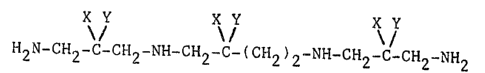Note: Descriptions are shown in the official language in which they were submitted.
PATENTS
i31,Eiff.``
GEM-DIHALO AND TETRAHALO-1!12-DIAMINO-4,9-DIAZA-DODECANES
8ACKGROUND AND DESCRIPTION
It is a well known observation that the biosynthesis
of natural polyamines, such as putrescine, spermidine and
5 spermine, is elevated in rapidl~ proli~erating cells
relative to norma~ quiescent cel1s. Conversely, it is also
known that the depletion of putrescine and spermidine leads
to a reduction in cell proliferation.
Ornithine is the metabolic precursor o~ putrescine,
10 which in turn, is the metabolic precursor of spermidine,
which in turn, is the metabolic precursor of spermin~.
Metabolically, these biochemical conversions are catalysed
by the enzymes ornithine decarboxylase, spermidine synthase
and spermine synthase, respectively. Additionally, spermi-
15 dine and spermine synthase enzymes utilize decarboxylated-
S-adenosyl-L-methionine as a co-substrate, the reaction
product of the S-adenosyl-~-methionine decarboxylase
enzyme. Inhibitors of these enzymes, including inhibitors
of S-adenosyl-L-methionine decarboxylase therefore, should
20 serve to prevent the biosynthesis of putrescine and the
higher polyamines derived therefrom, viz, spermidine and
spermine, and should, theoretically, be effective as
antiproliferative agents and~or an~itumor agents.
However, in the past, the use of irreversible
; 25 ornithine decarboxylase inhibitors or inhibitors of S-
adenosyl-L-methionine decarboxylase, spermidine synthase
and spermine synthase have not proven to b~ totally
effective. Thus, for example, putrescine and spermidine are
; C-34,435 US
PATENTS
not essential for the maintenance of cell viability as long
as the preexisting spermine pool i~ maintained above a
certain critical level. M~reover, a total in vivo
inhibi~ion of the decarboxylase enzymes is difficult due to
5 their rapid turnover.
Applicants have discovered a class of compounds which
antagonize spermine functions in the cell. These compounds
are highly effective inhibitors o~ cell growth in rapidly
proliferatiny cells. Accordingly, the compounds of this
10 invention are use~ul as antiproliferative and antitumor
agents.
SUMMARY OF THE INVENTION
The present invention relates to certain selective
gem-dihalo and tetrahalo derivatives of spermine. More
15 particularly this invention relates to gem-dihalo or
tetrahalo-1,12-diamino-4,9-diaza-dodecane derivatives
having the ~ormula
X Y X Y
~ / CH NH-~H -C-( CH -N \ ~
2 2 2 2~ 2)2H2 C CH2 NH2
X Y
(~)
wherein X and Y represent hydrogen or halogen, with the
proviso that in the case of the dihalo derivatives both
halogens are present on one and only one carbon atom, and
25 in the case of the ~etrahalo derivatives the compounds are
2,2,11,11-halo-substituted; and the pharmaceutically
acceptable salts thereof.
-- 2 --
C-34,435 ~S
~S~4 PATENTS
Additionally, certain aspects o~ this invention are
directed to a process for the preparation o~ the compounds
herein described, pharmaceutical compositions containing
the same, and the use o~ these compounds as antitumor
5 agents.
DETAILED DESCRIPTION OF THE INYENTION
As indicated in general formula ~1) above the
compounds o~ ~his invention ~orm a speci~ic class of gem-
dihalo or gem-tetrahalo-spermines together with their
10 pharmaceutically acceptable salts. As used throughout,
however, a more definitive nomenclature will be employed,
and the compounds will be designated as derivatives of
gem-dihalo or gem-tetrahalo-1,12-diamino-4t9-diaza-
dodecanes. When used herein, the term halo refers solely to
15 chloro and fluoro derivatives.
All of the compounds encompassed within this invention
are specific gem-dihalo derivatives or specific gem-
tetrahalo derivatives. That is to say, the compounds of
this invention are limited to either the 2,2-dihalo, the
20 6,6-dihalo or the 2,2,11,11 tetrahalo derivatives of 1,12-
diamino-4,9-diaza-dodecane, as indicated by the proviso
limitations. In view o~ the symmetrical nature of the
molecule, the 2,2-dihalo deriva~ives are identical with the
11,11-dihalo derivatives, and such derivatives shall be
25 designated throughout as 2,2-dihalo derivatives.
It will be noted that in the case the 2,2,11,11-
tetrahalo derivatives due to their method of preparation,
the substitutions at the 2 and 11 carbon atoms will be
symme~rical. That is to say, the same substitution occuring
30 at the 2-position must occur also at the ~1-position.
C-34,435 US
.,~.,~
65~ PATENTS
The compounds of this invention encompassed within the
scope of Claim 1 include:
2,2-difluoro-1,12-diamino-4,9-diaza-dodecane,
2,2-dichloro-1,12-diamino-4,9-diaza-dodecane,
2-chloro-2-~luoro-1,12-diamino-4,9-diaza-dodecane,
6,6-difluoro-1,12-diamino-4,9-diaza-dodecane,
6,6-dichloro-1,~2-diamino-4,9-diaza-dodecane,
6-chloro-6-fluoro-1,12-di~mino-4,9-diaza-dodecane,
2,2,11,11-tetra~luoro-1,12-diamino-4,9-diaza-
dodecane,
2,2,11,11-tetrachloro-1,12 diamino-4,9-diaza-
dodecane, and
2,11-dichloro-2,11-difluoro-1,12-diamino-4,9-
diaza-dodecane.
The pharmaceutically acceptable salts include those
non-toxic organic or inorganic acid addition salts of the
base compounds o~ Formula (1) above. Illustrative inorganic
acids include hydrochloric, hydrobromic, sulfuric and
phosphoric acids as well as metal salts such as sodium
20 monohydrogen orthophosphate and potassium hydrogen sulfate.
Illustrative organic acids which form suitable salts
include the mono, di and tricarboxylic acids, such as
acetic, glycolic, lactic, pyruvic, malonic, succinic,
glutaric, fumaricg malic, tartaric, citric, ascorbic,
25 maleic, hydroxymaleic, ben~oic, p-hydroxybenzoic, phenyl- -
acetic, cinnamic, salicylic, 2-phenoxybenzoic and sulfonic
acids such as methane sul~onic acid and 2 hydroxyethane
sulfonic acid. Such salts can exist in either a hydrated or
substantially anhydrous form.
All of the compounds of this invention are prepared in
a logical sequence starting with the correspondiny 2,2-
dihalo-1,4-butanediols having the ~ormula
C-34,435 US
PATE~TS
HO-C~2-C-(CH ) -OH
X1 Y1
(2)
wherein X1 and Y1 represent chlorine or fluorine. These
5 compounds are readily prepared by reacting the appropriate
2,2-dihalosuccinic acid with trifluoroacetic acid to form
the corresponding 2,2-dihalosuccinic anhydrides. Cleavage
of the anhydrides with methanol results in the formation of
the corresponding methyl 2,2-dihalosuccinates. Reduction of
10 the corresponding free acid function with borane-methyl
sulfide complex results in the formation of the
corresponding alcohols, i.e., the methyl 2,2-dihalo-4-
hydroxybutanoates. ~eduction of the ester function, for
example using sodium borohydride, results in the formation
15 of the desired starting materials, i.e., the 2,2-dihalo-
1,4-butanediols (2~.
The 6,6-dihalo-1,12-diamino-4,9-diaza-dodecane
derivatives of this invention can be prepared in accordance
with the following synthetic sequence wherein:
20 X1 and Y1 = chlorine or fluorine
O
Pht =
101
25 Mes = CH3-S-
O
Tos = CH3 ~ S
~ ~B C~34,435 US
PATENTS
X,~ Y~ X~ Y~ X~ Y1
~0-CH2-C-~ C~2 )2-8 - ~ MesO-CN2 C ~ CN2 )2 PhtN-C112-C-~ CN2 )2-NPht
2 3
2 X~ Y1 x~
IlN-cH2-c-~c~2)2N~8 t' N2N Cli2-~--~C82)2 N~2
Tos Tos
6 5
\Br( Cit2 )3NP-nt
3 PhtN-~ C82 )3--~--C~2--C-~ CN2 ~2-N ( C}12 )3
Tos Tos
4 H2N-~ CN2 )3--NU--CH2--C--~ CH2 )--NH--( CH2 )3-NH2
.
C-34,43~ US -6
; PATE~ITS
~ J
The compounds 2,2-dihalo-1,4'bu~anediol (2) are
treated wi-th two equivalents of methanesulfonyl chloride in
the presence of pyridine to form the corresponding 1,4-
bis-methanesul~onyloxy-2,2-dihalobutanes (3). Reaction of
5 (3) with two equivalen-ts of potassium phthalimide in a
solvent such as dimethylformamide results in the formation
of the corresponding 2,2-dihalo-1,4-diphthalimido-butanes
(_). Heating of (~) with hydrazine in a solvent such as
ethanol removes the phthaloyl protecting groups to form the
10 key in-termediates, 2,2-dihalo-1,4-diaminobutanes (5~, which
can be readily isolated as their dihydrohalide salts. The
compound 2,2-difluoro-1,4-diaminobutane is a key interme-
diate useful in the preparation of 6,6-difluoro-1,12-
diamino-4,9-diaza-dodecanes. Inasmuch as these 2,2-dihalo-
15 1,4-diaminobutanes, represent such useful and novel
compounds, they are specifically claimed herein as a part
of the instant invention.
To avoid obtaining higher alkylated products, the
primary amines of (5) are protected in a standard manner
20 with p-toluenesulfonyl chloride to yield the corresponding
2,2-dihalo-1,4-di-p-toluenesulfonylamino-butanes (6).
Alkylation of the protected amines (6) with two equivalents
of 3-bromopropylphthalimide under anhydrous conditions in a
suitable aprotic solvent, such as dimethylformamide in the
25 presence of sodium iodide and potassium tert-butoxide,
yields the corresponding 6,6-dihalo-1,12-diphthalimido-
4,9-di-p-toluenesulfonyl-4,9-diaza-dodecanes (7).
These 4,9-diaza-dodecane derivatives (7) are
deprotected in two steps, the first step involves heating
30 with hydrazine in a solvent such as ethanol to remove the
phthaloyl groups. The products so obtained are then heated
with aqueous HBr to remove the protecting tosyl moieties.
The final products obtained in this manner are the
corresponding 6,6-dihalo-1,12-diamino-4,9-diaza-dodecanes
g ~ C-34,435 US
~L~:~i53L~, PATENS3
t1), ~hich can b~ readlly ~sol~tet a~ thelr corresponding
tetrahydroh~lid~ ~lts, pre~erably a8 thelr tetrahydro-
brom:~d~ salt~.
The 2, 2-dlhalo-1 ,12-diamino~,9-diaza-dodecane deriYa-
tives o~ the present invention are prepared in accordanc~
wl~h the ~ollowin~ reactlon sequence, start~ng again with
the appropriate 2, -dihalo-1 "4-butanedio~ (2~. In this
react~o~ sequenc~ ~he symbcls X~, Y1, Pht D Mes and Tos have
the sa~e meanings previollsly indicated~
X~ ,Y1
~ cH2-c -( c~2 )~ sO-C~2--C-~ C~2 )2 OUe5 ~ i~esO-C~2~=c~2
2 3 e
I
~ /~ x~, y~ ~ y
BN-C~2-C~2 ~ - H211-C82-C-CN'c82 ~ Pb'tN-CH2-C-C~=CU2
~0.
11 1~ 9
~( Cfl2 )4NP~t
~tN-(C12)~ -~2-'C-C'd~A2 ~ ~2~ 12)~-r-~2-C-CN~2
rO,i, To~
12 13
-- 8
C-34,435 ~
0,
PAT~1TS
X~
2 )3 ;`J ( CH2 )4 ~-CH2-C-cH=cH2 ~r( CH2 )3~Pht H~-( CH ) -;~-CH2-C-CH=CH2
1`5 14
X1 Y1 x~ Y~
Pht~ ~ CH2 )3 ~ ( C~2 )4 ~ C 2 Pht~l--( CH2 )3--;\1--( CH2 )4--~--CH2--C--CH20H
16 17
Pht~-( CH2 )3-~-( CH2 )4-:1-CH2-C CH2
Tos Tos
18
X;l Y1
Pht~-( CH2 )3-~1-( CH2 )4-~1-CH2 C CH2 ;1
Tos Tos
19
~ , ~/1
35 H2;`1-( CH2 )3-;lH-( CH2 )4-~H-CH2 C CH2 ;~H2
_ g _
C-34, 435 US
. PATE~TS
6~
The 2,2-dihalo-1,4-butanediols t2) are treated with
two equivalents of methanesulfonyl chloride in the presence
of pyridine to form the corresponding 1,4-bis-methane-
sul~onyloxy-2,2-dihalobutanes (33. When (3) is heated with
5 diazabicycloundecen in a solvent such as dimethylformamide,
there is obtained the corresponding 1-methanesulfonyloxy-
2,2--dihalo-3-butenes (8).
Reaction of (8) with potassium phthalimide in a
solvent such as dimethyl~ormamide results in the formation
10 of the corresponding 1-phthalimido-2,2-dihalo-3-butenes
(9). Cleavage of the phthaloyl derivative (93 with
hydra~ine in an alcoholic solvent provides the correspond-
ing 1-amino-2,2-dihalo-3-butenes ~10).
The primary amines t10) are protected by reaction with
15 p-toluenesulfonyl chloride in a standard manner to obtain
the corresponding N-(2,2-dihalo-3-butenyl)-p-toluene-
sulfonamides ~11). Alkylation of the protected amine (11)
with 4-bromobutylphthalimide in the presence of potassium
tert.-butoxide under anhydrous conditions in a suitable
20 solvent, such as dimethylformamide results in the formation
o~ the corresponding N-(4-phthalimidobutyl)-N-(2,2-dihalo-
3-butenyl)-p~toluenesulfonamides (12).
The action of hydra~ine in a solvent such as ethanol
upon (12) ~orms the corresponding 9-amino-3,3-dihalo-5-p-
25 toluenesulfonyl-5-aza-1-nonenes (13), which upon treatment
with p~toluenesulfonyl chloride yield the corresponding 9-
p-toluenesulfonylamino-3,3-dihalo-5-p-toluenesulfonyl-5-
aza-l-nonenes (14).
Alkylation of the protected amines ~14~ takes place
30 with 3-bromopropylphthalimide in the presence of potassium
tert-butoxide under anhydrous conditions in a solvent such
as dimethylformam.ide to yield the corresponding 3,3-
- 10 -
C-34,435 U~
~26S~ PATENTS
" ~
dihalo-13-phthalimido-5/10-di-~-toluenesul~onyl-5,10-
diaza-1-tridecenes (15~. Oxidation o~ the double bond t15)
with KMnO4 in an aqueous acetic acid solution results in
the formation of the corresponding 2,2-dihalo-12-
5 phthalimido-4,9-di-p-toluenesulfonyl-4,9-diaza-dodecanoic
acids (16). Reduction o~ (16) with borane-methylsulfide
complex ~orms the corresponding 2,2-dihalo-12-phthalimido-
4,9-di-p~toluenesulfonyl-4,9~diaza-dodeeanols (17).
Reaction of (17) with methanesulfonyl chloride in the
10 presence of pyridine utilizing an anhydrous solvent such as
dichloromethane results in the ~ormation o~ the correspond-
ing 2,2-dihalo-1-methanesulfonyloxy-12-phthalimido-4,9-di-
p-toluenesulfonyl-4,9-diaza-dodecanes (183.
Further reaction of (18) with potassium phthalimide in
15 anhydrous dimethylformamide results in the ~ormation of the
corresponding 2,2-dihalo-1,12-diphthalimido-4,9-di-p-
toluenesulfonyl-4,9-diaza-dodecanes (193, which can be be
deprotected in two stages, first by heating with hydrazine
in a solvent such as ethanol to remo~e the phthaloyl
20 groups, and then followed by heating the products so
obtained with aqueous HBr in order to remove the protecting
tosyl moieties. The desired products resulting therefrom
are the corresponding 2,2-dihalo-1,12-diamino-4,9-diaza-
dodecanes (1), which can be isolated as their corresponding
25 tetrahydrohalide, and more particularly, as their tetra-
hydrobromide salts.
The 2~2,11,11-tetrahalo-1,12-diamino-4,9-diaza-
dodecane derivatives of the present invention can be
readily prepared in accordance with the following reaction
30 sequence. In this sequence, the symbols Xl1 Y~, Pht, Mes
and Tos have the same meanings as previously indicated with
the proviso that both values for X1 and bo~h ~alues for Y
must be the same.
-- 1 1
~-34,435 US
,.,
P ATENTS
r( C-H2 )1~r ) CH:2=CN-C-C82-~-( C~12 ).~ -CH2-C-C~I=CH2
11 ~19
X~ ~1 X~, Y~ X~ Y1 I X~ Y~
2H0CH2-C-C~I2-N-( CH2 )4--N--CH2 C CH208 ~ HOOC--C--CH2-N-( CN2 )4-N CH2 C
21 20
X,~ Y1 X;~ /Y1
Meso-cN2-c-c~32-N-( CH2 )4-N-CH2 C C82 e
Tos Tos
22
ll PhtN-CN2-C--C~2-~3-( CH2 )4--7-C82-C-CH2-WPht
Tos Tos
Xj~
H2~1--CH2--C~C~2~N8-~ CH2 )4-NH--CN2 C CH2 2
C-34, 435 US-12~.
PATENTS
,
Two equivalents of the compounds, N-( 2, 2-dihalo-3-
butenyl)-~-toluenesulfonylamide ~11), prepared as indicated
in accordance ~ith the previous reaction sequence, can be
symmetrically dialkylated with one e~uivalent of 1,4-
5 dibromobutane under anhydrous conditions in the presence ofpotassium tert.-butoxide, utilizing a solvent such as
dimethylformamide, to ~orm the corresponding 3,3,12,12-
tetrahalo-5,10-di-p-toluenesulfonyl-5,10-diaza-1,13-
tetradecadienes ( _ ).
Oxidation of the double bonds of ~19~, utilizin~ KMnO4
in an aqueous acetic acid solution results in the formation
of the corresponding 2,2,11,11-tetrahalo-4,9-di-~-toluene
sulfonyl-4,9-diaza-1,12-dodecanedioic acids (20). Reduction
of (20) with borane-methylsulfide complex forms the
15 corresponding 2,2,11,11-tetrahalo-4,9-di p-toluene-
sulfonyl-4,9-diaza-1,12-dodecanediols (21).
Reaction of (21) with methanesulfonyl chloride in the
presence of pyridine, utilizing an anhydrous solvent such
as dichloromethane, results in the preparation of the
20 corresponding 1,12-methanesul~onyloxy-2,2,11,11-tetrahalo-
4,9-di-p-toluenesul~onyloxy-4,9-diaza-dodecanes t22).
Fur~her reaction of (22) with potassium phthalimide in
anhydrous dimethylformamide yields the corresponding 1,12-
diphthali~ido-2,2,11,11-tetrahalo-4,9-di-p-toluene-
25 sulfonyl-4,9-diaza-dodecanes (23).
The compounds (23) can be deprotected in two stages,
~irst by hea~ing with hydrazine in a solvent such as
ethanol to remoYe the phthaloyl groups, and secondly by
heating the products so obtained with aqueous HBr so as to
30 remo~e the protecting tosyl moieties. The products so
obtained are the corresponding desired 2,2,11,11-
tetrahalo-1,12-diamino-4,9-diaza-dodecanes (1), which can
be readily isolated as their tetrahydrobromide salts ~1).
- 13 -
C 34,435 ~S
PATENTS
~ he compounds o~ the present invention are useful as
antiproliferative and antitumor agents. The mechanism by
which these compounds function is not known. What is known,
however, is that when the compounds o~ ~his invention are
5 added to a culture medium of growing rat hepa~oma tissue
culture (HTC) cells, a marked reduction o~ cell growth
occurs. In combination with known ornithine decarboxylase
inhibitors, such as 2-difluoromethyl~2,5-diaminopentanoic
acid (DFMO) or ~2R,5~ -6-heptyne-2,5-diamine (R,R-MAP), a
10 ~urther inhibition of cell growth occurs.
rhe compounds of this invention have also been found
to be capable of slowing neoplastic cell proliferation when
tested in standard animal tumor models. A preferred manner
of utilizing these compounds is in combination with DFMO or
15 ~R,R~-MAP, or in combination with other t~erapeutic methods
or agents ~nown to affect polyamine metabolism in the
treatment o~ neoplasms in animals. As used herein, the term
animals is taken to me~n warm blooded mammals, such as
mice, rats/ dogs, cats, guinea pigs, co~s, horses and
20 humans.
The compounds of this invention can be utilized both
prophylactically and therapeutically. The amount of active
ingredient ~or therapeutic administration can vary over a
wide range and is dependent upon such factors as the
25 species of animal to be treated, its age, health, sex,
weight, nature and the severity of the condition being
treated. Generally, a therapeutically ef~ective amount of
the active ingredient to be administered will range from
about O.2 to 5 grams, and pre~erably from O.5 to 3 grams
30 per day. For prophylactic administration, corresponding
lower doses of from 0.05 to 2 grams per day can be
utili 7.e~ ~ .
- 14 -
C-34,435 US
PATEN1'S
When administered in combination with other ornithine
decarboxylase inhibitors, such as DFM0 or CR,~-MAP, an
amount of from 0.1 to 4 grams o~ the particular gem-dihalo
or tetrahalo-1,12-diamino-4,9~diaza-dodecane and from 0,5
5 to 10 gr~ms o~ the ornithine decarboxylase inhibitor are
administer~d per day.
The compounds o~ this invention can be ora~ly admin-
istered. Illustrative dosage levels for oral a~ministration
range from 2 to 50 mg per kg of body weight. Preferably,
10 from 10 to 20 mg per kg o~ the gem-dihalo or tetrahalo-
1~12-diamino-4~9-diaza-dodecane are orally administered per
day in divided doses. In those instances where the drug is
administered via the parenteral route, corresponding lower
doses are employed~ When administered in combination with
15 ornithine decarboxylase inhibitors, the compounds can be
administered in standard dosage unit forms, such as
tablets, capsules, dragees, lozenges, elixirs, emulsions,
suspensions and various intravenous, intramuscular or
intradermal suspensions.
When administered orally, the preferred dosage form is
that of a tablet or capsule. The amount of active
ingredient contained in each dosage unit ~ill, of course,
vary depending upon the particular species of the gem-
dihalo or tetrahalo-1,12-diamino-4,9-diaza-dodecane
25 employed, and the particular dosage form utilized.
Generally, a given dosage unit will contain from 10 to
500 mg of the active ingredient in addition to the various
pharmaceutical excipients contained therein. Tablets
containing from 100 to 400 mg of the active ingredient, are
30 the preferred dosage unit and can be administered b.i.d.,
or t.i.d. or q.i.d.
C-34,~35 US
~Z~5~6~ PATENTS
In preparing solid dose forms such as tab~ets, the
active in~redient is ~enerally blended with conventional
pharmaceutical carriers or excipients such as gelatin,
various starches, lactose, calcium phosphate or powdered
5 sugar. ~he term pharmaceutical carrier as used herein also
includes lubricants employed to improve the ~low of tablet
granulations and which prevent adhesion of tablet material
to the surfaces of tablet dies and punches. Suitable lubri-
cants include, ~or example, talc, stearic acid, calcium
1~ stearate, magnesium stearate and zinc stearate. Also
included within the de~inition of a pharmaceutical carrier
as used herein, are disintegrating agents added to assist
the breakup and dissolution of tablets following adminis-
tration, as well as coloring and/or flavoring agents to
15 enhance the aesthetic qualities of the tablets and make
them more acceptable to the patient.
Suitable liquid excipients for the preparation of
liquid dosage unit forms include water and alcohols such as
ethanol, benzyl alcohol and the polyethylene glycols,
20 either with or without the addition of a surfactant. In
general, the preferred liquid excipients, particularly for
injectable preparations, include water9 saline solution,
dextrose and glycol solutions such as an aqueous propylene
glycol or an aqueous solution of polyethylene glycol.
Liquid preparations to be used as sterile injectable
solutions will ordinarily contain ~rom about 0.5 to about
25% by weightl and preferably from about 1 to about 10% by
weight, of the active ingredient in solution. In certain
topical and parenteral preparations, various oils can be
30 utilized as carriers or excipients. Illustrative o~ such
oils are mineral oils, glyceride oils such as lard oil, cod
liver oil, peanut oil, sesame oil, corn oil and soybean
oil. For insoluble compounds, suspending agents may be
added as well as agents to control the viscosity, as for
- 16 -
C-34,435 US
i.~S~60 PATENTS
example, magnesium aluminum silicat~ or carboxymethyl-
cellulose. In addition ~o these excipien~s, buf~ers,
preservatives and emulsi~ying agents may ~lso be added.
The proportion of the active ingredient employed in
5 parenteral dosage unit forms ranges ~rom 0.05 to about 20%
by ~eight, preferably from abou~ 0.1 to about 10% by weight
of the total liquid composition, the remaining component or
components comprising any of the various pharmaceutical
excipients previously mentioned. In order to minimize or
10 elimina~e irritation at the site of injection, such
compositions may contain a non-ionic surfactant ha~ing a
hydrophile-lipophile balance (HLB) of from about 12 to
about 17. The quantity of surfactant in such formulations
- ranges ~rom about 5 to about 15% by weight. The surfactant
15 can be a single component having the above-identified HLB,
or a mixture o~ two or more components having the desired
HLB. Illustrative of surfactants useful in parenteral .
formulations are the class of polyoxyethylene sorbitan
fatty acid esters as, for example, sorbitan monooleate and
20 the high molecular weight adducts of ethylene oxide with a
hydrophobic base, formed by the condensation of propylene
oxide with propylene glycol.
The invention described herein is more particularly
illustrated in conjun~tion with the followin~ specific
25 preparation, but is not necessarily limited thereto.
- 17
C-34,435 ~S
, :
~ PATENTS
PREPARATXO~ OF 6,6-DIFLUORO-1,12-DIAMINO-4~9-D~AZA-DODECANE
2,2-Difluoro-1,4-butanediol
. . .
2,2-Difluorosuccinic acid t120 g/ 0.78 moles) and
trifluoroacetic anhydride (540 mL) are reflux~d (bath
5 temperature 80C) for 2 hours. Most of the tri~luoroacetic
acid is distilled utilizing a short Vigreux column, the
~inal traces are removed under vacuum t12 mm Hg, 50C) and
finally by stripping twice with carbontetrachloride. The
- oily residue solidifies on scratching with petroleum ether.
10 Filtration and washing with petroleum ether yields 2,2-
difluorosuccinic anhydride as slightly violet crystals:
98 g (92~).
The 2,2-difluorosuccinic anhydride (98 g, 0.72 moles)
is dissolved in dichloromethane and slowly added with
15 stirring to methanol, cooled in an ice bath. The mixture is
kept at room temperature overnight, evaporated and stripped
twice with carbon tetrachloride to yield methyl 2, 2-
difluorosuccinate as a slightly brownish oil: 121 g t100%).
In a 4 L flask equipped with a reflux condenser and
20 1 L dropping funnel, a solution of BH3.Me2S complex (10 Ml
88 mL) in dry dichloromethane (1 L) is slowly added o~er a
2 hour period to a stirred solution o~ the methyl 2, 2-
di~luorosuccinate (120 g, 0.714 moles) in dry tetrahydro-
furan at 20C. After re~luxing (bath temperature 80C) for
25 about 15 hours, the mixture is allowed to cool to room
temperature and methanol t1 L) is slo~ly added. Evaporation
yields methyl 2,2-difluoro-4-hydroxybutyrate as an oil
which is stripped with methanol (1 L) and finally with CCl4
to yield a yellow oil: 100 g t92%).
- 18
C-34,4~5 US
~ O PATENTS
To a cold (0C) solution of sodium borohydride
(10.3 g, 0.272 moles) in ethanol, a solution of the methyl
~ difluoro-4-hydroxybutyrate (55 g, 0.36 moles) in
ethanol is slowly added, while maintaining the internal
5 temperature of the reaction mixture between - 5C and 0C.
The mixture is stirred ~or 1 hour at 0C, then, an approx-
imately 4 N solution of HCl gas in methanol (200 mL) is
carefully added. Sodium chloride is filtered, the methanol
is removed under vacuum, and the residue is dissolved in
10 ethanol. Additional NaCl is again removed by filtration
(membrane filter) and evaporation of the ~iltrate yields
the compound 2,2-difluoro-1,4-butanediol as a colorless oil
when distilled in a Kugelrohr at 150C/0.05 mm H~; 41 g
~ 90% ) .
15 2,2-Difluoro-1,4-diaminobutane
To a stirred mixture o~ 2,2-di~luoro-1,4-butanediol
(12.97 g, 103 mM), dry pyridine (65 mL) and dichloromethane
(200 mL), is slowly added a solution of methanesul~onyl
chloride (23.6 g) in dichloromethane (50 mL). Stirring is
20 continued overnight, and the mixture is washed with 2 N HCl
(twice), 10% aqueous NaHC03 until neutral, and finally with
water. The organic solution is dried (MgS04) and evaporated
to yield 1,4-bis-methanesulfonyloxy-2,2-difluorobutane as a
slightly yellow oil ~25.4 g, 87.5%) which crystallizes on
25 scratching with dry ether: white crystals, 22.3 g (77%).
The compound 1,4 bis-methanesulfonyloxy-2,2-difluoro-
butane (66 g, 234 m~), potassium phthalimide (97 g, 10%
excess) and dry DMF (700 mL) are stirred and heated (bath
temperature ~10C) under nitro~en for 110 hours. Most of
30 the DMF is removed under vacuum (oil pump) and water is
added to the residue. The precipitated material is
filtered, washed three times with water, dissolved in
chloroform, and washed with 1 N NaOH (twice) and brine
-- 19 --
C-3~,435 US
~ PATENTS
(twice). After dryin~ the organic solution (Na2S04) and
concentrating, the cornpound 2,2-di~luoro 1,4-
diphthalimido-butane begins t.p crystalliz~. Hexane is
added, the solid material which results i5 collected and
5 washed with ether to yield white crystals: 69.55 g t77%).
Under nitro3en and with e~ficient stirring a mixture
of 2,2-difluoro-1,4-diphthalimido-butane: (69.55 g
(180,6 mM), tetrahydrofuran (361 mL) and 361 mL of a 1 N
solution of hydrazine hydrate in ethanol are heated
10 overnight at 90C ~bath temperature~. After addition of 6 N
HCl (700 mL), stirring and heating are continued for
1~ hours. The reaction mixture is cooled tice bath), and
the precipitate is filtered (ethanol wash). The filtrate is
evaporated, the residue is taken up in water, ~iltered, and
15 evaporated again. This procedure is repeated once again
using a membrane filter (Millipore). The residue is
stripped with isopropanol (twice) and ethanol (twice) to
remove the final traces of water. Digestion with ethanol
and subsequent filtration of the product so obtained,
20 results in the formation o~ white crystals that are
successively washed with ethanol, acetone and ether: 30.9 g
(87%~. This material is recrystallized by dissolving in the
minimum amount of hot water (approximately 50 mL),
filtering and adding ethanol to form pure 2,2-difluoro-
25 1,4-diaminobutane as the dihydrochloride salt: 22 g
(61.8%).
Calc~d for C4H12C12F2N2 C,24.38; H 6 14; N 14 22
Found: C,24.33 H,5.19; N,14.24
- 20 -
C 34,435 US
..
~2~5~6~ PATENTS
6,6-Dl~luoro-1,12-dlphthalimido-4,9-di-E~-toluenesulfonyl-
4,9-diaza-dodecane
To a mixture o~ 2,2-difluoro-1,4-diaminobutane
dihydrochloride ~1.57 g, 8 mM), triethylamine (3.23 g,
5 4 equivalents) and dry dichloromethane is slowly added with
stirring a solution o~ toluenesulfonyl chloride ~3.04 g,
2 equivalentsl in dichloromethane. After stirring overnight
at room temperature, additional dichloromethane is added.
The reaction mixture is washed with 1 N HCl ( twice), brine
10 (twice), dried (Na2S04) and evaporated to yield white
crystals: 3 g. The resulting material is digested with
ether and thoroughly washed with ether to yield pure 2,2-
difluoro-1,4-di-p-toluenesulfonyl~mino-butane: 2.45 g
(71%).
Under nitrogen, potassium tert-butoxide (1.3 g,
2 equiYalents) is added to a stirred solution of 2,2-
difluoro-1,4-di-p-toluenesulfonylamino-butane (2.45 g,
5.67 mM) in dry dimethyl~ormamide (5 mL). After 30 minutes
at room temperature, additional dimethylformamide (5 mL),
20 N-3-bromopropylphthalimide (3.04 g, 2 equiYalents~, and
sodium iodide (170 mg) are added. The mixture is stirred
overnight, the dimethylformamide is removed under vacuum
(oil pump). Water ( 250 mL ) is added to the residue, and the
precipitate is filtered, washed with water and dried over
25 P205: 4.57 g. The crude material is recrystallized ~rom
chloroform/ether to yield pure 6,6-difluoro-1,12-
diphthalimido-4,9-di-p-toluenesulfonyl-4,~-diaza-dodecane
as whi~e crys~als: 3.45 g (75%).
6 ? 6-Difluoro-1,12-diamino-4,9-diaza-dodecane
A mixture of 6,6-difluoro-1,12-diphthalimido-4,9-di-
p-toluenesul~onyl-4,9-diaza-dodecane (3.4 g, 4.22 m~),
ethanol (8.5 mL) and 8.5 mL of a 1 N solution of hydrazine
- 21 -
C-34,435 US
~2~ PATENTS
hydrate in ethanol is s~irred and heated ~ bath temperature:
90C) overnight. Evaporation and stripping twice with
rnethanol yields a residue which is dissolved in a mixture
of water ~20 mL), methanol (20 mL), and concentrated HCl
(40 mL) t and heated ( bath temperature: 90C) under reflux
for 3 hours. The reaction mixture is cooled, filtered and
evaporated, and the residue i's dissolYed in hot 1 N HCl.
The solution is filtered, the filtrate is evaporated, and
the residue is stripped with isopropanol ~twice) and
10 ethanol (twice). The residue is dissolved i~ ethanol 9 ether
is added, and upon evaporation of the solvent, there is
obtained 1,12-diamino-6,6-difluoro-4,9-di-p-toluene-
sulfonyl-4,9-diaza-dodecane as a white crystalline ~oam:
2.7 g (quantitative yield).
The compound 1,12-diamino-6,6-difluoro-4,9-di-p-
toluenesulfonyl-4,9-diaza-dodecané (2.7 g, 4.36 mM) and 47%
aqueous HBr are refluxed (bath temperature: 110C) for
17 hours. After cooling to room temperature, the solution
is carefully extracted with ether (three times) and finally
20 with chloroform. Evaporation of the aqueous phase, followed
by stripping with isopropanol (twice~ and ethanol (twice)
produces a solid residue which is digested with acetone,
collected, and washed with acetone (three times), ethanol
(three times) and finally with ether: white crystals, 1.8 g
25 (74%). This materi~l is recrystallized by dissolving in hot
(80C) aqueous ethanol (100 mL, H20: ethanol 1:9),
filtering through paper, and adding additional ethanol
(10-20 mL) to yield the desired 6,6-difluoro-1,12-diamino-
4,9~diaza-dodecane as the tetrahydrobromide salt: white
30 crystals 1.2 g, (49%).
10~28Br4F2N4 C~21.37; H,5.02; N,9,g7
~ound: C,20.97; H,4.75; N,10,01
- 22 -
C-34,435 US
~ PATENTS
PREPARATION OF 2,2,11,11~TETRA~LUORO-4,12-DIAMINO-4~9-
DIAZA-OCTANE
, . _
1-Phthalimido-2,2-difluoro-3-butene
To a stirred mixture o~ 2,2-di~luoro-1,4-butanediol
5 (12097 g, 103 mM), dry pyridine t55 mL) and dichloromethane
(200 mL) is slowly added a solution of methanesulfonyl-
chloride (23.6 g) in dichloromethane (50 mL). Stirring is
continued overnight. The reaction mixture is wasbed with
2 N HCl (twice)l ~ollowed by 10~ aqueous NaHC03 until
10 neutral, and ~hen with water. The organic layer is dried
(MgS04) and upon evaporation o~ the solvents, a slightly
yellow oil is obtained. Crystallization is induced by
scratching the oil ~ith dry ether, to yield the compound
1,4-bis-methanesul~onyloxy-2,2-difluorobutane as white
15 crystals: 22.3 g (77%).
The compound 1,4-bis-methanesul~onyloxy-2,2-difluoro-
butane (20 g, 71 mM) and diazabicycloundecene (21.6 g,
142 mM) are stirred with dry tetrahydrofuran ~150 mL) and
heated overnight at 80C under nitrogen. The solvents are
20 removed under vacuum3 and the residual oil is dissolved in
dichloromethane, washed ~ith 1 N HCl (twice), brine (twice)
and dried (Na2S04). E~aporation of the solvents yields the
compound 1-methanesulfonyl-2,2-difluoro-3-butene as an oil:
11.2 g (85%).
The compound 1~methanesulfonyl-2,2-difluoro-3-butene
(11.2 g, 60.2 mM), potassium phthalide (12.3 g, 66.4 mM)
and dry dimethylformamide (30 mL) are stirred and heated
(bath temperature 110C) under nitrogen ~or 120 hours.
After cooling to room temperature, the crude reaction
30 product is precipitated by the addi~ion of water
(approximately 300 mL), ~iltered and dissolved in
- 23 -
C-34,435 US
, "
A~ ~ 5 ~ Q PATENTS
dichloromethane. The dichloromethane solution is washed
with l N potassium hydroxide ~twice3, water (twice), dried
(Na2S04) and evapora~ed to yield 1-phthalimido-2,2-
difluoro-3-butene as beige-colored crystals: 11.9 g (83%).
5 1-p-Toluenesul~onamino-2,2-dlfluoro-3-butene
The compound 1-phthalimido-2,2-difluoro-3-butene
(~1.4 g, 48.1 mM) is heated for 20 hours. After cooling in
an ice bath, the phthalic acid is removed via filtration
and the filtrate e~aporated. The residue so obtained is
10 dissolved in water, extracted with ether (twice),
evaporated to dryness and stripped with isopropanol.
Trituration with ether yields hygroscopic crystals of 1-
amino-2,2-di~luoro-3-butene as the hydrochloride salt:
6.12 g, (88%).
To a stirred mixture of 1-amino-2,2-difluoro-3-butene
hydrochloride (6.1 g, 42.5 ~M), 50 mL of dry dichloro-
methane and triethylamine t8.74 g, 2 equivalents), all of
which have been cooled in an ice bath, is slowly added a
solution of tosyl chloride (8.1 g, 1 eq~ivalent) in 50 mL
20 of dichloromethane. Stirring is continued overnight at room
temperature, additional dichloromethane is added to the
~ reaction mixture, and the resulting organic layer is washed
; with 1 N HCl (twice), water (twice) and dried (Na2S04).
Evaporation of the solvent yields a brown semi-solid
25 material (~.66 g~, which is purified via ~lash
chromatography on silica (300 g, eluent: ethyl
acetate/petroleum ether 20-80; fraction size 100 mL).
Fractions 17-25 are combined and evaporated to yield 1-p-
toluenesulfonamino-2,2-di~luoro-3-butene as white crystals;
30 4.8 g (43%). The desired compound can be recrystallized
from ether/petroleum ether to yield very ~ine, cotton-like,
needles.
- 24 -
C-34,435 US
~ ~ S ~ ~ ~ PATENTS
. .
Anal- Calc'd ~or C11H13F2N2S C,50.56; H95-02; N~5-36-
Found: C,50.93; H,5.02; N,5.45.
Following essentially the same procedure ~or the
preparation of the 2,2-difluoro-4912-diamino-4,9-diaza-
5 dodecane, two equivalents of the 1-~-toluenesulfonamino-
2,2-difluoro-3-butene, prepared above, is alkylated with
one equ~valent of 1,4-dibromobutane; the double bonds are
oxidized to the corresponding dicarboxylic acid with ~MnO4
in aqueous acetic acid; the resulting dicarboxylic acids
10 are reduced to the corresponding primary diols with
borane-methylsulfide complex; the diols are mesylated with
methanesulfonyl chloride; the mesyl derivative is reacted
with potassium phthalimide to obtain the corresponding
1,12-diphthalimido deri~ative; and the phthaloyl and tosyl
15 protecting groups are removed to obtain the desired
2,2,11,11-tetrafluoro-1,12-diamino-4 9 9-diaza-dodecane.
DEMONSTRATION OF THE ANTIPROLIFERATIVE EFFECT OF ~,6-
DIFLUORO-l,12-DIAMINO-4,9-DIAZA-DODECANE
.
Morris rat hepatoma rl288C (HTC) cells are routinely
20 grown as a suspension culture in Swim's 77 medium supple-
mented with 10% (V/V) dialysed horse serum, 11.0 mM
glucose, 2 mM glutamine, 0.057 mM cystine, 5.g mM NaHC03
and 50 mM o~ N-tris(hydroxymethyl)methylglycine. The HTC
cell cultures are incubated in the presence or absence of
25 1 m or 10 m of the compound 6,6-difluoro~1,12-diamino-
4,9-diaza-dodecane and observed ~or a period of 11 days.
The cell culture medium is changed at day 2, to
maintain cells in a logarithmic phase of gro~th. The actual
cell numbers are determined by cell-counting and the
- 25 -
C-34,435 US
.
",
~q ~ PATENTS
. i ~ !
relative cell growth is calculated taking into account the
various dilution factors employed. The per cent inhibition
of cell ~rowth is calculated according to the equation~
rNtn-NtO
100 - 100 . _
N n- N O
wherein
NcO is the relatiYe growth of control cultures at
time = 0,
Ncn is the relative growth of control cultures at
time = n,
NtO is the relative growth of test cultures at
time = 0, and
Ntn is the relative growth of test cultures at
time = n.
:
:; 15 Table I illustrates that administration of 1 ~ m of
6,6-difluoro-1,12-diamino-4,9-diaza-dodecane to the culture
medium inhibits cell growth by 54% at the end of 4 days,
whereas the administration of 10 ~m of the drug inhibits
cell growth by 77% at the end of the same period of time.
- 26 -
C-34,435 US
.
... .
o
___ ___ _______
o ~oo ~
C~J h P O ~9 o ~ a~
,_ C!s E~ o c~> u~ C'J cr~
Q ~ ~ N
l O O ~
~ ~ O co ~
O O
~ __ ___ _______
4~ ' ~1
_ O~
a _ ____~
-- 2 7
Another aspect o~ this invention i~ the u8e of the
fluorinated compounds disclosed herein a6 Nuclear Magnetic
Resonance (NMR) ima~ing agents useful for the detection
and diagnosis of tumor tissue by means of Fl9 NMR in ViYo
spectroscopy and tumor imaging by Fl9 NMR tomography.
Although all of the fluorinated compounds of this
invention are useful as such NMR agents ~or the detection
an~ for the accurate determination of the precise location
of any tumor existing in the mammal sought to be diagnosed
and/or treated, those most active compou~ds useful as
anti-proliferative and anti-tumor agents, are most
preferred as NMR tumor imaging agents. Another preferred
class of NMR imaging agents useful for the ~etection and
location of any mammalian tumors are the difluoro
intermediates used to prepare the fluorinated compounds of
formula I, especially 2,2-difluoro-1,4-butane diamine.
For this end-use application the compounds may be
administered in the range of 0~2 to 5 g.
C-3~,435 -28-
. .