Note: Descriptions are shown in the official language in which they were submitted.
~s7~a
Antibiotics, and their ~roduction
__ _ _ _ ______ _____ ___
The present invention relates to antibiotics and
to their production.
In the course of a search for new antibiotics, it
has been found that a Nocardia species indexed as SC-4710
in the collection in the Research Laboratory of Sumitomo
Chemical Company, Limited, Osaka, Japan, and on deposit
with the Fermentation Research Institute, Agency of
Industrial Science and Technology, Ibaraki-ken, Japan
under the deposition number FERM P-8233, produces
antibiotics. When this microorganism i5 grown in
a suitable nutrient medium, at least two different
antibiotics, which can be represented by the following
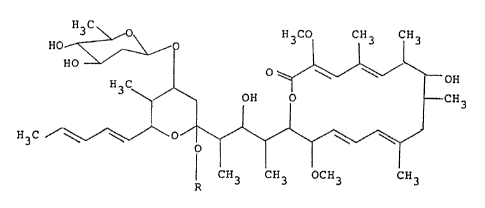
formula, are produced:
H~C
HO'~ , \ OH
! ~ OH O - CH3
H3C ~ / \~ ~ O ~ ~
CH3 CH3 OCH3 CH3
wherein R is a hydrogen atom or a methyl group~ When R is
a hydrogen atom, the antibiotic (I) is called "PC-766B".
~'
:' ' ~ ' "'; ' ''
. . .
i7~5 !3
When R is a methyl group, the antibiotic ~I) is called
"PC-766B"'.
Nocardia sp. SC-4710 was isolated from a soil
sample collected at Shiga-ken, Japan and shows the
following microbiological properties:
J 1) Morphological characteristics:-
On an agar medium and in a liquid medium, the sub-
strate mycelium elongates and branches well, and it is
sometimes divided into bacillus-like or branched short
rods. Good growth is observed on various media for
classification. On the substrate mycelium, white to
grayish white aerial mycelia are formed. ~erial mycelia
are lightly wavy or spiral and irregularly branched. On
observation of the grown culture by a scanning electron
microscope, 3 to 10 spores in chain are seen on the aerial
mycelium; the shape of the spores is ellipsoidal, the size
is from 0.3 x 0.8 micron to 0.4 x 1.0 micron and the
surfaces are smooth; no peculiar structures such as
sporangium and sclerotium are observed.
2) Cultural characteristics:-
Culture media were prepared according to the
Sharing and Gottlieb's method [International Journal of
Systematic Bacteriology, Vol. 16, page 313 (1966)], and
the corresponding test was carried out. Observation was
made after incubation for a period of three weeks at 27C.
The results are shown in Table 1 wherein the colors were
determined in comparison to the standard colors in "Color
Tone Mannual" edited by Japan Color Research Laboratory.
...r ~
": ;'' ; , ` ~ ; '
.
~2~i75~3
-- 3 --
1 tn ~ ~ P) (D ~ ~PJ U, H _ O ~ 3
UJ ~--1~ C H ~ q X (D~q U~ ~ H ~ ~ P) tD
PJ ~ Cr~ nu~ ~t P~ r~ ~ O t~ r~ P-
~ ~ n ~ ~ ~ o ~ ~ r~~ PJ n~ r~ r~ Ul ~-
I S OP~ O U~ Pl O ~ (D U~ r~ C
Pl ~n r~ _ n ~ ~ rD ~ rD ~
(D (D (D-- 3 H rt (DH ~ OH 01 ::~ `' P) H X ('G
1- 1 1(Dcn I I U~r~ t X
~ n ~ ~s r~
(D ~ P~It (D (DI t P~ ~ ~ ~3
~ O PJ ~~ n ~Q~ n ~ ~
UJ ~~ ~ P~~ rt n
t r~ IS rL tD
_._ ... ... , . .. . I
--~ ~ C~
O ~ O~: O O ~ O ~ O ~ O ~ O ~ ~
PJ Q It O(D RJ ~I tt ~1~ O1', 0 1~ 0 n o
1~ (D (D O (D ~ tt ~ ~ (D ~
(D ~ ~I~ It ~ ~ ~ ~ rt
P~ 0 P~ -
rt U~ rt Y rl U~
(D ~tl- (D tl~ (D ~ ~ :5 ~3 0
H~
~ ~
(D :5 ~ ~ ~
_ IJ- ~ (D
rt ~ 1-- rt rt rt ~
(D ~-- rt t~ (D (D ~-
rl (D -- -- -- p
(D ~_
_
p, G~ ~ o~ ~4 o~ ~, o~ ~ o~ ~ o~ ~ 0~ ~ 0~ ~ C~
C O ~t o ~ ~ C O ~ ~ ~ O ~ O (D O n o
~ tt 1~ (D 1~ tt 1~ t~ 1~ ~ 1~
1--rt
l O lJ- 5
~: U~ UJ rt 1~ ~ rl ~S tt C
(D :5 :J (D (D (D (D (D (D IJ- 3 O
t~ ~ ~ U~ ~h
P~ ~
O (D (D ~ - U)
I ~ u, u~ u ~ C
_ ~ ~ t
o o o u
~ It
-- -- (D (D (D ~D ~ p
_ rl
~
_
~ Z ~ Z ~: u
o o o o o o o o o
tD (D (D ~ (D tD ~D (D C Q~
n
. (D rt
.
~t
3 O
tD H~
rt
____
i
. :
:
., : .
~2~7S~3
~ 4 --
æ z
~S
_~0 ~ G~
O ~ O ~ ~
. 3~0 ~ o n o
1'~ 7t ~D ~
r~ ~D 1- rt
~D ~ ~3'
~3
(D ~
~-
_
G~
R- ~ O ~ O
~ ff ~D
,~ ,_
~ ~ ~ C'~
: o o ~o
~ . ~: ~::
: It ~ : ~ ;~
' : ~4
_ _
: ~ ~ tn ~d ~
~ O ~ ~O~
30 C~
: ~ _ ~ ~ :'
'~,.~`
:
" " .` ' " :
- 5
~2~75~3
3) Physioloyical characteristics:-
Observations were macle aecording to ~tandard
methods. The results are shown in Table 2.
Table 2
Test Result
Growth temperature 17 to 37C
Optimum temperature for growth 22 to 32C
Gelatin liquefaction Negative
Starch hydrolysis Negative
Nitrate reduetion Positive
Hydrogen sulfide production Slightly positive
~iilk peptonization Slightly positive
~lilk coagulation Slightly positive
Melanin-like pigment produetion Negative
4) Utilization of earbon sourees
The utilization of carbon sourees on the Pridham-
Gottlieb agar medium is shown in Table 3 wherein the marks
"+" and "++" indicate , respeetively, good and better
utilization and the mark "-" indieates no utilization.
Carbon souree Result
None
L-Arabinose
D-Xylose
D-Glucose +~
D~Fructose ~ +~
Sucrose
Inositol ~+
L-Rhamnose
::
- 6 ~ ~ 6 S7 ~ 8
Rha~finose
D-Mannitol -~
5) sacterial composition:-
Diaminopimelic acid in the bacterial cell is in the
meso form, and arabinose and galactose were detected as the
saccharides. By the analysis of the phopholipids according
to Lechevalier's method [Biochemical Systematics and
Ecology, Vol. 5, page 249 ~1977)], phosphatidylinositol,
phosphatidylinositol dimannoside, diphosphatidylglycerol and
phosphatidylethanolamine were detected, but phosphatidyl-
choline was not detected. In the analysis of micolic acid
according to Lechevalier's method [Canadian Journal o~
Microbiology, Vol. 19, page 965 (1973)] and Minikin's
~Journal of Chromatography, Vol. 188, page 221 (1980)], the
spot for nocardomicolic acid methyl ester was detected.
From the above obs~rvation results, the charac-
teristics of the mieroorganism SC-4710 are summarized as
fvllows: the eell wall type is Type IV, and the phospho-
lipid type is Type PII. Regarding the culture propertie~,
the substratum myeelium shows brown to pale yellow, and the
aerial myeelium shows white to grayish white. Morphologic-
ally, spores are formed in a ehain on the aerial myeelium,
and their sur~aces are smooth. Based on these eharaeteris-
ties, the mieroorganism SC-4710 has been elassified to
oeardia and named Nocardia ~ SC-4710 (FERM P-~233;
eorresponding to FERM BP-1203 used as the International
Deposition ~umber under the Budapest Treaty).
`- "~ ' ~ `"';
. ;,.
'-~ - ' ':
~L2~;57~;~
-- 7
The antibiotics (I) of the invention are produced
during cultivation oE a stanclard strain of r~_cardla sp.
SC-4710 or a natural or artificial variant or mutant
thereof in an aqueous nutrient medium. The composition of
this nutrient medium may be varied over a very wide range.
Essentially what is required is a carbon source, a nitrogen
source and trace inorganic elements. Examples of suitable
carbon sources are glucose, maltose, starch, dextrin,
glycerol, molasses, etc. Examples of suitable nitrogen
sources are soybean meal, corn steep liquor, cotton seed
flour, peptone, meat extract, yeast extract, casein
hydrolyzate, ammonium salts, nitrates~ etc. Examples of
suitable sources of inorganic elements are mineral salts,
e.~. chlorides, carbonates and phosphates of magnesium,
potassium, calcium, sodium, iron, manganese, etc.
Cultivation is usually carried out under aerobic
conditions, and preferably under aeration and agitation.
The temperature required for the cultivation may be
appropriately decided within the range in which the
microorganism grows and the antibiotics ~I) are produced.
A preferred temperature range is from about 25 to 30C.
The p~ is normally from about 7 to 8. The antibiotic
potency accumulated in the nutrient medium reaches usually
the highest ~ithin a cultivation period of about 80 to 120
hours.
For recovery of the antibiotics (I) from the
fermentation broth a~ter the cultivation, any conventional
procedure may be adopted. For example, a procedure
~` .
.. . . . .
: ~ ;`'" "' ,, '
- B -
utiLizing diEEerences ;n solubility between the clesired
substance and the impurities, a procedure utilizirlg
differences in adsorption a~finity onto various adsorbents
e.g. activated carbon, macroporous non-ionic resins, silica
gel and alumina, a procedure using ion exchan~e resins for
the removal of impurities, etc. may be adopted either
alone or in combination.
A typical example of the separation and purifica-
tion of the antibiotics (I) from the ~ermentation broth
after cultivation is as follows.
The fermentation broth may be separated into the
bacterial body and the supernatant or filtrate by centri-
fugation or filtration. The bacterial body can then be
extracted with acetone or methanol and the extract extrac-
ted with a suitable organic solvent for the antibiotics(I), e.g. ethyl acetate or n-butanol. Alternatively, the
supernatant or filtrate may be extracted with an organic
solvent which is not miscible with water and can dissolve
the antibiotics (I) therein, e.g. ethyl acetate, n-butanol
or methylisobutylketone The organic solvent extracts
comprising the antibiotics ~I) are combined together, and
a per se conventional procedure for purification of a
fat-soluble substance is applied thereto to recover the
antibiotics (I). For example, the ethyl acetate extract
may be washed with water and concentrated under reduced
pressure, ~ollowed by addition of n-hexane or the like to
precipitate the active component. 1~he precipltate can
then be collected by centriEugation or filtration to give
: ,~
'~
r~ ~
- g
a crude prod~ct com~rising the antibiotics (I).
For purification/ the above crude product may be
subjected to column chromatography using a carrier having a
molecular sieve effect, e.g. Sephadex LH-20 (trade mark,
manu~actured by Pharmacia, Sweden) and a developing solvent
e.g. alcohols (e.g. methanol), halogenated hydrocarbons
(e.g. chloroform), and their mixtures. For further purif-
ication, the resulting product may be subjected to adsorp-
tive chromatography, for example using a carrier generally
used for adsorptive separation of antibiotics (e.g. adsorp-
tive resins, silica gel, alumina) as an adsorbent. When
silica gel is used as the adsorbent, chromatographic separa-
tion may be effected by changing the mixing proportion of a
polar solvent, e.g. an alkanol (e.g. methanol), and a non-
polar solvent, e.g. halogenated alkanes (e.g. chloroform).
In order to isolate PC-766B and PC-766B~ from the
above purified product~ chromatography using the above-
mentioned adsorbent may be repeatedly applied thereto.
When~ for example, silica gel is used as the adsorbent, the
silica gel on which the antibiotics (I) are adsorbed may be
developed with a solvent system consistin9 of benzene and
e~hyl acetate, whereby PC-766B' and PC-766B are eluted in
this order. After assaying the purity by TLC (thin layer
chromatography) or HPLC ~high performance liquid chromatog-
raphy), the eluted fractions may be concentrated underreduced pressure to dryness so that PC-766B and PC-776B' can
be respectively obtained as powdery crystals.
`" '' '" '. '
' ''' ' ,' , '. ' '
' ~, ', ., ~ '
.~. . ..
~iL26~;7r~l~
-- 10 --
PLC~766B and PC-766B' can both be manu~actured by
cultivation of _ cardia ~ SC~q710. PC~766B' may also be
manu~actured by methylation of PC-766B under appropriate
conditions. This methylation can be effected, for example,
by maintaining a solution oE PC-766B in methanol at room
temperature (e.g. 20 to 25C).
The chemical structures of the antibiotics (I) were
determined by spectroscopic investigation. Some character-
istic physico-chemical properties of PC-766B and PC-766B'
are shown below, in which reEerence is made to the accompany-
ing drawings, wherein:
Figure 1 is a W absorption spectrum (in methanol)
of PC-766B;
Figure 2 is an Infra Red spectrum of PC-766B;
Figure 3 is an H-NMR spectrum of PC-766B;
Figure 4 is a C-NMR spectrum of PC-766B; and
Figure 5 is an ~-NMR spectrum of PC-766B'.
1. PC-766B
Appearance: colorless amorphous powder.
Elemental analysis: C, 61.29 ~; H, 8.11 %.
Molecular weight; 776.
Mass spectrographic analysis: FD, F~B-MS; m/z 799
(M -~Na), m/z 815 (M +K).
Molecular formula: Cg3H68l2.
Melting point: 132-134C.
Speci~ic ro~ation: [~]D = ~17 7a (C = 0.61,
methanol).
.
:;
,
~Z6~
- lOa -
Solubility: soluble in methanol, chrloro~orm and
ethyl. acetate; insoluble in n-hexane and water.
Color reaction: positive in I2 absorption and
anisaldehyde reaction; negative in ninhydrin reaction.
TLC (silica gel): chloroform-methanol (15 : 1 by
volume), Rf = Oal4; benzene-ethyl acetate (1 : 4 by volume),
Rf = 0.23.
UV absorption spectrum (in methanol): as shown in
:` ~ , .
': ' '`"
~6~
Fig. 1 of the accompanying drawings.
IR absorption spectrum (in l<sr tablet): as shown
in Fig. 2~
H-NMR spectrum (200 MHz, in CDC13, T~IS standard):
as shown in Fig. 3.
13c_N~1R spectrum (50.3 MHz, in CDC13, TkiS
standard): as shown in Fig. 4.
2. PC-766s'
Appearance: colorless amorphous powder.
Molecular weight: 790.
Molecular formula: C44H70O12.
Solubility: soluble in methanol, chloroform and
ethyl acetate; insoluble in n-hexane and water.
Color reaction: positive in I2 absorption and
anisaldehyde reaction; negative in ninhydrin reaction.
TLC (silica gel): chloroform-methanol (15 : 1 by
volume), Rf = 0.18; benzene-ethyl acetate ~1 : 4 by volume),
= 0.35.
H-NMR spectrum (90 MHz, in CDC13, TMS standard):
as shown in Fig. 5.
The antibiotics (I) exhibit significant anti-
microbial potency against various microorganisms. The
minimum inhibitory concentrations of PC-765B against various
microorgnisms as measured by the agar dilution method are
shown in Table 4. PC~766B' also shows similar antimicrobial
potency.
~ ,
- : : .:. ~ : :
~2~
-- 12 --
_, .. __ . . ... . .., ._
_ _ _ t~ t~ tr~ ~ ~ X ~ 0~ b~ ~n ~ u~ cn
~n ~t ~ p) tD U) ~ tn p) p) rt ~ ~ rt rt
1~ ~ ~ ~ tD O tD t~ ~1 t~ ~. ~ ~ ~ tl
tD t~ ~ Q- t~ ~:: rt tJ' ~ ~ I~ tD 1~ ~ ra 1 S
_ ~ 1-- P~ ~ tD U~ tD 1--~ ~ :~ :J O
~Q O O Q- rt O ~ ~' I S 1--1--rt tl ~< ~ O
IJ ~ P) 1' . 3 m tD IJ . ~:: ~:: O O I_ j_ ~
,_ ~ o ~ o ,_ n ~ ~ t~ t~ o o ~
~ ~ t~ ~ ~ ~ l_ ~ o t~ t~ t~ p,
Z; ~ rt ~ 1--3 P~ ~- ~ 1~ n tn t~ ~:: O O ::5
O V~ O 5 ~ P~ tn ~t P~ tD ~ t~ U~ ~ t~ 1
rt ~ tn 1~- It P~ ~ ~ tJ ~ t~ tl U~ t~
tD H) n t7 P~ ~ ~ o tD rl- Ul ~ C ~ 3 ~
.. ~ 3 ~ ~ tD tD 1-- tD O ~:: 1 ~ V~ U~ u~ tD
3 tD tD ~ ~n I S I--~ 1--~n I--1~ rt
~- ~ O rn t~ ~ IJ 3 IJ 1 ~ tD ¦ tD I P'
r~ ~h tD ~q tn O H ~n I O
~_ p- ~ o ~: ~ ~- ~ 1~ ~n l l ~
rt ~ t~ ~3 U~ :~ ~ ~- H ~ tD IQ-ltD
C ~t ~; C O !z ~ r ~ D ItD It-
m O p~ X u~ tD ::C ~ t~ltD ~ n
~1- ~ ~ pJ ~ O t~l~n t~
3 ::J tn ~1~ ~ ~ 'I ~ t~
~ ~: o o ~ t~ 1~ o
C rt ~ O O ~n t~ O
tD ~ 1-- ~ Iv ~ O ~I~n
P~ O ~ ~n r w :~ iP
t~ Q. Cl~ H
O IJ. O 3: 1-- 0 1:
~: I-h O ~ ~A) O 3
~t IJ. ~ C O ~,
P~ tD o
S~ ~ ~ ~~
u~
-~ o
~n ~: o
Y I_
'4 tD
t
.
tD :~
~ rt _
t- ~
Dl
~Q H
\~ \/ V V V V t~
~ l--
tD o o ~ o O o o o o 1~
~ ~ O O O o W r~ W ~ ~ ~4
1-~ ~ ......
~n ~ ~ ~ ~n ~ ~n
: 3 ~UIW~UI ,_
_
: :
X t~
t~ ~ G
* tD
.. _ _
, .. . .
., ~ .
- : :' ~ ' '.
: ~ .
. ~ .
- 13 --
57~
Practical and presently preferred embodiments of
this invention are illustratively shown in the following
Examples wherein part~s) and percenta~es are by weight, unless o~er-
wise indicated.
Example _1
A loopful of Nocardia sp. SC-4710 (FERM P-8233)
cultured on a slant agar was inoculated into a seed culture
medium, which was then subjectedto a shaking culture at 27C
~ for 5 days. The resulting seed culture was added to a
! 10 pre-culture medium in an amount of 2 % by volume, and a
shaking culture was carried out at 27C for 5 days. The
resulting pre-culture was added to a nutrient medium (50
liters) charged tQ a 90 liter volume jar fermenter in an
amount of 2 % by volume, and cultivation was carried out at
27C for 4 days under an aeration amount of 25 liters per
minute at an agitation rate of 200 r.p.m. All of the seed
culture medium, the pre-culture medi.um and the nutrient
medium comprised glucose (2.5 %), soybean meal (1.5 ~),
yeast extract (0.2 ~) and calcium carbonate ~0.4 %) and were
used after adjustment to pH 7.2 and sterili~ation at 121C
for 20 minutes.
Example 2
The fermentation broth after cultivation as in
Example 1 ~50 liters) was centrifuged to separate it into
the microbial body and the supernatant. The microbial body
in a wet state was shaken with acetone t5 liters~ and
filtered, and this operation was repeated twice. The
acetone extracts (15 liters) were combined together and
.~ il .. i
.. ...
,, : ' ~ ''
:. ..
,: : , :
:: . .
~6s~sa
concentrated under reduced pressure to make a volume of about
100 milliliters. 'I'he concentrate was shaken with a mixture
of water (1 liter) and ethyl acetate (1 litee) to extract the
active fraction. Separately, said supernatant was adjusted
to pH 7.0 and shaken with an equal volume of ethyl acetate.
The ethyl acetate extract was washed with water (30 liters),
combined with the extract comprising the active fraction and
concentrated under reduced pressure. n-~exane was added to
the concentrate. The precipitate was collected by filtration
and dissolved in a mixture of chloroform and methanol (1 : 1
by volume) (100 ml). The resultant solution was placed on
a column of Sephadex LH-20 ~trade mark, manufactured by
Pharmacia) (500 ml) equilibrated with a mixture of chloroform
and methanol (1 : 1 by volume) and eluted with a mixture of
chloroform and methanol to produce fractions of 50 milli-
liters each. The active ractions were concentrated to dry-
ness under reduced pressure, and the residue was dissolved
in chloroform (about 5~ ml). The resulting solution was
added to a column of silica gel ("Kiesel Gel 60" trade mark,
manufactured by E. Merck in W. Gemany, 63 to 200 microns)
(500 ml), and the column was eluted with chloroform and then
a mixture of chloroform and methanol (97 : 3 by volume) to
produce Eractions of 50 milliliters each. The active frac-
tions were concentrated under reduced pressure to dryness to
give a crude product (640 mg) of the antibiotic substance
"PC-766" comprising PC~766B as the major product with a
trace amount of PC-766B'.
. .
.. ~ . ., : : .
~` ' ', .:
, '';, '': ~ ' : ' :
~2G~
Example_3
The crude product of the antibiotic substance
PC-766 (640 mg) as obtained in ~xample 2 was purified by the
use of an apparatus for HPLC ~"System 500" trade mark, manu-
Eactured by Waters, U.S.A.) under the Eollowing operation
conditions:
Column: Preppack-500 (trade mark) silica gel column
~manuactured by Waters), two columns);
volume);
Flow rate: 100 ml/minute;
Detection: UV absorption at 254 nm;
Sample injection: the crude product "PC 766" (640
mg) dissolved in chloroform (30 ml) and then injected;
Fraction volume: 50 ml.
Fraction Nos. 27 and 28 were concentrated under
reduced pressure to dryness to give a mixture l2 mg) of
PC-766B and PC-766B'. Fraction Nos. 29 to 47 were
concentrated under reduced pressure to dryness to give
PC-766B (580 mg).
Example 4
The antibiotic substance PC-766B (50 mg) as
obtained in Example 3 was dissolved in methanol (100 ml) and
allowed to stand at room temperature (20 to 25C) for
methylation. After one week, the resultant solution was
concentrated under reduced pressure, subjec~ed to streak-
adsorption on a preparative thin layer chromatogram and
developped with a mixture of benzene and ethyl acetate (1 :
4 by volume). Observation was made under irradiation with
. .
,`, ;` `;
' ' ': ',. '
7~
- 16 -
UV ra~s. The bands or PC-766B and PC-766B' were respec-
tively scraped o~f, extracted with methanol and concentrated
under reduced pressure to yive unreacted PC-766B (34 mg) and
PC-766B' (3 mg). These products gave the physico-chemical
properties and biological properties as hereinabove
described.
,
. ' ' " :, ,
'