Note: Descriptions are shown in the official language in which they were submitted.
IMPLANTABBE ELECTROPHORETIC PUMP FOR
IONIC DRUGS AND ASSOCIATE~ METHODS
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to an implantable, molecular
electrophoretic pump for ionic drugs and, more specifically, it
relates to a durable and improved pump which is adapted to
- supply, on a predetermined basis, desired quantities of
medication.
2. Description of the Prior Art
It has beén known that medicines may, in many
instances, be employed more efficiently by providing a pump to
,
deliver the medication to a patient at a more uniform rate than
wouId be experienced by periodic injections through syringes.
Such~pumps have been provided with the capability of either being
mounted externally of the body or implanted in the body. See
generaliy Electronic Flow Rate Controller for Portable Insulin
Infusion Pump~by~R. T. Ferguson et~al., Diabetes Care, Vol. 3 No.
~: ~
2, March-April 1980 pp. 332-337; A Totally Implantable Drug
- Infusion Device:~ Laboratory and Clinical Experience Using a Model
~With~Single~Flow Rate and New Design for Modulated Insulin and
,
~ Infusion by Henry Buchwald et al., Diabetes Care, Vol. 3 No. 2,
~March-~April 1980, pP. 351~358~ Implantable Drug-Delivery Systems
by Perry J.`Blackshear pp. 66-73, Scientific American 241
(December 1979); and H. Buchwald, A Two-Phase Fluid Powered
`:
~Insulin Infusion Pump with Basal/Bolus Capability Which
,, :
:
Compensates for Pressure and Temperature Variability, Trans. Am.
Soc. Artif. Internal Organs, 27, 263-40 (1981).
U. S. patents 3,894,538 and 4,140,122 disclose multi-
chambered medicine supplying pumps which involve actual transfer
of solution and require the use of moving parts.
U. S. patent 4,140,121 discloses an implantable dosing
device which delivers' drugs plus liquid and involves a variable
volume medicine reservoir and a liquid chamber which is subject'ed
to variations in volume through transport of liquid by electro-
osmosis.
One of the problems experienced with the prior art
systems has been the need to use a system having movable parts,
thus rendering the pump susceptible to breakdowns and also
requiring large power consumption.
In spite of the foregoing teachings, there remains a
need for a self contained, refilla~le, externally programmable,
implantable drug infusion device.
- SUMMARY OF THE INVEN~ION
~ The present invention has solved the above-described
problems~by providing a self-contained, refillable, externally
~programmable, implantable ionic drug dispensing electrophoretic
~pump.~ It provides a sealed housing and a reservoir having filler
and~discharge openings. The filler opening may be sealed by a
self-sealing member which is adapted to be pierced by a reservoir
~charging instrument, such as a hypodermic'needle. The discharge
~opening is adapted to permit the flow of ions therethrough. The
discharge opening preferably has a passive diffusion membrane and
"` : :
2.
``: ~ ' ~:
. ~ .
i96~
-3~- 71548-20
a pair of associated electrodes. Battery means eneryize the
electrodes and cooperate with elec-tronic means so as to pro-
vide delivery of ions at the desired rate.
In one embodiment of the invention, diffusion will be
the principal source of ion distribution during periods when
electrodes are not energized. When the electrodes are energized,
depending upon the polarity of the electrodes as compared with
the nature of the ion, the rate of flcw by diffusion will
either be enhanced or retarded. ~lso, in a preferred embodi-
ment the amount of flow initiated by energizing the electrodescan be increased for periods of unusual need.
The invention also encompasses a method for accomp-
lishing distribution of ionic drugs~
~ n accordance with the present invention, there is
provided àn implantable electrophoretic pump for ionic drugs
comprising,
a sealed housing,
a reservoir having a filler opening and a discharge opening,
sai:d housing having opening means operatively reservoir
associated with.said filler opening and said discharge opening,
a filler member disposed within said filler opening and
being self~sealing and adapted to be pierced by a reservoir
filling member and upon removal of said filling member will
resist leakage therethrough.,
a reservoir discharge member disposed within said dischaxge
opening and having a diffusion membrane and a pair of assoc-
iated eIectrodes,
electrical energizing means for energizing said electrodes,
and
. ~
.. . . . ..
`` ~26~ 7l54g-20
the polarity of said e].ectrodes being adapted to be
connected such that when said electrodes are energized
increased flow of said ionic drug through said discharge member
will occur as compared with :Elow induced solely through
diffusion when said electrodes are not energized.
In accordance with another aspect of the invention,
there is provided a reservoir for an implantable electrophor-
etic pump including
a hollow body defining a storage chamber,
a filler opening defined within said body,
a self-sealing member sealingly secured within sai.d filler
opening,
a discharge opening deEined within said body,
a diffusion membrane sealingly secured within said dis-
eharge opening, and
a pair o~ eleetrodes operatively assoeiated with said
diffusion membrane.
In aecordanee with another aspect of the invention,
there is provided a method of delivering an ionic drug
eomprising
providing an implantable eleetrophoretie pump having a
housing within whieh is disposed a reservoir having a filler
opening sealed by a self-sealing membrane and a discharge
opening sealed by a diffusion membrane having eleetrode means
operatively assoeiated therewith,
introdueing an ionie drug into said reservoir,
implanting said pump,
ef~f~eeting basal rate delivery of said ionie drug by
diffusion, and
'
,
- 3a -
:
:
~X ~ 71548-20
periodically energizing said electrode to effect
alteration of the diffusion induced flow of ions out of said
reservoir.
It is an object of the present invention to provide a
reliable means for delivering efficiently, predetermined
quanti-ties of an ionic medication by means of an implantable
pump.
It is a further objec-t oE the invention to provide
such a pump and a method of using the same w~erein durability
and dependability of the pump are enhanced as a result of the
absence of moving parts.
It is another object of the present invention to
provide such an implantable pump which may be programmed
externally.
It is a further object oE the present invention to
provide such a pump which requires very modest electrical
energy to operate in the predetermined manner.
~ ~ ,
:: :
:: :
; ~ - 3b -
"
- . , .
~GS969L
It is yet another object of the invention to provide
such a pump which may have its reservoir replenished by means of
a hypodermic needle without the need for a surgical procedure.
It is a further object of the present invention to
provide such' a pump which is adapted to be used with a number of
different types of ionic drugs.
It is a further object of the present invention to
provide such a pump which is adapted to be employed in the
delivery o insulin to diabetic patients.
It is yet another object of the invention to provide
means for delivering an extra dose of the medication during
periods of unique needs.
These and other objects of the invention will be more
fully understood from the following description of the invention
on reference to the illustrations appended hereto.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure I is a schematic illustration of a principlé of
the present i~nvention.
Figure 2 is a plot of a relationship between diffusion
and electrophoresis.
.
Figure~3 is a plot of delivery rate versus current.
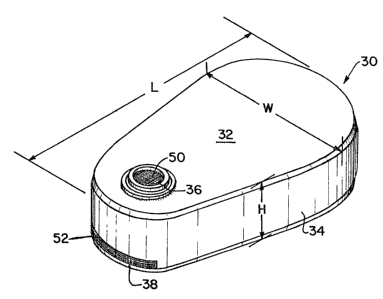
~ Figùre 4 is a perspective view o a form of pump of the
pr~esent invention.
~ Figure~5 is a partially schematic top plan view (with a
top wall not shown) of an embodiment of the present invention.
Figure 6 is a cross-sectional illustration of the
system of Figure 5 taken through 6-6.
~ 4.
:~
Figure 7 is an exploded view of a form of collar and
reservoir of the captioned invention.
Figure 8 is a generally cylindrical orm of reservoir
of the present invention.
Figure 9 is a partially exploded cross-sectional
illustration of a form of filler membrane assembly of the present
invention.
Figure lO is a fragmentary cross-sectional view showing
a portion of the discharge opening of the reservoir.
Figures ll through 13 show sequential stages of a
method of making a porous electrode of the present invention.
Figure 14 is a schematic illustration of another
embodiment wherein the reservoir is positioned exteriorly of the
housing.
Figure 15 is a schematic illustration of a portion of
the electronic components of the pump.
DESCRIPTION OF T~E PREFERRED EMBODIMENTS
As used herein, the term "ionic drug" means ionically
charged materials adapted for medical uses within a human or
anlmaI and shall expressly include, but not be limited to,
insulin, peptide hormones, blood thinners, neurotrophics,
antibiotias, analgesics, immunosuppresive agents and
pharmaoeutioal materials modified to carry a charge.
As used~herein the term "patient" shall be deemed to
include humans and animals.
Referring now to Figure 1 in greater detail there is
~shown~;schematically a reservoir of the present invention and the
'' ~ 5.
~.Z65~
manner in which the ionic drugs will be passed through the
discharge opening so as to emerge from the pump and be delivered
to the patient for absorption. The reservoir 2 is hermetically
sealed and has a reservoir chamber 4 which contains the ionic
drug or drugs to be dispensed. The drugs may conveniently be in
the form of a suspension. Discharge opening 6 is provided with a
passive membrane 8 which will permit ions to pass therethrough.
This membrane 8 preferably also resists passage of bacteria
therethrough The membrane 8 may be a cellulose membrane. Among
the preferred materials that are suitable for use as the membrane
52 are those made from cellulose esters, nylon polyvinylidene
flouride, polytetrafluoroethylene, cellulose nitrate and acetate
and mixtures thereof. The preferred membranes have pore sizes
- ~ from about 0.025 to 8 microns and are from about lO0 to 200lS microns thick. The membrane diameters are preferably between
about l3 and 293 millimeters. In general, many types of
microfi1tration membranes may be employed. Among the preferred
materials are those sold under the trade designations "MF"
.
tMillipore): "Ce1Otate" (Mill1pore); "~urapore" tMillipore);
"Diaflow"~ (Am1con); "Mitex" tMillipore); and "Fluoropore"
(Millipore).
The electodes are preferably composed o a material
selected from the group consisting of silver/silver chloride,
::
carbon, carbon mesh and platinum.
~ Disposed on opposite sides of the membrane 8 and
operatively associated therewith are a pair of porous electrodes
10,~12~which will be described in greater detail hereinafter. A
~;battery~14, by means of anode lead 16 and cathode lead 18,
:
~ 6.
~: :
,
2G~g~ ,
energizes the respective electrodes 10, 12. In this arrangement,
if insulin w~re contained within the chamber 4, as insulin is a
negative ion, the membrane 8 will permit passage of the ions
through the same. The direction of movement caused by
electrophoresis with the electrodes energized as shown indicated
by the arrow "E".
Under normal circumstances, the buildup of
concentration of ions in the reservoir 4 will result in passage
of the material through the membrane 8 in the direction indicated
by the arrow ~D~ even when the electrodes are not energized.
This diffusion flow may be relied upon, in some instances, as
establishing a basic rate for ongoing delivery of the ionic
drugs. In some cases, as may be true with insulin, it may be
desirable to provide a greater flow than would occur through
diffusion in which case energizing the electrodes 10, 12 serves
to increase the rate of delivery of the material. If desired,
for certain~materials, means may be provided for reversing
remotely the polarity of electrodes 10, 12 thereby causing the
~ electrophoresis to retard the amount of ionic flow effected ~-
through diffusion.
EXAMPLE
~ ~ ~In order~ to provide further understanding of the
invention the~following example is provided.
::
; A housing, made of titanium, having the shape
illustrated in Figure 4 is provided. The housing has a height H
of~about 18~mm.~,~a length L oP about 10 cm, an average width W of
, ~
~about 40 mm and a wall thickness of about 0.5 mm. The reservoir
~is made~;of titanium and has a continuous interior coating of
, ~ :
~ 7.
, . . . . .
, . . . . .
~26~
silicone rubber about l/lO mm. thick. The reservoir is
cylindrical in shape with a diameter of about 35 mm. and an
average height of about l9 mm. The reservoir is secured in the
housing and has about l gram of insulin in slurry form in its
chamber. The insulin may be crystalline insulin suspended in
~aCl based buffer with extra crystals suspended in a matrix of
trimethyl cellulose. The total insulin is present (slurry plus
undissolved solid) in quantities of about 25 mg/ml and the
reservoir holds about 25 ml. A unit of insulin weighs about 1/24
mg.
Two 2.7 volt lithium iodide batteries energize a pair
of carbon mesh porous electrodes which are on opposite sides of a
`cellulose acetate passive membrane having a thickness of about
150 microns and a cross sectional area of about 0.5 cm2. A
voltage of about 1.2 volts is imposed across the electrodes.
Direct current or pulsed direat current are preferably
employed. The filler membrane is composed of reinforced silicone
rubber and has an area of about 1 1/2 cm2.
The system, when the electrodes are energized, operates
at about 1-15 milliamperes.
The electronic unit turns the pump on and off during
~predetermined periods with diffusion induced flow continuing at a
basic~rate when the electrodes are not on.
~ Before turning to further details of the invention, the
backgrouad will be considered. The electrophoretic ion pump
~utilizes the phenomenon of electrodiffusion for the transport of
8.
~ .
~2~;96~
an ionic drug from a reservoir to the surrounding body tissue.
The phenomenon involves a combination of diffusion and
electrophoretic transport.
501utions consist of molecules dissolved in a
solvent. It sometimes is convenient to regard one or more
components as solute and the other the solvent. 30th solvent and
solute molecules are in constant motion with respect to each
other and in constant collision, as long as the solution remains
liquid. If an interface is formed between the solution and pure
liquid solvent and if this interface remains stable, solute
molecules will be transported across the interface from solution
to solvent. This process, if left undisturbed, will continue
until the concentration of solute becomes the same throughout.
Net transport will cease, and the system will be in
equilibrium. This mode of transport is called "free diffusion".
90metimes in the process of free diffusion, it is
difficult to stabilize the interface between solution and
solvent, Because this can be troublesome, a porous membrane is
sometimes placed between solution and the solvent, forming the
i~nterEace. Difusion, then, takes place within the pores of the
membrane. AIthough the rate of transport is usually diminished,
the phenomenon is still basically difusion.
If the solute molecules happen to be larger than the
~ ~ pores~of the membrane, they are excluded and will be retained in
the solution side of the boundary, If there is a mixture of
:
;~molecules~both larger and smaller than the pores, the smaller
ones w.ill pass through the membrane and the larger ones will be
ret~ained. In the~electrophoretic ion pump, the membrane will
~L2G5964
pass the solvent (water) of body fluids, the inorganic salts,
organic acids, sugars and most proteins. As only the ionic drug
will be supplied within the reservoir, all these components of
body fluids will quickly equilibrate with the reservoir so there
will be no or little net transport of these substances of body
fluids during operation of the pump. This concept will be
referred to herein as employing a membrane whiah will readily
pass the ionic drug therethrough. The pump of the present
invention may be considered a molecular pump as distinguished
from a bulk pump. The pump has pressure equilibrium with the
result that it does not induce the flow of fluids.
The process of diffusion is basically the same for
molecules that carry no net electrical charge as for ions. With
respect to molecules that are capable of penetrating the
membrane, if their rates of diffusion are diminished to the same
extent, the membrane is said to be passive. If, on the other
hand, the membrane affects different kinds of molecules to
different extents, the membrane is permselective (or an "active
membrane")O The most common kind of permselectivity arises with
ionic solute molecules and immobilized eiectric charges in or
near the pores ~of the membrane. In this case, the ion carrying a
;charge of the same sign as the immobilized ones will be inhibited ~;
or~exal~uded rom the membrane. The ion carrying a charge of the
opposite sign will be transported with little or no inhibition.
~ The pump~of the present invention preferably utilizes a passive
membrane, but permselective membranes may be used for transport
of particular ionic drugs.
... . .
~Z~5~6~
It is generally not satisfactory to rely solely on the
process of diffusion for a drug delivery system. This is because
the rate of delivery depends upon the physical and mechanical
properties of the membrane which are not alterable
conveniently. This me~ns that one has virtually no control of
the drug delivery rate. In ~he case of insulin delivery,
moreover, the pump may have to operate at two different delivery
rates, changing from one to the other several times a day such as
meals, for example. If ~he drug molecules are ions, however,
delivery can also be achieved through electrophoresis.
Electrophoresis can be best understood in terms of an
example. Consider a pair of parallel flat electrodes immersed in
a solution containing ions. If a certain voltage is applied to
the electrodes, a current will flow in the space between them.
The ions in solution move, those with positive charge moving
toward the cathode (cations) and those with negative charge
moving toward the anode (anions). This flow of ions constitutes
a flow of electric current. The rate of flow of the ions depends
on the slgn and magnitude of the electric charge that they carry,
the~ physical size of the ion, the voltage placed across the cell,
and the distance of separation of the electrodes. Because some
of~t~ese~properties are properties of the apparatus and not the
molecule~ being transported, it is customary to define a quantity
called mobility (u) as the velocity (v) imparted to a specific
~ion per unit of~electrical field strength E/L or ln symbols,
(1) u = vL/E
~ : `
.
,- ,
~26~4
Where E is the voltage drop and L is the separation distance
between electrodes~ Mobility is a property of the ion which can
be measured. If the mobility of the anion is u_ and the mobility
of the cathion is u+, then the fraction of the total current
carried by the ions is
(2) t = u /tu +u+) and t~ = u~/(u_+u+)
The fraction of the total current carried by the anions, t , and
cations t~, depends upon their respective mobilities. In
general, these are different numbers. However, the important
point is that the current is carried by ions in solution, the
fraction depending on their mobility, and that anions such as
insulin move to the anode and cations move to the cathode. The
ions are moving because they are being produced in the vicinity
of one of the electrodes and being consumed at the other,
resulting in a flow of ions. It is not proper to think of the
ions being attracted or repelled electrostatically to the
electrodes, as electrons are in space. This means also that if
an ion should~find itsel, for instance, on the side of the
anodej~facing away from the cathode, it will be neither attracted
nor~repelled from it because there is no current flowing in the
vlcinity: as current flows only between the electrodes.
The rate of movement of the ions depends upon the
voltage~across the cell, among other things. This, however, is a
very desi~rable~property because it allows one to control the rate
,
~ ~ ~of movement by controling a voltage. While it is difficult to
12.
:; :
-
:
~G~96~ 715~8-20
change the physical or mechanical properties of a device, it iB
usually easy to change the voltage across a cell.
It is a feature of the present invention to provide
an ionic drug delivery device working on the principles of both
diffusion and electrophoresis telectro~if~usion) wherein the
rate can be controlled electrically.
The transport of molecules from the combined forces
of electrical and diffusive forces has been described by Planck
and Nernst in the last century. For most situations, and
especially for passive membranes, the treatment is still
adequate (although now it is known to be inadequate for certain
cellular and subcellular membranes). The behavior of molecules
under free diffusion and electrophoresis can be summarized in
the following partial differential equation.
(3) ~c = uE ~c ~ D'
L ~ ~x
where c is concentration of the transported molecule at some
position, x, between the electrodes and at some time, t. For
the other variable, u is the mobility of the ion, D' is its
diffusion coefficient, and E is the electric field strength.
The diffusion coefficient is primed because with a membrane it
is different than for free diffusion D' = D(l-~) where the
factor (1-~) incorporates this. With resp~ct to the transport
of insulin values for c, u and D will be those for insulin.
Solving this differential equation for the case of
material transport across a membrane, for the steady state, the
so1ution is glven by equation (4).
- 13 -
~2659~ 71548-20
(4) J = uEc- ~ u2 ~
~ ~J
where J is the delivery rate (in g cm~2 o~ membrane/sec~l),
where ci is the concentration of the drug (insulinj in the
reservoir of the pump, and u is -the mobility of the ion being
transported, already defined. U is a function which depends
on some parameters of the drug and on the delivery system.
This is shown by equation (5).
(5) U = eU~h/2LD'
In this equation h is the thickness of the membrane.
For æero current density, equation (4) reduces to
that for diffusion only. For currents resulting in pumping the
drug out of the re~ervoir tnegative currents), and for high
current denslty, the function U in equation (5) is much larger
than 1 and equation 4 reduces to
(6) J = ~
Here, l~ l:S the current density (in amperes cm~2), and S i8 the
specific conductivity of the mediu~ containing the drug (in mho
cm~~ In this case, the specific de].ivery rate does not
depend on the di~fusion coefficient, D'. This means that, when
operated at high current density, transport is by
electrophoresis~only and diffusion is not important.
For small currents, equation (4) reduces to
(7) ~ J = cl ~ ~ hD )
14 -
.
, :.- .
~ 71548-20
This means that, for small current density, the spec~fic
delivery rate varies in a linear fashion with current density.
Figure 2 is a dimensionless plot of equation (4) in
which the specific deliver~ rate relative to the ~ero current
rate (i.e., diffusion alone) is plotted against the ~unction
Rx. Here Rx is a dimensionless function defined as
uhi/SD'. Rx depends upon constants of the system and current
density. When the constants in equation (4) are known, Figure
2 can be employed to calculate the fundamental relationships
describing the operation of the pump for various drugs and for
various operating conditions. It can be seen that for positive
current ("pumping in"), the delivery rate is less than for
diffusion alone and it rapidly approaches zero with increasing
current. For negative current ("pumping out"), the delivery
rate is larger than that for diffusion alone. In the linear
portion of the curve, the pump is operating by elec-trophoresis
primarily with diffusion not being significant.
A phenomenon called "polarization" generally prevents
the use of electrodes such as carbon to produce a direct
current although they would be acceptable with pulsed DC or
alternating current. As soon as current begins to flow, the
electrode products collect in the vicinity of the electrode
surface and create a back voltage. Sometimes this back voltage
is as high as the driving voltage and current will not flow.
We have developed a method whereby this problem can be avoided
in an electrophoretic pump. The degree of polarity depends
upon the current density in the electrode surface. The
half-time for decay of this polarity, however (with the
electrodes shorted) does not. Since the region of the polarity
about the electrode
~ - 15 -
. .
~2~ 715~8-20
surface is very thin, the decay time is very short . Thi6 means
that by making khe electrodes with a very high surf~ce area,
total curren-t can be high but current density at the electrode
surface kept low. Further, it means that polarization will
occur slowly and depolarization will be fast. Thus, we use a
pulsed DC power system (as described hereinafter) in the pump,
generating reasonable current while avoiding serious
polarization problems. The current will flow until the
polarization hits an upper limit, then will stop for a
relatively short time to allow depolariæation. Then, the cycle
will repeat. Our experiments with porous graphite electrodes
show that 5 milliamperes of current can flow for 1 minute
before the voltage rises to 1 volt. The electrodes depolarize
in about 10 seconds or less.
To create pulsed DC, the voltage across the membrane
may be monitored through a Comparator circuit. When the
voltage reaches a predetermined value, the Comparator output
changes. The Comparator output is used to turn off the DC
source for a predetermined time. When the time is exhausted,
the Comparator output is reset, and the cycle resumes from the
beginning. The circuit resembles in operation a free running
multivibrator (analog form) where the current charging a
capacitor while monitoring the voltage and starts to discharge
the capacitor when it reaches a certain value and stops
discharge when it reaches other values.
Shown in Figure 3 is the relationship between the
current resulting from a particular potential level applied to
the eleotrodes as plotted against the specific delivery rate of
- 16 -
., : ,
the ionic drug. Plot A is a theoretically developed
relationship. The minus current indication with respect to
current is to represent electrode polarity as shown in Figure 1
and the positive current is the reverse direction. When the
S current of the experimental system was at zero, diffusion was the
sole means of transport of ionic material out of the reservoir.
Ion transport was effected at the level B. In other words,
without energizing the electrodes an ionic flow equal to 0.5
units of the ionic drug, (which in the experimental case was
bovine serum albumen, which can readily be correlated with
insulin), per square centimeter of passive membrane area per hour
resulted. When a negative potential is imposed on the electrodes
a resultant current flows through the liquid between the
electrodes. As the magnitude of the current is increased in the
negative direction, the rate of delivery of the i~nic material
through the membrane is proportionately increased. When the
current reaches the level of -3 milliamperes the rate of delivery
increases from 0.5 units/cm2/hrs to 1 unit cm2/hr. Similarly,
when the polarity of the electrodes is reversed, the current
~0 imposes a retarding effect as electrophoresis action tends to
~oppose the;diffusion. As a result the net delivery rate drops
::
below the rate~for diffusion without any imposed current. It is
noted that the delivery rate did not reach zero even when the
current reached 6 milliamperes.
~ Referring now to Figure 4 there is shown the pump of
~ one embodlment of the present invention. It has an exterior
~: .
- ~ housing which is preferably sealed and provides suitable openings
or refilling and discharge of ions. The housing is preferably
17.
~:
~ 2~
made of a suitable inert material which will possess adequate
strength. Amon~ the suitable materials are titanium, medical
grade stainless steel and reinforced polymers. The size and
shape of the pump should be such that it is compatible with the
portions of the body in which it will be implanted. In the form
illustrated, the pump is generally triangular with rounded
corners and has a top wall 32, a lateral wall 34 and a bottom
wall (not shown) essentially identical to the top wall but
without an opening. The top wall has filler opening 36 and the
lateral wall defines discharge opening 38. In a preferred
embodiment of the invention the pump will have an overall length
L equal to about 90 mm to 110 mm, a height H equal to about lS mm
to 25 mm and width W equal to about 35 mm to 50 mm. Shown
positioned within the top opening 36 is a self-sealing filler
membrane 50 which will be described in greater detail
hereinafter. Disposed within discharge opening 38 is the passive
discharge membrane 52.
Referring now to Figures 5 and 6, the interior
arrangement of the pump will be considered in greater detail. In
general, in the form~illustrated, the pump interior has three
sections. The reservolr 56 is positioned at the narrow portion
of housing 30, the~electronic compartment 58 ls disposed adjacent
to~the reservoir 56 and the batteries 6n, 62 are positioned
within the enlarged;end of the housing. Electrical wires 64, 66
carry electricity from the batteries 60, 62 to the electronic
unit sa which includes a high value capacitor ton the order of
about 3 to 5 farads, for example). These capacitors function as
àn electrlcal energy reservoir. This is needed because lithium
18.
, :
.' ' ~,' "' ',
j59~
iodide batteries do not provide the required current during
periods of unusually high demand ("bolus") and they have a high
internal impedance. These capacitors would not necessarily be
required for batteries with low internal impedance such as Li-CuS
batteries, for example. Wires 68, 70 permit the batteries 60, 62
to energize the electrodes by way of electronics unit 58 (with
the electrical connection between the two not illustrated). As
is shown the wires 68, 70 are preferably wound around reservoir
56. These wires are used as antennas to transmit and receive
information from an external programmer. Internally the signals
may be fed through blocking capacitors to the communication
circuitry.
The reservoir 56 provides a sealed chamber for storage
of the ionic drugs. Preferably it has a storage capacity of
about 15 to 25 ml. In the form illustrated, the reservoir is of
generally cylindrical configuration and has an opening 72 in the
upper wall which sealingly receives filler membrane 50 and an
opening 74 in the Iateral wall which receives the passive~
membrane 52. As is shown in Figure 4, the membrane 50 is exposed
~0 through opening 36 in the top of the housing 32 and membrane 52
is exposed through opening 38 in the housing 32. In the form
illustrated the housing openings 36, 38 and the corresponding
resevoir openings 72, 74 are, respectively, disposed on surfaces
~oriented generally perpendicular to each other. This arrangement
prevents piercing of the delivery port by the filler needle,
thereby obviating the need for a needle target. For example,
assuming the embodiment illustrated in Figure 6 is surgically
implanted in the peritoneum of the patient with the membrane 50
~ ~: 19.
~ ~9~
closely adjacent to and facing the skin, a minimum path of travel
for a hypodermic syringe is provided for convenience in
filling. Also, discharge of ions through the opening 52 will
direct the ions into the surrounding tissue of the patient.
In order to minimize contact between the ionic drug or
ionic drug containing solution or slurry with the interior walls
of the reservoir 56 the interior may advantageously be provided
with a suitable coating material such as silicone rubber, for
example. A suitable material is that sold under the trade
designation Silastic. If desired, an immobilized ionic group
such as a suitable ion exchange resin may be employed to resist
precipitation and adhesion of the ionic drug to the reservoir
wall.
The present system is adapted to be operated at a low
current level. This provides several advantages. It enables the
use of batteries which under such service conditions will have a
very long life, thereby minimizing the frequency with which the
implantable device must be surgically removed in order to replace
the~batteries. Also, the pump housing may be maintained at the
desired small size. A suitable type of battery for use in the
present system is the lithium iodide cell which may have a
voltage of about 2.7 volts (beginning of life) to about 2.1 volts
` (end of life). ~These batteries have proven to be successful in
~ the~connea~tion with use in cardiac pacemakers. See generally,
~ The Lithium Iodide Cell - History by Dr. Alan A. Schneider et
~ ::
al.,~Medical Electronic ~ Data, January-February 1977, pp. 48-51.
..
20.
As the electronic means 58 may take the form of known
solid state systems or any alternate means which would be readily
apparent to ~hose skilled in the art, a detailed disclosure of
the same need not be provided herein. Also, numerous external
programmers are known to those skilled in the art. See generally
Christionsen et al., J. Clinical Lab. Invest. 41(z), pp. 674-654
(Nov. 1981); Geisen et al., Res. Exp. Med. (Bev); 179(2), pp.
103-111~ l9Bl; and W. Burns et al. Inn. Med. 36(17), pp. 625-627
(Sept. 1981). In general, such systems involve setting a basal
program based on timing principals such that the batteries 60, 62
of the present system will energize the electrodes adjacent
membrane 52 at a certain current level for a predetermined period
of time at predetermined times during the day. At times the
system will either be at an "off state~ wherein transport of ions
continues by diffusion only. Also, an emergency or larger demand
situation (bolus") may be provided for. In addition, in the
emergency situation the patient may activate the system so as to
provide a single cycle of operation at a predetermined emergency
or bolus level to dispense addltional quantiti~s of the ionic
drugs, soch as durin~ meal times, for example. One manner in
which this~might be accomplished is by having the patient hold a
magnet ln close proximity to the unit for a predetermined time.
~ As a convenient means for providing extra insulin, for
example to avert hyperglycemia, this externally activatible bolus
:~ :
~system may have a magnetic reed switch in the electrical circuit
of the~pump. ~The switch when in ~off" position may be moved to
the nonn position by application of a magnetic field of a
predetermined minimum intensity for a predetermined period This
21.
9~
. minimizes the risk of accidental triggering of the pump by stray
magnetic fields. In general, the programminy is so established
as to preclude emergency activation beyond the given number of
times within a twenty-four hour period in order to prevent
overdosing of the patient. It is also preferable, in general, to
have the programmer such that it can be programmed only by the
physician and not by the patient.
In providing insulin to a diabetic patient, for
example, the average daily dose will be about 1 unit /kg of body
weight. A unit of insulin is about 1/24 mg. A patient requires
about 40 to 80 units a day. The pump will be set to deliver a
portion of this by diffusion with the electrodes not energized
and a portion to be delivered electrophoretically, with possible
additional energizing amounts delivered by bolus action. The
reservoir will preferably hold about a 250 day supply for an
average patient.
~eferring once again to Figure 5, in the filler opening
72 a plurality of radially oriented reinforcement struts 78 are
preferahly molded into the membrane in order to reinforce the
same.
Figures 7 through 9 show further refinements of a
preferred reservoir arrangement. The reservoir 2 has the upper
wall 90 which contains opening 72 and the filler membrane 50. A
lateral wall 92 is provided with the discharge opening 74 and a
bottom wall ~not shown) which is substantially of the same size
and shape as the top wall 90 and may be imperforate. As is shown
in the exploded view of Figure 7, a collar member 94 is provided
with a tubular portion 96 and a lower radially extending flange
22~
.
6~
portion 98. The flange portion 98 is adapted to be secured to
the upper wall 90 such that the bore of tube 96 is generally
aligned with opening 72. After positioning of the membrane in
place and securement of the member 94, the upper portions of the
collar tube 96 are deformed downwardly and inwardly over the
membrane 50. This securement may be effected by any desired
means such as adhesive bonding. Collar 94 facilitates manual
location of membrane 50 for refilling.
Figure 9 shows a convenient means of creating the
membrane construction. A mold member 100 having an upwardly
facing recess is placed in underlying contacting relationship
with respect to wall 90. The strut members 78 are placed in the
desired reinforcing position and the membrane material in molten
state is poured into the assembly so as to create the desired
structure. This serves to seal the opening 72 while permitting
penetration therethrough by a hypodermic syringe employed to
refill the reservoir. As is shown in Figure 7, a membrane frame
110 is adapted to hold the electrode membrane assembly which
permits discharge of ions through opening 74.
~eferring to Figure 10, details of the preferred
assembly~ and the d~ischarge opening 74 will be considered. The
reservoir, which may advantageously be made of a suitable metal
such~ as titanium, has the protective titanium shroud 110 secured
to~the peripheral walI 92 thereof in surrounding relationship
with respect to the membrane 52. The assembly, which consists of
a pair of porous electrodes and interposed passive ionic membrane
which permits passage of ions, will be considered. A generally
channel shaped electrically insulative Çrame 112 which may be
.
~ 23
~.- :
/
9~
made of polytetraflouroethylene, secures a pair of metal frame
members 114, 116. A pair of carbon mesh electrodes 120, 122 are
positioned inwardly of the frame members 114, 116. The membrane
52 is separated from the electrodes 120, 122 by suitable annular
5 electrical insulators 124, 126. A suitable adhesive may serve to
both seal and secure the assembly lead. Electrical leads from the
battery 60, 62 by way of the electronics means 58 serve to
energize the electrodes with one lead functioning as a positive
electrode and the other as a negative electrode. In those
instances where a negative ionic drug is being dispensed by the
pump the innermost electrode 120 will have a negative charge and
the oatermost will have a positive charge. The electrodes will
produce an electrochemical potential gradient, as these ions
carry a net charge, they are subjected to forces which cause them
; 15 to move in the field. The anions move to the anode and cations
to the cathode.
Referring to Figures 11 through 13 a method of making a
porous electrode for use in the present invention-will be
consi~dered. The porous electrode provides the advantage of
increased surface area. A suitable electrically insulative
~support material~such as polytetrafluoruethylene, medical grade,
epoxy or~silicone rubber, for example, 120 is provided. A series
~f electrically conductive members 122, such as car~on fibers,
~: ,
preferably having a thickness of about l to lO microns are
positioned on the upper surface of support 120 with the fibers
being~generally parallel to each other. A second series of
electrically conductive fibers 124 are positioned in relative
~paral~lel relationship with respect to each other and are oriented
, ~ .
~ 24,
;9~
generally perpendicularly with respect to the other series df-
fibers 122. An electrically conductive frame member 130 composed
of a material such as platinum, medical grade stainless steel or
carbon paste, for example, is then secured in electrically
conductive overlying relationship with respect to underlying mesh
of fibers 122, 124. Lead wires 126, 128 from the electronics
unit 58 are electrically connected to the mesh of fibers 122, 124
through the conductive frame 130. A pair of such frames is used
in the construction shown in Figure 10.
Referring to Figure 14, an alternate embodiment of the
invention will now be considered. In this embodiment the
reservoir 140 rather than being positioned within the housing 142
is remotely positioned with respect thereto and is in electrical
communication with the electronics units and batteries disposed
within the housing 142 by means of electrical leads 144, 146. A
suitable covering layer 143 ~dotted) is provided on housing 142
and may be a silicone rubber, for example.
~As has been indicated hereinbefore, various means of
effecting electronically controlled triggering of the
electrophoretic~action to either boost or retard ionic drug
delivery~may be employed. A preferred approach of the present
invention;involves control of pumping of the ionic drug by means
~of~an~;essentially constant current applied to the electrodes for
~ ~a pr~edetermined time. The time-current combination may be
~ ~equated,~for a given ionic drug, with a predetermined quantity or
~; dosage to~be delivered. For example, if it has been determined
t~hat~O.lO~unlt of insulin will be delivered by the pump when the
curr`ent is~at a given current level such as about 1 to 10
,
::
~ . 25.
.
.,
~2 ~ 5~ 6 ~ 715~-20
milliamps, and the pump is energized for a given period of time
such as 25 to 35 seconds, the remote programmer will es-tablish
a 24 hour program for the pump wherein the given amount of
units desired to be delivered within that time will be
delivered by using multiples of the 0.10 unit. Further, this
is employed to space the dosage delivery in the desired
fashion.
Referring to Figure 15, a modular unit o the
electronic system of the pump and the paths of information flow
will be considered. The schematic illustrated may involve a
given unit of time such as two hours, for example, with twelve
duplicate units being provided to cover a day. In operation,
the programmer will send a radio signal which will be received
by the basal (regular) demand communication register 170 and
the bolus (unusual) communication register 176. The former
will be provided with a signal telling it how many pulses of
0.1 units of insulin to deliver with a given time period such
as two hours, for example. The latter will receive a signal
telling it how many pulses of 1.0 units of insulin to deliver
once it has been activated by the pa-tient as for example,
before a meal. The real time clock 210 serves to provide a
time reference for operation of the basal delivery system.
When the designated time for activation of the basal system has
been reached the information regarding the time period during
which the system will opera-te is transEerred from the basal
working reglster 172 by means of path 174. Each working
register 172, 178, 184 cooperates with an associated
communication register and communicates respectively by path
174, 180i 186 with registers 170, 176, 182, which in turn are
in receipt of signals from the external programmer.
.
.
~;,
This system will permit the batteries to energize the electrodes
so as to impose the current desired for the predetermined period
of time which will be governed by the real time clock 210. At
the end of the cycle of operation, energization of the electrodes
will be terminated. Information regarding the initial quantity
of the ionic drug present in the reservoir as well as the amount
di charged through basal or bolus action may be stored in a
separate counter. It is also desirable to have the programmer
receive, upon request, signals providing information regarding
the amount of material left in the reservoir. ThiS is determined
by counting the delivery pulses. This register is cleared only
after refilling of the reservoir in ordee to give a cumulative
reading of the amount of material remaining. In this way the
demands foe individual patients for insulin can be established.
. :~
The expressions Tbasal and ~bolus are the ti
required to deliver, respectively, the basal and bolus levels.
The times will generally consist of a certain number of pulses
such as, for example, 28 seconds for basal and 280 seconds for
bolus.
.
In a bolus situationj a magnetic field is applied to
the pump at a predetermined intensity for a given period of time
in~order~to initiate movement Oe the magnetic reed switch so as
to cause a signal to be transferred from bolus working register
178 to the output counter. Information is placed in the bolus
~flag communicatlon register 182 during programming and
~ tr~ansferred to the working register 178. The bolus flag working
,
register 184 permits the bolus system to be activated only a
predetermined number of times during the day in order to avoid
,
~ ~ 21.
the patient dispensing an excess amount of insulin by bolus
activation For example/ if it were determined that a patient
should be permitted a maximum of four bolus cycles per day, each
time the flag working register 184 is set by the programmer and
the magnet reed switch is activated by the patient, the bolus
delivery is initiated. The delivery is terminated after a
preselected number of pulses. The real time clock 210, is
connected to the respective working registers 172, 178, 184,
respectively by paths 196-200, 190-194-200 and 192-194-200. The
real time clock 210 will have a counter which serves to
automatically con~rol the duration of a bolus or basal cycle.
When the counter reaches zero the delivery by electrophoretic
action would be terminated.
If desired, a glucose sensor may be provided so as to
permit sensing of the glucose content of blood and providing a
servo control to adjust the operation of the pump for departures
from deslred glucose blood level ranges.
If~it is desired to provide a pump wherein diffusion is
either extinguished or substantially min:imized during periods
when the pump is not energized electrically, an active membrane
such as an ion exchange membrane, for example, may be used as the
dlffusion membrane.
~ While not preferred, if desirecl one could eliminate the
basal rate or reduce it to zero and rely solely on bolus
d~istribution.
It will be appreciated that the present invention
~provides~a self contained, refillable, externally programmable,
implantable ionic~drug diffusion device which is economical to
2Y,
::
' ~ ~ , . .
~L2~
use, will be of long duration through the low power required and
is of high reliability as a result of i~s lack of mechanical
moving parts which would be subject to wear and tear.
While for simplicity of disclosure reference has been
made previously to use of the pump with a single ionic drug, it
will be appreciated that it may be used with two or more
materials simultaneously.
Whereas particular embodiments of this invention have
been described above for purposes of illustration, it will be
evident to those skilled in the art that numerous variations of
the details may made without departing from tHe invention as
defined in the appended claims.
':
::
~:
'
::
: :
~: _
29
:
: