79. chamber for the blowdown
80~ barrier to prevent the blowdown from entering the condensing
chambers
81. chamber for the feed liquid
8æ chamber for the product
83. chamber for the feed liquid
84. inlet holes in the sheets for the feed liquid
86. ou~let holes in the sheets for the product
87. outlet holes in the sheets for the blowdown
88. inlet holes in the sheets for the blowdown
89. inlet holes in the sheets for the product
~lo outlet holes in the sheets for the feed liquid
92. assembly holes in the sheets
93. opening in the gasket to allow feed liquid to enter its chambers
94. opening in the end plate tl7) to allow feed liquid to enter the heat
exchanger
96. barrier to prevent the feed liquid from entering the chamber for the
blowdown
97. opening in the gasket (78) to allow the feed liquid to enter its chambers
98. opening in the end plate ~17) to allow the feed liquid to leave the
heat exchanger-
1 00. boiler
101. barrier to prevent the product from entering the chambe~r for theblowdown
102. opening in the end plate (17) to allow the product to enter the heat
exchanger
103. barrier to prevent the product from entering the chamber for the
feed liquid
104. openings in the ~asket (78,) to allow the product to enter its chambers
105. condenser
106. openin~ in the end plate (17) to allow the product to leave the heat
exchanger
~Z62 L08
63
109. opening in the end plate (17) to allow the blowdown to enter the
heat exchanger
110. gaske ts
111. openings in the gasket (7~) to allow the blowdown to enter its
chambers
112. opening in the end plate (17~ to allow the blowdown to leave the
heat exchanger
114. spiral core
11 5. gaskets
116. spiral heat exchanger
117. opening to allow the feed liquid to enter the heat exchanger
118. hose carrying feed liquid from the heat exchanger to the core
11~. metal tubes to spill the feed liquid near the top of the boiler
1 21. compressor
122. compressor motor
1 23. demister
124. compressor manifold
1 25. compressor
126. hose carrying product from the condenser to the heat exchanger
127. hose carrying blowdown from the boiler to the heat exchanger
128. core
1 29. Yat
130. gasket to form a condensing chamber
131, lid
132. compressor motor
133. tube to carry distilled water out of the condenser
134. demister
135. gasket to form a condensing chamber
136. hole to allow the distilled water to leave the cond~nslng chambers
137, barrier to prevent the distilled water from ~nterln~ the boiling
chambers
138. heat transfer sheet
139. case
140, spacers
14 1. compressor
`
~;21~38 -
.
142. opening in the case for inserting the solids to be dried
1 43. door
144. hole to allow vapors to leave the evaporator
145. compressor motor
14G. hole to allow distilled liquid to leave the condenser
147. evaporator
148. condenser
149. heat transf er sheet
150. hose connecting pressure switch with condenser
151. heat transfer sheet
152. gasket to form a boiling chamber
153. boiling chamber
lS4. front end plate
lS5. opening in the end plate through which hose connects pressure switch
to condenser
156. gasket to form a condensing chamber
lS7. condensing chamber
158. turbin.e
159. pressure switch
160. holes in the sheets connecting pressure switch to condenser
161. rear end plate
162. inlet hose for the salt water
1 63. heater
164. opening in the end plate to allow salt water to enter the core
165. holes in the shee~s connecting boiler to fresh water overflow
166. holes in the sheets to allow the salt water to enter the core
167. seal to hold liquids and vapors inside the system
168. barriers to prevent the salt water from entering ~he boiling chambers
169. openings in the sheets to allow the salt water to en~er the condensing
chambers
170. opening in the gasket to allow fresh water to flow toward spill tube
171. spill tube to regulate the height of salt wa.er in the condensin~
chambers
172. inlet hose for the fresh water
173. opening in the end plate (154) to allow fresh water to enter the core
174. holes in the sheets to allow the fresh water to enter the core
17S. hose connecting spill tube to boiler for pressure referent
.~ .
126Z~(18
176. barriers to prevent the fresh water from entering the condensing
chambers
177. openings in the gaskets ~15~) to allow the fresh water to enter the
boiling chambers
17S. spill tube to regulate the height of fresh water in the boiling chambers
179. opening in the end plate through which steam enters the top of the
spill tube assembly
180. opening in the end plate allowing fresh water to flow toward the
spill tube
1~1, hose to carry fresh water overflow out of the core
182. outlet manifold to allow steam to leave the boiling chambers
183. holes in the sheets to allow steam to leave the core
184. barrier to prevent steam from entering the condensing chambers
185. hose to carry fresh water toward the spill tube
1~6. opening in the end plate (154) to allow steam to leave the core
187. manifold to convey stearr toward the turbine
188. hose to carry steam toward the turbine
189. hose to carry steam away from the turbine
190. barrier to keep fresh water out of the condensing chambers
191. opening in the end plate (154) to allow steam to re-enter the core
192. holes in the sheets to allow steam to re-enter the core
193. barrier to prevent steam from entering the boilin~ chambers
194. inlet manifold to spread steam throughout the condensing chambers
195. hole in the sheets connecting boiler to spill tube
196. holes in the sheets to allow diluted salt water to leave the core
197. opening in the end plate (154) to allow diluted salt water to leave
the core
198. outlet hose for diluted salt water
199~ assembly holes in the sheets
200. assembly holes in the end plates
201. expander (includes 187, 188, 158, and 189)
202. barrier to keep the diluted salt watèr from entering the boiling
chambers
203~ opening in the gasket to allow diluted salt water to leave the
condensin~ chambers
204. spacers ~o keep the sheets apart