Note: Descriptions are shown in the official language in which they were submitted.
~2~;5~7~
The invention relates to an electrode of
reversible polarity for electrochemical use, expecially to
be used in an electro-flotation process, which electrode
comprises a carrier, an intermediate layer and an
electrocatalytic top layer.
The invention is directed to electrodes o
reversible polarity i.e. those which can work successively
as an anode and as a cathode within a given amount of
time. Applications of such electrodes are also taught
herein-
There are numerous electrochemical methods inwhich a certain content of calcium and magnesium can be
eliminated from the electrolyte.
An example of such a method is, firstly, the
production of hypochlorite by the electrolysis of sea
water, and secondly, the electrolysis of waste water
whereby oxygen and hydrogen are produced which, in turn,
are used for electro-flotation.
In such cases, compounds containing calcium
and/or magnesium often deposit on the cathode, thus
strongly restraining further flow of current through the
cathode and causing an intolerable increase in the cell
voltage.
Water softening is a third problem. However, in
this case the calcification of the cathodes is a desirable
efect since the depositing of calcium and or magnesium
compounds on the cathode leads to a decrease in water
hardness.
Various methods of the removal of calcium
containing deposits have been proposed thus far:
a) scrubbing the electrolysers with an acid
(preferably HCl) from time to time or periodical cleaning
with hydrochloric acid.
b) applying an auxiliary cathode to function a~
an anode against the actual cathode, wherein the auxiliary
~s~
-- 2 ~
cathodeand the actual cathoceare periodically automatically
interchanged in ihe electrical sense (West German
Offenlegungsschrift 3043571, issued July 1,1982, inventor ~.J. Jansen)
c) automatic mechanical cleaning of the
electrodes.
d) an electrode of reversible polarity, made of
a solid noble metal or provided with a noble-metal coating
of such a thickness that neither the electrolyte nor
daposits, if any, can penetrata the coating.
The method a) involves temporary interruption of
the operation and also storage as well as disposal of
hydrochloric acid. I~ is, therefore, practicable to use
this method only in l~rge plants where permanent staff is
available.
lS The method b) calls for complex mechanical and
electrical equipment; moreover, an increased corrosion of
the cathodes cannot be avoided.
The method c) is practicable only when the
content of calcium or magnesium in the solution is very
low.
As regards the method d), based on the prior art,
the reversible-polarity electrode would have had to be
made of solid noble metal such as Pt or Ir, since only
these metals have sufficient electrical conductivity to
match the required level of current and also sufficient
electro-chemical resistivity to be able to function
simultaneously alternately as anode or cathode. Such
electrodes, however, are very expensive.
It is further Xnown in the prior art to install
parallel wires made of, for instance, titanium coated with
an electrocatalytic layer, to function as anodes or
cathodes in the electrolysis of liquid sewage to produce
oxygen and hydrogen to be used, in turn, for
electro-flotation, or also in the electrolysis of other
low-conducting aqueous solutions for other purposes, e.g.
prèparation of drinking water.
-- 3
Due to low conductivity of the solutions used,
the transfer of current is concentrated on these parts of the
wires that are disposed directly opposite each other,
while those parts that are still opposite each other but
are more spaced from each other contribute almost nothing
to the transfer or current through the solution.
It is an object of the present invention to
provide a long-life ~Qlectrode from which the aforesaid
deposits can be removed in a simple manner and which is
stable under operatiny conditions. Moreover, elimination
of the potential drop between individual conductors of the
electrode over the cross-section ("current shadows") is
also desired.
According to the invention, an electrode of
reversible polarity is provided for electrochemical use,
particularly for the electro-flotation, comprising a
carrier, an intermediate layer and an electrocatalytically
active top layer, comprising in combination: a~ a carrier
made of valve metals and having the form of plates,
2~ gratings, lattices, nets, meshes, perforated disks,
sectional shaped elements, rods, bars, wires etc.; b) a
conductive ceramic layer as an intermediate layer, applied
by flame spraying or plasma spraying and selected from the
group consisting of an o~ide, nitride, boride, carbide,
and silicide of a valve metal; c) an electrocatalytic
active layer as a cover layer, made of metal or metals of
the platinum group, rhenium, mixtures or solid solutions
of the compounds of said metals with the said valve metal
compounds.
In one preferred embodiment, the electrode
constitutes an arrangement of rods or bars of rectangular
profile or cross-section, the rods being fastened to bus
bars in a comb-like fashion, wherein two such electrodes
face each other with their equal surfaces disposed
generally parallel to each other.
~ ~ ~ S4~ 7~
The application of rods of rectangular profile,
disposed substantially parallel and always at an equal
distance to each other results in the elimination of
"current shadows" and thus in a substantial increase of
5 the total e~ficiency of the electrode.
According to the invention, cell voltage is
prevented from rising since any cathodic build-up or
incrustation is in turn dissolved in anodic operation.
It is to be mentioned tha~ in the above examples
the electrolyte is acidic at the electrode that acts as an
anode and alkaline at the electrode that acts as a
cathode. The deposits under discussion build up in the
alkaline solution and dissolve in the acidic solution.
~ow, while a quick reversal of polarity hinders the
build-up of calcium and/or magnesium compounds on the
electrodes whereby the first and second of the aforesaid
problems are solved, a relatively slower reversal of
polarity makes the deposited compounds fall off so that
the compounds can be subsequently filtered off whereby the
third problem is eliminated.
The invention is explained below in more detail
in conjunction with figures 1-3 of the drawing.
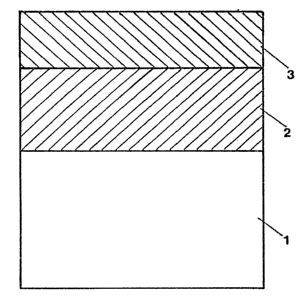
FIGURE l shows the layered structure of an
electrode of the invention; FIGURE 2A is a cross-sectional
view of two parallel round wires as electrodes, FIGURE 2B
is a cross-sectional view of two parallel electrodes of
rectangular cross-section; FIGURE 3 shows the layout of
individual electrode bars and their power supply means,
arranged horizontally or vertically in the electrolyzer.
Referring to FIGURE l, electrodes of reversible
polarity may be made of a valve metal that acts as a
mechanical carrier 1 and as an electric power supply
means. Onto the valve metal is applied a conductive
ceramic layer 2; further, the ceramic layer 2 is provided
with an electrocatalytic layer 3 which is active in both
anodic and cathodic mode.
-- 5
Examples of valve metals to make the carrier of
are Ti, Zr, Hf, Nb, Ta, W as well as their mixtures or
alloys inasmuch as they are anodically stable (in anodic
operation).
The materials suitable for the ceramic layer 2
are, for instance, partially reduced titanium dioxide
(TiO2 x) or TiC or also other compounds of such elements
as Ti, Nb, Ta, Zr, Hf, W and Mo on the one hand and 2'
~, B, Si or C on the other hand.
By way of example, the electrocatalytic active
layer 3 is a coatin~ that consists of oxides of valve
metals such as, eOg. Ti, ~b, Zr, Ta, Hf, W, M0 and oxides
of platinum group metals such as Pd, Pt, Rh, Ru, Ir and Os
and also of Re, wherein also matter not yet oxidic may be
lS present. Mixed oxides of the above-mentioned metals are
particularly active. Layers consisting only of metals
such as Pt or Ir may also be used.
Likewise, layers consisting of platinum-metal
oxides and platinum metals may be used, e.g. a layer
~0 comprising platinum oxide in addition to Pt and Ir.
The ceramic layer 2 can be applied, for instance,
by means of plasmatic spraying.
The electro-catalytic active layer 3 can be
~pplied in a following manner~
~5 a solution containing compounds of one or more
metals selected from the group of Ti, Nb, Zr, Hf, W, Mo
and of one or more elements selected from the group of Pd,
Pb, Rh, Ru, Ir, Os and Re is applied onto a suitable ceramlc coated
carrier, dried out and subsequently txeated at the
temperat~ure of ca. 500C for about 15 minutes under air.
The compounds suitable for this application are
e.g.chlorides; mixtures of butanol and hydrochloric acid
may be used as solvents. A conductive layer of partly
reduced titanium dioxide applied by p]asma spraying is an
example of a suitable substratum.
~265~73L
-- 6 --
The procedure is repeated until the
electrocatalytic layer 3 reaches a desired thickness.
This method is well known in the technology of
anode manufacturing for the chlorine-and-alkali industry.
Another method of forming the layer 3 involves
the step of applying a coating in the above described
manner, the coating subsequently being partly reduced
under the current of hydrogen at a temperature of ca.
400C, where~y some platinum metal oxides liberate the
metals. When this method is employed, the admixture of
the value-metal oxides is often renounced.
A third method of forming the layer 3 consists
of, e.g. the galvanic application of a platinum layer or a
coating of an alloy of platinum metals.
As shown in FIGURE 2A, anodes 4 and cathodes 5
are made o titanium wires of circular cross-section and
spaced a few millimeters from each other.
Due to the high electrical resistance, the
potential between the wire and the adjacent solution at
the contact places (current shadows) is lower by about
50-200 mV than at the reinforced peripheral areas 6,7 of
anode 4 and cathode 5. A resistivity of 50 Q cm was
assumed therefor as this is typical for such applications
as electroflo~tation and preparation of drinking water. It
is clear that in each case, only the reinforced peripheral
area 6,7 of the wires contributes actively to the transfer
of current.
As shown in FIGURE 2B, the electrodes 4', 5' are
made of rectangular titanium bars. Owing to the parallel, planar
arrangement of the ele trodes and the resulting uniform distance of
their facing surfaces, the entire area of the facing
surfaces of the electrodes contributes to the transfer of
current.
The total radius of action is thus
correspondingly enlarged.
~2~7~
-- 7 --
The wire-shaped electrodes 4,5, shown in FIG~RE
2A were spent after about eight weeks and had to be
provided with a new electrocatalytic layer, while the
electrocatalytic top layer on the rectangular titanium
bar electrodes 4', 5' of FIGURE 2B still did not require
to be renewed even after about thirty weeks. Other valve
metals may also be used instead of titanium.
It is expedient to provide isolating spacers
between the separate electrode bars since they are
disposed paxallel at a distance of mere few millimeters
rom each other.
It is advantageous to apply the layer that is
active both in anodic and cathodic operation onto the
entire surface of the bars, preferably by thermal spraying
lS of the o~ides of platinum group metals in a mixture with
the oxides of valve metals.
As shown in FIGURE 3, the rectangular electrodes
4', 5' are arranged ~s combs with meshing teeth wherein
the comb backs serve as bus bars 8,9 to supply power to
the electrodes 4', 5'.
In the course of experiments on electrodes of
reversible polarity it appeared unexpectedly that some
ceramic materials suitable for the conductive layer 2 are
capable to protect the valve metals that constitute the
carrier 1 from the formation of hydrides that tend to
develop in these valve metals in the cathodic operation.
Although such cexamic materials have been known
for a long time to be used in the production o~
electrodes, they have been deemed unsuitable for the
cathodic process; due to the porosity and presence of
crevices in the layers formed from these ceramic
materials, they were assumed as not being capable of
sufficient protection o~ the valve metal (carrier 1)
against the penetration by hydrogen.
~2~i5~7~3L
-- 8 --
The above holds true especially for plasma
sprayed ceramic materials because they are much more
porous than sintered materials or those applied by
baking. On the other hand, however, even metal mesh or
5 wires of titanium can be coated using plasma spraying
which is not possible by sintering.
The above-mentioned ceramic materials are
completely insufficient as electrocatalysts both in anodic
and in cathodic process. Therefore, it was necessary to
provide the materials with the active electrocatalytic
l~yer 3 as shown in FIGURE 1.
It appeared unexpectedly that anodic coatings,
known to be stable at high current densitites in the
chlorine-and-alkali electrolytic process, are also stable
in cathodic operation provided that they are prepared in
the above-described manner and that the density of current
is sufficiently low.
Moreover, it turned out unexpectedly that the
continuous alternation o~ charge does not damage the
electrocatalytic layer 3.
This is at variance with the prior art teaching,
se~ è.g. Juchnierwicz, Plat~ Met. Rev.6, 1962, 100-105, or
R. Doblhofer et al., Ber. Buns. Ges. 82, (1978~, 10~6.
Examples.
The following examples are meant to explain the
technical procedure in more detail; they do not constitute
any limitation of the above-described embodiments of the
electrodes.
A) A solution containing 8g NaCl per 1 liter of tap
water (~ardness 15) was pumped through a cartridge cell
such as described in West German Offenlegungsschrift
3138438 (issued April 14, 1983, inventors Fabian et al).
The volumetric flow as 6 l/h, the inner diameter of the
cartridge was 40 mm and the length was 25 cm. A 10 A current
(corresponding to about 600 A/M2) was passed through the
cell.
~iS~L7~
. g
In the first embodiment the current was
alternated every 15 minutes (the polarity was reversed);
the current was not alternated in the comparative
e~periment.
In the first experiment no deposit was visible on
either electrode even after 8 hours while in the
comparative experiment the cathode side became light-white
of calcium and/or magnesium compounds deposited thereon.
The cell voltage began to rise.
A continuous test was run in which the polarity
was reversed, the same cell worked several months
faultlessly under these conditions. The experiment was
terminated when it became clear that no calcification or
other damage to the electrodes would occur.
B) A similar system as in Example A was used, except
that the current was reversed only every 2-3 hours. The
electrolyte was tap water and the density of current was
only about 100 A/m . Elere, the calcium and/ or
magnesium compounds that deposited on the cathode blew off
~0 in a filterable form whereby the hardness of the effluent
water decreased correspondingly.
Should the cathodic deposits partly dissolve when
the process is reversed into anodic, then that part of water
which contains the dissolved material could be discarded and
~5 thus the amount of softened water reduced correspondingly.
C) Titanium rods were provided with a plasma sprayed
layer (intermediate layer) of partly reduced ~itanium
dioxide. The rods were subse~uently coated with an
electrolytically active coating as described in West German
~a Ofenlegungsschrift 2300422 (issued August 1, 1974, inventor
Hund et al). For comparison purposes, other titanium rods
were provided with the same electrocatalytic layer but the
intermediate TiO2 layer was not applied.
The rods with the intermediate layer were welded
together to form an electrode so that they were
comb-shaped. The rods were interpositioned with the other
~ 7~
-- 10 --
electrode rods while the backs of the rods served as
electric terminals ~power supply means~.
A direct voltage was applied to the terminals and
its polarity was reversed every 15 minutes. The density
of current was 100 A/m2. The electrodes worked in tap
water of hardness 15 and also in an industrial
wastewater. The cell voltage in the tap water was about
6-7 V; in the wastewater, about 3-4 V.
The comparative electrodes worked in an identical
system. The electrodes were considered out of duty when
the cell voltage rose to ca. 15 V. Following results were
obtained under these conditions.
Table
Type of electrodeElectrolyte Longevity (day~
15 with intermediate layertap water 102
with intermediate layerwaste water 30
w/o intermediate layertap water 38
w/o intermediate layerwaste water 14
It may be appreciated that the use of the
intermediate layer more than doubles the working life of
the electrodes.